Common Stock, $0.001 par value per share false 0001701108 0001701108 2024-01-05 2024-01-05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 5, 2024
SPERO THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-38266 |
|
46-4590683 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
| 675 Massachusetts Avenue, 14th Floor Cambridge, Massachusetts |
|
02140 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code (857) 242-1600
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.001 par value |
|
SPRO |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
As part of its press release, as described below, Spero Therapeutics, Inc. (the “Company”) disclosed that its cash and cash equivalents as of December 31, 2023 were approximately $76.3 million. The following information should be considered in connection with this preliminary result: The Company’s audited, consolidated financial statements as of December 31, 2023, are not yet available. Accordingly, the information presented above reflects the Company’s preliminary estimate, subject to the completion of the Company’s financial closing procedures and the annual audit of its financial statements by its auditors. As a result, this preliminary estimate may differ from the actual results that will be reflected in the Company’s audited, consolidated financial statements for the fiscal year ended December 31, 2023 when they are completed and publicly disclosed. This preliminary estimate may change, and that change may be material. The Company’s independent registered public accountants have not audited, reviewed or performed any procedures with respect to such preliminary estimate and accordingly do not express an opinion or any other form of assurance with respect thereto.
| Item 7.01. |
Regulation FD Disclosure. |
On January 5, 2024, the Company issued a press release entitled “Spero Therapeutics Provides Corporate Update and 2024 Outlook” (the “Press Release”). A copy of the Press Release is furnished as Exhibit 99.1 to this Current Report on Form 8-K (this “Current Report”).
In addition, on January 5, 2024, the Company updated its investor presentation (the “Investor Presentation”), which the Company expects to use in connection with general corporate presentations and will be made available on the Company’s website or distributed by the Company in hardcopy or electronic form. A copy of the Investor Presentation is attached as Exhibit 99.2 to this Current Report. The Investor Presentation is current as of January 5, 2024, and the Company disclaims any obligation to update the Investor Presentation after such date.
In accordance with General Instruction B.2 on Form 8-K, the information set forth in this Item 7.01, the Press Release, and the Investor Presentation is “furnished” and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section, nor shall such information be deemed incorporated by reference in any filing under the Securities Exchange Act of 1934, as amended, or the Securities Act of 1933, as amended.
Tebipenem HBr
As previously reported, the Company achieved first patient, first visit in PIVOT-PO, the global pivotal Phase 3 clinical trial of tebipenem HBr in patients with complicated urinary tract infections (cUTI), including acute pyelonephritis (AP), with a target enrollment of approximately 2,648 patients, which the Company expects to be completed in the second half of 2025.
Cash Runway Guidance
The Company believes its cash and cash equivalents as of December 31, 2023, together with the development milestone payments due under its license agreement with GlaxoSmithKline Intellectual Property (No. 3) Limited (GSK), will be sufficient to fund anticipated operating and capital expenditure requirements into late 2025.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: January 5, 2024 |
|
|
|
SPERO THERAPEUTICS, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Tamara Joseph |
|
|
|
|
|
|
Tamara Joseph |
|
|
|
|
|
|
Chief Legal Officer |
3
Exhibit 99.1
Spero Therapeutics Provides Corporate Update and 2024 Outlook
In 2H 2024, expect to provide Phase 2a topline
proof-of-concept data from our wholly-owned lead program SPR720 in NTM-PD patients
Initiated dosing in PIVOT-PO Phase 3 trial for tebipenem HBr in cUTI patients. Entitled to receive
$95 million in development milestones, payable over two years, as part of the GSK license agreement
Updated preliminary cash
balance and cash runway guidance into late 2025
CAMBRIDGE, Mass., January 5, 2023 — Spero Therapeutics, Inc. (Nasdaq: SPRO),
a multi-asset, clinical-stage, biopharmaceutical company, focused on identifying and developing novel therapies for rare diseases and multi-drug resistant (MDR) bacterial infections with high unmet need, today provided a corporate update
highlighting its recent accomplishments and anticipated milestones for 2024.
“We look forward to another productive year for Spero in 2024, having
achieved important clinical and regulatory milestones over the past twelve months,” said Sath Shukla, President, and CEO of Spero Therapeutics. “We are very pleased to move forward with the PIVOT-PO
clinical trial, evaluating tebipenem HBr in complicated urinary tract infections, as we recently began dosing patients. Our Phase 2a trial of SPR720 in nontuberculous mycobacterial pulmonary disease is on track, and we look forward to reporting
topline data, which is expected in the second half of 2024. Additionally, we ended 2023 with a strong cash balance, and believe our cash and cash equivalents, together with milestone payments from our tebipenem HBr commercial partner, GSK, will
provide runway into late 2025.”
2023 Pipeline Updates and 2024 Anticipated Milestones
SPR720 for Nontuberculous Mycobacterial Pulmonary Disease (NTM-PD)
| |
• |
|
In 2H 2024, the company expects to share topline data from the ongoing Phase 2a clinical trial of SPR720, which
is being developed as a novel first-line oral treatment for non-tuberculous mycobacterial pulmonary disease (NTM-PD). |
| |
• |
|
The trial is expected to enroll up to 35 treatment-naïve or
treatment-experienced non-refractory participants with NTM-PD, due to Mycobacterium avium complex. For more information on the trial, see ClinicalTrials.gov identifier
NCT05496374. |
Tebipenem HBr for complicated urinary tract infections (cUTI), including acute pyelonephritis (AP)
| |
• |
|
In December 2023, Spero achieved first patient, first visit in PIVOT-PO,
the global pivotal Phase 3 clinical trial of tebipenem HBr in patients with complicated urinary tract infections (cUTI), including acute pyelonephritis (AP), with a target enrollment of approximately 2,648 patients, which we expect to be completed
in 2H of 2025. |
| |
• |
|
The FDA has indicated that positive and persuasive results from PIVOT-PO,
supported with confirmatory evidence of efficacy, could be sufficient to support approval of tebipenem HBr as a treatment for cUTI, including pyelonephritis, for a limited use indication. |
| |
• |
|
As part of its license agreement with GSK, Spero is entitled to receive an additional $95 million in
development milestone payments, payable in four equal installments over two years. |
SPR206 for Bacterial Pneumonia
| |
• |
|
SPR206 is being developed in patients diagnosed with hospital-acquired or ventilator-associated bacterial
pneumonia caused by carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex (CRABC) or carbapenem-resistant Pseudomonas aeruginosa (CRPA). |
Financial Guidance
| |
• |
|
Cash and cash equivalents as of December 31, 2023, were approximately $76.3 million (unaudited). The
company believes its cash and cash equivalents, together with the development milestone payments due under its license agreement with GSK, will be sufficient to fund anticipated operating and capital expenditure requirements into late 2025.
|
The information presented above reflects the company’s preliminary estimate, subject to the completion of the
company’s financial closing procedures and the annual audit of its financial statements by its auditors. As a result, this preliminary estimate may differ from the actual results that will be reflected in the company’s audited,
consolidated financial statements for the fiscal year ended December 31, 2023, when they are completed and publicly disclosed.
Link: SPRO
Corporate Presentation
Tebipenem HBr Research Support
Select tebipenem HBr studies have been funded in part with federal funds from the U.S. Department of Health and Human Services; Administration for
Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority, under contract number HHSO100201800015C.
Government
Agency Research Support
The views expressed in this press release are those of the authors and may not reflect the official policy or position of the
Department of the Army, Department of Defense, or the U.S. Government. Select SPR206 studies are supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Joint Warfighter Medical Research Program under Award No.
W81XWH 19 1 0295. Opinions, interpretations, conclusions and recommendations are not necessarily endorsed by the Department of Defense.
National
Institute of Allergy and Infectious Disease
Select SPR206 studies have been funded in whole or in part with Federal funds from the National Institute
of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N93021C00022.
About Spero Therapeutics
Spero Therapeutics, headquartered in Cambridge, Massachusetts, is a multi-asset, clinical-stage biopharmaceutical company focused on identifying and developing
novel treatments for rare diseases and MDR bacterial infections.
| |
• |
|
Spero Therapeutics is developing SPR720 as a novel first-line oral therapy candidate for the treatment of a rare,
orphan pulmonary disease caused by NTM-PD. |
| |
• |
|
Tebipenem HBr is an investigational drug in the United States being developed for the treatment of cUTI,
including pyelonephritis, caused by certain bacteria, in adult patients who have limited treatment options; tebipenem HBr is not FDA-approved. |
| |
• |
|
Spero Therapeutics also has an IV-administered next generation polymyxin
product candidate, SPR206, developed from its potentiator platform, which is in development to treat MDR Gram-negative infections in the hospital setting. |
For more information, visit https://sperotherapeutics.com.
Forward Looking Statements
This press release may
contain forward-looking statements. These statements include, but are not limited to, statements about the design, initiation, timing, progress and results of Spero’s clinical trials and its research and development programs, as well as the
regulatory path forward for tebipenem HBr and potential FDA approval, the potential commercialization of tebipenem HBr, and the potential receipt under the GSK license agreement of milestone payments and royalties on future sales of tebipenem HBr,
and Spero’s cash forecast and cash runway. In some cases, forward-looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,”
“anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the
negative of these terms or other similar expressions. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including whether tebipenem HBr, SPR720 and SPR206 will
advance through the clinical trial process on a timely basis, or at all, taking into account the effects of possible regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, clinical trial design and clinical
outcomes; whether the results of such trials will warrant submission for approval from the FDA or equivalent foreign regulatory agencies; whether the FDA will ultimately approve tebipenem HBr and, if so, the timing of any such approval; whether the
FDA will require any additional clinical data that would delay approval of tebipenem HBr; whether results obtained in preclinical studies and clinical trials will be indicative of results obtained in future clinical trials; Spero’s reliance on
third parties to manufacture, develop, and commercialize its product candidates, if approved; Spero’s need for additional funding; Spero’s ability to retain key personnel; whether Spero’s cash resources will be sufficient to fund its
continuing operations for the periods and/or trials anticipated; changes to Spero’s financial results for the year ended December 31, 2023 due to the completion of financial closing procedures; and other factors discussed in the “Risk
Factors” set forth in filings that Spero periodically makes with the U.S. Securities and Exchange Commission. The forward-looking statements included in this press release represent Spero’s views as of the date of this press release. Spero
anticipates that subsequent events and developments will cause its views to change. However, while Spero may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These
forward-looking statements should not be relied upon as representing Spero’s views as of any date subsequent to the date of this press release.
