Form 8-K - Current report
February 22 2024 - 7:00AM
Edgar (US Regulatory)
false
0001645469
0001645469
2024-02-22
2024-02-22
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): February 22, 2024
|
MONOPAR THERAPEUTICS INC.
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
|
001-39070
|
|
32-0463781
|
|
(State or other jurisdiction
of incorporation)
|
|
(Commission
File Number)
|
|
(I.R.S. Employer
Identification No.)
|
|
1000 Skokie Blvd., Suite 350, Wilmette, IL
|
|
60091
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
(847) 388-0349
Registrant’s telephone number, including area code
N/A
(Former name or former address, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
|
Trading Symbol(s)
|
|
Name of each exchange on which registered
|
|
Common Stock, $0.001 par value
|
|
MNPR
|
|
The Nasdaq Stock Market LLC (Nasdaq Capital Market)
|
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
☐
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
☐
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
☐
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
☐
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 7.01 Regulation FD Disclosure.
On February 22, 2024, Monopar Therapeutics Inc. issued a press release announcing promising preclinical imaging and therapeutic efficacy data for its MNPR-101 radiopharmaceutical program targeting advanced cancers.
The press release is furnished as Exhibit 99.1 to this report and incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits
|
Exhibit No.
|
|
Description
|
|
99.1
|
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
Monopar Therapeutics Inc.
|
|
| |
|
|
|
|
Date: February 22, 2024
|
By:
|
/s/ Kim R. Tsuchimoto
|
|
| |
Name:
|
Kim R. Tsuchimoto
|
|
| |
Title:
|
Chief Financial Officer and Director
|
|
Exhibit 99.1

Monopar Announces Promising Preclinical Data for its MNPR-101 Radiopharma Program Targeting Advanced Cancers
WILMETTE, Ill., February 22, 2024 – Monopar Therapeutics Inc. (Nasdaq: MNPR), a clinical-stage biopharmaceutical company focused on developing innovative treatments for cancer patients, today announced promising preclinical imaging and therapeutic efficacy data for its MNPR-101 radiopharmaceutical program. This novel first-in-class radiopharma program targets cancers expressing the urokinase plasminogen activator receptor (uPAR), which include a majority of all triple-negative breast, colorectal, and pancreatic cancers.
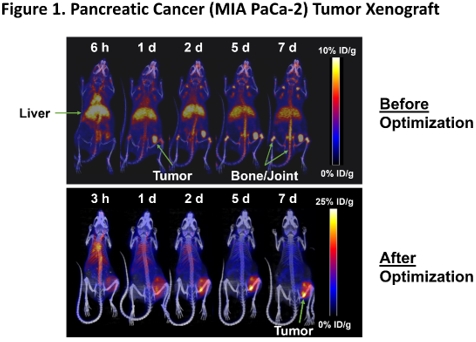
MNPR-101 Conjugated to Imaging Radioisotope
Maximizing the dose delivered to the tumor relative to normal tissue is of paramount importance in radiopharmaceutical therapy. Figure 1 below shows the before and after optimization of MNPR-101-Zr, Monopar’s radiopharmaceutical imaging agent for advanced solid tumors expressing uPAR. Monopar’s in-house radiopharmaceutical development team was able to significantly increase tumor uptake of MNPR-101-Zr while minimizing uptake in healthy tissue, as shown in this preclinical positron emission tomography (PET) sequential imaging time-series. The high specificity and durable tumor uptake are evident in the After Optimization panel below.
