
mesoblast Global Leader in Allogeneic Cellular Medicines for Inflammatory Diseases August 2023 ASX: MSB; Nasdaq: MESO Financial Results and Operational Update for the Year Ended June 30, 2023

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This presentation includes forward-looking statements that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. We make such forward- looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. All statements other than statements of historical facts contained in this presentation are forward-looking statements. Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “targets,” “likely,” “will,” “would,” “could,” and similar expressions or phrases identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and future events , recent changes in regulatory laws, and financial trends that we believe may affect our financial condition, results of operation, business strategy and financial needs. These statements may relate to, but are not limited to: expectations regarding the safety or efficacy of, or potential applications for, Mesoblast's adult stem cell technologies; expectations regarding the strength of Mesoblast's intellectual property, the timeline for Mesoblast's regulatory approval process, and the scalability and efficiency of manufacturing processes; expectations about Mesoblast's ability to grow its business and statements regarding its relationships with current and potential future business partners and future benefits of those relationships; statements concerning Mesoblast's share price or potential market capitalization; and statements concerning Mesoblast's capital requirements and ability to raise future capital, among others. Forward-looking statements should not be read as a guarantee of future performance or results, and actual results may differ from the results anticipated in these forward-looking statements, and the differences may be material and adverse. You should read this presentation together with our financial statements and the notes related thereto, as well as the risk factors, in our most recently filed reports with the SEC or on our website. Uncertainties and risks that may cause Mesoblast's actual results, performance or achievements to be materially different from those which may be expressed or implied by such statements, include, without limitation: risks inherent in the development and commercialization of potential products; uncertainty of clinical trial results or regulatory approvals or clearances; government regulation; the need for future capital; dependence upon collaborators; and protection of our intellectual property rights, among others. Accordingly, you should not place undue reliance on these forward-looking statements. We do not undertake any obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future developments or otherwise.

Our Mission Mesoblast is committed to bringing to market innovative cellular medicines to treat serious and life-threatening illnesses
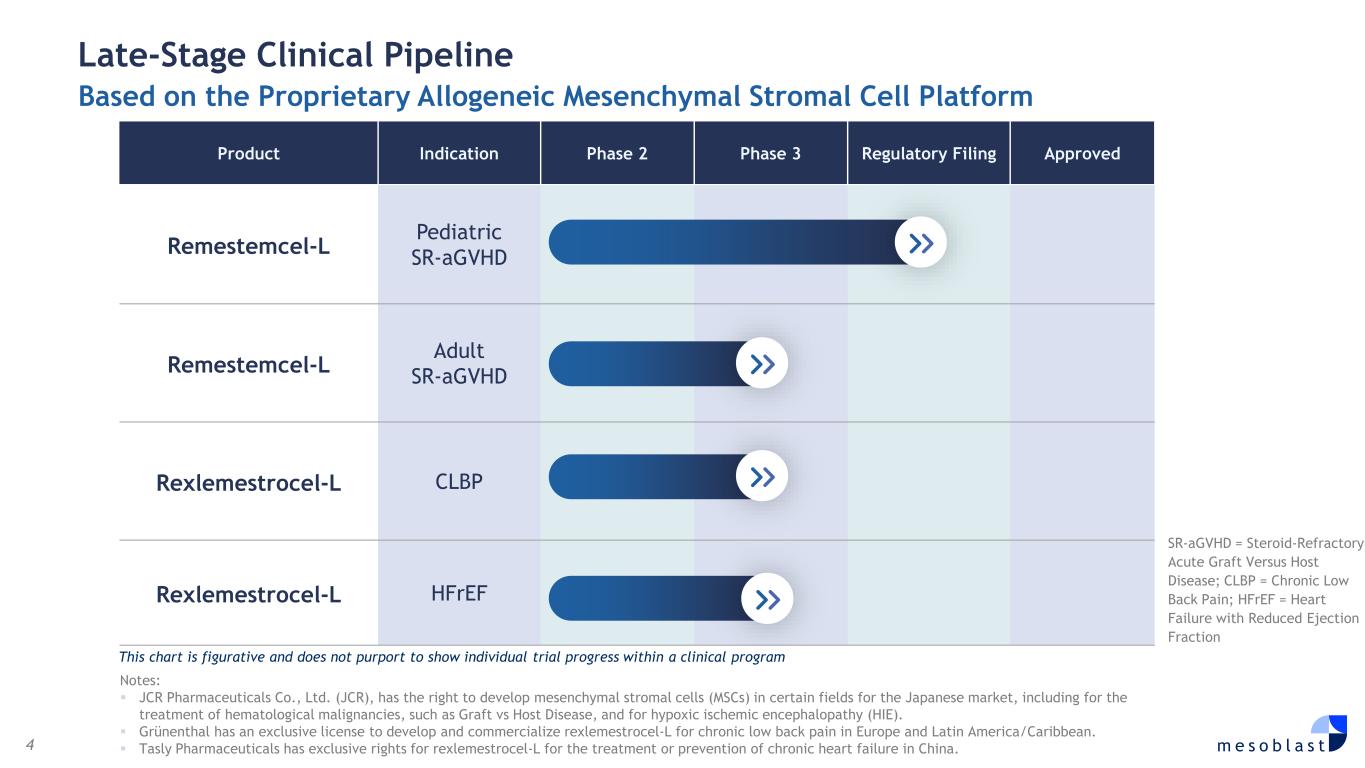
4 m e s o b l a s t Late-Stage Clinical Pipeline Based on the Proprietary Allogeneic Mesenchymal Stromal Cell Platform Product Indication Phase 2 Phase 3 Regulatory Filing Approved Remestemcel-L Pediatric SR-aGVHD Remestemcel-L Adult SR-aGVHD Rexlemestrocel-L CLBP Rexlemestrocel-L HFrEF SR-aGVHD = Steroid-Refractory Acute Graft Versus Host Disease; CLBP = Chronic Low Back Pain; HFrEF = Heart Failure with Reduced Ejection Fraction This chart is figurative and does not purport to show individual trial progress within a clinical program Notes: JCR Pharmaceuticals Co., Ltd. (JCR), has the right to develop mesenchymal stromal cells (MSCs) in certain fields for the Japanese market, including for the treatment of hematological malignancies, such as Graft vs Host Disease, and for hypoxic ischemic encephalopathy (HIE). Grünenthal has an exclusive license to develop and commercialize rexlemestrocel-L for chronic low back pain in Europe and Latin America/Caribbean. Tasly Pharmaceuticals has exclusive rights for rexlemestrocel-L for the treatment or prevention of chronic heart failure in China.

