
2024 Investor Presentation www.OrthoPediatrics.com

22 0 2 4 / / Disclaimer Forward-Looking Statements All statements, other than statements of historical facts, contained in this quarterly report, including statements regarding our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our business, operations and financial performance and condition, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward looking statements speak only as of the date of this report. Forward-looking statements involve known and unknown risks, uncertainties and other factors, such as the impact of widespread health emergencies, such as COVID 19 and respiratory syncytial virus, that may cause our results, activity levels, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements. Forward-looking statements may include, among other things, statements relating to: our ability to achieve or sustain profitability in the future; our ability to raise additional capital to fund our existing commercial operations, develop and commercialize new products and expand our operations; our ability to commercialize our products in development and to develop and commercialize additional products through our research and development efforts, and if we fail to do so we may be unable to compete effectively; our ability to generate sufficient revenue from the commercialization of our products to achieve and sustain profitability; our ability to comply with extensive government regulation and oversight both in the United States and abroad; our ability to maintain and expand our network of third-party independent sales agencies and distributors to market and distribute our products; and our ability to protect our intellectual property rights or if we are accused of infringing on the intellectual property rights of others; We cannot assure you that forward-looking statements will prove to be accurate, and you are encouraged not to place undue reliance on forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations expressed or implied by the forward-looking statements. You are urged to carefully review and consider the various disclosures made by us in our quarterly report, in our Annual Report on Form 10-K filed with the Securities and Exchange Commission (the "SEC") on March 8, 2024, and in other reports filed with the SEC that discuss the risks and factors that may affect our business. Other than as required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information, events or circumstances occurring after the date of this quarterly report. Use of Non-GAAP Financial Measures This press release includes certain non-GAAP financial measures such as adjusted diluted loss (earnings) per share and Adjusted EBITDA, which differ from financial measures calculated in accordance with U.S. generally accepted accounting principles (“GAAP”). Adjusted loss (earnings) per share in this press release represents diluted loss (earnings) per share on a GAAP basis, plus the accreted interest attributable to acquisition installment payables, the fair value adjustment of contingent consideration, trademark impairment, acquisition related costs, non-recurring Pega conversion fees, and minimum purchase commitment costs. The fair value adjustment of contingent consideration is associated with our estimates of the value of earn-outs in connection with certain acquisitions. We believe that providing the non-GAAP diluted loss (earnings) per share excluding these expenses, as well as the GAAP measures, assists our investors because such expenses are not reflective of our ongoing operating results. Adjusted EBITDA in this release represents net loss, plus interest expense, net plus other expense, provision for income taxes (benefit), depreciation and amortization, trademark impairment, stock-based compensation expense, fair value adjustment of contingent consideration, acquisition related costs, nonrecurring Pega conversion fees, and the cost of minimum purchase commitments. The Company believes the non-GAAP measures provided in this earnings release enable it to further and more consistently analyze the period-to-period financial performance of its core business operating performance. Management uses these metrics as a measure of the Company’s operating performance and for planning purposes, including financial projections. The Company believes these measures are useful to investors as supplemental information because they are frequently used by analysts, investors and other interested parties to evaluate companies in its industry. Adjusted EBITDA is a non-GAAP financial measure and should not be considered as an alternative to, or superior to, net income or loss as a measure of financial performance or cash flows from operations as a measure of liquidity, or any other performance measure derived in accordance with GAAP, and it should not be construed to imply that the Company’s future results will be unaffected by unusual or non-recurring items. In addition, the measure is not intended to be a measure of free cash flow for management’s discretionary use, as it does not reflect certain cash requirements such as debt service requirements, capital expenditures and other cash costs that may recur in the future. Adjusted EBITDA contains certain other limitations, including the failure to reflect our cash expenditures, cash requirements for working capital needs and other potential cash requirements. In evaluating these non-GAAP measures, you should be aware that in the future the Company may incur expenses that are the same or similar to some of the adjustments in this presentation. The Company’s presentation of non-GAAP diluted loss (earnings) per share or Adjusted EBITDA should not be construed to imply that its future results will be unaffected by any such adjustments. Management compensates for these limitations by primarily relying on the Company’s GAAP results in addition to using these adjusted measures on a supplemental basis. The Company’s definition of these measures is not necessarily comparable to other similarly tit led captions of other companies due to different methods of calculation. The schedules below contain reconciliations of reported GAAP diluted loss (earnings) per share to non-GAAP diluted loss (earnings) and net loss to non-GAAP Adjusted EBITDA.

32 0 2 4 / / pediatric patients treated since inception + 1,000,000 OrthoPediatrics was founded on the cause of impacting the lives of children with orthopedic conditions 1 1 Includes patients treated by MD Orthopaedics (MDO), Pega Medical (Pega), and Boston Orthotics & Prosthetics (Boston O&P) since inception

01 Children’s unique clinical conditions Existing solutions are re-purposed from adult implants Limited development of new technologies No specialized sales force in Pediatric Orthopedics Limited industry support of clinical education 02 03 04 05 42 0 2 4 / / Historical Challenges of Pediatric Orthopedics Re-Purposed Adult Plate Screws through growth plate