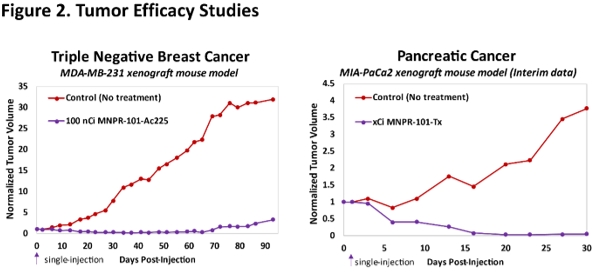
MNPR-101 Conjugated to Therapeutic Radioisotopes
Preclinical data to date demonstrate compelling and durable anti-tumor benefits with MNPR-101 conjugated to therapeutic radioisotopes. Figure 2 below shows preclinical efficacy data in a triple negative breast cancer as well as a pancreatic cancer human tumor xenograft mouse model utilizing two different therapeutic radioisotopes conjugated to MNPR-101; one of these radioisotopes has already been disclosed as being actinium-225 (Ac-225). The results in both show near complete elimination of the tumor after a single injection of the radiopharmaceutical agent. These studies demonstrate the potential of a MNPR-101 based radiopharmaceutical to provide a very meaningful clinical benefit to patients.
Monopar recently announced it received Human Research Ethics Committee (HREC) clearance in Australia to commence a Phase 1 dosimetry clinical trial for MNPR-101-Zr in advanced cancer patients. “As we prepare to launch this clinical trial, we are encouraged by the significant, precise, and durable accumulation we are seeing in tumors and the corresponding therapeutic benefit in preclinical human tumor xenograft models,” said Andrew Cittadine, Monopar’s Chief Operating Officer. “We are aiming to present these promising preclinical results at an upcoming scientific meeting.”
About Monopar Therapeutics Inc.
Monopar Therapeutics is a clinical-stage biopharmaceutical company focused on developing innovative treatments for cancer patients. Monopar's pipeline consists of Phase 1b-stage camsirubicin for the treatment of advanced soft tissue sarcoma; Phase 1-stage MNPR-101 for radiopharmaceutical use in advance cancers; and an early-stage camsirubicin analog, MNPR-202. For more information, visit: www.monopartx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of these forward-looking statements include: that these studies demonstrate the potential of an MNPR-101 based radiopharmaceutical to provide a very meaningful clinical benefit to patients; and that we are aiming to present these promising preclinical results at an upcoming scientific meeting. The forward-looking statements involve risks and uncertainties including, but not limited to: that future preclinical or clinical data will not be as promising as the data to date; not initiating and enrolling the Phase 1 clinical trial; that MNPR-101-Zr may cause unexpected serious adverse effects or fail to image or be effective against the cancer tumors in humans; the potential for the HREC to put the Phase 1 trial on clinical hold at any time; and the significant general risks and uncertainties surrounding the research, development, regulatory approval, and commercialization of imaging agents and therapeutics. Actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in Monopar's filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Monopar undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Any forward-looking statements contained in this press release represent Monopar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
CONTACT:
Monopar Therapeutics Inc.
Investor Relations
Kim R. Tsuchimoto
Chief Financial Officer
kimtsu@monopartx.com
Follow Monopar on social media for updates:
Twitter: @MonoparTx LinkedIn: Monopar Therapeutics
v3.24.0.1
Document And Entity Information
|
Feb. 22, 2024 |
| Document Information [Line Items] |
|
| Entity, Registrant Name |
MONOPAR THERAPEUTICS INC.
|
| Document, Type |
8-K
|
| Document, Period End Date |
Feb. 22, 2024
|
| Entity, Incorporation, State or Country Code |
DE
|
| Entity, File Number |
001-39070
|
| Entity, Tax Identification Number |
32-0463781
|
| Entity, Address, Address Line One |
1000 Skokie Blvd.
|
| Entity, Address, City or Town |
Wilmette
|
| Entity, Address, State or Province |
IL
|
| Entity, Address, Postal Zip Code |
60091
|
| City Area Code |
847
|
| Local Phone Number |
388-0349
|
| Title of 12(b) Security |
Common Stock, $0.001 par value
|
| Trading Symbol |
MNPR
|
| Security Exchange Name |
NASDAQ
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity, Emerging Growth Company |
true
|
| Entity, Ex Transition Period |
true
|
| Amendment Flag |
false
|
| Entity, Central Index Key |
0001645469
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Monopar Therapeutics (NASDAQ:MNPR)
Historical Stock Chart
From Apr 2024 to May 2024
Monopar Therapeutics (NASDAQ:MNPR)
Historical Stock Chart
From May 2023 to May 2024