Genzyme and Bone Care International Announce Completion of Hart-Scott-Rodino Review
June 15 2005 - 10:10AM
PR Newswire (US)
Genzyme and Bone Care International Announce Completion of
Hart-Scott-Rodino Review CAMBRIDGE, Mass. and MADISON, Wis., June
15 /PRNewswire-FirstCall/ -- Genzyme Corporation (NASDAQ:GENZ) and
Bone Care International, Inc. (NASDAQ:BCII) announced today that
the U.S. Federal Trade Commission has granted early termination of
the waiting period under the Hart-Scott-Rodino Antitrust
Improvements Act of 1976 with respect to the proposed merger of
Genzyme and Bone Care. As announced on May 4, 2005, Genzyme and
Bone Care have signed a definitive merger agreement under which
Genzyme will acquire Bone Care in an all cash transaction valued at
$33.00 per fully diluted share. The transaction remains subject to
approval by Bone Care's shareholders at a meeting on June 30, and
other customary closing conditions. About Genzyme One of the
world's leading biotechnology companies, Genzyme is dedicated to
making a major positive impact on the lives of people with serious
diseases. Founded in 1981, Genzyme has grown from a small start-up
to a diversified enterprise with annual revenues exceeding $2
billion and more than 7,000 employees in locations spanning the
globe. With many established products and services helping patients
in more than 80 countries, Genzyme is a leader in the effort to
develop and apply the most advanced technologies in the life
sciences. The company's products and services are focused on rare
inherited disorders, kidney disease, orthopedics, cancer,
transplant and immune diseases, and diagnostic testing. Genzyme's
commitment to innovation continues today with a substantial
development program focused on these fields, as well as heart
disease and other areas of unmet medical need. About Bone Care Bone
Care is a specialty pharmaceutical company engaged in the
discovery, development and commercialization of innovative
therapeutic products to treat the unmet medical needs of patients
with debilitating conditions and life- threatening diseases. The
company's current commercial and therapeutic focus is in
nephrology, utilizing Hectorol, a novel vitamin D hormone therapy,
to treat secondary hyperparathyroidism in patients with moderate to
severe chronic kidney disease and end-stage renal disease. In
addition to chronic kidney disease, the company is developing
vitamin D hormone therapies to treat hyperproliferative disorders
such as cancer and psoriasis. Genzyme's press releases and other
company information are available at http://www.genzyme.com/ and by
calling Genzyme's investor information line at 1-800-905-4369
within the United States or 1-703-797-1866 outside the United
States. Media Contact, Genzyme Investor Contact, Genzyme Dan Quinn
Kristen Galfetti (617) 768-6849 (617) 768-6563 Media Contact, Bone
Care International Brian Hayden (608) 662-7800 DATASOURCE: Genzyme
Corporation CONTACT: Dan Quinn, Media Contact, +1-617-768-6849, or
Kristen Galfetti, Investor Contact, +1-617-768-6563, both of
Genzyme; or Brian Hayden Media Contact of Bone Care International,
+1-608-662-7800 Web site: http://www.genzyme.com/ Company News
On-Call: http://www.prnewswire.com/comp/104284.html
Copyright
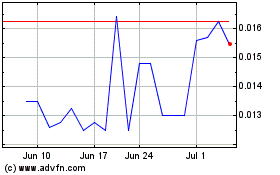
BCII Enterprises (PK) (USOTC:BCII)
Historical Stock Chart
From Apr 2024 to May 2024
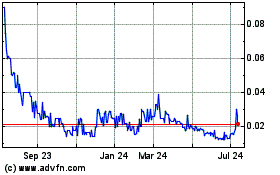
BCII Enterprises (PK) (USOTC:BCII)
Historical Stock Chart
From May 2023 to May 2024