0001739174
false
0001739174
2023-11-29
2023-11-29
0001739174
PHGE:UnitsEachConsistingOfOneShareOfCommonStock0.0001ParValueAndOneWarrantEntitlingHolderToReceiveOneHalfShareOfCommonStockMember
2023-11-29
2023-11-29
0001739174
PHGE:SharesOfCommonStock0.0001ParValueMember
2023-11-29
2023-11-29
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported):
November 29, 2023
| BiomX Inc. |
| (Exact Name of Registrant as Specified in its Charter) |
| Delaware |
|
001-38762 |
|
82-3364020 |
(State or other jurisdiction
of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification No.) |
22 Einstein St., Floor 4
Ness Ziona, Israel |
|
7414003 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: +972 723942377
| n/a |
| (Former name or former address, if changed since last report) |
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Units, each consisting of one share of Common Stock, $0.0001 par value, and one Warrant entitling the holder to receive one half share of Common Stock |
|
PHGE.U |
|
NYSE American |
| Shares of Common Stock, $0.0001 par value |
|
PHGE |
|
NYSE American |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
BiomX Inc., or the Company, from time to time, presents and/or distributes
to the investment community at various industry and other conferences slide presentations to provide updates and summaries of its business.
On November 29, 2023, the Company posted an updated corporate slide presentation in the “Investors” portion of its website
at www.biomx.com. A copy of the slide presentation is furnished pursuant to Item 7.01 as Exhibit 99.1 hereto. The Company undertakes no
obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1
Item 8.01 Other
Events.
On
November 29, 2023, the Company announced positive safety and efficacy results from Part 2 of the Phase 1b/2a trial evaluating the Company’s
novel phage cocktail, BX004, for the treatment of chronic pulmonary infections caused by Pseudomonas aeruginosa (or P. aeruginosa) in
patients with cystic fibrosis (“CF”). Highlights included:
| ● | Study
drug was safe and well-tolerated, with no related SAEs (serious adverse events) or related
APEs (acute pulmonary exacerbations) to study drug. |
| ● | BX004
vs. placebo showed a positive clinical effect in a predefined subgroup of patients with reduced
baseline lung function (FEV1<70%). Difference between groups at Day 17: relative FEV1
improvement of 5.67% (change from baseline +1.46 vs. -4.21) and +8.87 points in CFQR respiratory
symptom scale (change from baseline +2.52 vs. -6.35). |
| ● | In
the BX004 arm, 3 out of 21 (14.3%) patients converted to sputum culture negative for P.
aeruginosa after 10 days of treatment (including 2 patients after 4 days) compared to
0 out of 10 (0%) in the placebo arm. |
| ● | In
full population, BX004 vs. placebo P. aeruginosa levels were more variable in sputum,
potentially driven by the standard of care antibiotic treatment regimen. In a prespecified
subgroup of patients on standard-of-care inhaled antibiotics on continuous regimen, BX004
vs. placebo reduced sputum P. aeruginosa levels at Day 10: difference in change from
baseline between groups of -2.8 log10 CFU/g sputum (change from baseline -2.91
vs -0.11), exceeding Part 1 results. |
| ● | Alternating/cycling
background antibiotic regimen likely associated with fluctuations in P. aeruginosa
levels potentially confounding the ability to observe a P. aeruginosa reduction in
this subgroup. |
| ● | During
the study period, no evidence of treatment emergent phage resistance was observed in patients
treated with BX004 compared to placebo. |
| ● | The
Company plans to advance the BX004 program to a larger, pivotal Phase 2b/3 trial, subject
to regulatory feedback and availability of sufficient funding. |
Safe Harbor
This
Current Report on Form 8-K contains express or implied “forward-looking statements” within the meaning of the “safe
harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by
words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,”
“estimate,” “would,” “positioned,” “future,” and other similar expressions that predict
or indicate future events or trends or that are not statements of historical matters. For example, when the Company discusses the safety,
tolerability and efficacy of BX004 and its potential ability to treat CF patients, as well as the potential to advance the BX004 program
to a larger, pivotal Phase 2b/3 trial, including, among other things, timing, design, enrollment, regulatory feedback and approvals and
funding of such trial, it is making forward-looking statements. In addition, past and current pre-clinical and clinical results, as well
as compassionate use, are not indicative and do not guarantee future success of the Company’s clinical trials. Further, the Company
continues to analyze the results of the BX004 Phase 1b/2a Part 2 clinical trial and upon further analysis it may come to conclusions
that are different than the ones that are outlined in this presentation. Forward-looking statements are neither historical facts nor
assurances of future performance. Instead, they are based only on the Company’s management’s current beliefs, expectations
and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes
in circumstances that are difficult to predict and many of which are outside of the Company’s control. Actual results and outcomes
may differ materially from those indicated in the forward-looking statements. Therefore, investors should not rely on any of these forward-looking
statements and should review the risks and uncertainties described under the caption “Risk Factors” in the Company’s
Annual Report on Form 10-K filed with the Securities and Exchange Commission, or the SEC, on March 29, 2023 and additional disclosures
the Company makes in its other filings with the SEC, which are available on the SEC’s website at www.sec.gov. Forward-looking statements
are made as of the date of this Current Report on Form 8-K, and except as provided by law the Company expressly disclaims any obligation
or undertaking to update forward-looking statements.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
BIOMX INC. |
| |
|
|
| November 29, 2023 |
By: |
/s/ Jonathan Solomon |
| |
|
Name: |
Jonathan Solomon |
| |
|
Title: |
Chief Executive Officer |
3
Exhibit
99.1

Revolutionizing the treatment of Cystic Fibrosis through our unique BOLT Phage therapy platform Investor Presentation / November 2023

2 Safe Harbor Statement This presentation contains certain “forward - looking statements” within the meaning of the “safe harbor” provisions of the U . S . Private Securities Litigation Reform Act of 1995 . Forward - looking statements can be identified by words such as : “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would,” “positioned,” “future,” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters . Forward - looking statements are neither historical facts nor assurances of future performance . Instead, they are based only on BiomX management’s current beliefs, expectations and assumptions . For example, when we discuss future potential clinical trials, including their design, objectives, costs, endpoints, potential benefits and timing, the potential outcomes of discussions that we may have with the U . S . Food and Drug Administration and foreign regulatory agencies, potential commercial opportunities, our expected cash runway, our financial needs to fund future clinical trials and our ability to protect our intellectual property assets in the future we are making forward - looking statements . In addition, past and current pre - clinical and clinical results, as well as compassionate use, are not indicative and do not guarantee future success of BiomX clinical trials . Further, we continue to analyze the results of the BX 004 Phase 1 b/ 2 a Part 2 clinical trial results and upon further analysis we may come to conclusions that are different that the ones that are outlined in this presentation . Because forward - looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control . Actual results and outcomes may differ materially from those indicated in the forward - looking statements . Therefore, you should not rely on any of these forward - looking statements . You should review additional disclosures we make in our filings with the Securities and Exchange Commission (the “SEC”), which are available on the SEC’s website at www . sec . gov . Except as required by law, we are under no duty to (and expressly disclaim any such obligation to) update or revise any of the forward - looking statements, whether as a result of new information, future events or otherwise .
3 Unmet need in cystic fibrosis (‘CF’) • Improved treatment has shifted CF from being a disease of childhood to a disease of adulthood. As patients age, Pseudomonas aeruginosa (PsA) lung infections become the leading cause of morbidity and mortality • Prolonged antibiotic treatments lead to significant resistance, creating a large unmet need - an estimated 17,000 CF patients in the US and Western Europe with chronic PsA infections 1 BX004 – our lead program • BX004, our proprietary phage cocktail, has the potential to treat CF patients with chronic resistant PsA lung infections, providing a significant potential commercial opportunity of > $1 billion 2 • In a Phase 1b/2a, BX004 showed clinically meaningful improvement in pulmonary function vs. placebo, in relative FEV1 3 improvement (5.67% at Day 17, 1 week after EOT 3 ) and PRO 3 in patients with reduced lung function 4 • In the BX004 arm, 3 out of 21 (14.3%) patients converted to sputum culture negative for PsA after 10 days of treatment compared to 0 out of 10 (0%) in the placebo arm 5 • Plans to advance the BX004 program to a larger, pivotal Phase 2b/3 study 6 Our Bolt phage technology • Our proprietary BOLT phage technology platform - which is based on advanced machine learning – was used to design the BX004 phage cocktail that in vitro overcomes antibiotic resistance and biofilms • Publicly traded (NYSEAmerican:PHGE) • $23.4 million cash and cash equivalents as of September 30, 2023. Expected cash runway into the third quarter of 2024 • Backed by prominent biotech investors such as Orbimed, Johnson & Johnson and the CF Foundation Financing and investors 1. 2. 3. See slide 10 See slide 27 FEV1 or ppFEV1 – percent predicted forced expiratory volume, EOT – End of treatment, PRO – Patient reported outcome Executive Summary 4. 5. 6. Predefined group with Baseline FEV1<70% In patients that had quantitative CFU levels at study baseline Subject to regulatory feedback and availability of sufficient funding

4 Strong leadership and scientific team Management Board of Directors Jonathan Solomon - Chief Executive Officer, Director • Former co - Founder and CEO Proclara Merav Bassan, PhD - Chief Development Officer • 20 years drug and clinical development at Teva Assaf Oron - Chief Business Officer • Former EVP business development at Evogene Marina Wolfson, CPA - Chief Financial Officer • Former Bioview, Ernst & Young Inbal Benjamini - Elran – Chief HR Officer • Former HR roles at Teva and Herzog Law Russell Greig, PhD - Chairman of the Board • Former president of GSK Pharma International & SR one, GSK corporate venture group Scientific Team Prof. Rotem Sorek • Head of microbial genomics group at Weizmann Institute • Phage genomics and CRISPR research Prof. Timothy K. Lu • Associate professor leading synthetic biology group, MIT • Synthetic biology, biochemical engineering Prof. Eran Elinav • Principal investigator at Weizmann Institute • Immune system and intestinal microbiome interactions Prof. Eitan Kerem • Former Chairman of Pediatric Pulmonology Unit, Hadassah Medical Center • World leader in CF care and research Alan Moses, MD - Director • Former Global Chief Medical Officer of Novo Nordisk Lynne Sullivan - Director • Former Senior Vice President of Finance for Biogen Jason Marks - Director • Former Executive Vice President, Chief Legal and Compliance Officer at Amarin Corporation plc Michael Dambach - Director • Vice President and Treasurer of Biogen Inc. Eddie Williams - Director • Former special advisor to the CEO of Ascendis Pharma, Inc.

