Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to
be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.
x
If this Form is filed to register additional securities for
an offering pursuant to Rule 462(b) under the Securities Act of 1933, please check the following box and list the Securities Act
registration statement number of the earlier effective registration statement for the same offering.
¨
If this Form is a post-effective amendment filed pursuant to
Rule 462(c) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number
of the earlier effective registration statement for the same offering.
¨
If this Form is a post-effective amendment filed pursuant to
Rule 462(d) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number
of the earlier effective registration statement for the same offering.
¨
Indicate by check mark whether the registrant is a large accelerated
filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated
filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
TABLE OF CONTENTS
PROSPECTUS SUMMARY
This summary
highlights selected information contained elsewhere in this Prospectus. This summary does not contain all the information that
you should consider before investing in the common stock of Axxess Pharma, Inc. (referred to herein as “we,” “our,”
“us,” “Axxess Pharma, Inc.” or the “Company”). You should carefully read the entire Prospectus,
including “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
and the accompanying financial statements and the related notes to the Financial Statements before making an investment decision.
Business Overview
Axxess Pharma, Inc., a Nevada Corporation,
has in-licensed rights to manufacture and distribute several Health Canada-approved pharmaceutical and natural health products.
The Company, through its subsidiaries Axxess Pharma Canada, Inc. and Allstar Health Brands Inc., intends to manufacture and distribute
the products described below in Canada, the United States, and other international markets:
Soropon Medicated Shampoo
Soropon Medicated Shampoo is a treatment
for both infants and adults for fungal infections of the scalp such as sebhorreic dermatitis and cradle cap in infants. There are
currently several shampoos that treat similar conditions offered in the $20-24.00 price range. The Company plans to employ an aggressive
pricing strategy in order to effectively compete with the other medicated shampoos currently offered in this market segment. The
Company expects to re-launch Soropon Medicated Shampoo into the Canadian market in early 2015.
TapouT- branded Products
In 2013 the Company acquired a World-Wide
Exclusive License (the “License”) from ABG-Authentic Brands for various TapouT branded products in return for a royalty
rate of 5%. The TapouT-branded products for which the Company received the License include: TapouT Spray Pain Relief, TapouT Pain
Relief Towelettes, TapouT Hot & Cold Reusable Packs, TapouT Instant Cold Packs, TapouT Extreme Muscle Growth Supplement and
TapouT Muscle Recovery Supplement. In addition, the Company will launch: an Omega-3 Fish oil supplement, an all-natural testosterone
boost, a line of RTD’s (Ready-to-Drink) Protein meal replacement products, and a protein powder in both a two-pound and one
pound package. The Company intends to manufacture the TapouT products in the United States and possibly in Australia where some
of its formulation work is being done, and distribute them in Canada, the United States, Australia, New Zealand, South Korea, Indonesia
and other international markets.
Transaction with Beaufort
Investment Agreement
On June 9, 2014, the Company entered into
an Investment Agreement with Beaufort. The Investor Agreement provides that the Company may, from time to time in its sole discretion
when it determines appropriate in accordance with the terms and conditions of the Investment Agreement, during the Commitment Period
(defined below), deliver an advance notice (the “Advance Notice”) to Beaufort, which states the dollar amount of securities
that the Company intends to sell to Beaufort on a date specified in the Advance Notice (the “Advance”). The Company
will be entitled to Advance to Beaufort (the “Advance Amount”) the number of shares of common stock equal to a maximum
of two hundred fifty percent (250%) of the average daily volume (U.S. market only) of our common stock for the ten (10) trading
days prior to the applicable Advance Notice. The purchase price per share to be paid by Beaufort for each Advance Amount will be
calculated at a 30% discount to the median price for the average of the ten (10) closing daily prices and the ten (10) closing
bid prices of the Company’s Common Stock immediately prior to Beaufort’s receipt of the Advance Notice. The “Commitment
Period” begins on the trading day after a registration statement is declared effective as to the common stock to be subject
to the Advance, and ends 36 months after such date, unless earlier terminated in accordance with the Investment Agreement.
The Company has the right, pursuant to
the terms of the Investment Agreement to Advance up to $2,000,000 of common stock to Beaufort. If the Company was to draw down
on the entire $2,000,000, then the Company would have to issue approximately 11,428,572 shares of common stock based upon an assumed
purchase price under the Investment Agreement of $0.175 (equal to 70% of the closing price of our common stock of $0.25 on July
23, 2014), representing 18.86% of the outstanding common stock of the Company at the time the Company advances the maximum investment
amount of $2 million of shares of common stock.
The current registration statement covers
11,428,572 shares of our Common Stock under the Investment Agreement that would raise $2,000,000 assuming our Common Stock’s
closing bid price remains unchanged from its price as of July 23, 2014. In the event our Common Stock’s price decreases,
we may receive substantially less than $2,000,000. In that case, the Company may have to prepare and file one or more additional
registration statements registering the resale of these shares if this registration statement is unable to cover the remaining
amount of shares. These subsequent registration statements may be subject to review and comment by the staff of the SEC, and will
require the consent of our independent registered public accounting firm.
In addition, Beaufort will not be obligated
to purchase shares if Beaufort’s total number of shares beneficially held at that time would exceed 4.99% of the number of
shares of the Company’s common stock as determined in accordance with Rule 13d-1 of the Securities Exchange Act of 1934,
as amended. In addition, the Company is not permitted to draw on the facility unless there is an effective Registration Statement
(as further explained below) to cover the resale of the shares.
The Investment Agreement further provides
that Beaufort and the Company are each entitled to customary indemnification from the other for any losses or liabilities they
may suffer as a result of any breach by the other of any provisions of the Investment Agreement or Registration Rights Agreement
(as defined below).
Registration Rights Agreement
In connection with the Investment Agreement,
the Company and Beaufort entered into a registration rights agreement, or the Registration Rights Agreement. Under the Registration
Rights Agreement, the Company will use its commercially reasonable efforts to file, within thirty (30) days of the date of the
Investment Agreement, a Registration Statement on Form S-1 covering the resale of the common stock subject to the Investment Agreement.
The Company has agreed to have the Registration Statement declared effective with the SEC. Beaufort has agreed to pay all legal
costs and expenses associated with the Registration Rights Agreement. Beaufort has also agreed to extend the filing deadline
to July 25, 2014.
Promissory Note
On June 9, 2014, the Company issued Beaufort
a secured promissory note (the “Note”) in the principal amount of Two Hundred Fifty Thousand Dollars ($250,000.00).
The Note is to be funded in two equal tranches of $125,000. The first tranche of $125,000 has been funded at closing. Beaufort
has 30 days to fund the second tranche. If the second tranche is not funded, then the principal of the Note shall become $125,000.
The Note matures six months from the date the Company receives the full amount of the Note (the “Maturity Date”).
As collateral for the Note, Mr. Peter Daniel
Bagi, President of the Company, has agreed to pledge 2,000,000 shares of common stock of the Company (subject to adjustment) to
Beaufort as security for the payment in full of principal and performance under the Note (“Stock Pledge Agreement”).
Depending on our cash position, we may
not be able to repay the note. While we have been able to manage our working capital needs with current credit facilities, additional
financing or commencement of revenue generating from operations will be required in order to repay the note. To date, we have not
generated any revenue, and we cannot make any assurances that we will be successful in generating any in the future. Also, we cannot
predict whether we will be able to obtain new financing, nor do we know if it will be in the form of equity or debt. We may not
be able to obtain the necessary additional capital on a timely basis, on acceptable terms, or at all. Additional investments are
being sought, but we cannot guarantee that we will be able to obtain such investments. Moreover, the amount of indebtedness may
not be reduced or relieved by the issuance of shares under the equity line agreement.
Transaction with Seaside
On May 19, 2014, the Company entered into
a Securities Purchase Agreement with Seaside 88, LP, a Florida limited partnership, or Seaside, pursuant to which the Company will
issue and sell to Seaside up to 5,000,000 shares of its common stock.
The per share purchase price shall be an
amount equal to the lower of (a) the average of the high and low trading prices (measured to two decimal places) of the common
stock for the ten (10) consecutive trading days immediately prior to a closing date, multiplied by 0.50 and (b) the average of
the high and low trading prices (measured to two decimal places) of the common stock for the trading day immediately prior to a
closing date, multiplied by 0.55. However, in no event the per share purchase price shall be less than the floor price equal to
$0.14 per share in any subsequent closing, unless both parties agree to waive it.
In addition, Seaside will not be obligated
to purchase shares if Seaside’s total number of shares beneficially held at that time would exceed 9.99% of the number of
shares of the Company’s common stock as determined in accordance with Rule 13d-3 of the Securities Exchange Act of 1934,
as amended.
Seaside is entitled to piggyback registration
rights for all the shares issued or issuable under the Securities Purchase Agreement.
The parties conducted the initial closing
on May 20, 2014 and the Company issued a total of 584,350 shares at $0.15 per share. Two subsequent closings were took place on
June 20, 2014 and July 21. The Company issued 917,300 shares at $0.15 per share and 840,520 shares at $0.1195 per share, respectively.
The Company received total proceeds of $323,609.90 from these three closings.
Acquisition
On September 13, 2013 the Company through
its wholly owned subsidiary Allstar Health Brands, enter into an assets purchase agreement with Revive Bioscience Inc. The Company
acquired assets related to the distribution of Tapout Products including DINS of TapouT pain relief products as well as trademarks,
website, remaining finished goods inventory of Tapout products as well customer lists and intellectual products associated with
the Tapout brand name. The purchase price included $52,000 cash used to pay-off outstanding accounts payable of Revive Bioscience
as of the closing date and 6,450,000 shares of common stock valued at $0.48 per share at the closing date of the transaction.
Where You Can Find Us
Our principal executive offices are located
at 3250 Bloor Street West, Suite 613, Toronto, ON, M8X 2X9. Our telephone number is (416) 410-6006. Unless the context provides
otherwise, when we refer to “we,” “our,” “us,” “Axxess Pharma, Inc.” or the “Company”
in this Prospectus, we are referring to Axxess Pharma, Inc.
THE OFFERING
|
Common stock offered by Selling Stockholder
|
|
16,573,752 shares of common stock.
|
|
|
|
|
|
Common stock outstanding before the offering
|
|
49,167,009 shares of common stock as of July 23, 2014.
|
|
|
|
|
|
Common stock outstanding after the offering
|
|
65,740,761 shares of common stock.
|
|
|
|
|
|
Use of proceeds
|
|
We will receive proceeds from the sale of securities pursuant to the Investment Agreement. The proceeds received under the Investment Agreement will be used for general corporate and working capital purposes and acquisitions or assets, businesses or operations or for other purposes that the Board of Directors, in its good faith deem to be in the best interest of the Company.
|
|
|
|
|
|
OTC Pink Trading Symbol
|
|
AXXE
|
|
|
|
|
|
Risk Factors
|
|
The common stock offered hereby involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investment. See “Risk Factors”.
|
RISK FACTORS
You should
carefully consider the risks described below together with all of the other information included in this Prospectus before making
an investment decision with regard to our securities. The statements contained in or incorporated into this Prospectus that are
not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to
differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs,
our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock
could decline, and you may lose all or part of your investment.
Risks Related to the Operations and
Business
We have a history of losses and expect
to incur additional losses in the future. We are unable to predict the extent of future losses or when we will become profitable
.
For the years ended December 31, 2012 and
2013, we experienced net losses of $606,964 and $11,221,801, respectively. We reported revenue of $2,400 and a gross
profit of $1,656 from continuing operations for the year ended December 31, 2013. Until one or more of the products under development
is successfully brought to market, we do not anticipate generating significant revenue or gross profit.
We expect to continue to incur operating
losses for the near future. Our ability in the future to achieve or sustain profitability is based on a number of factors, many
of which are beyond our control. Even if we achieve profitability in the future, we may not be able to sustain profitability in
subsequent periods.
Our financial statements indicate
conditions exist that raise substantial doubt as to whether we will continue as a going concern.
Our audited financial statements for the
year ended December 31, 2013 indicate conditions exist that raise substantial doubt as to whether we will continue as a going concern.
Our continuation as a going concern is dependent upon our ability to obtain financing to fund the continued development of products
and working capital requirements. If we cannot continue as a going concern, our stockholders may lose their entire investment.
Funding
from our Investment Agreement with Beaufort may be limited or be insufficient to fund our operations or to implement our strategy.
Under our Investment
Agreement with Beaufort, upon effectiveness of the registration statement of which this Prospectus is a part, and subject to other
conditions, we may direct Beaufort to purchase up to $2,000,000 of our shares of common stock over a 36-month period.
The current registration statement covers
11,428,572 shares of our Common Stock under the Investment Agreement that would raise $2,000,000 assuming our Common Stock’s
closing bid price remains unchanged from its price as of July 23, 2014. In the event our Common Stock’s price decreases,
we may receive substantially less than $2,000,000. In that case, the Company may have to prepare and file one or more additional
registration statements registering the resale of these shares if this registration statement is unable to cover the remaining
amount of shares. These subsequent registration statements may be subject to review and comment by the staff of the SEC, and will
require the consent of our independent registered public accounting firm.
There can be no
assurance that we will be able to receive all or any of the total commitment from Beaufort because the Investment Agreement contains
certain limitations, restrictions, requirements, conditions and other provisions that could limit our ability to cause Beaufort
to buy common stock from us. For instance, the Company is prohibited from issuing a drawdown notice if the amount requested
in such drawdown notice exceeds the maximum drawdown amount which shall be equal to 250% of average daily trading volume of the
common stock during the pricing period or the sale of shares pursuant to the drawdown notice would cause the Company to sell or
Beaufort to purchase an aggregate number of shares of the Company’s common stock which would result in beneficial ownership
by Beaufort of more than 4.99% of the Company’s common stock (as calculated pursuant to Section 13(d) of the Securities Exchange
Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder). Also, as discussed above, there
must be an effective registration statement covering the resale of any shares to be issued pursuant to any drawdown under the Investment
Agreement, and the registration statement of which this Prospectus is a part covers the resale of only 11,428,572 shares that may
be issuable pursuant to drawdown under the Investment Agreement. These registration statements may be subject to review and
comment by the staff of the SEC, and will require the consent of our independent registered public accounting firm. Therefore,
the timing of effectiveness of these registration statements cannot be assured.
The extent to
which we rely on Beaufort as a source of funding will depend on a number of factors, including the amount of working capital needed,
the prevailing market price of our common stock and the extent to which we are able to secure working capital from other sources.
If obtaining sufficient funding from Beaufort were to prove unavailable or prohibitively dilutive, we would need to secure another
source of funding. Even if we sell all $2,000,000 of common stock under the Investment Agreement with Beaufort, we will still need
additional capital to fully implement our current business, operating and development plans.
Compliance with changing regulations
concerning corporate governance and public disclosure may result in additional expenses.
There have been changing laws, regulations
and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act, and new regulations promulgated
by the SEC. These new or changed laws, regulations and standards are subject to varying interpretations in many cases due to their
lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory
and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by
ongoing revisions to disclosure and governance practices. As a result, our efforts to comply with evolving laws, regulations and
standards are likely to continue to result in increased general and administrative expenses and a diversion of management time
and attention from revenue-generating activities to compliance activities. Our board members and executive officers could face
an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty
attracting and retaining qualified board members and executive officers, which could harm our business. If our efforts to comply
with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies, we could
be subject to liability under applicable laws or our reputation may be harmed.
We depend on key personnel to manage
our business effectively, and, if we are unable to hire, retain or motivate qualified personnel, our ability to design, develop,
market and sell our systems could be harmed.
Our future success depends, in part, on
certain key employees, including Peter Daniel Bagi, MD, our Director and President, and on our ability to attract and retain highly
skilled personnel. The loss of the services of any of our key personnel may seriously harm our business, financial condition and
results of operations. In addition, the inability to attract or retain qualified personnel, or delays in hiring required personnel,
particularly operations, finance, accounting, sales and marketing personnel, may also seriously harm our business, financial condition
and results of operations. Our ability to attract and retain highly skilled personnel will be a critical factor in determining
whether we will be successful in the future.
We will incur the expenses of complying
with public company reporting requirements.
After we become public, we will have an
obligation to comply with the applicable reporting requirements of the Exchange Act which includes the filing with the SEC of periodic
reports, proxy statements and other documents relating to our business, financial conditions and other matters, even though compliance
with such reporting requirements is economically burdensome.
Risks Related to Our Product Development
Efforts
We anticipate future losses and will
require additional financing, and our failure to obtain additional financing when needed could force us to delay, reduce or eliminate
our product development programs or commercialization efforts.
We anticipate future losses and therefore
may be dependent on additional financing to execute our business plan. In particular, we will require additional capital to continue
to conduct the research and development and obtain regulatory clearances and approvals necessary to bring any future products to
market and to establish effective marketing and sales capabilities for existing and future products. Our operating plan may change,
and we may need additional funds sooner than anticipated to meet our operational needs and capital requirements for product development,
clinical trials and commercialization. Additional funds may not be available when we need them on terms that are acceptable to
us, or at all. If adequate funds are not available on a timely basis, we may terminate or delay the development of one or more
of our products, or delay establishment of sales and marketing capabilities or other activities necessary to commercialize our
products.
Our future product development efforts
may not yield marketable products due to results of studies or trials, failure to achieve regulatory approvals or market acceptance,
proprietary rights of others or manufacturing issues.
