false
0001771910
00-0000000
0001771910
2024-01-04
2024-01-04
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of Earliest Event Reported):
January 4, 2024
ADC Therapeutics SA
(Exact Name of Registrant as Specified in Its Charter)
|
Switzerland
(State or Other Jurisdiction of Incorporation) |
001-39071
(Commission File Number) |
N/A
(IRS Employer Identification Number) |
|
Biopôle
Route de la Corniche 3B
1066 Epalinges
Switzerland
(Address of Principal Executive Offices) (Zip Code) |
+41 21 653 02 00
(Registrant’s Telephone Number) |
N/A
(Former Name or Former Address, if Changed Since
Last Report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2.
below):
| ☐ | Written communications pursuant to Rule 425 under the Securities
Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange
Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under
the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under
the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of Each Class |
Trading Symbol |
Name of Each Exchange on Which Registered |
| Common Shares, par value CHF 0.08 per share |
ADCT |
New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (17 C.F.R. §230.405) or Rule 12b-2 of the Securities Exchange Act of
1934 (17 C.F.R. §240.12b-2). Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act. ☐
Item 2.02. Results of Operations and Financial Condition.
Item 7.01. Regulation FD Disclosure.
On January 4, 2024, ADC Therapeutics SA (the “Company”)
issued a press release and made available a corporate presentation that include the preliminary ZYNLONTA net sales for the quarter ended
December 31, 2023 and the preliminary cash and cash equivalents as of December 31, 2023. A copy of the press release is attached as Exhibit
99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the corporate presentation is attached as Exhibit
99.1 to this Current Report on Form 8-K and incorporated by reference herein.
The ZYNLONTA net sales and cash and cash equivalents figures are preliminary
and unaudited and reflect the Company’s estimated financial results. In preparing this information, management made a number of
complex and subjective judgments and estimates about the appropriateness of certain reported amounts and disclosures. The Company’s
actual financial results for the quarter and year ended December 31, 2023 have not yet been finalized by management or audited or reviewed
by the Company’s independent auditors. The preliminary financial information is not a comprehensive statement of all financial results
for the quarter or year ended December 31, 2023. Subsequent information or events may lead to material differences between the foregoing
preliminary financial results and those reported in the Company’s subsequent SEC filings. Accordingly, investors should not place
undue reliance on these preliminary financial results.
The information contained in these Items and Exhibits 99.1 and 99.2
shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under
the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
ADC Therapeutics SA |
| Date: January 4, 2024 |
|
| |
By: |
/s/ Peter J. Graham |
| |
Name: |
Peter J. Graham |
| |
Title: |
Chief Legal Officer |
Exhibit 99.1

