false
0001425205
0001425205
2024-02-28
2024-02-28
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
Current Report
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (date of earliest event reported): February 28, 2024
IOVANCE BIOTHERAPEUTICS, INC.
(Exact Name of Registrant as Specified in
Charter)
| Delaware |
| (State of Incorporation) |
| |
| 001-36860 |
|
75-3254381 |
| Commission File Number |
|
(I.R.S. Employer Identification No.) |
| |
|
|
| 825
Industrial Road, Suite 400 |
|
|
| San Carlos, California |
|
94070 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
| |
|
|
| (650) 260-7120 |
| (Registrant’s Telephone Number, Including Area Code) |
| |
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425). |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12). |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)). |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)). |
Indicate
by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act
of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading
Symbol(s) |
Name of each exchange on which
registered |
| Common stock, par value $0.000041666 per value |
IOVA |
The Nasdaq Stock Market, LLC |
On February 28, 2024, Iovance Biotherapeutics,
Inc. (the “Company”) updated its corporate presentation that it uses for presentations at healthcare conferences and to analysts,
current stockholders, and others. A copy of the Company’s presentation that it intends to use at such events is attached as Exhibit
99.1 and incorporated herein by reference.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: February 29, 2024 |
IOVANCE BIOTHERAPEUTICS, INC. |
| |
|
|
| |
|
|
| |
By: |
/s/ Frederick G. Vogt |
| |
|
Frederick G. Vogt, Interim CEO & General Counsel |
Exhibit 99.1

1 © 2024, Iovance Biotherapeutics, Inc. © 2024, Iovance Biotherapeutics, Inc. Corporate Overview February 28, 2024 1

2 © 2024, Iovance Biotherapeutics, Inc. Forward - Looking Statements Certain matters discussed in this press release are “forward - looking statements” of Iovance Biotherapeutics, Inc. (hereinafter r eferred to as the “Company,” “we,” “us,” or “our”) within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”). Without limiting the foregoing, we may, in so me cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “forecast,” “guidance,” “outlook,” “may,” “c ould,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes and are intended to identify forward - looking statements. Forward - looking stateme nts are based on assumptions and assessments made in light of management’s experience and perception of historical trends, current conditions, expected future development s, and other factors believed to be appropriate. Forward - looking statements in this press release are made as of the date of this press release, and we undertake no duty to update or re vise any such statements, whether as a result of new information, future events or otherwise. Forward - looking statements are not guarantees of future performance and are subject to risks, uncertainties, and other factors, many of which are outside of our control, that may cause actual results, levels of activity, performance, achievements, and developments to be materially different from those expressed in or implied by these forward - looking statements. Important factors that could cause actual results, developments, and business decisions to dif fer materially from forward - looking statements are described in the sections titled "Risk Factors" in our filings with the U.S. Securities and Exchange Commission, including ou r m ost recent Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q, and include, but are not limited to, the following substantial known and unknown risks and uncertainties i nherent in our business: the risks related to our ability to successfully commercialize our products, including AMTAGVI, for which we obtain U.S. Food and Drug Administration (“FDA”), Eu rop ean Medicines Agency (“EMA”), or other regulatory authority approval; the risk that the EMA or other regulatory authorities may not approve or may delay approval for our biolo gic s license application (“BLA”) submission for lifileucel in metastatic melanoma; the acceptance by the market of our products, including AMTAGVI, and their potential pricing and/or reim bur sement by payors, if approved (in the case of our product candidates), in the U.S. and other international markets and whether such acceptance is sufficient to support continu ed commercialization or development of our products, including AMTAGVI, or product candidates, respectively; our ability or inability to manufacture our therapies using third par ty manufacturers or at our own facility may adversely affect our commercial launch; the results of clinical trials with collaborators using different manufacturing processes may not be refle cte d in our sponsored trials; the risk regarding the successful integration of the recent Proleukin acquisition; the risk that the successful development or commercialization of our product s, including AMTAGVI, may not generate sufficient revenue from product sales, and we may not become profitable in the near term, or at all; the risk that future competitive or other m ark et