false 0001829802 0001829802 2024-01-04 2024-01-04 0001829802 us-gaap:CommonStockMember 2024-01-04 2024-01-04 0001829802 us-gaap:SeriesAPreferredStockMember 2024-01-04 2024-01-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 4, 2024
Sensei Biotherapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-39980 |
|
83-1863385 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 1405 Research Blvd, Suite 125 Rockville, MD |
|
20850 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (240) 243-8000
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934:
|
|
|
|
|
| Title of each class |
|
Trading symbol |
|
Name of each exchange on which registered |
| Common Stock |
|
SNSE |
|
The Nasdaq Stock Market LLC |
| Series A Preferred Stock Purchase Rights |
|
|
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 4, 2024, Sensei Biotherapeutics, Inc. (the “Company”) updated its corporate presentation for use in meetings with investors, analysts and others. A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in Item 7.01 and the exhibit attached hereto are being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference into any filing under the Exchange Act or the Securities Act of 1933, as amended, whether filed before or after the date hereof and regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sensei Biotherapeutics, Inc. |
|
|
|
|
| Date: January 4, 2024 |
|
|
|
|
|
/s/ Christopher W. Gerry |
|
|
|
|
|
|
Christopher W. Gerry |
|
|
|
|
|
|
General Counsel and Secretary |
3
Conditionally Active Antibodies for
Immuno-oncology Corporate Deck | January 2024 Exhibit 99.1

Disclaimer This presentation has been
prepared by Sensei Biotherapeutics, Inc. (the “Company,” "we," "us") and is made for informational purposes only. The information set forth herein does not purport to be complete or to contain all of the information you may desire.
Statements contained herein are made as of the date of this presentation unless stated otherwise, and neither the delivery of this presentation at any time, nor any sale of securities, shall under any circumstances create an implication that the
information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This presentation
contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry. This presentation also contains "forward-looking" statements as that term is defined in the Private
Securities Litigation Reform Act of 1995 that are based on our management's beliefs and assumptions and on information currently available to management. These forward-looking statements include, without limitation, expectations regarding the
development and potential therapeutic benefits of our product candidates; the expected safety, pharmacokinetic and efficacy profile of our product candidates, including SNS-101; the expected timing of clinical data from our Phase 1/2 clinical trial
of SNS-101; the expansion of the Phase 1 clinical trial to include additional patients with specific tumor types; and our belief that our existing cash and cash equivalents will be sufficient to fund our operations at least into the fourth quarter
of 2025 and reach midway into Phase 2 clinical studies of SNS-101. When used in this presentation, the words and phrases "designed to," "may," "believes," "intends," "seeks," "anticipates," "plans," "estimates," "expects," "should," "assumes,"
"continues," "could," "will," "future" and the negative of these or similar terms and phrases are intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may
cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Risks and uncertainties that may cause actual results to
differ materially include uncertainties inherent in the development of therapeutic product candidates, such as preclinical discovery and development; conduct of clinical trials and related regulatory requirements, including the risk of delay or
cessation of any clinical trials of Sensei’s product candidates; the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical trials and early results from the clinical trial of SNS-101,
will not be replicated or will not continue in ongoing or future studies or clinical trials involving Sensei’s product candidates, including SNS-101; our reliance on third parties over which we may not always have full control; risks regarding
the accuracy of our estimates of expenses, capital requirements and needs for additional financing; and other risk and uncertainties that are described in our Quarterly Report on Form 10-Q filed with the SEC on or about November 7, 2023 and our
other Periodic Reports filed with the SEC. Forward-looking statements represent our management's beliefs and assumptions only as of the date of this presentation and include all matters that are not historical facts. Our actual future results may be
materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the
forward-looking statements, even if new information becomes available in the future. Certain information contained in this presentation relates to, or is based on, studies, publications, surveys and other data obtained from third-party
sources and the Company's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the
adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as
to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.

Company Highlights Sensei Bio’s
proprietary platform is designed to harness the unique acidic tumor microenvironment to widen the therapeutic window and enable druggability of promising oncology targets SNS-101, the company’s lead asset, targets VISTA, a critical negative
regulator of T-cell function and promising immune checkpoint target SNS-101 is currently in Phase 1 clinical testing with data to date displaying an attractive safety profile and potentially best-in-class pharmacokinetics Three additional
early-stage drug candidates Cash runway into the fourth quarter of 2025, which is expected to fund operations midway into Phase 2 studies of SNS-101 Anticipated near-term milestones include topline Phase 1 monotherapy data in Q2 2024

Leadership Team with History of
Antibody Oncology Success Erin Colgan Chief Financial Officer John Celebi, MBA President and CEO Christopher Gerry, J.D. VP, General Counsel Edward van der Horst, Ph.D. Chief Scientific Officer Stephanie Krebs, M.S., MBA Chief Business Officer Ron
Weitzman, M.D. Chief Medical Officer (part-time)
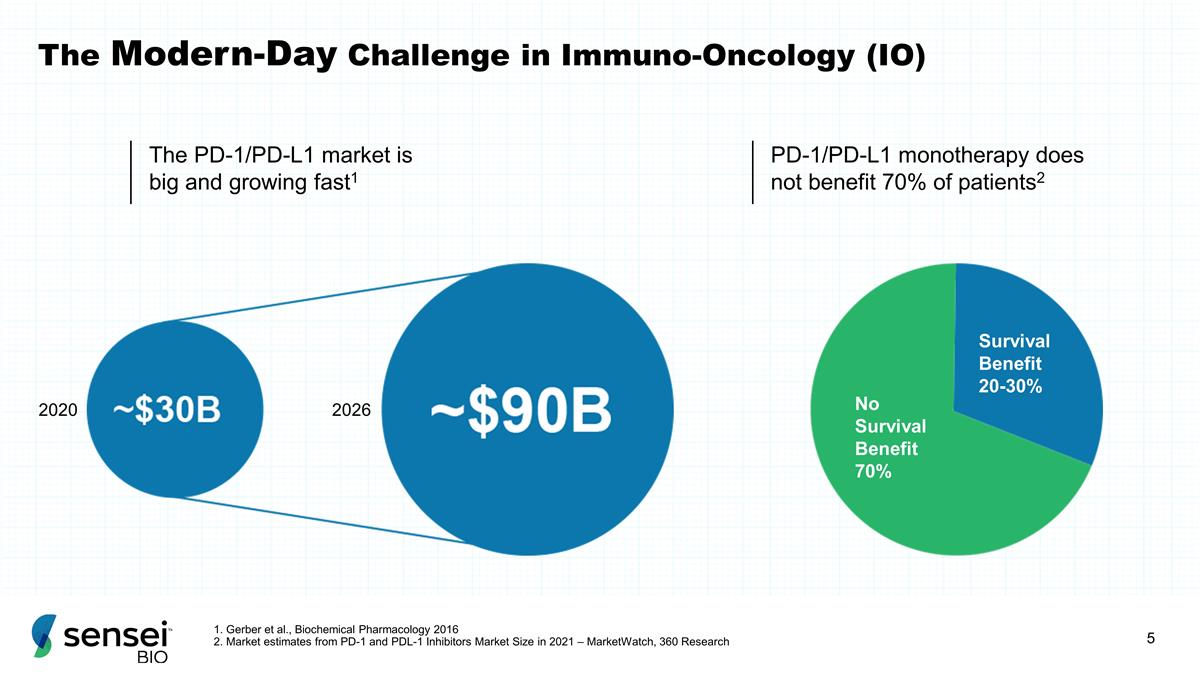
The PD-1/PD-L1 market is big and
growing fast1 PD-1/PD-L1 monotherapy does not benefit 70% of patients2 2020 2026 Survival Benefit 20-30% No Survival Benefit 70% The Modern-Day Challenge in Immuno-Oncology (IO) 1. Gerber et al., Biochemical Pharmacology 2016 2. Market estimates
from PD-1 and PDL-1 Inhibitors Market Size in 2021 – MarketWatch, 360 Research
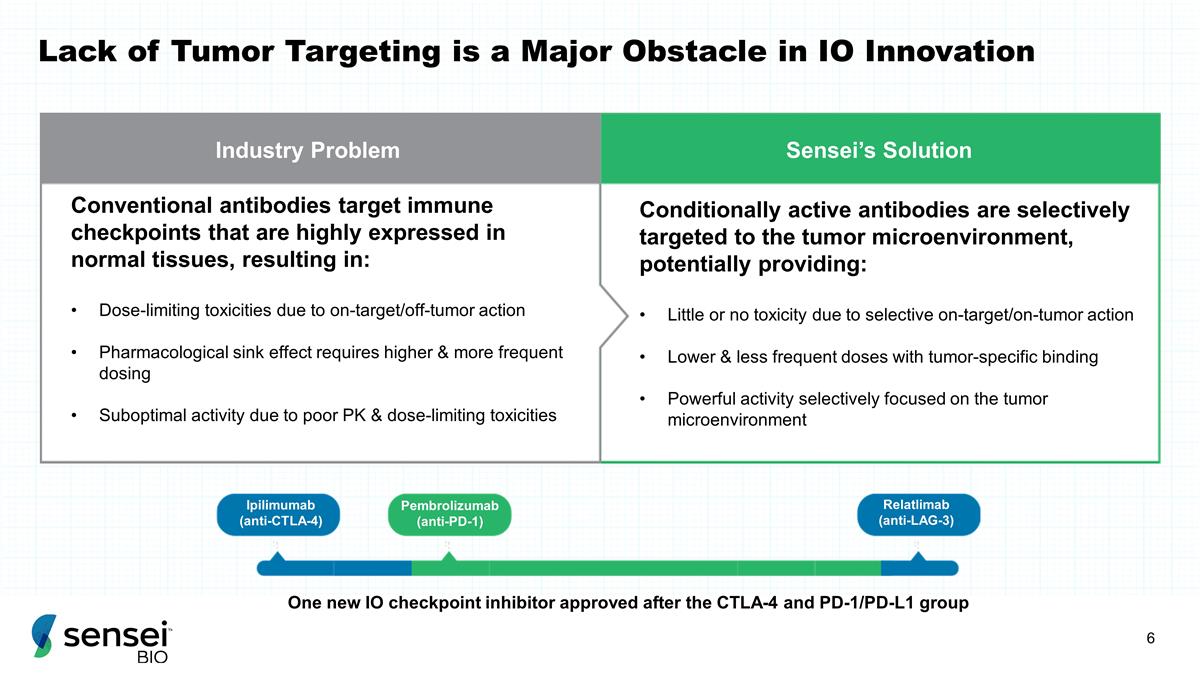
Industry Problem Sensei’s
Solution Conventional antibodies target immune checkpoints that are highly expressed in normal tissues, resulting in: Dose-limiting toxicities due to on-target/off-tumor action Pharmacological sink effect requires higher & more frequent dosing
Suboptimal activity due to poor PK & dose-limiting toxicities Conditionally active antibodies are selectively targeted to the tumor microenvironment, potentially providing: Little or no toxicity due to selective on-target/on-tumor action Lower
& less frequent doses with tumor-specific binding Powerful activity selectively focused on the tumor microenvironment One new IO checkpoint inhibitor approved after the CTLA-4 and PD-1/PD-L1 group 2011 2014 2022 Lack of Tumor Targeting is a
Major Obstacle in IO Innovation Ipilimumab (anti-CTLA-4) Pembrolizumab (anti-PD-1) Relatlimab (anti-LAG-3)
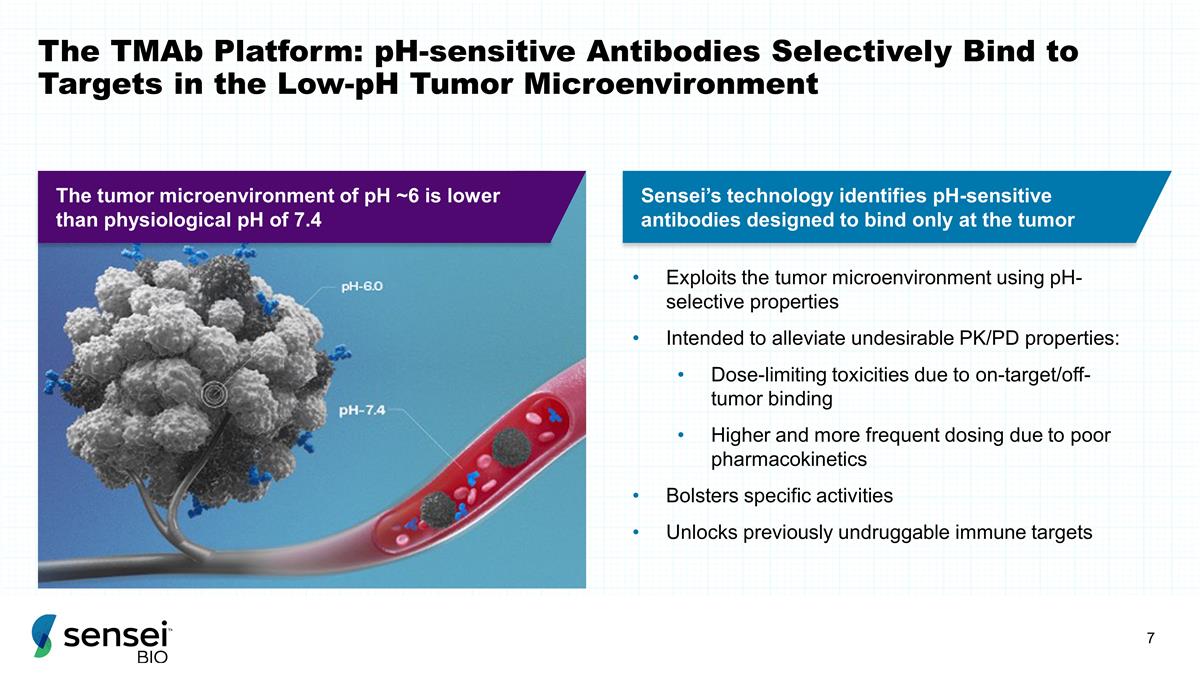
The TMAb Platform: pH-sensitive
Antibodies Selectively Bind to Targets in the Low-pH Tumor Microenvironment Exploits the tumor microenvironment using pH-selective properties Intended to alleviate undesirable PK/PD properties: Dose-limiting toxicities due to
on-target/off-tumor binding Higher and more frequent dosing due to poor pharmacokinetics Bolsters specific activities Unlocks previously undruggable immune targets Sensei’s technology identifies pH-sensitive antibodies designed to bind only at
the tumor The tumor microenvironment of pH ~6 is lower than physiological pH of 7.4
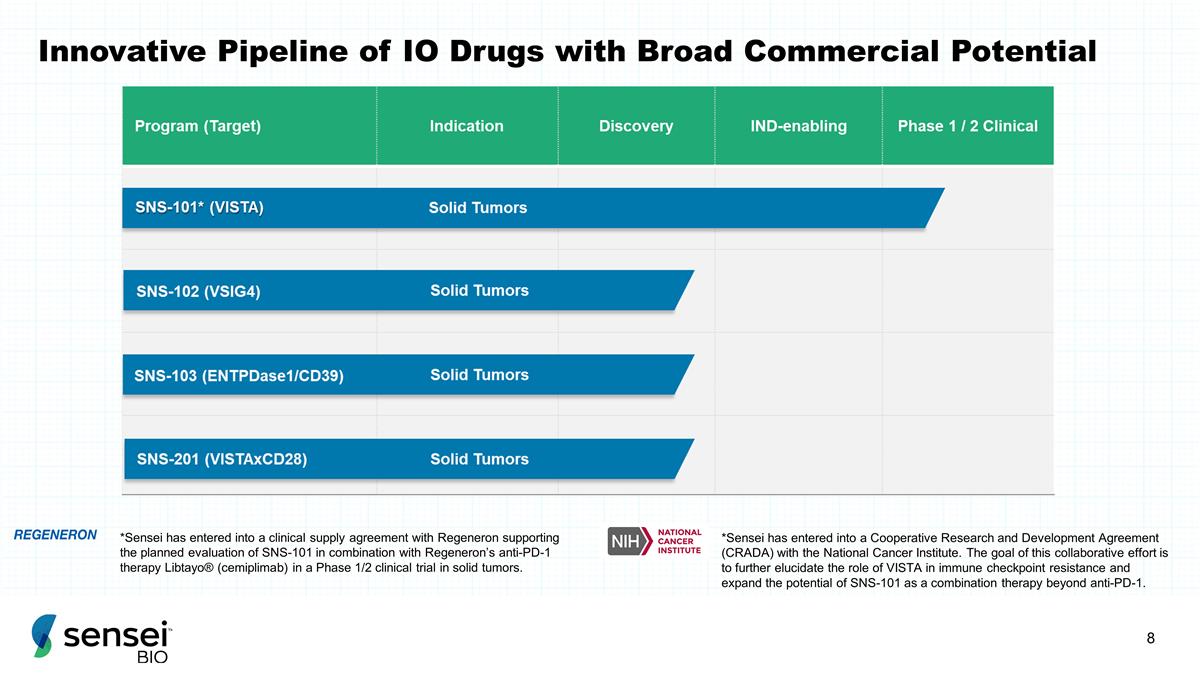
Innovative Pipeline of IO Drugs with
Broad Commercial Potential *Sensei has entered into a clinical supply agreement with Regeneron supporting the planned evaluation of SNS-101 in combination with Regeneron’s anti-PD-1 therapy Libtayo® (cemiplimab) in a Phase 1/2 clinical
trial in solid tumors. *Sensei has entered into a Cooperative Research and Development Agreement (CRADA) with the National Cancer Institute. The goal of this collaborative effort is to further elucidate the role of VISTA in immune checkpoint
resistance and expand the potential of SNS-101 as a combination therapy beyond anti-PD-1.