Investor Relations Contact:
Ted Jenkins
Vice President, Investor Relations and Strategic
Finance
IR@sperotherapeutics.com
(617) 798-4039
Media Inquiries:
Lora Grassilli, Health Media Relations
Zeno Group
lora.grassilli@zenogroup.com
646-932-3735

Corporate Presentation January 2024
Exhibit 99.2

Forward-looking Statement This
presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, the potential regulatory path forward for tebipenem HBr and the potential approval of tebipenem HBr by
the U.S. Food and Drug Administration (FDA) and the timing thereof; the potential commercialization of tebipenem HBr and its future value, the potential receipt of milestone payments and royalties on future sales of tebipenem HBr under the
GlaxoSmithKline Intellectual Property (No. 3) Limited (GSK) license agreement; the Company’s cash runway; the future development and commercialization of SPR206 and SPR720; the potential number of patients who could be treated by tebipenem HBr
and SPR720 and market demand for tebipenem HBr and SPR720 generally; the effectiveness of tebipenem HBr and its potential impact on healthcare resource utilizations; expected broad access across payer channels for tebipenem HBr; the anticipated
shift in treating patients from intravenous to oral administration; the initiation, timing, progress and results of the Company’s preclinical studies and clinical trials and its research and development programs, including management’s
assessment of such results; the timing of the availability of data from the Company’s clinical trials; the timing of the Company’s filings with regulatory agencies; product candidate benefits; competitive position; business
strategies; objectives of management; potential growth opportunities; potential market size; reimbursement matters; possible or assumed future results of operations; projected costs and the availability of additional non-dilutive funding from
governmental agencies beyond any initially funded awards. In some cases, forward-looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,”
“aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,”
“potential” or “continue” or the negative of these terms or other similar expressions. All statements other than statements of historical facts contained in this presentation are forward-looking statements. The
Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these
forward-looking statements as a result of various factors, including whether the FDA will ultimately approve tebipenem HBr and, if so, the timing of any such approval; whether the FDA will require any additional clinical data or place labeling
restrictions on the use of tebipenem HBr that would add costs for the Company, delay approval and/or reduce the commercial prospects of tebipenem HBr; the Company’s need for additional funding; the lengthy, expensive, and uncertain process of
clinical drug development; the Company’s reliance on third parties to manufacture, develop, and commercialize its product candidates, if approved; the ability to develop and commercialize the Company’s product candidates, if approved;
the potential impact of the COVID-19 pandemic; the Company’s ability to retain key personnel; whether results obtained in preclinical studies and clinical trials will be indicative of results obtained in future clinical trials and whether
preliminary data from the Company’s clinical trials will be predictive of final results from such trials; the Company’s dependence on raising capital and whether the Company’s product candidates will advance through the
preclinical development and clinical trial process on a timely basis, or at all, taking into account such factors as the effects of possible regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, clinical trial
design, clinical data requirements and clinical outcomes; whether the results of such clinical trials will warrant submission for approval from the FDA or equivalent foreign regulatory agencies; decisions made by the FDA and
equivalent foreign regulatory agencies with respect to the development and commercialization of the Company’s product candidates; the commercial potential of the Company’s product candidates; the Company’s ability to
obtain adequate third-party reimbursement for its product candidates; whether the Company will satisfy all of the pre-conditions to receipt of the milestone payments under its various license and collaboration agreements; whether BARDA elects to
exercise its second option under the Company’s agreement with BARDA; the Company’s ability to implement its strategic plans; the Company’s ability to obtain, maintain and enforce intellectual property and other proprietary rights
for its product candidates; the risks and uncertainties related to market conditions; whether the Company’s cash resources will be sufficient to fund its continuing operations for the periods and/or trials anticipated; and other factors
discussed in the “Risk Factors” section of the Company’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC), and risks described in other filings the Company may make with the SEC in the future. The
forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the
Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of
any date subsequent to the date of this presentation.
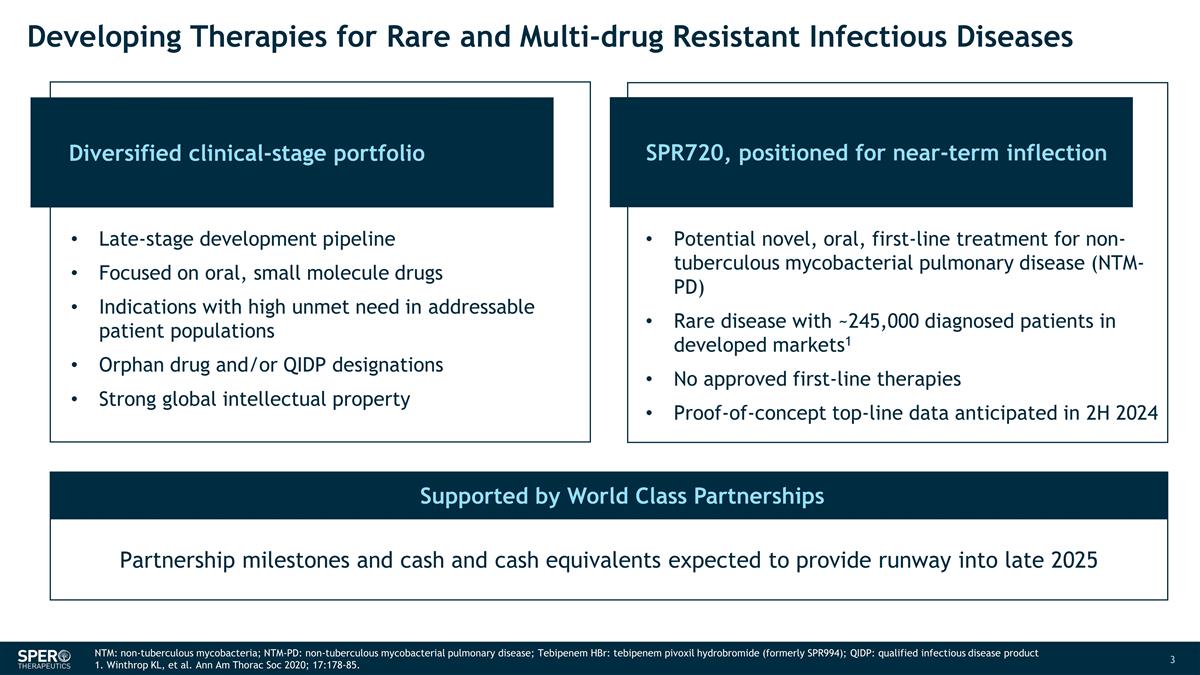
Diversified clinical-stage portfolio
Developing Therapies for Rare and Multi-drug Resistant Infectious Diseases SPR720, positioned for near-term inflection Partnership milestones and cash and cash equivalents expected to provide runway into late 2025 NTM:
non-tuberculous mycobacteria; NTM-PD: non-tuberculous mycobacterial pulmonary disease; Tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994); QIDP: qualified infectious disease product 1. Winthrop KL, et al. Ann Am Thorac Soc 2020;
17:178-85. Late-stage development pipeline Focused on oral, small molecule drugs Indications with high unmet need in addressable patient populations Orphan drug and/or QIDP designations Strong global intellectual property Potential
novel, oral, first-line treatment for non-tuberculous mycobacterial pulmonary disease (NTM-PD) Rare disease with ~245,000 diagnosed patients in developed markets1 No approved first-line therapies Proof-of-concept top-line data anticipated in 2H 2024
Supported by World Class Partnerships
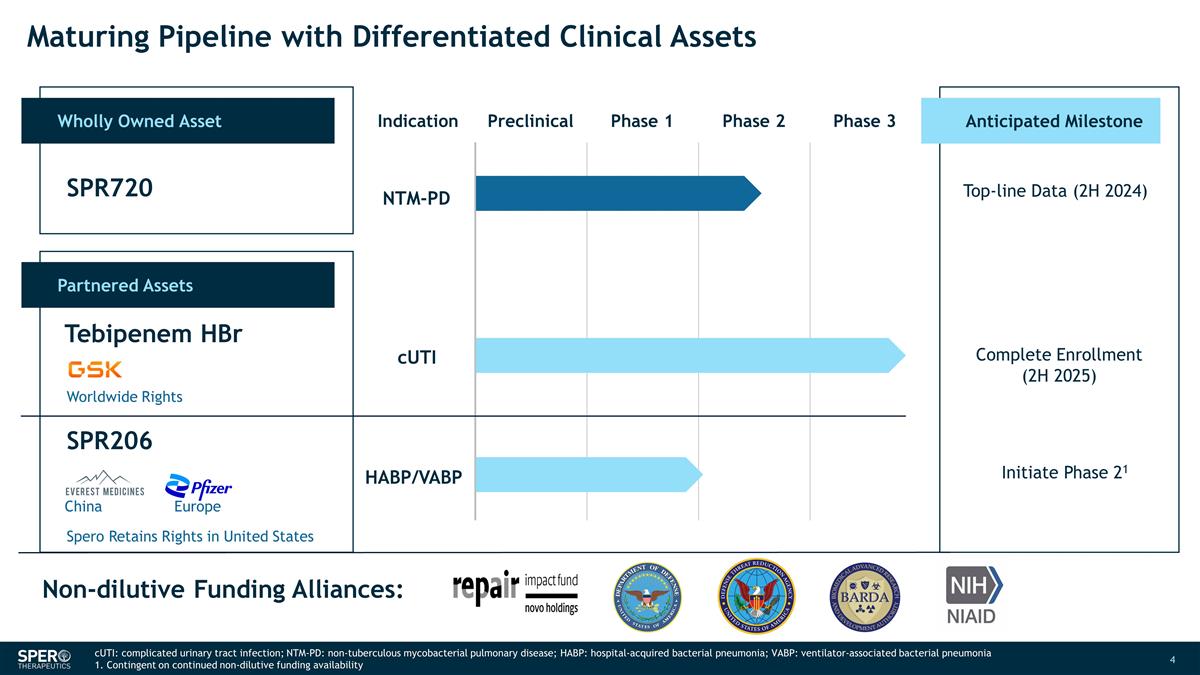
Maturing Pipeline with Differentiated
Clinical Assets Wholly Owned Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Partnered Assets SPR720 Tebipenem HBr SPR206 Anticipated Milestone NTM-PD Top-line Data (2H 2024) Complete Enrollment (2H 2025) Initiate Phase 21 Non-dilutive Funding
Alliances: Worldwide Rights Spero Retains Rights in United States cUTI HABP/VABP China Europe cUTI: complicated urinary tract infection; NTM-PD: non-tuberculous mycobacterial pulmonary disease; HABP: hospital-acquired bacterial pneumonia; VABP:
ventilator-associated bacterial pneumonia 1. Contingent on continued non-dilutive funding availability
SPR720
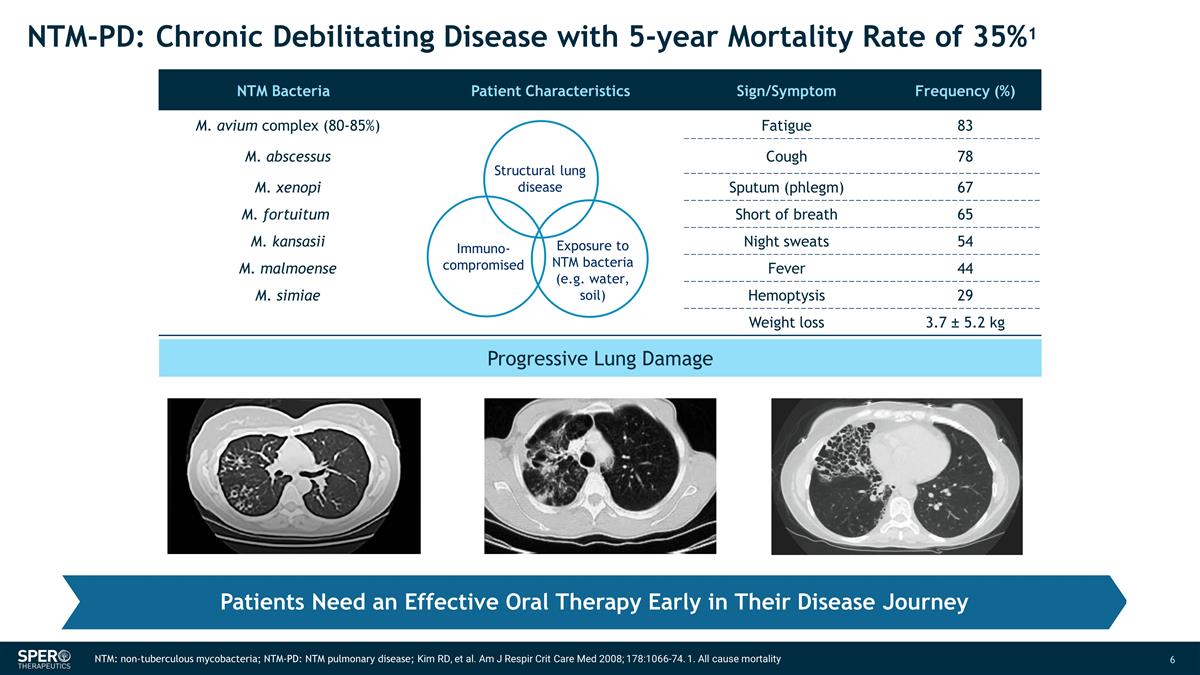
Progressive Lung Damage NTM:
non-tuberculous mycobacteria; NTM-PD: NTM pulmonary disease; Kim RD, et al. Am J Respir Crit Care Med 2008; 178:1066-74. 1. All cause mortality Patients Need an Effective Oral Therapy Early in Their Disease Journey NTM-PD: Chronic Debilitating
Disease with 5-year Mortality Rate of 35%1 NTM Bacteria Patient Characteristics Sign/Symptom Frequency (%) M. avium complex (80-85%) Fatigue 83 M. abscessus Cough 78 M. xenopi Sputum (phlegm) 67 M. fortuitum Short of breath 65 M.