Development of a product candidate requires
substantial technical, financial and human resources. Our potential product candidates may appear to be promising at various stages
of development yet fail to timely reach the market for a number of reasons, including: the lack of adequate quality or sufficient
prevention benefit; our or our collaborative development partners’ failure to receive necessary regulatory approvals on a
timely basis, or at all; the existence of proprietary rights of third parties; or the inability to develop manufacturing methods
that are efficient, cost-effective and capable of meeting stringent regulatory standards.
Our industry changes rapidly as a
result of technological and product developments, which may quickly render our product candidates less desirable or even obsolete.
If we are unable or unsuccessful in supplementing our product offerings, our revenue and operating results may be materially adversely
affected.
The industry in which we operate is subject
to rapid technological change. The introduction of new technologies in the market, including the delay in the adoption of these
technologies, as well as new alternatives for the delivery of products and services will continue to have a profound effect on
competitive conditions in this market. We may not be able to develop and introduce new products, services and enhancements that
respond to technological changes on a timely basis. If our product candidates are not accepted by the market as anticipated, if
at all, our business, operating results, and financial condition may be materially and adversely affected.
If we are unable to develop and later
market our products under development in a timely manner or at all, or if competitors develop or introduce similar products that
achieve commercialization before our products enter the market, the demand for our products may decrease or the products could
become obsolete.
Our products will operate in competitive
markets, where competitors may already be well established. We expect that competitors will continue to innovate and to develop
and introduce similar products that could be competitive in both price and performance. Competitors may succeed in developing or
introducing similar products earlier than, obtaining regulatory approvals and clearances for such products before our products
are approved and cleared, or developing more effective products. In addition, competitors may have products, which may achieve
commercialization before our products enter the market.
If a competitor’s products reach
the market before our products, they may gain a competitive advantage, impair the ability of us to commercialize the products,
or render the products obsolete. There can be no assurance that developments by competitors will not render our products obsolete
or noncompetitive. Our financial performance may be negatively impacted if a competitor’s successful product innovation reaches
the market before our products or gains broader market acceptance.
We believe that our products have certain
advantages, but maintaining these advantages will require continual investment in research and development, and later in sales
and marketing. There is no guarantee that we will be successful in maintaining these advantages. Nor is there any guarantee that
we will be successful in completing development of our products in any clinical trials or in achieving sales of our products, or
that future margins on such products will be acceptable.
We may never achieve market acceptance
or significant sales of our healthcare products or systems.
Through today, substantially all of our
healthcare products were under development and had generated only nominal revenue. We may never achieve market acceptance or more
than nominal or modest sales of these products and systems.
Risks Related to Our Common Stock
We do not anticipate declaring any
cash dividends on our common stock.
Any future determination with respect to
the payment of dividends will be at the discretion of the Board of Directors and will be dependent upon our financial condition,
results of operations, capital requirements, general business conditions, terms of financing arrangements and other factors that
our Board of Directors may deem relevant.
Our shares may be defined as "penny
stock," the rules imposed on the sale of the shares may affect your ability to resell any shares you may purchase, if at all.
Shares of our common stock may be defined
as a “penny stock” under the Exchange Act, and rules of the SEC. The Exchange Act and such penny stock rules
generally impose additional sales practice and disclosure requirements on broker-dealers who sell our securities to persons other
than certain accredited investors who are, generally, institutions with assets in excess of $5,000,000 or individuals with net
worth in excess of $1,000,000 or annual income exceeding $200,000, or $300,000 jointly with spouse, or in transactions not recommended
by the broker-dealer. For transactions covered by the penny stock rules, a broker-dealer must make a suitability determination
for each purchaser and receive the purchaser's written agreement prior to the sale. In addition, the broker-dealer must make certain
mandated disclosures in penny stock transactions, including the actual sale or purchase price and actual bid and offer quotations,
the compensation to be received by the broker-dealer and certain associated persons, and deliver certain disclosures required by
the SEC. Consequently, the penny stock rules may affect the ability of broker-dealers to make a market in or trade our common stock
and may also affect your ability to resell any shares you may purchase in this offering in the public markets.
Beaufort will pay less than the then-prevailing
market price for our common stock.
The common stock to be issued to Beaufort
pursuant to the Investment Agreement will be purchased at a 30% discount from the median price for the average of the ten (10)
closing daily prices and the ten (10) closing bid prices of the Company’s Common Stock immediately prior to Beaufort’s
receipt of the Advance Notice. Beaufort has a financial incentive to sell our common stock immediately upon receiving the shares
to realize the profit equal to the difference between the discounted price and the market price. If Beaufort sells the shares,
the price of our common stock could decrease. If our stock price decreases, Beaufort may have a further incentive to sell the shares
of our common stock that it holds. These sales may have a further impact on our stock price.
Your ownership interest may be diluted
and the value of our common stock may decline by exercising the drawdown right pursuant to the Investment Agreement.
Pursuant to the Investment Agreement, when
we deem it necessary, we may raise capital through the private sale of our common stock to Beaufort at a price equal to a 30% discount
from the median price for the average of the ten (10) closing daily prices and the ten (10) closing bid prices of the Company’s
Common Stock immediately prior to Beaufort’s receipt of the Advance Notice. Because the drawdown price is lower than the
prevailing market price of our common stock, to the extent that the drawdown right is exercised, your ownership interest may be
diluted.
We are registering an aggregate of
11,428,572 shares of common stock to be issued under the Investment Agreement. The sales of such shares could depress the market
price of our common stock.
We are registering an aggregate of 11,428,572
shares of common stock under this Prospectus, issuable to Beaufort pursuant to the drawdown notice under the Investment Agreement.
Notwithstanding Beaufort’s ownership limitation, the 11,428,572 shares would represent approximately 18.86% of our shares
of common stock outstanding immediately after our exercise of the drawdown right under the Investment Agreement. The sale of these
shares into the public market by Beaufort could depress the market price of our common stock.
The current registration statement covers
11,428,572 shares of our Common Stock under the Investment Agreement that would raise $2,000,000 assuming our Common Stock’s
closing bid price remains unchanged from its price as of July 23, 2014. Due to the floating offering price, we are not able to
determine the exact number of shares that we will issue under the Investment Agreement. In the event our Common Stock’s price
decreases, we may receive substantially less than $2,000,000. In that case, the Company may have to prepare and file one or more
additional registration statements registering the resale of these shares if this registration statement is unable to cover the
remaining amount of shares. These subsequent registration statements may be subject to review and comment by the staff of the SEC,
and will require the consent of our independent registered public accounting firm.
We may not have access to the full
amount available under the
Investment Agreement.
We have not drawn down funds and have not issued shares of our
common stock under the Investment Agreement. Our ability to drawdown funds and sell shares under the Investment Agreement requires
that the registration statement, of which this Prospectus is a part, be declared effective by the SEC, and that this registration
statement continue to be effective. In addition, the registration statement of which this Prospectus is a part registers 11,428,572
shares issuable under the Investment Agreement, and our ability to sell any remaining shares issuable under the Investment Agreement
is subject to our ability to prepare and file one or more additional registration statements registering the resale of these shares.
These registration statements may be subject to review and comment by the staff of the SEC, and will require the consent of our
independent registered public accounting firm. Therefore, the timing of effectiveness of these registration statements cannot be
assured. The effectiveness of these registration statements is a condition precedent to our ability to sell the shares of common
stock to Beaufort under the Investment Agreement. Even if we are successful in causing one or more registration statements registering
the resale of some or all of the shares issuable under the Investment Agreement to be declared effective by the SEC in a timely
manner, we may not be able to sell the shares unless certain other conditions are met. For instance, we are prohibited from issuing
a drawdown notice if the amount requested in such drawdown notice exceeds the maximum drawdown amount which shall be equal to 250%
of average daily trading volume of the common stock during the pricing period or the sale of shares pursuant to the drawdown notice
would cause us to sell or Beaufort to purchase an aggregate number of shares of our common stock which would result in beneficial
ownership by Beaufort of more than 4.99% of our common stock. Accordingly, because our ability to drawdown any amounts under the
Investment Agreement is subject to a number of conditions, there is no guarantee that we will be able to drawdown any portion or
all of the proceeds of $2,000,000 under the Investment Agreement.
Certain restrictions on the extent
of drawdowns and the delivery of advance notices may have little, if any, effect on the adverse impact of our issuance of shares
in connection with the Investment Agreement, and as such, Beaufort
may sell a large number of shares, resulting in
substantial dilution to the value of shares held by existing stockholders.
Beaufort has agreed, subject to certain
exceptions listed in the Investment Agreement, to refrain from holding an amount of shares which would result in Beaufort or its
affiliates owning more than 4.99% of the then-outstanding shares of our common stock at any one time. These restrictions, however,
do not prevent Beaufort from selling shares of common stock received in connection with a drawdown, and then receiving additional
shares of common stock in connection with a subsequent drawdown. In this way, Beaufort could sell more than 4.99% of the outstanding
common stock in a relatively short time frame while never holding more than 4.99% at one time.
SPECIAL NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus contains forward looking
statements that involve risks and uncertainties, principally in the sections entitled “Description of Business,” “Risk
Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
All statements other than statements of historical fact contained in this prospectus, including statements regarding future events,
our future financial performance, business strategy, and plans and objectives of management for future operations, are forward-looking
statements. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,”
“can,” “continue,” “could,” “estimates,” “expects,” “intends,”
“may,” “plans,” “potential,” “predicts,” “should,” or “will”
or the negative of these terms or other comparable terminology. Although we do not make forward looking statements unless we believe
we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve
known and unknown risks, uncertainties and other factors, including the risks outlined under “Risk Factors” or elsewhere
in this prospectus, which may cause our or our industry’s actual results, levels of activity, performance or achievements
expressed or implied by these forward-looking statements. Moreover, we operate in a highly regulated, very competitive, and rapidly
changing environment. New risks emerge from time to time and it is not possible for us to predict all risk factors, nor can we
address the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual
results to differ materially from those contained in any forward-looking statements.
We have based these forward-looking statements
largely on our current expectations and projections about future events and financial trends that we believe may affect our financial
condition, results of operations, business strategy, short term and long term business operations, and financial needs. These forward-looking
statements are subject to certain risks and uncertainties that could cause our actual results to differ materially from those reflected
in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to,
those discussed in this prospectus, and in particular, the risks discussed below and under the heading “Risk Factors”
and those discussed in other documents we file with the Securities and Exchange Commission. The following discussion should be
read in conjunction with the consolidated financial statements and notes included herewith. We undertake no obligation to revise
or publicly release the results of any revision to these forward-looking statements. In light of these risks, uncertainties and
assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ
materially and adversely from those anticipated or implied in the forward-looking statement.
You should not place undue reliance on
any forward-looking statement, each of which applies only as of the date of this prospectus. You should be aware that the occurrence
of the events described in the section entitled “Risk Factors” and elsewhere in this prospectus could negatively affect
our business, operating results, financial condition and stock price. Except as required by law, we undertake no obligation to
update or revise publicly any of the forward-looking statements after the date of this prospectus to conform our statements to
actual results or changed expectations.
USE OF PROCEEDS
We will receive
proceeds from the sale of shares to Beaufort pursuant to the Investment Agreement. We intend to use the net proceeds received from
any such sales of shares to Beaufort under the Investment Agreement for general corporate and working capital purposes and acquisitions
or assets, businesses or operations or for other purposes that the Board of Directors, in its good faith deem to be in the best
interest of the Company.
SELLING STOCKHOLDER
We are registering for resale shares of
our common stock that are issued and outstanding held by the selling stockholder identified below. We are registering the shares
to permit the selling stockholder to resell the shares when and as it deems appropriate in the manner described in the “Plan
of Distribution.” As of July 23, 2014, there were 49,167,009 shares of common stock issued and outstanding.
The selling stockholder has never served
as our officer or director or any of its predecessors or affiliates within the last three years, nor has the selling stockholder
had a material relationship with us. The selling stockholder is neither a broker-dealer nor an affiliate of a broker-dealer. The
selling stockholder did not have any agreement or understanding, directly or indirectly, to distribute any of the shares being
registered at the time of purchase.
The selling stockholder may offer for sale
all or part of the shares from time to time. The table below assumes that the selling stockholder will sell all of the shares offered
for sale. The selling stockholder is under no obligation, however, to sell any shares pursuant to this Prospectus.
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares of
|
|
|
|
|
|
|
|
Shares of
|
|
|
Maximum
|
|
|
Common
|
|
|
|
|
|
|
|
Common Stock
|
|
|
Number of
|
|
|
Stock
|
|
|
|
|
|
|
|
Beneficially
|
|
|
Shares of
|
|
|
Beneficially
|
|
|
Percent
|
|
|
|
|
Owned prior to
|
|
|
Common Stock
|
|
|
Owned after
|
|
|
Ownership
|
|
|
Name
|
|
Offering (1)
|
|
|
to be Offered
|
|
|
Offering
|
|
|
after Offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beaufort Capital Partners LLC (2)
|
|
|
0
|
|
|
|
11,428,572
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Seaside 88, LP (3)
|
|
|
2,342,170
|
|
|
|
2,342,170
|
|
|
|
0
|
|
|
|
0
|
%
|
|
MGP Architects (4)
|
|
|
41,028
|
|
|
|
41,028
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Calvin Cameron (4)
|
|
|
115,806
|
|
|
|
115,806
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Realty Capital Management Ltd. (4)
|
|
|
22,459
|
|
|
|
22,459
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Strategic Equity Capital Holdings Inc. (4)
|
|
|
599,870
|
|
|
|
599,870
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Daniel Gooding (4)
|
|
|
46,953
|
|
|
|
46,953
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Ian Hasinoff (4)
|
|
|
115,800
|
|
|
|
115,800
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Anna Henson (4)
|
|
|
59,737
|
|
|
|
59,737
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Andrew Houlton (4)
|
|
|
45,396
|
|
|
|
45,396
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Kirarv Capital (4)
|
|
|
467,556
|
|
|
|
467,556
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Rob Klein (4)
|
|
|
56,147
|
|
|
|
56,147
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Zosia Lancaster (4)
|
|
|
11,738
|
|
|
|
11,738
|
|
|
|
0
|
|
|
|
0
|
%
|
|
E52 Financial Ltd. (4)
|
|
|
413,356
|
|
|
|
413,356
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Earl Marek (4)
|
|
|
10,357
|
|
|
|
10,357
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Justin Marek (4)
|
|
|
61,483
|
|
|
|
61,483
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Tom Mcdonnell (4)
|
|
|
309,000
|
|
|
|
309,000
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Gordon Mezzomo (4)
|
|
|
6,095
|
|
|
|
6,095
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Liam O’Neil (4)
|
|
|
33,688
|
|
|
|
33,688
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Jason Reid (4)
|
|
|
43,794
|
|
|
|
43,794
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Farrukh Sair (4)
|
|
|
10,979
|
|
|
|
10,979
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Igor Sevic (4)
|
|
|
12,429
|
|
|
|
12,429
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Paul Shook
(4)
|
|
|
10,357
|
|
|
|
10,357
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Gordon Stratford
(4)
|
|
|
10,357
|
|
|
|
10,357
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Owen Ward
(4)
|
|
|
12,500
|
|
|
|
12,500
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Geoff Dover
(4)
|
|
|
7,500
|
|
|
|
7,500
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Roman Levy
(4)
|
|
|
5,000
|
|
|
|
5,000
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Realty Capital Management Ltd.
(4)
|
|
|
125,000
|
|
|
|
125,000
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Jeff Walna
(4)
|
|
|
10,979
|
|
|
|
10,979
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Cheming Yang
(4)
|
|
|
113,571
|
|
|
|
113,571
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Chad Zinn
(4)
|
|
|
11,229
|
|
|
|
11,229
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Terrence Joyce
(4)
|
|
|
561
|
|
|
|
561
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Edward Leon
(4)
|
|
|
561
|
|
|
|
561
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Samuel Sayson
(4)
|
|
|
11,724
|
|
|
|
11,724
|
|
|
|
0
|
|
|
|
0
|
%
|
|
Total
|
|
|
5,145,180
|
|
|
|
16,573,752
|
|
|
|
0
|
|
|
|
0
|
%
|
|
|
(1)
|
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In
computing the number of shares beneficially owned by a person and the percentage ownership of that person, securities that are
currently convertible or exercisable into shares of our common stock, or convertible or exercisable into shares of our common stock
within 60 days of the date hereof are deemed outstanding. Such shares, however, are not deemed outstanding for the purposes of
computing the percentage ownership of any other person.
|
|
|
(2)
|
Includes 11,428,572 shares of our common stock that we will put to Beaufort Capital Partners LLC
pursuant to that certain investment agreement dated June 9, 2014. Leib Schaeffer is a Managing Member at Beaufort Capital Partners
LLC and has voting and dispositive power over the shares owned by Beaufort.
|
|
|
(3)
|
On May 19, 2014, the Company entered into a Securities Purchase Agreement with Seaside 88, LP,
a Florida limited partnership, or Seaside, pursuant to which the Company will issue and sell to Seaside up to 5,000,000 shares
of its common stock. The Company issued 584,350 shares at $0.15 per share on May 20, 2014, 917,300 shares at $0.15 per share on
June 20, 2014, and 840,520 shares at $0.1195 per share on July 21, respectively.
|
|
|
(4)
|
Includes 2,803,010 shares issued to the shareholders of Revive Bioscience Inc. pursuant to that
certain assets purchase agreement dated September 13, 2013.
|
MARKET FOR COMMON EQUITY AND RELATED
STOCKHOLDER MATTERS
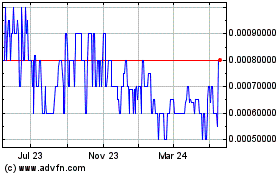
Our common stock is quoted on the OTC Pink
under the symbol “AXXE.” On July 23, 2014, the last reported bid price of our common stock was $0.25 per share. The
following table presents the high and low bid price for our common stock for the periods indicated:
|
Fiscal Year Ended December 31, 2014
|
|
High
|
|
|
Low
|
|
|
Quarter ended June 30, 2014
|
|
$
|
.27
|
|
|
$
|
.2505
|
|
|
Quarter ended March 31, 2014
|
|
$
|
.3861
|
|
|
$
|
.371
|
|
|
Fiscal Year Ended December 31, 2013
|
|
High
|
|
|
Low
|
|
|
Quarter ended December 31, 2013
|
|
$
|
.1709
|
|
|
$
|
.152
|
|
|
Quarter ended September 30, 2013
|
|
$
|
.278
|
|
|
$
|
.25
|
|
|
Quarter ended June 30, 2013
|
|
$
|
N/A
|
|
|
$
|
N/A
|
|
|
Quarter ended March 31, 2013
|
|
$
|
N/A
|
|
|
$
|
N/A
|
|
|
Fiscal Year Ended December 31, 2012
|
|
High
|
|
|
Low
|
|
|
Quarter ended December 31, 2012
|
|
$
|
N/A
|
|
|
$
|
N/A
|
|
|
Quarter ended September 30, 2012
|
|
$
|
N/A
|
|
|
$
|
N/A
|
|
|
Quarter ended June 30, 2012
|
|
$
|
N/A
|
|
|
$
|
N/A
|
|
|
Quarter ended March 31, 2012
|
|
$
|
N/A
|
|
|
$
|
N/A
|
|
Holders
As of July 23, 2014, there were approximately
657 holders of record of our common stock, which number does not reflect beneficial stockholders who hold their stock in nominee
or “street” name through various brokerage firms.
