false
0001904286
0.6
P2Y
0.6
P2Y
0001904286
2023-01-01
2023-09-30
0001904286
dei:BusinessContactMember
2023-01-01
2023-09-30
0001904286
2022-12-31
0001904286
2021-12-31
0001904286
us-gaap:NonrelatedPartyMember
2022-12-31
0001904286
us-gaap:NonrelatedPartyMember
2021-12-31
0001904286
us-gaap:RelatedPartyMember
2022-12-31
0001904286
us-gaap:RelatedPartyMember
2021-12-31
0001904286
2023-09-30
0001904286
us-gaap:NonrelatedPartyMember
2023-09-30
0001904286
us-gaap:RelatedPartyMember
2023-09-30
0001904286
2022-01-01
2022-12-31
0001904286
2021-01-01
2021-12-31
0001904286
2023-07-01
2023-09-30
0001904286
2022-07-01
2022-09-30
0001904286
2022-01-01
2022-09-30
0001904286
us-gaap:CommonStockMember
2020-12-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2020-12-31
0001904286
MIRA:StockSubscriptionReceivableMember
2020-12-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2020-12-31
0001904286
2020-12-31
0001904286
us-gaap:CommonStockMember
2021-12-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2021-12-31
0001904286
MIRA:StockSubscriptionReceivableMember
2021-12-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-12-31
0001904286
us-gaap:CommonStockMember
2022-03-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-03-31
0001904286
MIRA:StockSubscriptionReceivableMember
2022-03-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-03-31
0001904286
2022-03-31
0001904286
us-gaap:CommonStockMember
2022-06-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001904286
MIRA:StockSubscriptionReceivableMember
2022-06-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001904286
2022-06-30
0001904286
us-gaap:CommonStockMember
2022-12-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001904286
MIRA:StockSubscriptionReceivableMember
2022-12-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-12-31
0001904286
us-gaap:CommonStockMember
2023-03-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2023-03-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-03-31
0001904286
2023-03-31
0001904286
us-gaap:CommonStockMember
2023-06-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001904286
MIRA:StockSubscriptionReceivableMember
2023-06-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001904286
2023-06-30
0001904286
us-gaap:CommonStockMember
2021-01-01
2021-12-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2021-01-01
2021-12-31
0001904286
MIRA:StockSubscriptionReceivableMember
2021-01-01
2021-12-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-01-01
2021-12-31
0001904286
us-gaap:CommonStockMember
2022-01-01
2022-12-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-01-01
2022-12-31
0001904286
MIRA:StockSubscriptionReceivableMember
2022-01-01
2022-12-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-01-01
2022-12-31
0001904286
us-gaap:CommonStockMember
2022-01-01
2022-03-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-01-01
2022-03-31
0001904286
MIRA:StockSubscriptionReceivableMember
2022-01-01
2022-03-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-01-01
2022-03-31
0001904286
2022-01-01
2022-03-31
0001904286
us-gaap:CommonStockMember
2022-04-01
2022-06-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-04-01
2022-06-30
0001904286
MIRA:StockSubscriptionReceivableMember
2022-04-01
2022-06-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-04-01
2022-06-30
0001904286
2022-04-01
2022-06-30
0001904286
us-gaap:CommonStockMember
2022-07-01
2022-09-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-07-01
2022-09-30
0001904286
MIRA:StockSubscriptionReceivableMember
2022-07-01
2022-09-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-07-01
2022-09-30
0001904286
us-gaap:CommonStockMember
2023-01-01
2023-03-31
0001904286
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-03-31
0001904286
MIRA:StockSubscriptionReceivableMember
2023-01-01
2023-03-31
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-01-01
2023-03-31
0001904286
2023-01-01
2023-03-31
0001904286
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2023-04-01
2023-06-30
0001904286
MIRA:StockSubscriptionReceivableMember
2023-04-01
2023-06-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-04-01
2023-06-30
0001904286
2023-04-01
2023-06-30
0001904286
us-gaap:CommonStockMember
2023-07-01
2023-09-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2023-07-01
2023-09-30
0001904286
MIRA:StockSubscriptionReceivableMember
2023-07-01
2023-09-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-07-01
2023-09-30
0001904286
us-gaap:CommonStockMember
2022-09-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2022-09-30
0001904286
MIRA:StockSubscriptionReceivableMember
2022-09-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-09-30
0001904286
2022-09-30
0001904286
us-gaap:CommonStockMember
2023-09-30
0001904286
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001904286
MIRA:StockSubscriptionReceivableMember
2023-09-30
0001904286
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-09-30
0001904286
MIRA:BayShoreTrustMember
2023-01-01
2023-09-30
0001904286
MIRA:BayShoreTrustMember
us-gaap:CommonStockMember
2023-01-01
2023-09-30
0001904286
MIRA:MZGroupMember
2023-01-01
2023-09-30
0001904286
srt:RevisionOfPriorPeriodAccountingStandardsUpdateAdjustmentMember
2022-12-31
0001904286
2023-08-07
2023-08-07
0001904286
2023-08-07
0001904286
us-gaap:IPOMember
2023-08-07
2023-08-07
0001904286
us-gaap:PrivatePlacementMember
2022-01-01
2022-12-31
0001904286
MIRA:StarwoodTrustMember
2021-05-31
0001904286
MIRA:StarwoodTrustMember
2021-05-01
2021-05-31
0001904286
MIRA:BayShoreTrustMember
2021-04-30
0001904286
MIRA:BayShoreTrustMember
srt:MinimumMember
2021-05-31
0001904286
MIRA:BayShoreTrustMember
srt:MaximumMember
2021-04-30
0001904286
MIRA:BayShoreTrustMember
2021-05-31
0001904286
MIRA:BayShoreTrustMember
2023-04-01
2023-04-30
0001904286
MIRA:BayShoreTrustMember
2023-04-30
0001904286
MIRA:BayShoreTrustMember
2023-07-20
0001904286
MIRA:BayShoreTrustMember
2023-07-20
2023-07-20
0001904286
MIRA:BayShoreTrustMember
2023-08-14
0001904286
MIRA:BayShoreTrustMember
2023-08-14
2023-08-14
0001904286
2021-04-01
2021-04-30
0001904286
MIRA:UndesignatedPreferredStockMember
2022-12-31
0001904286
MIRA:OmnibusPlanMember
2022-12-31
0001904286
srt:BoardOfDirectorsChairmanMember
2022-01-01
2022-12-31
0001904286
srt:ExecutiveOfficerMember
2022-01-01
2022-12-31
0001904286
MIRA:EmployeeAndConsultantMember
2022-01-01
2022-12-31
0001904286
MIRA:UndesignatedPreferredStockMember
2023-09-30
0001904286
2023-06-28
2023-06-28
0001904286
us-gaap:IPOMember
us-gaap:CommonStockMember
2023-01-01
2023-09-30
0001904286
us-gaap:IPOMember
us-gaap:CommonStockMember
2023-09-30
0001904286
us-gaap:RestrictedStockMember
us-gaap:IPOMember
2023-01-01
2023-09-30
0001904286
srt:ExecutiveOfficerMember
2023-01-01
2023-09-30
0001904286
MIRA:EmployeeAndConsultantMember
2023-01-01
2023-09-30
0001904286
us-gaap:WarrantMember
2023-04-28
2023-04-28
0001904286
us-gaap:WarrantMember
2023-04-28
0001904286
us-gaap:WarrantMember
2023-09-30
0001904286
MIRA:UnderwriterWarrantsMember
2023-09-30
0001904286
us-gaap:EmployeeStockOptionMember
2023-01-01
2023-09-30
0001904286
us-gaap:WarrantMember
2023-01-01
2023-09-30
0001904286
us-gaap:EmployeeStockOptionMember
2022-01-01
2022-09-30
0001904286
us-gaap:WarrantMember
2022-01-01
2022-09-30
0001904286
us-gaap:EmployeeStockOptionMember
2023-07-01
2023-09-30
0001904286
us-gaap:WarrantMember
2023-07-01
2023-09-30
0001904286
us-gaap:EmployeeStockOptionMember
2022-07-01
2022-09-30
0001904286
us-gaap:WarrantMember
2022-07-01
2022-09-30
0001904286
us-gaap:WarrantMember
2022-01-01
2022-12-31
0001904286
MIRA:ConsultingAndEmploymentAgreementMember
2022-04-21
2022-04-22
0001904286
MIRA:ConsultingAndEmploymentAgreementMember
2022-04-22
0001904286
MIRA:ConsultantMember
2022-06-15
2022-06-15
0001904286
MIRA:ConsultantMember
2022-06-15
0001904286
srt:BoardOfDirectorsChairmanMember
2022-06-15
2022-06-15
0001904286
srt:BoardOfDirectorsChairmanMember
2022-06-15
0001904286
MIRA:EmploymentAgreementMember
MIRA:ErezAminovMember
2023-04-28
2023-04-28
0001904286
MIRA:EmploymentAgreementMember
MIRA:ErezAminovMember
2023-08-17
2023-08-17
0001904286
MIRA:EmploymentAgreementMember
MIRA:ErezAminovMember
srt:MinimumMember
2023-08-28
2023-08-28
0001904286
MIRA:EmploymentAgreementMember
MIRA:ErezAminovMember
srt:MaximumMember
2023-08-28
2023-08-28
0001904286
MIRA:EmploymentAgreementMember
MIRA:MichelleYanezMember
2023-04-28
2023-04-28
0001904286
MIRA:EmploymentAgreementMember
MIRA:MichelleYanezMember
2023-08-17
2023-08-17
0001904286
MIRA:EmploymentAgreementMember
MIRA:ChrisChapmanMember
2023-04-28
2023-04-28
0001904286
MIRA:EmploymentAgreementMember
MIRA:ChrisChapmanMember
2023-08-17
2023-08-17
0001904286
MIRA:EmploymentAgreementMember
MIRA:ChrisChapmanMember
2023-01-01
2023-09-30
0001904286
MIRA:EmploymentAgreementMember
MIRA:ChrisChapmanMember
2023-10-13
2023-10-13
0001904286
MIRA:EmploymentAgreementMember
MIRA:ChristosNicholoudisMember
2023-04-28
2023-04-28
0001904286
MIRA:EmploymentAgreementMember
MIRA:ChristosNicholoudisMember
2023-08-17
2023-08-17
0001904286
country:MD
2023-09-30
0001904286
country:MD
2023-01-01
2023-09-30
0001904286
stpr:FL
2023-09-30
0001904286
stpr:FL
2023-01-01
2023-09-30
0001904286
srt:MinimumMember
2023-01-01
2023-09-30
0001904286
srt:MaximumMember
2023-01-01
2023-09-30
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
utr:sqft
As
filed with the Securities and Exchange Commission on December 18, 2023
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
MIRA
PHARMACEUTICALS, INC.
(Exact
name of registrant as specified in its charter)
| Florida |
|
2834 |
|
85-3354547 |
|
(State
or other jurisdiction of
incorporation
or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
No.) |
855
N Wolfe Street, Suite 601
Baltimore,
Maryland 21205
(737)
289-0835
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Erez
Aminov
Chief
Executive Officer
MIRA
Pharmaceuticals, Inc.
855
N Wolfe Street, Suite 601
Baltimore,
Maryland 21205
(737)
289-0835
(Name,
address, including zip code, and telephone number including area code, of agent for service)
Copies
of all communications, including communications sent to agent for service, should be sent to:
Curt
P. Creely
Foley
& Lardner LLP
100
North Tampa Street, Suite 2700
Tampa,
Florida 33602
(813)
229-2300
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, as amended (the “Securities Act”), check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer ☐ |
Accelerated
filer ☐ |
| Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
Emerging
growth company ☒ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the
Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective on such date
as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell, and it
is not soliciting an offer to buy, these securities in any state where the offer or sale is not permitted.
Subject
to completion, dated 18, 2023
PROSPECTUS
1,700,000
Shares
of
Common Stock

This
prospectus relates to the resale by the selling stockholders named in this prospectus from time to time of up to 1,700,000 shares of
common stock of MIRA Pharmaceuticals, Inc., par value $0.0001 per share, consisting of (i) 1,000,000 shares that are issuable upon the
exercise of a warrant issued by us to Bay Shore Trust in April 2023, and (ii) 700,000 shares that are issuable upon the exercise of a
warrant issued by us to MIRALOGX, LLC in November 2023.
The
selling stockholders may sell shares from time to time in the open market, through privately negotiated transactions or a combination
of these methods, at market prices prevailing at the time of sale or at negotiated prices. The selling stockholders may offer shares
to or through underwriters, dealers or other agents, directly to investors or through any other manner permitted by law, on a continued
or delayed basis. We will bear all costs, expenses and fees in connection with the registration of the shares offered by this prospectus,
and the selling stockholders will bear all incremental selling expenses, including commissions and discounts, brokerage fees and other
similar selling expenses they incur in sale of the shares. See “Plan of Distribution”.
We
are not selling any common stock under this prospectus and will not receive any of the proceeds from the sale or other disposition of
shares by the selling stockholders. However, we will receive proceeds from the exercise of the warrants, if any of the selling stockholders
exercise a warrant for cash.
Our
common stock is traded on the Nasdaq Capital Market under the symbol “MIRA.” On December 15, 2023, the closing price
of one share of our common stock on the Nasdaq Capital Market was $1.30 per share.
We
are an “emerging growth company” as defined in the federal securities laws, and, as such, are subject to reduced public company
reporting requirements. See “Prospectus Summary — Implications of Being an Emerging Growth Company”.
Investing
in our securities is highly speculative and involves a significant degree of risk. See “Risk Factors” beginning
on page 31 of this prospectus for a discussion of information that should be considered before making a decision to purchase our
securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is , 2023
TABLE
OF CONTENTS
Please
read this prospectus carefully. It describes our business, financial condition, results of operations and prospects, among other things.
We are responsible for the information contained in this prospectus and in any free-writing prospectus we have authorized. Neither we
nor the selling stockholders have authorized anyone to provide you with different information, and neither we nor the selling stockholders
take responsibility for any other information others may give you. Neither we nor the selling stockholders are making an offer to sell
these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is accurate
only as of the date on the front of this prospectus, regardless of the time of delivery of this prospectus or any sale of securities.
You should not assume that the information contained in this prospectus is accurate as of any date other than its date.
INDUSTRY
AND MARKET DATA
We
are responsible for the disclosure in this prospectus. However, this prospectus includes industry data that we obtained from internal
surveys, market research, publicly available information, and industry publications. We did not fund and are not otherwise affiliated
with any of the sources cited in this prospectus. The market research, publicly available information, and industry publications that
we use generally state that the information contained therein has been obtained from sources believed to be reliable. The information
therein represents the most recently available data from the relevant sources and publications, and we believe remains reliable. However,
this data involves a number of assumptions and limitations regarding our industry which are necessarily subject to a high degree of uncertainty
and risk due to a variety of factors, including those described in the section titled “Risk Factors.” Forward-looking
information obtained from these sources is also subject to the same qualifications and additional uncertainties regarding the other forward-looking
statements in this prospectus.
TRADEMARKS
AND COPYRIGHTS
We
own or have rights to various trademarks, service marks and trade names that we use in connection with the operation of our business.
This prospectus may also contain trademarks, service marks and trade names of third parties, which are the property of their respective
owners. Our use or display of third parties’ trademarks, service marks and trade names or products in this prospectus is not intended
to, and does not imply a relationship with, or endorsement or sponsorship by us. Solely for convenience, the trademarks, service marks
and trade names referred to in this prospectus may appear without the ®, trademark (™) or servicemark (SM) symbols, but the
omission of such references is not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable
law, our rights or the right of the applicable owner of these trademarks, service marks and trade names.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,”
“will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,”
“target,” “project,” “contemplate,” “believe,” “estimate,” “predict,”
“potential”, or “continue” or the negative of these terms or other similar expressions. In particular, statements
about the markets in which we operate, including growth of our various markets, and our expectations, beliefs, plans, strategies, objectives,
prospects, assumptions, or future events or performance contained in this prospectus under the headings “Prospectus Summary,”
“Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
and “Business” are forward-looking statements.
We
have based these forward-looking statements on our current expectations, assumptions, estimates and projections. While we believe these
expectations, assumptions, estimates, and projections are reasonable, such forward-looking statements are only predictions and involve
known and unknown risks and uncertainties, many of which are beyond our control. These and other important factors, including those discussed
in this prospectus under the headings “Prospectus Summary,” “Risk Factors,” “Management’s Discussion
and Analysis of Financial Condition and Results of Operations” and “Business,” may cause our actual results, performance,
or achievements to differ materially from any future results, performance or achievements expressed or implied by these forward-looking
statements, or could affect our share price. Important factors that could cause actual results or events to differ materially from those
expressed in forward-looking statements include, but are not limited to, the following:
| |
● |
our
ability to obtain and maintain regulatory approval of our product candidates; |
| |
|
|
| |
● |
our
ability to successfully commercialize and market our product candidates, if approved; |
| |
● |
our
ability to contract with third-party suppliers, manufacturers and other service providers and their ability to perform adequately; |
| |
|
|
| |
● |
the
potential market size, opportunity, and growth potential for our product candidates, if approved; |
| |
|
|
| |
● |
our
ability to obtain additional funding for our operations and development activities; |
| |
|
|
| |
● |
the
accuracy of our estimates regarding expenses, capital requirements and needs for additional financing; |
| |
|
|
| |
● |
the
initiation, timing, progress and results of our pre-clinical studies and clinical trials, and our research and development programs; |
| |
● |
the
timing of anticipated regulatory filings; |
| |
|
|
| |
● |
the
timing of availability of data from our clinical trials; |
| |
|
|
| |
● |
our
future expenses, capital requirements, need for additional financing, and the period over which we believe that our existing cash
and cash equivalents will be sufficient to fund our operating expenses and capital expenditure requirements; |
| |
|
|
| |
● |
our
ability to retain the continued service of our key professionals and to identify, hire and retain additional qualified professionals; |
| |
|
|
| |
● |
our
ability to advance product candidates into, and successfully complete, clinical trials; |
| |
|
|
| |
● |
our
ability to recruit and enroll suitable patients in our clinical trials; |
| |
|
|
| |
● |
the
timing or likelihood of the accomplishment of various scientific, clinical, regulatory, and other product development objectives; |
| |
|
|
| |
● |
the
pricing and reimbursement of our product candidates, if approved; |
| |
|
|
| |
● |
the
rate and degree of market acceptance of our product candidates, if approved; |
| |
|
|
| |
● |
the
implementation of our business model and strategic plans for our business, product candidates, and technology; |
| |
|
|
| |
● |
the
scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology; |
| |
|
|
| |
● |
developments
relating to our competitors and our industry; |
| |
|
|
| |
● |
the
development of major public health concerns, including the novel coronavirus outbreak or other pandemics arising globally, and the
future impact of it and COVID-19 on our clinical trials, business operations and funding requirements; and |
| |
|
|
| |
● |
other
risks and factors listed under “Risk Factors” and elsewhere in this prospectus. |
Given
the risks and uncertainties set forth in this prospectus, you are cautioned not to place undue reliance on such forward-looking statements.
The forward-looking statements contained in this prospectus are not guarantees of future performance and our actual results of operations,
financial condition, and liquidity, and the development of the industry in which we operate, may differ materially from the forward-looking
statements contained in this prospectus. In addition, even if our results of operations, financial condition and liquidity, and events
in the industry in which we operate, are consistent with the forward-looking statements contained in this prospectus, they may not be
predictive of results or developments in future periods.
Any
forward-looking statement that we make in this prospectus speaks only as of the date of such statement. Except as required by federal
securities laws, we do not undertake any obligation to update or revise, or to publicly announce any update or revision to, any of the
forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus.
GLOSSARY
OF CERTAIN SCIENTIFIC TERMS
The
following medical and scientific terms used in this prospectus have the following meanings:
“Agonist”
is a substance which initiates a physiological response when combined with a receptor.
“Alpha-2a
adrenergic receptor”: A G protein-coupled receptor involved in modulating neurotransmitter release, related to the cardiovascular
and sedative effects of Ketamine.
“Anti-inflammatory
Effects”: The ability of a substance or treatment to reduce inflammation, characterized by swelling, redness, and pain, by inhibiting
the body’s inflammatory processes. Commonly used in treating conditions like arthritis and asthma.
“AMES
test” is a biological assay to assess the mutagenic potential of chemical compounds. It utilizes bacteria to test whether a given
chemical can cause mutations in the DNA of the test organism.
“AMPA
Receptors”: Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, a type of glutamate receptor involved in fast synaptic
transmission in the central nervous system, playing a key role in brain development and synaptic plasticity.
“API”
stands for Active Pharmaceutical Ingredient, which is the main ingredient in a medicine that causes the desired effect of the medicine.
“Area
Under the Concentration-Time Curve (AUC)”: A pharmacokinetic parameter representing the total exposure of the body to a drug, calculated
by plotting the concentration of the drug in blood plasma over time.
“BDNF
(Brain-Derived Neurotrophic Factor)”: A protein that plays a significant role in neuroplasticity – the brain’s ability
to reorganize and form new neural connections, implicated in the antidepressant effects of drugs like Ketamine.
“Bioavailability”:
The proportion of a drug that enters the systemic circulation when introduced into the body, crucial for determining a drug’s effectiveness.
“Biosensor
assay” is a biological assay used for the detection of a chemical substance that combines a biological component with a physicochemical
detector.
“BRD4
(Bromodomain-containing protein 4)”: A protein implicated in the regulation of gene expression and potentially involved in the
pathophysiology of depression.
“Caco-2
cell model”: A model originating from a human colorectal adenocarcinoma cell line, used in pharmaceutical research to estimate
intestinal absorption and indirectly, the bioavailability of drugs.
“cAMP”
is cyclic adenosine monophosphate, a messenger used for intracellular signal transduction in many different organisms.
“CBD”
is cannabidiol, the second most prevalent active ingredient in cannabis which does not have psychoactive properties.
“CDMO”
stands for Contract Development and Manufacturing Organization, a specialized type of supplier of development and production services
to the pharmaceutical industry.
“cGMP”
is the current Good Manufacturing Practices under the US Food and Drug Administration’s standards. cGMP contains the minimum requirements
for the methods, facilities, and controls used in the manufacturing, processing, and packing of a drug product. The regulations make
sure that a product is manufactured under conditions and tested to ensure that it meets standards of identity, strength, quality, and
purity.
“CNS”
or the central nervous system is the brain and spinal cord.
“CSA”
is the Controlled Substances Act, a U.S. regulatory framework that governs the classification of certain substances, and therefore the
market access available to such substances; based on the CSA, the Drug Enforcement Agency (DEA) determines if a compound should be considered
“Scheduled” or not. There are 5 levels of scheduling with certain substances such as marijuana categorized as Schedule 1,
with no currently acceptable medical use or high potential for abuse.
“DNA”
is the molecule that carries genetic information for the development and functioning of an organism.
“DRF”
is an initial part of the toxicity study aimed to find the dose that will produce tolerable levels of adverse toxic effects of tested
compounds.
“FDA”
is the U.S. Food and Drug Administration.
“Forced
Swim Test (FST)”: A behavioral test in rodents used to evaluate antidepressant-like activity, where immobility time is measured
as an index of depressive-like behavior.
“G
Protein-Coupled Receptors (GPCRs)”: A large family of cell surface receptors that respond to a variety of external signals and
activate internal signal transduction pathways, involved in numerous physiological processes.
“GABAergic
system”: Refers to the system of neurotransmission in the brain mediated by gamma-aminobutyric acid (GABA), which is involved in
mood regulation.
“GPCRs”
are G-protein-coupled receptors that form a large group of proteins which are expressed on the cell surface of eukaryotic cells to detect
molecules outside the cell and activate cellular responses.
“GMP”
is good manufacturing practice - a standard that is observed in regulated pharmaceutical-manufacturing facilities.
“GRIN1/GRIN2B
receptor subunit”: A specific subunit of the NMDA receptor, which plays a role in neuroplasticity and is associated with depression
and the action of antidepressants like Ketamine.
“HDRS”:
Hamilton Depression Rating Scale, a widely used clinician-administered depression assessment scale measuring the severity of depression
symptoms.
“HPA
Axis”: The Hypothalamic-Pituitary-Adrenal Axis, a complex set of interactions among the hypothalamus, pituitary gland, and adrenal
glands, regulating stress response, digestion, immune system, mood, and energy storage.
“In
Silico Analysis”: Computer-based techniques used in pharmaceutical research to analyze and predict the properties and behaviors
of pharmaceutical compounds.
“IND-enabling
studies”: Preclinical studies necessary for the submission of an Investigational New Drug (IND) application to regulatory bodies
such as the FDA. These studies include pharmacokinetics, pharmacodynamics, toxicology, and safety pharmacology.
“Intraperitoneal”
is within or through a thin, transparent membrane that lines the walls of the abdomen.
“JQ1”:
A small molecule inhibitor that targets bromodomain-containing protein 4 (BRD4), used in research to study gene expression regulation
and potential therapeutic applications in cancer and other diseases.
“Ketamine”
is an anesthetic used medically for induction and maintenance of anesthesia and treatment of depression.
“Ketamir”:
A novel chemical entity and an analog of Ketamine developed by Mira Pharmaceuticals, primarily for the treatment of Major Depressive
Disorder (MDD) refractory to treatment, known as Treatment-Resistant Depression (TRD).
“MADRS”:
Montgomery-Åsberg Depression Rating Scale, a diagnostic questionnaire used to measure the severity of depressive episodes in patients
with mood disorders.
“Major
Depressive Disorder (MDD)”: A significant global health concern characterized by persistent feelings of sadness and loss of interest
in activities. It affects millions worldwide and is a major cause of disability.
“MDSI”:
Major Depression Disorder with Suicidal Ideation.
“mTOR
pathway”: The mammalian target of rapamycin pathway, a key regulator of cell growth and survival, which is activated by Ketamine
and linked to increased synaptogenesis in the brain.
“Maximum
tolerated dose” is the highest dose of a drug or treatment that does not cause unacceptable side effects. The maximum tolerated
dose is determined in clinical trials by testing increasing doses on different subjects until the highest dose with acceptable side effects
is found.
“Metabolic
Profiling” is the measurement in biological systems of metabolites and their intermediates that reflects the dynamic response to
genetic modification and physiological, pathophysiological, and/or developmental stimuli.
“Metabolite”
is a substance made or used when the body breaks down food, drugs or chemicals, or its own tissue.
“Micronucleus
Assay” is used to determine if a compound causes DNA damage.
“Mu-Opioid
Receptor (MOR)”: A receptor targeted by endogenous opioids and exogenous opioid analgesics. Ketamine’s interaction with MOR
is implicated in its antidepressant effects and abuse potential.
“N-methyl-D-aspartate
(NMDA) receptor”: A type of glutamate receptor in the brain, which both Ketamir and Ketamine target as a non-competitive antagonist,
contributing to their rapid antidepressant effects.
“Neuroinflammation”
is the inflammation of nervous system.
“Phase
1 and Phase 2 clinical trials”: Early stages of clinical drug development focused on establishing safety, efficacy, and optimal
use of a drug in treating specific conditions.
“Post-Traumatic
Stress Disorder (PTSD)”: A psychiatric disorder that may develop after experiencing or witnessing traumatic events. Ketamir is
potentially considered for its treatment.
“Rapid
Onset of Action”: The speed at which a drug or therapy begins to exert its effect after administration.
“Sigma
Opioid Receptor”: Receptors implicated in the psychotomimetic and dissociative effects of ketamine.
“Synaptogenesis”:
The formation of synapses between neurons in the nervous system, a critical process in brain development and the remodeling of neural
circuits in response to experience.
“THC”
is tetrahydrocannabinol, a compound that is the main psychoactive ingredient of cannabis.
“Treatment-Resistant
Depression (TRD)”: A form of Major Depressive Disorder that does not respond adequately to standard antidepressant treatments.
PROSPECTUS
SUMMARY
The
following summary highlights selected information about our company and this offering that is included elsewhere in this prospectus in
greater detail. It does not contain all of the information that you should consider before investing in our common stock. Before investing
in our common stock, you should read this entire prospectus carefully, including the information presented under the heading “Risk
Factors” and in our financial statements and notes thereto.
Unless
otherwise noted, the share and per share information in this prospectus reflects a 1-for-5 reverse stock split of our common stock that
became effective as of June 28, 2023.
In
this prospectus, unless we indicate otherwise or the context requires, “MIRA,” “the company,” “our company,”
“we,” “our,” “ours” and “us” refer to MIRA Pharmaceuticals, Inc.
Our
Business
We
are a pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting a broad range of neurologic and
neuropsychiatric disorders. Our novel oral pharmaceutical marijuana, MIRA1a, is currently under investigation for treating adult patients
suffering from anxiety and cognitive decline, often associated with early-stage dementia. MIRA1a, if approved by the FDA, could mark
a significant advancement in addressing various neuropsychiatric, inflammatory, and neurologic diseases and disorders.
We
have an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to potentially
deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD) and major depressive
disorder with suicidal ideation (MDSI). The U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2
concluded that neither would be considered a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its
governing regulations.
Our
Product Candidates in Development
MIRA1a
Our
objective is to develop and commercialize new treatment options for neuropsychiatric, inflammatory, and neurologic diseases and disorders.
Cannabinoids are a class of chemical compounds that are naturally occurring and are primarily found in cannabis plant extracts. The two
major cannabinoids found in cannabis plant extracts include THC and CBD. These compounds bind to CB1 and CB2 cannabinoid receptors, which
are found throughout the body. Specifically, CB1 receptors are concentrated in the central nervous system (“CNS”), while
CB2 receptors are found mostly in peripheral organs and are associated with the immune system. When the chemical compounds bind to these
cannabinoid receptors, the process elicits certain physiological responses. Physiological responses to cannabinoids may vary among individuals.
Some of the effects of cannabinoids have been shown to impact nervous system functions, immune responses, muscular motor functions, gastrointestinal
maintenance, blood sugar management, and the integrity of ocular functions. Our product candidate, MIRA1a, has a strong selectivity for
CB2 versus CB1, and is designed to minimize the risk of psychoactive adverse events associated with CB1 activation.
Mechanism
of Action of MIRA1a
We
believe that the effects of MIRA1a at the cannabinoid receptors CB1 and CB2 is predicted to account for the majority of its potential
therapeutic effects, especially as it relates to its anti-anxiety, anti-pain and anti-inflammatory properties. For example, the difference
in the dose-response effects of MIRA1a compared with THC on CB1 receptors appears to coincide with its improved therapeutic profile.
If approved by the FDA, MIRA1a may potentially provide therapeutic effects for anxiety, pain and inflammation.
THC
has been demonstrated to have biphasic physiological effects, which have been described for over 40 years: at low levels THC has positive
effects while high doses cause the opposite, undesirable symptoms. Examples of biphasic effects at low versus high levels of THC include
the anti-anxiety versus pro-anxiety effects, respectively. We obtained the following dose-response effects for MIRA1a and THC at the
CB1 receptor (see below). In contrast to THC, which displays an initial maximally stimulatory and then inhibitory response at CB1, MIRA1a
appears to act as a monophasic partial agonist where it is stimulatory throughout its dose range, achieving a moderate activation of
the CB1 even at high doses. We believe that this accounts for the potential broad therapeutic efficacy of MIRA1a and the observed absence
of negative symptoms even at maximal doses of the drug.

Figure:
Compound activity with the selected GPCR Biosensor Assays:
THC
vs MIRA1a agonist activity at the CB1 Receptor.
In
pharmacology, “efficacy” or “Emax” refers to the maximum response that can be achieved with a drug or agent.
It represents the extent or magnitude of the response produced by the drug once it has bound to its target, typically referred to as
a receptor. The binding between a drug and its receptor is characterized by affinity, which quantifies the strength of their interaction.
Efficacy, however, assesses the action or effect of the drug following binding to the receptor.
The
dose-response curve is a commonly used graph in pharmacology that depicts the relationship between the effect of a drug and its dosage.
The X-axis represents the increasing doses of the drug, while the Y-axis represents the response produced by the drug. In the case of
the figure above, the term “% Efficacy” on the Y-axis refers to the maximum response that can be achieved with the agonist
(MIRA1a or THC) in relation to its ability to activate GPCR receptors (specifically CB1 receptors).
The
data presented in the figure above has been normalized to the maximal and minimal responses observed in the presence of a control compound
and vehicle, respectively. This normalization allows for a standardized comparison of the agonist’s efficacy.
Eurofins
DiscoverX has developed a panel of cell lines stably expressing non-tagged GPCRs that signal through cAMP. Hit Hunter® cAMP assays
are specialized tests that track the activation of a type of cell receptor known as GPCR. GPCRs play a crucial role in how cells respond
to external signals, and they are activated through two pathways: Gi and Gs secondary messenger signaling. These pathways are like internal
communication systems in cells that relay signals from the outside to trigger specific responses inside the cell. The assay is conducted
in a straightforward, uniform manner without the need for image-based analysis. This method uses a technology developed by DiscoverX
called Enzyme Fragment Complementation (EFC). In EFC, fragments of an enzyme, specifically β-galactosidase (β-Gal), are brought
together to become functional only when the GPCR is activated. β-Galactosidase, the enzyme used as a functional reporter in this
assay, is typically inactive in fragmented form and becomes active when the fragments reassemble, indicating the activation of the GPCR.
In this case, the GPCR target was CB1 receptor. Compounds were tested in agonist and antagonist mode with the requested GPCR Biosensor
Assays. For agonist assays, data was normalized to the maximal and minimal response observed in the presence of control ligand and vehicle.
This Eurofins DiscoverX system was used to test THC vs MIRA1a agonist activity at the CB1 receptor.
Unlike
CB1 receptors that mediate many of the psychotropic effects of cannabinoids on the CNS, CB2 receptors are predominantly present on cells
of the immune system. Based on preliminary results of our GPCR biosensor assays, the CB2 receptor agonistic effects of MIRA1a are 8-fold
more potent than THC and 30-fold more potent than CBD.
The
study regarding the ability of MIRA1a vs THC vs CBD to activate CB2Receptors and alter intracellular cAMP levels was performed by the
CRO Eurofins DiscoverX.
As
can be seen in the table below, the EC50 (i.e. concentration required to induce a half maximal response) for MIRA1a was 8 times more
potent than THC and at least 30 times more potent that CBD—i.e. it only took 1 uM of MIRA1a to induce the same response that required
8 uM of THC and >30 uM of CBD.
| Compound
Name |
|
Assay
Name |
|
Assay
Format |
|
Assay
Target |
|
Result
Type |
|
EC50 |
|
|
Unit |
|
| MIRA-1A |
|
cAMP |
|
Agonist |
|
CNR2/CB2 |
|
EC50 |
|
|
1.008462 |
|
|
|
uM |
|
| THC |
|
cAMP |
|
Agonist |
|
CNR2/CB2 |
|
EC50 |
|
|
8.209884 |
|
|
|
uM |
|
| CBD |
|
cAMP |
|
Agonist |
|
CNR2/CB2 |
|
EC50 |
|
|
>30 |
|
|
|
uM |
|
The
foregoing measurements were performed as follows:
DiscoverX
has developed a panel of cell lines that stably express non-tagged GPCRs (G-protein coupled receptors) capable of signaling through cAMP.
The Hit Hunter® assay platform is used to investigate the functionality and response of these GPCRs.
In
the case of the CB2 receptor, which is a GPCR involved in various physiological processes and has potential therapeutic implications,
the Hit Hunter® assay can be employed to study the effects of drug agonists on CB2 receptor activity.
To
measure the half maximal response (EC50) of CB2 receptor activation by a drug agonist that leads to a decrease in cAMP levels, an alternative
approach may be required. One common method involves using forskolin, an activator of adenylate cyclase, to stimulate cAMP production.
Forskolin bypasses the GPCR signaling and directly activates adenylate cyclase, resulting in increased cAMP levels.
In
the presence of forskolin, the drug agonist at the CB2 receptor can then be tested at various concentrations to determine its ability
to inhibit the forskolin-induced cAMP production. The drug’s concentration that leads to a 50% reduction in forskolin-stimulated
cAMP levels can be considered the half maximal response or EC50.
Pre-clinical
Developments and Studies
As
of the date of this prospectus, we completed several pre-clinical studies of MIRA1a, including, but not limited to, computational mutagenicity
analysis, radio-ligand binding assay, elevated plus maze (“EPM”) model of anxiety and hot plate model thermal sensitivity
testing.
We
have studied the effects of acute administration of MIRA1a on anxiety-related phenotypes in mice to model human conditions. An intraperitoneal
injection of Placebo [PBO] (e.g. saline) or MIRA1a (e.g. 50mg/kg = Treatment) was administered to C57Bl/6 mice (n=5/group) that were
8-12 weeks old. Thirty minutes following injection, mice were tested in anxiety related measures using the Elevated Plus Maze (EPM).
The EPM is a widely used pre-clinical behavioral assay for rodents and it has been validated to assess the anti-anxiety effects of pharmacological
agents. If determined and approved by the FDA or other regulatory agencies, MIRA1a has anti-anxiety effects at doses that lacked side
effects of sedation or intoxication in mice. The EPM is a test measuring anxiety in rodents as a screening test for putative anxiolytic
compounds and as a general research tool in neurobiological anxiety research such as Generalized Anxiety Disorder (GAD) or Post-Traumatic
Stress Disorder (PTSD). The model is based on the animal’s aversion to open spaces which are present in the open arms (Open Arm)
of the maze. Anti-anxiety effects of test agents are demonstrated by an increase in the percentage of time spent in the Open Arm with
treatment compared to placebo. The total distance traveled is a measure of the overall level of arousal and mobility of the mice undergoing
testing on the EPM and is used to rule out any sedating or intoxicating effects of the test agent.
Pre-clinical
studies also have shown the potential of MIRA1a for relieving pain. A number of clinically approved pharmacological agents used to treat
pain, including opioids, have been demonstrated to delay or ameliorate the onset of heat sensitivity upon paw exposure of mice to heat.
Thirty minutes after treatment with either a placebo (control) or MIRA1a, mice were placed on a heated plate to measure the time it took
for each mouse to lift its paw in response to the mild pain they felt from the heat. Mice treated with pain alleviating drugs took significantly
longer to become bothered by the heat and to lift their paws. Similarly, mice treated with MIRA1a statistically took significantly more
time to lift their legs, indicating MIRA1a’s potential effectiveness as a possible treatment for pain in this model. If approved
by the FDA, MIRA1a may potentially provide therapeutic effects for pain control.
MIRA1a
is a CB2 agonist which may be an optimal treatment for neurodegenerative diseases associated with neuroinflammation caused by microglial
activation. CB2 agonism has been shown in pre-clinical studies to regulate neuroinflammatory processes, reducing the neuronal damage
characteristic of degeneration. We believe there may be a strong rationale for CB2 agonism in neurodegenerative diseases, given increased
CB2 expression in patients with these diseases as well as preliminary results from animal models. We see potential for a potent CB2 agonist
to treat a range of neurodegenerative diseases. MIRA1a, through its robust activity at CB2 compared to CB1, was designed to minimize
the risk of psychotropic adverse events associated with CB1 activation. If approved by the FDA, MIRA1a may potentially provide therapeutic
effects for neurodegenerative and neuroinflammatory illnesses.
Our
pre-clinical development program for MIRA1a has included a variety of testing. Summarized below are the tests we have completed. Our
interpretation of results derived from pre-clinical data or our conclusions based on our pre-clinical data may prove inaccurate and are
not necessarily predictive indicators of future results.
| |
Completed
Pre-Clinical Tests* |
| ● |
EPM
model of anxiety |
| ● |
Thermal
Sensitivity Model of Pain |
| ● |
Context
Fear Conditioning Model of Cognition—Test of learning and memory. |
| ● |
Rat
Psychomotor Vigilance Test (“PVT”) of Cognition—Test of attention. |
| |
|
| *These
were non-human studies that were not powered for statistical significance and as such, no p-values are available. |
| |
● |
EPM
Model of Anxiety Test: |
| |
● |
Method:
We studied the effect of acute administration of MIRA1a on anxiety-related phenotypes in mice to model human conditions. |
| |
■ |
An
intraperitoneal (i.p.) injection of Placebo (e.g. saline) or MIRA1a (e.g. 50mg/kg = Treatment) was administered to C57Bl/6 mice (n=5/group)
that were 8-12 weeks old |
| |
■ |
30
minutes following injection, mice were tested in anxiety related measures using EPM |
| |
● |
Outcome:
The following chart demonstrates MIRA1a’s anti-anxiety effects: |
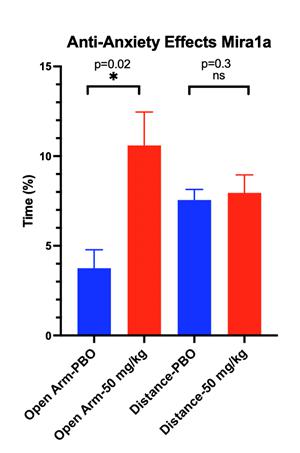
Figure:
Effects of MIRA1a vs Placebo Treatment on Mouse Behavior in the Elevated Plus Maze.
EPM
is a widely used behavioral test to assess anxiety-like behavior in rodents. Typically, rodents tend to avoid open spaces due to their
natural aversion to potentially dangerous areas. Therefore, spending more time in the open arms of the maze indicates decreased anxiety-like
behavior. Similarly, the total distance travelled can reflect general locomotor activity and exploratory behavior, which can be influenced
by the state of anxiety and the effect of drugs.
The
EPM apparatus consists of two open arms and two enclosed arms elevated above the floor. Blue Bars represent the percentage of time spent
in the open arms by mice in the placebo and drug-treated groups. Green Bars show the total distance travelled by mice in both groups
during the EPM test.
| |
● |
Thermal
Sensitivity Model of Pain: |
| |
● |
Method:
We studied the potential for pain reduction in pre-clinical models of heat tolerance using a hot plate methodology. |
| |
● |
Outcome:
MIRA1a provided significantly delayed thermal sensitivity and enhanced pain tolerance. |

Figure:
In this thermal sensitivity test, mice are placed on a heated metal plate (e.g. 52-55 degrees Celsius). The time taken for the mouse
to show a pain response - licking or shaking of the paws, jumping, or trying to escape from the hot plate - is measured. This time interval
is known as the “hot-plate latency”. A longer latency is indicative of reduced pain sensation or a higher pain tolerance.
The
Thermal Sensitivity Model of Pain in mice is a widely used experimental approach to study nociception, which is the perception of pain.
In this model, thermal stimuli are applied to the hind paws of mice to assess their sensitivity to heat-induced pain. The procedure typically
involves placing the mouse on a temperature-controlled surface, such as a hot plate or a radiant heat source. The temperature is gradually
increased, and the response of the mouse is measured, such as the latency to withdraw its paw from the heat source. The withdrawal latency
is considered an indicator of pain sensitivity, with shorter latencies indicating greater sensitivity. By comparing the response of normal
mice to that of mice with altered pain sensitivity, such as genetically modified mice or mice treated with analgesic drugs, researchers
can gain insights into the mechanisms underlying pain perception and potential therapeutic interventions. The Thermal Sensitivity Model
of Pain in mice provides a controlled and reproducible method for studying thermal nociception, allowing researchers to investigate the
effects of various genetic, pharmacological, and environmental factors on pain sensitivity. This model has contributed significantly
to our understanding of pain pathways and the development of novel analgesic treatments.
As
performed at Johns Hopkins, in our thermal sensitivity test, which measured sensitivity to thermal pain, MIRA1a significantly increased
the time it took mice to lift their legs in comparison to placebo (p=0.006) at 75mg/kg. This indicates that MIRA1a has an analgesic effect
and may be a potential treatment for pain. Each group (i.e. placebo and 75 mg/kg) was comprised of 9 mice, for a total of 18 mice.
The
issue of how to test the effect of MIRA1a on cognition was complicated by the following:1) MIRA1a has anti-anxiety (i.e. anxiolytic)
effects, 2) anxiolytics can potentially improve cognitive assessment outcomes by reducing anxiety levels that may otherwise hinder cognitive
functioning. Thus, in commonly performed tests of cognition in mice, such as novel object recognition and Morris water maze, anxiolytic
medications can indirectly result in improved performance by decreasing anxiety rather than by directly improving cognition. In order
to separate assessments of the impact of MIRA1a on cognitive performance from its demonstrated anti-anxiety effects, we employed a model
of context fear conditioning wherein we dosed the mice after training. Context fear conditioning in mice is a behavioral paradigm used
to measure cognitive processes related to associative learning and memory. Associative learning, where an individual learns to associate
specific stimuli or contexts with particular outcomes, in this case the mice associate being in a specific chamber with receiving a mild
foot shock that occurs during training the day before testing. This process of forming associations between stimuli, actions, and consequences
is involved in numerous skills and behaviors in everyday life: it underlies learning new skills, developing habits, and acquiring knowledge
through experiences and conditioning. The use of associating the chamber with the foot shock on day one, means that when the mice are
returned to the chamber on day 2 a measure of how much freezing they do corresponds to a read out of how well they can recall the experiences
they had during training on day 1 (i.e. the greater the freezing, the better the recollection of the association between the chamber
and food shock). Since the mice are given MIRA1a AFTER training that takes place on day 1, and only before testing on day 2, there is
no concern about the anxiolytic effects of MIRA1a on learning during training, but rather this model tests MIRA1a’s effects on
performance only—which in this case represents memory (i.e. the ability to recognize and recall the chamber where they had previously
been shocked) and to translate that into an associated behavior (i.e. freezing). As published in the Journal of Neuropharmacology in
2023, THC and cannabis impair context fear conditioning, both when given prior to training (because of its anti-anxiety effects) and
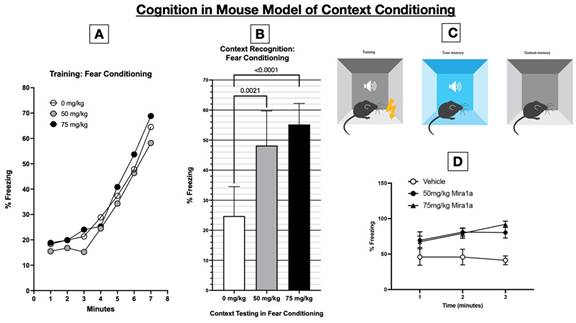
when given prior to testing (because of its cognitive impairing effects). As demonstrated in the figure below, MIRA1a resulted a dramatic
effect on cognitive performance in the context fear conditioning model: as shown in B, the second panel from the left, the percentage
of time spent freezing—that is a demonstration of their memory and association—in the mice who received MIRA1a at a dose
of 75 mg/kg was more than twice that of those who received 0 mg/kg=placebo (i.e. 55% vs 25%, p<0.0001). Thus, MIRA1a doubled the cognitive
performance of the mice compared to placebo. This degree of improvement in cognitive performance in healthy mice dosed just prior to
testing and after learning has not been demonstrated with any cannabinoid compound previously.
| |
● |
Trace
Fear Conditioning Model of Cognition: |
| |
● |
Method:
We studied the potential for improving recall in healthy mice using a fear conditioning model. |
| |
● |
Outcome:
MIRA1a sharply improves cognitive recall as dosage rises. |
The
Contextual Fear Conditioning Model of Cognition in mice is an experimental paradigm used to study associative learning and memory processes.
It focuses on the ability of mice to form an association between a specific environmental context and an aversive stimulus, which leads
to the acquisition and subsequent retrieval of contextual memories. During the acquisition phase of the model, mice are exposed to a
distinct context, such as a particular chamber or environment. In this context, they receive an aversive stimulus, typically a mild foot
shock. The presentation of the foot shock creates an association between the contextual cues and the aversive experience. Following the
acquisition phase, the mice undergo a testing phase to assess their memory of the association between the context where they received
the foot shock and the memory of the aversive stimulus. They are returned to the same context where the conditioning took place and their
behavioral responses, particularly fear-related behaviors such as freezing or defensive reactions, are measured.
These
behavioral responses serve as indicators of the mice’s ability to retrieve the associative memory formed during the acquisition
phase. The Contextual Fear Conditioning Model of Cognition in mice has been widely used in neuroscience research to explore the mechanisms
of associative learning, memory formation, and the neural circuits involved in fear-related associations. It has contributed to our understanding
of how animals, including humans, learn to associate environmental cues with aversive experiences, and has implications for understanding
and treating conditions related to associative learning, memory deficits, and emotional disorders.
As
performed at Johns Hopkins, in the Contextual Fear Conditioning Model the data shows that during training (in the absence of any treatment)
the mice learned as indicated by increased freezing over time. The following day, 30 minutes after MIRA1a administration, the mice were
tested in the context test, which showed significantly increased % freezing (p=<0.0001) in females given 50mg/kg or 75mg/kg MIRA1a.
The experiments were conducted with 10 mice in each group (placebo, 50 or 75 mg/kg MIRA1a) for a total of 30 mice.
In
the context conditioning figure above, mice learn to associate the neutral context (the chamber) with the aversive stimulus (the foot
shock), leading to a conditioned fear response (freezing). This is indicated by ‘freezing’ behavior - a fear-related response
in mice characterized by immobility except for respiratory movements.
A
timeline of the experimental procedure, indicating acclimatization, training (conditioning), and testing phases is shown above. Panel
A, the left-most panel, shows that on day 1 the pairing of a neutral context (the conditioning chamber shown in panel C) with an aversive
stimulus (a mild foot shock). With successive foot shocks the mice show increasing amounts of freezing, since they instinctively freeze
in anticipation of being shocked. Panel B, titled “Context Recognition: Fear Conditioning,” shows the percentage freezing
the mice did on day 2 after receiving placebo or MIRA1a just prior to being placed in the same chamber they had been shocked on day 1.
Since mice freeze in anticipation of receiving a shock, the relative amount of freezing in those mice given 0 mg/kg (placebo) vs either
50 or 75 mg/kg MIRA1a is a readout of (i.e. proportional to) how well the mice recalled that the chamber they were returned to was the
one in which they had been shocked. As shown in panel B, the mice who received 75 mg/kg of MIRA1a right before being placed into the
chamber showed 200% of the freezing than did the mice who received placebo (55% vs 25%, respectively. Panel D, in the lower right corner
of the figure, shows that at 1 min after being placed in the chamber on day 2, the mice that got vehicle (=0 mg/kg MIRA1a), relative
to those that got MIRA1a, have much less freezing, and in fact have less freezing over time. The mice given MIRA1a start off with better
recognition and recall of the chamber (demonstrated as increased freezing) at 1 minute and increase the association of the chamber with
the prior shocks (because they increase freezing over time).
Because
MIRA1a is an anxiolytic, the company decided to test whether it could impair cognitive function. The company therefore sought to determine
if MIRA1a could impair attention—a different aspect of cognition than memory, recall and associative learning, and one that is
affected negatively by sedating compounds (e.g. THC, Cannabis, benzodiazepine, etc.) and positively by stimulants (e.g. caffeine, nicotine,
amphetamine) In order to assess whether MIRA1a affected attention as compared to THC required a different testing model—Psychomotor
Vigilance Test (PVT). The rat Psychomotor Vigilance Test (rPVT) is a widely used method to measure sustained attention in rodents. In
the rPVT model, rats are trained to respond to a visual stimulus by pressing a lever, with shorter reaction times indicative of better
attentional performance. Mice with longer reaction times or higher variability in response times may be considered to have attention
deficits or altered vigilance. Data is shown as percentage accuracy at pressing the lever within the allowed reaction time vs dose of
drug used. In the figure below, it can be seen that at doses of THC that impair attention, MIRA1a had no negative effects on attention
(i.e. their accuracy at pressing a lever at the right amount of time after receiving a trained cue was not impaired at all).
| |
● |
Method:
We performed a PVT to evaluate simple reaction time. |
| |
● |
Outcome:
MIRA1a does not impair cognition. At 3 mg/kg and 10 mg/kg MIRA1a causes minimal impairment in rat PVT whereas THC has a clear
negative effect even at these low doses. |
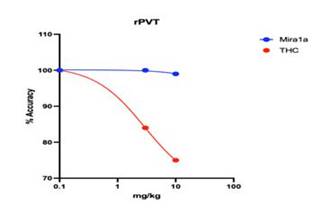
Figure:
Comparison of MIRA1a versus THC on Psychomotor Vigilance Test (PVT) Performance in Rats. The figure displays the percentage accuracy
of rats in the Psychomotor Vigilance Test (PVT) following administration of MIRA1a (blue) or THC (red). The y-axis represents the percentage
accuracy (% Accuracy), indicating the proportion of correct responses in the PVT task. The x-axis represents the treatment condition,
with increasing amount of compound being given to the rats before testing. The data shows that rats treated with MIRA1a exhibited no
decrease in percentage accuracy compared to the THC group (p < 0.05). The results indicate that administration of MIRA1a had no negative
impact on attention performance in the PVT task, as evidenced by the maintenance of 100% accuracy across the dosage range, compared to
THC that impaired attention leading to decreased accuracy more and more with increasing dosages.
The
Psychomotor Vigilance Test (PVT) is a behavioral test used in rats to assess attention and speed of response, providing insights into
their vigilance and cognitive performance. It is based on the measurement of reaction times to visual stimuli, typically presented in
a simple reaction time task paradigm.
In
the PVT, rats are typically placed in an operant chamber or testing apparatus equipped with a visual stimulus, such as a light or LED.
The rats are trained to perform a specific response, such as pressing a lever or nose-poking, when the visual stimulus appears. The timing
of the visual stimuli is randomized to prevent predictability and maintain the animals’ attention.
During
the test, the rats are required to pay attention to the visual stimuli and respond as quickly as possible when they appear. The reaction
time, which represents the time it takes for the rat to initiate the response upon stimulus presentation, is recorded. This measure reflects
the speed of response and can provide an indication of the rat’s attentional state and ability to sustain attention over time.
By analyzing the reaction time data, researchers can evaluate the rat’s attentional performance, including measures such as mean
reaction time, variability in response times, and the occurrence of lapses or errors. The PVT has been widely used to investigate the
effects of different manipulations, such as pharmacological interventions that cause sedation, sleep deprivation, or experimental treatments,
on attention, alertness, and cognitive performance in rats.
Therefore,
the combination of cognitive assessments demonstrated the following: despite having anxiolytic effects, 1) MIRA1a significantly improved
associative learning, memory and recall in the context fear conditioning model, and 2) MIRA1a had no negative effects on attention at
doses that THC showed significant impairment. This is the first time a cannabinoid has been shown to enhance (rather than inhibit) cognition
when given to normal healthy mice after training but before testing, demonstrating a specific cognitive improvement as a direct effect
on the brain that is independent of indirect effects—such as with acute administration by decreasing anxiety or with long term
administration by having anti-inflammatory effects in neurodegenerative diseases.
In
2023, our pre-clinical work will include the conduct of several other pre-clinical studies and initiation of a 7-day maximum tolerated
dose study of MIRA1a in rats and dogs.
| Status |
|
Planned
Activity |
| Drug
Substance Preparation |
●
●
● |
Analytical
Development
NonGMP
Production Refinement
GMP
Production Refinement |
| Testing |
●
●
●
●
●
●
●
●
●
|
MTD/7D
DRF Dog
MTD/7D
DRF Rat
Dog
28-day Toxicology
Rat
28-day Toxicology
Cardiovascular
Study Dog (Telemetry)
Respiratory
Study Rat
hERG
(Manual Patch-Clamp)
Neurobehavioral
Evaluation Rats
Neurobehavioral
Evaluation Mice |
We
further plan on neurobehavioral evaluation of orally and intraperitoneally administered MIRA1a in rats and mice, respiratory evaluation
of orally administered MIRA1a in rats, and in vitro testing for effects of MIRA1a on hERG (the human Ether-à-go-go-Related Gene)
channel currents. The hERG is an early in vitro assay required by the FDA to alert companies of any potential cardiac abnormalities by
the product before proceeding with dose studies in humans. hERG is a gene that codes for a protein known as the alpha subunit of a potassium
ion channel. This ion channel (sometimes simply denoted as ‘hERG’) is best known for its contribution to the electrical activity
of the heart: the hERG channel mediates the repolarizing current in the cardiac action potential, which helps coordinate the heart’s
beating. When this channel’s ability to conduct electrical current across the cell membrane is inhibited or compromised, either
by application of drugs or by rare mutations in some individuals, it can result in a potentially fatal disorder called long QT syndrome.
Testing
is anticipated to conclude in the first quarter of 2024. Additionally, a 28-day toxicology analysis for dogs and rats is expected to
begin at the end of the fourth quarter of 2023 and continue through the first quarter of 2024.
We
have started the analytical development and manufacturing of MIRA1a as of January 2023. By the third quarter of 2023, we anticipate our
suppliers will be developing MIRA1a at scale and manufactured under cGMP conditions, expanding on earlier non-GMP volumes of MIRA1a for
use in our initial testing programs. We plan to work closely with our suppliers to generate sufficient volumes of cGMP-grade MIRA1a materials
for the planned pre-clinical toxicity programs, expanded animal testing and human trials expected to be performed in 2024, subject to
FDA approval.
Our
Clinical Development Program
Following
the pre-clinical development plan outlined above, we plan to submit to the FDA an Investigational New Drug application (“IND”)
focused on investigating MIRA1a for the treatment of anxiety and cognitive decline in elderly patients.
Our
first IND application submission investigating MIRA1a for the treatment of elderly patients suffering from anxiety with some cognitive
decline is currently planned for the end of the third quarter of 2024, as we believe this is a patient population with unmet needs. If
allowed to proceed by the FDA, a Phase I trial will be initiated 30 days post-IND submission.
Our
second IND application will focus on investigating MIRA1a for the treatment of chronic pain.
All
development plans depend on FDA acceptance of our IND applications. As appropriate and pursuant to discussions with the FDA, we may periodically
adjust the timeline for certain filings and associated clinical trials. It is important to note that the process for conducting clinical
trials is uncertain and there is no assurance that our clinical development activities will meet the planned timelines set forth above.
Manufacture
of Product for Clinical Development Activities
Curia
Global (formerly AMRI), a leading global CDMO, is currently developing a large-scale synthesis protocol for us and will be supplying
quantities of MIRA1a needed for our pre-clinical and clinical development activities. We are currently in discussions with other partners
to have MIRA1a formulated into solid oral dosage forms for clinical trials.
Market
Opportunity
MIRA1a,
if approved, will compete in three key overlapping growth markets: the anxiety, cognitive decline (CNS/dementia), and chronic pain markets
where multiple products with varying safety and efficacy profiles are already on the market. MIRA1a competes at the intersection of these
three markets given the target patient profile for MIRA1a.
MIRA1a
will compete primarily within the CNS market that encapsulates anxiety, dementia, other pain, Alzheimer’s, migraines and related
conditions. Based on the market size of the CNS opportunity as set forth in IQVIA’s Global Use of Medicines 2023 analysis (the
“IQVIA Report”), we estimate that by 2027, the U.S. CNS market will be worth $48 billion, growing between two and five percent
during the period from 2023 to 2027. Within that market opportunity, anxiety is worth between approximately $10 billion and $15 billion
in annual sales. If approved by the FDA, MIRA1a may potentially provide therapeutic effects for anxiety, dementia and pain.
Anxiety
and pain are expected to grow approximately five percent over the same period according to the IQVIA Report, while Alzheimer’s
is expected to grow approximately twelve percent. This is critical given MIRA1a’s focus on early-stage patients with dementia,
as according to the Alzheimer’s Association 2023 Alzheimer’s Disease Facts and Figures analysis (the “Alzheimer
Association”), 0.5 million new Alzheimer cases emerge in the U.S. each year. According to the Alzheimer Association, about 60 to
80 percent of Alzheimer cases evolve into dementia. Thus, Alzheimer case directions are an important signal and gateway for MIRA1a-related
opportunities in dementia. Based on that epidemiology, the US Center for Disease Control (“CDC”) estimates that approximately
5.8 million Americans are living with Alzheimer’s, with that number expected to grow to 14 million by 2060 (“CDC Alzheimer”).
MIRA1a’s
other key market will be the traditional U.S. pain market, which the IQVIA Report estimates will be worth $42 billion in 2027 and grow
between three and six percent during the forecast period. Note that this sizing is inclusive of chronic and acute pain, and MIRA1a is
likely to only be used in the chronic segment of the market (approximately 40% to 50% of the market). Factors such as a rise in oncology
related pain, diabetic neuropathy, and pain associated with aging (e.g. joint pain) are among the key drivers of patient and prescription
growth. Opioid toxicity and related annual deaths suggest a novel non-opioid pain killer is needed. Given the overlap across indications
and the fact that the target patient is presenting across these markets.
Our
initial focus will be a dual path: potentially winning in traditional markets as well as the marijuana analog markets using a safe, effective
and, if determined by the FDA, an FDA-approved treatment option since safety and efficacy determinations are in the exclusive purview
of the FDA. Today, legal medical marijuana is a $13.2 billion industry whereas legal recreational marijuana is a $25.6 billion industry.
Both are sub-sets of the traditional pain and anxiety markets. However, in many patient populations, non-US legal, and cultural settings,
marijuana may not be the first or a viable option for treatment of neurological disorders. As a result, these patients will typically
use non-steroidal anti-inflammatory drugs (NSAIDs) or various mood management drugs, opening them up to a range of non-ideal outcomes.
The objective of MIRA1a is to offer physicians and patients an approved, viable synthetic option. Thus, if approved by the FDA, we believe
that MIRA1a may potentially provide a preferred alternative in such patient populations, as it is not derived from the marijuana plant.
Our
Strategy
Our
goal is to develop therapeutics targeting well-characterized CB1 and CB2 receptors with optimized pharmacological properties to transform
the lives of patients with neurological diseases. Key elements of our strategy to achieve this goal include:
| |
● |
Advance
our MIRA1a through clinical development and approval. Our product candidate, MIRA1a, is in pre-clinical studies. Existing
treatment options for neuropsychiatric disorders and neurological diseases have significant limitations, and, if approved, we believe
MIRA1a would represent a major therapeutic advancement for patients. |
| |
● |
Continue
pre-clinical development of MIRA1a across a range of CNS diseases associated with neurodegeneration and progress into clinical development.
MIRA1a is currently in IND-enabling studies for neurobehavioral disorders such as dementia, Post-Traumatic Stress Disorder
(PTSD), chronic pain, as well as neurodegenerative diseases such as Alzheimer’s and Parkinson’s Disease. We believe MIRA1a
may have potential in several diseases associated with neuroinflammation, including multiple sclerosis. |
| |
|
|
| |
● |
Identify
additional product candidates and expand current candidates into additional neurological diseases. We see potential for our
current product candidate to be evaluated in clinical trials outside of its initial indications and will evaluate additional indications
to maximize the potential of our drug development program. Our current product focus is on targets that are well characterized in
neurological diseases but for which there are limitations with currently available therapies. We also plan to continue to identify
and develop additional novel product candidates that align with our focus. |
| |
|
|
| |
● |
Explore
strategic collaborations to maximize the value of our product candidates. We plan to explore collaborations opportunistically
to maximize the value of our product candidates. We intend to retain significant economic and commercial rights to our programs in
key geographic areas that are core to our long-term strategy. |
KETAMIR-2
Major
Depressive Disorder (MDD) is a significant global health concern, affecting over 264 million people worldwide and ranking among the leading
causes of disability. In the United States alone, it impacts nearly 17.3 million adults, accounting for about 7.1% of the adult population.
This widespread mental health disorder not only undermines the quality of life and daily functioning of individuals but also imposes
a substantial economic burden, with costs in the U.S. amounting to tens of billions of dollars annually. MDD is also a major risk factor
for suicide, a leading cause of death globally, highlighting its profound impact on public health and the urgent need for effective treatment
and management strategies. If approved by the FDA, KETAMIR-2 may potentially provide antidepressant therapeutic effects.
Despite
the fact that antidepressants have been around for decades, with imipramine being the first FDA-approved antidepressant in 1959, the
need for a rapid-acting antidepressant that can help patients with Treatment-Resistant Depression (TRD) using a novel mechanism of action
(e.g. not a monoamine reuptake inhibitor) has been growing. In 2019, Ketamine was introduced but required a Risk Evaluation and Mitigation
Strategy (REMS) because of its: 1) poor oral availability requiring IV or IN administration, 2) ability to cause side effects including
dissociation, sedation and acute hypertension, and 3) potential abuse liability.
Ketamir-2
is a new chemical entity, analog of Ketamine that is designed to potentially preserve the same rapid antidepressant response but with
improved bioavailability. It may also have decreased side effects, and decreased abuse liability, though such conclusions are within
the sole authority of the FDA. This combination is intended to potentially facilitate safer and less cumbersome dosing requirements,
with the goal of obtaining an orally administered pill that can be taken at home.
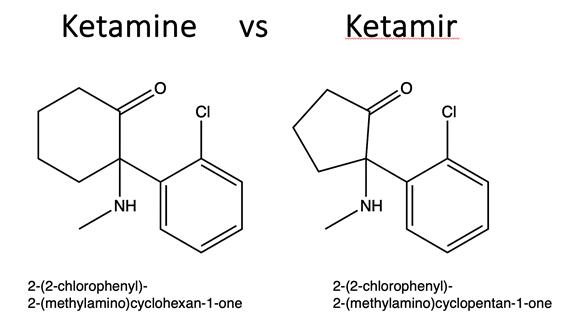
Figure
1: Structures of Ketamine and Ketamir-2 for comparison purposes.
The
Drug Enforcement Administration (DEA) conducted a scientific review of the Ketamir-2 “in accordance with the definitions within
the CSA and its implementing regulations. Based on this review, DEA determined that” Ketamir-2 is “not [a] controlled
substances or listed [chemical] under the CSA.”
Mechanism
of Action of Ketamir-2
Ketamir-2’s
mechanism of action (MOA) as a rapid acting antidepressant is the same as Ketamine’s, based on the fact that the two share a common
inhibitory effect on the NMDA receptor that is believed to be integral to the antidepressant effects of both of these drugs.1
In fact, Ketamir-2 and Ketamine differ in less than 2% in their antagonist activity at the GRIN1/GRIN2B receptor subunit of the NMDA
receptor (based in in silico analysis, see below). This subunit combination is prominently linked to neuroplasticity, believed
to be a key factor in depression and the action of antidepressants such as Ketamine.2 GRIN2B-containing NMDA receptors are
implicated in synaptic plasticity changes associated with depression and its treatment.
Ketamine’s
mechanism of action (MOA) as a rapidly acting antidepressant is multifaceted and distinct from traditional antidepressants like selective
serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants.1 While ketamine has shown promise as a rapid-acting antidepressant,
especially in treatment-resistant depression, its use is limited due to potential side effects and abuse potential that Ketamir-2 has
been targeted to minimize. Moreover, whereas Ketamine has a poor oral bioavailability and must therefore be given IV or IN, Ketamir-2
has a much better bioavailability suggesting it may be appropriate for oral use.3,4 Here’s a detailed synopsis of its
MOA of both Ketamir-2 and Ketamine:
| 1. | NMDA
Receptor Antagonism: Ketamine primarily acts as a non-competitive antagonist of the N-methyl-D-aspartate
(NMDA) receptor, a type of glutamate receptor. By inhibiting these receptors, ketamine modulates
the release of the neurotransmitter glutamate. This modulation leads to an increase in glutamatergic
signaling via activation of AMPA receptors, another type of glutamate receptor. This enhanced
signaling is believed to play a crucial role in ketamine’s rapid antidepressant effects.1 |
| | | |
| 2. | mTOR
Pathway Activation: Ketamine activates the mammalian target of rapamycin (mTOR) pathway,
a key regulator of cell growth and survival. This activation is linked to increased synaptogenesis
in the prefrontal cortex. The mTOR pathway plays a significant role in neural plasticity
and has been implicated in the pathophysiology of depression.1 |
| 3. | Effects
on GABAergic System: Recent research indicates that ketamine may also affect the gamma-aminobutyric
acid (GABAergic) system, which is responsible for inhibitory neurotransmission in the brain.
Alterations in GABAergic signaling have been associated with mood disorders.1 |
| | | |
| 4. | BDNF
Release and Synaptogenesis: The increased glutamatergic transmission leads to the activation
of downstream pathways that result in the release of Brain-Derived Neurotrophic Factor (BDNF).
BDNF is crucial for neuroplasticity – the brain’s ability to reorganize and form
new neural connections. Studies suggest that this increase in BDNF and subsequent synaptogenesis
(formation of new synapses) in brain areas like the prefrontal cortex is a key factor in
the antidepressant effects of ketamine.1 |
| | | |
| 5. | Anti-inflammatory
Effects: Depression is increasingly linked with chronic inflammation. Ketamine has been
shown to have anti-inflammatory properties, which might contribute to its antidepressant
effects.5 |
| | | |
| 6. | Neuroendocrine
Regulation: Ketamine may influence the hypothalamic-pituitary-adrenal (HPA) axis, which
is often dysregulated in depression. By modulating this axis, ketamine could exert additional
antidepressant effects.6 |
| | | |
| 7. | Rapid
Onset of Action: Unlike traditional antidepressants, which typically take weeks to exert
their effects, ketamine’s impact on mood can be noticed within hours of administration.
This rapid action is especially beneficial in acute management of severe depression and suicidal
ideation. |
In
summary, while Ketamir-2’s and ketamine’s antidepressant MOA are still being studied and explored, current evidence suggests
a complex and involved synergistic action on various neural pathways, primarily through the modulation of glutamatergic neurotransmission,
enhancement of neuroplasticity, and potentially through anti-inflammatory and neuroendocrine mechanisms. Both drugs rapid onset and efficacy
in treatment-resistant cases make them unique and valuable tools in psychiatry, but the potentially improved side effect profile and
oral bioavailability are what differentiate Ketamir-2 and ketamine as described below.
Preclinical
Research Findings
In
Silico Analysis of Targets of Ketamir-2 vs Ketamine
In
silico analysis, referring to computer-based techniques, has become an integral part of pharmaceutical research and development.7
This approach utilizes computational methods to analyze and predict the properties and behaviors of pharmaceutical compounds. The
use of in silico analysis is especially crucial in the early stages of drug development, as it aids in identifying potential drug targets
and elucidating differences between a new drug and its parent compound. By analyzing large datasets, such as genomic, proteomic, and
metabolomic data, researchers can predict how different compounds might interact with various biological targets. This approach helps
in understanding the mechanism of action of new drugs and can significantly reduce the time and cost associated with experimental screening.
InSilico Trials was contracted to provide a comparison between targets of Ketamir-2 vs Ketamine employing their target identification
protocol. The following characterize some of the unique targets that are predicted to interact with either Ketamir-2 or Ketamine, thereby
differentiating one drug from the next.
Ketamir-2
selective target:
BRD4,
or Bromodomain-containing protein 4, is a member of the bromodomain and extra-terminal (BET) family of proteins and has been implicated
in the regulation of gene expression, particularly those involved in cell cycle progression and inflammatory responses.8 In
the context of depression, research has started to explore the role of BRD4 and its potential impact.
| 1. | BRD4
and Neuroinflammation: Inflammation is increasingly recognized as a significant factor in
the pathophysiology of depression. BRD4 has been found to regulate the expression of inflammatory
genes. Its inhibition, therefore, might reduce neuroinflammation, which is thought to contribute
to depressive symptoms. |
| | | |
| 2. | Gene
Expression Regulation: BRD4 influences the transcription of genes involved in mood regulation
and stress response. Dysregulation of these genes can contribute to the development of depression.9 |
| | | |
| 3. | Pharmacological
Target: BRD4 is a target for new pharmacological interventions in depression. Inhibitors
of BRD4, such as JQ1, have shown promise in preclinical studies for their antidepressant
effects. These compounds can modulate the expression of genes associated with mood and stress
response. |
| 4. | Epigenetic
Mechanisms: As an epigenetic regulator, BRD4’s role in modifying the expression of
genes without changing the DNA sequence might be crucial in understanding the long-term impact
of environmental factors on depression.10 |
| | | |
| 5. | Animal
Studies: Research in animal models has provided some evidence that modulation of BRD4 activity
can influence behaviors related to depression. However, translating these findings to human
depression is complex and requires more research. |
| | | |
| 6. | Thus,
while BRD4 is not traditionally associated with depression like neurotransmitter systems
(e.g., serotonin or dopamine), emerging evidence suggests that it plays a role in the disease’s
pathophysiology. Its involvement in regulating gene expression, particularly related to inflammation
and stress response, positions it as a potential target for novel antidepressant therapies.9 |
Ketamine
selective targets:
Alpha-2a
adrenergic receptor: Alpha-2a adrenergic receptors are G protein-coupled receptors (GPCRs) involved in the modulation of neurotransmitter
release.11 They are generally thought to be inhibitory, reducing the release of norepinephrine when activated, which can lead
to various physiological effects.
| ● | Cardiovascular
Effects: Alpha-2a receptors play a role in cardiovascular regulation, which might explain
some of the blood pressure and heart rate changes seen with ketamine.12 |
| | | |
| ● | Sedation:
Activation of these receptors can lead to sedative effects, which is consistent with the
tranquilizing effects that ketamine can produce.13 |
Sigma
Opioid Receptor: Ketamine is known for its dissociative anesthetic properties, which are primarily attributed to its antagonism of the
N-methyl-D-aspartate (NMDA) receptor. However, the sigma receptors, particularly the sigma-1 receptor, have also been implicated in the
psychotomimetic and dissociative effects of ketamine.14 Here’s how ketamine’s interaction with sigma opioid receptors
might contribute to its dissociative side effects:
| ● | Cognitive
and Perceptual Processes: Activation of 15has been linked to modulating cognitive
and perceptual processes, which could be associated with the dissociative effects experienced
during ketamine administration. |
| | | |
| ● | Modulation
of NMDA Receptor Activity: Sigma-1 receptors are known to interact with NMDA receptors, and
this interaction might enhance or modulate the dissociative effects of ketamine, which primarily
acts as an NMDA receptor antagonist.16 |
Mu-Opioid
Receptor: The Mu-opioid receptor (MOR) is one of the principal targets within the central nervous system for endogenous opioids like
endorphins and enkephalins, as well as for exogenous opioid analgesics such as morphine and fentanyl.17 Activation of MOR
typically results in analgesic effects, reduced gastrointestinal motility, respiratory depression, and can influence the reward system
in the brain, which is associated with the pleasurable sensations or euphoria. Activation of the MOR by Ketamine could contribute to
side effects related to its abuse liability:
| ● | Euphoria
and Reward: MOR activation is heavily implicated in the reward pathway and can produce euphoria.
This effect is a key driver of the abuse potential of opioids. |
| | | |
| ● | Tolerance
and Dependence: Chronic activation of the MOR leads to tolerance (the need for increasing
doses to achieve the same effect) and physical dependence, contributing to the cycle of abuse. |
| | | |
| ● | Sedation:
MOR activation can also result in sedation, which might contribute to the overall sedative
effects of ketamine, particularly at higher doses. |
Opiate
Receptor Issue
One
topic that deserves explanation relates to the belief by some of the researchers in the field of ketamine antidepressant effects suggested
that mechanism of action of this drug is not by blocking the NMDA receptor but instead is mediated by its Mu-Opioid receptor (MOP) agonist
activity.18 This belief was generated by the observation that naltrexone, which blocks the MOP, inhibited the antidepressant
effects of ketamine. Since Ketamir-2 does not have MOP agonist activity, it might seem surprising that it does have antidepressant activity
in the same mouse model that first lead to the discovery of low-dose ketamine antidepressant properties (i.e. the Forced Swim Test FST).
We therefore take the time here to provide an explanation that resolves these seemingly paradoxical observations.
| 1. | Ketamir-2
and Its Pharmacology: |
| ○ | Ketamir-2
is a newly synthesized compound analogous to ketamine. Unlike ketamine, Ketamir-2 does not
bind to the mu-opioid receptor. This receptor is often linked with the addictive properties
of many drugs, including ketamine. |
| | | |
| ○ | This
lack of MOP agonist activity suggests that Ketamir-2 may have less addictive properties,
thus potentially improving its safety profile. |
| 2. | Ketamine,
Mu-Opioid Receptor, and Depression: |
| ○ | The
majority of people in the field of low-dose ketamine antidepressant activity believe the
evidence that suggests that it is working via NMDA receptor blockade. |
| | | |
| ○ | However,
Shatzberg et al at Stanford have suggested that Ketamine’s antidepressant effects might
be mediated through its interaction with the mu-opioid receptor.18 |
| | | |
| ○ | This
hypothesis was supported by the Stanford groups studies showing that the administration of
naltrexone, a mu-opioid receptor antagonist, reduces the antidepressant effects of ketamine.