kansasii Night sweats 54 M. malmoense Fever 44 M. simiae Hemoptysis 29 Weight loss 3.7 ± 5.2 kg Structural lung disease Exposure to NTM bacteria (e.g. water, soil) Immuno- compromised
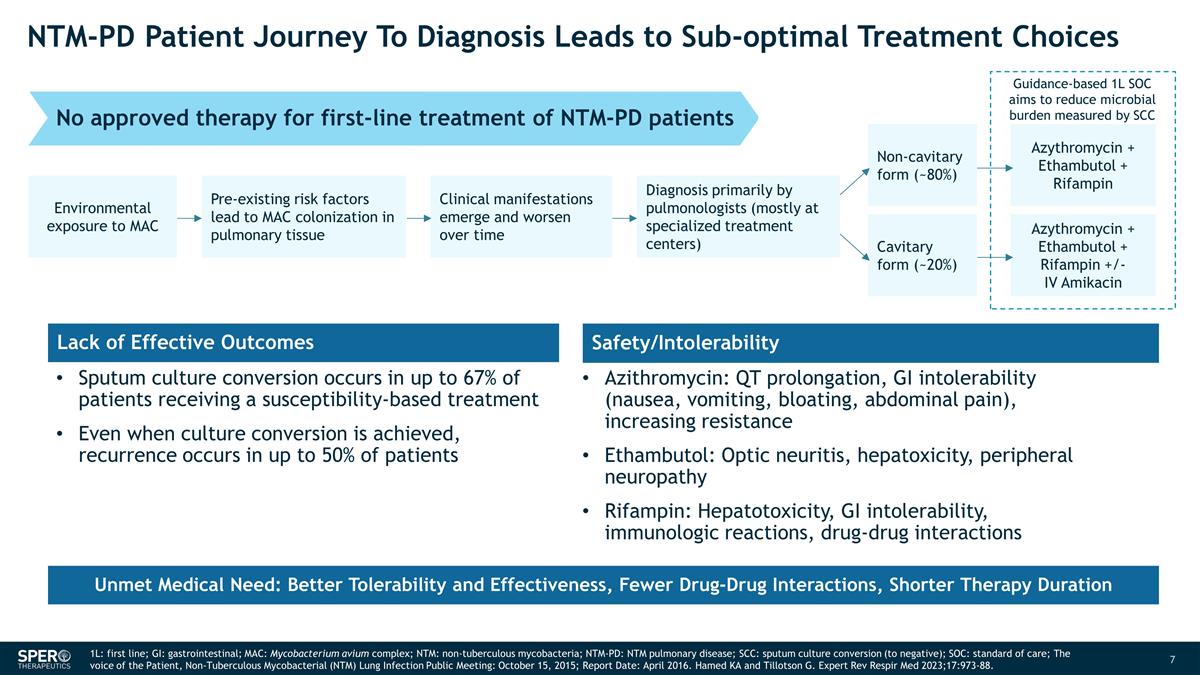
NTM-PD Patient Journey To Diagnosis
Leads to Sub-optimal Treatment Choices Guidance-based 1L SOC aims to reduce microbial burden measured by SCC Environmental exposure to MAC Pre-existing risk factors lead to MAC colonization in pulmonary tissue Clinical manifestations emerge and
worsen over time Diagnosis primarily by pulmonologists (mostly at specialized treatment centers) Non-cavitary form (~80%) Cavitary form (~20%) Azythromycin + Ethambutol + Rifampin Azythromycin + Ethambutol + Rifampin +/- IV Amikacin Sputum culture
conversion occurs in up to 67% of patients receiving a susceptibility-based treatment Even when culture conversion is achieved, recurrence occurs in up to 50% of patients Lack of Effective Outcomes Safety/Intolerability Azithromycin: QT
prolongation, GI intolerability (nausea, vomiting, bloating, abdominal pain), increasing resistance Ethambutol: Optic neuritis, hepatoxicity, peripheral neuropathy Rifampin: Hepatotoxicity, GI intolerability, immunologic reactions, drug-drug
interactions No approved therapy for first-line treatment of NTM-PD patients Unmet Medical Need: Better Tolerability and Effectiveness, Fewer Drug-Drug Interactions, Shorter Therapy Duration 1L: first line; GI: gastrointestinal; MAC:
Mycobacterium avium complex; NTM: non-tuberculous mycobacteria; NTM-PD: NTM pulmonary disease; SCC: sputum culture conversion (to negative); SOC: standard of care; The voice of the Patient, Non-Tuberculous Mycobacterial (NTM) Lung
Infection Public Meeting: October 15, 2015; Report Date: April 2016. Hamed KA and Tillotson G. Expert Rev Respir Med 2023;17:973-88.
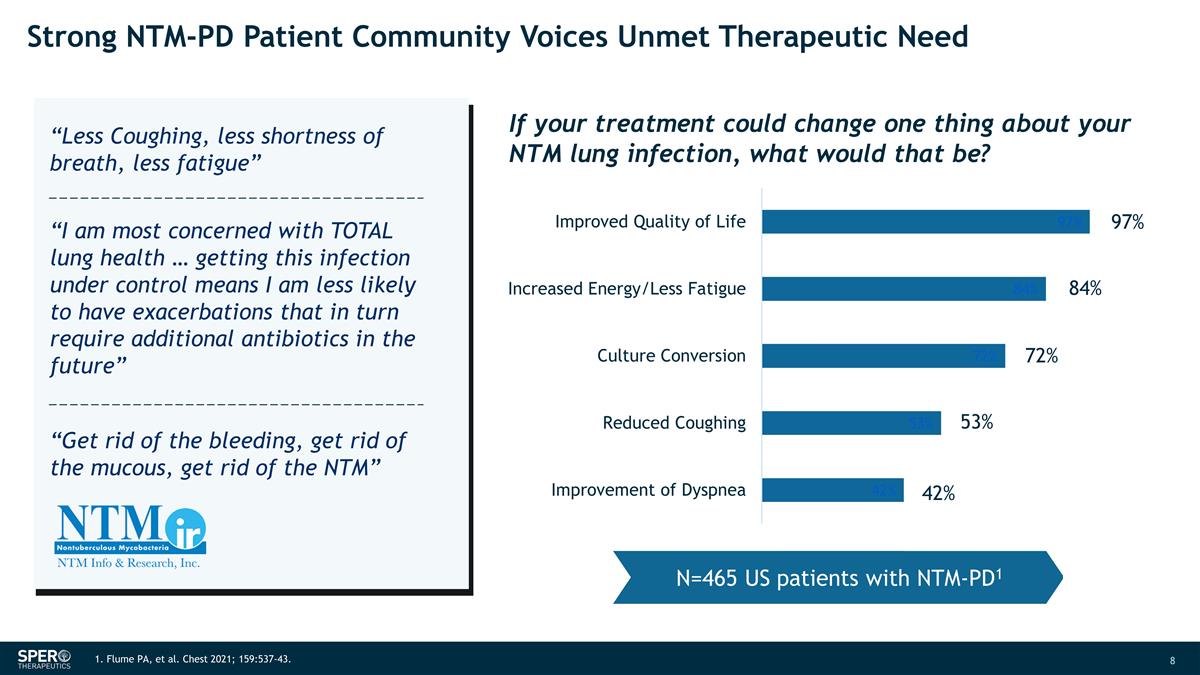
Strong NTM-PD Patient Community Voices
Unmet Therapeutic Need 97% 84% 72% 53% 42% 1. Flume PA, et al. Chest 2021; 159:537-43. N=465 US patients with NTM-PD1 If your treatment could change one thing about your NTM lung infection, what would that be? “Less Coughing, less shortness of
breath, less fatigue” “I am most concerned with TOTAL lung health … getting this infection under control means I am less likely to have exacerbations that in turn require additional antibiotics in the future” “Get rid
of the bleeding, get rid of the mucous, get rid of the NTM”
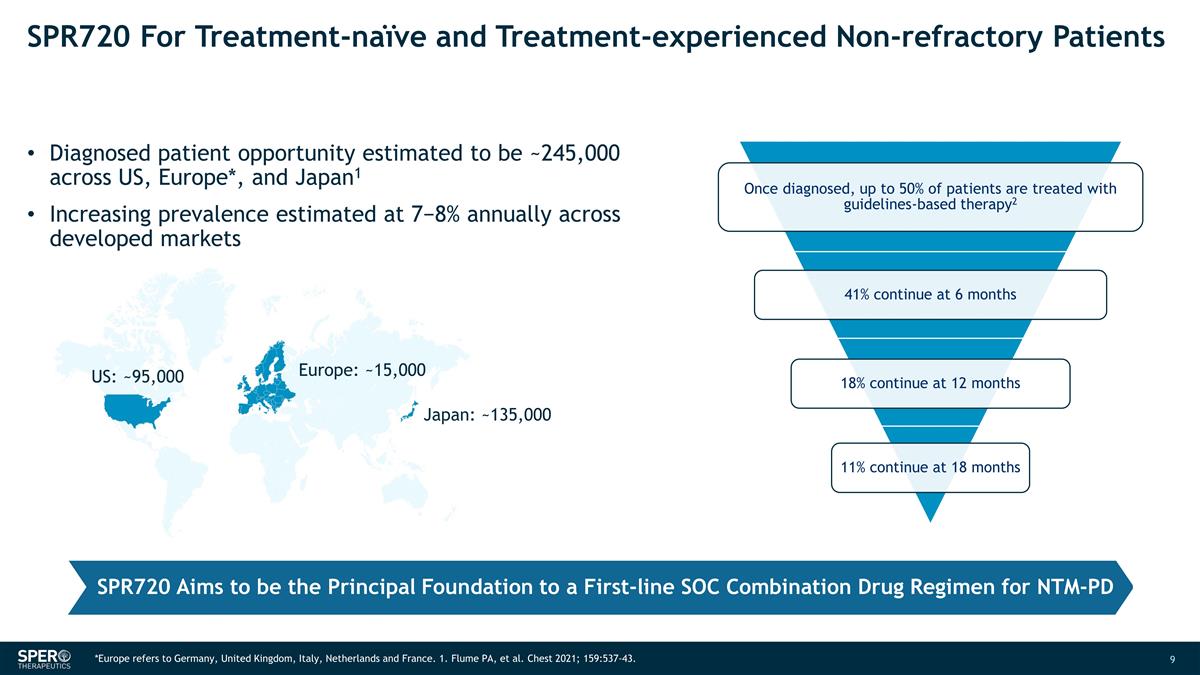
SPR720 For Treatment-naïve and
Treatment-experienced Non-refractory Patients Diagnosed patient opportunity estimated to be ~245,000 across US, Europe*, and Japan1 Increasing prevalence estimated at 7−8% annually across developed markets Europe: ~15,000 Japan: ~135,000 US:
~95,000 SPR720 Aims to be the Principal Foundation to a First-line SOC Combination Drug Regimen for NTM-PD *Europe refers to Germany, United Kingdom, Italy, Netherlands and France. 1. Flume PA, et al. Chest 2021; 159:537-43.