SNS-101 (VISTA)
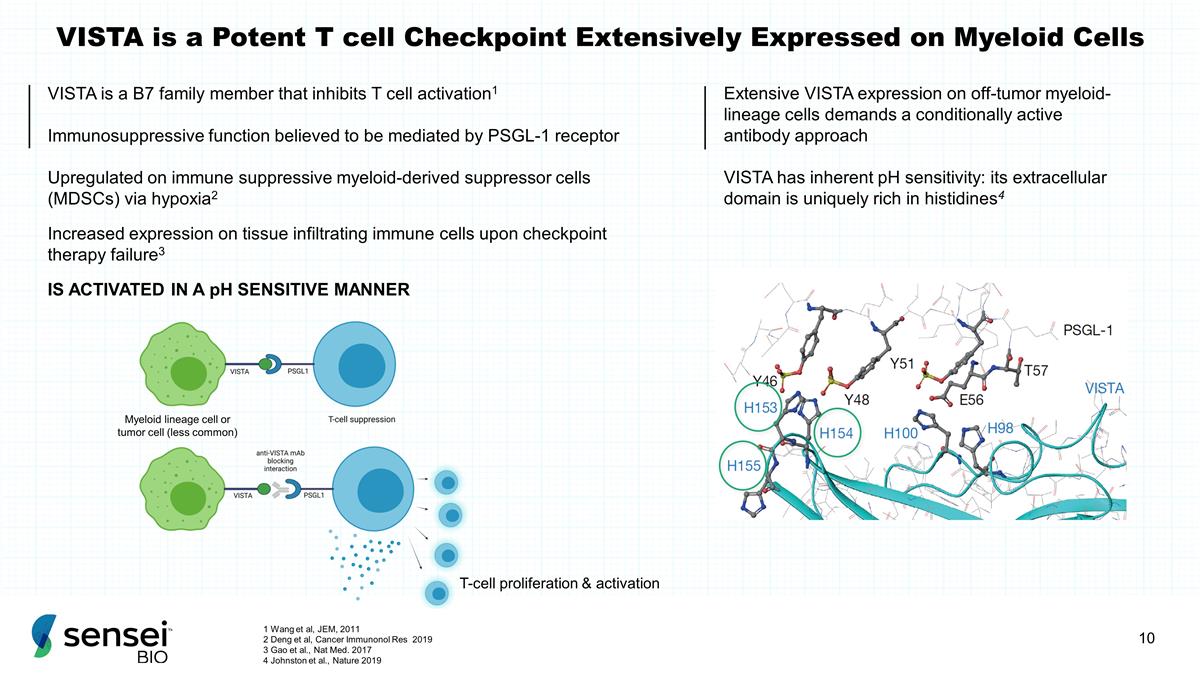
VISTA is a Potent T cell Checkpoint
Extensively Expressed on Myeloid Cells VISTA is a B7 family member that inhibits T cell activation1 Immunosuppressive function believed to be mediated by PSGL-1 receptor Upregulated on immune suppressive myeloid-derived suppressor cells (MDSCs)
via hypoxia2 Increased expression on tissue infiltrating immune cells upon checkpoint therapy failure3 IS ACTIVATED IN A pH SENSITIVE MANNER T-cell proliferation & activation Extensive VISTA expression on off-tumor myeloid-lineage cells demands
a conditionally active antibody approach VISTA has inherent pH sensitivity: its extracellular domain is uniquely rich in histidines4 Myeloid lineage cell or tumor cell (less common) 1 Wang et al, JEM, 2011 2 Deng et al, Cancer Immunonol Res
2019 3 Gao et al., Nat Med. 2017 4 Johnston et al., Nature 2019
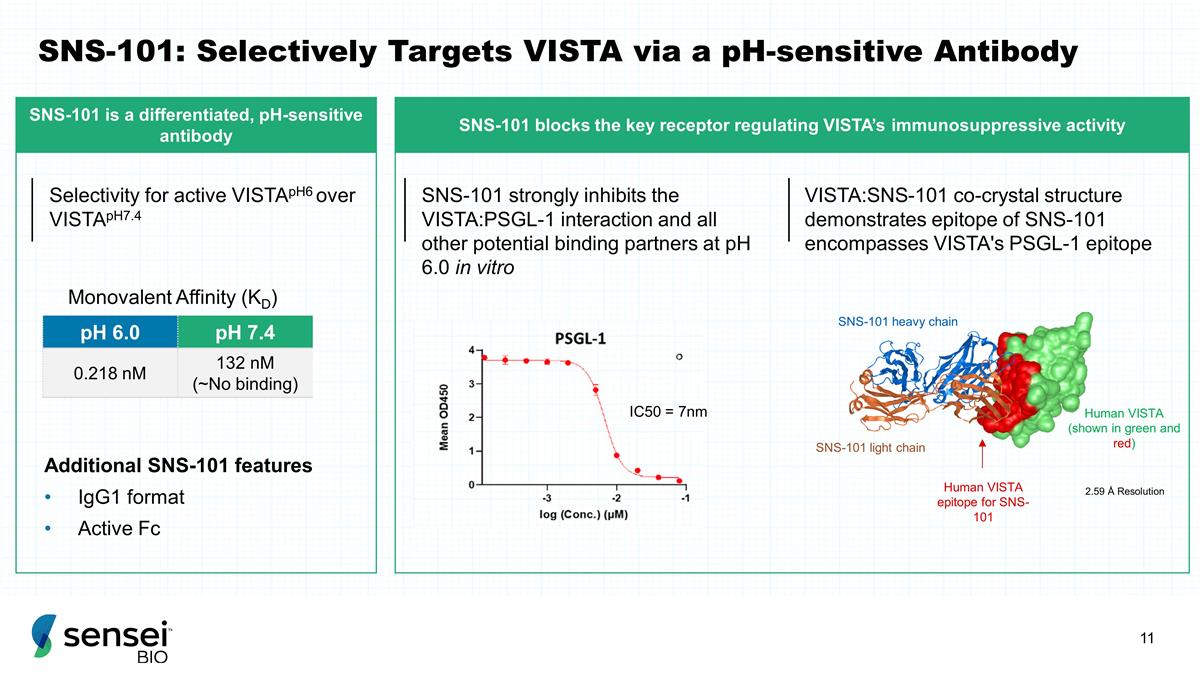
SNS-101: Selectively Targets VISTA
via a pH-sensitive Antibody pH 6.0 pH 7.4 0.218 nM 132 nM (~No binding) Additional SNS-101 features IgG1 format Active Fc Monovalent Affinity (KD) SNS-101 strongly inhibits the VISTA:PSGL-1 interaction and all other potential
binding partners at pH 6.0 in vitro IC50 = 7nm 2.59 Å Resolution SNS-101 heavy chain SNS-101 light chain Human VISTA (shown in green and red) Human VISTA epitope for SNS-101 Selectivity for active VISTApH6 over VISTApH7.4 VISTA:SNS-101
co-crystal structure demonstrates epitope of SNS-101 encompasses VISTA's PSGL-1 epitope SNS-101 is a differentiated, pH-sensitive antibody SNS-101 blocks the key receptor regulating VISTA’s immunosuppressive activity
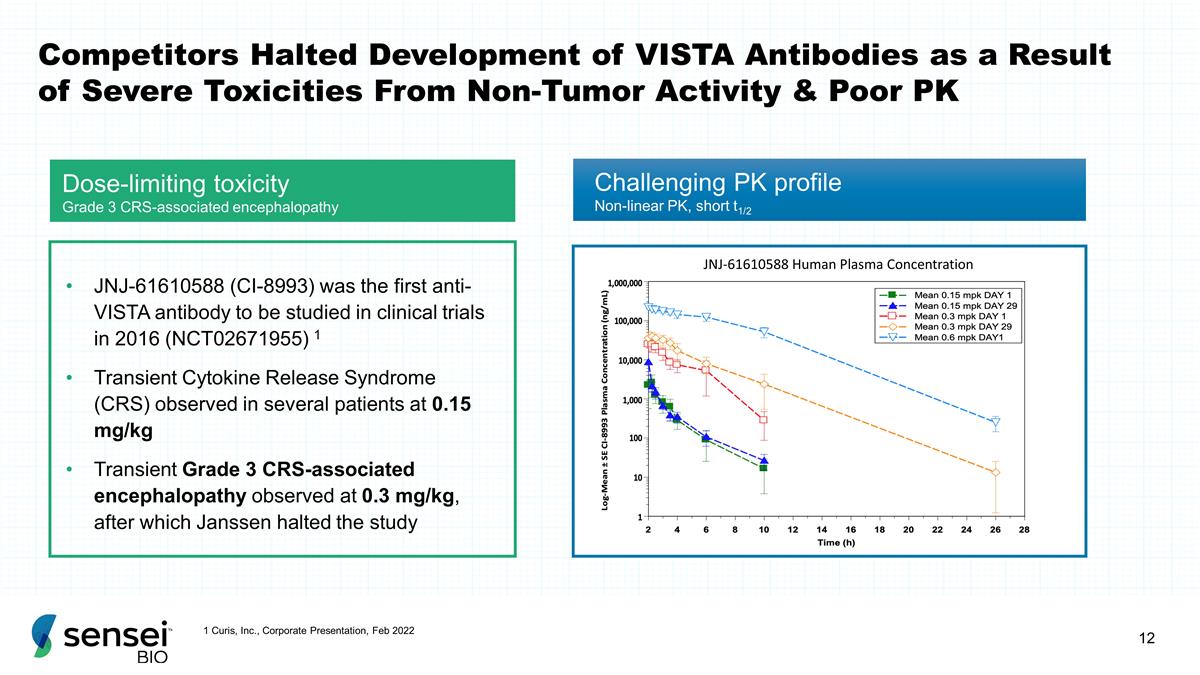
Competitors Halted Development of
VISTA Antibodies as a Result of Severe Toxicities From Non-Tumor Activity & Poor PK Dose-limiting toxicity Grade 3 CRS-associated encephalopathy JNJ-61610588 Human Plasma Concentration JNJ-61610588 (CI-8993) was the first anti-VISTA antibody to
be studied in clinical trials in 2016 (NCT02671955) 1 Transient Cytokine Release Syndrome (CRS) observed in several patients at 0.15 mg/kg Transient Grade 3 CRS-associated encephalopathy observed at 0.3 mg/kg, after which Janssen halted the study
Challenging PK profile Non-linear PK, short t1/2 1 Curis, Inc., Corporate Presentation, Feb 2022
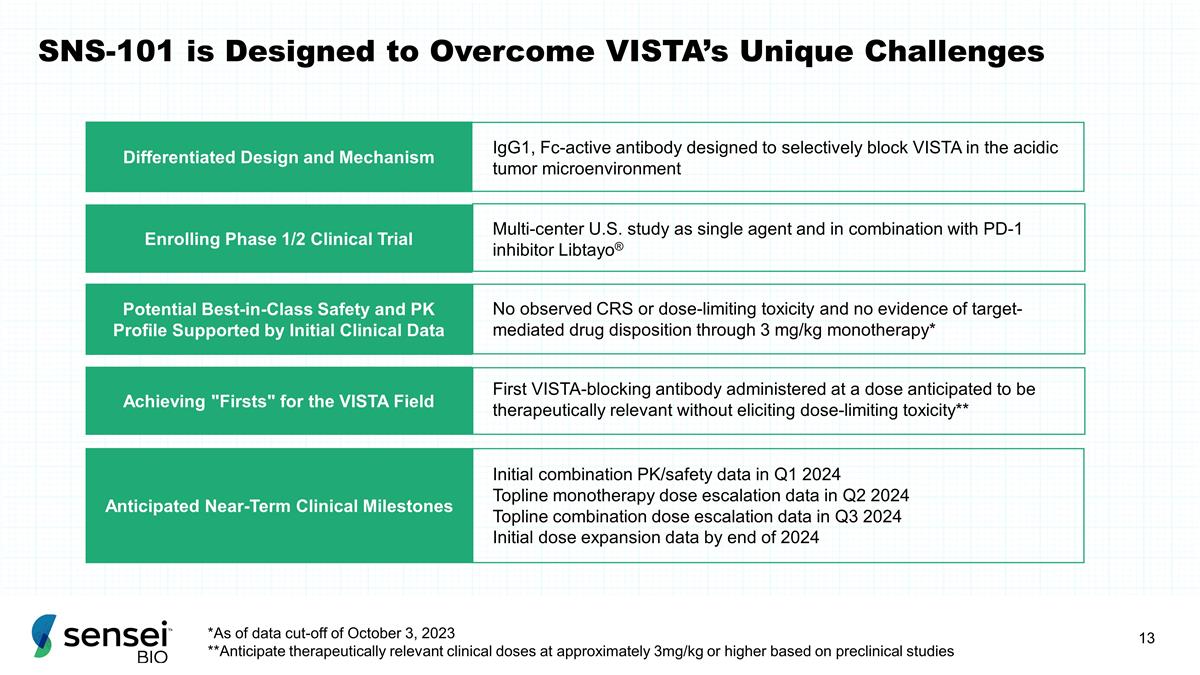
SNS-101 is Designed to Overcome
VISTA’s Unique Challenges Potential Best-in-Class Safety and PK Profile Supported by Initial Clinical Data No observed CRS or dose-limiting toxicity and no evidence of target-mediated drug disposition through 3 mg/kg monotherapy*
Enrolling Phase 1/2 Clinical Trial Multi-center U.S. study as single agent and in combination with PD-1 inhibitor Libtayo® First VISTA-blocking antibody administered at a dose anticipated to be therapeutically relevant without
eliciting dose-limiting toxicity** Achieving "Firsts" for the VISTA Field Differentiated Design and Mechanism IgG1, Fc-active antibody designed to selectively block VISTA in the acidic tumor microenvironment Anticipated Near-Term Clinical
