UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer Pursuant to Rule
13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the Month of April 2024
Commission File Number: 001-37353
SCINAI IMMUNOTHERAPEUTICS LTD.
(Translation of registrant’s name into English)
Jerusalem BioPark, 2nd Floor
Hadassah Ein Kerem Campus
Jerusalem, Israel
(Address of principal executive office)
Indicate by check mark whether the registrant files
or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒
Form 40-F ☐
Explanatory Note
Scinai Immunotherapeutics Ltd. (the “Company”)
has made available an updated presentation about its business, a copy of which is furnished herewith as Exhibit 99.1 and incorporated
by reference. The new updates in the presentation are not an admission as to the materiality of any information therein. The
information contained in the presentation is summary information that should be considered in the context of the Company’s
filings with the Securities and Exchange Commission and other public announcements the Company may make by press release or otherwise
from time to time.
Exhibit Index
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
Scinai Immunotherapeutics Ltd. |
| |
|
|
| Date: April 16, 2024 |
By: |
/s/ Amir Reichman |
| |
|
Amir Reichman |
| |
|
Chief Executive Officer |
3
Exhibit
99.1

CLICK TO EDIT MASTER TITLE STYLE Elevating dermatological drug development through advanced inflammatory marker targeting methodology - Focus of IL - 17 in the pathophysiology of psoriasis and unmet medical need – 5th DDDS Europe, April 17, 2024 Michael P. Schön Department of Dermatology , Venereology and Allergology Lower Saxony Institute of Occupational Dermatology University Medical Center Göttingen, Germany https://hautklinik.umg.eu Conflict of interest: AbbVie, Almirall, BMS, Celltrion, Janssen, Lilly, Novartis, UCB Tamar Ben - Yedidia Scinai Immunotherapeutics Ltd. Jerusalem, Israel https://www.scinai.com Presentation supported by Scinai Immunotherapeutics Ltd. (Nasdaq: SCNI)

CLICK TO EDIT MASTER TITLE STYLE What is Psoriasis? 1 - 4 • Systemic , chronic inflammatory disorder • Affects 2 – 3% of the fair - skinned population • Genetic and immunologic basis , can be triggered • Several comorbid diseases result in considerable impairment of quality of life • 2016: World Health Organization (WHO) publishes Global Report on Psoriasis 4 WHO: World Health Organisation. 1 National Psoriasis Foundation. About Psoriasis. Available at: https://www.psoriasis.org/about - psoriasis/. Accessed: 14/02/2024. 2 Schön MP and Boehncke WH. N Engl J Med. 2005;352:1899 – 912. 3 Boehncke WH and Schön MP. Lancet. 2015;386:983 – 94. 4 WHO Global Report on Psoriasis. Available at: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf?sequence=1&isAllowed= y. Accessed 14/02/2024.
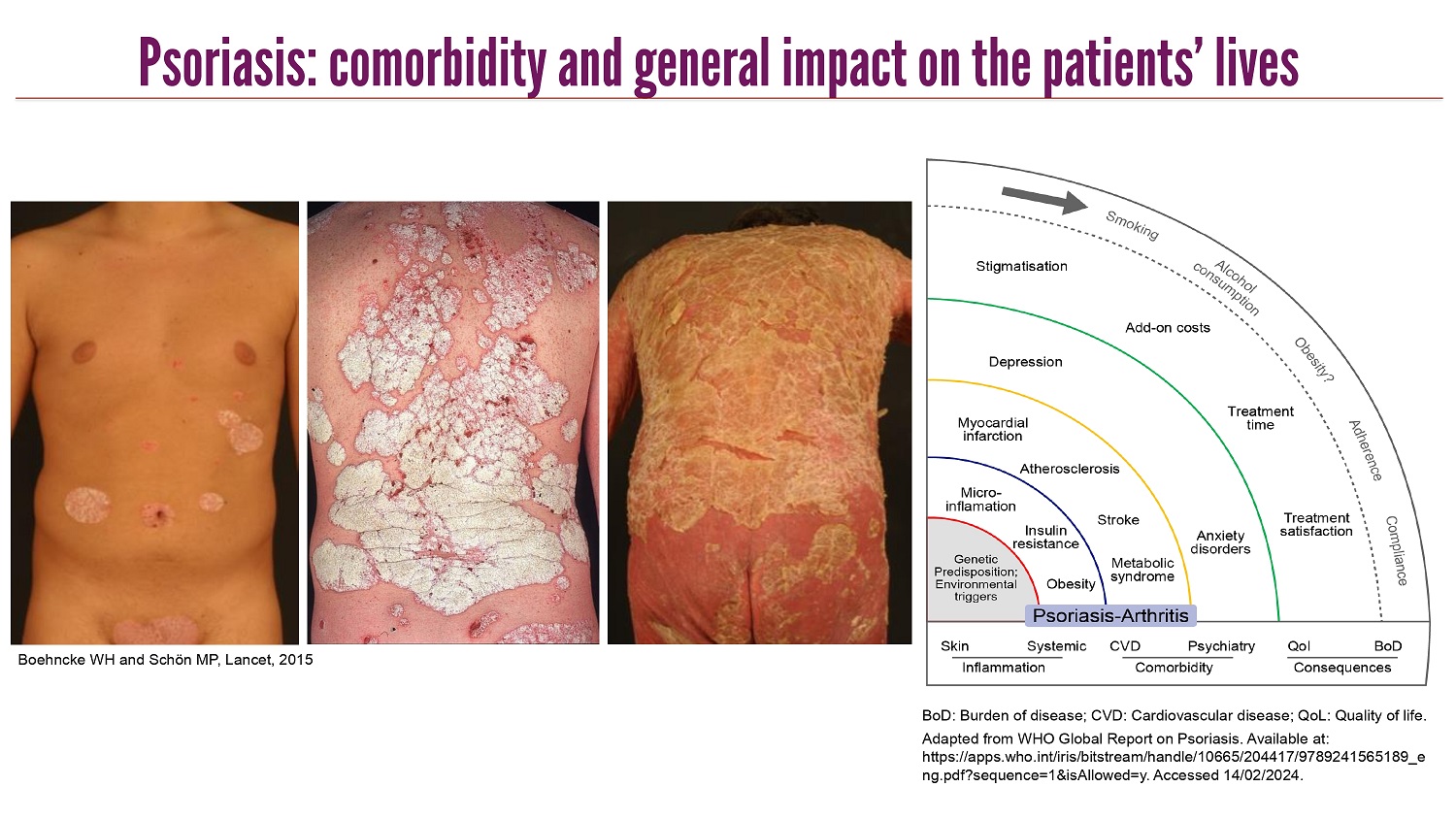
CLICK TO EDIT MASTER TITLE STYLE Psoriasis: comorbidity and general impact on the patients’ lives BoD : Burden of disease ; CVD: Cardiovascular disease ; QoL : Quality of life . Adapted from WHO Global Report on Psoriasis. Available at: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_e ng.pdf?sequence=1&isAllowed=y. Accessed 14/02/2024. Boehncke WH and Schön MP, Lancet, 2015

