4th Floor false 0001840439 0001840439 2024-01-09 2024-01-09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 9, 2024
Biomea Fusion, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-40335 |
|
82-2520134 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 900 Middlefield Road, 4th Floor Redwood City, California |
|
94063 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (650) 980-9099
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
BMEA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On January 9, 2024, Biomea Fusion, Inc., or the Company, presented a business update at the 42nd Annual J.P. Morgan Healthcare Conference. A copy of the Company’s presentation slides, which has been published on the Company’s website, is filed as Exhibit 99.1 to this current report on Form 8-K and is incorporated by reference herein.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Biomea Fusion, Inc. |
|
|
|
|
| Date: January 10, 2024 |
|
|
|
By: |
|
/s/ Thomas Butler |
|
|
|
|
|
|
Thomas Butler Principal Executive Officer |

Exhibit 99.1 JP Morgan 2024 Corporate Presentation Page 1

Disclaimer Legal Disclaimer & Forward-Looking Statements Certain
statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future business and financial performance of Biomea Fusion, Inc. (the “Company”) and
involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any
forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,”
“plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical
fact could be deemed forward-looking, including any projections of financial information or profitability, the initiation, timing and results of pending or future preclinical studies and clinical trials, the actual or potential actions of the FDA,
the status and timing of ongoing research, development and corporate partnering activities, any statements about historical results that may suggest trends for the Company's business; any statements of the plans, strategies, and objectives of
management for future operations; any statements of expectation or belief regarding future events, potential markets or market size, or technology developments, unfavorable global economic conditions, including inflationary pressures, market
volatility, acts of war and civil and political unrest, and other factors affecting the Company's financial condition or operations. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and
projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward- looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond
the Company's control. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled
Risk Factors in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the Securities
and Exchange Commission. The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to
conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data
about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future
performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Page 2
Excellent Science - Combining Validated Targets with Breakthrough
Chemistry We Aim to Cure Experienced Management Team TM Novel FUSION™ System Biomea Fusion is a clinical-stage biopharmaceutical company focused on the discovery and development of oral covalent small-molecule drugs to treat BMF-219 –
Phase II Stage patients with genetically defined cancers and metabolic diseases. We believe that our approach may lead to significant improvement and extension of life for patients. Our team is engaged in all phases of drug BMF-500 – Phase I
Stage discovery and development, including target selection, small molecule design, and preclinical and clinical studies to develop innovative medicines. Development of Combination Assets Page 3

Aiming to Develop Some of the Most Impactful Medicines of Our Time A
Long History of Developing Successful Drugs - Together Franco Valle Thomas Butler Ramses Erdtmann Naomi Cretcher Heow Tan Steve Morris, M.D. Juan Frías, M.D. Chief Financial President & COO Chairman & CEO Chief of People Chief Technical
& Chief Development Chief Medical Officer Quality Officer Officer Officer Co-Founder Co-Founder TM The FUSION SYSTEM * BMF-219 Co-Inventor Co-Inventor Page 4 *Note: BMF-219 is an investigational new drug