52 0 2 4 / / OrthoPediatrics Solution 01 02 03 04 05 Product development focused exclusively on pediatric patients Broadest pediatric specific portfolio in the industry Delivering first in market novel surgical solutions Only global commercial channel to market Leading provider of surgeon clinical education PediLoc Femur Screws parallel to growth plate Enhance surgeon confidence Increase surgical efficiency Improve surgical accuracy
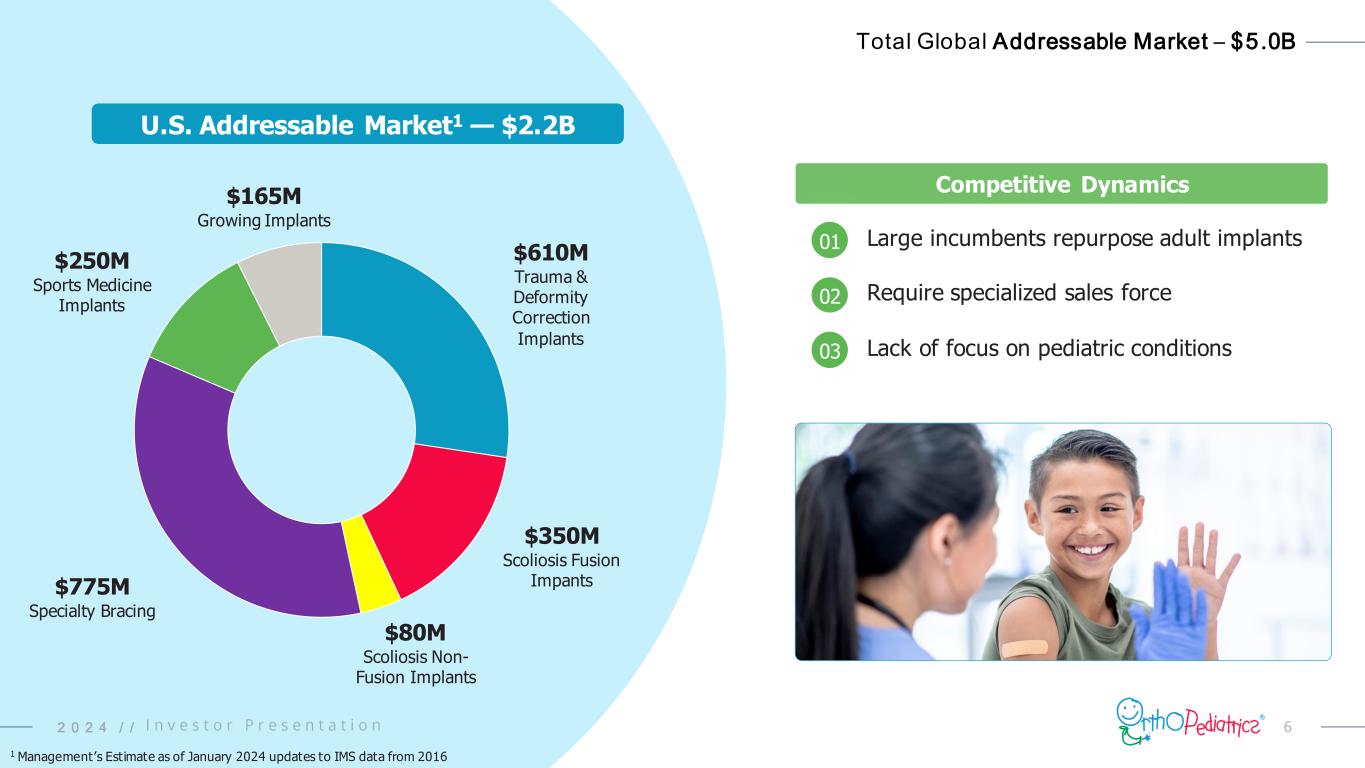
62 0 2 4 / / Total Global Addressable Market – $5.0B Competitive Dynamics $610M Trauma & Deformity Correction Implants $350M Scoliosis Fusion Impants $80M Scoliosis Non- Fusion Implants $775M Specialty Bracing $250M Sports Medicine Implants $165M Growing Implants Large incumbents repurpose adult implants Require specialized sales force Lack of focus on pediatric conditions 01 02 03 U.S. Addressable Market1 — $2.2B 2 0 2 4 / / 1 Management’s Estimate as of January 2024 updates to IMS data from 2016

