FALSE000168954800016895482024-03-052024-03-05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 5, 2024
PRAXIS PRECISION MEDICINES, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | 001-39620 | 47-5195942 |
(State or other jurisdiction of incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) |
Praxis Precision Medicines, Inc.
99 High Street, 30th Floor
Boston, Massachusetts 02110
(Address of principal executive offices, including zip code)
(617) 300-8460
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| | | | | |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | | | | |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | | | | |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | | | | |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trade Symbol(s) | | Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share | | PRAX | | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02. Results of Operations and Financial Condition.
On March 5, 2024, Praxis Precision Medicines, Inc. (the “Company”) announced its financial results for the quarter and full year ended December 31, 2023. A copy of the press release containing these announcements is furnished as Exhibit 99.1 to this Current Report on Form 8-K (the “Current Report”).
Item 7.01. Regulation FD Disclosure.
On March 5, 2024, the Company updated its corporate presentation for use in meetings with investors, analysts and others. The presentation is available in the “Investors + Media” portion of the Company’s website at investors.praxismedicines.com and a copy is furnished as Exhibit 99.2 to this Current Report.
The information in this Current Report under Items 2.02 and 7.01, including Exhibit 99.1 and Exhibit 99.2 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 8.01. Other Events.
As of February 29, 2024, the Company had cash and cash equivalents of $247.6 million, which is anticipated to fund operations into 2026. This cash and cash equivalents information is unaudited and is subject to completion of the Company’s financial closing procedures. The Company’s independent registered public accounting firm has not conducted an audit or review of, and does not express an opinion or any other form of assurance with respect to, this information.
Forward-Looking Statements
This Current Report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws, including statements regarding the Company’s cash and cash equivalents as of February 29, 2024 and its projected cash runway. The forward-looking statements included in this Current Report are subject to a number of risks, uncertainties and assumptions, including, without limitation known and unknown risks, uncertainties and other important factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the important factors discussed under the caption “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 and other filings with the Securities and Exchange Commission. These statements are based only on facts currently known by the Company and speak only as of the date of this Current Report. As a result, you are cautioned not to rely on these forward-looking statements and the Company undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| | | | | | | | |
Exhibit No. | | Description |
| | |
| | |
| | |
| | |
| | |
| 104 | | Cover Page Interactive Data File (embedded within the inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| PRAXIS PRECISION MEDICINES, INC. |
| | | |
Date: March 5, 2024 | By: | | /s/ Marcio Souza |
| | | Marcio Souza |
| | | Chief Executive Officer |
Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2023 Financial Results
Over 3,000 referrals received to date meet pre-qualifying eligibility criteria for ulixacaltamide Phase 3 studies in the Essential3 program for essential tremor (ET); enrollment on track to be completed in 1H 2024 with topline results in 2H 2024
Topline results from the PRAX-628 study in epilepsy patients with photo-paroxysmal response (PPR) expected in 1Q 2024; preliminary analysis of 15 mg cohort exceeded expectations
Tolerability and efficacy results from Part 1 of the EMBRAVE study of elsunersen were presented at the American Epilepsy Society 2023 Annual Meeting, showing 43% median reduction in seizures; received PRIME designation from the European Medicines Agency for the treatment of SCN2A gain-of-function developmental and epileptic encephalopathy (DEE)
Announced licensing partnership with Tenacia Biotechnology to develop and commercialize ulixacaltamide for ET in Greater China with total potential consideration of over $275 million
Completed underwritten public offering with over $160 million in net proceeds to extend cash runway into 2026
BOSTON, Mass., March 5, 2024 — Praxis Precision Medicines, Inc. (NASDAQ: PRAX), a clinical-stage biopharmaceutical company translating genetic insights into the development of therapies for central nervous system (CNS) disorders characterized by neuronal excitation-inhibition imbalance, today provided a corporate update and reported financial results for the fourth quarter and full-year 2023.
“2023 was a transformational year for Praxis. Living by our Dare for More™ motto, we made significant advancements across our portfolio of precision therapies for CNS disorders, established strategic collaborations and strengthened our financial position, which we believe will carry us beyond our upcoming milestones in the year ahead,” said Marcio Souza, president and chief executive officer of Praxis. “Notably, the strong participation we are seeing in our Essential3 Phase 3 studies in ulixacaltamide for essential tremor continues to highlight the significant unmet need for new therapies in essential tremor. We are also encouraged by the latest data supporting our highly differentiated epilepsy portfolio, including elsunersen and PRAX-628.”
Mr. Souza continued, “Looking ahead, we plan to report topline results from the PRAX-628 study in the first quarter, complete enrollment in Essential3 in the first half of this year and expect topline results from the Phase 2 EMBOLD study of PRAX-562 for the treatment of pediatric patients with DEEs mid-year. Together, these advancements, through patient-guided development strategies, will further position Praxis at the forefront of precision medicine for CNS disorders.”
Recent Highlights and Anticipated Milestones:
Cerebrum™ Small Molecule Platform
•Ulixacaltamide for ET: In November 2023, Praxis initiated Essential3, the Phase 3 program for ulixacaltamide. Enrollment in Essential3 is expected to complete in the first half of 2024, with topline results expected in the second half of 2024 to support a planned New Drug Application (NDA) submission in 2025.
oEssential3 is comprised of two simultaneous Phase 3 studies including a 12-week, parallel design study and a 12-week randomized withdrawal study for stable responders.
oEssential3 incorporates learnings from the Phase 2 Essential1 study including the use of a single 60 mg dose, using a modified Activities of Daily Living scale (mADL11) as the primary endpoint based on feedback from the U.S. Food and Drug Administration (FDA), and conducting the study in a decentralized manner. In the Phase 2 Essential1 study, mADL11 produced a statistically significant and clinically meaningful response in ulixacaltamide when compared to placebo after 8 weeks of treatment (p=0.042, nominal).
