Table of Contents
Registration No. 333-267039
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________
AMENDMENT NO. 6
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Cannabis Bioscience
International Holdings, Inc.
(formerly named China Infrastructure Construction
Corp.)
(Exact name of Registrant
as specified in its charter)
____________________
| Colorado |
8999; 8099 |
84-4901229 |
| (State or other jurisdiction of |
Primary Standard |
(I.R.S. Employer |
| Incorporation or organization) |
Industrial Classification |
Identification No.) |
| |
Code Numbers |
|
____________________
6201 Bonhomme Road, Suite 466S,
Houston, TX 77036
Telephone: (817) 528-2475
(Address, including zip
code and telephone number,
including area code, of Registrant’s principal executive offices)
____________________
Dante Picazo
Chief Executive Officer
Cannabis Bioscience International Holdings,
Inc.
6201 Bonhomme Road, Suite 466S
Houston, TX 91789
Telephone: (817) 528-2475
(Name, address, including zip code, and telephone
number,
including area code, of agent for service)
____________________
Copy to:
Barry J. Miller, Esq.
Barry J. Miller PLLC
7146 Pebble Park Drive
West Bloomfield, MI 48322
Telephone: (248) 232-8039
Fax: (248) 246-9524
____________________
Approximate date of commencement of proposed sale to the public:
As soon as practicable following the effective
date of this registration statement is declared effective by the Registrant and from time to time thereafter, as determined by the Selling
Stockholders.
If any of the securities being registered on
this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following
box: ☒
If this form is filed to register additional
securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
___________________
Indicate by check mark whether the Registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”
and “emerging growth company” in Rule 12b-2 of the Exchange Act. (check one)
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
| |
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check
mark if the Registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
___________________
The Registrant hereby amends this registration
statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which
specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities
Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting
pursuant to said Section 8(a), may determine.
The information in this prospectus
is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange
Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities
in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated November
13, 2023.
PROSPECTUS
Cannabis
Bioscience International Holdings, Inc.
10,087,154,885 SHARES OF COMMON
STOCK
This
Prospectus relates to the offer and sale of up to 10,087,154,885 shares of the common
stock, without par value (“Common Stock”), of Cannabis Bioscience International
Holdings, Inc., a Colorado corporation (the “Shares”), of which 6,250,000,000
shares are offered by the Company and 3,837,154,885 shares are offered by the Selling
Stockholders. The Company will receive the proceeds of sales of the shares that it sells,
but none of the proceeds of the sales of the shares that are sold by the Selling Stockholders.
The Company is offering the shares to be sold by it at an aggregate offering price of $5,000,000.
An investment
in Common Stock is speculative and involves a high degree of risk. Therefore, before purchasing Common Stock, investors should carefully
consider the risk factors and other uncertainties described in this Prospectus. See “Risk Factors.”
We are
an “emerging growth company” as defined under the U.S. federal securities laws and, as such, may elect to comply with reduced
public company reporting requirements for this Prospectus and future filings. See “Prospectus Summary – Implications
of Being an Emerging Growth Company.”
The Common Stock is quoted on the OTC Pink tier
of the alternate trading system operated by OTC Markets Group Inc. (“OTC”) under the trading symbol CBIH.
The Company and the Selling Stockholders will
offer their shares at $0.0008 per share (the “Fixed Offering Price”). See “Plan of Distribution”
for further information. The Selling Stockholders may sell any, all or none of their shares and the Company does not know when, in what
amounts or in what manner they may sell their shares.
Any investment in the shares offered herein
involves a high degree of risk. You should only purchase shares if you can afford a loss of your investment. Our independent
registered public accounting firm has issued an audit opinion for the Company’s consolidated financial statements for the
year ended May 31, 2022, that includes a statement expressing substantial doubt as to our ability to continue as a going concern.
On December 6, 2022, the Company changed its
corporate name from China Infrastructure Construction Corp. to Cannabis Bioscience International Holdings, Inc. On August 16, 2023,
the Financial Industry Regulatory Authority published the name change and a new trading symbol, CBIH.
Dante Picazo, the Company’s chief executive
officer and one of its directors, has voting control of the Company, with power to elect and remove its directors, and will continue
to have such control after the offering.
The Selling Stockholders and any broker-dealers
or agents involved in selling the Shares may be deemed to be underwriters within the meaning of the Securities Act of 1933, as amended
(the “Securities Act”), in connection with such sales. In such event, any commissions received by such broker-dealers or
agents and any profit on the resale of the Shares purchased by them may be deemed to be underwriting commissions or discounts under the
Securities Act.
The Selling
Stockholders and any other person participating in the sale of the Shares will be subject to the provisions of the Securities Exchange
Act of 1934 (the “Exchange Act”) and the rules and regulations promulgated thereunder. These rules include, without limitation,
Regulation M, which may limit the timing of purchases and sales of any of the Shares by the Selling Stockholders and any other person.
In addition, Regulation M may restrict the ability of any person engaged in the distribution of the Shares to engage in market-making
activities with respect to the particular shares being distributed, which may affect the marketability of the Shares and the ability
of any person or entity to engage in market-making activities with respect to the Shares.
Once sold
under the registration statement of which this Prospectus forms a part, the Shares will be freely tradeable in the hands of persons other
than our affiliates.
We have
paid and will pay all expenses incurred in registering the shares, whether offered by the Company or the Selling Stockholders, including
legal and accounting fees. See “Plan of Distribution.” For information regarding expenses of registration,
see “Use of Proceeds and Business Plan”
The Jumpstart
Our Business Startups Act, or the JOBS Act, was enacted in April 2012 to encourage capital formation in the United States and reduce
the regulatory burden on new-public companies that qualify as “emerging growth companies.” We are an “emerging growth
company” within the meaning of the JOBS Act. As an “emerging growth company,” we intend to take advantage of certain
exemptions from various public reporting requirements, including the requirement that our internal control over financial reporting be
audited by our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley
Act, certain requirements related to the disclosure of executive compensation in this Prospectus and our periodic reports and proxy statements,
and the requirement that we hold a non-binding advisory vote on executive compensation and any golden parachute payments. We may take
advantage of these exemptions until we are no longer an “emerging growth company.”
NEITHER THE SECURITIES AND EXCHANGE COMMISSION
NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE.
ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this Prospectus is November __,
2023.
TABLE OF CONTENTS
Through and including December__, 2023
(the 25th day after the date of this Prospectus), all dealers effecting transactions in these securities, whether or not participating
in this offering, may be required to deliver a prospectus, in addition to a dealer’s obligation to deliver a prospectus when acting
as an underwriter and with respect to an unsold allotment or subscription.
This Prospectus forms a part of a registration
statement on Form S-1 that we filed with the SEC. Under this registration statement, the Selling Stockholders may, from time to time,
sell their shares, as described in this Prospectus. We will not receive any proceeds from the sale of the Shares by any such Selling
Stockholders. See “Use of Proceeds and Business Plan.”
Neither we nor the Selling Stockholders have
authorized anyone to provide any information or make any representations other than those contained in this Prospectus or any free writing
prospectuses we have prepared. Neither we nor the Selling Stockholders take responsibility for and cannot assure as to the reliability
of any information that others may give you, other than the information contained in this Prospectus. This Prospectus is an offer
to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful. The information contained
in this Prospectus is current only as of its date, regardless of the time of delivery of this Prospectus or any sale of Common Stock.
For investors outside the United States: Neither
we nor the Selling Stockholders have taken any action that would permit this offering or possession or distribution of this Prospectus
in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who
come into possession of this Prospectus must inform themselves about and observe any restrictions relating to the offering of the shares
of Common Stock and the distribution of this Prospectus outside the United States.
PROSPECTUS SUMMARY
This summary highlights information contained
elsewhere in this Prospectus. Because this is only a summary, it does not contain all information that may be important to you. You should
read the entire Prospectus and should consider, among other things, the matters set forth under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the notes
thereto appearing elsewhere in this Prospectus before making an investment decision. This Prospectus contains forward-looking statements
and information relating to the Company. See “Cautionary Notes.”
The Company is based in Houston, Texas, and was
established in 2003. For more detailed information respecting its corporate history, see “Description of Business
– History.” The address of our principal executive office is 6201 Bonhomme Road, Suite 466S, Houston, TX 77036, and our
telephone number is (832) 606-7500. Its website is www.chnc-hdh.com. The information contained thereon is not intended to be incorporated
into this Prospectus or the registration statement of which it is a part.
We provide educational and other services to
the cannabis industry (the “Pharmacology University Business”) (see “Description of Business –
Business – Pharmacology University Business”) and clinical trial services to Sponsors and CROs and clinical trials
that we conduct as a Sponsor (the “Alpha Research Business”) (see “Description of Business –
Business – Alpha Research Business”) “Sponsor” means a person who takes responsibility for and initiates
a clinical investigation of a drug or medical device, including an individual or pharmaceutical company, governmental agency,
academic institution, private organization, or other organization. “CRO” means a person that assumes, as an independent
contractor with a Sponsor, one or more of the obligations of a Sponsor, such as the design of a protocol, selection or monitoring of
investigations, evaluation of reports, and preparation of materials to be submitted to the U.S. Food and Drug Administration (the
“FDA”).
Implications of Being an Emerging Growth Company
We are an “emerging growth company”
as defined in the Jumpstart Our Business Startups Act (known as the “JOBS Act”). Under the JOBS Act, we may utilize reduced
reporting requirements that are otherwise applicable to public companies, including delaying auditor attestation of internal control
over financial reporting, providing only two years of audited financial statements and related Management’s Discussion and Analysis
of Financial Condition and Results of Operations in this Prospectus and the reports that we will file with the U.S. Securities and Exchange
Commission (the “SEC”), including reduced executive compensation disclosures.
We are permitted to remain an emerging growth
company for up to five years from the date of the first sale in this offering. However, if certain events occur before the end of that
period, including our becoming a “large accelerated filer,” our annual gross revenue’s exceeding $1.07 billion or our
issuance of more than $1.0 billion of nonconvertible debt in any three-year period, we will cease to be an emerging growth company.
We have elected to take advantage of certain
of the reduced disclosure obligations in this Prospectus and the registration statement of which it is a part and may elect to take advantage
of other reduced reporting requirements in future filings. In particular, in this Prospectus, we have provided only two years of audited
financial statements and have not included all of the information relating to executive compensation that would be required if we were
not an emerging growth company. As a result, the information that we provide to our stockholders may be different from that which might
be received from public reporting companies that are not emerging growth companies. We have irrevocably elected to avail ourselves of
the extended transition period for complying with new or revised accounting standards and therefore, we will be subject to the same new
or revised accounting standards as private companies.
Recent Developments
The COVID-19 pandemic has harmed the Company.
Early in 2020, the COVID-19 pandemic resulted
in decreased business activity and restrictions on the conduct of businesses, including mandatory lockdowns. Because of these restrictions,
all our classrooms and public venues were closed and other Pharmacology University Business activities that required face-to-face contact,
such as its consulting services and franchising and marketing efforts, were sharply reduced or terminated. Among other things, the Pharmacology
University Business closed its seminars in Ecuador and the Dominican Republic; ceased holding classes at the University of Tadeo in Bogota,
Cartagena and Santa Marta, Colombia; and ceased all travel. The business conducted by the Alpha Research Business has also been adversely
affected because several of the clinical studies in which it was participating were deferred, shortened or canceled. These restrictions
have been reduced or eliminated in many jurisdictions, but if the pandemic resurges, they could be reimposed. We have not been able to
resume classroom teaching and seminars, consulting services, franchising and marketing efforts and the Alpha Research Business has continued
to be adversely impacted.
As a result of the pandemic, we experienced substantial
reductions in our revenues and our losses increased in our educational and clinical trial businesses. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Impact of the COVID-19 Pandemic.” To protect
our business from disruption caused by the COVID-19 pandemic and to enable our students to continue to be educated, we created online
courses. We currently have over 100 online videos in English, Spanish, Portuguese, Italian and Arabic. We also commenced the use of Zoom
meetings to hold virtual classes to teach students and be able to respond to their questions in real time. We believe that these measures
have helped us to manage our business prudently during the pandemic; nevertheless, much of our business depends on personal contacts,
and we have not been able to reduce the adverse effects of the pandemic’s reducing or eliminating personal contact.
Risk Factors Summary
Our business is subject to many risks and uncertainties
of which you should be aware before deciding whether to invest in Common Stock, in addition to general business risks. These risks are
more fully described in the section titled “Risk Factors” immediately following this Prospectus Summary. These risks include,
among others, the following:
| |
· |
The COVID-19 pandemic and the impact of actions to mitigate the COVID-19 pandemic have materially and adversely impacted and will continue materially and adversely to impact our business, results of operations and financial condition. In particular, our revenues have decreased and our losses have increased, in each case materially, since the onset of the pandemic. |
| |
|
|
| |
· |
The Company expects to encounter significant challenges in recovering from the adverse effects of the COVID-19 pandemic and can give no assurances respecting its success in meeting them. |
| |
|
|
| |
· |
The Company has incurred net losses each year since its inception
and may not be able to achieve profitability. It has incurred net losses of 1,032,579, $885,171 and $159,308 for the fiscal years ended
May 31, 2023, May 31, 2022, and May 31, 2021, respectively. Its accumulated deficit for the fiscal years ended May 31, 2023, May 31,
2022, and May 31, 2021, were 4,682,736, $3,650,156 and $2,764,985, respectively. |
| |
|
|
| |
· |
The Alpha Research Business is conducted in a highly competitive industry and may not be able to compete successfully with its current or future competitors. |
| |
|
|
| |
· |
Both the Pharmacology University Business and the Alpha Research Business are subject to a wide variety of complex, evolving, and, with respect to the Pharmacology University Business, sometimes inconsistent and ambiguous laws and regulations that may adversely impact their operations and could cause the Company to incur significant liabilities including fines and criminal penalties, which could have a material adverse effect on its business, results of operations, and financial condition. |
| |
|
|
| |
· |
The Alpha Research Business is conducted in a highly competitive industry and may not be able to compete successfully with its current or future competitors. |
| |
|
|
| |
· |
Following the Offering, there will be a large number of shares of Common Stock that may be sold in the public markets, which may substantially and adversely affect their market price. For further information, see “Risk Factors – Risks Related to the Common Stock and the Offering – There will be a larger number of shares of Common Stock that will be eligible to be sold in the public markets” and “Shares Eligible for Future Sale.” |
| |
|
|
| |
· |
The Company may not be able to sell all of the Shares at the Fixed Offering Price. See “Risk Factors – Risks Related to the Common Stock and the Offering – We may change the Fixed Offering Price. |
THE
OFFERING
| Amount of Offering by the Company: |
|
$5,000,000 |
| |
|
|
| Offering Price per Share: |
|
The shares offered by the Company will be sold at the Fixed Offering Price of $0.0008
per share for the duration of the offering (the “Fixed Offering Price”). The Selling Stockholders may offer their shares in different ways and at varying prices. See “Plan of Distribution.” |
| |
|
|
| Shares of Common Stock offered by the Company: |
|
6,250,000,000 shares |
| |
|
|
Shares
of Common Stock offered by the Selling Stockholders:
|
|
3,837,154,885 shares |
| |
|
|
Shares
of Common Stock outstanding prior to the Offering:
|
|
10,331,749,347 shares |
| |
|
|
Shares
of Common Stock outstanding after the Offering:
|
|
16,581,749,347 shares |
| |
|
|
| |
|
The number of shares of Common Stock to be outstanding after the Offering is based on 10,331,749,347
shares of Common Stock outstanding as of the date of this Prospectus. |
| |
|
|
| Voting rights: |
|
Each share of Common Stock and Series A Preferred is entitled to one vote per share. The Series B
Preferred has 60% of the voting power in the Company and all of the outstanding shares are held by the Company’s chief
executive officer, who is also a director. By virtue of his holdings of Series B Preferred, he has the power to control the outcome
of all matters submitted to stockholders for approval, including the election of directors and the approval of any change-of-control
transaction. See “Description of Capital Stock.” |
| |
|
|
| Use of Proceeds: |
|
The proceeds that we receive from sales of the shares offered by the Company will be used for the
purposes set forth under “Use of Proceeds and Business Plan.”. We will not receive any proceeds from the
sale of the Shares offered by the Selling Stockholders. |
| |
|
|
| Trading symbol: |
|
CBIH |
| |
|
|
| Risk Factors: |
|
An investment in Common Stock is highly speculative and involves a high degree of risk for the reasons
set forth in “Risk Factors” and elsewhere in this Prospectus. |
| |
|
|
| Fees and Expenses: |
|
We will pay all expenses incident to the registration of the shares offered by this Prospectus, except
for sales commissions and other expenses of the Selling Stockholders. |
RISK FACTORS
An investment in
Common Stock involves a high degree of risk. Prospective investors should carefully consider the risks described below and all of the
other information contained in this Prospectus, including the Company’s consolidated financial statements and the related notes
and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” before deciding whether
to invest in Common Stock. If any of the events described below occur, the Company’s business, business prospects, cash flow, results
of operations or financial condition could be materially and adversely harmed. In these events, the trading price of the Common Stock
could decline, and investors might lose all or part of their investments. Investors should read the section entitled “Forward-Looking Statements” for a discussion of what types of statements are forward-looking, as well as the significance of such statements
in the context of this Prospectus.
The following is a discussion of the risk
factors that the Company believes are currently material. These risks and uncertainties are not the only ones facing the Company,
and in addition to general business risks, there may be other matters of which the Company is not aware or that it currently considers
immaterial. All of these could adversely affect the Company’s business, business prospects, cash flow, results of operations
or financial condition.
Business-Related Risks
The COVID-19 pandemic
and the efforts to mitigate its impact may have an adverse effect on the Company’s business, liquidity, results of operations,
financial condition and price of its securities.
The COVID-19 pandemic has materially and adversely
impacted the Company and its results of operations, particularly as a result of limitations on the ability of the Pharmacology University
Business to conduct classes and other face-to-face activities due to lockdowns. Public health authorities and governments at local, national
and international levels have from time to time announced various measures of varying intensity to respond to this pandemic. Some measures
that have directly or indirectly impacted the Company’s business include voluntary or mandatory quarantines and business closures,
restrictions on travel and limiting gatherings of people in public places.
For detailed information respecting the impact
of the pandemic on the Company’s financial results, see “Management’s Discussion and Analysis of Financial
Condition and Results of Operations – Impact of the COVID-19 Pandemic.”
Although many of the measures introduced to combat
the COVID-19 pandemic have been relaxed and in some cases terminated, the Company does not know when it will be able to resume its normal
operations, particularly in the classroom, franchising and consulting activities of the Pharmacology University Business. However, we
expect that returning to normal operations will require time, will involve substantial costs and will involve uncertainties, including
(i) whether the pandemic will continue to abate, (ii) what measures governments will take if the pandemic intensifies and (iii) the ability
of our customers and suppliers to recover from the effects of the pandemic.
To the extent the pandemic has and may continue
to affect the Company’s business and financial results adversely, it may also have the effect of heightening many of the other
risks to which the Company is subject, whether or not described under “Risk Factors.” If the pandemic does
not continue to abate or it intensifies, the Company’s ability to execute its business plan on a timely basis or at all may be
materially impeded.
We have a limited operating history, making
it difficult to forecast our revenue and evaluate our business and prospects.
We have a limited operating history and as a
result, our ability to forecast our future results of operations and plan for growth is limited and subject to many uncertainties. We
have encountered and expect to continue to encounter risks and uncertainties frequently experienced by growing companies in rapidly evolving
industries, such as the risks and uncertainties described herein. Accordingly, we may be unable to prepare accurate internal financial
forecasts or replace anticipated revenue that we do not receive as a result of delays arising from these factors, and our results of
operations in future reporting periods may be below the expectations of investors. If we do not address these risks successfully, our
results of operations could differ materially from our estimates and forecasts or the expectations of investors, causing our business
to suffer and the market price of the Common Stock to decline.
We
have a history of net losses, we anticipate increasing operating expenses in the future, and we may not be able to achieve and, if achieved,
maintain profitability.
We have incurred significant net losses each
year since our inception (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations”).
We expect to continue to incur net losses for the foreseeable future and we may not achieve or maintain profitability in the future.
It is difficult for us to predict our future results of operations or the limits of our market opportunity. We expect our operating expenses
to significantly increase over the next several years as we hire additional personnel, particularly in sales and marketing, and expand
our operations, both domestically and internationally. We may also selectively pursue acquisitions. In addition, because we will become
subject to the reporting and other requirements of the Exchange Act as a result of the effectiveness of the registration statement of
which this Prospectus is a part, we will incur additional significant legal, accounting, and other expenses that we did not incur previously.
If our revenue does not increase to offset the expected increases in our operating expenses, we will not become profitable. Our growth
could be impeded for many reasons, including, but not limited to, those set forth under “Risk Factors.” Our failure to sustain
consistent profitability could cause the market price of the Common Stock to decline.
The Company requires substantial additional
capital. If the Company cannot raise capital, it may have to curtail its operations or it could fail.
The Company requires substantial additional capital through public
or private debt or equity financings to continue operating, as well as to fund its operating losses, increase its sales and marketing
capacity, take advantage of opportunities for internal expansion or acquisitions, hire, train and retain employees, develop and complete
existing services and new services and products and respond to economic and competitive pressures. The Company needs $5,000,000 to execute
its business plan and meet its other corporate expenses, some or all of which may be provided from the sale of the Shares. If it cannot
raise such capital, it may have to alter its business plan or curtail its operations, or it could fail. The financial condition of the
Company presents a material risk to investors and may make it difficult to attract additional capital or adversely affect the terms on
which the Company can obtain it. For further information, see “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – General Statement of Business – Going Concern”
and “– Liquidity and Capital Resources.”
The Company has received no commitment for financing
from investors or banks and no assurance can be given that any such commitment will be forthcoming or, if so, in what amount and on what
terms.
The preceding risk factors raise substantial
doubt about our ability to continue as a going concern and our independent registered public accounting firm has included an explanatory
paragraph relating to our ability to continue as a going concern in its report on our audited consolidated financial statements contained
in this Prospectus.
The consolidated financial statements contained
in the Prospectus were prepared on the assumption that we will continue as a going concern. Accordingly, the accompanying financial statements
do not include any adjustments that might be necessary should we be unable to continue as a going concern. We do not have adequate funds
available, and the Offering may not provide sufficient proceeds to fund our anticipated expenses without obtaining significant additional
financing. This raises substantial doubt about our ability to continue as a going concern. The perception that we may not be able to
continue as a going concern may materially limit our ability to raise additional funds through the issuance of new debt or equity securities
or otherwise and no assurance can be given that sufficient funding will be available when needed to allow us to continue as a going concern.
This perception may also make it more difficult to operate our business due to concerns about our ability to meet our contractual obligations.
Our ability to continue as a going concern is contingent upon, among other factors, our ability to sell shares of Common Stock, including
those that we are offering by this Prospectus, and obtaining additional capital. We cannot provide any assurance that we will be able
to raise additional capital. If we cannot secure additional capital, we may be required to curtail our operations and take measures to
reduce costs to conserve cash in amounts sufficient to sustain operations and meet our obligations. These measures could cause significant
delays in the realization of our business plan. It is not presently possible for us to predict the potential success of our business
plan. We cannot predict the revenue or the income potential of our proposed businesses and operations. If we cannot operate as a viable
entity, you may lose some or all of your investment.
In
addition, the report of our independent registered public accounting firm with respect to our consolidated financial statements appearing
elsewhere in this Prospectus contains an explanatory paragraph stating that the Company had negative working capital at May 31, 2021,
had incurred recurring losses and recurring negative cash flow from operating activities, and had an accumulated deficit, raising substantial
doubt about its ability to continue as a going concern. For information about Management’s evaluation of and plans regarding these
matters, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources” and Note 3 to its consolidated financial statements.
Delays in payments by Sponsors and CROs
have affected and may continue to affect the Company’s cash flows.
The Company has from time to time been affected
by delays in payments by Sponsors and CROs. More specifically, at May 31, 2023, and May 31, 2022, the Company had accounts receivable
from Sponsors and CROs of $10,549 and $5,614, respectively, of which $2,500 and $470, respectively, were not paid timely; all of these
accounts payable have been collected. At May 31, 2023, the balance of accounts receivable was 3.3% of the revenue of $316,825 for that
fiscal year and at May 31, 2022, the balance of accounts receivable was 2.6% of the revenue of $214,980 for that fiscal year. However,
at August 31, 2022, accounts receivable were $43,307, which was 31.3% of the revenue of $51,251 for the three months then ended due to
delays in payment; the Company has collected all of these receivables.
Although the Company was able to cope with
this delay, it may not be able to do so in the case of future delays. If such a delay were to recur, the proceeds of the Offering were
insufficient and other available resources were insufficient to pay its obligations, the Company would have to sell shares of Common
Stock, probably at below-market prices and/or borrow money at rates of interest that could be high. If the Company were unable to sell
shares or borrow money, it might have to alter its business plan or curtail its operations, or it could fail.
We May be Affected by Inflation.
Inflation rates have increased and may
continue to rise. Companies from which we purchase goods and services may raise their prices and we may be unable to pass these
increases on to our customers. Although we have not yet been affected, inflation could adversely affect our business,
including our competitive position, market share, revenues and operating income.
We May be Affected by Increasing
Interest Rates.
Interest rates have recently risen, but we
have not been materially affected. However, we cannot assure that rising interest rates will not affect us in the future, thereby reducing
our ability to borrow or increasing our expenses if we were to borrow, either of which would adversely affect our business plans and
growth, increase the cost of our borrowings and reduce our earnings.
Because the Pharmacology University Business
deals with persons that operate in the cannabis industry, it faces unique, unpredictable and evolving risks.
The Company does not grow or sell cannabis
or manufacture or sell cannabis-related paraphernalia and believes that it is not directly subject to the risks that may arise from violation
of the federal, state and foreign laws relating thereto. However, some of the Company’s customers and potential customers engage
in one or more of these activities, and are subject to these risks, the eventuation of which may adversely affect demand for the services
and products offered by the Pharmacology University Business and the Company’s ability to collect receivables from its customers.
Specific risks faced by these customers and potential customers include the following:
Cannabis is illegal
under federal law, the laws of certain states and certain foreign countries.
Cannabis is illegal under
federal law, as is growing, cultivating, selling or possessing it for any purpose or assisting or conspiring with those who do so. Additionally,
it is unlawful to knowingly open, lease, rent, use, or maintain any place, whether permanently or temporarily, for the purpose of manufacturing,
distributing, or using cannabis. The laws of some states and foreign countries in which the Company operates prohibit one or more of
these activities. Even in states in which the use of cannabis has been legalized, its use, growth, cultivation, sale and possession remain
violations of federal law, because federal law preempts state laws. Strict enforcement of these federal, state or foreign laws would
likely result in the inability of some or all of the Company’s U.S. customers and potential customers to operate, which could adversely
affect demand for the services offered by the Pharmacology University Business and the Company’s ability to collect receivables.
In particular, some of
the Company’s customers may be directly affected by these laws and as a result, the Company may be indirectly affected by the cannabis-related
laws of other jurisdictions in which it operates. These jurisdictions and summaries of the relevant laws are as follows:
| |
· |
Texas. Possession of (i) up to two ounces of cannabis
is a Class B misdemeanor, punishable by up to 180 days in jail and a fine of up to $2,000, (ii) from two up to four ounces is a Class
A misdemeanor, punishable by imprisonment of up to one year and a fine of up to $4,000, (iii) from four ounces up to five pounds
is a felony, punishable by imprisonment of up to two years and a fine of up to $10,000; (iv) from five pounds up to 50 pounds is
a felony, punishable by imprisonment of up to 10 years and a fine of up to $10,000; (v) from 50 pounds up to 2,000 pounds is a felony,
punishable by imprisonment of up to 20 years and a fine of up to $10,000; and more than 2,000 pounds is a felony, punishable by imprisonment
of up to 99 years and a fine of up to $50,000. The Texas Compassionate Use Act (TBCUA), passed in 2015, permits the use of low-THC
cannabis oil, containing no more than 0.5% THC and at least 10% CBD, for individuals diagnosed with intractable epilepsy, but this
legislation limits access to a small number of patients and requires physicians to register with the Texas Department of Public Safety
to prescribe the oil. |
| |
|
|
| |
· |
Colombia. In 2015, the Colombian Constitutional Court
ruled that personal possession and consumption of drugs, including cannabis, could not be criminal offenses. In 2016, legalized medical
cannabis and established a framework for its cultivation, production and export. In 2017, Colombia decriminalized the possession
of up to 20 grams of cannabis for personal use. Possession of more than 20 grams and any activity regarded as commercial cultivation,
sale, or export is punishable by imprisonment. |
| |
|
|
| |
· |
Mexico. The production, sale, and use of cannabis
for recreational and medical purposes is lawful. Possession of more than 28 grams up to 200 grams is subject to a fine of $500 and
possession of more than 200 grams is subject to imprisonment of up to six years. |
| |
|
|
| |
· |
Jordan. Recreational and medical use of cannabis is illegal.
Possession of cannabis is punishable by imprisonment, except that first-time offenders are placed in rehabilitation. The growth and
sale of cannabis are punishable by imprisonment or, in the case of the latter, death. |
| |
|
|
| |
· |
Ecuador. Cannabis is illegal in Ecuador for both medical
and recreational use. Possession, cultivation, sale, and trafficking are strictly prohibited, and persons violating these laws are
subject to imprisonment, except that the possession of up to 10 grams for personal use is legal, as is medical use. Cultivation and
sale are illegal. |
| |
|
|
| |
· |
Venezuela. The cultivation, sale, and possession of
cannabis for recreational purposes are illegal in Venezuela. However, the possession for personal use of up to 20 grams of cannabis
and 5 grams of genetically modified cannabis have been decriminalized. |
| |
|
|
| |
· |
Argentina. The cultivation, sale, and possession of
cannabis for recreational purposes are illegal and are punishable by imprisonment ranging from 4 to 15 years; personal recreational
use is lawful, as is medical use. |
| |
|
|
| |
· |
Brazil. Cannabis is illegal, except for possession
and cultivation of personal amounts and for private use and use of cannabis medications is allowed for terminally ill patients or
those who have exhausted other treatment options. |
| |
|
|
| |
· |
Canada. Canada permits the possession of cannabis
and the growth of cannabis in limited amounts. Medical cannabis is lawful for persons who have received authorization from their
healthcare providers. Penalties for possession over the legal limit, illegal distribution or sale, illegal production of cannabis,
illegal exportation and other cannabis-related offenses can be as high as imprisonment for 14 years. |
The Company does not
believe that it and its personnel are subject to direct risk under these laws, inasmuch as its operations in these jurisdictions involve
teaching, writing, editing, translating and other activities that do not involve the production, sale, use, cultivation or export of
cannabis. However, customers of the Company to whom it extends credit or who are required to make periodic payments, such as persons
to whom the Company provides consulting services or the Company’s franchises, could be directly affected by these laws, with the
result that their ability to pay amounts that they owe to the Company timely or all could be impaired or negated.
The Company may face
risks in the above foreign jurisdictions and others in which it may in the future have operations because of its lack of familiarity
and burdens of complying with foreign laws, legal standards and regulatory requirements relating to cannabis. See “Risk Factors
– Because our success depends in part on our ability to expand our operations outside the United States, our business will be susceptible
to risks associated with international operations” for information as to general risks that that the Company may encounter in conducting
foreign operations.
Uncertainties exist
respecting enforcement.
The enforcement of federal
laws relating to cannabis has varied and may continue to vary in intensity. Some administrations have indicated that they intend to enforce
such laws vigorously, while others have deprioritized enforcement to varying degrees, based, for example, on whether the laws of a state
in which an offense occurred have legalized cannabis or whether the offense relates to the recreational of medical use of cannabis. The
Company believes that the Department of Justice (the “DOJ”) under the Biden administration is not prioritizing enforcement
of the CSA, but the extent to which the DOJ will seek to enforce the CSA under a future administration and against whom enforcement will
be sought is unclear.
Since 2014, it has been
the policy of the Department of the Treasury to deprioritize enforcement of the Bank Secrecy Act against financial institutions and marijuana-related
businesses which utilize them. If the Department of the Treasury were to change this policy, it would be more difficult for our clients
and potential clients to access the U.S. banking systems and conduct financial transactions, which could in turn adversely affect our
operations.
Since 2014, it has been the
policy of the Department of the Treasury to deprioritize enforcement of the Bank Secrecy Act against financial institutions and marijuana-related
businesses that utilize them. If the Department of the Treasury were to change this policy, it would be more difficult for our clients
and potential clients to access the U.S. banking systems and conduct financial transactions, which could, in turn, adversely affect our
operations.
The Company cannot predict
the vigor with which state and foreign laws relating to cannabis are or may be enforced, but to the degree that they are enforced, its
customers and potential customers could be adversely affected, which in turn, could adversely affect demand for the Company’s services
and its ability to collect receivables.
On December 2, 2022,
the Medical Marijuana and Cannabidiol Research Expansion Act, which established a new, separate registration process to facilitate research
on marijuana, became law.
Recently, Congress has
considered several bills relating to cannabis:
| · | The
Marijuana Opportunity Reinvestment and Expungement Act (known as the “MORE Act”)
would among other things decriminalize cannabis by removing it from the list of scheduled
substances under the Controlled Substances Act, was passed by the House of Representatives
on in 2021 and 2022, , but was not taken up by the Senate. |
| | | |
| · | The
Cannabis Administration and Opportunity Act, which would decriminalize cannabis at the federal
level and expunge federal cannabis-related criminal records, was introduced in the Senate
in 2021 and 2022, but has not been acted on. |
| | | |
| · | The
Secure and Fair Enforcement (SAFE) Banking Act, which generally prohibits a federal banking
regulator from penalizing a depository institution for providing banking services to a legitimate
cannabis-related business, was passed by the House of Representatives on several occasions,
most recently in 2023, but has never been acted upon by the Senate. |
The Company believes that
these bills or similar legislation will be reintroduced in the Congress in the near future, but cannot predict whether any of
them will become law.
The Company cannot foresee
developments relating to the above matters and cannot predict how and the extent to which we could be affected by them; however, these
effects could be sudden and adverse.
The Company could
become subject to racketeering laws.
While the Company does not
grow, handle, process or sell cannabis or products derived from it, its receipt of money from clients that do so exposes it to risks
related to the Racketeer Influenced Corrupt Organizations Act (“RICO”). RICO is a federal statute providing civil and criminal
penalties for acts performed as part of an ongoing criminal organization. Under RICO, it is unlawful for any person who has received
income derived from a pattern of racketeering activity (which includes most felonious violations of the federal laws relating to cannabis)
to use or invest any of that income in the acquisition of any interest, or the establishment or operation of, any enterprise which is
engaged in interstate commerce. RICO also authorizes private parties whose properties or businesses are harmed by such patterns of racketeering
activity to initiate civil actions. A violation of RICO could result in fines, penalties, administrative sanctions, convictions or settlements
arising from civil or criminal proceedings, seizure of assets, disgorgement of profits, cessation of business activities or divestiture.
Banking regulations
could limit access to banking services and expose the Company to risk.
Receipt of payments from
clients engaged in the cannabis business could subject the Company to the consequences of federal laws and regulations relating to money
laundering, financial record keeping and proceeds of crime, including the Bank Secrecy Act, as amended by the “Patriot Act.”
Since the Company may receive money from persons whose activities are illegal, many banks and other financial institutions could be concerned
that their receipt of these funds from the Company could violate federal statutes such as those relating to money laundering, unlicensed
money remittances and the Bank Secrecy Act. As a result, banks may refuse to provide services to the Company. Such refusal could make
it difficult for the Company to operate. Additionally, some courts have denied cannabis-related businesses bankruptcy protection, thus
making it difficult for lenders to recoup their investments, which may make it more difficult for the Company to raise capital through
loans. While the Company has not encountered difficulty in obtaining banking services, no assurance can be given that it will be able
to do so.
Since
2014, the DOJ has de-prioritized enforcement of the Bank Secrecy Act against financial institutions and cannabis-related businesses that
utilize them. If such enforcement were to increase, it might become more difficult for the Company and its clients and potential
clients to access the U.S. banking systems and conduct financial transactions, which could adversely affect the Company’s operations.
Dividends and distributions
could be prevented if receipt of payments from clients is deemed to be proceeds of crime.
While the Company has no
intention to declare or pay dividends in the foreseeable future, if any of its revenues were found to have resulted from violations of
money laundering laws or otherwise the proceeds of crime, the Company might determine to or be required to suspend the declaration declaring
or payment of dividends.
Further legislative
developments beneficial to the Company’s operations are not assured.
The Pharmacology University
Business involves providing services to persons who may be directly or indirectly engaged in the cultivation, distribution, manufacture,
storage, transportation or sale of cannabis and cannabis products. Its success depends on the continued development of the cannabis industry.
Such development depends upon continued legislative and regulatory legalization of cannabis at the state level and either legalization
at the federal level or a continued “hands-off” approach by federal enforcement agencies. However, regulatory developments
beneficial to the industry cannot be assured. While there may be ample public support for legislative action, other factors, such as the
willingness of legislative bodies to act, election results, scientific findings or intangible events, could slow or halt progressive legislation
relating to cannabis and or reduce the current tolerance for the use of cannabis, which could adversely affect the demand for the Company’s
services.
The House of Representatives,
in its most recent term, passed bills that would decriminalize cannabis, remove it from the list of scheduled substances under the Controlled
Substances Act, eliminate criminal penalties for individuals who manufacture, distribute, or possess cannabis, and prohibit a federal
banking regulator from penalizing a depository institution for providing banking services to legitimate cannabis- or hemp-related businesses
or ordering a depository institution to terminate a customer account unless (i) the agency has a valid reason for doing so, and (ii) that
reason is not based solely on reputation risk. Neither of these bills became law because the Senate did not pass them. None of these bills
was adopted by Congress. No assurance can be given that any similar bill will be adopted by the present or any future Congress.
We may be subject
to risks relating to bankruptcy laws.
Some courts have denied
marijuana-related businesses bankruptcy protection, thus making it very difficult for lenders to recoup their investments, which may
limit the willingness of banks to lend to our clients and us. The lack of banking and financial services presents unique and significant
challenges to businesses in the cannabis industry. We could experience difficulties obtaining and maintaining regular banking and financial
services because of the activities of our clients.
Changes in legislation
or clients’ violations of law could adversely affect the Company.
The voters or legislatures
of states in which cannabis has been legalized could repeal or amend these laws, which could adversely affect the demand for the Company’s
services. In addition, changes to and interpretations of laws and regulations could detrimentally affect its clients and, in turn, result
in a material adverse effect on its operations. Violations of these laws, or allegations of such violations, could disrupt our clients’
business, thereby adversely affecting the Company.
Changes
in government regulation could affect the Alpha Research Business.
Governmental
agencies worldwide, including in the United States, strictly regulate the drug development process. The Alpha Research Business is subject
to regulation and its activities involve providing services helping pharmaceutical and biotechnology companies and CROs that are subject
to regulation. Changes in regulations, especially those that affect clinical trials, could adversely affect demand for our services.
Also, if government efforts to contain drug costs or changes in the practices of health insurers impact pharmaceutical and biotechnology
companies’ profits from new drugs, they may spend less or reduce their growth in spending on research and development, thereby
reducing the market for clinical trials.
Failure
to comply with existing regulations or contractual obligations could result in a loss of revenue or earnings or increased costs.
Failure
on the part of the Alpha Research Business to comply with applicable regulations, whether imposed directly or required to be complied
with by contract, could have adverse effects. If this were to happen, we could be contractually required to repeat the trial at no further
cost to our customer, but at substantial cost to us, or the contract could be terminated; in either case, we could be exposed to a lawsuit
seeking substantial monetary damages.
We may
bear financial losses because most of our clinical trial contracts are fixed price and may be delayed or terminated or reduced in scope
for reasons beyond our control.
Many of
our clinical trial contracts provide for services on a fixed-price or capped fee-for-service basis and they may be terminated or reduced
in scope either immediately or upon notice. Cancellations may occur for a variety of reasons, including the inefficacy of a drug or device;
its failure to meet safety requirements; unexpected or undesired results; insufficient patient enrollment; insufficient investigator
recruitment; a client’s decision to terminate the development of a product or to end a particular study; and our failure to perform
our duties under the contract properly.
The loss,
reduction in scope or delay of a contract or the loss, delay or conclusion of multiple contracts could materially adversely affect our
business, although our contracts often entitle us to receive the costs of winding down terminated projects, as well as all fees earned
by us up to the time of termination.
We may
suffer losses if we underprice our contracts or incur overrun costs.
Since
Alpha Research Institute’s contracts are often structured based on a fixed price or a fee for service with a cap, we would bear
the loss if we were to misestimate costs. Underpricing or cost overruns could have a material adverse effect on our business, results
of operations, financial condition, and cash flows.
The
potential loss or delay of a contract or multiple contracts could adversely affect our results.
Most of our contracts for clinical trials can
be terminated by our customers upon 30 to 90 days’ notice or immediately in certain circumstances. Our clients may delay, terminate
or reduce the scope of our contracts for a variety of reasons beyond our control, including but not limited to decisions to forego or
terminate a particular clinical trial; lack of available financing, budgetary limits or changing priorities; actions by regulatory authorities;
production problems resulting in shortages of the drug being tested; failure of products being tested to satisfy safety requirements
or efficacy criteria; unexpected or undesired clinical results for products; insufficient patient enrollment in a clinical trial; insufficient
investigator recruitment; shift of business to a competitor or internal resources; product withdrawal following market launch; shut down
of manufacturing facilities; or our failure to comply with the provisions of a contract.
In the event of termination, our contracts often
provide for fees for winding down the project, but these fees may not be sufficient for us to realize the full amount of revenues or
profits anticipated thereunder.
