false
0001888886
True
0001888886
2023-12-18
2023-12-18
0001888886
gpcr:AmericanDepositarySharesAdssEachRepresentingThreeOrdinarySharesParValue0.0001PerOrdinaryShareMember
2023-12-18
2023-12-18
0001888886
gpcr:OrdinarySharesParValue0.0001PerShareMember
2023-12-18
2023-12-18
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 18, 2023
Structure Therapeutics Inc.
(Exact name of registrant as specified in its
charter)
| Cayman Islands |
|
001-41608 |
|
98-1480821 |
|
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
|
601 Gateway Blvd., Suite 900
South San Francisco, California |
|
94080 |
| (Address of principal executive offices) |
|
(Zip Code) |
(Registrant’s telephone number, including area code): (628) 229-9277
Not Applicable
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2.
below):
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of
the Act:
| |
|
Name Of Each Exchange |
|
|
| Title of Each Class |
|
Trading Symbol(s) |
|
On Which Registered |
| American Depositary Shares (ADSs), each representing three ordinary shares, par value $0.0001 per ordinary share |
|
GPCR |
|
Nasdaq Global Market |
| |
|
|
|
|
| Ordinary shares, par value $0.0001 per share* |
|
True |
|
Nasdaq Global Market* |
* Not for trading, but only in connection with the registration of
the American Depositary Shares
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate
by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
On December 18, 2023, Structure Therapeutics
Inc. (the Company) issued a press release and will be hosting a conference call and webcast to discuss the results of its Phase 2a proof-of-concept
study of its oral GLP-1 agonist, GSBR-1290, in type 2 diabetes mellitus (T2DM) and obesity and provide a comprehensive program update.
Copies of the press release and investor presentation
the Company intends to use during the conference call and webcast are attached as Exhibits 99.1 and 99.2 to this Current Report on Form 8-K,
respectively.
The
information set forth in this Item 7.01 and in the press release and investor presentation attached hereto as Exhibits 99.1
and 99.2, respectively, is deemed to be “furnished” and shall not be deemed to be “filed” for purposes of Section 18
of the Securities Exchange Act of 1934, as amended (the Exchange Act), or otherwise subject to the liabilities of that Section. The
information set forth in this Item 7.01, including Exhibits 99.1 and 99.2, shall not be deemed incorporated by reference into any filing
under the Exchange Act or the Securities Act of 1933, as amended, except to the extent that the Company specifically incorporates it by
reference.
Item 8.01 Other Events.
GSBR-1290 – Phase 2a Study
The randomized, double-blind, 12-week placebo-controlled Phase 2a clinical
trial has enrolled a total of 94 participants to date, including 60 participants randomized to GSBR-1290. The T2DM cohort enrolled 54
participants, randomized to GSBR-1290 45 mg (n=10) or 90 mg (n=26), or placebo (n=18), dosed once daily. The obesity cohort initially
enrolled 40 individuals randomized to GSBR-1290 120 mg (n=24) or placebo (n=16), once-daily. An additional 24 participants are currently
being enrolled in the obesity arm as previously announced and will also be randomized 3:2 to GSBR-1290 or placebo.
The primary endpoint of the Phase 2a study is safety and tolerability
of GSBR-1290. Key secondary endpoints include reduction in weight for both cohorts, as well as reduction in HbA1c for the T2DM cohort.
Safety and Tolerability Results
GSBR-1290 demonstrated encouraging safety and tolerability following
repeated, daily dosing for all doses studied (up to 120 mg) in the obesity and T2DM cohorts.
| · | The majority (88 to 96%, depending on study arm) of adverse events (AEs) reported were mild to moderate. |
| · | There were no serious adverse events (SAEs) related to study drug. |
| · | As expected for this mechanism of action, leading AEs were gastrointestinal-related.
The two most common AEs were nausea and vomiting. |
| · | There were no cases of elevated liver enzymes in the obesity cohort. One
participant in the T2DM treatment group experienced an event of elevated liver
enzymes without an increase in bilirubin initially at day 8 while receiving 5 mg of study drug. This participant was diagnosed
with fatty liver disease while in the study. |
| · | Of the 60 participants dosed with GSBR-1290, only one participant discontinued
the study due to AEs related to study drug (none in the obesity cohort and one (2.8%) in the T2DM cohort). |
Table 1: Summary of Treatment Emergent Adverse Events (TEAEs)
| |
Phase 2a TDM Cohort
(12-week data) |
Phase 2a Obesity Cohort
(12-week interim data) |
| Event, N (%) |
GSBR-1290
45 mg
(n=10) |
GSBR-1290
90 mg
(n=26) |
Placebo
(n=18) |
GSBR-1290
120 mg (n=24) |
Placebo (n=16) |
| Any TEAE |
10 (100) |
25 (96.2) |
8 (44.4) |
23 (95.8) |
11 (68.8) |
| Any TEAE by maximum severity |
|
|
|
|
|
| Mild |
2 (20) |
6 (23.1) |
6 (33.3) |
6 (25) |
9 (56.3) |
| Moderate |
7 (70) |
17 (65.4) |
2 (11.1) |
17 (70.8) |
2 (12.5) |
| Severe |
0 |
2 (7.7) |
0 |
0 |
0 |
| Any SAEs |
1 (10) |
1 (3.8) |
0 |
0 |
0 |
| Any SAEs related to study drug |
0 |
0 |
0 |
0 |
0 |
Efficacy Results
GSBR-1290 demonstrated clinically meaningful activity in both T2DM
and obesity cohorts.
| · | In the T2DM cohort, there was a statistically significant HbA1c reduction
(- 1.01 to -1.02%, placebo-adjusted) at Week 12 (Table 2). The study demonstrated a statistically significant and clinically meaningful
reduction in weight at Week 12 (-3.26% to -3.51%, placebo-adjusted) (Table 3). Weight loss continued to decrease through Week 12. |
| · | Results of the interim analysis in the obesity cohort, showed a statistically
significant and clinically meaningful decrease in weight at Week 8 (-4.74%, placebo-adjusted) (Table 4). Weight loss continued to decrease
throughout the eight weeks of treatment. |
Table 2: Diabetes cohort least square means difference (LSM) change
in HbA1C from baseline to 12 weeks (%)
| |
GSBR-1290
45 mg
(n=10) |
GSBR-1290
90 mg
(n=26) |
Placebo
(n=18) |
| LSM
HbA1C change from baseline (%) |
-0.79 |
-0.84 |
0.18 |
| % HbA1C change placebo-adjusted (LSM, 95% confidence interval (CI)) |
-1.01
(-1.73, -0.29) |
-1.02
(-1.59, -0.44) |
|
| P-value vs. placebo |
p= 0.008 |
p= 0.001 |
|
* LSM, CI and p value from Mixed Model for Repeated Measures
Table 3: Diabetes cohort LSM change in weight from baseline (%)
| |
GSBR-1290
45 mg
(n=10) |
GSBR-1290
90 mg
(n=26) |
Placebo
(n=18) |
| LSM
weight change from baseline (%) |
-3.32 |
-3.22 |
0.04 |
| % weight change placebo-adjusted (LSM, 95% CI) |
-3.51
(-5.58, -1.43) |
-3.26
(-5.17, -1.36) |
- |
| P-value vs. placebo |
p= 0.0019 |
p= 0.0013 |
- |
* LSM, CI and p value from Mixed Model for Repeated Measures
Table 4: Obesity Cohort LSM change in weight from baseline (%) 8 week
interim results
| |
GSBR-1290
120 mg
(n=24) |
Placebo
(n=16) |
|
| LSM
weight change from baseline (%) |
-5.55 |
-0.82 |
|
| % weight change placebo-adjusted (LSM, 90% CI) |
-4.74
(-6.74, -3.10) |
|
|
| P-value vs. placebo |
p< 0.0001 |
|
|
* LSM, CI and p value from Mixed Model for Repeated Measures
Phase 1 Japanese Bridging Study
The 4-week Phase 1 Japanese ethnobridging study included healthy lean
Japanese participants randomized to GSBR-1290 (n=9) and placebo (n=3), and healthy lean non-Japanese participants receiving GSBR-1290
(n=6). GSBR-1290 demonstrated a substantial weight reduction in Japanese participants (-3.91% on GSBR-1290 vs -1.67% placebo) and in non-Japanese
participants (-5.13% not placebo-adjusted), with no discontinuations or dose reductions, and no SAEs. These data will be used for regulatory
interactions in Japan in preparation for potential future global studies of GSBR-1290.
6- and 9-Month Toxicology Studies
In preparation for Phase 2b development with longer durations of treatment, the Company has completed 6-month (rodent) and 9-month (non-human
primate) toxicology studies to evaluate the safety of GSBR-1290. No major findings were observed in either study, with no test article-related
changes observed in the liver, including ALT/AST, at all doses, and a >100 fold safety window at the 120 mg therapeutic dose.
GSBR-1290 Next Steps
Full 12-week results from the Phase 2a obesity cohort (n=64), including data from the additional 24 participants currently being enrolled,
are expected in the second quarter of 2024.
The Company plans to initiate a Phase 2b obesity study of GSBR-1290 in the second half of 2024. The study is planned to include at least
275 individuals across the United States and Europe and will include multiple modified dose titration regimens to optimize efficacy and
tolerability. An additional Phase 2 study in T2DM is also planned for the second half of 2024 to optimize the efficacy and tolerability
of GSBR-1290 in this patient population.
The ongoing formulation bridging and titration optimization study is evaluating capsule versus tablet pharmacokinetics (PK) and exploring
different titration regimens. This study has completed enrollment (n=54), and data are expected in the second quarter of 2024. Pending
supportive data from this bridging study, the tablet formulation would be used in future GSBR-1290 studies starting with the Phase 2b
studies.
Forward Looking Statements
All statements other than statements of historical fact are
statements that could be deemed forward-looking statements, including, without limitation, statements concerning the Company’s
future plans and prospects; any expectations regarding the safety, efficacy or tolerability of GSBR-1290 and other candidates under
development based on the topline and interim clinical data from the Phase 2a study of GSBR-1290 in patients with T2DM and obesity,
including the potential for increased efficacy with longer duration of treatment, the ability of GSBR-1290 to treat T2DM, obesity or
related indications, the planned initiation and study design of the Company’s Phase 2b studies for GSBR-1290 in patients with T2DM
and obesity and the timing thereof, the update from the PK/formulation study of GSBR-1290 and the planned timing thereof, the
planned timing of the Company’s data results and continued development of GSBR-1290 and next generation combination GLP-1R
candidates and expectations regarding a new tablet formulation GLP-1R. In addition, when or if used in this Form 8-K, the words
“may,” “could,” “should,” “anticipate,” “believe,”
“estimate,” “expect,” “intend,” “plan,” “predict” and similar
expressions and their variants, as they relate to the Company may identify forward-looking statements. Forward-looking statements
are neither historical facts nor assurances of future performance. Although the Company believes the expectations reflected in such
forward-looking statements are reasonable, the Company can give no assurance that such expectations will prove to be correct.
Readers are cautioned that actual results, levels of activity, safety, performance or events and circumstances could differ
materially from those expressed or implied in the Company’s forward-looking statements due to a variety of risks and
uncertainties, which include, without limitation, risks and uncertainties related to the preliminary nature of the results due to
length of the study and sample size, the risks that unblinded data is not consistent with blinded data, the risk that interim
results of a clinical trial do not predict final results and that one or more of the clinical outcomes may materially change as
patient enrollment continues, following more comprehensive reviews of the data, as follow-up on the outcome of any particular
patient continues, and as more patient data become available; the Company’s ability to advance GSBR-1290, LTSE-2578, ANPA-0073
and its other therapeutic candidates, obtain regulatory approval of and ultimately commercialize the Company’s therapeutic
candidates, the timing and results of preclinical and clinical trials, the impact of any data collection omissions at any of
its’ clinical sites, the Company’s ability to fund development activities and achieve development goals, the
Company’s reliance on third parties, including clinical research organizations, manufacturers, suppliers and collaborators,
over which it may not always have full control, the impact of any global pandemics, inflation, or supply chain issues on the
Company’s business, its ability to protect its intellectual property and other risks and uncertainties described in the
Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K
filed with the SEC on March 30, 2023, Quarterly Report on Form 10-Q filed with the SEC on November 17, 2023, and future reports the
Company may file with the SEC from time to time. All forward-looking statements contained in this Form 8-K speak only as of the date
on which they were made and are based on management’s assumptions and estimates as of such date. The Company undertakes no
obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were
made, except as required by law.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
Structure Therapeutics Inc. |
| |
|
|
| Date: December 18, 2023 |
By: |
/s/ Raymond Stevens |
| |
|
Raymond Stevens, Ph.D. |
| |
|
Chief Executive Officer |
Exhibit 99.1