oEssential3 has over 3,000 referrals who have met the pre-qualifying eligibility criteria from the ongoing recruitment campaign started in November 2023.
•PRAX-628 for Focal Epilepsy: The Phase 2a PPR study to evaluate the efficacy and safety of PRAX-628 across two cohorts, dosed at 15 mg and 45 mg, continues to advance, with plans to report topline results in the first quarter of 2024.
oPPR studies measure electroencephalogram (EEG) signatures after intermittent photic stimulation and are widely used as a marker of anti-seizure efficacy and to aid in dose determination.
oPreliminary analysis of the 15 mg cohort exceeded Praxis’ drug activity expectations and confirmed its decision to initiate a Phase 2b study of PRAX-628 in focal epilepsy in the second half of 2024.
oThe Phase 2a study builds on positive results from the Phase 1 dose escalation study in healthy volunteers.
•PRAX-628 was generally well-tolerated at all tested doses. Pharmacokinetic data demonstrated dose-dependent exposure supporting once-daily dosing without titration to achieve potentially therapeutically effective drug concentration levels.
•Further analysis of patients in the Phase 1 study using qEEG data showed a pharmacodynamic effect at all dose levels and was significantly different from placebo.
•PRAX-562 for SCN2A and SCN8A DEEs: Praxis expects topline results from the PRAX-562 Phase 2 EMBOLD study for the treatment of pediatric patients with DEEs in mid-2024.
oThe EMBOLD study is a randomized, double-blind, placebo-controlled Phase 2 clinical study to evaluate the safety, tolerability, efficacy (motor seizure frequency) and pharmacokinetics of PRAX-562 in pediatric patients aged 2 to 18 years with DEEs, followed by an open-label extension. Up to 20 participants with SCN2A-DEE or SCN8A-DEE are expected to be enrolled.
Solidus™ Antisense Oligonucleotide (ASO) Platform
•Elsunersen (PRAX-222) for SCN2A Gain-of-Function (GoF) Developmental Epilepsies: At the American Epilepsy Society 2023 Annual Meeting, Praxis shared data from Part 1 of the EMBRAVE study, where four patients were dosed once a month for a four-month period. Results showed a 43% median reduction in seizures from baseline, a 48% increase in seizure-free days from baseline and no drug-related treatment-emergent adverse events or serious adverse events.
oElsunersen received PRIME designation from the European Medical Agency (EMA) for the treatment of SCN2A GoF DEEs. The EMA’s PRIME designation provides enhanced development support for priority medicines that target an unmet need and was granted based on the Part 1 data from the EMBRAVE study that showed a reduction in seizures and improvement in seizure free days, as well as preclinical data.
oPraxis is completing multiple global regulatory interactions in the first quarter of 2024 in anticipation of starting the pivotal phase of the program later this year.
Corporate Update:
•In January 2024, Praxis completed an underwritten public offering. The net proceeds from the offering were approximately $161.7 million. As of February 29, 2024, Praxis had cash and cash equivalents of $247.6 million, which is anticipated to fund operations into 2026.
•In January 2024, Praxis announced a licensing partnership with Tenacia Biotechnology to develop and commercialize ulixacaltamide for the treatment of ET in Greater China, including mainland China, Hong Kong, Macau and Taiwan, with total potential consideration of over $275 million.
Fourth Quarter and Full Year 2023 Financial Results:
As of December 31, 2023, Praxis had $81.3 million in cash and cash equivalents, compared to $100.5 million in cash, cash equivalents and marketable securities as of December 31, 2022. This decrease of $19.2 million primarily reflects cash used in operations of $111.1 million during the year ended December 31, 2023, partially offset by $63.4 million in net proceeds from the June 2023 follow-on public offering and $28.2 million in net proceeds from at-the-market offerings.
The Company’s cash and cash equivalents as of December 31, 2023, combined with $161.7 million net proceeds from the January 2024 follow-on offering and $5.3 million from January 2024 at-the-market offerings, are expected to fund operations into 2026.
Praxis recognized $0.5 million and $2.4 million in collaboration revenue during the three months and year ended December 31, 2023, respectively, related to its Option and License Agreement with UCB which was entered into in December 2022.
Research and development expenses were $18.4 million for the fourth quarter of 2023, compared to $28.3 million for the fourth quarter of 2022. Research and development expenses were $86.8 million for the year ended December 31, 2023, compared to $155.0 million for the year ended December 31, 2022. The decrease in research and development expenses for full year 2023 of $68.2 million was primarily attributable to $61.4 million in decreased expenses related to the Company’s Cerebrum™ platform.
General and administrative expenses were $9.9 million for the fourth quarter of 2023, compared to $13.1 million for the fourth quarter of 2022. General and administrative expenses were $42.1 million for the year ended December 31, 2023, compared to $59.9 million for the year ended December 31, 2022. The decrease in general and administrative expenses for full year 2023 of $17.8 million was primarily attributable to a decrease of $7.6 million in professional fees and consulting expenses, $7.4 million in personnel-related expenses due to a decrease in headcount, and $2.9 million in other general and administrative expenses.
Praxis incurred a net loss of $26.9 million for the fourth quarter of 2023, including $5.7 million of stock-based compensation expense, compared to $41.2 million for the fourth quarter of 2022, including $6.4 million of stock-based compensation expense. Praxis reported a net loss of $123.3 million for the year ended December 31, 2023, including $24.9 million of stock-based compensation expense, compared to a net loss of $214.0 million for the year ended December 31, 2022, including $28.6 million of stock-based compensation expense.
As of December 31, 2023, Praxis had 8.8 million shares of common stock outstanding.
About Ulixacaltamide
Ulixacaltamide is a differentiated and highly selective small molecule inhibitor of T-type calcium channels designed to block abnormal neuronal burst firing in the Cerebello-Thalamo-Cortical (CTC) circuit correlated with tremor activity. Ulixacaltamide, the most advanced program within Praxis’ Cerebrum™ small molecule platform, is currently in late-stage development for the treatment of essential tremor, www.praxisessentialtremor.com.
About PRAX-628
PRAX-628 is a next-generation, functionally selective small molecule targeting the hyperexcitable state of sodium-channels in the brain that is currently being developed as a once daily, oral treatment for adult focal onset epilepsy. Preclinical data demonstrates PRAX-628 is differentiated from standard of care, with the potential to be best-in-class for focal epilepsy. In vitro, PRAX-628 has demonstrated superior selectivity for disease-state NaV channel hyperexcitability. In vivo studies of PRAX-628 have demonstrated unprecedented potency in the maximal electroshock seizure (MES) model, a highly predictive translational model for efficacy in focal epilepsy. Data from the PRAX-628-101 study demonstrated that PRAX-628 can be safely dosed in healthy subjects to greater than 15 times the predicted human equivalent of the rodent MES EC50.