If the Alpha Research Business fails to
perform services in accordance with contractual requirements, regulatory standards and ethical considerations, we could be subject to
significant costs or liability and our reputation could be harmed.
We contract
with Sponsors and CROs in performing clinical trials to assist them in bringing new drugs to market. Clinical trials are complex and
subject to contractual requirements, regulatory standards and ethical considerations. If we fail to perform in accordance with these
requirements, regulatory agencies may take action against us or customers may terminate contracts. Customers may also bring claims against
us for breach of our contractual obligations and patients in the clinical trials and patients taking drugs approved on the basis of those
clinical trials may bring personal injury claims against us for negligence. Any such action could have a material adverse effect on our
results of operations, financial condition and reputation. The occurrence of any of the foregoing could impact our ability to provide
the same level of service to our clients, require us to modify our services or increase our costs, which could materially and adversely
affect our operating results and financial condition.
We are subject to federal and state health
privacy laws and regulations. If we cannot comply or have not fully complied with such laws and regulations, we could face government
enforcement actions, civil penalties, criminal sanctions, or damages, which could harm our reputation and adversely affect our business.
The Health Insurance Portability and Accountability
Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act and their respective implementing regulations
(“collectively, HIPAA”), establishes federal privacy and security standards for the protection of individually identifiable
health information that applies to health plans, healthcare clearinghouses, and healthcare providers that submit certain covered transactions,
or “covered entities.” A subset of these standards also applies to “business associates,” which are persons or
entities that perform certain services for, or on behalf of, a covered entity that involve creating, receiving, maintaining, or transmitting
protected health information.
Some of our customers may be HIPAA-covered entities
and service providers, and in that context, we may function as a business associate under HIPAA. Among other things, this status means
that, for certain activities, we must comply with applicable administrative, technical, and physical safeguards as required by HIPAA,
including stringent data security obligations. Failure to comply with HIPAA can result in significant civil monetary penalties and, in
certain circumstances, criminal penalties with fines or imprisonment.
The HIPAA-covered entities and service providers
that we serve as business associates may require us to enter into HIPAA-compliant business associate agreements with them. If
we were unable to comply with our obligations as a HIPAA business associate, we could face contractual liability under the applicable
business associate agreement.
In addition, many state laws govern the privacy
and security of health information in certain circumstances, many of which differ from HIPAA. There may also be costs associated with
responding to government investigations regarding alleged violations of these and other laws and regulations, even if there are ultimately
no findings of violations or no penalties imposed. These costs could consume our resources and impact our business. Publicity from alleged
violations could harm our reputation.
If we are unable to meet the requirements of
HIPAA, our business associate agreements or state health privacy laws, we could face contractual liability or civil and criminal liability
under HIPAA, all of which could have an adverse impact on our business and generate negative publicity, which, in turn, could have an
adverse effect on our ability to attract new customers and adversely affect our business condition and prospects.
We may be adversely affected by client
concentration.
We derive the majority of our revenues from a
few customers. If any of them decreases or terminates its relationship with us, our business, results of operations or financial condition
could be materially adversely affected. For further information, see “Description of Business – Concentration of Revenues.”
Our business could incur liability if
a drug causes harm to a patient. While we are generally indemnified and insured against such risks, we may still suffer financial losses.
We could suffer liability for harm allegedly
caused by a drug or device for which we conduct a clinical trial, either as a result of a lawsuit against the Sponsor or CRO to which
we are joined or an action launched by a regulatory body. While we are generally indemnified for such harm under our agreements with
Sponsors and CROs, we could nonetheless incur financial losses, regulatory penalties or both. Further, the indemnification obligations
of Sponsors and CROS are enforceable by us only if specific facts, which may be difficult to prove or may be subject to dispute, exist.
Any claim could result in potential liability for us if the claim is outside the scope of such indemnification, the Sponsor or CRO does
not comply with its indemnification obligations or our liability exceeds applicable indemnification limits or available insurance coverage.
Further, we do not carry insurance to cover damages for which we are liable. Such a claim could have an adverse impact on our financial
condition and results of operations. Furthermore, the associated negative publicity could have an adverse effect on our business and
reputation.
If we fail to maintain an effective
system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements
or comply with applicable regulations could be impaired.
The Sarbanes-Oxley Act of 2002 (“SOX”)
requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting.
However, our independent registered public accounting has advised management that we have the following material weaknesses in internal
control: lack of in-house personnel with insufficient technical knowledge to identify and address certain accounting matters; insufficient
accounting personnel to perform duties over financial transaction processing, key account reconciliations and reporting; insufficient
written policies and procedures over accounting transaction processing such that routine transactions are recorded on an accrual basis
in a timely manner; and insufficient in-house knowledge on monitoring accounting standards for the impact of complex accounting standards.
Accordingly, we need to develop and refine
our disclosure controls and other procedures to ensure that information required to be disclosed by us in the reports that we will file
under the Exchange Act is accumulated and communicated to our principal executive and financial officers. We are also continuing to improve
our internal control over financial reporting. To maintain and improve effective disclosure controls and procedures and internal control
over financial reporting, we will need to expend significant resources, including accounting-related costs and significant management
oversight.
Our current controls and any new controls
that we develop may become inadequate because of changes in conditions in our business. Further, weaknesses in our disclosure controls
and internal control over financial reporting may be discovered. Any failure to develop or maintain effective controls or any difficulties
encountered in their implementation or improvement could adversely affect our results of operations or cause us to fail to meet our reporting
obligations and result in a restatement of our consolidated financial statements. Failure to implement and maintain effective internal
control over financial reporting could also adversely affect the results of periodic management evaluations and annual independent registered
public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we will
eventually be required to include in our periodic reports filed with the SEC. Ineffective disclosure controls and procedures and internal
control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which
could have a negative effect on the trading price of Common Stock. We are not currently required to comply with the SEC rules that implement
Section 404 of SOX and are therefore not required to make a formal assessment of the effectiveness of our internal control over financial
reporting for that purpose. After the registration statement of which this Prospectus forms a part is made effective, we will be required
to provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second
annual report on Form 10-K.
Our independent registered public accounting
firm will not be required to attest formally to the effectiveness of our internal control over financial reporting until after we cease
to be an “emerging growth company” as defined in the JOBS Act. At that time, our independent registered public accounting
firm may issue an adverse report if it is not satisfied with the level at which our internal control over financial reporting is documented,
designed or operating. Any failure to maintain effective disclosure controls and internal control over financial reporting could harm
our business, results of operations, and financial condition and could cause a decline in the price of Common Stock.
Our business and operations would suffer
in the event of computer system failures, cyber-attacks or deficiencies in our or related parties’ cyber security.
Our internal computer systems and those of
current and future third parties on which we rely may fail and are vulnerable to damage from computer viruses and unauthorized access.
Our information technology and other internal infrastructure systems, including corporate firewalls, servers, leased lines and connection
to the Internet, face the risk of systemic failure that could disrupt our operations. If such an event were to occur and cause interruptions
in our operations, it could result in a material disruption of our operations. Likewise, the failure, cyber-attack or deficiencies of
or on the computer systems of third parties on which we rely various products and services and similar events relating to their computer
systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result
in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could
incur liability, our competitive position could be harmed and the further development and commercialization of our products could be
hindered or delayed.
Risk Factors Related to Our CBD Products
We have no experience conducting clinical
trials as a Sponsor.
We have not commenced
clinical trials as a Sponsor (“Sponsored Trials”). These trials are subject to the risks inherent in the initial organization,
financing, expenditures, complications and delays in a new business. Further, cannabinoid research, clinical trials and product development
is highly speculative, involves a high degree of risk. Accordingly, investors should consider our prospects in light of the costs, uncertainties,
delays and difficulties frequently encountered by companies in the early stages of development, especially those relating engaging in
Sponsored Trials. Potential investors should carefully consider the risks and uncertainties that a company with a limited operating history
will face. In particular, potential investors should consider that we may be unable to:
| |
· |
successfully implement or execute our current business plan as it relates
to Sponsored Trials, or develop a business plan that is sound; |
| |
|
|
| |
· |
successfully complete clinical trials and obtain regulatory approval for the marketing of our
product candidates; |
| |
|
|
| |
· |
successfully contract for the manufacture of our CBD products and establish a commercial supply
of their components; |
| |
|
|
| |
· |
secure market exclusivity or adequate intellectual property protection for our product candidates; |
| |
|
|
| |
· |
attract and retain an experienced management and advisory team; or |
| |
|
|
| |
· |
raise sufficient funds in the capital markets to effectuate our
business plan, including clinical development, regulatory approval and commercialization for our product candidates. |
We have no CBD products sale and do not expect to have any
for a considerable period.
Our ability to obtain revenue and profits
from CBD products depends on identifying safe and effective products through clinical trials and commercializing them by selling or licensing
them. We presently have no such products and do not expect to have any for at least two years. We do not expect to generate significant
revenue unless and until we begin to sell one or more such products. Our ability to generate revenue depends on a number of factors,
including, but not limited to, our ability to:
| |
· |
successfully complete preclinical studies; |
| |
|
|
| |
· |
successfully enroll subjects in and complete clinical trials;
|
| |
|
|
| |
· |
receive regulatory approvals, if required, from applicable regulatory
authorities; |
| |
|
|
| |
· |
initiate and successfully complete all safety studies, if required,
to obtain marketing approval for our CBD products; |
| |
|
|
| |
· |
establish commercial manufacturing capabilities
or make arrangements with third-party manufacturers for clinical supply and commercial manufacturing; |
| |
|
|
| |
· |
obtain and maintain patent and trade secret
protection or regulatory exclusivity for our product candidates; |
| |
|
|
| |
· |
launch commercial sales of our CBD products,
whether alone or in collaboration with others; |
| |
|
|
| |
· |
obtain and maintain acceptance of the product
candidates, if and when approved, by patients, the medical community and third-party payors; |
| |
|
|
| |
· |
effectively compete with other therapies; |
| |
|
|
| |
· |
obtain and maintain healthcare coverage and
adequate reimbursement; |
| |
|
|
| |
· |
enforce and defend intellectual property rights
and claims; |
| |
|
|
| |
· |
maintain a continued acceptable safety profile
of the product candidates following approval. |
If we do not achieve one or more of these
factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product
candidates, which would materially harm our business. If regulatory approvals is required for any of our CBD products candidates, our
operations could be adversely affected.
We face competition from other companies
engaged in the development and commercial exploitation of CBD products and our operating results will suffer if we fail to compete effectively.
The CBD industry
is intensely competitive and subject to rapid and significant changes in technology and regulation. We have existing competitors and
potential new competitors in a number of jurisdictions, many of which have or will have substantially greater name recognition, commercial
infrastructures and financial, technical and personnel resources than we have. Established competitors may invest heavily to quickly
discover and develop novel compounds that could make any of our products obsolete or uneconomical. In addition, mergers and acquisitions
in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our
competitors, potentially reducing or eliminating our commercial opportunities. Further, such potential competitors may enter the market
before us, and their products may be designed to circumvent our granted patents and pending patent applications. They may also challenge,
narrow or invalidate our granted patents or our patent applications, and such patents and patent applications may fail to provide adequate
protection for our product candidates. Any new product that competes with an approved product may need to demonstrate compelling advantages
in efficacy, cost, convenience, tolerability and safety to be commercially successful. Other competitive factors, including generic competition,
could force us to lower prices or could result in reduced sales. In addition, new products developed by others could emerge as competitors
to our products. If we are not able to compete effectively against our current and future competitors, our business will not grow and
our financial condition and operations will suffer.
We may contract with third parties for
the manufacture and commercialization of our products. Such reliance increases the risk that we will not have sufficient quantities of
our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or
commercialization efforts.
We do not currently own or operate, nor do we
have any plans to establish in the future, any manufacturing facilities or personnel. We expect to rely on third parties for the manufacture
and, in some cases, the sale of our products. The failure of any such third party to manufacture products in accordance with our specifications,
fail to deliver or market products timely or otherwise fail to comply with it contract with us could have a material adverse effect on
us.
We may be unable to establish any agreements with
third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers,
reliance on third-party manufacturers entails additional risks, including:
| |
· |
the possible breach of the manufacturing agreement
by the third party; |
| |
|
|
| |
· |
the possible misappropriation of our proprietary
information, including our trade secrets and know-how; and |
| |
|
|
| |
· |
the possible termination or nonrenewal of the
agreement by the third party at a time that is costly or inconvenient for us. |
If we are unable to adequately protect
our products or obtain and maintain patent protection for them or if the scope of the patent protection obtained is not sufficiently
broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize
our products could be be impaired.
Our commercial success will depend in part
on our ability to obtain and maintain proprietary or intellectual property protection in the United States and other countries for our
product candidates, and our core technologies, including our novel target discovery technology and our proprietary compound library and
other know-how. We seek to protect our proprietary and intellectual property position by, among other methods, filing patent applications
in the United States and abroad related to our proprietary technology, inventions and improvements that are important to the development
and implementation of our business. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain
our proprietary and intellectual property position.
The patent position of CBD products
is highly uncertain, involves complex legal and factual questions.
The degree of patent protection we require
to successfully commercialize our products may be unavailable or severely limited in some cases and may not adequately protect our rights
or permit us to gain or keep any competitive advantage. We cannot provide assurance that any of pending patent application will mature
into issued patents that include claims with a scope sufficient to protect the patented product.. Furthermore, patents have a limited
lifespan. In the United States, the natural expiration of a patent is generally twenty years after it is filed. Various extensions may
be available; however, the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development,
and if required, the testing and regulatory review of new products, patents protecting such candidates might expire before or shortly
after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with adequate and continuing
patent protection sufficient to exclude others from commercializing products similar or identical to our product candidates, including
generic versions of such products.
Other parties have developed technologies
that may be related or competitive to our own, and such parties may have filed or may file patent applications, or may have received
or may receive patents, claiming inventions that may overlap or conflict with those claimed in our own patent applications, with respect
to either the same methods or formulations or the same subject matter, in either case that we may rely upon to dominate our patent position
in the market. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications
in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore,
we cannot know with certainty whether we were the first to make the inventions claimed in our owned or licensed pending patent applications,
or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability
and commercial value of our patent rights cannot be predicted with any certainty.
In addition, the patent prosecution process is
expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable
cost or in a timely manner. Further, with respect to most of the pending patent applications covering our product candidates, prosecution
has yet to commence. Patent prosecution is a lengthy process, during which the scope of the claims initially submitted for examination
by the U.S. Patent and Trademark Office (the “USPTO”), have been significantly narrowed by the time they issue, if
at all. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too
late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution
of patent applications, or to maintain the patents, covering technology that we license from third parties. Therefore, these patents
and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
Even if we acquire patent protection that
we expect should enable us to maintain such competitive advantage, third parties may challenge the validity, enforceability or scope
thereof, which may result in such patents being narrowed, invalidated or held unenforceable. The issuance of a patent is not conclusive
as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent
offices in the United States and abroad. For example, we may be subject to a third-party submission of prior art to the USPTO challenging
the priority of an invention claimed within one of our patents, which submissions may also be made prior to a patent’s issuance,
precluding the granting of any of our pending patent applications. We may become involved in opposition, derivation, reexamination, inter
parties review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others from whom
we have obtained licenses to such rights.
Competitors may claim that they invented the
inventions claimed in our issued patents or patent applications prior to us, or may file patent applications before we do. Competitors
may also claim that we are infringing on their patents and that we therefore cannot practice our technology as claimed under our patents,
if issued. Competitors may also contest our patents, if issued, by showing the patent examiner that the invention was not original, was
not novel or was obvious. In litigation, a competitor could claim that our patents, if issued, are not valid for a number of reasons.
If a court agrees, we would lose our rights to those challenged patents.
In addition, we may in the future be subject
to claims by our former employees or consultants asserting an ownership right in our patents or patent applications, as a result of the
work they performed on our behalf. Although we generally require all of our employees, consultants and advisors and any other third parties
who have access to our proprietary know-how, information or technology to assign or grant similar rights to their inventions to us, we
cannot be certain that we have executed such agreements with all parties who may have contributed to our intellectual property, nor can
we be certain that our agreements with such parties will be upheld in the face of a potential challenge, or that they will not be breached,
for which we may not have an adequate remedy.
An adverse determination in any such submission
or proceeding may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable,
in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products,
without payment to us, or could limit the duration of the patent protection covering our technology and product candidates. Such challenges
may also result in our inability to manufacture or commercialize our product candidates without infringing third party patent rights.
In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade
companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if they are unchallenged, our patent
portfolio may not provide us with any meaningful protection or prevent competitors from designing around our patent claims to circumvent
our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. For example,
a third party may develop a competitive product that provides benefits similar to one or more of our product candidates but that has
a different composition that falls outside the scope of our patent protection. If the patent protection provided by the patents and patent
applications we hold or pursue with respect to our product candidates is not sufficiently broad to impede such competition, our ability
to successfully commercialize our product candidates could be negatively affected, which would harm our business.
Obtaining and maintaining patent protection
depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent
agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental
patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent
application process. In addition, periodic maintenance fees on issued patents often must be paid to the USPTO and foreign patent agencies
over the lifetime of the patent. While an unintentional lapse can in many cases be cured by payment of a late fee or by other means in
accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or
patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could
result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions
within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain
the patents and patent applications covering our products or procedures, we may not be able to stop a competitor from marketing products
that are the same as or similar to our product candidates, which would have a material adverse effect on our business.
If we are unable to protect the confidentiality
of our trade secrets, our business and competitive position may be harmed.
In addition to the protection afforded by
patents, we rely upon unpatented trade secret protection, unpatented know-how and continuing technological innovation to develop and
maintain our competitive position. With respect to the building of our proprietary compound library, we consider trade secrets and know-how
to be our primary intellectual property. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality
agreements with our collaborators, scientific advisors, employees and consultants, and invention assignment agreements with our consultants
and employees. We may not be able to prevent the unauthorized disclosure or use of our technical know-how or other trade secrets by the
parties to these agreements, however, despite the existence generally of confidentiality agreements and other contractual restrictions.
Monitoring unauthorized uses and disclosures is difficult, and we do not know whether the steps we have taken to protect our proprietary
technologies will be effective. If any of the collaborators, scientific advisors, employees and consultants who are parties to these
agreements breaches or violates the terms of any of these agreements, we may not have adequate remedies for any such breach or violation,
and we could lose our trade secrets as a result. Enforcing a claim that a third party illegally obtained and is using our trade secrets,
like patent litigation, is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States
are sometimes less willing to protect trade secrets.
Our trade secrets could otherwise become known
or be independently discovered by our competitors. Competitors could purchase our product candidates and attempt to replicate some or
all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design
around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights.
If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent
them, or those to whom they communicate it, from using that technology or information to compete with us. If our trade secrets are not
adequately protected so as to protect our market against competitors’ products, our competitive position could be adversely affected,
as could our business.
Third parties may initiate legal proceedings
alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material
adverse effect on the success of our business.
Our commercial success depends upon our ability
and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies
without infringing the proprietary rights and intellectual property of third parties. The biotechnology and pharmaceutical industries
are characterized by extensive and frequent litigation regarding patents and other intellectual property rights. We may in the future
become party to, or threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our
product candidates and technology, including interference proceedings before the USPTO. Our competitors or other third parties may assert
infringement claims against us, alleging that our products or technologies are covered by their patents. Given the vast number of patents
in our field of technology, we cannot be certain that we do not infringe existing patents or that we will not infringe patents that may
be granted in the future. Some of these patent applications have already been allowed or issued, and others may issue in the future. Since
these areas are competitive and of strong interest to pharmaceutical and biotechnology companies, there will likely be additional patent
applications filed and additional patents granted in the future, as well as additional research and development programs expected in the
future. Furthermore, because patent applications can take many years to issue and may be confidential for 18 months or more after filing,
and because pending patent claims can be revised before issuance, there may be applications now pending which may later result in issued
patents that may be infringed by the manufacture, use or sale of our product candidates, or the practice of our technology. If a patent
holder believes our product or product candidate infringes on its patent, the patent holder may sue us even if we have received patent
protection for our technology. Moreover, we may face patent infringement claims from non-practicing entities that have no relevant product
revenue and against whom our own patent portfolio may thus have no deterrent effect.
If we are found to infringe a third party’s
intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our
product candidates and technology. We may choose to obtain a license, even in the absence of an action or finding of infringement. In
either case, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain
such a license, it could be granted on non-exclusive terms, thereby providing our competitors and other third parties access to the same
technologies licensed to us. Without such a license, we could be forced, including by court order, to cease developing and commercializing
the infringing technology or product candidates. In addition, we could be found liable for monetary damages, including treble damages
and attorneys’ fees if we are found to have willfully infringed such third-party patent rights. A finding of infringement could
prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm
our business. If we lose a foreign patent lawsuit, alleging our infringement of a competitor’s patents, we could be prevented from
marketing our products in one or more foreign countries, which would have a materially adverse effect on our business.
We may be subject to damages resulting
from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition
or non-solicitation agreements with our competitors.
We could in the future be subject to claims
that we or our employees have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of
former employers or competitors. Although we try to ensure that our employees and consultants do not use the intellectual property, proprietary
information, know-how or trade secrets of others in their work for us, we may in the future be subject to claims that we caused an employee
to breach the terms of his or her non-competition or non-solicitation agreement, or that we or these individuals have, inadvertently
or otherwise, used or disclosed the alleged trade secrets or other proprietary information of a former employer or competitor. Litigation
may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result
in substantial costs and could be a distraction to management. If our defenses to these claims fail, in addition to requiring us to pay
monetary damages, a court could prohibit us from using technologies or features that are essential to our product candidates, if such
technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former
employers. An inability to incorporate such technologies or features would have a material adverse effect on our business, and may prevent
us from successfully commercializing our product candidates. In addition, we may lose valuable intellectual property rights or personnel
as a result of such claims. Moreover, any such litigation or the threat thereof may adversely affect our ability to hire employees or
contract with independent sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to
commercialize our product candidates, which would have an adverse effect on our business, results of operations and financial condition.
We may become involved in lawsuits to
protect or enforce our patents and other intellectual property rights, which could be expensive, time consuming and unsuccessful.
Competitors and other third parties may infringe,
misappropriate or otherwise violate our patents and other intellectual property rights. To counter infringement or unauthorized use,
we may be required to file infringement claims. A court may disagree with our allegations, however, and may refuse to stop the other
party from using the technology at issue on the grounds that our patents do not cover the third-party technology in question. Further,
such third parties could counterclaim that we infringe their intellectual property or that a patent we have asserted against them is
invalid or unenforceable. In patent litigation in the United States, defendant counterclaims challenging the validity, enforceability
or scope of asserted patents are commonplace. In addition, third parties may initiate legal proceedings against us to assert such challenges
to our intellectual property rights. The outcome of any such proceeding is generally unpredictable. Grounds for a validity challenge
could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement.
Patents may be unenforceable if someone connected with prosecution of the patent withheld relevant information from the USPTO or made
a misleading statement during prosecution. It is possible that prior art of which we and the patent examiner were unaware during prosecution
exists, which could render any patents that may issue invalid. Moreover, it is also possible that prior art may exist that we are aware
of but do not believe is relevant to our future patents, should they issue, but that could nevertheless be determined to render our patents
invalid.
An adverse result in any litigation proceeding
could put one or more of our patents at risk of being invalidated or interpreted narrowly. If a defendant were to prevail on a legal
assertion of invalidity or unenforceability of our patents covering one of our product candidates, we would lose at least part, and perhaps
all, of the patent protection covering such product candidate. Competing products may also be sold in other countries in which our patent
coverage might not exist or be as strong.
Intellectual property litigation could
cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Litigation or other legal proceedings relating
to intellectual property claims, with or without merit, is unpredictable and generally expensive and time consuming and may divert
significant resources from our core business, including distracting our technical and management personnel from their normal responsibilities.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a
risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there
could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts
or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such
litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities
or any future sales, marketing or distribution activities.
We may not have sufficient financial or other
resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation
or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property
portfolios. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating or
from successfully challenging our intellectual property rights. Uncertainties resulting from the initiation and continuation of patent
litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
We may not be able to effectively enforce
our intellectual property rights throughout the world.
Filing, prosecuting and defending patents
on our product candidates in all countries throughout the world would be prohibitively expensive. The requirements for patentability
may differ in certain countries, particularly in developing countries. Moreover, our ability to protect and enforce our intellectual
property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, the patent laws
of some foreign countries do not afford intellectual property protection to the same extent as the laws of the United States. Many companies
have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The
legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property
rights. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual
property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to
third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the
United States. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own
products and, further, may export otherwise infringing products to territories where we have patent protection, if our ability to enforce
our patents to stop infringing activities is inadequate. These products may compete with our product candidates, and our patents or other
intellectual property rights may not be effective or sufficient to prevent them from competing.
Proceedings to enforce our patent rights in
foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and resources from other aspects
of our business. Furthermore, while we intend to protect our intellectual property rights in the major markets for our product candidates,
we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our
product candidates. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
We may need to license intellectual
property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property,
including patent rights, that are important or necessary to the development of our products. It may be necessary for us to use the patented
or proprietary technology of third parties to commercialize our products, in which case we would be required to obtain a license from
these third parties on commercially reasonable terms, or our business could be harmed, possibly materially. Although we believe that
licenses to these patents are available from these third parties on commercially reasonable terms, if we were not able to obtain a license,
or were not able to obtain a license on commercially reasonable terms, our business could be harmed, possibly materially.
If we fail to comply with our obligations
in the agreements under which we collaborate with or license intellectual property rights from third parties or otherwise experience
disruptions to our business relationships with our collaborators or licensors, we could lose rights that are important to our business.
We expect that our future license agreements will
impose, various development, diligence, commercialization, and other obligations on us in order to maintain the licenses. In spite of
our efforts, a future licensor might conclude that we have materially breached our obligations under such license agreements and seek
to terminate the license agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered
by these license agreements. If these in-licenses are terminated, or if the underlying patent rights licensed thereunder fail to provide
the intended exclusivity, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, products
identical to ours and we may be required to cease our development and commercialization of certain of our product candidates. Any of the
foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and
prospects.
Moreover, disputes may arise regarding intellectual
property subject to a licensing agreement, including:
| |
· |
the scope of rights granted under the license
agreement and other interpretation-related issues; |
| |
|
|
| |
· |
the extent to which our technology and processes
infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| |
|
|
| |
· |
the sublicensing of patent and other rights
under our collaborative development relationships; |
| |
|
|
| |
· |
our diligence obligations under the license
agreement and what activities satisfy those diligence obligations; |
| |
|
|
| |
· |
the inventorship and ownership of inventions
and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| |
|
|
| |
· |
the priority of invention of patented technology.
|
The agreements under which we may license
intellectual property or technology from third parties may be complex, and certain provisions in such agreements may be susceptible to
multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be
the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other
obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition,
results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability
to maintain our licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the
affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations,
and prospects.
Changes to the patent law in the United
States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
Our success with CBD products will be heavily
dependent on intellectual property, particularly patents. Obtaining and enforcing such patents involves both technological and legal
complexity and may be costly, time consuming and inherently uncertain. Recent patent reform legislation in the United States and other
countries, including the Leahy-Smith America Invents Act, or Leahy-Smith Act, signed into law on September 16, 2011, could increase
those uncertainties and costs. This act made a number of significant changes to U.S. patent law and included provisions that affect the
way patent applications are prosecuted, redefine prior art and provide more efficient and cost-effective avenues for competitors to challenge
the validity of patents. This legislation has transformed the U.S. patent system into a “first to file” system and could
make it more difficult to obtain patent protection and increase the uncertainties and costs surrounding the prosecution of our patent
applications and the enforcement or defense of our issued patents, all of which could harm our business, results of operations and financial
condition.
The U.S. Supreme Court has ruled on several patent
cases in recent years, either narrowing the scope of patent protection available in some circumstances and weakening the rights
of patent owners in others. Additionally, there have been recent proposals for additional changes to the patent laws of the United States
and other countries that, if adopted, could impact our ability to obtain patent protection for our proprietary technology or our ability
to enforce rights in our proprietary technology. Depending on future actions by the U.S. Congress, the U.S. courts, the USPTO and the
relevant law-making bodies in other countries, the laws and regulations governing patents could change in unpredictable ways that would
weaken our ability to obtain new patents or to enforce any patents that we may obtain in the future.
Intellectual property rights do not
necessarily address all potential threats.
The degree of future protection afforded by
our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our
business or permit us to maintain our competitive advantage. For example:
| |
· |
others may be able to make products that are
similar to our product candidates or utilize similar technology but that are not covered by the claims of the patents that we license
or may own; |
| |
|
|
| |
· |
we or our licensors or collaborators, might
not have been the first to make the inventions covered by the issued patent or pending patent application that we license or own
now or in the future; |
| |
|
|
| |
· |
we or our licensors or collaborators, might
not have been the first to file patent applications covering certain of our or their inventions; |
| |
|
|
| |
· |
others may independently develop similar or
alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights;
|
| |
|
|
| |
· |
it is possible that our present or future pending
patent applications (whether owned or licensed) will not lead to issued patents; |
| |
|
|
| |
· |
issued patents that we hold rights to may be
held invalid or unenforceable, including as a result of legal challenges by our competitors or other third parties; |
| |
|
|
| |
· |
our competitors or other third parties might
conduct research and development activities in countries where we do not have patent rights and then use the information learned
from such activities to develop competitive products for sale in our major commercial markets; |
| |
|
|
| |
· |
we may not develop additional proprietary technologies
that are patentable; |
| |
|
|
| |
· |
the patents of others may harm our business;
and |
| |
|
|
| |
· |
we may choose not to file a patent in order
to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
|
In any of these events, there be a material
adverse effect on our business, financial condition, results of operations and prospects.
The Company is an “emerging growth
company,” as defined in the Securities Act (an “EGC”), and a “smaller reporting company,” as defined in
Rule 405 promulgated under the Securities Act (an “SRC”) and intends to take advantage of certain exemptions from disclosure
requirements available to it. Doing so could make the Common Stock less attractive to investors and make it more difficult to compare
the performance of the Company with that of other public companies.
As long as the Company is an EGC, it intends to
utilize certain exemptions from reporting requirements that apply to public companies that are not EGCs. Among the reporting requirements
from which the Company is so exempted are the auditor attestation requirements of SOX, certain disclosures relating to executive compensation,
holding a nonbinding advisory vote on executive compensation and stockholder approval of “golden parachute” payments. The
Company is permitted to be an emerging growth company for up to five years or until the earliest of (i) the last day of the first
fiscal year in which its annual gross revenues exceed $1 billion, (ii) the date that it becomes a “large accelerated filer”
as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of Common Stock that is held by non-affiliates
exceeds $700 million as of the last business day of its most recently completed second fiscal quarter, or (iii) the date on which
it has issued more than $1 billion in nonconvertible debt during the preceding three-year period.
As an SRC, the Company intends to utilize certain
reduced disclosure requirements, including publishing two years of audited financial statements instead of three years, as required for
companies that are not SRCs. The Company will remain an SRC until the last day of the fiscal year in which it had (i) a public float
that exceeded $250 million or (ii) annual revenues of more than $100 million and a public float that exceeded $700 million. To the extent
the Company takes advantage of such reduced disclosure obligations, it may make comparison of its financial statements with those
of other public companies difficult or impossible.
After the Company ceases to be an EGC, it is
expected to incur additional management time and cost to comply with the more stringent reporting requirements applicable to companies
that are accelerated filers or large accelerated filers, including complying with the auditor attestation requirements of Section 404
of SOX.
Changes in existing financial accounting
standards or practices may harm our results of operations.
Changes in existing accounting rules or practices,
including generally accepted accounting principles in the United States (“GAAP”), new accounting pronouncements or varying
interpretations of current accounting pronouncements or practices could harm our results of operations or the manner in which we conduct
our business. Further, such changes could potentially affect our reporting of transactions completed before such changes are effective.
GAAP is subject to interpretation by the Financial Accounting Standards Board, FASB, the SEC and various bodies formed to promulgate and
interpret appropriate accounting principles. A change in these principles or interpretations could have a significant effect on our reported
financial results and affect the reporting of transactions completed before the announcement of a change. Any difficulties in implementing
these pronouncements could cause us to fail to meet our financial reporting obligations, which could result in regulatory discipline and
harm investors’ confidence in us.
If our estimates or judgments relating
to our critical accounting policies prove to be incorrect, our results of operations could be adversely affected.
Preparing financial statements in conformity
with GAAP requires management to make estimates and assumptions that affect the amounts reported in our consolidated financial statements
and related notes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under
the circumstances, as provided in “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
The results of these estimates form the basis for making assumptions and judgments affecting our consolidated financial statements, including
those related to revenue recognition, stock-based compensation, the fair value of Common Stock, valuation of strategic investments, periods
of benefit for deferred costs, and uncertain tax positions. Our results of operations may be adversely affected if our assumptions change
or if actual circumstances differ from those in our assumptions, which could cause our results of operations to fall below the expectations
of securities analysts and investors, resulting in a decline in the trading price of Common Stock.
The Company’s business depends substantially
on the continuing efforts of its executive officers, and its business may be severely disrupted if it were to lose the services rendered
by any of them.
The Company’s future success depends substantially
on the continued services of its executive officers. The Company does not maintain key-man life insurance on its executive officers.
If any of these executive officers were unable or unwilling to continue in their present positions, the Company might not be able to
replace them readily, if at all. The loss of any of these officers could cause the Company’s business to be disrupted, and it could
incur additional expenses to recruit and retain new officers.
This risk is increased because the Company has
no employment contracts with its officers and is paying them sporadically and in varying amounts as the Company’s financial condition
permits. Further, their salaries are not commensurate with their contributions and abilities. While none of these officers has indicated
when or whether he would terminate his employment if he continues to be paid on the basis set forth above, the Company believes that
they may not work for it indefinitely without appropriate and regularly paid compensation. If the Company were to lose any of its officers,
its ability to operate would be materially impaired.
The Company’s business depends substantially
on recruiting additional members of management and key personnel and its business could be severely disrupted if it were unable to hire
such personnel or lose their services.
The Company needs to attract, hire and retain
additional managers and key employees to implement its business plan. If it were unable to do so or if, after being hired, any
of the members of the Company’s management were lost, it would have to spend a considerable amount of time and resources searching,
recruiting, and integrating their replacements, which would substantially divert management’s attention from and severely disrupt
its business. The Company may face difficulties in attracting and retaining additional management and, if it were to lose any of them,
in attracting and retaining their replacements because it cannot presently pay competitive compensation and its future is uncertain.
Litigation could adversely affect the Company’s
business, financial condition and results of operations.
From time to time, the Company may become subject
to litigation that may result in liability materially adverse to its financial condition or may negatively affect its operating results
if changes to its business operation are required. The cost of defending such litigation could be significant and require the diversion
of its resources. Adverse publicity associated with litigation could negatively affect perceptions of the Company, regardless of whether
the allegations are valid or whether the Company is ultimately found not to be liable. As a result, litigation could adversely affect
the Company’s business, financial condition and results of operations.
Acquisitions, other strategic alliances
and investments could result in operating difficulties, dilution, and other harmful consequences that may adversely impact the Company’s
business and results of operations.
The Company may acquire other businesses and
these transactions could be material to its financial condition and results of operations. The areas where it may encounter risks in
connection with acquisitions include, but are not limited to, the failure to successfully further develop the acquired business, the
implementation or remediation of controls, procedures and policies at the acquired business, the transition of operations, users and
customers onto our existing platforms, and the challenges associated with integrating the acquired business and its employees into the
Company’s organization, as well as retaining employees of the acquired businesses. Failure to address these risks or other problems
encountered in connection with acquisitions successfully could cause the Company to fail to realize the anticipated benefits of such
acquisitions, investments or alliances, incur unanticipated liabilities, and harm its business generally.
Such acquisitions could also result in dilutive
issuances of the Company’s equity securities, the incurrence of debt, contingent liabilities or amortization expenses, impairment
of goodwill and purchased long-lived assets, or restructuring charges, any of which could adversely affect its financial condition, results
of operations and cash flows. Also, the anticipated benefits and synergies of acquisitions may not materialize.
Because our success depends in part on
our ability to expand our operations outside the United States, our business will be susceptible to risks associated with international
operations.
We currently maintain operations and have personnel
outside the United States in Mexico, Jordan, Ecuador, Columbia, Venezuela, Argentina and Brazil. We plan to expand our international
operations into Argentina, Chile, Peru, Panama and other countries where our activities are lawful. In the fiscal years ended May 31,
2023, and May 31, 2022, our non-U.S. revenue was approximately 5.2% and 4.2% of
our total revenue, respectively. We expect to continue to expand our international operations, but these efforts may not be successful.
In addition, conducting international operations subjects us to new risks, some of which we have not generally faced in the United States
or other countries where we currently operate. These risks include, among other things: lack of familiarity and burdens of complying
with foreign laws, legal standards, regulatory requirements and other barriers, and the risk of penalties to the Company, its management
and employees if its practices are deemed to be out of compliance; unexpected changes in regulatory requirements, taxes, trade laws,
tariffs, export quotas, customs duties, or other trade restrictions; longer accounts receivable payment cycles and difficulties in collecting
accounts receivable; increased financial accounting and reporting burdens and complexities; difficulties in managing and staffing international
operations including the proper classification of independent contractors and other contingent workers, differing employer/employee relationships,
and local employment laws; increased costs involved with recruiting and retaining an expanded employee population outside the United
States through cash- and equity-based incentive programs and unexpected legal costs and regulatory restrictions in issuing our shares
to employees outside the United States; global political and regulatory changes that may lead to restrictions on immigration and travel
for our employees outside the United States; potentially adverse tax consequences, including the complexities of foreign value added
tax (or other tax) systems, and restrictions on the repatriation of earnings; and permanent establishment risks and complexities in connection
with international payroll, tax, and social security requirements for international employees.
Additionally, operating in international markets
requires significant management attention and financial resources. There is no certainty that the investments and additional resources
required to establish operations in other countries will produce the desired revenue or profitability.
Compliance with laws and regulations applicable
to our global operations also substantially increases our cost of doing business in foreign jurisdictions. We have limited experience
in operating outside the United States, which increases the risk that any operations that we may undertake will not be successful. If
we invest substantial time and resources to expand our international operations and are unable to do so successfully and timely, our business,
results of operations, and financial condition will suffer. We may be unable to keep current with changes in government requirements as
they change from time to time. Failure to comply with these regulations could harm our business. In many countries, it is common for others
to engage in business practices that are prohibited by United States law and regulation or by our policies and procedures.
A portion
of our operations is conducted in foreign jurisdictions and is subject to the economic, political, legal and business environments of
the countries where we do business. Risks associated with such international operations could negatively affect our business, financial
condition, results of operations and cash flows.
We have operations outside the United
States and plan to expand them. International operations inherently subject us to a number of risks and uncertainties, including those
arising from compliance with governmental controls, trade restrictions, restrictions on direct investments, quotas, embargoes, import
and export restrictions, tariffs, duties, and regulatory and licensing requirements by domestic or foreign entities, including restrictions
administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury; difficulties in building, staffing and managing
foreign operations (including a geographically dispersed workforce) and maintaining compliance with foreign labor laws; burdens to comply
with, and different levels of protection offered by, multiple and potentially conflicting foreign laws and regulations, including those
relating to environmental, health and safety requirements and intellectual property; changes in laws, regulations, government controls
or enforcement practices with respect to our business and the businesses of our customers; political and social instability, including
crime, civil disturbance, terrorist activities, armed conflicts and natural and other disasters; ongoing instability or changes in a
country’s or region’s regulatory, economic or political conditions; local business and cultural factors that differ from
our standards and practices, including business practices prohibited by the Foreign Corrupt Practices Act and other anti-corruption laws
and regulations; longer payment cycles and increased exposure to counterparty risk; and differing needs of foreign customers.
The international nature of our business subjects
us to potential risks that various taxing authorities may challenge the pricing of our cross-border arrangements and subject us to additional
tax, adversely impacting our effective tax rate and tax liability.
In addition, international transactions may involve
increased financial and legal risks due to differing legal systems and customs. Compliance with these requirements may prohibit the import
or export of certain products and technologies or require us to obtain licenses before importing or exporting certain products or technology.
Our failure to comply with any of these laws, regulations or requirements could result in civil or criminal legal proceedings, monetary
or non-monetary penalties, or both, disruptions to our business, limitations on our ability to import and export products and services,
and damage to our reputation.
While the impact of these factors is difficult
to predict, any of them could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Changes in any of these laws, regulations or requirements, or the political environment in a particular country, may affect our ability
to engage in business transactions in certain markets, including investment, procurement and repatriation of earnings.
We are subject to anti-corruption, anti-bribery,
and similar laws, and noncompliance with them could subject us to criminal penalties or significant fines and harm our business and reputation.
We are subject to anti-corruption and anti-bribery
and similar laws, such as the Foreign Corrupt Practices Act of 1977 (the “FCPA”), and other anti-corruption, anti-bribery
and anti-money laundering laws in the United States and in the countries in which we conduct activities. These laws prohibit companies
and their employees and agents from promising, authorizing, making, or offering improper payments or other benefits to government officials
and others in the private sector. As we increase our international operations, our risks under these laws may increase. Anti-corruption
and anti-bribery laws have been enforced vigorously in recent years and interpreted broadly. Noncompliance with these laws could subject
us to investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages,
other civil and criminal penalties or injunctions, adverse media coverage, and other consequences. Any investigations, actions or sanctions
could harm our business, results of operations, and financial condition. Under some of these laws, we may be held liable for the corrupt
or other illegal activities of intermediaries, and our employees, representatives, contractors, partners, and agents, even if we do not
explicitly authorize such activities. We intend to implement an anti-corruption compliance program but cannot assure that all of these
persons will not take actions in violation of our policies and applicable law, for which we may be ultimately held responsible. Any violation
of the FCPA, other applicable anti-corruption laws or anti-money laundering laws could result in whistleblower complaints, negative
media coverage, investigations, loss of privileges and severe criminal or civil sanctions, any of which could have a materially adverse
effect on our reputation, business, results of operations, and prospects.