Milestones Initial combination PK/safety data in Q1 2024 Topline monotherapy dose escalation data in Q2 2024 Topline combination dose escalation data in Q3 2024 Initial dose expansion data by end of 2024 *As of data cut-off of October 3, 2023
**Anticipate therapeutically relevant clinical doses at approximately 3mg/kg or higher based on preclinical studies
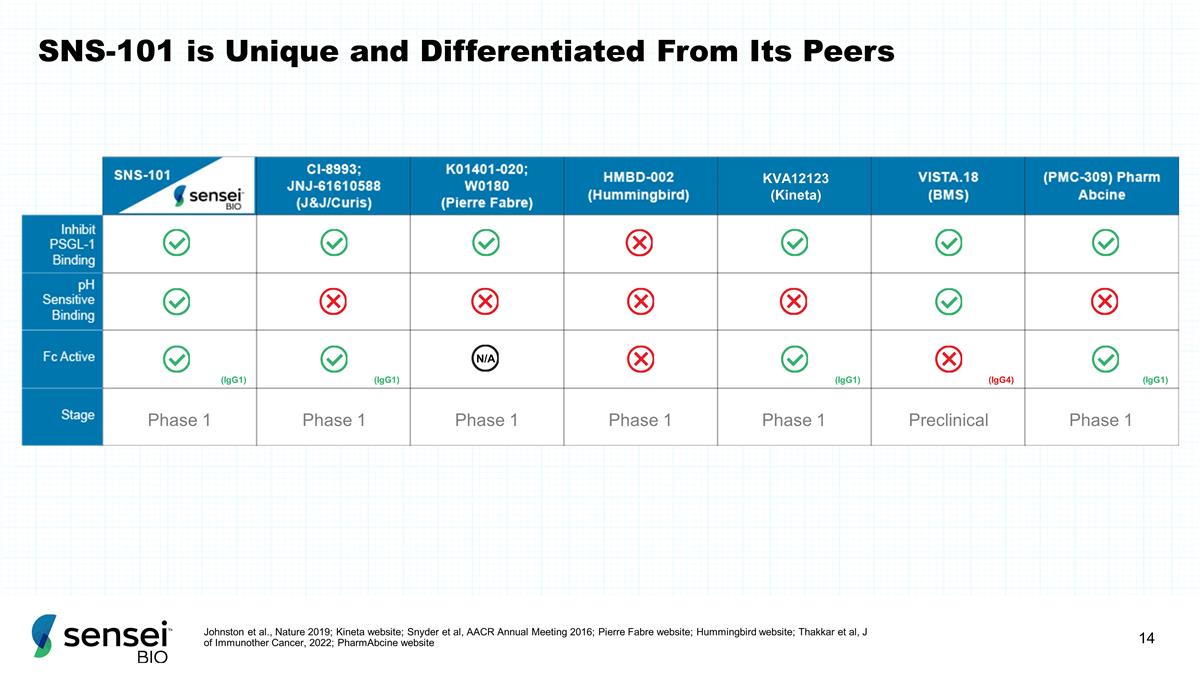
SNS-101 is Unique and
Differentiated From Its Peers Johnston et al., Nature 2019; Kineta website; Snyder et al, AACR Annual Meeting 2016; Pierre Fabre website; Hummingbird website; Thakkar et al, J of Immunother Cancer, 2022; PharmAbcine website KVA12123 (Kineta) Phase 1
Phase 1 Phase 1 Phase 1 Phase 1 Preclinical Phase 1 (IgG1) (IgG1) (IgG1) (IgG4) (IgG1)
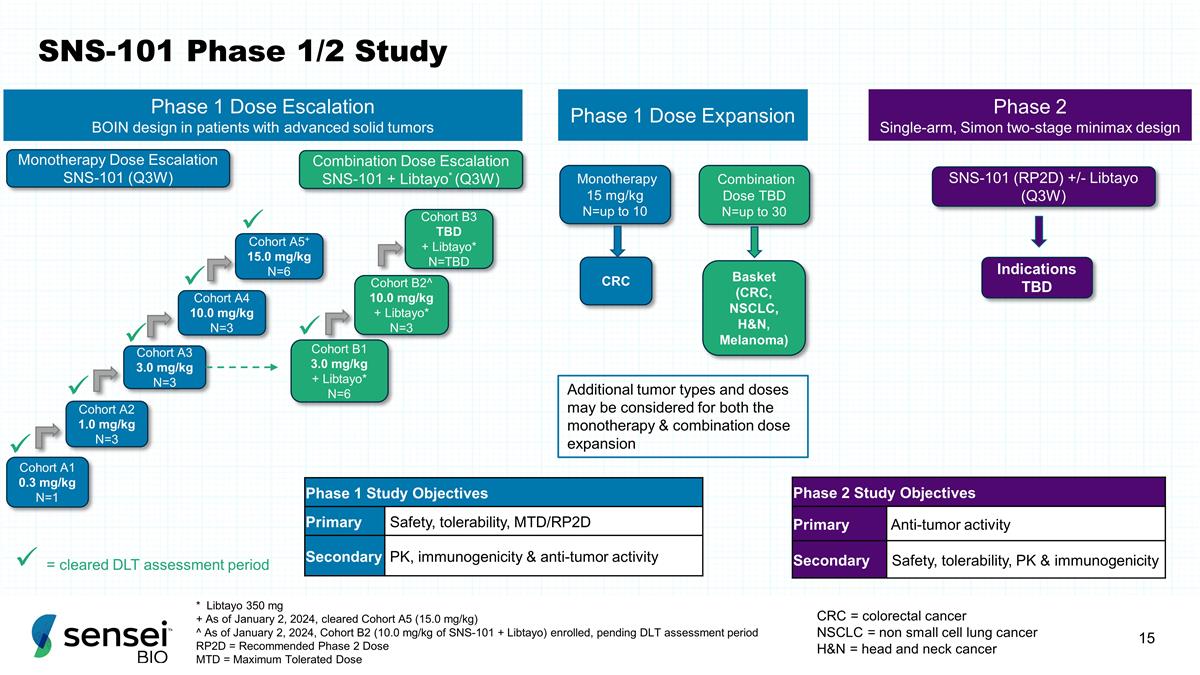
SNS-101 Phase 1/2 Study * Libtayo
350 mg + As of January 2, 2024, cleared Cohort A5 (15.0 mg/kg) ^ As of January 2, 2024, Cohort B2 (10.0 mg/kg of SNS-101 + Libtayo) enrolled, pending DLT assessment period RP2D = Recommended Phase 2 Dose MTD = Maximum Tolerated Dose Cohort A1 0.3
mg/kg N=1 Cohort B1 3.0 mg/kg + Libtayo* N=6 Cohort A2 1.0 mg/kg N=3 Cohort A3 3.0 mg/kg N=3 Cohort A4 10.0 mg/kg N=3 Cohort A5+ 15.0 mg/kg N=6 Cohort B2^ 10.0 mg/kg + Libtayo* N=3 Cohort B3 TBD + Libtayo* N=TBD Monotherapy 15 mg/kg N=up to 10
Combination Dose TBD N=up to 30 CRC Basket (CRC, NSCLC, H&N, Melanoma) Monotherapy Dose Escalation SNS-101 (Q3W) Combination Dose Escalation SNS-101 + Libtayo* (Q3W) Phase 1 Dose Expansion Phase 1 Dose Escalation BOIN design in patients with
advanced solid tumors ü ü ü ü Phase 1 Study Objectives Primary Safety, tolerability, MTD/RP2D Secondary PK, immunogenicity & anti-tumor activity Phase 2 Single-arm, Simon two-stage minimax design SNS-101 (RP2D) +/-
Libtayo (Q3W) Indications TBD Phase 2 Study Objectives Primary Anti-tumor activity Secondary Safety, tolerability, PK & immunogenicity CRC = colorectal cancer NSCLC = non small cell lung cancer H&N = head and neck cancer ü ü
ü = cleared DLT assessment period Additional tumor types and doses may be considered for both the monotherapy & combination dose expansion
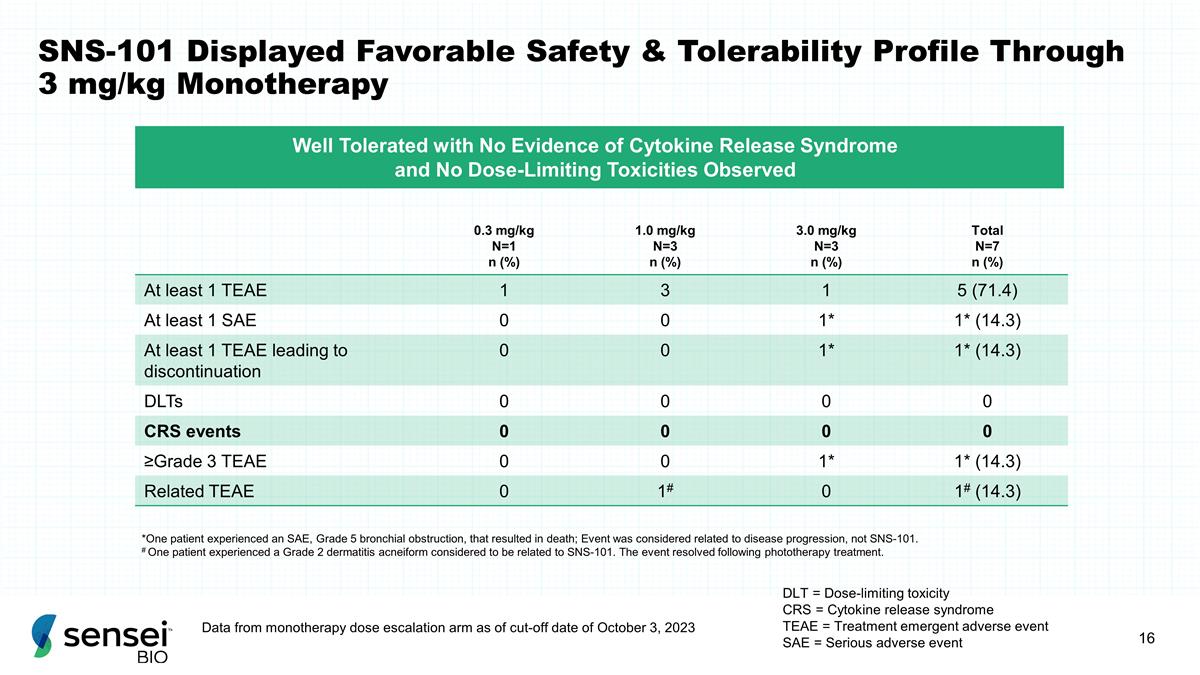
SNS-101 Displayed Favorable Safety
& Tolerability Profile Through 3 mg/kg Monotherapy DLT = Dose-limiting toxicity CRS = Cytokine release syndrome TEAE = Treatment emergent adverse event SAE = Serious adverse event 0.3 mg/kg N=1 n (%) 1.0 mg/kg N=3 n (%) 3.0 mg/kg N=3 n (%) Total
N=7 n (%) At least 1 TEAE 1 3 1 5 (71.4) At least 1 SAE 0 0 1* 1* (14.3) At least 1 TEAE leading to discontinuation 0 0 1* 1* (14.3) DLTs 0 0 0 0 CRS events 0 0 0 0 ≥Grade 3 TEAE 0 0 1* 1* (14.3) Related TEAE 0 1# 0 1# (14.3) *One patient
experienced an SAE, Grade 5 bronchial obstruction, that resulted in death; Event was considered related to disease progression, not SNS-101. # One patient experienced a Grade 2 dermatitis acneiform considered to be related to SNS-101. The event
resolved following phototherapy treatment. Well Tolerated with No Evidence of Cytokine Release Syndrome and No Dose-Limiting Toxicities Observed Data from monotherapy dose escalation arm as of cut-off date of October 3, 2023
