false 0001831915 0001831915 2023-11-28 2023-11-28
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
November 28, 2023
Cytek Biosciences, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-40632 |
|
47-2547526 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
| 47215 Lakeview Boulevard Fremont, California |
|
94538 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (877) 922-9835
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.001 per share |
|
CTKB |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure |
On November 28, 2023, Cytek Biosciences, Inc. (the “Company”) made available an updated corporate presentation, which can be found on the Company’s website. The presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference in this Item 7.01.
The information provided in this Item 7.01 of this Current Report on Form 8-K, including the exhibits hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any of the Company’s filings under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, regardless of any incorporation language in such a filing, except as expressly set forth by specific reference in such a filing.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Cytek Biosciences, Inc. |
|
|
|
|
| Date: November 28, 2023 |
|
|
|
By: |
|
/s/ Wenbin Jiang, Ph.D. |
|
|
|
|
|
|
Wenbin Jiang, Ph.D. |
|
|
|
|
|
|
President and Chief Executive Officer |

Corporate Presentation November 2023
Dr. Wenbin Jiang, CEO Patrik Jeanmonod, CFO Paul Goodson, Investor Relations Exhibit 99.1

Safe Harbor Statement This
presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, among others, statements regarding Cytek’s business and operational goals and strategies; Cytek’s
business development plans; Cytek’s estimated market size and opportunities; Cytek’s prospective products; objectives of management for future operations; and the expected key strategic benefits from the flow cytometry and imaging
business acquired from Luminex. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current expectations and projections about future events and financial trends that we
believe may affect our financial condition, results of operations, business strategy, and financial needs. All statements other than statements of historical facts contained in this presentation, including, without limitation, statements The words
“may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,”
“potential,” “continue” and other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to
numerous risks and uncertainties that could cause actual results to differ materially from currently anticipated results, including but not limited to, risks relating to global economic and market conditions; Cytek's ability to evaluate its
prospects for future viability and predict future performance; Cytek’s ability to accurately forecast customer demand and adoption of its products; Cytek’s ability to successfully integrate the acquired Luminex business and recognize the
anticipated benefits of the transaction; Cytek’s dependence on certain sole and single source suppliers; competition; market acceptance of Cytek’s current and potential products; Cytek’s ability to manage the growth and complexity
of its organization, maintain relationships with customers and suppliers and retain key employees; Cytek’s ability to maintain, protect and enhance its intellectual property; and Cytek’s ability to continue to stay in compliance with its
material contractual obligations, applicable laws and regulations. Information on these and additional risks and uncertainties and other information affecting Cytek’s business and operating results is contained in Cytek’s Quarterly
Report on Form 10-Q for the quarter ended September 30, 2023, and in its other filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof. Except as required by applicable law, Cytek does
not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made
about the accuracy of any such forward-looking statements. Certain information contained in this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and Cytek’s internal
estimates and research. While Cytek believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any
information obtained from third-party sources. While Cytek believes its internal research is reliable, such research has not been verified by any independent source. Cytek’s estimates are derived from publicly available information,
management’s knowledge of the Cytek’s industry and management’s assumptions based on such information and knowledge, which they believe to be reasonable. This data involves a number of assumptions and limitations which are
necessarily subject to a high degree of uncertainty and risk due to a variety of factors. This presentation includes certain financial information in accordance with U.S. GAAP and also on a non-GAAP basis for the twelve months ended September 30,
2023. Management believes that non-GAAP financial measures, including “Adjusted EBITDA”, taken in conjunction with GAAP financial measures, provide useful information for both management and investors by excluding certain non-cash and
other expenses that are not indicative of the company’s core operating results. Management uses non-GAAP measures to compare the company’s performance relative to forecasts and strategic plans and to benchmark the company’s
performance externally against competitors. Non-GAAP information is not prepared under a comprehensive set of accounting rules and should only be used to supplement an understanding of the company’s operating results as reported under U.S.
GAAP. Cytek encourages investors to carefully consider its results under GAAP, as well as its supplemental non-GAAP information and the reconciliation between these presentations, to more fully understand its business. Reconciliations between GAAP
and non-GAAP operating results are presented in the tables accompanying this presentation. Cytek, Full Spectrum Profiling, FSP, Northern Lights, Cytek Aurora, cFluor, Tonbo, Amnis, Guava, Muse, ImageStream, FlowSight, CellStream and easyCyte are
trademarks of Cytek Biosciences, Inc. Other trademarks appearing in this presentation are the property of their respective holders.
