
R&D Day SEPTEMBER 21, 2023

T h e s e s l i d e s c o n t a i n f o r w a r d - l o o k i n g s t a t e m e n t s w i t h i n t h e m e a n i n g o f t h e P r i v a t e S e c u r i t i e s L i t i g a t i o n R e f o r m A c t o f 1 9 9 5 . The words “may,” “will,” “estimate,” “plan”, “anticipate,” “expect,” “potential,” “could,” “project,” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding Cidara’s research and development efforts; preclinical and clinical development activities; plans, projections and expectations for and the potential effectiveness, safety and benefits of, its product candidates, including rezafungin, CD388, and other antiviral and oncology product candidates from the Cloudbreak platform; Cidara’s potential ability to achieve milestones under its respective collaborations with Melinta, Mundipharma and Janssen, and receipt of the related milestone payments; and advancement of its strategic plans. These slides are not intended to and do not constitute an offer to sell or the solicitation of an offer to subscribe for or buy or an invitation to purchase or subscribe for any securities in any jurisdiction, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Projections, assumptions and estimates of the future performance of the markets in which Cidara operates are necessarily subject to a high degree of uncertainty and risk, including, Cidara's ability to obtain additional financing; the success and timing of Cidara’s preclinical studies, clinical trials and other research and development activities; receipt of necessary regulatory approvals for development, as well as changes to applicable regulatory laws in the United States and foreign countries; changes in Cidara's plans to develop its product candidates; Cidara's ability to obtain and maintain intellectual property protection for its product candidates; and the loss of key scientific or management personnel. These and other risks and uncertainties are described more fully in Cidara's Form 10-K as most recently filed with the United States Securities and Exchange Commission ("SEC“’) under the heading “Risk Factors.” Additional risks and uncertainties may emerge from time to time, and it is not possible for Cidara’s management to predict all risk factors and uncertainties. F orwa rd - l o ok i ng statements

3 Agenda PresenterTopic Jeffrey Stein, PhDIntroduction Jeffrey Stein, PhDDrug-Fc Conjugates (DFCs) – a novel and clinically proven therapeutic class Jeffrey Stein, PhDUpdate on JNJ 0953 (CD388): a clinical stage universal influenza DFC in Phase 2 trials Nicole Davarpanah, MD, JDCloudbreak Pipeline Discussion - translating success in infectious disease to oncology Stephen Schoenberger, PhDWho is left behind by cancer immunotherapy? Les Tari, PhD and Nicole Davarpanah, MD, JD Harnessing DFCs to create targeted therapies that unleash the immune system Ezra Cohen, MDPerspective: The potential of DFCs to transform precision oncology Jeffrey Stein, PhDWrap up and Q&A

4 Today’s Speakers Jeffrey Stein, PhD President & CEO Cidara Therapeutics Stephen Schoenberger, PhD Professor, Immunology La Jolla Institute for Immunology Ezra Cohen, MD CMO, Oncology Tempus Les Tari, PhD CSO Cidara Therapeutics Nicole Davarpanah, MD, JD SVP, Translational R&D Cidara Therapeutics
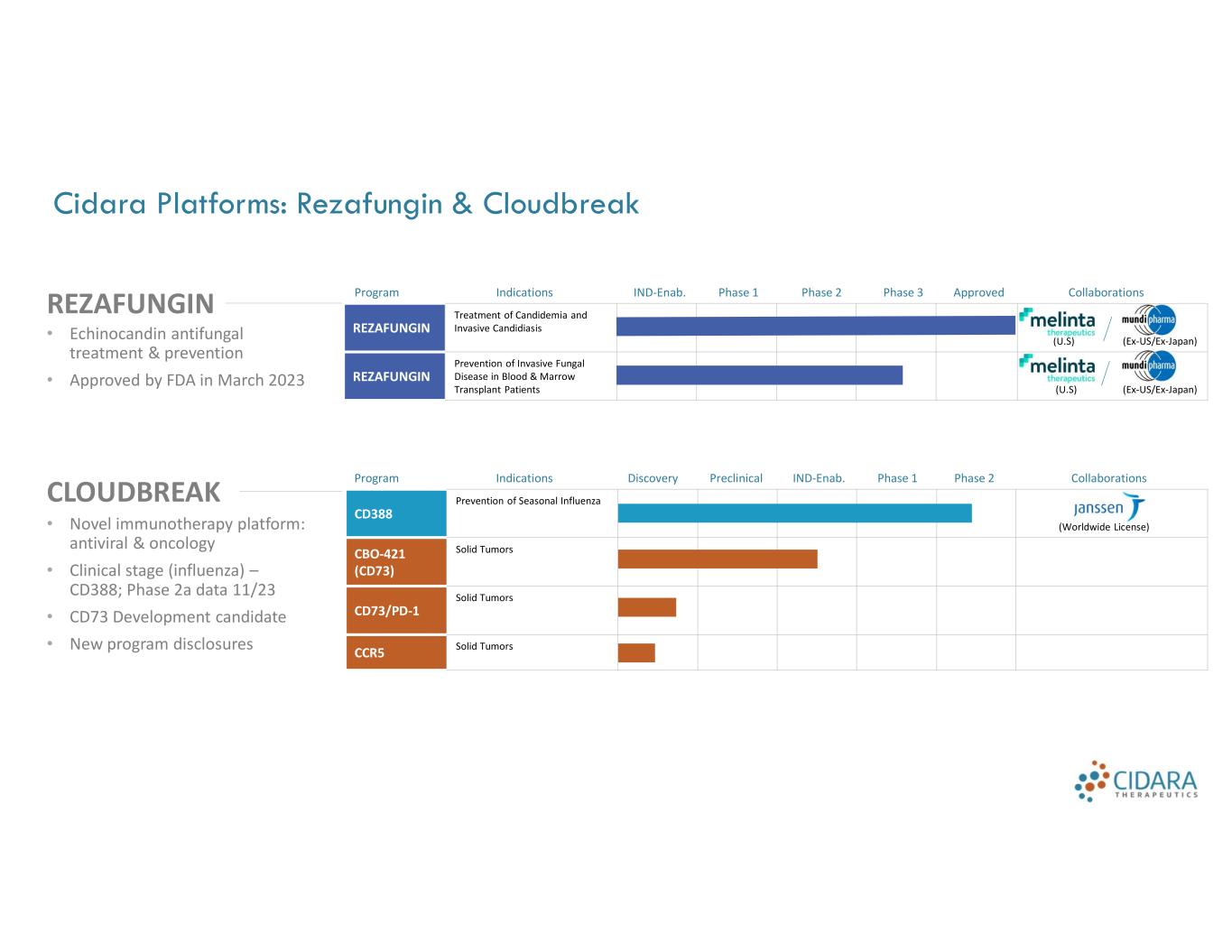
Cidara Platforms: Rezafungin & Cloudbreak REZAFUNGIN CLOUDBREAK • Echinocandin antifungal treatment & prevention • Approved by FDA in March 2023 • Novel immunotherapy platform: antiviral & oncology • Clinical stage (influenza) – CD388; Phase 2a data 11/23 • CD73 Development candidate • New program disclosures CollaborationsApprovedPhase 3Phase 2Phase 1IND-Enab.IndicationsProgram (U.S) (Ex-US/Ex-Japan) Treatment of Candidemia and Invasive CandidiasisREZAFUNGIN (U.S) (Ex-US/Ex-Japan) Prevention of Invasive Fungal Disease in Blood & Marrow Transplant Patients REZAFUNGIN CollaborationsPhase 2Phase 1IND-Enab.PreclinicalDiscoveryIndicationsProgram (Worldwide License) Prevention of Seasonal Influenza CD388 Solid TumorsCBO-421 (CD73) Solid Tumors CD73/PD-1 Solid TumorsCCR5

