As filed with the Securities and Exchange Commission on March 29, 2024
Registration No. 333-276367
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
POST-EFFECTIVE AMENDMENT No. 1
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
PANBELA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
|
2834
(Primary Standard Industrial
Classification Code Number)
|
|
88-2805017
(I.R.S. Employer
Identification No.)
|
712 Vista Blvd, Suite 305
Waconia, Minnesota 55387
(952) 479-1196
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Jennifer K. Simpson
Chief Executive Officer
712 Vista Blvd, Suite 305
Waconia, Minnesota 55387
(952) 479-1196
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
W. Morgan Burns, Joshua L. Colburn, W. Jason Deppen
Faegre Drinker Biddle & Reath LLP
90 South Seventh Street
2200 Wells Fargo Center
Minneapolis, Minnesota 55402-3901
Telephone: (612) 766-7000
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. ☑
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
Non-accelerated filer ☑
|
Smaller reporting company ☑
|
| |
Emerging growth company ☐
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Explanatory Note
Panbela Therapeutics, Inc. (the “Company”) previously filed a registration statement with the Securities and Exchange Commission (the “SEC”) on Form S-1 (Registration No. 333-276367), which was declared effective by the SEC on January 26, 2024 (the “Registration Statement”).
The Registration Statement, as amended by this Post-Effective Amendment No. 1 (this “Amendment”), pertains solely to the issuance by the Company of the remaining 8,750,000 shares of common stock, $0.001 par value per share, underlying Class E common stock purchase warrants and Class F common stock purchase warrants (collectively, the “Warrants”) previously issued by the Company to investors in a registered public offering. The shares of common stock issuable upon exercise of the Warrants were initially registered on the Registration Statement.
The Company is filing this Amendment to update the financial statements and other information, including pursuant to the undertakings in Item 17 of the Registration Statement, and to update and supplement the information contained in the Registration Statement with the information contained in the Company’s annual report on Form 10-K for the fiscal year ended December 31, 2023, that was filed with the SEC on March 26, 2024 and to update certain other information in the Registration Statement.
No additional securities are being registered under this Amendment. All applicable registration fees were paid at the time of the original filing of the Registration Statement.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell securities, and we are not soliciting offers to buy these securities, in any state where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT TO COMPLETION
|
DATED MARCH 29, 2024
|
8,750,000 Shares of Common Stock Underlying Previously Issued Warrants

This prospectus relates to the offer and sale by Panbela Therapeutics, Inc. of up to 8,750,000 shares of our common stock underlying Class E common stock purchase warrants and Class F common stock purchase warrants previously issued by us to investors in a registered public offering that are issuable at an exercise price of $2.06 per share, from time to time upon exercise of such warrants. We are not selling any shares of our common stock in this offering other than pursuant to the exercise of outstanding warrants. We will receive proceeds of up to approximately $18 million from the cash exercise of such warrants. To the extent that any of the warrants are exercised on a “cashless” basis, we will not receive any proceeds upon such exercise.
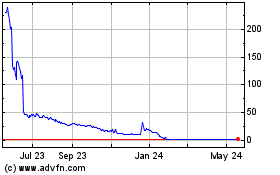
Our common stock is listed on the Nasdaq Capital Market under the symbol “PBLA.” On March 5, 2024, the Nasdaq Stock Market LLC (“Nasdaq”) notified us that the Nasdaq Hearings Panel has determined to delist our common stock and trading of our common stock on Nasdaq was suspended on March 7, 2024. Nasdaq will complete the delisting by filing a Form 25 Notification of Delisting with the U.S. Securities and Exchange Commission (the “SEC”) after applicable appeal periods have lapsed. In the interim, notwithstanding the suspension of trading on Nasdaq, we expect that our common stock will remain eligible for quotation on the OTC Pink Market under our existing symbol, “PBLA.” The last reported sale price on the OTC Pink Market for shares of our common stock on March 26, 2024 was $0.64 per share.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 10 of this prospectus, and under similar headings in any amendments or supplements to this prospectus, including our most recent annual report on Form 10-K and any similar section contained in any documents that are incorporated by reference into this prospectus.
The shares of common stock underlying the warrants will be offered on a continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended. We will deliver all securities to be issued in connection with this offering delivery versus payment upon receipt of investor funds received by us. Accordingly, there is no arrangement to receive or place investor funds in an escrow, trust or any similar account.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2024
TABLE OF CONTENTS
| |
Page |
| PROSPECTUS SUMMARY |
1 |
| THE OFFERING |
8 |
| RISK FACTORS |
9 |
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
21 |
| USE OF PROCEEDS |
22 |
| DIVIDEND POLICY |
22 |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
23 |
| FINANCIAL STATEMENTS |
23 |
| DESCRIPTION OF SECURITIES |
24 |
| SHARES ELIGIBLE FOR FUTURE SALE |
28 |
| PLAN OF DISTRIBUTION |
29 |
| LEGAL MATTERS |
29 |
| EXPERTS |
30 |
| WHERE YOU CAN FIND MORE INFORMATION |
30 |
| INCORPORATION OF DOCUMENTS BY REFERENCE |
30 |
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with any information other than that contained in this prospectus. We take no responsibility for and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus may only be used where it is legal to offer and sell our securities. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date. We are not making an offer of these securities in any jurisdiction where the offer is not permitted.
For investors outside the United States: We have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States must inform themselves about, and observe any restrictions relating to, the offering of securities and the distribution of this prospectus outside the United States.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We believe that the data obtained from these industry publications and third-party research, surveys and studies are reliable. We are ultimately responsible for all disclosure included in this prospectus.
You should rely only on the information contained in this prospectus, as supplemented and amended. We have not authorized anyone to provide you with information that is different. This prospectus may only be used where it is legal to sell these securities. The information in this prospectus may only be accurate on the date of this prospectus.
We urge you to read carefully this prospectus, as supplemented and amended, before deciding whether to invest in any of the securities being offered.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus, including our financial statements and the related notes and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in each case included elsewhere in this prospectus. Unless otherwise stated or the context requires otherwise, references in this prospectus to “Panbela,” the “Company,” “we,” “us,” “our” and similar references refer to Panbela Therapeutics, Inc. and its subsidiaries.
Business Overview
Panbela is a clinical stage biopharmaceutical company developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs. We are currently enrolling patients in our randomized double-blind placebo controlled clinical trial for the treatment of pancreatic cancer and we are a regulatory and commercial collaborator in a Phase III clinical trial funded by the National Cancer Institute (the “NCI”) for the study of colon cancer risk reduction and colon adenoma therapy (“CAT”), a preventative treatment approach for survivors of colorectal cancer or those who have high-risk colon polyps. In addition, the Company is designing a global protocol for a Phase III registration trial for familial adenomatous polyposis (“FAP”), a rare inherited condition that can cause the growth of thousands of colorectal adenomas (i.e., adenomatous polyps), which are recognized as a key risk factor for colon cancer. The global protocol will be submitted to the U.S. Federal Drug Administration (“FDA”) and European Medicines Agency (“EMA”) for agreement on the registration pathway. By leveraging Panbela’s extensive experience with FAP and in designing global registration trials, the team can develop a high-quality trial protocol that meets the standards of regulatory agencies and is designed to demonstrate the potential safety and efficacy of Flynpovi ™ efficiently and effectively in the treatment of FAP. We also support several investigator initiated trials and company sponsored preclinical trials including: (1) Phase II clinical trial for the treatment of early-onset type 1 diabetes funded by the Juvenile Diabetes Research Foundation; (2) Phase II clinical trial for treatment of gastric cancer funded by the NCI; (3) Phase I/II clinical trial for the treatment of non-small cell lung cancer (NSCLC) possessing the STK11 mutation; (4) Phase II program for the treatment of Metastatic Castration-Resistant Prostate Cancer; and (5) preclinical studies that we have sponsored in the orphan disease and cancer fields.
The Company’s lead assets are ivospemin (SBP-101), FlynpoviTM (eflornithine (CPP-1X) and sulindac), and eflornithine (CPP-1X) which provides a multi-targeted approach to reset dysregulated biology present in many types of diseases such as cancer and autoimmune disorders. Many tumors require greatly elevated levels of polyamines to support their growth and survival. These agents target the polyamine pathway at complementary junctions, which have been shown to be altered in disease. In particular, our lead assets have the potential to suppress and prevent tumor growth, enhance anti-tumor activity of other anti-cancer agents, and modulate the immune system.
Ivospemin is a proprietary polyamine analogue designed to induce polyamine metabolic inhibition. Ivospemin has demonstrated encouraging activity against metastatic disease in a clinical trial of patients with pancreatic cancer. The efficacy and safety results demonstrated in our completed Phase I clinical trial of ivospemin in combination with gemcitabine and nab-paclitaxel in the first line treatment of metastatic pancreatic cancer provide support for the current randomized, double-blind, placebo-controlled study of ivospemin in combination with gemcitabine and nab-paclitaxel in patients previously untreated for metastatic pancreatic cancer. We believe that ivospemin, if successfully developed, may represent a novel approach that effectively treats patients with pancreatic cancer and could become a dominant product in that market. In the past decade, two combination chemotherapy regimens, a quadruplet of fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) and a doublet, nab-paclitaxel and gemcitabine have been utilized as first-line standard of care. The first was based on a phase III trial but not Food and Drug Administration (“FDA”) approved and the latter based on a phase III trial which led to FDA approval. Most recently, the FDA approved Onivyde (irinotecan liposome injection) plus oxaliplatin, fluorouracil and leucovorin (NALIRIFOX) as a first-line treatment in adults living with metastatic pancreatic adenocarcinoma (“mPDAC”). This is the first FDA approval in first line mPDAC in over ten years. Ivospemin has received Fast Track status and orphan drug designation status for pancreatic cancer in the United States and we have also received orphan drug designation in Europe.
Our June 2022 acquisition of Cancer Prevention Pharmaceuticals, Inc. (“CPP”), added the Company’s second lead asset, eflornithine in multiple forms. First, an investigational new drug product, Flynpovi is a combination of the polyamine synthesis inhibitor eflornithine and the non-steroidal anti-inflammatory drug sulindac and then eflornithine as a single agent. Eflornithine is an enzyme-activated, irreversible inhibitor of the enzyme ornithine decarboxylase, the first rate-limiting enzyme in the biosynthesis of polyamines. Sulindac, a non-steroidal anti-inflammatory drug, facilitates the export and catabolism of polyamines. Flynpovi has a unique dual mechanism of action whereby it suppresses the synthesis of new polyamines and increases the export and catabolism of polyamines from the diet and microbiome. We believe Flynpovi is unique in that it is designed to treat the risk factors (e.g., polyps) that are hypothesized to lead to FAP surgeries and colon cancer and therefore may have the ability to prevent various types of colon cancer. In the FAP-310 Phase III trial, the efficacy and safety of the combination of Flynpovi (eflornithine and sulindac), as compared with either drug alone, in adults with FAP was conducted. While the study missed the primary composite endpoint (Burke et al. 2020), a post-hoc analysis showed that none of the patients in the combination arm progressed to a need for lower gastrointestinal (“LGI”) surgery for up to 48 months compared to 13.2% and 15.7% of patients in the sulindac and eflornithine arms (Balaguer et al. 2022). This data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy. Given the statistical significance of the LGI group, a new drug application (“NDA”) was filed with the FDA; however, since this was based on the results of an exploratory analysis, a complete response letter (“CRL”) was issued. To address the CRL, the Company is designing a Phase III registration trial and will advance this program while not increasing our current cash requirements. There are no currently approved pharmaceutical therapies for FAP.
Additional programs are evaluating a single agent tablet eflornithine or high dose powder eflornithine sachets for several indications including prevention of gastric cancer, recent onset Type 1 diabetes, metastatic castration-resistant prostate cancer, and STK-11 mutant NSCLC. Preclinical studies and Phase I or Phase II investigator-initiated trials suggest that eflornithine treatment is well tolerated and has potential activity.
Flynpovi has received Fast Track designation in the United States and orphan drug designation status for FAP in the United States and Europe. In addition, we have received orphan drug designation status for eflornithine as a single agent for Neuroblastoma in the United States and Europe and for gastric cancer in the United States.
Clinical Trials
Ivospemin (SBP-101)
In August 2015, the FDA accepted our Investigational New Drug (“IND”) application for our ivospemin product candidate. We have completed an initial clinical trial of ivospemin in patients with previously treated locally advanced or metastatic pancreatic cancer. This was a Phase I, first-in-human, dose-escalation, safety study. From January 2016 through September 2017, we enrolled twenty-nine patients into six cohorts, or groups, in the dose-escalation phase of the Phase I trial. No drug-related bone marrow toxicity or peripheral neuropathy was observed at any dose level. In addition to being evaluated for safety, 23 of the 29 patients were evaluable for preliminary signals of efficacy prior to or at the eight-week conclusion of their first cycle of treatment using the Response Evaluation Criteria in Solid Tumors (“RECIST”), the currently accepted standard for evaluating change in the size of tumors.
In 2018, we began enrolling patients in our second clinical trial, a Phase Ia/Ib study of the safety, efficacy, and pharmacokinetics of ivospemin administered in combination with two standard-of-care chemotherapy agents, gemcitabine and nab-paclitaxel. A total of 25 subjects were enrolled in four cohorts to evaluate the dosage level and schedule. An additional 25 subjects were enrolled in the expansion phase of the trial. Interim results were presented in January of 2022. Best response in evaluable subjects (cohorts 4 and Ib N=29) was a Complete Response in 1 (3%), Partial Response in 13 (45%), Stable Disease in 10 (34%) and Progressive Disease in 5 (17%). One subject did not have post baseline scans with RECIST tumor assessments. Median Progression Free Survival (“PFS”), now final at 6.5 months may have been negatively impacted by drug dosing interruptions to evaluate potential toxicity. Median overall survival in Cohort 4 + Phase Ib was 12.0 months when data was presented in January 2022 and is now final at 14.6 months. Two patients from cohort 2 have demonstrated long term survival. One at 30.3 months (final data) and one at 33.0 months and still alive. Seven subjects are still alive at this time, one from cohort 2 and six from cohort 4 plus Ib.