About Elsunersen (PRAX-222)
Elsunersen (PRAX-222) is an antisense oligonucleotide (ASO) designed to selectively decrease SCN2A gene expression, directly targeting the underlying cause of early-seizure-onset SCN2A-DEE to treat seizures and other symptoms in patients with gain-of-function SCN2A mutations. In vitro studies of PRAX-222 have demonstrated reduction in both SCN2A gene expression and protein levels. In vivo, PRAX-222 has demonstrated significant, dose-dependent reduction in seizures, improvement in behavioral and locomotor activity and increased survival in SCN2A mouse models, with potential to be the first disease-modifying treatment for SCN2A-DEE. PRAX-222 has received Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPD) from the FDA, and ODD and PRIME designations from the European Medicines Agency (EMA) for the treatment of SCN2A-DEE. The PRAX-222 program is ongoing under a collaboration with
Ionis Pharmaceuticals, Inc. (NASDAQ: IONS), and RogCon, Inc. To learn more about the EMBRAVE study, please visit https://www.embravestudy.com/.
About PRAX-562
PRAX-562 is a first-in-class small molecule in development for the treatment of developmental and epileptic encephalopathy (DEE) as a preferential inhibitor of persistent sodium current, shown to be a key driver of seizure symptoms in early onset SCN2A-DEE and SCN8A-DEE. PRAX-562’s mechanism of sodium channel block is consistent with superior selectivity for disease state sodium channel (NaV) channel hyperexcitability. In vivo studies of PRAX-562 have demonstrated dose-dependent inhibition of seizures up to complete control of seizure activity in SCN2A, SCN8A and other DEE mouse models. PRAX-562 has been generally well-tolerated in three Phase 1 studies and has demonstrated biomarker changes indicative of NaV channel blocking effects. PRAX-562 has received ODD and RPD from the FDA, and ODD from the European Medicines Agency for the treatment of SCN2A-DEE and SCN8A-DEE. To learn more about the EMBOLD study, please visit https://www.emboldstudy.org/.
About Praxis
Praxis Precision Medicines is a clinical-stage biopharmaceutical company translating insights from genetic epilepsies into the development of therapies for CNS disorders characterized by neuronal excitation-inhibition imbalance. Praxis is applying genetic insights to the discovery and development of therapies for rare and more prevalent neurological disorders through our proprietary small molecule platform, Cerebrum™, and antisense oligonucleotide (ASO) platform, Solidus™, using our understanding of shared biological targets and circuits in the brain. Praxis has established a diversified, multimodal CNS portfolio including multiple programs across movement disorders and epilepsy, with four clinical-stage product candidates. For more information, please visit www.praxismedicines.com and follow us on Facebook, LinkedIn and Twitter/X.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws, including express or implied statements regarding Praxis’ future expectations, plans and prospects, including, without limitation, statements regarding the anticipated timing of our clinical trials, the development of our product candidates, the anticipated timing of regulatory submissions and interactions and our projected cash runway, as well as other statements containing the words “anticipate,” “believe,” “continue,” “could,” “endeavor,” “estimate,” “expect,” “anticipate,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “target,” “will” or “would” and similar expressions that constitute forward-looking statements under the Private Securities Litigation Reform Act of 1995.
The express or implied forward-looking statements included in this press release are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: uncertainties inherent in clinical trials; preliminary analyses from ongoing studies differing materially from final data from preclinical studies and completed clinical trials; the expected timing of clinical trials, data readouts and the results thereof, and submissions for regulatory approval or review by governmental authorities; regulatory approvals to conduct trials; and other risks concerning Praxis’ programs and operations as described in its Annual Report on Form 10-K for the year ended December 31, 2023 to be filed and other filings made with the Securities and Exchange Commission. Although Praxis’ forward-looking statements reflect the good faith judgment of its management, these statements are based only on information and factors currently known by Praxis. As a result, you are cautioned not to rely on these forward-looking statements. Any forward-looking statement made in this press release speaks only as of the date on which it is made. Praxis undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Investor Contact:
Praxis Precision Medicines
investors@praxismedicines.com
857-702-9452
Media Contact:
Dan Ferry
Life Science Advisors
Daniel@lifesciadvisors.com
617-430-7576
PRAXIS PRECISION MEDICINES, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands)
(Unaudited)
| | | | | | | | | | | |
| December 31, 2023 | | December 31, 2022 |
| Assets | |
| Cash and cash equivalents | $ | 81,300 | | | $ | 61,615 | |
| Marketable securities | — | | | 38,874 | |
| Prepaid expenses and other current assets | 3,580 | | | 10,351 | |
| Property and equipment, net | 588 | | | 971 | |
| Operating lease right-of-use assets | 2,064 | | | 2,901 | |
| Other non-current assets | 416 | | | 416 | |
| Total assets | $ | 87,948 | | | $ | 115,128 | |
| Liabilities and stockholders’ equity | | |
| Accounts payable | $ | 5,815 | | | $ | 14,672 | |
| Accrued expenses | 7,416 | | | 15,850 | |
| Operating lease liabilities | 2,495 | | | 3,500 | |
| Deferred revenue | 2,553 | | | 5,000 | |
| Common stock | 13 | | | 5 | |
| Additional paid-in capital | 723,577 | | | 606,918 | |
| Accumulated other comprehensive loss | — | | | (173) | |
| Accumulated deficit | (653,921) | | | (530,644) | |
| Total liabilities and stockholders' equity | $ | 87,948 | | | $ | 115,128 | |
PRAXIS PRECISION MEDICINES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended December 31, | | Year Ended December 31, |
| 2023 | | 2022 | | 2023 | | 2022 |
| Collaboration revenue | $ | 515 | | | $ | — | | | $ | 2,447 | | | $ | — | |
| Operating expenses: | | | | | | | |
| Research and development | 18,388 | | | 28,329 | | | 86,766 | | | 155,040 | |
| General and administrative | 9,933 | | | 13,124 | | | 42,054 | | | 59,946 | |
| Total operating expenses | 28,321 | | | 41,453 | | | 128,820 | | | 214,986 | |
| Loss from operations | (27,806) | | | (41,453) | | | (126,373) | | | (214,986) | |
| Other income: | | | | | | | |
| Other income, net | 928 | | | 280 | | | 3,096 | | | 957 | |
| Total other income | 928 | | | 280 | | | 3,096 | | | 957 | |
| Net loss | $ | (26,878) | | | $ | (41,173) | | | $ | (123,277) | | | $ | (214,029) | |
| Net loss per share attributable to common stockholders, basic and diluted | $ | (2.97) | | | $ | (12.98) | | | $ | (18.69) | | | $ | (69.65) | |
| Weighted average common shares outstanding, basic and diluted | 9,060,813 | | 3,172,981 | | 6,594,316 | | 3,073,100 |

