Filed pursuant to Rule 424(b)(3)
Registration No. 333-220976
PROSPECTUS SUPPLEMENT
(To the Prospectus dated November 7, 2017)
OPIANT PHARMACEUTICALS, INC.
166,738 Shares of Common Stock
This prospectus relates to 166,738 shares of our common stock which may be sold from time to time by certain of our stockholders set forth in the “Selling Stockholders” section of this prospectus. The shares offered by this prospectus include 166,738 shares of common stock previously issued by us to certain of our Selling Stockholders in two separate private placements.
The prices at which the selling stockholders or their transferees may sell the shares will be determined by the prevailing market prices for the shares or in negotiated transactions. We will not receive any proceeds from the sale of the shares offered by this prospectus.
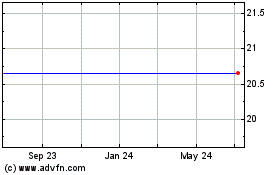
Our common stock is listed on the Nasdaq Capital Market under the symbol “OPNT.” The last reported sale price of our common stock on the Nasdaq Capital Market on November 1, 2018 was $18.79 per share.
Our business and an investment in our securities include significant risks. See “Risk Factors” beginning on page S-5 of this prospectus supplement and in our Transition Report on Form 10-KT for the five-month period ended December 31, 2017 and Form 10-Qs for the periods ended March 31, 2018 and June 30, 2018, which have been filed with the Securities and Exchange Commission ("SEC") and are incorporated by reference in this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus supplement is November 2, 2018
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus is part of a registration statement that we have filed with the Securities and Exchange Commission, or SEC, utilizing a “shelf” registration process. Under this shelf process, the selling stockholders may from time to time sell up to an aggregate amount of 166,738 shares of our common stock in one or more offerings and on terms to be determined by market conditions at the time of offering.
We provide information to you about this offering of shares of our common stock in two separate documents that are bound together: (1) this prospectus supplement, which describes the specific details regarding this offering; and (2) the accompanying base prospectus, which provides general information, some of which may not apply to this offering. Generally, when we refer to this “prospectus,” we are referring to both documents combined. If information in this prospectus supplement is inconsistent with the accompanying base prospectus, you should rely on this prospectus supplement. However, if any statement in one of these documents is inconsistent with a statement in a document having a later date incorporated by reference in this prospectus, the statement in the document incorporated by reference modifies or supersedes the earlier statement as our business, financial condition, results of operations and prospects may have changed since the earlier dates.
You should rely only on the information contained in, or incorporated by reference into, this prospectus supplement, the base prospectus, and in any free writing prospectus that we may authorize for use in connection with this offering. We have not, and the selling stockholders have not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the selling stockholders are not, making an offer to sell or soliciting an offer to buy our securities in any jurisdiction in which an offer or solicitation is not authorized or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation. You should assume that the information appearing in this prospectus supplement, the base prospectus, the documents incorporated by reference into this prospectus supplement and the base prospectus, and in any free writing prospectus that we may authorize for use in connection with this offering, is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this prospectus supplement, the base prospectus, the documents incorporated by reference into this prospectus supplement and the base prospectus, and any free writing prospectus that we may authorize for use in connection with this offering, in their entirety before making an investment decision. You should also read and consider the information in the documents to which we have referred you in the sections of this prospectus entitled “Where You Can Find More Information" and "Incorporation of Certain Documents by Reference.”
The selling stockholders are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus and the offering of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus outside the United States. This prospectus does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus and the information incorporated by reference in these documents contain certain statements that constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, statements about our plans, objectives, representations and contentions and are not historical facts and typically are identified by use of terms such as “anticipate,” “could,” “expect,” “believe,” “goal,” “plan,” “intend,” “estimate,” “may,” “seek,” “potential,” “predict,” “project,” “continue,” “should,” “would,” “will,” and similar expressions and variations thereof. Those statements appear in this prospectus supplement, any accompanying prospectus supplement and the documents incorporated herein and therein by reference, particularly in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” and include statements regarding the intent, belief or current expectations of the Company and management that are subject to known and unknown risks, uncertainties and assumptions and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to those discussed in the section titled “Risk Factors”.
You should be aware that this prospectus supplement, the accompanying prospectus and the information incorporated by reference in these documents also contain forward-looking statements that are based on management’s current expectations and beliefs, including estimates and projections about our company, industry, financial condition, results of operations and other matters. These statements are not guarantees of future performance and are subject to numerous risks, uncertainties, and assumptions that are difficult to predict.
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely upon forward-looking statements as predictions of future events. The events and circumstances reflected in the forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, including the securities laws of the United States and the rules and regulations of the SEC, we do not plan to publicly update or revise any forward-looking statements contained herein after we distribute this prospectus, whether as a result of any new information, future events or otherwise.
ABOUT THE COMPANY
This summary description about us and our business highlights selected information contained elsewhere in this prospectus supplement, the accompanying prospectus or incorporated in this prospectus by reference herein or therein. This summary does not contain all of the information you should consider before buying securities in this offering. You should carefully read this entire prospectus supplement, the corresponding base prospectus, and any free writing prospectus, including each of the documents incorporated herein or therein by reference, before making an investment decision. As used in this prospectus, “we,” “us,” “Opiant,” “the company” and “our” refer to Opiant Pharmaceuticals, Inc., a Delaware corporation.
Overview
Opiant Pharmaceuticals, Inc. is a specialty pharmaceutical company developing pharmacological treatments for addiction and drug overdose. We were incorporated in the State of Nevada in June 2005 as Madrona Ventures, Inc. and, in September 2009, we changed our name to Lightlake Therapeutics Inc. In January 2016, we again changed our name to Opiant Pharmaceuticals, Inc. Our fiscal year end is December 31.
We developed NARCAN® (naloxone hydrochloride) Nasal Spray, a treatment to reverse opioid overdose. This product was conceived, developed, licensed and approved by the U.S. Food and Drug Administration (“FDA”) in November 2015. It is commercialized by Adapt Pharma Operations Limited (“Adapt”), an Ireland based pharmaceutical company. We plan to replicate this relatively low cost business strategy primarily through developing pharmacological treatments for addiction and drug overdose. We primarily aim to identify and progress those drug development opportunities that have the potential to file additional New Drug Applications (“NDA”) with the FDA within three to five years, with larger market opportunities and with the potential to self-commercialize in the fields of addictions and related disorders.
We have not consistently attained profitable operations and have historically depended upon obtaining sufficient financing to fund our operations. We anticipate that if revenues are not sufficient, then additional funding will be required in the form of debt financing and/or equity financing from the sale of our common stock, $0.001 par value per share (the “Common Stock”), and/or financings from the sale of interests in our prospective products and/or royalty transactions. However, we may not be able to generate sufficient revenues or raise sufficient funding to fund our operations.
We have not had a bankruptcy, receivership or similar proceeding. We are required to comply with all regulations, rules and directives of governmental authorities and agencies applicable to the clinical testing and manufacturing and sale of pharmaceutical products.
Our current pipeline of product candidates includes pharmacological treatments for Bulimia Nervosa ("BN"), Alcohol Use Disorder ("AUD"), Opioid Use Disorder ("OUD") and a potent, long-acting Opioid Overdose Reversal ("OOR") product. We are also pursuing other treatment opportunities within the addiction space.
OPNT003 - Intranasal Nalmefene for OOR
On February 12, 2018, we announced positive data from a Phase I clinical study of our product candidate OPNT003 (intranasal nalmefene) and provided an update on a meeting held February 8, 2018 with the FDA regarding our planned development program. OPNT003 is in development as a long-lasting opioid antagonist for the treatment of opioid overdose. Based on feedback from the FDA in connection with this meeting, we intend to pursue a 505(b)(2) development path, with the potential to submit a NDA for the drug and intranasal delivery device combination in 2020. Nalmefene for injection was previously approved by the FDA for treating suspected or confirmed opioid overdose. The 505(b)(2) pathway allows companies to rely in part on the FDA’s findings of safety and efficacy for a previously approved product and to supplement these findings with a more limited set of their own studies to satisfy FDA requirements, as opposed to conducting the full array of preclinical and clinical studies that would typically be required.
Data generated in a Phase I study completed under a clinical trial agreement with the National Institute on Drug Abuse ("NIDA") provided the basis for the FDA meeting. These preliminary data demonstrate that our intranasal nalmefene formulation containing a proprietary absorption enhancer (Intravail®, from Aegis Therapeutics) resulted in rapid increases in plasma levels with an onset faster than an intramuscular injection and a comparatively long half-life (6.7-7.8 hours). Naloxone, the only FDA medication currently approved to treat opioid overdose, has a half-life of approximately two hours.
