false
0001035354
0001035354
2023-11-13
2023-11-13
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
November 13, 2023
Eloxx Pharmaceuticals, Inc.
(Exact name of registrant as specified
in its charter)
| Delaware |
|
001-31326 |
|
84-1368850 |
|
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
|
480
Arsenal Way, Suite 130, Watertown, MA |
|
02451 |
| (Address of principal executive offices) |
|
(Zip Code) |
(Registrant’s telephone number,
including area code): (781) 577-5300
N/A
(Former name or former address, if changed
since last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered
pursuant to Section 12(b) of the Act:
| Title
of each class |
Trading
Symbol(s) |
Name of each exchange on which
registered |
| Common Stock, $0.01 par value per share |
ELOX |
The Nasdaq Capital Market* |
*As previously reported, effective October 16, 2023, the registrant's common stock is being quoted on the OTC Pink Marketplace under the
symbol “ELOX.” Trading of the registrant's common stock will remain suspended on, but will not be delisted from, the Nasdaq
Capital Market during the pendency of the registrant's appeal from Nasdaq's delisting determination following the registrant's non-compliance
with Nasdaq Listing Rule 5550(b)(2). Nasdaq will not take any action to delist the Company's securities pending a final written decision
by the Nasdaq Listing Council.
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 2.02. |
Results of Operations and Financial Condition. |
On November 13, 2023, Eloxx Pharmaceuticals, Inc.
(the “Company”) issued a press release announcing its financial results for the third fiscal quarter ended September 30, 2023
and providing a business update. A copy of the Company’s press release is furnished as Exhibit 99.1 to this Current Report on Form
8-K and is incorporated herein by reference.
The information in this Current Report on Form
8-K, including the information contained in the press release furnished as Exhibit 99.1, shall not be deemed “filed” for purposes
of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities of that
section, and shall not be deemed incorporated by reference into any of the Company’s filings under the Securities Act of 1933, as
amended or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing,
except as shall be expressly set forth by specific reference in such filing.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| Date: November 13, 2023 |
ELOXX PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Sumit Aggarwal |
| |
Name: |
Sumit Aggarwal |
| |
Title: |
President and Chief Executive Officer |
Exhibit 99.1

Eloxx Pharmaceuticals Reports Third Quarter
2023 Financial and Operating Results and Provides Business Update
Kidney morphology improved in all three patients
with protein re-expression consistent with disease regression in its Phase 2 open-label clinical trial of ELX-02 for the treatment of
Alport Syndrome
ELX-02 improved podocyte foot process effacement
in all three treated patients by an average of 60% based on blinded biopsy analysis by NIPOKA GmbH
Clinical data from Phase 2 study of ELX-02
for Alport Syndrome included in presentations at the American Society of Nephrology (ASN) Kidney Week 2023
Significant progress toward completion of a
strategic partnership for ZKN-013
WATERTOWN, MA – November 13,
2023 – Eloxx Pharmaceuticals, Inc. (OTC: ELOX), a leader in ribosomal RNA-targeted genetic therapies for rare
diseases, today reported its financial results for the three months ended September 30, 2023, and provided a business update.
| · | Completed Phase 2 open label trial of ELX-02 for the treatment of Alport
Syndrome patients with nonsense mutations. |
| o | Analysis of biopsy samples provided unequivocal clinical evidence of both morphology and ELX-02’s disease modifying potential. |
| o | Collagen Alpha 5 protein expression observed in the glomerular basement membrane post treatment in all patients. |
| · | Data from the Phase 2 study of ELX-02 for Alport Syndrome was included in
two presentations at the American Society of Nephrology (ASN) Kidney Week 2023. |
| · | Significant progress towards completing a strategic partnership for ZKN-013 |
| · | Raised additional cash in ongoing efforts to strengthen balance sheet. |
“We are excited about the recent progress across our pipeline
and remain committed to advancing our programs for the benefit of our patients and stakeholders,” said Sumit Aggarwal, President
and Chief Executive Officer of Eloxx.