5 m e s o b l a s t Investment Highlights Remestemcel-L for SR-aGVHD Rexlemestrocel-L for CLBP Novel Allogeneic Cell Therapy Platform Finances Lead indication being developed for children with steroid-refractory acute graft versus host disease (SR-aGVHD) Upcoming Type A meeting with FDA to discuss strategy for product approval First Phase 3 completed for discogenic chronic low back pain (CLBP). RMAT granted by FDA. Initiation of second Phase 3 study Developing off-the-shelf, allogeneic cellular medicines based on proprietary mesenchymal stromal cell (MSC) technology platforms to enable treatment without the need for donor matching or immunosuppression Last 12 months revenue of US$7.5 million from royalties Cash-on-hand was US$71.3 million at June 30, 2023 Rexlemestrocel-L for HFrEF First Phase 3 completed for heart failure with reduced ejection fraction (HFrEF) Class II/III patients. RMAT granted by FDA for end-stage HFrEF patients with an LVAD BLA = Biologics License Application FDA = United States Food and Drug Administration PDUFA = Prescription Drug User Fee Act RMAT = Regenerative Medicine Advanced Therapy LVAD = Left Ventricular Assist Device
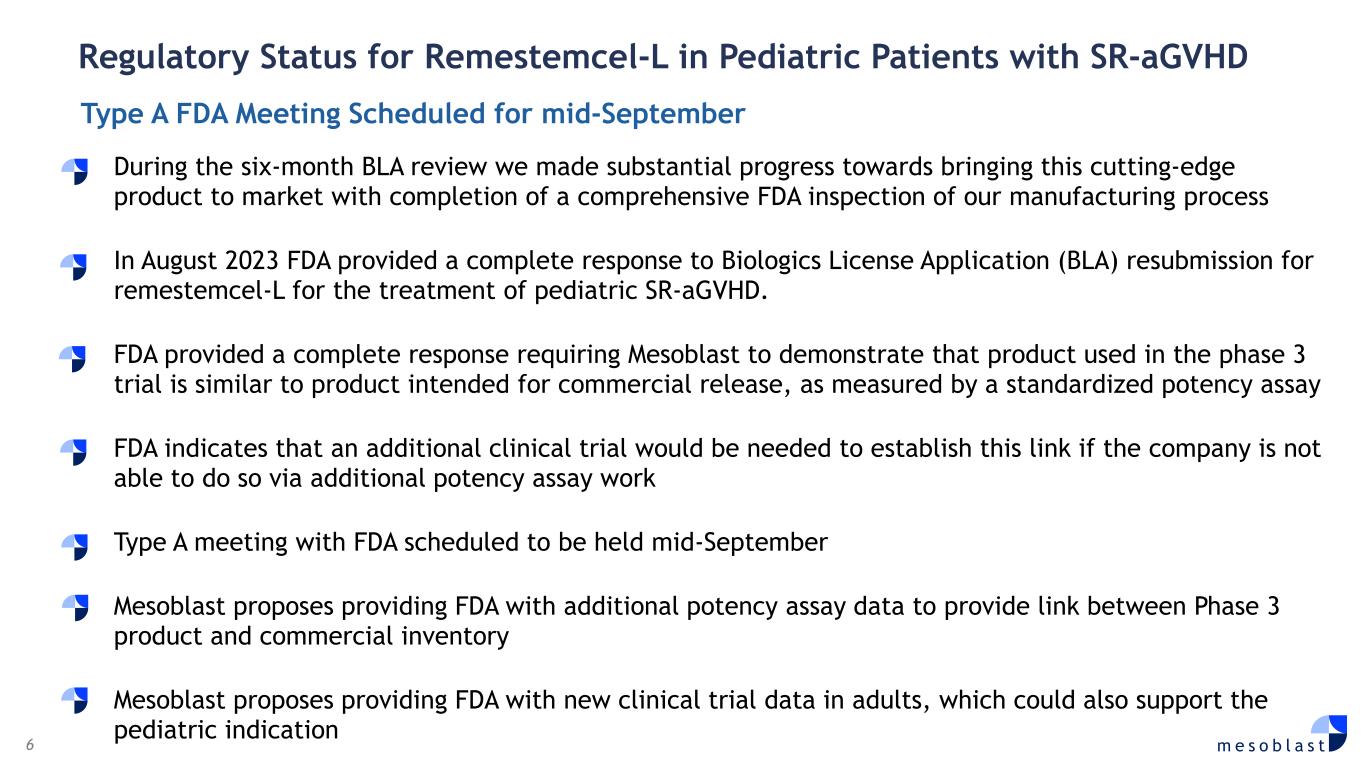
6 m e s o b l a s t During the six-month BLA review we made substantial progress towards bringing this cutting-edge product to market with completion of a comprehensive FDA inspection of our manufacturing process In August 2023 FDA provided a complete response to Biologics License Application (BLA) resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD. FDA provided a complete response requiring Mesoblast to demonstrate that product used in the phase 3 trial is similar to product intended for commercial release, as measured by a standardized potency assay FDA indicates that an additional clinical trial would be needed to establish this link if the company is not able to do so via additional potency assay work Type A meeting with FDA scheduled to be held mid-September Mesoblast proposes providing FDA with additional potency assay data to provide link between Phase 3 product and commercial inventory Mesoblast proposes providing FDA with new clinical trial data in adults, which could also support the pediatric indication Regulatory Status for Remestemcel-L in Pediatric Patients with SR-aGVHD Type A FDA Meeting Scheduled for mid-September

7 m e s o b l a s t In line with our overall commercial strategy to progress to adult patient populations, which make up approximately 5-fold larger numbers than children, Mesoblast intends to conduct a targeted, controlled study in adults with high mortality risk Survival in adults with SR-aGVHD who have failed at least one additional agent, such as ruxolitinib, remains as low as 20-30% by 100 days1,2 In contrast, 100-day survival was 63% after remestemcel-L treatment was used under expanded access in 71 patients aged 12 and older with SR-aGVHD who failed to respond to at least one additional agent, such as ruxolitinib Mesoblast is in discussions with world-leading investigators at the Blood and Marrow Clinical Trials Network (BM CTN), a body responsible for 80% of all US transplants, to conduct the new clinical trial The costs of this targeted study are expected to be covered by the planned spending reductions as outlined in the financial section Regulatory Status for Remestemcel-L in Patients with SR-aGVHD Generating New Clinical Data in Adults 1. Jagasia M et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020 May 14; 135(20): 1739–1749 2. Abedin S, et al. Ruxolitinib resistance or intolerance in steroid-refractory acute graft versus-host disease — a real-world outcomes analysis. British Journal of Haematology, 2021;195:429–43.

