false 0001840439 0001840439 2024-03-06 2024-03-06
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 6, 2024
Biomea Fusion, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-40335 |
|
82-2520134 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 900 Middlefield Road, 4th Floor Redwood City, CA |
|
94063 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (650) 980-9099
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value |
|
BMEA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On March 6, 2024, Biomea Fusion, Inc. (the “Company”) issued a press release titled, “Biomea Fusion Presents Patient Cohorts in COVALENT-111 Displaying a Durable Placebo-Adjusted Mean Reduction of up to 1.4% in HbA1c While Off Therapy at Week-26, after BMF-219’s 28-Day Treatment Cycle, Supporting Improved Pancreatic Function.” The information described in the press release was also presented in three poster presentations at the 17th International Conference on Advanced Technologies & Treatments for Diabetes, which is taking place March 6-9, 2024 in Florence, Italy.
Copies of the press release and the Company’s poster presentations are attached to this Current Report on Form 8-K as Exhibits 99.1 through 99.4 and incorporated herein by reference.
Forward-Looking Statements
Statements made or incorporated by reference in this Current Report on Form 8-K may include statements which are not historical facts and are considered forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words or similar expressions that are intended to identify forward-looking statements. Any such statements in this press release that are not statements of historical fact, including statements regarding the clinical and therapeutic potential of the Company’s product candidates and development programs, including BMF-219, the potential of BMF-219 as a treatment for type 1 and type 2 diabetes, the Company’s research, development and regulatory plans, including the Company’s pursuit of BMF-219 in metabolic diseases, the progress of the Company’s ongoing and upcoming clinical trials, including the Company’s Phase I/II COVALENT-111 study of BMF-219 in type 2 diabetes and the Company’s Phase II COVALENT-112 study of BMF-219 in type 1 diabetes, the anticipated enrollment of patients and availability of data from the Company’s clinical trials, the Company’s plans to continue the evaluation of BMF-219 for type 2 diabetes in the Company’s COVALENT-111 study, the Company’s plans to complete dose escalation, identify optimal dose levels, initiate dose expansion, explore longer duration of treatment and additional dosage forms and explore the potential utility of BMF-219 in type 1 diabetes, and the timing of such events, may be deemed to be forward-looking statements. The Company intends these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act and is making this statement for purposes of complying with those safe harbor provisions.
Any forward-looking statements made or incorporated by reference in this Current Report on Form 8-K are based on the Company’s current expectations, estimates and projections only as of the date of this Current Report on Form 8-K are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including the risk that the Company may encounter delays in patient enrollment and in the initiation, conduct and completion of its planned clinical trials and other research and development activities. These risks concerning the Company’s business and operations are described in additional detail in its periodic filings with the U.S. Securities and Exchange Commission (the “SEC”), including its most recent periodic report filed with the SEC and subsequent filings thereafter. The Company explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
|
|
|
| Exhibit Number |
|
Description |
|
|
| 99.1 |
|
Press release titled, “Biomea Fusion Presents Patient Cohorts in COVALENT-111 Displaying a Durable Placebo-Adjusted Mean Reduction of up to 1.4% in HbA1c While Off Therapy at Week-26, after BMF-219’s 28-Day Treatment Cycle, Supporting Improved Pancreatic Function.” |
|
|
| 99.2 |
|
Poster presentation titled, “Durable Glycemic Control With BMF-219 During Off-Treatment Period at Week 26: a Phase 1/2 Trial of BMF-219 in Patients with Type 2 Diabetes (Covalent-111).” |
|
|
| 99.3 |
|
Poster presentation titled, “Key Observations from the Dose-Escalation Portion of Covalent-111, a Phase 1/2 Trial of the Covalent Menin Inhibitor BMF-219 in Patients with Type 2 Diabetes.” |
|
|
| 99.4 |
|
Poster presentation titled, “Case Studies from Covalent-111, a Phase 1/2 Trial Of BMF-219, a Covalent Menin Inhibitor, in Patients with Type 2 Diabetes.” |
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
BIOMEA FUSION, INC. |
|
|
|
|
| Date: March 7, 2024 |
|
|
|
By: |
|
/s/ Thomas Butler |
| |
|
|
|
|
|
Thomas Butler |
| |
|
|
|
|
|
Principal Executive Officer |
Exhibit 99.1
Biomea Fusion Presents Patient Cohorts in COVALENT-111 Displaying a Durable Placebo-Adjusted Mean Reduction of up
to 1.4% in HbA1c While Off Therapy at Week-26, after BMF-219’s 28-Day Treatment Cycle, Supporting Improved Pancreatic
Function
Three Clinical Data Sets from the Dose Escalation Phase of COVALENT-111 to be Presented at the 17th Annual ATTD Conference Highlighting BMF-219’s Novel Mechanism of Action in Patients with Type 2 Diabetes
| |
• |
|
Patients in COVALENT-111 are displaying improved glycemic control while
off therapy out to Week 26 following the 28-day treatment with BMF-219, supporting enhanced pancreatic islet function as the mechanism of action |
| |
• |
|
BMF-219 was generally well tolerated with no serious adverse events and
no adverse event-related study discontinuations, and no symptomatic or clinically significant hypoglycemia |
| |
• |
|
