Current Report Filing (8-k)
January 13 2016 - 8:31AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): January 12, 2016
HALOZYME THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
001-32335 |
|
88-0488686 |
|
(State or other jurisdiction |
|
(Commission |
|
(IRS Employer |
|
of incorporation) |
|
File Number) |
|
Identification No.) |
|
11388 Sorrento Valley Road, San Diego, California |
|
92121 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (858) 794-8889
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
On January 12, 2016, Halozyme Therapeutics, Inc., a Delaware corporation (“Halozyme”) presented at the annual JP Morgan Healthcare Conference to provide a corporate update on certain strategic programs and to provide financial guidance for 2016. Attached hereto as Exhibit 99.1, and incorporated herein by reference, is a copy of certain slides used by Halozyme in making the presentation and that are expected to be used in subsequent presentations to interested parties, including analysts and stockholders.
This information is being furnished pursuant to Item 7.01 of this Report and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section and will not be incorporated by reference into any registration statement filed by Halozyme, under the Securities Act of 1933, as amended, unless specifically identified as being incorporated therein by reference. This Report will not be deemed an admission as to the materiality of any information in this Report that is being disclosed pursuant to Regulation FD.
Please refer to page 2 of the presentation attached hereto as Exhibit 99.1 for a discussion of certain forward-looking statements included therein and the risks and uncertainties related thereto.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
Halozyme Therapeutics, Inc. corporate update presentation, dated January 12, 2016 |
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
Halozyme Therapeutics, Inc. |
|
|
|
|
|
January 13, 2016 |
By: |
/s/ Harry J. Leonhardt,, Esq. |
|
|
|
Harry J. Leonhardt,, Esq. |
|
|
|
Senior Vice President, General
Counsel, Chief Compliance Officer
and Corporate Secretary |
3
Exhibit Index
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
Halozyme Therapeutics, Inc. corporate update presentation, dated January 12, 2016 |
4
Exhibit 99.1
34th Annual J.P. Morgan Healthcare Conference Platforms for Growth: Building a Premier Oncology Biotech Dr. Helen Torley President & CEO January 12, 2016
Forward-Looking Statements All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Examples of such statements include future product development and regulatory events and goals, anticipated clinical trial results and strategies, product collaborations, our business intentions and financial estimates and results. These statements are based upon management’s current plans and expectations and are subject to a number of risks and uncertainties which could cause actual results to differ materially from such statements. A discussion of the risks and uncertainties that can affect these statements is set forth in the Company’s annual and quarterly reports filed from time to time with the Securities and Exchange Commission under the heading “Risk Factors.” The Company disclaims any intention or obligation to revise or update any forward-looking statements, whether as a result of new information, future events, or otherwise. 1
Our Focus To discover, develop and commercialize biopharmaceuticals that target the extracellular matrix and tumor microenvironment 2

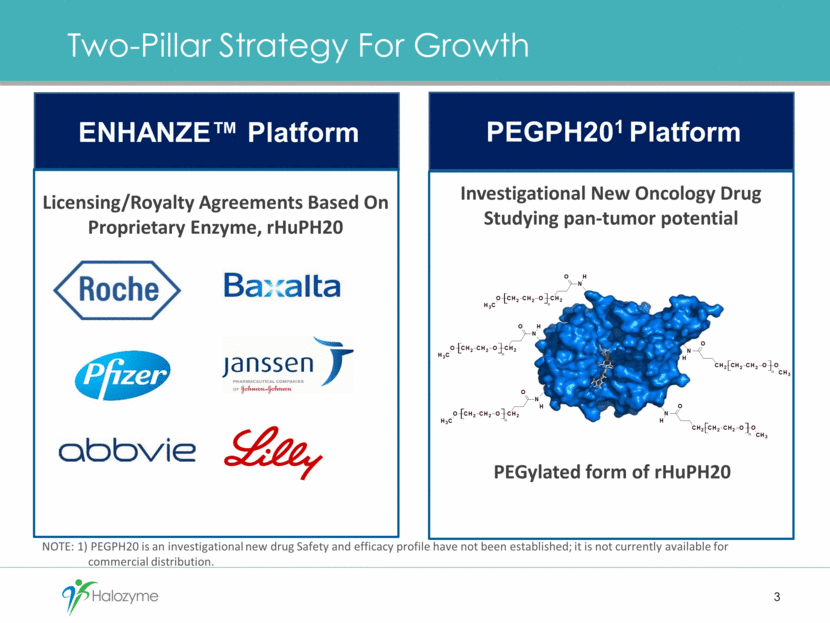
Two-Pillar Strategy For Growth 3 ENHANZE™ Platform PEGPH201 Platform PEGylated form of rHuPH20 Licensing/Royalty Agreements Based On Proprietary Enzyme, rHuPH20 Investigational New Oncology Drug Studying pan-tumor potential NOTE: 1) PEGPH20 is an investigational new drug Safety and efficacy profile have not been established; it is not currently available for commercial distribution.