TM Biomea Leverages the FUSION System to Create a Suite of Novel
Covalent Agents to Potentially Improve and Extend the Lives of Patients Biomea’s Development Principles Drugs pursuing Validated Disease Targets have a ~2x Validated Targets Breakthrough higher likelihood of approval than molecules pursuing
For Covalent Covalent Chemistry Validated a new mechanism of action Targets Sources: Nelson et al. (2015) Nat Genet.; Thomas et al. (2016) BIO; In a Landscape of 'Me Too' Drug Inhibition Validated Targets For Breakthrough Development, What Spurs
Radical Innovation? HBS Weekly Review (Jun 2018) Covalent Inhibition Covalent Chemistry Biology Chemistry Covalent Small Molecule Inhibitors provide deep target inactivation and a wider therapeutic window, allowing for longer duration on therapy
Covalent Sources: Singh et al. (2011) Nature Reviews Drug Discovery; Cheng et al. (2020) Journal of Hematology & Inhibitors Oncology; Strelow (2017) SLAS Discovery; Kalgutkar & Dalvie (2012) Expert Opin. Drug Discov.; Proprietary
Combinations Combination Therapy with non-overlapping Medicine resistance mechanisms results in more durable Proprietary Chemistry responses and better outcomes Combinations Sources: Palmer et al. (2019) eLife; Mokhtari et al. (2017) Oncotarget Page
5
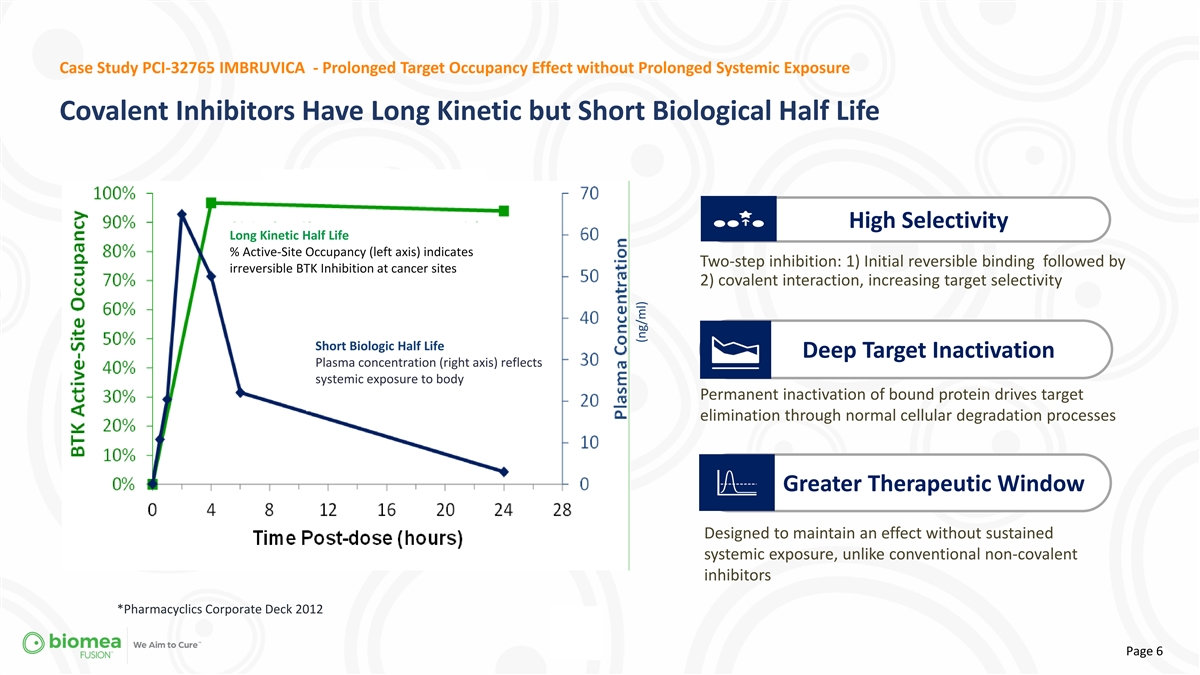
Case Study PCI-32765 IMBRUVICA - Prolonged Target Occupancy Effect
without Prolonged Systemic Exposure Covalent Inhibitors Have Long Kinetic but Short Biological Half Life High Selectivity Long Kinetic Half Life % Active-Site Occupancy (left axis) indicates Two-step inhibition: 1) Initial reversible binding
followed by irreversible BTK Inhibition at cancer sites 2) covalent interaction, increasing target selectivity Short Biologic Half Life Deep Target Inactivation Plasma concentration (right axis) reflects systemic exposure to body Permanent
inactivation of bound protein drives target elimination through normal cellular degradation processes Greater Therapeutic Window Designed to maintain an effect without sustained systemic exposure, unlike conventional non-covalent inhibitors
*Pharmacyclics Corporate Deck 2012 Page 6 (ng/ml)
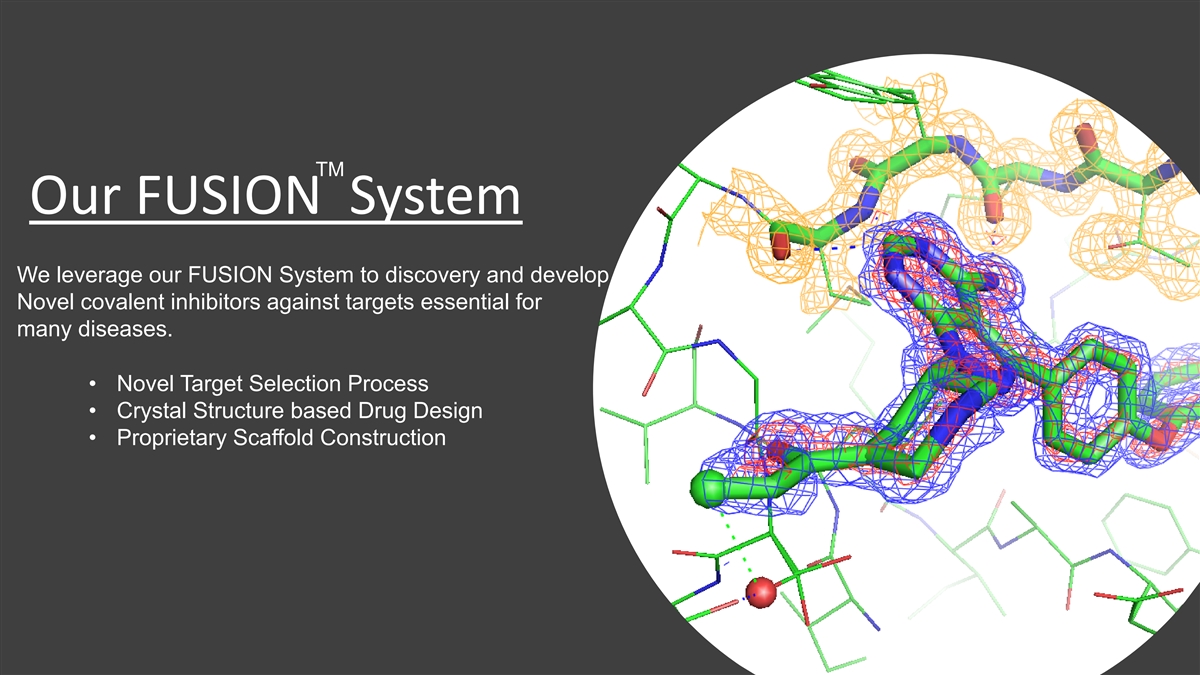
TM Our FUSION System We leverage our FUSION System to discovery and
develop Novel covalent inhibitors against targets essential for many diseases. • Novel Target Selection Process • Crystal Structure based Drug Design • Proprietary Scaffold Construction

Fusion System – Discovery and Development of Novel Covalent
Inhibitors Human Genome Wide Covalent Pocket Analysis CYS site spheres hydrophobic • 23,391 human genes as predicted • Top ranking pocket with sufficient • Analyze Apo structures without structures; 14,159 novel vs PDB hydrophobic
character ligands • Remove spurious N- and C-termini (blue)à Virtual screening for ligands • Pocket identification using • Analyze individual domains if needed – à Biomea Linker/Warhead established methods SiteMap
à potential artificial inter-domain pockets Determination Protocol “bindability” ranking • Manual curation for high interest targetsà Lead Molecule(s) CONFIDENTIAL Page 8