100mg and 200mg dose levels have been selected for the first 3 Arms of the Expansion Phase, which will dose
patients up to 12 weeks (compared to 4 weeks in the Escalation Phase) and extended follow-up to Week 52 |
| |
• |
|
The Expansion Phase of COVALENT-111 is currently enrolling on schedule
with initial 26-week data expected during 2H24 |
| |
• |
|
Biomea Fusion to announce an update on the first two patients with Type 1 Diabetes, from the COVALENT-112 Study, in the Q4 2023 Earnings Release |
REDWOOD CITY, Calif., March 6th, 2024 (GLOBE NEWSWIRE) – Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage biopharmaceutical company dedicated to discovering and developing oral covalent small
molecules to treat and improve the lives of patients with metabolic diseases and genetically defined cancers, today announced three poster presentations presenting long-term 26 week follow-up data from
patients treated with BMF-219, enrolled in the escalation portion of the ongoing Phase II clinical study (COVALENT-111), at the 17th International Conference on Advanced
Technologies & Treatments for Diabetes (ATTD) taking place in Florence, Italy from March 6-9, 2024. This clinical data from all dosing cohorts initiated to date as of February 12, 2024 from the
Escalation Phase of COVALENT-111 will be featured during a Poster Discussion Presentation and two Poster Viewing Presentations at ATTD. Biomea will showcase the following three
e-poster presentations:
| |
• |
|
Durable Glycemic Control with BMF-219 During Off-Treatment Period at Week 26: A Phase 1/2 Trial of BMF-219 in Patients with Type 2 Diabetes (COVALENT-111) (Poster Discussion
Session, March 7th, 10-10:30 am CET.) |
| |
• |
|
Case Studies from COVALENT-111, A Phase 1/2 Trial of BMF-219, a Covalent Menin Inhibitor, in Patients with Type 2 Diabetes (Poster Viewing Session) |
| |
• |
|
Key Observations from the Dose Escalation Portion of COVALENT-111, a
Phase 1/2 Trial of the Covalent Menin Inhibitor BMF-219 in Patients with Type 2 Diabetes (Poster Viewing Session) |
All e-Posters will be available for viewing through the conference virtual platform once the conference commences.
Please find a link here to our website where the poster presentations and discussion will be available.
“We aim to cure diabetes and believe we are
on the path to do so. Notably, after receiving a short, 4-week course of BMF-219, patients with type 2 diabetes are displaying durable glycemic control, and in some
cases displaying continued improvement in glycemic control while off therapy. The Escalation Phase of our first in human study was quite successful, generating strong clinical data with a novel mechanism of action and importantly providing us with proof-of-concept data to support the design of the Expansion Phase which is now enrolling. The Expansion Phase will dose patients for longer treatment periods with the goal of
broadening and deepening BMF-219’s effect across the type 2 patient population. As presented at the ATTD conference, we believe the observations from biomarkers including HbA1c, HOMA-B, and C-peptide analysis for responders vs. non-responders, together with pharmacokinetic dose response data all point to strong
evidence that BMF-219 is specifically proliferating beta cells in pancreatic islets of uncontrolled type 2 diabetes patients,” said Thomas Butler, Biomea Fusion’s Chief Executive Officer and Chairman
of the Board. “I am also excited about the potential this pathway may provide patients with type 1 diabetes. We are enrolling our open label arm (n=40) of our Phase II COVALENT-112 study in adults with
stage 3 type 1 diabetes first which will give us initial response data before embarking on a larger, potentially registrational study.”
Data
Highlights from ATTD Presentations
Efficacy Findings
| |
• |
|
Patients in COVALENT-111 are displaying improved glycemic control while
off therapy, supporting improved pancreatic function following BMF-219 treatment. Patients who demonstrated the greatest HbA1c reduction at Week 26 (22 weeks off treatment), had the greatest improvement in
beta cell function as measured by HOMA-B and C-peptide. |
| |
• |
|
In patients failing current standard of care medications, at Week 26, following a 28 day dose cycle of BMF-219, a general dose response was observed with placebo adjusted mean percent changes of HbA1c of -0.04% (50mg QD*), -0.2% (100mg QD
with food), - 0.8% (100mg), -0.4% (200mg QD), -0.4% (100mg BID), and -1.4% (200mg with food) (*50mg data out to Week 20, latest
data cut). |
| |
• |
|
The efficacy seen in the 200mg with food cohort is highlighting the direct benefits of an enhanced PD effect with
higher blood glucose and higher exposure, as seen in the human islet studies with BMF-219 (presented at WCIRD Dec. 2023). |
| |
• |
|
A higher proportion of patients treated with 200mg QD achieved a clinically significant reduction in HbA1c
compared to 100mg QD dosing. A durable glycemic response (≥1.0% HbA1C reduction) was seen in 20% and 36% of patients in once daily 100 mg and 200 mg cohorts, respectively. |
| |
• |
|
Across 100mg QD, 200mg QD, and 100mg BID cohorts (N=40), 38% of patients had ≥0.5% HbA1c reduction (with a
mean HbA1c reduction of 1.2%), and 23% of patients had ≥1.0% HbA1c reduction (with a mean HbA1c reduction of 1.5%) at Week 26. |
| |
• |
|
Patients with >7 years duration of diabetes and failing dual- or triple-agent therapy (including GLP1 RA
and/or SGLT2i) (n=2) also demonstrated improved glycemic control (HbA1c -0.4%, -1.1%, and -1.1% at Weeks 4, 12, and 26,
respectively) with BMF-219 dosed at 200mg with food. |
| |
• |
|
Increase in HOMA-B and C-peptide
generally correlated with glycemic control, consistent with BMF-219’s core mechanism of action: beta-cell proliferation and improved beta-cell function. |