8 m e s o b l a s t© Lonza, reproduced with permission Manufacturing Remestemcel-L for the Period Ended June 30, 2023

9 m e s o b l a s t Financial Highlights for the Year 1. TEMCELL® HS Inj. is a registered trademark of JCR Pharmaceuticals Co. Ltd. 2. TEMCELL sales by our Licensee are recorded in Japanese Yen before being translated into USD for the purposes of calculating the royalty paid to Mesoblast. Results have been adjusted for the movement of the USD to Japanese Yen exchange rate from 1USD:122.14 Yen for the year ended June 30, 2022 to 1USD:139.76 Yen for the year ended June 30, 2023. Net cash usage for operating activities in FY2023 was US$63.3 million; this represented a 37% reduction compared with FY2021 and a 4% reduction compared with FY2022.Cash Burn Royalty Revenue Revenue from royalties were US$7.5 million for the year ended June 30, 2023. On a constant currency basis, royalties on sales of TEMCELL® HS Inj.1 in Japan by our licensee were US$8.12 million for the year ended June 30, 2023, compared with US$8.7 million for the year ended June 30, 2022. At June 30, 2023, cash-on-hand was US$71.3 million, with up to an additional US$40.0 million from our existing financing facilities subject to both certain milestones and the extension of availability.Cash Reserves
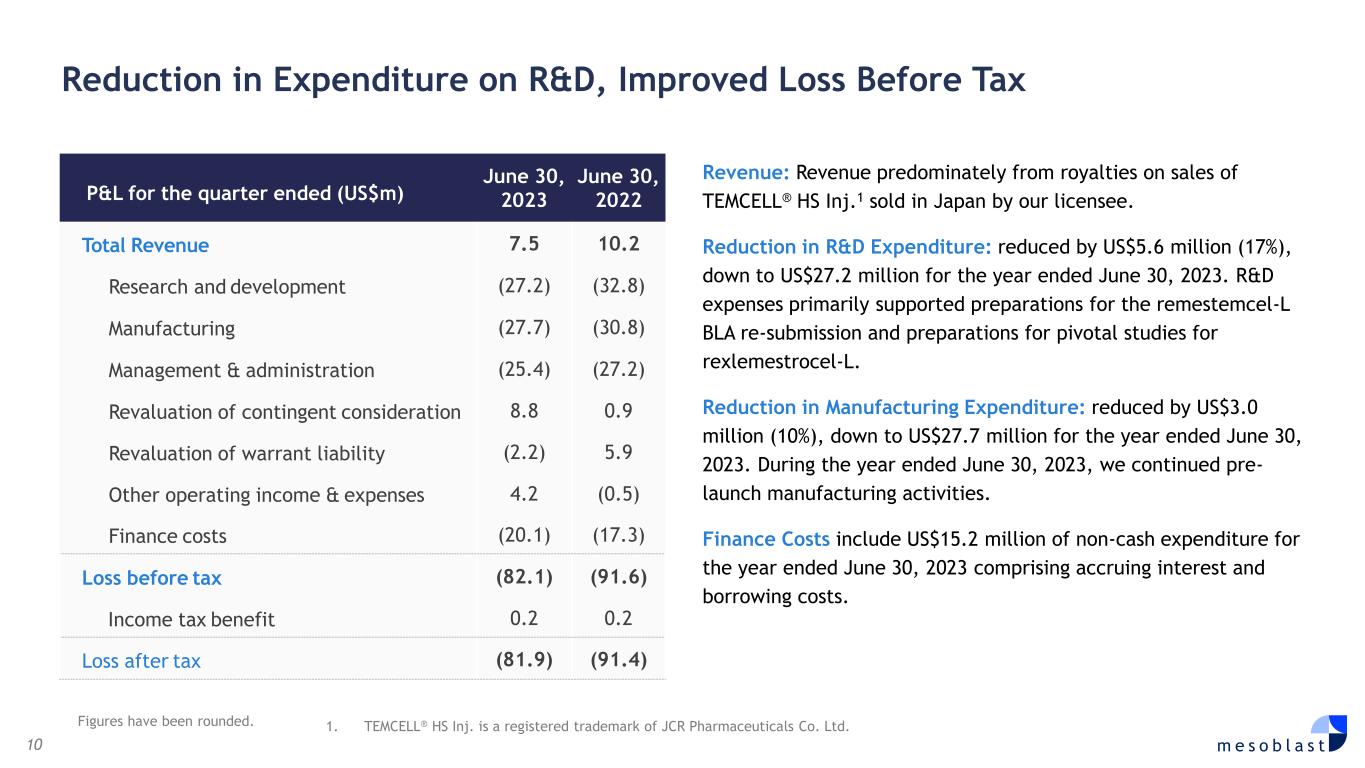
10 m e s o b l a s t Reduction in Expenditure on R&D, Improved Loss Before Tax Revenue: Revenue predominately from royalties on sales of TEMCELL® HS Inj.1 sold in Japan by our licensee. Reduction in R&D Expenditure: reduced by US$5.6 million (17%), down to US$27.2 million for the year ended June 30, 2023. R&D expenses primarily supported preparations for the remestemcel-L BLA re-submission and preparations for pivotal studies for rexlemestrocel-L. Reduction in Manufacturing Expenditure: reduced by US$3.0 million (10%), down to US$27.7 million for the year ended June 30, 2023. During the year ended June 30, 2023, we continued pre- launch manufacturing activities. Finance Costs include US$15.2 million of non-cash expenditure for the year ended June 30, 2023 comprising accruing interest and borrowing costs. P&L for the quarter ended (US$m) June 30, 2023 June 30, 2022 Total Revenue 7.5 10.2 Research and development (27.2) (32.8) Manufacturing (27.7) (30.8) Management & administration (25.4) (27.2) Revaluation of contingent consideration 8.8 0.9 Revaluation of warrant liability (2.2) 5.9 Other operating income & expenses 4.2 (0.5) Finance costs (20.1) (17.3) Loss before tax (82.1) (91.6) Income tax benefit 0.2 0.2 Loss after tax (81.9) (91.4) Figures have been rounded. 1. TEMCELL® HS Inj. is a registered trademark of JCR Pharmaceuticals Co. Ltd.