ADC Therapeutics Provides Business Updates
ZYNLONTA®1 4Q 2023 net sales expected
to be ~$16.5 million, a double-digit percentage increase as compared to 3Q 2023
LOTIS-7: Study of ZYNLONTA in combination with
bispecifics cleared first dosing cohort with no DLT and with early signs of efficacy
ADCT-601 (targeting AXL): Reached MTD and currently
in dose optimization; Early signs of antitumor activity in both monotherapy and in combination
Multiple data catalysts expected in 2024 and
with a cash runway now expected into 4Q 2025
Lausanne, Switzerland,
January 4, 2024 – ADC Therapeutics SA (NYSE: ADCT) today provided business updates.
“During 2023,
we took a number of decisive actions to help position the Company for success in 2024 and beyond. We prioritized our pipeline, strengthened
our organization and implemented a disciplined capital allocation model to generate cost efficiencies,” said Ameet Mallik, Chief
Executive Officer of ADC Therapeutics. “We believe we are starting to see signs of the commercial turnaround. We are also encouraged
to see positive initial signals in the LOTIS-7 trial of ZYNLONTA in combination with bispecifics as well as early signs of antitumor
activity in the Phase 1b trial of ADCT-601. We now expect our cash runway to extend into the fourth quarter of 2025 and believe we are
on a path to unlock the substantial value in the Company.”
Recent Highlights
and Developments
ZYNLONTA®
(loncastuximab tesirine-lpyl)
| • | ZYNLONTA
net sales for the fourth quarter of 2023 are expected to be approximately $16.5 million. |
| • | The
Phase 1 LOTIS-7 trial of ZYNLONTA in combination with bispecifics glofitamab or mosunetuzumab
for the treatment of patients with diffuse large B-cell lymphoma (DLBCL), follicular lymphoma
(FL) and marginal zone lymphoma (MZL) is actively enrolling patients. The dose-limiting toxicity
(DLT) period has been cleared for the first dosing level of ZYNLONTA 90 µg/kg in both
arms, and there have been no discontinuations due to adverse events (AEs). To date, each
of the first five patients eligible for assessment in this dosing level has shown a response
(partial response or complete response) at first scan. |
| • | An
oral presentation at the American Society of Hematology (ASH) 2023 Annual Meeting from the
University of Miami investigator-initiated trial exploring ZYNLONTA in combination with rituximab
in high-risk relapsed or refractory FL indicated a best overall response rate of 96.3% and
a complete response rate of 85.2% . After a median follow-up of 9.7 months, the
median |
| (1) | loncastuximab tesirine-lpyl;
DLT: Dose-Limiting Toxicity; MTD: Maximum-Tolerated Dose |
progression-free
survival (PFS) was not reached, and the 12-month PFS was 92.3% . The majority of AEs were grade 1. Grade 3 AEs included neutropenia
(n=2; 6.2%), and one case each (3.1%) of hyperglycemia, increased ALT, fatigue, dyspnea and skin infection. Neutropenia was the only
grade 4 AE (n=1; 3.1%).
Pipeline
| • | ADCT-601
(targeting AXL): In the Phase 1b trial, the maximum-tolerated dose has been reached,
and the study is currently in dose optimization. There have been early signs of antitumor
activity in both monotherapy and in combination. The dose-optimization/ expansion phase is
comprised of a monotherapy arm including patients with sarcoma, pancreatic cancer and AXL-expressing
non-small cell lung cancer (NSCLC) and a combination arm with gemcitabine in patients with
sarcoma and pancreatic cancer. |
| • | ADCT-901
(targeting KAAG1): The Company has decided to discontinue this program due to limited
signs of efficacy in the dose escalation phase and to reallocate capital to prioritized programs. |
| • | ADCT-602
(targeting CD22): Dose escalation and expansion in the Phase 1 trial in collaboration
with MD Anderson Cancer Center for patients with relapsed or refractory acute lymphoblastic
leukemia is progressing, and additional clinical trial sites are being added to accelerate
enrollment. |
| • | Early-stage
pipeline: The Company is advancing a portfolio of investigational ADCs including those
targeting Claudin-6, NaPi2b and PSMA. These candidates utilize exatecan with a novel hydrophilic
linker as a highly potent and differentiated payload. |
Balance Sheet
The Company ended
the fourth quarter of 2023 with cash and cash equivalents of ~$278.5 million.
Guidance
The Company expects
the following based on its current business plan:
| • | Decrease
in total operating expenses expected in full year 2023 and 2024 as compared to 2022 |
| • | Cash
runway expected into 4Q 20251 (previously: mid-2025) |
Expected Milestones
in 2024
ZYNLONTA
| • | Achieve
commercial brand profitability in 2024 |
| • | LOTIS-5:
Complete enrollment in 2024 |
| • | LOTIS-7:
Additional safety and efficacy data from the dose-escalation and dose-expansion portions
of the Phase 1 study in 2024 |
| (1) | Cash
runway assumes receipt of anticipated regulatory milestone payments under the Company’s
collaboration agreements and use of the amount it is required to maintain under its loan
agreement |
Pipeline
ADCT-601 (targeting
AXL)
| • | Additional
data updates from the Phase 1 study in patients with sarcoma, pancreatic cancer and NSCLC
in 2024 |
ADCT-602 (targeting
CD22)
| • | Additional
data from Phase 1 study in 2024 |
Please refer to
the Company’s Form 8-K and accompanying presentation filed with the Securities and Exchange Commission today for additional information.
About ZYNLONTA®
(loncastuximab tesirine-lpyl)
ZYNLONTA®
is a CD19-directed antibody drug conjugate (ADC). Once bound to a CD19-expressing cell, ZYNLONTA is internalized by the cell, where
enzymes release a pyrrolobenzodiazepine (PBD) payload. The potent payload binds to DNA minor groove with little distortion, remaining
less visible to DNA repair mechanisms. This ultimately results in cell cycle arrest and tumor cell death.
The U.S. Food and
Drug Administration (FDA) and the European Medicines Agency (EMA) have approved ZYNLONTA (loncastuximab tesirine-lpyl) for the treatment
of adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy, including diffuse
large B-cell lymphoma (DLBCL) not otherwise specified (NOS), DLBCL arising from low-grade lymphoma and also high-grade B-cell lymphoma.
The trial included a broad spectrum of heavily pre-treated patients (median three prior lines of therapy) with difficult-to-treat disease,
including patients who did not respond to first-line therapy, patients refractory to all prior lines of therapy, patients with double/triple
hit genetics and patients who had stem cell transplant and CAR-T therapy prior to their treatment with ZYNLONTA. This indication is approved
by the FDA under accelerated approval and in the European Union under conditional approval based on overall response rate and continued
approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. Please
see full prescribing information including important safety information about ZYNLONTA at www.ZYNLONTA.com.
ZYNLONTA is also
being evaluated as a therapeutic option in combination studies in other B-cell malignancies and earlier lines of therapy.
About ADC Therapeutics
ADC Therapeutics
(NYSE: ADCT) is a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs). The Company is advancing
its proprietary ADC technology to transform the treatment paradigm for patients with hematologic malignancies and solid tumors.
ADC Therapeutics’
CD19-directed ADC ZYNLONTA (loncastuximab tesirine-lpyl) received accelerated approval by the FDA and conditional approval from the European
Commission for the treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy. ZYNLONTA
is also in development in combination with other agents and in earlier lines of therapy. In addition to ZYNLONTA, ADC Therapeutics has
multiple ADCs in ongoing clinical and preclinical development.
ADC Therapeutics
is based in Lausanne (Biopôle), Switzerland and has operations in London, the San Francisco Bay Area and New Jersey. For more information,
please visit https://adctherapeutics.com/ and follow the Company on LinkedIn.
ZYNLONTA® is a registered
trademark of ADC Therapeutics SA.
Forward-Looking Statements
This
press release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. In some cases you can identify forward-looking statements by terminology such as “may”, “assumes”,
“will”, “should”, “would”, “expect”, “intend”, “plan”, “anticipate”,
“believe”, “estimate”, “predict”, “potential”, “seem”, “seek”,
“future”, “continue”, or “appear” or the negative of these terms or similar expressions, although
not all forward-looking statements contain these identifying words. Forward-looking statements are subject to certain risks and uncertainties
that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not
limited to: the actual Zynlonta revenue for 4Q 2023, the success of the Company’s updated corporate strategy including operating
efficiencies, capital deployment and portfolio prioritization; the Company’s ability to achieve the decrease in total operating
expenses for 2023 and 2024, the expected cash runway into 4Q 2025, the effectiveness of the new commercial go-to-market strategy, competition
from new technologies, and the Company’s ability to grow ZYNLONTA® revenue in the United States; Swedish Orphan
Biovitrum AB (Sobi®) ability to successfully commercialize ZYNLONTA® in the European Economic Area and
market acceptance, adequate reimbursement coverage, and future revenue from the same; approval by the NMPA of the BLA for ZYNLONTA®
in China submitted by Overland ADCT BioPharma and future revenue from the same, our strategic partners’, including Mitsubishi
Tanabe Pharma Corporation, ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions, and the timing
and amount of future revenue and payments to us from such partnerships; the impact, if any, from data reported by the University of Miami
for their IIT in FL; the timing and results of the Company’s or its partners’ clinical trials including LOTIS 5 and 7, ADCT
601 and 602 as well as the Company’s early-stage pipeline research projects, actions by the FDA or foreign regulatory authorities
with respect to the Company’s products or product candidates; projected revenue and expenses; the Company’s indebtedness,
including Healthcare Royalty Management and Oaktree and Blue Owl facilities, and the restrictions imposed on the Company’s activities
by such indebtedness, the ability to repay such indebtedness and the significant cash required to service such indebtedness; and the
Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities. Additional
information
concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements
is contained in the “Risk Factors” section of the Company's Annual Report on Form 20-F and in the Company's other periodic
reports and filings with the Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and
other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results,
performance, achievements or prospects expressed in or implied by such forward-looking statements. The Company cautions investors not
to place undue reliance on the forward-looking statements contained in this document. The Company undertakes no obligation to revise
or update these forward-looking statements to reflect events or circumstances after the date of this press release, except as required
by law.
CONTACTS:
Investors
Eugenia Litz
ADC Therapeutics
Eugenia.Litz@adctherapeutics.com
+44 7879 627205
+1 908-723-2350
Media
Nicole Riley
ADC Therapeutics
Nicole.Riley@adctherapeutics.com
+1 862-926-9040
Exhibit 99.2