Dividend Policy
The Company has not paid any cash dividends
to date, and has no intention of paying any cash dividends on the common stock in the foreseeable future. The declaration and payment
of dividends is subject to the discretion of the Company’s Board of Directors and to certain limitations imposed under Nevada
law. The timing, amount and form of dividends, if any, will depend upon, among other things, the Company’s results of operations,
financial condition, cash requirements, and other factors deemed relevant by the Board of Directors. The Company intends to retain
any future earnings for use in its business.
DESCRIPTION OF BUSINESS
History and Corporate Structure
The Company was incorporated in the state of Delaware on April
7, 1997 as CGI Communications Services, Inc. On July 26, 2008, the Company amended its certificate of incorporation to change
its name to Axxess Pharma, Inc. On December 6, 2012, the Company reincorporated in Nevada by merging into a newly formed Nevada
entity with the same name. The Company now operates as Axxess Pharma, Inc. with Axxess Pharma Canada, Inc. and Allstar Health
Brands Inc.as its wholly owned subsidiaries.
Axxess Pharma Canada Inc. was incorporated under the Laws of
the Province of Ontario. The Company is engaged in the acquisition of Drug Identification Numbers and the eventual sale of the
related products. All Star Health Brands Inc. was incorporated on October 1, 2013 under the Laws of the Province of Ontario. The
Company is engaged in the acquisition of Drug Identification Numbers and the eventual sale of the related products.
Overview
Axxess Pharma, Inc., a Nevada Corporation,
has in-licensed rights to manufacture and distribute several Health Canada-approved pharmaceutical and natural health products.
The Company intends to manufacture and distribute the products described below in Canada, the United States, and other international
markets:
Soropon Medicated Shampoo
Soropon Medicated Shampoo is a treatment
for both infants and adults for fungal infections of the scalp such as sebhorreic dermatitis and cradle cap in infants. There
are currently several shampoos that treat similar conditions offered in the $20-24.00 price range. The Company plans to employ
an aggressive pricing strategy in order to effectively compete with the other medicated shampoos currently offered in this market
segment. The Company expects to re-launch Soropon Medicated Shampoo into the Canadian market in early 2015.
Other Health Canada-Approved Products:
The Company will assess the market for and timing of manufacture and re-launch of several or all of its pharmaceutical and natural
health products, once further financing is secured.
TapouT- branded Products
In 2013 the Company acquired a World-Wide
Exclusive License (the “License”) from ABG-Authentic Brands for various TapouT branded products in return for a royalty
rate of 5%. The TapouT-branded products for which the Company received the License include: TapouT Spray Pain Relief, TapouT Pain
Relief Towelettes, TapouT Hot & Cold Reusable Packs, TapouT Instant Cold Packs, TapouT Extreme Muscle Growth Supplement and
TapouT Muscle Recovery Supplement. In addition, the Company will launch: an Omega-3 Fish oil supplement, an all-natural testosterone
boost, a line of RTD’s (Ready-to-Drink) Protein meal replacement products, and a protein powder in both a two-pound and
one pound package. The Company intends to manufacture the TapouT products in the United States and possibly in Australia where
some of its formulation work is being done, and distribute them in Canada, the United States, Australia, New Zealand, South Korea,
Indonesia and other international markets.
Sales, Marketing and Distribution
We intend to utilize a multi-pronged sales,
marketing and distribution plan for our healthcare products. The Company has already signed several spokespersons/ ambassadors
including Brittney Palmer, Cole Whitt and Ryan Jimmo to help promote the TapouT brand. The Company intends to continue to pursue
recognizable names in sports and fitness to help promote the TapouT brand. The company also advertises in the several different
media channels, including print media and social media.
The Company has begun sales through several
online venues, including: go4itnutrition.com, amazon.com, ronniecolemannutrition.com and others. The Company has also begun retail
sales in the United States to US military personnel through the Army Air Force Exchange Service (“AAFES”) and has
recently received a second order for its products from AAFES. The Company expects retail relationship with AAFES to grow substantially
as the TapouT product line becomes more diverse and adds additional products to the AAFES sales orders.
The Company intends to expand its retail
distribution in the US through large chain retailers such as Bi-Lo, Winn Dixie, Vitamin World, and Walgreen’s. The Company
has had preliminary meetings with several of the aforementioned retail chains, and received extremely positive feedback. The Company
expects to finalize negotiations with several of these retail chains over the course of 2014.
In International markets, the Company
has a distribution agreement with Hard Core Beverages (HCB) of Australia for distribution of its products into the Australia/Asia
region, and has been working closely with HCB to begin sales. HCB has a history of aggressive marketing and the Company expects
the addition of HCB to add significantly to its revenues. Additionally, the Company plans to begin distribution of its TapouT
products in the Australia/Asia markets and believes that the popularity of the TapouT brand will help to increase sales and profitability
in the future.
Manufacturing
The Company currently has its supplements
and topical pain relief products (spray and wipes) manufactured by Private Label Nutraceuticals, of Norcross, Georgia, a fully
compliant Good Manufacturing Practices contract manufacturing facility. Private Label Nutraceuticals has a long track record of
high-quality manufacturing under FDA guidelines. In addition, Private Label Nutraceuticals will also manufacture the Omega-3 Fish
Oil and the all-natural testosterone boost products as soon as those products are ready to be placed into production.
The Company’s RTD protein meal-replacement
products planned for a late 2014 launch, have been formulated in Australia, and the Company has yet to decide where the final
commercial batches will be manufactured.
The Company’s line of Instant Cold
Pack and Reusable Hot & Cold Pack products are currently manufactured in China, and have allowances to be sold in the US and
Canada. The Company anticipates continuing the manufacture of these products in China.
Supply Arrangements
Currently the Company has supply agreements
with Private Label Nutraceuticals LLC, for all its supplements, topical pain relief and vitamis & Minerals; Goldrich Printpak
Inc. for its packaging; Quality Tape and Label Company for its labelling design and production; Great Lakes Fulfillment Services
for part of its fulfillment services; Planet Fulfillment, LLC for the remainder of the Company’s fulfillment requirements;
Environmental Regulation
As the Company does not directly manufacture
the Company relies on its suppliers policies pertaining to environmental regulation. In addition, the Company’s products
are made with all-natural ingredients.
Government Regulation
The Company’s products are regulated
under FDA Guidelines and Regulations. The ingredients used by the Company for the production of its products are classified as
GRAS (Generally Regarded As Safe). To the best of the Company’s knowledge all the new products under development conform
to the FDA guidelines for safety, and quality manufacturing.
Competitive Conditions
The market for the Company’s products
contains products in many cases similar to the Company’s own products. The Company believes its pain relief products are
unique in that they use a patented process which eliminates the need for chemical binders to deliver trans-dermally, and therefore
is a totally natural product formulated with essential oils known to provide pain relief. While the Company strives to provide
products, including its supplements and protein based products, with the latest ingredient formulations based on the needs listed
in the most current scientific literature, the Company understands other companies may be doing the same. Therefore the Company’s
strategy is to differentiate its products based on its use of all-natural, high-quality ingredients, and especially on brand recognition,
using the power of the TapouT brand as a major selling feature.
Research and Development
The Company currently works with Private
Label Nutraceuticals LLC to formulate new products, including the Omega-3 Fish Oil and all-natural Testosterone boost. In addition
the Company intends to introduce an expanded line of vitamins and minerals, the exact formulations of which will be a collaborative
research and development effort between the Company and Private Label Nutraceuticals LLC.
The Company has been working with HCB
to formulate a line a protein supplement with a unique blend of L-Carnitine, an Amino Acid believed to burn fat even at rest.
The Company anticipates that the formulation will have the major added benefit of being good-tasting, a trait which seems to be
elusive among the competition. The Company is also developing a protein powder with HCB expected to be launched in the fall of
2014.
Employees
As of July, 2014, we had four full-time employees. We consider
our relationship with our employees to be satisfactory and have not experienced any interruptions of our operations as a result
of labor disagreements. None of our employees are represented by labor unions or covered by collective bargaining agreements.
DESCIRPTION OF PROPERTY
Our corporate headquarters is located
in Toronto, Canada, where we occupy approximately 500 square feet of office space, under a lease that expires in May 2015. The
monthly lease payment is approximately $1,243. Our wholly owned subsidiary AllStar Health Brands Inc. leases an office in Aventura,
Florida, with a monthly payment of approximately $395 and the lease expires in March 2015.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis
should be read in conjunction with our financial statements and the related notes. Our discussion includes forward-looking statements
based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions.
Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a
result of a number of factors, including those set forth under the Risk Factors, Special Note Regarding Forward-Looking Statements
and Business sections in this Prospectus. We use words such as “anticipate,” “estimate,” “plan,”
“project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,”
“may,” “will,” “should,” “could,” and similar expressions to identify forward-looking
statements.
Overview
Axxess Pharma, Inc., a Nevada Corporation,
has in-licensed rights to manufacture and distribute several Health Canada-approved pharmaceutical and natural health products.
The Company, through its subsidiaries, intends to manufacture and distribute the products described below in Canada, the United
States, and other international markets:
Soropon Medicated Shampoo
Soropon Medicated Shampoo is a treatment
for both infants and adults for fungal infections of the scalp such as sebhorreic dermatitis and cradle cap in infants. There are
several shampoos meant to treat similar offered in the $20-24.00 price range. The Company plans to employ an aggressive pricing
strategy to compete effectively with the other competitors in this market segment. The Company expects to re-launch Soropon Medicated
Shampoo into the Canadian market in early 2015.
TapouT- branded Products
In 2013 the Company acquired a World-Wide
Exclusive License from ABG-Authentic Brands for various TapouT branded products in return for a royalty rate of 5%. The TapouT-branded
products include TapouT Spray Pain Relief, TapouT Pain Relief Towelettes, TapouT Hot & Cold Reusable Packs, TapouT Instant
Cold Packs, TapouT Extreme Muscle Growth Supplement and TapouT Muscle Recovery Supplement. The Company intends to manufacture the
TapouT products in the United States and distribute them in Canada, the United States, and other international markets.
Results of Operations
The following table summarizes changes
in selected operating indicators of the Company, illustrating the relationship of various income and expense items to net sales
for the respective periods presented (components may not add or subtract to totals due to rounding):
|
|
|
Three Months Ended
|
|
|
Fiscal
|
|
|
|
|
2014
|
|
|
2013
|
|
|
2013
|
|
|
2012
|
|
|
Revenue
|
|
|
$4,994
|
|
|
|
$0
|
|
|
|
2,400
|
|
|
|
--
|
|
|
Cost of Revenue
|
|
|
3,495
|
|
|
|
0
|
|
|
|
744
|
|
|
|
--
|
|
|
Gross Profit
|
|
|
1,499
|
|
|
|
0
|
|
|
|
1,656
|
|
|
|
--
|
|
|
General and Administrative Expenses
|
|
|
233,588
|
|
|
|
49,188
|
|
|
|
608
,620
|
|
|
|
11,221,801
|
|
|
Total Expenses
|
|
|
233,588
|
|
|
|
49,188
|
|
|
|
608,620
|
|
|
|
11,221,801
|
|
|
Loss from Operations
|
|
|
232,090
|
|
|
|
52,563
|
|
|
|
606,964
|
|
|
|
11,221,801
|
|
|
Other Expense
|
|
|
20,361
|
|
|
|
2,977
|
|
|
|
6,326,433
|
|
|
|
41,800
|
|
|
Net Loss
|
|
|
252,451
|
|
|
|
52,165
|
|
|
|
6,933,397
|
|
|
|
11,263,601
|
|
Three Months Ended March 31, 2014
Compared with three Months Ended March 31, 2013
Revenue:
Revenues in the first three months of 2014
were $4,944, compared with $- in the first three months of 2013. The increase in revenues in the first three months of 2014 was
primarily attributable to sales of the TapOut line of products, consisting of topical muscle relief, hot and cold therapy packs,
selling both online and to primary distributor AAFES (Army Air Force Exchange Service).
he significant components of revenues
are as follows:
|
|
1)
|
Hot and cold therapy packs
|
|
|
4)
|
All natural supplements
|
In the future management is expecting a
significant increase in revenues based on the following considerations:
|
|
1)
|
Currently a protein is in development that is all natural and is expected to be released in the
coming months, AAFES and other chain stores are waiting for this product. Stores believe this protein product will anchor the supplements
that are currently being sold, as customers will purchase both the protein and the supplements. This will result in an increase
in sales volume for all the natural supplements line. The protein powder is an all natural product that customers are currently
looking for, by releasing this product it will round out the product line for individuals that work out consistently.
|
|
|
2)
|
The supplements line is sold at a higher price than the muscle relaxant line, the higher price
point combined with larger sales volumes will result in revenue trending upwards.
|
|
|
3)
|
Currently management is waiting for regulatory approval in Australia for all their products and
it is expected within the next few months. Once approval is given, this will allow a major retailer to sell in Australia, Idonesia,
New Zealand, and South Korea.
|
|
|
4)
|
Management believes the TapOut exclusive license and the brand recognition it brings will dramatically
increase sales in the future as the products are introduced to various merchandisers.
|
Cost of Revenue:
Cost of revenues in the first three months
of 2014 was $3,495, compared with $- in the first three months of 2013. The increase in cost of sales in the first three months
of 2014 was primarily attributable to an increase in revenue. With the introduction of Allstar Health Brands, the company began
selling various products resulting in an increase in both revenue and cost of revenue.
In the future management expects cost of
revenue to trend upwards; this is directly attributed to an increase in sales. As the company sells more products, cost of revenue
per product will decrease due to utilization of economies of scale.
Gross Profit:
Gross profit in the first three months
of 2014 was $1,498, compared with $- in the first three months of 2013. The increase in gross profit in the first three months
of 2014 was primarily attributable to an increase in sales volume. With the introduction of Allstar Health Brands., the company
saw an increase in revenue and cost of revenue, which resulted in an increase in gross profit.
In the future management expects gross
profit to trend upwards for the following:
|
|
1)
|
Management is expecting revenue to increase with more products are being introduced and increased
distribution through retailers.
|
|
|
2)
|
With larger volumes of sales, the company will achieve more efficient economies of scale for cost
of revenue; this will result in an increase of gross profit.
|
SG&A Expenses:
SG&A in the first three months of 2014
was $233,588, compared with $49,188 in the first three months of 2013, reflecting an increase of 375%. The increase
in SG&A in the first three months of 2014 was primarily attributable to more general expenses required for operations.
During the current year the following expenses
have a material increase:
|
|
1)
|
Royalty Fees have increased; these expenses are related to licensing TapOut for the product. The
fee is 5% of whole sale and with an increase in whole sale the expense has increased.
|
|
|
2)
|
Professional fees have increased; expenses have increased with more legal fees related to stock
sales, fees to ensure all regulations have been followed, and accounting fees. As the company matured into operations professional
fees went up do the nature of the business requiring specific regulations to be followed and the company requiring financing.
|
|
|
3)
|
Office and general has increased; there was more expense required for printing and for shipping
items.
|
In the future management is expecting an
overall increase in all expenses, this is related to an increase business operations. With more sales and growth management is
expecting to increase all expenses to accommodate the increase in sales. Management is expecting more travel, more professional
fees, and automotive expenses specifically for the following reasons:
|
|
1)
|
Travel will increase as management is required to go to the US to meet with sponsors and distributors
in order to increase revenue.
|
|
|
2)
|
Automotive will increase as management needs to drive to various locations in order to run day
to day operations.
|
|
|
3)
|
Professional fees will increase as the company will be applying for more trademarks, requiring
more legal advice with expansion into new markets and overall growth of revenue.
|
|
|
4)
|
Management also is expecting an increase in salaries and commissions, as revenues increase and
the company grows, they will be hiring a CFO and experienced salesman.
|
Total Expenses:
Total expenses in the first three months
of 2014 were $233,588, compared with $49,188 in the first three months of 2013, reflecting an increase of 375%. The
increase in SG&A in the first three months of 2014 was primarily attributable to more general expenses required for operations.
