UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT UNDER SECTION 13 OR
15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended March 31, 2024
or
☐ TRANSITION REPORT UNDER SECTION 13 OR
15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ___________ to ___________
Commission File Number: 001-41512
SILO PHARMA, INC.
(Exact name of registrant as specified in its charter)
| Delaware | | 27-3046338 |
(State or other jurisdiction of
incorporation or organization) | | (IRS Employer
Identification No.) |
| 560 Sylvan Avenue, Suite 3160, Englewood Cliffs, New Jersey | | 07632 |
| (Address of principal executive offices) | | (Zip code) |
Registrant’s telephone number, including
area code: (718) 400-9031
Securities registered pursuant to Section 12(b)
of the Exchange Act:
| Title of each class | | Trading Symbol(s) | | Name of exchange on which registered |
| Common Stock, par value $0.0001 per share | | SILO | | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registration was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | Emerging growth company | ☐ |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Number of shares of common stock, par value $0.0001
per share, outstanding as of May 13, 2024 was: 2,803,386 (355,710 shares held in treasury).
SILO PHARMA, INC. AND SUBSIDIARY
FORM 10-Q
MARCH 31, 2024
TABLE OF CONTENTS
CAUTIONARY NOTE ON FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking
statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this
report, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects,
plans and objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking
statements by terminology such as “may,” “could,” “will,” “would,” “should,”
“expect,” “plan,”, “anticipate,” “believe,” “estimate,” “intend,”
“predict,” “seek,” “contemplate,” “project,” “continue,” “potential,”
“ongoing” or the negative of these terms or other comparable terminology.
Any forward-looking statements are qualified in
their entirety by reference to the risk factors discussed throughout this Quarterly Report on Form 10-Q. Some of the risks, uncertainties
and assumptions that could cause actual results to differ materially from estimates or projections contained in the forward-looking statements
include, but are not limited to:
| |
● |
our ability to obtain additional funds for our operations; |
| |
● |
our financial performance; |
| |
● |
risks relating to the timing and costs of clinical trials and the timing and costs of other expenses; |
| |
● |
risks related to market acceptance of products; |
| |
● |
intellectual property risks; |
| |
● |
the impact of government regulation and developments relating to our competitors or our industry; |
| |
● |
our competitive position; |
| |
● |
our industry environment; |
| |
● |
our anticipated financial and operating results, including anticipated sources of revenues; |
| |
● |
assumptions regarding the size of the available market, benefits of our products, product pricing and timing of product launches; |
| |
● |
our estimates of our expenses, losses, future revenue and capital requirements, including our needs for additional financing; |
| |
● |
our ability to attract and retain qualified key management and technical personnel; |
| |
● |
statements regarding our goals, intensions, plans and expectations, including the introduction of new products and markets; |
| |
● |
our cash needs and financing plans. |
These statements relate to future events or our
future operational or financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual
results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied
by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among
other things, those listed under the section titled “Risk Factors” and elsewhere in this report.
Any forward-looking statement in this report reflects
our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our
business, results of operations, industry and future growth. Given these uncertainties, you should not place undue reliance on these forward-looking
statements. No forward-looking statement is a guarantee of future performance. You should read this report completely and with the understanding
that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements.
This report also contains estimates, projections
and other information concerning our industry, our business and our markets, including data regarding the estimated size of those markets
and their projected growth rates. Information that is based on estimates, forecasts, projections or similar methodologies is inherently
subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information.
Unless otherwise expressly stated, we obtained these industry, business, market and other data from reports, research surveys, studies
and similar data prepared by third parties, industry, and general publications, government data and similar sources. While we believe
that the reports, research surveys, studies and similar data prepared by third parties are reliable, we have not independently verified
the data contained in them.
You are cautioned not to place undue reliance
on any forward-looking statements, which speak only as of the date of this report. Except as required by law, we do not undertake any
obligation to update or release any revisions to these forward-looking statements to reflect any events or circumstances, whether as
a result of new information, future events, changes in assumptions or otherwise, after the date hereof. New factors emerge from time
to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on
our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained
in any forward-looking statements. We qualify all of the information presented in this Quarterly Report on Form 10-Q, and particularly
our forward-looking statements, by these cautionary statements.
PART I –
FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
SILO PHARMA, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
| | |
March 31, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| | |
(Unaudited) | | |
| |
| ASSETS | |
| | |
| |
| CURRENT ASSETS: | |
| | |
| |
| Cash and cash equivalents | |
$ | 2,668,273 | | |
$ | 3,524,308 | |
| Short-term investments | |
| 4,075,849 | | |
| 4,140,880 | |
| Prepaid expenses and other current assets | |
| 133,764 | | |
| 15,970 | |
| | |
| | | |
| | |
| Total Current Assets | |
| 6,877,886 | | |
| 7,681,158 | |
| | |
| | | |
| | |
| Prepaid expenses and other assets - non-current | |
| 63,523 | | |
| 64,983 | |
| | |
| | | |
| | |
| Total Assets | |
$ | 6,941,409 | | |
$ | 7,746,141 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| CURRENT LIABILITIES: | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 801,570 | | |
$ | 703,488 | |
| Deferred revenue - current portion | |
| 72,102 | | |
| 72,102 | |
| | |
| | | |
| | |
| Total Current Liabilities | |
| 873,672 | | |
| 775,590 | |
| | |
| | | |
| | |
| LONG TERM LIABILITIES: | |
| | | |
| | |
| Deferred revenue - long-term portion | |
| 775,654 | | |
| 793,680 | |
| | |
| | | |
| | |
| Total Long Term Liabilities | |
| 775,654 | | |
| 793,680 | |
| | |
| | | |
| | |
| Total Liabilities | |
| 1,649,326 | | |
| 1,569,270 | |
| | |
| | | |
| | |
| Commitment and Contingencies (see Note 7) | |
| | | |
| | |
| | |
| | | |
| | |
| STOCKHOLDERS’ EQUITY: | |
| | | |
| | |
| Preferred stock, $0.0001 par value, 5,000,000 shares authorized: none designated as of March 31, 2024 and December 31, 2023 | |
| | | |
| | |
| Common stock, $0.0001 par value, 100,000,000 shares authorized; 3,159,096 and 3,159,096 shares issued and 2,833,451 and 2,906,241 shares outstanding at March 31, 2024 and December 31, 2023, respectively | |
| 316 | | |
| 316 | |
| Additional paid-in capital | |
| 17,525,714 | | |
| 17,525,714 | |
| Treasury stock, at cost (325,645 and 252,855 shares on March 31, 2024 and December 31, 2023, respectively) | |
| (586,573 | ) | |
| (471,121 | ) |
| Accumulated other comprehensive income (loss) | |
| 26,104 | | |
| (6,227 | ) |
| Accumulated deficit | |
| (11,673,478 | ) | |
| (10,871,811 | ) |
| | |
| | | |
| | |
| Total Stockholders’ Equity | |
| 5,292,083 | | |
| 6,176,871 | |
| | |
| | | |
| | |
| Total Liabilities and Stockholders’ Equity | |
$ | 6,941,409 | | |
$ | 7,746,141 | |
See accompanying notes to unaudited consolidated financial statements.
SILO PHARMA, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Unaudited)
| | |
For the Three Months Ended | |
| | |
March 31, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| LICENSE FEE REVENUE | |
$ | 18,026 | | |
$ | 18,026 | |
| | |
| | | |
| | |
| COST OF REVENUES | |
| 1,460 | | |
| 1,460 | |
| | |
| | | |
| | |
| GROSS PROFIT | |
| 16,566 | | |
| 16,566 | |
| | |
| | | |
| | |
| OPERATING EXPENSES: | |
| | | |
| | |
| Compensation expense | |
| 173,346 | | |
| 162,257 | |
| Professional fees | |
| 318,427 | | |
| 365,270 | |
| Research and development | |
| 318,240 | | |
| 202,913 | |
| Insurance expense | |
| 21,704 | | |
| 24,645 | |
| Selling, general and administrative expenses | |
| 64,362 | | |
| 64,966 | |
| | |
| | | |
| | |
| Total operating expenses | |
| 896,079 | | |
| 820,051 | |
| | |
| | | |
| | |
| LOSS FROM OPERATIONS | |
| (879,513 | ) | |
| (803,485 | ) |
| | |
| | | |
| | |
| OTHER INCOME (EXPENSE): | |
| | | |
| | |
| Interest and dividend income, net | |
| 88,177 | | |
| 64,388 | |
| Interest expense | |
| (1,870 | ) | |
| (1,655 | ) |
| Net realized gain on short-term investments | |
| 234 | | |
| - | |
| Penalty from early termination of certificate of deposit | |
| - | | |
| (166,034 | ) |
| Net unrealized gain on equity investments | |
| - | | |
| 390 | |
| Foreign currency transaction loss | |
| (8,695 | ) | |
| - | |
| | |
| | | |
| | |
| Total other income (expense) | |
| 77,846 | | |
| (102,911 | ) |
| | |
| | | |
| | |
| LOSS BEFORE PROVISION FOR INCOME TAXES | |
| (801,667 | ) | |
| (906,396 | ) |
| | |
| | | |
| | |
| Provision for income taxes | |
| - | | |
| - | |
| | |
| | | |
| | |
| NET LOSS | |
$ | (801,667 | ) | |
$ | (906,396 | ) |
| | |
| | | |
| | |
| COMPREHENSIVE LOSS: | |
| | | |
| | |
| Net loss | |
$ | (801,667 | ) | |
$ | (906,396 | ) |
| | |
| | | |
| | |
| Other comprehensive income (loss): | |
| | | |
| | |
| Unrealized gain on short-term investments | |
| 32,331 | | |
| 5,239 | |
| | |
| | | |
| | |
| Total comprehensive loss | |
$ | (769,336 | ) | |
$ | (901,157 | ) |
| | |
| | | |
| | |
| NET LOSS PER COMMON SHARE: | |
| | | |
| | |
Basic and diluted | |
$ | (0.28 | ) | |
$ | (0.29 | ) |
| | |
| | | |
| | |
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | |
| | | |
| | |
Basic and diluted | |
| 2,866,934 | | |
| 3,158,797 | |
See accompanying notes to unaudited consolidated financial statements.
SILO PHARMA, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE THREE MONTHS ENDED MARCH 31, 2024 AND 2023
(Unaudited)
| | |
| | |
| | |
Additional | | |
| | |
| | |
Accumulated Other | | |
| | |
Total | |
| | |
Common Stock | | |
Paid In | | |
Treasury Stock | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Shares | | |
Amount | | |
Income (Loss) | | |
Deficit | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, December 31, 2023 | |
| 3,159,096 | | |
$ | 316 | | |
$ | 17,525,714 | | |
| 252,855 | | |
$ | (471,121 | ) | |
$ | (6,227 | ) | |
$ | (10,871,811 | ) | |
$ | 6,176,871 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Purchase of treasury stock | |
| - | | |
| - | | |
| - | | |
| 72,790 | | |
| (115,452 | ) | |
| - | | |
| - | | |
| (115,452 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accumulated other comprehensive loss - short-term investments | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 32,331 | | |
| - | | |
| 32,331 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (801,667 | ) | |
| (801,667 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, March 31, 2024 | |
| 3,159,096 | | |
$ | 316 | | |
$ | 17,525,714 | | |
| 325,645 | | |
$ | (586,573 | ) | |
$ | 26,104 | | |
$ | (11,673,478 | ) | |
$ | 5,292,083 | |
| | |
| | |
| | |
Additional | | |
| | |
| | |
Accumulated Other | | |
| | |
Total | |
| | |
Common Stock | | |
Paid In | | |
Treasury Stock | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Shares | | |
Amount | | |
Income | | |
Deficit | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, December 31, 2022 | |
| 3,158,797 | | |
$ | 316 | | |
$ | 17,511,589 | | |
| - | | |
$ | - | | |
$ | - | | |
$ | (7,171,128 | ) | |
$ | 10,340,777 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accretion of stock options expense to stock based compensation | |
| - | | |
| - | | |
| 4,237 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 4,237 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accumulated other comprehensive loss - short-term investments | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 5,239 | | |
| - | | |
| 5,239 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (906,396 | ) | |
| (906,396 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, March 31, 2023 | |
| 3,158,797 | | |
$ | 316 | | |
$ | 17,515,826 | | |
| - | | |
$ | - | | |
$ | 5,239 | | |
$ | (8,077,524 | ) | |
$ | 9,443,857 | |
See accompanying notes to unaudited consolidated financial statements.
SILO PHARMA, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| | |
For the Three Months Ended | |
| | |
March 31, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| CASH FLOWS FROM OPERATING ACTIVITIES: | |
| | |
| |
| Net loss | |
$ | (801,667 | ) | |
$ | (906,396 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities | |
| | | |
| | |
| Stock-based compensation and professional fees | |
| - | | |
| 4,237 | |
| Amortization of prepaid stock-based professional fees | |
| - | | |
| 33,775 | |
| Net realized gain on short-term investments | |
| (234 | ) | |
| - | |
| Net unrealized gain on equity investments | |
| - | | |
| (390 | ) |
| Change in operating assets and liabilities: | |
| | | |
| | |
| Prepaid expenses and other current assets | |
| (116,334 | ) | |
| (100,769 | ) |
| Interest receivable | |
| - | | |
| (1,183 | ) |
| Accounts payable and accrued expenses | |
| 98,082 | | |
| 296,064 | |
| Deferred revenue | |
| (18,026 | ) | |
| (18,026 | ) |
| | |
| | | |
| | |
| NET CASH USED IN OPERATING ACTIVITIES | |
| (838,179 | ) | |
| (692,688 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | |
| | | |
| | |
| Proceeds from sale of short-term investments | |
| 150,389 | | |
| - | |
| Purchase of short-term investments | |
| (52,793 | ) | |
| (6,008,339 | ) |
| | |
| | | |
| | |
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | |
| 97,596 | | |
| (6,008,339 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | |
| | | |
| | |
| Purchase of treasury stock | |
| (115,452 | ) | |
| - | |
| | |
| | | |
| | |
| NET CASH USED IN FINANCING ACTIVITIES | |
| (115,452 | ) | |
| - | |
| | |
| | | |
| | |
| NET DECREASE IN CASH AND CASH EQUIVALENTS | |
| (856,035 | ) | |
| (6,701,027 | ) |
| | |
| | | |
| | |
| CASH AND CASH EQUIVALENTS - beginning of the period | |
| 3,524,308 | | |
| 11,367,034 | |
| | |
| | | |
| | |
| CASH AND CASH EQUIVALENTS - end of the period | |
$ | 2,668,273 | | |
$ | 4,666,007 | |
| | |
| | | |
| | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | |
| | | |
| | |
| Cash paid during the period for: | |
| | | |
| | |
| Interest | |
$ | 1,870 | | |
$ | 167,689 | |
| Income taxes | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Non-cash investing and financing activities: | |
| | | |
| | |
| Change in accumulated other comprehensive income | |
$ | 32,331 | | |
$ | 5,239 | |
See accompanying notes to unaudited consolidated financial statements.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
NOTE 1 – ORGANIZATION AND BUSINESS
Silo Pharma, Inc. (the “Company”)
was incorporated in the State of New York on July 13, 2010, under the name Gold Swap, Inc. On January 24, 2013, the Company changed its
state of incorporation from New York to Delaware. On December 19, 2023, the Company changed its state of incorporation from the State
of Delaware to the State of Nevada.
The Company is a developmental stage biopharmaceutical
company focused on merging traditional therapeutics with psychedelic research. The Company seeks to acquire and/or develop intellectual
property or technology rights from leading universities and researchers to treat rare diseases, including the use of psychedelic drugs,
such as psilocybin, and the potential benefits they may have in certain cases involving depression, mental health issues and neurological
disorders. The Company is focused on merging traditional therapeutics with psychedelic research for people suffering from indications
such as depression, post-traumatic stress disorder (“PTSD”), Alzheimer’s, Parkinson’s, and other rare neurological
disorders. The Company’s mission is to identify assets to license and fund the research which the Company believes will be transformative
to the well-being of patients and the health care industry. The Company was previously engaged in the development of a streetwear
apparel brand, NFID (see below).
On May 21, 2019, the Company filed an amendment
to its Certificate of Incorporation with the State of Delaware to change its name from Point Capital, Inc. to Uppercut Brands, Inc. Thereafter,
on September 24, 2020, the Company filed an amendment to its Certificate of Incorporation with the State of Delaware to change its name
from Uppercut Brands, Inc. to Silo Pharma, Inc.
On April 8, 2020, the Company incorporated a new
wholly-owned subsidiary, Silo Pharma Inc., in the State of Florida. The Company has also secured the domain name www.silopharma.com.
The Company had been exploring opportunities to expand the Company’s business by seeking to acquire and/or develop intellectual
property or technology rights from leading universities and researchers to treat rare diseases, including the use of psychedelic drugs,
such as psilocybin, and the potential benefits they may have in certain cases involving depression, mental health issues and neurological
disorders. In July 2020, through the Company’s newly formed subsidiary, the Company entered into a commercial evaluation license
and option agreement with University of Maryland, Baltimore (“UMB”) (see Note 7) pursuant to which, among other things, UMB
granted the Company an exclusive, option to negotiate and obtain an exclusive, sublicensable, royalty-bearing license to certain technology.
The option was extended and exercised on January 13, 2021. On February 12, 2021, the Company entered into a Master License Agreement with
UMB (see Note 7). The Company plans to actively pursue the acquisition and/or development of intellectual property or technology rights
to treat rare diseases, and to ultimately expand the Company’s business to focus on this new line of business.