Launched July 31, 2023

Cloudbreak Programs REZAFUNGIN CLOUDBREAK • Echinocandin antifungal treatment & prevention • Approved by FDA in March 2023 • Novel immunotherapy platform: antiviral & oncology • Clinical stage (influenza) – CD388; Phase 2a data 11/23 • CD73 Development candidate • New program disclosures CollaborationsApprovedPhase 3Phase 2Phase 1IND-Enab.IndicationsProgram (U.S) (Ex-US/Ex-Japan) Treatment of Candidemia and Invasive CandidiasisREZAFUNGIN (U.S) (Ex-US/Ex-Japan) Prevention of Invasive Fungal Disease in Blood & Marrow Transplant Patients REZAFUNGIN CollaborationsPhase 2Phase 1IND-Enab.PreclinicalDiscoveryIndicationsProgram (Worldwide License) Prevention of Seasonal Influenza CD388 Solid TumorsCBO-421 (CD73) Solid Tumors CD73/PD-1 Solid TumorsCCR5

8 Cloudbreak® Creates a New Class of Drug Conjugates: “DFCs” DRUG Fc CONJUGATE Proprietary CH1-Fc hybrid domain hIgG1 Stable linker Small molecule Targeting Moiety (TM) DFCs are designed to engage extracellular targets

9 DFCs are Fundamentally Different from ADCs and Bispecifics SM Cytotoxin SM drug conjugate ADC Bispecific mAb DFC

Fc MOIETY Fc Moiety is Tailored to Specific Indications • IgG1 • Enhanced FcRn binding • Increased half-life compared to wild-type • IgG1 with reduced immune effector function PK extended Fc Immune silent Fc Wild type ANTIVIRALS ONCOLOGY

Fc MOIETY SMALL MOLECULES (SM) PEPTIDE FUSIONS Different Targeting Moieties (TMs) Attach to the Fc Moiety Directed against surface targets Example: Neuraminidase in CD388 Inhibit protein-protein interactions Example: PD-1 peptide

12 DFCs Can Improve Small Molecule Drug Potency and Safety DFC Long half-life, similar to mAbs Target Cell Target Cell Cyp450s, intracellular proteins DFC Unlike small molecules, DFCs don’t enter cells, reducing off-target risks Multivalent target engagement increases potency, reduces resistance potential

13 % hERG inhibition @10mM 77SM < 5CCR-DFC DFCs Have Reduced Potential for Off-Target Toxicity *EXPERT OPINION ON INVESTIGATIONAL DRUGS, 2016 VOL. 25, NO. 12, 1377–1392 http://dx.doi.org/10.1080/13543784.2016.1254615 DFCs can exploit drug targets that are difficult to drug with small molecules (SMs) Chemokine receptor DFC (CCR-DFC) Many CCR SM programs have failed to advance due to intracellular off-target toxicity (e.g. hepatotoxicity)* Cardiovascular toxicity due to hERG inhibition has impaired clinical development in many CCR programs* Target Cell Cyp450s, intracellular proteins DFC

CD388 Phase 2A Data Confirm the TPP CD388 is being developed for pre- exposure prophylaxis Neuraminidase inhibitor Single dose / ̴ 4-6 months Successful Phase 2a interim data* INFLUENZA Data available at https://www.cidara.com/cloudbreak/influenza/ and at https://clinicaltrials.gov/ct2/show/NCT05285137 * https://www.cidara.com/news/cidara-therapeutics-announces-promising-interim-phase-2a-data-assessing-the-safety-and-efficacy-of-a-single-dose-of-cd388-in-an-influenza-challenge-model/ Universal protection: all strains Potential to protect all high risk groups Potential for prevention and treatment Scale and cost High Yes Attractive DFCs Yes

H1N1 influenza virus JNJ0953 (CD388) Program Update 15

Influenza Worldwide: 650,000 deaths/year Influenza Vaccine Efficacy varies from year to year due to antigenic shift/drift and is consistently low in immunocompromised patients Patients with selected comorbidities are at increased risk of complications/hospitalizations of influenza-like illness despite vaccination General PopulationImmunocompromised 5% 2017-2018 19% 2014-15 US 38% 2017-18 US Source: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm; https://gis.cdc.gov/grasp/fluview/FluHospChars.html; Hughes et al. cid 2021:73 Unadjusted ORs (all-cause hospitalizations 30 days after influenza-like illness) against healthy controls in vaccinated individuals, Optum DS study 2022 Up to 20-fold increase in risk among severe kidney disease (healthy control) Chronic lung diseases increased risk up to 19-fold (healthy control) Chronic heart diseases increased risk up to 12-fold (healthy control) 16 *PrEP = Pre exposure prophylaxis 16 Despite influenza vaccination, a high disease burden and unmet need remains for influenza prevention in specific groups

17 Objectives of Early Clinical Development 17 Pharmacokinetics Safety Can this compound be used for seasonal prophylaxis of influenza? Is this compound well tolerated and potentially amenable for prophylactic use? Does the compound exhibit activity against virus in the respiratory tract of humans?Efficacy