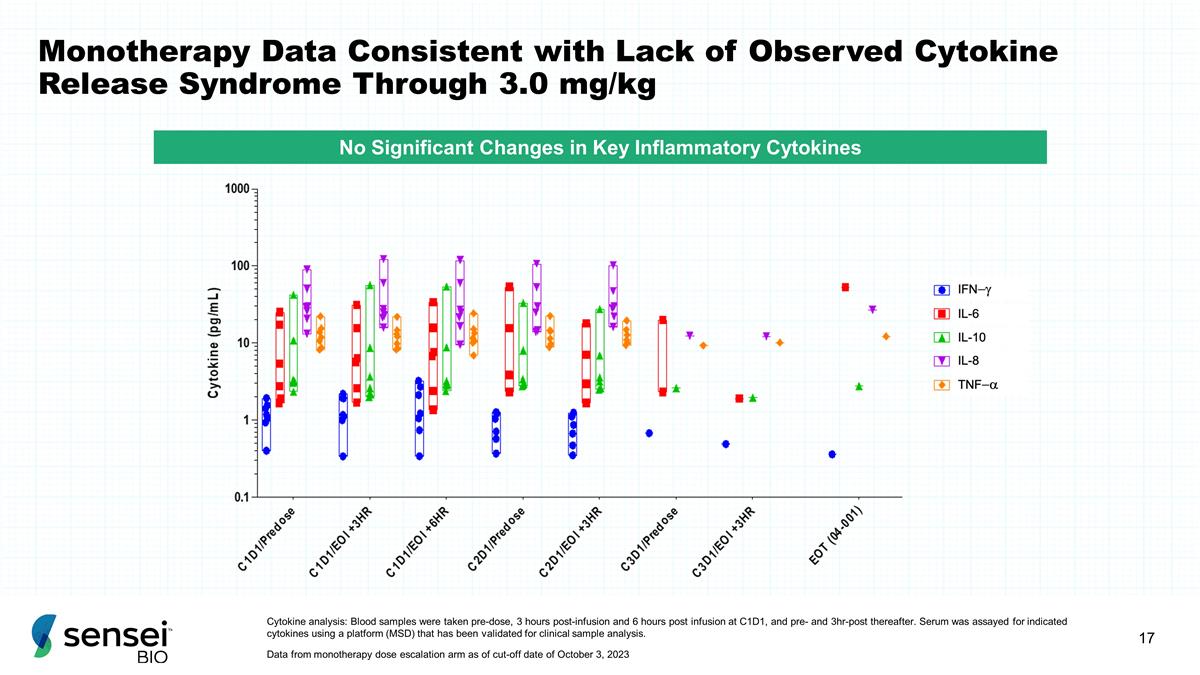
Monotherapy Data Consistent with
Lack of Observed Cytokine Release Syndrome Through 3.0 mg/kg Cytokine analysis: Blood samples were taken pre-dose, 3 hours post-infusion and 6 hours post infusion at C1D1, and pre- and 3hr-post thereafter. Serum was assayed for indicated cytokines
using a platform (MSD) that has been validated for clinical sample analysis. Data from monotherapy dose escalation arm as of cut-off date of October 3, 2023 No Significant Changes in Key Inflammatory Cytokines
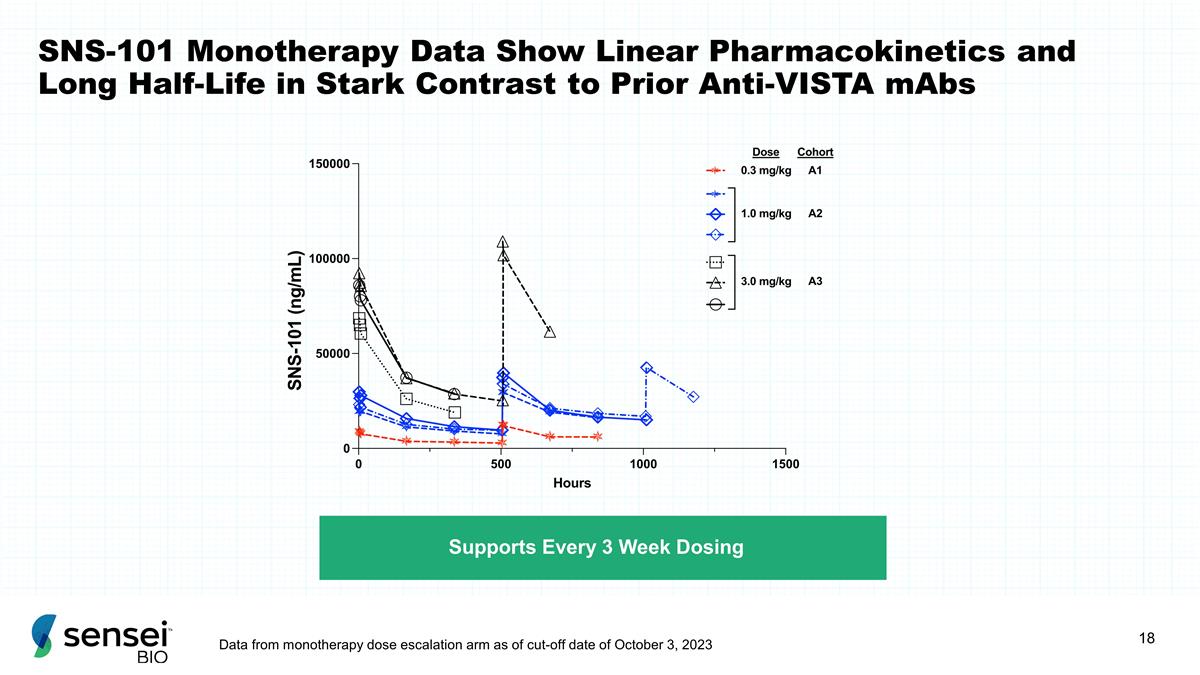
SNS-101 Monotherapy Data Show
Linear Pharmacokinetics and Long Half-Life in Stark Contrast to Prior Anti-VISTA mAbs Supports Every 3 Week Dosing Data from monotherapy dose escalation arm as of cut-off date of October 3, 2023
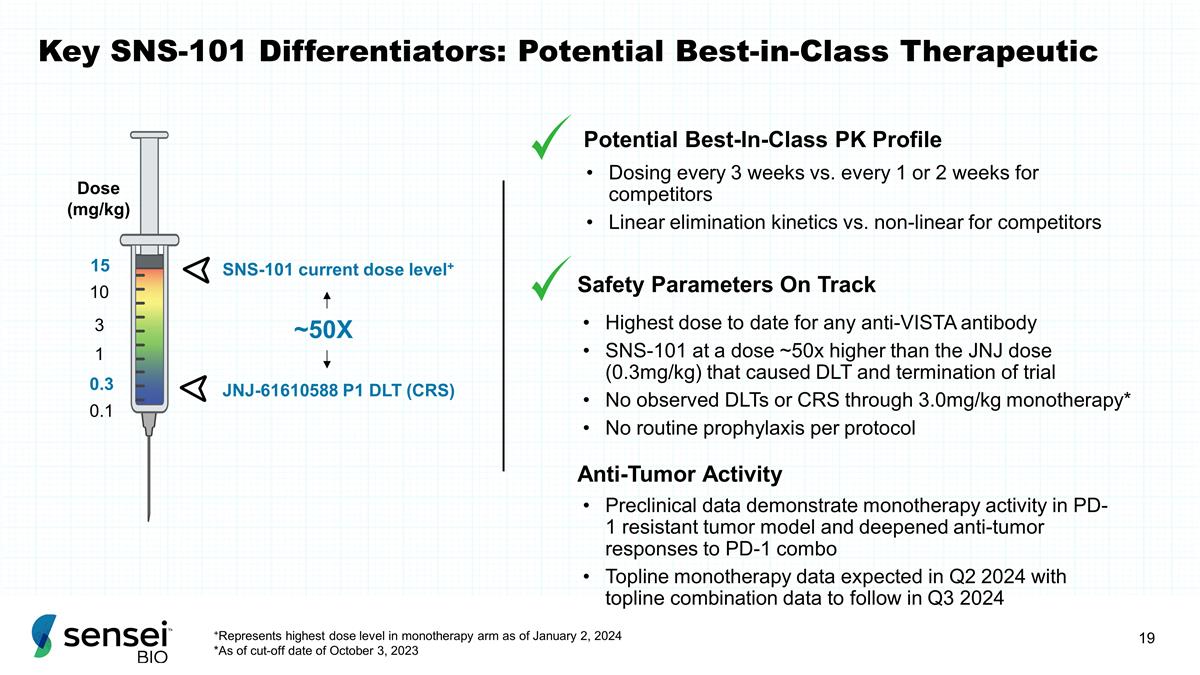
Key SNS-101 Differentiators:
Potential Best-in-Class Therapeutic 0.1 Dose (mg/kg) 0.3 3 10 1 JNJ-61610588 P1 DLT (CRS) SNS-101 current dose level+ ~50X 15 Dosing every 3 weeks vs. every 1 or 2 weeks for competitors Linear elimination kinetics vs. non-linear for competitors
Safety Parameters On Track Highest dose to date for any anti-VISTA antibody SNS-101 at a dose ~50x higher than the JNJ dose (0.3mg/kg) that caused DLT and termination of trial No observed DLTs or CRS through 3.0mg/kg monotherapy* No routine
prophylaxis per protocol Potential Best-In-Class PK Profile +Represents highest dose level in monotherapy arm as of January 2, 2024 *As of cut-off date of October 3, 2023 Anti-Tumor Activity Preclinical data demonstrate monotherapy activity in PD-1
resistant tumor model and deepened anti-tumor responses to PD-1 combo Topline monotherapy data expected in Q2 2024 with topline combination data to follow in Q3 2024
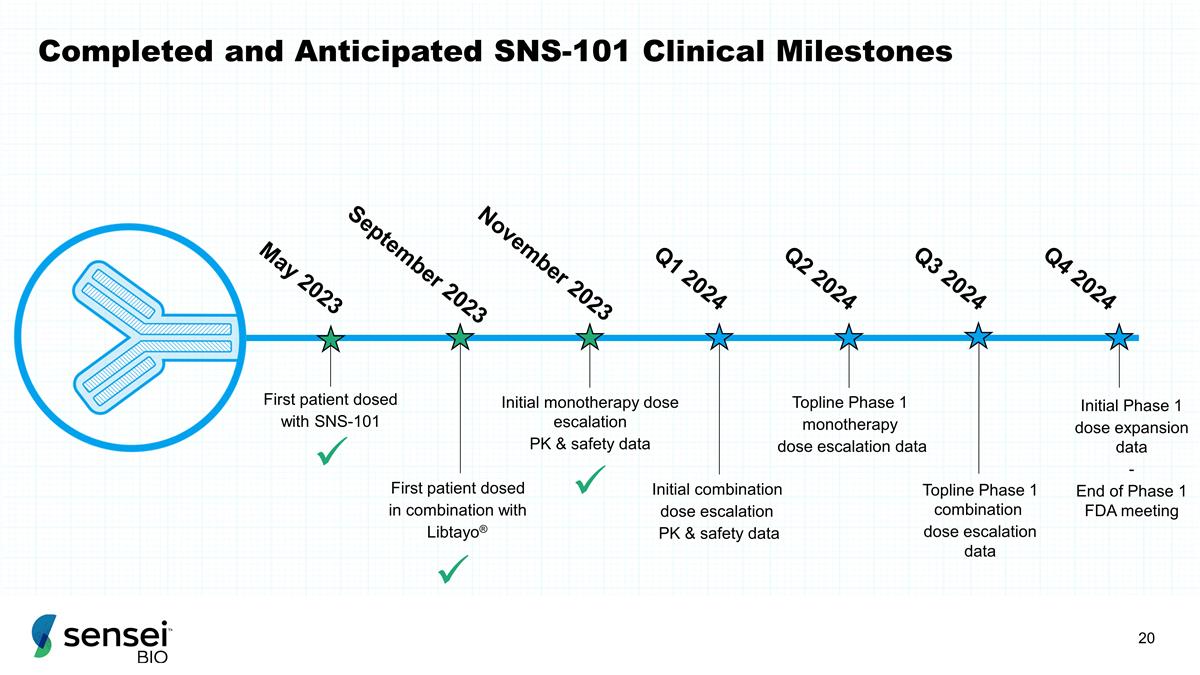
Completed and Anticipated SNS-101
Clinical Milestones First patient dosed with SNS-101 First patient dosed in combination with Libtayo® Initial monotherapy dose escalation PK & safety data Initial combination dose escalation PK & safety data
Topline Phase 1 monotherapy dose escalation data Topline Phase 1 combination dose escalation data Initial Phase 1 dose expansion data - End of Phase 1 FDA meeting May 2023 September 2023 November 2023 Q2 2024 Q3 2024 Q4 2024 Q1 2024 ü
ü ü
SNS-102 (VSIG-4)
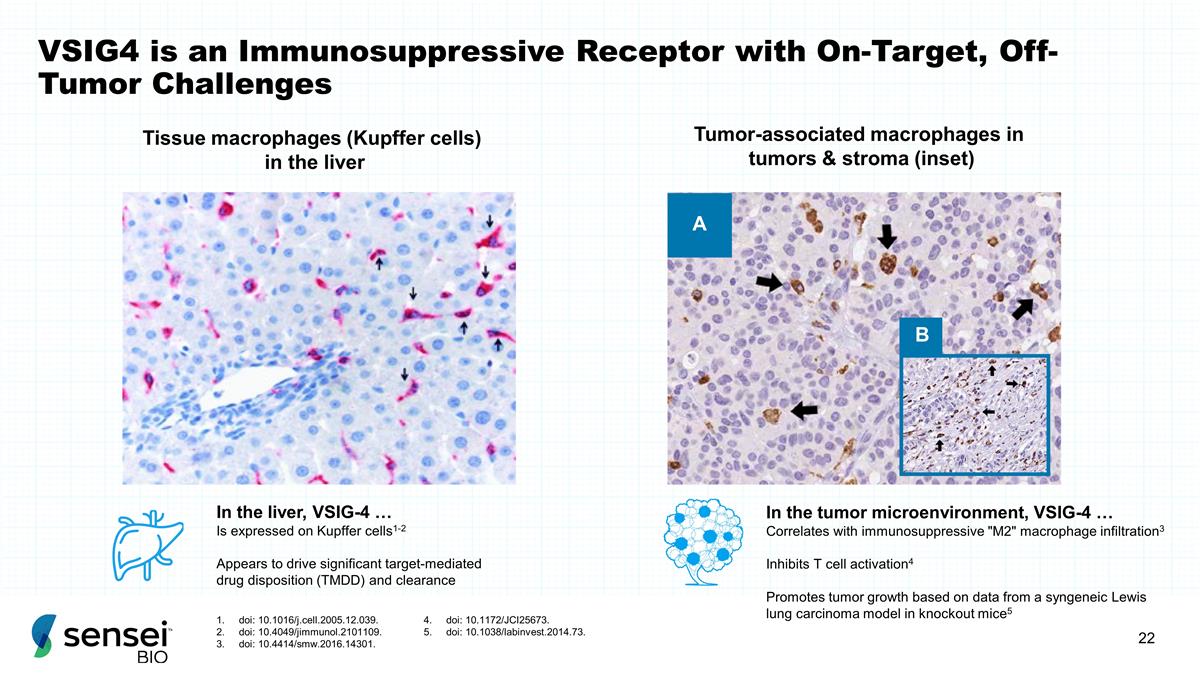
A B Tissue macrophages (Kupffer
cells) in the liver Tumor-associated macrophages in tumors & stroma (inset) In the liver, VSIG-4 … Is expressed on Kupffer cells1-2 Appears to drive significant target-mediated drug disposition (TMDD) and clearance In the tumor
microenvironment, VSIG-4 … Correlates with immunosuppressive "M2" macrophage infiltration3 Inhibits T cell activation4 Promotes tumor growth based on data from a syngeneic Lewis lung carcinoma model in knockout mice5 VSIG4 is an
Immunosuppressive Receptor with On-Target, Off-Tumor Challenges doi: 10.1016/j.cell.2005.12.039. doi: 10.4049/jimmunol.2101109. doi: 10.4414/smw.2016.14301. doi: 10.1172/JCI25673. doi: 10.1038/labinvest.2014.73.