Cytek’s Leadership Team Wenbin
Jiang, Ph.D. Chief Executive Officer Valerie Barnett General Counsel Allen Poirson, Ph.D. SVP, Corporate and Business Development Ming Yan, Ph.D. Chief Technology Officer Patrik Jeanmonod Chief Financial Officer Philippe Busque SVP, Global Sales and
Services Paul Goodson Head of Investor Relations Chris Williams Chief Operating Officer
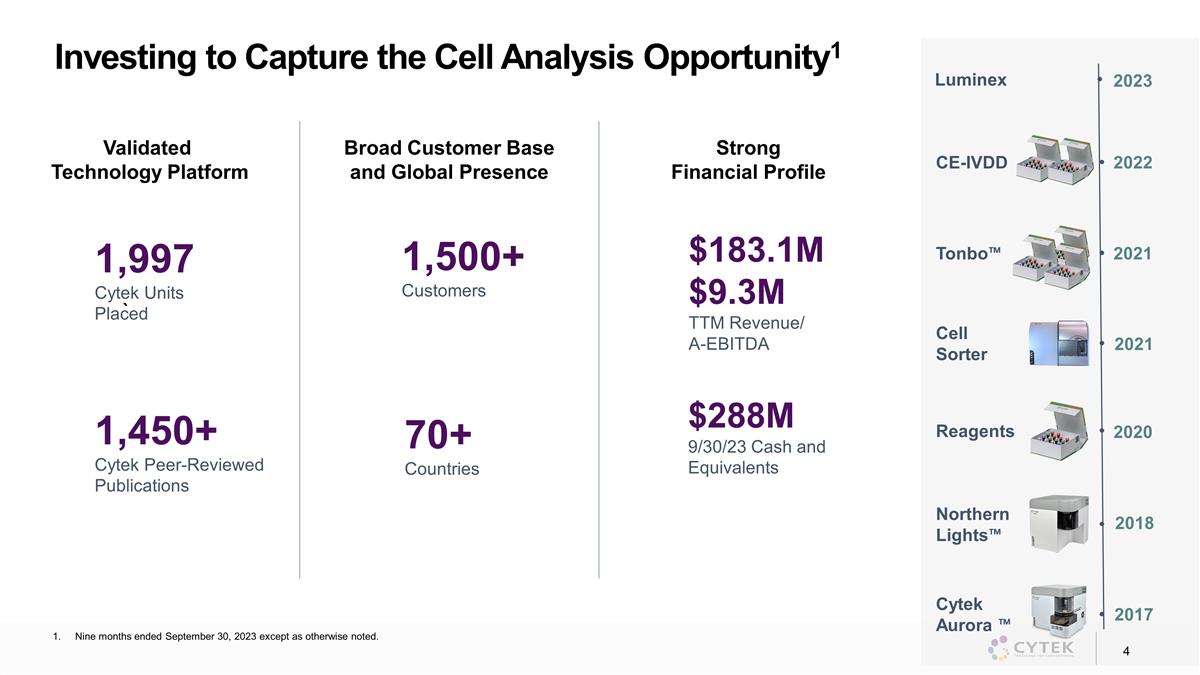
Investing to Capture the Cell Analysis
Opportunity1 2020 Reagents 2018 Northern Lights™ 2017 Cytek Aurora ™ 2021 Cell Sorter Strong Financial Profile Broad Customer Base and Global Presence Validated Technology Platform 1,500+ Customers 1,997 Cytek Units Placed $183.1M $9.3M
TTM Revenue/ A-EBITDA 70+ Countries 1,450+ Cytek Peer-Reviewed Publications $288M 9/30/23 Cash and Equivalents 2021 Tonbo™ 2022 CE-IVDD 2023 Luminex ` Nine months ended September 30, 2023 except as otherwise noted.