18 A Phase 1, Randomized, Double-Blind, Single- Dose and Repeat Single Dose, Dose-Escalation Study to Determine the Safety, Tolerability, and Pharmacokinetics of CD388 Intramuscular or Subcutaneous Administration in Healthy Subjects Primary endpoint: safety and tolerability Secondary endpoints: plasma pharmacokinetics A Phase 1, Randomized, Double-Blind, Single Ascending Dose Study to Determine the Safety, Tolerability, and Pharmacokinetics of CD388 Subcutaneous Administration in Healthy Japanese Subjects Primary endpoint: safety and tolerability Secondary endpoints: plasma pharmacokinetics A proof-of-concept, randomized, double-blind, placebo- controlled, Phase 2A study to assess the prophylactic antiviral activity against influenza, safety, tolerability, and pharmacokinetics of CD388 via a human viral challenge model Primary endpoint: prophylactic efficacy: reduction of area under the viral load-time curve (VL-AUC) after influenza challenge Secondary endpoints: • Prophylactic efficacy: additional endpoints • Safety and tolerability JNJ0953 Early clinical development studies

Safety Summary • No treatment-emergent SAEs and no discontinuation of study drug or withdrawals due to safety findings • No consistent AE patterns. • No hypersensitivity reactions • Most TEAEs were Grade 1 (90%), few Grade 2, all resolved • Incidence of TEAE not dose-dependent • Few injection site events (pain, IM route mainly), Grade 1, all resolved spontaneously • No clinically relevant ECG, vital signs or physical exam abnormalities TotalHCSJBSFIH 27271850 mg 5328718150 mg 250718450 mg 9009900 mg 114302163All Doses Aggregated Clinical Safety Data through end August 2023 from FIH, Japanese Bridging (JBS) and Human Challenge (HCS) studies 19 Number of participants that received one dose of JNJ- 0953 in Phase 1 and Phase 2a studies (as of Aug 2023) The JNJ-0953 PK profile is that of a long-acting compound, potentially enabling seasonal PrEP JNJ-0953 was safe and well-tolerated up to 900 mg
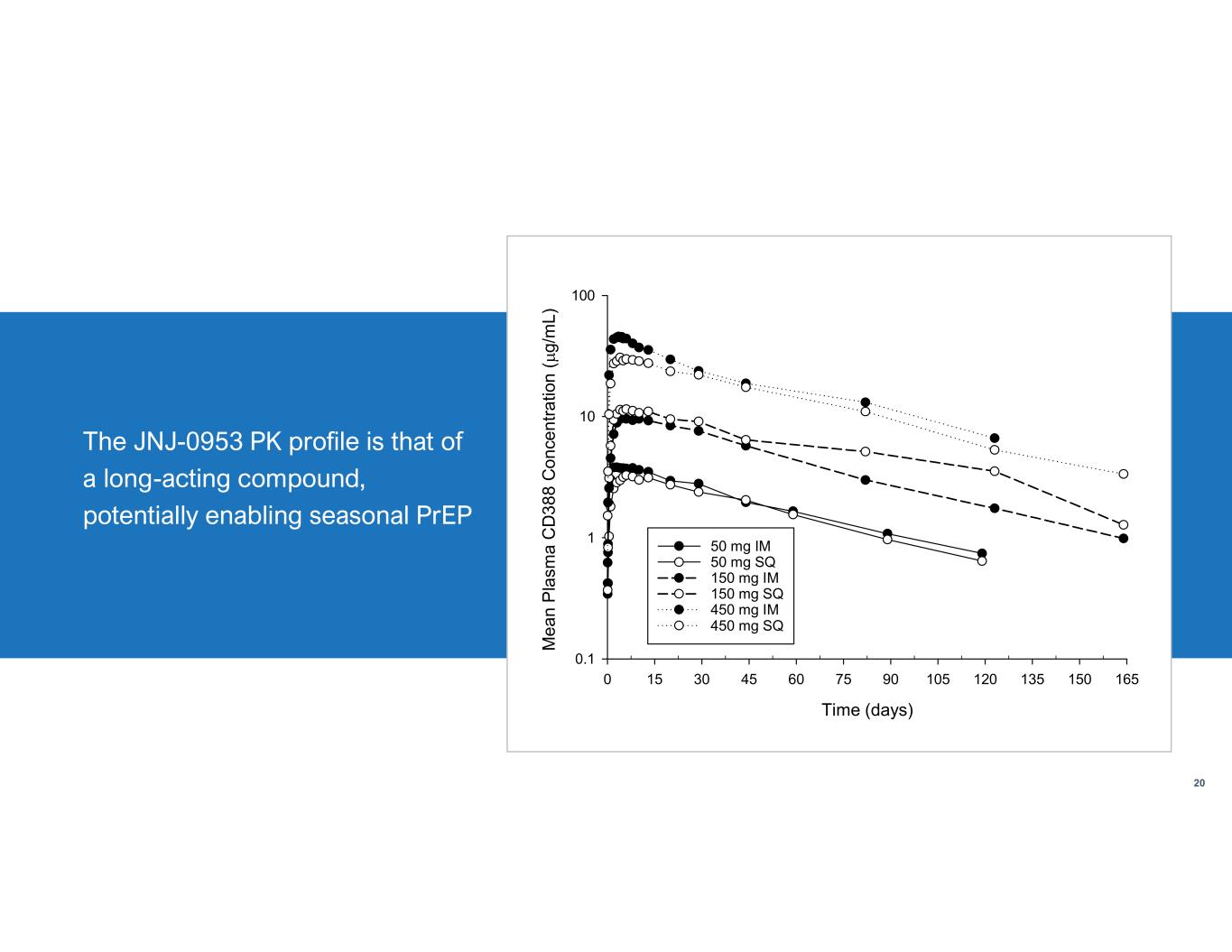
The JNJ-0953 PK profile is that of a long-acting compound, potentially enabling seasonal PrEP 20 Time (days) 0 15 30 45 60 75 90 105 120 135 150 165 M e a n P la sm a C D 3 8 8 C o n ce n tr a tio n ( g /m L) 0.1 1 10 100 50 mg IM 50 mg SQ 150 mg IM 150 mg SQ 450 mg IM 450 mg SQ