72 0 2 4 / / Only Focused Pediatric Orthopedic Company Consistent 20%+ Growth Since Inception 1 Excluding COVID-impacted 2020 71 unique pediatric systems Consistent cadence of innovative product launches Expanding suite of enabling technologies Internal R&D, acquisitions, and partnerships Only global sales & distribution channel Serve 100% of top children’s hospitals in the U.S. ~200 domestic field representatives Sell in over 70 countries around the world Commitment to clinical education Leading sponsor of critical pediatric medical societies >300 clinical product/education events per year Founder of Foundation of Advancing Pediatric Orthopedics Innovative Technology Commercial Execution Clinical Education 1
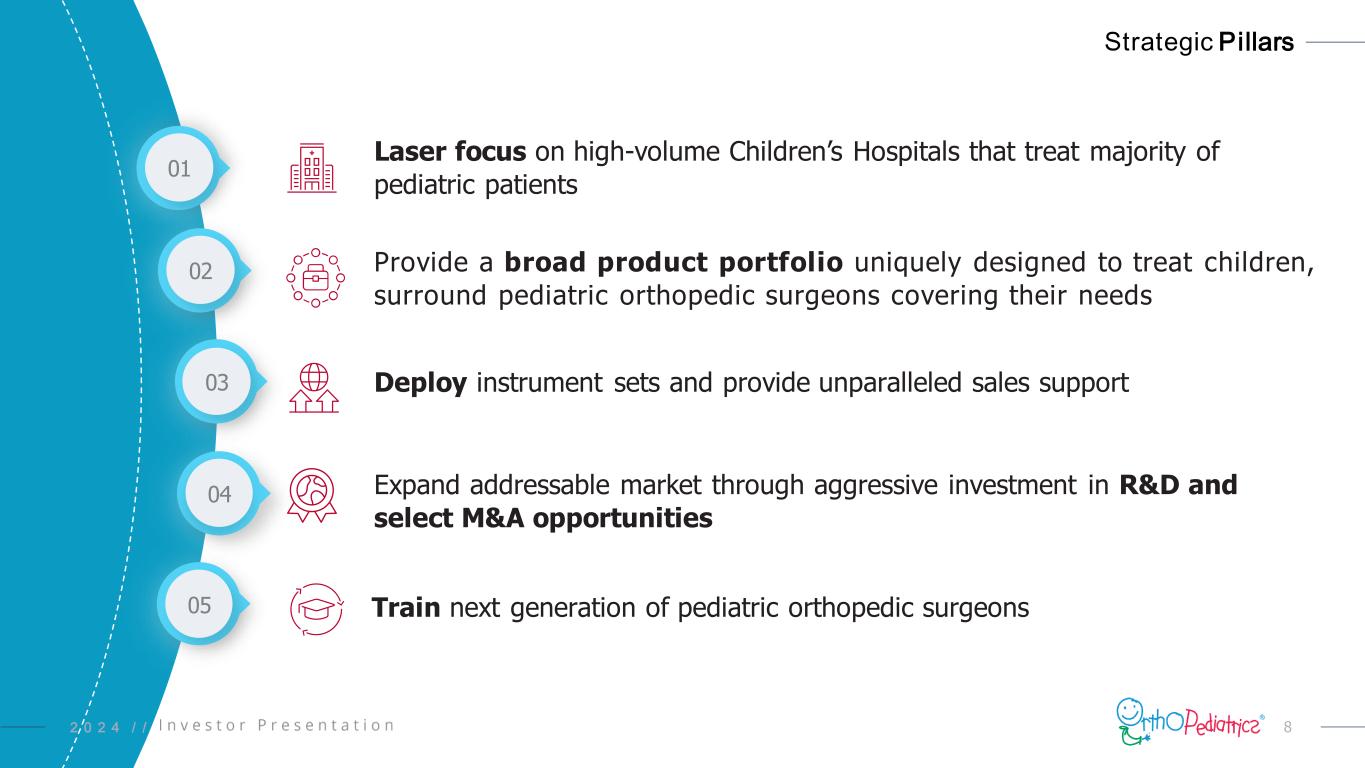
82 0 2 4 / / Strategic Pillars 02 Provide a broad product portfolio uniquely designed to treat children, surround pediatric orthopedic surgeons covering their needs 04 Expand addressable market through aggressive investment in R&D and select M&A opportunities 05 Train next generation of pediatric orthopedic surgeons 03 Deploy instrument sets and provide unparalleled sales support 01 Laser focus on high-volume Children’s Hospitals that treat majority of pediatric patients

92 0 2 4 / / Comments ~1,520 Fellowship Trained Pediatric Surgeons Majority of Pediatric Centers are Teaching Hospitals Centers Treat Most Complex Pediatric Conditions ~80% of Pediatric Surgeons time is Non-Surgical 01 02 03 $455M Trauma, Deformity Correction & Sports Medicine Implants $280M Scoliosis Fusion & Non-Fusion Implants $500M Specialty Bracing U.S. Current Target Market1 — $1.2B 01 Focus on High-Vol Children’s Hospitals Current Global Target Market – $2.8B 2 0 2 4 / / 04 1 Management’s Estimate as of January 2024 updates to IMS data from 2016 300 U.S. Pediatric Centers conduct ~62% of Trauma & Deformity Correction and Scoliosis Surgical Procedures

102 0 2 4 / / Pre-IPO Transitioned from Early Entry to Clinically Significant Cannulated Screws Response 5.5/6.0mm Fusion System PediPlate Physeal Tethering Hip Systems LCB / LPF ACL System BandLoc 5.5/6.0mm Banding Clavicle Plating System PediFlexPediLoc Femur PediLoc Tibia PediFragLocking Proximal Femur LPF Plate Distal Femoral Osteotomy System DFOS PAOSpica Table 02 Broad Product Portfolio 2008 2009 PediNail System 2012 2013 2014 2015 2016 2017

112 0 2 4 / / Accelerating Sales Growth Post-IPO Through Strategic Investment and Innovation $37.3 $45.6 $57.6 $72.6 $71.1 $98.0 $122.3 $148.7 2016 2017 2018 2019 2020 2021 2022 2023 Total Revenue ($M) 2016 2023 CAGR U.S. Independent Sales Consultants 90 205 13% Instrument Set Deployments $7M $22M 18% Unique Pediatric Systems 17 71 23% Intl. Independent Sales Agencies 0 14 Fav Increase Hospital PenetrationAccelerate Revenue Growth Improve Profitability Leverage Balance Sheet +22% CAGR 03 Expand Instr. Sets & Sales Personnel 1 1 Impacted by COVID

122 0 2 4 / / Post-IPO moved from Clinically Significant to Disruptive 202020192017 2018 FireFly Patient Specific Navigation Guides 2021 DFOS Distal Femoral Osteotomy System MPFL System Wrist Fusion Plates PNP Pediatric Nailing Platform Response 4.5/5.0mm Fusion System Orthex External Fixation Systems PediFoot System SCFE System QuickPack System ApiFix Non-Fusion Neuromuscular Scoliosis System 7D Intraoperative Navigation Enabling Technology 04 Expand Market with R&D Mini Rail System 2022 MD Ortho PonsetiTM Specialty Clubfoot Bracing PediFlexT M Advanced Interlock Clamp System Drive Rail External Fixation System Pega FD Telescopic IM Nail System 2023 MDO Move Brace Scoliosis Cannulated Screw System RESPONSE Power System GIRO System Developed by Pega DF2 Brace MP+ Bar PNP TIBIA System 2024 RESPONSE Rib & Pelvic Fixation System