We have full commercial rights to OPNT003 and on April 17, 2018 we were awarded a grant of approximately $7.4 million from the National Institutes of Health’s National Institute on Drug Abuse. The grant provides us with additional resources for the ongoing development of OPNT003 (intranasal nalmefene), a long-lasting opioid antagonist for the treatment
of opioid overdose. The grant includes approximately $2.6 million to be funded for the period ending March 31, 2019, with the balance to be funded over the subsequent two years, subject to available funds and satisfactory progress on the development of OPNT003. NIH leadership recently called for the development of “...stronger, longer-acting formulations of antagonists…to counteract the very high potency synthetic opioids that are now claiming thousands of lives each year.” (Volkow and Collins, NEJM 2017).
Synthetic opioids, such as fentanyl, are now responsible for more overdose deaths than either heroin or prescription opioids, with over 29,000 synthetic opioid overdose deaths in 2017. Fentanyl and derivatives, such as carfentanil, are especially dangerous because of a long half-life of seven to ten hours that may require continuous monitoring of overdose victims and repeated dosing to prevent relapse. A long-lasting overdose reversal drug may reduce this burden.
An easy-to-use nasal formulation of nalmefene with a rapid onset and long duration of action would be a ready-to-use tool for non-medically trained persons to administer. If approved by the FDA, OPNT003 may also be especially useful in rural areas, where a rapidly growing number of overdoses are occurring, and where access to emergency medical response may be delayed by hours.
OPNT001 - Intranasal Naloxone for Eating Disorders
Bulimia Nervosa ("BN") is a serious and potentially life-threatening eating disorder mainly affecting females. BN is characterized by binge eating followed by purging, fasting, and other strategies to prevent weight gain. It has a lifetime prevalence of 1-2%, and patients are at a heightened risk of other psychiatric disorders including depression, anxiety and substance abuse. Company analysis estimates that there are one million patients with BN, yet fluoxetine is currently the only FDA approved medication to treat BN. However, the remission rate with fluoxetine, both alone and combination with psychotherapy, ranges only between 19-41%.
The compulsive bingeing characteristic of BN has features in common with other addictive disorders, providing the basis for using opioid antagonists to mitigate their frequency.
In 2017 we initiated a Phase II clinical trial to evaluate OPNT001, nasal naloxone, as a potential treatment for BN. OPNT001 is being tested in a multi-site, Phase II randomized double-blind placebo controlled study in the United Kingdom with 80 patients suffering from BN. In September of 2018, we announced that we had completed patient enrollment in the Phase II trial, with 86 total patients enrolled. The primary endpoint is a reduction in binge eating days and a data readout is expected in the first quarter of 2019.
We have, in the past, and continue to review possible treatments for Binge Eating Disorder (“BED”). BED is the most common eating disorder in the United States. Approximately eight million Americans are diagnosed with BED and it is correlated with obesity, yet Vyvanse (lisdexamfetamine dimesylate) is the only FDA approved pharmacotherapy. We consider OPNT001 to be a potentially compelling pharmacological treatment for BED. It has a well-known safety profile and has the potential to block the reward that patients experience from bingeing.
OPNT002 - Nasal Naltrexone for Alcohol Use Disorder ("AUD")
We are developing OPNT002, nasal naltrexone for AUD. Alcohol triggers the release of naturally occurring endorphins, which then bind to the opioid receptors in the brain, leading to dopamine release in the reward center. Opioid antagonists are anticipated to reduce the risk of heavy drinking because they block these opioid receptors, which results in dampening of alcohol-induced dopamine release and reward.
Our product candidate OPNT002 will be taken nasally on an "as-needed" basis in anticipation of drinking and/or in high risk situations in order to reduce alcohol intake. We anticipate that taking our product on an as-needed basis could improve patient compliance and enable a patient to regain control of their drinking, especially in situations where heavy drinking is otherwise habitual. Furthermore, we expect patients to have high rates of adherence, because they will not be required to abstain and potentially go through detoxification and withdrawal prior to initiating OPNT002 therapy, unlike the typical situation with existing pharmacotherapies for AUD.
We have generated encouraging Phase I clinical data demonstrating rapid intranasal absorption of OPNT002, which confirms its suitability for use on an as needed basis. High levels of naltrexone can be delivered within minutes, which is critically important during a period of craving. The Company has also received feedback from the FDA on the proposed
505(b)(2) development plan, which accepts a harm reduction-based primary endpoint rather than a primary endpoint based on abstinence.
There are approximately 16.3 million people in the United States who suffer from some form of AUD. Alcohol misuse and alcohol use disorders cost the United States roughly $249 billion annually in lost productivity, healthcare expenses and criminal justice costs. Despite this, existing medications to treat AUD are not highly effective. Feedback from our primary market research strongly supports nasal naltrexone as a compelling product that could also be used in a primary care setting as well as by addiction specialists and in addiction treatment centers.
Corporate Information
We were incorporated in the State of Nevada on June 21, 2005 as Madrona Ventures, Inc., and on September 16, 2009, the Company changed its name to Lightlake Therapeutics Inc. On January 28, 2016, we again changed our name to Opiant Pharmaceuticals, Inc. On October 2, 2017, we changed our state of incorporation from the State of Nevada to the State of Delaware pursuant to an Agreement and Plan of Merger, dated October 2, 2017, whereby we merged with and into our recently formed, wholly-owned Delaware subsidiary. The merger and the Agreement and Plan of Merger were approved by our Board of Directors (the “Board”) and stockholders representing a majority of our outstanding Common Stock. On December 8, 2017, the Board, acting pursuant to Section 5.1 of the Company’s Bylaws, approved a resolution changing the Company’s fiscal year-end from July 31 to December 31.
Our principal executive offices are located at 201 Santa Monica Blvd., Suite 500, Santa Monica, CA 90401.
Our telephone number is (310) 598-5410. We maintain a website at www.opiant.com where general information about us is available. Our website, and the information contained therein, is not a part of this prospectus.
THE OFFERING
|
|
|
|
|
|
Common Stock offered by the selling stockholders
|
166,738 shares of our common stock.
|
|
Use of Proceeds
|
We will not receive any proceeds from the sale of shares of our common stock by the selling stockholders.
|
|
Risk Factors
|
Investing in our common stock involves significant risks. Please read the information contained in and incorporated by reference under the heading “Risk Factors” on page S-5 of this prospectus supplement and under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this prospectus, together with the other information included in or incorporated by reference into this prospectus, before deciding whether to invest in our common stock.
|
|
Nasdaq Capital Market symbol
|
“OPNT”
|
RISK FACTORS
An investment in our securities involves a high degree of risk. Prior to making a decision about investing in our securities, you should carefully consider all of the other information by reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under Item 1A, “Risk Factors,” in our Transition Report on Form 10-KT for the five-month period ended December 31, 2017 and Form 10-Qs for the periods ended March 31, 2018 and June 30, 2018, which are incorporated herein by reference, and may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these known or unknown risks might cause you to lose all or part of your investment in the offered securities.
Risks Associated with our Securities and this Offering
The price of our common stock has been highly volatile due to factors that will continue to affect the price of our stock.
Our Common Stock closed as high as $24.40 and as low as $5.35 per share between January 1, 2016 and August 28, 2017 on the OTCQB. On October 2, 2017 we changed our state of incorporation from the State of Nevada to the State of Delaware pursuant to an Agreement and Plan of Merger, dated October 2, 2017, whereby we merged with and into our recently formed, wholly-owned Delaware subsidiary. On August 24, 2017, we received approval for up-listing to the Nasdaq Capital Market and our Common Stock began trading on the Nasdaq Capital Market on August 29, 2017. Our Common Stock closed as high as $50.50 and as low as $11.51 per share between August 29, 2017 and November 1, 2018. On November 1, 2018 the closing price of our Common Stock, as reported on the Nasdaq Capital Market was $18.79. Historically, the over-the-counter markets for securities, such as our Common Stock, have experienced extreme price fluctuations. Some of the factors leading to this volatility include, but are not limited to:
|
|
|
|
•
|
fluctuations in our operating results;
|
|
|
|
|
•
|
announcements of product releases by us or our competitors;
|
|
|
|
|
•
|
announcements of acquisitions and/or partnerships by us or our competitors; and
|
|
|
|
|
•
|
general market conditions.
|
Although shares of our Common Stock currently trade on the Nasdaq Capital Market under the symbol “OPNT”, there is no assurance that our stock will not continue to be volatile while listed on the Nasdaq Capital Market in the future.
Certain of our executive officers and directors control the direction of our business by means of a significant collective beneficial ownership of our Common Stock. The concentrated beneficial ownership of our Common Stock may prevent other stockholders from influencing significant corporate decisions.
Dr. Roger Crystal, our Chief Executive Officer and a director, Dr. Michael Sinclair, a director, Ann MacDougall, a director, Thomas T. Thomas, a director, and Dr. Gabrielle Silver, the Lead Independent Director, collectively beneficially owned approximately 37.82% of our outstanding Common Stock as of June 30, 2018. As a result, such executive officers and directors effectively control the Company and have the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors, amendments to our Certificate of Incorporation, and any proposed merger, consolidation or sale of all or substantially all our assets and other corporate transactions. This concentration of ownership could be disadvantageous to other stockholders with differing interests from such executive officers and directors.