P-value JNJ-0953 150 mg N=28 Placebo N=28endpoint 0,02486 (21%)14 (50%) qRT-PCR confirmed influenza infection (“attack rate” see Placebo data) 0,10234 (14%)9 (32%) qRT-PCR confirmed symptomatic influenza infection 0,14773 (11%)7 (25%)qRT-PCR confirmed moderately to severe symptomatic influenza infection 21 Primary endpoint: AUC viral load- time_ qRT-PCR One sided p-value Wilcoxon rank sum test: 0.0390 Phase 2a Prophylactic Efficacy IA Results JNJ-0953 demonstrates prophylactic efficacy in reducing viral replication in the URT and the incidence of PCR-confirmed influenza infection
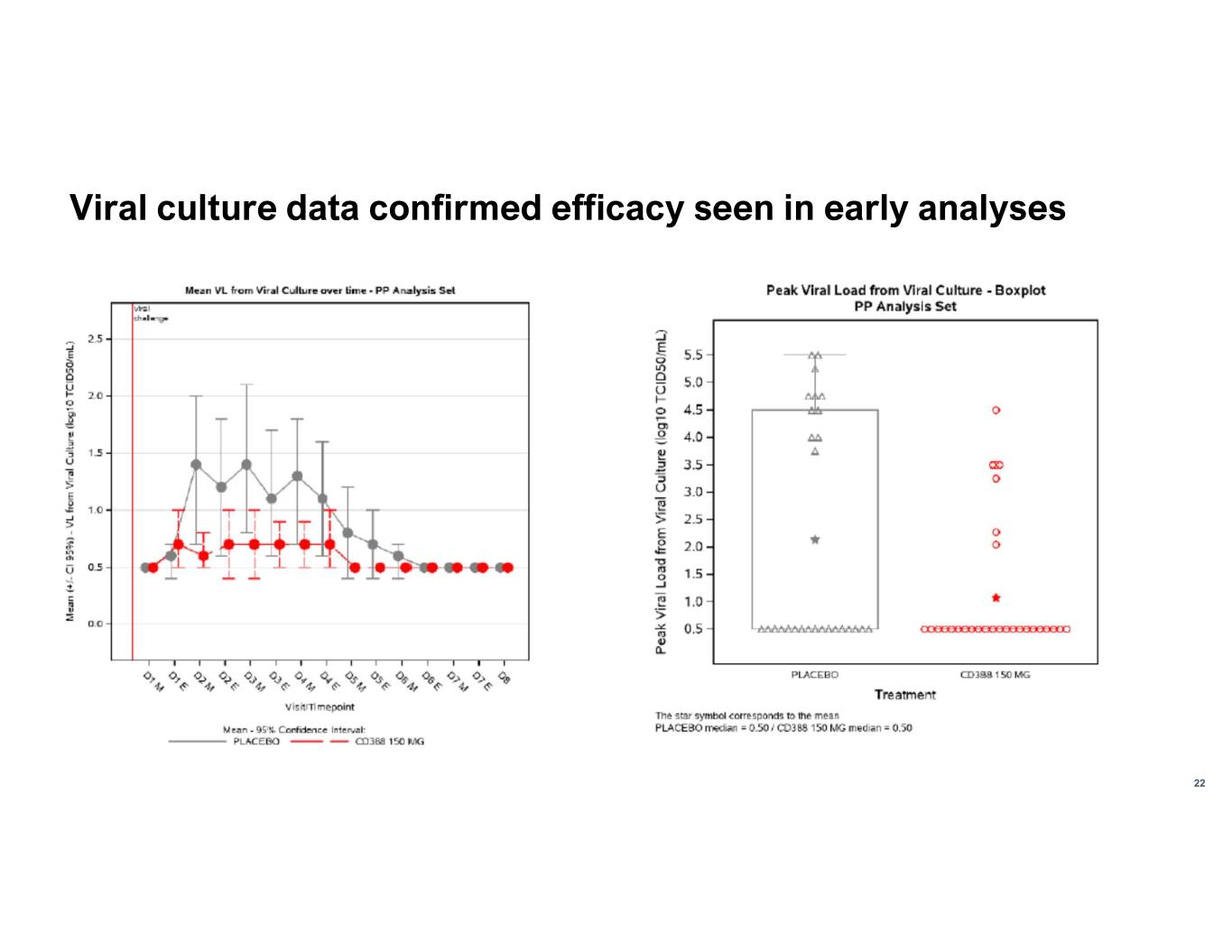
22 Viral culture data confirmed efficacy seen in early analyses

23 • Activity against all tested influenza viruses • FIH studies prove long-acting properties • Human Challenge Study demonstrates efficacy in preventing influenza infection • Safe and well tolerated 23 Summary First of its class antiviral conjugate, targeting influenza PrEP High recognition of unmet need Preclinical and clinical data show efficacy and long-acting properties

24 CD388 (JNJ-0953) Program Update

25 A Track Record of Forging Partnerships * Does not include royalties. Over $1.8 Billion in Potential Value* from Existing Licenses Rezafungin ~$568M P h a s e 2 d a t a $30M upfront $9M equity investment $42M in development support $487M clin/reg/comm milestones Double-digit royalties in the teens 2019 Rights: ex-US / Japan 2022 $30M upfront $60M regulatory milestones $370M commercial milestones Low double-digit to mid-teens royalties Rights: US ~$460M P h a s e 3 d a t a / N D A F i l e d 2021 • $27M upfront • $58M in R&D support • $695M clin/reg/comm milestones • Mid to high single digit royalties Program: Influenza--CD388 | Rights: Global ~$780M P r e c l i n i c a l d a t a Cloudbreak (CD388)

26 Cloudbreak Oncology DFC Programs CD73: CBO421 Development Candidate • Potential Best-in-Class • Complete responders in combination with PD-1 inhibitors • Excellent safety and COGS CD73/PD-1 Discovery Program • Unprecedented dual inhibitor of CD73 and PD-1 • Compelling preclinical data • Potential for more efficient clinical development CCR5 Discovery Program • Overcomes limitations of prior approaches • Proof of concept data against “undruggable” target • Opportunity to expand to other CCR targets

Nicole Davarpanah, MD, JD 27 S VP , T rans la t i ona l Resea r c h & Deve lo pmen t

28 Significant Opportunity to Address Unmet Drug Development Challenges and Clinical Needs in Immuno-Oncology Cloudbreak Oncology Immuno-Oncology • Only 1/5 patients show an initial response • Amongst responders, 85% develop resistance within 6m • Urgently need multi-modal therapies that address TME and mechanisms of immune evasion Small Molecules • Low response rates • Off-target binding contributes to toxicity, DDIs • Inadequate pharmacokinetics • Drug resistance Monoclonal Antibodies • Poor tumor penetration • Inhibition of small molecule receptors is challenging • High cost DFC Long half-life, similar to mAbs Target Cell Multivalent target engagement increases potency, reduces resistance potential Target Cell Cyp450s, intracellular proteins DFC Unlike small molecules, DFCs don’t enter cells, reducing off-target risks

29 DFCs Inhibit Multiple Tumor Immune Evasion Mechanisms CCR5 PD-1 Killer T-cell Sustain activated state Regulatory T-cell Prevent activation and migration to tumor Macrophage/MDSC Prevent migration to tumor A2AR A2AR CCR5 CD73 Tumor Prevent proliferation and metastasis CD73 targeting DFC CD73 CD73/PD-1 multispecific DFC CCR5 PD-L1 CD73 A2AR CCR5 targeting DFC

Stephen Schoenberger, PhD 30 Who i s l e f t beh ind i n cance r immuno the r apy?