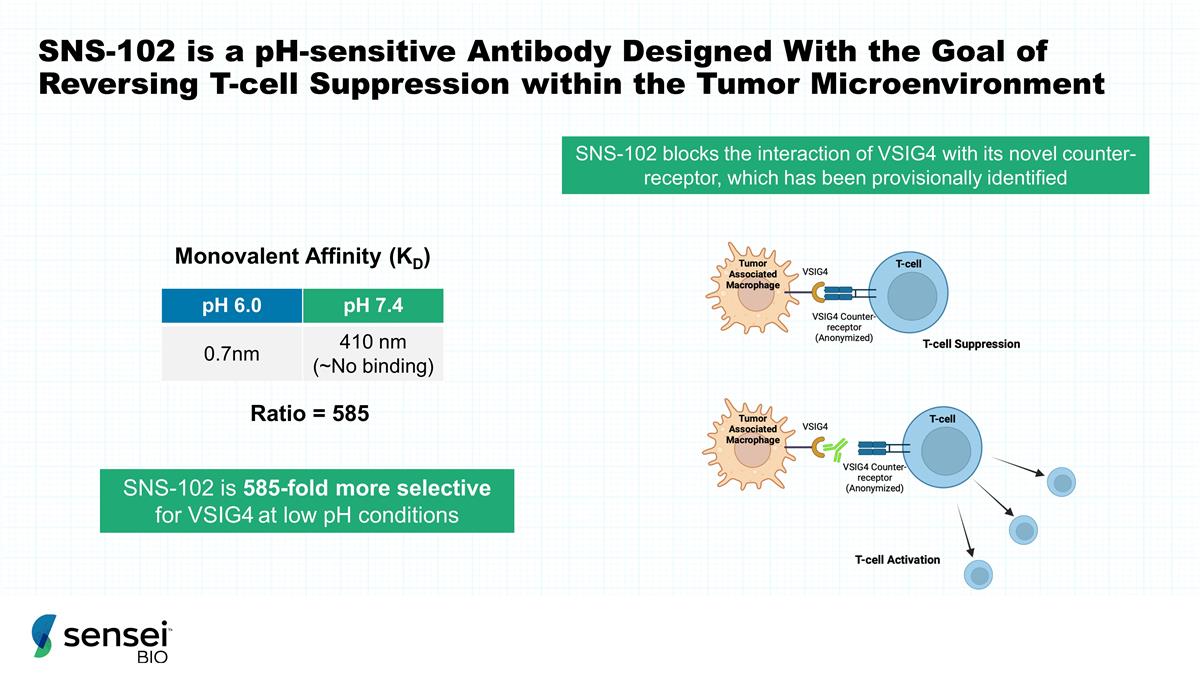
SNS-102 is a pH-sensitive Antibody
Designed With the Goal of Reversing T-cell Suppression within the Tumor Microenvironment SNS-102 blocks the interaction of VSIG4 with its novel counter-receptor, which has been provisionally identified SNS-102 is 585-fold more selective for VSIG4 at
low pH conditions pH 6.0 pH 7.4 0.7nm 410 nm (~No binding) Monovalent Affinity (KD) Ratio = 585
SNS-103 (ENTPDase1/CD39)
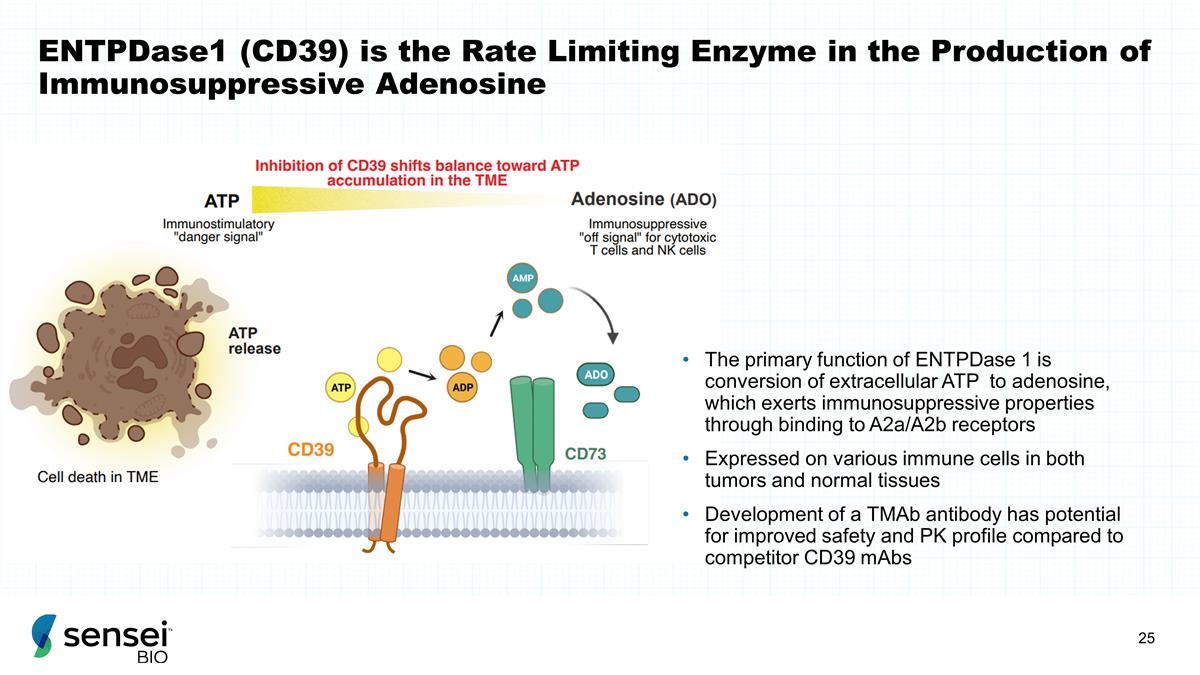
ENTPDase1 (CD39) is the Rate
Limiting Enzyme in the Production of Immunosuppressive Adenosine The primary function of ENTPDase 1 is conversion of extracellular ATP to adenosine, which exerts immunosuppressive properties through binding to A2a/A2b receptors Expressed on
various immune cells in both tumors and normal tissues Development of a TMAb antibody has potential for improved safety and PK profile compared to competitor CD39 mAbs
SNS-201 (VISTAxCD28)
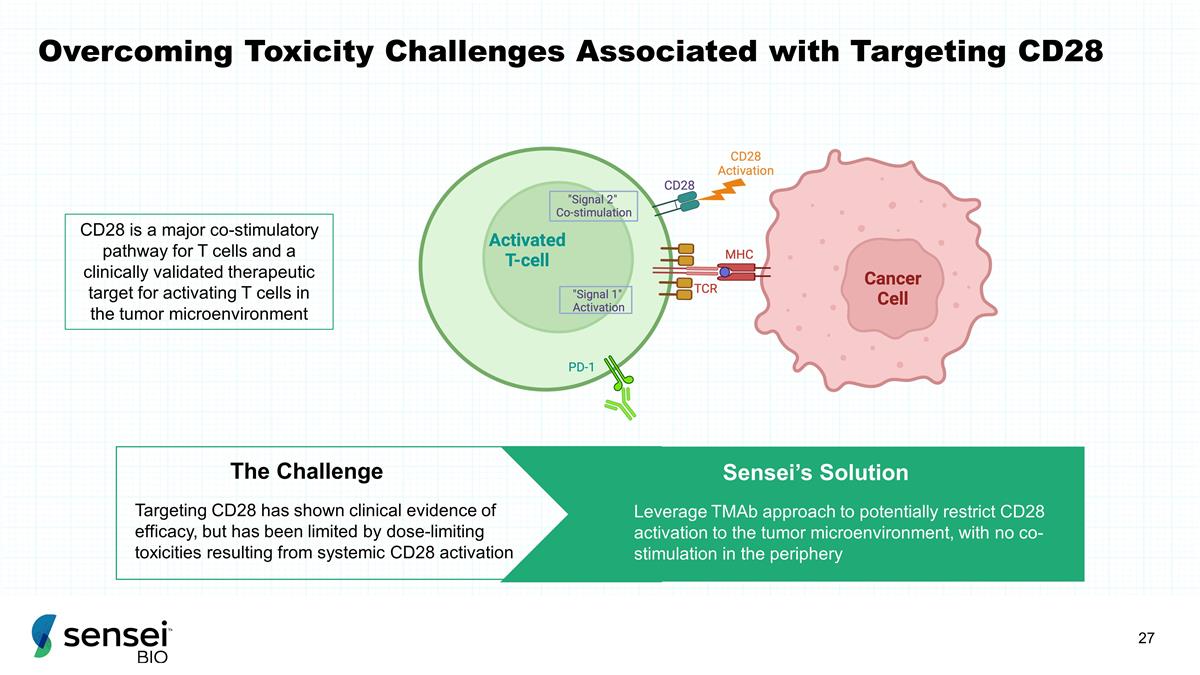
Overcoming Toxicity Challenges
Associated with Targeting CD28 Targeting CD28 has shown clinical evidence of efficacy, but has been limited by dose-limiting toxicities resulting from systemic CD28 activation The Challenge Sensei’s Solution Leverage TMAb approach to
potentially restrict CD28 activation to the tumor microenvironment, with no co-stimulation in the periphery CD28 is a major co-stimulatory pathway for T cells and a clinically validated therapeutic target for activating T cells in the tumor
microenvironment
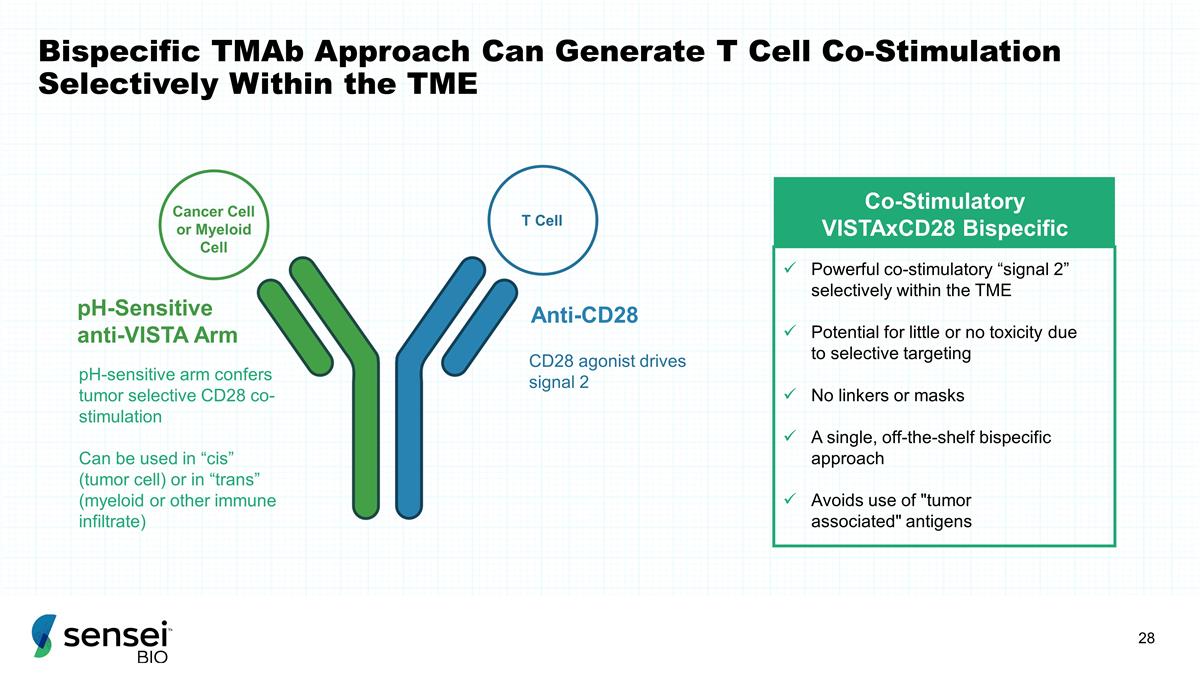
Bispecific TMAb Approach Can
Generate T Cell Co-Stimulation Selectively Within the TME Co-Stimulatory VISTAxCD28 Bispecific Powerful co-stimulatory “signal 2” selectively within the TME Potential for little or no toxicity due to selective targeting No linkers or
masks A single, off-the-shelf bispecific approach Avoids use of "tumor associated" antigens Anti-CD28 pH-Sensitive anti-VISTA Arm Cancer Cell or Myeloid Cell T Cell pH-sensitive arm confers tumor selective CD28 co-stimulation Can be used
in “cis” (tumor cell) or in “trans” (myeloid or other immune infiltrate) CD28 agonist drives signal 2
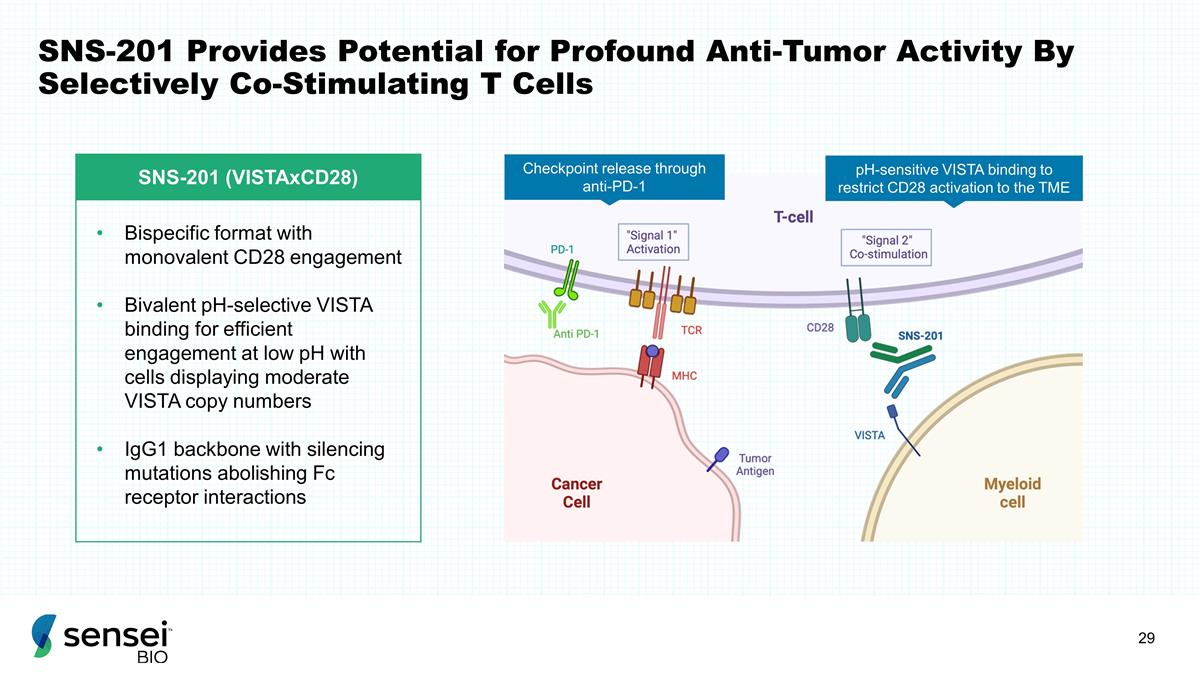
SNS-201 Provides Potential for
Profound Anti-Tumor Activity By Selectively Co-Stimulating T Cells SNS-201 (VISTAxCD28) Bispecific format with monovalent CD28 engagement Bivalent pH-selective VISTA binding for efficient engagement at low pH with cells displaying moderate VISTA