CYSTIC FIBROSIS The Unmet Need
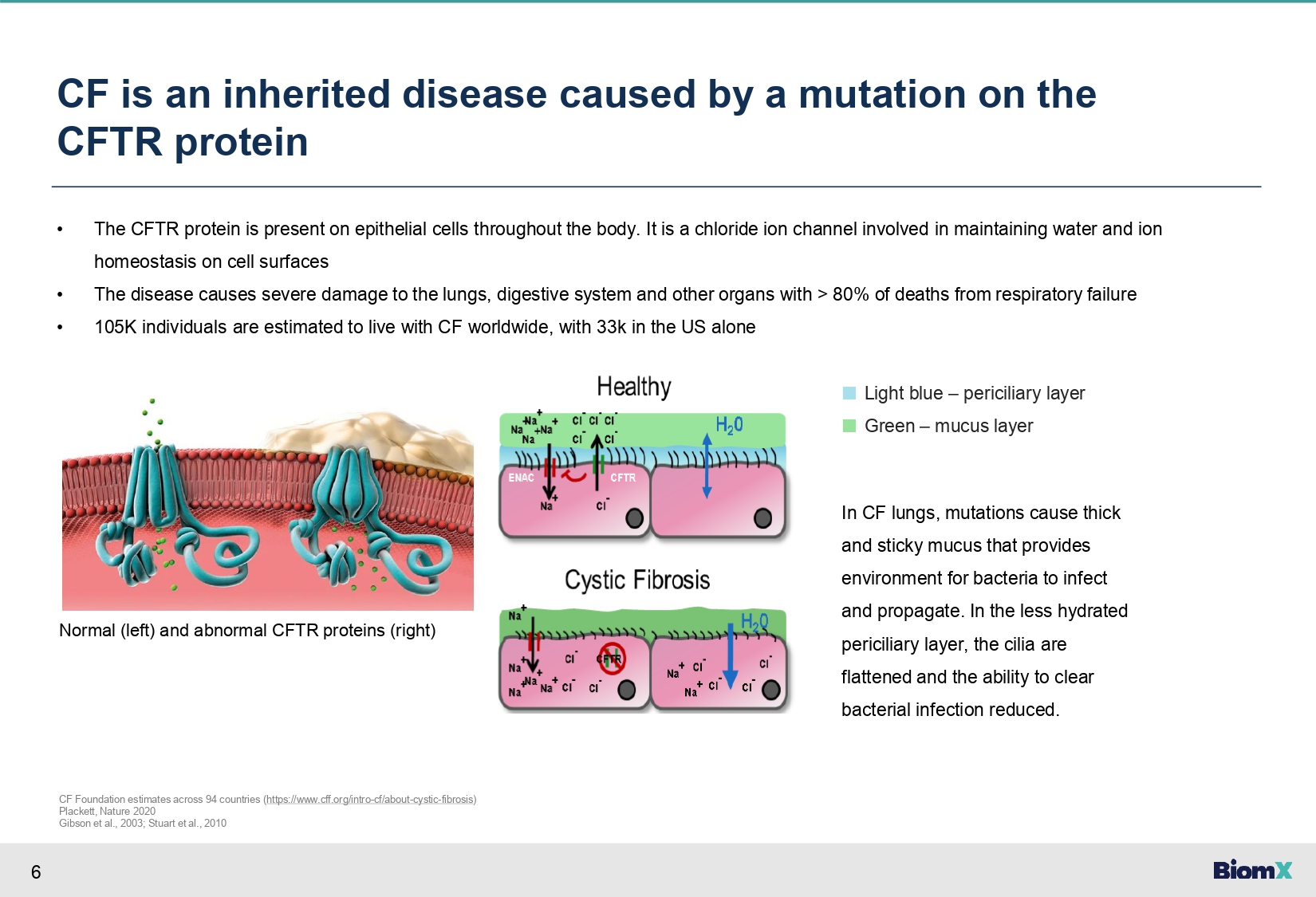
6 CF is an inherited disease caused by a mutation on the CFTR protein • The CFTR protein is present on epithelial cells throughout the body. It is a chloride ion channel involved in maintaining water and ion homeostasis on cell surfaces • The disease causes severe damage to the lungs, digestive system and other organs with > 80% of deaths from respiratory failure • 105K individuals are estimated to live with CF worldwide, with 33k in the US alone ■ Light blue – periciliary layer ■ Green – mucus layer In CF lungs, mutations cause thick and sticky mucus that provides environment for bacteria to infect and propagate. In the less hydrated periciliary layer, the cilia are flattened and the ability to clear bacterial infection reduced. CF Foundation estimates across 94 countries ( https://www.cff.org/intro - cf/about - cystic - fibrosis ) Plackett, Nature 2020 Gibson et al., 2003; Stuart et al., 2010 Normal (left) and abnormal CFTR proteins (right)

7 Declining incidence is offset by increased survival through improved treatment resulting in CF being shifted from being a disease of childhood to being a disease of adulthood >50 years 2022 2011 37.9 years 2016 42.6 years Improvements driven by introduction of life - changing medicines PREDICTED MEDIAN SURVIVAL AT BIRTH CFF 2021 patient registry annual data report , NACFC (North American CF Conference) Oct. 2021 plenary session
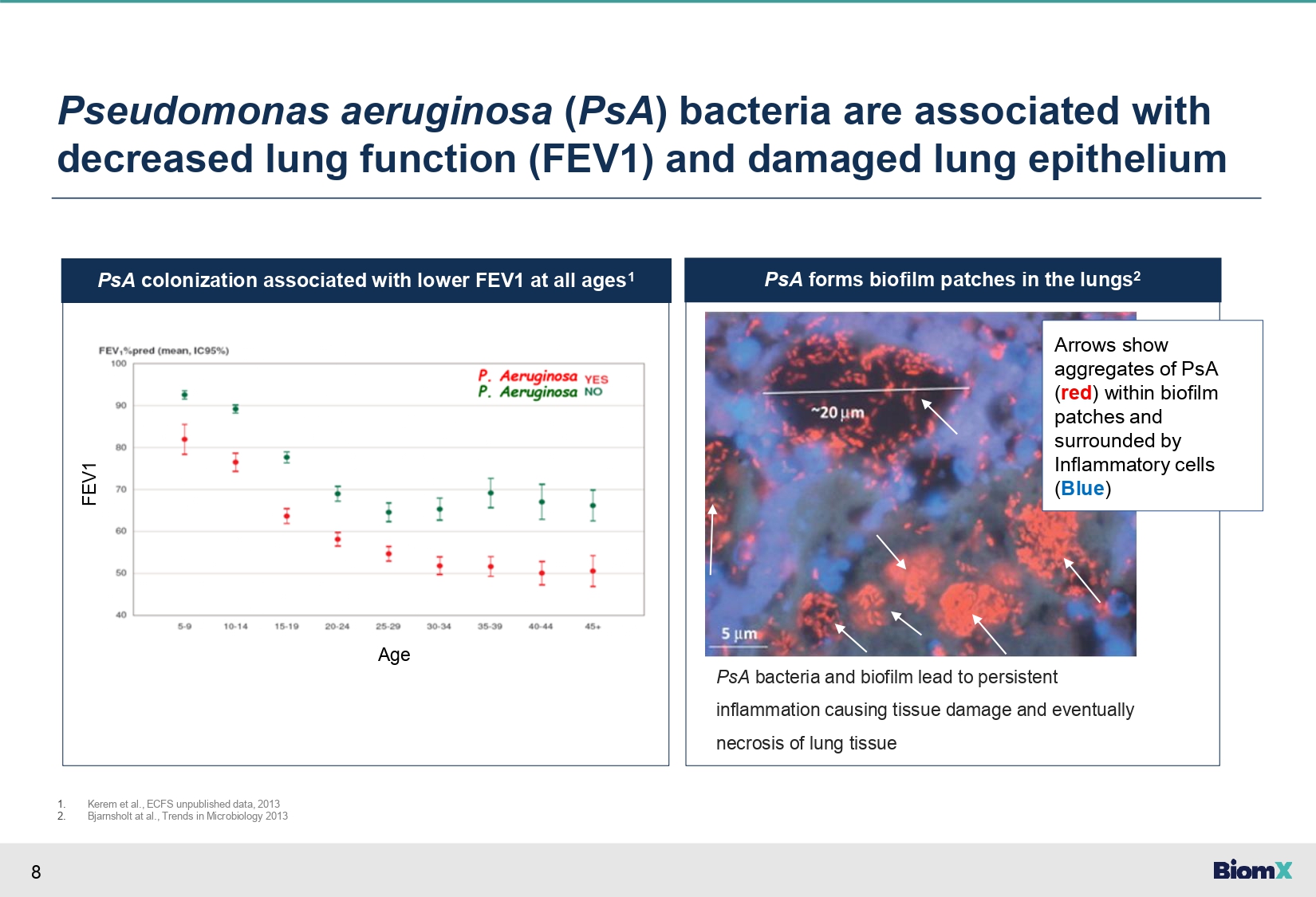
8 PsA forms biofilm patches in the lungs 2 PsA colonization associated with lower FEV1 at all ages 1 Pseudomonas aeruginosa ( PsA ) bacteria are associated with decreased lung function (FEV1) and damaged lung epithelium Age FEV1 PsA bacteria and biofilm lead to persistent inflammation causing tissue damage and eventually necrosis of lung tissue 1. 2. Kerem et al., ECFS unpublished data, 2013 Bjarnsholt at al., Trends in Microbiology 2013 Maya - Add image of biofilm formation by PsA in the lungs Check biofilm pathogenicity Arrows show aggregates of PsA ( red ) within biofilm patches and surrounded by Inflammatory cells ( Blue )
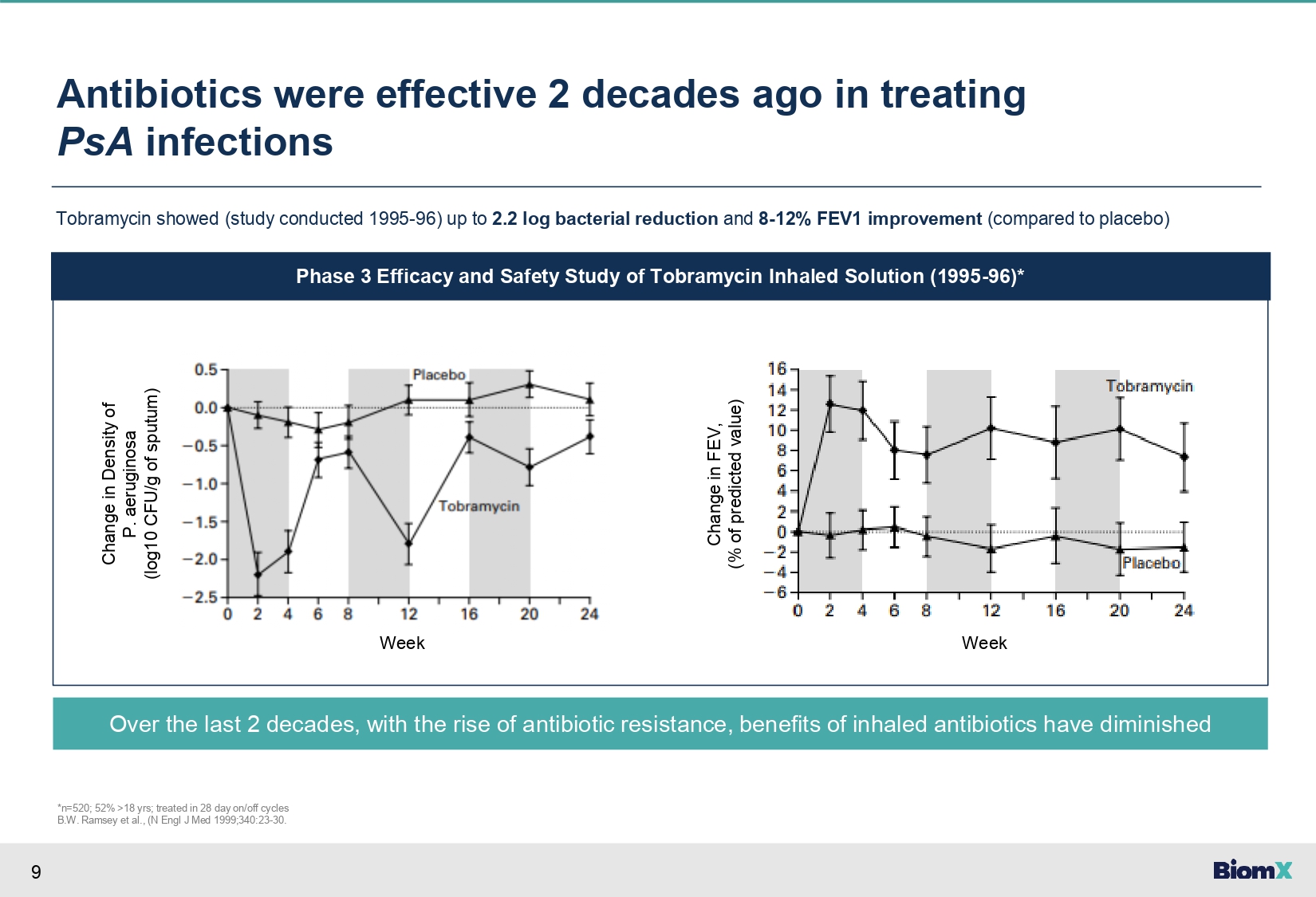
9 Antibiotics were effective 2 decades ago in treating PsA infections Tobramycin showed (study conducted 1995 - 96) up to 2.2 log bacterial reduction and 8 - 12% FEV1 improvement (compared to placebo) Phase 3 Efficacy and Safety Study of Tobramycin Inhaled Solution (1995 - 96)* Change in Density of P. aeruginosa (log10 CFU/g of sputum) Over the last 2 decades, with the rise of antibiotic resistance, benefits of inhaled antibiotics have diminished *n=520; 52% >18 yrs; treated in 28 day on/off cycles B.W. Ramsey et al., (N Engl J Med 1999;340:23 - 30. Change in FEV, (% of predicted value) Week Week
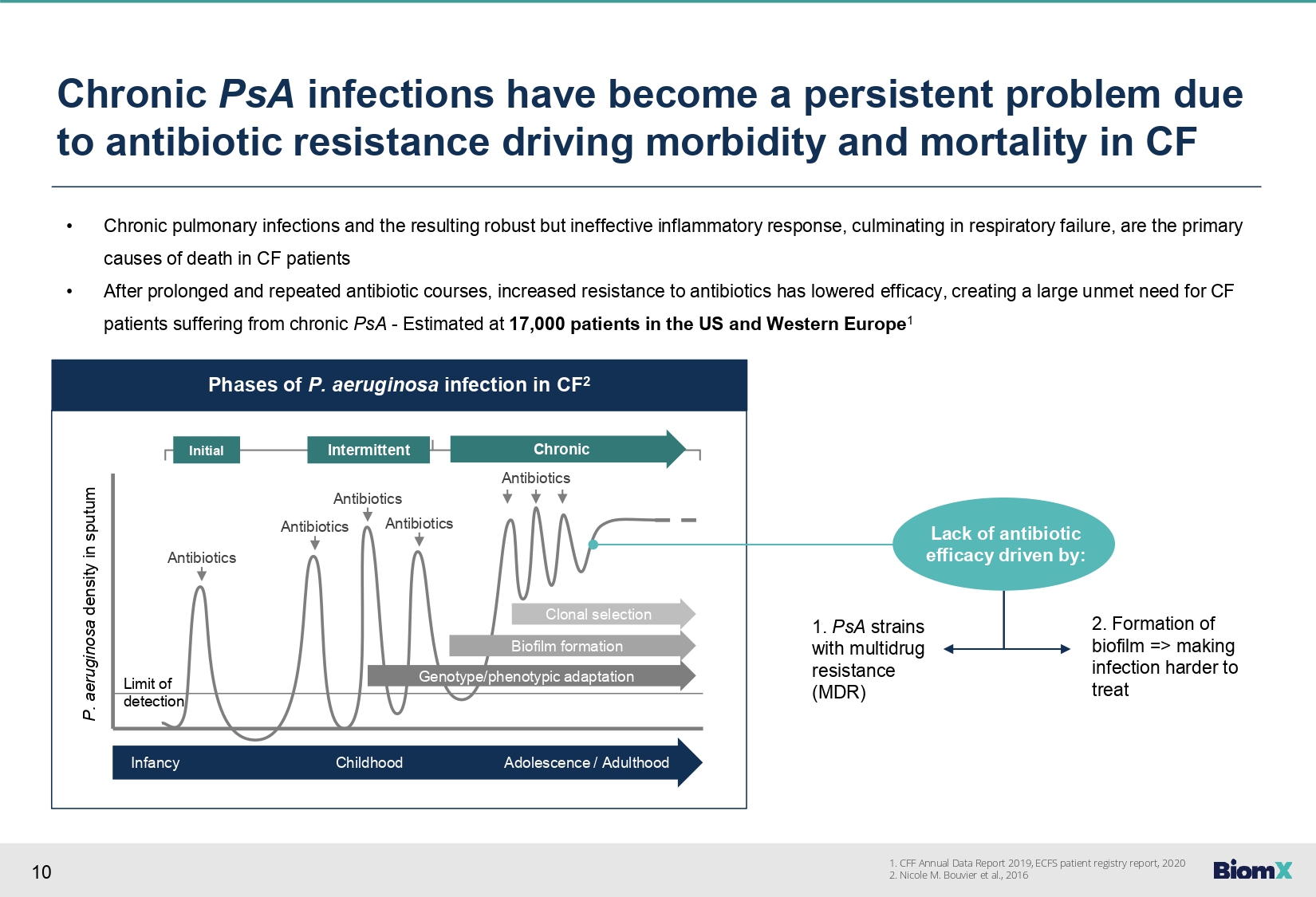
