UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of February 2024 (Report No. 4)
Commission File Number: 001-40303
Inspira Technologies Oxy B.H.N. Ltd.
(Translation of registrant’s name into
English)
2 Ha-Tidhar St.
Ra’anana 4366504, Israel
(Address of principal executive office)
Indicate by check mark whether the registrant
files or will file annual reports under cover of Form 20-F or Form 40-F:
☒ Form 20-F ☐ Form
40-F
CONTENTS
On February 27, 2024, Inspira
Technologies Oxy B.H.N. Ltd., or the Registrant, issued a press release titled “Inspira™ Announces Breakthrough Update: Nears
First FDA 510(k) Approval, 3 U.S. Patents Approved, $546 million in Summary Distribution Agreements,” a copy of which is furnished
as Exhibit 99.1 with this report of foreign private issuer on Form 6-K.
In addition, on February 27,
2024, the Registrant issued a press release titled “Inspira™ To Host Conference Call to Discuss Investor Presentation and
Announce Next Major Milestone,” a copy of which is furnished as Exhibit 99.2 with this report of foreign private issuer on Form
6-K.
This Report of Foreign Private
Issuer on Form 6-K also consists of the Registrant’s presentation that was posted to its website on February 27, 2024, and which
is attached hereto as Exhibit 99.3.
The
first two paragraphs and the section titled “Forward-Looking Statements” in the press release attached as Exhibit 99.1, and
the first paragraph and the section titled “Forward-Looking Statements” in the press release attached as Exhibit 99.2, are
incorporated by reference into the Registrant’s Registration Statements on Form
F-3 (Registration No. 333-266748) and Form S-8
(Registration No. 333-259057), filed with the Securities and Exchange Commission, to be a part thereof
from the date on which this report is submitted, to the extent not superseded by documents or reports subsequently filed or furnished.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
Inspira Technologies Oxy B.H.N. Ltd. |
| |
|
|
| Date: February 27, 2024 |
By: |
/s/ Dagi Ben-Noon |
| |
|
Name: |
Dagi Ben-Noon |
| |
|
Title: |
Chief Executive Officer |
2
Exhibit 99.1
Inspira™ Announces Breakthrough Update: Nears First FDA 510(k)
Approval, 3 U.S. Patents Approved, $546 million in Summary Distribution Agreements
Ra’anana, Israel, February 27, 2024 –
Inspira™ Technologies OXY B.H.N. Ltd. (Nasdaq: IINN, IINNW) (the “Company” or “Inspira Technologies”), a
breakthrough medical technology company, has announced that the Company is progressing towards 510(k) clearance from the U.S. Food &
Drug Administration (“FDA”) for its INSPIRA™ ART100 in the first half of 2024.
The Company plans additional FDA 510(k) submissions for the HYLA blood sensor in 2024 and for INSPIRA ART disposables in 2025, as part
of a scalable razor-blade business model. Three breakthrough patents have been approved by the United States Patent and Trademark Office
(“USPTO”) and an additional three patent applications are pending at the USPTO for certain core technologies. To date, the
Company has signed $546 million in pre-conditioned summary distribution agreements, subject to regulatory approvals and authorizing authorities.
Inspira has set out in its investor presentation
(link) its plans to revolutionize the $19 billion mechanical ventilator market with the next generation INSPIRA™
ART device, equipped with adaptive blood oxygenation technology. The INSPIRA ART device is the first INSPIRA device of its kind to be
embedded with our proprietary HYLA blood sensor and VORTX™, an orbiting blood oxygenation delivery
system. The INSPIRA ART device is designed to continuously scan blood parameters in real-time to deliver the needed oxygen volume straight
into the blood, elevating patient oxygen levels within minutes.
Joe Hayon, President and Co-founder, stated:
“The blood holds insights and valuable information needed when treating patients, so we believe that the HYLA blood sensor has a
vital role to play while the INSPIRA ART delivers the needed oxygen straight into the blood. We vision the INSPIRA ART is expected to
changing the medical landscape, with the VORTX orbiting blood oxygenation delivery system. This is the first patent of its kind, recently
approved by the USPTO.”