Structure
Therapeutics Provides Comprehensive GSBR-1290 Program Update Including Clinically Meaningful Proof-of-Concept Data From Phase 2a
Clinical Study
GSBR-1290 was
generally well-tolerated with no treatment-related serious adverse events over 12 weeks; 2.8% study discontinuation rate due to adverse
events related to study drug in diabetes and 0% study discontinuation rate due to adverse events in obesity
Topline
Phase 2a data from first study in type 2 diabetes mellitus (T2DM) demonstrate significant reductions in hemoglobin A1c (HbA1c) and
weight at 12 weeks
Interim Phase
2a data from obesity cohort demonstrate significant reduction in weight at 8 weeks; full 12-week obesity data expected in second quarter
2024 with Phase 2b study initiation on track for second half 2024
Program update
includes results from Japanese bridging study and findings from 6- and 9-month toxicology studies demonstrating encouraging safety to
support advancing into Phase 2b development
Company to host
conference call today at 8:30 a.m. ET
SAN
FRANCISCO – December 18, 2023 – Structure Therapeutics Inc. (NASDAQ: GPCR),
a clinical-stage global biopharmaceutical company developing novel oral small molecule therapeutics for metabolic and cardiopulmonary
diseases, today provided a comprehensive development program update for its highly selective oral GLP-1 receptor agonist, GSBR-1290.
“We are pleased that we have achieved
the objectives of our first Phase 2a clinical trial of GSBR-1290 in T2DM patients which were to demonstrate favorable safety, tolerability
and efficacy results and guide our plans to further optimize the already encouraging performance of GSBR-1290,” said Raymond Stevens,
Ph.D., Founder and CEO of Structure. “Our data demonstrated that once-daily GSBR-1290 has the potential to be a best-in-class compound
and a backbone for future combinations that could address large cardiometabolic indications.”
“GSBR-1290 has demonstrated proof
of concept in individuals with both obesity and T2DM, with clear effects on both weight loss and HbA1c that has the potential to increase
with longer duration of treatment,” said David D’Alessio, M.D., Chief of the Division of Endocrinology and Metabolism at
Duke University. “The unmet medical need for both T2DM and chronic weight management continues to be very large, and the GLP-1
receptor is a target with considerable potential. Safe and effective oral small molecule GLP-1 receptor agonists would be a significant
advance in that they could expand access for many patients for whom this is not now possible.”
Phase 2a Study in Diabetes and Obesity
The randomized, double-blind, 12-week
placebo-controlled Phase 2a clinical trial has enrolled a total of 94 participants to date, including 60 participants randomized to GSBR-1290.
The T2DM cohort enrolled 54 participants, randomized to GSBR-1290 45 mg (n=10) or 90 mg (n=26), or placebo (n=18), dosed once daily.
The obesity cohort initially enrolled 40 individuals randomized to GSBR-1290 120 mg (n=24) or placebo (n=16), once-daily. An additional
24 participants are currently being enrolled in the obesity arm as previously announced and will also be randomized 3:2 to GSBR-1290
or placebo.
The primary endpoint of the Phase 2a
study is safety and tolerability of GSBR-1290. Key secondary endpoints include reduction in weight for both cohorts, as well as reduction
in HbA1c for the T2DM cohort.
Safety and Tolerability Results
GSBR-1290 demonstrated encouraging safety
and tolerability following repeated, daily dosing for all doses studied (up to 120 mg) in the obesity and T2DM cohorts.
| · | The
majority (88 to 96%, depending on study arm) of adverse events (AEs) reported were mild to
moderate. |
| · | There
were no serious adverse events (SAEs) related to study drug. |
| · | As
expected for this mechanism of action, leading AEs were gastrointestinal-related. The two
most common AEs were nausea and vomiting. |
| · | There
were no cases of elevated liver enzymes in the obesity cohort. One participant in the T2DM
treatment group experienced
an event of elevated
liver enzymes without an increase in bilirubin initially
at day 8 while receiving 5 mg of study drug.
This participant was diagnosed with fatty liver disease while in the study. |
| · | Of
the 60 participants dosed with GSBR-1290, only one participant discontinued the study due
to AEs related to study drug (none in the obesity cohort and one (2.8%) in the T2DM cohort). |
Table 1: Summary of Treatment Emergent
Adverse Events (TEAEs)
| |
Phase 2a TDM Cohort
(12-week data) |
Phase 2a Obesity Cohort
(12-week interim data) |
| Event, N (%) |
GSBR-1290
45 mg
(n=10) |
GSBR-1290
90 mg
(n=26) |
Placebo
(n=18) |
GSBR-1290
120 mg
(n=24) |
Placebo
(n=16) |
| Any TEAE |
10 (100) |
25 (96.2) |
8 (44.4) |
23 (95.8) |
11 (68.8) |
| Any TEAE by maximum severity |
|
|
|
|
|
| Mild |
2 (20) |
6 (23.1) |
6 (33.3) |
6 (25) |
9 (56.3) |
| Moderate |
7 (70) |
17 (65.4) |
2 (11.1) |
17 (70.8) |
2 (12.5) |
| Severe |
0 |
2 (7.7) |
0 |
0 |
0 |
| Any SAEs |
1 (10) |
1 (3.8) |
0 |
0 |
0 |
| Any SAEs related to study drug |
0 |
0 |
0 |
0 |
0 |
Efficacy Results
GSBR-1290 demonstrated clinically meaningful
activity in both T2DM and obesity cohorts.
| · | In
the T2DM cohort, there was a statistically significant HbA1c reduction (- 1.01 to -1.02%,
placebo-adjusted) at Week 12 (Table 2). The study demonstrated a statistically significant
and clinically meaningful reduction in weight at Week 12 (-3.26% to 3.51%, placebo-adjusted)
(Table 3). Weight loss continued to decrease through Week 12. |
| · | Results
of the interim analysis in the obesity cohort, showed a statistically significant and clinically
meaningful decrease in weight at Week 8 (-4.74%, placebo-adjusted) (table 4). Weight loss
continued to decrease throughout the eight weeks of treatment. |
Table 2: Diabetes cohort least square
means difference (LSM) change in HbA1C from baseline to 12 weeks (%)
| |
GSBR-1290
45 mg
(n=10) |
GSBR-1290
90 mg
(n=26) |
Placebo
(n=18) |
| LSM HbA1C
change from baseline (%) |
-0.79 |
-0.84 |
0.18 |
| % HbA1C change placebo-adjusted (LSM, 95% confidence interval (CI)) |
-1.01
(-1.73, -0.29) |
-1.02
(-1.59, -0.44) |
|
| P-value vs. placebo |
p= 0.008 |
p= 0.001 |
|
* LSM, CI and p value
from Mixed Model for Repeated Measures
Table 3: Diabetes cohort LSM change
in weight from baseline (%)
| |
GSBR-1290
45 mg
(n=10) |
GSBR-1290
90 mg
(n=26) |
Placebo
(n=18) |
| LSM weight
change from baseline (%) |
-3.32 |
-3.22 |
0.04 |
| % weight change placebo-adjusted (LSM, 95% CI) |
-3.51
(-5.58, -1.43) |
-3.26
(-5.17, -1.36) |
- |
| P-value vs. placebo |
p= 0.0019 |
p= 0.0013 |
- |
* LSM, CI and p value
from Mixed Model for Repeated Measures
Table 4: Obesity Cohort LSM change in
weight from baseline (%) 8-week interim results
| |
GSBR-1290
120 mg
(n=24) |
Placebo
(n=16) |
| LSM weight
change from baseline (%) |
-5.55 |
-0.82 |
| % weight change placebo-adjusted (LSM, 90% CI) |
-4.74
(-6.74, -3.10) |
|
| P-value vs. placebo |
p< 0.0001 |
|
* LSM, CI and p value
from Mixed Model for Repeated Measures
Results from Phase 1 Japanese Bridging
Study
The 4-week Phase 1 Japanese ethnobridging
study included healthy lean Japanese participants randomized to GSBR-1290 (n=9) and placebo (n=3), and healthy lean non-Japanese participants
receiving GSBR-1290 (n=6). GSBR-1290 demonstrated a substantial weight reduction in Japanese participants (-3.91% on GSBR-1290 vs -1.67%
placebo) and in non-Japanese participants (-5.13% not placebo-adjusted), with no discontinuations or dose reductions, and no SAEs. These
data will be used for regulatory interactions in Japan in preparation for potential future global studies of GSBR-1290.
Results from 6- and 9-Month Toxicology
Studies
In
preparation for Phase 2b development with longer durations of treatment, Structure has completed 6-month (rodent) and 9-month (non-human
primate) toxicology studies to evaluate the safety of GSBR-1290. No major findings were observed in either study, with no test article-related
changes observed in the liver, including ALT/AST, at all doses, and a >100 fold safety window
at the 120 mg therapeutic dose.
GSBR-1290 Next Steps
Full 12-week results from the Phase
2a obesity cohort (n=64), including data from the additional 24 participants currently being enrolled, are expected in the second quarter
of 2024.
Structure plans to initiate a Phase
2b obesity study of GSBR-1290 in the second half of 2024. The study is planned to include at least 275 individuals across the United
States and Europe and will include multiple modified dose titration regimens to optimize efficacy and tolerability. An additional Phase
2 study in T2DM is also planned for the second half of 2024 to optimize the efficacy and tolerability of GSBR-1290 in this patient population.
The ongoing formulation bridging and
titration optimization study is evaluating capsule versus tablet pharmacokinetics (PK) and exploring different titration regimens. This
study has completed enrollment (n=54), and data are expected in the second quarter of 2024. Pending supportive data from this bridging
study, the tablet formulation would be used in future GSBR-1290 studies starting with the Phase 2b studies.
Conference Call and Webcast Information
Structure
will host a conference call and webcast today, December 18, 2023 at 8:30 a.m. Eastern Time. A live webcast of the call will
be available on the Investor Relations page of Structure’s website at https://ir.structuretx.com/events-presentations/events.
To access the call by phone, participants should visit this link (registration link) to receive
dial-in details. The webcast will be made available for replay on the company's website beginning approximately two hours after the live
event. The replay of the webcast will be available for 90 days.
About GSBR-1290 and Structure’s
Oral Metabolic Franchise
GSBR-1290 is an orally-available, small
molecule agonist of the glucagon-like-peptide-1 (GLP-1) receptor, a validated drug target for the treatment of type 2 diabetes and obesity.
GSBR-1290 was designed through the company’s structure-based drug discovery platform to be a biased GPCR agonist, which selectively
activates the G-protein signaling pathway. Beyond GSBR-1290, Structure is developing next generation combination GLP-1R candidates together
with GIP, amylin, glucagon and apelin.
About Structure Therapeutics
Structure
Therapeutics is a leading clinical-stage biopharmaceutical company focused on discovering and developing innovative oral treatments for
chronic metabolic and cardiopulmonary conditions with significant unmet medical needs. Utilizing its next generation structure-based
drug discovery platform, the company has established a scientifically-driven, GPCR-targeted pipeline, featuring two wholly-owned proprietary
clinical-stage small molecule compounds. These compounds are designed to surpass the limitations of traditional biologic and peptide
therapies and be accessible to more patients around the world. For additional information, please visit www.structuretx.com.
Forward Looking Statements
This
press release contains “forward-looking statements” within the meaning of the “safe harbor” provisions of the
Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact are statements that could be
deemed forward-looking statements, including, without limitation, statements concerning the Company’s future plans and prospects;
any expectations regarding the safety, efficacy or tolerability of GSBR-1290 and other candidates under development based on the topline
and interim clinical data from the Phase 2a study of GSBR-1290 in patients with T2DM and obesity, including the potential for increased
efficacy with longer duration of treatment, the ability of GSBR-1290 to treat T2DM, obesity or related indications, , the planned initiation
and study design of the Company’s Phase 2b studies for GSBR-1290 in patients with T2DM and obesity and the timing thereof, the
update from the PK/formulation study of GSBR-1290 and the planned timing thereof, the planned timing of the Company’s data results
and continued development of GSBR-1290 and next generation combination GLP-1R candidates and expectations regarding a new tablet formulation
GLP-1R. In addition, when or if used in this press release, the words “may,” “could,” “should,” “anticipate,”
“believe,” “estimate,” “expect,” “intend,” “plan,” “predict”
and similar expressions and their variants, as they relate to the Company may identify forward-looking statements. Forward-looking statements
are neither historical facts nor assurances of future performance. Although the Company believes the expectations reflected in such forward-looking
statements are reasonable, the Company can give no assurance that such expectations will prove to be correct. Readers are cautioned that
actual results, levels of activity, safety, performance or events and circumstances could differ materially from those expressed or implied
in the Company’s forward-looking statements due to a variety of risks and uncertainties, which include, without limitation, risks
and uncertainties related to the preliminary nature of the results due to length of the study and sample size, the risks that unblinded
data is not consistent with blinded data, the risk that interim results of a clinical trial do not predict final results and that one
or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data,
as follow-up on the outcome of any particular patient continues, and as more patient data become available; the Company’s ability
to advance GSBR-1290, LTSE-2578, ANPA-0073 and its other therapeutic candidates, obtain regulatory approval of and ultimately commercialize
the Company’s therapeutic candidates, the timing and results of preclinical and clinical trials, the impact of any data collection
omissions at any of its’ clinical sites, the Company’s ability to fund development activities and achieve development goals,
the Company’s reliance on third parties, including clinical research organizations , manufacturers, suppliers and collaborators,
over which it may not always have full control, the impact of any global pandemics, inflation, or supply chain issues on the Company’s
business, its ability to protect its intellectual property and other risks and uncertainties described in the Company’s filings
with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K filed with the SEC on
March 30, 2023, Quarterly Report on Form 10-Q filed with the SEC on November 17, 2023, and future reports the Company
may file with the SEC from time to time. All forward-looking statements contained in this press release speak only as of the date on
which they were made and are based on management’s assumptions and estimates as of such date. The Company undertakes no obligation
to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as
required by law.
CONTACTS
Investors:
Danielle Keatley
Structure Therapeutics Inc.
ir@structuretx.com
Media:
Dan Budwick
1AB
Dan@1abmedia.com
#####
Exhibit 99.2