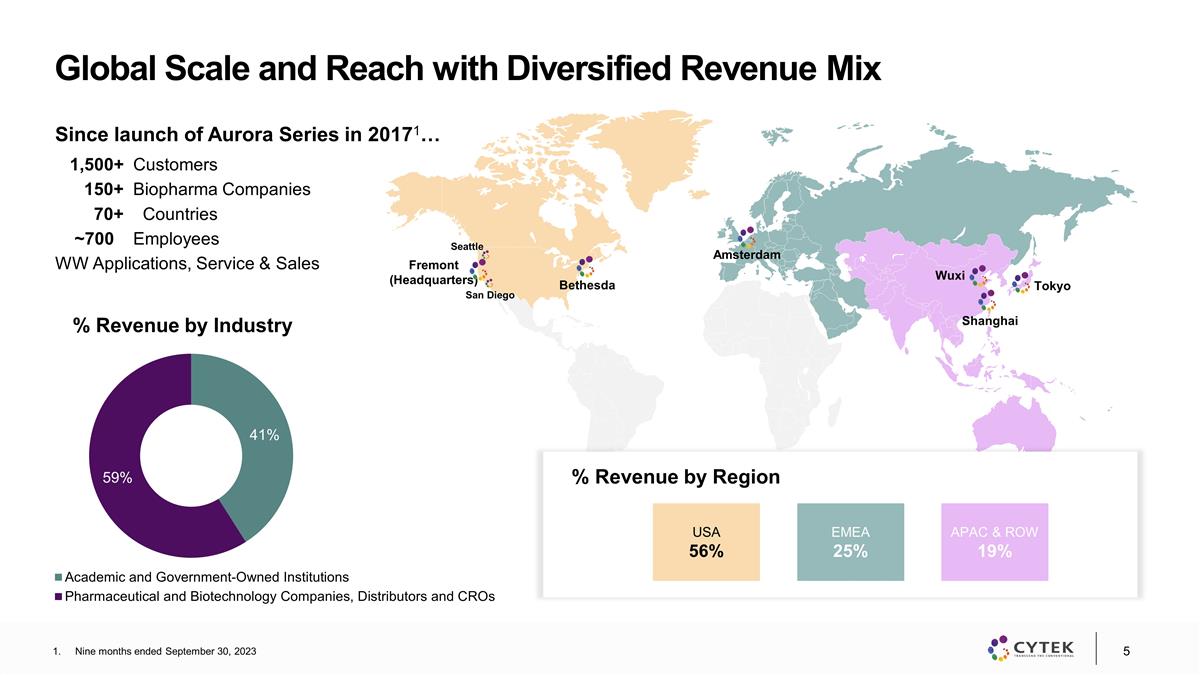
Global Scale and Reach with
Diversified Revenue Mix Nine months ended September 30, 2023 % Revenue by Industry Since launch of Aurora Series in 20171… 1,500+ Customers 150+ Biopharma Companies 70+ Countries ~700 Employees WW Applications, Service & Sales % Revenue by
Region Fremont (Headquarters) Bethesda Shanghai Wuxi Amsterdam Tokyo USA 56% EMEA 25% APAC & ROW 19% San Diego Seattle
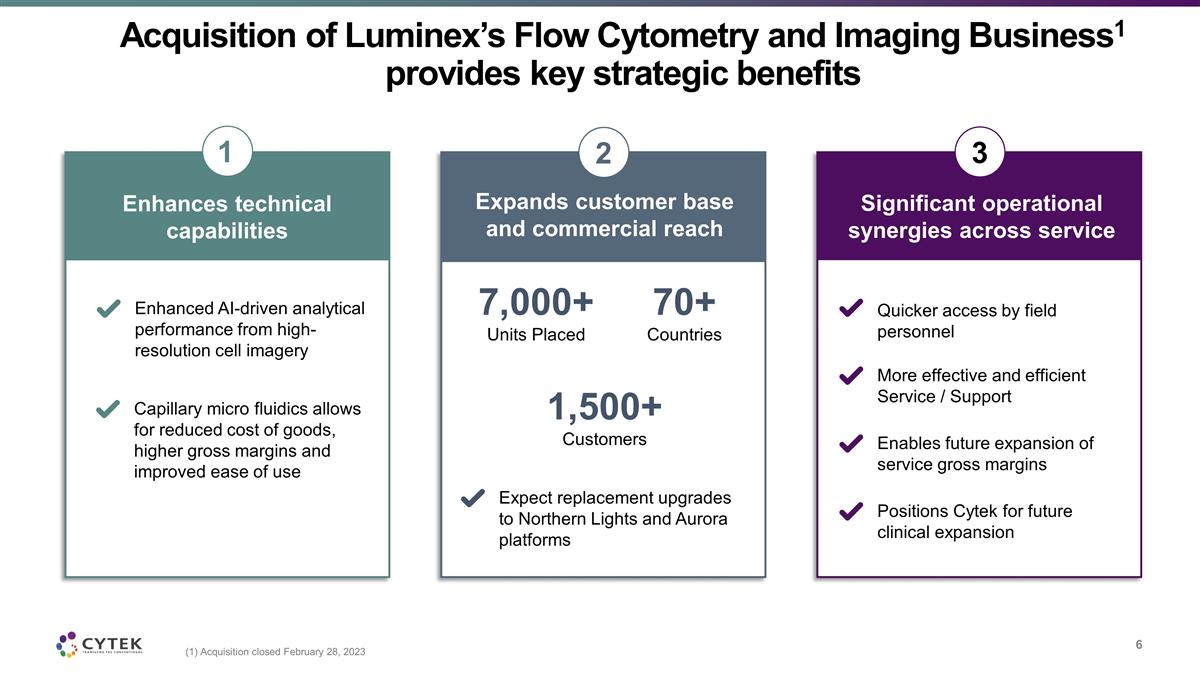
Acquisition of Luminex’s Flow
Cytometry and Imaging Business1 provides key strategic benefits (1) Acquisition closed February 28, 2023 Enhances technical capabilities Expands customer base and commercial reach Significant operational synergies across service 1 1,500+ Customers
70+ Countries 7,000+ Units Placed 2 3 Quicker access by field personnel More effective and efficient Service / Support Enables future expansion of service gross margins Positions Cytek for future clinical expansion Expect replacement upgrades to
