As filed with the Securities and Exchange Commission on January 22, 2016
Registration No. 333-208521
UNITED STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
Form S-4
REGISTRATION STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
CELLADON CORPORATION
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
2834 |
|
33-0971591 |
| (State or other jurisdiction of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
12707 High Bluff Drive, Suite 200
San Diego, CA 92130
(858)
350-4355
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Fredrik Wiklund
President
and Chief Executive Officer
Celladon Corporation
12707 High Bluff Drive, Suite 200
San Diego, CA 92130
(858)
350-4355
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
|
|
|
|
| Mike Hird
Patty M. DeGaetano
Pillsbury Winthrop Shaw
Pittman LLP 11255 El Camino Real, Suite 200
San Diego, CA 92130 (616)
234-5000 |
|
David A. Cory
President and Chief Executive Officer
Eiger BioPharmaceuticals, Inc.
350 Cambridge Ave., Suite 350
Palo Alto, CA 94306 (650)
272-6138 |
|
Glen Y. Sato
Michael E. Tenta Cooley
LLP 3175 Hanover Street
Palo Alto, CA (650)
328-4600 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement and the
satisfaction or waiver of all other conditions under the Merger Agreement described herein.
If the securities being registered on this Form are being
offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box: ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act
registration statement number of the earlier effective registration statement for the same offering. ¨
|
|
|
|
|
|
|
| Large accelerated filer |
|
¨ |
|
Accelerated filer |
|
¨ |
| Non-accelerated filer |
|
x (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
¨ |
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a
smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
Exchange Act Rule 13e-4(i) (Cross-Border Issuer Tender Offer) ¨
Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer) ¨
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall
file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become
effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this proxy statement/prospectus/information statement is not complete
and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This proxy statement/prospectus/information statement is not an offer to sell and it is not
soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JANUARY 22, 2016
PROPOSED MERGER
YOUR VOTE IS VERY IMPORTANT
To the Stockholders of Celladon
Corporation and Eiger BioPharmaceuticals, Inc.:
Celladon Corporation, or Celladon, and Eiger BioPharmaceuticals, Inc., or Eiger, have entered into an
Agreement and Plan of Merger and Reorganization, or the Merger Agreement, pursuant to which a wholly owned subsidiary of Celladon will merge with and into Eiger, with Eiger surviving as a wholly owned subsidiary of Celladon, or the merger. Eiger and
Celladon believe that the merger will result in a pharmaceutical company focused on the development and commercialization of proprietary, therapeutic product candidates in the fields of hepatitis delta virus, hyperinsulinemic hypoglycemia, pulmonary
arterial hypertension and lymphedema.
Immediately prior to the effective time of the merger, each share of Eiger preferred stock will be converted into
shares of Eiger common stock at a ratio determined in accordance with the Eiger certificate of incorporation then in effect and each outstanding warrant to purchase Eiger equity securities will be automatically exercised. At the effective time of
the merger, each share of Eiger common stock will be converted into the right to receive approximately 1.32 pre-split shares of Celladon common stock, subject to adjustment to account for the effect of a reverse stock split of Celladon common stock,
at a ratio of one new share for every fifteen shares outstanding, to be implemented prior to the consummation of the merger as discussed in this proxy statement/prospectus/information statement. The post-split exchange ratio is approximately 0.09.
Celladon will assume outstanding and unexercised options to purchase Eiger common stock, and they will be converted into options to purchase Celladon common stock. Celladon stockholders will continue to own and hold their existing shares of Celladon
common stock. Immediately after the merger, Eiger stockholders, warrantholders and optionholders will own approximately 78% of the fully-diluted common stock of Celladon, with Celladon optionholders and stockholders, whose shares of Celladon stock
will remain outstanding after the merger, holding approximately 22% of the fully-diluted common stock of Celladon. The exchange ratio is determined pursuant to a formula described in more detail in the Merger Agreement and in the attached proxy
statement/prospectus/information statement, and the 1.32 pre-split figure, 0.09 post-split figure and percentage ownership figures are estimates.
Shares of Celladon common stock are currently listed on The NASDAQ Global Market under the symbol “CLDN.” Prior to consummation of the merger,
Celladon intends to file an initial listing application for the combined company with The NASDAQ Global Market pursuant to NASDAQ “reverse merger” rules. After completion of the merger, Celladon will be renamed “Eiger
BioPharmaceuticals, Inc.” and expects to trade on The NASDAQ Global Market under the symbol “EIGR.” On , 2016, the
last trading day before the date of this proxy statement/prospectus/information statement, the closing sale price of Celladon common stock was $ per share.
Celladon is holding a special meeting of stockholders in order to obtain the stockholder approvals necessary to complete the merger and related matters. At the
Celladon special meeting, which will be held at , local time, on
, 2016 at 12255 El Camino Real, Suite 300, San Diego, California 92130, unless postponed or adjourned to a later date, Celladon
will ask its stockholders to, among other things, adopt the Merger Agreement thereby approving the merger and the issuance of Celladon common stock, and approve an amendment to the Celladon amended and restated certificate of incorporation effecting
a reverse stock split of Celladon common stock, at a ratio of 1-for-15, which is referred to herein as the 1-for-15 reverse stock split, and an amendment to the amended and restated certificate of incorporation changing the Celladon corporate name
to “Eiger BioPharmaceuticals, Inc.,” each as described in the accompanying proxy statement/prospectus/information statement.
As described in
the accompanying proxy statement/prospectus/information statement, certain Eiger stockholders who in the aggregate own approximately 88.7% of the outstanding shares of Eiger common stock on an as converted to common stock basis, and certain Celladon
stockholders who in the aggregate own 0.3% of the outstanding shares of Celladon common stock, are parties to support agreements with Celladon and Eiger, respectively, whereby such stockholders agreed to vote in favor of the adoption of the Merger
Agreement and the approval of the merger, and the merger and the issuance of Celladon common stock in the merger pursuant to the Merger Agreement, respectively, subject to the terms of the support agreements. In addition, following the registration
statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, being declared effective by the U.S. Securities and Exchange Commission and pursuant to the conditions of the Merger Agreement, the Eiger stockholders
who are party to the support agreements will each execute an action by written consent of the Eiger stockholders, referred to herein as the written consent, adopting the Merger Agreement, thereby approving the merger and related transactions.
Therefore, holders of a sufficient number of shares of Eiger capital stock required to adopt the Merger Agreement will adopt the Merger Agreement, and no meeting of Eiger stockholders to adopt the Merger Agreement and approve the merger and related
transactions will be held. Nevertheless, all Eiger stockholders will have the opportunity to elect to adopt the Merger Agreement, thereby approving the merger and related transactions, by signing and returning to Eiger a written consent.
After careful consideration, the Celladon and Eiger boards of directors have approved the Merger Agreement and the respective proposals referred to above, and
each of the Celladon and Eiger boards of directors has determined that it is advisable to enter into the merger. The board of directors of Celladon recommends that its stockholders vote “FOR” the proposals described in the accompanying
proxy statement/prospectus/information statement, and the board of directors of Eiger recommends that its stockholders sign and return the written consent indicating their approval of the merger and adoption of the Merger Agreement and related
transactions to Eiger.
More information about Celladon, Eiger and the proposed transaction is contained in this proxy statement/prospectus/information statement. Celladon and
Eiger urge you to read the accompanying proxy statement/prospectus/information statement carefully and in its entirety. IN PARTICULAR, YOU SHOULD CAREFULLY CONSIDER THE MATTERS DISCUSSED UNDER “RISK FACTORS”
BEGINNING ON PAGE 27.
Celladon and Eiger are excited about the opportunities the merger brings to both Celladon and Eiger stockholders, and thank
you for your consideration and continued support.
|
|
|
| Fredrik Wiklund |
|
David A. Cory |
| President and Chief Executive Officer |
|
President and Chief Executive Officer |
| Celladon Corporation |
|
Eiger BioPharmaceuticals, Inc. |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these
securities or passed upon the adequacy or accuracy of this proxy statement/prospectus/information statement. Any representation to the contrary is a criminal offense.
The accompanying proxy statement/prospectus/information statement is dated
, 2016, and is first being mailed to Celladon and Eiger stockholders on or about
, 2016.

CELLADON CORPORATION
12707 High Bluff Drive, Suite 200
San Diego, California 92130
(858) 350-4355
NOTICE
OF SPECIAL MEETING OF STOCKHOLDERS
To Be Held On
, 2016
Dear Stockholders of
Celladon:
On behalf of the board of directors of Celladon Corporation, a Delaware corporation, or Celladon, Celladon is pleased to deliver this proxy
statement/prospectus/information statement for the proposed merger between Celladon and Eiger BioPharmaceuticals, Inc., a Delaware corporation, or Eiger, pursuant to which Celladon Merger Sub, Inc., a wholly owned subsidiary of Celladon, will merge
with and into Eiger, with Eiger surviving as a wholly owned subsidiary of Celladon. The special meeting of stockholders of Celladon will be held on
, 2016 at , local time, at 12255 El Camino Real, Suite
300, San Diego, California 92130, for the following purposes:
1. To consider and vote upon a proposal to approve the merger and the issuance of Celladon
common stock pursuant to the Agreement and Plan of Merger and Reorganization, dated as of November 18, 2015, by and among Celladon, Celladon Merger Sub, Inc. and Eiger, a copy of which is attached as Annex A to the accompanying proxy
statement/prospectus/information statement;
2. To approve the amendment to the amended and restated certificate of incorporation of Celladon to effect a
reverse stock split of Celladon common stock, at a ratio of 1-for-15, in the form attached as Annex D to the accompanying proxy statement/prospectus/information statement;
3. To approve the amendment to the amended and restated certificate of incorporation of Celladon to change the name “Celladon Corporation” to
“Eiger BioPharmaceuticals, Inc.” in the form attached as Annex E to the accompanying proxy statement/prospectus/information statement;
4.
To consider and vote upon an adjournment of the Celladon special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Celladon Proposal Nos. 1, 2 and 3; and
5. To transact such other business as may properly come before the stockholders at the Celladon special meeting or any adjournment or postponement thereof.
The board of directors of Celladon has fixed
, 2016 as the record date for the determination of stockholders entitled to notice of, and to vote at, the Celladon special
meeting and any adjournment or postponement thereof. Only holders of record of shares of Celladon common stock at the close of business on the record date are entitled to notice of, and to vote at, the Celladon special meeting. At the close of
business on the record date, Celladon had shares of common stock outstanding and entitled to vote.
Your vote is important. The affirmative vote of the holders of a majority of the shares of Celladon common stock having voting power present in person or
represented by proxy at the Celladon special meeting, presuming a quorum is present, is required for approval of Celladon Proposal Nos. 1 and 4. The affirmative vote of the holders of a majority of shares of Celladon common stock having voting power
outstanding on the record date for the Celladon special meeting is required for approval of Celladon Proposal Nos. 2 and 3. Each of Proposal Nos. 1, 2 and 3 are conditioned upon each other. Therefore, the merger cannot be consummated without the
approval of Proposal Nos. 1, 2 and 3.
Even if you plan to attend the Celladon special meeting in person, Celladon requests that you sign and return
the enclosed proxy to ensure that your shares will be represented at the Celladon special meeting if you are unable to attend.
By Order of the
Celladon Board of Directors,
Fredrik Wiklund
President and
Chief Executive Officer
San Diego, California
, 2016
THE CELLADON BOARD OF DIRECTORS HAS DETERMINED AND BELIEVES THAT EACH OF THE PROPOSALS OUTLINED ABOVE IS ADVISABLE TO, AND IN THE BEST INTERESTS OF,
CELLADON AND ITS STOCKHOLDERS AND HAS APPROVED EACH SUCH PROPOSAL. THE CELLADON BOARD OF DIRECTORS RECOMMENDS THAT CELLADON STOCKHOLDERS VOTE “FOR” EACH SUCH PROPOSAL.
REFERENCES TO ADDITIONAL INFORMATION
This proxy statement/prospectus/information statement incorporates important business and financial information about Celladon that is not included in or
delivered with this document. You may obtain this information without charge through the Securities and Exchange Commission, or the SEC, website (www.sec.gov) or upon your written or oral request by contacting the Chief Financial Officer of Celladon
Corporation, 12707 High Bluff Drive, Suite 200, San Diego, California 92130 or by calling (858) 350-4355.
To ensure timely delivery of these
documents, any request should be made no later than , 2016 to receive them before the special meeting.
For additional details about where you can find information about Celladon, please see the section entitled “Where You Can Find More Information” in
this proxy statement/prospectus/information statement.
TABLE OF CONTENTS
i
ii
iii
QUESTIONS AND ANSWERS ABOUT THE MERGER
Except where specifically noted, the following information and all other information contained in this proxy statement/prospectus/information statement
does not give effect to the proposed 1-for-15 reverse stock split described in Celladon Proposal No. 2, beginning on page 142 in this proxy statement/prospectus/information statement.
The following section provides answers to frequently asked questions about the merger. This section, however, provides only summary information. For a more
complete response to these questions and for additional information, please refer to the cross-referenced sections.
| A: |
Celladon Corporation, or Celladon, and Eiger BioPharmaceuticals, Inc., or Eiger, have entered into an Agreement and Plan of Merger and Reorganization, dated as of November 18, 2015, or the Merger Agreement.
The Merger Agreement contains the terms and conditions of the proposed business combination of Celladon and Eiger. Under the Merger Agreement, Celladon Merger Sub, Inc., a wholly owned subsidiary of Celladon, or the Merger Sub, will merge with and
into Eiger, with Eiger surviving as a wholly owned subsidiary of Celladon. This transaction is referred to as “the merger” or “the Merger.” |
At the effective time of the merger, each share of Eiger common stock outstanding immediately prior to the effective time of the merger
(excluding certain shares to be canceled pursuant to the Merger Agreement, and shares held by stockholders who have exercised and perfected appraisal rights or dissenters’ rights as more fully described in “The Merger—Appraisal Rights
and Dissenters’ Rights” below) will be converted into the right to receive approximately 1.32 pre-split shares of Celladon common stock, subject to adjustment to account for a reverse stock split of Celladon common stock, at a ratio of one
new share for every fifteen outstanding shares, to be implemented prior to the consummation of the merger. The post-split exchange ratio is approximately 0.09. As a result of the merger, holders of Eiger stock, options and warrants are expected to
own in the aggregate approximately 78% of Celladon and the Celladon stockholders and optionholders are expected to own in the aggregate approximately 22% of Celladon. The exchange ratio is determined pursuant to a formula described in more detail in
the Merger Agreement and in this proxy statement/prospectus/information statement, and the 1.32 pre-split figure, 0.09 post-split figure and percentage ownership figures are estimates. After the completion of the merger, Celladon will change its
corporate name to “Eiger BioPharmaceuticals, Inc.” as required by the Merger Agreement.
| Q: |
What will happen to Celladon if, for any reason, the merger does not close? |
| A: |
If, for any reason, the merger does not close, the Celladon board of directors may elect to, among other things, attempt to complete another strategic transaction like the merger, attempt to sell or otherwise
dispose of the various assets of Celladon or continue to operate the business of Celladon. If Celladon decides to dissolve and liquidate its assets, Celladon would be required to pay all of its debts and contractual obligations, and to set aside
certain reserves for potential future claims, and there can be no assurances as to the amount or timing of available cash left to distribute to stockholders after paying the debts and other obligations of Celladon and setting aside funds for
reserves. |
If Celladon were to continue its business, it would need to identify, acquire and develop other products or
product candidates as it has no current plans to, and does not believe it is in the best interest of Celladon to, pursue development of its current product candidates. In addition, as of December 31, 2015, the Celladon workforce consisted of
three employees, all of whom are executive staff in support of the proposed merger. Celladon no longer has employees engaged in development and commercialization activities. If Celladon decides to reestablish its business, Celladon will need to
rebuild its senior management team and to hire managerial and other personnel to lead and staff all of its necessary functions, especially its research, development and commercialization areas.
1
| Q: |
Why are the two companies proposing to merge? |
| A: |
Following the merger, Celladon and Eiger believe that the combined organization will create a clinical-stage company with a diversified development portfolio of three well-characterized compounds addressing novel
targets for four distinct orphan diseases: hepatitis delta virus, or HDV, bariatric surgery-induced hyperinsulinemic hypoglycemia, pulmonary arterial hypertension, or PAH, and lymphedema. The lead Eiger product candidate, Sarasar® (lonafarnib), is a well-characterized, orally active compound. To date, over 50 HDV infected patients have been dosed with lonafarnib across international Phase 2 clinical trials. Lonafarnib
has demonstrated dose-related activity in reducing HDV viral load both as a monotherapy and in combination with other agents. HDV is considered to be the most severe form of viral hepatitis. Celladon and Eiger believe that the combined organization
will have the following potential advantages: (i) a diversified, clinical-stage product development pipeline; (ii) appropriate resources; (ii) a distinctive approach to developing novel products for the treatment of orphan diseases;
and (iv) an experienced management team. For a discussion of Celladon and Eiger reasons for the merger, please see the section entitled “The Merger—Celladon Reasons for the Merger” and “The Merger—Eiger Reasons for the
Merger” in this proxy statement/prospectus/information statement. |
| Q: |
Why am I receiving this proxy statement/prospectus/information statement? |
| A: |
You are receiving this proxy statement/prospectus/information statement because you have been identified as a stockholder of Celladon or Eiger as of the applicable record date, and you are entitled, as
applicable, to vote at the Celladon stockholder meeting to approve among other things the merger and the issuance of shares of Celladon common stock pursuant to the Merger Agreement, or sign and return the Eiger written consent to adopt the Merger
Agreement and approve the merger. This document serves as: |
| |
• |
|
a proxy statement of Celladon used to solicit proxies for its special meeting of stockholders; |
| |
• |
|
a prospectus of Celladon used to offer shares of Celladon common stock in exchange for shares of Eiger common stock in the merger and issuable upon exercise of Eiger options; and |
| |
• |
|
an information statement of Eiger used to solicit the written consent of its stockholders for the adoption of the Merger Agreement and the approval of the merger and related transactions. |
| Q: |
What is required to consummate the merger? |
| A: |
To consummate the merger, Celladon stockholders must approve the issuance of Celladon common stock pursuant to the Merger Agreement. In addition, the Merger Agreement anticipates approval of an amendment to the
amended and restated certificate of incorporation of Celladon effecting the 1-for-15 reverse stock split, and an amendment to the amended and restated certificate of incorporation to change Celladon’s name to “Eiger BioPharmaceuticals,
Inc.” Moreover, Eiger stockholders must approve the merger. |
The approval of the merger and the issuance of
Celladon common stock pursuant to the Merger Agreement by the stockholders of Celladon requires the affirmative vote of the holders of a majority of the shares of Celladon common stock having voting power present in person or represented by proxy at
the Celladon special meeting for the issuance of shares of Celladon common stock in the merger, presuming a quorum is present at the meeting. The approval of the 1-for-15 reverse stock split and the change of Celladon’s name require the
affirmative vote of the holders of a majority of shares of Celladon common stock having voting power outstanding on the record date for the Celladon special meeting. The approval of the 1-for-15 reverse stock split is required in order to authorize
Celladon to implement the reverse stock split and ensure that the post-merger trading price of Celladon’s common stock continues to meet the minimum bid price required by the listing requirements of The NASDAQ Global Market. Therefore, if the
requisite stockholders of Celladon approve the merger and the issuance of Celladon common stock pursuant to the Merger Agreement but do not approve the 1-for-15 reverse stock split, it is possible that the merger may not be consummated.
2
The adoption of the Merger Agreement and the approval of the merger and related transactions by
the stockholders of Eiger require the affirmative votes of the holders of (i) a majority of the outstanding Eiger common stock and preferred stock, voting together as one class and (ii) 60% of the shares of Eiger preferred stock. In
addition to the requirement of obtaining such stockholder approvals and appropriate regulatory approvals, each of the other closing conditions set forth in the Merger Agreement must be satisfied or waived.
Certain Eiger stockholders who in the aggregate own approximately 88.7% of the outstanding shares of Eiger common stock on an as converted to
common stock basis, and certain Celladon stockholders who in the aggregate own 0.3% of the outstanding shares of Celladon common stock, are parties to support agreements with Celladon and Eiger, respectively, whereby such stockholders agreed to vote
in favor of the adoption of the Merger Agreement and the issuance of Celladon common stock in the merger pursuant to the Merger Agreement, respectively, subject to the terms of the support agreements. In addition, following the registration
statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, being declared effective by the U.S. Securities and Exchange Commission and pursuant to the conditions of the Merger Agreement, Eiger stockholders who
are party to the support agreements will each execute written consents approving the merger and related transactions. Therefore, holders of a sufficient number of shares of Eiger capital stock required to adopt the Merger Agreement, thereby
approving the merger, have agreed to adopt the Merger Agreement via written consent. Stockholders of Eiger, including those who are parties to support agreements, are being requested to execute written consents providing such approvals.
For a more complete description of the closing conditions under the Merger Agreement, you are urged to read the section entitled “The
Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement.
| Q: |
What will Eiger stockholders, warrantholders and optionholders receive in the merger? |
| A: |
As a result of the merger, Eiger stockholders, warrantholders and optionholders will become entitled to receive shares of Celladon common stock equal to approximately 78% of the outstanding common stock of
Celladon. Eiger outstanding warrants to purchase shares of Eiger equity securities not terminated or exercised at or prior to the effective time of the merger will be automatically exercised immediately prior to the consummation of the merger.
Following the closing of the merger, Eiger optionholders will have their Eiger options converted into options to purchase Celladon common stock, with the number of shares and exercise price being appropriately adjusted to reflect the exchange ratio
between Celladon common stock and Eiger common stock determined in accordance with the Merger Agreement. |
For a more
complete description of what Eiger stockholders, warrantholders and optionholders will receive in the merger, please see the sections entitled “Market Price and Dividend Information” and “The Merger Agreement—Merger
Consideration” in this proxy statement/prospectus/information statement.
| Q: |
Who will be the directors of Celladon following the merger? |
| A: |
Immediately following the merger, the board of directors of Celladon is expected to be composed of seven directors to be designated solely by Eiger, five of whom are identified in the table below. Prior to the
effectiveness of the merger, Eiger intends to designate two additional directors who will be appointed effective as of the effective date of the merger. |
|
|
|
| Name |
|
Current Principal Affiliation |
| David A. Cory, RPH, MBA |
|
President and Chief Executive Officer, Eiger |
| Edgar G. Engleman, MD |
|
General Partner, Vivo Capital |
| Nina Kjellson |
|
General Partner, Canaan Partners |
| Thomas J. Dietz, PhD |
|
Chairman and Chief Executive Officer of Waypoint Holdings, LLC |
| Jeffrey S. Glenn, MD, PhD |
|
Associate Professor of Medicine, Gastroenterology & Hepatology, Stanford University |
3
| Q: |
Who will be the executive officers of Celladon immediately following the merger? |
| A: |
Immediately following the merger, the executive management team of Celladon is expected to be composed solely of the members of the Eiger executive management team prior to the merger as set forth below:
|
|
|
|
| Name |
|
Title |
| David A. Cory, RPH, MBA |
|
President and Chief Executive Officer |
| James H. Welch |
|
Chief Financial Officer |
| Joanne Quan, MD |
|
Chief Medical Officer |
| Eduardo Martins, MD, DPhil |
|
Senior Vice President, Liver and Infectious Diseases |
| James P. Shaffer, MBA |
|
Chief Business Officer |
| Q: |
What are the potential U.S. federal income tax consequences of the merger to Eiger stockholders? |
| A: |
Each of Celladon and Eiger intends the merger to qualify as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”). Assuming the merger
qualifies as a reorganization under the Code, then, in general, the material tax consequences to U.S. Holders (as defined herein) of Eiger common stock would be expected to be as follows: |
| |
• |
|
Each Eiger stockholder should not generally recognize gain or loss upon the exchange of Eiger common stock for Celladon common stock pursuant to the merger, except to the extent of cash received in lieu of a fractional
share of Celladon common stock as described below; and |
| |
• |
|
Each Eiger stockholder should recognize gain or loss to the extent any cash received in lieu of a fractional share of Celladon common stock exceeds or is less than the basis of such fractional share. |
Tax matters are very complicated, and the tax consequences of the merger to a particular Eiger stockholder will depend on such
stockholder’s circumstances. Accordingly, you should consult your tax advisor for a full understanding of the tax consequences of the merger to you, including the applicability and effect of federal, state, local and foreign income and other
tax laws. For more information, please see the section entitled “The Merger—Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger” beginning on page 112.
| Q: |
As a Celladon stockholder, how does the Celladon board of directors recommend that I vote? |
| A: |
After careful consideration, the Celladon board of directors recommends that Celladon stockholders vote: |
| |
• |
|
“FOR” Proposal No. 1 to approve the merger and the issuance of shares of common stock of Celladon in the merger; |
| |
• |
|
“FOR” Proposal No. 2 to approve the amendment to the amended and restated certificate of incorporation of Celladon to effect a reverse stock split of Celladon common stock, at a ratio of 1-for-15;
|
| |
• |
|
“FOR” Proposal No. 3 to approve the amendment to the amended and restated certificate of incorporation of Celladon to change the name of “Celladon Corporation” to “Eiger BioPharmaceuticals,
Inc.”; |
| |
• |
|
“FOR” Proposal No. 4 to adjourn the special meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1, 2 and 3.
|
| Q: |
As an Eiger stockholder, how does the Eiger board of directors recommend that I vote? |
| A: |
After careful consideration, the Eiger board of directors recommends that Eiger stockholders execute the written consent indicating their vote in favor of the adoption of the Merger Agreement and the approval of
the merger and the transactions contemplated thereby. |
4
| Q: |
What risks should I consider in deciding whether to vote in favor of the merger or to execute and return the written consent, as applicable? |
| A: |
You should carefully review the section of this proxy statement/prospectus/information statement entitled “Risk Factors,” which sets forth certain risks and uncertainties related to the merger, risks
and uncertainties to which the combined organization’s business will be subject, and risks and uncertainties to which each of Celladon and Eiger, as an independent company, is subject. |
| Q: |
When do you expect the merger to be consummated? |
| A: |
The merger is anticipated to occur sometime soon after the Celladon special meeting to be held on 2016, but the exact timing cannot be predicted. For more
information, please see the section entitled “The Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement. |
| Q: |
What do I need to do now? |
| A: |
Celladon and Eiger urge you to read this proxy statement/prospectus/information statement carefully, including its annexes, and to consider how the merger affects you. |
If you are a stockholder of Celladon, you may provide your proxy instructions in one of two different ways. First, you can mail your signed
proxy card in the enclosed return envelope. Second, you may also provide your proxy instructions via the Internet by following the instructions on your proxy card or voting instruction form. Please provide your proxy instructions only once, unless
you are revoking a previously delivered proxy instruction, and as soon as possible so that your shares can be voted at the special meeting of Celladon stockholders.
If you are a stockholder of Eiger, you may execute and return your written consent to Eiger in accordance with the instructions provided.
| Q: |
What happens if I do not return a proxy card or otherwise provide proxy instructions, as applicable? |
| A: |
If you are a Celladon stockholder, the failure to return your proxy card or otherwise provide proxy instructions will reduce the aggregate number of votes required to approve Celladon Proposals Nos. 1 and 4 and
will have the same effect as voting against Celladon Proposal Nos. 2 and 3, and your shares will not be counted for purposes of determining whether a quorum is present at the Celladon special meeting. |
| Q: |
May I vote in person at the special meeting of stockholders of Celladon? |
| A: |
If your shares of Celladon common stock are registered directly in your name with the Celladon transfer agent, you are considered to be the stockholder of record with respect to those shares, and the proxy
materials and proxy card are being sent directly to you by Celladon. If you are a Celladon stockholder of record, you may attend the special meeting of Celladon stockholders and vote your shares in person. Even if you plan to attend the Celladon
special meeting in person, Celladon requests that you sign and return the enclosed proxy to ensure that your shares will be represented at the Celladon special meeting if you are unable to attend. If your shares of Celladon common stock are held in
a brokerage account or by another nominee, you are considered the beneficial owner of shares held in “street name,” and the proxy materials are being forwarded to you by your broker or other nominee together with a voting instruction card.
As the beneficial owner, you are also invited to attend the special meeting of Celladon stockholders. Because a beneficial owner is not the stockholder of record, you may not vote these shares in person at the Celladon special meeting unless you
obtain a proxy from the broker, trustee or nominee that holds your shares, giving you the right to vote the shares at the meeting. |
5
| Q: |
When and where is the special meeting of Celladon stockholders being held? |
| A: |
The special meeting of Celladon stockholders will be held at 12255 El Camino Real, Suite 300, San Diego, California 92130, at local time, on
2016. Subject to space availability, all Celladon stockholders as of the record date, or their duly appointed proxies, may
attend the meeting. Since seating is limited, admission to the meeting will be on a first-come, first-served basis. |
| Q: |
If my Celladon shares are held in “street name” by my broker, will my broker vote my shares for me? |
| A: |
Unless your broker has discretionary authority to vote on certain matters, your broker will not be able to vote your shares of Celladon common stock on matters requiring discretionary authority without
instructions from you. Brokers are not expected to have discretionary authority to vote for Celladon Proposal No. 1. To make sure that your vote is counted, you should instruct your broker to vote your shares, following the procedures provided by
your broker. |
| Q: |
May I change my vote after I have submitted a proxy or provided proxy instructions? |
| A: |
Celladon stockholders of record, other than those Celladon stockholders who are parties to support agreements, may change their vote at any time before their proxy is voted at the Celladon special meeting in one
of three ways. First, a stockholder of record of Celladon can send a written notice to the Secretary of Celladon stating that it would like to revoke its proxy. Second, a stockholder of record of Celladon can submit new proxy instructions either on
a new proxy card or via the Internet. Third, a stockholder of record of Celladon can attend the Celladon special meeting and vote in person. Attendance alone will not revoke a proxy. If a Celladon stockholder of record or a stockholder who owns
Celladon shares in “street name” has instructed a broker to vote its shares of Celladon common stock, the stockholder must follow directions received from its broker to change those instructions. |
| Q: |
Who is paying for this proxy solicitation? |
| A: |
Celladon and Eiger will share equally the cost of printing and filing of this proxy statement/prospectus/information statement and the proxy card. Arrangements will also be made with brokerage firms and other
custodians, nominees and fiduciaries who are record holders of Celladon common stock for the forwarding of solicitation materials to the beneficial owners of Celladon common stock. Celladon will reimburse these brokers, custodians, nominees and
fiduciaries for the reasonable out-of-pocket expenses they incur in connection with the forwarding of solicitation materials. Celladon has retained Advantage Proxy to assist it in soliciting proxies using the means referred to above. Celladon will
pay the fees of Advantage Proxy, which Celladon expects to be approximately $10,000, plus reimbursement of out-of-pocket expenses. |
| Q: |
Who can help answer my questions? |
| A: |
If you are a Celladon stockholder and would like additional copies, without charge, of this proxy statement/prospectus/information statement or if you have questions about the merger, including the procedures for
voting your shares, you should contact Celladon’s proxy solicitor: |
ADVANTAGE PROXY
(877) 870-8565 (toll free)
(206) 870-8565 (collect)
If you are an Eiger stockholder and would like additional copies, without charge, of this proxy statement/prospectus/information statement or
if you have questions about the merger, including the procedures for voting your shares, you should contact:
Eiger BioPharmaceuticals,
Inc.
350 Cambridge Ave., Suite 350
Palo Alto, California 94306
Tel:
(650) 272-6138
Attn: David A. Cory, President and Chief Executive Officer
dcory@eiger.com
6
Prospectus Summary
This summary highlights selected information from this proxy statement/prospectus/information statement and may not contain all of the information that is
important to you. To better understand the merger, the proposals being considered at the Celladon special meeting and the Eiger stockholder actions that are the subject of the written consent, you should read this entire proxy
statement/prospectus/information statement carefully, including the Merger Agreement and the other annexes to which you are referred herein. For more information, please see the section entitled “Where You Can Find More Information” in
this proxy statement/prospectus/information statement.
The Companies
Celladon Corporation
12707 High Bluff Drive, Suite
200
San Diego, California 92130
(858) 350-4355
Celladon Corporation, or Celladon, is a biotechnology company that has been focused on the development of cardiovascular gene therapy. As a consequence of the
negative results from the Phase 2b clinical trial of its lead product candidate, MYDICAR® (AAV1/SERCA2a), referred to as the CUPID 2 trial, Celladon suspended further research and development
of MYDICAR and its pre-clinical programs. Celladon’s current development activities are limited to the oversight of the long-term follow up period in the CUPID 2 trial, which is expected to continue through February 2016.
Eiger BioPharmaceuticals, Inc.
350 Cambridge
Ave., Suite 350
Palo Alto, California 94306
(650) 272-6138
Eiger BioPharmaceuticals, Inc., or Eiger, is a clinical stage biopharmaceutical company focused on bringing to market novel product candidates for the
treatment of orphan diseases. Since its founding in 2008, Eiger has worked with investigators at Stanford University and has evaluated a number of potential development candidates from pharmaceutical companies to comprise a pipeline of novel product
candidates. Eiger’s resulting pipeline includes three Phase 2 candidates addressing four distinct orphan diseases. The programs have several aspects in common: the disease targets represent conditions of high medical need which are inadequately
treated by current standard of care; the therapeutic approaches are supported by an understanding of disease biology and mechanism as elucidated by Eiger’s academic research relationships; prior clinical experience with the product candidates
guides an understanding of safety; and the development paths leverage the experience and capabilities of Eiger’s experienced, commercially focused management team. The pipeline includes
Sarasar® (lonafarnib) for HDV, exendin (9-39) for severe hypoglycemia, and Bestatin™ (ubenimex) for PAH and lymphedema. Lonafarnib and ubenimex (for PAH) have been granted orphan drug
designation by the U.S. Food and Drug Administration, or the FDA, and European Medicines Agency, or the EMA. Eiger plans to deliver Phase 2 data on all four programs over the course of the next one to three years beginning in 2016.
Lonafarnib is Eiger’s most advanced program and to date, over 50 HDV infected patients have been dosed with lonafarnib across international Phase 2
clinical trials. The National Institutes of Health, or the NIH, conducted a 14-patient, double blind, placebo-controlled, proof of concept study, which was the first ever to evaluate lonafarnib in patients
infected with HDV. Doses of 100 mg and 200 mg of lonafarnib administered twice daily demonstrated a dose dependant decrease in viral loads of 0.73 and 1.54 log decline, respectively, in 28 days. The results of this study were
published in The Lancet Infectious Disease Journal (Koh, C. et al. “Oval prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomized, double-blind, placebo-controlled phase 2A trial,” Lancet
Infect Dis, 2015; 15:10).
7
Celladon Merger Sub, Inc.
12707 High Bluff Drive, Suite 200
San Diego, California 92130
(858) 350-4355
Celladon Merger Sub, Inc., or Merger Sub,
is a wholly owned subsidiary of Celladon and was formed solely for the purposes of carrying out the merger.
The
Merger (see page 80)
If the merger is completed, Merger Sub will merge with and into Eiger, with Eiger surviving as a wholly owned
subsidiary of Celladon.
Immediately after the merger, subject to adjustments to reflect certain events that could occur prior to closing of the merger,
Eiger stockholders, option holders and warrant holders will own approximately 78% of the fully-diluted common stock of post-merger Celladon, with Celladon stockholders, option holders and warrant holders holding approximately 22% of the
fully-diluted common stock of post-merger Celladon. Eiger outstanding warrants to purchase shares of Eiger equity securities not terminated or exercised at or prior to the effective time of the merger will be automatically exercised immediately
prior to the consummation of the merger. Celladon will assume outstanding and unexercised options to purchase Eiger common stock, and they will be converted into options to purchase Celladon common stock. The exchange ratio is determined pursuant to
a formula described in more detail in the Merger Agreement and in this proxy statement/prospectus/information statement, and the percentage ownership figures are estimates. The foregoing percentages assume that the exchange ratio is not adjusted, as
described in “The Merger—Merger Consideration and Adjustment” below.
For a more complete description of the merger exchange ratio, please
see the section entitled “The Merger Agreement” in this proxy statement/prospectus/information statement.
The closing of the merger will occur
no later than the second business day after the last of the conditions to the merger has been satisfied or waived, or at another time as Celladon and Eiger agree. Celladon and Eiger anticipate that the consummation of the merger will occur promptly
after the Celladon special meeting. However, because the merger is subject to a number of conditions, neither Celladon nor Eiger can predict exactly when the closing will occur or if it will occur at all. After completion of the merger, assuming
that Celladon receives the required stockholder approval of Celladon Proposal No. 3, Celladon will be renamed “Eiger BioPharmaceuticals, Inc.”
Reasons for the Merger (see pages 91 and 94)
Following the merger, the combined organization will be a clinical-stage company with a diversified development portfolio of product candidates addressing
novel targets for four distinct orphan diseases: HDV, bariatric surgery-induced hyperinsulinemic hypoglycemia, PAH and lymphedema. The lead Eiger product candidate, Sarasar® (lonafarnib), is a
well-characterized, orally active compound currently being investigated in a Phase 2 clinical trial for potential use in patients infected with HDV, which is considered to be the most severe form of viral hepatitis in humans. Celladon and Eiger
believe that the combined organization will have the following potential advantages:
| |
• |
|
Diversified, Clinical-Stage Product Development Pipeline. Eiger’s pipeline includes four development programs that comprise a
diverse, clinical-stage portfolio of product candidates with the potential to address orphan diseases for which the unmet medical need is high, the biology for treatment is identified, and an effective therapy is urgently needed. Since Eiger’s
founding in 2008, Eiger has worked with investigators and inventors at Stanford University, identified potentially promising |
8
| |
candidates from pharmaceutical companies, and in-licensed four programs which represent novel potential therapeutic approaches for four different orphan diseases:
|
| |
• |
|
Lonafarnib—Eiger is conducting multiple Phase 2 studies of Sarasar at U.S. and international sites, and data read-outs from results of these Phase 2 studies are expected in 2016.
|
| |
• |
|
Exendin (9-39)—Eiger is developing exendin (9-39), a glucagon-like peptide-1 or GLP-1 receptor antagonist as a treatment for hyperinsulinemic hypoglycemia associated with bariatric surgery. Eiger is
planning to initiate a Phase 2 dose-ranging trial in affected patients with exendin (9-39) in a novel subcutaneous formulation in the second quarter of 2016. |
| |
• |
|
Ubenimex in PAH—Eiger is developing ubenimex for PAH. Eiger has filed an Investigational New Drug application (IND) with the United States Food and Drug Administration (FDA) for ubenimex in PAH which
has been approved, and Eiger intends to begin enrollment in a Phase 2 clinical trial in the first quarter of 2016. |
| |
• |
|
Ubenimex in lymphedema—Eiger is also developing ubenimex for lymphedema. Eiger filed an IND for ubenimex in lymphedema with the FDA in December 2015, which was approved to proceed. Eiger plans to begin
enrollment in a Phase 2 clinical trial in the first half of 2016. |
| |
• |
|
Approach. Eiger’s approach to orphan diseases is distinct in two important ways: first, Eiger pursues opportunities in which disease biology has been elucidated by academic collaborators or other research
efforts; and second, these are opportunities in which Eiger’s team has identified a well characterized, clinical stage product candidate that can be tested in clinical proof-of-concept studies in Eiger’s targeted indication.
|
| |
• |
|
Management Team. It is expected that the combined organization will be led by experienced senior management from Eiger and a board of directors of seven members designated by Eiger. |
| |
• |
|
Resources. Eiger has commitments for $33.5 million to fund Eiger’s development pipeline from an investor syndicate that includes its existing, founding venture investors, Vivo Capital and InterWest
Partners, as well as new investors. This investment, in addition to $6.0 million of funding provided to Eiger prior to execution of the definitive merger agreement along with the existing Celladon cash, is expected to provide sufficient funding to
advance all four pipeline programs. At least two of the planned Phase 2 programs are expected to generate potentially meaningful clinical data during 2016, although further funding may be required to complete Phase 2 studies in the other two
programs. Each of Eiger’s Phase 2 programs has the potential, if successful, to create value for the stockholders of the merged company and present the combined organization with additional fundraising opportunities in the future.
|
Each of the board of directors of Celladon and Eiger also considered other reasons for the merger, as described herein. For example, the
board of directors of Celladon considered, among other things:
| |
• |
|
the consequences of the negative results from the Phase 2b CUPID 2 clinical trial of Celladon’s lead product candidate, MYDICAR (AAV1/SERCA2a), and the likelihood that the resulting circumstances for the company
would not change for the benefit of the Celladon stockholders in the foreseeable future on a stand-alone basis; |
| |
• |
|
the strategic alternatives of Celladon to the merger, including potential transactions that could have resulted from discussions that Celladon’s management conducted with other potential merger partners;
|
| |
• |
|
the risks associated with, and the uncertain value, time and costs to stockholders of, liquidating Celladon or effecting a sale of all or some of its assets and thereafter distributing the proceeds; |
| |
• |
|
the risks of continuing to operate Celladon on a stand-alone basis, including the need to rebuild the company’s product development programs, infrastructure and management to continue its operations;
|
9
| |
• |
|
the risks and costs associated with litigation, including defending against claims made in the three putative class action complaints filed in July 2015 following Celladon’s announcements regarding the negative
CUPID 2 clinical trial data and the suspension of further research and development activities, and the subsequent decline of the price of Celladon’s common stock; |
| |
• |
|
the opportunity as a result of the merger for Celladon stockholders to participate in the potential value that may result from development of the Eiger product candidate portfolio and the potential increase in value of
the combined organization following the merger; and |
| |
• |
|
the analyses of Wedbush Securities Inc., and its opinion to the board of directors of Celladon as to the fairness to Celladon, from a financial point of view and as of the date of such opinion, of the exchange ratio for
the conversion of Eiger capital stock into Celladon common stock. |
In addition, the board of directors of Eiger approved the merger based on
a number of factors, including the following:
| |
• |
|
the potential increased access to sources of capital and a broader range of investors to support the clinical development of its product candidate portfolio than it could otherwise obtain if it continued to operate as a
privately held company; |
| |
• |
|
the potential to provide its current stockholders with greater liquidity by owning stock in a public company; |
| |
• |
|
the board’s belief that no alternatives to the merger were reasonably likely to create greater value for Eiger’s stockholders after reviewing the various strategic options to enhance stockholder value that
were considered by Eiger’s board; |
| |
• |
|
the cash resources of the combined organization expected to be available at the closing of the merger; and |
| |
• |
|
the expectation that the merger should be treated as a reorganization for U.S. federal income tax purposes, with the result that the Eiger stockholders should generally not recognize taxable gain or loss for U.S.
federal income tax purposes. |
Opinion of the Celladon Financial Advisor (see page 95)
Celladon’s board of directors engaged Wedbush Securities Inc., or Wedbush, to provide financial advisory and investment banking services to consider and
evaluate potential strategic transactions, and ultimately requested that Wedbush render an opinion as to whether the exchange ratio in connection with the merger, as provided in the Merger Agreement, was fair to Celladon from a financial point of
view. At the November 16, 2015 meeting of Celladon’s board of directors, Wedbush rendered its oral opinion, subsequently confirmed by delivery of a written opinion dated November 16, 2015, to Celladon’s board of directors that,
as of the date of such opinion, and based upon the assumptions made, procedures followed, matters considered, and qualifications and limitations of the review set forth in its written opinion, the exchange ratio in connection with the merger, as
provided in the Merger Agreement, was fair to Celladon from a financial point of view.
The full text of Wedbush’s written opinion, which sets
forth the procedures followed, assumptions made, matters considered, and limitations and qualifications of the review undertaken in connection with the opinion, is attached as Annex B and is incorporated herein by reference.
Wedbush’s opinion was intended for the use and benefit of Celladon’s board of directors (in its capacity as such) in connection with its evaluation of the merger. Wedbush’s opinion does not address Celladon’s underlying business
decision to enter into the Merger Agreement or complete the merger or the relative merits of the merger compared to any alternative transactions or strategies that may be available to Celladon. Wedbush’s opinion did not
10
constitute a recommendation to Celladon’s board of directors as to how to act or to any Celladon stockholder or any other person as to how to vote with respect to the merger or any other
matter.
Overview of the Merger Agreement and Agreements Related to the Merger Agreement
Merger Consideration (see page 119)
Immediately prior to the closing of the financing contemplated by the Subscription Agreement, dated as of November 18, 2015, by and among Eiger, certain
current stockholders of Eiger and certain new investors in Eiger (the “Subscription Agreement”), each share of Eiger preferred stock outstanding at such time will be converted into shares of Eiger common stock at a ratio determined in
accordance with the Eiger certificate of incorporation then in effect. Immediately prior to the closing of the financing contemplated by the Subscription Agreement, the $6.0 million in aggregate principal amount outstanding under, and all interest
accrued on, the convertible promissory notes of Eiger will be converted into shares of Eiger common stock pursuant to the Subscription Agreement. Additionally, immediately prior to the closing of the effective time of the merger, each outstanding
warrant to purchase shares of Eiger’s common stock or preferred stock will be automatically exercised. At the effective time of the merger:
| |
• |
|
each share of Eiger common stock outstanding immediately prior to the effective time of the merger will automatically be converted into the right to receive a number of shares of Celladon common stock pursuant to an
exchange ratio of approximately 1.32 pre-split, herein referred to as the exchange ratio, (which is subject to adjustment to account for the proposed 1-for-15 reverse stock split, resulting in a post-split exchange ratio of approximately 0.09); and
|
| |
• |
|
each option to purchase shares of Eiger common stock outstanding and unexercised immediately prior to the effective time of the merger will be assumed by Celladon and will become an option to purchase shares of Celladon
common stock, with the number of shares and exercise price being adjusted by the exchange ratio (which is subject to adjustment to account for the proposed 1-for-15 reverse stock split). |
Immediately after the merger, based on the exchange ratio, Eiger stockholders, warrantholders and optionholders will own approximately 78% of the
fully-diluted common stock of Celladon with Celladon stockholders and optionholders holding approximately 22% of the fully-diluted common stock of Celladon. The exchange ratio is determined pursuant to a formula described in more detail in the
Merger Agreement and in this proxy statement/prospectus/information statement, and the 1.32 pre-split figure, 0.09 post-split figure and percentage ownership figures are estimates.