Significant Milestones Achieved in 2015 4 PEGPH20 Program Interim clinical data at ASCO Reached targeted enrollment in Study 202 Expanded pan-tumor study of PEGPH20 in clinic Formed clinical collaboration with Eisai Selected Ventana for CDx development ✓ ✓ ✓ ✓ ✓ ✓ ENHANZE Platform Substantial ramp in royalties AbbVie collaboration agreement Lilly collaboration agreement Janssen in clinic with daratumumab Pfizer in clinic with rivipansel ✓ ✓ ✓ ✓
A Journey of Transformation and Growth 5 2014 - 2016 2016 - 2018 2018 - 2020 Establishing the Foundation for Growth Investing for Growth Accelerating Long-Term Growth Interim PEGPH20 data presented Value-generating licensing agreements Initiate PEGPH20 pivotal trial in pancreatic cancer Readout of Ph. 2 pancreatic study Clinical studies in multiple tumors Expand ENHANZE franchise value Multiple Phase 2 readouts possible Potential regulatory submissions:PEGPH20 in pancreatic cancer Expand ENHANZE franchise value
PEGPH20 Goal: Improve Targeting Of Co-Administered Cancer Therapies 6
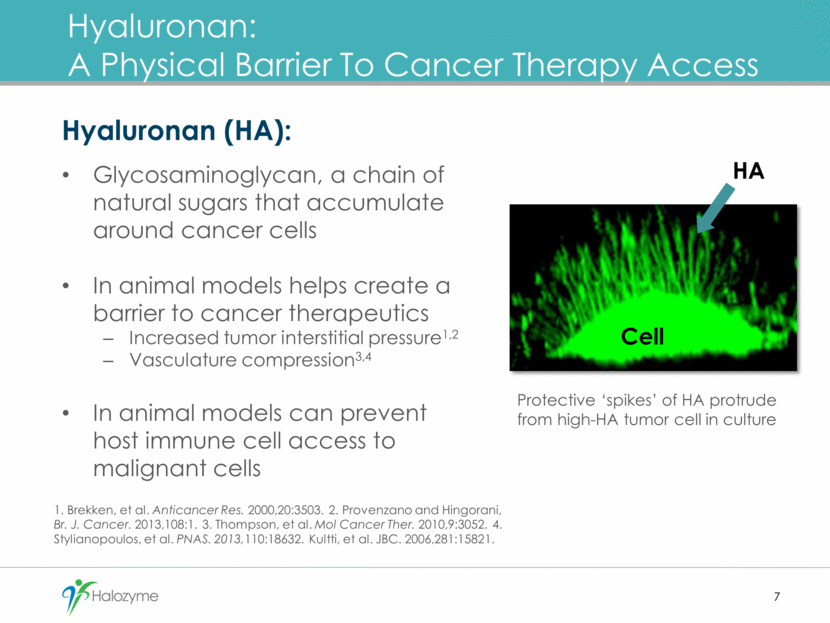
Hyaluronan: A Physical Barrier To Cancer Therapy Access 7 Hyaluronan (HA): Glycosaminoglycan, a chain of natural sugars that accumulate around cancer cells In animal models helps create a barrier to cancer therapeutics Increased tumor interstitial pressure1,2 Vasculature compression3,4 In animal models can prevent host immune cell access to malignant cells 1. Brekken, et al. Anticancer Res. 2000,20:3503. 2. Provenzano and Hingorani, Br. J. Cancer. 2013,108:1. 3. Thompson, et al. Mol Cancer Ther. 2010,9:3052. 4. Stylianopoulos, et al. PNAS. 2013,110:18632. Kultti, et al. JBC. 2006,281:15821. Protective ‘spikes’ of HA protrude from high-HA tumor cell in culture HA Cell