In addition to the SG&A expenses, there
was an increase in the following:
|
|
1)
|
Advertising has increased with more expenses given for a marketing campaign in the US and overall
attempt to increase growth.
|
|
|
2)
|
Distribution costs have increased as companies helped the obtain contracts in the US for online
selling and other retail distribution.
|
In the future, management expects the following
trends:
|
|
1)
|
There will be an increase in advertising as the company will be sponsoring more athletes, advertising
on TV and in magazines, and general increase in order to increase brand awareness.
|
|
|
2)
|
The distribution costs will increase marginally in comparison to revenue, as the company has already
established channels for distribution.
|
Loss from Operations:
Loss from operations in the first three
months of 2014 was $232,090, compared with $49,188 in the first three months of 2013, reflecting an increase of 372%. The
increase in loss from operations in the first three months of 2014 was primarily attributable to more expenses required to establish
the company, while products are being introduced.
Management expects loss from operations
to decrease in the future, as there should be an upward trend in both revenue and gross profit. Management is expecting a significant
increase in sales with more products being introduced and more distribution in the marketplace.
Net loss:
We incurred a net loss of $252,451,
or 5,055% of revenues, in the first three months of 2014 compared to a net loss of $52,165 in the first three months of
2013.
With an expected increase in revenue and
gross profit, management expects net loss to trend downwards; this is consistent with the expectation in loss from operations.
Fiscal 2013 Compared With Fiscal
2012
Revenue:
Revenues in fiscal 2013 were $2,400, compared
with $- in fiscal 2012. The increase in revenues in fiscal 2013 was primarily attributable to Allstar Brands selling TapOut products.
Cost of Revenue:
Cost of revenues in fiscal 2013 were $744
compared with $- in fiscal 2012. The increase in cost of revenue in fiscal 2013 was primarily attributable to the increase
in revenue from selling and producing more products.
Gross Profit:
Gross profit in fiscal 2013 was $1,656
compared with $- in fiscal 2012. The increase was primarily attributable to increased sales. With the introduction of Allstar Health
Brands., the company saw an increase in revenue and cost of revenue, which resulted in an increase in gross profit.
SG&A Expenses:
SG&A in fiscal 2013 was $608,620, compared
with $11,221,801in fiscal 2012, reflecting a decrease of 36%. The decrease in SG&A in fiscal 2013 was primarily
attributable to a decrease in consulting expense by $10,771,641 and an increase of travel and entertainment of $30,147 and an increase
in advertising of 21,466.
Management expects this trend to continue,
with more revenue and growth expected, management expects all other general expenses to increase.
Other Income (Expense):
Other Income (Expense) increased in fiscal
2013 to (6,326,433) compared to (41,800) in 2012. The increase was primarily due to recording of Excess purchase price over fair
value of assets acquired of ($3,079,731) and extinguishment of debt o ($3,196,000) compare to $ - in fiscal 2012.
Loss from Operations:
Losses from operations in fiscal 2013 were
$606,620, compared with $11,221,801 in fiscal 2012, reflecting a decrease of 95%. The increase in loss from operations
is primarily attributed to more general and admin expenses required to get the company operating. The company did not start selling
any products until later in the year and has begun to see an upward trend in revenue.
Management expects loss from operations
to decrease in the future, as revenue is expected to increase with the introduction of new products and increased distribution.
Net loss:
We incurred a net loss of $6,933,397 in
fiscal 2013 compared to a net loss of $11,263,601, in fiscal 2012.
Liquidity and Capital Resources
As of March 31, 2014, we had cash and cash
equivalents of approximately $62,682. The following table provides a summary of our net cash flows from operating, investing, and
financing activities. We have historically financed our operations primarily through net cash flow from issuance of
capital stock sales and loans.
|
|
|
Three Months Ended
|
|
|
Fiscal
|
|
|
|
|
2014
|
|
|
2013
|
|
|
2013
|
|
|
2012
|
|
|
Net cash provided by (used in) operating activities
|
|
$
|
(184,596
|
)
|
|
$
|
45,836
|
)
|
|
$
|
(264,478
|
)
|
|
$
|
(88,207
|
))
|
|
Net cash used in investing activities
|
|
|
--
|
|
|
|
|
|
|
|
--
|
|
|
|
(5,086
|
)
|
|
Net cash provided by (used in) financing activities
|
|
|
194,014
|
|
|
|
(58,895
|
)
|
|
|
(95,207
|
)
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
57,498
|
|
|
|
--
|
|
|
|
--
|
|
|
|
(1,914
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
5,061
|
|
|
|
--
|
|
|
|
-
|
|
|
|
--
|
|
|
Cash and cash equivalents, end of period
|
|
|
62,559
|
|
|
|
--
|
|
|
|
5,061
|
|
|
|
--
|
|
Net cash flow from operating activities
Net Cash provided by operating activities
decreased $230,432from the first three months of 2014 compared to the first three months of 2013 due to an increase in general
and administrative expenses 2014. Net cash provided by operating activities in 2013 d increased by $72,276 from Fiscal
2012. The increase was primarily due to a larger net loss in 2012.
Net cash flow from investing activities
Net Cash provided by investing activities
was $ - for the first three months of 2014 compared to the first three months of 2013 due to the company not requiring any capital
expenditures for operations. Net cash provided by investing activities decreased by $5,086 from Fiscal 2012. This
was due to the company not requiring any capital expenditures for operations in 2013.
Net cash flow from financing activities
Net Cash provided by financing activities
has increased by $135,119from the first three months of 2014 compared to the first three months of 2013 due to the company issuing
capital stock in order to raise cash increase in note payable to a related party. Net cash provided by investing activities
increased by 127,724l from Fiscal 2012. This was due to increase in note payable to a related party.
Critical Accounting Policies and Estimates
The preparation of financial statements
in conformity with accounting principles generally accepted in the United States of America requires management to make estimates
and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities
at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period.
Cash and Cash Equivalents
All short-term investments with original
maturities of three months or less at date of purchase are considered cash equivalents.
Accounts receivable
The Company considers the entire accounts
receivable to be fully collectible. The accounts receivable consist of trade receivable to wholesale customer. Management believes
all receivables are fully collectible and therefore no allowance for bad debt has been recorded as of December 31, 2013 and 2012.
Foreign Currency Transactions
The Company's functional currency is the
Canadian dollar and the reporting currency is the U.S. dollar. Assets and liabilities are translated from the functional to the
reporting currency at the exchange rate in effect at the balance sheet date and equity at the historical exchange rates. Revenue
and expenses are translated at rates in effect at the time of the transactions. Resulting translation gains and losses are accumulated
in a separate component of stockholders' equity - other comprehensive income (loss). Realized foreign currency transaction gains
and losses are credited or charged directly to operations.
Intangible Assets
Licensing as it relates to Drug Identification
Numbers, is accounted for as an indefinite life intangible asset until the product is abandoned at which point the intangible asset
will be written off.
Other intangible assets consist of websites,
trademarks, domain names etc. and are amortized over estimated useful lives.
Revenue recognition
The Company derives revenues from sale
of merchandise and upon the following: (1) there is persuasive evidence that an arrangement exists; (2) delivery has occurred or
services have been rendered, (3) the seller's price to the buyer is fixed or determinable, and (4) collectability is reasonably
assured.
Comprehensive Income
ASC Topic 220, "Comprehensive Income",
establishes standards for reporting and display of comprehensive income and its components in a full set of general-purpose financial
statements. Comprehensive Income consists of foreign currency translation.
Going Concern
The Company currently has limited working
capital, and has not completed its efforts to establish a stabilized source of revenues sufficient to cover operating costs over
an extended period of time. Management anticipates that the Company will be dependent, for the near future, on additional investment
capital to fund operating expenses The Company intends to position itself so that it may be able to raise additional funds through
the capital markets. In light of management’s efforts, there are no assurances that the Company will be successful in this
or any of its endeavors or become financially viable and continue as a going concern.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Contractual Obligations
We do not have any contractual obligations.
DIRECTORS AND EXECUTIVE OFFICERS AND
CORPORATE GOVERNANCE
Directors and Officers
Our sole director and officer, his age and business experience,
as of July 25, 2014, are set forth below:
|
Name
|
|
Age
|
|
Positions with the Company
|
|
Peter Daniel Bagi, MD
|
|
60
|
|
President, Chief Executive Officer, Chief Financial Officer and Director
|
Peter Daniel Bagi, MD
, is
a pharmaceutical and biotech professional with almost 25 years’ experience. Dr. Bagi has been the sole director and officer
of the Company since inception.
Dr. Bagi graduated from the Faculty of
Medicine at the University of Monterrey, in 1990, and subsequently successfully passed the US foreign-medical graduate licensing
exam in 1991. Dr. Bagi began in the Biotech industry in 1990 as the Associate Medical Director of a Canadian Biotech company, which
has since undergone several name changes over the years, but is currently known as Lorus Therapeutics, and as such Dr. Bagi’s
responsibilities included overseeing the cancer clinical trial program in Canada, US and Mexico. During his five-year tenure at
Lorus, Dr. Bagi was asked to participate in presentations at medical and scientific conferences to highlight the progress of its
lead cancer-fighting compound: Virulizin. In addition, Dr. Bagi was able to spearhead the approval of Virulizin in Mexico as a
second-line treatment for metastatic pancreatic cancer. Since Lorus was a small Biotech company, Dr. Bagi was also asked to present
the scientific progress of Lorus during fund-raising efforts Lorus periodically undertook.
After leaving Lorus, Dr. Bagi freelanced
as a biotech analyst at several of Toronto’s brokerage firms writing reports on pharmaceutical and biotech companies the
brokerages were considering investments in.
Dr. Bagi also was able to recruit World-Class
Advisory Boards for several of the small biotech companies he has consulted with over the years, such as Alpha Rx. Dr. Bagi also
worked as a Business Development consultant for various small biotech companies, and was instrumental in out-licensing several
medicinal products between Canadian and foreign pharmaceutical companies.
We believe that Dr. Bagi’s experience
across several aspects of the operations of a small pharmaceutical company will serve the Company well in the execution of its
business plan.
Code of Business Conduct and Ethics
The Company has not adopted any Code of
Business Conduct and Ethics.
EXECUTIVE COMPENSATION
The following table sets forth information
regarding compensation earned in or with respect to our fiscal year 2013 and 2012 by our directors and officers:
Summary Compensation Table
Name and
Principal Position
|
|
Year
|
|
|
Salary
($)
|
|
|
Bonus
($)
|
|
|
Stock
Awards
($)
|
|
|
Option
Awards
($)
|
|
|
Non-Equity
Incentive
Plan
Compensation
($)
|
|
|
All Other
Compensation
($)
|
|
|
Total
($)
|
|
|
Peter Daniel Bagi
|
|
|
2013
|
|
|
|
22,600
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
22,600
|
|
|
CEO and CFO
|
|
|
2012
|
|
|
|
7,652
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
7,652
|
|
Executive Employment Arrangements
On April 30, 2012, the Company entered into a Consulting Agreement
with Peter Daniel Bagi to provide consulting services to advise and assist in the launch of medicinal products in Mexico, Canada
and other territories. The Agreement has a term of two years and shall continue on a three-month basis until completion of the
services, subject to early termination as described in the agreement. The Company pays a monthly consultant fees of $5,000.
Outstanding Equity Awards as Of December 31, 2013
None.
Equity Compensation Plan Information
None.
SECURITY OWNERSHIP
OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain
information known to us regarding beneficial ownership of shares of our common stock as of July 23, 2014 by:
|
|
●
|
each of our named executive officers;
|
|
|
●
|
all of our executive officers and directors as a group; and
|
|
|
●
|
each person, or group of affiliated persons, known to us to be the beneficial owner of more than
5% of our outstanding shares of common stock.
|
Beneficial ownership is determined in accordance
with the rules and regulations of the SEC and includes voting and investment power with respect to the securities. In computing
the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject
to options or warrants held by that person that are currently exercisable or exercisable within 60 days of July 23, 2014 are deemed
outstanding. Such shares, however, are not deemed outstanding for purposes of computing the percentage ownership of any other person.
To our knowledge, except as indicated in the footnotes to this table and subject to community property laws where applicable, the
persons named in the table have sole voting and investment power with respect to all shares of our common stock shown opposite
such person’s name. The percentage of beneficial ownership is based on 49,167,009 shares of our common stock outstanding
as of July 23, 2014. Unless otherwise noted below, the address of the persons and entities listed in the table is c/o Axxess Pharma,
Inc., 3250 Bloor Street West, Suite 613, Toronto, ON, M8X 2X9.
|
Name and Address of Beneficial Owner
|
|
Number of
Shares
Beneficially
Owned (#)
|
|
|
Percent of
Outstanding
Shares (%)
|
|
|
Named Executive Officers and Directors:
|
|
|
|
|
|
|
|
|
|
Peter Daniel Bagi
|
|
|
5,000,000
|
|
|
|
10.17
|
%
|
|
5% Stockholders:
|
|
|
|
|
|
|
|
|
|
Blue Ivory International Holdings, Ltd. (1)
|
|
|
12,500,000
|
|
|
|
25.42
|
%
|
|
|
( 1 )
|
Blue Ivory International Holdings, Ltd., or Blue Ivory,
or Blue Ivory’s President Alan Cole may be deemed to beneficially own shares of common stock beneficially owned by Blue
Ivory. Voting and dispositive power with respect to the shares owned by Blue Ivory is exercised by Alan Cole, President.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS,
AND DIRECTOR INDEPENDENCE
Since the beginning of our fiscal year
2011, there has not been, and there is not currently proposed any transaction or series of similar transactions in which the amount
involved exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets at year end for the last
two completed fiscal years and in which any related person, including any director, executive officer, holder of more than 5% of
our capital stock during such period, or entities affiliated with them, had a material interest, other than as described in the
transactions set forth below.
PLAN OF DISTRIBUTION
On June 9, 2014, we entered into the Investment
Agreement with Beaufort. Pursuant to the terms of the Investment Agreement, Beaufort committed to purchase up to $2,000,000 of
our common stock over a period of up to thirty-six (36) months. From time to time during the thirty-six (36) month period commencing
on the day immediately following the effectiveness of this registration statement, we may deliver a drawdown notice to Beaufort
which states the dollar amount that we intend to sell to Beaufort on a date specified in the drawdown notice. The maximum investment
amount per notice shall be equal to two hundred fifty percent (250%) of the average daily volume of the common stock for the ten
consecutive trading days immediately prior to date of the applicable drawdown notice so long as such amount does not exceed 4.99%
of the outstanding shares of our common stock. The purchase price per share to be paid by Beaufort shall be calculated at a thirty
percent (30%) discount to the average of the median price for the average of the ten (10) closing daily prices and the ten (10)
closing bid prices of the Company’s Common Stock immediately prior to Beaufort’s receipt of the Advance Notice.
In connection with the Investment Agreement,
we also entered into the Registration Rights Agreement, pursuant to which we will use our commercially reasonable efforts to file,
within thirty (30) days of the date of the Investment Agreement, a Registration Statement on Form S-1 covering the resale of the
common stock subject to the Investment Agreement. We have agreed to have the Registration Statement declared effective with the
SEC. Beaufort has agreed to pay all legal costs and expenses associated with the Registration Rights Agreement. Beaufort has also
agreed to extend the filing deadline to July 25, 2014.
The current registration statement covers
11,428,572 shares of our Common Stock under the Investment Agreement that would raise $2,000,000 assuming our Common Stock’s
closing bid price remains unchanged from its price as of July 23, 2014. In the event our Common Stock’s price decreases,
we may receive substantially less than $2,000,000. In that case, the Company may have to prepare and file one or more additional
registration statements registering the resale of these shares if this registration statement is unable to cover the remaining
amount of shares. Beaufort has agreed to refrain from holding an amount of shares which would result in Beaufort owning more than
4.99% of the then-outstanding shares of our common stock at any one time.
The selling stockholder may, from time
to time, sell any or all of its shares of common stock on any stock exchange, market or trading facility on which the shares are
traded or in private transactions. These sales may be at fixed or negotiated prices. The selling stockholder may use any one or
more of the following methods when selling shares:
|
|
·
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
·
|
block trades in which the broker-dealer will attempt to sell the shares as agent, but may position
and resell a portion of the block as principal to facilitate the transaction
|
|
|
·
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
·
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
·
|
privately negotiated transactions;
|
|
|
·
|
short sales after this Prospectus becomes effective;
|
|
|
·
|
broker-dealers may agree with the selling stockholder to sell a specified number of such shares
at a stipulated price per share;
|
|
|
·
|
through the writing of options on the shares;
|
|
|
·
|
a combination of any such methods of sale; and
|
|
|
·
|
any other method permitted pursuant to applicable law.
|
The selling stockholder may also sell the
shares directly to market makers acting as principals and/or broker-dealers acting as agents for themselves or their customers.