Inspira Technologies OXY B.H.N. Ltd.
Inspira™ Technologies is an innovative medical
technology company in the respiratory treatment arena. The Company has developed a breakthrough Augmented Respiration Technology (INSPIRA
ART), designed to rebalance patient oxygen saturation levels. This technology potentially allows patients to remain awake during treatment
while reducing the need for highly invasive, risky, and costly mechanical ventilation systems that require intubation and medically induced
coma. The Company’s products have not yet been tested or used in humans and has not been approved by any regulatory entity.
For more information, please visit our corporate
website: https://inspira-technologies.com
Forward-Looking Statement Disclaimer
This press release contains express or implied
forward-looking statements pursuant to U.S. Federal securities laws. These forward-looking statements and their implications are based
on the current expectations of the management of the Company only and are subject to a number of factors and uncertainties that could
cause actual results to differ materially from those described in the forward-looking statements. For example, the Company is using forward-looking
statements when it discusses the expected timing of its 510(k) clearance from the FDA, that it intends to submit additional 510(k) submissions,
the value and potential of its summary distribution agreements, each subject to regulatory approvals and authorizing authorities, the
belief that the HYLA blood sensor has a vital role to play while the INSPIRA ART delivers the needed oxygen straight into the blood, the
belief that the INSPIRA ART is expected to change the medical landscape, and the potential benefits of its products. These forward-looking
statements and their implications are based solely on the current expectations of the Company’s management and are subject to a
number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements.
Except as otherwise required by law, the Company undertakes no obligation to publicly release any revisions to these forward-looking statements
to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information
about the risks and uncertainties affecting the Company is contained under the heading “Risk Factors” in the Company’s
annual report on Form 20-F for the fiscal year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission (the “SEC”),
which is available on the SEC’s website, www.sec.gov
For more details:
Public Relations Manager
Adi Shmueli
Inspira Technologies
info@inspirao2.com
+972-9-9664485
MRK-ARS-088
Copyright © 2018-2024 Inspira Technologies
OXY B.H.N. LTD., All rights reserved.
Exhibit 99.2
Inspira™ To Host Conference Call to Discuss
Investor Presentation and Announce Next Major Milestone
Ra’anana, Israel, February 27, 2024 –
Inspira™ Technologies OXY B.H.N. Ltd. (Nasdaq: IINN, IINNW) (the “Company” or “Inspira Technologies”),
a breakthrough medical technology company, announced today that the Company President, Director and co-founder, Mr. Joe Hayon, will present
an Investor Presentation and announce the Company’s next major milestone. The conference call is to be held at 9:00am Eastern Time
on Monday, March 4, 2024.
To attend the conference call, please enter: link
Inspira Technologies OXY B.H.N. Ltd.
Inspira™ Technologies is an innovative medical
technology company in the respiratory treatment arena. The Company has developed a breakthrough Augmented Respiration Technology (INSPIRA
ART), designed to rebalance patient oxygen saturation levels. This technology potentially allows patients to remain awake during treatment
while reducing the need for highly invasive, risky, and costly mechanical ventilation systems that require intubation and medically induced
coma. The Company’s products have not yet been tested or used in humans and has not been approved by any regulatory entity.
For more information, please visit our corporate
website: https://inspira-technologies.com
Forward-Looking Statement Disclaimer
This press release contains express or implied
forward-looking statements pursuant to U.S. Federal securities laws. These forward-looking statements and their implications are based
on the current expectations of the management of the Company only and are subject to a number of factors and uncertainties that could
cause actual results to differ materially from those described in the forward-looking statements. For example, the Company is using forward-looking
statements when it discusses the expected release of the investor presentation and announcement of its next major milestone. These forward-looking
statements and their implications are based solely on the current expectations of the Company’s management and are subject to a
number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements.