There will be no adjustment to the total number of shares of Celladon common stock that Eiger stockholders will be entitled to receive for changes in the
market price of Celladon common stock. Accordingly, the market value of the shares of Celladon common stock issued pursuant to the merger will depend on the market value of the shares of Celladon common stock at the time the merger closes, and could
vary significantly from the market value on the date of this proxy statement/prospectus/information statement.
Treatment of Celladon Stock
Options and Warrants (see page 121)
As of the effective time of the reverse stock split, Celladon will adjust and proportionately decrease
the number of shares of Celladon’s common stock reserved for issuance upon exercise of, and adjust and proportionately increase the exercise price of, all options and warrants to acquire Celladon’s common stock outstanding immediately
prior to the closing date at a fifteen (15) to one (1) ratio. All stock options and warrants to acquire shares of Celladon’s common stock that are outstanding immediately prior to the effective time of the merger will remain
outstanding following the effective time of the merger. In addition, as of the effective time of the reverse stock split, Celladon will adjust and proportionately decrease the total number of shares of Celladon’s common stock that may be the
subject of future grants under Celladon’s stock option plans at a fifteen (15) to one (1) ratio.
11
Treatment of Eiger Stock Options and Warrants (see page 121)
At the effective time of the merger, each option to purchase Eiger common stock that is outstanding and unexercised immediately prior to the effective time of
the merger under the Eiger 2009 Equity Incentive Plan, whether or not vested, will be converted into an option to purchase Celladon common stock. Celladon will assume the Eiger 2009 Equity Incentive Plan. All rights with respect to Eiger common
stock under Eiger options assumed by Celladon will be converted into rights with respect to Celladon common stock. Accordingly, from and after the effective time of the merger, each Eiger stock option assumed by Celladon may be exercised for such
number of shares of Celladon common stock as is determined by multiplying the number of shares of Eiger common stock subject to the option by the exchange ratio (which is subject to adjustments to account for the effect of the proposed 1-for-15
reverse stock split prior to the closing of the merger) and rounding that result down to the nearest whole number of shares of Celladon common stock. The per share exercise price of the converted option will be determined by dividing the existing
exercise price of the option by the exchange ratio (which is subject to adjustments to account for the effect of the proposed 1-for-15 reverse stock split prior to the closing of the merger) and rounding that result up to the nearest whole cent. Any
restrictions on the exercise of any Eiger option assumed by Celladon will continue following the conversion and the term, exercisability, vesting schedules and other provisions of assumed Eiger options will generally remain unchanged; provided, that
any Eiger options assumed by Celladon may be subject to adjustment to reflect changes in Celladon capitalization after the effective time of the merger and that the Celladon board of directors will succeed to the authority of the Eiger board of
directors with respect to each assumed Eiger option.
Eiger has issued warrants to purchase shares of its equity securities at an exercise price of $0.01
per share. Each outstanding warrant to purchase shares of Eiger equity securities not terminated or exercised at or prior to the effective time of the merger will be automatically exercised immediately prior to the consummation of the merger.
Conditions to the Completion of the Merger (see page 123)
To consummate the merger, Celladon stockholders must approve the merger and the issuance of shares of Celladon common stock in the merger. In addition, the
Merger Agreement anticipates approval of an amendment to the amended and restated certificate of incorporation of Celladon effecting the proposed 1-for-15 reverse stock split, and an amendment to the amended and restated certificate of incorporation
effecting a change of the Celladon name to “Eiger BioPharmaceuticals, Inc.” Moreover, the Eiger stockholders must adopt the Merger Agreement thereby approving the merger. In addition to obtaining such stockholder approvals and appropriate
regulatory approvals, each of the other closing conditions set forth in the Merger Agreement must be satisfied or waived.
No
Solicitation (see page 127)
Each of Celladon and Eiger agreed that, subject to limited exceptions, Celladon and Eiger and any of their
respective subsidiaries will not, nor will either party or any of its subsidiaries authorize or permit any of the officers, directors, employees, investment bankers, attorneys, accountants, representatives, consultants, or other agents retained by
it or any of its subsidiaries to, directly or indirectly:
| |
• |
|
solicit, initiate, encourage, induce or knowingly facilitate the communication, making, submission or announcement of, any “acquisition proposal,” as defined in the Merger Agreement, or inquiry, indication of
interest or request for information that could reasonably be expected to lead to an acquisition proposal or take any action that could reasonably be expected to lead to an acquisition proposal or an inquiry, indication of interest or request for
information that could reasonably be expected to lead to an acquisition proposal; |
12
| |
• |
|
furnish any information with respect to it to any person in connection with or in response to an acquisition proposal or inquiry, indication of interest or request for information that could reasonably be expected to
lead to an acquisition proposal; |
| |
• |
|
engage in discussions or negotiations with any person with respect to any acquisition proposal or inquiry, indication of interest or request for information that could reasonably be expected to lead to an acquisition
proposal; |
| |
• |
|
approve, endorse or recommend an acquisition proposal; |
| |
• |
|
execute or enter into any letter of intent or similar document or any contract contemplating or otherwise relating to an “acquisition transaction,” as defined in the Merger Agreement; or |
| |
• |
|
grant any waiver or release under any confidentiality, standstill or similar agreement, other than to either Celladon or Eiger. |
However, before obtaining the applicable Celladon or Eiger stockholder approvals required to consummate the merger, each party may furnish nonpublic
information regarding such party to, and may enter into discussions or negotiations with, any third party in response to a bona fide written acquisition proposal made or received after the date of the Merger Agreement, which such party’s board
of directors determines in good faith, after consultation with a nationally recognized independent financial advisor and its outside legal counsel, constitutes or is reasonably likely to result in a “superior offer,” as defined in the
Merger Agreement, if:
| |
• |
|
neither such party nor any representative of such party has breached the no solicitation provisions of the Merger Agreement described above; |
| |
• |
|
such party’s board of directors concludes in good faith, based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to result in a breach of the fiduciary duties of such
board of directors under applicable legal requirements; |
| |
• |
|
such party gives the other party at least five business days’ prior notice of the identity of the third party and of that party’s intention to furnish information to, or enter into discussions or negotiations
with, such third party before furnishing any information or entering into discussions or negotiations with such third party; |
| |
• |
|
such party receives from the third party an executed confidentiality agreement containing provisions at least as favorable to such party as those contained in the confidentiality agreement between Celladon and Eiger;
and |
| |
• |
|
at least five business days’ prior to the furnishing of any information to a third party, such party furnishes the same information to the other party to the extent not previously furnished. |
Termination of the Merger Agreement (see page 131)
Either Celladon or Eiger can terminate the Merger Agreement under certain circumstances, which would prevent the merger from being consummated.
Termination Fee (see page 134)
If the Merger
Agreement is terminated under certain circumstances, Celladon or Eiger will be required to pay the other party a termination fee of $3.0 million or, in some circumstances, reimburse the other party for expenses incurred in connection with the
merger, up to a maximum of $1.0 million.
13
Subscription Agreement (see page 137)
On November 18, 2015, prior to the execution of the Merger Agreement, Eiger entered into the Subscription Agreement with certain current stockholders of
Eiger and certain new investors in Eiger pursuant to which Eiger agreed to sell, and the purchasers listed therein agreed to purchase, shares of Eiger common stock for an aggregate purchase price of $39.5 million, including the conversion of the
$6.0 million in aggregate principal amount outstanding under, and all interest accrued on, the convertible promissory notes of Eiger. The merger is conditioned upon the closing of the financing contemplated by the Subscription Agreement.
The consummation of the financing contemplated by the Subscription Agreement is subject to certain conditions, including the satisfaction or waiver of each of
the conditions to the consummation of the merger set forth in the Merger Agreement and the parties to the Merger Agreement being ready, willing and able to consummate the merger immediately after the closing of the financing, the U.S. Securities and
Exchange Commission, or SEC, having declared effective the registration statement of which this proxy statement/prospectus/information statement is a part and no stop order suspending the effectiveness of the registration statement of which this
proxy statement/prospectus/information statement is a part having been issued and remain pending, and the adoption of the Merger Agreement and the approval of the merger by Eiger’s stockholders.
Celladon has certain termination and amendment consent rights under the Subscription Agreement and is also an express third-party beneficiary of the
Subscription Agreement, with the right to specifically enforce its terms, including the obligations of the parties to consummate the financing contemplated by the Subscription Agreement if the conditions to consummation have been satisfied.
Bridge Loan (see page 138)
On
November 12, 2015, certain of the purchasers under the Subscription Agreement agreed to loan Eiger $6.0 million in a bridge loan in order to provide the financing required by Eiger to complete the merger. In connection with the bridge loan,
Eiger issued unsecured convertible promissory notes and warrants exercisable for shares of Eiger’s equity securities, referred to here as the Bridge Notes and Bridge Warrants.
The Bridge Notes accrue interest at a rate of 6.0% per year and have a maturity date of March 31, 2016. In the event Eiger consummates the sale of
its equity securities, either in the form of preferred stock or common stock, for aggregate proceeds of at least $25.0 million (excluding the conversion of the Bridge Notes) which is referred to as a Qualified Financing, the outstanding principal
and all accrued interest under the Bridge Notes will automatically convert into the class of equity securities issued in such qualified financing at the price per share of such equity securities sold in such qualified financing.
In connection with the bridge loan, Eiger also issued Bridge Warrants exercisable for shares of the equity securities issued by Eiger in any qualified
financing as described in the Bridge Notes, or if no qualified financing is consummated, then into shares of Eiger’s common stock. The exercise price of the equity securities issuable under the Bridge Warrants is $0.01 per share. The number of
shares of equity securities exercisable pursuant to the Bridge Warrants equals fifteen percent (15%) of the principal amount of such holder’s Bridge Note divided by (a) the price per share of the equity securities sold by Eiger in any
qualified financing (as defined in the Bridge Notes) or, (b) if no qualified financing is consummated, the price per shares of equity securities sold by Eiger in its next bona fide equity financing with aggregate proceeds to Eiger of at least
$1.0 million, provided, however, if no qualified financing, or next financing has occurred by January 1, 2017, then the per share price Eiger last sold shares of its Series A-1 Preferred Stock.
In the event Eiger consummates the transactions contemplated by the Subscription Agreement in connection with the closing of the merger, the outstanding
principal amount and all accrued interest (other than the portion of the interest used to automatically exercise the Bridge Warrants) under the Bridge Notes will convert into shares of
14
Eiger’s common stock at a price of $1.5002 per share and the Bridge Warrants will be automatically exercised for shares of Eiger’s common stock in exchange for cancellation of interest
under the Bridge Notes equal to the aggregate exercise price for the share of common stock issuable under the Bridge Warrants.
Support
Agreements and Written Consent (see page 139)
Certain Eiger stockholders are each party to a support agreement with Celladon pursuant to
which, among other things, each of these stockholders agreed, solely in its capacity as a stockholder, to vote all of its shares of Eiger capital stock in favor of the adoption of the Merger Agreement and the approval of any other matter necessary
to consummate the transactions contemplated by the Merger Agreement that are considered and voted upon by Eiger’s stockholders and against any “Acquisition Proposal,” as defined in the Merger Agreement. The parties to the support
agreements with Celladon are: Eiger Group International, Inc., InterWest Partners X L.P., Vivo Ventures Fund VI, L.P., Vivo Ventures VI Affiliates Fund, L.P., and the directors and officers of Eiger.
The stockholders of Eiger that are party to a support agreement with Celladon owned an aggregate of 2,461,505 shares of Eiger common stock and 26,754,308
shares of Eiger preferred stock, representing approximately 88.7% of the outstanding shares of Eiger capital stock on an as converted to common stock basis, in each case as of December 31, 2015. Therefore, holders of the number of shares of Eiger
stock required to adopt the Merger Agreement and approve the merger and related transactions are contractually obligated to adopt the Merger Agreement. Following the effectiveness of the registration statement of which this proxy
statement/prospectus/information statement is a part and pursuant to the Merger Agreement, stockholders of Eiger holding a sufficient number of shares to adopt the Merger Agreement and approve the merger and related transactions will execute written
consents providing for such adoption and approval.
Certain Celladon stockholders are each party to a support agreement with Eiger pursuant to which,
among other things, each of these stockholders agreed, solely in its capacity as a stockholder, to vote all of its shares of Celladon common stock in favor of the issuance of Celladon common stock in the merger pursuant to the Merger Agreement, the
adoption of the Merger Agreement if submitted for adoption, the approval of any proposal to adjourn or postpone the meeting to a later date, if there are not sufficient votes for the issuance of Celladon common stock in the merger pursuant to the
Merger Agreement on the date on which such meeting is held, and any other matter necessary to consummate the transactions contemplated by the Merger Agreement that are considered and voted upon by Celladon’s stockholders and against any
“Acquisition Proposal,” as defined in the Merger Agreement.
The stockholders of Celladon that are party to a support agreement with Eiger
owned an aggregate of 82,500 shares of Celladon common stock, representing 0.3% of the outstanding Celladon common stock as of December 31, 2015. These stockholders include executive officers and directors of Celladon.
Management Following the Merger (see page 223)
Effective as of the closing of the merger, Celladon’s executive officers are expected to be the current Eiger management team:
|
|
|
| Name |
|
Title |
| David A. Cory, RPh, MBA |
|
President and Chief Executive Officer |
| James H. Welch, MBA |
|
Chief Financial Officer |
| Joanne Quan, MD |
|
Chief Medical Officer |
| Eduardo Martins, MD, DPhil |
|
Senior VP, Liver and Infectious Disease |
| James P. Shaffer, MBA |
|
Chief Business Officer |
15
Interests of Certain Directors, Officers and Affiliates of Celladon and
Eiger (see pages 104 and 107)
In considering the recommendation of the Celladon board of directors with respect to issuing shares of Celladon common
stock pursuant to the Merger Agreement and the other matters to be acted upon by Celladon stockholders at the Celladon special meeting, Celladon stockholders should be aware that certain members of the Celladon board of directors and executive
officers of Celladon have interests in the merger that may be different from, or in addition to, interests they have as Celladon stockholders. For example, Celladon has entered into agreements with each of Fredrik Wiklund, Celladon’s President
and Chief Executive Officer, Andrew Jackson, Celladon’s Chief Financial Officer, and Elizabeth Reed, Celladon’s Vice President and General Counsel, the sole remaining executive officers and employees of Celladon, providing for a cash bonus
payment to each of them in an amount equal to the cash severance and retention benefit payments provided in each officer’s previously existing employment and retention agreements, which cash bonus payment replaces and supersedes each
officer’s right to any cash severance or retention benefits under any prior agreements and is not contingent upon such officer’s continued employment with Celladon through the completion of the proposed merger. The incentive cash bonus
payments for Messrs. Wiklund and Jackson and Ms. Reed are $454,190, $386,618 and $527,234, respectively. In addition, in connection with the proposed merger, Messrs. Wiklund and Jackson and Ms. Reed are eligible to receive incentive payments
upon the achievement of the following key milestones: (i) filing of this registration statement on Form S-4 in respect of the proposed merger; (ii) mailing of the proxy statement/prospectus/information statement portion of such
registration statement on Form S-4 to Celladon’s stockholders; and (iii) approval of the merger by Celladon’s stockholders. Subject to achievement and provided that such officer remains employed with Celladon as of the date of such
milestone achievement, Mr. Wiklund, will be eligible to receive a payment of $50,000 for each milestone, and each of Mr. Jackson and Ms. Reed will be eligible to receive a payment of $40,000 for each milestone. The employment of
Messrs. Wiklund and Jackson and Ms. Reed is expected to terminate no later than the consummation of the merger.
As of December 31, 2015, directors
and executive officers of Celladon owned 0.3% of the outstanding shares of Celladon common stock. Celladon directors and executive officers have entered into support agreements in connection with the merger. The support agreements are discussed in
greater detail in the section entitled “Agreements Related to the Merger—Support Agreements and Written Consent” in this proxy statement/prospectus/information statement.
In considering the recommendation of the Eiger board of directors with respect to approving the merger and related transactions by written consent, Eiger
stockholders should be aware that certain members of the board of directors and executive officers of Eiger have interests in the merger that may be different from, or in addition to, interests they have as Eiger stockholders. For example, certain
of Eiger’s officers received a grant of shares of Eiger common stock prior to the execution of the Merger Agreement, certain of Eiger’s directors and executive officers have options, subject to vesting, to purchase shares of Eiger common
stock which will be converted into and become options to purchase shares of Celladon common stock, certain of Eiger’s directors and executive officers are expected to become directors and executive officers of Celladon upon the closing of the
merger and all of Eiger’s directors and executive officers are entitled to certain indemnification and liability insurance coverage pursuant to the terms of the Merger Agreement.
As of December 31, 2015, directors and executive officers of Eiger, together with their affiliates, owned approximately 88.5% of the outstanding shares of
Eiger capital stock, on an as converted to common stock basis. Eiger officers and directors, and their affiliates, have also entered into support agreements in connection with the merger. The support agreements are discussed in greater detail in the
section entitled “Agreements Related to the Merger—Support Agreements and Written Consent” in this proxy statement/prospectus/information statement.
Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger (see page 112)
Each of Celladon and Eiger intends the merger to qualify as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as
amended (the “Code”). Assuming the merger qualifies
16
as a reorganization under the Code, then, in general and subject to the qualifications and limitations set forth in the section entitled “The Merger—Considerations with Respect to U.S.
Federal Income Tax Consequences of the Merger,” the material tax consequences to U.S. Holders (as defined herein) of Eiger common stock should be as follows:
| |
• |
|
an Eiger stockholder should not recognize gain or loss upon the exchange of Eiger common stock for Celladon common stock pursuant to the merger, except to the extent of cash received in lieu of a fractional share of
Celladon common stock as described below; |
| |
• |
|
an Eiger stockholder who receives cash in lieu of a fractional share of Celladon common stock in the merger should recognize capital gain or loss in an amount equal to the difference between the amount of cash received
instead of a fractional share and the stockholder’s tax basis allocable to such fractional share; |
| |
• |
|
an Eiger stockholder’s aggregate tax basis for the shares of Celladon common stock received in the merger (including any fractional share interest for which cash is received) should equal the stockholder’s
aggregate tax basis in the shares of Eiger common stock surrendered upon completion of the merger; and |
| |
• |
|
the holding period of the shares of Celladon common stock received by an Eiger stockholder in the merger should include the holding period of the shares of Eiger common stock surrendered in exchange therefor.
|
Tax matters are very complicated, and the tax consequences of the merger to a particular Eiger stockholder will depend on such
stockholder’s circumstances. Accordingly, you should consult your tax advisor for a full understanding of the tax consequences of the merger to you, including the applicability and effect of federal, state, local and foreign income and other
tax laws. For more information, please see the section entitled “The Merger—Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger” beginning on page 112.
Risk Factors (see page 27)
Both Celladon and Eiger are subject to various risks associated with their businesses and their industries. In addition, the merger, including the possibility
that the merger may not be completed, poses a number of risks to each company and its respective stockholders, including the following risks:
| |
• |
|
The exchange ratio is not adjustable based on the market price of Celladon common stock so the merger consideration at the closing may have a greater or lesser value than at the time the Merger Agreement was signed;
|
| |
• |
|
Failure to complete the merger may result in Celladon and Eiger paying a termination fee or expenses to the other and could harm the common stock price of Celladon and future business and operations of each company;
|
| |
• |
|
If the conditions to the merger are not met, the merger may not occur; |
| |
• |
|
The merger may be completed even though material adverse changes may result from the announcement of the merger, industry-wide changes and other causes; |
| |
• |
|
Some Celladon and Eiger executive officers and directors have interests in the merger that are different from yours and that may influence them to support or approve the merger without regard to your interests;
|
| |
• |
|
The market price of the combined organization common stock may decline as a result of the merger; |
| |
• |
|
Celladon and Eiger stockholders may not realize a benefit from the merger commensurate with the ownership dilution they will experience in connection with the merger; |
17
| |
• |
|
During the pendency of the merger, Celladon and Eiger may not be able to enter into a business combination with another party at a favorable price (subject to certain exceptions) because of restrictions in the Merger
Agreement, which could adversely affect their respective businesses; |
| |
• |
|
Certain provisions of the Merger Agreement may discourage third parties from submitting alternative takeover proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement;
and |
| |
• |
|
Because the lack of a public market for Eiger shares makes it difficult to evaluate the fairness of the merger, the stockholders of Eiger may receive consideration in the merger that is less than the fair market value
of the Eiger shares and/or Celladon may pay more than the fair market value of the Eiger shares. |
These risks and other risks are discussed
in greater detail under the section entitled “Risk Factors” in this proxy statement/prospectus/information statement. Celladon and Eiger both encourage you to read and consider all of these risks carefully.
Regulatory Approvals (see page 112)
In the United States, Celladon must comply with applicable federal and state securities laws and the rules and regulations of The NASDAQ Global Market in
connection with the issuance of shares of Celladon common stock and the filing of this proxy statement/prospectus/information statement with the SEC. As of the date hereof, the registration statement of which this proxy
statement/prospectus/information statement is a part has not become effective.
NASDAQ Stock Market Listing (see page
115)
Celladon has filed an initial listing application for the combined company with The NASDAQ Global Market pursuant to NASDAQ Stock Market LLC
“reverse merger” rules. If such application is accepted, Celladon anticipates that Celladon’s common stock will be listed on The NASDAQ Global Market following the closing of the merger under the trading symbol “EIGR.”
Anticipated Accounting Treatment (see page 115)
The merger will be treated by Celladon as a reverse merger under the acquisition method of accounting in accordance with accounting principles generally
accepted in the United States. For accounting purposes, Eiger is considered to be acquiring Celladon in the merger.
Appraisal Rights and Dissenters’ Rights (see page 115)
Holders of Celladon common stock are not entitled to appraisal rights in connection with the merger. Eiger stockholders are entitled to appraisal rights in
connection with the merger under Delaware law. For more information about such rights, see the provisions of Section 262 of the Delaware General Corporation Law, or the DGCL, attached hereto as Annex C, and the section entitled “The
Merger—Appraisal Rights and Dissenters’ Rights” in this proxy statement/prospectus/information statement.
Comparison of Stockholder Rights (see page 253)
Both Celladon and Eiger are incorporated under the laws of the State of Delaware and, accordingly, the rights of the stockholders of each are currently, and
will continue to be, governed by the DGCL. If the merger is completed, Eiger stockholders will become stockholders of Celladon, and their rights will be governed by the
18
DGCL, the bylaws of Celladon and, assuming Celladon Proposal No. 2 is approved by Celladon stockholders at the Celladon special meeting, the amended and restated certificate of
incorporation, as amended, of Celladon attached to this proxy statement/prospectus/information statement as Annex D. The rights of Celladon stockholders contained in the amended and restated certificate of incorporation, as amended, and
bylaws of Celladon differ from the rights of Eiger stockholders under the amended and restated certificate of incorporation and bylaws of Eiger, as more fully described under the section entitled “Comparison of Rights of Holders of Celladon
Stock and Eiger Stock” in this proxy statement/prospectus/information statement.
19
SELECTED HISTORICAL AND UNAUDITED PRO FORMA
CONDENSED COMBINED FINANCIAL INFORMATION AND DATA
The following tables present summary historical financial data for Celladon and Eiger, summary unaudited pro forma condensed combined financial data for
Celladon and Eiger, and comparative historical and unaudited pro forma per share data for Celladon and Eiger.
Selected
Historical Consolidated Financial Data of Celladon
The selected consolidated statements of operations data for the years ended December 31, 2014,
2013 and 2012 and the selected consolidated balance sheet data as of December 31, 2014 and 2013 are derived from Celladon’s audited consolidated financial statements included elsewhere in this proxy statement/prospectus/information
statement. The selected consolidated statements of operations data for the year ended December 31, 2012 are derived from Celladon’s audited consolidated financial statements which are not included in this proxy
statement/prospectus/information statement. The selected consolidated statements of operations data for the nine months ended September 30, 2015 and 2014 and the selected consolidated balance sheet data as of September 30, 2015 are derived
from Celladon’s unaudited interim consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement. Celladon’s unaudited interim consolidated financial statements have been prepared in
accordance with U.S. generally accepted accounting principles on the same basis as its audited annual consolidated financial statements and, in the opinion of management, reflect all adjustments, consisting only of normal, recurring adjustments,
necessary for the fair presentation of those unaudited interim consolidated financial statements. Celladon’s historical results are not necessarily indicative of the results that may be expected in any future period and the results for the nine
months ended September 30, 2015 are not necessarily indicative of results to be expected for the full year ending December 31, 2015 or any other period.
20
The selected historical consolidated financial data below should be read in conjunction with the section
titled “Celladon Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Risk Factors—Risks Related to Celladon” and Celladon’s consolidated financial statements and related notes
included elsewhere in this proxy statement/prospectus/information statement.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Year ended December 31, |
|
|
Nine Months Ended
September 30, |
|
| |
|
2014 |
|
|
2013 |
|
|
2012 |
|
|
2015 |
|
|
2014 |
|
| |
|
|
|
|
(unaudited) |
|
| |
|
(in thousands, except per share and share data) |
|
Consolidated Statements of Operations
Data: |
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
22,676 |
|
|
$ |
16,927 |
|
|
$ |
13,314 |
|
|
$ |
21,757 |
|
|
$ |
15,515 |
|
| General and administrative |
|
|
10,342 |
|
|
|
3,037 |
|
|
|
2,631 |
|
|
|
10,805 |
|
|
|
6,545 |
|
| Restructuring charges |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,862 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
33,018 |
|
|
|
19,964 |
|
|
|
15,945 |
|
|
|
37,424 |
|
|
|
22,060 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(33,018 |
) |
|
|
(19,964 |
) |
|
|
(15,945 |
) |
|
|
(37,424 |
) |
|
|
(22,060 |
) |
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest income |
|
|
118 |
|
|
|
69 |
|
|
|
35 |
|
|
|
60 |
|
|
|
65 |
|
| Interest expense |
|
|
(741 |
) |
|
|
(59 |
) |
|
|
(108 |
) |
|
|
(2,302 |
) |
|
|
(330 |
) |
| Other income (expense |
|
|
(29 |
) |
|
|
25 |
|
|
|
147 |
|
|
|
(1 |
) |
|
|
(4 |
) |
| Change in fair value of warrant liability |
|
|
(183 |
) |
|
|
(162 |
) |
|
|
— |
|
|
|
— |
|
|
|
(183 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Consolidated net loss |
|
|
(33,853 |
) |
|
|
(20,091 |
) |
|
|
(15,871 |
) |
|
|
(39,667 |
) |
|
|
(22,512 |
) |
| Net loss attributable to noncontrolling interest |
|
|
— |
|
|
|
96 |
|
|
|
154 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss attributable to Celladon Corporation |
|
|
(33,853 |
) |
|
|
(19,995 |
) |
|
|
(15,717 |
) |
|
|
(39,667 |
) |
|
|
(22,512 |
) |
| Accretion to redemption value of redeemable convertible preferred stock |
|
|
— |
|
|
|
— |
|
|
|
(343 |
) |
|
|
— |
|
|
|
— |
|
| Change in fair value of noncontrolling interest |
|
|
— |
|
|
|
(3,105 |
) |
|
|
(154 |
) |
|
|
— |
|
|
|
— |
|
| Deemed dividend |
|
|
— |
|
|
|
(856 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss attributable to common stockholders |
|
$ |
(33,853 |
) |
|
$ |
(23,956 |
) |
|
$ |
(16,214 |
) |
|
$ |
(39,667 |
) |
|
$ |
(22,512 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share attributable to common stockholders, basic and diluted(1) |
|
$ |
(1.82 |
) |
|
$ |
(27.09 |
) |
|
$ |
(19.74 |
) |
|
$ |
(1.66 |
) |
|
$ |
(1.32 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted-average shares outstanding, basic |
|
|
18,603,605 |
|
|
|
884,179 |
|
|
|
821,568 |
|
|
|
23,830,668 |
|
|
|
16,999,766 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of December 31, |
|
|
As of
September 30, |
|
| |
|
2014 |
|
|
2013 |
|
|
2012 |
|
|
2015 |
|
| |
|
|
|
|
(unaudited) |
|
| |
|
(in thousands) |
|
| Selected Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
14,435 |
|
|
$ |
7,903 |
|
|
$ |
13,841 |
|
|
$ |
37,092 |
|
| Short-term and long-term investments |
|
|
70,513 |
|
|
|
10,467 |
|
|
|
21,670 |
|
|
|
— |
|
| Total assets |
|
|
89,110 |
|
|
|
21,154 |
|
|
|
35,929 |
|
|
|
38,236 |
|
| Working capital |
|
|
81,477 |
|
|
|
11,990 |
|
|
|
31,159 |
|
|
|
36,296 |
|
| Common stock and additional-paid-in-capital |
|
|
218,551 |
|
|
|
61,593 |
|
|
|
64,166 |
|
|
|
222,516 |
|
| Accumulated Deficit |
|
|
(146,439 |
) |
|
|
(112,586 |
) |
|
|
(92,591 |
) |
|
|
(186,106 |
) |
| Total stockholders’ equity (deficit) |
|
|
72,104 |
|
|
|
(50,991 |
) |
|
|
(28,416 |
) |
|
|
36,410 |
|
| (1) |
See Note 1 to Celladon’s audited consolidated financial statements and Note 1 to Celladon’s unaudited interim consolidated financial statements included elsewhere in this proxy statement/prospectus/information
statement for an explanation of the calculations of its basic and diluted net loss per share and the weighted-average number of shares used in the computation of the per share amounts. |
Selected Historical Consolidated Financial Data of Eiger
The selected consolidated statements of operations data for the years ended December 31, 2014 and 2013 and the selected consolidated balance sheet data as
of December 31, 2014 and 2013 are derived from Eiger’s audited consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement. The audit report on the consolidated financial statements for the
year ended December 31, 2014, which appears elsewhere herein, includes an explanatory paragraph related to Eiger’s ability to continue as a going concern. The selected consolidated statements of operations data for the nine months ended
September 30, 2015 and 2014 and the selected consolidated balance sheet data as of September 30, 2015 are derived from Eiger’s unaudited interim condensed consolidated financial statements included elsewhere in this proxy
statement/prospectus/information statement. Eiger’s unaudited interim condensed consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles on the same basis as its audited annual
consolidated financial statements and, in the opinion of management, reflect all adjustments, consisting only of normal, recurring adjustments, necessary for the fair presentation of those unaudited interim condensed consolidated financial
statements. Eiger’s historical results are not necessarily indicative of the results that may be expected in any future period and the results for the nine months ended September 30, 2015 are not necessarily indicative of results to be
expected for the full year ending December 31, 2015 or any other period.
22
The selected historical consolidated financial data below should be read in conjunction with the section
titled “Eiger Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Risk Factors—Risks Related to Eiger’s Financial Condition and Capital Requirements” and Eiger’s
consolidated financial statements and related notes included elsewhere in this proxy statement/prospectus/information statement.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
|
|
Nine Months Ended
September 30, |
|
| |
|
2014 |
|
|
2013 |
|
|
2015 |
|
|
2014 |
|
| |
|
|
|
|
(unaudited) |
|
| |
|
(in thousands, except per share and share amounts) |
|
| Consolidated Statements of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
644 |
|
|
$ |
426 |
|
|
$ |
4,493 |
|
|
$ |
320 |
|
| General and administrative |
|
|
872 |
|
|
|
526 |
|
|
|
1,768 |
|
|
|
403 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
1,516 |
|
|
|
952 |
|
|
|
6,261 |
|
|
|
723 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(1,516 |
) |
|
|
(952 |
) |
|
|
(6,261 |
) |
|
|
(723 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(1,516 |
) |
|
$ |
(952 |
) |
|
$ |
(6,261 |
) |
|
$ |
(723 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per common share, basic and diluted |
|
$ |
(0.68 |
) |
|
$ |
(0.43 |
) |
|
$ |
(2.82 |
) |
|
$ |
(0.33 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares used in computing net loss per common share, basic and diluted |
|
|
2,214,958 |
|
|
|
2,214,958 |
|
|
|
2,222,561 |
|
|
|
2,214,958 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of December 31, |
|
|
As of
September 30,
2015 |
|
| |
|
2014 |
|
|
2013 |
|
|
| |
|
|
|
|
|
|
|
(unaudited) |
|
| |
|
(in thousands) |
|
| Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash |
|
$ |
777 |
|
|
$ |
145 |
|
|
$ |
2,136 |
|
| Working capital |
|
|
522 |
|
|
|
93 |
|
|
|
1,711 |
|
| Total assets |
|
|
817 |
|
|
|
176 |
|
|
|
2,516 |
|
| Convertible preferred stock |
|
|
15,366 |
|
|
|
13,452 |
|
|
|
22,567 |
|
| Accumulated deficit |
|
|
(15,949 |
) |
|
|
(14,433 |
) |
|
|
(22,210 |
) |
| Total stockholders’ equity |
|
|
530 |
|
|
|
106 |
|
|
|
1,756 |
|
23
Selected Unaudited Pro Forma Condensed Combined Financial Data of Celladon and
Eiger
(In thousands, except per share amounts)
The following information does not give effect to the proposed reverse stock split of Celladon common stock described in Celladon Proposal No. 2.
|
|
|
|
|
|
|
|
|
| |
|
Year Ended
December 31,
2014 |
|
|
Nine Months
Ended
September 30,
2015 |
|
| |
|
(in thousands except per share amount) |
|
| Unaudited Pro Forma Combined Statement of Operations Data: |
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
23,320 |
|
|
$ |
26,250 |
|
| General and administrative |
|
|
11,214 |
|
|
|
11,971 |
|
| Restructuring charges |
|
|
— |
|
|
|
4,862 |
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
34,534 |
|
|
|
43,083 |
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(34,534 |
) |
|
|
(43,083 |
) |
|
|
|
|
|
|
|
|
|
| Interest expense |
|
|
(741 |
) |
|
|
(2,302 |
) |
| Interest and other income (expense), net |
|
|
(94 |
) |
|
|
59 |
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(35,369 |
) |
|
$ |
(45,326 |
) |
|
|
|
|
|
|
|
|
|
| Basic and diluted net loss per share |
|
$ |
(0.84 |
) |
|
$ |
(0.70 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of September 30, 2015 |
|
| |
|
(in thousands) |
|
| Unaudited Pro Forma Combined Balance Sheet Data: |
|
|
|
|
| Cash and cash equivalents |
|
$ |
78,728 |
|
| Working capital |
|
|
71,530 |
|
| Total assets |
|
|
80,252 |
|
| Total stockholders’ equity |
|
|
71,689 |
|
24
Comparative Historical and Unaudited Pro Forma Per Share Data
The information below reflects the historical net loss and book value per share of Celladon common stock and the historical net loss and book value per share
of Eiger common stock in comparison with the unaudited pro forma net loss and book value per share after giving effect to the proposed merger of Celladon with Eiger on a pro forma basis. The unaudited pro forma net loss and book value per share does
not give effect to the proposed reverse stock split of Celladon common stock described in Celladon Proposal No. 2.
You should read the tables below
in conjunction with the audited and unaudited financial statements of Celladon included in this proxy statement/prospectus/information statement and the audited and unaudited financial statements of Eiger included in this proxy
statement/prospectus/information statement and the related notes and the unaudited pro forma condensed combined financial information and notes related to such financial statements included elsewhere in this proxy statement/prospectus/information
statement.
CELLADON
|
|
|
|
|
|
|
|
|
| |
|
Nine Months
Ended
September 30,
2015 |
|
|
Year Ended
December 31,
2014 |
|
| Historical Per Common Share Data: |
|
|
|
|
|
|
|
|
| Basic and diluted net loss per share |
|
$ |
(1.66 |
) |
|
$ |
(1.82 |
) |
| Book value per share |
|
$ |
1.52 |
|
|
$ |
3.07 |
|
EIGER
|
|
|
|
|
|
|
|
|
| |
|
Nine Months
Ended
September 30,
2015 |
|
|
Year Ended
December 31,
2014 |
|
| Historical Per Common Share Data: |
|
|
|
|
|
|
|
|
| Basic and diluted net loss per share |
|
$ |
(2.82 |
) |
|
$ |
(0.68 |
) |
| Book value per share |
|
$ |
0.57 |
|
|
$ |
0.24 |
|
CELLADON AND EIGER
|
|
|
|
|
|
|
|
|
| |
|
Nine Months
Ended
September 30,
2015 |
|
|
Year Ended
December 31,
2014 |
|
| Combined Company Pro Forma Data: |
|
|
|
|
|
|
|
|
| Basic and diluted net loss per share |
|
$ |
(0.70 |
) |
|
$ |
(0.84 |
) |
| Book value per share |
|
$ |
0.69 |
|
|
$ |
N/A |
|
25
MARKET PRICE AND DIVIDEND INFORMATION
Celladon common stock is listed on The NASDAQ Global Market under the symbol “CLDN.” The following table presents, for the periods indicated, the
range of high and low per share sales prices for Celladon common stock as reported on The NASDAQ Global Market for each of the periods set forth below. Eiger is a private company and its common stock and preferred stock are not publicly traded.
These per share sales prices do not give effect to the proposed 1-for-15 reverse stock split of Celladon common stock to be implemented prior to the consummation of the merger.
Celladon Common Stock
|
|
|
|
|
|
|
|
|
| |
|
High |
|
|
Low |
|
| Year Ended December 31, 2014 |
|
|
|
|
|
|
|
|
| First Quarter (from January 30, 2014) |
|
$ |
17.16 |
|
|
$ |
7.45 |
|
| Second Quarter |
|
$ |
16.47 |
|
|
$ |
7.82 |
|
| Third Quarter |
|
$ |
16.72 |
|
|
$ |
9.20 |
|
| Fourth Quarter |
|
$ |
20.85 |
|
|
$ |
9.20 |
|
| Year Ended December 31, 2015 |
|
|
|
|
|
|
|
|
| First Quarter |
|
$ |
28.25 |
|
|
$ |
15.51 |
|
| Second Quarter |
|
$ |
19.20 |
|
|
$ |
1.24 |
|
| Third Quarter |
|
$ |
1.29 |
|
|
$ |
1.00 |
|
| Fourth Quarter |
|
$ |
1.89 |
|
|
$ |
1.00 |
|
On
, 2016, the last reported sale price of Celladon’s common stock on the NASDAQ Global Market was
$ per share.
Because the market price of Celladon common stock is subject to fluctuation, the market
value of the shares of Celladon common stock that Eiger stockholders will be entitled to receive in the merger may increase or decrease.
Assuming
approval of Celladon Proposal No. 3 and successful application for initial listing with The NASDAQ Global Market, following the consummation of the merger, Celladon common stock will be listed on The NASDAQ Global Market and will trade under
Celladon’s new name, “Eiger BioPharmaceuticals, Inc.” and new trading symbol, “EIGR.”
As of December 31, 2015, Celladon had
approximately 135 holders of record of its common stock. For detailed information regarding the beneficial ownership of certain stockholders of Celladon upon consummation of the merger, see the section entitled “Principal Stockholders of
Combined Company” in this proxy statement/prospectus/information statement.
Dividend Policy
Celladon has never paid or declared, and does not anticipate declaring, or paying in the foreseeable future, any cash dividends on its common stock. Future
determination as to the declaration and payment of dividends, if any, will be at the discretion of Celladon’s board of directors and will depend on then existing conditions, including its operating results, financial conditions, contractual
restrictions, capital requirements, business prospects and other factors its board of directed may deem relevant.
Eiger has never paid or declared any
cash dividends on its common stock. If the merger does not occur, Eiger does not anticipate paying any cash dividends on its common stock in the foreseeable future, and Eiger intends to retain all available funds and any future earnings to fund the
development and expansion of its business. Any future determination to pay dividends will be at the discretion of Eiger’s board of directors and will depend upon a number of factors, including its results of operations, financial condition,
future prospects, contractual restrictions, restrictions imposed by applicable law and other factors Eiger’s board of directors deems relevant.
26
RISK FACTORS
The combined organization will be faced with a market environment that cannot be predicted and that involves significant risks, many of which will be
beyond its control. In addition to the other information contained in this proxy statement/prospectus/information statement, you should carefully consider the material risks described below before deciding how to vote your shares of stock. In
addition, you should read and consider the risks associated with the business of Celladon because these risks may also affect the combined company—these risks can be found in Celladon’s Annual Report on Form 10-K, as updated by subsequent
Quarterly Reports on Form 10-Q, all of which are filed with the SEC. You should also read and consider the other information in this proxy statement/prospectus/information statement and the other documents incorporated by reference into this proxy
statement/prospectus/information statement. Please see the section entitled “Where You Can Find More Information” in this proxy statement/prospectus/information statement.
Risks Related to the Merger
The exchange ratio is not adjustable based on the market price of Celladon common stock so the merger consideration at the closing may have a greater or
lesser value than at the time the Merger Agreement was signed.
The Merger Agreement has set the exchange ratio for the Eiger common stock, and
the exchange ratio is only adjustable upward or downward if the outstanding capital stock of Eiger or the outstanding common stock of Celladon changes based upon certain events, including the proposed 1-for-15 reverse stock split, prior to
completion of the merger as described in “The Merger—Merger Consideration and Adjustment.” The pre-split exchange ratio is approximately 1.32, and the post-split exchange ratio is approximately 0.09. Any changes in the market price of
Celladon common stock before the completion of the merger will not affect the number of shares Eiger securityholders will be entitled to receive pursuant to the Merger Agreement. Therefore, if before the completion of the merger the market price of
Celladon common stock declines from the market price on the date of the Merger Agreement, then Eiger securityholders could receive merger consideration with substantially lower value. Similarly, if before the completion of the merger the market
price of Celladon common stock increases from the market price on the date of the Merger Agreement, then Eiger securityholders could receive merger consideration with substantially more value for their shares of Eiger capital stock than the parties
had negotiated for in the establishment of the exchange ratio. The Merger Agreement does not include a price-based termination right, however, Eiger’s obligation to consummate the Merger is conditioned upon Celladon having “Net Cash”
of at least $24 million, as defined and described under “The Merger Agreement—Conditions to the Completion of the Merger.” Because the exchange ratio does not adjust as a result of changes in the value of Celladon common stock, for
each one percentage point that the market value of Celladon common stock rises or declines, there is a corresponding one percentage point rise or decline, respectively, in the value of the total merger consideration issued to Eiger securityholders.
Failure to complete the merger may result in Celladon and Eiger paying a termination fee or expenses to the other party and could harm the
common stock price of Celladon and future business and operations of each company.
If the merger is not completed, Celladon and Eiger are subject
to the following risks:
| |
• |
|
if the Merger Agreement is terminated under certain circumstances, Celladon or Eiger will be required to pay certain transaction expenses of the other party, up to a maximum of $1.0 million; |
| |
• |
|
if the Merger Agreement is terminated under certain circumstances, Celladon or Eiger will be required to pay the other party a termination fee of $3.0 million; |
| |
• |
|
the price of Celladon stock may decline and remain volatile; and |
| |
• |
|
costs related to the merger, such as legal and accounting fees which Celladon and Eiger estimate will total approximately $1.3 million and $0.9 million, respectively, some of which must be paid even if the merger is not
completed. |
27
In addition, if the Merger Agreement is terminated and the board of directors of Celladon or Eiger determines to
seek another business combination, there can be no assurance that either Celladon or Eiger will be able to find a partner willing to provide equivalent or more attractive consideration than the consideration to be provided by each party in the
merger.
If the conditions to the merger are not met, the merger may not occur.
Even if the merger is approved by the stockholders of Celladon and Eiger, specified conditions must be satisfied or waived to complete the merger. These
conditions are set forth in the Merger Agreement and described in the section entitled “The Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement. Celladon and Eiger
cannot assure you that all of the conditions will be satisfied or waived. If the conditions are not satisfied or waived, the merger may not occur or will be delayed, and Celladon and Eiger each may lose some or all of the intended benefits of the
merger.
The merger may be completed even though material adverse changes may result from the announcement of the merger, industry-wide changes and
other causes.