This implies that blocking this receptor diminishes ketamine’s efficacy.18 |
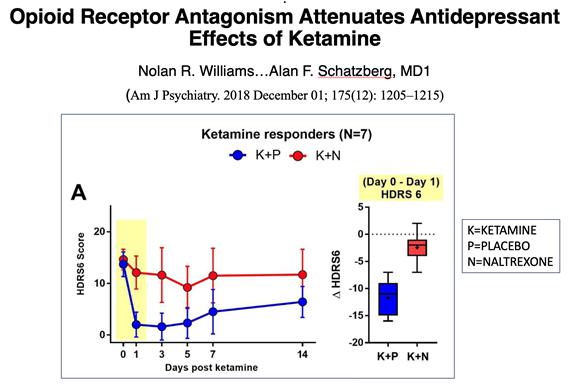
Figure
2: Study in humans demonstrating that IV Ketamine (K) pluse Placebo (P) had rapid antidepressant effects that were blocked by Naltrexone
(N).
| 3. | Wang
and Kaplin’s Counterargument: |
| ○ | Adam
Kaplin, MD, PhD is the president and CSO of MIRA. At Johns Hopkins he and his student Michael
Wang published a plausible scenario that explains one possible way in which naltrexone could
block Ketamine’s antidepressant response in a manner that has nothing to do with Ketamine’s
MOP agonist activity.19 |
| | | |
| ○ | Wang
and Kaplin proposed an alternative interpretation of the interaction between ketamine, naltrexone,
and the mu-opioid receptor. They argue that the interference of naltrexone with ketamine’s
antidepressant effects might not be due to its action at the mu-opioid receptor. Instead,
they suggest a complex interaction involving the N-methyl-D-aspartate (NMDA) receptor signaling
pathway. |
| ○ | According
to their hypothesis, naltrexone’s antagonism at the mu-opioid receptor increases cyclic
AMP (cAMP) levels. This elevation in cAMP interferes with the activation of the mammalian
target of rapamycin (mTOR) pathway by ketamine, a crucial mediator of its antidepressant
effects. This suggests a more intricate mechanism beyond direct opioid receptor activity. |
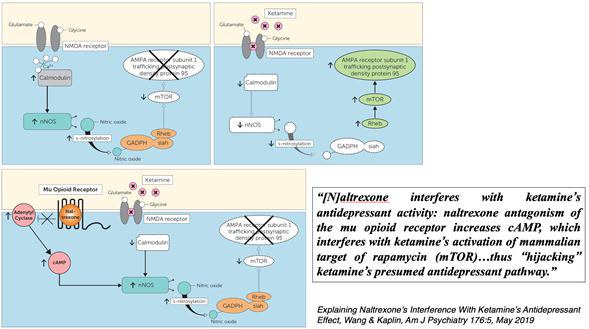
Figure
3: Plausible mechanism whereby naltrexone—by blocking constitutively active signalling from the Mu-Opioid Receptor—interferes
with the downstream signaling produced by Ketamine at the NMDA receptor that is independent of any effect of Ketamine on the opioid receptor.
| 4. | Ketamir-2’s
Antidepressant Effects in Animal Models: |
| ◌ | Despite
not targeting the mu-opioid receptor, Ketamir-2 exhibited antidepressant effects in the mouse
FST model of antidepressant effects. This finding is significant because it demonstrates
that the mu-opioid receptor activity of both Ketamir-2 (which has none) and Ketamine (which
is a MOP agonist) is not necessary for their antidepressant effects, challenging the Stanford
understanding of ketamine’s mechanism of action in treating depression. |
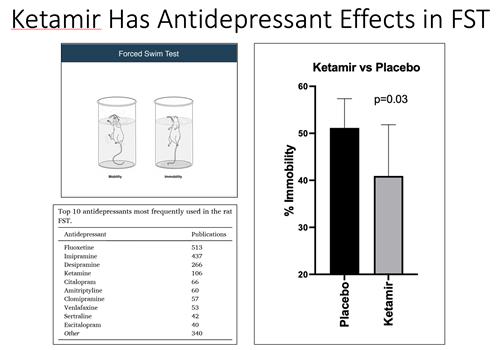
Figure
4: The Forced Swim Test, how many publications that involve antidepressants that have used it, and the statistically significant effect
of Ketamir-2 in the test demonstrating its antidepressant properties.
In
conclusion, the finding that Ketamir-2 has antidepressant effects despite having no MOP agonist activity supports the model by Wang and
Kaplin that suggests that naltrexone can have effects on second messenger signaling that interferes with Ketamine’s intracellular
effects, which has nothing to do with Ketamine’s antidepressant activity via NMDA receptor blockade. The end result is the development
of a Ketamine analog (i.e. Ketamir-2) that maintains Ketamir-2’s antidepressant effects but without it MOP agonist activity, thereby
creating a potentially safer treatment alternative.
Bioavailability:
The
Caco-2 cell model, originating from a human colorectal adenocarcinoma cell line, plays a significant role in pharmaceutical research
for estimating the intestinal absorption and indirectly the bioavailability of drugs.20 Bioavailability, the proportion of
a drug that enters the systemic circulation when introduced into the body, is crucial for determining a drug’s effectiveness. Traditionally,
bioavailability is determined through in vivo studies, including human and animal trials, as well as in vitro models like the Caco-2
cell model and in silico computational approaches.
The
Caco-2 model involves culturing cells that differentiate into a monolayer mimicking the intestinal epithelium, complete with tight junctions
and microvilli. This model is pivotal in permeability studies to assess how well drugs can pass through the intestinal barrier and in
understanding both active and passive drug transport mechanisms. While primarily used for estimating drug absorption, the Caco-2 model
also serves to predict potential drug-drug interactions within the gastrointestinal system.
The
Caco-2 model offers a high-throughput, cost-effective, and human-relevant system, making it a preferred choice for initial screening
of multiple compounds. In pharmaceutical research, the Caco-2 model often serves as an initial study to predict the absorption properties
of new drugs and is typically validated against clinical data once that becomes available.21 It plays a crucial role in the
early stages of drug development, influencing decisions on which compounds to advance.
CaCO-2
cells are human epithelial colorectal adenocarcinoma cells that are widely used as an in vitro model of the intestinal barrier.20
The CaCO-2 assay is employed to study the absorption and transport of orally administered drugs across the intestinal epithelium.
The assay evaluates the permeability of a drug from the apical (AP) side, representative of the intestinal lumen, to the basolateral
(BL) side, representative of the blood side, and vice versa.
The
bidirectional transport assays conducted with CaCO-2 cells can provide the following insights about two different drugs:
| 1. | Absorption
Potential: The AP to BL (A→B) transport rate can indicate a drug’s ability to
be absorbed through the intestines into systemic circulation. Higher transport rates suggest
better absorption potential. |
| | | |
| 2. | Efflux
Ratio: By comparing the BL to AP (B→A) transport rate with the A→B transport rate,
one can determine the efflux ratio. If the efflux ratio is significantly greater than 1,
this implies that there are active efflux mechanisms, such as P-glycoprotein, that are pumping
the drug back into the intestinal lumen, thus reducing its absorption. |
| | | |
| 3. | Permeability
Classification: The transport rates can be used to classify the drugs according to their
permeability. High permeability drugs are absorbed more completely and are likely to have
a more reliable and faster onset of action. |
| | | |
| 4. | Influence
of Efflux and Influx Transporters: Differences in the AB-BA values between two drugs can
indicate the involvement of different efflux or influx transporters, suggesting that the
drugs have different affinities for these transporters. |
| | | |
| 5. | Impact
of Metabolism: If a drug is extensively metabolized by the intestinal wall before reaching
systemic circulation, this will be reflected in a low A→B permeability. |
| | | |
| 6. | Predicting
Oral Bioavailability: Generally, drugs that exhibit high permeability in CaCO-2 assays are
expected to have good oral bioavailability, although this is not always the case due to other
factors such as solubility and first-pass metabolism.20 |
In
summary, the CaCO-2 intestinal absorption (AB-BA) assay is a valuable tool for predicting the intestinal absorption and oral bioavailability
of drugs. Differences in the assay results between two drugs can provide important information about their absorption characteristics,
potential interactions with transporters, overall oral bioavailability, and possible drug-drug interactions.
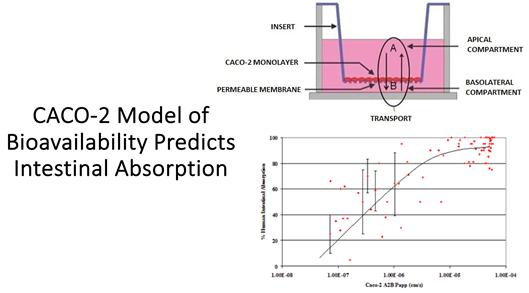
Figure
5: Model of the CaCO-2 model of drug intestinal absorption and how well it correlates with actual measures of human intestinal absorption.

Figure
6: Data obtained from the CaCO-2 model of intestinal absorption. Propranolol, a commonly prescribed beta-blocker that is taken orally
and used to treat hypertension, is included as a positive control. The intestinal absorption (AB), Intestinal efflux (BA) and net absorption
(AB-BA) are shown.
As
can be seen in Figure 6 of the Bioavailability of Ketamir-2 vs Ketamine, as determined by the CaCO-2 model, comparing the rate for Ketamir-2
vs Ketamine, the absorption from the intestinal lumen into the blood that is 80% greater (80.6 vs 44.5), the rate of efflux back into
the intestinal lumen that is 35% less (-38.7 vs -59.6), and the net absorption (AB-BA) rate is 3.77 fold greater [(41.9+15.1)/15.1=3.77],
respectively. Since the reported oral bioavailability of Ketamine has been reported to be between 16-30% (average of 23%),3
then the predicted oral bioavailability of Ketamir-2 could be as high as 87% (i.e. Ketamir-2’s oral bioavailability is 3.77 fold
greater than Ketamine’s = 23%*3.77=87%).
This
is just an approximation, and when sufficient Ketamir-2 has been synthesized to do in vivo animal initially and then human Pharmacokinetic
(PK) studies, it will be possible to get a more precise estimate of Ketamir-2’s Oral Bioavailability compared to Ketamine by testing
and calculating the area under the concentration-time curve (AUCoral) for oral dosing divided by the AUC for IV dosing (i.e. AUCoral/AUCiv).22
But based on the available preliminary estimates, it appears highly likely that the oral bioavailability of Ketamir-2 in humans
is going to be substantially larger than that of Ketamine. Orally available Ketamir-2, as opposed to IV or IN Ketamine, would be much
easier to be patient self-delivered at home, thereby improving on the ease and availability of this rapid acting antidepressant for TRD
& MDSI.
Our
Clinical Development Program
The
clinical development plan for Ketamir-2 involves a series of methodically structured phases, starting with IND-enabling studies and progressing
through Phase 1 and Phase 2 clinical trials. These trials aim to establish the safety, efficacy, and optimal use of Ketamir-2 in treating
psychiatric conditions like TRD, Major Depressive Episode with Suicidal Ideation (MDSI), and potentially Post-Traumatic Stress Disorder
(PTSD). The strategy underscores patient safety while evaluating Ketamir-2’s therapeutic benefits and risks. The successful development
of Ketamir-2 could significantly impact the treatment landscape for depression, offering a novel approach that addresses the shortcomings
of current therapies.
Initially,
the development process begins with completion of all necessary IND-enabling studies. These preclinical studies, encompassing pharmacokinetics,
pharmacodynamics, toxicology, and safety pharmacology, are crucial for ensuring that the investigational drug meets regulatory standards.
The successful completion of these studies allows for the submission of an Investigational New Drug (IND) application to the FDA, specifically
targeting TRD.
Upon
FDA approval, the plan progresses to Phase 1 clinical trials. These trials are designed to assess the safety and tolerability of Ketamir-2
in healthy volunteers. They are typically randomized, double-blind, and placebo-controlled, and aim to determine the appropriate dosing
while closely monitoring for adverse effects. Key to this phase is the collection of pharmacokinetic and pharmacodynamic data, which
guides the dosing strategies for subsequent trials.
Phase
1: Safety and Dosage Determination in Healthy Volunteers
| ○ | A
randomized, double-blind, placebo-controlled trial. |
| ○ | Primary
objective: Assess safety and tolerability of Ketamir-2. |
| ○ | Secondary
objectives: Determine pharmacokinetics and pharmacodynamics. |
| ○ | Enroll
healthy volunteers, ensuring a diverse demographic representation. |
| ○ | Exclude
individuals with a history of psychiatric illness, substance abuse, or significant medical
conditions. |
| 3. | Dosing
and Administration: |
| ○ | Start
with a low dose, escalating gradually to higher doses. |
| ○ | Monitor
participants closely for adverse effects. |
| ○ | Safety
assessments: Vital signs, laboratory tests, ECG, adverse event monitoring. |
| ○ | PK/PD
assessments: Blood sampling for drug levels, brain imaging for receptor binding (if feasible). |
Following
the establishment of safety and initial dosing parameters in Phase 1, the development plan moves into Phase 2. This phase involves trials
with patients diagnosed with TRD. The primary goal here is to evaluate the optimal dose and tolerability of Ketamir-2 in this specific
patient population. Additionally, these trials provide preliminary data on the efficacy of Ketamir-2 for the treatment of TRD. Safety
remains a priority, with close monitoring for any adverse events and detailed assessments using depression rating scales.
Phase
2: Dose, Tolerability, and Early Efficacy in TRD
| ○ | A
randomized, controlled trial with TRD patients. |
| ○ | Primary
objective: Evaluate the optimal dose and tolerability. |
| ○ | Secondary
objective: Obtain preliminary efficacy data. |
| ○ | Enroll
patients diagnosed with TRD. |
| ○ | Utilize
standardized diagnostic criteria and severity scales. |
| ○ | Implement
a dose range based on Phase 1 findings. |
| ○ | Consider
flexible dosing or fixed-dose regimen based on safety and tolerability data. |
| ○ | Tolerability
assessment: Adverse event monitoring, patient-reported outcomes. |
| ○ | Efficacy
assessment: Depression rating scales (e.g., HDRS, MADRS). |
As
the development of Ketamir-2 progresses, there is potential to expand its indications. One such area is MDSI, where Ketamir-2’s
application could be particularly beneficial given ketamine’s established efficacy in this domain.24 This would involve
designing a trial specifically targeting MDSI, with a focus on the rapid onset of action and short-term safety considerations.
Furthermore,
given the emerging research suggesting ketamine’s therapeutic potential in PTSD, a similar approach could be considered for Ketamir-2.25
Developing a trial protocol for PTSD treatment requires a careful balance, considering the complexity of the disorder, potential
comorbidities, and the need for robust safety and efficacy data.
Pursuing
Additional INDs:
| 1. | Major
Depressive Episode with Suicidal Ideation (MDSI): |
| ○ | Following
successful Phase 2 outcomes, pursue an IND for MDSI, leveraging existing data and research
on ketamine.26 |
| ○ | Design
a trial specifically targeting MDSI, focusing on rapid onset of action and short-term safety. |
| 2. | Post-Traumatic
Stress Disorder (PTSD): |
| ○ | Based
on early research suggesting ketamine’s efficacy in PTSD, consider developing a clinical
trial protocol for Ketamir-2 in PTSD.25 |
| ○ | Prioritize
safety and efficacy, given the complex nature of PTSD and potential comorbidities. |
In
summary, the clinical development plan for Ketamir-2 is a meticulous, multi-phase strategy that prioritizes patient safety while exploring
the drug’s potential in treating complex psychiatric conditions. Each phase is carefully designed to address specific research
questions and regulatory requirements, ensuring a thorough evaluation of Ketamir-2’s therapeutic benefits and risks.
Market
Opportunity
The
market opportunity for Ketamir-2 is substantial. The U.S. has a large patient pool looking for effective treatments, with diagnosed prevalence
rates of 3.1% for MDSI and 2.4% for TRD, translating to total addressable populations of 4.9 million and 3.8 million patients respectively.28
Based on a total estimates of MDSI and TRD together, this represents a Total Diagnosed Prevalence rate of 12.3 million patients
and, assuming a Treatment Rate of 65%, the Total Addressable Population is 8.7 million patients This represents a significant market,
especially considering the current limitations and side effects associated with existing treatments.
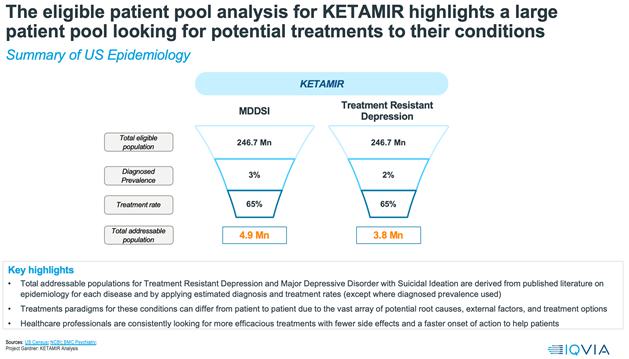
Figure
7: Estimates by IQVIA of the total addressable populations affected with MDSI and TRD.
Our
Strategy
MIRA,
following its successful IPO and the licensing of Ketamir-2 from Miralogx, is positioned to advance the development of Ketamir-2. The
ultimate goal is to continue develop Ketamir-2 as an orally administered medication with potentially fewer side effects, freed from the
restrictions such as those imposed by Ketamine’s REMS, to fill the current clinical need for a rapid acting antidepressant to manage
TRD and MDSI in patients who are able to take Ketamir-2 at home. The strategic plan for Ketamir-2’s development encompasses several
critical stages, from scaling up manufacturing to exploring effective exit strategies.
Scaling
Up Manufacturing at Recipharm and Benuvia (both third party vendors): The first step for us is to scale up the manufacturing process
of Ketamir-2. Small-scale synthesis has already been achieved at Recipharm, which will be essential for refining the process and identifying
potential challenges. This will be followed by a phase of process optimization, focusing on improving yield, purity, and cost-efficiency.
In order to determine the best manufacturer, we contracted with two manufacturers: Recipharm and Benuvia. Whichever company successfully
demonstrates the ability to manufacture 100 grams of Ketamir-2 efficiently and with a good purity will be the one that is contracted
to make kilogram quantities needed by Frontage for IND enabling safety and toxicology studies. Of note, Benuvia has expressed some interest
in not only manufacturing Ketamir-2, but potentially partnering in the development of this drug through their investment and collaboration.
This would obviously affect the decision about the ultimate manufacturer of Ketamir-2. We will then transition to pilot-scale production
to validate the manufacturing process under real-world conditions. Once the process is established, large-scale manufacturing can commence,
ensuring compliance with Good Manufacturing Practices (GMP) standards. Integral to this stage is the development of a robust supply chain
strategy to manage the consistent availability of raw materials and distribution.
IND-Enabling
Research at Frontage: Prior to IND submission, we must conduct comprehensive IND-enabling research. This includes pharmacokinetics/pharmacodynamics
(PK/PD) studies to understand how Ketamir-2 is absorbed, distributed, metabolized, and excreted, along with its mechanism of action.
Toxicology and tolerability studies are also crucial, encompassing both acute and chronic toxicology assessments in relevant animal models,
including 7 and 28-day studies in rats and dogs. Additionally, the development of a stable and effective formulation for Ketamir-2’s
oral administration is necessary.
IND
Submission for TRD Indication: With the data from preclinical studies in hand, we will prepare and submit an Investigational New
Drug (IND) application to the FDA. This submission will include all preclinical data and a proposed plan for clinical trials. A well-thought-out
regulatory strategy is essential to address potential queries and concerns from regulatory bodies.
Clinical
Trials - Phase 1 and 2: Upon IND approval, clinical development will proceed with Phase 1 trials, focusing on assessing the safety,
tolerability, and optimal dosing in a small group of healthy volunteers. This will be followed by Phase 2 trials, where the efficacy
of Ketamir-2 will be evaluated in a larger group of patients, along with further safety assessments.
Exit
Strategy: Given that we have already undergone an IPO, the exit strategy will focus on partnerships and licensing. We can explore
partnerships with larger pharmaceutical companies for further development and commercialization of Ketamir-2. Licensing agreements can
also be considered, allowing other companies to market Ketamir-2 in different regions or for varied indications. The following options
could be considered depending on the available opportunities:
| 1. | Strategic
Partnerships and Collaborations: This can involve partnering with larger pharmaceutical
companies, which brings the benefit of their extensive resources, global market reach, and
regulatory expertise. However, such partnerships often mean sharing profits and relinquishing
some control over the drug. Collaborations with biotechnology firms in similar therapeutic
areas can also be beneficial, offering synergistic research efforts and niche expertise,
though these firms may not provide as much financial support as larger pharma companies. |
| 2. | Licensing
Agreements: We could choose to license the drug to another company for further development
and commercialization. Out-licensing can provide an immediate capital infusion and reduce
the risk and investment required for later-stage trials. However, this often leads to losing
direct control over the development and commercialization processes. Co-development and co-marketing
deals are another form of licensing where the development, marketing, and commercialization
responsibilities are shared, which can combine strengths and reduce individual risks but
requires aligned objectives and effective collaboration. |
| 3. | Additional
Funding and Investment: Seeking additional capital through a Secondary Public Offering
(SPO) is a way to fund Phase 3 trials and marketing efforts but can dilute existing shareholders’
equity. Private investments and venture capital are also viable options, offering large sums
of capital and business expertise, though they may lead to a potential loss of autonomy and
come with high expectations for returns. |
Additionally,
the potential for a buyout from a larger pharmaceutical company remains a viable exit strategy, especially if Ketamir-2 demonstrates
substantial promise.
Throughout
this process, it is crucial for us to maintain a robust intellectual property strategy, regularly assess the antidepressant market landscape,
especially for treatment-resistant depression (TRD), and engage with key stakeholders. Implementing a risk management plan is also essential
to navigate potential development and commercialization challenges. This strategic plan must be adaptable, capable of responding to new
data, regulatory feedback, and changes in the market. Regular assessments and checkpoints will ensure the project aligns with our strategic
goals and the evolving landscape of pharmaceutical development.
Intellectual
Property
Our
company owns U.S. Patent 10,787,675 B2, titled “Purified Synthetic Marijuana and Methods of Treatment by Administering Same,”
which covers the MIRA1a compound per se as a racemic mixture, an isolated R-enantiomer, or an isolated S-enantiomer, as well as
pharmaceutical formulations of the compound. This patent also covers MIRA1a in methods of treating Alzheimer’s disease, anxiety,
depression, and addictions and expires on February 11, 2039.
Foreign
patents covering MIRA1a, and its therapeutic uses have issued in Australia, Belgium, Canada, Czech Republic, France, Germany, Greece,
Netherlands, Hungary, Ireland, Israel, Italy, Malta, Poland, Portugal, Romania, South Korea, Spain, Sweden, and the United Kingdom, and
corresponding applications are pending in China and Japan. MyMD currently owns these foreign patents and patent applications. We currently
have no plans to develop the MIRA1a compound for approval and commercialization outside of the United States or for manufacture outside
of the United States, including in the foreign jurisdictions in which MyMD has patent rights. We may in the future seek an agreement
to license or purchase all or a portion of such foreign patent rights from MyMD, but we have no current plans to do so and there is no
assurance that we would be able to successfully conclude such an agreement. MyMD’s foreign patent rights would not preclude us
from pursuing the development, manufacture, approval, or commercialization of the MIRA1a compound in foreign jurisdictions in which MyMD
does not have patent rights, such as India, if we chose in the future to pursue such activities. See “Risk Factors— Risks
Related to Our Intellectual Property— We own the rights associated with our patents in the United States, but we do not own the
rights to patents covering MIRA1a in foreign jurisdictions.”
Notwithstanding
the foregoing, we have a worldwide perpetual, royalty free, non-exclusive license from MyMD to use MyMD’s Supera-CBD™, a
different compound from MIRA1a, as a synthetic intermediate in the manufacture of MIRA1a for all purposes (including clinical development
and commercial production). In consideration of this license, we agreed to share with MyMD technical information and know-how that pertains
to the synthetic manufacture and/or formulation of our MIRA1a product candidate and granted a license to MyMD to use improvements to
MIRA1a made under the agreement, and the agreement does not involve any prior or future cash payments by us. Except for this license,
we do not license any patent rights or other intellectual property for MIRA1a from third parties. Although we believe that Supera-CBD
is currently the best available synthetic intermediate for the manufacture of MIRA1a, we believe that other intermediates and/or processes
could be used to manufacture MIRA1a.
Besides
relying on patents, we also rely on trade secrets, proprietary know-how and continuing innovation to develop and maintain our competitive
position, especially when we do not believe that patent protection is appropriate or can be obtained. We seek protection of these trade
secrets, proprietary know-how and any continuing innovation, in part, through confidentiality and proprietary information agreements.
However, these agreements may not provide meaningful protection for, or adequate remedies to protect, our technology in the event of
unauthorized use or disclosure of information. Furthermore, our trade secrets may otherwise become known to, or be independently developed
by, our competitors. We intend to seek appropriate patent protection for technology in our research and development programs, where applicable,
and their uses by filing patent applications in the United States and other selected countries. We intend for these patent applications
to cover, where possible, claims for compositions of matter, medical uses, processes for preparation and formulations.
We
license the U.S., Canadian, and Mexican patent rights for the use of KETAMIR-2 in human applications from MIRALOGX LLC, an intellectual
property development and holding company established by Jonnie R. Williams, Sr., the founder of our company and the sole inventor of
KETAMIR-2. MIRALOGX is owned by the Bay Shore Trust, an irrevocable trust established by Mr. Williams. MIRALOGX filed U.S. Provisional
App. No. 63/537,744 on September 11, 2023 and U.S. Provisional App. No. 63/451,891, on March 13, 2023, both titled, ANTIDEPRESSANT COMPOUNDS,
PHARMACEUTICAL COMPOSITIONS, AND METHODS OF TREATING DEPRESSION AND OTHER DISORDERS. MIRALOGX plans to file a corresponding international
application under the Patent Cooperation Treaty (PCT) in 2024 and in due course enter the national phase in the United States, Canada
and Mexico, among other countries. These applications, if granted and subject to payment of patent maintenance fees, would offer protection
extending through at least March 13, 2044. The patent rights for KETAMIR-2 outside of the United States, Canada, and Mexico are not included
in our current patent rights.
Our
license from MIRALOGX is set forth in the Exclusive License Agreement, dated November 15, 2023, pursuant to which the licensed field
of use includes therapeutic treatments and other medical or health uses in humans, and related preclinical studies and activities conducted
in furtherance of obtaining regulatory approval for and commercialization of human therapeutic treatments and uses (the “MIRALOGX
License Agreement”). “Licensed Product” is defined as a drug product containing as an active agent 2-(2-chlorophenyl)-2-(methylamino)cyclopentan-1-one
or a pharmaceutically acceptable salt or ester thereof. We also have the right to grant corresponding sublicenses under the licensed
patent rights. The MIRALOGX License Agreement provides for the payment to MIRALOGX of an 8% royalty (payable quarterly) on our net sales
of Licensed Products by us or our sublicensees and on non-royalty bearing milestone revenue, with the royalty obligation ceasing upon
the later of the expiration of the last-to-expire licensed patent. The agreement also provides for an up-front Cost Reimbursement of
$100,000 payable to MIRALOGX to cover the already-incurred costs associated with the patent rights. The Cost Reimbursement is the only
payment made to date under the agreement. MIRALOGX may terminate the agreement upon insolvency, an uncured breach including the failure
to make any payment owed under the agreement or the failure to use commercially reasonable efforts to develop the licensed product, or
upon a default of the November 15, 2023 Promissory Note and Loan Agreement. The MIRALOGX License Agreement provides that MIRALOGX will
have sole control over the filing, prosecution, maintenance, and management of the licensed patent rights, provided that we will be responsible
for the cost of prosecuting and maintaining the licensed patents. The agreement grants to us the primary right, but not the obligation,
to enforce the licensed patent rights.
Summary
Risk Factors
There
are a number of risks that you should understand before making an investment decision regarding this offering. These risks are discussed
more fully in the section entitled “Risk Factors” following this prospectus summary. If any of these risks actually occur,
our business, financial condition, or results of operations would likely be materially and adversely affected. In such a case, the trading
price of our common stock would likely decline, and you may lose all or part of your investment. These risks include, but are not limited
to:
| |
● |
We
are development-stage company that has no revenues and has incurred losses since our inception. We expect to incur losses for the
foreseeable future and may never achieve or maintain profitability. |
| |
|
|
| |
● |
Our
limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future
viability. |
| |
|
|
| |
● |
We
are dependent on the success of our product candidates, some of which may not receive regulatory approval or be successfully commercialized. |
| |
|
|
| |
● |
We
will need additional funds to complete the further development of our business plan, and there is no assurance that additional financing
will be available or will be available on terms acceptable to us. |
| |
|
|
| |
● |
Certain
of our executive officers are not employed by us on a full-time basis, and certain directors and officers may have actual or potential
conflicts of interest because of their positions with MyMD Pharmaceuticals, Inc. |
| |
|
|
| |
● |
Although
we own the patent relating to MIRA1a in the United States, foreign patents covering MIRA1a and its therapeutic uses held by MyMD
Pharmaceuticals, Inc. have issued in Australia, Belgium, Canada, Czech Republic, France, Germany, Greece, Netherlands, Hungary, Ireland,
Israel, Italy, Malta, Poland, Portugal, Romania, South Korea, Spain, Sweden, and the United Kingdom, and corresponding applications
are pending in China and Japan. |
| |
|
|
| |
● |
We
face risks related to health, pandemics, epidemics, and outbreaks, including the novel coronavirus (“COVID-19”), which
could significantly disrupt our pre-clinical studies and clinical trials, commercialization efforts, supply chain, regulatory and
clinical development activities, and other business operations, in addition to the impact of a global economic slowdown. |
| |
|
|
| |
● |
Results
of pre-clinical studies and future early clinical trials are not necessarily predictive indicators of future results. |
| |
|
|
| |
● |
We
may fail to expand our anticipated outsourced manufacturing capability in time to meet market demand for our products and product
candidates, and the FDA may refuse to accept the facilities of our contract manufacturers as being suitable to produce our products
and product candidates. Any problems in our manufacturing process could have a material adverse effect on our business, results of
operations and financial condition. |
| |
● |
Our
future success will largely depend on the success of our product candidates, which development will require significant capital resources
and years of clinical development effort. |
| |
|
|
| |
● |
There
is a high rate of failure for drug candidates proceeding through clinical trials. |
| |
|
|
| |
● |
The
legalization and use of medical and recreational marijuana in the U.S. and elsewhere may impact our business. |
| |
|
|
| |
● |
We
rely on, and expect to continue to rely on, third parties to conduct clinical trials for our product candidates. If these third parties
do not successfully carry out their contractual duties, comply with regulatory requirements or meet expected deadlines, we may not
be able to obtain marketing approval for or commercialize our product candidates, and our business could be substantially harmed. |
| |
|
|
| |
● |
We
rely on third parties to manufacture our clinical product supplies, and we intend to rely on third parties for at least a portion
of the manufacturing process of our product candidates, if approved. Our business could be harmed if those third parties fail to
provide us with sufficient quantities of product or fail to do so at acceptable quality levels or prices or fail to maintain or achieve
satisfactory regulatory compliance. |
| |
|
|
| |
● |
Even
if any of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians,
patients, third-party payors, and others in the medical community necessary for commercial success. |
| |
|
|
| |
● |
If
we are unable to obtain and maintain intellectual property protection for our technology and products, or if the scope of the intellectual
property protection obtained is not sufficiently broad, our competitors could commercialize technology and products similar or identical
to ours, and our ability to successfully commercialize our technology and products may be impaired. |
| |
|
|
| |
● |
Certain
recent initial public offerings of companies with relatively small public floats comparable to our anticipated public float have
experienced extreme volatility that was seemingly unrelated to the underlying performance of the respective company, and our securities
may potentially experience rapid and substantial price volatility, which may make it difficult for prospective investors to assess
the value of our securities. |
Implications
of Being an Emerging Growth Company
As
a company with less than $1.235 billion in annual gross revenue during our last fiscal year, we qualify as an “emerging growth
company” as defined in Section 2(a) of the Securities Act of 1933, as amended (the “Securities Act”). An emerging growth
company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies.
These provisions include:
| |
● |
we
are required to present only two years of audited financial statements and related management’s discussion and analysis of
financial condition and results of operations in the registration statement of which this prospectus is a part; |
| |
|
|
| |
● |
we
are exempt from compliance with the requirement that our independent registered public accounting firm provide an attestation report
on the effectiveness of our internal control over financial reporting; |
| |
|
|
| |
● |
we
are exempt from compliance with any requirement that the Public Company Accounting Oversight Board (the “PCAOB”) has
adopted regarding communication of critical accounting matters and may adopt regarding mandatory audit firm rotation or a supplement
to the auditor’s report providing additional information about the audit and the financial statements; |
| |
● |
we
are exempt from the “say on pay,” “say when on pay,” and “say on golden parachute” non-binding
advisory vote requirements; and |
| |
|
|
| |
● |
we
can provide reduced disclosures about our executive compensation arrangements. |
We
currently intend to take advantage of each of the exemptions described above. It is possible, therefore, that some investors will find
our common stock less attractive, which may result in a less active trading market for our common stock and higher volatility in our
stock price.
We
may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the completion of our
initial public offering or such an earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth
company upon the earliest of: (i) the last day of the first fiscal year in which our annual gross revenues are $1.235 billion or more;
(ii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt securities;
or (iii) the date on which we are deemed to be a “large accelerated filer,” which will occur as of the end of any fiscal
year in which we (x) have an aggregate market value of our common stock held by non-affiliates of $700 million or more as of the last
business day of our most recently completed second fiscal quarter, (y) have been required to file annual and quarterly reports under
the Securities Exchange Act of 1934, as amended (the “Exchange Act”), for a period of at least 12 months and (z) have filed
at least one annual report pursuant to the Exchange Act.
In
addition, emerging growth companies may take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities
Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain
accounting standards until those standards would otherwise apply to private companies. We intend to take advantage of the benefits of
this extended transition period. For risks related to our status as an emerging growth company, see “Risk Factors — Risks
Related to Ownership of Our Common Stock — Taking advantage of the reduced disclosure requirements applicable to
“emerging growth companies” may make our common stock less attractive to investors.”
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage
of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We
will remain a smaller reporting company until the last day of any fiscal year for so long as either: (i) the market value of our shares
of common stock held by non-affiliates does not equal or exceed $250 million as of the prior June 30th; or (ii) our annual
revenues did not equal or exceed $100 million during such completed fiscal year. To the extent we take advantage of such reduced disclosure
obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
Corporate
Information
Our
corporate headquarters is located at 855 N Wolfe Street, Suite 601, Baltimore, Maryland 21205. Our telephone number is 737-289-0835.
Our
principal website address is www.mirapharmaceuticals.com. The information contained on, or that can be accessed through, our website
is deemed not to be incorporated in this prospectus or to be part of this prospectus. You should not consider information contained on
our website to be part of this prospectus.
The
Offering
The
selling stockholders identified in this prospectus may offer and sell up to 1,700,000 shares of our common stock, as more fully described
below.
| Shares
of Common Stock being Offered by the Selling Stockholders |
|
Up
to 1,700,000 shares of common stock of MIRA Pharmaceuticals, Inc., par value $0.0001 per share, consisting of (i) 1,000,000 shares
that are issuable upon the exercise of a warrant issued by us to Bay Shore Trust in April 2023, and (ii) 700,000 shares that are
issuable upon the exercise of a warrant issued by us to MIRALOGX, LLC in November 2023. |
| |
|
|
| Shares
of Common Stock Outstanding prior to this Offering |
|
14,780,885 |
| |
|
|
| Shares
of Common Stock to be Outstanding after this Offering (1) |
|
16,480,885 |
| |
|
|
| Use
of Proceeds |
|
We
will not receive any proceeds from the sale by the selling stockholders of the shares of Common Stock offered by this prospectus.
We will receive proceeds in the event that the warrants are exercised at the respective exercise prices per share for cash, which
will result in gross proceeds of up to approximately $6.4 million. Any proceeds that we receive from the exercise of the warrants
will be used for general corporate purposes and for working capital purposes. See “Use of Proceeds” for additional information. |
| |
|
|
| Trading
Symbol of Common Stock |
|
MIRA |
| |
|
|
| Risk
Factors |
|
Investing
in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 31 of this prospectus for
a discussion of factors you should carefully consider before investing in our common stock. |
| (1) | The
number of shares of common stock to be outstanding after this offering is based on 14,780,885
shares of our common stock outstanding as of December 15, 2023, and excludes: |
| ● | 1,210,001
shares
of our common stock issuable upon the exercise of stock options outstanding as of December
15, 2023, under our 2022 Omnibus Incentive Plan (the “2022 Omnibus Plan”)
at a weighted-average exercise price of $5.00 per share; |
| ● | 789,999
shares
of our common stock reserved for future issuance under the 2022 Omnibus Plan, as well as
any automatic increases in the number of shares of common stock reserved for future issuance
under the plan; |
Summary
Financial Data
The
following tables summarize our financial data as of the dates and for the periods presented. We have derived the summary statements of
operations data for the years ended December 31, 2022 and 2021, and the balance sheet data as of December 31, 2022 and 2021, from our
audited financial statements included elsewhere in this prospectus. We have derived the summary statement of operations data for the
nine months ended September 30, 2023 and 2022, and the balance sheet data as of September 30, 2023, from our unaudited financial statements
included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the
future.
The
following summary financial and other data should be read in conjunction with the section titled “Management’s Discussion
and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere
in this prospectus.
Selected
Statement of Operations data:
| | |
Nine months ended September 30, | | |
Year ended December 31, | |
| | |
2023 | | |
2022 | | |
2022 | | |
2021 | |
| | |
(Unaudited) | | |
(Audited) | |
| Revenues | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating costs: | |
| | | |
| | | |
| | | |
| | |
| General and administrative expenses | |
| 2,144,832 | | |
| 736,059 | | |
| 2,992,125 | | |
| 770,115 | |
| Related party travel costs | |
| - | | |
| 357,350 | | |
| 1,704,350 | | |
| 697,600 | |
| Research and development expenses | |
| 1,015,252 | | |
| 714,968 | | |
| 2,351,465 | | |
| 684,447 | |
| Total operating costs | |
| 3,160,084 | | |
| 1,808,377 | | |
| 7,047,940 | | |
| 2,152,162 | |
| | |
| | | |
| | | |
| | | |
| | |
| Interest expense, net | |
| (427,732 | ) | |
| (2,307 | ) | |
| (10,250 | ) | |
| (24,374 | ) |
| Net loss | |
$ | (3,587,816 | ) | |
$ | (1,810,684 | ) | |
$ | (7,058,190 | ) | |
$ | (2,176,536 | ) |
Balance
Sheet data:
| | |
September 30, | | |
December 31, | | |
| |
| | |
2023 | | |
2022 | | |
2021 | |
| | |
(Unaudited) | | |
(Audited) | | |
| |
| ASSETS | |
| | | |
| | | |
| | |
| Current assets: | |
| | | |
| | | |
| | |
| Cash | |
$ | 5,868,330 | | |
$ | 350,978 | | |
$ | 2,809,552 | |
| Deferred offering costs | |
| - | | |
| 143,427 | | |
| 100,000 | |
| Prepaid expenses | |
| 202,817 | | |
| - | | |
| - | |
| Total current assets | |
| 6,071,147 | | |
| 494,405 | | |
| 2,909,552 | |
| | |
| | | |
| | | |
| | |
| Deferred financing costs, net | |
| 2,782,708 | | |
| - | | |
| - | |
| Operating lease, right of use assets | |
| 114,357 | | |
| 164,910 | | |
| - | |
| Related party operating lease, right of use assets | |
| - | | |
| 198,759 | | |
| - | |
| Due from related party | |
| 50,000 | | |
| - | | |
| - | |
| Advances to affiliates | |
| - | | |
| - | | |
| 445,612 | |
| Total assets | |
$ | 9,018,212 | | |
$ | 858,074 | | |
$ | 3,355,164 | |
| | |
| | | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | | |
| | |
| Trade accounts payable and accrued liabilities | |
$ | 779,573 | | |
$ | 811,738 | | |
$ | 228,406 | |
| Related party accounts payable | |
| - | | |
| 116,350 | | |
| 547,600 | |
| Related party line of credit | |
| - | | |
| 133,062 | | |
| 293,062 | |
| Related party accrued interest | |
| 14,472 | | |
| 34,987 | | |
| 24,738 | |
| Advances from affiliates | |
| - | | |
| - | | |
| - | |
| Current portion of operating lease liabilities | |
| 74,328 | | |
| 75,143 | | |
| - | |
| Related party current portion of operating lease liabilities | |
| - | | |
| 198,759 | | |
| - | |
| Total current liabilities | |
| 868,373 | | |
| 1,370,039 | | |
| 1,093,806 | |
| | |
| | | |
| | | |
| | |
| Non-current operating lease liabilities | |
| 34,528 | | |
| 84,267 | | |
| - | |
| | |
| | | |
| | | |
| | |
| Total liabilities | |
| 902,901 | | |
| 1,454,306 | | |
| 1,093,806 | |
| | |
| | | |
| | | |
| | |
| Stockholders’ Equity (Deficit) | |
| | | |
| | | |
| | |
| Preferred Stock, $0.0001 par value, 10,000,000 shares authorized and none issued or outstanding. - | |
| - | | |
| - | | |
| | |
| Common Stock, $0.0001 par value; 100,000,000 shares authorized, 14,780,885, 13,313,000 and 12,673,874 issued and outstanding at September 30, 2023, December 31, 2022 and December 31, respectively. | |
| 1,478 | | |
| 6,657 | | |
| 6,337 | |
| Additional paid-in capital | |
| 23,611,517 | | |
| 8,699,830 | | |
| 4,499,550 | |
| Accumulated deficit | |
| (15,497,684 | ) | |
| (9,302,719 | ) | |
| (2,244,529 | ) |
| Total stockholders’ equity (deficit) | |
| 8,115,311 | | |
| (596,232 | ) | |
| 2,261,358 | |
| Total liabilities and stockholders’ equity (deficit) | |
$ | 9,018,212 | | |
$ | 858,074 | | |
$ | 3,355,164 | |
RISK
FACTORS
Investing
in shares of our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below,
the section of this prospectus entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
and our financial statements and related notes included elsewhere in this prospectus before investing in shares of our common stock.
The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of,
or that we currently believe are not material, may also become important factors that affect us. If any of the following risks occur,
our business, operating results and prospects could be materially harmed. In that event, the price of our common stock could decline,
and you could lose part or all of your investment.
Risks
Related to Our Operations and Financial Condition
We
are an early development-stage company with no revenues.
As
an early development-stage enterprise that is focused on the development of a pre-clinical pharmaceutical product, we have generated
no revenue and have an accumulated deficit of $15.5 million through September 30, 2023 and $9.3 million through December 31, 2022. There
can be no assurance that sufficient funds required to pursue our development program will be generated from operations or that funds
will be available from external sources, such as debt or equity financings or other potential sources. The lack of additional capital
resulting from the inability to generate cash flow from operations, or to raise capital from external sources would force us to substantially
curtail or cease operations and would, therefore, have a material adverse effect on business. Furthermore, there can be no assurance
that any such required funds, if available, will be available on attractive terms or that they will not have a significant dilutive effect
on our existing stockholders.
We
seek to overcome the circumstances that impact our ability to remain a going concern in the future through the growth of revenues with
interim cash flow deficiencies being addressed through additional equity and debt financing. We anticipate raising additional funds through
public or private financing, strategic relationships, or other arrangements in the near future to support our business operations; however,
we may not have commitments from third parties for a sufficient amount of additional capital. We cannot be certain that any such financing
will be available on acceptable terms, or at all, and our failure to raise capital when needed could limit our ability to continue operations.
Our ability to obtain additional funding will determine our ability to continue as a going concern. Failure to secure additional financing
in a timely manner and on favorable terms would have a material adverse effect on our financial performance, results of operations and
stock price and require us to curtail or cease operations, sell off our assets, seek protection from our creditors through bankruptcy
proceedings, or otherwise. Furthermore, additional equity financing may be dilutive to the holders of our common stock, and debt financing,
if available, may involve restrictive covenants, and strategic relationships, if necessary, to raise additional funds, and may require
that we relinquish valuable rights.
Because
we have a limited operating history, you may not be able to accurately evaluate our operations.
We
have had limited operations to date. Therefore, we have a limited operating history upon which to evaluate the merits of investing in
our company. Potential investors should be aware of the difficulties normally encountered by new companies and the high rate of failure
of such enterprises. The likelihood of success must be considered in light of the problems, expenses, difficulties, complications, and
delays encountered in connection with the operations that we plan to undertake. These potential problems include, but are not limited
to, unanticipated problems relating to the ability to generate sufficient cash flow to operate our business, and additional costs and
expenses that may exceed current estimates. We expect to continue to incur significant losses into the foreseeable future. We recognize
that if the effectiveness of our business plan is not forthcoming, we will not be able to continue business operations. There is no history
upon which to base any assumption as to the likelihood that we will prove successful, and it is doubtful that we will generate any operating
revenues or ever achieve profitable operations. If we are unsuccessful in addressing these risks, our business will most likely fail.
We
are dependent on additional financing for the continuation of our operations.
Because
we have generated no revenues and currently operate at a loss, we are completely dependent on the continued availability of financing
in order to continue our business operations. There can be no assurance that financing sufficient to enable us to continue our operations
will be available to us in the future.
We
will need additional funds to complete further development of our business plan to achieve a sustainable level where ongoing operations
can be funded out of revenues. We expect that we have adequate resources to fund our operations and initial clinical development programs
through at least the fourth quarter of 2024. We will require further funding to fully implement our business plan to its fullest potential
and achieve our growth plans. There is no assurance that any additional financing will be available or if available, on terms that will
be acceptable to us.
Our
failure to obtain future financing or to produce levels of revenue to meet our financial needs could result in our inability to continue
as a going concern in the future and, as a result, our investors could lose their entire investment.
Our
operating results may fluctuate, which could have a negative impact on our ability to grow our client base, establish sustainable revenues
and succeed overall.
Our
results of operations may fluctuate as a result of a number of factors, some of which are beyond our control including but not limited
to:
| |
● |
general
economic conditions in the geographies and industries where we sell our services and conduct operations; legislative policies where
we sell our services and conduct operations; |
| |
|
|
| |
● |
the
budgetary constraints of our customers; seasonality; |
| |
|
|
| |
● |
success
of our strategic growth initiatives; |
| |
|
|
| |
● |
costs
associated with the launching or integration of new or acquired businesses; timing of new product introductions by us, our suppliers
and our competitors; product and service mix, availability, utilization and pricing; |
| |
|
|
| |
● |
the
mix, by state and country, of our revenues, personnel, and assets; movements in interest rates or tax rates; |
| |
|
|
| |
● |
changes
in, and application of, accounting rules; changes in the regulations applicable to us; and litigation matters. |
As
a result of these factors, we may not succeed in our business, and we could go out of business.
We
have yet to achieve a profit and may not achieve a profit in the near future, if at all.
We
have not yet produced any revenues or profit and may not in the near future, if at all. We cannot be certain that we will be able to
realize sufficient revenue to achieve profitability. Further, many of our competitors have a significantly larger industry presence and
revenue stream but have yet to achieve profitability. Our ability to continue as a going concern in the future is dependent upon raising
capital from financing transactions, increasing revenue and keeping operating expenses below our revenue levels in order to achieve positive
cash flows, none of which can be assured.
Certain
of our executive officers will not be employed by us on a full-time basis.
Erez
Aminov, our Chief Executive Officer, Dr. Adam Kaplin, our President and Chief Scientific Officer’, and Dr. Chris Chapman, our Executive
Chairman, will not be employed by our company on full-time basis. As provided in their respective employment agreements with our company,
Dr. Chapman, and Mr. Aminov are expected to devote approximately fifty percent (50%) of their business time to the affairs of our company.
Dr. Kaplin is a non-employee consultant to our company and provides consulting services and advice to our company on an at-will and as-needed
basis, and he is not obligated to expend a specific minimum number of hours on matters relating to our company. Because each of these
officers will not work full time for our company, instances may occur where he may not be immediately available to provide solutions
to problems or address concerns that arise in the course of us conducting our business and thus adversely affect our business. In addition,
they can become subject to conflicts of interest because they devote part of their working time to other business endeavors, including
consulting relationships with other entities, and have responsibilities to these other entities. Although such officers are aware of
their duties and accountability to our company and to applicable laws and policies relating to corporate opportunity and conflicts of
interest, such conflicts of interest may include deciding how much time to devote to our affairs, as well as what business opportunities
should be presented to us.
Certain
of our directors and officers may have actual or potential conflicts of interest because of their positions with MyMD.
Dr.
Adam Kaplin, our President and Chief Scientific Officer, continues to serve as the Chief Scientific Officer of MyMD. In addition, Dr.
Chris Chapman, our Executive Chairman, continue to serve as a director, President, and Chief Medical Officer of MyMD. Also, our CEO,
Erez Aminov, has provided services to MyMD from time to time. Although these persons are not full-time employees of MyMD, it is possible
that the amount of time that they expend on their work for MyMD may adversely impact the amount of time that they can spend on their
work for our company. These persons also own MyMD common stock and options to purchase MyMD common stock. Their respective positions
at MyMD and the ownership of any MyMD equity or equity awards creates, or may create the appearance of, conflicts of interest when these
individuals are faced with decisions that could have different implications for MyMD than the decisions have for us. Furthermore, as
MyMD holds the patent rights to the MIRA1a compound in foreign jurisdictions and in light of the license agreement we have with MyMD,
if a dispute were to arise between MyMD and our company relating to our past or future relationship with MyMD or with respect to intellectual
property matters, these potential conflicts of interest may make it more difficult for us to favorably resolve such disputes.
Risks
Relating to Our Business and Our Industry
Our
future success will largely depend on the success of MIRA1a and any future product candidates, which development will require significant
capital resources and years of clinical development effort.
We
currently have no drug products on the market, and all of our drug development projects are in a pre-clinical stage of development. Our
business depends almost entirely on the successful pre-clinical and clinical development, FDA regulatory approval, and commercialization
of our product candidates, principally MIRA1a. Investors need to be aware that substantial additional investments including pre-clinical
and clinical development and FDA regulatory submission and approval efforts will be required before we are permitted to undertake clinical
studies and market and commercialize our product candidates, if ever. It may be several years before we can commence clinical trials,
if ever. Any clinical trial will be subject to extensive and rigorous review and regulation by numerous government authorities in the
United States and other jurisdictions where we intend, if approved, to market our product candidates. Before obtaining regulatory approvals
for any of our product candidates, we must demonstrate through pre-clinical testing and clinical trials that the product candidate is
safe and effective for its specific application. This process can take many years and may include post- marketing studies and surveillance,
which would require the expenditure of substantial resources. Of the large number of drugs in development for approval in the United
States (and the rest of the world), only a small percentage will successfully complete the FDA regulatory approval financing to fund
our planned research, development, and clinical programs, we cannot assure you that any of our product candidates will be successfully
developed or commercialized.
We
may be unable to formulate or scale up any or all of our product candidates. There is no guarantee that any of the product candidates
will be or are able to be manufactured or produced in a manner to meet the FDA’s criteria for product stability, content uniformity
and all other criteria necessary for product approval in the United States and other markets. Any of our product candidates may fail
to achieve their specified endpoints in clinical trials.
Furthermore,
product candidates may not be approved even if they achieve their specified endpoints in clinical trials. The FDA may disagree with our
trial design and our interpretation of data from clinical trials or may change the requirements for approval even after it has reviewed
and commented on the design for our clinical trials. The FDA may also approve a drug for fewer or more limited indications than we request
or may grant approval contingent on the performance of costly post-approval clinical trials (i.e., Phase IV trials). In addition, the
FDA may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of our product
candidates.
If
we are unable to obtain regulatory approval for MIRA1a within the timeline we anticipate, we will not be able to execute our business
strategy effectively and our ability to substantially grow our revenues will be limited, which would have a material adverse impact on
our long-term business, results of operations, financial condition, and prospects.
We
are dependent on the success of our current and future product candidates, some of which may not receive regulatory approval or be successfully
commercialized.
Our
success will depend on our ability to successfully commercialize our product candidates. Our ability to successfully commercialize our
product candidates will depend on, among other things, our ability to:
| |
● |
successfully
complete pre-clinical and other nonclinical studies and clinical trials; |
| |
|
|
| |
● |
receive
regulatory approvals from the FDA; |
| |
|
|
| |
● |
produce,
through a validated process, in manufacturing facilities inspected and approved by regulatory authorities, including the FDA, sufficiently
large quantities of product candidates to permit successful commercialization; |
| |
|
|
| |
● |
obtain
reimbursement from payers such as government health care programs and insurance companies and achieve commercially attractive levels
of pricing; |
| |
|
|
| |
● |
secure
acceptance of our product candidates from physicians, health care payers, patients, and the medical community; |
| |
|
|
| |
● |
create
positive publicity surrounding our product candidates; |
| |
|
|
| |
● |
manage
our spending as costs and expenses increase due to clinical trials and commercialization; and |
| |
|
|
| |
● |
obtain
and enforce sufficient intellectual property for our product candidates. |
Our
failure or delay with respect to any of the factors above could have a material adverse effect on our business, results of operations
and financial condition.
Our
business may be materially and adversely affected in the future by the evolving effects of the COVID-19 pandemic as a result of the current
and potential future impacts on our commercialization efforts, supply chain, regulatory and clinical development activities, and other
business operations, in addition to the impact of a global economic slowdown.
Our
business could be materially and adversely affected in the future by the evolving effects of the COVID-19 pandemic. If we are unable
to obtain adequate supplies of personal protective equipment due to shortages or encounter other challenges related to the evolving COVID-19
pandemic, we may have to place or may experience additional limitations on our in-person activities. In addition, our increased reliance
on personnel working from home may negatively impact productivity or disrupt, delay or otherwise adversely impact our business. This
could also increase our cybersecurity risk, create data accessibility concerns, and make us more susceptible to communication disruptions,
any of which could adversely impact our business operations. Impacts related to the COVID-19 pandemic could materially and adversely
affect our business, our ability to generate sales of and revenues from our approved products, and our ability to advance the development
of our products and product candidates, as described elsewhere in this “Risk Factors” section. The magnitude of such impacts
will depend, in large part, on the ultimate duration and severity of the evolving effects of the COVID-19 pandemic.
The
effects of the COVID-19 pandemic continue to rapidly evolve. These effects have increased market volatility and could result in a significant
long-term disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect
our liquidity. In addition, market corrections resulting from the effects of the COVID-19 pandemic could materially affect our business
and the value of our common stock. The extent to which the evolving effects of the COVID-19 pandemic impact our business, our ability
to generate sales of and revenues from our approved products, and our clinical development and regulatory efforts will depend on future
developments that are highly uncertain and cannot be predicted with confidence, such as the ultimate duration and severity of the pandemic,
government actions, such as travel restrictions, quarantines and social distancing requirements in the U.S. and in other countries, business
closures or business disruptions and the effectiveness of actions taken in the U.S. and in other countries to contain and treat the disease.
Accordingly, we do not yet know the full extent of potential delays or impacts on our business, sales of our products, our clinical and
regulatory activities, our research programs, healthcare systems or the global economy as a whole. However, these effects could materially
and adversely affect our business, financial condition, results of operations and growth prospects. In addition, to the extent the evolving
effects of the COVID-19 pandemic adversely affect our business, financial condition, results of operations and growth prospects, they
may also have the effect of heightening many of the other risks and uncertainties described elsewhere in this “Risk Factors”
section. It is also possible that future global pandemics could also occur and also materially and adversely affect our business, financial
condition, results of operations and growth prospects.
Results
of pre-clinical studies and earlier clinical trials are not necessarily predictive indicators of future results.
Any
positive results from future pre-clinical testing of our product candidates and potential future clinical trials may not necessarily
be predictive of the results from Phase I, Phase II or Phase III clinical trials. In addition, our interpretation of results derived
from clinical data or our conclusions based on our pre-clinical data may prove inaccurate. Frequently, pharmaceutical and biotechnology
companies have suffered significant setbacks in clinical trials after achieving positive results in pre-clinical testing and early phase
clinical trials, and we cannot be certain that we will not face similar setbacks. These setbacks may be caused by the fact that pre-clinical
and clinical data can be susceptible to varying interpretations and analyses. Furthermore, certain product candidates may perform satisfactorily
in pre-clinical studies and clinical trials, but nonetheless fail to obtain FDA approval or appropriate approvals by the appropriate
regulatory authorities in other countries. If we fail to produce positive results in our clinical trials for our product candidates,
the development timeline and regulatory approval and commercialization prospects for them and as a result our business and financial
prospects, would be materially adversely affected.
We
have limited marketing experience, and we do not anticipate at this time establishing a sales force or distribution and reimbursement
capabilities, and we may not be able to successfully commercialize any of our product candidates if they are approved in the future.
Our
ability to generate revenues ultimately depends on our ability to sell our approved products and secure adequate third-party reimbursement.
We currently have limited experience in marketing and selling our products. We currently do not have any products approved for sale in
the United States or in any other country.
The
commercial success of our product candidates will depend on a number of factors beyond our control, including the willingness of physicians
to prescribe our products to patients, payers’ willingness and ability to pay for the drugs, the level of pricing achieved, patients’
response to our drugs and the ability of our marketing partners to generate sales. There can be no guarantee that we will be able to
establish or maintain the personnel, systems, arrangements and capabilities necessary to successfully commercialize MIRA1a or any product
candidate approved by the FDA in the future. If we fail to establish or maintain successful marketing, sales and reimbursement capabilities
or fail to enter into successful marketing arrangements with third parties, our product revenues may suffer.
Should
we later determine it is in our best interest to develop a sales force we may be unable to effectively train and equip our sales force,
therefore our ability to successfully commercialize our products may be harmed.
We
will be required to expend significant time and resources to train our sales force to be credible, persuasive and compliant with applicable
laws in marketing MIRA1a or our other product candidates to physicians for their approved uses. In addition, we must continue to train
our sales force to ensure that a consistent and appropriate message about MIRA1a or our other product candidates are being delivered
to our potential customers. If we are unable to effectively train our sales force and equip them with effective materials, including
medical and sales literature to help them inform and educate potential customers about the benefits of MIRA1a and our product candidates
and its proper administration, our efforts to successfully commercialize MIRA1a and our product candidates could be jeopardized, which
would negatively impact our ability to generate product revenues.
We
will need to further increase the size and complexity of our organization in the future, and we may experience difficulties in managing
our growth and executing our growth strategy.
Our
management and personnel, systems, and facilities currently in place may not be adequate to support our business plan and future growth.
As a result, we may need to further expand certain areas of our organization.
Our
need to effectively manage our operations, growth and various projects requires that we:
| |
● |
continue
to improve our operational, financial, management and regulatory compliance controls and reporting systems and procedures; |
| |
|
|
| |
● |
attract
and retain enough talented employees; |
| |
|
|
| |
● |
manage
our clinical trials effectively; |
| |
|
|
| |
● |
manage
our external manufacturing operations with contract research organizations effectively and in a cost-effective manner; |
| |
|
|
| |
● |
manage
our development efforts effectively while carrying out our contractual obligations to contractors and other third parties; and |
In
addition, we may utilize the services of part-time outside consultants and contractors to perform several tasks for us, including tasks
related to compliance programs, clinical trial management, regulatory affairs, formulation development and other drug development functions.
Our growth strategy may entail expanding our use of consultants and contractors to implement these and other tasks going forward. If
we are not able to effectively expand our organization by hiring new employees and expanding our use of consultants and contractors,
we may be unable to successfully implement the tasks necessary to effectively execute on our planned research, development, manufacturing,
and commercialization activities and, accordingly, may not achieve our research, development and commercialization goals.
Our
product candidates, if approved, may be unable to achieve the expected market acceptance and, consequently, limit our ability to generate
revenue from new products.
Even
when product development is successful and regulatory approval has been obtained, our ability to generate sufficient revenue depends
on the acceptance of our products by physicians and patients. We cannot assure you that our product candidates will achieve the expected
level of market acceptance and revenue if and when they obtain the requisite regulatory approvals. The market acceptance of any product
depends on a number of factors, including the indication statement and warnings required by regulatory authorities in the product label.
Market acceptance can also be influenced by continued demonstration of efficacy and safety in commercial use, physicians’ willingness
to prescribe the product, reimbursement from third-party payers such as government health care programs and private third-party payers,
the price of the product, the nature of any post-approval risk, management activities mandated by regulatory authorities, competition,
and marketing and distribution support. Further, an ineffective or inefficient distribution model at launch may lead to the inability
to fulfill demand, and consequently a loss of revenue. Any factors preventing or limiting the market acceptance of our products could
have a material adverse effect on our business, results of operations and financial condition.
If
the price for any future approved products decreases or if government and other third-party payers do not provide coverage and adequate
reimbursement levels, our revenue and prospects for profitability will suffer.
Patients
who are prescribed medicine for the treatment of their conditions generally rely on third-party payers to reimburse all or part of the
costs associated with their prescription drugs. Reimbursement systems in international markets vary significantly by country and by region,
and reimbursement approvals generally must be obtained on a country-by-country basis. Coverage and adequate reimbursement from governmental
healthcare programs, such as Medicare and Medicaid, and commercial payers is critical to new product acceptance. Coverage decisions may
depend upon clinical and economic standards that disfavor new drug products when more established or lower-cost therapeutic alternatives
are already available or subsequently become available. Even if we obtain coverage for products we may market, the resulting reimbursement
payment rates may require co-payments that patients find unacceptably high. Patients may not use our products if coverage is not provided,
or reimbursement is inadequate to cover a significant portion of its cost.
In
addition, the market for our products will depend significantly on access to third-party payers’ drug formularies or lists of medications
for which third-party payers provide coverage and reimbursement. The industry competition to be included in such formularies often leads
to downward pricing pressures on pharmaceutical companies. Also, third-party payers may refuse to include a particular branded drug in
their formularies or otherwise restrict patient access to a branded drug when a less costly generic equivalent or other alternative is
available, even if not approved for the indications for which our products are approved.
Third-party
payers or governmental or commercial entities are developing increasingly sophisticated methods of controlling healthcare costs. The
current environment is putting pressure on companies to price products below what they may feel is appropriate. Selling our products
at less than an optimized price could impact our revenues and overall success as a company. It will be difficult to determine the optimized
price for our products. In addition, in the U.S., no uniform policy of coverage and reimbursement for drug products exists among third-party
payers. Therefore, coverage and reimbursement for our products may differ significantly from payer to payer. As a result, the coverage
determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for
the use of our products to each payer separately, with no assurance that coverage will be obtained. If we are unable to obtain coverage
of, and adequate payment levels for, products we may market to third-party payers, physicians may limit how much or under what circumstances
they will prescribe or administer them, and patients may decline to purchase them. This in turn could affect our ability to successfully
commercialize products we may market, and thereby adversely impact our profitability, results of operations, financial condition, and
future success.
In
addition, where we have chosen to collaborate with a third party on product candidate development and commercialization, our partner
may elect to reduce the price of our products in order to increase the likelihood of obtaining reimbursement approvals. In many countries,
products cannot be commercially launched until reimbursement is approved and the negotiation process in some countries can exceed 12
months. In addition, pricing and reimbursement decisions in certain countries can be affected by decisions taken in other countries,
which can lead to mandatory price reductions and/or additional reimbursement restrictions across a number of other countries, which may
thereby adversely affect our sales and profitability. In the event that countries impose prices that are not sufficient to allow us or
our partners to generate a profit, our partners may refuse to launch the product in such countries or withdraw the product from the market,
which would adversely affect sales and profitability. Events, such as price decreases, government mandated rebates or unfavorable reimbursement
decisions, could affect the pricing and reimbursement of MIRA1a and our other product candidates and could have a material adverse effect
on our business, reputation, results of operations and financial condition.
We
expect to face intense competition, often from companies with greater resources and experience than we have.
Demand
for synthetic cannabinoids such as MIRA1a, will likely be dependent on a number of social, political, legislative, and economic factors
that are beyond our control. While we believe that there will be a demand for such drugs, and that the demand will grow, there is no
assurance that such demand will happen, that we will benefit from any demand or that our business, in fact, will ever generate revenues
from our drug development programs or become profitable.
The
emerging markets for synthetic cannabinoids and medical research and development is and will likely remain competitive. The development
and commercialization of drugs and medicines is highly competitive. We compete with a variety of multinational pharmaceutical companies
and specialized biotechnology companies, as well as products and processes being developed by universities and other research institutions.
Many of our competitors have developed, are developing, or will develop drugs and processes which may be competitive with our drug candidates.
Competitive therapeutic treatments include those that have already been approved by medicines regulators and accepted by the medical
community and any new treatments that may enter the market. For some of our drug development programs / areas of therapeutic interest,
other treatment options are currently available, under development, and may become commercially available in the future. If any of our
product candidates are approved for the diseases and conditions we are currently pursuing, they may compete with a range of medicines
or therapeutic treatments that are either in development, will be developed in the future or currently marketed.
Established
companies may have a competitive advantage over us due to their size and experiences, financial resources, and institutional networks.
Many of our competitors may have significantly greater financial, technical, and human resources than we do. Due to these factors, our
competitors may have an advantage in marketing their approved drugs and may obtain regulatory approval of their drug candidates before
we are able to, which may limit our ability to develop or commercialize our drug candidates. Our competitors may also develop drugs /
medicines that are safer, more effective, more widely used and less expensive than ours. These advantages could materially impact our
ability to develop and, if approved, commercialize our product candidates successfully. Furthermore, some of these competitors may make
acquisitions or establish collaborative relationships among themselves or with third parties to increase their ability to rapidly gain
market share.
Our
product candidates may compete with other synthetic cannabinoids, as well as with cannabinoid or cannabis-based drugs, in addition to
competing with state-licensed medical and recreational marijuana, in markets where the recreational and/or medical use of marijuana is
legal. There is continuing support in the U.S. for further state legalization of marijuana. In markets where recreational and/or medical
marijuana is not legal, our product candidates, once approved by regulators, may compete with marijuana or marijuana-based products purchased
in the illegal drug market. This may or may not affect the commercial price that we may be able to achieve for our synthetic regulatory-approved
medicines, should they be approved by the FDA.
Moreover,
as generic versions of drug products enter the market, the price for such medicines may be expected to decline rapidly and substantially.
Even if we are the first to obtain FDA approval of one of our product candidates, the future potential approval of generics could adversely
affect the price we are able to charge, and the profitability of our product(s) will likely decline.
Mergers
and acquisitions in the pharmaceutical and biotechnology industries may result in more resources being concentrated among a smaller number
of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative
arrangements with large and established companies.
These
companies may compete with us in recruiting and retaining qualified scientific, management and commercial personnel, utilizing contract
manufacturing facilities or contract research organizations (CROs), or establishing clinical trial sites and subject registration for
clinical trials, as well as in acquiring technologies complementary to our research projects.
Product
shipment delays could have a material adverse effect on our business, results of operations and financial condition.
The
shipment, import and export of MIRA1a and our other product candidates require import and export licenses. In the U.S., FDA, U.S. Customs
and Border Protection and the DEA, and in other countries similar regulatory authorities, regulate the import and export of pharmaceutical
products that contain controlled substances. Specifically, the import and export process require the issuance of import and export licenses
by the relevant controlled substance authority in both the importing and exporting country. We may not be granted, or if granted, maintain,
such licenses from the authorities in certain countries. Even if we obtain the relevant licenses, shipments of MIRA1a and our product
candidates may be held up in transit, which could cause significant delays and may lead to product batches being stored outside required
temperature ranges. Inappropriate storage may damage the product shipment resulting in a partial or total loss of revenue from one or
more shipments of MIRA1a or our other product candidates. A partial or total loss of revenue from one or more shipments of MIRA1a or
our other product candidates could have a material adverse effect on our business, results of operations and financial condition. Even
though the DEA has confirmed in writing that it conducted a scientific review of the chemical structure of MIRA1a in accordance with
the definitions within the CSA and its implementing regulations and determined that MIRA1a is not a controlled substance or listed chemical,
there is no assurance that the DEA may not change its position.
Problems
in our manufacturing process, failure to comply with manufacturing regulations or unexpected increases in our manufacturing costs could
harm our business, results of operations and financial condition.
The
manufacturing of our product candidates necessitates compliance with cGMP and other regulatory requirements in jurisdictions internationally.
We must ensure chemical consistency among our batches of products, including clinical batches and, if approved, marketing batches. Demonstrating
such consistency may require typical manufacturing controls as well as clinical data. We must also ensure that our batches conform to
complex release specifications. If we are unable to manufacture our product candidates in accordance with regulatory specifications,
including cGMP, or if there are disruptions in our manufacturing process due to damage, loss or otherwise, or failure to pass regulatory
inspections of our manufacturing facilities, we may not be able to meet current demand or supply sufficient product for use in clinical
trials, and this may also harm our ability to commercialize our product candidates on a timely or cost-competitive basis, if at all.
We
may fail to expand our manufacturing capability in time to meet market demand for our products and product candidates, and the FDA may
refuse to accept our facilities or those of our contract manufacturers as being suitable for the production of our products and product
candidates. Any problems in our manufacturing process could have a material adverse effect on our business, results of operations and
financial condition.
In
addition, before we can begin commercial manufacture of any product candidates for sale in the U.S., we must obtain FDA regulatory approval
for the product, which requires a successful FDA inspection of our manufacturing facilities and those of our contract manufacturers,
processes, and quality systems in addition to other product-related approvals. Although we may successfully navigate this pre-approval
inspection process as it relates in the U.S., pharmaceutical manufacturing facilities are continuously subject to post-approval inspection
by the FDA and foreign regulatory authorities. Due to the complexity of the processes used to manufacture our product candidates, we
may be unable to initially or continue to pass federal, state or international regulatory inspections in a cost-effective manner. If
we are unable to comply with manufacturing regulations, we may be subject to fines, unanticipated compliance expenses, recall or seizure
of any approved products, total or partial suspension of production and/or enforcement actions, including injunctions, and criminal or
civil prosecution. These possible sanctions would adversely affect our business, results of operations and financial condition.
Business
interruptions could delay us in the process of developing our product candidates and could disrupt our product sales.
Our
research and development activities are conducted through outside contractors and manufacturers. Loss of our contracted manufacturing
facilities, stored inventory or laboratory facilities through fire, theft or other causes, or loss of our raw material, could have an
adverse effect on our ability to continue product development activities and to conduct our business. Failure to supply our partners
with commercial product may lead to adverse consequences, including the right of partners to take over responsibility for product supply.
We currently do not have insurance coverage to compensate us for such business interruptions. Our contract manufacturers and suppliers
provide that in their separate operations; however, such coverage may prove insufficient to fully compensate us for the damage to our
business resulting from any significant property or casualty loss to those facilities.
We
have significant and increasing liquidity needs and may require additional funding.
Our
operations have consumed substantial amounts of cash since inception. For the nine months ended September 30, 2023, we reported a net
operating cash outflow of $3.5 million and a net cash inflow from investing activities of $9.0 million. For the year ended December 31,
2022, we reported a net operating cash outflow of $5.6 million and a net cash inflow from investing activities of $3.1 million. For the
year ended December 31, 2021, we reported a net operating cash outflow of $1.4 million and a net cash inflow from investing activities
of $4.2 million.
Research
and development, and general and administrative expenses, and cash used for operations will continue to be significant and may increase
substantially in the future in connection with new research and development initiatives and continued product commercialization efforts.
We may need to raise additional capital to fund our operations, continue to conduct clinical trials to support potential regulatory approval
of marketing applications and to fund commercialization of our products.
The
amount and timing of our future funding requirements will depend on many factors, including, but not limited to:
| ● |
the
timing of FDA approval, if any; |
| |
|
| ● |
the
DEA continuing to classify MIRA1a as a substance not subject to CSA; |
| |
|
| ● |
the
timing and amount of revenue from sales of our products, or revenue from grants or other sources; |
| |
|
| ● |
the
rate of progress and cost of our clinical trials and other product development programs; |
| |
|
| ● |
costs
of establishing or outsourcing sales, marketing, and distribution capabilities; |
| |
|
| ● |
costs
and timing of completion of expanded in-house manufacturing facilities as well as any outsourced commercial manufacturing supply
arrangements for our product candidates; |
| |
|
| ● |
costs
of filing, prosecuting, defending, and enforcing any patent claims and other intellectual property rights associated with our product
candidates; |
| |
|
| ● |
costs
of operating as a U.S. public company; |
| |
|
| ● |
the
effect of competing technological and market developments; |
| |
|
| ● |
personnel,
facilities, and equipment requirements; and |
| |
|
| ● |
the
terms and timing of any additional collaborative, licensing, co-promotion, or other arrangements that we may establish. |
While
we expect to fund our future capital requirements from a number of sources including existing cash balances, future cash flows from operations
and the proceeds from further public offerings, we cannot assure you that any of these funding sources will be available to us on favorable
terms, or at all. Further, even if we can raise funds from all of the above sources, the amounts raised may not be sufficient to meet
our future capital requirements.
Operating
results may vary significantly in future periods.
Our
expenses and operating results have fluctuated in the past and our revenues, expenses, and operating results are likely to fluctuate
significantly in the future. Our financial results are unpredictable and may fluctuate, for among other reasons, due to:
| |
● |
commercial
sales of our products; |
| |
|
|
| |
● |
our
achievement of product development objectives and milestones; |
| |
|
|
| |
● |
clinical
trial enrollment and expenses; |
| |
|
|
| |
● |
research
and development expenses; and |
| |
|
|
| |
● |
the
timing and nature of contract manufacturing and contract research payments. |
A
high portion of our costs are predetermined on an annual basis, due in part to our significant research and development costs. Thus,
small declines in revenue could disproportionately affect financial results in a quarter. Because of these factors, our financial results
in one or more future quarters may fail to meet the expectations of securities analysts or investors, which could cause our share price
to decline.
If
product liability lawsuits are successfully brought against us, we will incur substantial liabilities and may be required to limit the
commercialization of MIRA1a and our product candidates.
Although
we have never had any product liability claims or lawsuits brought against us, we face potential product liability exposure related to
the testing of our product candidates in human clinical trials. We may face exposure to claims by an even greater number of persons when
we begin to market and distribute our products commercially in the U.S., Europe and elsewhere. Now, and in the future, an individual
may bring a liability claim against us alleging that MIRA1a or one of our product candidates caused an injury. While we continue to take
what we believe are appropriate precautions, we may be unable to avoid significant liability if any product liability lawsuit is brought
against us. Large judgments have been awarded in class action or individual lawsuits based on drugs that had unanticipated side effects.
If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities. Regardless of merit
or eventual outcome, liability claims may result in:
| |
● |
decreased
demand for MIRA1a and our product candidates if such product candidates are approved; |
| |
|
|
| |
● |
injury
to our reputation; |
| |
|
|
| |
● |
withdrawal
of clinical trial participants; |
| |
|
|
| |
● |
costs
of related litigation; |
| |
|
|
| |
● |
substantial
monetary awards to patients and others; |
| |
|
|
| |
● |
increased
cost of liability insurance; |
| |
|
|
| |
● |
loss
of revenue; and |
| |
|
|
| |
● |
the
inability to successfully commercialize our products. |
Counterfeit
versions of our products could harm our business.
Counterfeiting
activities and the presence of counterfeit products in a number of markets and over the Internet continue to be a challenge for maintaining
a safe drug supply for the pharmaceutical industry. Counterfeit products are frequently unsafe or ineffective and can be life-threatening.
To distributors and users, counterfeit products may be visually indistinguishable from the authentic version. Reports of adverse reactions
to counterfeit drugs along with increased levels of counterfeiting could be mistakenly attributed to the authentic product, affect patient
confidence in the authentic product and harm the business of companies such as ours. If our products were to be the subject of counterfeits,
we could incur reputational and financial harm.
We
depend upon our key personnel and our ability to attract and retain employees.
Our
future growth and success depend on our ability to recruit, retain, manage, and motivate our employees. The inability to hire or retain
experienced management personnel could adversely affect our ability to execute our business plan and harm our operating results. Due
to the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific,
technical, and managerial personnel. The competition for qualified personnel in the pharmaceutical field is intense. Due to this intense
competition, we may be unable to continue to attract and retain the qualified personnel necessary for the development of our business
or to recruit suitable replacement personnel.
Our
employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We
are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with
FDA or foreign regulations, provide accurate information to FDA or other regulatory authorities, comply with applicable manufacturing
standards, comply with other foreign, federal, and state laws and regulations, report information or data accurately or disclose unauthorized
activities to us. Employee misconduct could also involve the improper use of information, including information obtained during clinical
trials, or illegal appropriation of drug products, which could result in government investigations and serious harm to our reputation.
The precautions we take to detect and prevent these prohibited activities may not be effective in controlling unknown or unmanaged risks
or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such
laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our
rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
We
are subject to the U.S. Foreign Corrupt Practices Act and other anti-corruption laws, as well as export control laws, customs laws, sanctions
laws and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties,
other remedial measures, and legal expenses, which could adversely affect our business, results of operations and financial condition.
Our
operations are subject to anti-corruption laws, including the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”),
and other anti-corruption laws that apply in countries where we do business. The FCPA and these other laws generally prohibit us and
our employees and intermediaries from bribing, being bribed or making other prohibited payments to government officials or other persons
to obtain or retain business or gain some other business advantage. We and our commercial partners operate in a number of jurisdictions
that pose a high risk of potential FCPA violations, and we participate in collaborations and relationships with third parties whose actions
could potentially subject us to liability under the FCPA or local anti-corruption laws. In addition, we cannot predict the nature, scope,
or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws
might be administered or interpreted.
We
are also subject to other laws and regulations governing our international operations, including regulations administered by the government
of the U.S. and other countries in which we operate or plan to operate, including applicable export control regulations, economic sanctions
on countries and persons, customs requirements, and currency exchange regulations, (collectively referred to as the “Trade Control
laws”).
However,
there is no assurance that we will be completely effective in ensuring our compliance with all applicable anti-corruption laws, including
the FCPA or other legal requirements, including Trade Control laws. If we are not in compliance with the FCPA and other anti-corruption
laws or Trade Control laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures,
and legal expenses, which could have an adverse impact on our business, financial condition, results of operations and liquidity, as
well as our reputation. Likewise, any investigation of any potential violations of the FCPA, other anti-corruption laws or Trade Control
laws by the U.S. or other authorities could also have an adverse impact on our reputation, our business, results of operations and financial
condition.
Our
proprietary information, or that of our customers, suppliers, and business partners, may be lost or we may suffer security breaches.
In
the ordinary course of our business, we will collect and store sensitive data, including valuable and commercially sensitive intellectual
property, clinical trial data, our proprietary business information and that of our customers, suppliers and business partners, and personally
identifiable information of our customers, clinical trial subjects and employees, and patients, in our data centers, on our networks,
and with our third-party cloud service providers. The secure processing, maintenance and transmission of this information is critical
to our operations. Despite our security measures, our information technology and infrastructure, and that of our third parties, may be
vulnerable to attacks by hackers or breached due to employee error, malfeasance, or other disruptions. Any breach could compromise our
networks and the information stored there could be accessed, publicly disclosed, lost, or stolen. Any such access, disclosure or other
loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information,
regulatory penalties, disrupt our operations, damage our reputation, and cause a loss of confidence in our products and our ability to
conduct clinical trials, which could adversely affect our business and reputation and lead to delays in gaining regulatory approvals
for MIRA1a or other product candidates.
Failure
of our information technology systems, including cybersecurity attacks or other data security incidents, could significantly disrupt
the operation of our business.
Our
business is increasingly dependent on critical, complex, and interdependent information technology (“IT”) systems, including
internet-based systems, some of which are managed or hosted by third parties, to support business processes as well as internal and external
communications. The size and complexity of our IT systems make us potentially vulnerable to IT system breakdowns, malicious intrusion,
and computer viruses, which may result in the impairment of our ability to operate our business effectively.