Our business is exposed to domestic and
foreign currency fluctuations that could have a material adverse effect on our business, financial condition, results of operations and
cash flows.
In the fiscal years ended May 31, 2023,
and May 31, 2022, our non-U.S. revenue was approximately 5.2% and 4.2% of our total revenue, respectively. With the abatement of
the COVID-19 pandemic permitting the reopening of classrooms in Latin America, this percentage may increase. Changes in non-U.S.
currencies relative to the U.S. dollar impact our revenues, profits, assets and liabilities. In addition, the weakening or
strengthening of the U.S. dollar may result in significant favorable or unfavorable translation effects when the operating results
of our non-U.S. business activity are translated into U.S. dollars and could cause our results of operations to differ from our
expectations and the expectations of our investors. For our international sales denominated in U.S. dollars, an increase in the
value of the U.S. dollar relative to foreign currencies could make our products and services less competitive in international
markets. Alternatively, a weakening of the currencies in which sales are generated relative to those in which costs are denominated
would decrease operating profits and cash flow. Changes in currency exchange rates may also affect the relative prices at which we
provide services in foreign markets. In addition, the impact of currency devaluations in countries experiencing high inflation rates
or significant currency exchange fluctuations could negatively impact our operating results. While we may use financial instruments
to mitigate the impact of fluctuations in currency exchange rates on our cash flows, unhedged exposures would continue to be subject
to currency fluctuations.
If we fail to manage our growth effectively,
we may be unable to execute our business plan or maintain high service levels and customer satisfaction.
We hope to attain rapid growth. Doing so will
place significant demands on our management and operational and financial resources. We have established international operations, including
Mexico, Peru, Ecuador, Columbia and the Dominican Republic. We plan to expand into Argentina, Chile, Brazil, Panama and other countries
where its activities are lawful. In addition, our organizational structure will become more complex as we grow, as will our operational,
financial and management controls and reporting systems and procedures. To manage growth in our operations, we will need to continue
to grow and improve our operational, financial and management controls and reporting systems and procedures. We will require significant
capital expenditures and the allocation of valuable management resources to grow and change in these areas. Our growth will place a significant
strain on our management and may distract management from other important functions. If we cannot manage our growth effectively, our
reputation, as well as our business, results of operations and financial condition, could be harmed.
We may not be able to compete effectively.
While we believe that the market served by the
Pharmacology University Business has few participants, if one or more competitors were to enter this market, we might not be able to compete
effectively for many reasons, including a competitor’s greater financial resources, better services, a more effective sales organization
or a superior website.
Many of our existing competitors have, and some
of our potential competitors could have, substantial competitive advantages such as greater recognition and longer operating histories,
larger sales and marketing budgets and resources, and, especially in the case of the Alpha Research Business, established relationships
with customers, greater resources to make acquisitions, lower labor costs and substantially greater financial and other resources. Competitors
with greater financial and operating resources may be able to respond more quickly and effectively than we can to new or changing opportunities,
developments or customer requirements. Conditions relating to the Alpha Research Business could also change rapidly and significantly,
potentially adversely, as a result of changes in the laws relating to cannabis, especially at the federal level.
If we do not compete effectively with established
companies as well as new market entrants, our business, results of operations, and financial condition could be harmed. Competitive pressures
could result in price reductions; fewer customers; reduced revenue, gross profit and gross margins; increased net losses; and loss of
market share.
Risks Related to the Common Stock and This
Offering
There are risks, including stock market
volatility, inherent in owning Common Stock.
The market price and volume of the Common Stock
have been, and may continue to be, subject to significant fluctuations and trading in Common Stock has often been sporadic. These fluctuations
may arise from general stock market conditions, the impact of risk factors described herein on our results of operations and financial
position, or a change in opinion in the market regarding our business prospects or other factors, many of which may be outside our control.
We believe that this has and may continue to materially and adversely affect our ability to fund our business through sales of equity
securities and could adversely affect the retentive power of our 2022 Equity Incentive Plan. The lack of an active market for Common
Stock may impair investors’ ability to sell their shares when they wish to sell them or at prices that they consider reasonable,
may reduce the fair market value of their shares and may impair the Company’s ability to raise capital to continue to fund operations
by selling shares and may impair its ability to acquire additional intellectual property assets by using our shares as consideration.
We may change the Fixed Offering Price.
If we cannot sell the Shares at the Fixed
Offering Price, we may amend the Registration Statement of which this Prospectus is a part to reduce it one or more times. In any such
event, investors who had purchased Shares before the reduction would suffer an immediate and perhaps permanent loss in the market value
of their Shares.
There will be a large number of shares
of Common Stock that will be eligible to be sold in the public markets.
In addition to the 10,087,154,885 shares of
Common Stock that are offered by this Prospectus, (i) approximately 1.7 billion shares of restricted Common Stock held by persons who
are not deemed to be affiliates of the Company will be permitted to be sold after January 13, 2024, under Rule 144 promulgated by the
SEC under the Securities Act (“Rule 144”) without notice to the SEC in unlimited amounts and without restriction as to the
manner of sale, (ii) approximately 2.4 billion share of Common Stock that are freely tradable, (iii) approximately 4,.6 billion shares
of restricted Common Stock held by persons who are deemed to be affiliates of the Company will be permitted to be sold under Rule 144
after January 13, 2024, in limited amounts, subject to notice to the SEC and subject to restriction as to the manner of sale and (iv)
up to 600,000,000 shares of Common Stock that may be issued under the Company's 2022 Equity Incentive Plan may be sold in the public
markets, subject to limitations in the case of shares issued under that plan to affiliates of the Company, upon the filing of a registration
statement on Form S-8 with respect thereto or without registration under Rule 144 after being held by for the period required by that
rule. The sale of these shares or the perception that they may be sold may substantially and adversely affect the market price of the
Common Stock, with the result that persons who acquire shares of Common Stock in the Offering may be able to resell them only at substantial
losses.
The Company may fail to comply with
its reporting obligations.
The Company is required to file reports with
the SEC, including annual and quarterly reports. These reports are required to contain information about the Company’s business
and operations, as well as financial statements (which will be audited by the Company’s public accounting firm in the case of annual
reports and reviewed by such firm in the case of quarterly reports) and provide other information upon which investors will rely in making
investment decisions about the Company. The Company, under prior management, failed to file these reports. The Company’s current
management intends to cause it to comply with its reporting obligations, but investors should consider such failure in making a decision
as to whether to purchase the shares offered by this Prospectus. The Company may also become delinquent if its funding were to become
insufficient to pay the legal and accounting costs of preparing these reports. If the Company were again to become delinquent, investors
would be deprived of material information about the Company, which could adversely affect the market price for the Common Stock, such
that holders of Common Stock could lose some or all of their investment and could encounter limited or no markets for the sale of their
securities.
If the Company issues additional equity
or equity-linked securities, investors may incur immediate and substantial dilution in the book value of their shares.
If the Company issues additional shares of Common
Stock (including under stock options or warrants) or securities convertible into or exchangeable or exercisable for shares of Common
Stock, its stockholders, including investors who purchase shares of Common Stock in the Offering, may experience additional dilution.
Any such issuances may result in downward pressure on the price of the Common Stock. No assurance can be given that investors will be
able to sell shares sold pursuant to this Prospectus at a price per share that is equal to or greater than the prices that they pay.
The Company has sold substantial amounts of shares at below-market prices. During the year ended May 31, 2023, it sold 2,042,146,825
shares of Common Stock at below-market prices for proceeds totaling $801,966 and during the year ended May 31, 2022, it sold 798,760,199
shares of Common Stock at below-market prices for proceeds totaling $825,290. Since May 31, 2023, it has sold 221,071,428 shares of Common
Stock at below-market prices for proceeds totaling $75,000. To the extent that the Company does not sell all of the shares of Common
Stock offered by this Prospectus, it may continue this practice. If it does so, shareholders will suffer substantial dilution.
The Company does not intend to pay dividends
for the foreseeable future and investors must rely on increases in the market price of the Common Stock for returns on their investments.
For the foreseeable future, the Company intends
to retain its earnings, if any, to finance the development and expansion of our business, and the Company does not anticipate paying any
cash dividends on the Common Stock. Accordingly, investors must be prepared to rely on sales of their Common Stock after price appreciation
to earn an investment return, but no assurance can be given that the price of the Common Stock will appreciate, or if it does, that it
will remain at or rise above the level to which it has appreciated. Any determination to pay dividends in the future will be made at the
discretion of the Company’s board of directors (the “Board”) and will depend on our results of operations, financial
condition, capital needs, contractual restrictions, restrictions imposed by applicable law and other factors the Board deems relevant.
Because the Common Stock is subject to
the penny stock rules, it may be more difficult to sell.
The SEC has adopted rules that regulate broker-dealer
practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00
(subject to exceptions that do not apply to the Common Stock). The penny stock rules require a broker-dealer, at least two business days
prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver to the customer a standardized risk disclosure
document containing specified information and to obtain from the customer a signed and date an acknowledgment of receipt of that
document. In addition, these rules require that, prior to effecting any transaction in a penny stock not otherwise exempt from those rules,
a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive:
(i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions
involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These requirements may have the effect of
reducing the trading activity in Common Stock, and therefore, stockholders may have difficulty selling their shares.
One person has voting control of the company
and may authorize or prevent corporate actions to the detriment of other stockholders.
One person, who is an officer and director of
the Company, through his ownership of Series B Preferred, has voting control of the Company. Accordingly, he has the power to determine
the outcome of all matters requiring the approval of the stockholders, including the election of directors and the approval of mergers
and other significant corporate transactions. His interests could conflict with the interests of other stockholders.
CAUTIONARY NOTES
Regarding Forward-Looking Statements
This Prospectus contains forward-looking statements
about us and our industry that involve substantial risks and uncertainties. All statements other than statements of historical facts
contained in this Prospectus, including statements regarding our strategy, future financial condition, future operations, projected costs,
prospects, plans, objectives of management, and expected market growth, are forward-looking statements. In some cases, you can identify
forward-looking statements because they contain words such as “may,” “will,” “shall,” “should,”
“expects,” “plans,” “anticipates,” “could,” “intends,” “target,”
“projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential,”
“goal,” “objective,” “seeks,” or “continue” or the negative of these words or other similar
terms or expressions that concern our expectations, strategy, plans, or intentions. Forward-looking statements contained in this Prospectus
include, but are not limited to, statements about the effects of the COVID-19 pandemic on our business and the U.S. and global economies
generally; our expectations regarding our financial performance; our expectations regarding future operating performance; our ability
to attract and retain customers; our ability to compete in our industries; our ability to meet our liquidity needs; our ability to effectively
manage our exposure to fluctuations in foreign currency exchange rates; the increased expenses associated with being a public company;
the size of our addressable markets, market share, and market trends, including our ability to grow our business in the countries we
have identified as near- term priorities; anticipated trends, developments, and challenges in our industry, business, and the highly
competitive markets in which we operate; our ability to anticipate market needs or develop new or enhanced offerings and services to
meet those needs; our ability to manage expansion into international markets and new industries; our ability to comply with laws and
regulations, including laws affecting the cannabis and pharmaceutical industries, that currently apply or may become applicable to our
business both in the United States and internationally; our ability to effectively manage our growth and expand our infrastructure and
maintain our corporate culture; our ability to identify, recruit, and retain skilled personnel, including key members of senior management;
our ability to successfully defend litigation brought against us; our ability to successfully identify, manage, and integrate any existing
and potential acquisitions; our ability to maintain, protect, and enhance our intellectual property; and our intended use of the net
proceeds from this offering.
You should not rely upon forward-looking statements
as predictions of future events. We have based the forward-looking statements in this Prospectus primarily on our current expectations,
estimates, forecasts, and projections about future events and trends that we believe may affect our business, results of operations, financial
condition, and prospects. Although we believe that we have a reasonable basis for each such forward-looking statement, we cannot guarantee
that the future results, activity levels, performance, or events and circumstances reflected in the forward-looking statements will be
achieved. The outcome of the events described or discussed in these forward-looking statements is subject to risks, uncertainties, and
other factors described in the section titled “Risk Factors” and elsewhere in this Prospectus. Moreover, we operate in a very
competitive and rapidly changing environment. New risks and uncertainties emerge from time to time, and we cannot predict all of
them that could have an impact on the forward-looking statements contained in this Prospectus. The results, events, and circumstances
reflected in the forward-looking statements may not be achieved or occur, and actual results, events or circumstances could differ materially
from those described in the forward-looking statements.
The forward-looking statements in this Prospectus
relate only to events or circumstances as of the date on which they are made. We undertake no obligation to update any forward-looking
statement in this Prospectus to reflect events or circumstances after the date of this Prospectus or to reflect new information or the
occurrence of unanticipated events, except as required by law. We may not achieve the plans, intentions, or expectations disclosed in
our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Our forward-looking statements
do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, or investments we may make.
In addition, statements that “we believe”
and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available
to us as of the date of this Prospectus, and while we believe such information forms a reasonable basis for such statements, such information
may be limited or incomplete, and such statements should not be read to indicate that we have conducted an exhaustive inquiry into, or
review of, all potentially available relevant information. These statements are inherently uncertain, and you should not unduly rely
upon them.
You should read this Prospectus and the documents
referred to in it completely and with the understanding that our actual future results may be materially different from what we expect.
All forward-looking statements in this Prospectus are qualified by these cautionary statements.
Third-Party Information
This Prospectus includes information and estimates
based on reports and other publications, sources from industry analysts, market research firms and other independent sources that were
generally available to the public and not commissioned by us, in addition to management’s good-faith estimates and analyses.
We believe that such reports and publications are reliable but have not independently verified them or their underlying data sources,
methodologies or assumptions. They contain information and estimates that are based on estimates, forecasts, projections, market research,
or similar methodologies and are inherently subject to uncertainties. Actual events or circumstances may differ materially from events
and circumstances reflected in these reports.
Descriptions of Contracts
This Prospectus may contain descriptions of contracts
and instruments to which the Company or its officers and directors are parties or by which it is affected. These contracts and instruments
are exhibits to the Registration Statement of which this Prospectus is a part and are identified in Item 16, Exhibits, Financial Statement
Schedules. Where any such contract or instrument is described in this Prospectus, you are referred to the related exhibit, which
may be found on the SEC’s website, and the description thereof is qualified by such reference.
USE OF PROCEEDS AND BUSINESS
PLAN
This Prospectus relates in part to shares of
Common Stock that may be offered and sold from time to time by the Company and in part to shares being offered and sold by the Selling
Shareholders. We will receive proceeds from sales of the shares that we are offering and none of the proceeds of sales of shares offered
by the Selling Stockholders. See “Plan of Distribution.”
We will realize gross proceeds from the Offering
of $1,250,000 if 25% of the shares offered by us are sold, $2,500,000 if 50% of such shares are sold, $3,750,000 if 75% of such shares
are sold and $5,000,000 if 100% of such shares are sold.
Use of Proceeds
The following table shows how we expect to use
the net proceeds from our sales of the shares in executing our business plan, which is discussed below. Further details as to such use
appear in “Business Plan.” The table does not represent the order of priority in which such proceeds may be applied.
Estimated
Use of Proceeds for 25%, 50%, 75%, and 100% of Offering
| |
|
25% of Offering |
|
|
50% of Offering |
|
|
75% of Offering |
|
|
100% of Offering |
|
| |
|
Dollar
Amount |
|
|
|
% of Gross Proceeds |
|
|
|
Dollar Amount |
|
|
|
% of Gross Proceeds |
|
|
Dollar
Amount |
|
|
% of Gross Proceeds |
|
|
Dollar
Amount |
|
|
% of Gross Proceeds |
|
| Alpha Research Institute |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Increase employees from 8 to 35 |
|
$ |
131,250 |
|
|
|
10.5% |
|
|
$ |
262,500 |
|
|
|
10.5% |
|
|
$ |
393,750 |
|
|
|
10.5% |
|
|
$ |
525,000 |
|
|
|
10.5% |
|
| Obtain new contacts with health professionals,
sponsors and CROs |
|
$ |
7,500 |
|
|
|
0.6% |
|
|
$ |
15,000 |
|
|
|
0.6% |
|
|
$ |
22,500 |
|
|
|
0.6% |
|
|
$ |
30,000 |
|
|
|
0.6% |
|
| Contract with at least ten new principal
investigators specializing in various areas of medicine |
|
$ |
25,000 |
|
|
|
2.0% |
|
|
$ |
50,000 |
|
|
|
2.0% |
|
|
$ |
75,000 |
|
|
|
2.0% |
|
|
$ |
100,000 |
|
|
|
2.0% |
|
| Conduct at least six seminars with
the expectation of generating relationships |
|
$ |
5,000 |
|
|
|
0.4% |
|
|
$ |
10,000 |
|
|
|
0.4% |
|
|
$ |
15,000 |
|
|
|
0.4% |
|
|
$ |
20,000 |
|
|
|
0.4% |
|
| Expand to be capable of performing
clinical trials with cannabinoids |
|
$ |
200,000 |
|
|
|
16.0% |
|
|
$ |
400,000 |
|
|
|
16.0% |
|
|
$ |
600,000 |
|
|
|
16.0% |
|
|
$ |
800,000 |
|
|
|
16.0% |
|
| Add additional equipment for research
|
|
$ |
143,750 |
|
|
|
11.5% |
|
|
$ |
287,500 |
|
|
|
11.5% |
|
|
$ |
431,250 |
|
|
|
11.5% |
|
|
$ |
575,000 |
|
|
|
11.5% |
|
| Total Alpha Research Institute |
|
$ |
512,500 |
|
|
|
41.0% |
|
|
$ |
1,025,000 |
|
|
|
41.0% |
|
|
$ |
1,537,500 |
|
|
|
41.0% |
|
|
$ |
2,050,000 |
|
|
|
41.0% |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Pharmacology University |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Increase the number of annual paid
subscriptions to Cannabis World Journals to at least 5,000 |
|
$ |
12,500 |
|
|
|
1.0% |
|
|
$ |
25,000 |
|
|
|
1.0% |
|
|
$ |
37,500 |
|
|
|
1.0% |
|
|
$ |
50,000 |
|
|
|
1.0% |
|
| Sell educational materials to third
parties |
|
$ |
25,000 |
|
|
|
2.0% |
|
|
$ |
50,000 |
|
|
|
2.0% |
|
|
$ |
75,000 |
|
|
|
2.0% |
|
|
$ |
100,000 |
|
|
|
2.0% |
|
| Resume and increase classroom and
seminar teaching |
|
$ |
31,250 |
|
|
|
2.5% |
|
|
$ |
62,500 |
|
|
|
2.5% |
|
|
$ |
93,750 |
|
|
|
2.5% |
|
|
$ |
125,000 |
|
|
|
2.5% |
|
| Increase our portfolio of cannabis-related
educational material |
|
$ |
25,000 |
|
|
|
2.0% |
|
|
$ |
50,000 |
|
|
|
2.0% |
|
|
$ |
75,000 |
|
|
|
2.0% |
|
|
$ |
100,000 |
|
|
|
2.0% |
|
| Total Pharmacology University |
|
$ |
93,750 |
|
|
|
7.5% |
|
|
$ |
187,500 |
|
|
|
7.5% |
|
|
$ |
281,250 |
|
|
|
7.5% |
|
|
$ |
375,000 |
|
|
|
7.5% |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Corporate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating costs |
|
$ |
243,750 |
|
|
|
19.5% |
|
|
$ |
487,500 |
|
|
|
19.5% |
|
|
$ |
731,250 |
|
|
|
19.5% |
|
|
$ |
975,000 |
|
|
|
19.5% |
|
| Overhead |
|
$ |
150,000 |
|
|
|
12.0% |
|
|
$ |
300,000 |
|
|
|
12.0% |
|
|
$ |
450,000 |
|
|
|
12.0% |
|
|
$ |
600,000 |
|
|
|
12.0% |
|
| Legal and accounting |
|
$ |
50,000 |
|
|
|
4.0% |
|
|
$ |
100,000 |
|
|
|
4.0% |
|
|
$ |
150,000 |
|
|
|
4.0% |
|
|
$ |
200,000 |
|
|
|
4.0% |
|
| Operating capital |
|
$ |
200,000 |
|
|
|
16.0% |
|
|
$ |
400,000 |
|
|
|
16.0% |
|
|
$ |
600,000 |
|
|
|
16.0% |
|
|
$ |
800,000 |
|
|
|
16.0% |
|
| Total Corporate |
|
$ |
643,750 |
|
|
|
51.5% |
|
|
$ |
1,287,500 |
|
|
|
51.5% |
|
|
$ |
1,931,250 |
|
|
|
51.5% |
|
|
$ |
2,575,000 |
|
|
|
51.5% |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Use of Proceeds |
|
$ |
1,250,000 |
|
|
|
100% |
|
|
$ |
2,500,000 |
|
|
|
100% |
|
|
$ |
3,750,000 |
|
|
|
100% |
|
|
$ |
5,000,000 |
|
|
|
100% |
|
The foregoing represents our best estimate as
to how the proceeds of the shares offered by the Company will be expended. We reserve the right to redirect any portion of the funds either
among the items referred to above or to such other projects as our management considers to be in our best interest.
Business Plan
Background
Since the year ended on
May 31, 2020, the Company has striven to grow and improve in the following ways:
Alpha Research Institute
Alpha Research Institute
has devoted time and attention to bettering interinstitutional relationships with the pharmaceutical industry; improving operational
values; creating and re-establishing alliances with clinical study contractors; understanding the needs of its staff and its patients,
improving documentation and internal and external communications, offering transportation to patients and increasing participation in
clinical trials by addressing potential patients’ Covid-related concerns. As discussed under the caption “Clinical Trials
in which we will be the Sponsor,” the Company also intends to conduct clinical trials of cannabinoids.
Pharmacology University
As a result of the COVID-19
pandemic, which made classroom education impossible, Pharmacology University has focused on the production of educational materials for
sale on online platforms (including those operated by Amazon, Zinio, Apple, Walmart/Kobo, Barnes & Noble and Google Books), while
maintaining its relationships with academic venues. During the pandemic, in an effort to continue to provide cannabis-related education,
Pharmacology University recorded over 100 online classes that were available on our platform as well as third-party platforms.
With the abatement of
the COVID-19 pandemic, it has resumed classroom education by holding a class in Austin, Texas, on April 22, 2023, and mixed-mode (personal
and virtual) classes in Cartagena, Colombia, beginning on April 14, 2023. The Company believes that over time, but subject to the availability
of capital, it will be successful in resuming classroom education.
We have published 50
cannabis-related eBooks in five languages, have produced videos to offer online and have recorded over 13,000 minutes of audio in five
languages. We have also engaged artificial intelligence services to generate translations of these materials in up to 100 additional
languages; while this activity has resulted in increased expenses while producing minimal revenue and no profit, we believe that it will
become profitable and become a significant segment of our business.
Several of our online publications
have been unified into a single magazine, Cannabis World Journals, which began publication in five languages, beginning in the
third and fourth quarters of the year ending May 31, 2022.
Operating Goals
The Company has established
the following principal goals, for the attainment of which it expects to expend approximately $2,425,000 for the period ending May 31,
2024:
Alpha Research Institute
The Company’s principal
goals for Alpha Research Institute are:
| |
· |
to increase its revenue from clinical trials from $196,637 in the year ended May 31, 2022, to $1,000,000 in the year ending May 31, 2024.. To achieve this goal, the number of Alpha Research Institute’s personnel employees will be increased from its present seven to approximately 35, comprising approximately 25 employees in the Houston office and approximately ten patient recruiters, for which the Company will need to spend approximately $525,000. |
| |
|
|
| |
· |
to obtain new contacts with health professionals, sponsors and CROs to obtain new and diverse clinical trials at an approximate cost of $30,000. |
| |
|
|
| |
· |
to contract with at least ten new principal investigators, specializing in various areas of medicine, including cancer, PTSD, rare diseases and glaucoma, and organize six training programs on clinical research for health professionals at an approximate cost of $100,000. |
| |
|
|
| |
· |
to conduct at least six seminars in Houston with the expectation of generating relationships with personnel in the U.S. pharmaceutical industry in charge of finding new clinical trials at the cost of approximately $20,000. |
| |
|
|
| |
· |
to create the capacity to conduct cannabinoid-related clinical trials at a cost of approximately $800,000 |
| |
|
|
| |
· |
to purchase additional research equipment at a cost of approximately $575,000. |
The total cost of these
goals is approximately $2,050,000.
Pharmacology University
The Company’s principal
goals for Pharmacology University are:
| |
· |
to continue to resume and increase classroom and seminar teaching, which involved approximately four classrooms in two countries, generating revenues of approximately $38,440, to 10 classrooms in 4 countries, generating revenues of $200,000, at an approximate cost of $125,000. |
| |
|
|
| |
· |
to increase the number of annual paid subscriptions to Cannabis World Journals to at least 5,000, which will require better positioning and improving traffic on the internet and social media by using search engine optimization (SEO) and search engine marketing (SEM) strategists at an approximate cost of $50,000. |
| |
|
|
| |
· |
to increase our portfolio of cannabis-related educational material from 50 eBooks in five languages, 154 online videos in five languages, and over 13,000 minutes of audio in 5 languages to 150 eBooks in five languages, 300 online videos in more than 100 languages, and over 40,000 minutes of audio in five languages, generating revenues of $200,000 at an approximate cost of $100,000. |
| |
|
|
| |
· |
to sell the above educational materials to third parties, who would resell them worldwide on their platforms, we will need to increase our sales staff and supervisors. We believe that this activity could generate revenues of $500,000 at an approximate cost of $100,000. |
The total cost of these
goals is approximately $375,000.
The Company intends to devote
its manpower and capital resources to execute its business plan, which it believes will enable it to become profitable. No assurance can
be given, however, that the Company can obtain any of the goals set forth above, in whole or in part.
Even if the Company sells all of the shares of
Common Stock that the Company is offering under this Prospectus, it will need to obtain additional financing to attain the above
goals. For further information regarding the Company’s capital needs and its ability to meet them, see “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources.”
DIVIDEND POLICY
We intend to retain any future earnings and do
not anticipate declaring or paying cash dividends in the foreseeable future. If we raise capital through borrowing, the terms of the
related instruments may restrict our ability to pay dividends or make distributions. Future determination to declare cash dividends will
be made at the discretion of our Board, subject to applicable laws, and will depend on many factors, including our financial condition,
results of operations, capital requirements, contractual restrictions, general business conditions and such other factors as the Board
may deem relevant.
CAPITALIZATION
The following table sets forth our capitalization
as of May 31, 2023, and as adjusted at that date to give effect to the issuance of all of the shares offered by this Prospectus
at the Fixed Offering Price.
| | |
As of May 31, 2023 | |
| | |
Actual | | |
As Adjusted | |
| Long-term debt: | |
$ | 249,500 | | |
$ | 234,210 | |
| Stockholders’ equity: | |
| | | |
| | |
| Common Stock | |
| – | | |
| – | |
| Series A Preferred | |
| – | | |
| – | |
| Series B Preferred | |
| – | | |
| – | |
| Additional paid-in capital | |
| 4,091,071 | | |
| 8,676,771 | |
| Accumulated deficit | |
| (4,682,736 | ) | |
| (4,082,072 | ) |
| Total capitalization (stockholders’ deficit) | |
$ | (342,165 | ) | |
$ | 4,828,909 | |
DILUTION
If you invest in the shares offered by this
Prospectus, your ownership interest will be diluted to the extent of the difference between the offering price per share of Common Stock
and the pro forma as adjusted net tangible book value per share of Common Stock immediately after this offering. Dilution results from
the fact that the per share offering price of the Common Stock is substantially higher than the book value per share attributable to
our existing stockholders. Pro forma net tangible book value per share represents the amount of stockholders’ equity (deficit),
excluding intangible assets, divided by the number of shares of Common Stock outstanding at that date, without giving effect to the conversion
of the Series A Convertible Preferred Stock, which is convertible into shares of Common Stock, but cannot be so converted, now or for
the foreseeable future, on an economically rational basis.
Our historical net tangible book value deficit
at May 31, 2023, was $615,585, or $0.00006 per share of Common Stock. This deficit is our total tangible assets minus our total liabilities.
Historical net tangible book value (deficit) per share is historical net tangible book value (deficit) divided by the number of shares
of Common Stock outstanding as of May 31, 2023.
After giving effect to our sale in the offering
of the shares of Common Stock offered by the Company at an assumed initial public offering price of $0.0008 per share, the midpoint of
the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated
offering expenses payable by us, our pro forma as adjusted net tangible book value as of May 31, 2023, would have been approximately
4,258,335, or $0.00026 per share of Common Stock. This represents an immediate dilution of $0.00054 per share, or 67.5% per share, to
investors purchasing shares in the Offering.
The following table illustrates such dilution.
| Assumed initial public offering price per share | |
$ | 0.0008 | |
| Net tangible book value per share as of May 31, 2023 | |
| (0.00006 | ) |
| | |
| | |
| Increase in net tangible book value per share attributable
to investors in the Offering | |
| 0.00078 | |
| Net tangible book value per share after
the Offering | |
$ | 0.00028 | |
| | |
| | |
| Dilution in net tangible book value
per share to investors in the Offering | |
$ | 0.00052 | |
The following table summarizes, on a pro forma
as adjusted basis described above as of May 31, 2023, the total cash consideration paid and the average price per share paid by
our existing stockholders and by our new investors purchasing shares in this offering at the Offering Price of $0.0008 per share, before
deducting the estimated offering expenses payable by us:
| |
|
Shares
Purchased |
|
|
Total
Consideration |
|
|
Average
Price |
|
| |
|
Number |
|
|
Percent |
|
|
Amount |
|
|
Percent |
|
|
Per Share |
|
| Existing stockholders1 |
|
|
10,059,677,919 |
|
|
|
61.68 |
|
|
$ |
(615,585 |
) |
|
|
(13.89 |
) |
|
$ |
(0.00006 |
) |
| New investors1 |
|
|
6,250,000,000 |
|
|
|
38.32 |
|
|
|
4,850,000 |
|
|
|
113.89 |
|
|
|
0.00078 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
16,309,677,919 |
|
|
|
100 |
|
|
|
4,234,415 |
|
|
|
100 |
|
|
|
0.00026 |
|
___________
1 After estimated offering
expenses of $150,000.
The foregoing tables and calculations (other
than the historical net tangible book value calculation) are based on 16,581,749,347 shares of Common Stock outstanding after the Offering
and exclude (i) 272,071,428 shares of Common Stock that have been issued since May 31, 2023, at below market prices, for the effect of
which on dilution, see the next paragraph, (ii) 600,000,000 shares of Common Stock that may be issued under the Incentive Plan and (iii)
shares of Common Stock that may be issued upon the conversion of the Series A Convertible Preferred Stock, which is convertible into
shares of Common Stock, but cannot be so converted, now or for the foreseeable future, on an economically rational basis. To the extent
that shares of Common Stock are issued under the Incentive Plan or we issue additional shares of Common Stock in the future, there will
be further dilution to investors participating in the Offering. If we raise additional capital through the sale of equity or convertible
debt securities, the issuance of these securities could result in further dilution to our stockholders.
The Company received $75,000 for the 272,071,428
shares of Common Stock described in the previous paragraph. The following table summarizes, on a pro forma as adjusted basis described
above as of May 31, 2023, the total cash consideration paid and the average price per share that would be paid by our existing stockholders
and by our new investors purchasing shares in the Offering at the Offering Price of $0.0008 per share, before deducting the estimated
offering expenses payable by us, if these shares had been issued and paid for and the Offering had occurred on that date:
| |
|
Shares
Purchased |
|
|
Total
Consideration |
|
|
Average
Price |
|
| |
|
Number |
|
|
Percent |
|
|
Amount |
|
|
Percent |
|
|
Per Share |
|
| Existing stockholders1 |
|
|
10,331,749,347 |
|
|
|
62.31 |
|
|
$ |
(540,585 |
) |
|
|
(12.54 |
) |
|
$ |
(0.00005 |
) |
| New investors1 |
|
|
6,250,000,000 |
|
|
|
37.69 |
|
|
|
4,850,000 |
|
|
|
12.54 |
|
|
|
0.00078 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
16,581,749,347 |
|
|
|
100.00 |
|
|
|
4,309,415 |
|
|
|
100 |
|
|
|
0.00026 |
|
___________
1 After estimated offering
expenses of $150,000.
MANAGEMENT’S DISCUSSION
AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our
financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes
that appear in this Prospectus. In addition to historical consolidated financial information, the following discussion may contain forward-looking
statements that reflect our plans, estimates, and beliefs. These forward-looking statements are subject to numerous risks and uncertainties,
including, but not limited to, the risks and uncertainties described under “Risk Factors” and elsewhere
in this Prospectus. Our actual results may differ materially from those contained in or implied by any forward-looking statements.
Information about the Company
The Company, headquartered in Houston, Texas, conducts
clinical trials for Sponsors and CROs and as a Sponsor through Alpha Research Institute and cannabis-related education in classrooms,
seminars and online through Pharmacology University. For detailed information about the Company and its operations, see “Description of Business.”
The Company’s fiscal year begins on June 1 in
each year and ends on May 31 in the following year.
Going Concern
As indicated in Note 3 of the notes to the audited
consolidated financial statements for the year ended May 31, 2023, and the report thereon of the Company’s independent auditing
firm, there is substantial doubt as to the ability of the Company to continue as a going concern. The Company has incurred recurring
losses and recurring negative cash flow from operating activities and has an accumulated deficit, and its ability to continue as a going
concern depends on the successful execution of its operating plan, which includes the resumption of services that were interrupted by
the COVID-19 pandemic, increasing sales of existing services and introducing new services, as well as raising either debt or equity financing.
The Company needs substantial additional capital to
fund its business, including the completion of its business plan and repayment of its debts. No assurance can be given that any additional
capital can be obtained or, if obtained, will be adequate to meet its needs, and the Company may need to take measures to remain a going
concern. If adequate capital cannot be obtained on a timely basis and satisfactory terms, the Company’s operations could be materially
negatively impacted, or it could be forced to terminate its operations.
Impact of the COVID-19 Pandemic
The COVID-19 pandemic has adversely impacted the
Company and its financial results in different ways, depending on the particular business operation, as follows:
Pharmacology University Business. The
Company encountered quarantines, restrictions on gatherings and other governmental regulations that precluded classroom education, as
well as restrictions on travel that reduced consulting activities. The Company reduced the impact of the pandemic by developing online
educational programs and transitioning its workforce to a remote working environment without reducing its workforce. Revenue from this
operation was increased from $18,323 in the year ended May 31, 2019 (unaudited), to $44,799 and $38,440 in the years ended May 31, 2020,
and May 31, 2021, respectively; revenue for the year ended May 31, 2022, was $18,341 and for May 31, 2023, was $42,655. Revenue for the
quarter ended August 31, 2023, was $1,580.
Clinical Trials. Quarantines,
restrictions on gatherings and other governmental regulations, amplified by potential patients’ fears of contracting COVID-19 at
the Company’s clinics, negatively affected clinical trials. In addition, these clinics were subject to closure if cases of the
virus were detected. Revenue from this operation changed from $165,666 in the year ended May 31, 2019 (unaudited), to $84,979 and $706,008
in the years ended May 31, 2020, and May 31, 2021, respectively; revenue for the year ended May 31, 2022, was $196,637; revenue for the
year ended May 31, 2023, was $266,280. Revenue for the quarter ended August 31, 2023, was $72,821.
The Company believes that it may have been negatively
impacted by the association of the pandemic with the People’s Republic of China because “China” appeared in its former
corporate name. Although the Company has no operations in or any relationship with China, the Company believes that potential investors
may have been deterred from considering the Company because of concerns related to that country. For this reason, and because the Company’s
corporate name does not reflect its activities, it changed its name to Cannabis Bioscience International Holdings, Inc. on December 6,
2022.
Overview
During the quarter ended August 31, 2023, the
Company provided educational systems focused on medical cannabis in the United States and Latin America, as well as worldwide through
online education; services in therapeutic areas of clinical trials; and services relating to sleep disorders through its Sleep Center
in Houston, Texas. The Company’s operating units and their activities were:
| |
· |
Alpha Research Institute – Clinical trials and medical research. |
| |
|
|
| |
· |
Pharmacology University: – Education, consulting, digital publishing, marketing, and franchising related to medical cannabis. |
The Company operated a Sleep Center, which diagnosed
sleep-related disorders and its operations on April 30, 2023.
For further information concerning the Company and
its business, see “Description of Business.”
Results of Operations
Comparison of the Quarter Ended August
31, 2023, and the Quarter Ended August 31, 2022
The following table sets forth information from
the consolidated statements of operations for the quarters ended August 31, 2023, and August 31, 2022.
| |
|
Quarter Ended August
31, |
|
| |
|
2023 |
|
|
2022 |
|
| Revenues |
|
$ |
72,821 |
|
|
$ |
143,752 |
|
| Cost of revenues |
|
|
8,820 |
|
|
|
33,170 |
|
| Gross profit |
|
|
64,001 |
|
|
|
110,582 |
|
| |
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
240,785 |
|
|
|
302,388 |
|
| Operating loss |
|
|
(176,784 |
) |
|
|
(191,806 |
) |
| |
|
|
|
|
|
|
|
|
| Non-operating income (expense): |
|
|
|
|
|
|
|
|
| Interest |
|
|
(5,005 |
) |
|
|
(12,235 |
) |
| Other income |
|
|
0 |
|
|
|
41,666 |
|
| Net income (loss) |
|
$ |
(181,789 |
) |
|
$ |
(162,375 |
) |
Revenues
Revenues were $72,821 and $143,752 for the
quarter ended August 31, 2023, and August 31, 2022, respectively, primarily due to a decrease of $61,958 in revenues from clinical trial
contracts, which were $124,916 in the earlier period and $62,958 in the later because no new Sponsors were added. Revenues from cannabis-related
educational classes and seminars decreased by $9,915, from $9,915 for the quarter ended August 31, 2022, to $0 for the quarter ended
August 31, 2023, primarily due to a reduction of staff and marketing to promote them. Consulting fees were $8,333 for the quarter ended
August 31, 2023, versus $0 for the quarter ended August 31, 2022. Online sales of educational materials decreased by $7,391, from $8,921
in the quarter ended August 31, 2022, to $1,530 in the quarter ended August 31, 2023
Operating Expenses
Operating expenses for the quarters ended August
31, 2023, and August 31, 2022, consisted of the following:
| |
|
Quarter Ended August
31, |
|
| |
|
2023 |
|
|
2022 |
|
| General and administrative |
|
$ |
48,083 |
|
|
$ |
29,304 |
|
| Contract labor |
|
|
95,654 |
|
|
|
173,849 |
|
| Professional fees |
|
|
62,394 |
|
|
|
67,322 |
|
| Officer compensation |
|
|
12,000 |
|
|
|
12,000 |
|
| Rent |
|
|
20,953 |
|
|
|
18,246 |
|
| Travel |
|
|
1,701 |
|
|
|
1,667 |
|
| Total operating expenses |
|
$ |
240,785 |
|
|
$ |
302,388 |
|
The reduction in operating expenses was primarily
due to a decrease in contract labor of $78,915 as the result of reductions in the staffing used to write, translate, and produce audiobooks,
e-books, and online videos and a reduction of $10,303 in general and administrative as the result of a $5,000 reduction in advertising,
a $2,000 reduction in job posting and a $3,000 reduction in office supplies.
Operating Loss
For the reasons set forth above, operating
loss decreased from $191,806 in the quarter ended August 31, 2022, to $176,784 in the quarter ended August 31, 2023.
Interest
Interest was $12,235 in the quarter ended
August 31, 2022, and $5,005 in the quarter ended August 31, 2023.
Other Income
In the quarters ended August 31, 2023, and
August 31, 2022, respectively, the Company recorded other income of $0 and $41,666 from the forgiveness of PPP loans.
Net Loss
Net loss for the quarter ended August 31,
2023, was $181,789, compared with a net loss of $162,375 for the quarter ended August 31, 2022, for the reasons set forth above in relation
to loss from operations and the effect of other income received in the quarter ended August 31, 2022.
Comparison of the Year Ended May 31,
2023, and the Year Ended May 31, 2022
The following table sets forth information from
the consolidated statements of operations for the years ended May 31, 2023, and May 31, 2022.
| | |
Year Ended May 31, | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | 316,825 | | |
$ | 214,981 | |
| Cost of revenues | |
| 93,450 | | |
| 46,763 | |
| Gross profit | |
| 223,375 | | |
| 168,217 | |
| | |
| | | |
| | |
| Total operating expenses | |
| 1,206,746 | | |
| 1,056,275 | |
| Operating loss | |
| (983,371 | ) | |
| (888,058 | ) |
| | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | |
| Interest | |
| (90,973 | ) | |
| (51,036 | ) |
| Other income | |
| 41,765 | | |
| 53,923 | |
| Net income (loss) | |
$ | (1,032,579 | ) | |
$ | (885,171 | ) |
Revenues
Revenues were $316,825 and $214,981 for the
years ended May 31, 2023, and May 31, 2022, respectively, primarily due to an increase of $69,643 in revenues from clinical trial contracts,
which were $196,637 in the earlier period and $266,280 in the later. Revenues from cannabis-related educational classes and seminars
increased by $24,314, from $18,341 for the year ended May 31, 2022, to $42,655 for the year ended May 31, 2023, primarily due to the
effects of the COVID-19 pandemic’s abating. Consulting fees were $8,333 for the year ended May 31, 2023, versus $0 for the year
ended May 31, 2022.