GSBR - 1290 Comprehensive Program Update CONFIDENTIAL December 18, 2023

2 Attendees • Raymond Stevens, Ph.D., Chief Executive Officer • Mark Bach, M.D., Ph.D., Chief Medical Officer • Blai Coll, M.D., Ph.D., VP Clinical Development • Jun Yoon, Chief Financial Officer • Danielle Keatley, Investor Relations Confidential

3 Forward looking statements This presentation contains “forward - looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Re form Act of 1995. All statements other than statements of historical fact are statements that could be deemed forward - looking statements, includin g, without limitation, statements concerning the Company’s future plans and prospects, any expectations regarding the safety, efficacy or tolerability of GSBR - 1290 and othe r candidates under development based on the topline and interim clinical data from the Phase 2a study of GSBR - 1290 in patients with T2DM and obesi ty, including the potential for maintained or increased efficacy results with longer duration of treatment, the ability of GSBR - 1290 to treat type 2 diabetes, o besity or related indications, the planned initiation and study design of the Company’s Phase 2b studies for GSBR - 1290 in patients with T2DM and obesity and the ti ming thereof; the update from the pharmacokinetic (PK)/formulation study of GSBR - 1290 and the planned timing thereof; the planned timing of the Company’s data res ults and continued development of GSBR - 1290 and next generation combination GLP - 1R candidates and expectations regarding a new tablet formulation targeting GLP - 1R. In addition, when or if used in this presentation, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “p red ict” and similar expressions and their variants, as they relate to the Company may identify forward - looking statements. Forward - looking statements are neither historic al facts nor assurances of future performance. Although the Company believes the expectations reflected in such forward - looking statements are reasonable, the Com pany can give no assurance that such expectations will prove to be correct. Readers are cautioned that actual results, levels of activity, safety, performanc e o r events and circumstances could differ materially from those expressed or implied in the Company’s forward - looking statements due to a variety of risks and uncertainti es, which include, without limitation, risks and uncertainties related to the preliminary nature of the results due to length of the study and sample size, the risk s t hat unblinded data is not consistent with blinded data, the risk that interim results of a clinical trial do not predict final results and that one or more of the clin ica l outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data, as follow - up on the outcome of any particular patient co ntinues, and as more patient data become available, the Company’s ability to advance GSBR - 1290, LTSE - 2578, ANPA - 0073 and its other therapeutic candidates, obtain regulatory approval of and ultimately commercialize the Company’s therapeutic candidates, the timing and results of preclinical and clinical trials, the im pact of any data collection omissions at any of our clinical trial sites, the Company’s ability to fund development activities and achieve development goals, the Comp any 's reliance on third parties, including clinical research organizations , manufacturers, suppliers and collaborators, over which it may not always have full control, th e impact of any global pandemics, inflation and supply chain issues on the Company’s business, its ability to protect its intellectual property and other risks an d uncertainties described in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10 - K for the year ended December 31, 2022 filed with the SEC on March 30, 2023, Quarterly Report on Form 10 - Q for the quarter ended September 30, 2023 filed with the SEC on November 17, 2023, and future reports the Company may file with the SEC from time to time. All forward - looking statements contained in this presentation spea k only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. The Company undertakes no obligation to up dat e such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.

4 Agenda • Opening Remarks and Overview (Ray Stevens) • GSBR - 1290 Program Update (Blai Coll) − Phase 2a Safety and Tolerability Summary − Phase 2a Efficacy Summary − Phase 2b - enabling studies − Japanese Bridging Study − 6 and 9 month Toxicology Update • Overall Profile and Next Steps ( Mark Bach ) • GSBR - 1290 Closing (Ray Stevens) • Q&A Confidential
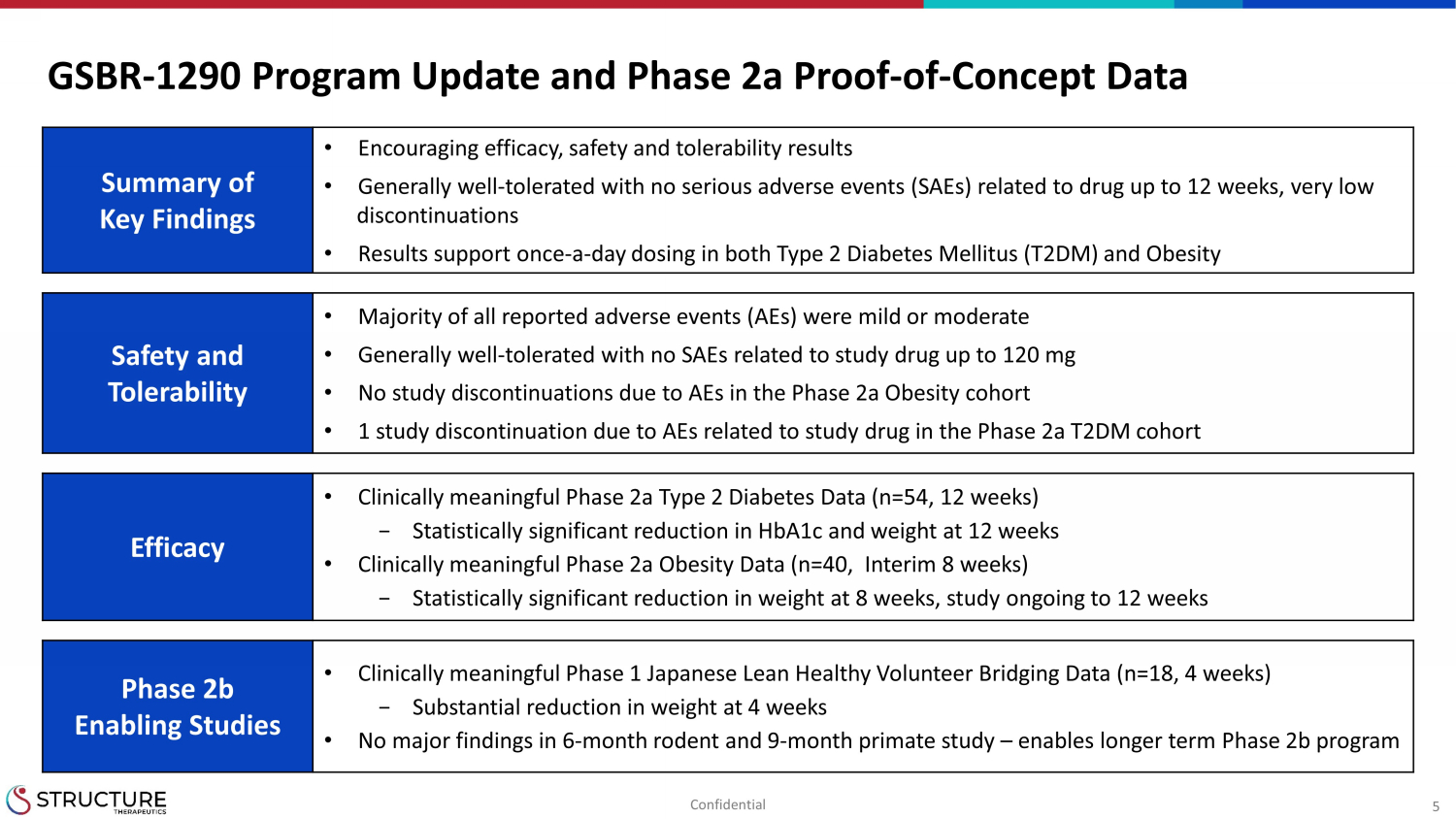
5 GSBR - 1290 Program Update and Phase 2a Proof - of - Concept Data Summary of Key Findings • Encouraging efficacy, safety and tolerability results • Generally well - tolerated with no serious adverse events (SAEs) related to drug up to 12 weeks, very low discontinuations • Results support once - a - day dosing in both Type 2 Diabetes Mellitus (T2DM) and Obesity Safety and Tolerability • Majority of all reported adverse events (AEs) were mild or moderate • Generally well - tolerated with no SAEs related to study drug up to 120 mg • No study discontinuations due to AEs in the Phase 2a Obesity cohort • 1 study discontinuation due to AEs related to study drug in the Phase 2a T2DM cohort Efficacy • Clinically meaningful Phase 2a Type 2 Diabetes Data (n=54, 12 weeks) − Statistically significant reduction in HbA1c and weight at 12 weeks • Clinically meaningful Phase 2a Obesity Data (n=40, Interim 8 weeks) − Statistically significant reduction in weight at 8 weeks, study ongoing to 12 weeks Phase 2b Enabling Studies • Clinically meaningful Phase 1 Japanese Lean Healthy Volunteer Bridging Data (n=18, 4 weeks) − Substantial reduction in weight at 4 weeks • No major findings in 6 - month rodent and 9 - month primate study – enables longer term Phase 2b program Confidential