Except as otherwise required by law, the Company undertakes no obligation to publicly release any revisions to these forward-looking statements
to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information
about the risks and uncertainties affecting the Company is contained under the heading “Risk Factors” in the Company’s
annual report on Form 20-F for the fiscal year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission (the “SEC”),
which is available on the SEC’s website, www.sec.gov
For more details:
Public Relations Manager
Adi Shmueli
Inspira Technologies
info@inspirao2.com
+972-9-9664485
MRK-ARS-089
Copyright © 2018-2024 Inspira Technologies
OXY B.H.N. LTD., All rights reserved.
Exhibit 99.3

Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 1 INVESTOR DECK I FEBRUARY 2024 EMPOWERED BREATHING WITHOUT LUNGS

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 2 This presentation contains express or implied forward - looking statements pursuant to U . S . Federal securities laws . These forward - looking statements and their implications are based on the current expectations of the management of the Company only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements . For example, the Company is using forward - looking statements when it discusses the projected size of and the Mechanical ventilation Market and Perfusion Systems Market, the potential market sizes of its future products, the potential outcome that its technology can eliminate complications associated with Mechanical Ventilation, its aim to replace the use of Mechanical Ventilators, the intended uses and potential benefits of its products and technology,, its prospective targeting of current procedural terminology codes that the Company could potentially use for charging and reimbursement of its products, potential revenues models, revenue sources and streams that may be realized pursuant to various distribution agreements, strategic opportunities and based on software licensing, its prospective target consumers and end - users for its products, its projected milestone timelines for each of its products, its strategy for market penetration and market share gain, its go - to - market strategy, its regulatory strategy and anticipation of software licensing and U . S Food and Drug Administration (FDA) clearance, its potential commercialization of its products, and its prospective and ongoing regulatory approval