Operating Expenses
Operating expenses for the years ended May 31,
2023, and May 31, 2022, consisted of the following:
| | |
Years Ended May 31, | |
| | |
2023 | | |
2022 | |
| General and administrative | |
$ | 177,110 | | |
$ | 134,348 | |
| Contract labor | |
| 659,651 | | |
| 544,760 | |
| Professional fees | |
| 245,691 | | |
| 222,535 | |
| Officer compensation | |
| 45,735 | | |
| 70,983 | |
| Rent | |
| 71,942 | | |
| 75,226 | |
| Travel | |
| 6,617 | | |
| 8,420 | |
| Total operating expenses | |
$ | 1,206,746 | | |
$ | 1,056,272 | |
Increased contract labor was due to adding staff
to write, translate, and produce audiobooks, e-books, and online videos. Professional fees increased due to increases in auditing costs
incurred principally in connection with the preparation of the Company’s financial statements for the year ended May 31, 2023, and
legal expenses incurred in connection with the preparation of the registration statement of which this Prospectus is a part, as well in
the preparation of reports that the Company filed with OTC Markets Group Inc. Officer compensation decreased because an officer left the
Company and was not replaced.
Operating Loss
For the reasons set forth above, operating loss
increased from $888,058 in the year ended May 31, 2022, to $983,371 in the year ended May 31, 2023.
Interest
Interest was $51,036 in the year ended May 31,
2022, and $90,973 in the year ended May 31, 2023.
Other Income
In the years ended May 31, 2023, and May 31,
2022, respectively, the Company recorded other income of $41,765 and $53,923, of which $41,765 and $46,695 respectively resulted from
the forgiveness of PPP loans.
Net Loss
Net loss for the year ended May 31, 2023, was
$1,032,579, compared with a net loss of $885,171 for the year ended May 31, 2022, for the reasons set forth above in relation to income
(loss) from operations and the effect of other income received in these years.
Liquidity and Capital Resources
At August 31, 2023, the Company had $4,750
in cash and cash equivalents and accounts receivable of $27,990, negative working capital of $482,547 and no commitments for capital
expenditures. At May 31, 2023, the Company had $8,913 in cash and cash equivalents and accounts receivable of $10,549, negative working
capital of $366,085 and no commitments for capital expenditures. The Company had cash in the amount of $_______ on the date of this Prospectus.
During the quarters ended August 31, 2023,
and August 31, 2022, the Company had net cash used in operations of $74,620 and $195,969, respectively, and cash flow provided by financing
activities of $70,453 and $165,859, respectively. During the years ended May 31, 2023, and May 31, 2022, the Company had net cash used
in operations of $898,367 and $870,704, respectively, and net cash provided by financing activities of $875,298 and $861,364, respectively.
The Company had accumulated deficits of $4,855,403 at August 31, 2023, and $4,682,736 at May 31, 2023.
For information regarding delays in payments by Sponsors
and CROs that have affected, and if they were to recur, could affect, the Company’s cash flows, see “Risk Factors –
Delays in payments by Sponsors and CROs have affected and may continue to affect the Company’s cash flows.”
Since June 1, 2021, the Company has raised capital
as follows:
| |
· |
In the years ended May 31, 2023, and the year ended May 31, 2022,
the Company received $801,956 and $825,290, respectively, from sales of
Common Stock to private investors. Since June 1, 2023, it has received $75,000 from such sales. |
| |
|
|
| |
· |
In the years ended May 31, 2023, and May 31, 2022, the Company received
loans of $73,332 and $48,074, respectively. Since June 1, 2023, it has received $33,481 from loans. |
The Company believes that it will require $2,425,000
to attain the goals described under “Business Plan” and estimates that other capital needs, including operating costs of $975,000,
legal/accounting costs of $200,000, overhead of $600,000 and a reserve for contingencies of $800,000 for the next two years, will approximate
$2,575,000, totaling $5,000,000.
To the extent that capital needs cannot be met by
revenue from operations, profits and the proceeds of the Offering, the Company will need to raise additional capital through the sale
of debt or equity securities to public and private investors. There is no assurance that such funding will be available on acceptable
terms or at all or that the Company will attain profitability. If the Company cannot raise sufficient funds when required or on acceptable
terms, it may have to reduce its operations significantly or discontinue them entirely. To the extent that funds are raised by issuing
equity securities or securities that are convertible into the Company’s equity securities, its stockholders may experience significant
dilution.
As indicated in this Prospectus, the Company was materially
and adversely impacted by the COVID-19 pandemic. With the lifting of the restrictions imposed in response to the pandemic, the Company
is resuming normal operations in its Pharmacology University and Alpha Research Businesses. If the Company is successful in carrying out
its business plan, it expects to become profitable in the year ending May 31, 2024, and beyond.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
DESCRIPTION OF BUSINESS
History
The Company was formed in the State of Colorado
on February 28, 2003, as a limited liability company under the name Fidelity Aircraft Partners LLC. On December 16, 2009, it converted
to a corporation under the name Fidelity Aviation Corporation, and on August 24, 2009, it changed its name to China Infrastructure Construction
Corp. On February 28, 2018, the Company changed its name to Hippocrates Direct Healthcare, Inc. and on July 4, 2018, it resumed its present
name. On December 6, 2022, it changed its corporate name to Cannabis Bioscience International Holdings, Inc.
From its inception to 2009, the Company
sold used aircraft parts and airframe components salvaged from non-flying jet aircraft. Beginning on October 8, 2009, the Company
terminated that business and entered into concrete production in the People’s Republic of China and Hong Kong through
subsidiaries, which were sold to an unrelated party in 2016. No information about the Company’s operations or management was
available from early 2012 to early 2015, but the current management believes that the Company was dormant during that period and
that its directors and officers abandoned their positions. In February 2015, an independent investor obtained control of the
Company. On July 25, 2016, the Company disposed of its subsidiaries and on January 6, 2017, transferred control of the Company to
another independent investor. On February 5, 2018, control of the Company was acquired by a former member of its management. On
December 20, 2019, the present management acquired control of the Company as a result of the acquisition of Pharmacology University,
Inc. (see below). The Company began to file reports with OTC in 2018 under its Alternate Reporting Standard and has been a
“Pink Sheet” company since then. Market prices for the Common Stock have been volatile and its trading volume has
fluctuated extremely. See “Risks Related to the Common Stock and This Offering – There are risks, including stock market
volatility, inherent in owning Common Stock.” In the past, the Company failed to comply with its reporting requirements under
the Securities Exchange Act of 1934. See “Risk Factors – Risk Factors Related to the Common Stock and to This Offering
– The Company has failed to comply with its reporting obligations.”
Acquisition of Hippocrates
On December 17, 2017, the Company acquired
Hippocrates Direct Healthcare, LLC, a Texas limited liability company (“Hippocrates”). Before this acquisition, the Company
had no operations and no or nominal assets (a “Rule 144 Shell Company”). As a result of this acquisition, the Company ceased
to be a Rule 144 Shell Company. The business of Hippocrates (the “Hippocrates Business”), which was terminated on October
31, 2020, was concierge healthcare.
Acquisition of Pharmacology University Inc.
Pharmaceutical University Inc.(“PUI”) was
incorporated in the State of Delaware on January 5, 2017, under the corporate name Canna-Pharmacology University Inc.; on March 15,
2017, its certificate of incorporation was amended to change its corporate name to Pharmacology University Inc. On December 20,
2019, PUI was merged with and into the Company, such that the shareholders of PUI received 4,875,000,000 shares of Common Stock and
2,000,000 shares of the Company’s Series A Convertible Preferred Stock (“Series A Preferred”) as merger
consideration. The Company conducts the business acquired by this merger (the “Pharmacology University Business”) under
the trade name Pharmacology University. The Pharmacology University Business is generally cannabis-related research and education.
For a more detailed description of the Pharmacology University Business, see “Description of Business –
Pharmacology University Business.” For a description of the interest of certain members of the management of the Company
in this merger, “Certain Relationships and Related Party Transactions – Merger with Pharmacology
University, Inc.”
Acquisition of Precision Research Institute
On March 31, 2019, the Company entered into the
Alpha Research Business by acquiring all of the outstanding units in Precision Research Institute, LLC, a Texas limited liability company
(“PRI”), which was formed on May 18, 2016, from the Company’s then president. On August 20, 2020, PRI was merged with
and into the Company. The Company conducts the Alpha Research Business under the trade name Alpha Research Institute. As indicated
under that caption, the Company intends to conduct clinical trials of cannabinoids as a Sponsor. For a detailed description of the
Alpha Research Business, see “Description of Business – Alpha Research Business.”
Businesses
The Company has three operations, namely,
the Pharmacology University Business, the Alpha Research Business and the Hippocrates Business. Its vision is to provide superior
services, while adhering to its core values of integrity, respect, compassion, inclusiveness, social responsibility, excellence and innovation.
Pharmacology University Business
The Cannabis Industry
The cannabis industry is
fast-growing, increasingly complex, and rapidly changing. The Company believes that the growing cannabis industry in numerous U.S. states
and other countries represents a significant market opportunity for the Pharmacology University Business, as persons involved in the
industry need the educational and other services that it furnishes, as more fully described below.
The U.S. cannabis industry
is undergoing rapid growth and change, particularly with the recent opening of opportunities for federally sanctioned research on cannabis
in partnership with the Drug Enforcement Administration (the “DEA”), as well as the federal legalization of hemp and corresponding
state and federal hemp research programs.
The cannabis market generally
is large and growing. In 2023, according to Brightfield’s 2023 US Cannabis Market Forecast, the USA cannabis market had approximately
$27 million in sales in 2022 and is forecast to reach $50.7 by 2028. According to a report by New Frontier Data, the U.S. legal cannabis
market is predicted to reach $41.5 billion in sales by 2025.
In the medical market,
the demand for cannabis for research is likely to increase significantly over the next few years and decades, due to the increasing number
of states legalizing cannabis and the strong public support for cannabis legalization. By 2025, 5.4 million Americans, or 2.4% of U.S.
adults, are predicted to be registered patients in medical cannabis states, according to a report by New Frontier Data (“New Frontier”).
New Frontier also projects that the medical cannabis market will nearly double to over $16 billion in that time, taking into account
more geographies within the U.S. legalizing cannabis, which will lead to market expansion, the normalization of cannabis which will increase
the number of consumers, and medical cannabis patients turning to cannabis as an alternative to prescription drugs. The global medical
cannabis market is projected to reach $87.4 billion by 2027, according to Global Market Insights (“GMI”). The DEA’s
aggregate production quotas for cannabis were 3,200 kg in 2022 for dried flower (an estimated $35 million market) and 1,000 kg for cannabis
extract (an estimated $100 million market). These aggregate production quotas are expected to continue increasing to meet increasing
demand for cannabis research in the U.S. In addition to government funding, some institutions are already receiving private investment
in cannabis research. For example, Harvard and MIT recently received a $9 million donation to fund research into cannabis’ influence
on brain health and behavior. Additionally, Skylight Health Group (formerly named “CB2 Insights”) has noted that average
prescriptions for qualifying conditions such as chronic pain, PTSD, sleep disorders, epilepsy and anxiety saw a decline of 11% in favor
of medical cannabis replacement, leading the company to estimate that more than $4 billion in sales that currently go to pharmaceutical
products could be redirected towards medical cannabis. Further research on cannabis legalization and its impact on public health is needed
and is likely to take place over the coming years, as the DEA has recognized the increased need for cannabis-related research.
In 2019, large pharmaceutical
companies in the U.S. spent $83 billion on drug research and development. The private research market, like the federal DEA research program,
has an interest in investigating the uses and risks of cannabis and hemp derivatives, not only in states that have legalized medical cannabis,
but also in anticipation of potential full legalization.
The high prevalence of cancer
is expected to be one of the factors driving the demand for legal cannabis. For instance, according to the World Health Organization
(WHO), cancer is the second leading cause of death worldwide and was responsible for about 8.8 million deaths in 2015. In addition, the
growing disease burden of chronic pain and significant side effects associated with opioid usage is expected to drive the demand for
medical cannabis, which has proved to be a potent product for chronic pain management. The Company believes that these and other applications
will lead to increased demand.
Expanding Legalization of Cannabis
The 2018 Farm Bill in the
U.S. created an opportunity for hemp-derived cannabidiol (CBD) products in retail and pharmaceutical channels. Also, many countries,
including Canada, China, Italy, Australia, and South Korea, have legalized hemp for growth and export. In the United States, CBD is widely
available from retailers, including online, drug and convenience stores, natural products, beauty, grocery, and pet stores. According
to the Grand View Research Industrial Hemp Market Analysis, the global CBD market was valued at $4.6 billion in 2018 and is expected
to grow at a CAGR of 22.2% from 2019 to 2025. Additionally, the global industrial hemp market size was estimated at $4.71 billion in
2019 and is expected to show a revenue-based compound annual growth rate of 15.8%.
A recent CBS News poll found
that 88% of Americans support the legal use of medical cannabis when recommended by a doctor. Sales in the cannabis industry are projected
by Cannabis Business Daily to be $15.5 billion and $20.3 billion in 2020 and 2021, respectively and sales could be as high as $37 billion
in 2024. The size of the industry was only $3.4 billion industry in 2015. Sharp sales increases in recently launched medical cannabis
programs – as well as continued gains in adult-use markets – are expected to fuel much of the industry’s growth over
the coming years.
Thirty-eight U.S. states,
the District of Columbia, Puerto Rico and Guam have legalized some form of whole-plant cannabis cultivation, sales and use for certain
medical purposes. Eighteen of those states and the District of Columbia and Northern Mariana have also legalized cannabis for adults for
non-medical purposes (sometimes referred to as adult use). Under federal law, however, those activities are illegal. Cannabis, other than
hemp (defined by the U.S. government as Cannabis sativa L. with a THC concentration of not more than 0.3% on a dry weight basis), is a
Schedule I controlled substance under the CSA. Even in states or territories that have legalized cannabis to some extent, the cultivation,
possession, and sale of cannabis, whether in-state or interstate, violate the CSA and are punishable by imprisonment, substantial fines
and forfeiture. Moreover, individuals and entities may violate federal law if they aid and abet another in violating the CSA or conspire
with another to violate the law. Violation of the CSA is a predicate for violation of other criminal laws, including money laundering
laws and RICO. The U.S. Supreme Court has ruled that the federal government has the authority to regulate and criminalize the sale, possession
and use of cannabis, even for individual medical purposes, regardless of whether it is legal under state law.
While the U.S. government
has not enforced these laws against companies complying with state cannabis laws, it retains the authority to do so, and therefore, the
likelihood of any future adverse enforcement against companies complying with state cannabis laws remains uncertain. See “Risk Factors--Because
the Pharmacology University Business deals with persons that operate in the cannabis industry, it faces unique, unpredictable and evolving
risks.” U.S. Attorneys can prosecute violations of the CSA, including cannabis activities that comply with state law; however, U.S.
Attorneys have not targeted state-law-compliant entities in recent years. The Company believes that the policy of not prosecuting such
entities is likely to continue under current U.S. Attorney General Merrick Garland.
Since 2014, versions of
the U.S. omnibus spending bill have included provisions prohibiting the DOJ, which includes the DEA, from using appropriated funds to
prevent states from implementing their medical-use cannabis laws. In 2016, the U.S. Court of Appeals for the Ninth Circuit held that this
provision prohibits the DOJ from spending funds to prosecute individuals who engage in conduct permitted by state medical-use cannabis
laws and who strictly comply with such laws and other courts that have considered the issue have ruled similarly. However, the court noted
that if these provisions were not continued, prosecutors could prosecute conduct that occurred even while the provision was previously
in force. This decision does not apply to adult-use businesses.
Despite the ongoing federal
illegality of cannabis, the DEA has authorized certain institutions to conduct research using cannabis. Between January 2017 and January
2019, the DEA’s projections for federally approved cannabis research projects increased dramatically: the number of federally registered
cannabis researchers increased from 384 to 542. In 2019, the DEA announced that it would further facilitate and expand scientific and
medical research for cannabis in the United States, including registering additional entities to produce cannabis for researchers and
increasing the amount and variety of cannabis available for research in order “facilitate research, advance scientific understanding
about the effects of marijuana, and potentially aid in the development of safe and effective drug products that may be approved for marketing
by the Food and Drug Administration.” Further, this announcement acknowledged the possibility that medical cannabis or related
products may, in the future, require FDA approval and come under the FDA’s FDCA jurisdiction.
On December 18,
2020, the DEA finalized regulations pertaining to applications by entities seeking to become registered with the DEA to grow
cannabis as bulk manufacturers for authorized purposes. Under these and other applicable regulations, applicants are responsible for
demonstrating they have met various requirements, including requirements to possess appropriate state authority, document that their
customers are licensed to perform research, and employ adequate safeguards to prevent diversion.
On May 14, 2021, the DEA
announced that memorandums of agreement were provided to an unspecified and unnamed number of companies to collaborate with the DEA “to
facilitate the production, storage, packaging, and distribution of marijuana under the new regulations as well as other applicable legal
standards and relevant laws.” To the extent these memorandums of agreement are finalized, DEA anticipates issuing DEA registrations
to these manufacturers. Each applicant will then be authorized to cultivate cannabis – up to an allotted quota – in support
of the more than 575 DEA-licensed researchers nationwide. As of 2022, six companies have been granted DEA registrations to bulk-manufacture
cannabis.
If the DEA continues the
above policies and activities (as to which no assurance can be given), the Company believes that demand for medical cannabis, and in turn,
the Company’s products and services, will increase.
According to the Biden
campaign website: “A Biden Administration will support the legalization of cannabis for medical purposes and reschedule cannabis
as a CSA Schedule II drug so researchers can study its positive and negative impacts. This will include allowing the VA to research the
use of medical cannabis to treat veteran-specific health needs.”
As discussed under the
caption “Alpha Research Business,” recent legislation has made research in product candidates containing hemp-derived cannabinoids
possible without FDA approvals.
The Company believes
that the anticipated growth of the cannabis industry, propelled in significant part by the increasing legalization of cannabis, offers
the Company opportunities to expand. The industry requires skilled and educated cannabis professionals to operate.
Overview of the Pharmacology
University Business
Through the Pharmacology
University Business, the Company provides knowledge and promotes professionalism in the rapidly growing worldwide cannabis industry through
education in and research about the medical properties and healing virtues of this substance. The Company does not cultivate, sell or
distribute cannabis or cannabis-infused products and has no plans to do so. Pharmacology University is not an institution of higher education,
is not chartered, regulated or accredited by any governmental or private agency and does not offer training that qualifies recipients
to become pharmacists or pharmacologists.
The Pharmacology University
Business and its prospects depend on the growth of the cannabis industry and the need for experienced, educated professional persons to
lead and grow that industry ethically and responsibly in the United States and other countries where the Company’s activities are
legal. While the Company embraces the legal cannabis industry generally, its primary focus is on educating cannabis industry workers and
leaders and scientific research and development of hemp and cannabis for medicinal and commercial applications. One of the Company’s
most important assets is the close relationship of its personnel to and cooperation with law enforcement agencies in the locations where
it does business. Police agencies in several countries have appeared as guest speakers at the Company’s cannabis seminars.
In the United States,
the Company has conducted instructional seminars and cannabis classes in the states of Texas, Arkansas, Florida, Illinois, Missouri,
Oklahoma and Georgia, as well as Puerto Rico, and is planning to do likewise in the remaining states. Currently, the Company is
holding seminars and classes only in the state of Texas. The Company has conducted instructional seminars and cannabis classes in
Mexico, Peru, Ecuador, Columbia and the Dominican Republic, but is presently conducting them only in Colombia. With the COVID-19
pandemic having abated, it plans to resume these activities in Mexico, Peru, Ecuador and the Dominican Republic, and to expand into
Argentina, Chile, Brazil, Panama and other Latin American countries where its activities are lawful.
Before the pandemic,
the Company offered, and with the abatement of the pandemic, it intends to offer, opportunities for learning, discovery and engagement
to students, doctors, scientists, entrepreneurs and others in a real-world setting. The Company offers a full range of educational
programs at all levels and pursues a broad agenda of research, innovative and creative activities and builds partnerships with other
educational institutions, community organizations, government agencies and the private sector in many jurisdictions, including Jorge
Tadeo Lozano University in Bogota, Cartagena, and Santa Marta, Colombia; Clayton State University, Atlanta, Georgia; Autonomous University
of Santo Domingo, Dominican Republic; EUFLORIA Medical Cannabis Dispensary, Tulsa, Oklahoma; the Polytechnic University of Puerto Rico
in San Juan, Dispensarios 420, Puerto Rico; Cannapolis Scientific Farm in Colombia; Hemp Ecuador in Ecuador.
Educational Services
The Company has offered,
and after the abatement of the pandemic, intends to offer, multilevel educational services to entrepreneurs, medical and legal professionals,
cultivators, dispensary technicians, manufacturers, patients and others who desire to participate in the cannabis industry or who are
otherwise interested in cannabis. These services include:
| · | Continuing
medical education courses for physicians |
| | | |
| · | Continuing
legal education courses for attorneys |
| | | |
| · | Certification
courses for physicians |
| | | |
| · | Certification
for industry workers |
| | | |
| · | General
education seminars |
| | | |
| · | On-site
training |
These courses cover all
aspects of the medical cannabis industry. For the general public, they focus on the history of cannabis, its medicinal value, dispensary
concepts, legal issues and ethics, production, growing and extracts, security, operations and economics. For doctors, our courses and
seminars cover subjects such as medicinal uses of cannabis, the biochemistry of cannabis, functions of the endocannabinoid system, pharmacology,
cannabis use and abuse, and administration and dosage of cannabis medications. The cultivation course focuses on germination, cultivation
practices, cloning, growth stages and harvesting, drying and curing, and the manufacturing course covers the chemical composition of
cannabis plants, extraction of oils, laboratory practices, the manufacture of cannabis products and marketing. Overall, we have certified
and graduated several thousand students in our courses in the United States, Puerto Rico and Colombia.
Courses are taught and seminars
are led by degreed professionals, university professors, and industry experts with at least two years of commercial experience in the
particular subject. For example, the cultivation course might be taught by a professor of horticulture, an individual with an M.S. degree
in agriculture, or a master grower with three years’ experience growing crops of at least 500 plants. Before the COVID-19 pandemic,
classes were usually held at local colleges and universities in classrooms with projectors, screens and microphones. Among these colleges
and universities were the University of Texas, Houston; Texas Women’s University; University of Oklahoma; Oklahoma State University;
Clayton State University, Atlanta; Polytechnic University, San Juan; Texas A&M University; and Jorge Tadeo Lozano University in Bogota,
Cartagena, and Santa Marta, Colombia.
Students learn about Pharmacology
University through its website, social media, ticket venues, and local cannabis groups. Upon completing a course of study, students receive
certifications of completion, which are not certifications of their ability to work in a particular field, but recognition of their completion
of a non-accredited class. A 130-hour course lasting a semester was available at the University of Tadeo in Colombia, and the students
who completed it received a certificate entitled “Diplomado en Cannabis.” In addition, CME and CLE credits were available
for doctors and lawyers taking the classes. The Company has received CLE approvals for courses that it has offered in Arkansas (Office
of Professional Programs), Oklahoma (Oklahoma MCLE Commission) and Texas (State Bar of Texas) and received CME approvals for courses
that it has offered in Arkansas (University of Arkansas for Medical Sciences Office of Continuing Education), Texas and Florida (Ponce
Medical School Foundation). We were the first company approved by the Department of Health of the Commonwealth of Puerto Rico as
a provider of all training certifications, including medical education, agriculture and manufacturing education, dispensary education,
and others in the medicinal cannabis industry.
After the advent of the COVID-19
pandemic, all of our classrooms and public venues were forced to close. We also canceled all travel plans to further our expansion. To
meet this pandemic, we created online courses. We currently have more than 100 videos available online in English, Spanish, Portuguese,
Italian and Arabic and we plan to add other languages. Additionally, we have used Zoom to hold virtual classes to teach students and be
able to respond to their questions in real time during the courses. However, revenue received from online courses has not replaced the
revenue that we believe we would have generated if our classrooms and public venues had remained open. The Company intends to resume its
former courses and add new courses as the COVID-19 pandemic abates.
With the restrictions imposed
in response to the pandemic being lifted and increasing activity in the cannabis industry, we are resuming classroom and seminar activities.
We expect to hold two classes and one seminar during the year ending May 31, 2023, producing revenue of $18,000. We are continuing to
expand our online business by promoting products across 30 platforms and we are continuing to promote Cannabis World Journals.
Digital Products
As a result of the COVID-19
pandemic, which made classroom education impossible, Pharmacology University has focused on the production of educational materials for
sale on online platforms (including those operated by Amazon, Zinio, Apple, Walmart/Kobo, Barnes & Noble and Google Books), which
maintaining its relationships with academic venues where it expects to resume classroom teaching when the pandemic abates (including ICESI,
TADEO and UTB). It also focuses on entering into subscription and commercial agreements with universities and e-commerce platforms.
We have published 50
cannabis-related eBooks in five languages, have produced videos to offer online and have recorded over 13,000 minutes of audio in 5 languages.
We have also engaged artificial intelligence services to generate translations of these materials in up to 100 additional languages;
while this activity has resulted in increased expenses, while producing minimal revenue and no profit, we believe that it will become
profitable and be a significant component of our business.
We have aimed to publish
our educational content on different marketplaces that host products in languages commonly used worldwide. We work with platforms from
Brazil, Spain, England, Mexico, Canada, the United States, Germany, and more. We currently have four types of products published on different
platforms:
| · | E-Books:
We publish fifty titles in Spanish, English, Portuguese, Italian, and Arabic on Amazon,
Kobo and Google Books. In addition, Smashwords distributes our content on Barnes & Noble,
Apple, Baker & Taylor's Axis 360, OverDrive, Scribd, cloudLibrary, Gardners Extended
Retail, Odilo and Gardners Library. |
| | | |
| · | Audiobooks:
Findawayvoices distributes our content on 3Leaf Group, Axiell, Baker & Taylor, Bibliotheca,
Bidi, EBSCO, Follett, hoopla, LOL, dilo, Overdrive, Perma-Bound, Ulverscroft and Wheelers,
as well as on 24symbols, Anyplay, Apple, Audiobooks.com, AudiobooksNow, AudiobooksNZ, BajaL,
BingeBooks, Bokus Play, Bookmate, Chirp, Cliq, Downpour, eStories, Google Play, Hummingbird,
Instaread, Leamos, Libro.FM, Milkbox, Nextory, NOOK, Scribd, and Ubook. |
| | | |
| · | Video
courses: We publish 161 titles on Amazon (6 courses), Sympla (17 courses), Teachlr
(62 courses), Edusity (13 courses), Simplivlearning (16 courses), Alugha (40 courses), Aprendum (4 courses), and Unihance (105 courses). |
| | | |
| · | Cannabis
Worlds. We publish Cannabis Worlds, our digital magazine, on Google books (25
issues in five languages), Zinio (25 issues in Spanish and English), Pocketmags (25 issues in English) and Magzter (25 issues in five
languages). |
The Company believes that the amount and scope
of its digital products exceed those offered by any of its competitors in cannabis-related education.
A staff of 29 independent
contractors in Venezuela, Argentina, Colombia, Brazil, Mexico, Jordan, Canada and Texas, hold classes; research, edit and materials;
translate materials; prepare audio-visual materials; and engage in web development.
Franchising
We offered, until the COVID-19
pandemic, educational programs to franchisees worldwide. A franchisee purchased the right to provide our courses in its particular city.
In addition to an initial franchise fee, a franchisee paid a 10% franchise fee and a 2% advertising fee on all gross sales. We assisted
in creating and registering a franchisee’s business identity; developing and activating its websites; creating its social media
platforms; providing it with marketing plans; assisting in finding venues for their classes; explaining how to find qualified instructors;
providing PowerPoint presentations as well as books for students and instructors; and providing one week of one-on-one training relating
to the operation of the franchise. In addition, we provided one month of marketing assistance. Before the pandemic, we had four franchisees
that produced revenue of $0 and $17,361 in the years ended May 31, 2021, and May 31, 2020, respectively. As a result of the pandemic,
we received no revenue from these franchisees in the years ended May 31, 2023, and May 31, 2022. Although the COVID-19 pandemic
has abated, we have not resumed franchise operations because we have not completed the revision of our franchise model, which we expect
will include more online classes.
Consulting
Prior to the pandemic,
the Company offered consulting services, which included: These services may include:
| · | creating
and presenting advertising material for campaigns in traditional and digital media, including
publicity strategy, campaign creation, design of flyers, advertising social networks, newspapers
and magazines and creation of audiovisual content. |
| | | |
| · | consulting services to entrepreneurs
who are considering entering the cannabis industry, manufacturers and growers, including
preparation of business plans, guidance in business structure, guidance in seeking investment,
preparation of license and other applications and development of operating procedures. |
The cost of these services
were based on the nature of each assignment.
The Company provided
these services in many states and Puerto Rico and assisted in obtaining over 40 licenses for its clients for dispensaries,
cultivation, manufacturing and a full analytical laboratory.
In the year ended May 31,
2023, the Company began to offer these services again and recorded revenue of $8,333 therefrom, for that year and $8,333 for the quarter
ended August 31, 2023.
Alpha Research Business
Through the Alpha Research
Business, based in Houston, Texas, the Company offers specialized services in all therapeutic areas of clinical trials and has conducted
over 20 clinical trials for Sponsors and CROs. These trials have included drugs relating to diseases in the areas of asthma, allergies,
renal disorders, neurology disorders, cardiac and vascular disorders, nutrition/metabolism, obstetrics/gynecology, dermatology, oncology,
ophthalmology, orthopedics, gastroenterology, psychiatric disorders, infectious diseases, pulmonary and respiratory diseases, urology
and COVID-19, as well as devices for orthopedic and cardiovascular problems. Our clients have included Sponsors such as Pfizer Inc.,
Merck & Co, Inc., Shionogi & Co., Ltd., Medtronic plc, Novartis, GlaxoSmithKline plc, Gilead Sciences, Inc. and Johnson &
Johnson, and CROs, such as PPD, Inc., Icon plc, Parexel, PRA Health Sciences, Inc., Covance, IQVIA Holdings Inc. and Medpace Holdings,
Inc. In the near future, Alpha Research intends to conduct cannabinoid clinical trials, in which it will be the Sponsor.
Clinical trials are a clinical
research method designed to evaluate and test new drugs or devices. They are typically conducted in four phases, each of which has a different
purpose and helps scientists answer different questions.
| · | Phase
I. Researchers test an experimental drug or treatment in a small group of people for
the first time. The researchers evaluate the treatment’s safety, determine a safe dosage
range, and identify side effects. |
| | | |
| · | Phase II. The experimental
drug or treatment is given to a larger group of people to ascertain whether it is effective
and to evaluate its safety further. |
| | | |
| · | Phase III. The experimental
study drug or treatment is administered to large groups of people. Researchers confirm its
effectiveness, monitor side effects, compare it to commonly used treatments, and collect
information that will allow the experimental drug or treatment to be used safely. |
| | | |
| · | Phase IV. Post-marketing
studies, which are conducted after a treatment is approved for use by the FDA, provide additional
information, including information relating to treatment, risks, benefits and best use. |
The Company’s facilities
are equipped with examination and blood drawing rooms, storage for investigational medication and study-related equipment. The Company
employs only clinical research coordinators (“CRCs”) with at least five years of experience. CRCs are involved in supervising
drug trials and medical research, which involves recruiting patients for medical and drug trials and screening them to ensure that they
meet the guidelines of the trial, as well as following good clinical practice, overseeing the progress of the clinical trial and ensuring
that it is properly conducted, recorded, and reported.
The recruitment of subjects
from minority, rural and economically disadvantaged groups is important to clinical trials because the benefits and risks of new drugs
with respect to them may differ from other groups due to genetic, environmental and other factors. To enhance such recruitment, the Company
has worked with community organizations, churches, social services and public agencies and has provided transportation services.
The Alpha Research Business
is staffed by seven personnel responsible for regulatory and Investigational Review Board (“IRB”) processes and a staff of
two auditors. An IRB is an independent body required by federal regulation, comprising medical, scientific, and nonscientific members,
the responsibility of which is to ensure the protection of the rights, safety, and well-being of human subjects involved in a clinical
trial. An IRB reviews and approves clinical trials, protocols, amendments, methods and materials to be used in obtaining and documenting
informed consents from the trial subjects.
We have more than
six principal investigators, who are physicians who prepare and perform or oversee clinical trials, usually in conjunction with
their medical practices. These investigators are independent contractors. In addition, we have seven professional personnel who
analyze data and report the results of trials to Sponsors and CROs, all of whom are independent contractors; they are encouraged to
keep up to date on good clinical practices and regulations relating to clinical research and a part-time accountant.
Clinical Trials for
Sponsors and CROs
In connection with these
clinical trials, the Company will contract with a Sponsor or CRO to provide services in connection with a clinical trial after it has
provided information respecting its ability to provide them and after a visit by the Sponsor or CRO to our facilities to confirm our ability
to conduct the trial and to establish communications procedures. After further measures, which include establishing a budget and providing
additional information about the Company and a second visit to our facilities, we will enter into a contract with the Sponsor or CRO,
which will issue a “Site Activation Letter.” When we receive this letter, we begin enrolling volunteer test subjects.
Alpha Research finds
Sponsors and CROs in three ways:
| · | Recruitment
websites. On these websites, we search for trials that are within our competence and
contact the related Sponsors or CROs, providing relevant information about ourselves, who
will respond if they are interested in our services. The Sponsor or CRO will consider entering
into a contract for the study only after it has met with our personnel and has visited our
facilities and if the Sponsor or CRO is satisfied that we can conduct the trial and comply
with the terms of its contract, which, as indicated above, are complex. Even then, the Sponsor
or CRO may award the contract to a firm that it considers better qualified. |
| | | |
| · | Sponsor
or CRO websites. The process is similar to that described above for recruitment websites. |
| | | |
| · | Personal
contact. |
We are currently conducting
clinical trials for Sponsors and CROs, in non-cirrhotic non-alcoholic steatohepatitis, chronic obstructive pulmonary disease, a multivalent
pneumococcal vaccine, iron deficiency anemia and the collection of biospecimen collections and samples across all ages and various therapeutic
areas, and multiple medical conditions. We are actively seeking contracts, have bid on four and believe that we will be successful in
obtaining some of them. We produced revenue of approximately $276,000 from our clinical trials business for the year ending May 31, 2023,
and $73,000 for the quarter ended August 31, 2023.
Clinical Trial
in Which We Will be the Sponsor
Beginning in the near future, Alpha
Research intends to conduct clinical trials of hemp-derived cannabidiol (“CBD”) medical products, in which it will be the
Sponsor. CBD derived from hemp containing less than 0.3% of tetrahydrocannabinol (“THC”) was legalized at the federal level
by the Agriculture Improvement Act of 2018 and its sale and use of CBD products containing less than 0.3% of THC is legal in all states
except for 18, which restrict sale and use for various reasons, including the age of the purchaser, non-medical use and the THC content.
The Company does not intend to conduct clinical trials of products that require any approval of the FDA.
CBD is a naturally-produced
cannabinoid from the cannabis plant that differs from other cannabinoids such as THC for its structure and mechanism of action. CBD does
not have psychoactive effects and does not alter the conscience and perception of the user. In contrast, CBD been proven to clinically
have anti-inflammatory, analgesic, anti-seizure, immunomodulatory and anxiolytic activity.
According to an article in
Harvard Health Publishing, the consumer health education division of Harvard Medical School (“Cannabidiol (CBD): What we know
and what we don't,” published on September 24, 2021), there is strong evidence for the use of CBD in treating certain childhood
epilepsy syndromes, and one CBD-based drug, Epidolex, has been approved by the Federal Drug Administration for treating these conditions.
This article states that animal studies and human research and self-reporting suggest that CBD may help with anxiety, insomnia, chronic
pain and treatment of addiction. Other articles state, with varying degrees of certainty, that CBD may be used to treat other conditions,
including mental disorders, such as depression, psychosis and PTSD; cancer-related symptoms, such as nausea, vomiting and pain; neurological
conditions, such as Parkinson’s disease, Huntington’s disease, autism spectrum disorder and motor disorders; substance abuse;
glioblastoma; high blood pressure; and sleep disorders. Initially, the Company intends to conduct research in the areas of endocannabinoid
system physiology to study the normal endocannabinoid range in healthy individuals, the medical consequences of low endocannabinoid levels
and the effects of cannabinoids administration in the organism. These stdies will involve various different methodologies which will
include blood tests, breathalyzer tests and electroencephalograms (EEGs) to determined the impact of cannabinoids on pathologies that
have been linked to clinical endocannabinoid deficiencies such as irritable bowel syndrome, migraine and fibromyalgia. Research will
also be conducted in oncology regarding the influence of cannabinoid-based treatments of cancer prevention, tumor size and viability,
cancer-related symptoms and chemotherapy-induced symptoms. Finally, the Company will conduct research in anxiety disorders with a view
to determining whether cannabinoids can reduce anxiety-related symptoms and improve quality of life.
The Company determined
to conduct these clinical trials for the following reasons:
| · | According
to Forbes Business Institute, the global CBD oil and CBD consumer health market is expected
to grow from $39.54 billion in 2022 to $55.79 billion in 2028 at a compound annual growth
rate of 47.49%. |
| · | According
to a Forbes Health Survey conducted by OnePoll, 60% of respondents reported that they have
tried a cannabidiol (CBD) product and believe CBD has health and wellness benefits, including
the potential to improve sleep, reduce anxiety and/or relieve pain. |
Based on these factors
and the expertise of the Company in conducting clinical trials, it believes that it can develop and test CBD products.
These trials will be
conducted in the Company’s facilities, adding additional CRCs to handle the new studies. It will contract with an IRB to ensure
all protocols are met and approved.
If a clinical trial indicates
that a product is safe and effective, the Company intends to exploit it by selling or licensing it. Where possible, it will seek protection
of intellectual property related to and the use of a product through patents and trademarking.
Sleep Center Business
In July 2022, the Company opened its Sleep
Center, which served Houston-area patients who wereinterested in improving their sleep quality and enhancing their physical and mental
well-being. The Sleep Center utilized state-of-the-art equipment. Its goal was to assess, diagnose, and treat sleep problems and provide
patients with convenient and flexible care. During the year ended May 31, 2023, the Sleep Center treated only six patients and produced
revenue of $900. The Company closed the Sleep Center in April 2023, because it was unable to obtain patients in the quantity needed to
justify its operating and the Company believed that its manpower and financial resources would more effectively be used in its other
activities.
The Company has two employees, namely its executive
officers, who serve on a full-time basis. It meets its other manpower needs through approximately 34
independent contractors; the number of these personnel changes from time to time in accordance with the Company’s staffing needs.
For a description of the services provided by these independent contractors, see the descriptions of the three segments of the Company’s
business.
Concentration of Revenues
The Company has depended on a few customers
of the Alpha Research Business for substantial portions of its revenue. For the year ended May 31, 2023, the Company had revenues of
$316,825, of which 63.9%, 27.2% and 5.1% were received from three customers. For the year ended May 31, 2022, the Company had revenues
of $214,980, of which 36.7%, 14.8%, 10.1% and 7.6% were received from four customers. As the Company implements its business plan, the
Company expects, but cannot assure, that such dependence may be reduced.
Description of Property
The Company leases premises of approximately
4,500 square feet located at 6201 Bonhomme Road, Suites 460S and 466S, Houston, Texas. The lease provides for a base rent of $3,381.96
per month, increasing to (i) $3,529 per month on July 1, 2020, (ii) $3,676 per month on July 1, 2021, and (iii) $3,823 per month
on July 1, 2022, subject to CPI increase. The lease was to expire on June 30, 2023; however, on March 23, 2023, it was amended to extend
its term to June 30, 2024, at a base rent of $4,779 per month. On September 5, 2023, the lease was amended to extend its term to June
30, 2025, at rentals of $0 per month for the two months ended August 31, 2023, $$4,779 per month for the 10 months ending June 30, 2024,
and $4,926 per month for the 12 months ending June 30, 2025. This space is shared by PUI and the Alpha Research Institute.
Two of the Company’s officers leased
1,400 square feet in Houston, Texas, at 1625 Main St., Houston, Texas, under a lease the term of which commenced on March 15, 2023, and
expired on September 14, 2023, at a rent of $3,168 per month; these officers have made a portion of these premises available to the Company
for use as office space, for which the Company pays them $2,817 per month. These officers have entered into a new lease for these premises,
which commenced on September 15, 2023, and will expire on September 14, 2024, at a rent of $3,164 per month and they are making a portion
of these premises available to the Company for use as office space, for which the Company is paying them $2,817 per month. For further
information, see “Certain Relationships and Related Party Transactions—Lease.”
Legal Proceedings
The Company has not been and is not a party to
any litigation and is not aware of any threatened litigation.
Off-Balance Sheet Arrangements
We have no off-balance-sheet arrangements.
MANAGEMENT
The following table presents information with
respect to our officers and directors:
| Name |
|
Age |
|
Position |
| Dante Picazo |
|
67 |
|
Chief Executive Officer and Director |
| Henry Levinski |
|
71 |
|
Vice President and Director |
| Jose Torres |
|
64 |
|
Secretary and Director |
Each of our directors serves until his
death, resignation or removal or until his successor is elected and qualified. Each of our officers is elected by the Board to a
term of one year and serves until his successor is duly elected and qualified or until he dies, resigns or is removed. Members of
the Board receive no compensation for their services as such.
Biographical Information Regarding Officers and Directors
Dante Picazo
Mr. Picazo has been the chief executive officer
and a director of the Company since the merger of PUI into the company on December 19, 2019, and was the co-founder of PUI, serving as
one of its directors and as its chief executive officer and president from its incorporation in 2009 to that merger.
He has 45 years of experience in operating and
growing from concept to profitability, originating marketing and branding efforts, leading to initial public offerings for three companies.