In January of 2022, the Company announced the initiation of a new pancreatic cancer clinical trial. Referred to as ASPIRE, the trial is a randomized double-blind placebo-controlled trial in combination with gemcitabine and nab-paclitaxel, a standard pancreatic cancer treatment regimen, in patients previously untreated for metastatic pancreatic cancer. The trial will be conducted globally at approximately 95 sites in the United States, Europe and Asia – Pacific.
The ASPIRE trial will evaluate overall survival as the primary endpoint and will also be examined at the interim analysis. PFS will also be analyzed to provide additional efficacy evidence. This trial design was supported by the final data from the Phase Ia/Ib first line metastatic pancreatic cancer trial which completed enrollment in December of 2020. The ASPIRE study will enroll 600 subjects and is anticipated to take 36 months to complete enrollment with the interim analysis available in mid- 2024. The Independent Data Safety Monitoring Board (“DSMB”) has met twice, the most recent taking place in November 2023. The DSMB members evaluated the safety of 214 patients. The result of both DSMB meetings confirmed no safety concerns and the trial continuing without modification. On January 25, 2024, the Company announced that the ASPIRE trial had surpassed fifty percent enrollment and expects that the trial will be fully enrolled by the first quarter of 2025.
If we successfully complete all FDA recommended clinical studies, we intend to seek marketing authorization from the FDA, the European Medicines Agency (“EMA”) (European Union), and Therapeutic Goods Administration (“TGA”) (Australia). The submission fees in the US and Europe may be waived for ivospemin as it has been designated an orphan drug in these geographic regions.
In early April 2023, the Company announced a poster presentation highlighting the results for ivospemin as a polyamine metabolism modulator in ovarian cancer at the American Association for Cancer Research Annual Conference The poster concludes that the ivospemin treatment of C57Bl/6 mice injected with VDID8+ ovarian cancer cells significantly prolonged survival and decreased overall tumor burden. The results suggest that ivospemin may have a role in the clinical management of ovarian cancer, and the Company intends to continue pre-clinical and clinical studies in ovarian cancer.
Additional preclinical work is underway evaluating ivospemin (also known as SBP-101) and eflornithine (also known as CPP-1X or DFMO) in multiple myeloma (cell lines). Data published in the November 2023, supplemental issue of the Journal Blood investigated the effects of polyamine inhibition by ivospemin and CPP-1X on myeloma cell lines growth and viability in vitro. Results showed that ivospemin and CPP-1X treatment significantly decreased cell proliferation and induced apoptosis in a panel of multiple myeloma cell lines. When ivospemin and CPP-1X were combined an almost complete abolition of cell growth occurred. These results demonstrate the anti-neoplastic potential of ivospemin and CPP-1X and offer a compelling rationale for its clinical development as a potentially promising treatment option for multiple myeloma. The work reflects the Company’s on-going collaboration with researchers from The University of Texas MD Anderson Cancer Center for the evaluation of polyamine metabolic inhibitor therapies in combination with CAR-T cell therapies in preclinical models.
FLYNPOVI
In December 2009, the FDA accepted our IND application for the combination product, Flynpovi. Flynpovi showed promising results in an NCI supported randomized, placebo-controlled Phase IIb/III clinical trial to prevent recurrent colon adenomas, particularly high-risk pre-cancerous polyps in which 375 subjects who had resected sporadic adenoma were treated for 3 years with eflornithine (500 mg once a day) + sulindac (150 mg once a day [N = 191]) or matched placebo/placebo (N = 184). Results demonstrated a marked risk reduction (70%) in developing metachronous adenomas, 92% risk reduction in developing advanced adenomas, and 95% risk reduction in developing multiple adenomas with the active combination regimen compared to placebo (Meyskens et al. 2008). This combination regimen was generally well tolerated.
Given the similar mechanism of disease in sporadic and FAP-associated adenomatous polyposis, and the mechanism of action of Flynpovi in prevention of progressive polyposis in both the general population with sporadic adenomas and in patients with FAP, a Phase III program in FAP, and a Phase III program to study colon cancer risk reduction in partnership with the Southwest Oncology Group (SWOG) and the NCI were initiated.
In the FAP-310 Phase III study completed in 2019, the efficacy and safety of the combination of eflornithine and sulindac, as compared with either drug alone, in adults with familial adenomatous polyposis was conducted (Burke et al. 2020). The patients were randomly assigned in a 1:1:1 ratio to receive eflornithine, sulindac, or both once daily for up to 48 months. The primary end point, assessed in a time-to-event analysis, was disease progression, defined as a composite of major surgery, endoscopic excision of advanced adenomas, diagnosis of high-grade dysplasia in the rectum or pouch, or progression of duodenal disease. A total of 171 patients underwent randomization. Disease progression occurred in 18 of 56 patients (32%) in the eflornithine-sulindac group, 22 of 58 (38%) in the sulindac group, and 23 of 57 (40%) in the eflornithine group, with a hazard ratio of 0.71 (95% confidence interval [CI], 0.39 to 1.32) for eflornithine-sulindac as compared with sulindac (P = 0.29) and 0.66 (95% CI, 0.36 to 1.23) for eflornithine-sulindac as compared with eflornithine (Burke et al. 2020). Adverse and serious adverse events were similar across the treatment groups. In a post-hoc analysis, none of the patients in the combination arm progressed to a need for LGI surgery for up to 48 months compared with 7 (13.2%) and 8 (15.7%) patients in the sulindac and eflornithine arms (Balaguer et al. 2022). These data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy with HR = 0.00 (95% CI, 0.00-0.48; p = 0.005) for combination versus sulindac and HR = 0.00 (95% CI, 0.00-0.44; p = 0.003) for combination versus eflornithine. Given the statistical significance of the LGI group, an NDA was filed with the FDA. As the study failed to meet the primary endpoint, and the NDA was based on the results of an exploratory analysis, a complete response letter was issued. To address this deficiency concern, the Company must submit the results of one or more adequate and well-controlled clinical trials which demonstrate an effect on a clinical endpoint.
In collaboration with the NCI, and SWOG, a Phase III clinical trial has been initiated to study the benefits of Flynpovi as a therapeutic treatment for use by colon cancer survivors. The trial is named PACES for “Prevention of Adenomas and Cancer with eflornithine and sulindac.” The PACES trial is funded by the NCI and managed by the Southwest Oncology Group (“SWOG”). This is an ongoing double-blind placebo-controlled trial of Flynpovi to prevent recurrence of high risk adenomas and second primary colorectal cancers in patients with stage 0-III colon or rectal cancer, Phase III – Preventing Adenomas of the Colon With Eflornithine and Sulindac (“PACES”). The purpose of this study is to assess whether Flynpovi (compared to corresponding placebos) has a reduced rate of cancer or high-risk adenoma recurrence compared to comparator arms after three years of daily dosing. We have exclusive rights to the data that comes from the trial for regulatory and commercial purposes. The Company is evaluating its options for CAT in the European Union and Asia.
In April 2023, the Company announced that it regained the North American rights to develop and commercialize Flynpovi in patients with FAP, as a result of the termination of the license agreement between CPP and One-Two Therapeutics Assets Limited effective July 4, 2023.
Eflornithine (CPP-1X) and eflornithine sachets (CPP-1X-S)
For the single agent eflornithine, there is a Phase I/II trial in STK11 mutation patients with non-small cell lung cancer and Phase II trial in Recent Onset Type I diabetes with eflornithine have been initiated and are enrolling. Recently, a phase II trial evaluating eflornithine and High Dose Testosterone With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer started enrolling. Lastly, a Phase II trial evaluating eflornithine for the prevention of gastric cancer was completed in 2021 with data analysis ongoing.
Recent Developments
Nasdaq Staff Determination Letters
On March 5, 2024, Nasdaq notified us that the Nasdaq Hearings Panel had determined to delist our common stock and trading of our common stock was suspended on March 7, 2024. Nasdaq will complete the delisting by filing a Form 25 Notification of Delisting with the SEC after applicable appeal periods have lapsed. The panel reached its decision because our Company was in violation of the minimum $2.5 million stockholders equity requirement in Listing Rule 5550(b)(1) and unable to comply with any of the alternative requirements in Listing Rule 5550(b) (collectively, the “Minimum Stockholders’ Equity Requirement”). The period during which we could appeal the decision to the Nasdaq Listing and Hearing Review Council has lapsed, but the Council may, on its own motion, determine to review the panel’s decision within 45 calendar days after the Company was notified of the decision. Although we are seeking all possible opportunities to regain compliance with the Minimum Stockholders’ Equity Requirement or to obtain an alternative listing on a national securities exchange, we believe that even if we were able to regain compliance with all applicable Nasdaq continued listing requirements, it is likely that Nasdaq will proceed with delisting our common stock.
As previously disclosed, in the past we have received notices from Nasdaq’s Listing Qualifications Department indicating that, for 30 consecutive business days, our common stock had not maintained a minimum closing bid price of $1.00 per share as required by Nasdaq Listing Rule 5550(a)(2) (“Minimum Bid Price Requirement”); (ii) the Minimum Stockholders’ Equity Requirement; and (iii) the minimum requirement of 500,000 publicly held shares as required by Nasdaq Listing Rule 5550(a)(4) (the “Minimum Float Requirement”). In February 2024, we received a letter from Nasdaq confirming that we had cured the most recently identified deficiencies under the Minimum Bid Price Requirement and Minimum Float Requirement.
We are evaluating all available opportunities to obtain a listing on another national securities exchange. In the interim, we may seek eligibility for quotation on the OTCQB Market.
Issuances of common stock and warrants after December 31, 2023
On January 31, 2024, the Company completed a registered public offering and issued an aggregate of 794,000 shares of its common stock, pre-funded warrants to purchase up to an aggregate of 3,581,000 shares of common stock at an exercise price of $0.001 per shares and warrants to purchase up to an aggregate of 8,750,000 shares of its common stock. The initial exercise price of the warrants is $2.06 per underlying share. The securities were issued for a combined offering price of $2.06 per share of common stock and warrants to purchase up to two additional shares of common stock, or $2.059 per pre-funded warrant and warrants. Net proceeds from the offering totaled approximately $8.2 million. As of March 7, 2024 no pre-funded warrants remained outstanding. The securities were offered pursuant to an effective registration statement on Form S-1.
Reverse Stock Splits
Effective January 18, 2024, we completed a 1-for-20 reverse split of our outstanding shares of common stock. Unless specifically provided herein, the share and per-share information that follows in this prospectus, other than in the historical financial statements and related notes included elsewhere in this prospectus, assumes the effect of the reverse stock split.
Our primary objective in effecting the reverse stock split was to attempt to raise the per-share trading price of our common stock to regain compliance with the Minimum Bid Price Requirement.
On March 25, 2024, we filed a preliminary proxy statement for a special meeting of stockholders to seek approval of a reverse stock split of our outstanding common stock at a ratio ranging from any whole number between 1-for-10 and 1-for-45, subject to and as determined by our Board of Directors. Our primary objective to effect the proposed reverse stock split is to attempt to raise the per-share trading price of our common stock to (1) exceed the minimum per share bid price requirements for initial and continued listing on a national securities exchange and (2) exceed minimum criteria to avoid “penny stock” classification. Assuming our common stock is delisted from Nasdaq, we will need to maintain a minimum closing bid price of greater than $1.00, and as much as $3.00 or $4.00 in order to be eligible for uplisting to a national securities exchange.
Although we expect that the reverse stock split would increase the bid price per share of our common stock above any applicable per share minimum price for any required number of days, thereby avoiding a deficiency or regaining compliance with the listing requirement, there can be no assurance that the reverse stock split would have that effect, initially or in the future, or that it would enable us to uplist or maintain the listing of our common stock on a national securities exchange for any particular duration.
There can be no assurance that any reverse stock split will achieve any of the desired results. There also can be no assurance that the price per share of our common stock immediately after any reverse stock split would increase proportionately with the reverse stock split, or that any increase would be sustained for any period of time, as evidenced by the Company’s past reverse stock splits.