132 0 2 4 / / Strategic Acquisitions & Partnerships Partnership Acquisition 202020192017 2022 04 Expand Market with M&A 2021 2023 2024

142 0 2 4 / / Acquired Innovative Technologies Boston Orthotics & Prosthetics • Pioneered the original patient-specific, custom Boston Scoliosis Brace • Currently has 5 disease state focuses with 17 different product offerings • Custom manufacturing and fabrication center based outside of Boston, MA • Newly established headquarters for the OrthoPediatrics Specialty Bracing (OPSB) division • Owns and operates 26 pediatric / adolescent focused O&P clinics (w/CPOs) in 10 states, mainly New England area Terms: • Closed January 5, 2024 • $22M Cash 04 Expand Market with M&A State-of-the-Art Products - - That Better Each Patient’s Life

152 0 2 4 / / Comments ~80% of Pediatric Surgeons Time is Non-Surgical Same Surgeons Who Use OP Surgical Products Relationship with OP Sales Channel Surrounds the Surgeon with all the Products They Need 01 02 03 $200M Lower Limb Extremity Specialty Bracing $125M Hip / Cranial / Other Speciality Bracing $175M Scoliosis Speciality Bracing 300 U.S. Pediatric Centers U.S. Specialty Bracing Market 1 — $0.5B Current Global Target Market – $1.1B 2 0 2 4 / / 04 1 Management’s Estimate as of January 2024 04 Expand Market with M&A

162 0 2 4 / / Motion Assist Devices Fracture Fixation Devices Specialty Casting Spine Halo Traction, Other Specialty Solutions 300 U.S. Pediatric Centers U.S. Potential Target Market 1 — $1.0B Potential Global Target Market – $2.2B 2 0 2 4 / / 1 Management’s Estimate as of January 2024 Levity Device for Gait Assist Dynamic Femur Fracture (DF2) Brace 04 Expand Market with M&A

172 0 2 4 / / PLAYBOOK Workflow & Care Optimization for the OR DIGITAL WORK INSTRUCTIONS define best practices for each user’s role throughout the entire surgical procedure DATA ANALYTICS Surgical Debrief provides real-time PERFORMANCE VISUALIZATION metrics INTERACTIVE LIVE SURGERY VIEW for remote support, education and training DYNAMIC PRE-OP PLANNING offers coordination and communication across CPD, Rep and Care Team REAL-TIME SURGEON PREF CARD UPDATES with notifications to drive accountability for changes QUALITY CHECKLISTS are initiated based on surgery phase and step completion Better care requires improved planning, communication & support to deliver reproducible outcomes 04 Expand Market with M&A Acquired Innovative Technologies

182 0 2 4 / / Terms: • Closed April 1, 2022 • $8.2M cash, $8.9M shares, $2.5M RSA MD Orthopaedics • Develops, manufactures and sells the patented Mitchell Ponseti Ankle-Foot Orthosis (AFO) to treat clubfoot • Dr. Ignacio Ponseti developed the gold standard for treating clubfoot which has >90% success rate • Casting is used from 0-3mos then bracing from 3mos–4 years. Requires multiple sizes as child grows creating repeat revenue. • Products sold in 90 countries including e-commerce platform direct to consumers • Approximately 80% of a pediatric surgeon's treatment time is non-surgical • Creates a profitable platform business for OP to develop and manufacture best-in-class specialty bracing with speed to market (class 1 device) as well as no consignment inventory required to grow the business Acquired Innovative Technologies04 Expand Market with M&A

192 0 2 4 / / Terms: • Closed July 5, 2022 • $31M cash, $2M stock Pega Medical • Developed the Fassier-Duval Telescopic Intramedullary Nail System (FD Nail) • FD Nail is cutting-edge implant designed to treat bone deformities in children with Osteogenesis Imperfecta without disrupting their normal growth • Pega offers 7 products in total, 6 of which focus on limb deformity correction, and 1 trauma • Products sold in 70 countries • Approximately 35,000 children suffer from Osteogenesis Imperfecta in the U.S. Acquired Innovative Technologies04 Expand Market with M&A

202 0 2 4 / / ApiFix • Disruptive non-fusion technology • Viable alternative to failed bracing & spinal fusion • Posterior, minimally invasive approach • Motion preserving capabilities • Granted FDA HDE approval Orthex • Disruptive software complements ex-fix frame • Expands addressable market • Serve 85% of procedures, up from 65% • Significantly simplifies surgical planning and alignment • Enables participation in most complex surgeries Acquired software-based and non-fusion technologies Significant sales synergies with legacy portfolio Expands critical KOL network Provides surgeons broadest product portfolio Acquired Innovative Technologies04 Expand Market with M&A

212 0 2 4 / / FIREFLY® Pedicle Screw Navigation Guides FireFly S2/Alar Unique patient specific 3D printed bone models and drill guides, can be used with any Spinal Deformity Correction system. • 99.7% screw placement accuracy • Preoperative concierge surgical planning drives intraoperative efficiency • Minimal intraoperative radiation • Simplifies S2AI approach Enabling Technology Partnerships 7D Surgical Intraoperative Navigation Eliminates Radiation exposure to staff & patients Cuts Registration from 30 min to < 30 sec Improves Accuracy to improve surgical outcomes Reduces Costs & improve hospital economic value 04 Expand Market with M&A