He graduated from Cornell University School of
Hotel Administration, AMP in Ithaca, N.Y., and is fluent in three languages.
Mr. Picazo’s control of the Company through
his ownership of its capital stock, together with his knowledge of the Pharmacology University Business and his extensive experience
in international business and finance, led to the conclusion that he should serve as a member of the board.
Henry Levinski
Mr. Levinski has served as treasurer and a director
of the Company since the merger of PUI into the company on December 19, 2019, and was the co-founder of PUI, serving as one of its directors
and its chief executive officer from its incorporation in 2009. He has over 40 years of experience in operations, marketing, purchasing
and training.
Mr. Levinski’s knowledge of the Pharmacology
University Business, together with his prior experience, led to the conclusion that he should serve as a board member.
Jose Torres
Dr. Torres has served as a director and national
medical director of the Company since the merger of PUI into the company on December 19, 2019. He served in like positions with PUI until
the merger. He is board-certified in General and internal medicine and is an Anti-aging medicine Specialist with 35 years of medical
practice experience.
He received his medical degree from the Autonomous
University of Guerrero in Chilpancingo, Guerrero, Mexico, and completed a residency in internal medicine residency at Caguas Regional
Hospital in Puerto Rico. He is certified in urgent care and by World Link Medical. He is a Member of the American College of Physicians,
the Puerto Rico College of Physicians and the American Academy of Cannabinoid Medicine. He is an expert in the medical uses of cannabis
and is involved in research respecting its use in treating several medical conditions, including sleep disorders, pain management, treatment
of nausea and vomiting associated with cancer and chemotherapy, asthma and other bronchial ailments, and decreased libido.
Mr. Torres’ experience with the medicinal
use of cannabis and with sleep disorders led to the conclusion that he should serve as a member of the board.
EXECUTIVE COMPENSATION
Compensation of Officers
The following table sets forth information concerning
all compensation awarded to, earned by, or paid to our principal executive officer and our only other executive officer, who were serving
as such on May 31, 2023, for the fiscal years ended May 31, 2023, and May 31, 2022.
SUMMARY COMPENSATION TABLE
| Name and principal
position |
|
Year |
|
Salary
($) |
|
Bonus
($) |
|
Stock
Awards
($) |
|
Option
Awards
($) |
|
Non-equity
incentive plan compensation
($) |
|
Change in pension
value and nonqualified deferred compensation earnings
($) |
|
All Other
Compensation
($) |
|
Total
($) |
| (a) |
|
(b) |
|
(c) |
|
(d) |
|
(e) |
|
(f) |
|
(g) |
|
(h) |
|
(i) |
|
(j) |
| Dante Picazo |
|
2023 |
|
24,500 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
24,500 |
| CEO |
|
2022 |
|
24,000 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
24,000 |
Henry Levinski
|
|
2023 |
|
21,375 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
21,375 |
| VP |
|
2022 |
|
24,000 |
|
– |
|
– |
|
– |
|
– |
|
– |
|
– |
|
24,000 |
Compensation Discussion and Analysis
The Company has determined the amount paid as
salary to the persons named in the above table based on the Company’s ability to pay. The Company believes that these salaries
are lower than those that these persons could earn in equivalent positions in other companies and that these persons have elected to
receive these salaries and remain with the Company because of their equity positions in the Company, their belief in the prospects of
the Company and intangible reasons of which the Company may not be aware. In the case of Mr. Levinski, the Company has provided additional
compensation in the form of shares of Common Stock. The Company believes that it needs to be able to provide competitive compensation
to these persons, as well as persons that it hires in the future, but will not be able to do so until it can generate materially increased
revenue. Until then, the Company is subject to the risk that one or more of these persons will seek employment elsewhere. The Company
has adopted its 2022 Equity Incentive Plan (see “Incentive Plan”) and may explore the adoption of plans that will enable
it to reward and retain the loyalty of these and other employees through awards of share-based compensation, such as stock options, restricted
stock and restricted stock units.
Incentive Plan
General Information
On July 20, 2022, the Board
adopted, and the shareholders approved, the 2022 Equity Incentive Plan (the “Incentive Plan”), which provides for the grant
of stock options, stock appreciation rights, restricted stock, unrestricted stock, restricted stock units, and performance awards to directors,
officers, employees and consultants (“Grantees”). The Incentive Plan is administered by the Board, which has the authority,
among other things, to select eligible persons to receive awards and determine the terms of awards.
The Company will recognize
as share-based compensation expense all share-based payments to Grantees over the requisite service period (generally the vesting period)
in its consolidated statements of income based on the fair values of the awards that are ultimately expected to vest. As a result, for
most awards, recognized share-based compensation expense will be reduced for estimated forfeitures prior to vesting, primarily based initially
on the judgment of management and thereafter, estimated forfeitures will be reassessed in subsequent periods based on facts and circumstances.
As no awards were made under the Incentive Plan during the periods covered by the consolidated financial statements included in this Prospectus,
no expense for share-based compensation was recorded therein.
The Company adopted the
Incentive Plan because it believes that long-term incentives for Grantees will be a significant factor in generating returns for its
shareholders based upon the Incentive Plan’s ability to focus on long-term performance. By providing grantees with opportunities
to acquire a meaningful equity stake in the Company, it can better align their interests with those of its shareholders and create value
for them.
The Company expects to make
periodic awards to its executive officers, employees and consultants, as well as awards in connection with promotions or new hires, the
occurrence of significant events or to promote retention of employees.
Awards will generally be
subject to time- or performance-based vesting over periods determined by the Board. Performance-based goals will be determined by the
Board. We believe that performance-based awards will encourage Grantees to achieve key strategic objectives and maximize value creation
for our shareholders.
No awards have been made
as of the date of this Prospectus.
Provisions of the
Incentive Plan
The following is a description
of the material terms of the Incentive Plan, which is not a complete description and is qualified in its entirety by reference to the
Incentive Plan, which is filed as an exhibit to the registration statement of which this Prospectus is a part.
Authorized shares.
Subject to adjustment in certain events, the maximum number of shares of Common Stock that may be issued in satisfaction of awards
is 600,000,000. As of the date of this Prospectus, no awards had been granted.
Eligibility. The
Board may select participants from among employees and directors of and consultants to the Company.
Types of awards; vesting.
The Incentive Plan provides for various awards, including incentive stock options (“ISOs”), nonstatutory stock options,
stock appreciation rights, restricted and unrestricted stock and stock units, performance awards and cash. The Board has the authority
to determine the vesting schedule applicable to each award and to accelerate the vesting or exercisability of any award.
Termination of awards.
Unless otherwise provided
in an award agreement, upon termination of employment or service, a participant’s options and SARS will terminate and the participant
will have no further right, title or interest therein, the shares of Common Stock subject thereto or any consideration in respect thereof.
If employment or service terminates otherwise than for cause, the Participant may exercise his Option or SAR to the extent vested,
but only within the following period or, if applicable, such other period provided in the Award Agreement.
Except as otherwise provided
in the Award Agreement or other written agreement, if a Participant’s continuous service terminates for any reason, (i) the Company
may receive through a forfeiture condition or a repurchase right any or all of the shares of Common Stock held by the participant under
his restricted stock award that have not vested as of the date of such termination as set forth in such agreement and (ii) any portion
of his RSU award that has not vested shall terminate upon such termination and he shall have no further right, title or interest in the
RSU award, the shares of Common Stock issuable pursuant thereto the RSU Award or any consideration in respect thereof the RSU.
Except as provided in an
award agreement, in the event of a dissolution or liquidation of the Company, outstanding awards (other than those consisting of vested
and outstanding shares of Common Stock not subject to a forfeiture condition or the Company’s right of repurchase) shall terminate
prior to the completion of such dissolution or liquidation, and the shares of Common Stock subject to the Company’s repurchase
rights or subject to a forfeiture condition may be repurchased or reacquired by the Company, provided that the Board may cause some or
all expired or terminated Awards to become fully vested, exercisable or no longer subject to repurchase or forfeiture before the dissolution
or liquidation is completed but contingent on its completion.
Transferability.
Options and SARs may not
be transferred to financial institutions for value and the Board may impose such additional limitations on the transferability of an
option or SAR as it determines. In the absence of any such determination, the following restrictions shall apply (provided that, except
as explicitly provided in the Incentive Plan, an option or a SAR may not be transferred for consideration and, if an option is an ISO,
it may be deemed to be a nonstatutory stock option as a result of such transfer):
An option or SAR shall not
be transferable, except by will or by the laws of descent and distribution, and shall be exercisable during the lifetime of a participant
only by him (provided that, in certain cases, the Board may permit the transfer of an Option or SAR in a manner that is not prohibited
by applicable tax and securities laws upon the Participant’s request, including to a trust if the Participant is considered to
be the sole beneficial owner of such trust (as determined under Section 671 of the U.S. Internal Revenue Code of 1986, as amended (the
“Code”), and applicable state law) while such Option or SAR is held in such trust, provided that the Participant and the
trustee enter into a transfer and other agreements required by the Company.
Subject to the execution
of transfer documentation in a format acceptable to the Company and subject to the approval of the Board or a duly authorized officer,
an Option or SAR may be transferred pursuant to a domestic relations order.
Corporate transactions.
In the event of certain corporate transactions (including merger, consolidation, reorganization, recapitalization, reincorporation,
stock dividend, dividend in property other than cash, large nonrecurring cash dividend, stock split, reverse stock split, liquidating
dividend, combination of shares, exchange of shares, change in corporate structure), the Board shall appropriately and proportionately
adjust (a) the class or classes and the maximum number of shares of Common Stock subject to the Plan, (b) the class or classes and the
maximum number of shares that may be issued pursuant to the exercise of ISOs and (c) the class or classes and the number of securities
and exercise price, strike price or purchase price of Common Stock subject to outstanding Awards.
Acceleration.
The Board may accelerate the time at which an award may first be exercised or the time during which an award or any part thereof will
vest.
Change in control.
In the event of a change in control of the Company (as defined in the Incentive Plan), the Board shall have discretion (i) settle
awards for an amount of cash or securities equal to their value, where in the case of options and SARs, the value of such Awards, if
any, shall be equal to their in-the-money spread value (if any), as determined in the sole discretion of the Board, (ii) arrange for
the surviving corporation or acquiring corporation (or its parent company) to assume or continue the award or to substitute a substantially
similar award, (iii) arrange for the assignment of any reacquisition or repurchase rights held by the Company in respect of Common Stock
issued pursuant to the award to the surviving corporation or acquiring corporation (or its parent company), (iv) modify the terms of
awards to add events, conditions or circumstances (including termination of employment within any specified period after a change in
control) upon which the vesting of such awards or lapse of restrictions thereon shall accelerate or deem any performance conditions satisfied
at target, maximum or actual performance through closing or provide for the performance conditions to continue after closing, (v) arrange
for the lapse, in whole or in part, of any reacquisition or repurchase rights held by the Company with respect to awards, (vi) cancel
or arrange for the cancellation of awards, to the extent not vested or not exercised prior to the effective time of the change in control,
in exchange for such cash consideration, if any, as the Board may consider appropriate, or(vii) provide that, for at least 20 days
prior to the change in control, any Options or SARs that would not otherwise become exercisable prior thereto shall be exercisable as
to all shares of Common Stock subject thereto, contingent upon and subject to the occurrence of the change in control, and that any options
or SARs not exercised prior to the consummation of the change in control shall terminate and be of no further force and effect as of
the consummation thereof.
Amendment and termination.
The Board may amend the Incentive Plan or outstanding awards, except that it may not materially impair the rights and obligations
under any award except with the written consent of the affected participant.
Retirement, Resignation or Termination Plans
We have or sponsor no plan, whether written or
verbal, that would provide compensation or benefits of any type to an executive upon retirement or any plan that would provide payment
for retirement, resignation, or termination as a result of a change in control of our company or as a result of a change in the responsibilities
of an executive following a change in control of our company.
Pension Benefits
The Company has no plan under which retirement
payments and benefits, or payments and benefits that will be provided primarily following retirement may be or have been or may be paid.
Nonqualified Defined Contribution and Other
Nonqualified Deferred Compensation Plans
The Company has no defined contribution or other
plan that provides for the deferral of compensation.
Potential Payments upon Termination or Change-in-Control
The Company is not a party to any contract, agreement,
plan or arrangement, whether written or unwritten, that provides for payment to any of its executive officers at, following or in connection
with any termination, including without limitation resignation, severance, retirement or constructive termination, or a change in control
of the Company or a change in any of their responsibilities.
Compensation of Directors
Our directors receive no compensation in their
capacities as such.
CERTAIN RELATIONSHIPS AND
RELATED PARTY TRANSACTIONS
Issuances of Shares
On December 19, 2019, Pharmacology University,
Inc., a Delaware corporation (“PUI”), with and into the Company pursuant to an Agreement and Plan of Merger, dated as of November
7, 2019, under which PUI was merged with and into the Company (the “PU Merger Agreement”). Pursuant to this agreement, the
Company issued 4,595,467,025 shares of Common Stock to the former holders of the common stock of PUI and also issued 2,000,000 shares
of its Series A Preferred to Dante Picazo, who became the Company’s chief executive officer president upon the closing of the PU
Merger Agreement. As a result of these issuances, Mr. Picazo acquired sole control of the Company.
On January 5, 2020, the Company issued 50,000,000
shares of Common Stock to Henry Levinski, a director and vice president of the Company in consideration of his employment; these shares
had a market value of $5,000 on the date of their issuance.
On January 5, 2020, the Company issued 40,000,000
shares of Common Stock to Jose Torres, a director and secretary of the Company, in consideration of $40,000. Based on the closing price
for the Common Stock on January 3, 2020, which was the most recent date on which it was traded, these shares had a market value of $8,000.
The Company and Henry Levinski entered into an
agreement, dated as of November 1, 2022, under which he agreed, in consideration of the issuance to him of 50,000,000 shares of Common
Stock, to provide services in connection with causing the registration statement of which this Prospectus is a part to be declared effective
by the SEC and, after it is declared effective, in assisting the Company in the marketing of the shares of the Common Stock registered
thereunder for a period of the earlier of (i) two years after the registration statement is made effective or (ii) the date on which
all of the shares offered by the Company under the registration statement have been sold. He also agreed that he would not resign as
an officer or director of the Company during such period. A quorum of the board of directors, which did not include Mr. Levinski, approved
this transaction. These shares were not issued under June 30, 2023, and had a market value of $60,000 on that date.
Lease
Dante Picazo and Henry Levinski, two of the
Company’s officers, leased 1,400 square feet in Houston, Texas, at 1625 Main St, Houston, Texas, under a lease, the term of which
commenced on February 29, 2020, and expired on September 30, 2022, at a rent of $3,449 per month. These officers have made a portion
of these premises available to the Company for office space, for which the Company pays them $2,817 per month. On September 15, 2022,
these officers re-leased these premises under a lease that expired on March 14, 2023, at a rent of $3,038 per month, and they continued
to make a portion of these premises available to the Company for use as office space, for which the Company paid them $2,817 per month.
On March 2, 2023, these officers entered into a new lease for the same premises, which expired on September 14, 2023, at a rent of $3,168
per month, and they continued to make a portion of these premises available to the Company for use as office space, for which the Company
paid them $2,817 per month. These officers have entered into a new lease for these premises, which commenced on September 15, 2023, and
will expire on September 14, 2024, at a rent of $3,164 per month and they are making a portion of these premises available to the Company
for use as office space, for which the Company is paying them $2,817 per month. The Company believes that these rentals are the fair
market value of the space rented.
Exchange of Shares
On August 15, 2022, pursuant to resolutions
of the Board, Mr. Picazo exchanged 595,467,205 shares of Common Stock for 1,000 shares of the Company’s Series B Preferred Stock
(“Series B Preferred”). By his ownership of these shares, Mr. Picazo has voting control of the Company. Since Mr. Picazo
had an interest in this exchange, he did not vote on the adoption of this resolution. A quorum of the board of directors, which did not
include Mr. Picazo, approved this transaction.
Advances
The Company has received advances from Messrs.
Picazo and Levinski from time to time. All of these advances are non-interest-bearing and have no set maturity date. The Company expects
to repay these advances when funds become available. During the years ended May 31, 2023, and May 31, 2022, the Company received and
repaid advances as follows:
| | |
Dante Picazo | | |
Henry Levinski | |
| Balance at May 31, 2021 |
|
$ |
1,455 |
|
|
$ |
10,808 |
|
| Year ended May 31, 2022: |
|
|
|
|
|
|
|
|
| Amounts advanced |
|
|
850 |
|
|
|
58,000 |
|
| Amounts repaid |
|
|
(2,350 |
) |
|
$ |
(52,925 |
) |
| Balance at May 31, 2022 |
|
$ |
(45 |
) |
|
|
15,883 |
|
| Year months ended May 31, 2023: |
|
|
|
|
|
|
|
|
| Amounts advanced |
|
|
12,530 |
|
|
|
86,440 |
|
| Amounts repaid |
|
|
– |
|
|
|
(9,860 |
) |
| Balance at May 31, 2023 |
|
$ |
12,485 |
|
|
$ |
92,643 |
|
| Quarter ended August 31, 2023 |
|
|
|
|
|
|
|
|
| Amounts advanced |
|
|
100 |
|
|
|
4,420 |
|
| Amounts repaid |
|
|
0 |
|
|
|
0 |
|
| Balance at August 31, 2023 |
|
$ |
12,585 |
|
|
$ |
97,063 |
|
Since August 31, 2023, Mr. Picazo has advanced
$0 to the Company and has been repaid $0 and Mr. Levinski has advanced $3,132 to the Company and has been repaid $0. At November 10,
2023, the balances that the Company owed to Messrs. Picazo and Levinski were $12,585 and $100,195, respectively.
PRINCIPAL AND SELLING STOCKHOLDERS
The table below sets forth the beneficial ownership
of Common Stock as of the date of this Prospectus. At that date, 10,331,749,347 shares of Common Stock were issued and outstanding.
The following table provides information with
respect to the beneficial ownership of Common Stock by the following (i) each of our named executive officers, (ii) each of our directors,
(ii) all directors and executive officers as a group, (iii) each person known to beneficially own more than 5% of Common Stock (excluding
the Selling Stockholders) and (iv) the Selling Stockholders.
The amounts and percentages of shares beneficially
owned are reported as required by the SEC’s rules respecting the determination of beneficial ownership of securities. Under these
rules, a person is deemed to be a “beneficial owner” of a security if he has or shares voting power or investment power, which
includes the power to dispose of or to direct the disposition of such security; and is also deemed to be a beneficial owner of any securities
of which he has a right to acquire beneficial ownership within 60 days after the determination date. Securities that can be so acquired
are deemed to be outstanding for purposes of determining such person’s ownership percentage, but not for purposes of determining
any other person’s ownership percentage. Under these rules, more than one person may be deemed to be a beneficial owner of
the same securities and a person may be deemed to be a beneficial owner of securities in which he has no economic interest.
| |
|
Shares Beneficially
Owned Prior to the Offering |
|
|
|
|
|
Shares Beneficially
Owned After the Offering1 |
|
| Name and Address of Beneficial
Owner2 |
|
Title of Class or Series |
|
Number |
|
Percent of
Outstanding Shares3 |
|
|
Shares Being Offered |
|
|
Number |
|
|
Percent of
Outstanding Shares4 |
|
| Named Executive Officers and Directors: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dante Picazo |
|
Common Stock |
|
4,002,611,700 |
4, 5 |
|
38.7 |
|
|
|
490,500,000 |
|
|
|
3,512,111,700 |
5, 6 |
|
|
21.2 |
|
| |
|
Series A Preferred |
|
2,000,000 |
|
|
80.00 |
|
|
|
– |
|
|
|
2,000,000 |
|
|
|
80.00 |
|
| |
|
Series B Preferred |
|
1,000 |
|
|
100.00 |
|
|
|
– |
|
|
|
1,000 |
|
|
|
100.00 |
|
| Henry Levinski |
|
Common Stock |
|
100,000,000 |
|
|
<1 |
|
|
|
100,000,000 |
|
|
|
– |
|
|
|
– |
|
| Jose A. Torres |
|
Common Stock |
|
40,000,000 |
|
|
<1 |
|
|
|
40,000,000 |
|
|
|
– |
|
|
|
– |
|
| All directors and executive officers as a group (3 persons): |
|
Common Stock |
|
4,142,611,700 |
|
|
40.1 |
|
|
|
630,500,000 |
|
|
|
3,512,111,700 |
5, 6 |
|
|
21.2 |
|
| |
|
Series A Preferred |
|
2,000,000 |
|
|
100.0 |
|
|
|
– |
|
|
|
2,500,000 |
|
|
|
100.00 |
|
| |
|
Series B Preferred |
|
1,000 |
|
|
100.0 |
|
|
|
– |
|
|
|
1,000 |
|
|
|
100.00 |
|
| The Selling Stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| John Jones |
|
Common Stock |
|
1,103,888,888 |
|
|
10.7 |
|
|
|
1,103,888,888 |
|
|
|
– |
|
|
|
– |
|
| Ibeth Corrales |
|
Common Stock |
|
625,000,000 |
|
|
6.0 |
|
|
|
625,000,000 |
|
|
|
– |
|
|
|
– |
|
| Julian J. Gonzalez |
|
Common Stock |
|
321,428,572 |
|
|
3.1 |
|
|
|
65,285,714 |
|
|
|
256,142,858 |
|
|
|
1.5 |
|
| John Neville |
|
Common Stock |
|
300,000,000 |
|
|
2.9 |
|
|
|
300,000,000 |
|
|
|
– |
|
|
|
– |
|
| Mark Herbert |
|
Common Stock |
|
203,000,000 |
|
|
2.0 |
|
|
|
203,000,000 |
|
|
|
– |
|
|
|
– |
|
| Harry Feinberg |
|
Common Stock |
|
164,705,881 |
|
|
1.6 |
|
|
|
164,705,881 |
|
|
|
– |
|
|
|
– |
|
| Tony Brown |
|
Common Stock |
|
75,892,857 |
|
|
<1 |
|
|
|
15,178,572 |
|
|
|
60,714,285 |
|
|
|
<1 |
|
| Richard Meikle and Laurie Meikle |
|
Common Stock |
|
62,500,000 |
|
|
<1 |
|
|
|
62,500,000 |
|
|
|
– |
|
|
|
– |
|
| Asad H. Shalami and Razia T. Quareshi-Shalami |
|
Common Stock |
|
62,500,000 |
|
|
<1 |
|
|
|
62,500,000 |
|
|
|
– |
|
|
|
– |
|
| Jeffery Lien |
|
Common Stock |
|
55,000,000 |
|
|
<1 |
|
|
|
55,000,000 |
|
|
|
– |
|
|
|
– |
|
| Esteban Berberian |
|
Common Stock |
|
51,470,588 |
|
|
<1 |
|
|
|
10,294,118 |
|
|
|
41,176,470 |
|
|
|
<1 |
|
| Stephen A. Khoury |
|
Common Stock |
|
50,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
30,000,000 |
|
|
|
<1 |
|
| Casaro, S.A. |
|
Common Stock |
|
50,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
30,000,000 |
|
|
|
<1 |
|
| Paola Cedano |
|
Common Stock |
|
50,000,000 |
|
|
<1 |
|
|
|
10,000,000 |
|
|
|
40,000,000 |
|
|
|
<1 |
|
| Clifford Miller |
|
Common Stock |
|
41,025,641 |
|
|
<1 |
|
|
|
20,512,820 |
|
|
|
20,512,821 |
|
|
|
<1 |
|
| Katerin Osuna Robles |
|
Common Stock |
|
39,285,714 |
|
|
<1 |
|
|
|
39,285,714 |
|
|
|
– |
|
|
|
– |
|
| Alfonso Campos |
|
Common Stock |
|
35,000,000 |
|
|
<1 |
|
|
|
33,000,000 |
|
|
|
2,000,000 |
|
|
|
<1 |
|
| Leroy Wilits |
|
Common Stock |
|
31,904,762 |
|
|
<1 |
|
|
|
6,380,953 |
|
|
|
25,523,809 |
|
|
|
<1 |
|
| Maria Madelena Pinedo |
|
Common Stock |
|
33,571,428 |
|
|
<1 |
|
|
|
28,571,428 |
|
|
|
5,000,000 |
|
|
|
– |
|
| Dwight Gaddis |
|
Common Stock |
|
25,000,000 |
|
|
|
|
|
|
25,000,000 |
|
|
|
– |
|
|
|
– |
|
| Richard Royall |
|
Common Stock |
|
25,000,000 |
|
|
|
|
|
|
25,000,000 |
|
|
|
– |
|
|
|
– |
|
| Fabian Ortega |
|
Common Stock |
|
25,000,000 |
|
|
<1 |
|
|
|
25,000,000 |
|
|
|
– |
|
|
|
– |
|
| Jeremy Szeto |
|
Common Stock |
|
25,000,000 |
|
|
<1 |
|
|
|
25,000,000 |
|
|
|
– |
|
|
|
– |
|
| Miriam Maciel Rivera |
|
Common Stock |
|
22,500,000 |
|
|
<1 |
|
|
|
22,500,000 |
|
|
|
– |
|
|
|
– |
|
| Nicola Abate |
|
Common Stock |
|
22,222,222 |
|
|
<1 |
|
|
|
22,222,222 |
|
|
|
– |
|
|
|
– |
|
| Shane Leupold |
|
Common Stock |
|
21,250,000 |
|
|
<1 |
|
|
|
21,250,000 |
|
|
|
– |
|
|
|
– |
|
| Jorge Verar |
|
Common Stock |
|
20,000,000 |
|
|
<1 |
|
|
|
5,000,000 |
|
|
|
15,000,000 |
|
|
|
<1 |
|
| Guadeloupe G. Ramirez |
|
Common Stock |
|
20,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
– |
|
|
|
– |
|
| Brandon Milatovic |
|
Common Stock |
|
20,000,000 |
|
|
<1 |
|
|
|
20,000,000 |
|
|
|
– |
|
|
|
– |
|
| Rosa Casares |
|
Common Stock |
|
16,975,703 |
|
|
<1 |
|
|
|
3,395,141 |
|
|
|
13,580,562 |
|
|
|
<1 |
|
| Frank and Maria Hernandez |
|
Common Stock |
|
16,071,428 |
|
|
<1 |
|
|
|
3,214,285 |
|
|
|
12,857,143 |
|
|
|
<1 |
|
| Jonathan Eisner |
|
Common Stock |
|
16,000,000 |
|
|
<1 |
|
|
|
16,000,000 |
|
|
|
– |
|
|
|
– |
|
| Adriane Kearney |
|
Common Stock |
|
15,000,000 |
|
|
<1 |
|
|
|
3,000,000 |
|
|
|
12,000,000 |
|
|
|
<1 |
|
| Ludvina Martinez |
|
Common Stock |
|
14,705,882 |
|
|
<1 |
|
|
|
2,941,177 |
|
|
|
11,764,705 |
|
|
|
<1 |
|
| Andres Mesa |
|
Common Stock |
|
14,285,715 |
|
|
<1 |
|
|
|
2,857,143 |
|
|
|
11,428,572 |
|
|
|
<1 |
|
| Laura and Jesus Grimaldo |
|
Common Stock |
|
13,161,764 |
|
|
<1 |
|
|
|
2,632,353 |
|
|
|
10,529,411 |
|
|
|
<1 |
|
| Yoselet Isamark Ortiz |
|
Common Stock |
|
12,500,000 |
|
|
<1 |
|
|
|
12,500,000 |
|
|
|
– |
|
|
|
– |
|
| Lucy Caldera |
|
Common Stock |
|
12,500,000 |
|
|
<1 |
|
|
|
12,500,000 |
|
|
|
– |
|
|
|
– |
|
| Manny Escobedo |
|
Common Stock |
|
12,500,000 |
|
|
<1 |
|
|
|
12,500,000 |
|
|
|
– |
|
|
|
– |
|
| David Ward |
|
Common Stock |
|
10,080,645 |
|
|
<1 |
|
|
|
2,016,129 |
|
|
|
8,064,516 |
|
|
|
<1 |
|
| Dianely Heredia |
|
Common Stock |
|
10,000,000 |
|
|
<1 |
|
|
|
10,000,000 |
|
|
|
– |
|
|
|
– |
|
| Will Morey | |
Common Stock | |
10,000,000 | |
| <1 | | |
| 10,000,000 | | |
| – | | |
| – | |
| Robert A. Fleming | |
Common Stock | |
10,000,000 | |
| <1 | | |
| 2,000,000 | | |
| 8,000,000 | | |
| <1 | |
| Dolores Diaz | |
Common Stock | |
8,928,857 | |
| <1 | | |
| 1,785,772 | | |
| 7,143,085 | | |
| <1 | |
| Shawna M. Heisler | |
Common Stock | |
7,000,000 | |
| <1 | | |
| 1,400,000 | | |
| 5,600,000 | | |
| <1 | |
| Stephen Joshua Bertrand | |
Common Stock | |
7,000,000 | |
| <1 | | |
| 1,400,000 | | |
| 5,600,000 | | |
| <1 | |
| Lourdes Perez Ruiz and Cesar A. Oliver Canabal | |
Common Stock | |
7,000,000 | |
| <1 | | |
| 7,000,000 | | |
| – | | |
| – | |
| Ana and Raul Hernandez | |
Common Stock | |
6,944,444 | |
| <1 | | |
| 1,388,889 | | |
| 5,555,555 | | |
| <1 | |
| Cannapolis Scientific Farm SAS | |
Common Stock | |
6,429,000 | |
| <1 | | |
| 1,285,800 | | |
| 5,143,200 | | |
| <1 | |
| Juan de Dios Martinez | |
Common Stock | |
6,250,000 | |
| <1 | | |
| 1,250,000 | | |
| 5,000,000 | | |
| <1 | |
| Rosa Galindo | |
Common Stock | |
6,250,000 | |
| <1 | | |
| 1,250,000 | | |
| 5,000,000 | | |
| <1 | |
| Paola Perales | |
Common Stock | |
6,000,000 | |
| <1 | | |
| 1,200,000 | | |
| 4,800,000 | | |
| <1 | |
| David Esparza | |
Common Stock | |
5,882,353 | |
| <1 | | |
| 1,176,471 | | |
| 4,705,882 | | |
| <1 | |
| George and Sky Noel | |
Common Stock | |
5,018,939 | |
| <1 | | |
| 1,003,788 | | |
| 4,015,151 | | |
| <1 | |
| Alex J. Cruz Valez | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Eduardo Ibarra | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 5,000,000 | | |
| – | | |
| <1 | |
| Bob Wood | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Brian Cuban | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Victor Montanez | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 1,000,000 | | |
| 4,000,000 | | |
| <1 | |
| Marianna Jazmin Sardi Nobles | |
Common Stock | |
5,000,000 | |
| <1 | | |
| 5,000,000 | | |
| – | | |
| – | |
| Teresa Lafond | |
Common Stock | |
4,000,000 | |
| <1 | | |
| 4,000,000 | | |
| – | | |
| – | |
| Ericka and Marcos Nava | |
Common Stock | |
3,750,000 | |
| <1 | | |
| 750,000 | | |
| 3,000,000 | | |
| <1 | |
| Wyntrea Cunningham | |
Common Stock | |
3,571,428 | |
| <1 | | |
| 714,286 | | |
| 2,857,142 | | |
| <1 | |
| Shana Rodriguez | |
Common Stock | |
3,125,000 | |
| <1 | | |
| 685,000 | | |
| 2,440,000 | | |
| <1 | |
| Dante Rodriguez | |
Common Stock | |
3,000,000 | |
| <1 | | |
| 600,000 | | |
| 2,400,000 | | |
| <1 | |
| Eugenio E. Ibarra Pereira | |
Common Stock | |
3,000,000 | |
| <1 | | |
| 600,000 | | |
| 2,400,000 | | |
| <1 | |
| Leidy Marulanda Escudero | |
Common Stock | |
3,000,000 | |
| <1 | | |
| 600,000 | | |
| 2,400,000 | | |
| <1 | |
| Arturo Gomez | |
Common Stock | |
2,500,000 | |
| <1 | | |
| 500,000 | | |
| 2,000,000 | | |
| <1 | |
| Teresa Serrano-Lamm | |
Common Stock | |
2,500,000 | |
| <1 | | |
| 500,000 | | |
| 2,000,000 | | |
| <1 | |
| Cardamom Export Company SAS | |
Common Stock | |
2,143,000 | |
| <1 | | |
| 428,600 | | |
| 1,714,400 | | |
| <1 | |
| Billy and Krista Foxworth | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Cecil Bishop, Jr. | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Martina A Cortez | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Presly Schoenman | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Tom Tusing | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 400,000 | | |
| 1,600,000 | | |
| <1 | |
| Fernando and Ramon Najera | |
Common Stock | |
2,000,000 | |
| <1 | | |
| 2,000,000 | | |
| – | | |
| – | |
| Lizeth Vega | |
Common Stock | |
1,893,939 | |
| <1 | | |
| 378,788 | | |
| 1,515,151 | | |
| <1 | |
| Steven and Sonia Flores | |
Common Stock | |
1,562,500 | |
| <1 | | |
| 312,500 | | |
| 1,250,000 | | |
| <1 | |
| Eric Dangler, Timothy Borgmann and David Farmos | |
Common Stock | |
1,500,000 | |
| <1 | | |
| 300,000 | | |
| 1,200,000 | | |
| <1 | |
| Leavery Y. Davidson | |
Common Stock | |
1,500,000 | |
| <1 | | |
| 300,000 | | |
| 1,200,000 | | |
| <1 | |
| Akil Thomas | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Annabel Velasquez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Jeanette Cantu and Ricardo Beltran | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Maryanne Velasquez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Ricardo Delacruz | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Robert Gomez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Shanner Fugett | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Tommy Hampton | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Victor and Rene Gonzalez | |
Common Stock | |
1,470,588 | |
| <1 | | |
| 294,117 | | |
| 1,176,471 | | |
| <1 | |
| Carrie Ray | |
Common Stock | |
1,177,000 | |
| <1 | | |
| 235,400 | | |
| 941,600 | | |
| <1 | |
| Denise Rodriguez Steidel | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Jill Rocha | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Kristina Gallegos Martinez | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Monique Lucy Castillo Velosa | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 200,000 | | |
| 800,000 | | |
| <1 | |
| Rosangel del Valle Andrades Fuentes | |
Common Stock | |
1,000,000 | |
| <1 | | |
| 1,000,000 | | |
| – | | |
| – | |
| Barbara Collazo Cortes | |
Common Stock | |
500,000 | |
| <1 | | |
| 500,000 | | |
| – | | |
| – | |
| Daniela Montana Arevalo | |
Common Stock | |
500,000 | |
| <1 | | |
| 500,000 | | |
| – | | |
| – | |
| Erika Daniel | |
Common Stock | |
500,000 | |
| <1 | | |
| 100,000 | | |
| 400,000 | | |
| <1 | |
| Travis Slater | |
Common Stock | |
500,000 | |
| <1 | | |
| 100,000 | | |
| 400,000 | | |
| <1 | |
| Brenda Gonzalez | |
Common Stock | |
250,000 | |
| <1 | | |
| 250,000 | | |
| – | | |
| – | |
| Sara and Maria Jaramillo Castillo | |
Common Stock | |
250,000 | |
| <1 | | |
| 50,000 | | |
| 200,000 | | |
| <1 | |
| Alexis Marie Molina | |
Common Stock | |
150,000 | |
| <1 | | |
| 30,000 | | |
| 120,000 | | |
| <1 | |
___________
(1) Assumes the
sale of all of the shares of Common Stock being offered by the Company, which would result in 16,581,749,347 shares of outstanding
Common Stock.
(2) The address
for each person is c/o Cannabis Bioscience International Holdings, Inc., 6201 Bonhomme Road, Suite 466S, Houston, TX 91789.
(3) Applicable percentage
of ownership is based on 10,331,749,347 shares of Common Stock outstanding on the date of this Prospectus, plus the 2,500,000 shares
of Common Stock into which the outstanding shares of Series A Preferred Stock are convertible, totaling 10,334,249,347 shares of Stock.
(4) Applicable percentage
of ownership is based on 16,581,749,347 shares of Common Stock to be outstanding, assuming that all of the shares offered by the Company
pursuant to this Prospectus are sold, plus the 2,500,000 shares of Common Stock into which the outstanding shares of Series A Preferred
Stock are convertible, totaling 16,584,249347 shares of Common Stock.
(5) Assumes the
conversion of all of the shares of Series A Preferred.
(6) Includes 117,000
shares of Common Stock beneficially owned together with Henry Levinski.
Corporate Governance
Director Independence
OTC defines “independent director”
as a person other than an executive officer or employee of a company or any other person having a relationship which, in the opinion
of the Board, would interfere with the exercise of independent judgment in carrying out their responsibilities as a director. The persons
who are not considered independent for purposes of this definition are (i) a director who is, or at any time during the past three
years was, employed by the company; (ii) a director who accepted or has a family member who accepted any compensation from the company
in excess of $120,000 during any fiscal year within the three years preceding the determination of independence, other than compensation
for board or board committee service; compensation paid to a family member who is an employee (other than an executive officer) of the
company or benefits under a tax-qualified retirement plan, or non-discretionary compensation or (iii) a director who is the family member
of a person who is, or at any time during the past three years was, employed by the Company as an executive officer.
Inasmuch as all of the directors of the Company
are employed by the Company as its officers, none of them is an independent director.
A director is not considered independent if he
is also an executive officer or employee of the corporation.
Compensation Committee
The Company does not have a standing compensation
committee or a committee performing similar functions because the Board believes that, in light of the Company’s early stage of
development and the fact that its compensation structure is not complex, such a committee is not presently warranted. Accordingly, the
whole Board participates in considering executive compensation and will do so if, in the future, directors are compensated for their services
as such.
MARKET PRICE FOR OUR COMMON
EQUITY
AND RELATED SHAREHOLDER MATTERS
The Common Stock is quoted on the OTC Pink tier
of the alternate trading system operated by OTC under the trading symbol CBIH; prior to August 17, 2023, the trading symbol was CHNC.
Market quotations for shares of Common Stock shown on OTC’s quotation system reflect inter-dealer prices without retail mark-up,
mark-down or commission and may not necessarily represent actual transactions.
On the date of this Prospectus, the closing price
for the Common Stock quoted by OTC was $_____.
As of August 31, 2023, there were 419
record holders of the shares of the Common Stock, of which 2,335,975,553 shares were freely tradable.
The exemption from registration afforded by Rule
144 will not be available until January 13, 2024, at the earliest.
DESCRIPTION OF CAPITAL STOCK
Our authorized capital stock comprises 20,000,000,000
shares of Common Stock, without par value, of which 10,331,749,347 shares are outstanding, and 10,000,000 shares of preferred stock,
without par value, issuable in series, of which 2,500,000 shares have been designated Series A Convertible Preferred Stock (“Series
A Preferred”), all of which are outstanding, and 1,000 shares have been designated Series B Convertible Preferred Stock (“Series
B Preferred”), all of which are outstanding. The rights of the holders of each class and series are as follows:
Common Stock
Holders of Common Stock are entitled to cast
one vote for each share of Common Stock on all matters submitted to a vote of the stockholders; to receive, on a pro-rata basis, dividends
and distributions, if any, that the Board may declare out of legally available funds, subject to preferences that are applicable to the
Series A Preferred and Series B Preferred, and, if any, to series of preferred stock that may be designated in the future; and upon liquidation,
dissolution or winding up, to share equally and ratably in any assets remaining after the payment of all debts and other liabilities,
subject to the prior rights of the holders of the Series A Preferred.
We do not expect to declare or pay dividends
on Common Stock for the foreseeable future. See “Dividend Policy.”
The holders of Common Stock do not have any preemptive,
cumulative voting, subscription, conversion, redemption or sinking fund rights. The Common Stock is not subject to calls or assessments.
The rights and privileges of holders of the Common Stock are subject to those of the Series A Preferred, which are described below, and
to any other series of preferred stock that we may issue in the future.
The Common Stock is quoted on the OTC Pink tier
of the alternate trading system operated by OTC under the symbol “CBIH.”
Preferred Stock
The Company has authorized 10,000,000 shares
of preferred stock, of which 2,500,000 shares have been designated Series A Convertible Preferred Stock and 1,000 shares have been designated
Series B Preferred Stock (“Series B Preferred”). The rights and preferences of the Series A Preferred Stock are the Series
B Preferred Stock are as follows:
Series A Preferred
The Series A Preferred Stock
is senior to the Common Stock and subordinate to all other series of preferred stock.
Each share of Series A Preferred
is entitled to receive out of the funds of the Company legally available therefor, on the date on which such dividend or other distribution
is paid or made to the holders of Common Stock, a dividend or distribution equal to the dividend or distribution that would be paid on
the number of shares of Common Stock into which such share of Series A Preferred Stock is convertible immediately prior to the record
date for such dividend.
In the event of any liquidation,
dissolution or winding up of the Company, the holders of the outstanding shares of Series A Preferred shall be entitled to be paid out
of the assets of the Corporation available for distribution to its shareholders, whether from capital, surplus funds or earnings, and
before any payment is made in respect of the shares of Common Stock, an amount equal to the greater of (i) the Market Price (as defined
in the restated articles of incorporation) of the Series A Preferred Stock on the date of the liquidation, or (ii) ten cents ($0.10) per
share of Series A Preferred, plus accrued but unpaid dividends.
The Series A Preferred may
be redeemed, as a whole or in part, at any time or from time to time, as determined by the Board in its discretion. Upon redemption,
each share of Series A Preferred shall receive as the full redemption payment the number of shares of Common Stock into which it is then
convertible. The Board shall select the shares of Series A Preferred to be redeemed in its sole and unfettered discretion and need not
do so on a pro-rata basis. The Series A Preferred is not redeemable at the option of the holders.
Each share of Series A Preferred
is entitled to one vote for each share of Common Stock into which it is convertible, and except as otherwise required by law, vote as
a group with the holders of Common Stock.