31 Stephen P. Schoenberger, PhD Professor, Center for Immunotherapy, La Jolla Institute for Immunology Adj. Professor, Division of Hematology & Oncology, UCSD Moores Cancer Center Co-Director, San Diego Center for Precision Immunotherapy

• Research Focus: Translational Immuno-Oncology Personalized Cancer Vaccines Adoptive Cell Therapy Preclinical and Clinical immune and biomarker analysis Fundamental T cell biology 1) Developed NeoAg ID platform & 1st/2nd gen. vaccines 2) Phase Ib NeoAg vaccine trial (NCT03568058) 3) Phase Ib NeoAg ACT (TIL)trial (NCT03991741) 4) Phase I/II NeoAg vaccine trial (NCT05153304) Stephen P. Schoenberger, Ph.D Professor, Center for Immunotherapy, La Jolla Institute for Immunology Adj. Professor, Division of Hematology & Oncology, UCSD Moores Cancer Center Co-Director, San Diego Center for Precision Immunotherapy
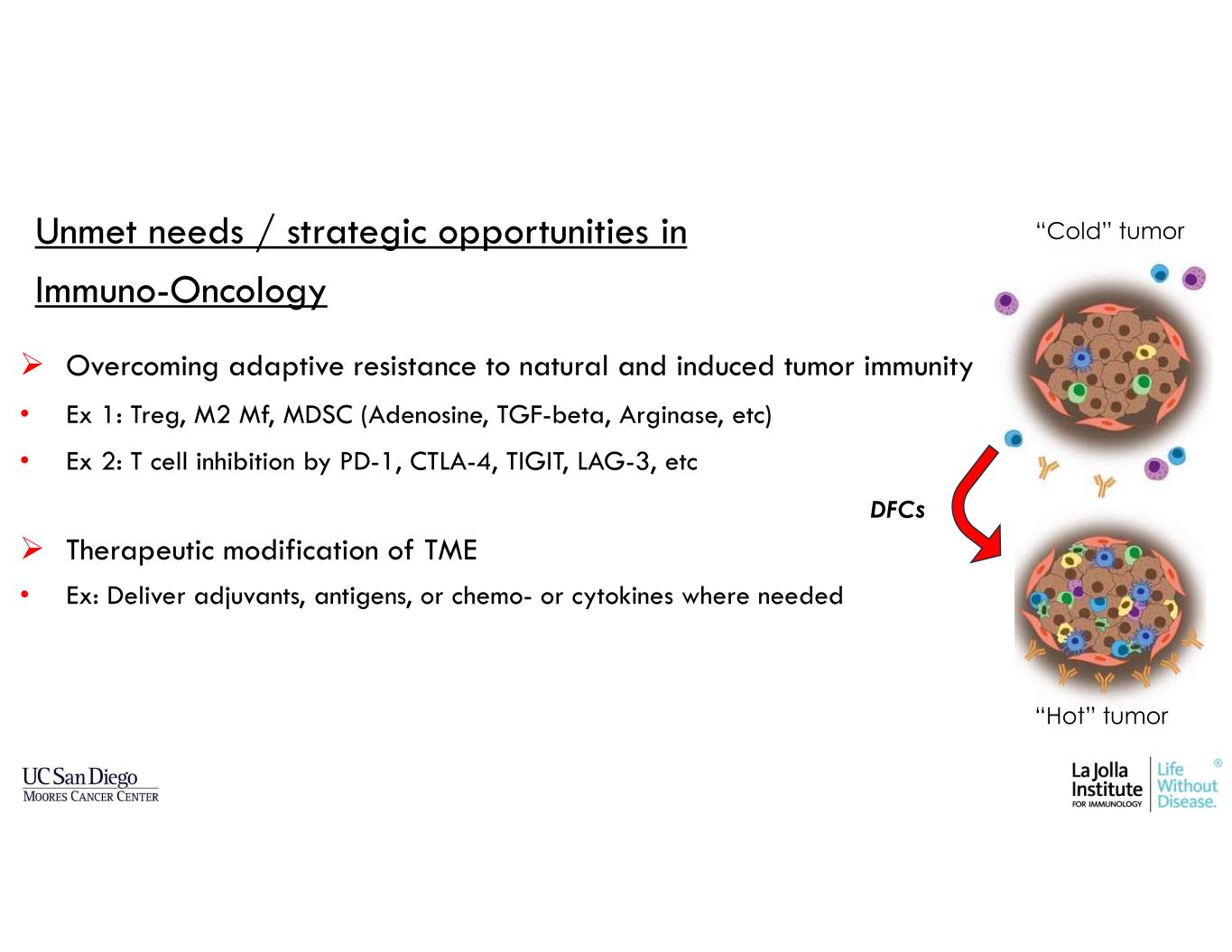
Unmet needs / strategic opportunities in Immuno-Oncology Overcoming adaptive resistance to natural and induced tumor immunity • Ex 1: Treg, M2 Mf, MDSC (Adenosine, TGF-beta, Arginase, etc) • Ex 2: T cell inhibition by PD-1, CTLA-4, TIGIT, LAG-3, etc Therapeutic modification of TME • Ex: Deliver adjuvants, antigens, or chemo- or cytokines where needed “Cold” tumor “Hot” tumor DFCs

Unmet needs / strategic opportunities - Which patients/cancer types? - Incomplete ICB: Lung, Skin, RCC, HNSCC (20-40% ORR) Little or no ICB signal: CRC, BrCa, PrCa, Liver, others

Why DFC? Leverages a proven “winner” in immunobiology protein structure with many impressive features (T1/2, bioavailability, neglible immune response, etc.) Unique biology and target interaction features Facile and efficient conjugation/derivatization platform Unique PK/ADME characteristics Proven efficacy in humans in influenza setting and impressive preclinical data in oncology models

inhibitor 3 tor Functional Payload Inhibitors Agonists Peptides Targeting Moeity Tumor APC Accessory cell by specific molecular target The DFC platform offer an unprecedented capacity for delivering custom small molecule response modifiers to cell surface targets with unique and desirable pharmacological properties that are well-suited to the IO setting

37 CCR5 Discovery Program • Overcomes limitations of prior approaches • Proof of concept data against “undruggable” target • Opportunity to expand to other CCR targets Cloudbreak Oncology DFC Programs CD73: CBO421 Development Candidate • Potential Best-in-Class • Complete responders in combination with PD-1 inhibitors • Excellent safety and COGS CD73/PD-1 Discovery Program • Unprecedented dual inhibitor of CD73 and PD-1 • Promising preclinical data • Potential for more efficient clinical development

38 CBO421 Is Cidara’s First DFC Oncology Development Candidate CBO421 Targets CD73, which mediates Immunosuppression and Resistance via Adenosine (Ado) Production CBO421 CD73 AMP ADO X Tumor Immunosuppression Mast Cells M2 Macrophage NK Cells MDSCs Dendritic Cells Tregs Effector T Cells TME remodeling and Angiogenesis Antigen Presentation Adenosine Image reprinted from Harvey JB, Phan LH, Villarreal OE and Bowser JL (2020) CD73's Potential as an Immunotherapy Target in Gastrointestinal Cancers. Front. Immunol. 11:508.