222 0 2 4 / / 7D Real World Experience Chris Comstock, MD & Eric Wait, MD Driscoll Children’s Hospital First Pediatric Deformity Installation in US I have noticed we are seeing shorter stays for our patients with complex spinal surgeries since we have started using the 7D technology. It used to be children would stay 3-5 days at Driscoll following surgery. Now what we are seeing is most of them are going home after 3 days. And that is better for kids and their families What we are seeing with this technology is surgeries which might have taken up to 5-6 hours are often being reduced to 3.5 hours Dr. Eric Wait Driscoll Children’s Hospital 04 Expand Market with M&A

232 0 2 4 / / Physician Education and Awareness OP Hands-on sales training and support • Annually invests 3% of sales on clinical education • Conducts >300 product/training sessions per year Continuous education • Major Sponsor of the prominent pediatric orthopedic societies Market development • Fosters early relationships with young surgeons and fellows to drive sustainable growth 02 03 01 As a surgeon educator, I have always appreciated and valued OrthoPediatrics’ commitment to education. Ryan Goodwin, MD, MBA, FAOA The Cleveland Clinic 05 Train Next-Gen Surgeons

242 0 2 4 / / Catalysts & Pipeline Enabling Technologies Scoliosis • Orthex surgical software • Firefly patient-specific planning/guides • 7D spinal interoperative navigation • PediPortal app • Medtech Concepts – Acquired May 1, 2023 • Advancing non-fusion treatment • Early-onset scoliosis innovations • Innovation in highly-complex fusion • Manual growing, rib based, etc. • Custom Scoliosis Bracing T&D • Expanding intramedullary nailing portfolio • Expanding of external fixation portfolio • Expanding specialty bracing portfolio • Solutions for rare bone disease