We
are continuously evaluating and, where appropriate, enhancing our IT systems to address our planned growth, including to support our
planned manufacturing operations. There are inherent costs and risks associated with implementing the enhancements to our IT systems,
including potential delays in access to, or errors in, critical business and financial information, substantial capital expenditures,
additional administrative time and operating expenses, retention of sufficiently skilled personnel to implement and operate the enhanced
systems, demands on management time, and costs of delays or difficulties in transitioning to the enhanced systems, any of which could
harm our business and results of operations. In addition, the implementation of enhancements to our IT systems may not result in productivity
improvements at a level that outweighs the costs of implementation, or at all. In addition, our systems and the systems of our third-party
providers and collaborators are potentially vulnerable to data security breaches which may expose sensitive data to unauthorized persons
or to the public. Such data security breaches could lead to the loss of confidential information, trade secrets or other intellectual
property, could lead to the public exposure of personal information (including personally identifiable information or individually identifiable
health information) of our employees, clinical trial patients, customers, business partners, and others, could lead to potential identity
theft, or could lead to reputational harm. Data security breaches could also result in loss of clinical trial data or damage to the integrity
of that data. In addition, the increased use of social media by our employees and contractors could result in inadvertent disclosure
of sensitive data or personal information, including but not limited to, confidential information, trade secrets and other intellectual
property.
Any
such disruption or security breach, as well as any action by us or our employees or contractors that might be inconsistent with the rapidly
evolving data privacy and security laws and regulations applicable within the United States and elsewhere where we conduct business,
could result in enforcement actions by U.S. states, the U.S. federal government or foreign governments, liability or sanctions under
data privacy laws, including healthcare laws such as HIPAA, that protect certain types of sensitive information, regulatory penalties,
other legal proceedings such as but not limited to private litigation, the incurrence of significant remediation costs, disruptions to
our development programs, business operations and collaborations, diversion of management efforts and damage to our reputation, which
could harm our business and operations. Because of the rapidly moving nature of technology and the increasing sophistication of cybersecurity
threats, our measures to prevent, respond to and minimize such risks may be unsuccessful.
Security
breaches, loss of data and other disruptions could compromise sensitive information related to our business, prevent us from accessing
critical information or expose us to liability, which could adversely affect our business and our reputation.
In
the ordinary course of our business, we, our vendors, and our third-party cloud service providers may collect and store sensitive data,
including legally protected patient health information, credit card information, personally identifiable information about our employees
and patients, intellectual property, and proprietary business information. We manage and maintain our applications and data utilizing
cloud-based and on-site systems. These applications and data encompass a wide variety of business-critical information including research
and development information, commercial information and business and financial information.
The
secure processing, storage, maintenance, and transmission of this critical information is vital to our operations and business strategy,
and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized
access or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers, or viruses, breaches, or
interruptions due to employee error, malfeasance or other disruptions, or lapses in compliance with privacy and security mandates. Any
such virus, breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties,
publicly disclosed, lost or stolen. We have measures in place that are designed to prevent, and if necessary to detect and respond to
such security incidents, breaches of privacy, and security mandates. However, in the future, any such access, disclosure or other loss
of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such
as HIPAA in the United States and the General Data Protection Regulation in the European Union, or GDPR, government enforcement actions
and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to process
samples, provide test results, share and monitor safety data, bill payers or patients, provide customer support services, conduct research
and development activities, process and prepare company financial information, manage various general and administrative aspects of our
business and may damage our reputation, any of which could adversely affect our business, financial condition and results of operations.
Legislative
or regulatory reform of the health care system in the U.S. may affect our ability to profitably sell our products, if approved.
Our
ability to commercialize our future products successfully, alone or with collaborators, will depend in part on the extent to which coverage
and reimbursement for the products will be available from government and health administration authorities, private health insurers and
other third-party payers. The continuing efforts of the U.S. government, insurance companies, managed care organizations and other payers
for health care services to contain or reduce health care costs may adversely affect our ability to set prices for our products which
we believe are fair, and our ability to generate revenues and achieve and maintain profitability.
Specifically,
in the U.S., there have been a number of legislative and regulatory proposals to change the health care system in ways that could affect
our ability to sell our products profitably. For example, certain states in the U.S. are proposing legislation mandating publicly funded
health program coverage of medical cannabis. In addition, the 2010 Affordable Care Act, or the ACA, substantially changed the way healthcare
is financed by both governmental and private insurers. Both Congress and the U.S. President have already taken some actions that are
intended to significantly limit the ACA, and we expect efforts to further modify or repeal the ACA to continue. The success and potential
effects of these efforts to repeal or modify the ACA are not clear.
We
expect additional federal and state legislative proposals for health care reform, which could limit the prices that can be charged for
the products we develop and may limit our commercial opportunity.
The
continuing efforts of government and other third-party payers to contain or reduce the costs of health care through various means may
limit our commercial opportunity. It will be time-consuming and expensive for us to go through the process of seeking coverage and reimbursement
from Medicare, Medicaid, and other governmental health programs and from private payers. Our products may not be considered cost-effective,
and government and third-party private health insurance coverage and reimbursement may not be available to patients for any of our future
products or sufficient to allow us to sell our products on a competitive and profitable basis. Our results of operations could be adversely
affected by ACA, changes to the ACA, and by other health care reforms that may be enacted or adopted in the future. In addition, increasing
emphasis on managed care in the U.S. will continue to put downward pressure on the pricing of pharmaceutical products. Cost-control initiatives
could decrease the price that we or any potential collaborators could receive for any of our future products and could adversely affect
our ability to generate revenue in the U.S. market and maintain profitability.
We
may acquire other companies which could divert our management’s attention, result in additional dilution to our shareholders and
otherwise disrupt our operations and harm our operating results.
We
may in the future seek to acquire businesses, products, or technologies that we believe could complement or expand our product offerings,
enhance our technical capabilities or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention
of management and cause us to incur various expenses in identifying, investigating, and pursuing suitable acquisitions, whether or not
they are consummated. If we acquire additional businesses, we may not be able to integrate the acquired personnel, operations and technologies
successfully, effectively manage the combined business following the acquisition or realize anticipated cost savings or synergies. We
also may not achieve the anticipated benefits from the acquired business due to a number of factors, including:
| |
● |
incurrence
of acquisition-related costs; |
| |
|
|
| |
● |
diversion
of management’s attention from other business concerns; |
| |
● |
unanticipated
costs or liabilities associated with the acquisition; |
| |
|
|
| |
● |
harm
to our existing business relationships with collaboration partners as a result of the acquisition; |
| |
|
|
| |
● |
harm
to our brand and reputation; |
| |
|
|
| |
● |
the
potential loss of key employees; |
| |
|
|
| |
● |
use
of resources that are needed in other parts of our business; and |
| |
|
|
| |
● |
use
of substantial portions of our available cash to consummate the acquisition. |
In
the future, if our acquisitions do not yield expected returns, we may be required to take charges to our operating results arising from
the impairment assessment process. Acquisitions may also result in dilutive issuances of equity securities or the incurrence of debt,
which could adversely affect our operating results. In addition, if an acquired business fails to meet our expectations, our business,
results of operations and financial condition may be adversely affected.
Risks
Related to Development and Regulatory Approval of Our Product Candidates
Clinical
trials for our product candidates are expensive, time-consuming, uncertain, and susceptible to change, delay or termination. The results
of clinical trials are open to differing interpretations.
Clinical
trials are expensive, time consuming and difficult to design and implement. Regulatory agencies may analyze or interpret the results
differently than us. Even if the results of our clinical trials are favorable, the clinical trials for a number of our product candidates
are expected to continue for several years and may take significantly longer to complete. In addition, we, the FDA, or other regulatory
authorities, including state and local authorities, or an Institutional Review Board, or IRB, with respect to a trial at its institution,
may suspend, delay or terminate our clinical trials at any time, require us to conduct additional clinical trials, require a particular
clinical trial to continue for a longer duration than originally planned, require a change to our development plans such that we conduct
clinical trials for a product candidate in a different order, e.g., in a step-wise fashion rather than running two trials of the same
product candidate in parallel, or the DEA could suspend or terminate the registrations and quota allotments we require in order to procure
and handle controlled substances, for various reasons, including:
| |
● |
lack
of effectiveness of any product candidate during clinical trials; |
| |
|
|
| |
● |
discovery
of serious or unexpected toxicities or side effects experienced by trial participants or other safety issues, such as drug interactions,
including those which cause confounding changes to the levels of other concomitant medications; |
| |
|
|
| |
● |
slower
than expected rates of subject recruitment and enrollment rates in clinical trials; |
| |
|
|
| |
● |
difficulty
in retaining subjects who have initiated a clinical trial but may withdraw at any time due to adverse side effects from the therapy,
insufficient efficacy, fatigue with the clinical trial process or for any other reason; |
| |
|
|
| |
● |
the
evolving effects of the COVID-19 pandemic; |
| |
|
|
| |
● |
delays
or inability in manufacturing or obtaining sufficient quantities of materials for use in clinical trials due to regulatory and manufacturing
constraints; |
| |
|
|
| |
● |
inadequacy
of or changes in our manufacturing process or product formulation; |
| |
● |
delays
in obtaining regulatory authorization to commence a trial, including “clinical holds” or delays requiring suspension
or termination of a trial by a regulatory agency, such as the FDA, before or after a trial is commenced; |
| |
|
|
| |
● |
changes
in applicable regulatory policies and regulation, including changes to requirements imposed on the extent, nature, or timing of studies; |
| |
|
|
| |
● |
delays
or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective clinical trial sites; |
| |
|
|
| |
● |
uncertainty
regarding proper dosing; |
| |
|
|
| |
● |
delay
or failure to supply product for use in clinical trials which conforms to regulatory specification; |
| |
|
|
| |
● |
unfavorable
results from ongoing pre-clinical studies and clinical trials; |
| |
|
|
| |
● |
failure
of our contract research organizations, or CROs, or other third-party contractors to comply with all contractual requirements or
to perform their services in a timely or acceptable manner; |
| |
|
|
| |
● |
failure
by us, our employees, our CROs or their employees to comply with all applicable FDA or other regulatory requirements relating to
the conduct of clinical trials or the handling, storage, security, and recordkeeping; |
| |
|
|
| |
● |
scheduling
conflicts with participating clinicians and clinical institutions; |
| |
|
|
| |
● |
failure
to design appropriate clinical trial protocols; |
| |
|
|
| |
● |
regulatory
concerns with cannabinoid products generally and the potential for abuse; |
| |
|
|
| |
● |
insufficient
data to support regulatory approval; |
| |
|
|
| |
● |
inability
or unwillingness of medical investigators to follow our clinical protocols; or |
| |
|
|
| |
● |
difficulty
in maintaining contact with patients during or after treatment, which may result in incomplete data. |
Any
of the foregoing could have a material adverse effect on our business, results of operations and financial condition.
Clinical
trials of synthetic cannabinoid drug candidates are novel with very limited or non-existing history; we face a significant risk that
the trials will not result in commercially viable drugs and treatments.
At
present, there is only a very limited documented clinical trial history from which we can derive any scientific conclusions for our product
candidates or prove that our present assumptions for the current and planned research are scientifically compelling. The API content
of the Investigational New Drug applications (INDs) can vary from one IMD to another – hence it is not necessarily possible to
extrapolate results from studies with one product and predict efficacy of safety with another product containing a similar API and different
source. Whilst the principal synthetic cannabinoid component may be similar, the APIs may differ in terms of minor cannabinoid content,
impurity profiles or degradant profiles. While we are encouraged by the results of clinical trials by others (where they exist), there
can be no assurance that any pre-clinical study or clinical trial will result in in commercially viable drugs or treatments.
Clinical
trials are expensive, time consuming and difficult to design and implement. We, as well as the regulatory authorities may suspend, delay
or terminate our clinical trials at any time, may require us, for various reasons, to conduct additional clinical trials, or may require
a particular clinical trial to continue for a longer duration than originally planned, including, among others:
| |
● |
lack
of effectiveness of any API, formulation, or delivery system during clinical trials; |
| |
● |
discovery
of serious or unexpected toxicities or side effects experienced by trial participants or other safety issues; |
| |
|
|
| |
● |
slower
than expected rates of subject recruitment and enrollment rates in clinical trials; |
| |
|
|
| |
● |
delays
or inability in manufacturing or obtaining sufficient quantities of GMP-grade materials for use in clinical trials due to regulatory
and manufacturing constraints; |
| |
|
|
| |
● |
delays
in obtaining regulatory authorization to commence a trial, including Institutional Review Board (“IRB”) approvals or
DEA approvals, licenses required for obtaining and using synthetic cannabinoids or cannabinoid-like substances for research, either
before or after a trial is commenced; |
| |
|
|
| |
● |
unfavorable
results from ongoing pre-clinical studies and clinical trials; |
| |
|
|
| |
● |
patients
or investigators failing to comply with clinical trial protocols; |
| |
|
|
| |
● |
patients
failing to return for post-treatment follow-up at the expected rate; |
| |
|
|
| |
● |
sites
participating in an ongoing clinical trial withdraw, requiring us to engage new sites; |
| |
|
|
| |
● |
third-party
clinical investigators decline to participate in our clinical trials, do not perform the clinical trials on the anticipated schedule,
or act in ways inconsistent with the established investigator agreement, clinical trial protocol, good clinical practices, and other
IRB requirements; |
| |
|
|
| |
● |
third-party
entities do not perform data collection and analysis in a timely or accurate manner or at all; or |
| |
|
|
| |
● |
regulatory
inspections of our clinical trials require us to undertake corrective action or suspend or terminate our clinical trials. |
Any
of the foregoing could have a material adverse effect on our business, results of operations and financial condition.
Any
failure by us to comply with existing regulations could harm our reputation and operating results.
We
are subject to extensive regulation by U.S. federal and state governments in each of the markets where we have product candidates progressing
through the approval process.
We
must also adhere to all regulatory requirements including FDA’s Good Laboratory Practice, Good Clinical Practice, and current Good
Manufacturing Practices requirements (“cGMP”) pharmacovigilance requirements, advertising, and promotion restrictions, reporting
and recordkeeping requirements. If we or our suppliers fail to comply with applicable regulations, including FDA pre-or post-approval
cGMP requirements, then FDA could sanction us. Even if a drug is FDA-approved, regulatory authorities may impose significant restrictions
on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly post-marketing trials. MIRA1a,
and any of our product candidates that may be approved in the U.S. in the future, will be subject to ongoing regulatory requirements
for manufacturing, labeling, packaging, storage, distribution, import, export, advertising, promotion, sampling, recordkeeping and submission
of safety and other post-market information, including both federal and state requirements in the U.S. In addition, manufacturers and
manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing
procedures conform to GMP. As such, we, and our contract manufacturers (in the event contract manufacturers are appointed in the future)
are subject to continual review and periodic inspections to assess compliance with GMP. Accordingly, we and others with whom we work
must continue to spend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, quality control
and quality assurance. We will also be required to report certain adverse reactions and production problems, if any, to the FDA, and
to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription
drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s
approved label.
If
a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency,
or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of the product,
it may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply
with applicable regulatory requirements, a regulatory agency or enforcement authority may:
| |
● |
issue
untitled or warning letters; |
| |
|
|
| |
● |
seek
to enjoin our activities; |
| |
|
|
| |
● |
impose
civil or criminal penalties; |
| |
|
|
| |
● |
suspend
regulatory approval; |
| |
|
|
| |
● |
suspend
any of our ongoing clinical trials; |
| |
|
|
| |
● |
refuse
to approve pending applications or supplements to approved applications submitted by us; |
| |
|
|
| |
● |
impose
restrictions on our operations, including by requiring us to enter into a Corporate Integrity Agreement or closing our contract manufacturers’
facilities, if any; or |
| |
|
|
| |
● |
seize
or detain products or require a product recall. |
In
addition, any government investigation of alleged violations of law could require us to expend significant time and resources in response
and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect
our ability to commercialize and generate revenue from our product candidates. If regulatory sanctions are applied or if regulatory approval
is withdrawn, the value of our business and our operating results may be adversely affected.
Any
action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses,
divert our management’s attention from the operation of our business and damage our reputation. We expend significant resources
on compliance efforts and such expenses are unpredictable and might adversely affect our results. Changing laws, regulations and standards
might also create uncertainty, higher expenses and increase insurance costs. As a result, we intend to invest all reasonably necessary
resources to comply with evolving standards, and this investment might result in increased management and administrative expenses and
a diversion of management time and attention from revenue-generating activities to compliance activities.
We
are subject to federal and state healthcare laws and regulations and implementation of or changes to such healthcare laws and regulations
could adversely affect our business and results of operations.
In
the United States, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could
impact our ability to sell our product candidates. If we are found to be in violation of any of these laws or any other federal or state
regulations, we may be subject to administrative, civil and/or criminal penalties, damages, fines, individual imprisonment, exclusion
from federal health care programs and the restructuring of our operations. Any of these could have a material adverse effect on our business
and financial results. Since many of these laws have not been fully interpreted by the courts, there is an increased risk that we may
be found in violation of one or more of their provisions. Any action against us for violation of these laws, even if we ultimately are
successful in our defense, will cause us to incur significant legal expenses and divert our management’s attention away from the
operation of our business.
We
expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage
criteria and in additional downward pressure on the price that we may receive for any approved product. There have been judicial challenges
to certain aspects of the ACA and numerous legislative attempts to repeal and/or replace the ACA in whole or in part, and we expect there
will be additional challenges and amendments to the ACA in the future. At this time, the full effect that the ACA will have on our business
in the future remains unclear. An expansion in the government’s role in the U.S. healthcare industry may cause general downward
pressure on the prices of prescription drug products, lower reimbursements, or any other product for which we obtain regulatory approval,
reduce product utilization, and adversely affect our business and results of operations. Any reduction in reimbursement from Medicare
or other government programs may result in a similar reduction in payments from private payers. The implementation of cost containment
measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize products
for which we may receive regulatory approval.
The
regulatory approval processes with the FDA are lengthy and inherently unpredictable.
We
are not permitted to market our drug candidates as medicines in the United States or other countries until we receive approval of a New
Drug Application (“NDA”) from the FDA or in any foreign countries until we receive the approval from the regulatory authorities
of such countries. Prior to submitting an NDA to the FDA for approval of our drug candidates we will need to have completed our pre-clinical
studies and clinical trials and demonstrate that our products meet all applicable standards of identity, strength, quality, and purity
throughout their expiration date. Successfully completing any clinical program and obtaining approval of an NDA is a complex, lengthy,
expensive, and uncertain process, and the FDA (or other country medicines regulatory body) may delay, limit, or deny approval of product
candidates for many reasons, including, among others, because:
| |
● |
an
inability to demonstrate that our product candidates are safe and effective in treating patients to the satisfaction of the FDA; |
| |
|
|
| |
● |
results
of clinical trials that may not meet the level of statistical or clinical significance required by the FDA; |
| |
|
|
| |
● |
disagreements
with the FDA with respect to the number, design, size, conduct or implementation of clinical trials; |
| |
|
|
| |
● |
requirements
by the FDA to conduct additional clinical trials; |
| |
|
|
| |
● |
disapproval
by the FDA of certain formulations, labeling or specifications of product candidates; |
| |
|
|
| |
● |
findings
by the FDA that the data from pre-clinical studies and clinical trials are insufficient; |
| |
|
|
| |
● |
findings
by the FDA that our API or finished products do not meet all applicable standards of identity, strength, quality, and purity; |
| |
|
|
| |
● |
the
FDA may disagree with the interpretation of data from pre-clinical studies and clinical trials; and |
| |
|
|
| |
● |
the
FDA may change their approval policies or adopt new regulations. |
Any
of these factors, many of which are beyond our control, could increase development time and / or costs or jeopardize our ability to obtain
regulatory approval for our drug candidates.
There
is a high rate of failure for drug candidates proceeding through clinical trials.
Generally,
there is a high rate of failure for drug candidates proceeding through clinical trials. We may suffer significant setbacks in our clinical
trials similar to the experience of a number of other companies in the pharmaceutical and biotechnology industries, even after receiving
promising results in earlier trials. Further, even if we view the results of a clinical trial to be positive, FDA may disagree with our
interpretation of the data. In the event that we obtain negative results from clinical trials for product candidates or other problems
related to potential chemistry, manufacturing and control issues or other hurdles occur and our product candidates are not approved,
we may not be able to generate sufficient revenue or obtain financing to continue our operations, our ability to execute on our current
business plan may be materially impaired, our reputation in the industry and in the investment community might be significantly damaged
and the price of our common stock could decrease significantly. In addition, our inability to properly design, commence and complete
clinical trials may negatively impact the timing and results of our clinical trials and ability to seek approvals for our drug candidates.
If
we are found in violation of federal or state “fraud and abuse” laws, we may be required to pay a penalty and/or be suspended
from participation in federal or state health care programs, which may adversely affect our business, financial condition, and results
of operations.
In
the United States, we are subject to various federal and state health care “fraud and abuse” laws, including anti-kickback
laws, false claims laws and other laws intended to reduce fraud and abuse in federal and state health care programs, which could affect
us particularly upon successful commercialization of our products in the U.S. The Medicare and Medicaid Patient Protection Act of 1987,
or federal Anti-Kickback Statute, makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its
behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce the referral of business,
including the purchase, order or prescription of a particular drug for which payment may be made under a federal health care program,
such as Medicare or Medicaid. Under federal law, some arrangements, known as safe harbors, are deemed not to violate the federal Anti-Kickback
Statute. Although we seek to structure our business arrangements in compliance with all applicable requirements, it is often difficult
to determine precisely how the law will be applied in specific circumstances. Accordingly, it is possible that our practices may be challenged
under the federal Anti-Kickback Statute and Federal False Claims Act. Violations of fraud and abuse laws may be punishable by criminal
and/or civil sanctions, including fines and/or exclusion or suspension from federal and state health care programs such as Medicare and
Medicaid and debarment from contracting with the U.S. government. In addition, private individuals have the ability to bring actions
on behalf of the government under the federal False Claims Act as well as under the false claims laws of several states.
Many
states have adopted laws similar to the federal anti-kickback statute, some of which apply to the referral of patients for health care
services reimbursed by any source, not just governmental payers. There are ambiguities as to what is required to comply with these state
requirements and if we fail to comply with an applicable state law requirement, we could be subject to penalties.
Neither
the government nor the courts have provided definitive guidance on the application of fraud and abuse laws to our business. Law enforcement
authorities are increasingly focused on enforcing these laws, and it is possible that some of our practices may be challenged under these
laws. While we believe we have structured our business arrangements to comply with these laws, it is possible that the government could
allege violations of, or convict us of violating, these laws. If we are found in violation of one of these laws, we could be required
to pay a penalty and could be suspended or excluded from participation in federal or state health care programs, and our business, results
of operations and financial condition may be adversely affected.
Serious
adverse events or other safety risks could require us to abandon development and preclude, delay or limit approval of our product candidates,
limit the scope of any approved label or market acceptance, or cause the recall or loss of marketing approval of products that are already
marketed.
If
any of our product candidates prior to or after any approval for commercial sale, cause serious or unexpected side effects, or are associated
with other safety risks such as misuse, abuse or diversion, a number of potentially significant negative consequences could result, including:
| |
● |
regulatory
authorities may interrupt, delay or halt clinical trials; |
| |
|
|
| |
● |
regulatory
authorities may deny regulatory approval of our product candidates; |
| |
|
|
| |
● |
regulatory
authorities may require certain labeling statements, such as warnings or contraindications or limitations on the indications for
use, and/or impose restrictions on distribution in the form of a REMS in connection with approval or post-approval; |
| |
● |
regulatory
authorities may withdraw their approval, require more onerous labeling statements, impose a more restrictive Risk Evaluation and
Mitigation Strategy (“REMS”), or require us to recall any product that is approved; |
| |
|
|
| |
● |
we
may be required to change the way the product is administered or conduct additional clinical trials; |
| |
|
|
| |
● |
our
relationships with our collaboration partners may suffer; |
| |
|
|
| |
● |
we
could be sued and held liable for harm caused to patients; or |
| |
|
|
| |
● |
our
reputation may suffer. The reputational risk is heightened with respect to those of our product candidates that are being developed
for pediatric indications. |
We
may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to participants
or if preliminary data demonstrate that our product candidates are unlikely to receive regulatory approval or unlikely to be successfully
commercialized. Following receipt of approval for commercial sale of a product we may voluntarily withdraw or recall that product from
the market if at any time we believe that its use, or a person’s exposure to it, may cause adverse health consequences or death.
To date we have not withdrawn, recalled, or taken any other action, voluntary or mandatory, to remove an approved product from the market.
In addition, regulatory agencies, IRBs, or data safety monitoring boards may at any time recommend the temporary or permanent discontinuation
of our clinical trials or request that we cease using investigators in the clinical trials if they believe that the clinical trials are
not being conducted in accordance with applicable regulatory requirements, or that they present an unacceptable safety risk to participants.
Although we have never been asked by a regulatory agency, IRB, or data safety monitoring board to discontinue a clinical trial temporarily
or permanently, if we elect or are forced to suspend or terminate a clinical trial of any of our product candidates, the commercial prospects
for that product will be harmed and our ability to generate product revenue from that product may be delayed or eliminated. Furthermore,
any of these events may result in labeling statements such as warnings or contraindications. In addition, such events or labeling could
prevent us or our partners from achieving or maintaining market acceptance of the affected product and could substantially increase the
costs of commercializing our product candidates and impair our ability to generate revenue from the commercialization of these products
either by us or by our collaboration partners.
Risks
Related to Our Reliance Upon Third Parties
Our
existing collaboration arrangements and any that we may enter into in the future may not be successful, which could adversely affect
our ability to develop and commercialize our product candidates.
We
may seek additional collaboration arrangements with pharmaceutical or biotechnology companies for the development or commercialization
of our product candidates. We may, with respect to our product candidates, enter into new arrangements on a selective basis depending
on the merits of retaining commercialization rights for ourselves as compared to entering into selective collaboration arrangements with
leading pharmaceutical or biotechnology companies for each product candidate, both in the U.S. and internationally. To the extent that
we decide to enter into collaboration agreements, we will face significant competition in seeking appropriate collaborators and the terms
of any collaboration or other arrangements that we may establish may not be favorable to us.
Any
existing or future collaboration that we enter may not be successful. The success of our collaboration arrangements will depend heavily
on the efforts and activities of our collaborators. Collaborators generally have significant discretion in determining the efforts and
resources that they will apply to these collaborations. Disagreements between parties to a collaboration arrangement regarding development,
intellectual property, regulatory or commercialization matters can lead to delays in the development process or commercialization of
the applicable product candidate and, in some cases, termination of the collaboration arrangement. These disagreements can be difficult
to resolve if neither of the parties has final decision-making authority. Any such termination or expiration could harm our business
reputation and may adversely affect us financially.
We
depend on a limited number of suppliers for materials and components required to manufacture our product candidates. The loss of these
suppliers, or their failure to supply us on a timely basis, could cause delays in our current and future capacity and adversely affect
our business.
We
depend on a limited number of suppliers for the materials and components required to manufacture our product candidates. As a result,
we may not be able to obtain sufficient quantities of critical materials and components in the future. A delay or interruption by our
suppliers may also harm our business, results of operations and financial condition. In addition, the lead time needed to establish a
relationship with a new supplier can be lengthy, and we may experience delays in meeting demand in the event we must switch to a new
supplier. The time and effort to qualify for and, in some cases, obtain regulatory approval for a new supplier could result in additional
costs, diversion of resources or reduced manufacturing yields, any of which would negatively impact our operating results. Our dependence
on single-source suppliers exposes us to numerous risks, including the following: our suppliers may cease or reduce production or deliveries,
raise prices or renegotiate terms; our suppliers may become insolvent or cease trading; we may be unable to locate a suitable replacement
supplier on acceptable terms or on a timely basis, or at all; and delays caused by supply issues may harm our reputation, frustrate our
customers and cause them to turn to our competitors for future needs.
We
maintain our cash at financial institutions, at times in balances that exceed federally insured limits. The failure of financial institutions
could adversely affect our ability to pay operational expenses or make other payments.
Our
cash held in non-interest-bearing and interest-bearing accounts can at times exceed the Federal Deposit Insurance Corporation (“FDIC”)
insurance limits. If such banking institutions were to fail, we could lose all or a portion of those amounts held in excess of such insurance
limitations. In addition, even if account holders are ultimately made whole with respect to a future bank failure, account holders’
access to their accounts and assets held in their accounts may be substantially delayed. Any material loss that we may experience in
the future or inability for a material time period to access our cash and cash equivalents could have an adverse effect on our ability
to pay our operational expenses or make other payments, which could adversely affect our business.
Risks
Related to Our Intellectual Property
We
may not be able to adequately protect our product candidates or our proprietary technology in the marketplace.
Our
success will depend, in part, on our ability to obtain patents, protect our trade secrets and operate without infringing on the proprietary
rights of others. We may rely upon a combination of patents, trade secret protection (i.e., know-how), trademarks, licenses, and confidentiality
agreements to protect the intellectual property of our product candidates. The strengths of patents in the pharmaceutical field involve
complex legal and scientific questions and can be uncertain. Where appropriate, we seek patent protection for certain aspects of our
products and technology. However, patent protection for naturally occurring compounds is exceedingly difficult to obtain, defend and
enforce. Filing, prosecuting and defending patents throughout the world would be prohibitively expensive, so our policy is to look to
patent technologies with commercial potential in jurisdictions with significant commercial opportunities. However, patent protection
may not be available for some of the products or technology we are developing. If we must spend significant time and money protecting,
defending, or enforcing our patents, designing around patents held by others or licensing, potentially for large fees, patents or other
proprietary rights held by others, our business, results of operations and financial condition may be harmed. We may not develop additional
proprietary products that are patentable.
The
patent positions of pharmaceutical products are complex and uncertain. The scope and extent of patent protection for our product candidates
are particularly uncertain. To date, our principal product candidates have been based on specific formulations of certain previously
known cannabinoids found in nature in the cannabis sativa plant. While we have sought patent protection, where appropriate, directed
to, among other things, composition-of-matter for our specific formulations, their methods of use, and methods of manufacture, we do
not have and will not be able to obtain composition of matter protection on these previously known cannabinoids per se. We anticipate
that the products we develop in the future will continue to be based on the same or other naturally occurring compounds, as well as additional
synthetic compounds we may discover. Although we have sought and expect to continue to seek patent protection for our product candidates,
their methods of use, and methods of manufacture, any, or all of them may not be subject to effective patent protection. If any of our
products are approved and marketed for an indication for which we do not have an issued patent, our ability to use our patents to prevent
a competitor from commercializing a non-branded version of our commercial products for that non-patented indication could be significantly
impaired or even eliminated.
Publication
of information related to our product candidates by us, or others may prevent us from obtaining or enforcing patents relating to these
products and product candidates. Furthermore, others may independently develop similar products, may duplicate our products, or may design
around our patent rights. In addition, any of our issued patents may be opposed and/or declared invalid or unenforceable. If we fail
to adequately protect our intellectual property, we may face competition from companies who attempt to create a generic product to compete
with our product candidates. We may also face competition from companies who develop a substantially similar product to one of our product
candidates that is not covered by any of our patents.
If
third parties claim that the Company’s intellectual property, products, processes, or anything else used by us infringes upon their
intellectual property, our operating profits could be adversely affected.
There
is a substantial amount of litigation, both within and outside the U.S., involving patent and other intellectual property rights in the
pharmaceutical industry. We may, from time to time, be notified of claims that we are infringing upon patents, trademarks, copyrights,
or other intellectual property rights owned by third parties, and we cannot provide assurances that other companies will not, in the
future, pursue such infringement claims against us, our commercial partners or any third-party proprietary technologies we have licensed.
If we were found to infringe upon a patent or other intellectual property right, or if we failed to obtain or renew a license under a
patent or other intellectual property right from a third party, or if a third party that we were licensing technologies from was found
to infringe upon a patent or other intellectual property rights of another third party, we may be required to pay damages, including
damages of up to three times the damages found or assessed, if the infringement is found to be willful, suspend the manufacture of certain
products or reengineer or rebrand our products, if feasible, or we may be unable to enter certain new product markets. Any such claims
could also be expensive and time consuming to defend and divert management’s attention and resources. Our competitive position
could suffer as a result. In addition, if we have declined or failed to enter into a valid non-disclosure or assignment agreement for
any reason, we may not own the invention or our intellectual property, and our products may not be adequately protected. Thus, we cannot
guarantee that our product candidates, or our commercialization thereof, does not and will not infringe any third party’s intellectual
property.
We
own the rights associated with our patents in the United States, but we do not own the rights to patents covering MIRA1a in foreign jurisdictions.
We
own the patent relating to MIRA1a in the United States. Foreign patents covering MIRA1a and its therapeutic uses have issued in Australia,
Belgium, Canada, Czech Republic, France, Germany, Greece, Netherlands, Hungary, Ireland, Israel, Italy, Malta, Poland, Portugal, Romania,
South Korea, Spain, Sweden, and the United Kingdom, and corresponding applications are pending in China and Japan. MyMD Pharmaceuticals,
Inc. (Nasdaq: MYMD, “MyMD”), a publicly traded New Jersey corporation, currently owns these foreign patents and patent applications.
We currently have no plans to develop the MIRA1a compound for approval and commercialization outside of the United States or for manufacture
outside of the United States, including in the foreign jurisdictions in which MyMD has patent rights. We may in the future seek an agreement
to license or purchase all or a portion of such foreign patent rights from MyMD, but we have no current plans to do so and there is no
assurance that we would be able to successfully conclude such an agreement. If we are unable to obtain foreign patent rights to MIRA1a
from MyMD, MyMD may retain such patent rights, in which case we would not have the ability to commercialize MIRA1a outside of the United
States in jurisdictions in which MyMD has foreign patent rights, and MyMD potentially could develop a competing product for such jurisdictions
outside of the United States.
Risks
Relating to our Common Stock
Because
of the speculative nature of investment risk, you may lose your entire investment.
An
investment in our securities carries a high degree of risk and should be considered as a speculative investment. We have a limited operating
history, no revenues, have not paid dividends, and are unlikely to pay dividends in the immediate or near future. The likelihood of our
success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection
with the establishment of any business. An investment in our securities may result in the loss of an investor’s entire investment.
Only potential investors who are experienced in high-risk investments and who can afford to lose their entire investment should consider
an investment in our securities.
The
requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract
and retain executive management and qualified board members.
As
a reporting issuer, we are subject to the reporting requirements of applicable securities legislation of the jurisdiction in which we
are a reporting issuer, the listing requirements of Nasdaq and other applicable securities rules and regulations. Compliance with these
rules and regulations increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly
and increase demand on its systems and resources. Applicable securities laws require us to, among other things, file certain annual and
quarterly reports with respect to its business and results of operations. In addition, applicable securities laws require us to, among
other things, maintain effective disclosure controls and procedures and internal control over financial reporting.
In
order to maintain and, if required, improve its disclosure controls and procedures and internal control over financial reporting to meet
this standard, significant resources and management oversight are required and, as a result, management’s attention may be diverted
from other business concerns, which could harm our business and results of operations. To comply with these requirements, we may need
to hire more employees in the future or engage outside consultants, which will increase its costs and expenses.
In
addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for
public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations
and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application
in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty
regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to
continue to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general
and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance
activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing
bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us,
which could adversely affect our business and financial results.
As
a public company subject to these rules and regulations, it may be more expensive to obtain director and officer liability insurance,
and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make
it more difficult for us to attract and retain qualified members of the Board, particularly to serve on the Audit Committee and Compensation
Committee, and qualified executive officers.
We
are an “emerging growth company,” and any decision on our part to comply only with certain reduced reporting and disclosure
requirements applicable to emerging growth companies could make shares of our common stock less attractive to investors.
We
are an “emerging growth company,” as defined in Section 2(a) of the Securities Act. For as long as we continue to be an emerging
growth company, we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies
that are not emerging growth companies, including, but not limited to, not being required to have our independent registered public accounting
firm audit our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations
regarding executive compensation in our periodic reports and exemptions from the requirements of holding a nonbinding advisory vote on
executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth
company until the fifth anniversary of the fiscal year end date following the completion of our initial public offering, however, our
status would change more quickly if we have more than US$1.235 billion in annual revenue, if the market value of our shares of common
stock held by non-affiliates equals or exceeds US$700 million as of June 30 of any year, or we issue more than US$1.0 billion of non-convertible
debt over a three-year period before the end of that period.
Investors
could find our shares less attractive if we choose to rely on these exemptions. If some investors find shares less attractive as a result
of any choice to reduce future disclosure, there may be a less active trading market for our shares and our share price may be more volatile.
For
as long as we are an “emerging growth company”, our independent registered public accounting firm will not be required to
attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404. We could be an “emerging
growth company” until the fifth anniversary of the fiscal year end date following the completion of our initial public offering.
An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment
might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur
the expense of remediation.
If
we identify material weaknesses in our internal control over financial reporting, or if we are unable to comply with the requirements
of Section 404 in a timely manner or assert that our internal control over financial reporting is effective, or if our independent registered
public accounting firm is unable to express an opinion as to the effectiveness of our internal control over financial reporting when
required, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our securities
could be negatively affected, and we could become subject to investigations by the stock exchange on which our securities are listed,
the SEC, or other regulatory authorities, which could require additional financial and management resources.
Additionally,
we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take
advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements.
We will remain a smaller reporting company until the last day of any fiscal year for so long as either: (i) the market value of our shares
of common stock held by non-affiliates does not equal or exceed $250 million as of the prior June 30th; or (ii) our annual
revenues did not equal or exceed $100 million during such completed fiscal year. To the extent we take advantage of such reduced disclosure
obligations, it may also make the comparison of our financial statements with other public companies difficult or impossible.
If
we fail to maintain compliance with Nasdaq Listing Rules, our shares may be delisted from Nasdaq, which would result in a limited trading
market for our shares and make obtaining future debt or equity financing more difficult for the Company.
Our
common stock is listed on the Nasdaq Capital Market under the symbol “MIRA”. However, there is no assurance that we will
be able to continue to maintain our compliance with the Nasdaq continued listing requirements. If we fail to do so, our securities may
lose their status on Nasdaq and they would likely be traded on the over-the-counter markets, including the Pink Sheets market. As a result,
selling our securities could be more difficult because smaller quantities of shares or warrants would likely be bought and sold, transactions
could be delayed, and security analysts’ coverage of us may be reduced. In addition, in the event our securities are delisted,
broker dealers would bear certain regulatory burdens which may discourage broker dealers from effecting transactions in the securities
and further limit the liquidity of the securities. These factors could result in lower prices and larger spreads in the bid and ask prices
for the securities. Such delisting from Nasdaq and continued or further declines in the share price of the securities could also greatly
impair our ability to raise additional necessary capital through equity or debt financing and could significantly increase the ownership
dilution to shareholders caused by our issuing equity in financing or other transactions.
If
our shares were to be delisted from Nasdaq, they may become subject to the SEC’s “penny stock” rules.
Delisting
from Nasdaq may cause the securities of the Company to become subject to the SEC’s “penny stock” rules. The SEC generally
defines a penny stock as an equity security that has a market price of less than $5.00 per share or an exercise price of less than $5.00
per share, subject to certain exemptions. One such exemption is to be listed on Nasdaq. Therefore, if shares of our common stock were
to be delisted from Nasdaq, the securities of the Company could become subject to the SEC’s “penny stock” rules. These
rules require, among other things, that any broker engaging in a purchase or sale of our securities provide its customers with: (i) a
risk disclosure document, (ii) disclosure of market quotations, if any, (iii) disclosure of the compensation of the broker and its salespersons
in the transaction, and (iv) monthly account statements showing the market values of our securities held in the customer’s accounts.
A broker would be required to provide the bid and offer quotations and compensation information before effecting the transaction. This
information must be contained in the customer’s confirmation. Generally, brokers are less willing to effect transactions in penny
stocks due to these additional delivery requirements. These requirements may make it more difficult for shareholders to purchase or sell
the shares of our common stock. Since the broker, not us, prepares this information, we would not be able to assure that such information
is accurate, complete or current.
Some
provisions of Florida law and our amended and restated articles of incorporation and amended and restated bylaws may have anti-takeover
effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our shareholders, and may
prevent attempts by our shareholders to replace or remove our current management.
Our
status as a Florida corporation and the anti-takeover provisions of the Florida Business Corporation Act, which we sometimes refer to
as the FBCA, may discourage, delay or prevent a change in control even if a change in control would be beneficial to our shareholders.
The
control share acquisition statute, Section 607.0902 of the FBCA, generally provides that in the event a person acquires voting shares
of the company in excess of 20% of the voting power of all of our issued and outstanding shares, such acquired shares will not have any
voting rights unless such rights are restored by the holders of a majority of the votes of each class or series entitled to vote separately,
excluding shares held by the person acquiring the control shares or any of our officers or employees who are also directors of the company.
Certain acquisitions of shares are exempt from these rules, such as shares acquired pursuant to the laws of intestate succession or pursuant
to a gift or testamentary transfer, pursuant to a merger or share exchange effected in compliance with the FBCA if we are a party to
the agreement, or pursuant to an acquisition of our shares if the acquisition has been approved by our board of directors before the
acquisition. The control share acquisition statute generally applies to any “issuing public corporation,” which means a Florida
corporation which has:
| ● |
One
hundred or more shareholders; |
| |
|
| ● |
Its
principal place of business, its principal office, or substantial assets within Florida; and |
| |
|
| ● |
Either
(i) more than 10% of its shareholders are resident in Florida; (ii) more than 10% of its shares are owned by residents of Florida;
or (iii) one thousand shareholders are resident in Florida. |
The
affiliated transaction (or so-called “business combination”) statute, Section 607.0901 of the FBCA, provides that we may
not engage in certain mergers, consolidations, sales of assets, issuances of stock, reclassifications, recapitalizations, and other affiliated
transactions with any “interested shareholder” for a period of three years following the time that such shareholder became
an interested shareholder, unless:
| ● |
Prior
to the time that such shareholder became an interested shareholder, our board of directors approved either the affiliated transaction
or the transaction which resulted in the shareholder becoming an interested shareholder; or |
| |
|
| ● |
Upon
consummation of the transaction that resulted in the shareholder becoming an interested shareholder, the interested shareholder owned
at least 85% of our voting shares outstanding at the time the transaction commenced; or |
| |
|
| ● |
At
or subsequent to the time that such shareholder became an interested shareholder, the affiliated transaction is approved by our board
of directors and authorized at an annual or special meeting of shareholders, and not by written consent, by the affirmative vote
of at least two-thirds of the outstanding voting shares which are not owned by the interested shareholder. |
An
“interested shareholder” is generally defined as any person who is the beneficial owner of more than 15% of our outstanding
voting shares.
The
voting requirements set forth above do not apply to a particular affiliated transaction if one or more conditions are met, including,
but not limited to, the following: if the affiliated transaction has been approved by a majority of our disinterested directors; if we
have not had more than 300 shareholders of record at any time during the three years preceding the date the affiliated transaction is
announced; if the interested shareholder has been the beneficial owner of at least 80% of our outstanding voting shares for at least
three years preceding the date the affiliated transaction is announced; or if the consideration to be paid to the holders of each class
or series of voting shares in the affiliated transaction meets certain requirements of the statute with respect to form and amount, among
other things.
Both
the control share acquisition statute and the affiliated transactions statute may have the effect of discouraging or preventing certain
change of control or takeover transactions involving us.
In
addition, our amended and restated articles of incorporation and amended and restated bylaws contain provisions that may make it more
difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our shareholders, including
transactions in which shareholders might otherwise receive a premium for their shares. These provisions include:
| ● |
nothing
in our amended and restated articles of incorporation precludes future issuances without shareholder approval of the authorized but
unissued shares of our common stock; |
| |
|
| ● |
advance
notice procedures apply for shareholders to nominate candidates for election as directors or to bring matters before an annual meeting
of shareholders; |
| |
|
| ● |
a
special meeting of shareholders can only be called by our chairman of the board of directors, our chief executive officer, our president
(in the absence of a chief executive officer), a majority of our board of directors or the holders of 10% or more of all of our votes
entitled to be cast on any issue proposed to be considered at the special meeting of shareholders; |
| |
|
| ● |
no
provision in our amended and restated articles of incorporation or amended and restated bylaws provides for cumulative voting, which
limits the ability of minority shareholders to elect director candidates; |
| |
|
| ● |
directors
will only be able to be removed for cause; |
| |
|
| ● |
our
amended and restated articles of incorporation authorizes undesignated preferred stock, the terms of which may be established and
shares of which may be issued, without the approval of the holders of our capital stock; and |
| |
|
| ● |
certain
litigation against us can only be brought in Florida. |
These
provisions could discourage, delay or prevent a transaction involving a change in control of our company. These provisions could also
discourage proxy contests and make it more difficult for you and other shareholders to elect directors of your choosing and cause us
to take corporate actions other than those you desire. See “Description of Capital Stock.”
Our
amended and restated bylaws designates the state courts located within the state of Florida as the exclusive forum for substantially
all disputes between us and our shareholders and the federal district courts as the exclusive forum for Securities Act claims, which
could limit our shareholders’ ability to obtain a favorable judicial forum for disputes with us.
Our
amended and restated bylaws provide that, unless we consent in writing to the selection of an alternative forum, the sole and exclusive
forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty
owed by any of our current or former directors, officers or other employees to us or our shareholders, (iii) any action arising pursuant
to any provision of the FBCA, our amended and restated articles of incorporation or our amended and restated bylaws, or (iv) any other
action asserting a claim that is governed by the internal affairs doctrine shall be a state court located within the state of Florida
(or, if a state court located within the state of Florida does not have jurisdiction, the federal district court for the Middle District
of Florida); provided that, the exclusive forum provision will not apply to suits brought to enforce any liability or duty created by
the Exchange Act, or to any claim for which the federal courts have exclusive jurisdiction. Our amended and restated bylaws also provide
that, unless we consent in writing to the selection of an alternative forum, the U.S. federal district courts shall be the exclusive
forum for the resolution of any claims arising under the Securities Act. Under the Securities Act, federal and state courts have concurrent
jurisdiction over all suits brought to enforce any duty or liability created by the Securities Act, and investors cannot waive compliance
with the federal securities laws and the rules and regulations thereunder. Accordingly, there is uncertainty as to whether a court would
enforce such a forum selection provision as written in connection with claims arising under the Securities Act.
By
becoming a shareholder in our company, you will be deemed to have notice of and have consented to the provisions of our amended and restated
bylaws related to choice of forum. The choice of forum provisions in our amended and restated bylaws may limit our shareholders’
ability to obtain a favorable judicial forum for disputes with us. Additionally, the enforceability of choice of forum provisions in
other companies’ governing documents has been challenged in legal proceedings, and it is possible that, in connection with any
applicable action brought against us, a court could find the choice of forum provisions contained in our amended and restated bylaws
to be inapplicable or unenforceable in such action. If so, we may incur additional costs associated with resolving such action in other
jurisdictions, which could harm our business, results of operations, and financial condition.
Securities
or industry analysts may not regularly publish reports on us, which could cause the price of our securities or trading volumes to decline.
The
trading market for our securities could be influenced by research and reports that industry and/or securities analysts may publish us,
our business, the market or our competitors. We do not have any control over these analysts and cannot be assured that such analysts
will cover us or provide favorable coverage. If any of the analysts who may cover our business change their recommendation regarding
our securities adversely, or provide more favorable relative recommendations about our competitors, the price of our securities would
likely decline. If any analysts who may cover our business were to cease coverage or fail to regularly publish reports on us, we could
lose visibility in the financial markets, which in turn could cause the price of our securities or trading volumes to decline.
We
will likely conduct further offerings of our equity securities in the future, in which case your proportionate interest may become diluted.
We
will likely be required to conduct equity offerings in the future to finance our current projects or to finance subsequent projects that
we decide to undertake. If our common stock shares are issued in return for additional funds, the price per share could be lower than
that paid by our current shareholders. We anticipate continuing to rely on equity sales of our common stock shares in order to fund our
business operations. If we issue additional common stock shares or securities convertible into shares of our common stock, your percentage
interest in us could become diluted.
We
may issue shares of preferred stock in the future, which could make it difficult for another company to acquire us or could otherwise
adversely affect holders of our common stock, which could depress the price of our common stock.
Our
certificate of incorporation authorizes us to issue one or more series of preferred stock. Our board of directors will have the authority
to determine the preferences, limitations and relative rights of the shares of preferred stock and to fix the number of shares constituting
any series and the designation of such series, without any further vote or action by our shareholders. Our preferred stock could be issued
with voting, liquidation, dividend and other rights superior to the rights of our common stock. The potential issuance of preferred stock
may delay or prevent a change in control of us, discouraging bids for our common stock at a premium to the market price, and materially
adversely affect the market price and the voting and other rights of the holders of our common stock.
We
have never declared or paid any cash dividends or distributions on our capital stock. We do not anticipate paying any cash dividends
on our common stock in the foreseeable future.
We
have never declared or paid any cash dividends or distributions on our capital stock. We currently intend to retain our future earnings,
if any, to support operations and to finance expansion and therefore we do not anticipate paying any cash dividends on our common stock
in the foreseeable future.
The
declaration, payment and amount of any future dividends will be made at the discretion of the board of directors, and will depend upon,
among other things, the results of our operations, cash flows and financial condition, operating and capital requirements, and other
factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and, if dividends are
paid, there is no assurance with respect to the amount of any such dividend.
USE
OF PROCEEDS
We
will not receive any proceeds from the sale by the selling stockholders of the shares of common stock offered by this prospectus. However,
we may receive up to $6.4 million in aggregate gross proceeds from the cash exercise of the warrants, based on the per share exercise
price of each warrant. We will use the net proceeds, if any, for general corporate purposes and working capital purposes.
The
selling stockholders will pay any underwriting discounts and commissions and expenses incurred by them for brokerage, accounting, tax
or legal services or any other expenses incurred by them in disposing of the shares. We will bear all other costs, fees and expenses
incurred in effecting the registration of the shares covered by this prospectus, including, without limitation, all registration and
filing fees and fees and expenses of our counsel and our accountants.
DIVIDEND
POLICY
We
have never declared or paid cash dividends on our capital stock. We currently intend to retain all available funds and future earnings,
if any, for use in the operation of our business and do not anticipate paying any cash dividends on our common stock in the foreseeable
future. Investors should not purchase our common stock with the expectation of receiving cash dividends.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The
following discussion and analysis provide information which our management believes is relevant to an assessment and understanding of
our results of operations and financial condition. You should read the following discussion and analysis of our results of operations
and financial condition together with our financial statements and related notes and other information included elsewhere in this prospectus.
In
addition to historical financial information, this discussion contains forward-looking statements based upon our current expectations
that involve risks and uncertainties. Our actual results could differ materially from such forward-looking statements as a result of
various factors, including those set forth under “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements”
included elsewhere in this prospectus. Additionally, our historical results are not necessarily indicative of the results that may be
expected for any period in the future.
Overview
We
are a pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting a broad range of neurologic and
neuropsychiatric disorders. Our novel oral pharmaceutical marijuana, MIRA1a, is currently under investigation for treating adult patients
suffering from anxiety and cognitive decline, often associated with early-stage dementia. MIRA1a, if approved by the FDA, could mark
a significant advancement in addressing various neuropsychiatric, inflammatory, and neurologic diseases and disorders.
We
have an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to potentially
deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD) and major depressive
disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered
a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
We
had net losses of $6.2 million and $5.7 million for the nine months ended September 30, 2023 and September 30, 2022, respectively, and
losses of $7.1 million and $2.2 million for the years ended December 31, 2022 and December 31, 2021, respectively.
Reverse
Stock Split
Effective
June 28, 2023, we completed a 1-for-5 reverse stock split of our outstanding common stock. Unless otherwise noted, the share and per
share information in this prospectus reflects the reverse stock split.
Supply
Chain Disruption / COVID-19 Business Update
Due
to the residual impact of the global COVID-19 pandemic, we have taken measures to secure our research and development activities, while
work in laboratories and facilities has been organized to reduce the risk of COVID-19 transmission. The extent of the impact of the COVID-19
pandemic on our business, operations and clinical development timelines and plans remains uncertain, and will depend on certain developments,
including the duration and spread of the outbreak and its impact on our clinical trials, CROs, manufacturing process, supply chain, and
other third parties with whom we do business, as well as its impact on regulatory authorities and our key scientific and management personnel.
While we are experiencing limited financial impacts at this time, given the global economic slowdown, the overall disruption of global
supply chains and the other risks and uncertainties associated with the pandemic, our business, financial condition, and results of operations
ultimately could be materially adversely affected. Some of our suppliers have experienced delays in securing critical raw materials;
while this has not materially impacted their services, we have observed delays in certain activities. Therefore, we continue to closely
monitor the COVID-19 pandemic as we evolve our business continuity plans, clinical development plans and response strategy.
Components
of our Results of Operations
Research
and Development Expenses
Research
and development expenses represent costs incurred to conduct research and development of our product candidate. We recognize all research
and development costs as they are incurred. Research and development expenses consist primarily of the following:
| |
● |
salaries
and benefits; |
| |
|
|
| |
● |
contracted
research and manufacturing; |
| |
● |
consulting
arrangements; and |
| |
|
|
| |
● |
other
expenses incurred to advance the Company’s research and development activities. |
Our
operating expenses have historically been the costs associated with our patent prosecution and initial investment in pre-clinical research
and development activities. We expect research and development expenses will increase in the future as we advance MIRA1a and Ketamir-2
into and through clinical trials and pursue regulatory approvals, which will require a significant investment in costs of clinical trials,
regulatory support, and contract manufacturing. In addition, we will evaluate opportunities to acquire or in-license additional product
candidates and technologies, which may result in higher research and development expenses due to license fee and/or milestone payments,
as well as added clinical development costs.
The
process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. We may never succeed in timely
development and achieving regulatory approval for our product candidates. The probability of success of our product candidates may be
affected by numerous factors, including clinical data, competition, manufacturing capability and commercial viability. As a result, we
are unable to determine the duration and completion costs of our development projects or when and to what extent we will generate revenue
from the commercialization and sale of our product candidates.
General
and Administrative Expenses
General
and administrative expenses consist of employee-related expenses, including salaries, benefits, and travel, and other administrative
functions, as well as fees paid for legal, accounting and tax services, consulting fees and facilities costs not otherwise included in
research and development expense. Legal costs include general corporate legal fees and patent costs. We expect to incur additional expenses
as a result of becoming a public company, including expenses related to compliance with the rules and regulations of the SEC and Nasdaq,
additional insurance, investor relations and other administrative expenses and professional services.
Interest
expense
Interest
expense, net consists of accrued interest on a related party line of credit, net of earned interest income.
Results
of Operations for the three months ended September 30, 2023 and 2022
| | |
Three months ended September 30, | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Operating costs: | |
| | | |
| | |
| General and administrative expenses | |
| 2,144,832 | | |
| 736,059 | |
| Related party travel costs | |
| - | | |
| 357,350 | |
| Research and development expenses | |
| 1,015,252 | | |
| 714,968 | |
| Total operating costs | |
| 3,160,084 | | |
| 1,808,377 | |
| | |
| | | |
| | |
| Interest expense, net | |
| (427,732 | ) | |
| (2,307 | ) |
| Net loss attributable to common stockholders | |
$ | (3,587,816 | ) | |
$ | (1,810,684 | ) |
| Basic and diluted loss per share | |
$ | (0.26 | ) | |
$ | (0.14 | ) |
| Weighted average common stock shares outstanding | |
| 13,639,197 | | |
| 13,168,556 | |
General
and Administrative Expenses. We incurred $2.1 million and $0.7 million in general and administrative expenses during the three months
ended September 30, 2023 and September 30, 2022, respectively. General and administrative expenses are composed primarily of compensation,
insurance, professional fees, stock-based compensation, administration and other related costs. The increase is primarily due to an increase
in stock-based compensation, debt issuance costs, and compensation related to the IPO efforts of the executive team.
Related
Party Travel Costs. We incurred $0.4 million in related party travel costs during the three months ended September 30, 2022. There
was no such expense incurred during the same period ended September 30, 2023. Related party travel costs consisted of a lease and use
of an airplane with an entity under common control. The decrease in related party travel costs is due to the termination of the lease
in March 2023.
Interest
expense. We incurred $0.43 million net in interest expense and interest income during the three months ended September 30, 2023 and
$0.002 million interest expense during the three months September 30, 2022, respectively. Interest expense during 2023 included $0.44
million of debt issuance costs and $0.02 million of interest income. The remaining 2023 and 2022 interest expense consists of accrued
interest on a related party line of credit.
Research
and Development Expenses. During the three months ended September 30, 2023, we incurred $1.0 million in research and development
expenses, which were primarily related to initial payments for pre-clinical research projects. We incurred $0.7 million in research and
development expenses during the three months ended September 30, 2022, relating to initial payment for toxicology study costs. Research
and development expenses include pre-clinical, toxicology and consultant expenses. Major components of research and development expenses
during the nine months ended September 30, 2023 are as follows:
| R&D
Category |
|
|
Expense |
|
| R&D
consultants |
|
$ |
0.1
million |
|
| R&D
preclinical research |
|
$ |
0.5
million |
|
| R&D
stock compensation |
|
$ |
0.4
million |
|
Results
of Operations for the nine months ended September 30, 2023 and 2022
| | |
Nine months ended September 30, | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Operating costs: | |
| | | |
| | |
| General and administrative expenses | |
| 3,830,303 | | |
| 2,940,469 | |
| Related party travel costs | |
| 453,550 | | |
| 1,293,050 | |
| Research and development expenses | |
| 1,185,839 | | |
| 1,466,708 | |
| Total operating costs | |
| 5,469,692 | | |
| 5,700,227 | |
| | |
| | | |
| | |
| Interest expense, net | |
| (725,273 | ) | |
| (8,484 | ) |
| Net loss attributable to common stockholders | |
$ | (6,194,965 | ) | |
$ | (5,708,711 | ) |
| Basic and diluted loss per share | |
$ | (0.45 | ) | |
$ | (0.43 | ) |
| Weighted average common stock shares outstanding | |
| 13,639,197 | | |
| 13,166,200 | |
General
and Administrative Expenses. We incurred general and administrative expenses of $3.8 million and $2.9 million during the nine months
ended September 30, 2023 and September 30, 2022, respectively, which consisted of payroll, consulting fees, IT-related costs, legal and
accounting costs, office and rent expenses, and expenses related to investor relations. We incurred $1.7 million and $1.1 million of
general and administrative stock compensation for the nine months ended September 30, 2023 and September 30, 2022, respectively.
Related
party travel costs. During the nine months ended September 30, 2023, we incurred related-party travel costs of $0.5 million compared
to $1.3 million during the nine months ended September 30, 2022. Our related party travel costs consist of payments made in connection
with an airplane lease which began in May 2021. We leased an aircraft under an operating lease with Supera Aviation I, LLC, (Supera Aviation)
with monthly rental of $0.05 million plus certain operating expenses. The Supera Aviation lease took effect on April 20, 2021 for a term
of 24 months. However, we and Supera terminated the lease on March 31, 2023. The decrease in related party travel during the nine months
ended September 30, 2023 is due to the termination of the lease in 2023.
Interest
expense. We incurred $0.72 million net in interest expense and interest income during the nine months ended September 30, 2023 and
$0.008 million September 30, 2022, respectively. Interest expense during 2023 included $0.73 million of debt issuance costs and $0.02
million of interest income. The remaining 2023 and 2022 interest expense consists of accrued interest on a related party line of credit.
Research
and Development Expenses. We incurred research and development expenses of $1.2 million during the nine months ended September 30,
2023, compared to $1.5 million during the nine months ended September 30, 2022. We incurred research and development stock compensation
of $0.6 million for the nine months ended September 30, 2023. There was no such related stock compensation expense for the same period
in 2022. The decrease in research and development expenses during the nine months ended September 30, 2023 compared to September 30,
2022 is due to higher upfront costs of the expansion in pre-clinical programs during 2022. Major components of research and development
expenses during the nine months ended September 30, 2023 are as follows:
| R&D
Category |
|
|
Expense |
|
| R&D
consultants |
|
$ |
0.2
million |
|
| R&D
preclinical research |
|
$ |
0.4
million |
|
| R&D
stock compensation |
|
$ |
0.6
million |
|
Results
of Operations for years ended December 31, 2022 and 2021
| | |
Year ended December 31, | |
| | |
2022 | | |
2021 | |
| Revenues | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Operating costs: | |
| | | |
| | |
| General and administrative expenses | |
| 2,992,125 | | |
| 770,115 | |
| Related party travel costs | |
| 1,704,350 | | |
| 697,600 | |
| Research and development expenses | |
| 2,351,465 | | |
| 684,447 | |
| Total operating costs | |
| 7,047,940 | | |
| 2,152,162 | |
| | |
| | | |
| | |
| Interest expense | |
| (10,250 | ) | |
| (24,374 | ) |
| Net loss | |
$ | (7,058,190 | ) | |
$ | (2,176,536 | ) |
General
and Administrative Expenses. We incurred general and administrative expenses of $3.0 million during the year ended December 31,
2022, which consisted of payroll, consulting fees, IT-related costs, legal and accounting costs, office and rent expenses, and expenses
related to investor relations, compared to $0.8 million during the year ended December 31, 2021, which consisted of payroll, consulting
fees, IT-related costs and investor relations costs. We incurred general and administrative stock compensation of $0.7 million for the
year ended December 31, 2022. There was no such related stock compensation expense for the year ended 2021.
Related
party travel costs. We incurred related party travel costs of $1.7 million during the year ended December 31, 2022, compared
to $0.7 million during the year ended December 31, 2021. Our related party travel costs consist of payments made in connection with an
airplane lease which began in May 2021. We lease an aircraft under an operating lease with Supera Aviation I, LLC, (Supera Aviation)
with monthly rental of approximately $50,000 plus certain operating expenses. The Supera Aviation lease took effect on April 20, 2021
for a term of 24 months, but it was mutually terminated effective March 31, 2023. The increase in related party travel during the year
ended December 31, 2022 is due to an increase in related to the expansion of pre-clinical programs, existing and potential vendor visits
and preparation for manufacturing, and ongoing Company fund raising efforts.
Research
and Development Expenses. We incurred research and development expenses of $2.4 million during the year ended December 31, 2022,
compared to $0.7 million during the year ended December 31, 2021, as our contract research organizations (“CROs”) began substantive
pre-clinical efforts on MIRA1a, primarily in the fourth quarter of 2021. We incurred research and development stock compensation of $0.5
million for the year ended December 31, 2022. There was no such related stock compensation expense for the year ended 2021. The increase
in research and development expenses during 2022 compared to 2021 is due to the expansion of pre-clinical programs during 2022. Major
components of research and development expenses during 2022 is as follows:
| R&D
Category |
|
|
Expense |
|
| Toxicology |
|
$ |
1.1
million |
|
| Pre-clinical
research |
|
$ |
0.4
million |
|
| R&D
consultants |
|
$ |
0.3
million |
|
| R&D
laboratory costs |
|
$ |
0.1
million |
|
| R&D
stock compensation |
|
$ |
0.5
million |
|
Liquidity
and Capital Resources
Since
the Company’s inception in September 2020, we have financed our operations primarily through an unsecured line of credit with a
major shareholder and an affiliated company and through a private placement of shares of our common stock that occurred during the fourth
quarter 2021 and during 2022. We intend to finance our clinical development programs and working capital needs from existing cash, potential
new sources of debt and equity financing, including the proceeds from our completed IPO in August 2023. We may enter into new licensing
and commercial partnership agreements.
On
April 28, 2023, we entered into a Promissory Note and Loan Agreement with the Bay Shore Trust, a trust established by our founder, Jonnie
R. Williams, Sr., and under which various of his family members are beneficiaries (the “Bay Shore Trust”). Under this Promissory
Note and Loan Agreement (the “Bay Shore Note”), we have the right to borrow up to an aggregate of $5,000,000 from the Bay
Shore Trust at any time up to the second anniversary of the issuance of the Bay Shore Note or, if earlier, upon the completion of our
initial public offering. Our right to borrow funds under the Bay Shore Note is subject to the absence of a material adverse change in
our assets, operations, or prospects. The Bay Share Note, together with accrued interest, will become due and payable on the second anniversary
of the issuance of the note, provided that it may be prepaid at any time without penalty. The Bay Shore Note will accrue interest at
a rate equal 7% per annum, simple interest, during the first year that the note is outstanding and 10% per annum, simple interest, thereafter.
The Bay Shore Note is unsecured. As of June 30, 2023, the Bay Shore Note had an outstanding principal balance of $1.8 million and accrued
and unpaid interest of $0.04 million. The Bay Shore Note replaced a Line of Credit Agreement that we entered into with The Starwood Trust,
a separate trust established by our founder, in May 2021 and pursuant to which we had an outstanding principal balance of $0.2 as of
the date of the Bay Shore Note (which outstanding balance was retired with an advance under the Bay Shore Note). In consideration of
the loan facility provided by the Bay Shore Trust, we issued to the Bay Shore Trust a common stock purchase warrant on April 28, 2023
giving the Bay Shore Trust the right to purchase up to 1,000,000 shares of common stock at an exercise price of $5.00 per share, which
warrant will expire five years after the date of grant.
Since
January 1, 2023, MIRALOGX, LLC, an intellectual property development and holding company owned by Bay Shore Trust (“MIRALOGX”),
has advanced funds on behalf of Bay Shore Trust to our company in order to fund operating activities. The total amount advanced and outstanding
from MIRALOGX was $1.6 million immediately prior to being consolidated into the Bay Shore Note on June 30, 2023, and such amounts become
a part of the outstanding balance of the Bay Shore Note as of June 30, 2023 and are payable under the terms of the Bay Shore Note.
On
July 20, 2023, we entered into a conversion agreement with the Bay Shore Trust under which the Bay Shore Trust has agreed to convert,
upon the completion of our initial public offering, $1,100,190 of the outstanding principal balance of the Bay Shore Note into shares
of our common stock at a conversion price equal to our initial public offering price, which resulted in the issuance of 157,170 shares
to the Bay Shore Trust upon the completion of our initial public offering (the “Bay Shore Trust Conversion Agreement”).
In
August 2023, we completed our IPO of common stock selling 1,275,000 shares at an offering price of $7.00 per share, resulting in gross
proceeds of $8.9 million. Net proceeds received after underwriting fees and offering expenses were $8.1 million. We raised $3.2 million
in 2022. Substantially all our equity capital had been raised at $1.00 per share (pre-reverse split).
We
used $3.5 million in operating activities during the nine months ended September 30, 2023, compared to $4.7 million in operating activities
during the nine months ended September 30, 2022. We used $5.6 million in operating activities during the year ended December 31, 2022,
compared to $1.4 million in operating activities during the year ended December 31, 2021.
We
have incurred significant losses and negative cash flows from operations since inception and expect to incur additional losses until
such time that we can generate significant revenue and profit. We had negative cash flow from operations of approximately $3.5 million
for the nine months ended September 30, 2023 and an accumulated deficit of approximately $15.5 million as of September 30, 2023. As of
September 30, 2023, we had cash and cash equivalents of approximately $5.9 million and working capital of $8.1 million. We currently
expect that our cash and cash equivalents be sufficient to fund our operations, development plans, and capital expenditures through at
least the fourth quarter of 2024.
We
did not have any material non-cancellable contractual obligations as of September 30, 2023.
Cash
Flows
The
following table provides information regarding our cash flows for the periods presented:
| | |
Nine Months Ended September 30, | |
| | |
2023 | | |
2022 | |
| Net cash provided by (used in): | |
| | | |
| | |
| Operating activities | |
$ | (3,497,388 | ) | |
$ | (4,629,323 | ) |
| Financing activities | |
| 9,014,740 | | |
| 2,007,186 | |
| Net change in cash | |
$ | 5,517,352 | | |
$ | (2,622,137 | ) |
Net
Cash Used in Operating Activities
The
cash used in operating activities resulted primarily from our net losses, stock-based compensation expense, amortization of debt issuance
costs and changes in components of accounts payable and accrued liabilities.
For
the nine months ended September 30, 2023, operating activities used $3.5 million of cash, primarily due to a net loss of $6.2 million,
a $0.4 million change in accounts payable, accrued and prepaid expenses, offset by $2.3 million in stock-based compensation expense and
$0.7 million in amortization of debt issuance costs. Accounts payable, accrued and prepaid expenses was primarily composed of research
and development payables, consultant costs, insurance costs and investor relations expenses.
For
the nine months ended September 30, 2022, operating activities used $4.6 million of cash, primarily due to a net loss of $5.7 million,
a $0.07 million change in accounts payable, accrued and prepaid expenses, offset by $1.1 million in stock-based compensation expense.
Accounts payable, accrued and prepaid expenses was primarily composed of research and development payables, consultant costs, insurance
costs and investor relations expenses.
Net
Cash Provided by Financing Activities
For
the nine months ended September 30, 2023, financing activities provided $9.0 million of cash, resulting primarily from $7.7 million in
proceeds from sale of common stock, less offering costs, $1.4 million of issuance of common stock for conversion of debt and issuance
of common stock in lieu of investor relation fees, and offset by $0.1 million of repayments under related party line of credit.
For
the nine months ended September 30, 2022, financing activities provided $2.0 million of cash, resulting primarily from $2.6 million in
proceeds from sale of common stock, less offering costs, offset by $0.1 million of repayments under related party line of credit.
We
currently anticipate that we will seek to monetize our product candidates, MIRA1a and Ketamir-2, at the end of our planned Phase 2 studies.
Prior to that time, we anticipate that additional capital may be required to support ongoing activities and further phases of development.
Should that be required, our available capital may be consumed more rapidly than currently anticipated, resulting in the need for additional
funding. In addition, there can be no assurance that additional funding, when and if required, will be available at commercially favorable
terms, if at all.
Accordingly,
we may need to raise additional capital, which may be available to us through a variety of sources, including:
| |
● |
public
equity markets; |
| |
|
|
| |
● |
private
equity financings; |
| |
|
|
| |
● |
commercialization
agreements and collaborative arrangements; |
| |
|
|
| |
● |
sale
of product royalty; |
| |
|
|
| |
● |
grants
and new license revenues; |
| |
|
|
| |
● |
bank
loans; and |
| |
|
|
| |
● |
public
or private debt. |
Additional
funding, capital, or loans (including, without limitation, milestone, or other payments from potential commercialization agreements)
may be unavailable on favorable terms, if at all. If adequate funds are not available, we may be required to significantly reduce or
refocus our operations or to obtain funds through arrangements that may require us to relinquish rights to certain technologies and drug
formulations or potential markets, any of which could have a material adverse effect on us, our financial condition, and our results
of operations. To the extent that additional capital is raised through the sale of equity or convertible debt securities or exercise
of warrants and options, the issuance of such securities would result in ownership dilution to existing stockholders.
If
we are unable to attract additional funds on commercially acceptable terms, it may adversely affect our ability to achieve our development
and commercialization goals, which could have a material and adverse effect on our business, results of operations and financial condition.
Recently
Issued and Adopted Accounting Pronouncements
A
description of recently issued and adopted accounting pronouncements that may potentially impact our financial position and results of
operations is disclosed in Note 8 to our financial statements appearing at the end of this prospectus.
Off-Balance
Sheet Arrangements
During
the periods presented, we did not have, nor do we currently have, any off-balance sheet arrangements as defined under SEC rules.
Quantitative
and Qualitative Disclosures about Market Risk
We
are exposed to market risks in the ordinary course of our business. These risks primarily include interest rate risks and inflation risks.
Periodically, we maintain deposits in accredited financial institutions in excess of the FDIC federally insured limits. We deposit our
cash in financial institutions that we believe have high credit quality and have not experienced any losses on such accounts and do not
believe we are exposed to any unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Interest
Rate Risk
Our
cash consists of cash in readily available checking accounts. We may also invest in short-term money market fund investments. Such interest-earning
instruments carry a degree of interest rate risk; however, historical fluctuations in interest income have not been significant.
Inflation
Risk
Inflation
generally affects us by increasing our cost of labor and research and development contract costs. We do not believe inflation has had
a material effect on our results of operations during the periods presented.
BUSINESS
Overview
We
are a pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting a broad range of neurologic and
neuropsychiatric disorders. Our novel oral pharmaceutical marijuana, MIRA1a, is currently under investigation for treating adult patients
suffering from anxiety and cognitive decline, often associated with early-stage dementia. MIRA1a, if approved by the FDA, could mark
a significant advancement in addressing various neuropsychiatric, inflammatory, and neurologic diseases and disorders.
We
have an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to potentially
deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD) and major depressive
disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered
a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
We
were organized as a Florida corporation in September 2020 and commenced substantive operations in late 2020, at which time we commenced
our pharmaceutical development program.
We
had net losses of $6.2 million and $5.7 million for the nine months ended September 30, 2023 and September 30, 2022, respectively, and
losses of $7.1 million and $2.2 million for the years ended December 31, 2022 and December 31, 2021, respectively.
Our
Product Candidates in Development
MIRA1a
Our
objective is to develop and commercialize new treatment options for neuropsychiatric, inflammatory, and neurologic diseases and disorders.
Cannabinoids are a class of chemical compounds that are naturally occurring and are primarily found in cannabis plant extracts. The two
major cannabinoids found in cannabis plant extracts include THC and CBD. These compounds bind to CB1 and CB2 cannabinoid receptors, which
are found throughout the body. Specifically, CB1 receptors are concentrated in the central nervous system (“CNS”), while
CB2 receptors are found mostly in peripheral organs and are associated with the immune system. When the chemical compounds bind to these
cannabinoid receptors, the process elicits certain physiological responses. Physiological responses to cannabinoids may vary among individuals.
Some of the effects of cannabinoids have been shown to impact nervous system functions, immune responses, muscular motor functions, gastrointestinal
maintenance, blood sugar management, and the integrity of ocular functions. Our product candidate, MIRA1a, has a strong selectivity for
CB2 versus CB1, and is designed to minimize the risk of psychoactive adverse events associated with CB1 activation.
Mechanism
of Action of MIRA1a
We
believe that the effects of MIRA1a at the cannabinoid receptors CB1 and CB2 is predicted to account for the majority of its potential
therapeutic effects, especially as it relates to its anti-anxiety, anti-pain and anti-inflammatory properties. For example, the difference
in the dose-response effects of MIRA1a compared with THC on CB1 receptors appears to coincide with its improved therapeutic profile.
If approved by the FDA, MIRA1a may potentially provide therapeutic effects for anxiety, pain and inflammation.
THC
has been demonstrated to have biphasic physiological effects, which have been described for over 40 years: at low levels THC has positive
effects while high doses cause the opposite, undesirable symptoms. Examples of biphasic effects at low versus high levels of THC include
the anti-anxiety versus pro-anxiety effects, respectively. We obtained the following dose-response effects for MIRA1a and THC at the
CB1 receptor (see below). In contrast to THC, which displays an initial maximally stimulatory and then inhibitory response at CB1, MIRA1a
appears to act as a monophasic partial agonist where it is stimulatory throughout its dose range, achieving a moderate activation of
the CB1 even at high doses. We believe that this accounts for the potential broad therapeutic efficacy of MIRA1a and the observed absence
of negative symptoms even at maximal doses of the drug.

Figure:
Compound activity with the selected GPCR Biosensor Assays:
THC
vs MIRA1a agonist activity at the CB1 Receptor.
In
pharmacology, “efficacy” or “Emax” refers to the maximum response that can be achieved with a drug or agent.
It represents the extent or magnitude of the response produced by the drug once it has bound to its target, typically referred to as
a receptor. The binding between a drug and its receptor is characterized by affinity, which quantifies the strength of their interaction.
Efficacy, however, assesses the action or effect of the drug following binding to the receptor.
The
dose-response curve is a commonly used graph in pharmacology that depicts the relationship between the effect of a drug and its dosage.
The X-axis represents the increasing doses of the drug, while the Y-axis represents the response produced by the drug. In the case of
the figure above, the term “% Efficacy” on the Y-axis refers to the maximum response that can be achieved with the agonist
(MIRA1a or THC) in relation to its ability to activate GPCR receptors (specifically CB1 receptors).
The
data presented in the figure above has been normalized to the maximal and minimal responses observed in the presence of a control compound
and vehicle, respectively. This normalization allows for a standardized comparison of the agonist’s efficacy.
Eurofins
DiscoverX has developed a panel of cell lines stably expressing non-tagged GPCRs that signal through cAMP. Hit Hunter® cAMP assays
are specialized tests that track the activation of a type of cell receptor known as GPCR. GPCRs play a crucial role in how cells respond
to external signals, and they are activated through two pathways: Gi and Gs secondary messenger signaling. These pathways are like internal
communication systems in cells that relay signals from the outside to trigger specific responses inside the cell. The assay is conducted
in a straightforward, uniform manner without the need for image-based analysis. This method uses a technology developed by DiscoverX
called Enzyme Fragment Complementation (EFC). In EFC, fragments of an enzyme, specifically β-galactosidase (β-Gal), are brought
together to become functional only when the GPCR is activated. β-Galactosidase, the enzyme used as a functional reporter in this
assay, is typically inactive in fragmented form and becomes active when the fragments reassemble, indicating the activation of the GPCR.
In this case, the GPCR target was CB1 receptor. Compounds were tested in agonist and antagonist mode with the requested GPCR Biosensor
Assays. For agonist assays, data was normalized to the maximal and minimal response observed in the presence of control ligand and vehicle.
This Eurofins DiscoverX system was used to test THC vs MIRA1a agonist activity at the CB1 receptor.
Unlike
CB1 receptors that mediate many of the psychotropic effects of cannabinoids on the CNS, CB2 receptors are predominantly present on cells
of the immune system. Based on preliminary results of our GPCR biosensor assays, the CB2 receptor agonistic effects of MIRA1a are 8-fold
more potent than THC and 30-fold more potent than CBD.
The
study regarding the ability of MIRA1a vs THC vs CBD to activate CB2Receptors and alter intracellular cAMP levels was performed by the
CRO Eurofins DiscoverX.