6 GSBR - 1290 Program Update Blai Coll, M.D., Ph.D., VP Clinical Development Confidential
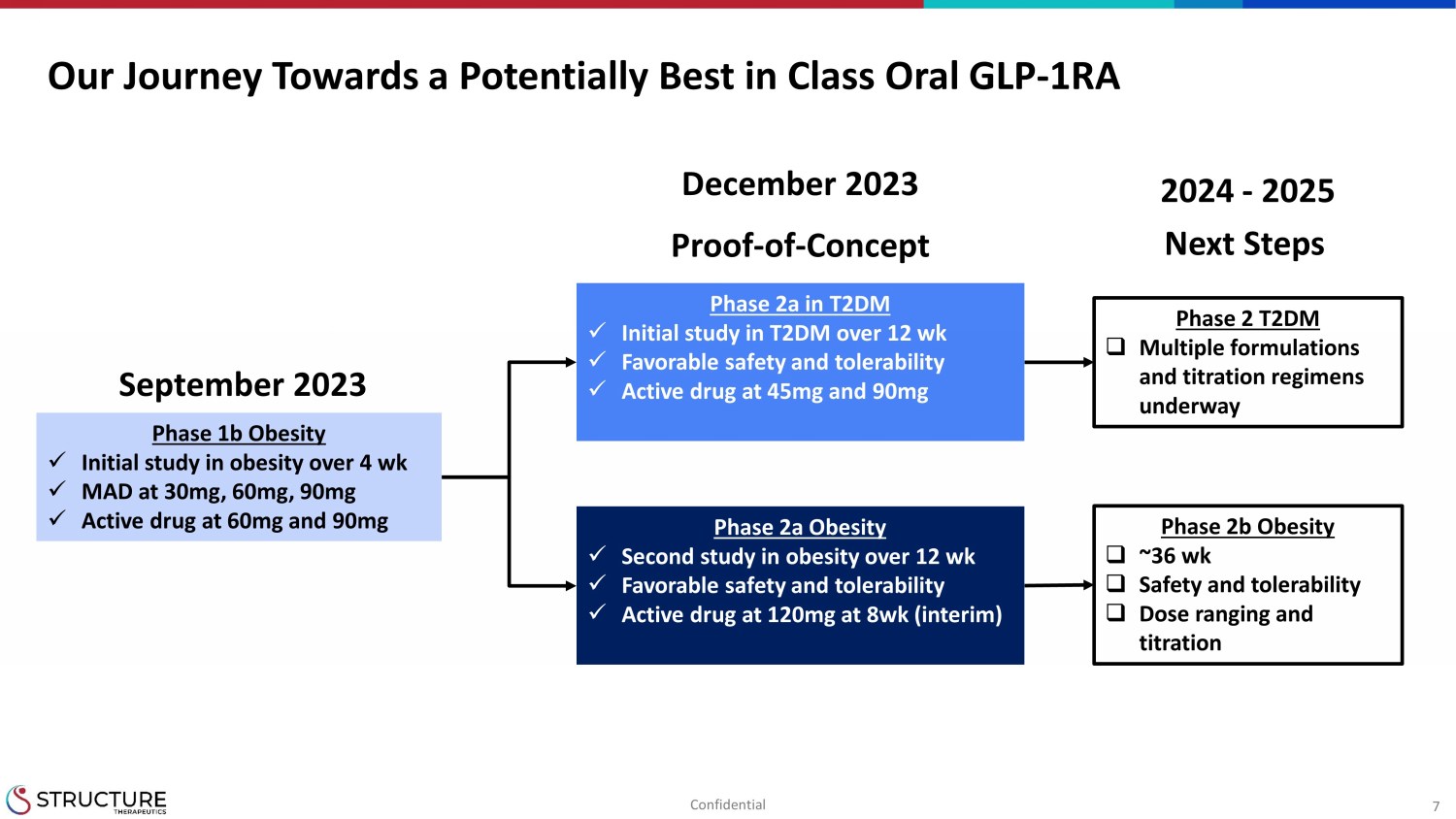
7 Our Journey Towards a Potentially Best in Class Oral GLP - 1RA Phase 2a in T2DM x Initial study in T2DM over 12 wk x Favorable s afety and tolerability x Active drug at 45mg and 90mg Phase 2a Obesity x Second study in obesity over 12 wk x Favorable s afety and tolerability x Active drug at 120mg at 8wk (interim) Phase 1b Obesity x Initial study in obesity over 4 wk x MAD at 30mg, 60mg, 90mg x Active drug at 60mg and 90mg September 2023 Proof - of - Concept December 2023 Phase 2 T2DM □ Multiple formulations and titration regimens underway Phase 2b Obesity □ ~36 wk □ Safety and tolerability □ Dose ranging and titration 2024 - 2025 Next Steps Confidential

8 GSBR - 1290 Phase 2a Study Design in T2DM and Obesity Top line data at 12 weeks Primary endpoint: S afety and tolerability Secondary endpoints: Demonstrate decrease in HbA1c Demonstrate decrease in weight Demonstrate changes in metabolic parameters after a Mixed Meal Tolerance Test Interim results at 8 weeks/12 weeks Primary endpoint: Safety and tolerability Secondary endpoint: Demonstrate decrease in weight Type 2 Diabetes Key Eligibility Criteria • T2DM of ≥ 6 months adult men and women • BMI ≥27.0 and ≤40.0 kg/m2 • Stable dose of metformin • HbA1c ≥7.0% and ≤10.5% • Age ≥18 and ≤75 years Healthy Overweight/Obese Key Eligibility Criteria • Healthy overweight/obese adult men and women • BMI ≥27.0 and ≤40.0 kg/m2 • HbA1c ≤6.5% • Age ≥18 and ≤75 years Confidential

9 Phase 2a (12 wk) Phase 2a (12 wk) Characteristics N (%) T2DM Obesity without T2DM 45 mg (N=10) 90 mg (N=26) Placebo (N=18) 120 mg (N=24) Placebo (N=16) Age, years 60.5 (7.5) 55.9 (11.0) 59.4 (9.3) 45.8 (14) 46 (14) Sex, female N (%) 4 (40) 12 (46) 7 (39) 13 (54) 4 (25) Hispanic or Latino, N(%) 8 (80) 19 (73) 12 (66) 10 (41) 7 (43) Weight, Kg 94.3 (13.7) 90.5 (13.6) 92.8 (15.8) 90.3 (11.4) 93.4 (13.9) BMI, kg/m 2 33.7 (4.7) 32.6 (3.5) 34 (4.2) 31.5 (3.4) 31.2 (3.2) Duration of diabetes, years 12 11.6 12.7 - - Dose of metformin, mg/day 1490 (561) 1796 (400) 1563 (611) - - HbA1c,% 8.08 (0.95) 7.98 (0.83) 7.96 (0.86) 5.5 (0.3) 5.4 (0.4) Fasting plasma glucose, mmol/L 9.61 (2.23) 8.76 (1.86) 9.43 (2.65) 5.3 (0.4) 5.1 (0.4) Heart rate, bpm 67.1 (9.2) 72.3 (13) 73.1 (11) 68.1 (9.3) 70.6 (6.3) Systolic blood pressure, mmHg 124.3 (14) 124 (11) 124 (11) 124.8 (10.7) 127.8 (12.6) Diastolic blood pressure, mmHg 75.4 (8.9) 76.3 (6.3) 76.7 (6.8) 80.1 (7.6) 83.1 (8) GSBR - 1290 Phase 2a Study: Demographics and baseline characteristics Data are mean (SD) unless otherwise indicated. Confidential

10 GSBR - 1290 Phase 2a Study: Participant disposition Phase 2a (12 wk) Phase 2a (12 wk) N (%) T2DM Obesity without T2DM 45 mg (N=10) 90mg (N=26) Placebo (N=18) 120mg (N=24) Placebo (N=16) Discontinued study due to AEs 2 (20)* 0 0 0 0 Discontinued study due to AEs related to study drug 1 (10)** 0 0 0 0 Dose discontinuation, down titrated or hold 4 (40) 11 (42) 0 9 (37) 0 Completed study 8 (80) 26 (100) 17 (89.5) Study still on going GSBR - 1290 Phase 2a T2DM study Randomized (N=54) GSBR - 1290 Phase 2a Obesity study Randomized (N=40) Interim * 1 subject discontinued due to COVID - 19 and 1 subject discontinued due to GI - related AEs ** 1 subject discontinued study due to GI - related AEs Confidential