On September 30, 2021, the Company entered
into and closed on an Asset Purchase Agreement (the “Asset Purchase Agreement) with NFID, LLC, a Florida limited liability
company (the “Buyer”), whereby the Buyer purchased from the Company certain assets, properties, and rights in connection
with the Company’s NFID trademark name, logos, domain, and apparel clothing and accessories for a purchase price of $60,000 in
the form of a promissory note amounting to $60,000. The promissory note bore 8% interest per annum and matured on October 1, 2023.
On November 8, 2023 and effective on October 1, 2023, the Company and the Buyer entered into a First Amendment Promissory Note which
increased the interest rate to 9% per annum and extended the maturity date to December 30, 2023 for no consideration. On December
30, 2023, the buyer defaulted on the promissory note (See Note 4).
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING
POLICIES
Basis of Presentation and Principles of Consolidation
The accompanying unaudited consolidated financial
statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America
(the “U.S. GAAP”) for interim financial information and with the instructions Article 8-03 of Regulation S-X. Operating results
for interim periods are not necessarily indicative of results that may be expected for the fiscal year as a whole. Certain information
and note disclosure normally included in financial statements prepared in accordance with U.S. GAAP has been condensed or omitted from
these statements pursuant to such accounting principles and, accordingly, they do not include all the information and notes necessary
for comprehensive financial statements. These unaudited consolidated financial statements should be read in conjunction with the summary
of significant accounting policies and notes to the consolidated financial statements for the year ended December 31, 2023 included in
the Company’s Annual Report on Form 10-K as filed with the Securities and Exchange Commission on March 25, 2024.
The Company’s consolidated financial statements
include financial statements for Silo Pharma, Inc. and its inactive wholly-owned subsidiary with the same name as the parent entity, Silo
Pharma, Inc. All intercompany transactions and balances have been eliminated in consolidation. Management acknowledges its responsibility
for the preparation of the accompanying unaudited consolidated financial statements which reflect all adjustments, consisting of normal
recurring and non-recurring adjustments, considered necessary in its opinion for a fair statement of its consolidated financial position
and the consolidated results of its operations for the periods presented.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
Liquidity
As reflected in the accompanying unaudited consolidated
financial statements, the Company generated a net loss of $801,667 and used cash in operations of $838,179 during the three months ended
March 31, 2024. Additionally, the Company has an accumulated deficit of $11,673,478 on March 31, 2024. As of March 31, 2024, the Company
had working capital of $6,004,214.
The positive working capital serves to mitigate
the conditions that historically raised substantial doubt about the Company’s ability to continue as a going concern. The Company
believes that the Company has sufficient cash and liquid short-term investments to meet its obligations for a minimum of twelve months
from the date of this filing.
Use of Estimates
The preparation of consolidated financial statements
in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities
and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses
during the reporting period. Making estimates requires management to exercise significant judgment. It is at least reasonably possible
that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements,
which management considered in formulating its estimate could change in the near term due to one or more future events. Accordingly, the
actual results could differ significantly from estimates. Significant estimates during the three months ended March 31, 2024 and 2023
include the collectability of notes receivable, the percentage of completion of research and development projects, valuation of equity
investments, valuation allowances for deferred tax assets, and the fair value of shares and stock options issued for services.
Cash and Cash Equivalents
The Company considers all highly liquid investments
with a maturity of three months or less when acquired to be cash equivalents. The Company places its cash with high credit quality financial
institutions. The Company’s accounts at these institutions are insured by the Federal Deposit Insurance Corporation (“FDIC”)
up to $250,000 or by the Securities Investor Protection Corporation up to $250,000. To reduce its risk associated with the failure of
such financial institutions, the Company evaluates at least annually the rating of the financial institutions in which it holds deposits.
On March 31, 2024 and December 31, 2023, the Company had cash in excess of FDIC limits of approximately $1,971,000 and $2,805,000, respectively.
In connection with the early termination of a certificate of deposit, during the three months ended March 31, 2023, the Company paid a
penalty of $166,034, which is reflected on the accompanying unaudited consolidated statement of operations and comprehensive loss. Any
material loss that we may experience in the future could have an adverse effect on our ability to pay our operational expenses or make
other payments.
Short-Term Investments
The Company’s portfolio of short-term investments
consists of marketable debt securities which are comprised solely of highly rated U.S. government securities with maturities of more than
three months, but less than one year. The Company classifies these as available-for-sale at purchase date and will reevaluate such designation
at each period end date. The Company may sell these marketable debt securities prior to their stated maturities depending upon changing
liquidity requirements. These debt securities are classified as current assets in the unaudited consolidated balance sheet and recorded
at fair value, with unrealized gains or losses included in accumulated other comprehensive income and as a component of the unaudited
consolidated statements of comprehensive loss. Gains and losses are recognized when realized. Gains and losses are determined using the
specific identification method and are reported in other income (expense), net in the unaudited consolidated statements of operations
and comprehensive loss.
An impairment loss may be recognized when the
decline in fair value of the debt securities is determined to be other-than-temporary. The Company evaluates its investments for other-than-temporary
declines in fair value below the cost basis each quarter, or whenever events or changes in circumstances indicate that the cost basis
of the short-term investments may not be recoverable. The evaluation is based on a number of factors, including the length of time and
the extent to which the fair value has been below the cost basis, as well as adverse conditions related specifically to the security,
such as any changes to the credit rating of the security and the intent to sell or whether the Company will more likely than not be required
to sell the security before recovery of its amortized cost basis.
The Company recorded $32,331 and $5,239 of unrealized
income on short-term investments as a component of other comprehensive income (loss) for the three months ended March 31, 2024 and 2023,
respectively.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
Equity Investments, at Fair Value
Realized gain or loss is recognized when an investment
is disposed of and is computed as the difference between the Company’s carrying value and the net proceeds received from such disposition.
Realized gains and losses on investment transactions are determined by specific identification. Net unrealized gains or losses are computed
as the difference between the fair value of the investment and the cost basis of such investment. Net unrealized gains or losses for equity
investments are recognized in operations as the difference between the carrying value at the beginning of the period and the fair value
at the end of the period.
Note Receivable
The Company recognizes an allowance for losses
on notes receivable in an amount equal to the estimated probable losses net of recoveries. The allowance is based on an analysis of historical
bad debt experience, current note receivable aging, and expected future write-offs, as well as an assessment of specific identifiable
accounts considered at risk or uncollectible. The expense associated with the allowance for doubtful accounts is recorded as part of general
and administrative expenses. As of December 31, 2023, the Company recognized an allowance for loss on the note receivable and accrued
interest receivable in an amount equal to the estimated probable losses, and accordingly, the Company recorded bad debt expense of $69,600,
which represents the note receivable principal balance of $60,000 and accrued interest receivable of $9,600. As of March 31, 2024, there
were no subsequent collections of previously written-off notes receivable.
Prepaid Expenses
Prepaid expenses and other current assets of $133,764
and $15,970 on March 31, 2024 and December 31, 2023, respectively, consist primarily of costs paid for future services which will occur
within a year. On March 31, 2024 and December 31, 2023, prepaid expenses and other assets – non-current amounted to $63,523 and
$64,983, respectively, and consist primarily of costs paid for future services which will occur after a year. Prepaid expenses may include
prepayments in cash and equity instruments for consulting, research and development, license fees, public relations and business advisory
services, and legal fees which are being amortized over the terms of their respective agreements, which may exceed a year of service.
Revenue Recognition
The Company applies ASC Topic 606, Revenue from
Contracts with Customers (“ASC 606”). ASC 606 establishes a single comprehensive model for entities to use in accounting for
revenue arising from contracts with customers and supersedes most of the existing revenue recognition guidance. This standard requires
an entity to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those goods or services and also requires certain additional disclosures.
The Company records interest and dividend income
on an accrual basis to the extent that the Company expects to collect such amounts.
For the license and royalty income, revenue is
recognized when the Company satisfies the performance obligation based on the related license agreement. Payments received from the licensee
that are related to future periods are recorded as deferred revenue to be recognized as revenues over the term of the related license
agreement (see Note 7).
Cost of Revenues
The primary components of cost of revenues on
license fees includes the cost of the license fees. Payments made to the licensor that are related to future periods are recorded as prepaid
expense to be amortized over the term of the related license agreement (see Note 7).
Stock-Based Compensation
Stock-based compensation is accounted for based
on the requirements of ASC 718 – “Compensation – Stock Compensation”, which requires recognition in the financial
statements of the cost of employee, director, and non-employee services received in exchange for an award of equity instruments over the
period the employee, director, or non-employee is required to perform the services in exchange for the award (presumptively, the vesting
period). The ASC also requires measurement of the cost of employee, director, and non-employee services received in exchange for an award
based on the grant-date fair value of the award. The Company has elected to recognize forfeitures as they occur as permitted under Accounting
Standards Update (“ASU”) 2016-09 Improvements to Employee Share-Based Payment.
Income Taxes
Deferred income tax assets and liabilities arise
from temporary differences between the financial statements and tax basis of assets and liabilities, as measured by the enacted tax rates,
which are expected to be in effect when these differences reverse. Deferred tax assets and liabilities are classified as current or non-current,
depending upon the classification of the asset or liabilities to which they relate. Deferred tax assets and liabilities not related to
an asset or liability are classified as current or non-current depending on the periods in which the temporary differences are expected
to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
The Company follows the provisions of Financial
Accounting Standards Board (“FASB”) ASC 740-10, “Uncertainty in Income Taxes”. Certain recognition thresholds
must be met before a tax position is recognized in the financial statements. An entity may only recognize or continue to recognize tax
positions that meet a “more-likely-than-not” threshold. The Company does not believe it has any uncertain tax positions as
of March 31, 2024 and December 31, 2023 that would require either recognition or disclosure in the accompanying unaudited consolidated
financial statements.
Research and Development
In accordance with ASC 730-10, “Research
and Development-Overall,” research and development costs are expensed when incurred. During the three months ended March 31,
2024 and 2023, research and development costs were $318,240 and $202,913, respectively.
Leases
Leases are accounted for using ASU 2016-02, “Leases
(Topic 842)”. ASU 2016-02 sets out the principles for the recognition, measurement, presentation and disclosure of leases for
both parties to a contract (i.e., lessees and lessors). The standard requires lessees to apply a dual approach, classifying leases as
either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee.
This classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis
over the term of the lease. A lessee is also required to recognize a right-of-use asset and a lease liability for all leases with a term
of greater than 12 months regardless of their classification. Leases with a term of 12 months or less will be accounted for similar to
existing guidance for operating leases today. As of March 31, 2024 and December 31, 2023, the Company has no leases. The Company will
analyze any lease to determine if it would be required to record a lease liability and a right of use asset on its unaudited consolidated
balance sheets at fair value upon adoption of ASU 2016-02. The Company has elected not to recognize right-of-use assets and lease liabilities
for short-term leases that have a term of 12 months or less.
Net Loss per Common Share
Basic loss per share is computed by dividing net
loss allocable to common shareholders by the weighted average number of shares of common stock outstanding during each period. Diluted
loss per share is computed by dividing net loss available to common shareholders by the weighted average number of shares of common stock,
common stock equivalents and potentially dilutive securities outstanding during the period using the as-if converted method. Potentially
dilutive securities which include stock options and stock warrants are excluded from the computation of diluted shares outstanding if
they would have an anti-dilutive impact on the Company’s net losses.
The following potentially dilutive shares have
been excluded from the calculation of diluted net loss per share as their effect would be anti-dilutive for the three months ended March
31, 2024 and 2023:
| |
|
March 31, |
|
March 31, |
|
| |
|
2024 |
|
2023 |
|
| Stock options |
|
|
28,850 |
|
|
28,850 |
|
| Warrants |
|
|
404,580 |
|
|
404,580 |
|
| |
|
|
433,430 |
|
|
433,4230 |
|
Recent Accounting Pronouncements
Management does not believe that any recently
issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the Company’s unaudited consolidated
financial statements.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
NOTE 3 – FAIR VALUE OF FINANCIAL INSTRUMENTS
AND FAIR VALUE MEASUREMENTS
Fair Value Measurements and Fair Value of Financial
Instruments
FASB ASC 820 - Fair Value Measurements and
Disclosures, defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date. FASB ASC 820 requires disclosures about the fair value of all financial
instruments, whether or not recognized, for financial statement purposes. Disclosures about the fair value of financial instruments are
based on pertinent information available to the Company on March 31, 2024 and December 31, 2023. Accordingly, the estimates presented
in these financial statements are not necessarily indicative of the amounts that could be realized on disposition of the financial instruments.
FASB ASC 820 specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or
unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions.
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement)
and the lowest priority to unobservable inputs (Level 3 measurement).
| |
Level 1 - |
Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| |
|
|
| |
Level 2 - |
Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| |
|
|
| |
Level 3 - |
Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The carrying value of certain financial instruments,
including cash and cash equivalents, prepaid expenses and other current assets, notes receivable, and accounts payable and accrued expenses
are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments.
The Company analyzes all financial instruments
with features of both liabilities and equity under the Financial Accounting Standard Board’s (the “FASB”) accounting
standard for such instruments. Under this standard, financial assets and liabilities are classified in their entirety based on the lowest
level of input that is significant to the fair value measurement.
The following table represents the Company’s
fair value hierarchy of its financial assets and liabilities measured at fair value on a recurring basis as of March 31, 2024 and December
31, 2023.
| | |
March 31, 2024 | | |
December 31, 2023 | |
| Description | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Short-term investments | |
$ | 4,075,849 | | |
$ | - | | |
$ | - | | |
$ | 4,140,880 | | |
$ | - | | |
$ | - | |
The Company’s short-term investments and
equity investments are level 1 measurements and are based on redemption value at each date.
Short-Term Investments – Debt Securities,
at Fair Value
The following table summarizes activity in the
Company’s short-term investments, at fair value for the periods presented:
| | |
Three Months
Ended
March 31, | | |
Three Months
Ended March 31, | |
| | |
2024 | | |
2023 | |
| Balance, beginning of period | |
$ | 4,140,880 | | |
$ | - | |
| Additions | |
| 52,793 | | |
| 6,008,339 | |
| Sales at original cost | |
| (150,389 | ) | |
| - | |
| Net realized gain on short-term investments | |
| 234 | | |
| - | |
| Realized and Unrealized gains | |
| 32,331 | | |
| 5,239 | |
| Balance, end of period | |
$ | 4,075,849 | | |
$ | 6,013,578 | |
ASC 825-10 “Financial Instruments”
allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair
value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value
option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent
reporting date. The Company did not elect to apply the fair value option to any outstanding equity instruments.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
NOTE 4 – NOTE RECEIVABLE
On March 31, 2024 and December 31, 2023, note receivable consisted
of the following:
| | |
March 31, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Principal amount of note receivable | |
$ | 60,000 | | |
$ | 60,000 | |
| Accrued interest receivable | |
| 9,600 | | |
| 9,600 | |
| Subtotal | |
| 69,600 | | |
| 69,600 | |
| Less: allowance for doubtful accounts | |
| (69,600 | ) | |
| (69,600 | ) |
| Note receivable – current | |
| - | | |
| - | |
As of December 31, 2023, the Company recognized
an allowance for loss on the note receivable and accrued interest receivable in an amount equal to the estimated probable losses of $69,600,
which represents the note receivable principal balance of $60,000 and accrued interest receivable of $9,600. As of March 31, 2024, there
were no subsequent collections of previously written-off notes receivable.
NOTE 5 – STOCKHOLDERS’ EQUITY
Shares Authorized
On December 4, 2023, stockholders of the Company
approved a decrease to the number of authorized shares of the Company’s common stock from 500,000,000 shares to 100,000,000 shares.
On December 4, 2023, the Company filed a Certificate of Amendment (the “Amendment”) to its Certificate of Incorporation with
the Delaware Secretary of State to decrease its authorized shares of common stock from 500,000,000 shares to 100,000,000 shares. On December
19, 2023, the Company reincorporated as a Nevada corporation and filed Articles of Incorporation with the Nevada Secretary of State on
such date. The Company has 105,000,000 shares authorized which consist of 100,000,000 shares of common stock and 5,000,000 shares of preferred
stock.
Common Stock Issued for Services
On August 29, 2022, the Company entered into a
one-year consulting agreement with an entity for investor relations services. In connection with this consulting agreement, the Company
issued 20,000 restricted common shares of the Company to the consultant. These shares vest immediately. These shares were valued at $135,100,
or $6.755 per common share, based on contemporaneous common share sales by the Company. In connection with this consulting agreement,
during the three months ended March 31, 2024 and 2023, the Company recorded stock-based professional fees of $0 and $33,775, respectively.
Stock Repurchase Plan
On January 26, 2023, the Company’s Board
of Directors authorized a stock repurchase plan to repurchase up to $1 million of the Company’s issued and outstanding common
stock, from time to time, with such plan to be in place until December 31, 2023. On January 9, 2024, the Board of Directors of the Company
approved an extension of the previously announced stock repurchase program authorizing the purchase of up to $1 million of the Company’s
common stock until March 31, 2024 and on April 4, 2024, the stock Repurchase Plan was extended to April 30, 2024. During the year ended
December 31, 2023, the Company purchased 252,855 shares of common stock for a cost of $471,121, which is reflected in treasury stock on
the accompanying unaudited consolidated balance sheet. During the three months ended March 31, 2024, the Company purchased 72,790 shares
of common stock for a cost of $115,452. As of March 31, 2024, the Company have repurchased an aggregate of 325,645 shares of its common
stock for a total cost of $586,573 pursuant to its Stock Repurchase Program.