CLICK TO EDIT MASTER TITLE STYLE Gro a TARC RANTES MIP - 3 a TNF a IL - 10 IFN g VEGF IL - 1 IL - 6 neutrophils in epidermis , microabscesses hyperkeratosis , parakeratosis epidermal T cell recruitment altered differentiation of many cell types keratinocyte hyperproliferation , acanthosis macrophage activation and increase mast cell increase inflammatory activation of keratinocytes and dermal cells angiogenesis und vasodilation leukocyte extravasation T cell activation activation of cutaneous nerves TGF a GM - CSF NGF IL - 8 CTACK MDC MCP - 1 IP10 MIG Cytokines Chemokines IL - 12 IL - 17 IL - 6 TGF a GM - CSF IL - 1 IL - 12 IL - 17A, F IL - 22 IL - 23 VEGF IFN g TNF a IL - 10 NGF Gro - a IL - 8 MIP - 3 a CTACK TARC MDC RANTES MCP - 1 IP10 MIG pro - inflammatory mediators reduced apoptosis Microenvironment in psoriasis (I) • altered expression of many cytokines and chemokines in psoriatic lesions • known activities can explain many pathological tissue changes • increased expression alone is not sufficient to predict the therapeutic efficacy of the inhibition Adapted from : Schön MP. Akt Dermatologie , 2006;32:169 – 75. Boehncke WH and Schön MP. Lancet. 2015;386:983 – 94. Schön MP and Wilsmann - Theis D: J Dtsch Dermatol Ges 2023;21(4):363 - 73
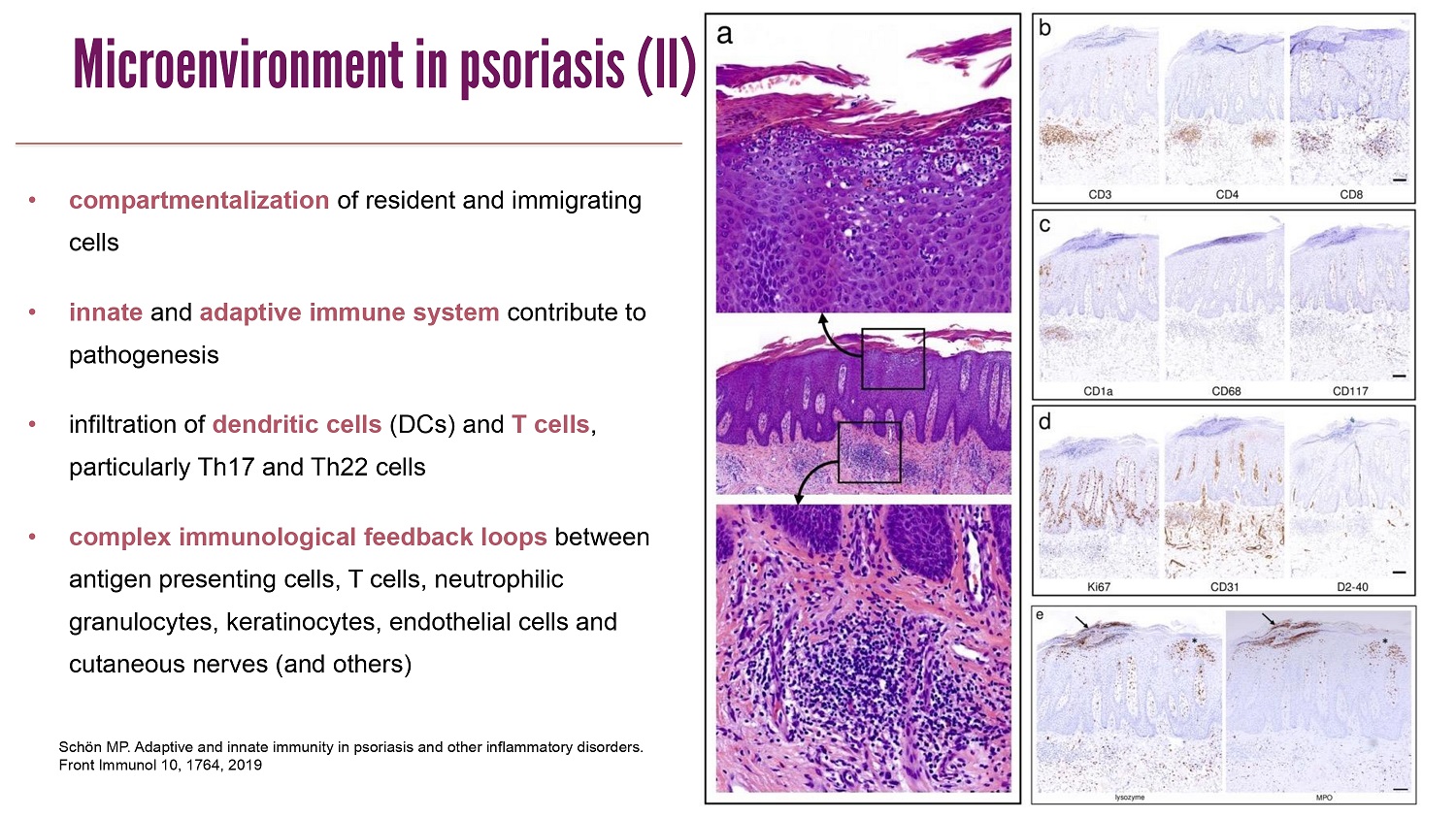
CLICK TO EDIT MASTER TITLE STYLE Schön MP. Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol 10, 1764, 2019 • compartmentalization of resident and immigrating cells • innate and adaptive immune system contribute to pathogenesis • infiltration of dendritic cells (DCs) and T cells , particularly Th17 and Th22 cells • complex immunological feedback loops between antigen presenting cells , T cells , neutrophilic granulocytes , keratinocytes , endothelial cells and cutaneous nerves (and others ) Microenvironment in psoriasis (II)
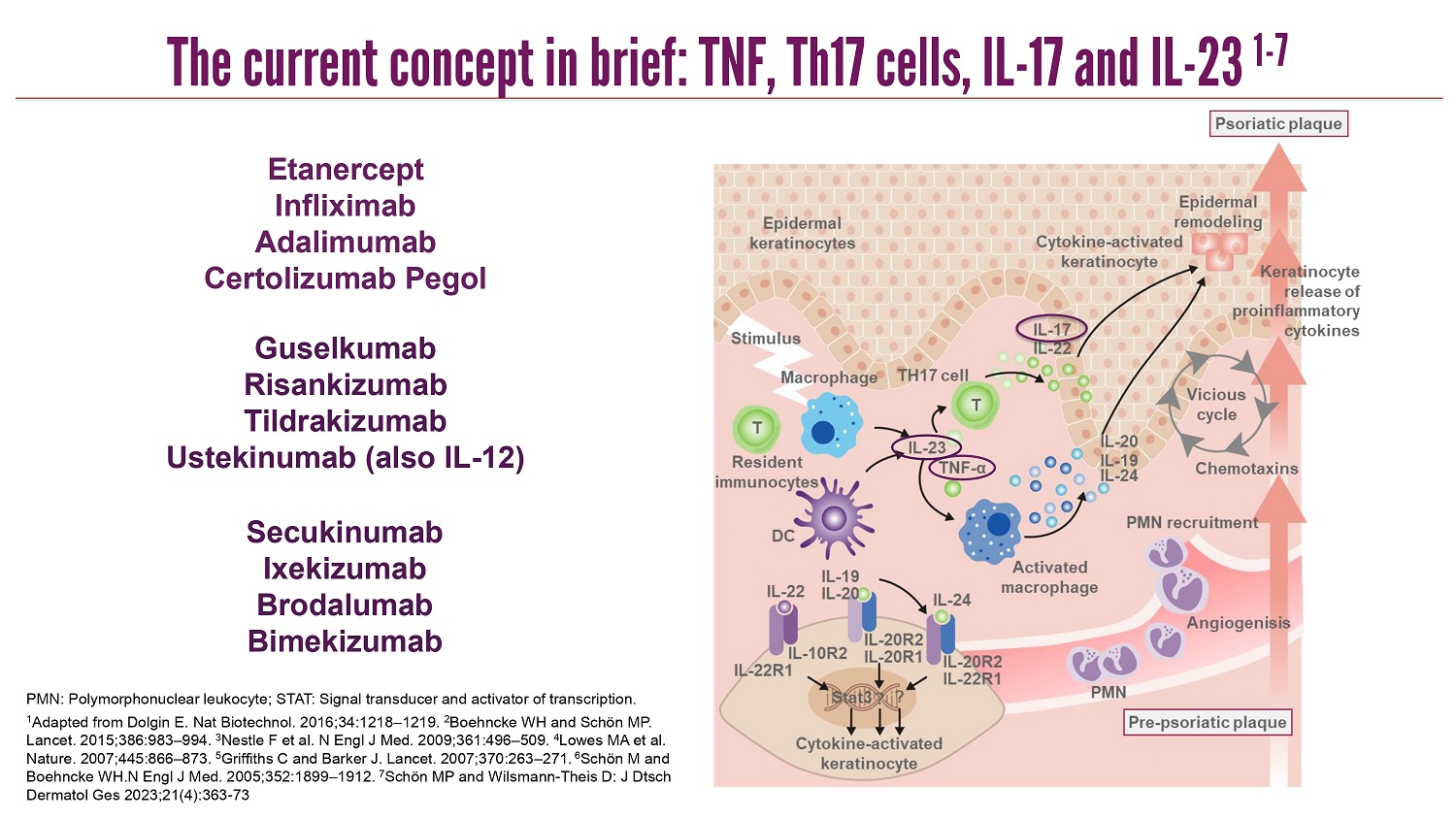
CLICK TO EDIT MASTER TITLE STYLE PMN: Polymorphonuclear leukocyte; STAT: Signal transducer and activator of transcription. 1 Adapted from Dolgin E. Nat Biotechnol. 2016;34:1218 – 1219. 2 Boehncke WH and Schön MP. Lancet. 2015;386:983 – 994. 3 Nestle F et al. N Engl J Med. 2009;361:496 – 509. 4 Lowes MA et al. Nature. 2007;445:866 – 873. 5 Griffiths C and Barker J. Lancet. 2007;370:263 – 271. 6 Schön M and Boehncke WH.N Engl J Med. 2005;352:1899 – 1912. 7 Schön MP and Wilsmann - Theis D: J Dtsch Dermatol Ges 2023;21(4):363 - 73 Secukinumab Ixekizumab Brodalumab Bimekizumab Etanercept Infliximab Adalimumab Certolizumab Pegol Guselkumab Risankizumab Tildrakizumab Ustekinumab (also IL - 12) The current concept in brief: TNF, Th17 cells, IL - 17 and IL - 23 1 - 7
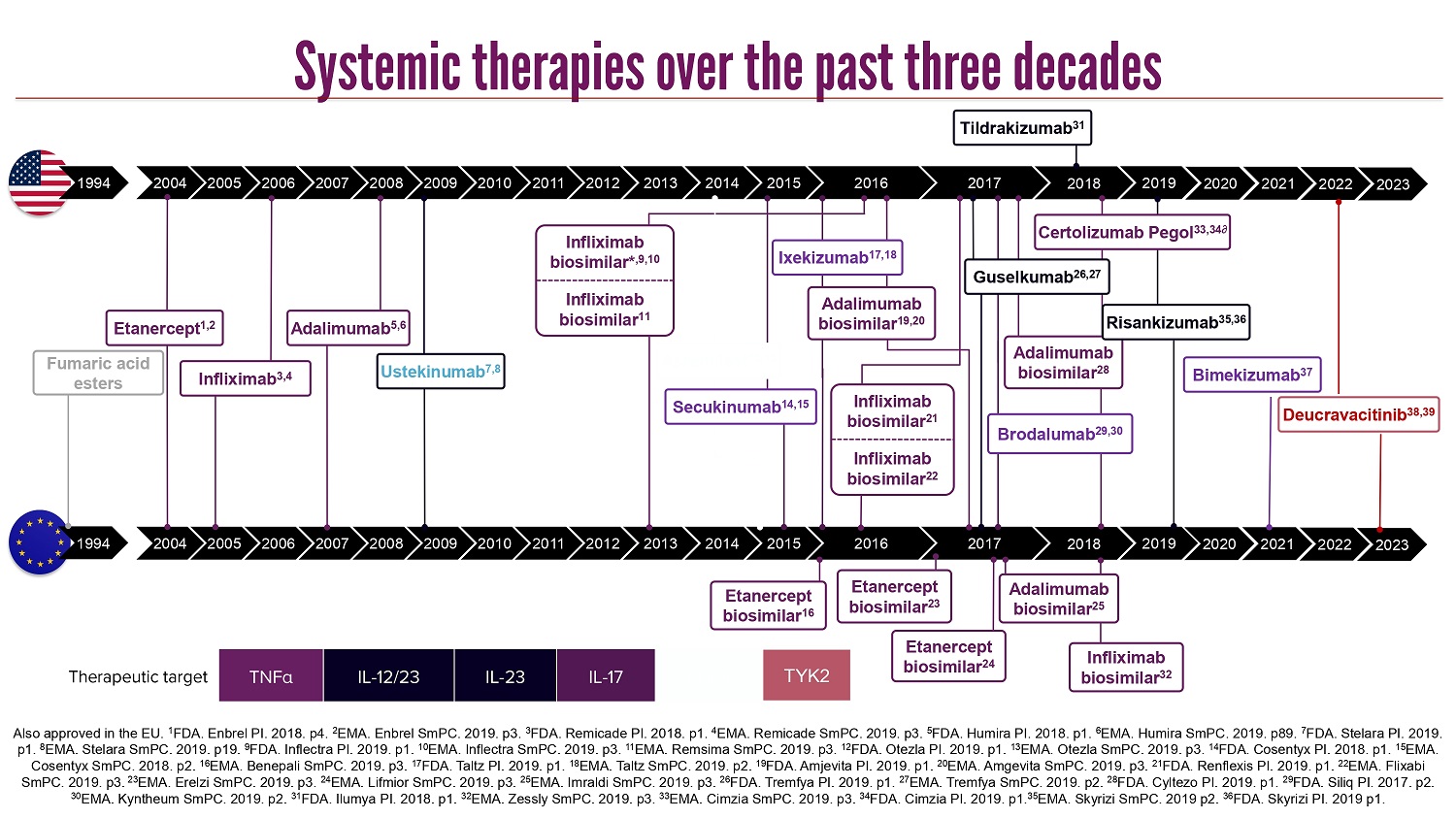
CLICK TO EDIT MASTER TITLE STYLE Also approved in the EU. 1 FDA. Enbrel PI. 2018. p4. 2 EMA. Enbrel SmPC. 2019. p3. 3 FDA. Remicade PI. 2018. p1. 4 EMA. Remicade SmPC. 2019. p3. 5 FDA. Humira PI. 2018. p1. 6 EMA. Humira SmPC. 2019. p89. 7 FDA. Stelara PI. 2019. p1. 8 EMA. Stelara SmPC. 2019. p19. 9 FDA. Inflectra PI. 2019. p1. 10 EMA. Inflectra SmPC. 2019. p3. 11 EMA. Remsima SmPC. 2019. p3. 12 FDA. Otezla PI. 2019. p1. 13 EMA. Otezla SmPC. 2019. p3. 14 FDA. Cosentyx PI. 2018. p1. 15 EMA. Cosentyx SmPC. 2018. p2. 16 EMA. Benepali SmPC. 2019. p3. 17 FDA. Taltz PI. 2019. p1. 18 EMA. Taltz SmPC. 2019. p2. 19 FDA. Amjevita PI. 2019. p1. 20 EMA. Amgevita SmPC. 2019. p3. 21 FDA. Renflexis PI. 2019. p1. 22 EMA. Flixabi SmPC. 2019. p3. 23 EMA. Erelzi SmPC. 2019. p3. 24 EMA. Lifmior SmPC. 2019. p3. 25 EMA. Imraldi SmPC. 2019. p3. 26 FDA. Tremfya PI. 2019. p1. 27 EMA. Tremfya SmPC. 2019. p2. 28 FDA. Cyltezo PI. 2019. p1. 29 FDA. Siliq PI. 2017. p2. 30 EMA. Kyntheum SmPC. 2019. p2. 31 FDA. Ilumya PI. 2018. p1. 32 EMA. Zessly SmPC. 2019. p3. 33 EMA. Cimzia SmPC. 2019. p3. 34 FDA. Cimzia PI. 2019. p1. 35 EMA. Skyrizi SmPC. 2019 p2. 36 FDA. Skyrizi PI. 2019 p1. PDE4 IL - 17 IL - 23 IL - 12/23 TNF α Therapeutic target TYK2 2019 2016 2018 2018 2004 2005 2006 2007 2008 2009 2010 2011 2015 2014 2013 2012 2019 2017 2004 2005 2006 2007 2008 2009 2010 2011 2015 2014 2013 2012 2017 2016 Ustekinumab 7,8 Etanercept 1,2 Infliximab 3,4 Adalimumab 5,6 Etanercept biosimilar 16 Adalimumab biosimilar 19,20 Ixekizumab 17,18 Brodalumab 29,30 Guselkumab 26,27 Tildrakizumab 31 Infliximab biosimilar 32 Infliximab biosimilar* ,9,10 Infliximab biosimilar 11 Apremilast 12,13 Secukinumab 14,15 Etanercept biosimilar 23 Adalimumab biosimilar 28 Etanercept biosimilar 24 Adalimumab biosimilar 25 Certolizumab Pegol 33,34∂ Infliximab biosimilar 21 Infliximab biosimilar 22 Risankizumab 35,36 2020 2020 Bimekizumab 37 2021 2021 Deucravacitinib 38,39 1994 1994 Fumaric acid esters 2022 2022 2023 2023 Systemic therapies over the past three decades