In general, either Celladon or Eiger can refuse to complete the merger if there is a material adverse change affecting the other
party between November 18, 2015, the date of the Merger Agreement, and the closing. However, certain types of changes do not permit either party to refuse to complete the merger, even if such change could be said to have a material adverse
effect on Celladon or Eiger, including:
| |
• |
|
any effect, change, event, circumstance or development in the conditions generally affecting the industries in which Eiger and Celladon operate or the United States or global economy or capital markets as a whole;
|
| |
• |
|
any natural disaster or any acts of terrorism, sabotage, military action or war or any escalation of worsening thereof; |
| |
• |
|
any change in accounting requirements or principles or any change in applicable laws, rules or regulations or the interpretation thereof; |
| |
• |
|
any effect resulting from the announcement or pendency of the merger or any related transactions; |
| |
• |
|
any failure by Celladon or Eiger to meet internal projections or forecasts or third party revenue or earnings predictions for any period ending on or after November 18, 2015; |
| |
• |
|
any changes in GAAP or applicable legal requirements after November 18, 2015; |
| |
• |
|
with respect to Celladon, any change in the price or trading volume of Celladon Common Stock; |
| |
• |
|
with respect to Celladon, the resignation or termination of any officer or director; |
| |
• |
|
with respect to Eiger, any rejection by a governmental body of a registration or filing by Eiger relating to certain intellectual property rights; or |
| |
• |
|
with respect to Eiger, any change in the cash position of Eiger which results from operations in the ordinary course of business. |
If adverse changes occur and Celladon and Eiger still complete the merger, the combined organization stock price may suffer. This in turn may reduce the value
of the merger to the stockholders of Celladon, Eiger or both.
Some Celladon and Eiger executive officers and directors have interests in the merger
that are different from yours and that may influence them to support or approve the merger without regard to your interests.
Certain officers and
directors of Celladon and Eiger participate in arrangements that provide them with interests in the merger that are different from yours, including, among others, the continued service as an officer or
28
director of the combined organization, severance and retention benefits, incentive payments upon achievement of key milestones relating to the merger, the acceleration of stock option vesting,
continued indemnification and the potential ability to sell an increased number of shares of common stock of the combined organization in accordance with Rule 144 under the Securities Act of 1933, as amended, or the Securities Act.
For example, Celladon has entered into employment agreements with each of its executive officers that provide for severance benefits. In addition, pursuant to
a retention program adopted on April 26, 2015 and amended on May 13, 2015, Celladon entered into retention agreements with certain current and former executive officers which provided for retention payments if such officers remained
employed with Celladon until December 31, 2015 or were terminated without cause prior to that date. In connection with execution of the Merger Agreement, effective November 18, 2015, Celladon entered into agreements with each of Messrs.
Wiklund and Jackson and Ms. Reed, the sole remaining employees of Celladon, providing for a cash bonus payment (an “Executive Bonus Payment”) to each of them in an amount equal to the cash severance and retention benefit payments
provided in each officer’s previously existing agreements. The Executive Bonus Payment for each officer equals the cash payment amount that each such officer would have been entitled to under the terms of his or her employment agreement, as
amended, and his or her retention agreement dated May 27, 2015 that was entered into pursuant to the retention program adopted on April 26, 2015 and amended on May 13, 2015 (collectively, the “Prior Agreements”) had such
officer been terminated without cause or resigned for good reason (or, with respect to the retention agreements, remained employed by Celladon on December 31, 2015), and replaces and supersedes each officer’s right to any cash severance or
retention benefits under the Prior Agreements. Each officer’s Executive Bonus Payment will be subject to his or her delivery of an effective waiver and release of claims and is expected to be paid in late 2015 or early 2016. Each of Messrs.
Wiklund’s and Jackson’s and Ms. Reed’s employment relationship with Celladon remains at-will and continued employment with Celladon is not a requirement to receive the Executive Bonus Payment. The closing of the merger will also
result in the acceleration of vesting of options to purchase shares of Celladon common stock held by the Celladon directors. For more information concerning the treatment of Celladon options in connection with the merger, see the section entitled
“The Merger Agreement—Treatment of Celladon Stock Options and Warrants” in this proxy statement/prospectus/information statement. In addition, Celladon adopted an incentive bonus program for Celladon’s remaining three officers,
who are the sole remaining employees of Celladon, pursuant to which they will be eligible to receive incentive payments upon the achievement of the following key milestones: (i) filing of the registration statement on Form S-4 in respect of the
proposed merger; (ii) mailing of the proxy statement/prospectus/information statement portion of such registration statement on Form S-4 to the Celladon stockholders; and (iii) approval of the Merger by Celladon’s stockholders.
Subject to achievement and provided that such officer remains employed with Celladon as of the date of such milestone achievement, Mr. Wiklund will be eligible to receive a payment of $50,000 for each milestone, and each of Mr. Jackson and
Ms. Reed will be eligible to receive a payment of $40,000 for each milestone.
In addition, certain of Eiger’s officers received grants of
shares of Eiger common stock prior to the execution of the Merger Agreement, certain of Eiger’s directors and executive officers have options, subject to vesting, to purchase shares of Eiger common stock which will be converted into and become
options to purchase shares of Celladon common stock, certain of Eiger’s directors and executive officers are expected to become directors and executive officers of Celladon upon the closing of the merger and all of Celladon’s and
Eiger’s directors and executive officers are entitled to certain indemnification and liability insurance coverage pursuant to the terms of the Merger Agreement. These interests, among others, may influence the officers and directors of Celladon
and Eiger to support or approve the merger. For more information concerning the interests of Celladon and Eiger executive officers and directors, see the sections entitled “The Merger—Interests of the Celladon Directors and Executive
Officers in the Merger” and “The Merger—Interests of the Eiger Directors and Executive Officers in the Merger” in this proxy statement/prospectus/information statement.
29
The market price of Celladon common stock following the merger may decline as a result of the merger.
The market price of Celladon common stock may decline as a result of the merger for a number of reasons including if:
| |
• |
|
investors react negatively to the prospects of the combined organization’s business and prospects from the merger; |
| |
• |
|
the effect of the merger on the combined organization’s business and prospects is not consistent with the expectations of financial or industry analysts; or |
| |
• |
|
the combined organization does not achieve the perceived benefits of the merger as rapidly or to the extent anticipated by financial or industry analysts. |
Celladon and Eiger stockholders may not realize a benefit from the merger commensurate with the ownership dilution they will experience in connection
with the merger.
If the combined organization is unable to realize the full strategic and financial benefits currently anticipated from the
merger, Celladon and Eiger stockholders will have experienced substantial dilution of their ownership interests in their respective companies without receiving any commensurate benefit, or only receiving part of the commensurate benefit to the
extent the combined organization is able to realize only part of the strategic and financial benefits currently anticipated from the merger.
During
the pendency of the merger, Celladon and Eiger may not be able to enter into a business combination with another party at a favorable price because of restrictions in the Merger Agreement, which could adversely affect their respective businesses.
Covenants in the Merger Agreement impede the ability of Celladon and Eiger to make acquisitions, subject to certain exceptions relating to
fiduciaries duties, as set forth below, or complete other transactions that are not in the ordinary course of business pending completion of the merger. As a result, if the merger is not completed, the parties may be at a disadvantage to their
competitors during that period. In addition, while the Merger Agreement is in effect, each party is generally prohibited from soliciting, initiating, encouraging or entering into certain extraordinary transactions, such as a merger, sale of assets
or other business combination outside the ordinary course of business, with any third party, subject to certain exceptions described below. Any such transactions could be favorable to such party’s stockholders.
Certain provisions of the Merger Agreement may discourage third parties from submitting alternative takeover proposals, including proposals that may be
superior to the arrangements contemplated by the Merger Agreement.
The terms of the Merger Agreement prohibit each of Celladon and Eiger from
soliciting alternative takeover proposals or cooperating with persons making unsolicited takeover proposals, except in limited circumstances when such party’s board of directors determines in good faith that an unsolicited alternative takeover
proposal is or is reasonably likely to lead to a superior takeover proposal and is reasonably capable of being consummated and that failure to cooperate with the proponent of the proposal is reasonably likely to result in a breach of the
board’s fiduciary duties. In addition, if Celladon or Eiger terminate the Merger Agreement under certain circumstances, including terminating because of a decision of a board of directors to recommend a superior proposal, Celladon or Eiger
would be required to pay a termination fee of $3.0 million to the other party. If the Merger Agreement is terminated under certain circumstances, Celladon or Eiger will be required to pay the other party a termination fee of $3.0 million or, in some
circumstances, reimburse the other party for expenses incurred in connection with the merger, up to a maximum of $1.0 million. This termination fee may discourage third parties from submitting alternative takeover proposals to Celladon or Eiger or
their stockholders, and may cause the respective boards of directors to be less inclined to recommend an alternative proposal.
30
Because the lack of a public market for Eiger shares makes it difficult to evaluate the fairness of the
merger, the stockholders of Eiger may receive consideration in the merger that is less than the fair market value of the Eiger shares and/or Celladon may pay more than the fair market value of the Eiger shares.
The outstanding capital stock of Eiger is privately held and is not traded in any public market. The lack of a public market makes it extremely difficult to
determine the fair market value of Eiger. Because the percentage of Celladon equity to be issued to Eiger stockholders was determined based on negotiations between the parties, it is possible that the value of the Celladon common stock to be
received by Eiger stockholders will be less than the fair market value of Eiger, or Celladon may pay more than the aggregate fair market value for Eiger.
Risks Related to Celladon
Celladon’s business to date has been almost entirely dependent on the success of MYDICAR, which recently failed to show a treatment effect in the
Phase 2b clinical trial known as CUPID 2. Following the analysis of the data from CUPID 2, Celladon decided to substantially suspend further research and development while seeking a merger or sale, and there is no guarantee that this strategic path
will be successful.
On April 26, 2015, Celladon announced that the CUPID 2 trial did not meet its primary and secondary endpoints. CUPID 2
was a 250-patient randomized, double-blind, placebo-controlled multinational Phase 2b trial that was designed to evaluate MYDICAR in patients with heart failure with reduced ejection fraction, or HFrEF, also referred to as systolic heart failure.
Celladon had previously devoted substantially all of its research, development and clinical efforts and financial resources toward the development of MYDICAR. As a result of the negative results from CUPID 2, Celladon has suspended further research
and development of MYDICAR and its pre-clinical programs to reduce operating expenses while seeking a merger or sale. There can be no assurance that the proposed merger transaction will be approved or consummated, or if consummated, that it would
enhance shareholder value. If the merger is not consummated, there also can be no assurance that Celladon will conduct drug development activities in the future.
There is no assurance that the proposed merger between Celladon and Eiger will be completed in a timely manner or at all. If the merger with Eiger is
not consummated, Celladon’s business could suffer materially and its stock price could decline.
The consummation of the proposed merger
between Celladon and Eiger is subject to a number of closing conditions, including the approval by Celladon’s stockholders and other customary closing conditions. The parties are targeting a closing of the transaction in the first half of 2016,
however, there can be no assurance that the proposed merger will be consummated on their desired timeframe, or at all.
If the proposed merger between
Celladon and Eiger is not consummated, Celladon may be subject to a number of material risks, and its business and stock price could be adversely affected, as follows:
| |
• |
|
Celladon has incurred and expects to continue to incur significant expenses related to the proposed merger with Eiger even if the merger is not consummated; |
| |
• |
|
Celladon could be obligated to pay Eiger a $3.0 million termination fee or up to $1.0 million in merger related expenses in connection with the termination of the Merger Agreement, depending on the reason for the
termination; |
| |
• |
|
The market price of Celladon’s common stock may decline to the extent that the current market price reflects a market assumption that the proposed merger will be completed; and |
| |
• |
|
With the November 2015 termination of all of Celladon’s remaining employees other than its President and Chief Executive Officer, its Chief Financial Officer and its Vice President and General Counsel, Celladon may
not pursue an alternate merger transaction if the proposed merger with Eiger is not completed. |
31
If the merger is not completed, Celladon’s board of directors may decide to pursue a dissolution and
liquidation of the company. In such an event, the amount of cash available for distribution to its stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and
contingent liabilities.
There can be no assurance that the merger will be completed. If the merger is not completed, Celladon’s board of
directors may decide to pursue a dissolution and liquidation of the company. In such an event, the amount of cash available for distribution to its stockholders will depend heavily on the timing of such decision, as with the passage of time the
amount of cash available for distribution will be reduced as Celladon continues to fund its operations. In addition, if Celladon’s board of directors were to approve and recommend, and its stockholders were to approve, a dissolution and
liquidation of the company, it would be required under Delaware corporate law to pay its outstanding obligations, as well as to make reasonable provision for contingent and unknown obligations, prior to making any distributions in liquidation to its
stockholders. Celladon’s commitments and contingent liabilities may include: (i) regulatory and clinical obligations remaining under its CUPID 2 trial, which is currently in the long-term follow up stage until February 2016; (ii) the
pending litigation against Celladon, and other various claims and legal actions arising in the ordinary course of business; and (iii) non-cancelable lease obligations. As a result of this requirement, a portion of Celladon’s assets may
need to be reserved pending the resolution of such obligations. In addition, Celladon may be subject to litigation or other claims related to a dissolution and liquidation of its company. If a dissolution and liquidation were pursued,
Celladon’s board of directors, in consultation with its advisors, would need to evaluate these matters and make a determination about a reasonable amount to reserve. Accordingly, holders of its common stock could lose all or a significant
portion of their investment in the event of a liquidation, dissolution or winding up of its company.
If Celladon fails to continue to meet all
applicable NASDAQ Global Market requirements and NASDAQ determines to delist Celladon’s common stock, the delisting could adversely affect the value of the merger, market liquidity of its common stock and the market price of its common stock
could decrease.
Celladon’s common stock is listed on The NASDAQ Global Market. In order to maintain the listing, Celladon must meet
minimum financial and other requirements, including requirements for a minimum amount of capital, a minimum price per share and continued business operations so that it is not characterized as a “public shell company.” If Celladon is
unable to comply with NASDAQ’s listing standards, NASDAQ may determine to delist its common stock from The NASDAQ Global Market. If its common stock is delisted for any reason, it could reduce the value of its common stock and its liquidity.
Delisting could also adversely affect the ability to obtain financing for the continuation of Celladon’s operations, if Celladon chooses to reestablish its business, or to use its common stock in acquisitions, including the merger. Delisting
could result in the loss of confidence by suppliers and employees. Delisting or failing to maintain the continuous listing of Celladon’s common stock or otherwise meet NASDAQ’s listing requirements would prevent Celladon from satisfying a
closing condition for the merger, and, in such event, Eiger may elect not to consummate the merger and may elect to terminate the Merger Agreement if the merger is not consummated by March 31, 2016. In addition, the combined organization must submit
a new application for listing on The NASDAQ Global Market after the merger pursuant to the reverse merger rules, and the combined organization will need to meet NASDAQ’s minimum listing requirements.
As a result of the CUPID 2 data and the reductions in Celladon’s workforce announced in April 2015, June 2015 and November 2015, Celladon has
only three employees remaining. If Celladon is unable to retain the remaining three employees, Celladon’s ability to consummate the planned merger transaction may be delayed or seriously jeopardized.
On April 30, 2015, June 26, 2015 and November 18, 2015, Celladon announced workforce reductions, which have reduced the headcount to three
remaining employees. Celladon’s cash conservation activities may yield unintended consequences, such as attrition beyond the planned reductions in workforce and reduced employee morale which may cause the remaining three employees to seek
alternate employment. Competition among biotechnology companies for qualified employees is intense, and the ability to retain the remaining employees is
32
critical to Celladon’s ability to effectively manage its resources following the CUPID 2 data and to consummate the planned merger transaction. Additional attrition could have a material
adverse effect on Celladon’s business. In addition, as a result of the reduction in Celladon’s workforce, Celladon faces an increased risk of employment litigation.
Risks Related to Celladon’s Financial Condition
Celladon has incurred significant losses since its inception and anticipates that it will continue to incur significant losses for the foreseeable
future.
Celladon is a biotechnology company and it has not yet generated any revenues. Celladon has incurred net losses in each year since its
inception in December 2000, including consolidated net losses of $33.9 million for the year ended December 31, 2014. As of September 30, 2015, Celladon had an accumulated deficit of approximately $186.1 million. Its prior losses, combined
with expected future losses, have had and may continue to have an adverse effect on its stockholders’ equity and working capital.
Prior to the
recent suspension of its further research and clinical development activities, Celladon had devoted most of its financial resources to research and development, including developing its manufacturing capabilities and preclinical and clinical
development activities. To date, Celladon has financed its operations primarily through the sale of equity securities and convertible debt. The amount of its future net losses will depend, in part, on the rate of its future expenditures and its
ability to obtain funding through equity or debt financings or strategic collaborations.
The net losses Celladon incurs may fluctuate significantly from
quarter-to-quarter and year-to-year, such that a period-to-period comparison of its results of operations may not be a good indication of its future performance. In any particular quarter or quarters, its operating results could be below the
expectations of securities analysts or investors, which could cause its stock price to decline.
If the merger is not completed, Celladon would need
to raise substantial additional funding to the extent it continues its product development efforts, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force it to delay, limit or
terminate its product development efforts or other operations.
Celladon’s operations have consumed substantial amounts of cash since
inception. As of September 30, 2015, its cash, cash equivalents and investments were approximately $37.1 million. Celladon’s research and development expenses were $21.8 million and $15.5 million for the nine months ended
September 30, 2015 and 2014, respectively. Celladon believes that its existing cash, cash equivalents and investments will enable it to fund its operations for at least the next 12 months. However, drug development is expensive and
time-consuming and if Celladon undertakes development efforts, it will need to raise substantial additional capital to fund future activities.
Any
additional fundraising efforts may divert Celladon’s management from their day-to-day activities, which may adversely affect its ability to develop and commercialize product candidates. In addition, it cannot guarantee that future financing
will be available in sufficient amounts or on terms acceptable to Celladon, if at all. It could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than would otherwise be ideal and it may
be required to relinquish rights to some of its technologies, product candidates, or otherwise agree to terms unfavorable to it, any of which may have a material adverse effect on its business, operating results and prospects.
If Celladon is unable to obtain funding on a timely basis, it may be required to significantly curtail, delay or discontinue one or more of its research or
development programs or the commercialization of any approved products, or be unable to deploy the capital necessary to refocus or expand its operations or otherwise capitalize on its business opportunities, as desired, any of which could materially
adversely affect its business, financial condition and results of operations and could even require it to cease operations entirely.
33
Celladon has never generated any revenue from product sales and may never be profitable.
Celladon’s ability to generate meaningful revenue and achieve profitability depends on its ability, and the ability of any third party with which it may
partner, to successfully complete the development of, and obtain the regulatory approvals necessary to, commercialize product candidates. Celladon does not anticipate generating revenues from product sales for the foreseeable future, if ever. If any
future product candidates fail in clinical trials or do not gain regulatory approval, or if any product candidates, if approved, fail to achieve market acceptance, Celladon may never become profitable. Even if it achieves profitability in the
future, Celladon may not be able to sustain profitability in subsequent periods. Celladon’s ability to generate future revenues from product sales will depend heavily on its success in:
| |
• |
|
completing research and preclinical and clinical development of product candidates; |
| |
• |
|
seeking and obtaining regulatory and marketing approvals for product candidates for which it completes clinical trials; |
| |
• |
|
developing a sustainable, scalable, reproducible, and transferable manufacturing process for product candidates; |
| |
• |
|
establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products and services to support clinical development and, if approved, the market
demand for its product candidates; |
| |
• |
|
launching and commercializing product candidates for which it obtains regulatory and marketing approval, either by establishing a sales force, marketing and distribution infrastructure, or by collaborating with a
partner; |
| |
• |
|
obtaining market acceptance of any approved products; |
| |
• |
|
addressing any competing technological and market developments; |
| |
• |
|
implementing additional internal systems and infrastructure, as needed; |
| |
• |
|
identifying and validating new product candidates; |
| |
• |
|
negotiating favorable terms in any collaboration, licensing or other arrangements into which it may enter; |
| |
• |
|
maintaining, protecting and expanding its portfolio of intellectual property rights, including patents, trade secrets and know-how; and |
| |
• |
|
attracting, hiring and retaining qualified personnel. |
Even if one or more product candidates is approved for
commercial sale, Celladon anticipates incurring significant costs associated with commercializing any approved product. Its expenses could increase beyond expectations if it is required by the U.S. Food and Drug Administration, or FDA, the European
Medicines Agency, or EMA, or other foreign regulatory authorities to perform clinical trials and other studies in addition to those that it originally anticipated. Even if it is able to generate revenues from the sale of any approved products,
Celladon may not become profitable and may need to obtain additional funding to continue operations.
Raising additional funds through debt or
equity financing is likely to be difficult, could be dilutive and may cause the market price of Celladon’s common stock to decline further.
To the extent that Celladon raises additional capital through the sale of equity or convertible debt securities, the issuance of those securities could result
in substantial dilution for Celladon’s current stockholders and the terms may include liquidation or other preferences that adversely affect the rights of its current stockholders. Furthermore, the issuance of additional securities, whether
equity or debt, by Celladon, or the possibility of such issuance, may cause the market price of its common stock to decline further and existing stockholders may not agree with its financing plans or the terms of such financings.
34
Even if Celladon resumes or initiates and completes any necessary preclinical studies and clinical trials
for any product candidates it may choose to develop, it cannot predict when, or if, it will obtain regulatory approval to commercialize a product candidate or the approval may be for a more narrow indication than Celladon expects.
Even if Celladon resumes or initiates any necessary preclinical studies and clinical trials for any product candidates it may choose to develop, it cannot
commercialize a product until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if Celladon’s product candidates demonstrate safety and efficacy in clinical trials, the regulatory agencies may not
complete their review processes in a timely manner, or Celladon may not be able to obtain regulatory approval. Additional delays may result if an FDA advisory committee or other regulatory authority recommends non-approval or restrictions on
approval. In addition, Celladon may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development,
clinical trials and the review process. Regulatory agencies also may approve a treatment candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition,
regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of Celladon’s treatment candidates.
Even if Celladon obtains regulatory approval for a product candidate, its products will remain subject to regulatory scrutiny.
Even if Celladon obtains regulatory approval in a jurisdiction, regulatory authorities may still impose significant restrictions on the indicated uses or
marketing of its product candidates, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance.
In
addition, product manufacturers and their facilities are subject to payment of user fees and continual review and periodic inspections by the FDA and other regulatory authorities. If Celladon or a regulatory agency discovers previously unknown
problems with a product, such as adverse events of unanticipated severity or frequency or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing
facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If Celladon fails to comply with
applicable regulatory requirements following approval of any of its product candidates, a regulatory agency may:
| |
• |
|
issue a warning letter asserting that Celladon is in violation of the law; |
| |
• |
|
seek an injunction or impose civil or criminal penalties or monetary fines; |
| |
• |
|
suspend or withdraw regulatory approval; |
| |
• |
|
suspend any ongoing clinical trials; |
| |
• |
|
refuse to approve a pending Biologic License Application or supplements to a Biologic License Application submitted by Celladon; |
| |
• |
|
refuse to allow Celladon to enter into supply contracts, including government contracts. |
Any government
investigation of alleged violations of law could require Celladon to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit Celladon’s ability
to commercialize its product candidates and generate revenues.
35
Risks Related to Celladon’s Reliance on Third Parties
Celladon relies on third parties to conduct, supervise and monitor its business operations. If these third parties do not successfully carry out their
contractual duties or meet expected deadlines, Celladon may not be able to conduct its business and operations and its status as a publicly traded company could be substantially harmed.
Celladon has historically relied on third party vendors for its development work, and since the termination of MYDICAR as a product candidate and the
announcement of the merger, certain administrative functions. Celladon’s vendors are not its employees, and there can be no assurance of any ability to directly monitor whether or not the vendors devote sufficient time and resources in
furtherance of the company. These vendors may also have relationships with other parties that receive priority over the activities of Celladon. If its vendors do not successfully carry out their contractual duties or obligations, fail to meet
expected deadlines, or if the quality or accuracy of the work required to complete the merger and maintain Celladon’s status as a NASDAQ-listed company is not sufficient, its financial results and the commercial prospects for its business would
be harmed, its costs to regain compliance could increase, and its ability to identify alternatives for its business and generate revenues would be delayed or significantly impaired.
Healthcare reform measures may have a material adverse effect on Celladon’s business and results of operations.
In the United States, the legislative landscape continues to evolve. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health
Care and Education Reconciliation Act, or the Affordable Care Act, was passed, which has the potential to substantially change health care financing by both governmental and private insurers, and significantly impact the U.S. pharmaceutical
industry. The Affordable Care Act, among other things, subjects biological products to potential competition by lower-cost biosimilars, revised the methodology by which rebates owed by manufacturers for covered outpatient drugs under the Medicaid
Drug Rebate Program are calculated, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate Program to utilization of prescriptions of individuals enrolled in
Medicaid managed care organizations, subjected manufacturers to new annual fees and taxes for certain branded prescription drugs, and provided incentives to programs that increase the federal government’s comparative effectiveness research.
In addition, other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. On August 2,
2011, the Budget Control Act of 2011 among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the
years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers of up to 2% per
fiscal year, which went into effect on April 1, 2013 and will remain in effect through 2024 unless additional Congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or
the ATRA, which, among other things, further reduced Medicare payments to certain providers, including physicians, hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover
overpayments to providers from three to five years. Celladon expects that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for
healthcare products and services, which could result in reduced demand for any product candidates Celladon seeks to develop or additional pricing pressures.
Risks Related to Celladon’s Business Operations
Celladon is the subject of securities class action lawsuits, and additional securities litigation may be brought against it in the future.
In July 2015, following Celladon’s announcements of the negative CUPID 2 data and the suspension of further research and development activities and the
subsequent declines of the price of its common stock, three putative
36
class actions were filed in the U.S. District Court for the Southern District of California against Celladon and certain of its current and former officers. The complaints generally allege that
the defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, by making materially false and misleading statements regarding the clinical trial program for MYDICAR, thereby artificially
inflating the price of Celladon’s common stock. The complaints seek unspecified monetary damages and other relief, including attorneys’ fees. On September 1, 2015, six stockholders (or groups of stockholders) filed motions to
consolidate the three putative securities class actions and to appoint lead plaintiffs (the “Motions to Consolidate”). A hearing on the Motions to Consolidate was held on December 3, 2015. On December 9, 2015, the Court
consolidated the three putative securities class actions and appointed a lead plaintiff to represent the putative class. Celladon expects the lead plaintiff to file a consolidated complaint. It is possible that additional suits will be filed, or
allegations made by stockholders, with respect to these same or other matters and also naming Celladon and/or its officers and directors as defendants. Celladon believes that it has meritorious defenses and intends to defend these lawsuits
vigorously. Due to the early stage of these proceedings, Celladon is not able to predict or reasonably estimate the ultimate outcome or possible losses relating to these claims. While Celladon has directors’ and officers’ liability
insurance, there is no assurance that the coverage will be sufficient. In addition, any such litigation could result in substantial costs and a diversion of management’s attention and resources, which could harm its business.
Celladon’s employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including
non-compliance with regulatory standards and requirements and insider trading.
Celladon is exposed to the risk of fraudulent conduct or other
illegal activity by its employees, independent contractors, principal investigators, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with
the regulations of the FDA and non-U.S. regulators, provide accurate information to the FDA and non-U.S. regulators, comply with healthcare fraud and abuse laws in the United States and abroad, report financial information or data accurately or
disclose unauthorized activities to Celladon. In particular, promotion, sales, marketing and certain business arrangements in the healthcare industry are subject to extensive laws intended to prevent fraud, misconduct, kickbacks, self-dealing and
other abusive practices. These laws may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the
improper use of information obtained in the course of patient recruitment or clinical trials, which could result in regulatory sanctions and cause serious harm to Celladon’s reputation. Celladon has adopted a code of business conduct and ethics
applicable to all of its employees, but it is not always possible to identify and deter employee misconduct, and the precautions Celladon takes to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or
losses or in protecting it from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against Celladon, and it is not successful in defending
itself or asserting its rights, those actions could have a significant impact on its business, including the imposition of significant fines or other sanctions.
Celladon faces potential product liability, and, if successful claims are brought against it, it may incur substantial liability and costs. If the use
or misuse of Celladon’s product candidates harms patients, or is perceived to harm patients even when such harm is unrelated to its product candidates, its regulatory approvals could be revoked or otherwise negatively impacted and it could be
subject to costly and damaging product liability claims.
The use or misuse of Celladon’s product candidates in clinical trials and the sale
of any products for which it obtains marketing approval exposes Celladon to the risk of product liability claims. Product liability claims might be brought against Celladon by consumers, healthcare providers, pharmaceutical companies or others
selling or otherwise coming into contact with its products. There is a risk that its product candidates may induce adverse
37
events. If Celladon cannot successfully defend against product liability claims, it could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product
liability claims may result in:
| |
• |
|
impairment of Celladon’s business reputation; |
| |
• |
|
initiation of investigations by regulators; |
| |
• |
|
withdrawal of clinical trial participants; |
| |
• |
|
costs due to related litigation; |
| |
• |
|
distraction of management’s attention from Celladon’s primary business; |
| |
• |
|
substantial monetary awards to patients or other claimants; |
| |
• |
|
the inability to commercialize Celladon’s product candidates; |
| |
• |
|
product recalls, withdrawals or labeling, marketing or promotional restrictions; and |
| |
• |
|
decreased demand for Celladon’s product candidates, if approved for commercial sale. |
Celladon carries
product liability insurance of $10.0 million per occurrence and a $10.0 million aggregate limit. Celladon believes its product liability insurance coverage is appropriate in light of its current clinical programs; however, it may not be able to
maintain insurance coverage at a reasonable cost or in sufficient amounts to protect itself against losses due to liability. If and when Celladon obtains marketing approval for product candidates, it intends to expand its insurance coverage to
include the sale of commercial products; however, it may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded in class action lawsuits based on drugs
or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against Celladon could cause its stock price to decline and, if judgments exceed its insurance coverage, could adversely
affect its results of operations and business.
Patients with the diseases targeted by Celladon’s product candidates are often already in severe and
advanced stages of disease and have both known and unknown significant pre-existing and potentially life-threatening health risks. During the course of treatment, patients may suffer adverse events, including death, for reasons that may or may not
be related to its product candidates. Such events could subject Celladon to costly litigation, require it to pay substantial amounts of money to injured patients, delay, negatively impact or end its opportunity to receive or maintain regulatory
approval to market its products, or require it to suspend or abandon its commercialization efforts. Even in a circumstance in which Celladon does not believe that an adverse event is related to its products, the investigation into the circumstance
may be time-consuming or inconclusive. These investigations may interrupt its sales efforts, delay its regulatory approval process in other countries, or impact and limit the type of regulatory approvals its product candidates receive or maintain.
As a result of these factors, a product liability claim, even if successfully defended, could have a material adverse effect on its business, financial condition or results of operations.
Celladon may not be successful in identifying or discovering additional product candidates.
Celladon’s research programs, if resumed, may fail to identify other potential product candidates for clinical development for a number of reasons. For
example, Celladon’s research methodology may be unsuccessful in identifying potential product candidates or its potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products
unmarketable or unlikely to receive marketing approval.
If any of these events occur, Celladon may be forced to abandon its development efforts for a
program or programs, which may have a material adverse effect on its business and could potentially cause Celladon to cease operations. Research programs to identify new product candidates require substantial technical, financial and human
resources. Celladon may focus its efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
38
Unfavorable global economic conditions could adversely affect Celladon’s business, financial condition
or results of operations.
Celladon’s results of operations could be adversely affected by general conditions in the global economy and in the
global financial markets. The recent global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn, such as the recent global financial crisis, could result in a variety
of risks to Celladon’s business, including, weakened demand for its product candidates and a decreased ability to raise additional capital when needed on acceptable terms, if at all. This is particularly true in Europe, which is undergoing a
continued severe economic crisis. A weak or declining economy could also strain Celladon’s suppliers, possibly resulting in supply disruption, or cause its customers to delay making payments for its services. Any of the foregoing could harm
Celladon’s business and it cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact its business.
Celladon’s business and operations would suffer in the event of system failures.
Despite the implementation of security measures, Celladon’s internal computer systems and those of its current and any future contract research
organizations, or CROs, and other contractors, consultants and potential collaborators are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While
Celladon has not experienced any such material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in its operations, it could result in a material disruption of its development programs and
its business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in regulatory approval efforts and significantly increase Celladon’s costs to recover or reproduce the data.
To the extent that any disruption or security breach were to result in a loss of, or damage to, Celladon’s data or applications, or inappropriate disclosure of confidential or proprietary information, Celladon could incur liability and the
further development and commercialization of product candidates could be delayed.
Interruptions in the supply of product or inventory loss may
adversely affect Celladon’s operating results and financial condition.
Many product candidates are manufactured and distributed using
technically complex processes requiring specialized facilities, highly specific raw materials and other production constraints. The complexity of these processes, as well as strict company and government standards for the manufacture and storage of
Celladon’s products, subjects Celladon to production risks. While product batches released for use in clinical trials or for commercialization undergo sample testing, some defects may only be identified following product release. In addition,
process deviations or unanticipated effects of approved process changes may result in these intermediate products not complying with stability requirements or specifications. Most of Celladon’s product candidates must be stored and transported
at temperatures within a certain range. If these environmental conditions deviate, Celladon’s product candidates’ remaining shelf-lives could be impaired or their efficacy and safety could become adversely affected, making them no longer
suitable for use. The occurrence or suspected occurrence of production and distribution difficulties can lead to lost inventories, and in some cases product recalls, with consequential reputational damage and the risk of product liability. The
investigation and remediation of any identified problems can cause production delays, substantial expense, lost sales and delays of new product launches. Any interruption in the supply of finished products or the loss thereof could hinder
Celladon’s ability to timely distribute its products and satisfy customer demand. Any unforeseen failure in the storage of the product or loss in supply could delay its clinical trials and, if its product candidates are approved, result in a
loss of its market share and negatively affect its revenues and operations.
39
Celladon or the third parties upon whom it depends may be adversely affected by earthquakes or other
natural disasters and its business continuity and disaster recovery plans may not adequately protect Celladon from a serious disaster.
Earthquakes
or other natural disasters could severely disrupt Celladon’s operations, and have a material adverse effect on its business, results of operations, financial condition and prospects. A majority of Celladon’s management operates in its
principal executive offices located in San Diego, California. If Celladon’s San Diego offices were affected by a natural or man-made disaster, particularly those that are characteristic of the region, such as wildfires and earthquakes, or other
business interruption, its ability to manage its domestic and foreign operations could be impaired, which could materially and adversely affect its results of operations and financial condition. If a natural disaster, power outage or other event
occurred that prevented Celladon from using all or a significant portion of its headquarters, that damaged critical infrastructure, such as the manufacturing facilities of Celladon’s third-party contract manufacturers, or that otherwise
disrupted operations, it may be difficult or, in certain cases, impossible for Celladon to continue its business for a substantial period of time. The disaster recovery and business continuity plans Celladon has in place currently are limited and
are unlikely to prove adequate in the event of a serious disaster or similar event. Celladon may incur substantial expenses as a result of the limited nature of its disaster recovery and business continuity plans, which, particularly when taken
together with its lack of earthquake insurance, could have a material adverse effect on its business.
Risks Related to
Celladon’s Intellectual Property
If Celladon is unable to obtain or protect intellectual property rights related to any future product
candidates, Celladon may not be able to compete effectively in its markets.
Biotechnology companies typically rely upon a combination of patents,
trade secret protection and confidentiality agreements to protect the intellectual property related to product candidates. The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can
be uncertain. The patent applications that Celladon owns or in-licenses may fail to result in issued patents with claims that cover its product candidates in the United States or in other foreign countries. There is no assurance that all of the
potentially relevant prior art relating to Celladon’s patents and patent applications will be found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and
even if such patents cover Celladon’s product candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated. Furthermore, even if they are unchallenged,
Celladon’s patents and patent applications may not adequately protect its intellectual property, provide exclusivity for its product candidates or prevent others from designing around its claims.
If the patent applications Celladon holds or in-licenses with respect to its programs and product candidates fail to issue, if their breadth or strength of
protection is threatened, or if they fail to provide meaningful exclusivity for Celladon’s product candidates, it could dissuade companies from collaborating with Celladon to develop product candidates, and threaten Celladon’s ability to
commercialize future products. Celladon cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties.
Any successful opposition to patents owned by or licensed to Celladon could deprive it of rights necessary for the successful commercialization of any product candidates that it may develop. Further, if it encounters delays in regulatory approvals,
the period of time during which Celladon could market a product candidate under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some
remain so until issued, Celladon cannot be certain that it was the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United
States can be initiated by a third party to determine who was the first to invent any of the subject matter covered by the patent claims of Celladon’s applications. In addition, patents have a limited lifespan. In the United States, the natural
expiration of a patent is generally 20 years after it is filed. Various extensions may be available; however the life of a patent, and the protection it
40
affords, is limited. Even if patents covering Celladon’s product candidates are obtained, once the patent life has expired for a product, Celladon may be open to competition from generic
medications.
In addition to the protection afforded by patents, Celladon relies on trade secret protection and confidentiality agreements to protect
proprietary know-how that is not patentable or that it elects not to patent, processes for which patents are difficult to enforce and any other elements of its product candidates discovery and development processes that involve proprietary know-how,
information or technology that is not covered by patents. However, trade secrets can be difficult to protect. Celladon seeks to protect its proprietary technology and processes, in part, by entering into confidentiality agreements with those who
have access to its confidential information, including employees, consultants, scientific advisors and contractors. Celladon also seeks to preserve the integrity and confidentiality of its data and trade secrets by maintaining physical security of
its premises and physical and electronic security of its information technology systems. While Celladon has confidence in these individuals, organizations and systems, agreements or security measures may be breached, and Celladon may not have
adequate remedies for any breach. In addition, Celladon’s trade secrets may otherwise become known or be independently discovered by competitors.
Although Celladon expects all of its employees and consultants to assign their inventions to Celladon, and all of its employees, consultants, advisors and any
third parties who have access to its proprietary know-how, information or technology to enter into confidentiality agreements, Celladon cannot provide any assurances that all such agreements have been duly executed or that its trade secrets and
other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to its trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized
disclosure of Celladon’s trade secrets could impair its competitive position and may have a material adverse effect on its business. Additionally, if the steps taken to maintain its trade secrets are deemed inadequate, Celladon may have
insufficient recourse against third parties for misappropriating the trade secret. In addition, others may independently discover Celladon’s trade secrets and proprietary information. For example, the FDA, as part of its Transparency
Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that Celladon may consider to be trade secrets or other proprietary information, and it is not clear at the
present time how the FDA’s disclosure policies may change in the future, if at all. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a
result, Celladon may encounter significant problems in protecting and defending its intellectual property both in the United States and abroad. If Celladon is unable to prevent material disclosure of the non-patented intellectual property related to
its technologies to third parties, and there is no guarantee that Celladon will have any such enforceable trade secret protection, Celladon may not be able to establish or maintain a competitive advantage in its market, which could materially
adversely affect its business, results of operations and financial condition.
Third-party claims of intellectual property infringement may prevent
or delay Celladon’s development and commercialization efforts.
Celladon’s commercial success depends in part on avoiding infringement of
the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries,
including patent infringement lawsuits, interferences, oppositions and inter partes review proceedings before the U.S. Patent and Trademark Office, or U.S. PTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued
patents and pending patent applications, which are owned by third parties, exist in the fields in which Celladon is pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk
increases that Celladon’s product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may
assert that Celladon is employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture
41
or methods for treatment related to the use or manufacture of its product candidates. Because patent applications can take many years to issue, third parties may have currently pending patent
applications which may later result in issued patents that Celladon’s product candidates may infringe, or which such third parties claim are infringed by the use of Celladon’s technologies. If any third-party patents are held by a court of
competent jurisdiction to cover any aspect of the manufacturing process for any of Celladon’s product candidates, any molecules formed during the manufacturing process, or any final product candidate, including the formulation or method of use
of such product candidate, the holders of any such patents may be able to block Celladon’s ability to commercialize such product candidate unless Celladon obtained a license under the applicable patents, or until such patents expire. In any
such case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against Celladon for infringement of
their intellectual property rights may obtain injunctive or other equitable relief, which could effectively block Celladon’s ability to further develop and commercialize one or more of its product candidates. Defense of these claims, regardless
of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from Celladon’s business. In the event of a successful claim of infringement against Celladon, it could be required to
redesign its infringing products or obtain a license from such third party to continue developing and commercializing its products and technology. However, Celladon may not be able to obtain any required license on commercially reasonable terms, or
at all. Even if Celladon is able to obtain a license, it may be non-exclusive, thereby giving its competitors access to the same technologies licensed to Celladon. It may be impossible to redesign Celladon’s products and technology, or it may
require substantial time and monetary expenditure, which could force Celladon to cease commercialization of one or more of its product candidates, or some of its business operations, which could materially harm its business. In addition, in any such
proceeding, Celladon may be required to pay substantial damages, including treble damages and attorneys’ fees in the event Celladon is found liable for willful infringement.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of Celladon’s patent applications and the
enforcement or defense of its issued patents.
In September 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law.
The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The U.S. PTO is currently developing regulations
and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, were enacted in March 2013. It is not clear what,
if any, impact the Leahy-Smith Act will have on the operation of Celladon’s business. Moreover, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of Celladon’s patent
applications and the enforcement or defense of its issued patents, all of which could have a material adverse effect on Celladon’s business and financial condition.
Celladon may be subject to claims that its employees, consultants or independent contractors have wrongfully used or disclosed confidential information
of third parties or that Celladon’s employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Although
Celladon seeks to protect its ownership of intellectual property rights by ensuring that its agreements with its employees, collaborators and other third parties with whom it does business include provisions requiring such parties to assign rights
in inventions to Celladon, Celladon may also be subject to claims that former employees, collaborators or other third parties have an ownership interest in its patents or other intellectual property. Celladon may be subject to ownership disputes in
the future arising, for example, from conflicting obligations of consultants or others who are involved in developing its product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership.
If Celladon fails in defending any such claims, in addition to paying monetary damages, Celladon may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome
42
could have a material adverse effect on Celladon’s business. Even if Celladon is successful in defending against such claims, litigation could result in substantial costs and be a
distraction to management and other employees.
Celladon may be subject to claims challenging the inventorship or ownership of its patents and other
intellectual property.
Celladon may also be subject to claims that former employees, collaborators or other third parties have an ownership
interest in its patents or other intellectual property. Celladon may be subject to ownership disputes in the future arising, for example, from conflicting obligations of consultants or others who are involved in developing its product candidates.
Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If Celladon fails in defending any such claims, in addition to paying monetary damages, Celladon may lose valuable intellectual property
rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on Celladon’s business. Even if Celladon is successful in defending against such claims, litigation
could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining Celladon’s patent
protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and Celladon’s patent protection could be reduced or eliminated for non-compliance with
these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will
be due to be paid to the U.S. PTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. Celladon has systems in place to remind it to pay these fees, and it
employs an outside firm and relies on its outside counsel to pay these fees due to non-U.S. patent agencies. The U.S. PTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and
other similar provisions during the patent application process. Celladon employs reputable law firms and other professionals to help it comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in
accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
In such an event, Celladon’s competitors might be able to enter the market and this circumstance would have a material adverse effect on its business.
Risks Related to Ownership of Celladon’s Common Stock
The market price of Celladon’s common stock may continue to be highly volatile, and you may not be able to resell some or all of your shares at a
desired market price.
The market price of Celladon’s common stock has been and is likely to continue to be volatile. Since
Celladon’s initial public offering in January 2014 at a price of $8.00 per share, the sale price of stock as reported on The NASDAQ Global Market has ranged from $1.00 to $28.25, through December 31, 2015. Celladon’s stock price could
be subject to wide fluctuations in response to a variety of factors, including the following:
| |
• |
|
announcements related to the merger; |
| |
• |
|
adverse results or delays in preclinical studies or clinical trials; |
| |
• |
|
Celladon’s decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial; |
| |
• |
|
announcements of significant changes in Celladon’s business or operations, including the decision not to pursue one or more drug development programs; |
| |
• |
|
unanticipated serious safety concerns related to the use of any of Celladon’s product candidates; |
| |
• |
|
inability to obtain additional funding; |
43
| |
• |
|
sales or potential sales of Celladon’s common stock by Celladon or Celladon’s stockholders in the future; |
| |
• |
|
failure to successfully develop, manufacture and commercialize Celladon’s product candidates; |
| |
• |
|
failure to enter into collaborations; |
| |
• |
|
failure by Celladon or its licensors to prosecute, maintain or enforce intellectual property rights; |
| |
• |
|
Celladon’s dependence on third parties, including, commercial manufactures and CROs; |
| |
• |
|
changes in laws or regulations applicable to future products; |
| |
• |
|
inability to obtain adequate clinical and commercial product supply for Celladon’s product candidates or the inability to do so at acceptable prices; |
| |
• |
|
adverse regulatory decisions; |
| |
• |
|
introduction of new products, services or technologies by Celladon’s competitors; |
| |
• |
|
failure to meet or exceed financial projections Celladon may provide to the public; |
| |
• |
|
failure to meet or exceed the financial projections of the investment community; |
| |
• |
|
the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community; |
| |
• |
|
announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by Celladon or Celladon’s competitors; |
| |
• |
|
announcement of a strategic transaction, including the acquisition of Celladon or its assets; |
| |
• |
|
disputes or other developments relating to proprietary rights, including patents, litigation matters and Celladon’s ability to obtain patent protection for its technologies; |
| |
• |
|
additions or departures of key personnel; |
| |
• |
|
significant lawsuits, including patent or stockholder litigation; |
| |
• |
|
changes in the market valuations of similar companies; |
| |
• |
|
overall performance of the equity markets and other factors that may be unrelated to Celladon’s operating performance or the operating performance of Celladon’s competitors, including changes in market
valuations of similar companies; and |
| |
• |
|
trading volume of Celladon’s common stock. |
In addition, companies trading in the stock market in
general, and the NASDAQ Global Market in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may
negatively affect the market price of Celladon’s common stock, regardless of its actual operating performance.