Les Tari, PhD 39 C h i ef S c i e nt i f i c O f f i c e r
40 CBO421 demonstrates outstanding preclinical performance • Best-in-class activity • Highly stable with excellent pharmaceutical properties • ~65 kDa (vs > 150 kDa for mAbs) – better tumor penetration • Inhibits both membrane bound and soluble CD73, downregulates CD73 via internalization • Robust manufacturing process CBO421 CD73 AMP ADO X

41 Potent Tumor Activity MC38 – murine colorectal carcinoma Potential Best-in-Class Activity EC50 [nM] Target/ClassTest article CD8+CD25+CD4+CD25+ 5113CD73/DFCCBO421 7339CD73/small moleculeAB680* >1,000>1,000CD73/mAbOleclumab PBMC rescue assay (ATP) vs clinical stage adenosine pathway inhibitors >1,000>1,000CD39/mAbIPH5201 >1,000>1,000A2AR/small moleculeAB928 >1,000>1,000A2AR/small moleculeCPI-444 P=0.0052 CBO421 CBO421 Exhibits Exceptional Preclinical Performance AB680 – Arcus Biosciences CD73 inhibitor Oleclumab – Astra Zeneca biosimilar CD73 inhibitor IPH5201 – Innate Pharma biosimilar CD39 inhibitor AB928 – Arcus Biosciences A2AR inhibitor CPI-444 – Corvus A2AR inhibitor

42 Tumor Control: CBO421 Exhibits Superior Tumor Penetration Vs. mAbs CD421 Oleclumab 0 50 100 150 200 250 300 0.0 0.1 0.2 0.3 0.4 BO421 Re la tiv e Fl uo re sc en ce In te ns ity Edge Center MDA-MB-231 Spheroids Study conducted by PhenoVista Biosciences

43 MC38 – murine colorectal carcinoma CBO421 Enhances Anti-tumor Activity Of PD-1 Inhibitors *RMP1-14 CBO421 + Anti-PD-1 combination improves response rates versus monotherapies *Defined as mice that demonstrate cessation of tumor growth or reduction in tumor volume across two or more timepoints % Responders*Study Arm 0Vehicle 27CBO421 47Anti-PD-1 60CBO421 + Anti-PD-1

44 CBO421 Elicits Complete Responses with PD-1 Inhibitors *RMP1-14 CBO421 + Anti-PD-1 combination improves survival and induces immunological memory D+62 (re-challenge) Last dose (D+22) 40-day washout period Study start (D+0) D+73 EMT6 – murine mammary carcinoma 40% of mice in combination arm demonstrated complete responses All complete responses persisted Survivor rechallenge EMT6 tumor cellsSurvival (Day 62) Compounds dosed 2x weekly for 22 days

45 Potential for minimal off-target effects Potential for improved toxicity profile Superior specificity vs clinically available molecules Potential to fully eradicate tumor Potential to control micro-metastatic disease Enhanced tumor penetration vs. mAbs Demonstrate formation of immunologic memory Potential to control disease with fewer doses Potential to achieve durable responses – large unmet need in IO Optimized manufacturing process Low COGS Ease of manufacturing Ability to optimize avidity, size, and pharmacokinetic properties Multispecific DFCsModularity of DFC platform Broad clinical development Lower toxicity = patient centric; ability to achieve efficacious dose Lower DDI potential = better combinability with chemotherapy Enhanced efficacy with IO inhibitors CBO421 Preclinical Summary

46 202820272026202520242023 Q4Q3Q2Q1Q4Q3Q2Q1Q4Q3Q2Q1Q4Q3Q2Q1Q4Q3Q2Q1Q4Q3Q2Q1 R E G U L A T O R Y C L I N I C A L Dose Expansion & Efficacy 2L/3L Advanced Solid tumors (NSCLC, MSS CRC, Gastric, RCC) Dose 1 Dose 2 Dose 3 Dose Escalation Monotherapy cohort IO Combination Cohort at 2 dose levels Interim Efficacy Analysis will inform Ph2 Phase 1: FIH, Dose-Escalation, Safety & PK study Phase 2 Phase 3 IND SubmissionPre-IND Meeting Optimal Dose Interim Analysis CBO421 Clinical Development Timeline