Stock Options
On January 18, 2021, the Company’s board
of directors (“Board”) approved the Silo Pharma, Inc. 2020 Omnibus Equity Incentive Plan (the “2020 Plan”) to
incentivize employees, officers, directors and consultants of the Company and its affiliates. 170,000 shares of common stock are reserved
and available for issuance under the 2020 Plan, provided that certain exempt awards (as defined in the 2020 Plan), shall not count against
such share limit. The 2020 Plan provides for the grant, from time to time, at the discretion of the Board or a committee thereof, of cash,
stock options, including incentive stock options and nonqualified stock options, restricted stock, dividend equivalents, restricted stock
units, stock appreciation units and other stock or cash-based awards. The 2020 Plan shall terminate on the tenth anniversary of the
date of adoption by the Board. Subject to certain restrictions, the Board may amend or terminate the Plan at any time and for any reason.
An amendment of the 2020 Plan shall be subject to the approval of the Company’s stockholders only to the extent required by applicable
laws, rules or regulations. On March 10, 2021, the 2020 Plan was approved by the stockholders. On September 15, 2023, our Board of
Directors adopted the Silo Pharma, Inc. Amended and Restated 2020 Omnibus Equity Incentive Plan which was approved by the Company’s
stockholders on December 4, 2023. The Amended and Restated Omnibus Equity Incentive Plan (i) increases the number of shares of common
stock that may be issued under such plan by 300,000 shares to 470,000 shares and (ii) includes clawback provisions to comply with recent
developments of applicable law.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
During the three months ended March 31, 2024 and
2023, the Company amortized $0 and $4,237 of the deferred compensation which was recorded as compensation expense in the accompanying
unaudited consolidated statement of operations and comprehensive loss, respectively. As of March 31, 2024 and December 31, 2023, there
were no remaining deferred compensation costs.
Stock option activities for the three months ended
March 31, 2024 are summarized as follows:
| |
|
Number of
Options |
|
|
Weighted
Average
Exercise
Price |
|
|
Weighted
Average
Remaining
Contractual Term
(Years) |
|
|
Aggregate
Intrinsic
Value |
|
| Balance Outstanding, December 31, 2023 |
|
|
28,850 |
|
|
$ |
7.28 |
|
|
|
5.31 |
|
|
$ |
8,610 |
|
| Granted |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
| Balance Outstanding, March 31, 2024 |
|
|
28,850 |
|
|
$ |
7.28 |
|
|
|
5.06 |
|
|
$ |
11,669 |
|
| Exercisable, March 31, 2024 |
|
|
28,850 |
|
|
$ |
7.28 |
|
|
|
5.06 |
|
|
$ |
11,669 |
|
Stock Warrants
Warrant activities for the three months ended
March 31, 2024 are summarized as follows:
| | |
Number of
Warrants | | |
Weighted
Average
Exercise
Price | | |
Weighted
Average
Remaining
Contractual Term
(Years) | | |
Aggregate
Intrinsic
Value | |
| Balance Outstanding, December 31, 2023 | |
| 404,580 | | |
$ | 14.05 | | |
| 2.31 | | |
$ | - | |
| Granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Balance Outstanding, March 31, 2024 | |
| 404,580 | | |
$ | 14.05 | | |
| 2.06 | | |
$ | - | |
| Exercisable, March 31, 2024 | |
| 404,580 | | |
$ | 14.05 | | |
| 2.06 | | |
$ | - | |
NOTE 6 – CONCENTRATIONS
Customer concentration
For the three months ended March 31, 2024 and
2023, one licensee accounted for 100% total revenues from customer license fees.
Vendor concentrations
For the three months ended March 31, 2024 and
2023, one licensor accounted for 100% of the Company’s vendor license agreements (see Note 7) related to the Company’s biopharmaceutical
operations.
NOTE 7 – COMMITMENTS AND CONTINGENCIES
Employment Agreements
Eric Weisblum
On April 17, 2020, the Company entered into an
employment agreement (“Employment Agreement”) with Eric Weisblum to serve as Chief Executive Officer and Chief Financial Officer
of the Company. The term of the Employment Agreement continued for a period of one year from the date of execution date thereof and automatically
renewed for successive one-year periods at the end of each term until either party delivers written notice of their intent not to renew
at least six months prior to the expiration of the then effective term. The Employment Agreement provided for a base salary of $120,000
and 152,619 vested shares of the Company’s common stock in April 2020. On January 18, 2021, the Company and Mr. Weisblum entered
into the first amendment (the “Amendment”) to the Employment Agreement, effective as of January 1, 2021. Pursuant to the Amendment
Mr. Weisblum’s base salary was increased from $120,000 per year to $180,000 per year and all the terms and provisions of the Employment
Agreement remained in full force and effect.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
On October 12, 2022, the Company entered into
a new employment agreement with Eric Weisblum (the “2022 Weisblum Employment Agreement”) pursuant to which Mr. Weisblum’s
(i) base salary will be $350,000 per year, (ii) Mr. Weisblum was paid a one-time signing bonus of $100,000, and (iii) Mr. Weisblum shall
be entitled to receive an annual bonus of up to $350,000, subject to the sole discretion of the Compensation Committee of the Board of
Directors of the Company (the “Compensation Committee”), and upon the achievement of additional criteria established by the
Compensation Committee from time to time (the “Annual Bonus”). In addition, pursuant to the 2022 Weisblum Employment Agreement,
upon termination of Mr. Weisblum’s employment for death or Total Disability (as defined in the 2022 Weisblum Employment Agreement),
in addition to any accrued but unpaid compensation and vacation pay through the date of his termination and any other benefits accrued
to him under any Benefit Plans (as defined in the 2022 Weisblum Employment Agreement) outstanding at such time and the reimbursement of
documented, unreimbursed expenses incurred prior to such termination date (collectively, the “Weisblum Payments”), Mr. Weisblum
shall also be entitled to the following severance benefits: (i) 24 months of his then base salary; (ii) if Mr. Weisblum elects continuation
coverage for group health coverage pursuant to COBRA Rights (as defined in the 2022 Weisblum Employment Agreement), then for a period
of 24 months following Mr. Weisblum’s termination he will be obligated to pay only the portion of the full COBRA Rights cost of
the coverage equal to an active employee’s share of premiums (if any) for coverage for the respective plan year; and (iii) payment
on a pro-rated basis of any Annual Bonus or other payments earned in connection with any bonus plan to which Mr. Weisblum was a participant
as of the date of his termination (together with the Weisblum Payments, the “Weisblum Severance”). Furthermore, pursuant to
the 2022 Weisblum Employment Agreement, upon Mr. Weisblum’s termination (i) at his option (A) upon 90 days prior written notice
to the Company or (B) for Good Reason (as defined in the 2022 Weisblum Employment Agreement), (ii) termination by the Company without
Cause (as defined in the 2022 Weisblum Employment Agreement) or (iii) termination of Mr. Weisblum’s employment within 40 days of
the consummation of a Change in Control Transaction (as defined in the Weisblum Employment Agreement), Mr. Weisblum shall receive the
Weisblum Severance; provided, however, Mr. Weisblum shall be entitled to a pro-rated Annual Bonus of at least $200,000. In addition, any
equity grants issued to Mr. Weisblum shall immediately vest upon termination of Mr. Weisblum’s employment by him for Good Reason
or by the Company at its option upon 90 days prior written notice to Mr. Weisblum, without Cause. In September 2023 and October 2022,
the Company paid a bonus of $200,000 and $100,000 to Mr. Weisblum, respectively.
Daniel Ryweck
On September 27, 2022, the Board appointed Daniel
Ryweck as Chief Financial Officer of the Company. On September 28, 2022, the Company entered into an employment agreement (the “Ryweck
Employment Agreement”) with Mr. Ryweck. Pursuant to the terms of the Ryweck Employment Agreement, which was amended on October 12,
2022, Mr. Ryweck will (i) receive a base salary at an annual rate of $60,000 (the “Base Compensation”) payable in equal monthly
installments, and (ii) be eligible to receive an annual discretionary bonus. The term of Mr. Ryweck’s engagement under the Ryweck
Employment Agreement commenced on September 28, 2022 and continued until September 28, 2023, unless earlier terminated in accordance with
the terms of the Ryweck Employment Agreement. The term of Mr. Ryweck’s Employment Agreement was automatically renewed until September
28, 2024 and will automatically renew for successive one-year periods until terminated by Mr. Ryweck or the Company.
Dr. James Kuo
On January 27, 2022, the Company and Dr. James
Kuo entered into an employment agreement (“Kuo Employment Agreement”) for Dr. Kuo to serve as the Vice President of Research
& Development. The Kuo Employment Agreement shall be effective as of the date of the agreement and shall automatically renew for a
period of one year at every anniversary of the effective date, with the same terms and conditions, unless either party provides written
notice of its intention not to extend the term of the Kuo Employment Agreement at least thirty days prior to the applicable renewal date.
Dr. Kuo shall be paid an annual base salary of $30,000. For each twelve-month period of his employment, Dr. Kuo shall be entitled to a
bonus whereby amount and terms shall be in the sole and absolute discretion of the Board of Directors (“Board”) and shall
be payable at the Company’s sole option in stock or in cash. In addition, an aggregate of 16,000 incentive stock options were granted
under the 2020 Plan to Dr. Kou, exercisable at $10.00 per share and expires on January 31, 2032. The stock options vested as follows:
(i) 6,000 stock options upon issuance; (ii) 5,000 vested on October 31, 2022 and; (iii) 5,000 vested on October 31, 2023. The 16,000 stock
options had a fair value of $94,915 which valued at grant date using Binomial Lattice option pricing model with the following assumptions:
risk-free interest rate of 1.18%, expected dividend yield of 0%, expected term of 2 years using the simplified method and expected volatility
of 117% based on calculated volatility. The Company recorded the fair value of the stock options, in the amount of $94,915, as deferred
compensation which is being amortized over the vesting period. During the three months ended March 31, 2024 and 2023, the Company amortized
$0 and $4,237 of the deferred compensation which was recorded as compensation expenses in the unaudited consolidated statement of operations
and comprehensive loss, respectively. As of March 31, 2024 and December 31, 2023, there were no remaining deferred compensation related
to these issuances. (see Note 5).
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
License Agreements between the Company and
Vendors
University of Maryland, Baltimore - License
Agreement for Development and Use of Central Nervous System-Homing Peptides
Commercial Evaluation License and Option Agreement
with the University of Maryland, Baltimore
Effective as of July 15, 2020, the Company, through
its wholly-owned subsidiary, Silo Pharma, Inc. (see Note 1) and University of Maryland, Baltimore (“UMB”) (collectively as
“Parties”), entered into a commercial evaluation license and option agreement (“License Agreement”), granting
the Company an exclusive, non-sublicensable, non-transferable license to with respect to the exploration of the potential use of central
nervous system-homing peptides in vivo and their use for the investigation and treatment of multiple sclerosis and other neuroinflammatory
pathology. The License Agreement also granted the Company an exclusive option to negotiate and obtain an exclusive, sublicensable, royalty-bearing
license (“Exclusive Option”) with respect to the subject technology. The License Agreement had a term of six months from the
effective date however if the Company exercises the Exclusive Option, the License Agreement shall expire at the end of the negotiation
period (as defined in the License Agreement) or upon execution of a master license agreement, whichever occurs first. The Company exercised
its Exclusive Option on January 13, 2021 and entered into a Master License Agreement on February 12, 2021. Both parties may terminate
this agreement within thirty days by giving written notice.
University of Maryland, Baltimore - License
Agreement for Development and Use of Joint-Homing Peptides
Commercial Evaluation License and Option Agreement
with the University of Maryland, Baltimore
Effective as of February 26, 2021, the Company,
through its wholly-subsidiary, Silo Pharma, Inc., and University of Maryland, Baltimore (“UMB”) (collectively as “Parties”),
entered into a commercial evaluation license and option agreement (“License Agreement”), which granted the Company an exclusive,
non-sublicensable, non-transferable license with respect to the exploration of the potential use of joint-homing peptides for use in the
investigation and treatment of arthritogenic processes. The License Agreement also granted the Company an exclusive option to negotiate
and obtain an exclusive, sublicensable, royalty-bearing license (“Exclusive Option”) with respect to the subject technology.
On July 6, 2021, the Company entered into a First Amendment Agreement (“First Amendment”) with UMB to extend the term of the
original License Agreement by an additional six months such that the First Amendment was effective until February 25, 2022. On January
28, 2022, the Parties entered into a second amendment to the commercial evaluation and license agreement dated February 26, 2021 (“Second
Amendment”) to extend the term of the original license agreement until December 31, 2022. On June 22, 2022, the Parties entered
into a third amendment to the commercial evaluation and license agreement dated February 26, 2021 (“Third Amendment”). The
Third Amendment expanded the scope of the license granted in the License Agreement to add additional patent rights with respect to an
invention generally known as Peptide-Targeted Liposomal Delivery for Treatment Diagnosis, and Imaging of Diseases and Disorders.
In consideration of the licenses granted under this Third Amendment, the Company agreed to pay a one-time, non-refundable fee of $2,500
which was recorded as research and development expense in the unaudited consolidated statement of operations and comprehensive loss during
the year ended December 31, 2022. On December 16, 2022, the Company and UMB entered into a fourth amendment to License Agreement (the
“Fourth Amendment”) to extend the term of the License Agreement until March 31, 2023. In addition, the parties agreed in the
Fourth Amendment to allow the Company to extend the term of the License Agreement to June 30, 2023 by paying UMB a fee of $1,000 on or
before February 28, 2023. This fee was paid and the term of the License Agreement was extended to June 30, 2023. In February 2023, upon
payment of the extension fee of $1,000, the Company recorded license fees of $1,000 which are included in research and development expenses
on the accompanying unaudited consolidated statement of operations and comprehensive loss. On June 28, 2023, the Company and UMB entered
into a fifth amendment to License Agreement (the “Fifth Amendment”) to extend the term of the License Agreement until September
30, 2023. The Company may at its option extend this Agreement until December 31, 2023, by providing written notice to University on or
before August 31, 2023, and by paying an additional license fee of $2,500. This fee was paid and the term of the License Agreement was
extended to December 31, 2023. In August 2023, upon payment of the extension fee of $2,500, the Company recorded license fees of $2,500
which are included in research and development expense on the accompanying unaudited consolidated statement of operations and comprehensive
loss. As of December 31, 2023, the Company decided not to extend this License Agreement and will not continue to pursue this license.
Master License Agreement with the University
of Maryland, Baltimore
As disclosed above, effective as of February 12,
2021, the Company and University of Maryland, Baltimore (“UMB”), entered into the Master License Agreement (“Master
License Agreement”) which grants the Company an exclusive, worldwide, sublicensable, royalty-bearing license to certain intellectual
property: (i) to make, have made, use, sell, offer to sell, and import certain licensed products and: (ii) to use the invention titled,
“Central nervous system-homing peptides in vivo and their use for the investigation and treatment of multiple sclerosis and other
neuroinflammatory pathology” and UMB’s confidential information to develop and perform certain licensed processes for the
therapeutic treatment of neuroinflammatory disease.
The Master License Agreement will remain in effect
on a Licensed Product-by-Licensed Product basis and country-by-country basis until the later of: (a) the last patent covered under the
Master License Agreement expires, (b) the expiration of data protection, new chemical entity, orphan drug exclusivity, regulatory exclusivity,
or other legally enforceable market exclusivity, if applicable, or (c) 10 years after the first commercial sale of a Licensed Product
in that country, unless earlier terminated in accordance with the provisions of the Master License Agreement. The term of the Master License
Agreement shall expire 15 years after the Master License Agreement Effective Date in which (a) there were never any patent rights, (b)
there was never any data protection, new chemical entity, orphan drug exclusivity, regulatory exclusivity, or other legally enforceable
market exclusivity or (c) there was never a first commercial sale of a Licensed Product.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
The Company may assign, sublicense, grant, or
otherwise convey any rights or obligations under the Master License Agreement to a Company affiliate, without obtaining prior written
consent from UMB provided that it meets the terms defined in the Master License Agreement. The Company may grant sublicenses of some or
all of the rights granted by the Master License Agreement, provided that there is no uncured default or breach of any material term or
condition under the Master License Agreement, by Company, at the time of the grant, and that the grant complies with the terms and conditions
of the Master License Agreement. The Company shall be and shall remain responsible for the performance by each of the Company’s
sublicensee. Any sublicense shall be consistent with and subject to the terms and conditions of the Master License Agreement and shall
incorporate terms and conditions sufficient to enable Company to comply with the Master License Agreement. The Company or Company affiliates
shall pay to UMB a percentage of all income received from its sublicensee as follows: (i) 25% of the Company’s sublicense income
which is receivable with respect to any sublicense that is executed before the filing of an NDA (or foreign equivalent) for the first
licensed product; and (b) 15% of the Company’s sublicense income which is receivable with respect to any sublicense that is executed
after the filing of an NDA (or foreign equivalent) for the first licensed product.