11 GSBR - 1290 Program Update Safety and Tolerability Summary Phase 2a – Topline data from first study in T2DM Phase 2a – Interim results in Obesity Confidential
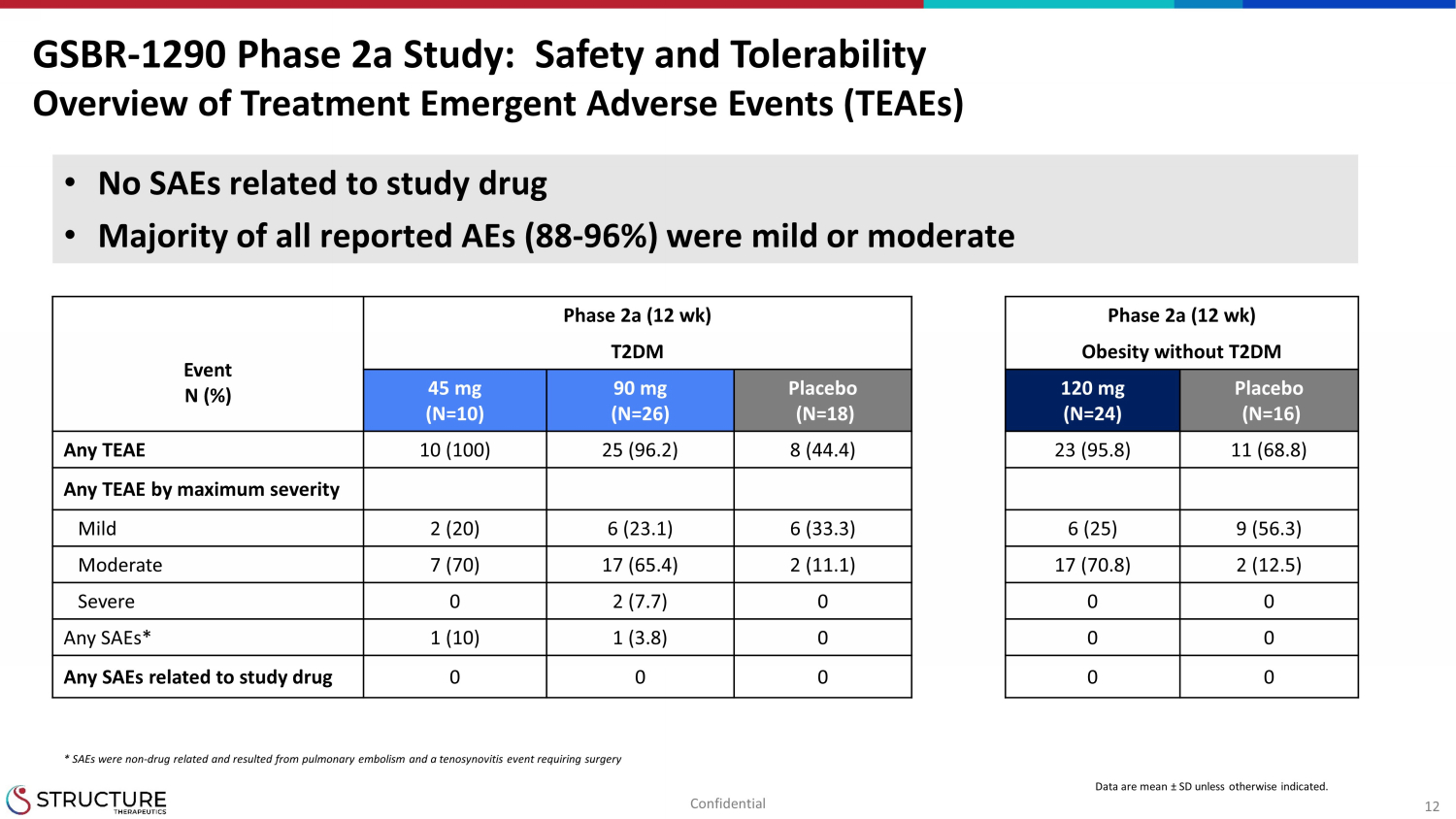
12 Phase 2a (12 wk) Phase 2a (12 wk) Event N (%) T2DM Obesity without T2DM 45 mg (N=10) 90 mg (N=26) Placebo (N=18) 120 mg (N=24) Placebo (N=16) Any TEAE 10 (100) 25 (96.2) 8 (44.4) 23 (95.8) 11 (68.8) Any TEAE by maximum severity Mild 2 (20) 6 (23.1) 6 (33.3) 6 (25) 9 (56.3) Moderate 7 (70) 17 (65.4) 2 (11.1) 17 (70.8) 2 (12.5) Severe 0 2 (7.7) 0 0 0 Any SAEs* 1 (10) 1 (3.8) 0 0 0 Any SAEs related to study drug 0 0 0 0 0 GSBR - 1290 Phase 2a Study: Safety and Tolerability Overview of Treatment Emergent Adverse Events (TEAEs) Data are mean ± SD unless otherwise indicated. • No SAEs related to study drug • Majority of all reported AEs (88 - 96%) were mild or moderate * SAEs were non - drug related and resulted from pulmonary embolism and a tenosynovitis event requiring surgery Confidential
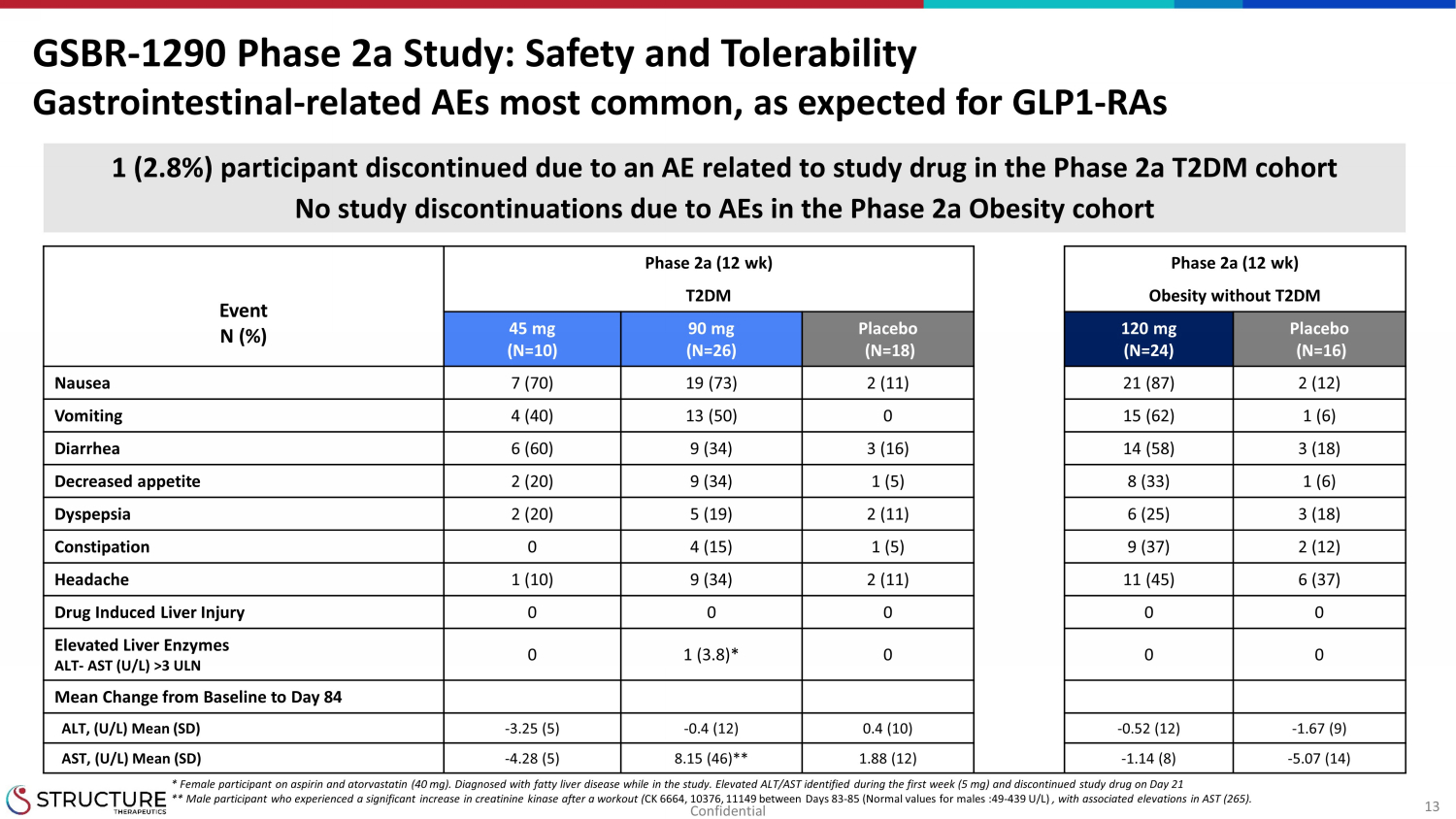
13 Phase 2a (12 wk) Phase 2a (12 wk) Event N (%) T2DM Obesity without T2DM 45 mg (N=10) 90 mg (N=26) Placebo (N=18) 120 mg (N=24) Placebo (N=16) Nausea 7 (70) 19 (73) 2 (11) 21 (87) 2 (12) Vomiting 4 (40) 13 (50) 0 15 (62) 1 (6) Diarrhea 6 (60) 9 (34) 3 (16) 14 (58) 3 (18) Decreased appetite 2 (20) 9 (34) 1 (5) 8 (33) 1 (6) Dyspepsia 2 (20) 5 (19) 2 (11) 6 (25) 3 (18) Constipation 0 4 (15) 1 (5) 9 (37) 2 (12) Headache 1 (10) 9 (34) 2 (11) 11 (45) 6 (37) Drug Induced Liver Injury 0 0 0 0 0 Elevated Liver Enzymes ALT - AST (U/L) >3 ULN 0 1 (3.8)* 0 0 0 Mean Change from Baseline to Day 84 ALT, (U/L) Mean (SD) - 3.25 (5) - 0.4 (12) 0.4 (10) - 0.52 (12) - 1.67 (9) AST, (U/L) Mean (SD) - 4.28 (5) 8.15 (46)** 1.88 (12) - 1.14 (8) - 5.07 (14) GSBR - 1290 Phase 2a Study : Safety and Tolerability Gastrointestinal - related AEs most common, as expected for GLP1 - RAs 1 (2.8%) participant discontinued due to an AE related to study drug in the Phase 2a T2DM cohort No study discontinuations due to AEs in the Phase 2a Obesity cohort * Female participant on aspirin and atorvastatin (40 mg). Diagnosed with fatty liver disease while in the study. Elevated ALT/A ST identified during the first week (5 mg) and discontinued study drug on Day 21 ** Male participant who experienced a significant increase in creatinine kinase after a workout ( CK 6664, 10376, 11149 between Days 83 - 85 (Normal values for males :49 - 439 U/L) , with associated elevations in AST (265). Confidential
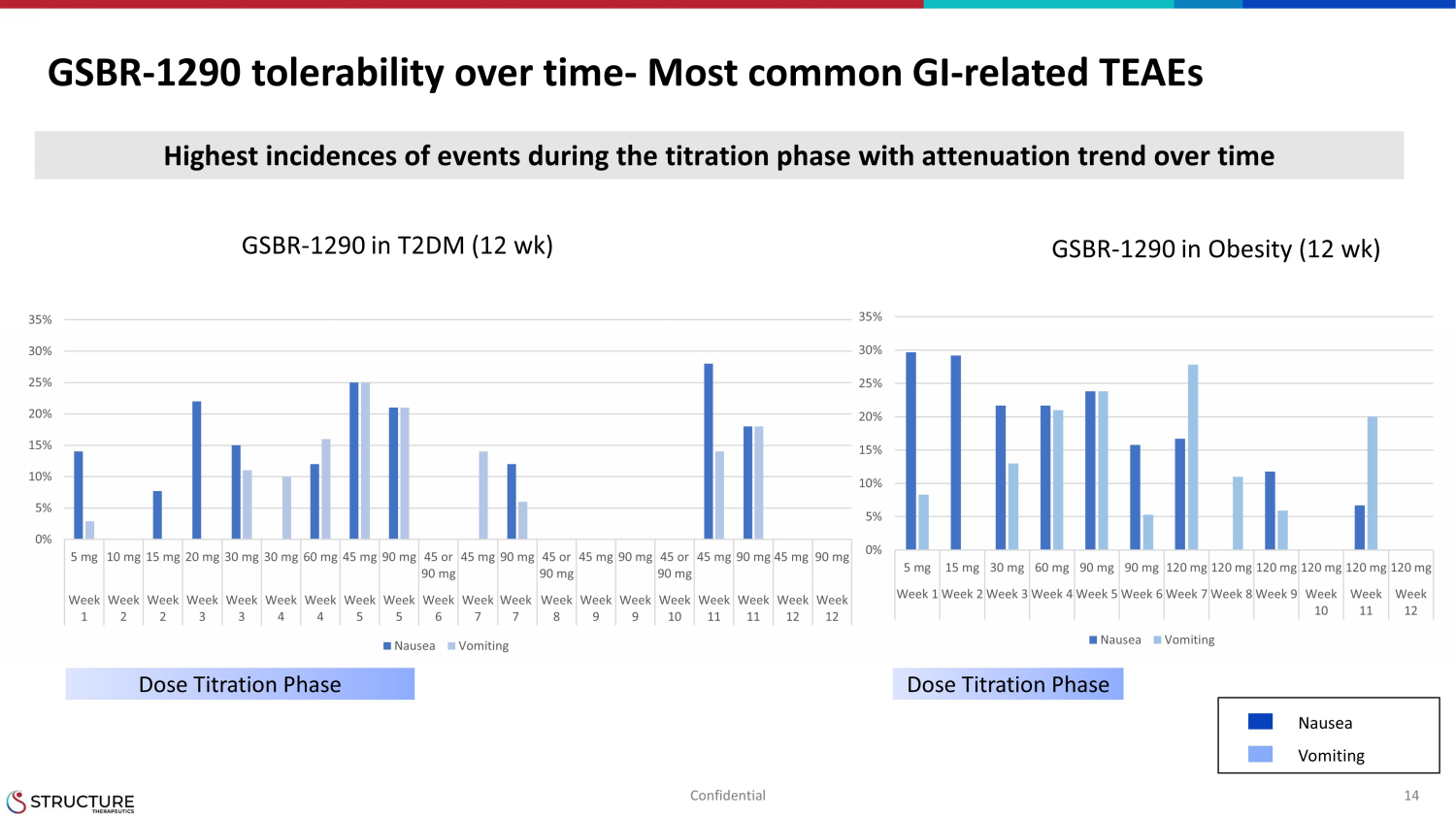
GSBR - 1290 tolerability over time - Most common GI - related TEAEs 14 Confidential Highest incidences of events during the titration phase with attenuation trend over time GSBR - 1290 in T2DM (12 wk) GSBR - 1290 in Obesity (12 wk) Dose Titration Phase Dose Titration Phase Nausea Vomiting 0% 5% 10% 15% 20% 25% 30% 35% 5 mg 15 mg 30 mg 60 mg 90 mg 90 mg 120 mg 120 mg 120 mg 120 mg 120 mg 120 mg Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Week 7 Week 8 Week 9 Week 10 Week 11 Week 12 Nausea Vomiting 0% 5% 10% 15% 20% 25% 30% 35% 5 mg 10 mg 15 mg 20 mg 30 mg 30 mg 60 mg 45 mg 90 mg 45 or 90 mg 45 mg 90 mg 45 or 90 mg 45 mg 90 mg 45 or 90 mg 45 mg 90 mg 45 mg 90 mg Week 1 Week 2 Week 2 Week 3 Week 3 Week 4 Week 4 Week 5 Week 5 Week 6 Week 7 Week 7 Week 8 Week 9 Week 9 Week 10 Week 11 Week 11 Week 12 Week 12 Nausea Vomiting