Total Sites 45 (US only) Enrollment Stage 1 (interim data) 146 patients Target enrollment Stage 2 114 patients Estimated top line data Q4 2016 Primary endpoints: Progression Free Survival (PFS) Rate of TE events Secondary endpoints: PFS by Hyaluronan (HA) level Overall Response Rate (ORR) Overall Survival (OS) 8 PEGPH20 + ABRAXANE® + gemcitabine (PAG) ABRAXANE® + gemcitabine (AG) Stage IV Metastatic PDA N=260 HALO-202 HALO 202 Pancreatic: Target Enrollment Achieved
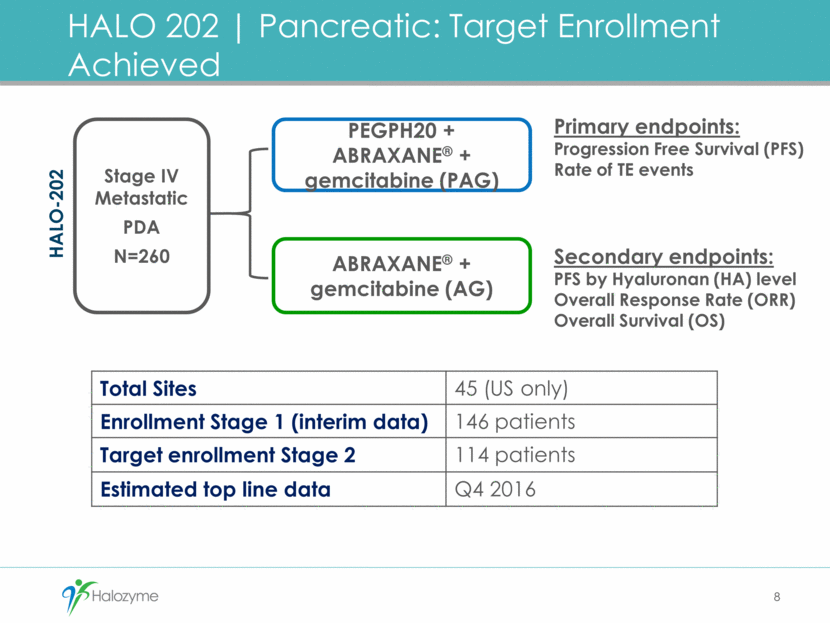
HALO 202 Pancreatic: Stage 1 Interim Results 9 PFS in HA-High2 Patients At Risk PAG 23 14 10 6 5 2 1 0 AG 21 14 7 4 0 0 0 0 AG 4.3 months PAG 9.2 months HR 0.39 (0.15, 1.04) p=0.05 1) Hingorani, et al. ASCO 2015 (Abstract #4006). 2) HA measured using Halozyme prototype companion diagnostic. Presented at ASCO 20151 0 2 4 6 8 10 12 14 0 50 100 Study Duration (months) K - M E s t i m a t e o f P r o g r e s s i o n F r e e S u r v i v a l ( % ) AG PAG
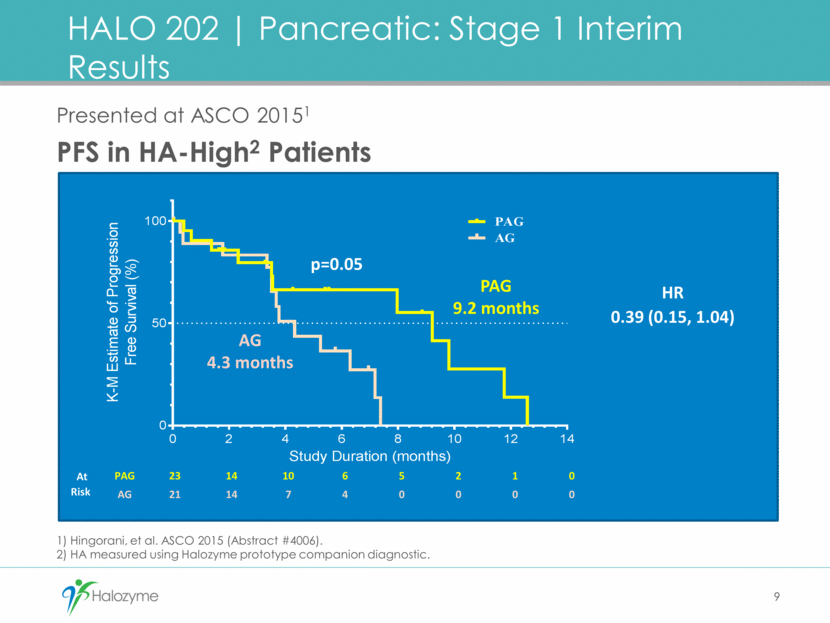
PAG N=74 Patients, n (%) AG N=61 Patients, n (%) Preferred Term Grade 3+ Any Grade Grade 3+ Any Grade Any AE 61 (82.4) 73 (98.6) 45 (73.8) 57 (93.4) Fatigue 13 (17.6) 50 (67.6) 11 (18.0) 42 (68.9) Nausea 5 (6.8) 41 (55.4) 2 (3.3) 27 (44.3) Anemia 14 (18.9) 31 (41.9) 10 (16.4) 32 (52.5) Edema peripheral 2 (2.7) 43 (58.1) 4 (6.6) 19 (31.1) Diarrhea 5 (6.8) 31 (41.9) 2 (3.3) 24 (39.3) Alopecia 0 24 (32.4) 0 25 (41.0) Decreased appetite 4 (5.4) 26 (35.1) 2 (3.3) 15 (24.6) Muscle spasms 6 (8.1) 41 (55.4) 0 1 (1.6) Platelet count decreased 5 (6.8) 22 (29.7) 4 (6.6) 18 (29.5) Vomiting 4 (5.4) 23 (31.1) 0 16 (26.2) Neutropenia 18 (24.3) 24 (32.4) 9 (14.8) 11 (18.0) 10 HALO 202 Pancreatic: Stage 1 Overall Safety Profile Treatment Related AEs >25%1 Note: 1) Hingorani, et al. ASCO 2015 (Abstract #4006).