copy numbers IgG1 backbone with silencing mutations abolishing Fc receptor interactions pH-sensitive VISTA binding to restrict CD28 activation to the TME Checkpoint release through anti-PD-1
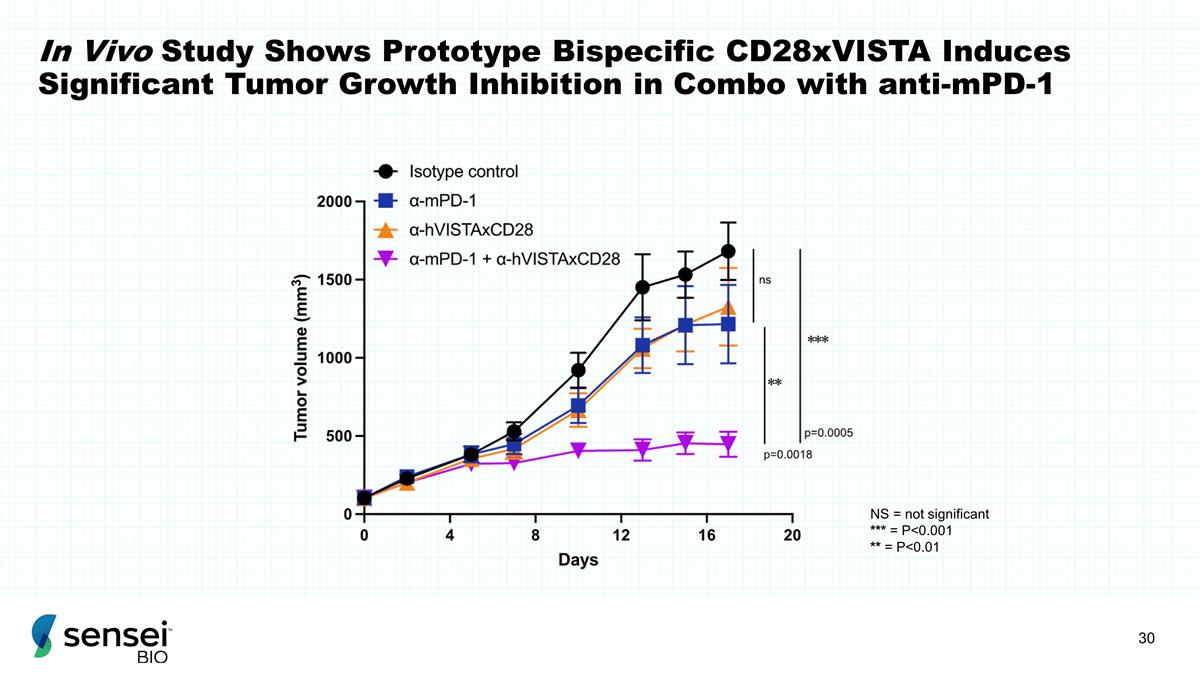
In Vivo Study Shows Prototype
Bispecific CD28xVISTA Induces Significant Tumor Growth Inhibition in Combo with anti-mPD-1 NS = not significant *** = P<0.001 ** = P<0.01
*Consists of cash, cash equivalents
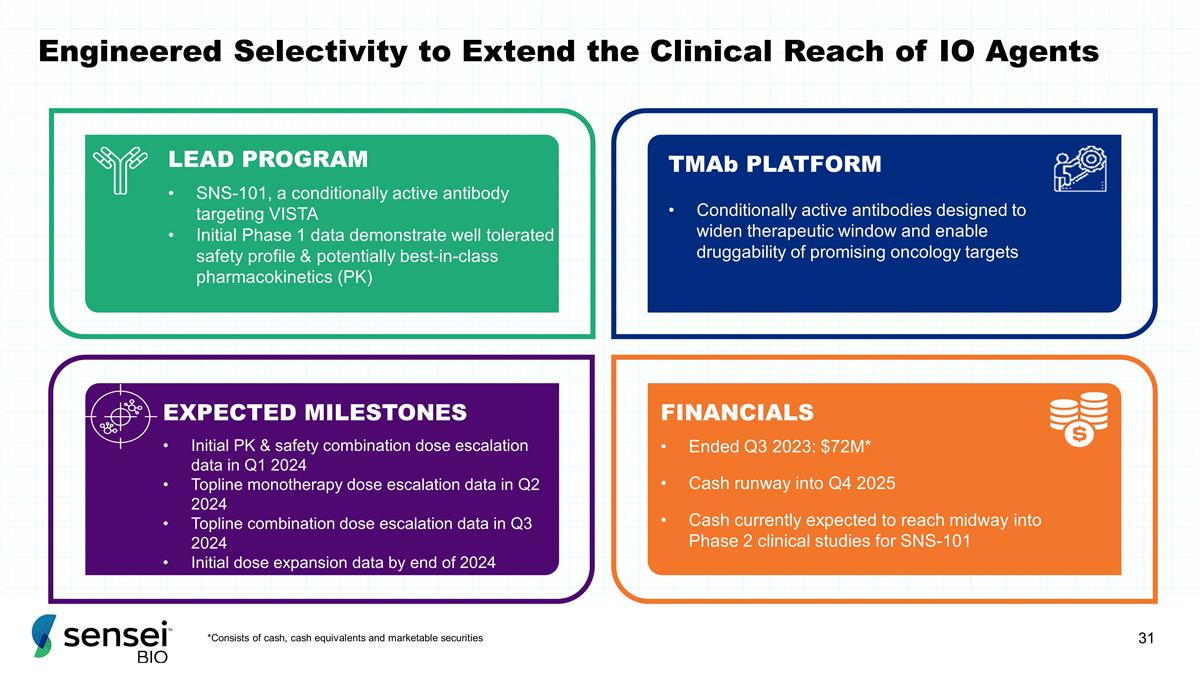
and marketable securities Engineered Selectivity to Extend the Clinical Reach of IO Agents LEAD PROGRAM SNS-101, a conditionally active antibody targeting VISTA Initial Phase 1 data demonstrate well tolerated safety profile & potentially
best-in-class pharmacokinetics (PK) TMAb PLATFORM Conditionally active antibodies designed to widen therapeutic window and enable druggability of promising oncology targets EXPECTED MILESTONES Initial PK & safety combination dose escalation data
in Q1 2024 Topline monotherapy dose escalation data in Q2 2024 Topline combination dose escalation data in Q3 2024 Initial dose expansion data by end of 2024 FINANCIALS Ended Q3 2023: $72M* Cash runway into Q4 2025 Cash currently expected to reach
midway into Phase 2 clinical studies for SNS-101

HQ: 1405 Research Blvd, Suite 125,
Rockville, MD 20850 / MA: 22 Boston Wharf Rd, 7th floor, Boston, MA 02210 senseibio.com
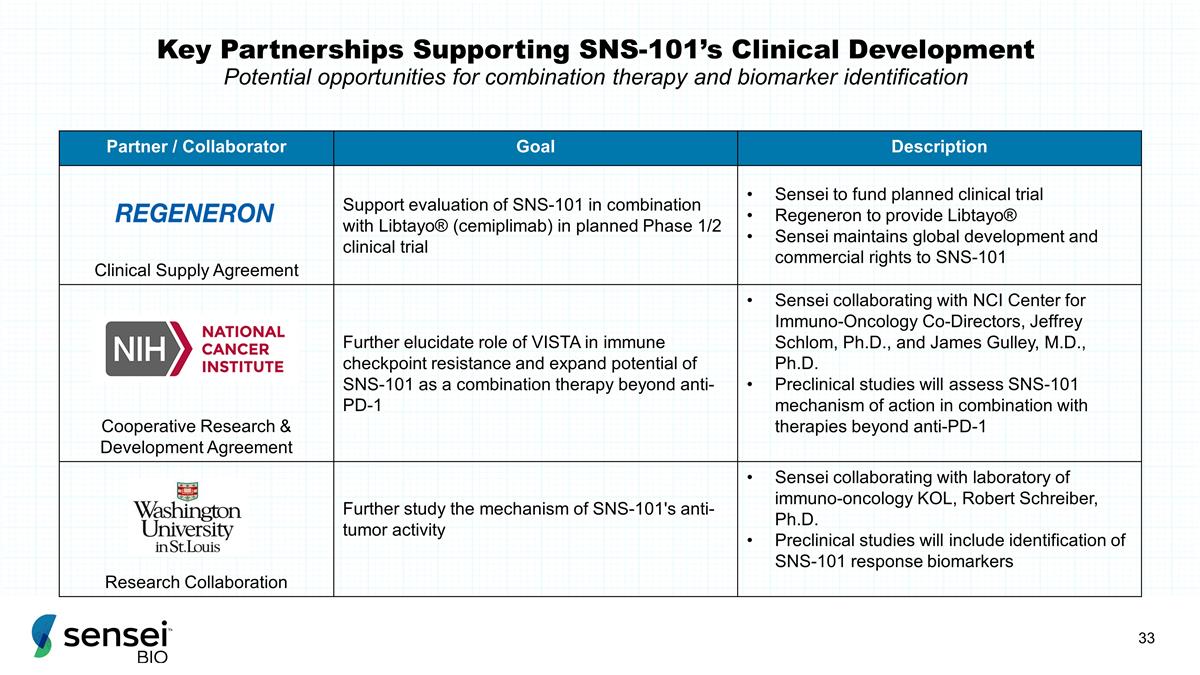
Key Partnerships Supporting
SNS-101’s Clinical Development Potential opportunities for combination therapy and biomarker identification Partner / Collaborator Goal Description Clinical Supply Agreement Support evaluation of SNS-101 in combination with
Libtayo® (cemiplimab) in planned Phase 1/2 clinical trial Sensei to fund planned clinical trial Regeneron to provide Libtayo® Sensei maintains global development and commercial rights to SNS-101 Cooperative Research & Development
Agreement Further elucidate role of VISTA in immune checkpoint resistance and expand potential of SNS-101 as a combination therapy beyond anti-PD-1 Sensei collaborating with NCI Center for Immuno-Oncology Co-Directors, Jeffrey Schlom, Ph.D., and
James Gulley, M.D., Ph.D. Preclinical studies will assess SNS-101 mechanism of action in combination with therapies beyond anti-PD-1 Research Collaboration Further study the mechanism of SNS-101's anti-tumor activity Sensei collaborating with
laboratory of immuno-oncology KOL, Robert Schreiber, Ph.D. Preclinical studies will include identification of SNS-101 response biomarkers