As
can be seen in the table below, the EC50 (i.e. concentration required to induce a half maximal response) for MIRA1a was 8 times more
potent than THC and at least 30 times more potent that CBD—i.e. it only took 1 uM of MIRA1a to induce the same response that required
8 uM of THC and >30 uM of CBD.
| Compound
Name |
|
Assay
Name |
|
Assay
Format |
|
Assay
Target |
|
Result
Type |
|
EC50 |
|
Unit |
|
| MIRA-1A |
|
cAMP |
|
Agonist |
|
CNR2/CB2 |
|
EC50 |
|
|
1.008462 |
|
|
uM |
|
| THC |
|
cAMP |
|
Agonist |
|
CNR2/CB2 |
|
EC50 |
|
|
8.209884 |
|
|
uM |
|
| CBD |
|
cAMP |
|
Agonist |
|
CNR2/CB2 |
|
EC50 |
|
|
>30 |
|
|
uM |
|
The
foregoing measurements were performed as follows:
DiscoverX
has developed a panel of cell lines that stably express non-tagged GPCRs (G-protein coupled receptors) capable of signaling through cAMP.
The Hit Hunter® assay platform is used to investigate the functionality and response of these GPCRs.
In
the case of the CB2 receptor, which is a GPCR involved in various physiological processes and has potential therapeutic implications,
the Hit Hunter® assay can be employed to study the effects of drug agonists on CB2 receptor activity.
To
measure the half maximal response (EC50) of CB2 receptor activation by a drug agonist that leads to a decrease in cAMP levels, an alternative
approach may be required. One common method involves using forskolin, an activator of adenylate cyclase, to stimulate cAMP production.
Forskolin bypasses the GPCR signaling and directly activates adenylate cyclase, resulting in increased cAMP levels.
In
the presence of forskolin, the drug agonist at the CB2 receptor can then be tested at various concentrations to determine its ability
to inhibit the forskolin-induced cAMP production. The drug’s concentration that leads to a 50% reduction in forskolin-stimulated
cAMP levels can be considered the half maximal response or EC50.
Pre-clinical
Developments and Studies
As
of the date of this prospectus, we completed several pre-clinical studies of MIRA1a, including, but not limited to, computational mutagenicity
analysis, radio-ligand binding assay, elevated plus maze (“EPM”) model of anxiety and hot plate model thermal sensitivity
testing.
We
have studied the effects of acute administration of MIRA1a on anxiety-related phenotypes in mice to model human conditions. An intraperitoneal
injection of Placebo [PBO] (e.g. saline) or MIRA1a (e.g. 50mg/kg = Treatment) was administered to C57Bl/6 mice (n=5/group) that were
8-12 weeks old. Thirty minutes following injection, mice were tested in anxiety related measures using the Elevated Plus Maze (EPM).
The EPM is a widely used pre-clinical behavioral assay for rodents and it has been validated to assess the anti-anxiety effects of pharmacological
agents. If determined and approved by the FDA or other regulatory agencies, MIRA1a has anti-anxiety effects at doses that lacked side
effects of sedation or intoxication in mice. The EPM is a test measuring anxiety in rodents as a screening test for putative anxiolytic
compounds and as a general research tool in neurobiological anxiety research such as Generalized Anxiety Disorder (GAD) or Post-Traumatic
Stress Disorder (PTSD). The model is based on the animal’s aversion to open spaces which are present in the open arms (Open Arm)
of the maze. Anti-anxiety effects of test agents are demonstrated by an increase in the percentage of time spent in the Open Arm with
treatment compared to placebo. The total distance traveled is a measure of the overall level of arousal and mobility of the mice undergoing
testing on the EPM and is used to rule out any sedating or intoxicating effects of the test agent.
Pre-clinical
studies also have shown the potential of MIRA1a for relieving pain. A number of clinically approved pharmacological agents used to treat
pain, including opioids, have been demonstrated to delay or ameliorate the onset of heat sensitivity upon paw exposure of mice to heat.
Thirty minutes after treatment with either a placebo (control) or MIRA1a, mice were placed on a heated plate to measure the time it took
for each mouse to lift its paw in response to the mild pain they felt from the heat. Mice treated with pain alleviating drugs took significantly
longer to become bothered by the heat and to lift their paws. Similarly, mice treated with MIRA1a statistically took significantly more
time to lift their legs, indicating MIRA1a’s potential effectiveness as a possible treatment for pain in this model. If approved
by the FDA, MIRA1a may potentially provide therapeutic effects for pain control.
MIRA1a
is a CB2 agonist which may be an optimal treatment for neurodegenerative diseases associated with neuroinflammation caused by microglial
activation. CB2 agonism has been shown in pre-clinical studies to regulate neuroinflammatory processes, reducing the neuronal damage
characteristic of degeneration. We believe there may be a strong rationale for CB2 agonism in neurodegenerative diseases, given increased
CB2 expression in patients with these diseases as well as preliminary results from animal models. We see potential for a potent CB2 agonist
to treat a range of neurodegenerative diseases. MIRA1a, through its robust activity at CB2 compared to CB1, was designed to minimize
the risk of psychotropic adverse events associated with CB1 activation. If approved by the FDA, MIRA1a may potentially provide therapeutic
effects for neurodegenerative and neuroinflammatory illnesses.
Our
pre-clinical development program for MIRA1a has included a variety of testing. Summarized below are the tests we have completed. Our
interpretation of results derived from pre-clinical data or our conclusions based on our pre-clinical data may prove inaccurate and are
not necessarily predictive indicators of future results.
| |
Completed
Pre-Clinical Tests* |
| |
● |
EPM
model of anxiety |
| |
● |
Thermal
Sensitivity Model of Pain |
| |
● |
Context
Fear Conditioning Model of Cognition—Test of learning and memory. |
| |
● |
Rat
Psychomotor Vigilance Test (“PVT”) of Cognition—Test of attention. |
| |
|
|
| |
*These
were non-human studies that were not powered for statistical significance and as such, no p-values are available. |
| |
● |
EPM
Model of Anxiety Test: |
| |
● |
Method:
We studied the effect of acute administration of MIRA1a on anxiety-related phenotypes in mice to model human conditions. |
| |
■ |
An
intraperitoneal (i.p.) injection of Placebo (e.g. saline) or MIRA1a (e.g. 50mg/kg = Treatment) was administered to C57Bl/6 mice (n=5/group)
that were 8-12 weeks old |
| |
■ |
30
minutes following injection, mice were tested in anxiety related measures using EPM |
| |
● |
Outcome:
The following chart demonstrates MIRA1a’s anti-anxiety effects: |
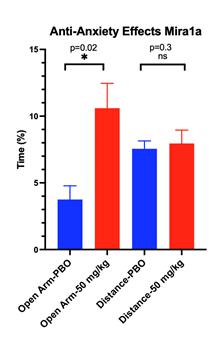
Figure:
Effects of MIRA1a vs Placebo Treatment on Mouse Behavior in the Elevated Plus Maze.
EPM
is a widely used behavioral test to assess anxiety-like behavior in rodents. Typically, rodents tend to avoid open spaces due to their
natural aversion to potentially dangerous areas. Therefore, spending more time in the open arms of the maze indicates decreased anxiety-like
behavior. Similarly, the total distance travelled can reflect general locomotor activity and exploratory behavior, which can be influenced
by the state of anxiety and the effect of drugs.
The
EPM apparatus consists of two open arms and two enclosed arms elevated above the floor. Blue Bars represent the percentage of time spent
in the open arms by mice in the placebo and drug-treated groups. Green Bars show the total distance travelled by mice in both groups
during the EPM test.
| |
● |
Thermal
Sensitivity Model of Pain: |
| |
● |
Method:
We studied the potential for pain reduction in pre-clinical models of heat tolerance using a hot plate methodology. |
| |
● |
Outcome:
MIRA1a provided significantly delayed thermal sensitivity and enhanced pain tolerance. |
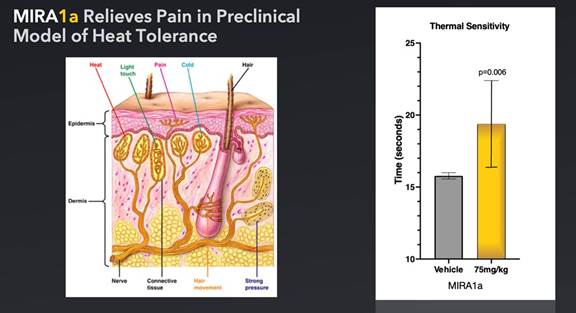
Figure:
In this thermal sensitivity test, mice are placed on a heated metal plate (e.g. 52-55 degrees Celsius). The time taken for the mouse
to show a pain response - licking or shaking of the paws, jumping, or trying to escape from the hot plate - is measured. This time interval
is known as the “hot-plate latency”. A longer latency is indicative of reduced pain sensation or a higher pain tolerance.
The
Thermal Sensitivity Model of Pain in mice is a widely used experimental approach to study nociception, which is the perception of pain.
In this model, thermal stimuli are applied to the hind paws of mice to assess their sensitivity to heat-induced pain. The procedure typically
involves placing the mouse on a temperature-controlled surface, such as a hot plate or a radiant heat source. The temperature is gradually
increased, and the response of the mouse is measured, such as the latency to withdraw its paw from the heat source. The withdrawal latency
is considered an indicator of pain sensitivity, with shorter latencies indicating greater sensitivity. By comparing the response of normal
mice to that of mice with altered pain sensitivity, such as genetically modified mice or mice treated with analgesic drugs, researchers
can gain insights into the mechanisms underlying pain perception and potential therapeutic interventions. The Thermal Sensitivity Model
of Pain in mice provides a controlled and reproducible method for studying thermal nociception, allowing researchers to investigate the
effects of various genetic, pharmacological, and environmental factors on pain sensitivity. This model has contributed significantly
to our understanding of pain pathways and the development of novel analgesic treatments.
As
performed at Johns Hopkins, in our thermal sensitivity test, which measured sensitivity to thermal pain, MIRA1a significantly increased
the time it took mice to lift their legs in comparison to placebo (p=0.006) at 75mg/kg. This indicates that MIRA1a has an analgesic effect
and may be a potential treatment for pain. Each group (i.e. placebo and 75 mg/kg) was comprised of 9 mice, for a total of 18 mice.
The
issue of how to test the effect of MIRA1a on cognition was complicated by the following:1) MIRA1a has anti-anxiety (i.e. anxiolytic)
effects, 2) anxiolytics can potentially improve cognitive assessment outcomes by reducing anxiety levels that may otherwise hinder cognitive
functioning. Thus, in commonly performed tests of cognition in mice, such as novel object recognition and Morris water maze, anxiolytic
medications can indirectly result in improved performance by decreasing anxiety rather than by directly improving cognition. In order
to separate assessments of the impact of MIRA1a on cognitive performance from its demonstrated anti-anxiety effects, we employed a model
of context fear conditioning wherein we dosed the mice after training. Context fear conditioning in mice is a behavioral paradigm used
to measure cognitive processes related to associative learning and memory. Associative learning, where an individual learns to associate
specific stimuli or contexts with particular outcomes, in this case the mice associate being in a specific chamber with receiving a mild
foot shock that occurs during training the day before testing. This process of forming associations between stimuli, actions, and consequences
is involved in numerous skills and behaviors in everyday life: it underlies learning new skills, developing habits, and acquiring knowledge
through experiences and conditioning. The use of associating the chamber with the foot shock on day one, means that when the mice are
returned to the chamber on day 2 a measure of how much freezing they do corresponds to a read out of how well they can recall the experiences
they had during training on day 1 (i.e. the greater the freezing, the better the recollection of the association between the chamber
and food shock). Since the mice are given MIRA1a AFTER training that takes place on day 1, and only before testing on day 2, there is
no concern about the anxiolytic effects of MIRA1a on learning during training, but rather this model tests MIRA1a’s effects on
performance only—which in this case represents memory (i.e. the ability to recognize and recall the chamber where they had previously
been shocked) and to translate that into an associated behavior (i.e. freezing). As published in the Journal of Neuropharmacology in
2023, THC and cannabis impair context fear conditioning, both when given prior to training (because of its anti-anxiety effects) and
when given prior to testing (because of its cognitive impairing effects). As demonstrated in the figure below, MIRA1a resulted a dramatic
effect on cognitive performance in the context fear conditioning model: as shown in B, the second panel from the left, the percentage
of time spent freezing—that is a demonstration of their memory and association—in the mice who received MIRA1a at a dose
of 75 mg/kg was more than twice that of those who received 0 mg/kg=placebo (i.e. 55% vs 25%, p<0.0001). Thus, MIRA1a doubled the cognitive
performance of the mice compared to placebo. This degree of improvement in cognitive performance in healthy mice dosed just prior to
testing and after learning has not been demonstrated with any cannabinoid compound previously.
| |
● |
Trace
Fear Conditioning Model of Cognition: |
| |
● |
Method:
We studied the potential for improving recall in healthy mice using a fear conditioning model. |
| |
● |
Outcome:
MIRA1a sharply improves cognitive recall as dosage rises. |
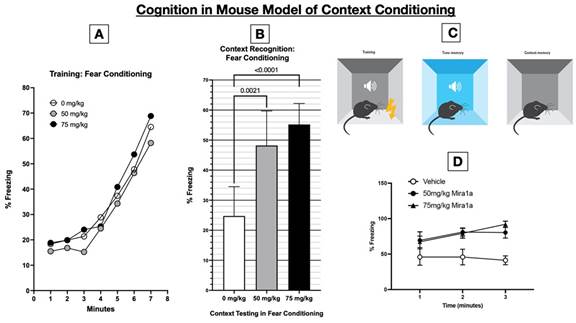
The
Contextual Fear Conditioning Model of Cognition in mice is an experimental paradigm used to study associative learning and memory processes.
It focuses on the ability of mice to form an association between a specific environmental context and an aversive stimulus, which leads
to the acquisition and subsequent retrieval of contextual memories. During the acquisition phase of the model, mice are exposed to a
distinct context, such as a particular chamber or environment. In this context, they receive an aversive stimulus, typically a mild foot
shock. The presentation of the foot shock creates an association between the contextual cues and the aversive experience. Following the
acquisition phase, the mice undergo a testing phase to assess their memory of the association between the context where they received
the foot shock and the memory of the aversive stimulus. They are returned to the same context where the conditioning took place and their
behavioral responses, particularly fear-related behaviors such as freezing or defensive reactions, are measured.
These
behavioral responses serve as indicators of the mice’s ability to retrieve the associative memory formed during the acquisition
phase. The Contextual Fear Conditioning Model of Cognition in mice has been widely used in neuroscience research to explore the mechanisms
of associative learning, memory formation, and the neural circuits involved in fear-related associations. It has contributed to our understanding
of how animals, including humans, learn to associate environmental cues with aversive experiences, and has implications for understanding
and treating conditions related to associative learning, memory deficits, and emotional disorders.
As
performed at Johns Hopkins, in the Contextual Fear Conditioning Model the data shows that during training (in the absence of any treatment)
the mice learned as indicated by increased freezing over time. The following day, 30 minutes after MIRA1a administration, the mice were
tested in the context test, which showed significantly increased % freezing (p=<0.0001) in females given 50mg/kg or 75mg/kg MIRA1a.
The experiments were conducted with 10 mice in each group (placebo, 50 or 75 mg/kg MIRA1a) for a total of 30 mice.
In
the context conditioning figure above, mice learn to associate the neutral context (the chamber) with the aversive stimulus (the foot
shock), leading to a conditioned fear response (freezing). This is indicated by ‘freezing’ behavior - a fear-related response
in mice characterized by immobility except for respiratory movements.
A
timeline of the experimental procedure, indicating acclimatization, training (conditioning), and testing phases is shown above. Panel
A, the left-most panel, shows that on day 1 the pairing of a neutral context (the conditioning chamber shown in panel C) with an aversive
stimulus (a mild foot shock). With successive foot shocks the mice show increasing amounts of freezing, since they instinctively freeze
in anticipation of being shocked. Panel B, titled “Context Recognition: Fear Conditioning,” shows the percentage freezing
the mice did on day 2 after receiving placebo or MIRA1a just prior to being placed in the same chamber they had been shocked on day 1.
Since mice freeze in anticipation of receiving a shock, the relative amount of freezing in those mice given 0 mg/kg (placebo) vs either
50 or 75 mg/kg MIRA1a is a readout of (i.e. proportional to) how well the mice recalled that the chamber they were returned to was the
one in which they had been shocked. As shown in panel B, the mice who received 75 mg/kg of MIRA1a right before being placed into the
chamber showed 200% of the freezing than did the mice who received placebo (55% vs 25%, respectively. Panel D, in the lower right corner
of the figure, shows that at 1 min after being placed in the chamber on day 2, the mice that got vehicle (=0 mg/kg MIRA1a), relative
to those that got MIRA1a, have much less freezing, and in fact have less freezing over time. The mice given MIRA1a start off with better
recognition and recall of the chamber (demonstrated as increased freezing) at 1 minute and increase the association of the chamber with
the prior shocks (because they increase freezing over time).
Because
MIRA1a is an anxiolytic, the company decided to test whether it could impair cognitive function. The company therefore sought to determine
if MIRA1a could impair attention—a different aspect of cognition than memory, recall and associative learning, and one that is
affected negatively by sedating compounds (e.g. THC, Cannabis, benzodiazepine, etc.) and positively by stimulants (e.g. caffeine, nicotine,
amphetamine) In order to assess whether MIRA1a affected attention as compared to THC required a different testing model—Psychomotor
Vigilance Test (PVT). The rat Psychomotor Vigilance Test (rPVT) is a widely used method to measure sustained attention in rodents. In
the rPVT model, rats are trained to respond to a visual stimulus by pressing a lever, with shorter reaction times indicative of better
attentional performance. Mice with longer reaction times or higher variability in response times may be considered to have attention
deficits or altered vigilance. Data is shown as percentage accuracy at pressing the lever within the allowed reaction time vs dose of
drug used. In the figure below, it can be seen that at doses of THC that impair attention, MIRA1a had no negative effects on attention
(i.e. their accuracy at pressing a lever at the right amount of time after receiving a trained cue was not impaired at all).
| |
● |
Method:
We performed a PVT to evaluate simple reaction time. |
| |
● |
Outcome:
MIRA1a does not impair cognition. At 3 mg/kg and 10 mg/kg MIRA1a causes minimal impairment in rat PVT whereas THC has a clear
negative effect even at these low doses. |
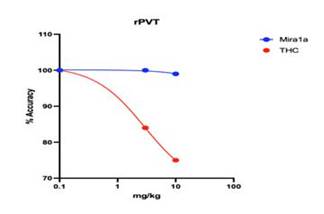
Figure:
Comparison of MIRA1a versus THC on Psychomotor Vigilance Test (PVT) Performance in Rats. The figure displays the percentage accuracy
of rats in the Psychomotor Vigilance Test (PVT) following administration of MIRA1a (blue) or THC (red). The y-axis represents the percentage
accuracy (% Accuracy), indicating the proportion of correct responses in the PVT task. The x-axis represents the treatment condition,
with increasing amount of compound being given to the rats before testing. The data shows that rats treated with MIRA1a exhibited no
decrease in percentage accuracy compared to the THC group (p < 0.05). The results indicate that administration of MIRA1a had no negative
impact on attention performance in the PVT task, as evidenced by the maintenance of 100% accuracy across the dosage range, compared to
THC that impaired attention leading to decreased accuracy more and more with increasing dosages.
The
Psychomotor Vigilance Test (PVT) is a behavioral test used in rats to assess attention and speed of response, providing insights into
their vigilance and cognitive performance. It is based on the measurement of reaction times to visual stimuli, typically presented in
a simple reaction time task paradigm.
In
the PVT, rats are typically placed in an operant chamber or testing apparatus equipped with a visual stimulus, such as a light or LED.
The rats are trained to perform a specific response, such as pressing a lever or nose-poking, when the visual stimulus appears. The timing
of the visual stimuli is randomized to prevent predictability and maintain the animals’ attention.
During
the test, the rats are required to pay attention to the visual stimuli and respond as quickly as possible when they appear. The reaction
time, which represents the time it takes for the rat to initiate the response upon stimulus presentation, is recorded. This measure reflects
the speed of response and can provide an indication of the rat’s attentional state and ability to sustain attention over time.
By analyzing the reaction time data, researchers can evaluate the rat’s attentional performance, including measures such as mean
reaction time, variability in response times, and the occurrence of lapses or errors. The PVT has been widely used to investigate the
effects of different manipulations, such as pharmacological interventions that cause sedation, sleep deprivation, or experimental treatments,
on attention, alertness, and cognitive performance in rats.
Therefore,
the combination of cognitive assessments demonstrated the following: despite having anxiolytic effects, 1) MIRA1a significantly improved
associative learning, memory and recall in the context fear conditioning model, and 2) MIRA1a had no negative effects on attention at
doses that THC showed significant impairment. This is the first time a cannabinoid has been shown to enhance (rather than inhibit) cognition
when given to normal healthy mice after training but before testing, demonstrating a specific cognitive improvement as a direct effect
on the brain that is independent of indirect effects—such as with acute administration by decreasing anxiety or with long term
administration by having anti-inflammatory effects in neurodegenerative diseases.
In
2023, our pre-clinical work will include the conduct of several other pre-clinical studies and initiation of a 7-day maximum tolerated
dose study of MIRA1a in rats and dogs.
| Status |
|
Planned
Activity |
| Drug
Substance Preparation |
●
●
● |
Analytical
Development
NonGMP
Production Refinement
GMP
Production Refinement |
| Testing |
●
●
●
●
●
●
●
●
●
|
MTD/7D
DRF Dog
MTD/7D
DRF Rat
Dog
28-day Toxicology
Rat
28-day Toxicology
Cardiovascular
Study Dog (Telemetry)
Respiratory
Study Rat
hERG
(Manual Patch-Clamp)
Neurobehavioral
Evaluation Rats
Neurobehavioral
Evaluation Mice |
We
further plan on neurobehavioral evaluation of orally and intraperitoneally administered MIRA1a in rats and mice, respiratory evaluation
of orally administered MIRA1a in rats, and in vitro testing for effects of MIRA1a on hERG (the human Ether-à-go-go-Related Gene)
channel currents. The hERG is an early in vitro assay required by the FDA to alert companies of any potential cardiac abnormalities by
the product before proceeding with dose studies in humans. hERG is a gene that codes for a protein known as the alpha subunit of a potassium
ion channel. This ion channel (sometimes simply denoted as ‘hERG’) is best known for its contribution to the electrical activity
of the heart: the hERG channel mediates the repolarizing current in the cardiac action potential, which helps coordinate the heart’s
beating. When this channel’s ability to conduct electrical current across the cell membrane is inhibited or compromised, either
by application of drugs or by rare mutations in some individuals, it can result in a potentially fatal disorder called long QT syndrome.
Testing
is anticipated to conclude in the first quarter of 2024. Additionally, a 28-day toxicology analysis for dogs and rats is expected to
begin at the end of the fourth quarter of 2023 and continue through the first quarter of 2024.
We
have started the analytical development and manufacturing of MIRA1a as of January 2023. By the third quarter of 2023, we anticipate our
suppliers will be developing MIRA1a at scale and manufactured under cGMP conditions, expanding on earlier non-GMP volumes of MIRA1a for
use in our initial testing programs. We plan to work closely with our suppliers to generate sufficient volumes of cGMP-grade MIRA1a materials
for the planned pre-clinical toxicity programs, expanded animal testing and human trials expected to be performed in 2024, subject to
FDA approval.
Our
Clinical Development Program
Following
the pre-clinical development plan outlined above, we plan to submit to the FDA an Investigational New Drug application (“IND”)
focused on investigating MIRA1a for the treatment of anxiety and cognitive decline in elderly patients.
Our
first IND application submission investigating MIRA1a for the treatment of elderly patients suffering from anxiety with some cognitive
decline is currently planned for the end of the third quarter of 2024, as we believe this is a patient population with unmet needs. If
allowed to proceed by the FDA, a Phase I trial will be initiated 30 days post-IND submission.
Our
second IND application will focus on investigating MIRA1a for the treatment of chronic pain.
All
development plans depend on FDA acceptance of our IND applications. As appropriate and pursuant to discussions with the FDA, we may periodically
adjust the timeline for certain filings and associated clinical trials. It is important to note that the process for conducting clinical
trials is uncertain and there is no assurance that our clinical development activities will meet the planned timelines set forth above.
Manufacture
of Product for Clinical Development Activities
Curia
Global (formerly AMRI), a leading global CDMO, is currently developing a large-scale synthesis protocol for us and will be supplying
quantities of MIRA1a needed for our pre-clinical and clinical development activities. We are currently in discussions with other partners
to have MIRA1a formulated into solid oral dosage forms for clinical trials.
Market
Opportunity
MIRA1a,
if approved, will compete in three key overlapping growth markets: the anxiety, cognitive decline (CNS/dementia), and chronic pain markets
where multiple products with varying safety and efficacy profiles are already on the market. MIRA1a competes at the intersection of these
three markets given the target patient profile for MIRA1a.
MIRA1a
will compete primarily within the CNS market that encapsulates anxiety, dementia, other pain, Alzheimer’s, migraines and related
conditions. Based on the market size of the CNS opportunity as set forth in IQVIA’s Global Use of Medicines 2023 analysis (the
“IQVIA Report”), we estimate that by 2027, the U.S. CNS market will be worth $48 billion, growing between two and five percent
during the period from 2023 to 2027. Within that market opportunity, anxiety is worth between approximately $10 billion and $15 billion
in annual sales. If approved by the FDA, MIRA1a may potentially provide therapeutic effects for anxiety, dementia and pain.
Anxiety
and pain are expected to grow approximately five percent over the same period according to the IQVIA Report, while Alzheimer’s
is expected to grow approximately twelve percent. This is critical given MIRA1a’s focus on early-stage patients with dementia,
as according to the Alzheimer’s Association 2023 Alzheimer’s Disease Facts and Figures analysis (the “Alzheimer
Association”), 0.5 million new Alzheimer cases emerge in the U.S. each year. According to the Alzheimer Association, about 60 to
80 percent of Alzheimer cases evolve into dementia. Thus, Alzheimer case directions are an important signal and gateway for MIRA1a-related
opportunities in dementia. Based on that epidemiology, the US Center for Disease Control (“CDC”) estimates that approximately
5.8 million Americans are living with Alzheimer’s, with that number expected to grow to 14 million by 2060 (“CDC Alzheimer”).
MIRA1a’s
other key market will be the traditional U.S. pain market, which the IQVIA Report estimates will be worth $42 billion in 2027 and grow
between three and six percent during the forecast period. Note that this sizing is inclusive of chronic and acute pain, and MIRA1a is
likely to only be used in the chronic segment of the market (approximately 40% to 50% of the market). Factors such as a rise in oncology
related pain, diabetic neuropathy, and pain associated with aging (e.g. joint pain) are among the key drivers of patient and prescription
growth. Opioid toxicity and related annual deaths suggest a novel non-opioid pain killer is needed. Given the overlap across indications
and the fact that the target patient is presenting across these markets.
Our
initial focus will be a dual path: potentially winning in traditional markets as well as the marijuana analog markets using a safe, effective
and, if determined by the FDA, an FDA-approved treatment option since safety and efficacy determinations are in the exclusive purview
of the FDA. Today, legal medical marijuana is a $13.2 billion industry whereas legal recreational marijuana is a $25.6 billion industry.
Both are sub-sets of the traditional pain and anxiety markets. However, in many patient populations, non-US legal, and cultural settings,
marijuana may not be the first or a viable option for treatment of neurological disorders. As a result, these patients will typically
use non-steroidal anti-inflammatory drugs (NSAIDs) or various mood management drugs, opening them up to a range of non-ideal outcomes.
The objective of MIRA1a is to offer physicians and patients an approved, viable synthetic option. Thus, if approved by the FDA, we believe
that MIRA1a may potentially provide a preferred alternative in such patient populations, as it is not derived from the marijuana plant.
Our
Market Advantage
MIRA1a
is being developed as the first manufactured prescription drug to potentially target the CB1 and CB2 receptors for chronic pain and anxiety
without the impurities of marijuana or its side effects, such as increased appetite and paranoia. MIRA1a has demonstrated the ability
to rapidly and significantly improve cognitive performance with acute use—i.e. doubling cognitive performance after a single dose
in normal mice (see figure on page 4 and 51). MIRA1a is a novel synthetic cannabinoid analog directed at potentially treating patients
with dementia associated cognitive decline and anxiety diagnoses. Unlike other cannabinoids in the market, MIRA1a is not derived from
plants. Plants generate alkaloids as a defense mechanism, and it has been speculated that plant-derived cannabinoids have adverse side
effects in humans.
Furthermore,
in animal studies conducted by us, MIRA1a has preliminarily demonstrated more than 30-fold increased CB2 activation compared to CBD.
Our
Strategy
Our
goal is to develop therapeutics targeting well-characterized CB1 and CB2 receptors with optimized pharmacological properties to transform
the lives of patients with neurological diseases. Key elements of our strategy to achieve this goal include:
| |
● |
Advance
our MIRA1a through clinical development and approval. Our product candidate, MIRA1a, is in pre-clinical studies. Existing
treatment options for neuropsychiatric disorders and neurological diseases have significant limitations, and, if approved, we believe
MIRA1a would represent a major therapeutic advancement for patients. |
| |
● |
Continue
pre-clinical development of MIRA1a across a range of CNS diseases associated with neurodegeneration and progress into clinical development.
MIRA1a is currently in IND-enabling studies for neurobehavioral disorders such as dementia, Post-Traumatic Stress Disorder
(PTSD), chronic pain, as well as neurodegenerative diseases such as Alzheimer’s and Parkinson’s Disease. We believe MIRA1a
may have potential in several diseases associated with neuroinflammation, including multiple sclerosis. |
| |
|
|
| |
● |
Identify
additional product candidates and expand current candidates into additional neurological diseases. We see potential for our
current product candidate to be evaluated in clinical trials outside of its initial indications and will evaluate additional indications
to maximize the potential of our drug development program. Our current product focus is on targets that are well characterized in
neurological diseases but for which there are limitations with currently available therapies. We also plan to continue to identify
and develop additional novel product candidates that align with our focus. |
| |
|
|
| |
● |
Explore
strategic collaborations to maximize the value of our product candidates. We plan to explore collaborations opportunistically
to maximize the value of our product candidates. We intend to retain significant economic and commercial rights to our programs in
key geographic areas that are core to our long-term strategy. |
Competition
We
are subject to competition from pharmaceutical and biotechnology companies and academic and research institutions. We believe our future
success will depend, in large part, on our ability to maintain a first mover advantage and competitive lead in our industry.
Competition
arises mainly from two sources, traditional cell-based in vitro culture approaches and traditional in vivo animal models and testing.
We also face future competition from companies developing cannabinoid therapies, as summarized in the table below:
Sativex
(delta-9-tetrahydrocannibinol and cannabidiol in the EU) is an oromucosal spray indicated as treatment for symptom improvement in adult
patients with moderate to severe spasticity due to multiple sclerosis (MS) who have not responded adequately to other anti-spasticity
medication and who demonstrate clinically significant improvement in spasticity related symptoms during an initial trial of therapy.
Sativex is not assigned a schedule in the U.S. by the DEA as it is not approved but is a Class B controlled drug under the Misuse of
Drugs Act 1971 and is placed in Schedule 4 to the Misuse of Drug Regulations 2001 in the United Kingdom.
Marinol
(dronabinol) is an oral cannabinoid indicated in adults for the treatment of: Anorexia associated with weight loss in patients with AIDS
and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic
treatments. Marinol is a Schedule III controlled substance.
Cesamet
(Nabilone) is a synthetic cannabinoid for oral administration that are indicated for the treatment of the nausea and vomiting associated
with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. Cesamet contains nabilone,
which is a controlled in Schedule II of the Controlled Substances Act (CSA).
KETAMIR-2
Major
Depressive Disorder (MDD) is a significant global health concern, affecting over 264 million people worldwide and ranking among the leading
causes of disability. In the United States alone, it impacts nearly 17.3 million adults, accounting for about 7.1% of the adult population.
This widespread mental health disorder not only undermines the quality of life and daily functioning of individuals but also imposes
a substantial economic burden, with costs in the U.S. amounting to tens of billions of dollars annually. MDD is also a major risk factor
for suicide, a leading cause of death globally, highlighting its profound impact on public health and the urgent need for effective treatment
and management strategies. If approved by the FDA, KETAMIR-2 may potentially provide antidepressant therapeutic effects.
Despite
the fact that antidepressants have been around for decades, with imipramine being the first FDA-approved antidepressant in 1959, the
need for a rapid-acting antidepressant that can help patients with Treatment-Resistant Depression (TRD) using a novel mechanism of action
(e.g. not a monoamine reuptake inhibitor) has been growing. In 2019, Ketamine was introduced but required a Risk Evaluation and Mitigation
Strategy (REMS) because of its: 1) poor oral availability requiring IV or IN administration, 2) ability to cause side effects including
dissociation, sedation and acute hypertension, and 3) potential abuse liability.
Ketamir-2
is a new chemical entity, analog of Ketamine that is designed to potentially preserve the same rapid antidepressant response but with
improved bioavailability. It may also have decreased side effects, and decreased abuse liability, though such conclusions are within
the sole authority of the FDA. This combination is intended to potentially facilitate safer and less cumbersome dosing requirements,
with the goal of obtaining an orally administered pill that can be taken at home.
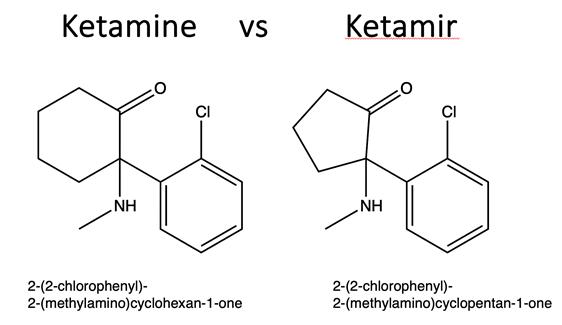
Figure
1: Structures of Ketamine and Ketamir-2 for comparison purposes.
The
Drug Enforcement Administration (DEA) conducted a scientific review of the Ketamir-2 “in accordance with the definitions within
the CSA and its implementing regulations. Based on this review, DEA determined that” Ketamir-2 is “not [a] controlled
substances or listed [chemical] under the CSA.”
Mechanism
of Action of Ketamir-2
Ketamir-2’s
mechanism of action (MOA) as a rapid acting antidepressant is the same as Ketamine’s, based on the fact that the two share a common
inhibitory effect on the NMDA receptor that is believed to be integral to the antidepressant effects of both of these drugs.1
In fact, Ketamir-2 and Ketamine differ in less than 2% in their antagonist activity at the GRIN1/GRIN2B receptor subunit of the NMDA
receptor (based in in silico analysis, see below). This subunit combination is prominently linked to neuroplasticity, believed
to be a key factor in depression and the action of antidepressants such as Ketamine.2 GRIN2B-containing NMDA receptors are
implicated in synaptic plasticity changes associated with depression and its treatment.
Ketamine’s
mechanism of action (MOA) as a rapidly acting antidepressant is multifaceted and distinct from traditional antidepressants like selective
serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants.1 While ketamine has shown promise as a rapid-acting antidepressant,
especially in treatment-resistant depression, its use is limited due to potential side effects and abuse potential that Ketamir-2 has
been targeted to minimize. Moreover, whereas Ketamine has a poor oral bioavailability and must therefore be given IV or IN, Ketamir-2
has a much better bioavailability suggesting it may be appropriate for oral use.3,4 Here’s a detailed synopsis of its
MOA of both Ketamir-2 and Ketamine:
| 8. | NMDA
Receptor Antagonism: Ketamine primarily acts as a non-competitive antagonist of the N-methyl-D-aspartate
(NMDA) receptor, a type of glutamate receptor. By inhibiting these receptors, ketamine modulates
the release of the neurotransmitter glutamate. This modulation leads to an increase in glutamatergic
signaling via activation of AMPA receptors, another type of glutamate receptor. This enhanced
signaling is believed to play a crucial role in ketamine’s rapid antidepressant effects.1 |
| 9. | mTOR
Pathway Activation: Ketamine activates the mammalian target of rapamycin (mTOR) pathway,
a key regulator of cell growth and survival. This activation is linked to increased synaptogenesis
in the prefrontal cortex. The mTOR pathway plays a significant role in neural plasticity
and has been implicated in the pathophysiology of depression.1 |
| 10. | Effects
on GABAergic System: Recent research indicates that ketamine may also affect the gamma-aminobutyric
acid (GABAergic) system, which is responsible for inhibitory neurotransmission in the brain.
Alterations in GABAergic signaling have been associated with mood disorders.1 |
| 11. | BDNF
Release and Synaptogenesis: The increased glutamatergic transmission leads to the activation
of downstream pathways that result in the release of Brain-Derived Neurotrophic Factor (BDNF).
BDNF is crucial for neuroplasticity – the brain’s ability to reorganize and form
new neural connections. Studies suggest that this increase in BDNF and subsequent synaptogenesis
(formation of new synapses) in brain areas like the prefrontal cortex is a key factor in
the antidepressant effects of ketamine.1 |
| 12. | Anti-inflammatory
Effects: Depression is increasingly linked with chronic inflammation. Ketamine has been
shown to have anti-inflammatory properties, which might contribute to its antidepressant
effects.5 |
| 13. | Neuroendocrine
Regulation: Ketamine may influence the hypothalamic-pituitary-adrenal (HPA) axis, which
is often dysregulated in depression. By modulating this axis, ketamine could exert additional
antidepressant effects.6 |
| 14. | Rapid
Onset of Action: Unlike traditional antidepressants, which typically take weeks to exert
their effects, ketamine’s impact on mood can be noticed within hours of administration.
This rapid action is especially beneficial in acute management of severe depression and suicidal
ideation. |
In
summary, while Ketamir-2’s and ketamine’s antidepressant MOA are still being studied and explored, current evidence suggests
a complex and involved synergistic action on various neural pathways, primarily through the modulation of glutamatergic neurotransmission,
enhancement of neuroplasticity, and potentially through anti-inflammatory and neuroendocrine mechanisms. Both drugs rapid onset and efficacy
in treatment-resistant cases make them unique and valuable tools in psychiatry, but the potentially improved side effect profile and
oral bioavailability are what differentiate Ketamir-2 and ketamine as described below.
Preclinical
Research Findings
In
Silico Analysis of Targets of Ketamir-2 vs Ketamine
In
silico analysis, referring to computer-based techniques, has become an integral part of pharmaceutical research and development.7
This approach utilizes computational methods to analyze and predict the properties and behaviors of pharmaceutical compounds. The
use of in silico analysis is especially crucial in the early stages of drug development, as it aids in identifying potential drug targets
and elucidating differences between a new drug and its parent compound. By analyzing large datasets, such as genomic, proteomic, and
metabolomic data, researchers can predict how different compounds might interact with various biological targets. This approach helps
in understanding the mechanism of action of new drugs and can significantly reduce the time and cost associated with experimental screening.
InSilico Trials was contracted to provide a comparison between targets of Ketamir-2 vs Ketamine employing their target identification
protocol. The following characterize some of the unique targets that are predicted to interact with either Ketamir-2 or Ketamine, thereby
differentiating one drug from the next.
Ketamir-2
selective target:
BRD4,
or Bromodomain-containing protein 4, is a member of the bromodomain and extra-terminal (BET) family of proteins and has been implicated
in the regulation of gene expression, particularly those involved in cell cycle progression and inflammatory responses.8 In
the context of depression, research has started to explore the role of BRD4 and its potential impact.
| 7. | BRD4
and Neuroinflammation: Inflammation is increasingly recognized as a significant factor in
the pathophysiology of depression. BRD4 has been found to regulate the expression of inflammatory
genes. Its inhibition, therefore, might reduce neuroinflammation, which is thought to contribute
to depressive symptoms. |
| 8. | Gene
Expression Regulation: BRD4 influences the transcription of genes involved in mood regulation
and stress response. Dysregulation of these genes can contribute to the development of depression.9 |
| 9. | Pharmacological
Target: BRD4 is a target for new pharmacological interventions in depression. Inhibitors
of BRD4, such as JQ1, have shown promise in preclinical studies for their antidepressant
effects. These compounds can modulate the expression of genes associated with mood and stress
response. |
| 10. | Epigenetic
Mechanisms: As an epigenetic regulator, BRD4’s role in modifying the expression of
genes without changing the DNA sequence might be crucial in understanding the long-term impact
of environmental factors on depression.10 |
| 11. | Animal
Studies: Research in animal models has provided some evidence that modulation of BRD4 activity
can influence behaviors related to depression. However, translating these findings to human
depression is complex and requires more research. |
| 12. | Thus,
while BRD4 is not traditionally associated with depression like neurotransmitter systems
(e.g., serotonin or dopamine), emerging evidence suggests that it plays a role in the disease’s
pathophysiology. Its involvement in regulating gene expression, particularly related to inflammation
and stress response, positions it as a potential target for novel antidepressant therapies.9 |
Ketamine
selective targets:
Alpha-2a
adrenergic receptor: Alpha-2a adrenergic receptors are G protein-coupled receptors (GPCRs) involved in the modulation of neurotransmitter
release.11 They are generally thought to be inhibitory, reducing the release of norepinephrine when activated, which can lead
to various physiological effects.
| ● | Cardiovascular
Effects: Alpha-2a receptors play a role in cardiovascular regulation, which might explain
some of the blood pressure and heart rate changes seen with ketamine.12 |
| ● | Sedation:
Activation of these receptors can lead to sedative effects, which is consistent with the
tranquilizing effects that ketamine can produce.13 |
Sigma
Opioid Receptor: Ketamine is known for its dissociative anesthetic properties, which are primarily attributed to its antagonism of the
N-methyl-D-aspartate (NMDA) receptor. However, the sigma receptors, particularly the sigma-1 receptor, have also been implicated in the
psychotomimetic and dissociative effects of ketamine.14 Here’s how ketamine’s interaction with sigma opioid receptors
might contribute to its dissociative side effects:
| ● | Cognitive
and Perceptual Processes: Activation of 15has been linked to modulating cognitive
and perceptual processes, which could be associated with the dissociative effects experienced
during ketamine administration. |
| ● | Modulation
of NMDA Receptor Activity: Sigma-1 receptors are known to interact with NMDA receptors, and
this interaction might enhance or modulate the dissociative effects of ketamine, which primarily
acts as an NMDA receptor antagonist.16 |
Mu-Opioid
Receptor: The Mu-opioid receptor (MOR) is one of the principal targets within the central nervous system for endogenous opioids like
endorphins and enkephalins, as well as for exogenous opioid analgesics such as morphine and fentanyl.17 Activation of MOR
typically results in analgesic effects, reduced gastrointestinal motility, respiratory depression, and can influence the reward system
in the brain, which is associated with the pleasurable sensations or euphoria. Activation of the MOR by Ketamine could contribute to
side effects related to its abuse liability:
| ● | Euphoria
and Reward: MOR activation is heavily implicated in the reward pathway and can produce euphoria.
This effect is a key driver of the abuse potential of opioids. |
| ● | Tolerance
and Dependence: Chronic activation of the MOR leads to tolerance (the need for increasing
doses to achieve the same effect) and physical dependence, contributing to the cycle of abuse. |
| ● | Sedation:
MOR activation can also result in sedation, which might contribute to the overall sedative
effects of ketamine, particularly at higher doses. |
Opiate
Receptor Issue
One
topic that deserves explanation relates to the belief by some of the researchers in the field of ketamine antidepressant effects suggested
that mechanism of action of this drug is not by blocking the NMDA receptor but instead is mediated by its Mu-Opioid receptor (MOP) agonist
activity.18 This belief was generated by the observation that naltrexone, which blocks the MOP, inhibited the antidepressant
effects of ketamine. Since Ketamir-2 does not have MOP agonist activity, it might seem surprising that it does have antidepressant activity
in the same mouse model that first lead to the discovery of low-dose ketamine antidepressant properties (i.e. the Forced Swim Test FST).
We therefore take the time here to provide an explanation that resolves these seemingly paradoxical observations.
| 5. | Ketamir-2
and Its Pharmacology: |
| ○ | Ketamir-2
is a newly synthesized compound analogous to ketamine. Unlike ketamine, Ketamir-2 does not
bind to the mu-opioid receptor. This receptor is often linked with the addictive properties
of many drugs, including ketamine. |
| ○ | This
lack of MOP agonist activity suggests that Ketamir-2 may have less addictive properties,
thus potentially improving its safety profile. |
| 6. | Ketamine,
Mu-Opioid Receptor, and Depression: |
| ○ | The
majority of people in the field of low-dose ketamine antidepressant activity believe the
evidence that suggests that it is working via NMDA receptor blockade. |
| ○ | However,
Shatzberg et al at Stanford have suggested that Ketamine’s antidepressant effects might
be mediated through its interaction with the mu-opioid receptor.18 |
| ○ | This
hypothesis was supported by the Stanford groups studies showing that the administration of
naltrexone, a mu-opioid receptor antagonist, reduces the antidepressant effects of ketamine.
This implies that blocking this receptor diminishes ketamine’s efficacy.18 |
Figure
2: Study in humans demonstrating that IV Ketamine (K) pluse Placebo (P) had rapid antidepressant effects that were blocked by Naltrexone
(N).
| 7. | Wang
and Kaplin’s Counterargument: |
| ○ | Adam
Kaplin, MD, PhD is the president and CSO of MIRA. At Johns Hopkins he and his student Michael
Wang published a plausible scenario that explains one possible way in which naltrexone could
block Ketamine’s antidepressant response in a manner that has nothing to do with Ketamine’s
MOP agonist activity.19 |
| ○ | Wang
and Kaplin proposed an alternative interpretation of the interaction between ketamine, naltrexone,
and the mu-opioid receptor. They argue that the interference of naltrexone with ketamine’s
antidepressant effects might not be due to its action at the mu-opioid receptor. Instead,
they suggest a complex interaction involving the N-methyl-D-aspartate (NMDA) receptor signaling
pathway. |
| ○ | According
to their hypothesis, naltrexone’s antagonism at the mu-opioid receptor increases cyclic
AMP (cAMP) levels. This elevation in cAMP interferes with the activation of the mammalian
target of rapamycin (mTOR) pathway by ketamine, a crucial mediator of its antidepressant
effects. This suggests a more intricate mechanism beyond direct opioid receptor activity. |
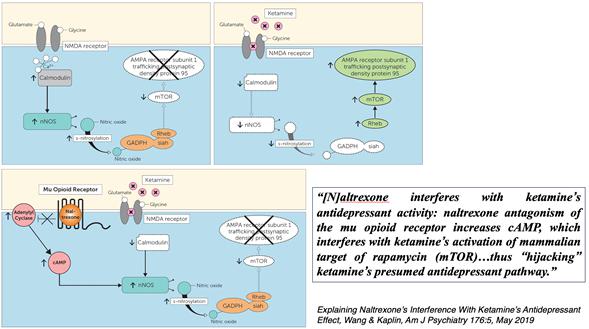
Figure
3: Plausible mechanism whereby naltrexone—by blocking constitutively active signalling from the Mu-Opioid Receptor—interferes
with the downstream signaling produced by Ketamine at the NMDA receptor that is independent of any effect of Ketamine on the opioid receptor.
| 8. | Ketamir-2’s
Antidepressant Effects in Animal Models: |
| ○ | Despite
not targeting the mu-opioid receptor, Ketamir-2 exhibited antidepressant effects in the mouse
FST model of antidepressant effects. This finding is significant because it demonstrates
that the mu-opioid receptor activity of both Ketamir-2 (which has none) and Ketamine (which
is a MOP agonist) is not necessary for their antidepressant effects, challenging the Stanford
understanding of ketamine’s mechanism of action in treating depression. |
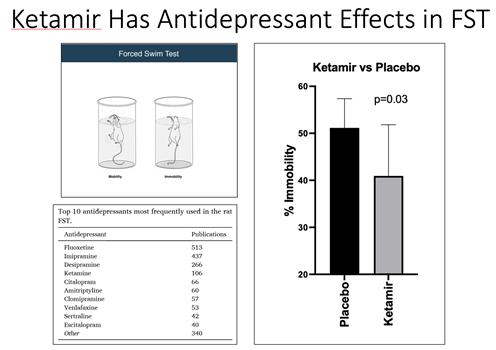
Figure
4: The Forced Swim Test, how many publications that involve antidepressants that have used it, and the statistically significant effect
of Ketamir-2 in the test demonstrating its antidepressant properties.
In
conclusion, the finding that Ketamir-2 has antidepressant effects despite having no MOP agonist activity supports the model by Wang and
Kaplin that suggests that naltrexone can have effects on second messenger signaling that interferes with Ketamine’s intracellular
effects, which has nothing to do with Ketamine’s antidepressant activity via NMDA receptor blockade. The end result is the development
of a Ketamine analog (i.e. Ketamir-2) that maintains Ketamir-2’s antidepressant effects but without it MOP agonist activity, thereby
creating a potentially safer treatment alternative.
Bioavailability:
The
Caco-2 cell model, originating from a human colorectal adenocarcinoma cell line, plays a significant role in pharmaceutical research
for estimating the intestinal absorption and indirectly the bioavailability of drugs.20 Bioavailability, the proportion of
a drug that enters the systemic circulation when introduced into the body, is crucial for determining a drug’s effectiveness. Traditionally,
bioavailability is determined through in vivo studies, including human and animal trials, as well as in vitro models like the Caco-2
cell model and in silico computational approaches.
The
Caco-2 model involves culturing cells that differentiate into a monolayer mimicking the intestinal epithelium, complete with tight junctions
and microvilli. This model is pivotal in permeability studies to assess how well drugs can pass through the intestinal barrier and in
understanding both active and passive drug transport mechanisms. While primarily used for estimating drug absorption, the Caco-2 model
also serves to predict potential drug-drug interactions within the gastrointestinal system.
The
Caco-2 model offers a high-throughput, cost-effective, and human-relevant system, making it a preferred choice for initial screening
of multiple compounds. In pharmaceutical research, the Caco-2 model often serves as an initial study to predict the absorption properties
of new drugs and is typically validated against clinical data once that becomes available.21 It plays a crucial role in the
early stages of drug development, influencing decisions on which compounds to advance.
CaCO-2
cells are human epithelial colorectal adenocarcinoma cells that are widely used as an in vitro model of the intestinal barrier.20
The CaCO-2 assay is employed to study the absorption and transport of orally administered drugs across the intestinal epithelium.
The assay evaluates the permeability of a drug from the apical (AP) side, representative of the intestinal lumen, to the basolateral
(BL) side, representative of the blood side, and vice versa.
The
bidirectional transport assays conducted with CaCO-2 cells can provide the following insights about two different drugs:
| 7. | Absorption
Potential: The AP to BL (A→B) transport rate can indicate a drug’s ability to
be absorbed through the intestines into systemic circulation. Higher transport rates suggest
better absorption potential. |
| 8. | Efflux
Ratio: By comparing the BL to AP (B→A) transport rate with the A→B transport rate,
one can determine the efflux ratio. If the efflux ratio is significantly greater than 1,
this implies that there are active efflux mechanisms, such as P-glycoprotein, that are pumping
the drug back into the intestinal lumen, thus reducing its absorption. |
| 9. | Permeability
Classification: The transport rates can be used to classify the drugs according to their
permeability. High permeability drugs are absorbed more completely and are likely to have
a more reliable and faster onset of action. |
| 10. | Influence
of Efflux and Influx Transporters: Differences in the AB-BA values between two drugs can
indicate the involvement of different efflux or influx transporters, suggesting that the
drugs have different affinities for these transporters. |
| 11. | Impact
of Metabolism: If a drug is extensively metabolized by the intestinal wall before reaching
systemic circulation, this will be reflected in a low A→B permeability. |
| 12. | Predicting
Oral Bioavailability: Generally, drugs that exhibit high permeability in CaCO-2 assays are
expected to have good oral bioavailability, although this is not always the case due to other
factors such as solubility and first-pass metabolism.20 |
In
summary, the CaCO-2 intestinal absorption (AB-BA) assay is a valuable tool for predicting the intestinal absorption and oral bioavailability
of drugs. Differences in the assay results between two drugs can provide important information about their absorption characteristics,
potential interactions with transporters, overall oral bioavailability, and possible drug-drug interactions.
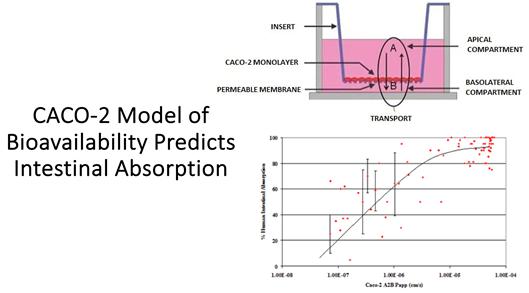
Figure
5: Model of the CaCO-2 model of drug intestinal absorption and how well it correlates with actual measures of human intestinal absorption.
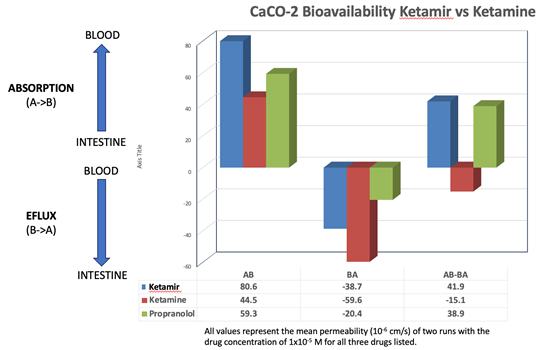
Figure
6: Data obtained from the CaCO-2 model of intestinal absorption. Propranolol, a commonly prescribed beta-blocker that is taken orally
and used to treat hypertension, is included as a positive control. The intestinal absorption (AB), Intestinal efflux (BA) and net absorption
(AB-BA) are shown.
As
can be seen in Figure 6 of the Bioavailability of Ketamir-2 vs Ketamine, as determined by the CaCO-2 model, comparing the rate for Ketamir-2
vs Ketamine, the absorption from the intestinal lumen into the blood that is 80% greater (80.6 vs 44.5), the rate of efflux back into
the intestinal lumen that is 35% less (-38.7 vs -59.6), and the net absorption (AB-BA) rate is 3.77 fold greater [(41.9+15.1)/15.1=3.77],
respectively. Since the reported oral bioavailability of Ketamine has been reported to be between 16-30% (average of 23%),3
then the predicted oral bioavailability of Ketamir-2 could be as high as 87% (i.e. Ketamir-2’s oral bioavailability is 3.77 fold
greater than Ketamine’s = 23%*3.77=87%).
This
is just an approximation, and when sufficient Ketamir-2 has been synthesized to do in vivo animal initially and then human Pharmacokinetic
(PK) studies, it will be possible to get a more precise estimate of Ketamir-2’s Oral Bioavailability compared to Ketamine by testing
and calculating the area under the concentration-time curve (AUCoral) for oral dosing divided by the AUC for IV dosing (i.e. AUCoral/AUCiv).22
But based on the available preliminary estimates, it appears highly likely that the oral bioavailability of Ketamir-2 in humans
is going to be substantially larger than that of Ketamine. Orally available Ketamir-2, as opposed to IV or IN Ketamine, would be much
easier to be patient self-delivered at home, thereby improving on the ease and availability of this rapid acting antidepressant for TRD
& MDSI.
Our
Clinical Development Program
The
clinical development plan for Ketamir-2 involves a series of methodically structured phases, starting with IND-enabling studies and progressing
through Phase 1 and Phase 2 clinical trials. These trials aim to establish the safety, efficacy, and optimal use of Ketamir-2 in treating
psychiatric conditions like TRD, Major Depressive Episode with Suicidal Ideation (MDSI), and potentially Post-Traumatic Stress Disorder
(PTSD). The strategy underscores patient safety while evaluating Ketamir-2’s therapeutic benefits and risks. The successful development
of Ketamir-2 could significantly impact the treatment landscape for depression, offering a novel approach that addresses the shortcomings
of current therapies.
Initially,
the development process begins with completion of all necessary IND-enabling studies. These preclinical studies, encompassing pharmacokinetics,
pharmacodynamics, toxicology, and safety pharmacology, are crucial for ensuring that the investigational drug meets regulatory standards.
The successful completion of these studies allows for the submission of an Investigational New Drug (IND) application to the FDA, specifically
targeting TRD.
Upon
FDA approval, the plan progresses to Phase 1 clinical trials. These trials are designed to assess the safety and tolerability of Ketamir-2
in healthy volunteers. They are typically randomized, double-blind, and placebo-controlled, and aim to determine the appropriate dosing
while closely monitoring for adverse effects. Key to this phase is the collection of pharmacokinetic and pharmacodynamic data, which
guides the dosing strategies for subsequent trials.
Phase
1: Safety and Dosage Determination in Healthy Volunteers
| ○ | A
randomized, double-blind, placebo-controlled trial. |
| ○ | Primary
objective: Assess safety and tolerability of Ketamir-2. |
| ○ | Secondary
objectives: Determine pharmacokinetics and pharmacodynamics. |
| ○ | Enroll
healthy volunteers, ensuring a diverse demographic representation. |
| ○ | Exclude
individuals with a history of psychiatric illness, substance abuse, or significant medical
conditions. |
| 7. | Dosing
and Administration: |
| ○ | Start
with a low dose, escalating gradually to higher doses. |
| ○ | Monitor
participants closely for adverse effects. |
| ○ | Safety
assessments: Vital signs, laboratory tests, ECG, adverse event monitoring. |
| ○ | PK/PD
assessments: Blood sampling for drug levels, brain imaging for receptor binding (if feasible). |
Following
the establishment of safety and initial dosing parameters in Phase 1, the development plan moves into Phase 2. This phase involves trials
with patients diagnosed with TRD. The primary goal here is to evaluate the optimal dose and tolerability of Ketamir-2 in this specific
patient population. Additionally, these trials provide preliminary data on the efficacy of Ketamir-2 for the treatment of TRD. Safety
remains a priority, with close monitoring for any adverse events and detailed assessments using depression rating scales.
Phase
2: Dose, Tolerability, and Early Efficacy in TRD
| ○ | A
randomized, controlled trial with TRD patients. |
| ○ | Primary
objective: Evaluate the optimal dose and tolerability. |
| ○ | Secondary
objective: Obtain preliminary efficacy data. |
| ○ | Enroll
patients diagnosed with TRD. |
| ○ | Utilize
standardized diagnostic criteria and severity scales. |
| ○ | Implement
a dose range based on Phase 1 findings. |
| ○ | Consider
flexible dosing or fixed-dose regimen based on safety and tolerability data. |
| ○ | Tolerability
assessment: Adverse event monitoring, patient-reported outcomes. |
| ○ | Efficacy
assessment: Depression rating scales (e.g., HDRS, MADRS). |
As
the development of Ketamir-2 progresses, there is potential to expand its indications. One such area is MDSI, where Ketamir-2’s
application could be particularly beneficial given ketamine’s established efficacy in this domain.24 This would involve
designing a trial specifically targeting MDSI, with a focus on the rapid onset of action and short-term safety considerations.
Furthermore,
given the emerging research suggesting ketamine’s therapeutic potential in PTSD, a similar approach could be considered for Ketamir-2.25
Developing a trial protocol for PTSD treatment requires a careful balance, considering the complexity of the disorder, potential
comorbidities, and the need for robust safety and efficacy data.
Pursuing
Additional INDs:
| 3. | Major
Depressive Episode with Suicidal Ideation (MDSI): |
| ○ | Following
successful Phase 2 outcomes, pursue an IND for MDSI, leveraging existing data and research
on ketamine.26 |
| ○ | Design
a trial specifically targeting MDSI, focusing on rapid onset of action and short-term safety. |
| 4. | Post-Traumatic
Stress Disorder (PTSD): |
| ○ | Based
on early research suggesting ketamine’s efficacy in PTSD, consider developing a clinical
trial protocol for Ketamir-2 in PTSD.25 |
| ○ | Prioritize
safety and efficacy, given the complex nature of PTSD and potential comorbidities. |
In
summary, the clinical development plan for Ketamir-2 is a meticulous, multi-phase strategy that prioritizes patient safety while exploring
the drug’s potential in treating complex psychiatric conditions. Each phase is carefully designed to address specific research
questions and regulatory requirements, ensuring a thorough evaluation of Ketamir-2’s therapeutic benefits and risks.
Market
Opportunity and Our Market Advantage
Ketamir-2’s
market opportunity and market advantage was analyzed by IQVIA27 who were contracted to perform an independent Market Characterization
and Drug Valuation Analysis. The following is a summary of their findings: MIRA Pharmaceuticals is developing Ketamir-2, a novel ketamine
derivative, for the treatment of Treatment-Resistant Depression (TRD) and Major Depressive Disorder with Suicidal Ideation (MDSI). These
indications represent areas of high unmet medical need, with significant disease burden and limited effective treatments available. Ketamir-2’s
formulate on as a once-daily oral medication addresses shortcomings in existing treatments, such as route of administration (RoA) and
time to effectiveness, which are issues with Spravato, a current ketamine-based therapy.
The
market opportunity for Ketamir-2 is substantial. The U.S. has a large patient pool looking for effective treatments, with diagnosed prevalence
rates of 3.1% for MDSI and 2.4% for TRD, translating to total addressable populations of 4.9 million and 3.8 million patients respectively.28
Based on a total estimates of MDSI and TRD together, this represents a Total Diagnosed Prevalence rate of 12.3 million patients
and, assuming a Treatment Rate of 65%, the Total Addressable Population is 8.7 million patients This represents a significant market,
especially considering the current limitations and side effects associated with existing treatments.

Figure
7: Estimates by IQVIA of the total addressable populations affected with MDSI and TRD.
Ketamir-2’s
market advantage lies in its unique profile and potential to address these unmet needs. As a synthetic ketamine derivative, it offers
an improved mechanism to treat disease, building on the success of Spravato but with upgrades to the base molecules to reduce unwanted
side effects. Its oral formulation, not requiring health care professional (HCP) supervision, contrasts with Spravato’s administration
requirements, potentially improving patient compliance and ease of use.
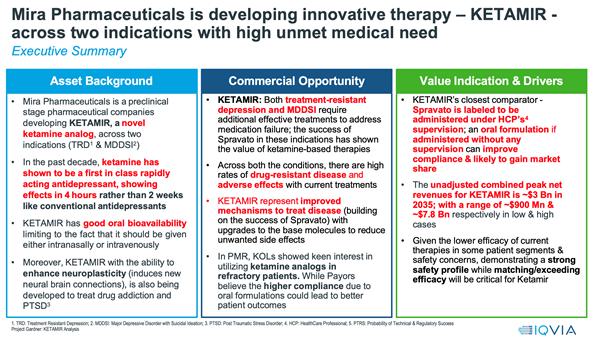
Figure
8: Summary of assessment by IQVIA of valuation of Ketamir-2, including the background, commercial opportunity, and drivers of valuation
assessment.
The
financial projections for Ketamir-2 are promising. If approved by the FDA and deemed safe, peak annual net sales in the U.S. are estimated
to potentially reach approximately $3 billion across both indications, with a base case eNPV (expected net present value) of around $92
million. In the high case scenario, the unadjusted peak revenue opportunity could go up to about $7.8 billion by 2035, with the eNPV
potentially reaching $324 million. The estimated patient pool for Ketamir-2 treatment may reach approximately 0.2 million patients in
the U.S. by 2036. The NPV (net present value) ranges from approximately $270 million to $4.6 billion, with the base case being around
$1.4 billion.
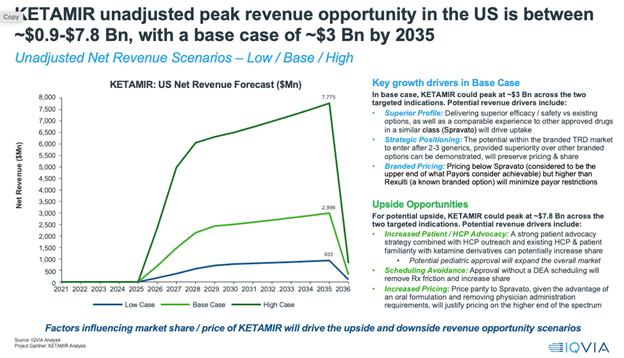
Figure
9: Actual valuation of Ketamir-2 over time, including base and peak revenue opportunities in the US.
These
estimates are based on several key assumptions, including the market share Ketamir-2 might achieve, the years to peak sales, gross price
per dose, and the Probability of Technical & Regulatory Success (PTRS). Feedback from key opinion leaders (KOLs) and payors suggests
that there is a significant unmet need in behavioral health, particularly for treatments like Ketamir-2 with fewer adverse effects and
more consistent outcomes. However, issues such as pricing, insurance coverage, and potential DEA scheduling are important considerations
that could affect Ketamir-2’s market penetration.
In
summary, Ketamir-2 presents a significant market opportunity in the treatment of TRD and MDSI, offering a novel approach with potential
advantages over existing therapies in terms of efficacy, safety, and patient compliance. The financial outlook is positive, contingent
upon successful market penetration and realization of its therapeutic potential.
Our
Strategy
MIRA,
following its successful IPO and the licensing of Ketamir-2 from Miralogx, is positioned to advance the development of Ketamir-2. The
ultimate goal is to continue develop Ketamir-2 as an orally administered medication with potentially fewer side effects, freed from the
restrictions such as those imposed by Ketamine’s REMS, to fill the current clinical need for a rapid acting antidepressant to manage
TRD and MDSI in patients who are able to take Ketamir-2 at home. The strategic plan for Ketamir-2’s development encompasses several
critical stages, from scaling up manufacturing to exploring effective exit strategies.
Scaling
Up Manufacturing at Recipharm and Benuvia (both third party vendors): The first step for us is to scale up the manufacturing process
of Ketamir-2. Small-scale synthesis has already been achieved at Recipharm, which will be essential for refining the process and identifying
potential challenges. This will be followed by a phase of process optimization, focusing on improving yield, purity, and cost-efficiency.
In order to determine the best manufacturer, we contracted with two manufacturers: Recipharm and Benuvia. Whichever company successfully
demonstrates the ability to manufacture 100 grams of Ketamir-2 efficiently and with a good purity will be the one that is contracted
to make kilogram quantities needed by Frontage for IND enabling safety and toxicology studies. Of note, Benuvia has expressed some interest
in not only manufacturing Ketamir-2, but potentially partnering in the development of this drug through their investment and collaboration.
This would obviously affect the decision about the ultimate manufacturer of Ketamir-2. We will then transition to pilot-scale production
to validate the manufacturing process under real-world conditions. Once the process is established, large-scale manufacturing can commence,
ensuring compliance with Good Manufacturing Practices (GMP) standards. Integral to this stage is the development of a robust supply chain
strategy to manage the consistent availability of raw materials and distribution.
IND-Enabling
Research at Frontage: Prior to IND submission, we must conduct comprehensive IND-enabling research. This includes pharmacokinetics/pharmacodynamics
(PK/PD) studies to understand how Ketamir-2 is absorbed, distributed, metabolized, and excreted, along with its mechanism of action.
Toxicology and tolerability studies are also crucial, encompassing both acute and chronic toxicology assessments in relevant animal models,
including 7 and 28-day studies in rats and dogs. Additionally, the development of a stable and effective formulation for Ketamir-2’s
oral administration is necessary.
IND
Submission for TRD Indication: With the data from preclinical studies in hand, we will prepare and submit an Investigational New
Drug (IND) application to the FDA. This submission will include all preclinical data and a proposed plan for clinical trials. A well-thought-out
regulatory strategy is essential to address potential queries and concerns from regulatory bodies.
Clinical
Trials - Phase 1 and 2: Upon IND approval, clinical development will proceed with Phase 1 trials, focusing on assessing the safety,
tolerability, and optimal dosing in a small group of healthy volunteers. This will be followed by Phase 2 trials, where the efficacy
of Ketamir-2 will be evaluated in a larger group of patients, along with further safety assessments.
Exit
Strategy: Given that we have already undergone an IPO, the exit strategy will focus on partnerships and licensing. We can explore
partnerships with larger pharmaceutical companies for further development and commercialization of Ketamir-2. Licensing agreements can
also be considered, allowing other companies to market Ketamir-2 in different regions or for varied indications. The following options
could be considered depending on the available opportunities:
| 4. | Strategic
Partnerships and Collaborations: This can involve partnering with larger pharmaceutical
companies, which brings the benefit of their extensive resources, global market reach, and
regulatory expertise. However, such partnerships often mean sharing profits and relinquishing
some control over the drug. Collaborations with biotechnology firms in similar therapeutic
areas can also be beneficial, offering synergistic research efforts and niche expertise,
though these firms may not provide as much financial support as larger pharma companies. |
| 5. | Licensing
Agreements: We could choose to license the drug to another company for further development
and commercialization. Out-licensing can provide an immediate capital infusion and reduce
the risk and investment required for later-stage trials. However, this often leads to losing
direct control over the development and commercialization processes. Co-development and co-marketing
deals are another form of licensing where the development, marketing, and commercialization
responsibilities are shared, which can combine strengths and reduce individual risks but
requires aligned objectives and effective collaboration. |
| 6. | Additional
Funding and Investment: Seeking additional capital through a Secondary Public Offering
(SPO) is a way to fund Phase 3 trials and marketing efforts but can dilute existing shareholders’
equity. Private investments and venture capital are also viable options, offering large sums
of capital and business expertise, though they may lead to a potential loss of autonomy and
come with high expectations for returns. |
Additionally,
the potential for a buyout from a larger pharmaceutical company remains a viable exit strategy, especially if Ketamir-2 demonstrates
substantial promise.
Throughout
this process, it is crucial for us to maintain a robust intellectual property strategy, regularly assess the antidepressant market landscape,
especially for treatment-resistant depression (TRD), and engage with key stakeholders. Implementing a risk management plan is also essential
to navigate potential development and commercialization challenges. This strategic plan must be adaptable, capable of responding to new
data, regulatory feedback, and changes in the market. Regular assessments and checkpoints will ensure the project aligns with our strategic
goals and the evolving landscape of pharmaceutical development.
Competition
Ketamine,
originally known as a dissociative anesthetic, has emerged as a significant breakthrough in the treatment of depression, particularly
due to its rapid-acting antidepressant properties. The FDA approved in 2019 eskteamine delivered IN developed by Janssen with the brand
name Spravato.28 This has opened new avenues in psychiatric treatment, especially for patients who do not respond to traditional
antidepressants, have depression with suicidal ideation, or require rapid antidepressant responses.
In
contrast to most novel antidepressants, which are multi-billion dollar drugs annually, 2023 Janssen is projected to bring in $600M.29
The primary reason for this discrepancy is because Spravato’s REMS requires Spravato to be patient administered but clinician
observed for 2 hours, with the patient unable to drive for the rest of the day. This is quite challenging for both patients and clinicians,
which has severely restricted the use of this form of Ketamine from patients who would benefit from this treatment (e.g. those with TRD
and Major Depression with Suicidal Ideation—MDSI).
Thus,
the principle competitor of Ketamir-2 is Ketamine.
Niche
Filled by Ketamine
| 1. | Treatment-Resistant
Depression: Ketamine has shown efficacy in cases where conventional antidepressants fail,
addressing a significant gap in mental health treatment. |
| 2. | Rapid
Onset of Action: Unlike traditional antidepressants that may take weeks to show effects,
ketamine can produce noticeable antidepressant effects within hours to days, providing immediate
relief in acute cases of depression. |
| 3. | Suicidality:
It has shown promise in rapidly reducing suicidal thoughts, which is crucial in acute psychiatric
emergencies. |
Limitations
of Ketamine Due to Side Effects
| 1. | Psychotomimetic
Effects: Ketamine can induce dissociative symptoms, hallucinations, and other psychotomimetic
effects, limiting its use to controlled settings.30 |
| 2. | Potential
for Abuse: Given its history as a recreational drug, there are concerns about its potential
for abuse and addiction. |
| 3. | Short
Duration of Effect: The antidepressant effect of ketamine can be transient, requiring
repeated administrations, which may increase the risk of side effects. |
| 4. | Physical
Side Effects: These may include increased heart rate, elevated blood pressure, nausea,
and dizziness. |
Requirements
of Ketamine under the REMS (Risk Evaluation and Mitigation Strategy)31
The
use of ketamine, especially Esketamine (a nasal spray form of ketamine approved for treatment-resistant depression), is regulated under
the Risk Evaluation and Mitigation Strategy (REMS) program to ensure safe use:
| 1. | Healthcare
Setting Administration: Esketamine must be administered in a certified healthcare setting
under the supervision of a healthcare provider. |
| 2. | Patient
Monitoring: Patients must be monitored for at least two hours after administration due
to the risk of sedation and dissociation. |
| 3. | Restricted
Distribution: The drug is not available for take-home use and can only be dispensed to
healthcare facilities and pharmacies enrolled in the REMS program. |
| 4. | Patient
Education and Consent: Patients must be informed about the risks and provide written
consent. |
| 5. | Follow-up
and Reporting: Healthcare providers are required to report any serious adverse effects
and ensure follow-up to monitor the patient’s response to treatment. |
Conclusion
Ketamine’s
role as a rapid-acting antidepressant fills a crucial niche in the management of treatment-resistant depression and acute suicidality.
However, its use is tempered by significant side effects and the stringent requirements of the REMS program, which necessitate careful
patient selection and monitoring to optimize safety and efficacy.
The
finding of up to 80% oral bioavailability with the potential for decreased abuse liability (e.g. because of the lack of opiate agonist
activity) and potentially decreased side effects (e.g. fewer dissociative experiences and less hypertension) puts Ketamir-2 in a prime
situation to potentially offer the same antidepressant effects but with fewer restrictions, perhaps even permitting patients to take
it orally at home.
Regulation
The
U.S. Food and Drug Administration (FDA) and comparable regulatory authorities in state and local jurisdictions impose substantial and
burdensome requirements upon companies involved in the clinical development, manufacture, marketing, and distribution of drugs. These
agencies and other federal, state, and local entities regulate, among other things, the research and development, testing, manufacture,
quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, distribution, post-approval
monitoring and reporting, sampling and export and import of our drug candidates.
U.S.
Government Regulation
In
the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations.
The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes
and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements
at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative
or judicial sanctions, such as the FDA’s refusal to approve pending New Drug Applications (NDAs), withdrawal of an approval, imposition
of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution,
injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The
process required by the FDA before a drug may be marketed in the United States generally involves the following:
| |
● |
completion
of pre-clinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice
(“GLP”) regulations; |
| |
|
|
| |
● |
submission
to the FDA of an Investigational New Drug (“IND”) application, which must become effective before human clinical trials
may begin; |
| |
● |
approval
by an independent Institutional Review Board (“IRB”), at each clinical site before each trial may be initiated; |
| |
|
|
| |
● |
performance
of adequate and well-controlled human clinical trials in accordance with good clinical practices (“GCP”) requirements
to establish the safety and efficacy of the proposed drug product for each indication; |
| |
|
|
| |
● |
demonstration
that the API and finished product are manufactured under cGMP conditions and meet all applicable standards of identity, strength,
quality, and purity; |
| |
|
|
| |
● |
submission
to the FDA of an NDA; |
| |
|
|
| |
● |
satisfactory
completion of an FDA advisory committee review, if applicable; |
| |
|
|
| |
● |
satisfactory
completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance
with cGMP requirements and to assure that the facilities, methods, and controls are adequate to preserve the drug’s identity,
strength, quality, and purity; |
| |
|
|
| |
● |
FDA
review and approval of the NDA, including consideration of the views of any FDA advisory committee, prior to commercial marketing
or sale of the drug in the United States; and |
| |
|
|
| |
● |
compliance
with any post-approval requirements, including the potential requirement to implement a Risk Evaluation and Mitigation Strategy (“REMS”)
or to conduct a post-approval study. |
Pre-clinical
studies
Before
testing any drug or biological product candidate in humans, the product candidate must undergo rigorous pre-clinical testing. The pre-clinical
developmental stage generally involves laboratory evaluations of drug chemistry, formulation, and stability, as well as studies to evaluate
toxicity in animals, to assess the potential for adverse events (“AEs”) and, in some cases, to establish a rationale for
therapeutic use. The conduct of pre-clinical studies is subject to federal regulations and requirements, including GLP regulations for
safety/toxicology studies. An IND sponsor must submit the results of the pre-clinical studies, together with manufacturing information,
analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND.
An
IND is a request for authorization from the FDA to ship an investigation product and then administer it to humans and must be allowed
to proceed by the FDA before human clinical trials may begin. Some long-term pre-clinical testing, such as animal tests of reproductive
AEs and carcinogenicity, may continue after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the
FDA, unless the FDA raises concerns or questions before that time related to one or more proposed clinical trials and places the trial
on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin.
As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical
trials
The
clinical stage of development involves the administration of the investigational product to healthy volunteers or patients under the
supervision of qualified investigators, generally physicians not employed by, or under control of, the trial sponsor, in accordance with
GCPs, which include the requirement that all research patients provide their informed consent for their participation in any clinical
trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures,
subject selection and exclusion criteria and the parameters to be used to monitor subject safety and assess efficacy. Each protocol,
and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Furthermore, each clinical trial must
be reviewed and approved by an IRB for each institution at which the clinical trial will be conducted to ensure that the risks to individuals
participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the
informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical
trial until completed. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trial results
to public registries. Information about most clinical trials must be submitted within specific timeframes for publication on the www.clinicaltrials.gov
website. Information related to the product, patient population, phase of investigation, study sites and investigators and other aspects
of the clinical trial is made public as part of the registration of the clinical trial. Sponsors are also obligated to disclose the results
of their clinical trials after completion. Disclosure of the results of these trials can be delayed in some cases for up to two years
after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress
of development programs.
Human
clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
| |
● |
Phase
I clinical trials generally involve a small number of healthy volunteers or disease-affected patients who are initially exposed to
a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism,
pharmacologic action, side effect tolerability and safety of the drug. |
| |
|
|
| |
● |
Phase
II clinical trials involve studies in disease-affected patients to determine the dose required to produce the desired benefits. At
the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, possible adverse effects and safety
risks are identified, and a preliminary evaluation of efficacy is conducted. |
| |
|
|
| |
● |
Phase
III clinical trials generally involve a larger number of patients at multiple sites and are designed to provide the data necessary
to demonstrate the effectiveness of the product for its intended use, its safety in use and to establish the overall benefit/risk
relationship of the product and provide an adequate basis for product approval. These trials may include comparisons with placebo
and/or other comparator treatments. The duration of treatment is often extended to mimic the actual use of a product during marketing. |
Post-approval
trials, sometimes referred to as Phase IV clinical trials, may be conducted after initial marketing approval. These trials are used to
gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow
up. In certain instances, the FDA may mandate the performance of Phase IV clinical trials as a condition of approval of an NDA or a Biologics
License Application (“BLA”).