11 m e s o b l a s t Net operating cash usage in FY2023 was a 37% reduction compared with FY2021 and 4% reduction compared with FY2022 Further targeted 23% reduction (US$15 million) from US$63.3 million in FY2023 to US$48.3 million in projected FY2024 annual net operating cash spend through reduced spend across research, sales & marketing, commercial inventory, and payroll, which will be partially offset by investment in our Phase 3 programs for SR-aGVHD and CLBP 40% annualized reduction in payroll by February 2024 which includes base salaries, STI payments and contractor fees o CEO and CMO have deferred their entire FY23 short-term incentives (STI), have voluntarily reduced their base salaries for FY24 by 30% to preserve cash and will instead receive long-term non-cash incentives (LTIs) to further align with shareholders o FY23 short-term incentives (STI) have been entirely deferred for all employees o Management are eligible to receive LTIs in lieu of a 30% reduction in salary Non-Executive Directors have voluntarily deferred 100% of the cash payment of their fees and agreed to receive 50% of the value of their compensation in long-term non-cash incentives (LTI) Shift from quarterly to half yearly reporting of Financial Statements from FY2024 with continued quarterly Appendix 4C cash and operational reports, in-line with ASX-listed entities Cost Containment Plan for Next 12 Months Reduction in Spend on Operational Activities And Payroll

12 m e s o b l a s t Steroid-Refractory Acute Graft Versus Host Disease (SR-aGVHD)

13 m e s o b l a s t Acute Graft Versus Host Disease (aGVHD) Serious and Fatal Complication of Allogeneic Bone Marrow Transplantation (BMT) PHASE 1 Host Tissue Damage by BMT Conditioning PHASE 2 Immune Cell Activation & Cytokine Storm PHASE 3 Inflammation and End Organ Damage Conditioning regimen, chemotherapy, or radiation Tissue Damage Activation of CD4 & CD8 T-cells Cytokine Storm TNF, IL-1, IL-6 IFNγ, IL-2, IL-12, IL-21, IL-22, IL-23 Macrophage input to cytokine storm Macrophage Modified from Blazar et al., Nature Reviews Immunology 12: 443 – 458

14 m e s o b l a s t 1. Westin, J., Saliba, RM., Lima, M. (2011) Steroid-refractory acute GVHD: predictors and outcomes. Advances in Hematology. 2. Anthem-HealthCore/Mesoblast claims analysis (2016). Data on file 3. Niederwieser D, Baldomero H, Szer J. (2016) Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. 4. HRSA Transplant Activity Report, CIBMTR, 2020 5. Axt L, Naumann A, Toennies J (2019) Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation. More than 30,000 allogeneic BMTs performed globally (>20K US/EU) annually, ~20% pediatric3,4 Approx. 9,000 -10,000 allogeneic BMTs performed in the US annually Approx. 1,500 allogenic BMTs are in children and adolescents in US4 Remestemcel-L: Steroid-Refractory Acute Graft Versus Host Disease (SR-aGVHD) SR-aGVHD is associated with mortality rates as high as 90% Acute GVHD is a life- threatening complication that occurs in ~50% of patients receiving allogeneic bone marrow transplants (BMTs)1 Acute GVHD primarily affects skin, GI tract, and liver Steroid-refractory aGVHD is associated with mortality rates as high as 90%1,5 and significant extended hospital stay costs2 Treatment Options Burden of Illness Market Opportunity Corticosteroids are first-line therapy for aGVHD There is only one approved treatment for disease refractory to steroids and no approved treatment in the US for children under 12 years old In Japan, Mesoblast’s licensee has received the only product approval for SR-aGVHD in both children and adults