Pursuant to the Master License Agreement, the
Company shall pay UMB; (i) a license fee, (ii) certain event-based milestone payments (see below for payment terms), (iii) royalty payments
depending on net revenues (see below for payment terms), and (iv) a tiered percentage of sublicense income. The Company paid to UMB a
license fee of $75,000, which was paid as follows: (a) $25,000 was due and paid within 30 days following the effective date; and (b) $50,000
on or before the first anniversary of the effective date, which was paid in February 2022. The license fee is non-refundable and is not
creditable against any other fee, royalty or payment. The Company shall be responsible for payment of all patent expenses in connection
with preparing, filing, prosecution and maintenance of patents or patent applications relating to the patent rights. The $75,000 license
fee was recorded as a prepaid expense and is being amortized over the 15-year term. During the three months ended March 31, 2024 and 2023,
the Company recognized license fees of $1,250 and $1,250, respectively, from the amortization of prepaid license fees, which is included
in costs of revenues on the accompanying unaudited consolidated statements of operations. On March 31, 2024, prepaid expense and other
current assets – current amounted to $5,000 and prepaid expense – non-current amounted to $54,375. On December 31, 2023, prepaid
expense and other current assets – current amounted to $5,000 and prepaid expense – non-current amounted to $55,625 as reflected
in the unaudited consolidated balance sheets.
| Milestone | |
Payment | |
| Filing of an Investigational New Drug (or any foreign equivalent) for a Licensed Product | |
$ | 50,000 | |
| Dosing of first patient in a Phase 1 Clinical Trial of a Licensed Product | |
$ | 100,000 | |
| Dosing of first patient in a Phase 2 Clinical Trial of a Licensed Product | |
$ | 250,000 | |
| Receipt of New Drug Application (“NDA”) (or foreign equivalent) approval for a Licensed Product | |
$ | 500,000 | |
| Achievement of First Commercial Sale of Licensed Product | |
$ | 1,000,000 | |
Royalty Payments Terms:
| (i) | 3% on sales of licensed products (as defined in the Master License Agreement) during the applicable calendar year for sales less than $50,000,000; and |
| (ii) | 5% on sales of licensed products during the applicable calendar year for sales greater than $50,000,000; and |
| (iii) | minimum annual royalty payments, as follows: |
| Years | |
Minimum
Annual
Royalty | |
| Prior to First Commercial Sale | |
$ | N/A | |
| Year of First Commercial Sale | |
$ | N/A | |
| First calendar year following the First Commercial Sale | |
$ | 25,000 | |
| Second calendar year following the First Commercial Sale | |
$ | 25,000 | |
| Third calendar year following the First Commercial Sale | |
$ | 100,000 | |
On November 10, 2023, the Company entered into
a Third Amendment to Master License Agreement (the “Third Amendment”) with UMB, pursuant to which the parties agreed to an
amended and restated schedule of diligence milestones for the Master License Agreement.
In April 2021, in connection with the Company’s
Sublicense Agreement with Aikido Pharma Inc. (see below - Patent License Agreement with Aikido Pharma Inc.), the Company paid 25%
of its sublicense income to UMB, pursuant to the Master License Agreement, which amounted to $12,500. During the three months ended March
31, 2024 and 2023, the Company recognized license fees of $210 and $210, respectively, from the amortization of the sublicense fee. On
March 31, 2024, prepaid expense and other current assets – current amounted to $838 and prepaid expenses – non-current amounted
to $9,148. On December 31, 2023, prepaid expense and other current assets – current amounted to $838 and prepaid expenses –
non-current amounted to $9,358 as reflected in the unaudited consolidated balance sheets.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
License Agreements between the Company and
Customer
Customer Patent License Agreement with Aikido
Pharma Inc.
On January 5, 2021, the Company and its subsidiary
Silo Pharma, Inc., entered into a patent license agreement (“License Agreement”) (collectively, the “Licensor”)
with Aikido Pharma Inc. (“Aikido” or the “Customer”), as amended on April 12, 2021, pursuant to which the Licensor
granted Aikido an exclusive, worldwide (“Territory”), sublicensable, royalty-bearing license to certain intellectual property:
(i) to make, have made, use, provide, import, export, lease, distribute, sell, offer for sale, develop and advertise certain licensed
products and (ii) to develop and perform certain licensed processes for the treatment of cancer and symptoms caused by cancer (“Field
of Use”).
The License Agreement also provided that, if the
Licensor exercised the option granted to it pursuant to its commercial evaluation license and option agreement with UMB, effective as
of July 15, 2020, it would grant Aikido a non-exclusive sublicense (“Right”) to certain UMB patent rights in the field of
neuroinflammatory diseases occurring in patients diagnosed with cancer (“Field”). Pursuant to the License Agreement, Aikido
agreed to pay the Licensor, among other things, (i) a one-time non-refundable cash payment of $500,000 and (ii) royalty payments equal
to 2% of net sales (as defined in the License Agreement) in the Field of Use in the Territory. In addition, Aikido agreed to issue the
Licensor 500 shares of Aikido’s newly designated Series M Convertible Preferred Stock which were to be converted into an aggregate
of 625,000 shares of Aikido’s common stock. On April 12, 2021, the Company entered into an amendment to the License Agreement (“Amended
License Agreement”) with Aikido dated January 5, 2021 whereby Aikido issued an aggregate of 625,000 restricted shares of Aikido’s
common stock instead of the 500 shares of the Series M Convertible Preferred Stock.
Pursuant to the License Agreement, the Company
is required to prepare, file, prosecute, and maintain the licensed patents. Unless earlier terminated, the term of the license to the
licensed patents will continue until the expiration or abandonment of all issued patents and filed patent applications within the licensed
patents. The Company may terminate the License Agreement upon 30 day written notice if Aikido fails to pay any amounts due and payable
to the Company or if Aikido or any of its affiliates brings a patent challenge against the Company, assists others in bringing a legal
or administrative challenge to the validity, scope, or enforceability of or opposes any of the licensed patents (“Patent Challenge”)
against the Company (except as required under a court order or subpoena). Aikido may terminate the Agreement at any time without cause,
and without incurring any additional penalty, (i) by providing at least 30 days’ prior written notice and paying the Company all
amounts due to it through such termination effective date. Either party may terminate the Agreement for material breaches that have failed
to be cured within 60 days after receiving written notice. The Company collected the non-refundable cash payment of $500,000 on January
5, 2021 which was recorded as deferred revenue to be recognized as revenues over 15 years, the estimated term of the UMB Master License
Agreement.
Prior to the April 12, 2021, issuance of the common
stock in lieu of the Series M Convertible Preferred Stock as discussed above, the Company valued the 500 Series M Convertible Preferred
stock which was equivalent into Aikido’s 625,000 shares of common stock at a fair value of $0.85 per common share or $531,250 based
quoted trading price of Aikido’s common stock on the date of grant. The Company recorded an equity investment of $531,250 (see Note
3) and deferred revenue of $531,250 to be recognized as revenues over the estimated term of the UMB Master License. Accordingly, the Company
recorded a total deferred revenue of $1,031,250 ($500,000 cash received and $531,250 value of equity securities received) to be recognized
as revenues over the 15-year term.
During the three months ended March 31, 2024 and
2023, the Company recognized license fee revenues of $17,188 and $17,188, respectively. On March 31, 2024, deferred revenue – current
portion amounted to $68,750 and deferred revenue – long-term portion amounted to $739,063. On December 31, 2023, deferred revenue
– current portion amounted to $68,750 and deferred revenue – long-term portion amounted to $756,250 as reflected in the unaudited
consolidated balance sheets.
The Right shall be, to the full extent permitted
by and on terms and conditions required by UMB, for a term consistent with the term of patent and technology licenses that UMB normally
grants. In the event that the Company exercises its option and executes a license with UMB to the UMB patent rights within 40 days after
the execution of such UMB license, for consideration to be agreed upon and paid by Aikido, which consideration shall in no event exceed
110% of any fee payable by the Company to UMB for the right to sublicense the UMB patent rights. The Company shall grant Aikido a nonexclusive
sublicense in the United States to the UMB patent rights in the Field, subject to the terms of any UMB license Licensor obtains, including
any royalty obligations on sublicensees required under any such sublicense. The option was exercised on January 13, 2021. Accordingly,
on April 6, 2021, the Company entered into the Sublicense Agreement with Aikido pursuant to which it granted Aikido a worldwide exclusive
sublicense to its licensed patents under the Master License Agreement.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
Customer Sublicense Agreement with
Aikido Pharma Inc.
On April 6, 2021 (the “Sublicense Agreement
Effective Date”), the Company entered into the Sublicense Agreement with Aikido pursuant to which the Company granted Aikido an
exclusive worldwide sublicense to (i) make, have made, use, sell, offer to sell and import the Licensed Products (as defined below) and
(ii) in connection therewith to (A) use an invention known as “Central nervous system-homing peptides in vivo and their use for
the investigation and treatment of multiple sclerosis and other neuroinflammatory pathology” which was sublicensed to the Company
pursuant to the Master License Agreement and (B) practice certain patent rights (“Patent Rights”) for the therapeutic
treatment of neuroinflammatory disease in cancer patients. “Licensed Products” means any product, service, or process, the
development, making, use, offer for sale, sale, importation, or providing of which: (i) is covered by one or more claims of the Patent
Rights; or (ii) contains, comprises, utilizes, incorporates, or is derived from the Invention or any technology disclosed in the Patent
Rights.
Pursuant to the Sublicense Agreement, Aikido agreed
to pay the Company (i) an upfront license fee of $50,000, (ii) the same sales-based royalty payments that the Company is subject
to under the Master License Agreement and (iii) total milestone payments of up to $1.9 million. The Sublicense Agreement shall continue
on a Licensed Product-by-Licensed Product and country-by-country basis until the later of (i) the date of expiration of the last to expire
claim of the Patent Rights covering such Licensed Product in such country, (ii) the expiration of data protection, new chemical entity,
orphan drug exclusivity, regulatory exclusivity or other legally enforceable market exclusivity, if applicable and (iii) 10 years after
the first commercial sale of a Licensed Product in that country, unless terminated earlier pursuant to the terms of the Sublicense Agreement.
Furthermore, the Sublicense Agreement shall expire 15 years after the Sublicense Agreement Effective Date with respect to any country
in which (i) there were never any Patent Rights, (ii) there was never any data protection, new chemical entity, orphan drug exclusivity,
regulatory exclusivity or other legally enforceable market exclusivity with respect to a Licensed Product and (ii) there was never a commercial
sale of a Licensed Product, unless such agreement is earlier terminated pursuant to its terms. The Company collected the upfront license
fee of $50,000 in April 2021. During the three months ended March 31, 2024 and 2023, the Company recognized revenue of $838 and $838,
respectively. On March 31, 2024, deferred revenue – current portion amounted to $3,352 and deferred revenue – long-term portion
amounted to $36,592, and on December 31, 2023, deferred revenue – current portion amounted to $3,352 and deferred revenue –
long-term portion amounted to $37,430 as reflected in the unaudited consolidated balance sheets.
Sponsored Study and Research Agreements
between the Company and Vendors
Investigator-Sponsored Study Agreement with
University of Maryland, Baltimore
On January 5, 2021, the Company entered into an
investigator-sponsored study agreement (“Sponsored Study Agreement”) with the University of Maryland, Baltimore. The research
project is a clinical study to examine a novel peptide-guided drug delivery approach for the treatment of multiple sclerosis (“MS”).
More specifically, the study is designed to evaluate (1) whether MS-1-displaying liposomes can effectively deliver dexamethasone to the
CNS and (2) whether MS-1-displaying liposomes are superior to plain liposomes, also known as free drug, in inhibiting the relapses and
progression of experimental autoimmune encephalomyelitis. Pursuant to the Sponsored Study Agreement, the research shall commence on March
1, 2021 and will continue until substantial completion, subject to renewal upon mutual written consent of the parties. The total cost
under the Sponsored Study Agreement shall not exceed $81,474 which was payable in two equal installments of $40,737 upon execution of
the Sponsored Study Agreement and $40,737 upon completion of the project with an estimated project timeline of nine months. In 2021, the
Company paid $40,737 and recorded research and development expense of $40,737. This project has been postponed until further notice and
the second payment is not due.
Sponsored Research Agreement with The Regents
of the University of California
On June 1, 2021 (the “Effective Date”),
the Company entered into a sponsored research agreement (the “Sponsored Research Agreement”) with The Regents of the University
of California, on behalf of its San Francisco Campus (“UCSF”) pursuant to which UCSF shall conduct a study to examine psilocybin’s
effect on inflammatory activity in humans to accelerate its implementation as a potential treatment for Parkinson’s Disease, chronic
pain, and bipolar disorder. Pursuant to the Agreement, the Company shall pay UCSF a total fee of $342,850 to conduct the research over
the two-year period. The Agreement was effective for a period of two years from the Effective Date, subject to renewal or earlier termination
as set forth in the Sponsored Research Agreement. During the years ended December 31, 2022 and 2021, pursuant to the Sponsored Research
Agreement, the Company paid to UCSF $181,710 and $100,570, respectively, which were recorded to prepaid expense and other current assets
– current to be amortized over the two-year period. During the year ended December 31, 2023, the Company paid the remaining amount
due of $60,570. During the three months ended March 31, 2024 and 2023, the Company recorded research and development expenses of $0 and
$42,856, respectively, from the amortization of the prepaid research and development fees. On March 31, 2024 and December 31, 2023, there
were no remaining prepaid research and development fees relating to this agreement.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
Sponsored Research Agreement with University
of Maryland, Baltimore
On July 6, 2021, the Company and University of
Maryland, Baltimore (“UMB”) entered into a sponsored research agreement (“July 2021 Sponsored Research Agreement”)
pursuant to which UMB shall evaluate the pharmacokinetics of dexamethasone delivered to arthritic rats via liposome. The research
pursuant to the July 2021 Sponsored Research Agreement shall commence on September 1, 2021 and will continue until the substantial completion
thereof, subject to renewal upon written consent of the parties. The July 2021 Sponsored Research Agreement may be terminated by either
party upon 30 days’ prior written notice to the other party. In addition, if either party commits any material breach of or defaults
with respect to any terms or conditions of the July 2021 Sponsored Research Agreement and fails to remedy such default or breach within
10 business days after written notice from the other party, the party giving notice may terminate the July 2021 Sponsored Research Agreement
as of the date of receipt of such notice by the other party. If the Company terminates the July 2021 Sponsored Research Agreement for
any reason other than an uncured material breach by UMB, the Company shall relinquish any and all rights it may have in the Results (as
defined in the July 2021 Sponsored Research Agreement) to UMB. In addition, if the July 2021 Sponsored Research Agreement is terminated
early, the Company, among other things, will pay all costs incurred and accrued by UMB as of the date of termination. On June 7, 2022,
the Company and UMB amended the July 2021 Sponsored Research Agreement whereby both parties agreed to make changes to the original project
work and budget. The amendment had no effect on the unaudited consolidated financial statements.
Pursuant to the terms of the July 2021 Sponsored
Research Agreement, UMB granted the Company an option (the “Option”) to negotiate and obtain an exclusive license to any UMB
Arising IP (as defined in the July 2021 Sponsored Research Agreement) and UMB’s rights in any Joint Arising IP (as defined in the
July 2021 Sponsored Research Agreement) (collectively, the “UMB IP”). The Company may exercise the Option by giving UMB written
notice within 60 days after it receives notice from UMB of the UMB IP. Pursuant to the July 2021 Sponsored Research Agreement, the Company
shall pay UMB the fees below:
| | | |
Payment | | |
|
| 1 | | |
$ | 92,095 | | |
Paid upon execution of the July 2021 Sponsored Research Agreement |
| 2 | | |
$ | 92,095 | | |
Paid six months after the start of project work as outlined in the July 2021 Sponsored Research Agreement |
| 3 | | |
$ | 92,095 | | |
Upon completion of the project work as outlined in the July 2021 Sponsored Research Agreement |
The Company paid the first payment of $92,095
on September 1, 2021 and on August 31, 2022, the Company paid the second payment of $92,095. These payments were recorded to prepaid expense
and other current assets – current to be amortized into research and development expense during the years ended December 31, 2022
and 2021. During the three months ended March 31, 2024 and 2023, there were no recorded research and development expenses from the amortization
of these prepaid research and development fees and other expenses. On March 31, 2024 and December 31, 2023, the Company owed UMB $92,095
which was included in accounts payable on the accompanying unaudited consolidated balance sheets.
Sponsored Research Agreement with Columbia
University
On October 1, 2021, the Company entered into a
sponsored research agreement with Columbia University pursuant to which the Company has been granted an option to license certain assets
currently under development, including assets related to the potential treatment of patients suffering from Alzheimer’s disease.
The term of the option will commence on the effective date of this agreement and will expire upon the earlier of (i) 90 days after the
date of the Company’s receipt of a final research report for each specific research proposal as defined in the agreement or (ii)
termination of the research. If the Company elects to exercise the option, both parties will commence negotiation of a license agreement
and will execute a license agreement no later than 3 months after the date of the exercise of the option. Columbia University and the
Company will work towards developing a therapeutic treatment for patients suffering from Alzheimer’s disease to post-traumatic stress
disorder. During a one-year period from the date of this agreement, the Company shall pay a total of $1,436,082 to Columbia University
for the support of the research according to the payment schedule as follows: (i) 30% at signing, (ii) 30% at four and half months after
the start of the project, (iii) 30% at nine months after the start of the project and, (iv)10% at completion of the project. The Company
paid the first payment of $430,825 in November 2021 and the second payment of $430,825 in July 2022, which were recorded to prepaid expense
and other current assets – current to be amortized over the estimated project timeline of twelve months. On October 13, 2022, the
Company entered into an amendment to the Columbia Agreement (the “Columbia Amendment”), pursuant to which the parties agreed
to extend the payment schedule, whereby the third payment of $430,825 was due in March 2023. In August 2023, the Company paid $100,000
of this balance and $330,825 of such payment remains unpaid as of March 31, 2024 and December 31, 2023, which is included in accounts
payable on the accompanying unaudited consolidated balance sheet. The remaining payment of $143,607 is due upon completion.