Such broker-dealers may receive compensation in the form of discounts, concessions or commissions from the selling stockholder
and/or the purchasers of shares for whom such broker-dealers may act as agents or to whom they sell as principal or both, which
compensation as to a particular broker-dealer might be in excess of customary commissions. Market makers and block purchasers purchasing
the shares will do so for their own account and at their own risk. It is possible that a selling stockholder will attempt to sell
shares of common stock in block transactions to market makers or other purchasers at a price per share which may be below the then
market price. The selling stockholder cannot assure that all or any of the shares offered in this Prospectus will be issued to,
or sold by, the selling stockholder. The selling stockholder and any brokers, dealers or agents, upon effecting the sale of any
of the shares offered in this Prospectus, are “underwriters” as that term is defined under the Securities Act, or the
Exchange Act, or the rules and regulations under such acts. In such event, any commissions received by such broker-dealers or agents
and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the
Securities Act.
Discounts, concessions, commissions and
similar selling expenses, if any, attributable to the sale of shares will be borne by the selling stockholder. The selling stockholder
may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the shares if liabilities
are imposed on that person under the Securities Act.
The selling stockholder shall acquire the
securities offered hereby in the ordinary course of business and has advised us that it has not entered into any agreements, understandings
or arrangements with any underwriters or broker-dealers regarding the sale of its shares of common stock, nor is there an underwriter
or coordinating broker acting in connection with a proposed sale of shares of common stock by any selling stockholder. If we are
notified by any selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of shares
of common stock, if required, we will file a supplement to this Prospectus.
If the selling stockholder uses this Prospectus
for any sale of the shares of common stock, it will be subject to the prospectus delivery requirements of the Securities Act.
Regulation M
The anti-manipulation rules of Regulation
M under the Exchange Act may apply to sales of our common stock and activities of the selling stockholder.
During such time as it may be engaged in
a distribution of any of the shares we are registering by this registration statement, Beaufort is required to comply with Regulation
M. In general, Regulation M precludes any selling security holder, any affiliated purchasers and any broker-dealer or other person
who participates in a distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase, any
security which is the subject of the distribution until the entire distribution is complete. Regulation M defines a "distribution"
as an offering of securities that is distinguished from ordinary trading activities by the magnitude of the offering and the presence
of special selling efforts and selling methods. Regulation M also defines a "distribution participant" as an underwriter,
prospective underwriter, broker, dealer, or other person who has agreed to participate or who is participating in a distribution.
Regulation M under the Exchange Act prohibits,
with certain exceptions, participants in a distribution from bidding for or purchasing, for an account in which the participant
has a beneficial interest, any of the securities that are the subject of the distribution. Regulation M also governs bids and purchases
made in order to stabilize the price of a security in connection with a distribution of the security. We have informed Beaufort
that the anti-manipulation provisions of Regulation M may apply to the sales of their shares offered by this Prospectus, and we
have also advised Beaufort of the requirements for delivery of this Prospectus in connection with any sales of the common stock
offered by this Prospectus.
Pursuant to the Investment Agreement, Beaufort
shall not sell stock short, either directly or indirectly through its affiliates, principals or advisors, our common stock during
the term of the agreement.
DESCRIPTION OF SECURITIES TO BE REGISTERED
Authorized
Capital Stock
The authorized
capital stock of the Company consists of, 100,000,000 shares of the common stock and 20,000,000 shares of preferred stock, of which
as of the date hereof, 49,167,009 shares of common stock, par value $0.0001 per share, are issued and outstanding, and 20,000,000
shares of preferred stock, par value $0.0001 per share, are issued and outstanding.
Common Stock
As of July 23,
2014, 49,167,009 shares of common stock are issued and outstanding. The Company completed a 750-for-1 reverse split in April 2012.
The holders of
our common stock have equal ratable rights to dividends from funds legally available if and when declared by our board of directors
and are entitled to share ratably in all of our assets available for distribution to holders of common stock upon liquidation,
dissolution or winding up of our affairs. Our common stock does not provide the right to a preemptive, subscription or conversion
rights and there are no redemption or sinking fund provisions or rights. Our common stock holders are entitled to one non-cumulative
vote per share on all matters on which stockholders may vote.
All shares of
common stock now outstanding are fully paid for and non-assessable. We refer you to our Articles of Incorporation, Bylaws and the
applicable statutes of the state of Nevada for a more complete description of the rights and liabilities of holders of our securities.
All material terms of our common stock have been addressed in this section.
Holders of shares
of our common stock do not have cumulative voting rights, which means that the holders of more than 50% of the outstanding shares,
voting for the election of directors, can elect all of the directors to be elected, if they so choose, and, in that event, the
holders of the remaining shares will not be able to elect any of our directors.
Preferred Stock
Our Board of Directors has the authority,
without further action by the stockholders, to issue up to 20,000,000 shares of preferred stock in one or more series, and to fix
the designations, powers, preferences and relative, participating, optional and other special rights, if any, of each such class
or series and the qualifications, limitations and restrictions thereof, including dividend rights, conversion rights, voting rights,
sinking-fund provisions, terms of redemption, liquidation preferences, preemption rights, and the number of shares constituting
any series or the designation of such series, without any further vote or action by the stockholders. The issuance of preferred
stock could adversely affect the voting power of holders of our common stock and could have the effect of delaying, deferring or
preventing a change in control of us.
Series A Preferred Stock
As of the date
hereof, 20,000,000 shares of Series A Preferred Stock are issued and outstanding. The preferred stock has no voting rights.
Registration
Rights
In connection
with the execution of the Investment Agreement, on June 9, 2014, the Company and Beaufort also entered into the Registration
Rights Agreement. Pursuant to the Registration Rights Agreement, the Company has agreed to file the registration statement of which
this Prospectus is a part with the SEC to register the shares. The effectiveness of the registration statement of which this
Prospectus is a part is a condition precedent to our ability to sell common stock to Beaufort under the Securities Purchase Agreement.
The Company has
agreed to file with the SEC one or more additional registration statements to cover all of the securities required to be registered
under the Registration Rights Agreement that are not covered by this Prospectus, in each case, as soon as practicable, for such
additional registration statements as provided in the Registration Rights Agreement.
Transfer Agent
The transfer agent
and registrar for our common stock is Corporate Stock Transfer, Inc., located at 3200 Cherry Creek South Drive, Suite 430, Denver,
Colorado 80209, and its telephone number is (303) 282-4800.
LEGAL MATTERS
The validity of
the common stock offered by this Prospectus will be passed upon for us by Szaferman Lakind Blumstein & Blader, P.C., Lawrenceville,
New Jersey.
EXPERTS
The consolidated
balance sheets of Axxess Pharma, Inc. as of December 31, 2013 and 2012, and the related consolidated statements of operations,
stockholders’ equity (deficit) and cash flows for each of the years in the two-year period ended December 31, 2013, included
in this Prospectus and in the registration statement, have been audited by KLJ & Associates, LLP, independent registered public
accounting firm, as stated in their report which is incorporated herein, in reliance on the report of such firm, given upon their
authority of experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement,
of which this Prospectus is a part, with the SEC under the Securities Act with respect to our common stock. This Prospectus, which
constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement,
parts of which are omitted as permitted by the rules and regulations of the SEC. Statements contained in this Prospectus as to
the contents of any contract or other document are not necessarily complete. For further information pertaining to us and our common
stock, we refer you to our registration statement and the exhibits thereto, copies of which may be inspected without charge at
the SEC’s Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549. Information concerning the operation of the
SEC’s Public Reference Room is available by calling the SEC at 1-800-SEC-0330. Copies of all or any part of the registration
statement may be obtained at prescribed rates from the SEC. The SEC also makes our filings available to the public on its Internet
site (http://www.sec.gov). Quotations relating to our common stock appear on the OTC Pink, and such reports, proxy statements and
other information concerning us can also be inspected at the offices of the National Association of Securities Dealers, Inc., 1735
K Street N.W., Washington, D.C. 20006.
We file annual, quarterly and special reports, proxy statements
and other information with the SEC. Such periodic reports, proxy and information statements and other information are available
for inspection and copying at the public reference facilities and Internet site of the SEC referred to above.
AXXESS
PHARMA INC. & SUBSIDIARIES
CONSOLIDATED
FINANCIAL STATEMENTS
Including Independent Accountants’
Audit Report
For the Fiscal Years Ended
December 31, 2013 and 2012
Table of Contents

REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors and
Stockholders Axxess Pharma, Inc.
We have
audited the accompanying consolidated balance sheets of Axxess Pharma, Inc. & Subsidiaries (the “Company”) as of
December 31, 2013 and 2012 and the related statements of operations, comprehensive income, stockholders’ equity, and cash
flows for the years ended. Axxess Pharma, Inc.’s management is responsible for these consolidated financial statements. Our
responsibility is to express an opinion on these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards
require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material
misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial
reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures
that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s
internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test
basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our
audits provide a reasonable basis for our opinion.
In
our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position
of Axxess Pharma, Inc. & Subsidiaries as of December 31, 2013 and 2012, and the results of its operations and its cash flows
for years ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial
statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial
statements, The Company has suffered net losses and has had negative cash flows from operating activities during the years ended
December 31, 2013 and 2012. These matters raise substantial doubt about the Company’s ability to continue as a going concern.
Management’s plans concerning these matters are also described in Note 2. The financial statements do not include any adjustments
to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might
result should the Company be unable to continue as a going concern.
|
/s/ KLJ & Associates, LLP
|
|
|
|
KLJ & Associates, LLP
|
|
St. Louis Park, MN
|
|
July 25 , 2014
|
1660 South Highway 100
Suite 500
St. Louis Park, MN 55416
630.277.2330
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
|
|
December 31, 2013
|
|
|
December 31, 2012
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
5,061
|
|
|
$
|
-
|
|
|
Accounts receivable
|
|
|
2,400
|
|
|
|
-
|
|
|
Inventory
|
|
|
20,525
|
|
|
|
-
|
|
|
Prepaid expenses and other
|
|
|
100
|
|
|
|
11,663
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL CURRENT ASSETS
|
|
|
28,086
|
|
|
|
11,663
|
|
|
|
|
|
|
|
|
|
|
|
|
INTANGIBLE ASSETS
|
|
|
250,529
|
|
|
|
224,733
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
$
|
278,615
|
|
|
$
|
236,396
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
$
|
387,105
|
|
|
$
|
258,136
|
|
|
Notes payable – related party
|
|
|
309,171
|
|
|
|
195,800
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL CURRENT LIABILITIES
|
|
|
696,276
|
|
|
|
453,936
|
|
|
|
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES
|
|
|
|
|
|
|
|
|
|
Note Payable – Related Party
|
|
|
505,120
|
|
|
|
418,952
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LONG-TERM LIABILITIES
|
|
|
505,120
|
|
|
|
418,952
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
|
1,201,396
|
|
|
|
872,888
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY
|
|
|
|
|
|
|
|
|
|
Preferred stock $0.0001 par value: 20,000,000 Authorized shares: 20,000,0000 shares Issued and outstanding
|
|
|
2,000
|
|
|
|
2,000
|
|
|
Common Stock, $0.0001 par value: 100,000,000Authorized shares: 50,734,863 and 39,609,863 Issued and outstanding shares, respectively
|
|
|
5,074
|
|
|
|
3,961
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
17,733,526
|
|
|
|
11,134,139
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive Income (loss)
|
|
|
44,694
|
|
|
|
(1,914
|
)
|
|
Retained earnings
|
|
|
(18,708,075
|
)
|
|
|
(11,774,678
|
)
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL STOCKHOLDERS’ EQUITY
|
|
|
(922,781
|
)
|
|
|
(642,386
|
)
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
$
|
278,615
|
|
|
$
|
236,396
|
|
The accompanying notes are an integral
part of these consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
|
Year Ended December 31,
|
|
|
|
|
2013
|
|
|
2012
|
|
|
SALES
|
|
|
2,400
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF SALES
|
|
|
744
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT
|
|
|
1,656
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
GENERAL & ADMINISTRATIVE EXPENSES
|
|
|
|
|
|
|
|
|
|
Professional fees
|
|
|
176,873
|
|
|
|
115,572
|
|
|
Office
|
|
|
34,474
|
|
|
|
14,272
|
|
|
Consulting
|
|
|
228,359
|
|
|
|
11,000,000
|
|
|
Management fees
|
|
|
60,160
|
|
|
|
60,012
|
|
|
Advertising
|
|
|
33,960
|
|
|
|
8,764
|
|
|
Travel and entertainment
|
|
|
44,647
|
|
|
|
23,181
|
|
|
Other
|
|
|
30,147
|
|
|
|
-
|
|
|
|
|
|
608,620
|
|
|
|
11,221,801
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS FORM OPERATIONS
|
|
|
(606,964
|
)
|
|
|
(11,221,801
|
)
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME/(EXPENSE)
|
|
|
|
|
|
|
|
|
|
Excess purchase price over fair value of assets acquired
|
|
|
(3,079,731
|
)
|
|
|
-
|
|
|
Extinguishment of debt
|
|
|
(3,196,000
|
)
|
|
|
-
|
|
|
Interest expense
|
|
|
(50,702
|
)
|
|
|
(41,800
|
)
|
|
TOTAL OTHER ITEMS
|
|
|
(6,326,433
|
)
|
|
|
(41,800
|
)
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS BEFORE INCOME TAXES
|
|
|
(6,933,397
|
)
|
|
|
(11,263,601
|
)
|
|
|
|
|
|
|
|
|
|
|
|
INCOME TAX EXPENSE
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
|
(6,933,397
|
)
|
|
|
(11,263,601
|
)
|
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME
|
|
|
46,608
|
|
|
|
(1,914
|
)
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS & COMPREHENSIVE INCOME
|
|
$
|
(6,886,789
|
)
|
|
$
|
(11,265,515
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Basic & Diluted earnings per share
|
|
$
|
(0.15
|
)
|
|
$
|
(0.46
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average basis & diluted shares outstanding
|
|
|
44,531,712
|
|
|
|
24,531,668
|
|
The accompanying notes are an integral
part of these consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’
EQUITY & COMPREHENSIVE INCOME
|
|
|
Preferred
Shares
|
|
|
Amount
|
|
|
Common
Shares
|
|
|
Amount
|
|
|
Additional
Paid-In
Capital
|
|
|
Retained
Earnings
|
|
|
Other
Comprehensive
Income
|
|
|
Total
|
|
|
Balance, December 31, 2011
|
|
|
20,000,000
|
|
|
$
|
2,000
|
|
|
|
109,688
|
|
|
$
|
11
|
|
|
$
|
13,089
|
|
|
$
|
(511,077
|
)
|
|
$
|
|
|
|
$
|
(495,977
|
)
|
|
Shares issued for cash
|
|
|
-
|
|
|
|
-
|
|
|
|
750,000
|
|
|
|
75
|
|
|
|
74,925
|
|
|
|
-
|
|
|
|
-
|
|
|
|
75,000
|
|
|
Shares issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
13,750,175
|
|
|
|
1,375
|
|
|
|
10,998,625
|
|
|
|
-
|
|
|
|
-
|
|
|
|
11,000,000
|
|
|
Shares issued as collateral on licensing agreement
|
|
|
-
|
|
|
|
-
|
|
|
|
25,000,000
|
|
|
|
2,500
|
|
|
|
(2,500
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Related party debt converted to equity
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
50,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
50,000
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(11,263,601
|
)
|
|
|
-
|
|
|
|
(11,263,601
|
)
|
|
Foreign currency Translation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,914
|
)
|
|
|
(1,914
|
)
|
|
Balance, December 31, 2012
|
|
|
20,000
|
|
|
|
2,000
|
|
|
|
39,609,863
|
|
|
|
3,961
|
|
|
|
11,134,139
|
|
|
|
(11,774,678
|
)
|
|
|
(1,914
|
)
|
|
|
(636,492
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued for cash
|
|
|
-
|
|
|
|
-
|
|
|
|
400,000
|
|
|
|
40
|
|
|
|
69,960
|
|
|
|
-
|
|
|
|
-
|
|
|
|
70,000
|
|
|
Shares issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
275,000
|
|
|
|
28
|
|
|
|
234,472
|
|
|
|
-
|
|
|
|
-
|
|
|
|
234,500
|
|
|
Related party debt converted to equity
|
|
|
-
|
|
|
|
-
|
|
|
|
2,000,000
|
|
|
|
200
|
|
|
|
1,599,800
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,600,000
|
|
|
Debt converted to equity
|
|
|
-
|
|
|
|
-
|
|
|
|
2,000,000
|
|
|
|
200
|
|
|
|
1,599,800
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,600,000
|
|
|
Record shares issued Acquisition
|
|
|
-
|
|
|
|
-
|
|
|
|
6,450,000
|
|
|
|
645
|
|
|
|
3,095,355
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,096,000
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(6,933,397
|
)
|
|
|
-
|
|
|
|
(6,933,397
|
)
|
|
Foreign currency Translation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
46,608
|
|
|
|
46,608
|
|
|
Balance, December 31, 2013
|
|
|
20,000,000
|
|
|
$
|
2,000
|
|
|
|
50,734,863
|
|
|
$
|
5,074
|
|
|
$
|
17,733,526
|
|
|
$
|
(18,708,075
|
)
|
|
$
|
44,694
|
|
|
$
|
(922,781
|
)
|
The accompanying notes are an integral
part of these consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
For the Year Ended December 31,
|
|
|
|
|
2013
|
|
|
2012
|
|
|
OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(6,933,397
|
)
|
|
$
|
(11,263,601
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|
|
|
|
|
|
|
Stock issued for services
|
|
|
234,500
|
|
|
|
11,050,000
|
|
|
Conversion of debt to equity
|
|
|
3,200,000
|
|
|
|
-
|
|
|
Stock issued for acquisition
|
|
|
3,096,000
|
|
|
|
-
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
-
|
|
|
Accounts receivable
|
|
|
(2,400
|
)
|
|
|
-
|
|
|
Inventory
|
|
|
287
|
|
|
|
-
|
|
|
Prepaid expenses and other assets
|
|
|
11,563
|
|
|
|
(11,663
|
)
|
|
Accounts payable & accrued liabilities
|
|
|
128,969
|
|
|
|
137,057
|
|
|
Net cash provided by operating activities
|
|
|
(264,478
|
)
|
|
|
(88,207
|
)
|
|
|
|
|
|
|
|
|
|
|
|
INVESTING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Purchase of fixed assets
|
|
|
-
|
|
|
|
(5,086
|
)
|
|
Net cash (used) by investing activities
|
|
|
-
|
|
|
|
(5,086
|
)
|
|
|
|
|
|
|
|
|
|
|
|
FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Issuance of common stock
|
|
|
70,000
|
|
|
|
75,000
|
|
|
Proceeds from note payable – related party
|
|
|
152,931
|
|
|
|
20,207
|
|
|
Net cash (used) by financing activities
|
|
|
222,931
|
|
|
|
95,207
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of foreign currency translation
|
|
|
46,608
|
|
|
|
(1,914
|
)
|
|
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH
|
|
|
5,061
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH
|
|
|
|
|
|
|
|
|
|
Beginning of year
|
|
|
-
|
|
|
|
-
|
|
|
End of year
|
|
$
|
5,061
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Interest paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-CASH ACTIVITY
|
|
|
|
|
|
|
|
|
|
Stock issued for acquisition
|
|
|
3,096,000
|
|
|
|
-
|
|
|
Acquisition financed through note payable
|
|
|
52,000
|
|
|
|
-
|
|
The accompanying notes are an integral
part of these consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
NOTE 1—THE COMPANY AND BASIS OF PRESENTATION
Nature of operations
Axxess Pharma, Inc. was incorporated in the state of Delaware
on April 7, 1997 as CGI Communications Services Inc. On July 26, 2008, the Company amended its certificate of incorporation to
change its name to Axxesss Pharma, Inc. On December 6, 2012 the Company reincorporated in Nevada by merging into a newly formed
Nevada entity with the name Axxess Pharma, Inc. The Company now operates as Axxess Pharma, Inc. with Axxess Pharma Canada, Inc.
and Allstar Health Brands Inc.as its wholly owned subsidiaries.