CLICK TO EDIT MASTER TITLE STYLE Current therapeutic targets in psoriasis Schön MP and Wilsmann - Theis D: Current developments and perspectives in psoriasis . J Dtsch Dermatol Ges 2023;21(4):363 - 73

CLICK TO EDIT MASTER TITLE STYLE Adapted from Dolgin E. Nat Biotechnol. 2016;34:1218 – 1219. 2 Boehncke WH and Schön MP. Lancet. 2015;386:983 – 994. 3 Nestle F et al. N Engl J Med. 2009;361:496 – 509. 4 Lowes MA et al. Nature. 2007;445:866 – 873. 5 Griffiths C and Barker J. Lancet. 2007;370:263 – 271. 6 Schön MP and Boehncke WH. N Engl J Med. 2005;352:1899 – 1912.; 7 Ivanov S and Linden A. Trends Pharmacol Sci . 2008;30:95 - 103; 8 Kolls J, Linden A. Immunity . 2004;21:467 - 476; 9 Gaffen S. Nat Rev Immunology . 2009/9:556 - 567 Inhibition of specific mediators: IL - 23 and IL - 17 1 - 9 Interferons Colony - stimulating Factors Tumor Necrosis Factor Interleukins Cytokine families Transforming Growth Factor IL - 17 family* * IL - 17F isoforms 1 and 2 have strongest homology with IL - 17A (55% and 40%, resp.), followed by IL - 17B (29%), IL - 17D (25%), IL - 17C (23%), and IL - 17E (17%) 7 IL - 17B IL - 17E (aka IL - 25) IL - 17C IL - 17A IL - 17D IL - 17F
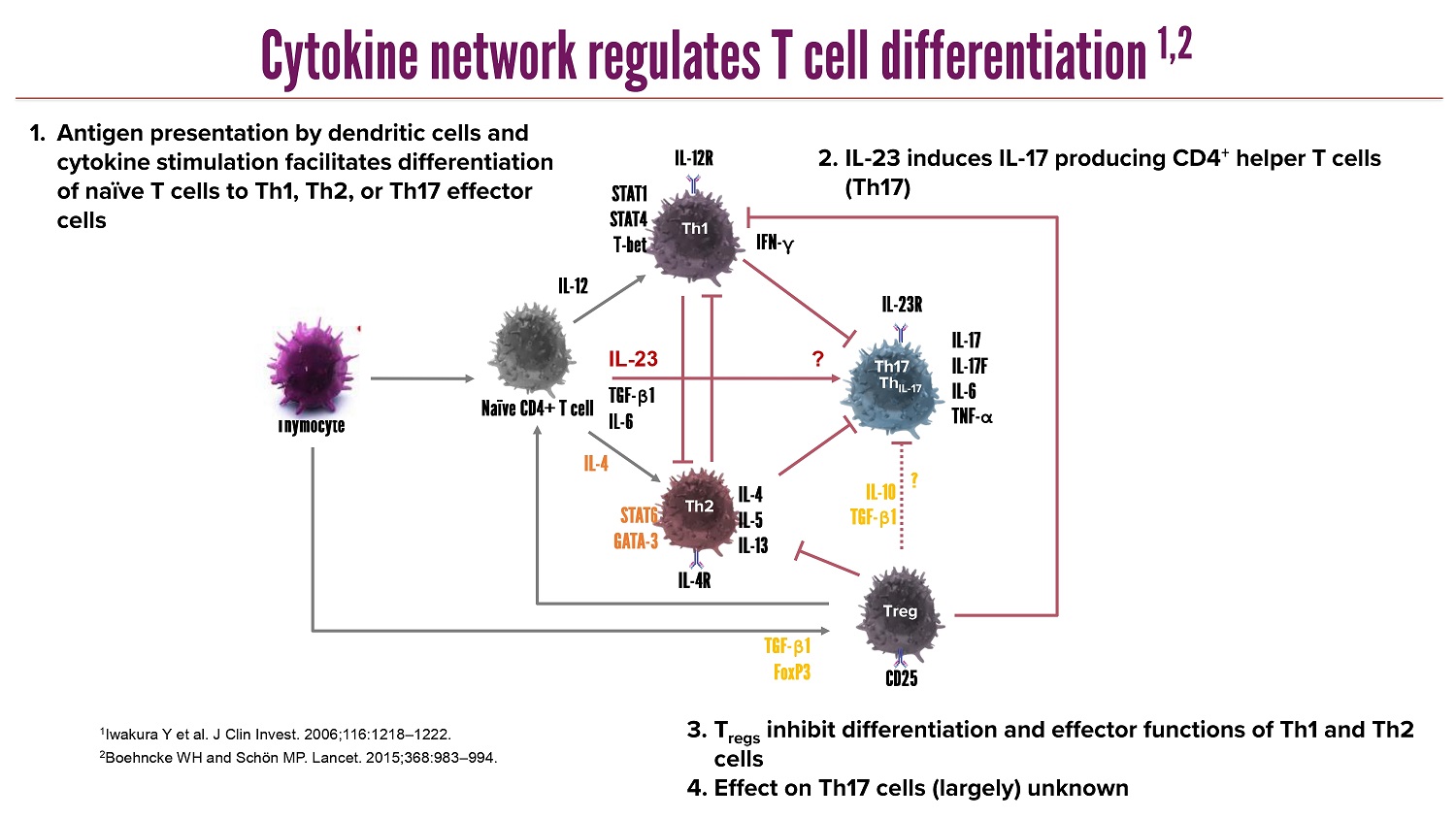
CLICK TO EDIT MASTER TITLE STYLE 1 Iwakura Y et al. J Clin Invest. 2006;116:1218 – 1222. 2 Boehncke WH and Schön MP. Lancet. 2015;368:983 – 994. Th1 Th2 Th17 Treg IL - 17 IL - 17F IL - 6 TNF - α IL - 23R IL - 4R IL - 12R IFN - γ IL - 23 IL - 12 IL - 4 TGF - β 1 IL - 6 ? TGF - β 1 FoxP3 CD25 IL - 4 IL - 5 IL - 13 ? IL - 10 TGF - β 1 Thymocyte Naïve CD4+ T cell STAT1 STAT4 T - bet STAT6 GATA - 3 Th IL - 17 1. Antigen presentation by dendritic cells and cytokine stimulation facilitates differentiation of naïve T cells to Th1, Th2, or Th17 effector cells 2. IL - 23 induces IL - 17 producing CD4 + helper T cells (Th17) 3. T regs inhibit differentiation and effector functions of Th1 and Th2 cells 4. Effect on Th17 cells (largely) unknown Cytokine network regulates T cell differentiation 1,2
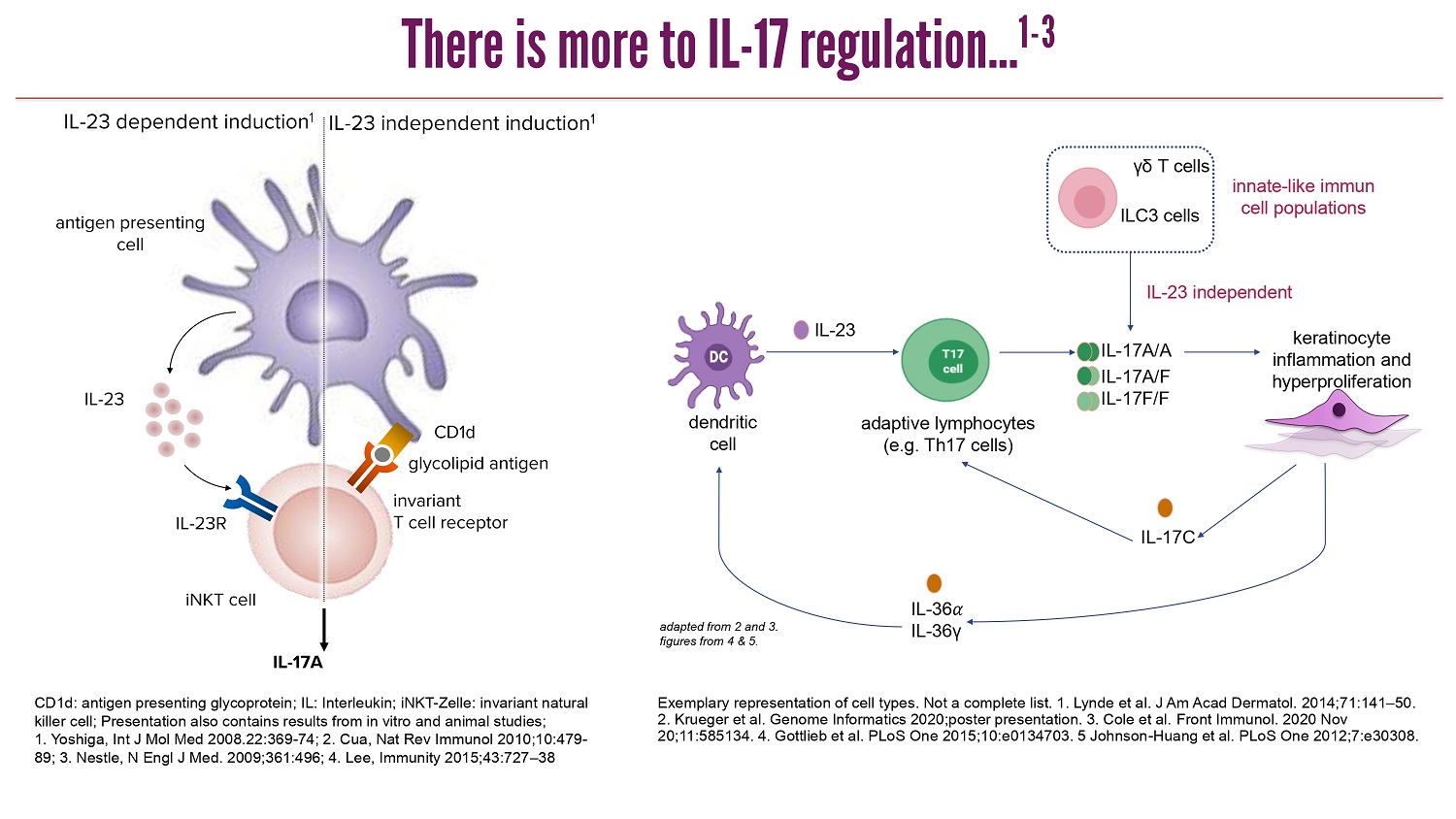
CLICK TO EDIT MASTER TITLE STYLE There is more to IL - 17 regulation... 1 - 3 d endritic c ell keratinocyte inflammation a nd h yperproliferation a daptive l ymphocytes ( e.g . Th17 cells ) ILC3 cells γδ T cells i nnate - like i mmun cell p opulations IL - 23 independent IL - 23 IL - 17C IL - 36 ߙ IL - 36γ IL - 17A/A IL - 17A/F IL - 17F/F a dapted from 2 a nd 3. figures from 4 & 5. Exemplary representation of cell types . Not a complete list . 1. Lynde et al. J Am Acad Dermatol. 2014;71:141 – 50. 2. Krueger et al. Genome Informatics 2020;poster presentation. 3. Cole et al. Front Immunol . 2020 Nov 20;11:585134 . 4. Gottlieb et al. PLoS One 2015;10:e0134703. 5 Johnson - Huang et al. PLoS One 2012;7:e30308. IL - 23 IL - 23R invariant T cell receptor CD1d IL - 23 dependent induction 1 IL - 17A glycolipid antigen antigen presenting cell iNKT cell IL - 23 independent induction 1 CD1d: antigen presenting glycoprotein; IL: Interleukin; iNKT - Zelle : invariant natural killer cell; Presentation also contains results from in vitro and animal studies; 1. Yoshiga , Int J Mol Med 2008.22:369 - 74; 2. Cua , Nat Rev Immunol 2010;10:479 - 89; 3. Nestle, N Engl J Med. 2009;361:496; 4. Lee, Immunity 2015;43:727 – 38