CORPORATE OVERVIEW March 2024

2 Forward-looking statements This presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to express or implied statements regarding the current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our preclinical and clinical results and other future conditions. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation, including, without limitation, risks relating to: (i) the success and timing of our ongoing clinical trials, (ii) the success and timing of our product development activities and initiating clinical trials, (iii) the success and timing of our collaboration partners’ product development activities, (iv) the timing of and our ability to obtain and maintain regulatory approval of any of our product candidates, (v) our plans to research, discover and develop additional product candidates, (vi) our ability to enter into collaborations for the development of new product candidates, (vii) our ability to establish manufacturing capabilities, and our collaboration partners’ abilities to manufacture our product candidates and scale production, (viii) our ability to meet any specific milestones set forth herein, and (ix) the potential addressable market sizes for product candidates. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. For further information regarding the risks, uncertainties and other factors that may cause differences between our expectations and actual results, you should review the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2023 to be filed and other filings with the Securities and Exchange Commission. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.

3 GENETICS Focus on therapeutic targets identified through human genetics TRANSLATIONAL TOOLS Translational tools validate potential of target and product candidate and can provide early proof of biology EFFICIENT & RIGOROUS Efficient, rigorous clinical development paths to proof- of-concept in humans applying an agile way of working PATIENT-GUIDED Patient-guided development strategies to deliver on what patients actually need Four pillars guide how we develop medicines

4 4 >$7B 4 2 2026 Praxis is positioned to bring more innovation to patients Assets in clinical trials Commercial opportunity across the portfolio Trial readouts in next twelve months Discovery platforms to optimize drug development Cash runway into

5 CEREBRUM SMALL MOLECULE PLATFORM Cerebrum utilizes deep understanding of neuronal excitability and neuronal networks and applies a series of computational and experimental tools to develop orally available precision therapies SOLIDUS ANTISENSE OLIGONUCLEOTIDE (ASO) PLATFORM Solidus is an efficient, targeted precision medicine discovery and development engine for ASOs anchored on proprietary, computational methodology Two platforms to generate optimized therapies for defined patient populations Molecule Indication Mechanism ulixacaltamide Essential Tremor T-type calcium channel modulator PRAX-628 Focal Epilepsy Sodium channel functional state modulator for broad use PRAX-562 DEE Epilepsies Sodium channel functional state modulator for pediatric use PRAX-020* KCNT1 Epilepsy KCNT1 specific inhibitor PRAX-050 Not disclosed Not disclosed Molecule Indication Mechanism elsunersen SCN2A GoF Gapmer ASO PRAX-080+ PCDH19 Mosaic expression Gapmer ASO PRAX-090+ SYNGAP1 LoF Splice switching ASO PRAX-100+ SCN2A LoF Splice switching ASO *PRAX-020 (KCNT1) is a research collaboration with UCB +PRAX-080 (PCDH19 ), PRAX-090 (SYNGAP1) & PRAX-100 (SCN2A-LoF) ASOs are a collaboration with The Florey Institute of Neuroscience and Mental Health