Safety and Tolerability Findings
| |
• |
|
BMF-219 was generally well tolerated with no serious adverse events and
no adverse event-related study discontinuations, and no symptomatic or clinically significant hypoglycemia. |
Next Steps
| |
• |
|
The Expansion Phase of COVALENT-111 is designed to further explore BMF-219’s potential for long-term glycemic control by dosing BMF-219 for up to 12 weeks at various dosing levels with follow-up of
26 and 52 weeks. The Expansion Phase is currently enrolling on schedule with initial data expected in the second half of 2024. |
| |
• |
|
A PK study further assessing the optimal use of BMF-219 to ensure minimal
variability of exposure is currently under way. |
| |
• |
|
Biomea is currently awaiting the read out and analysis of an additional 400 mg cohort, which will also help
inform further inclusion into the Expansion Phase. |
About COVALENT-111
COVALENT-111 is a multi-site, randomized, double-blind, placebo-controlled Phase I/II study. In the completed Phase I
portion of the trial, healthy patients were enrolled in single ascending dose cohorts to evaluate safety at the prospective dosing levels for type 2 diabetic patients. Phase II consists of multiple ascending dose cohorts and includes adult patients
with type 2 diabetes uncontrolled by standard of care medicines. Once the Escalation Phase of COVALENT-111 completes, the study advances into an Expansion Phase (n>200) consisting of multiple cohorts dosing
type 2 diabetes patients for longer dose durations. Additional information about the Phase I/II clinical trial of BMF-219 in type 2 diabetes can be found at ClinicalTrials.gov using the identifier NCT05731544.
About COVALENT-112
COVALENT-112 is a multi-site, randomized, double-blind, placebo-controlled Phase II study in adults with stage 3 type 1
diabetes. This stage describes the period following clinical diagnosis of type 1 diabetes when symptoms are present due to significant beta cell loss. COVALENT-112 will be a
multi-arm trial comparing two different doses of BMF-219 to placebo control (1:1:1) to evaluate the safety, tolerability, and efficacy of
BMF-219 in persons with type 1 diabetes. Approximately 150 patients will be enrolled in the trial and will receive either BMF-219 or placebo for 12 weeks, followed by a
40 week “off-treatment” period.
This trial will also include an open label portion for adults with type
1 diabetes up to 15 years since diagnosis. The open label portion (n=40) will examine the safety, efficacy, and durability of BMF-219 at two oral dose levels, 100 mg and 200 mg for 12-weeks of treatment followed by a 40 week off-treatment period. Additional information about the Phase II clinical trial of BMF-219
in type 1 diabetes can be found at ClinicalTrials.gov using the identifier NCT06152042.
About Menin’s Role in Diabetes
Loss of functional beta cell mass is a core component of the natural history in both types of diabetes — type 1 diabetes (mediated by autoimmune
dysfunction) and type 2 diabetes (mediated by metabolic dysfunction). Beta cells are found in the pancreas and are responsible for the synthesis and secretion of insulin. Insulin is a hormone that helps the body use glucose for energy and helps
control blood glucose levels. In patients with diabetes, beta cell mass and function have been observed to be diminished, leading to insufficient insulin secretion and hyperglycemia. Menin is thought to act as a brake on beta-cell turnover and
growth, supporting the notion that inhibition of menin could lead to the regeneration of normal, healthy beta cells. Based on these and other scientific findings, Biomea is exploring the potential for BMF-219-mediated menin inhibition as a viable therapeutic approach to potentially halt or reverse progression of type 2 diabetes.
About Type 2 Diabetes
Diabetes is considered a chronic health condition that affects how the body turns food into energy and results in too much sugar in the bloodstream. Over time,
this can cause serious health problems and damage vital organs. Most people with diabetes have a shorter life expectancy than people without this disease. The CDC estimates about 2 in 5 of the adult population in the USA are now expected to develop
diabetes during their lifetime. More than 37 million people of all ages (about 11% of the US population) have diabetes today. 96 million adults (more than 1 in 3) have pre-diabetes, blood sugars that
are higher than normal but not high enough to be classified as diabetes. Diabetes is also one of the largest economic burdens on the United States health care system with $1 out of every $4 in US health care costs being spent on caring for people
with diabetes. Despite the current availability of many diabetes medications, there remains a significant need in the treatment and care of patients with diabetes.
About Biomea Fusion
Biomea Fusion is a clinical stage
biopharmaceutical company focused on the discovery and development of oral covalent small molecules to treat patients with metabolic diseases and genetically defined cancers. A covalent small molecule is a synthetic compound that forms a permanent
bond to its target protein and offers a number of potential advantages over conventional non-covalent drugs, including greater target selectivity, lower drug exposure, and the ability to drive a deeper, more
durable response.
We are utilizing our proprietary FUSION™ System to discover, design and
develop a pipeline of next-generation covalent-binding small molecule medicines designed to maximize clinical benefit for patients. We aim to have an outsized impact on the treatment of disease for the patients we serve. We aim to cure.
Visit us at biomeafusion.com and follow us on LinkedIn, Twitter and Facebook.