Third Quarter 2023 and Subsequent Highlights
ELX-02 in Nonsense Mutation Alport Syndrome
| · | Confirmed
that all three patients showed an improvement in morphology and proteinuria in its proof-of-concept
Phase 2 open-label clinical trial (NCT05448755) of ELX-02 for the treatment of Alport
Syndrome after eight weeks of treatment. |
| o | All patients had Autosomal Recessive Alport Syndrome with a nonsense mutation on one allele. No collagen 4 alpha 5 was expressed in
the glomerular basement membrane (GBM) at baseline. |
| o | Collagen 4 Alpha 5 expression in the GBM post-treatment was reported post-treatment based on immunofluorescence staining of patient
biopsies |
| o | ELX-02 increased the filtration slit density (FSD) by an average of 60% as compared to baseline levels, consistent with reduction
of podocyte foot process effacement. |
| § | Biopsies were analyzed on a blinded basis by NIPOKA GmbH. FSD was estimated
for 15 to 20 glomeruli per sample. |
| § | Data supporting FSD as a precise histopathological estimator of podocyte
health and its inverse correlation with proteinuria was presented at the ASN meeting on November 2, 2023. |
| o | Visual assessment of improvement in foot process effacement in Transmission Electron Micrography (TEM) images was independently confirmed
by highly regarded renal pathologist and TEM expert. |
| o | All three treated patients had reduced proteinuria variability and two patients had a reduction in proteinuria compared to baseline
during or in 2 months post treatment consistent with improvement in kidney morphology. |
| o | ELX-02 was well-tolerated in the study with no discontinuations. |
| · | Data from the Phase 2 study of ELX-02 for Alport Syndrome was included in
two presentations at the ASN Kidney Week 2023. |
ZKN-013 in nonsense mutation Recessive Dystrophic Epidermolysis
Bullosa (RDEB), Junctional Epidermolysis Bullosa (JEB) and Familial Adenomatous Polyposis
| · | Eloxx made significant progress in completing a strategic transaction for
ZKN-013. |
| o | Following FDA clearance to begin a single ascending dose trial in healthy volunteers for ZKN-013 for the treatment of recessive RDEB
and JEB, Eloxx received significant strategic interest in ZKN-013. |
| o | Should these discussions lead to a transaction, it will allow Eloxx to remain focused on fully maximizing the potential of ELX-02
in rare kidney diseases and continue funded discovery efforts on our TURBO-ZM platform. |
Third Quarter 2023 Financial Results
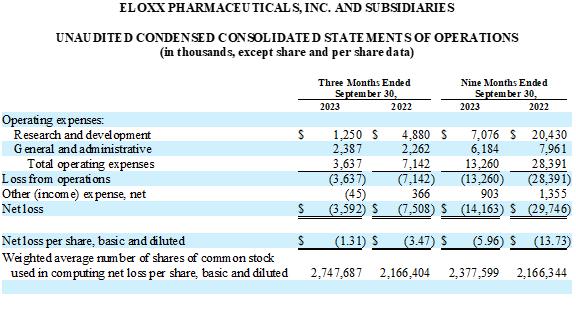
For the three months ended September 30, 2023, we incurred a net
loss of $3.6 million, or $1.31 per share, which included $0.4 million in stock-based compensation. For the same period in the prior year,
we incurred a net loss of $7.5 million, or $3.47 per share, which included $0.7 million in stock-based compensation.

R&D expenses were $1.3 million for the three months ended September 30,
2023, which included $0.1 million in stock-based compensation. For the same period in the prior year, R&D expenses were $4.9 million,
which included $0.3 million of stock-based compensation. The decrease was primarily related to a decrease in expenses related to subcontractors,
advisors, and laboratory supplies in connection with preclinical research and development activities related to inhaled delivery of ELX-02
in CF, a decrease in CFF funded activities, a decrease in salaries and other personnel costs, a decrease related to stock-based compensation,
and a decrease in facility and overhead expenses.
General and administrative (G&A) expenses were $2.4 million for
the three months ended September 30, 2023, which included $0.4 million in stock-based compensation. For the same period in the prior
year, G&A expenses were $2.3 million, which included $0.4 million of stock-based compensation. The increase was primarily related
to a $0.2 million increase in expenses attributable to professional and consulting fees, offset by a decrease of $0.1 million related
to facility and overhead expenses.
As of September 30, 2023, we had unrestricted cash and cash equivalents
of $4.8 million. During the quarter, we raised gross proceeds of $2.0 million from the sale of shares of common stock, pre-funded warrants
and warrants. Additionally, we sold shares of common stock through our ATM program during the quarter for gross proceeds of $1.8 million.
Eloxx remains focused on its liquidity position and raising additional capital in the near term in order to fund its operating plan through
the end of 2023 and beyond. Assuming that we initiate Phase 3 clinical trial activities when sufficient funding allows, and that we maintain compliance with our debt covenants, we believe that our current cash position will
be sufficient to fund our operations through the end of the fourth quarter of 2023.
As previously reported, we received a delisting determination from
the Listing Qualifications Department of the Nasdaq Stock Market LLC as the company did not regain compliance with the Nasdaq Listing
Rule 5550(b)(2), which requires a listed company to have at least $35 million in market value of listed securities. Effective October 16,
2023, trading of our common stock was suspended on the Nasdaq Capital Market and began trading on the OTC Pink Marketplace under the symbol
“ELOX.” On October 26, 2023, we requested a review of the Nasdaq delisting determination by the Nasdaq Listing Council.
Trading of or common stock on the Nasdaq Capital Market will remain suspended pending a decision by the Nasdaq Listing Council.
About Nonsense Mutation Alport Syndrome
Nonsense Mutation Alport syndrome is a rare Type IV Collagenopathy
characterized by mutations in the genes (COL4A3, COL4A4, and COL4A5) that result in a less than full length (truncated) Type 4 Collagen.
This disorder mostly affects children with a median age at diagnosis of 9 to 20 years. It is characterized by rapid and progressive damage
to the kidneys, ear and eyes, starting with worsening of kidney morphology to proteinuria and finally kidney failure, hearing loss and
eye abnormalities. It is estimated that there are approximately 7,500 patients in the US and 20,000 patients in US, Europe, Japan and
China with Nonsense Mutation Alport Syndrome. These patients have no approved treatment options.