Product Developments
Through March 26, 2024, we had:
| |
●
|
secured an orphan drug designation for ivospemin from the FDA;
|
| |
●
|
submitted and received acceptance from the FDA for an IND application for ivospemin;
|
| |
●
|
completed a Phase Ia monotherapy safety study of ivospemin in the treatment of patients with metastatic pancreatic ductal adenocarcinoma;
|
| |
●
|
received “Fast Track” designation from the FDA for ivospemin for metastatic pancreatic cancer;
|
| |
●
|
completed enrollment and released interim results in our second trial a Phase Ia /Ib clinical study of ivospemin, a first-line study with ivospemin given in combination with a current standard of care in patients with pancreatic ductal adenocarcinoma who were previously untreated for metastatic disease; a total of 50 subjects were enrolled in this study, 25 in the Phase Ia and 25 in the Phase Ib or expansion phase;
|
| |
●
|
secured a two-year research agreement with Johns Hopkins School of Medicine led by Professor Robert Casero, an internationally recognized researcher in polyamine biology;
|
| |
●
|
completed process improvement measures expected to be scalable for commercial use and received issue notification for a patent covering this new shorter synthesis of ivospemin in several territories;
|
| |
●
|
initiated a randomized, double-blind, placebo-controlled study, referred to as ASPIRE, with ivospemin given in combination with gemcitabine and nab-paclitaxel in patients with pancreatic ductal adenocarcinoma who are previously untreated for metastatic disease;
|
| |
●
|
completed preclinical evaluation of ivospemin for use as neoadjuvant therapy in resectable pancreatic cancer prior to surgery;
|
| |
●
|
obtained early, preclinical, indication of tumor growth inhibition activity in ovarian cancer and presented the results at ASCO-GI and AACR conferences;
|
| |
●
|
received USAN adoption of the nonproprietary name of ivospemin for SBP-101;
|
| |
●
|
acquired and integrated CPP, adding a second lead asset in multiple forms and an expansive clinical development program ranging from pre-clinical to registration level clinical trials;
|
| |
●
|
EMA Committee for Orphan Medicinal Products issued a positive opinion on Panbela’s application for orphan designation of ivospemin in combination with gemcitabine and nab-Paclitaxel in patients with metastatic pancreatic ductal adenocarcinoma;
|
| |
●
|
announced the initiation of Phase II program through Indiana University for early onset Type I diabetes utilizing eflornithine;
|
| |
●
|
ASPIRE is open to enrollment in every planned country within NA, EMEA, and APAC, Completed two Independent DSMB meetings for ASPIRE with no safety concerns or modifications to study design;
|
| |
●
|
announced the initiation of the Phase I/II clinical trial for the treatment of NSCLC possessing the STK11 mutation through Moffitt Cancer Center;
|
| |
●
|
entered into a sponsored research agreement with The University of Texas MD Anderson Cancer Center for the evaluation of polyamine metabolic inhibitor therapies in combination with CAR-T cell therapies in preclinical models;
|
| |
●
|
announced the SWOG Cancer Research Network’s PACES S0820 Phase III trial passed a single planned futility analysis and will continue;
|
| |
●
|
announced the approval of US WorldMeds’ NDA Approval for Eflornithine (DFMO) in Pediatric Neuroblastoma, the first polyamine approval in oncology; and
|
| |
●
|
exceeded 50% enrollment in ASPIRE global clinical trial.
|
Risks Associated with Our Company
Our business is subject to many significant risks, as more fully described in the section titled “Risk Factors” immediately following this prospectus summary. You should read and carefully consider these risks, together with the risks set forth under the section titled “Risk Factors” and all of the other information in this prospectus, including the financial statements and the related notes included elsewhere in this prospectus, before deciding whether to invest in our securities. If any of the risks discussed in this prospectus actually occur, our business, financial condition or operating results could be materially and adversely affected. In particular, our risks include, but are not limited to, the following:
| |
●
|
our ability to obtain additional capital, on acceptable terms or at all, required to implement our business plan;
|
| |
●
|
our lack of diversification and the corresponding risk of an investment in our Company;
|
| |
●
|
our ability to uplist and maintain the listing of our common stock on a national securities exchange;
|
| |
●
|
progress and success of our randomized Phase II/III clinical trial;
|
| |
●
|
our ability to demonstrate the safety and effectiveness of our product candidates: ivospemin ( SBP-101 ), Flynpovi, and eflornithine (CPP-1X);
|
| |
●
|
our ability to obtain regulatory approvals for our product candidates, ivospemin, Flynpovi and eflornithine in the United States, the European Union or other international markets;
|
| |
●
|
the market acceptance and level of future sales of our product candidates, ivospemin, Flynpovi and eflornithine ;
|
| |
●
|
the cost and delays in product development that may result from changes in regulatory oversight applicable to our product candidates, ivospemin, Flynpovi and eflornithine;
|
| |
●
|
the rate of progress in establishing reimbursement arrangements with third-party payors;
|
| |
●
|
the effect of competing technological and market developments;
|
| |
●
|
the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims; and
|
| |
●
|
other risk factors included under the caption “Risk Factors” starting on page Error! Bookmark not defined. of this prospectus.
|
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined in Rule 12b-2 of the Exchange Act and have elected to take advantage of certain scaled disclosure available to smaller reporting companies.
Corporate History
The primary business underlying Panbela Therapeutics, Inc., was originally incorporated under the laws of the State of Delaware under the name “Sun BioPharma, Inc.” in September 2011. In 2015, it became a public company by completing a merger transaction with a wholly owned subsidiary of a public company then organized under the laws of the State of Utah. In 2016, it was reincorporated under the laws of the State of Delaware via a merger with our operating subsidiary. That company changed its name to “Panbela Therapeutics, Inc.” on December 2, 2020. On June 15, 2022, we became a successor issuer to Panbela Therapeutics, Inc. and adopted its name, pursuant to a holding company reorganization via merger by operation of Rule 12g-3(a) promulgated under the Exchange Act, resulting in our current structure – consisting of two wholly owned subsidiaries: Panbela Research, Inc. and Cancer Prevention Pharmaceuticals, Inc.
Corporate Information
Our corporate mailing address is 712 Vista Blvd, #305, Waconia, MN 55387. Our telephone number is (952) 479-1196, and our website is www.panbela.com. The information on our website is not part of this prospectus. We have included our website address as a factual reference and do not intend it to be an active link to our website. The information contained in or connected to our website is not incorporated by reference into, and should not be considered part of, this prospectus. The trade names, trademarks, and service marks of other companies appearing in this prospectus are the property of the respective holders.
THE OFFERING
|
Common stock offered by us
|
Up to 8,750,000 shares of our common stock issuable upon exercise of outstanding warrants with an exercise price of $2.06 per share (collectively, the “Warrants”). Each Warrant is exercisable at any time for the purchase of one share of our common stock and is scheduled to expire on January 30, 2028.
|
| |
|
|
Common stock outstanding as of the date of this prospectus
|
4,845,861 shares
|
| |
|
|
Common stock to be outstanding immediately after completion of this offering
|
13,595,861 shares (assuming full exercise of the Warrants for cash)
|
| |
|
|
Use of proceeds
|
We will receive up to approximately $18 million if all of the currently outstanding Warrants are exercised for cash. We intend to use the net proceeds, if any, from this offering for the continued clinical development of our product candidates ivospemin and eflornithine and for working capital, and other general corporate purposes, which may include repayment of debt. The Warrants contain cashless exercise provisions. As a result, we may receive significantly less in net proceeds. See “Use of Proceeds” on page 22.
|
| |
|
|
Risk Factors
|
You should read the “Risk Factors” section of this prospectus beginning on page 10 for a discussion of factors to consider carefully before deciding to invest in our securities.
|
| |
|
|
Trading symbol
|
“PBLA”
|
| |
|
The number of shares of our common stock outstanding as of the date of this prospectus and after this offering is based on 4,854,861 shares of our common stock outstanding as of March 26, 2024, and excludes:
| |
●
|
all shares issuable in this offering;
|
| |
●
|
607 shares of common stock issuable upon the exercise of outstanding stock options at a weighted average exercise price of $14,410.38 per share;
|
| |
●
|
163,800 additional shares of common stock reserved and available for future issuances under our equity plans; and
|
| |
●
|
345,943 shares of common stock issuable upon exercise of stock purchase warrants not related to this offering at a weighted average exercise price of $39.08 per share.
|
Unless otherwise indicated, all information in this prospectus assumes no exercise of the outstanding options or warrants.
RISK FACTORS
Any investment in our securities involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to purchase our securities. Our business, financial condition or results of operations could be materially adversely affected by these risks if any of them actually occur. This prospectus also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face as described below and elsewhere in this prospectus.
Risks Related to Our Business and Financial Position
We are a pre revenue company with a history of negative operating cash flow.
We have experienced negative cash flows for our operating activities since inception, primarily due to the investments required to commercialize our primary drug candidates. Our financing cash flows historically have been positive due to proceeds from the sale of equity securities and promissory notes issuances. Our net cash used in operating activities was $25.2 million and $15.3 million for the years ended December 31, 2023, and 2022, respectively, and we had negative working capital of $9.3 million on December 31, 2023, and negative working capital of 6.0 million as December 31, 2022. Working capital is defined as current assets less current liabilities.
Our operations are subject to all the risks, difficulties, complications and delays frequently encountered in connection with the development of new products, as well as those risks that are specific to the pharmaceutical and biotechnology industries in which we compete. Investors should evaluate us considering the delays, expenses, problems and uncertainties frequently encountered by companies developing markets for new products, services and technologies. We may never overcome these obstacles.
As a result of our limited financial liquidity, we and our auditors have expressed substantial doubt regarding our ability to continue as a “going concern.”
As a result of our limited financial liquidity, our auditors’ report for our 2023 financial statements, which is incorporated by reference herein, contains a statement concerning our ability to continue as a “going concern.” Our limited liquidity could make it more difficult for us to secure additional financing or enter into strategic relationships on terms acceptable to us, if at all, and may materially and adversely affect the terms of any financing that we may obtain and our public stock price generally.
Our continuation as a “going concern” is dependent upon, among other things, achieving positive cash flow from operations and, if necessary, augmenting such cash flow using external resources to satisfy our cash needs. Our plans to achieve positive cash flow primarily include engaging in offerings of securities. Additional potential sources of funds include negotiating up-front and milestone payments on our current and potential future product candidates or royalties from sales of our products that secure regulatory approval and any milestone payments associated with such approved products. These cash sources could, potentially, be supplemented by financing or other strategic agreements. However, we may be unable to achieve these goals or obtain required funding on commercially reasonable terms, or at all, and therefore may be unable to continue as a going concern.
We may be unable to obtain the additional capital that is required to execute our business plan, which could restrict our ability to grow.
Our current capital and our other existing resources will be sufficient only to provide a limited amount of working capital and will not be sufficient to fund our expected continuing opportunities. While we project that our current capital resources will fund our operations, including increased clinical trial costs, into the second quarter of 2024, we will require additional capital to continue to operate our business and complete our clinical development plans.
Future research and development, including clinical trial cost, capital expenditures and possible acquisitions, and our administrative requirements, such as salaries, insurance expenses and general overhead expenses, as well as legal compliance costs and accounting expenses, will require a substantial amount of additional capital and cash flow. There is no guarantee that we will be able to raise the additional capital required to fund our ongoing business on commercially reasonable terms or at all.
We intend to pursue sources of additional capital through various financing transactions or arrangements, including collaboration arrangements, debt financing, equity financing or other means. We may not be successful in locating suitable financing transactions on commercially reasonable terms, in the time period required or at all, and we may not obtain the capital we require by other means. If we do not succeed in raising additional capital, our resources will not be sufficient to fund our operations going forward.
Any additional capital raised through the sale of equity may dilute the ownership percentage of our stockholders. This could also result in a decrease in the fair market value of our equity securities because our assets would be owned by a larger pool of outstanding equity. The terms of securities we issue in future capital transactions may be more favorable to our new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities which may have a further dilutive effect.
Our ability to obtain needed financing may be impaired by such factors as the capital markets, both generally and in the pharmaceutical and other drug development industries in particular, the limited diversity of our activities and/or the loss of key personnel. If the amount of capital we are able to raise from financing activities is not sufficient to satisfy our capital needs, even to the extent that we reduce our operations, we may be required to cease our operations.
We may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs, which may adversely impact our financial condition.
The markets for our product candidates are highly competitive and are subject to rapid scientific change, which could have a material adverse effect on our business, results of operations and financial condition.
The pharmaceutical and biotechnology industries in which we compete are highly competitive and characterized by rapid and significant technological change. We face intense competition from organizations such as pharmaceutical and biotechnology companies, as well as academic and research institutions and government agencies. Some of these organizations are pursuing products based on technologies similar to our technology. Other of these organizations have developed and are marketing products or are pursuing other technological approaches designed to produce products that are competitive with our product candidates in the therapeutic effect these competitive products have on the diseases targeted by our product candidates. Our competitors may discover, develop or commercialize products or other novel technologies that are more effective, safer or less costly than any that we may develop. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for our product candidates.
Many of our competitors are substantially larger than we are and have greater capital resources, research and development staff and facilities than we have. In addition, many of our competitors are more experienced in drug discovery, development and commercialization, obtaining regulatory approvals and drug manufacturing and marketing.
We anticipate that the competition with our product candidates and technology will be based on a number of factors including product efficacy, safety, availability and price. The timing of market introduction of our planned future product candidates and competitive products will also affect competition among products. We expect the relative speed with which we can develop our product candidates, complete the required clinical trials, establish strategic partners and supply appropriate quantities of the product candidate for late-stage trials, if required, to be important competitive factors. Our competitive position will also depend upon our ability to attract and retain qualified personnel, to obtain patent protection in non-U.S. markets, which we currently do not have, or otherwise develop proprietary products or processes and to secure sufficient capital resources for the period between technological conception and commercial sales or out-license to pharmaceutical partners. If we fail to develop and deploy a proposed product candidate in a successful and timely manner, we will in all likelihood not be competitive.
Our lack of diversification increases the risk of an investment in our Company and our financial condition and results of operations may deteriorate if we fail to diversify.
Our Board of Directors has centered our attention on our drug development activities, which are currently focused on a limited number of product candidates. Our ability to diversify our investments will depend on our access to additional capital and financing sources and the availability and identification of suitable opportunities.
Larger companies have the ability to manage their risk by diversification. However, we lack and expect to continue to lack diversification, in terms of both the nature and geographic scope of our business. As a result, we will likely be impacted more acutely by factors affecting pharmaceutical and biotechnology industries in which we compete than we would if our business were more diversified, enhancing our risk profile. If we cannot diversify our operations, our financial condition and results of operations could deteriorate.
Our business may suffer if we do not attract and retain talented personnel.
Our success will depend in large measure on the abilities, expertise, judgment, discretion, integrity and good faith of our management and other personnel in conducting our business. We have a small management team, and the loss of a key individual or inability to attract suitably qualified staff could materially adversely impact on our business.
Our success depends on the ability of our management, employees, consultants and strategic partners, if any, to interpret market data correctly and to interpret and respond to economic market and other conditions in order to locate and adopt appropriate investment opportunities, monitor such investments, and ultimately, if required, to successfully divest such investments. Further, no assurance can be given that our key personnel will continue their association or employment with us or that replacement personnel with comparable skills can be found. We will seek to ensure that management and any key employees are appropriately compensated; however, their services cannot be guaranteed. If we are unable to attract and retain key personnel, our business may be adversely affected.
We may be required to defend lawsuits or pay damages for product liability claims.
Product liability is a major risk in testing and marketing biotechnology and pharmaceutical products. We may face substantial product liability exposure in human clinical trials and in the sale of products after regulatory approval. Product liability claims, regardless of their merits, could exceed policy limits, divert management’s attention and adversely affect our reputation and the demand for our product. In any such event, your investment in our securities could be materially and adversely affected.