6 PHASE TWO *PRAX-020 (KCNT1) is a research collaboration with UCB +PRAX-080 (PCDH19 ), PRAX-090 (SYNGAP1) & PRAX-100 (SCN2A-LoF) ASOs are a collaboration with The Florey Institute of Neuroscience and Mental Health PRECLINICAL PHASE ONE PHASE THREEPLATFORM PRAX-020* KCNT1 PRAX-050 Undisclosed PRAX-090+ SYNGAP1 PRAX-100+ SCN2A LoF PRAX-080+ PCDH19 CEREBRUM SMALL MOLECULE PLATFORM SOLIDUS ASO PLATFORM Elsunersen SCN2A GoF DEE Ulixacaltamide Essential Tremor PRAX-562 DEEs PRAX-628 Focal Epilepsy Four clinical stage assets and multitude of early-stage programs UPCOMING MILESTONES Elsunersen • 1H: Regulatory alignment on registrational program PRAX-562 • Mid-year: Ph 2 EMBOLD Study topline Results PRAX-628 • Q1: Phase 2 PPR study topline results • 2H: Initiate Focal Epilepsy Phase 2 Study Ulixacaltamide • 1H: Complete enrollment • 2H: Topline results

7 CEREBRUM SMALL MOLECULE PLATFORM

8 Ulixacaltamide Milestones 1H 2024: Enrollment complete 2H 2024: Topline results 2025: File NDA

9 Essential tremor market is significantly underserved and ready for disruption $4bn+ US ET Market SOURCE: Vetterick, C., Lyons, K.E., Matthews, L.G. et al. The Hidden Burden of Disease and Treatment Experiences of Patients with Essential Tremor: A Retrospective Claims Data Analysis. Adv Ther (2022). https://doi.org/10.1007/s12325-022-02318-8 1. GHOSH (2016) (P.231, C.1, PH.1, L.1-2), 2. Elble RJ. Curr Neurol Neurosci Rep. 2013 Jun;13(6):353. 3. Putzke JD, et al. J Neurol Neurosurg Psychiatry. 2006 Nov;77(11):1235-7. 4. Vetterick, C., Lyons, K.E., Matthews, L.G. et al. The Hidden Burden of Disease and Treatment Experiences of Patients with Essential Tremor: A Retrospective Claims Data Analysis. Adv Ther (2022). https://doi.org/10.1007/s12325-022-02318-8 • Essential Tremor (ET) is the most prevalent movement disorder • People with ET experience significant disruption of their daily activities • Hallmark feature of ET is action tremor that primarily affects the hands2,3 • Almost all ET patients suffer from at least one comorbid condition (e.g., depression, anxiety, sleep disorders, cognitive dysfunction)4 Vast majority of the patients are left without any treatment option • <30% of patients are eligible to receive propranolol due to other medications/health conditions • Of those who start propranolol >50% discontinue after only 1 month • Of those who start propranolol <20% still receive propranolol after 2 years ~2M ET patients seeking treatment 7M US Prevalence1

10 Speaking Dressing Using Keys Hygiene Pouring Working Writing Drinking from a glass Feeding with a spoon Carrying food trays, plates or similar items Overall disability with most affected task • Improvement based on regaining function • Each point reduction provides benefit to a patient • ADL assessment performed by a physician • Aligned as primary endpoint for Essential3 studies with FDA Modified Activities of Daily Living 11 (mADL11) as Primary Endpoint TETRAS = TRG Essential Tremor Rating Assessment Scale; ADL = Activities of Daily Living Each item is individually scored, up to a total of 33 0 = Slightly abnormal. Tremor is present but does not interfere with __. 1 = Mildly abnormal. Spills a little. 2 = Moderately abnormal. Spills a lot or changes strategy to complete task. 3 = Severely abnormal. Cannot drink from a glass or uses straw or sippy cup. 11 items from the well-established TETRAS ADL* scale

11 • Alignment with FDA on dose and primary endpoint • Phase 3 program design structured around the patient needs • Robust recruitment strategy Essential1 Phase 2b set foundation for the Essential3 Phase 3 program Sets up a clear path to registration • Strong efficacy signal with robust endpoint (mADL11) • Early clinical benefit in 8-Week Study • Long-term, durable benefit • Well-tolerated with a differentiated safety profile • Tested Phase 3 design concepts Validated the clinical hypothesis p = 0.042 ULIXACALTAMIDE (n=78) PLACEBO (n=38) Improvement in mADL11 in 8 weeks -2.69 -0.88 Im pr ov em en t Results from Essential1 study, p value is nominal

12 31% 55% 64% 0% 10% 20% 30% 40% 50% 60% 70% Placebo ulixacaltamide ulixacaltamide maintained Majority of patients achieved at least a 3-point change in mADL11 at 8 weeks Durable response in extension study patients who continued through 14 weeks Results from Essential1 study measuring participants achieving meaningful change at 8 and 14 weeks based on ≥3-point improvement from baseline https://praxismedicines.com/wp-content/uploads/2023/09/Giroux_MDS2023_E1_MSD_SUBMIT.pdf Essential1 Study – 8 weeks Essential1: % participants achieving > 3-point change in mADL11 Essential1 Extension–14 weeks p =.023