Biomea’s Pipeline Expands into 5 Clinical Trials with 2 Novel
Agents Multiple Upcoming Milestones in the Near Term Study Indications Milestones Expected Timeline COVALENT-111 Type 2 Diabetes Phase II - Dose Escalation Completion, ATTD 1Q 2024 2024 Phase II - Initial Proof of Concept COVALENT-112 Type 1
Diabetes BMF-219 Menin Liquid Tumors COVALENT-101 Phase I - Dose Escalation Completion, RP2D 2024 Program COVALENT-102 Solid Tumors Phase I - Dose Escalation Completion, RP2D 2024 BMF-500 AML/ALL FLT3 COVALENT-103 Phase I - Dose Escalation
Completion, RP2D 2024 (acute leukemia) Program Additional Progress Update 2024 Target # 3 TBA Program Page 9

Aiming to Develop Some of the Most Impactful Medicines of Our Time Juan
Pablo Frías, M.D. is Appointed as Biomea’s Chief Medical Officer August 31, 2023 Dr. Frías is a board-certified endocrinologist who has served as principal investigator on over 250 clinical diabetes studies, with over half of those
being Phase III studies, and has participated in the clinical development of more than 20 approved diabetic agents • Previous Pharmaceutical Leadership Positions: in Clinical and Medical Affairs at Eli Lilly, Amylin Pharmaceuticals, Pfizer,
and Johnson & Johnson, where he served as CMO and Global Vice President of Clinical and Medical Affairs, Diabetes Care. • Academic Positions: • University of Colorado Health Sciences Center, Barbara Davis Center for Diabetes •
Clinical Faculty at University of California San Diego School of Medicine • Published over 125 articles in peer reviewed journals Page 10
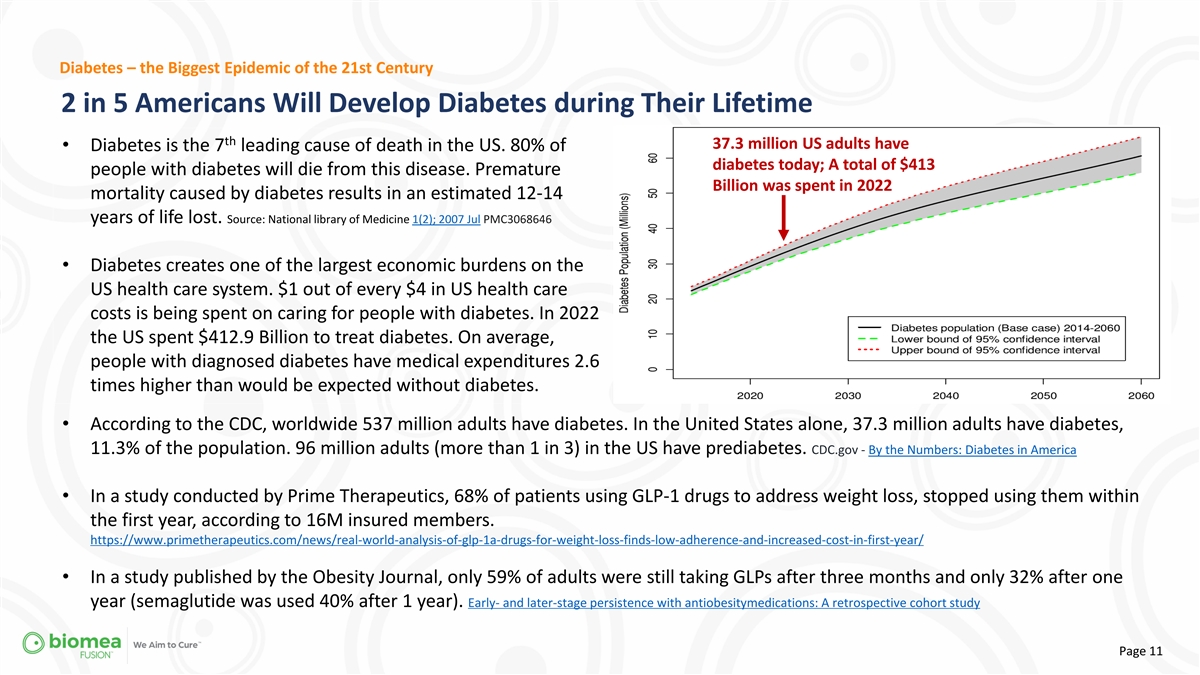
Diabetes – the Biggest Epidemic of the 21st Century 2 in 5
Americans Will Develop Diabetes during Their Lifetime th 37.3 million US adults have • Diabetes is the 7 leading cause of death in the US. 80% of diabetes today; A total of $413 people with diabetes will die from this disease. Premature
Billion was spent in 2022 mortality caused by diabetes results in an estimated 12-14 years of life lost. Source: National library of Medicine 1(2); 2007 Jul PMC3068646 • Diabetes creates one of the largest economic burdens on the US health
care system. $1 out of every $4 in US health care costs is being spent on caring for people with diabetes. In 2022 the US spent $412.9 Billion to treat diabetes. On average, people with diagnosed diabetes have medical expenditures 2.6 times higher
than would be expected without diabetes. • According to the CDC, worldwide 537 million adults have diabetes. In the United States alone, 37.3 million adults have diabetes, 11.3% of the population. 96 million adults (more than 1 in 3) in the US
have prediabetes. CDC.gov - By the Numbers: Diabetes in America • In a study conducted by Prime Therapeutics, 68% of patients using GLP-1 drugs to address weight loss, stopped using them within the first year, according to 16M insured members.
https://www.primetherapeutics.com/news/real-world-analysis-of-glp-1a-drugs-for-weight-loss-finds-low-adherence-and-increased-cost-in-first-year/ • In a study published by the Obesity Journal, only 59% of adults were still taking GLPs after
three months and only 32% after one year (semaglutide was used 40% after 1 year). Early- and later-stage persistence with antiobesitymedications: A retrospective cohort study Page 11
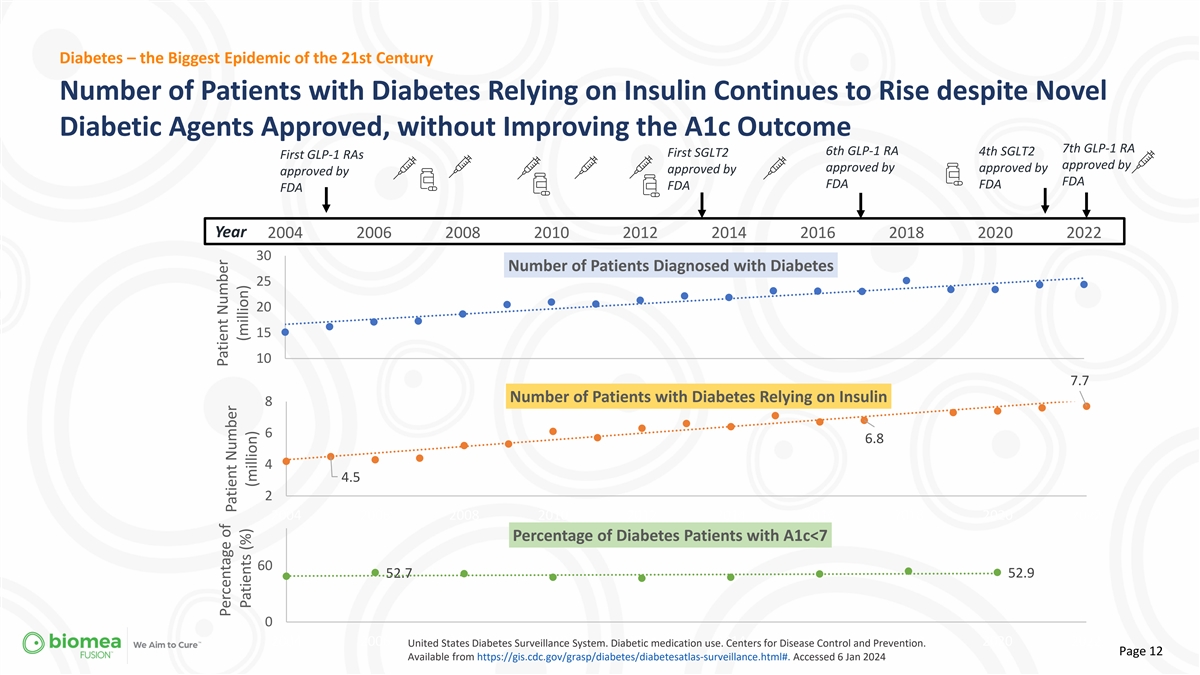
Diabetes – the Biggest Epidemic of the 21st Century Number of
Patients with Diabetes Relying on Insulin Continues to Rise despite Novel Diabetic Agents Approved, without Improving the A1c Outcome 7th GLP-1 RA 6th GLP-1 RA 4th SGLT2 First SGLT2 First GLP-1 RAs approved by approved by approved by approved by
approved by FDA FDA FDA FDA FDA Year 2004 2006 2008 2010 2012 2014 2016 2018 2020 2022 30 Number of Patients Diagnosed with Diabetes 25 20 15 10 7.7 Number of Patients with Diabetes Relying on Insulin 8 6 6.8 4 4.5 2 2004 2006 2008 2010 2012 2014
2016 2018 2020 2022 Percentage of Diabetes Patients with A1c<7 60 52.7 52.9 0 2004 2006 2008 2010 2012 2014 2016 2018 2020 2022 United States Diabetes Surveillance System. Diabetic medication use. Centers for Disease Control and Prevention. Page
12 Available from https://gis.cdc.gov/grasp/diabetes/diabetesatlas-surveillance.html#. Accessed 6 Jan 2024 Patient Number Percentage of Patient Number (million) Patients (%) (million)