processes in various jurisdictions . This presentation also contains estimates of the Company’s health economics model . These forward - looking statements and their implications are based solely on the current expectations of the Company’s management and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements . Except as otherwise required by law, the Company undertakes no obligation to publicly release any revisions to these forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events . More detailed information about the risks and uncertainties affecting the Company is contained under the heading “Risk Factors” in the Company’s annual report on Form 20 - F for the fiscal year ended December 31 , 2022 filed with the U . S . Securities and Exchange Commission (the “SEC”), which is available on the SEC’s website, www . sec . gov . Forward Looking Statement Disclaimer

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 3 • The reference to INSPIRA TM ART is a series of planned devices . The use of the term INSPIRA TM ART can refer to the INSPIRA TM ART 500 . INSPIRA TM ART 100 was previously known as the ALICE TM . Mechanical Ventilation/Ventilators or MV refers to Invasive Mechanical Ventilation or IMV . • Planned timelines, milestones, estimates or assumptions are subject to change . • To - date the Augmented Respiration Technology, INSPIRA TM AI, HYLA TM , INSPIRA TM ART 100 , INSPIRA TM ART 500 , INSPIRA TM ART, VORTX TM and any other Inspira products, devices, disposables, components, software or technologies are still in development and have not been tested or used on humans . Product intent of uses and regulatory pathways are yet to be defined or finalized . The products have not been cleared or approved by the U . S . Food and Drug Administration (FDA) or any other regulatory or authorizing authority . Estimated date/time of regulatory clearance or approval may be subject to change . Approval or clearance by the FDA, CE or any other authorizing entity may not be granted or may require different study parameters or data or validation from those that are intended or were included in the submission . In addition, there is inherent risk and variability in the overall regulatory process and no guarantee as to the success of any trial or regulatory approval or clearance .. Some or all the clinical studies may be conducted outside of the U . S . • INSPIRA TM ART 100 , INSPIRA TM ART 500 , INSPIRA TM