Anti-takeover provisions in our charter documents and under Delaware law could prevent or delay transactions that our stockholders may favor and may prevent stockholders from changing the direction of our business or management.
After giving effect to our merger into our wholly-owned Delaware subsidiary, provisions of our Certificate of Incorporation, as amended and restated, and Bylaws may discourage, delay or prevent a merger or acquisition that our stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares, and may also frustrate or prevent any attempt by stockholders to change our direction or management. For example, these provisions:
|
|
|
|
•
|
prohibit stockholder action by written consent;
|
|
|
|
|
•
|
establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings;
|
|
|
|
|
•
|
establish a staggered board of directors such that all members of the Board are not elected at one time;
|
|
|
|
|
•
|
allow only the Board to fill any vacancy in the Board by reason of death, resignation or otherwise, or if the number of directors shall be increased; and
|
|
|
|
|
•
|
require a vote of a majority of the shares of our outstanding stock entitled to vote at an election of directors to remove a director.
|
Compliance with changing corporate governance and public disclosure regulations may result in additional expense.
Keeping abreast of, and in compliance with, changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, and any new SEC regulations will require an increased amount of management attention and external resources. We intend to continue to invest all reasonably necessary resources to comply with evolving standards, which may result in increased general and administrative expense and a diversion of management time and attention from revenue-generating activities to compliance activities.
Our common stock is thinly traded on the Nasdaq Capital Market, and no assurances can be made about stock performance, liquidity, or maintenance of our Nasdaq listing.
On August 24, 2017, our Common Stock was approved for trading on the Nasdaq Capital Market. Beginning on August 29, 2017, our Common Stock began trading on the Nasdaq Capital Market under the symbol “OPNT.” Although currently listed on the Nasdaq Capital Market, there can be no assurance that we will continue to meet the Nasdaq Capital Market’s minimum listing requirements or that of any other national exchange. In addition, there can be no assurances that a liquid market will be created for our Common Stock. If we are unable to maintain listing on the Nasdaq Capital Market or if a liquid market for our Common Stock does not develop, our common stock may remain thinly traded.
USE OF PROCEEDS
We will not receive any proceeds from the sale of shares of our common stock by the selling stockholders.
SELLING STOCKHOLDERS
The shares may be offered by the selling stockholders or by pledges, donees, transferees or other successors in interest that receive such shares as a gift or through a private sale or transfer. We may amend or supplement this prospectus from time to time to update information provided in the table.
We have prepared this prospectus supplement to allow the selling stockholders or their successors, assignees or other permitted transferees to sell or otherwise dispose of, from time to time, up to 166,738 shares of our common stock.
The shares to be offered hereby by Torreya Partners (Europe) LLP (“Torreya”) were issued in connection with the provision of financial advisory services pursuant to an engagement agreement by and between the company and Torreya, dated as of December 18, 2014, as amended by that certain Supplemental Agreement, dated as of September 8, 2017, and as further amended by that certain Waiver and Amended dated as of October 13, 2017 (as amended, the “Torreya Agreement”). Pursuant to the terms of the Torreya Agreement and as of the date hereof, we have issued an aggregate total of 6,738 shares of common Stock to Torreya, and further agreed to register the 6,738 shares of its common stock pursuant to the Torreya Agreement. These shares were issued in reliance on the exemption from securities registration in Section 4(a)(2) under the Securities Act and Rule 506 promulgated thereunder, as well as the safe harbor provided by Regulation S under the Securities Act.
The shares to be offered hereby by Valour Fund LLC ("Valour") were issued in connection with an agreement dated September, 22 2015, whereby we received $1.6 million, in exchange for Valour receiving a 2.13% interest in any pre-tax revenue received by us that was derived from the sale of the Certain Studies Products less any and all expenses incurred by and payments made by us in connection with the Certain Studies Products. The agreement provide that if an aforementioned treatment is not introduced to the market by September 22, 2018, Valour had a 60-day option to exchange its 2.13% interest for shares of our Common Stock at an exchange rate of one-tenth of a share for every dollar of its investment. In September 2018, Valour elected to exchange its 2.13% interest for stock and accordingly we issued 160,000 shares of our common stock to Valour. These shares were issued in reliance on the exemption from securities registration in Section 4(a)(2) under the Securities Act and Rule 506 promulgated thereunder, as well as the safe harbor provided by Regulation S under the Securities Act.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. The Registrant believes these transactions were exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated under the Securities Act as transactions by an issuer not involving any public offering. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. All recipients had adequate access, through their relationships with us or otherwise, to information about the company.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock Beneficially
Owned After the
Offering
(1)
|
|
|
|
|
Common Stock
Beneficially Owned
Prior to the
Offering
(1)
|
|
|
|
|
|
|
Common Stock
Registered for
Resale
|
|
|
Name of Stockholder
|
|
Number
(2)
|
|
Percentage
(2)
|
|
|
Torreya Partners (Europe) LLP
|
|
|
6,738
|
|
|
6,738
|
|
|
0
|
|
|
0
|
%
|
|
|
Valour Fund LLC
|
|
|
160,000
|
|
|
160,000
|
|
|
0
|
|
|
0
|
%
|
|
|
Total
|
|
|
166,738
|
|
|
166,738
|
|
|
|
|
|
|
|
(1)
Pursuant to Securities and Exchange Commission rules, a person is deemed to beneficially own a security if that person has the right to acquire beneficial ownership of such security within 60 days.
(2)
Assumes sale of all shares available for sale under this prospectus supplement and no further acquisitions of shares of common stock by the selling stockholder.
PLAN OF DISTRIBUTION
We are registering the shares of common stock issuable to the selling stockholders to permit the resale of these shares of common stock by the selling stockholders from time to time after the date of this prospectus supplement. We will not receive any of the proceeds from the sale by the selling stockholders of the shares of common stock. We will bear all fees and expenses incident to our obligation to register the shares of common stock.
Each selling stockholder may sell all or a portion of the shares of common stock held by it and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers, the selling stockholders will be responsible for underwriting discounts or commissions or agent's commissions. The shares of common stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions, pursuant to one or more of the following methods:
|
|
|
|
•
|
on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale;
|
•
in the over-the-counter market;
•
in transactions otherwise than on these exchanges or systems or in the over-the-counter market;
•
through the writing or settlement of options, whether such options are listed on an options exchange or otherwise;
•
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
|
•
|
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
•
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
•
an exchange distribution in accordance with the rules of the applicable exchange;
•
privately negotiated transactions;
|
|
|
|
•
|
broker-dealers may agree with selling stockholders to sell a specified number of such shares at a stipulated price per share;
|
•
a combination of any such methods of sale; and
•
any other method permitted pursuant to applicable law.
The selling stockholders may also sell shares of common stock under Rule 144 promulgated under the Securities Act, if available, rather than under this prospectus supplement. In addition, the selling stockholders may transfer the shares of common stock by other means not described in this prospectus supplement. If the selling stockholders effects such transactions by selling shares of common stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the shares of common stock for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved).
In connection with sales of the shares of common stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares of common stock short and deliver shares of common stock covered by this prospectus supplement to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loan or pledge shares of common stock to broker-dealers that in turn may sell such shares.
The selling stockholders may pledge or grant a security interest in some or all of the shares of common stock owned by it and, if it defaults in the performance of its secured obligations, the pledgees or secured parties may offer and sell the shares of common stock from time to time pursuant to this prospectus supplement or any amendment to this prospectus supplement under Rule 424(b)(3) or other applicable provision of the Securities Act amending, if necessary, the prospectus supplement to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus supplement. The selling stockholders also may transfer and donate the shares of common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus supplement.
To the extent required by the Securities Act and the rules and regulations thereunder, the selling stockholders and any broker-dealer participating in the distribution of the shares of common stock may be deemed to be "underwriters" within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement, if required, will be distributed, which will set forth the
aggregate amount of shares of common stock being offered and the terms of the offering, including the name or names of any underwriter, dealer or agent, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or re-allowed or paid to dealers.
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that the selling stockholders will sell any or all of the shares of common stock registered pursuant to the registration statement, of which this prospectus supplement forms a part.
The selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder, including, without limitation, to the extent applicable, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the selling stockholders and any other participating person. To the extent applicable, Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of common stock. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
We will pay all expenses of the registration of the resale of the shares of common stock, including, without limitation, SEC filing fees and expenses of compliance with state securities or "blue sky" laws; provided, however, the selling stockholders will pay all underwriting discounts and selling commissions, if any.
LEGAL MATTERS
The validity of the shares of common stock offered hereby will be passed upon for us by Wilson Sonsini Goodrich & Rosati, P.C. 650 Page Mill Road, Palo Alto, California 94304.