CLICK TO EDIT MASTER TITLE STYLE IL - 17 and IL - 17 receptors 1 - 5 1 Lauffer F, ..., Schön MP: J Dtsch Dermatol Ges. 18(7), 675 - 681, 2020 2 Kolbinger et al. J Allergy Clin Immunol 2017;139:923 – 32 3 Reich K, et al. N Engl J Med 2021;385:142 - 52; 4 Reich K, et al. Lancet 2021;397:487 - 98; 5 Warren RB, et al. N Engl J Med 2021;385:130 - 41. † Data from maintenance group . In BE SURE switch in BKZ Q4W/Q8W arm in week 16 from Q4W to BKZ Q8W, and in the adalimumab BKZ Q4W arm in week 24 von adalimumab to BKZ Q4W. BKZ, Bimekizumab ; PASI 100, 100% improvement compared to baseline in PASI Q4W, every 4 weeks ; Q8W, every 8 weeks . 9.8 317 0 50 100 150 200 250 300 350 IL-17A IL-17F (N=8) (N=8) IL - 17 Zytokinkonzentration ( pg /ml) PASI 100 response , % weeks 0.0 58.6 64.2 * 20.9 38.0 0 20 40 60 80 100 0 4 8 12 16 20 24 28 32 36 40 44 48 52 8 16 52 *p < 0.001 vs ustekinumab 67.4 66.0 * 57.8 73.5 * 50.8 48.3 0 20 40 60 80 100 0 4 8 12 16 20 24 28 32 36 40 44 48 *p < 0.001 vs secukinumab weeks 16 0 8 48 Bimekizumab vs Secukinumab * BE RADIANT † 1 Bimekizumab vs Ustekinumab * BE VIVID 2 Ustekinumab; N=163 BKZ 320 mg Q4W; N=321 Placebo; N=83 BKZ 320 mg Q4W/Q4W (N=147) BKZ 320 mg Q4W/Q8W (N=215) SEC 300 mg (N=354) 29.6 66.7 67.7 72.2 65.8 70.2 0 20 40 60 80 100 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 weeks 16 24 56 8 0 Bimekizumab vs Adalimumab* BE SURE 3 Adalimumab BKZ 320 mg Q4W; N=159 BKZ 320 mg Q4W/Q4W; N=158 BKZ 320 mg Q4W/Q8W; N=161 0
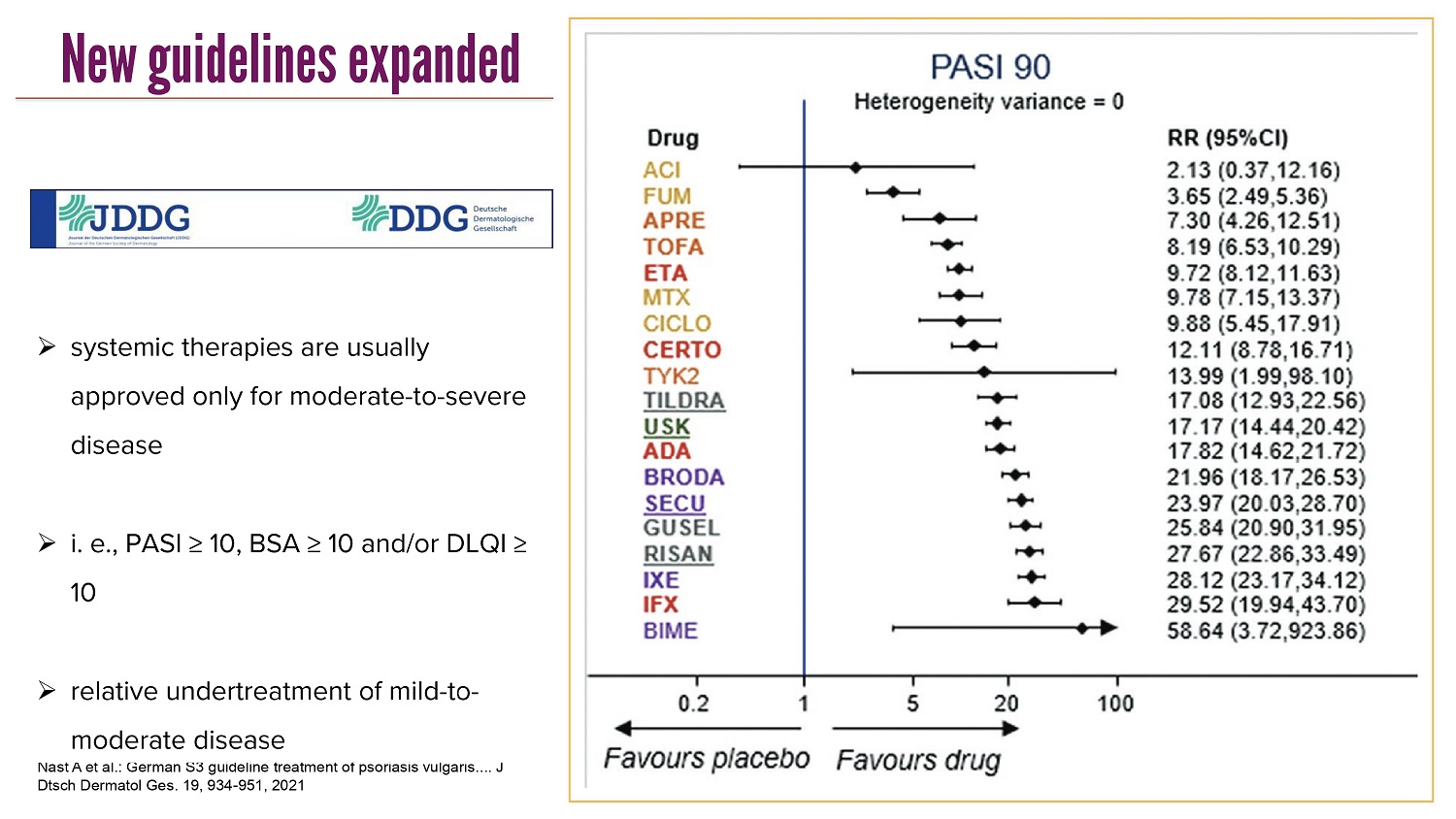
CLICK TO EDIT MASTER TITLE STYLE New guidelines expanded Nast A et al.: German S3 guideline treatment of psoriasis vulgaris .... J Dtsch Dermatol Ges. 19, 934 - 951, 2021 » systemic therapies are usually approved only for moderate - to - severe disease » i. e., PASI ≥ 10, BSA ≥ 10 and/ or DLQI ≥ 10 » relative undertreatment of mild - to - moderate disease

CLICK TO EDIT MASTER TITLE STYLE Why do we need more? » not all patients achieve complete clearance (PASI90 or PASI100) • recalcitrant lesions that do not respond adequately » some patients have psoriasis in difficult - to - treat areas • e.g. hands , feet , scalp , genitals ... » most patients are not eligible for systemic therapy ( only moderate - to - severe disease ) • lesions in visible or sensitive areas still cause considerable burden of disease » individual preferences

CLICK TO EDIT MASTER TITLE STYLE Hard - to - treat lesions: scalp pat . #1 pat . #2 pat . #3

CLICK TO EDIT MASTER TITLE STYLE pat . #4 pat . #5 Hard - to - treat lesions: scalp