Diabetes – the Biggest Epidemic of the 21st Century
Investigational BMF-219 - A Unique Value Proposition: Beta Cell Health st BMF-219: 1 in Class Potential for Differentiated Profile Complementary Agent Well-Tolerated Profile Non-Chronic Oral Small Molecule to Available Diabetes After First Read Out
Dosing Therapies Continued Glycemic Control Even After Disease Modifying Potential Cessation of Dosing Addressing the Root Cause of Diabetes Addressable Market May Include All Diabetic Patients Page 13

Role of Beta Cells in Diabetes Beta Cells Proliferate - Just not enough
to overcome pre-existing metabolic disorder “We conclude that during pregnancy, placental hormones act through the prolactin receptor to increase beta cell mass by up regulating beta cell “In nondiabetic obesity, an expansion in
proliferation by engaging Jak2, Akt, menin/p18, beta cell mass occurs to provide sufficient and p21.” insulin and to prevent hyperglycemia. This Hughs et al. Endocrinology, March 2011, 152(3):847–855 expansion is at least in part due to
beta cell proliferation. Linnmann et al. American Society for Nutrition. Adv. Nutr. 5: 278–288, 2014 “This quantitative morphological study shows a marked enlargement of the islets of Langerhans in pregnant women.” F. A. Van Assche
et al. British Jornal of Obstetrics and Gynaecology, 1978 November Page 14