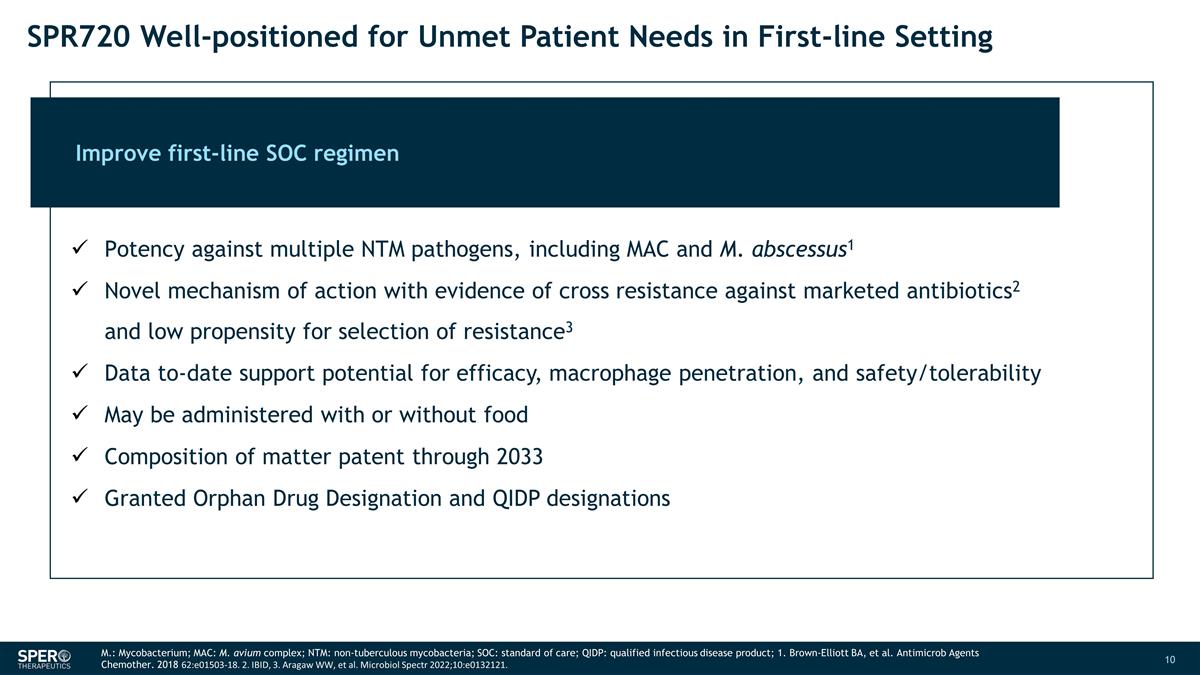
SPR720 Well-positioned for Unmet
Patient Needs in First-line Setting M.: Mycobacterium; MAC: M. avium complex; NTM: non-tuberculous mycobacteria; SOC: standard of care; QIDP: qualified infectious disease product; 1. Brown-Elliott BA, et al. Antimicrob Agents Chemother. 2018
62:e01503-18. 2. IBID, 3. Aragaw WW, et al. Microbiol Spectr 2022;10:e0132121. Improve first-line SOC regimen Potency against multiple NTM pathogens, including MAC and M. abscessus1 Novel mechanism of action with evidence of cross resistance against
marketed antibiotics2 and low propensity for selection of resistance3 Data to-date support potential for efficacy, macrophage penetration, and safety/tolerability May be administered with or without food Composition of matter patent through
2033 Granted Orphan Drug Designation and QIDP designations
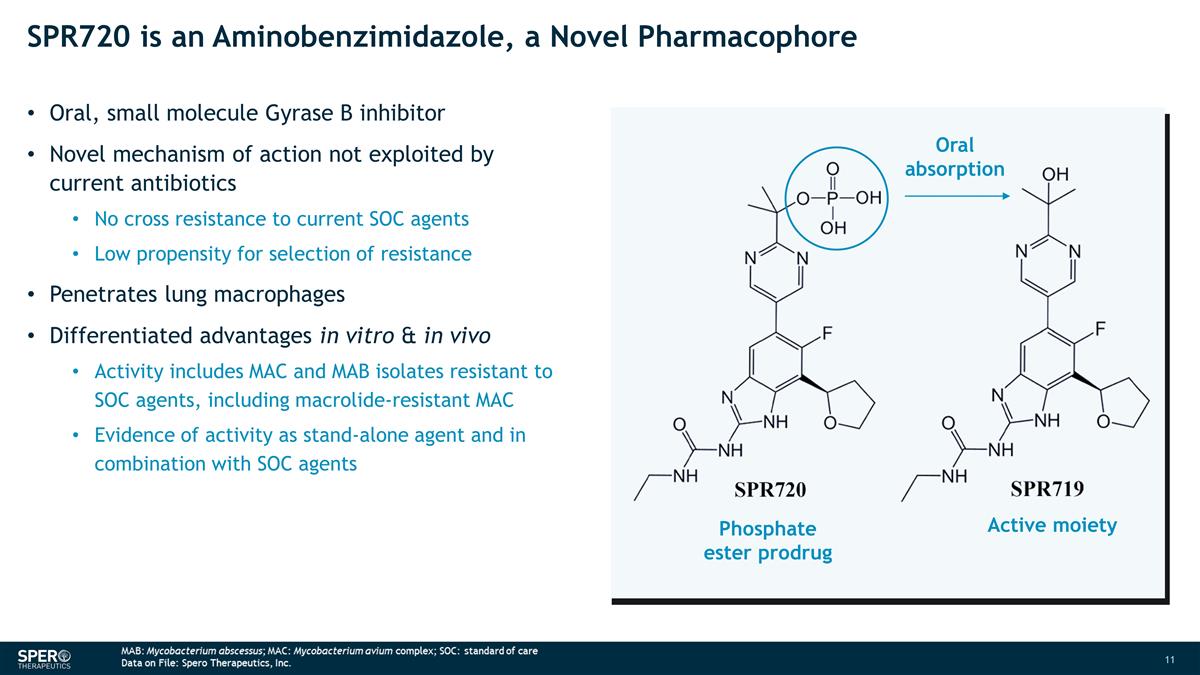
SPR720 is an Aminobenzimidazole, a
Novel Pharmacophore Oral, small molecule Gyrase B inhibitor Novel mechanism of action not exploited by current antibiotics No cross resistance to current SOC agents Low propensity for selection of resistance Penetrates lung macrophages
Differentiated advantages in vitro & in vivo Activity includes MAC and MAB isolates resistant to SOC agents, including macrolide-resistant MAC Evidence of activity as stand-alone agent and in combination with SOC agents Phosphate ester
prodrug Active moiety Oral absorption
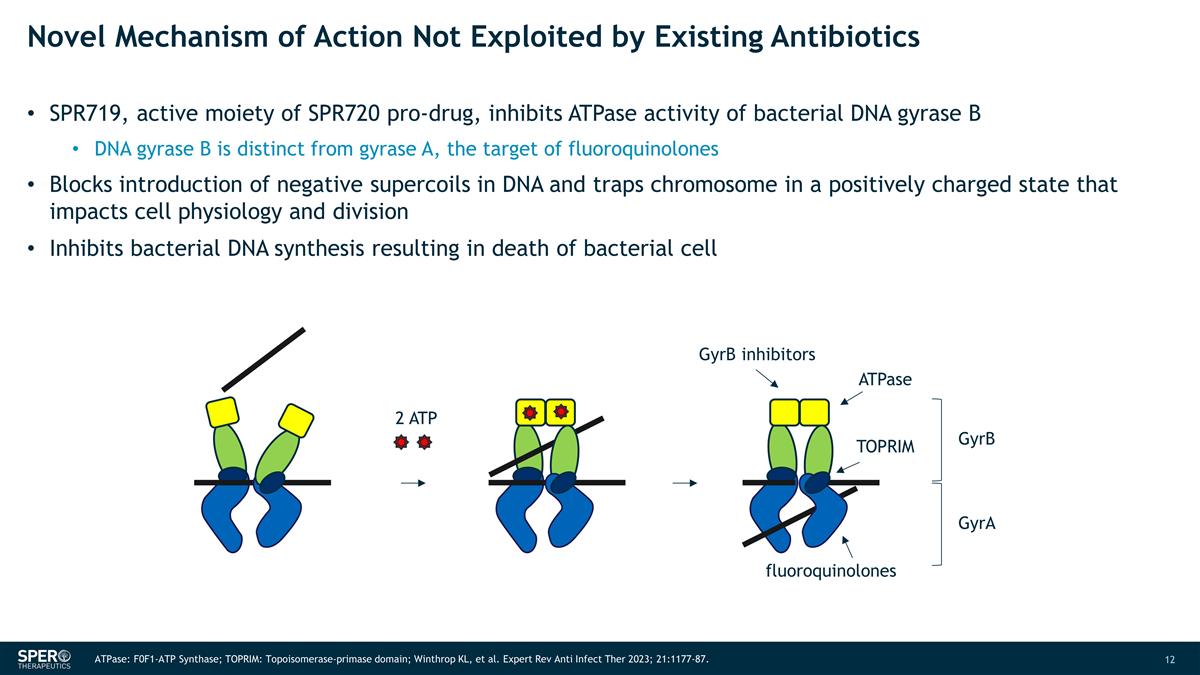
Novel Mechanism of Action Not
Exploited by Existing Antibiotics SPR719, active moiety of SPR720 pro-drug, inhibits ATPase activity of bacterial DNA gyrase B DNA gyrase B is distinct from gyrase A, the target of fluoroquinolones Blocks introduction of negative supercoils in DNA
and traps chromosome in a positively charged state that impacts cell physiology and division Inhibits bacterial DNA synthesis resulting in death of bacterial cell 2 ATP GyrB inhibitors ATPase TOPRIM fluoroquinolones GyrB GyrA ATPase: F0F1-ATP
Synthase; TOPRIM: Topoisomerase-primase domain; Winthrop KL, et al. Expert Rev Anti Infect Ther 2023; 21:1177-87.