CLICK TO EDIT MASTER TITLE STYLE Visible areas with high burden of disease: face pat . #6 pat . #7 pat . #8 pat . #9
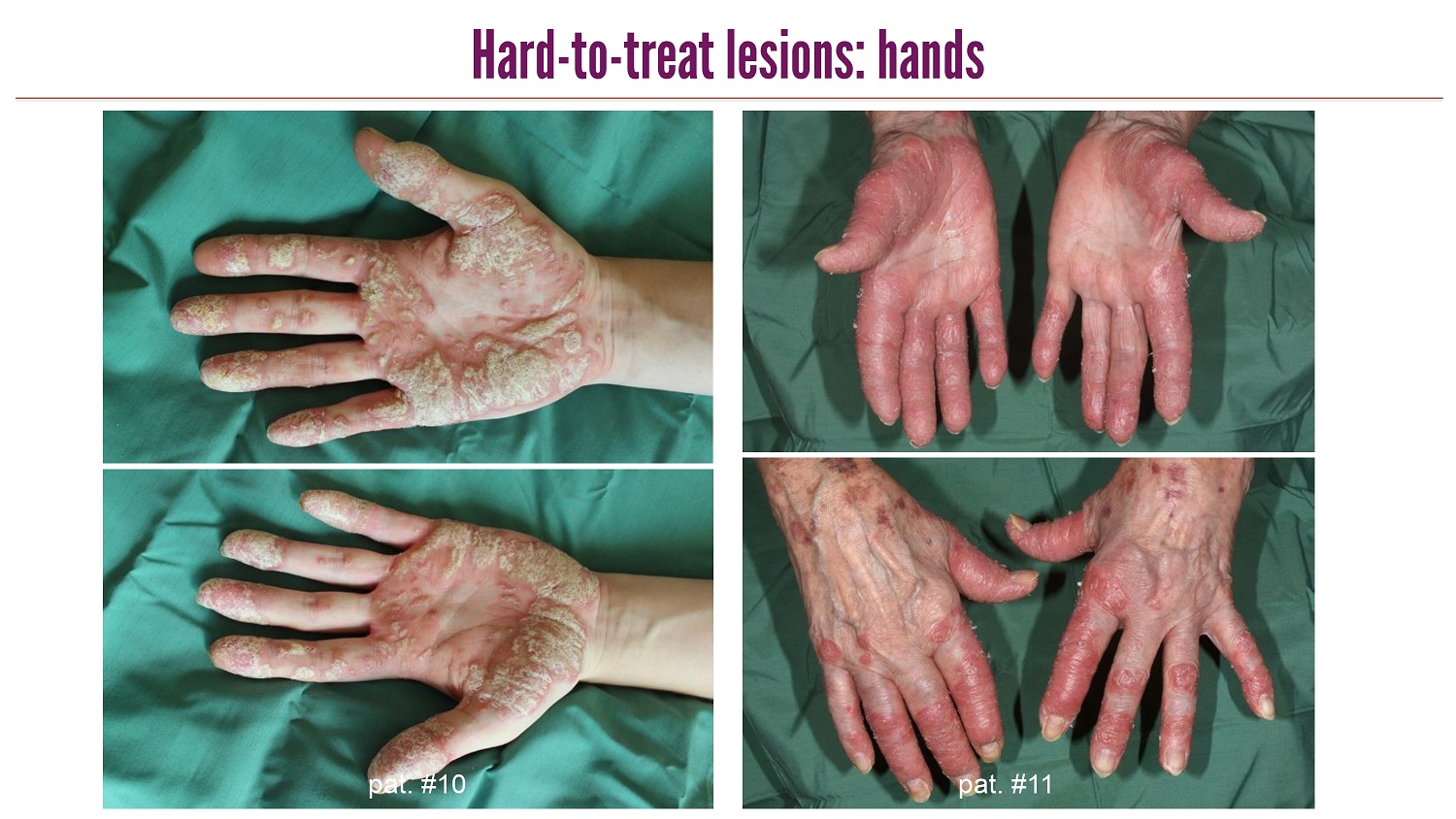
CLICK TO EDIT MASTER TITLE STYLE Hard - to - treat lesions: hands pat . #10 pat . #11
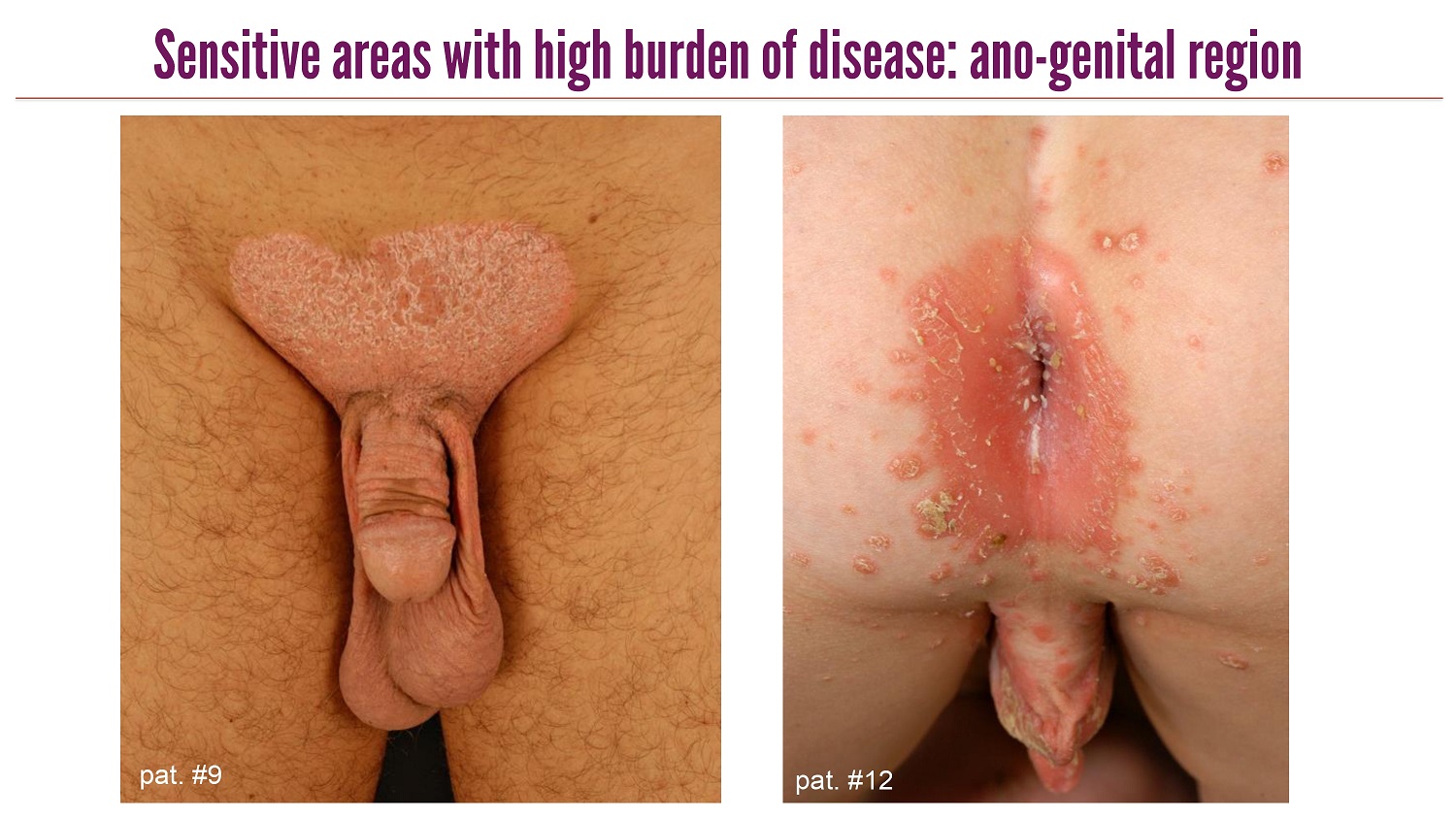
CLICK TO EDIT MASTER TITLE STYLE Sensitive areas with high burden of disease: ano - genital region pat . #9 pat . #12
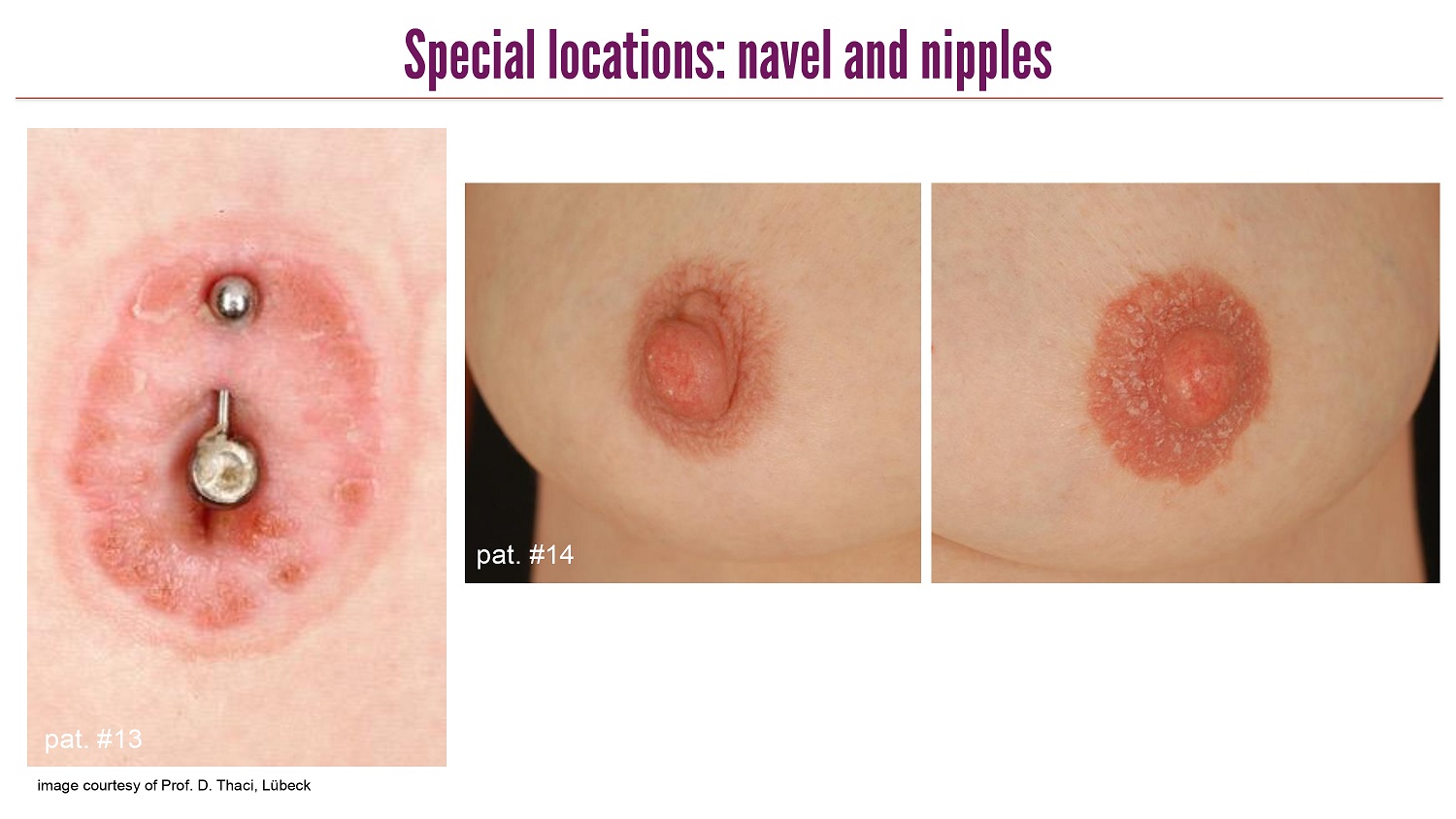
CLICK TO EDIT MASTER TITLE STYLE Special locations: navel and nipples image courtesy of Prof. D. Thaci, Lübeck pat . #13 pat . #14
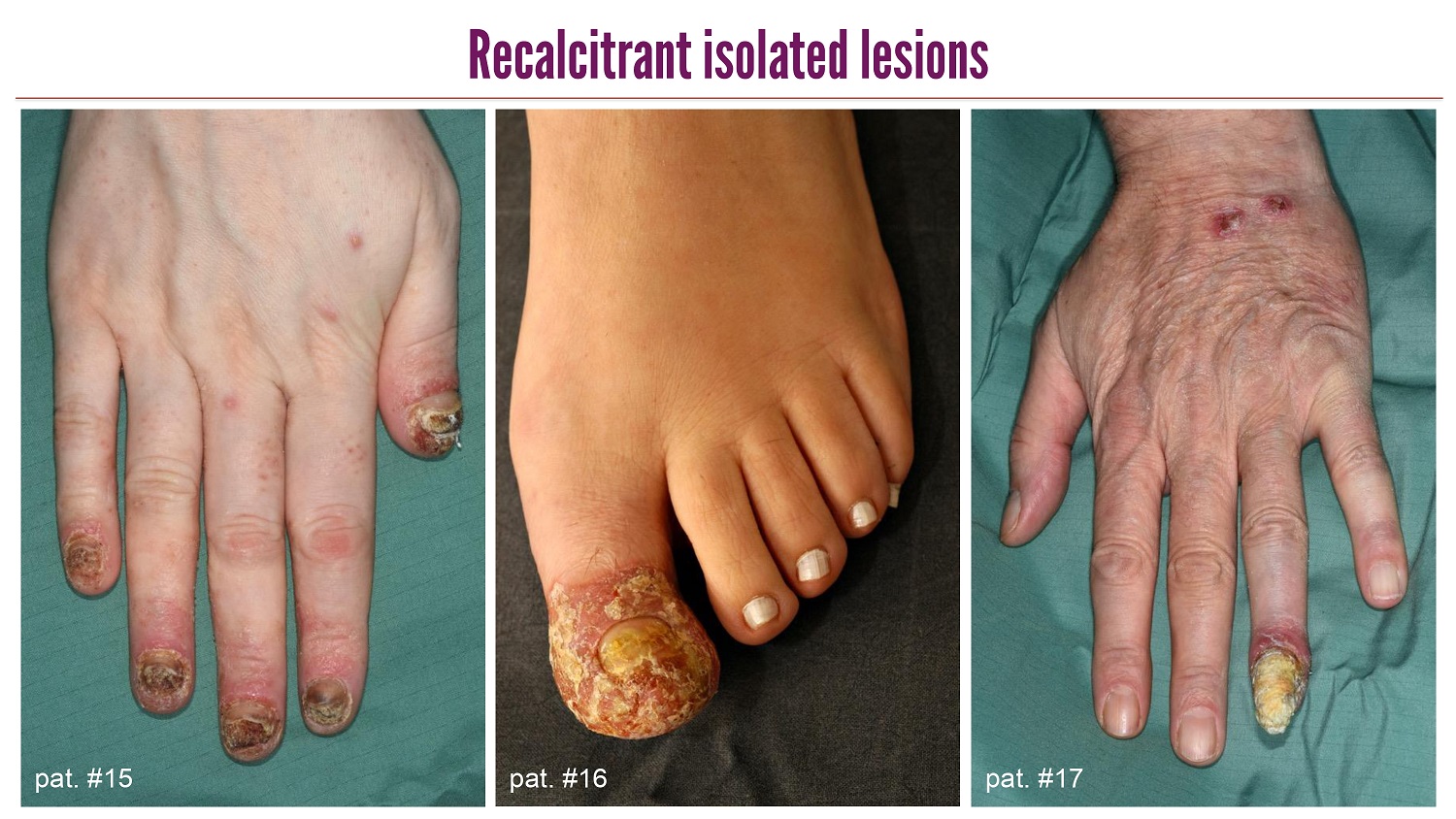
CLICK TO EDIT MASTER TITLE STYLE Recalcitrant isolated lesions pat . #15 pat . #16 pat . #17

CLICK TO EDIT MASTER TITLE STYLE Pretreated lesions with therapy side effects: soles pat . #18 pat . #19

CLICK TO EDIT MASTER TITLE STYLE Summary » psoriasis is a systemic chronic inflammatory disorder with high burden of disease » complex ( immuno ) pathogenesis with involvement of both innate and adaptive immune mechanisms , prominent role of IL - 23/IL - 17 axis » pathogenesis - oriented therapies have greatly improved therapy » yet , need for further developments , especially for certain localized treatments

CLICK TO EDIT MASTER TITLE STYLE Thank you !