Role of Menin in Diabetes Menin Controls Beta-Cell Proliferation and
Mass § Menin is a transcriptional scaffold protein that controls the expression of proteins that regulate beta-cell proliferation. - Menin has been found to control islet growth in pregnant mice. Pregnancy § Menin is thought to act as a
brake on beta stimulated proliferation of maternal cell turnover / beta cell growth, supporting pancreatic islet b-cells was accompanied by the notion that inhibition of menin could reduced islet levels of menin and its targets. - Prolactin, a
hormonal regulator of lead to the reactivation, protection, and pregnancy, repressed islet menin levels and regeneration of beta cells, which could be a stimulated b-cell proliferation. disease-modifying approach to treat type 2 Dr. Kim, S.K. et
al., Science. 2007 Nov 2. doi: 10.1126/science.1146812. diabetes. BMF-219 is a small molecule designed by the Biomea Fusion Team to covalently inhibit menin. Preclinical studies have shown that the inhibition of menin leads to the overall
rehabilitation of beta cell health and function, and thereby to increased insulin production and glycemic control. Clinical trials with BMF-219 are under way to investigate oral dosing for a limited time only until the pool of healthy beta cells are
restored. The goal is to address diabetes with BMF-219 at the root cause. Page 15
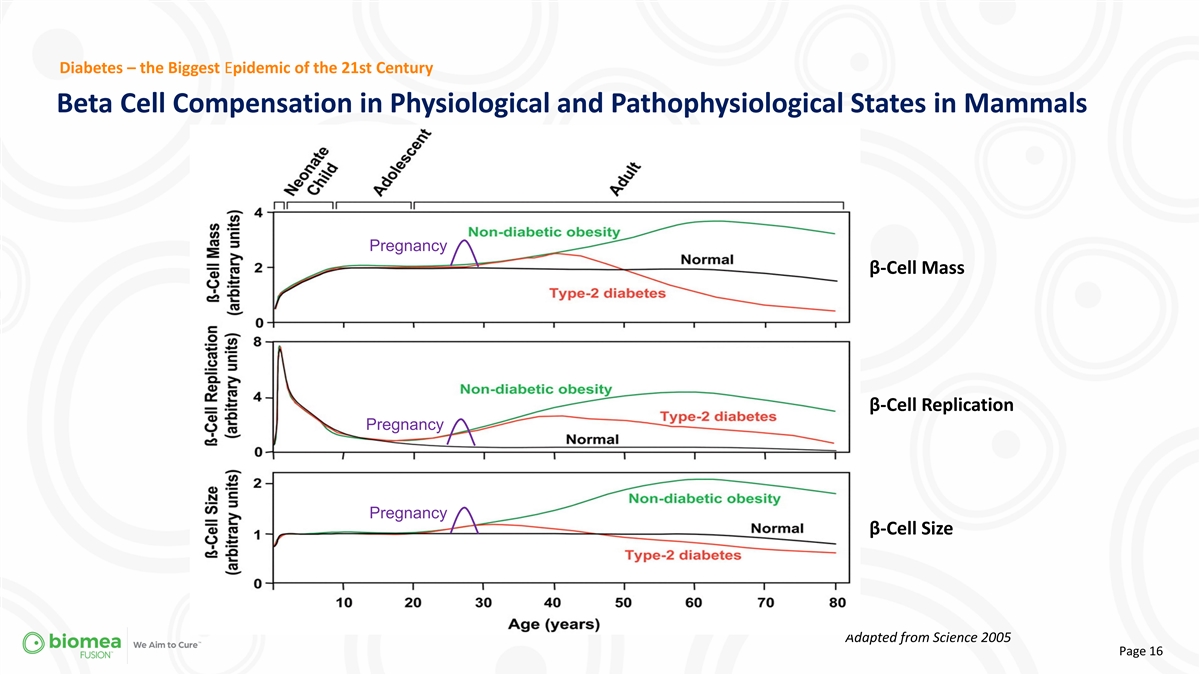
Diabetes – the Biggest Epidemic of the 21st Century Beta Cell
Compensation in Physiological and Pathophysiological States in Mammals Pregnancy β-Cell Mass β-Cell Replication Pregnancy Pregnancy β-Cell Size Adapted from Science 2005 Page 16

BMF-219 – Mechanism of Action The Goal for BMF-219 is to Improve
Glycemic Control without Continuous Medication BMF-219 is aimed to increase beta cell mass and function, thereby increase insulin Proposed effect of BMF-219 production in order to achieve glycemic control Proposed effect of BMF-219 - without the
need of continuous medication. Page 17 *Int. J. Mol. Sci. 2016, 17, 744; doi:10.3390/ijms17050744

BMF-219 – Impact on Beta Cell Proliferation and Insulin Secretion
in Ex Vivo Models BMF-219 Induced a Glucose-Dependent Enhancement in β-Cell Proliferation Proliferating beta cells as a fraction of total beta cells Donor 2 Standard glucose High glucose Donor 2; Day 14, High glucose Proliferating β-cell
Fraction Day 14 Day 14 Day 21 Day 21 0.3 * 0.2 0.1 0.0 BMF-219 BMF-219 BMF-219 BMF-219 Data represent mean ±SEM of 1 donor with n = 9-12 technical replicates. One-way ANOVA with Dunnett’s post hoc test rel. to DMSO control. *p < 0.05,
**p < 0.01, ***p < 0.001 Donor 2 Age BMI HbA1c Proliferation observed only under elevated glucose conditions, which mimic diabetic levels. Caucasian 32 25.0 5.2 Page 18 + + + EdU NKX6.1 / NKX6.1 % DMSO 0.075 μM 0.15 μM 0.3 μM
Harmine 10 μM DMSO 0.075 μM 0.15 μM 0.3 μM Harmine 10 μM DMSO 0.075 μM 0.15 μM 0.3 μM Harmine 10 μM DMSO 0.075 μM 0.15 μM 0.3 μM Harmine 10 μM