About Eloxx Pharmaceuticals
Eloxx Pharmaceuticals, Inc. is
engaged in the science of ribosome modulation, leveraging its innovative TURBO-ZM™ chemistry technology platform in an effort to
develop novel Ribosome Modulating Agents (RMAs) and its library of Eukaryotic Ribosome Selective Glycosides (ERSGs). Eloxx’s lead
investigational product candidate, ELX-02, is a small molecule drug candidate designed to restore production of full-length functional
proteins. ELX-02 is in Phase 2 clinical development for the treatment of Alport syndrome in patients with nonsense mutations. For more
information, please visit www.eloxxpharma.com.
Forward-looking Statements
This press release contains forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of present
and historical facts contained in this press release, including without limitation, statements regarding our cash runway to fund our operating
plan, our plans to raise additional capital, our ability to comply with the covenants in our debt agreement, the expected timing of and
results from trials of our product candidates, the potential of our product candidate to treat nonsense mutations, the outcome of the
Nasdaq Listing Council’s review of the delisting determination; the strategic partnership for the clinical development of
ZKN-013 are forward-looking statements. Forward-looking statements can be identified by the words “aim,” “may,”
“will,” “would,” “should,” “expect,” “explore,” “plan,” “anticipate,”
“could,” “intend,” “target,” “project,” “contemplate,” “believe,”
“estimate,” “predict,” “potential,” “seeks,” or “continue” or the negative
of these terms similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based
on management's current plans, estimates, assumptions and projections based on information currently available to us. Forward-looking
statements are subject to known and unknown risks, uncertainties and assumptions, and actual results or outcomes may differ materially
from those expressed or implied in the forward-looking statements due to various important factors, including, but not limited to: our
ability to obtain the capital necessary to fund our operations; our ability to regain and maintain compliance with the continued listing
requirements of the Nasdaq Capital Market; our ability to progress any product candidates in preclinical or clinical trials; the uncertainty
of clinical trial results and the fact that positive results from preclinical studies are not always indicative of positive clinical results;
the scope, rate and progress of our preclinical studies and clinical trials and other research and development activities; the competition
for patient enrollment from drug candidates in development; the impact of the global COVID-19 pandemic on our clinical trials, operations,
vendors, suppliers, and employees;; the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual
property rights; our ability to obtain financial in the future through product licensing, public or private equity or debt financing or
otherwise;; general business conditions, regulatory environment, competition and market for our products; and business ability and judgment
of personnel, and the availability of qualified personnel and other important factors discussed under the caption “Risk Factors”
in our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2023, as any such factors may be updated from
time to time in our other filings with the SEC, accessible on the SEC’s website at www.sec.gov and the “Financials &
Filings” page of our website at https://investors.eloxxpharma.com/financials-filings.
All forward-looking statements speak only as of the date of this press
release and, except as required by applicable law, we have no obligation to update or revise any forward-looking statements contained
herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Contact
Investors
John Woolford
john.woolford@westwicke.com
443.213.0506
Media
Laureen Cassidy
laureen@outcomescg.com
Source: Eloxx Pharmaceuticals