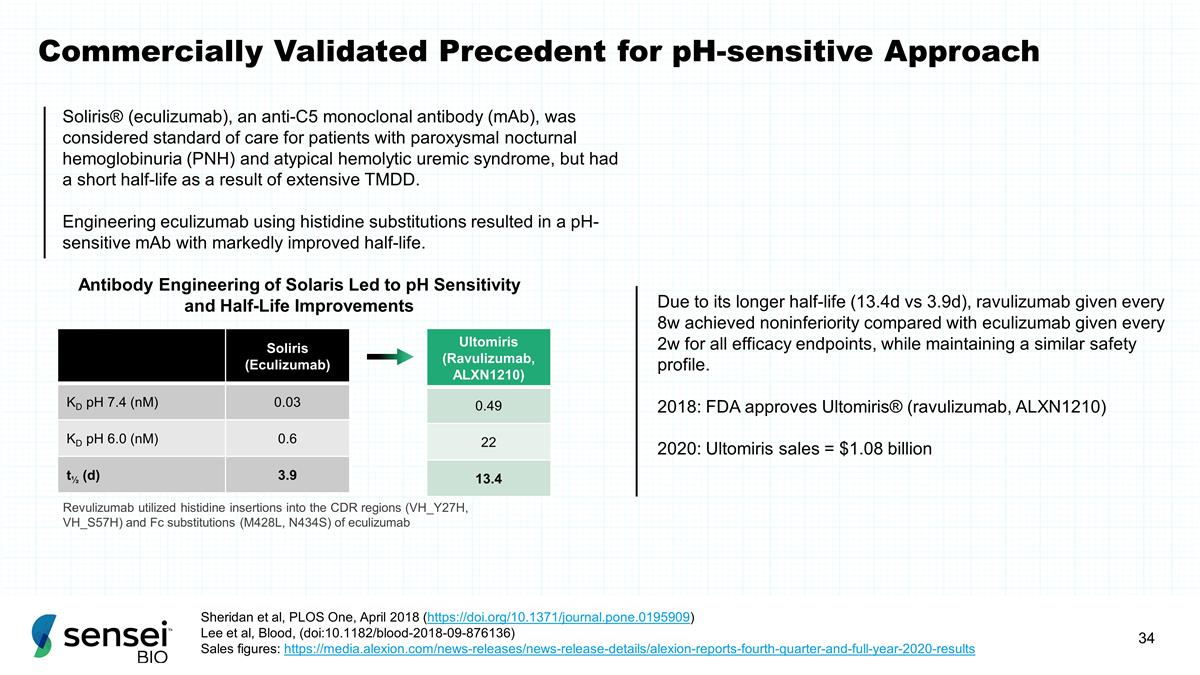
Commercially Validated Precedent
for pH-sensitive Approach Soliris (Eculizumab) KD pH 7.4 (nM) 0.03 KD pH 6.0 (nM) 0.6 t½ (d) 3.9 Ultomiris (Ravulizumab, ALXN1210) 0.49 22 13.4 Due to its longer half-life (13.4d vs 3.9d), ravulizumab given every 8w achieved noninferiority
compared with eculizumab given every 2w for all efficacy endpoints, while maintaining a similar safety profile. 2018: FDA approves Ultomiris® (ravulizumab, ALXN1210) 2020: Ultomiris sales = $1.08 billion Revulizumab utilized histidine
insertions into the CDR regions (VH_Y27H, VH_S57H) and Fc substitutions (M428L, N434S) of eculizumab Soliris® (eculizumab), an anti-C5 monoclonal antibody (mAb), was considered standard of care for patients with paroxysmal nocturnal
hemoglobinuria (PNH) and atypical hemolytic uremic syndrome, but had a short half-life as a result of extensive TMDD. Engineering eculizumab using histidine substitutions resulted in a pH-sensitive mAb with markedly improved half-life. Sheridan et
al, PLOS One, April 2018 (https://doi.org/10.1371/journal.pone.0195909) Lee et al, Blood, (doi:10.1182/blood-2018-09-876136) Sales figures:
https://media.alexion.com/news-releases/news-release-details/alexion-reports-fourth-quarter-and-full-year-2020-results Antibody Engineering of Solaris Led to pH Sensitivity and Half-Life Improvements
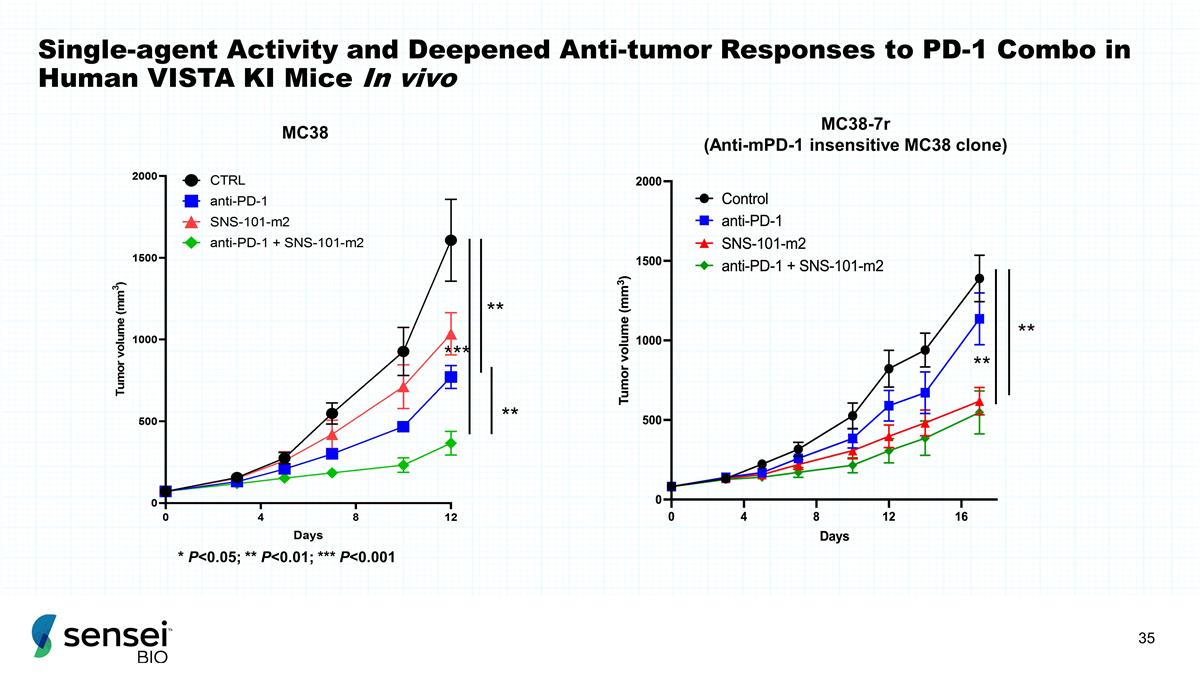
Single-agent Activity and Deepened
Anti-tumor Responses to PD-1 Combo in Human VISTA KI Mice In vivo ** ** MC38-7r (Anti-mPD-1 insensitive MC38 clone) *** ** ** * P<0.05; ** P<0.01; *** P<0.001 MC38
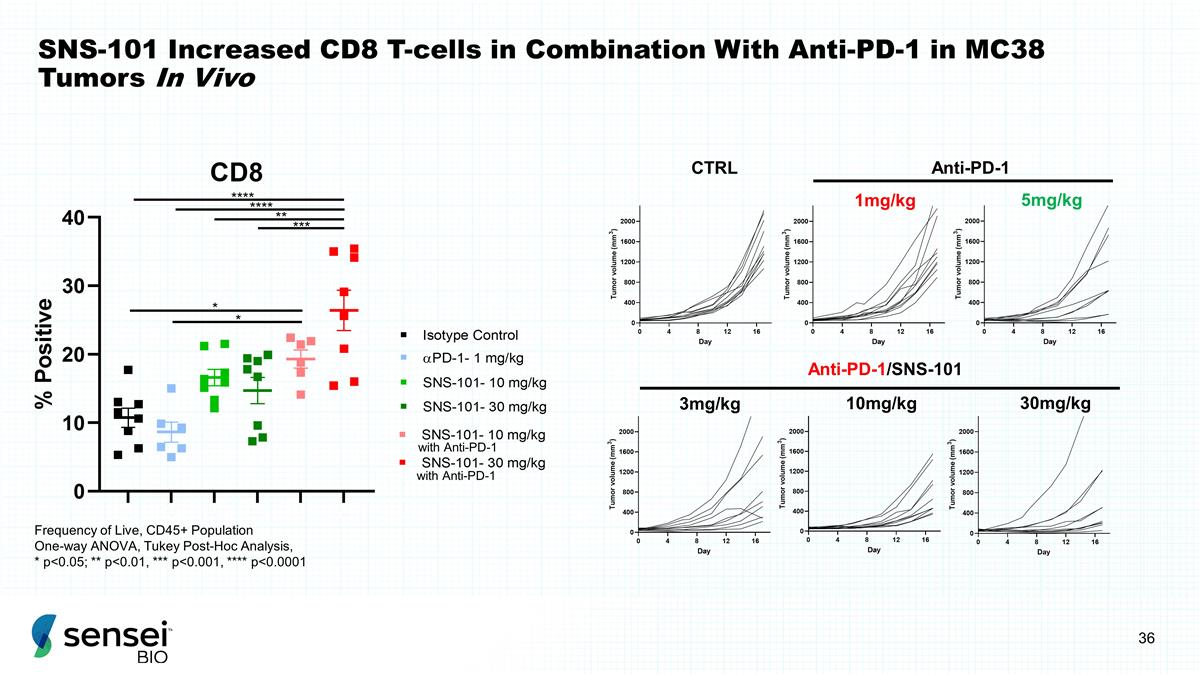
SNS-101 Increased CD8 T-cells in
Combination With Anti-PD-1 in MC38 Tumors In Vivo CTRL Anti-PD-1 1mg/kg 5mg/kg Anti-PD-1/SNS-101 3mg/kg 10mg/kg 30mg/kg Frequency of Live, CD45+ Population One-way ANOVA, Tukey Post-Hoc Analysis, * p<0.05; ** p<0.01, *** p<0.001, ****
p<0.0001 with Anti-PD-1 with Anti-PD-1
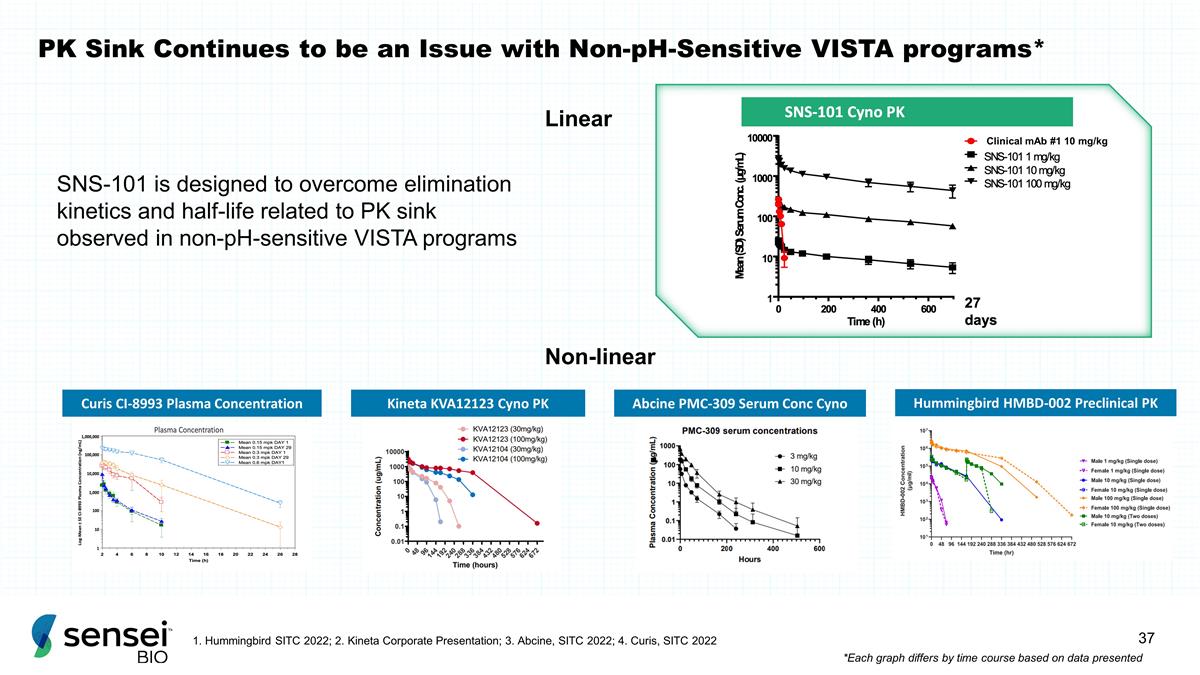
PK Sink Continues to be an Issue
with Non-pH-Sensitive VISTA programs* SNS-101 is designed to overcome elimination kinetics and half-life related to PK sink observed in non-pH-sensitive VISTA programs Curis CI-8993 Plasma Concentration Kineta KVA12123 Cyno PK Abcine PMC-309 Serum
Conc Cyno Hummingbird HMBD-002 Preclinical PK Linear Non-linear 1. Hummingbird SITC 2022; 2. Kineta Corporate Presentation; 3. Abcine, SITC 2022; 4. Curis, SITC 2022 *Each graph differs by time course based on data presented Clinical mAb #1 10 mg/kg