Progress
reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if significant
adverse events (“SAEs”) occur. The FDA or the sponsor may suspend or terminate a clinical trial at any time, or the FDA may
impose other sanctions on various grounds, including a finding that the research patients are being exposed to an unacceptable health
risk. Similarly, an IRB can refuse, suspend, or terminate approval of a clinical trial at its institution if the clinical trial is not
being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Concurrently
with clinical trials, companies usually complete additional pre-clinical studies and must also develop additional information about the
physical characteristics of the drug or biological product as well as finalize a process for manufacturing the product in commercial
quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches
of the product candidate and, among other things, the sponsor must develop methods for testing the identity, strength, quality, potency,
and purity of the final biological product. Additionally, appropriate packaging must be selected and tested, and stability studies must
be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life.
Marketing
Approval
Assuming
successful completion of the required clinical testing, the results of the pre-clinical studies and clinical trials, together with detailed
information relating to the product’s chemistry, manufacture, controls, and proposed labeling, among other things, are submitted
to the FDA as part of an NDA requesting approval to market the product for one or more indications. In most cases, the submission of
an NDA is subject to a substantial application user fee.
The
review process typically takes twelve months from the date the NDA is submitted to the FDA. The FDA conducts a preliminary review of
all NDAs within the first 60 days after submission to determine whether they are sufficiently complete to permit substantive review before
accepting them for “filing.” The FDA may request additional information rather than accept an NDA for filing. In this event,
the application must be resubmitted with the additional information and may be subject to an additional application user fee. The resubmitted
application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins
an in-depth substantive review. The FDA reviews an NDA to determine, among other things, whether the drug is safe and effective and whether
the facility in which it is manufactured, processed, packaged, or held meets standards designed to assure the product’s continued
safety, quality and purity. Under the current guidelines in effect in the Prescription Drug User Fee Act (PDUFA), the FDA has a goal
to review and act on the submission within ten months from the completion of the preliminary review of a standard NDA for a new molecular
entity.
The
FDA also may require submission of a REMS plan to ensure that the benefits of the drug outweigh its risks. The REMS plan could include
medication guides, physician communication plans, assessment plans, and/or elements to assure safe use, such as restricted distribution
methods, patient registries, or other risk minimization tools.
The
FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including
clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be
approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations
carefully when making decisions.
Before
approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve
an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate
to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect
one or more clinical trial sites to assure compliance with GCP requirements.
After
evaluating the NDA and all related information, including the advisory committee recommendation, if any, and inspection reports regarding
the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter, or, in some cases, a complete response letter.
A complete response letter generally contains a statement of specific conditions that must be met in order to secure final approval of
the NDA and may require additional clinical trials or pre-clinical studies in order for FDA to reconsider the application. Even with
submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria
for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
Post-approval
requirements
Drugs
manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among
other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion
and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications
or other labeling claims are subject to prior FDA review and approval. There also are continuing annual user fee requirements for any
marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications
with clinical data.
Intellectual
Property
Our
company owns U.S. Patent 10,787,675 B2, titled “Purified Synthetic Marijuana and Methods of Treatment by Administering Same,”
which covers the MIRA1a compound per se as a racemic mixture, an isolated R-enantiomer, or an isolated S-enantiomer, as well as
pharmaceutical formulations of the compound. This patent also covers MIRA1a in methods of treating Alzheimer’s disease, anxiety,
depression, and addictions and expires on February 11, 2039.
Foreign
patents covering MIRA1a, and its therapeutic uses have issued in Australia, Belgium, Canada, Czech Republic, France, Germany, Greece,
Netherlands, Hungary, Ireland, Israel, Italy, Malta, Poland, Portugal, Romania, South Korea, Spain, Sweden, and the United Kingdom, and
corresponding applications are pending in China and Japan. MyMD currently owns these foreign patents and patent applications. We currently
have no plans to develop the MIRA1a compound for approval and commercialization outside of the United States or for manufacture outside
of the United States, including in the foreign jurisdictions in which MyMD has patent rights. We may in the future seek an agreement
to license or purchase all or a portion of such foreign patent rights from MyMD, but we have no current plans to do so and there is no
assurance that we would be able to successfully conclude such an agreement. MyMD’s foreign patent rights would not preclude us
from pursuing the development, manufacture, approval, or commercialization of the MIRA1a compound in foreign jurisdictions in which MyMD
does not have patent rights, such as India, if we chose in the future to pursue such activities. See “Risk Factors— Risks
Related to Our Intellectual Property— We own the rights associated with our patents in the United States, but we do not own the
rights to patents covering MIRA1a in foreign jurisdictions.”
Notwithstanding
the foregoing, we have a worldwide perpetual, royalty free, non-exclusive license from MyMD to use MyMD’s Supera-CBD™, a
different compound from MIRA1a, as a synthetic intermediate in the manufacture of MIRA1a for all purposes (including clinical development
and commercial production). In consideration of this license, we agreed to share with MyMD technical information and know-how that pertains
to the synthetic manufacture and/or formulation of our MIRA1a product candidate and granted a license to MyMD to use improvements to
MIRA1a made under the agreement, and the agreement does not involve any prior or future cash payments by us. Except for this license,
we do not license any patent rights or other intellectual property for MIRA1a from third parties. Although we believe that Supera-CBD
is currently the best available synthetic intermediate for the manufacture of MIRA1a, we believe that other intermediates and/or processes
could be used to manufacture MIRA1a.
Besides
relying on patents, we also rely on trade secrets, proprietary know-how and continuing innovation to develop and maintain our competitive
position, especially when we do not believe that patent protection is appropriate or can be obtained. We seek protection of these trade
secrets, proprietary know-how and any continuing innovation, in part, through confidentiality and proprietary information agreements.
However, these agreements may not provide meaningful protection for, or adequate remedies to protect, our technology in the event of
unauthorized use or disclosure of information. Furthermore, our trade secrets may otherwise become known to, or be independently developed
by, our competitors. We intend to seek appropriate patent protection for technology in our research and development programs, where applicable,
and their uses by filing patent applications in the United States and other selected countries. We intend for these patent applications
to cover, where possible, claims for compositions of matter, medical uses, processes for preparation and formulations.
We
license the U.S., Canadian, and Mexican patent rights for the use of KETAMIR-2 in human applications from MIRALOGX LLC, an intellectual
property development and holding company established by Jonnie R. Williams, Sr., the founder of our company and the sole inventor of
KETAMIR-2. MIRALOGX is owned by the Bay Shore Trust, an irrevocable trust established by Mr. Williams. MIRALOGX filed U.S. Provisional
App. No. 63/537,744 on September 11, 2023 and U.S. Provisional App. No. 63/451,891, on March 13, 2023, both titled, ANTIDEPRESSANT COMPOUNDS,
PHARMACEUTICAL COMPOSITIONS, AND METHODS OF TREATING DEPRESSION AND OTHER DISORDERS. MIRALOGX plans to file a corresponding international
application under the Patent Cooperation Treaty (PCT) in 2024 and in due course enter the national phase in the United States, Canada
and Mexico, among other countries. These applications, if granted and subject to payment of patent maintenance fees, would offer protection
extending through at least March 13, 2044. The patent rights for KETAMIR-2 outside of the United States, Canada, and Mexico are not included
in our current patent rights.
Our
license from MIRALOGX is set forth in the Exclusive License Agreement, dated November 15, 2023, pursuant to which the licensed field
of use includes therapeutic treatments and other medical or health uses in humans, and related preclinical studies and activities conducted
in furtherance of obtaining regulatory approval for and commercialization of human therapeutic treatments and uses (the “MIRALOGX
License Agreement”). “Licensed Product” is defined as a drug product containing as an active agent 2-(2-chlorophenyl)-2-(methylamino)cyclopentan-1-one
or a pharmaceutically acceptable salt or ester thereof. We also have the right to grant corresponding sublicenses under the licensed
patent rights. The MIRALOGX License Agreement provides for the payment to MIRALOGX of an 8% royalty (payable quarterly) on our net sales
of Licensed Products by us or our sublicensees and on non-royalty bearing milestone revenue, with the royalty obligation ceasing upon
the later of the expiration of the last-to-expire licensed patent. The agreement also provides for an up-front Cost Reimbursement of
$100,000 payable to MIRALOGX to cover the already-incurred costs associated with the patent rights. The Cost Reimbursement is the only
payment made to date under the agreement. MIRALOGX may terminate the agreement upon insolvency, an uncured breach including the failure
to make any payment owed under the agreement or the failure to use commercially reasonable efforts to develop the licensed product, or
upon a default of the November 15, 2023 Promissory Note and Loan Agreement. The MIRALOGX License Agreement provides that MIRALOGX will
have sole control over the filing, prosecution, maintenance, and management of the licensed patent rights, provided that we will be responsible
for the cost of prosecuting and maintaining the licensed patents. The agreement grants to us the primary right, but not the obligation,
to enforce the licensed patent rights.
Properties
Our
corporate headquarters and executive offices are in Baltimore, Maryland. Our Baltimore location, which comprises approximately 150 square
feet, is under a lease that us due to expire on April 4, 2024. We believe that this facility will be sufficient for our current and planned
operations, although we may require additional office and laboratory space in Baltimore for our planned operations as we progress our
programs.
Employees
As
of December 15, 2023, we had two full-time employees and two part-time employees. None of our employees are represented by a labor
union or are covered by a collective bargaining agreement. We consider our relationship with our employees to be satisfactory. In addition,
we utilize the services of part-time outside consultants and contractors to perform several tasks for us.
Legal
Proceedings
From
time to time, we may be named in claims arising in the ordinary course of business. Currently, no legal proceedings, government actions,
administrative actions, investigations, or claims are pending against us or involve us that, in the opinion of our management, could
reasonably be expected to have a material adverse effect on our business and financial condition.
We
anticipate that we will expend significant financial and managerial resources in the defense of our intellectual property rights in the
future if we believe that our rights have been violated. We also anticipate that we will expend significant financial and managerial
resources to defend against claims that our products and services infringe upon the intellectual property rights of third parties.
Corporation
Information
Our
corporate headquarters is located at 855 N Wolfe Street, Suite 601, Baltimore, Maryland 21205. Our telephone number is 737-289-0835.
Our
principal website address is www.mirapharmaceuticals.com. The information contained on, or that can be accessed through, our website
is deemed not to be incorporated in this prospectus or to be part of this prospectus. You should not consider information contained on
our website to be part of this prospectus.
MANAGEMENT
Executive
Officers and Directors
The
following table sets forth information about our current executive officers and directors, including their ages as of December 15,
2023. With respect to our directors, each biography includes information regarding the experience, qualifications, attributes, or
skills that caused our board of directors to determine that such person should serve as a director of our company.
| Name | |
Age | | |
Position |
| Erez Aminov | |
| 45 | | |
Chief Executive Officer and Director |
| Michelle Yanez | |
| 52 | | |
Chief Financial Officer, Secretary and Treasurer |
| Adam Kaplin, MD, PhD | |
| 57 | | |
President and Chief Scientific Officer |
| Chris Chapman, MD | |
| 71 | | |
Executive Chairman and Director |
| Christos Nicholoudis, Esq. | |
| 34 | | |
Director and General Counsel |
| Michael Jerman | |
| 40 | | |
Director |
| Brad Kroenig | |
| 44 | | |
Director |
| Talhia Tuck | |
| 45 | | |
Director |
| Hugh McColl III | |
| 63 | | |
Director |
The
following is a brief biography of each of our current executive officers and directors:
Executive
Officers and Directors
Erez
Aminov has served as a director and our Chief Executive Officer since April 2023. From April 2022 to March 2023, Mr. Aminov was
a consultant to our company, providing support on fundraising and investor relations matters. Mr. Aminov is an experienced biotechnology
investor and adviser with over 18 years of experience. Since September 2021, Mr. Aminov was the founder of Locate Venture Corp, a strategy
and investment consulting firm which has advised multiple, early-stage life sciences companies including MyMD Pharmaceuticals (Nasdaq:
MYMD), Telomir Pharmaceuticals and Tyna Pharmaceuticals on fund raising and strategic partnerships. Mr. Aminov’s work has generally
focused on assisting clients with structuring private investment opportunities, designing new clinical partnerships, and negotiating
access to new markets. From February 2015 to September 2020, Mr. Aminov served as the President of Finds4less Inc., a global distributor
of electronics and gaming products. In this role, Mr. Aminov provided strategic oversight and direction for all aspects of the company’s
operations, while also spearheading new business development initiatives to capitalize on emerging market opportunities. Mr. Aminov received
his B.A. in accounting in 2004 from Touro University in New York. We believe that Mr. Aminov is qualified to serve as one of our directors
based on his finance and investment experience, particularly with early-stage life sciences companies.
Michelle
Yanez has served as our Chief Financial Officer since April 2023, prior to which she served as our Corporate Controller since
May 2022. Ms. Yanez is a senior financial executive with over 25 years of experience in public and privately held biotech, pharmaceutical,
and life science companies. Ms. Yanez’ experience includes a broad range of responsibilities in a highly complex and regulated
market. She also brings deep corporate governance experience through her work with corporate boards, including audit and finance committees.
Since May 2022, Ms. Yanez is part-time Corporate Controller at Telomir Pharmaceuticals, Inc., a privately held biotech company. From
May 2002 until its acquisition in April 2022, Ms. Yanez held various positions, including the Director of Financial Reporting, of BioDelivery
Sciences International, Inc. (Nasdaq:BDSI). In her role, she led financial offerings, managed due diligence for product acquisitions
and financings and managed finance documents and filings for the tender offer, leading to the acquisition of BioDelivery Sciences in
April 2022. Ms. Yanez also serves as a non-employee director of Inhibitor Therapeutics, Inc. (OTCQB: INTI), a publicly traded pharmaceutical
development company focused on therapeutics for certain cancers and certain non-cancerous proliferation disorders, since December 2022.
Ms. Yanez is a member of the Institute of Management Accountants and a member of the SEC Professionals Group. Ms. Yanez received her
MBA degree cum laude from Rutgers Business School.
Adam
Kaplin, MD, PhD has served as our President and Chief Scientific Officer since May 2022. Dr. Kaplin serves and will continue
to serve in such capacity as a non-employee consultant to our company on an at-will and as-needed basis. Dr. Kaplin currently serves
as the Chief Scientific Officer of MyMD Pharmaceuticals, Inc. (Nasdaq: MYMD), a publicly traded Delaware corporation focused on the development
and commercialization of an immunometabolic regulator, and previously served as the Chief Scientific Officer of MyMD’s predecessor
company, MyMD Pharmaceuticals, Inc., a Florida corporation (“MyMD Florida”), since December 18, 2020. Since 2002, Dr. Kaplin
has served in a number of positions at Johns Hopkins University, including Principal Neuro-Psychiatric Consultant to the Johns Hopkins
Multiple Sclerosis Center of Excellence, Director of the Johns Hopkins Ketamine Clinic and the Departments of Psychiatry and Neurology
at Johns Hopkins University School of Medicine, positions he has held at various times from 2002 to present. In addition, since 2019,
Dr. Kaplin has served as Adjunct Faculty at the George Mason University Department of Global and Community Health. Dr. Kaplin has also
served as Co-Founder of numerous healthcare related startups, including, from 2018 to present, REWARD Pathways Inc., a company devoted
to addiction treatment development focused on a combined eHealth and medicine approach to curing addiction, and from 2016 to present,
Hollinger Kaplin Benjamin & Bond, an eHealth software development company. Dr. Kaplin’s research focuses on the investigation
of the biological basis of immune mediated depression and cognitive impairment by using multiple sclerosis as the model. Dr. Kaplin has
also been active for over a decade in the development and application of health information technology to mental health, combining this
work with providing neuropsychiatric consultation and ongoing care of patients with multiple sclerosis spectrum disorders. Dr. Kaplin’s
original research has been published over 40 times in several different publications, and he has authored or co-authored numerous review
articles and textbooks. Dr. Kaplin received his B.S. in Biology from Yale University, graduating cum laude in 1988, and received
his M.D. and Ph.D. degrees from the Johns Hopkins University School of Medicine in 1996. Because of his research and scholastic accomplishments,
as well as his executive experience in the pharmaceutical industry, we believe Dr. Kaplin is qualified to serve as one of our directors.
Chris
Chapman, MD was appointed to serve as our Executive Chairman in April 2023. As Executive Chairman, Dr. Chapman’s duties
include those that are customarily associated with the position of Chairman of the Board, as well as oversight of the regulatory affairs
and drug development activities of the Company. Dr. Chapman has also served as one of our directors since November 1, 2021, and served
as a consultant with respect to regulatory affairs and drug development from November 1, 2021 until he began serving as our Executive
Chairman in April 2023. Dr. Chapman also serves as the President, Chief Medical Officer, and a director of MyMD. Dr. Chapman previously
served as President and Chief Medical Officer of MyMD Florida effective as of November 1, 2020. Prior to joining MyMD Florida and since
1999, Dr. Chapman has also served as the Chief Executive Officer of Chapman Pharmaceutical Consulting, Inc., a consulting organization
that provides support to pharmaceutical and biotechnology companies in North America, Europe, Japan, India and Africa on issues such
as product safety, pharmacovigilance, medical devices, clinical trials and regulatory issues. In addition, from 2003-2004, Dr. Chapman
served as the Associate Director of Drug Safety, Pharmacovigilance, and Clinical Operations for Organon Pharmaceuticals, where he was
responsible for the supervision of four fellow M.D.s and 10 drug safety specialists. Prior to his time at Organon, Dr. Chapman served
as Director, Medical Affairs, Drug Safety and Medical Writing Departments at Quintiles (currently known as IQVIA), from 1995 to 2003,
where he grew the division from no employees to forty employees, including eight board certified physicians, four RNs, two pharmacists,
eight medical writers and supporting staff. Dr. Chapman has also served on the board of directors of Rock Creek Pharmaceuticals, Inc.
(formerly, Star Scientific, Inc.) from 2007 to 2016, including as a member of the Audit Committee from 2007 to 2014, chairperson of the
Compensation Committee from 2007 to 2014, and chairperson of the Executive Search Committee from 2007 to 2014. Dr. Chapman is an experienced
executive and global medical expert and has extensive experience in providing monitoring and oversight for ongoing clinical trials including
both adult and pediatric subjects. Dr. Chapman is also the founder of the Chapman Pharmaceutical Health Foundation, an IRS Section 501(c)(3)
nonprofit organization established to solicit public funds and to support healthcare needs such as AIDS, diabetes, hypertension, lupus,
sickle cell anemia, malaria and tuberculosis, which was organized in 2006. Dr. Chapman earned an Executive Certificate in Nonprofit Financial
Stewardship from the Harvard Kennedy School in 2020. Dr. Chapman received his M.D. degree from Georgetown University in Washington, D.C.
in 1987, and completed his internship in Internal Medicine, a residency in Anesthesiology and a fellowship in Cardiovascular and Obstetric
Anesthesiology at Georgetown. We believe Dr. Chapman is qualified to serve as one of our directors due to his executive experience in
the pharmaceutical and biotechnology industries, as well as his medical expertise.
Christos
Nicholoudis joined our company as a director in April 2023, and he was initially appointed under an agreement between our company
and our largest stockholder, the Bay Shore Trust, to serve as the designated representative of the Bay Shore Trust on our board of directors.
Mr. Nicholoudis was also named our General Counsel in April 2023, although he is not deemed to be an executive officer of our company.
Mr. Nicholoudis is an attorney who has practiced with his own firm, The Law Firm of Christos Nicholoudis PLLC, since February, 2022,
where he handles a wide range of legal matters including contract work, personal injury, real estate, wills trusts and estates and criminal
law. Prior to that, from July of 2019 to February of 2022, Mr. Nicholoudis was employed by the State of Florida as a Public Defender
for the 12th Judicial Circuit and from July 2012 to February of 2020, Mr. Nicholoudis owned and operated a restaurant franchise
under Cortez Roadhouse, LLC. Mr. Nicholoudis is a 2012 graduate of Cornell University’s School of Hotel Administration where he
received a B.S. in hospitality and a 2017 graduate of Stetson College of Law where he received his J.D. degree. He is admitted to the
bar in New York, Florida, Texas, and Washington D.C. We believe that Mr. Nicholoudis is qualified to serve as one of our directors based
on his legal experience and training and his diverse business management experience.
Michael
Jerman joined our company as a director in December 2023. He also serves as a member of the board of directors of Inhibitor Therapeutics,
Inc. (OTC:INTI). Mr. Jerman has served as the managing partner at Hollywell Partners, a professional accounting and finance consulting
firm, since May 2019, and has provided chief financial officer and other services to multiple private equity-backed companies in the
energy, SaaS, and manufacturing industries. Prior to his role with Hollywell Partners, he was a Director with PwC in the US and UK from
January 2007 to August of 2019 and was a Captain with the United States Air Force from July 2003 to June 2015. He has led global public
and private client engagements in the industries of retail and consumer, energy, utilities and mining, and transportation and logistics.
Mr. Jerman has significant experience in client equity and debt offerings, business combinations inclusive of public listing and reporting
requirements, initial valuations and ongoing goodwill impairment analyses, share-based awards, restructuring, and global taxes, as well
as stakeholder management, specifically with board and management presentation experience to include annual and quarterly requirements,
fee negotiations, technical accounting and finance discussions, and fraud and non-compliance investigations. Mr. Jerman has specialized
in rapid project mobilization and deployment of skilled resources for emergency issues, design, and implementation of small to large
scale assurance requirements and advisory projects. Mr. Jerman’s additional experience includes leading PwC’s data acquisition
methods and tools, client acquisitions and systems implementations to include new SOX-compliant control plan implementations across multiple
systems, leading co-sourced internal audit projects, and time spent driving PwC’s lean efficiency initiatives. Mr. Jerman was a
member of the PwC national office within the SEC PCAOB quality group supporting Europe and the EMEA regions with complex accounting and
audit consultations. He earned a B.S. in accounting from the University of South Florida, an M.S. in accounting from the University of
Tampa, and an M.B.A. from the University of Oxford.
Brad
Kroenig has served as one of our directors since November 1, 2021. Since 2000, Mr. Kroenig’s principal occupation has been
serving as one of the world’s leading fashion models. Mr. Kroenig was the face of Ralph Lauren, The Gap, Tommy Hilfiger, Chanel,
Fendi, Peter Millar, and many other top brands. Models.com ranked him the #1 male model in the world from 2004 to 2006, and Vogue magazine
ranked him the #3 male model of all time. Mr. Kroenig also serves as a business and strategy consultant for many private firms and early-stage
companies, where as a part of his consulting business he advises companies regarding building management teams and managing relationships
with investors. Mr. Kroenig is an experienced investor and business executive with significant experience in collaborating with executive-level
and cross-functional teams, analyzing business situations, and developing and implementing practical investor strategies. Mr. Kroenig
attended Florida International University on a NCAA Division I soccer scholarship. We believe that Mr. Kroenig’s business experience
in the modeling industry as a business executive qualifies him to serve as one of our directors.
Talhia
Tuck has served as one of our directors since November 1, 2021. She has worked in the higher education field for over a decade,
including her most recent position as an Admissions and Recruitment Counselor for Georgetown Law School in 2023. From 2019-2023, Ms.
Tuck was a Project Director with Georgetown Law School’s Center for Innovations in Community Safety, formerly the Innovative Policing
Program, which identifies new approaches to long-standing issues in policing. Ms. Tuck served as an Associate Director of Admissions
at Georgetown University from 2015-2019, where she evaluated applications for the undergraduate schools and chaired several admissions
committees. Prior to 2015, Ms. Tuck worked in the investment relations and communications field as Vice President for Communications
and Investor Relations at Star Scientific, Inc. (OTC: STSC) where she was responsible for coordinating communications with shareholders,
the financial community, and the media. She also has experience in the legal industry, as she participated in the Ropes & Gray New
Alternatives Program as a Fellow at the Office of the State’s Attorney for Montgomery County, Maryland, and subsequently worked
in the Corporate Department at Ropes & Gray LLP in Washington, D.C. Prior to attending law school, Ms. Tuck was a journalist with
MSNBC, NBC News, ABC News, and the CBS affiliate, WINK-TV, and worked as an admissions officer for Harvard College at Harvard University.
She also served as a financial analyst at Goldman Sachs in the Investment Management Division from July 2000 until April 2001. She received
her A.B. degree from Harvard College, cum laude, and received her J.D. degree from Harvard Law School. We believe that Ms. Tuck’s
experience in public policy and investor relations qualifies her to serve as one of our directors.
Hugh
McColl III has served as one of our directors since November 1, 2021. Mr. McColl has served as Co-Managing Member of Collwick
Capital LLC, a fund of funds, since 2010 and Managing Member of McColl Brothers Lockwood LLC, a family investment office, since 2006.
Since June 2015, he has served as a Senior Advisor at Brown Brothers Harriman Capital Partners where he assists in sourcing, investment
evaluation, transaction execution, and providing post-investment, value-added oversight to portfolio companies. Before co-founding Collwick
Capital LLC, Mr. McColl spent 14 years in the hedge fund industry, where he was a private investments portfolio manager for Round Table
Investment Management and McColl Brothers Lockwood LLC, served as the Chief Operating Officer for M&M Partners LLC and was the Chief
Executive Officer for McColl Partners LLC. Mr. McColl has served on the boards of directors of Heritage Brands Inc. since 2019 and Fintag
Holdings Inc. since 2022. Mr. McColl received a B.S. degree in Business Administration from the University of North Carolina at Chapel
Hill in 1982 and an MBA degree from the University of Virginia Darden School of Business in 1987. We believe that Mr. McColl’s
investment management and executive experience qualifies him to serve as a member of our board of directors. We believe that Mr. McColl’s
investment management and executive experience qualifies him to serve as a member of our board of directors.
Board
Composition
Our
business and affairs are managed under the direction of our board of directors, which currently consists of seven members. The number
of directors is determined by our board of directors, subject to the terms of our amended and restated articles of incorporation and
bylaws that. Our directors are elected for one-year terms.
Family
Relationships
There
are no family relationships among any of our directors and executive officers. Erez Aminov, our Chief Executive Officer, is the spouse
of the daughter of our company’s founder, Jonnie R. Williams, Sr.
Director
Independence
Our
board of directors has undertaken a review of the independence of each director. Based on information provided by each director concerning
his or her background, employment, and affiliations, our board of directors has determined that Michael Jerman, Brad Kroenig, Talhia
Tuck, and Hugh McColl III do not have any relationship that would interfere with the exercise of independent judgment in carrying out
the responsibilities of a director and are independent directors under the Nasdaq Listing Rules.
In
making these determinations, our board of directors considered the current and prior relationships that each non-employee director has
with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including
the transactions described in the section of this prospectus titled “Certain Relationships and Related Party Transactions.”
Committees
of the Board of Directors
Our
board of directors has established an audit committee, a compensation committee, and a nominating and corporate governance committee.
The functions of these committees are described below. Members will serve on these committees until their resignation or until otherwise
determined by our board of directors. Our board of directors may establish other committees as it deems necessary or appropriate from
time to time.
Audit
Committee
Our
audit committee consists of Michael Jerman, Brad Kroenig and Hugh McColl III, with Michael Jerman serving as the chair of the audit committee.
Each member of the committee meets the requirements for independence under the listing standards of Nasdaq and SEC rules and regulations,
including Rule 10A-3(b)(1) under the Exchange Act. Each member of our audit committee also meets the financial literacy requirements
of the listing standards of Nasdaq. In addition, our board of directors has determined that Michael Jerman is an audit committee financial
expert within the meaning of Item 407(d) of Regulation S-K under the Securities Act.
The
audit committee’s main purpose is to oversee our corporate accounting and financial reporting process. Our audit committee is responsible
for, among other things:
| |
● |
selecting
a qualified firm to serve as the independent registered public accounting firm to audit our financial statements; |
| |
|
|
| |
● |
helping
to ensure the independence and performance of the independent registered public accounting firm; |
| |
|
|
| |
● |
discussing
the scope and results of the audit with the independent registered public accounting firm, and reviewing, with management and the
independent registered public accounting firm, our interim and year-end results of operations; |
| |
|
|
| |
● |
developing
procedures for employees to submit concerns anonymously about questionable accounting or audit matters; |
| |
|
|
| |
● |
reviewing
our policies on risk assessment and risk management; |
| |
|
|
| |
● |
reviewing
related party transactions; |
| |
● |
reviewing
and pre-approving, as required, all audit and all permissible non-audit services to be performed by the independent registered public
accounting firm; and |
| |
|
|
| |
● |
assisting
our board of directors in monitoring the performance of our internal audit function. |
Our
audit committee operates under a written charter that satisfies the applicable rules and regulations of the SEC and the listing standards
of Nasdaq, a copy of which is available on our website at www.mirapharmaceuticals.com.
Compensation
Committee
Our
compensation committee consists of Talhia Tuck, Brad Kroenig, and Michael Jerman, with Talhia Tuck serving as the chair of the compensation
committee. Each member of the committee meets the requirements for independence under the listing standards of Nasdaq and SEC rules and
regulations. Each member of our compensation committee is also a non-employee director, as defined pursuant to Rule 16b-3 promulgated
under the Exchange Act, or Rule 16b-3. In arriving at these determinations, our board of directors examined all factors relevant to determining
whether any compensation committee member has a relationship to us that is material to that member’s ability to be independent
from management in connection with carrying out such member’s duties as a compensation committee member.
The
compensation committee’s main purpose is to review and recommend policies relating to compensation and benefits of our officers
and employees. Our compensation committee is responsible for, among other things:
| |
● |
reviewing,
approving, and determining, or making recommendations to our board of directors regarding, the compensation and compensation arrangements
of our executive officers; |
| |
|
|
| |
● |
administering
our equity compensation plans; |
| |
|
|
| |
● |
reviewing
and approving, or making recommendations to our board of directors regarding, incentive compensation and equity compensation plans;
and |
| |
|
|
| |
● |
establishing
and reviewing general policies relating to compensation and benefits of our employees. |
Our
compensation committee operates under a written charter that satisfies the applicable rules and regulations of the SEC and the listing
standards of Nasdaq, a copy of which is available on our website.
Nominating
and Corporate Governance Committee
Our
nominating and corporate governance committee consists of Talhia Tuck, Brad Kroenig and Hugh McColl III, with Talhia Tuck serving as
the chair of the nominating and corporate governance committee. Each member of the committee meets the requirements for independence
under the listing standards of Nasdaq and SEC rules and regulations.
Our
nominating and corporate governance committee is responsible for, among other things:
| |
● |
identifying,
evaluating, and selecting, or making recommendations to our board of directors regarding, nominees for election to our board of directors
and its committees; |
| |
|
|
| |
● |
developing
and overseeing the annual evaluation of our board of directors and of its committees; |
| |
|
|
| |
● |
considering
and making recommendations to our board of directors regarding the composition of our board of directors and its committees; |
| |
|
|
| |
● |
overseeing
our corporate governance practices; and |
| |
|
|
| |
● |
making
recommendations to our board of directors regarding corporate governance guidelines. |
Our
nominating and corporate governance committee operates under a written charter that satisfies the applicable listing standards of Nasdaq,
a copy of which is available on our website.
Compensation
Committee Interlocks and Insider Participation
None
of the members of our compensation committee is a current or former executive officer or employee of our company. None of our executive
officers serves as a member of the compensation committee of any entity that has one or more executive officers serving on our compensation
committee.
Risk
Oversight
One
of the key functions of our board of directors is informed oversight of our risk management process. Our board of directors administers
this oversight function directly through our board of directors as a whole, and through various standing committees of our board of directors
that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring
and assessing strategic risk exposure, including risks associated with cybersecurity and data protection, and our audit committee has
the responsibility to consider our major financial risk exposures and the steps our management has taken to monitor and control these
exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. Our audit committee
will review legal, regulatory, and compliance matters that could have a significant impact on our financial statements. Our nominating
and corporate governance committee will monitor the effectiveness of our corporate governance practices, including whether they are successful
in preventing illegal or improper liability-creating conduct. Our compensation committee will assess and monitor whether any of our compensation
policies and programs has the potential to encourage excessive risk taking. While each committee is responsible for evaluating certain
risks and overseeing the management of such risks, our entire board of directors will be regularly informed through committee reports
about such risks.
Board
Diversity
Our
nominating and corporate governance committee is responsible for reviewing with the board of directors, on an annual basis, the appropriate
characteristics, skills, and experience required for the board of directors as a whole and its individual members. Although our board
of directors does not have a formal written diversity policy with respect to the evaluation of director candidates, in its evaluation
of director candidates, our nominating and corporate governance committee will consider factors including, without limitation, issues
of character, integrity, judgment, potential conflicts of interest, other commitments, and diversity, and with respect to diversity,
such factors as gender, race, ethnicity, experience, and area of expertise, as well as other individual qualities and attributes that
contribute to the total diversity of viewpoints and experience represented on the board of directors.
The
nominating and corporate governance committee will ensure compliance with the new rule by Nasdaq for board diversity (the “Nasdaq
Diversity Rule”), on or before the date required under the Nasdaq Diversity Rule. The Nasdaq Diversity Rule requires, assuming
our shares of common stock are listed on the Nasdaq Capital Market and that we are a smaller reporting company, that we will have at
least two directors serving on our board of directors, at least one of which identifies as female and the second of which identifies
as female, underrepresented minority or LGBTQ+, by December 31, 2026, unless our board of directors is comprised of five or less directors.
Code
of Business Conduct and Ethics
Our
board of directors has adopted a code of business conduct and ethics applicable to all of our directors, officers (including our principal
executive officer, principal financial officer, and principal accounting officer) and all global employees in accordance with applicable
federal securities laws and corporate governance rules of the Nasdaq Capital Market. Our code of business conduct and ethics is available
on our website. Any amendments to the code of business conduct and ethics, or waivers of its requirements, will, if required, be disclosed
on our website.
Corporate
Governance Guidelines
Our
board of directors has adopted corporate governance guidelines, a copy of which is available on our website.
Director
Compensation
We
did not provide any cash compensation to any of our directors during the year ended December 31, 2022 in their capacity as directors.
However, on June 15, 2022, each of our non-employee directors was granted an option to purchase up to 20,000 shares of our common stock
under our 2022 Omnibus Plan at an exercise price of $5.00 per share, and on April 28, 2023, each non-employee director was granted an
additional option to purchase up to 10,000 shares of our common stock under the 2022 Omnibus Plan. Each such option was immediately vested
in full upon grant and has a 10-year term.
Certain
of our directors have received option grants as a result of their service to our company in a non-director capacity. Prior to his appointment
as Executive Chairman, Dr. Chapman was a party to a consulting agreement with our company entered into in April 2022 and was granted
additional options in his capacity as a consultant on June 15, 2022. Dr. Chapman also received employee related grants in April 2023
and August 2023. Mr. Kroenig previously provided consulting services to our company in 2022 and received an additional option grant on
June 15, 2022 under which he has the right to purchase up to 10,000 shares of our common stock.. Upon his appointment as the company’s
General Counsel, Mr. Nicholoudis was granted an option to purchase shares of our common of 15,000 shares in April 2023, and 10,000 shares
in August 2023.
EXECUTIVE
COMPENSATION
This
section discusses the material components of the executive compensation program for the following persons: (i) all persons serving as
our principal executive officers during 2022 and (ii) the most highly compensated of our other executive officers who received compensation
during 2022 of at least $100,000 and who were executive officers on December 31, 2022. We refer to these persons as our “named
executive officers” elsewhere in this prospectus. Our “named executive officers” and their positions are as follows:
| |
● |
Jude
Uzonwanne, Former Chief Executive Officer and President; |
| |
● |
James
A. McNulty, CPA, Former Chief Financial Officer; and |
| |
● |
Adam
Kaplin, MD, PhD, President and Chief Scientific Officer. |
In
April 2023, Mr. Aminov succeeded Mr. Uzonwanne as our Chief Executive Officer and President, and Ms. Yanez succeeded Mr. McNulty as our
Chief Financial Officer.
Summary
Compensation Table
The
following table shows the compensation paid by us during the 2022 and 2021 fiscal years to our named executive officers.
| Name and principal position | |
Year | |
Salary | | |
Bonus | | |
Stock Awards | | |
Option
Awards (6) | | |
All Other Compensation | | |
Total ($) | |
| Jude Uzonwanne, Former | |
2022 | |
$ | 125,000 | | |
$ | 50,000 | (1) | |
| | | |
| 739,000 | | |
$ | 8,385 | (3) | |
$ | 922,385 | |
| Chief Executive Officer and President | |
2021 | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
$ | - | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| James A. McNulty, CPA, | |
2022 | |
$ | 266,868 | | |
$ | 100,000 | (4) | |
| | | |
| | | |
| - | | |
$ | 366,868 | |
| Former Chief Financial Officer | |
2021 | |
$ | 43,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
$ | 43,000 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Adam Kaplin, MD, PhD | |
2022 | |
| - | | |
$ | 50,000 | (5) | |
| | | |
| 739,000 | | |
| - | | |
$ | 789,000 | |
| President and Chief Scientific Officer | |
2021 | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
$ | - | |
| |
(1) |
The
bonus represents a paid sign-on amount. |
| |
(2) |
Of
these 2022 option grants, 75% were cancelled and non-exercisable as of April 2023, pursuant to the termination of Mr. Uzonwanne. |
| |
(2) |
Amount
represents health insurance premiums paid. |
| |
(4) |
The
bonus represents a milestone payment pursuant to a prior employment agreement with Mr. McNulty. |
| |
(5) |
The
bonus represents a milestone payment pursuant to a prior employment agreement with Dr. Kaplin. |
| |
(6) |
The
reported amounts represent the aggregate grant date fair value of the awards computed in accordance with Financial Accounting Standards
Board Account Standards Codification Topic 718, Stock Compensation, as modified or supplemented, or FASB ASC Topic 718. The assumptions
used in calculating the grant date fair value of the stock options reported in this column are set forth in Note 8 to our Consolidated
Financial Statements for the year ended December 31, 2022 included in this Report. In April 2023, we entered into an agreement with
Mr. Uzonwanne in which the number of shares subject to his option agreement was reduced from 200,000 to 40,000. |
Executive
Compensation Arrangements
Below
is a more detailed summary of the elements of our current executive compensation program as it relates to our continuing named executive
officers, as well as our current executive officers who were not executive officers as of the end of 2022, including our Executive Chairman.
Employment
Agreements
Erez
Aminov
Effective
April 28, 2023, we entered into an employment agreement with Mr. Aminov, as amended on August 28, 2023, pursuant to which Mr. Aminov
will serve as our Chief Executive Officer. Under his employment agreement, as amended, Mr. Aminov has agreed to devote at least 50% of
his business time to the affairs of the Company. Mr. Aminov’s employment agreement provides that his employment will be on an at-will
basis and can be terminated by either Mr. Aminov or our company at any time and for any reason. Under the agreement, Mr. Aminov will
receive a base salary of $200,000 per year, effective August 1, 2023. In the event that Mr. Aminov’s employment is terminated by
our company without “Cause” or is terminated by Mr. Aminov for “Good Reason”, Mr. Aminov will be entitled to
severance compensation in the form of salary continuation for a period of three months (subject to Mr. Aminov executing and delivering
a customary general release in favor of the company). “Cause” is defined in the agreement to include dishonesty, misappropriation,
willful misconduct, breach of the agreement, and other customary matters. “Good Reason” is defined to include a material
adverse change in Mr. Aminov’s compensation or duties and level of responsibility. The employment agreement also contains customary
confidentiality and invention-assignment covenants to which Mr. Aminov is subject.
On
August 17, 2023, Mr. Aminov received a $120,000 cash bonus net of federal, state, local and income taxes related to the successful completion
of the IPO.
Michelle
Yanez
On
April 28, 2023, we entered into an employment agreement with Ms. Yanez pursuant to which Ms. Yanez will serve as our Chief Financial
Officer on a full-time basis. Ms. Yanez’s employment agreement provides that her employment will be on an at-will basis and can
be terminated by either Ms. Yanez or our company at any time and for any reason. Under the agreement, Ms. Yanez will receive an initial
base salary of $165,000 per year. In the event that her employment is terminated by our company without “Cause” or is terminated
by Ms. Yanez for “Good Reason”, Ms. Yanez will be entitled to severance compensation in the form of salary continuation for
a period of three months (subject to Ms. Yanez executing and delivering a customary general release in favor of the company). “Cause”
is defined in the agreement to include dishonesty, misappropriation, willful misconduct, breach of the agreement, and other customary
matters. “Good Reason” is defined to include a material adverse change in Ms. Yanez’s compensation or duties and level
of responsibility. The employment agreement also contains customary confidentiality and invention-assignment covenants to which Ms. Yanez
is subject.
On
August 17, 2023, Ms. Yanez received a $50,000 cash bonus net of federal, state, local and income taxes related to the successful completion
of the IPO.
Chris
Chapman
On
April 28, 2023, we entered into an employment agreement with Dr. Chapman, as amended on August 28, 2023, and October 13, 2023, pursuant
to which Dr. Chapman will serve as our Executive Chairman. Dr. Chapman’s employment agreement, as amended, provides that his employment
will be on a part-time basis whereby Dr. Chapman will devote time and effort to the business and affairs of the company on an as needed
basis, and it further provides that such employment will be on an at-will basis and can be terminated by either Dr. Chapman or our company
at any time and for any reason. Under the agreement, Dr. Chapman will receive a base salary of $50,000 per year for a period of 90 days
following the October 13, 2023 amendment, and following the 90-day period, Dr. Chapman’s base salary will increase to $150,000.
In the event that Dr. Chapman’s employment is terminated by our company without “Cause” or is terminated by Dr. Chapman
for “Good Reason”, Dr. Chapman will be entitled to severance compensation in the form of salary continuation for a period
of three months (subject to Dr. Chapman executing and delivering a customary general release in favor of the company). “Cause”
is defined in the agreement to include dishonesty, misappropriation, willful misconduct, breach of the agreement, and other customary
matters. “Good Reason” is defined to include a material adverse change in Dr. Chapman’s compensation or duties and
level of responsibility. The employment agreement also contains customary confidentiality and invention-assignment covenants to which
Dr. Chapman is subject.
On
August 17, 2023, Dr. Chapman received a $50,000 cash bonus net of federal, state, local and income taxes related to the successful completion
of the IPO.
Consulting
Relationship with Adam Kaplin
Dr.
Kaplin is a paid non-employee consultant to our company under which he provides services and consultation on an as-needed basis. Dr.
Kaplin is paid $9,166 a month for his services. We do not currently have a written consulting agreement with Dr. Kaplin.
Base
Salaries
The
base salaries of our employed executive officers are specified in their respective employment agreements, as summarized above.
Bonuses
We
paid bonuses to three named executive officers in 2022. See Summary Compensation Table for details.
Equity
Compensation
In
June 2022, Dr. Kaplin was granted an option to purchase 200,000 shares of our common stock. In June 2022, prior to becoming our Chief
Financial Officer, Ms. Yanez was granted an option to purchase 10,000 shares of our common stock. In June 2022, Dr. Chapman was granted
an option to purchase 220,000 shares of our common stock.
In
April 2023, we granted additional options to the following current executive officers for the following number shares of our common stock:
Mr. Aminov, 150,000 shares; Ms. Yanez, 46,667 shares; Dr. Kaplin, 40,000 shares; and Dr. Chapman, 60,000 shares.
The
foregoing options were granted under our 2022 Omnibus Plan and have an exercise price of $5.00 per share. These options vest as to one-third
of the option shares on the date of option grant and will vest as to one-third of the option shares on the succeeding two anniversaries
of the date of option grant. Any unvested portion of the option will vest in full upon a “change of control” of our company
within the meaning of the 2022 Omnibus Plan. The options have a term of 10-years, subject to earlier termination upon termination of
employment.
Retirement
Plans
We
do not currently maintain any retirement plans for our employees.
Outstanding
Equity Awards at Fiscal Year-End
There
were a cumulative 750,000 stock options granted and outstanding as of December 31, 2022. Of the aforementioned amount, 280,000 stock
options were vested at December 31, 2022.
2022
Omnibus Incentive Plan
Our
board of directors has adopted, and our stockholders have approved, our 2022 Omnibus Incentive Plan, or the 2022 Omnibus Plan. The 2022
Omnibus Plan authorizes the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, to our
employees and any of our parent and subsidiary corporations’ employees, and the grant of nonstatutory stock options, restricted
stock, restricted stock units, stock appreciation rights, performance units and performance shares to our employees, directors, and consultants
and any of our future subsidiary corporations’ employees and consultants. The following is a summary of certain terms and conditions
of the 2022 Omnibus Plan. This summary is qualified in its entirety by reference to the 2022 Omnibus Plan attached as an exhibit to the
registration statement of which this prospectus forms a part. You are encouraged to read the full text of the 2022 Omnibus Plan.
As
of December 15, 2023, there are options to purchase an aggregate of 1,210,001 shares of our common stock outstanding under the
2022 Omnibus Plan.
Administration
The
2022 Omnibus Plan is administered by our board of directors or our compensation committee, or any other committee or subcommittee or
one or more of our officers to whom authority has been delegated (collectively, the “Administrator”). The Administrator has
the authority to interpret the 2022 Omnibus Plan and award agreements entered into with respect to the 2022 Omnibus Plan; to make, change
and rescind rules and regulations relating to the 2022 Omnibus Plan; to make changes to, or reconcile any inconsistency in, the 2022
Omnibus Plan or any award agreement covering an award; and to take any other actions needed to administer the 2022 Omnibus Plan.
Eligibility
The
Administrator may designate any of the following as a participant under the 2022 Omnibus Plan: any officer or employee, or individuals
engaged to become an officer or employee, of our company or our affiliates; and consultants of our company or our affiliates, and our
directors, including our non-employee directors.
Types
of Awards
The
2022 Omnibus Plan permits the Administrator to grant stock options, stock appreciation rights (“SARs”), performance shares,
performance units, shares of common stock, restricted stock, restricted stock units (“RSUs”), cash incentive awards, dividend
equivalent units, or any other type of award permitted under the 2022 Omnibus Plan. The Administrator may grant any type of award to
any participant it selects, but only our employees or our subsidiaries’ employees may receive grants of incentive stock options
within the meaning of Section 422 of the Internal Revenue Code. Awards may be granted alone or in addition to, in tandem with, or (subject
to the repricing prohibition described below) in substitution for any other award (or any other award granted under another plan of our
company or any affiliate, including the plan of an acquired entity).
Shares
Reserved Under the 2022 Omnibus Incentive Plan
The
2022 Omnibus Plan provides that 2,000,000 shares of our common stock are reserved for issuance under the 2022 Omnibus Plan, all of which
may be issued pursuant to the exercise of incentive stock options. The number of shares available for issuance under our 2022 Omnibus
Plan will also include an annual increase on the first day of each fiscal year equal to the lesser of:
| |
● |
200,000
shares; |
| |
|
|
| |
● |
1.0%
of the outstanding shares of all class of our common stock as of the last day of the immediately preceding fiscal year; or |
| |
|
|
| |
● |
such
other amount as our board of directors may determine. |
The
number of shares reserved for issuance under the 2022 Omnibus Plan will be reduced on the date of the grant of any award by the maximum
number of shares, if any, with respect to which such award is granted. However, an award that may be settled solely in cash will not
deplete the 2022 Omnibus Plan’s share reserve at the time the award is granted. If (a) an award expires, is canceled, or terminates
without issuance of shares or is settled in cash, (b) the Administrator determines that the shares granted under an award will not be
issuable because the conditions for issuance will not be satisfied, (c) shares are forfeited under an award, (d) shares are issued under
any award and we reacquire them pursuant to our reserved rights upon the issuance of the shares, (e) shares are tendered or withheld
in payment of the exercise price of an option or as a result of the net settlement of outstanding stock appreciation rights or (f) shares
are tendered or withheld to satisfy federal, state or local tax withholding obligations, then those shares are added back to the reserve
and may again be used for new awards under the 2022 Omnibus Plan. However, shares added back to the reserve pursuant to clauses (d),
(e) or (f) in the preceding sentence may not be issued pursuant to incentive stock options.
Options
The
Administrator may grant stock options and determine all terms and conditions of each stock option, which include the number of stock
options granted, whether a stock option is to be an incentive stock option or non-qualified stock option, and the grant date for the
stock option. However, the exercise price per share of common stock may never be less than the fair market value of a share of common
stock on the date of grant and the expiration date may not be later than 10 years after the date of grant. Stock options will be exercisable
and vest at such times and be subject to such restrictions and conditions as are determined by the Administrator, including with respect
to the manner of payment of the exercise price of such stock options.
Stock
Appreciation Rights
The
Administrator may grant SARs, which represent the right of a participant to receive cash in an amount, or common stock with a fair market
value, equal to the appreciation of the fair market value of a share of common stock during a specified period of time. The 2022 Omnibus
Plan provides that the Administrator will determine all terms and conditions of each SAR, including, among other things: (a) whether
the SAR is granted independently of a stock option or relates to a stock option, (b) the grant price, which may never be less than the
fair market value of our common stock as determined on the date of grant, (c) a term that must be no later than 10 years after the date
of grant, and (d) whether the SAR will settle in cash, common stock or a combination of the two.
Performance
and Stock Awards
The
Administrator may grant awards of shares of common stock, restricted stock, RSUs, performance shares or performance units. Restricted
stock means shares of common stock that are subject to a risk of forfeiture or restrictions on transfer, which may lapse upon the achievement
or partial achievement of performance goals (as described below) or upon the completion of a period of service. An RSU grants the participant
the right to receive cash or shares of common stock the value of which is equal to the fair market value of one share of common stock,
to the extent performance goals are achieved or upon the completion of a period of service. Performance shares give the participant the
right to receive shares of common stock to the extent performance goals are achieved. Performance units give the participant the right
to receive cash or shares of common stock valued in relation to a unit that has a designated dollar value or the value of which is equal
to the fair market value of one or more shares of common stock, to the extent performance goals are achieved.
The
Administrator will determine all terms and conditions of the awards including (a) whether performance goals must be achieved for the
participant to realize any portion of the benefit provided under the award, (b) the length of the vesting or performance period and,
if different, the date that payment of the benefit will be made, (c) with respect to performance units, whether to measure the value
of each unit in relation to a designated dollar value or the fair market value of one or more shares of common stock, and (d) with respect
to performance shares, performance units, and RSUs, whether the awards will settle in cash, in shares of common stock (including restricted
stock), or in a combination of the two.
Cash
Incentive Awards
The
Administrator may grant cash incentive awards. An incentive award is the right to receive a cash payment to the extent one or more performance
goals are achieved. The Administrator will determine all terms and conditions of a cash incentive award, including, but not limited to,
the performance goals (described below), the performance period, the potential amount payable, and the timing of payment. While the 2022
Omnibus Plan permits cash incentive awards to be granted under the 2022 Omnibus Plan, we may also make cash incentive awards outside
of the 2022 Omnibus Plan.
Performance
Goals
For
purposes of the 2022 Omnibus Plan, the Administrator may establish objective or subjective performance goals which may apply to any performance
award. Such performance goals may include, but are not limited to, one or more of the following measures with respect to our company
or any one or more of our subsidiaries, affiliates, or other business units: net sales; cost of sales; gross income; gross revenue; revenue;
operating income; earnings before taxes; earnings before interest and taxes; earnings before interest, taxes, depreciation and amortization;
earnings before interest, taxes, depreciation, amortization and exception items; income from continuing operations; net income; earnings
per share; diluted earnings per share; total stockholder return; fair market value of a share of common stock; cash flow; net cash provided
by operating activities; net cash provided by operating activities less net cash used in investing activities; ratio of debt to debt
plus equity; return on stockholder equity; return on invested capital; return on average total capital employed; return on net capital
employed; return on assets; return on net assets employed before interest and taxes; operating working capital; average accounts receivable
(calculated by taking the average of accounts receivable at the end of each month); average inventories (calculated by taking the average
of inventories at the end of each month); economic value added; succession planning; manufacturing return on assets; manufacturing margin;
and customer satisfaction. Performance goals may also relate to a participant’s individual performance. The Administrator reserves
the right to adjust any performance goals or modify the manner of measuring or evaluating a performance goal.
Dividend
Equivalent Units
The
Administrator may grant dividend equivalent units. A dividend equivalent unit gives the participant the right to receive a payment, in
cash or shares of common stock, equal to the cash dividends or other distributions that we pay with respect to a share of common stock.
We determine all terms and conditions of a dividend equivalent unit award, except that dividend equivalent units may not be granted in
connection with a stock option or SAR, and dividend equivalent unit awards granted in connection with another award cannot provide for
payment until the date such award vests or is earned, as applicable.
Other
Stock-Based Awards
The
Administrator may grant to any participant shares of unrestricted stock as a replacement for other compensation to which such participant
is entitled, such as in payment of director fees, in lieu of cash compensation, in exchange for cancellation of a compensation right
or as a bonus.
Transferability
Awards
are not transferable, including to any financial institution, other than by will or the laws of descent and distribution, unless the
Administrator allows a participant to (a) designate in writing a beneficiary to exercise the award or receive payment under the award
after the participant’s death, (b) transfer an award to a former spouse as required by a domestic relations order incident to a
divorce, or (c) transfer an award without receiving any consideration.
Adjustments
If
(a) we are involved in a merger or other transaction in which our shares of common stock are changed or exchanged; (b) we subdivide or
combine shares of common stock or declare a dividend payable in shares of common stock, other securities, or other property (other than
stock purchase rights issued pursuant to a stockholder rights agreement); (c) we effect a cash dividend that exceeds 10% of the fair
market value of a share of common stock or any other dividend or distribution in the form of cash or a repurchase of shares of common
stock that our board of directors determines is special or extraordinary, or that is in connection with a recapitalization or reorganization;
or (d) any other event occurs that in the Administrator’s judgment requires an adjustment to prevent dilution or enlargement of
the benefits intended to be made available under the 2022 Omnibus Plan, then the Administrator will, in a manner it deems equitable,
adjust any or all of (1) the number and type of shares subject to the 2022 Omnibus Plan and which may, after the event, be made the subject
of awards; (2) the number and type of shares of common stock subject to outstanding awards; (3) the grant, purchase, or exercise price
with respect to any award; and (4) the performance goals of an award. In any such case, the Administrator may also provide for a cash
payment to the holder of an outstanding award in exchange for the cancellation of all or a portion of the award, subject to the terms
of the 2022 Omnibus Plan.
The
Administrator may, in connection with any merger, consolidation, acquisition of property or stock, or reorganization, authorize the issuance
or assumption of awards upon terms and conditions we deem appropriate without affecting the number of shares of common stock otherwise
reserved or available under the 2022 Omnibus Plan.
Change
of Control
Upon
a change of control (as defined in the 2022 Omnibus Plan), the successor or surviving corporation may agree to assume some or all outstanding
awards or replace them with the same type of award with similar terms and conditions, without the consent of any participant, subject
to the following requirements:
| |
● |
Each
award that is assumed must be appropriately adjusted, immediately after such change of control, to apply to the number and class
of securities that would have been issuable to a participant upon the consummation of such change of control had the award been exercised,
vested, or earned immediately prior to such change of control, and other appropriate adjustment to the terms and conditions of the
award may be made. |
| |
|
|
| |
● |
If
the securities to which the awards relate after the change of control are not listed and traded on a national securities exchange,
then (a) each participant must be provided the option to elect to receive, in lieu of the issuance of such securities, cash in an
amount equal to the fair value of the securities that would have otherwise been issued, and (b) no reduction may be taken to reflect
a discount for lack of marketability, minority, or any similar consideration, for purposes of determining the fair value of such
securities. |
| |
|
|
| |
● |
If
a participant is terminated from employment without cause, or due to death or disability, or the participant resigns employment for
good reason (as defined in any award or other agreement between the participant and our company or an affiliate) within two years
following the change of control, then upon such termination, all of the participant’s awards in effect on the date of such
termination will vest in full or be deemed earned in full. |
If
the purchaser, successor, or surviving entity does not assume the awards or issue replacement awards, then immediately prior to the change
of control date, unless the Administrator otherwise determines:
| |
● |
Each
stock option or SAR then held by a participant will become immediately and fully vested, and all stock options and SARs will be cancelled
on the change of control date in exchange for a cash payment equal to the excess of the change of control price of the shares of
common stock over the purchase or grant price of such shares under the award. |
| |
|
|
| |
● |
Unvested
restricted stock and RSUs (that are not performance awards) will vest in full. |
| |
|
|
| |
● |
All
performance shares, performance units and cash incentive awards for which the performance period has expired will be paid based on
actual performance, and all such awards for which the performance period has not expired will be cancelled in exchange for a cash
payment equal to the amount that would have been due under such awards, valued assuming achievement of target performance goals at
the time of the change of control, prorated based on the number of full months elapsed in the performance period. |
| |
|
|
| |
● |
All
unvested dividend equivalent units will vest (to the same extent as the award granted in tandem with such units) and be paid. |
| |
|
|
| |
● |
All
other unvested awards will vest and any amounts payable will be paid in cash. |
Term
of Plan
Unless
earlier terminated by our board of directors, the 2022 Omnibus Plan will terminate on, and no further awards may be granted, after the
tenth (10th) anniversary of its effective date.
Termination
and Amendment of Plan
Our
board of directors or the Administrator may amend, alter, suspend, discontinue, or terminate the 2022 Omnibus Plan at any time, subject
to the following limitations:
| |
● |
Our
board of directors must approve any amendment to the 2022 Omnibus Plan if we determine such approval is required by prior action
of our board of directors, applicable corporate law, or any other applicable law; |
| |
|
|
| |
● |
Stockholders
must approve any amendment to the 2022 Omnibus Plan, which may include an amendment to materially increase the number of shares reserved
under the 2022 Omnibus Plan, if we determine that such approval is required by Section 16 of the Exchange Act, the Code, the listing
requirements of any principal securities exchange or market on which the shares are then traded, or any other applicable law; and |
| |
|
|
| |
● |
Stockholders
must approve any amendment to the 2022 Omnibus Plan that would diminish the protections afforded by the participant award limits
or repricing and backdating prohibitions. |
Amendment,
Modification, Cancellation and Disgorgement of Awards
Subject
to the requirements of the 2022 Omnibus Plan, the Administrator may modify or amend any award or waive any restrictions or conditions
applicable to any award or the exercise of the award, or amend, modify, or cancel any terms and conditions applicable to any award, in
each case, by mutual agreement of the Administrator and the participant or any other person that may have an interest in the award, so
long as any such action does not increase the number of shares of common stock issuable under the 2022 Omnibus Plan.
We
do not need to obtain participant (or other interested party) consent for any such action (a) that is permitted pursuant to the adjustment
provisions of the 2022 Omnibus Plan; (b) to the extent we deem the action necessary to comply with any applicable law or the listing
requirements of any principal securities exchange or market on which our common stock is then traded; (c) to the extent we deem the action
is necessary to preserve favorable accounting or tax treatment of any award for us; or (d) to the extent we determine that such action
does not materially and adversely affect the value of an award or that such action is in the best interest of the affected participant
or any other person as may then have an interest in the award.
The
Administrator can cause a participant to forfeit any award, and require the participant to disgorge any gains attributable to the award,
if the participant engages in any action constituting, as determined by the Administrator in its discretion, cause for termination, or
a breach of a material company policy, any award agreement or any other agreement between the participant and us or one of our affiliates
concerning noncompetition, nonsolicitation, confidentiality, trade secrets, intellectual property, nondisparagement or similar obligations.
Any
awards granted under the 2022 Omnibus Plan, and any shares of common stock issued or cash paid under an award, will be subject to any
recoupment or clawback policy that we adopt, or any recoupment or similar requirement otherwise made applicable by law, regulation or
listing standards to us.
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The
following is a description of transactions within the last three years to which we have been a party, in which the amount involved exceeded
or will exceed $120,000, and in which any of our executive officers, directors or holders of more than 5% of our voting securities, or
an immediate family member thereof, had or will have a direct or indirect material interest. We believe the terms obtained or consideration
that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or amounts
that would be paid or received, as applicable, in arm’s-length transactions with unrelated third parties.
Confirmatory
Patent Assignment and Royalty Agreement
On
November 1, 2021, we entered into a Confirmatory Patent Assignment and Royalty Agreement with SRQ Patent Holdings II, LLC (“Patent
Assignor”), and the founder of our company, Jonnie R. Williams, Sr., pursuant to which we granted a royalty of 8% of any net sales,
royalties, or other revenue received by us with respect to the sale, commercialization, or disposition of MIRA1a, with such royalty being
paid to Patent Assignor in consideration for Patent Assignor’s assignment to us of U.S. Patent 10,787,675 B2, which is the patent
for MIRA1a.
Line
of Credit and Promissory Note with the Bay Shore Trust
On
April 28, 2023, we entered into the Bay Shore Note with the Bay Shore Trust, under which we have the right to borrow up to an aggregate
of $5,000,000 from the Bay Shore Trust at any time up to the second anniversary of the issuance of the Bay Shore Note or, if earlier,
upon the completion of our initial public offering. Our right to borrow funds under the Bay Shore Note is subject to the absence of a
material adverse change in our assets, operations, or prospects. The Bay Share Note, together with accrued interest, will become due
and payable on the second anniversary of the issuance of the note, provided that it may be prepaid at any time without penalty. The Bay
Shore Note will accrue interest at a rate equal 7% per annum, simple interest, during the first year that the note is outstanding and
10% per annum, simple interest, thereafter. The Bay Shore Note is unsecured. As of June 30, 2023, the Bay Shore Note had an outstanding
principal balance of $1.8 million and accrued and unpaid interest of $0.04 million. Under the Bay Shore Trust Conversion Agreement, the
Bay Shore Trust agreed to convert, upon the completion of our initial public offering, $1,100,190 of the outstanding principal balance
of the Bay Shore Note into shares of our common stock at a conversion price equal to our initial public offering price, which resulted
in the issuance of 157,170 shares to the Bay Shore Trust upon the completion of our initial public offering.
In
consideration of the loan facility provided by the Bay Shore Trust, we issued to the Bay Shore Trust a common stock purchase warrant
on April 28, 2023 giving the Bay Shore Trust the right to purchase up to 1,000,000 shares of common stock at an exercise price of $5.00
per share, which warrant will expire five years after the date of grant. Pursuant to a registration rights agreement, we have granted
to Bay Shore Trust the right to require us, at any time after one year following our initial public offering, to register for resale
the shares issuable upon the exercise of the warrant, with such registration rights being in the form of demand and “piggyback”
registration rights that are subject to customary limitations and restrictions. Upon issuance, the warrant met the criteria to be classified
as equity based on an analysis under Accounting Standards Codification (480) ASC 480, “Distinguishing Liabilities from Equity”
and will be measured at fair value, resulting in an initial fair value of approximately $3.5 million upon issuance of the warrant using
Black-Scholes valuation techniques.
Transactions
with MIRALOGX LLC
Since
January 1, 2023, MIRALOGX has advanced funds on behalf of Bay Shore Trust to our company in order to fund operating activities. The total
amount advanced and outstanding from MIRALOGX was $1.6 million immediately prior to being consolidated into the Bay Shore Note on June
30, 2023, and such amounts became a part of the outstanding balance of the Bay Shore Note as of June 30, 2023 and are payable under the
terms of the Bay Shore Note.
We
are also a party to an Agreement for Shared Lease Costs, dated April 1, 2023, with MIRALOGX under which we have agreed to pay our pro
rata share of the operating usage costs owing by MIRALOGX under an aircraft lease agreement between MIRALOGX and Supera Aviation I LLC
(“Supera Aviation”) based on our usage of the leased aircraft each month. No amounts are payable by us under this agreement
unless and to the extent we choose to utilize the leased aircraft, and we may discontinue the use of the aircraft and terminate this
agreement at any time. Prior to entering into this agreement, we were a party to an aircraft lease agreement with Supera Aviation from
April 20, 2021, through March 31, 2023. During the term of such lease agreement, we paid Supera Aviation an aggregate of $0.5 million
during the first quarter of 2023, $1.7 million in 2022, and $0.7 million in 2021. Supera Aviation is a company owned by Starwood Trust,
a trust established by Mr. Williams.
On
November 15, 2023, we entered into an exclusive license agreement in with MIRALOGX to develop and commercialize a drug product containing
2-(2-chlorophenyl)-2-(methylamino) cyclopentan-1-one (sometimes referred to by the Parties as “M209” or “KETAMIR-2”)
as an active agent in North America. The exclusive license in the license agreement includes our right to sublicense the licensed intellectual
property. Pursuant to the terms of the license agreement, and subject to the conditions set forth therein, we paid MIRALOGX a one-time,
nonrefundable payment of $100,000 upon the signing of the Agreement and will be obligated to pay quarterly royalty payments on sales
of the Product in the Territory of 8% of net sales and 8% of other revenue (such as milestone or sublicense payments) from licensed products.
Also, in consideration of License Agreement, we issued to MIRALOGX a common stock purchase warrant to purchase up to 700,000 shares of
our common stock. The MIRALOGX Warrants are exercisable, in whole or in part, any time prior to November 15, 2028 at a cash exercise
price of $2.00 per share.
On
November 15, 2023, we entered into a promissory note and loan agreement with MIRALOGX. Pursuant to the loan agreement, we may borrow
up to $3.0 million from MIRALOGX to fund the development of licensed products under the license agreement. Together with any advance
request, we will deliver to the Lender a budget for the requested advance. The budget may only include costs directly associated with
preparing an IND application for KETAMIR-2, exclusive of personnel costs. Any advances made by the Lender to us pursuant to this note
may be repaid by us (together with any and all interest accrued thereon) at any time without penalty or premium in accordance with the
terms hereof. Amounts repaid hereunder may not be reborrowed. The loan agreement has a one-year term, and all outstanding principal and
accrued but unpaid interest must be repaid in full on November 15, 2023. Interest on the amounts borrowed under the loan agreement accrues
at an annual fixed rate of 8%. We may prepay all or a portion of the outstanding principal and accrued unpaid interest under the loan
agreement at any time without a prepayment fee.
Amended
and Restated Limited License Agreement with MyMD Pharmaceuticals
On
June 27, 2022, we entered into an Amended and Restated Limited License Agreement with MyMD, having an effective date of April 26, 2022.
The license, as amended on April 20, 2023, grants our company a perpetual, worldwide, royalty-free non-exclusive right to use MyMD’s
Supera-CBD compound, a different compound than MIRA1a, as a synthetic intermediate in the manufacture of MIRA1a for all purposes (including
clinical development and commercial production). This license is perpetual, and MyMD does not have a right to terminate it. In consideration
of this license, we agreed to share with MyMD technical information and know-how that pertains to the synthetic manufacture and/or formulation
of our MIRA1a product candidate and granted a license to MyMD to use improvements to MIRA1a made under the agreement, agreement, and
the agreement does not involve any prior or future cash payments by us. Although we believe that Supera-CBD is currently the best available
synthetic intermediate for the manufacture of MIRA1a, we believe that other intermediates and/or processes could be used to manufacture
MIRA1a.
Consulting
and Employment Agreements with Dr. Chapman
On
April 1, 2022, we entered into a Consulting Agreement with Dr. Chapman pursuant to which he provided regulatory and drug development
consulting services to the Company on an as-requested basis. Pursuant to the Consulting Agreement, he was to be paid a one-time fee of
$100,000 upon the completion of our initial public offering (of which $50,000 was prepaid in in the first quarter of 2022) plus a monthly
fee of $20,000 thereafter. The monthly fee was to begin upon the completion of our initial public offering. He was also reimbursed for
reasonable out-of-pocket expenses incurred in connection with his duties under the Consulting Agreement. The agreement had a term of
one year with an automatic one-year extension, provided that either party could terminate the agreement without cause upon 30-days prior
written notice.
In
his capacity as a consultant, Dr. Chapman was also granted on June 15, 2022, an option to purchase up to 200,000 shares of our common
stock at an exercise price of $5.00 per share. Upon Dr. Chapman becoming Executive Chairman, he has received or will receive additional
compensation in that capacity, and his employment agreement will at such time replace his Consulting Agreement. See “Executive
Compensation” above.
Consulting
Relationship with Mr. Kroenig
In
his capacity as a consultant, Mr. Kroenig was also granted on June 15, 2022, an option to purchase up to 10,000 shares of our common
stock at an exercise price of $5.00 per share. This option was granted under our 2022 Omnibus Plan and vested as to 33.33% of the option
shares on the date of grant, with the balance vesting in one-half increments on each of the two successive anniversaries of the grant
date. The option has a term of 10 years, subject to earlier termination upon certain terminations of Kroenig’s position as a consultant
to the Company and may be accelerated upon a change in control.
Prior
Consulting Agreement with Dr. Kaplin
Prior
to Dr. Kaplin becoming our President and Chief Scientific Officer in May 2022, Dr. Kaplin was a party to a consulting agreement with
our company pursuant to which Dr. Kaplin was paid $100,000 in 2021. This agreement was terminated in May 2022.
Review
and Approval of Related Party Transactions
Our
board of directors has adopted a written policy regarding the review and approval of related party transactions. Our audit committee
charter provides that the audit committee shall review and approve or disapprove any related party transactions, which are transactions
between us and related persons in which the aggregate amount involved exceeds or may be expected to exceed $120,000 and in which a related
person has or will have a direct or indirect material interest. Our policy regarding transactions between us and related persons provides
that a related person is defined as a director, executive officer, nominee for director or greater than 5% beneficial owner of our common
stock, in each case since the beginning of the most recently completed year, and any of their immediate family members.
Certain
of the foregoing disclosures are summaries of certain provisions of our related party agreements and are qualified in their entirety
by reference to all of the provisions of such agreements. Because these descriptions are only summaries of the applicable agreements,
they do not necessarily contain all of the information that you may find useful. Copies of certain of the agreements have been filed
as exhibits to the registration statement of which this prospectus is a part and are available electronically on the website of the SEC
at www.sec.gov.
PRINCIPAL
SHAREHOLDERS
The
following table sets forth information as of December 15, 2023 (the “Beneficial Ownership Date”) with respect to the
beneficial ownership of our common stock by:
| |
● |
each
of our named executive officers; |
| |
|
|
| |
● |
each
of our directors; |
| |
|
|
| |
● |
all
of our current directors and executive officers as a group; and |
| |
|
|
| |
● |
each
person known by us to be the beneficial owner of more than 5% of the outstanding shares of our common stock. |
We
have determined beneficial ownership in accordance with the rules of the SEC, and thus it represents sole or shared voting or investment
power with respect to our securities. In computing the number of shares beneficially owned by a person and the percentage ownership of
that person, shares of common stock subject to options or warrants held by that person that are currently exercisable or exercisable
within 60 days of the Beneficial Ownership Date are deemed outstanding but are not deemed outstanding for computing the percentage ownership
of any other person. Unless otherwise indicated below, to our knowledge, the persons and entities named in the table have sole voting
and sole investment power with respect to all shares that they beneficially owned, subject to community property laws where applicable.
The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Sections 13(d) and
13(g) of the Securities Act.
In
the table below, the applicable percentage ownership is based on shares of our common stock outstanding as of the Beneficial Ownership
Date. Unless otherwise indicated in the footnotes to the table below, the address of each beneficial owner listed in the table below
is 855 N Wolfe Street Suite 601, Baltimore, MD 21205.
| | |
Shares of Common Stock Beneficially Owned | |
| Name of beneficial owner | |
Number of Shares | | |
Percentage | |
| Directors and Executive Officers | |
| | | |
| | |
| Erez Aminov | |
| 573,500 | | |
| 3.83 | % |
| Michelle Yanez | |
| 42,223 | | |
| * | |
| Adam Kaplin, MD, PhD | |
| 313,334 | | |
| 2.10 | % |
| Chris Chapman, MD | |
| 390,000 | | |
| 2.61 | % |
| Christos Nicholoudis, Esq. | |
| 15,000 | | |
| * | |
| Michael Jerman | |
| - | | |
| * | |
| Brad Kroenig | |
| 86,667 | | |
| * | |
| Talhia Tuck | |
| 50,000 | | |
| * | |
| Hugh McColl III | |
| 70,000 | | |
| * | |
| All current directors and officers as a group (9 persons) (1) | |
| 1,540,724 | | |
| 10.32 | % |
| | |
| | | |
| | |
| 5% Stockholders | |
| | | |
| | |
| Brian McNulty(2) | |
| 5,110,270 | | |
| 34.57 | % |
| | |
| | | |
| | |
| *Represents beneficial ownership of less than 1% | |
| | | |
| | |
| |
(1) |
Includes
shares subject to options granted under our 2022 Omnibus Plan that are exercisable as of the Beneficial Ownership Date or within
60 days of the Beneficial Ownership Date held as follows: Mr. Aminov, 200,000 shares; Ms. Yanez, 42,223 shares; Dr. Kaplin, 113,334
shares; Dr. Chapman, 190,000 shares; Mr. Nicholoudis, 15,000 shares; Mr. Kroenig, 36,667 shares; Ms. Tuck, 30,000 shares; Mr. McColl,
30,000 shares; and all current officers and directors as a group, 457,224 shares. Excludes shares subject to options granted under
our 2022 Omnibus Plan that are not exercisable within 60 days of the Beneficial Ownership Date. |
| |
(2) |
Includes
(i) 10,000 shares held directly by Mr. McNulty, (ii) 2,740,270 shares held by the Bay Shore Trust, (iii) 660,000 shares held by the
Celeste J Williams Lifetime QTIP Trust, (iv) 1,000,000 shares issuable pursuant to warrants held by the Bay Shore Trust that are
immediately exercisable, and (v) 700,000 shares issuable pursuant to warrants held by MIRALOGX LLC, that are immediately exercisable.
As trustee of the Bay Shore Trust and the Celeste J Williams Lifetime QTIP Trust, Mr. McNulty has sole voting and dispositive
power over the shares held by each trust, and, as a result is deemed to have beneficial ownership (as determined under Section 13(d)
of the Exchange Act) of the securities held by the trusts. |
SELLING
STOCKHOLDERS
This
prospectus covers the possible resale by the selling stockholders identified in the table below of up to 1,700,000 shares of our common
stock (the “Resale Shares”). When we refer to the “selling stockholders” in this prospectus, we are referring
to the persons listed in the table below, and the transferees, pledgees or donees, or their respective successors-in-interest, that may
come to hold any of the selling stockholders’ interest in the Resale Shares after the date of this prospectus, and that may be
identified in a supplement to this prospectus or, if required, a post-effective amendment to the registration statement of which this
prospectus is a part. The transactions by which the selling stockholders acquired their securities from us were exempt under the registration
provisions of the Securities Act.
The
selling stockholders may sell some, all, or none of the Resale Shares. Unless otherwise indicated in the footnotes to the table below,
the selling stockholders have not had any material relationship with us or any of our affiliates within the past three years other than
as a security holder.
We
have prepared the following table based on written representations and information furnished to us by or on behalf of the selling stockholders.
Unless otherwise indicated in the footnotes to the table below, we believe that (i) none of the selling stockholders are broker-dealers
or affiliates of broker-dealers, and (ii) no selling stockholder has direct or indirect agreements or understandings with any person
to distribute their Resale Shares. To the extent any selling stockholder identified below is, or is affiliated with, a broker-dealer,
it could be deemed, individually, but not severally, to be an “underwriter” within the meaning of the Securities Act. Information
about the selling stockholders may change over time.
The
table below lists the selling stockholders and other information regarding the beneficial ownership of the shares of common stock by
the selling stockholders. The second column lists the number of shares of common stock beneficially owned by the selling stockholders,
based on its ownership of Resale Shares as of December 15, 2023.
The
column entitled “Number of Shares Being Offered” lists the shares of common stock being offered by this prospectus by the
selling stockholders.
The
column entitled “Number of Shares Beneficially Owned After Offering” assumes the sale of all of the shares offered by the
selling stockholders pursuant to this prospectus.
| Selling Stockholder | |
Number of Shares Beneficially
Owned
Before
Offering | | |
Percentage
of Shares
Beneficially
Owned
Before this
Offering | | |
Number of Shares Being Offered | | |
Number of
Shares
Beneficially
Owned
After
Offering (1) | | |
Percentage
of Shares
Beneficially
Owned
After
Offering
(%) | |
| Bay Shore Trust | |
| 3,740,270 | | |
| 25.30 | % | |
| 1,000,000 | | |
| 2,740,270 | | |
| 18.54 | % |
| MIRALOGX, LLC | |
| 700,000 | | |
| 4.74 | % | |
| 700,000 | | |
| - | | |
| - | |
*Less
than 1%
| (1) |
Applicable
percentage ownership after this offering is based on 14,780,885 shares of common stock deemed to be outstanding as of December
15, 2023. |
DESCRIPTION
OF CAPITAL STOCK
The
following description of the material terms of our amended and restated articles of incorporation and our amended and restated bylaws
is a summary, does not purport to be complete and is qualified in its entirety by reference to our third amended and restated articles
of incorporation and amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus
is a part and are incorporated by reference into this prospectus.
After
giving effect to the 1-for-5 reverse stock split that we completed on June 28, 2023, the total number of shares of common stock our company
is authorized to issue is presently 100,000,000, $0.0001 par value per share. The total number of shares of preferred stock our company
is authorized to issue is 10,000,000, $0.0001 par value per share.
Corporate
Governance
We
are a corporation organized under the laws of the state of Florida and are governed by the Florida Business Corporation Act, which we
sometimes refer to as the FBCA, our amended and restated articles of incorporation and our amended and restated bylaws.
Common
Stock
Holders
of shares of our common stock are entitled to one vote for each share held on all matters submitted to a vote of shareholders. Accordingly,
holders of a majority of the shares of our common stock entitled to vote in any election of directors may elect all of the directors
standing for election. Holders of shares of our common stock are entitled to receive proportionately any dividends if and when such dividends
are declared by our board of directors, subject to any preferential dividend rights of outstanding preferred stock. Upon the liquidation,
dissolution or winding up of the company, the holders of our common stock are entitled to receive ratably net assets available after
the payment of all debts and other liabilities and subject to the prior rights of holders of any outstanding preferred stock. The rights,
preferences, and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders
of shares of any series of preferred stock that we may designate and issue in the future.