Forward-Looking Statements
Statements we make in this
press release may include statements which are not historical facts and are considered forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E
of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,”
“expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words or similar
expressions that are intended to identify
forward-looking statements. Any such statements in this press release that are not statements of historical fact, including statements regarding the clinical and therapeutic potential of our
product candidates and development programs, including BMF-219, the potential of BMF-219 as a treatment for type 1 and type 2 diabetes, our research, development and
regulatory plans, including our pursuit of BMF-219 in metabolic diseases, the progress of our ongoing and upcoming clinical trials, including our Phase I/II COVALENT-111
study of BMF-219 in type 2 diabetes and our Phase II COVALENT-112 study of BMF-219 in type 1 diabetes, the anticipated enrollment
of patients and availability of data from our clinical trials, our plans to continue the evaluation of BMF-219 for type 2 diabetes in our COVALENT-111 study, our plans
to complete dose escalation, identify optimal dose levels, initiate dose expansion, explore longer duration of treatment and additional dosage forms and explore the potential utility of BMF-219 in type 1
diabetes, and the timing of such events, may be deemed to be forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the
Securities Act and Section 21E of the Exchange Act and are making this statement for purposes of complying with those safe harbor provisions.
Any
forward-looking statements in this press release are based on our current expectations, estimates and projections only as of the date of this release and are subject to a number of risks and uncertainties that could cause actual results to differ
materially and adversely from those set forth in or implied by such forward-looking statements, including the risk that initial results may not be indicative of final results in later clinical trials, we may encounter delays, regulatory challenges
or unforeseen and/or adverse results in preclinical or clinical development, we may face difficulties in patient enrollment and in the initiation, conduct and completion of our ongoing and planned clinical trials and other research, development and
regulatory activities. These risks concerning Biomea Fusion’s business and operations are described in additional detail in its periodic filings with the U.S. Securities and Exchange Commission (the “SEC”), including its most recent
periodic report filed with the SEC and subsequent filings thereafter. Biomea Fusion explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Investor Relations
Chunyi Zhao, PhD
Associate Director of Investor Relations & Corporate Development
czhao@biomeafusion.com
Media Relations
Neera Chaudhary
nchaudhary@biomeafusion.com

DURABLE GLYCEMIC CONTROL WITH BMF-219 DURING OFF-TREATMENT PERIOD AT
WEEK 26: Exhibit 99.2 A PHASE 1/2 TRIAL OF BMF-219 IN PATIENTS WITH TYPE 2 DIABETES (COVALENT-111) 1 2 3 4 5 6 7 7 7 7 7 7 Jose Rodriguez , Alexander Abitbol , Douglas Denham , Rizwana Mohseni , Janice Faulknor , Cesar Perez , Brian Munneke ,
Courtney Follit , Juan Frias , Sanchita Mourya , Thomas Butler , Steve Morris ; 1 2 3 4 5 6 South West General Healthcare Center, FL, United States of America, LCM Clinical Research, Canada, Clinical Trials of Texas, TX, United States of America,
Catalina Research Institute, CA, United States of America, BioPharma Services, Canada, Sunbright Health Medical Center, Clinical Trial Investigator, FL, 7 United States of America, Biomea Fusion, CA, United States of America Background Glycemic
Results Summary at Week 26 (22 Weeks After Last Dose of BMF-219) Case Study BMF-219 100mg BMF-219 200mg BMF-219 100mg BMF-219 200mg • T2D is characterized by hyperglycemia due to a progressive decline in beta- Placebo • 51-year-old man
with 5-year history of T2D • BMF-219 100mg QD without food for 4 weeks Without Food Without Food With Food With Food N = 6 • Metformin 500mg BID • Metformin continued N = 10 N = 9 N = 10 N = 2 cell function 2 • HbA 8.9%; FPG
184mg/dL; BMI 32.1 kg/m • No adverse events reported 1c Mean change in HbA1c -0.5% -0.1% 0.1% -1.1% 0.3% • Menin, a scaffold protein, is an important regulator of glycemic control, Placebo Adjusted Mean -0.8% -0.4% -0.2% -1.4% -- Change