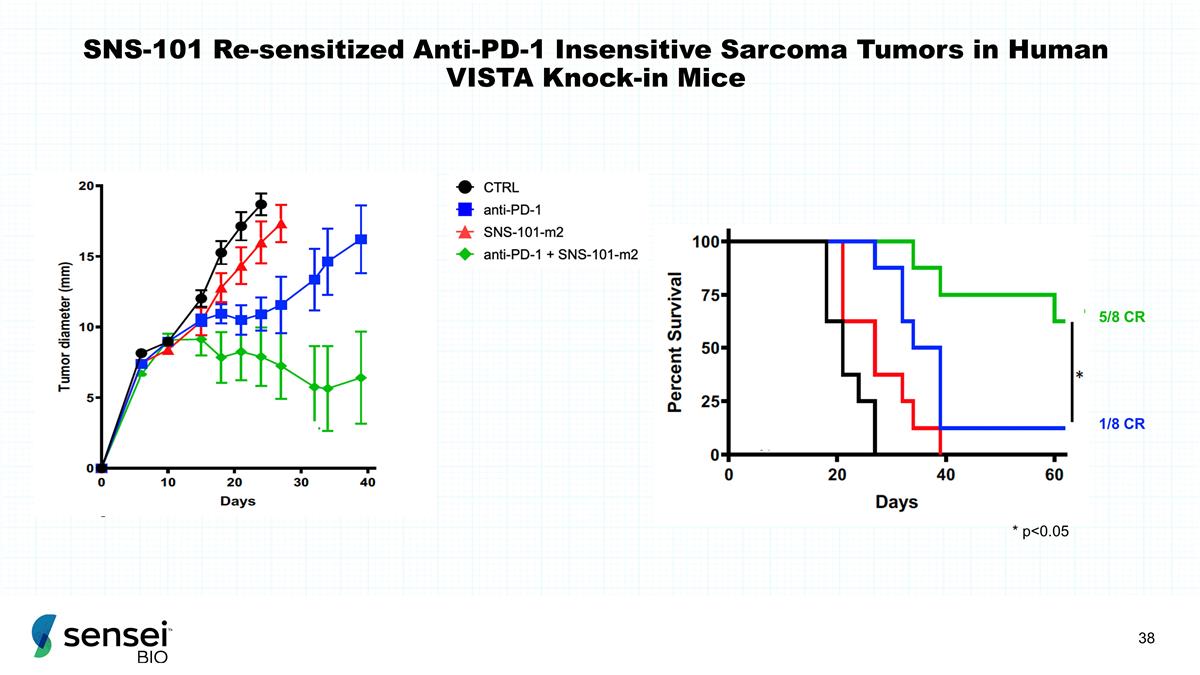
SNS-101 Re-sensitized Anti-PD-1
Insensitive Sarcoma Tumors in Human VISTA Knock-in Mice 5/8 CR 1/8 CR * p<0.05
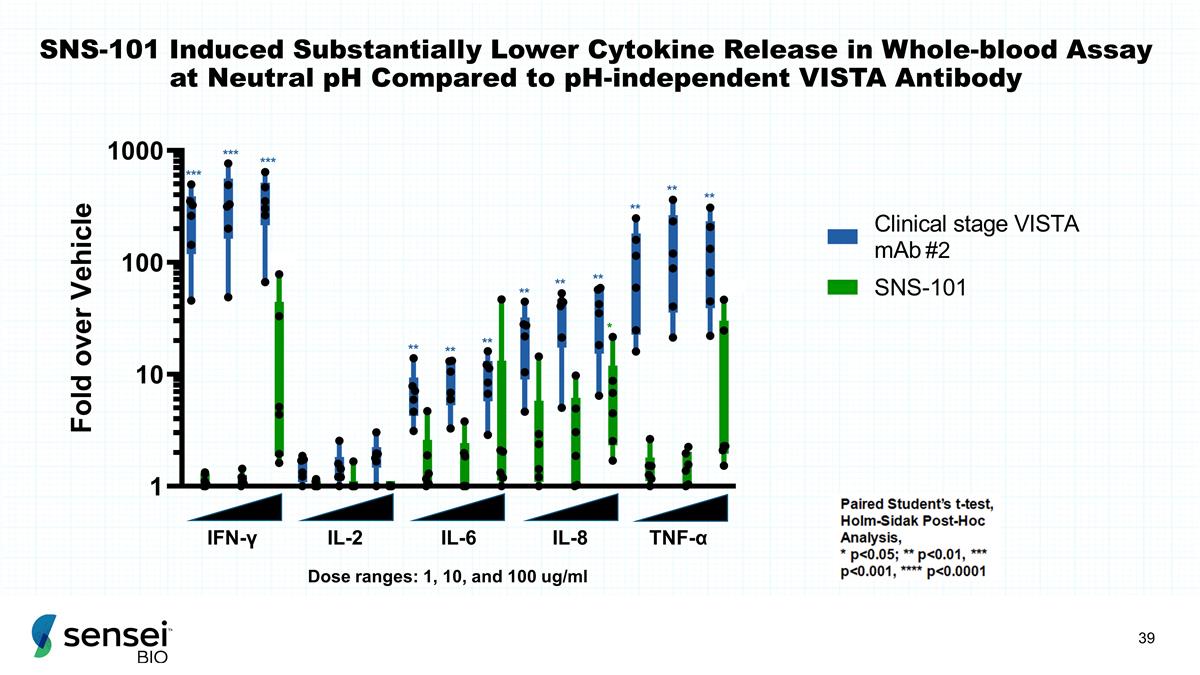
SNS-101 Induced Substantially Lower
Cytokine Release in Whole-blood Assay at Neutral pH Compared to pH-independent VISTA Antibody IFN-γ IL-8 TNF-α IL-6 IL-2 *** *** *** ** * ** ** ** ** ** ** ** ** ** ** Dose ranges: 1, 10, and 100 ug/ml #2
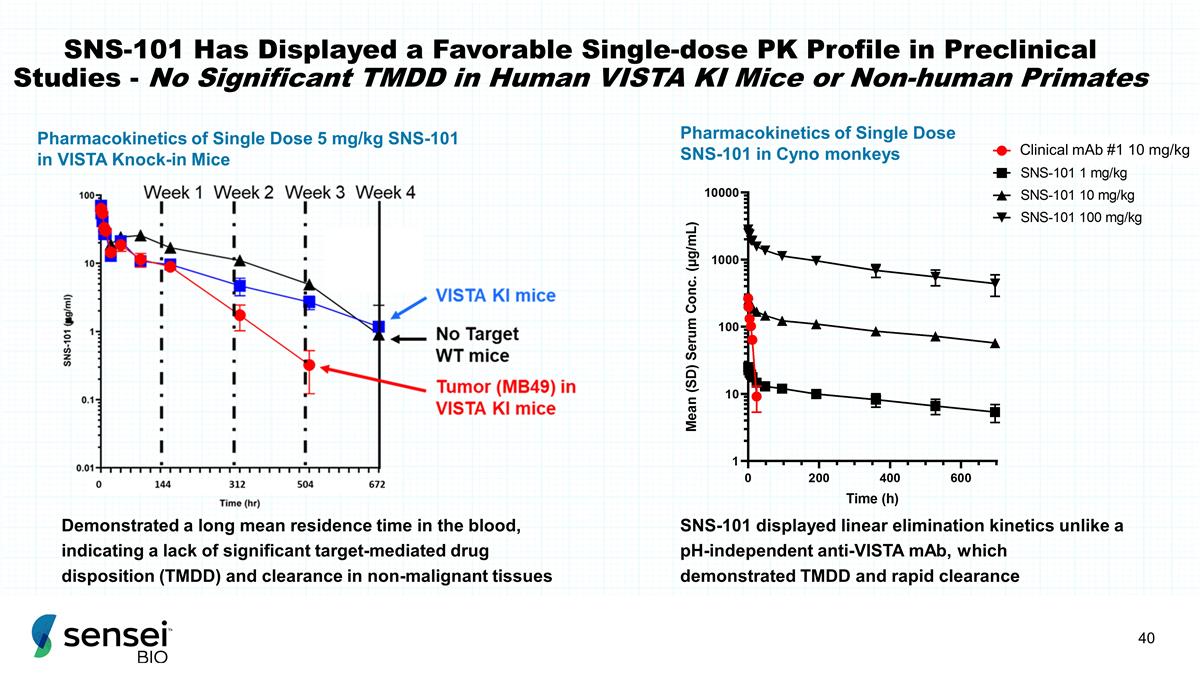
SNS-101 Has Displayed a Favorable
Single-dose PK Profile in Preclinical Studies - No Significant TMDD in Human VISTA KI Mice or Non-human Primates Pharmacokinetics of Single Dose 5 mg/kg SNS-101 in VISTA Knock-in Mice Demonstrated a long mean residence time in the blood, indicating
a lack of significant target-mediated drug disposition (TMDD) and clearance in non-malignant tissues SNS-101 displayed linear elimination kinetics unlike a pH-independent anti-VISTA mAb, which demonstrated TMDD and rapid clearance Pharmacokinetics
of Single Dose SNS-101 in Cyno monkeys Clinical mAb #1 10 mg/kg
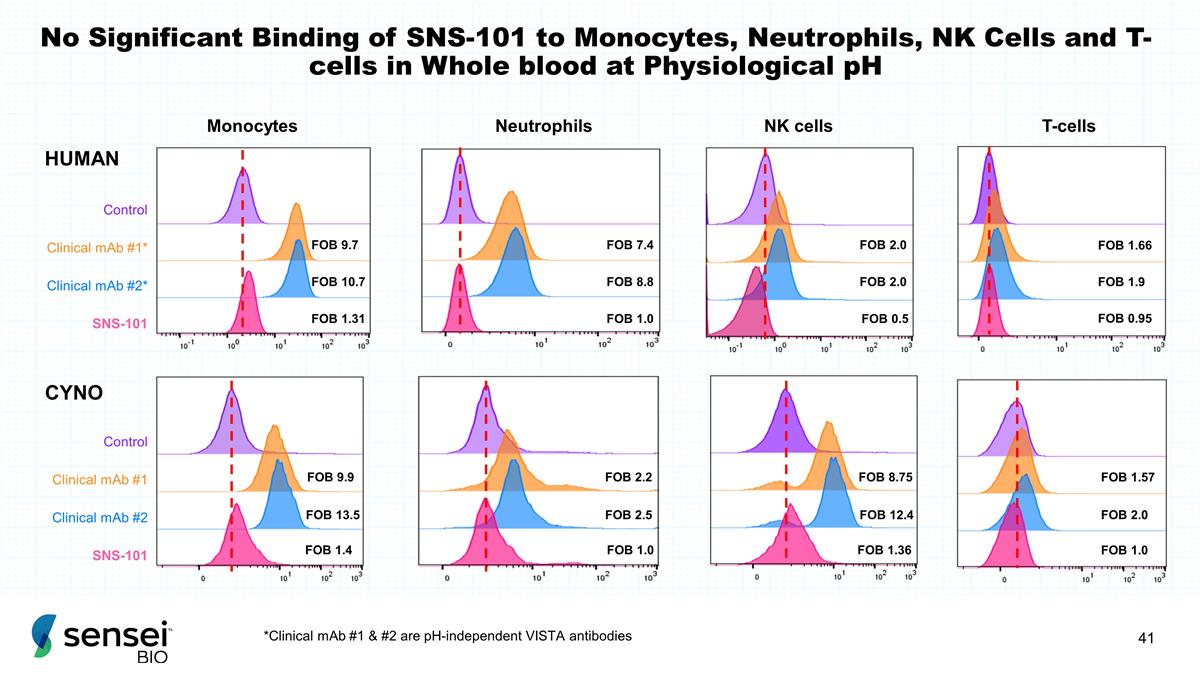
No Significant Binding of SNS-101
to Monocytes, Neutrophils, NK Cells and T-cells in Whole blood at Physiological pH T-cells NK cells FOB 1.57 FOB 2.0 FOB 1.0 FOB 8.75 FOB 12.4 FOB 1.36 Monocytes FOB 9.9 FOB 13.5 FOB 1.4 FOB 2.2 FOB 2.5 FOB 1.0 Neutrophils CYNO HUMAN FOB 7.4 FOB 8.8
FOB 1.0 Clinical mAb #1* Clinical mAb #2* SNS-101 Control FOB 2.0 FOB 2.0 FOB 0.5 Clinical mAb #1 Clinical mAb #2 SNS-101 Control FOB 1.66 FOB 1.9 FOB 0.95 FOB 9.7 FOB 10.7 FOB 1.31 *Clinical mAb #1 & #2 are pH-independent VISTA
antibodies
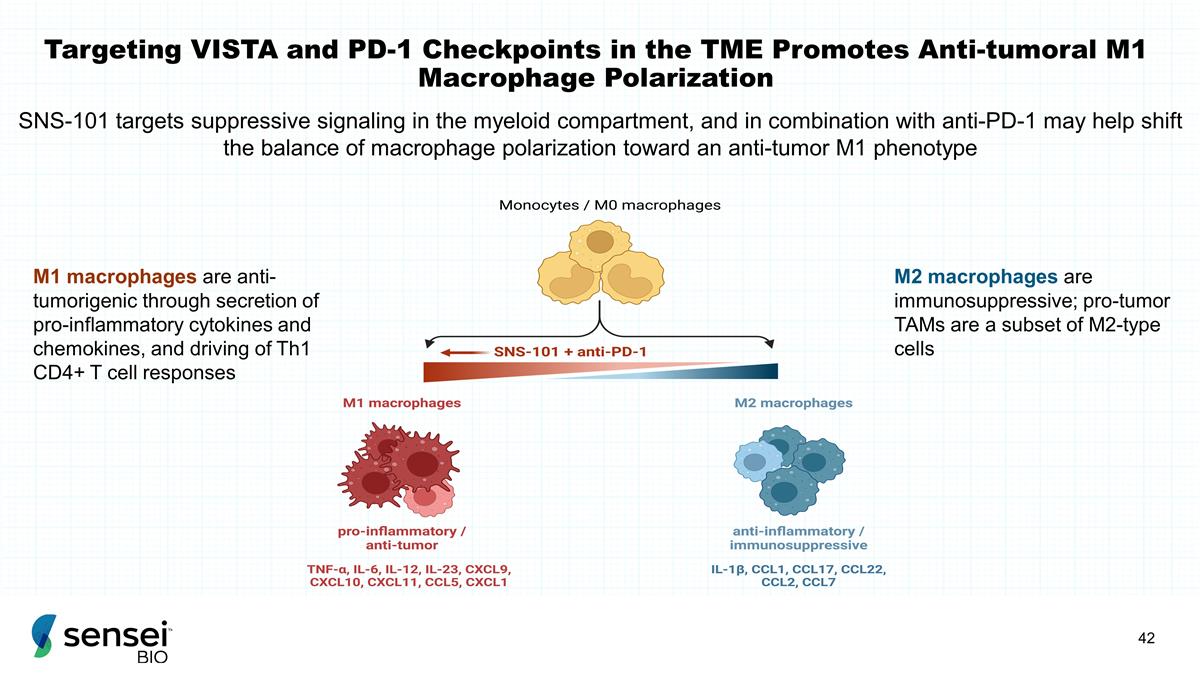
Targeting VISTA and PD-1
Checkpoints in the TME Promotes Anti-tumoral M1 Macrophage Polarization M1 macrophages are anti-tumorigenic through secretion of pro-inflammatory cytokines and chemokines, and driving of Th1 CD4+ T cell responses M2 macrophages are
immunosuppressive; pro-tumor TAMs are a subset of M2-type cells SNS-101 targets suppressive signaling in the myeloid compartment, and in combination with anti-PD-1 may help shift the balance of macrophage polarization toward an anti-tumor M1
phenotype
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesAPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
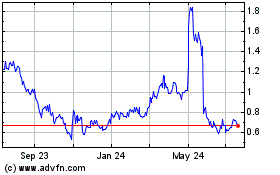
Sensei Biotherapeutics (NASDAQ:SNSE)
Historical Stock Chart
From Mar 2024 to Apr 2024
Sensei Biotherapeutics (NASDAQ:SNSE)
Historical Stock Chart
From Apr 2023 to Apr 2024