ART or HYLA TM may either have embedded or integrated INSPIRA TM AI or other software's with selective levels or functionality, yet to be decided by the company . • While the Company intends to execute on the summary distribution agreements, there is no guarantee any sales will occur pursuant to those existing agreements, and agreements that may be executed in the future . The pre - conditional summary distribution agreements are for a period of up to seven years and are subject to the completion of the development and the required approvals by regulatory and other authorizing authorities . The timeline also assumes that Inspira’s existing summary distribution agreements and arrangements, or any future similar types of agreements if and when they may be executed, will be initiated as planned, and that Inspira’s planned devices will qualify for reimbursement pursuant to existing Current Procedural Terminology (CPT) codes or other reimbursement mechanisms . The company or distributor may decide to terminate the agreements at any given time . The reference in this deck to summary distribution agreements and or to distribution agreements are to be considered one and the same . • There is no guarantee that the patents or patent families will be granted . Intellectual property is subject to development, testing validation and approval by regulatory or other authorizing authorities . • Our future business model includes a future revenue model based on the disposable razorblade model and software licensing fees . • “Inspira targets to revolutionize the $ 19 B Mechanical Ventilation Market” refers to our belief that we can provide a better solution then current legacy solutions . • “Patients can often experience legacy complications delirium, lung infections and injury or possible death!“ refers to known associated risks and complications associated with Invasive Mechanical Ventilation • “Inspira Oxygen Delivery straight into the blood” refers to our INSPIRA ART that is designed to directly oxygenate the blood that has been circulated through the device . • “ADAPTIVE Blood Oxygenation” refers to the INSPIRA TM ART or to the INSPIRA TM ART 500 which we believe has the potential to be a game - changing solution . “delivering needed oxygen volume” refers to the level/volume/flow/concentration of oxygen performed by device or defined by operator . • “We aim to replace mechanical ventilators” refers to our intention