Preferred
Stock
Under
the terms of our amended and restated articles of incorporation, which we sometimes refer to as the articles, the board of directors
is authorized to designate and issue up to 10,000,000 shares of preferred stock in one or more series without shareholder approval. Our
board of directors will have discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend
rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
It
is not possible to state the actual effect of the issuance of any shares of preferred stock upon the rights of holders of our common
stock until the board of directors determines the specific rights of the holders of the preferred stock. However, these effects might
include:
| ● |
restricting
dividends on the common stock; |
| |
|
| ● |
diluting
the voting power of the common stock; |
| |
|
| ● |
impairing
the liquidation rights of the common stock; and |
| |
|
| ● |
delaying
or preventing a change in control of the company. |
There
are no shares of preferred stock outstanding and, at present, we have no plans to issue any shares of preferred stock.
Dividends
and Other Distributions
The
holders of our common stock will be entitled to receive proportionately any cash or stock dividends if and when such dividends are declared
by the board of directors, subject to any preferential dividend rights of outstanding preferred stock. In the event of the dissolution
or liquidation of the company, after the full preferential rights, if any, on any outstanding preferred stock has been paid to or set
aside for the holders of such preferred stock, the holders of our common stock will be entitled to receive proportionately all of our
remaining assets.
The
declaration and payment of any dividend will be subject to the discretion of our board of directors, subject to applicable laws. The
time and amount of any dividend will depend on a number of factors, including our financial condition, results of operations, capital
requirements, contractual restrictions, general business conditions, and any other factors that our board of directors may deem relevant.
We
currently intend to retain all available funds and any future earnings for general corporate purposes, including working capital, operating
expenses, and capital expenditures, and do not anticipate declaring or paying any cash dividends on our common stock in the foreseeable
future. See “Dividend Policy.”
Number
and Election of Directors
Our
Board consists of seven members. The holders of common stock and any other class of stock of our company, to the extent they shall have
the right to vote, shall retain the right to elect and remove all members of the board of directors.
Quorum/Voting
At
all meetings of our board of directors, a majority of the total number of directors constitutes a quorum. If there is a quorum, a vote
of the majority of the directors present at the meeting is considered an act of our board of directors.
Removal
of Directors
Our
amended and restated articles provide that any director may be removed from office, but only for cause by the affirmative vote of not
less than a majority of our shareholders entitled to vote in the election of directors. “Cause” is construed to exist only
if the director whose removal is proposed has been convicted of a felony or has been adjudged to be liable for willful misconduct in
the performance of his or her duties to us in a matter which has a material adverse effect on our business.
Vacancies
on the Board of Directors
A
vacancy on our board of directors may be filled by a vote of a majority of the remaining members of the board of directors, even if less
than a quorum, at any meeting of the board of directors. A person so elected by the board of directors to fill a vacancy shall hold office
for the remainder of the full term of the director for which the vacancy was created or occurred and until such director’s successor
shall have been duly elected and qualified.
Voting
by Shareholders
Each
holder of our common stock is entitled to one vote per share for the election of directors and for all other corporate purposes.
Amendment
of Articles
The
FBCA allows us to amend our amended and restated articles at any time to add or change a provision that is required or permitted to be
included in the articles of incorporation or to delete a provision that is not required to be included in the articles of incorporation.
Our board of directors can propose one or more amendments for submission to shareholders and may condition its submission of the proposed
amendment on any basis if it provides certain notice and includes certain information regarding the proposed amendment in that notice.
The provisions in our articles that require a greater voting requirement than provided in the FBCA may only be amended by the same vote
required to take action under that voting requirement.
Amendment
of Bylaws
Our
bylaws may be amended or repealed, and new bylaws may be adopted by our shareholders at any annual or special meetings at which a quorum
is present. The bylaws may also be amended or repealed, and new bylaws may be adopted by our board of directors by affirmative vote of
a majority of the number of directors present at any meeting at which a quorum is in attendance. Notwithstanding the foregoing, pursuant
to our articles, the provisions of our bylaws that require a greater voting requirement than provided in the FBCA may only be amended
by the same vote required to take action under that voting requirement.
Anti-Takeover
Effects of Various Provisions of Florida Law, Our Amended and Restated Articles of Incorporation and Our Bylaws
Provisions
of Florida law have certain anti-takeover effects. Our amended and restated articles of incorporation and bylaws also contain provisions
that may have similar effects.
Florida
Anti-Takeover Statutes
The
control share acquisition statute, Section 607.0902 of the FBCA, generally provides that in the event a person acquires voting shares
of the company in excess of 20% of the voting power of all of our issued and outstanding shares, such acquired shares will not have any
voting rights unless such rights are restored by the holders of a majority of the votes of each class or series entitled to vote separately,
excluding shares held by the person acquiring the control shares or any of our officers or employees who are also directors of the company.
Certain acquisitions of shares are exempt from these rules, such as shares acquired pursuant to the laws of intestate succession or pursuant
to a gift or testamentary transfer, pursuant to a merger or share exchange effected in compliance with the FBCA if we are a party to
the agreement, or pursuant to an acquisition of our shares if the acquisition has been approved by our board of directors before the
acquisition. The control share acquisition statute generally applies to any “issuing public corporation,” which means a Florida
corporation which has:
| |
● |
One
hundred or more shareholders; |
| |
|
|
| |
● |
Its
principal place of business, its principal office, or substantial assets within Florida; and |
| |
|
|
| |
● |
Either
(i) more than 10% of its shareholders are resident in Florida; (ii) more than 10% of its shares are owned by residents of Florida;
or (iii) one thousand shareholders are resident in Florida. |
The
affiliated transaction (or so-called “business combination”) statute, Section 607.0901 of the FBCA, provides that we may
not engage in certain mergers, consolidations, sales of assets, issuances of stock, reclassifications, recapitalizations, and other affiliated
transactions with any “interested shareholder” for a period of three years following the time that such shareholder became
an interested shareholder, unless:
| |
● |
Prior
to the time that such shareholder became an interested shareholder, our board of directors approved either the affiliated transaction
or the transaction which resulted in the shareholder becoming an interested shareholder; or |
| |
|
|
| |
● |
Upon
consummation of the transaction that resulted in the shareholder becoming an interested shareholder, the interested shareholder owned
at least 85% of our voting shares outstanding at the time the transaction commenced; or |
| |
|
|
| |
● |
At
or subsequent to the time that such shareholder became an interested shareholder, the affiliated transaction is approved by our board
of directors and authorized at an annual or special meeting of shareholders, and not by written consent, by the affirmative vote
of at least two-thirds of the outstanding voting shares which are not owned by the interested shareholder. |
An
“interested shareholder” is generally defined as any person who is the beneficial owner of more than 15% of our outstanding
voting shares.
The
voting requirements set forth above do not apply to a particular affiliated transaction if one or more conditions are met, including,
but not limited to, the following: if the affiliated transaction has been approved by a majority of our disinterested directors; if we
have not had more than 300 shareholders of record at any time during the three years preceding the date the affiliated transaction is
announced; if the interested shareholder has been the beneficial owner of at least 80% of our outstanding voting shares for at least
three years preceding the date the affiliated transaction is announced; or if the consideration to be paid to the holders of each class
or series of voting shares in the affiliated transaction meets certain requirements of the statute with respect to form and amount, among
other things.
No
Cumulative Voting
The
FBCA provides that shareholders do not have the right to cumulate votes in the election of directors unless the articles of incorporation
provide otherwise. Our articles do not provide for cumulative voting.
Advance
Notice Requirements for Shareholder Proposals and Director Nominations; Calling a Special Meeting
Our
amended and restated bylaws provide that shareholders seeking to bring business before an annual meeting must provide timely notice of
their proposal in writing to the corporate secretary. To be timely, a shareholder’s notice must have been received on or before
December 31 of the year immediately preceding the annual meeting; provided, however, that in the event that the date of the annual meeting
is on or after May 1 in any year, notice by the shareholder to be timely must be received not later than the close of business on the
day which is determined by adding to December 31 of the year immediately preceding such annual meeting the number of days starting with
May 1 and ending on the date of the annual meeting in such year. The amended and restated bylaws also specify requirements as to the
form and content of a shareholder’s notice. These provisions may impede shareholders’ ability to bring matters before an
annual meeting of shareholders or make nominations for directors at an annual meeting of shareholders.
Our
amended and restated bylaws also provide that a special meeting of shareholders can only be called by our chairman of the board of directors,
our chief executive officer, our president (in the absence of a chief executive officer), a majority of our board of directors or the
holders of 10% or more of all of our votes entitled to be cast on any issue proposed to be considered at the special meeting of shareholders.
Authorized
But Unissued Shares
Our
authorized but unissued shares of common stock and preferred stock will be available for future issuance without shareholder approval.
We could use these additional shares for a variety of corporate purposes, including future public offerings to raise additional capital,
acquisitions of other businesses or entities and issuances under employee benefit plans. Additionally, we could issue a series of preferred
stock that could, depending on its terms, impede the completion of a merger, tender offer or other takeover attempt. Our board of directors
will make any determination to issue such shares based on its judgment as to the best interests of us and our shareholders. The board
of directors, in so acting, could issue preferred stock having terms that could discourage an acquisition attempt through which an acquiror
may be able to change the composition of the board of directors, including a tender offer or other transaction that some, or a majority,
of our shareholders might believe to be in their best interests or in which shareholders might receive a premium over the then-current
market price of the common stock.
Exclusive
Jurisdiction
Our
amended and restated bylaws provide that, unless we consent in writing to the selection of an alternative forum, the sole and exclusive
forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty
owed by any of our current or former directors, officers or other employees to us or our shareholders, (iii) any action arising pursuant
to any provision of the FBCA, our amended and restated articles of incorporation or our amended and restated bylaws, or (iv) any other
action asserting a claim that is governed by the internal affairs doctrine shall be a state court located within the state of Florida
(or, if a state court located within the state of Florida does not have jurisdiction, the federal district court for the Middle District
of Florida); provided that, the exclusive forum provision will not apply to suits brought to enforce any liability or duty created by
the Exchange Act, or to any claim for which the federal courts have exclusive jurisdiction. Our bylaws also provide that, unless we consent
in writing to the selection of an alternative forum, the U.S. federal district courts shall be the exclusive forum for the resolution
of any claims arising under the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in our securities
shall be deemed to have notice of and consented to these provisions. Although we believe these provisions benefit us by providing increased
consistency in the application of law for the specified types of actions and proceedings, the provisions may have the effect of discouraging
lawsuits against us or our directors and officers. We note that investors cannot waive compliance with the federal securities laws and
the rules and regulations thereunder. Please also see the section titled “Risk Factors—Risks Related to Ownership of our
Common Stock—Our amended and restated bylaws designates the state courts located within the state of Florida as the exclusive forum
for substantially all disputes between us and our shareholders and the federal district courts as the exclusive forum for Securities
Act claims, which could limit our shareholders’ ability to obtain a favorable judicial forum for disputes with us.”
Preemptive
Rights
No
holder of our common stock has any preemptive or subscription rights to acquire shares of our capital stock.
Liability
and Indemnification of Officers and Directors
Our
amended and restated articles of incorporation and bylaws provide that we shall indemnify any and all persons whom we shall have power
to indemnify under the FBCA to the fullest extent permitted by law.
Section
607.0831 of the FBCA, provides that a director is not personally liable for monetary damages to the corporation or any other person for
any statement, vote, decision to take or not to take action, or any failure to take any action, as a director, unless (1) the director
breached or failed to perform his or her duties as a director and (2) the director’s breach of, or failure to perform, those duties
constitutes (a) a violation of the criminal law, unless the director had reasonable cause to believe his or her conduct was lawful or
had no reasonable cause to believe his or her conduct was unlawful, (b) a transaction from which the director derived an improper personal
benefit, either directly or indirectly, (c) a circumstance under which the liability provisions of Section 607.0834 of the FBCA are applicable,
(d) in a proceeding by or in the right of the corporation to procure a judgment in its favor or by or in the right of a shareholder,
conscious disregard for the best interest of the corporation, or willful or intentional misconduct, or (e) in a proceeding by or in the
right of someone other than the corporation or a shareholder, recklessness or an act or omission which was committed in bad faith or
with malicious purpose or in a manner exhibiting wanton and willful disregard of human rights, safety, or property. A judgment or other
final adjudication against a director in any criminal proceeding for a violation of the criminal law estops that director from contesting
the fact that his or her breach, or failure to perform, constitutes a violation of the criminal law; but does not estop the director
from establishing that he or she had reasonable cause to believe that his or her conduct was lawful or had no reasonable cause to believe
that his or her conduct was unlawful.
Under
Section 607.0851 of the FBCA, a corporation has power to indemnify any person who is a party to any proceeding (other than an action
by, or in the right of the corporation), because he or she is or was a director or officer of the corporation against liability incurred
in connection with such proceeding, including any appeal thereof, if he or she acted in good faith and in a manner he or she reasonably
believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had
no reasonable cause to believe his or her conduct was unlawful. The termination of any proceeding by judgment, order, settlement or conviction
or upon a plea of nolo contendere or its equivalent shall not, of itself, create a presumption that the person did not act in good faith
and in a manner which he or she reasonably believed to be in, or not opposed to, the best interests of the corporation or, with respect
to any criminal action or proceeding, has reasonable cause to believe that his or her conduct was unlawful.
For
purposes of the indemnification provisions of the FBCA, “director” or “officer” means an individual who is or
was a director or officer, respectively, of a corporation or who, while a director or officer of the corporation, is or was serving at
the corporation’s request as a director or officer, manager, partner, trustee, employee, or agent of another domestic or foreign
corporation, limited liability company, partnership, joint venture, trust, employee benefit plan, or another enterprise or entity and
the terms include, unless the context otherwise requires, the estate, heirs, executors, administrators, and personal representatives
of a director or officer.
In
addition, under Section 607.0851 of the FBCA, a corporation has the power to indemnify any person, who was or is a party to any proceeding
by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director or
officer, against expenses and amounts paid in settlement not exceeding, in the judgment of the board of directors, the estimated expense
of litigating the proceeding to conclusion, actually and reasonably incurred in connection with the defense or settlement of such proceeding,
including any appeal thereof. Such indemnification shall be authorized if such person acted in good faith and in a manner he or she reasonably
believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be made under this
subsection in respect of any claim, issue, or matter as to which such person shall have been adjudged to be liable unless, and only to
the extent that, the court in which such proceeding was brought, or any other court of competent jurisdiction, shall determine upon application
that, despite the adjudication of liability but in view of all circumstances of the case, such person is fairly and reasonably entitled
to indemnity for such expenses which such court shall deem proper.
Section
607.0852 of the FBCA provides that a corporation must indemnify an individual who is or was a director or officer who was wholly successful,
on the merits or otherwise, in the defense of any proceeding to which the individual was a party because he or she is or was a director
or officer of the corporation against expenses incurred by the individual in connection with the proceeding.
Section
607.0853 of the FBCA provides that a corporation may, before final disposition of a proceeding, advance funds to pay for or reimburse
expenses incurred in connection with the proceeding by an individual who is a party to the proceeding because that individual is or was
a director or an officer if the director or officer delivers to the corporation a signed written undertaking of the director or officer
to repay any funds advanced if (a) the director or officer is not entitled to mandatory indemnification under Section 607.0852; and (b)
it is ultimately determined under Section 607.0854 or Section 607.0855 (as described below) that the director or officer has not met
the relevant standard of conduct described in Section 607.0851 or the director or officer is not entitled to indemnification under Section
607.0859 (as described below).
Section
607.0854 of the FBCA provides that, unless the corporation’s articles of incorporation provide otherwise, notwithstanding the failure
of a corporation to provide indemnification, and despite any contrary determination of the board of directors or of the shareholders
in the specific case, a director or officer of the corporation who is a party to a proceeding because he or she is or was a director
or officer may apply for indemnification or an advance for expenses, or both, to a court having jurisdiction over the corporation which
is conducting the proceeding, or to a circuit court of competent jurisdiction. Our amended and restated articles of incorporation do
not provide any such exclusion. After receipt of an application and after giving any notice it considers necessary, the court may order
indemnification or advancement of expenses upon certain determinations of the court.
Section
607.0855 of the FBCA provides that, unless ordered by a court under Section 607.0854, a corporation may not indemnify a director or officer
under Section 607.0851 unless authorized for a specific proceeding after a determination has been made that indemnification is permissible
because the director or officer has met the relevant standard of conduct set forth in Section 607.0851.
Section
607.0857 of the FBCA also provides that a corporation shall have the power to purchase and maintain insurance on behalf of and for the
benefit of any person who is or was a director or officer of the corporation against any liability asserted against the person and incurred
by him or her in any such capacity or arising out of his or her status as such, whether or not the corporation would have the power to
indemnify or advance expenses to the individual against such liability under the provisions of Section 607.0857.
Section
607.0858 of the FBCA provides that the indemnification provided pursuant to Section 607.0851 and Section 607.0852, and the advancement
of expenses provided pursuant to Section 607.0853, are not exclusive. A corporation may, by a provision in its articles of incorporation,
bylaws, or any agreement, or by vote of shareholders or disinterested directors, or otherwise, obligate itself in advance of the act
or omission giving rise to a proceeding to provide any other or further indemnification or advancement of expenses to any of its directors
or officers.
Section
607.0859 of the FBCA provides that, unless ordered by a court under the provisions of Section 607.0854 of the FBCA, a corporation may
not indemnify a director or officer under Section 607.0851 or Section 607.0858, or advance expenses to a director or officer under Section
607.0853 or Section 607.0858, if a judgment or other final adjudication establishes that his or her actions, or omissions to act, were
material to the cause of action so adjudicated and constitute: (a) willful or intentional misconduct or a conscious disregard for the
best interests of the corporation in a proceeding by or in the right of the corporation to procure a judgment in its favor or in a proceeding
by or in the right of a shareholder; (b) a transaction in which a director or officer derived an improper personal benefit; (c) a violation
of the criminal law, unless the director or officer had reasonable cause to believe his or her conduct was lawful or had no reasonable
cause to believe his or her conduct was unlawful; or (d) in the case of a director, a circumstance under which the liability provisions
of Section 607.0834 are applicable (relating to unlawful distributions).
These
provisions may have the practical effect in certain cases of eliminating the ability of shareholders to collect monetary damages from
our directors and officers. We believe that these provisions are necessary to attract and retain qualified persons to serve as our directors
and officers. There is currently no pending material litigation or proceeding involving any of our directors, officers or employees for
which indemnification is sought.
Transfer
Agent and Registrar
American
Stock Transfer (also known as Equiniti) will be the transfer agent and registrar for our common stock. The transfer agent’s address
is 6201 15th Avenue, Brooklyn, NY 11219.
Listing
Our
common stock is listed on the Nasdaq Capital Market under the symbol “MIRA”.
MATERIAL
U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S.
HOLDERS
OF OUR COMMON STOCK
The
following discussion is a summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the
ownership and disposition of shares of our common stock issued pursuant to this offering but is not intended to be a complete analysis
of all potential tax consequences. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state,
local or non-U.S. tax laws are not discussed. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”),
final, temporary, and proposed Treasury Regulations, judicial decisions, and published rulings and administrative pronouncements of the
U.S. Internal Revenue Service (the “IRS”), in each case as in effect as of the date of this prospectus. These authorities
may change or be subject to differing interpretations, and any such change or differing interpretation may be applied retroactively in
a manner that could adversely affect a non-U.S. holder of our common stock. We have not sought and will not seek any rulings from the
IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position to that discussed
below regarding the tax consequences of the ownership and disposition of our common stock.
This
discussion is limited to a non-U.S. holder that holds our common stock as a “capital asset” within the meaning of Section
1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences
relevant to a non-U.S. holder’s particular circumstance, including the impact of the alternative minimum tax, the special tax accounting
rules in Section 451(b) of the Code or the Medicare surtax on net investment income provided by Section 1411 of the Code. In addition,
it does not address consequences relevant to Non-U.S. Holders subject to special rules, including, without limitation:
| |
● |
U.S.
expatriates and former citizens or long-term residents of the United States; |
| |
|
|
| |
● |
persons
holding shares of our common stock as part of a straddle, or other risk reduction strategy or as part of a conversion transaction
or other integrated investment; |
| |
|
|
| |
● |
banks,
insurance companies, and other financial institutions; |
| |
|
|
| |
● |
brokers,
dealers, or certain electing traders in securities that use a mark-to-market method of tax accounting for their securities positions; |
| |
|
|
| |
● |
“controlled
foreign corporations”, “passive foreign investment companies” , as defined in Sections 957 and Section 1297 of
the Code, respectively, and corporations that accumulate earnings to avoid U.S. federal income tax under Section 531 and 532 of the
Code; |
| |
|
|
| |
● |
partnerships
or other entities or arrangements treated as partnerships for U.S. federal income tax purposes and other pass-through entities (and
investors in such entities); |
| |
|
|
| |
● |
tax-exempt
organizations or governmental organizations; |
| |
|
|
| |
● |
persons
deemed to sell our common stock under the constructive sale provisions of the Code; |
| |
|
|
| |
● |
tax-qualified
retirement plans; and |
| |
|
|
| |
● |
“qualified
foreign pension funds” as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified
foreign pension funds. |
If
an entity treated as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in the
partnership will depend on the status of the partner, the activities of the partnership, and certain determinations made at the partner
level. Partnerships holding our common stock and the partners in such partnerships should consult their tax advisors regarding the U.S.
federal income tax consequences to them.
THIS
DISCUSSION IS FOR INFORMATIONAL PURPOSES ONLY AND IS NOT TAX ADVICE. INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE
APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP,
AND DISPOSITION OF SHARES OF OUR COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE,
LOCAL, OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Definition
of a Non-U.S. Holder
For
purposes of this discussion, a “non-U.S. holder” is any beneficial owner of our common stock that is an individual, corporation,
estate or trust and is not a “U.S. person.” A U.S. person is any person that, for U.S. federal income tax purposes, is or
is treated as any of the following:
| |
● |
an
individual who is a citizen or resident of the United States; |
| |
|
|
| |
● |
a
corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| |
|
|
| |
● |
an
estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| |
|
|
| |
● |
a
trust that (1) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons”
(within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a United States person
for U.S. federal income tax purposes. |
Distributions
As
described in the section entitled “Dividend Policy,” we do not anticipate declaring or paying dividends to holders of our
common stock in the foreseeable future. However, if we do make distributions of cash or property on our common stock, such distributions
will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits,
as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute
a nontaxable return of capital and first be applied against and reduce a Non-U.S. Holder’s adjusted tax basis in its common stock,
but not below zero, and any excess will be treated as capital gain and will be treated as described below under “— Sale or
Other Taxable Disposition”.
Subject
to the discussion below on effectively connected income, dividends paid to a Non-U.S. Holder of our common stock will be subject to U.S.
federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax
treaty, provided the Non-U.S. Holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification
for the lower treaty rate of withholding). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies
for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the
IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
If
dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within
the United States (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the
United States to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described
above. To claim the exemption, the non-U.S. holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying
that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States.
Any
such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the rates applicable to U.S.
persons. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified
by an applicable income tax treaty) on such effectively connected dividends, as adjusted for certain items. Non-U.S. Holders should consult
their tax advisors regarding any applicable tax treaties that may provide for different rules.
Sale
or Other Taxable Disposition
Subject
to the discussion below under “— Information Reporting and Backup Withholding” and “— Additional Withholding
Tax Under FATCA”, a Non-U.S. Holder will not be subject to U.S. federal income tax on any gain realized upon the sale or other
taxable disposition of our common stock unless:
| |
● |
the
gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required
by an applicable income tax treaty, the non-U.S. holder maintains a permanent establishment in the United States to which such gain
is attributable);or |
| |
|
|
| |
● |
the
non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the
disposition and certain other requirements are met; |
Gain
described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the rates applicable
to U.S. persons. A non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower
rate specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items.
Gain
described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified
by an applicable income tax treaty), which may be offset by U.S. source capital losses of the non-U.S. Holder (even though the individual
is not considered a resident of the United States), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with
respect to such losses.
Non-U.S.
Holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Information
Reporting and Backup Withholding
Information
returns are required to be filed with the IRS in connection with any dividends on our common stock paid to a non-U.S. holder whether
or not withholding is required. Copies of the information returns reporting such interest, dividends, and withholding may also be made
available to the tax authorities in the country in which a non-U.S. holder resides under the provisions of an applicable income tax treaty.
Payments of dividends on our common stock will not be subject to backup withholding, provided the applicable withholding agent does not
have actual knowledge or reason to know the beneficial owner is a United States person and the Non-U.S. Holder either certifies its non-U.S.
status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or other applicable documentation, or otherwise establishes
an exemption. Proceeds of the sale or other taxable disposition of our common stock within the United States or conducted through certain
U.S.-related brokers generally will not be subject to backup withholding or information reporting, if the applicable withholding agent
receives the certification described above and does not have actual knowledge or reason to know that such beneficial owner is a United
States person, or otherwise establishes an exemption. Proceeds of a disposition of our common stock conducted through a non-U.S. office
of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Backup
withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit
against a non-U.S. Holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional
Withholding Tax Under FATCA
Sections
1471 to 1474 of the Code (such sections commonly referred to as the Foreign Account Tax Compliance Act, or “FATCA”) and the
Treasury Regulations and administrative guidance thereunder impose a 30% withholding tax on certain types of payments made to a “foreign
financial institution” or a “non-financial foreign entity” (each as defined in the Code), including, in some cases,
when such foreign financial institution or non-financial foreign entity acts as an intermediary, unless (1) the foreign financial institution
has entered into an agreement with the U.S. government to withhold on certain payments and to undertake certain diligence and reporting
obligations regarding U.S. account holders (including certain account holders that are non-U.S. entities with U.S. owners), (2) the non-financial
foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes
identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign
entity otherwise qualifies for an exemption from these rules. Foreign financial institutions located in jurisdictions that have an intergovernmental
agreement with the United States governing FATCA may be subject to different rules.
Under
the applicable Treasury Regulations and administrative guidance, withholding under FATCA generally applies to payments of dividends on
our common stock. While withholding under FATCA would have applied also to payments of gross proceeds from the sale or other disposition
of stock on or after January 1, 2019, recently proposed Treasury Regulations eliminate FATCA withholding on payments of gross proceeds
entirely. Taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued.
Prospective
investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our
common stock.
PLAN
OF DISTRIBUTION
We
are registering the Resale Shares to permit the resale of the Resale Shares by the selling stockholders from time to time after the date
of this prospectus. We will not receive any of the proceeds from the sale of the Resale Shares. We will pay all expenses (other than
discounts, commissions, and transfer taxes, if any) relating to the registration of the Resale Shares in the registration statement of
which this prospectus forms a part.
The
selling stockholders may sell all or a portion of the Resale Shares beneficially owned by them and offered hereby from time to time directly
or through one or more underwriters, broker-dealers, or agents. If the Resale Shares are sold through underwriters or broker-dealers,
the selling stockholders will be responsible for any underwriter discounts or commissions and any applicable transfer taxes. The Resale
Shares may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices
determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block
transactions,
| ● |
on
any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| |
|
| ● |
in
the over-the-counter market; |
| |
|
| ● |
in
transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| |
|
| ● |
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
|
| ● |
block
trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block
as principal to facilitate the transaction; |
| |
|
| ● |
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
|
| ● |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
|
| ● |
privately
negotiated transactions; |
| |
|
| ● |
short
sales; |
| |
|
| ● |
in
transactions through broker-dealers that agree with the selling stockholders to sell a specified number of such securities at a stipulated
price per security; |
| |
|
| ● |
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
|
| ● |
a
combination of any such methods of sale; or |
| |
|
| ● |
any
other method permitted pursuant to applicable law |
The
selling stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available,
rather than under this prospectus. The selling stockholders may also sell securities under Rule 144 or any other exemption from registration
under the Securities Act, if available, rather than under this prospectus.
Broker-dealers
engaged by the selling stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions
or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser)
in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in
excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or
markdown in compliance with FINRA Rule 2121.
In
connection with the sale of the securities or interests therein, the selling stockholders may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they
assume. The selling stockholders may also sell securities short and deliver these securities to close out their short positions, or loan
or pledge the securities to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into option
or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the
delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The
selling stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. Each selling stockholders has informed us that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
SELLING
RESTRICTIONS
Other
than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered
by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be
offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with
the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result
in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are
advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus.
This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in
any jurisdiction in which such an offer or a solicitation is unlawful.
European
Economic Area
In
relation to each member state of the European Economic Area that has implemented the Prospectus Directive (each, a relevant member state),
with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state (the relevant
implementation date), an offer of shares described in this prospectus may not be made to the public in that relevant member state other
than:
| |
● |
to
any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| |
|
|
| |
● |
to
fewer than 100 or, if the relevant member state has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural
or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive,
subject to obtaining the prior consent of the relevant Dealer or Dealers nominated by us for any such offer; or |
| |
|
|
| |
● |
in
any other circumstances falling within Article 3(2) of the Prospectus Directive, |
| |
|
|
| |
● |
provided
that no such offer of shares shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus
Directive. |
For
purposes of this provision, the expression an “offer of securities to the public” in any relevant member state means the
communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to
enable an investor to decide to purchase or subscribe for the shares, as the expression may be varied in that member state by any measure
implementing the Prospectus Directive in that member state, and the expression “Prospectus Directive” means Directive 2003/71/EC
(and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the relevant member state) and includes
any relevant implementing measure in the relevant member state. The expression 2010 PD Amending Directive means Directive 2010/73/EU.
The
sellers of the shares have not authorized and do not authorize the making of any offer of shares through any financial intermediary on
their behalf, other than offers made by the underwriters with a view to the final placement of the shares as contemplated in this prospectus.
Accordingly, no purchaser of the shares, other than the underwriters, is authorized to make any further offer of the shares on behalf
of the sellers or the underwriters.
United
Kingdom
This
prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the
meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the
Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (ii) high net worth entities, and
other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (each such person being referred
to as a “relevant person”). This prospectus and its contents are confidential and should not be distributed, published or
reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom
that is not a relevant person should not act or rely on this document or any of its contents.
Canada
The
securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors,
as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted
clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale
of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements
of applicable securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus
(including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by
the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser
should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars
of these rights or consult with a legal advisor.
LEGAL
MATTERS
The
validity of the common stock covered by this prospectus will be passed upon by Foley & Lardner LLP.
EXPERTS
The
financial statements of MIRA as of and for the years ended December 31, 2022 and 2021 included in this prospectus have been audited by
Cherry Bekaert LLP, an independent registered public accounting firm, appearing elsewhere herein, given on the authority of said firm
as experts in auditing and accounting.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered
by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all the information set
forth in the registration statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that
is included in the registration statement. Statements made in this prospectus concerning the contents of any contract, agreement, or
other document are summaries of all material information about the documents summarized but are not complete descriptions of all terms
of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document itself
for a complete description of its terms.
You
may read and copy the registration statement, including the related exhibits and schedules, and any document we file with the SEC without
charge at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, DC 20549. You may also obtain copies of
the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington,
DC 20549. You may call the SEC at 1-800-SEC-0330 for further information on the public reference room. The SEC also maintains an Internet
website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC
are also available to the public through the SEC’s website at http://www.sec.gov.
We
are subject to the information reporting requirements of the Exchange Act, and we file periodic reports, proxy statements and other information
with the SEC. These periodic reports, and other information are available for inspection and copying at the website of the SEC referred
to above. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, reports on Form 8-K and amendments to those
reports filed pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably
practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be
accessed through, our website is not incorporated by reference in, and is not part of, this prospectus. A copy of the registration statement
and the exhibits filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F
Street, NE, Washington, DC 20549, and copies of all or any part of the registration statement may be obtained from that office. Please
call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains
reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address
of the website is www.sec.gov.
We
maintain a corporate website at www.mirapharmaceuticals.com. Information contained in, or that can be accessed through, our website does
not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
We will post on our website any materials required to be so posted on such website under applicable corporate or securities laws and
regulations.
INDEX
TO FINANCIAL STATEMENTS
MIRA
Pharmaceuticals, Inc. Financial Statements
As
of and For the Years Ended December 31, 2022 and 2021
MIRA
Pharmaceuticals, Inc. Condensed Financial Statements
As
of September 30, 2023 and December 31, 2022
For
the Three and Nine Months Ended September 30, 2023 and 2022
Report
of Independent Registered Public Accounting Firm
To
the Board of Directors and Stockholders
MIRA
Pharmaceuticals, Inc.
Tampa,
Florida
Opinion
on the Financial Statements
We
have audited the accompanying balance sheets of MIRA Pharmaceuticals, Inc. (f/k/a MIRA1a Therapeutics, Inc.) (the “Company”)
as of December 31, 2022 and 2021, and the related statements of operations, stockholders’ equity (deficit) and cash flows for the
years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial
statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the
results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in
the United States of America.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial
statements based on our audits. We are required to be independent with respect to the Company in accordance with the relevant ethical
requirements relating to our audit.
We
conducted our audits in accordance with the auditing standards of the Public Company Accounting Oversight Board (United States) and in
accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform
the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error
or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether
due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence
regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used
and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe
that our audits provide a reasonable basis for our opinion.
Emphasis
of Matter
As
more fully described in Note 2 to the financial statements, the Company has incurred historical net losses and sustained substantial
cash losses. Our opinion is not modified with respect to this matter.
/s/
Cherry Bekaert LLP
We
have served as the Company’s auditor since 2022.
Tampa,
Florida
April
4, 2023, except for the 2nd paragraph of Note 10, and its related effects to the financial statements, which is as of July 14, 2023
MIRA
Pharmaceuticals, Inc.
BALANCE
SHEETS
DECEMBER
31, 2022 AND DECEMBER 31, 2021
| | |
December 31, | | |
December 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 350,978 | | |
$ | 2,809,552 | |
| Deferred offering costs | |
| 143,427 | | |
| 100,000 | |
| Prepaid expenses | |
| - | | |
| - | |
| Total current assets | |
| 494,405 | | |
| 2,909,552 | |
| | |
| | | |
| | |
| Deferred financing costs, net | |
| - | | |
| - | |
| Operating lease, right of use assets | |
| 164,910 | | |
| - | |
| Related party operating lease, right of use assets | |
| 198,759 | | |
| - | |
| Operating lease, right of use assets | |
| 198,759 | | |
| - | |
| Due from related party | |
| - | | |
| - | |
| Advances to affiliates | |
| - | | |
| 445,612 | |
| Total assets | |
$ | 858,074 | | |
$ | 3,355,164 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Trade accounts payable and accrued liabilities | |
$ | 811,738 | | |
$ | 228,406 | |
| Related party accounts payable | |
| 116,350 | | |
| 547,600 | |
| Related party line of credit | |
| 133,062 | | |
| 293,062 | |
| Related party accrued interest | |
| 34,987 | | |
| 24,738 | |
| Current portion of operating lease liabilities | |
| 75,143 | | |
| - | |
| Related party current portion of operating lease liabilities | |
| 198,759 | | |
| | |
| Current
portion of operating lease liabilities | |
| 198,759 | | |
| | |
| Total current liabilities | |
| 1,370,039 | | |
| 1,093,806 | |
| | |
| | | |
| | |
| Non-current operating lease liabilities | |
| 84,267 | | |
| - | |
| | |
| | | |
| | |
| Total liabilities | |
| 1,454,306 | | |
| 1,093,806 | |
| | |
| | | |
| | |
| Stockholders’ (Deficit) Equity | |
| | | |
| | |
| Preferred Stock, $0.0001 par value, 5,000,000 shares authorized and none issued or outstanding. | |
| - | | |
| - | |
| Common Stock, $0.0001 par value; 95,000,000 shares authorized, 13,313,000 and 12,673,800 issued and outstanding at December 31, 2022 and December 31, 2021, respectively. | |
| 6,657 | | |
| 6,337 | |
| Additional paid-in capital | |
| 8,699,830 | | |
| 4,499,550 | |
| Accumulated deficit | |
| (9,302,719 | ) | |
| (2,244,529 | ) |
| Total stockholders’ (deficit) equity | |
| (596,232 | ) | |
| 2,261,358 | |
| Total liabilities and stockholders’ (deficit) equity | |
$ | 858,074 | | |
$ | 3,355,164 | |
The
accompanying notes to the financial statements are an integral part of these statements.
MIRA
Pharmaceuticals, Inc.
STATEMENTS
OF OPERATIONS
YEAR
ENDED DECEMBER 31, 2022 AND DECEMBER 31, 2021
| | |
2022 | | |
2021 | |
| | |
Year ended December 31, | |
| | |
2022 | | |
2021 | |
| Revenues | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Operating costs: | |
| | | |
| | |
| General and administrative expenses | |
| 2,992,125 | | |
| 770,115 | |
| Related party travel costs | |
| 1,704,350 | | |
| 697,600 | |
| Research and development expenses | |
| 2,351,465 | | |
| 684,447 | |
| Total operating costs | |
| 7,047,940 | | |
| 2,152,162 | |
| | |
| | | |
| | |
| Interest expense | |
| (10,250 | ) | |
| (24,374 | ) |
| Net loss | |
$ | (7,058,190 | ) | |
$ | (2,176,536 | ) |
| Basic and diluted loss per share | |
| - | | |
| - | |
| Weighted average common stock shares outstanding | |
| - | | |
| - | |
The
accompanying notes to the financial statements are an integral part of these statements.
MIRA
Pharmaceuticals, Inc.
STATEMENTS
OF STOCKHOLDERS’ EQUITY (DEFICIT)
YEAR
ENDED DECEMBER 31, 2022 AND DECEMBER 31, 2021
| | |
Shares | | |
Amount | | |
Capital | | |
Receivable | | |
Deficit | | |
(Deficit) | |
| | |
Common Stock | | |
Additional Paid-In | | |
Stock Subscription | | |
Accumulated | | |
Total
Stockholders’ Equity | |
| | |
Shares | | |
Amount | | |
Capital | | |
Receivable | | |
Deficit | | |
(Deficit) | |
| Balances, January 1, 2021 | |
| 11,773,800 | | |
| 5,887 | | |
| - | | |
| (5,887 | ) | |
| (67,993 | ) | |
| (67,993 | ) |
| Sale of common stock | |
| 900,000 | | |
| 450 | | |
| 4,499,550 | | |
| - | | |
| - | | |
| 4,500,000 | |
| Collection of stock subscription receivable | |
| - | | |
| - | | |
| - | | |
| 5,887 | | |
| - | | |
| 5,887 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,176,536 | ) | |
| (2,176,536 | ) |
| Balances, December 31, 2021 | |
| 12,673,800 | | |
$ | 6,337 | | |
$ | 4,499,550 | | |
$ | - | | |
$ | (2,244,529 | ) | |
$ | 2,261,358 | |
| Balance | |
| 12,673,800 | | |
$ | 6,337 | | |
$ | 4,499,550 | | |
$ | - | | |
$ | (2,244,529 | ) | |
$ | 2,261,358 | |
| Sale of common stock, net | |
| 639,200 | | |
| 320 | | |
| 2,903,680 | | |
| - | | |
| - | | |
| 2,904,000 | |
| Stock-based compensation | |
| - | | |
| - | | |
| 1,296,600 | | |
| - | | |
| - | | |
| 1,296,600 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (7,058,190 | ) | |
| (7,058,190 | ) |
| Balances, December 31, 2022 | |
| 13,313,000 | | |
$ | 6,657 | | |
$ | 8,699,830 | | |
$ | - | | |
$ | (9,302,719 | ) | |
$ | (596,232 | ) |
| Balance | |
| 13,313,000 | | |
$ | 6,657 | | |
$ | 8,699,830 | | |
$ | - | | |
$ | (9,302,719 | ) | |
$ | (596,232 | ) |
The
accompanying notes to the financial statements are an integral part of these statements.
MIRA
Pharmaceuticals, Inc.
STATEMENTS
OF CASH FLOWS
YEAR
ENDED DECEMBER 31, 2022 AND DECEMBER 31, 2021
| | |
| | |
| |
| | |
Year Ended December 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Cash flows from Operating activities | |
| | | |
| | |
| Net loss | |
$ | (7,058,190 | ) | |
$ | (2,176,536 | ) |
| Adjustments to reconcile net loss to net cash from operations | |
| | | |
| | |
| Non-cash interest expense | |
| 10,250 | | |
| 24,374 | |
| (Interest Expense)/Income- Accrued, net | |
| - | | |
| - | |
| Amortization of debt issuance costs | |
| - | | |
| - | |
| Stock-based compensation expense | |
| 1,296,600 | | |
| - | |
| Change in operating assets and liabilities: | |
| | | |
| | |
| Right of use lease, net | |
| (5,500 | ) | |
| - | |
| Accounts payable and accrued expenses | |
| 152,081 | | |
| 776,006 | |
| Prepaid expenses | |
| - | | |
| - | |
| Net cash flows from operating activities | |
| (5,604,759 | ) | |
| (1,376,156 | ) |
| | |
| | | |
| | |
| Financing activities: | |
| | | |
| | |
| Advances to affiliates | |
| 445,612 | | |
| (426,732 | ) |
| Payment of deferred offering costs | |
| (43,427 | ) | |
| (100,000 | ) |
| Net (repayments) borrowings under related party line of credit | |
| (160,000 | ) | |
| 203,062 | |
| Collection of stock subscription receivable | |
| - | | |
| 5,887 | |
| Repayments under related party line of credit | |
| - | | |
| - | |
| Proceeds from sale of common stock, less offering costs | |
| 2,904,000 | | |
| 4,500,000 | |
| Issuance of Common Stock Conversion of Debt | |
| - | | |
| - | |
| Issuance of Common Stock in lieu of fees | |
| - | | |
| - | |
| Net cash flows from financing activities | |
| 3,146,185 | | |
| 4,182,217 | |
| | |
| | | |
| | |
| Net change in cash | |
| (2,458,574 | ) | |
| 2,806,061 | |
| Cash, beginning of year | |
| 2,809,552 | | |
| 3,491 | |
| Cash, end of year | |
$ | 350,978 | | |
$ | 2,809,552 | |
| Cash paid for interest | |
| - | | |
| - | |
Non-cash
Financing and Investing Activities:
The
Company recorded a right of use asset and a corresponding liability in the amount of $1.0 million in exchange for an operating lease
liability as a result of the adoption of Accounting Standards Codification, (“ASC”), Topic 842, Leases, on January 1, 2022.
The
accompanying notes to the financial statements are an integral part of these statements.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Note
1. Description of business and summary of significant accounting policies:
Overview
MIRA
Pharmaceuticals, Inc. (“MIRA” or the “Company” and formerly known as MIRA1a Therapeutics, Inc.) was formed in
September 2020 and is a Florida-based pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting
a broad range of neurologic and neuropsychiatric disorders. The Company’s novel oral pharmaceutical marijuana, MIRA1a, is currently
under investigation for treating adult patients suffering from anxiety and cognitive decline, often associated with early-stage dementia.
MIRA1a, if approved by the FDA, could mark a significant advancement in addressing various neuropsychiatric, inflammatory, and neurologic
diseases and disorders.
The
Company has an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to
potentially deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD)
and major depressive disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered
a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
Substantive
operations began in late 2020 and the Company’s Investigative New Drug application is anticipated to be filed with the U.S. Food
and Drug Administration (“FDA”) end of first quarter 2024. The Company owns U.S. Patent 10,787,675 B2, titled “Purified
Synthetic Marijuana and Methods of Treatment by Administering Same,” which covers the MIRA1a compound as a new molecular entity
as well as pharmaceutical formulations of the compound and methods of treating Alzheimer’s disease, anxiety, depression, and addictions.
Foreign patent applications covering MIRA1a, and its therapeutic uses are pending in Australia, Canada, China, Europe, Israel, Japan,
and South Korea.
The
accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America
(“GAAP”).
As
used herein, the Company’s Common Stock, par value $0.0001 per share, is referred to as the “Common Stock” and the
Company’s preferred stock, par value $0.0001 per share, is referred to as the “Preferred Stock”.
Pending
transactions
The
Company is in the process of preparing for an initial public offering and expects to be listed under the NASDAQ symbol “MIRA.”
The transaction is expected to be complete in second half of 2023. The Company incurred $0.04 million and $0.1 million of legal costs,
during the years ended December 31, 2022 and December 31, 2021, respectively, associated with the offering, which have been recorded
as deferred offering costs in the accompanying balance sheets. These deferred offering costs will be derecognized as a reduction in offering
proceeds when the offering closes. However, there can be no guarantees that the Company will be successful in completing the proposed
transaction and ultimately listing on the NASDAQ.
Income
taxes
The
Company is a C corporation. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences
between the financial statement carrying amount of existing assets and liabilities and their respective tax bases. Deferred tax assets
are recognized for temporary differences that will result in deductible amounts in future years and for loss carryovers. A valuation
allowance is recognized regarding deferred tax assets, if any, if it is more likely than not that some portion of the deferred tax asset
will not be realized.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Research
and development expenses
Research
and development costs are expensed in the period in which they are incurred and include the expenses paid to third parties, such as contract
research organizations and consultants, who conduct research and development activities on behalf of the Company.
Use
of estimates
The
preparation of financial statements in accordance with generally accepted accounting principles in the United States of America
requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and
liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported
amounts of expenses during the reporting period. Actual results may differ from such estimates and such differences could be
material.
Cash
The
Company maintains cash balances with financial institutions that management believes are of high credit quality. The Company’s
cash account at times may exceed federally insured limits. The Company has not experienced any losses in such accounts. The Company believes
it is not exposed to any significant credit risk from its cash account.
Stock-based
compensation
The
Company accounts for stock-based compensation under the provisions of FASB ASC 718, “Compensation - Stock Compensation”,
which requires the measurement and recognition of compensation expense for all stock-based awards made to employees, directors and consultants
based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using
the Black-Scholes model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the
requisite service periods using the straight-line method. The Company has elected to account for forfeiture of stock-based awards as
they occur.
Change
in Accounting Principle
In
February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes existing guidance for accounting for leases under Topic
840, Leases. The FASB also subsequently issued additional ASUs which amend and clarify Topic 842. The most significant change in the
new leasing guidance is the requirement to recognize right-to-use (ROU) assets and lease liabilities for operating leases on the balance
sheet.
The
Company adopted these ASUs effective January 1, 2022 using the modified retrospective approach. As a result of adopting these ASUs, the
Company recorded ROU assets and lease liabilities of approximately $1.0 million and $0.4 million, respectively. Adoption of the new standard
did not materially impact the Company’s net income and had no impact on cash flows.
Note
2. Liquidity and capital resources:
As
of December 31, 2022, the Company had cash of approximately $0.4 million. The Company used approximately $5.6 million of cash in operations
during the year ended December 31, 2022 and had stockholders’ (deficit) of approximately $0.6 million, versus stockholders’
equity of approximately $2.3 million at December 31, 2021. During the year ended December 31, 2022, the Company raised approximately
$3.2 million to finance its research and development and working capital needs, through a private placement of the Company’s common
stock and collections on amounts previously advanced to affiliates of the Company.
Historically,
the Company has been primarily engaged in developing MIRA1a. During these activities, the Company sustained substantial losses. The Company’s
ability to fund ongoing operations and future clinical trials required for FDA approval is dependent on the Company’s ability to
obtain significant additional external funding in the near term. Since inception, the Company financed its operations through the sale
of Common Stock and related party financings. See Note 4 for details of a related party line of credit established in 2021. Additional
sources of financing may be sought by the Company. However, there can be no assurance that any fundraising will be achieved on commercially
reasonable terms, if at all.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
The
Company expects to be able to fund operations through the anticipated initial public offering, or through the first quarter of 2024,
with available borrowings on the related party line of credit (Note 4). Should actual cash expenditures exceed management’s budget,
the Company may be forced to curtail operations along with implementing other cost-saving measures, such as a reduction in staff, reducing
the use of outside professional service providers, or significantly modifying or delaying the development of our product candidates.
Note
3. License agreement, related party:
On
April 28, 2022, and subsequently amended and restated on June 27, 2022 (the “Effective Date”), the Company and MyMD Pharmaceuticals,
Inc. (“MYMD”) entered into a non-exclusive, royalty-free license (the “Agreement”) to use MYMD’s Supera-CBD
as a synthetic intermediate in the manufacture of MIRA1a for research and development activities relating to our planned pre-clinical
and clinical studies.
This
Agreement was amended on April 17, 2023 to extend its original one-year term through December 31, 2024. The term of agreement may be
extended by mutual agreement of the parties for an additional period that is reasonably necessary to complete the manufacture of quantities
of MIRA1a needed for pre-clinical or clinical studies.
Either
party may terminate this Agreement without cause upon forty-five (45) calendar days prior written notice to the other Party.
The
Company and MYMD have similar members of the Board, as well as officers from the respective companies.
Note
4. Line of credit, related party:
In
May 2021, the Company entered into a revolving credit facility which allows for borrowings of up to $5,000,000 with a shareholder. The
facility has an initial term of 24 months (extended to 36 months in March 2023), with a new maturity date of May 10, 2024, at which time
all outstanding borrowings and accrued interest, if any, are due in full. Borrowings accrue interest at a rate of 5% per annum. The Company
anticipates repaying the line of credit through proceeds from the anticipated initial public offering.
Note
5. Related party transactions:
Advances
to affiliates – During the year ended December 31, 2022, and December 31, 2021, the Company made working capital advances to
companies under common control. These advances were due on demand and were non-interest bearing. As of December 31, 2022, such advances
were repaid in full.
Related
party accounts payable – Amounts due to related parties as of December 31, 2022 and December 31, 2021, are recorded as Accounts
payable related parties, in the accompanying balance sheets.
Travel
expenses – In April 2021, the Company entered into an airplane lease with an entity under common control that the Company incurs
approximately $0.05 million of lease charges per month. The lease is renewable, at the Company’s discretion, for an additional
one to three years, however, the Company intends to terminate the lease upon the date of its initial public offering, as allowed in the
lease agreement. During the year ended December 31, 2022 and 2021, the Company incurred $1.7 million and $0.7 million, respectively,
for travel-related expenses to the related party for monthly rental charges and airplane-related expenses.
License
agreement - See Note 3.
Line
of credit - See Note 4.
Lease
and lease reimbursements - See Note 6.
Consulting
and employment agreements – See Note 9.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Note
6. Lease:
Leases
The
Company leases certain office space and an airplane. The Company determines whether a contract contains a lease at inception by determining
if the contract conveys the right to control the use of identified property, plant or equipment for a period of time in exchange for
consideration. The Company has lease agreements with lease and non-lease components, which are generally accounted for separately with
amounts allocated to the lease and non-lease components based on relative stand-alone prices.
Right-of-use
(“ROU”) assets and lease liabilities are recognized at the commencement date based on the present value of the future minimum
lease payments over the lease term. Renewal and termination clauses that are factored into the determination of the lease term if it
is reasonably certain that these options would be exercised by the Company. Lease assets are amortized over the lease term unless there
is a transfer of title or purchase option reasonably certain of exercise, in which case the asset life is used. Certain of our lease
agreements include variable payments. Variable lease payments not dependent on an index or rate primarily consist of common area maintenance
charges and are not included in the calculation of the ROU asset and lease liability and are expensed as incurred. In order to determine
the present value of lease payments, the Company uses the implicit rate when it is readily determinable. As most of the Company’s
leases do not provide an implicit rate, management uses the Company’s incremental borrowing rate based on the information available
at lease commencement to determine the present value of lease payments.
Our
lease agreements do not contain any material residual value guarantees or material restrictive covenants. The Company does not have leases
where it is involved with the construction or design of an underlying asset. The Company has no material obligation for leases signed
but not yet commenced as of December 31, 2022. The Company does not have any material sublease activities.
Practical
Expedients Elected
| |
● |
The
Company elected the three transition practical expedients that permit an entity to (a) not reassess whether expired or existing contracts
contain leases, (b) not reassess lease classification for existing or expired leases, and (c) not consider whether previously capitalized
initial direct costs would be appropriate under the new standard. |
| |
● |
The
Company has elected to account for lease and non-lease components as a single component. |
Variable
lease costs
Variable
lease costs primarily include utilities, property taxes, and other operating costs that are passed on from the lessor. Variable lease
costs related to the aircraft include usage expenses, which includes pilot expenses, jet fuel and general flight expenses.
The
components of lease expense were as follows:
Schedule of Lease Expense
| |
2022 | | |
2021 | |
| | |
Year ended December 31, | |
| Lease Costs | |
2022 | | |
2021 | |
| Operating Lease Cost | |
| | | |
| | |
| Operating Lease | |
$ | 657,797 | | |
$ | - | |
| Variable Lease Costs | |
| 1,112,913 | | |
| - | |
| Total Lease Cost | |
$ | 1,770,710 | | |
$ | - | |
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Supplemental
cash flow information related to leases were as follows:
Schedule
of Cash Flow Information Related to Leases
| Other Lease Information | |
2022 | | |
2021 | |
| | |
Year ended December 31, | |
| Other Lease Information | |
2022 | | |
2021 | |
| Cash paid for amounts included in the measurement of lease liabilities | |
| | | |
| | |
| Operating cash flows from operating leases | |
$ | 626,304 | | |
$ | - | |
Schedule
of Remaining Weighted-average Lease Term and Weighted-average Discount Rate
| | |
Year
ended December 31, | |
| | |
2022 | | |
2021 | |
| Lease Term and Discount | |
| | | |
| | |
| Weighted Average remaining lease term | |
| 0.53 years | | |
| - | |
| Weighted Average discount rate | |
| 5.0 | % | |
| - | |
Maturity
of Lease Liabilities
Future
minimum lease payments under non-cancellable leases as of December 31, 2022 were as follows:
Schedule
of Maturity
of Lease Liabilities
| Maturity of Lease Liabilities | |
| |
| | |
December 31, 2022 | |
| 2023 | |
| 281,050 | |
| 2024 | |
| 69,309 | |
| 2025 | |
| 17,444 | |
| Total Lease payments | |
| 367,803 | |
| Less: Interest | |
| (9,634 | ) |
| Present Value of Lease Liabilities | |
| 358,169 | |
Note
7. Income taxes:
The
significant components of the Company’s net deferred tax assets are as follows as of December 31:
Schedule
of Components of Net Deferred Tax Assets
| | |
2022 | | |
2021 | |
| | |
December 31, | |
| | |
2022 | | |
2021 | |
| Deferred tax assets | |
| | | |
| | |
| Net operating loss carry-forward | |
$ | 1,061,300 | | |
$ | 572,355 | |
| Section 174 Qualified Research Expenditures | |
| 388,230 | | |
| - | |
| Stock compensation | |
| 330,633 | | |
| - | |
| ROU liability | |
| 91,333 | | |
| - | |
| Other | |
| 6,120 | | |
| - | |
| Deferred tax assets Gross | |
| 1,877,616 | | |
| 572,355 | |
| Less: valuation allowance | |
| (1,784,880 | ) | |
| (572,355 | ) |
| Total deferred tax asset | |
| 92,736 | | |
| - | |
| Deferred tax liabilities | |
| | | |
| | |
| ROU asset | |
| (92,736 | ) | |
| - | |
| Total net deferred tax asset | |
$ | - | | |
$ | - | |
Beginning
in 2022, in accordance with Internal Revenue Code Section 174, Qualified Research Expenditures are capitalized for tax purposes and amortized
over a period of five years. Accordingly, for income tax purposes, the Company has recorded a deferred tax asset totaling approximately
$0.4 million related to the timing difference between GAAP and Tax recognition of these expenditures.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
The
components of the provision for income taxes consist of the following:
Schedule
of Components of Provision For Income Taxes
| | |
2022 | | |
2021 | |
| Deferred tax: | |
| | | |
| | |
| Deferred | |
| (1,212,525 | ) | |
| (555,017 | ) |
| Change in valuation allowance | |
| 1,212,525 | | |
| 555,017 | |
| Total deferred | |
| - | | |
| - | |
| Total provision for income taxes | |
$ | - | | |
$ | - | |
ASC
Topic 740 requires that a deferred tax amount be reduced by a valuation allowance if, based on the weight of available evidence it is
more likely than not (a likelihood of more than 50%) that some portion or all of the deferred tax assets will not be realized. The valuation
allowance should be sufficient to reduce the deferred tax asset to the amount that is more likely than not to be realized. The Company
has recorded a full valuation allowance against its deferred tax assets generated by net operating loss carryforwards as it has determined
that such amounts may not be recognizable, given the historical losses of the Company to date. As of December 31, 2022, the Company has
a cumulative federal net operating loss carryforward of approximately $4.2 million. The net operating loss carryforwards have no expiry
date.
Note
8. Stockholders’ equity:
Capital
stock
The
Company has the authority to issue 110,000,000 shares of capital stock, consisting of 100,000,000 shares of Common Stock and 10,000,000
shares of undesignated preferred stock, whose rights and privileges will be defined by the Board of Directors when a series of preferred
stock is designated.
Private
placement
During
the year ended December 31, 2022, the Company sold 3.2 million shares of Common Stock at $1.00 per share, net of offering costs of $0.3
million, resulting in net proceeds of $2.9 million.
2022
Omnibus Incentive Plan
In
June 2022, the Company’s Board of Directors adopted, and its stockholders approved, the Company’s 2022 Omnibus Incentive
Plan, (“2022 Omnibus Plan”). The 2022 Omnibus Plan authorizes the grant of incentive stock options, within the meaning of
Section 422 of the Internal Revenue Code, to the Company’s employees and any of its parent and subsidiary corporations’ employees,
and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units
and performance shares to the Company’s employees, directors, and consultants and any of its future subsidiary corporations’
employees and consultants.
The
2022 Omnibus Plan provides that 10,000,000 shares of the Company’s Common Stock are reserved for issuance under the 2022 Omnibus
Plan, all of which may be issued pursuant to the exercise of incentive stock options.
Stock-based
compensation
During
the year ended December 31, 2022, a total of 750,000 options to purchase Common Stock, with an aggregate fair market value of approximately
$2.7 million were granted to the Company’s Board of Directors, executive officers and management, and a consultant of the Company.
Options have a term of 10 years from the grant date. The Company’s option vesting structure is the following: (i) Board of Director
options vest 100% on date of grant, (ii) executive officer options vest 25% on date of grant and the remaining vest ratably over a three-year
period, and (iii) management, employee and consultant options vest 33.3% on date of grant and the remaining vest ratably over a two-year
period.
The
fair value of each option award is estimated on the grant date using the Black-Scholes valuation model that uses assumptions for expected
volatility, expected dividends, expected term, and the risk-free interest rate.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Expected
price volatility is based on the historical volatilities of a peer group as the Company does not have a trading history for its shares.
Industry peers consist of several public companies in the biotech industry similar to the Company in size, stage of life cycle and product
indications. The Company intends to continue to consistently apply this process using the same or similar public companies until a sufficient
amount of historical information regarding the volatility of the Company’s own stock price becomes available, or unless circumstances
change such that the identified companies are no longer similar to the Company, in which case, more suitable companies whose share prices
are publicly available would be utilized in the calculation.
Expected
term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of
the vesting term plus contract term. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for
the period of the expected term.
The
key assumptions used in determining the fair value of options granted during the year ended December 31, 2022 follows:
Schedule of Key Assumptions Used to Value Warrants
| Expected price volatility | |
| 84.42 | % |
| Risk-free interest rate | |
| 3.38 | % |
| Fair value of common stock | |
$ | 1.00 | |
| Minimum and maximum average expected life in years | |
| 5-6.50 years | |
| Dividend yield | |
| - | |
Option
activity during the year ended December 31, 2022 was as follows:
Schedule of Stock
option Activity
| | |
Number of shares | | |
Weighted average exercise price per share | | |
Aggregate intrinsic value | |
| Outstanding as January 1, 2022 | |
| - | | |
| | | |
| | |
| Options granted | |
| 750,000 | | |
$ | 5.00 | | |
| | |
| Outstanding as December 31, 2022 | |
| 750,000 | | |
$ | 5.00 | | |
| - | |
As
of December 31, 2022, options exercisable totaled 280,000. There are approximately $1.4 million of unrecognized compensation costs related
to non-vested share-based compensation awards, which will be expensed through 2025.
Note
9 – Consulting and employment agreements:
Employment Agreements
On
April 1, 2022, the Company entered into a Consulting Agreement with Dr. Chapman pursuant to which he provided regulatory and drug development
consulting services to the Company on an as-requested basis. Pursuant to the Consulting Agreement, he was to be paid a one-time fee of
$0.1 million upon the completion of the anticipated offering (of which $0.05 million was prepaid in in the first quarter of 2022) plus
a monthly fee of $0.02 million thereafter. The monthly fee was to begin upon the completion of the offering. He was also reimbursed for
reasonable out-of-pocket expenses incurred in connection with his duties under the Consulting Agreement. The agreement had a term of
one year with an automatic one-year extension, provided that either party could terminate the agreement without cause upon 30-days prior
written notice.
In
his capacity as a consultant, Dr. Chapman was also granted on June 15, 2022, an option to purchase up to 200,000 shares of the Company’s
common stock at an exercise price of $5.00 per share. This option was granted under the Company’s 2022 Omnibus Plan and vested
as to 25% of the option shares on the date of grant, with the balance vesting in one-third increments on each of the three successive
anniversaries of the grant date. Any unvested portion of the option will vest in full upon a “change of control” of our company
within the meaning of the 2022 Omnibus Plan. The option has a term of 10-years, subject to earlier termination upon certain terminations
of Dr. Chapman’s position as a consultant to the Company. In his capacity as a Board Director, Dr. Chapman was also granted on
June 15, 2022, an option to purchase up to 20,000 shares of the Company’s common stock at an exercise price of $5.00 per share.
This option was granted under the Company’s 2022 Omnibus Plan and vested as to 100% of the option shares on the date of grant.
The option has a term of 10-years, subject to earlier termination upon certain terminations of Dr. Chapman’s position as a director
of the Company.
Note
10 – Subsequent events:
The
Company has evaluated subsequent events through April 4, 2023, in connection with the preparation of these financial statements, which
is the date the financial statements were available to be issued.
Reverse
Stock Split
Effective
June 28, 2023, the Company completed a reverse stock split of its outstanding common stock upon the filing of the Company’s Third
Amended and Restated Articles of Incorporation with the Florida Secretary of State. No fractional shares were or will be issued in connection
with the reverse stock split, and all such fractional shares resulting from the reverse stock split were and will be rounded up to the
nearest whole number. The shares issuable upon the exercise of our outstanding options and warrants, and the exercise prices of such
options and warrants, have been adjusted to reflect the reverse stock split. Unless otherwise noted, the share and per share information
in this prospectus reflects the reverse stock split.
MIRA
PHARMACEUTICALS, INC.
CONDENSED
BALANCE SHEETS
AS
OF SEPTEMBER 30, 2023 AND DECEMBER 31, 2022
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| | |
| (Unaudited) | | |
| | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 5,868,330 | | |
$ | 350,978 | |
| Deferred offering costs | |
| - | | |
| 143,427 | |
| Prepaid expenses | |
| 202,817 | | |
| - | |
| Total current assets | |
| 6,071,147 | | |
| 494,405 | |
| | |
| | | |
| | |
| Deferred financing costs, net | |
| 2,782,708 | | |
| - | |
| Operating lease, right of use assets | |
| 114,357 | | |
| 164,910 | |
| Related party operating lease, right of use assets | |
| - | | |
| 198,759 | |
| Operating lease, right of use assets | |
| - | | |
| 198,759 | |
| Due from related party | |
| 50,000 | | |
| - | |
| Total assets | |
$ | 9,018,212 | | |
$ | 858,074 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Trade accounts payable and accrued liabilities | |
$ | 779,573 | | |
$ | 811,738 | |
| Related party accounts payable | |
| - | | |
| 116,350 | |
| Related party line of credit | |
| - | | |
| 133,062 | |
| Related party accrued interest | |
| 14,472 | | |
| 34,987 | |
| Current portion of operating lease liabilities | |
| 74,328 | | |
| 75,143 | |
| Related party current portion of operating lease liabilities | |
| - | | |
| 198,759 | |
| Current
portion of operating lease liabilities | |
| - | | |
| 198,759 | |
| Total current liabilities | |
| 868,373 | | |
| 1,370,039 | |
| | |
| | | |
| | |
| Non-current operating lease liabilities | |
| 34,528 | | |
| 84,267 | |
| | |
| | | |
| | |
| Total liabilities | |
| 902,901 | | |
| 1,454,306 | |
| | |
| | | |
| | |
| Stockholders’ Equity (deficit) | |
| | | |
| | |
| Preferred Stock, $0.0001 par value, 10,000,000 shares authorized and none issued or outstanding at September 30, 2023 and 5,000,000 authorized and none issued or outstanding at December 31, 2022. | |
| - | | |
| - | |
| Common Stock, $0.0001
par value; 100,000,000 shares authorized,
at September 30, 2023 and 14,780,885 shares
issued and outstanding. 95,000,000 shares authorized at December 31, 2022 and 13,313,000 shares issued and outstanding. | |
| 1,478 | | |
| 6,657 | |
| Additional paid-in capital | |
| 23,611,517 | | |
| 8,699,830 | |
| Accumulated deficit | |
| (15,497,684 | ) | |
| (9,302,719 | ) |
| Total stockholders’ equity (deficit) | |
| 8,115,311 | | |
| (596,232 | ) |
| Total liabilities and stockholders’ equity (deficit) | |
$ | 9,018,212 | | |
$ | 858,074 | |
See
notes to condensed financial statements
MIRA
PHARMACEUTICALS, INC.
CONDENSED
STATEMENTS OF OPERATIONS
FOR
THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2023 AND 2022
(Unaudited)
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
Three months ended September 30, | | |
Nine months ended
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Revenues | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating costs: | |
| | | |
| | | |
| | | |
| | |
| General and administrative expenses | |
| 2,144,832 | | |
| 736,059 | | |
| 3,830,303 | | |
| 2,940,469 | |
| Related party travel costs | |
| - | | |
| 357,350 | | |
| 453,550 | | |
| 1,293,050 | |
| Research and development expenses | |
| 1,015,252 | | |
| 714,968 | | |
| 1,185,839 | | |
| 1,466,708 | |
| Total operating costs | |
| 3,160,084 | | |
| 1,808,377 | | |
| 5,469,692 | | |
| 5,700,227 | |
| | |
| | | |
| | | |
| | | |
| | |
| Interest expense, net | |
| (427,732 | ) | |
| (2,307 | ) | |
| (725,273 | ) | |
| (8,484 | ) |
| Net loss attributable to common stockholders | |
$ | (3,587,816 | ) | |
$ | (1,810,684 | ) | |
$ | (6,194,965 | ) | |
$ | (5,708,711 | ) |
| Basic and diluted loss per share | |
$ | (0.26 | ) | |
$ | (0.14 | ) | |
$ | (0.45 | ) | |
$ | (0.43 | ) |
| Weighted average common stock shares outstanding | |
| 13,639,197 | | |
| 13,168,556 | | |
| 13,639,197 | | |
| 13,166,200 | |
See
notes to condensed financial statements
MIRA
PHARMACEUTICALS, INC.
CONDENSED
STATEMENTS OF STOCKHOLDERS’ EQUITY
FOR
THE NINE MONTHS AND THREE MONTHS ENDED SEPTEMBER 30, 2023 AND 2022
(Unaudited)
| | |
Shares | | |
Amount | | |
Capital | | |
Receivable | | |
Deficit | | |
(Deficit) | |
| | |
Common
Stock | | |
Additional
Paid-In | | |
Stock
Subscription | | |
Accumulated | | |
Total
Stockholders’ Equity | |
| | |
Shares | | |
Amount | | |
Capital | | |
Receivable | | |
Deficit | | |
(Deficit) | |
| Balances,
January 1, 2022 | |
| 12,673,800 | | |
| 6,337 | | |
| 4,499,550 | | |
| - | | |
| (2,244,529 | ) | |
| 2,261,358 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Sale
of common stock, net | |
| 402,200 | | |
| 201 | | |
| 1,718,799 | | |
| (135,000 | ) | |
| - | | |
| 1,584,000 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,475,046 | ) | |
| (1,475,046 | ) |
| Balances,
March 31, 2022 | |
| 13,076,000 | | |
$ | 6,538 | | |
$ | 6,218,349 | | |
$ | (135,000 | ) | |
$ | (3,719,575 | ) | |
$ | 2,370,312 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based
compensation | |
| - | | |
| - | | |
| 1,001,000 | | |
| - | | |
| - | | |
| 1,001,000 | |
| Collection
of stock subscription receivable | |
| - | | |
| - | | |
| - | | |
| 135,000 | | |
| - | | |
| 135,000 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,422,979 | ) | |
| (2,422,979 | ) |
| Balances,
June 30, 2022 | |
| 13,076,000 | | |
$ | 6,538 | | |
$ | 7,219,349 | | |
$ | - | | |
$ | (6,142,554 | ) | |
$ | 1,083,333 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Sale
of Common Stock | |
| 180,000 | | |
| 90 | | |
| 899,910 | | |
| - | | |
| - | | |
| 900,000 | |
| Stock-based
compensation | |
| - | | |
| - | | |
| 147,800 | | |
| - | | |
| - | | |
| 147,800 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,810,684 | ) | |
| (1,810,684 | ) |
| Balances,
September 30, 2022 | |
| 13,256,000 | | |
$ | 6,628 | | |
$ | 8,267,059 | | |
$ | - | | |
$ | (7,953,238 | ) | |
$ | 320,449 | |
| | |
Common
Stock | | |
Additional
Paid-In | | |
Stock
Subscription | | |
Accumulated | | |
Total
Stockholders’ Equity | |
| | |
Shares | | |
Amount | | |
Capital | | |
Receivable | | |
Deficit | | |
(Deficit) | |
| Balances,
January 1, 2023 | |
| 13,313,000 | | |
| 6,657 | | |
| 8,699,830 | | |
| - | | |
$ | (9,302,719 | ) | |
$ | (596,232 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Sale
of common stock, net | |
| - | | |
| - | | |
| 147,800 | | |
| - | | |
| - | | |
| 147,800 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,341,044 | ) | |
| (1,341,044 | ) |
| Balances,
March 31, 2023 | |
| 13,313,000 | | |
$ | 6,657 | | |
$ | 8,847,630 | | |
$ | | | |
$ | (10,643,763 | ) | |
$ | (1,789,476 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based
compensation | |
| - | | |
| - | | |
| 737,200 | | |
| - | | |
| - | | |
| 737,200 | |
| Issuance
of Warrants | |
| - | | |
| - | | |
| 3,515,000 | | |
| - | | |
| - | | |
| 3,515,000 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,266,107 | ) | |
| (1,266,107 | ) |
| Balances,
June 30, 2023 | |
| 13,313,000 | | |
$ | 6,657 | | |
$ | 13,099,830 | | |
$ | - | | |
$ | (11,909,868 | ) | |
$ | 1,196,619 | |
| Balance | |
| 13,313,000 | | |
$ | 6,657 | | |
$ | 13,099,830 | | |
$ | - | | |
$ | (11,909,868 | ) | |
$ | 1,196,619 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based
compensation | |
| - | | |
$ | (5,326 | ) | |
| 1,457,459 | | |
| - | | |
| - | | |
| 1,452,133 | |
| Issuance
of common stock at IPO, net | |
| 1,275,000 | | |
| 128 | | |
| 7,704,152 | | |
| - | | |
| - | | |
| 7,704,279 | |
| Issuance
of common stock Related to conversion of debt | |
| 157,170 | | |
| 16 | | |
| 1,100,080 | | |
| - | | |
| - | | |
| 1,100,096 | |
| Issuance
of common stock | |
| 35,715 | | |
| 4 | | |
| 249,996 | | |
| - | | |
| - | | |
| 250,000 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,587,816 | ) | |
| (3,587,816 | ) |
Balances,
September 30, 2023 | |
| 14,780,885 | | |
$ | 1,478 | | |
$ | 23,611,517 | | |
$ | - | | |
$ | (15,497,684 | ) | |
$ | 8,115,311 | |
Balance | |
| 14,780,885 | | |
$ | 1,478 | | |
$ | 23,611,517 | | |
$ | - | | |
$ | (15,497,684 | ) | |
$ | 8,115,311 | |
See
notes to condensed financial statements
MIRA
PHARMACEUTICALS, INC.
CONDENSED
STATEMENTS OF CASH FLOWS
FOR
THE NINE MONTHS ENDED SEPTEMBER 30, 2023 AND 2022
(Unaudited)
| | |
2023 | | |
2022 | |
| | |
Nine Months Ended September 30, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Cash flows from Operating activities | |
| | | |
| | |
| Net loss | |
$ | (6,194,965 | ) | |
$ | (5,708,711 | ) |
| Adjustments to reconcile net loss to net cash from operations | |
| | | |
| | |
| (Interest Expense)/Income- Accrued, net | |
| (20,515 | ) | |
| 8,484 | |
| Amortization of debt issuance costs | |
| 732,292 | | |
| - | |
| Stock-based compensation expense | |
| 2,337,133 | | |
| 1,148,800 | |
| Change in operating assets and liabilities: | |
| | | |
| | |
| Right of use lease, net | |
| - | | |
| (5,500 | ) |
| Accounts payable and accrued expenses | |
| (148,516 | ) | |
| (20,300 | ) |
| Prepaid expenses | |
| (202,817 | ) | |
| (52,096 | ) |
| Net cash flows used in operating activities | |
| (3,497,388 | ) | |
| (4,629,323 | ) |
| | |
| | | |
| | |
| Financing activities: | |
| | | |
| | |
| Advances to affiliates | |
| (50,000 | ) | |
| (463,236 | ) |
| Payment of deferred offering costs | |
| 143,427 | | |
| (38,578 | ) |
| Repayments under related party line of credit | |
| (133,062 | ) | |
| (110,000 | ) |
| Proceeds from sale of common stock, less offering costs | |
| 7,704,279 | | |
| 2,619,000 | |
| Issuance of Common Stock Conversion of Debt | |
| 1,100,096 | | |
| - | |
| Issuance of Common Stock in lieu of fees | |
| 250,000 | | |
| - | |
| Net cash flows provided by financing activities | |
| 9,014,740 | | |
| 2,007,186 | |
| | |
| | | |
| | |
| Net change in cash | |
| 5,517,352 | | |
| (2,622,137 | ) |
| Cash, beginning of year | |
| 350,978 | | |
| 2,809,552 | |
| Cash, end of period | |
$ | 5,868,330 | | |
$ | 187,415 | |
| Cash paid for interest | |
| - | | |
| - | |
See
notes to condensed financial statements
SUPPLEMENTAL
CASH FLOW INFORMATION
Non-cash
Operating, Financing and Investing Activities:
The
Company recorded the fair value of a total of 1,000,000 shares of common stock issued to Bay Shore Trust during the nine months ended
September 30, 2023 totaling approximately $3.5 million to deferred finance costs. The Company has amortized approximately $0.7 million
of deferred offering costs as non-cash amortization of debt issuances costs in accordance with Generally Accepted Accounting Principles.
The Company recorded the
fair value of a total of 157,170 shares of common stock issued to Bay Shore Trust during the nine months ended September 30, 2023 totaling
approximately $1.1 million to record Bay Shore Trust conversions of a line of credit and interest to shares of common stock.
The Company recorded the fair value of a total of 35,715 shares of common stock issued to the MZ Group during the
nine months ended September 30, 2023 totaling $0.25 million in lieu of fees for investor relation services.
Note
1. Description of business and summary of significant accounting policies:
Overview
MIRA
Pharmaceuticals, Inc. (“MIRA” or the “Company” and formerly known as MIRA1a Therapeutics, Inc.) was formed in
September 2020 and is a Florida-based pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting a broad range of neurologic and neuropsychiatric disorders. The Company’s
novel oral pharmaceutical marijuana, MIRA1a, is currently under investigation for treating adult patients suffering from anxiety and cognitive decline,
often associated with early-stage dementia. MIRA1a, if approved by the FDA, could mark a significant advancement in addressing various
neuropsychiatric, inflammatory, and neurologic diseases and disorders.
The
Company has an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to
potentially deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD)
and major depressive disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered a controlled
substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
Substantive
operations began in late 2020 and the Company’s Investigative New Drug application is anticipated to be filed with the U.S. Food
and Drug Administration (“FDA”) end of third quarter 2024. The Company owns U.S. Patent 10,787,675 B2, titled “Purified
Synthetic Marijuana and Methods of Treatment by Administering Same,” which covers the MIRA1a compound as a new molecular entity
as well as pharmaceutical formulations of the compound and methods of treating Alzheimer’s disease, anxiety, depression, and addictions.
The
accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America
(“GAAP”).
As
used herein, the Company’s Common Stock, par value $0.0001 per share, is referred to as the “Common Stock” and the
Company’s preferred stock, par value $0.0001 per share, is referred to as the “Preferred Stock”.
Initial
Public Offering
On
August 7, 2023, the Company closed its initial public offering consisting of 1,275,000 shares at a price of $7.00 per share for approximately
$8.9 million in gross proceeds. After deducting the underwriting commission and other deferred offering expenses totaling $1.2 million,
the net proceeds to the Company were $7.7 million (the “IPO”).
The
shares were offered and sold pursuant to the Company’s Registration Statement on Form S-1, as amended (File No. 333-273024), originally
filed with the Securities and Exchange Commission (the “SEC”) on June 29, 2023 (the “Registration Statement”)
and the final quarterly report filed with the Commission pursuant to Rule 424(b)(4) of the Securities Act of 1933, as amended. The Registration
Statement was declared effective by the Commission on August 2, 2023. The common stock began trading on The Nasdaq Capital Market on
August 3, 2023 under the symbol “MIRA”. The closing of the IPO occurred on August 7, 2023.
As
of the completion of the IPO, among other things, certain of the Company’s then-outstanding convertible debt was converted into
shares of common stock. See Note 5 for more information.
Income
taxes
The
Company is taxed as a C corporation. Deferred tax assets and liabilities are recognized for the future tax consequences attributable
to differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases. Deferred
tax assets are recognized for temporary differences that will result in deductible amounts in future years and for loss carryovers. A
valuation allowance is recognized regarding deferred tax assets, if any, if it is more likely than not that some portion of the deferred
tax asset will not be realized.
Research
and development expenses
Research
and development costs are expensed in the period in which they are incurred and include the expenses paid to third parties, such as contract
research organizations and consultants, who conduct research and development activities on behalf of the Company. Patent-related costs,
including registration costs, documentation costs and other legal fees associated with the application, are expensed in the period in
which they are incurred.