Each share of Series A Preferred
may be converted, at the option of the holder, into the number of shares of Common Stock equal to the quotient obtained by dividing the
current Series A Preference Price by the Series A Conversion Price, which is the greater of (i) $0.10 or (ii) 75% of the Market
Price of the Common Stock on the Conversion Date.
Series B Preferred
The Series B Preferred is
senior to the Common Stock and the Series A Preferred.
In the event of liquidation,
the shares of Series B Preferred shall not be entitled to receive any distribution of cash or other property whatsoever.
The Series B Preferred is
not redeemable at the option of the holder or the Company.
The holders of the Series
B Preferred vote as a group with the holders of all other classes and series of the Corporation’s capital stock and have 60% of
the voting power of the Company on all matters, except that the holders of the Series B Preferred vote as a separate voting group on
all matters affecting their rights as such or as otherwise specified by law. No series of preferred stock having voting rights equal
or superior to the voting rights of the Series B Preferred may designated without the unanimous vote of all of the holders thereof.
The holders of Series B
Preferred have no conversion rights.
Anti-Takeover Effects of the Series B Preferred
The provisions of the restated articles of incorporation
designating the Series B Preferred vest 60% of the voting power of the Company in the holders thereof. These provisions prevent the holders
of Common Stock from taking any action without the approval of the holders of the Series B Preferred. These provisions may have an anti-takeover
effect and may delay, deter or prevent a tender offer, takeover attempt or other transaction that might be in a stockholder’s best
interest, including an attempt that might result in the receipt of a premium over the market price for shares of Common Stock.
Indemnification
The Company’s amended and restated articles
of incorporation require it to indemnify, to the full extent permitted by law, any person who is or was a director or officer of the Company
and may indemnify any other person against any claim, liability or expense arising against or incurred by such person made a party to
a proceeding because he is or was a director, officer, agent, fiduciary or employee of the Company or because he is or was serving another
entity as a director, officer, partner, trustee, employee, fiduciary or agent at the Company’s request.
Elimination of Personal Liability
The Company’s amended and restated articles
of incorporation provide that the personal liability of the Company’s directors to the Company or its stockholders is limited to
the full extent permitted by the CBCA.
Annual Stockholders Meeting
Our amended and restated by-laws provide that
annual stockholder meetings will be held at a date, time and place selected by resolution adopted by a majority of our entire Board or,
if duly authorized by the affirmative vote of a majority of our entire Board, by a committee thereof, or by the chairman of our Board
(if delegated such authority by resolution adopted by a majority of our entire Board). We are permitted to conduct stockholder meetings
by remote communications.
The affirmative vote of holders of a majority
of the outstanding shares of our capital stock present, in person or by proxy, at any annual or special meeting of stockholders and entitled
to vote will decide all matters voted on by stockholders at such meeting, provided that such shares constitute a quorum, unless the question
is one upon which, by express provision of law, under our amended and restated certificate of incorporation, or under our amended and
restated by-laws, a different vote is required, in which case such provision will control.
SHARES ELIGIBLE FOR FUTURE
SALE
Future sales of substantial amounts of Common
Stock, including shares issued upon the exercise of outstanding options or warrants, in the public market after the Offering, or the
perception that such sales may occur, could cause the market price for the Common Stock to fall or impair our ability to raise capital
through sales of our equity securities.
Upon the termination of the Offering, assuming
that all of the shares offered by the Company are sold, there will be 16,581,749,347 shares of Common Stock outstanding.
In addition to the 10,087,154,885 shares of
Common Stock that are offered by this Prospectus, (i) approximately 1.7 billion shares of restricted Common Stock held by persons who
are not deemed to be affiliates of the Company will be permitted to be sold after January 13, 2024, under Rule 144 promulgated by the
SEC under the Securities Act (“Rule 144”) without notice to the SEC in unlimited amounts and without restriction as to the
manner of sale, (ii) approximately 2.4 billion share of Common Stock that are freely tradable, (iii) approximately 4,.6 billion shares
of restricted Common Stock held by persons who are deemed to be affiliates of the Company will be permitted to be sold under Rule 144
after January 13, 2024, in limited amounts, subject to notice to the SEC and subject to restriction as to the manner of sale and (iv)
up to 600,000,000 shares of Common Stock that may be issued under the Company's 2022 Equity Incentive Plan may be sold in the public
markets, subject to limitations in the case of shares issued under that plan to affiliates of the Company, upon the filing of a registration
statement on Form S-8 with respect thereto or without registration under Rule 144 after being held by for the period required by that
rule. The sale of these shares or the perception that they may be sold may substantially and adversely affect the market price of the
Common Stock, with the result that persons who acquire shares of Common Stock in the Offering may be able to resell them only at substantial
losses.
Rule 144
In general, under Rule 144, beginning on January
13, 2024, any person who is not our affiliate and has held their shares for at least six months, including the holding period of any
prior owner, except for our affiliates, may sell shares without restriction, subject to the availability of current public information
about us. In addition, under Rule 144, any person who is not and has not been our affiliate at any time during the preceding three months
and has held his shares for at least one year, including the holding period of any prior owner, except for our affiliates, would be entitled
to sell an unlimited number of shares immediately in the event that no current public information about us is available. Beginning on
January 13, 2024, a person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially
owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates,
will be entitled to sell a number of shares within any three-month period that does not exceed the greater of: (i) 1% of the number
of shares of Common Stock outstanding, which 1% will be approximately 166 million shares immediately after the termination of
the Offering, assuming that all of the shares offered by the Company are sold, and (ii) the average weekly trading volume of Common
Stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. Such sales will be subject
to certain manner-of-sale provisions, notice requirements and the availability of current public information about us.
Incentive Plan Registration Statement
We intend to file with the SEC a registration
statement on Form S-8 under the Securities Act covering the shares of common stock that are issuable to existing and future awards under
the Incentive Plan. Shares covered by such registration statement will be available for sale in the open market following its effective
date, subject to limitations applicable to shares of Common Stock held by our affiliates.
Registration rights
Persons to whom we sell shares of Common Stock
or securities convertible into Common Stock pursuant to exemptions from registration under the Securities Act may acquire these shares
or securities under agreements pursuant to which they may demand that we register the sale of the purchased shares under the Securities
Act or, if we file a registration statement under the Securities Act other than a registration statement on Form S-8 covering securities
issuable under the Incentive Plan or on Form S-4, may have the right to include their shares in such registration. Following such registered
sales, these shares will be freely tradable without restriction under the Securities Act, unless they are held by our affiliates.
MATERIAL U.S. FEDERAL INCOME
TAX CONSEQUENCES TO NON-U.S. HOLDERS OF COMMON STOCK
The following is a summary of the material U.S.
federal income tax consequences to non-U.S. holders (as defined below) of the acquisition, ownership, and disposition of Common Stock
issued pursuant to this offering. This discussion is not a complete analysis of all potential U.S. federal income tax consequences relating
thereto, does not address the potential application of the Medicare contribution tax on net investment income, the alternative minimum
tax, or the special tax accounting rules under Section 451(b) of the Code, and does not address any estate or gift tax consequences or
any tax consequences arising under any state, local, or foreign tax laws, or any other U.S. federal tax laws. This discussion is based
on the Code and applicable Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative
pronouncements of the Internal Revenue Service, or IRS, all as in effect as of the date hereof. These authorities are subject to differing
interpretations and may change, possibly retroactively, resulting in U.S. federal income tax consequences different from those discussed
below. We have not requested a ruling from the IRS with respect to the statements made and the conclusions reached in the following summary,
and there can be no assurance that the IRS or a court will agree with such statements and conclusions.
This discussion is limited to non-U.S. holders
who purchase Common Stock pursuant to this offering and who hold Common Stock as a “capital asset” within the meaning of
Section 1221 of the Code (generally, property held for investment). This discussion does not address all of the U.S. federal income tax
consequences that may be relevant to a particular holder in light of such holder’s particular circumstances. This discussion also
does not consider any specific facts or circumstances that may be relevant to holders subject to special rules under the U.S. federal
income tax laws, including:
| · |
|
certain former citizens or long-term residents of the United States;
|
| |
|
|
| · |
|
“controlled foreign corporations”; |
| |
|
|
| · |
|
“passive foreign investment companies”; |
| |
|
|
| · |
|
corporations that accumulate earnings to avoid U.S. federal income
tax; |
| |
|
|
| · |
|
banks, financial institutions, investment funds, insurance companies,
brokers, dealers, or traders in securities; |
| |
|
|
| · |
|
tax-exempt organizations and governmental organizations; |
| |
|
|
| · |
|
tax-qualified retirement plans; |
| |
|
|
| · |
|
“qualified foreign pension funds” as defined in Section 897(l)(2)
of the Code and entities, all of the interests of which are held by qualified foreign pension funds; |
| |
|
|
| · |
|
persons that own, or have owned, actually or constructively, more than
5% of Common Stock at any time; |
| |
|
|
| · |
|
persons who have elected to mark securities to market. |
If an entity or arrangement that is classified
as a partnership for U.S. federal income tax purposes holds Common Stock, the U.S. federal income tax treatment of the partnership and
the partners thereof generally depend on the status of the partner and the activities
of the partnership. Partnerships holding Common Stock and the partners in such partnerships are urged to consult their tax advisors about
the particular U.S. federal income tax consequences to them of holding and disposing of Common Stock.
THIS DISCUSSION IS FOR INFORMATIONAL PURPOSES
ONLY AND IS NOT TAX ADVICE. PROSPECTIVE INVESTORS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX
CONSEQUENCES TO THEM OF ACQUIRING, OWNING, AND DISPOSING OF COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL,
OR FOREIGN TAX LAWS AND ANY OTHER U.S. FEDERAL TAX LAWS.
Definition of non-U.S. holder
For purposes of this discussion, the term “non-U.S.
holder” means any beneficial owner of Common Stock that is not a “U.S. person” or a partnership (including any entity
or arrangement treated as a partnership) for U.S. federal income tax purposes. A U.S. person is any person that, for U.S. federal income
tax purposes, is or is treated as any of the following:
| · |
|
an individual who is a citizen or resident of the United States; |
| |
|
|
| · |
|
a corporation (or entity treated as a corporation for U.S. federal
income tax purposes) created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| |
|
|
| · |
|
an estate, the income of which is subject to U.S. federal income tax
regardless of its source; or |
| |
|
|
| · |
|
a trust (1) whose administration is subject to the primary supervision
of a U.S. court and which has one or more U.S. persons who have the authority to control all substantial decisions of the trust or
(2) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Distributions on Common Stock
We have not paid dividends on Common Stock and
do not anticipate paying dividends on Common Stock for the foreseeable future. However, if we make cash or other property distributions
on Common Stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current
or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S.
federal income tax purposes will constitute a return of capital and will first be applied against and reduce a holder’s tax basis
in Common Stock, but not below zero. Any excess will be treated as gain realized on the sale or other disposition of Common Stock and
will be treated as described under the section titled “— Gain on disposition of Common Stock” below.
Subject to the discussions below regarding effectively
connected income, backup withholding and Sections 1471 through 1474 of the Code (commonly referred to as FATCA), dividends paid to a
non-U.S. holder generally will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends or such
lower rate specified by an applicable income tax treaty. To receive the benefit of a reduced treaty rate, a non-U.S. holder must furnish
a valid IRS Form W-8BEN or IRS Form W-8BEN-E (or applicable successor form) and satisfy applicable certification and other requirements.
This certification must be provided before the payment of dividends and must be updated periodically. If the non-U.S. holder holds the
stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required
to provide appropriate documentation to the agent, which then will be required to provide certification, either directly or through other
intermediaries.
If a non-U.S. holder holds Common Stock in connection
with the conduct of a trade or business in the United States, and dividends paid on Common Stock are effectively connected with such
holder’s U.S. trade or business (and are attributable to such holder’s permanent establishment in the United States if required
by an applicable tax treaty), the non-U.S. holder will be exempt from U.S. federal withholding tax. To claim the exemption, the non-U.S.
holder must generally furnish a valid IRS Form W-8ECI (or applicable successor form) to us or our paying agent. However, any such effectively
connected dividends paid on Common Stock generally will be subject to U.S. federal income tax on a net income basis at the regular U.S.
federal income tax rates in the same manner as if such holder were a resident of the United States. A non-U.S. holder that is a foreign
corporation also may be subject to an additional branch profits tax equal to 30% (or such lower rate specified by an applicable income
tax treaty) of its effectively connected earnings and profits for the taxable year, as adjusted for certain items. Non-U.S. holders should
consult their tax advisors regarding any applicable income tax treaties that may provide for different rules.
Non-U.S. holders that do not provide the required
certification on a timely basis, but that qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely
filing an appropriate claim for refund with the IRS.
Gain on disposition of Common Stock
Subject to the discussions below regarding backup
withholding and FATCA, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized on the sale or
other disposition of Common Stock unless:
| · |
|
the gain is effectively connected with the non-U.S. holder’s
conduct of a trade or business in the United States and, if required by an applicable income tax treaty, is attributable to a permanent
establishment maintained by the non-U.S. holder in the United States; |
| |
|
|
| · |
|
the non-U.S. holder is a nonresident alien individual present in the
United States for 183 days or more during the taxable year of the disposition, and certain other requirements are met; or |
| |
|
|
| · |
|
Common Stock constitutes a “United States real property interest,”
or USRPI, by reason of our status as a United States real property holding corporation, or USRPHC, for U.S. federal income tax purposes
at any time within the shorter of the five-year period preceding the disposition or the non-U.S. holder’s holding period for
Common Stock. |
The determination of whether we are a USRPHC
depends on the fair market value of our USRPIs relative to the fair market value of worldwide real property interests and our other assets
used or held for use in a trade or business. We believe that we are not and do not anticipate becoming a USRPHC for U.S. federal
income tax purposes, although there can be no assurance that we will not become a USRPHC. Even if we are or were to become a USRPHC,
gain arising from the sale or other taxable disposition of Common Stock by a non-U.S. holder will not be subject to U.S. federal income
tax if Common Stock is “regularly traded” (as defined by applicable Treasury Regulations) on an established securities market,
and such non-U.S. holder owned, actually and constructively, 5% or less of Common Stock throughout the shorter of the five-year period
ending on the date of the sale or other taxable disposition or the non-U.S. holder’s holding period. Prospective investors are
encouraged to consult their own tax advisors regarding the possible consequences to them if we are, or were to become, a USRPHC.
Gain described in the first bullet point above
generally will be subject to U.S. federal income tax on a net income basis at the regular U.S. federal income tax rates in the same manner
as if such holder were a resident of the United States. A non-U.S. holder that is a foreign corporation also may be subject to an additional
branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of its effectively connected earnings
and profits for the taxable year, as adjusted for certain items. Gain described in the second bullet point above will be subject to U.S.
federal income tax at a flat 30% rate (or such lower rate specified by an applicable income tax treaty), but may be offset by certain
U.S.-source capital losses (even though the individual is not considered a resident of the United States), provided that the non-U.S.
holder has timely filed U.S. federal income tax returns with respect to such losses. Non-U.S. holders should consult their tax advisors
regarding any applicable income tax treaties that may provide for different rules.
Information reporting and backup withholding
Annual reports are required to be filed with
the IRS and provided to each non-U.S. holder indicating the amount of distributions on Common Stock paid to such holder and the amount
of any tax withheld with respect to those distributions. These information reporting requirements apply regardless of whether such distributions
constitute dividends and even if no withholding was required. This information also may be made available under a specific treaty or
agreement with the tax authorities in the country in which the non-U.S. holder resides or is established. Backup withholding, currently
at a 24% rate, generally will not apply to payments to a non-U.S. holder of dividends on or the gross proceeds of a disposition of Common
Stock provided the non-U.S. holder furnishes the required certification for its non-U.S. status, such as by providing a valid IRS Form
W-8BEN, IRS Form W-8BEN-E, or IRS Form W-8ECI, or certain other requirements are met. Backup withholding may apply if the payor has actual
knowledge, or reason to know, that the holder is a U.S. person who is not an exempt recipient.
Backup withholding is not an additional tax.
If any amount is withheld under the backup withholding rules, the non-U.S. holder should consult with a U.S. tax advisor regarding the
possibility of and procedure for obtaining a refund or a credit against the non-U.S. holder’s U.S. federal income tax liability,
if any.
FATCA
FATCA imposes a U.S. federal withholding tax
of 30% on certain payments made to a “foreign financial institution” (as specially defined under these rules) unless such
institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S.
tax authorities substantial information regarding certain U.S. account holders of such institution (which includes certain equity and
debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or an exemption applies.
FATCA also generally will impose a U.S. federal withholding tax of 30% on certain payments made to a non-financial foreign entity unless
such entity provides the withholding agent a certification identifying certain direct and indirect U.S. owners of the entity or an exemption
applies. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Under
certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. FATCA currently applies to dividends
paid on Common Stock. Under applicable Treasury Regulations and administrative guidance, withholding under FATCA would have applied to
payments of gross proceeds from the sale or other disposition of stock, but under proposed regulations (the preamble to which specifies
that taxpayers are permitted to rely on such proposed regulations pending finalization), no withholding would apply with respect to payments
of gross proceeds.
Prospective investors are encouraged to consult
with their own tax advisors regarding the possible implications of this legislation on their investment in Common Stock.
PLAN OF DISTRIBUTION
By the Company
The Company is offering up to 6,250,000,000
shares of Common Stock at the Fixed Offering Price, unless modified by a post-effective amendment to the registration statement of which
this Prospectus is a part.
Each time we offer and sell such shares, we
will, if required, make available a Prospectus that will describe the method of distribution and set forth the terms of the offering.
We may terminate the offering before all shares
are sold. There is no minimum number of shares that must be sold before we may use the proceeds. Proceeds will not be returned to investors
if we sell less than all of the shares offered by this Prospectus. The proceeds from the sales of the shares will not be placed in an
escrow account.
The offering by the Company will be conducted
by the executive officers of the Company. Under Rule 3a 4-1 of the Exchange Act, an issuer may conduct a direct offering of its securities
without registration as a broker-dealer using officers who perform substantial duties for or on behalf of the issuer otherwise than in
connection with securities transactions and who were not brokers or dealers or associated persons of brokers or dealers within the preceding
12 months and who have not participated in selling an offering of securities for any issuer more than once every 12 months, with certain
exceptions. Furthermore, such persons may not be subject to statutory disqualification under Section 3(a)(39) of the Securities Exchange
Act and may not be compensated in connection with securities offerings by payment of commission or other remuneration based either directly
or indirectly on transactions in securities and at the time of offering our shares may not be associated persons of a broker or dealer.
Mr. Picazo and our other executive officers meet these requirements.
During the Offering, the Company may offer
unregistered shares of Common Stock to investors in private placements at prices per share that may be higher or lower than the
public offering price, but only to the extent that it is permitted to do so by the federal securities laws, including Rule 152
promulgated under the Securities Act.
By the Selling Stockholders
The Selling Stockholders identified in this Prospectus
may offer, from time to time, up to 3,860,369,171 shares of Common Stock up to the respective amounts set forth in this Prospectus.
We will not receive any of the proceeds of such sales. There can be no assurance that the Selling Stockholders will offer or sell any
or all of such Common Stock.
Messrs. Picazo, Levinski and Torres, who are
officers and directors of the Company, and Mr. John Jones, who owns more than ten percent of the outstanding shares of Common Stock,
may be regarded as underwriters. In addition, Mr. Picazo has indicated that he may reinvest all or a portion of the proceeds of sales
of his shares, in the form of equity or debt, on terms to be approved by the Board in the manner provided by Colorado law respecting
transactions in which officers and directors of the Company have an interest and may be regarded as an underwriter in respect of such
reinvestments.
The Selling Stockholders and their successors,
including their transferees, may sell all or a portion of their shares directly to purchasers or through broker-dealers
or agents, who may receive compensation in the form of discounts, concessions or commissions from the Selling Stockholders or the purchasers
of the shares. These discounts, concessions or commissions as to any particular underwriter, broker-dealer or agent may be in excess of
those customary in the types of transactions involved.
These shares may be sold in one or more transactions
on any national securities exchange or alternate trading system on which the shares may be listed or quoted at the time of sale or in
the over-the-counter market or transactions otherwise than on these exchanges or systems in one or more transactions. The shares will
be sold at the Fixed Offering Price. These sales may be effected in transactions, which may involve crosses or block transactions.
Additionally, the Selling Stockholders may enter into derivative transactions with third parties or sell securities not covered by this
Prospectus to third parties in privately negotiated transactions. The Selling Stockholders may use any one or more of the following methods
when selling shares:
| · | on
any national securities exchange or alternated trading system on which the shares may be
listed or quoted at the time of sale, including NASDAQ; |
| | | |
| · | in
transactions otherwise than on these exchanges or services or in the over-the-counter market; |
| · | through
the writing or settlement of options, whether the options are listed on an options exchange
or otherwise; |
| · | ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block
trades in which the broker-dealer will attempt to sell the shares as agent but may position
and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | an
exchange distribution in accordance with the rules of the applicable exchange; |
| · | a
debt-for-equity exchange; |
| · | privately
negotiated transactions; |
| · | settlement
of short sales entered into after the effective date of the registration statement of which
this Prospectus forms a part; |
| · | broker-dealers
may agree with the Selling Stockholders to sell a specified number of such shares at a stipulated
price per share; |
| · | a
combination of any such methods of sale; and |
| · | any
other method permitted by applicable law. |
The anti-manipulation rules of Regulation M under
the Exchange Act may apply to sales of the shares of Common Stock offered pursuant to this Prospectus and the activities of the
Selling Stockholders. In addition, we will make copies of this Prospectus available to the Selling Stockholders to satisfy the prospectus
delivery requirements of the Securities Act. To the extent applicable, Regulation M may also restrict the ability of any person engaged
in the distribution of the Common Stock to engage in market-making activities with respect to the Common Stock. All of the foregoing
may affect the marketability of the Common Stock and the ability of any person or entity to engage in market-making activities with respect
to the Common Stock.
In addition, any securities that qualify for sale
pursuant to Rule 144, Regulation S under the Securities Act or Section 4(1) under the Securities Act may be sold under such rules rather
than pursuant to this Prospectus.
The Selling Stockholders may sell short
the shares and deliver Common Stock to close out short positions, or loan or pledge the shares to broker-dealers that in turn may
sell these shares. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial
institutions or the creation of one or more derivative securities that require the delivery to such broker-dealer or other financial
institution of shares offered by this Prospectus, which shares such broker-dealer or other financial institution may resell pursuant
to this Prospectus. The Selling Stockholders also may transfer and donate the shares in other circumstances, in which case the
transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this
Prospectus.
The aggregate proceeds to the Selling Stockholders
from the sale of the shares of Common Stock will be the sale price for the shares, less discounts and commissions, if any.
In offering the shares of Common Stock covered
by this Prospectus, the Selling Stockholders and any broker-dealers who execute sales for the Selling Stockholders may be deemed to be
“underwriters” within the meaning of Section 2(a)(11) of the Securities Act in connection with such sales. Any profits realized
by the Selling Stockholders and the compensation of any broker-dealer may be deemed to be underwriting discounts and commissions. Selling
Stockholders who are “underwriters” within the meaning of Section 2(a)(11) of the Securities Act will be subject to the prospectus
delivery requirements of the Securities Act and may be subject to certain statutory and regulatory liabilities, including liabilities
imposed pursuant to Sections 11, 12 and 17 of the Securities Act and Rule 10b-5 under the Exchange Act.
To comply with the securities laws of certain
states, if applicable, the shares of Common Stock must be sold in such jurisdictions only through registered or licensed brokers or dealers.
In addition, in certain states the shares may not be sold unless the shares are registered or qualified for sale in the applicable state
or an exemption from the registration or qualification requirement is available and is complied with.
LEGAL MATTERS
The validity of the shares of Common Stock
offered by this Prospectus has been passed upon by Barry J, Miller, Esq., of West Bloomfield, Michigan. Mr. Miller is the indirect beneficial
holder of 50,000,000 shares of Common Stock.
EXPERTS
The consolidated financial statements of the
Company for the year ended on May 31, 2023, have been included in this Prospectus and in the registration statement of which it forms
a part in reliance upon the report of Victor Mokuolu, CPA PLLC, appearing elsewhere in herein, and the consolidated financial statements
of the Company for the year ended on May 31, 2022, have been included in this Prospectus and in the registration statement of which it
forms a part in reliance upon the report of PWR CPA, LLP, appearing elsewhere herein, each firm being the Company’s independent
registered public accounting firm for the year to which its report relates, and upon the authority of said firms as experts in accounting
and auditing.
CHANGE IN INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
On March 21, 2023, PWR CPA, LLP (“PWR CPA”)
gave notice of its resignation as the Company’s independent auditor and on July 21, 2023, it retained Victor Mokuolu, CPA PLLC (“VM
CPA”) as its independent registered public accounting firm to audit its consolidated financial statements for the fiscal year ended
May 31, 2023. The decision to retain VM CPA was approved by the Company’s board of directors.
PWR CPA issued reports on the Company’s
financial statements for the fiscal years ended May 31, 2022, and May 31, 2021. Except for a statement as to substantial doubt as to the
Company’s ability to continue as a going concern, these reports did not contain an adverse opinion or a disclaimer of opinion and
were not qualified or modified as to uncertainty, audit scope, or accounting principle. PWR CPA did not audit the Company’s consolidated
financial statements for any period subsequent to May 31, 2022.
During the two most recent fiscal years and any
subsequent interim period preceding the resignation of PWR CPA (the “Relevant Period”), (i) the Company had no disagreements
with PWR CPA on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure (“Disagreements”),
which, if not resolved to the satisfaction of PWR CPA, would have caused it to make reference in connection with its reports to the subject
matter of the Disagreement during the two audits immediately preceding its dismissal and (ii) there were no “reportable events,”
as that term is defined in Item 304(a)(1)(v) of Regulation S-K (each a “Reportable Event”).
The Company has provided PWR CPA with a copy of
the disclosures set forth under the caption “Changes in Independent Registered Public Accounting Firm” included in this Prospectus
and has requested that it furnish a letter addressed to the SEC stating whether or not PWR CPA agrees with statements related to them
under that caption. A copy of that letter has been filed as Exhibit 16.1 to the registration statement of which this Prospectus forms
a part.
During the Relevant Period, neither the
Company nor someone acting on its behalf consulted with VM CPA regarding the application of accounting principles to a specified
transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial
statements and neither a written report nor oral advice was provided to the Company that VM CPA concluded was an important factor
considered by the Company in reaching a decision as to (i) any accounting, auditing, or financial reporting issue or (ii) any matter
that was either the subject of a Disagreement or a Reportable Event.
The Company has requested that VM CPA review
the disclosures set forth under the aforesaid caption and has provided it with the opportunity to furnish the Company with a letter
addressed to the Commission containing any new information, clarification of the Company’s expression of its views, or the
respects in which it does not agree with such disclosures.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement
on Form S-1, including exhibits and schedules, under the Securities Act, with respect to the shares of Common Stock being offered by
this Prospectus. This Prospectus, which constitutes part of the registration statement, does not contain all of the information in the
registration statement and its exhibits. For further information with respect to us and the Common Stock offered by this Prospectus,
we refer you to the registration statement and its exhibits. Statements contained in this Prospectus as to the contents of any contract
or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other
document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration
statement, free of charge, over the Internet at the SEC’s website at www.sec.gov.
We will be subject to the information reporting
requirements of the Exchange Act and we will file reports and other information with the SEC. You may access these materials free of
charge on the SEC’s website as soon as they are filed with the SEC.
Information on or accessible through our website
is not a part of this Prospectus, and the inclusion of our website address in this Prospectus is an inactive textual reference only.
INDEX
TO CONSOLIDATED FINANCIAL STATEMENTS
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED BALANCE SHEETS
| | |
August 31, 2023 | | |
May 31, 2023 | |
| | |
(Unaudited) | | |
(Audited) | |
| ASSETS |
|
|
|
|
|
|
|
|
| CURRENT ASSETS | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 4,745 | | |
$ | 8,913 | |
| Accounts receivable | |
| 27,990 | | |
| 10,549 | |
| Related party receivable | |
| – | | |
| – | |
| TOTAL CURRENT ASSETS | |
| 32,735 | | |
| 19,462 | |
| Right-of-use asset | |
| – | | |
| 23,920 | |
| TOTAL ASSETS | |
$ | 32,735 | | |
$ | 43,382 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT LIABILITIES | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 169,952 | | |
$ | 111,299 | |
| Deferred revenue | |
| 20,308 | | |
| 28,641 | |
| Related party payables | |
| 155,541 | | |
| 105,173 | |
| Short-term loans (net of amortization of loan fees) | |
| 154,888 | | |
| 121,407 | |
| SBA loan – current | |
| 14,593 | | |
| 14,592 | |
| Lease liabilities – current | |
| – | | |
| 4,435 | |
| TOTAL CURRENT LIABILITIES | |
| 515,282 | | |
| 385,547 | |
| | |
| | | |
| | |
| LONG-TERM LIABILITIES | |
| | | |
| | |
| SBA loan | |
| 234,908 | | |
| 249,500 | |
| TOTAL LONG-TERM LIABILITIES | |
| 234,908 | | |
| 249,500 | |
| TOTAL LIABILITIES | |
| 750,190 | | |
| 635,047 | |
| | |
| | | |
| | |
| STOCKHOLDERS’ DEFICIENCY | |
| | | |
| | |
| Authorized 10,000,000 shares of preferred stock, without par
value, of which 2,500,000 shares have been designated Series A Convertible Preferred Stock and 1,000 shares have been designated
Series B Preferred Stock | |
| – | | |
| – | |
| | |
| | | |
| | |
| Common Stock, without par value: 20,000,000,000 shares authorized; 10,331,749,347 and 10,059,677,919 shares issued and outstanding at August 31, 2023, and May 31, 2023, respectively. | |
| – | | |
| – | |
| | |
| | | |
| | |
| Additional paid-in capital | |
| 4,147,071 | | |
| 4,091,071 | |
| Accumulated deficit | |
| (4,864,525 | ) | |
| (4,682,736 | ) |
| TOTAL STOCKHOLDERS’ DEFICIENCY | |
| (717,454 | ) | |
| (591,665 | ) |
| | |
| | | |
| | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | |
$ | 32,735 | | |
$ | 43,382 | |
See accompanying notes to condensed
consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS
OF OPERATIONS
(Unaudited)
| | |
Three Months Ended August 31, | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | 72,821 | | |
$ | 143,752 | |
| Cost of revenues | |
| 8,820 | | |
| 33,170 | |
| Gross profit | |
| 64,001 | | |
| 110,582 | |
| | |
| | | |
| | |
| Cost and expenses | |
| | | |
| | |
| General and administrative | |
| 48,083 | | |
| 29,304 | |
| Contract labor | |
| 95,654 | | |
| 173,849 | |
| Professional fees | |
| 62,394 | | |
| 67,322 | |
| Officer compensation | |
| 12,000 | | |
| 12,000 | |
| Rent and lease | |
| 20,953 | | |
| 18,246 | |
| Travel | |
| 1,701 | | |
| 1,667 | |
| Total operating expenses | |
| 240,785 | | |
| 302,388 | |
| | |
| | | |
| | |
| Loss from operations | |
| (176,784 | ) | |
| (191,806 | ) |
| | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | |
| Forgiveness of debt | |
| – | | |
| 41,666 | |
| Interest expense-short and SBA loans | |
| (5,005 | ) | |
| (12,235 | ) |
| Total other income (expense) | |
| (5,042 | ) | |
| 29,431 | |
| | |
| | | |
| | |
| Net loss | |
$ | (181,789 | ) | |
$ | (162,375 | ) |
| | |
| | | |
| | |
| Average common stock outstanding | |
| 10,331,749,347 | | |
| 8,559,051,075 | |
| | |
| | | |
| | |
| Average loss per share | |
$ | (0.00002 | ) | |
$ | (0.00002 | ) |
See accompanying notes to condensed
consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS
OF CASH FLOWS
(Unaudited)
| | |
Three Months Ended August 31, | |
| | |
2023 | | |
2022 | |
| CASH FLOW FROM OPERATING ACTIVITIES: | |
| | | |
| | |
| Net loss | |
$ | (181,789 | ) | |
$ | (162,375 | ) |
| Adjustments to reconcile net loss: | |
| | | |
| | |
| Amortization of right-of-use-asset and liability | |
| 23,920 | | |
| (1,810 | ) |
| Forgiveness of PPP loan | |
| – | | |
| (41,666 | ) |
| Changes in assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| (17,444 | ) | |
| (43,364 | ) |
| Accounts payable and accrued expenses | |
| 58,654 | | |
| 53,256 | |
| Deferred revenue | |
| (8,333 | ) | |
| – | |
| Related party payable | |
| 50,373 | | |
| – | |
| Other current assets | |
| – | | |
| (10 | ) |
| NET CASH USED IN OPERATIONS | |
| (74,620 | ) | |
| (195,969 | ) |
| | |
| | | |
| | |
| CASH FLOW FROM FINANCING ACTIVITIES: | |
| | | |
| | |
| Proceeds from sales of common stock | |
| 75,000 | | |
| 75,000 | |
| Repayment of shareholder | |
| (19,000 | ) | |
| – | |
| Change in lease liability | |
| (4,435 | ) | |
| – | |
| Proceeds of short-term loans | |
| 33,481 | | |
| 47,209 | |
| Repayment of SBA loan | |
| (14,592 | ) | |
| 43,650 | |
| Proceeds from related party loan | |
| – | | |
| 43,650 | |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | |
| 70,453 | | |
| 165,859 | |
| | |
| | | |
| | |
| NET DECREASE IN CASH | |
| (4,167 | ) | |
| (30,110 | ) |
| | |
| | | |
| | |
| CASH AT BEGINNING OF PERIOD | |
| 8,913 | | |
| 31,982 | |
| | |
| | | |
| | |
| CASH AT END OF PERIOD | |
$ | 4,746 | | |
$ | 1,872 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information | |
| | | |
| | |
| Cash paid for interest | |
$ | 5,005 | | |
$ | 5,524 | |
| Cash paid for taxes | |
$ | – | | |
$ | – | |
See accompanying notes to condensed
consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ DEFICIENCY
FOR THE THREE MONTHS ENDED AUGUST 31, 2023,
AND AUGUST 31, 2022
(Unaudited)
| | |
Series
A Convertible Preferred Stock | | |
Series B Convertible
Preferred Stock | | |
Common Stock | | |
Additional Paid-In | | |
Accumulated | | |
| |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Capital | | |
Deficit | | |
Total | |
| Balance - May 31, 2023 | |
| 2,500,000 | | |
$ | – | | |
| 1,000 | | |
$ | – | | |
| 10,059,677,919 | | |
$ | 4,091,071 | | |
$ | (4,682,736 | ) | |
$ | (591,665 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 272,071,428 | | |
| 75,000 | | |
| – | | |
| 75,000 | |
| Net loss for the quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (181,789 | ) | |
| (181,789 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance - August 31, 2023 | |
| 2,500,000 | | |
$ | – | | |
| 1,000 | | |
$ | – | | |
| 10,331,749,347 | | |
$ | 4,147,071 | | |
$ | (4,864,525 | ) | |
$ | (717,454 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance - May 31, 2022 | |
| 2,500,000 | | |
$ | 2,500 | | |
| 1,000 | | |
$ | – | | |
| 8,612,998,299 | | |
$ | 3,286,605 | | |
$ | (3,650,156 | ) | |
$ | (361,052 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 125,000,000 | | |
| 75,000 | | |
| – | | |
| 75,000 | |
| Change in par value of common stock | |
| – | | |
| (2,500 | ) | |
| – | | |
| – | | |
| – | | |
| 2,500 | | |
| – | | |
| – | |
| Exchange of Series B Preferred Stock for common stock | |
| – | | |
| – | | |
| – | | |
| – | | |
| (595,467,205 | ) | |
| – | | |
| – | | |
| – | |
| Net loss for the quarter | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (212,030 | ) | |
| (212,030 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance - August 31, 2022 | |
| 2,500,000 | | |
$ | – | | |
| 1,000 | | |
$ | – | | |
| 8,142,531,094 | | |
$ | 3,364,105 | | |
$ | (3,862,186 | ) | |
$ | (498,082 | ) |
See accompanying notes to condensed
consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
August 31, 2023
(Unaudited)
Note 1 – Organization and Business
Organization and Operations
Cannabis Bioscience International Holdings, Inc.,
a Colorado corporation (the “Company”), was formed on February 28, 2003, as a limited liability company under the name Fidelity
Aircraft Partners LLC. On December 16, 2009, it converted to a corporation under the name Fidelity Aviation Corporation, and on August
24, 2009, it changed its name to China Infrastructure Construction Corp. On February 28, 2018, the Company changed its name to Hippocrates
Direct Healthcare, Inc.; on July 4, 2018, it resumed the name China Infrastructure Construction Corp. On December 6, 2022, it changed
its name to its present name. The Company provides educational systems focused on medical cannabis in cities throughout the United States
and six countries in Latin America. The Company provides services to third parties in therapeutic areas of clinical trials and conducts
clinical trials relating to cannabinoids for its own account. The Company has one non-operating subsidiary, Alpha Fertility and Sleep
Center, LLC, a Texas limited liability company, through which it conducted its sleep center business until April 30, 2023.
Note 2 – Summary of Significant Accounting
Policies Accounting Principles
The accompanying unaudited consolidated financial
statements have been prepared by management using the accrual basis of accounting in accordance with accounting principles generally
accepted in the United States of America (“U.S. GAAP”) for interim financial statements and with the instructions to Article
10 of Regulation S-X of the U.S. Securities and Exchange Commission (the “SEC”). Accordingly, they do not contain all of the
information and footnotes required by U.S. GAAP for annual financial statements. In the opinion of the Company’s management, the
accompanying unaudited consolidated financial statements contain all the adjustments necessary (consisting only of normal recurring
accruals) to present the financial position of the Company at August 31, 2023, and the results of operations and cash flows for the periods
presented. The results of operations for the three months ended August 31, 2023, are not necessarily indicative of the operating results
for the full fiscal year or any future period. These unaudited consolidated financial statements should be read in conjunction with
the audited financial statements and related notes thereto for the year ended May 31, 2023.
Use of Estimates
The preparation of financial statements in conformity
with U.S. GAAP requires management to make significant estimates and assumptions that affect the reported amounts of assets and liabilities
and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenue and expenses
during the reporting periods. Making estimates requires management to exercise significant judgment. Certain of these estimates could
be affected by external conditions, including those unique to the Company’s businesses, and general economic conditions. These
external conditions could have an effect on the Company’s estimates that could cause actual results to differ materially from its
estimates. Actual results could differ from those estimates. The Company reevaluates all of its accounting estimates at least quarterly
based on these conditions and records adjustments when necessary. Significant estimates relied upon in preparing these statements include
revenue recognition, accounts receivable reserves, accrued expenses, share-based compensation and the recoverability of the Company’s
net deferred tax assets and any related valuation allowance.
Principles of Consolidation
The consolidated financial statements include
the accounts of the Company and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Reclassification
Certain amounts in the prior consolidated financial statements have
been reclassified to conform to the presentation of the current period financial statements. These reclassifications had no impact on
the results of operations, changes in equity, or cash flows.
Cash and Cash Equivalents
Cash equivalents are short-term, highly liquid
investments that are readily convertible to cash with original maturities of three months or less at the date acquired. The Company had
zero investment securities that were deemed cash equivalents at August 31, 2023, and May 31, 2023, respectively.
Accounts Receivable
Included in accounts receivable on the balance
sheets are amounts primarily related to customers. The Company estimates losses on receivables based on known troubled accounts and historical
experience of losses incurred. Receivables are considered impaired and written off when it is probable that all contractual payments
due will not be collected in accordance with the terms of the related agreement. Based upon experience and the judgment of management,
there was no allowance for doubtful accounts at August 31, 2023, and May 31, 2023.
Revenue Recognition
The Company follows the Financial Accounting Standards
Board’s (“FASB”) Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers
(Topic 606), as amended. This standard requires a company to recognize revenues when it transfers goods or services to customers in
an amount that reflects the consideration that it expects to receive for them.
Under ASU No. 2014-09, the Company recognizes
revenue when a customer obtains control of promised goods or services, or when they are shipped to a customer, in an amount that reflects
the consideration that it expects to receive in exchange for them. The Company recognizes revenues following the five-step model prescribed
under ASU No. 2014-09: (a) it identifies a contract with a customer; (b) it identifies the performance obligations in the contract; (c)
it determines the transaction price; (d) it allocates the transaction price to the performance obligations in the contract; and (e) it
recognizes revenues when (or as) it satisfies its performance obligation.
The Company generates revenue from multiple streams,
namely, clinical trials, consulting fees, seminars and merchandise sales. Revenues from product sales are recognized when a customer
obtains control of the Company’s product, which occurs at a point in time or over time, typically upon shipment to the customer
or when services are fulfilled and the customer receives benefit from such services. Revenue is deferred and a liability is established
to the extent that the Company receives payments from customers in advance of goods being shipped or services being rendered.
The Company expenses incremental costs of obtaining
a contract as and when incurred if the expected amortization period of the asset in which it would have been recognized is one year or
less or the amount is immaterial.
A performance obligation is a contractual promise
to transfer a distinct product or service to a customer and is the unit of account in the new revenue standard. The contract transaction
price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied.
Each contract has a single performance obligation as the promise to transfer the individual goods or services is not separately identifiable
from other promises in the contracts and, therefore, not distinct. Revenue from contracts that satisfy the criteria for overtime recognition
is recognized as the work progresses. The majority of the Company’s revenue is derived from services provided to customers and is
executed typically over a period that is typically between 1 to 12 months, based on evaluation of when these services are rendered. Contracts
will continue to be recognized over time because of the continuous transfer of control to the customer as services are rendered to customers.