Northern Lights and Aurora platforms Enhanced AI-driven analytical performance from high-resolution cell imagery Capillary micro fluidics allows for reduced cost of goods, higher gross margins and improved ease of use
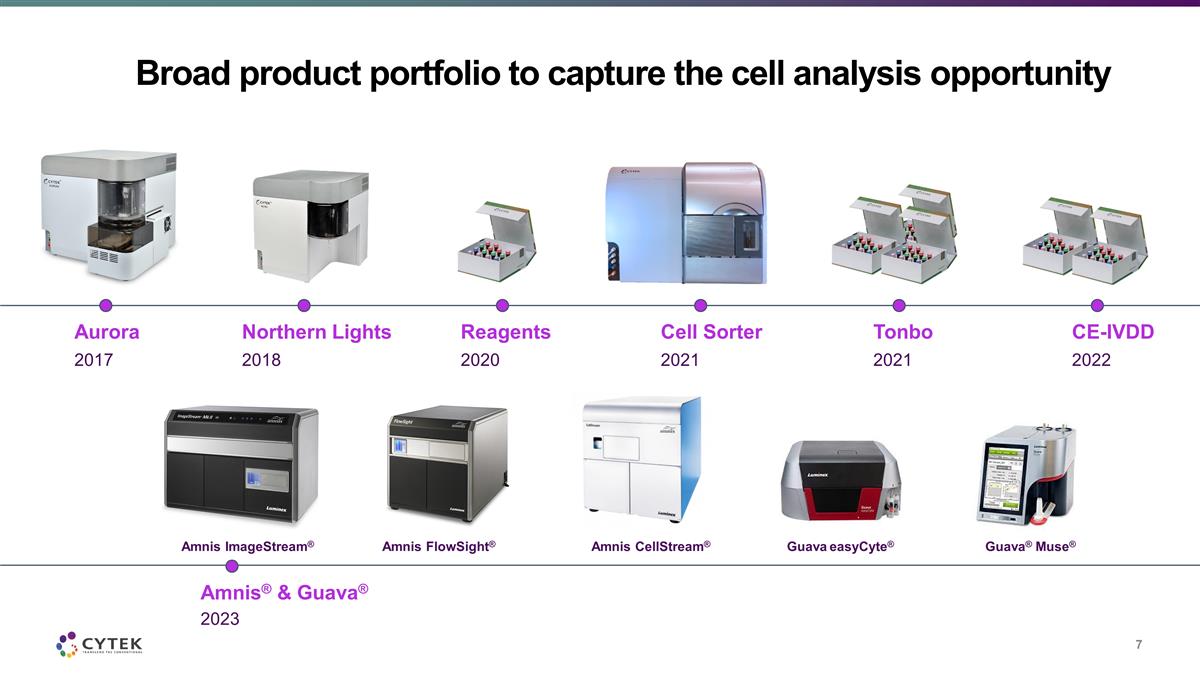
Broad product portfolio to capture the
cell analysis opportunity 2017 Aurora 2018 Northern Lights 2021 Cell Sorter 2020 Reagents 2021 Tonbo 2022 CE-IVDD 2023 Amnis® & Guava® Amnis ImageStream® Amnis FlowSight® Amnis CellStream® Guava easyCyte®
Guava® Muse®
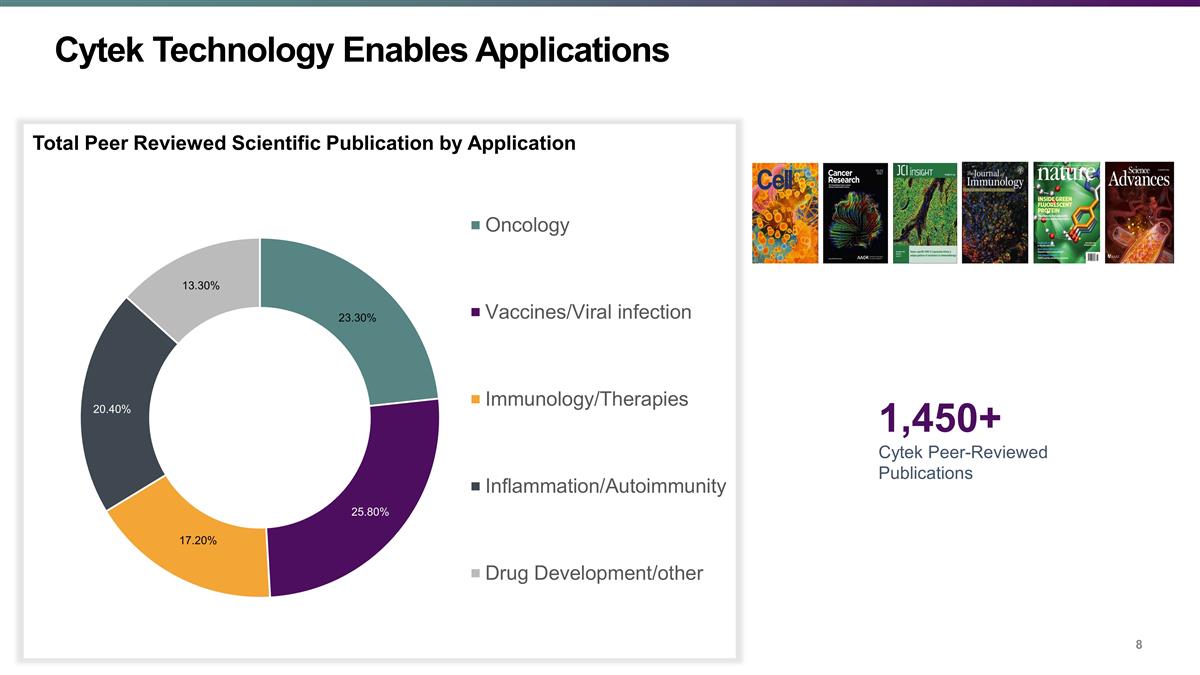
Cytek Technology Enables Applications
FOOTNOTE Total Peer Reviewed Scientific Publication by Application 1,450+ Cytek Peer-Reviewed Publications
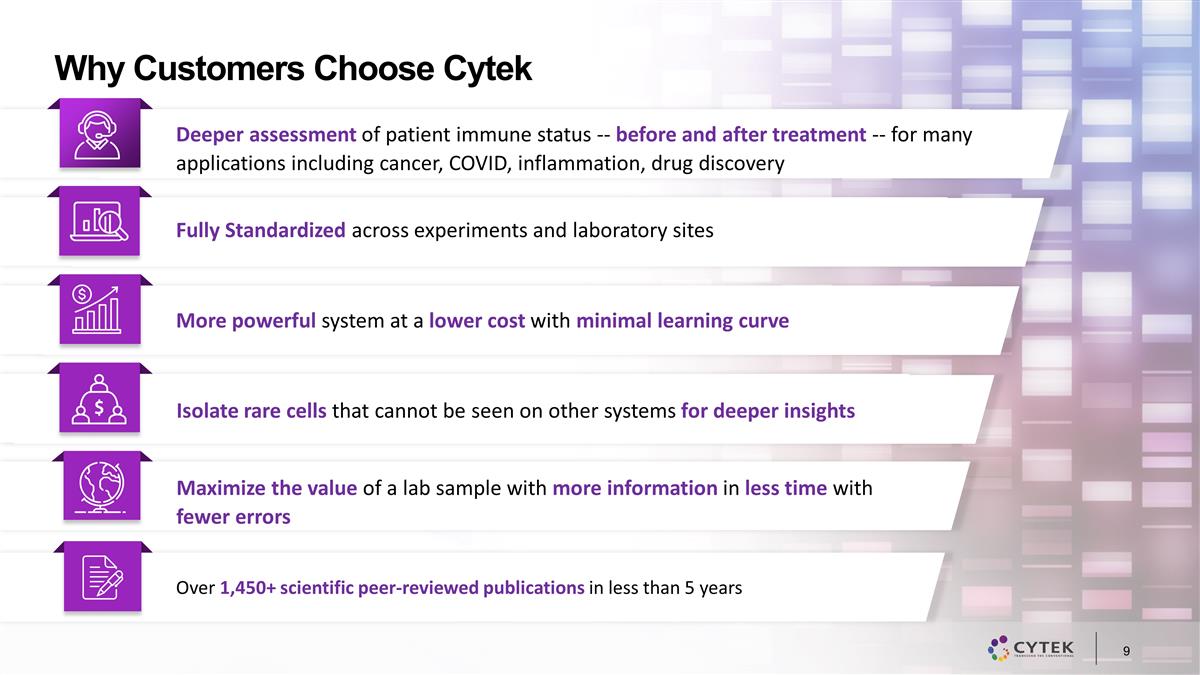
Why Customers Choose Cytek Deeper