Leases
The
Company accounts for leases under the provisions of FASB ASC Topic 842, “Leases”, which requires the Company to recognize
right-to-use (ROU) assets and lease liabilities for operating leases on the balance sheet.
Use
of estimates
The
preparation of financial statements in accordance with generally accepted accounting principles in the United States of America requires
the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the
disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the
reporting period. Actual results may differ from such estimates and such differences could be material.
Cash
The
Company maintains cash balances with financial institutions that management believes are of high credit quality. The Company’s
cash account at times may exceed federally insured limits. The Company has not experienced any losses in such accounts. The Company believes
it is not exposed to any significant credit risk from its cash account.
Stock-based
compensation
The
Company accounts for stock-based compensation under the provisions of FASB ASC 718, “Compensation - Stock Compensation”,
which requires the measurement and recognition of compensation expense for all stock-based awards made to employees, directors and consultants
based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using
the Black-Scholes model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the
requisite service periods using the straight-line method. The Company has elected to account for forfeiture of stock-based awards as
they occur.
Fair
Value of Financial Instruments
The
Company measures the fair value of financial instruments in accordance with GAAP which defines fair value, establishes a framework for
measuring fair value, and expands disclosures about fair value measurements.
GAAP
defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
GAAP also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use
of unobservable inputs when measuring fair value. The Company considers the carrying amount of deferred offering costs to approximate
fair value due to short-term nature of this instrument. GAAP describes three levels of inputs that may be used to measure fair value:
Level
1 – quoted prices in active markets for identical assets or liabilities.
Level
2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable.
Level
3 – inputs that are unobservable (for example cash flow modeling inputs based on assumptions).
Note
2. Liquidity and capital resources:
As
of September 30, 2023, the Company had cash of approximately $5.9 million. The Company used approximately $3.5 million of cash in operations
during the nine months ended September 30, 2023 and had stockholders’ equity of approximately $8.1 million, versus stockholders’
deficit of approximately $0.6 million at December 31, 2022
Historically,
the Company has been primarily engaged in developing MIRA1a. During these activities, the Company sustained substantial losses. The Company’s
ability to fund ongoing operations and future clinical trials required for FDA approval is dependent on the Company’s ability to
obtain significant additional external funding in the near term. Since inception, the Company financed its operations through the sale
of Common Stock, the IPO and related party financings. Additional sources of financing may be sought by the Company. The Company believes
that current cash and the proceeds of the August 2023 IPO are sufficient to fund operations until approximately Q4 2024. Additional financing
will be needed by the Company to fund its operations after such date to complete clinical developments and to commercially develop its
product candidate. However, there can be no assurance that any fundraising will be achieved on commercially reasonable terms, if at all.
Note
3 Accounts payable and accrued liabilities:
The
following table represents the components of accounts payable and accrued liabilities as of:
Schedule
of Accounts Payable And Accrued Liabilities
| | |
September 30, 2023 | | |
December 31, 2022 | |
| Trade accounts payable | |
$ | 510,688 | | |
$ | 789,204 | |
| Pre-clinical research and toxicology studies | |
| 254,293 | | |
| - | |
| Accrued other | |
| 14,592 | | |
| 22,534 | |
| Accounts
payable and accrued liabilities | |
$ | 779,573 | | |
$ | 811,738 | |
Note
4. License agreement, related party:
Effective
April 26, 2023 (the “Effective Date”), the Company and MyMD Pharmaceuticals, Inc. (“MYMD”) entered into an Amended
and Restated Limited License Agreement with MyMD. The license grants our company a perpetual, worldwide, royalty-free non-exclusive right
to use MyMD’s Supera-CBD compound, a different compound than MIRA1a, as a synthetic intermediate in the manufacture of MIRA1a for
all purposes (including clinical development and commercial production). This license is perpetual, and MyMD does not have the right to
terminate it. In consideration of this license, we agreed to share with MyMD technical information and know-how that pertains to the
synthetic manufacture and/or formulation of our MIRA1a product candidate and granted a license to MyMD to use improvements to MIRA1a
made under the agreement, agreement, and the agreement does not involve any prior or future cash payments by us.
The
Company and MYMD have similar members of the Board, as well as officers from the respective companies.
Note
5. Line of credit, related party:
In
May 2021, the Company entered into a revolving credit facility which allowed for borrowings of up to $5 million from Starwood Trust,
a shareholder of the Company. The facility had an initial term of 24 months (extended to 36 months in March 2023), with a new maturity
date of May 10, 2024, at which time all outstanding borrowings and accrued interest, if any, were due in full. Borrowings accrued interest
at a rate of 5% per annum.
In
April 2023, the Company entered into a Promissory Note and Loan Agreement with the Bay Shore Trust, a trust established by a shareholder
of the Company. Under this Promissory Note and Loan Agreement (the “Bay Shore Note”), the Company has the right to borrow
up to an aggregate of $5 million from the Bay Shore Trust at any time up to the second anniversary of the issuance of the Bay Shore Note
or, if earlier, upon the completion of the Company’s IPO. The Company’s right to borrow funds under the Bay Shore Note is
subject to the absence of a material adverse change in the Company’s assets, operations, or prospects. The Bay Share Note, together
with accrued interest, will become due and payable on the second anniversary of the issuance of the note, provided that it may be prepaid
at any time without penalty. The Bay Shore Note will accrue interest at a rate equal 7% per annum, simple interest, during the first
year that the note is outstanding and 10% per annum, simple interest, thereafter. The Bay Shore Note is unsecured.
The
Bay Shore Note replaced the revolving credit facility that the Company entered into with Starwood Trust, a separate trust established
by a shareholder of the Company, in May 2021 and pursuant to which the Company had an outstanding principal balance of $0.2 million as
of the date of the Bay Shore Note (which outstanding balance was retired with an advance under the Bay Shore Note).
In
consideration of the loan facility provided by the Bay Shore Trust, in April 2023, the Company issued to the Bay Shore Trust a common
stock purchase warrant giving the Bay Shore Trust the right to purchase up to 1,000,000 shares of common stock at an exercise price of
$5.00 per share, which warrant will expire five years after the date of grant. Pursuant to a registration rights agreement, the Company
has granted to Bay Shore Trust the right to require the Company, at any time after one year following the Company’s IPO, to register
for resale the shares issuable upon the exercise of the warrant, with such registration rights being in the form of demand and “piggyback”
registration rights that are subject to customary limitations and restrictions. See Note 8 for additional details related to these warrants.
On
July 20, 2023, the Company entered into a conversion agreement with the Bay Shore Trust under which the Bay Shore Trust had agreed to
convert, upon the completion of the IPO, $1.1 million of the outstanding principal balance of the Bay Shore Note into shares of the Company’s
common stock at a conversion price equal to the Company’s IPO price, which resulted in the issuance of 157,170 shares to the Bay
Shore Trust. On August 14, 2023, the Company paid $1.0 million in full to Bay Shore Trust, which was the amount due. The company also
paid accrued interest of $0.03 million. There is a remaining amount of $0.01 in accrued interest due to Bay Shore Trust as of September
30, 2023.
Note
6. Related party transactions:
Due
from related parties – As of the nine months ended September 30, 2023, the Company paid $0.05 million in accounts payable on
behalf of a related party.
Advances
from affiliates – During the nine months ended September 30, 2023, the Company received working capital advances in the amount
of $1.06 million from the Bay Shore Trust LOC, which was used to pay off advances from affiliates. As of September 30, 2023, all advances
have been repaid in full.
Related
party accounts payable – Amounts due to related parties as of September 30, 2023 and December 31, 2022, are recorded as Accounts
payable related parties, in the accompanying balance sheets.
Travel
expenses – In April 2021, the Company entered into an airplane lease with an entity under common control that the Company incurs
approximately $0.05 million of lease charges per month. The lease was renewable, at the Company’s discretion, for an additional
one to three years, however, the Company terminated the lease at March 31, 2023, without any penalties. The Company may continue to incur
related party travel-related expenses as they occur, which will be recorded in Related Party Travel Costs, in the condensed statement
of operations. During the nine months ended September 30, 2023, the Company incurred $0.5 million, for travel-related expenses to the
related party for monthly rental charges and airplane-related expenses.
License
agreement - See Note 4.
Line
of credit - See Note 5.
Note
7. Leases:
The
Company’s corporate headquarters is in Baltimore, Maryland, which includes a lease for office space. This lease began in November
2021 and was amended in April 2023. This space is approximately 550 square feet and has a remaining base rent of $0.01 million payable
through April 2024. Rent is payable in monthly installments and is subject to yearly price increases.
The
Company had leased an office in Tampa, Florida, for its finance and general operations, which began in March 2022 for 37 months. This
space is approximately 2,300 square feet and has a remaining base rent of $0.1 million payable through March 2025. Rent is payable in
monthly installments and is subject to yearly price increases. As of August 1, 2023, a related party to the Company began paying the
monthly lease expenses on behalf of the Company, directly to the landlord. This is conjunction with the Company’s IPO and the resignation
of its former Tampa employees. As such, the Tampa, Florida location is no longer needed. The related party who has been paying for the
lease since August 1, 2023 is in the process of amending the lease to remove the Company from further obligations. Until such time, the
Company will continue to reflect the right of use assets and liabilities on the condensed balance sheets.
The
Company also leased a jet (Note 5) from a related party, which lease the Company terminated on March 31 2023.
Variable
lease costs
Variable
lease costs primarily include utilities, property taxes, and other operating costs that are passed on from the lessor. Variable lease
costs related to the aircraft include usage expenses, which includes pilot expenses, jet fuel and general flight expenses.
The
components of lease expense were as follows:
Schedule of Lease Expense
| | |
| | |
| |
| | |
Nine months ended September 30, | |
| Lease Costs | |
2023 | | |
2022 | |
| Operating Lease Cost | |
| | | |
| | |
| Operating Lease | |
$ | 200,283 | | |
$ | 333,046 | |
| Variable Lease Costs | |
| 311,126 | | |
| 637,420 | |
| Total Lease Cost | |
$ | 511,409 | | |
$ | 970,466 | |
Supplemental
cash flow information related to leases were as follows:
Schedule
of Cash Flow Information Related to Leases
| | |
| | |
| |
| | |
Nine months ended September 30, | |
| Other Lease Information | |
2023 | | |
2022 | |
| Cash paid for amounts included in the measurement of lease liabilities | |
| | | |
| | |
| Operating cash flows from operating leases | |
$ | 511,409 | | |
$ | 970,466 | |
Schedule
of Remaining Weighted-average Lease Term and Weighted-average Discount Rate
| | |
Nine months ended September 30, | |
| | |
2023 | | |
2022 | |
| Lease Term and Discount | |
| | | |
| | |
| Weighted Average remaining lease term | |
| 1.79 years | | |
| 3 years | |
| Weighted Average discount rate | |
| 5.0 | % | |
| 5.0 | % |
Maturity
of Lease Liabilities
Future
minimum lease payments under non-cancellable leases as of September 30, 2023 were as follows:
Schedule
of Maturity
of Lease Liabilities
Maturity
of Lease Liabilities
| | |
September 30, 2023 | |
| Remainder of 2023 | |
$ | 20,722 | |
| 2024 | |
| 74,402 | |
| 2025 | |
| 17,444 | |
| Total Lease payments | |
| 112,568 | |
| Less: Interest | |
| (3,711 | ) |
| Present Value of Lease Liabilities | |
$ | 108,857 | |
On
April 1, 2023 the Company entered into an Agreement For Shared Lease Costs with MIRALOGX, LLC, (the “Shared Agreement”) who
is a related party for the jet usage. Under the Shared Agreement, the Company agrees to make monthly contributions or payments in accordance
with its monthly use of shared aircraft toward rent payments. However, the Company has not used the aircraft after the termination of
the lease and there are no minimum payments due without usage.
Note
8. Stockholders’ equity:
Capital
stock
The
Company has the authority to issue 110,000,000 shares of capital stock, consisting of 100,000,000 shares of Common Stock and 10,000,000
shares of undesignated preferred stock (as amended and restated on June 28, 2023), whose rights and privileges will be defined by the
Board of Directors when a series of preferred stock is designated.
Reverse
stock-split
Effective
June 28, 2023, the Company completed a 1-for-5 reverse stock split of its outstanding common stock upon the filing of the Company’s
Third Amended and Restated Articles of Incorporation with the Florida Secretary of State. No fractional shares were issued in connection
with the reverse stock split, and all such fractional shares resulting from the reverse stock split were rounded up to the nearest whole
number. The shares issuable upon the exercise of our outstanding options and warrants, and the exercise prices of such options and warrants,
have been adjusted to reflect the reverse stock split.
IPO
stock issuances
At
IPO, 1,275,000 shares of the Company’s common stock were issued at a price of $7.00 per share which resulted in gross proceeds
of $8.9 million and net proceeds of $7.7 million to the Company after the underwriter discount but before other IPO related expenses.
Additionally,
the Company issued its investor relations firm $0.25 million worth of restricted common stock upon closing of the IPO, which resulted
in issuance of 35,715 shares of stock.
Stock-based
compensation
The
Company may grant options under its 2022 Omnibus Incentive Plan, as amended and restated (the “2022 Omnibus Plan”). The 2022
Omnibus Plan authorizes the grant of “incentive stock options” within the meaning of Section 422 of the Internal Revenue
Code, to the Company’s employees and any of its parent and subsidiary corporations’ employees, and for the grant of nonstatutory
stock options, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to the Company’s
employees, directors, and consultants and any of its future subsidiary corporations’ employees and consultants.
The
fair value of each option award is estimated on the grant date using the Black-Scholes valuation model that uses assumptions for expected
volatility, expected dividends, expected term, and the risk-free interest rate. Expected price volatility is based on the historical
volatilities of a peer group as the Company does not have a trading history for its shares prior to its IPO. Industry peers consist of
several public companies in the biotech industry similar to the Company in size, stage of life cycle and product indications. The Company
intends to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical
information regarding the volatility of the Company’s own stock price becomes available, or unless circumstances change such that
the identified companies are no longer similar to the Company, in which case, more suitable companies whose share prices are publicly
available would be utilized in the calculation.
Expected
term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of
the vesting term plus contract term. The risk-free rate is based on the 5-year U.S. Treasury yield curve in effect at the time of grant.
During
the nine months ended September 30, 2023, a total of 635,001 options to purchase Common Stock, with an aggregate fair market value of
approximately $2.75 million were granted to the Company’s executive officers and management, and consultants of the Company. Options
have a term of 10 years from the grant date. These option vest as follows: (i) executive officer options vested 100% on date of grant
and (ii) employee and consultant options vest 33.33% at 6 month anniversary of date of grant, 33.33% at 1 year anniversary at date of
grant and the remaining vest at two-year anniversary of date of grant.
The
following is option activity during the nine months ended September 30, 2023.
Schedule of Stock
option Activity
| | |
Number of shares | | |
Weighted average exercise price per share | | |
Aggregate intrinsic value | |
| Outstanding as January 1, 2023 | |
| 750,000 | | |
$ | 5.00 | | |
| | |
| Options granted | |
| 635,001 | | |
$ | 5.00 | | |
| | |
| Forfeitures | |
| (170,000 | ) | |
$ | 5.00 | | |
| | |
| Outstanding as September 30, 2023 | |
| 1,215,001 | | |
$ | 5.00 | | |
$ | - | |
The
estimated fair value of stock options on date of grant was $1.3 million. As of September 30, 2023, options exercisable totaled 760,004.
There are approximately $1.5 million of unrecognized compensation costs related to non-vested share-based compensation awards, which
will be expensed through 2025.
Key
assumptions used to value stock options during the nine months ended September 30, 2023 are as follows:
Schedule of Key Assumptions Used to Value Stock Options
| Expected price volatility | |
| 116.64 | % |
| Risk-free interest rate | |
| 4.42 | % |
| Weighted average fair values | |
$ | 5.384
- $ 5.631 | |
| Weighted average expected life in years | |
| 5-6 years | |
| Dividend yield | |
| - | |
Warrants
Bay
Shore Trust warrants
In
consideration of the line of credit provided by the Bay Shore Trust, the Company issued to the Bay Shore Trust a common stock purchase
warrant on April 28, 2023 giving the Bay Shore Trust the right to purchase up to 1,000,000 shares of common stock at an exercise price
of $5.00 per share. This warrant will expire five years after the date of grant.
The
fair value of the warrants were estimated on the grant date using the Black-Scholes valuation model and level 3 inputs based on assumptions
for expected volatility, expected dividends, expected term, and the risk-free interest rate, which resulted in $3.5 million of deferred
financing costs. This cost was recorded as deferred financing costs and additional paid in capital on the accompanying condensed balance
sheet and is amortized straight-line over the term of the line of credit (which is 24 months). Associated amortization of deferred finance
costs is recorded to interest expense on the condensed income statement of operations.
Key
assumptions used to value warrants during the nine months ended September 30, 2023 are as follows:
Schedule of Key Assumptions Used to Value Warrants
| Expected price volatility | |
| 88.01 | % |
| Risk-free interest rate | |
| 3.51 | % |
| Weighted average fair values | |
$ | 0.703 | |
| Weighted average expected life in years | |
| 5 years | |
| Dividend yield | |
| - | |
Underwriter
warrants
In
connection with the IPO, the Company issued 63,750 warrants to purchase common stock to the IPO underwriter (or its designees) at an
exercise price of $7.00 which will expire in the four-and-a-half-year period commencing six months after the commencement of sales in
the IPO. The warrants will be exercisable at any time and from time to time, in whole or in part, during the four-and-a-half-year period
commencing six months after the commencement of sales in the IPO. The warrants provide for registration rights (including a one-time
demand registration right and piggyback registration rights that expire 5 years from the commencement of sales of the offering) and customary
anti-dilution provisions as permitted under FINRA Rule 5110(g)(8).
Earnings
Per Share
During
the nine months ended September 30, 2023 and 2022, outstanding stock options and warrants of 2,215,001 and 750,000, respectively, were
not included in the computation of diluted earnings per share, because to do so would have had an antidilutive effect.
During
the three months ended September 30, 2023 and 2022, outstanding stock options, and warrants of 1,235,001 and 750,000, respectively, were
not included in the computation of diluted earnings per share, because to do so would have had an antidilutive effect.
Note
9. Employment Agreements:
Erez
Aminov
On
April 28, 2023, the Company entered into an employment agreement with Mr. Erez Aminov pursuant to which Mr. Aminov serves as the Company’s
Chief Executive Officer on a full-time basis. Mr. Aminov’s employment agreement provides that his employment will be on an at-will
basis and can be terminated by either Mr. Aminov or the Company at any time and for any reason. Under the agreement, Mr. Aminov will
receive an initial base salary of $0.11 million per year. In the event that Mr. Aminov’s employment is terminated by the company
without “Cause” or is terminated by Mr. Aminov for “Good Reason”, Mr. Aminov will be entitled to severance compensation
in the form of salary continuation for a period of three months (subject to Mr. Aminov executing and delivering a customary general release
in favor of the company).
On
August 17, 2023, Mr. Aminov received a $0.12
million cash bonus net of federal, state, local
and income taxes related to the successful completion of the IPO.
On
August 28, 2023, the Company amended Mr. Aminov’s employment agreement to increase his yearly compensation from its current amount
of $0.11 million to $0.2 million per year, effective August 1, 2023.
Michelle
Yanez
On
April 28, 2023, the Company entered into an employment agreement with Ms. Michelle Yanez pursuant to which Ms. Yanez serves as the Company’s
Chief Financial Officer on a full-time basis. Ms. Yanez’s employment agreement provides that her employment will be on an at-will
basis and can be terminated by either Ms. Yanez or the company at any time and for any reason. Under the agreement, Ms. Yanez will receive
an initial base salary of $0.17 million per year. In the event that her employment is terminated by the company without “Cause”
or is terminated by Ms. Yanez for “Good Reason”, Ms. Yanez will be entitled to severance compensation in the form of salary
continuation for a period of three months (subject to Ms. Yanez executing and delivering a customary general release in favor of the
company).
On
August 17, 2023, Ms. Yanez received a $0.05 million cash bonus net of federal, state, local and income taxes related to the
successful completion of the IPO.
Chris
Chapman
On
April 28, 2023, the Company entered into an employment agreement with Dr. Chris Chapman pursuant to which Dr. Chapman serves as the Company’s
Executive Chairman. Dr. Chapman’s employment agreement provides that his employment will be on a part-time basis whereby Dr. Chapman
will devote 50% of his full business time and effort to the business and affairs of the company, and it further provides that such employment
will be on an at-will basis and can be terminated by either Dr. Chapman or the company at any time and for any reason. Under the agreement,
Dr. Chapman will receive an initial base salary of $0.15 million per year. In the event that Dr. Chapman’s employment is terminated
by the company without “Cause” or is terminated by Dr. Chapman for “Good Reason”, Dr. Chapman will be entitled
to severance compensation in the form of salary continuation for a period of three months (subject to Dr. Chapman executing and delivering
a customary general release in favor of the company).
On
August 17, 2023, Dr. Chapman received a $0.05
million cash bonus net of federal, state, local
and income taxes related to the successful completion of the IPO.
On
August 28, 2023, the Company amended Dr. Chapman’s employment agreement to indicate that he works part-time on an as needed
basis for the Corporation, rather than fifty percent (50%) of the time, effective August 1st, 2023.
On October 13, 2023, the Company amended Dr. Chapman’s employment agreement to reflect a temporary reduction
in his compensation from $0.15 million per year to $0.05 million per year, to extend for a period of 90 days. After the 90-day period,
Dr. Chapman’s compensation shall be reinstated to the amount in his employment agreement of $0.15 million per year.
Christos
Nicholoudis
On
April 28, 2023, the Company entered into an employment agreement with Christos Nicholoudis pursuant to which Mr. Nicholoudis serves as
the Company’s General Counsel. Mr. Nicholoudis’ employment agreement provides that Mr. Nicholoudis will devote 50% of his
full business time and effort to the business and affairs of the company, and it further provides that such employment will be on an
at-will basis and can be terminated by either Mr. Nicholoudis or the company at any time and for any reason. Under the agreement, Mr.
Nicholoudis will receive an initial base salary of $0.075 million per year. In the event that Mr. Nicholoudis employment is terminated
by the company without “Cause” or is terminated by Mr. Nicholoudis for “Good Reason”, Mr. Nicholoudis will be
entitled to severance compensation in the form of salary continuation for a period of three months (subject to Mr. Nicholoudis executing
and delivering a customary general release in favor of the company).
On
August 17, 2023, Mr. Nicholoudis received a $0.025 million cash bonus net of federal, state, local and income taxes related to the successful
completion of the IPO.
1,700,000
Shares
Common
Stock

MIRA
Pharmaceuticals, Inc.
Prospectus
You
should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information
that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities
in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is correct only as of the
date of this prospectus, regardless of the time of the delivery of this prospectus or the sale of these securities.
, 2023
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution
The
following table sets forth all the costs and expenses to be paid by us in connection with the sale of the shares of common stock being
registered hereby. All amounts shown below are estimates, except the SEC registration fee:
| | |
Amount | |
| SEC registration fee | |
$ | 381.40 | |
| Printing expenses | |
| 5,000.00 | |
| Legal fees and expenses | |
| 15,000.00 | |
| Accounting fees and expenses | |
| 7,500.00 | |
| Miscellaneous expenses | |
| 5,000.00 | |
| Total | |
$ | 32,881.40 | |
Item
14. Indemnification of Directors and Officers
MIRA
Pharmaceuticals, Inc. is incorporated under the laws of the state of Florida. Section 607.0831 of the Florida Business Corporation Act,
as amended (the “FBCA”), provides that a director is not personally liable for monetary damages to the corporation or any
other person for any statement, vote, decision to take or not to take action, or any failure to take any action, as a director, unless
(1) the director breached or failed to perform his or her duties as a director and (2) the director’s breach of, or failure to
perform, those duties constitutes (a) a violation of the criminal law, unless the director had reasonable cause to believe his or her
conduct was lawful or had no reasonable cause to believe his or her conduct was unlawful, (b) a transaction from which the director derived
an improper personal benefit, either directly or indirectly, (c) a circumstance under which the liability provisions of Section 607.0834
of the FBCA are applicable, (d) in a proceeding by or in the right of the corporation to procure a judgment in its favor or by or in
the right of a shareholder, conscious disregard for the best interest of the corporation, or willful or intentional misconduct, or (e)
in a proceeding by or in the right of someone other than the corporation or a shareholder, recklessness or an act or omission which was
committed in bad faith or with malicious purpose or in a manner exhibiting wanton and willful disregard of human rights, safety, or property.
A judgment or other final adjudication against a director in any criminal proceeding for a violation of the criminal law estops that
director from contesting the fact that his or her breach, or failure to perform, constitutes a violation of the criminal law; but does
not estop the director from establishing that he or she had reasonable cause to believe that his or her conduct was lawful or had no
reasonable cause to believe that his or her conduct was unlawful.
Under
Section 607.0851 of the FBCA, a corporation has power to indemnify any person who is a party to any proceeding (other than an action
by, or in the right of the corporation), because he or she is or was a director or officer of the corporation against liability incurred
in connection with such proceeding, including any appeal thereof, if he or she acted in good faith and in a manner he or she reasonably
believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had
no reasonable cause to believe his or her conduct was unlawful. The termination of any proceeding by judgment, order, settlement or conviction
or upon a plea of nolo contendere or its equivalent shall not, of itself, create a presumption that the person did not act in good faith
and in a manner which he or she reasonably believed to be in, or not opposed to, the best interests of the corporation or, with respect
to any criminal action or proceeding, has reasonable cause to believe that his or her conduct was unlawful.
For
purposes of the indemnification provisions of the FBCA, “director” or “officer” means an individual who is or
was a director or officer, respectively, of a corporation or who, while a director or officer of the corporation, is or was serving at
the corporation’s request as a director or officer, manager, partner, trustee, employee, or agent of another domestic or foreign
corporation, limited liability company, partnership, joint venture, trust, employee benefit plan, or another enterprise or entity and
the terms include, unless the context otherwise requires, the estate, heirs, executors, administrators, and personal representatives
of a director or officer.
In
addition, under Section 607.0851 of the FBCA, a corporation has the power to indemnify any person, who was or is a party to any proceeding
by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director or
officer, against expenses and amounts paid in settlement not exceeding, in the judgment of the board of directors, the estimated expense
of litigating the proceeding to conclusion, actually and reasonably incurred in connection with the defense or settlement of such proceeding,
including any appeal thereof. Such indemnification shall be authorized if such person acted in good faith and in a manner he or she reasonably
believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be made under this
subsection in respect of any claim, issue, or matter as to which such person shall have been adjudged to be liable unless, and only to
the extent that, the court in which such proceeding was brought, or any other court of competent jurisdiction, shall determine upon application
that, despite the adjudication of liability but in view of all circumstances of the case, such person is fairly and reasonably entitled
to indemnity for such expenses which such court shall deem proper.
Section
607.0852 of the FBCA provides that a corporation must indemnify an individual who is or was a director or officer who was wholly successful,
on the merits or otherwise, in the defense of any proceeding to which the individual was a party because he or she is or was a director
or officer of the corporation against expenses incurred by the individual in connection with the proceeding.
Section
607.0853 of the FBCA provides that a corporation may, before final disposition of a proceeding, advance funds to pay for or reimburse
expenses incurred in connection with the proceeding by an individual who is a party to the proceeding because that individual is or was
a director or an officer if the director or officer delivers to the corporation a signed written undertaking of the director or officer
to repay any funds advanced if (a) the director or officer is not entitled to mandatory indemnification under Section 607.0852; and (b)
it is ultimately determined under Section 607.0854 or Section 607.0855 (as described below) that the director or officer has not met
the relevant standard of conduct described in Section 607.0851 or the director or officer is not entitled to indemnification under Section
607.0859 (as described below).
Section
607.0854 of the FBCA provides that, unless the corporation’s articles of incorporation provide otherwise, notwithstanding the failure
of a corporation to provide indemnification, and despite any contrary determination of the board of directors or of the shareholders
in the specific case, a director or officer of the corporation who is a party to a proceeding because he or she is or was a director
or officer may apply for indemnification or an advance for expenses, or both, to a court having jurisdiction over the corporation which
is conducting the proceeding, or to a circuit court of competent jurisdiction. Our amended and restated articles of incorporation do
not provide any such exclusion. After receipt of an application and after giving any notice it considers necessary, the court may order
indemnification or advancement of expenses upon certain determinations of the court.
Section
607.0855 of the FBCA provides that, unless ordered by a court under Section 607.0854, a corporation may not indemnify a director or officer
under Section 607.0851 unless authorized for a specific proceeding after a determination has been made that indemnification is permissible
because the director or officer has met the relevant standard of conduct set forth in Section 607.0851.
Section
607.0857 of the FBCA also provides that a corporation shall have the power to purchase and maintain insurance on behalf of and for the
benefit of any person who is or was a director or officer of the corporation against any liability asserted against the person and incurred
by him or her in any such capacity or arising out of his or her status as such, whether or not the corporation would have the power to
indemnify or advance expenses to the individual against such liability under the provisions of Section 607.0857.
Section
607.0858 of the FBCA provides that the indemnification provided pursuant to Section 607.0851 and Section 607.0852, and the advancement
of expenses provided pursuant to Section 607.0853, are not exclusive. A corporation may, by a provision in its articles of incorporation,
bylaws or any agreement, or by vote of shareholders or disinterested directors, or otherwise, obligate itself in advance of the act or
omission giving rise to a proceeding to provide any other or further indemnification or advancement of expenses to any of its directors
or officers.
Section
607.0859 of the FBCA provides that, unless ordered by a court under the provisions of Section 607.0854 of the FBCA, a corporation may
not indemnify a director or officer under Section 607.0851 or Section 607.0858, or advance expenses to a director or officer under Section
607.0853 or Section 607.0858, if a judgment or other final adjudication establishes that his or her actions, or omissions to act, were
material to the cause of action so adjudicated and constitute: (a) willful or intentional misconduct or a conscious disregard for the
best interests of the corporation in a proceeding by or in the right of the corporation to procure a judgment in its favor or in a proceeding
by or in the right of a shareholder; (b) a transaction in which a director or officer derived an improper personal benefit; (c) a violation
of the criminal law, unless the director or officer had reasonable cause to believe his or her conduct was lawful or had no reasonable
cause to believe his or her conduct was unlawful; or (d) in the case of a director, a circumstance under which the liability provisions
of Section 607.0834 are applicable (relating to unlawful distributions).
Our
amended and restated articles of incorporation and bylaws provide that we shall indemnify any and all persons whom it shall have power
to indemnify under the FBCA to the fullest extent permitted by law.
We
also maintain director and officer liability insurance against certain claims and liabilities which may be made against our former, current
or future directors and officers. In addition, we have individual indemnification agreements with our directors.
Item
15. Recent Sales of Unregistered Securities
In
the preceding three years, we have issued and sold the following securities that were not registered under the Securities Act:
| |
1. |
From
November 2021 to December 2022, we undertook a private placement solely to accredited investors pursuant to which we issued and sold
an aggregate of 1,539,200 shares of our common stock at a price of $5.00 per share, for an aggregate purchase price of approximately
$7.7 million to 90 investors. |
| |
2. |
In
June 2022 and April 2023, we granted to 16 directors, employees, or other service providers stock options to purchase an aggregate
of 1,000,000 shares of our common stock at an exercise price of $5.00 per share pursuant to our 2022 Omnibus Plan. |
| |
3. |
In
April 2023, we granted to Bay Shore Trust a warrant to purchase up to 1,000,000 shares of our common stock at an exercise price of
$5.00 per share in consideration of making a credit facility available to the Company. |
| |
4. |
In
November 2023, we granted to MIRALOGX a warrant to purchase up to 700,000 shares of our common stock at an exercise price of $2.00
per share in consideration for the license agreement between the Company and MIRALOGX, dated November 15, 2023. |
We
claimed exemption from registration under the Securities Act of 1933, as amended, or the Securities Act, for the sale and issuance of
securities in the transaction described in paragraphs 1 and 3 above by virtue of Section 4(a)(2) and/or Regulation D promulgated thereunder
as a transaction not involving any public offering. All the purchasers of unregistered securities for which we relied on Section 4(a)(2)
and/or Regulation D represented that they were accredited investors as defined in Rule 501(a) under the Securities Act. We claimed such
exemption on the basis that (a) the purchasers in each case represented that they intended to acquire the securities for investment only
and not with a view to the distribution thereof and that they either received adequate information about the registrant or had access,
through employment or other relationships, to such information and (b) appropriate legends were affixed to the stock certificates issued
in such transactions.
We
claimed exemption from registration under the Securities Act for the sales and issuances of securities in the transactions described
in paragraph 2 above under Section 4(a)(2) of the Securities Act in that such sales and issuances did not involve a public offering or
under Rule 701 promulgated under the Securities Act, in that they were offered and sold either pursuant to written compensatory plans
or pursuant to a written contract relating to compensation, as provided by Rule 701.
Item
16. Exhibits and Financial Statement Schedules
(A)
Exhibits.
INDEX
TO EXHIBITS
| Exhibit
No. |
|
Exhibit
Description |
| 3.1 |
|
Third Amended and Restated Articles of Incorporation of MIRA Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1 to Form S-1 filed July 28, 2023). |
| 3.2 |
|
Amended and Restated Bylaws of MIRA Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.3 to Form S-1 filed July 28, 2023). |
| 4.1 |
|
Common Stock Purchase Warrant, dated April 28, 2023, between MIRA Pharmaceuticals, Inc. and Bay Shore Trust (incorporated by reference to Exhibit 4.2 to Form S-1 filed July 28, 2023). |
| 4.2 |
|
Common Stock Purchase Warrant from the Company to MIRALOGX, dated November 15, 2023 (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed November 20, 2023). |
| 5.1 |
|
Opinion of Foley & Lardner LLP |
| 10.1+ |
|
2022 Omnibus Incentive Plan, as amended and restated (incorporated by reference to Exhibit 10.1 to Form S-1 filed July 28, 2023). |
| 10.2+ |
|
Form of Stock Option Award under 2022 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.2 to Form S-1 filed July 28, 2023). |
| 10.3 |
|
Form of Indemnification Agreement (incorporated by reference to Exhibit 10.3 to Form S-1 filed July 28, 2023). |
| 10.4 |
|
Confirmatory Patent Assignment and Royalty Agreement, dated November 1, 2021, between SRQ Patent Holdings II, LLC and MIRA Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.4 to Form S-1 filed July 28, 2023). |
| 10.5 |
|
Amended and Restated Limited License Agreement, dated June 27, 2022, between MIRA Pharmaceuticals, Inc. and MyMD Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.5 to Form S-1 filed July 28, 2023). |
| 10.6 |
|
Amendment No. 1, dated April 20, 2023, to Amended and Restated Limited License Agreement between MIRA Pharmaceuticals, Inc. and MyMD Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.6 to Form S-1 filed July 28, 2023). |
| 10.7+ |
|
Employment Agreement, dated April 28, 2023, between MIRA Pharmaceuticals, Inc. and Erez Aminov (incorporated by reference to Exhibit 10.7 to Form S-1 filed July 28, 2023). |
| 10.8+ |
|
Amendment to Employment Agreement, August 28, 2023, between MIRA Pharmaceuticals, Inc. and Erez Aminov (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed August 31, 2023). |
| 10.9+ |
|
Employment Agreement, dated April 28, 2023, between MIRA Pharmaceuticals, Inc. and Michele Yanez (incorporated by reference to Exhibit 10.8 to Form S-1 filed July 28, 2023). |
| 10.10+ |
|
Employment Agreement, dated April 28, 2023 between MIRA Pharmaceuticals, Inc. and Chris Chapman (incorporated by reference to Exhibit 10.9 to Form S-1 filed July 28, 2023). |
| 10.11+ |
|
Amendment to Employment Agreement, dated August 28, 2023, between MIRA Pharmaceuticals and Dr. Chris Chapman (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed August 31, 2023). |
| 10.12+ |
|
Amendment to Employment Agreement, dated October 13, 2023, between MIRA Pharmaceuticals and Dr. Chris Chapman. |
| 10.13 |
|
Promissory Note and Loan Agreement, dated April 28, 2023, between MIRA Pharmaceuticals, Inc. and Bay Shore Trust (incorporated by reference to Exhibit 10.10 to Form S-1 filed July 28, 2023). |
| 10.14 |
|
Registration Rights Agreement, dated April 28, 2023, between MIRA Pharmaceuticals, Inc. and Bay Shore Trust (incorporated by reference to Exhibit 10.11 to Form S-1 filed July 28, 2023). |
| 10.15 |
|
Agreement for Shared Lease Costs, dated April 1, 2023, between MIRA Pharmaceuticals, Inc., Telomir Pharmaceuticals, Inc., and MIRALOGX LLC (incorporated by reference to Exhibit 10.12 to Form S-1 filed July 28, 2023). |
| 10.16 |
|
Master Collaboration Agreement, dated November 1, 2021, between MIRA Pharmaceuticals, Inc. and The Johns Hopkins University (incorporated by reference to Exhibit 10.13 to Form S-1 filed July 28, 2023). |
| 10.17 |
|
Conversion Agreement, dated July 20, 2023, between MIRA Pharmaceuticals, Inc. and the Bay Shore Trust (incorporated by reference to Exhibit 10.14 to Form S-1 filed July 28, 2023). |
| 10.18 |
|
Exclusive License Agreement, by and between the Company and MIRALOGX, dated as of November 30, 2023 (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed November 20, 2023). |
| 10.19 |
|
Promissory Note and Loan Agreement, by and between the Company and MIRALOGX, dated as of November 15, 2023 (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed November 20, 2023). |
| 14.1 |
|
Code of Business Conduct and Ethics (incorporated by reference to Exhibit 14.1 to Form S-1 filed July 28, 2023). |
| 21.1 |
|
List of Subsidiaries of Registrant (incorporated by reference to Exhibit 21.1 to Form S-1 filed July 28, 2023). |
| 23.1 |
|
Consent of Cherry Bekaert LLP |
| 23.2 |
|
Consent of Foley & Lardner LLP (included in Exhibit 5.1) |
| 24.1 |
|
Power of Attorney (included on signature page). |
| 99.1 |
|
Audit Committee Charter (incorporated by reference to Exhibit 99.1 to Form S-1 filed July 28, 2023). |
| 99.2 |
|
Nominating and Corporate Governance Committee Charter (incorporated by reference to Exhibit 99.2 to Form S-1 filed July 28, 2023). |
| 99.3 |
|
Compensation Committee Charter (incorporated by reference to Exhibit 99.3 to Form S-1 filed July 28, 2023). |
| 99.4 |
|
Corporate Governance Guidelines (incorporated by reference to Exhibit 99.4 to Form S-1 filed July 28, 2023). |
| 99.5 |
|
Insider Trading Policy (incorporated by reference to Exhibit 99.5 to Form S-1 filed July 28, 2023). |
| 99.6 |
|
Related Person Transaction Policy and Procedures (incorporated by reference to Exhibit 99.6 to Form S-1 filed July 28, 2023). |
| 107 |
|
Filing Fee Table |
+ Denotes management contract or compensatory plan or arrangement.
(B)
Financial Statement Schedules.
Not
applicable.
Item
17. Undertakings
The
undersigned Registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended.
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume
and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement.
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or
any material change to such information in the registration statement.
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering.
The
undersigned registrant hereby undertakes that:
(1)
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed
as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant
to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time
it was declared effective.
(2)
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of
prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons
of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities
and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the
event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid
by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted
by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the
opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question
whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of
such issue.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement to be signed
on its behalf by the undersigned, thereunto duly authorized, in the City of Tampa, Florida, on this 18 day of December,
2023.
| |
MIRA
Pharmaceuticals, Inc. |
| |
|
|
| |
By: |
/s/
Erez Aminov |
| |
|
Erez
Aminov |
| |
|
Chief
Executive Officer |
KNOW
ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Erez Aminov and Michelle
Yanez, and each of them individually, his or her true and lawful attorneys-in-fact and agents, with full powers of substitution and resubstitution,
for him or her and in his or her name, place and stead, in any and all capacities, to sign any or all amendments (including post effective
amendments) to this registration statement and any subsequent registration statement filed pursuant to Rule 462 under the Securities
Act of 1933, as amended, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities
and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform
each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or
she might or could do in person, and hereby ratifying and confirming all that either of the said attorneys-in-fact and agents, or his
or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act of 1933, as amended, this Amendment No. 5 to the Registration Statement has been signed below
by the following persons in the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Erez Aminov |
|
Chief
Executive Officer and Director |
|
December
18, 2023 |
| Erez
Aminov |
|
(Principal
Executive Officer) |
|
|
| |
|
|
|
|
| /s/
Michelle Yanez |
|
Chief
Financial Officer |
|
December
18, 2023 |
| Michelle
Yanez |
|
(Principal
Financial Officer and Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/
Chris Chapman |
|
Executive
Chairman and Director |
|
December
18, 2023 |
| Chris
Chapman |
|
|
|
|
| |
|
|
|
|
| /s/
Christos Nicholoudis |
|
Director |
|
December
18, 2023 |
| Christos
Nicholoudis, Esq. |
|
|
|
|
| |
|
|
|
|
| /s/
Michael Jerman |
|
Director |
|
December
18, 2023 |
| Michael
Jerman |
|
|
|
|
| |
|
|
|
|
| /s/
Brad Kroenig |
|
Director |
|
December
18, 2023 |
| Brad
Kroenig |
|
|
|
|
| |
|
|
|
|
| /s/
Talhia Tuck |
|
Director |
|
December
18, 2023 |
| Talhia
Tuck |
|
|
|
|
| |
|
|
|
|
| /s/
Hugh McColl III |
|
Director |
|
December
18, 2023 |
| Hugh
McColl III |
|
|
|
|
Exhibit
5.1
|
ATTORNEYS
AT LAW
100
North Tampa Street, Suite 2700
Tampa, FL 33602-5810
P.O. Box 3391
Tampa,
FL 33601-3391
813.229.2300
TEL
813.221.4210
FAX
www.foley.com |
December
18, 2023
MIRA
Pharmaceuticals, Inc.
855
N Wolfe Street
Suite
601
Baltimore,
Maryland 21205
Ladies
and Gentlemen:
We
have acted as counsel to MIRA Pharmaceuticals, Inc., a Florida corporation (the “Company”), in connection with the
preparation of a Registration Statement on Form S-1 (the “Registration Statement”), including the Prospectus constituting
a part thereof (the “Prospectus”), filed on the date hereof with the Securities and Exchange Commission (the “SEC”)
under the Securities Act of 1933, as amended (the “Securities Act”), in connection with the public offering by the
selling stockholders identified in the Registration Statement of up to 1,700,000 shares (the “Warrant Shares”) of
the Company’s common stock, par value $0.0001 per share, issuable upon exercise of outstanding common stock purchase warrants (the
“Warrants”).
In
connection with our representation, we have examined: (i) the forms of the Warrants, (ii) the Registration Statement and the Prospectus,
(iii) the Amended and Restated Certificate of Incorporation of the Company, (iv) the Amended and Restated Bylaws of the Company, and
(v) certain proceedings and actions taken by the Board of Directors of the Company in connection with the issuance and sale of the Warrants
and Warrant Shares. We have also considered such matters of law and of fact, including the examination of originals or copies, certified
or otherwise identified to our satisfaction, of such records and documents of the Company, certificates of officers, directors and representatives
of the Company, certificates of public officials, and such other documents as we have deemed appropriate as a basis for the opinions
set forth below. In our examination of the above-referenced documents, we have assumed the genuineness of all signatures, the authenticity
of all documents, certificates, and instruments submitted to us as originals and the conformity with the originals of all documents submitted
to us as copies.
The
opinions expressed herein are limited in all respects to the federal laws of the United States of America and the applicable provisions
of the Florida Business Corporation Act, and no opinion is expressed with respect to the laws of any other jurisdiction or any effect
which such laws may have on the opinions expressed herein. This opinion is limited to the matters stated herein, and no opinion is implied
or may be inferred beyond the matters expressly stated herein.
Based
upon the foregoing examination and in reliance thereon, and subject to the assumptions stated and in reliance on the statements of fact
contained in the documents that we have examined, we are of the opinion that the Warrant Shares, when issued and paid for in accordance
with the terms of the Warrants, will be validly issued, fully paid and nonassessable.
AUSTIN
Boston
CHICAGO
dallas
DENVER |
DETROIT
houston
JACKSONVILLE
LOS
ANGELES
MADISON |
MEXICO
CITY
MIAMI
MILWAUKEE
NEW
YORK
ORLANDO |
SACRAMENTO
Salt
Lake City
SAN
DIEGO
SAN
FRANCISCO
SILICON
VALLEY |
TALLAHASSEE
TAMPA
WASHINGTON,
D.C.
BRUSSELS
TOKYO |
December
18, 2023
This
opinion is issued as of the date hereof, and we assume no obligation to supplement this opinion if any applicable law changes after the
date hereof or if we become aware of any fact that might change the opinion expressed herein after the date hereof. This opinion is limited
to the matters set forth herein, and no other opinion should be inferred beyond the matters expressly stated.
We
consent to your filing this opinion as an exhibit to the Registration Statement and to the reference to our firm in the Prospectus under
the heading “Legal Matters.” In giving such consent, we do not thereby admit that we are in the category of persons whose
consent is required under Section 7 of the Securities Act or the rules and regulations of the SEC thereunder.
| |
Very
truly yours, |
| |
|
| |
/s/
Foley & Lardner LLP |
| |
|
| |
Foley
& Lardner LLP |
Exhibit 10.12


Exhibit
23.1

Exhibit
107
Calculation
of Filing Fee Tables
FORM
S-1
(Form
Type)
MIRA
Pharmaceuticals, Inc.
(Exact
name of Registrant as specified in its charter)
Table
1: Newly Registered and Carry Forward Securities
| | |
Security Type | |
Security Class Title | |
Fee Calculation or Carry Forward Rule | | |
Amount Registered | | |
Proposed Maximum Offering Price Per Unit | | |
Maximum Aggregate Offering Price | | |
Fee Rate | | |
Amount of Registration Fee | |
| Fees to be Paid | |
Equity | |
Common Stock, par value $0.0001 per share | |
| 457 | (c) | |
| 1,700,000 | (1) | |
$ | 1.52 | (2) | |
$ | 2,584,000.00 | | |
| 0.00014760 | | |
$ | 381.40 | |
| | |
Total Offering Amounts | |
| | | |
$ | 2,584,000.00 | | |
| | | |
$ | 381.40 | |
| | |
Total Fees Previously Paid | |
| | | |
| | | |
| | | |
| — | |
| | |
Total Fee Offsets | |
| | | |
| | | |
| | | |
| — | |
| | |
Net Fee Due | |
| | | |
| | | |
| | | |
$ | 381.40 | |
| (1) |
The
amount registered consists of up to 1,700,000 shares of common stock, par value $0.0001 per share, that are issuable upon the exercise
of outstanding warrants, to be offered by the selling stockholder named herein. Pursuant to Rule 416 under the Securities Act of
1933, as amended (the “Securities Act”), this registration statement also covers such an indeterminate amount of shares
of common stock as may become issuable to prevent dilution resulting from stock splits, stock dividends and similar events. |
| |
|
| (2) |
The
proposed maximum offering price per share is estimated solely for purposes of calculating the registration fee according to Rule
457(c) under the Securities Act based on the average of the high and low prices of the registrant’s common stock quoted on
The Nasdaq Capital Market on December 15, 2023. |
v3.23.4
Cover
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Entity Addresses [Line Items] |
|
| Document Type |
S-1
|
| Amendment Flag |
false
|
| Entity Registrant Name |
MIRA
PHARMACEUTICALS, INC.
|
| Entity Central Index Key |
0001904286
|
| Entity Tax Identification Number |
85-3354547
|
| Entity Incorporation, State or Country Code |
FL
|
| Entity Address, Address Line One |
855
N Wolfe Street
|
| Entity Address, Address Line Two |
Suite 601
|
| Entity Address, City or Town |
Baltimore
|
| Entity Address, State or Province |
MD
|
| Entity Address, Postal Zip Code |
21205
|
| City Area Code |
(737)
|
| Local Phone Number |
289-0835
|
| Entity Filer Category |
Non-accelerated Filer
|
| Entity Small Business |
true
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
true
|
| Business Contact [Member] |
|
| Entity Addresses [Line Items] |
|
| Entity Address, Address Line One |
855
N Wolfe Street
|
| Entity Address, Address Line Two |
Suite 601
|
| Entity Address, City or Town |
Baltimore
|
| Entity Address, State or Province |
MD
|
| Entity Address, Postal Zip Code |
21205
|
| Contact Personnel Name |
Erez
Aminov
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
dei_EntityAddressesLineItems |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Condensed Balance Sheets - USD ($)
|
Sep. 30, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Current assets: |
|
|
|
| Cash |
$ 5,868,330
|
$ 350,978
|
$ 2,809,552
|
| Deferred offering costs |
|
143,427
|
100,000
|
| Prepaid expenses |
202,817
|
|
|
| Total current assets |
6,071,147
|
494,405
|
2,909,552
|
| Deferred financing costs, net |
2,782,708
|
|
|
| Total assets |
9,018,212
|
858,074
|
3,355,164
|
| Current liabilities: |
|
|
|
| Trade accounts payable and accrued liabilities |
779,573
|
811,738
|
228,406
|
| Related party accounts payable |
|
116,350
|
547,600
|
| Total current liabilities |
868,373
|
1,370,039
|
1,093,806
|
| Non-current operating lease liabilities |
34,528
|
84,267
|
|
| Total liabilities |
902,901
|
1,454,306
|
1,093,806
|
| Stockholders’ Equity (deficit) |
|
|
|
| Preferred Stock, $0.0001 par value, 10,000,000 shares authorized and none issued or outstanding at September 30, 2023 and 5,000,000 authorized and none issued or outstanding at December 31, 2022. |
|
|
|
| Common Stock, $0.0001 par value; 100,000,000 shares authorized, at September 30, 2023 and 14,780,885 shares issued and outstanding. 95,000,000 shares authorized at December 31, 2022 and 13,313,000 shares issued and outstanding. |
1,478
|
6,657
|
6,337
|
| Additional paid-in capital |
23,611,517
|
8,699,830
|
4,499,550
|
| Accumulated deficit |
(15,497,684)
|
(9,302,719)
|
(2,244,529)
|
| Total stockholders’ equity (deficit) |
8,115,311
|
(596,232)
|
2,261,358
|
| Total liabilities and stockholders’ equity (deficit) |
9,018,212
|
858,074
|
3,355,164
|
| Nonrelated Party [Member] |
|
|
|
| Current assets: |
|
|
|
| Operating lease, right of use assets |
114,357
|
164,910
|
|
| Current liabilities: |
|
|
|
| Current portion of operating lease liabilities |
74,328
|
75,143
|
|
| Related Party [Member] |
|
|
|
| Current assets: |
|
|
|
| Operating lease, right of use assets |
|
198,759
|
|
| Due from related party |
50,000
|
|
|
| Advances to affiliates |
|
|
445,612
|
| Current liabilities: |
|
|
|
| Related party line of credit |
|
133,062
|
293,062
|
| Related party accrued interest |
14,472
|
34,987
|
$ 24,738
|
| Current portion of operating lease liabilities |
|
$ 198,759
|
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of deferred costs capitalized at the end of the reporting period that are expected to be charged against earnings within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredCostsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated amortization, of debt issuance costs. Includes, but is not limited to, legal, accounting, underwriting, printing, and registration costs. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
| Name: |
us-gaap_DeferredFinanceCostsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe carrying value as of the balance sheet date of the current portion of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_LinesOfCreditCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of receivable classified as other and noncurrent. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherReceivableAfterAllowanceForCreditLossNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount due from parties in nontrade transactions, classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(5)(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Condensed Balance Sheets (Parenthetical) - $ / shares
|
Sep. 30, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Statement of Financial Position [Abstract] |
|
|
|
| Preferred stock, par value |
$ 0.0001
|
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares authorized |
10,000,000
|
5,000,000
|
5,000,000
|
| Preferred stock, shares issued |
0
|
0
|
0
|
| Preferred stock, shares outstanding |
0
|
0
|
0
|
| Common stock, par value |
$ 0.0001
|
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized |
100,000,000
|
95,000,000
|
95,000,000
|
| Common stock, shares issued |
14,780,885
|
13,313,000
|
12,673,800
|
| Common stock, shares outstanding |
14,780,885
|
13,313,000
|
12,673,800
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Condensed Statements of Operations - USD ($)
|
3 Months Ended |
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Income Statement [Abstract] |
|
|
|
|
|
|
| Revenues |
|
|
|
|
|
|
| Operating costs: |
|
|
|
|
|
|
| General and administrative expenses |
2,144,832
|
736,059
|
3,830,303
|
2,940,469
|
2,992,125
|
770,115
|
| Related party travel costs |
|
357,350
|
453,550
|
1,293,050
|
1,704,350
|
697,600
|
| Research and development expenses |
1,015,252
|
714,968
|
1,185,839
|
1,466,708
|
2,351,465
|
684,447
|
| Total operating costs |
3,160,084
|
1,808,377
|
5,469,692
|
5,700,227
|
7,047,940
|
2,152,162
|
| Interest expense, net |
(427,732)
|
(2,307)
|
(725,273)
|
(8,484)
|
(10,250)
|
(24,374)
|
| Net loss attributable to common stockholders |
$ (3,587,816)
|
$ (1,810,684)
|
$ (6,194,965)
|
$ (5,708,711)
|
$ (7,058,190)
|
$ (2,176,536)
|
| Basic loss per share |
$ (0.26)
|
$ (0.14)
|
$ (0.45)
|
$ (0.43)
|
|
|
| Diluted loss per share |
$ (0.26)
|
$ (0.14)
|
$ (0.45)
|
$ (0.43)
|
|
|
| Weighted average common stock shares outstanding - basic |
13,639,197
|
13,168,556
|
13,639,197
|
13,166,200
|
|
|
| Weighted average common stock shares outstanding - diluted |
13,639,197
|
13,168,556
|
13,639,197
|
13,166,200
|
|
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Condensed Statements of Stockholders' Equity - USD ($)
|
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Stock Subscription Receivable [Member] |
AOCI Attributable to Parent [Member] |
Total |
| Balance at Dec. 31, 2020 |
$ 5,887
|
|
$ (5,887)
|
$ (67,993)
|
$ (67,993)
|
| Balance, shares at Dec. 31, 2020 |
11,773,800
|
|
|
|
|
| Sale of common stock, net |
$ 450
|
4,499,550
|
|
|
4,500,000
|
| Sale of common stock, net, shares |
900,000
|
|
|
|
|
| Collection of stock subscription receivable |
|
|
5,887
|
|
5,887
|
| Net loss |
|
|
|
(2,176,536)
|
(2,176,536)
|
| Balance at Dec. 31, 2021 |
$ 6,337
|
4,499,550
|
|
(2,244,529)
|
2,261,358
|
| Balance, shares at Dec. 31, 2021 |
12,673,800
|
|
|
|
|
| Sale of common stock, net |
$ 201
|
1,718,799
|
(135,000)
|
|
1,584,000
|
| Sale of common stock, net, shares |
402,200
|
|
|
|
|
| Net loss |
|
|
|
(1,475,046)
|
(1,475,046)
|
| Balance at Mar. 31, 2022 |
$ 6,538
|
6,218,349
|
(135,000)
|
(3,719,575)
|
2,370,312
|
| Balance, shares at Mar. 31, 2022 |
13,076,000
|
|
|
|
|
| Balance at Dec. 31, 2021 |
$ 6,337
|
4,499,550
|
|
(2,244,529)
|
2,261,358
|
| Balance, shares at Dec. 31, 2021 |
12,673,800
|
|
|
|
|
| Net loss |
|
|
|
|
(5,708,711)
|
| Balance at Sep. 30, 2022 |
$ 6,628
|
8,267,059
|
|
(7,953,238)
|
320,449
|
| Balance, shares at Sep. 30, 2022 |
13,256,000
|
|
|
|
|
| Balance at Dec. 31, 2021 |
$ 6,337
|
4,499,550
|
|
(2,244,529)
|
2,261,358
|
| Balance, shares at Dec. 31, 2021 |
12,673,800
|
|
|
|
|
| Sale of common stock, net |
$ 320
|
2,903,680
|
|
|
2,904,000
|
| Sale of common stock, net, shares |
639,200
|
|
|
|
|
| Net loss |
|
|
|
(7,058,190)
|
(7,058,190)
|
| Stock-based compensation |
|
1,296,600
|
|
|
1,296,600
|
| Balance at Dec. 31, 2022 |
$ 6,657
|
8,699,830
|
|
(9,302,719)
|
(596,232)
|
| Balance, shares at Dec. 31, 2022 |
13,313,000
|
|
|
|
|
| Balance at Mar. 31, 2022 |
$ 6,538
|
6,218,349
|
(135,000)
|
(3,719,575)
|
2,370,312
|
| Balance, shares at Mar. 31, 2022 |
13,076,000
|
|
|
|
|
| Collection of stock subscription receivable |
|
|
135,000
|
|
135,000
|
| Net loss |
|
|
|
(2,422,979)
|
(2,422,979)
|
| Stock-based compensation |
|
1,001,000
|
|
|
1,001,000
|
| Balance at Jun. 30, 2022 |
$ 6,538
|
7,219,349
|
|
(6,142,554)
|
1,083,333
|
| Balance, shares at Jun. 30, 2022 |
13,076,000
|
|
|
|
|
| Sale of common stock, net |
$ 90
|
899,910
|
|
|
900,000
|
| Sale of common stock, net, shares |
180,000
|
|
|
|
|
| Net loss |
|
|
|
(1,810,684)
|
(1,810,684)
|
| Stock-based compensation |
|
147,800
|
|
|
147,800
|
| Balance at Sep. 30, 2022 |
$ 6,628
|
8,267,059
|
|
(7,953,238)
|
320,449
|
| Balance, shares at Sep. 30, 2022 |
13,256,000
|
|
|
|
|
| Balance at Dec. 31, 2022 |
$ 6,657
|
8,699,830
|
|
(9,302,719)
|
(596,232)
|
| Balance, shares at Dec. 31, 2022 |
13,313,000
|
|
|
|
|
| Sale of common stock, net |
|
147,800
|
|
|
147,800
|
| Net loss |
|
|
|
(1,341,044)
|
(1,341,044)
|
| Balance at Mar. 31, 2023 |
$ 6,657
|
8,847,630
|
|
(10,643,763)
|
(1,789,476)
|
| Balance, shares at Mar. 31, 2023 |
13,313,000
|
|
|
|
|
| Balance at Dec. 31, 2022 |
$ 6,657
|
8,699,830
|
|
(9,302,719)
|
(596,232)
|
| Balance, shares at Dec. 31, 2022 |
13,313,000
|
|
|
|
|
| Net loss |
|
|
|
|
(6,194,965)
|
| Balance at Sep. 30, 2023 |
$ 1,478
|
23,611,517
|
|
(15,497,684)
|
8,115,311
|
| Balance, shares at Sep. 30, 2023 |
14,780,885
|
|
|
|
|
| Balance at Mar. 31, 2023 |
$ 6,657
|
8,847,630
|
|
(10,643,763)
|
(1,789,476)
|
| Balance, shares at Mar. 31, 2023 |
13,313,000
|
|
|
|
|
| Net loss |
|
|
|
(1,266,107)
|
(1,266,107)
|
| Stock-based compensation |
|
737,200
|
|
|
737,200
|
| Issuance of Warrants |
|
3,515,000
|
|
|
3,515,000
|
| Balance at Jun. 30, 2023 |
$ 6,657
|
13,099,830
|
|
(11,909,868)
|
1,196,619
|
| Balance, shares at Jun. 30, 2023 |
13,313,000
|
|
|
|
|
| Net loss |
|
|
|
(3,587,816)
|
(3,587,816)
|
| Stock-based compensation |
(5,326)
|
1,457,459
|
|
|
1,452,133
|
| Issuance of common stock at IPO, net |
$ 128
|
7,704,152
|
|
|
7,704,279
|
| Issuance of common stock at IPO, net, shares |
1,275,000
|
|
|
|
|
| Issuance of common stock Related to conversion of debt |
$ 16
|
1,100,080
|
|
|
1,100,096
|
| Issuance of common stock Related to conversion of debt, shares |
157,170
|
|
|
|
|
| Issuance of common stock |
$ 4
|
249,996
|
|
|
250,000
|
| Issuance of common stock, shares |
35,715
|
|
|
|
|
| Balance at Sep. 30, 2023 |
$ 1,478
|
$ 23,611,517
|
|
$ (15,497,684)
|
$ 8,115,311
|
| Balance, shares at Sep. 30, 2023 |
14,780,885
|
|
|
|
|
| X |
- DefinitionCollection of stock subscription receivable.
| Name: |
MIRA_AdjustmentsToAdditionalPaidInCapitalCollectionOfStockSubscriptionReceivable |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in additional paid in capital (APIC) resulting from the issuance of warrants. Includes allocation of proceeds of debt securities issued with detachable stock purchase warrants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 20
-Section 25
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481284/470-20-25-2
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalWarrantIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued during the period as a result of the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe gross value of stock issued during the period upon the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued in lieu of cash for services contributed to the entity. Value of the stock issued includes, but is not limited to, services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodValueIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodValueOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-24
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 34: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 38: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 40: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 41: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-15
Reference 42: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-16
Reference 43: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4I
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.4
Condensed Statements of Cash Flows - USD ($)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Cash flows from Operating activities |
|
|
|
|
| Net loss |
$ (6,194,965)
|
$ (5,708,711)
|
$ (7,058,190)
|
$ (2,176,536)
|
| Adjustments to reconcile net loss to net cash from operations |
|
|
|
|
| Non-cash interest expense |
|
|
10,250
|
24,374
|
| (Interest Expense)/Income- Accrued, net |
(20,515)
|
8,484
|
|
|
| Amortization of debt issuance costs |
732,292
|
|
|
|
| Stock-based compensation expense |
2,337,133
|
1,148,800
|
1,296,600
|
|
| Change in operating assets and liabilities: |
|
|
|
|
| Right of use lease, net |
|
(5,500)
|
(5,500)
|
|
| Accounts payable and accrued expenses |
(148,516)
|
(20,300)
|
152,081
|
776,006
|
| Prepaid expenses |
(202,817)
|
(52,096)
|
|
|
| Net cash flows used in operating activities |
(3,497,388)
|
(4,629,323)
|
(5,604,759)
|
(1,376,156)
|
| Financing activities: |
|
|
|
|
| Advances to affiliates |
(50,000)
|
(463,236)
|
445,612
|
(426,732)
|
| Payment of deferred offering costs |
143,427
|
(38,578)
|
(43,427)
|
(100,000)
|
| Net (repayments) borrowings under related party line of credit |
|
|
(160,000)
|
203,062
|
| Collection of stock subscription receivable |
|
|
|
5,887
|
| Repayments under related party line of credit |
(133,062)
|
(110,000)
|
|
|
| Proceeds from sale of common stock, less offering costs |
7,704,279
|
2,619,000
|
2,904,000
|
4,500,000
|
| Issuance of Common Stock Conversion of Debt |
1,100,096
|
|
|
|
| Issuance of Common Stock in lieu of fees |
250,000
|
|
|
|
| Net cash flows provided by financing activities |
9,014,740
|
2,007,186
|
3,146,185
|
4,182,217
|
| Net change in cash |
5,517,352
|
(2,622,137)
|
(2,458,574)
|
2,806,061
|
| Cash, beginning of year |
350,978
|
2,809,552
|
2,809,552
|
3,491
|
| Cash, end of period |
5,868,330
|
187,415
|
350,978
|
2,809,552
|
| Cash paid for interest |
|
|
|
|
| X |
- DefinitionCollection of stock subscription receivable.
| Name: |
MIRA_CollectionOfStockSubscriptionReceivable |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease decrease right of use lease net.
| Name: |
MIRA_IncreaseDecreaseRightOfUseLeaseNet |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionInterest expense income accrued net.
| Name: |
MIRA_InterestExpenseincomeAccruedNet |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPayment of deferred offering costs.
| Name: |
MIRA_PaymentOfDeferredOfferingCosts |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPayments for proceeds from affiliates advances.
| Name: |
MIRA_PaymentsForProceedsFromAffiliatesAdvances |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from issuance of common stock in lieu of fees.
| Name: |
MIRA_ProceedsFromIssuanceOfCommonStockInLieuOfFees |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense attributable to debt issuance costs. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_AmortizationOfFinancingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionInterest paid other than in cash for example by issuing additional debt securities. As a noncash item, it is added to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_PaidInKindInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of a long-term debt instrument which can be exchanged for a specified amount of another security, typically the entity's common stock, at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for the settlement of obligation drawn from a contractual arrangement with the lender, including letter of credit, standby letter of credit and revolving credit arrangements, under which borrowings can be made up to a specific amount at any point in time with maturities due beyond one year or the operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfLongTermLinesOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.4
Condensed Statements of Cash Flows (Parenthetical) - USD ($)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Right to use asset and liability |
|
$ 1,000,000.0
|
| Amortization of debt issuance costs |
$ 732,292
|
|
| Bay Shore Trust [Member] |
|
|
| Shares issued |
1,000,000
|
|
| Deferred finance cost |
$ 3,500,000
|
|
| Amortization of debt issuance costs |
$ 700,000
|
|
| Bay Shore Trust [Member] | Common Stock [Member] |
|
|
| Shares issued |
157,170
|
|
| Deferred finance cost |
$ 1,100,000
|
|
| MZ Group [Member] |
|
|
| Shares issued |
35,715
|
|
| Deferred finance cost |
$ 250,000
|
|
| X |
- DefinitionAmount of amortization expense attributable to debt issuance costs. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_AmortizationOfFinancingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in lease obligation from new lease. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
| Name: |
us-gaap_CapitalLeaseObligationsIncurred |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=MIRA_BayShoreTrustMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=MIRA_MZGroupMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
Description of business and summary of significant accounting policies
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Accounting Policies [Abstract] |
|
|
| Description of business and summary of significant accounting policies |
Note
1. Description of business and summary of significant accounting policies:
Overview
MIRA
Pharmaceuticals, Inc. (“MIRA” or the “Company” and formerly known as MIRA1a Therapeutics, Inc.) was formed in
September 2020 and is a Florida-based pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting a broad range of neurologic and neuropsychiatric disorders. The Company’s
novel oral pharmaceutical marijuana, MIRA1a, is currently under investigation for treating adult patients suffering from anxiety and cognitive decline,
often associated with early-stage dementia. MIRA1a, if approved by the FDA, could mark a significant advancement in addressing various
neuropsychiatric, inflammatory, and neurologic diseases and disorders.
The
Company has an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to
potentially deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD)
and major depressive disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered a controlled
substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
Substantive
operations began in late 2020 and the Company’s Investigative New Drug application is anticipated to be filed with the U.S. Food
and Drug Administration (“FDA”) end of third quarter 2024. The Company owns U.S. Patent 10,787,675 B2, titled “Purified
Synthetic Marijuana and Methods of Treatment by Administering Same,” which covers the MIRA1a compound as a new molecular entity
as well as pharmaceutical formulations of the compound and methods of treating Alzheimer’s disease, anxiety, depression, and addictions.
The
accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America
(“GAAP”).
As
used herein, the Company’s Common Stock, par value $0.0001 per share, is referred to as the “Common Stock” and the
Company’s preferred stock, par value $0.0001 per share, is referred to as the “Preferred Stock”.
Initial
Public Offering
On
August 7, 2023, the Company closed its initial public offering consisting of 1,275,000 shares at a price of $7.00 per share for approximately
$8.9 million in gross proceeds. After deducting the underwriting commission and other deferred offering expenses totaling $1.2 million,
the net proceeds to the Company were $7.7 million (the “IPO”).
The
shares were offered and sold pursuant to the Company’s Registration Statement on Form S-1, as amended (File No. 333-273024), originally
filed with the Securities and Exchange Commission (the “SEC”) on June 29, 2023 (the “Registration Statement”)
and the final quarterly report filed with the Commission pursuant to Rule 424(b)(4) of the Securities Act of 1933, as amended. The Registration
Statement was declared effective by the Commission on August 2, 2023. The common stock began trading on The Nasdaq Capital Market on
August 3, 2023 under the symbol “MIRA”. The closing of the IPO occurred on August 7, 2023.
As
of the completion of the IPO, among other things, certain of the Company’s then-outstanding convertible debt was converted into
shares of common stock. See Note 5 for more information.
Income
taxes
The
Company is taxed as a C corporation. Deferred tax assets and liabilities are recognized for the future tax consequences attributable
to differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases. Deferred
tax assets are recognized for temporary differences that will result in deductible amounts in future years and for loss carryovers. A
valuation allowance is recognized regarding deferred tax assets, if any, if it is more likely than not that some portion of the deferred
tax asset will not be realized.
Research
and development expenses
Research
and development costs are expensed in the period in which they are incurred and include the expenses paid to third parties, such as contract
research organizations and consultants, who conduct research and development activities on behalf of the Company. Patent-related costs,
including registration costs, documentation costs and other legal fees associated with the application, are expensed in the period in
which they are incurred.
Leases
The
Company accounts for leases under the provisions of FASB ASC Topic 842, “Leases”, which requires the Company to recognize
right-to-use (ROU) assets and lease liabilities for operating leases on the balance sheet.
Use
of estimates
The
preparation of financial statements in accordance with generally accepted accounting principles in the United States of America requires
the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the
disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the
reporting period. Actual results may differ from such estimates and such differences could be material.
Cash
The
Company maintains cash balances with financial institutions that management believes are of high credit quality. The Company’s
cash account at times may exceed federally insured limits. The Company has not experienced any losses in such accounts. The Company believes
it is not exposed to any significant credit risk from its cash account.
Stock-based
compensation
The
Company accounts for stock-based compensation under the provisions of FASB ASC 718, “Compensation - Stock Compensation”,
which requires the measurement and recognition of compensation expense for all stock-based awards made to employees, directors and consultants
based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using
the Black-Scholes model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the
requisite service periods using the straight-line method. The Company has elected to account for forfeiture of stock-based awards as
they occur.
Fair
Value of Financial Instruments
The
Company measures the fair value of financial instruments in accordance with GAAP which defines fair value, establishes a framework for
measuring fair value, and expands disclosures about fair value measurements.
GAAP
defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
GAAP also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use
of unobservable inputs when measuring fair value. The Company considers the carrying amount of deferred offering costs to approximate
fair value due to short-term nature of this instrument. GAAP describes three levels of inputs that may be used to measure fair value:
Level
1 – quoted prices in active markets for identical assets or liabilities.
Level
2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable.
Level
3 – inputs that are unobservable (for example cash flow modeling inputs based on assumptions).
|
Note
1. Description of business and summary of significant accounting policies:
Overview
MIRA
Pharmaceuticals, Inc. (“MIRA” or the “Company” and formerly known as MIRA1a Therapeutics, Inc.) was formed in
September 2020 and is a Florida-based pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting
a broad range of neurologic and neuropsychiatric disorders. The Company’s novel oral pharmaceutical marijuana, MIRA1a, is currently
under investigation for treating adult patients suffering from anxiety and cognitive decline, often associated with early-stage dementia.
MIRA1a, if approved by the FDA, could mark a significant advancement in addressing various neuropsychiatric, inflammatory, and neurologic
diseases and disorders.
The
Company has an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to
potentially deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD)
and major depressive disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered
a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
Substantive
operations began in late 2020 and the Company’s Investigative New Drug application is anticipated to be filed with the U.S. Food
and Drug Administration (“FDA”) end of first quarter 2024. The Company owns U.S. Patent 10,787,675 B2, titled “Purified
Synthetic Marijuana and Methods of Treatment by Administering Same,” which covers the MIRA1a compound as a new molecular entity
as well as pharmaceutical formulations of the compound and methods of treating Alzheimer’s disease, anxiety, depression, and addictions.
Foreign patent applications covering MIRA1a, and its therapeutic uses are pending in Australia, Canada, China, Europe, Israel, Japan,
and South Korea.
The
accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America
(“GAAP”).
As
used herein, the Company’s Common Stock, par value $0.0001 per share, is referred to as the “Common Stock” and the
Company’s preferred stock, par value $0.0001 per share, is referred to as the “Preferred Stock”.
Pending
transactions
The
Company is in the process of preparing for an initial public offering and expects to be listed under the NASDAQ symbol “MIRA.”
The transaction is expected to be complete in second half of 2023. The Company incurred $0.04 million and $0.1 million of legal costs,
during the years ended December 31, 2022 and December 31, 2021, respectively, associated with the offering, which have been recorded
as deferred offering costs in the accompanying balance sheets. These deferred offering costs will be derecognized as a reduction in offering
proceeds when the offering closes. However, there can be no guarantees that the Company will be successful in completing the proposed
transaction and ultimately listing on the NASDAQ.
Income
taxes
The
Company is a C corporation. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences
between the financial statement carrying amount of existing assets and liabilities and their respective tax bases. Deferred tax assets
are recognized for temporary differences that will result in deductible amounts in future years and for loss carryovers. A valuation
allowance is recognized regarding deferred tax assets, if any, if it is more likely than not that some portion of the deferred tax asset
will not be realized.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Research
and development expenses
Research
and development costs are expensed in the period in which they are incurred and include the expenses paid to third parties, such as contract
research organizations and consultants, who conduct research and development activities on behalf of the Company.
Use
of estimates
The
preparation of financial statements in accordance with generally accepted accounting principles in the United States of America
requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and
liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported
amounts of expenses during the reporting period. Actual results may differ from such estimates and such differences could be
material.
Cash
The
Company maintains cash balances with financial institutions that management believes are of high credit quality. The Company’s
cash account at times may exceed federally insured limits. The Company has not experienced any losses in such accounts. The Company believes
it is not exposed to any significant credit risk from its cash account.
Stock-based
compensation
The
Company accounts for stock-based compensation under the provisions of FASB ASC 718, “Compensation - Stock Compensation”,
which requires the measurement and recognition of compensation expense for all stock-based awards made to employees, directors and consultants
based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using
the Black-Scholes model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the
requisite service periods using the straight-line method. The Company has elected to account for forfeiture of stock-based awards as
they occur.
Change
in Accounting Principle
In
February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes existing guidance for accounting for leases under Topic
840, Leases. The FASB also subsequently issued additional ASUs which amend and clarify Topic 842. The most significant change in the
new leasing guidance is the requirement to recognize right-to-use (ROU) assets and lease liabilities for operating leases on the balance
sheet.
The
Company adopted these ASUs effective January 1, 2022 using the modified retrospective approach. As a result of adopting these ASUs, the
Company recorded ROU assets and lease liabilities of approximately $1.0 million and $0.4 million, respectively. Adoption of the new standard
did not materially impact the Company’s net income and had no impact on cash flows.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the business description and accounting policies concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Accounting policies describe all significant accounting policies of the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Liquidity and capital resources
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Liquidity And Capital Resources |
|
|
| Liquidity and capital resources |
Note
2. Liquidity and capital resources:
As
of September 30, 2023, the Company had cash of approximately $5.9 million. The Company used approximately $3.5 million of cash in operations
during the nine months ended September 30, 2023 and had stockholders’ equity of approximately $8.1 million, versus stockholders’
deficit of approximately $0.6 million at December 31, 2022
Historically,
the Company has been primarily engaged in developing MIRA1a. During these activities, the Company sustained substantial losses. The Company’s
ability to fund ongoing operations and future clinical trials required for FDA approval is dependent on the Company’s ability to
obtain significant additional external funding in the near term. Since inception, the Company financed its operations through the sale
of Common Stock, the IPO and related party financings. Additional sources of financing may be sought by the Company. The Company believes
that current cash and the proceeds of the August 2023 IPO are sufficient to fund operations until approximately Q4 2024. Additional financing
will be needed by the Company to fund its operations after such date to complete clinical developments and to commercially develop its
product candidate. However, there can be no assurance that any fundraising will be achieved on commercially reasonable terms, if at all.
|
Note
2. Liquidity and capital resources:
As
of December 31, 2022, the Company had cash of approximately $0.4 million. The Company used approximately $5.6 million of cash in operations
during the year ended December 31, 2022 and had stockholders’ (deficit) of approximately $0.6 million, versus stockholders’
equity of approximately $2.3 million at December 31, 2021. During the year ended December 31, 2022, the Company raised approximately
$3.2 million to finance its research and development and working capital needs, through a private placement of the Company’s common
stock and collections on amounts previously advanced to affiliates of the Company.
Historically,
the Company has been primarily engaged in developing MIRA1a. During these activities, the Company sustained substantial losses. The Company’s
ability to fund ongoing operations and future clinical trials required for FDA approval is dependent on the Company’s ability to
obtain significant additional external funding in the near term. Since inception, the Company financed its operations through the sale
of Common Stock and related party financings. See Note 4 for details of a related party line of credit established in 2021. Additional
sources of financing may be sought by the Company. However, there can be no assurance that any fundraising will be achieved on commercially
reasonable terms, if at all.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
The
Company expects to be able to fund operations through the anticipated initial public offering, or through the first quarter of 2024,
with available borrowings on the related party line of credit (Note 4). Should actual cash expenditures exceed management’s budget,
the Company may be forced to curtail operations along with implementing other cost-saving measures, such as a reduction in staff, reducing
the use of outside professional service providers, or significantly modifying or delaying the development of our product candidates.
|
| X |
- References
+ Details
| Name: |
MIRA_DisclosureLiquidityAndCapitalResourcesAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLiquidity And Capital Resources [Text Block]
| Name: |
MIRA_LiquidityAndCapitalResourcesTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
License agreement, related party
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| License Agreement Related Party |
|
|
| License agreement, related party |
Note
4. License agreement, related party:
Effective
April 26, 2023 (the “Effective Date”), the Company and MyMD Pharmaceuticals, Inc. (“MYMD”) entered into an Amended
and Restated Limited License Agreement with MyMD. The license grants our company a perpetual, worldwide, royalty-free non-exclusive right
to use MyMD’s Supera-CBD compound, a different compound than MIRA1a, as a synthetic intermediate in the manufacture of MIRA1a for
all purposes (including clinical development and commercial production). This license is perpetual, and MyMD does not have the right to
terminate it. In consideration of this license, we agreed to share with MyMD technical information and know-how that pertains to the
synthetic manufacture and/or formulation of our MIRA1a product candidate and granted a license to MyMD to use improvements to MIRA1a
made under the agreement, agreement, and the agreement does not involve any prior or future cash payments by us.