BMF-219 – Mechanism of Action BMF-219 Preserved, Reactivated and
Regenerated Beta Cells in Preclinical Studies Reactivation Preservation Regeneration +96% Normal (Adequate) State +351% Quantitative Analysis of pancreatic islet tissue BMF-219 demonstrated a significant level of beta BMF-219 increased HOMA-B by 96%
in a type 2 cross sections shows BMF-219 treated ZDF animals cell function compared to vehicle at day 31 in an animal model (STZ = 50% Beta Cell destruction). show novel effects in Beta Cell Mass growth and insulin resistant type 2 diabetes animal
model Homa B, a measurement of Beta Cell function, was maintenance. BMF-219 was able to maintain Beta (ZDF). Homa B, a measurement of Beta Cell analyzed using 4 h fasting glucose and insulin levels. Cell function and prevent Beta Cell Mass loss in a
function, was analyzed using 4 h fasting glucose BMF-219 in ex-vivo Human Donor Islets (Ex-Vivo) model of insulin resistance. Importantly, Beta Cell and insulin levels. It increased up to ~351% versus statistically significant increased beta cells
with BMF-219. Mass is maintained, despite cessation of dosing. vehicle, despite cessation of therapy. Page 19 Butler et al. Oral long-acting menin inhibitor normalizes type 2 diabetes in two rat models; Ex-vivo Human Islets data EASD 2022

BMF-219 Mechanism of Action BMF-219 is a Potential First-in-Class
Diabetic Agent – Addressing the Root Cause of Disease BMF-219: Menin Inhibition a Potential New Class of Diabetes Agents Beta cell reactivation, preservation and proliferation Beta Cell Mass ↑ Beta Cell Health ↑ Nat Rev Endocrinol
12, 337–346 (2016). https://doi.org/10.1038/nrendo.2016.51 Control of Glycemia even after Cessation of Dosing BMF-219 represents a potential new class of diabetes Currently approved therapies are primarily targeting the agents addressing the:
Root Cause of Diabetes Symptoms of Type 2 Diabetes: Hyperglycemia - Loss of Beta Cell Mass and Function - Page 20

COVALENT-111 Study Design (Type 2 Diabetes Patients Failing Standard of
Care) Additional Dose Levels and Various Dosing Durations Are Being Explored in the Escalation and Expansion Portion of COVALENT-111 Part 1 Dose Escalation, Part 2 Dose Expansion, n=216 – 288 incl. 4 weeks dosing+ 12 weeks follow up 12 weeks
dosing + 40 weeks follow-up Healthy Volunteers n=16 100 mg Arm A* 50 mg QD, n=10 x 8 wks x 4 wks 100 mg Arm B x 12 wks 100 mg QD, n=20 x 4 wks 100 mg 200 mg Arm C x 8 wks x 4 wks 200 mg QD / 100 mg BID, n=22 Anticipated to be added based on data
from x 4 wks Arm D 400 mg cohort of escalation portion 400 mg QD 200 mg QD, x 2 wks, n=10 x 2 wks *Redosing if required at Week 22 for another 4 weeks. Page 21