During the three months ended March 31, 2024 and
2023, the Company recorded research and development expense of $0 and $143,608, respectively, from the amortization of the prepaid research
and development fees. As of March 31, 2024, the Company estimates that this research project is approximately 90% complete.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
Research Agreement with Reprocell
On October 25, 2022, (the “Effective Date”),
the Company entered into a research agreement (the “Reprocell Research Agreement”) with Reprocell Europe Ltd. (“Reprocell”)
pursuant to which Reprocell shall conduct a study to assess the binding of a peptide on healthy and rheumatoid arthritis synovial tissue.
Pursuant to the Reprocell Research Agreement, the Company shall pay Reprocell a total fee of $41,306 to conduct the research over a three-month
period. During the year ended December 31, 2022, pursuant to the Reprocell Research Agreement, the Company paid to Reprocell $21,172 which
was recorded as prepaid expense and other current assets – current to be amortized over the three-month period. During the three
months ended March 31, 2024 and 2023, the Company recorded research and development expense of $0 and $8,054 from the amortization of
the prepaid research and development fees and the payment of other fees. As of March 31, 2024 and December 31, 2023, accounts payable
and accrued expenses related to this research agreement were $0 and $5,891, respectively.
Research Agreements with Upperton Pharma Solutions
On February 28, 2023 and October 16, 2023, (the
“Effective Dates”), the Company entered into research agreements (the “Upperton Research Agreements”) with Upperton
Pharma Solutions (“Upperton”) pursuant to which Upperton shall conduct a study to support the development and feasibility
of Prucalopride nasal solutions. Pursuant to the October 16, 2023 Upperton Research Agreement, the Company shall pay Upperton an aggregate
total fee of approximately 216,210 British Pound (GBP) (approximately $272,620) to conduct the research, which will expensed over the
research period. During the year ended December 31, 2023, pursuant to the Upperton Research Agreements, the Company paid Upperton $177,903.
During the three months ended March 31, 2024 and 2023, the Company recorded research and development expense of $113,023 and $7,395, respectively,
from the amortization of the prepaid research and development fees. As of March 31, 2024 and December 31, 2023, accounts payable and accrued
expenses related to Upperton research agreements were $63,820 and $34,525, respectively.
Research Agreement with AmplifyBio
On October 16, 2023, (the “Effective Date”),
the Company entered into a research agreement (the “AmplifyBio Research Agreement”) with AmplifyBio, LLC. (“AmplifyBio”)
pursuant to which AmplifyBio shall conduct a study in rates to investigate intranasal administration of a novel drug product. Pursuant
to the AmplifyBio Research Agreement, the Company shall pay AmplifyBio a total fee of $229,000 to conduct the research. During the year
ended December 31, 2023, pursuant to the AmplifyBio Research Agreement, the Company paid AmplifyBio $182,980 which was recorded as prepaid
expense and other current assets – current, which was amortized into research and development expense during 2023. During the three
months ended March 31, 2024 and 2023, pursuant to this agreement, the Company paid and recorded research and development expense of $36,280
and $0, respectively. As of March 31, 2024, $9,740 of research remains to be billed by AmplifyBio. As of March 31, 2024 and December 31,
2023, accounts payable related to this research agreement were $0.
Research Agreement with Sever Pharma Solutions
On April 26, 2023, (the “Effective Date”),
the Company entered into a research agreement (the “Sever Pharma Research Agreement”) with Foster Delivery Science, Inc, dba
as Sever Pharma Solutions (“Sever Pharma”) pursuant to which Sever Pharma shall conduct a study of pre-clinical extrusion
of proof-of-concept of PLGA/Ketamine Implants. Pursuant to the Sever Pharma Research Agreement, the Company shall pay Sever Pharma a total
fee of $231,688 to conduct the research plus the cost of change orders, as needed. During the three months ended March 31, 2024 and year
ended December 31, 2023, the Company paid Sever Pharma $84,157 and $26,612, respectively. During the three months ended March 31, 2024
and 2023, pursuant to this agreement, the Company recorded research and development expense of $150,466 and $0, respectively. As of March
31, 2024 and December 31, 2023, accounts payable related to this research agreement were $66,309 and $0, respectively.
During the three months ended March 31, 2024 and
2023, the Company entered into other research and development agreements. In connection with such agreements, the Company recorded research
and development expenses of $18,471 and $1,000, respectively.
SILO PHARMA, INC. AND
SUBSIDIARY
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
March 31, 2024
(UNAUDITED)
On March 31, 2024, future amounts due under sponsored
study and research agreements between the Company and vendors is as follows:
| Year ended March 31, | |
Amount | |
| 2025 | |
$ | 827,125 | |
| Total | |
$ | 827,125 | |
Joint Venture Agreement with Zylö Therapeutics,
Inc.
On April 22, 2021 (“Effective Date”),
the Company entered into a Joint Venture Agreement (“JV Agreement”) with Zylö Therapeutics, Inc. (“ZTI”)
pursuant to which the parties agreed to form a joint venture entity, to be named Ketamine Joint Venture, LLC (“Joint Venture”),
to, among other things, focus on the clinical development of ketamine using ZTI’s Z-pod™ technology (“Venture”).
Pursuant to the JV Agreement, the Company shall act as the manager (“Manager”) of the Joint Venture. The Joint Venture shall
terminate if the development program does not meet certain specifications and milestones as set forth in the JV Agreement within 30 days
of the date set forth in the JV Agreement. Notwithstanding the foregoing, the Manager may, in its sole discretion, terminate the Joint
Venture at any time.
Pursuant to the terms of the JV Agreement, (A)
the Company shall contribute (1) $225,000 and (2) its expertise and the expertise of its science advisory board and (B) ZTI shall contribute
(1) certain rights to certain of its patented technology as set forth in the JV Agreement, (2) a license to the know-how and trade secrets
with respect to its Z-pod™ technology for the loading and release of ketamine, (3) ketamine to be used for clinical purposes, (4)
reasonable use of its facilities and permits and (5) its expertise and know-how. Pursuant to the JV Agreement, 51% of the interest in
the Joint Venture shall initially be owned by the Company and 49% of the interest in the Joint Venture shall initially be owned by ZTI,
subject to adjustment in the event of additional contributions by either party. Notwithstanding the foregoing, in no event shall either
party own more than 60% of the interest in the Joint Venture. As of March 31, 2024 and December 31, 2023 and as of the current date of
this report, the joint venture entity has not been formed yet.
Furthermore, pursuant to the terms of the JV Agreement,
ZTI shall grant the Joint Venture a sublicense pursuant to its license agreement (the “License Agreement”) with Albert Einstein
College of Medicine dated November 27, 2017, in the event that the Company or a third party makes a request indicating that the patented
technology (the “Patented Technology”) licensed to ZTI pursuant to the License Agreement is needed to advance the development
of the Joint Venture or it is contemplated or determined that the Patented Technology will be sold. Furthermore, pursuant to the JV Agreement,
ZTI granted the Company an exclusive option to enter into a separate joint venture for the clinical development of psilocybin using ZTI’s
Z-pod™ technology on the same terms and conditions set forth in the JV Agreement, which option expired 24 months after the JV Effective
Date.
Amended Service Agreement
On September 10, 2021 (“Effective Date”),
the Company entered into an Amendment Agreement (“Amended Service Agreement”) to a certain service agreement dated on September
8, 2020 with the University of Texas (“University”) at Austin whereby the University will provide advisory service and assist
the Company on identifying license and sponsored research opportunities for the Company. The Company shall pay the University $5,000 per
quarter starting on the Effective Date. Any cost incurred will be reimbursed only after prior written consent by the Company. The term
of the Amended Service Agreement is for 36 months unless earlier terminated by either party upon giving a written notice as defined in
the agreement. During the three months ended March 31, 2024 and 2023, the Company paid $5,000 and $5,000, respectively, related to this
agreement which in included in professional fees on the accompanying unaudited consolidated statements of operations.
NOTE 8 – SUBSEQUENT EVENTS
Stock Repurchase Plan
On April
4, 2024, the Company’s Board of Directors approved an extension of the previously announced stock repurchase program authorizing
the purchase of up to $1.0 million of the Company’s common stock until April 30, 2024. From April 1, 2024 to April 30, 2024,
the Company purchased 30,065 shares of common stock for a cost of $57,661, or average cost of $1.92.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our
financial condition and results of operations should be read together with the unaudited financial statements and related notes appearing
elsewhere in this Quarterly Report on Form 10-Q and the audited financial statements and related notes for the year ended December 31,
2023 included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission , or SEC. In addition to historical
information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual
results may differ materially from those anticipated in these forward-looking statements as a result of certain factors. We discuss factors
that we believe could cause or contribute to these differences below and elsewhere in this Quarterly Report on Form 10-Q, including those
factors set forth in the section entitled “Cautionary Note Regarding Forward-Looking Statements and Industry Data” and in
the section entitled “Risk Factors” in Part II, Item 1A.
Overview
We are a developmental stage biopharmaceutical
company developing novel therapeutics that address underserved conditions including PTSD, stress-induced anxiety disorders, fibromyalgia,
and central nervous system (CNS) diseases. We are focused on developing novel therapies that include conventional drugs and psychedelic
formulations. The Company’s lead program, SPC-15, is an intranasal drug targeting PTSD and stress-induced anxiety disorders. SP-26
is a time-release ketamine-based loaded implant for fibromyalgia and chronic pain relief. Silo’s two preclinical programs are SPC-14,
an intranasal compound for the treatment of Alzheimer’s disease, and SPU-16, a CNS-homing peptide targeting the central nervous
system with initial research indication in multiple sclerosis (MS).
Rare Disease Therapeutics
We seek to acquire and/or develop intellectual
property or technology rights from leading universities and researchers to treat rare diseases, including the use of psychedelic drugs,
such as psilocybin, ketamine, and the potential benefits they may have in certain cases involving depression, mental health issues and
neurological disorders. We are focused on developing traditional therapeutics and psychedelic medicine. The company concentrates on the
development and commercialization of therapies for unmet needs from indications such as depression, post-traumatic stress disorder (“PTSD”),
, and other rare neurological disorders. Our mission is to identify assets to license and fund the research which we believe will be transformative
to the well-being of patients and the health care industry.
Psilocybin is considered a serotonergic hallucinogen
and is an active ingredient in some species of mushrooms. Recent industry studies using psychedelics, such as psilocybin, have been promising,
and we believe there is a large unmet need with many people suffering from depression, mental health issues and neurological disorders.
While classified as a Schedule I substance under the Controlled Substances Act (“CSA”), there is an accumulating body of evidence
that psilocybin may have beneficial effects on depression and other mental health conditions. Therefore, the U.S. Food and Drug Administration
(“FDA”) and U.S. Drug Enforcement Agency (“DEA”) have permitted the use of psilocybin in clinical studies for
the treatment of a range of psychiatric conditions.
The potential of psilocybin therapy in mental
health conditions has been demonstrated in a number of academic-sponsored studies over the last decade. In these early studies, it was
observed that psilocybin therapy provided rapid reductions in depression symptoms after a single high dose, with antidepressant effects
lasting for up to at least six months for a number of patients. These studies assessed symptoms related to depression and anxiety through
a number of widely used and validated scales. The data generated by these studies suggest that psilocybin is generally well-tolerated
and may have the potential to treat depression when administered with psychological support.
We have engaged in discussions with a number of
world-renowned educational institutions and advisors regarding potential opportunities and have formed a scientific advisory board that
is intended to help advise management regarding potential acquisition and development of products.
In addition, as more fully described below, we
have entered into a license agreement with the University of Maryland, Baltimore, and developing a Ketamine polymer implant. In addition,
we have recently entered into a sponsored research agreement with Columbia University pursuant to which we have been granted an option
to license certain patents and inventions relating to the treatment of Alzheimer’s disease and stress-induced affective disorders
using Ketamine in combination with certain other compounds.
We plan to actively pursue the acquisition and/or
development of intellectual property or technology rights to treat rare diseases, and to ultimately expand our business to focus on this
new line of business.
Product Candidates
We are currently focusing on four product candidates:
(i) SPC-15 for treatment of depression disorders; (ii) SP-26 for treatments of chronic pain; (iii)SPC-14 for the treatment of Alzheimer’s
disease and (iv) SPU-16 for the treatment of CNS disorders with an initial indication for multiple sclerosis.
SPC-15
On October 1, 2021, the Company entered into a
sponsored research agreement with Columbia University pursuant to which the Company has been granted an option to license certain assets
currently under development, including assets related to SPC-15 for the treatment of depression disorders. On September 22, 2022, we entered
into a First Amendment to Sponsored Research Agreement with Columbia to extend the term of the Columbia Agreement to conduct further research
studies, which extension runs through March 31, 2024. On April 11, 2023 the assets under development for which we have the option to license
as described above were issued a patent from the U.S. Patent & Trademark Office (USPTO) for “Biomarkers for Efficacy of Prophylactic
Treatments Against Stress-Induced Affective Disorders” (US 11,622,948, B2). The Company exercised its option for an exclusive license
agreement for SPC-15, a prophylactic treatment for stress-induced affective disorders including anxiety and PTSD pursuant to which the
Company will be granted an exclusive license to further develop, manufacture, and commercialize SPC-15 worldwide. The Company expects
to have the complete license agreement with Columbia completed in the first half of 2024.
SPC-15 is a targeted prophylactic therapeutic
composition for the treatment and prevention for stress-induced affective disorders including PTSD. The treatment predicts levels of severity
or progression of such disorders and their metabolomic biomarkers’ response to pharmacological treatments. We intend to develop
SPC-15 under the Section 505(b)(2) regulatory pathway of the FDA rules. Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act
(“FDCA”) was enacted to enable sponsors to seek New Drug Application (“NDA”) approval for novel repurposed drugs
without the need for such sponsors to undertake time consuming and expensive pre-clinical safety studies and Phase 1 safety studies. Proceeding
under this regulatory pathway, we will be able to rely upon publicly available data with respect to our active ingredient in our NDA submission
to the FDA for marketing approval.
On November 15, 2023, the Company entered an exclusive
license agreement with Medspray Pharma BV for its proprietary patented soft mist nasal spray technology, as the delivery mechanism for
SPC-15, which agreement has an effective date of October 31, 2023. Preclinical and formulation studies are expected to be completed in
the first quarter of 2024 and the Company intends to submit a pre-IND meeting request to FDA in the first half of 2024.
SP-26
In March 2023, the Company filed a provisional
patent application with the USPTO to use SP-26 for treatment of chronic pain, including fibromyalgia Fibromyalgia is a chronic condition
causing pain to the connective tissues through the body including muscles, ligaments, and tendons. Musculoskeletal pain is often accompanied
by sleep difficulties, fatigue, mood disorders, and problems with memory and concentration. Fibromyalgia affects about 4 million American
adults, or about 2% of the adult population.
We intend to develop SP-26 following the Section
505(b)(2) regulatory pathway of the FDA rules. Section 505(b)(2) of the FDCA was enacted to enable sponsors to seek NDA approval for novel
repurposed drugs without the need for such sponsors to undertake time consuming and expensive pre-clinical safety studies and Phase 1
safety studies. Proceeding under this regulatory pathway, we will be able to rely upon publicly available data with respect to our active
ingredient in our NDA submission to the FDA for marketing approval.
SPC-14
On October 1, 2021, the Company entered into a
sponsored research agreement with Columbia University (“Columbia”) pursuant to which Columbia shall conduct two different
studies related to the use of SPC-14 for the treatment of Alzheimer’s. See “Investigator-Sponsored Study Agreements between
the Company and Vendors---Sponsored Research Agreement with Columbia University for the Study of Ketamine in Combination with Other Drugs
for Treatment of Alzheimer’s and Depression Disorders.” for additional details. In addition, Company has been granted an option
to license certain assets currently under development, including SPC-14 for the treatment of Alzheimer’s disease. The Company exercised
its option for an exclusive license agreement for SPC-15, a prophylactic treatment for stress-induced affective disorders including anxiety
and PTSD pursuant to which the Company will be granted an exclusive license to further develop, manufacture, and commercialize SPC-15
worldwide. The Company expects to have the complete license agreement with Columbia completed in the first half of 2024.
SPC-14 is a novel drug combining two approved
therapeutics, so we intend to develop SPC-14 following the Section 505(b)(2) regulatory pathway of the FDA rules. Section 505(b)(2) of
the FDCA was enacted to enable sponsors to seek NDA approval for novel repurposed drugs without the need for such sponsors to undertake
time consuming and expensive pre-clinical safety studies and Phase 1 safety studies. Proceeding under this regulatory pathway, we will
be able to rely upon publicly available data with respect to our active ingredient in our NDA submission to the FDA for marketing approval.
On October 13, 2022, the Company extended the
term of the sponsored research agreement with Columbia to conduct further research studies into the mechanism of action of SPC-14 in the
treatment of Alzheimer’s disease. We expect the results from further preclinical studies in 2024.
SPU-16
On February 12, 2021, we entered into a Master
License Agreement (the “UMB License Agreement”) with the University of Maryland, Baltimore (“UMB”) pursuant to
which UMB granted us an exclusive, worldwide, sublicensable, royalty-bearing license to certain intellectual property (i) to make, have
made, use, sell, offer to sell, and import certain licensed products and (ii) to use the invention titled “Central nervous system-homing
peptides in vivo and their use for the investigation and treatment of multiple sclerosis and other neuroinflammatory pathology,”
or SPU-16. See “License Agreements between the Company and Vendors--Vendor License Agreement with the University of Maryland, Baltimore
for CNS Homing Peptide” for additional details. On April 11, 2023 certain intellectual property under the UMB License Agreement
described above were issued a patent from the U.S. Patent & Trademark Office (USPTO) for “Peptide-Targeted Liposomal Delivery
For Treatment, Diagnosis, and Imaging of Diseases and Disorders” (US 11,766,403, B2).