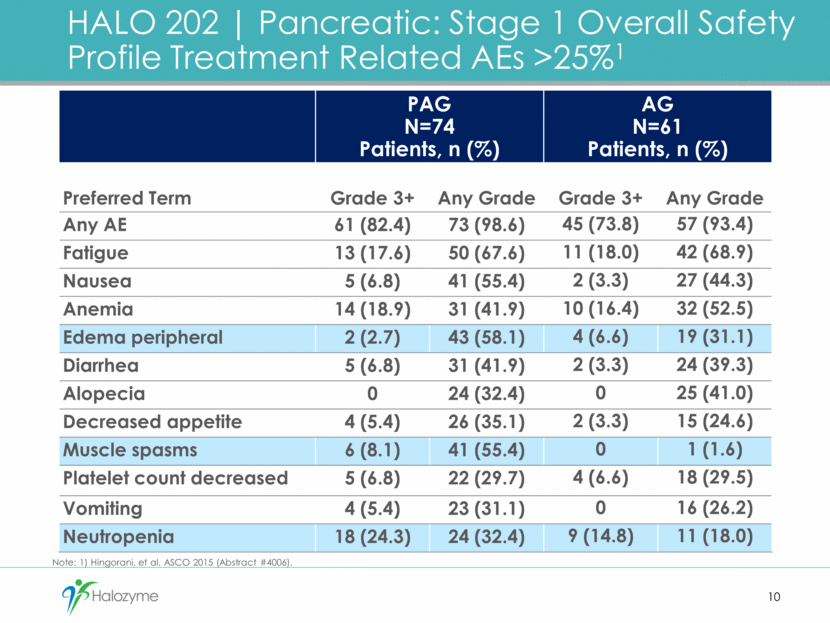
11 Enoxaparin Prophylaxis Dose TE Event Rate PAG AG Stage 1 (through Dec. 5, 2014) N/A 42% (n=74) 25% (n=61) Stage 2 (through Dec. 15, 2015) 40 mg/day; or, 40 mg/day increased to 1 mg/kg/day 29% (n=17) 29% (n=7) Started on 1 mg/kg/day 7% (n=56) 4% (n=27) TOTAL – Stage 2 12% (n=73) 9% (n=34) HALO 202 Pancreatic: Updated Thromboembolic (TE) Events With Enoxaparin Prophylaxis
12 HALO-301 Pancreatic: Phase 3 Design Randomized (2:1 PAG:AG), double-blind, placebo-controlled, global Plan to initiate March 2016, approximately 200 sites in 20 countries Interim analysis when target number of PFS events reached PFS powered with a hazard ratio of 0.59 (to detect a 41% risk reduction for progression) PEGPH20 + ABRAXANE® + gemcitabine (PAG) ABRAXANE® + gemcitabine (AG) + placebo Stage IV Metastatic PDA High-HA patients N=420 Primary Endpoints: Progression-Free Survival (PFS) Overall Survival (OS)
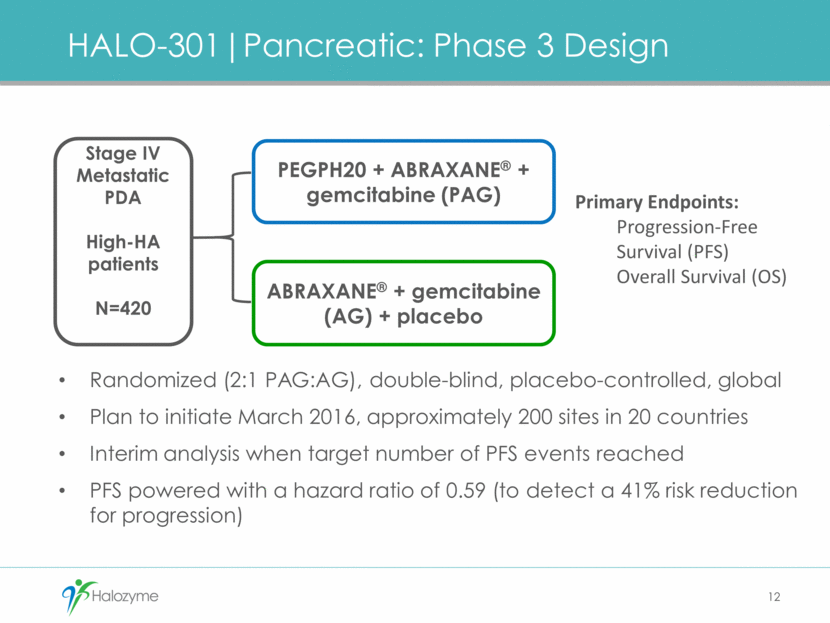