assessment of patient immune status -- before and after treatment -- for many applications including cancer, COVID, inflammation, drug discovery Fully Standardized across experiments and laboratory sites Isolate rare cells that cannot be seen on
other systems for deeper insights More powerful system at a lower cost with minimal learning curve Maximize the value of a lab sample with more information in less time with fewer errors Over 1,450+ scientific peer-reviewed publications in less than
5 years
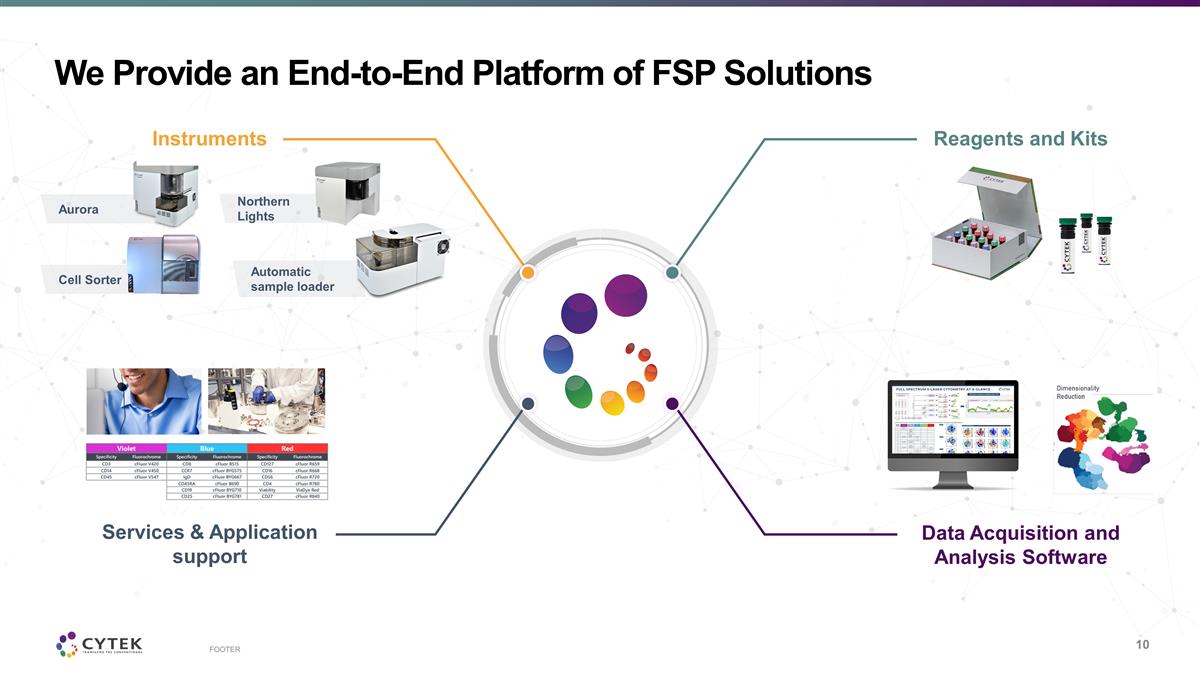
FOOTER Aurora Northern Lights Cell
Sorter Automatic sample loader We Provide an End-to-End Platform of FSP Solutions Instruments Services & Application support Reagents and Kits Data Acquisition and Analysis Software
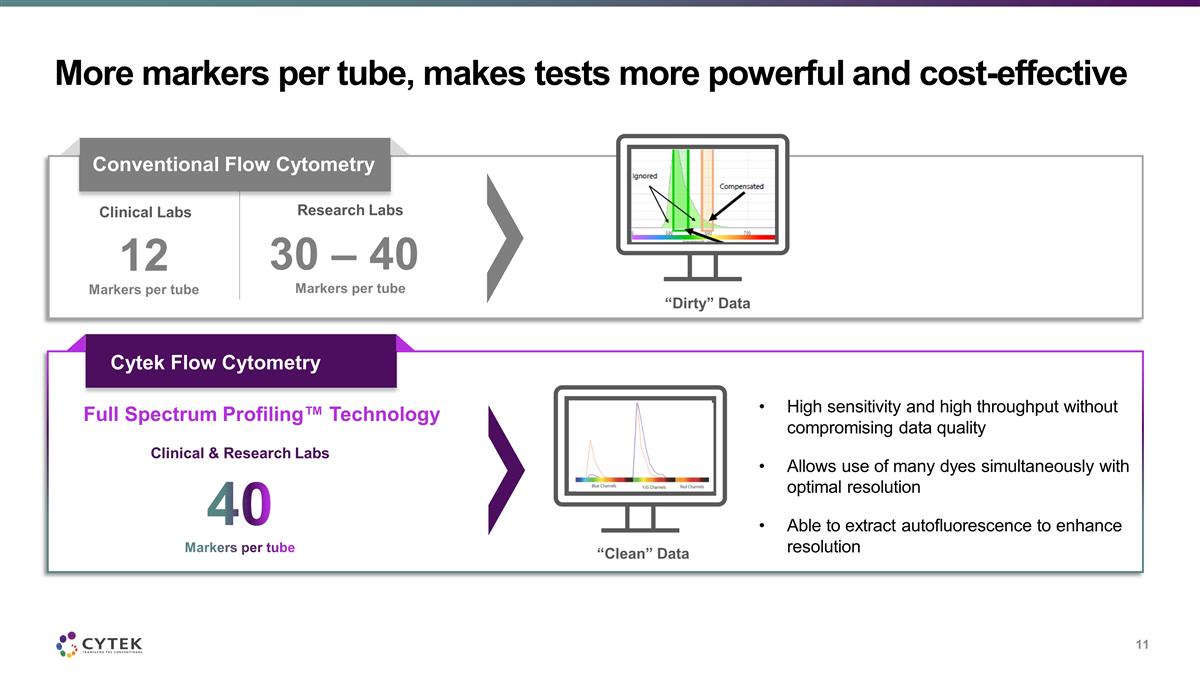
More markers per tube, makes tests
more powerful and cost-effective Clinical Labs 12 Markers per tube “Dirty” Data Research Labs 30 – 40 Markers per tube Conventional Flow Cytometry Cytek Flow Cytometry Full Spectrum Profiling™ Technology 40 Markers per tube