Risks Related to the Development and Approval of New Drugs
Clinical trials required for our product candidates are expensive and time-consuming, and their outcome is highly uncertain. If any of our drug trials are delayed or yield unfavorable results, we will have to delay or may be unable to obtain regulatory approval for our product candidate.
We must conduct extensive testing of each product candidate before we can obtain regulatory approval to market and sell it. We need to conduct both preclinical animal testing and human clinical trials. Conducting these trials is a lengthy, time-consuming and expensive process. These tests and trials may not achieve favorable results for many reasons, including, among others, failure of the product candidate to demonstrate safety or efficacy, the development of serious or life-threatening adverse events, or side effects, caused by or connected with exposure to the product candidate, difficulty in enrolling and maintaining subjects in the clinical trial, lack of sufficient supplies of the product candidate or comparator drug, and the failure of clinical investigators, trial monitors, contractors, consultants, or trial subjects to comply with the trial protocol. A clinical trial may fail because it did not include a sufficient number of patients to detect the endpoint being measured or reach statistical significance. A clinical trial may also fail because the dose(s) of the investigational drug included in the trial were either too low or too high to determine the optimal effect of the investigational drug in the disease setting. Many clinical trials are conducted under the oversight of Independent Data Monitoring Committees (“IDMCs”) also known as DSMB’s. These independent oversight bodies are made up of external experts who review the progress of ongoing clinical trials, including available safety and efficacy data, and make recommendations concerning a trial’s continuation, modification, or termination based on interim, unblinded data. Any of our ongoing clinical trials may be discontinued or amended in response to recommendations made by responsible IDMCs based on their review of such interim trial results.
We will need to reevaluate our product candidates if they do not test favorably and either conduct new trials, which are expensive and time consuming, or abandon our drug development program. Even if we obtain positive results from preclinical or clinical trials, we may not achieve the same success in future trials. Many companies in the biopharmaceutical industry have suffered significant setbacks in clinical trials, even after promising results have been obtained in earlier trials. The failure of clinical trials to demonstrate safety and effectiveness for the desired indication could harm the development of our product candidate and our business, financial condition and results of operations may be materially harmed.
We face significant risks in our product candidate development efforts.
Our business depends on the successful development and commercialization of our product candidates. We are currently focused on developing our initial product candidate, SBP-101, for the treatment of PDA and are not permitted to market it in the United States until we receive approval of an NDA from the FDA, or in any foreign jurisdiction until we receive the requisite approvals from such jurisdiction. The process of developing new drugs and/or therapeutic products is inherently complex, unpredictable, time-consuming, expensive and uncertain. We must make long-term investments and commit significant resources before knowing whether our development programs will result in drugs that will receive regulatory approval and achieve market acceptance. A product candidate that appears to be promising at all stages of development may not reach the market for a number of reasons that may not be predictable based on results and data from the clinical program. A product candidate may be found ineffective or may cause harmful side effects during clinical trials, may take longer to progress through clinical trials than had been anticipated, may not be able to achieve the pre-defined clinical endpoints even though clinical benefit may have been achieved, may fail to receive necessary regulatory approvals, may prove impracticable to manufacture in commercial quantities at reasonable cost and with acceptable quality, or may fail to achieve market acceptance.
We cannot predict whether or when we will obtain regulatory approval to commercialize our product candidates and we cannot, therefore, predict the timing of any future revenues from this or other product candidates, if any. The FDA has substantial discretion in the drug approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. For example, the FDA:
| |
●
|
could determine that the information provided by us was inadequate, contained clinical deficiencies or otherwise failed to demonstrate the safety and effectiveness of any of our product candidates for any indication;
|
| |
●
|
may not find the data from clinical trials sufficient to support the submission of an NDA or to obtain marketing approval in the United States, including any findings that the clinical and other benefits of our product candidates outweigh their safety risks;
|
| |
●
|
may disagree with our trial design or our interpretation of data from preclinical studies or clinical trials or may change the requirements for approval even after it has reviewed and commented on the design for our trials;
|
| |
●
|
may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for the manufacturing of our product candidates;
|
| |
●
|
may approve our product candidates for fewer or more limited indications than we request or may grant approval contingent on the performance of costly post-approval clinical trials;
|
| |
●
|
may change its approval policies or adopt new regulations; or
|
| |
●
|
may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of our product candidates.
|
Any failure to obtain regulatory approval of our initial product candidate or future product candidates we develop, if any, would significantly limit our ability to generate revenues, and any failure to obtain such approval for all of the indications and labeling claims we deem desirable could reduce our potential revenues.
Our product candidates are based on a new formulation of an existing technology which has never been approved for the treatment of any cancer and, consequently, is inherently risky. Concerns about the safety and efficacy of our product candidate could limit our future success.
We are subject to the risks of failure inherent in the development of product candidates based on new technologies. These risks include the possibility that any product candidates we create will not be effective, that our current product candidate will be unsafe, ineffective or otherwise fail to receive the necessary regulatory approvals or that our product candidate will be hard to manufacture on a large scale or will be uneconomical to market.
Many pharmaceutical products cause multiple potential complications and side effects, not all of which can be predicted with accuracy and many of which may vary from patient to patient. Long-term follow-up data may reveal additional complications associated with our product candidate. The responses of potential physicians and others to information about complications could materially affect the market acceptance of our product candidate, which in turn would materially harm our business.
Due to our reliance on third parties to conduct our clinical trials, we are unable to directly control the timing, conduct, expense and quality of our clinical trials, which could adversely affect our clinical data and results and related regulatory approvals.
We extensively outsource our clinical trial activities and expect to directly perform only a small portion of the preparatory stages for planned trials. We rely on independent third-party CROs to perform most of our clinical trials, including document preparation, site identification, screening and preparation, pre-study visits, training, program management and bio-analytical analysis. Many important aspects of the services performed for us by the CROs are out of our direct control. If there is any dispute or disruption in our relationship with our CROs, our clinical trials may be delayed. Moreover, in our regulatory submissions, we rely on the quality and validity of the clinical work performed by third-party CROs. If a CRO’s processes, methodologies or results are determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals could be adversely affected or invalidated.
We rely on third-party suppliers and other third parties for production of our product candidate and our dependence on these third parties may impair the advancement of our research and development programs and the development of our product candidates.
We rely on, and expect to continue to rely on, third parties for the supply of raw materials and manufacture of drug supplies necessary to conduct our preclinical studies and clinical trials. During 2021 the Company, in collaboration with our manufacturing partner confirmed a new shorter and less expensive synthesis of the active drug substance. However, delays in production by third parties could delay our clinical trials or have an adverse impact on any commercial activities. In addition, the fact that we are dependent on third parties for the manufacture of and formulation of our product candidates means that we are subject to the risk that the products may have manufacturing defects that we have limited ability to prevent or control. Although we oversee these activities to ensure compliance with our quality standards, budgets and timelines, we have had and will continue to have less control over the manufacturing of our product candidates than potentially would be the case if we were to manufacture our product candidates. Further, the third parties we deal with could have staffing difficulties, might undergo changes in priorities or may become financially distressed, which would adversely affect the manufacturing and production of our product candidates.
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials.
Pre-clinical studies and Phase I clinical trials are not primarily designed to test the efficacy of a product candidate in the general population, but rather to test initial safety, to study pharmacokinetics and pharmacodynamics, to study limited efficacy in a small number of study patients in a selected disease population, and to identify and attempt to understand the product candidate’s side effects at various doses and dosing schedules. Success in pre-clinical studies or completed clinical trials does not ensure that later studies or trials, including continuing pre-clinical studies and large-scale clinical trials, will be successful nor does it necessarily predict future results. Favorable results in early studies or trials may not be repeated in later studies or trials, and product candidates in later stage trials may fail to show acceptable safety and efficacy despite having progressed through earlier trials.
Risks Related to the Regulation of our Business
Federal and state pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
The Food and Drug Administration Modernization Act (the “FDMA”) established a public registry of open clinical trials involving drugs intended to treat serious or life-threatening diseases or conditions in order to promote public awareness of and access to these clinical trials. Under the FDMA, pharmaceutical manufacturers and other trial sponsors are required to post the general purpose of these trials, as well as the eligibility criteria, location and contact information of the trials. Failure to comply with any clinical trial posting requirements could expose us to negative publicity, fines and other penalties, all of which could materially harm our business.
In recent years, several states, including California, Vermont, Maine, Minnesota, New Mexico and West Virginia, have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs and file periodic reports on sales, marketing, pricing and other activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and available guidance is limited. Unless we are in full compliance with these laws, we could face enforcement actions and fines and other penalties and could receive adverse publicity, all of which could harm our business.
If the product candidate we develop becomes subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, our ability to successfully commercialize our product candidate may be impaired.
Our future revenues, profitability and access to capital will be affected by the continuing efforts of governmental and private third-party payors to contain or reduce the costs of health care through various means. We expect several federal, state and foreign proposals to control the cost of drugs through government regulation. We are unsure of the impact recent health care reform legislation may have on our business or what actions federal, state, foreign and private payors may take in response to the recent reforms. Therefore, it is difficult to predict the effect of any implemented reform on our business. Our ability to commercialize our product candidate successfully will depend, in part, on the extent to which reimbursement for the cost of such product candidate and related treatments will be available from government health administration authorities, such as Medicare and Medicaid in the United States, private health insurers and other organizations. Significant uncertainty exists as to the reimbursement status of newly approved health care products, particularly for indications for which there is no current effective treatment or for which medical care typically is not sought. Adequate third-party coverage may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product research and development. If adequate coverage and reimbursement levels are not provided by government and third-party payors for the use of our product candidates, our product candidates may fail to achieve market acceptance and our results of operations will be harmed.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
Legislative and regulatory actions affecting government prescription drug procurement and reimbursement programs occur relatively frequently. In the United States, the ACA was enacted in 2010 to expand healthcare coverage. Since then, numerous efforts have been made to repeal, amend or administratively limit the ACA in whole or in part. For example, the Tax Cuts and Jobs Act, signed into law by President Trump in 2017, repealed the individual health insurance mandate, which is considered a key component of the ACA. In December 2018, a Texas federal district court struck down the ACA on the grounds that the individual health insurance mandate is unconstitutional, although this ruling has been stayed pending appeal. The ongoing challenges to the ACA and new legislative proposals have resulted in uncertainty regarding the ACA's future viability and destabilization of the health insurance market. The resulting impact on our business is uncertain and could be material.
Efforts to control prescription drug prices could also have a material adverse effect on our business. For example, in 2018, President Trump and the Secretary of the U.S. Department of Health and Human Services (“HHS”) released the "American Patients First Blueprint" and have begun implementing certain portions. The initiative includes proposals to increase generic drug and biosimilar competition, enable the Medicare program to negotiate drug prices more directly and improve transparency regarding drug prices and ways to lower consumers' out-of-pocket costs. The Trump administration also proposed to establish an “international pricing index” that would be used as a benchmark to determine the costs and potentially limit the reimbursement of drugs under Medicare Part B. Among other pharmaceutical manufacturer industry-related proposals, Congress has proposed bills to change the Medicare Part D benefit to impose an inflation-based rebate in Medicare Part D and to alter the benefit structure to increase manufacturer contributions in the catastrophic phase. The volume of drug pricing-related bills has dramatically increased under the current Congress, and the resulting impact on our business is uncertain and could be material.
In addition, many states have proposed or enacted legislation that seeks to regulate pharmaceutical drug pricing indirectly or directly, such as by requiring biopharmaceutical manufacturers to publicly report proprietary pricing information or to place a maximum price ceiling on pharmaceutical products purchased by state agencies. For example, in 2017, California’s governor signed a prescription drug price transparency state bill into law, requiring prescription drug manufacturers to provide advance notice and explanation for price increases of certain drugs that exceed a specified threshold. Both Congress and state legislatures are considering various bills that would reform drug purchasing and price negotiations, allow greater use of utilization management tools to limit Medicare Part D coverage, facilitate the import of lower-priced drugs from outside the United States and encourage the use of generic drugs. Such initiatives and legislation may cause added pricing pressures on our products.
Changes to the Medicaid program at the federal or state level could also have a material adverse effect on our business. Proposals that could impact coverage and reimbursement of our products, including giving states more flexibility to manage drugs covered under the Medicaid program and permitting the re-importation of prescription medications from Canada or other countries, could have a material adverse effect by limiting our products’ use and coverage. Furthermore, state Medicaid programs could request additional supplemental rebates on our products as a result of an increase in the federal base Medicaid rebate. To the extent that private insurers or managed care programs follow Medicaid coverage and payment developments, they could use the enactment of these increased rebates to exert pricing pressure on our products, and the adverse effects may be magnified by their adoption of lower payment schedules.
Other proposed regulatory actions affecting manufacturers could have a material adverse effect on our business. It is difficult to predict the impact, if any, of any such proposed legislative and regulatory actions or resulting state actions on the use and reimbursement of our products in the United States, but our results of operations may be adversely affected.
Risks Related to our Intellectual Property
If we are unable to obtain, maintain and enforce our proprietary rights, we may not be able to compete effectively or operate profitably.
For ivospemin, we are party to a license agreement with the University of Florida Research Foundation (“UFRF”) and for Flynpovi, we are party to a license agreement with the Arizona Board of Regents of the University of Arizona. The patents underlying the licensed intellectual property and those of other biopharmaceutical companies are generally uncertain and involve complex legal, scientific and factual questions.
Our ability to develop and commercialize drugs depends in significant part on our ability to: (i) obtain and/or develop broad, protectable intellectual property; (ii) obtain additional licenses, if required, to the proprietary rights of others on commercially reasonable terms; (iii) operate without infringing upon the proprietary rights of others; (iv) prevent others from infringing on our proprietary rights; and (v) protect our corporate know-how and trade secrets.
Patents that we may acquire, and those that might be issued in the future, may be challenged, invalidated or circumvented, and the rights granted thereunder may not provide us with proprietary protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies or duplicate any technology we develop. Because of the extensive time required for development, testing and regulatory review of potential product candidates, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a brief period following commercialization, thus reducing any advantage of the patent.