to provide a new form of Life Support that can be administered to patients deteriorating (with acute respiratory failure) and that would be potential candidates for Invasive Mechanical Ventilation . “Potentially reduces hospital days”, refers to the belief that with the INSPIRA ART 500 expecting to treat patients that are awake and without intubation, the belief is that this will potentially reduce legacy complications and the need for weaning, therefore potentially reducing hospital days . “No lung infection I No lung injury”, refers to our belief that our delivering oxygen straight into the blood (without intubation and going through lungs), reduces legacy complications such as Ventilator induced infections and lung injury . • “Targeting Excising CPT Codes”, refers to there potentially being existing CPT Codes (Current Procedural Terminology) for coding medical services that we could potentially use for charging and reimbursement of Inspira products and services Disclaimers

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 4 Inspira aims to revolutionize the $19 Billion Mechanical Ventilation Market We aspire waves of change in the world of life support Source: $19 Billion Predicted Global Mechanical Ventilation Market By 2030

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 5 See Disclaimers on slides 1 - 16 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved ц 20 Million/Year on Mechanical Ventilation Source: ц 20M Patients/Year on MV
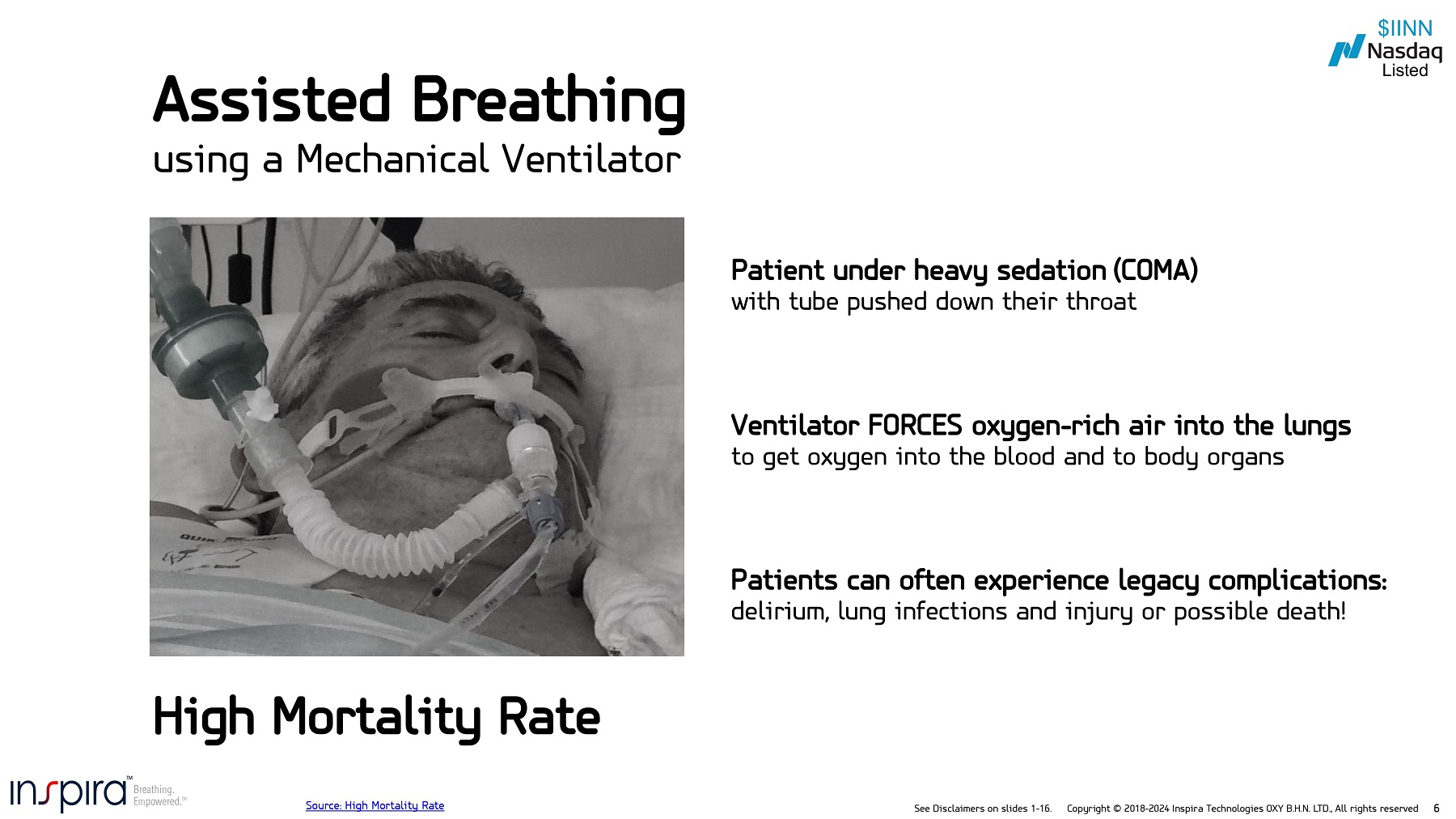
See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 6 Patients can often experience legacy complications: delirium, lung infections and injury or possible death! Patient under heavy sedation (COMA) with tube pushed down their throat Ventilator FORCES oxygen - rich air into the lungs to get oxygen into the blood and to body organs High Mortality Rate Assisted Breathing using a Mechanical Ventilator Source: High Mortality Rate

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 7 Oxygen Blood Body Organs Straight into the Blood Inspira
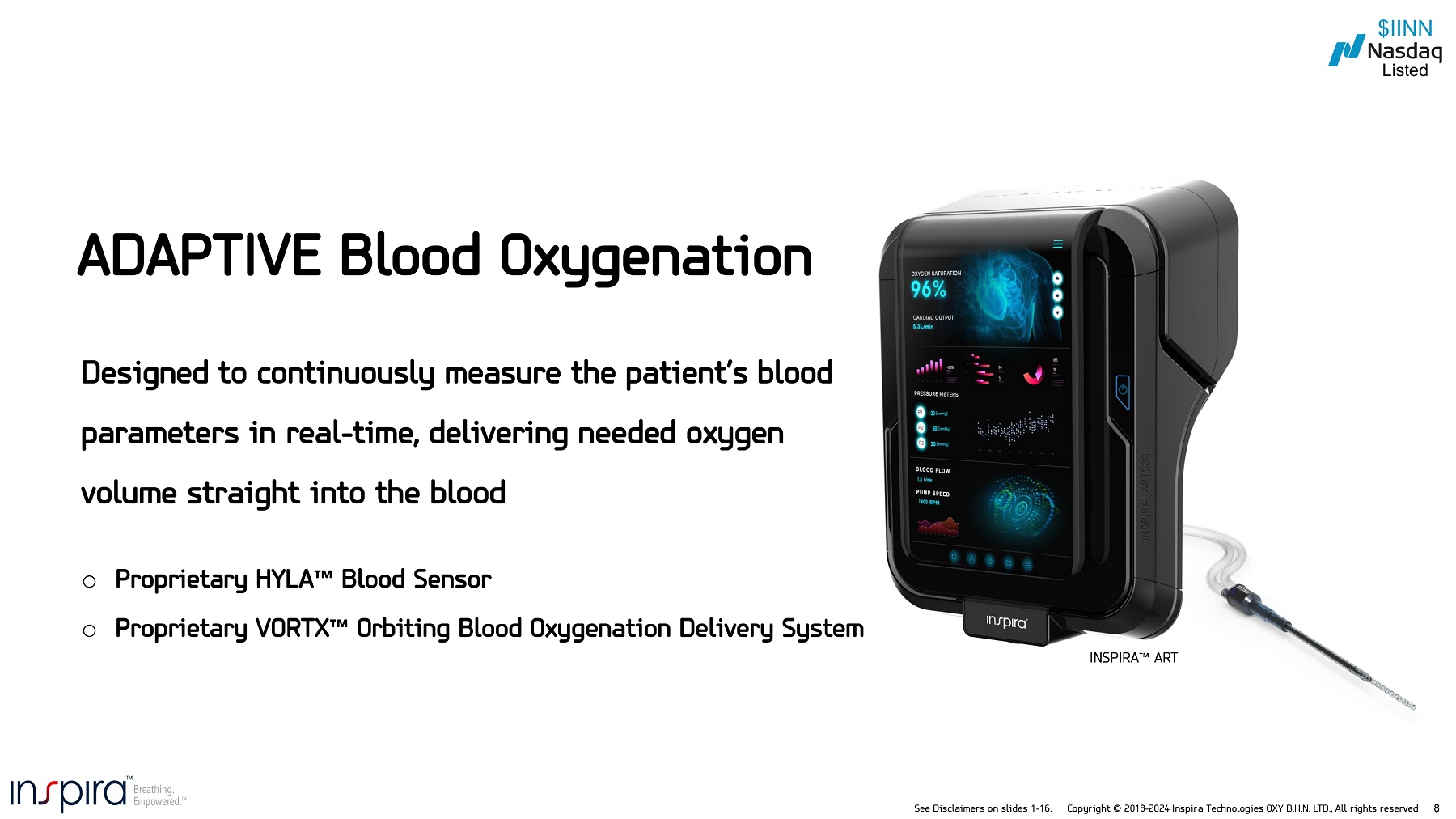
See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 8 Designed to continuously measure the patient’s blood parameters in real - time, delivering needed oxygen volume straight into the blood o Proprietary HYLA o Proprietary VORTX Blood Sensor Orbiting Blood Oxygenation Delivery System ADAPTIVE Blood Oxygenation INSPIRA ART