CLICK TO EDIT MASTER TITLE STYLE 25 Plaque Psoriasis: a large unmet need with the mild and moderate Psoriasis prevalence and severity SEVERE MODERATE MILD 22% of patients. 28% of patients. 50% of patients. >10% of the body Typically has a severe impact on quality of life 3 - 10% of the body Generally affects quality of life <3% of the body Typically occasional effect on quality of life e.g., aesthetic discomfort Sources: Canadian Psoriasis Network; National Psoriasis Foundation; https://link.springer.com/article/10.1007/s13555 - 021 - 00518 - 8 • 125 million patients globally, of them 15.7 million in the 7 major markets (US, EU5 and Japan) • 80 - 90% with plaque psoriasis • Mild patients may suffer from considerable and visible lesions which may be uncomfortable, painful, and impact social and mental well - being • Mild patients are ineligible for biological treatments in most cases; and moderate psoriatic patients are often reluctant to receive systemic biological treatments due to side effects and costs. Current biological therapies targeted to moderate & severe patients and are administered systemically

CLICK TO EDIT MASTER TITLE STYLE 26 CURRENT PLAQUE PSORIASIS TREATMENTS Corticosteroid creams, vitamin E+A, vitamin D derivatives Phototherapy 1 st line systemic immunosuppressants (Methotrexate and Cyclosporine) & Immunomodulators (Otezla) 2 nd line systemic Immunomodulators (e.g., Sotyktu ) Injectable biologics (anti - TNF α , IL - 17, IL - 23)

CLICK TO EDIT MASTER TITLE STYLE 27 NANOABs ADDRESS UNMET NEED Designed to be convenient, safe, affordable, effective biologic for mild and moderate patients Current treatment shortcomings Corticosteroids • Side effects including: Skin thinning (bruising) & Lightening of skin color • Development of tolerance • An ointment that is inconvenient for use Phototherapy • Requires 20 - 35 sessions, 3 times a week 1st line systemic immunosuppressants & Immunomodulators • E.g. Methotrexate (5.8M prescriptions in the USA in 2020) and Cyclosporin (2.2 million prescriptions) come with concerns for health risks and adverse effects. Otezla (PDE4 Inhibitor) has limited efficacy and requires daily dosing. 2nd line systemic Immunomodulators (e.g. Sotyktu ) • Expensive • Limited efficacy (lower than Biologics) • Systemic and chronic, with systemic side effects Injectable Biologics (mAbs) • Limited to moderate - to - severe patients • Very expensive • Systemic and chronic; Increased risk of infections especially oral candida and developing side effects such as psychological illness (suicidal thoughts) and inflammatory bowel disease.

CLICK TO EDIT MASTER TITLE STYLE 28 The need for a novel local therapeutic • T here ha s not been any novel local treatment in the last 25 years. • Significant patient preference for local therapies, compared to systemic drugs. • Corticosteroids are not suitable for long - term management of PsO due to adverse safety profiles, development of tolerance and inconvenient treatment procedure for the patients. • If innovator local treatments could provide increased efficacy with a superior safety profile early in the disease progression, PsO patients may never need to escalate to the injectable biologics. Source: Global Data Plaque Psoriasis Market Analysis 2021 - 2030
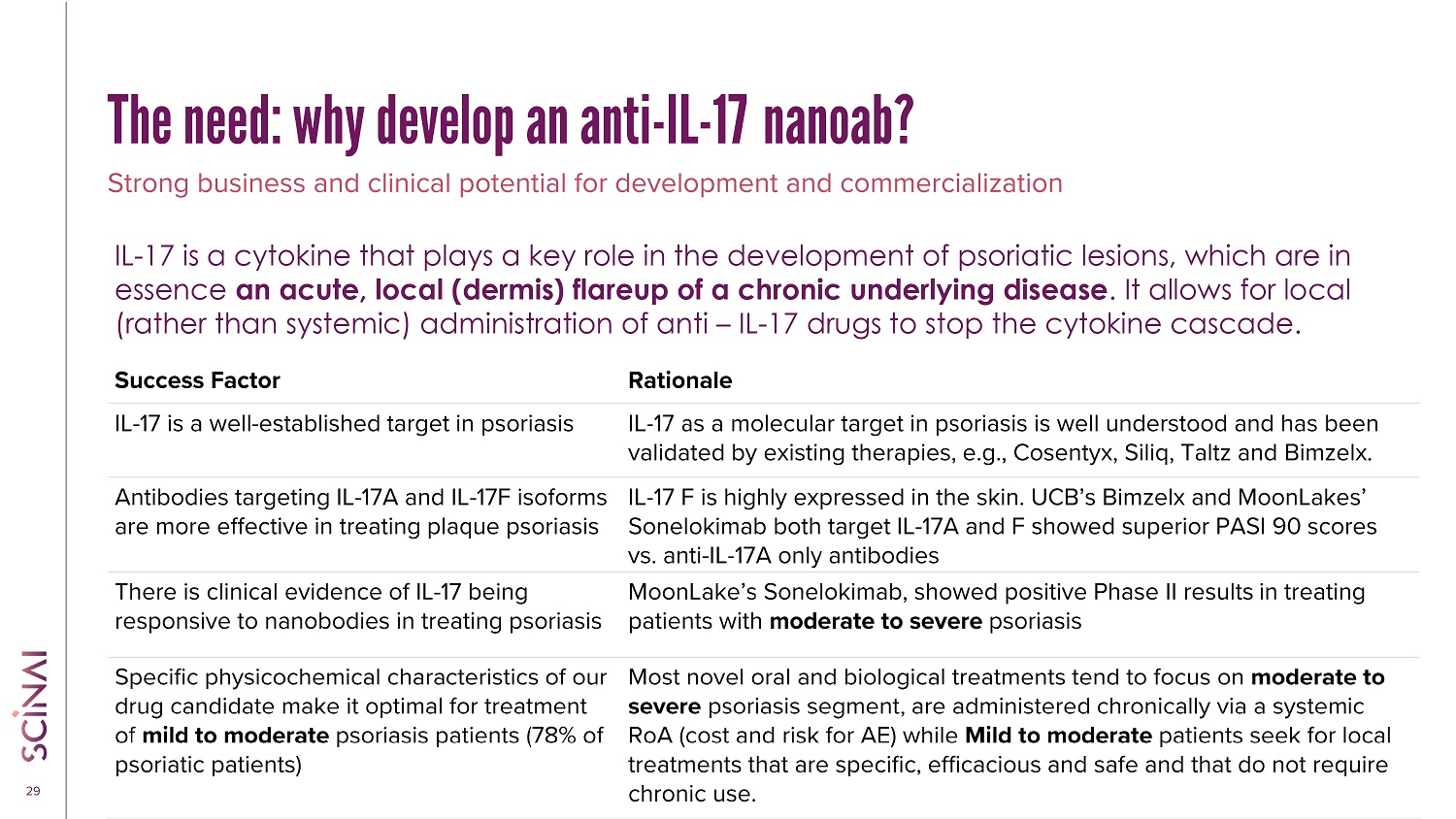
CLICK TO EDIT MASTER TITLE STYLE 29 Rationale Success Factor IL - 17 as a molecular target in psoriasis is well understood and has been validated by existing therapies, e.g., Cosentyx, Siliq, Taltz and Bimzelx . IL - 17 is a well - established target in psoriasis IL - 17 F is highly expressed in the skin. UCB’s Bimzelx and MoonLakes’ Sonelokimab both target IL - 17A and F showed superior PASI 90 scores vs. anti - IL - 17A only antibodies Antibodies targeting IL - 17A and IL - 17F isoforms are more effective in treating plaque psoriasis MoonLake’s Sonelokimab , showed positive Phase II results in treating patients with moderate to severe psoriasis There is clinical evidence of IL - 17 being responsive to nanobodies in treating psoriasis Most novel oral and biological treatments tend to focus on moderate to severe psoriasis segment , are administered chronically via a systemic RoA (cost and risk for AE) while Mild to moderate patients seek for local treatments that are specific, efficacious and safe and that do not require chronic use. Specific physicochemical characteristics of our drug candidate make it optimal for treatment of mild to moderate psoriasis patients (78% of psoriatic patients) Strong business and clinical potential for development and commercialization IL - 17 is a cytokine that plays a key role in the development of psoriatic lesions, which are in essence an acute, local (dermis) flareup of a chronic underlying disease . It allows for local (rather than systemic) administration of anti – IL - 17 drugs to stop the cytokine cascade. The need: why develop an anti - IL - 17 nanoab?