Clinical & Research Labs High sensitivity and high throughput without compromising data quality Allows use of many dyes simultaneously with optimal resolution Able to extract autofluorescence to enhance resolution “Clean” Data
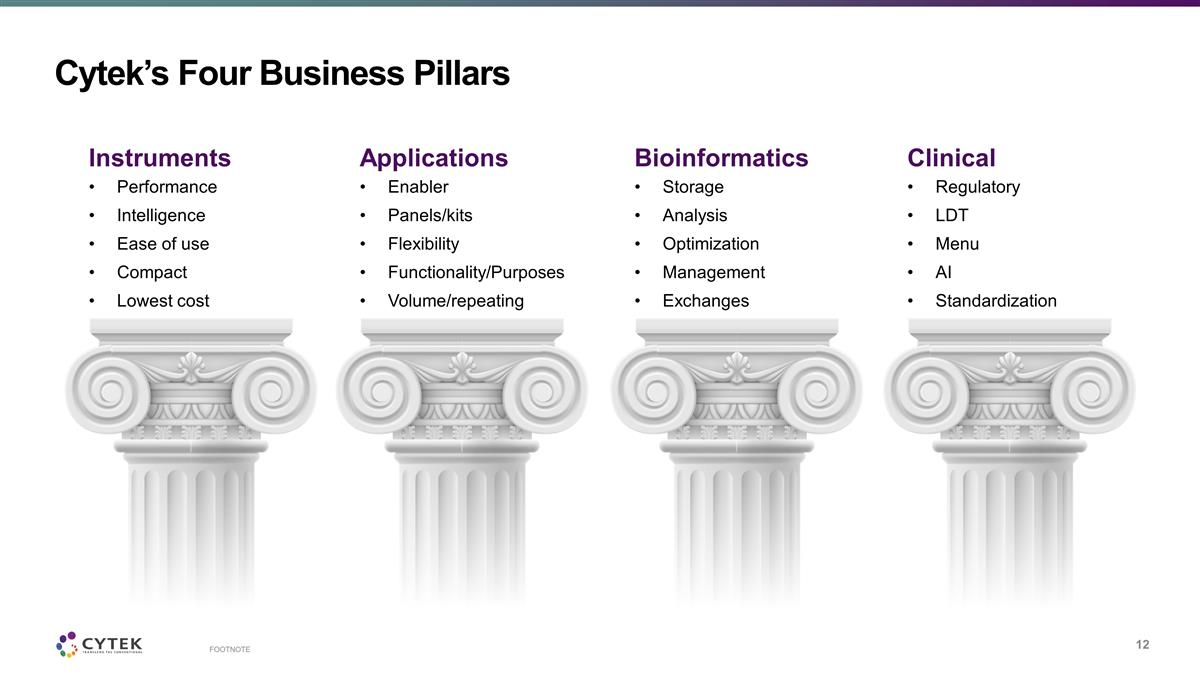
Cytek’s Four Business Pillars
FOOTNOTE Instruments Performance Intelligence Ease of use Compact Lowest cost Applications Enabler Panels/kits Flexibility Functionality/Purposes Volume/repeating Bioinformatics Storage Analysis Optimization Management Exchanges Clinical
Regulatory LDT Menu AI Standardization
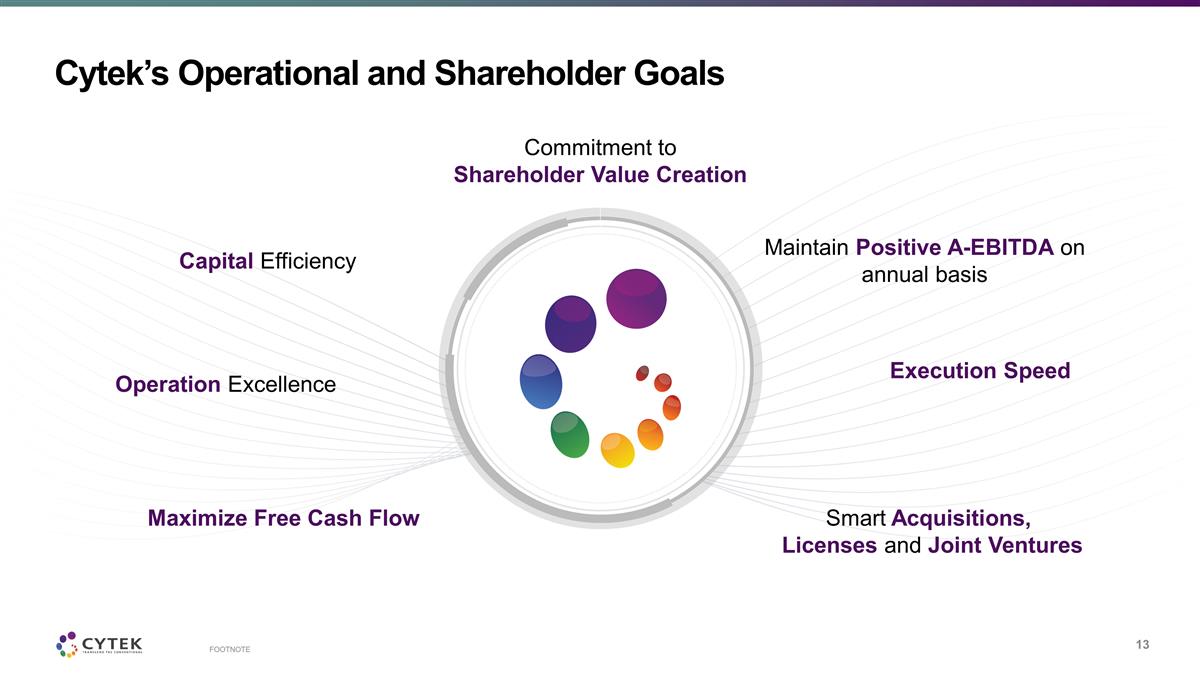
Cytek’s Operational and
Shareholder Goals Commitment to Shareholder Value Creation Capital Efficiency Operation Excellence Maximize Free Cash Flow Maintain Positive A-EBITDA on annual basis Execution Speed Smart Acquisitions, Licenses and Joint Ventures FOOTNOTE