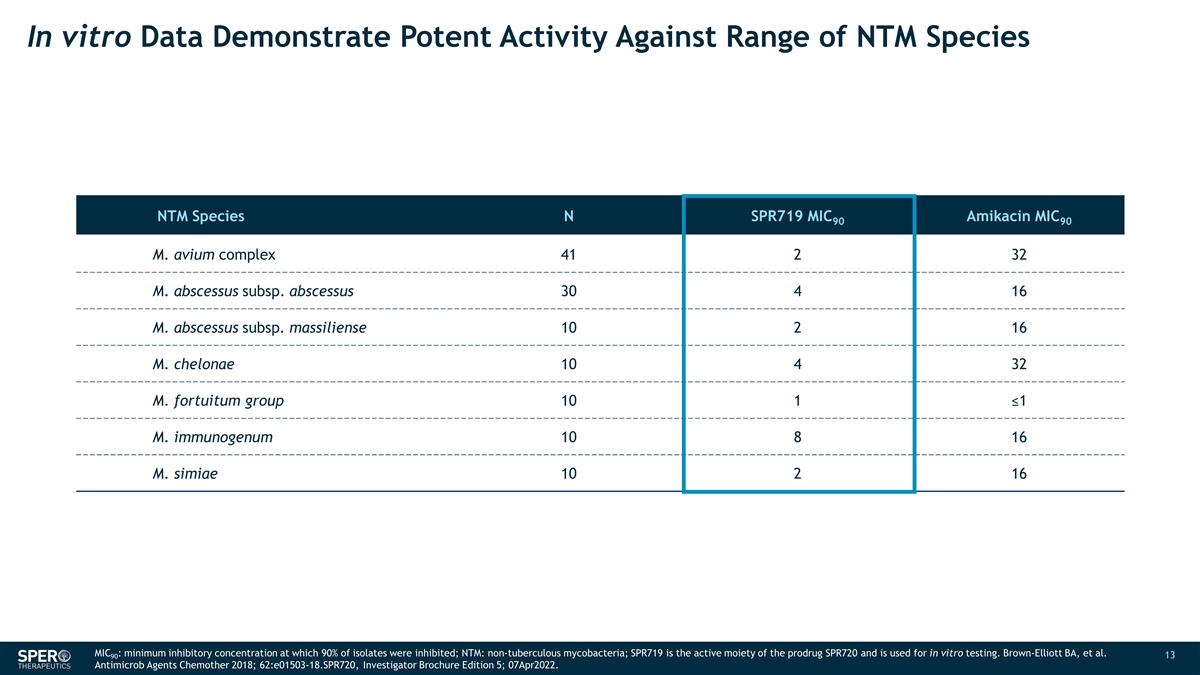
In vitro Data Demonstrate Potent
Activity Against Range of NTM Species NTM Species N SPR719 MIC90 Amikacin MIC90 M. avium complex 41 2 32 M. abscessus subsp. abscessus 30 4 16 M. abscessus subsp. massiliense 10 2 16 M. chelonae 10 4 32 M. fortuitum group 10 1 ≤1 M.
immunogenum 10 8 16 M. simiae 10 2 16 MIC90: minimum inhibitory concentration at which 90% of isolates were inhibited; NTM: non-tuberculous mycobacteria; SPR719 is the active moiety of the prodrug SPR720 and is used for in vitro testing.
Brown-Elliott BA, et al. Antimicrob Agents Chemother 2018; 62:e01503-18.SPR720, Investigator Brochure Edition 5; 07Apr2022.
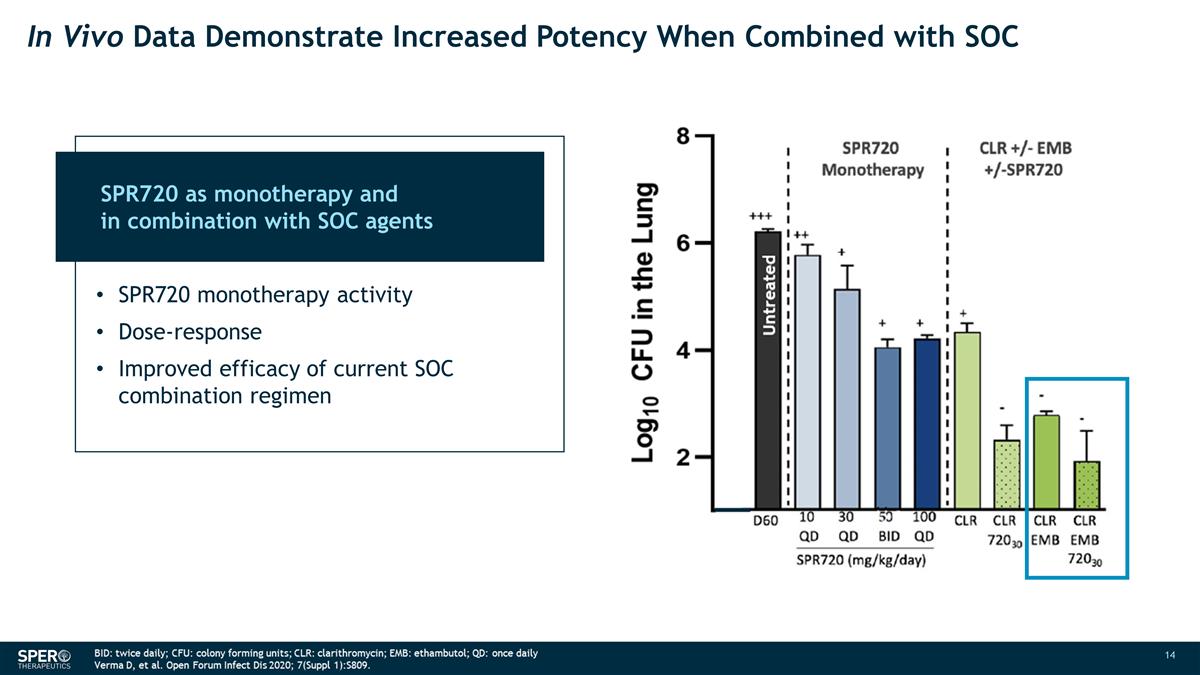
In Vivo Data Demonstrate Increased
Potency When Combined with SOC Untreated SPR720 as monotherapy and in combination with SOC agents SPR720 monotherapy activity Dose-response Improved efficacy of current SOC combination regimen
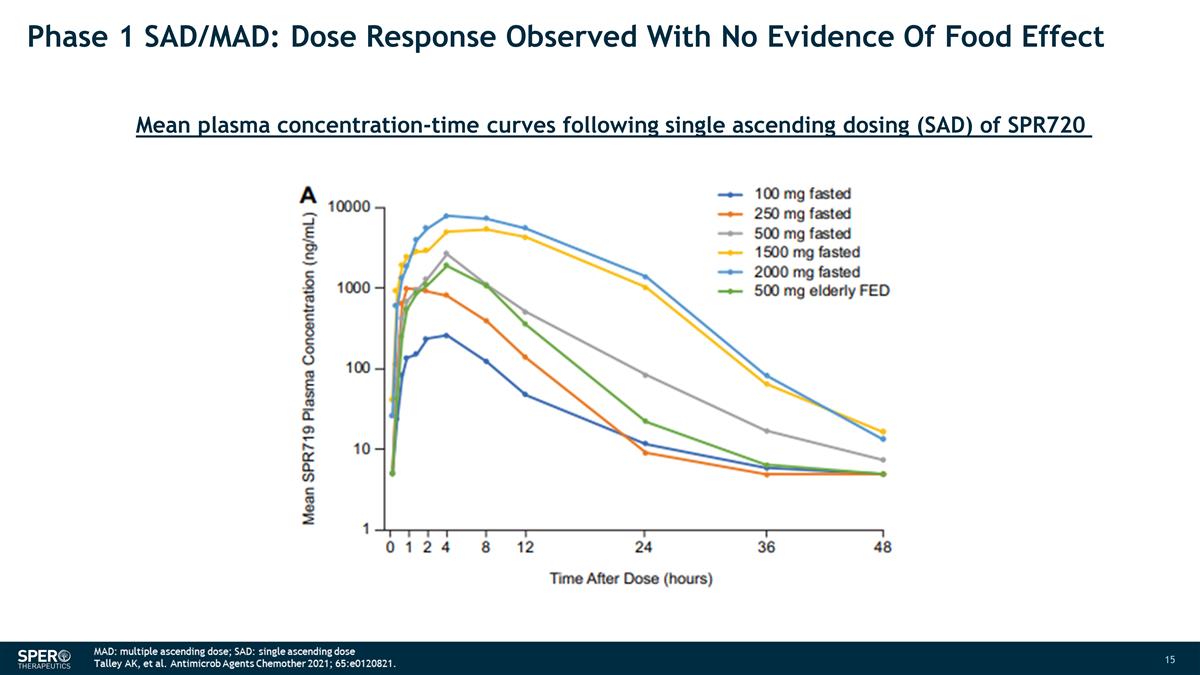
Phase 1 SAD/MAD: Dose Response
Observed With No Evidence Of Food Effect Mean plasma concentration-time curves following single ascending dosing (SAD) of SPR720
Phase 1 SAD/MAD: Favorable
Safety/Tolerability Profile 500 mg and 1000 mg doses evaluated over 7- and 14-days Dose-dependent increase in plasma exposure No SAEs reported; mild GI symptoms at ≥1500mg doses One discontinuation at 1500 mg dose: Grade 2 pancreatic enzyme
elevation that was asymptomatic, monitorable and reversible Phase 1 Results Show SPR720 was Well-tolerated with Exposures above Predicted Therapeutic Levels
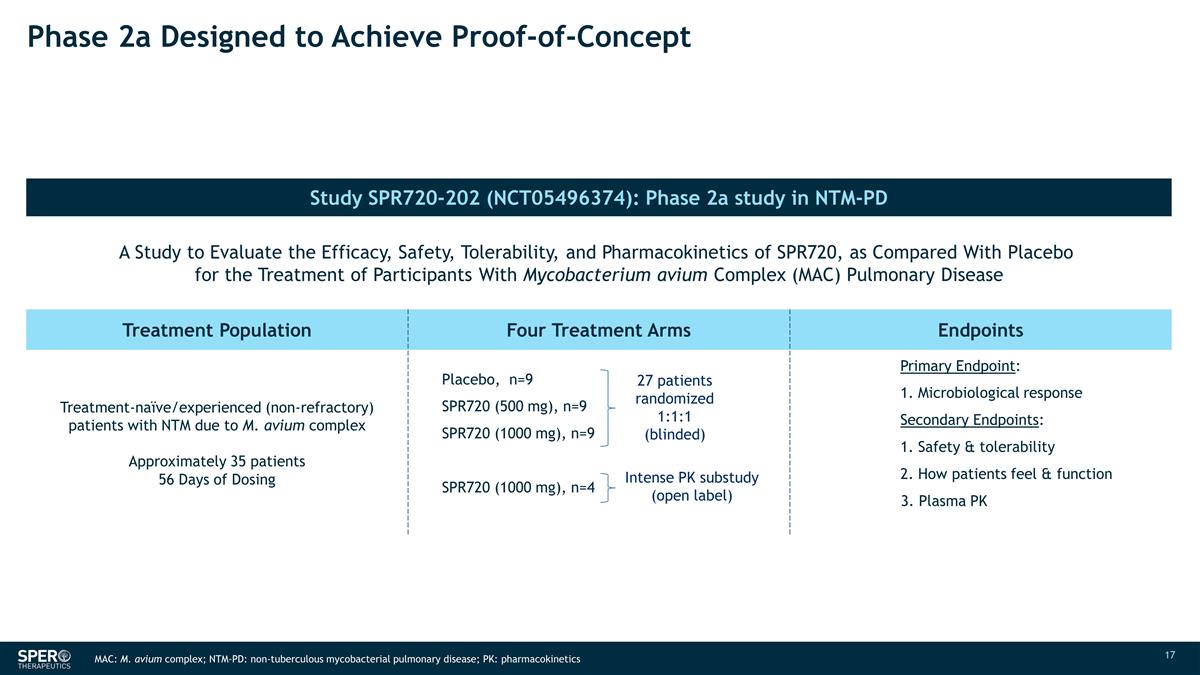
Phase 2a Designed to Achieve