in HbA1c by Study Week CGM Across Study Visits whereby inhibition of menin enhances beta cell proliferation and function Change in HbA1c Percent of Participants with • BMF-219 is an oral covalent menin inhibitor in clinical development for the
≥ 1.0 reduction in HbA1c at 2/10 (20%) 2/9 (22%) 2/10 (20%) 2/2 (100%) 0/6 (0%) Week 26 management of T2D and T1D • In preclinical diabetes ZDF and STZ rat models, BMF-219 showed durable Increase in HOMA-B and C-Peptide at Week 26 1,2
glycemic control following short-term treatment % Change in HOMA-B % Change in C-Peptide • In multiple ascending dose (MAD) cohorts in patients with T2D, 4 weeks of .0 (100mg and 200mg cohorts combined) (100mg and 200mg cohorts combined)
BMF-219 100 and 200mg once daily dosing improved glycemic control at Responders* Non-responders Responders* Non-responders 3 Week 26 (22 weeks after the final dose) Aim Week 12 Week 26 Week 4 Baseline Week 12 Week 26 A case study demonstrating
continued improvement in HbA1C and improved Time In Range on CGM (after • To assess the safety and efficacy of 4 weeks of once daily BMF-219 at completion of 4 weeks of once daily oral treatment), indicating a durable glycemic control. Week 26
(22 weeks after final dose) Summary and Conclusion Methods • At Week 26 (22 weeks after completion of a 4-week regimen) 100 and 200 mg • COVALENT-111 (NCT05731544) is an ongoing Phase 1/2 randomized, double- N = 8 N = 3 N = 3 N = 17
BMF-219 resulted in: blind, placebo controlled, MAD study evaluating BMF-219 in patients with N = 8 N = 3 inadequately controlled T2D who receive once-daily BMF-219 (50, 100, 200, • Durable glycemic response (≥1.0% HbA1C reduction in 20%
and 36% of N = 18 N = 3 patients in once daily 100 and 200mg cohorts, respectively) 400mg) for 4 weeks and are followed until Week 26. • Key eligibility criteria: Adults with T2D treated with up to 3 antidiabetic • Durable increase in
C-peptide at Week 26 (22 weeks off treatment) for Placebo Placebo Active Placebo Active Active Active Placebo agents (excluding SU, insulin), HbA1c 7%-10.5%, T2D duration ≤ 15 years BMF-219 responders After 4 weeks of once-daily dosing,
responders across both 100 and 200mg cohorts had a greater increase in HOMA-B and • Primary endpoint: Safety and tolerability • Patients who demonstrated the greatest HbA1c reduction at Week 26, had C-peptide AUC when compared to
non-responders and placebo. • Secondary endpoints: Measures of glycemic control (HbA1c, CGM), beta cell * Responders have baseline HOMA-B <200 and have achieved at least 0.5% decrease in HbA1c at Week 26 greatest improvement in beta cell
function as measured by HOMA-B and function (HOMA-B and C-peptide), and durability of glycemic response C-peptide 200mg Cohorts (N=11) 200mg Cohorts (N=11) Study Design • A generally well tolerated safety profile with no serious adverse events
Day 1 BMF-219 Week 4 and no adverse event-related study discontinuations Week 26 (100mg or 200 mg) Daily • No symptomatic or clinically significant hypoglycemia Dosing Period Off-Treatment Period • Robust durable responses seen in many
patients after 4 weeks of BMF-219 and Baseline Characteristics and Demographics the demonstration of improvement in beta-cell function which correlates with 100mg without 200mg without 100mg with 200mg with Placebo this glycemic response, support
the assessment of longer duration of therapy 1 Characteristics Food Food Food Food (8-12 weeks) with BMF-219 N = 10 N = 10 N = 10 N = 2 N = 6 Age (yrs) 52 (38,63) 50 (25,64) 51 (35,60) 61 (60,61) 46 (31,61) • Subsequent study cohorts are
currently assessing BMF-219 administration for Female 4 (40%) 4 (40%) 3 (30%) 2 (100%) 0 (0%) Male 6 (60%) 6 (60%) 7 (70%) 0 (0%) 6 (100%) N = 6 N = 4 N = 3 N = 3 N = 5 N = 6 N = 5 N = 4 up to 12 weeks, with follow-up until Week 52 Duration of
diabetes (yrs) 4.3 (0.3,9.3) 4.9 (0.5,11.7) 8.3 (2.6,13.6) 11.3 (9.7,12.8) 3.9 (0.7,9.5) Baseline HbA1c (%) 8.1 (0.92) 7.85 (0.82) 7.96 (0.62) 8.35 (0.64) 8.25 (0.71) Any Decrease in Any Decrease At least 0.5% At least 0.5% Any Decrease in Any
Decrease in At least 0.5% At least 0.5% Baseline therapy HbA1c in HbA1c and decrease in Decrease and HbA1c HbA1c and decrease in Decrease and References Baseline HbA1c Baseline Baseline HbA1c Baseline Metformin only 9 (90%) 6 (60%) 5 (50%) 0 (0%) 5