10 Phases of P. aeruginosa infection in CF 2 Antibiotics Antibiotics Antibiotics Antibiotics Antibiotics Initial Intermittent Chronic Clonal selection Biofilm formation Genotype/phenotypic adaptation Infancy Childhood Adolescence / Adulthood Limit of detection P. aeruginosa density in sputum Chronic PsA infections have become a persistent problem due to antibiotic resistance driving morbidity and mortality in CF • Chronic pulmonary infections and the resulting robust but ineffective inflammatory response, culminating in respiratory failure, are the primary causes of death in CF patients • After prolonged and repeated antibiotic courses, increased resistance to antibiotics has lowered efficacy, creating a large unmet need for CF patients suffering from chronic PsA - Estimated at 17,000 patients in the US and Western Europe 1 Lack of antibiotic efficacy driven by: 1. PsA strains with multidrug resistance (MDR) 2. Formation of biofilm => making infection harder to treat 1. CFF Annual Data Report 2019, ECFS patient registry report, 2020 2. Nicole M. Bouvier et al., 2016
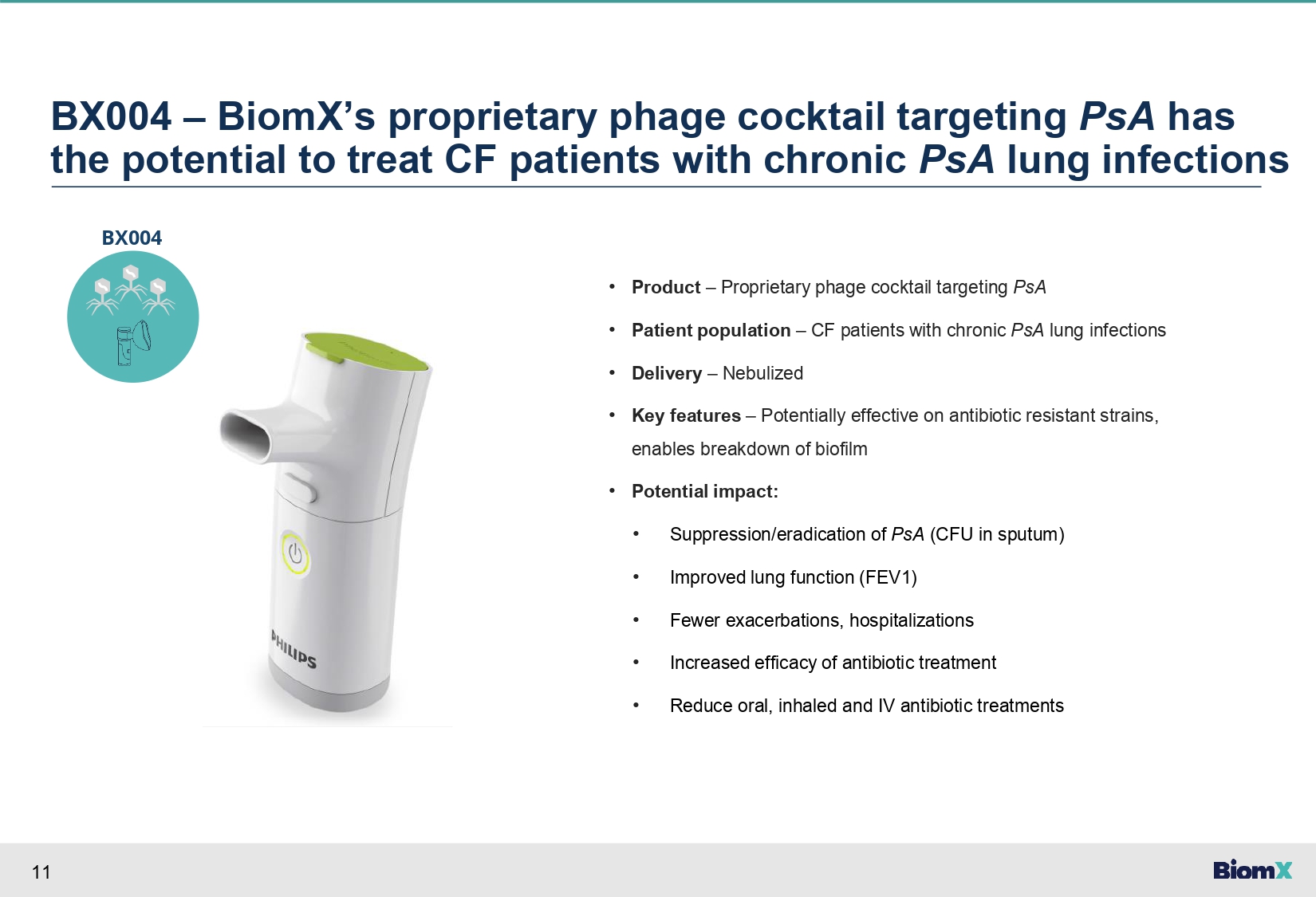
11 BX004 – BiomX’s proprietary phage cocktail targeting PsA has the potential to treat CF patients with chronic PsA lung infections • Product – Proprietary phage cocktail targeting PsA • Patient population – CF patients with chronic PsA lung infections • Delivery – Nebulized • Key features – Potentially effective on antibiotic resistant strains, enables breakdown of biofilm • Potential impact: • Suppression/eradication of PsA (CFU in sputum) • Improved lung function (FEV1) • Fewer exacerbations, hospitalizations • Increased efficacy of antibiotic treatment • Reduce oral, inhaled and IV antibiotic treatments BX004

Intro To PHAGE
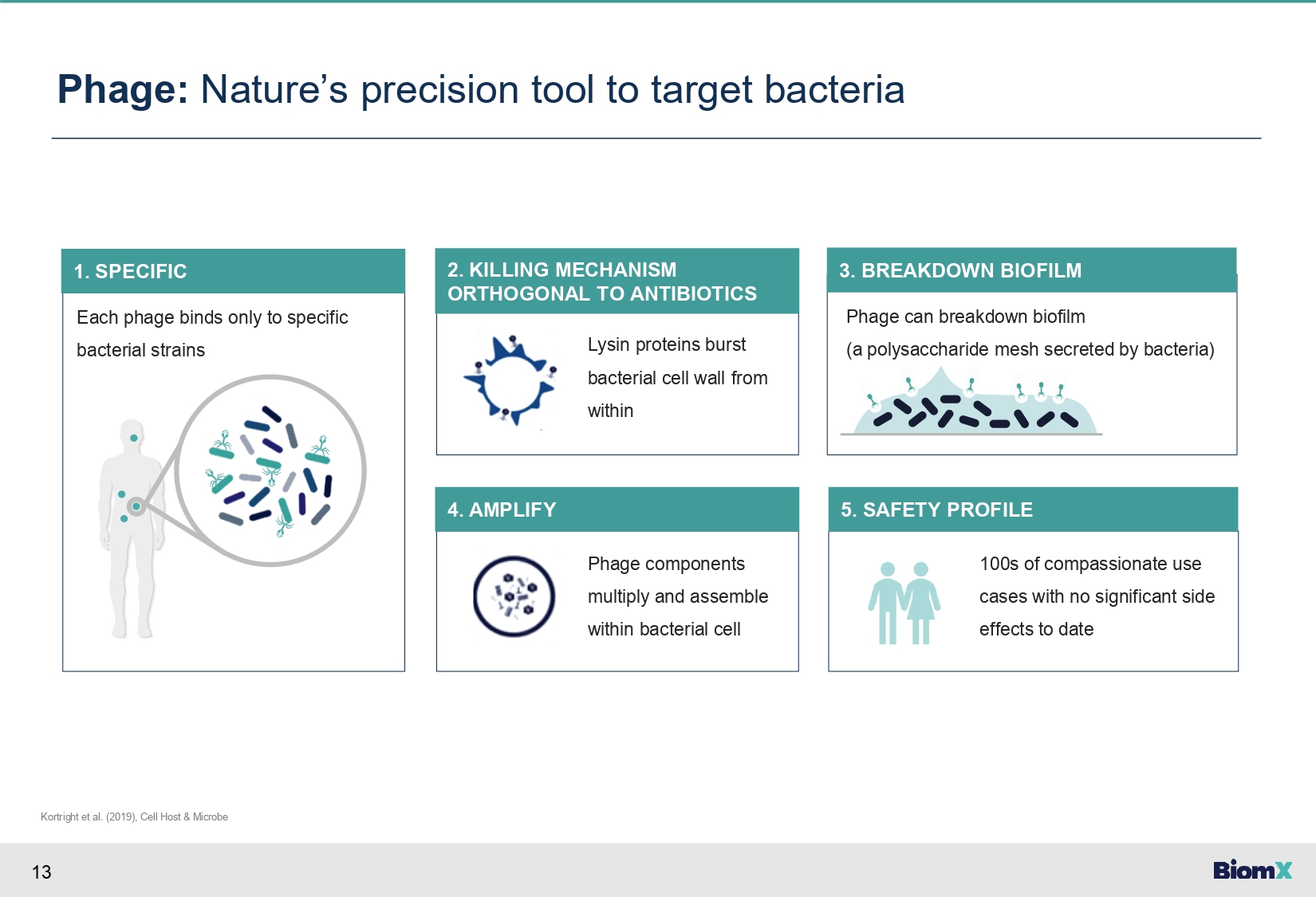
13 Each phage binds only to specific bacterial strains 1. SPECIFIC Phage: Nature’s precision tool to target bacteria Kortright et al. (2019), Cell Host & Microbe Lysin proteins burst bacterial cell wall from within 2. KILLING MECHANISM ORTHOGONAL TO ANTIBIOTICS Phage components multiply and assemble within bacterial cell 4. AMPLIFY 100s of compassionate use cases with no significant side effects to date 5. SAFETY PROFILE Phage can breakdown biofilm (a polysaccharide mesh secreted by bacteria) 3. BREAKDOWN BIOFILM
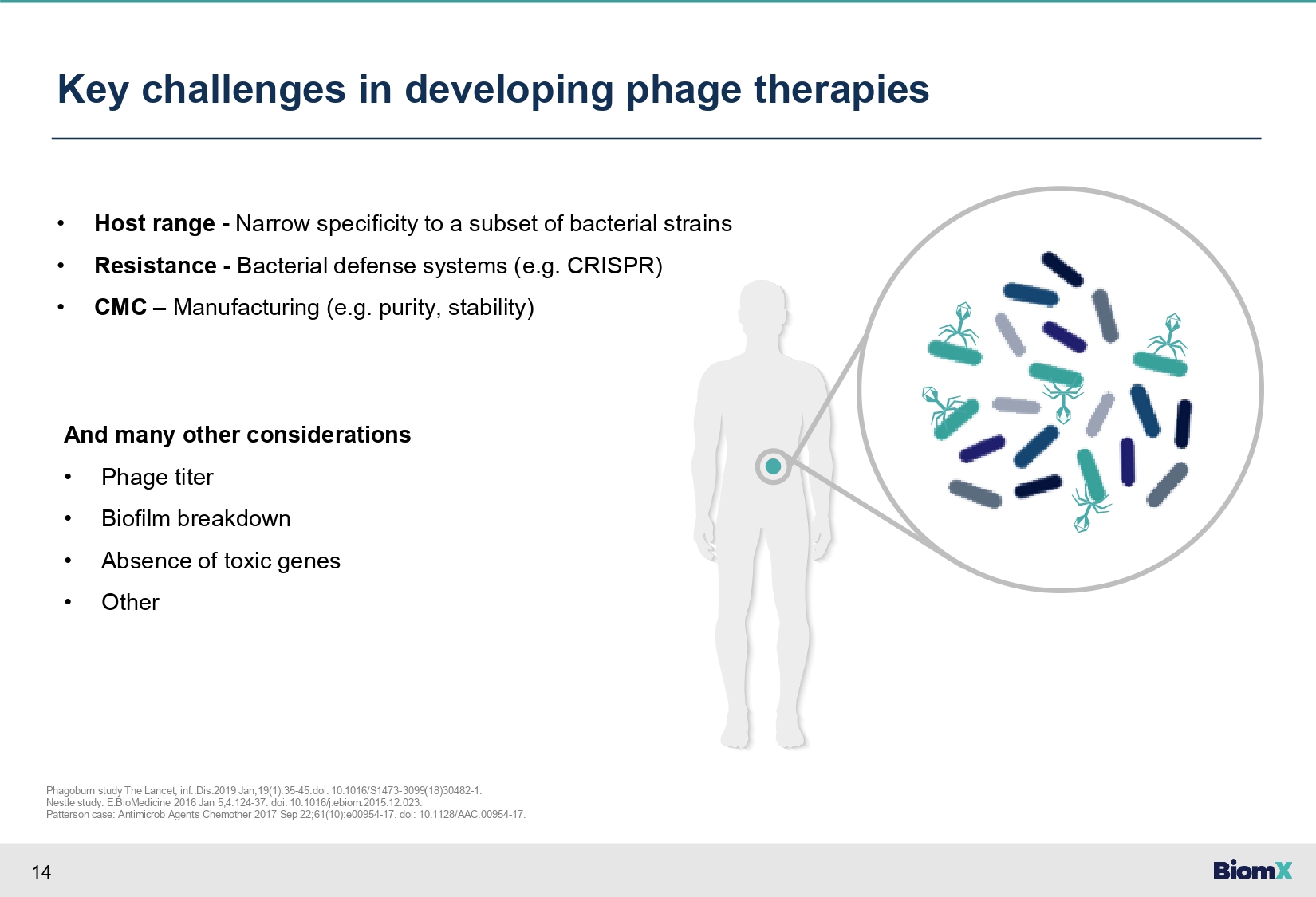
14 Key challenges in developing phage therapies • Host range - Narrow specificity to a subset of bacterial strains • Resistance - Bacterial defense systems (e.g. CRISPR) • CMC – Manufacturing (e.g. purity, stability) And many other considerations • Phage titer • Biofilm breakdown • Absence of toxic genes • Other Phagoburn study The Lancet, inf..Dis.2019 Jan;19(1):35 - 45.doi: 10.1016/S1473 - 3099(18)30482 - 1. Nestle study: E.BioMedicine 2016 Jan 5;4:124 - 37. doi: 10.1016/j.ebiom.2015.12.023. Patterson case: Antimicrob Agents Chemother 2017 Sep 22;61(10):e00954 - 17. doi: 10.1128/AAC.00954 - 17.
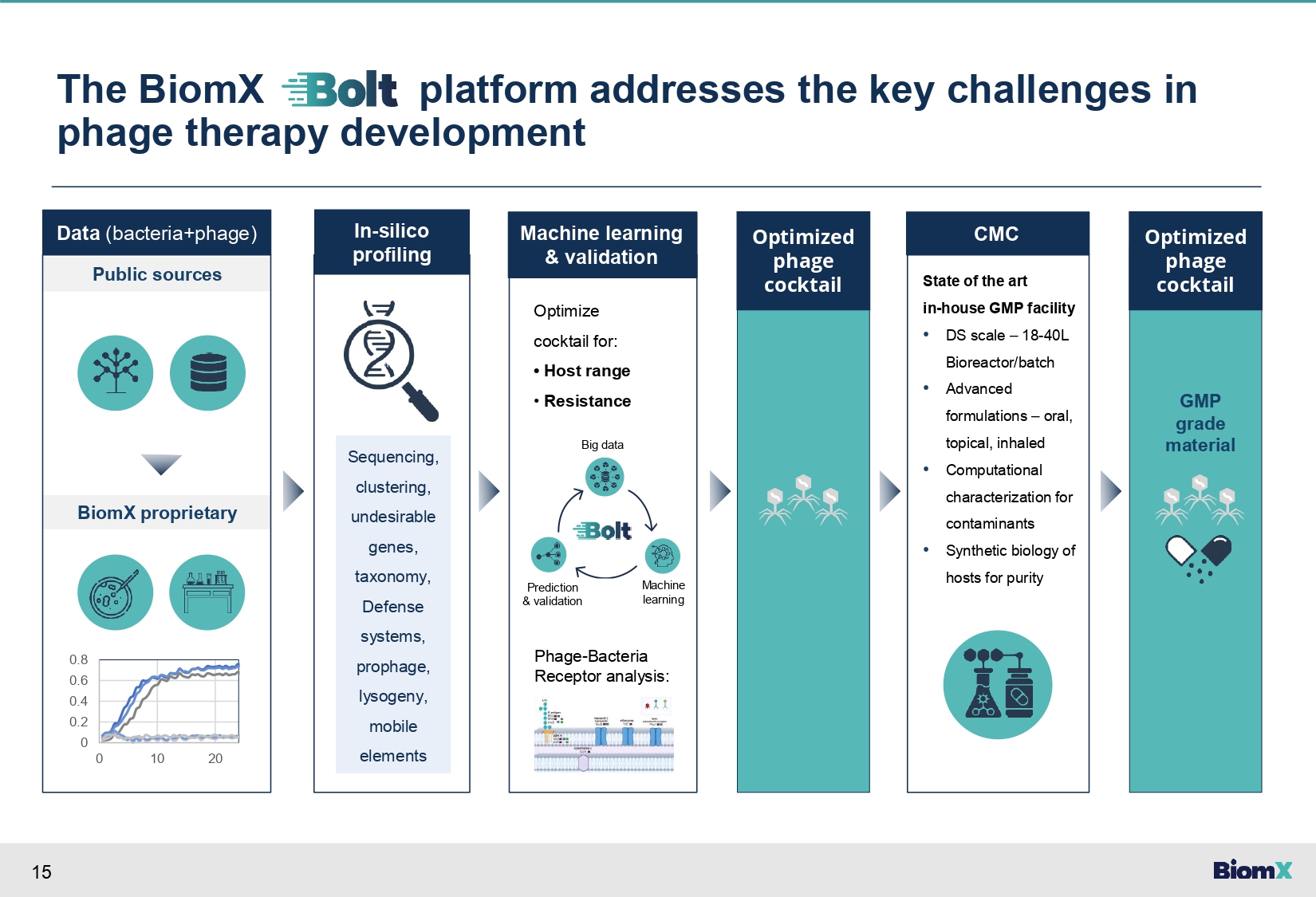
15 The BiomX platform addresses the key challenges in phage therapy development Data (bacteria+phage) Public sources BiomX proprietary 0.8 0.6 0.4 0.2 0 0 10 20 Machine learning & validation In - silico profiling Phage - Bacteria Receptor analysis: Optimize cocktail for: • Host range • Resistance Big data Optimized phage cocktail CMC State of the art in - house GMP facility • DS scale – 18 - 40L Bioreactor/batch • Advanced formulations – oral, topical, inhaled • Computational characterization for contaminants • Synthetic biology of hosts for purity Optimized phage cocktail Machine learning Prediction & validation Sequencing, clustering, undesirable genes, taxonomy, Defense systems, prophage, lysogeny, mobile elements GMP grade material