Because patent applications in the U.S. and many foreign jurisdictions are typically not published until at least 12 months after filing, or in some cases not at all, and because publications of discoveries in the scientific literature often lag behind actual discoveries, neither we nor our licensors can be certain that either we or our licensors were the first to make the inventions claimed in issued patents or pending patent applications, or that we were the first to file for protection of the inventions set forth in these patent applications.
Additionally, UFRF previously elected to seek protection for certain elements of the licensed technology only in the United States, and the time to file for international patent protection has expired. This limits the strength of the Company’s intellectual property position in certain markets and could affect the overall value of the Company to a potential corporate partner.
Obtaining and maintaining our patent protection depends upon compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The United States Patent and Trademark Office and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
We may be exposed to infringement or misappropriation claims by third parties, which, if determined adversely to us, could cause us to pay significant damage awards.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. We may become a party to various types of patent litigation or other proceedings regarding intellectual property rights from time to time even under circumstances where we are not using and do not intend to use any of the intellectual property involved in the proceedings.
The cost of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the cost of such litigation or proceedings more effectively than we will be able to because our competitors may have substantially greater financial resources. If any patent litigation or other proceeding is resolved against us, we or our collaborators may be enjoined from developing, manufacturing, selling or importing our drugs without a license from the other party and we may be held liable for significant damages. We may not be able to obtain any required license(s) on commercially acceptable terms or at all.
Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
Confidentiality agreements with employees and others may not adequately prevent disclosure of our know-how, trade secrets and other proprietary information and may not adequately protect our intellectual property, which could impede our ability to compete.
Because we operate in the highly technical field of medical technology development, we rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with all of our employees, consultants and corporate partners, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties any confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements from these parties which provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology industry, we employ individuals who were previously employed at other biotechnology companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Associated with this Offering and Ownership of Our Common Stock
Failure to maintain the listing of our common stock on a national securities exchange could seriously harm the liquidity of our stock and our ability to raise capital.
On March 5, 2024, Nasdaq notified us that the Nasdaq Hearings Panel had determined to delist our common stock and trading of our common stock was suspended on March 7, 2024. Nasdaq will complete the delisting by filing a Form 25 Notification of Delisting with the SEC after applicable appeal periods have lapsed. The panel reached its decision because our Company was in violation of the minimum $2.5 million stockholders equity requirement in Listing Rule 5550(b)(1) and unable to comply with any of the alternative requirements in Listing Rule 5550(b) (collectively, the “Minimum Stockholders’ Equity Requirement”). The period during which we could appeal the decision to the Nasdaq Listing and Hearing Review Council has lapsed, but the Council may, on its own motion, determine to review the panel’s decision within 45 calendar days after the Company was notified of the decision. Although we are seeking all possible opportunities to regain compliance with the Minimum Stockholders’ Equity Requirement or to obtain an alternative listing on a national securities exchange, we believe that even if we were able to regain compliance with all applicable Nasdaq continued listing requirements, it is likely that Nasdaq will proceed with delisting our common stock.
As previously disclosed, in the past we have received notices from Nasdaq’s Listing Qualifications Department indicating that, for 30 consecutive business days, our common stock had not maintained a minimum closing bid price of $1.00 per share as required by Nasdaq Listing Rule 5550(a)(2) (“Minimum Bid Price Requirement”); (ii) the Minimum Stockholders’ Equity Requirement; and the minimum requirement of 500,000 publicly held shares as required by Nasdaq Listing Rule 5550(a)(4) (the “Minimum Float Requirement”). In February 2024, we received a letter from Nasdaq confirming that we had cured the most recently identified deficiencies under the Minimum Bid Price Requirement and Minimum Float Requirement.
While we have regained compliance in the past, if, for any reason, Nasdaq were to delist our securities from trading on the Nasdaq Capital Market and we were unable to obtain listing on another reputable national securities exchange, a reduction in some or all of the following may occur, each of which could materially adversely affect our stockholders:
| |
●
|
the liquidity and marketability of our common stock;
|
| |
●
|
the market price of our common stock;
|
| |
●
|
our ability to obtain financing for the continuation of our operations;
|
| |
●
|
the number of institutional and general investors that will consider investing in our common stock;
|
| |
●
|
the number of market makers in our common stock;
|
| |
●
|
the availability of information concerning the trading prices and volume of our common stock; and
|
| |
●
|
the number of broker-dealers willing to execute trades in shares of our common stock.
|
In addition, if we cease to be listed on the Nasdaq Capital Market, we may have to pursue trading on a less recognized or accepted market, such as the over the counter markets, our stock may be traded as a “penny stock”, which would make transactions in our stock more difficult and cumbersome, and we may be unable to access capital on favorable terms or at all, as companies trading on alternative markets may be viewed as less attractive investments with higher associated risks, such that existing or prospective institutional investors may be less interested in, or prohibited from, investing in our common stock. This may also cause the market price of our common stock to further decline.
Raising additional capital may cause dilution to our stockholders or restrict our operations.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, stockholders’ ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect their rights as stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. Any of these events could adversely affect our ability to achieve our product development and commercialization goals and harm our business. We do not anticipate any adverse effects stemming from the lack of available credit facilities at this time.
Issuance of common stock in offerings or pursuant to the exercise of rights to purchase shares may cause the price of our common stock to decline and cause investors to lose a significant portion of their investment.
If our stockholders sell substantial amounts of our common stock in the public market or upon the expiration of any statutory holding period under Rule 144, or upon expiration of lock-up periods applicable to outstanding shares or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether sales have occurred or are occurring, also could make our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate more difficult. As of March 22, 2024, we had outstanding options to purchase 607 shares of our common stock at a weighted-average exercise price of $14,410.38 per share with a remaining contractual life of 8.50 years and outstanding warrants to purchase 9,095,943 shares of common stock at a weighted-average exercise price of $3.13 per share and a remaining exercise period of 5.23 years.
Securities analysts may not initiate coverage or continue to cover our common stock, and this may have a negative impact on the market price of our common stock.
Common stock prices are often significantly influenced by the research and reports that securities analysts publish about companies and their business. We do not have any control over these analysts. There is no guarantee that securities analysts will cover, or continue to cover, our common stock. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of our common stock. If our common stock is covered by securities analysts and our stock is downgraded, our stock price will likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, we can lose visibility in the financial markets, which can cause our stock price or trading volume to decline.
Our charter documents and Delaware law may inhibit a takeover that stockholders consider favorable.
The provisions of our certificate of incorporation and bylaws and applicable provisions of Delaware law may make it more difficult for or prevent a third party from acquiring control of us without the approval of our board of directors. These provisions:
| |
●
|
set limitations on the removal of directors;
|
| |
●
|
limit who may call a special meeting of stockholders;
|
| |
●
|
establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon at stockholder meetings;
|
| |
●
|
do not permit cumulative voting in the election of our directors, which would otherwise permit less than a majority of stockholders to elect directors;
|
| |
●
|
establish a classified board of directors limiting the number of directors that are elected each year; and
|
| |
●
|
provide our board of directors with the ability to designate the terms of and issue preferred stock without stockholder approval.
|
In addition, Section 203 of the Delaware General Corporation Law generally limits our ability to engage in any business combination with certain persons who own 15% or more of our outstanding voting stock or any of our associates or affiliates who at any time in the past three years have owned 15% or more of our outstanding voting stock unless our board of directors has pre-approved the acquisitions that lead to such ownership. These provisions may have the effect of entrenching our management team and may deprive stockholders of the opportunity to sell their shares to potential acquirers at a premium over prevailing prices. This potential inability to obtain a control premium could reduce the price of our common stock.
We cannot assure that a reverse stock split will increase our stock price for the required time period or maintain our listing on a national securities exchange.
Our recent reverse stock splits were each accompanied by an initial increase in the market price of our common stock; however, the ongoing effect of reverse stock splits on the market price of our common stock cannot be predicted with any certainty, and the history of reverse stock splits for other companies is varied. Some investors may view one or more reverse stock splits negatively.
While the rules of national securities exchanges do not impose specific limits on the number of times a listed company may effect a reverse stock split, certain exchanges, such as Nasdaq, have stated that a series of reverse stock splits may undermine investor confidence, especially where the reverse stock splits follow dilutive transactions. Accordingly, a national securities exchange may determine that it is not in the public interest to maintain or accept our listing, even if we comply with all applicable initial or continued listing standards.
Furthermore, a reverse stock split may not result in a per share price that attracts investors who do not trade in lower priced stocks. Although we believe a reverse stock split may enhance the marketability of our common stock to certain potential investors, we cannot assure you that our common stock will be more attractive to investors following the reverse stock split. Even though we have implemented a reverse stock split, the market price of our common stock may decrease due to factors unrelated to the reverse stock split, including our future performance or general market trends. If the trading price of the common stock declines, the percentage declines as an absolute number and as a percentage of our overall market capitalization may be greater than would occur in the absence of a reverse stock split.
Recent and future reverse stock splits may decrease the liquidity of our common stock and result in higher transaction costs.
The liquidity of our common stock may be negatively impacted by a reverse stock split, given the reduced number of shares that are outstanding after such a reverse stock split, particularly if the stock price does not increase as a result of the reverse stock split. Additionally, if the reverse stock split is implemented, it will increase the number of our stockholders who own “odd lots” of fewer than 100 shares of common stock. Brokerage commissions and other costs of transactions in odd lots are generally higher than the costs of transactions of more than 100 shares of common stock. Accordingly, reverse stock splits may not achieve the desired results of increasing marketability of our common stock as described above.
Recent reverse stock splits have not been accompanied by decreases in our authorized shares.
Although the reverse stock splits have not had have any directly dilutive effects on our stockholders, the reduction in outstanding shares that resulted from the reverse stock split reduced the proportion of shares owned by our stockholders relative to the number of shares authorized for issuance, giving the Board of Directors an effective increase in the relative number of authorized shares available for issuance, in its discretion. The Board of Directors from time to time may deem it to be in the best interests of the Company and its stockholders to enter into transactions and other ventures that may include the issuance of shares of our common stock. If the Board of Directors authorizes the issuance of additional shares of common stock subsequent to a reverse stock split, the dilution to the ownership interest of our existing stockholders may be greater than would occur had such reverse stock split not been effected.
If we issue preferred stock, the rights of the holders of our common stock and the value of such common stock could be adversely affected.
Our Board of Directors is authorized to issue classes or series of preferred stock, without any action on the part of the stockholders. The Board of Directors also has the power, without stockholder approval, to set the terms of any such classes or series of preferred stock, including voting rights, dividend rights and preferences over the common stock with respect to dividends or upon the liquidation, dissolution or winding-up of our business and other terms. If we issue preferred stock in the future that has a preference over the common stock with respect to the payment of dividends or upon liquidation, dissolution or winding-up, or if we issue preferred stock with voting rights that dilute the voting power of the common stock, the rights of holders of the common stock or the value of the common stock would be adversely affected.
If we fail to maintain effective internal control over financial reporting, the price of our common stock may be adversely affected.
We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. Any failure of these controls could also prevent us from maintaining accurate accounting records and discovering accounting errors and financial fraud.
Management’s assessment of internal controls over financial reporting may identify weaknesses that need to be addressed or other potential matters that may raise concerns for investors. Any actual or perceived weaknesses that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
We will need to raise additional capital in the future to finance our operations, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
We have had recurring losses from operations, negative operating cash flow and have an accumulated deficit. We must raise additional funds in order to continue financing our operations. If additional capital is not available to us when needed or on acceptable terms, we may not be able to continue to operate our business pursuant to our business plan or we may have to discontinue our operations entirely. Any additional capital raised through the sale of equity or equity-backed securities may dilute our stockholders’ ownership percentages and could also result in a decrease in the market value of our equity securities. The terms of any securities issued by us in future capital transactions may be more favorable to new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect on the holders of any of our securities then outstanding.
If we are unable to secure additional funds when needed or on acceptable terms, we may be required to defer, reduce or eliminate significant planned expenditures, restructure, curtail or eliminate some or all of our operations, dispose of technology or assets, pursue an acquisition of our company by a third party at a price that may result in a loss on investment for our stockholders, file for bankruptcy or cease operations altogether. Any of these events could have a material adverse effect on our business, financial condition and results of operations. Moreover, if we are unable to obtain additional funds on a timely basis, there will be substantial doubt about our ability to continue as a going concern and increased risk of insolvency and up to a total loss of investment by our stockholders.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
In some cases, you can identify forward-looking statements by the following words: “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. Forward-looking statements are not a guarantee of future performance or results and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. Forward-looking statements are based on information available at the time the statements are made and involve known and unknown risks, uncertainties and other factors that may cause our results, levels of activity, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements in this prospectus. These factors include:
| |
●
|
our lack of diversification and the corresponding risk of an investment in our Company;
|
| |
●
|
potential deterioration of our financial condition and results due to failure to diversify;
|
| |
●
|
our ability to successfully complete acquisitions and integrate operations for new product candidates;
|
| |
●
|
our ability to obtain additional capital, on acceptable terms or at all, required to implement our business plan;
|
| |
●
|
final results of our Phase Ia/Ib clinical trial;
|
| |
●
|
progress and success of our randomized Phase II/III clinical trial;
|
| |
●
|
our ability to demonstrate safety and effectiveness of our product candidate;
|
| |
●
|
our ability to obtain regulatory approvals for our product candidate in the United States, the European Union, or other international markets;
|
| |
●
|
the market acceptance and future sales of our product candidate;
|
| |
●
|
the cost and delays in product development that may result from changes in regulatory oversight applicable to our product candidate;
|
| |
●
|
the rate of progress in establishing reimbursement arrangements with third-party payors;
|
| |
●
|
the effect of competing technological and market developments;
|
| |
●
|
the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims; and
|
| |
●
|
other risk factors included under the caption “Risk Factors” starting on page 10 of this prospectus.
|
You should read the matters described in “Risk Factors” and the other cautionary statements made in this prospectus as being applicable to all related forward-looking statements wherever they appear in this prospectus. We cannot assure you that the forward-looking statements in this prospectus will prove to be accurate and therefore you are encouraged not to place undue reliance on forward-looking statements. You should read this prospectus completely. Other than as required by law, we undertake no obligation to update or revise these forward-looking statements, even though our situation may change in the future.