The
Company and MYMD have similar members of the Board, as well as officers from the respective companies.
|
Note
3. License agreement, related party:
On
April 28, 2022, and subsequently amended and restated on June 27, 2022 (the “Effective Date”), the Company and MyMD Pharmaceuticals,
Inc. (“MYMD”) entered into a non-exclusive, royalty-free license (the “Agreement”) to use MYMD’s Supera-CBD
as a synthetic intermediate in the manufacture of MIRA1a for research and development activities relating to our planned pre-clinical
and clinical studies.
This
Agreement was amended on April 17, 2023 to extend its original one-year term through December 31, 2024. The term of agreement may be
extended by mutual agreement of the parties for an additional period that is reasonably necessary to complete the manufacture of quantities
of MIRA1a needed for pre-clinical or clinical studies.
Either
party may terminate this Agreement without cause upon forty-five (45) calendar days prior written notice to the other Party.
The
Company and MYMD have similar members of the Board, as well as officers from the respective companies.
|
| X |
- DefinitionLicense Agreement Related Party [Text Block]
| Name: |
MIRA_LicenseAgreementRelatedPartyTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Line of credit, related party
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Line Of Credit Related Party |
|
|
| Line of credit, related party |
Note
5. Line of credit, related party:
In
May 2021, the Company entered into a revolving credit facility which allowed for borrowings of up to $5 million from Starwood Trust,
a shareholder of the Company. The facility had an initial term of 24 months (extended to 36 months in March 2023), with a new maturity
date of May 10, 2024, at which time all outstanding borrowings and accrued interest, if any, were due in full. Borrowings accrued interest
at a rate of 5% per annum.
In
April 2023, the Company entered into a Promissory Note and Loan Agreement with the Bay Shore Trust, a trust established by a shareholder
of the Company. Under this Promissory Note and Loan Agreement (the “Bay Shore Note”), the Company has the right to borrow
up to an aggregate of $5 million from the Bay Shore Trust at any time up to the second anniversary of the issuance of the Bay Shore Note
or, if earlier, upon the completion of the Company’s IPO. The Company’s right to borrow funds under the Bay Shore Note is
subject to the absence of a material adverse change in the Company’s assets, operations, or prospects. The Bay Share Note, together
with accrued interest, will become due and payable on the second anniversary of the issuance of the note, provided that it may be prepaid
at any time without penalty. The Bay Shore Note will accrue interest at a rate equal 7% per annum, simple interest, during the first
year that the note is outstanding and 10% per annum, simple interest, thereafter. The Bay Shore Note is unsecured.
The
Bay Shore Note replaced the revolving credit facility that the Company entered into with Starwood Trust, a separate trust established
by a shareholder of the Company, in May 2021 and pursuant to which the Company had an outstanding principal balance of $0.2 million as
of the date of the Bay Shore Note (which outstanding balance was retired with an advance under the Bay Shore Note).
In
consideration of the loan facility provided by the Bay Shore Trust, in April 2023, the Company issued to the Bay Shore Trust a common
stock purchase warrant giving the Bay Shore Trust the right to purchase up to 1,000,000 shares of common stock at an exercise price of
$5.00 per share, which warrant will expire five years after the date of grant. Pursuant to a registration rights agreement, the Company
has granted to Bay Shore Trust the right to require the Company, at any time after one year following the Company’s IPO, to register
for resale the shares issuable upon the exercise of the warrant, with such registration rights being in the form of demand and “piggyback”
registration rights that are subject to customary limitations and restrictions. See Note 8 for additional details related to these warrants.
On
July 20, 2023, the Company entered into a conversion agreement with the Bay Shore Trust under which the Bay Shore Trust had agreed to
convert, upon the completion of the IPO, $1.1 million of the outstanding principal balance of the Bay Shore Note into shares of the Company’s
common stock at a conversion price equal to the Company’s IPO price, which resulted in the issuance of 157,170 shares to the Bay
Shore Trust. On August 14, 2023, the Company paid $1.0 million in full to Bay Shore Trust, which was the amount due. The company also
paid accrued interest of $0.03 million. There is a remaining amount of $0.01 in accrued interest due to Bay Shore Trust as of September
30, 2023.
|
Note
4. Line of credit, related party:
In
May 2021, the Company entered into a revolving credit facility which allows for borrowings of up to $5,000,000 with a shareholder. The
facility has an initial term of 24 months (extended to 36 months in March 2023), with a new maturity date of May 10, 2024, at which time
all outstanding borrowings and accrued interest, if any, are due in full. Borrowings accrue interest at a rate of 5% per annum. The Company
anticipates repaying the line of credit through proceeds from the anticipated initial public offering.
|
| X |
- DefinitionLine Of Credit Related Party [Text Block]
| Name: |
MIRA_LineOfCreditRelatedPartyTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Related party transactions
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Related Party Transactions [Abstract] |
|
|
| Related party transactions |
Note
6. Related party transactions:
Due
from related parties – As of the nine months ended September 30, 2023, the Company paid $0.05 million in accounts payable on
behalf of a related party.
Advances
from affiliates – During the nine months ended September 30, 2023, the Company received working capital advances in the amount
of $1.06 million from the Bay Shore Trust LOC, which was used to pay off advances from affiliates. As of September 30, 2023, all advances
have been repaid in full.
Related
party accounts payable – Amounts due to related parties as of September 30, 2023 and December 31, 2022, are recorded as Accounts
payable related parties, in the accompanying balance sheets.
Travel
expenses – In April 2021, the Company entered into an airplane lease with an entity under common control that the Company incurs
approximately $0.05 million of lease charges per month. The lease was renewable, at the Company’s discretion, for an additional
one to three years, however, the Company terminated the lease at March 31, 2023, without any penalties. The Company may continue to incur
related party travel-related expenses as they occur, which will be recorded in Related Party Travel Costs, in the condensed statement
of operations. During the nine months ended September 30, 2023, the Company incurred $0.5 million, for travel-related expenses to the
related party for monthly rental charges and airplane-related expenses.
License
agreement - See Note 4.
Line
of credit - See Note 5.
|
Note
5. Related party transactions:
Advances
to affiliates – During the year ended December 31, 2022, and December 31, 2021, the Company made working capital advances to
companies under common control. These advances were due on demand and were non-interest bearing. As of December 31, 2022, such advances
were repaid in full.
Related
party accounts payable – Amounts due to related parties as of December 31, 2022 and December 31, 2021, are recorded as Accounts
payable related parties, in the accompanying balance sheets.
Travel
expenses – In April 2021, the Company entered into an airplane lease with an entity under common control that the Company incurs
approximately $0.05 million of lease charges per month. The lease is renewable, at the Company’s discretion, for an additional
one to three years, however, the Company intends to terminate the lease upon the date of its initial public offering, as allowed in the
lease agreement. During the year ended December 31, 2022 and 2021, the Company incurred $1.7 million and $0.7 million, respectively,
for travel-related expenses to the related party for monthly rental charges and airplane-related expenses.
License
agreement - See Note 3.
Line
of credit - See Note 4.
Lease
and lease reimbursements - See Note 6.
Consulting
and employment agreements – See Note 9.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Leases
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Leases |
|
|
| Leases |
Note
7. Leases:
The
Company’s corporate headquarters is in Baltimore, Maryland, which includes a lease for office space. This lease began in November
2021 and was amended in April 2023. This space is approximately 550 square feet and has a remaining base rent of $0.01 million payable
through April 2024. Rent is payable in monthly installments and is subject to yearly price increases.
The
Company had leased an office in Tampa, Florida, for its finance and general operations, which began in March 2022 for 37 months. This
space is approximately 2,300 square feet and has a remaining base rent of $0.1 million payable through March 2025. Rent is payable in
monthly installments and is subject to yearly price increases. As of August 1, 2023, a related party to the Company began paying the
monthly lease expenses on behalf of the Company, directly to the landlord. This is conjunction with the Company’s IPO and the resignation
of its former Tampa employees. As such, the Tampa, Florida location is no longer needed. The related party who has been paying for the
lease since August 1, 2023 is in the process of amending the lease to remove the Company from further obligations. Until such time, the
Company will continue to reflect the right of use assets and liabilities on the condensed balance sheets.
The
Company also leased a jet (Note 5) from a related party, which lease the Company terminated on March 31 2023.
Variable
lease costs
Variable
lease costs primarily include utilities, property taxes, and other operating costs that are passed on from the lessor. Variable lease
costs related to the aircraft include usage expenses, which includes pilot expenses, jet fuel and general flight expenses.
The
components of lease expense were as follows:
Schedule of Lease Expense
| | |
| | |
| |
| | |
Nine months ended September 30, | |
| Lease Costs | |
2023 | | |
2022 | |
| Operating Lease Cost | |
| | | |
| | |
| Operating Lease | |
$ | 200,283 | | |
$ | 333,046 | |
| Variable Lease Costs | |
| 311,126 | | |
| 637,420 | |
| Total Lease Cost | |
$ | 511,409 | | |
$ | 970,466 | |
Supplemental
cash flow information related to leases were as follows:
Schedule
of Cash Flow Information Related to Leases
| | |
| | |
| |
| | |
Nine months ended September 30, | |
| Other Lease Information | |
2023 | | |
2022 | |
| Cash paid for amounts included in the measurement of lease liabilities | |
| | | |
| | |
| Operating cash flows from operating leases | |
$ | 511,409 | | |
$ | 970,466 | |
Schedule
of Remaining Weighted-average Lease Term and Weighted-average Discount Rate
| | |
Nine months ended September 30, | |
| | |
2023 | | |
2022 | |
| Lease Term and Discount | |
| | | |
| | |
| Weighted Average remaining lease term | |
| 1.79 years | | |
| 3 years | |
| Weighted Average discount rate | |
| 5.0 | % | |
| 5.0 | % |
Maturity
of Lease Liabilities
Future
minimum lease payments under non-cancellable leases as of September 30, 2023 were as follows:
Schedule
of Maturity
of Lease Liabilities
Maturity
of Lease Liabilities
| | |
September 30, 2023 | |
| Remainder of 2023 | |
$ | 20,722 | |
| 2024 | |
| 74,402 | |
| 2025 | |
| 17,444 | |
| Total Lease payments | |
| 112,568 | |
| Less: Interest | |
| (3,711 | ) |
| Present Value of Lease Liabilities | |
$ | 108,857 | |
On
April 1, 2023 the Company entered into an Agreement For Shared Lease Costs with MIRALOGX, LLC, (the “Shared Agreement”) who
is a related party for the jet usage. Under the Shared Agreement, the Company agrees to make monthly contributions or payments in accordance
with its monthly use of shared aircraft toward rent payments. However, the Company has not used the aircraft after the termination of
the lease and there are no minimum payments due without usage.
|
Note
6. Lease:
Leases
The
Company leases certain office space and an airplane. The Company determines whether a contract contains a lease at inception by determining
if the contract conveys the right to control the use of identified property, plant or equipment for a period of time in exchange for
consideration. The Company has lease agreements with lease and non-lease components, which are generally accounted for separately with
amounts allocated to the lease and non-lease components based on relative stand-alone prices.
Right-of-use
(“ROU”) assets and lease liabilities are recognized at the commencement date based on the present value of the future minimum
lease payments over the lease term. Renewal and termination clauses that are factored into the determination of the lease term if it
is reasonably certain that these options would be exercised by the Company. Lease assets are amortized over the lease term unless there
is a transfer of title or purchase option reasonably certain of exercise, in which case the asset life is used. Certain of our lease
agreements include variable payments. Variable lease payments not dependent on an index or rate primarily consist of common area maintenance
charges and are not included in the calculation of the ROU asset and lease liability and are expensed as incurred. In order to determine
the present value of lease payments, the Company uses the implicit rate when it is readily determinable. As most of the Company’s
leases do not provide an implicit rate, management uses the Company’s incremental borrowing rate based on the information available
at lease commencement to determine the present value of lease payments.
Our
lease agreements do not contain any material residual value guarantees or material restrictive covenants. The Company does not have leases
where it is involved with the construction or design of an underlying asset. The Company has no material obligation for leases signed
but not yet commenced as of December 31, 2022. The Company does not have any material sublease activities.
Practical
Expedients Elected
| |
● |
The
Company elected the three transition practical expedients that permit an entity to (a) not reassess whether expired or existing contracts
contain leases, (b) not reassess lease classification for existing or expired leases, and (c) not consider whether previously capitalized
initial direct costs would be appropriate under the new standard. |
| |
● |
The
Company has elected to account for lease and non-lease components as a single component. |
Variable
lease costs
Variable
lease costs primarily include utilities, property taxes, and other operating costs that are passed on from the lessor. Variable lease
costs related to the aircraft include usage expenses, which includes pilot expenses, jet fuel and general flight expenses.
The
components of lease expense were as follows:
Schedule of Lease Expense
| |
2022 | | |
2021 | |
| | |
Year ended December 31, | |
| Lease Costs | |
2022 | | |
2021 | |
| Operating Lease Cost | |
| | | |
| | |
| Operating Lease | |
$ | 657,797 | | |
$ | - | |
| Variable Lease Costs | |
| 1,112,913 | | |
| - | |
| Total Lease Cost | |
$ | 1,770,710 | | |
$ | - | |
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Supplemental
cash flow information related to leases were as follows:
Schedule
of Cash Flow Information Related to Leases
| Other Lease Information | |
2022 | | |
2021 | |
| | |
Year ended December 31, | |
| Other Lease Information | |
2022 | | |
2021 | |
| Cash paid for amounts included in the measurement of lease liabilities | |
| | | |
| | |
| Operating cash flows from operating leases | |
$ | 626,304 | | |
$ | - | |
Schedule
of Remaining Weighted-average Lease Term and Weighted-average Discount Rate
| | |
Year
ended December 31, | |
| | |
2022 | | |
2021 | |
| Lease Term and Discount | |
| | | |
| | |
| Weighted Average remaining lease term | |
| 0.53 years | | |
| - | |
| Weighted Average discount rate | |
| 5.0 | % | |
| - | |
Maturity
of Lease Liabilities
Future
minimum lease payments under non-cancellable leases as of December 31, 2022 were as follows:
Schedule
of Maturity
of Lease Liabilities
| Maturity of Lease Liabilities | |
| |
| | |
December 31, 2022 | |
| 2023 | |
| 281,050 | |
| 2024 | |
| 69,309 | |
| 2025 | |
| 17,444 | |
| Total Lease payments | |
| 367,803 | |
| Less: Interest | |
| (9,634 | ) |
| Present Value of Lease Liabilities | |
| 358,169 | |
|
| X |
- References
+ Details
| Name: |
MIRA_DisclosureLeasesAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Income taxes
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income taxes |
Note
7. Income taxes:
The
significant components of the Company’s net deferred tax assets are as follows as of December 31:
Schedule
of Components of Net Deferred Tax Assets
| | |
2022 | | |
2021 | |
| | |
December 31, | |
| | |
2022 | | |
2021 | |
| Deferred tax assets | |
| | | |
| | |
| Net operating loss carry-forward | |
$ | 1,061,300 | | |
$ | 572,355 | |
| Section 174 Qualified Research Expenditures | |
| 388,230 | | |
| - | |
| Stock compensation | |
| 330,633 | | |
| - | |
| ROU liability | |
| 91,333 | | |
| - | |
| Other | |
| 6,120 | | |
| - | |
| Deferred tax assets Gross | |
| 1,877,616 | | |
| 572,355 | |
| Less: valuation allowance | |
| (1,784,880 | ) | |
| (572,355 | ) |
| Total deferred tax asset | |
| 92,736 | | |
| - | |
| Deferred tax liabilities | |
| | | |
| | |
| ROU asset | |
| (92,736 | ) | |
| - | |
| Total net deferred tax asset | |
$ | - | | |
$ | - | |
Beginning
in 2022, in accordance with Internal Revenue Code Section 174, Qualified Research Expenditures are capitalized for tax purposes and amortized
over a period of five years. Accordingly, for income tax purposes, the Company has recorded a deferred tax asset totaling approximately
$0.4 million related to the timing difference between GAAP and Tax recognition of these expenditures.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
The
components of the provision for income taxes consist of the following:
Schedule
of Components of Provision For Income Taxes
| | |
2022 | | |
2021 | |
| Deferred tax: | |
| | | |
| | |
| Deferred | |
| (1,212,525 | ) | |
| (555,017 | ) |
| Change in valuation allowance | |
| 1,212,525 | | |
| 555,017 | |
| Total deferred | |
| - | | |
| - | |
| Total provision for income taxes | |
$ | - | | |
$ | - | |
ASC
Topic 740 requires that a deferred tax amount be reduced by a valuation allowance if, based on the weight of available evidence it is
more likely than not (a likelihood of more than 50%) that some portion or all of the deferred tax assets will not be realized. The valuation
allowance should be sufficient to reduce the deferred tax asset to the amount that is more likely than not to be realized. The Company
has recorded a full valuation allowance against its deferred tax assets generated by net operating loss carryforwards as it has determined
that such amounts may not be recognizable, given the historical losses of the Company to date. As of December 31, 2022, the Company has
a cumulative federal net operating loss carryforward of approximately $4.2 million. The net operating loss carryforwards have no expiry
date.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Stockholders’ equity
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Equity [Abstract] |
|
|
| Stockholders’ equity |
Note
8. Stockholders’ equity:
Capital
stock
The
Company has the authority to issue 110,000,000 shares of capital stock, consisting of 100,000,000 shares of Common Stock and 10,000,000
shares of undesignated preferred stock (as amended and restated on June 28, 2023), whose rights and privileges will be defined by the
Board of Directors when a series of preferred stock is designated.
Reverse
stock-split
Effective
June 28, 2023, the Company completed a 1-for-5 reverse stock split of its outstanding common stock upon the filing of the Company’s
Third Amended and Restated Articles of Incorporation with the Florida Secretary of State. No fractional shares were issued in connection
with the reverse stock split, and all such fractional shares resulting from the reverse stock split were rounded up to the nearest whole
number. The shares issuable upon the exercise of our outstanding options and warrants, and the exercise prices of such options and warrants,
have been adjusted to reflect the reverse stock split.
IPO
stock issuances
At
IPO, 1,275,000 shares of the Company’s common stock were issued at a price of $7.00 per share which resulted in gross proceeds
of $8.9 million and net proceeds of $7.7 million to the Company after the underwriter discount but before other IPO related expenses.
Additionally,
the Company issued its investor relations firm $0.25 million worth of restricted common stock upon closing of the IPO, which resulted
in issuance of 35,715 shares of stock.
Stock-based
compensation
The
Company may grant options under its 2022 Omnibus Incentive Plan, as amended and restated (the “2022 Omnibus Plan”). The 2022
Omnibus Plan authorizes the grant of “incentive stock options” within the meaning of Section 422 of the Internal Revenue
Code, to the Company’s employees and any of its parent and subsidiary corporations’ employees, and for the grant of nonstatutory
stock options, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to the Company’s
employees, directors, and consultants and any of its future subsidiary corporations’ employees and consultants.
The
fair value of each option award is estimated on the grant date using the Black-Scholes valuation model that uses assumptions for expected
volatility, expected dividends, expected term, and the risk-free interest rate. Expected price volatility is based on the historical
volatilities of a peer group as the Company does not have a trading history for its shares prior to its IPO. Industry peers consist of
several public companies in the biotech industry similar to the Company in size, stage of life cycle and product indications. The Company
intends to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical
information regarding the volatility of the Company’s own stock price becomes available, or unless circumstances change such that
the identified companies are no longer similar to the Company, in which case, more suitable companies whose share prices are publicly
available would be utilized in the calculation.
Expected
term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of
the vesting term plus contract term. The risk-free rate is based on the 5-year U.S. Treasury yield curve in effect at the time of grant.
During
the nine months ended September 30, 2023, a total of 635,001 options to purchase Common Stock, with an aggregate fair market value of
approximately $2.75 million were granted to the Company’s executive officers and management, and consultants of the Company. Options
have a term of 10 years from the grant date. These option vest as follows: (i) executive officer options vested 100% on date of grant
and (ii) employee and consultant options vest 33.33% at 6 month anniversary of date of grant, 33.33% at 1 year anniversary at date of
grant and the remaining vest at two-year anniversary of date of grant.
The
following is option activity during the nine months ended September 30, 2023.
Schedule of Stock
option Activity
| | |
Number of shares | | |
Weighted average exercise price per share | | |
Aggregate intrinsic value | |
| Outstanding as January 1, 2023 | |
| 750,000 | | |
$ | 5.00 | | |
| | |
| Options granted | |
| 635,001 | | |
$ | 5.00 | | |
| | |
| Forfeitures | |
| (170,000 | ) | |
$ | 5.00 | | |
| | |
| Outstanding as September 30, 2023 | |
| 1,215,001 | | |
$ | 5.00 | | |
$ | - | |
The
estimated fair value of stock options on date of grant was $1.3 million. As of September 30, 2023, options exercisable totaled 760,004.
There are approximately $1.5 million of unrecognized compensation costs related to non-vested share-based compensation awards, which
will be expensed through 2025.
Key
assumptions used to value stock options during the nine months ended September 30, 2023 are as follows:
Schedule of Key Assumptions Used to Value Stock Options
| Expected price volatility | |
| 116.64 | % |
| Risk-free interest rate | |
| 4.42 | % |
| Weighted average fair values | |
$ | 5.384
- $ 5.631 | |
| Weighted average expected life in years | |
| 5-6 years | |
| Dividend yield | |
| - | |
Warrants
Bay
Shore Trust warrants
In
consideration of the line of credit provided by the Bay Shore Trust, the Company issued to the Bay Shore Trust a common stock purchase
warrant on April 28, 2023 giving the Bay Shore Trust the right to purchase up to 1,000,000 shares of common stock at an exercise price
of $5.00 per share. This warrant will expire five years after the date of grant.
The
fair value of the warrants were estimated on the grant date using the Black-Scholes valuation model and level 3 inputs based on assumptions
for expected volatility, expected dividends, expected term, and the risk-free interest rate, which resulted in $3.5 million of deferred
financing costs. This cost was recorded as deferred financing costs and additional paid in capital on the accompanying condensed balance
sheet and is amortized straight-line over the term of the line of credit (which is 24 months). Associated amortization of deferred finance
costs is recorded to interest expense on the condensed income statement of operations.
Key
assumptions used to value warrants during the nine months ended September 30, 2023 are as follows:
Schedule of Key Assumptions Used to Value Warrants
| Expected price volatility | |
| 88.01 | % |
| Risk-free interest rate | |
| 3.51 | % |
| Weighted average fair values | |
$ | 0.703 | |
| Weighted average expected life in years | |
| 5 years | |
| Dividend yield | |
| - | |
Underwriter
warrants
In
connection with the IPO, the Company issued 63,750 warrants to purchase common stock to the IPO underwriter (or its designees) at an
exercise price of $7.00 which will expire in the four-and-a-half-year period commencing six months after the commencement of sales in
the IPO. The warrants will be exercisable at any time and from time to time, in whole or in part, during the four-and-a-half-year period
commencing six months after the commencement of sales in the IPO. The warrants provide for registration rights (including a one-time
demand registration right and piggyback registration rights that expire 5 years from the commencement of sales of the offering) and customary
anti-dilution provisions as permitted under FINRA Rule 5110(g)(8).
Earnings
Per Share
During
the nine months ended September 30, 2023 and 2022, outstanding stock options and warrants of 2,215,001 and 750,000, respectively, were
not included in the computation of diluted earnings per share, because to do so would have had an antidilutive effect.
During
the three months ended September 30, 2023 and 2022, outstanding stock options, and warrants of 1,235,001 and 750,000, respectively, were
not included in the computation of diluted earnings per share, because to do so would have had an antidilutive effect.
|
Note
8. Stockholders’ equity:
Capital
stock
The
Company has the authority to issue 110,000,000 shares of capital stock, consisting of 100,000,000 shares of Common Stock and 10,000,000
shares of undesignated preferred stock, whose rights and privileges will be defined by the Board of Directors when a series of preferred
stock is designated.
Private
placement
During
the year ended December 31, 2022, the Company sold 3.2 million shares of Common Stock at $1.00 per share, net of offering costs of $0.3
million, resulting in net proceeds of $2.9 million.
2022
Omnibus Incentive Plan
In
June 2022, the Company’s Board of Directors adopted, and its stockholders approved, the Company’s 2022 Omnibus Incentive
Plan, (“2022 Omnibus Plan”). The 2022 Omnibus Plan authorizes the grant of incentive stock options, within the meaning of
Section 422 of the Internal Revenue Code, to the Company’s employees and any of its parent and subsidiary corporations’ employees,
and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units
and performance shares to the Company’s employees, directors, and consultants and any of its future subsidiary corporations’
employees and consultants.
The
2022 Omnibus Plan provides that 10,000,000 shares of the Company’s Common Stock are reserved for issuance under the 2022 Omnibus
Plan, all of which may be issued pursuant to the exercise of incentive stock options.
Stock-based
compensation
During
the year ended December 31, 2022, a total of 750,000 options to purchase Common Stock, with an aggregate fair market value of approximately
$2.7 million were granted to the Company’s Board of Directors, executive officers and management, and a consultant of the Company.
Options have a term of 10 years from the grant date. The Company’s option vesting structure is the following: (i) Board of Director
options vest 100% on date of grant, (ii) executive officer options vest 25% on date of grant and the remaining vest ratably over a three-year
period, and (iii) management, employee and consultant options vest 33.3% on date of grant and the remaining vest ratably over a two-year
period.
The
fair value of each option award is estimated on the grant date using the Black-Scholes valuation model that uses assumptions for expected
volatility, expected dividends, expected term, and the risk-free interest rate.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
Expected
price volatility is based on the historical volatilities of a peer group as the Company does not have a trading history for its shares.
Industry peers consist of several public companies in the biotech industry similar to the Company in size, stage of life cycle and product
indications. The Company intends to continue to consistently apply this process using the same or similar public companies until a sufficient
amount of historical information regarding the volatility of the Company’s own stock price becomes available, or unless circumstances
change such that the identified companies are no longer similar to the Company, in which case, more suitable companies whose share prices
are publicly available would be utilized in the calculation.
Expected
term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of
the vesting term plus contract term. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for
the period of the expected term.
The
key assumptions used in determining the fair value of options granted during the year ended December 31, 2022 follows:
Schedule of Key Assumptions Used to Value Warrants
| Expected price volatility | |
| 84.42 | % |
| Risk-free interest rate | |
| 3.38 | % |
| Fair value of common stock | |
$ | 1.00 | |
| Minimum and maximum average expected life in years | |
| 5-6.50 years | |
| Dividend yield | |
| - | |
Option
activity during the year ended December 31, 2022 was as follows:
Schedule of Stock
option Activity
| | |
Number of shares | | |
Weighted average exercise price per share | | |
Aggregate intrinsic value | |
| Outstanding as January 1, 2022 | |
| - | | |
| | | |
| | |
| Options granted | |
| 750,000 | | |
$ | 5.00 | | |
| | |
| Outstanding as December 31, 2022 | |
| 750,000 | | |
$ | 5.00 | | |
| - | |
As
of December 31, 2022, options exercisable totaled 280,000. There are approximately $1.4 million of unrecognized compensation costs related
to non-vested share-based compensation awards, which will be expensed through 2025.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Employment Agreements
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Employment Agreements |
|
|
| Employment Agreements |
Note
9. Employment Agreements:
Erez
Aminov
On
April 28, 2023, the Company entered into an employment agreement with Mr. Erez Aminov pursuant to which Mr. Aminov serves as the Company’s
Chief Executive Officer on a full-time basis. Mr. Aminov’s employment agreement provides that his employment will be on an at-will
basis and can be terminated by either Mr. Aminov or the Company at any time and for any reason. Under the agreement, Mr. Aminov will
receive an initial base salary of $0.11 million per year. In the event that Mr. Aminov’s employment is terminated by the company
without “Cause” or is terminated by Mr. Aminov for “Good Reason”, Mr. Aminov will be entitled to severance compensation
in the form of salary continuation for a period of three months (subject to Mr. Aminov executing and delivering a customary general release
in favor of the company).
On
August 17, 2023, Mr. Aminov received a $0.12
million cash bonus net of federal, state, local
and income taxes related to the successful completion of the IPO.
On
August 28, 2023, the Company amended Mr. Aminov’s employment agreement to increase his yearly compensation from its current amount
of $0.11 million to $0.2 million per year, effective August 1, 2023.
Michelle
Yanez
On
April 28, 2023, the Company entered into an employment agreement with Ms. Michelle Yanez pursuant to which Ms. Yanez serves as the Company’s
Chief Financial Officer on a full-time basis. Ms. Yanez’s employment agreement provides that her employment will be on an at-will
basis and can be terminated by either Ms. Yanez or the company at any time and for any reason. Under the agreement, Ms. Yanez will receive
an initial base salary of $0.17 million per year. In the event that her employment is terminated by the company without “Cause”
or is terminated by Ms. Yanez for “Good Reason”, Ms. Yanez will be entitled to severance compensation in the form of salary
continuation for a period of three months (subject to Ms. Yanez executing and delivering a customary general release in favor of the
company).
On
August 17, 2023, Ms. Yanez received a $0.05 million cash bonus net of federal, state, local and income taxes related to the
successful completion of the IPO.
Chris
Chapman
On
April 28, 2023, the Company entered into an employment agreement with Dr. Chris Chapman pursuant to which Dr. Chapman serves as the Company’s
Executive Chairman. Dr. Chapman’s employment agreement provides that his employment will be on a part-time basis whereby Dr. Chapman
will devote 50% of his full business time and effort to the business and affairs of the company, and it further provides that such employment
will be on an at-will basis and can be terminated by either Dr. Chapman or the company at any time and for any reason. Under the agreement,
Dr. Chapman will receive an initial base salary of $0.15 million per year. In the event that Dr. Chapman’s employment is terminated
by the company without “Cause” or is terminated by Dr. Chapman for “Good Reason”, Dr. Chapman will be entitled
to severance compensation in the form of salary continuation for a period of three months (subject to Dr. Chapman executing and delivering
a customary general release in favor of the company).
On
August 17, 2023, Dr. Chapman received a $0.05
million cash bonus net of federal, state, local
and income taxes related to the successful completion of the IPO.
On
August 28, 2023, the Company amended Dr. Chapman’s employment agreement to indicate that he works part-time on an as needed
basis for the Corporation, rather than fifty percent (50%) of the time, effective August 1st, 2023.
On October 13, 2023, the Company amended Dr. Chapman’s employment agreement to reflect a temporary reduction
in his compensation from $0.15 million per year to $0.05 million per year, to extend for a period of 90 days. After the 90-day period,
Dr. Chapman’s compensation shall be reinstated to the amount in his employment agreement of $0.15 million per year.
Christos
Nicholoudis
On
April 28, 2023, the Company entered into an employment agreement with Christos Nicholoudis pursuant to which Mr. Nicholoudis serves as
the Company’s General Counsel. Mr. Nicholoudis’ employment agreement provides that Mr. Nicholoudis will devote 50% of his
full business time and effort to the business and affairs of the company, and it further provides that such employment will be on an
at-will basis and can be terminated by either Mr. Nicholoudis or the company at any time and for any reason. Under the agreement, Mr.
Nicholoudis will receive an initial base salary of $0.075 million per year. In the event that Mr. Nicholoudis employment is terminated
by the company without “Cause” or is terminated by Mr. Nicholoudis for “Good Reason”, Mr. Nicholoudis will be
entitled to severance compensation in the form of salary continuation for a period of three months (subject to Mr. Nicholoudis executing
and delivering a customary general release in favor of the company).
On
August 17, 2023, Mr. Nicholoudis received a $0.025 million cash bonus net of federal, state, local and income taxes related to the successful
completion of the IPO.
|
Note
9 – Consulting and employment agreements:
Employment Agreements
On
April 1, 2022, the Company entered into a Consulting Agreement with Dr. Chapman pursuant to which he provided regulatory and drug development
consulting services to the Company on an as-requested basis. Pursuant to the Consulting Agreement, he was to be paid a one-time fee of
$0.1 million upon the completion of the anticipated offering (of which $0.05 million was prepaid in in the first quarter of 2022) plus
a monthly fee of $0.02 million thereafter. The monthly fee was to begin upon the completion of the offering. He was also reimbursed for
reasonable out-of-pocket expenses incurred in connection with his duties under the Consulting Agreement. The agreement had a term of
one year with an automatic one-year extension, provided that either party could terminate the agreement without cause upon 30-days prior
written notice.
In
his capacity as a consultant, Dr. Chapman was also granted on June 15, 2022, an option to purchase up to 200,000 shares of the Company’s
common stock at an exercise price of $5.00 per share. This option was granted under the Company’s 2022 Omnibus Plan and vested
as to 25% of the option shares on the date of grant, with the balance vesting in one-third increments on each of the three successive
anniversaries of the grant date. Any unvested portion of the option will vest in full upon a “change of control” of our company
within the meaning of the 2022 Omnibus Plan. The option has a term of 10-years, subject to earlier termination upon certain terminations
of Dr. Chapman’s position as a consultant to the Company. In his capacity as a Board Director, Dr. Chapman was also granted on
June 15, 2022, an option to purchase up to 20,000 shares of the Company’s common stock at an exercise price of $5.00 per share.
This option was granted under the Company’s 2022 Omnibus Plan and vested as to 100% of the option shares on the date of grant.
The option has a term of 10-years, subject to earlier termination upon certain terminations of Dr. Chapman’s position as a director
of the Company.
|
| X |
- References
+ Details
| Name: |
MIRA_DisclosureEmploymentAgreementsAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEmployment Agreements Disclosure [Text Block]
| Name: |
MIRA_EmploymentAgreementsDisclosureTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Subsequent events
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent events |
Note
10 – Subsequent events:
The
Company has evaluated subsequent events through April 4, 2023, in connection with the preparation of these financial statements, which
is the date the financial statements were available to be issued.
Reverse
Stock Split
Effective
June 28, 2023, the Company completed a reverse stock split of its outstanding common stock upon the filing of the Company’s Third
Amended and Restated Articles of Incorporation with the Florida Secretary of State. No fractional shares were or will be issued in connection
with the reverse stock split, and all such fractional shares resulting from the reverse stock split were and will be rounded up to the
nearest whole number. The shares issuable upon the exercise of our outstanding options and warrants, and the exercise prices of such
options and warrants, have been adjusted to reflect the reverse stock split. Unless otherwise noted, the share and per share information
in this prospectus reflects the reverse stock split.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Accounts payable and accrued liabilities
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Payables and Accruals [Abstract] |
|
| Accounts payable and accrued liabilities |
Note
3 Accounts payable and accrued liabilities:
The
following table represents the components of accounts payable and accrued liabilities as of:
Schedule
of Accounts Payable And Accrued Liabilities
| | |
September 30, 2023 | | |
December 31, 2022 | |
| Trade accounts payable | |
$ | 510,688 | | |
$ | 789,204 | |
| Pre-clinical research and toxicology studies | |
| 254,293 | | |
| - | |
| Accrued other | |
| 14,592 | | |
| 22,534 | |
| Accounts
payable and accrued liabilities | |
$ | 779,573 | | |
$ | 811,738 | |
|
| X |
- DefinitionThe entire disclosure for accounts payable and accrued liabilities at the end of the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a),20,24)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Description of business and summary of significant accounting policies (Policies)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Accounting Policies [Abstract] |
|
|
| Overview |
Overview
MIRA
Pharmaceuticals, Inc. (“MIRA” or the “Company” and formerly known as MIRA1a Therapeutics, Inc.) was formed in
September 2020 and is a Florida-based pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting a broad range of neurologic and neuropsychiatric disorders. The Company’s
novel oral pharmaceutical marijuana, MIRA1a, is currently under investigation for treating adult patients suffering from anxiety and cognitive decline,
often associated with early-stage dementia. MIRA1a, if approved by the FDA, could mark a significant advancement in addressing various
neuropsychiatric, inflammatory, and neurologic diseases and disorders.
The
Company has an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to
potentially deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD)
and major depressive disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered a controlled
substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
Substantive
operations began in late 2020 and the Company’s Investigative New Drug application is anticipated to be filed with the U.S. Food
and Drug Administration (“FDA”) end of third quarter 2024. The Company owns U.S. Patent 10,787,675 B2, titled “Purified
Synthetic Marijuana and Methods of Treatment by Administering Same,” which covers the MIRA1a compound as a new molecular entity
as well as pharmaceutical formulations of the compound and methods of treating Alzheimer’s disease, anxiety, depression, and addictions.
The
accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America
(“GAAP”).
As
used herein, the Company’s Common Stock, par value $0.0001 per share, is referred to as the “Common Stock” and the
Company’s preferred stock, par value $0.0001 per share, is referred to as the “Preferred Stock”.
|
Overview
MIRA
Pharmaceuticals, Inc. (“MIRA” or the “Company” and formerly known as MIRA1a Therapeutics, Inc.) was formed in
September 2020 and is a Florida-based pre-clinical-stage pharmaceutical development company with two neuroscience programs targeting
a broad range of neurologic and neuropsychiatric disorders. The Company’s novel oral pharmaceutical marijuana, MIRA1a, is currently
under investigation for treating adult patients suffering from anxiety and cognitive decline, often associated with early-stage dementia.
MIRA1a, if approved by the FDA, could mark a significant advancement in addressing various neuropsychiatric, inflammatory, and neurologic
diseases and disorders.
The
Company has an exclusive licensing agreement for Ketamir-2, a unique, patent pending novel oral ketamine analog under investigation to
potentially deliver ultra-rapid antidepressant effects, providing hope for individuals battling treatment-resistant depression (TRD)
and major depressive disorder with suicidal ideation (MDSI).
The
U.S. Drug Enforcement Administration (DEA)’s scientific review of MIRA1a and Ketamir-2 concluded that neither would be considered
a controlled substance or listed chemical under the Controlled Substances Act (CSA) and its governing regulations.
Substantive
operations began in late 2020 and the Company’s Investigative New Drug application is anticipated to be filed with the U.S. Food
and Drug Administration (“FDA”) end of first quarter 2024. The Company owns U.S. Patent 10,787,675 B2, titled “Purified
Synthetic Marijuana and Methods of Treatment by Administering Same,” which covers the MIRA1a compound as a new molecular entity
as well as pharmaceutical formulations of the compound and methods of treating Alzheimer’s disease, anxiety, depression, and addictions.
Foreign patent applications covering MIRA1a, and its therapeutic uses are pending in Australia, Canada, China, Europe, Israel, Japan,
and South Korea.
The
accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America
(“GAAP”).
As
used herein, the Company’s Common Stock, par value $0.0001 per share, is referred to as the “Common Stock” and the
Company’s preferred stock, par value $0.0001 per share, is referred to as the “Preferred Stock”.
|
| Pending transactions |
|
Pending
transactions
The
Company is in the process of preparing for an initial public offering and expects to be listed under the NASDAQ symbol “MIRA.”
The transaction is expected to be complete in second half of 2023. The Company incurred $0.04 million and $0.1 million of legal costs,
during the years ended December 31, 2022 and December 31, 2021, respectively, associated with the offering, which have been recorded
as deferred offering costs in the accompanying balance sheets. These deferred offering costs will be derecognized as a reduction in offering
proceeds when the offering closes. However, there can be no guarantees that the Company will be successful in completing the proposed
transaction and ultimately listing on the NASDAQ.
|
| Income taxes |
Income
taxes
The
Company is taxed as a C corporation. Deferred tax assets and liabilities are recognized for the future tax consequences attributable
to differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases. Deferred
tax assets are recognized for temporary differences that will result in deductible amounts in future years and for loss carryovers. A
valuation allowance is recognized regarding deferred tax assets, if any, if it is more likely than not that some portion of the deferred
tax asset will not be realized.
|
Income
taxes
The
Company is a C corporation. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences
between the financial statement carrying amount of existing assets and liabilities and their respective tax bases. Deferred tax assets
are recognized for temporary differences that will result in deductible amounts in future years and for loss carryovers. A valuation
allowance is recognized regarding deferred tax assets, if any, if it is more likely than not that some portion of the deferred tax asset
will not be realized.
MIRA
Pharmaceuticals, Inc.
NOTES
TO THE FINANCIAL STATEMENTS
DECEMBER
31, 2022, AND DECEMBER 31, 2021
|
| Research and development expenses |
Research
and development expenses
Research
and development costs are expensed in the period in which they are incurred and include the expenses paid to third parties, such as contract
research organizations and consultants, who conduct research and development activities on behalf of the Company. Patent-related costs,
including registration costs, documentation costs and other legal fees associated with the application, are expensed in the period in
which they are incurred.
|
Research
and development expenses
Research
and development costs are expensed in the period in which they are incurred and include the expenses paid to third parties, such as contract
research organizations and consultants, who conduct research and development activities on behalf of the Company.
|
| Use of estimates |
Use
of estimates
The
preparation of financial statements in accordance with generally accepted accounting principles in the United States of America requires
the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the
disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the
reporting period. Actual results may differ from such estimates and such differences could be material.
|
Use
of estimates
The
preparation of financial statements in accordance with generally accepted accounting principles in the United States of America
requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and
liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported
amounts of expenses during the reporting period. Actual results may differ from such estimates and such differences could be
material.
|
| Cash |
Cash
The
Company maintains cash balances with financial institutions that management believes are of high credit quality. The Company’s
cash account at times may exceed federally insured limits. The Company has not experienced any losses in such accounts. The Company believes
it is not exposed to any significant credit risk from its cash account.
|
Cash
The
Company maintains cash balances with financial institutions that management believes are of high credit quality. The Company’s
cash account at times may exceed federally insured limits. The Company has not experienced any losses in such accounts. The Company believes
it is not exposed to any significant credit risk from its cash account.
|
| Stock-based compensation |
Stock-based
compensation
The
Company accounts for stock-based compensation under the provisions of FASB ASC 718, “Compensation - Stock Compensation”,
which requires the measurement and recognition of compensation expense for all stock-based awards made to employees, directors and consultants
based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using
the Black-Scholes model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the
requisite service periods using the straight-line method. The Company has elected to account for forfeiture of stock-based awards as
they occur.
|
Stock-based
compensation
The
Company accounts for stock-based compensation under the provisions of FASB ASC 718, “Compensation - Stock Compensation”,
which requires the measurement and recognition of compensation expense for all stock-based awards made to employees, directors and consultants
based on estimated fair values on the grant date. The Company estimates the fair value of stock-based awards on the date of grant using
the Black-Scholes model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the
requisite service periods using the straight-line method. The Company has elected to account for forfeiture of stock-based awards as
they occur.
|
| Change in Accounting Principle |
|
Change
in Accounting Principle
In
February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes existing guidance for accounting for leases under Topic
840, Leases. The FASB also subsequently issued additional ASUs which amend and clarify Topic 842. The most significant change in the
new leasing guidance is the requirement to recognize right-to-use (ROU) assets and lease liabilities for operating leases on the balance
sheet.
The
Company adopted these ASUs effective January 1, 2022 using the modified retrospective approach. As a result of adopting these ASUs, the
Company recorded ROU assets and lease liabilities of approximately $1.0 million and $0.4 million, respectively. Adoption of the new standard
did not materially impact the Company’s net income and had no impact on cash flows.
|
| Initial Public Offering |
Initial
Public Offering
On
August 7, 2023, the Company closed its initial public offering consisting of 1,275,000 shares at a price of $7.00 per share for approximately
$8.9 million in gross proceeds. After deducting the underwriting commission and other deferred offering expenses totaling $1.2 million,
the net proceeds to the Company were $7.7 million (the “IPO”).
The
shares were offered and sold pursuant to the Company’s Registration Statement on Form S-1, as amended (File No. 333-273024), originally
filed with the Securities and Exchange Commission (the “SEC”) on June 29, 2023 (the “Registration Statement”)
and the final quarterly report filed with the Commission pursuant to Rule 424(b)(4) of the Securities Act of 1933, as amended. The Registration
Statement was declared effective by the Commission on August 2, 2023. The common stock began trading on The Nasdaq Capital Market on
August 3, 2023 under the symbol “MIRA”. The closing of the IPO occurred on August 7, 2023.
As
of the completion of the IPO, among other things, certain of the Company’s then-outstanding convertible debt was converted into
shares of common stock. See Note 5 for more information.
|
|
| Leases |
Leases
The
Company accounts for leases under the provisions of FASB ASC Topic 842, “Leases”, which requires the Company to recognize
right-to-use (ROU) assets and lease liabilities for operating leases on the balance sheet.
|
|
| Fair Value of Financial Instruments |
Fair
Value of Financial Instruments
The
Company measures the fair value of financial instruments in accordance with GAAP which defines fair value, establishes a framework for
measuring fair value, and expands disclosures about fair value measurements.
GAAP
defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
GAAP also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use
of unobservable inputs when measuring fair value. The Company considers the carrying amount of deferred offering costs to approximate
fair value due to short-term nature of this instrument. GAAP describes three levels of inputs that may be used to measure fair value:
Level
1 – quoted prices in active markets for identical assets or liabilities.
Level
2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable.
Level
3 – inputs that are unobservable (for example cash flow modeling inputs based on assumptions).
|
|
| X |
- DefinitionChange in Accounting Principle [Policy Text Block]
| Name: |
MIRA_ChangeInAccountingPrinciplePolicyTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionInitial Public Offering [Policy Text Block]
| Name: |
MIRA_InitialPublicOfferingPolicyTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPending Transactions [Policy Text Block]
| Name: |
MIRA_PendingTransactionsPolicyTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for salaries, bonuses, incentive awards, postretirement and postemployment benefits granted to employees, including equity-based arrangements; discloses methodologies for measurement, and the bases for recognizing related assets and liabilities and recognizing and reporting compensation expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (b),(f(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_CompensationRelatedCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Leases (Tables)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Leases |
|
|
| Schedule of Lease Expense |
The
components of lease expense were as follows:
Schedule of Lease Expense
| | |
| | |
| |
| | |
Nine months ended September 30, | |
| Lease Costs | |
2023 | | |
2022 | |
| Operating Lease Cost | |
| | | |
| | |
| Operating Lease | |
$ | 200,283 | | |
$ | 333,046 | |
| Variable Lease Costs | |
| 311,126 | | |
| 637,420 | |
| Total Lease Cost | |
$ | 511,409 | | |
$ | 970,466 | |
|
The
components of lease expense were as follows:
Schedule of Lease Expense
| |
2022 | | |
2021 | |
| | |
Year ended December 31, | |
| Lease Costs | |
2022 | | |
2021 | |
| Operating Lease Cost | |
| | | |
| | |
| Operating Lease | |
$ | 657,797 | | |
$ | - | |
| Variable Lease Costs | |
| 1,112,913 | | |
| - | |
| Total Lease Cost | |
$ | 1,770,710 | | |
$ | - | |
|
| Schedule of Cash Flow Information Related to Leases |
Supplemental
cash flow information related to leases were as follows:
Schedule
of Cash Flow Information Related to Leases
| | |
| | |
| |
| | |
Nine months ended September 30, | |
| Other Lease Information | |
2023 | | |
2022 | |
| Cash paid for amounts included in the measurement of lease liabilities | |
| | | |
| | |
| Operating cash flows from operating leases | |
$ | 511,409 | | |
$ | 970,466 | |
|
Supplemental
cash flow information related to leases were as follows:
Schedule
of Cash Flow Information Related to Leases
| Other Lease Information | |
2022 | | |
2021 | |
| | |
Year ended December 31, | |
| Other Lease Information | |
2022 | | |
2021 | |
| Cash paid for amounts included in the measurement of lease liabilities | |
| | | |
| | |
| Operating cash flows from operating leases | |
$ | 626,304 | | |
$ | - | |
|
| Schedule of Remaining Weighted-average Lease Term and Weighted-average Discount Rate |
Schedule
of Remaining Weighted-average Lease Term and Weighted-average Discount Rate
| | |
Nine months ended September 30, | |
| | |
2023 | | |
2022 | |
| Lease Term and Discount | |
| | | |
| | |
| Weighted Average remaining lease term | |
| 1.79 years | | |
| 3 years | |
| Weighted Average discount rate | |
| 5.0 | % | |
| 5.0 | % |
|
Schedule
of Remaining Weighted-average Lease Term and Weighted-average Discount Rate
| | |
Year
ended December 31, | |
| | |
2022 | | |
2021 | |
| Lease Term and Discount | |
| | | |
| | |
| Weighted Average remaining lease term | |
| 0.53 years | | |
| - | |
| Weighted Average discount rate | |
| 5.0 | % | |
| - | |
|
| Schedule of Maturity of Lease Liabilities |
Future
minimum lease payments under non-cancellable leases as of September 30, 2023 were as follows:
Schedule
of Maturity
of Lease Liabilities
Maturity
of Lease Liabilities
| | |
September 30, 2023 | |
| Remainder of 2023 | |
$ | 20,722 | |
| 2024 | |
| 74,402 | |
| 2025 | |
| 17,444 | |
| Total Lease payments | |
| 112,568 | |
| Less: Interest | |
| (3,711 | ) |
| Present Value of Lease Liabilities | |
$ | 108,857 | |
|
Future
minimum lease payments under non-cancellable leases as of December 31, 2022 were as follows:
Schedule
of Maturity
of Lease Liabilities
| Maturity of Lease Liabilities | |
| |
| | |
December 31, 2022 | |
| 2023 | |
| 281,050 | |
| 2024 | |
| 69,309 | |
| 2025 | |
| 17,444 | |
| Total Lease payments | |
| 367,803 | |
| Less: Interest | |
| (9,634 | ) |
| Present Value of Lease Liabilities | |
| 358,169 | |
|
| X |
- References
+ Details
| Name: |
MIRA_DisclosureLeasesAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLease Quantitative Disclosure [Table Text Block]
| Name: |
MIRA_LeaseQuantitativeDisclosureTableTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLessee Cash Flow Information Of Leases [Table Text Block]
| Name: |
MIRA_LesseeCashFlowInformationOfLeasesTableTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Income taxes (Tables)
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Schedule of Components of Net Deferred Tax Assets |
The
significant components of the Company’s net deferred tax assets are as follows as of December 31:
Schedule
of Components of Net Deferred Tax Assets
| | |
2022 | | |
2021 | |
| | |
December 31, | |
| | |
2022 | | |
2021 | |
| Deferred tax assets | |
| | | |
| | |
| Net operating loss carry-forward | |
$ | 1,061,300 | | |
$ | 572,355 | |
| Section 174 Qualified Research Expenditures | |
| 388,230 | | |
| - | |
| Stock compensation | |
| 330,633 | | |
| - | |
| ROU liability | |
| 91,333 | | |
| - | |
| Other | |
| 6,120 | | |
| - | |
| Deferred tax assets Gross | |
| 1,877,616 | | |
| 572,355 | |
| Less: valuation allowance | |
| (1,784,880 | ) | |
| (572,355 | ) |
| Total deferred tax asset | |
| 92,736 | | |
| - | |
| Deferred tax liabilities | |
| | | |
| | |
| ROU asset | |
| (92,736 | ) | |
| - | |
| Total net deferred tax asset | |
$ | - | | |
$ | - | |
|
| Schedule of Components of Provision For Income Taxes |
The
components of the provision for income taxes consist of the following:
Schedule
of Components of Provision For Income Taxes
| | |
2022 | | |
2021 | |
| Deferred tax: | |
| | | |
| | |
| Deferred | |
| (1,212,525 | ) | |
| (555,017 | ) |
| Change in valuation allowance | |
| 1,212,525 | | |
| 555,017 | |
| Total deferred | |
| - | | |
| - | |
| Total provision for income taxes | |
$ | - | | |
$ | - | |
|
| X |
- DefinitionTabular disclosure of the components of income tax expense attributable to continuing operations for each year presented including, but not limited to: current tax expense (benefit), deferred tax expense (benefit), investment tax credits, government grants, the benefits of operating loss carryforwards, tax expense that results from allocating certain tax benefits either directly to contributed capital or to reduce goodwill or other noncurrent intangible assets of an acquired entity, adjustments of a deferred tax liability or asset for enacted changes in tax laws or rates or a change in the tax status of the entity, and adjustments of the beginning-of-the-year balances of a valuation allowance because of a change in circumstances that causes a change in judgment about the realizability of the related deferred tax asset in future years. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 9
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
| Name: |
us-gaap_ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Stockholders’ equity (Tables)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Equity [Abstract] |
|
|
| Schedule of Key Assumptions Used to Value Warrants |
Key
assumptions used to value warrants during the nine months ended September 30, 2023 are as follows:
Schedule of Key Assumptions Used to Value Warrants
| Expected price volatility | |
| 88.01 | % |
| Risk-free interest rate | |
| 3.51 | % |
| Weighted average fair values | |
$ | 0.703 | |
| Weighted average expected life in years | |
| 5 years | |
| Dividend yield | |
| - | |
|
The
key assumptions used in determining the fair value of options granted during the year ended December 31, 2022 follows:
Schedule of Key Assumptions Used to Value Warrants
| Expected price volatility | |
| 84.42 | % |
| Risk-free interest rate | |
| 3.38 | % |
| Fair value of common stock | |
$ | 1.00 | |
| Minimum and maximum average expected life in years | |
| 5-6.50 years | |
| Dividend yield | |
| - | |
|
| Schedule of Stock option Activity |
The
following is option activity during the nine months ended September 30, 2023.
Schedule of Stock
option Activity
| | |
Number of shares | | |
Weighted average exercise price per share | | |
Aggregate intrinsic value | |
| Outstanding as January 1, 2023 | |
| 750,000 | | |
$ | 5.00 | | |
| | |
| Options granted | |
| 635,001 | | |
$ | 5.00 | | |
| | |
| Forfeitures | |
| (170,000 | ) | |
$ | 5.00 | | |
| | |
| Outstanding as September 30, 2023 | |
| 1,215,001 | | |
$ | 5.00 | | |
$ | - | |
|
Option
activity during the year ended December 31, 2022 was as follows:
Schedule of Stock
option Activity
| | |
Number of shares | | |
Weighted average exercise price per share | | |
Aggregate intrinsic value | |
| Outstanding as January 1, 2022 | |
| - | | |
| | | |
| | |
| Options granted | |
| 750,000 | | |
$ | 5.00 | | |
| | |
| Outstanding as December 31, 2022 | |
| 750,000 | | |
$ | 5.00 | | |
| - | |
|
| Schedule of Key Assumptions Used to Value Stock Options |
Key
assumptions used to value stock options during the nine months ended September 30, 2023 are as follows:
Schedule of Key Assumptions Used to Value Stock Options
| Expected price volatility | |
| 116.64 | % |
| Risk-free interest rate | |
| 4.42 | % |
| Weighted average fair values | |
$ | 5.384
- $ 5.631 | |
| Weighted average expected life in years | |
| 5-6 years | |
| Dividend yield | |
| - | |
|
|
| X |
- DefinitionSchedule Of Share Based Payment Award Warrant Valuation Assumptions [Table Text Block]
| Name: |
MIRA_ScheduleOfShareBasedPaymentAwardWarrantValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Accounts payable and accrued liabilities (Tables)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Payables and Accruals [Abstract] |
|
| Schedule of Accounts Payable And Accrued Liabilities |
The
following table represents the components of accounts payable and accrued liabilities as of:
Schedule
of Accounts Payable And Accrued Liabilities
| | |
September 30, 2023 | | |
December 31, 2022 | |
| Trade accounts payable | |
$ | 510,688 | | |
$ | 789,204 | |
| Pre-clinical research and toxicology studies | |
| 254,293 | | |
| - | |
| Accrued other | |
| 14,592 | | |
| 22,534 | |
| Accounts
payable and accrued liabilities | |
$ | 779,573 | | |
$ | 811,738 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the (a) carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business (accounts payable); (b) other payables; and (c) accrued liabilities. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). An alternative caption includes accrued expenses.
| Name: |
us-gaap_ScheduleOfAccountsPayableAndAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.4
Description of business and summary of significant accounting policies (Details Narrative) - USD ($)
|
|
12 Months Ended |
|
Aug. 07, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
Sep. 30, 2023 |
| Finite lived patents gross |
|
$ 10,787,675
|
|
$ 10,787,675
|
| Common stock, par value |
|
$ 0.0001
|
$ 0.0001
|
$ 0.0001
|
| Preferred stock, par value |
|
$ 0.0001
|
$ 0.0001
|
$ 0.0001
|
| Legal fees |
|
$ 40,000.00
|
$ 100,000
|
|
| Operating lease liabilites |
|
358,169
|
|
$ 108,857
|
| Issuance of shares |
1,275,000
|
|
|
|
| Share price |
$ 7.00
|
|
|
|
| Proceeds of issuance of IPO |
$ 8,900,000
|
|
|
|
| IPO [Member] |
|
|
|
|
| Proceeds of issuance of IPO |
7,700,000
|
|
|
|
| Underwriting commission and other offering expenses |
$ 1,200,000
|
|
|
|
| Revision of Prior Period, Accounting Standards Update, Adjustment [Member] |
|
|
|
|
| Operating lease asset |
|
1,000,000.0
|
|
|
| Operating lease liabilites |
|
$ 400,000
|
|
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionGross carrying amount before accumulated amortization as of the balance sheet date of the costs pertaining to the exclusive legal rights granted to the owner of the patent to exploit an invention or a process for a period of time specified by law. Such costs may have been expended to directly apply and receive patent rights, or to acquire such rights. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedPatentsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense provided in the period for legal costs incurred on or before the balance sheet date pertaining to resolved, pending or threatened litigation, including arbitration and mediation proceedings. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_LegalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash outflow for cost incurred directly with the issuance of an equity security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RestatementAxis=srt_RevisionOfPriorPeriodAccountingStandardsUpdateAdjustmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
Liquidity and capital resources (Details Narrative) - USD ($)
|
3 Months Ended |
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
| Cash |
$ 5,868,330
|
|
$ 5,868,330
|
|
$ 350,978
|
$ 2,809,552
|
| Net cash in operations |
|
|
3,497,388
|
$ 4,629,323
|
5,604,759
|
1,376,156
|
| Stockholders deficit |
8,115,311
|
|
8,115,311
|
|
(596,232)
|
2,261,358
|
| Research and development expense |
$ 1,015,252
|
$ 714,968
|
$ 1,185,839
|
$ 1,466,708
|
2,351,465
|
$ 684,447
|
| Private Placement [Member] |
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
| Research and development expense |
|
|
|
|
$ 3,200,000
|
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_PrivatePlacementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
Line of credit, related party (Details Narrative) - USD ($)
|
|
|
1 Months Ended |
|
Aug. 14, 2023 |
Jul. 20, 2023 |
Apr. 30, 2023 |
May 31, 2021 |
Apr. 30, 2021 |
| Starwood Trust [Member] |
|
|
|
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
|
|
|
| Outstanding principal balance |
|
|
|
$ 5,000,000
|
|
| Debt instrument, maturity date |
|
|
|
May 10, 2024
|
|
| Interest rate |
|
|
|
5.00%
|
|
| Bay Shore Trust [Member] |
|
|
|
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
|
|
|
| Outstanding principal balance |
$ 1,000,000.0
|
$ 1,100,000
|
|
$ 200,000
|
$ 5,000,000
|
| Issuance of common stock |
|
157,170
|
1,000,000
|
|
|
| Exercise price |
|
|
$ 5.00
|
|
|
| Payment of accrued interest |
30,000.00
|
|
|
|
|
| Payment of accrued interest |
$ 0.01
|
|
|
|
|
| Bay Shore Trust [Member] | Minimum [Member] |
|
|
|
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
|
|
|
| Interest rate |
|
|
|
7.00%
|
|
| Bay Shore Trust [Member] | Maximum [Member] |
|
|
|
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
|
|
|
| Interest rate |
|
|
|
|
10.00%
|
| X |
- DefinitionRemaining debt instrument periodic payment interest.
| Name: |
MIRA_RemainingDebtInstrumentPeriodicPaymentInterest |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued in exchange for the original debt being converted in a noncash (or part noncash) transaction. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_DebtConversionConvertedInstrumentSharesIssued1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe price per share of the conversion feature embedded in the debt instrument. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-5
| Name: |
us-gaap_DebtInstrumentConvertibleConversionPrice1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionEffective interest rate for the funds borrowed under the debt agreement considering interest compounding and original issue discount or premium. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-6
| Name: |
us-gaap_DebtInstrumentInterestRateEffectivePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDate when the debt instrument is scheduled to be fully repaid, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(2))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the required periodic payments applied to interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentPeriodicPaymentInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_DefinedBenefitPlanDisclosureLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
Related party transactions (Details Narrative) - USD ($)
|
1 Months Ended |
9 Months Ended |
12 Months Ended |
Apr. 30, 2021 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Related Party Transaction [Line Items] |
|
|
|
|
|
| Lease charges |
$ 50,000.00
|
$ 511,409
|
$ 970,466
|
$ 1,770,710
|
|
| Related party. travel cost |
|
500,000
|
|
$ 1,700,000
|
$ 700,000
|
| Working capital |
|
1,060,000.00
|
|
|
|
| Related Party [Member] |
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
| Accounts payable |
|
$ 50,000.00
|
|
|
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lease cost recognized by lessee for lease contract. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.4
Schedule of Lease Expense (Details) - USD ($)
|
1 Months Ended |
9 Months Ended |
12 Months Ended |
Apr. 30, 2021 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Leases |
|
|
|
|
|
| Operating Lease |
|
$ 200,283
|
$ 333,046
|
$ 657,797
|
|
| Variable Lease Costs |
|
311,126
|
637,420
|
1,112,913
|
|
| Total Lease Cost |
$ 50,000.00
|
$ 511,409
|
$ 970,466
|
$ 1,770,710
|
|
| X |
- References
+ Details
| Name: |
MIRA_DisclosureLeasesAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lease cost recognized by lessee for lease contract. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of variable lease cost, excluded from lease liability, recognized when obligation for payment is incurred for finance and operating leases. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_VariableLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.4
| X |
- References
+ Details
| Name: |
MIRA_DisclosureLeasesAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.4
| X |
- References
+ Details
| Name: |
MIRA_DisclosureLeasesAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.4
Schedule of Maturity of Lease Liabilities (Details) - USD ($)
|
Sep. 30, 2023 |
Dec. 31, 2022 |
| Leases |
|
|
| Remainder of 2023 |
$ 20,722
|
$ 281,050
|
| 2024 |
74,402
|
69,309
|
| 2025 |
17,444
|
17,444
|
| Total Lease payments |
112,568
|
367,803
|
| Less: Interest |
(3,711)
|
(9,634)
|
| Present Value of Lease Liabilities |
$ 108,857
|
$ 358,169
|
| X |
- References
+ Details
| Name: |
MIRA_DisclosureLeasesAbstract |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease having initial or remaining lease term in excess of one year to be paid in remainder of current fiscal year. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.4
Schedule of Components of Net Deferred Tax Assets (Details) - USD ($)
|
Dec. 31, 2022 |
Dec. 31, 2021 |
| Income Tax Disclosure [Abstract] |
|
|
| Net operating loss carry-forward |
$ 1,061,300
|
$ 572,355
|
| Section 174 Qualified Research Expenditures |
388,230
|
|
| Stock compensation |
330,633
|
|
| ROU liability |
91,333
|
|
| Other |
6,120
|
|
| Deferred tax assets Gross |
1,877,616
|
572,355
|
| Less: valuation allowance |
(1,784,880)
|
(572,355)
|
| Total deferred tax asset |
92,736
|
|
| ROU asset |
(92,736)
|
|
| Total net deferred tax asset |
|
|
| X |
- DefinitionDeferred tax assets qualified research expenditures.
| Name: |
MIRA_DeferredTaxAssetsQualifiedResearchExpenditures |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDeferred tax assets tax deferred expense compensation and benefits right of use liability
| Name: |
MIRA_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsRightOfUseLiability |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsLiabilitiesNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of valuation allowance, of deferred tax asset attributable to deductible temporary differences, classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from share-based compensation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax liability attributable to taxable temporary differences from long-lived assets other than property, plant, and equipment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxLiabilitiesOtherFiniteLivedAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.4
v3.23.4
| X |
- DefinitionAmount, before allocation of valuation allowance, of deferred tax asset attributable to deductible interest carryforward. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetInterestCarryforward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.4
| X |
- DefinitionShare based compensation arrangement by share based payment award fair value assumptions average expected life years maximum.
| Name: |
MIRA_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsAverageExpectedLifeYearsMaximum |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare based compensation arrangement by share based payment award fair value assumptions average expected life yearsmMinimum.
| Name: |
MIRA_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsAverageExpectedLifeYearsMinimum |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare based compensation arrangement by share based payment award fair value assumptions weighted average fair values.
| Name: |
MIRA_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsWeightedAverageFairValues |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAgreed-upon price for the exchange of the underlying asset relating to the share-based payment award.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
Schedule of Stock option Activity (Details) - USD ($)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Equity [Abstract] |
|
|
| Number of shares, Outstanding beginning balance |
750,000
|
|
| Number of shares, Options granted |
635,001
|
750,000
|
| Weighted average exercise price per share, Options granted |
$ 5.00
|
$ 5.00
|
| Number of shares, Outstanding ending balance |
1,215,001
|
750,000
|
| Weighted average exercise price per share, outstanding ending balance |
$ 5.00
|
$ 5.00
|
| Aggregate intrinsic value, outstanding |
|
|
| Weighted average exercise price per share, Outstanding beginning balance |
$ 5.00
|
|
| Number of shares, forfeitures |
(170,000)
|
|
| Weighted average exercise price per share, forfeitures |
$ 5.00
|
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIntrinsic value of outstanding award under share-based payment arrangement. Excludes share and unit options.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.4
Stockholders’ equity (Details Narrative) - USD ($)
|
|
|
|
|
3 Months Ended |
9 Months Ended |
12 Months Ended |
Aug. 07, 2023 |
Jun. 28, 2023 |
Apr. 28, 2023 |
Jun. 15, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Authorized capital shares |
|
|
|
|
110,000,000
|
|
110,000,000
|
|
|
|
| Common stock, shares authorized |
|
|
|
|
100,000,000
|
|
100,000,000
|
|
95,000,000
|
95,000,000
|
| Undesignated preferred stock, shares authorized |
|
|
|
|
10,000,000
|
|
10,000,000
|
|
5,000,000
|
5,000,000
|
| Share-based compensation arrangement, options, outstanding |
|
|
|
|
|
|
635,001
|
|
750,000
|
|
| Share-based compensation options, granted value |
|
|
|
|
$ 2,750,000
|
|
$ 2,750,000
|
|
$ 2,700,000
|
|
| Option expiration period |
|
|
|
|
|
|
10 years
|
|
10 years
|
|
| Options exercisable |
|
|
|
|
760,004
|
|
760,004
|
|
280,000
|
|
| Unrecognized compensation costs |
|
|
|
|
$ 1,500,000
|
|
$ 1,500,000
|
|
$ 1,400,000
|
|
| Reverse stock split |
|
Effective
June 28, 2023, the Company completed a 1-for-5 reverse stock split of its outstanding common stock upon the filing of the Company’s
Third Amended and Restated Articles of Incorporation with the Florida Secretary of State
|
|
|
|
|
|
|
|
|
| Issuance of common stock, shares |
1,275,000
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance of common stock |
|
|
|
|
|
|
7,704,279
|
$ 2,619,000
|
$ 2,904,000
|
$ 4,500,000
|
| Shares issued, value |
|
|
|
|
$ 250,000
|
|
|
|
|
|
| Share-based compensation options, granted |
|
|
|
|
|
|
$ 1,300,000
|
|
|
|
| Share-Based Payment Arrangement, Option [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Computation of diluted earnings per share |
|
|
|
|
1,235,001
|
750,000
|
2,215,001
|
750,000
|
|
|
| IPO [Member] | Restricted Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock, shares |
|
|
|
|
|
|
35,715
|
|
|
|
| Shares issued, value |
|
|
|
|
|
|
$ 250,000
|
|
|
|
| Board of Directors Chairman [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Share-based payment award, vesting rights, percentage |
|
|
|
100.00%
|
|
|
|
|
100.00%
|
|
| Share-based payment award, vesting period |
|
|
|
10 years
|
|
|
|
|
|
|
| Executive Officer [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Share-based payment award, vesting rights, percentage |
|
|
|
|
|
|
100.00%
|
|
25.00%
|
|
| Employee And Consultant [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Share-based payment award, vesting rights, percentage |
|
|
|
|
|
|
33.33%
|
|
33.30%
|
|
| Share-based payment award, vesting period |
|
|
|
|
|
|
2 years
|
|
2 years
|
|
| Omnibus Plan [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Exercise of incentive stock options |
|
|
|
|
|
|
|
|
10,000,000
|
|
| Undesignated Preferred Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Undesignated preferred stock, shares authorized |
|
|
|
|
10,000,000
|
|
10,000,000
|
|
10,000,000
|
|
| Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Authorized capital shares |
|
|
|
|
|
|
|
|
110,000,000
|
|
| Common stock, shares authorized |
|
|
|
|
|
|
|
|
100,000,000
|
|
| Proceeds from Issuance or Sale of Equity |
|
|
|
|
|
|
|
|
$ 3,200,000
|
|
| Per share |
|
|
|
|
|
|
|
|
$ 1.00
|
|
| Offering costs |
|
|
|
|
|
|
|
|
$ 300,000
|
|
| Proceeds from Issuance of Private Placement |
|
|
|
|
|
|
|
|
$ 2,900,000
|
|
| Issuance of common stock, shares |
|
|
|
|
35,715
|
|
|
|
|
|
| Shares issued, value |
|
|
|
|
$ 4
|
|
|
|
|
|
| Common Stock [Member] | IPO [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock, shares |
|
|
|
|
|
|
1,275,000
|
|
|
|
| Shares issued price per share |
|
|
|
|
$ 7.00
|
|
$ 7.00
|
|
|
|
| Gross proceeds from IPO |
|
|
|
|
|
|
$ 8,900,000
|
|
|
|
| Proceeds from issuance of common stock |
|
|
|
|
|
|
7,700,000
|
|
|
|
| Warrant [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Share-based compensation arrangement, options, outstanding |
|
|
1,000,000
|
|
|
|
|
|
|
|
| Exercise price per share |
|
|
$ 5.00
|
|
|
|
|
|
|
|
| Deferred financing costs |
|
|
|
|
$ 3,500,000
|
|
$ 3,500,000
|
|
|
|
| Computation of diluted earnings per share |
|
|
|
|
1,235,001
|
750,000
|
2,215,001
|
750,000
|
|
|
| Underwriter Warrants [Member] |
|
|
|
|
|
|
|
|
|
|
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Exercise price per share |
|
|
|
|
$ 7.00
|
|
$ 7.00
|
|
|
|
| Warrants to purchase common stock |
|
|
|
|
63,750
|
|
63,750
|
|
|
|
| X |
- DefinitionProceeds from issuance initial public offering gross.
| Name: |
MIRA_ProceedsFromIssuanceInitialPublicOfferingGross |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of authorized capital units or capital shares. This element is relevant to issuers of face-amount certificates and registered investment companies.
| Name: |
us-gaap_CapitalUnitsAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred cost, excluding capitalized cost related to contract with customer; classified as noncurrent. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_DeferredCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSpecific incremental costs directly attributable to a proposed or actual offering of securities which are deferred at the end of the reporting period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 5.A)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480341/340-10-S99-1
| Name: |
us-gaap_DeferredOfferingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThis item represents the aggregate value of each identified investment accounted for under the equity method of accounting based on the quoted market price for those investments in common stock for which a quoted market price is available. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
| Name: |
us-gaap_EquityMethodInvestmentQuotedMarketValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's raising of capital via private rather than public placement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares authorized for issuance under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of fully vested and expected to vest exercisable options that may be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares purchased for issuance under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesPurchasedForAward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of vesting of award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionValue, after forfeiture, of shares granted under share-based payment arrangement. Excludes employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 30
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480513/718-10-30-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 30
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480843/718-30-35-1
| Name: |
us-gaap_StockGrantedDuringPeriodValueSharebasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the reverse stock split arrangement. Also provide the retroactive effect given by the reverse split that occurs after the balance sheet date but before the release of financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SAB Topic 4.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-4
| Name: |
us-gaap_StockholdersEquityReverseStockSplit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_BoardOfDirectorsChairmanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ExecutiveOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=MIRA_EmployeeAndConsultantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=MIRA_OmnibusPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=MIRA_UndesignatedPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=MIRA_UnderwriterWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
Employment Agreements (Details Narrative) - USD ($)
|
|
|
|
|
|
|
9 Months Ended |
12 Months Ended |
|
Oct. 13, 2023 |
Aug. 28, 2023 |
Aug. 17, 2023 |
Apr. 28, 2023 |
Jun. 15, 2022 |
Apr. 22, 2022 |
Sep. 30, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
Aug. 07, 2023 |
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Prepaid expense |
|
|
|
|
|
|
$ 202,817
|
|
|
|
| Share price |
|
|
|
|
|
|
|
|
|
$ 7.00
|
| Initial base salary per year |
|
|
|
|
|
|
|
$ (1,212,525)
|
$ (555,017)
|
|
| Consultant [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Purchase shares |
|
|
|
|
200,000
|
|
|
|
|
|
| Share price |
|
|
|
|
$ 5.00
|
|
|
|
|
|
| Share-based payment award, vesting rights, percentage |
|
|
|
|
25.00%
|
|
|
|
|
|
| Option termination period |
|
|
|
|
10 years
|
|
|
|
|
|
| Board of Directors Chairman [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Purchase shares |
|
|
|
|
20,000
|
|
|
|
|
|
| Share price |
|
|
|
|
$ 5.00
|
|
|
|
|
|
| Share-based payment award, vesting rights, percentage |
|
|
|
|
100.00%
|
|
|
100.00%
|
|
|
| Option termination period |
|
|
|
|
10 years
|
|
|
|
|
|
| Consulting And Employment Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| One time fee |
|
|
|
|
|
$ 100,000
|
|
|
|
|
| Prepaid expense |
|
|
|
|
|
50,000.00
|
|
|
|
|
| Monthly fee |
|
|
|
|
|
$ 20,000.00
|
|
|
|
|
| Employment Agreement [Member] | Erez Aminov [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Initial base salary per year |
|
|
|
$ 110,000
|
|
|
|
|
|
|
| Initial base salary per year |
|
|
$ 120,000
|
|
|
|
|
|
|
|
| Employment Agreement [Member] | Erez Aminov [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Officers compensation |
|
$ 110,000
|
|
|
|
|
|
|
|
|
| Employment Agreement [Member] | Erez Aminov [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Officers compensation |
|
$ 200,000
|
|
|
|
|
|
|
|
|
| Employment Agreement [Member] | Michelle Yanez [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Initial base salary per year |
|
|
|
170,000
|
|
|
|
|
|
|
| Initial base salary per year |
|
|
50,000.00
|
|
|
|
|
|
|
|
| Employment Agreement [Member] | Chris Chapman [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Initial base salary per year |
$ 150,000
|
|
|
150,000
|
|
|
|
|
|
|
| Initial base salary per year |
$ 50,000.00
|
|
50,000.00
|
|
|
|
$ 150,000
|
|
|
|
| Employment Agreement [Member] | Christos Nicholoudis [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Initial base salary per year |
|
|
|
$ 75,000.000
|
|
|
|
|
|
|
| Initial base salary per year |
|
|
$ 25,000.000
|
|
|
|
|
|
|
|
| X |
- DefinitionRepresents the amortization to expense from discounts on short-term negotiable time drafts drawn on and accepted by an institution (also known as Banker's Acceptances) which have been resold in the secondary market. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph 14
-SubTopic 210
-Topic 942
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_FeesPaidOnAcceptancesResold |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for salary and wage arising from service rendered by officer. Excludes allocated cost, labor-related nonsalary expense, and direct and overhead labor cost included in cost of good and service sold. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OfficersCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense for salary and wage arising from service rendered by nonofficer employee. Excludes allocated cost, labor-related nonsalary expense, and direct and overhead labor cost included in cost of good and service sold. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SalariesAndWages |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of the monthly rental payments due under the lease entered into in connection with the transactions involving the sale of property to another party and the lease of the property back to the seller. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481295/840-40-50-1
| Name: |
us-gaap_SaleLeasebackTransactionMonthlyRentalPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of vesting of award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued during the period as part of a transaction to acquire assets that do not qualify as a business combination.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesPurchaseOfAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=MIRA_ConsultantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_BoardOfDirectorsChairmanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=MIRA_ConsultingAndEmploymentAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=MIRA_EmploymentAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=MIRA_ErezAminovMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=MIRA_MichelleYanezMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=MIRA_ChrisChapmanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=MIRA_ChristosNicholoudisMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
Schedule of Accounts Payable And Accrued Liabilities (Details) - USD ($)
|
Sep. 30, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Payables and Accruals [Abstract] |
|
|
|
| Trade accounts payable |
$ 510,688
|
$ 789,204
|
|
| Pre-clinical research and toxicology studies |
254,293
|
|
|
| Accrued other |
14,592
|
22,534
|
|
| Accounts payable and accrued liabilities |
$ 779,573
|
$ 811,738
|
$ 228,406
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section 45
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-8
| Name: |
us-gaap_AccountsPayableTradeCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for insurance that provides economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidInsurance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.4
| X |
- DefinitionShare based compensation arrangement by share based payment award fair value assumptions weighted average fair values.
| Name: |
MIRA_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsWeightedAverageFairValues |
| Namespace Prefix: |
MIRA_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 50
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479468/405-50-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 50
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479468/405-50-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 50
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479468/405-50-50-3
| Name: |
us-gaap_SupplierFinanceProgramLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.4
| X |
- DefinitionRental expense for the reporting period incurred under operating leases, including minimum and any contingent rent expense, net of related sublease income. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481501/840-20-50-1
| Name: |
us-gaap_OperatingLeasesRentExpenseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=country_MD |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=stpr_FL |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
MIRA Pharmaceuticals (NASDAQ:MIRA)
Historical Stock Chart
From Apr 2024 to May 2024

MIRA Pharmaceuticals (NASDAQ:MIRA)
Historical Stock Chart
From May 2023 to May 2024