(83%) HOMA-B* <200 HOMA-B* <200 HOMA-B* <200 HOMA-B* <200 Other 1 (10%) 2 (20%) 4 (40%) 2 (100%) 1 (17%) 1. Butler T. et al. Oral Long-Acting Menin Inhibitor Normalizes Type 2 Diabetes Mellitus (T2DM) in Two Rat Models. Diabetes 1 June
2022; 71 (Supplement_1): 851–P. 2. Somanath P. et al. Oral Menin Inhibitor, BMF-219, Displays a Significant and Durable Reduction in HbA1c in a Type 2 Diabetes Mellitus Rat Model. Diabetes 1 June 2022; None 0 (0%) 2 (20%) 1 (10%) 0 (0%) 0 (0%)
HOMA-B and C-peptide at Week 26 increases with magnitude of reduction in HbA1C and in patients with baseline HOMA-B <200 71 (Supplement_1): 113–LB. 1 with BMF-219 200 mg once daily dosing for 4 weeks. Mean (Minimum, Maximum); n (%); Mean
(SD) 3. Frias J. et al. BMF-219: A Novel Therapeutic Agent to Re-Establish Functional Beta Cells and Provide Long-Term Glycemic Control. Metabolism May 2023; 142 (Supplement): Abstract#0088 * HOMA-B <200 is considered beta cell deficient Mean %
Change in HOMA-B Mean % Change in HOMA-B Mean % Change in C-Peptide AUC Mean % Change in C-Peptide AUC Change in HbA1c (%) % Time in Glucose Range
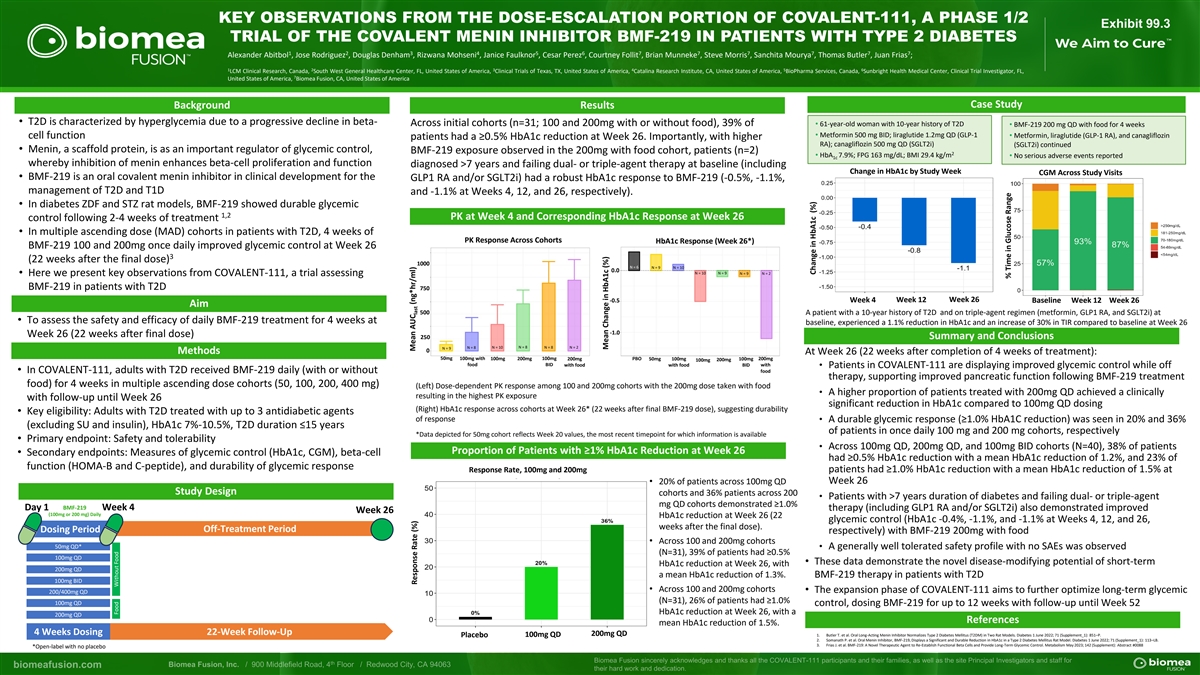
KEY OBSERVATIONS FROM THE DOSE-ESCALATION PORTION OF COVALENT-111, A
PHASE 1/2 Exhibit 99.3 TRIAL OF THE COVALENT MENIN INHIBITOR BMF-219 IN PATIENTS WITH TYPE 2 DIABETES 1 2 3 4 5 6 7 7 7 7 7 7 Alexander Abitbol , Jose Rodriguez , Douglas Denham , Rizwana Mohseni , Janice Faulknor , Cesar Perez , Courtney Follit ,
Brian Munneke , Steve Morris , Sanchita Mourya , Thomas Butler , Juan Frias ; 1 2 3 4 5 6 LCM Clinical Research, Canada, South West General Healthcare Center, FL, United States of America, Clinical Trials of Texas, TX, United States of America,
Catalina Research Institute, CA, United States of America, BioPharma Services, Canada, Sunbright Health Medical Center, Clinical Trial Investigator, FL, 7 United States of America, Biomea Fusion, CA, United States of America Case Study Background
Results • T2D is characterized by hyperglycemia due to a progressive decline in beta- Across initial cohorts (n=31; 100 and 200mg with or without food), 39% of • 61-year-old woman with 10-year history of T2D • BMF-219 200 mg QD
with food for 4 weeks • Metformin 500 mg BID; liraglutide 1.2mg QD (GLP-1 cell function • Metformin, liraglutide (GLP-1 RA), and canagliflozin patients had a ≥0.5% HbA1c reduction at Week 26. Importantly, with higher RA);
canagliflozin 500 mg QD (SGLT2i) (SGLT2i) continued • Menin, a scaffold protein, is as an important regulator of glycemic control, BMF-219 exposure observed in the 200mg with food cohort, patients (n=2) 2 • HbA 7.9%; FPG 163 mg/dL; BMI
29.4 kg/m • No serious adverse events reported 1c whereby inhibition of menin enhances beta-cell proliferation and function diagnosed >7 years and failing dual- or triple-agent therapy at baseline (including Change in HbA1c by Study Week
CGM Across Study Visits • BMF-219 is an oral covalent menin inhibitor in clinical development for the GLP1 RA and/or SGLT2i) had a robust HbA1c response to BMF-219 (-0.5%, -1.1%, management of T2D and T1D and -1.1% at Weeks 4, 12, and 26,
respectively). • In diabetes ZDF and STZ rat models, BMF-219 showed durable glycemic 1,2 PK at Week 4 and Corresponding HbA1c Response at Week 26 control following 2-4 weeks of treatment • In multiple ascending dose (MAD) cohorts in
patients with T2D, 4 weeks of PK Response Across Cohorts HbA1c Response (Week 26*) BMF-219 100 and 200mg once daily improved glycemic control at Week 26 3 (22 weeks after the final dose) 1000 N = 6 N = 9 N = 10 0.0 N = 10 N = 9 N = 9 N = 2 •
Here we present key observations from COVALENT-111, a trial assessing BMF-219 in patients with T2D 750 -0.5 Week 4 Week 12 Week 26 Baseline Week 12 Week 26 Aim 500 A patient with a 10-year history of T2D and on triple-agent regimen (metformin, GLP1
RA, and SGLT2i) at • To assess the safety and efficacy of daily BMF-219 treatment for 4 weeks at baseline, experienced a 1.1% reduction in HbA1c and an increase of 30% in TIR compared to baseline at Week 26 -1.0 Week 26 (22 weeks after final
dose) 250 Summary and Conclusions N = 8 N = 10 N = 8 N = 8 N = 2 N = 9 0 Methods At Week 26 (22 weeks after completion of 4 weeks of treatment): 50mg 100mg with 100mg 200mg 100mg 200mg PBO 50mg 100mg 100mg 200mg 100mg 200mg food BID with food with
food BID with • Patients in COVALENT-111 are displaying improved glycemic control while off • In COVALENT-111, adults with T2D received BMF-219 daily (with or without food therapy, supporting improved pancreatic function following