13 25% 48% 0% 10% 20% 30% 40% 50% Week 8 Propanolol + Placebo Propanolol + ulixacaltamide Adding ulixacaltamide benefited more patients on propranolol with > 3-point improvement Results from Essential1 study showing % of participants on stable propranolol dose achieving meaningful change at 8 weeks based on a meaningful score difference of ≥3 points Essential1: % of patients achieving a >3-point mADL11 Among patients who could tolerate propranolol, adding ulixacaltamide led to ~2-fold increase in the proportion achieving at least a 3-point improvement in mADL11

14 Essential3: An innovative Phase 3 program that optimizes all aspects of study conduct Single Recruiting Expert Review for Eligibility Screening Blinded Study Randomization Study 1: Placebo-controlled Parallel Group Study ulixacaltamide Placebo Study 2: Randomized Withdrawal Study ulixacaltamide Randomization 60 mg Placebo Long-term Safety Study ulixacaltamide CT.gov NCT06087276 8 weeks 4 weeks 12 weeks

15 Essential3 Program is well powered Study Study 1 – Parallel Design Study 2 – Randomized Withdrawal Participants 400 200 Primary endpoint and power mADL11 Change from Baseline to Week 12 between ulixacaltamide and placebo 90% power to detect difference Difference in maintenance of response rate during the 4- week RW period between ulixacaltamide and placebo 90% power to detect difference Stratification Intention tremor status, family history, and propranolol use Main Secondary endpoints o TETRAS-ADL o CGI o PGI CGI-S = Clinical Global Impression of Severity; PGI-S/C = Patient Global Impression of Severity/Change

16 Path to success De-risked Trial design based on key learnings from Essential1 Regulatory alignment based on successful End-of-Phase 2 meeting Efficient Focused execution Single protocol: Optimized screening, enrollment, analysis Streamlined Design Decentralized study to expand reach and reduce study burden to participants Patient-driven Approach mADL11 as a clinically meaningful primary endpoint NDA Readiness Clear path to filing in 2025

17 PRAX-628 Milestones 1Q 2024: Topline results of Phase 2a PPR 2H 2024: Initiate Phase 2b in focal epilepsy

18 The Praxis epilepsy portfolio targets significant unmet need in both the common and rare epilepsy markets >$3B+ US Common and Developmental Epilepsy Market Opportunity 3.5M US Prevalence for Common Epilepsy 14K+ US Prevalence for Rare Epilepsies covered by Praxis’ Portfolio1 1 SCN2A Gof, SCN2A LoF, SYNGAP1, PCDH19, SCN8A, KCNT1 developmental epilepsies 2 PRAX-020 (KCNT1) is a research collaboration with UCB 3 PRAX-080 (PCDH19 ), PRAX-090 (SYNGAP1) & PRAX-100 (SCN2A-LoF) ASOs are a collaboration with The Florey Institute of Neuroscience and Mental Health Praxis Epilepsy Portfolio PRAX-628 PRAX-0202 PRAX-0803 PRAX-0903 PRAX-1003 PRAX-222 PRAX-562