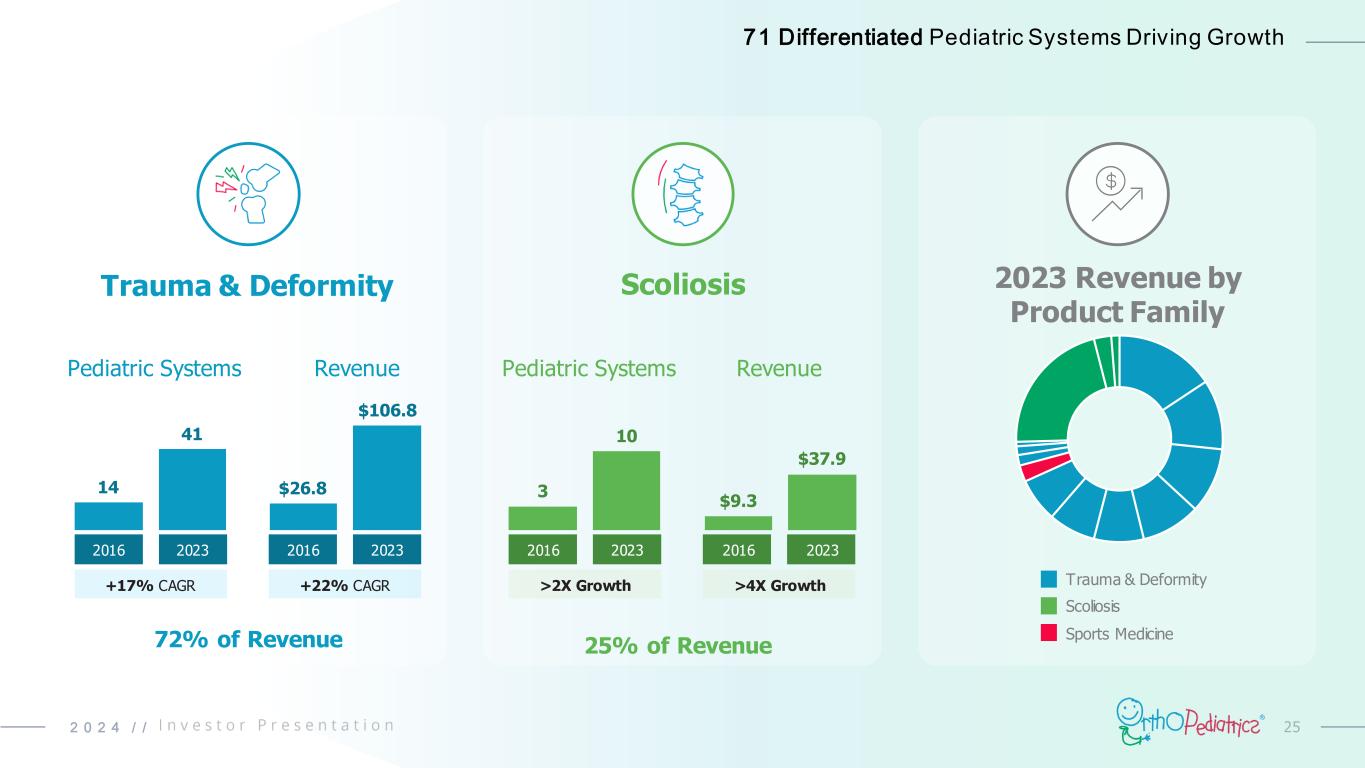
252 0 2 4 / / Trauma & Deformity +17% CAGR 14 41 2016 2023 $26.8 $106.8 2016 2023 Pediatric Systems Revenue +22% CAGR Scoliosis >2X Growth 3 10 2016 2023 $9.3 $37.9 2016 2023 Pediatric Systems Revenue >4X Growth 2023 Revenue by Product Family Trauma & Deformity Scoliosis Sports Medicine72% of Revenue 25% of Revenue 71 Differentiated Pediatric Systems Driving Growth
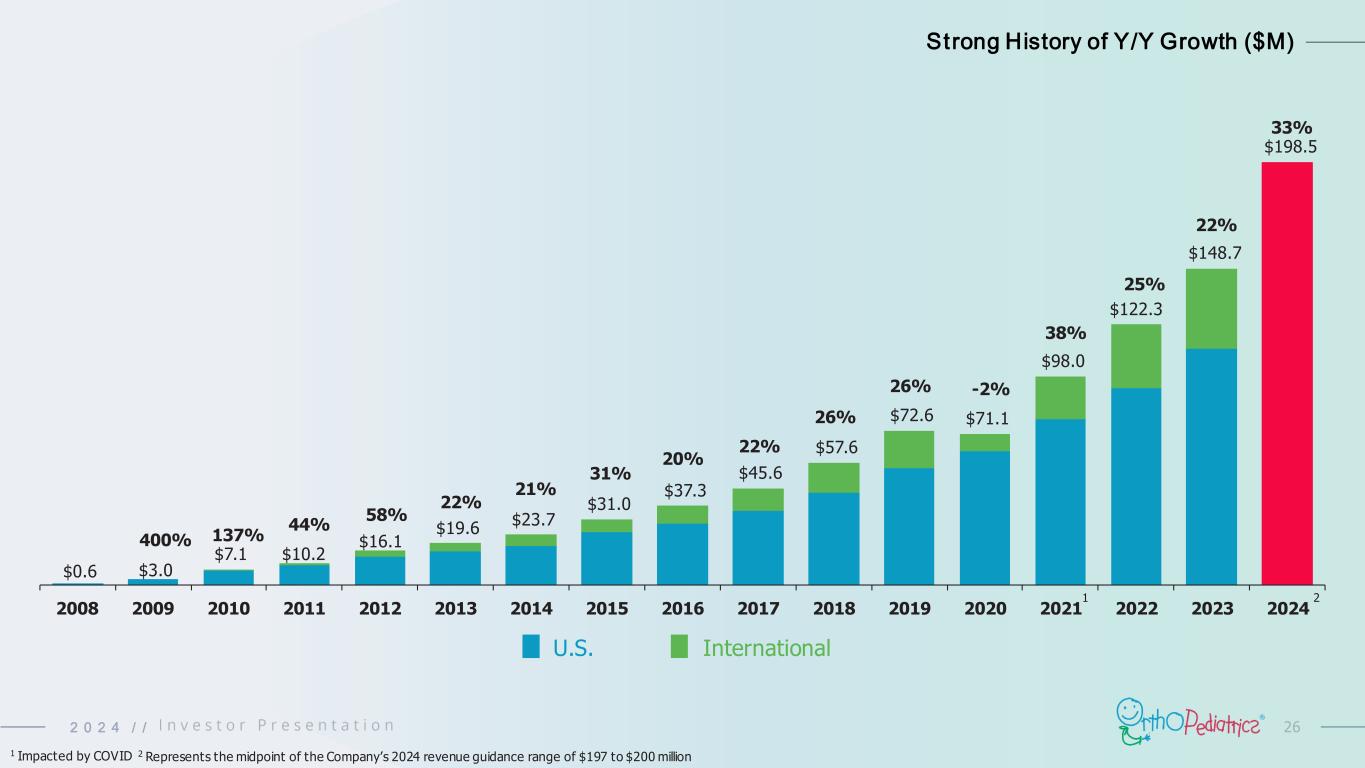
262 0 2 4 / / $0.6 $3.0 $7.1 $10.2 $16.1 $19.6 $23.7 $31.0 $37.3 $45.6 $57.6 $72.6 $71.1 $98.0 $122.3 $148.7 $198.5 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 Strong History of Y/Y Growth ($M) 400% 137% 44% 58% 22% 21% 31% 20% 22% 26% 26% -2% 38% U.S. International 1 25% 1 Impacted by COVID 22% 33% 2 2 Represents the midpoint of the Company’s 2024 revenue guidance range of $197 to $200 million
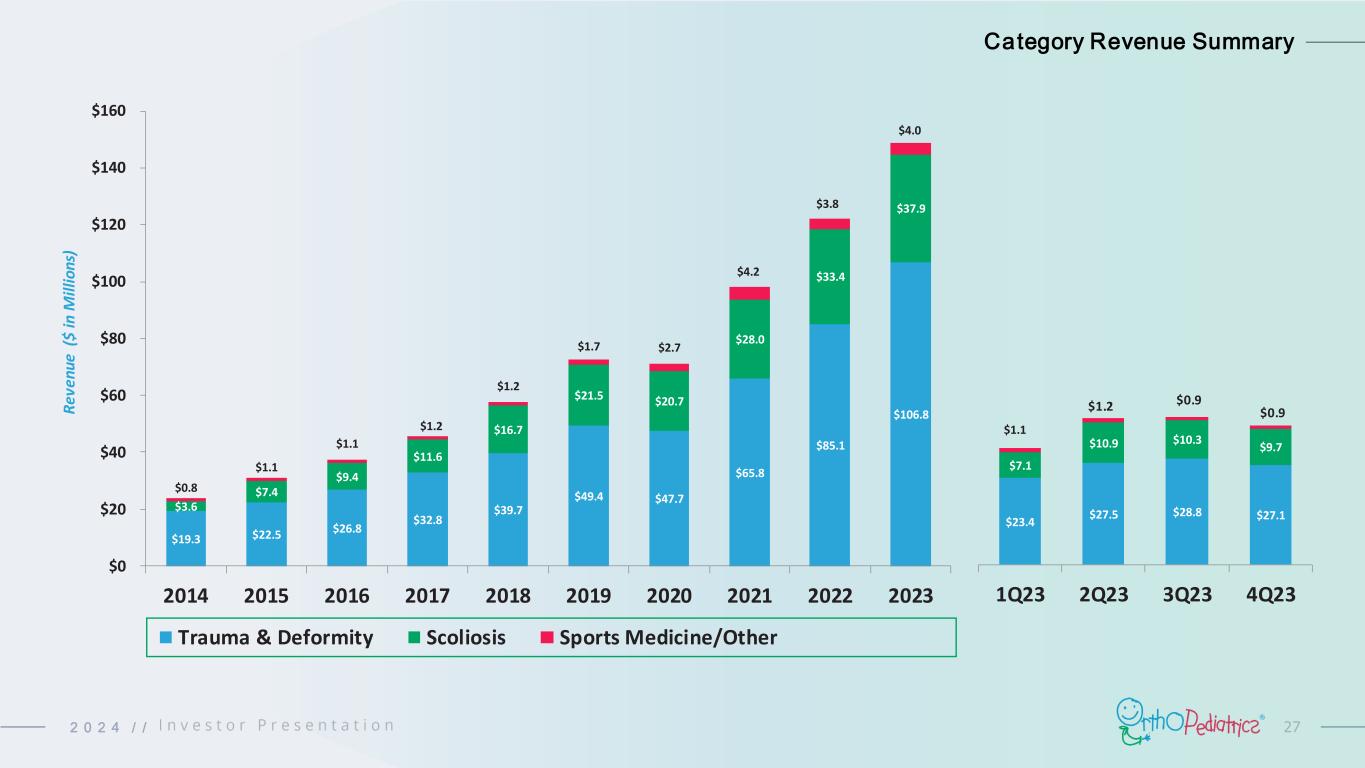
272 0 2 4 / / $19.3 $22.5 $26.8 $32.8 $39.7 $49.4 $47.7 $65.8 $85.1 $106.8 $3.6 $7.4 $9.4 $11.6 $16.7 $21.5 $20.7 $28.0 $33.4 $37.9 $0.8 $1.1 $1.1 $1.2 $1.2 $1.7 $2.7 $4.2 $3.8 $4.0 $0 $20 $40 $60 $80 $100 $120 $140 $160 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 R ev en ue ( $ in M ill io ns ) Trauma & Deformity Scoliosis Sports Medicine/Other $23.4 $27.5 $28.8 $27.1 $7.1 $10.9 $10.3 $9.7 $1.1 $1.2 $0.9 $0.9 1Q23 2Q23 3Q23 4Q23 Category Revenue Summary