EXPERTS
The financial statements of the Company as of and for the five-month period ended December 31, 2017 and the years ended July 31, 2017 and July 31, 2016, appearing in the Company’s Transition Report on Form 10-KT for the five-month period ended December 31, 2017, have been audited by MaloneBailey, LLP, the Company’s independent registered public accounting firm, as set forth in their reports thereon, included therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such reports given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly current and other reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. You may also read and copy any document we file at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K, including any amendments to those reports, and other information that we file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act can also be accessed free of charge through the Internet. These filings will be available as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
We have filed with the SEC a registration statement on Form S-3 under the Securities Act relating to the offering of these securities offered by this prospectus supplement. The registration statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus supplement does not contain all of the information set forth in the registration statement. You can obtain a copy of the registration statement, at prescribed rates, from the SEC at the address listed above. The registration statement and the documents referred to below under “Incorporation of Certain Documents by Reference” are also available on our website, www.opiant.com. We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” much of the information we file with them under Commission File No. 001-38193, which means that we can disclose important information to you by referring you to other documents we have filed or will file with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and any of our subsequent filings with the SEC will automatically update and supersede this information. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any statements in the prospectus or any document previously incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents set forth below that we have previously filed with the SEC, and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, except for information furnished under Items 2.02 or 7.01 of Current Report on Form 8-K, or exhibits related thereto, until the filing of a post-effective amendment to this prospectus which indicates that all securities registered have been sold or which deregisters all securities then remaining unsold:
|
|
|
|
•
|
our annual report on Form 10-K for the fiscal year ended July 31, 2017, filed with the SEC on October 13, 2017;
|
|
|
|
|
•
|
our quarterly report on Form 10-Q for the quarter ended October 31, 2017, filed with the SEC on December 4, 2017;
|
|
|
|
|
•
|
our transition report on From 10-KT for the five-month period ended December 31, 2017, filed with the SEC on March 7, 2018;
|
|
|
|
|
•
|
our quarterly report on Form 10-Q for the quarter ended March 31, 2018, filed with the SEC on May 8, 2018;
|
|
|
|
|
•
|
our proxy statement for our annual meeting of stockholders, filed with the SEC on April 17, 2018;
|
|
|
|
|
•
|
our quarterly report on Form 10-Q for the quarter ended June 30, 2018, filed with the SEC on August 9, 2018;
|
|
|
|
|
•
|
our current reports on Form 8-K, filed with the SEC on August 10, 2017, August 28, 2017, August 29, 2017, September 5, 2017, September 5, 2017, September 11, 2017, September 11, 2017, September 14, 2017, October 6, 2017, October 19, 2017, December 1, 2017, December 8, 2017, December 13, 2017, December 27, 2017, January 2, 2018, January 16, 2018, February 12, 2018, February 27, 2018, March 5, 2018, April 18, 2018, May 10, 2018, June 12, 2018, June 13, 2018, August 9, 2018, September 10, 2018, September 20, 2018, September 25, 2018, September 27, 2018 and October 29, 2018;
|
|
|
|
|
•
|
our description of our Common Stock contained in the Registration Statement on Form 8-A12B filed with the SEC on August 25, 2017; and
|
|
|
|
|
•
|
all other reports filed pursuant to Section 13(a) or 15(d) of the Exchange Act after the date of this registration statement and prior to the effectiveness of the registration statement.
|
We also incorporate by reference all documents we file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the sale of all the securities covered by this prospectus (including all such documents filed with the SEC after the date of the initial filing of the registration statement that contains this prospectus and prior to effectiveness of the registration statement or after such effectiveness), except in each case the information contained in such document to the extent “furnished” and not “filed.”
We will provide, upon written or oral request, to each person to whom a prospectus is delivered, a copy of any or all of the information that has been incorporated by reference in the prospectus but not delivered with the prospectus. You may request a copy of these filings, at no cost, by writing or calling us at:
Opiant Pharmaceuticals, Inc.
201 Santa Monica Boulevard, Suite 500
Santa Monica, CA 90401
Attn: Chief Financial Officer
(310) 598-5410
You may also access the documents incorporated by reference in this prospectus through our website at www.opiant.com. Except for the specific incorporated documents listed above, no information available on or through our website shall be deemed to be incorporated in this prospectus or the registration statement of which it forms a part. You should rely only on the information incorporated by reference or provided in this prospectus or any supplement. We have not authorized anyone else to provide you with different information. You should not assume that information in this prospectus or any supplement is accurate as of any date other than the date on the front of these documents.
PROSPECTUS
OPIANT PHARMACEUTICALS, INC.
$150,000,000
Common Stock
Warrants
Opiant Pharmaceuticals, Inc., a Delaware corporation (“Opiant”), may offer and sell from time to time, in one or more series or issuances and on terms that Opiant will determine at the time of the offering, any combination of the securities described in this prospectus, up to an aggregate amount of $150,000,000.
This prospectus provides a general description of the securities we may offer. Each time we sell securities, we will provide specific terms of any offering in a supplement to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. Any prospectus supplement and any related free writing prospectus may also add, update, or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as the documents incorporated or deemed to be incorporated by reference in this prospectus before you purchase any of the securities offered hereby. This prospectus may not be used to consummate a sale of securities by us unless accompanied by the applicable prospectus supplement.
These securities may be offered and sold in the same offering or in separate offerings; to or through underwriters, dealers, and agents; or directly to institutional purchasers. The names of any underwriters, dealers, or agents involved in the sale of our securities, their compensation and any over-allotment options held by them will be described in the applicable prospectus supplement. For a more complete description of the plan of distribution of these securities, see the section entitled “Plan of Distribution” beginning on page 16 of this prospectus.
Our common stock, $0.001 par value per share (“Common Stock”), is listed on the Nasdaq Capital Market under the symbol “OPNT.” The last reported sale price of our Common Stock on the Nasdaq Capital Market on October 13, 2017 was $37.98 per share. We will provide information in any applicable prospectus supplement regarding any listing of securities other than shares of our Common Stock on any securities exchange.
Investing in our securities involves a high degree of risk. You should carefully read and consider the risk factors described in, and incorporated by reference under, “Risk Factors” beginning on page 6 of this prospectus and in the applicable prospectus supplement before investing in any securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is November 7, 2017
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-3 that we filed with the United States Securities and Exchange Commission (the “SEC”), using a “shelf” registration process. Under this shelf process, we may, from time to time, offer and sell any combination of the securities described in this prospectus in one or more offerings up to a total amount of $150,000,000.
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities under this shelf registration, we will, to the extent required by law, provide a prospectus supplement that will contain specific information about the terms of that offering. The prospectus supplement may also add to, update or change information contained in this prospectus and, accordingly, to the extent there are inconsistencies between any prospectus supplement, this prospectus and any documents incorporated by reference, the document with the most recent date will control.
The prospectus supplement to be attached to the front of this prospectus may describe, as applicable: the terms of the securities offered; the initial public offering price; the price paid for the securities; net proceeds; and the other specific terms related to the offering of the securities. This prospectus may not be used by us to consummate sales of our securities, unless it is accompanied by a prospectus supplement.
You should only rely on the information contained in, or incorporated by reference into, this prospectus and any prospectus supplement or issuer free writing prospectus relating to a particular offering. We have not authorized any dealer, salesman or other person to give any information or make any representations in connection with this offering other than those contained or incorporated by reference in this prospectus, any accompanying prospectus supplement and any related issuer free writing prospectus in connection with the offering described herein and therein, and, if given or made, such information or representations must not be relied upon as having been authorized by us. Neither this prospectus nor any prospectus supplement nor any related issuer free writing prospectus shall constitute an offer to sell or a solicitation of an offer to buy offered securities in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation. This prospectus does not contain all of the information included in the registration statement. For a more complete understanding of the offering of the securities, you should refer to the registration statement, including its exhibits.
You should carefully read this entire prospectus, the applicable prospectus supplement and any related issuer free writing prospectus, as well as the documents incorporated by reference into this prospectus or any prospectus supplement or any related issuer free writing prospectus, before making an investment decision. Neither the delivery of this prospectus or any prospectus supplement or any issuer free writing prospectus nor any sale made hereunder shall under any circumstances imply that the information contained or incorporated by reference herein or in any prospectus supplement or issuer free writing prospectus is correct as of any date subsequent to the date hereof or of such prospectus supplement or issuer free writing prospectus, as applicable. You should assume that the information appearing in this prospectus, any prospectus supplement or any document incorporated by reference is accurate only as of the date of the applicable documents, regardless of the time of delivery of this prospectus or any sale of securities. Our business, financial condition, results of operations and prospects may have changed since that date.
ABOUT THE COMPANY
This summary description about us and our business highlights selected information contained elsewhere in this prospectus or incorporated in this prospectus by reference. This summary does not contain all of the information you should consider before buying securities in this offering. You should carefully read this entire prospectus and any applicable prospectus supplement, including each of the documents incorporated herein or therein by reference, before making an investment decision. As used in this prospectus, “we,” “us,” “Opiant,” “the Company” and “our” refer to Opiant Pharmaceuticals, Inc., a Delaware corporation.