Companion Diagnostic Goals Achieved with Ventana Assay, Algorithm and Scoring approach Halozyme Prototype Ventana Specificity for HA Good Better Regulatory approval path Limited Yes FDA-approved platform widely available in Clinical Labs No Yes Consistent pathologist-based read for approval and broad adoption No Yes 13
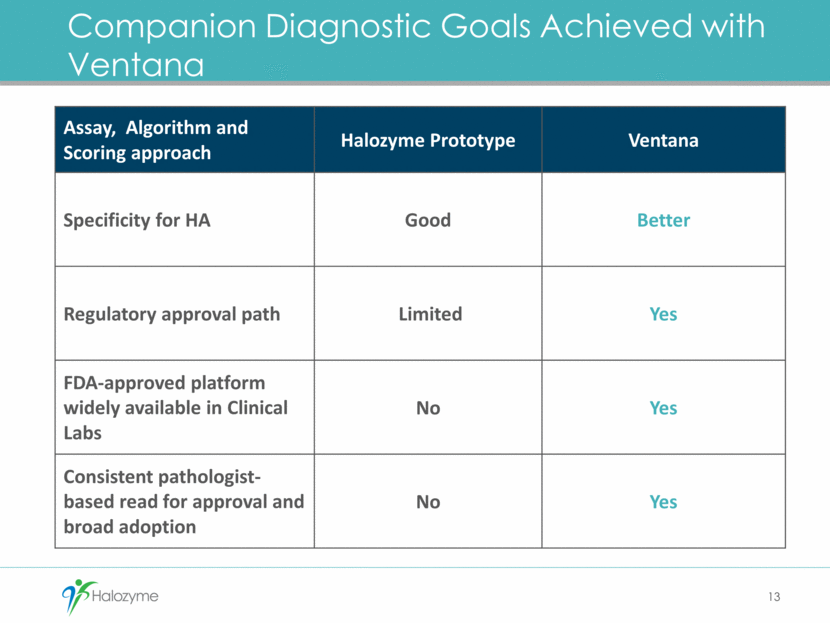
Ventana PEGPH20 Companion Diagnostic 14 Approach Potential Benefits Detection Rabbit Fc version HTI-601 Specific for HA Adapted for Ventana platform Scoring Pathologist Read 35-40% of HA-high population Regulatory U.S. Europe IDE submission February 2016 European Conformity Mark in planning Commercial Ventana Globally approved and available platform
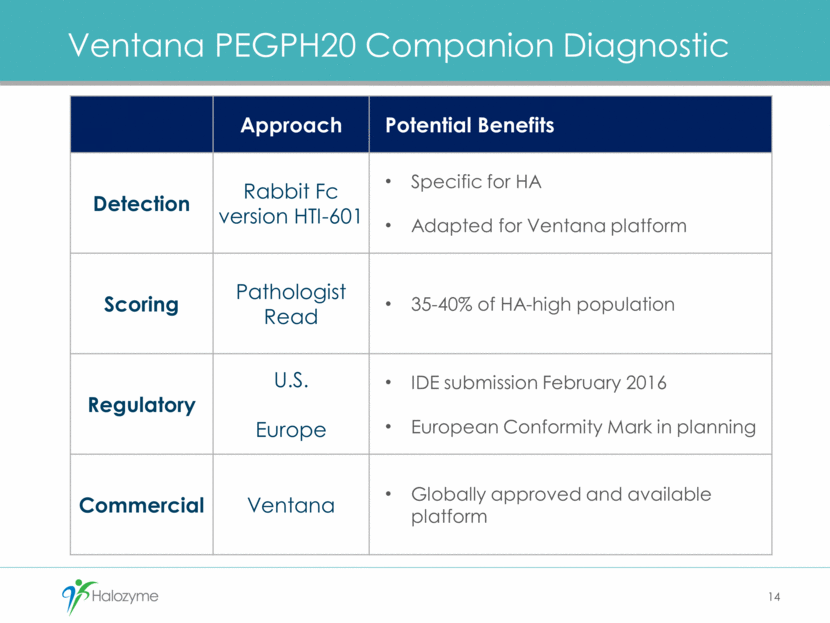
PFS Benefit and Hazard Ratio on Ventana Platform Support Design of Phase 3 Study 15 15 AG 6.3 months PAG 9.2 months Halozyme Prototype CDx (Stage 1 202: presented ASCO 2015) Ventana CDx (Stage1 202) HR 0.48 (0.16, 1.48) HR 0.39 (0.15, 1.01) AG 4.3 months PAG 9.2 months 0 2 4 6 8 10 12 14 0 2 4 6 8 10 12 14 Risk 23 14 10 6 5 2 1 0 21 14 7 4 0 0 0 0 PAG AG At 22 12 10 7 5 2 0 0 21 14 8 5 1 0 0 0 PAG AG
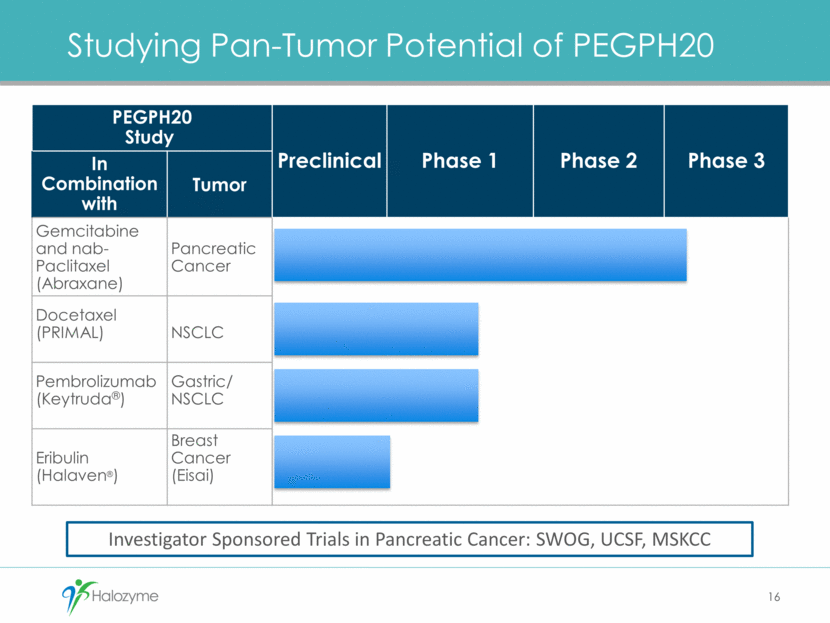