282 0 2 4 / / Seasonality Drives Stronger Performance in Summer Months and Holiday Periods 21% 26% 27% 25% 20% 25% 29% 26% 23% 19% 31% 27% 22% 27% 26% 25% 19% 27% 29% 25% 21% 27% 27% 25% 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q R e ve n u e a s % o f To ta l Ye ar 2018 2019 2020 2021 2022 Revenue Seasonality 2023
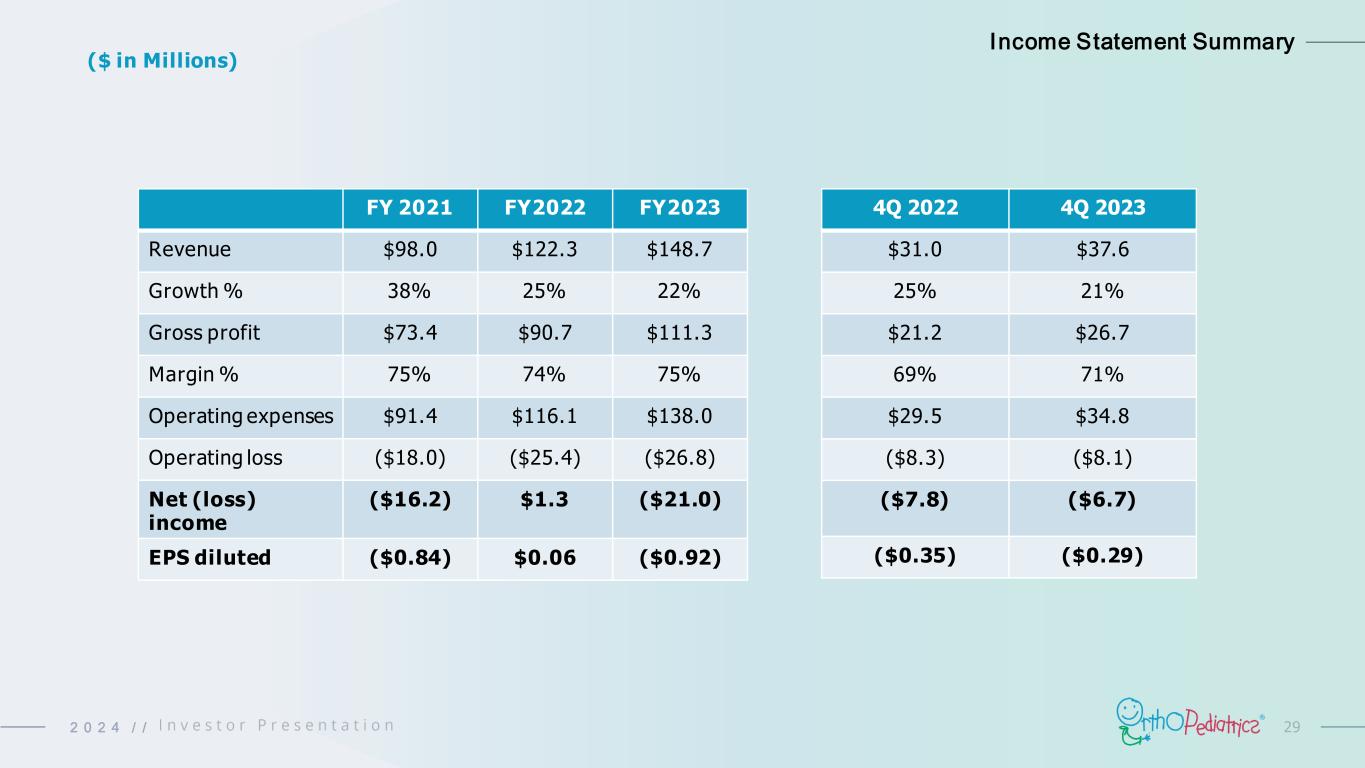
292 0 2 4 / / FY 2021 FY2022 FY2023 Revenue $98.0 $122.3 $148.7 Growth % 38% 25% 22% Gross profit $73.4 $90.7 $111.3 Margin % 75% 74% 75% Operating expenses $91.4 $116.1 $138.0 Operating loss ($18.0) ($25.4) ($26.8) Net (loss) income ($16.2) $1.3 ($21.0) EPS diluted ($0.84) $0.06 ($0.92) Income Statement Summary ($ in Millions) 4Q 2022 4Q 2023 $31.0 $37.6 25% 21% $21.2 $26.7 69% 71% $29.5 $34.8 ($8.3) ($8.1) ($7.8) ($6.7) ($0.35) ($0.29)