BX004
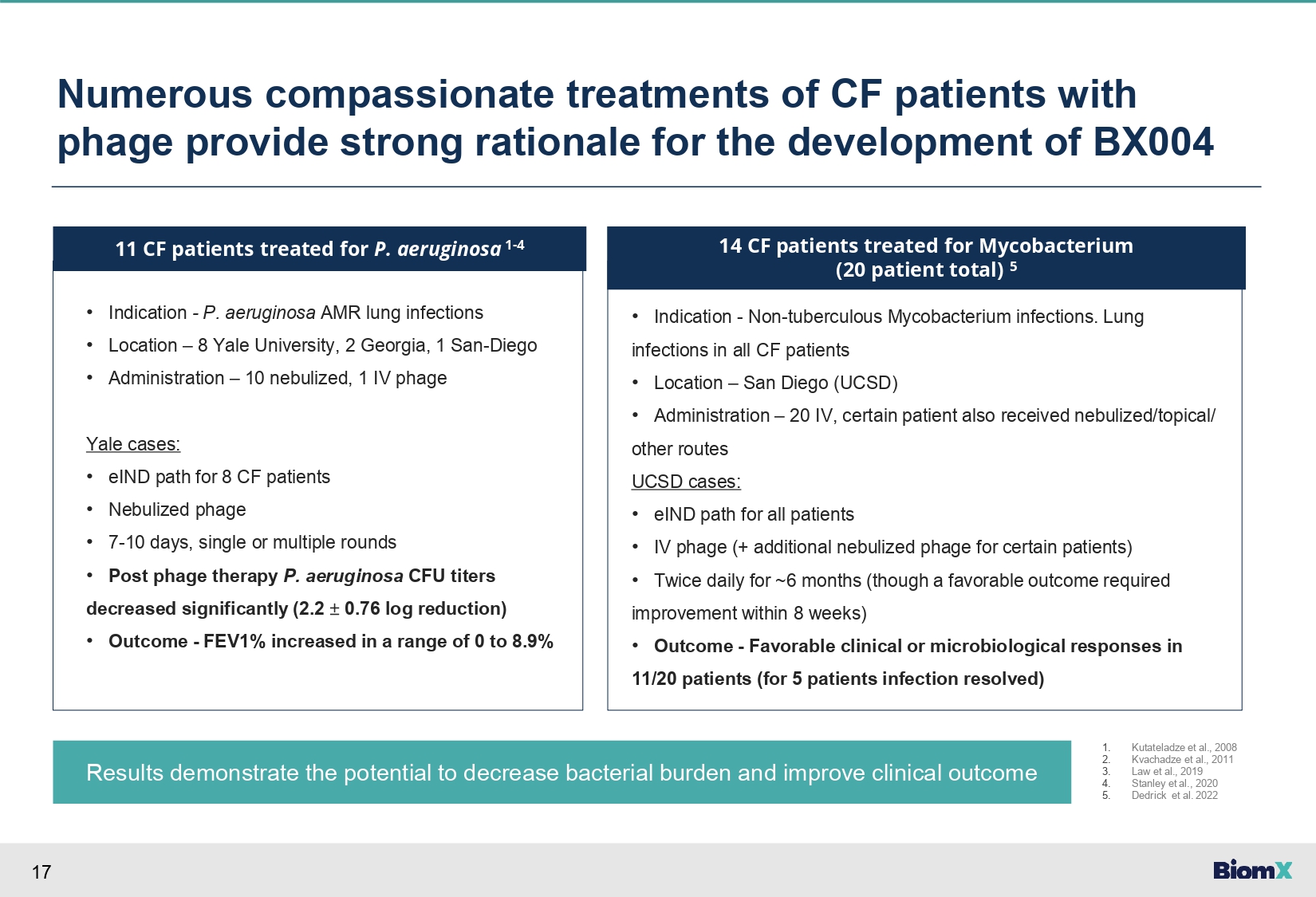
17 Numerous compassionate treatments of CF patients with phage provide strong rationale for the development of BX004 Results demonstrate the potential to decrease bacterial burden and improve clinical outcome • Indication - P. aeruginosa AMR lung infections • Location – 8 Yale University, 2 Georgia, 1 San - Diego • Administration – 10 nebulized, 1 IV phage Yale cases: • eIND path for 8 CF patients • Nebulized phage • 7 - 10 days, single or multiple rounds • Post phage therapy P. aeruginosa CFU titers decreased significantly (2.2 “ 0.76 log reduction) • Outcome - FEV1% increased in a range of 0 to 8.9% 11 CF patients treated for P. aeruginosa 1 - 4 • Indication - Non - tuberculous Mycobacterium infections. Lung infections in all CF patients • Location – San Diego (UCSD) • Administration – 20 IV, certain patient also received nebulized/topical/ other routes UCSD cases: • eIND path for all patients • IV phage (+ additional nebulized phage for certain patients) • Twice daily for ~6 months (though a favorable outcome required improvement within 8 weeks) • Outcome - Favorable clinical or microbiological responses in 11/20 patients (for 5 patients infection resolved) 14 CF patients treated for Mycobacterium (20 patient total) 5 1. 2. 3. 4. 5. Kutateladze et al., 2008 Kvachadze et al., 2011 Law et al., 2019 Stanley et al., 2020 Dedrick et al. 2022
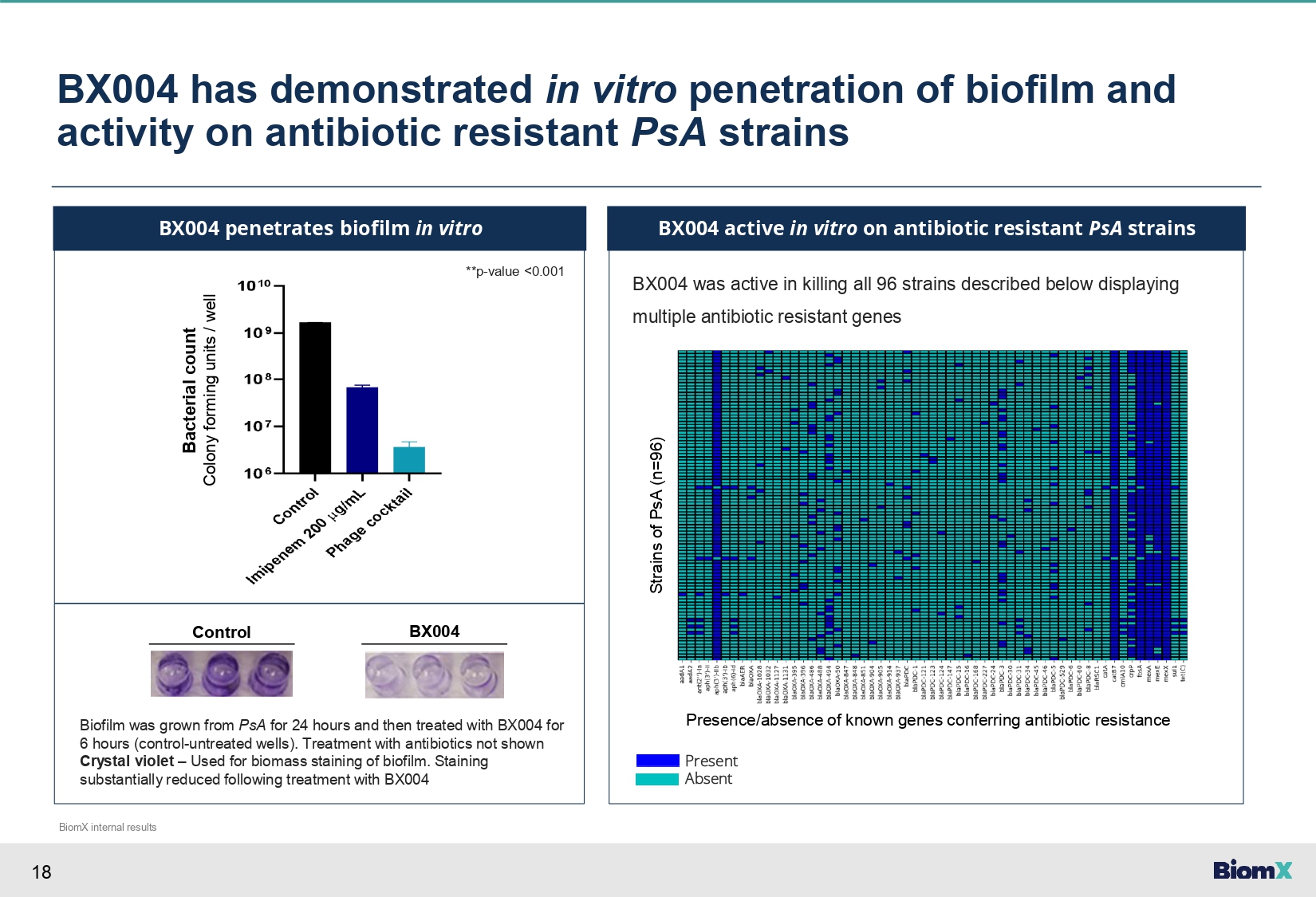
18 BX004 has demonstrated in vitro penetration of biofilm and activity on antibiotic resistant PsA strains BX004 penetrates biofilm in vitro BX004 was active in killing all 96 strains described below displaying multiple antibiotic resistant genes BX004 active in vitro on antibiotic resistant PsA strains **p - value <0.001 Bacterial count Colony forming units / well Control BX004 Biofilm was grown from PsA for 24 hours and then treated with BX004 for 6 hours (control - untreated wells). Treatment with antibiotics not shown Crystal violet – Used for biomass staining of biofilm. Staining substantially reduced following treatment with BX004 Strains of PsA (n=96) Presence/absence of known genes conferring antibiotic resistance Present Absent BiomX internal results

19 Study design informed by input from the CF Foundation Phase 1b/2a study Part 1 – Study design Part 1 (n=9) Objectives • Safety, PK and microbiologic/clinical activity Endpoints • Safety and tolerability (Primary endpoint) • Decrease in PsA burden • Sputum pharmacokinetics • FEV1 (forced expiratory volume) • CFQ - R (CF Questionnaire - Revised) and CRISS Study Population • CF patients with chronic PsA infection • Physician choice of inhaled antibiotic regimen (continuous or alternating or cycling); on tobramycin, aztreonam or colistin during study drug • No restriction on CFTR modulators 9 Subjects • 7 received nebulized BX004 phage therapy • 2 received nebulized placebo • 7 days duration (3 ascending, 4 multiple dosing) Key Design Features • Single ascending dose followed by multiple doses Completed