Proof-of-Concept Study SPR720-202 (NCT05496374): Phase 2a study in NTM-PD N SPR719 MIC50 A Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of SPR720, as Compared With Placebo for the Treatment of Participants With
Mycobacterium avium Complex (MAC) Pulmonary Disease 30 2 Treatment Population Four Treatment Arms Endpoints Treatment-naïve/experienced (non-refractory) patients with NTM due to M. avium complex Approximately 35 patients 56 Days of
Dosing Placebo, n=9 SPR720 (500 mg), n=9 SPR720 (1000 mg), n=9 SPR720 (1000 mg), n=4 Primary Endpoint: 1. Microbiological response Secondary Endpoints: 1.
Safety & tolerability 2. How patients feel & function 3. Plasma PK 27 patients randomized 1:1:1 (blinded) Intense PK substudy (open label) MAC: M. avium complex; NTM-PD: non-tuberculous mycobacterial pulmonary disease; PK:
pharmacokinetics
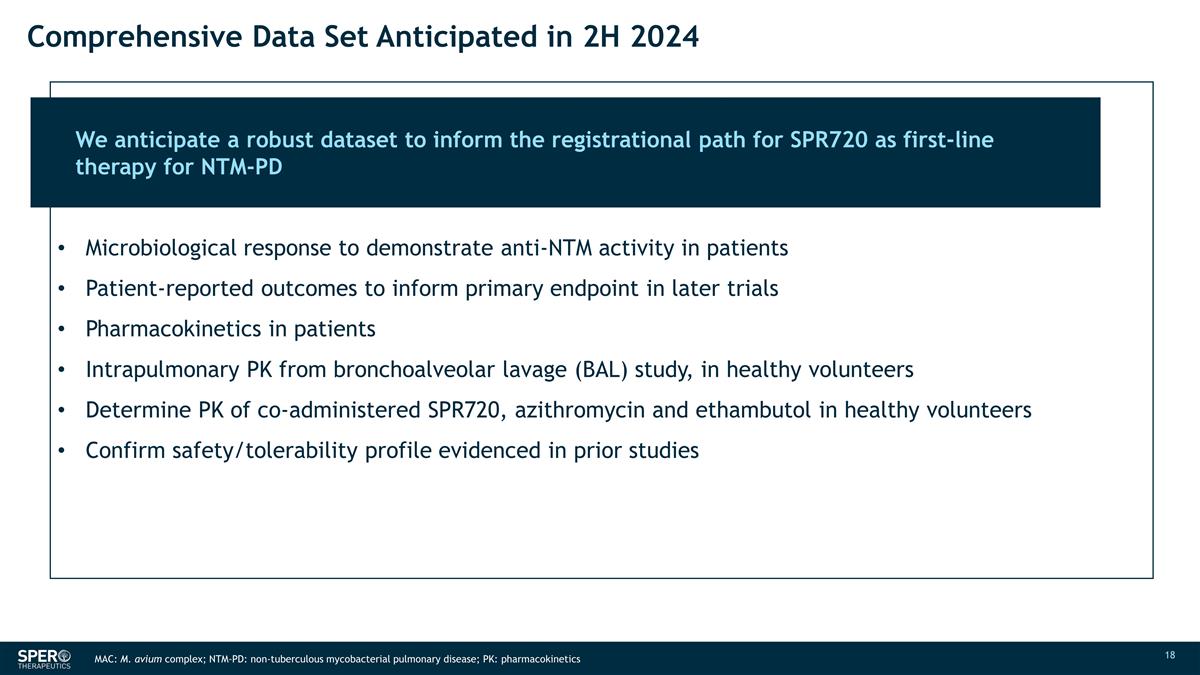
Comprehensive Data Set Anticipated
in 2H 2024 We anticipate a robust dataset to inform the registrational path for SPR720 as first-line therapy for NTM-PD Microbiological response to demonstrate anti-NTM activity in patients Patient-reported outcomes to inform primary endpoint in
later trials Pharmacokinetics in patients Intrapulmonary PK from bronchoalveolar lavage (BAL) study, in healthy volunteers Determine PK of co-administered SPR720, azithromycin and ethambutol in
healthy volunteers Confirm safety/tolerability profile evidenced in prior studies MAC: M. avium complex; NTM-PD: non-tuberculous mycobacterial pulmonary disease; PK: pharmacokinetics
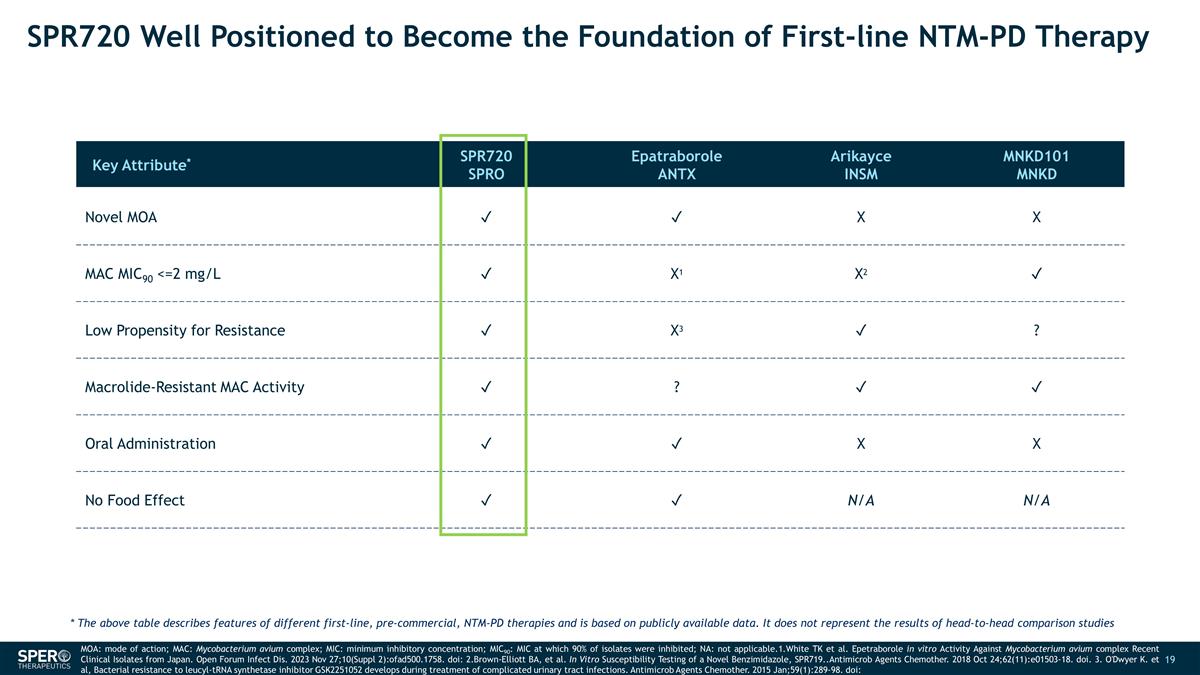
SPR720 Well Positioned to Become
the Foundation of First-line NTM-PD Therapy Key Attribute* SPR720 SPRO Epatraborole ANTX Arikayce INSM MNKD101 MNKD Novel MOA ✓ ✓ X X MAC MIC90 <=2 mg/L ✓ X1 X2 ✓ Low Propensity for Resistance ✓ X3 ✓ ?