47 Expanding The DFC Platform: Modularity and properties of DFCs allows for inhibition of multiple tumor immune evasion mechanisms, novel combinations, and exploitation of ‘difficult to drug’ targets Killer T-cell CCR5 Regulatory T-cell CD73 PD-1 CD73 CBO421 CD73 Inhibitors Macrophage/MDSC CCR5 CCR5 Regulatory T-cell CCR Antagonists
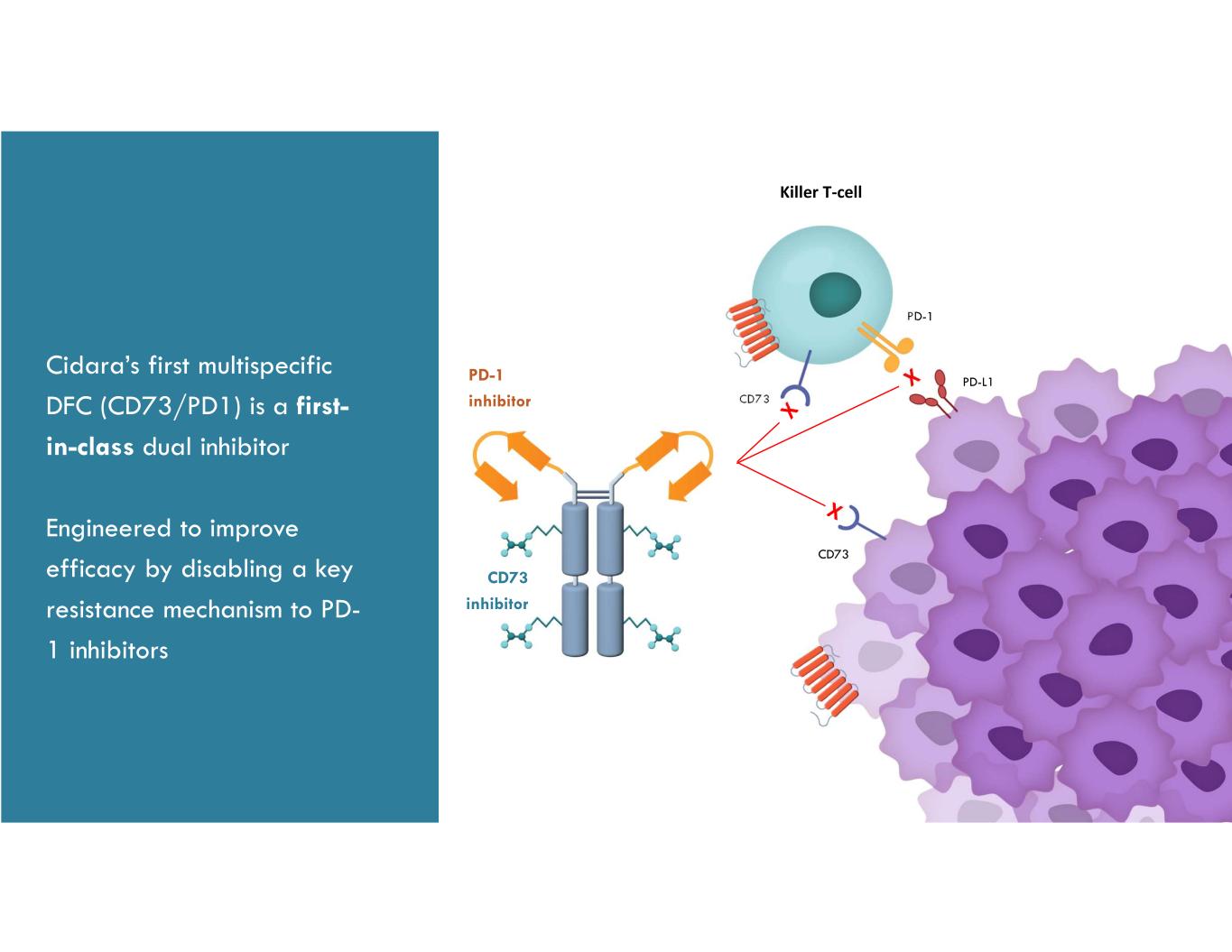
Cidara’s first multispecific DFC (CD73/PD1) is a first- in-class dual inhibitor Engineered to improve efficacy by disabling a key resistance mechanism to PD- 1 inhibitors CD73 inhibitor Killer T-cell PD-1 CD73 CD73 PD-1 inhibitor PD-L1

49 CD73/PD-1 DFC Potently Inhibits Both PD-1 and CD73 Receptors CTP-PD1 CD73/PD-1 DFC PD-1 inhibitor CD73 inhibitor CD73 DFC PD-1 Binding (IC50, nM) – 0.056 T-cell reactivation (EC50, nM) – 12 PD-1 Binding (IC50, nM) – 0.064 T-cell reactivation (EC50, nM) – 9

50 CD73/PD-1 DFC Outperforms Monotherapies In Humanized Tumor Models Veh icl e (P BS) Pem br ol izu m ab (1 0 m g/ kg ) CBO42 1 (1 0 m g/ kg ) CD73 /P D-1 D FC (3 m g/ kg ) 0 500 1000 1500 2000 Tumor Volume (MC38, hPD-L1), day 15 hPD-1/hPD-L1 BALB/c mice 2X weekly dosing T um or v ol um e (m m 3 ) p = 0.007 p = 0.03

51 Cloudbreak Oncology DFC Programs CD73: CBO421 Development Candidate • Potential Best-in-Class • Complete responders in combination with PD-1 inhibitors • Excellent safety and COGS CD73/PD-1 Discovery Program • Unprecedented dual inhibitor of CD73 and PD-1 • Promising preclinical data • Potential for more efficient clinical development CCR5 Discovery Program • Overcomes limitations of prior approaches • Proof of concept data against “undruggable” target • Opportunity to expand to other CCR targets

CCRs are a historically difficult to drug receptor family Next generation DFCs allow us to exploit validated but difficult targets like CCR5 which promote metastasis, invasiveness, migration, recruitment of immunosuppressive cells to TME, and render tumors resistant to DNA damaging agents CCR5-DFC CCR5 Antagonist CCR5-Signaling Assay* CCR5-DFC EC50 – 3.4 nM CCR5 signaling induces migration of tumor suppressive Macrophages / MDSCs and TREGS to the tumor Autocrine CCR5 signaling induces tumor matrix remodeling, migration, metastasis, chemotherapy resistance X CCR5 CCL3/5 CCR5 * Pathhunter® eXpress b-arrestin CCR5 GPCR assay

53 CCR5-DFC treatment accomplishes a similar degree of tumor reduction MC38 tumors are unable to proliferate in CCL5 KO mice (MC38 = murine colorectal carcinoma) V ol um e ( m m 3 ) CCR5-DFC Shows Strong Tumor Control In Preclinical Model Zhang et al. Cell Death and Disease (2018) 9:766 DOI 10.1038/s41419-018-0796-2 p=0.0052 20 MPK 2X/week WT mice CCL5 KO mice

54 Cloudbreak Oncology DFC Programs CD73: CBO421 Development Candidate • Potential Best-in-Class • Complete responders in combination with PD-1 inhibitors • Excellent safety and COGS CD73/PD-1 Discovery Program • Unprecedented dual inhibitor of CD73 and PD-1 • Compelling preclinical data • Potential for more efficient clinical development CCR5 Discovery Program • Overcomes limitations of prior approaches • Proof of concept data against “undruggable” target • Opportunity to expand to other CCR targets

Ezra Cohen, MD 55 Per spec t ive : The Po ten t ia l o f DFCs t o T r ans fo r m P r e c i s i on Onco logy