SPU-16 is a novel peptide homing specifically
to inflamed CNS areas. It may be used to diagnose neuroinflammation in patients, and targeted delivery of drugs into the spinal cord.
The initial indication is for multiple sclerosis (MS). The peptides have been tested in the EAE mouse model of human MS, where they show
homing specifically to inflamed CNS areas.
Product Development Pipeline
The following table summarizes our product development
pipeline.
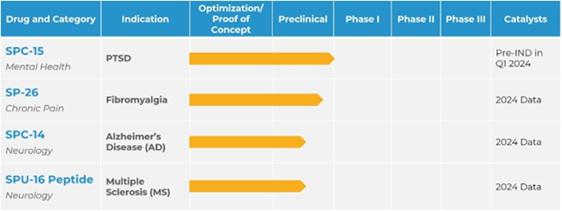
License Agreements between the Company and
Vendor
Vendor License Agreement with the University
of Maryland, Baltimore for CNS Homing Peptide
On February 12, 2021, we entered into a Master
License Agreement (the “UMB License Agreement”) with the University of Maryland, Baltimore (“UMB”) pursuant to
which UMB granted us an exclusive, worldwide, sublicensable, royalty-bearing license to certain intellectual property (i) to make, have
made, use, sell, offer to sell, and import certain licensed products and (ii) to use the invention titled, “Central nervous system-homing
peptides in vivo and their use for the investigation and treatment of multiple sclerosis and other neuroinflammatory pathology”
(the “Invention”) and UMB’s confidential information to develop and perform certain licensed processes for the therapeutic
treatment of neuroinflammatory disease. The term of the License Agreement shall commence on the UMB Effective Date and shall continue
until the latest of (i) ten years from the date of First Commercial Sale (as defined in the Sublicense Agreement) of the Licensed Product
in such country and (ii) the date of expiration of the last to expire claim of the Patent Rights (as defined in the UMB License Agreement)
covering such Licensed Product in such country, or (iii) the expiration of data protection, new chemical entity, orphan drug exclusivity,
regulatory exclusivity, or other legally enforceable market exclusivity, if applicable, unless terminated earlier pursuant to the terms
of the agreement. Pursuant to the UMB License Agreement, we agreed to pay UMB (i) a license fee of $75,000, (ii) certain event-based milestone
payments, (iii) royalty payments, depending on net revenues, (iv) minimum royalty payments, and (v) a tiered percentage of sublicense
income. The UMB License Agreement will remain in effect until the later of: (a) the last patent covered under the UMB License Agreement
expires, (b) the expiration of data protection, new chemical entity, orphan drug exclusivity, regulatory exclusivity, or other legally
enforceable market exclusivity, if applicable, or (c) ten years after the first commercial sale of a licensed product in that country,
unless earlier terminated in accordance with the provisions of the UMB License Agreement. The term of the UMB License Agreement shall
expire 15 years after the effective date in which (a) there were never any patent rights, (b) there was never any data protection, new
chemical entity, orphan drug exclusivity, regulatory exclusivity, or other legally enforceable market exclusivity or (c) there was never
a first commercial sale of a licensed product.
As described below, the Company has entered into
an investigator sponsored research agreement with UMB related to a clinical study to examine a novel peptide-guided drug delivery approach
for the treatment of Multiple Sclerosis.
Commercial Evaluation License and Option Agreement
with UMB for Joint Homing Peptide
Effective as of February 26, 2021, the Company,
through its wholly-subsidiary, Silo Pharma, Inc., and University of Maryland, Baltimore (“UMB”), entered into a commercial
evaluation license and option agreement (“License Agreement”), which granted the Company an exclusive, non-sublicensable,
non-transferable license to with respect to the exploration of the potential use of joint-homing peptides for use in the investigation
and treatment of arthritogenic processes. The License Agreement also granted the Company an exclusive option to negotiate and obtain an
exclusive, sublicensable, royalty-bearing license (“Exclusive Option”) to with respect to the subject technology. The License
Agreement had a term of six months from the effective date. Both parties could have terminated the License Agreement within thirty days
by giving a written notice.
On July 6, 2021, the Company entered into a First
Amendment Agreement (“Amended License Agreement”) with UMB to extend the term of the original License Agreement by an additional
six months such that the Amended License Agreement was effective until February 25, 2022 however, if the Company exercises the Exclusive
Option, the License Agreement shall expire at the end of the negotiation period (as defined in the License Agreement) or upon execution
of a master license agreement, whichever occurs first. The Company paid a license fee of $10,000 to UMB in March 2021 pursuant to the
License Agreement, which was expensed, since the Company could not conclude that such costs would be recoverable for this early-stage
venture.
On January 28, 2022, the Company and University
of Maryland, Baltimore entered into a second amendment to the License Agreement dated February 26, 2021 (“Second Amendment”).
The Second Amendment extended the term of the License Agreement until December 31, 2022. However, if the Company exercises the Exclusive
Option, the License Agreement shall expire at the end of the negotiation period (as defined in the License Agreement) or upon execution
of a master license agreement, whichever occurs first.
On June 22, 2022, the Company and UMB entered
into a third amendment to the License Agreement dated February 26, 2021 under which UMB agreed, to expand the scope of the license granted
in the CELA to add additional Patent Rights with respect to an invention generally known as “Peptide-Targeted Liposomal Delivery
for Treatment Diagnosis, and Imaging of Diseases and Disorders.” On December 16, 2022, the Company and UMB entered into a fourth
amendment to License Agreement (the “Fourth Amendment”) dated February 26, 2021 to extend the term of the License Agreement
until March 31, 2023. In addition, the parties agreed in the Fourth Amendment to allow the Company to extend the term of the License Agreement
to June 30, 2023 by paying UMB a fee of $1,000 on or before February 28, 2023. This fee was paid and thus the term of the License Agreement
was extended to June 30, 2023. We let this license expire by its terms on December 31, 2023.
Joint Venture Agreement with Zylö Therapeutics,
Inc. for Z-pod™ Technology
On April 22, 2021, the Company entered into a
Joint Venture Agreement with Zylö Therapeutics, Inc. (“ZTI”) pursuant to which the parties agreed to form a joint venture
entity, to be named Ketamine Joint Venture, LLC, to, among other things, focus on the clinical development of ketamine using ZTI’s
Z-pod™ technology. Pursuant to the Joint Venture Agreement, the Company shall act as the manager of the Joint Venture. The Venture
shall terminate if the development program does not meet certain specifications and milestones as set forth in the Joint Venture Agreement
within 30 days of the date set forth in the Joint Venture Agreement. Notwithstanding the foregoing, the Manager may, in its sole discretion,
terminate the Venture at any time.
Pursuant to the terms of the Joint Venture Agreement, (A)
the Company shall contribute (1) $225,000 and (2) its expertise and the expertise of its science advisory board and (B) ZTI shall contribute
(1) certain rights to certain of its patented technology as set forth in the JV Agreement, (2) a license to the know-how and trade secrets
with respect to its Z-pod™ technology for the loading and release of ketamine, (3) ketamine to be used for clinical purposes, (4)
reasonable use of its facilities and permits and (5) its expertise and know-how. Pursuant to the Joint Venture Agreement, 51% of the interest
in the Joint Venture shall initially be owned by the Company and 49% of the interest in the Joint Venture shall initially be owned by
ZTI, subject to adjustment in the event of additional contributions by either party. Notwithstanding the foregoing, in no event shall
either party own more than 60% of the interest in the Joint Venture. As of March 31, 2024 and as of the current date of this Annual Report,
the joint venture entity has not been formed yet.
Furthermore, pursuant to the terms of the JV Agreement,
ZTI shall grant the Joint Venture a sublicense pursuant to its license agreement (the “License Agreement”) with Albert Einstein
College of Medicine dated November 27, 2017, in the event that the Company or a third party makes a request indicating that the patented
technology (the “Patented Technology”) licensed to ZTI pursuant to the License Agreement is needed to advance the development
of the Joint Venture or it is contemplated or determined that the Patented Technology will be sold. Furthermore, pursuant to the JV Agreement,
ZTI granted the Company an exclusive option to enter into a separate joint venture for the clinical development of psilocybin using ZTI’s
Z-pod™ technology on the same terms and conditions set forth in the JV Agreement, which option shall expire 24 months after the
JV Effective Date. We do not intend to continue with Zylo and have entered into an agreement to develop a polymer implant for dosage and
time release of Ketamine for chronic pain and Fibromyalgia.
Exclusive License Agreement between Medspray
Pharma BV and the Company
On November 15, 2023, we entered into an Exclusive
License Agreement (the “Medspray License Agreement”) with Medspray Pharma BV (“Medspray”) pursuant to which Medspray
granted us an exclusive, non-revocable, worldwide royalty bearing license for Medspray’s proprietary patented soft mist nasal spray
technology for marketing, promotion, sale and distribution of the products licensed by Medspray to us under the Medspray License Agreement.
The Medspray License Agreement has an effective date of October 31, 2023 and expires on the earlier of (i) termination of the Medspray
License Agreement or expiry of all Medspray license rights in the United States, Germany, United Kingdom, Spain, Italy and France. In
consideration of the exclusive rights granted by Medspray to us, we agreed to pay Medspray a royalty on a quarterly basis equal to 5%
of net sales. The term of the agreement commences on the effective date and continues until the earlier of (i) expiration of the last
to expire of Medspray’s patent rights or (ii) December 31, 2023 (the “Initial Term”) at which time, the Medspray License
Agreement will automatically renew for a successive period of three (3) years, unless terminated by either party upon one year prior written
notice prior to the end of any term; provided, however, the Medspray may terminate the Medspray License Agreement immediately if fail
to have any licensed product under the Medspray License Agreement registered with the FDA or EMA by July 1, 2028 or has filed to reach
the point of first sale of any licensed product under the Medspray License Agreement by July 1, 2028.
Investigator-Sponsored Study Agreements between
the Company and Vendors
Sponsored Research Agreement with Columbia
University for the Study of Ketamine in Combination with Other Drugs for Treatment of Alzheimer’s and Depression Disorders
On October 1, 2021, the Company entered into a
sponsored research agreement with Columbia University (“Columbia”) pursuant to which Columbia shall conduct two different
studies related to all uses of Ketamine or its metabolites in combination with Prucalopride, one of which is related to Alzheimer’s
and the other of which is related to Depression, PTSD and Stress Projects. In addition, Company has been granted an option to license
certain assets currently under development, including Alzheimer’s disease. The term of the option will commence on the effective
date of this agreement and will expire upon the earlier of (i) 90 days after the date of the Company’s receipt of a final research
report for each specific research proposal as defined in the agreement or (ii) termination of the research. If the Company elects to exercise
the option, both parties will commence negotiation of a license agreement and will execute a license agreement no later than 3 months
after the dated of the exercise of the option. The Company exercised its option for an exclusive license agreement for SPC-15, a prophylactic
treatment for stress-induced affective disorders including anxiety and PTSD pursuant to which the Company will be granted an exclusive
license to further develop, manufacture, and commercialize SPC-15 worldwide. The Company expects to have the complete license agreement
with Columbia completed in the first half of 2024. Columbia University and the Company will work towards developing a therapeutic treatment
for patients suffering from Alzheimer’s disease to posttraumatic stress disorder. During a one-year period from the date of this
agreement, the Company shall pay a total of $1,436,082 to Columbia University for the support of the research according to the payment
schedule as follows: (i) 30% at signing, (ii) 30% at four and half months after the start of the project, (iii) 30% at nine months after
the start of the project and, (iv)10% at completion of the project. On October 13, 2022, the Company entered into an amendment of the
sponsored research agreement pursuant to which the parties agreed to extend the payment schedule until March 31, 2024. The Company paid
the first payment of $430,825 in November 2021 and the second payment of $430,825 in July 2022.
Sponsored Research Agreement with University
of Maryland, Baltimore for the Study of Targeted liposomal drug delivery for rheumatoid arthritis
On July 6, 2021, we entered into a sponsored research
agreement (the “July 2021 Sponsored Research Agreement”) with UMB pursuant to which UMB shall evaluate the pharmacokinetics
of dexamethasone delivered to arthritic rats via liposome. The research pursuant to the July 2021 Sponsored Research Agreement commenced
on September 1, 2021 and will continue until the substantial completion thereof, subject to renewal upon written consent of the parties
with a project timeline of twelve months. The July 2021 Sponsored Research Agreement may be terminated by either party upon 30 days’
prior written notice to the other party. In addition, if either party commits any material breach of or defaults with respect to any terms
or conditions of the July 2021 Sponsored Research Agreement and fails to remedy such default or breach within 10 business days after written
notice from the other party, the party giving notice may terminate the July 2021 Sponsored Research Agreement as of the date of receipt
of such notice by the other party. If the Company terminates the July 2021 Sponsored Research Agreement for any reason other than an uncured
material breach by UMB, we shall relinquish any and all rights it may have in the Results (as defined in the July 2021 Sponsored Research
Agreement) to UMB. In addition, if the July 2021 Sponsored Research Agreement is terminated early, we, among other things, will pay all
costs incurred and accrued by UMB as of the date of termination. Pursuant to the terms of the July 2021 Sponsored Research Agreement,
UMB granted us an option (the “Option”) to negotiate and obtain an exclusive license to any UMB Arising IP (as defined in
the July 2021 Sponsored Research Agreement) and UMB’s rights in any Joint Arising IP (as defined in the July 2021 Sponsored Research
Agreement) (collectively, the “UMB IP”). We may exercise the Option by giving UMB written notice within 60 days after it receives
notice from UMB of the UMB IP. We shall pay total fees of $276,285 as set forth in the July 2021 Sponsored Research Agreement. The Company
paid the first payment of $92,095 on September 1, 2021 and on August 31, 2022, the Company paid the second payment of $92,095.
Sponsored Research Agreement with The Regents
of the University of California for the Effect of Psilocybin on Inflammation in the Blood
On June 1, 2021, the Company entered into a sponsored
research agreement (“Sponsored Research Agreement”) with The Regents of the University of California, on behalf of its San
Francisco Campus (“UCSF”) pursuant to which UCSF shall conduct a study to examine psilocybin’s effect on inflammatory
activity in humans to accelerate its implementation as a potential treatment for Parkinson’s Disease, chronic pain, and bipolar
disorder. The purpose of this is to show what effect psilocybin has on inflammation in the blood. The Company believe that this study
will help support the UMB homing peptide study. Pursuant to the Agreement, we shall pay UCSF a total fee of $342,850 to conduct the research
over the two-year period. The Agreement shall be effective for a period of two years from the effective date, subject to renewal or earlier
termination as set forth in the Sponsored Research Agreement. During the years ended December 31, 2022 and 2021, pursuant to the Sponsored
Research Agreement, the Company paid to UCSF $181,710 and $100,570, respectively. We have notified UCSF we do not plan to continue this
study.
Investigator-Sponsored Study Agreement with
UMB for CNS Homing Peptide
On January 5, 2021, we entered into an investigator-sponsored
study agreement with UMB. The research project is a clinical study to examine a novel peptide-guided drug delivery approach for the treatment
of Multiple Sclerosis (“MS”). More specifically, the study is designed to evaluate (1) whether MS-1-displaying liposomes can
effectively deliver dexamethasone to the central nervous system and (2) whether MS-1-displaying liposomes are superior to plain liposomes,
also known as free drug, in inhibiting the relapses and progression of Experimental Autoimmune Encephalomyelitis. Pursuant to the agreement,
the research commenced on March 1, 2021 and will continue until substantial completion, subject to renewal upon mutual written consent
of the parties. The total cost under the investigator-sponsored study agreement shall not exceed $81,474. which is payable in two
equal installments of $40,737 upon execution of the Sponsored Study Agreement and $40,737 upon completion of the project with an estimated
project timeline of nine months. The Company paid $40,737 on January 13, 2021. This project is postponed until further notice and the
second payment is not due.
Stock Repurchase Plan
On January 26, 2023, the Company’s Board
of Directors authorized a stock repurchase plan to repurchase up to $1,000,000 of our issued and outstanding common stock, from time to
time, with such program to be in place until December 31, 2023. On January 9, 2024, the Board of Directors of the Company approved an
extension of the previously announced stock repurchase program authorizing the purchase of up to $1 million of the Company’s common
stock until March 31, 2024 and on April 4, 2024, the stock Repurchase Plan was extended to April 30, 2024. During the year ended December
31, 2023, the Company purchased 252,855 shares of common stock for a cost of $471,121, which is reflected in treasury stock on the accompanying
unaudited consolidated balance sheet During the three months ended March 31, 2024, the Company purchased 72,790 shares of common stock
for a cost of $115,452. As of March 31, 2024, the Company had repurchased an aggregate of 325,645 shares of its common stock for a total
cost of $586,573 pursuant to its Stock Repurchase Program.
Short-Term Investments
Our portfolio of short-term investments consists
of marketable debt securities which are comprised solely of that are all highly rated U.S. government securities with maturities of more
than three months, but less than one year. We classify these as available-for-sale at purchase date and will reevaluate such designation
at each period end date. We may sell these marketable debt securities prior to their stated maturities depending upon changing liquidity
requirements. These debt securities are classified as current assets in the unaudited consolidated balance sheet and recorded at fair
value, with unrealized gains or losses included in accumulated other comprehensive gain and as a component of the unaudited consolidated
statements of comprehensive loss. Gains and losses are recognized when realized. Gains and losses are determined using the specific identification
method and are reported in other income (expense), net in the unaudited consolidated statements of operations.