BMF-219 treatment food) for 4 weeks in multiple ascending dose cohorts (50, 100, 200, 400 mg) (Left) Dose-dependent PK response among 100 and 200mg cohorts with the 200mg dose taken with food • A higher proportion of patients treated with
200mg QD achieved a clinically resulting in the highest PK exposure with follow-up until Week 26 significant reduction in HbA1c compared to 100mg QD dosing (Right) HbA1c response across cohorts at Week 26* (22 weeks after final BMF-219 dose),
suggesting durability • Key eligibility: Adults with T2D treated with up to 3 antidiabetic agents of response • A durable glycemic response (≥1.0% HbA1C reduction) was seen in 20% and 36% (excluding SU and insulin), HbA1c 7%-10.5%,
T2D duration ≤15 years of patients in once daily 100 mg and 200 mg cohorts, respectively *Data depicted for 50mg cohort reflects Week 20 values, the most recent timepoint for which information is available • Primary endpoint: Safety and
tolerability • Across 100mg QD, 200mg QD, and 100mg BID cohorts (N=40), 38% of patients Proportion of Patients with ≥1% HbA1c Reduction at Week 26 • Secondary endpoints: Measures of glycemic control (HbA1c, CGM), beta-cell had
≥0.5% HbA1c reduction with a mean HbA1c reduction of 1.2%, and 23% of function (HOMA-B and C-peptide), and durability of glycemic response Response Rate, 100mg and 200mg patients had ≥1.0% HbA1c reduction with a mean HbA1c reduction of
1.5% at • 20% of patients across 100mg QD Week 26 Study Design cohorts and 36% patients across 200 • Patients with >7 years duration of diabetes and failing dual- or triple-agent mg QD cohorts demonstrated ≥1.0% BMF-219 Day 1
Week 4 therapy (including GLP1 RA and/or SGLT2i) also demonstrated improved Week 26 (100mg or 200 mg) Daily HbA1c reduction at Week 26 (22 glycemic control (HbA1c -0.4%, -1.1%, and -1.1% at Weeks 4, 12, and 26, weeks after the final dose). Dosing
Period Off-Treatment Period respectively) with BMF-219 200mg with food • Across 100 and 200mg cohorts 50mg QD* • A generally well tolerated safety profile with no SAEs was observed (N=31), 39% of patients had ≥0.5% 100mg QD •
These data demonstrate the novel disease-modifying potential of short-term HbA1c reduction at Week 26, with 200mg QD a mean HbA1c reduction of 1.3%. BMF-219 therapy in patients with T2D 100mg BID • Across 100 and 200mg cohorts • The
expansion phase of COVALENT-111 aims to further optimize long-term glycemic 200/400mg QD (N=31), 26% of patients had ≥1.0% 100mg QD control, dosing BMF-219 for up to 12 weeks with follow-up until Week 52 HbA1c reduction at Week 26, with a
200mg QD References mean HbA1c reduction of 1.5%. 4 Weeks Dosing 22-Week Follow-Up 200mg QD 1. Butler T. et al. Oral Long-Acting Menin Inhibitor Normalizes Type 2 Diabetes Mellitus (T2DM) in Two Rat Models. Diabetes 1 June 2022; 71 (Supplement_1):
851–P. Placebo 100mg QD 2. Somanath P. et al. Oral Menin Inhibitor, BMF-219, Displays a Significant and Durable Reduction in HbA1c in a Type 2 Diabetes Mellitus Rat Model. Diabetes 1 June 2022; 71 (Supplement_1): 113–LB. 3. Frias J. et
al. BMF-219: A Novel Therapeutic Agent to Re-Establish Functional Beta Cells and Provide Long-Term Glycemic Control. Metabolism May 2023; 142 (Supplement): Abstract #0088 *Open-label with no placebo Food Without Food Mean AUC (ng*hr/ml) Response
Rate (%) last Mean Change in HbA1c (%) Change in HbA1c (%) % Time in Glucose Range

CASE STUDIES FROM COVALENT-111, A PHASE 1/2 TRIAL OF BMF-219, A COVALENT
MENIN Exhibit 99.4 INHIBITOR, IN PATIENTS WITH TYPE 2 DIABETES 1 2 3 4 5 6 7 7 7 7 7 7 Douglas Denham , Alexander Abitbol , Rizwana Mohseni , Jose Rodriguez , Cesar Perez , Janice Faulknor , Courtney Follit , Brian Munneke , Steve Morris , Juan
Frias , Thomas Butler , Sanchita Mourya ; 1 2 3 4 5 Clinical Trials of Texas, TX, United States of America, LCM Clinical Research, Canada, Catalina Research Institute, CA, United States of America, South West General Healthcare Center, FL, United
States of America, Sunbright Health Medical Center, Clinical Trial Investigator, FL, United States of America, 6 7 BioPharma Services, Canada, Biomea Fusion, CA, United States of America Background Results • T2D is characterized by
hyperglycemia due to a gradual decline in beta-cell Case Study 1 Case Study 2 function • 29-year-old man with 4-year history of T2D• BMF-219 200 mg QD without food for 4 weeks• BMF-219 100 mg QD with food for 4 weeks •