CLICK TO EDIT MASTER TITLE STYLE 30 Local ID injection of Anti - IL - 17A/F VHH antibody fragment A novel way to using VHH antibodies - Most other entities working with VHHs (nanobodies®) aim to mimic mAbs “playbook” and hence are competing for the same patient populations and are using the same routes of administration . Making biologics available for the mild to moderate patients : Current biologics treatments are approved only for moderate to severe psoriasis patients since they are provided systemically and come with associated risks for severe adverse effects (e . g . infections, exacerbation of IBD, heart diseases) . Scinai’s nanoAb is for local administration for local action . No systemic impact Improves patient’s convenience by sparing the need for twice a day application of creams and ointments that makes day to day activities cumbersome (e . g . wearing cloths after application or getting into bed without getting bed sheets dirty) or the need to attend three times a week a phototherapy center for 10 weeks long .
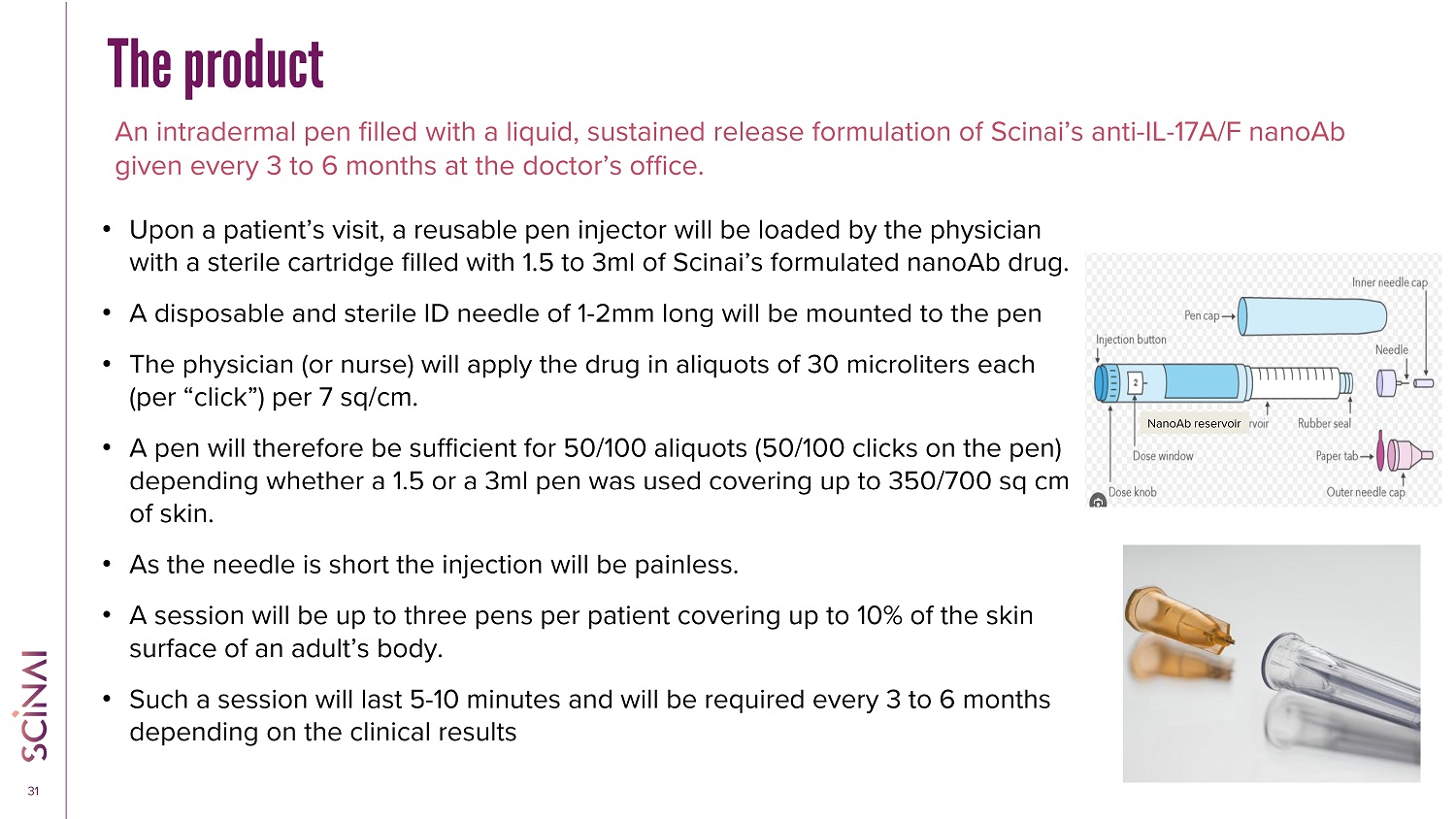
CLICK TO EDIT MASTER TITLE STYLE 31 The product An intradermal pen filled with a liquid, sustained release formulation of Scinai’s anti - IL - 17A/F nanoAb given every 3 to 6 months at the doctor’s office. • Upon a patient’s visit, a reusable pen injector will be loaded by the physician with a sterile cartridge filled with 1.5 to 3ml of Scinai’s formulated nanoAb drug. • A disposable and sterile ID needle of 1 - 2mm long will be mounted to the pen • The physician (or nurse) will apply the drug in aliquots of 30 microliters each (per “click”) per 7 sq/cm. • A pen will therefore be sufficient for 50/100 aliquots (50/100 clicks on the pen) depending whether a 1.5 or a 3ml pen was used covering up to 350/700 sq cm of skin. • As the needle is short the injection will be painless. • A session will be up to three pens per patient covering up to 10% of the skin surface of an adult’s body. • Such a session will last 5 - 10 minutes and will be required every 3 to 6 months depending on the clinical results NanoAb reservoir
CLICK TO EDIT MASTER TITLE STYLE 32 Generating incentives for the customers – the three P’s Patients: Mild to moderate plaque psoriasis patients. • Currently treated with corticosteroids and are unhappy : • Inconvenience of use (e.g. twice a day, use of ointments/creams). • Development of tolerance • Development of side effects – thinning of the skin and changes in color of the skin. • Cannot do phototherapy: • Location of lesion • Low compliance with phototherapy schedule • Are pushing the physician to receive biologics • Do not want to take daily systemic orals (Otezla or Sotyktu ) • Prefer a local, non painful treatment 2 - 3 times per year that saves daily treatments and at lower costs than once a month systemic biologics and without the risks of systemic immunosuppressants. Providers • Dermatologists • Don’t want to prescribe biologics off label to mild patients (risks). • Prefer a solution that would allow them to charge for the visit, the drug dispensing and the injection. Payers • Prefer lower costs vs. systemic biologics especially when used off label • Provide their clients a superior solution vs. corticosteroids and safer than systemic biologics at a lower deductible to the patient.

CLICK TO EDIT MASTER TITLE STYLE 33 Where is the money? • Total sales of drugs in the 7MM for mild psoriasis expected to be $1.8B in 2030 • $1.3B is expected to come from prescription of biologics and $259M from TYK2 inhibitor • This is the market where the topicals and phototherapy do not help. • This is the unmet need, which represents approx. 300K monthly prescriptions of expensive drugs not planned for use with mild patients • Pay attention that topicals and immunosuppressants sell altogether $250M annually in the 7MM. Source: GlobalData

CLICK TO EDIT MASTER TITLE STYLE 34 The Max Planck Institute & UMG 1 bring… • Recombinant protein drug development experience from lab to Phase 3 clinical trial • Manufacturing, quality, international regulatory experience • GMP biologics manufacturing facility • Best - in - class equipped labs • Top - tier big pharma & biotech leadership expertise • World - class science & access to leading scientists • NanoAb platform for the development of promising potent therapeutics • Patents covering NanoAbs & their manufacturing Professor Dr Dirk Görlich Director of Max Planck Institute for Multidisciplinary Sciences Winner of inaugural World Laureates Association (WLA) Prize in Life Sciences or Medicine Professor Dr Matthias Dobbelstein Fellow at Max Planck Institute for Multidisciplinary Sciences UMG Head of Department Covering discovery and initial characterization of NanoAbs aimed at predefined list of molecular targets. Designed to create significant clinical and commercial advantages. Scinai brings… MAX PLANCK , UMG, SCINAI COLLABORATION 1. Max Planck Institute for Multidisciplinary Sciences and the University Medical Center Göttingen (UMG)

CLICK TO EDIT MASTER TITLE STYLE 35 Alpaca - derived nanosized antibodies (NanoAbs) are also known as VHH antibodies or nanobodies 1 mAb therapeutic market size is ~ $205 billion 2 including Cosentyx for psoriasis $4.8 billion (2022) 3 NanoAbs: Human monoclonal antibody (mAb)’s biobetter 1. VHH antibody is trademarked by ABLYNX N.V., a wholly owned subsidiary of Sanofi, as Nanobody. Scinai has no affiliation wi th and is not endorsed by Sanofi. 2. https://www.researchandmarkets.com/reports/5791212/monoclonal - antibody - therapeutics - market - source (accessed 14.Aug.2023) 3. https://www.reporting.novartis.com/2022/novartis - in - society/performance - in - 2022/financial - performance.html (Accessed 7.Jan.20 24) HUMAN ANTIBODY (mAb) ALPACA - DERIVED ANTIBODY (NanoAb) NANOSIZED ANTIBOD Y PIPELINE: HUGE OPPORTUNITY