We caution readers not to place undue reliance on any forward-looking statement that speaks only as of the date made and to recognize that forward-looking statements are predictions of future results, which may not occur as anticipated. Actual results could differ materially from those anticipated in the forward-looking statements and from historical results due to the risks and uncertainties described under the heading “Risk Factors” in this prospectus, as well as others that we may consider immaterial or do not anticipate at this time. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. Our expectations reflected in our forward-looking statements can be affected by inaccurate assumptions that we might make or by known or unknown risks and uncertainties, including those described under the heading “Risk Factors” in this prospectus. The risks and uncertainties described i under the heading “Risk Factors” in this prospectus are not exclusive and further information concerning us and our business, including factors that potentially could materially affect our financial results or condition, may emerge from time to time. We assume no obligation to update forward-looking statements to reflect actual results or changes in factors or assumptions affecting such forward-looking statements. We advise stockholders and investors to consult any further disclosures we may make on related subjects in our subsequent annual reports on Form 10-K, quarterly reports on Form 10-Q, and current reports on Form 8-K that we file with or furnish to the U.S. Securities and Exchange Commission (the “SEC”).
USE OF PROCEEDS
Assuming the issuance of all 8,750,000 shares of common stock pursuant to cash exercise of all Warrants, we will receive an aggregate of approximately $18 million. There is no assurance that the holders of the Warrants will elect to exercise any or all of such Warrants. The exercise price of the Warrants is $2.06 per share of common stock. We believe the likelihood that Warrant holders will exercise their Warrants, and therefore the amount of cash proceeds that we would receive, is highly dependent upon the trading price of our common stock. The last reported sales price on the OTC Pink Market for shares of our common stock on March 26, 2024 was $0.64 per share. If the trading price for our common stock is less than the exercise price of the Warrants, we believe holders of the Warrants will be unlikely to exercise their Warrants for cash. There is no assurance that the holders of the Warrants will elect to exercise any or all of such Warrants. To the extent that shares of common stock are issued pursuant to the exercise of Warrants on a “cashless basis,” the amount of cash we would receive from the exercise of the Warrants will decrease.
We intend to use the net proceeds from the sale of any securities for (i) the continued clinical development of our initial product candidate ivospemin, (ii) cost of drug product for use in clinical development with collaboration partners of CPP assets, and (iii) general corporate purposes unless otherwise indicated in the applicable prospectus supplement. General corporate purposes may include the repayment of outstanding indebtedness, working capital, general and administrative expenses. We may also use a portion of the net proceeds to invest in or acquire businesses or technologies that we believe are complementary to our own, although we have no current plans, commitments or agreements with respect to any acquisitions as of the date of this prospectus supplement. We have not yet determined the amount of net proceeds to be used specifically for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds from the sale of these securities.
We are party to a guaranty (the “Guaranty”) pursuant to which we have agreed to guarantee the payment obligations of CPP, under a promissory note in favor of Sucampo GmbH dated as of June 15, 2022, (the “Note”), which had a principal balance of approximately $5.4 million as of December 31, 2023. CPP is required to make three remaining payments of $1 million, plus accrued but unpaid interest, on January 31st of each of 2025 and 2026, with the remaining balance due on January 31, 2027. The first two installments of $1.0 million plus accrued interest were as paid in February 2023 and March 2024.
Our expected use of net proceeds from this offering represents our intentions based on our present plans and business conditions, which could change as our plans and business conditions evolve. The amount and timing of our actual expenditures will depend on numerous factors, including the timing and success of clinical studies or clinical studies we may commence in the future, the timing of regulatory submissions and the feedback from regulatory authorities. As a result, our management will have broad discretion over the use of the net proceeds from this offering. Pending our use of the net proceeds from this offering, we may temporarily invest the net proceeds in investment-grade, interest-bearing securities.
We currently estimate the funds will allow us to make progress in the conduct of our randomized double-blind, placebo-controlled clinical trial (known as the ASPIRE trial) for the treatment of pancreatic ductile adenocarcinoma. Continuation of the current trial, if the interim analysis is positive, will be required for FDA or other similar approvals. The cost and timing of additional clinical trials are highly dependent on the number of indications we pursue and the nature and size of the trials. It is estimated that the completion of the randomized clinical trial and other steps in the approval process for ivospemin in pancreatic cancer could cost between $60 and $80 million.
Predicting the cost necessary to develop product candidates can be difficult and we anticipate we will need additional funds to complete the development work generally required for obtaining regulatory approval to commercialize a drug. We have based these estimates on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect.
DIVIDEND POLICY
We have never declared or paid cash dividends on our capital stock. Following the completion of this offering, we intend to retain our future earnings, if any, to finance the further development and expansion of our business and do not expect to pay cash dividends in the foreseeable future. Payment of future cash dividends, if any, will be at the discretion of our Board of Directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, outstanding indebtedness, plans for expansion and restrictions imposed by lenders, if any.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The disclosure appearing in Part II, Item 7, of our annual report on Form 10-K for the year ended December 31, 2023, is hereby incorporated by reference in its entirety. The Company is eligible to incorporate this information by reference pursuant to General Instruction VII of Form S-1.
FINANCIAL STATEMENTS
Our audited financial statements for the years ended December 31, 2022 and December 31, 2023, appearing in our annual report on Form 10-K for the year ended December 31, 2023, are hereby incorporated by reference in their entirety. The Company is eligible to incorporate this information by reference pursuant to General Instruction VII of Form S-1.
DESCRIPTION OF SECURITIES
The summary of the general terms and provisions of the common stock, par value $0.001 per share (“Common Stock”), of Panbela set forth below does not purport to be complete and is subject to and qualified by reference to the Corporation’s Certificate of Incorporation, as amended (the “Certificate”), and Bylaws of the Corporation, as amended (the “Bylaws”). For additional information, please read the Certificate, Bylaws and the applicable provisions of the General Corporation Law of Delaware (the “DGCL”).
Authorized Shares
The Corporation is authorized to issue up to 110,000,000 shares of capital stock, of which 100,000,000 constitute shares of Common Stock and 10,000,000 constitute shares of preferred stock, par value $0.001 per share (“Preferred Stock”).
Common Stock
No outstanding shares of common stock is entitled to preference over any other share, and each share is equal to any other share in all respects. Holders of shares of common stock are entitled to one vote for each share held of record at each meeting of shareholders. Holders of shares of common stock are not entitled to any preemptive, subscription, conversion, redemption or sinking fund rights. The absence of preemptive rights could result in a dilution of the interest of shareholders should additional common shares be issued.
Subject to any prior rights of any Preferred Stock then outstanding, holders of common stock are entitled to receive dividends in the form of cash, property or shares of capital stock of the Corporation, when and as declared by the board of directors, provided there are sufficient net profits or surplus legally available for that purpose. In any distribution of capital assets, such as liquidation, whether voluntary or involuntary, holders of shares of common stock are entitled to receive pro rata the assets remaining after creditors have been paid in full. All of the issued and outstanding shares of common stock are non-assessable.
Anti-Takeover Provisions
The Charter Documents and the DGCL contain certain provisions that may discourage an unsolicited takeover of the Company or make an unsolicited takeover of the Company more difficult. The following are some of the more significant anti-takeover provisions that are applicable to the Company:
Delaware Anti-Takeover Law
In general, Section 203 of the DGCL prohibits a Delaware corporation with a class of voting stock listed on a national securities exchange or held of record by 2,000 or more stockholders from engaging in a Business Combination (as defined below) with an Interested Stockholder (as defined below) for a three-year period following the time that this stockholder becomes an interested stockholder, unless the Business Combination is approved in a prescribed manner. A “Business Combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the Interested Stockholder. An “Interested Stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of Interested Stockholder status, 15% or more of the corporation’s voting stock. Under Section 203, a Business Combination between a corporation and an Interested Stockholder is prohibited for three years unless it satisfies one of the following conditions:
| |
●
|
Before the stockholder became an Interested Stockholder, the board of directors approved either the Business Combination or the transaction which resulted in the stockholder becoming an Interested Stockholder;
|
| |
●
|
Upon consummation of the transaction which resulted in the stockholder becoming an Interested Stockholder, the Interested Stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances; or
|
| |
●
|
At or after the time the stockholder became an Interested Stockholder, the Business Combination was approved by the board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the Interested Stockholder.
|
Requirements for Advance Notification of Stockholder Nominations and Proposals
The Bylaws establish advance-notice procedures with respect to stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors.
Special Meetings of Stockholders
The Certificate and Bylaws provide that a special meeting of stockholders may be called only by the board of directors, the Chairman of the Board or the Chief Executive Officer of the Corporation.
Classified Board of Directors
The Certificate provides that directors are divided into three classes and elected for staggered terms. At each annual meeting, approximately one third of the directors will be elected to serve a three-year term. Directors serving staggered terms can be removed from office only for cause and only by the affirmative vote of the holders of 75% or more of the outstanding shares of stock then entitled to vote at an election of directors.
Authority of the Board of Directors
The board of directors has the power to issue any or all of the shares of the Corporation’s capital stock, including the authority to establish one or more series of Preferred Stock and to fix the powers, preferences, rights and limitations of such class or series, without seeking stockholder approval. The board of directors has the authority to adopt and change Bylaws, subject to the right of holders of at least 66.67% of the voting power of all then-outstanding shares entitled to vote generally in the election of directors to adopt, amend or repeal Bylaws.
Preferred Stock
Our Board of Directors has the authority, without first obtaining the approval of our stockholders, to establish one or more series of preferred stock and to fix:
| |
●
|
the number of shares of such series;
|
| |
●
|
the designations, preferences and relative rights, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences; and
|
| |
●
|
any qualifications, limitations or restrictions.
|
We believe that the ability of our Board of Directors to issue one or more series of preferred stock provides flexibility in structuring possible future financings and acquisitions, and in meeting other corporate needs that may arise. The authorized shares of preferred stock, as well as authorized and unissued shares of common stock, are available for issuance without action by the holders of common stock, unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded.
Our Board of Directors may authorize, without stockholder approval, the issuance of preferred stock with voting and conversion rights that could adversely affect the voting power and other rights of holders of common stock. Although our Board of Directors has no current intention of doing so, it could issue a series of preferred stock that could, depending on the terms of such series, impede the completion of a merger, tender offer or other takeover attempt of our Company. Our Board of Directors could also issue preferred stock having terms that could discourage an acquisition attempt through which an acquirer may be able to change the composition of our Board of Directors, including a tender offer or other transaction that some, or a majority, of the stockholders might believe to be in their best interests or in which stockholders might receive a premium for their stock over the then-current market price. Any issuance of preferred stock therefore could have the effect of decreasing the market price of our common stock.
Our Board of Directors will make any determination to issue such shares based on its judgment as to the best interests of our Company and stockholders. We have no current plans to issue any preferred stock.
Options
The 2016 Plan initially authorized the issuance of up to 116 shares of our common stock pursuant to awards granted thereunder and 164,246 shares have been added pursuant to its annual evergreen feature. As of March 26, 2024, options to purchase 561 shares of our common stock were outstanding under the 2016 Plan with a weighted average price of $14,420.00 per share. Approximately 163,800 shares of common stock remained available for future grants under the 2016 Plan as of the same date.
As of March 26, 2024, options to purchase 5 shares of our common stock remained outstanding under the 2011 Plan with a weighted average price of $76,200.00 per share. We ceased making awards under the 2011 Plan upon stockholder approval of the 2016 Plan.
As of March 26, 2024, options to purchase 41 shares of our common stock remained outstanding under CPP’s 2010 Equity Incentive Plan with a weighted average price of $6,743.41, all of which were assumed by us in connection with the acquisition of CPP.
Warrants Outstanding
As of March 26, 2024, we had issued outstanding warrants to purchase 9,095,943 shares of common stock and no warrants to purchase shares of preferred stock outstanding. As of the same date, the outstanding warrants had a weighted average exercise price of $3.13 per share and an average remaining exercise period of 4.86 years. Under no circumstances (other than a forward stock split) will any outstanding warrants be exercisable for a greater number of shares of Common Stock.
October 2022 Warrants
In October 2022, we issued common stock purchase warrants to purchase up to an aggregate of 1,256 shares of our Common Stock (the “October 2022 Warrants”) in connection with a public offering of shares of our Common Stock and warrants. The October 2022 Warrants had an initial exercise price of $108,960.00 per share (after adjusting for subsequent reverse stock splits), were exercisable upon issuance and through October 4, 2027. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. As of March 26, 2024 there remained 285 shares underlying outstanding October 2022 Warrants, each having an adjusted exercise price of $1.099 per share.
January 2023 Warrants
In January 2023, we issued common stock purchase warrants to purchase up to an aggregate of 22,250 shares of our Common Stock (the “January 2023 Warrants”) in connection with a public offering of shares of our Common Stock and warrants. The January Warrants had an initial exercise price of $1,350.00 per share (after adjusting for subsequent reverse stock splits), were exercisable upon issuance and through January 30, 2028. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. On or after the thirty-day anniversary of their issuance, a holder of common warrants may also provide notice and elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal to the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise and (y) 0.75. As of March 26, 2024 there remained 128 shares underlying outstanding January 2023 Warrants, each having an adjusted cash exercise price of $1.099 per share.