15 m e s o b l a s t 1. GVHD001 had 55 randomized patients, however one patient dropped out before receiving any dose of remestemcel-L; 2. Mount Sinai Acute GVHD International Consortium (MAGIC) - a group of ten BMT centers throughout the US and Europe whose purpose is to conduct ground-breaking clinical trials in GVHD, including developing informative biorepositories that assist in developing treatments that can guide GVHD therapy; 3. Two subjects in the MAGIC cohort had follow-up <100 days; these subjects are excluded from the respective survival analyses; 4. Data on file Remestemcel-L for Children with SR-aGVHD Improved Early Survival Across Three Studies involving more than 300 Treated Children Day 100 Survival Remestemcel-L Protocol Remestemcel-L Matched Controls Matched Control Protocol First Line Therapy after Steroids Treatment Setting Pediatric Subset of Protocol 280: randomized controlled P3, n=27 w/SR-aGVHD 79% 54% Study Control Arm (n=13) Study 001, open-label P3, n=541 with 89% Grade C/D disease 74% 57% MAGIC2 cohort, n=303 propensity- controlled subset Salvage Therapy Treatment Setting Expanded Access Protocol (EAP275), n=241 66% na EAP275, n=51 Grade D subset 51% 31% CIBMTR dbase, n=3274 propensity controlled subset
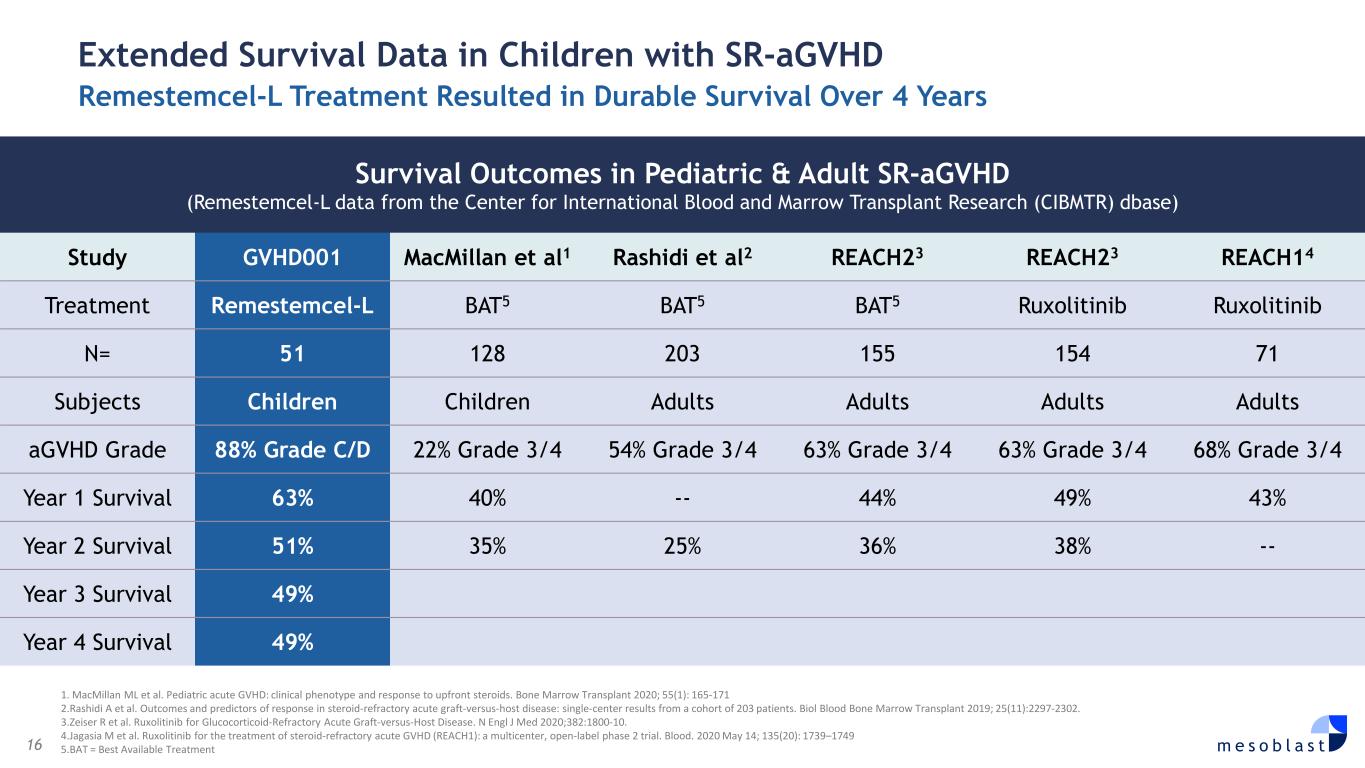
16 m e s o b l a s t 1. MacMillan ML et al. Pediatric acute GVHD: clinical phenotype and response to upfront steroids. Bone Marrow Transplant 2020; 55(1): 165-171 2.Rashidi A et al. Outcomes and predictors of response in steroid-refractory acute graft-versus-host disease: single-center results from a cohort of 203 patients. Biol Blood Bone Marrow Transplant 2019; 25(11):2297-2302. 3.Zeiser R et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med 2020;382:1800-10. 4.Jagasia M et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020 May 14; 135(20): 1739–1749 5.BAT = Best Available Treatment Extended Survival Data in Children with SR-aGVHD Remestemcel-L Treatment Resulted in Durable Survival Over 4 Years Survival Outcomes in Pediatric & Adult SR-aGVHD (Remestemcel-L data from the Center for International Blood and Marrow Transplant Research (CIBMTR) dbase) Study GVHD001 MacMillan et al1 Rashidi et al2 REACH23 REACH23 REACH14 Treatment Remestemcel-L BAT5 BAT5 BAT5 Ruxolitinib Ruxolitinib N= 51 128 203 155 154 71 Subjects Children Children Adults Adults Adults Adults aGVHD Grade 88% Grade C/D 22% Grade 3/4 54% Grade 3/4 63% Grade 3/4 63% Grade 3/4 68% Grade 3/4 Year 1 Survival 63% 40% -- 44% 49% 43% Year 2 Survival 51% 35% 25% 36% 38% -- Year 3 Survival 49% Year 4 Survival 49%
17 m e s o b l a s t Commercial strategy is to progress to adults who have failed steroids and a first-line agent, including ruxolitinib Market opportunity approximately five times larger than pediatric Approximately 45% of ruxolitinib patients are non-responders 1 Survival in adults with SR-aGVHD who have failed at least one additional agent, such as ruxolitinib, is 20-30% by 100 days 1,2 In contrast, 100-day survival was 63% after remestemcel-L treatment was used under compassionate care in 71 patients aged 12 and older with SR-aGVHD who failed to respond to at least one additional agent, such as ruxolitinib Mesoblast is in discussions with world-leading investigators at the Blood and Marrow Clinical Trials Network (BM CTN), a body responsible for 80% of all US transplants, to conduct the new clinical trial The costs of this targeted study are expected to be covered by the planned spending reductions as outlined in the financial section Remestemcel-L for Adults with SR-aGVHD 1. Jagasia M et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020 May 14; 135(20): 1739–1749 2. Abedin S, et al. Ruxolitinib resistance or intolerance in steroid-refractory acute graft versus-host disease — a real-world outcomes analysis. British Journal of Haematology, 2021;195:429–43.
18 m e s o b l a s t Chronic Low Back Pain due to Degenerative Disc Disease (CLBP)