factors may adversely affect the commercial potential for AMTAGVI; the risks related to the timing of and our ability to successfully develop, submit, obtain, or maintain FDA, EMA, or ot her regulatory authority approval of, or other action with respect to, our product candidates; whether clinical trial results from our pivotal studies and cohorts, and meetings with th e F DA, EMA, or other regulatory authorities may support registrational studies and subsequent approvals by the FDA, EMA, or other regulatory authorities, including the risk that the pl anned single arm Phase 2 IOV - LUN - 202 trial may not support registration; preliminary and interim clinical results, which may include efficacy and safety results, from ongoing c lin ical trials or cohorts may not be reflected in the final analyses of our ongoing clinical trials or subgroups within these trials or in other prior trials or cohorts; the risk that enrollment ma y need to be adjusted for our trials and cohorts within those trials based on FDA and other regulatory agency input; the risk that the changing landscape of care for cervical cancer patients may im pact our clinical trials in this indication; the risk that we may be required to conduct additional clinical trials or modify ongoing or future clinical trials based on feedback from the FDA , EMA, or other regulatory authorities; the risk that our interpretation of the results of our clinical trials or communications with the FDA, EMA, or other regulatory authorities may di ffer from the interpretation of such results or communications by such regulatory authorities (including from our prior meetings with the FDA regarding our non - small cell lung cancer clinical trials); the risk that clinical data from ongoing clinical trials of AMTAGVI will not continue or be repeated in ongoing or planned clinical trials or may not support reg ulatory approval or renewal of authorization; the risk that unanticipated expenses may decrease our estimated cash balances and forecasts and increase our estimated capital requirements ; t he effects of the COVID - 19 pandemic; and other factors, including general economic conditions and regulatory developments, not within our control.
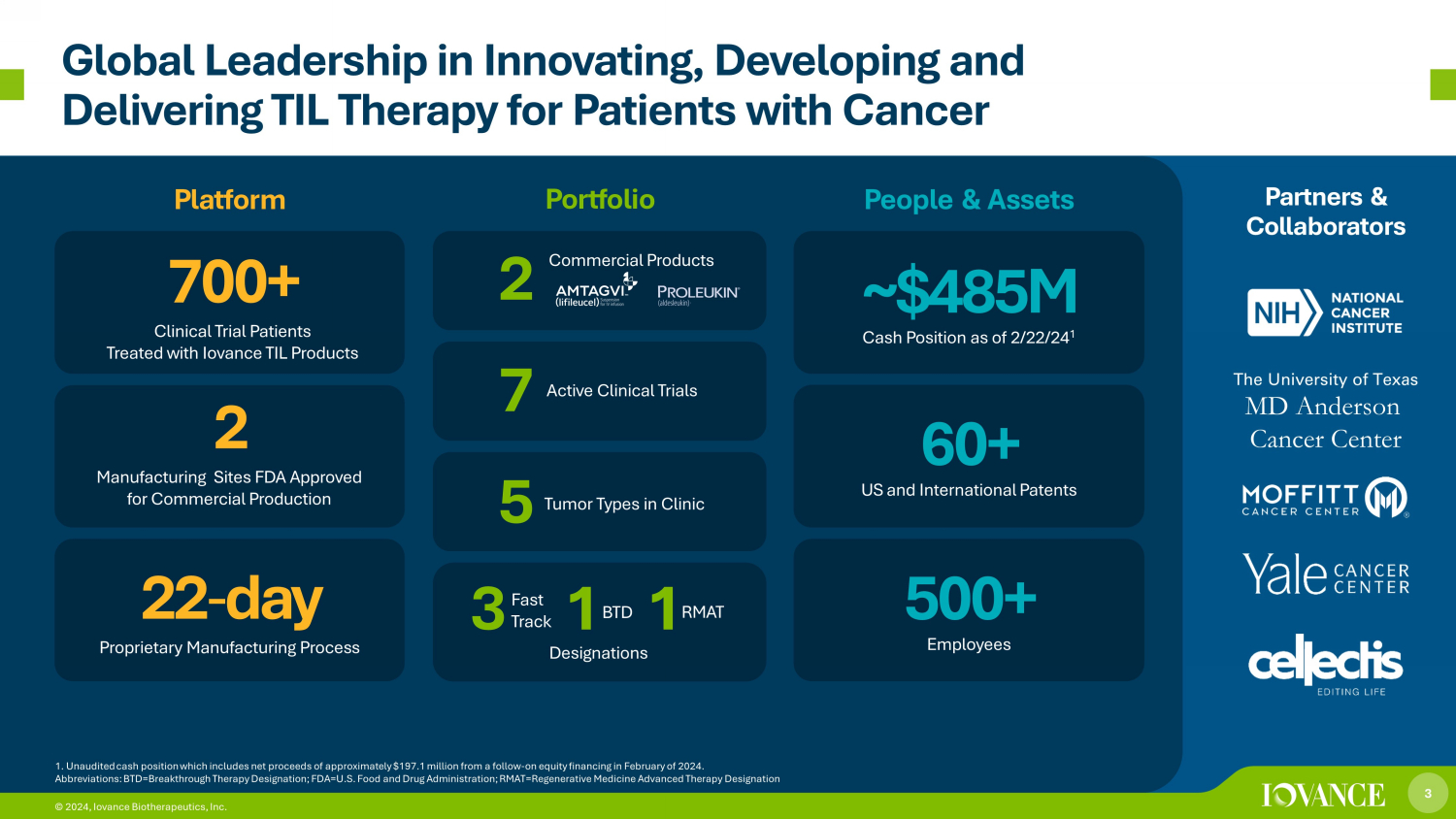
3 © 2024, Iovance Biotherapeutics, Inc. Global Leadership in Innovating, Developing and Delivering TIL Therapy for Patients with Cancer Platform Portfolio People & Assets Partners & Collaborators 1. Unaudited cash position which includes net proceeds of approximately $197.1 million from a follow - on equity financing in Febr uary of 2024. Abbreviations: BTD=Breakthrough Therapy Designation; FDA=U.S. Food and Drug Administration; RMAT =Regenerative Medicine Advanced Therapy Designation 22 - day Proprietary Manufacturing Process 2 Manufacturing Sites FDA Approved for Commercial Production 7 00+ Clinical Trial Patients Treated with Iovance TIL Products ~$ 485 M Cash Position as of 2/22/24 1 60+ US and International Patents 500+ Employees The University of Texas MD Anderson Cancer Center 3 © 2024, Iovance Biotherapeutics, Inc. 3 1 1 Designations Active Clinical Trials 7 Tumor Types in Clinic 5 Commercial Products Fast Track BTD RMAT 2
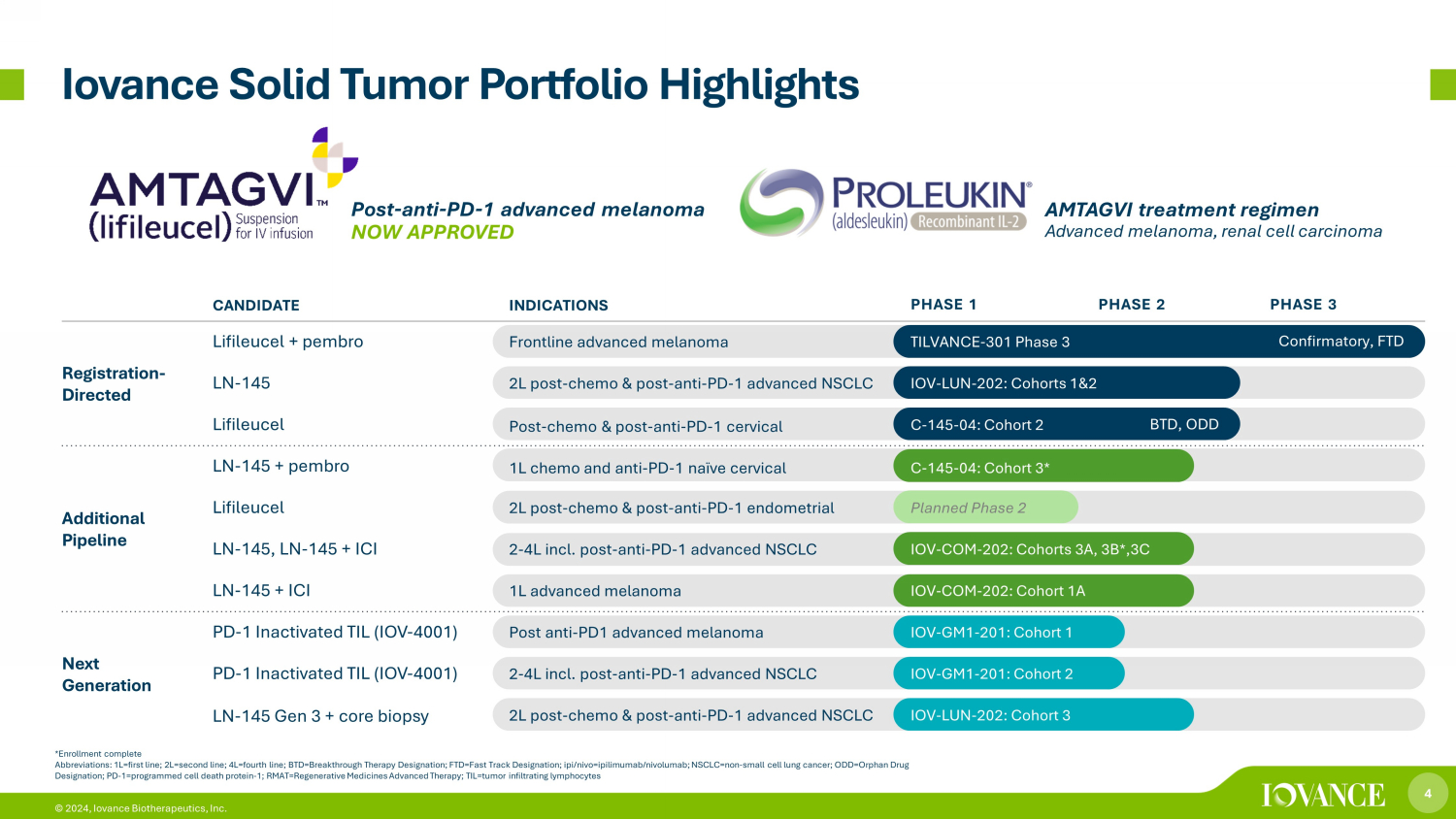
4 © 2024, Iovance Biotherapeutics, Inc. *Enrollment complete Abbreviations: 1L=first line; 2L=second line; 4L=fourth line; BTD=Breakthrough Therapy Designation; FTD=Fast Track Designatio n; ipi/nivo=ipilimumab/nivolumab; NSCLC=non - small cell lung cancer; ODD=Orphan Drug Designation; PD - 1=programmed cell death protein - 1; RMAT=Regenerative Medicines Advanced Therapy; TIL=tumor infiltrating lymphocy tes Iovance Solid Tumor Portfolio Highlights Confirmatory, FTD BTD, ODD AMTAGVI treatment regimen A dvanced melanoma, renal cell carcinoma Post - anti - PD - 1 advanced melanoma NOW APPROVED CANDIDATE INDICATIONS PHASE 1 PHASE 2 PHASE 3 Registration - Directed Lifileucel + pembro Frontline advanced melanoma TILVANCE - 301 Phase 3 LN - 145 2L post - chemo & post - anti - PD - 1 advanced NSCLC IOV - LUN - 202: Cohorts 1&2 Lifileucel Post - chemo & post - anti - PD - 1 cervical C - 145 - 04: Cohort 2 Additional Pipeline LN - 145 + pembro 1L chemo and anti - PD - 1 naïve cervical C - 145 - 04: Cohort 3* Lifileucel 2L post - chemo & post - anti - PD - 1 endometrial Planned Phase 2 LN - 145, LN - 145 + ICI 2 - 4L incl. post - anti - PD - 1 advanced NSCLC IOV - COM - 202: Cohorts 3A, 3B*,3C LN - 145 + ICI 1L advanced melanoma IOV - COM - 202: Cohort 1A Next Generation PD - 1 Inactivated TIL (IOV - 4001) Post anti - PD1 advanced melanoma IOV - GM1 - 201: Cohort 1 PD - 1 Inactivated TIL (IOV - 4001) 2 - 4L incl. post - anti - PD - 1 advanced NSCLC IOV - GM1 - 201: Cohort 2 LN - 145 Gen 3 + core biopsy 2L post - chemo & post - anti - PD - 1 advanced NSCLC IOV - LUN - 202: Cohort 3
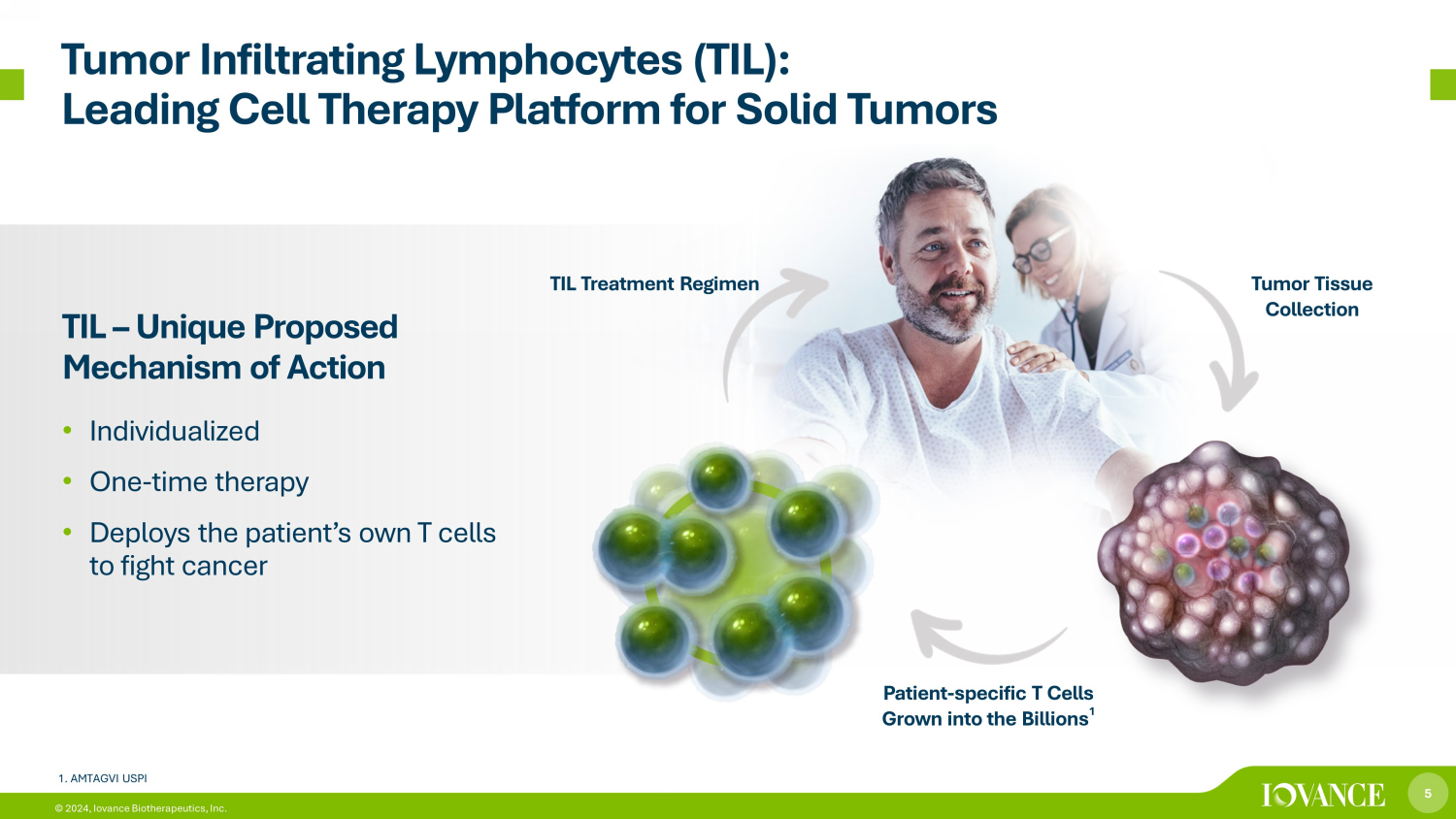
5 © 2024, Iovance Biotherapeutics, Inc. Tumor Infiltrating Lymphocytes (TIL): Leading Cell Therapy Platform for Solid Tumors TIL – Unique Proposed Mechanism of Action • Individualized • One - time therapy • Deploys the patient’s own T cells to fight cancer Tumor Tissue Collection Patient - specific T Cells Grown into the Billions 1 TIL Treatment Regimen 1. AMTAGVI USPI