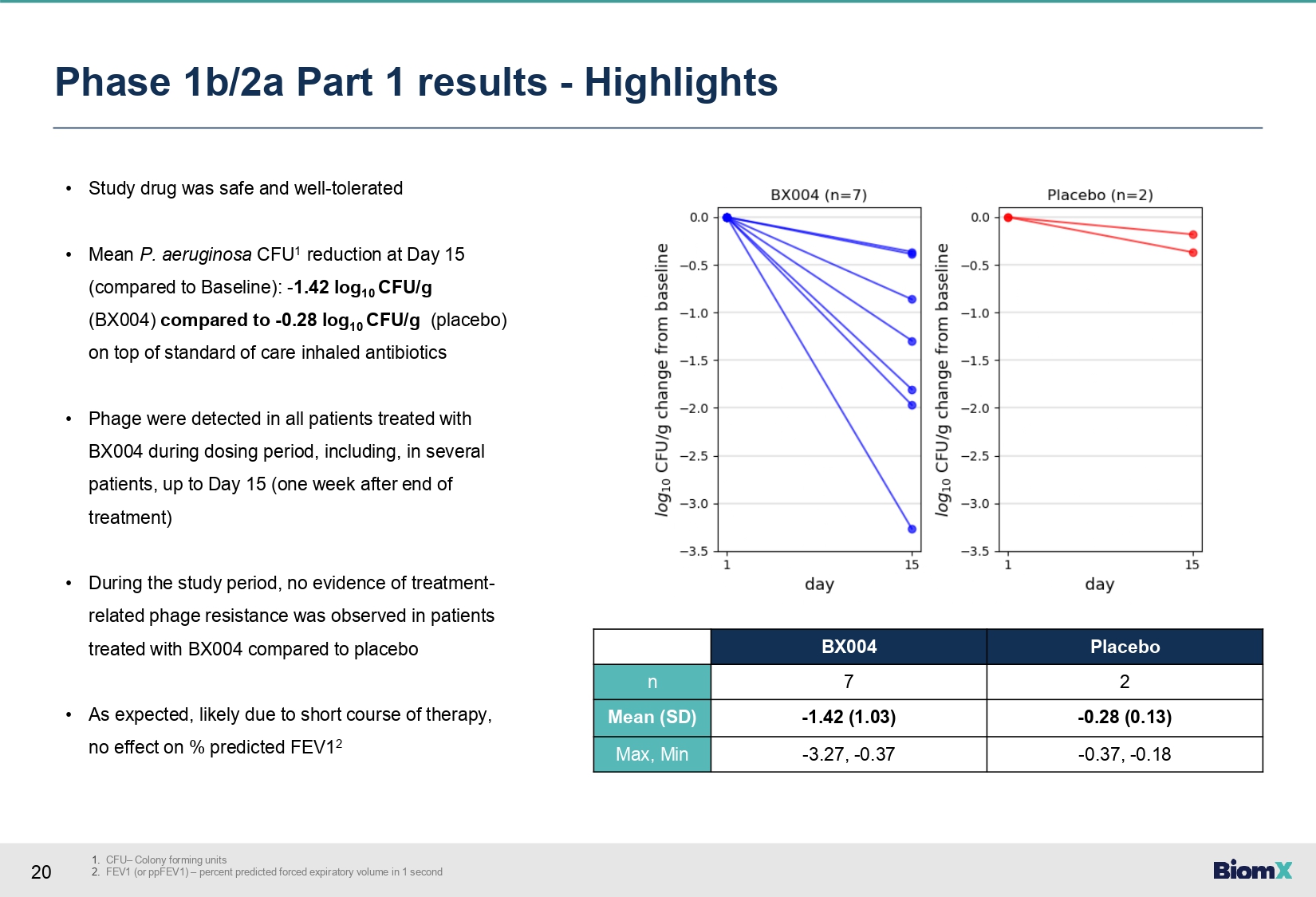
20 1. CFU – Colony forming units 2. FEV1 (or ppFEV1) – percent predicted forced expiratory volume in 1 second Phase 1b/2a Part 1 results - Highlights • Study drug was safe and well - tolerated • Mean P. aeruginosa CFU 1 reduction at Day 15 (compared to Baseline): - 1.42 log 10 CFU/g (BX004) compared to - 0.28 log 10 CFU/g (placebo) on top of standard of care inhaled antibiotics • Phage were detected in all patients treated with BX004 during dosing period, including, in several patients, up to Day 15 (one week after end of treatment) • During the study period, no evidence of treatment - related phage resistance was observed in patients treated with BX 004 compared to placebo • As expected, likely due to short course of therapy, no effect on % predicted FEV 1 2 Placebo BX004 2 7 n - 0.28 (0.13) - 1.42 (1.03) Mean (SD) - 0.37, - 0.18 - 3.27, - 0.37 Max, Min
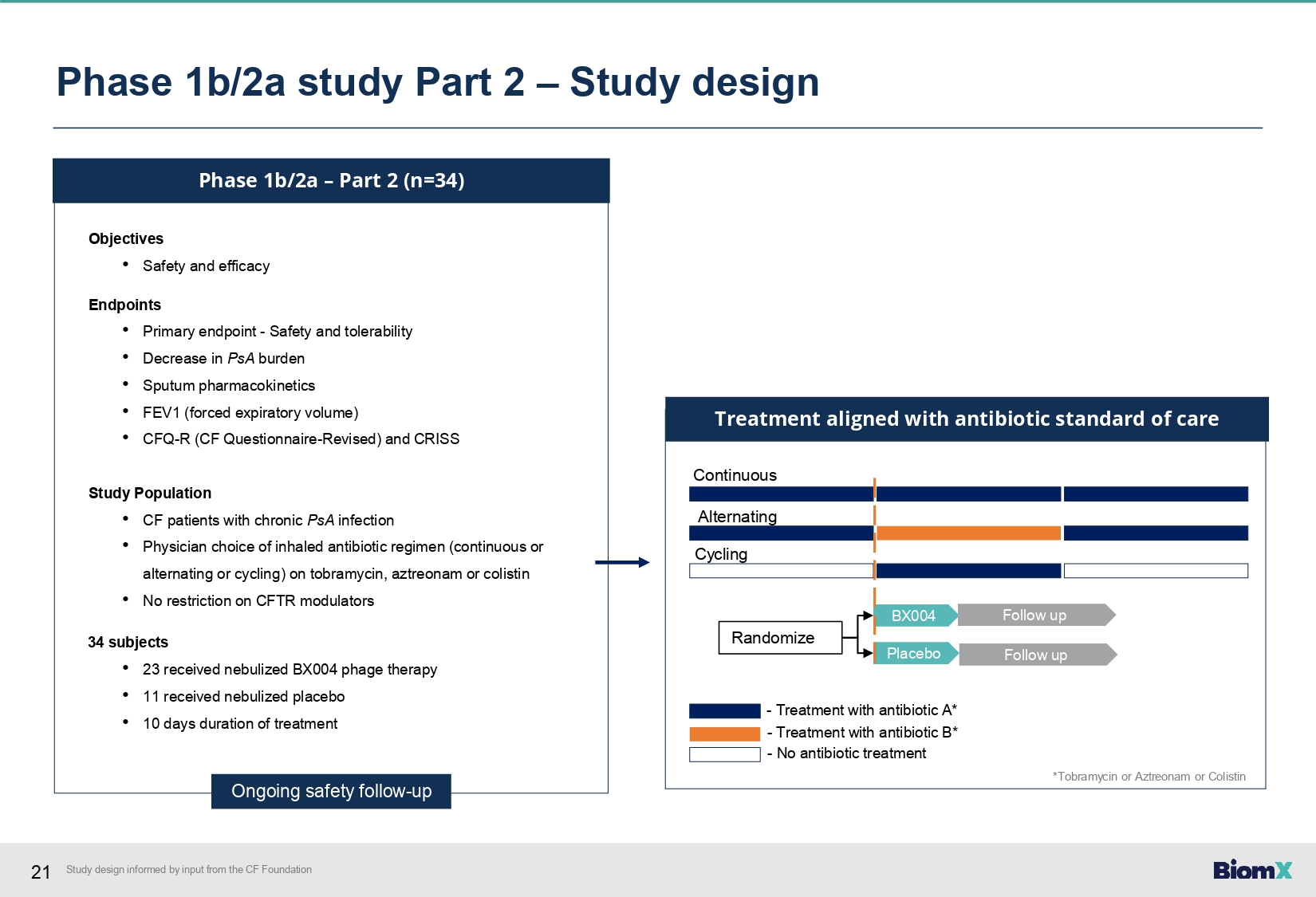
21 Phase 1b/2a – Part 2 (n=34) Objectives • Safety and efficacy Endpoints • Primary endpoint - Safety and tolerability • Decrease in PsA burden • Sputum pharmacokinetics • FEV1 (forced expiratory volume) • CFQ - R (CF Questionnaire - Revised) and CRISS Study Population • CF patients with chronic PsA infection • Physician choice of inhaled antibiotic regimen (continuous or alternating or cycling) on tobramycin, aztreonam or colistin • No restriction on CFTR modulators 34 subjects • 23 received nebulized BX004 phage therapy • 11 received nebulized placebo • 10 days duration of treatment Ongoing safety follow - up BX004 Placebo Continuous Alternating Cycling Randomize Treatment aligned with antibiotic standard of care Follow up Follow up - Treatment with antibiotic A* - Treatment with antibiotic B* - No antibiotic treatment Phase 1b/2a study Part 2 – Study design *Tobramycin or Aztreonam or Colistin Study design informed by input from the CF Foundation
22 Phase 1b/2a study Part 2 – Highlights 1. 2. SAE – Serious Adverse Event, APE – Acute Pulmonary Exacerbation FEV1 (or ppFEV1) – percent predicted forced expiratory volume in 1 second, CF Questionnaire - Revised Respiratory – a PRO (Patient reported outcome) for respiratory parameters in CF aptients, EOT – End of treatment, SOC – standard of care 3. 4. Predefined group with Baseline FEV1<70% In patients that had quantitative CFU levels at study baseline • Study drug was well - tolerated, no related SAE 1 s or related APE 1 s to study drug were observed • BX004 showed clinically meaningful improvement in pulmonary function vs. placebo: Relative FEV1 2 improvement (5.67%) and CF Questionnaire - Revised respiratory 2 (8.87 points) at Day 17 (1 week after EOT 2 ) in subgroup of patients with reduced lung function 3 • In the BX004 arm, 3 out of 21 (14.3%) patients converted to sputum culture negative for P. aeruginosa after 10 days of treatment compared to 0 out of 10 (0%) in the placebo arm 4 • In full population, BX004 vs. placebo P. aeruginosa levels were more variable. In a prespecified subgroup of patients on SOC 2 inhaled antibiotics