Payments made by customers in advance of services being rendered are recorded as deferred revenue.
Our significant payment terms for customer contracts
vary based on the revenue stream. Franchising business clients are required to advance a percentage of the franchise fee upon acceptance
of the contract. These advances, when received, are accounted for as contract liabilities on the consolidated balance sheet and are subsequently
recognized in revenue when they are earned. Contracts for clinical trials typically provide for progress payments based on the number
of patients seen, with final payments generally due within 30 days upon completion of work or the termination of the contract. Revenue
is recognized when all performance obligations under the terms of a contract are satisfied. The Company requires advance payments from
its consulting customers and these payments are recorded as contract liabilities on the consolidated balance sheet until service is performed
and revenue is recognized. These advance payments are not treated as financing component based on the guidance in ASC 606-10-32-196-16
and -17, whereby the timing of when services are provided are at the discretion of the customers or a substantial amount of the consideration
promised by the customer is variable and not in the control of the customer or the Company. There is no significant financing component
to any of the Company’s contracts.
Contracts for educational services require nonrefundable
payment in advance and are recorded as revenue when received. There is no significant financing component to any contracts.
Contract Modifications
Contracts for the Company’s clinical trial
business are subject to modification. These modifications may create new, or change existing, enforceable rights and obligations of the
parties thereto. Modifications are generally effected pursuant to an amendment or addendum to the original contract. A contract modification
is accounted for as a new contract if it reflects an increase in scope that is regarded as distinct from the original contract and is
priced in line with the standalone price for the related services. If a contract modification is not considered a new contract, the modification
is combined with the original contract and the impact on revenue recognition will depend on whether the remaining services are distinct
from the original contract. If they are distinct from those in the original contract, all remaining performance obligations will be accounted
for on a prospective basis, with unrecognized consideration allocated to the remaining performance obligations. If the remaining goods
or services are not distinct, the modification will be treated as if it were a part of the existing contract and the effect that the contract
modification has on the transaction price and the measure of progress toward satisfaction of the performance obligations are recognized
as an adjustment to revenue (either as an increase in or a reduction of revenue) at the date of the contract modification on a cumulative
catch-up basis.
Remaining Performance Obligations
The Company follows ASC 606, which requires the
allocation of the transaction price to the remaining performance obligations of a contract and applies a practical expedient allowing
it not to disclose the amount of the transaction price allocated to the remaining performance obligations for contracts with an original
expected duration of one year or less. At August 31, 2023, and May 31, 2023, the Company had no remaining performance obligations.
Share-Based Payments
ASC 718, “Compensation – Stock
Compensation,” prescribes accounting and reporting standards for all share-based payment transactions. In June 2018, FASB issued
ASU No. 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which
aligns accounting for share-based payments issued to non-employees to that of employees under the existing guidance of Topic 718, with
certain exceptions. This update supersedes previous guidance for share-based payments to non-employees under Subtopic 505-50, Equity
– Equity-Based Payments to Non-Employees. This guidance became effective for the Company on January 1, 2019. Based on its completed
analysis, the Company has determined that adopting this guidance will not have a material impact on its financial statements. The Company
follows FASB guidance related to equity-based payments, which requires that equity-based compensation be accounted for using a fair value
method and recognized as expense in the accompanying statements of operations. Equity-based compensation expense will be recognized as
compensation expense.
Leases
The Company has adopted ASU 2016-02, Leases
(Topic 842), along with related clarifications and improvements, under which lessees are required to recognize a lease liability,
which represents the discounted obligation to make future minimum lease payments and a corresponding right-of-use asset on the balance
sheet for most leases. The guidance retains the historical accounting for lessors and does not make significant changes to the recognition,
measurement, and presentation of expenses and cash flows by a lessee. Enhanced disclosures are also required to give financial statement
users the ability to assess the amount, timing and uncertainty of cash flows arising from leases.
Cash Flows
The Company follows ASU 2016-18, “Statement
of Cash Flows (Topic 230),” requiring that the statement of cash flows explain the change in the total cash, cash equivalents,
and amounts generally described as restricted cash or restricted cash equivalents. The provisions of this guidance are to be applied using
a retrospective approach, which requires application of the guidance for all periods presented.
Fair Value Measurements
The Company has adopted ASC Topic 820, Fair Value Measurements,
which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value and expands
disclosure of fair-value measurements.
The estimated fair value of certain financial
instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses, is carried at historical
cost basis, which approximates their fair values because of the short-term nature of these instruments. The carrying amounts of the Company’s
short- and long-term credit obligations approximate fair value because the effective yields on these obligations, which include contractual
interest rates taken together with other features, such as concurrent issuances of warrants and/or embedded conversion options, are comparable
to rates of returns for instruments of similar credit risk.
ASC Topic 820 defines fair value as the exchange
price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market
for the asset or liability in an orderly transaction between market participants on the measurement date. ASC Topic 820 also establishes
a fair-value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs
when measuring fair value. ASC Topic 820 describes three levels of inputs that may be used to measure fair value:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Quoted prices for similar assets and liabilities in active
markets or inputs that are observable. Level 3: Inputs that are unobservable (for example, cash flow modeling inputs based on assumptions).
Income Taxes
The Company accounts for income taxes in accordance
with Accounting Standards Codification No. 740, “Income Taxes” (“ASC 740”). This codification prescribes
the use of the asset and liability method whereby deferred tax asset and liability account balances are determined based on differences
between financial reporting and tax bases of assets and liabilities and for carryforward tax losses. Deferred taxes are measured using
the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance,
if necessary, to reduce deferred tax assets to their estimated realizable value if it is more likely than not that some portion or all
of the deferred tax asset will not be realized.
Deferred tax liabilities and assets are classified
as current or noncurrent based on the classification of the related asset or liability for financial reporting or according to the expected
reversal dates of the specific temporary differences, if not related to an asset or liability for financial reporting.
The Company accounts for uncertain tax positions
in accordance with the provisions of ASC 740, which provides guidance as to the determination of whether tax benefits claimed or expected
to be claimed on a tax return should be recorded in its financial statements, under which a company may recognize the tax benefit from
an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities,
based on the technical merits of the position.
The tax benefits recognized in financial statements
from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate
settlement. Accordingly, the Company would report a liability for unrecognized tax benefits resulting from uncertain tax positions taken
or expected to be taken in a tax return. The Company elects to recognize any interest and penalties, if any, related to unrecognized tax
benefits in tax expense.
Loss per Share
The Company computes basic earnings per share
amounts in accordance with Accounting Standards Codification Topic 260, “Earnings per Share.” Basic earnings per share
is calculated by dividing net income (loss) available to common stockholders by the weighted average number of common shares outstanding
during the reporting period. Diluted loss per share is computed by dividing net loss by the weighted average number of shares of common
stock, common stock equivalents and potentially dilutive securities outstanding during the period. At August 31, 2023, and August 31,
2022, the Company had no dilutive securities.
Recently Issued Accounting Standards
The Company does not believe there are any other
recently issued, but not yet effective, accounting standards that would have a significant impact on the Company’s financial position
or results of operations.
Note 3 – Going Concern
The accompanying consolidated financial statements
have been prepared in conformity with U.S. GAAP, which contemplate the Company’s continuation as a going concern in accordance with
ASC 240-40-50. The Company’s history of recurring losses, negative working capital and negative cash flows from operating activities
raises substantial doubt about its ability to continue as a going concern. The Company has not generated any profits since inception and
its current cash balances will not meet its working capital needs. During the quarter ended August 31, 2023, the Company had a net loss
from operations of $181,789, net cash used in operations of $74,620,
a working capital deficit of $482,546 and an accumulated deficit of $4,864,525.
The ability of the Company to continue as a going
concern depends on the successful execution of its operating plan, which includes expanding its operations and raising either debt or
equity financing. There is no assurance that the Company will be able to expand its operations or obtain such financing on satisfactory
terms or at all. If the Company is unsuccessful in these endeavors, it may be required to curtail or cease its operations.
The accompanying financial statements do not include any adjustments
related to the recoverability or classification of asset carrying amounts or the amounts and classification of liabilities that may result
should the Company be unable to continue as a going concern.
Note 4 – Debt PPP Loans
During the years ended May 31, 2021, and May 31,
2020, the Company received one loan of $31,750, two loans of $20,833 each and three loans of $5,000 each under the Payroll Protection
Program (the “PPP”). The PPP was established in 2020 as part of the Coronavirus Aid, Relief and Economic Security Act (the
“CARES Act”) to provide loans to qualifying businesses for amounts up to 2.5 times their average monthly payroll expenses.
At May 31, 2022, the Company’ s outstanding PPP loans of $41,666 were recorded as current liabilities; these loans were forgiven
on June 21, 2022. On May 5, 2021, pursuant to the CARES Act, the Company received forgiveness of the loan of $31,750, on December 30,
2021; it received forgiveness of a loan of $5,000; on March 16, 2021, it received forgiveness for a loan of $5,000; and on March 18, 2021,
it received forgiveness for a loan of $5,000, Each such forgiveness was recorded as other income during the year in which it received
official notice that it was forgiven.
EIDL Loans
In June 2020, the Company received proceeds of
$106,200 from the Small Business Administration through a second EIDL loan to help fund its operations during the COVID-19 pandemic.
The loan bears interest at the rate of 3.75% per annum and is payable in monthly installments of $518 over a 30-year period. An additional
$4,000 borrowed under EIDL, which was provided for payroll, was forgiven and recorded as Other Income during 2022. The Company’s
EIDL loans were recorded in the balance sheet as follows:
| | |
August 31, 2023 | | |
May 31, 2023 | |
| SBA (EIDL) current portion | |
$ | 14,592 | | |
$ | 14,592 | |
| SBA (EIDL) noncurrent portion | |
| 234,908 | | |
| 249,500 | |
| | |
$ | 249,500 | | |
$ | 264,092 | |
Short-Term Loans
The Company has entered into agreements under
which it sold receivables to third parties. In accordance with ASC 470, these transactions are treated as loans encumbering the receivables
of the Company in the event of default and are accounted for as a debt, such that payments are allocated to principal and interest expense
as they are made. These transactions are as follows:
| · |
In October 2019, the Company entered into a business line of credit with an unrelated party for a loan of $15,000,
bearing interest at the rate of 53.47% per annum, to be repaid at the rate of $1,644 per month. The outstanding balance at August 31,
2023, was $15,727. |
| |
|
| · |
In August 2022, the Company entered into a financing agreement with an unrelated party for a loan of $25,000, bearing
interest at the rate of 14% per annum, to be repaid at the rate of $259 per week. The outstanding balance at August 31, 2023, was $15,072. |
| |
|
| · |
In October 2022, the Company entered into a financing agreement with an unrelated party for a loan of $70,000, bearing
interest at the rate of 26.4% per annum, to be repaid at the rate of $1,218 per week. The outstanding balance at August 31, 2023, was
$54,331. |
| |
|
| · |
In March 2023, the Company entered into a financing agreement with the same unrelated party for a loan of $47,000,
bearing interest at the rate of 49% per annum, to be repaid at the rate of $6,000 per week. The outstanding balance at August 31, 2023,
was $41,583. |
| |
|
| · |
In January 2023, the Company entered into a financing agreement with an unrelated party for a loan of $20,000, bearing
interest at the rate of 33.5% per annum, to be repaid at the rate of $1,874 per week. The outstanding balance at August 31, 2023, was
$1,874. |
| |
|
| · |
In April 2023, the Company entered into a financing agreement with an unrelated party for a loan of $25,000, bearing
interest at the rate of 32.3% per annum, to be repaid $1,718 per week. The outstanding balance at August 31, 2023, was $26,300. |
Note 5 – Right-of-Use Assets and Lease
Liabilities
The Company leases real property from unrelated
parties under leases that are classified as operating leases. The right-of-use assets for operating leases are included in right-of-use
assets on the balance sheets, with the corresponding lease liability in liabilities. Lease expense is recognized on a straight-line basis
over the lease term. Renewals and terminations are included in the calculation of right-of-use assets and lease liabilities when they
are considered reasonably certain to be exercised. When the implicit rate is unknown, the incremental borrowing rate, based on the commencement
date, is used in determining the present value of lease payments.
The following amounts related to leases were recorded
in the balance sheets:
| | |
August 31, 2023 | | |
May 31, 2023 | |
| Right-of-use asset | |
$ | 155,387 | | |
$ | 155,387 | |
| Less: Accumulated amortization | |
| (155,387 | ) | |
| (131,467 | ) |
| Right-of-use asset, net | |
$ | – | | |
$ | 23,920 | |
| | |
| | | |
| | |
| Lease liabilities – current | |
$ | – | | |
$ | 4,435 | |
| Lease liabilities – noncurrent | |
| – | | |
| – | |
| | |
$ | – | | |
$ | 4,435 | |
The Company reimburses related parties for an
office space operating lease under a month-to-month arrangement, payable at the discretion of management. See Note 10.
The Company’s total operating lease expense
was $20,953 and $18,246 during the three months ended August 31, 2023, and August 31, 2022, respectively. See Note 10 for additional lease
information.
Note 6 -- Revenue
Most of the Company’s revenue is generated
by the performance of services to customers and recognized at a point in time based on the evaluation of when the customer obtains control
of the products. Revenue is recognized when all performance obligations under the terms of a contract are satisfied, net of certain taxes
and gain/loss resulting from changes in foreign currency. Revenue is recorded when customer acceptance is received and all performance
obligations have been satisfied. Sales of goods typically do not include multiple products and/or service elements.
The table below summarizes the Company’s disaggregated revenue
information:
| | |
Quarter Ended August 31, | |
| | |
2023 | | |
2022 | |
| Clinical trials | |
$ | 62,958 | | |
$ | 124,916 | |
| Consulting fees | |
| 8,333 | | |
| – | |
| Video courses | |
| – | | |
| – | |
| Seminar fees | |
| – | | |
| 9,915 | |
| Royalties | |
| – | | |
| – | |
| Merchandise | |
| 1,530 | | |
| 8,921 | |
| Total revenue | |
$ | 72,821 | | |
$ | 143,752 | |
Cost of revenue consists of third-party costs
associated with patient stipends, sleep study fees and audio/video fees. At August 31, 2023, and August 31, 2022, cost of revenue totaled
$8,820 and $33,170, respectively.
Note 7 – Stockholders’ Deficiciency
The Company is authorized to issue
20,010,000,000 of capital stock, of which 20,000,000,000 shares are common stock, without par value, and 10,000,000 are preferred
stock, without par value, issuable in series.
Preferred Stock
The Company has designated 2,500,000 shares of
preferred stock as Series A Convertible Preferred Stock (the “Series A Stock”). Until July 20, 2022, each share had a par
value of $0.001; on that date, the Company amended its articles of incorporation to provide that each such share has no par value. Under
this amendment, (i) Series A Stock is entitled to receive dividends on the shares of Common Stock into which such shares are convertible,
(ii) has the voting power of the number of shares of Common Stock into which such shares are convertible, (iii) is redeemable at the option
of the Company for a redemption price equal to the number of shares of Common Stock into which the redeemed shares are convertible and
(iv) are senior to the Common Stock and junior to the Series B Convertible Preferred Stock described below. At August 31, 2023, and May
31, 2023, there were 2,500,000 shares of Series A Stock issued and outstanding.
On July 20, 2022, the Company designated a series
of preferred stock, named Series B Preferred Convertible Preferred Stock, comprising 1,000 shares (“Series B Preferred”).
The shares of this series have no par value, are not entitled to dividends, have no liquidation rights, are not redeemable, are not convertible,
have 60% of the Company’s voting power and rank senior to the Common Stock and Series A Convertible Preferred Stock. The 1,000 preferred
shares were issued in exchange for Common Stock to an existing common shareholder. The Company has deemed the value of the preferred and
common shares to be the same, resulting in no change to additional paid capital.
Common Stock
During the quarter
ended August 31, 2023, the Company sold 272,071,428 shares of Common Stock for $75,000, net of a
rescission.
At August 31, 2023, and May 31, 2023, there were
respectively 10,331,749,347 and 10,059,677,919 shares of Common Stock issued and outstanding.
Note 8 – Share-Based Compensation
On July 20, 2022, the Company adopted its 2022
Equity Incentive Plan, which provides for the grant of incentive and non-statutory stock options, stock appreciation rights, restricted
stock, unrestricted stock, restricted stock units and performance awards to directors, officers, employees and consultants, as determined
by the Board, as plan administrator. The Company will recognize as share-based compensation expense all share-based payments to employees
over the requisite service period (generally the vesting period) in its consolidated statements of operations based on the fair values
of the awards that are issued.
Note 9 – Income Taxes
The Company provides for income taxes under ASC
740. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recorded based on the differences between
the financial statement and tax basis of assets and liabilities and the tax rates in effect when these differences are expected to reverse.
A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax
assets through future operations.
On December 22, 2017, the 2017 Tax Cuts and Jobs
Act (the “Tax Act”) was enacted into law, making significant changes to the Code. These changes included a federal corporate
tax rate decrease from 35% to 21% for tax years beginning after December 31, 2017, the transition of U.S. international taxation from
a worldwide tax system to a territorial system and a one-time transition tax on the mandatory deemed repatriation of foreign earnings.
The Company is required to recognize the effect of the tax law changes in the period of enactment, such as re-measuring its U.S. deferred
tax assets and liabilities as well as reassessing the net realizability of its deferred tax assets and liabilities. The Tax Act did not
give rise to any material impact on the balance sheets and statements of operations due to the Company’s historical worldwide loss
position and the full valuation allowance on its net U.S. deferred tax assets.
Due to changes in ownership provisions of the
income tax laws of the United States of America, net operating loss carryforwards of approximately $4,864,525 and $2,909,738 at August
31, 2023, and August 31, 2022, respectively, for federal income tax reporting purposes are subject to annual limitations. When a change
in ownership occurs, the use of net operating loss carryforwards may be limited in future years. They generally expire 20 years from when
incurred.
Income taxes for 2017 to 2022 remain subject to
examination.
Note 10 – Commitments and Contingencies
The Company leases premises of approximately 4,500
square feet located at 6201 Bonhomme Road, Suites 460S and 466S, Houston, Texas. The lease currently provides for the base rent of $3,382
per month, increasing to (i) $3,529 per month on July 1, 2020, (ii) $3,676.04 per month on July 1, 2021, and (iii) $3,823 per month on
July 1, 2022, subject to CPI increase. On March 23, 2023, the Company amended the lease to extend its term to June 30, 2024, at a base
rent of $4,779 per month. On September 5, 2023, the lease was amended to extend its term to June 30, 2025, at rentals of $0 per month
for the two months ended August 31, 2023, $$4,779 per month for the 10 months ending June 30, 2024, and $4,926 per month for the 12 months
ending June 30, 2025. For information regarding the recording of the right-of-use asset and the lease liability in the balance sheets
in respect of this lease, see Note 5.
Two of the Company’s officers leased 1,400
square feet in Houston, Texas (the “Officers’ Leased Property”), under a lease, the term of which commenced on February
29, 2020, and expired on March 14, 2022, at a rent of $3,449 per month. These officers made a portion of these premises available to the
Company for office space on a month-to-month basis, for which the Company paid them $2,817 per month. On March 15, 2022, these officers
entered into a new lease for the same premises, which expired on September 14, 2022, at a rent of $3,008 per month, and these officers
continued to make a portion of these premises available to the Company for use as office space, for which the Company is paying them $2,817
per month on a month-to-month basis. On September 15, 2022, the officers that leased the Officers’ Leased Property entered into
a new lease for these premises, which expired on March 14, 2023, at a rent of $3,038 per month, and these officers continued to make a
portion of these premises available to the Company for use as office space, for which the Company paid them $2,817 per month. On March
2, 2023, these officers entered into a new lease for the same premises, which expires on September 14, 2023, at a rent of $3,168 per month;
they are continuing to make a portion of these premises available to the Company for use as office space, for which the Company is paying
them $2,817 per month.
Note 11 – Related Party Transactions
See Note 10 for information respecting the lease
of real property to the Company by two of its officers.
At May 31, 2023, and August 31, 2023, the Company
was indebted to related parties for cash advances made by them for use as working capital in the respective amounts of $155,541 and $105,173.
During the year ended May 31, 2023, the Company
wrote off indebtedness $12,000 owed by a former related party.
Note 12 – Off-Balance-Sheet Arrangements
The Company has no off-balance sheet arrangements.
Note 13 – Concentration of Risk
The Company had revenue, net of taxes and foreign
currency gain/loss of $72,821 and $143,752 for the three months ending August 31, 2023, and August 31, 2022, respectively.
The Company had four customers that provided 89%
of gross revenue for the quarter ended August 31, 2023, and two customers that provided 82% of gross revenue for the quarter ended August
31, 2022.
Note 14 – Subsequent Events
During the quarters ended August 31, 2023, and
August 31, 2022, the COVID-19 pandemic had a material adverse effect on the Company’s educational business because governmental
measures that we imposed to control it resulted in the closing of classrooms and other educational venues, and also hindered the Company’s
franchising and consulting activities. As the pandemic has abated, many of these restrictions have been removed and the Company is beginning
to resume normal operations. If the pandemic does not continue to abate, because of infections resulting from emerging virus variants
or for other reasons, restrictions could be reimposed or increased. The ultimate impact of the pandemic will depend on future developments,
which are highly uncertain and cannot be predicted.
On September 6, 2023, the officers who leased
the Officers’ Leased Property entered into a new lease therefor, which commenced on September 15, 2023, and will expire on September
14, 2024, at a rent of $3,164 per month and they are making a portion of these premises available to the Company for use as office space,
for which the Company is paying them $2,817 per month.
Since August 31, 2023, officers of the Company
have made advances to it of $3,132 and have been repaid $0. At November 10, 2023, the balance that the Company owed to these officers
was $112,780.
Management has evaluated all other subsequent
events when these consolidated financial statements were issued and has determined that none of them requires disclosure herein.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
For Year Ended May 31, 2023
To the Board of Directors and Stockholders
Cannabis Bioscience International Holdings, Inc. (formerly China Infrastructure
Construction Corp.)
6201 Bonhomme Rd, Ste 466S
Houston, TX 77036
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheet of Cannabis Bioscience International Holdings, Inc. (formerly China Infrastructure Construction Corp.) (the Company) as
of May 31, 2023, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for the year ended
May 31, 2023, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present
fairly, in all material respects, the financial position of the Company as of May 31, 2023, and the results of its operations and its
cash flows for the year ended May 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Substantial doubt about the Company's ability
to continue as a going concern
The accompanying financial statements have been
prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has
suffered recurring losses since inception, has a stockholders’ deficit, and the Company has not generated sufficient revenues
to date to cover its operating costs – these factors raise substantial doubt about its ability to continue as a going concern. Management's
plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result
from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required
to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations
of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding
of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
A critical audit matter is a matter arising from
the current period audit of the financial statements that was communicated or required to be communicated to the audit committee or the
Company’s governance and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved
our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our
opinion on the financial statements, taken as a whole, and we are not, by communicating a critical audit, providing separate opinions
on the critical audit matters or on the accounts or disclosures to which they relate. We determined that there are no critical audit matters
communicated or required to be communicated to the audit committee.
Other Matters
The audit of the accompanying consolidated balance
sheet of Cannabis Bioscience International Holdings, Inc. (formerly China Infrastructure Construction Corp.) as of May 31, 2022, and the
related consolidated statements of operations, stockholders’ deficit, and cash flows for the year ended May 31, 2022, and the related
notes (collectively referred to as the financial statements) was performed by another Independent Registered Public Accounting Firm, PWR
CPA, LLP.
/s/ Victor Mokuolu, CPA PLLC
We have served as the Company’s auditor
since 2023.
Houston, Texas
September 18, 2023
PCAOB ID: 6771
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
For Year Ended May 31, 2022
To the Board of Directors and
Stockholders of China Infrastructure Construction
Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of China Infrastructure Construction Corp. (the Company) as of May 31, 2022, and 2021, and the related consolidated statements
of operations, cash flows and stockholders’ equity (deficit) for each of the years in the two-year period ended May 31, 2022, and
the related notes (collectively referred to as the financial statements). In our opinion, the consolidated financial statements present
fairly, in all material respects, the financial position of the Company as of May 31, 2022, and 2021, and the results of its operations
and its cash flows for each of the two years in the two-year ended May 31, 2022, in conformity with accounting principles generally accepted
in the United States of America.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States)(PCAOB) and are required
to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations
of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding
of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 3 to the financial statements,
the Company’s recurring losses from operations, working capital deficit, negative cash flows from operating activities, and its
need for additional financing in order to fund its projected loss in 2022 raise substantial doubt about its ability to continue as a going
concern. These 2022 and 2021 financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Going Concern
The accompanying financial statements have been
prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company had
negative working capital at May 31, 2022, has incurred recurring losses and recurring negative cash flow from operating activities, and
has an accumulated deficit which raises substantial doubt about its ability to continue as a going concern. Management’s plans concerning
these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome
of this uncertainty.
Critical Audit Matters
Critical audit matters arising from the current
period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate
to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex
judgments. We determined that there were no critical audit matters.
/s/ PWR CPA, LLP
Houston, Texas
PCAOB #6686
We have served as the Company’s auditor
since 2021.
November 28, 2022
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED BALANCE SHEETS
| |
|
May 31, |
|
| |
|
2023 |
|
|
2022 |
|
| ASSETS |
| CURRENT ASSETS |
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
8,913 |
|
|
$ |
31,982 |
|
| Accounts receivable |
|
|
10,549 |
|
|
|
5,614 |
|
| Related party receivable |
|
|
– |
|
|
|
12,000 |
|
| TOTAL CURRENT ASSETS |
|
|
19,462 |
|
|
|
49,596 |
|
| Right-of-use asset |
|
|
23,920 |
|
|
|
60,298 |
|
| TOTAL ASSETS |
|
$ |
43,382 |
|
|
$ |
109,894 |
|
| |
|
|
|
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
| |
|
|
|
|
|
|
|
|
| CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
| Accounts payable and accrued expenses |
|
$ |
111,299 |
|
|
$ |
68,210 |
|
| Deferred revenue |
|
|
28,641 |
|
|
|
– |
|
| Related party payables |
|
|
105,173 |
|
|
|
15,838 |
|
| Short-term loans (net of amortization of loan fees) |
|
|
121,407 |
|
|
|
48,074 |
|
| SBA loan – current |
|
|
14,592 |
|
|
|
5,561 |
|
| PPP loan |
|
|
– |
|
|
|
41,666 |
|
| Lease liabilities – current |
|
|
4,435 |
|
|
|
44,054 |
|
| TOTAL CURRENT LIABILITIES |
|
|
385,547 |
|
|
|
223,403 |
|
| |
|
|
|
|
|
|
|
|
| LONG-TERM LIABILITIES |
|
|
|
|
|
|
|
|
| SBA loan |
|
|
249,500 |
|
|
|
243,738 |
|
| Lease liabilities |
|
|
– |
|
|
|
3,804 |
|
| TOTAL LONG-TERM LIABILITIES |
|
|
249,500 |
|
|
|
247,542 |
|
| TOTAL LIABILITIES |
|
|
635,047 |
|
|
|
470,945 |
|
| |
|
|
|
|
|
|
|
|
| STOCKHOLDERS’ DEFICIENCY |
|
|
|
|
|
|
|
|
| Authorized 10,000,000 shares of preferred stock, of which 2,500,000 shares have been designated Series A Convertible Preferred Stock and 1,000 shares have been designated Series B Preferred Stock |
|
|
– |
|
|
|
2,500 |
|
| |
|
|
|
|
|
|
|
|
| Common Stock, without par value: 20,000,000,000 shares authorized; 10,059,677,919 and 8,612,998,299 shares issued and outstanding at May 31, 2023, and May 31, 2022, respectively. |
|
|
– |
|
|
|
– |
|
| |
|
|
|
|
|
|
|
|
| Additional paid-in capital |
|
|
4,091,071 |
|
|
|
3,286,605 |
|
| Accumulated deficit |
|
|
(4,682,736 |
) |
|
|
(3,650,156 |
) |
| TOTAL STOCKHOLDERS’ DEFICIENCY |
|
|
(591,665 |
) |
|
|
(361,051 |
) |
| |
|
|
|
|
|
|
|
|
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIENCY |
|
$ |
43,382 |
|
|
$ |
109,894 |
|
The accompanying notes are an integral part of
these consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS OF
OPERATIONS
| | |
May 31, | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | 316,825 | | |
$ | 214,980 | |
| Cost of revenues | |
| 93,450 | | |
| 46,763 | |
| Gross profit | |
| 223,375 | | |
| 168,217 | |
| | |
| | | |
| | |
| Cost and expenses | |
| | | |
| | |
| General and administrative | |
| 177,110 | | |
| 134,351 | |
| Contract labor | |
| 659,651 | | |
| 544,760 | |
| Professional fees | |
| 245,691 | | |
| 222,535 | |
| Officer compensation | |
| 45,735 | | |
| 70,983 | |
| Rent and lease | |
| 71,942 | | |
| 75,226 | |
| Travel | |
| 6,617 | | |
| 8,420 | |
| Total operating expenses | |
| 1,206,746 | | |
| 1,056,275 | |
| | |
| | | |
| | |
| Operating loss | |
| (983,371 | ) | |
| (888,058 | ) |
| | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | |
| Forgiveness of debt | |
| 41,765 | | |
| 53,923 | |
| Interest expense-short and SBA loans | |
| (90,973 | ) | |
| (51,036 | ) |
| Total other income | |
| (49,208 | ) | |
| 2,887 | |
| | |
| | | |
| | |
| Net loss | |
$ | (1,032,579 | ) | |
$ | (885,171 | ) |
| | |
| | | |
| | |
| Average common stock outstanding | |
| 9,001,539,324 | | |
| 8,090,501,599 | |
| | |
| | | |
| | |
| Average earnings (loss) per share | |
$ | (0.00011 | ) | |
$ | (0.00011 | ) |
The accompanying notes are
an integral part of these consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS
OF CASH FLOWS
| | |
May 31, | |
| | |
2023 | | |
2022 | |
| OPERATING ACTIVITIES: | |
| | | |
| | |
| Net loss | |
$ | (1,032,579 | ) | |
$ | (885,171 | ) |
| Adjustments to reconcile net income: | |
| | | |
| | |
| Amortization of right-of-use-asset and liability | |
| 36,378 | | |
| 33,874 | |
| Share-based compensation | |
| 12,000 | | |
| 12,000 | |
| Forgiveness of PPP loan | |
| (41,666 | ) | |
| (46,965 | ) |
| Changes in assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| (4,935 | ) | |
| (4,319 | ) |
| Accounts payable and accrued expenses | |
| 43,089 | | |
| 51,864 | |
| Deferred revenue | |
| 28,641 | | |
| – | |
| Lease liability | |
| (43,423 | ) | |
| (37,017 | ) |
| Accrued interest on SBA Loan | |
| 21,571 | | |
| – | |
| Interest on SBA loan | |
| (6,778 | ) | |
| | |
| Advances from related party | |
| 99,015 | | |
| 5,030 | |
| Repayments of related party advances | |
| (9,680 | ) | |
| | |
| NET CASH USED IN OPERATIONS | |
| (898,367 | ) | |
| (870,704 | ) |
| | |
| | | |
| | |
| FINANCING ACTIVITIES: | |
| | | |
| | |
| Proceeds from sales of common stock | |
| 801,966 | | |
| 813,290 | |
| Proceeds of short-term loans | |
| 73,332 | | |
| 48,074 | |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | |
| 875,298 | | |
| 861,364 | |
| | |
| | | |
| | |
| NET DECREASE IN CASH | |
| (23,069 | ) | |
| (9,340 | ) |
| | |
| | | |
| | |
| CASH AT BEGINNING OF PERIOD | |
| 31,982 | | |
| 41,322 | |
| | |
| | | |
| | |
| CASH AT END OF PERIOD | |
$ | 8,914 | | |
$ | 31,982 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information | |
| | | |
| | |
| Cash paid for interest | |
$ | 93,472 | | |
$ | 3,104 | |
| Cash paid for taxes | |
$ | – | | |
$ | – | |
The accompanying notes are
an integral part of these consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
CONSOLIDATED STATEMENTS OF
STOCKHOLDERS’ DEFICIT
| | |
Series A Convertible Preferred Stock | | |
Series B Preferred Convertible Stock | | |
Common Stock | | |
Additional Paid-In | | |
Accumulated | | |
| |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Capital | | |
Deficit | | |
Total | |
| Balance - May 31, 2021 | |
| 2,500,000 | | |
$ | 2,500 | | |
| | | |
$ | – | | |
| 7,814,238,100 | | |
$ | 2,461,315 | | |
$ | (2,764,985 | ) | |
$ | (301,170 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 798,760,199 | | |
| 825,290 | | |
| – | | |
| 825,290 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (885,172 | ) | |
| (885,172 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance - May 31, 2022 | |
| 2,500,000 | | |
$ | 2,500 | | |
| – | | |
$ | – | | |
| 8,612,998,299 | | |
$ | 3,286,605 | | |
$ | (3,650,157 | ) | |
$ | (361,052 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance - May 31, 2022 | |
| 2,500,000 | | |
$ | 2,500 | | |
| – | | |
$ | – | | |
| 8,612,998,299 | | |
$ | 3,286,605 | | |
$ | (3,650,157 | ) | |
$ | (361,052 | ) |
| Sales of common stock for cash | |
| – | | |
| – | | |
| – | | |
| – | | |
| 2,042,146,825 | | |
| 801,966 | | |
| – | | |
| 801,966 | |
| Change in par value of common stock | |
| – | | |
| (2,500 | ) | |
| – | | |
| – | | |
| – | | |
| 2,500 | | |
| – | | |
| – | |
| Exchange of Series B Preferred Stock for common stock | |
| – | | |
| – | | |
| 1,000 | | |
| – | | |
| (595,467,205 | ) | |
| – | | |
| – | | |
| – | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (1,032,579 | ) | |
| (1,032,579 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance - May 31, 2023 | |
| 2,500,000 | | |
$ | – | | |
| 1,000 | | |
$ | – | | |
| 10,059,677,919 | | |
$ | 4,091,071 | | |
$ | (4,682,736 | ) | |
$ | (591,665 | ) |
The accompanying notes are
an integral part of these consolidated financial statements.
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS,
INC.
(formerly named China Infrastructure Construction
Corp.)
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
May 31, 2023
Note 1 – Organization and Business
Organization and Operations
Cannabis Bioscience International Holdings, Inc.,
a Colorado corporation (the “Company”), was formed on February 28, 2003, as a limited liability company under the name Fidelity
Aircraft Partners LLC. On December 16, 2009, it converted to a corporation under the name Fidelity Aviation Corporation, and on August
24, 2009, it changed its name to China Infrastructure Construction Corp. On February 28, 2018, the Company changed its name to Hippocrates
Direct Healthcare, Inc.; on July 4, 2018, it resumed the name China Infrastructure Construction Corp. On December 6, 2022, it changed
its name to its present name. The Company provides educational systems focused on medical cannabis in cities throughout the United States
and six countries in Latin America. The Company offered concierge medicine at an affordable price through a membership-based model through
its wholly owned subsidiary, Hippocrates Direct Healthcare, LLC, a Texas limited liability company, formed on September 11, 2017; this
business was discontinued during the quarter ended August 31, 2020. The Company has one subsidiary, Alpha Fertility and Sleep Center,
LLC, a Texas limited liability company, through which it conducted its Sleep Center and which ceased operations in April 2023.
Note 2 – Summary of Significant Accounting
Policies
Accounting Principles
These consolidated financial statements and notes
thereto have been prepared by management using the accrual basis of accounting in accordance with accounting principles generally accepted
in the United States of America (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in conformity
with U.S. GAAP requires management to make significant estimates and assumptions that affect the reported amounts of assets and liabilities
and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenue and expenses
during the reporting periods. Making estimates requires management to exercise significant judgment. Certain of these estimates could
be affected by external conditions, including those unique to the Company’s businesses, and general economic conditions. These external
conditions could have an effect on the Company’s estimates that could cause actual results to differ materially from its estimates.
Actual results could differ from those estimates. The Company re-evaluates all of its accounting estimates at least quarterly based on
these conditions and records adjustments when necessary. Significant estimates relied upon in preparing these statements include revenue
recognition, accounts receivable reserves, accrued expenses, share-based compensation and the recoverability of the Company’s net
deferred tax assets and any related valuation allowance.
Principles of Consolidation
The consolidated financial statements include
the accounts of the Company and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Reclassification
Certain amounts in the prior consolidated financial
statements have been reclassified to conform to the presentation of the current period financial statements. These reclassifications had
no impact on the results of operations, changes in equity, or cash flows.
Cash and Cash Equivalents
Cash equivalents are short-term, highly liquid
investments that are readily convertible to cash with original maturities of three months or less at the date acquired. The Company had
zero investment securities that were deemed cash equivalents at May 31, 2023, and May 31, 2022, respectively.
Accounts Receivable
Included in accounts receivable on the balance
sheets are amounts primarily related to customers. The Company estimates losses on receivables based on known troubled accounts and historical
experience of losses incurred. Receivables are considered impaired and written off when it is probable that all contractual payments due
will not be collected in accordance with the terms of the related agreement. Based on experience and the judgment of management, there
was no allowance for doubtful accounts at May 31, 2023, and May 31, 2022.
Revenue Recognition
The Company follows the Financial Accounting Standards
Board’s (“FASB”) Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers
(Topic 606), as amended. This standard requires a company to recognize revenues when it transfers goods or services to customers in
an amount that reflects the consideration that it expects to receive for them.
Under ASU No. 2014-09, the Company recognizes
revenue when a customer obtains control of promised goods or services, or when they are shipped to a customer, in an amount that reflects
the consideration that it expects to receive in exchange for them. The Company recognizes revenues following the five-step model prescribed
under ASU No. 2014-09: (a) it identifies a contract with a customer; (b) it identifies the performance obligations in the contract;
(c) it determines the transaction price; (d) it allocates the transaction price to the performance obligations in the contract; and (e)
it recognizes revenues when (or as) it satisfies its performance obligation.
The Company generates revenue from multiple streams,
namely, clinical trials, consulting fees, seminars and merchandise sales. Revenues from product sales are recognized when a customer obtains
control of the Company’s product, which occurs at a point in time or over time, typically upon shipment to the customer or when
services are fulfilled and the customer receives benefit from such services. Revenue is deferred and a liability is established to the
extent that the Company receives payments from customers in advance of goods being shipped or services being rendered.
The Company expenses incremental costs of obtaining
a contract as and when incurred if the expected amortization period of the asset in which it would have been recognized is one year or
less or the amount is immaterial.
A performance obligation is a contractual promise
to transfer a distinct product or service to a customer and is the unit of account in the new revenue standard. The contract transaction
price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied.
Each contract has a single performance obligation as the promise to transfer the individual goods or services is not separately identifiable
from other promises in the contracts and, therefore, not distinct. Revenue from contracts that satisfy the criteria for overtime recognition
is recognized as the work progresses. The majority of the Company’s revenue is derived from services provided to customers and is
executed typically over a period that is typically between 1 to 12 months, based on evaluation of when these services are rendered. Contracts
will continue to be recognized over time because of the continuous transfer of control to the customer as services are rendered to customers.
Payments made by customers in advance of services being rendered are recorded as deferred revenue.
Our significant payment terms for customer contracts
vary based on the revenue stream. Franchising business clients are required to advance a percentage of the franchise fee upon acceptance
of the contract. These advances, when received, are accounted for as contract liabilities on the consolidated balance sheet and are subsequently
recognized in revenue when they are earned. Contracts for clinical trials typically provide for progress payments based on the number
of patients seen, with final payments generally due within 30 days upon completion of work or the termination of the contract. Revenue
is recognized when all performance obligations under the terms of a contract are satisfied. The Company requires advance payments from
its consulting customers and these payments are recorded as contract liabilities on the consolidated balance sheet until service is performed
and revenue is recognized. These advance payments are not treated as financing component based on the guidance in ASC 606-10-32-196-16
and -17, whereby the timing of when services are provided are at the discretion of the customers or a substantial amount of the consideration
promised by the customer is variable and not in the control of the customer or the Company. There is no significant financing component
to any of the Company’s contracts.
Contracts for educational services require nonrefundable
payment in advance and are recorded as revenue when received.
There is no significant financing component to
any contracts.
Contract Modifications
Contracts for the Company’s clinical trial
business are subject to modification. These modifications may create new, or change existing, enforceable rights and obligations of the
parties thereto. Modifications are generally effected pursuant to an amendment or addendum to the original contract. A contract modification
is accounted for as a new contract if it reflects an increase in scope that is regarded as distinct from the original contract and is
priced in line with the standalone price for the related services. If a contract modification is not considered a new contract, the modification
is combined with the original contract and the impact on revenue recognition will depend on whether the remaining services are distinct
from the original contract. If they are distinct from those in the original contract, all remaining performance obligations will be accounted
for on a prospective basis, with unrecognized consideration allocated to the remaining performance obligations. If the remaining goods
or services are not distinct, the modification will be treated as if it were a part of the existing contract and the effect that the contract
modification has on the transaction price and the measure of progress toward satisfaction of the performance obligations are recognized
as an adjustment to revenue (either as an increase in or a reduction of revenue) at the date of the contract modification on a cumulative
catch-up basis.
Remaining Performance Obligations
The Company follows ASC 606, which requires the
allocation of the transaction price to the remaining performance obligations of a contract and applies a practical expedient allowing
it not to disclose the amount of the transaction price allocated to the remaining performance obligations for contracts with an original
expected duration of one year or less. As of May 31, 2023, and May 31, 2022, the Company had no remaining performance obligations.