Thank You

Appendix
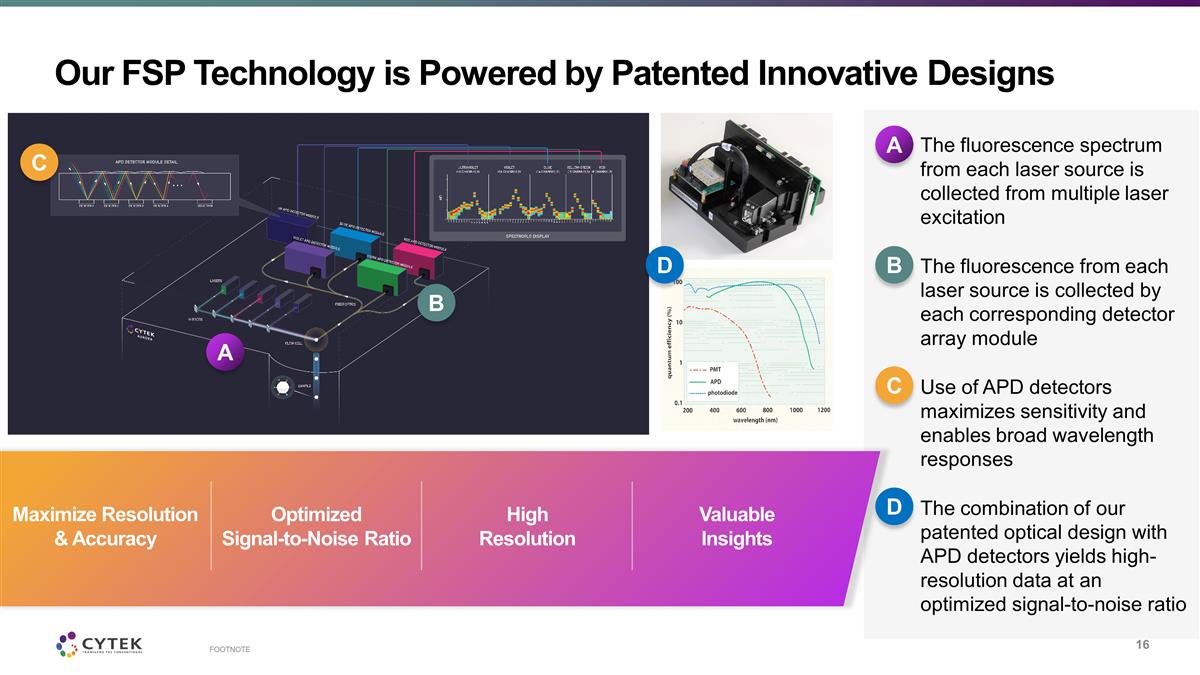
Our FSP Technology is Powered by
Patented Innovative Designs FOOTNOTE The fluorescence spectrum from each laser source is collected from multiple laser excitation The fluorescence from each laser source is collected by each corresponding detector array module Use of APD detectors
maximizes sensitivity and enables broad wavelength responses The combination of our patented optical design with APD detectors yields high-resolution data at an optimized signal-to-noise ratio A B C Maximize Resolution & Accuracy Optimized
Signal-to-Noise Ratio High Resolution A B C D D Valuable Insights
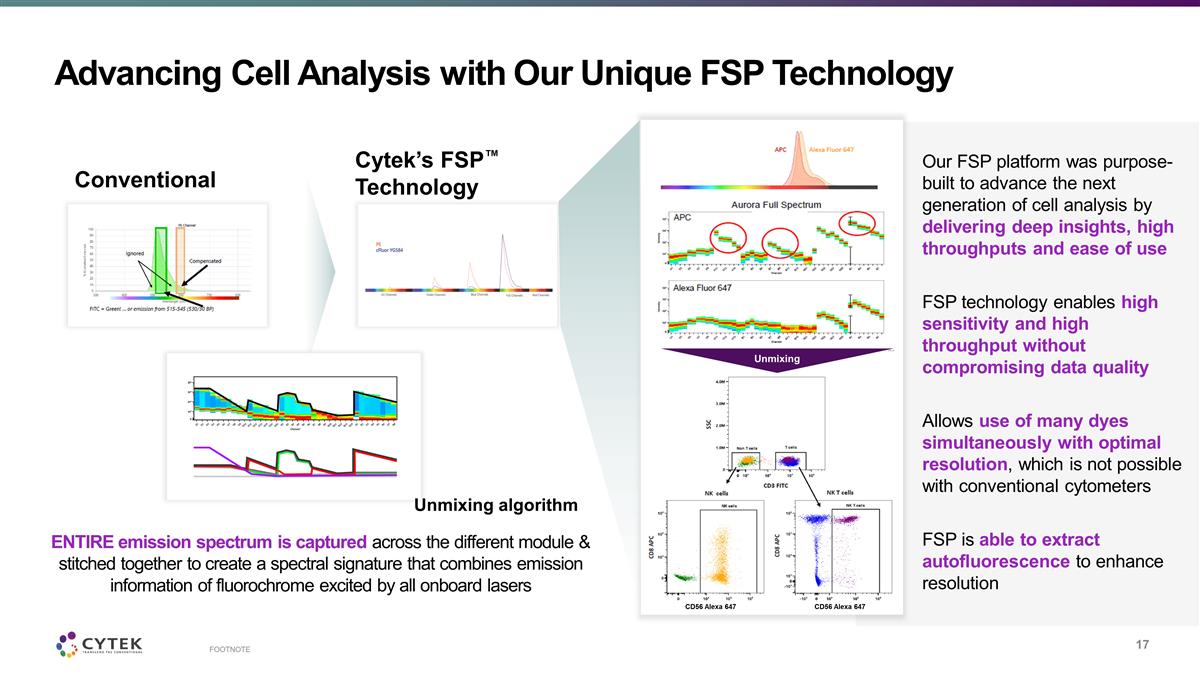
Advancing Cell Analysis with Our
Unique FSP Technology FOOTNOTE Conventional Cytek’s FSP™ Technology Our FSP platform was purpose-built to advance the next generation of cell analysis by delivering deep insights, high throughputs and ease of use FSP technology enables