on continuous regimen, BX004 vs. placebo showed bacterial reduction of 2.8 log 10 CFU/g at EOT 2 , exceeding Part 1 results • Alternating/cycling background antibiotic regimen likely associated with fluctuations in P. aeruginosa levels potentially confounding the ability to observe a P. aeruginosa reduction in this subgroup • During the study period, based on current available data, no evidence of treatment - related phage resistance was observed in patients treated with BX004 compared to placebo • Plans to advance the BX004 program to a larger, pivotal Phase 2b/3 trial, subject to regulatory feedback and availability of sufficient funding We believe this better - than - expected clinical effect in a short treatment duration de - risks planned pivotal P2b/3
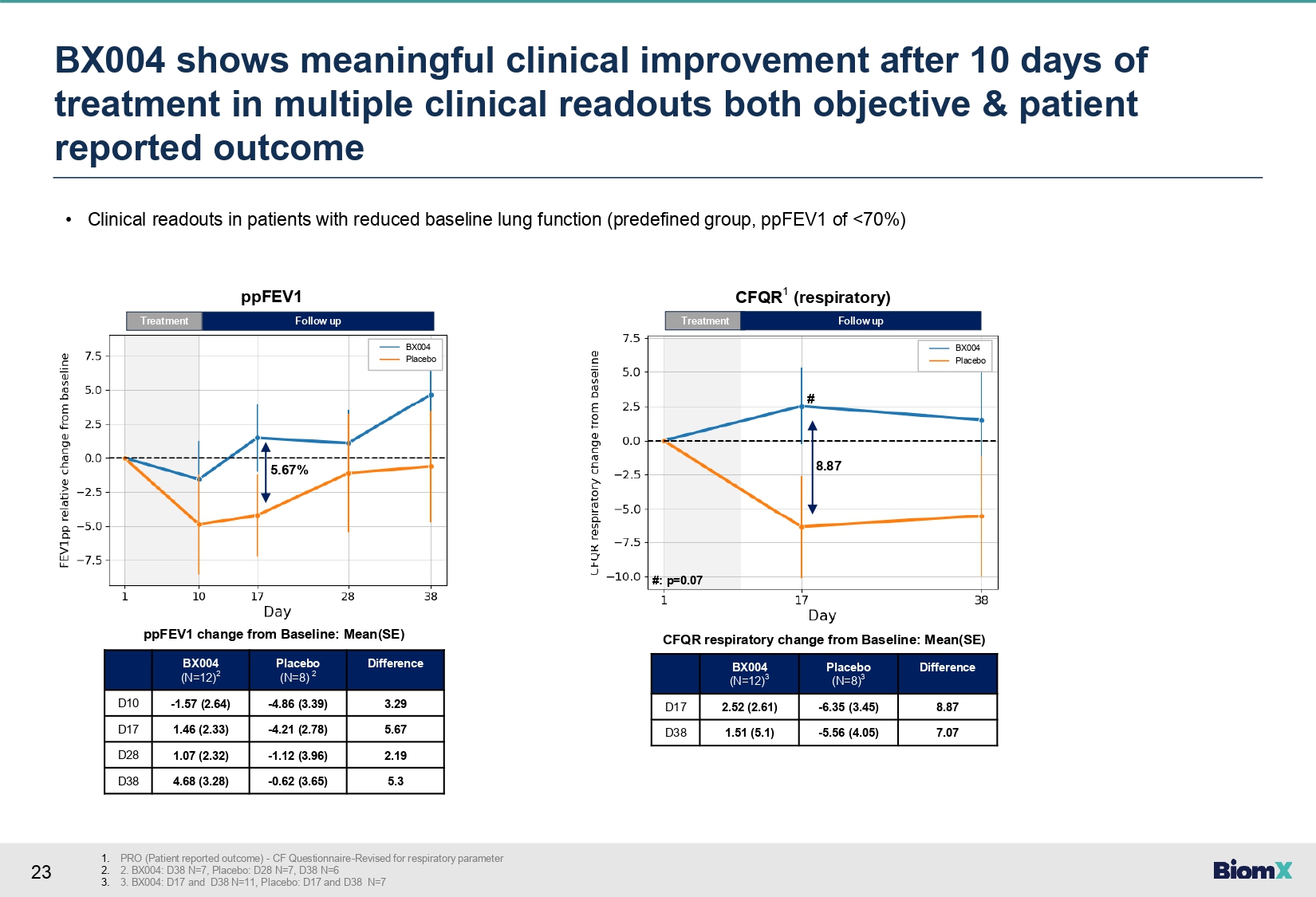
23 • Clinical readouts in patients with reduced baseline lung function (predefined group, ppFEV1 of <70%) Difference Placebo (N=8) 2 BX004 (N=12) 2 3.29 - 4.86 (3.39) - 1.57 (2.64) D10 5.67 - 4.21 (2.78) 1.46 (2.33) D17 2.19 - 1.12 (3.96) 1.07 (2.32) D28 5.3 - 0.62 (3.65) 4.68 (3.28) D38 Difference Placebo (N=8) 3 BX004 (N=12) 3 8.87 - 6.35 (3.45) 2.52 (2.61) D17 7.07 - 5.56 (4.05) 1.51 (5.1) D38 ppFEV1 change from Baseline: Mean(SE) CFQR respiratory change from Baseline: Mean(SE) Treatment 5.67% Follow up ppFEV1 Treatment Follow up CFQR 1 (respiratory) 8.87 BX004 Placebo BX004 Placebo # #: p=0.07 BX 004 shows meaningful clinical improvement after 10 days of treatment in multiple clinical readouts both objective & patient reported outcome 1. PRO (Patient reported outcome) - CF Questionnaire - Revised for respiratory parameter 2. 2. BX004: D38 N=7, Placebo: D28 N=7, D38 N=6 3. 3. BX004: D17 and D38 N=11, Placebo: D17 and D38 N=7

24 BX004 showed greater conversion (bacterial culture turned negative) in treatment over placebo • In the BX004 arm 3 out of 21 (14.3%) patients converted to sputum culture negative for P. aeruginosa after 10 days of treatment (2 already after 4 days) 2 Baseline PsA 1 in sputum (CFU/g) Duration of PsA infection (years) Patient 2.40x10 3 18 1 5.60x10 7 13 2 1.09x10 7 35 3* *Subject had negative sputum culture for P. aeruginosa at D4, D10, D28, D38, and at most recent standard of care clinic visit (D63) • In the placebo arm 0 out of 10 (0%) 2 • In addition, in Part 1 of the study, one subject in the BX004 arm (1/7: 14.3%) who was persistently positive for P. aeruginosa for at least 13 years had a 3.3 log reduction at D15 later converted to sputum negative 1. PsA – Pseudomonas aeruginosa, CFU/g – Colony forming units per gram 2. In patients that had quantitative CFU levels at study baseline
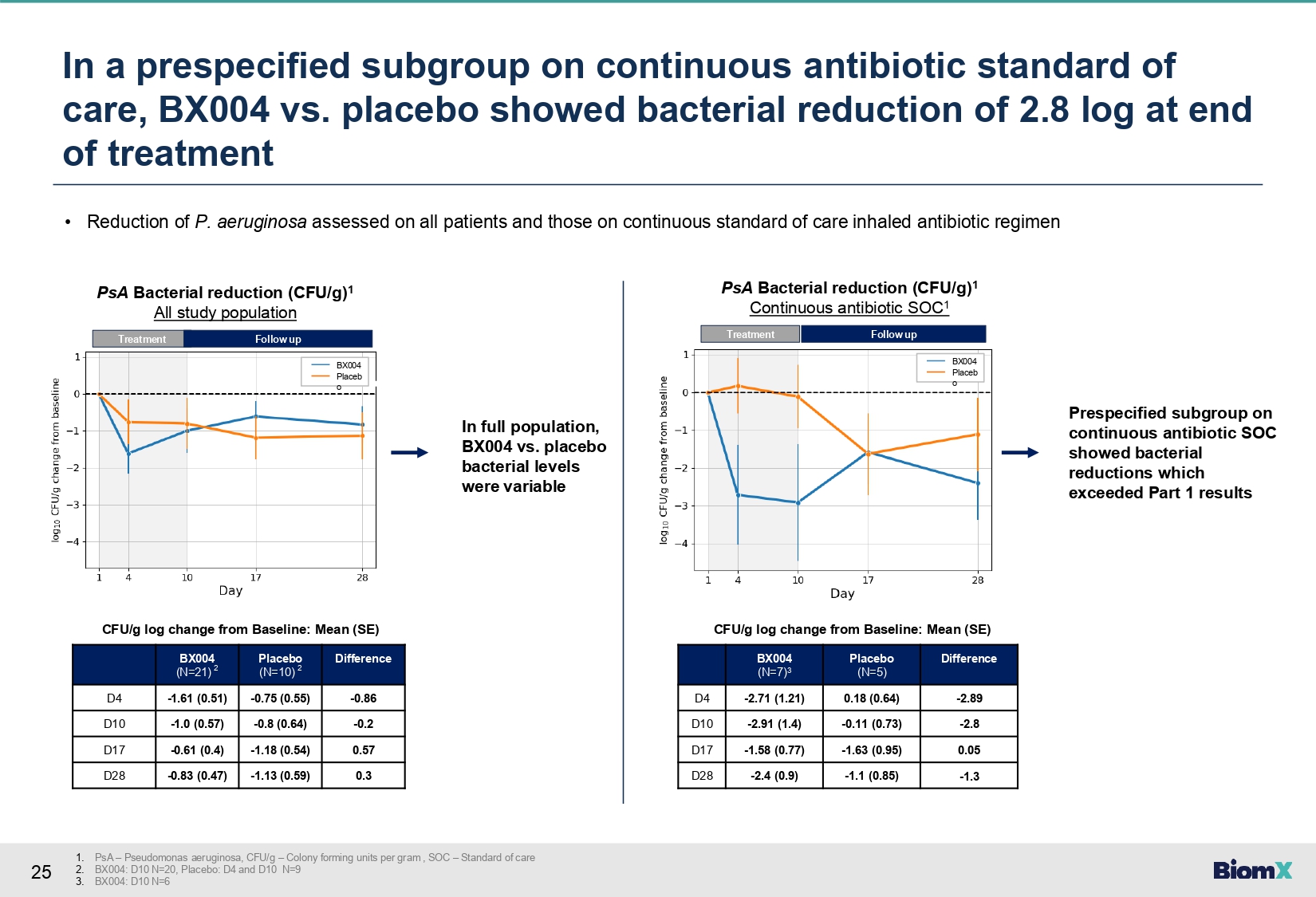