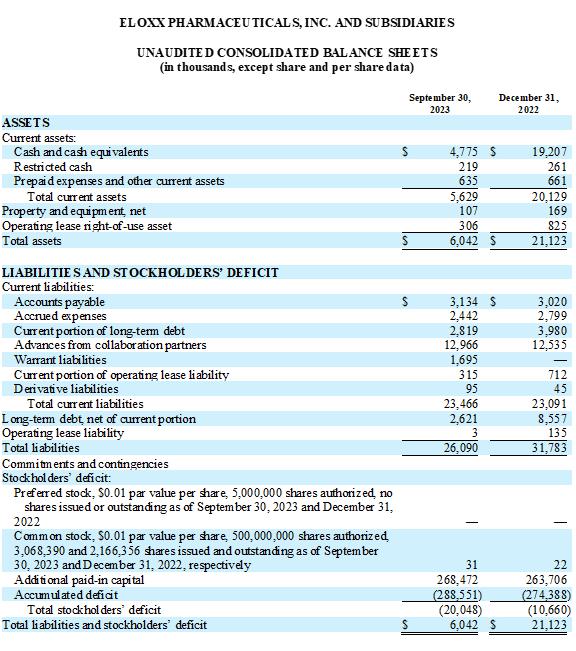

ELOXX PHARMACEUTICALS, INC. AND SUBSIDIARIES
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share and per share data)
| | |
September 30,
2023 | | |
December 31,
2022 | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 4,775 | | |
$ | 19,207 | |
| Restricted cash | |
| 219 | | |
| 261 | |
| Prepaid expenses and other current assets | |
| 635 | | |
| 661 | |
| Total current assets | |
| 5,629 | | |
| 20,129 | |
| Property and equipment, net | |
| 107 | | |
| 169 | |
| Operating lease right-of-use asset | |
| 306 | | |
| 825 | |
| Total assets | |
$ | 6,042 | | |
$ | 21,123 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 3,134 | | |
$ | 3,020 | |
| Accrued expenses | |
| 2,442 | | |
| 2,799 | |
| Current portion of long-term debt | |
| 2,819 | | |
| 3,980 | |
| Advances from collaboration partners | |
| 12,966 | | |
| 12,535 | |
| Warrant liabilities | |
| 1,695 | | |
| — | |
| Current portion of operating lease liability | |
| 315 | | |
| 712 | |
| Derivative liabilities | |
| 95 | | |
| 45 | |
| Total current liabilities | |
| 23,466 | | |
| 23,091 | |
| Long-term debt, net of current portion | |
| 2,621 | | |
| 8,557 | |
| Operating lease liability | |
| 3 | | |
| 135 | |
| Total liabilities | |
| 26,090 | | |
| 31,783 | |
| Total stockholders’ deficit: | |
| (20,048 | ) | |
| (10,660 | ) |
| Total liabilities and stockholders’ deficit | |
$ | 6,042 | | |
$ | 21,123 | |
ELOXX PHARMACEUTICALS, INC. AND SUBSIDIARIES
UNAUDITED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share data)
| | |
Three Months Ended
September 30, | | |
Nine Months Ended
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Research and development | |
$ | 1,250 | | |
$ | 4,880 | | |
$ | 7,076 | | |
$ | 20,430 | |
| General and administrative | |
| 2,387 | | |
| 2,262 | | |
| 6,184 | | |
| 7,961 | |
| Total operating expenses | |
| 3,637 | | |
| 7,142 | | |
| 13,260 | | |
| 28,391 | |
| Loss from operations | |
| (3,637 | ) | |
| (7,142 | ) | |
| (13,260 | ) | |
| (28,391 | ) |
| Other (income) expense, net | |
| (45 | ) | |
| 366 | | |
| 903 | | |
| 1,355 | |
| Net loss | |
$ | (3,592 | ) | |
$ | (7,508 | ) | |
$ | (14,163 | ) | |
$ | (29,746 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per share, basic and diluted | |
$ | (1.31 | ) | |
$ | (3.47 | ) | |
$ | (5.96 | ) | |
$ | (13.73 | ) |
| Weighted
average number of shares of common stock used in computing net loss per share, basic and diluted | |
| 2,747,687 | | |
| 2,166,404 | | |
| 2,377,599 | | |
| 2,166,344 | |
v3.23.3
Cover
|
Nov. 13, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Nov. 13, 2023
|
| Entity File Number |
001-31326
|
| Entity Registrant Name |
Eloxx Pharmaceuticals, Inc.
|
| Entity Central Index Key |
0001035354
|
| Entity Tax Identification Number |
84-1368850
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
480
Arsenal Way, Suite 130
|
| Entity Address, City or Town |
Watertown
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02451
|
| City Area Code |
781
|
| Local Phone Number |
577-5300
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.01 par value per share
|
| Trading Symbol |
ELOX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Eloxx Pharmaceuticals (PK) (USOTC:ELOX)
Historical Stock Chart
From Apr 2024 to May 2024

Eloxx Pharmaceuticals (PK) (USOTC:ELOX)
Historical Stock Chart
From May 2023 to May 2024