If securities or industry
analysts issue an adverse or misleading opinion regarding Celladon’s stock, Celladon’s stock price and trading volume could decline.
The
trading market for Celladon’s common stock will be influenced by the research and reports that industry or securities analysts publish about Celladon or its business. If any of the analysts who cover Celladon issue an adverse or misleading
opinion regarding Celladon, its business model, its intellectual property or its stock performance, or if Celladon’s clinical trials and operating results fail to meet the expectations of analysts, its stock price would likely decline. If one
or more of these analysts cease coverage of Celladon or fail to publish reports on it regularly, Celladon could lose visibility in the financial markets, which in turn could cause its stock price or trading volume to decline.
44
Celladon is an “emerging growth company,” and cannot be certain if the reduced reporting
requirements applicable to emerging growth companies will make its common stock less attractive to investors.
Celladon is an “emerging growth
company,” as defined in the JOBS Act. For as long as Celladon continues to be an emerging growth company, it may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not
“emerging growth companies,” including exemption from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley Act), reduced disclosure obligations regarding executive
compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation. Celladon will remain an emerging growth company until the earlier of (1) the last
day of the fiscal year (a) following the fifth anniversary of the completion of its initial public offering, (b) in which it has total annual gross revenue of at least $1.0 billion, or (c) in which it is deemed to be a large
accelerated filer, which means the market value of Celladon’s common stock that is held by non-affiliates exceeded $700.0 million as of the prior June 30th, and (2) the date on which Celladon has issued more than $1.0 billion in
non-convertible debt during the prior three-year period.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting
standards until such time as those standards apply to private companies. Celladon has irrevocably elected not to avail itself of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised
accounting standards as other public companies that are not emerging growth companies.
Celladon’s quarterly operating results may fluctuate
significantly or may fall below the expectations of investors or securities analysts, each of which may cause its stock price to fluctuate or decline.
Celladon expects its operating results to be subject to quarterly fluctuations. Celladon’s net loss and other operating results will be affected by
numerous factors, including:
| |
• |
|
variations in the level of expenses related to its product candidates, future development programs or general and administrative expenses (including as the result of a significant decrease or increase in employee
headcount); |
| |
• |
|
if any product candidates receives regulatory approval, the level of underlying demand for these product candidates and wholesalers’ buying patterns; |
| |
• |
|
addition or termination of clinical trials or funding support; |
| |
• |
|
Celladon’s execution of any collaborative, licensing or similar arrangements, and the timing of payments Celladon may make or receive under these arrangements; |
| |
• |
|
any intellectual property infringement lawsuit in which Celladon may become involved; and |
| |
• |
|
regulatory developments affecting its product candidates or those of its competitors. |
If Celladon’s
quarterly operating results fall below the expectations of investors or securities analysts, the price of its common stock could decline substantially. Furthermore, any quarterly fluctuations in its operating results may, in turn, cause the price of
its stock to fluctuate substantially. Celladon believes that quarterly comparisons of its financial results are not necessarily meaningful and should not be relied upon as an indication of its future performance.
If Celladon fails to maintain an effective system of internal control over financial reporting, it may not be able to accurately report its financial
results or prevent fraud. As a result, stockholders could lose confidence in its financial and other public reporting, which would harm its business and the trading price of its common stock.
Effective internal controls over financial reporting are necessary for Celladon to provide reliable financial reports and, together with adequate disclosure
controls and procedures, are designed to prevent fraud. Any failure to
45
implement required new or improved controls, or difficulties encountered in their implementation could cause Celladon to fail to meet its reporting obligations. In addition, any testing by
Celladon conducted in connection with Section 404 of the Sarbanes-Oxley Act, or the subsequent testing by Celladon’s independent registered public accounting firm, may reveal deficiencies in its internal controls over financial reporting
that are deemed to be material weaknesses or that may require prospective or retroactive changes to its consolidated financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause
investors to lose confidence in Celladon’s reported financial information, which could have a negative effect on the trading price of its common stock.
Sales of a substantial number of shares of Celladon’s common stock in the public market could cause its stock price to fall.
Sales of a substantial number of shares of Celladon’s common stock in the public market or the perception that these sales might occur, could depress the
market price of Celladon’s common stock and could impair its ability to raise capital through the sale of additional equity securities. Celladon is unable to predict the effect that sales may have on the prevailing market price of its common
stock.
Certain holders of Celladon’s securities are entitled to rights with respect to the registration of their shares under the Securities Act.
Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by Celladon’s affiliates as defined in Rule 144 under the Securities
Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of Celladon’s common stock.
Future sales and issuances of Celladon’s common stock or rights to purchase common stock, including pursuant to its equity incentive plans, could
result in additional dilution of the percentage ownership of Celladon’s stockholders and could cause its stock price to fall.
Celladon
expects that significant additional capital will be needed in the future to continue operations, should it decide to continue its historical business operations. To the extent Celladon raises additional capital by issuing equity securities, its
stockholders may experience substantial dilution. Celladon may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner it determines from time to time. If Celladon sells common
stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. These sales may also result in material dilution to its existing stockholders, and new investors could
gain rights superior to existing stockholders.
Pursuant to Celladon’s 2013 equity incentive plan, or the 2013 plan, Celladon’s management is
authorized to grant stock options and other equity-based awards to its employees, directors and consultants. The number of shares available for future grant under the 2013 plan will automatically increase on January 1 of each year by 5% of the
total number of shares of Celladon’s common stock outstanding on December 31 of the preceding calendar year, subject to the ability of Celladon’s board of directors to take action to reduce the size of the increase in any given year.
In addition, Celladon may grant or provide for the grant of rights to purchase shares of its common stock pursuant to its 2013 employee stock purchase plan, or ESPP. The number of shares of Celladon’s common stock reserved for issuance under
the ESPP will automatically increase on January 1 of each calendar year by the lesser of 1% of the total number of shares of its common stock outstanding on December 31 of the preceding calendar year and 384,307 shares, subject to the
ability of Celladon’s board of directors to take action to reduce the size of the increase in any given year. Currently, Celladon plans to register the increased number of shares available for issuance under the 2013 plan and ESPP each year.
Increases in the number of shares available for future grant or purchase may result in additional dilution, which could cause Celladon’s stock price to decline. In addition, Celladon has in the past and may in the future grant inducement grants
to prospective employees and consultants, which may result in further dilution and cause Celladon’s stock price to decline.
46
Celladon does not intend to pay dividends on its common stock so any returns will be limited to the value
of its stock.
Celladon has never declared or paid any cash dividends on its common stock. Celladon currently anticipates that it will retain
future earnings for the development, operation and expansion of its business and does not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, Celladon’s ability to pay dividends is currently restricted by
the terms of its loan agreement with Hercules. Any return to stockholders will therefore be limited to the appreciation of their stock.
Provisions
in Celladon’s amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire Celladon or increase the cost of acquiring it, even if doing so would
benefit Celladon’s stockholders or remove its current management.
Some provisions of Celladon’s charter documents and Delaware law may
have anti-takeover effects that could discourage an acquisition of Celladon by others, even if an acquisition would be beneficial to its stockholders and may prevent attempts by Celladon’s stockholders to replace or remove current management.
These provisions include:
| |
• |
|
authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
| |
• |
|
limiting the removal of directors by the stockholders; |
| |
• |
|
creating a staggered board of directors; |
| |
• |
|
prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of Celladon’s stockholders; |
| |
• |
|
eliminating the ability of stockholders to call a special meeting of stockholders; and |
| |
• |
|
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
These provisions may frustrate or prevent any attempts by Celladon’s stockholders to replace or remove current management by making it more difficult for
stockholders to replace members of the board of directors, which is responsible for appointing the members of management. In addition, Celladon is subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a
Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are
approved by Celladon’s board of directors. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to Celladon’s stockholders. Further, other provisions of Delaware
law may also discourage, delay or prevent someone from acquiring Celladon or merging with it.
Risks Related to Eiger’s
Financial Condition and Capital Requirements
Eiger has incurred losses since its inception, has a limited operating history on which to assess
its business, and anticipates that it will continue to incur significant losses for the foreseeable future.
Eiger is a clinical development-stage
biopharmaceutical company with a limited operating history. Eiger has incurred net losses in each year since its inception in 2008, including net losses of $1.5 million and $1.0 million for the years ended December 31, 2014 and 2013,
respectively, and $6.3 million for the nine months ended September 30, 2015. As of September 30, 2015, Eiger had an accumulated deficit of $22.2 million.
The audit report on Eiger’s consolidated financial statements for the year ended December 31, 2014, which appears elsewhere herein, includes an
explanatory paragraph related to Eiger’s ability to continue as a going concern. As of September 30, 2015, Eiger had cash of $2.1 million. In November 2015, Eiger received
47
$6.0 million in financing under bridge promissory notes which is due and payable on March 31, 2016. Eiger’s current capital resources, if the transactions contemplated by this
proxy statement/prospectus/information statement are not completed, are insufficient to fund its planned operations for a 12-month period, and therefore, raise substantial doubt about its ability to continue as a going concern. Eiger will continue
to require substantial additional capital to continue its clinical development and potential commercialization activities. Accordingly, Eiger will need to raise substantial additional capital to continue to fund its operations. The amount and timing
of its future funding requirements will depend on many factors, including the pace and results of its clinical development efforts. Failure to raise capital as and when needed, on favorable terms or at all, would have a negative impact on its
financial condition and its ability to develop its product candidates.
Eiger has devoted substantially all of its financial resources to identify,
acquire, and develop its product candidates, including conducting clinical studies and providing general and administrative support for its operations. To date, Eiger has financed its operations primarily through the sale of equity securities and
convertible promissory notes. The amount of its future net losses will depend, in part, on the rate of its future expenditures and its ability to obtain funding through equity or debt financings, strategic collaborations, or grants.
Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. Eiger expects losses to increase as it advances three clinical candidates into Phase 2 development for potentially four indications.
While Eiger has not yet commenced pivotal clinical studies for any product candidate and it may be several years, if ever, before Eiger completes pivotal clinical studies and has a product candidate approved for commercialization, Eiger expects to
invest significant funds into these clinical candidates to determine the potential to advance these compounds to regulatory approval.
If Eiger obtains
regulatory approval to market a product candidate, its future revenue will depend upon the size of any markets in which its product candidates may receive approval, and its ability to achieve sufficient market acceptance, pricing, reimbursement from
third-party payors, and adequate market share for its product candidates in those markets. Even if Eiger obtains adequate market share for its product candidates, because the potential markets in which its product candidates may ultimately receive
regulatory approval could be very small, Eiger may never become profitable despite obtaining such market share and acceptance of its products.
Eiger
expects to continue to incur significant expenses and increasing operating losses for the foreseeable future and its expenses will increase substantially if and as Eiger:
| |
• |
|
continues the clinical development of its product candidates; |
| |
• |
|
undertakes the manufacturing or has manufactured its product candidates; |
| |
• |
|
advances its programs into larger, more expensive clinical studies; |
| |
• |
|
initiates additional nonclinical, clinical, or other studies for its product candidates; |
| |
• |
|
identifies, educates and develops potential commercial opportunities, such as hepatitis D virus biology for its lonafarnib product candidate; |
| |
• |
|
seeks regulatory and marketing approvals and reimbursement for its product candidates; |
| |
• |
|
establishes a sales, marketing, and distribution infrastructure to commercialize any products for which Eiger may obtain marketing approval and market for itself; |
| |
• |
|
seeks to identify, assess, acquire, and/or develop other product candidates; |
| |
• |
|
makes milestone, royalty or other payments under third party license agreements; |
| |
• |
|
seeks to maintain, protect, and expand its intellectual property portfolio; |
| |
• |
|
seeks to attract and retain skilled personnel; |
| |
• |
|
creates additional infrastructure to support its operations as a public company and its product development and planned future commercialization efforts; and |
48
| |
• |
|
experiences any delays or encounters issues with the development and potential for regulatory approval of its clinical candidates such as safety issues, clinical trial accrual delays, longer follow-up for planned
studies, additional major studies, or supportive studies necessary to support marketing approval. |
Further, the net losses Eiger incurs may
fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of its results of operations may not be a good indication of its future performance.
Eiger has never generated any revenue from product sales and may never be profitable.
Eiger has no products approved for commercialization and has never generated any revenue. Eiger’s ability to generate revenue and achieve profitability
depends on its ability, alone or with strategic collaboration partners, to successfully complete the development of, and obtain the regulatory and marketing approvals necessary to commercialize one or more of its product candidates. Eiger does not
anticipate generating revenue from product sales for the foreseeable future. Eiger’s ability to generate future revenue from product sales depends heavily on its success in many areas, including but not limited to:
| |
• |
|
completing research and development of its product candidates; |
| |
• |
|
obtaining regulatory and marketing approvals for its product candidates; |
| |
• |
|
manufacturing product candidates and establishing and maintaining supply and manufacturing relationships with third parties that meet regulatory requirements and Eiger’s supply needs in sufficient quantities to
meet market demand for its product candidates, if approved; |
| |
• |
|
marketing, launching and commercializing product candidates for which Eiger obtains regulatory and marketing approval, either directly or with a collaborator or distributor; |
| |
• |
|
gaining market acceptance of its product candidates as treatment options; |
| |
• |
|
addressing any competing products; |
| |
• |
|
protecting and enforcing its intellectual property rights, including patents, trade secrets, and know-how; |
| |
• |
|
negotiating favorable terms in any collaboration, licensing, or other arrangements into which Eiger may enter; |
| |
• |
|
obtaining reimbursement or pricing for its product candidates that supports profitability; and |
| |
• |
|
attracting, hiring, and retaining qualified personnel. |
Even if one or more of the product candidates that
Eiger develops is approved for commercial sale, Eiger anticipates incurring significant costs associated with commercializing any approved product candidate. Its current pipeline of product candidates has been in-licensed from third parties and
Eiger will have to develop or acquire manufacturing capabilities in order to continue development and potential commercialization of its product candidates. Additionally, if Eiger is not able to generate revenue from the sale of any approved
products, Eiger may never become profitable.
Raising additional capital may cause dilution to Eiger’s stockholders, restrict its operations or
require Eiger to relinquish rights.
To the extent that Eiger raises additional capital through the sale of equity, debt or other securities
convertible into equity, your ownership interest will be diluted, and the terms of these new securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing, if available at all,
would likely involve agreements that include covenants limiting or restricting Eiger’s ability to take specific actions, such as incurring additional debt, making capital expenditures, making additional product acquisitions, or declaring
dividends. If Eiger raises additional funds through collaborations, strategic alliances or licensing arrangements with third parties, Eiger may have to relinquish valuable rights to its product candidates or future
49
revenue streams or grant licenses on terms that are not favorable to Eiger. Eiger cannot assure you that it will be able to obtain additional funding if and when necessary to fund its entire
portfolio of product candidates to meet its projected plans. If Eiger is unable to obtain funding on a timely basis, Eiger may be required to delay or discontinue one or more of its development programs or the commercialization of any product
candidates or be unable to expand its operations or otherwise capitalize on potential business opportunities, which could materially affect Eiger’s business, financial condition, and results of operations.
Risks Related to the Development of Eiger’s Product Candidates
Eiger is heavily dependent on the success of its product candidates, which are in the early stages of clinical development. Certain of its product
candidates have produced results in academic settings to date or for other indications than those that Eiger contemplates and Eiger cannot give any assurance that it will generate data for any of its product candidates sufficient to receive
regulatory approval in its planned indications, which will be required before they can be commercialized.
To date, Eiger has invested
substantially all of its efforts and financial resources to identify, acquire, and develop its portfolio of product candidates. Its future success is dependent on its ability to successfully further develop, obtain regulatory approval for, and
commercialize one or more product candidates. Eiger currently generates no revenue from sales of any drugs, and Eiger may never be able to develop or commercialize a product candidate.
Eiger currently has three product candidates in or ready for four Phase 1/2 or Phase 2 clinical studies. One of the Eiger product candidates, exendin (9-39)
has only generated data in an academic setting and Eiger may not be able to replicate or develop additional data to satisfy regulatory requirements for approval. For ubenimex, data to date has been developed for use in indications other than those
that Eiger has rights to or in which Eiger plans to develop the product candidate. There can be no assurance that the data that Eiger develops for its product candidates in its planned indications will be sufficient to obtain regulatory approval.
In addition, none of its product candidates have advanced into a pivotal study for Eiger’s proposed indications and it may be years before such
study is initiated and completed, if at all. Eiger is not permitted to market or promote any of its product candidates before it receives regulatory approval from the FDA or comparable foreign regulatory authorities, and Eiger may never receive such
regulatory approval for any of its product candidates. Eiger cannot be certain that any of its product candidates will be successful in clinical studies or receive regulatory approval. Further, its product candidates may not receive regulatory
approval even if they are successful in clinical studies. If Eiger does not receive regulatory approvals for its product candidates, Eiger may not be able to continue its operations.
Eiger’s business strategy is based upon obtaining orphan drug designation for its product candidates, which is an uncertain process. The regulatory
approval processes of the FDA and comparable foreign authorities are lengthy, time consuming, and inherently unpredictable. If Eiger is unable to obtain orphan drug designation or regulatory approval for its product candidates, its business will be
substantially harmed.
Eiger’s approach to identifying and developing product candidates depends, in large part, on its ability to obtain
orphan drug designation from regulatory authorities in major markets. Without the protection of this regulatory exclusivity, many of its product candidates would otherwise not justify investment as they are not protected by patents or they are
otherwise marketed or generic products. While Eiger assesses the potential for obtaining orphan drug designation at the time that Eiger contemplates the acquisition of product candidates and Eiger intends to timely file for such designation, there
can be no assurance that Eiger will obtain orphan drug designation or be able to successfully meet the regulatory requirements to maintain that designation with the planned clinical trials for its product candidates. Failure to obtain orphan drug
designation would make its product candidates significantly less competitive and potentially not viable investments for further development.
The time
required to obtain approval by the FDA and comparable foreign authorities is unpredictable, typically takes many years following the commencement of clinical studies, and depends upon numerous factors. In
50
addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and
may vary among jurisdictions, which may cause delays in the approval or the decision not to approve an application. Eiger has not obtained regulatory approval for any product candidate, and it is possible that none of its existing product candidates
or any product candidates Eiger may seek to develop in the future will ever obtain regulatory approval.
Applications for Eiger’s product candidates
could fail to receive regulatory approval for many reasons, including but not limited to the following:
| |
• |
|
the FDA or comparable foreign regulatory authorities may disagree with the design, size or implementation of its clinical studies; |
| |
• |
|
the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which Eiger seeks approval; |
| |
• |
|
the FDA or comparable foreign regulatory authorities may disagree with Eiger’s interpretation of data from its development efforts; |
| |
• |
|
the data collected from clinical studies of Eiger’s product candidates may not be sufficient to support the submission of a new drug application, or NDA, or other submission or to obtain regulatory approval in the
United States or foreign jurisdictions; |
| |
• |
|
the FDA or comparable foreign regulatory authorities may find failures in Eiger’s manufacturing processes, validation procedures and specifications, or facilities of its third-party manufacturers with which Eiger
contracts for clinical and commercial supplies that may delay or limit Eiger’s ability to obtain regulatory approval for its product candidates; and |
| |
• |
|
the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering Eiger’s NDA or other submission insufficient for approval.
|
The lengthy and uncertain regulatory approval process, as well as the unpredictability of the results of clinical studies, may result in
Eiger’s failing to obtain regulatory approval to market any of its product candidates, which would significantly harm its business, results of operations, and prospects. In addition, although Eiger has obtained orphan drug designation for two
of its product candidates in its planned indications to date, there can be no assurance that the FDA will grant Eiger’s similar status for its other proposed development indications or other product candidates in the future.
Drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies may not be predictive of future
study results.
Clinical testing is expensive and generally takes many years to complete, and the outcome is inherently uncertain. Failure can
occur at any time during the clinical study process. The results of preclinical studies and early clinical studies of Eiger’s product candidates may not be predictive of the results of larger, later-stage controlled clinical studies. Product
candidates that have shown promising results in early-stage clinical studies may still suffer significant setbacks in subsequent clinical studies. Eiger’s clinical studies to date have been conducted on a small number of patients in limited
numbers of clinical sites and in academic settings or for other indications. Eiger will have to conduct larger, well-controlled studies in its proposed indications to verify the results obtained to date and to support any regulatory submissions for
further clinical development. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical studies due to lack of efficacy or adverse safety profiles despite promising results in earlier, smaller
clinical studies. Moreover, clinical data are often susceptible to varying interpretations and analyses. Eiger does not know whether any Phase 2, Phase 3, or other clinical studies Eiger may conduct will demonstrate consistent or adequate efficacy
and safety with respect to the proposed indication for use sufficient to obtain regulatory approval to receive regulatory approval or market its drug candidates.
51
Eiger may find it difficult to enroll patients in its clinical studies given the limited number of patients
who have the diseases for which its product candidates are being studied. Difficulty in enrolling patients could delay or prevent clinical studies of its product candidates.
Identifying and qualifying patients to participate in clinical studies of Eiger’s product candidates is essential to its success. The timing of
Eiger’s clinical studies depends in part on the rate at which Eiger can recruit patients to participate in clinical trials of its product candidates, and Eiger may experience delays in its clinical studies if Eiger encounters difficulties in
enrollment.
The eligibility criteria of Eiger’s planned clinical studies may further limit the available eligible study participants as Eiger
expects to require that patients have specific characteristics that Eiger can measure or meet the criteria to assure their conditions are appropriate for inclusion in its clinical studies. Eiger may not be able to identify, recruit, and enroll a
sufficient number of patients to complete its clinical studies in a timely because of the perceived risks and benefits of the product candidate under study, the availability and efficacy of competing therapies and clinical studies, and the
willingness of physicians to participate in its planned clinical studies. If patients are unwilling to participate in Eiger’s clinical studies for any reason, the timeline for conducting studies and obtaining regulatory approval of its product
candidates may be delayed.
If Eiger experiences delays in the completion of, or termination of, any clinical study of its product candidates, the
commercial prospects of its product candidates could be harmed, and its ability to generate product revenue from any of these product candidates could be delayed or prevented. In addition, any delays in completing its clinical studies would likely
increase its overall costs, impair product candidate development and jeopardize its ability to obtain regulatory approval relative to its current plans. Any of these occurrences may harm its business, financial condition, and prospects
significantly.
Clinical studies are costly, time consuming and inherently risky, and Eiger may fail to demonstrate safety and efficacy to the
satisfaction of applicable regulatory authorities.
Clinical development is expensive, time consuming and involves significant risk. Eiger cannot
guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of development. Events that may prevent successful or timely completion of
clinical development include but are not limited to:
| |
• |
|
inability to generate satisfactory preclinical, toxicology, or other in vivo or in vitro data or diagnostics to support the initiation or continuation of clinical studies; |
| |
• |
|
delays in reaching agreement on acceptable terms with CROs and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical study sites;
|
| |
• |
|
delays in obtaining required Institutional Review Board, or IRB, approval at each clinical study site; |
| |
• |
|
failure to permit the conduct of a study by regulatory authorities, after review of an investigational new drug, or IND, or equivalent foreign application or amendment; |
| |
• |
|
delays in recruiting qualified patients in its clinical studies; |
| |
• |
|
failure by clinical sites or its CROs or other third parties to adhere to clinical study requirements; |
| |
• |
|
failure to perform in accordance with the FDA’s good clinical practices requirements, or applicable foreign regulatory guidelines; |
| |
• |
|
patients dropping out of Eiger’s clinical studies; |
| |
• |
|
occurrence of adverse events associated with Eiger’s product candidates; |
| |
• |
|
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
52
| |
• |
|
the cost of clinical studies of Eiger’s product candidates; |
| |
• |
|
negative or inconclusive results from Eiger’s clinical trials which may result in Eiger’s deciding, or regulators requiring Eiger, to conduct additional clinical studies or abandon development programs in
other ongoing or planned indications for a product candidate; and |
| |
• |
|
delays in in reaching agreement on acceptable terms with third manufacturers and the time for manufacture of sufficient quantities of its product candidates for use in clinical studies. |
Any inability to successfully complete clinical development and obtain regulatory approval could result in additional costs to Eiger or impair its ability to
generate revenue. In addition, if Eiger makes manufacturing or formulation changes to its product candidates, such as its plan to manufacture a new subcutaneous formulation of exendin (9-39), Eiger may need to conduct additional studies or the
results obtained from such new formulation may not be consistent with previous results obtained. Clinical study delays could also shorten any periods during which its products have patent protection and may allow competitors to develop and bring
products to market before Eiger does, which could impair its ability to obtain orphan drug designation exclusivity and to successfully commercialize its product candidates and may harm its business and results of operations.
Eiger’s product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit
the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side
effects caused by its product candidates could cause Eiger or regulatory authorities to interrupt, delay, or terminate clinical studies or even if approved, result in a restrictive label or delay regulatory approval by the FDA or comparable foreign
authorities.
In addition, while Eiger’s lonafarnib product candidate has been studied in thousands of oncology patients and the most common
non-hematologic adverse events of any grade were gastrointestinal system disorders (nausea, anorexia, diarrhea and vomiting), fatigue and rash, treatment discontinuation across the lonafarnib clinical studies conducted in oncology has been in the
range of approximately 20-25% and Eiger may experience comparable or higher rates of discontinuation in testing in its anti-viral, hepatitis D virus studies. There is no guarantee that additional or more severe side effects will not be identified
through ongoing clinical studies by other licensees of lonafarnib for other indications or its own clinical trials. Merck & Co., Inc., or Merck, its licensor, has granted rights to develop lonafarnib in progeria, a rare, fatal rapid aging
disease, to The Progeria Research Foundation. Additionally, while Eiger has a license to another farnesyltranferase inhibitor compound, tipifarnib, from Janssen Pharmaceutica, N.V., or Janssen, Janssen has granted rights to tipifarnib to Kura
Oncology, Inc., or Kura, in oncology (as tipifarnib) and negative results or undesirable side effects from Kura’s clinical trials for a compound with a similar mechanism of action may negatively impact the perception of lonafarnib for
anti-viral indications. Merck may also grant rights to other anti-viral or potentially other indications to other third parties. Undesirable side effects and negative results for other indications may negatively impact the development and potential
for approval of Eiger’s product candidates for its proposed indications.
Additionally, even if one or more of its product candidates receives
marketing approval, and Eiger or others later identify undesirable side effects caused by such products, potentially significant negative consequences could result, including but not limited to:
| |
• |
|
regulatory authorities may withdraw approvals of such products; |
| |
• |
|
regulatory authorities may require additional warnings on the label; |
| |
• |
|
Eiger may be required to create a Risk Evaluation and Mitigation Strategy, or REMS, plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication
plan for healthcare providers, and/or other elements to assure safe use; |
| |
• |
|
Eiger could be sued and held liable for harm caused to patients; and |
| |
• |
|
its reputation may suffer. |
53
Any of these events could prevent Eiger from achieving or maintaining market acceptance of a product candidate,
even if approved, and could significantly harm its business, results of operations, and prospects.
Even if Eiger obtains regulatory approval for a
product candidate, Eiger will remain subject to ongoing regulatory requirements.
If Eiger’s product candidates are approved, they will be
subject to ongoing regulatory requirements with respect to manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies, and submission of safety, efficacy and other post-approval
information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to continuously comply with FDA and comparable foreign regulatory authority requirements,
including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices, or cGMP, regulations and corresponding foreign regulatory manufacturing requirements. As such, Eiger and its contract manufacturers
will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any NDA or marketing authorization application, or MAA.
Any regulatory approvals that Eiger receives for its product candidates may be subject to limitations on the approved indicated uses for which the product
candidate may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase IV clinical trials, and surveillance to monitor the safety and efficacy of the product candidate.
Eiger will be required to report certain adverse reactions and production problems, if any, to the FDA and comparable foreign regulatory authorities. Any new legislation addressing drug safety issues could result in delays in product development or
commercialization, or increased costs to assure compliance. If its original marketing approval for a product candidate was obtained through an accelerated approval pathway, Eiger could be required to conduct a successful post-marketing clinical
study in order to confirm the clinical benefit for its products. An unsuccessful post-marketing study or failure to complete such a study could result in the withdrawal of marketing approval.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with
the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, the regulatory agency may impose restrictions on that product or Eiger, including requiring withdrawal of the product from the
market. If Eiger fails to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
| |
• |
|
impose civil or criminal penalties; |
| |
• |
|
suspend or withdraw regulatory approval; |
| |
• |
|
suspend any of Eiger’s ongoing clinical studies; |
| |
• |
|
refuse to approve pending applications or supplements to approved applications submitted by Eiger; |
| |
• |
|
impose restrictions on Eiger’s operations, including closing its contract manufacturers’ facilities; or |
| |
• |
|
require a product recall. |
Any government investigation of alleged violations of law would be expected to
require Eiger to expend significant time and resources in response and could generate adverse publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect its ability to develop and commercialize its
products and the value of Eiger and its operating results would be adversely affected.
54
Eiger relies on third parties to conduct its clinical studies, manufacture its product candidates and
perform other services. If these third parties do not successfully perform and comply with regulatory requirements, Eiger may not be able to successfully complete clinical development, obtain regulatory approval or commercialize its product
candidates and its business could be substantially harmed.
Eiger has relied upon and plans to continue to rely upon third-party CROs to conduct,
monitor and manage its ongoing clinical programs. Eiger relies on these parties for execution of clinical studies and manages and controls only certain aspects of their activities. Eiger remains responsible for ensuring that each of its studies is
conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards and its reliance on the CROs does not relieve Eiger of its regulatory responsibilities. Eiger and its CROs and other vendors are required to comply all
applicable laws, regulations and guidelines, including those required by the FDA and comparable foreign regulatory authorities for all of its product candidates in clinical development. If Eiger or any of its CROs or vendors fail to comply with
applicable laws, regulations and guidelines, the results generated in its clinical studies may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require Eiger to perform additional studies before approving its
marketing applications. Eiger cannot assure you that its CROs and other vendors will meet these requirements, or that upon inspection by any regulatory authority, such regulatory authority will determine that efforts, including any of its clinical
studies, comply with applicable requirements. Its failure to comply with these laws, regulations and guidelines may require Eiger to repeat clinical studies, which would be costly and delay the regulatory approval process.
If any of Eiger’s relationships with these third-party CROs terminate, Eiger may not be able to enter into arrangements with alternative CROs in a timely
manner or do so on commercially reasonable terms. In addition, Eiger’s CROs may not prioritize Eiger’s clinical studies relative to those of other customers and any turnover in personnel or delays in the allocation of CRO employees by the
CRO may negatively affect its clinical studies. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, Eiger’s clinical studies may be delayed or terminated and Eiger may not be able to meet
its current plans with respect to its product candidates. CROs may also involve higher costs than anticipated, which could negatively affect Eiger’s financial condition and operations.
In addition, Eiger does not currently have, nor does Eiger plan to establish the capability to manufacture product candidates for use in the conduct of its
clinical studies, and Eiger lacks the resources and the capability to manufacture any of its product candidates on a clinical or commercial scale without the use of third party manufacturers. Eiger plans to rely on third party manufacturers and
their responsibilities will include purchasing from third-party suppliers the materials necessary to produce its product candidates for its clinical studies and regulatory approval. There are expected to be a limited number of suppliers for the
active ingredients and other materials that Eiger expects to use to manufacture its product candidates, and Eiger may not be able to identify alternative suppliers to prevent a possible disruption of the manufacture its product candidates for its
clinical studies, and, if approved, ultimately for commercial sale. Although Eiger generally does not expect to begin a clinical study unless Eiger believes it has a sufficient supply of a product candidate to complete the study, any significant
delay or discontinuity in the supply of a product candidate, or the active ingredient or other material components in the manufacture of the product candidate could delay completion of its clinical studies and potential timing for regulatory
approval of its product candidates, which would harm its business and results of operations.
With respect to its lonafarnib and ubenimex product
candidates, Eiger relies on Merck and Nippon Kayaku to supply its clinical study materials and Eiger does not have long-term supply agreements or commitments from those parties to supply its materials. Moreover, even if Eiger has a longer term
supply arrangement, Eiger may be precluded from entering into a back-up or alternative supplier arrangement which may increase the risk for further development, regulatory approval, or commercialization of its product candidates. Its current
agreement with Nippon Kayaku provides for such party to be Eiger’s exclusive supplier for its ubenimex product candidate, which may increase its cost of goods and may not support FDA or other regulatory authority approval for manufacturing.
55
Eiger relies and expects to continue to rely on third parties to manufacture its clinical product supplies,
and Eiger intends to rely on third parties to produce and process its product candidates, if approved, and Eiger’s commercialization of any of its product candidates could be stopped, delayed or made less profitable if those third parties fail
to obtain approval of government regulators, fail to provide Eiger with sufficient quantities of drug product, or fail to do so at acceptable quality levels or prices.
Eiger does not currently have nor does it plan to acquire the infrastructure or capability internally to manufacture its clinical supplies for use in the
conduct of Eiger’s clinical trials, and Eiger lacks the resources and the capability to manufacture any of its product candidates on a clinical or commercial scale. Eiger currently relies on outside vendors to manufacture its clinical supplies
of its product candidates and plan to continue relying on third parties to manufacture its product candidates on a commercial scale, if approved.
The
facilities used by Eiger’s contract manufacturers to manufacture its product candidates must be approved by the FDA pursuant to inspections that will be conducted after Eiger submits its marketing applications to the FDA. Eiger does not control
the manufacturing process of, and is completely dependent on, its contract manufacturing partners for compliance with the regulatory requirements, known as cGMPs, for manufacture of Eiger’s product candidates. If Eiger’s contract
manufacturers cannot successfully manufacture material that conforms to Eiger’s specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their
manufacturing facilities. In addition, Eiger has no control over the ability of its contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparable foreign regulatory authority does
not approve these facilities for the manufacture of its product candidates or if it withdraws any such approval in the future, Eiger may need to find alternative manufacturing facilities, which would significantly impact Eiger’s ability to
develop, obtain regulatory approval for or market its product candidates, if approved.
Eiger does not yet have sufficient information to reliably
estimate the cost of the commercial manufacturing of its product candidates, and the actual cost to manufacture its product candidates could materially and adversely affect the commercial viability of its product candidates. As a result, Eiger may
never be able to develop a commercially viable product.
In addition, Eiger’s reliance on third-party manufacturers exposes Eiger to the following
additional risks:
| |
• |
|
Eiger may be unable to identify manufacturers on acceptable terms or at all. |
| |
• |
|
Eiger’s third-party manufacturers might be unable to timely formulate and manufacture Eiger’s product or produce the quantity and quality required to meet Eiger’s clinical and commercial needs, if any.
|
| |
• |
|
Contract manufacturers may not be able to execute Eiger’s manufacturing procedures appropriately. |
| |
• |
|
Eiger’s future contract manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply its clinical trials or to successfully produce, store and
distribute its products. |
| |
• |
|
Manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMP and other government regulations and corresponding foreign
standards. Eiger does not have control over third-party manufacturers’ compliance with these regulations and standards. |
| |
• |
|
Eiger may not own, or may have to share, the intellectual property rights to any improvements made by Eiger’s third-party manufacturers in the manufacturing process for its product candidates. |
| |
• |
|
Eiger’s third-party manufacturers could breach or terminate their agreement with Eiger. |
Each of these
risks could delay Eiger’s clinical trials, the approval, if any of its product candidates by the FDA or the commercialization of its product candidates or result in higher costs or deprive Eiger of potential product revenue. In addition, Eiger
relies on third parties to perform release testing on its product candidates prior to
56
delivery to patients. If these tests are not appropriately conducted and test data are not reliable, patients could be put at risk of serious harm and could result in product liability suits.
The manufacture of medical products is complex and requires significant expertise and capital investment, including the development of advanced
manufacturing techniques and process controls. Manufacturers of biologic products often encounter difficulties in production, particularly in scaling up and validating initial production and absence of contamination. These problems include
difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign
regulations. Furthermore, if contaminants are discovered in Eiger’s supply of its product candidates or in the manufacturing facilities, such manufacturing facilities may need to be closed for an extended period of time to investigate and
remedy the contamination. Eiger cannot assure you that any stability or other issues relating to the manufacture of its product candidates will not occur in the future. Additionally, Eiger’s manufacturers may experience manufacturing
difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If Eiger’s manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations,
Eiger’s ability to provide its product candidates to patients in clinical trials would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs
associated with maintaining clinical trial programs and, depending upon the period of delay, require Eiger to commence new clinical trials at additional expense or terminate clinical trials completely.
If the market opportunities for its product candidates are smaller than Eiger believes they are, Eiger may not meet its revenue expectations and,
assuming approval of a product candidate, its business may suffer. Because the patient populations in the market for its product candidates may be small, Eiger must be able to successfully identify patients and acquire a significant market share to
achieve profitability and growth.
Eiger focuses its research and product development on treatments for orphan diseases. Given the small number of
patients who have the diseases that Eiger is targeting, its eligible patient population and pricing estimates may differ significantly from the actual market addressable by its product candidate. Its projections of both the number of people who have
these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with its product candidates, are based on its beliefs and estimates. These estimates have been derived from a variety of sources,
including the scientific literature, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower
than expected. For example, for lonafarnib, HDV is related to hepatitis B virus, and there is limited scientific literature in support of a causal link between these two viruses. Although Eiger believes that the data are supportive of the increased
severity of hepatitis in the presence of hepatitis D with hepatitis B virus, there can be no assurance that its clinical trials will successfully address this recently observed condition. Likewise, the potentially addressable patient population for
each of its product candidates may be limited or may not be amenable to treatment with its product candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect its results of operations
and its business.
Eiger faces intense competition and rapid technological change and the possibility that Eiger’s competitors may develop
therapies that are similar, more advanced, or more effective than Eiger’s, may adversely affect its financial condition and its ability to successfully commercialize its product candidates.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Eiger is currently aware
of various existing therapies that may compete with its product candidates. For example, Eiger has competitors both in the United States and internationally, including multinational pharmaceutical companies, specialty pharmaceutical companies and
biotechnology companies. Some of the pharmaceutical and biotechnology companies Eiger expects to compete with include Gilead, Replicor, Novartis, Xoma and Arena as well as other smaller companies or biotechnology startups and large
57
multinational pharmaceutical companies. Many of its competitors have substantially greater financial, technical, and other resources, such as larger research and development staff and experienced
marketing and manufacturing organizations. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in Eiger’s competitors. As a result, these companies may
obtain regulatory approval more rapidly than Eiger is able to and may be more effective in selling and marketing their products as well. Smaller or early-stage companies may also prove to be significant competitors, particularly through
collaborative arrangements with large, established companies. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries.
Eiger’s competitors may succeed in developing, acquiring, or licensing on an exclusive basis, products that are more effective or less costly than any product candidate that Eiger may develop, or achieve earlier patent protection, regulatory
approval, product commercialization, and market penetration than Eiger does. Additionally, technologies developed by Eiger’s competitors may render its potential product candidates uneconomical or obsolete, and Eiger may not be successful in
marketing its product candidates against competitors.
Eiger currently has limited marketing and sales experience. If Eiger is unable to establish
sales and marketing capabilities or enter into agreements with third parties to market and sell its product candidates, Eiger may be unable to generate any revenue.
Although certain of its employees may have marketed, launched, and sold other pharmaceutical products in the past while employed at other companies, Eiger has
no recent experience selling and marketing its product candidates and Eiger currently has no marketing or sales organization. To successfully commercialize any products that may result from its development programs, Eiger will need to invest in and
develop these capabilities, either on its own or with others, which would be expensive, difficult and time consuming. Any failure or delay in the timely development of Eiger’s internal commercialization capabilities could adversely impact the
potential for success of its products.
Further, given its lack of prior experience in marketing and selling biopharmaceutical products, Eiger may rely on
future collaborators to commercialize its products. If collaborators do not commit sufficient resources to commercialize its future products and Eiger is unable to develop the necessary marketing and sales capabilities on its own, Eiger will be
unable to generate sufficient product revenue to sustain or grow its business. Eiger may be competing with companies that currently have extensive and well-funded marketing and sales operations, in particular in the markets its product candidates
are intended to address. Without appropriate capabilities, whether directly or through third party collaborators, Eiger may be unable to compete successfully against these more established companies.
The commercial success of any of Eiger’s current or future product candidate will depend upon the degree of market acceptance by physicians,
patients, third-party payors, and others in the medical community.
Even with the approvals from the FDA and comparable foreign regulatory
authorities, the commercial success of Eiger’s products will depend in part on the health care providers, patients, and third-party payors accepting its product candidates as medically useful, cost-effective, and safe. Any product that Eiger
brings to the market may not gain market acceptance by physicians, patients, third-party payors and other health care providers. The degree of market acceptance of any of Eiger’s products will depend on a number of factors, including without
limitation:
| |
• |
|
the efficacy of the product as demonstrated in clinical studies and potential advantages over competing treatments; |
| |
• |
|
the prevalence and severity of the disease and any side effects; |
| |
• |
|
the clinical indications for which approval is granted, including any limitations or warnings contained in a product’s approved labeling; |
58
| |
• |
|
the convenience and ease of administration; |
| |
• |
|
the willingness of the patients and physicians to accept these therapies; |
| |
• |
|
the marketing, sales and distribution support for the product; |
| |
• |
|
the publicity concerning its products or competing products and treatments; and |
| |
• |
|
the pricing and availability of third-party insurance coverage and reimbursement. |
Even if a product displays
a favorable efficacy and safety profile upon approval, market acceptance of the product remains uncertain. Efforts to educate the medical community and third-party payors on the benefits of the products may require significant investment and
resources and may never be successful. If its products fail to achieve an adequate level of acceptance by physicians, patients, third-party payors, and other health care providers, Eiger will not be able to generate sufficient revenue to become or
remain profitable.
Failure to obtain or maintain adequate reimbursement or insurance coverage for new or current products could limit Eiger’s
ability to market those products and decrease its ability to generate revenue.
The pricing, coverage, and reimbursement of Eiger’s products
must be sufficient to support its commercial efforts and other development programs and the availability and adequacy of coverage and reimbursement by governmental and private payors are essential for most patients to be able to afford expensive
treatments, particularly in orphan drug designated indications where the eligible patient population is small. Sales of Eiger’s product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of its
product candidates will be paid for or reimbursed by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or government authorities, private health insurers, and other third-party payors. If coverage
and reimbursement are not available, or are available only in limited amounts, Eiger may have to subsidize or provide products for free or Eiger may not be able to successfully commercialize its products.
In addition, there is significant uncertainty related to the insurance coverage and reimbursement for newly approved products. In the United States, the
principal decisions about coverage and reimbursement for new drugs are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to
what extent a new drug will be covered and reimbursed under Medicare. Private payors tend to follow the coverage reimbursement policies established by CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to
reimbursement for products such as Eiger’s and what reimbursement codes its products may receive.
Outside the United States, international
operations are generally subject to extensive governmental price controls and other price-restrictive regulations, and Eiger believes the increasing emphasis on cost-containment initiatives in Europe, Canada, and other countries has and will
continue to put pressure on the pricing and usage of products. In many countries, the prices of products are subject to varying price control mechanisms as part of national health systems. Price controls or other changes in pricing regulation could
restrict the amount that Eiger is able to charge for its products. Accordingly, in markets outside the United States, the potential revenue may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to limit or reduce healthcare costs may result in
restrictions on coverage and the level of reimbursement for new products and, as a result, they may not cover or provide adequate payment for its products. Eiger expects to experience pricing pressures in connection with products due to the
increasing trend toward managed healthcare, including the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs has and is
expected to continue to increase in the future. As a result, profitability of Eiger’s products may be more difficult to achieve even if they receive regulatory approval.
59
Eiger intends to rely on a combination of exclusivity from orphan drug designation as well as patent rights
for its product candidates and any future product candidates. If Eiger is unable to obtain or maintain exclusivity from the combination of these approaches, Eiger may not be able to compete effectively in its markets.
The Eiger business strategy is to focus on product candidates for which orphan drug designation may be obtained in the major markets of the world. In addition,
Eiger relies or will upon a combination of patents, trade secret protection, and confidentiality agreements to protect the intellectual property related to its technologies and product candidates. For example, the portfolio of patents licensed from
Merck expires before the anticipated launch date of the lonafarnib product candidate. Its success depends in large part on its and its licensors’ ability to obtain regulatory exclusivity and maintain patent and other intellectual property
protection in the United States and in other countries with respect to its proprietary technology and products.
Under the Orphan Drug Act, the FDA may
designate a product as an orphan drug if it is intended to treat a rare disease or condition, defined as a patient population of fewer than 200,000 in the United States, or a patient population greater than 200,000 in the United States where there
is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. In the European Union, the EMA’s Committee for Orphan Medicinal Products, or COMP, grants orphan drug designation to promote
the development of products that are intended for the diagnosis, prevention, or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the European Union. Additionally, designation is
granted for products intended for the diagnosis, prevention, or treatment of a life-threatening, seriously debilitating or serious and chronic condition when, without incentives, it is unlikely that sales of the drug in the European Union would be
sufficient to justify the necessary investment in developing the drug or biological product or where there is no satisfactory method of diagnosis, prevention, or treatment, or, if such a method exists, the medicine must be of significant benefit to
those affected by the condition.