19 m e s o b l a s t 1. Williams, J., NG, Nawi, Pelzter, K. (2015) Risk factors and disability associated with low back pain in older adults in low-and middle-income countries. Results from the WHO Study on global ageing and adult health (SAGE). PloS One. 2015; 10(6): e0127880., 2.Decision Resources: Chronic Pain December 2015., 3. LEK & NCI opinion leader interviews, and secondary analysis., 4. Navigant: Commercial Assessment for a Proprietary Cell-Based Therapy for DDD in the U.S. and the EU3 – August 2014. Chronic Low Back Pain Due to Degenerative Disc Disease (CLBP) Impacts 7M+ Rexlemestrocel-L represents a potential new paradigm for the treatment of CLBP Over 7m patients are estimated to suffer from CLBP due to degenerative disc disease (DDD) in each of the U.S. and E.U.5 2-4 Minimal treatment options for patients with chronic low back pain (CLBP) who fail conservative therapy include opioids and surgery 50% of opioid prescriptions are for CLBP2 Durable improvement in pain has potential to reduce opioid use and prevent surgical intervention Burden of Illness Treatment Options Market Opportunity Back pain causes more disability than any other condition1 Inflicts substantial direct and indirect costs on the healthcare system,1 including excessive use of opioids in this patient population
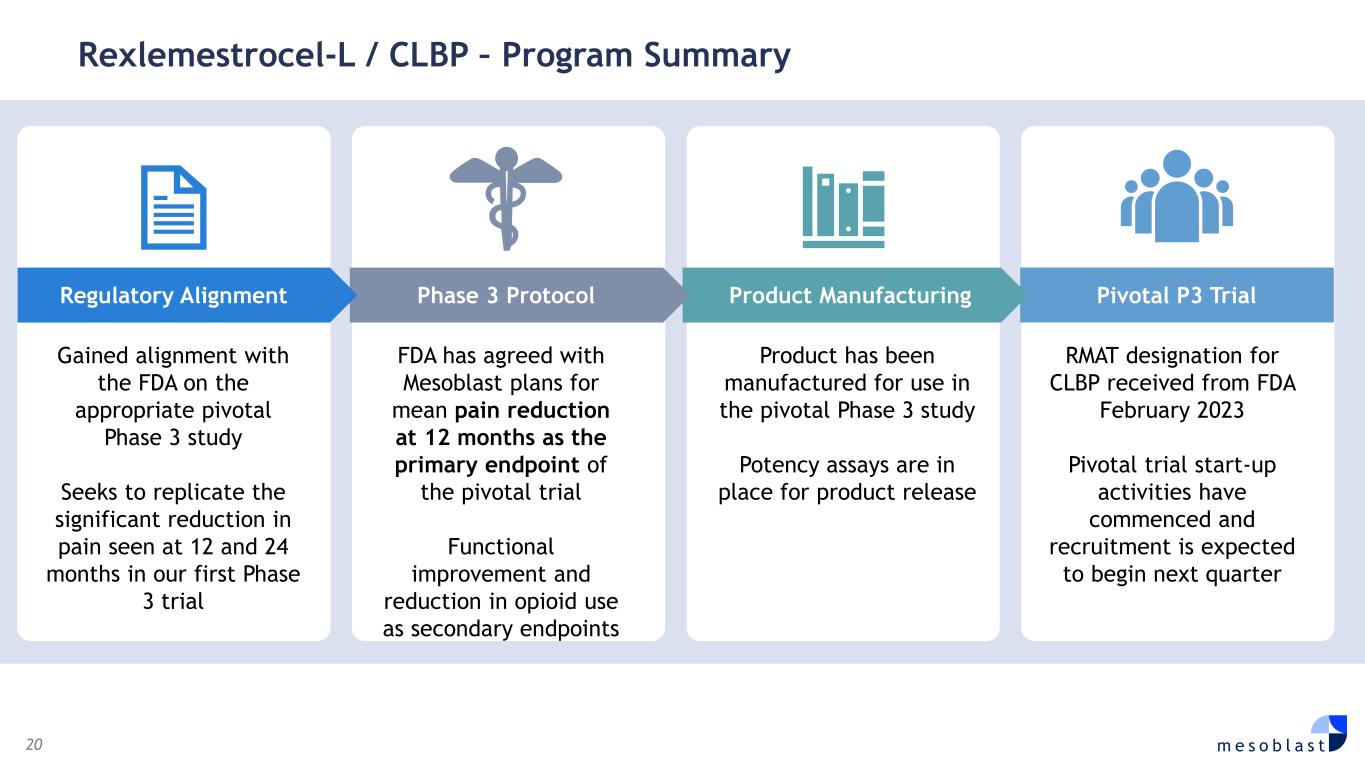
20 m e s o b l a s t Rexlemestrocel-L / CLBP – Program Summary Gained alignment with the FDA on the appropriate pivotal Phase 3 study Seeks to replicate the significant reduction in pain seen at 12 and 24 months in our first Phase 3 trial FDA has agreed with Mesoblast plans for mean pain reduction at 12 months as the primary endpoint of the pivotal trial Functional improvement and reduction in opioid use as secondary endpoints Product has been manufactured for use in the pivotal Phase 3 study Potency assays are in place for product release RMAT designation for CLBP received from FDA February 2023 Pivotal trial start-up activities have commenced and recruitment is expected to begin next quarter Pivotal P3 Trial Product Manufacturing Phase 3 Protocol Regulatory Alignment

21 m e s o b l a s t RMAT designation provides all the benefits of Breakthrough and Fast Track designations, including rolling review and eligibility for priority review on filing of a Biologics License Application (BLA) Results from the trial showed that: A single injection of rexlemestrocel-L+HA into the lumbar disc resulted in significant reduction in pain compared with saline control at 12 and 24 months across all subjects (n=404) Pain reduction through 36 months was seen in the subset of patients using opioids at baseline (n=168) with the rexlemestrocel-L+HA group having substantially greater reduction at all time points compared with saline controls Among patients on opioids at baseline, despite instructions to maintain existing therapies throughout the trial, at 36 months 28% who received rexlemestrocel-L+HA were not taking an opioid compared with 8% of saline treated controls Regenerative Medicine Advanced Therapy (RMAT) Designation Granted by FDA for Rexlemestrocel-L in the treatment of CLBP

22 m e s o b l a s t LS Mean VAS Change in Low Back Pain from Baseline - Duration CLBP < 68 Month Median Baseline Duration (n=202) Phase 3 Trial Outcomes based on a Single Injection of Rexlemestrocel-L + HA Results in More than Three Years of Pain Reduction Greatest pain reduction was observed in the pre-specified population of subjects with CLBP duration shorter than the baseline study median of 68 months (n=202) with significantly greater reduction (nominal p-value < 0.05) at all time points analyzed over 36 months compared with saline controls VAS=Visual Analog Score; HA=Hyaluronic Acid