Business Update January 4, 2024

2 Forward - Looking Statements This presentation and any accompanying oral presentation have been prepared by ADC Therapeutics SA ("ADC Therapeutics“, “we” or “us”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or ADC Therapeutics or any officer, director, employee, agent or advisor of ADC Therapeutics. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Information provided in this presentation an d a ny accompanying oral presentation speak only as of the date hereof. This presentation contains forward - looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. In some cases you can identify forward - looking statements by terminology such as “may”, “assumes”, “will”, “should”, “would”, “expect”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “seem”, “ see k”, “future”, “continue”, or “appear” or the negative of these terms or similar expressions, although not all forward - looking statements contain these identifying words. Forward - looking state ments are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the actual Zynlonta revenue for 4Q 2023 , the success of the Company’s updated corporate strategy including operating efficiencies, capital deployment and portfolio prioritization; the expected cash runway into the beginning of Q4 2025, the effectiveness of th e new commercial go - to - market strategy, competition from new technologies, the Company’s ability to grow ZYNLONTA® revenue in the United States; Swedish Orphan Biovitrum AB (Sobi®) ability to successfully commercialize ZYNLONTA® in the European Economic Area and market acceptance, adequate reim bu rsement coverage, and future revenue from the same; approval by the NMPA of the BLA for ZYNLONTA® in China submitted by Overland ADCT BioPharma an d future revenue from the same, our strategic partners’, including Mitsubishi Tanabe Pharma Corporation, ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions, and the timing and amount of futur e r evenue and payments to us from such partnerships; the timing and results of the Company’s or its partners’ research projects or clinical trials including early research in certain solid tumors with different targets, linke rs and payloads, LOTIS 5 and 7, ADCT 601 and 602 as well as IITs in FL and MZL, the timing and results of research the timing and outcome of regulatory submissions for the Company’s products or product candidates; actions by the FDA or fore ign regulatory authorities, the ability to complete clinical trials on expected timelines, if at all, and the results of the same; projected revenue and expenses; the Company’s indebtedness, including Healthcare Royalty Management and Blu e Owl and Oaktree facilities, and the restrictions imposed on the Company’s activities by such indebtedness, the ability to repay such indebtedness and the significant cash required to service such indebtedness; and the Com pany’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities. Additional information concerning these and other factors that may cause actual results to differ mate ria lly from those anticipated in the forward - looking statements is contained in the “Risk Factors” section of the Company's Annual Report on Form 20 - F and in the Company's other periodic and current reports and filings with the U.S. Secur ities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any f utu re results, performance, achievements or prospects expressed in or implied by such forward - looking statements. The Company cautions investors not to place undue reliance on the forward - looking statements contained in this docum ent. Forward - looking statements are based on our management’s beliefs and assumptions and on information currently available to our m anagement. No assurance can be given that such future results will be achieved. Such forward - looking statements contained in this presentation speak only as of the date of this presentation. The Company expressly discl aim any obligation or undertaking to update these forward - looking statements contained in this presentation to reflect any change in our expectations or any change in events, conditions, or circumstances on which such st ate ments are based unless required to do so by applicable law. No representations or warranties (expressed or implied) are made about the accuracy of any such forward - looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data d eri ved from third - party sources and our own internal estimates and research. While we believe these third - party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no represen tat ion as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this presentation involve a number of assumptions and limitati ons , and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, although we believe our own internal research is reliable, such research has not been verified by any independent source.

3 ADC Therapeutics at a Glance Solid Tumors Advancing ADCT - 601 targeting AXL in the clinic and multiple investigational ADCs Hematology Maximizing ZYNLONTA® in 3L+ DLBCL and expanding into earlier lines of DLBCL and indolent lymphomas; advancing ADCT - 602 targeting CD22 Corporate Cash runway into 4Q 2025 with multiple catalysts in 2024 Platform Pioneering ADC field with robust technology toolbox and specialized end - to - end capabilities ADC: Antibody Drug Conjugate; DLBCL: Diffuse Large B - Cell Lymphoma

4 Clinical Strong track record including an approved product with robust lifecycle management and multiple clinical assets Platform Validated and differentiated ADC platform with multiple payloads and targets Regulatory Proven capabilities securing FDA and EMA approvals for ZYNLONTA and multiple INDs Platform and Capabilities for Developing Optimized ADCs CMC: Chemistry, Manufacturing and Controls; PBD: Pyrrolobenzodiazepines Discovery Cutting - edge research to select the optimal targeting moiety, linker, and payload Development Swiftly moving from research to clinical trials with an optimized development strategy Manufacturing Robust in - house CMC capabilities enhanced by a top - tier external manufacturing network Commercialization Integrated go - to - market model with experienced team

5 2023: Positioning the Company for Success ▪ Management team with extensive commercial, development, and corporate expertise ▪ Board members with biotech and large pharma leadership experience; larger number of independents ▪ Established new go - to - market model with upgraded talent ▪ Refined brand positioning and enhanced data generation ▪ Hematology: ZYNLONTA Life Cycle Management, ADCT - 602 ▪ Solid tumors: ADCT - 601, multiple novel ADCs ▪ Advancing a range of payloads, linkers, and conjugation technologies against multiple targets ▪ Expanded internal capabilities to enable partnerships for early - stage assets ▪ Right - sized organizational structure; trimmed consulting and contractors ▪ Reduced 3rd party spending, optimized tax, and improved return on investments Upgraded Organization En hanced ZYNLONTA Commercialization Prioritized Portfolio Validated Research Platform Realized Cost Efficiencies

6 Key Business Updates DLT: Dose - Limiting Toxicity; MTD: Maximum Tolerated Dose. Note: Financials are unaudited. ▪ Balance sheet with ~$ 278.5M cash at end of FY 2023 ▪ Cash runway expected to extend into 4Q 2025 ; double - digit Opex reduction in 2023 compared to 2022 ▪ LOTIS - 7 (ZYNLONTA with bispecifics ): Cleared first dosing cohort with no DLT and early signs of efficacy ▪ LOTIS - 5 (ZYNLONTA with rituximab): Accelerated enrollment in 2023, over 2/3rd enrollment completed ▪ ADCT - 601 (Targeting AXL): R eached MTD and currently in dose optimization; e arly signs of anti - tumor activity in both monotherapy and in combination ▪ ADCT - 901 (Targeting KAAG1): Discontinuing due to limited signs of efficacy in dose escalation, reallocating capital to prioritized programs ▪ 4Q 2023 revenues expected to be ~$16.5M , a double - digit percentage increase compared to 3Q 2023 Pipeline Corporate ZYNLONTA (loncastuximab tesirine - lpyl)

7 Unlocking Value of Robust ADC Portfolio in Hematology and Solid Tumors DLBCL: Diffuse Large B - Cell Lymphoma; FL: Follicular Lymphoma; MZL: Marginal Zone Lymphoma; NSCLC: Non Small - Cell Lung Cancer; ALL: Acute Lymphoblastic Leukemia. Short - Mid Term Mid - long Term Hematology Portfolio Solid Tumor Portfolio ZYNLONTA ▪ Maximize ZYNLONTA in 3L+ DLBCL ▪ Seek to expand ZYNLONTA to earlier lines of DLBCL and other indolent lymphomas (e.g., FL, MZL) as single agent and combination agent of choice ‒ ZYNLONTA ‒ ZYNLONTA + rituximab (LOTIS - 5) ‒ ZYNLONTA + bispecifics (LOTIS - 7) ADCT - 602 (CD22) ▪ Escalating and expanding Phase 1 dose in r/r ALL ADCT - 601 (AXL) ▪ Optimizing dose for expansion as single agent and / or in combination in sarcoma, pancreatic, and NSCLC Next - generation ADCs ▪ Advancing a portfolio of investigational ADCs ‒ Differentiated exatecan - based payload with novel hydrophilic linker ‒ Targeting Claudin - 6, NaPi2b, PSMA ▪ Continuing research with a range of payloads, linkers, and conjugation technologies against undisclosed targets