In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant
funding towards clinical trial costs, tax advantages, and user-fee waivers. In addition, if a product receives the first FDA approval for the indication for which it has orphan drug designation, the product is entitled to orphan drug exclusivity,
which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan
exclusivity or where the manufacturer is unable to assure sufficient product quantity. In the European Union, orphan drug designation entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market
exclusivity following drug or biological product approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify
maintenance of market exclusivity.
Because the extent and scope of patent protection for its products may in some cases be limited, orphan drug
designation is especially important for Eiger’s products for which orphan drug designation may be available. For eligible drugs, Eiger plans to rely on the exclusivity period under the Orphan Drug Act to maintain a competitive position. If
Eiger does not obtain orphan drug exclusivity for its drug products and biologic products that do not have broad patent protection, its competitors may then sell the same drug to treat the same condition sooner than if Eiger had obtained orphan drug
exclusivity and its revenue will be reduced.
Even though Eiger has orphan drug designation for lonafarnib in the United States and Europe, Eiger may not
be the first to obtain marketing approval for any particular orphan indication due to the uncertainties associated with developing pharmaceutical products. Further, even if Eiger obtains orphan drug exclusivity for a product, that exclusivity may
not effectively protect the product from competition because different drugs with different active moieties can be approved for the same condition. Even after an orphan drug is approved, the FDA or EMA can subsequently approve the same drug with the
same active moiety for the same condition if the FDA or EMA concludes that the later drug is safer, more effective, or makes a major contribution to patient care. Orphan drug designation neither shortens the development time or regulatory review
time of a product candidate nor gives the product candidate any advantage in the regulatory review or approval process.
60
Eiger has sought to protect its proprietary position by filing patent applications in the United States and
abroad related to its product candidates that are important to its business. This process is expensive and time consuming, and Eiger may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a
timely manner. It is also possible that Eiger will fail to identify patentable aspects of its research and development output before it is too late to obtain patent protection.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which
legal principles remain unsolved. The patent applications that Eiger owns or in-licenses may fail to result in issued patents with claims that cover its product candidates in the United States or in other foreign countries. There is no assurance
that all potentially relevant prior art relating to its patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even
if such patents cover Eiger’s product candidates, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are
unchallenged, Eiger’s patents and patent applications may not adequately protect its intellectual property, provide exclusivity for its product candidates, or prevent others from designing around the Eiger claims. Any of these outcomes could
impair Eiger’s ability to prevent competition from third parties, which may have an adverse impact on its business.
Eiger, independently or together
with its licensors, has filed several patent applications covering various aspects of its product candidates. Eiger cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents
will be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to Eiger after patent issuance could deprive Eiger of rights necessary for the
successful commercialization of any product candidates that Eiger may develop. Further, if Eiger encounters delays in regulatory approvals, the period of time during which Eiger could market a product candidate under patent protection could be
reduced.
Although Eiger has licensed a number of patents covering methods of use and certain compositions of matter, Eiger does not have complete patent
protection for its product candidates. For example, the patent coverage for lonafarnib expires before the anticipated launch date. Likewise, most of the patents covering products that Eiger has licensed in from Stanford University have limited
protection outside of the United States. Therefore, a competitor could develop the same or similar product that may compete with its product candidate.
Certain of Eiger’s product licenses are limited to specified indications or therapeutic areas which may result in the same compound being developed and
commercialized by a third party whom Eiger has no control over or rights against. This may result in safety data, pricing or off label uses from that third party’s product that may negatively affect the development and commercialization of its
product candidates. For example, Kura has an exclusive license to tipifarnib for use in cancer indications while Eiger has a license for anti-viral indications. As a result of Kura’s right to use the same compound in a different indication, it
is possible that development and sales may impact the Eiger product development and commercialization efforts. If Eiger cannot obtain and maintain effective protection of exclusivity from its regulatory efforts and intellectual property rights,
including patent protection, for its product candidates, Eiger may not be able to compete effectively and its business and results of operations would be harmed.
Eiger may not have sufficient patent term protections for its products to effectively protect its business.
Patents have a limited term. In the United States, the statutory expiration of a patent is generally 20 years after it is filed. Although various extensions
may be available, the life of a patent, and the protection it affords, is limited. Even if patents covering its product candidates are obtained, once the patent life has expired for a product, Eiger may be open to competition from generic
medications. In addition, upon issuance in the United States any patent term can be adjusted based on certain delays caused by the applicant(s) or the United States
61
Patent and Trademark Office, or USPTO. For example, a patent term can be reduced based on certain delays caused by the patent applicant during patent prosecution.
Patent term extensions under the Hatch-Waxman Act in the United States and under supplementary protection certificates in Europe may be available to extend
the patent or data exclusivity terms of products. With respect to ubenimex, lonafarnib and exendin (9-39), a substantial portion of the potential commercial opportunity will likely rely on patent term extensions, and Eiger cannot provide any
assurances that any such patent term extensions will be obtained and, if so, for how long. As a result, Eiger may not be able to maintain exclusivity for its products for an extended period after regulatory approval, which would negatively impact
its business and results of operations. If Eiger does not have sufficient patent terms or regulatory exclusivity to protect its products, its business and results of operations will be adversely affected.
Patent laws and rule changes could increase the uncertainties and costs surrounding the prosecution of Eiger’s patent applications and the
enforcement or defense of its issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States and
other countries may diminish the value of its patents or narrow the scope of Eiger’s patent protection. The laws of foreign countries may not protect its rights to the same extent as the laws of the United States. Publications of discoveries in
the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Eiger therefore cannot be
certain that it or its licensors were the first to make the invention claimed in its owned and licensed patents or pending applications, or that Eiger or its licensor were the first to file for patent protection of such inventions. Assuming the
other requirements for patentability are met, in the United States prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is entitled
to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act, or the Leahy-Smith Act, enacted on September 16, 2011, the United States has moved to a first to file system. The Leahy-Smith Act also includes a number of
significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. The effects of these changes are currently unclear as the USPTO must still implement various regulations, the courts have yet to
address any of these provisions and the applicability of the act and new regulations on specific patents discussed herein have not been determined and would need to be reviewed. In general, the Leahy-Smith Act and its implementation could increase
the uncertainties and costs surrounding the prosecution of Eiger’s patent applications and the enforcement or defense of Eiger’s issued patents, all of which could have a material adverse effect on Eiger’s business and financial
condition.
If Eiger is unable to maintain effective proprietary rights for its product candidates or any future product candidates, Eiger may not
be able to compete effectively in its markets.
In addition to the protection afforded by patents, Eiger relies on trade secret protection and
confidentiality agreements to protect proprietary know-how that is not patentable or that Eiger elects not to patent, processes for which patents are difficult to enforce and any other elements of its product candidate discovery and development
processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. Eiger seeks to protect its proprietary technology and processes, in part, by entering into
confidentiality agreements with its employees, consultants, scientific advisors, and contractors. Eiger also seeks to preserve the integrity and confidentiality of its data and trade secrets by maintaining physical security of its premises and
physical and electronic security of its information technology systems. While Eiger has confidence in these individuals, organizations and systems, agreements or security measures may be breached, and Eiger may not have adequate remedies for any
breach. In addition, its trade secrets may otherwise become known or be independently discovered by competitors.
Although Eiger expects all of its
employees and consultants to assign their inventions to Eiger, and all of its employees, consultants, advisors, and any third parties who have access to its proprietary know-how,
62
information, or technology to enter into confidentiality agreements, Eiger cannot provide any assurances that all such agreements have been duly executed or that its trade secrets and other
confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to its trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized
disclosure of Eiger’s trade secrets could impair its competitive position and may have a material adverse effect on its business. Additionally, if the steps taken to maintain its trade secrets are deemed inadequate, Eiger may have insufficient
recourse against third parties for misappropriating the trade secret.
Third-party claims of intellectual property infringement may prevent or delay
Eiger’s development and commercialization efforts.
Eiger’s commercial success depends in part on its avoiding infringement of the
patents and proprietary rights of third parties. There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits,
interferences, oppositions, and reexamination proceedings before the USPTO and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in
which Eiger is developing product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that its product candidates may be subject to claims of infringement of the patent rights of
third parties.
Third parties may assert that Eiger is employing their proprietary technology without authorization. There may be third-party patents or
patent applications with claims to materials, formulations, methods of manufacture, or methods for treatment related to the use or manufacture of its product candidates. Eiger has conducted freedom to operate analyses with respect to only certain of
its product candidates, and therefore Eiger does not know whether there are any third-party patents that would impair its ability to commercialize these product candidates. Eiger also cannot guarantee that any of its analyses are complete and
thorough, nor can Eiger be sure that Eiger has identified each and every patent and pending application in the United States and abroad that is relevant or necessary to the commercialization of its product candidates. Because patent applications can
take many years to issue, there may be currently pending patent applications that may later result in issued patents that its product candidates may infringe.
In addition, third parties may obtain patents in the future and claim that use of its technologies infringes upon these patents. If any third-party patents
were held by a court of competent jurisdiction to cover aspects of its formulations, the manufacturing process of any of its product candidates, methods of use, any molecules formed during the manufacturing process or any final product itself, the
holders of any such patents may be able to block its ability to commercialize such product candidate unless Eiger obtained a license under the applicable patents, or until such patents expire or are finally determined to be invalid or unenforceable.
Such a license may not be available on commercially reasonable terms, or at all.
Parties making claims against Eiger may obtain injunctive or other
equitable relief, which could effectively block its ability to further develop and commercialize one or more of its product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a
substantial diversion of employee resources from its business. In the event of a successful claim of infringement against Eiger, Eiger may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement,
pay royalties, redesign its infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
63
Eiger may not be successful in meeting its diligence obligations under its existing license agreements
necessary to maintain its product candidate licenses in effect. In addition, if required in order to commercialize its product candidates, Eiger may be unsuccessful in obtaining or maintaining necessary rights to its product candidates through
acquisitions and in-licenses.
Eiger currently has rights to the intellectual property, through licenses from third parties and under patents that
Eiger does not own, to develop and commercialize its product candidates. Because its programs may require the use of proprietary rights held by third parties, the growth of its business will likely depend in part on its ability to maintain in effect
these proprietary rights. For example, Eiger has certain specified diligence obligations under its Stanford University license agreements for its ubenimex and lonafarnib product candidates. Eiger may not be able to achieve the required diligence
milestones in a timely manner, which may result in a right of termination by Stanford University, and Eiger may be unable to successfully negotiate an extension or waiver of those termination rights. Any termination of license agreements with third
parties with respect to its product candidates would be expected to negatively impact its business prospects.
Eiger may be unable to acquire or
in-license any compositions, methods of use, processes, or other third-party intellectual property rights from third parties that Eiger identifies as necessary for its product candidates. The licensing and acquisition of third-party intellectual
property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that Eiger may consider attractive. These established companies may have a
competitive advantage over Eiger due to their size, cash resources, and greater clinical development and commercialization capabilities. In addition, companies that perceive Eiger to be a competitor may be unwilling to assign or license rights to
Eiger. Even if Eiger is able to license or acquire third-party intellectual property rights that are necessary for its product candidates, there can be no assurance that they will be available on favorable terms.
Eiger collaborates with U.S. and foreign academic institutions to identify product candidates, accelerate its research and conduct development. Typically,
these institutions have provided Eiger with an option to negotiate an exclusive license to any of the institution’s rights in the patents or other intellectual property resulting from the collaboration. Regardless of such option, Eiger may be
unable to negotiate a license within the specified timeframe or under terms that are acceptable to Eiger. If Eiger is unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking its ability to
pursue a program of interest to Eiger.
If Eiger is unable to successfully obtain and maintain rights to required third-party intellectual property, Eiger
may have to abandon development of that product candidate or pay additional amounts to the third party, and its business and financial condition could suffer.
Eiger’s product candidates may be subject to generic competition.
Under the Hatch-Waxman Act, a pharmaceutical manufacturer may file an abbreviated new drug application, or ANDA, seeking approval of a generic copy of an
approved innovator product. Under the Hatch-Waxman Act, a manufacturer may also submit an NDA under section 505(b)(2) that references the FDA’s finding of safety and effectiveness of a previously approved drug. A 505(b)(2) NDA product may be
for a new or improved version of the original innovator product. Innovative small molecule drugs may be eligible for certain periods of regulatory exclusivity (e.g., five years for new chemical entities, three years for changes to an approved drug
requiring a new clinical study, seven years for orphan drugs), which preclude FDA approval (or in some circumstances, FDA filing and review of) an ANDA or 505(b)(2) NDA relying on the FDA’s finding of safety and effectiveness for the innovative
drug. In addition to the benefits of regulatory exclusivity, an innovator NDA holder may have patents claiming the active ingredient, product formulation or an approved use of the drug, which would be listed with the product in the FDA publication,
“Approved Drug Products with Therapeutic Equivalence Evaluations,” known as the “Orange Book.” If there are patents listed in the Orange Book, a generic applicant that seeks to market its product before expiration of the patents
must include in the ANDA or 505(b)(2) what is known as a
64
“Paragraph IV certification,” challenging the validity or enforceability of, or claiming non-infringement of, the listed patent or patents. Notice of the certification must be given to
the innovator, too, and if within 45 days of receiving notice the innovator sues to protect its patents, approval of the ANDA is stayed for 30 months, or as lengthened or shortened by the court.
If there are patents listed for its product candidates in the Orange Book, ANDAs and 505(b)(2) NDAs with respect to those product candidates would be required
to include a certification as to each listed patent indicating whether the ANDA applicant does or does not intend to challenge the patent. Eiger cannot predict whether any patents issuing from its pending patent applications will be eligible for
listing in the Orange Book, how any generic competitor would address such patents, whether Eiger would sue on any such patents, or the outcome of any such suit.
Eiger may not be successful in securing or maintaining proprietary patent protection for products and technologies Eiger develops or licenses. Moreover, if
any patents that are granted and listed in the Orange Book are successfully challenged by way of a Paragraph IV certification and subsequent litigation, the affected product could more immediately face generic competition and its sales would likely
decline materially. Should sales decline, Eiger may have to write off a portion or all of the intangible assets associated with the affected product and its results of operations and cash flows could be materially and adversely affected.
The patent protection and patent prosecution for some of Eiger’s product candidates is dependent on third parties.
While Eiger normally seeks and gains the right to fully prosecute the patents relating to its product candidates, there may be times when patents relating to
its product candidates are controlled by its licensors. This is the case with its agreements with Stanford University and Nippon Kayaku, each of whom is primarily responsible for the prosecution of patents and patent applications licensed to Eiger
under the applicable collaboration agreements. If they or any of its future licensors fail to appropriately and broadly prosecute and maintain patent protection for patents covering any of its product candidates, its ability to develop and
commercialize those product candidates may be adversely affected and Eiger may not be able to prevent competitors from making, using, importing, and selling competing products. In addition, even where Eiger now has the right to control patent
prosecution of patents and patent applications Eiger has licensed from third parties, Eiger may still be adversely affected or prejudiced by actions or inactions of its licensors in effect from actions prior to Eiger assuming control over patent
prosecution.
If Eiger fails to comply with obligations in the agreements under which Eiger licenses intellectual property and other rights from
third parties or otherwise experience disruptions to its business relationships with its licensors, Eiger could lose license rights that are important to its business.
Eiger is a party to a number of intellectual property license and supply agreements that are important to its business and expects to enter into additional
license agreements in the future. Eiger’s existing agreements impose, and Eiger expects that future license agreements will impose, various diligence, milestone payment, royalty, purchasing, and other obligations on it. If Eiger fails to comply
with its obligations under these agreements, or Eiger is subject to a bankruptcy, its agreements may be subject to termination by the licensor, in which event Eiger would not be able to develop, manufacture, or market products covered by the license
or subject to supply commitments.
Although Eiger is not currently involved in any litigation, Eiger may be involved in lawsuits to protect or
enforce its patents or the patents of its licensors, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe
Eiger’s patents or the patents of its licensors. Although Eiger is not currently involved in any litigation, if Eiger or one of its licensing partners were to initiate legal proceedings against a third party to enforce a patent covering one of
its product candidates, the defendant could counterclaim that the patent covering
65
its product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for
a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with
prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Interference proceedings provoked by third parties or brought by Eiger or declared by the USPTO may be necessary to determine the priority of inventions with
respect to Eiger’s patents or patent applications or those of its licensors. An unfavorable outcome could require Eiger to cease using the related technology or to attempt to license rights to it from the prevailing party. Eiger’s business
could be harmed if the prevailing party does not offer Eiger a license on commercially reasonable terms. Its defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract its
management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on its ability to raise the funds necessary to continue its clinical trials, continue its research programs, license
necessary technology from third parties, or enter into development partnerships that would help Eiger bring its product candidates to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of Eiger
confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or
investors perceive these results to be negative, it could have a material adverse effect on the price of its common stock.
Eiger may be subject to
claims that its employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that its employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Eiger employs individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including its
competitors or potential competitors. Although Eiger has written agreements and make every effort to ensure that its employees, consultants, and independent contractors do not use the proprietary information or intellectual property rights of others
in their work for Eiger, and Eiger is not currently subject to any claims that its employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties, Eiger may in the future be subject to
such claims. Litigation may be necessary to defend against these claims. If Eiger fails in defending any such claims, in addition to paying monetary damages, Eiger may lose valuable intellectual property rights or personnel, which could adversely
impact its business. Even if Eiger is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Eiger may not be able to protect its intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and its intellectual
property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state
laws in the United States. Competitors may use Eiger’s technologies in jurisdictions where Eiger has not obtained patent protection to develop its own products and may also export infringing products to territories where Eiger has patent
protection, but enforcement is not as strong as that in the United States. These products may compete with its products and its patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of
certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those
66
relating to biotechnology products, which could make it difficult for Eiger to stop the infringement of its patents or marketing of competing products in violation of its proprietary rights
generally. Proceedings to enforce Eiger’s patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert Eiger’s efforts and attention from other aspects of its business, could put
Eiger’s patents at risk of being invalidated or interpreted narrowly and its patent applications at risk of not issuing and could provoke third parties to assert claims against Eiger. Eiger may not prevail in any lawsuits that Eiger initiates
and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, its efforts to enforce its intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the
intellectual property that Eiger develops or licenses.
Risks Related to Eiger’s Business Operations
Eiger has identified a material weakness in its internal control over financial reporting and may identify additional material weaknesses in the future
or otherwise fail to maintain an effective system of internal control, which may result in material misstatements of Eiger’s financial statements or cause Eiger to fail to meet its periodic reporting obligations.
Prior to the merger, Eiger was a private company and had limited accounting and financial reporting personnel and other resources with which to address its
internal controls and procedures. In connection with the audit of Eiger’s consolidated financial statements for the years ended December 31, 2014 and 2013, Eiger and its independent auditors identified a material weakness in Eiger’s
internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the subject
company’s annual or interim financial statements will not be prevented or detected on a timely basis. Eiger’s lack of sufficient accounting personnel has resulted in the identification of a material weakness in Eiger’s internal
control over financial reporting. Specifically, the material weakness that was identified related to a lack of sufficient accounting resources and personnel that has limited Eiger’s ability to adequately segregate duties, establish defined
accounting policies and procedures and perform timely reviews of account reconciliations.
To address this material weakness, Eiger plans to hire
additional accounting personnel, establish and document accounting policies and procedures, and implement management review controls. While Eiger intends to implement a plan to remediate this material weakness, Eiger cannot predict the success of
such plan or the outcome of Eiger’s assessment of these plans at this time. Eiger can give no assurance that this implementation will remediate this deficiency in internal control or that additional material weaknesses or significant
deficiencies in Eiger’s internal control over financial reporting will not be identified in the future. Eiger’s failure to implement and maintain effective internal control over financial reporting could result in errors in Eiger’s
financial statements that could result in a restatement of Eiger’s financial statements or cause Eiger to fail to meet its reporting obligations.
Eiger’s future success depends in part on its ability to retain its President and Chief Executive Officer and to attract, retain, and motivate
other qualified personnel.
Eiger is highly dependent on David Cory, its President and Chief Executive Officer, the loss of whose services may
adversely impact the achievement of its objectives. Mr. Cory could leave Eiger’s employment at any time, as he is an “at will” employee. Recruiting and retaining other qualified employees, consultants, and advisors for
Eiger’s business, including scientific and technical personnel, will also be critical to Eiger success. There is currently a shortage of highly qualified personnel in Eiger’s industry, which is likely to continue. As a result, competition
for personnel is intense and the turnover rate can be high. Eiger may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for individuals with similar skill
sets. In addition, failure to succeed in development and commercialization of Eiger’s product candidates may make it more challenging to recruit and retain qualified personnel. The inability to recruit and retain qualified personnel, or the
loss of the services of Mr. Cory may
67
impede the progress of Eiger’s research, development, and commercialization objectives and would negatively impact Eiger’s ability to succeed in its in-licensing strategy.
Eiger will need to expand its organization and Eiger may experience difficulties in managing this growth, which could disrupt its operations.
As of September 30, 2015, Eiger had seven full-time employees. As Eiger’s development and commercialization plans and strategies
develop, Eiger expects to need additional managerial, operational, sales, marketing, financial, legal, and other resources. Its management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a
substantial amount of time to managing these growth activities. Eiger may not be able to effectively manage the expansion of its operations, which may result in weaknesses in its infrastructure, operational mistakes, loss of business opportunities,
loss of employees, and reduced productivity among remaining employees. Eiger’s expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product
candidates. If its management is unable to effectively manage its growth, its expenses may increase more than expected, its ability to generate and/or grow revenue could be reduced and Eiger may not be able to implement its business strategy.
Eiger’s future financial performance and its ability to commercialize product candidates and compete effectively will depend, in part, on its ability to effectively manage any future growth.
Failure in Eiger’s information technology and storage systems could significantly disrupt the operation of Eiger’s business.
Eiger’s ability to execute its business plan and maintain operations depends on the continued and uninterrupted performance of its information technology,
or IT, systems. IT systems are vulnerable to risks and damages from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of
Eiger’s and its vendors’ servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite precautionary measures to prevent unanticipated problems that could affect its IT
systems, sustained or repeated system failures that interrupt its ability to generate and maintain data could adversely affect its ability to operate its business.
Eiger may not be successful in any efforts to identify, license, discover, develop, or commercialize additional product candidates.
Although a substantial amount of Eiger’s effort will focus on the continued clinical testing, potential approval, and commercialization of its existing
product candidates, the success of Eiger’s business is also expected to depend in part upon its ability to identify, license, discover, develop, or commercialize additional product candidates. Research programs to identify new product
candidates require substantial technical, financial, and human resources. Eiger may focus its efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. Eiger’s research programs or licensing
efforts may fail to yield additional product candidates for clinical development and commercialization for a number of reasons, including but not limited to the following:
| |
• |
|
Eiger’s research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates; |
| |
• |
|
Eiger may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates; |
| |
• |
|
its product candidates may not succeed in preclinical or clinical testing; |
| |
• |
|
its potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval; |
| |
• |
|
competitors may develop alternatives that render Eiger’s product candidates obsolete or less attractive; |
68
| |
• |
|
product candidates Eiger develops may be covered by third parties’ patents or other exclusive rights; |
| |
• |
|
the market for a product candidate may change during Eiger’s program so that such a product may become unreasonable to continue to develop; |
| |
• |
|
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
| |
• |
|
a product candidate may not be accepted as safe and effective by patients, the medical community, or third-party payors. |
If any of these events occur, Eiger may be forced to abandon its development efforts for a program or programs, or Eiger may not be able to identify, license,
discover, develop, or commercialize additional product candidates, which would have a material adverse effect on its business and could potentially cause Eiger to cease operations.
Healthcare legislative reform measures may have a material adverse effect on Eiger’s business and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the
Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the Health Care Reform Law, was passed, which substantially changes the way health care is financed by both governmental and private
insurers, and significantly impacts the U.S. pharmaceutical industry. The Health Care Reform Law, among other things, addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs
that are inhaled, infused, instilled, implanted, or injected, increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care
organizations, establishes annual fees and taxes on manufacturers of certain branded prescription drugs, and promotes a new Medicare Part D coverage gap discount program.
In addition, other legislative changes have been proposed and adopted in the United States since the Health Care Reform Law was enacted and Eiger expects that
additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand or
lower pricing for its product candidates, or additional pricing pressures.
Eiger may be subject, directly or indirectly, to federal and state
healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws. If Eiger is unable to comply, or has not fully complied, with such laws, it could face substantial penalties.
If Eiger obtains FDA approval for any of its product candidates and begins commercializing those products in the United States, its operations may be subject
to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations. These laws may impact, among other things, its proposed
sales, marketing, and education programs. In addition, Eiger may be subject to patient privacy regulation by both the federal government and the states in which Eiger conduct its business. The laws that may affect its ability to operate include:
| |
• |
|
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for,
the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| |
• |
|
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from
Medicare, Medicaid, or other third-party payors that are false or fraudulent; |
69
| |
• |
|
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false
statements relating to healthcare matters; |
| |
• |
|
HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security, and transmission of
individually identifiable health information; |
| |
• |
|
the federal physician sunshine requirements under the Health Care Reform Laws requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human
Services information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate
family members and applicable group purchasing organizations; and |
| |
• |
|
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, state laws
that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to
healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and
state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of Eiger’s
business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, the Health Care Reform Law, among other things, amends the intent
requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the Health Care Reform Law provides that the
government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If Eiger’s operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to Eiger, Eiger
may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of its
operations, any of which could adversely affect its ability to operate Eiger’s business and its results of operations.
If Eiger fails to
comply with environmental, health and safety laws and regulations, Eiger could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of its business.
Eiger’s research and development activities and its third-party manufacturers’ and suppliers’ activities involve the controlled storage, use,
and disposal of hazardous materials, including the components of its product candidates and other hazardous compounds. Eiger and its manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling,
and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at Eiger’s and its manufacturers’ facilities pending their use and disposal. Eiger cannot eliminate
the risk of contamination, which could cause an interruption of its commercialization efforts, research and development efforts and business operations, environmental damage resulting in costly clean-up and liabilities under applicable laws and
70
regulations governing the use, storage, handling, and disposal of these materials and specified waste products. Although Eiger believes that the safety procedures utilized by it and its
third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, Eiger cannot guarantee that this is the case or eliminate the risk of accidental contamination or
injury from these materials. In such an event, Eiger may be held liable for any resulting damages and such liability could exceed its resources and state or federal or other applicable authorities may curtail Eiger’s use of certain materials
and/or interrupt its business operations. Furthermore, environmental laws and regulations are complex, change frequently, and have tended to become more stringent. Eiger cannot predict the impact of such changes and cannot be certain of its future
compliance. Eiger does not currently carry biological or hazardous waste insurance coverage.
Eiger or the third parties upon whom it depends may be
adversely affected by earthquakes or other natural disasters and its business continuity and disaster recovery plans may not adequately protect Eiger from a serious disaster.
Its corporate headquarters are located in the San Francisco Bay Area which has in the past experienced severe earthquakes and other natural disasters. Eiger
does not carry earthquake insurance. Earthquakes or other natural disasters could severely disrupt its operations or those of its collaborators, and have a material adverse effect on its business, results of operations, financial condition, and
prospects. If a natural disaster, terrorist attack, power outage, or other event occurred that prevented Eiger from using or damaged critical elements of its business and operations (such as the manufacturing facilities of its third-party contract
manufacturers) its business may be disrupted for a substantial period of time. Eiger has limited or no disaster recovery and business continuity plans in place currently and its business would be impaired in the event of a serious disaster or
similar event. Eiger may incur substantial expenses to develop and implement any disaster recovery and business continuity plans, which could have a material adverse effect on its business.
Eiger’s principal stockholders own a significant percentage of its stock and will be able to exert significant control over matters subject to
stockholder approval.
Eiger’s principal stockholders and their affiliates currently beneficially own in excess of 70% of Eiger’s
outstanding voting stock. Therefore, these stockholders have the ability and may continue to have the ability to influence Eiger through this ownership position. These stockholders may be able to determine some or all matters requiring stockholder
approval. For example, these stockholders, acting together, may be able to control elections of directors, amendments of organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or
discourage unsolicited acquisition proposals or offers for Eiger’s common stock that you may believe are in your best interest as one of Eiger’s stockholders.
Risks Related to the Combined Organization
In determining whether you should approve the merger, the issuance of shares of Celladon common stock and other matters related to the merger, as the case
may be, you should carefully read the following risk factors in addition to the risks described above.
Celladon’s stock price is expected
to be volatile, and the market price of its common stock may drop following the merger.
The market price of Celladon’s common stock following
the merger could be subject to significant fluctuations following the merger. Market prices for securities of early-stage pharmaceutical, biotechnology and other life sciences companies have historically been particularly volatile. Some of the
factors that may cause the market price of Celladon’s common stock to fluctuate include:
| |
• |
|
the ability of the combined organization to obtain regulatory approvals for lonafarnib or other product candidates, and delays or failures to obtain such approvals; |
71
| |
• |
|
failure of any of the combined organization’s product candidates, if approved, to achieve commercial success; |
| |
• |
|
failure to obtain orphan drug designation; |
| |
• |
|
failure to maintain its existing third party license and supply agreements; |
| |
• |
|
failure by Celladon or its licensors to prosecute, maintain, or enforce its intellectual property rights; |
| |
• |
|
changes in laws or regulations applicable to its product candidates; |
| |
• |
|
any inability to obtain adequate supply of its product candidates or the inability to do so at acceptable prices; |
| |
• |
|
adverse regulatory authority decisions; |
| |
• |
|
introduction of new products, services, or technologies by its competitors; |
| |
• |
|
failure to meet or exceed financial and development projections Eiger may provide to the public; |
| |
• |
|
failure to meet or exceed the financial and development projections of the investment community; |
| |
• |
|
the perception of the pharmaceutical industry by the public, legislatures, regulators, and the investment community; |
| |
• |
|
announcements of significant acquisitions, strategic partnerships, joint ventures, or capital commitments by Celladon or its competitors; |
| |
• |
|
disputes or other developments relating to proprietary rights, including patents, litigation matters, and its ability to obtain patent protection for its technologies; |
| |
• |
|
additions or departures of key personnel; |
| |
• |
|
significant lawsuits, including patent or stockholder litigation; |
| |
• |
|
if securities or industry analysts do not publish research or reports about its business, or if they issue an adverse or misleading opinions regarding its business and stock; |
| |
• |
|
changes in the market valuations of similar companies; |
| |
• |
|
general market or macroeconomic conditions; |
| |
• |
|
sales of its common stock by Celladon or its stockholders in the future; |
| |
• |
|
trading volume of its common stock. |
| |
• |
|
announcements by commercial partners or competitors of new commercial products, clinical progress or the lack thereof, significant contracts, commercial relationships or capital commitments; |
| |
• |
|
adverse publicity relating to the hepatitis market generally, including with respect to other products and potential products in such markets; |
| |
• |
|
the introduction of technological innovations or new therapies that compete with potential products of the combined organization; |
| |
• |
|
changes in the structure of health care payment systems; and |
| |
• |
|
period-to-period fluctuations in the combined organization’s financial results. |
Moreover, the stock
markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of the combined
organization’s common stock.
72
In the past, following periods of volatility in the market price of a company’s securities, stockholders
have often instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm the combined
organization’s profitability and reputation.
The combined organization will incur costs and demands upon management as a result of complying
with the laws and regulations affecting public companies.
The combined organization will incur significant legal, accounting and other expenses
that Eiger did not incur as a private company, including costs associated with public company reporting requirements. The combined organization will also incur costs associated with corporate governance requirements, including requirements under the
Sarbanes-Oxley Act, as well as new rules implemented by the SEC and The NASDAQ Stock Market LLC. These rules and regulations are expected to increase the combined organization’s legal and financial compliance costs and to make some activities
more time-consuming and costly. For example, the combined organization’s management team will consist of certain executive officers of Eiger prior to the merger, some of whom have not previously managed and operated a public company. These
executive officers and other personnel will need to devote substantial time to gaining expertise regarding operations as a public company and compliance with applicable laws and regulations. These rules and regulations may also make it difficult and
expensive for the combined organization to obtain directors’ and officers’ liability insurance. As a result, it may be more difficult for the combined organization to attract and retain qualified individuals to serve on the combined
organization’s board of directors or as executive officers of the combined organization, which may adversely affect investor confidence in the combined organization and could cause the combined organization’s business or stock price to
suffer.
Anti-takeover provisions in the combined organization’s charter documents and under Delaware law could make an acquisition of the
combined organization more difficult and may prevent attempts by the combined organization stockholders to replace or remove the combined organization management.
Provisions in the combined organization’s certificate of incorporation and bylaws may delay or prevent an acquisition or a change in management. These
provisions include a classified board of directors, a prohibition on actions by written consent of the combined organization’s stockholders and the ability of the board of directors to issue preferred stock without stockholder approval. In
addition, because the combined organization will be incorporated in Delaware, it is governed by the provisions of Section 203 of the DGCL, which prohibits stockholders owning in excess of 15% of the outstanding combined organization voting
stock from merging or combining with the combined organization. Although Celladon and Eiger believe these provisions collectively will provide for an opportunity to receive higher bids by requiring potential acquirors to negotiate with the combined
organization’s board of directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by the combined organization’s stockholders to
replace or remove then current management by making it more difficult for stockholders to replace members of the board of directors, which is responsible for appointing the members of management.
Celladon and Eiger do not anticipate that the combined organization will pay any cash dividends in the foreseeable future.
The current expectation is that the combined organization will retain its future earnings to fund the development and growth of the combined
organization’s business. As a result, capital appreciation, if any, of the common stock of the combined organization will be your sole source of gain, if any, for the foreseeable future.
Future sales of shares by existing stockholders could cause the combined organization’s stock price to decline.
If existing stockholders of Celladon and Eiger sell, or indicate an intention to sell, substantial amounts of the combined organization’s common stock in
the public market after legal restrictions on resale discussed in this
73
proxy statement/prospectus/information statement lapse, the trading price of the common stock of the combined organization could decline. Based on shares outstanding as of December 31, 2015 and
shares expected to be issued upon completion of the merger, the combined organization is expected to have outstanding a total of approximately 7.0 million shares of common stock (after giving effect to the proposed 1-for-15 reverse stock split)
immediately following the completion of the merger. Approximately 2.9 million of such shares of common stock will be freely tradable, without restriction, in the public market. Approximately 4.0 million of such shares will be held by
directors, executive officers of the combined organization and other affiliates and will be subject to volume limitations under Rule 144 under the Securities Act and various vesting agreements. In addition, shares of common stock that are subject to
outstanding options of Eiger will become eligible for sale in the public market to the extent permitted by the provisions of various vesting agreements and Rules 144 and 701 under the Securities Act. If these additional shares are sold, or if it is
perceived that they will be sold, in the public market, the trading price of the combined organization common stock could decline.
If the
ownership of the combined organization common stock is highly concentrated, it may prevent you and other stockholders from influencing significant corporate decisions and may result in conflicts of interest that could cause the combined organization
stock price to decline.
Executive officers and directors of the combined organization and their affiliates are expected to beneficially own or
control approximately 49.3% of the outstanding shares of the combined organization common stock following the completion of the merger. Accordingly, these executive officers, directors and their affiliates, acting as a group, will have substantial
influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of the combined organization assets or any other significant corporate
transactions. These stockholders may also delay or prevent a change of control of the combined organization, even if such a change of control would benefit the other stockholders of the combined organization. The significant concentration of stock
ownership may adversely affect the trading price of the combined organization’s common stock due to investors’ perception that conflicts of interest may exist or arise.
Because the merger will result in an ownership change under Section 382 of the Internal Revenue Code for Celladon and Eiger, pre-merger net
operating loss carryforwards and certain other tax attributes will be subject to limitations.
If a corporation undergoes an “ownership
change” within the meaning of Section 382 of the Internal Revenue Code of 1986, as amended, or Section 382, the corporation’s net operating loss carryforwards and certain other tax attributes arising from before the ownership
change are subject to limitations on use after the ownership change. In general, an ownership change occurs if there is a cumulative change in the corporation’s equity ownership by certain stockholders that exceeds fifty percentage points over
a rolling three-year period. Similar rules may apply under state tax laws. The merger will result in an ownership change for Celladon and, accordingly, Celladon’s net operating loss carryforwards and certain other tax attributes will be subject
to limitations on their use after the merger. The contemporaneous financing taken together with the merger is expected to limit Eiger’s net operating loss carryforwards. Additional ownership changes in the future could result in additional
limitations on Celladon’s, Eiger’s and the combined organization’s net operating loss carryforwards, even if the Tax Benefit Preservation Plan adopted by the Celladon board of directors in September 2013 remains in place.
Consequently, even if the combined organization achieves profitability, it may not be able to utilize a material portion of Celladon’s, Eiger’s or the combined organization’s net operating loss carryforwards and other tax attributes,
which could have a material adverse effect on cash flow and results of operations.
74
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
This proxy statement/prospectus/information statement and the documents incorporated by reference into this proxy statement/prospectus/information statement
contain forward-looking statements. These forward-looking statements are based on current expectations and beliefs and involve numerous risks and uncertainties that could cause actual results to differ materially from expectations. These
forward-looking statements should not be relied upon as predictions of future events as Celladon cannot assure you that the events or circumstances reflected in these statements will be achieved or will occur. You can identify forward-looking
statements by the use of forward-looking terminology including “believes,” “expects,” “may,” “will,” “should,” “seeks,” “intends,” “plans,” “pro forma,”
“estimates,” or “anticipates” or the negative of these words and phrases or other variations of these words and phrases or comparable terminology. All statements other than statements of historical fact are statements that could
be deemed forward-looking statements. For example, forward-looking statements include, but are not limited to statements about:
| |
• |
|
the expected benefits of and potential value created by the merger for the stockholders of Celladon and Eiger; |
| |
• |
|
any statements of the plans, strategies and objectives of management for future operations, including the execution of integration and restructuring plans and the anticipated timing of filings; |
| |
• |
|
likelihood of the satisfaction of certain conditions to the completion of the merger and whether and when the merger will be consummated; |
| |
• |
|
statements of the plans, strategies and objectives of management with respect to the approval and closing of the merger, and Celladon’s ability to solicit a sufficient number of proxies to approve matters related
to the consummation of the merger; |
| |
• |
|
any statements concerning proposed new products, services or developments; |
| |
• |
|
any statements regarding future economic conditions or performance; and |
| |
• |
|
statements of belief and any statement of assumptions underlying any of the foregoing. |
For a discussion
of the factors that may cause Celladon, Eiger or the combined organization’s actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied in such forward-looking
statements, or for a discussion of risk associated with the ability of Celladon and Eiger to complete the merger and the effect of the merger on the business of Celladon, Eiger and the combined organization, see “Risk Factors” beginning on
page 27.
Additional factors that could cause actual results to differ materially from those expressed in the forward-looking statements are discussed in
reports filed with the SEC by Celladon. See “Where You Can Find More Information” beginning on page 265.
If any of these risks or
uncertainties materializes or any of these assumptions proves incorrect, the results of Celladon, Eiger or the combined organization could differ materially from the forward-looking statements. All forward-looking statements in this proxy
statement/prospectus/information statement are current only as of the date on which the statements were made. Celladon and Eiger do not undertake any obligation to publicly update any forward-looking statement to reflect events or circumstances
after the date on which any statement is made or to reflect the occurrence of unanticipated events.
75
THE SPECIAL MEETING OF CELLADON STOCKHOLDERS
Date, Time and Place
The
special meeting of Celladon stockholders will be held on 2016, at 12255 El Camino Real, Suite 300, San Diego, California 92130
commencing at local time. Celladon is sending this proxy statement/prospectus/information statement to its stockholders in connection with the solicitation of proxies by the
Celladon board of directors for use at the Celladon special meeting and any adjournments or postponements of the special meeting. This proxy statement/prospectus/information statement is first being furnished to stockholders of Celladon on or about
, 2016.
Purposes of the Celladon Special Meeting
The purposes of the Celladon special meeting are:
| 1. |
To consider and vote upon a proposal to approve the merger and the issuance of Celladon common stock in the merger pursuant to the Agreement and Plan of Merger and Reorganization, dated as of November 18, 2015, by
and among Celladon, Celladon Merger Sub, Inc. and Eiger, a copy of which is attached as Annex A to this proxy statement/prospectus/information statement; |
| 2. |
To approve the amendment to the amended and restated certificate of incorporation of Celladon to effect a reverse stock split of Celladon common stock, at a ratio of one new share for every 15 shares outstanding, in the
form attached as Annex D to this proxy statement/prospectus/information statement; |
| 3. |
To approve the amendment to the amended and restated certificate of incorporation of Celladon to change the name “Celladon Corporation” to “Eiger BioPharmaceuticals, Inc.” in the form attached as
Annex E to this proxy statement/prospectus/information statement; |
| 4. |
To consider and vote upon an adjournment of the Celladon special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Celladon Proposal Nos. 1, 2 and 3; and
|
| 5. |
To transact such other business as may properly come before the Celladon special meeting or any adjournment or postponement thereof. |
Recommendation of the Celladon Board of Directors
| |
• |
|
The Celladon board of directors has determined and believes that the merger and the issuance of shares of Celladon common stock pursuant to the merger is in the best interests of, Celladon and its stockholders and has
approved such items. The Celladon board of directors recommends that Celladon stockholders vote “FOR” Celladon Proposal No. 1 to approve the merger and the issuance of shares of Celladon common stock in the merger. |
| |
• |
|
The Celladon board of directors has determined and believes that it is advisable to, and in the best interests of, Celladon and its stockholders to approve the amendment to the amended and restated certificate of
incorporation of Celladon effecting the proposed 1-for-15 reverse stock split, as described in this proxy statement/prospectus/information statement. The Celladon board of directors recommends that Celladon stockholders vote “FOR” Celladon
Proposal No. 2 to approve the amendment to the amended and restated certificate of incorporation of Celladon effecting the proposed 1-for-15 reverse stock split, as described in this proxy statement/prospectus/information statement.
|
| |
• |
|
The Celladon board of directors has determined and believes that the amendment to the amended and restated certificate of incorporation of Celladon to change the name of Celladon to “Eiger BioPharmaceuticals,
Inc.” is advisable to, and in the best interests of, Celladon and its stockholders and has approved such name change. The Celladon board of directors recommends that Celladon stockholders vote “FOR” Celladon Proposal No. 3 to
approve the name change. |
76
| |
• |
|
The Celladon board of directors has determined and believes that adjourning the Celladon special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Celladon Proposal Nos.
1, 2 and 3 is advisable to, and in the best interests of, Celladon and its stockholders and has approved and adopted the proposal. The Celladon board of directors recommends that Celladon stockholders vote “FOR” Celladon Proposal
No. 4 to adjourn the Celladon special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Celladon Proposal Nos. 1, 2 and 3. |
Record Date and Voting Power
Only holders of record of Celladon common stock at the close of business on the record date,
, 2016, are entitled to notice of, and to vote at, the Celladon special meeting. At the close of business on the record date,
shares of Celladon common stock were issued and outstanding. Each share of Celladon common stock entitles the holder thereof to one vote on each matter submitted for
stockholder approval. See the section entitled “Principal Stockholders of Celladon” in this proxy statement/prospectus/information statement for information regarding persons known to the management of Celladon to be the beneficial owners
of more than 5% of the outstanding shares of Celladon common stock.
Voting and Revocation of Proxies
The proxy accompanying this proxy statement/prospectus/information statement is solicited on behalf of the board of directors of Celladon for use at the
Celladon special meeting.
If you are a stockholder of record of Celladon as of the record date referred to above, you may vote in person at the Celladon
special meeting or vote by proxy using the enclosed proxy card. Whether or not you plan to attend the Celladon special meeting, Celladon urges you to vote by proxy to ensure your vote is counted. You may still attend the Celladon special meeting and
vote in person if you have already voted by proxy. As a stockholder of record:
| |
• |
|
to vote in person, come to the Celladon special meeting and Celladon will give you a ballot when you arrive. |
| |
• |
|
to vote using the proxy card, simply mark, sign and date your proxy card and return it promptly in the postage-paid envelope provided. If you return your signed proxy card to Celladon before the Celladon special
meeting, Celladon will vote your shares as you direct. |
| |
• |
|
to vote on the Internet, go to the website on the proxy card or voting instruction form to complete an electronic proxy card. You will be asked to provide the company number and control number from the enclosed proxy
card. Your vote must be received by , 2016, Pacific Time to be counted. |
If your Celladon shares are held by your broker as your nominee, that is, in “street name,” the enclosed voting instruction card is sent by the
institution that holds your shares. Please follow the instructions included on that proxy card regarding how to instruct your broker to vote your Celladon shares. If you do not give instructions to your broker, your broker can vote your Celladon
shares with respect to “discretionary” items but not with respect to “non-discretionary” items. Discretionary items are proposals considered routine under the rules of The NASDAQ Global Market on which your broker may vote shares
held in “street name” in the absence of your voting instructions. On non-discretionary items for which you do not give your broker instructions, the Celladon shares will be treated as broker non-votes. It is anticipated that Celladon
Proposal No. 1 will be a non-discretionary item.