high sensitivity and high throughput without compromising data quality Allows use of many dyes simultaneously with optimal resolution, which is not possible with conventional cytometers FSP is able to extract autofluorescence to enhance resolution
ENTIRE emission spectrum is captured across the different module & stitched together to create a spectral signature that combines emission information of fluorochrome excited by all onboard lasers Unmixing CD56 Alexa 647 CD56 Alexa 647 Unmixing
algorithm
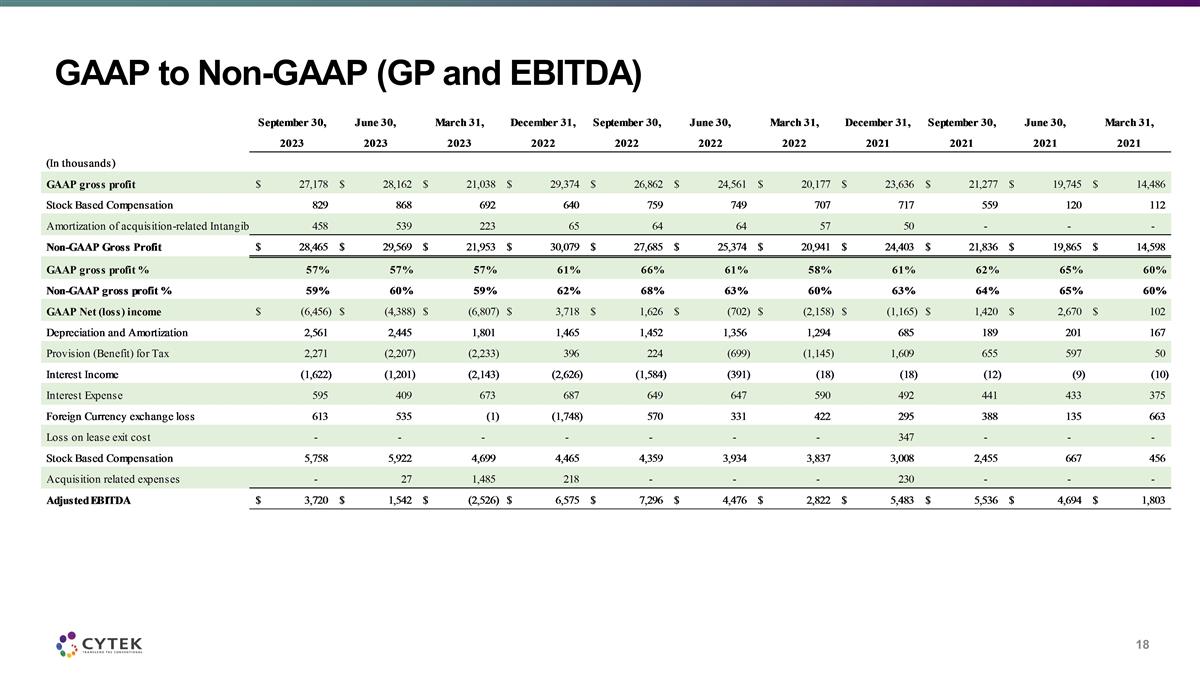
GAAP to Non-GAAP (GP and EBITDA)
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
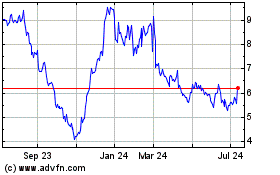
Cytek Biosciences (NASDAQ:CTKB)
Historical Stock Chart
From Oct 2024 to Nov 2024
Cytek Biosciences (NASDAQ:CTKB)
Historical Stock Chart
From Nov 2023 to Nov 2024