8 Focused Pipeline in Hematology and Solid Tumors Anticipated milestones set forth in this chart are subject to further future adjustment. NTE: Non - Transplant Eligible. 1. DLBCL, FL, MZL 2. Non - selected advanced / metastatic NSCLC completed. Moving forward with AXL expressing NSCLC contingent on in - house IHC a ssay. AA: Accelerated Approval. Preclinical Phase 1a Phase 1b Phase 2 ZYNLONTA | Targeting CD19 ADCT - 602 | Targeting CD22 Expected Milestones LOTIS - 5: Complete enrollment in 2024 LOTIS - 7: Additional dose escalation/ expansion data in 2024 Phase 3 / Confirmatory Hematology Confirmatory LOTIS - 5 with rituximab in 2L+ NTE DLBCL LOTIS - 2 in 3L+ DLBCL NaPi2b Candidate selected FDA approved (AA) LOTIS - 7 in combination with glofitamab in r/r NHL 1 Solid Tumors Single agent and with gemcitabine in adv. / met. Sarcoma Mipasetamab uzoptirine ( ADCT - 601) | Targeting AXL Additional data from Phase 1 study in 2024 Acute Lymphoblastic Leukemia Additional data from Phase 1 study in 2024 Claudin - 6 PSMA Undisclosed target Candidate selection in 2024 Candidate selection in 2024 Candidate selection in 2024 Single agent and with gemcitabine and others in adv. / met. Pancreatic Single agent and other combinations in adv. / met. NSCLC 2 LOTIS - 7 in combination with mosunetuzumab in r/r NHL 1 Next - Gen ADCs | Exatecan Payload with Novel Linker

9 Proportion of patients by line - of - therapy* DLBCL, FL & MZL account for ~60% of mature B - cell lymphomas 1 * 1L (~70%) 1L (~65%) 1L (~61%) DLBCL FL MZL Key: Current Approval Current Development Areas Advancing ZYNLONTA Development in B - Cell Lymphomas 2L (~24%) 2L (~27%) 3L+ (~11%) 3L+ (~12%) 2023 U.S. Market Value 2 , 5 - year prevalence 3 ▪ LOTIS - 5 and LOTIS - 7 potential to move ZYNLONTA into 2L+ DLBCL Current Development Areas ▪ IIT suggests ZYNLONTA regimen could provide benefit in 2L+ high - risk FL (96% ORR, 85% CR, N=27); IIT studying ZYNLONTA in 2L+ MZL ‒ Unmet need is significant in these populations ‒ Assessing regulatory path and compendial strategy ▪ ZYNLONTA combination with bispecifics (LOTIS - 7) is currently being studied in r/r FL and r/r MZL $3.1b 2 , ~109 K patients $2.6b 2 , ~61 K patients $1.4b 2 , ~38 K patients 1. As per Leukemia & Lymphoma Society data; 2. Clarivate & Global Data used to size US market value; 3. Cerner Enviza CancerMPact database, 2023. Note: Distribution by line of therapy is based on the incident, drug - treated population .

10 ZYNLONTA: W ell - positioned to Lead in 3L/3L+ DLBCL with Product Profile Ideally Suited Across Treatment Settings Community Centers/ Hospitals Academic Centers PRODUCT PROFILE ▪ Off - the - shelf option for R/R patients with rapid, deep and durable single - agent efficacy ‒ Fast time to response (median 41 days) with no step - up dose required ‒ Median DoR not yet reached for patients in CR at 2 - year follow - up ▪ Manageable safety profile with no CRS, ideal for community care ▪ Simple dosing, with no REMS or inpatient stay requirements OPPORTUNITY ▪ For patients who will remain in community care and will not get to CAR - T or bispecifics ▪ For patients post CAR - T or bispecifics

11 ZYNLONTA in 3L+ DLBCL: Current and Forward Looking GtN : Gross - to - Net Implemented new commercial model focused on: ▪ Increasing / leveraging advocacy from the academic centers ▪ Growing community awareness and utilization, where the majority of the opportunity is for ZYNLONTA Rationale for change: ▪ Increasing competition in fragmented r/r DLBCL market particularly in the academic setting where complicated therapies are administered ▪ Increasing influence of academic centers to community and need for tighter coordination in local healthcare markets Impact of execution since implementation in August 2023: ▪ Average vials per day in November / December in line with 1H, partially offset by high single digit percentage point increase in GtN from 1H to 2H 2023 ▪ Increase in the number of accounts and volume from community ▪ Average calls per month back to 1Q 2023 level Increase Key Thought Leader Advocacy 1 Drive Awareness of ZYNLONTA’s Differentiated Profile 2 Ensure a Positive Patient Experience 3 STRATEGIC IMPERATIVES 2023 2024

12 BsAbs : Bispecific Antibodies; R : Rituximab ; ORR: Overall Response Rate; CR: Complete Response, REMS: Risk Evaluation and Mitigation Strategies, SCT: Stem - Cell Transplant ▪ ZYNLONTA combinations deliver simple dosing with no REMS ▪ ZYNLONTA combinations have potential to expand to 2L+ populations ‒ LOTIS - 5 (ZYNLONTA + R) results to date show 80% ORR and 50% CR in 2L+ SCT - ineligible DLBCL (N=20) ‒ Combos with bispecifics may elevate efficacy to levels of CAR - T , given high single agent activity of both drugs and non - overlapping toxicities (excluding neutropenia) ▪ ZYNLONTA combinations have the potential to deliver manageable safety profiles that are well - suited to both the academic and community settings ACCESSIBILITY EFFICACY SAFETY Potential for ZYNLONTA Combinations in 2L+ DLBCL