Overview
We are a specialty pharmaceutical company which develops pharmacological treatments for substance use, addictive and eating disorders. Our strategy is to develop pharmacological treatments for substance use, addictive, and eating disorders based on our expertise using opioid antagonists. We have developed a treatment for reversing opioid overdoses in collaboration with the National Institute on Drug Abuse (NIDA), part of the National Institutes of Health (NIH). This treatment, now known as NARCAN® (naloxone hydrochloride) Nasal Spray (“NARCAN®”), was approved by the U.S. Food and Drug Administration (“FDA”) in November 2015, and is marketed by Adapt Pharma Operations Limited, a wholly owned subsidiary of Adapt Pharma Limited, an Ireland-based pharmaceutical company.
We have not consistently attained profitable operations and have historically depended upon obtaining sufficient financing to fund our operations. We anticipate that if revenues are not sufficient, then additional funding will be required in the form of debt financing and/or equity financing from the sale of our Common Stock, $0.001 par value per share (the “Common Stock”), and/or financings from the sale of interests in our prospective products and/or royalty transactions. However, we may not be able to generate sufficient revenues or raise sufficient funding to fund our operations.
We developed NARCAN®, a treatment to reverse opioid overdoses, which was conceived, licensed, developed, approved by the FDA, and commercialized in less than three years. We plan to replicate this relatively low cost, successful business strategy primarily through developing nasal opioid antagonists in the field of developing pharmacological treatments for substance use, addictive, and eating disorders. We also plan to identify and progress drug development opportunities with potentially larger markets, potentially larger addressable patient populations, and greater revenue potential. In addition, we plan to invest in long-term development opportunities by identifying early stage product candidates with novel modes of action.
Our current pipeline of product candidates includes a treatment for Bulimia Nervosa, a treatment for Binge Eating Disorder, a treatment for Alcohol Use Disorder and a heroin vaccine. We are also focused on other treatment opportunities.
Corporate Information
We were incorporated in the State of Nevada on June 21, 2005 as Madrona Ventures, Inc. and on September 16, 2009, the Company changed its name to Lightlake Therapeutics Inc. On January 28, 2016, we again changed our name to Opiant Pharmaceuticals, Inc. On October 2, 2017, we changed our state of incorporation from the State of Nevada to the State of Delaware pursuant to an Agreement and Plan of Merger, dated October 2, 2017, wherby we merged with and into our recently formed, wholly-owned Delaware subsidiary (the “Reincorporation Merger”). The merger and the Agreement and Plan of Merger were approved by our Board of Directors (the “Board”) and stockholders representing a majority of our outstanding common stock, par value $0.001 per share (the “Common Stock”). The Company’s fiscal year end is July 31.
Our principal executive offices are located at 201 Santa Monica Blvd., Suite 500, Santa Monica, CA 90401.
Our telephone number is (310) 598-5410. We maintain a website at www.opiant.com where general information about us is available. Our website, and the information contained therein, is not a part of this prospectus.
Description of the Securities We May Offer
The descriptions of the securities contained in this prospectus, together with the applicable prospectus supplements, summarize the material terms and provisions of the various types of securities that we may offer. We will describe in the applicable prospectus supplement relating to any securities the particular amounts, prices and other terms of the securities offered by that prospectus supplement. If we so indicate in the applicable prospectus supplement, the terms of the securities may differ from the terms we have summarized below. We will also include in the prospectus supplement information, where applicable, about material U.S. federal income tax considerations relating to the securities, and the securities exchange, if any, on which the securities will be listed.
In this prospectus, we refer to the Common Stock and warrants, or any combination of Common Stock and warrants, to be sold by us in a primary offering collectively as “securities.” The total dollar amount of all securities that we may issue under this prospectus will not exceed $150,000,000.
This prospectus may not be used by us to consummate a sale of securities unless it is accompanied by a prospectus supplement.
RISK FACTORS
An investment in our securities involves a high degree of risk. The prospectus supplement applicable to each offering of our securities will contain a discussion of the risks applicable to an investment in our securities. Prior to making a decision about investing in our securities, you should carefully consider the specific factors discussed under the heading “Risk Factors” in the applicable prospectus supplement, together with all of the other information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under Item 1A, “Risk Factors,” in our Annual Report on Form 10-K for the fiscal year ended July 31, 2017 and any updates described in our Quarterly Reports on Form 10-Q, all of which are incorporated herein by reference, and may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future and any prospectus supplement related to a particular offering. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these known or unknown risks might cause you to lose all or part of your investment in the offered securities.
FORWARD-LOOKING STATEMENTS
This prospectus, each prospectus supplement and the information incorporated by reference in this prospectus and each prospectus supplement contain certain statements that constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, statements about our plans, objectives, representations and contentions and are not historical facts and typically are identified by use of terms such as “anticipate,” “could,” “expect,” “believe,” “goal,” “plan,” “intend,” “estimate,” “may,” “seek,” “potential,” “predict,” “project,” “continue,” “should,” “would,” “will,” and similar expressions and variations thereof. Those statements appear in this prospectus, any accompanying prospectus supplement and the documents incorporated herein and therein by reference, particularly in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” and include statements regarding the intent, belief or current expectations of the Company and management that are subject to known and unknown risks, uncertainties and assumptions and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to those discussed in the section titled “Risk Factors” set forth above.
You should be aware that this prospectus, any prospectus supplement and the information incorporated by reference in this prospectus and any prospectus supplement also contain forward-looking statements that are based on management’s current expectations and beliefs, including estimates and projections about our company, industry, financial condition, results of operations and other matters. These statements are not guarantees of future performance and are subject to numerous risks, uncertainties, and assumptions that are difficult to predict.
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely upon forward-looking statements as predictions of future events. The events and circumstances reflected in the forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, including the securities laws of the United States and the rules and regulations of the SEC, we do not plan to publicly update or revise any forward-looking statements contained herein after we distribute this prospectus, whether as a result of any new information, future events or otherwise.
USE OF PROCEEDS
Unless otherwise indicated in the prospectus supplement, we will use the net proceeds from the sale of securities offered by this prospectus for general corporate purposes, which may include working capital, capital expenditures, other corporate expenses and acquisitions of complementary products, product candidates, technologies or businesses. However, we currently have no present agreements or commitments for any such acquisitions. The timing and amount of our actual expenditures will be based on many factors, including cash flows from operations and the anticipated growth of our business. As a result, unless otherwise indicated in the prospectus supplement, our management will have broad discretion to allocate the net proceeds of the offerings. Pending their ultimate use, we intend to invest the net proceeds in short-term, investment-grade, interest-bearing instruments. We believe it is prudent to have an effective shelf registration statement on file with the SEC to preserve the flexibility to raise capital if and when needed.
DESCRIPTION OF CAPITAL STOCK
The following description of our Common Stock, together with the additional information we include in any applicable prospectus supplements, summarizes the material terms and provisions of the Common Stock that we may offer under this prospectus. It may not contain all the information that is important to you. For the complete terms of our Common Stock, please refer to our amended and restated certificate of incorporation (the “Certificate of Incorporation”) and bylaws, as may be amended from time to time, and which are incorporated by reference into the registration statement which includes this prospectus. The Delaware General Corporation Law (the “DGCL”) may also affect the terms of these securities. While the terms we have summarized below will apply generally to any future Common Stock that we may offer, we will describe the particular terms of the Common Stock in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement, the terms of any security we offer under that prospectus supplement may differ from the terms we describe below.
Common Stock
Under our Certificate of Incorporation, we have the authority to issue 200,000,000 shares of our Common Stock. As of October 10, 2017, there were 2,037,888 shares of Common Stock issued and outstanding. When we issue shares of our Common Stock under this prospectus, the shares will be fully paid and nonassessable and, unless specified in the applicable prospectus supplement, will not have or be subject to any rights of first refusal or similar rights.
Voting
. For all matters submitted to a vote of stockholders, each holder of our Common Stock is entitled to one vote for each share registered in his or her name. Except as may be required by law and in connection with some significant actions, such as mergers, consolidations, or amendments to our Certificate of Incorporation that affect the rights of stockholders, holders of our Common Stock vote together as a single class. There is no cumulative voting in the election of our directors, which means that a plurality of the votes cast at a meeting of stockholders at which a quorum is present is sufficient to elect a director. As such, the holders of more than 50% of the outstanding shares of Common Stock, in a vote for the election of directors, may elect all of the directors to be elected, if they so choose, and, in that event, the holders of the remaining shares of Common Stock will not be able to elect any of the Company’s directors.
Dividends
. The holders of shares of our Common Stock are entitled to receive dividends, including dividends of our stock, as and when declared by our Board, subject to any limitations under the DGCL. We have never declared or paid any cash dividends on our Common Stock. We do not anticipate paying any cash dividends to stockholders in the foreseeable future. In addition, any future determination to pay cash dividends will be at the discretion of our Board and will be dependent upon our financial condition, results of operations, capital requirements, and such other factors as our Board deems relevant.
Liquidation
. In the event we are liquidated, dissolved or our affairs are wound up, after we pay or make adequate provision for all of our known debts and liabilities, each holder of our Common Stock will be entitled to share ratably in all assets that remain.
