Pfizer, BioNTech Get Order for 300 Million Doses of Covid-19 Vaccine from U.S. Government
July 22 2020 - 7:55AM
Dow Jones News
By Chris Wack
Pfizer Inc. and BioNTech SE said Wednesday they have signed an
agreement with the U.S. Department of Health and Human Services and
the Department of Defense to meet the federal government's
Operation Warp Speed program goal to begin delivering 300 million
doses of a Covid-19 vaccine in 2021.
Under the agreement, the U.S. government would receive 100
million doses of BNT162, the Covid-19 vaccine candidate jointly
developed by Pfizer and BioNTech, after it receives approval or
emergency use authorization from Food and Drug Administration.
Pfizer would make the vaccine.
The U.S. government would pay the companies $1.95 billion upon
the receipt of the first 100 million doses. The U.S. government
also can acquire as much as 500 million extra doses. Americans
would receive the vaccine for free.
The BNT162 program is based on BioNTech's proprietary mRNA
technology and supported by Pfizer's global vaccine development and
manufacturing capabilities. The BNT162 vaccine candidates are
undergoing clinical studies and aren't currently approved for
distribution anywhere in the world.
The Pfizer/BioNTech vaccine development program is evaluating at
least four experimental vaccines. If the studies are successful,
Pfizer and BioNTech expect to seek emergency use authorization or
some form of regulatory approval as early as October.
The companies currently expect to manufacture globally up to 100
million doses by the end of 2020 and potentially more than 1.3
billion doses by the end of 2021, subject to final dose selection
from their clinical trial.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
July 22, 2020 07:40 ET (11:40 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
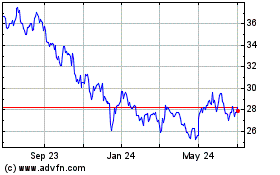
Pfizer (NYSE:PFE)
Historical Stock Chart
From Mar 2024 to Apr 2024
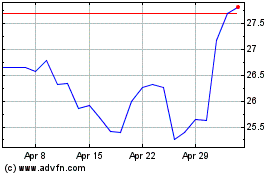
Pfizer (NYSE:PFE)
Historical Stock Chart
From Apr 2023 to Apr 2024