Class A and B Warrants
In June 2023, we issued Class A common stock purchase warrants to purchase up to an aggregate of 113,500 shares of our Common Stock (the “Class A Warrants”) and Class B common stock purchase warrants to purchase up to an aggregate of 113,500 shares of our Common Stock (the “Class B Warrants” and, together with the Class A Warrant, the “June Warrants”) in connection with a public offering of shares of our Common Stock and warrants. The June Warrants had an initial exercise price of $75.00 per share (after adjusting for the subsequent reverse stock split), were exercisable upon issuance and through June 16, 2028. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. With respect to the Class A Warrants only, on or after the thirty-day anniversary of their issuance, a holder of common warrants may also provide notice and elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal to the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise and (y) 0.24. As of March 26, 2024 there remained 2,595 shares underlying outstanding Class A Warrants and 7,000 shares underlying outstanding Class B Warrants, each having an adjusted cash exercise price of $1.099 per share.
Class C Warrants
On November 2, 2023, we issued Class C common stock purchase warrants to purchase up to an aggregate of 213,000 shares of our Common Stock (the “Class C Warrants”) in connection with the inducement of exercises of existing warrants to purchase shares of our Common Stock. The Class C Warrants had an initial exercise price of $15.60 per share (after adjusting for the subsequent reverse stock split) and were exercisable upon the date of stockholder approval through the date that is five years from the date of any stockholder approvals necessary under the listing rules of Nasdaq. Our stockholders approved the issuance of the underlying shares of Common Stock at a special meeting held on December 19, 2023. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. As of March 26, 2024 there remained 80,200 shares underlying outstanding Class C Warrants, each having a cash exercise price of $1.099 per share.
We agreed to file a registration statement covering the resale of the shares underlying the Class C Warrants issued or issuable upon the exercise of the Class C Warrants and a registration statement on Form S-1 (File No. 333-275733) was declared effective by the SEC on December 20, 2023.
Class D Warrants
On December 21, 2023, we issued Class D common stock purchase warrants to purchase up to an aggregate of 255,600 shares of our Common Stock (the “Class D Warrants”) in connection with the inducement of exercises of existing warrants to purchase shares of our Common Stock. The Class D Warrants had an initial exercise price of $19.00 per share (after adjusting for the subsequent reverse stock split) but may not be exercised by the holders thereof until or unless we obtain such approval from our stockholders as may be required by the applicable rules and regulations of the Nasdaq (or any successor entity) with respect to the transactions contemplated by the associated inducement agreement (referred to as the “Stockholder Approval”). The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. The date upon which the Class D Warrant first become exercisable is referred to herein as the “Initial Exercise Date”. As of March 26, 2024 there remained 80,200 shares underlying outstanding Class C Warrants, each having a cash exercise price of $1.099 per share.
The Company has agreed to hold an annual or special meeting of stockholders on or prior to the date that is withing six months of the associated inducement agreement for the purpose of obtaining the Stockholder Approval, with the recommendation of the Company’s board of directors that such proposals be approved. If the Company does not obtain the Stockholder Approval at the first meeting, the Company has agreed to call a meeting to seek Stockholder Approval every sixty days until the one-year anniversary of the date of the inducement letters unless Stockholder Approval is obtained prior to such one-year anniversary and, if Stockholder Approval is not so obtained, every six months thereafter until the earlier of the date on which Stockholder Approval is obtained or the two-year anniversary of the date of the Inducement Letters.
We have agreed that as soon as practicable after we file our annual report, we would file a registration statement providing for the resale of the shares of common stock issuable upon exercise of the Class D Warrants and to use commercially reasonable efforts to keep such registration statement effective at all times until the investor owns no Class D Warrants or shares of common stock issuable upon exercise thereof. At our request, the selling securityholders agreed to an extension of the filing deadline, and we have filed a registration statement on Form S-1 (File No. [●]) to fulfill that obligation.
Class E and F Warrants
In January 2024, we issued Class E common stock purchase warrants to purchase up to an aggregate of 1,093,750 shares of our Common Stock (the “Class E Warrants”) and Class F common stock purchase warrants to purchase up to an aggregate of 7,656,250 shares of our Common Stock (the “Class F Warrants” and, together with the Class E Warrant, the “January 2024 Warrants”) The January 2024 Warrants are exercisable during the period commencing on the date of their issuance and ending five years from such date at an exercise price of $2.06 per share of common stock (100% of the original public offering price per share and warrants).
Duration and Exercise Price. The January 2024 Warrants were immediately exercisable and will expire on the fifth anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability. The January 2024 Warrants are exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the January 2024 Warrants to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the January 2024 Warrants. No fractional shares of common stock will be issued in connection with the exercise of any January 2024 Warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. If, at the time a holder exercises its January 2024 Warrants, a registration statement registering the issuance of the shares of common stock underlying the warrants under the Securities Act is not then effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the warrants.
Transferability. Subject to applicable laws, a January 2024 Warrant in book entry form may be transferred at the option of the holder through the facilities of the Depository Trust Company and warrants in physical form may be transferred upon surrender of the warrant to the warrant agent together with the appropriate instruments of transfer. Pursuant to a warrant agency agreement between us and VStock Transfer, our warrant agent, the warrants initially were issued in book-entry form and are represented by one or more global certificates deposited with The Depository Trust Company (“DTC”) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Transfer Agent, Registrar, and Warrant Agent
The transfer agent and registrar for our common stock, warrant agent for the October 2022 Warrants, January 2023 Warrants, and Class A, B, E, and F Warrants is VStock Transfer, which can be contacted at 18 Lafayette Place, Woodmere, New York, 11598, info@vstocktransfer.com, or +1 (212) 828-8436.
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options and warrants, or the anticipation of these sales, could adversely affect prevailing market prices from time to time and could impair our ability to raise equity capital in the future.
Upon the completion of this offering, we will have a total of 13,595,931 shares of our common stock outstanding assuming the Warrants are exercised in full for cash, based on the 4,854,931 shares of our common stock outstanding as of March 26, 2024. Of these outstanding shares, all of the shares sold in the offering will be freely tradable, except that any shares purchased in this offering by our affiliates, as that term is defined in Rule 144 under the Securities Act, would only be able to be sold in compliance with the limitations described below. In addition, we expect that the Warrants and the shares issued upon exercise of the Warrants will be freely tradeable except for any such shares issued to our affiliates, which would also only be able to be sold in compliance with Rule 144.
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares upon expiration of the lock-up agreements described below, without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described below, within any three-month period, a number of shares that does not exceed the greater of:
| |
●
|
1% of the number of shares of our common stock then outstanding; or
|
| |
●
|
the average weekly trading volume of our common stock on the Nasdaq Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
|
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Stock Options and Warrants
See “Description of Securities” above for a discussion of our outstanding options and warrants to purchase shares of common stock.
PLAN OF DISTRIBUTION
We are registering the issuance by us of an aggregate of up to 8,750,000 shares of common stock issuable upon exercise of outstanding Warrants with a per share exercise price of $2.06, subject to adjustment. We will receive proceeds upon exercise of Warrants for cash, to the extent any Warrants are exercised. Pursuant to the terms of the Warrants, the shares of common stock will be distributed to those holders who deliver a duly executed exercise notice and payment in full in immediately available funds for the number of shares of common stock purchased upon such exercise.
Upon receipt of proper notice by any of the holders of the Warrants that such holder desires to exercise a Warrant, we will, within the time allotted by the agreement governing the Warrant, issue instructions to our transfer agent to issue to the holder shares of common stock, free of a restrictive legend. Shares of common stock issued to affiliates upon exercise of the Warrants will be issued free of legend but will be deemed control securities.
A holder does not have the right to exercise any portion of a Warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
LEGAL MATTERS
The validity of the shares of common stock being offered by this prospectus has been passed upon for us by Faegre Drinker Biddle & Reath LLP.
EXPERTS
The financial statements of Panbela as of December 31, 2023 and 2022 and for the two years in the period ended December 31, 2023 incorporated in this prospectus by reference from the Company’s Annual Report on Form 10-K have been audited by Cherry Bekaert LLP, an independent registered public accounting firm, as stated in their report, which is incorporated herein by reference. Such financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act that registers the distribution of the securities offered under this prospectus. The registration statement, including the attached exhibits and schedules, contains additional relevant information about us and the securities. The rules and regulations of the SEC allow us to omit from this prospectus certain information included in the registration statement.
We are subject to the informational requirements of the Securities Exchange Act and are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. Any information we file with the SEC, including the documents incorporated by reference into this prospectus, is also available on the SEC’s website at www.sec.gov. We also make these documents publicly available, free of charge, on our website at www.panbela.com as soon as reasonably practicable after filing such documents with the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
INCORPORATION OF DOCUMENTS BY REFERENCE
We have elected to incorporate by reference certain information in this prospectus pursuant to General Instruction VII of Form S-1. We have previously filed the following documents with the SEC and are incorporating them by reference into this prospectus, except for information furnished under Item 2.02 or Item 7.01 of Form 8-K, and any exhibits relating to such information, which is neither deemed filed nor incorporated by reference herein:
| |
●
|
the description of our common stock contained in Exhibit 4.1 to the Annual Report on Form 10-K for the year ended December 31, 2020.
|
As a smaller reporting company, we also are incorporating by reference any future information filed (rather than furnished) by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, after the date of the initial filing of the registration statement of which this prospectus is a part and before the effective date of the registration statement and after the date of this prospectus until the termination of the offering. Any statements contained in a previously filed document incorporated by reference into this prospectus is deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, or in a subsequently filed document also incorporated by reference herein, modifies or supersedes that statement
Our internet address is www.panbela.com. We make available free of charge, on or through the investor relations section of our website, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. You may access the documents incorporated by reference in this prospectus through our website at www.neurometrix.com. Except for the specific incorporated documents listed above, no information available on or through our website shall be deemed to be incorporated by reference in this prospectus or the registration statement of which it forms a part.
We hereby undertake to provide without charge to each person, including any beneficial owner, to whom a prospectus is delivered, upon written or oral request of any such person, a copy of any and all of the information that has been incorporated by reference in this prospectus, but not delivered with the prospectus. Requests for such copies should be sent to us at the following address:
Panbela Therapeutics, Inc.
712 Vista Blvd #305
Waconia, MN 55387
Attention: Investor Relations
(952) 479-1196
8,750,000 Shares of Common Stock Underlying Previously Issued Warrants
Panbela Therapeutics, Inc.
PROSPECTUS
, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses payable by Panbela Therapeutics, Inc. (the “Company”) in connection with the offering and sale of the common stock being registered. All amounts shown are estimates, except the Securities and Exchange Commission (the “Commission”) registration fee.0
|
U.S. Securities and Exchange Commission registration fee
|
|
$ |
- |
|
|
Accounting fees and expenses
|
|
$ |
10,000 |
|
|
Legal fees and expenses
|
|
$ |
40,000 |
|
|
Transfer agent and registrar fees
|
|
$ |
5,000 |
|
|
Printing expenses
|
|
$ |
5,000 |
|
|
Miscellaneous
|
|
$ |
5,000 |
|
|
Total
|
|
$ |
65,000 |
|
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive officer, employee or agent of the corporation or is or was serving at the request of the corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation.
The Company’s certificate of incorporation and amended and restated bylaws limit the liability of its directors to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| |
●
|
breach of their duty of loyalty to the Company or its stockholders;
|
| |
●
|
act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
|
| |
●
|
unlawful payment of dividends or redemption of shares as provided in Section 174 of the Delaware General Corporation Law; or
|
| |
●
|
transaction from which the directors derived an improper personal benefit.
|
These limitations of liability do not apply to liabilities arising under federal securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission. The Company’s amended and restated bylaws provide that it will indemnify its directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law.
As permitted by the Delaware General Corporation Law, the Company has entered into indemnification agreements with each of the Company’s directors and executive officers that require the Company to indemnify such persons against expenses, judgments, penalties, fines, settlements and other amounts actually and reasonably incurred, including expenses of a derivative action, in connection with an actual or threatened proceeding if any of the Company’s directors or executive officers may be made a party because he or she is or was one of the Company’s directors. The Company will be obligated to pay such amounts only if the director acted in good faith and in a manner that he or she reasonably believed to be in or not opposed to the Company’s best interests. With respect to any criminal proceeding, the Company will be obligated to pay such amounts only if the director had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification.
Section 145(g) of the Delaware General Corporation Law permits a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee, or agent of the corporation arising out of his or her actions in connection with their services to the Company, regardless of whether its amended and restated bylaws permit indemnification. The Company has purchased and intends to maintain insurance on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
Item 15. Recent Sales of Unregistered Securities.
During the three months ended March 31, 2021, the Company issued 2 shares of common stock as a result of exercises of outstanding warrants. The shares were issued pursuant to net, cashless, exercises of warrants to purchase 8 shares.
The net cash proceeds for each of the foregoing sales of securities were used for the continued clinical development of our initial product candidate ivospemin and for working capital and other general corporate purposes.
On June 15, 2022, pursuant to the Merger Agreement, Panbela sold and issued the following securities to the holders of CPP securities: (a) 301 shares of Panbela Common Stock, (iv) replacement options to purchase up to 42 shares of Panbela Common Stock at a weighted average purchase price of $6,743.41.00 per share, and (v) replacement warrants to purchase up to 4 shares of Panbela Common Stock at a weighted average purchase price of $6,720.00 per share.
On April 14, 2023, the Company entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with Michael T. Cullen (the “Purchaser”), Chairman of the Company’s Board of Directors, pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series A Preferred Stock, par value $0.001 per share, to the Purchaser for $10 in cash. The sale closed on April 14, 2023. The share of Series A Preferred Stock will have 100,000,000 votes and will vote together with the outstanding shares of the Company’s common stock as a single class exclusively with respect to any proposal to amend the Company’s Amended and Restated Certificate of Incorporation to effect a reverse stock split of the Company’s common stock. The Series A Preferred Stock will be voted, without action by the holder, on any such proposal in the same proportion as shares of common stock are voted. The Series A Preferred Stock otherwise has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware. The Series A Preferred Stock is not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the Company. The Series A Preferred Stock has no rights with respect to any distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder of the Series A Preferred Stock will not be entitled to receive dividends of any kind. The outstanding share of Series A Preferred Stock shall be redeemed in whole, but not in part, at any time (i) if such redemption is ordered by the Board of Directors in its sole discretion or (ii) automatically upon the approval by the Company’s stockholders of a reverse stock split at any meeting of stockholders. Upon such redemption, which occurred on May 25, 2023, the holder of the Series A Preferred Stock received consideration of $10 in cash. No shares of Series A Preferred Stock are currently outstanding.