Macrolide-Resistant MAC Activity ✓ ? ✓ ✓ Oral Administration ✓ ✓ X X No Food Effect ✓ ✓ N/A N/A MOA: mode of action; MAC: Mycobacterium avium complex; MIC: minimum inhibitory concentration; MIC90: MIC
at which 90% of isolates were inhibited; NA: not applicable.1.White TK et al. Epetraborole in vitro Activity Against Mycobacterium avium complex Recent Clinical Isolates from Japan. Open Forum Infect Dis. 2023 Nov 27;10(Suppl
2):ofad500.1758. doi: 2.Brown-Elliott BA, et al. In Vitro Susceptibility Testing of a Novel Benzimidazole, SPR719..Antimicrob Agents Chemother. 2018 Oct 24;62(11):e01503-18. doi. 3. O'Dwyer K. et al, Bacterial resistance to leucyl-tRNA
synthetase inhibitor GSK2251052 develops during treatment of complicated urinary tract infections. Antimicrob Agents Chemother. 2015 Jan;59(1):289-98. doi: * The above table describes features of different first-line, pre-commercial, NTM-PD
therapies and is based on publicly available data. It does not represent the results of head-to-head comparison studies
Tebipenem HBr Oral
Carbapenem
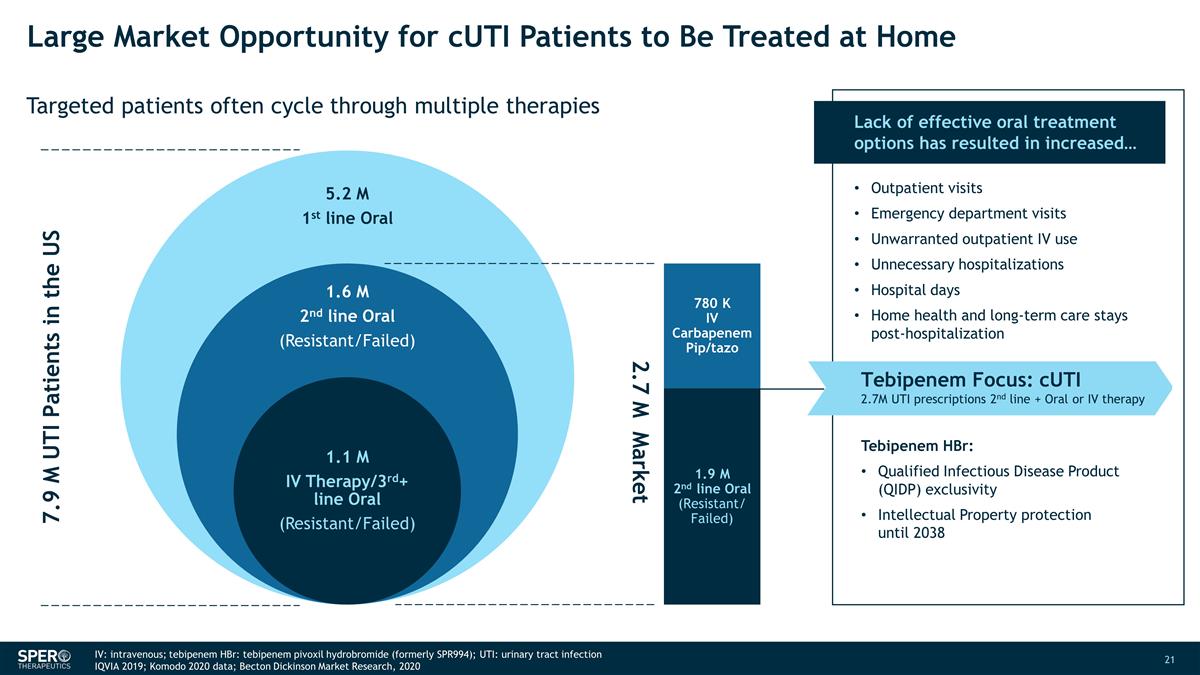
Large Market Opportunity for cUTI
Patients to Be Treated at Home Targeted patients often cycle through multiple therapies 7.9 M UTI Patients in the US 2.7 M Market 780 K IV Carbapenem Pip/tazo 1.9 M 2nd line Oral (Resistant/ Failed) Lack of effective oral treatment options has
resulted in increased… Outpatient visits Emergency department visits Unwarranted outpatient IV use Unnecessary hospitalizations Hospital days Home health and long-term care stays post-hospitalization Tebipenem Focus: cUTI 2.7M UTI
prescriptions 2nd line + Oral or IV therapy Tebipenem HBr: Qualified Infectious Disease Product (QIDP) exclusivity Intellectual Property protection until 2038 IV: intravenous; tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994);
UTI: urinary tract infection IQVIA 2019; Komodo 2020 data; Becton Dickinson Market Research, 2020
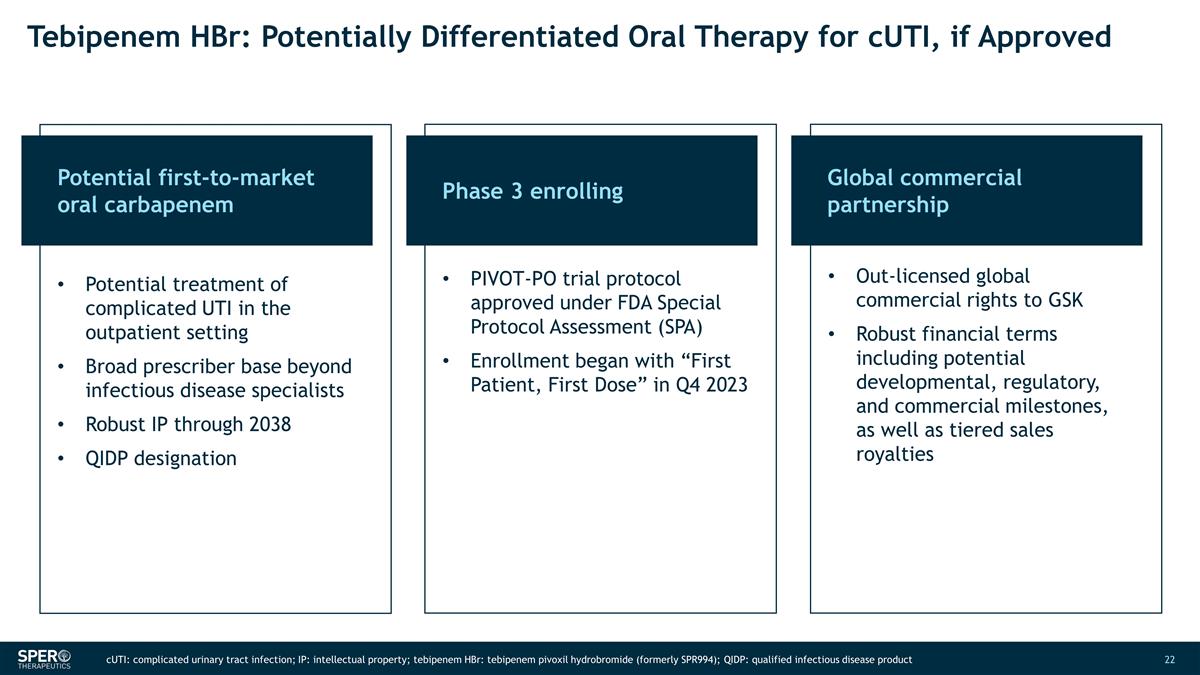
Tebipenem HBr: Potentially
Differentiated Oral Therapy for cUTI, if Approved Potential first-to-market oral carbapenem Phase 3 enrolling Global commercial partnership Potential treatment of complicated UTI in the outpatient setting Broad prescriber base beyond infectious
disease specialists Robust IP through 2038 QIDP designation PIVOT-PO trial protocol approved under FDA Special Protocol Assessment (SPA) Enrollment began with “First Patient, First Dose” in Q4 2023 Out-licensed global commercial rights
to GSK Robust financial terms including potential developmental, regulatory, and commercial milestones, as well as tiered sales royalties cUTI: complicated urinary tract infection; IP: intellectual property; tebipenem HBr: tebipenem pivoxil
hydrobromide (formerly SPR994); QIDP: qualified infectious disease product
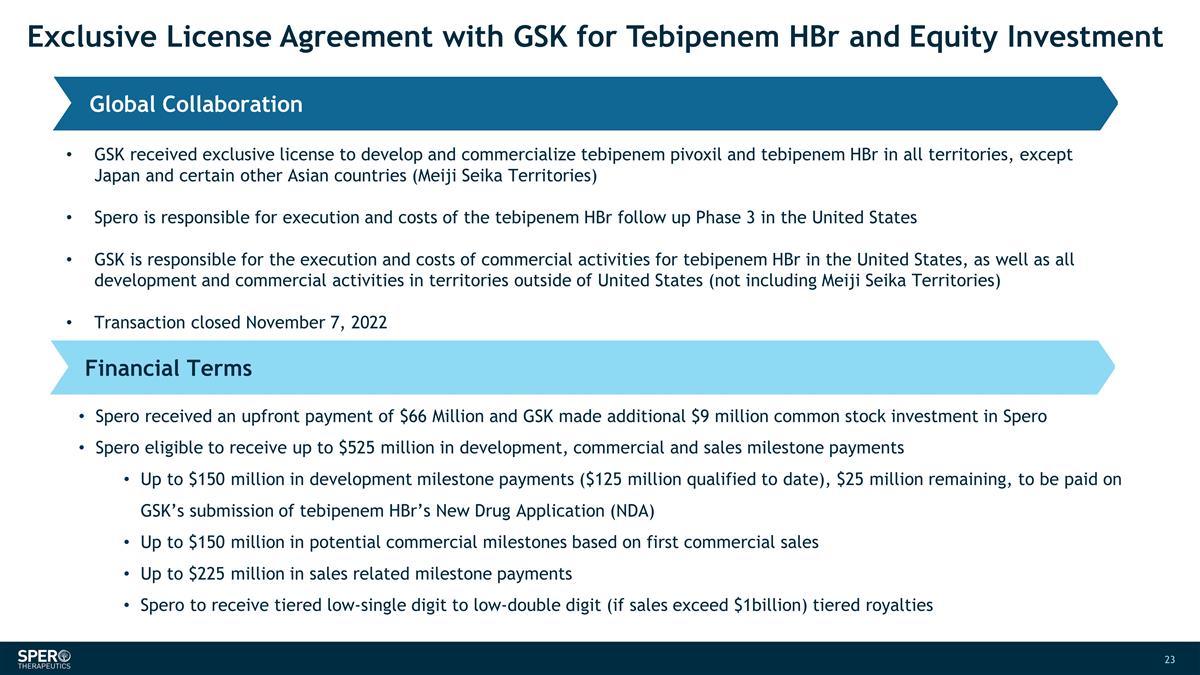
Exclusive License Agreement with
GSK for Tebipenem HBr and Equity Investment Global Collaboration Financial Terms GSK received exclusive license to develop and commercialize tebipenem pivoxil and tebipenem HBr in all territories, except Japan and certain other Asian countries
(Meiji Seika Territories) Spero is responsible for execution and costs of the tebipenem HBr follow up Phase 3 in the United States GSK is responsible for the execution and costs of commercial activities for tebipenem HBr in the United States, as
well as all development and commercial activities in territories outside of United States (not including Meiji Seika Territories) Transaction closed November 7, 2022 Spero received an upfront payment of $66 Million and GSK made additional $9 million
common stock investment in Spero Spero eligible to receive up to $525 million in development, commercial and sales milestone payments Up to $150 million in development milestone payments ($125 million qualified to date), $25 million remaining, to be
paid on GSK’s submission of tebipenem HBr’s New Drug Application (NDA) Up to $150 million in potential commercial milestones based on first commercial sales Up to $225 million in sales related milestone payments Spero to receive tiered
low-single digit to low-double digit (if sales exceed $1billion) tiered royalties
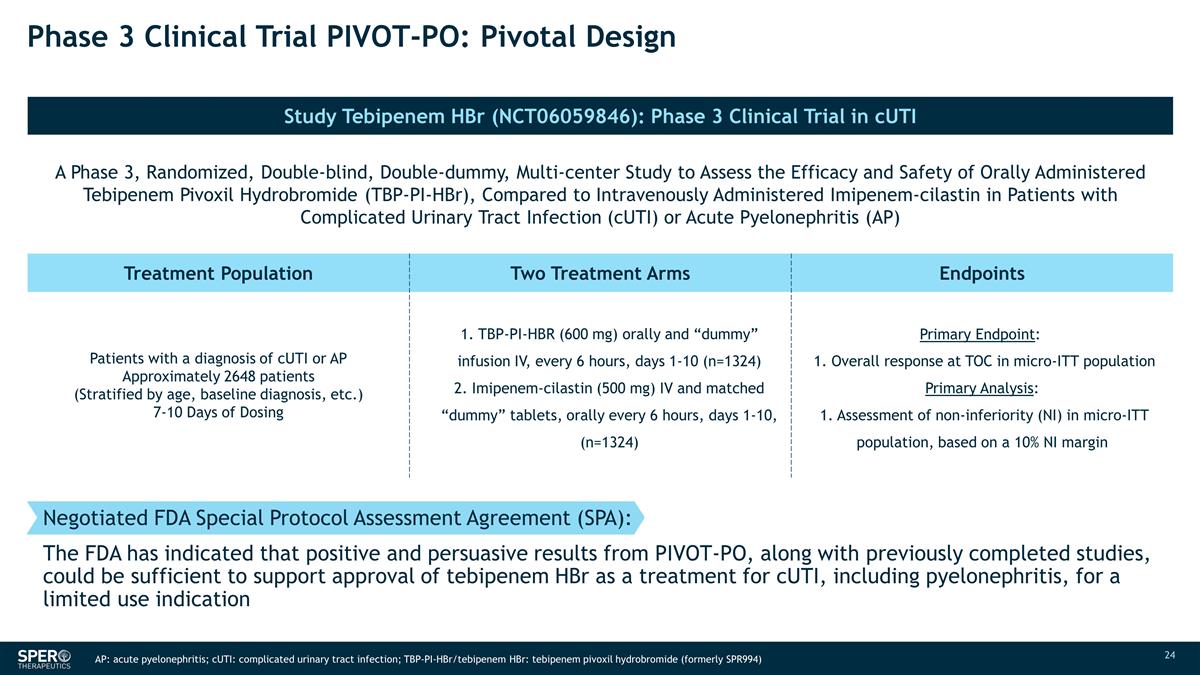
Phase 3 Clinical Trial PIVOT-PO:
Pivotal Design Negotiated FDA Special Protocol Assessment Agreement (SPA): The FDA has indicated that positive and persuasive results from PIVOT-PO, along with previously completed studies, could be sufficient to support approval of tebipenem HBr as