25 In a prespecified subgroup on continuous antibiotic standard of care, BX004 vs. placebo showed bacterial reduction of 2.8 log at end of treatment 1. PsA – Pseudomonas aeruginosa, CFU/g – Colony forming units per gram , SOC – Standard of care 2. BX004: D10 N=20, Placebo: D4 and D10 N=9 3. BX004: D10 N=6 • Reduction of P. aeruginosa assessed on all patients and those on continuous standard of care inhaled antibiotic regimen Difference Placebo (N=10) 2 BX004 (N=21) 2 - 0.86 - 0.75 (0.55) - 1.61 (0.51) D4 - 0.2 - 0.8 (0.64) - 1.0 (0.57) D10 0.57 - 1.18 (0.54) - 0.61 (0.4) D17 0.3 - 1.13 (0.59) - 0.83 (0.47) D28 CFU/g log change from Baseline: Mean (SE) Difference Placebo (N=5) BX004 (N=7) 3 - 2.89 0.18 (0.64) - 2.71 (1.21) D4 - 2.8 - 0.11 (0.73) - 2.91 (1.4) D10 0.05 - 1.63 (0.95) - 1.58 (0.77) D17 - 1.3 - 1.1 (0.85) - 2.4 (0.9) D28 CFU/g log change from Baseline: Mean (SE) In full population, BX004 vs. placebo bacterial levels were variable Treatment BX004 Placeb o PsA Bacterial reduction (CFU/g) 1 All study population Follow up Treatment Follow up BX004 Placeb o PsA Bacterial reduction (CFU/g) 1 Continuous antibiotic SOC 1 Prespecified subgroup on continuous antibiotic SOC showed bacterial reductions which exceeded Part 1 results
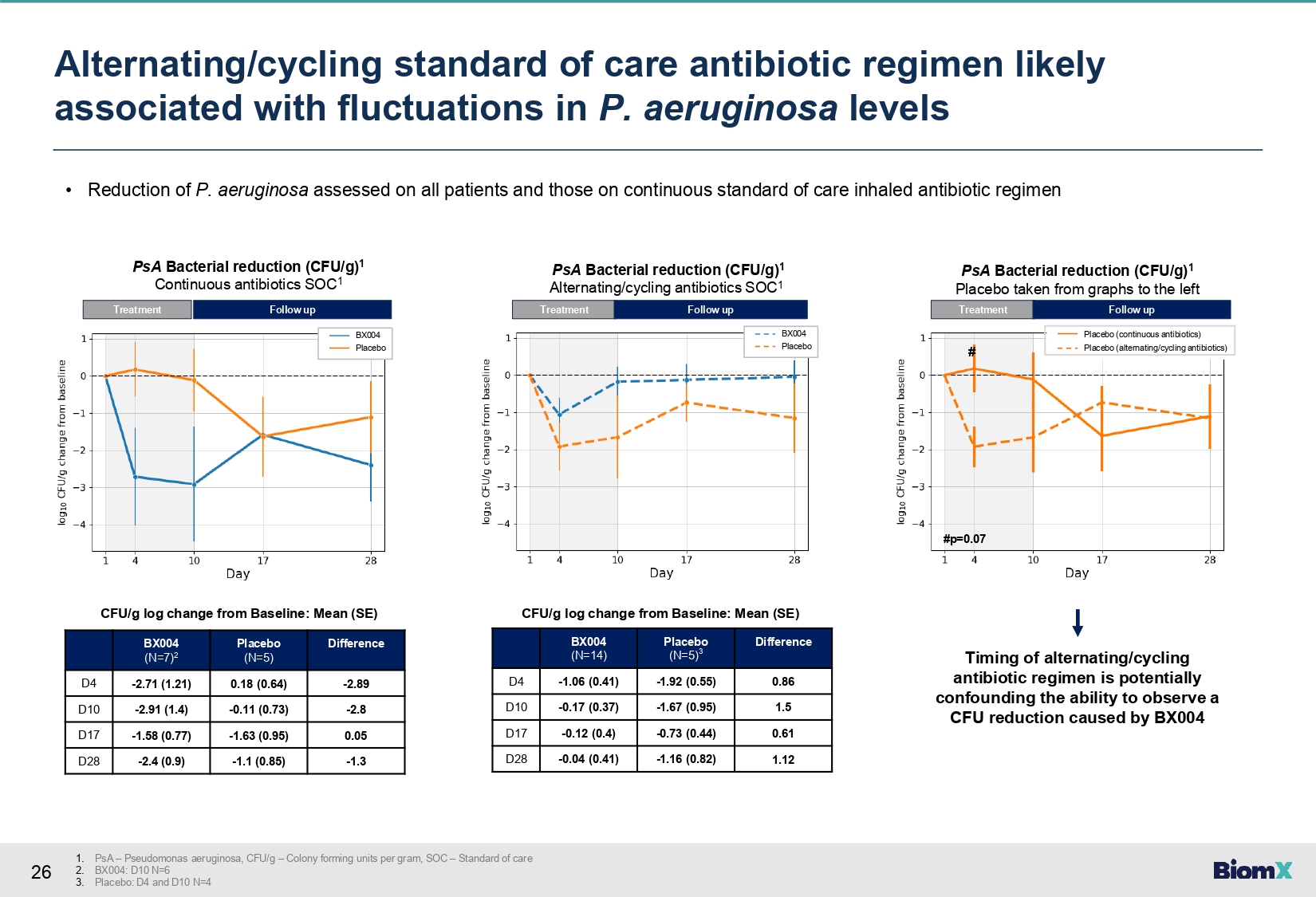
26 • Reduction of P. aeruginosa assessed on all patients and those on continuous standard of care inhaled antibiotic regimen Difference Placebo (N=5) BX004 (N=7) 2 - 2.89 0.18 (0.64) - 2.71 (1.21) D4 - 2.8 - 0.11 (0.73) - 2.91 (1.4) D10 0.05 - 1.63 (0.95) - 1.58 (0.77) D17 - 1.3 - 1.1 (0.85) - 2.4 (0.9) D28 CFU/g log change from Baseline: Mean (SE) PsA Bacterial reduction (CFU/g) 1 Continuous antibiotics SOC 1 Treatment Follow up Difference Placebo (N=5) 3 BX004 (N=14) 0.86 - 1.92 (0.55) - 1.06 (0.41) D4 1.5 - 1.67 (0.95) - 0.17 (0.37) D10 0.61 - 0.73 (0.44) - 0.12 (0.4) D17 1.12 - 1.16 (0.82) - 0.04 (0.41) D28 CFU/g log change from Baseline: Mean (SE) PsA Bacterial reduction (CFU/g) 1 Alternating/cycling antibiotics SOC 1 Treatment Follow up PsA Bacterial reduction (CFU/g) 1 Placebo taken from graphs to the left Treatment Follow up # #p=0.07 Placebo (continuous antibiotics) Placebo (alternating/cycling antibiotics) Timing of alternating/cycling antibiotic regimen is potentially confounding the ability to observe a CFU reduction caused by BX004 BX004 Placebo BX004 Placebo 1. PsA – Pseudomonas aeruginosa, CFU/g – Colony forming units per gram, SOC – Standard of care 2. BX004: D10 N=6 3. Placebo: D4 and D10 N=4 Alternating/cycling standard of care antibiotic regimen likely associated with fluctuations in P. aeruginosa levels
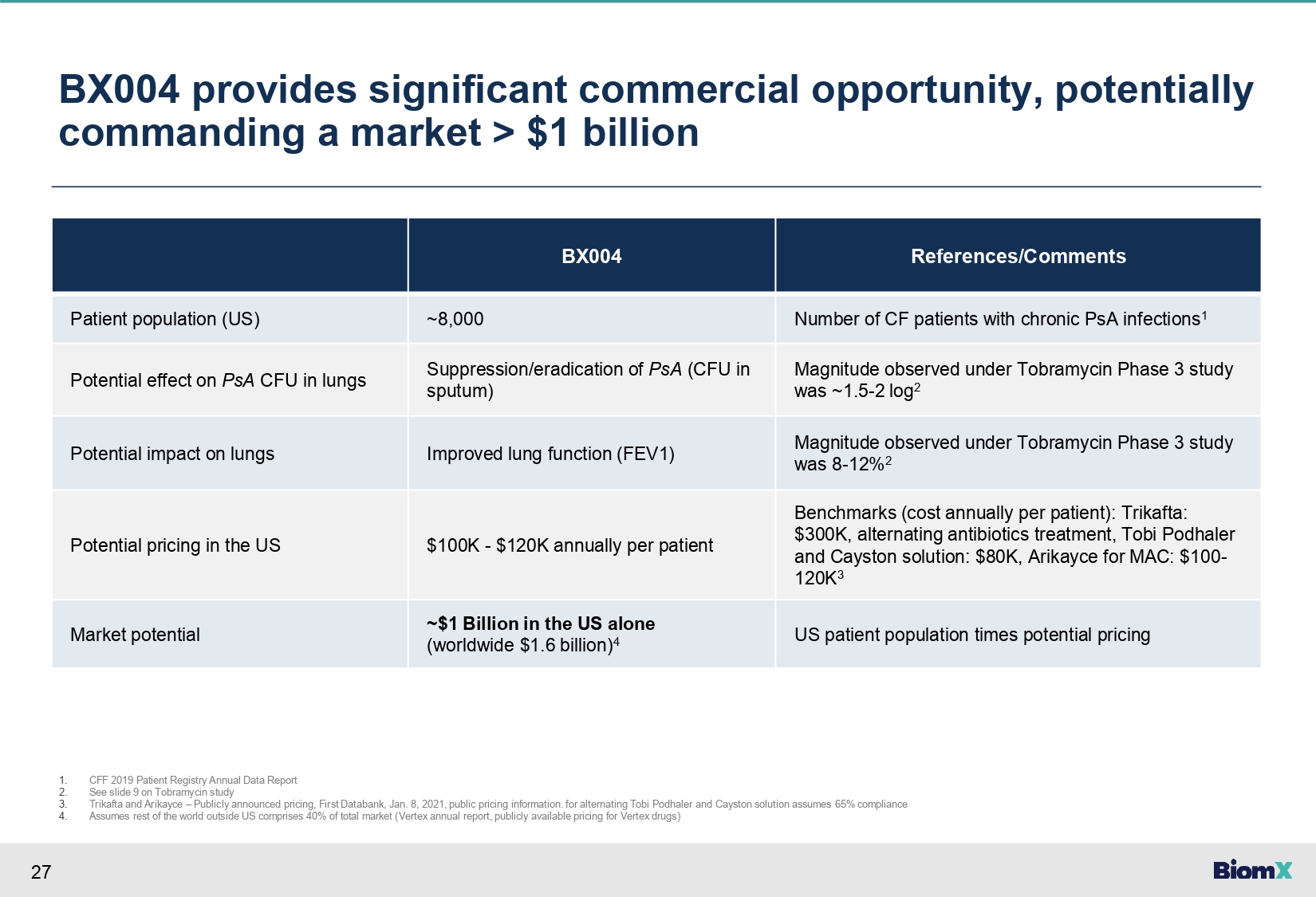
27 BX004 provides significant commercial opportunity, potentially commanding a market > $1 billion References/Comments BX004 Number of CF patients with chronic PsA infections 1 ~8,000 Patient population (US) Magnitude observed under Tobramycin Phase 3 study was ~1.5 - 2 log 2 Suppression/eradication of PsA (CFU in sputum) Potential effect on PsA CFU in lungs Magnitude observed under Tobramycin Phase 3 study was 8 - 12% 2 Improved lung function (FEV1) Potential impact on lungs Benchmarks (cost annually per patient): Trikafta: $300K, alternating antibiotics treatment, Tobi Podhaler and Cayston solution: $80K, Arikayce for MAC: $100 - 120K 3 $100K - $120K annually per patient Potential pricing in the US US patient population times potential pricing ~$1 Billion in the US alone (worldwide $1.6 billion) 4 Market potential 1. 2. 3. 4. CFF 2019 Patient Registry Annual Data Report See slide 9 on Tobramycin study Trikafta and Arikayce – Publicly announced pricing, First Databank, Jan. 8, 2021, public pricing information. for alternating Tobi Podhaler and Cayston solution assumes 65% compliance Assumes rest of the world outside US comprises 40% of total market (Vertex annual report, publicly available pricing for Vertex drugs)
