Report of Foreign Issuer (6-k)
March 08 2018 - 6:10AM
Edgar (US Regulatory)
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
Pursuant
to Rule 13a-16 or 15d-16 of the
Securities
Exchange Act of 1934
For
the month of March 2018
Commission
File Number: 001-36581
Vascular
Biogenics Ltd.
(Translation
of registrant’s name into English)
8 HaSatat St.
Modi’in
Israel
7178106
(Address
of principal executive offices)
Indicate
by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form
20-F [X] Form 40-F [ ]
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): [ ]
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): [ ]
Indicate
by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information
to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes
[ ] No [X]
If
“Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-
EXPLANATORY
NOTE
Attached
hereto and incorporated by reference herein is the registrant’s press release issued on March 8, 2018, titled “VBL
Therapeutics Announces Top-Line Results from Pivotal Phase 3 GLOBE Study in Patients with Recurrent Glioblastoma”.
This Report of Foreign Private Issuer on Form 6-K shall be incorporated by reference into the Company’s registration statement
on Form F-3 (File No. 333-207250), filed with the Securities and Exchange Commission (the “SEC”) on October 2, 2015
and registration statement on Form F-3 (File No. 333-222138) filed on December 18, 2017, to the extent not superseded by information
subsequently filed or furnished (to the extent the Company expressly states that it incorporates such furnished information by
reference) by the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended.
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf
by the undersigned, thereunto duly authorized.
|
|
VASCULAR
BIOGENICS LTD.
|
|
|
|
|
|
Date:
March 8, 2018
|
By:
|
/s/
Dror Harats
|
|
|
Name:
|
Dror
Harats
|
|
|
Title:
|
Chief
Executive Officer
|
VBL
Therapeutics Announces Top-Line Results from
Pivotal Phase 3 GLOBE Study in Patients with
Recurrent Glioblastoma
Conference
Call and Webcast @ 8.30am ET Today
Tel
Aviv, March 8, 2018 – VBL Therapeutics (Nasdaq: VBLT), today reported top-line results from its pivotal Phase 3 GLOBE study
in patients with recurrent glioblastoma (rGBM) which was designed to evaluate VB-111 in combination with bevacizumab (Avastin
®
),
compared to the bevacizumab control arm. The study did not meet its pre-specified primary endpoint of overall survival (OS).
Dror
Harats, M.D., Chief Executive Officer of VBL Therapeutics, said "We are disappointed that our encouraging Phase 2 data were
not replicated in the GLOBE Phase 3 study, and once we receive the full and final data we will be analyzing them carefully to
better understand the outcome of the study. We are grateful to the trial investigators, site personnel, patients and caregivers
who participated in GLOBE. We believe that VB-111 may still hold promise for other indications we currently study or may study
in the future”.
|
Conference
Call
|
|
Thursday,
March 8 at 8:30am Eastern Time
|
|
US
Investors:
|
|
800-239-9838
|
|
International
Investors:
|
|
323-794-2551
|
|
Conference
ID:
|
|
3511679
|
|
Webcast:
|
|
https://edge.media-server.com/m6/p/f7whg6sk
|
About
the GLOBE study
The
GLOBE pivotal Phase 3 trial is a randomized, controlled, double-arm, open-label study of VB-111 dosed every two months in combination
with bevacizumab dosed every two weeks, compared to bevacizumab monotherapy. Key inclusion criteria include first or second progression
of glioblastoma following standard of care treatment with temozolomide and radiation, a histologically confirmed diagnosis of
glioblastoma and measurable disease by RANO criteria at progression.
The
study is conducted under a Special Protocol Assessment (SPA) granted by the FDA, with full endorsement by the Canadian Brain Tumor
Consortium (CBTC). VB-111 has received orphan drug designation in the United States and Europe and was granted Fast Track designation
by the FDA for promising and meaningful long-term survival in patients with glioblastoma that has recurred following treatment
with standard chemotherapy and radiation.
About
Glioblastoma (GBM)
GBM
is the most common and most aggressive form of primary brain tumors. In 2017, it is estimated there were approximately 12,000-13,000
new cases diagnosed in the United States. Median OS from diagnosis averages 12 to 15 months with patients treated usually with
surgery, chemotherapy and radiation. Progression occurs within approximately 6 months in virtually all patients, and upon progression
median OS is about 6-8 months. Although significant research and clinical efforts have focused on improving treatments for recurrent
GBM, no systemic therapy has shown an OS benefit, resulting in a significant unmet medical need.
About
VB-111 (ofranergene obadenovec)
VB-111,
a potential first-in-class anticancer therapeutic candidate, is the Company’s lead oncology product currently being studied
in a Phase 3 trial for ovarian cancer. VB-111 has demonstrated statistically significant OS and PFS in a Phase 2 trial in patients
with rGBM, versus current standard of care. VB-111 has received orphan drug designation in both the US and Europe, and fast track
designation in the US for prolongation of survival in patients with rGBM. In addition, VB-111 successfully demonstrated proof-of-concept
and survival benefit in Phase 2 clinical trials in radioiodine-refractory thyroid cancer and recurrent platinum resistant ovarian
cancer. VB-111 has received an Orphan Designation for the treatment of ovarian cancer by the European Medicines Agency (EMA).
About
VBL
Vascular
Biogenics Ltd., operating as VBL Therapeutics, is a clinical stage biopharmaceutical company focused on the discovery, development
and commercialization of first-in-class treatments for cancer. The Company’s lead oncology product candidate, ofranergene
obadenovec (VB-111), is a first-in-class, targeted anti-cancer gene-therapy agent that is positioned to treat a wide range of
solid tumors. It is conveniently administered as an IV infusion once every two months. It has been observed to be well-tolerated
in >300 cancer patients and demonstrated efficacy signals in an “all comers” Phase 1 trial as well as in three
tumor-specific Phase 2 studies. Ofranergene obadenovec is currently being studied in a Phase 3 trial for platinum-resistant ovarian
cancer.
Forward
Looking Statements
This
press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking
statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,”
“expect,” “goal,” “intend,” “look forward to”, “may,” “plan,”
“potential,” “predict,” “project,” “should,” “will,” “would”
and similar expressions. These forward-looking statements include, but are not limited to, statements regarding projected cash
resources and the clinical development of ofranergene obadenovec (VB-111), including our expectations for its therapeutic potention
in any other future indications we may study. These forward-looking statements are not promises or guarantees and involve substantial
risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected
herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews
and approvals, and the risk that historical clinical trial results may not be predictive of future trial results. . A further
list and description of these risks, uncertainties and other risks can be found in the Company’s regulatory filings with
the U.S. Securities and Exchange Commission, including in our annual report on Form 20-F for the year ended December 31, 2016.
Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak
only as of the date hereof. VBL Therapeutics undertakes no obligation to update or revise the information contained in this press
release, whether as a result of new information, future events or circumstances or otherwise.
INVESTOR
CONTACT:
Michael
Rice
LifeSci
Advisors, LLC
(646)
597-6979
MEDIA
CONTACT:
Matt
Middleman, M.D.
LifeSci
Public Relations
matt@lifescipublicrelations.com
(646)
627-8384

Vascular Biogenics (NASDAQ:VBLT)
Historical Stock Chart
From Mar 2024 to Apr 2024
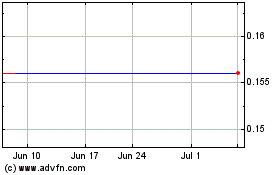
Vascular Biogenics (NASDAQ:VBLT)
Historical Stock Chart
From Apr 2023 to Apr 2024