BioNTech, Pfizer Phase 2/3 Trial for Covid-19 Vaccine Approved in Germany
September 07 2020 - 10:22AM
Dow Jones News
By Cecilia Butini
BioNTech SE and Pfizer Inc. said Monday that they have received
approval from the German regulatory authority to commence the phase
2/3 trial in Germany for their Covid-19 vaccine.
The two pharmaceutical companies said their vaccine candidate,
identified as BNT162b2, is being tested for safety and efficacy in
a placebo-controlled trial with up to 30,000 participants globally,
including in Germany. The trial for the vaccine--which isn't yet
approved for distribution anywhere in the world--will be conducted
in about 120 sites worldwide, the companies said.
Pfizer and BioNTech are expecting to get regulatory review for
the vaccine as early as October 2020. If regulatory approval is
obtained, they plan to be able to supply up to 100 million vaccine
doses globally by the end of 2020 and roughly 1.3 billion by the
end of 2021, they said.
Write to Cecilia Butini at cecilia.butini@wsj.com
(END) Dow Jones Newswires
September 07, 2020 10:07 ET (14:07 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
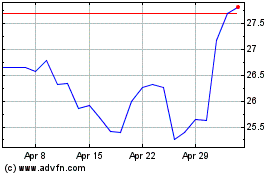
Pfizer (NYSE:PFE)
Historical Stock Chart
From Aug 2024 to Sep 2024
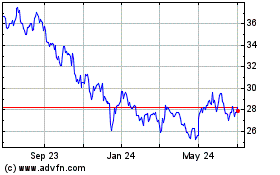
Pfizer (NYSE:PFE)
Historical Stock Chart
From Sep 2023 to Sep 2024