6 © 2024, Iovance Biotherapeutics, Inc. 6 AMTAGVI ¯ (lifileucel): First and only one - time, individualized T cell therapy approved by FDA for a solid tumor cancer

7 © 2024, Iovance Biotherapeutics, Inc. 1. AMTAGVI USPI
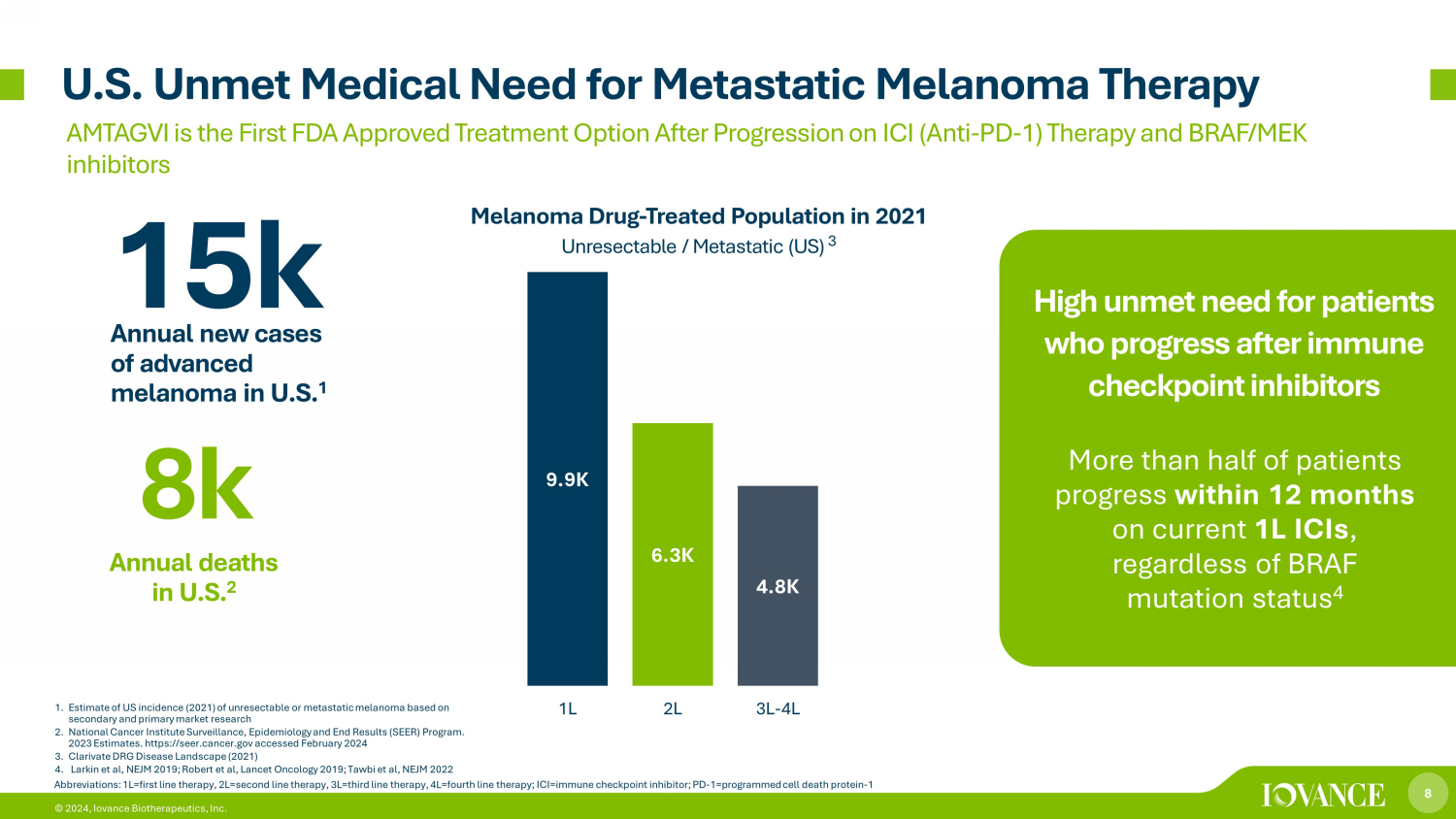
8 © 2024, Iovance Biotherapeutics, Inc. U.S. Unmet Medical Need for Metastatic Melanoma Therapy AMTAGVI is th e First FDA Approved Treatment Option After Progression on ICI (Anti - PD - 1) Therapy and BRAF/MEK inhibitors More than half of patients progress within 12 months on current 1L ICIs , regardless of BRAF mutation status 4 High unmet need for patients who progress after immune checkpoint inhibitors 9.9K 6.3K 4.8K 1L 2L 3L-4L Melanoma Drug - Treated Population in 2021 Unresectable / Metastatic (US) 3 Abbreviations: 1L=first line therapy, 2L=second line therapy, 3L=third line therapy, 4L=fourth line therapy; ICI=immune check poi nt inhibitor; PD - 1=programmed cell death protein - 1 15k Annual new cases of advanced melanoma in U.S. 1 8k Annual deaths in U.S. 2 1. Estimate of US incidence (2021) of unresectable or metastatic melanoma based on secondary and primary market research 2. National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program. 2023 Estimates. https://seer.cancer.gov accessed February 2024 3. Clarivate DRG Disease Landscape (2021) 4. Larkin et al, NEJM 2019; Robert et al, Lancet Oncology 2019; Tawbi et al, NEJM 2022
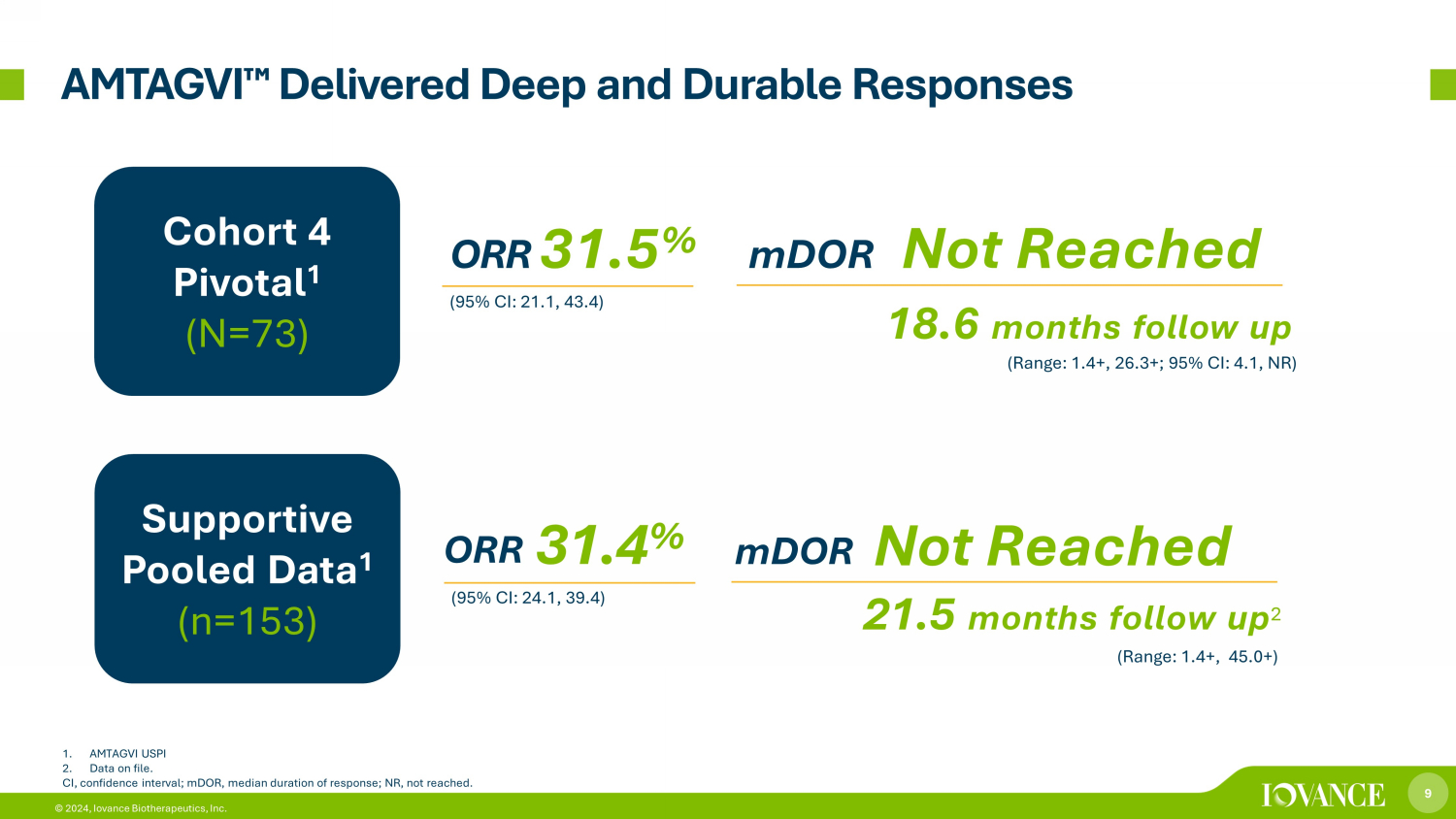
9 © 2024, Iovance Biotherapeutics, Inc. AMTAGVI ¯ Delivered Deep and Durable Responses ORR 31.5 % mDOR Not Reached (95% CI: 21.1, 43.4) mDOR Not Reached ORR 31.4 % (95% CI: 24.1, 39.4) (Range: 1.4+, 45.0+) (Range: 1.4+, 26.3+; 95% CI: 4.1, NR) 18.6 months follow up 21.5 months follow up 2 1. AMTAGVI USPI 2. Data on file. CI, confidence interval; mDOR, median duration of response; NR, not reached. Cohort 4 Pivotal 1 (N=73) Supportive Pooled Data 1 (n=153)
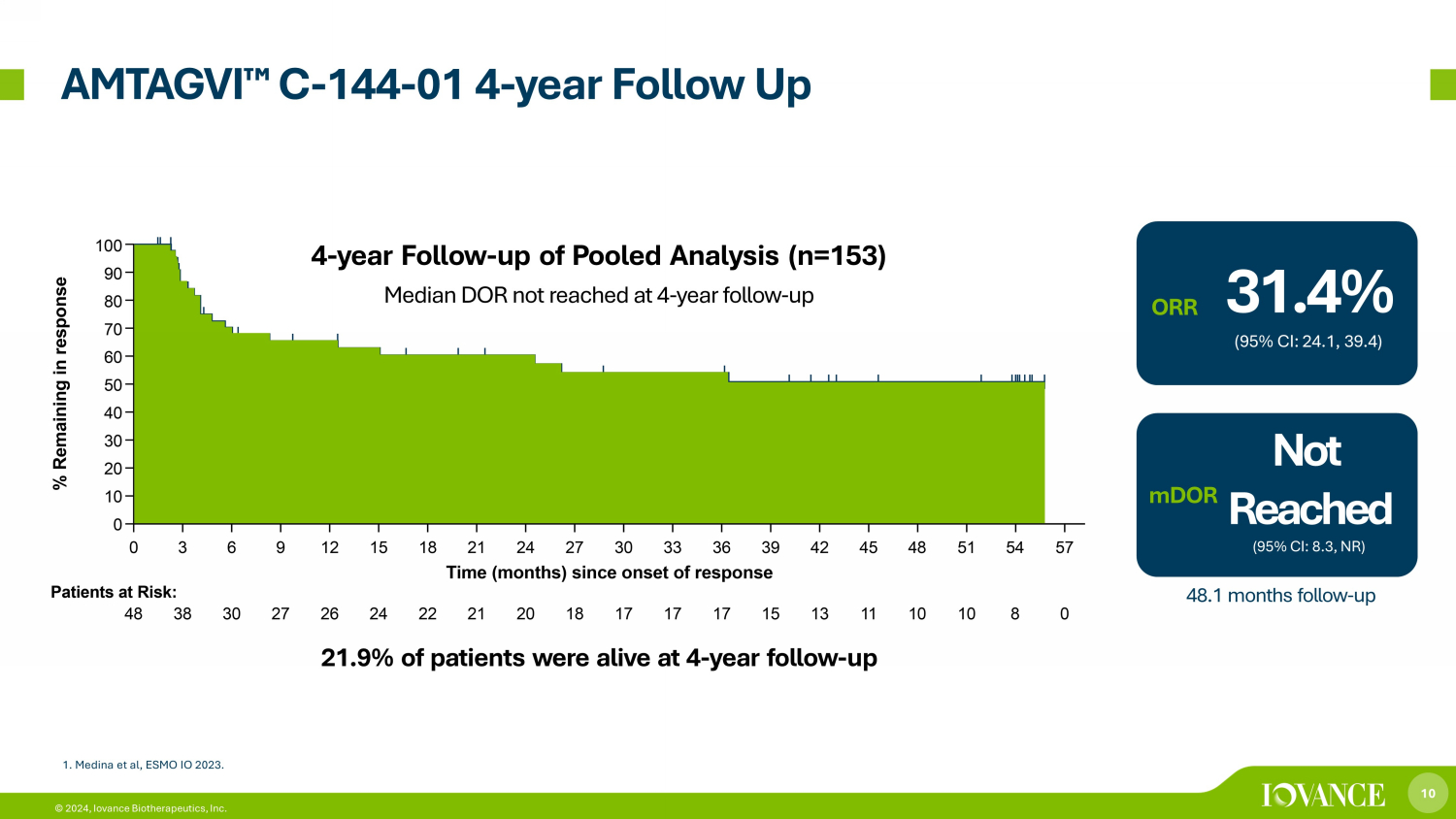
10 © 2024, Iovance Biotherapeutics, Inc. AMTAGVI ¯ C - 144 - 01 4 - year Follow Up Median DOR not reached at 4 - year follow - up 4 - year Follow - up of Pooled Analysis (n=153) 31.4% (95% CI: 24.1, 39.4) ORR Not Reached (95% CI: 8.3, NR) mDOR 48.1 months follow - up 21.9% of patients were alive at 4 - year follow - up 1. Medina et al, ESMO IO 2023 .
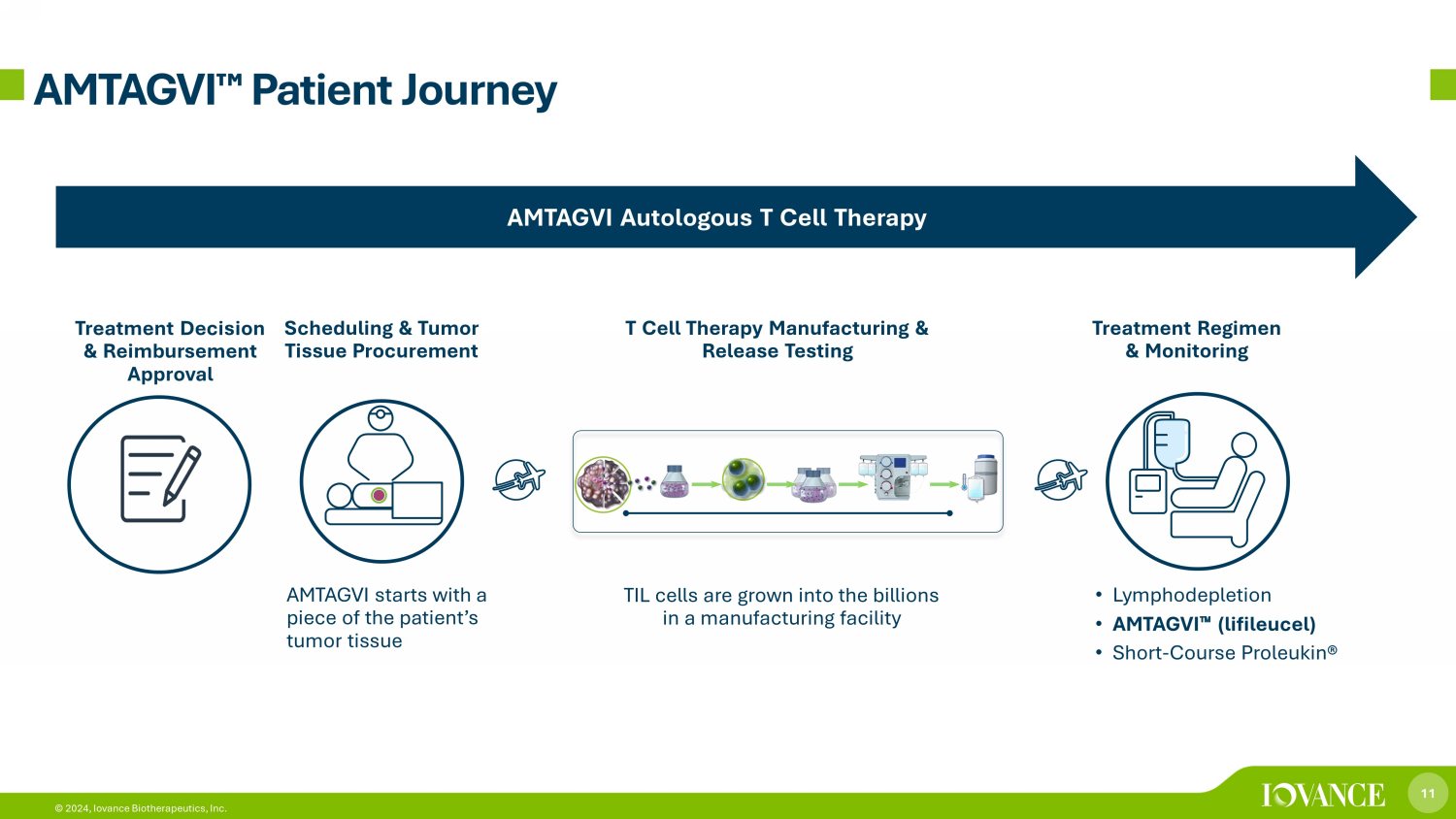
11 © 2024, Iovance Biotherapeutics, Inc. AMTAGVI ¯ Patient Journey AMTAGVI Autologous T Cell Therapy ) AMTAGVI starts with a piece of the patient’s tumor tissue • Lymphodepletion • AMTAGVI 䉼 (lifileucel) • Short - Course Proleukin® Scheduling & Tumor Tissue Procurement Treatment Regimen & Monitoring Treatment Decision & Reimbursement Approval T Cell Therapy Manufacturing & Release Testing TIL cells are grown into the billions in a manufacturing facility