56 Spotlight on Colorectal Carcinoma (CRC) • 3rd most common cancer and the 2nd cause of cancer-related deaths worldwide. Bray et al. CA Cancer J Clin. (2018) 68:394–424 • OS remains poor for metastatic disease ~ 30 months. • Mortality has increased by 0.5%–3% annually in individuals younger than 50 years. American Cancer Society, 2021 • IO therapy was recently approved for refractory MMR- deficient/microsatellite instability high (MSI-H) CRC but applies to less than 5% of metastatic diagnoses. • Great unmet need remains, particularly for MMR-proficient/ microsatellite stable (MSS) CRC. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-2520.
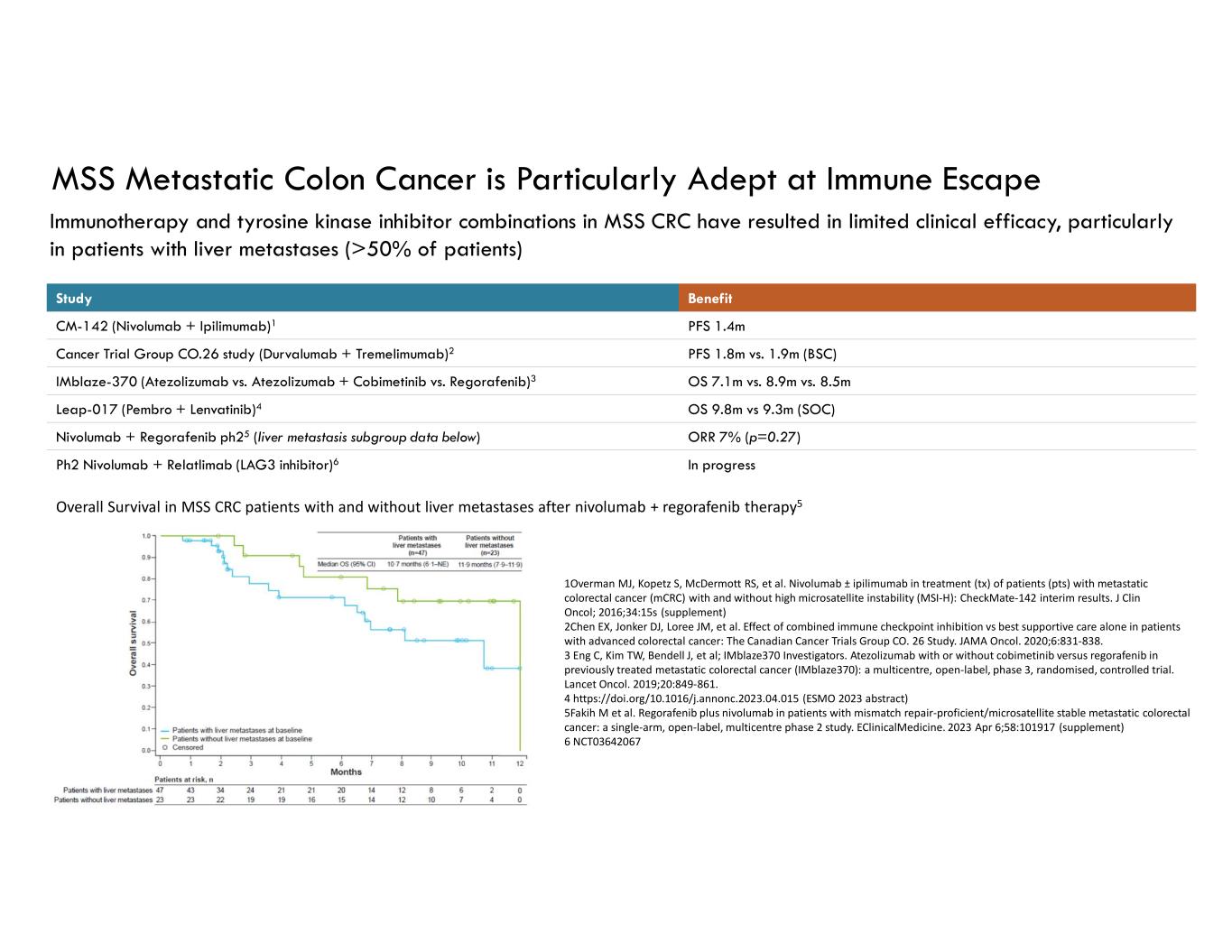
MSS Metastatic Colon Cancer is Particularly Adept at Immune Escape Immunotherapy and tyrosine kinase inhibitor combinations in MSS CRC have resulted in limited clinical efficacy, particularly in patients with liver metastases (>50% of patients) BenefitStudy PFS 1.4mCM-142 (Nivolumab + Ipilimumab)1 PFS 1.8m vs. 1.9m (BSC)Cancer Trial Group CO.26 study (Durvalumab + Tremelimumab)2 OS 7.1m vs. 8.9m vs. 8.5mIMblaze-370 (Atezolizumab vs. Atezolizumab + Cobimetinib vs. Regorafenib)3 OS 9.8m vs 9.3m (SOC)Leap-017 (Pembro + Lenvatinib)4 ORR 7% (p=0.27) Nivolumab + Regorafenib ph25 (liver metastasis subgroup data below) In progressPh2 Nivolumab + Relatlimab (LAG3 inhibitor)6 1Overman MJ, Kopetz S, McDermott RS, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol; 2016;34:15s (supplement) 2Chen EX, Jonker DJ, Loree JM, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: The Canadian Cancer Trials Group CO. 26 Study. JAMA Oncol. 2020;6:831-838. 3 Eng C, Kim TW, Bendell J, et al; IMblaze370 Investigators. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849-861. 4 https://doi.org/10.1016/j.annonc.2023.04.015 (ESMO 2023 abstract) 5Fakih M et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023 Apr 6;58:101917 (supplement) 6 NCT03642067 Overall Survival in MSS CRC patients with and without liver metastases after nivolumab + regorafenib therapy5

DFCs offer opportunity to uniquely target tumor microenvironment of MSS CRC which is distinct from its MSI-H counterpart 1Messaoudi N et al. Prognostic value of CD73 expression in resected colorectal cancer liver metastasis. Oncoimmunology. 2020 Apr 23;9(1):1746138. Preclinical model CCR5-DFC accomplished similar degree of tumor reduction as CCR5 deficient CRC cell First CCR5 targeted molecule specifically designed for oncology needs DFC platform allows for multi-targeted mechanisms of action Potential for both monotherapy and synergistic activity in combination with approved checkpoint inhibitors Promising non-GLP animal safety data Anticipate benign toxicity profile Only CD73 biologic that targets both soluble and membrane- bound CD73 High levels of soluble CD73 in patients with CRC liver metastases linked to shorter survival1 Preclinical model demonstrated enhanced tumor penetration compared to mAbs Potential to achieve complete responses and prevent seeding of micro-metastatic disease Preclinical models demonstrated immunologic memory Durability of response significantly lacking in current treatment regimens