Axxess Pharma Canada, Inc. was incorporated under the Laws of
the Province of Ontario. The Company is engaged in the acquisition of Drug Identification Numbers and the eventual sale of the
related products. All Star Health Brands Inc. was incorporated on October 1, 2013 under the Laws of the Province of Ontario. The
Company is engaged in the acquisition of Drug Identification Numbers and the eventual sale of the related products
The consolidated financial statements included the results of
Axxess Pharma Inc. Axxess Pharma Canada and its wholly owned subsidiary Allstar Health Brands Inc. All intercompany accounts have
been eliminated during consolidation.
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of financial statements in conformity with accounting
principles generally accepted in the United States of America requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial
statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those
estimates.
The Company’s most significant areas of estimation and
assumption are:
|
|
•
|
determination of the appropriate amount and timing of markdowns to clear unproductive or slow-moving retail inventory and overall
inventory obsolescence
|
|
|
•
|
estimation of future cash flows used to assess the recoverability of long-lived assets
|
Cash and Cash Equivalents
All short-term investments with original maturities of three
months or less at date of purchase are considered cash equivalents.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company
to concentrations of credit risk consist of cash and accounts receivable. The Company maintains their cash in bank deposit accounts
at high credit quality financial institutions. The balances, at times, may exceed federally insured limits. The Company also maintains
a certain amount of cash on hand at the retail store locations. The Company has not experienced any significant losses with respect
to its cash. At both December 31, 2013 and 2012, the Company did not exceed the insured limit of a depository institution.
Accounts receivable
The Company considers the entire accounts receivable to be fully
collectible. The accounts receivable consist of trade receivable to wholesale customer. Management believes all receivables are
fully collectible and therefore no allowance for bad debt has been recorded as of December 31, 2013 and 2012.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
Inventory
Inventory consists of finished product acquired for resale and
is valued at the lower-of-cost-or-market with cost determined on a specific item basis.
Fair value of financial instruments
The carrying amounts of certain of the Company’s financial
instruments, including cash, accounts receivable, accounts payable and accrued liabilities approximate fair value due to their
relatively short maturities.
Foreign Currency Transactions
The Company's functional currency is the
Canadian dollar and the reporting currency is the U.S. dollar. Assets and liabilities are translated from the functional to the
reporting currency at the exchange rate in effect at the balance sheet date and equity at the historical exchange rates. Revenue
and expenses are translated at rates in effect at the time of the transactions. Resulting translation gains and losses are accumulated
in a separate component of stockholders' equity - other comprehensive income (loss). Realized foreign currency transaction gains
and losses are credited or charged directly to operations.
Intangible Assets
Licensing as it relates to Drug Identification Numbers, is accounted
for as an indefinite life intangible asset until the product is abandoned at which point the intangible asset will be written off.
Other intangible assets consist of websites, trademarks, domain
names etc. and are amortized over estimated useful lives.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including property and
equipment, for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable
based on undiscounted cash flows. If long-lived assets are impaired, an impairment loss is recognized and is measured as the amount
by which the carrying value exceeds the estimated fair value of the assets. The estimation of future undiscounted cash flows from
operating activities requires significant estimates of factors that include future sales growth and gross margin performance. Management
believes they have appropriately determined future cash flows and operating performance; however, should actual results differ
from those expected, additional impairment may be required.
Cost of sales
Cost of sales of approximately $744 and $0 million for the years
ended December 31, 2013 and 2012, respectively, consist primarily of merchandise costs of sales.
Revenue recognition
The Company derives revenues from sale of merchandise and upon
the following: (1) there is persuasive evidence that an arrangement exists; (2) delivery has occurred or services have been rendered,
(3) the seller's price to the buyer is fixed or determinable, and (4) collectability is reasonably assured.
Advertising / Marketing
Advertising costs are charged to expense when incurred. The
Company’s advertising method is primarily print, web based and word of mouth. Advertising / marketing costs were approximately
$33,960 and $8,764 for the years ended December 31, 2013 and 2012, respectively.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
Comprehensive Income
ASC Topic 220, "Comprehensive Income",
establishes standards for reporting and display of comprehensive income and its components in a full set of general-purpose financial
statements. Comprehensive Income consists of foreign currency translation.
NOTE 3 – GOING CONCERN
The accompanying financial statements have
been prepared in conformity with generally accepted accounting principle, which contemplate continuation of the Company as a going
concern. The Company currently has limited working capital, and has not completed its efforts to establish a stabilized source
of revenues sufficient to cover operating costs over an extended period of time.
Management anticipates that the Company
will be dependent, for the near future, on additional investment capital to fund operating expenses The Company intends to position
itself so that it may be able to raise additional funds through the capital markets. In light of management’s efforts, there
are no assurances that the Company will be successful in this or any of its endeavors or become financially viable and continue
as a going concern.
NOTE 4—NOTES PAYABLE –RELATED PARTY
Loan from stockholders are non interest bearing and due on demand.
On October 25, 2010, the Company entered into a note payable
with Ramos and Ramos Investments Inc. The note bears interest at a 7% per annum interest rate. The note matures December 31, 2014.
The holder of the note has the right to convert the full or any portion of the principal and accrued but unpaid interest into shares
of common stock. Interest expense for the years ended December 31, 2013 and 2012 was $7,000 and $7,000, respectively.
The Company entered into a note payable with maximum lending
amount of $500,000 with Ramos and Ramos Investments Inc. a maximum of lending amount of $500,000 could be advanced within the first
six months of the note. The note bears interest at a 12% per annum interest rate. The note cannot be paid off in full before January
1, 2015 or the completion of a public offering by the Company. In the event of default of the repayment, the note shall automatically
be converted to shares of the Company’s common stock at the price of $0.001 per share. The holder of the note has the right
to convert the full or any portion of the principal and accrued but unpaid interest into shares of common stock. Interest expense
for the years ended December 31, 2013 and 2012 was $45,311 and $34,792, respectively.
The Company entered into a note payable with Canadian Heritage
Ltd. The loan is not interest bearing and cannot be called prior to January 1, 2015.
On October 3, 2013 the Company entered into a $200,000 note
payable with RAMM Venture Developments Inc. The note bears interest at 12% annually The earliest the note can become due is December
31, 2014 if a private placement funds are received at which time the note repayment can be accelerated. In the event of default
of the repayment, the note shall automatically be converted to shares of the Company’s common stock at the price of $0.001
per share.
NOTE 5 – EQUITY
At December 31, 2013, the Company’s authorized stock consists
of 20,000,000 shares of $.0001 par value preferred stock and 100,000,000 shares of $.0001 par value common stock. The preferred
stock has no voting rights. The common stock has voting rights and entitle to one vote per share.
Of the 20,000,000 shares of preferred stock 20,000,000 shares
are issued and outstanding as of December 31, 2013 and 2012.
The following common stock transactions occurred during the
period:
On September 1, 2012 the Company issued 500,000 shares valued
@ $0.80 per share for consulting services
On April 30, 2012 the Company issued 5,000,000 shares valued
@ $0.80 per share for consulting service to its Chief Executive Officer.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
On September 11, 2012 the Company issued 2,000,000 shares valued
@ $0.80 per share for consulting services.
On January 1, 2012 the Company issued 2,500,000 shares valued
@ $0.80 per share for consulting services.
On June 11, 2012 the Company issued 750,000 shares valued @
$0.10 per share for cash.
On June 1, 2012 the Company issued 200,000 shares valued @ $0.80
per share for consulting services.
On May 15, 2012 the Company issued 500,000 shares valued @ $0.80
per share for consulting services
On June 11, 2012 the Company issued 2,000,000 shares valued
@ $0.80 per share for consulting services
On June 11, 2012 the Company issued 1,000,000 shares valued
@ $0.80 per share for consulting services.
On September 1, 2012 the Company issued 50,000 shares valued
@ $0.80 per share for consulting services.
On July 11, 2013 the Company issued 250,000 shares valued @
$0.85 per share for consulting services.
On July 11, 2013 the Company issued 200,000 shares valued @
$0.125 per share for cash.
On August 8, 2013 the Company issued 100,000 shares valued @
$0.20 per share for cash.
On May 2, 2013 the Company issued 4,000,000 shares valued @
$0.80 per share to convert $4,000 of principal on loan from related party to equity.
On May 8, 2013 the Company issued 1000,000 shares valued @ $0.25
per share for cash.
On August 15, 2013 the Company issued 25,000 shares valued @
$0.88 per share for consulting services.
On September 13, 2013 the Company issued 6,450,000 shares valued
@ $0.48 in relation to asset acquisition See note 5
NOTE 6 – ACQUISITION
On September 13, 2013 the Company through
its wholly owned subsidiary Allstar Health Brands, enter into an assets purchase agreement with Revive Bioscience Inc. The Company
acquired assets related to the distribution of Tapout Products including DINS of TapouT pain relief products as well as trademarks,
website, remaining finished goods inventory of Tapout products as well customer lists and intellectual products associated with
the Tapout brand name. The purchase price included $52,000 cash used to pay-off outstanding accounts payable of Revive Bioscience
as of the closing date and 6,450,000 shares of common stock valued at $0.48 per share at the closing date of the transaction. The
purchase price allocation is as follows:
|
Inventory
|
|
$
|
21,269
|
|
|
DINS
|
|
$
|
20,000
|
|
|
Patents
|
|
$
|
20,000
|
|
Based on the fair value of assets received
compared to the fair market value of the consideration give the $52,000 cash and fair value of common stock $3,096,000 ( 6,450,000
X $0.48) The company recorded expenses of as excess consideration paid over fair value of assets received of $3,079,731.
Under the terms of the agreement the seller
can receive an additional 2,500,000 shares of common stock is certain private placement funding levels are reached as well as specified
revenue goals related to the sale of TapouT products achieved with a eighteen months period commencing September 13, 2013.
The company is in the process of negotiation
of cancelling approximately 3,250,000 shares which has not been finalized as of the date of these financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
NOTE 7— INCOME TAXES
As of December 31, 2013, the Company had
net operating loss carry forwards of approximately $18,562,000 that may be available to reduce future years’ taxable income
in varying amounts through 2033. Future tax benefits which may arise as a result of these losses have not been recognized in these
financial statements, as their realization is determined not likely to occur and accordingly, the Company has recorded a valuation
allowance for the deferred tax asset relating to these tax loss carry-forwards.
The provision for Federal income tax consists of the following:
|
|
|
December 31, 2013
|
|
|
December 31, 2012
|
|
|
Federal income tax benefit attributable to:
|
|
|
|
|
|
|
|
|
|
Current operations
|
|
$
|
6,933,397
|
|
|
|
11,263,601
|
|
|
Less: valuation allowance
|
|
|
(6,933,397
|
)
|
|
|
(11,263,601
|
)
|
|
Net provision for Federal income taxes
|
|
$
|
-
|
|
|
|
-
|
|
The cumulative tax effect at the expected rate of 34% of significant
items comprising our net deferred tax amount is as follows:
|
|
|
December 31, 2013
|
|
|
December 31, 2012
|
|
|
Deferred tax asset attributable to:
|
|
|
|
|
|
|
|
|
|
Net operating loss carryover
|
|
$
|
2,357,355
|
|
|
|
3,829,624
|
|
|
Less: valuation allowance
|
|
|
(2,357,355
|
)
|
|
|
(3,829,624
|
)
|
|
Net deferred tax asset
|
|
$
|
-
|
|
|
|
-
|
|
NOTE 8— LICENSING AGREEMENT
On October 1, 2012, the Company entered into a licensing agreement
with Blue Ivory Holdings Inc. The Licensing agreement relates to the development, market, make, use and sell, certain drug formulations
know as DINS. The terms of the agreement require a total payment of $5,000,000 consisting of being paid quarterly payments of $125,000
over ten year period as well as royalty payment to Blue Holdings of 5% on all net sales by the Company and a 10% royalty fee for
Sublicense fees paid to Blue Ivory Holding on account of sublicenses for the sale of licensed products. As of December 31, 2012
and 2013 no payments have been made.
NOTE 9— CONSULTING AGREEMENT
On October 1, 2013 The Company entered into a license agreement
with ABG Tapout, LLC to sell various products. The license term is for 5 years ending December 31, 2018 and provided the Company
is not in breach of the agreement shall have the option to extend the licenses agreement for two five year terms. Pursuant to the
agreement the Company must pay ABG Tapout, LLC 5% royalty on net sales, the company must also maintain certain agreed upon sales
levels set out in the agreement in order to maintain the license.
On July 1, 2013 the Company entered into a consulting agreement
with Global Health Link Corp (“Consultants”). The Consultants will perform the duties of a Vice President of Marketing
for the Company. The term of the agreement is for three years with the option of a three year renewal. The agreement can be cancelled
by either party by giving a thirty day notice. Upon signing this agreement the Company will issue to the Consultants 250,000 shares
of the Company’s common stock.
Once the Company receives outside investments of $1,000,000
then the Company will begin paying the consultants $2,000.00 a month. If the Company receives a cumulative amount equal to $3,000,000
then the Company will pay the consultants an monthly fee of $5,000.00.
Finally, upon the Company obtaining $3,000,000 in external investments
or on July 1.2014, which ever comes first, the Company will issue and additional 250,000 shares of the Company’s common stock
to the Consultants.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
December 31, 2013 and 2012
NOTE 10— SUBSEQUENT EVENTS
On January 22, 2014 the Company entered into a Stock Purchase
Agreement with Equity Portfolio Funding, Inc. whereby the Company sold 750,000 shares of common stock at a purchase price of $0.10
per share for a total purchase price of $75,000.00. The agreement also calls for the Company to issue to Equity Portfolio Funding,
Inc. a half warrant to purchase further common stock of the Company for up to two years at $0.50 per share.
Other than event mentioned above we evaluated all events or
transactions that occurred after December 31, 2013 up through the date we issued these financial statements. During this period
we did not have any other material subsequent events that impacted our financial statements.