Studying Pan-Tumor Potential of PEGPH20 16 PEGPH20 Study Preclinical Phase 1 Phase 2 Phase 3 In Combination with Tumor Gemcitabine and nab-Paclitaxel (Abraxane) Pancreatic Cancer Docetaxel (PRIMAL) NSCLC Pembrolizumab (Keytruda®) Gastric/ NSCLC Eribulin (Halaven®) Breast Cancer (Eisai) Investigator Sponsored Trials in Pancreatic Cancer: SWOG, UCSF, MSKCC
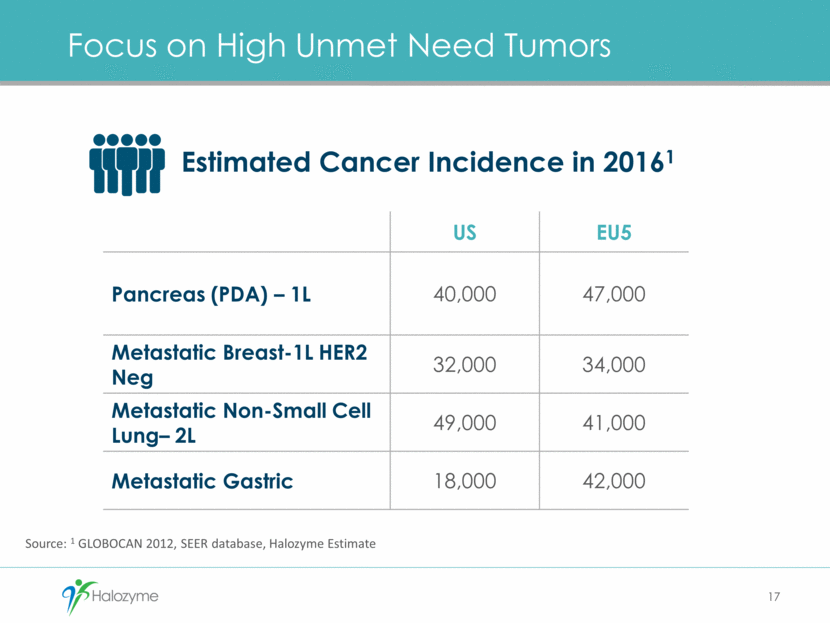
Focus on High Unmet Need Tumors 17 US EU5 Pancreas (PDA) – 1L 40,000 47,000 Metastatic Breast-1L HER2 Neg 32,000 34,000 Metastatic Non-Small Cell Lung– 2L 49,000 41,000 Metastatic Gastric 18,000 42,000 Source: 1 GLOBOCAN 2012, SEER database, Halozyme Estimate Estimated Cancer Incidence in 20161
ENHANZE™ Technology 18

ENHANZE: Our Novel Delivery Platform Potential Benefits: Facilitate transition of IV therapies to subcutaneous administration Reduce multiple injections Enable large volume of biologics to be delivered subcutaneously Reduce time required for drug administration Life cycle management, including potential patent life extension 19