13 LOTIS - 5 Overview ▪ Patient Population: 2L+ DLBCL, ASCT ineligible ▪ Summary: Ph3 confirmatory trial in combination with rituximab ▪ Status: ‒ Enrollment ongoing in randomized portion, target 350 patients ‒ IDMC met in July 2023 and reviewed more than 100 pts , there were no safety concerns and recommended for the study to move forward as planned ▪ Support: Updated safety lead - in results at SOHO 2023: ORR of 80%, CR of 50% with no new safety signals ▪ Next steps: Enrollment expected to complete in 2024 Accelerated Enrollment in 2023 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 Q2 2023 Q3 2023 Q4 2023 > 2/3 rd Pts Enrollment 2022 Enrollment 2023 Enrollment ~1.5X Pace LOTIS - 5: Developing ZYNLONTA to be the Combination Agent of Choice in Earlier Lines of Therapy Target Positioning Competitive 2L+ efficacy with favorable safety and convenient dosing schedule, well - suited for use in both academic and community settings
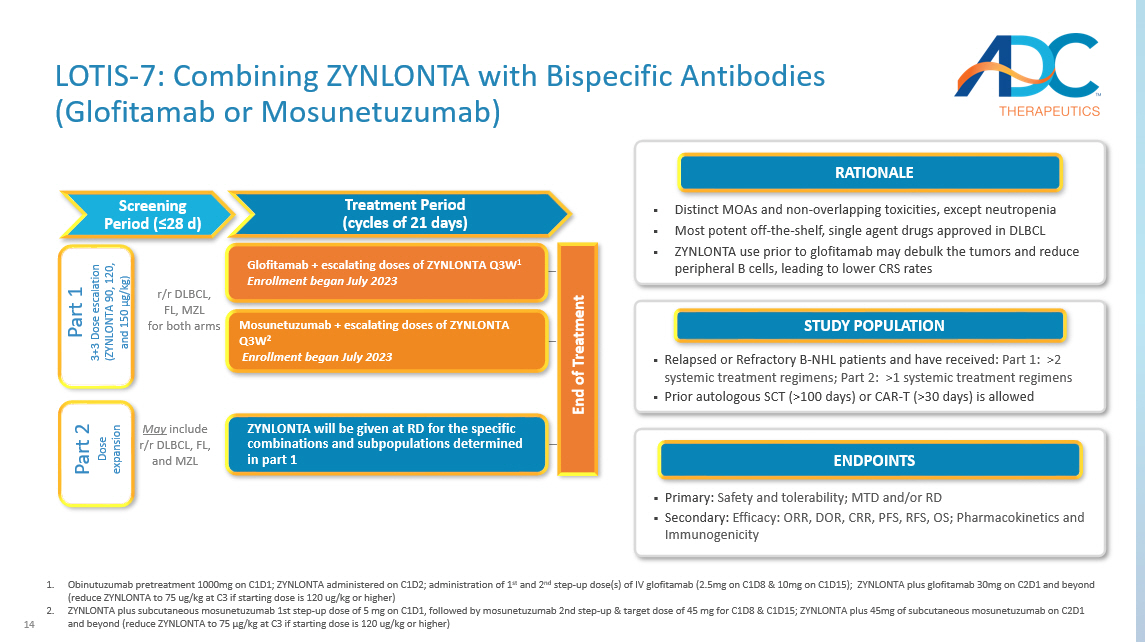
14 Arm E Arm F Treatment Period (cycles of 21 days) r/r DLBCL, FL, MZL for both arms May include r/r DLBCL, FL, and MZL ZYNLONTA will be given at RD for the specific combinations and subpopulations determined in part 1 End of Treatment Part 1 3+3 Dose escalation (ZYNLONTA 90, 120, and 150 μg /kg) Part 2 Dose expansion Screening Period (≤28 d) Mosunetuzumab + escalating doses of ZYNLONTA Q3W 2 Enrollment began July 2023 Glofitamab + escalating doses of ZYNLONTA Q3W 1 Enrollment began July 2023 1. Obinutuzumab pretreatment 1000mg on C1D1; ZYNLONTA administered on C1D2; administration of 1 st and 2 nd step - up dose(s) of IV glofitamab (2.5mg on C1D8 & 10mg on C1D15); ZYNLONTA plus glofitamab 30mg on C2D1 and beyond (reduce ZYNLONTA to 75 ug/kg at C3 if starting dose is 120 ug/kg or higher) 2. ZYNLONTA plus subcutaneous mosunetuzumab 1st step - up dose of 5 mg on C1D1, followed by mosunetuzumab 2nd step - up & target dose of 45 mg for C1D8 & C1D15; ZYNLONTA plus 45mg of subcutaneous mosunetuzumab on C2D1 and beyond (reduce ZYNLONTA to 75 μg /kg at C3 if starting dose is 120 ug/kg or higher) LOTIS - 7: Combining ZYNLONTA with Bispecific Antibodies ( Glofitamab or Mosunetuzumab ) ENDPOINTS ▪ Primary: Safety and tolerability; MTD and/or RD ▪ Secondary: Efficacy: ORR, DOR, CRR, PFS, RFS, OS; Pharmacokinetics and Immunogenicity STUDY POPULATION ▪ Relapsed or Refractory B - NHL patients and have received: Part 1: >2 systemic treatment regimens; Part 2: >1 systemic treatment regimens ▪ Prior autologous SCT (>100 days) or CAR - T (>30 days) is allowed RATIONALE ▪ Distinct MOAs and non - overlapping toxicities, except neutropenia ▪ Most potent off - the - shelf, single agent drugs approved in DLBCL ▪ ZYNLONTA use prior to glofitamab may debulk the tumors and reduce peripheral B cells, leading to lower CRS rates

15 IIT on FL Note: 1 Data as of December 21, 2023. MOA: Mechanism of Action; CRS: Cytokine Release Syndrome LOTIS - 7: Preliminary Results and Next Steps Expecting additional data to be shared in 2024 NEXT STEPS ▪ Expect to continue dose escalation up to 150 µg/kg ▪ In parallel, expect to expand in 120 µg/kg with glofitamab in DLBCL once the DLT period has been cleared ▪ Plan to expand to other dosing cohorts in 3L+ and move to expansion in 2L+ ▪ Adding multiple new sites to accelerate enrollment EARLY SAFETY UPDATES (DL1) ▪ DLT period has been cleared for DL1 (90 µg/kg ZYNLONTA) in both arms (+ glofitamab and + mosunetuzumab ) ▪ No cases of CRS in the glofitamab arm, Grade 1 CRS (fever only) observed in the mosunetuzumab arm ▪ No discontinuations due to AEs EARLY EFFICACY UPDATE (DL1) ▪ To date, each of the first five patients eligible for assessment in this dosing level has shown response (PR or CR) at first scan

16 ZYNLONTA + Rituximab Showed Strong Initial Signals in High - risk r/r FL with 96% ORR and 85% CR rates Patient Population – 2L+ FL U of Miami IIT in FL - ASH 2023 Oral Presentation 1L 1L Phase 2 study of loncastuximab tesirine with rituximab in patients with high - risk relapsed/refractory follicular lymphoma. Highlights of the data presented at ASH include: ▪ N = 33 patients enrolled out of a targeted 39, 27 patients evaluable for efficacy and 32 patients evaluable for toxicity ▪ Best overall response rate of 96% and CR rate of 85% ▪ After a median follow - up of 9.7 months, the median progression - free survival (PFS) was not reached, and the 12 - month PFS was 92% ▪ Majority of adverse events (AEs) were grade 1. Grade 3 AEs included neutropenia (n=2; 6%), and one case each (3%) of hyperglycemia, increased ALT, fatigue, dyspnea and skin infection. Neutropenia was the only grade 4 AE (n=1; 3%) 1.Ghione et al., Blood (2022); 2.Salles et al., Blood Adv (2022); 3. Budde E, et al. Lancet Oncol, 2022; 4. Sehn L, et al. Lancet Oncol 2016; 17: 1081 – 93 ; 5. Leonard J, et al. J Clin Oncol, 2019 37:1188 - 1199; 6. Cerner Enviza CancerMPact database, 2023 Note: Distribution by line of therapy is based on the incident, drug - treated population . 0 - 100 - 90 - 70 - OR R Len + Rituximab (R2) (2L+) 5 78% Benda + Obin (2L+) 4 79% % 80 - Mosun label (3L+) 3 80% 0 - 100 - 80 - CR Mosun label (3L+) 3 60% Benda + Obin (2L+) 4 17% Len + Rituximab (R2) (2L+) 5 34% % 60 - 40 - 20 - Current r/r FL Competitive Landscape ~21 K 2L+ FL patients U.S. 5 - year Prevalence 6 3L+ Key: 2L 1L ▪ University of Miami plans to expand the number of trial participants up to 100 and add other cancer research centers ▪ Data is expected to be published at a medical conference ▪ Path forward will be discussed with regulatory authorities and compendia Next Steps Axi - cel (3L+) 1 94% Axi - cel (3L+) 1 Tisa - cel (3L+) 2 69% Tisa - cel (3L+) 2 86% 79%