AXXESS
PHARMA INC. & SUBSIDIARIES
_________,
________
CONSOLIDATED
FINANCIAL STATEMENTS
For the Three Months Ended
March 31, 2014
Table of Contents
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
|
|
March 31, 2014
|
|
|
December 31, 2013
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
62,559
|
|
|
$
|
5,061
|
|
|
Accounts receivable
|
|
|
6,683
|
|
|
|
2,400
|
|
|
Inventory
|
|
|
24,342
|
|
|
|
20,525
|
|
|
Prepaid expenses and other
|
|
|
90
|
|
|
|
100
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL CURRENT ASSETS
|
|
|
93,674
|
|
|
|
28,086
|
|
|
|
|
|
|
|
|
|
|
|
|
INTANGIBLE ASSETS
|
|
|
264,038
|
|
|
|
250,529
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
$
|
357,712
|
|
|
$
|
278,615
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
|
419,578
|
|
|
|
387,105
|
|
|
Notes payable – related party
|
|
|
215,602
|
|
|
|
309,171
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL CURRENT LIABILITIES
|
|
|
635,180
|
|
|
|
696,276
|
|
|
|
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES
|
|
|
|
|
|
|
|
|
|
Note Payable – Related Party
|
|
|
613,646
|
|
|
|
505,120
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LONG-TERM LIABILITIES
|
|
|
613,646
|
|
|
|
505,120
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
|
1,248,826
|
|
|
|
1,201,396
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY
|
|
|
|
|
|
|
|
|
|
Preferred stock $0.0001 par value: 20,000,000 Authorized shares: 20,000,0000 shares Issued and outstanding
|
|
|
2,000
|
|
|
|
2,000
|
|
|
Common Stock, $0.0001 par value: 100,000,000Authorized shares: 49,588,314 and 50,734,863 Issued and outstanding shares, respectively
|
|
|
4,959
|
|
|
|
5,074
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
17,969,679
|
|
|
|
17,733,526
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive Income (loss)
|
|
|
92,774
|
|
|
|
44,694
|
|
|
Retained earnings
|
|
|
(18,960,526
|
)
|
|
|
(18,708,075
|
)
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL STOCKHOLDERS’ EQUITY
|
|
|
(891,114
|
)
|
|
|
(922,781
|
)
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
$
|
357,712
|
|
|
$
|
278,615
|
|
The accompanying notes are an integral
part of these consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
|
|
|
Three Months Ended March 31,
|
|
|
|
|
2014
|
|
|
2013
|
|
|
SALES
|
|
|
4,993
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF SALES
|
|
|
3,495
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT
|
|
|
1,498
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
GENERAL & ADMINISTRATIVE EXPENSES
|
|
|
|
|
|
|
|
|
|
Professional fees
|
|
|
16,750
|
|
|
|
12,108
|
|
|
Office
|
|
|
23,510
|
|
|
|
7,233
|
|
|
Stock compensation expense
|
|
|
31,038
|
|
|
|
-
|
|
|
Management fees
|
|
|
13,614
|
|
|
|
16,821
|
|
|
Consulting
|
|
|
30,000
|
|
|
|
-
|
|
|
Royalty
|
|
|
23,274
|
|
|
|
-
|
|
|
Advertising
|
|
|
13,273
|
|
|
|
5,224
|
|
|
Travel and entertainment
|
|
|
13,968
|
|
|
|
7,802
|
|
|
Other
|
|
|
68,161
|
|
|
|
-
|
|
|
|
|
|
(233,588
|
)
|
|
|
49,188
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS FORM OPERATIONS
|
|
|
(232,090
|
)
|
|
|
(49,188
|
)
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME/(EXPENSE)
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(20,361
|
)
|
|
|
(2,977
|
)
|
|
TOTAL OTHER ITEMS
|
|
|
(20,361
|
)
|
|
|
(2,977
|
)
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS BEFORE INCOME TAXES
|
|
|
(252,451
|
)
|
|
|
(52,165
|
)
|
|
|
|
|
|
|
|
|
|
|
|
INCOME TAX EXPENSE
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
|
(252,451
|
)
|
|
|
(52,165
|
)
|
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME
|
|
|
48,080
|
|
|
|
9,217
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS & COMPREHENSIVE INCOME
|
|
$
|
(204,371
|
)
|
|
$
|
(42,948
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Basic & Diluted earnings per share
|
|
$
|
(0.00
|
)
|
|
$
|
(0.00
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average basis & diluted shares outstanding
|
|
|
50,589,456
|
|
|
|
50,734,863
|
|
The accompanying notes are an integral
part of these consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’
EQUITY & COMPREHENSIVE INCOME (Unaudited)
|
|
|
Preferred
Shares
|
|
|
Amount
|
|
|
Common
Shares
|
|
|
Amount
|
|
|
Additional
Paid-In
Capital
|
|
|
Retained
Earnings
|
|
|
Other
Comprehensive
Income
|
|
|
Total
|
|
|
Balance, December 31, 2011
|
|
|
20,000,000
|
|
|
$
|
2,000
|
|
|
|
109,688
|
|
|
$
|
11
|
|
|
$
|
13,089
|
|
|
$
|
(511,077
|
)
|
|
$
|
|
|
|
$
|
(495,977
|
)
|
|
Shares issued for cash
|
|
|
-
|
|
|
|
-
|
|
|
|
750,000
|
|
|
|
75
|
|
|
|
74,925
|
|
|
|
-
|
|
|
|
-
|
|
|
|
75,000
|
|
|
Shares issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
13,750,175
|
|
|
|
1,375
|
|
|
|
10,998,625
|
|
|
|
-
|
|
|
|
-
|
|
|
|
11,000,000
|
|
|
Shares issued as collateral on licensing agreement
|
|
|
-
|
|
|
|
-
|
|
|
|
25,000,000
|
|
|
|
2,500
|
|
|
|
(2,500
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Related party debt converted to equity
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
50,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
50,000
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(11,263,601
|
)
|
|
|
-
|
|
|
|
(11,263,601
|
)
|
|
Foreign currency Translation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,914
|
)
|
|
|
(1,914
|
)
|
|
Balance, December 31, 2012
|
|
|
20,000,000
|
|
|
|
2,000
|
|
|
|
39,609,863
|
|
|
|
3,961
|
|
|
|
11,134,139
|
|
|
|
(11,774,678
|
)
|
|
|
(1,914
|
)
|
|
|
(636,492
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued for cash
|
|
|
-
|
|
|
|
-
|
|
|
|
400,000
|
|
|
|
40
|
|
|
|
69,960
|
|
|
|
-
|
|
|
|
-
|
|
|
|
70,000
|
|
|
Shares issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
275,000
|
|
|
|
28
|
|
|
|
234,472
|
|
|
|
-
|
|
|
|
-
|
|
|
|
234,500
|
|
|
Related party debt converted to equity
|
|
|
-
|
|
|
|
-
|
|
|
|
2,000,000
|
|
|
|
200
|
|
|
|
1,599,800
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,600,000
|
|
|
Debt converted to equity
|
|
|
-
|
|
|
|
-
|
|
|
|
2,000,000
|
|
|
|
200
|
|
|
|
1,599,800
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,600,000
|
|
|
Record shares issued Acquisition
|
|
|
-
|
|
|
|
-
|
|
|
|
6,450,000
|
|
|
|
645
|
|
|
|
3,095,355
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,096,000
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(6,933,397
|
)
|
|
|
-
|
|
|
|
(6,933,397
|
)
|
|
Foreign currency Translation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
46,608
|
|
|
|
46,608
|
|
|
Balance, December 31, 2013
|
|
|
20,000,000
|
|
|
$
|
2,000
|
|
|
|
50,734,863
|
|
|
$
|
5,074
|
|
|
$
|
17,733,526
|
|
|
$
|
(18,708,075
|
)
|
|
$
|
44,694
|
|
|
$
|
(922,781
|
)
|
|
Shares issued for cash
|
|
|
-
|
|
|
|
-
|
|
|
|
1,850,000
|
|
|
|
185
|
|
|
|
174,815
|
|
|
|
-
|
|
|
|
-
|
|
|
|
175,000
|
|
|
Warrant expense
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
31,038
|
|
|
|
-
|
|
|
|
-
|
|
|
|
31,038
|
|
|
Cancellation of shares
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,096,549
|
)
|
|
|
(310
|
)
|
|
|
310
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Shares issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
100,000
|
|
|
|
10
|
|
|
|
29,990
|
|
|
|
-
|
|
|
|
-
|
|
|
|
30,000
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(252,451
|
)
|
|
|
-
|
|
|
|
(252,451
|
)
|
|
Foreign Currency Translation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
48,080
|
|
|
|
48,080
|
|
|
Balance, March 31, 2014
|
|
|
20,000,000
|
|
|
|
2,000
|
|
|
|
49,588,314
|
|
|
|
4,959
|
|
|
|
17,969,679
|
|
|
|
(18,960,526
|
)
|
|
|
92,774
|
|
|
|
(891,114
|
)
|
The accompanying notes are an integral
part of these unaudited consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
For the Three Months Ended March 31,
|
|
|
|
|
2014
|
|
|
2013
|
|
|
OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(252,451
|
)
|
|
$
|
(52,165
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|
|
|
|
|
|
|
Stock issued for services
|
|
|
30,000
|
|
|
|
-
|
|
|
Stock compensation expense
|
|
|
31,038
|
|
|
|
-
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(4,283
|
)
|
|
|
-
|
|
|
Inventory
|
|
|
(3,187
|
)
|
|
|
-
|
|
|
Prepaid expenses and other assets
|
|
|
10
|
|
|
|
242
|
|
|
Accounts payable & accrued liabilities
|
|
|
14,277
|
|
|
|
97,759
|
|
|
Net cash provided by operating activities
|
|
|
(184,596
|
)
|
|
|
45,836
|
|
|
|
|
|
|
|
|
|
|
|
|
FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Issuance of common stock
|
|
|
175,000
|
|
|
|
-
|
|
|
Proceeds from note payable – related party
|
|
|
19,014
|
|
|
|
|
|
|
Payments on notes payable – related party
|
|
|
-
|
|
|
|
(58,895
|
)
|
|
Net cash (used) by financing activities
|
|
|
194,014
|
|
|
|
(58,895
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Effect of foreign currency translation
|
|
|
48,080
|
|
|
|
13,059
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH
|
|
|
57,498
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH
|
|
|
|
|
|
|
|
|
|
Beginning of year
|
|
|
5,061
|
|
|
|
-
|
|
|
End of year
|
|
$
|
62,559
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Interest paid
|
|
$
|
-
|
|
|
$
|
-
|
|
The accompanying notes are an integral
part of these consolidated financial statements.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
(Unaudited)
March 31, 2014
NOTE 1—THE COMPANY AND BASIS OF PRESENTATION
Nature of operations
Axxess Pharma, Inc. Was incorporated in the state of Delaware
on April 7, 1997 as CGI Communications Services. Inc. On July 26, 2008, the Company amended its certificate of incorporation to
change its name to Axxesss Pharma, Inc. On December 6, 2012 the Company reincorporated in Nevada by merging into a newly formed
Nevada entity with the name Axxess Pharma, Inc. The Company now operates as Axxess Pharma, Inc. with Axxess Pharma Canada, Inc.
and Allstar Health Brands Inc.as its wholly owned subsidiaries.
Axxess Pharma Canada, Inc. was incorporated under the Laws of
the Province of Ontario. The Company is engaged in the acquisition of Drug Identification Numbers and the eventual sale of the
related products. All Star Health Brands Inc. was incorporated on October 1, 2013 under the Laws of the Province of Ontario. The
Company is engaged in the acquisition of Drug Identification Numbers and the eventual sale of the related products
The consolidated financial statements included the results of
Axxess Pharma Inc. Axxess Pharma Canada and its wholly owned subsidiary Allstar Health Brands Inc. All intercompany accounts have
been eliminated during consolidation.
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of financial statements in conformity with accounting
principles generally accepted in the United States of America requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial
statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those
estimates.
The Company’s most significant areas of estimation and
assumption are:
|
|
•
|
determination of the appropriate amount and timing of markdowns to clear unproductive or slow-moving retail inventory and overall
inventory obsolescence
|
|
|
•
|
estimation of future cash flows used to assess the recoverability of long-lived assets
|
Cash and Cash Equivalents
All short-term investments with original maturities of three
months or less at date of purchase are considered cash equivalents.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company
to concentrations of credit risk consist of cash and accounts receivable. The Company maintains their cash in bank deposit accounts
at high credit quality financial institutions. The balances, at times, may exceed federally insured limits. The Company also maintains
a certain amount of cash on hand at the retail store locations. The Company has not experienced any significant losses with respect
to its cash. At both March 31, 2014 and December 31, 2013, the Company did not exceed the insured limit of a depository institution.
Accounts receivable
The Company considers the entire accounts receivable to be fully
collectible. The accounts receivable consist of trade receivable to wholesale customer. Management believes all receivables are
fully collectible and therefore no allowance for bad debt has been recorded as of March 31, 2014 and December 31, 2013.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
(Unaudited)
March 31, 2014
Inventory
Inventory consists of finished product acquired for resale and
is valued at the lower-of-cost-or-market with cost determined on a specific item basis.
Fair value of financial instruments
The carrying amounts of certain of the Company’s financial
instruments, including cash, accounts receivable, accounts payable and accrued liabilities approximate fair value due to their
relatively short maturities.
Foreign Currency Transactions
The Company's functional currency is the
Canadian dollar and the reporting currency is the U.S. dollar. Assets and liabilities are translated from the functional to the
reporting currency at the exchange rate in effect at the balance sheet date and equity at the historical exchange rates. Revenue
and expenses are translated at rates in effect at the time of the transactions. Resulting translation gains and losses are accumulated
in a separate component of stockholders' equity - other comprehensive income (loss). Realized foreign currency transaction gains
and losses are credited or charged directly to operations.
Intangible Assets
Licensing as it relates to Drug Identification Numbers, is accounted
for as an indefinite life intangible asset until the product is abandoned at which point the intangible asset will be written off.
Other intangible assets consist of websites, trademarks, domain
names etc. and are amortized over estimated useful lives.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including property and
equipment, for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable
based on undiscounted cash flows. If long-lived assets are impaired, an impairment loss is recognized and is measured as the amount
by which the carrying value exceeds the estimated fair value of the assets. The estimation of future undiscounted cash flows from
operating activities requires significant estimates of factors that include future sales growth and gross margin performance. Management
believes they have appropriately determined future cash flows and operating performance; however, should actual results differ
from those expected, additional impairment may be required.
Cost of sales
Cost of sales of approximately $3,495 and $0 for the three months
ended March 31, 2014 and 2013, respectively, consist primarily of merchandise costs of sales.
Revenue recognition
The Company derives revenues from sale of merchandise and upon
the following: (1) there is persuasive evidence that an arrangement exists; (2) delivery has occurred or services have been rendered,
(3) the seller's price to the buyer is fixed or determinable, and (4) collectability is reasonably assured.
Advertising / Marketing
Advertising costs are charged to expense when incurred. The
Company’s advertising method is primarily print, web based and word of mouth. Advertising / marketing costs were approximately
$13,273 and $5,224 for the three months ended March 31, 2014 and 2013, respectively.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
(Unaudited)
March 31, 2014
Comprehensive Income
ASC Topic 220, "Comprehensive Income",
establishes standards for reporting and display of comprehensive income and its components in a full set of general-purpose financial
statements. Comprehensive Income consists of foreign currency translation.
NOTE 3 – GOING CONCERN
The accompanying financial statements have
been prepared in conformity with generally accepted accounting principle, which contemplate continuation of the Company as a going
concern. The Company currently has limited working capital, and has not completed its efforts to establish a stabilized source
of revenues sufficient to cover operating costs over an extended period of time.
Management anticipates that the Company
will be dependent, for the near future, on additional investment capital to fund operating expenses The Company intends to position
itself so that it may be able to raise additional funds through the capital markets. In light of management’s efforts, there
are no assurances that the Company will be successful in this or any of its endeavors or become financially viable and continue
as a going concern.
NOTE 4—NOTES PAYABLE –RELATED PARTY
Loan from stockholders are non interest bearing and due on demand.
On October 25, 2010, the Company entered into a note payable
with Ramos and Ramos Investments Inc. The note bears interest at a 7% per annum interest rate. The note matures December 31, 2014.
The holder of the note has the right to convert the full or any portion of the principal and accrued but unpaid interest into shares
of common stock.
The Company entered into a note payable with maximum lending
amount of $500,000 with Ramos and Ramos Investments Inc. a maximum of lending amount of $500,000 could be advanced within the first
six months of the note. The note bears interest at a 12% per annum interest rate. The note cannot be paid off in full before January
1, 2015 or the completion of a public offering by the Company. In the event of default of the repayment, the note shall automatically
be converted to shares of the Company’s common stock at the price of $0.001 per share. The holder of the note has the right
to convert the full or any portion of the principal and accrued but unpaid interest into shares of common stock.
The Company entered into a note payable with Canadian Heritage
Ltd. The loan is not interest bearing and cannot be called prior to January 1, 2015.
On October 3, 2013 the Company entered into a $200,000 note
payable with RAMM Venture Developments Inc. The note bears interest at 12% annually. The earliest the note can become due is December
31, 2014 if a private placement funds are received at which time the note repayment can be accelerated. In the event of default
of the repayment, the note shall automatically be converted to shares of the Company’s common stock at the price of $0.001
per share.
NOTE 5 – EQUITY
At December 31, 2013, the Company’s authorized stock consists
of 20,000,000 shares of $.0001 par value preferred stock and 100,000,000 shares of $.0001 par value common stock. The preferred
stock has no voting rights. The common stock has voting rights and entitle to one vote per share.
Of the 20,000,000 shares of preferred stock 20,000,000 shares
are issued and outstanding as of March 31, 2014 and December 31, 2013.
The following common stock transactions occurred during the
period:
On September 1, 2012 the Company issued 500,000 shares valued
@ $0.80 per share for consulting services
On April 30, 2012 the Company issued 5,000,000 shares valued
@ $0.80 per share for consulting service to its Chief Executive Officer.
On September 11, 2012 the Company issued 2,000,000 shares valued
@ $0.80 per share for consulting services.
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
(Unaudited)
March 31, 2014
On January 1, 2012 the Company issued 2,500,000 shares valued
@ $0.80 per share for consulting services.
On June 11, 2012 the Company issued 750,000 shares valued @
$0.10 per share for cash.
On June 1, 2012 the Company issued 200,000 shares valued @ $0.80
per share for consulting services.