Share-Based Payments
ASC 718, “Compensation – Stock
Compensation,” prescribes accounting and reporting standards for all share-based payment transactions. In June 2018, FASB issued
ASU No. 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which
aligns accounting for share-based payments issued to non-employees to that of employees under the existing guidance of Topic 718, with
certain exceptions. This update supersedes previous guidance for share-based payments to non-employees under Subtopic 505-50, Equity
– Equity-Based Payments to Non-Employees. This guidance became effective for the Company on January 1, 2019. Based on its completed
analysis, the Company has determined that adopting this guidance will not have a material impact on its financial statements. The Company
follows FASB guidance related to equity-based payments, which requires that equity-based compensation be accounted for using a fair value
method and recognized as expense in the accompanying statements of operations. Equity-based compensation expense will be recognized as
compensation expense.
Leases
The Company has adopted ASU 2016-02, Leases
(Topic 842), along with related clarifications and improvements, under which lessees are required to recognize a lease liability,
which represents the discounted obligation to make future minimum lease payments and a corresponding right-of-use asset on the balance
sheet for most leases. The guidance retains the historical accounting for lessors and does not make significant changes to the recognition,
measurement, and presentation of expenses and cash flows by a lessee. Enhanced disclosures are also required to give financial statement
users the ability to assess the amount, timing and uncertainty of cash flows arising from leases.
Cash Flows
The Company follows ASU 2016-18, “Statement
of Cash Flows (Topic 230),” requiring that the statement of cash flows explain the change in the total cash, cash equivalents,
and amounts generally described as restricted cash or restricted cash equivalents. The provisions of this guidance are to be applied using
a retrospective approach, which requires application of the guidance for all periods presented.
Fair Value Measurements
The Company has adopted ASC Topic 820, Fair
Value Measurements, which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring
fair value and expands disclosure of fair-value measurements.
The estimated fair value of certain financial
instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses, is carried at historical
cost basis, which approximates their fair values because of the short-term nature of these instruments. The carrying amounts of the Company’s
short- and long-term credit obligations approximate fair value because the effective yields on these obligations, which include contractual
interest rates taken together with other features, such as concurrent issuances of warrants and/or embedded conversion options, are comparable
to rates of returns for instruments of similar credit risk.
ASC Topic 820 defines fair value as the exchange
price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market
for the asset or liability in an orderly transaction between market participants on the measurement date. ASC Topic 820 also establishes
a fair-value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs
when measuring fair value. ASC Topic 820 describes three levels of inputs that may be used to measure fair value:
Level 1: Quoted prices in active markets for identical assets
or liabilities.
Level 2: Quoted prices for similar assets and liabilities
in active markets or inputs that are observable.
Level 3: Inputs that are unobservable (for example, cash
flow modeling inputs based on assumptions).
Income Taxes
The Company accounts for income taxes in accordance
with Accounting Standards Codification No. 740, “Income Taxes” (“ASC 740”). This codification prescribes
the use of the asset and liability method whereby deferred tax asset and liability account balances are determined based on differences
between financial reporting and tax bases of assets and liabilities and for carryforward tax losses. Deferred taxes are measured using
the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance,
if necessary, to reduce deferred tax assets to their estimated realizable value if it is more likely than not that some portion or all
of the deferred tax asset will not be realized.
Deferred tax liabilities and assets are classified
as current or noncurrent based on the classification of the related asset or liability for financial reporting or according to the expected
reversal dates of the specific temporary differences, if not related to an asset or liability for financial reporting.
The Company accounts for uncertain tax positions
in accordance with the provisions of ASC 740, which provides guidance as to the determination of whether tax benefits claimed or expected
to be claimed on a tax return should be recorded in its financial statements, under which a company may recognize the tax benefit from
an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities,
based on the technical merits of the position.
The tax benefits recognized in financial statements
from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate
settlement. Accordingly, the Company would report a liability for unrecognized tax benefits resulting from uncertain tax positions taken
or expected to be taken in a tax return. The Company elects to recognize any interest and penalties, if any, related to unrecognized tax
benefits in tax expense.
Loss per Share
The Company computes basic earnings per share
amounts in accordance with Accounting Standards Codification Topic 260, “Earnings per Share.” Basic earnings per share
is calculated by dividing net income (loss) available to common stockholders by the weighted average number of common shares outstanding
during the reporting period. Diluted loss per share is computed by dividing net loss by the weighted average number of shares of common
stock, common stock equivalents and potentially dilutive securities outstanding during the period. At May 31, 2023, and May 31, 2022,
the Company had no dilutive securities.
Recently Issued Accounting Standards
The Company does not believe there are any other
recently issued, but not yet effective, accounting standards that would have a significant impact on the Company’s financial position
or results of operations.
Note 3 – Going Concern
The accompanying consolidated financial statements
have been prepared in conformity with U.S. GAAP, which contemplate the Company’s continuation as a going concern in accordance with
ASC 240-40-50. The Company’s history of recurring losses, negative working capital and negative cash flows from operating activities
raises substantial doubt about its ability to continue as a going concern. The Company has not generated any profits since inception and
its current cash balances will not meet its working capital needs. During the year ended May 31, 2023, the Company had a net loss from
operations of $1,032,579, net cash used in operations of $913,160, a working capital deficit of $366,085 and an accumulated deficit of
$4,682,736.
The ability of the Company to continue as a going
concern depends on the successful execution of its operating plan, which includes expanding its operations and raising either debt or
equity financing. There is no assurance that the Company will be able to expand its operations or obtain such financing on satisfactory
terms or at all. If the Company is unsuccessful in these endeavors, it may be required to curtail or cease its operations.
The accompanying financial statements do not include
any adjustments related to the recoverability or classification of asset carrying amounts or the amounts and classification of liabilities
that may result should the Company be unable to continue as a going concern.
Note 4 – Debt
PPP Loans
During the years ended May 31, 2021, and May 31,
2020, the Company received one loan of $31,750, two loans of $20,833 each and three loans of $5,000 each under the Payroll Protection
Program (the “ PPP” ). The PPP was established in 2020 as part of the Coronavirus Aid, Relief and Economic Security Act (the
“ CARES Act” ) to provide loans to qualifying businesses for amounts up to 2.5 times their average monthly payroll expenses.
At May 31, 2022, the Company’ s outstanding PPP loans of $41,666 were recorded as current liabilities; these loans were forgiven
on June 21, 2022. On May 5, 2021, pursuant to the CARES Act, the Company received forgiveness of the loan of $31,750, on December 30,
2021; it received forgiveness of a loan of $5,000; on March 16, 2021, it received forgiveness for a loan of $5,000; and on March 18, 2021,
it received forgiveness for a loan of $5,000, Each such forgiveness was recorded as other income during the year in which it received
official notice that it was forgiven.
EIDL Loans
In May 2020, the Company received $143,100 from
the Small Business Administration as an Economic Injury Disaster Loan (“EIDL”) to help fund its operations during the COVID-19
pandemic. The loan bears interest at the rate of 3.75% per annum and is payable in monthly installments of $698 over a 30-year period,
with deferral of payments for the first 12 months. An additional $10,000 borrowed under EIDL, which was provided for payroll, was forgiven
and recorded as Other Income during 2022.
In June 2020, the Company received proceeds of
$106,200 from the Small Business Administration through a second EIDL loan to help fund its operations during the COVID-19 pandemic. The
loan bears interest at the rate of 3.75% per annum and is payable in monthly installments of $518 over a 30-year period. An additional
$4,000 borrowed under EIDL, which was provided for payroll, was forgiven and recorded as Other Income during 2022.
The Company’s EIDL loans were recorded in
the balance sheet as follows:
| | |
May 31, | |
| | |
2023 | | |
2022 | |
| SBA (EIDL) current portion | |
$ | 14,592 | | |
$ | 5,561 | |
| SBA (EIDL) noncurrent portion | |
| 234,210 | | |
| 243,738 | |
| | |
$ | 248,802 | | |
$ | 249,299 | |
Short-Term Loans
The Company has entered into agreements under
which it sold receivables to third parties. In accordance with ASC 470, these transactions are treated as loans encumbering the receivables
of the Company in the event of default and are accounted for as a debt, such that payments are allocated to principal and interest expense
as they are made. These transactions are as follows:
| |
· |
In May 2022, the Company entered into a financing agreement with an unrelated party for a loan of $50,000 at an annual interest rate of 20.9%, to be repaid at the rate of $1,218 per week for one year. At May 31, 2023, the outstanding balance, including interest, was $55,569. |
| |
|
|
| |
· |
On August 8, 2022, the Company entered into a financing agreement with an unrelated party for a loan of $45,000 at an annual interest rate of 26.4%, to be repaid at the rate of $3,057 per week for 20 weeks. On October 17, 2022, this loan was refinanced to include an additional $10,000, such that it bears interest at an annual interest rate of 26.4% and was to be repaid at the rate of $3,057 per week for four weeks. |
| |
|
|
| |
· |
On December 20, 2022, the Company increased the loan to $76,000 and modified the financing agreement such that the loan bears interest at an annual interest rate of 26.4% and is to be repaid at the rate of $6,114 per week for 17 weeks. The outstanding balance as of May 31, 2023, including interest, was $33,873. |
On June 29, 2022, the Company borrowed $12,500
from an unrelated party at an annual interest rate of 14%. This loan is payable at the weekly rate of $589 for 24 weeks. On October 13,
2022, an additional loan of $6,304 was obtained with a weekly payment of $297 for 24 weeks. At May 31, 2023, the outstanding balance of
this loan, including interest, was $15,152.
On August 3, 2022, the Company borrowed $15,000
from an unrelated party at an annual interest rate of 42.5%, repayable at the rate of $1,188 per month for 18 months. At May 31, 2023,
the outstanding balance of this loan, including interest, was $15,141.
Related Party Advances
The Company has received advances from related
parties from time to time. All of these advances are non-interest-bearing and have no set maturity date. The Company expects to repay
these advances when funds become available. During the years ended May 31, 2023, and May 31, 2022, the Company received and repaid advances
as follows:
| |
|
|
|
|
|
|
| Balance at May 31, 2021 |
|
$ |
12,263 |
|
|
|
|
|
| Year ended May 31, 2022: |
|
|
|
|
|
|
|
|
| Amounts advanced |
|
|
58,850 |
|
|
|
|
|
| Amounts repaid: |
|
|
(55,275 |
) |
|
|
|
|
| Balance at May 31, 2022 |
|
$ |
(15,838 |
) |
|
|
|
|
| Year months ended May 31, 2023: |
|
|
|
|
|
|
|
|
| Amounts advanced |
|
|
98,470 |
|
|
|
|
|
| Amounts repaid |
|
|
(9,860 |
) |
|
|
|
|
| Balance at May 31, 2023 |
|
$ |
105,128 |
|
|
|
|
|
Note 5 – Right-of-Use Assets and Lease
Liabilities
The Company leases real property from unrelated
parties under leases that are classified as operating leases. The right-of-use assets for operating leases are included in right-of-use
assets on the balance sheets, with the corresponding lease liability in liabilities. Lease expense is recognized on a straight-line basis
over the lease term. Renewals and terminations are included in the calculation of right-of-use assets and lease liabilities when they
are considered reasonably certain to be exercised. When the implicit rate is unknown, the incremental borrowing rate, based on the commencement
date, is used in determining the present value of lease payments.
The following amounts related to leases were recorded in the balance
sheets:
| | |
May 31, | |
| | |
2023 | | |
2022 | |
| Right-of-use asset | |
$ | 155,387 | | |
$ | 63,213 | |
| Less: Accumulated amortization | |
| (131,467 | ) | |
| (2,915 | ) |
| Right-of-use asset, net | |
$ | 23,920 | | |
$ | 60,298 | |
| | |
| | | |
| | |
| Lease liabilities – current | |
$ | 4,435 | | |
$ | 44,054 | |
| Lease liabilities – noncurrent | |
| – | | |
| 3,804 | |
| | |
$ | 4,435 | | |
$ | 47,858 | |
The Company reimburses for an office space operating
lease under a month-to-month arrangement, payable at the discretion of management.
The Company’s total operating lease expense
was $71,942 and $75,226 during the years ended May 31, 2023, and May 31, 2022, respectively. See Note 10 for additional lease information.
Note 6 -- Revenue
Most of the Company’s revenue is generated
by the performance of services to customers and recognized at a point in time based on the evaluation of when the customer obtains control
of the products. Revenue is recognized when all performance obligations under the terms of a contract are satisfied, net of certain taxes
and gain/loss resulting from changes in foreign currency. Revenue is recorded when customer acceptance is received and all performance
obligations have been satisfied. Sales of goods typically do not include multiple products and/or service elements.
The table below summarizes the Company’s
disaggregated revenue information:
| | |
Year Ended May 31, | |
| | |
2023 | | |
2022 | |
| Clinical trials | |
$ | 267,220 | | |
$ | 196,637 | |
| Consulting fees | |
| 8,333 | | |
| – | |
| Video courses | |
| 2,497 | | |
| – | |
| Seminar fees | |
| 16,433 | | |
| 13,985 | |
| Royalties | |
| 42 | | |
| 1,678 | |
| Merchandise | |
| 22,300 | | |
| 2,680 | |
| Total revenue | |
$ | 316,825 | | |
$ | 214,980 | |
Cost of revenue consists of third-party costs
associated with the patient stipend, sleep study fees and audio/video fees. At May 31, 2023, and May 31, 2022, cost of revenue totaled
$93,450 and $46,763, respectively.
Note 7 – Stockholders’ Deficit
The Company is authorized to issue 20,010,000,000
of capital stock, of which 20,000,000,000 shares are common stock, without par value, and 10,000,000 are preferred stock, issuable in
series.
Preferred Stock
The Company has designated 2,500,000 shares of
preferred stock as Series A Convertible Preferred Stock (the “Series A Stock”). Until July 20, 2022, each share had a par
value of $0.001; on that date, the Company amended its articles of incorporation to provide that each such share has no par value. Under
this amendment, (i) Series A Stock is entitled to receive dividends on the shares of Common Stock into which such shares are convertible,
(ii) has the voting power of the number of shares of Common Stock into which such shares are convertible, (iii) is redeemable at the option
of the Company for a redemption price equal to the number of shares of Common Stock into which the redeemed shares are convertible and
(iv) are senior to the Common Stock and junior to the Series B Convertible Preferred Stock described below. At May 31, 2023, and May 31,
2022, there were 2,500,000 shares of Series A Stock issued and outstanding.
On July 20, 2022, the Company designated a series
of preferred stock, named Series B Preferred Convertible Preferred Stock, comprising 1,000 shares (“Series B Preferred”).
The shares of this series have no par value, are not entitled to dividends, have no liquidation rights, are not redeemable, are not convertible,
have 60% of the Company’s voting power and rank senior to the Common Stock and Series A Convertible Preferred Stock. The 1,000 preferred
shares were issued in exchange for Common Stock to an existing common shareholder. The Company has deemed the value of the preferred and
common shares to be the same, resulting in no change to additional paid capital.
Common Stock
During the year ended May 31, 2023, the
Company sold 2,042,146,825 shares of Common Stock for $801,966 and during the year ended May 31, 2022, the Company sold 798,760,199
shares of Common Stock for $825,290.
During the year ended May 31, 2023, the Company
did not issue shares of Common Stock for services rendered. During the year ended May 31, 2022, the Company issued 20,000,000 shares of
Common Stock for services rendered; these shares had a market value of $12,000 on the date of their issuance.
At May 31, 2023, and May 31, 2023, there were
respectively 10,059,677,919 and 8,612,998,299 shares of Common Stock issued and outstanding.
Note 8 – Share-Based Compensation
On July 20, 2022, the Company adopted its 2022
Equity Incentive Plan, which provides for the grant of incentive and non-statutory stock options, stock appreciation rights, restricted
stock, unrestricted stock, restricted stock units and performance awards to directors, officers, employees and consultants, as determined
by the Board, as plan administrator. The Company will recognize as share-based compensation expense all share-based payments to employees
over the requisite service period (generally the vesting period) in its consolidated statements of operations based on the fair values
of the awards that are issued.
Note 9 – Income Taxes
The Company provides for income taxes under ASC
740. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recorded based on the differences between
the financial statement and tax basis of assets and liabilities and the tax rates in effect when these differences are expected to reverse.
A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax
assets through future operations.
On December 22, 2017, the 2017 Tax Cuts and Jobs
Act (the “Tax Act”) was enacted into law, making significant changes to the Code. These changes included a federal corporate
tax rate decrease from 35% to 21% for tax years beginning after December 31, 2017, the transition of U.S. international taxation
from a worldwide tax system to a territorial system and a one-time transition tax on the mandatory deemed repatriation of foreign earnings.
The Company is required to recognize the effect of the tax law changes in the period of enactment, such as re-measuring its U.S. deferred
tax assets and liabilities as well as reassessing the net realizability of its deferred tax assets and liabilities. The Tax Act did not
give rise to any material impact on the balance sheets and statements of operations due to the Company’s historical worldwide loss
position and the full valuation allowance on its net U.S. deferred tax assets. The reconciliation of taxes at the federal and state statutory
rate to the Company’s provision for income taxes for the years ended May 31, 2023, and May 31, 2022, was as follows:
| May 31, 2023 |
| Income tax expense (benefit) at the statutory rate |
|
$ |
979,658 |
|
| Valuation allowance |
|
|
(979,658 |
) |
| Income tax expense per books |
|
$ |
– |
|
| |
|
|
|
|
| May 31, 2022 |
|
| Income tax expense (benefit) at the statutory rate |
|
$ |
611,045 |
|
| Valuation allowance |
|
|
(611,045 |
) |
| Income tax expense per books |
|
$ |
– |
|
Due to changes in ownership provisions of the
income tax laws of the United States of America, net operating loss carryforwards of approximately $4,665,040 and $2,909,738 at May 31,
2023, and May 31, 2022, respectively, for federal income tax reporting purposes are subject to annual limitations. When a change in ownership
occurs, the use of net operating loss carryforwards may be limited in future years. They generally expire 20 years from when incurred.
Income taxes for 2017 to 2022 remain subject to
examination.
Note 10 – Commitments and Contingencies
The Company leases premises of approximately 4,500
square feet located at 6201 Bonhomme Road, Suites 460S and 466S, Houston, Texas. The lease currently provides for the base rent of $3,382
per month, increasing to (i) $3,529 per month on July 1, 2020, (ii) $3,676.04 per month on July 1, 2021, and (iii) $3,823 per
month on July 1, 2022, subject to CPI increase. On March 23, 2023, the Company amended the lease to extend its term to June 30, 2024,
at a base rent of $4,779 per month. On September 5, 2023, the lease was amended to extend its term to June 30, 2025, at rentals of $0
per month for the two months ended August 31, 2023, $$4,779 per month for the 10 months ending June 30, 2024, and $4,926 per month for
the 12 months ending June 30, 2025. For information regarding the recording of the right-of-use asset and the lease liability in the balance
sheets in respect of this lease, see Note 5.
Two of the Company’s officers leased 1,400
square feet in Houston, Texas (the “Officers’ Leased Property”), under a lease, the term of which commenced on February
29, 2020, and expired on March 14, 2022, at a rent of $3,449 per month. These officers made a portion of these premises available to the
Company for office space on a month-to-month basis, for which the Company paid them $2,817 per month. On March 15, 2022, these officers
entered into a new lease for the same premises, which expired on September 14, 2022, at a rent of $3,008 per month, and these officers
continued to make a portion of these premises available to the Company for use as office space, for which the Company is paying them $2,817
per month on a month-to-month basis. On September 15, 2022, the officers that leased the Officers’ Leased Property entered into
a new lease for these premises, which expired on March 14, 2023, at a rent of $3,038 per month, and these officers continued to make a
portion of these premises available to the Company for use as office space, for which the Company paid them $2,817 per month. On March
2, 2023, these officers entered into a new lease for the same premises, which expires on September 14, 2023, at a rent of $3,168 per month;
they are continuing to make a portion of these premises available to the Company for use as office space, for which the Company is paying
them $2,817 per month.
Note 11 – Related Party Transactions
See Note 10 for information respecting the lease
of real property to the Company by two of its officers.
During the year ended May 31, 2023, the Company
received cash advances from related parties of $101,335 for use as working capital.
The balance of related party liabilities outstanding
to certain shareholders totaling $105,173 and $15,838 at May 31, 2023, and May 31, 2022, respectively.
During the year ended May 31, 2023, the Company
wrote off $12,000 owed by a former related party.
Note 12 – Off-Balance-Sheet Arrangements
The Company has no off-balance sheet arrangements.
Note 13 – Concentration of Risk
The Company had revenue, net of taxes and foreign
currency gain/loss of $314,825 and $214,980 for the years ending May 31, 2023, and May 31, 2022, respectively.
The Company had three customers that provided
84 % of gross revenue for the year ended May 31, 2023, and three customers that provided 46% of gross revenue for the year ended May 31,
2022.
Note 14 – Subsequent Events
During the years ended May 31, 2023, and May 31,
2022, the COVID-19 pandemic had a material adverse effect on the Company’s educational business because governmental measures that
we imposed to control it resulted in the closing of classrooms and other educational venues, and also hindered the Company’s franchising
and consulting activities. As the pandemic has abated, many of these restrictions have been removed and the Company is beginning to resume
normal operations. If the pandemic does not continue to abate, because of infections resulting from emerging virus variants or for other
reasons, restrictions could be reimposed or increased. The ultimate impact of the pandemic will depend on future developments, which are
highly uncertain and cannot be predicted.
On June 5, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party for $5,000.
On June 23, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party for $10,000.
On June 26, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party for $10,000.
On June 30, 2023, the Company issued 10,000,000
shares of Common Stock to an unrelated party for $5,000.
On June 30, 2023, the Company issued 26,000,000
shares of Common Stock to three employees without consideration as employee benefits. On that date, these shares had a fair market value
of $13,000.
On June 30, 2023, the Company issued 50,000,000
shares of Common Stock to an officer of the Company in consideration of his agreement to remain employed by the Company. On that date,
these shares had a fair market value of $25,000.
On July 5, 2023, the Company issued 12,500,000
shares of Common Stock to an unrelated party for $5,000.
On July 11, 2023, the Company issued 25,000,000
shares of Common Stock to an unrelated party for $10,000.
On July 17, 2023, the Company issued 61,071,428
shares of Common Stock to three employees without consideration as employee benefits. On that date, these shares had a fair market value
of $30,536.
On August 1, 2023, the Company issued 12,500,000
shares of Common Stock to an unrelated party for $5,000.
On September 6, 2023, the officers who leased
the Officers’ Leased Property entered into a new lease therefor, which commenced on September 15, 2023, and will expire on September
14, 2024, at a rent of $3,164 per month and they are making a portion of these premises available to the Company for use as office space,
for which the Company is paying them $2,817 per month.
Since May 31, 2023, related parties have advanced
$4,520 to the Company and have been repaid $0. As of the date on which these financial statements were issued, the balance that the Company
owed to these related parties was $109,943.
Management has evaluated all other subsequent
events when these consolidated financial statements were issued and has determined that none of them requires disclosure herein.
PART II — INFORMATION NOT REQUIRED IN
THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the expenses expected
to be incurred by us in connection with the issuance and distribution of the securities being registered. No portion of such expenses
will be borne by the Selling Stockholders.
| SEC Registration | |
$ | 907 | |
| Legal Fees and Expenses* | |
$ | 75,000 | |
| Accounting Fees* | |
$ | 62,000 | |
| Miscellaneous* | |
$ | 12,093 | |
| Total | |
$ | 150,000 | |
* In the above table, numbers that are marked
with an asterisk are estimated.
Item 14. Indemnification of Directors and Officers.
Under Section 7-109-102 of the Colorado Business
Corporation Act (the “CBCA”), a corporation may indemnify a person made a party to a proceeding because he is or was a director
against liability incurred in the proceeding if (a) his conduct was in good faith and (b) he reasonably believed (i) in
the case of conduct in an official capacity with the corporation, that such conduct was in the corporation’s best interests; and
(ii) in all other cases, that such conduct was at least not opposed to the corporation’s best interests and (c) in the
case of any criminal proceeding, the person had no reasonable cause to believe that his conduct was unlawful. However, a corporation
may not indemnify a director under this section (a) in connection with a proceeding by or in the right of the corporation in which
he was adjudged liable to the corporation; or (b) in connection with any other proceeding charging that he derived an improper personal
benefit, whether or not involving action in an official capacity, in which proceeding he was adjudged liable on the basis that he derived
an improper personal benefit. The termination of a proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere
or its equivalent is not, of itself, determinative that the director did not meet the requisite standard of conduct. Indemnification
permitted under this section in connection with a proceeding by or in the right of the corporation is limited to reasonable expenses
incurred in connection with the proceeding.
The CBCA further provides that, unless limited
by its articles of incorporation, a corporation shall indemnify a person who was wholly successful, on the merits or otherwise, in the
defense of any proceeding to which the person was a party because the person is or was a director or officer of the corporation, against
reasonable expenses incurred by the person in connection with the proceeding. The Registrant’s articles of incorporation contain
no such limitation. In addition, a director or officer, who is or was a party to a proceeding, may apply for indemnification to the court
conducting the proceeding or to another court of competent jurisdiction. The CBCA allows a corporation to indemnify and advance expenses
to an officer, employee, fiduciary or agent of the corporation to the same extent as a director.
Pursuant to the foregoing, the Registrant’s
amended and restated articles of incorporation require it to indemnify, to the full extent permitted by law, any person who is or was
a director or officer of the Registrant and may indemnify any other person against any claim, liability or expense arising against or
incurred by such person made a party to a proceeding because he is or was a director, officer, agent, fiduciary or employee of the Registrant
or because he is or was serving another entity as a director, officer, partner, trustee, employee, fiduciary or agent at the Company’s
request.
Under Section 7-108-402 of the CBCA, a corporation
may, in its articles of incorporation, eliminate or limit the personal liability of a director to the corporation or its shareholders
for monetary damages for breach of his fiduciary duty as a director, except that such provision may not eliminate or limit the liability
of a director to the corporation or its shareholders for monetary damages for any breach of his duty of loyalty to the corporation or
its shareholders, acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, unlawful
distributions or any transaction from which he directly or indirectly derived an improper personal benefit. No such provision may eliminate
or limit the liability of a director to the corporation or to its shareholders for monetary damages for any act or omission occurring
before the date when such provision became effective. As permitted by the CBCA, the Registrant’s amended and restated articles of
incorporation provide that the personal liability of the Company’s directors to the Company or its stockholders is limited to the
full extent permitted by the CBCA.
In addition, Section 7-108-402 provides that no director or officer
shall be personally liable for any injury to person or property arising out of a tort committed by an employee unless he was personally
involved in the situation giving rise to the litigation or unless he committed a criminal offense in connection with such situation,
without restricting other common-law protections and rights that he may have.
Section 7-109-108 of the CBCA provides that a
corporation may purchase and maintain insurance on behalf of a person who is or was a director, officer, employee, fiduciary or agent
of the corporation, or who, while a director, officer, employee, fiduciary or agent of the corporation, is or was serving at the request
of the corporation as a director, officer, partner, trustee, employee, fiduciary or agent of another entity or an employee benefit plan,
against liability asserted against or incurred by the person in that capacity or arising from the person’s status as a director,
officer, employee, fiduciary or agent, whether or not the corporation would have power to indemnify the person against the same liability
under the CBCA. The Registrant has not purchased such insurance.
Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to directors, officers or persons controlling the Registrant pursuant to the foregoing
provisions, the Registrant has been informed that in the opinion of the Securities and Exchange Commission, such indemnification is against
public policy as expressed in the Act and is therefore unenforceable.
As indicated in the Prospectus which is part of the Registration
Statement under the caption “Description of Capital Stock – Elimination of Personal Liability,” the personal liability
of the Registrant’s directors has been eliminated to the extent set forth.
Item 15. Recent sales of unregistered securities.
Within the past three years, the Company has
issued securities which were not registered under the Securities Act as follows:
| Date | |
No. of Shares | | |
Class of Securities | |
Value ($) | | |
Transaction Type | |
Exemption Claimed |
| 03/15/21 | |
| 50,000,000 | | |
Common Stock | |
| 150,000 | | |
Settlement of litigation | |
4(a)(2) of the Securities Act; foreign |
| 03/15/21 | |
| 7,500,000 | | |
Common Stock | |
| 22,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/26/21 | |
| 13,392,857 | | |
Common Stock | |
| 3,750 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 1,893,939 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 8,928,571 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 8,152,174 | | |
Common Stock | |
| 15,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/09/21 | |
| 10,080,645 | | |
Common Stock | |
| 25,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/21/21 | |
| 3,750,000 | | |
Common Stock | |
| 9,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/28/21 | |
| 10,714,286 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/29/21 | |
| 178,571,429 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/01/21 | |
| 6,944,444 | | |
Common Stock | |
| 15,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/08/21 | |
| 2,500,000 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/10/21 | |
| 36,764,706 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/18/21 | |
| 2,500,000 | | |
Common Stock | |
| 5,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/21/21 | |
| 12,500,000 | | |
Common Stock | |
| 2,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 05/24/21 | |
| 3,750,000 | | |
Common Stock | |
| 7,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/03/21 | |
| 8,928,857 | | |
Common Stock | |
| 9,800 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/11/21 | |
| 14,705,882 | | |
Common Stock | |
| 20,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/25/21 | |
| 6,250,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/26/21 | |
| 6,250,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 09/21/21 | |
| 10,000,000 | | |
Common Stock | |
| 40,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 11/30/21 | |
| 40,000,000 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 11/30/21 | |
| 1,893,939 | | |
Common Stock | |
| 2,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 55,555,555 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 27,777,778 | | |
Common Stock | |
| 25,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 10,000,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/04/22 | |
| 200,000,000 | | |
Common Stock | |
| 200,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/07/22 | |
| 30,000,000 | | |
Common Stock | |
| 30,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/21/22 | |
| 20,000,000 | | |
Common Stock | |
| 20,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/24/22 | |
| 10,000,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 01/31/22 | |
| 10,000,000 | | |
Common Stock | |
| 10,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/02/22 | |
| 20,000,000 | | |
Common Stock | |
| 12,000 | | |
Services | |
4(a)(2) of the Securities Act |
| 03/03/22 | |
| 94,117,647 | | |
Common Stock | |
| 84,700 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/09/22 | |
| 11,111,111 | | |
Common Stock | |
| 1,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/11/22 | |
| 55,000,000 | | |
Common Stock | |
| 38,500 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/28/22 | |
| 70,588,234 | | |
Common Stock | |
| 70,600 | | |
Cash | |
4(a)(2) of the Securities Act |
| 03/28/22 | |
| 41,025,641 | | |
Common Stock | |
| 41,025 | | |
Cash | |
4(a)(2) of the Securities Act |
| 04/01/22 | |
| 55,555,555 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 06/26/22 | |
| 125,000,000 | | |
Common Stock | |
| 50,000 | | |
Cash | |
4(a)(2) of the Securities Act |
| 08/10/22 |
|
|
1,000 |
|
|
Series B Preferred |
|
|
-- |
|
|
1 |
|
4(a)(2) of the Securities Act |
| 09/07/22 |
|
|
62,500,000 |
|
|
Common Stock |
|
|
54,200 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 09/07/22 |
|
|
16,888,889 |
|
|
Common Stock |
|
|
12,667 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 09/30/22 |
|
|
11,250,000 |
|
|
Common Stock |
|
|
-- |
|
|
Services |
|
4(a)(2) of the Securities Act |
| 10/17/22 |
|
|
625,000,000 |
|
|
Common Stock |
|
|
250,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 11/01/22 |
|
|
70,000,000 |
|
|
Common Stock |
|
|
-- |
|
|
Services Agreements |
|
4(a)(2) of the Securities Act |
| 11/01/22 |
|
|
16,000,000 |
|
|
Common Stock |
|
|
-- |
|
|
Employee benefit |
|
4(a)(2) of the Securities Act |
| 12/01/22 |
|
|
4,000,000 |
|
|
Common Stock |
|
|
2,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 12/09/22 |
|
|
20,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 12/12/22 |
|
|
22,222,222 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 01/09/23 |
|
|
100,000,000 |
|
|
Common Stock |
|
|
25,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 01/09/23 |
|
|
16,000,000 |
|
|
Common Stock |
|
|
4,800 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 01/18/23 |
|
|
300,000,000 |
|
|
Common Stock |
|
|
150,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 02/23/23 |
|
|
14,285,714 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 03/02/23 |
|
|
12,500,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 03/09/23 |
|
|
150,000,000 |
|
|
Common Stock |
|
|
50,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 03/18/23 |
|
|
62,500,000 |
|
|
Common Stock |
|
|
25,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 04/15/23 |
|
|
150,000,000 |
|
|
Common Stock |
|
|
50,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 04/25/23 |
|
|
200,000,000 |
|
|
Common Stock |
|
|
50,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 05/31/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 05/31/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/20/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/22/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/29/23 |
|
|
10,000,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 06/20/23 |
|
|
28,571,428 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 07/01/23 |
|
|
12,500,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 07/01/23 |
|
|
12,500,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 07/11/23 |
|
|
25,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 07/17/23 |
|
|
20,000,000 |
|
|
Common Stock |
|
|
10,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
| 08/01/23 |
|
|
12,500,000 |
|
|
Common Stock |
|
|
5,000 |
|
|
Cash |
|
4(a)(2) of the Securities Act |
______________
1 Issued in exchange for 595,467,205 shares of Common
Stock.
The proceeds of the securities issued for cash were used
for general corporate purposes.
Item 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Exhibits.
Exhibit
Number
| Description |
| 3.1 | Amended
and Restated Articles of Organization, filed with the Secretary of State of the State of Colorado on July
20, 2022. ** |
| 3.2 | Amendment
to the Articles of Incorporation, filed with the Secretary of State of the State of Colorado on December 6, 2022. ** |
| 3.3 | By-Laws. ** |
| 5 | Opinion of Barry J. Miller ** |
| 10.1 | 2022
Incentive Award Plan.+ ** |
| 10.2 | Lease,
dated July 1, 2016, by and between 6201 Bonhomme, L.P. as landlord and Precision Research Institute, L.L.C., as tenant
(includes amendments). ** |
| 10.3 | Apartment Lease, dated March 2, 2023, by and between SPUSG HSTN North Tower, as Lessor, and Dante Picazo and Henry Levinski, as tenants.** |
| 10.4 | U.S.
Small Business Note, dated April 16, 2021, made by Elizabeth Hernandez and assumed by the Registrant. ** |
| 10.5 | Forward
Purchase Agreement (Fixed ACH Delivery), dated May 13, 2022, by and between Kapitos LLC and the Registrant. ** |
| 10.6 | First
Electronic Bank Revolving Credit Agreement, dated December 10, 2020, by and between Registrant and First Electronic Bank.
** |
| 10.7 | Business
Line of Credit Agreement, dated October 8, 2019, by and between Headway Capital, LLC and Pharmacology University, Inc.
** |
| 10.8 | Future
Receivables Sale and Purchase Agreement, dated as of August 8, 2022, by and between Park Avenue Funding and the Registrant ** |
| 10.9 | Agreement, dated as of November 1, 2022, by and between the Registrant and Henry Levinski.+ ** |
| 10.10 | Agreement, dated as of August 1, 2019, by and between Universidad de Bogata Jorge Tadeo Lozano and the Registrant.** |
| 10.11 | Clinical
Trial Agreement, dated as of August 19, 2022, by and between Alpha Research Institute, LLC and Pharmaceutical Research
Associates, Inc. ** |
| 10.12 | Master
Research Services Agreement, dated as of June 9, 2021, by and between the Registrant and SeraTrials, LLC and amendments
thereto ** |
| 10.13 | Third
Amendment to Office Lease, by and between Precision Research Institute, LLC and 6201 Bonhomme, LP ** |
| 10.14 | Future
Receipts Sale and Purchase Agreement, dated April 20, 2023, by and between Cloudfund LLC and the Registrant. ** |
| 10.15 | Future
Receivables Sale and Purchase Agreement, dated March 30, 2023, by and between Amerifund Group LLC and the Registrant.
** |
| 10.16 | Fourth Amendment to Office Lease, dated September 5, 2023, by and between Precision Research Institute, LLC and 6201 Bonhomme, LP ** |
| 10.17 | Apartment Lease, dated September 6, 2023, by and between SPUSG HSTN North Tower, as Lessor, and Dante Picazo and Henry Levinski, as tenants ** |
| 16 | Letter Regarding Change in Independent Registered Public Accounting Firm.* |
| 21 | Subsidiaries
of the Registrant. ** |
| 23.1 | Consent of PWR CPA, LLP.* |
| 23.2 | Consent of Victor Mokuolu, CPA PLLC * |
| 23.3 | Consent of Barry J. Miller. Included in Exhibit 5. |
| 24 | Power
of Attorney. Included on the signature page of the registration statement filed on August 24, 2022. |
| 107 | Filing Fee Table (Amended). ** |
__________
* Filed herewith
** Filed previously
+ Management contract or Compensatory
Plan.
(b) Financial
Statement Schedules.
All schedules are omitted because the required
information is either not present, not present in material amounts or is presented within the consolidated financial statements included
in the Prospectus that is part of this registration statement.
Item 17. Undertakings.
The undersigned hereby undertakes:
(1) To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (i) | To include any prospectus required by Section 10(a)(3) of
the Securities Act of 1933; |
| (ii) | To reflect in the prospectus
any facts or events arising after the effective date of the registration statement (or the
most recent post-effective amendment thereof) which, individually or in the aggregate, represent
a fundamental change in the information set forth in the registration statement. Notwithstanding
the foregoing, any increase or decrease in volume of securities offered (if the total dollar
value of securities offered would not exceed that which was registered) and any deviation
from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate,
the changes in volume and price represent no more than a 20 percent change in the maximum
aggregate offering price set forth in the “Calculation of Registration Fee” table
in the effective registration statement; and |
| (iii) | To include any material information
with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement. |
(2) That,
for the purpose of determining liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a
new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed
to be the initial bona fide offering thereof.
(3) To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
(4) That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b)
as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses
filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used
after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration
statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is
part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify
any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such
document immediately prior to such date of first use.
(5) That,
for the purpose of determining liability of the Registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to
the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus or prospectus
of the undersigned Registrant relating to the offering required to be filed pursuant to Rule
424; |
| (ii) | Any free writing prospectus relating
to the offering prepared by or on behalf of the undersigned Registrant or used or referred
to by the undersigned Registrant; |
| (iii) | The portion of any other free writing
prospectus relating to the offering containing material information about the undersigned
Registrant or its securities provided by or on behalf of the undersigned Registrant;
and |
| (iv) | Any other communication that is an offer in the offering made
by the undersigned Registrant to the purchaser. |
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1933, the Registrant has duly caused this amendment to the registration statement to be signed on its behalf by the undersigned,
thereunto duly authorized, in the City of Houston, Texas.
Date: November 13, 2023
| |
CANNABIS BIOSCIENCE INTERNATIONAL HOLDINGS, INC. |
| |
|
| |
By: /s/ Dante Picazo |
| |
Dante Picazo |
| |
Chief Executive Officer |
Pursuant to the requirements of the Securities
Act, this registration statement has been signed below by the following persons in the capacities set forth opposite their names and
on the dates indicated.
| Person |
|
Title |
|
Date |
| |
|
|
|
|
| * |
|
Chief Executive Officer and Director |
|
November 13, 2023 |
| Dante Picazo |
|
(Principal Executive Officer and Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/ Henry Levinski |
|
Director |
|
November 13, 2023 |
| Henry Levinski |
|
|
|
|
| |
|
|
|
|
| * |
|
Director |
|
November 13, 2023 |
| Jose Torres |
|
|
|
|
| *By: |
/s/
Henry Levinski |
| |
Henry Levinski
Attorney-in-Fact |
Exhibit 16

November 9, 2023
Securities and Exchange Commission
Washington, DC 20549
Ladies and Gentlemen:
We have read the disclosures to be made by Cannabis
Bioscience International Holdings, Inc. in the section of Amendment No. 6 to its the Registration Statement on Form S-1 (Registration
No. 333-26703) captioned “Changes in Independent Registered Public Accounting Firm” and are in agreement with the statements
contained therein in response to Item 304(a) of Regulation S-K.
Very truly yours,
/s/ PWR CPA, LLP
PWR CPA, LLP
Houston, TX
Exhibit 23.1

To Whom It May Concern:
We hereby consent
to the use in this Registration Statement to form S-1 Amendment No. 6 of our audit opinion report dated November 28, 2022, with respect
to the audited financial statements of Cannabis Bioscience International Holdings, Inc (formerly China Infrastructure Construction Corp) included
in form S-1 for the period ended May 31, 2022. We also consent to the references to us under the headings “Experts” in
such Registration Statement.
Very truly yours,
/s/ PWR CPA, LLP
PWR CPA, LLP
Houston, TX
Exhibit 23.3
|
VICTOR MOKUOLU, CPA PLLC
Accounting | Advisory | Assurance & Audit
| Tax |
|
|
|
CONSENT OF INDEPENDENT ACCOUNTANTS
We hereby consent to the incorporation by reference
in this Registration Statement on Form S-1, Amendment No. 6 of Cannabis Bioscience International Holdings, Inc. (formerly; China Infrastructure
Construction Corp) of our report dated September 18, 2023 relating to the consolidated financial statements for the year ended May 31,
2023 listed in the accompanying index.
We also consent to the reference to us under the
caption “Experts” in the Registration Statement.
|

|
| Houston, Texas |
| November 10, 2023 |
China Infrastructure Con... (PK) (USOTC:CHNC)
Historical Stock Chart
From Mar 2024 to Apr 2024
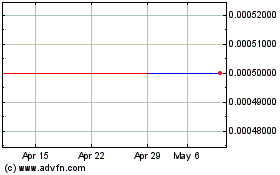
China Infrastructure Con... (PK) (USOTC:CHNC)
Historical Stock Chart
From Apr 2023 to Apr 2024