Other Rights and Restrictions
. All shares of our Common Stock have equal dividend, distribution, liquidation and other rights, and have no preference, appraisal or exchange rights, except for any appraisal rights provided by the DGCL. Furthermore, holders of our Common Stock have no conversion, sinking fund or redemption rights, or preemptive rights to subscribe for any of our securities. Our Certificate of Incorporation and bylaws do not restrict the ability of a holder of our Common Stock to transfer his or her shares of our Common Stock.
Listing
. Our Common Stock is listed on the Nasdaq Capital Market under the symbol “OPNT.”
Transfer Agent and Registrar
. The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company (AST), LLC, 6201 15th Avenue, Brooklyn, NY 11219. AST's telephone number is
Certain Effects of Authorized but Unissued Stock
We have shares of Common Stock available for future issuance without stockholder approval. We may issue these additional shares for a variety of corporate purposes, including future public offerings to raise additional capital or facilitate corporate acquisitions or for payment as a dividend on our capital stock. The existence of unissued and
unreserved Common Stock may enable our Board to issue shares to persons friendly to current management, thereby protecting the continuity of our management.
Delaware Law and Certificate of Incorporation and Bylaws Provisions
Board of Directors.
Our Bylaws provide that:
|
|
|
|
•
|
any directors, or the entire Board, may be removed from office at any time, but only with cause, by the affirmative vote of at least seventy-five percent (75%) of all eligible votes present in person or by proxy at a meeting of stockholders at which a quorum is present; and
|
|
|
|
|
•
|
vacancies in the Board resulting from such removal may be filled by a majority of the directors then in office, though less than a quorum, or by the sole remaining director. Directors so chosen shall hold office until the next annual meeting of stockholders at which the term of office of the class to which they have been elected expires.
|
These provisions could discourage, delay or prevent a change in control of our Company or an acquisition of our Company at a price which many stockholders may find attractive. The existence of these provisions could limit the price that investors might be willing to pay in the future for shares of our Common Stock. These provisions may also have the effect of discouraging a third party from initiating a proxy contest, making a tender offer or attempting to change the composition or policies of our Board.
Stockholder Action
;
Special Meeting of Stockholders
. Our Bylaws also provide that:
|
|
|
|
•
|
stockholder action may be taken only at a duly called and convened annual or special meeting of stockholders and then only if properly brought before the meeting;
|
|
|
|
|
•
|
stockholder action may not be taken by written action in lieu of a meeting;
|
|
|
|
|
•
|
special meetings of stockholders may be called only by our Board, the Chairman of the Board or the Chief Executive Officer; and
|
|
|
|
|
•
|
in order for any matter to be considered “properly brought” before a meeting, a stockholder must comply with requirements regarding specified information and advance notice to us.
|
These provisions could delay, until the next stockholders’ meeting, actions which are favored by the holders of a majority of our outstanding voting securities. These provisions may also discourage another person or entity from making a tender offer for our Common Stock, because a person or entity, even if it acquired a majority of our outstanding voting securities, would be able to take action as a stockholder only at a duly called stockholders’ meeting, and not by written consent.
Indemnification
. Our Certificate of Incorporation provides that we shall, to the fullest extent permitted by, and in accordance with the provisions of, the DGCL, indemnify each of our directors or officers or employees against expenses (including attorneys’ fees), judgments, taxes, fines and amounts paid in settlement, incurred by him in connection with, and shall advance expenses (including attorneys’ fees) incurred by him in defending, any threatened, pending or completed action, suit or proceeding (whether civil, criminal, administrative or investigative) to which he is, or is threatened to be made, a party by reason of the fact that he is or was a director or officer or employee of ours, or is or was serving at the request of us as a director, officer, partner, employee or agent of another domestic or foreign corporation, partnership, joint venture, trust or other enterprise. Advancement of expenses shall be made upon receipt of an undertaking, with such security, if any, as the Board or stockholders may reasonably require, by or on behalf of the person seeking indemnification to repay amounts advanced if it shall ultimately be determined that he is not entitled to be indemnified us as authorized therein.
DESCRIPTION OF WARRANTS
General
We may issue warrants for the purchase of our Common Stock. Warrants may be issued independently or together with our Common Stock and may be attached to or separate from any offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and one or more banks or trust companies, as warrant agent, reflecting the particular terms and provisions of a series of offered warrants. The warrant agent will act solely as our agent in connection with the warrants. The warrant agent will not have any obligation or relationship of agency or trust for or with any holders or beneficial owners of warrants.
The following briefly summarizes the material provisions of the warrant agreements and the warrants. This summary of certain provisions of the warrants is not complete. For the terms of a particular series of warrants, you should refer to the prospectus supplement for that series of warrants and the warrant agreement for that particular series, which description may modify or replace the general terms described in this section. If there are differences between the prospectus supplement and this prospectus, the prospectus supplement will control. Thus, the statements made in this section may not apply to your warrant. You can obtain a copy of any form of warrant agreement when it has been filed with the SEC or by following the directions outlined in “Where You Can Find More Information” and “Incorporation of Certain Documents by Reference” or by contacting the applicable warrant agent.
As of October 13, 2017, there were 768,800 shares of Common Stock underlying outstanding warrants which expire between December 31, 2017 and December 15, 2024. Each of these warrants entitles the holder to purchase Common Stock at prices ranging between $8 and $15 per share of Common Stock. Each of these warrants also contains provisions for the adjustment of the exercise price and the aggregate number of shares issuable upon the exercise of the warrant in the event of dividends, share splits, reorganizations and reclassification and consolidations.
Warrants
The prospectus supplement relating to a particular series of warrants to purchase our Common Stock will describe the terms of the warrants, including the following:
|
|
|
|
•
|
the title of the warrants;
|
|
|
|
|
•
|
the offering price for the warrants, if any;
|
|
|
|
|
•
|
the aggregate number of warrants;
|
|
|
|
|
•
|
the designation and terms of the Common Stock that may be purchased upon exercise of the warrants;
|
|
|
|
|
•
|
if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each security;
|
|
|
|
|
•
|
if applicable, the date from and after which the warrants and any securities issued with the warrants will be separately transferable;
|
|
|
|
|
•
|
the number of shares of Common Stock that may be purchased upon exercise of a warrant and the exercise price for the warrants;
|
|
|
|
|
•
|
whether the exercise price may be paid in cash, by the exchange of warrants or other securities or both, and the method of exercising the warrants;
|
|
|
|
|
•
|
whether the warrants will be settled by delivery of the underlying securities or other property or in cash;
|
|
|
|
|
•
|
the dates on which the right to exercise the warrants shall commence and expire;
|
|
|
|
|
•
|
whether and under what circumstances we may cancel the warrants prior to their expiration date, in which case the holders will be entitled to receive only the applicable cancellation amount, which may be either a fixed amount or an amount that varies during the term of the warrants in accordance with a schedule or formula;
|
|
|
|
|
•
|
if applicable, the minimum or maximum amount of the warrants that may be exercised at any one time;
|
|
|
|
|
•
|
the currency or currency units in which the offering price, if any, and the exercise price are payable;
|
|
|
|
|
•
|
whether the warrants will be issued in global or non-global form;
|
|
|
|
|
•
|
the identities of the warrant agent, any depositaries and any paying, transfer, calculation or other agents for the warrants;
|
|
|
|
|
•
|
any securities exchange or quotation system on which the warrants or any securities deliverable upon exercise of the warrants may be listed;
|
|
|
|
|
•
|
whether the warrants are to be sold separately or with other securities, and if the warrants are to be sold with the securities of another company or other companies, certain information regarding such company or companies;
|
|
|
|
|
•
|
if applicable, a discussion of material U.S. federal income tax considerations;
|
|
|
|
|
•
|
the antidilution provisions of the warrants, if any;
|
|
|
|
|
•
|
the redemption or call provisions, if any, applicable to the warrants;
|
|
|
|
|
•
|
any provisions with respect to the holder’s right to require us to repurchase the warrants upon a change in control or similar event; and
|
|
|
|
|
•
|
any additional terms of the warrants, including procedures, and limitations relating to the exchange, exercise and settlement of the warrants.
|
Holders of equity warrants will not be entitled:
|
|
|
|
•
|
to vote, consent or receive dividends;
|
|
|
|
|
•
|
receive notice as stockholders with respect to any meeting of stockholders for the election of our directors or any other matter; or
|
|
|
|
|
•
|
exercise any rights as stockholders of us.
|
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to the specified time on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information, and paying the required amount to the warrant agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate and in the applicable prospectus supplement the information that the holder of the warrant will be required to deliver to the warrant agent in connection with the exercise of the warrant.
Upon receipt of the required payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate for the remaining amount of warrants. If we so indicate in the applicable prospectus supplement, holders of the warrants may surrender securities as all or part of the exercise price for the warrants.
No Limit on Issuance of Warrants
The warrant agreements will not limit the number of warrants or other securities that we may issue, except for the limitation of the number of shares authorized underlying such warrants.