12 © 2024, Iovance Biotherapeutics, Inc. Iovance Cell Therapy Center: i CTC • Built - to - suit custom facility in Navy Yard Philadelphia • Annual capacity for up to several thousand patients as built • Expansion underway for additional capacity within iCTC over next few years • Additional CDMO capacity • Control to optimize capacity, quality & COGS FDA - Approved Cell Therapy Manufacturing Facility Dedicated to Commercial and Clinical TIL Cell Therapies
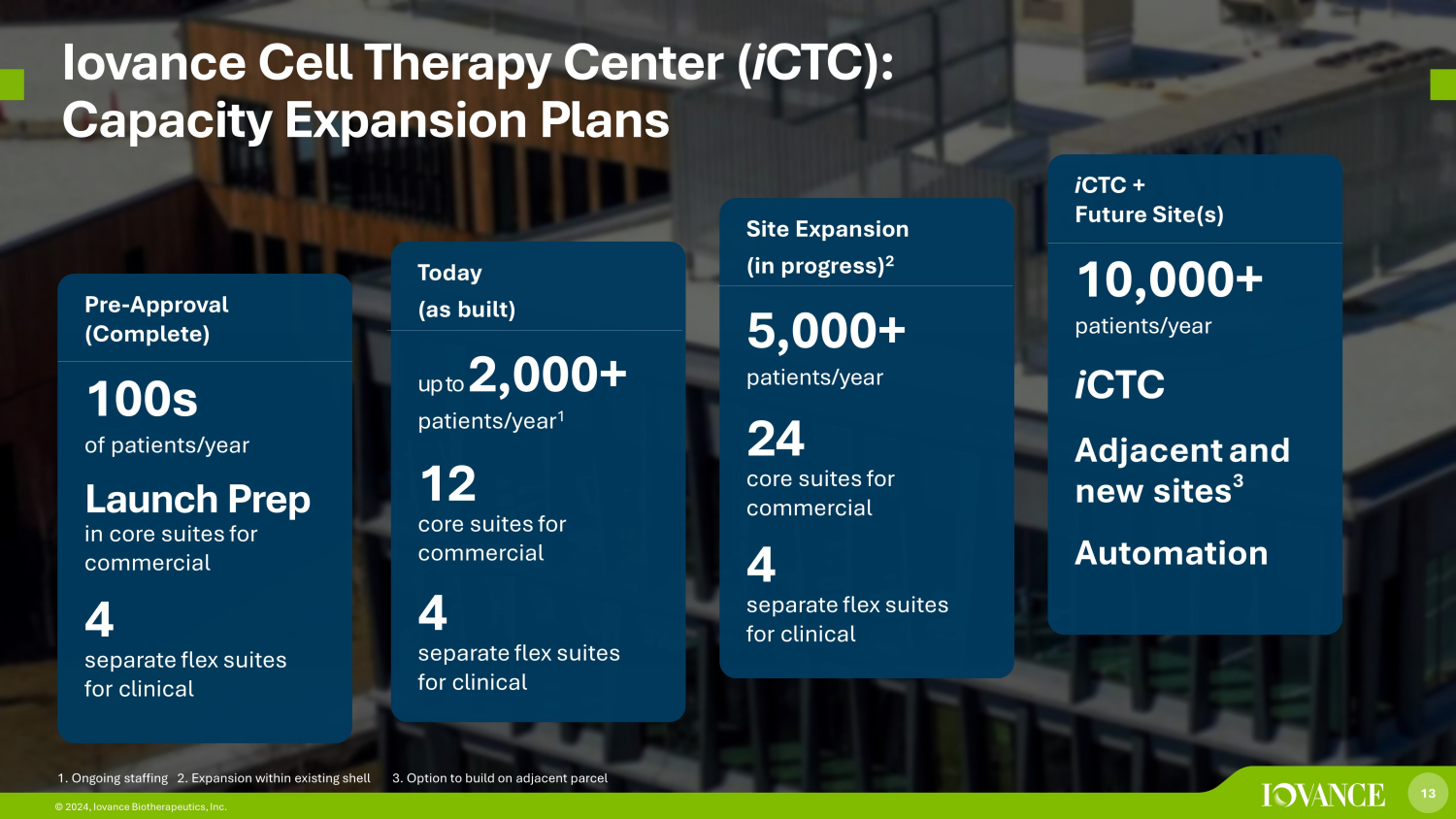
13 © 2024, Iovance Biotherapeutics, Inc. Iovance Cell Therapy Center ( i CTC): Capacity Expansion Plans Pre - Approval (Complete) 100s of patients/year Launch Prep in core suites for commercial 4 separate flex suites for clinical Today (as built) up to 2,000 + patients/year 1 12 core suites for commercial 4 separate flex suites for clinical Site Expansion (in progress) 2 5,000+ patients/year 24 core suites for commercial 4 separate flex suites for clinical i CTC + Future Site(s) 10,000+ patients/year i CTC Adjacent and new sites 3 Automation 13 © 2024, Iovance Biotherapeutics, Inc. 1. Ongoing staffing 2. Expansion within existing shell 3. Option to build on adjacent parcel

14 © 2024, Iovance Biotherapeutics, Inc. Proleukin® (aldesleukin) Strategic Benefits • Significant revenue anticipated with AMTAGVI ¯ launch • IL - 2 supply chain secured for AMTAGVI regimen • Lower clinical trial costs and COGS anticipated £167.7M Upfront investment Short course Proleukin® is administered after AMTAGVI® to promote T cell growth in the body Key Transaction Figures £41.7M Following first lifileucel approval Global Rights Acquired in May of 2023

15 © 2024, Iovance Biotherapeutics, Inc. Targeting Potential Authorized Treatment Centers (ATCs) 1. Amtagvi.com (Last accessed February 28, 2024) Abbreviations : ATC= Authorized Treatment Centers; NCCN=National Comprehensive Cancer Network; KOL=Key Opinion Leaders; BMT=Bone Marrow Transplant Targeting Considerations • Patient volume • NCCN status, KOLs • Existing cell therapy / BMT • Inpatient capacity • Iovance clinical trial (s) Drive Demand • Top account prioritization • Community referrals 30 Active ATCs at Approval; ~50 ATCs Expected 90 Days Post - PDUFA