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 9 Patient treated awake and without Mechanical Ventilation No Coma I No Tube in throat I Potentially reduces hospital days Oxygen is delivered straight into the blood, with carbon dioxide removed No lung infection I No lung injury Blood parameters measured continuously and in real - time Early detection of changes I Provides decision - making assistance data We Aim to Prevent Mechanical Ventilation

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 10 VORTX - Orbiting blood oxygenation delivery system: A blood - gas exchange device and methods of use Dual lumen cannula and methods of use o Granted in the U.S., 2023 o Filed in Europe, China, Korea, Japan, Israel Inspira Patent Portfolio A cannula fixation device Extracorporeal oxygenation system for low flow rates and methods of use o Granted in the U.S., 2023* o Filed in the U.S., Canada, Europe, China, Korea, Japan, Australia, Brazil, Israel o Filed in the U.S., Europe o Granted in Israel, 2022 *Initiation module o Granted in the U.S. 2024

11 We believe our devices will revolutionize the medical landscape Gen 1 Blood Oxygenation (Cardio - Pulmonary Bypass) HYLA 1 Blood Sensor (Integrated with INSPIRA ART100) Gen 2 Adaptive Blood Oxygenation VORTX Orbiting Blood Oxygenation HYLA 2 Blood Sensor o Continuous blood monitoring o Real - time detection of changes o Provides decision - making assistance data And aiming much higher… The timeline assumes certain approvals by regulatory and or other authorizing authorities and are subject to change. There is inherent risk and variability in the overall regulatory process and no guarantee as to the success of any device or the approval or clearance of such devices by any regulatory authorities and or other authorizing authorities. See Disclaimers on slides 1 - 16 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved INSPIRA ART100 Submitted to the FDA in 2023 INSPIRA ART
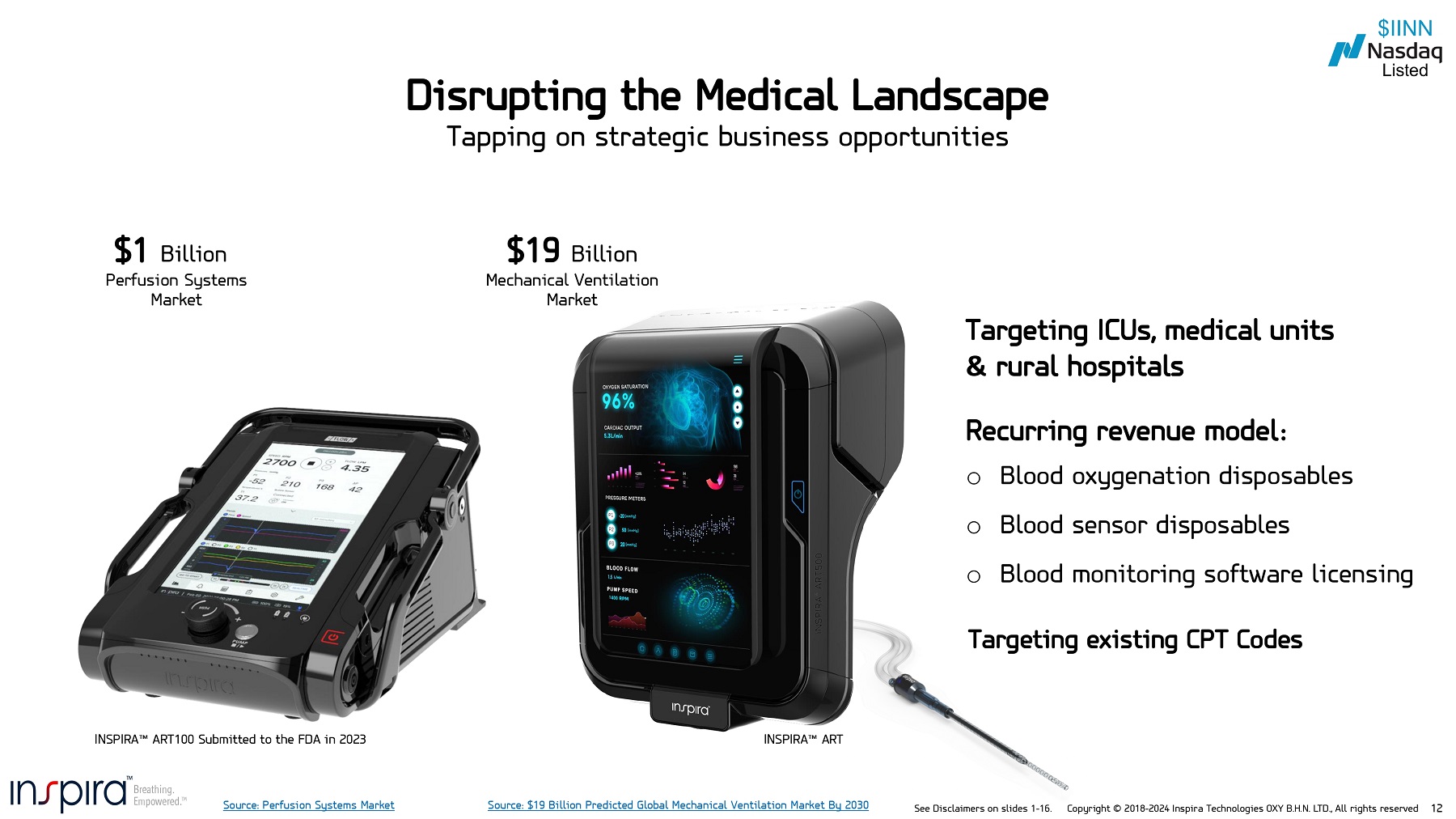
See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 12 Disrupting the Medical Landscape Tapping on strategic business opportunities $1 Billion Perfusion Systems Market $19 Billion Mechanical Ventilation Market INSPIRA ART100 Submitted to the FDA in 2023 Targeting ICUs, medical units & rural hospitals Recurring revenue model: o Blood oxygenation disposables o Blood sensor disposables o Blood monitoring software licensing Targeting existing CPT Codes Source: Perfusion Systems Market INSPIRA ART Source: $19 Billion Predicted Global Mechanical Ventilation Market By 2030