23 m e s o b l a s t Chronic Heart Failure Reduced Ejection Fraction (HFrEF)
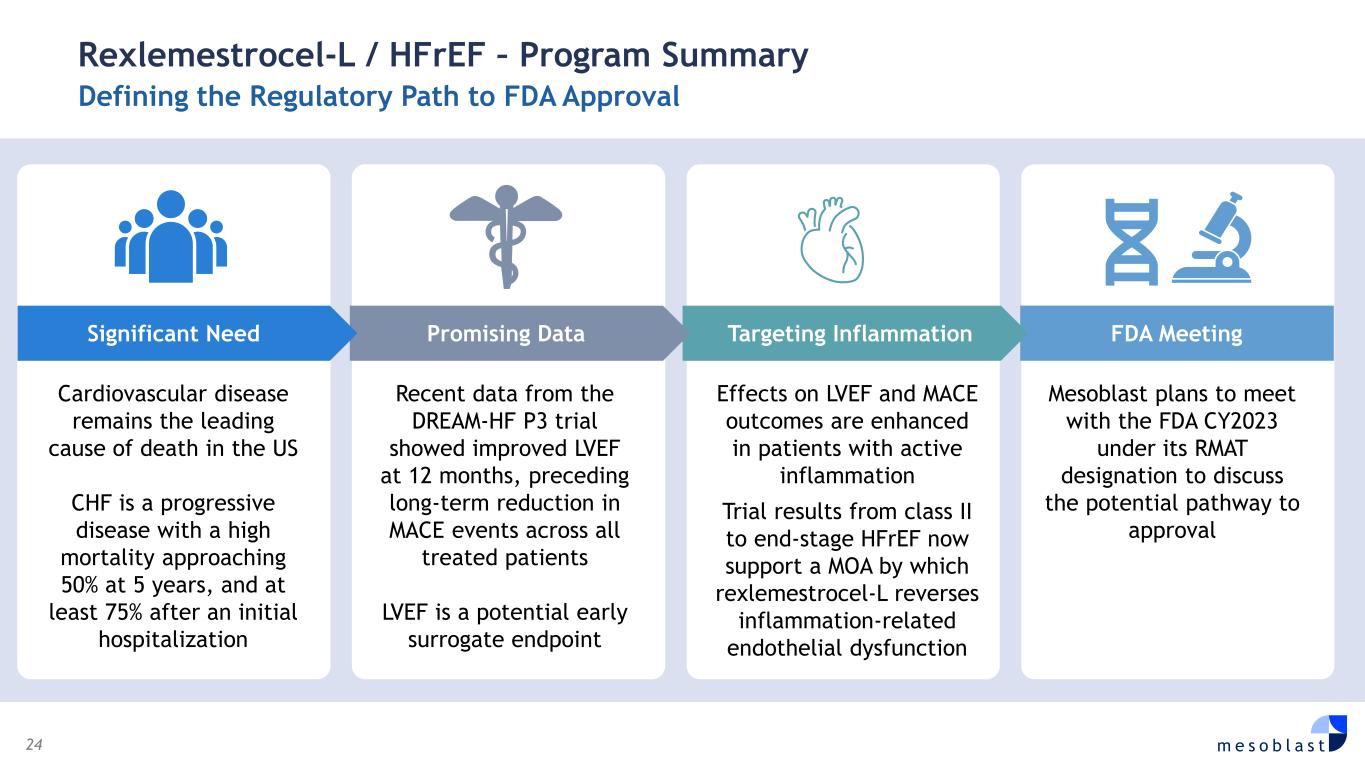
24 m e s o b l a s t Rexlemestrocel-L / HFrEF – Program Summary Defining the Regulatory Path to FDA Approval Cardiovascular disease remains the leading cause of death in the US CHF is a progressive disease with a high mortality approaching 50% at 5 years, and at least 75% after an initial hospitalization Recent data from the DREAM-HF P3 trial showed improved LVEF at 12 months, preceding long-term reduction in MACE events across all treated patients LVEF is a potential early surrogate endpoint Effects on LVEF and MACE outcomes are enhanced in patients with active inflammation Trial results from class II to end-stage HFrEF now support a MOA by which rexlemestrocel-L reverses inflammation-related endothelial dysfunction Mesoblast plans to meet with the FDA CY2023 under its RMAT designation to discuss the potential pathway to approval FDA Meeting Targeting Inflammation Promising DataSignificant Need

