Milestone Pharmaceuticals Shares Sink 24% After Receiving Rejection from FDA
December 26 2023 - 8:54AM
Dow Jones News
By Denny Jacob
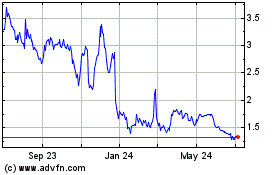
Milestone Pharmaceuticals shares sank 24% to $2.20 in premarket
trading after it received a refusal to file letter from the Food
and Drug Administration.
The stock is down 27% on the year.
The biopharmaceutical company said it received the letter over
its new drug application for its etripamil nasal spray to treat a
type of abnormal heart rhythm. It added that the FDA determined
that the application for etripamil nasal spray as a treatment of
paroxysmal supraventricular tachycardia wasn't sufficiently
complete to permit a substantive review based on a preliminary
review.
The regulator requested clarification about the time of data
recorded for adverse events in Phase 3 clinical trials, said
Milestone.
Milestone said the FDA didn't express concerns about the nature
or severity of adverse events. It will seek clarification and is
planning a meeting with the FDA.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
December 26, 2023 08:39 ET (13:39 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
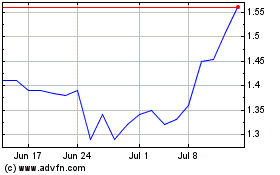
Milestone Pharmaceuticals (NASDAQ:MIST)
Historical Stock Chart
From Mar 2024 to Apr 2024
Milestone Pharmaceuticals (NASDAQ:MIST)
Historical Stock Chart
From Apr 2023 to Apr 2024