28 IP protection of phage cocktails CORE IP APPLICATIONS: Natural phage cocktails • Composition claims on combinations of phage in cocktail/s based on additive/synergistic effects (e.g. combinations of phage avoiding development of resistance due to multiple MOAs) • Method claims for use of the combinations against the infecting bacteria Synthetic phage cocktails • Composition claims on new synthetic matter on each specific synthetic phage and the phage combination of the cocktail (e.g. a synthetic phage where a heterologous gene was added conferring traits, such as improved biofilm breakdown capabilities) SUPPLEMENT IP APPLICATIONS: • Claim product aspects invented in later product development such as effective formulations, delivery device features, manufacturing methods, synthetic engineering of manufacturing host or other + new gene (payload) Novel combination of natural phage Synthetically engineered phage
PIPELINE
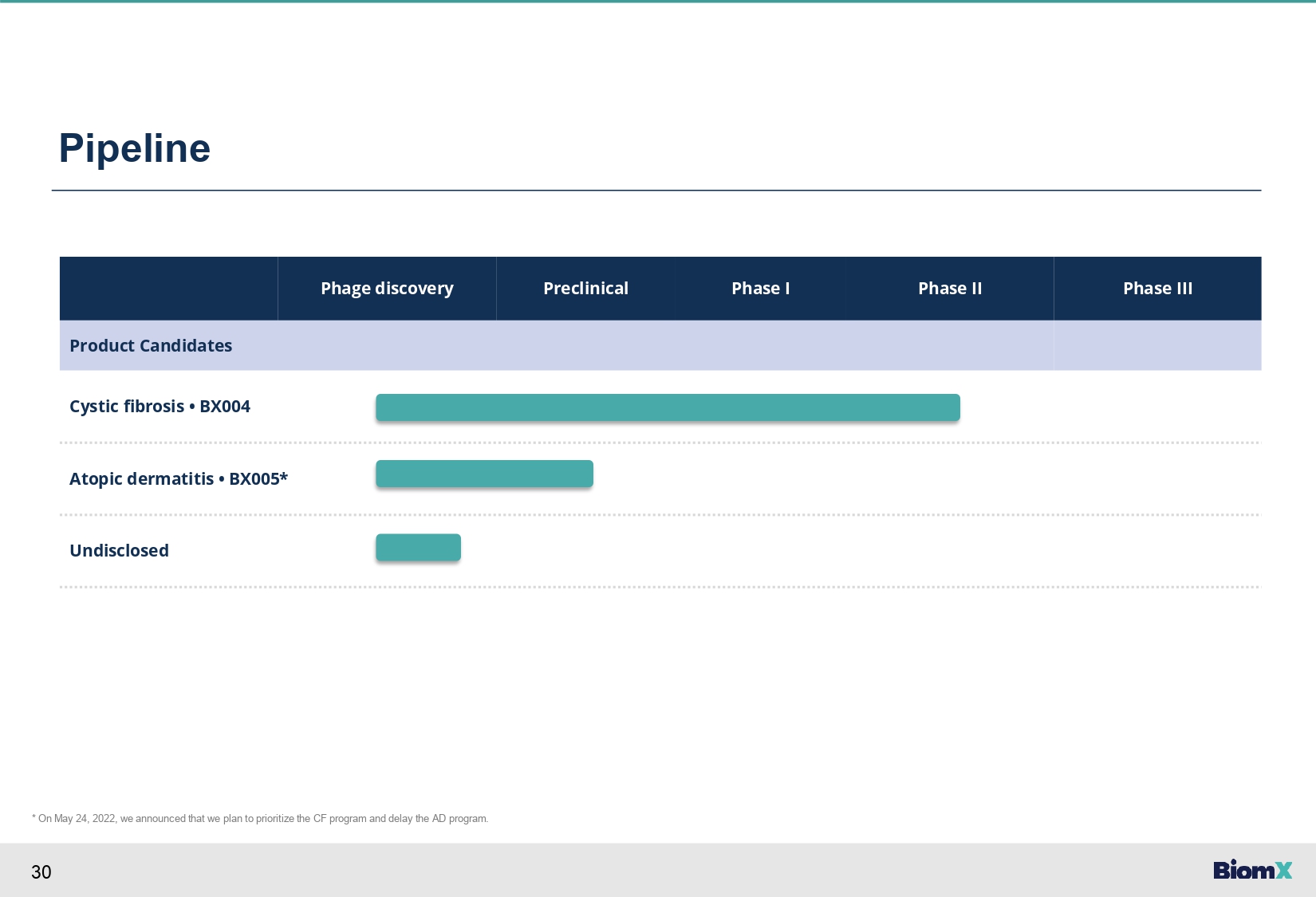
30 Pipeline Phase III Phase II Phase I Preclinical Phage discovery Product Candidates Cystic fibrosis • BX004 Atopic dermatitis • BX005* Undisclosed * On May 24, 2022, we announced that we plan to prioritize the CF program and delay the AD program.

Thank you
v3.23.3
Cover
|
Nov. 29, 2023 |
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Nov. 29, 2023
|
| Entity File Number |
001-38762
|
| Entity Registrant Name |
BiomX Inc.
|
| Entity Central Index Key |
0001739174
|
| Entity Tax Identification Number |
82-3364020
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
22 Einstein St.
|
| Entity Address, Address Line Two |
Floor 4
|
| Entity Address, City or Town |
Ness Ziona
|
| Entity Address, Country |
IL
|
| Entity Address, Postal Zip Code |
7414003
|
| City Area Code |
+972
|
| Local Phone Number |
723942377
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
true
|
| Units, each consisting of one share of Common Stock, $0.0001 par value, and one Warrant entitling the holder to receive one half share of Common Stock |
|
| Title of 12(b) Security |
Units, each consisting of one share of Common Stock, $0.0001 par value, and one Warrant entitling the holder to receive one half share of Common Stock
|
| Trading Symbol |
PHGE.U
|
| Security Exchange Name |
NYSEAMER
|
| Shares of Common Stock, $0.0001 par value |
|
| Title of 12(b) Security |
Shares of Common Stock, $0.0001 par value
|
| Trading Symbol |
PHGE
|
| Security Exchange Name |
NYSEAMER
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHGE_UnitsEachConsistingOfOneShareOfCommonStock0.0001ParValueAndOneWarrantEntitlingHolderToReceiveOneHalfShareOfCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHGE_SharesOfCommonStock0.0001ParValueMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
BiomX (AMEX:PHGE)
Historical Stock Chart
From Mar 2024 to Apr 2024
BiomX (AMEX:PHGE)
Historical Stock Chart
From Apr 2023 to Apr 2024