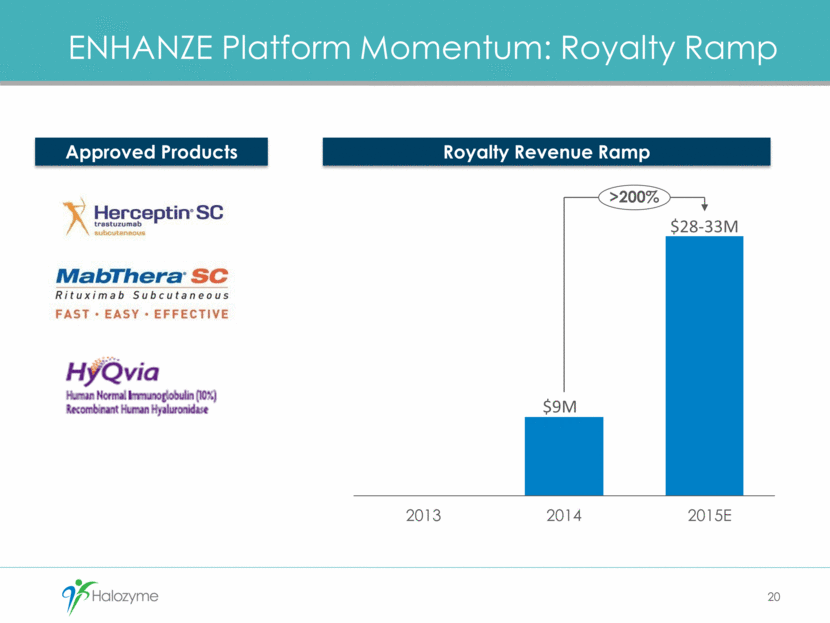
ENHANZE Platform Momentum: Royalty Ramp Royalty Revenue Ramp Approved Products 2013 2014 2015E >200% $9M $28-33M 20
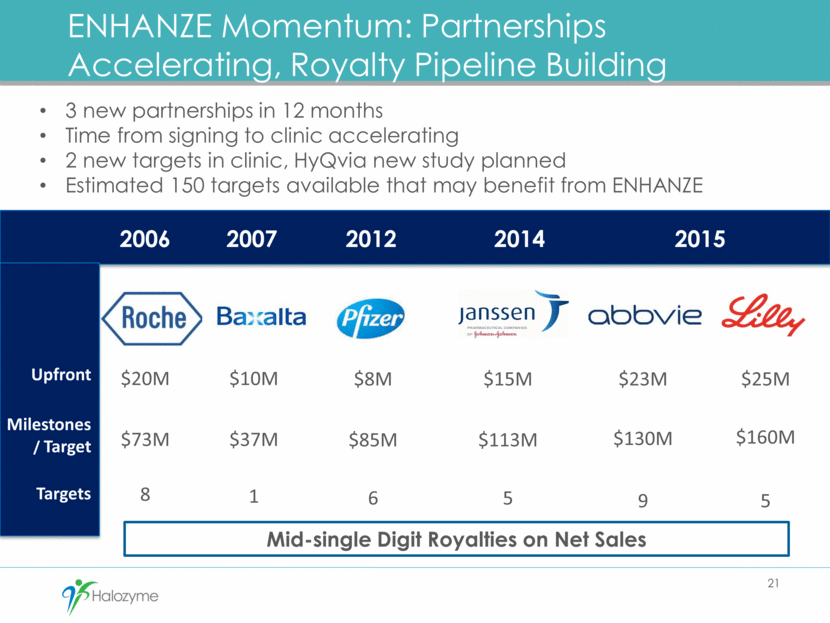
ENHANZE Momentum: Partnerships Accelerating, Royalty Pipeline Building 21 $25M $160M 5 $23M $130M 9 $15M $113M 5 $8M $85M 6 $10M $37M 1 $20M $73M 8 Upfront Milestones/ Target Targets 3 new partnerships in 12 months Time from signing to clinic accelerating 2 new targets in clinic, HyQvia new study planned Estimated 150 targets available that may benefit from ENHANZE 2015 2007 2012 2014 2006 Mid-single Digit Royalties on Net Sales
Financial Update 22

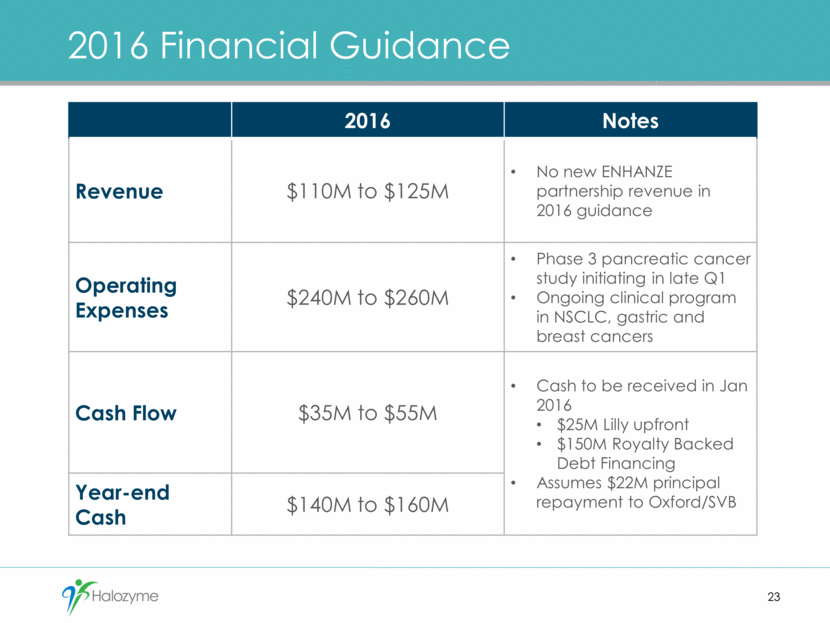
2016 Financial Guidance 23 2016 Notes Revenue $110M to $125M No new ENHANZE partnership revenue in 2016 guidance Operating Expenses $240M to $260M Phase 3 pancreatic cancer study initiating in late Q1 Ongoing clinical program in NSCLC, gastric and breast cancers Cash Flow $35M to $55M Cash to be received in Jan 2016 $25M Lilly upfront $150M Royalty Backed Debt Financing Assumes $22M principal repayment to Oxford/SVB Year-end Cash $140M to $160M