An impairment loss may be recognized when the
decline in fair value of the debt securities is determined to be other-than-temporary. The Company evaluates its investments for other-than-temporary
declines in fair value below the cost basis each quarter, or whenever events or changes in circumstances indicate that the cost basis
of the short-term investments may not be recoverable. The evaluation is based on a number of factors, including the length of time and
the extent to which the fair value has been below the cost basis, as well as adverse conditions related specifically to the security,
such as any changes to the credit rating of the security and the intent to sell or whether the Company will more likely than not be required
to sell the security before recovery of its amortized cost basis.
On March 31, 2024 and December 31, 2023, short-term
investments, at fair value, amounted to $ 4,075,849 and $4,140,880, respectively.
Results of Operations
Comparison of Our Results of Operations for the Three Months Ended
March 31, 2024 and 2023
The following table summarizes the results of
operations for the three months ending March 31, 2024 and 2023 and were based primarily on the comparative unaudited consolidated financial
statements, footnotes and related information for the periods identified and should be read in conjunction with the consolidated financial
statements and the notes to those statements that are included elsewhere in this report.
| | |
Three Months Ended
March 31, | |
| | |
2024 | | |
2023 | |
| Revenues | |
$ | 18,026 | | |
$ | 18,026 | |
| Cost of revenues | |
| 1,460 | | |
| 1,460 | |
| Gross profit | |
| 16,566 | | |
| 16,566 | |
| Operating expenses | |
| 896,079 | | |
| 820,051 | |
| Loss from operations | |
| (879,513 | ) | |
| (803,485 | ) |
| Other income (expense), net | |
| 77,846 | | |
| (102,911 | ) |
| Net loss | |
$ | (801,667 | ) | |
$ | (906,396 | ) |
Revenues
During the three months ended March 31, 2024 and
2023, we generated minimal revenues from operations. For the three months ended March 31, 2024 and 2023, revenues amounted to $18,026
and $18,026, respectively. Such revenues are related to the Aikido License and Sublicense Agreement and are recognized over the term of
the related license agreement.
Cost of Revenues
During the three months ended March 31, 2024 and
2023, cost of revenues amounted to $1,460 and $1,460, respectively, and consisted of license fees related to the UMB License and Sublicense
Agreement, which are being amortized into cost of revenues over the terms of their respective agreement.
Operating Expenses
For the three months ended March 31, 2024 and
2023, total operating expenses consisted of the following:
| | |
For the Three Months Ended
March 31, | |
| | |
2024 | | |
2023 | |
| Compensation expense | |
$ | 173,346 | | |
$ | 162,257 | |
| Professional fees | |
| 318,427 | | |
| 365,270 | |
| Research and development | |
| 318,240 | | |
| 202,913 | |
| Insurance expense | |
| 21,704 | | |
| 24,645 | |
| Selling, general and administrative expenses | |
| 64,362 | | |
| 64,966 | |
| Total | |
$ | 896,079 | | |
$ | 820,051 | |
| |
|
For the three months ended March 31, 2024 and 2023, compensation expense was $173,346 and $162,257, respectively, an increase of $11,089, or 6.8%. This increase resulted from an increase in payroll expense and related benefits of $15,326, offset by a decrease in stock-based compensation of $4,237. |
| |
|
For the three months ended March 31, 2024 and 2023, professional fees were $318,427 and $365,270 and, respectively, a decrease of $46,843, or 12.8%. The decrease was primarily attributable to a decrease in other consulting fees of $38,819, a decrease in stock-based consulting fees of $33,775 related to the amortization of prepaid expense on previously issued shares to consultants for business advisory and strategic planning services, and a decrease in investor relations fees of $2,888, offset by an increase in legal fees of $25,908, and an increase in accounting and auditing fees of $2,731. During the three months ended March 31, 2024, we reimbursed Columbia University $11,467 related to patent related legal fees incurred by them on our behalf. |
| |
● |
Research and Development: |
| |
|
For the three months ended March 31, 2024 and 2023, we incurred research and development expense of $318,240 and $202,913, respectively, an increase of $115,327, or 56.8%. The increase was a result of an increase in research and development costs in connection with the Investigator-sponsored Study Agreement with UCSF, UMB, Columbia University, and other parties. |
| |
|
For the three months ended March 31, 2024 and 2023, insurance expense was $21,704 and $24,645, respectively, a decrease of $2,941, or 11.9%. This decrease was a result of decrease in the cost of renewal of the D&O insurance policy. |
| |
● |
Selling, General and Administrative Expenses: |
| |
|
Selling, general and administrative expenses include advertising and promotion, patent related expenses, public company expenses, custodian fees, bank service charges, travel, and other office expenses. |
| |
|
For the three months ended March 31, 2024 and 2023, selling, general and administrative expenses were $64,362 and $64,966, respectively, a decrease of $604, or 0.9%. The decrease was primarily attributed to a decrease in Delaware franchise taxes of $14,967 resulting from a reincorporation of the Company in Nevada, offset by a net increase in other general and administrative expenses of $14,363. |
Loss from Operations
For the three months ended March 31, 2024 and
2023, loss from continuing operations amounted to $879,513 and $803,485, respectively, an increase of $76,028, or 9.5%. The increase
was primarily a result of the changes in operating expenses discussed above.
Other Income (Expenses), net
For the three months ended March 31, 2024 and
2023, other income (expense), net amounted to $77,846 and $(102,911), respectively, a positive change of $180,757, or 175.6%. The positive
change in other income (expenses), net was primarily due to a decrease in penalty expense of $166,034 which was incurred due to the early
termination of a certificate of deposit, an increase in interest and dividend income of $23,789, an increase in net realized gain on short-term
investments of $234, offset by an increase in foreign currency transaction loss of $8,695, a decrease in net unrealized gain on equity
investment of $390, and an increase in interest expense of $215.
Net Loss
For the three months ended March 31, 2024, net
loss amounted to $801,667 or $0.28 per common share (basic and diluted), as compared to net loss of $906,396, or $0.29 per common share
(basic and diluted) for the three months ended March 31, 2023, a decrease of $104,729, or 11.6%. The change was primarily a result of
the changes discussed above.
Liquidity and Capital Resources
Liquidity is the ability of an enterprise to generate
adequate amounts of cash to meet its needs for cash requirements. We had working capital of $6,004,214, $4,075,849 in short-term investments,
and $2,668,273 in cash and cash equivalents as of March 31, 2024, and working capital of $6,905,568, $4,140,880 in short-term investments
and $3,524,308 in cash and cash equivalents as of December 31, 2023, respectively.
| | |
March 31,
2024 | | |
December 31,
2023 | | |
Working
Capital
Change | | |
Percentage
Change | |
| Working capital: | |
| | |
| | |
| | |
| |
| Total current assets | |
$ | 6,877,886 | | |
$ | 7,681,158 | | |
$ | (803,272 | ) | |
| (10 | )% |
| Total current liabilities | |
| (873,672 | ) | |
| (775,590 | ) | |
| (98,082 | ) | |
| (13 | )% |
| Working capital: | |
$ | 6,004,214 | | |
$ | 6,905,568 | | |
$ | (901,354 | ) | |
| (13 | )% |
The decrease in working capital of $901,354 was
primarily attributable to a decrease in current assets of $803,272 primarily due to a decrease in cash of $856,035, a decrease in short-term
investments of $65,031, a decrease in prepaid expenses and other current assets of $117,794, and an increase in current liabilities of
$98,082.
Cash Flows
A summary of cash flow activities is summarized as follows:
| | |
Three Months Ended March 31, | |
| | |
2024 | | |
2023 | |
| Net cash used in operating activities | |
$ | (838,179 | ) | |
$ | (692,688 | ) |
| Net cash provided by (used in) investing activities | |
| 97,596 | ) | |
| (6,008,339 | ) |
| Net cash used in financing activities | |
| (115,452 | ) | |
| - | |
| Net decrease in cash and cash equivalents | |
$ | (856,035 | ) | |
$ | (6,701,027 | ) |
Net Cash Used in Operating Activities
Net cash used in operating activities for the
three months ended March 31, 2024 and 2023 were $838,179 and $692,688, respectively, an increase of $145,491, or 21%.
| |
● |
Net cash used in operating activities for the three months ended March 31, 2024 primarily reflected a net loss of $801,667, adjusted for the add-back of non-cash items such as net realized gain on short-term investments of $234, and changes in operating asset and liabilities primarily consisting of an increase in prepaid expenses and other current assets of $116,334, an increase in accounts payable and accrued expenses of $98,082, and a decrease in deferred revenue of $18,026. |
| |
● |
Net cash used in operating activities for the three months ended March 31, 2023 primarily reflected a net loss of $906,396, adjusted for the add-back of non-cash items such as net unrealized gain on equity investments of $390, stock-based compensation of $4,237, and amortization of prepaid stock-based professional fees of $33,775, and changes in operating asset and liabilities primarily consisting of an increase in prepaid expenses and other current assets of $100,769, an increase of interest receivable of $1,183, an increase in accounts payable and accrued expenses of $296,064 and a decrease in deferred revenue of $18,026. |
Net Cash Provided by (Used in) Investing Activities
Net cash provided by (used in) investing activities
for the three months ended March 31, 2024 and 2023 were $97,596 and $(6,008,339), respectively, a change of $6,105,935, or 102%.
| |
● |
Net cash provided by investing activities for the three months ended March 31, 2024 was $97,596 which consisted of proceeds from sale of short-term investments of $150,389, offset by aggregate payments for the purchase of short-term investments of $52,793. |
| |
● |
Net cash used in investing activities for the three months ended March 31, 2023 was $6,008,339 which consisted of aggregate payments for the purchase of short-term investments. |
Net cash Used in Financing Activities
Net cash used in financing activities for the
three months ended March 31, 2024 was $115,452 which solely consisted of the repurchase of shares of our common stock under our stock
repurchase plan. We did not have any cash provided by or used in financing activities for the three months ended March 31, 2023.
Cash Requirements
We believe that our current cash and cash equivalent
amount and short-term investment amount will provide sufficient cash required to meet our obligations for a minimum of twelve months from
the date of this filing.
Other than cash requirements pursuant to research
and development agreements, we currently have no other material commitments for any capital expenditures.
Liquidity
As reflected in the accompanying consolidated
financial statements, we generated a net loss of $801,667 and used cash in operations of $838,179 during the three months ended March
31, 2024. Additionally, we have an accumulated deficit of $11,673,478 on March 31, 2024. As of March 31, 2024, we had working capital
of $6,004,214.
The positive working capital serves to mitigate
the conditions that historically raised substantial doubt about our ability to continue as a going concern. We believe that the Company
has sufficient cash to meet its obligations for a minimum of twelve months from the date of this filing.
Risks and Uncertainties
On August 16, 2022, the Inflation Reduction Act
of 2022 (the “IR Act”) was signed into federal law. The IR Act provides for, among other things, a new U.S. federal 1% excise
tax on certain repurchases of stock by publicly traded U.S. domestic corporations and certain U.S. domestic subsidiaries of publicly traded
foreign corporations occurring on or after January 1, 2023. The excise tax is imposed on the repurchasing corporation itself, not its
shareholders from which shares are repurchased. The amount of the excise tax is generally 1% of the fair market value of the shares repurchased
at the time of the repurchase. However, for purposes of calculating the excise tax, repurchasing corporations are permitted to net the
fair market value of certain new stock issuances against the fair market value of stock repurchases during the same taxable year. In addition,
certain exceptions apply to the excise tax. The U.S. Department of the Treasury (the “Treasury”) has been given authority
to provide regulations and other guidance to carry out and prevent the abuse or avoidance of the excise tax.
Off-Balance Sheet Arrangements
None.
Critical Accounting Estimates
Stock-Based Compensation
Stock-based compensation is accounted for based
on the requirements of ASC 718 – “Compensation – Stock Compensation”, which requires recognition in the financial
statements of the cost of employee, director, and non-employee services received in exchange for an award of equity instruments over the
period the employee, director, or non-employee is required to perform the services in exchange for the award (presumptively, the vesting
period). The ASC also requires measurement of the cost of employee, director, and non-employee services received in exchange for an award
based on the grant-date fair value of the award. The Company has elected to recognize forfeitures as they occur as permitted under Accounting
Standards Update (“ASU”) 2016-09 Improvements to Employee Share-Based Payment.
Research and Development
In accordance with ASC 730-10, “Research
and Development-Overall,” research and development costs are expensed when incurred.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES
ABOUT MARKET RISK
We are not required to provide the information
required by this Item as we are a “smaller reporting company,” as defined in Rule 12b-2 of the Exchange Act.
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures
that are designed to ensure that material information required to be disclosed in our periodic reports filed under the Securities Exchange
Act of 1934, as amended, or 1934 Act, is recorded, processed, summarized, and reported within the time periods specified in the SEC’s
rules and forms and to ensure that such information is accumulated and communicated to our management, including our chief executive officer
and chief financial officer as appropriate, to allow timely decisions regarding required disclosure. We carried out an evaluation, under
the supervision and with the participation of our management, including the principal executive officer and the principal financial officer
(principal financial officer), of the effectiveness of the design and operation of our disclosure controls and procedures, as defined
in Rule 13(a)-15(e) under the 1934 Act, as of the end of the period covered by this report. Based on this evaluation, because of the Company’s
limited resources and limited number of employees, management concluded that our disclosure controls and procedures were not effective
as of March 31, 2024.
The ineffectiveness of our internal control over
financial reporting was due to the following material weaknesses which we identified in our internal control over financial reporting:
| |
● |
We lack segregation of duties within accounting functions duties as a result of our limited financial resources to support hiring of personnel; and. |
| |
|
|
| |
● |
We have not implemented adequate system and manual controls. |
Until such time as we expand our staff to include
additional accounting personnel, it is likely we will continue to report material weaknesses in our internal control over financial reporting.
A material weakness is a deficiency or a combination
of control deficiencies in internal control over financial reporting such that there is a reasonable possibility that a material misstatement
of our annual or interim financial statements will not be prevented or detected on a timely basis.
Changes in Internal Control Over Financial
Reporting
There have been no changes in our internal control
over financial reporting that occurred during the period covered by this Quarterly Report that have materially affected, or are reasonably
likely to materially affect, our internal control over financial reporting.
PART II – OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
From time to time, we may be subject to litigation
and claims arising in the ordinary course of business. We are not currently a party to any material legal proceedings, and we are not
aware of any pending or threatened legal proceeding against us that we believe could have a material adverse effect on our business, operating
results, cash flows or financial condition.
ITEM 1A. RISK FACTORS
Risk factors that affect our business and financial
results are discussed in Part I, Item 1A “Risk Factors,” in our Annual Report on Form 10-K for the year ended December 31,
2023 as filed with the SEC on March 25, 2024 (“Annual Report”). There have been no material changes in our risk factors from
those previously disclosed in our Annual Report. You should carefully consider the risks described in our Annual Report, which could materially
affect our business, financial condition or future results. The risks described in our Annual Report are not the only risks we face. Additional
risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our
business, financial condition, and/or operating results. If any of the risks actually occur, our business, financial condition, and/or
results of operations could be negatively affected.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES
AND USE OF PROCEEDS
None.
Issuer Purchases of Equity Securities
On January 26, 2023, our Board of Directors authorized
a stock repurchase plan to repurchase up to $1,000,000 of our issued and outstanding common stock, from time to time, with such program
to be in place until December 31, 2023. On January 9, 2024, our Board of Directors approved an extension of the previously announced stock
repurchase program authorizing the purchase of up to $1 million of the Company’s common stock until March 31, 2024 and on April
4, 2024, the stock Repurchase Plan was extended to April 30, 2024. During the year ended December 31, 2023, we purchased 252,855 shares
of common stock for a cost of $471,121, which is reflected in treasury stock on the accompanying unaudited consolidated balance sheet.
During the three months ended March 31, 2024, , we purchased 72,790 shares of common stock for a cost of $115,452. As of March 31, 2024,
we have repurchased an aggregate of 325,645 shares of our common stock for a total cost of $586,573 pursuant to its Stock Repurchase Program.
The following is a summary of our common stock
repurchases during the quarterly period ended March 31, 2024:
| Period | |
Total
number of
shares
purchased | | |
Average
price paid
per share | | |
Total
number of
shares
purchased
as part of
publicly
announced
program | | |
Maximum
number (or
approximate
dollar value)
of shares
that may
yet be
purchased
under the
program | |
| January 1, 2024 through January 31, 2024 | |
| 28,944 | | |
$ | 1.45 | | |
| 28,944 | | |
| | |
| February 1, 2024 through February 29, 2024 | |
| 21,318 | | |
$ | 1.59 | | |
| 21,318 | | |
| | |
| March 1, 2024 through March 31, 2024 | |
| 22,528 | | |
$ | 1.79 | | |
| 22,528 | | |
| | |
| Total | |
| 72,790 | | |
$ | 1.59 | | |
| 72,790 | | |
$ | 413,427 | |
On April
4, 2024, our Board of Directors of Silo Pharma, Inc. (the “Company”) approved an extension of the previously announced stock
repurchase program authorizing the purchase of up to $1.0 million of the Company’s common stock until April 30, 2024. From April
1, 2024 to April 30, 2024, the Company purchased 30,065 shares of common stock for a cost of $57,661, or average cost of $1.92.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
10b5-1 Trading Plans
During the fiscal quarter ended March 31,
2024, no Section 16 director or officer adopted, modified, or terminated a “Rule 10b5-1 trading arrangement” (as
defined in Item 408 of Regulation S-K of the Exchange Act).