16 © 2024, Iovance Biotherapeutics, Inc. 55% Commercial Medicare IPPS Exempt 4% 17% Medicare Advantage 8% Medicare FFS 7% Medicaid 9% Other Market Access Metastatic Melanoma Payer Mix 1 All Treatment Settings and Lines of Therapy 1. Metastatic Melanoma Insurance Claims Analysis, TIL - eligible patients treated in the ATC setting(1/1/2018 – 6/30/2021). Medicai d is 6% Medicaid Advantage and 1% Medicaid Fee - For - Service; For the 12% Medicare FFS lives, 11 PPS - exempt hospitals are reimbursed by Medicare FFS on a cost - basis (~4%), with the remaining Medicare FFS lives (~8%) reimbursed under DRG - 018 payment methodology, NTAP/Outlier payments may add to the total Medicare reimbursement. Other segment includes cash, self - insured, VA, and other unidentifiable claims. Abbreviations: FFS=Fee - For - Service; ICD - 10 PCS=International Classification of Diseases, 10 th Revision, Procedure Coding System; NTAP = New Technology Add - on Payment Payers appreciate the high unmet need, lack of treatment options, and AMTAGVI clinical value Anticipated Access • Engagement with payers responsible for ~90% of covered lives • Strong hospital reimbursement expected ̶ Inpatient payment methodologies are established ̶ Key payers expected to reimburse majority of provider costs • Expect similar coverage to CAR - Ts
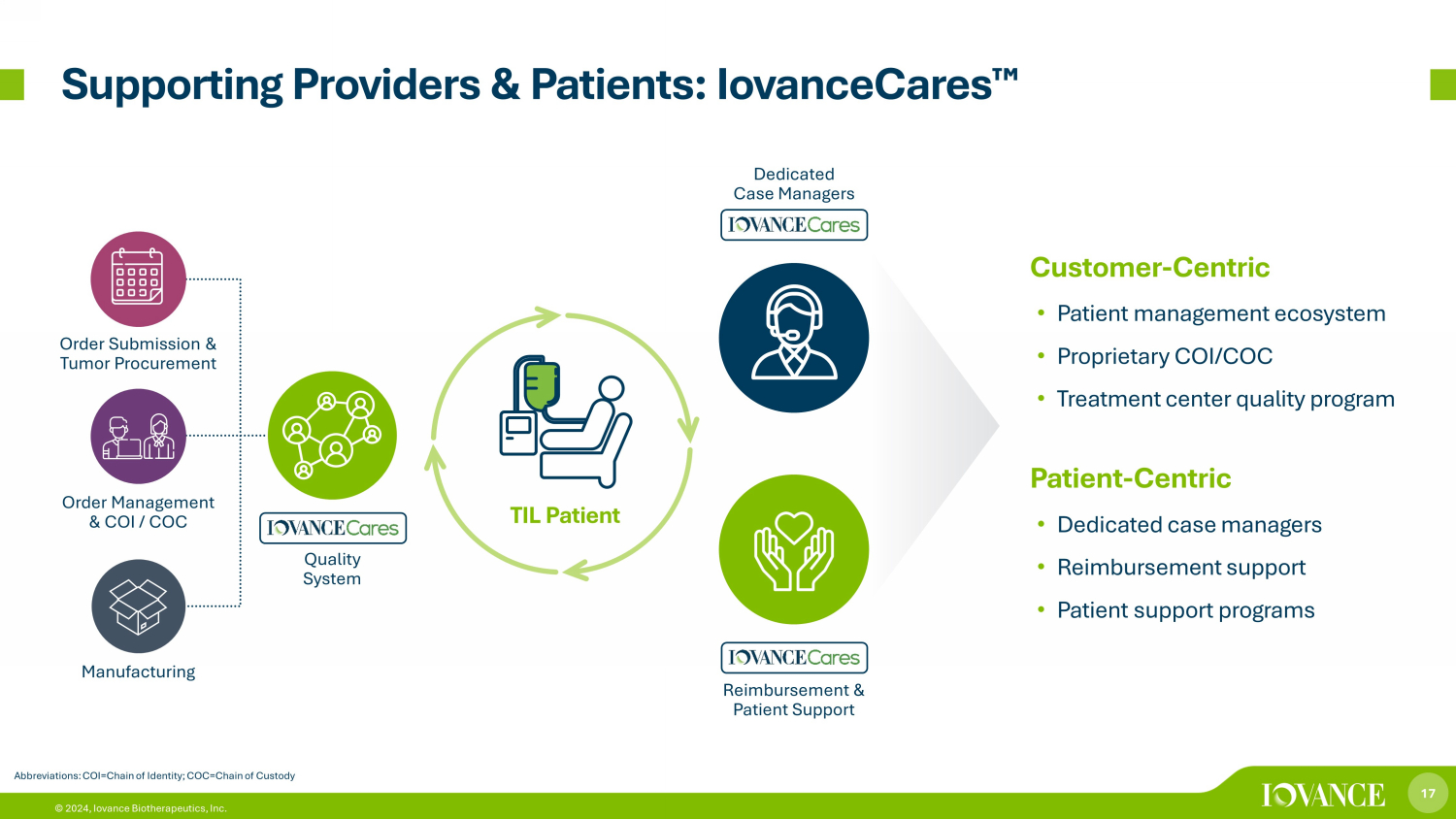
17 © 2024, Iovance Biotherapeutics, Inc. Supporting Providers & Patients: IovanceCares ¯ Abbreviations: COI=Chain of Identity; COC=Chain of Custody Customer - Centric • Patient management ecosystem • Proprietary COI/COC • Treatment center quality program Patient - Centric • Dedicated case managers • Reimbursement support • Patient support programs Quality System Dedicated Case Managers Reimbursement & Patient Support Order Submission & Tumor Procurement Order Management & COI / COC Manufacturing TIL Patient

18 © 2024, Iovance Biotherapeutics, Inc. 18 AMTAGVI ¯ Expansion Plans in Advanced Melanoma
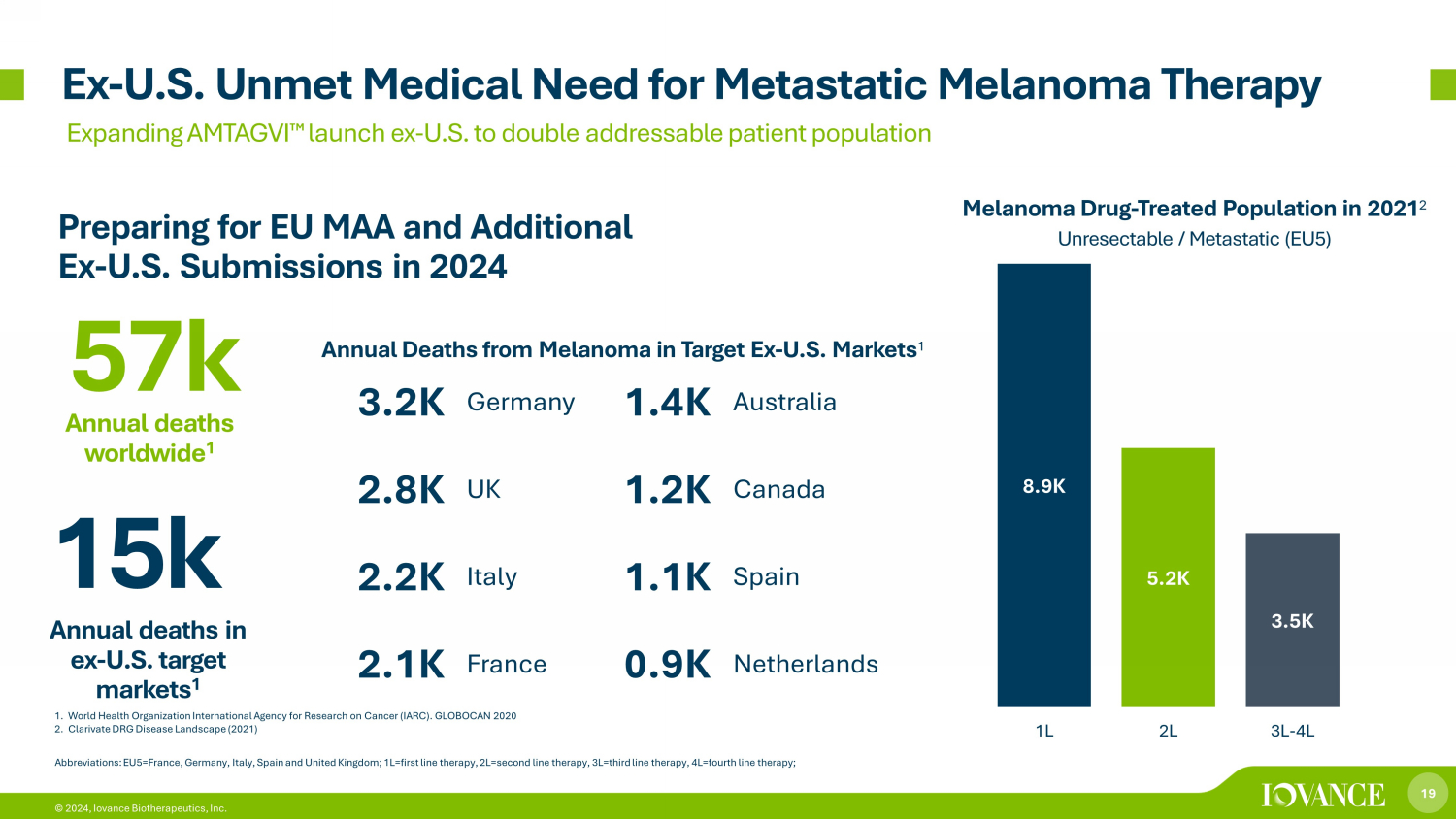
19 © 2024, Iovance Biotherapeutics, Inc. 1. World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2020 2. Clarivate DRG Disease Landscape (2021) Ex - U.S. Unmet Medical Need for Metastatic Melanoma Therapy Expanding AMTAGVI ¯ launch ex - U.S. to double addressable patient population 8.9K 5.2K 3.5K 1L 2L 3L-4L Melanoma Drug - Treated Population in 2021 2 Unresectable / Metastatic (EU5) Abbreviations: EU5=France, Germany, Italy, Spain and United Kingdom; 1L=first line therapy, 2L=second line therapy, 3L=third lin e therapy, 4L=fourth line therapy; 15k Annual deaths in ex - U.S. target markets 1 3.2K Germany 1.4K Australia 2.8K UK 1.2K Canada 2.2K Italy 1.1K Spain 2.1K France 0.9K Netherlands 57k Annual deaths worldwide 1 Annual Deaths from Melanoma in Target Ex - U.S. Markets 1 Preparing for EU MAA and Additional Ex - U.S. Submissions in 2024

20 © 2024, Iovance Biotherapeutics, Inc. 1:1 Randomization TILVANCE - 301 Global Phase 3 and Confirmatory Trial LIFILEUCEL + PEMBROLIZUMAB IN FRONTLINE ADVANCED MELANOMA Abbreviations: BIRC, blinded independent review committee; ORR=objective response rate; PD=progressive disease; PD - 1, programme d cell death protein - 1; PFS=progression free survival Arm A: lifileucel plus pembrolizumab Long - term follow up Patient Population Unresectable or metastatic melanoma; no prior therapy for metastatic disease N=670 Arm B: pembrolizumab alone Study Design with FDA Agreement • Dual Primary Endpoints: ORR & PFS • Registrational for frontline melanoma • Confirmatory for full approval of AMTAGVI in post - anti - PD - 1 melanoma • First patient randomized 2Q23 Option to crossover to lifileucel after BIRC - confirmed PD Randomized, multicenter study with optional crossover to offer all patients potential to receive Iifileucel (NCT05727904)

21 © 2024, Iovance Biotherapeutics, Inc. Lifileucel in combination with anti - PD - 1/PD - L1 therapy in ICI - naïve patients (IOV - COM - 202 Cohort 1A, N=12 ) 1 Iovance TIL Clinical Highlights in Combination with Pembrolizumab in Metastatic Melanoma IOV - COM - 202 COHORT 1A MELANOMA COMBINATION (TIL+PEMBROLIZUMAB) • 8 / 12 patients had a confirmed objective response per RECIST v1.1 (3 CRs & 5 PRs) • 6 / 8 responders had ongoing response • 5 responders had DOR >1 year • FDA Fast Track Designation 66.7% ORR 1. As assessed by investigator using RECIST 1.1 (January 20, 2022 data cutoff) 2. Each bar is presented for each patient starting from date of TIL infusion up to date of new anti - cancer therapy, end of assessme nt, death, or data cutoff date, whichever occurs earlier. Abbreviations: CR=complete response; ICI=immune checkpoint inhibitor; ORR=objective response rate; PR=partial response; SD=st abl e disease; pembro=pembrolizumab; RECIST=Response Evaluation Criteria in Solid Tumors Time (months) since TIL Infusion 1A-12 1A-11 1A-04 1A-07 1A-06 1A-05 1A-01 1A-03 S u b j e c t s 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Time (months) since TIL Infusion Pembro Infusion Progression Ongoing on Study PR Start CR Start Time (months) since TIL Infusion 1A - 03 1A - 01 1A - 05 1A - 06 1A - 07 1A - 04 1A - 11 1A - 12 Patient ID Time to Response for Responders 2 Patient ID CRPRSD Best Overall Response -30 1A-09 1A-02 1A-08 1A-01 1A-11 1A-12 1A-04 1A-05 1A-03 1A-06 1A-07 -100 -80 -60 -40 -20 0 20 % C h a n g e f r o m B a s e l i n e N=0 N=11 Cohort 1A % Change from Baseline SD PR CR 1A - 09 1A - 02 1A - 08 1A - 01 1A - 11 1A - 12 1A - 04 1A - 05 1A - 03 1A - 06 1A - 07 Patient ID Best Overall Response for Evaluable Patients