17 ZYNLONTA has Potential to Address Significant Unmet Need in MZL Given Low CR Rates with Approved Therapies 1. Sehn L, et al. Lancet Oncol 2016; 17: 1081 – 93. 2. Opat S, et.al - ASH Oral Presentation December 2022, New Orleans, LA. 3. Leonard J, et al. J Clin Oncol, 2019 37:1188 - 1199. 4. Cerner Enviza CancerMPact database, 2023. Note: Distribution by line of therapy is based on the incident, drug - treated population . 1L 1L ▪ It is expected that the futility analysis for this study will have 19 patients and it will be conducted in the first half of 2024. The investigator plans to share the results at an upcoming scientific congress ▪ University of Miami plans to add other cancer research centers in order to accelerate enrollment of up to 50 trial participants ▪ Study Title: A Phase 2, Open - label, Study Evaluating Safety and Efficacy of the Loncastuximab in Relapsed/Refractory Marginal Zone Lymphoma ▪ Line of Therapy: Relapsed/Refractory ▪ Status: 15 patients enrolled as of December 2023 U of Miami IIT in MZL Overview Next Steps 0 - 80 - OR R Benda + Obin (2L+) 1 Zanubritinib (2L+) 2 68% % 79% 60 - Len + Rituximab (R2) (2L+) 3 64% 0 - 30 - CR % 20 - Len + Rituximab (R2) (2L+) 3 29% 10 - Current r/r MZL Competitive Landscape 40 - 20 - Zanubritinib (2L+) 2 26% Benda + Obin (2L+) 1 17% Patient Population – 2L+ MZL ~15 K 2L+ MZL patients U.S. 5 - year Prevalence 4 3L+ Key: 2L 1L
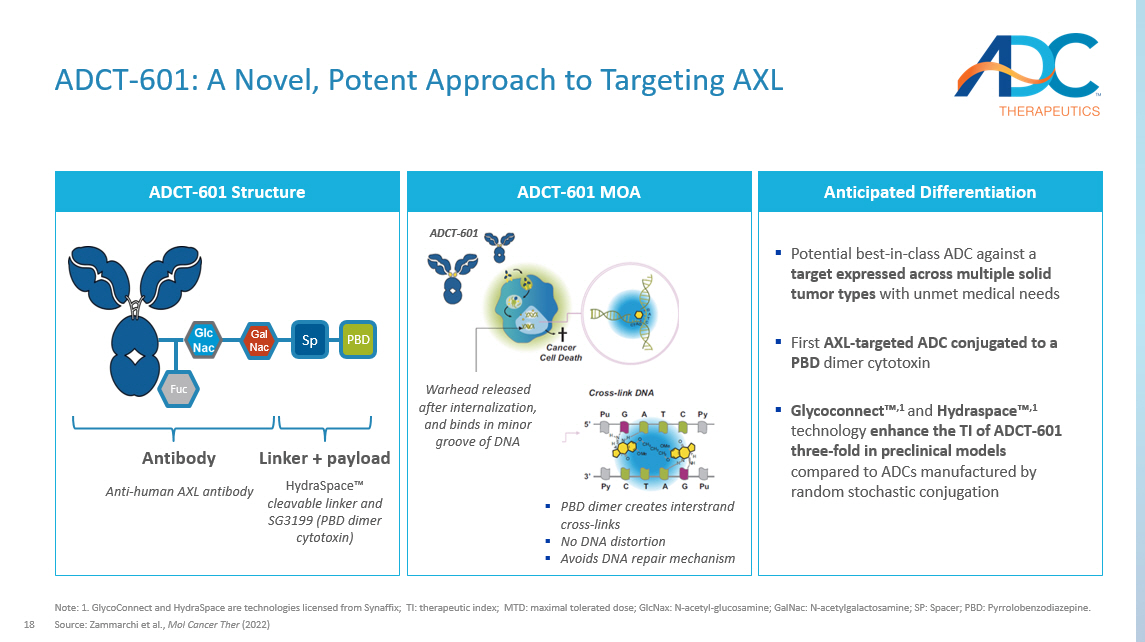
18 ADCT - 601: A Novel, Potent Approach to Targeting AXL Note: 1. GlycoConnect and HydraSpace are technologies licensed from Synaffix ; TI: therapeutic index; MTD: maximal tolerated dose; GlcNax : N - acetyl - glucosamine; GalNac : N - acetylgalactosamine ; SP: Spacer; PBD: Pyrrolobenzodiazepine . Source: Zammarchi et al., Mol Cancer Ther (2022) ADCT - 601 Structure Anticipated Differentiation ▪ Potential best - in - class ADC against a target expressed across multiple solid tumor types with unmet medical needs ▪ First AXL - targeted ADC conjugated to a PBD dimer cytotoxin ▪ Glycoconnect Ρ ,1 and Hydraspace Ρ ,1 technology enhance the TI of ADCT - 601 three - fold in preclinical models compared to ADCs manufactured by random stochastic conjugation PBD Sp Gal Nac Fuc HydraSpace Ρ cleavable linker and SG3199 (PBD dimer cytotoxin) Anti - human AXL antibody Antibody Linker + payload ADCT - 601 MOA Warhead released after internalization, and binds in minor groove of DNA ▪ PBD dimer creates interstrand cross - links ▪ No DNA distortion ▪ Avoids DNA repair mechanism ADCT - 601
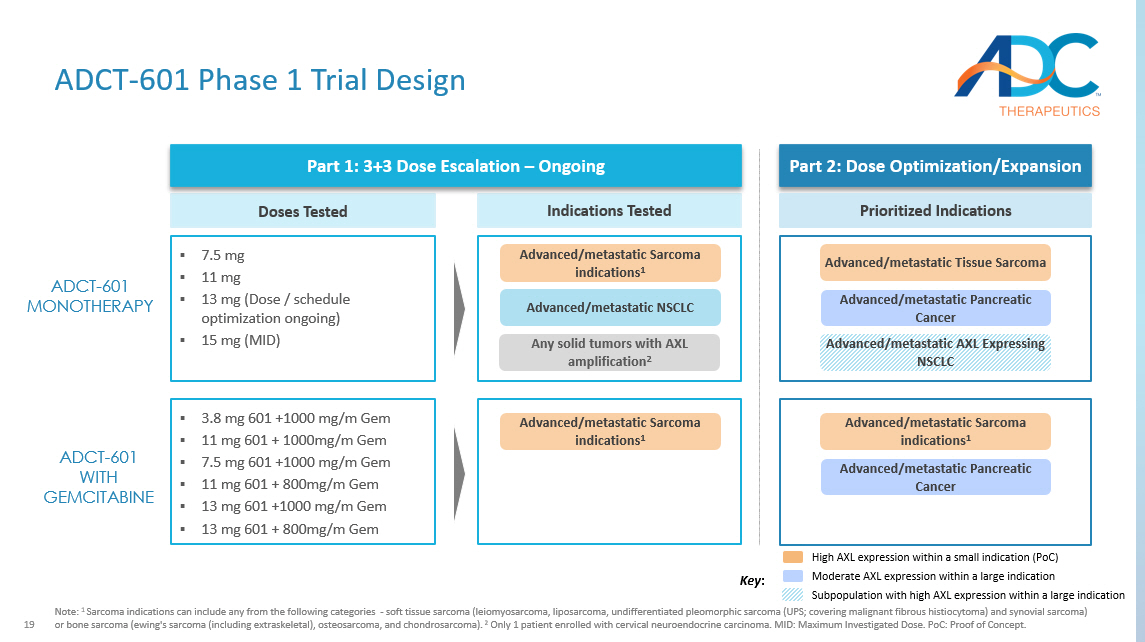
19 ADCT - 601 Phase 1 Trial Design Note: 1 Sarcoma indications can include any from the following categories - soft tissue sarcoma (leiomyosarcoma, liposarcoma, undiffere ntiated pleomorphic sarcoma (UPS; covering malignant fibrous histiocytoma) and synovial sarcoma) or bone sarcoma ( ewing's sarcoma (including extraskeletal ), osteosarcoma, and chondrosarcoma). 2 Only 1 patient enrolled with cervical neuroendocrine carcinoma. MID: Maximum Investigated Dose. PoC: Proof of Concept. ADCT - 601 MONOTHERAPY Advanced/metastatic AXL Expressing NSCLC Advanced/metastatic Pancreatic Cancer Advanced/metastatic Tissue Sarcoma Part 2: Dose Optimization/Expansion Prioritized Indications Indications Tested Advanced/ metastatic NSCLC Any solid tumors with AXL amplification 2 Advanced/metastatic Sarcoma indications 1 ▪ 7.5 mg ▪ 11 mg ▪ 13 mg (Dose / schedule optimization ongoing) ▪ 15 mg (MID) Doses Tested Part 1: 3+3 Dose Escalation – Ongoing ▪ 3.8 mg 601 +1000 mg/m Gem ▪ 11 mg 601 + 1000mg/m Gem ▪ 7.5 mg 601 +1000 mg/m Gem ▪ 11 mg 601 + 800mg/m Gem ▪ 13 mg 601 +1000 mg/m Gem ▪ 13 mg 601 + 800mg/m Gem Advanced/metastatic Sarcoma indications 1 Advanced/metastatic Sarcoma indications 1 Advanced/metastatic Pancreatic Cancer ADCT - 601 WITH GEMCITABINE Key : High AXL expression within a small indication (PoC) Moderate AXL expression within a large indication Subpopulation with high AXL expression within a large indication