45-year-old man with 10-year history of T2D • Menin, a scaffold protein, is as an important regulator of glycemic control, • Metformin 500 mg BID, empagliflozin 25 mg BID• Metformin and SGLT2i continued • Metformin 500 mg
BID• Metformin continued 2 • No adverse events reported 2 whereby inhibition of menin enhances beta cell proliferation and function• HbA 9.5%; FPG 134 mg/dL; BMI 25.6 kg/m• No adverse events reported 1c • HbA 8.6%; FPG
235 mg/dL; BMI 29.6 kg/m 1c • At (Week 26), HbA1c 7.0% (change from baseline [CFB], • CGM TIR 34% • CGM TIR 4%• At (Week 26), HbA1c 7.5% (CFB, -1.1%), FPG 144 • BMF-219 is an oral covalent menin inhibitor in clinical
development for the -2.2%), FPG 105 mg/dL (CFB, -24 mg/dL), TIR 90% (CFB, mg/dL (CFB, -91 mg/dL), TIR 79% (CFB, +73%), HOMA +65%). B (CFB, 12-fold increase). management of T2D and T1D • In preclinical diabetes ZDF and STZ rat models, BMF-219
showed durable Change in HbA1c by Study Week CGM Across Study Visits Change in HbA1c by Study Week CGM Across Study Visits 1,2 glycemic control following short-term treatment • In a multiple ascending dose (MAD) cohorts in patients with T2D, 4
weeks of BMF-219 100 and 200mg once daily dosing improved glycemic control at 3 Week 26 and was generally safe and well tolerated • Here, we highlight two BMF-219-treated T2D patients who demonstrated significant efficacy in a double blind
randomized-controlled trial Aim • To assess the safety and efficacy of BMF-219 once daily treatment for 4 Week 4 Week 12 Week 26 Week 4 Week 12 Week 26 Baseline Week 12 Week 20 Baseline Week 12 Week 26 weeks at Week 26 (22 weeks after final
dose) % Change HOMA-B at Week 26 % Change C-Peptide at Week 26 % Change HOMA-B at Week 26 % Change C-Peptide at Week 26 Methods • In COVALENT-111, adults with T2D receiving up to 3 antidiabetic agents received BMF-219 with or without food once
daily for 4 weeks in MAD cohorts (50, 100, 200, and 400mg), with follow-up until Week 26 • Key eligibility criteria: Adults with T2D treated with up to 3 antidiabetic agents (excluding insulin secretagogues and insulin), HbA1c 7%-10.5%,
diabetes duration ≤15 years • Primary endpoint: Safety and tolerability • Secondary endpoints: Measures of glycemic control (HbA1c, CGM), beta cell function (HOMA-B and C-peptide), and durability of glycemic response At Week 26, a
45-year-old male participant with 10-year history of T2D experienced a 1.1% reduction in HbA1c and an At Week 26, a 29-year-old male participant with a 4-year history of T2D experienced a 2.5% reduction in HbA1c and an increase of 75% TIR compared
to baseline after 4 weeks BMF-219 100mg QD with food, indicating a durable effect on Study Design increase of 56% TIR compared to baseline after 4 weeks BMF-219 200mg QD without food, indicating a durable effect glycemic control. on glycemic
control. BMF-219 Day 1 Week 4 Week 26 (100mg or 200 mg) Daily Summary and Conclusions References Dosing Period Off-Treatment Period 50mg QD* 1. Butler T. et al. Oral Long-Acting Menin Inhibitor Normalizes Type 2 Diabetes Mellitus • These case
studies illustrate the potential disease-modifying and durable effect 100mg QD (T2DM) in Two Rat Models. Diabetes 1 June 2022; 71 (Supplement_1): 851–P. of short-term BMF-219 treatment in patients with uncontrolled T2D 200mg QD 2. Somanath P.
et al. Oral Menin Inhibitor, BMF-219, Displays a Significant and Durable 100mg BID • HbA1c and Time-In-Range (TIR) on CGM continue to improve while off treatment Reduction in HbA1c in a Type 2 Diabetes Mellitus Rat Model. Diabetes 1 June 2022;
200/400mg QD 71 (Supplement_1): 113–LB. 100mg QD • Increase in HOMA-B and C-peptide correlates with glycemic control, consistent 200mg QD 3. Frias J. et al. BMF-219: A Novel Therapeutic Agent to Re-Establish Functional Beta with
BMF-219's core mechanism of action: beta-cell proliferation and improved Cells and Provide Long-Term Glycemic Control. Metabolism May 2023; 142 beta-cell function 4 Weeks Dosing 22-Week Follow-Up (Supplement): Abstract #0088 *Open-label with no
placebo Food Without Food Change in HbA1c (%) % Time in Glucose Range Change in HbA1c (%) % Time in Glucose Range
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Biomea Fusion (NASDAQ:BMEA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Biomea Fusion (NASDAQ:BMEA)
Historical Stock Chart
From Apr 2023 to Apr 2024