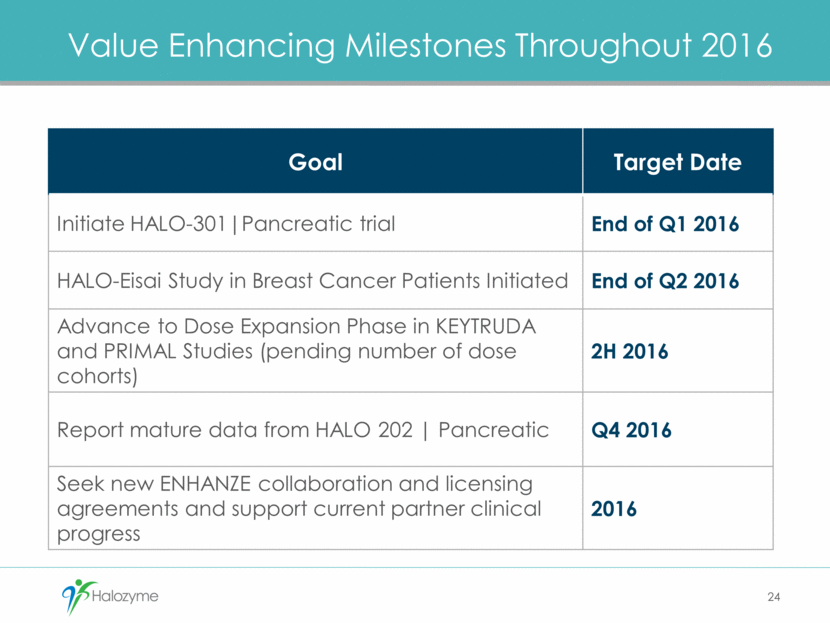
Value Enhancing Milestones Throughout 2016 24 Goal Target Date Initiate HALO-301 Pancreatic trial End of Q1 2016 HALO-Eisai Study in Breast Cancer Patients Initiated End of Q2 2016 Advance to Dose Expansion Phase in KEYTRUDA and PRIMAL Studies (pending number of dose cohorts) 2H 2016 Report mature data from HALO 202 Pancreatic Q4 2016 Seek new ENHANZE collaboration and licensing agreements and support current partner clinical progress 2016
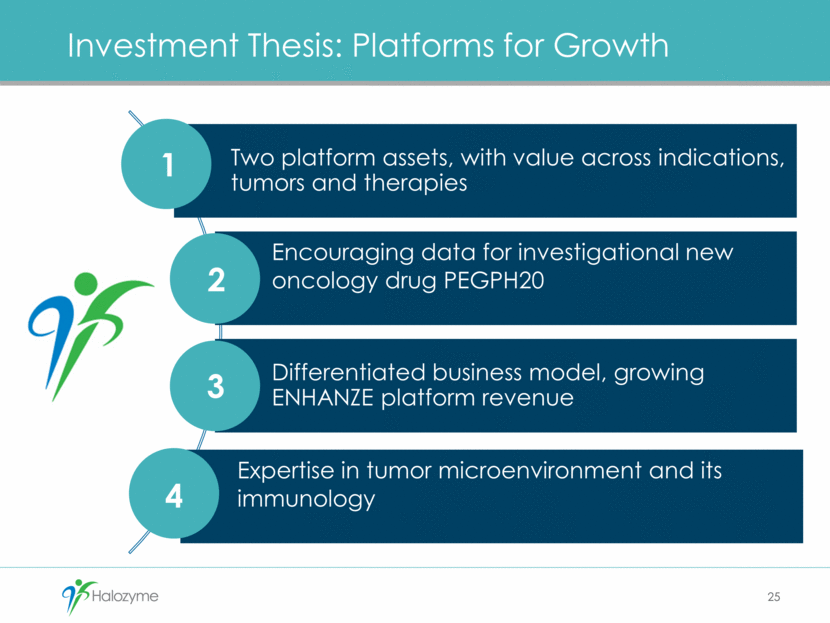
Investment Thesis: Platforms for Growth 25 1 2 3 4 Two platform assets, with value across indications, tumors and therapies Encouraging data for investigational new oncology drug PEGPH20 Differentiated business model, growing ENHANZE platform revenue Expertise in tumor microenvironment and its immunology
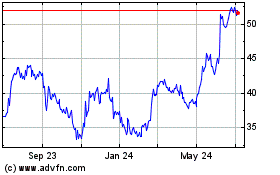
Halozyme Therapeutics (NASDAQ:HALO)
Historical Stock Chart
From Mar 2024 to Apr 2024
Halozyme Therapeutics (NASDAQ:HALO)
Historical Stock Chart
From Apr 2023 to Apr 2024