22 © 2024, Iovance Biotherapeutics, Inc. 22 TIL Therapy Pipeline
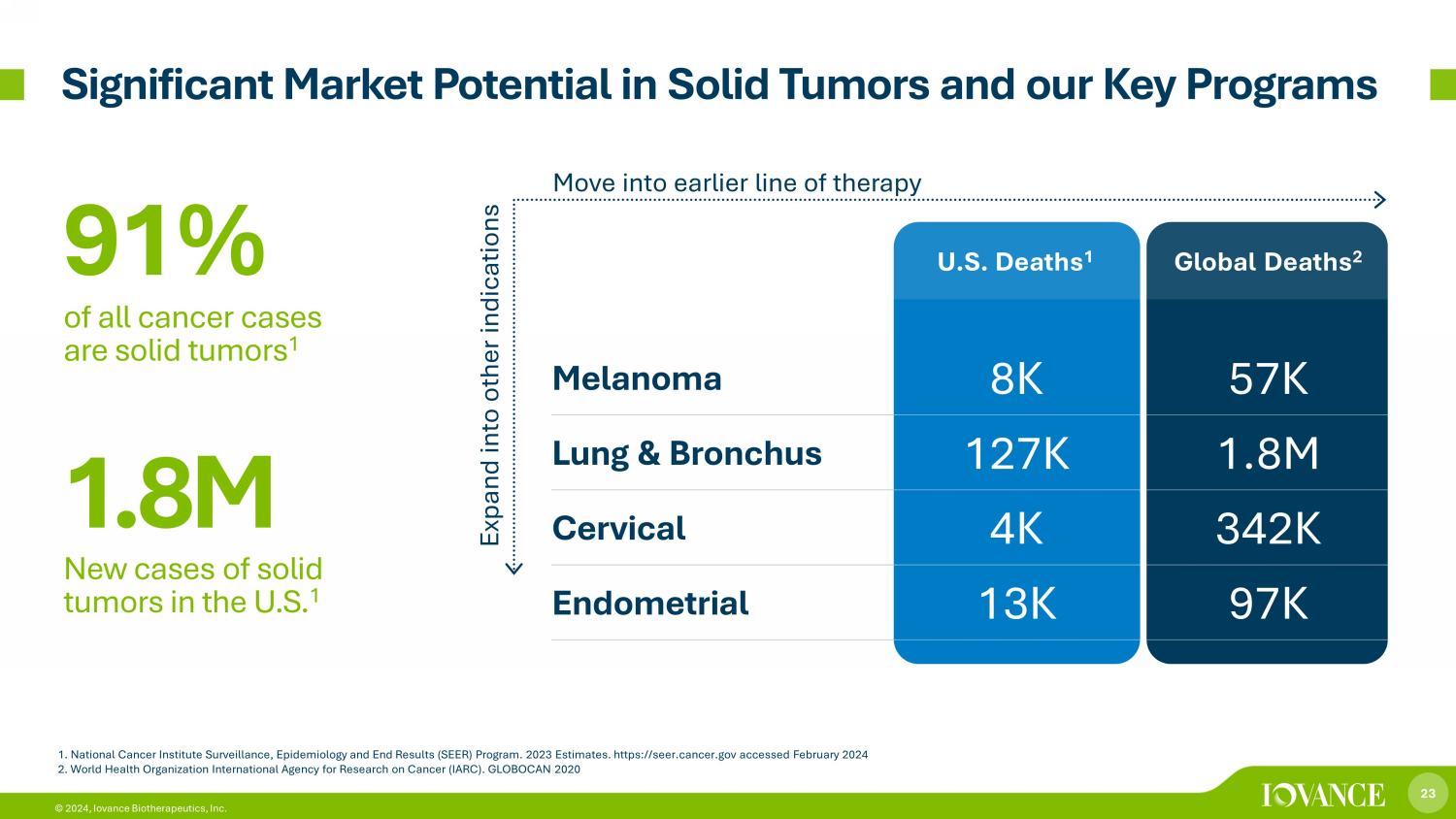
23 © 2024, Iovance Biotherapeutics, Inc. 1. National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program. 2023 Estimates. https://seer.cancer.g ov accessed February 2024 2. World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2020 U.S. Deaths 1 Global Deaths 2 Melanoma 8K 57K Lung & Bronchus 127K 1.8M Cervical 4K 342K Endometrial 13K 97K 91% of all cancer cases are solid tumors 1 1 .8 M New cases of solid tumors in the U.S. 1 Expand into other indications Move into earlier line of therapy Significant Market Potential in Solid Tumors and our Key Programs

24 © 2024, Iovance Biotherapeutics, Inc. 1. National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program. 2023 Estimates. https://seer.cancer.gov acc essed February 2024 2. American Cancer Society, Lung Cancer. https://www.cancer.org/cancer/types/lung - cancer/about.html accessed July 2023 3. National Cancer Database, NSCLC survival from >1 million patients assessed. Lou Y et al. Survival trends among non - small - cell lu ng cancer patients over a decade: impact of initial therapy at academic centers. Cancer Med. 2018. 4. Clarivate DRG Disease Landscape (2021) Abbreviations: EU5=France, Germany, Italy, Spain and United Kingdom; 1L=first line therapy, 2L=second line therapy, 3L=third lin e therapy, 4L=fourth line therapy; mOS=median overall survival Potential Market for Advanced Non - Small Cell Lung Cancer (NSCLC) Addressing a Substantial Unmet Need in Metastatic NSCLC Iovance TIL clinical program: • 6 cohorts across 3 trials • Multiple treatment regimens • Various populations and stages of disease Annual Deaths in U.S. 2 127K annual deaths in U.S. 1 107K 51.6K 19.0K 116K 46.2K 12.3K 1L 2L 3L US EU5 NSCLC Drug - Treated Population in 2022 Stage IV (US and EU5) 4 Leading cause of U.S. cancer deaths, accounting for ~1 in 5 cancer - related deaths 2 9% 5 - year survival rate 2 and real - world overall survival <6 months 3 in U.S.
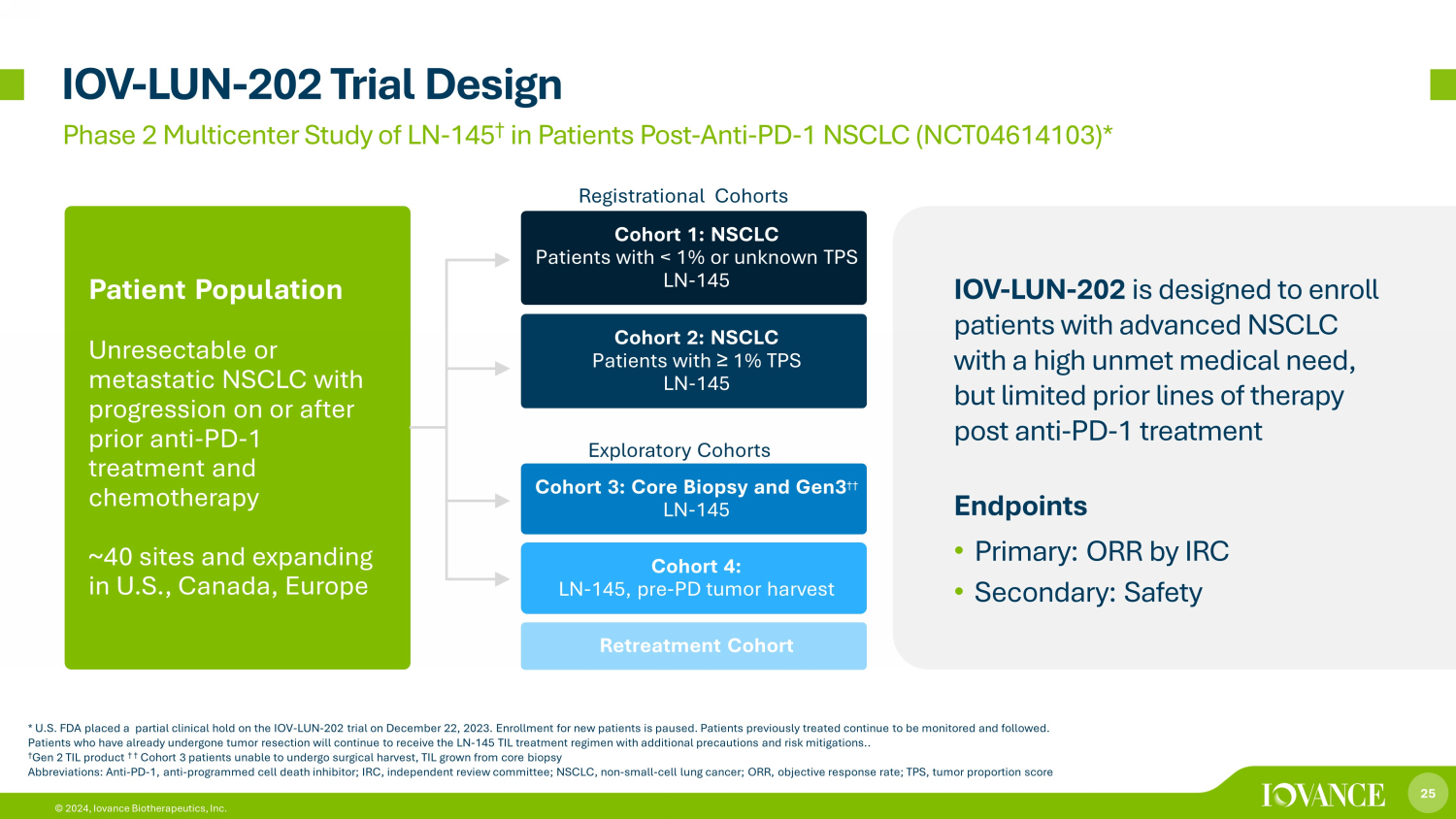
25 © 2024, Iovance Biotherapeutics, Inc. IOV - LUN - 202 Trial Design Phase 2 Multicenter Study of LN - 145 † in Patients Post - Anti - PD - 1 NSCLC (NCT04614103)* Endpoints • Primary: ORR by IRC • Secondary: Safety * U.S. FDA placed a partial clinical hold on the IOV - LUN - 202 trial on December 22, 2023 . Enrollment for new patients is paused. Patients previously treated continue to be monitored and followed. Patients who have already undergone tumor resection will continue to receive the LN - 145 TIL treatment regimen with additional pr ecautions and risk mitigations.. † Gen 2 TIL product † † Cohort 3 patients unable to undergo surgical harvest, TIL grown from core biopsy Abbreviations: Anti - PD - 1, anti - programmed cell death inhibitor; IRC, independent review committee; NSCLC, non - small - cell lung ca ncer; ORR, objective response rate; TPS, tumor proportion score Iovance TIL Therapy LN - 145 in NSCLC IOV - LUN - 202 is designed to enroll patients with advanced NSCLC with a high unmet medical need, but limited prior lines of therapy post anti - PD - 1 treatment Patient Population Unresectable or metastatic NSCLC with progression on or after prior anti - PD - 1 treatment and chemotherapy ~40 sites and expanding in U.S., Canada, Europe Cohort 1: NSCLC Patients with < 1% or unknown TPS LN - 145 Cohort 2: NSCLC Patients with ≥ 1% TPS LN - 145 Cohort 3: Core Biopsy and Gen3 †† LN - 145 Cohort 4: LN - 145, pre - PD tumor harvest Retreatment Cohort Exploratory Cohorts Registrational Cohorts
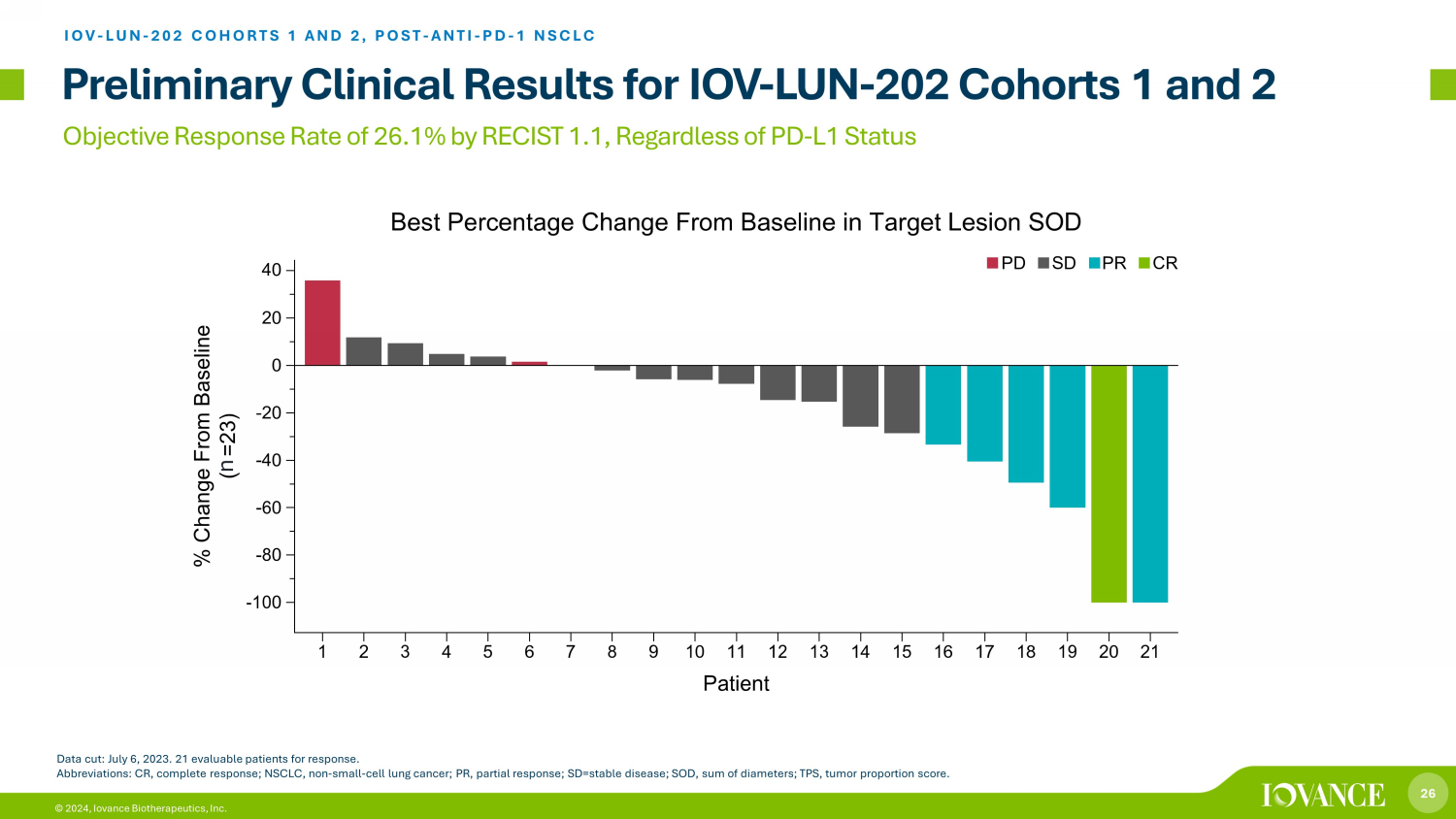
26 © 2024, Iovance Biotherapeutics, Inc. Objective Response Rate of 26.1% by RECIST 1.1, Regardless of PD - L1 Status Preliminary Clinical Results for IOV - LUN - 202 Cohorts 1 and 2 IOV - LUN - 202 COHORTS 1 AND 2, POST - ANTI - PD - 1 NSCLC Data cut: July 6, 2023. 21 evaluable patients for response. Abbreviations: CR, complete response; NSCLC, non - small - cell lung cancer; PR, partial response; SD=stable disease; SOD, sum of di ameters; TPS, tumor proportion score. n