There were no “non-Rule 10b5-1 trading
arrangements” (as defined in Item 408 of Regulation S-K of the Exchange Act) adopted, modified or terminated during the fiscal
quarter ended March 31, 2024 by our directors and Section 16 officers.
ITEM 6. EXHIBITS
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| |
SILO PHARMA, INC. |
| |
|
|
| Dated: May 13, 2024 |
By: |
/s/ Eric Weisblum |
| |
Name: |
Eric Weisblum |
| |
Title: |
Chairman and Chief Executive Officer
(Principal Executive Officer) |
| Dated: May 13, 2024 |
By: |
/s/ Daniel Ryweck |
| |
Name: |
Daniel Ryweck |
| |
Title: |
Chief Financial Officer
(Principal Financial and Accounting Officer) |
-33-
--12-31
0.28
0.29
2866934
3158797
4334230
false
Q1
0001514183
0001514183
2024-01-01
2024-03-31
0001514183
2024-05-13
0001514183
2024-03-31
0001514183
2023-12-31
0001514183
2023-01-01
2023-03-31
0001514183
us-gaap:CommonStockMember
2023-12-31
0001514183
us-gaap:AdditionalPaidInCapitalMember
2023-12-31
0001514183
us-gaap:TreasuryStockCommonMember
2023-12-31
0001514183
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-12-31
0001514183
us-gaap:RetainedEarningsMember
2023-12-31
0001514183
us-gaap:CommonStockMember
2024-01-01
2024-03-31
0001514183
us-gaap:AdditionalPaidInCapitalMember
2024-01-01
2024-03-31
0001514183
us-gaap:TreasuryStockCommonMember
2024-01-01
2024-03-31
0001514183
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-01-01
2024-03-31
0001514183
us-gaap:RetainedEarningsMember
2024-01-01
2024-03-31
0001514183
us-gaap:CommonStockMember
2024-03-31
0001514183
us-gaap:AdditionalPaidInCapitalMember
2024-03-31
0001514183
us-gaap:TreasuryStockCommonMember
2024-03-31
0001514183
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2024-03-31
0001514183
us-gaap:RetainedEarningsMember
2024-03-31
0001514183
us-gaap:CommonStockMember
2022-12-31
0001514183
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001514183
us-gaap:TreasuryStockCommonMember
2022-12-31
0001514183
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-12-31
0001514183
us-gaap:RetainedEarningsMember
2022-12-31
0001514183
2022-12-31
0001514183
us-gaap:CommonStockMember
2023-01-01
2023-03-31
0001514183
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-03-31
0001514183
us-gaap:TreasuryStockCommonMember
2023-01-01
2023-03-31
0001514183
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-01-01
2023-03-31
0001514183
us-gaap:RetainedEarningsMember
2023-01-01
2023-03-31
0001514183
us-gaap:CommonStockMember
2023-03-31
0001514183
us-gaap:AdditionalPaidInCapitalMember
2023-03-31
0001514183
us-gaap:TreasuryStockCommonMember
2023-03-31
0001514183
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-03-31
0001514183
us-gaap:RetainedEarningsMember
2023-03-31
0001514183
2023-03-31
0001514183
2021-09-30
2021-09-30
0001514183
2021-09-30
0001514183
2023-10-01
0001514183
us-gaap:BankTimeDepositsMember
2024-03-31
0001514183
us-gaap:BankTimeDepositsMember
2023-12-31
0001514183
2023-01-01
2023-12-31
0001514183
us-gaap:StockOptionMember
2024-01-01
2024-03-31
0001514183
us-gaap:StockOptionMember
2023-01-01
2023-03-31
0001514183
us-gaap:WarrantMember
2024-01-01
2024-03-31
0001514183
us-gaap:WarrantMember
2023-01-01
2023-03-31
0001514183
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001514183
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001514183
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2024-03-31
0001514183
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2023-12-31
0001514183
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2023-12-31
0001514183
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2023-12-31
0001514183
us-gaap:NotesReceivableMember
2023-12-31
0001514183
srt:MinimumMember
2023-12-04
0001514183
srt:MaximumMember
2023-12-04
0001514183
srt:MinimumMember
silo:AmendmentMember
2023-12-04
0001514183
srt:MaximumMember
silo:AmendmentMember
2023-12-04
0001514183
us-gaap:CommonStockMember
2024-01-01
2024-03-31
0001514183
us-gaap:PreferredStockMember
2024-01-01
2024-03-31
0001514183
2022-08-29
2022-08-29
0001514183
srt:DirectorMember
silo:StockRepurchasePlanMember
2022-01-26
2022-01-26
0001514183
2024-01-09
2024-01-09
0001514183
silo:StockRepurchasePlanMember
2024-01-01
2024-03-31
0001514183
silo:StockRepurchasePlanMember
2024-03-31
0001514183
silo:OmnibusEquityIncentivePlanMember
2021-01-18
0001514183
srt:MinimumMember
srt:BoardOfDirectorsChairmanMember
2024-01-01
2024-03-31
0001514183
srt:MaximumMember
srt:BoardOfDirectorsChairmanMember
2024-01-01
2024-03-31
0001514183
silo:StockOptionsMember
2023-12-30
0001514183
silo:StockOptionsMember
2023-12-31
2023-12-31
0001514183
silo:StockOptionsMember
2024-01-01
2024-03-31
0001514183
silo:StockOptionsMember
2024-03-31
0001514183
us-gaap:WarrantMember
2023-12-30
0001514183
us-gaap:WarrantMember
2023-12-31
2023-12-31
0001514183
us-gaap:WarrantMember
2024-01-01
2024-03-31
0001514183
us-gaap:WarrantMember
2024-03-31
0001514183
silo:CustomerConcentrationMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2024-01-01
2024-03-31
0001514183
us-gaap:SalesRevenueNetMember
us-gaap:SupplierConcentrationRiskMember
silo:VendorMember
2024-01-01
2024-03-31
0001514183
silo:CustomerConcentrationMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2023-01-01
2023-03-31
0001514183
us-gaap:SalesRevenueNetMember
us-gaap:SupplierConcentrationRiskMember
silo:VendorMember
2023-01-01
2023-03-31
0001514183
silo:EricWeisblumMember
2020-04-17
2020-04-17
0001514183
srt:MinimumMember
silo:EricWeisblumMember
2024-01-01
2024-03-31
0001514183
srt:MaximumMember
silo:EricWeisblumMember
2024-01-01
2024-03-31
0001514183
silo:EricWeisblumMember
2022-10-12
2022-10-12
0001514183
silo:EricWeisblumMember
2022-10-12
0001514183
srt:BoardOfDirectorsChairmanMember
silo:EricWeisblumMember
2022-10-12
0001514183
silo:MrWeisblumMember
2022-10-12
0001514183
silo:MrWeisblumMember
2023-09-01
2023-09-30
0001514183
silo:MrWeisblumMember
2022-10-01
2022-10-31
0001514183
silo:DanielRyweckMember
2024-01-01
2024-03-31
0001514183
silo:DrJamesKuoMember
2022-01-27
2022-01-27
0001514183
silo:DrJamesKuoMember
2022-01-27
0001514183
silo:DrJamesKuoMember
2022-10-31
0001514183
silo:DrJamesKuoMember
2023-10-31
0001514183
silo:DrJamesKuoMember
2024-01-01
2024-03-31
0001514183
us-gaap:MeasurementInputRiskFreeInterestRateMember
silo:DrJamesKuoMember
2024-01-01
2024-03-31
0001514183
us-gaap:MeasurementInputExpectedDividendRateMember
silo:DrJamesKuoMember
2024-01-01
2024-03-31
0001514183
us-gaap:MeasurementInputExpectedTermMember
2024-01-01
2024-03-31
0001514183
us-gaap:MeasurementInputPriceVolatilityMember
silo:DrJamesKuoMember
2024-01-01
2024-03-31
0001514183
silo:StockOptionsMember
silo:DrJamesKuoMember
2024-03-31
0001514183
silo:ThirdAmendmentMember
silo:UniversityOfMarylandBaltimoreMember
2022-01-01
2022-12-31
0001514183
silo:UniversityOfMarylandBaltimoreMember
2023-06-30
2023-06-30
0001514183
silo:ExtensionFeeMember
silo:UniversityOfMarylandBaltimoreMember
2023-02-01
2023-02-28
0001514183
us-gaap:LicenseMember
silo:UniversityOfMarylandBaltimoreMember
2023-02-01
2023-02-28
0001514183
silo:UniversityOfMarylandBaltimoreMember
2023-08-31
2023-08-31
0001514183
silo:UniversityOfMarylandBaltimoreMember
2023-08-01
2023-08-31
0001514183
us-gaap:LicenseMember
silo:UniversityOfMarylandBaltimoreMember
2023-08-01
2023-08-31
0001514183
silo:MasterLincenseAgreementMember
2024-01-01
2024-03-31
0001514183
us-gaap:LicenseMember
silo:MasterLincenseAgreementMember
2024-01-01
2024-03-31
0001514183
silo:MasterLincenseAgreementMember
2022-02-01
2022-02-28
0001514183
us-gaap:LicenseMember
2024-01-01
2024-03-31
0001514183
silo:MasterLincenseAgreementMember
2024-03-31
0001514183
us-gaap:LicenseMember
silo:SponsoredResearchAgreementWithColumbiaUniversityMember
2024-01-01
2024-03-31
0001514183
silo:MasterLincenseAgreementMember
2023-12-31
0001514183
silo:SublicenseAgreementMember
2021-04-01
2021-04-30
0001514183
silo:MasterLincenseAgreementMember
silo:SublicenseAgreementMember
2024-03-31
0001514183
silo:SublicenseAgreementMember
2023-12-31
0001514183
silo:CustomerPatentLicenseAgreementWithAikidoPharmaIncMember
2024-01-01
2024-03-31
0001514183
silo:CustomerPatentLicenseAgreementWithAikidoPharmaIncMember
2021-01-01
2021-01-05
0001514183
silo:SeriesMConvertiblePreferredMember
2021-04-12
0001514183
silo:CustomerPatentLicenseAgreementWithAikidoPharmaIncMember
2021-04-01
2021-04-12
0001514183
silo:CustomerPatentLicenseAgreementWithAikidoPharmaIncMember
2021-04-12
0001514183
silo:CustomerPatentLicenseAgreementWithAikidoPharmaIncMember
2024-03-31
0001514183
us-gaap:LicenseMember
silo:CustomerPatentLicenseAgreementWithAikidoPharmaIncMember
2024-01-01
2024-03-31
0001514183
us-gaap:LicenseMember
2023-01-01
2023-03-31
0001514183
silo:CustomerPatentLicenseAgreementWithAikidoPharmaIncMember
2023-12-31
0001514183
silo:CustomerSublicenseAgreementWithAikidoPharmaIncMember
2024-01-01
2024-03-31
0001514183
silo:CustomerSublicenseAgreementWithAikidoPharmaIncMember
2021-04-01
2021-04-30
0001514183
silo:CustomerSublicenseAgreementWithAikidoPharmaIncMember
2023-01-01
2023-03-31
0001514183
silo:CustomerSublicenseAgreementWithAikidoPharmaIncMember
2024-03-31
0001514183
silo:CustomerSublicenseAgreementWithAikidoPharmaIncMember
2023-12-31
0001514183
silo:SponsoredStudyAgreementMember
2021-01-05
0001514183
silo:InvestigatorSponsoredStudyAgreementWithUniversityOfMarylandBaltimoreMember
silo:SponsoredStudyAgreementMember
2021-01-05
0001514183
silo:InvestigatorSponsoredStudyAgreementWithUniversityOfMarylandBaltimoreMember
2021-01-05
0001514183
silo:InvestigatorSponsoredStudyAgreementWithUniversityOfMarylandBaltimoreMember
2021-01-01
2021-12-31
0001514183
silo:SponsoredResearchAgreementWithTheRegentsOfTheUniversityOfCaliforniaMember
2021-06-01
2021-06-01
0001514183
silo:SponsoredResearchAgreementWithTheRegentsOfTheUniversityOfCaliforniaMember
2022-12-31
0001514183
silo:SponsoredResearchAgreementWithTheRegentsOfTheUniversityOfCaliforniaMember
2021-12-31
0001514183
silo:SponsoredResearchAgreementWithColumbiaUniversityMember
2024-01-01
2024-03-31
0001514183
silo:SponsoredResearchAgreementWithColumbiaUniversityMember
2023-01-01
2023-03-31
0001514183
silo:SponsoredResearchAgreementWithUniversityOfMarylandBaltimoreMember
2021-09-01
2021-09-01
0001514183
silo:SponsoredResearchAgreementWithUniversityOfMarylandBaltimoreMember
2022-08-31
2022-08-31
0001514183
silo:SponsoredResearchAgreementWithUniversityOfMarylandBaltimoreMember
2024-03-31
0001514183
silo:SponsoredResearchAgreementWithUniversityOfMarylandBaltimoreMember
2023-12-31
0001514183
silo:FirstPaymentMember
silo:SponsoredResearchAgreementWithColumbiaUniversityMember
2021-11-01
2021-11-30
0001514183
silo:SecondPaymentMember
silo:SponsoredResearchAgreementWithColumbiaUniversityMember
2022-07-01
2022-07-31
0001514183
silo:SponsoredResearchAgreementWithColumbiaUniversityMember
2023-08-01
2023-08-31
0001514183
silo:SponsoredResearchAgreementWithColumbiaUniversityMember
2024-03-31
0001514183
silo:ResearchAgreementWithReprocellMember
2022-10-25
2022-10-25
0001514183
silo:ResearchAgreementWithReprocellMember
2022-10-25
0001514183
silo:ResearchAgreementWithReprocellMember
2024-01-01
2024-03-31
0001514183
silo:ResearchAgreementWithReprocellMember
2023-01-01
2023-03-31
0001514183
silo:ResearchAgreementsWithUppertonPharmaSolutionsMember
2023-10-16
2023-10-16
0001514183
silo:UppertonResearchAgreementsMember
2023-01-01
2023-12-31
0001514183
silo:PrucaloprideNasalSolutionsMember
2024-01-01
2024-03-31
0001514183
silo:PrucaloprideNasalSolutionsMember
2023-01-01
2023-03-31
0001514183
silo:ResearchAgreementWithReprocellMember
2024-03-31
0001514183
silo:ResearchAgreementWithReprocellMember
2023-12-31
0001514183
silo:AmplifyBioResearchAgreementMember
2023-10-16
2023-10-16
0001514183
silo:SponsoredResearchAgreementWithTheRegentsOfTheUniversityOfCaliforniaMember
2023-12-31
0001514183
silo:AmplifyBioResearchAgreementMember
2024-01-01
2024-03-31
0001514183
silo:AmplifyBioResearchAgreementMember
2023-01-01
2023-03-31
0001514183
2023-04-26
2023-04-26
0001514183
silo:FosterDeliveryScienceIncMember
2024-01-01
2024-03-31
0001514183
silo:FosterDeliveryScienceIncMember
2023-01-01
2023-03-31
0001514183
silo:ResearchAgreementWithAmplifyBioMember
2024-01-01
2024-03-31
0001514183
silo:ResearchAgreementWithAmplifyBioMember
2023-01-01
2023-03-31
0001514183
silo:JVAgreementMember
2024-01-01
2024-03-31
0001514183
silo:ZylTherapeuticsIncMember
2024-01-01
2024-03-31
0001514183
silo:AmendedServiceAgreementMember
2021-09-10
2021-09-10
0001514183
silo:AmendedServiceAgreementMember
2024-01-01
2024-03-31
0001514183
silo:AmendedServiceAgreementMember
2023-01-01
2023-03-31
0001514183
silo:PaymentOneMember
2024-01-01
2024-03-31
0001514183
silo:PaymentTwoMember
2024-01-01
2024-03-31
0001514183
silo:PaymentThreeMember
2024-01-01
2024-03-31
0001514183
silo:PaymentFourMember
2024-01-01
2024-03-31
0001514183
silo:PaymentFiveMember
2024-01-01
2024-03-31
0001514183
silo:PaymentOneMember
2024-01-01
2024-03-31
0001514183
silo:PaymentTwoMember
2024-01-01
2024-03-31
0001514183
silo:PaymentThreeMember
2024-01-01
2024-03-31
0001514183
2024-04-04
0001514183
2024-01-01
2024-04-30
0001514183
us-gaap:SubsequentEventMember
2024-01-01
2024-04-30
xbrli:shares
iso4217:USD
iso4217:USD
xbrli:shares
xbrli:pure
iso4217:GBP
In connection with this quarterly report on Form
10-Q of Silo Pharma, Inc. (the “Company”) for the period ended March 31, 2024, as filed with the Securities and Exchange Commission
(the “Report”), I, Eric Weisblum, Chief Executive Officer of the Company, certify, pursuant to Section 1350 of Title 18 of
the United States Code as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
A signed original of this written statement required
by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission
or its staff upon request.
In connection with this quarterly report on Form
10-Q of Silo Pharma, Inc. (the “Company”) for the period ended March 31, 2024 as filed with the Securities and Exchange Commission
(the “Report”), I, Daniel Ryweck, Chief Financial Officer of the Company, certify, pursuant to Section 1350 of Title 18 of
the United States Code as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
A signed original of this written statement required
by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission
or its staff upon request.