Inovio Shares Rise 8% After FDA Feedback for INO-3107
October 10 2023 - 10:51AM
Dow Jones News
By Chris Wack
Inovio shares were up 8% to 39 cents after the company received
feedback from the U.S. Food and Drug Administration that data from
its completed Phase 1/2 trial of INO-3107 for the treatment of
recurrent respiratory papillomatosis could support Inovio's
submission of a biological license application for review under the
FDA's accelerated approval program.
The FDA also advised that the company's previously planned Phase
3 randomized, placebo-controlled trial wouldn't be required to
support this submission.
Inovio will be required to initiate a confirmatory trial prior
to BLA submission for accelerated approval and satisfy all other
FDA filing requirements. The design of the confirmatory trial
hasn't been finalized yet.
If approved, INO-3107 would be the first DNA medicine in the
U.S. and the first Inovio candidate to receive regulatory
approval.
INO-3107 is Inovio's lead candidate and one of three
clinical-stage DNA medicine candidates targeting HPV-related
disease.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
October 10, 2023 10:36 ET (14:36 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
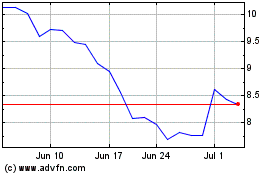
Inovio Pharmaceuticals (NASDAQ:INO)
Historical Stock Chart
From Mar 2024 to Apr 2024
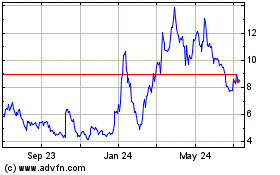
Inovio Pharmaceuticals (NASDAQ:INO)
Historical Stock Chart
From Apr 2023 to Apr 2024