CLICK TO EDIT MASTER TITLE STYLE 36 Anti - IL - 17 Nanoab: A better neutralizer Secukinumab* NanoAb Cytokine 0.2nM 0.56nM IL17A m M 1.00nM IL17F 2.0nM 0.25nM IL17AF Single NanoAb neutralizes IL - 17 A, F, and AF complex IL - 17 A IL - 17 F IL - 17 AF COMPLEX * Cosentyx, INN - sekukinumab (europa.eu) Mode of Action: By Blocking IL - 17, the interactions with the IL - 17 receptors expressed on keratinocytes, fibroblast - like synoviocytes , endothelial cells, chondrocytes and osteoblasts downstream cascade of events in the epidermis and expression of Psoriasis - related markers is avoided. The NanoAb neutralizes IL - 17 isoforms at nM concentrations, x100 better than the mAb IL - 17 A Whereas the affinity for NanoAb is comparable to that of Cosentyx (for IL - 17A), the neutralization shows a significantly better efficacy of the NanoAb Neutralization assays
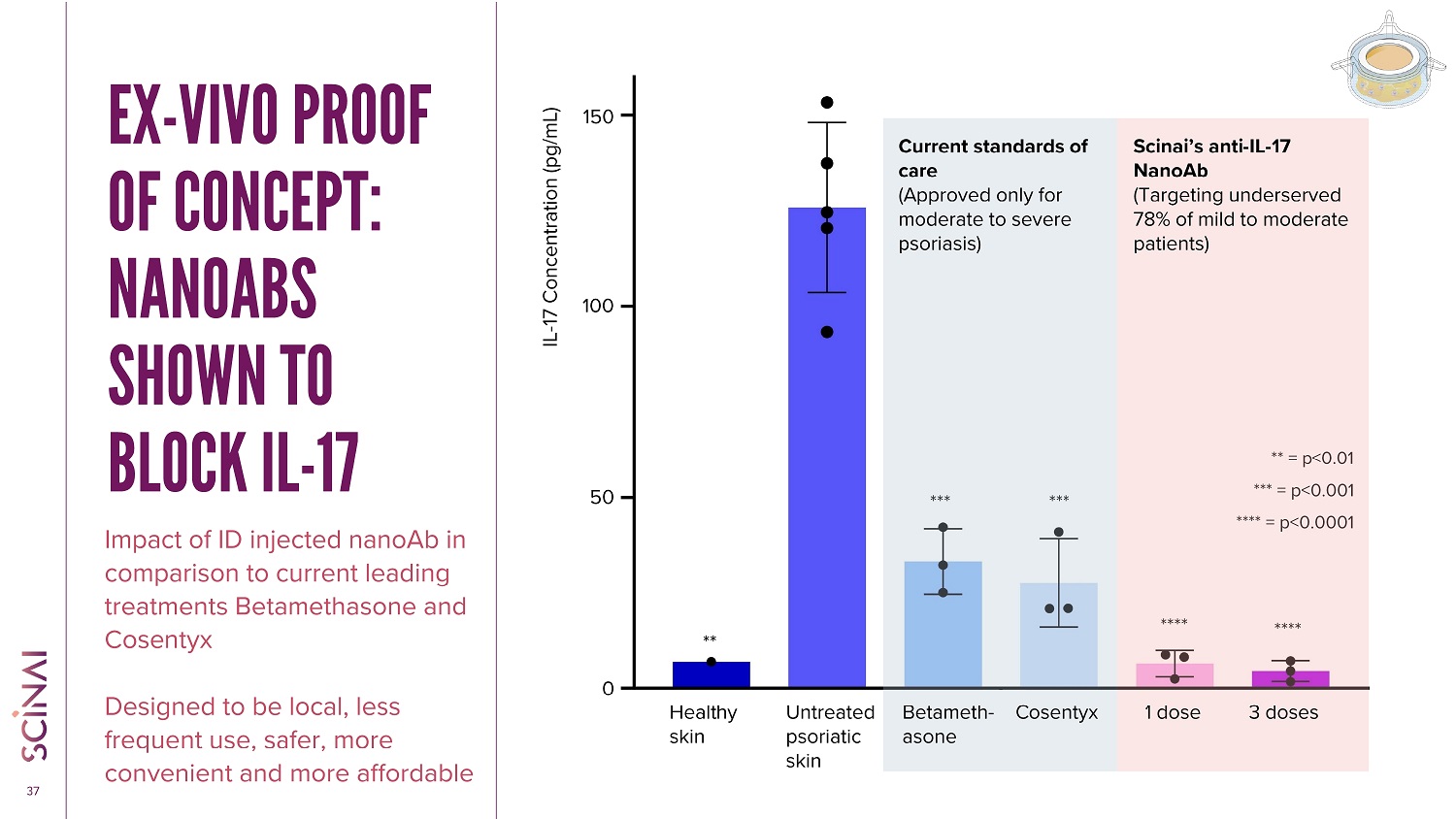
CLICK TO EDIT MASTER TITLE STYLE 37 EX - VIVO PROOF OF CONCEPT: NANOABS SHOWN TO BLOCK IL - 17 Impact of ID injected nanoAb in comparison to current leading treatments Betamethasone and Cosentyx Designed to be local, less frequent use, safer, more convenient and more affordable *** = p<0.001 **** = p<0.0001 ** = p<0.01 **** *** **** *** Healthy skin Betameth - asone Cosentyx Scinai’s anti - IL - 17 NanoAb (Targeting underserved 78% of mild to moderate patients) IL - 17 Concentration (pg/mL) Untreated psoriatic skin Current standards of care (Approved only for moderate to severe psoriasis) 1 dose 3 doses ** 0 50 100 150
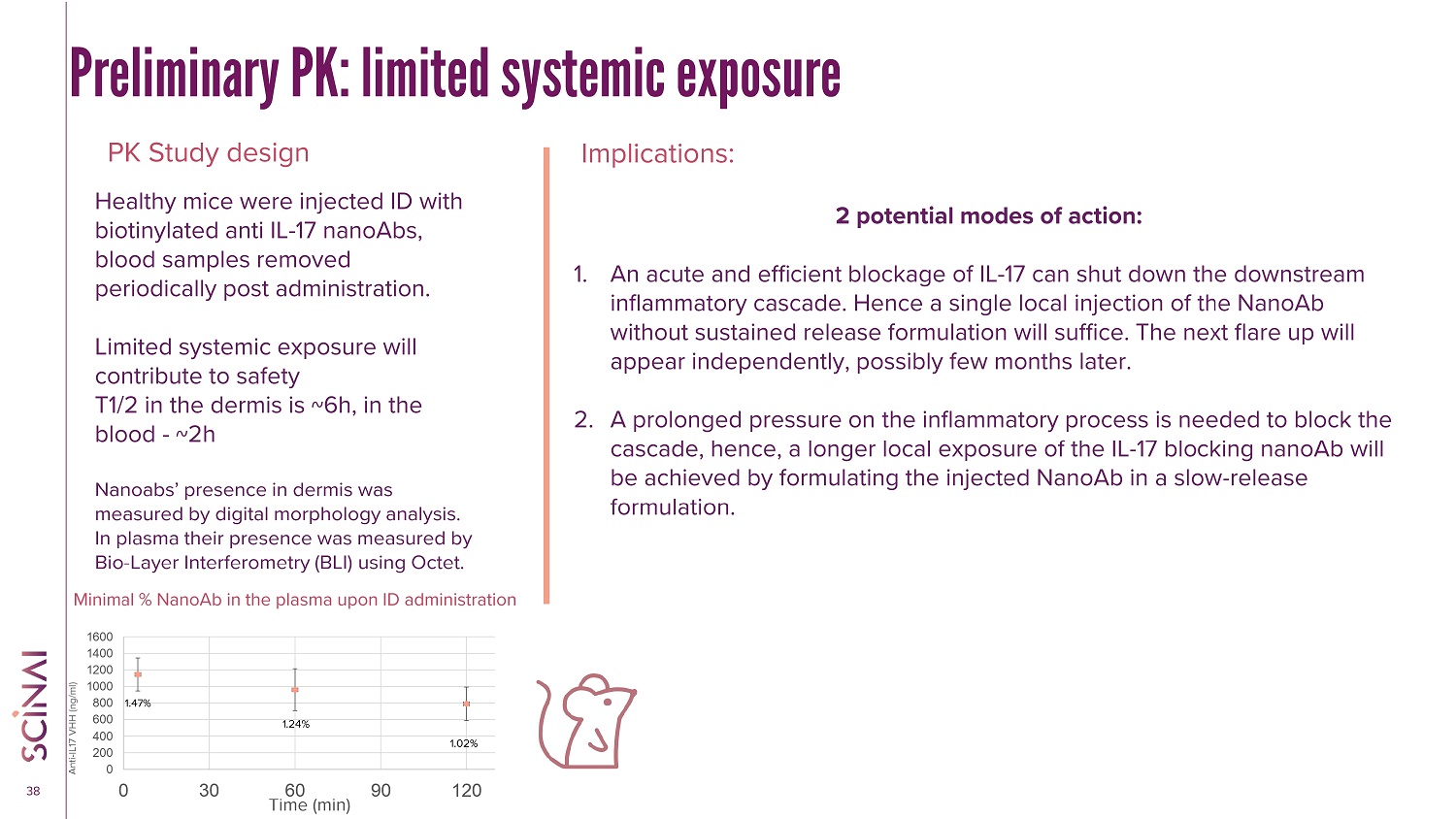
CLICK TO EDIT MASTER TITLE STYLE 38 Preliminary PK: limited systemic exposure PK Study design Implications: Healthy mice were injected ID with biotinylated anti IL - 17 nanoAbs, blood samples removed periodically post administration. Limited systemic exposure will contribute to safety T1/2 in the dermis is ~6h, in the blood - ~2h Nanoabs ’ presence in dermis was measured by digital morphology analysis. In plasma their presence was measured by Bio - Layer Interferometry (BLI) using Octet. 2 potential modes of action: 1. An acute and efficient blockage of IL - 17 can shut down the downstream inflammatory cascade. Hence a single local injection of the NanoAb without sustained release formulation will suffice. The next flare up will appear independently, possibly few months later. 2. A prolonged pressure on the inflammatory process is needed to block the cascade, hence, a longer local exposure of the IL - 17 blocking nanoAb will be achieved by formulating the injected NanoAb in a slow - release formulation. 0 200 400 600 800 1000 1200 1400 1600 0 30 60 90 120 Anti - IL17 VHH (ng/ml) Time (min) 1.47% 1.24% 1.02% Minimal % NanoAb in the plasma upon ID administration
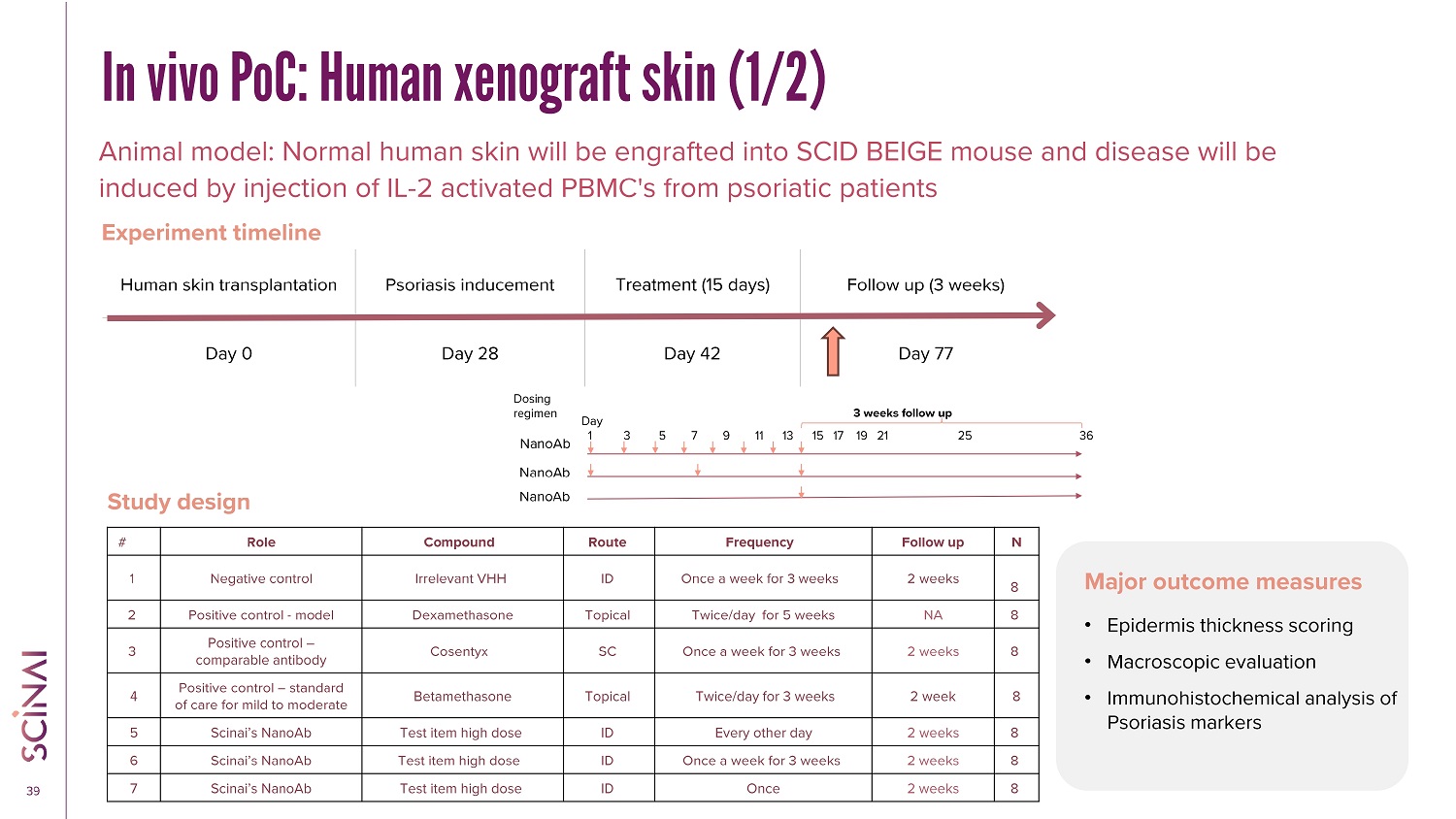
CLICK TO EDIT MASTER TITLE STYLE 39 In vivo PoC: Human xenograft skin (1/2) Animal model: Normal human skin will be engrafted into SCID BEIGE mouse and disease will be induced by injection of IL - 2 activated PBMC's from psoriatic patients Study design Major outcome measures Follow up (3 weeks) Treatment (15 days) Psoriasis inducement Human skin transplantation Day 77 Day 42 Day 28 Day 0 Experiment timeline • Epidermis thickness scoring • Macroscopic evaluation • Immunohistochemical analysis of Psoriasis markers N Follow up Frequency Route Compound Role # 8 2 weeks Once a week for 3 weeks ID Irrelevant VHH Negative control 1 8 NA Twice/day for 5 weeks Topical Dexamethasone Positive control - model 2 8 2 weeks Once a week for 3 weeks SC Cosentyx Positive control – comparable antibody 3 8 2 week Twice/day for 3 weeks Topical Betamethasone Positive control – standard of care for mild to moderate 4 8 2 weeks Every other day ID Test item high dose Scinai’s NanoAb 5 8 2 weeks Once a week for 3 weeks ID Test item high dose Scinai’s NanoAb 6 8 2 weeks Once ID Test item high dose Scinai’s NanoAb 7 Day 1 3 5 7 9 11 13 15 17 19 21 25 36 3 weeks follow up Dosing regimen NanoAb NanoAb NanoAb
CLICK TO EDIT MASTER TITLE STYLE 40 In vivo PoC: Human xenograft skin (2/2) What is being studied? The impact of local psoriasis treatments by efficacy parameters: x Clinical evaluation by medical photographs. x Macroscopic evaluation, epidermis thickness scoring & Histological analysis. x IHC analysis for Ki - 67, (proliferative marker of keratinocytes). x IHC analysis: HLA - DR (High DR characterizes Psoriasis), epidermal human beta - defensin - 2 (BD - 2 serum levels correlate with IL - 17A and PASI, it is decreased after IL - 17A blockade). x Psoriasin (S100A7), CD8 & CD4 (increase in inflammation), IL - 17, IL - 22 (parallel to IL17), TNF - a, CD31 (angiogenesis marker) x Results expected in Q2 2024 Source: IL - 17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa - Fletcher - 2020 - Clinical & Experimental Immunology - Wiley Online Library

CLICK TO EDIT MASTER TITLE STYLE 41 Summary • T here is a need for a better treatment for patients with mild to moderate Psoriasis and for specific lesions that are hard to treat with current therapies. • Biological drugs are the safest and most efficient, yet – they are administered systemically and are expensive . • Blocking IL - 17A and IL - 17F isoforms is an effective mechanism to control Psoriasis • Scinai’s NanoAbs, administered locally ID already showed superior neutralization of IL - 17 in cell culture, and ex - vivo in human Psoriatic skin. • Scinai’s upcoming in vivo study will compare schedules of administration and show the duration of the therapeutic effect • Next steps: Toxicology and First in Human clinical trial

CLICK TO EDIT MASTER TITLE STYLE THANK YOU
BiondVax Pharmaceuticals (NASDAQ:BVXV)
Historical Stock Chart
From Mar 2024 to Apr 2024
BiondVax Pharmaceuticals (NASDAQ:BVXV)
Historical Stock Chart
From Apr 2023 to Apr 2024