25 m e s o b l a s t Patients Experience Progressive Vascular Dysfunction and Heart Failure Rexlemestrocel-L has the potential to improve endothelial dysfunction in patients from Class II thru IV DEATH Early • Statins • Beta blockers • Re-vascularization or valvular surgery • RAAS antagonists • Diuretics for fluid retention • Hydralazine / isosorbide dinitrate • Digitalis NYHA Class I NYHA Class IIIB/IVNYHA Class II NYHA Class IIB/IIIA NYHA Class IIIB/IV Pts with end-stage HFrEF • Optimal medical management • LVAD implantation • Heart transplant • Artificial Heart NYHA Class IIB or IIIA Persistent HFrEF Patients • Cardioverter Defibrillator (ICD) +/- • CRT-D or Wearable Cardioverter Defibrillator if Indicated Traditional Early Therapies for HFrEF DREAM HF-1 Trial 537 Patients Recent New Oral Therapies for Decompensated HFrEF Hospitalizations and Fluid Overload • sacubitril / valsartan • SGLT2 inhibitors • Vericiguat Guideline Directed Medical Therapies (GDMT) LVAD MPC Studies 189 Patients Continuum of Cardiovascular Disease Risk Mesoblast’s Development Programs
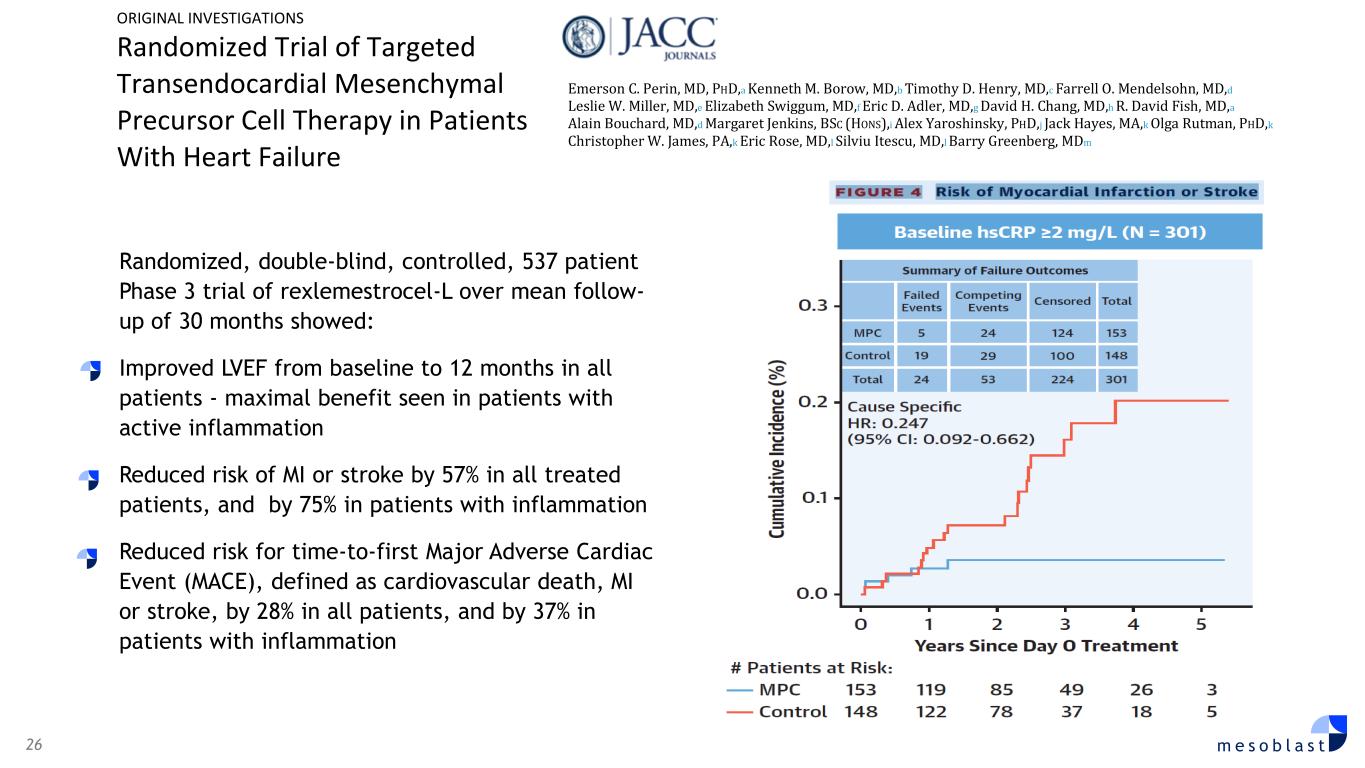
26 m e s o b l a s t Randomized, double-blind, controlled, 537 patient Phase 3 trial of rexlemestrocel-L over mean follow- up of 30 months showed: Improved LVEF from baseline to 12 months in all patients - maximal benefit seen in patients with active inflammation Reduced risk of MI or stroke by 57% in all treated patients, and by 75% in patients with inflammation Reduced risk for time-to-first Major Adverse Cardiac Event (MACE), defined as cardiovascular death, MI or stroke, by 28% in all patients, and by 37% in patients with inflammation ORIGINAL INVESTIGATIONS Randomized Trial of Targeted Transendocardial Mesenchymal Precursor Cell Therapy in Patients With Heart Failure Emerson C. Perin, MD, PHD,a Kenneth M. Borow, MD,b Timothy D. Henry, MD,c Farrell O. Mendelsohn, MD,d Leslie W. Miller, MD,e Elizabeth Swiggum, MD,f Eric D. Adler, MD,g David H. Chang, MD,h R. David Fish, MD,a Alain Bouchard, MD,d Margaret Jenkins, BSC (HONS),i Alex Yaroshinsky, PHD,j Jack Hayes, MA,k Olga Rutman, PHD,k Christopher W. James, PA,k Eric Rose, MD,l Silviu Itescu, MD,l Barry Greenberg, MDm
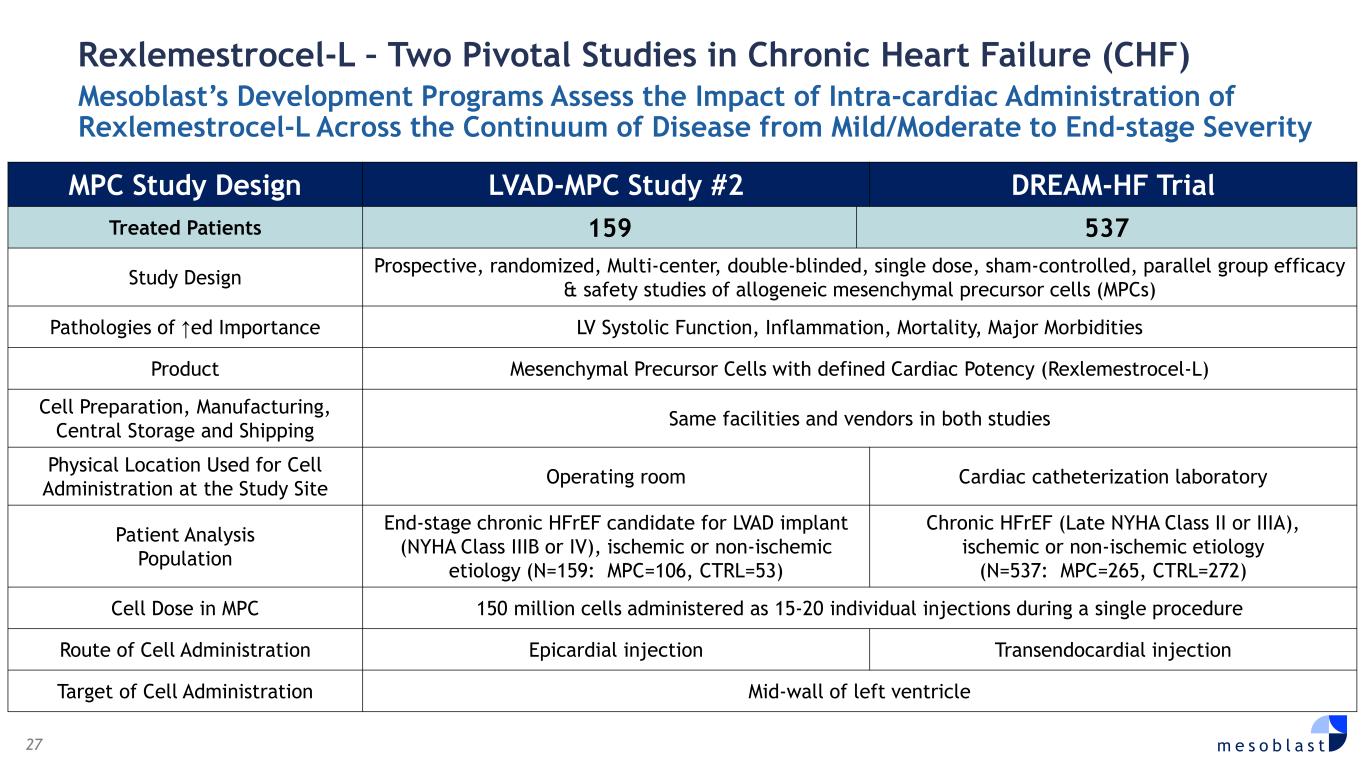
27 m e s o b l a s t Rexlemestrocel-L – Two Pivotal Studies in Chronic Heart Failure (CHF) Mesoblast’s Development Programs Assess the Impact of Intra-cardiac Administration of Rexlemestrocel-L Across the Continuum of Disease from Mild/Moderate to End-stage Severity MPC Study Design LVAD-MPC Study #2 DREAM-HF Trial Treated Patients 159 537 Study Design Prospective, randomized, Multi-center, double-blinded, single dose, sham-controlled, parallel group efficacy & safety studies of allogeneic mesenchymal precursor cells (MPCs) Pathologies of ↑ed Importance LV Systolic Function, Inflammation, Mortality, Major Morbidities Product Mesenchymal Precursor Cells with defined Cardiac Potency (Rexlemestrocel-L) Cell Preparation, Manufacturing, Central Storage and Shipping Same facilities and vendors in both studies Physical Location Used for Cell Administration at the Study Site Operating room Cardiac catheterization laboratory Patient Analysis Population End-stage chronic HFrEF candidate for LVAD implant (NYHA Class IIIB or IV), ischemic or non-ischemic etiology (N=159: MPC=106, CTRL=53) Chronic HFrEF (Late NYHA Class II or IIIA), ischemic or non-ischemic etiology (N=537: MPC=265, CTRL=272) Cell Dose in MPC 150 million cells administered as 15-20 individual injections during a single procedure Route of Cell Administration Epicardial injection Transendocardial injection Target of Cell Administration Mid-wall of left ventricle

28 m e s o b l a s t mesoblast Thank You