All properly executed proxies that are not revoked will be voted at the Celladon special meeting
and at any adjournments or postponements of the Celladon special meeting in accordance with the instructions contained in
77
the proxy. If a holder of Celladon common stock executes and returns a proxy and does not specify otherwise, the shares represented by that proxy will be voted “FOR” Celladon Proposal
No. 1 to approve the merger and the issuance of shares of Celladon common stock in the merger; “FOR” Celladon Proposal No. 2 to approve the amendment to the amended and restated certificate of incorporation of Celladon effecting
the proposed 1-for-15 reverse stock split; “FOR” Celladon Proposal No. 3 to approve the amendment to the amended and restated certificate of incorporation of Celladon to change the name of “Celladon Corporation” to
“Eiger BioPharmaceuticals, Inc.”; and “FOR” Celladon Proposal No. 4 to adjourn the Celladon special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Celladon Proposal Nos. 1,
2 and 3 in accordance with the recommendation of the Celladon board of directors.
Celladon stockholders of record, other than those Celladon stockholders
who have executed support agreements, may change their vote at any time before their proxy is voted at the Celladon special meeting in one of three ways. First, a stockholder of record of Celladon can send a written notice to the Secretary of
Celladon stating that the stockholder would like to revoke its proxy. Second, a stockholder of record of Celladon can submit new proxy instructions either on a new proxy card or via the Internet. Third, a stockholder of record of Celladon can attend
the Celladon special meeting and vote in person. Attendance alone will not revoke a proxy. If a Celladon stockholder of record or a stockholder who owns Celladon shares in “street name” has instructed a broker to vote its shares of
Celladon common stock, the stockholder must follow directions received from its broker to change those instructions.
Required Vote
The presence, in
person or represented by proxy, at the Celladon special meeting of the holders of a majority of the shares of Celladon common stock outstanding and entitled to vote at the Celladon special meeting is necessary to constitute a quorum at the meeting.
Abstentions and broker non-votes will be counted towards a quorum. Approval of Celladon Proposal Nos. 1 and 4 requires the affirmative vote of the holders of a majority of the shares of Celladon common stock having voting power present in person or
represented by proxy at the Celladon special meeting. Approval of Celladon Proposal Nos. 2 and 3 requires the affirmative vote of holders of a majority of the Celladon common stock having voting power outstanding on the record date for the Celladon
special meeting. Each of Proposal Nos. 1, 2 and 3 are conditioned upon each other. Therefore, the merger cannot be consummated without the approval of Proposal Nos. 1, 2 and 3.
Votes will be counted by the inspector of election appointed for the meeting, who will separately count “FOR” and “AGAINST” votes,
abstentions and broker non-votes. Abstentions will be counted towards the vote total for each proposal and will have the same effect as “AGAINST” votes. Broker non-votes will have the same effect as “AGAINST” votes for Celladon
Proposal No. 1. Celladon Proposal Nos. 2, 3 and 4 are matters on which a broker or other nominee are generally empowered to vote, and therefore, broker non-votes are not expected with respect to those proposals.
As of December 31, 2015, the directors and executive officers of Celladon owned 0.3% of the outstanding shares of Celladon common stock entitled to vote at
the Celladon special meeting. The directors and executive officers of Celladon owning these shares are subject to support agreements. Each stockholder that entered into a support agreement has agreed to vote all shares of Celladon common stock owned
by him as of the record date in favor of the merger and the issuance of Celladon common stock in the merger pursuant to the Merger Agreement, the adoption of the Merger Agreement if submitted for adoption, the approval of any proposal to adjourn or
postpone the meeting to a later date, if there are not sufficient votes for the merger and the issuance of Celladon common stock in the merger pursuant to the Merger Agreement on the date on which such meeting is held, and any other matter necessary
to consummate the transactions contemplated by the Merger Agreement that are considered and voted upon by Celladon’s stockholders and against any “acquisition proposal,” as defined in the Merger Agreement. As of December 31, 2015,
Celladon is not aware of any affiliate of Eiger owning any shares of Celladon common stock entitled to vote at the Celladon special meeting.
78
Solicitation of Proxies
In addition to solicitation by mail, the directors, officers, employees and agents of Celladon may solicit proxies from Celladon stockholders by personal
interview, telephone, telegram or otherwise. Celladon and Eiger will share equally the costs of printing and filing this proxy statement/prospectus/information statement and proxy card. Arrangements will also be made with brokerage firms and other
custodians, nominees and fiduciaries who are record holders of Celladon common stock for the forwarding of solicitation materials to the beneficial owners of Celladon common stock. Celladon will reimburse these brokers, custodians, nominees and
fiduciaries for the reasonable out-of-pocket expenses they incur in connection with the forwarding of solicitation materials. Celladon has retained Advantage Proxy to assist it in soliciting proxies using the means referred to above. Celladon will
pay the fees of Advantage Proxy, which Celladon expects to be approximately $10,000, plus reimbursement of out-of-pocket expenses.
Other Matters
As of the date of this proxy statement/prospectus/information statement, the Celladon board of directors does not know of any
business to be presented at the Celladon special meeting other than as set forth in the notice accompanying this proxy statement/prospectus/information statement. If any other matters should properly come before the Celladon special meeting, it is
intended that the shares represented by proxies will be voted with respect to such matters in accordance with the judgment of the persons voting the proxies.
79
THE MERGER
This section and the section entitled “The Merger Agreement” in this proxy statement/prospectus/information statement describe the material
aspects of the merger, including the Merger Agreement. While Celladon and Eiger believe that this description covers the material terms of the merger and the Merger Agreement, it may not contain all of the information that is important to you. You
should read carefully this entire proxy statement/prospectus/information statement for a more complete understanding of the merger and the Merger Agreement, including the Merger Agreement, and the other documents to which you are referred herein.
See the section entitled “Where You Can Find More Information” in this proxy statement/prospectus/information statement.
Background of the Merger
Historical Background for Celladon
Celladon is currently in the long-term follow-up stage of a 250-patient randomized, double-blind, placebo-controlled multinational Phase 2b trial that was
designed to evaluate MYDICAR® (AAV1/SERCA2a) in patients with heart failure for reduced ejection fraction, or HFrEF (also referred to as systolic heart failure). This Phase 2b trial is
referred to as the CUPID 2 trial. Celladon had previously devoted substantially all of its research, development and clinical efforts and financial resources toward the development of MYDICAR. CUPID 2 evaluated a single, one-time, intracoronary
infusion of the cardiovascular gene therapy agent MYDICAR versus placebo, in each case added to a maximal, optimized heart failure drug and device regimen. Celladon completed enrollment of CUPID 2 in February 2014 and un-blinded the results from the
active observation period in late April 2015.
On April 26, 2015, Celladon announced that the CUPID 2 trial did not meet its primary and secondary
endpoints and failed to show any treatment effect. No safety issues were noted. Following analysis of the CUPID 2 data and in light of these results, on April 26, 2015, the Celladon board of directors approved an approximately 50% reduction of
Celladon’s then-current full-time workforce of 34 employees in order to reduce operating expenses and conserve cash resources. Celladon’s board of directors also suspended further research and development activities for MYDICAR and
Celladon’s pre-clinical programs, and implemented cost-cutting measures including termination of certain contracts related to MYDICAR and the pre-clinical programs. Celladon’s continuing development activities are currently limited to the
oversight of the long-term follow up period in the CUPID 2 trial, which is expected to continue through February 2016.
As a consequence of the negative
results from the CUPID 2 trial, Celladon’s board of directors began evaluating its strategic opportunities to maximize stockholder value, including the possibility of seeking a merger, a sale of the company or all or some of its assets, and/or
a liquidation. Celladon’s management provided the Celladon board of directors with management’s preliminary assessment of a variety of strategic alternatives that Celladon could pursue to maximize stockholder value, including engaging in a
reverse merger process, a sale of some or all of Celladon’s assets, or distributing some or all of Celladon’s remaining cash through either a dividend or a liquidation of Celladon.
On May 14, 2015, Celladon announced that it was evaluating its strategic options in order to determine the best path forward to maximize stockholder
value.
On May 28, 2015, Celladon executed an engagement letter with Wedbush as its exclusive financial advisor in connection with a potential
merger, reorganization or other business combination transaction or potential alternatives thereto, including a liquidation and dissolution of Celladon.
On May 29, 2015, the Celladon board of directors approved a strategic plan which was announced on June 1, 2015, pursuant to which Celladon would
immediately commence a process to seek a merger or sale in order to maximize stockholder value. The company also announced that it had retained Wedbush as its exclusive financial
80
advisor. The Celladon board of directors directed management to explore, identify, evaluate, and if applicable recommend strategic transactions available to Celladon, in consultation with Wedbush
and legal counsel.
Also on May 29, 2015, Krisztina M. Zsebo, Ph.D., resigned as the Chief Executive Officer and as a director of Celladon, and
Patrick Y. Yang, Ph.D. resigned as a director of Celladon. Concurrently with Dr. Zsebo’s resignation, the Celladon board of directors appointed Paul Cleveland as Chief Executive Officer and as a director, in addition to his role as
President. At that same time, Andrew Jackson was appointed as Celladon’s Chief Financial Officer.
Beginning in May 2015 and continuing through
November 2015, Celladon conducted a process of identifying and evaluating potential strategic combinations with biotechnology companies. In its review, Celladon focused on biotechnology companies possessing (i) a portfolio of product
development candidates with the potential for significant value appreciation, (ii) resources sufficient to achieve potentially meaningful development milestones within such portfolio, including resources to be obtained through financing
activities consummated prior to the effectiveness of a combination with Celladon as well as the resources that would result from a combination with Celladon, (iii) an ability to enter into an agreement in the near-term for a combination with a
public company (i.e., Celladon) and thereafter proceed in an orderly manner toward implementing the combination (necessitating, for example, the availability of the requisite financial statements to accompany a registration statement on Form S-4),
and (iv) a management team with the breadth and skills to accomplish the foregoing. Working with Wedbush, Celladon identified and screened approximately 160 companies and set management calls and meetings with 29 companies. These activities
resulted in indications of interest in a potential combination with 19 biotechnology companies. In evaluating these indications of interest, including in certain cases through discussions and diligence activities with potential counterparties (see
in this regard the discussion below with respect to Celladon’s engagement with Parties 1, 2, 3, 4, 5 and 6), Celladon ultimately concluded in each instance (except for Eiger) that (x) one or more desired elements were missing from a
potential combination (for example, that the counterparty did not have sufficient resources to achieve potentially meaningful development milestones within its portfolio of product development candidates or an ability to enter into an agreement in
the near-term for a combination with a public company), (y) the terms expected to be available to Celladon and its stockholders in a potential combination, including as represented by the potential share of the combined company that might be
owned by the pre-combination Celladon stockholders immediately following a combination and any concurrent financing, would likely not be fair or appropriate to the pre-combination Celladon stockholders, and/or (z) Celladon should pursue a
combination with Eiger to the exclusion of other possibilities. In the course of its process, Eiger is the only party with which Celladon ultimately reached a mutual understanding on deal terms, including the potential share of the combined company
that would be owned by the pre-combination Celladon stockholders immediately following a combination and any concurrent financing, and moved forward with negotiating a definitive merger agreement.
On June 1, 2015, Wedbush contacted a major investor in Eiger to explore the possibility of a potential business combination between Celladon and Eiger,
and in response to a positive indication of interest, on June 7, 2015, Wedbush sent Eiger a non-confidential corporate presentation slide deck on Celladon. On June 17, 2015, Celladon and Eiger entered into a nondisclosure agreement.
On June 23, 2015, the Celladon board of directors approved the voluntary prepayment of the outstanding amounts due under Celladon’s Loan and
Security Agreement with Hercules Technology III, L.P. and Hercules Technology Growth Capital, Inc. dated July 31, 2014, with such prepayment to be effected on August 3, 2015. Celladon paid the lenders (i) the $10,000,000 outstanding
principal balance borrowed in 2014, (ii) $75,625 in accrued and unpaid interest, and (iii) an end of term charge of $1,750,000, for a total payment of $11,825,625. Upon the prepayment on August 3, 2015, Celladon’s obligations,
covenants, debts and liabilities under such Loan and Security Agreement were satisfied in full and the lender’s commitments to extend further credit to Celladon were terminated.
81
On June 24, 2015, the management teams of Celladon (i.e., Messrs. Cleveland and Jackson and Fredrik
Wiklund, Celladon’s Vice President, Corporate Development and Investor Relations) and Eiger (i.e., David Cory, the President and Chief Executive Officer of Eiger, James Welch, Eiger’s Chief Financial Officer, and James Shaffer,
Eiger’s Chief Business Officer) met in person to discuss a potential business combination. In follow-up, on June 27, 2015, Mr. Wiklund discussed the potential combination with Eiger director Nina Kjellson.
On June 26, 2015, Celladon confirmed its plans to suspend further research or development of its MYDICAR (AAV1/SERCA2a) program and its pre-clinical
programs including the Stem Cell Factor (mSCF) gene therapy and SERCA2b small molecule programs. Celladon further announced (i) that it was continuing to consider its strategic options, which could include the sale of the company or some or all
of its assets and/or a liquidation and distribution of the remaining cash to stockholders, and (ii) its estimate that, if it were to liquidate in the third quarter of 2015, the net cash available for distribution to Celladon’s stockholders
would be approximately $25-$30 million.
Also on June 26, 2015, Celladon announced a second workforce reduction which had been approved by the
Celladon board of directors on June 23, with approximately half of the employees not previously notified of termination of employment being terminated in the third quarter, and bringing the aggregate reductions to that time to approximately 70%
of Celladon’s peak workforce of 34 employees at April 30, 2015, in order to reduce operating expenses and further conserve cash resources.
On July 9, 2015, Celladon granted Eiger and its financial and legal advisors access to Celladon’s virtual data room for purposes of reviewing due
diligence materials.
On July 10, 2015, Wedbush received from a thirty party (“Party 1”) a preliminary proposal for a potential business
combination between Celladon and Party 1. Also on that date, Mr. Cleveland provided an update to the Celladon board of directors regarding the potential timing and financial implications for a hypothetical potential reverse merger transaction
with a private biotechnology company, for planning purposes, as well as an overview regarding various potential transactions being explored.
In July
2015, following Celladon’s announcements of the negative CUPID 2 data and the suspension of further research and development activities and the subsequent declines of the price of Celladon’s common stock, three putative securities class
action complaints (captioned Fialkov v. Celladon Corporation, Case No. 15-cv-1458-AJB-DHB, Lorusso v. Celladon Corporation, Case No. 15-cv-1501-L-JLB and Jacobs v. Celladon Corporation, Case No. 15-cv-1529-AJB-MDD ) were filed in the
U.S. District Court for the Southern District of California against Celladon and certain of Celladon’s current and former officers. The complaints generally allege that the defendants violated Sections 10(b) and 20(a) of the Securities Exchange
Act of 1934 by making materially false and misleading statements regarding the clinical trial program for MYDICAR, thereby artificially inflating the price of Celladon’s common stock. The complaints seek unspecified monetary damages and other
relief, including attorneys’ fees. On September 1, 2015, six stockholders (or groups of stockholders) filed motions to consolidate the three putative securities class actions and to appoint lead plaintiffs (the “Motions to
Consolidate”). A hearing on the Motions to Consolidate was held on December 3, 2015. On December 9, 2015, the Court consolidated the three putative securities class actions and appointed a lead plaintiff to represent the putative class.
Celladon expects the lead plaintiff to file a consolidated complaint. It is possible that additional suits will be filed, or allegations made by stockholders, with respect to these same or other matters and also naming Celladon and/or
Celladon’s officers and directors as defendants. Celladon believes that it has meritorious defenses and intends to defend these lawsuits vigorously. Due to the early stage of these proceedings, Celladon is not able to predict or reasonably
estimate the timing of resolving, or the ultimate outcome or possible losses relating to, these claims.
On July 16 and 20, 2015, Mr. Cleveland
provided updates to the Celladon board of directors regarding the nature and status of various biotechnology companies being explored as possible counterparties for a potential combination with Celladon, including Party 1 and another third party
(“Party 2”), as well as the expressed interest by certain possible counterparties in such a combination.
82
On July 21, 2015, the Celladon board of directors met to review with its financial advisor, Wedbush, and its
legal advisor, Pillsbury Winthrop Shaw Pittman LLP, or Pillsbury, the status and nature of ongoing discussions with various possible counterparties regarding a potential business combination, as well as potential transaction structures, timing and
legal considerations.
On July 22, 2015, Party 1 and Party 2 granted Celladon and its financial and legal advisors access to their respective
data rooms for purposes of reviewing due diligence materials.
On July 23, 2015, members of Celladon’s management and its legal and
financial advisors held a process and due diligence telephone conference with Party 1, and Party 1’s legal advisor transmitted to Pillsbury on behalf of Celladon a draft term sheet for a pre-merger financing of Party 1 and a draft securities
purchase agreement relating thereto.
On July 24, 2015, on behalf of Celladon, Pillsbury provided to legal counsel for Party 1 a preliminary term
sheet for a potential business combination between Celladon and Party 1.
Also on July 24, 2015, members of Celladon’s management and its legal
and financial advisors held a process and due diligence telephone conference with Party 2, and Pillsbury transmitted to legal counsel for Party 2 a preliminary term sheet for a potential business combination between Celladon and Party 2.
On the same date, members of Celladon’s management and its legal and financial advisors held a process and due diligence telephone conference with
another third party (“Party 3”), Pillsbury transmitted to legal counsel for Party 3 a preliminary term sheet for a potential business combination between Celladon and Party 3, and Party 3 granted Celladon and its financial and legal
advisors access to Party 3’s data room for purposes of reviewing due diligence materials.
On July 29, 2015, Pillsbury received on behalf
of Celladon a revised preliminary term sheet from legal counsel for Party 1, and on that same date, Celladon and Party 3 held a financial due diligence call.
On July 31, 2015 and August 3 and 4, 2015, Mr. Cleveland provided updates to the Celladon board of directors regarding discussions with various
possible counterparties to a potential business combination with Celladon.
On August 3, 2015, Mr. Cleveland and the chief executive officer of
Party 2 discussed by telephone the proposed transaction between Celladon and Party 2.
On August 4, 2015, the Celladon board of directors held a
meeting to review the status of discussions with Party 1 and Party 2 and due diligence information relating thereto, and Mr. Cleveland provided a post-meeting update to the board members.
On August 6, 2015, Celladon and Party 1 held a clinical due diligence call. Also, on August 6, 2015, Celladon received information on certain
potential terms for a potential business combination from Party 2.
On August 7, 2015, Mr. Cleveland had a telephone conference with the chief
executive officer of Party 2 regarding the status of Party 2’s acquisition of certain clinical development assets and Party 2’s pre-merger financing.
On August 14, 2015, Mr. Cleveland had a telephone discussion with the chief executive officer of Party 2 regarding a potential business combination
between the parties and the status of Party 2’s pending pre-merger financing.
On August 17 and 18, 2015, Mr. Cleveland provided updates to
the Celladon board of directors regarding the status of discussions with Party 1 as well as a summary of Celladon’s due diligence activities with Party 1, together with Celladon management’s recommendation that, due to the absence of
certain desired elements in a potential combination with Party 1, further discussions be terminated with Party 1, subject to discussion with the board of directors at an upcoming meeting.
83
On August 18, 2015, Celladon received a revised preliminary term sheet from Party 2. On August 20,
2015, Mr. Cleveland had a telephone discussion with the chief executive officer of Party 2 to discuss the status of Party 2’s pending pre-merger financing and pre-merger business activities.
On August 19, 2015, the Celladon board of directors held a meeting to discuss the status and nature of discussions and due diligence activities with
various counterparties, including the decision not to pursue further negotiations with Party 1.
On August 20, 2015, Mr. Cleveland notified the
chief executive officer of Party 1 of Celladon’s election to terminate further discussions with respect to a potential business combination with Party 1. Also on that date, Mr. Cleveland had a telephone discussion with the chief executive
officer of Party 2 regarding the status of Party 2’s pre-merger financing as well as timing for a potential business combination.
On August 21,
2015, members of the Celladon’s management team held a telephone conference with finance personnel from Party 2 regarding a potential business combination. On August 26, 2015, the chief executive officer of Party 2 notified
Mr. Cleveland by email that Party 2 had completed its planned pre-merger business activities.
On August 27, 2015, Mr. Cleveland and the
chief executive officer of Party 2 discussed by telephone a potential business combination, and Mr. Cleveland transmitted information regarding certain terms for a potential business combination between Celladon and Party 2.
On September 9, 2015, Messrs. Cleveland and Wiklund met in person with Mr. Cory to continue discussions regarding a potential business combination
between Celladon and Eiger.
On September 10, 2015, in light of the scale-down of certain operations, Celladon terminated the lease agreement
for office space in San Diego, California, effective November 13, 2015, in order to reduce Celladon’s office space and corresponding rent obligations. In the third quarter of 2015, Celladon also terminated its Seattle, Washington leases.
On September 11, 2015, Mr. Cleveland provided an update to the Celladon board of directors regarding the status of discussions with various
possible counterparties, and Celladon’s management engaged with the board of directors regarding various potential business combination transactions that Celladon could pursue.
On September 14, 2015, Mr. Cleveland provided a preliminary term sheet to Mr. Cory outlining certain terms for a potential business
combination. This preliminary term sheet provided that the pre-combination Celladon securityholders would collectively receive a share of the combined company equal to the percentage represented by $28 million divided by the sum of the pre-money
value of Eiger established in a financing concurrent with the proposed combination (capped at $100 million) plus the sum of the proceeds raised in such financing plus Celladon’s $28 million allocation, with (i) such $28 million figure to be
adjusted up or down depending upon whether Celladon possessed more or less than $26.5 million in “net cash” at the time of the proposed combination, (ii) Celladon believing that it would have approximately $26.5 million in net cash at the
time of the proposed combination, and (iii) net cash being defined as the sum (at the time of the proposed combination) of Celladon’s cash, cash equivalents and other current assets, plus reimbursement owed to Celladon by Eiger for 50% of
certain transaction fees (such as printer and stock exchange fees), less the amount of Celladon’s accounts payable and accrued expenses, less the amount of any change in control, severance or retention payments owed or potentially payable by
Celladon, less Celladon’s merger-related transaction fees and expenses, less a reserve equal to the remaining retention amount for any ongoing litigation involving Celladon, and less any remaining amounts to prepay a D&O tail policy for
Celladon, all remaining lease obligations of Celladon and a third-party contractor to manage remaining activities for the CUPID 2 trial. This preliminary term sheet also provided that Eiger would conduct a financing concurrent with the merger for at
least $50 million in proceeds, Celladon and Eiger would propose one member and four members, respectively, for the post-merger board of directors, and the two-way termination fee payable by the parties in certain circumstances would be $3 million
plus up to $1 million in expense reimbursement.
84
On September 17, 2015, members of Celladon’s management held a telephone conference with a third party
(“Party 4”) to discuss a potential business combination, and Mr. Cleveland transmitted to the chief executive officer of Party 4 a preliminary term sheet for a potential business combination between Celladon and Party 4.
Also on September 17, 2015, on behalf of Celladon, Wedbush transmitted to the financial advisor for a third party (“Party 5”) a preliminary
term sheet for a potential business combination between Celladon and Party 5.
On September 18, 2015, Eiger provided comments to Celladon on
Celladon’s proposed terms for a potential business combination. These comments sought clarification on Celladon’s “net cash” definition (for purposes of establishing the share of the combined company that would be owned by the
pre-combination Celladon securityholders), lowered to $30 million the minimum proceeds for an Eiger financing concurrent with the proposed combination, sought support agreements for the proposed combination from Celladon’s board of directors
and major stockholders, sought a period of exclusivity for Eiger to continue discussions with Celladon regarding a proposed combination, voided any termination fee obligation for Eiger if it did not close upon a financing otherwise expected to be
concurrent with the proposed combination, and included a placeholder for discussion regarding post-combination board of director composition.
On
September 21, 2015, Messrs. Cleveland and Wiklund discussed by telephone with Mr. Cory the status of discussions and diligence activities between the parties.
On September 22, 2015, members of Celladon’s management (i.e., Messrs. Cleveland, Jackson and Wiklund and Ms. Reed) and members of Eiger’s
management (i.e., Messrs. Cory, Welch and Shaffer) as well as representatives from each company’s financial advisors (Wedbush for Celladon and Jefferies for Eiger) and each company’s legal advisors (Pillsbury for Celladon and Cooley LLP,
or Cooley, for Eiger) held a telephonic meeting to discuss various aspects of a potential business combination between the parties. Topics addressed included the respective shares of the combined company that would be owned by the pre-combination
securityholders of each company as well as participants in an Eiger financing expected to be concurrent with the proposed combination, the “net cash” definition used for purposes of establishing the ownership share for the pre-combination
securityholders of Celladon, the status and potential terms of a concurrent Eiger financing, support agreements for the proposed combination from affiliates of each party, the composition of the post-merger board of directors, and the termination
fee and expense reimbursement payable by either party in certain termination events. Also on September 22, 2015, Mr. Wiklund spoke with a potential investor in Eiger to discuss Eiger’s proposed pre-merger financing arrangements.
On September 23 and 25, 2015, the Celladon board of directors held meetings to discuss a potential business combination with Eiger. On September 23,
2015, Celladon received additional due diligence materials from Eiger regarding its clinical development programs, and on September 24 and 25, 2015, members of Celladon’s management (i.e., Messrs. Cleveland, Jackson and Wiklund and Ms.
Reed) participated in legal due diligence and financial due diligence calls with Eiger management (i.e., Messrs. Cory, Welch and Shaffer) as well as KPMG, its accountants, and Cooley.
On September 26, 2015, Cooley transmitted to Pillsbury a draft exclusivity letter between Eiger and Celladon, although no such letter was ever finalized
or implemented.
On October 6, 2015, members of Celladon’s management team met with the chief executive officer of a third party (“Party
6”) regarding a potential business combination between Celladon and Party 6, and Party 6 transmitted to Celladon due diligence information regarding Party 6.
On October 8, 2015, Mr. Cory called Mr. Cleveland to convey that Eiger had received a draft term sheet from a syndicate to provide pre-merger
financing to Eiger.
On October 12, 2015, Mr. Cleveland provided an update to the Celladon board of directors on the proposed merger transaction
with Eiger as well as various potential alternative business combinations that remained under evaluation by Celladon.
85
On October 13, 2015, Mr. Cory and Mr. Cleveland discussed by telephone the status of discussions
between the parties as well as the status of Eiger’s pre-merger financing.
On October 15, 2015, Eiger granted Celladon and its financial
and legal advisors access to Eiger’s data room for purposes of reviewing additional due diligence materials.
On October 16, 2015, Celladon
received a draft term sheet from Eiger relating to Eiger’s proposed pre-merger financing. This draft term sheet anticipated a financing for Eiger of $40 million in proceeds at a pre-money value for Eiger of $55 million, with the pre-combination
Celladon securityholders holding a share of the combined company equal on a relative basis to $25.5 million. Mr. Cleveland and Mr. Cory exchanged email correspondence regarding the draft financing term sheet.
Also on October 16, 2015, Celladon provided a preliminary term sheet to Party 6 with respect to a potential business combination between Celladon and
Party 6.
On October 19, 2015, Pillsbury had a telephone conference with Cooley to discuss the draft term sheet for Eiger’s pre-merger
financing.
On October 20, 2015, on behalf of Celladon, Pillsbury transmitted to Cooley, on behalf of Eiger, a term sheet with respect to a
potential business combination between Celladon and Eiger. This term sheet provided that the pre-combination Celladon securityholders would collectively receive a share of the combined company equal to the percentage represented by $28 million
divided by the sum of $55 million (as the pre-money value of Eiger in a financing expected to be concurrent with the proposed combination) plus the sum of $40 million in proceeds expected to be raised in such financing plus Celladon’s $28
million allocation, with such $28 million figure to be adjusted up or down depending upon whether Celladon possessed more or less than $26.5 million in “net cash” (pursuant to the definition previously proposed by Celladon and detailed
above) at the time of the proposed combination. This term sheet also provided that Eiger would conduct a financing concurrent with the proposed combination for at least $40 million in proceeds, Eiger would propose all members for the post-merger
board of directors (assuming that the composition of directors would be compliant with the listing standards of The Nasdaq Stock Market), support agreements would be provided by the officers and directors of both parties as well as their respective
affiliates, the two-way termination fee payable by the parties in certain circumstances would be $3 million plus up to $1 million in expense reimbursement, and no exclusivity would exist with respect to negotiations toward a potential combination
unless and until a definitive merger agreement is executed. Also on October 20, 2015, Cooley transmitted to Pillsbury a draft subscription agreement for Eiger’s pre-merger financing.
On October 21, 2015, Cooley transmitted to Pillsbury comments on the proposed term sheet with respect to a potential business combination between
Celladon and Eiger. These comments lowered to $26 million the relative allocation for the pre-combination Celladon securityholders, deleted the potential adjustment in such allocation based upon Celladon’s net cash at the time of the merger,
and lowered to $30 million the minimum proceeds for Eiger’s financing concurrent with the merger.
On October 23, 2015, Mr. Cleveland
provided an update to the Celladon board of directors regarding the status of negotiations with Eiger. Mr. Cleveland also provided due diligence information on Eiger for review by the board together with the current draft of the term sheet with
respect to a potential business combination between Celladon and Eiger. Also on that date, Mr. Cleveland talked by telephone with Mr. Cory about the status of negotiations, including features of the formula for determining the exchange
ratio for the conversion of shares of Eiger capital stock into shares of Celladon common stock, as well as the status of Eiger’s pre-merger financing.
On October 25, 2015, the Celladon board of directors held a meeting to review the term sheet with respect to a potential business combination between
Celladon and Eiger. Following discussion regarding the term sheet, the status of Eiger’s pre-merger financing and due diligence information regarding Eiger, the Celladon board of directors authorized Celladon’s management to provide a
draft Merger Agreement to Eiger and to continue negotiations with Eiger regarding a potential business combination between the parties.
86
On October 26, 2015, members of Celladon’s management (i.e., Messrs. Cleveland, Jackson and Wiklund
and Elizabeth Reed, Celladon’s Vice President and General Counsel) and members of Eiger’s management (i.e., Mr. Cory, James Welch, Eiger’s Chief Financial Officer, and James Shaffer, Eiger’s Chief Business Officer) as well as
representatives from each company’s financial advisors (Wedbush for Celladon and Jefferies for Eiger) and each company’s legal advisors (Pillsbury for Celladon and Cooley for Eiger), held a meeting to discuss the potential business
combination transaction between Celladon and Eiger. Topics addressed included the allocation of ownership in the combined company for the pre-combination securityholders of Celladon and Eiger, the status and expected size of a concurrent Eiger
financing and the allocation of ownership in the combined company for investors in such financing, and a potential timeline for the parties to finalize negotiations and diligence activities as well as for Eiger to finalize arrangements with respect
to its financing.
On October 27, 2015, on behalf of Celladon, Pillsbury sent a draft Merger Agreement to Cooley for review, on behalf of Eiger. This
draft Merger Agreement provided that the pre-combination Celladon securityholders would collectively receive a share of the combined company equal to the percentage represented by $27 million divided by the sum of $55 million (as the pre-money value
of Eiger in its financing expected to be concurrent with the proposed combination) plus the sum of the gross proceeds raised in such financing plus Celladon’s $27 million allocation. This draft Merger Agreement also provided that Eiger would
conduct a financing concurrent with the proposed combination for at least $30 million in proceeds, in addition to the conversion of any promissory notes issued in anticipation of such financing, and that the two-way termination fee payable by the
parties in certain circumstances would be $3 million plus up to $1 million in expense reimbursement.
On October 29, 2015, Mr. Cory
provided an update to Mr. Cleveland regarding the status of Eiger’s pre-merger financing, and Messrs. Cory and Cleveland discussed potential timing for a proposed business combination transaction between the parties.
On October 30, 2015, the chief executive officer of Party 6 called Mr. Cleveland to discuss moving forward with discussions regarding a potential
business combination between Celladon and Party 6. Also on that date, Messrs. Cory and Cleveland discussed by telephone the status of Eiger’s pre-merger financing as well as certain terms with respect to a proposed business combination between
the parties.
On November 1, 2015, Cooley transmitted to Pillsbury comments from Eiger on Celladon’s draft Merger Agreement. These comments
included that the pre-combination Celladon securityholders would collectively receive a share of the combined company equal to the percentage represented by $26.75 million divided by the sum of $55 million (as the pre-money value of Eiger in its
financing expected to be concurrent with the proposed combination) plus the sum of the gross proceeds raised in such financing plus Celladon’s $26.75 million allocation, that Celladon would need to have at least $26.5 million in net cash at the
time of the proposed combination (with the net cash definition approximating the definition originally proposed by Celladon as discussed above) as a condition to Eiger’s obligation to proceed with the proposed combination, and that the
obligation for any fee plus any reimbursement of expenses payable by a party in certain termination events would be capped at $3 million in total.
On November 2, 2015, Messrs. Cleveland and Cory spoke by telephone regarding the terms of the proposed business combination transaction between the
parties and Eiger’s comments to the draft Merger Agreement.
On November 3, 2015, Mr. Cleveland and the chief executive officer of Party 6
discussed by telephone a potential business combination between Celladon and Party 6.
On November 4, 2015, members of Celladon’s management
(i.e., Messrs. Cleveland, Jackson and Wiklund and Elizabeth Reed, Celladon’s Vice President and General Counsel) and members of Eiger’s management (i.e., Mr. Cory, James Welch, Eiger’s Chief Financial Officer, and James Shaffer,
Eiger’s Chief Business Officer) as well as representatives from each company’s legal advisors (Pillsbury for Celladon and Cooley for Eiger) held a conference call to review and discuss the draft Merger Agreement and proposed business
combination
87
transaction between the parties. Also on that date, Mr. Cleveland provided an update to the Celladon board of directors regarding the proposed business combination transaction between
Celladon and Eiger, including materials prepared by Wedbush regarding certain financial aspects of the proposed transaction. Pillsbury provided Celladon’s board of directors with a current draft of the Merger Agreement for review and
discussion.
On November 5, 2015, Party 6 transmitted to Celladon a revised term sheet for a proposed business combination between Celladon and
Party 6. Also on that date, Pillsbury transmitted to Cooley a revised draft Merger Agreement with respect to the proposed business combination between Celladon and Eiger. This draft Merger Agreement provided that the pre-combination Celladon
securityholders would collectively receive a share of the combined company equal to the percentage represented by $26.75 million divided by the sum of $55 million (as the pre-money value of Eiger in its financing expected to be concurrent with the
proposed combination) plus the sum of the gross proceeds raised in such financing plus Celladon’s $26.75 million allocation, reflecting concurrence between the parties with respect to the exchange ratio for the proposed combination, and that
Celladon would need to have at least $24 million in net cash at the time of the proposed combination (as opposed to the $26 million in net cash most recently proposed by Eiger) as a condition to Eiger’s obligation to proceed with the proposed
combination.
On November 5 and 9, 2015, the Celladon board of directors held meetings to discuss the terms of the proposed business combination
transaction between Celladon and Eiger, the status of Eiger’s pre-merger financing, and due diligence information regarding Eiger. At these meetings, representatives of Wedbush discussed with the board of directors of Celladon certain
preliminary financial information regarding the proposed business combination transaction between Celladon and Eiger. Celladon’s management also provided the board of directors with its assessment of the amount potentially available for
distribution to stockholders if Celladon were to pursue an orderly liquidation (see “The Merger—Opinion of the Celladon Financial Advisor—Future Liquidation Value—Celladon”), noting that various circumstances rendered the
expected timing and amount of any such distribution, and thus its value to stockholders, uncertain and/or adversely affected. These circumstances include the pending lawsuits which followed Celladon’s announcements of the negative CUPID 2 data
and the suspension of further research and development activities as well as the subsequent declines of the price of Celladon’s common stock. At the November 5 and 9, 2015 meetings of the board of directors, Celladon management also
provided the Celladon board of directors with an outline of proposed terms with respect to, and an update on discussions regarding, a potential business combination with Party 6.
On November 6, 2015, Pillsbury discussed various non-substantive revisions to the proposed draft Merger Agreement between Celladon and Eiger with Cooley,
and on November 7, 2015, Mr. Jackson discussed certain non-substantive provisions of the draft Merger Agreement with Mr. Welch.
On
November 8, 2015, on behalf of Celladon, Pillsbury transmitted to Cooley, on behalf of Eiger, a revised draft Merger Agreement with respect to a proposed business combination transaction between Celladon and Eiger which reflected concurrence
between the parties on the substantive aspects of the proposed transaction.
Between November 10 and 18, 2015, Pillsbury and Cooley exchanged
multiple additional drafts of the Merger Agreement with respect to a proposed business combination transaction between Celladon and Eiger to reflect ongoing negotiations on various non-substantive issues between the parties.
On November 13, 2015, Mr. Cleveland provided an update to the Celladon board of directors regarding the status of negotiations between Celladon and
Eiger with respect to a Merger Agreement regarding a proposed business combination transaction as well as the status of Eiger’s pre-merger financing.
On November 16, 2015, members of Celladon’s management held a telephonic meeting with Eiger and KPMG to review the status of Eiger’s financial
statements and KPMG’s audit of certain of those financial statements.
Also on November 16, 2015, Mr. Cleveland distributed to the Celladon
board of directors, in advance of a meeting scheduled for later that day, a current draft of the Merger Agreement with respect to a proposed business
88
combination transaction between Celladon and Eiger, proposed resolutions for adoption by the Celladon board of directors if it elected to authorize Celladon’s management to proceed with such
transaction, and a presentation to the Celladon board of directors prepared by Wedbush containing certain financial analyses relating to the proposed transaction which assumed, among other things, that Eiger would receive a minimum of $30 million in
pre-merger financing.
Further on November 16, 2015, the Celladon board of directors held a meeting which included Celladon’s legal and
financial advisors. During the meeting, members of Celladon’s management reviewed the key features of the proposed business combination between Celladon and Eiger, including: structure and timing considerations; the exchange ratio for the
conversion of Eiger capital stock into Celladon common stock as well as the relative percentages of ownership of the existing Celladon stockholders, on the one hand, and the Eiger stockholders (including investors in Eiger’s planned pre-merger
financing), on the other hand, following the completion of the merger; the planned pre-merger financing of Eiger; the terms of a bridge loan to be made to Eiger by certain of its investors in such planned pre-merger financing; the terms of support
agreements from certain Eiger directors, officers, stockholders and affiliates, as well as Celladon executive officers and directors, to vote in favor of the proposed business combination; the closing conditions in the proposed Merger Agreement as
well as the subscription agreement for Eiger’s planned pre-merger financing; and the termination provisions and termination fees set forth in the proposed Merger Agreement. In addition, representatives of Wedbush reviewed with the board of
directors Wedbush’s financial analysis of the exchange ratio for the conversion of Eiger capital stock into Celladon common stock and delivered an oral opinion, which was confirmed by delivery of a written opinion dated November 16, 2015,
to the effect that, as of such date and based upon and subject to assumptions made, procedures followed, matters considered and qualifications and limitations set forth therein, the exchange ratio was fair, from a financial point of view, to
Celladon. Representatives from Pillsbury reviewed with the Celladon Board of Directors the fiduciary duties of the board members in the context of the proposed business combination. During the various presentations, the board of directors of
Celladon asked questions and discussed the terms and features of the proposed business combination, including provisions of the proposed Merger Agreement and related documentation. After the presentations and discussion among the Celladon board of
directors, the board unanimously (i) determined that the proposed merger and the other transactions contemplated by the Merger Agreement are fair to and in the best interests of Celladon and its stockholders, (ii) approved and adopted the
proposed Merger Agreement and the transactions contemplated thereby, subject to finalization of the proposed Merger Agreement and ancillary documents by Celladon’s management in consultation with Celladon’s legal counsel, with such changes
thereto as Celladon’s management deems to be in the best interests of Celladon and its stockholders, (iii) approved a reverse split of Celladon’s common stock in a ratio to be determined by Celladon’s Chief Executive Officer or
Chief Financial Officer, and (iv) resolved to recommend that the Celladon stockholders vote to approve the merger, adopt the Merger Agreement, approve a reverse split of Celladon’s common stock and approve and/or adopt the other
transactions and arrangements as contemplated by the Merger Agreement, including the issuance of shares of Celladon common stock in the merger.
At the
November 16, 2015 meeting, the Celladon board of directors also ratified and approved a further reduction in force affecting all remaining employees and officers of Celladon other than Messrs. Jackson and Wiklund and Ms. Reed, with such
terminations effective on or before November 15, 2015, with the exception of Mr. Cleveland whose termination was to be effective as of midnight on the day after the signing of the definitive Merger Agreement. Mr. Cleveland’s
separation was ultimately effective at midnight on November 19, 2015, and he resigned as a director at that time. The Celladon board of directors appointed Mr. Wiklund to succeed Mr. Cleveland as President and Chief Executive Officer
upon Mr. Cleveland’s separation.
Following the November 16, 2015 board meeting, Mr. Cleveland requested that Wedbush include
certain additional information in its presentation materials to reflect a scenario addressed in the November 16, 2015 board meeting in which Eiger would have $39.5 million in proceeds from its planned pre-merger financing activities, in
addition to the scenario presented in the Wedbush presentation delivered to the Celladon board of directors on November 16, 2015, in which Eiger’s pre-merger financing activities were assumed to result in only the $30 million minimum
requirement contemplated by the Merger Agreement, with such supplement not
89
affecting the opinion of Wedbush provided to the Celladon board of directors on November 16, 2015. Such updated materials were provided to the members of the Celladon board of directors on
November 17, 2015, and their view of the merger was unaffected.
On November 18, 2015, members of the Celladon and Eiger management teams met,
together with representatives of Pillsbury and Cooley, to finalize the proposed Merger Agreement. After finalization, Celladon and Eiger entered into the Merger Agreement.
Historical Background for Eiger
Eiger’s
board of directors and executive management regularly review Eiger’s operating and strategic plans, both near-term and long-term, as well as potential partnerships in an effort to enhance stockholder value, including debt and/or equity
financing, mergers and acquisitions, and other strategic transactions, and engaged in discussions with numerous potential strategic partners, lenders and investors, including then current investors in Eiger and potential new investors. On or about
September 17, 2015, Eiger retained Jeffries to provide advice and review the proposed terms of a transaction with Celladon as well as to identify and complete the syndicate for the pre-merger contemplated by a transaction with Celladon.
On September 18, 2015, Eiger’s Chief Executive Officer, Mr. Cory and Jefferies updated Eiger’s board of directors in a telephone meeting
regarding the proposed business combination of Eiger and Celladon and the proposed term sheet drafted by Celladon. Following the review of the negotiations, the Eiger board of directors provided Mr. Cory guidance regarding acceptable terms and
instructed Mr. Cory to continue discussions with Celladon toward acceptable terms.
Throughout the next two weeks Mr. Cory provided regular
updates to the Board as Eiger pursued the negotiations with Celladon as well as finalization of terms for a potential private financing as an alternative available in the event the business combination negotiations with Celladon failed to proceed.
On October 9, 2015, the Eiger board of directors discussed by telephone the status of the proposed business combination with Celladon. During the
meeting Mr. Cory and representative of Cooley LLP, legal counsel to Eiger, updated the board regarding the ongoing negotiations and the main issues that were being negotiated as the final major terms regarding a potential business combination.
On October 16, 2015, the Eiger board of directors held a telephonic meeting, in which Eiger’s management and legal counsel updated the board of
directors regarding the status of the financing term sheet, bridge loan documents, timeline for the potential transaction with Celladon, the support of Jeffries and Eiger’s three-year budget by program. During the meeting Mr. Tom Dietz was
appointed as new independent board member and Mr. Eduardo Martins was approved as Senior Vice President of Clinical Development.
On October 25,
2015, the Eiger board of directors held a telephonic meeting, during which the Eiger management and legal counsel updated the board of directors regarding the negotiations with Celladon of the Merger Agreement, discussions with potential investors
in the Celladon transaction as well as an alternative financing to remain private, and the status of the bridge financing.
On November 15, 2015, the
Eiger board of directors held a telephonic meeting with Eiger’s legal and financial advisors to review the finally negotiated major business terms of the Celladon transaction. The directors acknowledged and discussed that they had met and
discussed on numerous occasions, both formerly and informally, the potential merits and risks to Eiger and its stockholders of the financing contemplated by the Subscription Agreement and of the merger, the chronology of events leading to the
proposals to approve such financing and the merger, the negotiations with the investors in such financing and with Celladon with respect to the merger, the requirements for a bridge financing to proceed with the merger and the terms and conditions
of such financing and the merger and related timeline. Eiger’s legal counsel summarized the terms and conditions of
90
the proposed financing and merger, advised the directors on their fiduciary duties and answered directors’ questions. After discussion, Eiger’s board of directors (i) authorized
and approved the financing contemplated by the Subscription Agreement and the related bridge financing from the three largest investors in the financing, (ii) approved and adopted the Subscription Agreement, (iii) determined that the
Merger Agreement and the transactions contemplated thereby, including the merger, are advisable and fair to, and in the best interests of, Eiger and its stockholders, (iv) authorized and approved the merger, (v) approved and adopted the
Merger Agreement, (vi) resolved to recommend that the stockholders of Eiger approve and adopt the Merger Agreement and (vii) approved certain other related matters, including the terms of a financing alternative to remain private in the
event the merger is unsuccessful.