a treatment for cUTI, including pyelonephritis, for a limited use indication Study Tebipenem HBr (NCT06059846): Phase 3 Clinical Trial in cUTI N SPR719 MIC50 A Phase 3, Randomized, Double-blind, Double-dummy, Multi-center Study to Assess the
Efficacy and Safety of Orally Administered Tebipenem Pivoxil Hydrobromide (TBP-PI-HBr), Compared to Intravenously Administered Imipenem-cilastin in Patients with Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP) 30 2 Treatment
Population Two Treatment Arms Endpoints Patients with a diagnosis of cUTI or AP Approximately 2648 patients (Stratified by age, baseline diagnosis, etc.) 7-10 Days of Dosing 1. TBP-PI-HBR (600 mg) orally and “dummy” infusion IV, every 6
hours, days 1-10 (n=1324) 2. Imipenem-cilastin (500 mg) IV and matched “dummy” tablets, orally every 6 hours, days 1-10, (n=1324) Primary Endpoint: 1. Overall response at TOC in micro-ITT population Primary Analysis: 1.
Assessment of non-inferiority (NI) in micro-ITT population, based on a 10% NI margin AP: acute pyelonephritis; cUTI: complicated urinary tract infection; TBP-PI-HBr/tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994)
SPR206 Direct Acting IV
Potentiator
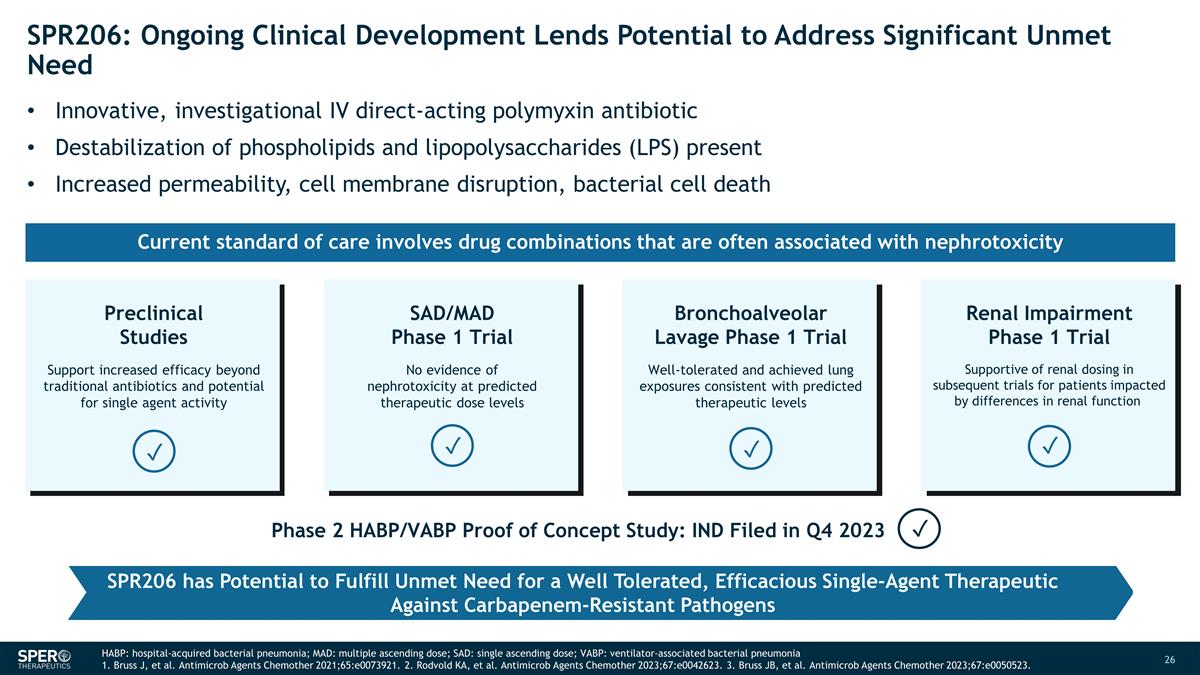
SPR206: Ongoing Clinical
Development Lends Potential to Address Significant Unmet Need Innovative, investigational IV direct-acting polymyxin antibiotic Destabilization of phospholipids and lipopolysaccharides (LPS) present Increased permeability, cell membrane disruption,
bacterial cell death SAD/MAD Phase 1 Trial No evidence of nephrotoxicity at predicted therapeutic dose levels Bronchoalveolar Lavage Phase 1 Trial Well-tolerated and achieved lung exposures consistent with predicted therapeutic levels Preclinical
Studies Support increased efficacy beyond traditional antibiotics and potential for single agent activity Renal Impairment Phase 1 Trial Supportive of renal dosing in subsequent trials for patients impacted by differences in renal function
✓ ✓ ✓ ✓ Phase 2 HABP/VABP Proof of Concept Study: IND Filed in Q4 2023 ✓ SPR206 has Potential to Fulfill Unmet Need for a Well Tolerated, Efficacious Single-Agent Therapeutic Against Carbapenem-Resistant Pathogens
Current standard of care involves drug combinations that are often associated with nephrotoxicity HABP: hospital-acquired bacterial pneumonia; MAD: multiple ascending dose; SAD: single ascending dose; VABP: ventilator-associated bacterial pneumonia
1. Bruss J, et al. Antimicrob Agents Chemother 2021;65:e0073921. 2. Rodvold KA, et al. Antimicrob Agents Chemother 2023;67:e0042623. 3. Bruss JB, et al. Antimicrob Agents Chemother 2023;67:e0050523.

Leadership Team Timothy Keutzer
Chief Operating Officer Previously, Spero’s Chief Development Officer Prior VP Program and Portfolio Management, Cubist Extensive antibiotic development experience from pre-clinical to approval Over 30 years in the pharmaceutical
industry James Brady Chief Human Resource Officer Prior CHRO at uniQure Therapeutics; Vice President, Human Resources at Intarcia Therapeutics Over 30 years of senior human resources leadership with over 17 years in the life science space Sath
Shukla President and Chief Executive Officer Prior CFO at Spero Therapeutics; Prior CFO at Ziopharm Oncology; VP and Global Head of Corporate Finance at Vertex Over 20 years of financial leadership, executing within commercial and clinical
companies Tamara Joseph, JD, LLM Chief Legal Officer Over 20 years of leadership and legal experience in the biotech sector Prior General Counsel at several biotechnology companies including Millendo Therapeutics, Enzyvant Therapeutics, InVivo
Therapeutics, and Cubist Kamal Hamed, MD, MPH, MBA Chief Medical Officer Prior CMO (Lysovant Sciences), Head of Development & Medical Affairs (Basilea) Therapeutic Area Head (Novartis) Led various anti-infective clinical
development programs in antibacterials, antivirals, antimalarials, and antifungals Over 20 years in the pharmaceutical industry Esther Rajavelu Chief Financial Officer Chief Business Officer Over two decades of life science sector experience,
combining equities research, investment banking, strategy consulting, and M&A. Prior CFO at Fulcrum Therapeutics. Senior equity research analyst at UBS, Oppenheimer and Deutsche Bank. Healthcare Investment Banker at Bank of America.
*Trademarks are properties of their respective owners

Thank You
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Spero Therapeutics (NASDAQ:SPRO)
Historical Stock Chart
From Apr 2024 to May 2024
Spero Therapeutics (NASDAQ:SPRO)
Historical Stock Chart
From May 2023 to May 2024