13 $546 Million in Pre - conditional Signed Summary Distribution Agreements Source: inspira - technologies.com/news/ While the Company intends to execute on the signed summary distribution agreements, there is no guarantee any sales will occur pursuant to the existing agreements. The potential pre - conditional summary distribution agreements are subject to signing definitive agreements, targeting $271 Million North America $28 Million Central America $200 Million Europe $47 Million Israel, Gulf States Minimum Order Quantities for a period of up to seven years and are subject to the completion of the development and the required approvals by regulatory and other authorizing authorities. The company or distributor may decide to terminate the agreements at any given time. See Disclaimers on slides 1 - 16 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 14 Dr. Daniella Yeheskely - Hayon, PhD CTO Renowned Expert in the field of Artificial Lung Development Yafit Tehila, CPA CFO & Legal Serial Financial management experience in public companies Dagi Ben - Noon, BSc Co - Founder & CEO Co - founded Nano Dimension Nasdaq: NNMD Joe Hayon, MBA Co - Founder & President M & A experience & track record Elscint Technologies, Arazim Avi Shabtay COO Serial New - tech development & delivery track record Prof. Benad Goldwasser, MD, MBA Executive Chairman Urologic surgeon, inventor & entrepreneur. Multiple well - known industry exits Dr. Dekel Stavi, MD Medical Director Senior Intensive Care physician at Tel Aviv Sourasky Medical Center. Chairman of the Israeli ECMO Society Team

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 15 INTUITION o Strong execution team o We believe there is a $19 billion market opportunity o FDA Clearance anticipated in H1 - 2024 for INSPIRA ART100 o Potential recurring revenue model based on disposables and software licensing INSPIRA ART Source: $19 Billion Predicted Global Mechanical Ventilation Market By 2030

See Disclaimers on slides 1 - 16. Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 16 Inspira has a life print... Thank You
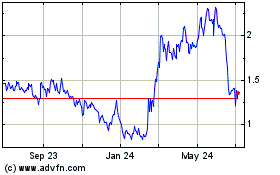
Inspira Technologies Oxy... (NASDAQ:IINN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Inspira Technologies Oxy... (NASDAQ:IINN)
Historical Stock Chart
From Apr 2023 to Apr 2024