GSBR - 1290 Phase 2a study (12 wk): Changes in heart rate 15 Confidential • Higher pulse rate observed (5 to 8 bpm) with GSBR - 1290 as expected for the class • Increases consistent with other GLP - 1RAs 1,2 1,2 Granhall C, Donsmark M, Blicher TM, et al. Clin Pharmacokinet. 2019;58(6):781 - 791. Pratt E, Ma X, Liu R, et al. Diabetes Obes Me tab. 2023;25:2634 – 2641. Beats per minute (BPM) 0 1 2 3 4 5 6 7 8 9 GSBR-1290 45 mg GSBR-1290 90 mg GSBR-1290 120 mg Change from baseline in HR (bpm) to Week 12, placebo adjusted T2DM Obesity without T2DM

16 GSBR - 1290 Program Update Efficacy Summary Phase 2a – Topline data from first study in T2DM patients Phase 2a – Interim results in Obesity patients Confidential
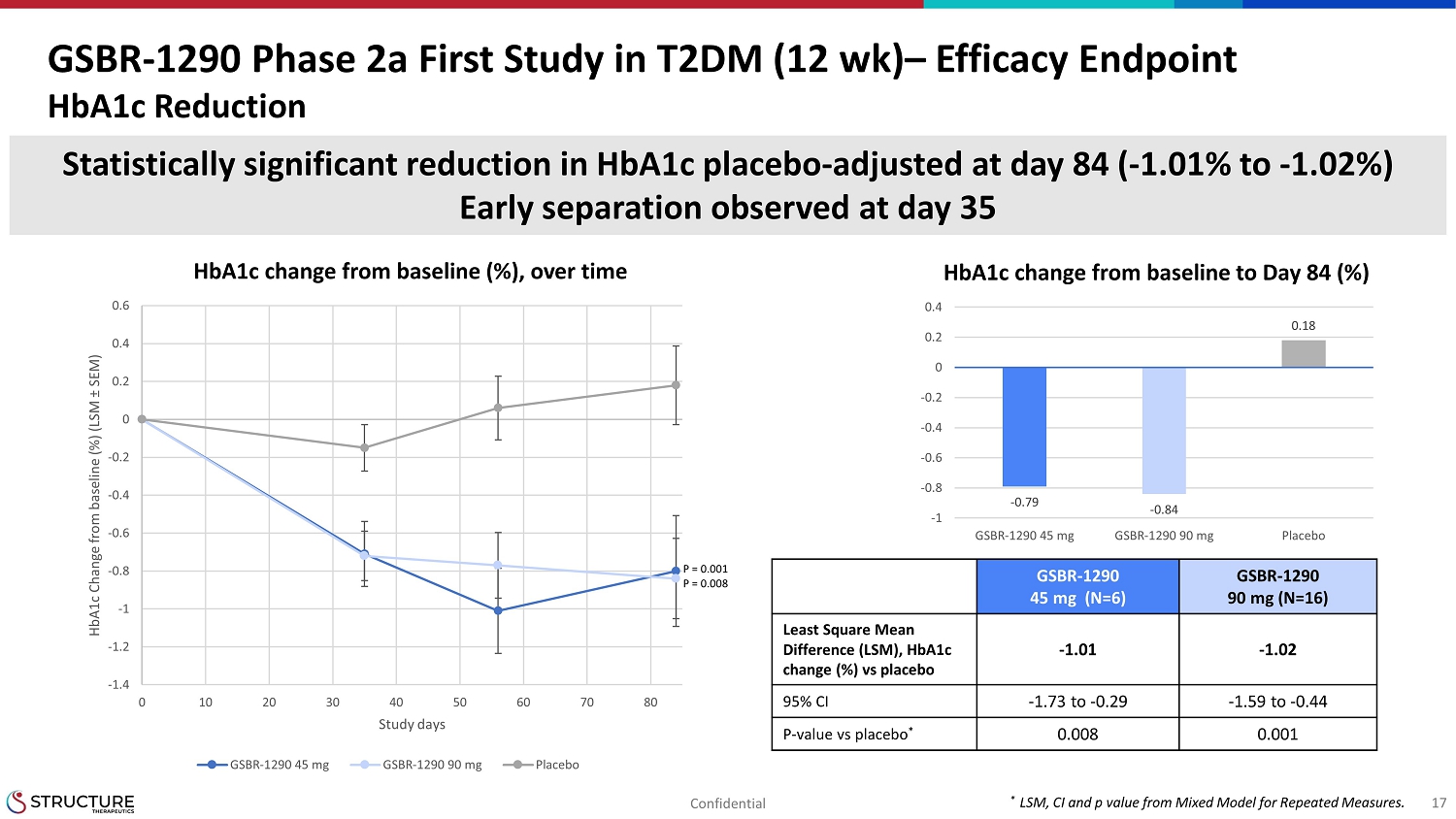
GSBR1290 Phase 2a First Study in T2DM (12 wk) Efficacy EndpointHbA1c ReductionStatisticallysignificant reduction in HbA1c placebo adjusted at day 84 ( 1.01% to 1.02%)Early separation observed at day 3517HbA1c change from baseline to Day 84 (%)HbA1c change from baseline (%), over time-1.4-1.2-1-0.8-0.6-0.4-0.200.20.40.60 10 20 30 40 50 60 70 80HbA1c Change from baseline (%) (LSM SEM)Study daysGSBR-1290 45 mg GSBR-1290 90 mg PlaceboGSBR129045 mg (N=6)GSBR129090 mg (N=16)Least Square MeanDifference (LSM), HbA1cchange (%) vs placebo-1.01 -1.0295% CI-1.73 to 0.29 -1.59 to 0.44Pvalue vs placebo 0.0080.001*LSM, CI and p value from Mixed Model for Repeated Measures.-0.79-0.840.18-1-0.8-0.6-0.4-0.200.20.4GSBR-1290 45 mg GSBR-1290 90 mg Placebo, N=17P = 0.001P = 0.008Confidential

18GSBR129045 mg (N=6)GSBR129090 mg (N=16)Least Square MeanDifference (LSM), Changein BW (%) vs Placebo-3.51 -3.2695% CI-5.58 to 1.43 -5.17 to 1.36Pvalue vs placebo 0.00190.0013Weight change from baseline (kg), over timeWeight change from baseline (%), Day 84*LSM, CI and p value from Mixed Model for Repeated Measures-3.32 -3.220.04-3.50-3.00-2.50-2.00-1.50-1.00-0.500.000.50GSBR-1290 45 mg GSBR-1290 90 mg PlaceboStatistically significant reduction in weight placeboadjusted at d ay 84 ( 3.26% to 3.51%)Continuing decrease in weight at day 84GSBR1290 Phase 2a First Study in T2DM (12 wk) Efficacy EndpointWeight Reduction-5-4-3-2-10120 10 20 30 40 50 60 70 80Weight change from baseline (kg) (LSM SEM)Study daysGSBR-1290 45 mg GSBR-1290 90 mg PlaceboP = 0.0005P = 0.0007Confidential

-1.61-1.281.21-2.00-1.50-1.00-0.500.000.501.001.50GSBR 45 mg GSBR 90 mg Placebo-4-3-2-10120 20 40 60 80Fasting plasma glucose change from baseline (mmol/L)(LSM SEM)Study daysGSBR1290 45 mg GSBR-1290 90 mg PlaceboGSBR1290 Phase 2a: First Study in T2DM (12 wk) Efficacy EndpointFasting Plasma GlucoseStatistically significant reduction in fasting plasma glucose19Fasting plasma glucose change from baseline (mmol/L), Day 84Fasting plasma glucose change from baseline (mmol/L), over timeGSBR129045 mg (N=6)GSBR129090 mg (N=16)Least Square MeanDifference (LSM), Changein FPG (mmol/L) vs Placebo-2.70 -2.5095% CI-4.82 to 0.56 -3.90 to 1.08Pvalue vs placebo 0.010.0008P = 0.0008P = 0.01*LSM, CI and p value from Mixed Model for Repeated Measures Confidential