27 © 2024, Iovance Biotherapeutics, Inc. All Patients Progressed on or After Anti - PD - 1 Therapy and Chemotherapy Preliminary Clinical Results for IOV - LUN - 202 Cohorts 1 and 2 1. Data cut: July 6, 2023. Responses were assessed by investigator. 2. Patients who have progressed on or after chemotherapy and anti - PD - 1 therapy for advanced (unresectable or metastatic) NSCLC with out EGFR, ROS or ALK genomic mutations and had received at least one line of an FDA - approved targeted therapy if indicated by other actionable tumor mutations. Abbreviations: AE, adverse event; CI, confidence interval; CR, complete response; ICI, immune checkpoint inhibitor; NE, not e val uable; NMA - LD, non - myeloablative lymphodepletion; NSCLC, non - small - cell lung cancer; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; TEAE, trea tme nt - emergent AE. Iovance TIL Therapy LN - 145 in NSCLC Cohort 1 + 2 (n=23) 2 Objective Response Rate, n (%) 1 6 (26.1) (95% CI) (10.2, 48.4) Best overall response, n (%) CR 1 (4.3) PR 5 (21.7) SD 13 (56.5) PD 2 (8.7) NE 2 (8.7) IOV - LUN - 202 COHORTS 1 AND 2, POST - ANTI - PD - 1 NSCLC TEAEs were consistent with the underlying disease and known AE profiles of NMA - LD and IL - 2

28 © 2024, Iovance Biotherapeutics, Inc. All Responses Remain Ongoing at Time of Data Cut Data cut: July 6, 2023. A bar is presented for each patient starting from date of LN - 145 infusion up to date of new anti - cancer therapy, end of assessme nt, death, or data cutoff date, whichever occurs earlier. Abbreviations: CR, complete response; DOR, duration of response; NSCLC, non - small - cell lung cancer; PR, partial response. IOV - LUN - 202 COHORTS 1 AND 2, POST - ANTI - PD - 1 NSCLC Preliminary Clinical Results for IOV - LUN - 202 Cohorts 1 and 2
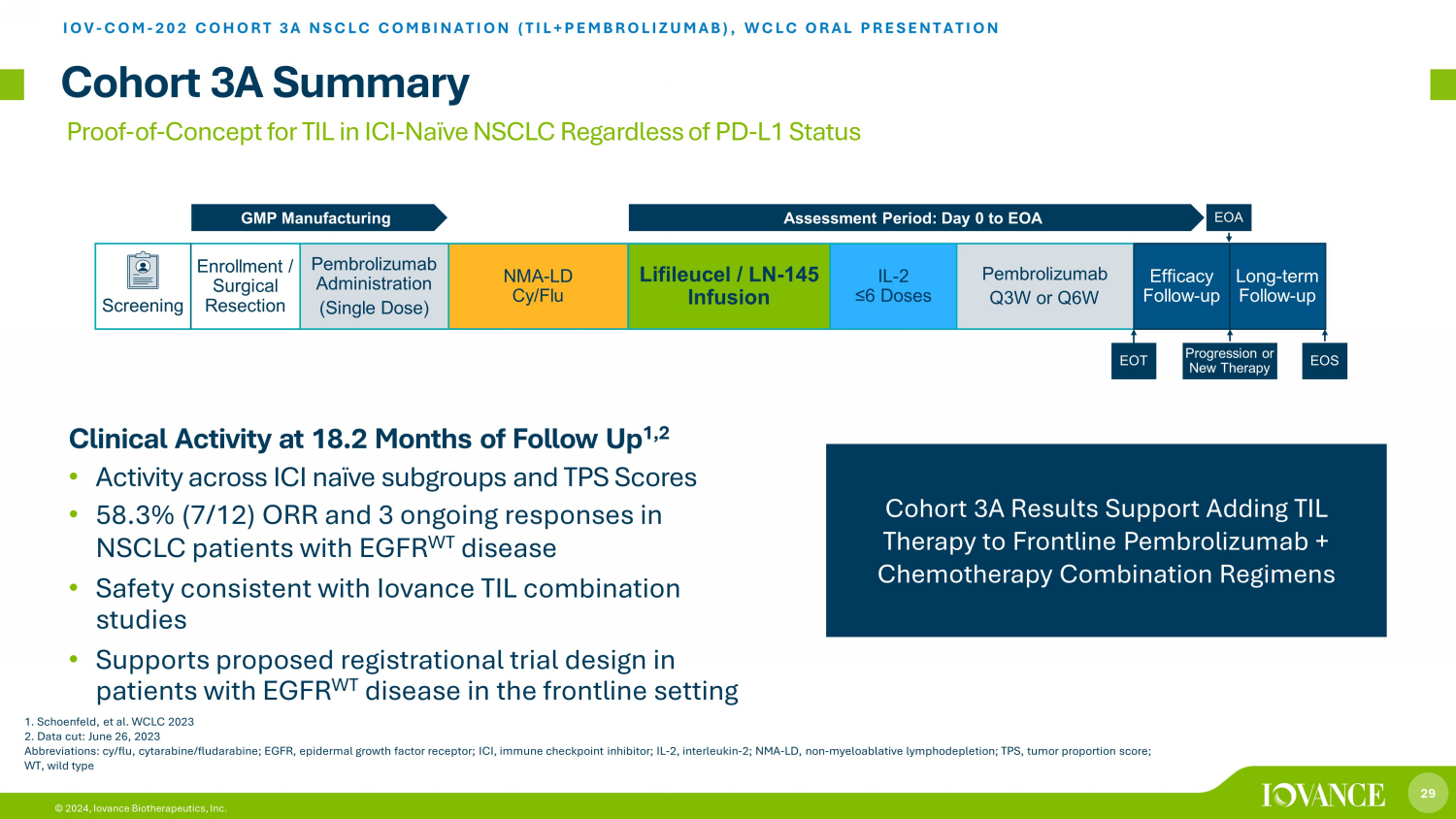
29 © 2024, Iovance Biotherapeutics, Inc. Proof - of - Concept for TIL in ICI - Naïve NSCLC Regardless of PD - L1 Status Cohort 3A Summary IOV - COM - 202 COHORT 3A NSCLC COMBINATION (TIL+PEMBROLIZUMAB ), WCLC ORAL PRESENTATION Cohort 3A Results Support Adding TIL Therapy to Frontline Pembrolizumab + Chemotherapy Combination Regimens Clinical Activity at 18.2 Months of Follow Up 1,2 • Activity across ICI naïve subgroups and TPS Scores • 58.3% (7/12) ORR and 3 ongoing responses in NSCLC patients with EGFR WT disease • Safety consistent with Iovance TIL combination studies • Supports proposed registrational trial design in patients with EGFR WT disease in the frontline setting Screening Pembrolizumab Administration (Single Dose) NMA - LD Cy/Flu Lifileucel / LN - 145 Infusion IL - 2 ≤6 Doses Pembrolizumab Q3W or Q6W Efficacy Follow - up Enrollment / Surgical Resection Assessment Period: Day 0 to EOA GMP Manufacturing EOA Progression or New Therapy EOS EOT Long - term Follow - up 1. Schoenfeld, et al. WCLC 2023 2. Data cut: June 26, 2023 Abbreviations: cy/flu, cytarabine/fludarabine; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; IL - 2, interleukin - 2; NMA - LD, non - myeloablative lymphodepletion; TPS, tumor proportion score; WT, wild type