Modifications
We and the relevant warrant agent may, without the consent of the holders, amend each warrant agreement and the terms of each issue of warrants, for the purpose of curing any ambiguity or of correcting or supplementing any defective or inconsistent provision, or in any other manner that we may deem necessary or desirable and that will not adversely affect the interests of the holders of the outstanding unexercised warrants in any material respect.
We and the relevant warrant agent also may, with the consent of the holders of at least a majority in number of the outstanding unexercised warrants affected, modify or amend the warrant agreement and the terms of the warrants. No such modification or amendment may, without the consent of each holder of an affected warrant:
•
reduce the amount receivable upon exercise, cancellation or expiration;
•
shorten the period of time during which the warrants may be exercised;
|
|
|
|
•
|
otherwise materially and adversely affect the exercise rights of the beneficial owners of the warrants; or
|
|
|
|
|
•
|
reduce the percentage of outstanding warrants whose holders must consent to modification or amendment of the applicable warrant agreement or the terms of the warrants.
|
Merger and Similar Transactions Permitted; No Restrictive Covenants or Events of Default
The warrant agreements will not restrict our ability to merge or consolidate with, or sell our assets to, another firm or to engage in any other transactions. If at any time there is a merger or consolidation involving us or a sale or other disposition of all or substantially all of our assets, the successor or assuming company will be substituted for us, with the same effect as if it had been named in the warrant agreement and in the warrants. We will be relieved of any further obligation under the warrant agreement or warrants, and, in the event of any such merger, consolidation, sale or other disposition, we as the predecessor corporation may at any time thereafter be dissolved, wound up or liquidated.
The warrant agreements will not include any restrictions on our ability to put liens on our assets, including our interests in our subsidiaries, nor will they provide for any events of default or remedies upon the occurrence of any events of default.
Warrant Agreements Will Not Be Qualified under Trust Indenture Act
No warrant agreement will be qualified as an indenture, and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders of warrants issued under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
Governing Law
Unless we provide otherwise in the applicable prospectus supplement, the warrants and warrant agreements, and any claim, controversy or dispute arising or related to the warrants or the warrant agreements, will be governed by and constructed in accordance with the laws of the State of Delaware.
Enforceability of Rights by Holders of Warrants
Each warrant agent will act solely as our agent under the applicable warrant agreement and not assume any obligations or relationship of agency or trust for or with any registered holder of or owner of a beneficial interest in any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder a a warrant may, without the consent of the applicable warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise their warrants, and to receive payment, if any, for their warrants, in the case of universal warrants.
LEGAL OWNERSHIP OF SECURITIES
We can issue securities in registered form or in the form of one or more global securities. We describe global securities in greater detail below. We refer to those persons who have securities registered in their own names on the books that we or any applicable trustee or depositary maintain for this purpose as the “holders” of those securities. These persons are the legal holders of the securities. We refer to those persons who, indirectly through others, own beneficial interests in securities that are not registered in their own names, as “indirect holders” of those securities. As we discuss below, indirect holders are not legal holders, and investors in securities issued in book-entry form or in street name will be indirect holders.
Book-Entry Holders
We may issue securities in book-entry form, as we will specify in the applicable prospectus supplement. This means securities may be represented by one or more global securities registered in the name of a financial institution that holds them as depositary on behalf of other financial institutions that participate in the depositary’s book-entry system. These participating institutions, which are referred to as participants, in turn, hold beneficial interests in the securities on behalf of themselves or their customers.
Subject to applicable law, only the person in whose name a security is registered is recognized as the holder of that security. Global securities will be registered in the name of the depositary or its participants. Consequently, for global securities, we will recognize only the depositary as the holder of the securities, and we will make all payments on the securities to the depositary. The depositary passes along the payments it receives to its participants, which in turn pass the payments along to their customers who are the beneficial owners. The depositary and its participants do so under agreements they have made with one another or with their customers; they are not obligated to do so under the terms of the securities.
As a result, investors in a global security will not own securities directly. Instead, they will own beneficial interests in a global security, through a bank, broker or other financial institution that participates in the depositary’s book-entry system or holds an interest through a participant. As long as the securities are issued in global form, investors will be indirect holders, and not legal holders, of the securities.
Street Name Holders
A global security may be terminated in certain situations as described under “-Special Situations When A Global Security Will Be Terminated,” or we may issue securities that are not issued in global form. In these cases, investors may choose to hold their securities in their own names or in “street name.” Securities held by an investor in street name would be registered in the name of a bank, broker or other financial institution that the investor chooses, and the investor would hold only a beneficial interest in those securities through an account he or she maintains at that institution.
For securities held in street name, we or any applicable trustee or depositary will recognize only the intermediary banks, brokers and other financial institutions in whose names the securities are registered as the holders of those securities, and we or any such trustee or depositary will make all payments on those securities to them. These institutions pass along the payments they receive to their customers who are the beneficial owners, but only because they agree to do so in their customer agreements or because they are legally required to do so. Investors who hold securities in street name will be indirect holders, not holders, of those securities.
Legal Holders
Subject to applicable law, our obligations, as well as the obligations of any applicable trustee and of any third parties employed by us or a trustee, run only to the legal holders of the securities. We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any other indirect means. This will be the case whether an investor chooses to be an indirect holder of a security or has no choice because we are issuing the securities only in global form.
For example, once we make a payment or give a notice to the holder, we have no further responsibility for the payment or notice even if that holder is required, under agreements with depositary participants or customers or by law, to pass it along to the indirect holders but does not do so. Similarly, we may want to obtain the approval of the holders to amend an indenture, to relieve us of the consequences of a default or of our obligation to comply with a particular provision of the indenture or for other purposes. In such an event, we would seek approval only from the holders, and not the indirect holders, of the securities. Whether and how the holders contact the indirect holders is up to the holders.
Special Considerations for Indirect Holders
If you hold securities through a bank, broker or other financial institution, either in book-entry form because the securities are represented by one or more global securities or in street name, you should check with your own institution to find out:
|
|
|
|
•
|
how it handles securities payments and notices;
|
|
|
|
|
•
|
whether it imposes fees or charges;
|
|
|
|
|
•
|
how it would handle a request for the holders’ consent, if ever required;
|
|
|
|
|
•
|
whether and how you can instruct it to send you securities registered in your own name so you can be a holder, if that is permitted in the future;
|
|
|
|
|
•
|
how it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to protect their interests; and
|
|
|
|
|
•
|
if the securities are in book-entry form, how the depositary’s rules and procedures will affect these matters.
|
Global Securities
A global security is a security that represents one or any other number of individual securities held by a depositary. Generally, all securities represented by the same global securities will have the same terms.
Each security issued in book-entry form will be represented by a global security that we issue to, deposit with and register in the name of a financial institution or its nominee that we select. The financial institution that we select for this purpose is called the depositary. Unless we specify otherwise in the applicable prospectus supplement, The Depository Trust Company, New York, New York, known as DTC, will be the depositary for all securities issued in book-entry form.
In general, a global security may not be transferred to or registered in the name of anyone other than the depositary, its nominee or a successor depositary, unless special termination situations arise. We describe those situations below under “-Special Situations When A Global Security Will Be Terminated.” As a result of these arrangements, the depositary, or its nominee, will be the sole registered owner and legal holder of all securities represented by a global security, and investors will be permitted to own only beneficial interests in a global security. Beneficial interests must be held by means of an account with a broker, bank or other financial institution that in turn has an account with the depositary or with another institution that does. Thus, an investor whose security is represented by a global security will be an indirect holder of a beneficial interest in the global security.
If the prospectus supplement for a particular security indicates that the security will be issued as a global security, then the security will be represented by a global security at all times unless and until the global security is terminated or applicable laws require otherwise. If termination occurs, we may issue the securities through another book-entry clearing system or decide that the securities may no longer be held through any book-entry clearing system.
Special Considerations For Global Securities
As an indirect holder, an investor’s rights relating to a global security will be governed by the account rules of the investor’s financial institution and of the depositary, as well as general laws relating to securities transfers. We do not recognize an indirect holder as a holder of securities and instead deal only with the depositary that holds the global security.
If securities are issued only as global securities, an investor should be aware of the following:
|
|
|
|
•
|
unless applicable laws provide otherwise, an investor cannot cause the securities to be registered in his or her name, and cannot obtain non-global certificates for his or her interest in the securities, except in the special situations we describe below;
|
|
|
|
|
•
|
an investor will be an indirect holder and must look to his or her own bank or broker for payments on the securities and protection of his or her legal rights relating to the securities, as we describe above;
|
|
|
|
|
•
|
an investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are required by law to own their securities in non-book-entry form;
|
|
|
|
|
•
|
an investor may not be able to pledge his or her interest in the global security in circumstances where certificates representing the securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective; and
|
|
|
|
|
•
|
the depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating to an investor’s interest in the global security. We and any applicable trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership interests in a global security. We and the trustee also do not supervise the depositary in any way;
|
|
|
|
|
•
|
the depositary may, and we understand that DTC will, require that those who purchase and sell interests in a global security within its book entry system use immediately available funds, and your broker or bank may require you to do so as well; and
|
|
|
|
|
•
|
financial institutions that participate in the depositary’s book entry system, and through which an investor holds its interest in a global security, may also have their own policies affecting payments, notices and other matters relating to the securities. There may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries.
|
There may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries.