GSBR - 1290 Phase 2a: First Study in T2DM (12 wk) – Efficacy Endpoint Mixed Meal Tolerance Test placebo adjusted change from BL at Day 84 (LSM) GSBR - 1290 demonstrated improvements in postprandial glucose, insulin and marker of insulin resistance Plasma glucose (mmol/L*hour), AUC 0 - 4 Insulin (mIU/L*hour), AUC 0 - 4 HOMA - IR, AUC 0 - 4 - 19.4 - 113.7 - 78.3 - 20.4 - 47.5 - 49.0 -120 -100 -80 -60 -40 -20 0 GSBR-1290 45 mg, N=6 GSBR-1290 90 mg, N=16 P value = 0.0008 P value = 0.02 P value = 0.05 P value <0.0001 P value <0.0001 P value = 0.025 20 Confidential
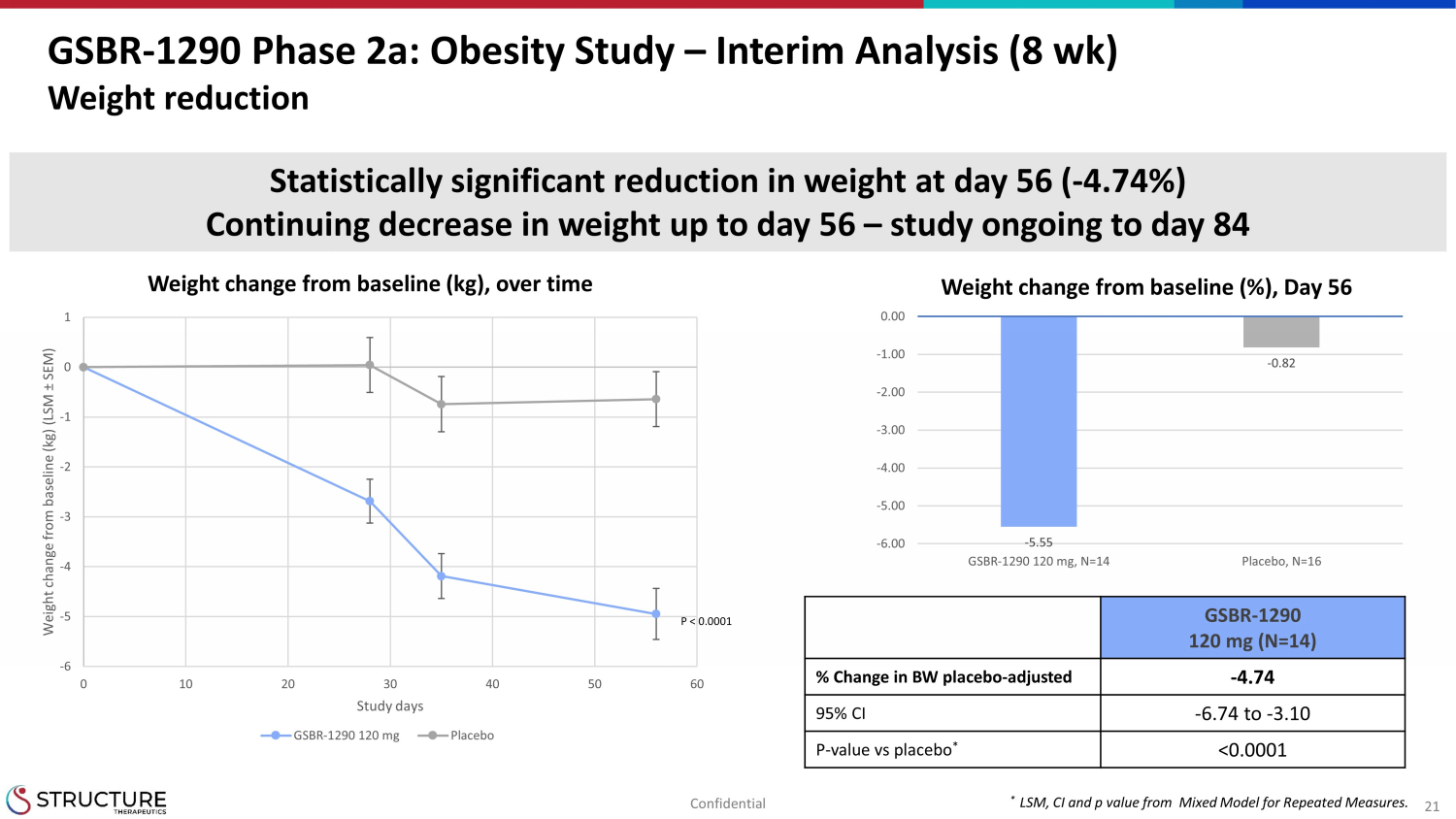
21 GSBR - 1290 Phase 2a: Obesity Study – Interim Analysis (8 wk) Weight reduction Weight change from baseline (kg), over time * LSM, CI and p value from Mixed Model for Repeated Measures. -6 -5 -4 -3 -2 -1 0 1 0 10 20 30 40 50 60 Weight change from baseline (kg) (LSM ± SEM) Study days GSBR-1290 120 mg Placebo - 5.55 - 0.82 -6.00 -5.00 -4.00 -3.00 -2.00 -1.00 0.00 GSBR-1290 120 mg, N=14 Placebo, N=16 Weight change from baseline (%), Day 56 Statistically significant reduction in weight at day 56 ( - 4.74%) Continuing decrease in weight up to day 56 – study ongoing to day 84 GSBR - 1290 120 mg (N=14) % Change in BW placebo - adjusted - 4.74 95% CI - 6.74 to - 3.10 P - value vs placebo * <0.0001 P < 0.0001 Confidential

22 GSBR - 1290 Summary of Weight Reduction Clinically meaningful and statistically significant weight reduction observed in T2DM and Obesity 22 % change from baseline Confidential -6 -5 -4 -3 -2 -1 0 1 0 10 20 30 40 50 60 70 80 90 Weight change from baseline (%) Obesity 90mg (Ph1b) Obesity 120mg (Ph2a) Study days Non - T2DM Placebo (Ph2a T2DM) Placebo (Ph1b) Placebo (Ph2a obesity) Placebo (Japanese Bridging) Obesity 30mg (Ph1b) Placebo T2DM 90mg (Ph2a) T2DM 45mg (Ph2a) T2DM Lean non - Japanese 60mg (Bridging) Lean Japanese 60mg (Bridging) Obesity 60mg (Ph1b)

23GSBR1290 in Context of Oral Small Molecule GLP 1RA-14-12-10-8-6-4-2020.0 4.0 8.0 12.0 16.0 20.0 24.0 28.0 32.0 36.0Body weight change from baseline (%)Study Time (weeks)Weight change from baseline (%)GSBR1290 120mgObesityGSBR1290 45/90mgT2DMOrforglipron 24/45mg2ObesityOrforglipron 12mg1T2DMOrforglipron 12mg2ObesityOrforglipron 24/45mg1T2DMPlaceboGSBR1290 120mg in obesity is competitive at 8 weeks vs Orforglipron in obesityAdapted from1 The Lancet ( https:// doi.org /10.1016/S0140 6736(23)01302 8 ) and 2 New England Journal Medicine 10.1056/NEJMoa2302392*No head to head study has been conducted evaluating GSBR 1290 against Orforglipron included herein. Differences exist between study designs and conditions, and caution shouldbe excised when comparing data across studies

GSBR - 1290 Program Update Phase 2b - enabling Activities Phase 1 – Japanese Bridging Study Preclinical GLP - Toxicology Studies 24 Confidential

25 GSBR - 1290 Phase 1 (4 wk): Japanese and Non - Japanese Bridging Study • All participants completed the study • No discontinuations or dose reductions • No SAEs N (%) Japanese cohort Non - Japanese cohort 60mg (N=9) Placebo (N=3) 60mg (N=6) Nausea 6 (66.7) 0 3 (50.0) Decreased appetite 6 (66.7) 0 1 (16.7) Early Satiety 3 (33.3) 0 1 (16.7) Vomiting 3 (33.3) 0 0 Diarrhea 1 (11.1) 0 0 Elevated liver enzymes 0 0 0 - 3.91 - 5.13 - 1.67 -6 -5 -4 -3 -2 -1 0 GSBR-1290 60mg Japanese (n=9) GSBR-1290 60mg Non-Japanese (n=6) Japanese Placebo (N=3) -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 0 5 10 15 20 25 30 Mean weight change from baseline (kg) Study days GSBR-1290 Japanese (n=9) GSBR-1290 Non-Japanese (n=6) Japanese Placebo (N=3) Significant weight loss observed in healthy volunteers at 4 weeks Weight change from baseline to Day 28 (%) Confidential Participants • Lean participants with baseline BMI~ 22 to 23 kg/m 2 • Ages: 34 to 46 years • Predominantly female (67%)

26 6/9 Month GLP - Toxicology Study Preliminary Results x 6 - month study in rodents (N=216) • Daily oral dosing for 6 - month (10, 100, 1000 mg/kg/day), plus 1 month recovery • Animals per group (treatment + recovery): N=15+5/group/sex • NOAEL is 1000 mg/kg/day, leading to >100 fold safety window at 120 mg therapeutic dose • No increase in ALT/AST and no test - article related changes in the liver x 9 - month study in healthy n on - human primates (N=60) • Daily oral dosing for 9 - month (3, 10, 30 mg/kg/day), plus 1 month recovery • Animals per group (treatment + recovery): N=5+4/group/sex (high dose) and N=4+2/group/sex (other doses) • Dose - dependent body weight reduction up to - 20 % vs baseline • No increase in ALT/AST and no test - article related changes in the liver NOAEL: No Observed Adverse Effect Level Confidential

27 GSBR - 1290 Overall Profile and Next Steps Mark Bach, M.D., Ph.D., CMO Confidential
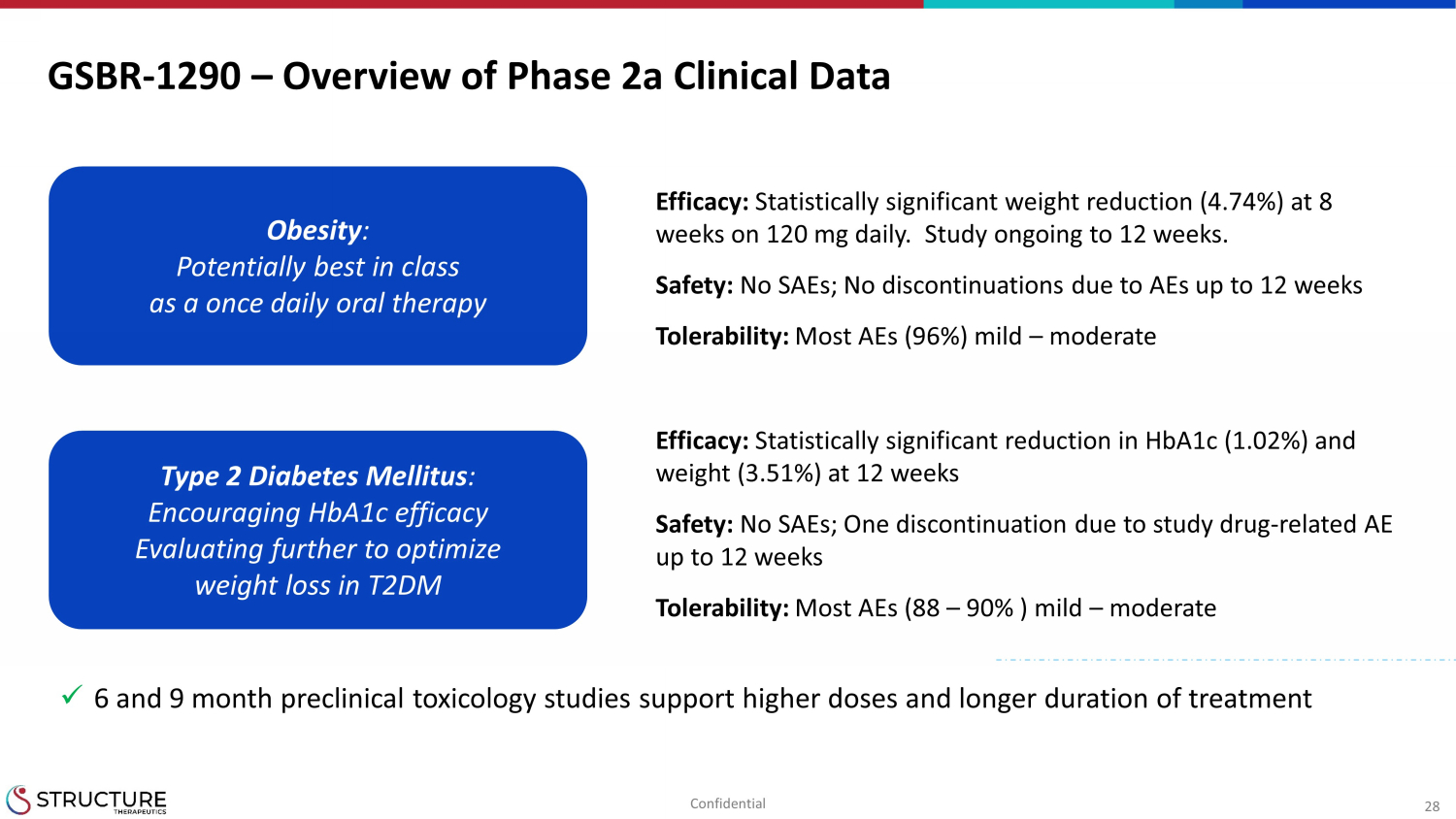
28 GSBR - 1290 – Overview of Phase 2a Clinical Data Efficacy: Statistically significant weight reduction (4.74%) at 8 weeks on 120 mg daily. Study ongoing to 12 weeks. Safety: No SAEs; No discontinuations due to AEs up to 12 weeks Tolerability: Most AEs (96%) mild – moderate Confidential Obesity : Potentially best in class as a once daily oral therapy Type 2 Diabetes Mellitus : Encouraging HbA1c efficacy Evaluating further to optimize weight loss in T2DM Efficacy: Statistically significant reduction in HbA1c (1.02%) and weight (3.51%) at 12 weeks Safety: No SAEs; One discontinuation due to study drug - related AE up to 12 weeks Tolerability: Most AEs (88 – 90% ) mild – moderate x 6 and 9 month preclinical toxicology studies support higher doses and longer duration of treatment