COVALENT-111 Phase 2 Study (Type 2 Diabetes) Baseline Characteristics
and Demographics BMF-219 100mg QD B MF -219 100mg QD without food with food Placebo (n=10) (n=10) (n=6) Age (year, min-max) 52 (38-63) 51 (35-60) 46 (31-61) Sex (n, M/F) 6/4 7/3 6/0 Duration of diabetes (year, min-max) 4.2 (0.5-9.0) 8.7 (4.0-14.0)
4.2 (1.0, 10.0) HbA (%-point, SD) 8.1 (0.9) 8.0 (0.6) 8.3 (0.7) 1c Diet and exercise alone (n, %) 0 (0%) 1 (10%) 0 (0%) 1 antihyperglycemic agent (n, %) 9 (90%) 7 (70%) 5 (83%) 2 antihyperglycemic agent (n, %) 0 (0%) 2 (20%) 1 (17%) 3
antihyperglycemic agent (n, %) 1 (10%) 0 0 (0%) Page 22
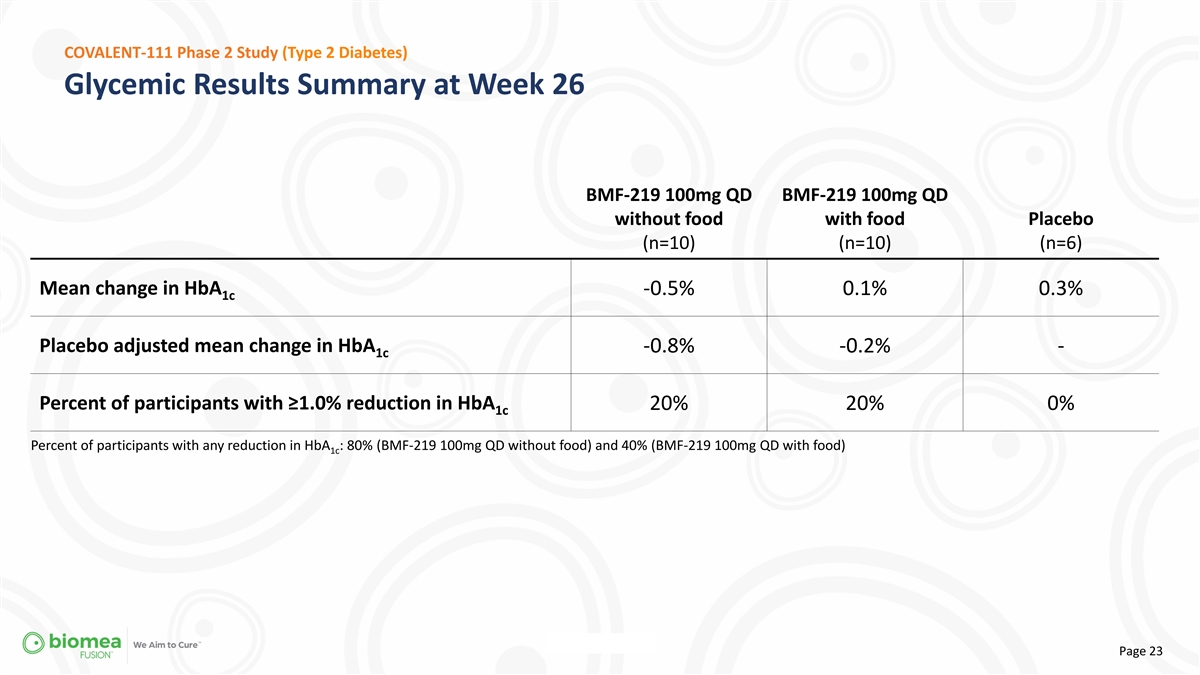
COVALENT-111 Phase 2 Study (Type 2 Diabetes) Glycemic Results Summary
at Week 26 BMF-219 100mg QD BMF-219 100mg QD without food with food Placebo (n=10) (n=10) (n=6) Mean change in HbA -0.5% 0.1% 0.3% 1c Placebo adjusted mean change in HbA -0.8% -0.2% - 1c Percent of participants with ≥1.0% reduction in HbA 20%
20% 0% 1c Percent of participants with any reduction in HbA : 80% (BMF-219 100mg QD without food) and 40% (BMF-219 100mg QD with food) 1c Page 23
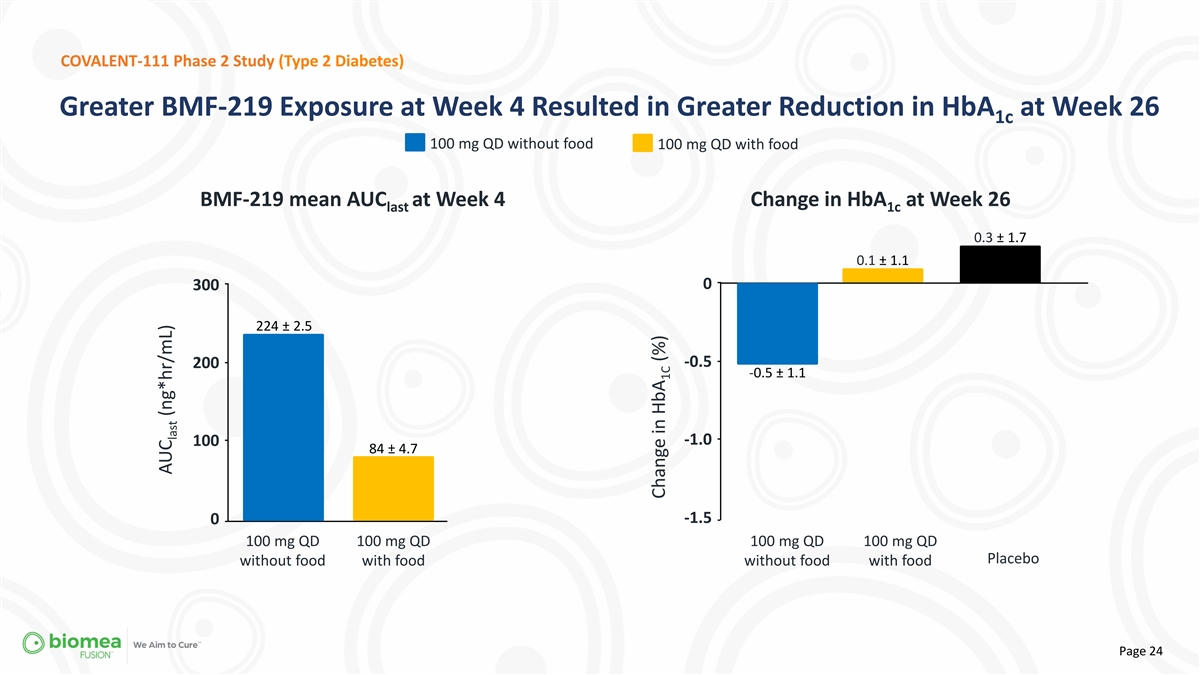
COVALENT-111 Phase 2 Study (Type 2 Diabetes) Greater BMF-219 Exposure
at Week 4 Resulted in Greater Reduction in HbA at Week 26 1c 100 mg QD without food 100 mg QD with food BMF-219 mean AUC at Week 4 Change in HbA at Week 26 last 1c 0.3 ± 1.7 0.1 ± 1.1 0 300 224 ± 2.5 -0.5 200 -0.5 ± 1.1 -1.0 100
84 ± 4.7 -1.5 0 100 mg QD 100 mg QD 100 mg QD 100 mg QD Placebo without food with food without food with food Page 24 AUC (ng*hr/mL) last Change in HbA (%) 1C

COVALENT-111 Phase 2 Study (Type 2 Diabetes) % Increase in HOMA-B and
C-peptide AUC in Responders Patients with HbA reduction ≥0.5% at Week 26 and baseline HOMA-B <200 1c % change HOMA-B % change C-peptide AUC Page 25