20 ADCT - 601 Early Safety Results in Advanced / Metastatic Sarcoma Safety – ADCT - 601 Monotherapy ( STS ) Total (N=18) N (%) Any TEAE ≥ Gr3 9 (50.0) Total Discontinued (%) Discontinuation due to Treatment (%) 15 (83.3%) 3 (16.7) Preferred Term Any TEAE ≥ Gr3 (%) Cheilitis 1 (5.6) Abdominal pain 1 (5.6) Drug - induced liver injury 1 (5.6) Dyspnoea 1 (5.6) Eczema 1 (5.6) Generalized oedema 1 (5.6) Oedema peripheral 1 (5.6) Pulmonary embolism 1 (5.6) Thrombocytopenia 1 (5.6) Skin infections 1 (5.6) Photosensitivity reactions 1 (5.6) Lymphoedema 1 (5.6) LFT increased 1 (5.6) Hypotension 1 (5.6) Alanine aminotransferase increased 1 (5.6) Aspartate aminotransferase increased 1 (5.6) Gamma - glutamyltransferase increased 2 (11.1) Pleural effusion 1 (5.6) Uncoded 1 (5.6) Safety – ADCT - 601 With Gemcitabine (Sarcoma) Total (N=9) N (%) Any TEAE ≥ Gr3 8 (88.9) Total Discontinued (%) Discontinuation due to Treatment (%) 9 (100) 0 Preferred Term Any TEAE ≥ Gr3 (%) Anaemia 4 (44.4) Pleural effusion 2 (22.2) Thrombocytopenia 4 (44.4) Alanine aminotransferase increased 1 (11.1) Neutropenia 3 (33.3) Neutrophil count decreased 3 (33.3) Platelet count decreased 2 (22.2) Gamma - glutamyltransferase increased 2 (22.2) Arthralgia 1 (11.1) Aspartate aminotransferase increased 1 (11.1) Fatigue 1 (11.1) Hypertension 1 (11.1) Pleuritic pain 1 (11.1) Pneumonia 1 (11.1) Note: Data as of 12/04/2023. The data captured includes individual patients who experienced multiple AEs while on study.

21 ADCT - 601 Shows Early Signs of Anti - Tumor Activity as Monotherapy and in Combination in Sarcoma Patients Note: Data as of 12/04/2023. STS: Soft Tissue Sarcoma; NSCLC: Non - small cell lung cancer. ADCT - 601 Monotherapy (STS) -60% -50% -40% -30% -20% -10% 0% 10% 20% 30% 40% Baseline Week 6 Week 12 Week 21 15mg 13mg 11mg 7.5mg ADCT - 601 + Gemcitabine (Sarcoma) -60% -50% -40% -30% -20% -10% 0% 10% 20% 30% 40% 50% 60% Baseline Week 6 Week 12 Week 21 Week 30 Week 39 Week 48 % Change in Tumor Size 11mg 7.5 mg 3.8 mg Dose and schedule optimization at 13 mg initiated in STS and planned in Pancreatic cancer; dose expansion in NSCLC contingent on assay development Dose optimization in combination with gemcitabine planned in Sarcoma and Pancreatic cancer Change in Tumor Size Over Time Change in Tumor Size Over Time PR PR PR PR
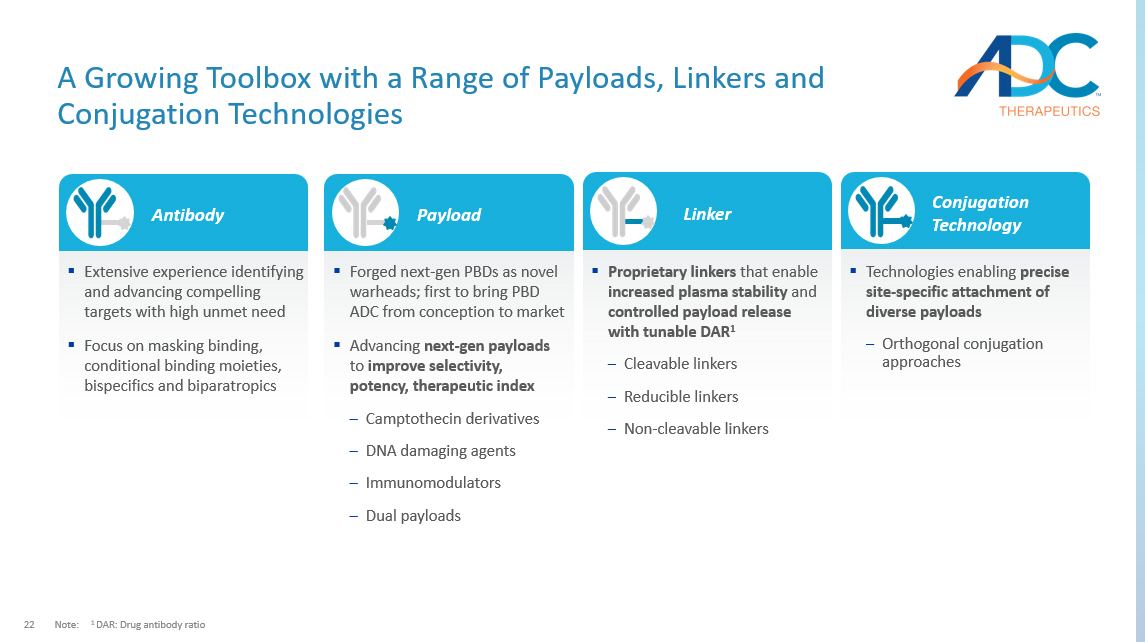
22 A Growing Toolbox with a Range of Payloads, Linkers and Conjugation Technologies Note: 1 DAR: Drug antibody ratio Antibody ▪ Extensive experience identifying and advancing compelling targets with high unmet need ▪ Focus on masking binding, conditional binding moieties, bispecifics and biparatropics Linker ▪ Proprietary linkers that enable increased plasma stability and controlled payload release with tunable DAR 1 ‒ Cleavable linkers ‒ Reducible linkers ‒ Non - cleavable linkers Payload ▪ Forged next - gen PBDs as novel warheads; first to bring PBD ADC from conception to market ▪ Advancing next - gen payloads to improve selectivity, potency, therapeutic index ‒ Camptothecin derivatives ‒ DNA damaging agents ‒ Immunomodulators ‒ Dual payloads Conjugation Technology ▪ Technologies enabling precise site - specific attachment of diverse payloads ‒ Orthogonal conjugation approaches