29 Next Steps: Formulation Bridging and Titration Optimization Study Capsule to Tablet Formulation and Explore Additional Titration Schemes Part 2: • To assess the tolerability at different titration schemes with the tablet • To study the comparative bioavailability of capsules and tablets at a therapeutic dose (60 mg) Status: Protocol finalization Projected timelines: - Top Line results by Q2’24 Screening Check - in Dose Check - out Phone call Check - in Dose Check - out Follow - up call EOS visit PK assessment PK assessment Day - 28 - 2 - 1 1 2 3 4 5 7 8 9 10 11 17 // // Capsule Tablet Tablet Capsule Study Confinement Period Study Outpatient Period Healthy Overweight/ Obese Participants N= 54 Cohort 3 12:3 Screening 5mg 15mg 30mg 60mg capsule 60mg tablet Follow up 60mg tablet 60mg capsule - 4 - 3 - 2 - 1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Cohort 2 12:3 Screening 15mg 30mg 60mg 90mg 120mg Follow up Cohort 1 12:3 Screening 10mg 20mg 30mg 60mg 90mg 120mg Follow up Part 1: To compare the PK of capsule to tablet (10 mg dose), N=16 x Enrollment completed □ Top - line 12 - week study results anticipated in Q2 2024 Confidential

30 GSBR - 1290 Closing Raymond Stevens, Ph.D., CEO Confidential

31 GSBR - 1290: Program Progress and Anticipated Milestones 2023 2024 x Phase 1b/MAD data (4 wk) • N=24, healthy overweight/obese participants, up to 90 mg • No adverse event - related discontinuations up to 90 mg • Statistically significant reductions in weight (up to 4.9% placebo - adjusted) at 60 and 90 mg x Phase 2a T2DM data (12 wk) • N=54, T2D participants, up to 90 mg • 1 study discontinuation (2.8%) due to AEs related to study drug • Statistically significant reductions in weight (up to 3.51% placebo - adjusted) at 45mg and 90mg x Phase 2a Obesity data (interim 8 wk) • N=40, healthy overweight/obese participants, up to 120 mg • No adverse event - related discontinuations up to 120 mg • Statistically significant reductions in weight (4.74% placebo - adjusted) at 120 mg at 8wks x Japan PK/ethno - bridging data (4 wk) • N=18 non - obese, healthy adult Japanese and non - Japanese participants, up to 60 mg • No adverse event - related discontinuations up to 60 mg • Substantial reductions in weight (3.91% to 5.13%, not placebo - adjusted) at 60 mg at 4wks x Clean 6/9 month GLP - Tox report □ Phase 2a Obesity data (12 wk) • N=64 participants, up to 120 mg • Enrolling 24 replacement participants • Completion anticipated in Q2 2024 □ Capsule to tablet PK/Formulation data (12 wk) • N= 54 participants, up to 120 mg • Fully enrolled and completion anticipated in Q2 2024 □ Obesity IND submission • Submit IND for Chronic Weight Management to FDA in Q2 2024 □ Phase 2b Obesity clinical study (~36 wk) • Modified dose titration regimens to optimize tolerability • Approximately 275 participants in US and Europe • Initiation planned in 2H 2024 □ Additional Phase 2 T2DM clinical study • Evaluate potential use of higher doses, longer titration to increase percent of patients on target dose, alternate formulations to optimize efficacy in T2DM • Initiation planned in 2H 2024 Confidential

32 Our Journey Towards a Potentially Best - in - Class Oral GLP - 1R Agonist x Phase 2a T2DM (12wk) x Phase 2a Obesity (interim) x Phase 1b MAD (4wk) Sep 2023 Phase 2b Obesity (~36wk) Proof - of - Concept Phase 2b T2DM (~26wk) Dose - range finding/ Optimization Phase 3 Obesity (>52wk) Phase 3 T2DM (>52wk) Pivotal Studies / Adjacent Indications Dec 2023 2024 - 2025 2026 and beyond Including Additional Formulations, Titrations, Dosing Regimens M ASH Chronic Kidney Disease Heart failure Addiction Alzheimer’s Phase 2 T2DM Significant Opportunity to Increase Accessibility and Treat Type 2 Diabetes & Obesity * Note: Represents Company's current anticipated future development plans, which are subject to change including based on stu dy results Confidential
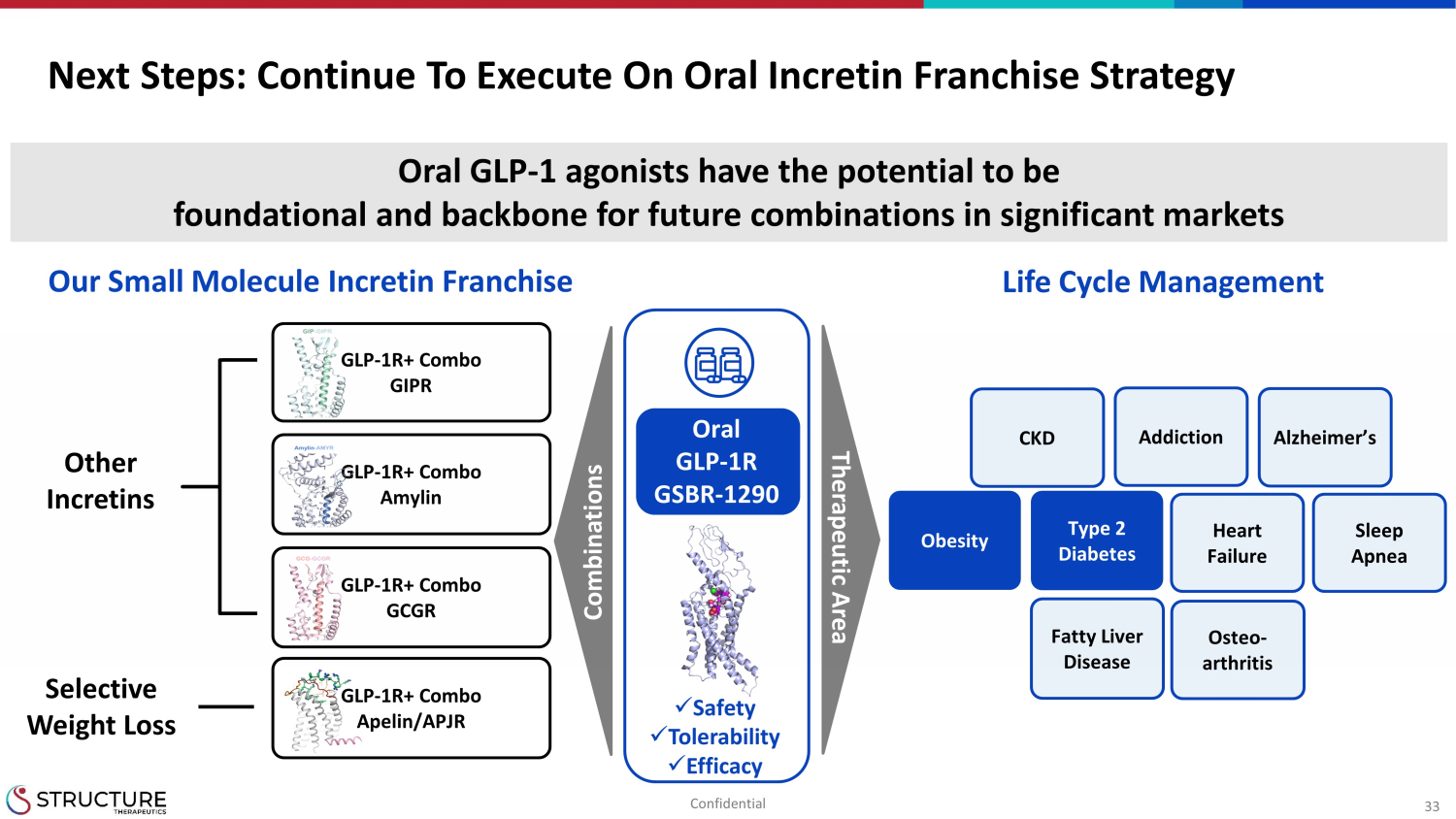
33 Next Steps: Continue To Execute On Oral Incretin Franchise Strategy GLP - 1R+ Combo GIPR GLP - 1R+ Combo Amylin GLP - 1R+ Combo Apelin/APJR GLP - 1R+ Combo GCGR Osteo - arthritis Fatty Liver Disease Obesity Type 2 Diabetes CKD Alzheimer’s Heart Failure Sleep Apnea Other Incretins Selective Weight Loss x Safety x Tolerability x Efficacy Addiction Oral GLP - 1 agonists have the potential to be foundational and backbone for future combinations in significant markets Combinations Therapeutic Area Life Cycle Management Our Small Molecule Incretin Franchise Oral GLP - 1R GSBR - 1290 Confidential

34 GSBR - 1290 in context • Significant future market opportunity for oral GLP - 1 agonists to treat cardiometabolic diseases such as obesity, type 2 diabetes, chronic kidney disease, MASH and others • Oral GLP - 1 agonists have the potential to be foundational and a backbone for future combinations – Safety and tolerability are key requirements to combine with different mechanisms of action and allow optimization of therapy for efficacy, safety and tolerability – Promising mechanisms include other incretins and muscle maintenance targets • Based on today’s comprehensive update, GSBR - 1290 appears to have the characteristics of a promising oral GLP - 1 agonist in this important marketplace x Generally well - tolerated with no serious adverse events (SAEs) related to study drug up to 120 mg x No study discontinuations due to AEs in the Phase 2a Obesity Study x 1 study discontinuation (2.8%) due to AEs related to study drug in the Phase 2a Type 2 Diabetes Study x No major findings in 6 - month rodent and 9 - month primate study – enables longer term evaluation in Phase 2b program x Clinically meaningful and statistically significant weight reductions in Obesity and Type 2 Diabetes x Optimize promising safety, tolerability, and efficacy profile with additional dosing and titration regimens in future Phase 2 b studies Confidential

Thank you! CONTACT US FOR ADDITIONAL INFORMATION: Email: ir@structuretx.com http://www.structuretx.com Confidential
v3.23.4
Cover
|
Dec. 18, 2023 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Dec. 18, 2023
|
| Entity File Number |
001-41608
|
| Entity Registrant Name |
Structure Therapeutics Inc.
|
| Entity Central Index Key |
0001888886
|
| Entity Tax Identification Number |
98-1480821
|
| Entity Incorporation, State or Country Code |
E9
|
| Entity Address, Address Line One |
601 Gateway Blvd.
|
| Entity Address, Address Line Two |
Suite 900
|
| Entity Address, City or Town |
South San Francisco
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94080
|
| City Area Code |
628
|
| Local Phone Number |
229-9277
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| American Depositary Shares (ADSs), each representing three ordinary shares, par value $0.0001 per ordinary share |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
American Depositary Shares (ADSs), each representing three ordinary shares, par value $0.0001 per ordinary share
|
| Trading Symbol |
GPCR
|
| Security Exchange Name |
NASDAQ
|
| Ordinary shares, par value $0.0001 per share* |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Ordinary shares, par value $0.0001 per share*
|
| No Trading Symbol Flag |
true
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a security having no trading symbol.
| Name: |
dei_NoTradingSymbolFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:trueItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=gpcr_AmericanDepositarySharesAdssEachRepresentingThreeOrdinarySharesParValue0.0001PerOrdinaryShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=gpcr_OrdinarySharesParValue0.0001PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Structure Therapeutics (NASDAQ:GPCR)
Historical Stock Chart
From Mar 2024 to Apr 2024
Structure Therapeutics (NASDAQ:GPCR)
Historical Stock Chart
From Apr 2023 to Apr 2024