COVALENT-111 Phase 2 Study (Type 2 Diabetes) Case Study 2: 29-Year-Old
Man with 4-Year History of T2D • 29-year-old man with 4-year history of T2D • BMF-219 200 mg once daily without food for 4 weeks • Metformin and empagliflozin • CGM at Week 21 with ~90% TIR 70-180 mg/dL 2 • HbA 9.5%;
FPG 146 mg/dL; BMI 25.6 kg/m • No tolerability issues or related adverse events 1c Change in HbA (%) Continuous Glucose Monitoring 1c Page 26

COVALENT-111 Phase 2 Study (Type 2 Diabetes) Case Study 2: 29-Year-Old
Man with 4-Year History of T2D • 29-year-old man with 4-year history of T2D • BMF-219 200 mg once daily without food for 4 weeks • Metformin and empagliflozin • CGM at Week 21 with ~90% TIR 70-180 mg/dL 2 • HbA 9.5%;
FPG 146 mg/dL; BMI 25.6 kg/m • No tolerability issues or related adverse events 1c Page 27

WE AIM TO CURE 2023 Accomplishments DIABETES COVALENT-111: Type 2
Diabetes Patients failing standard of care (Metformin, SGLT2, GLP-1, DPP-4) • 84% of patients responded to BMF-219 while on treatment (any reduction in HbA1c at Week 4) • 74% of patients continued to respond to BMF-219 despite
off-treatment (any reduction in HbA1c at Week 12) • 20% of patients achieved at least a 1% reduction in HbA1c, 5 months off treatment (100mg @ Week 26) • 36% of patients achieved at least a 1% reduction in HbA1c, 5 months off treatment
(200mg @ Week 26) • Expansion Cohorts initiated – exploring 8 and 12 weeks of dosing COVALENT-112: Type 1 Diabetes IND (FDA) & CTA (Health Canada) cleared ONCOLOGY COVALENT-101: Relapsed/ Refractory Acute Leukemia • Initial
Phase I topline data with first Complete Responses, including MRD- COVALENT-103: Relapsed/ Refractory Acute Leukemia • IND for BMF-500 accepted and first patient in FLT-3 Leukemia enrolled FUSION™ SYSTEM • New lab facilities built
out to expand in-house capabilities • Continued development of the Biomea FUSION™ Platform Technology Page 28

WE AIM TO CURE 2024 Anticipated Milestones DIABETES - COVALENT-111
Phase II - BMF-219 in type 2 diabetes – Dose Escalation Completed - COVALENT-111 Phase II - BMF-219 in type 2 diabetes - Expansion cohorts fully enrolled (n=216+) - COVALENT-112 Phase II - BMF-219 in type 1 diabetes - Open Label cohorts fully
enrolled (n=40) - COVALENT-112 Phase II - BMF-219 in type 1 diabetes - Initial proof of concept established ONCOLOGY - COVALENT-101 Phase I - BMF-219 in liquid tumors - Dose Escalation Completed and Recommended Phase II Dose established -
COVALENT-102 Phase I - BMF-219 in solid tumors - Dose Escalation Completed and Recommended Phase II Dose established - COVALENT-103 Phase I - BMF-500 in AML – Dose Escalation Completed and Recommended Phase II Dose established FUSION SYSTEM -
Third pipeline asset from FUSION™ Platform Technology announced Page 29

WE AIM TO CURE Our Development Plan: Next 4 Years BMF-219 in Diabetes
2024 Phase II Expansion Phase Completed in type 2 diabetes Proof of concept in type 1 diabetes established 2025 Pivotal Phase III study in type 1 diabetes initiated Pivotal Phase III studies in type 2 diabetes initiated 2026 NDA for BMF-219 in type
1 diabetes filed 2027 NDA for BMF-219 in type 2 diabetes filed Page 30

As of September 30, 2023 Company Financials (NASDAQ: BMEA) Three Months
Ended September 30, 2023 Operating expenses: R&D $ 25,347 G&A 5,772 Total Operating Expenses 31,119 Loss from operations (31,119) Interest and other income, net 2,690 Net loss $ (28,429) Other comprehensive loss: Changes in unrealized gain
on short term investments, net — Comprehensive loss $ (28,429) Net loss per common share, basic and diluted $ (0.80) Weighted-average number of common shares used to compute basic and diluted net loss per common share 35,653,988 Q3 Operating
Expenses minus Stock Based Comp $24.8 M Cash, Cash Equivalents, Investments, and Restricted Cash as of 30 September 2023 $199.5M Page 31

THANK YOU Biomea Fusion 900 Middlefield Road, 4th floor Redwood City,
CA, 94063 biomeafusion.com Page 32
v3.23.4
Document and Entity Information
|
Jan. 09, 2024 |
| Cover [Abstract] |
|
| Entity Address, Address Line Two |
4th Floor
|
| Amendment Flag |
false
|
| Entity Central Index Key |
0001840439
|
| Document Type |
8-K
|
| Document Period End Date |
Jan. 09, 2024
|
| Entity Registrant Name |
Biomea Fusion, Inc.
|
| Entity Incorporation State Country Code |
DE
|
| Entity File Number |
001-40335
|
| Entity Tax Identification Number |
82-2520134
|
| Entity Address, Address Line One |
900 Middlefield Road, 4th Floor
|
| Entity Address, City or Town |
Redwood City
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94063
|
| City Area Code |
(650)
|
| Local Phone Number |
980-9099
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre Commencement Tender Offer |
false
|
| Pre Commencement Issuer Tender Offer |
false
|
| Security 12b Title |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
BMEA
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Biomea Fusion (NASDAQ:BMEA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Biomea Fusion (NASDAQ:BMEA)
Historical Stock Chart
From Apr 2023 to Apr 2024