23 Exatecan has the Potential to be Differentiated over Commercial - Stage Toxins such as DXd Note: 1 PgP : P - glycoprotein; 2 Cell line derived xenograft (CDX) model is a renal leiomyoblastoma model, a ll arms dosed intravenously qdx1 Source: Weng et al., Cancer Discov (2023) EXATECAN V. DXD ▪ Better potency ▪ No PgP 1 transport, enabling enhanced intracellular presence ▪ Increased bystander effect , leading to more cell death and enhancing therapeutic impact over DXd Exatecan DXd Vehicle Isotype (10 mg/kg) Antibody - D Xd (15 mg/kg) Antibody - exatecan (6.6 mg/kg) Antibody - exatecan (10 mg/kg) ▪ ADCT has developed a novel hydrophilic linker that enables efficient conjugation of exatecan ▪ Our exatecan - based ADCs enable traceless release of exatecan after internalization ▪ Superior therapeutic index driven by strong in vivo efficacy and excellent tolerability in cynomolgus monkey without any signs of ILD EXATECAN ADC V. DXD ADC in CDX MODEL 2 EXATECAN ADVANTAGE ADC THERAPEUTICS PLATFORM ADVANTAGE

24 Target description Phosphate transporter Adhesion protein Enzymatic glycoprotein Amino acid transporter Tumor types of interest NSCLC Ovarian cancer Ovarian cancer Endometrial cancer Prostate cancer NSCLC Colorectal cancer Payload Exatecan Exatecan Exatecan Exatecan Validation data in vitro characterization in vivo efficacy NHP toxicology in vitro characterization in vivo efficacy NHP toxicology Ongoing in vitro characterization in vivo efficacy NHP toxicology 2 Candidate selection 1 Complete 2024 2024 2024 Advancing ADC Assets Against Validated Targets in Indications with High Unmet Need Note: 1 IND enabling studies can be completed within 18 months after selection of the candidate ; 2 NHP study done with chimeric candidate NaPi2b Claudin - 6 PSMA Undisclosed target

25 Corporate Business Development Strategy for ADCT Portfolio HEMATOLOGY ZYNLONTA, ADCT - 602 SOLID TUMOR S ADCT - 601, Research Assets Business Development to Unlock Value Business Development Goals: U.S. EX - U.S. Co - development & Co - promote* Royalty & Milestones to ADCT Accelerate & Expand Asset Development 3 Non - dilutive Financing 2 Maximize Deal Value & Value Split 1 Direct Partnership *Flexible for alternative construct depending on economics. OVERLAND JOINT VENTURE
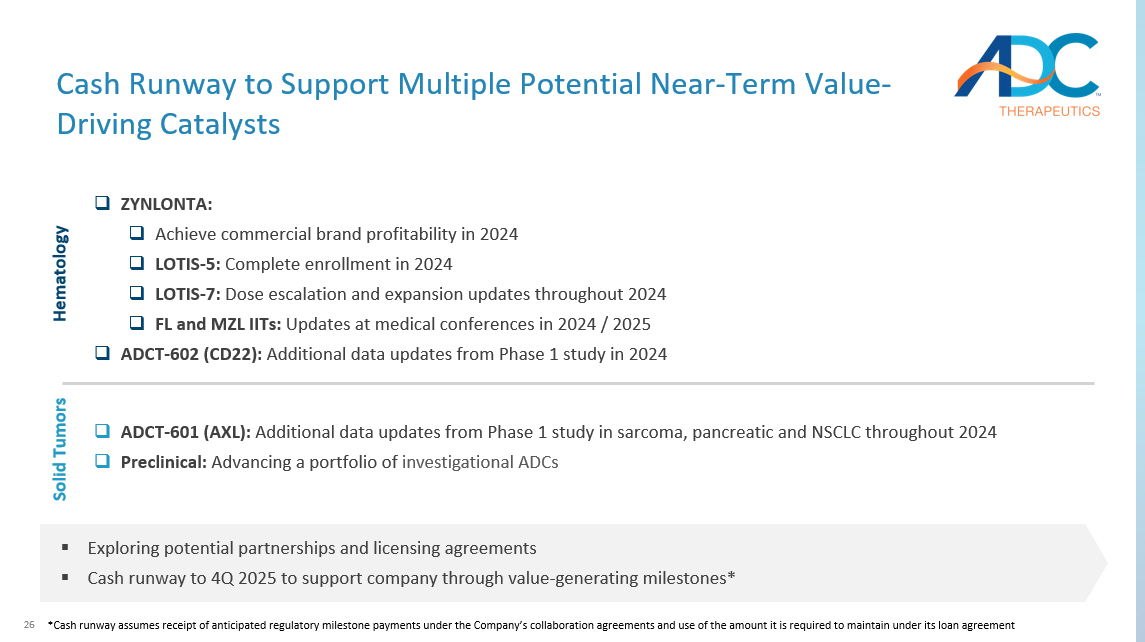
26 Cash Runway to Support Multiple Potential Near - Term Value - Driving Catalysts □ ZYNLONTA: □ Achieve commercial brand profitability in 2024 □ LOTIS - 5: Complete enrollment in 2024 □ LOTIS - 7: Dose escalation and expansion updates throughout 2024 □ FL and MZL IITs: Updates at medical conferences in 2024 / 2025 □ ADCT - 602 (CD22): Additional data updates from Phase 1 study in 2024 ▪ Exploring potential partnerships and licensing agreements ▪ Cash runway to 4Q 2025 to support company through value - generating milestones* Hematology □ ADCT - 601 (AXL): Additional data updates from Phase 1 study in sarcoma, pancreatic and NSCLC throughout 2024 □ Preclinical: Advancing a portfolio of investigational ADCs Solid Tumors * Cash runway assumes receipt of anticipated regulatory milestone payments under the Company’s collaboration agreements and use of the amount it is required to maintain under its loan agreement
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
ADC Therapeutics (NYSE:ADCT)
Historical Stock Chart
From Mar 2024 to Apr 2024
ADC Therapeutics (NYSE:ADCT)
Historical Stock Chart
From Apr 2023 to Apr 2024