On May 15, 2012 the Company issued 500,000 shares valued @ $0.80
per share for consulting services
On June 11, 2012 the Company issued 2,000,000 shares valued
@ $0.80 per share for consulting services
On June 11, 2012 the Company issued 1,000,000 shares valued
@ $0.80 per share for consulting services.
On September 1, 2012 the Company issued 50,000 shares valued
@ $0.80 per share for consulting services.
On July 11, 2013 the Company issued 250,000 shares valued @
$0.85 per share for consulting services.
On July 11, 2013 the Company issued 200,000 shares valued @
$0.125 per share for cash.
On August 8, 2013 the Company issued 100,000 shares valued @
$0.20 per share for cash.
On May 2, 2013 the Company issued 4,000,000 shares valued @
$0.80 per share to convert $4,000 of principal on loan from related party to equity.
On May 8, 2013 the Company issued 1,000,000 shares valued @
$0.25 per share for cash.
On August 15, 2013 the Company issued 25,000 shares valued @
$0.88 per share for consulting services.
On September 13, 2013 the Company issued 6,450,000 shares valued
@ $0.48 in relation to asset acquisition. See note 5
In January 2014 the Company issued 1,000,000 valued @ $0.10
in relation to distribution agreement.
On January 22, 2014 the Company issued 850,000 shares valued
@ $0.10 per share for cash.
On February 22, 2014 the Company issued 100,000 shares valued
@ $0.30 per share for consulting services.
The company cancelled 3,096,549 shares
in relation to an amendment related to revive acquisition.
NOTE 7 – WARRANTS
On January 22, 2014 the company issued warrants to purchase
350,000 shares of common stock at an exercise price of $0.50 per share and vested immediately. The fair value of the warrant was
determined to be $0.08 using Black Scholes analysis assuming risk free interest rate of 3.49%,expected volatility of 97%, expected
term 24 months, and $0 expected dividends . The warrants can be exercise over the next twenty four months from the grant date.
NOTE 8— CONSULTING AGREEMENT
On February 27, 2014 the Company entered into a consulting agreement
with SG Business development will perform consulting services related to distribution channels regarding the Company’s nutritional
supplement product lines
AXXESS PHARMA, INC. & SUBSIDIARIES
Notes to Consolidated Financial Statements
(Unaudited)
March 31, 2014
NOTE 9— SUBSEQUENT EVENTS
Other than event mentioned above we evaluated all events or
transactions that occurred after March 31, 2014 up through the date we issued these financial statements. During this period we
did not have any other material subsequent events that impacted our financial statements.
PART II — INFORMATION NOT REQUIRED
IN THE PROSPECTUS
Item. 13 Other Expenses Of Issuance And Distribution.
|
Securities and Exchange Commission registration fee
|
|
$
|
500.21
|
|
|
Transfer Agent Fees
|
|
$
|
0
|
|
|
Accounting fees and expenses
|
|
$
|
0
|
|
|
Legal fees and expense
|
|
$
|
25,000
|
|
|
Blue Sky fees and expenses
|
|
$
|
0
|
|
|
Miscellaneous
|
|
$
|
0
|
|
|
Total
|
|
$
|
25,500.21
|
|
All amounts are estimates other than the
SEC’s registration fee. Beaufort is paying all of the registration expenses incurred in connection with the registration
of the shares except for accounting fees and expenses. No portion of these expenses will be borne by the selling stockholders.
The selling stockholders, however, will pay any other expenses incurred in selling their common stock, including any brokerage
commissions or costs of sale.
Item 14. Indemnification of Directors
and Officers.
Articles of Incorporation
The Company’s Articles of Incorporation
do not address the indemnification or insurance of controlling persons, directors or officers against liability in their capacity
as such.
Bylaws
The Company’s Bylaws provide as follows
with respect to the indemnification and insurance of controlling persons, directors or officers against liability in their capacity
as such.
The Company must indemnify any person made
a party to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative
(“Proceeding”) by reason of the fact that he is or was a director, against judgments, penalties, fines, settlements
and reasonable expenses (including attorney’s fees) (“Expenses”) actually incurred by him in connection with
such Proceeding if:(a) he conducted himself in good faith, and: (i) in the case of conduct in his own official capacity with
the Company, he reasonably believed his conduct to be in the Company’s best interests, or (ii) in all other cases, he
reasonably believes his conduct to be at least not opposed to the Company’s best interests; and (b) in the case of any criminal
Proceeding, he had no reasonable cause to believe his conduct was unlawful.
The Company must indemnify any person made
a party to any Proceeding by or in the right of the Company, by reason of the fact that he is or was a director, against reasonable
expenses actually incurred by him in connection with such proceeding if he conducted himself in good faith, and: (a) in the case
of conduct in his official capacity with the Company, he reasonably believed his conduct to be in its best interests; or (b) in
all other cases, he reasonably believed his conduct to be at least not opposed to its best interests; provided that no such indemnification
may be made in respect of any proceeding in which such person shall have been adjudged to be liable to the Company.
A director will not be indemnified in respect
to any Proceeding charging improper personal benefit to him, whether or not involving action in his official capacity, in which
he shall have been adjudged to be liable on the basis that personal benefit was improperly received by him. No indemnity
will indemnify any director from or on account of acts or omissions of such director finally adjudged to be intentional misconduct
or a knowing violation of law, or from or on account of conduct of such director finally adjudged to be in violation of, from or
on account of any transaction with respect to which it was finally adjudged that such director personally received a benefit in
money, property, or services to which the director was not legally entitled.
No indemnification will be made by unless
authorized in the specific case after a determination that indemnification of the director is permissible in the circumstances
because he has met the applicable standard of conduct.
Reasonable expenses incurred by a director
who is party to a proceeding may be paid or reimbursed by the Company in advance of the final disposition of such Proceeding in
certain cases.
The Company has the power to purchase and
maintain insurance on behalf of any person who is or was a director, officer, employee, or agent of the Company or is or was serving
at the request of the Company as an officer, employee or agent of another corporation, partnership, joint venture, trust, other
enterprise, or employee benefit plan against any liability asserted against him and incurred by him in any such capacity or arising
out of his status as such, whether or not the Company would have the power to indemnify him against such liability under the provisions
of the Bylaws.
Nevada Law
Nevada law provides as follows with respect
to the indemnification and insurance of controlling persons, directors or officers against liability in their capacity as such.
Indemnification. Pursuant to
NRS 78.7502 (Discretionary and mandatory indemnification of officers, directors, employees and agents: General provisions), a corporation
may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action,
suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation,
by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving
at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture,
trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually
and reasonably incurred by the person in connection with the action, suit or proceeding if the person: (a) is not liable pursuant
to Nevada Revised Statutes 79.138 (breach of good faith); or (b) acted in good faith and in a manner which he or she reasonably
believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding,
had no reasonable cause to believe the conduct was unlawful. The termination of any action, suit or proceeding by judgment,
order, settlement, conviction or upon a plea of nolo contendere or its equivalent, does not, of itself, create a presumption that
the person is liable pursuant to Nevada Revised Statutes 79.138 or did not act in good faith and in a manner which he or she reasonably
believed to be in or not opposed to the best interests of the corporation, or that, with respect to any criminal action or proceeding,
he or she had reasonable cause to believe that the conduct was unlawful.
A corporation may also indemnify any person
who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right
of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee
or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of
another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement
and attorneys’ fees actually and reasonably incurred by the person in connection with the defense or settlement of the action
or suit if the person: (a) is not liable pursuant to Nevada Revised Statutes 79.138; or (b) acted in good faith and in a manner
which he or she reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be
made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion
of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only
to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application
that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses
as the court deems proper.
To the extent that a director, officer,
employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding
referred to above, or in defense of any claim, issue or matter therein, the corporation must indemnify him or her against expenses,
including attorneys’ fees, actually and reasonably incurred by him or her in connection with the defense.
Insurance. Pursuant to NRS 78.752
(Insurance and other financial arrangements against liability of directors, officers, employees and agents), a corporation may
purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer,
employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or
agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against the person
and liability and expenses incurred by the person in his or her capacity as a director, officer, employee or agent, or arising
out of his or her status as such, whether or not the corporation has the authority to indemnify such a person against such liability
and expenses. No such financial arrangement may provide protection for a person adjudged by a court of competent jurisdiction,
after exhaustion of all appeals therefrom, to be liable for intentional misconduct, fraud or a knowing violation of law, except
with respect to the advancement of expenses or indemnification ordered by a court.
The SEC’s Position on Indemnification
for Securities Act Liabilities
Insofar as indemnification for liabilities
arising under the Securities Act of 1933, as amended, may be permitted to the Company’s directors, officers or controlling
persons, the Company has been advised that in the opinion of the Commission this indemnification is against public policy as expressed
in the Securities Act of 1933, as amended, and is, therefore, unenforceable.
Item 15. Recent Sales of Unregistered
Securities.
Common Stock
On October 29, 2012, the Company issued 25,000,000
shares to Blue Ivory International Holdings, Ltd. in connection with an agreement for the licensing of Drug Identification Numbers.
On January 1, 2012 the Company issued 2,500,000
shares valued at $0.80 per share for consulting services.
On April 30, 2012 the Company issued 5,000,000
shares valued at $0.80 per share for consulting service to its Chief Executive Officer.
On May 15, 2012 the Company issued 500,000
shares valued at $0.80 per share for consulting services.
On June 11, 2012 the Company issued 750,000
shares valued at $0.10 per share for cash.
On June 1, 2012 the Company issued 200,000
shares valued at $0.80 per share for consulting services.
On June 11, 2012 the Company issued 2,000,000
shares valued at $0.80 per share for consulting services.
On June 11, 2012 the Company issued 1,000,000
shares valued at $0.80 per share for consulting services.
On September 1, 2012 the Company issued
50,000 shares valued at $0.80 per share for consulting services.
On September 1, 2012 the Company issued
500,000 shares valued at $0.80 per share for consulting services.
On September 11, 2012 the Company issued
2,000,000 shares valued at $0.80 per share for consulting services.
On May 2, 2013 the Company issued 4,000,000
shares valued at $0.80 per share to convert $4,000 of principal on loan from related party to equity.
On May 8, 2013 the Company issued 1000,000
shares valued at $0.25 per share for cash.
On July 11, 2013 the Company issued 250,000
shares valued at $0.85 per share for consulting services.
On July 11, 2013 the Company issued 200,000
shares valued at $0.125 per share for cash.
On August 8, 2013 the Company issued 100,000
shares valued at $0.20 per share for cash.
On August 15, 2013 the Company issued 25,000
shares valued at $0.88 per share for consulting services.
On September 13, 2013 the Company issued
6,450,000 shares valued at $0.48 to Revive shareholders in relation to asset acquisition.
On January 22, 2014, the Company issued
750,000 shares valued at $0.10 pursuant to certain stock purchase agreement to Equity Portfolio Funding, Inc., and a half warrant
to purchase further common stock of the Company for up to two years at $0.50 per share.
On May 19, 2014, the Company entered into
a Securities Purchase Agreement with Seaside 88, LP, a Florida limited partnership, or Seaside, pursuant to which the Company will
issue and sell to Seaside up to 5,000,000 shares of its common stock. The Company issued 584,350 shares at $0.15 per share on May
20, 2014, 917,300 shares at $0.15 per share on June 20, 2014, and 840,520 shares at $0.1195 per share on July 21, respectively.
Preferred Stock
On October 29, 2012, the Company issued 20,000,000
shares of preferred stock to Blue Ivory International Holdings, Inc.
Note
On October 25, 2010, the Company entered
into a note payable with Ramos and Ramos Investments Inc. The note bears interest at a 7% per annum interest rate. The note matures
December 31, 2014. The holder of the note has the right to convert the full or any portion of the principal and accrued but unpaid
interest into shares of common stock. Interest expense for the years ended December 31, 2013 and 2012 was $7,000 and $7,000, respectively.
The Company entered into a note payable
with maximum lending amount of $500,000 with Ramos and Ramos Investments Inc. a maximum of lending amount of $500,000 could be
advanced within the first six months of the note. The note bears interest at a 12% per annum interest rate. The note cannot be
paid off in full before January 1, 2015 or the completion of a public offering by the Company. In the event of default of the repayment,
the note shall automatically be converted to shares of the Company’s common stock at the price of $0.001 per share. The holder
of the note has the right to convert the full or any portion of the principal and accrued but unpaid interest into shares of common
stock. Interest expense for the years ended December 31, 2013 and 2012 was $45,311 and $34,792, respectively.
The Company entered into a note payable
with Canadian Heritage Ltd. The loan is not interest bearing and cannot be called prior to January 1, 2015.
On October 3, 2013 the Company entered into a $200,000 note
payable with RAMM Venture Developments Inc. The note bears interest at 12% annually. The earliest the note can become due is December
31, 2014 if a private placement funds are received at which time the note repayment can be accelerated. In the event of default
of the repayment, the note shall automatically be converted to shares of the Company’s common stock at the price of $0.001
per share.
The above securities were issued pursuant
to the registration exemption afforded the Company under Section 4(2) of the Securities Act of 1933, as amended.
Item 16 Exhibits and Financial Statement Schedules.
Exhibit
No.
|
|
Description
|
|
3.1
|
|
Articles of Incorporation
|
|
3.2
|
|
Bylaws
|
|
5.1
|
|
Opinion of Szaferman, Lakind, Blumstein & Blader, P.C.*
|
|
10.1
|
|
Asset Purchase Agreement with Revive Bioscience Inc. dated September 13, 2013
|
|
10.2
|
|
Amendment to the Asset Purchase Agreement with Revive Bioscience Inc. dated January 30, 2014
|
|
10.3
|
|
Consulting Agreement with Peter Daniel Bagi dated April 30, 2012
|
|
10.4
|
|
Distribution Agreement with Hard Core Beverages LLC dated March 18, 2014
|
|
10.5
|
|
Service Agreement with Great Lakes Fulfillment Services dated October 23, 2013
|
|
10.6
|
|
Sponsorship Agreement with Wide-Open Sports Marketing, Inc. dated June 11, 2014
|
|
10.7
|
|
Manufacturing Agreement with Private Label Nutraceuticals, Inc. dated May 1, 2014
|
|
10.8
|
|
Sales Management and Service Agreement with Nutritional Products International, Inc. dated January 16, 2014
|
|
10.9
|
|
Sales Representation Agreement with Acosta Military Sales, LLC dated February 13, 2014
|
|
10.10
|
|
Securities Purchase Agreement with Seaside 88, LP dated May 19, 2014
|
|
10.11
|
|
Investment Agreement with Beaufort Capital Partners LLC dated June 9, 2014
|
|
10.12
|
|
Registration Rights Agreement with Beaufort Capital Partners LLC dated June 9, 2014
|
|
10.13
|
|
Secured Promissory Note issued to Beaufort Capital Partners LLC dated June 9, 2014
|
|
10.14
|
|
Stock Pledge Agreement with Beaufort Capital Partners LLC dated June 9, 2014
|
|
10.15
|
|
Escrow Agreement with Beaufort Capital Partners LLC dated June 9, 2014
|
|
23.1
|
|
Consent of KLJ & Associates, LLP
|
|
23.2
|
|
Consent of Szaferman, Lakind, Blumstein & Blader, P.C. (filed as Exhibit 5.1)
|
* To be filed by amendment.
Item 17. Undertakings.
The undersigned
registrant hereby undertakes:
(1) To file, during
any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i.
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii.
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high
end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b)
if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price
set forth in the “Calculation of Registration Fee” table in the effective registration statement.
iii.
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement;
(2) That, for
the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to
be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
(3) To remove
from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
(4)
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and
controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that
in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act
and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment
by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful
defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities
being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed
in the Act and will be governed by the final adjudication of such issue.
(5) Each prospectus
filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying
on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration
statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement
or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference
into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of
contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus
that was part of the registration statement or made in any such document immediately prior to such date of first use.
SIGNATURES
Pursuant to the
requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf
by the undersigned, thereunto duly authorized in Toronto, Canada, on July 25, 2014.
|
|
AXXESS PHARMA, INC.
|
|
|
|
|
|
|
By:
|
/s/ Peter Daniel Bagi, MD
|
|
|
|
Peter D. Bagi, MD
|
|
|
|
President and Director
|
KNOW ALL MEN BY
THESE PRESENTS, that each person whose signature appears below constitutes and appoints Peter D. Bagi, MD, his true and lawful
attorney-in-fact and agent, with full power of substitution and resubstitution, for him and in his name, place and stead, in any
and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, including
any related registration statement filed pursuant to Rules 413 or 462 under the Securities Act of 1933, as amended, and to file
the same, with all exhibits thereto, and all other documents in connection therewith, with the SEC, granting unto said attorney-in-fact
and agent full power and authority to do and perform each and every act in person, hereby ratifying and confirming all that said
attorney-in-fact and agent or his substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities
Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ Peter Daniel Bagi, MD
|
|
President, Chief Executive Officer,
|
|
July 25, 2014
|
|
Peter D. Bagi, MD
|
|
Chief Financial Officer and Director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allstar Health Brands (PK) (USOTC:ALST)
Historical Stock Chart
From Mar 2024 to Apr 2024
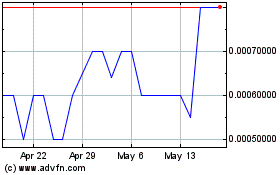
Allstar Health Brands (PK) (USOTC:ALST)
Historical Stock Chart
From Apr 2023 to Apr 2024