Special Situations When A Global Security Will Be Terminated
In a few special situations described below, a global security will terminate and interests in it will be exchanged for physical certificates representing those interests. After that exchange, the choice of whether to hold securities directly or in street name will be up to the investor. Investors must consult their own banks or brokers to find out how to have their interests in securities transferred to their own names, so that they will be direct holders. We have described the rights of holders and street name investors above.
A global security will terminate when the following special situations occur:
|
|
|
|
•
|
if the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security and we do not appoint another institution to act as depositary within 90 days;
|
|
|
|
|
•
|
if we notify any applicable trustee that we wish to terminate that global security; or
|
|
|
|
|
•
|
if an event of default has occurred with regard to securities represented by that global security and has not been cured or waived.
|
The applicable prospectus supplement may also list additional situations for terminating a global security that would apply only to the particular series of securities covered by the prospectus supplement. When a global security terminates, the depositary, and neither we nor any applicable trustee, is responsible for deciding the names of the institutions that will be the initial direct holders.
PLAN OF DISTRIBUTION
We may sell the securities from time to time pursuant to underwritten public offerings, negotiated transactions, block trades or a combination of these methods. We may sell the securities to or through underwriters or dealers, through agents, or directly to one or more purchasers. We may distribute securities from time to time in one or more transactions:
|
|
|
|
•
|
at a fixed price or prices, which may be changed;
|
|
|
|
|
•
|
at market prices prevailing at the time of sale;
|
|
|
|
|
•
|
at prices related to such prevailing market prices;
|
|
|
|
|
•
|
at negotiated prices; or
|
|
|
|
|
•
|
a combination of these pricing methods.
|
We may also sell equity securities covered by this registration statement in an “at the market offering” as defined in Rule 415(a)(4) under the Securities Act. Such offering may be made into an existing trading market for such securities in transactions at other than a fixed price on or through the facilities of NASDAQ or any other securities exchange or quotation or trading service on which such securities may be listed, quoted or traded at the time of sale.
Such at-the-market offerings, if any, may be conducted by underwriters acting as principal or agent.
A prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the securities, including, to the extent applicable:
|
|
|
|
•
|
the name or names of any underwriters, dealers or agents, if any;
|
|
|
|
|
•
|
the purchase price of the securities and the proceeds we will receive from the sale;
|
|
|
|
|
•
|
any over-allotment options under which underwriters may purchase additional securities from us;
|
|
|
|
|
•
|
any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation;
|
|
|
|
|
•
|
any public offering price;
|
|
|
|
|
•
|
any discounts or concessions allowed or reallowed or paid to dealers; and
|
|
|
|
|
•
|
any securities exchange or market on which the securities may be listed.
|
Only underwriters named in the prospectus supplement are underwriters of the securities offered by the prospectus supplement.
If underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement. In connection with the sale of the securities, we, or the purchasers of securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts or commissions. The underwriter may sell the securities to or through dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters and/or commissions from the purchasers for which they may act as agent. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may change from time to time. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
Any compensation paid to underwriters, dealers or agents in connection with the offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers will be provided in the applicable prospectus supplement. Underwriters, dealers and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions.
We may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement so indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be named in the applicable prospectus supplement (or a post-effective amendment). In addition, we may otherwise loan or pledge securities to a financial
institution or other third party that in turn may sell the securities short using this prospectus and an applicable prospectus supplement. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities, and we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
We may provide agents, dealers and underwriters with indemnification against civil liabilities related to this offering, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities and to reimburse those persons for certain expenses. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business for which they receive compensation.
All securities we offer, other than our Common Stock, will be new issues of securities with no established trading market. Any underwriters to whom securities offered by this prospectus are sold by us for public offering and sale may make a market in the securities offered by this prospectus, but the underwriters will not be obligated to do so and may discontinue any market making at any time without notice. No assurance can be given as to the liquidity of the trading market for any securities offered by this prospectus.
The securities may or may not be listed on a national securities exchange. To facilitate the offering of securities, any underwriter may engage in overallotment, stabilizing transactions, short covering transactions and penalty bids. Overallotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time. These transactions may be effected on any exchange or over-the-counter market or otherwise.
Any underwriters who are qualified market makers on NASDAQ may engage in passive market making transactions in the securities on NASDAQ in accordance with Rule 103 of Regulation M, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
We will bear all costs, expenses and fees in connection with the registration of the securities as well as the expense of all commissions and discounts, if any, attributable to the sales of any of our securities by us.
To the extent required, this prospectus may be amended or supplemented from time to time to describe a specific plan of distribution.
LEGAL MATTERS
The validity of the shares of Common Stock offered hereby will be passed upon for us by DLA Piper LLP (US), Short Hills, New Jersey. Additional legal matters may be passed upon for us or any underwriters, dealers or agents by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The financial statements of the Company as of and for the years ended July 31, 2017 and July 31, 2016, appearing in the Company’s Annual Report on Form 10-K for the year ended July 31, 2017, have been audited by MaloneBailey, LLP, the Company’s independent registered public accounting firm, as set forth in their reports thereon, included therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such reports given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly current and other reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. You may also read and copy any document we file at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K, including any amendments to those reports, and other information that we file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act can also be accessed free of charge through the Internet. These filings will be available as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
We have filed with the SEC a registration statement under the Securities Act relating to the offering of these securities. The registration statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus does not contain all of the information set forth in the registration statement. You can obtain a copy of the registration statement, at prescribed rates, from the SEC at the address listed above. The registration statement and the documents referred to below under “Incorporation of Certain Documents by Reference” are also available on our website, www.opiant.com. We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” much of the information we file with them under Commission File No. 000-31719, which means that we can disclose important information to you by referring you to other documents we have filed or will file with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and any of our subsequent filings with the SEC will automatically update and supersede this information. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any statements in the prospectus or any document previously incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents set forth below that we have previously filed with the SEC, and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, except for information furnished under Items 2.02 or 7.01 of Current Report on Form 8-K, or exhibits related thereto, until the filing of a post-effective amendment to this prospectus which indicates that all securities registered have been sold or which deregisters all securities then remaining unsold:
|
|
|
|
•
|
our Annual Report on Form 10-K for the fiscal year ended, filed with the SEC on October 13, 2017, in which there is set forth the audited financial statements for our fiscal year ended July 31, 2017;
|
|
|
|
|
•
|
our proxy statement for our annual meeting of stockholders, filed with the SEC on July 27, 2017;
|
|
|
|
|
•
|
our current reports on Form 8-K, filed with the SEC on August 10, 2017, August 28, 2017, August 29, 2017, September 5, 2017, September 5, 2017, September 11, 2017, September 11, 2017, September 14, 2017 and October 6, 2017;
|
|
|
|
|
•
|
our description of our Common Stock contained in the Registration Statement on Form 8-A12B filed with the SEC on August 25, 2017; and
|
|
|
|
|
•
|
all other reports filed pursuant to Section 13(a) or 15(d) of the Exchange Act after the date of this registration statement and prior to the effectiveness of the registration statement.
|
We also incorporate by reference all documents we file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the sale of all the securities covered by this prospectus (including all such documents filed with the SEC after the date of the initial filing of the registration statement that contains this prospectus and prior to effectiveness of the registration statement or after such effectiveness), except in each case the information contained in such document to the extent “furnished” and not “filed.”
We will provide, upon written or oral request, to each person to whom a prospectus is delivered, a copy of any or all of the information that has been incorporated by reference in the prospectus but not delivered with the prospectus. You may request a copy of these filings, at no cost, by writing or calling us at:
Opiant Pharmaceuticals, Inc.
201 Santa Monica Boulevard, Suite 500
Santa Monica, CA 90401
Attn: Chief Financial Officer
(310) 598-5410
You may also access the documents incorporated by reference in this prospectus through our website at www.opiant.com. Except for the specific incorporated documents listed above, no information available on or through our website shall be deemed to be incorporated in this prospectus or the registration statement of which it forms a part.
You should rely only on the information incorporated by reference or provided in this prospectus or any supplement. We have not authorized anyone else to provide you with different information. You should not assume that information in this prospectus or any supplement is accurate as of any date other than the date on the front of these documents.
166,738 Shares
Opiant Pharmaceuticals, Inc.
Common Stock
PROSPECTUS SUPPLEMENT
November 2, 2018
Opiant Pharmaceuticals (NASDAQ:OPNT)
Historical Stock Chart
From Mar 2024 to Apr 2024
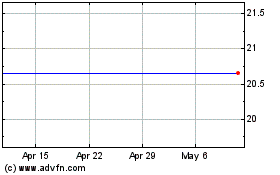
Opiant Pharmaceuticals (NASDAQ:OPNT)
Historical Stock Chart
From Apr 2023 to Apr 2024