19 Differentiated Profile Goal: Preferential action against neuronal hyperexcitability PRAX-628: Precision medicine therapeutic for Focal Epilepsy Next generation, functionally selective small molecule targeting the hyperexcitable states of sodium channels in the brain, with the potential to address limitations of current treatments • Ideal safety/tolerability profile • Achieves brain penetration • Rapidly achieves therapeutic concentrations without titration • Favorable half-life and PK profile • Optimized efficacy STIMULATION LEVEL epileptic range IDEAL DRUG healthy neuron non preferring drug NE UR AL A CT IV IT Y Untreated neuron
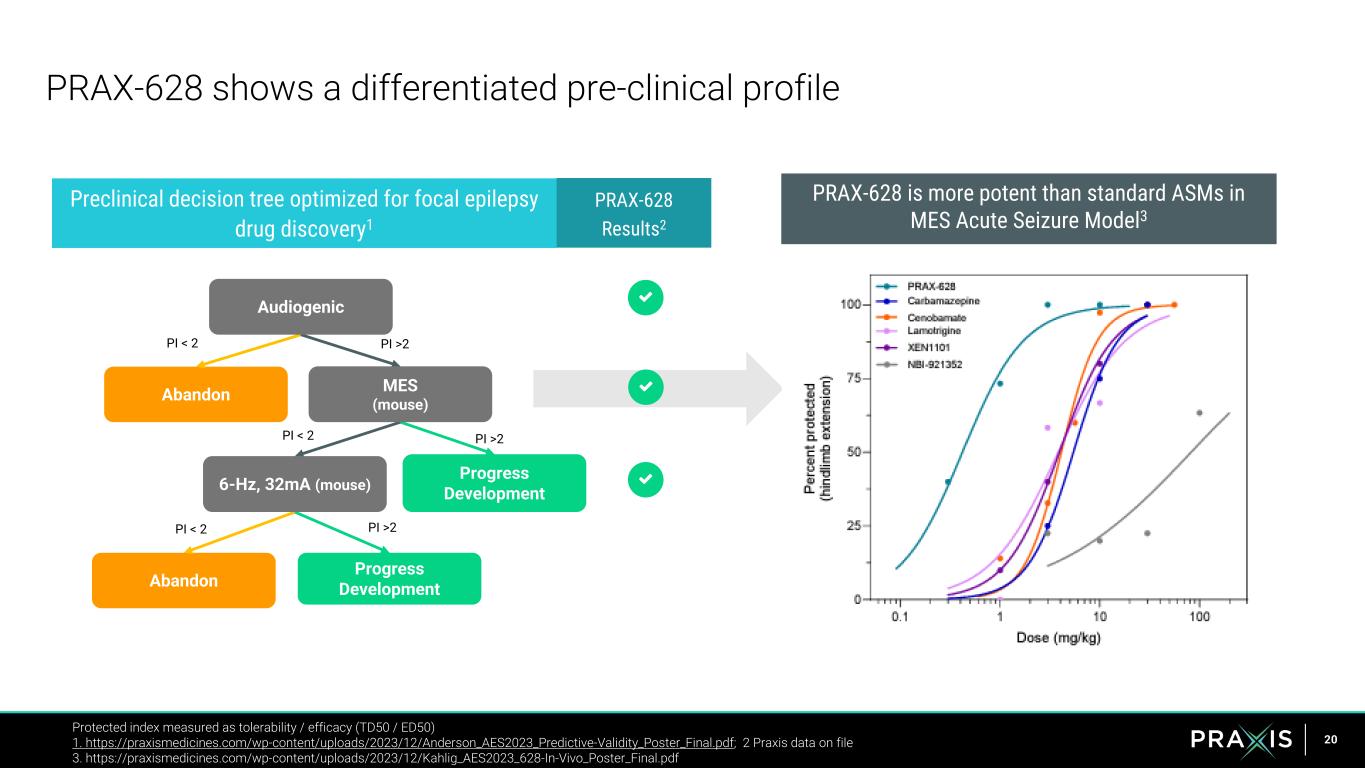
20 PRAX-628 shows a differentiated pre-clinical profile Protected index measured as tolerability / efficacy (TD50 / ED50) 1. https://praxismedicines.com/wp-content/uploads/2023/12/Anderson_AES2023_Predictive-Validity_Poster_Final.pdf; 2 Praxis data on file 3. https://praxismedicines.com/wp-content/uploads/2023/12/Kahlig_AES2023_628-In-Vivo_Poster_Final.pdf PRAX-628 is more potent than standard ASMs in MES Acute Seizure Model3 Preclinical decision tree optimized for focal epilepsy drug discovery1 Progress Development Abandon Progress DevelopmentAbandon PI >2 PI >2 PI >2 PI < 2 PI < 2 PI < 2 Audiogenic MES (mouse) 6-Hz, 32mA (mouse) PRAX-628 Results2

21 PRAX-628 has unprecedented margins over best-in-class ASMs based on MES efficacy and clinical tolerability in humans Source: Praxis data on file (Ph1 study), Cenobamate Cmax: >46,100 ng/mL, 400 mg Cmax (Vernillet et al 2020), XEN1101 Cmax: >107 ng/mL (Phase 1 data) x MES EC50 = multiple of predicted human EC50 based on the rodent MES model 4.8 2.5 15.5 0 2 4 6 8 10 12 14 16 CENOBOMATE XEN-1101 PRAX-628 xM ES EC 50 Additional tolerated margin for PRAX-628 No tolerability concerns toxicity and tolerability levels 400 mg 25 mg 30 mg Clinically Tolerated Exposures A

22 0 5 23 0 4 8 12 16 20 24 Cenobamate XEN1101 PRAX-628 Phase 1 study demonstrated brain activity and rapid achievement of therapeutic concentrations - 5 10 15 20 25 0 1 2 3 4 5 6 7 8 QE EG C OM PO SI TI VE C HA NG E HOURS POST DOSE • Composite endpoint from qEEG showed separation of drug versus placebo in the SAD and MAD cohorts • Difference between PRAX-628 and placebo significant for all doses at first point measured • Effect consistent with known PK profile • PRAX-628 achieves nearly complete coverage on Day 1 400 mg 25 mg 30 mg PRAX-628 composite qEEG change Hours of day 1 drug coverage

23 PRAX-628 has presented an ideal precision ASM profile through Phase 1 https://praxismedicines.com/wp-content/uploads/2023/12/Kahlig_AES2023_628-In-Vivo_Poster_Final.pdf https://praxismedicines.com/wp-content/uploads/2023/09/IEC2023_628-SAD-MAD.pdf Ability to provide maximum effect Rapidly achieves therapeutic concentrations Brain activity evidence Therapeutic level of coverage achieved continuously Tolerable safety profile Ideal Treatment

24 The Phase 2 PRAX-628 Photo Paroxysmal Response (PPR) study will provide insight into efficacy and inform dose selection for pivotal studies 1 Source: First Pub: C.D. Binnie Electroencephalography and clinical neurophysiology A, 1986, 63, 35-41; LEV paper: DGA Kasteleijn-Nolst Trenité Epilepsy Research 25(1996) 225-230; DGA Kasteleijn-Nolst Trenité Neurology 93(6) 2019 e559-e567 cenobamate paper 18 Hz 60 Hz 2 Hz BASELINE: PLACEBO WITH DRUG LI GH T FR EQ UE NC Y PPR present PPR eliminated RESPONSE • The PPR Photosensitivity Model has been used to assess many ASMs1 • Reduction of PPR photosensitivity range correlates to success in larger studies • Expected results from 15 and 45mg single-dose cohorts in Q1 2024 Study Compares Ranges Which Trigger PPR Response