Celladon Reasons for the Merger
The Celladon board of directors considered the following factors in reaching its conclusion to approve and adopt the Merger Agreement and the transactions
contemplated thereby and to recommend that the Celladon stockholders approve the merger, adopt the Merger Agreement and approve the other transactions contemplated by the Merger Agreement, including the issuance of shares of Celladon common stock in
the merger, all of which the Celladon board of directors viewed as supporting its decision to approve the business combination with Eiger:
| |
• |
|
The Celladon board of directors believes, based in part on the judgment, advice and analysis of Celladon management with respect to the potential strategic, financial and operational benefits of the merger (which
judgment, advice and analysis was informed in part on the business, technical, financial, accounting and legal due diligence investigation performed with respect to Eiger), that: |
(i) the combined organization will be a clinical-stage company with a diversified development portfolio of three well-characterized compounds
addressing novel targets for four distinct orphan diseases. To date, over 50 HDV infected patients have been dosed with lonafarnib, which is Eiger’s most advanced program, across international Phase 2 clinical trials. Lonafarnib has
demonstrated dose-related activity in reducing HDV viral load both as monotherapy and in combination with other agents;
(ii) Eiger’s
approach to orphan diseases is distinct in two important ways: first, Eiger pursues opportunities in which disease biology has been elucidated by academic collaborators or other research efforts; and second, these are opportunities in which
Eiger’s team has identified a well characterized, clinical stage product candidate that can be tested in clinical proof-of-concept studies in Eiger’s targeted indication;
(iii) the combined organization will be led by experienced senior management from Eiger and a board of directors of seven members designated
by Eiger; and
(iv) Eiger has commitments for $33.5 million to fund Eiger’s development pipeline from an investor syndicate that
includes its existing, founding venture investors, Vivo Capital and InterWest Partners, as well as new investors. This investment, in addition to $6.0 million of funding provided to Eiger prior to execution of the definitive Merger Agreement along
with the existing Celladon cash, is expected to provide sufficient funding to advance all four pipeline programs. At least two of the planned Phase 2 programs are expected to generate potentially meaningful clinical data during 2016, although
further funding may be required to complete Phase 2 studies in the other two programs. Each of Eiger’s Phase 2 programs has the potential, if successful, to create value for the stockholders of the merged company and present the combined
organization with additional fund raising opportunities in the future.
| |
• |
|
The Celladon board of directors also reviewed with the management of Celladon the current plans of Eiger for developing its product candidates to
confirm the likelihood that the combined organization would possess sufficient financial resources to allow the management team to focus initially on the continued development of its product candidates. The Celladon board of directors also
considered the |
91
| |
possibility that the combined organization would be able to take advantage of the potential benefits resulting from the combination of Celladon and Eiger to raise additional funds in the future.
|
| |
• |
|
The Celladon board of directors considered the opportunity as a result of the merger for Celladon stockholders to participate in the potential value that may result from development of the Eiger product candidate
portfolio and the potential increase in value of the combined organization following the merger. |
| |
• |
|
The Celladon board of directors concluded that the merger would provide the existing Celladon stockholders with a significant opportunity to participate in the potential increase in value of the combined organization
following the merger. |
| |
• |
|
The Celladon board of directors considered the analyses of Wedbush, and its opinion to the board of directors of Celladon as to the fairness to Celladon, from a financial point of view and as of the date of such
opinion, of the exchange ratio for the conversion of Eiger capital stock into Celladon common stock, as more fully described below under the caption “The Merger—Opinion of the Celladon Financial Advisor.” |
| |
• |
|
The Celladon board of directors also reviewed various factors impacting the financial condition, results of operations and prospects for Celladon, including: |
(i) the strategic alternatives of Celladon to the merger, including potential transactions that could have resulted from discussions that
Celladon’s management conducted with other potential merger partners;
(ii) the consequences of the negative results from the Phase
2b CUPID 2 clinical trial of Celladon’s lead product candidate, MYDICAR (AAV1/SERCA2a), and the likelihood that the resulting circumstances for the company would not change for the benefit of the Celladon stockholders in the foreseeable future
on a stand-alone basis;
(iii) the risks associated with, and the uncertain value, timing and costs to stockholders of, liquidating
Celladon or effecting a sale of all or some of its assets and thereafter distributing the proceeds;
(iv) the risks of continuing to
operate Celladon on a stand-alone basis, including the need to rebuild the company’s product candidate development programs, infrastructure and management to continue its operations; and
(v) the risks and costs associated with litigation, including defending against claims made in the three putative class action complaints
filed in July 2015 following Celladon’s announcements regarding the negative Phase 2b CUPID 2 clinical trial data and the suspension of further research and development activities, and the subsequent decline of the price of Celladon’s
common stock.
The Celladon board of directors also reviewed the terms and conditions of the proposed Merger Agreement and associated transactions, as
well as the safeguards and protective provisions included therein intended to mitigate risks, including:
| |
• |
|
the exchange ratio used to establish the number of shares of Celladon common stock to be issued in the merger, and the expected relative percentage ownership of Celladon stockholders and Eiger stockholders immediately
following the completion of the merger; |
| |
• |
|
the planned pre-merger financing in Eiger, the limited number and nature of conditions to the obligation of the proposed investors in Eiger to consummate the planned pre-merger financing, and the ability of Celladon to
specifically enforce the obligations of the investors to complete the investment in Eiger if all of such conditions have been satisfied; |
| |
• |
|
the limited number and nature of the conditions to the Eiger obligation to consummate the merger and the limited risk of non-satisfaction of such conditions as well as the likelihood that the merger will be consummated
on a timely basis; |
92
| |
• |
|
the respective rights of, and limitations on, Celladon and Eiger under the Merger Agreement to consider certain unsolicited acquisition proposals under certain circumstances should Celladon or Eiger receive a superior
proposal; |
| |
• |
|
the reasonableness of the potential termination fee of $3.0 million or the reimbursement of certain transaction expenses of up to $1.0 million, which could become payable by either Celladon or Eiger if the Merger
Agreement is terminated in certain circumstances; |
| |
• |
|
the support agreements, pursuant to which certain directors, officers and affiliated stockholders of Eiger agreed, solely in their capacity as stockholders, to vote all of their shares of Eiger capital stock in favor of
adoption of the Merger Agreement; |
| |
• |
|
the agreement of Eiger to provide written consent of its stockholders necessary to adopt the Merger Agreement thereby approving the merger and related transactions within two business days of the registration statement
on Form S-4, of which this proxy statement/prospectus/information statement is a part, becoming effective; and |
| |
• |
|
the belief that the terms of the Merger Agreement, including the parties’ representations, warranties and covenants, and the conditions to their respective obligations, are reasonable under the circumstances.
|
In the course of its deliberations, the Celladon board of directors also considered a variety of risks and other countervailing factors
related to entering into the merger, including:
| |
• |
|
the $3.0 million termination fee or the reimbursement of certain transaction expenses of up to $1.0 million that may be payable to Eiger upon the occurrence of certain events, and the potential effect of such
termination fee or reimbursement of transaction expenses in deterring other potential acquirors from proposing an alternative transaction that may be more advantageous to Celladon stockholders; |
| |
• |
|
the substantial expenses to be incurred in connection with the merger; |
| |
• |
|
the possible volatility, at least in the short term, of the trading price of the Celladon common stock resulting from the merger announcement; |
| |
• |
|
the risk that the merger might not be consummated in a timely manner or at all and the potential adverse effect of the public announcement of the merger or on the delay or failure to complete the merger on the
reputation of Celladon; |
| |
• |
|
the risk to Celladon’s business, operations and financial results in the event that the merger is not consummated; |
| |
• |
|
the strategic direction of the continuing entity following the completion of the merger, which will be determined by a board of directors initially designated entirely by Eiger; |
| |
• |
|
the fact that the merger would give rise to substantial limitations on the utilization of Celladon’s NOLs; and |
| |
• |
|
various other risks associated with the combined organization and the merger, including those described in the section entitled “Risk Factors” in this proxy statement/prospectus/information statement.
|
The foregoing information and factors considered by the Celladon board of directors are not intended to be exhaustive but are believed to
include all of the material factors considered by the Celladon board of directors. In view of the wide variety of factors considered in connection with its evaluation of the merger and the complexity of these matters, the Celladon board of directors
did not find it useful to attempt, and did not attempt, to quantify, rank or otherwise assign relative weights to these factors. In considering the factors described above, individual members of the Celladon board of directors may have given
different weight to different factors. The Celladon board of directors conducted an overall analysis of the factors described above, including thorough discussions with, and questioning of, the Celladon management team and the legal and financial
advisors of Celladon, and considered the factors overall to be favorable to, and to support, its determination.
93
Eiger Reasons for the Merger
In the course of reaching its decision to approve the merger, the Eiger board of directors consulted with its senior management, financial advisor and legal
counsel, reviewed a significant amount of information and considered a number of factors, including, among others:
| |
• |
|
the potential to provide its current stockholders with greater liquidity by owning stock in a public company; |
| |
• |
|
the potential to access of public market capital, including sources of capital from a broader range of investors to support the clinical development of its product candidates than it could otherwise obtain if it
continued to operate as a privately held company; |
| |
• |
|
the cash resources of the combined organization, including the concurrent financing, expected to be available at the closing of the merger would provide funding to continue or initiate clinical development of all four
development programs with the opportunity to receive Phase 2 data from at least two of the four programs in 2016; |
| |
• |
|
the potential increased access to sources of capital and a broader range of investors to support the clinical development of its product candidates than it could otherwise obtain if it continued to operate as a
privately held company; |
| |
• |
|
the expectation that the merger with Celladon would be a more time- and cost-effective means to access capital than other options considered, including an initial public offering which Eiger was alternatively planning
to pursue; |
| |
• |
|
value to the principal investors under the Subscription Agreement of Eiger’s business being operated as a public company as a condition to their investment; |
| |
• |
|
the fact that shares of Celladon common stock issued to Celladon stockholders will be registered pursuant to a Form S-4 registration statement by Celladon and will become freely tradable for Eiger’s stockholders
who are not affiliates of Eiger; |
| |
• |
|
the likelihood that the merger will be consummated on a timely basis; |
| |
• |
|
the terms and conditions of the Merger Agreement, including, without limitation, the following: |
| |
• |
|
the determination that an exchange ratio that is fixed and not subject to adjustment based on trading prices is appropriate to reflect the expected relative percentage ownership of Celladon securityholders, Eiger
securityholders and securityholders of those shares sold in the concurrent financing was appropriate based, in the judgment of the Eiger’s board of directors; |
| |
• |
|
the expectation that the merger should be treated as a reorganization for U.S. federal income tax purposes, with the result that the Eiger stockholders will generally not recognize taxable gain or loss for U.S. federal
income tax purposes; |
| |
• |
|
the limited number and nature of the conditions of the obligation of Celladon to consummate the merger and the limited risk of non-satisfaction of such conditions; |
| |
• |
|
the rights of Eiger under the Merger Agreement to consider certain unsolicited acquisition proposals under certain circumstances should Eiger receive a superior proposal; and |
| |
• |
|
the conclusion of Eiger’s board of directors that the potential termination fee of $3.0 million, or in some situations the reimbursement of certain transaction expenses incurred in connection with the merger of up
to $1.0 million, payable by Celladon to Eiger and the circumstances when such fee may be payable, were reasonable. |
94
Eiger’s board of directors also considered a number of uncertainties and risks in its deliberations
concerning the merger and the other transactions contemplated by the Merger Agreement, including the following:
| |
• |
|
the possibility that the merger might not be completed and the potential adverse effect of the public announcement of the merger on the reputation of Eiger and the ability of Eiger to obtain financing in the future in
the event the merger is not completed; |
| |
• |
|
the termination fee of $3.0 million or in some situations the reimbursement of certain transaction expenses incurred in connection with the merger of up to $1.0 million, payable by Eiger to Celladon upon the occurrence
of certain events, and the potential effect of such termination fee in deterring other potential acquirers from proposing an alternative transaction that may be more advantageous to Eiger’s stockholders; |
| |
• |
|
the risk that the merger might not be consummated in a timely manner or at all; |
| |
• |
|
the expenses to be incurred in connection with the merger and related administrative challenges associated with combining the companies; |
| |
• |
|
the additional public company expenses and obligations that Eiger’s business will be subject to following the merger that it has not previously been subject to; and |
| |
• |
|
various other risks associated with the combined organization and the merger, including the risks described in the section entitled “Risk Factors” in this proxy statement/prospectus/information statement.
|
The foregoing information and factors considered by the Eiger board of directors are not intended to be exhaustive but are believed to
include all of the material factors considered by the Eiger board of directors. In view of the wide variety of factors considered in connection with its evaluation of the merger and the complexity of these matters, the Eiger board of directors did
not find it useful, and did not attempt, to quantify, rank or otherwise assign relative weights to these factors. In considering the factors described above, individual members of the Eiger board of directors may have given different weight to
different factors. The Eiger board of directors conducted an overall analysis of the factors described above, including thorough discussions with, and questioning of, Eiger’s management and Eiger’s legal and financial advisors, and
considered the factors overall to be favorable to, and to support, its determination.
Opinion of the Celladon Financial
Advisor
Scope of the Assignment
In May
2015, Celladon’s board of directors engaged Wedbush to provide financial advisory and investment banking services in connection with evaluating and considering potential strategic transactions, and ultimately requested that Wedbush render an
opinion as to whether the exchange ratio in connection with the merger, as provided in the Merger Agreement, was fair to Celladon from a financial point of view. At the November 16, 2015 meeting of Celladon’s board of directors, Wedbush
rendered its oral opinion, subsequently confirmed by delivery of a written opinion dated November 16, 2015, to Celladon’s board of directors that, as of the date of such opinion, and based upon the assumptions made, procedures followed,
matters considered, and qualifications and limitations of the review set forth in its written opinion, the exchange ratio in connection with the merger, as provided in the Merger Agreement, was fair to Celladon from a financial point of view.
The full text of Wedbush’s written opinion, which sets forth the procedures followed, assumptions made, matters considered, and qualifications and
limitations of the review undertaken in connection with such opinion, is attached as Annex B and is incorporated herein by reference. Wedbush’s opinion was intended for the use and benefit of Celladon’s board of directors (in its
capacity as such) in connection with its evaluation of the merger. Wedbush’s opinion did not address Celladon’s underlying business decision to enter into the Merger Agreement or complete the merger or the relative merits of the merger
compared to any alternative transactions or strategies
95
that may be available to Celladon and did not constitute a recommendation to Celladon’s board of directors as to how to act or to any Celladon stockholder or any other person as to how to
vote with respect to the merger or any other matter. The following summary of Wedbush’s opinion is qualified in its entirety by reference to the full text of such opinion.
For purposes of its opinion and in connection with its review of the exchange ratio in connection with the merger, as provided in the Merger Agreement,
Wedbush, among other things:
| |
• |
|
reviewed a draft of the Merger Agreement dated November 13, 2015; |
| |
• |
|
reviewed certain publicly available business and financial information relating to Celladon and Eiger, respectively; |
| |
• |
|
reviewed certain internal information, primarily financial in nature, furnished to Wedbush by the managements of Celladon and Eiger, respectively, and approved for Wedbush’s use by Celladon; |
| |
• |
|
reviewed certain publicly available information with respect to other companies in the biopharmaceutical industry that Wedbush believed to be comparable in certain respects to Eiger; and |
| |
• |
|
considered the financial terms, to the extent publicly available, of selected recent business combinations and initial public offerings involving companies in the biopharmaceutical industry that Wedbush believed to be
comparable in certain respects to Eiger, in whole or in part, and to the merger. |
In addition, Wedbush held discussions with the management
of Celladon and Eiger concerning their views as to the financial and other information described in the bullet points above. Wedbush also conducted such other analyses and examinations and considered such other financial, economic and market
criteria as Wedbush deemed appropriate to arrive at its opinion.
In rendering its opinion, Wedbush relied upon and assumed, without independent
verification, the accuracy and completeness of all information that was publicly available or was furnished to or discussed with Wedbush by Celladon, Eiger or any other party to the Merger Agreement or otherwise reviewed by Wedbush. With respect to
information provided to or reviewed by it, Wedbush was advised by management of Celladon and Eiger that such information was reasonably prepared on bases reflecting the best currently available estimates and judgments of management of Celladon or
Eiger, as applicable. Wedbush did not express any view as to the reasonableness of such financial information or the assumptions on which it was based.
Wedbush further relied on the assurances of Celladon’s management that they were unaware of any facts that would make the information provided to Wedbush
incomplete or misleading. Except for certain estimates of liabilities expected to be incurred by Celladon in connection with a potential liquidation of Celladon prepared by management of Celladon and estimated equity values of Celladon upon
liquidation prepared by management of Celladon, Wedbush did not make and was not provided with any independent evaluations or appraisals of any of the assets, properties, liabilities (including any contingent, derivative or off-balance-sheet assets
or liabilities) or securities, nor did Wedbush make any physical inspection of the properties or assets, of Celladon or Eiger or any of their respective subsidiaries. Further, as Celladon’s board of directors was aware, Eiger’s management
did not provide Wedbush with, and Wedbush did not otherwise have access to, financial forecasts regarding Eiger’s business, other than certain expense forecasts for the three years ended December 31, 2018, and, accordingly, Wedbush did not
perform either a discounted cash flow analysis or any multiples-based analyses with respect to Eiger. Wedbush did not evaluate the solvency or fair value of Celladon, Eiger, or any of their subsidiaries (or the impact of the transactions
contemplated by the Merger Agreement thereon) under any law relating to bankruptcy, insolvency or similar matters.
Wedbush’s opinion was based on
economic, market and other conditions as in effect on, and the information made available to Wedbush as of, the date of such opinion. Wedbush also relied on the accuracy and completeness of Celladon’s and Eiger’s representations and
warranties in the Merger Agreement, without regard to any qualifications that may be set forth in disclosure schedules or any other such qualifications. In addition,
96
Wedbush assumed that the merger will be consummated in accordance with the terms set forth in the Merger Agreement without any waiver, amendment or delay of any terms or conditions that would be
material to Wedbush’s analysis. Representatives of Celladon advised Wedbush that, and Wedbush further assumed that, the final terms of the Merger Agreement would not differ from the terms set forth in the draft reviewed by Wedbush in any
respect material to Wedbush’s analysis. In addition, Wedbush assumed that Eiger’s contemplated issuance of at least $30,000,000 of Eiger common stock would be consummated prior to the consummation of the merger in accordance with the terms
of the Merger Agreement. Wedbush noted that events occurring after the date of its opinion could materially affect the assumptions used in preparing its opinion. Wedbush did not undertake any obligation to reaffirm or revise its opinion or otherwise
comment upon any events occurring after the date of such opinion.
Wedbush is not a legal, tax or regulatory advisor, and did not express any opinion as
to any tax or other consequences that may arise from the transactions contemplated by the Merger Agreement, nor does its opinion address any legal, regulatory or accounting matters, as to which Wedbush understood that Celladon had obtained such
advice as it deemed necessary from qualified professionals. Wedbush is a financial advisor only and relied upon, without independent verification, the assessment of Celladon and Eiger and their legal, tax or regulatory advisors with respect to
legal, tax or regulatory matters. Wedbush assumed that the merger will have the tax effects contemplated by the Merger Agreement.
Wedbush is an
investment banking firm and a member of The New York Stock Exchange and other principal stock exchanges in the United States, and is regularly engaged as part of its business in the valuation of businesses and their securities in connection with
mergers and acquisitions, negotiated underwritings, private placements, secondary distributions of listed and unlisted securities, and valuations for corporate, estate and other purposes. Wedbush was selected by Celladon based on Wedbush’s
experience, expertise, reputation and familiarity with Celladon. Celladon’s board of directors did not impose any limitations on Wedbush with respect to the investigations made or procedures followed in rendering its opinion. Wedbush’s
opinion was approved by a fairness committee at Wedbush in accordance with the requirements of FINRA Rule 5150.
In rendering its opinion, Wedbush
expressed no opinion as to the amount or nature of any compensation to any officers, directors, or employees of Celladon, or any class of such persons, whether relative to the exchange ratio or otherwise, or with respect to the fairness of any such
compensation.
Wedbush was not asked to, nor did it, offer any opinion as to the terms, other than the exchange ratio in connection with the merger to the
extent expressly set forth in Wedbush’s opinion, of the Merger Agreement or the form of the merger. Wedbush did not express any opinion with respect to the terms of any other agreement entered into or to be entered into in connection with the
merger. Wedbush expressed no opinion as to the price at which shares of common stock of Celladon may trade at any time subsequent to the announcement or consummation of the merger. Wedbush also assumed that all governmental, regulatory or other
consents and approvals necessary for the consummation of the merger will be obtained without imposition of any terms or conditions that would be material to Wedbush’s analysis.
Celladon paid Wedbush a $20,000 retainer upon execution of its engagement letter and has agreed to pay Wedbush a fee of $100,000 for rendering its opinion,
which became payable upon the delivery of Wedbush’s opinion. Celladon has also agreed to pay Wedbush as additional fee of $630,000, contingent upon closing of the merger. In addition, Celladon has agreed to indemnify Wedbush for certain
liabilities arising out of its engagement and has agreed to reimburse Wedbush for its expenses, including attorney’s fees and disbursements. In the two years prior to the date of its opinion, Wedbush has provided to Celladon services unrelated
to the merger in connection with (i) Celladon’s initial public offering in January 2014 and (ii) Celladon’s follow-on equity offering in August 2014. Wedbush received aggregate fees in the amount of approximately $0.5 million for
the foregoing referenced services that were unrelated to the merger. In the two years prior to the date of its opinion, Wedbush has not provided any services to Eiger. Wedbush may in the future provide investment banking and financial advisory
services to Celladon, Eiger and their respective affiliates for which services Wedbush would expect to receive compensation.
97
In the ordinary course of its business, Wedbush and its affiliates may actively trade the common stock of
Celladon or other instruments or obligations of Celladon for their own accounts and for the accounts of their customers and, accordingly, Wedbush and its affiliates may at any time hold a long or short position in the common stock of Celladon or
such other instruments or obligations of Celladon.
Summary of Analyses
The following is a summary of the material financial analyses performed by Wedbush in connection with reaching its opinion:
| |
• |
|
Public Market Equity Value Analysis with respect to Celladon |
| |
• |
|
Future Liquidation Value with respect to Celladon |
| |
• |
|
Public Company Market Valuation Analysis with respect to Eiger |
| |
• |
|
Merger and Acquisition Transaction Analysis with respect to Eiger |
| |
• |
|
Initial Public Offering Analysis with respect to Eiger |
The following summaries are not a comprehensive
description of Wedbush’s opinion or the analyses and examinations conducted by Wedbush, and the preparation of an opinion necessarily is not susceptible to partial analysis or summary description. Wedbush believes that such analyses and the
following summaries must be considered as a whole and that selecting portions of such analyses and of the factors considered, without considering all such analyses and factors, would create an incomplete view of the process underlying the analyses.
The order in which the analyses are described below does not represent the relative importance or weight given to the analyses by Wedbush. Some of the summaries of financial analyses below include information presented in tabular format. In order to
fully understand the analyses, the tables must be read together with the text of each summary. The tables alone do not constitute a complete description of Wedbush’s analyses. Considering the data described below without considering the full
narrative description of the financial analyses, including the methodologies and assumptions underlying the analyses, could create a misleading or incomplete view of the analyses.
In performing its analyses, Wedbush made numerous assumptions with respect to industry performance and general business and economic conditions such as
industry growth, inflation, interest rates and many other matters, many of which are beyond the control of Celladon, Eiger and Wedbush. Any estimates contained in Wedbush’s analyses are not necessarily indicative of actual values or future
results, which may be significantly more or less favorable than suggested by such analyses.
Wedbush noted that it was Celladon management’s view
that a discounted cash flow analysis was not an appropriate method of valuing Celladon because Celladon had ceased all product research and development and therefore did not have any anticipated future revenues to form a basis for such an analysis.
Accordingly, Wedbush did not conduct a discounted cash flow analysis and instead relied on the other analyses described herein.
Wedbush did not perform a
discounted cash flow analysis or any multiples-based analyses for Eiger because Wedbush was not provided, and Wedbush did not otherwise have access to, financial forecasts regarding Eiger’s business, other than certain expense forecasts for the
three years ending December 31, 2018. Further, Wedbush believed that such analyses were not appropriate because Eiger is a clinical stage company with no marketed products and it will not have any revenues until its product candidates are
approved for marketing by the FDA, which will require the successful completion of ongoing or planned phase 2 trials and future phase 3 trials.
Except as
otherwise noted, the following quantitative information, to the extent that it is based on market data, is based on market data as it existed on or before November 13, 2015 and is not necessarily indicative of current market conditions.
98
Public Market Equity Value Analysis—Celladon
Using publicly available information, Wedbush noted that the volume weighed average trading price for the Celladon common stock was $1.07 per share on
November 13, 2015, $1.11 per share for the one week ended November 13, 2015 and $1.13 per share for the one month ended November 13, 2015. Based upon these volume weighted average trading prices for the Celladon common stock and the
number of fully diluted outstanding shares of Celladon common stock as provided by management of Celladon, Wedbush calculated Celladon’s equity value as approximately $25.7 million to $26.9 million.
Future Liquidation Value – Celladon
Wedbush
reviewed information prepared by Celladon management regarding Celladon’s liquidation value. Based upon Celladon’s cash balance of approximately $36.0 million as of October 2015 and Celladon management’s estimates of future
liabilities with respect to clinical obligations, pending litigation, insurance and legal costs, other corporate expenses, lease expenses and compensation and severance expenses, Wedbush noted that Celladon management estimated that Celladon would
have a liquidation value of approximately $26.1 million as of June 30, 2016 and, that, taking into account Celladon management estimates of monthly cash expenses after such date, Celladon would have a liquidation value of approximately $23.7
million as of June 30, 2017.
Public Company Market Valuation Analysis—Eiger
Wedbush reviewed publicly available information relating to the following publicly-traded companies with an aggregate market capitalization under $1 billion in
the biopharmaceutical industry which had multiple Phase 2 product candidates and no product candidates beyond Phase 2 (the “Phase 2 Companies”):
| |
• |
|
Aldeyra Therapeutics, Inc. |
| |
• |
|
Atara Biotherapeutics, Inc. |
| |
• |
|
Minerva Neurosciences, Inc. |
| |
• |
|
OncoMed Pharmaceuticals, Inc. |
| |
• |
|
Viking Therapeutics, Inc. |
Wedbush also reviewed publicly available information relating to the following publicly-traded
companies with an aggregate market capitalization under $1 billion in the biopharmaceutical industry which had a therapeutic focus in antivirals (the “Antiviral Companies”):
| |
• |
|
Arbutus Biopharma Corporation |
| |
• |
|
Arrowhead Research Corporation |
| |
• |
|
Assembly Biosciences, Inc. |
| |
• |
|
Conatus Pharmaceuticals Inc. |
| |
• |
|
ContraVir Pharmaceuticals, Inc. |
| |
• |
|
Regulus Therapeutics Inc. |
| |
• |
|
Sangamo Biosciences, Inc. |
| |
• |
|
Tobira Therapeutics, Inc. |
Wedbush noted that, although such companies had certain financial and operating
characteristics that could be considered similar to those of Eiger, none of the companies had the same management, make-up, technology, size or mix of business as Eiger and, accordingly, there were inherent limitations on the applicability of such
companies to the valuation analysis of Eiger.
99
Wedbush calculated the aggregate market capitalization of each of the selected companies based upon the closing
price of the common stock of each selected company on November 13, 2015 and the fully-diluted number of shares outstanding, using the treasury stock method. For purposes of this analysis, Wedbush calculated the “trimmed mean” without
taking into account the companies with the highest and lowest market capitalizations. The results of this analysis are summarized as follows:
|
|
|
|
|
|
|
|
|
| |
|
Market Capitalization at November 13, 2015
($ in millions) |
|
| |
|
Phase 2 Companies |
|
|
Antiviral Companies |
|
| Trimmed Mean |
|
$ |
346.8 |
|
|
$ |
261.3 |
|
| Median |
|
$ |
333.4 |
|
|
$ |
245.4 |
|
Wedbush calculated the implied ownership of holders of Celladon common stock in the combined company based upon the $26.75
million value attributed to the Celladon common stock pursuant to the exchange ratio formula in the Merger Agreement, the trimmed mean and median values described above and taking into account $30.0 million in additional financing to be raised by
Eiger prior to closing of the merger. In addition, following the Celladon board meeting on November 16, 2015, Wedbush also calculated such implied ownership percentages taking into account $39.5 million of pre-closing financing. Wedbush
compared these implied ownership percentages with the implied ownership percentages of holders of Celladon common stock in the combined company based upon the $26.75 million value attributed to the Celladon common stock and the $55.0 million value
attributed to the Eiger common stock pursuant to the exchange ratio formula in the Merger Agreement.
The results of this analysis are summarized as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Implied Ownership Based on Phase 2 Companies |
|
| |
|
$30.0M Pre-Closing Financing |
|
|
$39.5M Pre-Closing Financing |
|
| |
|
Trimmed
Mean
($346.8M) |
|
|
Median
($333.4M) |
|
|
Merger
Agreement
($55M) |
|
|
Trimmed
Mean
($346.8M) |
|
|
Median
($333.4M) |
|
|
Merger
Agreement
($55M) |
|
| Eiger |
|
|
85.9 |
% |
|
|
85.5 |
% |
|
|
49.2 |
% |
|
|
84.0 |
% |
|
|
83.4 |
% |
|
|
45.4 |
% |
| Celladon |
|
|
6.6 |
% |
|
|
6.9 |
% |
|
|
23.9 |
% |
|
|
6.5 |
% |
|
|
6.7 |
% |
|
|
22.1 |
% |
| Pre-closing financing investors |
|
|
7.4 |
% |
|
|
7.7 |
% |
|
|
26.8 |
% |
|
|
9.6 |
% |
|
|
9.9 |
% |
|
|
32.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Implied Ownership Based on Antiviral Companies |
|
| |
|
$30.0M Pre-Closing Financing |
|
|
$39.5M Pre-Closing Financing |
|
| |
|
Trimmed
Mean
($261.3M) |
|
|
Median
($245.4M) |
|
|
Merger
Agreement
($55M) |
|
|
Trimmed
Mean
($261.3M) |
|
|
Median
($245.4M) |
|
|
Merger
Agreement
($55M) |
|
| Eiger |
|
|
82.2 |
% |
|
|
81.2 |
% |
|
|
49.2 |
% |
|
|
79.8 |
% |
|
|
78.7 |
% |
|
|
45.4 |
% |
| Celladon |
|
|
8.4 |
% |
|
|
8.9 |
% |
|
|
23.9 |
% |
|
|
8.2 |
% |
|
|
8.6 |
% |
|
|
22.1 |
% |
| Pre-closing financing investors |
|
|
9.4 |
% |
|
|
9.9 |
% |
|
|
26.8 |
% |
|
|
12.1 |
% |
|
|
12.7 |
% |
|
|
32.6 |
% |
Wedbush noted that the implied ownership percentage of holders of Celladon common stock based upon the $26.75 million value
attributed to the Celladon common stock and the $55.0 million value attributed to the Eiger common stock pursuant to the exchange ratio formula in the Merger Agreement was higher than the implied ownership percentages derived based upon the trimmed
mean and median equity values attributed to Eiger described above.
Merger and Acquisition Transaction Analysis—Eiger
Wedbush reviewed publicly available information relating to the following acquisitions of companies in the biopharmaceutical industry which had either a
therapeutic focus in antivirals or multiple phase 2 product candidates with no product candidates beyond Phase 2 at the time of announcement of the transaction, in each
100
case, with an aggregate valuation (based solely upon upfront payments and excluding contingent value rights or other post-closing payments) of less than $1.0 billion announced between January
2005 and November 2015 (the “Selected Transactions”):
|
|
|
|
|
| Announcement Date |
|
Target |
|
Acquiror |
| January 11, 2015 |
|
OnCore Biopharma, Inc. |
|
Tekmira Pharmaceuticals Corporation |
| May 12, 2014 |
|
Lumena Pharmaceuticals, Inc. |
|
Shire plc |
| May 29, 2013 |
|
Okairos AG |
|
GlaxoSmithKline plc |
| May 7, 2013 |
|
Inviragen, Inc. |
|
Takeda Pharmaceutical Company Limited |
| October 17, 2011 |
|
Anadys Pharmaceuticals, Inc. |
|
Roche Holding AG |
| March 3, 2009 |
|
ViroChem Pharma Inc. |
|
Vertex Pharmaceuticals Incorporated |
| January 31, 2007 |
|
Arrow Therapeutics Ltd. |
|
AstraZeneca PLC |
Other than Anadys Pharmaceuticals, Inc., which was publicly traded, all of the target companies in such transactions were
privately-held, like Eiger. Wedbush noted that although the companies that were acquired in the Selected Transactions had certain financial and operating characteristics that could be considered similar to those of Eiger, none of such companies had
the same management, make-up, technology, size or mix of business as Eiger and, accordingly, there were inherent limitations on the applicability of such companies to the valuation analysis of Eiger. In addition, none of the acquiring companies in
such transactions was in the process of winding down its operations at the time of the relevant transaction. Wedbush also noted that market conditions have varied over the precedent time periods.
Wedbush calculated the aggregate value of each of the target companies in the Selected Transactions (based solely on upfront payments). For purposes of this
analysis, Wedbush calculated the “trimmed mean” without taking into account the companies with the highest and lowest valuations. The results of this analysis are summarized as follows:
|
|
|
| |
|
Valuation ($ in millions) |
| Trimmed Mean |
|
$276.9 |
| Median |
|
$300.3 |
Wedbush calculated the implied ownership of holders of Celladon common stock in the combined company based upon the $26.75
million value attributed to the Celladon common stock pursuant to the exchange ratio formula in the Merger Agreement, the trimmed mean and median values described above and taking into account $30.0 million in additional financing to be raised by
Eiger prior to closing of the merger. In addition, following the Celladon board meeting on November 16, 2015, Wedbush also calculated such implied ownership percentages taking into account $39.5 million of pre-closing financing. Wedbush
compared these implied ownership percentages with the implied ownership percentages of holders of Celladon common stock in the combined company based upon the $26.75 million value attributed to the Celladon common stock and the $55.0 million value
attributed to the Eiger common stock pursuant to the exchange ratio formula in the Merger Agreement.
The results of this analysis are summarized as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Implied Ownership |
|
| |
|
$30.0M Pre-Closing Financing |
|
|
$39.5M Pre-Closing Financing |
|
| |
|
Trimmed
Mean
($276.9M) |
|
|
Median
($300.3M) |
|
|
Merger
Agreement
($55M) |
|
|
Trimmed
Mean
($276.9M) |
|
|
Median
($300.3M) |
|
|
Merger
Agreement
($55M) |
|
| Eiger |
|
|
83.0 |
% |
|
|
84.1 |
% |
|
|
49.2 |
% |
|
|
80.7 |
% |
|
|
81.9 |
% |
|
|
45.4 |
% |
| Celladon |
|
|
8.0 |
% |
|
|
7.5 |
% |
|
|
23.9 |
% |
|
|
7.8 |
% |
|
|
7.3 |
% |
|
|
22.1 |
% |
| Pre-closing financing investors |
|
|
9.0 |
% |
|
|
8.4 |
% |
|
|
26.8 |
% |
|
|
11.5 |
% |
|
|
10.8 |
% |
|
|
32.6 |
% |
101
Wedbush noted that the implied ownership percentage of holders of Celladon common stock based upon the $26.75
million value attributed to the Celladon common stock and the $55.0 million value attributed to the Eiger common stock pursuant to the exchange ratio formula in the Merger Agreement was higher than the implied ownership percentages derived based
upon the trimmed mean and median equity values attributed to Eiger described above.
Initial Public Offering Analysis—Eiger
Wedbush reviewed publicly available information relating to the following initial public offerings of companies in the biopharmaceutical industry which had
multiple Phase 2 product candidates and no product candidates beyond Phase 2 at the time of the initial public offering and which raised a minimum of $30.0 million between January 2011 and November 2011 (the “Phase 2 IPOs”):
|
|
|
| Pricing Date |
|
Issuer |
| May 7, 2014 |
|
Alder BioPharmaceuticals, Inc. |
| February 11, 2014 |
|
Flexion Therapeutics, Inc. |
| January 30, 2014 |
|
Ultragenyx Pharmaceutical Inc. |
Wedbush also reviewed publicly available information relating to the following initial public offerings of companies in
the biopharmaceutical industry which had a therapeutic focus in antivirals and which raised a minimum of $30 million between January 2011 and November 2011 (the “Antiviral IPOs”):
|
|
|
| Pricing Date |
|
Issuer |
| February 6, 2014 |
|
Argos Therapeutics, Inc. |
| February 4, 2014 |
|
Genocea Biosciences, Inc. |
| July 24, 2013 |
|
Conatus Pharmaceuticals Inc. |
| April 10, 2013 |
|
Chimerix, Inc. |
| March 21, 2013 |
|
Enanta Pharmaceuticals, Inc. |
| October 4, 2012 |
|
Regulus Therapeutics Inc. |
Wedbush noted that although such companies had certain financial and operating characteristics that could be considered
similar to those of Eiger, none of the companies had the same management, make-up, technology, size or mix of business as Eiger and, accordingly, there were inherent limitations on the applicability of such companies to the valuation analysis of
Eiger. Wedbush also noted that market conditions have varied over the precedent time periods.
Wedbush calculated the fully diluted pre-money valuation of
each of the companies that participated in the Phase 2 IPOs and Antiviral IPOs at the time of pricing of its initial public offering. For purposes of this analysis, Wedbush calculated the “trimmed mean” without taking into account the
companies with the highest and lowest pre-money valuations. The results of this analysis are summarized as follows:
|
|
|
|
|
|
|
|
|
| |
|
Pre-Money
Valuation
($ in millions) |
|
| |
|
Phase 2
IPOs |
|
|
Antiviral
IPOs |
|
| Trimmed Mean |
|
$ |
235.6 |
|
|
$ |
145.4 |
|
| Median |
|
$ |
235.6 |
|
|
$ |
134.8 |
|
Wedbush calculated the implied ownership of holders of Celladon common stock in the combined company based upon the $26.75
million value attributed to the Celladon common stock pursuant to the exchange ratio formula in the Merger Agreement, the trimmed mean and median values described above and taking into account $30.0 million in additional financing to be raised by
Eiger prior to closing of the merger. In addition, following
102
the Celladon board meeting on November 16, 2015, Wedbush also calculated such implied ownership percentages taking into account $39.5 million of pre-closing financing. Wedbush compared these
implied ownership percentages with the implied ownership percentages of holders of Celladon common stock in the combined company based upon the $26.75 million value attributed to the Celladon common stock and the $55.0 million value attributed to
the Eiger common stock pursuant to the exchange ratio formula in the Merger Agreement.
The results of this analysis are summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Implied Ownership Based on Phase 2 IPOs |
|
| |
|
$30.0M Pre-Closing Financing |
|
|
$39.5M Pre-Closing Financing |
|
| |
|
Trimmed
Mean
($235.6M) |
|
|
Median
($235.6M) |
|
|
Merger
Agreement
($55M) |
|
|
Trimmed
Mean
($235.6M) |
|
|
Median
($235.6M) |
|
|
Merger
Agreement
($55M) |
|
| Eiger |
|
|
80.6 |
% |
|
|
80.6 |
% |
|
|
49.2 |
% |
|
|
78.0 |
% |
|
|
78.0 |
% |
|
|
45.4 |
% |
| Celladon |
|
|
9.2 |
% |
|
|
9.2 |
% |
|
|
23.9 |
% |
|
|
8.9 |
% |
|
|
8.9 |
% |
|
|
22.1 |
% |
| Pre-closing financing investors |
|
|
10.3 |
% |
|
|
10.3 |
% |
|
|
26.8 |
% |
|
|
13.1 |
% |
|
|
13.1 |
% |
|
|
32.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Implied Ownership Based on Antiviral IPOs |
|
| |
|
$30.0M Pre-Closing Financing |
|
|
$39.5M Pre-Closing Financing |
|
| |
|
Trimmed
Mean
($145.4M) |
|
|
Median
($134.8M) |
|
|
Merger
Agreement
($55M) |
|
|
Trimmed
Mean
($145.4M) |
|
|
Median
($134.8M) |
|
|
Merger
Agreement
($55M) |
|
| Eiger |
|
|
71.9 |
% |
|
|
70.4 |
% |
|
|
49.2 |
% |
|
|
68.7 |
% |
|
|
67.1 |
% |
|
|
45.4 |
% |
| Celladon |
|
|
13.2 |
% |
|
|
14.0 |
% |
|
|
23.9 |
% |
|
|
12.6 |
% |
|
|
13.3 |
% |
|
|
22.1 |
% |
| Pre-closing financing investors |
|
|
14.8 |
% |
|
|
15.7 |
% |
|
|
26.8 |
% |
|
|
18.7 |
% |
|
|
19.6 |
% |
|
|
32.6 |
% |
Wedbush noted that the implied ownership percentage of holders of Celladon common stock based upon the $26.75 million value
attributed to the Celladon common stock and the $55.0 million value attributed to the Eiger common stock pursuant to the exchange ratio formula in the Merger Agreement was higher than the implied ownership percentages derived based upon the trimmed
mean and median equity values attributed to Eiger described above.
Miscellaneous
This summary is not a complete description of Wedbush’s opinion or the underlying analyses and factors considered in connection with Wedbush’s
opinion. The preparation of a fairness opinion is a complex process involving the application of subjective business and financial judgment in determining the most appropriate and relevant methods of financial analysis and the application of those
methods to the particular circumstances and, therefore, is not readily susceptible to partial analysis or summary description. Wedbush believes that its analyses described above must be considered as a whole and that considering any portion of such
analyses and of the factors considered without considering all analyses and factors could create a misleading view of the process underlying its opinion. Selecting portions of the analyses or summary set forth above, without considering the analyses
as a whole, could create an incomplete view of the processes underlying the Wedbush opinion. In arriving at its fairness determination, Wedbush considered the results of all of its analyses and did not attribute any particular weight to any factor
or analysis. Rather, it made its fairness determination on the basis of its experience and professional judgment after considering the results of all of its analyses. No company or transaction in the analyses described above is identical to
Celladon, Eiger or the merger.
In conducting its analyses and arriving at its opinion, Wedbush utilized a variety of valuation methods. The analyses were
prepared solely for the purpose of enabling Wedbush to provide its opinion to the Celladon board of directors as to the fairness of the exchange ratio in connection with the merger, as provided in the Merger Agreement, from a financial point of
view, to Celladon as of the date of the opinion and do not purport to be an appraisal or necessarily reflect the prices at which businesses or securities actually may be sold, which are inherently subject to uncertainty.
103
The terms of the merger were determined through arm’s-length negotiations between Celladon and Eiger and
were approved by Celladon’s board of directors. Although Wedbush provided advice to the Celladon board of directors during the course of these negotiations, the decision to enter into the Merger Agreement was solely that of Celladon’s
board of directors. Wedbush did not recommend any specific consideration to Celladon or Celladon’s board of directors, or that any specific amount or type of consideration constituted the only appropriate consideration for the merger. As
described above, the opinion of Wedbush and its presentation to Celladon’s board of directors were among a number of factors taken into consideration by the Celladon board of directors in making its determination to approve the Merger
Agreement, the merger and the other transactions contemplated by the Merger Agreement.
Interests of the Celladon Directors
and Executive Officers in the Merger
In considering the recommendation of the Celladon board of directors with respect to issuing shares of Celladon
common stock as contemplated by the Merger Agreement and the other matters to be acted upon by the Celladon stockholders at the Celladon special meeting, the Celladon stockholders should be aware that certain members of the board of directors and
executive officers of Celladon have interests in the merger that may be different from, or in addition to, the interests of the Celladon stockholders. These interests relate to or arise from the matters described below. The board of directors of
each of Celladon and Eiger was aware of these potential conflicts of interest and considered them, among other matters, in reaching their respective decisions to approve the Merger Agreement and the merger, and to recommend, as applicable, that the
Celladon stockholders approve the Celladon proposals to be presented to the Celladon stockholders for consideration at the Celladon special meeting as contemplated by this proxy statement/prospectus/information statement, and that the Eiger
stockholders sign and return the written consent as contemplated by this proxy statement/prospectus/information statement.
Severance and Bonus
Payments
Under the original terms of the employment agreements for each of Fredrik Wiklund, Celladon’s President and Chief Executive Officer,
Andrew Jackson, Celladon’s Chief Financial Officer, Elizabeth Reed, Celladon’s Vice President and General Counsel, and Paul Cleveland, Celladon’s former President and Chief Executive Officer, upon the executive’s termination
without “cause,” or resignation for “good reason,” each as defined in the employment agreements, each executive officer was eligible to receive continued base salary payments and COBRA premium payments for nine months (and, upon
his appointment as Chief Executive Officer on May 29, 2015, 12 months for Mr. Cleveland). If the executive officer’s termination without cause or resignation for good reason were to occur within the three month period before or 12
month period following a change of control, as defined under the 2013 Plan (as defined below), the executive officer was eligible to receive (1) continued base salary payments and COBRA premium payments for 12 months (and, upon his appointment
as Chief Executive Officer on May 29, 2015, 18 months for Mr. Cleveland), (2) a lump sum payment equal to the named executive officer’s target bonus for the year of termination and (3) full vesting acceleration of all
outstanding equity awards that are subject to time-based vesting. All severance benefits under the letter agreements are contingent upon the named executive officer executing an effective release and waiver of claims against Celladon.
In light of the unfavorable CUPID 2 results, on April 26, 2015, Celladon’s board of directors approved a reduction of Celladon’s full-time
workforce and committed to retention payments payable to certain key employees, if such employees remain with Celladon until December 31, 2015 or are terminated by Celladon without cause prior to such date (the “Retention Program”).