302 0 2 4 / / Revenue By Geography and Product Category Three Months Ended December 31, Product Sales by geography 2022 2023 U.S. $22.7 $28.3 International 8.3 9.3 Total Revenue $31.0 $37.6 Three Months Ended December 31, Product Sales by category 2022 2023 Trauma and deformity $22.1 $27.1 Scoliosis 8.0 9.6 Sports medicine/other 0.9 0.9 Total Revenue $31.0 $37.6 ($ in Millions) Twelve Months Ended December 31, Product Sales by geography 2022 2023 U.S. $92.4 $111.0 International 29.9 37.7 Total Revenue $122.3 $148.7 Twelve Months Ended December 31, Product Sales by category 2022 2023 Trauma and deformity $85.1 $106.8 Scoliosis 33.4 37.9 Sports medicine/other 3.8 4.0 Total Revenue $122.3 $148.7

312 0 2 4 / / Adjusted EBITDA Reconciliation Three Months Ended December 31, 2022 2023 Net (loss) income ($7.8) ($6.7) Interest (income) expense, net (0.1) (0.3) Other (income) expense 0.1 (0.9) Provision for income taxes (benefit) 0.0 (0.2) Depreciation and amortization 3.8 5.5 Stock-based compensation 1.6 2.5 Trademark impairment - - Fair value adjustment of contingent consideration (0.5) 0.0 Acquisition related costs - 0.5 Nonrecurring Pega conversion fees - - Minimum purchase commitment cost 0.7 0.9 Adjusted EBITDA ($2.2) $1.3 ($ in Millions) Twelve Months Ended December 31, 2022 2023 $1.3 ($21.0) 2.4 (0.2) 1.8 (2.3) (4.9) (0.3) 13.4 17.4 6.7 10.5 3.6 1.0 (25.9) (3.0) 0.8 0.7 - 0.3 1.1 2.0 $0.2 $5.0

322 0 2 4 / / Adjusted EPS Reconciliation Three Months Ended December 31, 2022 2023 Loss (income) per share, diluted (GAAP) ($0.35) ($0.29) Accretion of interest attributable to acquisition installment payable 0.02 0.01 Fair value adjustment of contingent consideration (0.02) - Trademark impairment - - Acquisition related costs - 0.02 Nonrecurring Pega conversion fees - - Minimum purchase commitment cost 0.03 0.04 Adjusted loss per share, diluted (non-GAAP) ($0.32) ($0.22) Twelve Months Ended December 31, 2022 2023 $0.06 ($0.92) 0.11 0.05 (1.25) (0.13) 0.17 0.04 0.04 0.03 - 0.01 0.05 0.09 ($0.82) ($0.83)

332 0 2 4 / / Balance Sheet Assets Cash, cash equivalents & short-term investments $82.3 Account receivable 34.6 Inventory (net) 105.8 Other current assets 3.8 Total Current Assets 226.5 PP&E (net) 41.0 Intangibles and goodwill 171.2 Total Assets $438.7 Liabilities Accounts payable $12.6 Debt 10.0 Accrued comp. & other liab. 25.3 Acquisition pay. & cont. consideration 13.7 Paid-in capital 580.3 Accumulated deficit (net) (197.7) Accumulated other comprehensive loss (5.5) Total Liabilities / Equity $438.7 ($ in Millions) As of December 31, 2023

342 0 2 4 / / 2024 Guidance FY2024 Revenue $197.0 to $200.0 Adjusted EBITDA $8.0 to $9.0 FY2024 2024 Total Revenue Growth % 32% to 34% Set Deployment <$20.0 Assumptions Full Year 2024 Guidance ($ in Millions)

352 0 2 4 / / Investment Summary Only diversified company focused exclusively on pediatric orthopedics Large, underpenetrated market opportunity in pediatrics Highly concentrated customer base with targeted commercial strategy Broad product portfolio with innovative solutions Only provider committed to pediatric clinical education Dynamic, award-winning corporate culture Proven commercial execution and attractive financial profile 01 04 02 05 03 06 07

2850 Frontier Drive Warsaw, IN 465852 ph: 574.268.6379 or 877.268.6339 fax: 574.268.6302 www.OrthoPediatrics.com2 0 2 4 / /