25 PRAX-562 Milestones Mid-2024: Topline results in Phase 2 EMBOLD Study

26 Preclinical and emerging clinical data demonstrate PRAX-562 has the potential to be a first- and best-in-class small molecule for DEEs Superior selectivity for disease-state NaV channel hyperexcitability Convenient auto-titration regimen with stable PK Unprecedented therapeutic window with potential for superior safety and efficacy PRAX-562 SCN2A, SCN8A FORMULATED FOR PEDIATRIC USE SMALL MOLECULE FUNCTIONAL STATE MODULATOR

27 PRAX-562 Phase 1 summary Source: Praxis data on file; https://investors.praxismedicines.com/news-releases/news-release-details/praxis-precision-medicines-provides-corporate-update-and-5 * Co-administration of supra-therapeutic doses of PRAX-562 and oxcarbazepine led to additive sodium blocking effects, including resulting in SAEs All TEAEs mild to moderate as stand-alone therapy*, with headache & dizziness most common TEAEs Significant changes observed between placebo and PRAX-562 on qEEG biomarkers PRAX-562 has been generally well tolerated in over 130 healthy volunteers No MTD at exposures multiple fold above therapeutic range indicates potential for superior therapeutic index

28 PRAX-562 Phase 2 EMBOLD study topline data expected in mid-2024 + Participants receive either 0.5 mg/kg/day PRAX-562 QD for 16 weeks or 0.5 mg/kg/day PRAX-562 QD for 12 weeks & matching placebo QD for 4 weeks. Participants in the PRAX-562/placebo arm will receive placebo for 4 consecutive weeks during the 16-week treatment period, with timing of placebo administration blinded for both participants and investigator. Dose adjustment is permitted to a max of 1.0 mg/kg/day and a min of 0.25 mg/kg/day. Safety Follow- Up SAFETY FOLLOW- UP PERIOD (4 WEEKS) DOUBLE-BLIND TREATMENT PERIOD (16 WEEKS) PRAX-562 1:1 Randomization N=~20 (SCN2A/SCN8A) OLE TREATMENT PERIOD (48 WEEKS) PRAX-562 0.5 mg/kg/day Placebo for 4 weeks/PRAX-562 for 12 weeks+ 0.5 mg/kg/day PRIMARY ENDPOINT: Incidence and severity of treatment-emergent adverse events (TEAEs) KEY SECONDARY: Change from baseline in monthly (28 day) motor seizure frequency

29 Elsunersen (PRAX-222) SOLIDUS ASO PLATFORM

30 Elsunersen specifically designed for SCN2A GoF patients DISEASE OVERVIEW • Epilepsy and developmental impairment before the age of 16 years occurs in 1 in 340 children • The majority of these cases result from sporadic de novo genetic variation • In both de novo and familial form of epilepsy some 900 genes have been identified • Many of these genes also represent opportunities for intervention in other neurological disorders • Patients with SCN2A-DEE have a debilitating and ultimately fatal trajectory • Affects approximately 1,500 patients in the US RESEARCH APPROACH Modifying gene expression to address both gain and loss of function disorders is achieved through various ASO modalities to either silence harmful genes or enhance expression of reduced function or missing genes

31 Significant reduction in seizures observed for SCN2A patients Data from Part 1 of EMBRAVE Study, https://praxismedicines.com/wp-content/uploads/2023/12/Frizzo_AES2023_EMBRAVE_Poster_Final.pdf -39% -43% Im pr ov em en t Mean Median 52% 48% Mean Median Im pr ov em en t • No TEAEs or SAEs considered related to study drug • All TEAEs recovered/resolved Overall % reduction in seizures from 28-day baseline (n=4) Overall relative % increase in seizure-free days from 28-day baseline (n=4)

32 What to expect from Praxis during 2024 1H2024 2H24PLATFORM CEREBRUM SMALL MOLECULE PLATFORM SOLIDUS ASO PLATFORM PRAX-562 Phase 2 EMBOLD Study Topline Results DEEs PRAX-628 Phase 2 PPR study Topline Results Focal Epilepsy Ulixacaltamide Phase 3 Complete Enrollment Essential Tremor Elsunersen Regulatory Meetings SCN2A GoF DEE Ulixacaltamide Phase 3 Topline Results Essential Tremor Initiate PRAX-628 Focal Epilepsy Phase 2 Study Focal Epilepsy

34 Appendix

35 Essential1 Phase 2b study evaluating the efficacy and safety of ulixacaltamide for essential tremor DBLI = double blind lead-in, OLE = open label extension ClinicalTrials.gov NCT05021991 Safety Follow-up Essential 1 Study: Weeks: 1–8 ulixacaltamide Placebo Or DBLI Weeks 9–14 OLE Week 15+ Ulixa Placebo 1: 1 Ra nd om iz at io n 3 weeks 3 weeks Placebo Crossover Sub-study ESSENTIAL1 DESIGN Ulixa Ulixa Extension Period
v3.24.0.1
Cover
|
Mar. 05, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Document Period End Date |
Mar. 05, 2024
|
| Entity Registrant Name |
PRAXIS PRECISION MEDICINES, INC.
|
| Amendment Flag |
false
|
| Entity Central Index Key |
0001689548
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-39620
|
| Entity Tax Identification Number |
47-5195942
|
| Entity Address, Address Line One |
99 High Street
|
| Entity Address, Address Line Two |
30th Floor
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02110
|
| City Area Code |
617
|
| Local Phone Number |
300-8460
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value per share
|
| Trading Symbol |
PRAX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Praxis Precision Medicines (NASDAQ:PRAX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Praxis Precision Medicines (NASDAQ:PRAX)
Historical Stock Chart
From Apr 2023 to Apr 2024