30 © 2024, Iovance Biotherapeutics, Inc. Frontline NSCLC Registrational Trial: Design Supported by Cohort 3A Data Long - Term Follow Up SOC Maintenance Treatment +/ - TIL SOC Chemo - IO Treatment* Adding TIL Therapy to Standard - of - Care Therapy TIL Patient Population Treatment naïve metastatic NSCLC EGFR WT Disease All PD - L1 TPS Scores Chemotherapy Platinum Doublet * SOC Chemo - IO is 4 - 6 cycles of pembro + platinum - based chemotherapy doublet 1. KEYTRUDA USPI 2. Ghandi et al, NEJM 2018 Benchmarks EGFR/ALK status ORR mDOR (mos) mPFS (mos.) Prior IO Prior Chemo PD - L1 (%) SQ or NSQ Keynote - 189 1,2 WT 48% 11.2 8.8 No No All NSQ PD - L1 <1 Subgroup 1,2 WT 32% No No <1 NSQ Keynote - 407 1 N/A 58% 7.2 6.4 No No All SQ Experimental Arm Pembrolizumab Comparator Arm Abbreviations: EGFR, epidermal growth factor receptor; mDOR, median duration of response; NSCLC, non - small - cell lung cancer; NSQ, nonsquamous; platinum doublet, pemetrexed and cisplatin or carboplatin; PD - L1, programmed death ligand 1; PR, partial response; Pt, patient; SOC, standard of care; SQ, squamous; TPS, tumor proportion score; WT, wild - type Chemotherapy Platinum Doublet Pembrolizumab • FDA meeting in 2024 to discuss frontline trial to be registrational in treatment naïve EGFR WT NSCLC patients • Confirmatory trial for accelerated approval in post anti - PD - 1 NSCLC
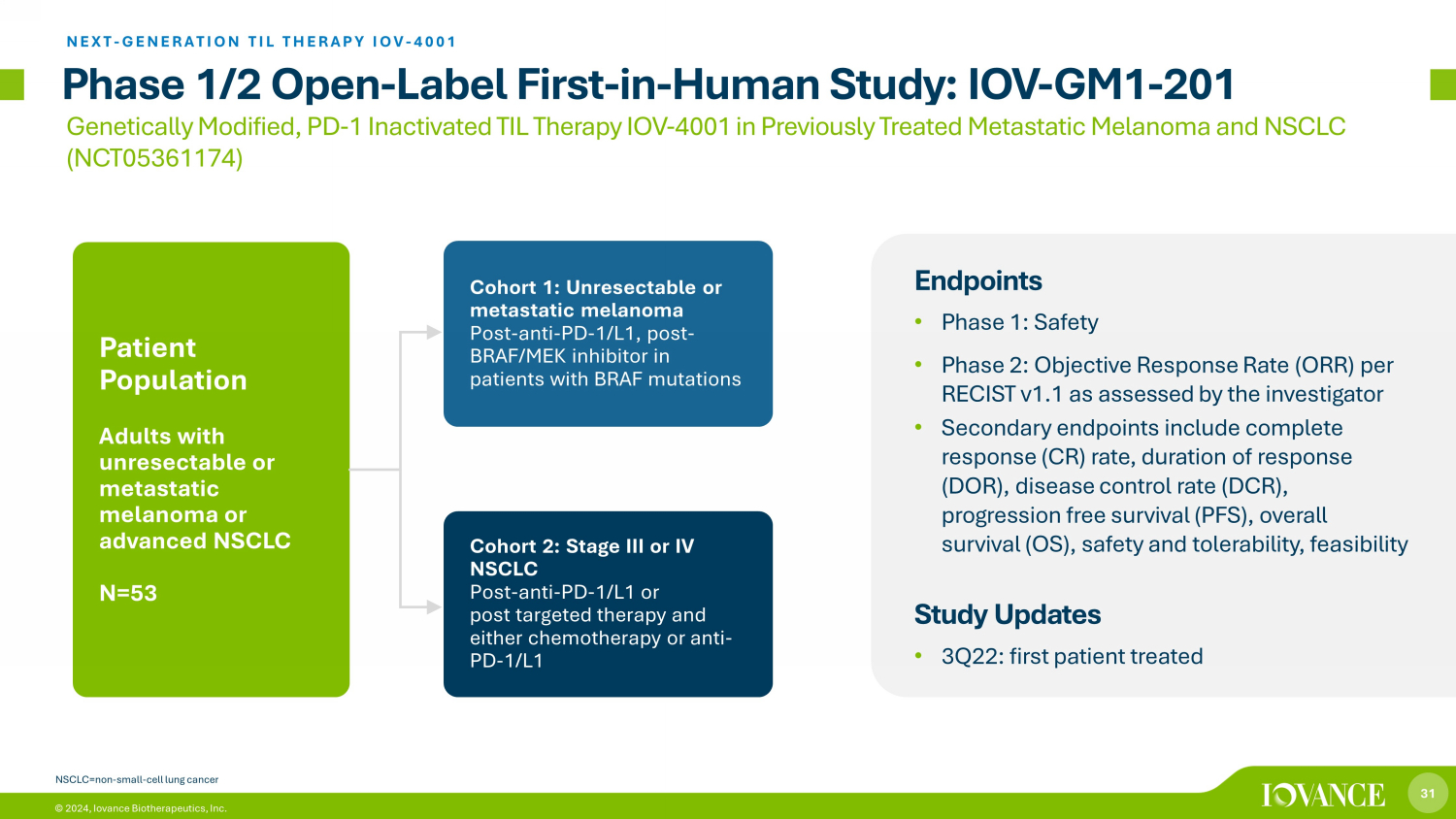
31 © 2024, Iovance Biotherapeutics, Inc. Phase 1/2 Open - Label First - in - Human Study: IOV - GM1 - 201 Endpoints • Phase 1: Safety • Phase 2: Objective Response Rate (ORR) per RECIST v1.1 as assessed by the investigator • Secondary endpoints include complete response (CR) rate, duration of response (DOR), disease control rate (DCR), progression free survival (PFS), overall survival (OS), safety and tolerability, feasibility Study Updates • 3Q22: first patient treated Cohort 1: Unresectable or metastatic melanoma Post - anti - PD - 1/L1, post - BRAF/MEK inhibitor in patients with BRAF mutations Cohort 2: Stage III or IV NSCLC Post - anti - PD - 1/L1 or post targeted therapy and either chemotherapy or anti - PD - 1/L1 Patient Population Adults with unresectable or metastatic melanoma or advanced NSCLC N=53 Genetically Modified, PD - 1 Inactivated TIL Therapy IOV - 4001 in Previously Treated Metastatic Melanoma and NSCLC (NCT05361174) NEXT - GENERATION TIL THERAPY IOV - 4001 NSCLC=non - small - cell lung cancer

32 © 2024, Iovance Biotherapeutics, Inc. Genetically modify TIL Optimize TIL composition Next - generation processes Expand TIL into new regimens Cellectis gene - editing TALEN ® collaboration 1,2 PD - 1 and other immune checkpoint targets (single and multiple knockouts) Cytokine - tethered TILs PD - 1+ selected TIL CD39/69 double n egative TILs 3 Gen 3 (16 - day) process Core biopsy IOV - 3001 IL - 2 analog licensed from Novartis: IND enabling studies Trailblazing Next - Generation TIL Programs 1. Ritthipichai et al., ESMO 2020 2. Natarajan, et al., AACR 2022 3. Cubas et al., ESMO IO 2021 32 © 2024, Iovance Biotherapeutics, Inc.

33 © 2024, Iovance Biotherapeutics, Inc. 33 Corporate Summary & Milestones

34 © 2024, Iovance Biotherapeutics, Inc. February 22, 2024 (in millions) Cash, cash equivalents, investments, restricted cash $485.2 1 Common shares outstanding 279.3 Preferred shares outstanding 2.9 2 Stock options and restricted stock units outstanding 22.0 1. Unaudited cash position which includes net proceeds of approximately $197.1 million from a follow - on equity financing in Februar y of 2024. Includes Restricted Cash of $66.4 million. 2. Preferred shares are shown on an as - converted basis 3. Includes anticipated revenue from Amtagvi ¯ and Proleukin ® Well - Capitalized in Pursuit of TIL Commercialization Cash runway is sufficient well into second half of 2025 3

35 © 2024, Iovance Biotherapeutics, Inc. REGULATORY □ Obtain FDA approval for lifileucel in advanced melanoma (approved on Feb. 16, 2024) □ Submit EMA regulatory dossier (1H24) □ Submit additional ex - US dossiers (2H24) □ Meet with FDA to discuss NSCLC registrational path/frontline study PIPELINE □ Report clinical and pre - clinical data □ Resume enrollment in IOV - LUN - 202 □ Initiate Phase 2 trial in endometrial cancer □ Continue to enroll patients in clinical trials for advanced melanoma, NSCLC and gynecological cancers □ Advance new products toward clinic, including additional genetically - modified TIL therapies MANUFACTURING □ Fulfill patient demand for commercial launch and clinical trials □ Further expand capacity to meet US and ex - US demand COMMERCIAL □ Execute commercial launch (1Q24) □ On - board 50 ATCs within 90 days of PDUFA date Anticipated 2024 Milestones

36 © 2024, Iovance Biotherapeutics, Inc. Corporate Highlights Pioneering a Transformational Approach to Cure Cancer Large Market Opportunity in High Unmet Need Cancers First FDA Approved T Cell Therapy for a Solid Tumor Cancer Efficient and Scalable Proprietary Manufacturing Facility Fully - Integrated for Commercial Success • Initial focus in post - ICI solid tumors • Expansion into combinations, earlier lines of therapy and genetic modifications • Key late - stage trials in melanoma, NSCLC and cervical cancer • First - in - human trial of genetically modified PD - 1 inactivated TIL • FDA accelerated approval for AMTAGVI ¯ in advanced melanoma • TILVANCE - 301 Phase 3 confirmatory trial in frontline advanced melanoma (FTD) • Defined registration strategy in NSCLC and cervical cancer (BTD) • Iovance Cell Therapy Center ( i CTC) in - house manufacturing • Additional capacity with contract manufacturers • Rapid 22 - day Gen 2 manufacturing • >700 patients treated with Iovance proprietary process • Experienced cross - functional cell therapy team • TIL service - line capabilities established with leading U.S. cancer centers • IovanceCares ¯ proprietary platform 36 © 2024, Iovance Biotherapeutics, Inc. Abbreviations: BLA, Biologics License Application; BTD, breakthrough therapy designation; FTD, fast track designation; ICI, i mmu ne checkpoint inhibitor; NSCLC, non - small cell lung cancer; PD - 1, programmed cell death protein - 1; RMAT, Regenerative Medicines Advanced Therapy; TIL, tumor inf iltrating lymphocytes.

37 © 2024, Iovance Biotherapeutics, Inc. © 2024, Iovance Biotherapeutics, Inc. Thank You
v3.24.0.1
Cover
|
Feb. 28, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Feb. 28, 2024
|
| Entity File Number |
001-36860
|
| Entity Registrant Name |
IOVANCE BIOTHERAPEUTICS, INC.
|
| Entity Central Index Key |
0001425205
|
| Entity Tax Identification Number |
75-3254381
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
825
Industrial Road
|
| Entity Address, Address Line Two |
Suite 400
|
| Entity Address, City or Town |
San Carlos
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94070
|
| City Area Code |
650
|
| Local Phone Number |
260-7120
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common stock, par value $0.000041666 per value
|
| Trading Symbol |
IOVA
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Iovance Biotherapeutics (NASDAQ:IOVA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Iovance Biotherapeutics (NASDAQ:IOVA)
Historical Stock Chart
From Apr 2023 to Apr 2024