On November 2, 2023, the Company entered into warrant exercise inducement offer letters with certain holders of existing warrants to purchase our common stock, pursuant to which the holders agreed to exercise for cash their existing warrants to purchase 106,500 shares of our common stock, in the aggregate, at a reduced exercise price of $15.60 per share, in exchange for our agreement to issue new Class C common stock purchase warrants to purchase up to an aggregate of 213,000 shares of our common stock. The Company received aggregate gross proceeds of approximately $1.9 million from the exercise of the existing warrants and purchase of the new warrants. The new warrants had an initial exercise price of $15.60 per share and were exercisable upon the date of stockholder approval through the date that is five years from the date of any stockholder approvals necessary under the listing rules of Nasdaq. Our stockholders approved the issuance of the underlying shares of common stock at a special meeting held on December 19, 2023.
On December 21, 2023, we entered into warrant exercise inducement offer letters, each an “Inducement Agreement,” with each selling securityholder, pursuant to which the selling securityholders agreed to exercise for cash their existing Class C Warrants to purchase 127,800 shares of our common stock, in the aggregate, at their existing exercise price of $15.60 per share, in exchange for our agreement to issue new Class D common stock purchase warrants, referred to herein as the “Inducement Warrants,” to purchase up to an aggregate of 255,600 shares of our common stock. The Company received aggregate gross proceeds of approximately $2.0 million from the exercise of the existing Class C Warrants. The Inducement Warrants had an initial exercise price of $19.00 per share and will only be exercisable contingent upon and after receiving stockholder approval as required by listing rules of Nasdaq and may be exercised until five years from the date of such stockholder approval, if any.
Unless otherwise indicated, for all of the foregoing transactions, we relied on exemptions from registration set forth in Section 4(a)(2) of the Securities Act, without the use of any general solicitations or advertising to market or otherwise offer the securities for sale and all participants were “accredited investors,” as defined in Rule 501 of Regulation D as promulgated by the SEC under the Securities Act.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
|
Exhibit
No.
|
|
Description
|
|
3.1
|
|
Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to current report on Form 8-K filed January 17, 2023)
|
|
3.2
|
|
Certificate of Amendment to Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to current report on Form 8-K filed May 31, 2023)
|
|
3.3
|
|
Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to current report on Form 8-K filed April 18, 2023)
|
|
3.4
|
|
Certificate of Amendment to Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to current report on Form 8-K filed January 19, 2024)
|
|
4.1
|
|
Description of Securities (incorporated by reference to Exhibit 4.1 to annual report on Form 10-K for fiscal year ended December 31, 2020)
|
|
4.2
|
|
Form of Common Stock Warrant issued December 2018 and January 2019 (incorporated by reference to Exhibit 10.3 to current report on Form 8-K filed December 28, 2018)
|
|
4.3
|
|
Form of Common Stock Warrant issued August through October 2019 (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed August 29, 2019)
|
|
4.4
|
|
Form of Warrants issued May 22, June 5, June 15, and June 22, 2020 (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed June 11, 2020)
|
|
4.5
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated September 1, 2020 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed September 1, 2020)
|
|
4.6
|
|
Form of Common Stock Purchase Warrant (included in Exhibit 4.5)
|
|
4.7
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated as of October 4, 2022 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on October 4, 2022)
|
|
4.8
|
|
Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.3 to current report on Form 8-K filed October 4, 2022)
|
|
4.9
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated as of January 30, 2023 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on January 31. 2023)
|
|
4.10
|
|
Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.3 to current report on Form 8-K filed on January 31, 2023)
|
|
4.11
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated as of June 21, 2023 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on June 21, 2023)
|
|
4.12++
|
|
Form of Warrant Agency Agreement by and between Panbela Therapeutics, Inc. and VStock Transfer, LLC (previously Exhibit 4.15)
|
|
4.13
|
|
Form of Class A Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.3 to current report on Form 8-K filed on June 21, 2023)
|
|
4.14
|
|
Form of Class B Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.4 to current report on Form 8-K filed on June 21, 2023)
|
|
4.15
|
|
Form of Class C Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on November 3, 2023)
|
|
4.16
|
|
Form of Class D Common Stock Purchase Warrant (“Inducement Warrant”) (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on December 21, 2023)
|
|
4.17
|
|
Form of Class E Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.3 to current report on Form 8-K filed on January 29, 2024)
|
|
4.18
|
|
Form of Class F Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.4 to current report on Form 8-K filed on January 29, 2024)
|
|
5.1++
|
|
Opinion of Faegre Drinker Biddle & Reath LLP
|
|
10.1*
|
|
2011 Stock Option Plan, as amended through January 1, 2015 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed September 11, 2015)
|
|
10.2*
|
|
Form of Incentive Stock Option Agreement for awards under 2011 Plan (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed September 11, 2015)
|
|
10.3*
|
|
2016 Omnibus Incentive Plan as amended and restated through April 9, 2020 (incorporated by reference to Exhibit 99.1 to current report on Form 8-K filed May 26, 2020)
|
|
10.4*
|
|
Form of Incentive Stock Option Agreement for awards under 2016 Plan (incorporated by reference to Exhibit 10.4 to quarterly report on Form 10-Q for quarter ended June 30, 2016)
|
|
10.5*
|
|
Form of Non-Qualified Stock Option Agreement for awards under 2016 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.5 to quarterly report on Form 10-Q for quarter ended June 30, 2016)
|
|
10.6*
|
|
Form of Performance-Based Stock Option Agreement for awards under 2016 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.7 to annual report on Form 10-K for fiscal year ended December 31, 2016)
|
|
10.7**
|
|
Standard Exclusive License Agreement with University of Florida Research Foundation, Inc., dated December 22, 2011 (incorporated by reference to Exhibit 10.5 to current report on Form 8-K filed September 11, 2015)
|
|
10.8
|
|
Form of First Amendment to License Agreement with University of Florida Research Foundation, Inc. dated December 12, 2016 (incorporated by reference to Exhibit 10.10 to annual report on Form 10-K for fiscal year ended December 31, 2019)
|
|
10.9
|
|
Second Amendment to License Agreement with University of Florida Research Foundation, Inc., dated October 3, 2019 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed October 9, 2019)
|
|
10.10*
|
|
Employment Agreement with Susan Horvath, dated April 17, 2018 (incorporated by reference to Exhibit 10.4 to quarterly report on Form 10-Q for quarter ended March 31, 2018)
|
|
10.11*
|
|
Employment Agreement with Jennifer K. Simpson, dated July 15, 2020 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed July 16, 2020)
|
|
10.12
|
|
Form of Securities Purchase Agreement, dated December 21 and 31, 2018, January 14, 25, and 31, 2019 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed December 28, 2018)
|
|
10.13*
|
|
Cancer Prevention Pharmaceuticals, Inc. 2010 Equity Incentive Plan, as amended (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed June 16, 2022)
|
|
10.14*
|
|
Form of Stock Option Assumption Notice (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed June 16, 2022)
|
|
10.15
|
|
Form of Replacement Warrant (incorporated by reference to Exhibit 10.3 to current report on Form 8-K filed June 16, 2022)
|
|
10.16
|
|
Convertible Promissory Note in favor of Sucampo GmbH (f/k/a Sucampo AG), dated as of September 6, 2017, as amended through April 7, 2022 (incorporated by reference to Exhibit 10.4 to current report on Form 8-K filed June 16, 2022)
|
|
10.17
|
|
Guaranty in favor of Sucampo GmbH (f/k/a Sucampo AG), dated June 15, 2022 (incorporated by reference to Exhibit 10.5 to current report on Form 8-K filed June 16, 2022)
|
|
10.18
|
|
Separation and Release Agreement with Jeffrey E. Jacobs, dated June 15, 2022 (incorporated by reference to Exhibit 10.6 to current report on Form 8-K filed June 16, 2022)
|
|
10.19
|
|
Form of Securities Purchase Agreement (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed on October 4, 2022)
|
|
10.20
|
|
Placement Agency Agreement with Roth Capital Partners, LLC dated as of September 29, 2022 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed on October 4, 2022)
|
|
10.21
|
|
Placement Agency Agreement with Roth Capital Partners, LLC dated as of January 26, 2023 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed January 31, 2023)
|
|
10.22
|
|
Securities Purchase Agreement dated January 26, 2023 (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed January 31, 2023)
|
|
10.23
|
|
Placement Agency Agreement with Roth Capital Partners, LLC dated as of June 16, 2023 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed June 21, 2023)
|
|
10.24
|
|
Securities Purchase Agreement dated June 16, 2023 (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed June 21, 2023)
|
|
10.25
|
|
Form of Inducement Letters dated November 2, 2023 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed November 3, 2023).
|
|
10.26
|
|
Form of Inducement Letters dated December 21, 2023 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed December 21, 2023)
|
|
10.27
|
|
Placement Agency Agreement dated as of January 28, 2024 by and between Panbela Therapeutics, Inc. and Roth Capital Partners, LLC (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed on January 29, 2024)
|
|
10.28
|
|
Form of Securities Purchase Agreement by and between Panbela Therapeutics, Inc. and the purchasers named therein (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed January 29, 2024)
|
|
10.29*
|
|
Form of Retention Agreements dated February 7, 2024 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed February 13, 2024)
|
|
21.1
|
|
List of Subsidiaries (incorporated by reference to Exhibit 21.1 to annual report on Form 10-K for fiscal year ended December 31, 2023)
|
|
23.1+
|
|
Consent of Independent Registered Public Accounting Firm
|
|
23.2++
|
|
Consent of Faegre Drinker Biddle & Reath LLP (included in Exhibit 5.1)
|
|
24.1++
|
|
Powers of Attorney (see signature page to original filing)
|
|
107++
|
|
Filing Fee Table
|
| + |
Filed herewith |
| ++ |
Previously filed. |
| * |
Management compensatory plan or arrangement required to be filed as an exhibit to this prospectus. |
| ** |
Portions of exhibit omitted pursuant to order granting confidential treatment issued by the Securities and Exchange Commission. |
(b) Financial Statement Schedules.
All schedules are omitted as the required information is inapplicable or the information is presented in the financial statements or related notes.
Schedule II. Valuation and Qualifying Accounts
All other schedules are omitted as the required information is inapplicable or the information is presented in the financial statements or related notes.
Item 17. Undertakings.
The registrant hereby undertakes:
| |
(1)
|
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
| |
(i)
|
To include any prospectus required by Section 10(a)(3) of the Securities Act;
|
| |
(ii)
|
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
|
| |
(iii)
|
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in this registration statement.
|
| |
provided, however, that paragraphs (a)(1)(i), (ii) and (iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section 15(d) of the Exchange Act, that are incorporated by reference in the registration statement.
|
| |
(2)
|
That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered herein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
| |
(3)
|
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
|
| |
(4)
|
That, for the purpose of determining liability under the Securities Act to any purchaser: each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
|
| |
(5)
|
That, for the purpose of determining liability under the Securities Act to any purchaser the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective; and for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
| |
(6)
|
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
|
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Minneapolis, State of Minnesota on March 29, 2024.
| |
|
PANBELA THERAPEUTICS, INC.
|
| |
|
|
| |
|
By:
|
/s/ Jennifer K. Simpson
|
| |
|
|
Jennifer K. Simpson
President and Chief Executive Officer
|
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
| |
|
|
|
|
|
/s/ Jennifer K. Simpson
|
|
President and Chief Executive Officer
|
|
March 29, 2024
|
|
Jennifer K. Simpson
|
|
(Principal Executive Officer), and Director
|
|
|
| |
|
|
|
|
|
/s/ Susan Horvath
|
|
Vice President of Finance, Chief Financial Officer, Treasurer
|
|
March 29, 2024
|
|
Susan Horvath
|
|
and Secretary (Principal Financial and Accounting Officer)
|
|
|
| |
|
|
|
|
|
*
|
|
Chair of the Board and Director
|
|
March 29, 2024
|
|
Michael T. Cullen
|
|
|
|
|
| |
|
|
|
|
|
*
|
|
Director
|
|
March 29, 2024
|
|
Daniel J. Donovan
|
|
|
|
|
| |
|
|
|
|
|
*
|
|
Director
|
|
March 29, 2024
|
|
Arthur J. Fratamico
|
|
|
|
|
| |
|
|
|
|
|
*
|
|
Director
|
|
March 29, 2024
|
|
Jeffrey E. Jacob
|
|
|
|
|
| |
|
|
|
|
|
*
|
|
Director
|
|
March 29, 2024
|
|
Jeffrey S. Mathiesen
|
|
|
|
|
| |
|
|
|
|
|
*
|
|
Director
|
|
March 29, 2024
|
|
D. Robert Schemel
|
|
|
|
|
|
*
|
Jennifer K. Simpson, by signing her name hereto, does hereby sign this document on behalf of each of the above-named directors of the registrant pursuant to powers of attorney duly executed by such persons.
|
|
|
By:
|
/s/ Jennifer K. Simpson
|
|
|
|
|
Jennifer K. Simpson
|
|
|
|
|
Attorney-in-Fact
|
|
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in this Post-Effective Amendment No.1 to the Registration Statement on Form S-1 (Registration No. 333-276367) of our report dated March 26, 2024 relating to the consolidated financial statements of Panbela Therapeutics, Inc. (the “Company”) and its subsidiaries, as of and for the years ended December 31, 2023 and 2022, and to the reference of our firm under the caption “Experts” in the Registration Statement.
/s/ Cherry Bekaert LLP
Tampa, Florida
March 29, 2024
Panbela Therapeutics (NASDAQ:PBLA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Panbela Therapeutics (NASDAQ:PBLA)
Historical Stock Chart
From Apr 2023 to Apr 2024