The Retention Program was amended on May 13, 2015 to add certain current and former executive officers, including Andrew Jackson, Rebecque Laba, Elizabeth Reed and Fredrik Wiklund, and on May 27, 2015, Celladon entered into an agreement
with each of the foregoing executive officers (the “Retention Agreements”) to memorialize the terms of the amended Retention Program.
On
September 25, 2015, Celladon’s board of directors approved modifications to the severance arrangements of the following current or former executive officers: Paul Cleveland, Andrew Jackson, Rebecque Laba, Elizabeth
104
Reed and Fredrik Wiklund and on October 20, 2015, Celladon entered into an amendment to the employment agreement of each such executive officer to memorialize the modified severance
arrangements. In connection with Celladon’s process of seeking a merger, sale or other disposition of the company, Celladon’s board of directors deemed it advisable, and in the best interests of Celladon’s stockholders, to incentivize
the executive officers identified above to continue their employment with Celladon for an additional period of time. Accordingly, the modified severance arrangements for such executive officers provided certainty that, if terminated by Celladon
without “cause” or upon a resignation for “good reason,” each executive officer would receive the enhanced severance benefits previously provided for in the event of such a qualifying termination occurring within three months
prior to or twelve months following a change of control transaction (including a liquidation of Celladon).
Bonus Payments in Lieu of Severance and
Retention Payments
Effective November 18, 2015, Celladon entered into agreements with each of Messrs. Wiklund and Jackson and Ms. Reed, the
sole remaining executive officers and employees of Celladon, providing for a cash bonus payment (an “Executive Bonus Payment”) to each of them in an amount equal to the cash severance and retention benefit payments provided in each
officer’s previously existing employment and Retention Agreements. The Executive Bonus Payment for each officer equals the cash payment amount that each such officer would have been entitled to under the terms of his or her employment
agreement, as amended, and his or her Retention Agreement (collectively, the “Prior Agreements”) had such officer been terminated without cause or resigned for good reason (or, with respect to the Retention Agreements, remained employed by
Celladon on December 31, 2015), and replaces and supersedes each officer’s right to any cash severance or retention benefits under the Prior Agreements. Each officer’s Executive Bonus Payment will be subject to his or her delivery of
an effective waiver and release of claims and is expected to be paid in late 2015 or early 2016. Each of Messrs. Wiklund’s and Jackson’s and Ms. Reed’s employment relationship with Celladon remains at-will and continued
employment with Celladon is not a requirement to receive the Executive Bonus Payment.
The Executive Bonus Payments for Messrs. Wiklund and Jackson and
Ms. Reed are set forth in the table below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Employee |
|
Severance |
|
|
Retention |
|
|
Health
Insurance
Premiums |
|
|
Total
Executive
Bonus
Payment |
|
| Fredrik Wiklund |
|
$ |
326,850 |
|
|
$ |
125,712 |
|
|
$ |
1,629 |
|
|
$ |
454,190 |
|
| Andrew Jackson |
|
$ |
275,000 |
|
|
$ |
97,936 |
|
|
$ |
13,682 |
|
|
$ |
386,618 |
|
| Elizabeth Reed |
|
$ |
364,650 |
|
|
$ |
140,250 |
|
|
$ |
22,334 |
|
|
$ |
527,234 |
|
Incentive Bonus Program
In connection with the proposed merger, on November 16, 2015, Celladon’s board of directors adopted an incentive bonus program for Messrs. Wiklund
and Jackson and Ms. Reed, pursuant to which they will be eligible to receive incentive payments upon the achievement of the following key milestones: (i) filing of this registration statement on Form S-4 in respect of the proposed merger;
(ii) mailing of the proxy statement/prospectus/ information statement portion of such registration statement on Form S-4 to Celladon’s stockholders; and (iii) approval of the merger by Celladon’s stockholders. Subject to such
achievements and provided that such officer remains employed with Celladon as of the date of such milestone achievement, Mr. Wiklund will be eligible to receive a payment of $50,000 for each milestone, and each of Mr. Jackson and
Ms. Reed will be eligible to receive a payment of $40,000 for each milestone. The incentive bonus payments and the corresponding milestones are set forth in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
|
| Employee |
|
Filing of
Form S-4 |
|
|
Mailing
of Proxy
Statement |
|
|
Stockholder
Approval of
Merger |
|
| Fredrik Wiklund |
|
$ |
50,000 |
|
|
$ |
50,000 |
|
|
$ |
50,000 |
|
| Andrew Jackson |
|
$ |
40,000 |
|
|
$ |
40,000 |
|
|
$ |
40,000 |
|
| Elizabeth Reed |
|
$ |
40,000 |
|
|
$ |
40,000 |
|
|
$ |
40,000 |
|
105
The employment of Messrs. Wiklund and Jackson and Ms. Reed is expected to terminate no later than the
consummation of the merger.
Named Executive Officer Compensation
The following table and the related footnotes present information about the compensation payable to Celladon’s named executive officers included in
Celladon’s most recent filing under the Exchange Act that required disclosure pursuant to Item 402(c) of Regulation S-K. The compensation shown in the table below is intended to comply with Item 402(t) of Regulation S-K, which
requires disclosure of information about compensation for each named executive officer that is based on or otherwise relates to the merger. Krisztina Zsebo, Ph.D., Celladon’s former Chief Executive Officer and a named executive officer,
resigned effective May 29, 2015 and is not receiving any compensatory payments in connection with the merger. The employment of Paul Cleveland, Celladon’s former President and Chief Executive Officer and a named executive officer, was
terminated without cause effective November 19, 2015.
The named executive officers are not entitled to any pension or non-qualified deferred
compensation benefits enhancements or any tax reimbursements in connection with the merger. Further, all unvested stock options held by the named executive officers are currently out of the money.
Golden Parachute Compensation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
|
Cash(1) |
|
|
Perquisites/
Benefits(2) |
|
|
Other(3) |
|
|
Total |
|
| Krisztina Zsebo |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Paul Cleveland |
|
$ |
1,060,348 |
|
|
$ |
53,440 |
|
|
|
— |
|
|
$ |
1,113,788 |
|
| Elizabeth Reed |
|
$ |
504,900 |
|
|
$ |
22,334 |
|
|
$ |
120,000 |
|
|
$ |
647,234 |
|
| (1) |
The amount in this column for Mr. Cleveland represents (a) $1,060,348 in cash severance payments that Mr. Cleveland received in connection with his termination without cause effective November 19,
2015 and reflects his amended severance arrangement described above under “Severance and Bonus Payments.” The amount in this column for Ms. Reed includes $364,650 in severance payments and $140,250 in retention payments provided for
in her previously existing employment and Retention Agreement, which amounts are included in Ms. Reed’s Executive Bonus Payment described above. |
| (2) |
The amounts in this column reflect 18 months of health insurance premium payments for Mr. Cleveland and 12 months of health insurance premium payments for Ms. Reed. |
| (3) |
The amount in this column represents the full amount of cash payments Ms. Reed is eligible to receive pursuant to the incentive bonus program described above under “Severance and Bonus Payments—Incentive
Bonus Program.” As described above, the amount reflects the potential payment of $40,000 to Ms. Reed upon the achievement of each of the following three milestones: (i) filing of this registration statement on Form S-4 in respect of
the proposed merger; (ii) mailing of the proxy statement/prospectus/information statement portion of such registration statement on Form S-4 to Celladon’s stockholders; and (iii) approval of the merger by Celladon’s stockholders,
provided that Ms. Reed remains employed with Celladon as of the date the milestone is achieved. |
Acceleration of Unvested Option
Awards
In connection with the consummation of the merger, the unvested stock option awards held by the Celladon board members will vest in full.
All unvested stock option awards held by the Celladon board members are currently out of the money.
Ownership Interests
As of December 31, 2015, directors and executive officers of Celladon owned 0.3% of the outstanding shares of Celladon common stock. Celladon directors
and executive have entered into support agreements in connection
106
with the merger. For a more detailed discussion of the support agreements see the section entitled “Agreements Related to the Merger—Support Agreements and Written Consent” in this
proxy statement/prospectus/information statement.
Indemnification of the Celladon Officers and Directors
Under the Merger Agreement, from the closing of the merger through the sixth anniversary of the closing, Celladon shall, jointly and severally, indemnify and
hold harmless each person who is or has served as a director or officer of Celladon against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees and disbursements,
incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that such person is or was a director or officer of Celladon, to the
fullest extent permitted under the DGCL for directors or officers of Delaware corporations. In addition, each such director and officer, or former director and officer, is entitled to advancement of expenses incurred in the defense of any such
claim, action, suit, proceeding or investigation.
Under the Merger Agreement, the certificate of incorporation and bylaws of Celladon shall contain
provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers of each of Celladon and Eiger than are presently set forth in the certificate of incorporation and
bylaws of Celladon and Eiger, as applicable, which provisions shall not be amended, modified or repealed for a period of six years’ time from the closing of the merger in a manner that would adversely affect the rights thereunder of individuals
who, at or prior to the closing, were officers or directors of Celladon or Eiger.
The Merger Agreement also provides that Celladon shall purchase an
insurance policy, which maintains in effect for six years from the closing the current directors’ and officers’ liability insurance policies maintained by Celladon or substitute policies of at least the same coverage containing terms and
conditions that are not materially less favorable.
Interests of Certain Eiger Directors, Executive Officers and Affiliates
in the Merger
In considering the recommendation of the Eiger board of directors with respect to adopting the Merger Agreement, Eiger stockholders
should be aware that certain members of the board of directors and executive officers of Eiger have interests in the merger that may be different from, or in addition to, interests they may have as Eiger stockholders. Each of the Celladon and Eiger
board of directors was aware of these potential conflicts of interest and considered them, among other matters, in reaching their respective decisions to approve the Merger Agreement and the merger, and to recommend, as applicable, that the Celladon
stockholders approve the Celladon proposals to be presented to the Celladon stockholders for consideration at the Celladon special meeting as contemplated by this proxy statement/prospectus/information statement, and that the Eiger stockholders sign
and return the written consent as contemplated by this proxy statement/prospectus/information statement.
107
Ownership Interests. Certain of Eiger’s directors and executive officers currently hold shares of
Eiger’s common stock, Series A Preferred Stock and Series A-1 Preferred Stock, which such shares of Series A Preferred Stock and Series A-1 Preferred Stock will be converted into shares of Eiger common stock prior to the closing of the merger.
In addition, certain of Eiger’s directors will acquire additional shares of common stock prior to the closing of the merger by purchasing such shares pursuant to the Subscription Agreement and/or the conversion of their senior secured
promissory notes into shares of common stock pursuant to the Subscription Agreement. The table below sets forth the ownership of Eiger’s common stock, Series A Preferred Stock and Series A-1 Preferred Stock as of December 31, 2015 by
Eiger’s directors and executive officers and their anticipated ownership of Eiger common stock immediately prior to the closing of the merger following their purchase of shares of common stock pursuant to the Subscription Agreement and/or the
conversion of their senior secured promissory notes into shares of common stock pursuant to the Subscription Agreement.
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stockholder Name |
|
Number of
Shares of
Common
Stock as of
December 31,
2015 |
|
|
Number of
Shares of
Preferred
Stock as of
December 31,
2015 |
|
|
Number of
Shares of
Common
Stock
Immediately
Prior to the
Merger |
|
| David Cory |
|
|
649,999 |
|
|
|
— |
|
|
|
649,999 |
|
| Ed Engleman(1) |
|
|
— |
|
|
|
13,377,154 |
|
|
|
18,043,198 |
|
| Nina Kjellson(2) |
|
|
— |
|
|
|
13,377,154 |
|
|
|
18,043,198 |
|
| Jeffrey Glenn(3) |
|
|
1,741,506 |
|
|
|
— |
|
|
|
1,741,506 |
|
| (1) |
Vivo Ventures Fund VI, L.P. directly holds 13,279,868 shares of common stock issuable upon conversion of preferred stock. Vivo Ventures VI Affiliates Fund, L.P. directly holds 97,286 shares of common stock issuable upon
conversion of preferred stock. Vivo Ventures Fund VI, L.P. will acquire 4,632,111 shares of common in connection with the Subscription Agreement and Vivo Ventures VI Affiliates Fund, L.P. will acquire 33,933 shares in connection with the
Subscription Agreement. Vivo Ventures VI, LLC, the sole general partner of both Vivo Ventures Fund VI, L.P. and Vivo Ventures VI Affiliates Fund, L.P., has shared voting power and shared investment power over such securities, may be deemed to
beneficially own such shares, and disclaims beneficial ownership of the shares except to the extent of its pecuniary interests therein. Dr. Engleman, one of Eiger’s board members, is a managing partner at Vivo Ventures VI, LLC, the general
partner of both Vivo Ventures Fund VI, L.P. and Vivo Ventures VI Affiliates Fund, L.P., Dr. Engleman has shared voting or investment power over the shares held by Vivo Ventures Fund VI, L.P. and Vivo Ventures VI Affiliates Fund, L.P. and
disclaims beneficial ownership of these shares except to the extent of any pecuniary interest therein. |
| (2) |
Represents 13,377,154 shares of common stock issuable upon conversion of preferred stock held by InterWest Partners X, LP, and 4,666,044 shares of common stock InterWest Partners X, LP will acquire in connection with
the subscription agreement. InterWest Management Partners X, LLC has sole voting and investment control over the shares owned by InterWest X, LP. The Managing Directors and Venture Members of InterWest Management Partners X, LLC have shared voting
and investment control over the shares owned by InterWest Partners X, LP. The managing directors of InterWest Management Partners X, LLC are Bruce A. Cleveland, Philip T. Gianos, W. Stephen Holmes, Gilbert H. Kilman, Arnold L. Oronsky and Douglas A.
Pepper and its venture members are Keval Desai and Khaled A. Nasr. Each of the foregoing individuals disclaims beneficial ownership of the shares owned by InterWest Partners X, LP, except to the extent of their pro rata partnership interest therein.
Nina Kjellson is a member of InterWest Management Partners X, LLC. Nina Kjellson disclaims beneficial ownership of the shares owned by InterWest Partners X, LP, except to the extent of her pro rata partnership interest therein. |
| (3) |
Includes 1,726,024 shares held by Eiger Group International. Jeffrey Glenn is the Chief Executive Officer of Eiger Group International, Inc. Dr. Glenn has sole power to vote and sole power to dispose of shares
directly owned by Eiger Group International, Inc. |
108
Stock Options and Warrants. Certain of Eiger’s directors and executive officers currently hold
options, subject to vesting, to purchase shares of Eiger common stock, which pursuant to the Merger Agreement will be converted into and become options to purchase shares of Celladon common stock. The table below sets forth certain information with
respect to such options
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Optionholder Name |
|
Grant Date |
|
|
Expiration Date |
|
|
Exercise
Price |
|
|
Number of
Shares of
Common
Stock
Underlying
Option as of
December 31,
2015 |
|
|
Number of
Vested
Shares of
Common
Stock
Underlying
Option as of
December 31,
2015 |
|
| David Cory |
|
|
September 24, 2013 |
|
|
|
September 24, 2023 |
|
|
$ |
0.12 |
|
|
|
200,000 |
|
|
|
112,500 |
|
|
|
|
September 22, 2015 |
|
|
|
September 22, 2025 |
|
|
|
0.18 |
|
|
|
849,999 |
|
|
|
0 |
|
| James Welch |
|
|
September 22, 2015 |
|
|
|
September 22, 2025 |
|
|
|
0.18 |
|
|
|
335,737 |
|
|
|
0 |
|
| Joanne Quan |
|
|
September 22, 2015 |
|
|
|
September 22, 2025 |
|
|
|
0.18 |
|
|
|
300,000 |
|
|
|
0 |
|
| James Shaffer |
|
|
September 22, 2015 |
|
|
|
September 22, 2025 |
|
|
|
0.18 |
|
|
|
300,000 |
|
|
|
0 |
|
Management Following the Merger. As described elsewhere in this proxy statement/prospectus/information statement,
including in “Management Following the Merger,” all of Eiger’s directors and executive officers are expected to become the directors and executive officers of Celladon upon the closing of the merger.
Indemnification and Insurance. Under the Merger Agreement, from the closing of the merger through the sixth anniversary of the closing, Celladon and
Eiger, as the surviving corporation in the merger, shall, jointly and severally, indemnify and hold harmless each person who is or has served as a director or officer of Eiger against all claims, losses, liabilities, damages, judgments, fines and
reasonable fees, costs and expenses, including attorneys’ fees and disbursements, incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or
pertaining to the fact that such person is or was a director or officer of Eiger, to the fullest extent permitted under the DGCL for directors or officers of Delaware corporations. In addition, each such director and officer, or former director and
officer, is entitled to advancement of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation.
Under the Merger
Agreement, the certificate of incorporation and bylaws of each of Celladon and Eiger shall contain provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers of
each of Celladon and Eiger than are presently set forth in the certificate of incorporation and bylaws of Celladon and Eiger, as applicable, which provisions shall not be amended, modified or repealed for a period of six years’ time from the
closing of the merger in a manner that would adversely affect the rights thereunder of individuals who, at or prior to the closing, were officers or directors of Celladon or Eiger.
The Merger Agreement also provides that Celladon shall purchase an insurance policy, which maintains in effect for six years from the closing the current
directors’ and officers’ liability insurance policies maintained by Celladon or substitute policies of at least the same coverage containing terms and conditions that are not materially less favorable.
Limitations of Liability and Indemnification
In addition to the indemnification required in the amended and restated certificate of incorporation and amended and restated bylaws of Celladon, Celladon
entered into indemnification agreements with each of its directors and officers. These agreements provide for the indemnification of the directors and officers of Celladon for all reasonable expenses and liabilities incurred in connection with any
action or proceeding brought against them by reason of the fact that they are or were agents of Celladon. Celladon believes that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors
and officers.
109
Stock Options and Warrants
As of December 31, 2015, an aggregate of 2,902,860 shares of Eiger common stock were issuable upon the exercise of outstanding stock options under Eiger’s
2009 Equity Incentive Plan at a weighted average exercise price of $0.17 per share. Such options will be converted into and become options to purchase shares of Celladon common stock pursuant to the Merger Agreement.
As of December 31, 2015, shares of Eiger capital stock were issuable upon the exercise of outstanding warrants at an exercise price of $0.01 per share, which
warrants will be automatically exercised immediately prior to the closing of the merger. For a discussion of the terms of such warrants and the number of shares of Eiger capital stock issuable upon the exercise of such warrants, see “Agreements
Related to the Merger—Bridge Loan.”
Form of the Merger
The Merger Agreement provides that at the effective time, Merger Sub will be merged with and into Eiger. Upon the consummation of the merger, Eiger will
continue as the surviving corporation and will be a wholly owned subsidiary of Celladon.
After completion of the merger, assuming Celladon Proposal
No. 3 is approved by Celladon stockholders at the Celladon special meeting, Celladon will be renamed “Eiger BioPharmaceuticals, Inc.” and expects to trade on The NASDAQ Global Market under the symbol “EIGR.”
Merger Consideration
Immediately prior to the closing of the financing contemplated by the Subscription Agreement, each share of Eiger preferred stock outstanding at such time will
be converted into shares of Eiger common stock at a ratio determined in accordance with the Eiger certificate of incorporation then in effect and each outstanding Eiger warrant to purchase equity securities will automatically be exercised.
Immediately prior to the closing of the financing contemplated by the Subscription Agreement, the $6.0 million in aggregate principal amount outstanding under, and all interest accrued on, senior secured promissory notes of Eiger will be converted
into shares of Eiger common stock pursuant to the note. At the effective time of the merger:
| |
• |
|
each share of Eiger common stock outstanding immediately prior to the effective time of the merger will automatically be converted into the right to receive approximately 1.32 pre-split (or 0.09 post-split) shares of
Celladon common stock, or the exchange ratio, subject to adjustment to account for the proposed 1-for-15 reverse stock split, and |
| |
• |
|
each option to purchase shares of Eiger common stock outstanding and unexercised immediately prior to the effective time of the merger will be assumed by Celladon and will become an option, subject to vesting, to
purchase shares of Celladon common stock. |
Immediately after the merger, based on the exchange ratio, it is expected that Eiger
stockholders, warrantholders and optionholders will own approximately 78% of the fully-diluted common stock of Celladon with Celladon stockholders and optionholders holding approximately 22% of the fully-diluted common stock of Celladon. The
exchange ratio is determined pursuant to a formula described in more detail in the Merger Agreement and in this proxy statement/prospectus/information statement, and the 1.32 pre-split figure, 0.09 post-split figure and percentage ownership figures
are estimates.
There will be no adjustment to the total number of shares of Celladon common stock that Eiger stockholders will be entitled to
receive for changes in the market price of Celladon common stock. Accordingly, the market value of the shares of Celladon common stock issued pursuant to the merger will depend on the market value of the shares of Celladon common stock at the time
the merger closes, and could vary significantly from the market value on the date of this proxy statement/prospectus/information statement.
110
No fractional shares of Celladon common stock will be issuable pursuant to the merger to Eiger stockholders.
Instead, each Eiger stockholder who would otherwise be entitled to receive a fraction of a share of Celladon common stock, after aggregating all fractional shares of Celladon common stock issuable to such stockholder, will be entitled to receive in
cash the dollar amount, rounded to the nearest whole cent, without interest, determined by multiplying such fraction by the closing price of a share of Celladon common stock as quoted on The NASDAQ Global Market, on the date the merger becomes
effective.
The Merger Agreement provides that, at the effective time of the merger, Celladon will deposit with an exchange agent acceptable to Celladon
and Eiger stock certificates representing the shares of Celladon common stock issuable to the Eiger stockholders and a sufficient amount of cash to make payments in lieu of fractional shares.
The Merger Agreement provides that, promptly after the effective time of the merger, the exchange agent will mail to each record holder of Eiger capital stock
immediately prior to the effective time of the merger a letter of transmittal and instructions for surrendering and exchanging the record holder’s Eiger stock certificates for shares of Celladon common stock. Upon surrender of an Eiger stock
certificate for exchange to the exchange agent, together with a duly signed letter of transmittal and such other documents as the exchange agent or Celladon may reasonably require, the Eiger stock certificate surrendered will be cancelled and the
holder of the Eiger stock certificate will be entitled to receive the following:
| |
• |
|
a certificate representing the number of whole shares of Celladon common stock that such holder has the right to receive pursuant to the provisions of the Merger Agreement; |
| |
• |
|
cash in lieu of any fractional share of Celladon common stock; and |
| |
• |
|
dividends or other distributions, if any, declared or made with respect to Celladon common stock with a record date after the effective time of the merger. |
At the effective time of the merger, all holders of certificates representing shares of Eiger common stock or Eiger preferred stock that were outstanding
immediately prior to the effective time of the merger will cease to have any rights as stockholders of Eiger. In addition, no transfer of Eiger common stock or Eiger preferred stock after the effective time of the merger will be registered on the
stock transfer books of Eiger.
If any Eiger stock certificate has been lost, stolen or destroyed, Celladon may, in its discretion, and as a condition to
the delivery of any shares of Celladon common stock, require the owner of such lost, stolen or destroyed certificate to deliver an affidavit claiming such certificate has been lost, stolen or destroyed and post a bond indemnifying Celladon against
any claim suffered by Celladon related to the lost, stolen or destroyed certificate or any Celladon common stock issued in exchange for such certificate as Celladon may reasonably request.
From and after the effective time of the merger, until it is surrendered, each certificate that previously evidenced Eiger common stock or Eiger preferred
stock will be deemed to represent only the right to receive shares of Celladon common stock, and cash in lieu of any fractional share of Celladon common stock.
Celladon will not pay dividends or other distributions on any shares of Celladon common stock to be issued in exchange for any unsurrendered Eiger stock
certificate until the Eiger stock certificate is surrendered as provided in the Merger Agreement.
Effective Time of the
Merger
The Merger Agreement requires the parties to consummate the merger after all of the conditions to the consummation of the merger contained in
the Merger Agreement are satisfied or waived, including the adoption of the Merger Agreement by the stockholders of Eiger and the approval by the Celladon stockholders of the issuance of Celladon common stock, the amendment to the amended and
restated certificate of incorporation of Celladon effecting the proposed 1-for-15 reverse stock split and the amendment to the amended and restated certificate of incorporation of Celladon effecting the name change from “Celladon
Corporation” to “Eiger
111
BioPharmaceuticals, Inc.” The merger will become effective upon the filing of a certificate of merger with the Secretary of State of the State of Delaware or at such later time as is agreed
by Celladon and Eiger and specified in the certificate of merger. Neither Celladon nor Eiger can predict the exact timing of the consummation of the merger.
Regulatory Approvals
In the
United States, Celladon must comply with applicable federal and state securities laws and the rules and regulations of The NASDAQ Global Market in connection with the issuance of shares of Celladon common stock and the filing of this proxy
statement/prospectus/information statement with the SEC.
Tax Treatment of the Merger
Celladon and Eiger intend the merger to qualify as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended,
or the Code. Each of Celladon and Eiger will use its commercially reasonable efforts to cause the merger to qualify as a reorganization within the meaning of Section 368(a) of the Code, and not to permit or cause any affiliate or any subsidiary
of Celladon or Eiger to, take any action or cause any action to be taken which would cause the merger and the second merger, taken together, to fail to qualify as a reorganization under Section 368(a) of the Code. For a description of certain
of the considerations regarding U.S. federal tax consequences of the merger, see the section entitled “Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger” below.
Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger
In the opinion of Pillsbury Winthrop Shaw Pittman LLP, counsel to Celladon, and Cooley LLP, counsel to Eiger, the following is a discussion of the potentially
material U.S. federal income tax consequences of the merger applicable to U.S. Holders (as defined below) who exchange their Eiger common stock for Celladon common stock in the merger, but does not purport in any manner to be a complete or otherwise
material analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local, or foreign tax laws are not discussed. This discussion is based on the Internal Revenue
Code of 1986, as amended (the “Code”), U.S. Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the Internal Revenue Service (the “IRS”) in effect as of the
date of the merger. These authorities may change or be subject to differing interpretations. Any such change may be applied retroactively in a manner that could adversely affect a holder of Eiger common stock.
This discussion assumes and is limited to U.S. Holders who hold their Eiger common stock as a “capital asset” within the meaning of
Section 1221 of the Code (generally, property held for investment). This discussion is an overview of certain potential tax treatment and does not address all U.S. federal income tax consequences relevant to the particular circumstances of an
Eiger common stockholder, including the impact of the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to holders of Eiger common stock that are subject to particular U.S. or foreign tax
rules, including, without limitation:
| |
• |
|
persons subject to the alternative minimum tax or Medicare contribution tax; |
| |
• |
|
persons whose functional currency is not the U.S. dollar; |
| |
• |
|
persons holding Eiger common stock as part of a hedge, straddle, or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| |
• |
|
persons who are not U.S. Holders; |
| |
• |
|
banks, insurance companies, and other financial institutions; |
| |
• |
|
real estate investment trusts or regulated investment companies; |
| |
• |
|
brokers, dealers, or traders in securities; |
| |
• |
|
partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
112
| |
• |
|
tax-exempt organizations or governmental organizations; |
| |
• |
|
persons deemed to sell Eiger common stock under the constructive sale provisions of the Code; |
| |
• |
|
persons who hold or receive Eiger common stock pursuant to the exercise of any employee stock options or otherwise as compensation; |
| |
• |
|
persons holding Eiger common stock who exercise dissenters’ rights; and |
| |
• |
|
tax-qualified retirement plans. |
For purposes of this discussion, a “U.S. Holder” is a beneficial
owner of Eiger common stock that, for U.S. federal income tax purposes, is or is treated as:
| |
• |
|
an individual who is a citizen or resident of the United States; |
| |
• |
|
a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| |
• |
|
an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| |
• |
|
a trust if either a court within the United States is able to exercise primary supervision over the administration of such trust and one or more United States persons (within the meaning of Section 7701(a)(30) of
the Code) have the authority to control all substantial decisions of such trust, or the trust has a valid election in effect under applicable Treasury Regulations to be treated as a United States person for U.S. federal income tax purposes.
|
If an entity treated as a partnership for U.S. federal income tax purposes holds Eiger common stock, the tax treatment of a partner in the
partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding Eiger common stock and the partners in such partnerships should consult
their tax advisors regarding the U.S. federal income tax consequences to them.
In addition, the following discussion does not address the tax
consequences of the merger under state, local and foreign tax laws. Furthermore, the following discussion does not address any tax consequences of transactions effectuated before, after or at the same time as the merger, whether or not they are in
connection with the merger, including, without limitation, transactions in which Eiger common stock is acquired (including, but not limited to, pursuant to the Subscription Agreement) or Eiger preferred stock is converted to Eiger common stock.
STOCKHOLDERS AND INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR
SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE MERGER ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
In connection with the filing of the registration statement of which this proxy statement/prospectus/information statement is a part, Pillsbury Winthrop Shaw
Pittman LLP will deliver to Celladon and Cooley LLP will deliver to Eiger opinions that the statements under the caption “The Merger—Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger” constitute the
opinions of Pillsbury Winthrop Shaw Pittman LLP and Cooley LLP, respectively.
In rendering their opinions, counsel will assume that the statements and
facts concerning the merger set forth in this proxy statement/prospectus/information statement and in the Merger Agreement, are true and accurate in all respects, that the merger will be completed in accordance with this proxy
statement/prospectus/information statement and the Merger Agreement, and that the merger qualifies as a reorganization within the meaning of section 368(a) of the Code. In addition, the tax opinions will be based on the law in effect on the
date of the opinions. If any of these assumptions is inaccurate, the tax consequences of the merger could differ from those described in this proxy statement/prospectus/information statement.
113
No ruling from the IRS or opinion of counsel has been or will be requested in connection with the merger with
respect to the tax treatment. Assuming that the merger will be treated for U.S. federal income tax purposes as a reorganization within the meaning of Section 368(a) of the Code and subject to the qualifications and assumptions described in this
proxy statement/prospectus/information statement, the tax consequences to U.S. Holders of Eiger common stock will be as follows:
| |
• |
|
a U.S. Holder will not recognize gain or loss upon the exchange of Eiger common stock for Celladon common stock pursuant to the merger, except to the extent of cash received in lieu of a fractional share of Celladon
common stock as described below; |
| |
• |
|
a U.S. Holder who receives cash in lieu of a fractional share of Celladon common stock in the merger will generally recognize capital gain or loss in an amount equal to the difference between the amount of cash received
instead of a fractional share and the stockholder’s tax basis allocable to such fractional share; |
| |
• |
|
a U.S. Holder’s aggregate tax basis for the shares of Celladon common stock received in the merger (including any fractional share interest for which cash is received) will equal the stockholder’s aggregate
tax basis in the shares of Eiger common stock surrendered upon completion of the merger; and |
| |
• |
|
the holding period of the shares of Celladon common stock received by a U.S. Holder in the merger will include the holding period of the shares of Eiger common stock surrendered in exchange therefor. |
Capital gains or losses recognized in the merger as described above generally will constitute long-term capital gain or loss if the U.S. Holder’s holding
period in the Eiger common stock surrendered in the merger is more than one year as of the effective date of the merger. The deductibility of capital losses is subject to limitations. In addition, for purposes of the above discussion of the bases
and holding periods for shares of Eiger common stock and Celladon common stock, stockholders who acquired different blocks of Eiger common stock at different times for different prices must calculate their gains and losses and holding periods
separately for each identifiable block of such stock exchanged in the merger.
U.S. Holders who owned at least one percent (by vote or value) of the total
outstanding stock of Eiger are required to attach a statement to their tax returns for the year in which the merger is consummated that contains the information listed in Treasury Regulation Section 1.368-3(b). Such statement must include the
stockholder’s tax basis in the stockholder’s Eiger common stock and the fair market value of such stock.
If the merger fails to qualify as a
reorganization within the meaning of Section 368(a) of the Code, then a U.S. Holder would recognize gain or loss upon the exchange of Eiger common stock for Celladon common stock equal to the difference between the fair market value, at the
time of the merger, of the Celladon common stock received in the merger (including any cash received in lieu of a fractional share) and such U.S. Holder’s tax basis in the Eiger common stock surrendered in the merger. Such gain or loss
would be long-term capital gain or loss if the Eiger common stock was held for more than one year at the time of the merger. In such event, the tax basis of Celladon common stock received in the merger would equal its fair market value at the
time of the merger and the holding period of such Celladon common stock would commence the day after the merger.
THE PRECEDING DISCUSSION DOES NOT
PURPORT TO BE A COMPLETE ANALYSIS OR DISCUSSION OF ALL OF THE MERGER’S POTENTIAL TAX EFFECTS. U.S. HOLDERS OF EIGER STOCK SHOULD CONSULT THEIR TAX ADVISORS AS TO THE SPECIFIC TAX CONSEQUENCES TO THEM OF THE MERGER, INCLUDING TAX RETURN
REPORTING REQUIREMENTS, AND THE APPLICABILITY AND EFFECT OF FEDERAL, STATE, LOCAL AND OTHER APPLICABLE TAX LAWS.
114
NASDAQ Stock Market Listing
Celladon common stock currently is listed on The NASDAQ Global Market under the symbol “CLDN”. Celladon has agreed to use commercially reasonable
efforts to maintain its existing listing on The NASDAQ Global Market, and to obtain approval for listing on The NASDAQ Global Market of the shares of Celladon common stock that Eiger stockholders will be entitled to receive pursuant to the merger.
In addition, under the Merger Agreement, each party’s obligation to complete the merger is subject to the satisfaction or waiver by each of the parties, at or prior to the merger, of various conditions, including that the existing shares of
Celladon common stock must have been continually listed on The NASDAQ Global Market, and Celladon must have caused the shares of Celladon common stock to be issued in the merger to be approved for listing on The NASDAQ Global Market as of the
closing of the merger.
Celladon has filed an initial listing application for the combined company with The NASDAQ Global Market pursuant to NASDAQ
“reverse merger” rules. If such application is accepted, Celladon anticipates that its common stock will be listed on The NASDAQ Global Market following the closing of the merger under the trading symbol “EIGR.”
Anticipated Accounting Treatment
The merger will be treated by Celladon as a reverse merger under the acquisition method of accounting in accordance with accounting principles generally
accepted in the United States. For accounting purposes, Eiger is considered to be acquiring Celladon in this transaction. Management of Celladon and Eiger have made a preliminary estimate of the purchase price calculated as described in Note 1 to
the unaudited pro forma condensed combined financial statements. The net tangible and intangible assets acquired and liabilities assumed in connection with the transaction are recorded at their estimated acquisition date fair values. The acquisition
method of accounting is dependent upon certain valuations and other studies that have yet to commence or progress to a stage where there is sufficient information for a definitive measurement. A final determination of these estimated fair values,
which cannot be made prior to the completion of the transaction, will be based on the actual net tangible and intangible assets of Celladon that exist as of the date of completion of the transaction.
Appraisal Rights and Dissenters’ Rights
Delaware Law
If the merger is completed, Eiger
stockholders who do not deliver a written consent approving the merger are entitled to appraisal rights under Section 262 of the DGCL, or Section 262, provided that they comply with the conditions established by Section 262. Holders
of Celladon common stock are not entitled to appraisal rights under Delaware law in connection with the merger.
The discussion below is not a complete
summary regarding an Eiger stockholder’s appraisal rights under Delaware law and is qualified in its entirety by reference to the text of the relevant provisions of Delaware law, which are attached to this proxy statement/prospectus/information
statement as Annex C. Stockholders intending to exercise appraisal rights should carefully review Annex C. Failure to follow precisely any of the statutory procedures set forth in Annex C may result in a termination or waiver of
these rights. This summary does not constitute legal or other advice, nor does it constitute a recommendation that Eiger stockholders exercise their appraisal rights under Delaware law.
Under Section 262, where a merger is adopted by stockholders by written consent in lieu of a meeting of stockholders pursuant to Section 228 of the
DGCL, either the constituent corporation before the effective date of the merger or the surviving corporation, within 10 days after the effective date of the merger, must notify each stockholder of the constituent corporation entitled to appraisal
rights of the approval of the merger, the effective date of the merger and that appraisal rights are available.
115
If the merger is completed, within 10 days after the effective date of the merger Eiger will notify its
stockholders that the merger has been approved, the effective date of the merger and that appraisal rights are available to any stockholder who has not approved the merger. Holders of shares of Eiger capital stock who desire to exercise their
appraisal rights must deliver a written demand for appraisal to Eiger within 20 days after the date of mailing of that notice, and that stockholder must not have delivered a written consent approving the merger. A demand for appraisal must
reasonably inform Eiger of the identity of the stockholder and that such stockholder intends thereby to demand appraisal of the shares of Eiger capital stock held by such stockholder. Failure to deliver a written consent approving the merger will
not in and of itself constitute a written demand for appraisal satisfying the requirements of Section 262. All demands for appraisal should be addressed to Eiger BioPharmaceuticals, Inc., 350 Cambridge Ave. Suite 350, Palo Alto, California
94306, Attention: Corporate Secretary, and should be executed by, or on behalf of, the record holder of shares of Eiger capital stock. ALL DEMANDS MUST BE RECEIVED BY EIGER WITHIN TWENTY (20) DAYS AFTER THE DATE EIGER MAILS A NOTICE TO ITS
STOCKHOLDERS NOTIFYING THEM THAT THE MERGER HAS BEEN APPROVED, THE EFFECTIVE DATE OF THE MERGER AND THAT APPRAISAL RIGHTS ARE AVAILABLE TO ANY STOCKHOLDER WHO HAS NOT APPROVED THE MERGER.
If you fail to deliver a written demand for appraisal within the time period specified above, you will be entitled to receive the merger consideration for
your shares of Eiger capital stock as provided for in the Merger Agreement, but you will have no appraisal rights with respect to your shares of Eiger capital stock.
To be effective, a demand for appraisal by a holder of shares of Eiger capital stock must be made by, or in the name of, the registered stockholder, fully and
correctly, as the stockholder’s name appears on the stockholder’s stock certificate(s). Beneficial owners who do not also hold the shares of record may not directly make appraisal demands to Eiger. The beneficial owner must, in these
cases, have the registered owner, such as a broker, bank or other custodian, submit the required demand in respect of those shares. If shares are owned of record in a fiduciary capacity, such as by a trustee, guardian or custodian, execution of a
demand for appraisal should be made by or for the fiduciary; and if the shares are owned of record by more than one person, as in a joint tenancy or tenancy in common, the demand should be executed by or for all joint owners. An authorized agent,
including an authorized agent for two or more joint owners, may execute the demand for appraisal for a stockholder of record; however, the agent must identify the record owner or owners and expressly disclose the fact that, in executing the demand,
he or she is acting as agent for the record owner. A record owner, such as a broker, who holds shares as a custodian for others, may exercise the record owner’s right of appraisal with respect to the shares held for one or more beneficial
owners, while not exercising this right for other beneficial owners. In that case, the written demand should state the number of shares as to which appraisal is sought. Where no number of shares is expressly mentioned, the demand will be presumed to
cover all shares held in the name of the record owner. In addition, the stockholder must continuously hold the shares of record from the date of making the demand through the effective time of the merger.
If you hold your shares of Eiger capital stock in a brokerage account or in other custodian form and you wish to exercise appraisal rights, you should consult
with your bank, broker or other custodian to determine the appropriate procedures for the making of a demand for appraisal by the custodian.
At any time
within 60 days after the effective time of the merger, any stockholder who has demanded an appraisal, but has neither commenced an appraisal proceeding or joined an appraisal proceeding as a named party, has the right to withdraw such
stockholder’s demand and accept the terms of the merger by delivering a written withdrawal to Eiger. If, following a demand for appraisal, you have withdrawn your demand for appraisal in accordance with Section 262, you will have the right
to receive the merger consideration for your shares of Eiger capital stock.
Within 120 days after the effective date of the merger, any stockholder who
has delivered a demand for appraisal in accordance with Section 262 will, upon written request to the surviving corporation, be entitled to receive a written statement setting forth the aggregate number of shares not voted in favor of the
Merger Agreement and with respect to which demands for appraisal rights have been received and the aggregate number of holders of
116
these shares. This written statement will be mailed to the requesting stockholder within 10 days after the stockholder’s written request is received by the surviving corporation or within
ten days after expiration of the period for delivery of demands for appraisal, whichever is later. Within 120 days after the effective date of the merger, either the surviving corporation or any stockholder who has delivered a demand for appraisal
in accordance with Section 262 may file a petition in the Delaware Court of Chancery demanding a determination of the fair value of the shares held by all such stockholders. Upon the filing of the petition by a stockholder, service of a copy of
the petition must be made upon the surviving corporation. The surviving corporation has no obligation to file a petition in the Delaware Court of Chancery in the event there are dissenting stockholders, and Eiger, which is expected to be the
surviving corporation, has no present intent to file a petition in the Delaware Court of Chancery. Accordingly, the failure of a stockholder to file a petition within the period specified could nullify the stockholder’s previously written
demand for appraisal.
If a petition for appraisal is duly filed by a stockholder and a copy of the petition is delivered to the surviving corporation,
the surviving corporation will then be obligated, within 20 days after receiving service of a copy of the petition, to provide the Delaware Court of Chancery with a duly verified list containing the names and addresses of all stockholders who have
demanded an appraisal of their shares and with whom agreements as to the value of their shares have not been reached by the surviving corporation. After notice to dissenting stockholders who demanded appraisal of their shares, the Delaware Court of
Chancery is empowered to conduct a hearing upon the petition, and to determine those stockholders who have complied with Section 262 and who have become entitled to the appraisal rights provided thereby. The Delaware Court of Chancery may
require the stockholders who have demanded appraisal for their shares to submit their stock certificates to the Register in Chancery for notation thereon of the pendency of the appraisal proceedings; and if any stockholder fails to comply with that
direction, the Delaware Court of Chancery may dismiss the proceedings as to that stockholder.
After determination of the stockholders entitled to
appraisal of their shares, the Delaware Court of Chancery will appraise the “fair value” of the shares owned by those stockholders. This value will be exclusive of any element of value arising from the accomplishment or expectation of the
merger, but may include a fair rate of interest, if any, upon the amount determined to be the fair value. When the value is determined, the Delaware Court of Chancery will direct the payment of the value, with interest thereon accrued during the
pendency of the proceeding, if the Delaware Court of Chancery so determines, to the stockholders entitled to receive the same, upon surrender by the holders of the certificates representing those shares.
In determining fair value, and, if applicable, a fair rate of interest, the Delaware Court of Chancery is required to take into account all relevant factors.
In Weinberger v. UOP, Inc., the Delaware Supreme Court discussed the factors that could be considered in determining fair value in an appraisal proceeding, stating that “proof of value by any techniques or methods which are generally
considered acceptable in the financial community and otherwise admissible in court” should be considered, and that “fair price obviously requires consideration of all relevant factors involving the value of a company.”
Section 262 provides that fair value is to be “exclusive of any element of value arising from the accomplishment or expectation of the merger.”
In Cede & Co. v. Technicolor, Inc., the Delaware Supreme Court stated that this exclusion is a “narrow exclusion [that] does not encompass known elements of value,” but which rather applies only to the speculative elements
of value arising from such accomplishment or expectation. In Weinberger, the Delaware Supreme Court construed Section 262 to mean that “elements of future value, including the nature of the enterprise, which are known or susceptible
of proof as of the date of the merger and not the product of speculation, may be considered.”
You should be aware that the fair value of your shares
as determined under Section 262 could be more than, the same as, or less than the value that you are entitled to receive under the terms of the Merger Agreement.
Costs of the appraisal proceeding may be imposed upon the surviving corporation and the stockholders participating in the appraisal proceeding by the Delaware
Court of Chancery as the Court deems equitable in the circumstances. Upon the application of a stockholder, the Delaware Court of Chancery may order all or a portion
117
of the expenses incurred by any stockholder in connection with the appraisal proceeding, including, without limitation, reasonable attorneys’ fees and the fees and expenses of experts, to be
charged pro rata against the value of all shares entitled to appraisal. In the absence of such a determination of assessment, each party bears its own expenses. Any stockholder who had demanded appraisal rights will not, after the effective time of
the merger, be entitled to vote shares subject to that demand for any purpose or to receive payments of dividends or any other distribution with respect to those shares, other than with respect to payment as of a record date prior to the effective
time; however, if no petition for appraisal is filed within 120 days after the effective time of the merger, or if the stockholder delivers a written withdrawal of his or her demand for appraisal and an acceptance of the terms of the merger within
60 days after the effective time of the merger, then the right of that stockholder to appraisal will cease and that stockholder will be entitled to receive the merger consideration for shares of his or her Celladon capital stock pursuant to the
Merger Agreement. Any withdrawal of a demand for appraisal made more than 60 days after the effective time of the merger may only be made with the written approval of the surviving corporation. No appraisal proceeding in the Delaware Court of
Chancery will be dismissed as to any stockholder without the approval of the court.
Failure to follow the steps required by Section 262 for
perfecting appraisal rights may result in the loss of appraisal rights. In view of the complexity of Section 262, stockholders who may wish to dissent from the merger and pursue appraisal rights should consult their legal advisors.
118
THE MERGER AGREEMENT
The following is a summary of the material terms of the Merger Agreement. A copy of the Merger Agreement is attached as Annex A to this proxy
statement/prospectus/information statement and is incorporated by reference into this proxy statement/prospectus/information statement. The Merger Agreement has been attached to this proxy statement/prospectus/information statement to provide you
with information regarding its terms. It is not intended to provide any other factual information about Celladon, Eiger or Merger Sub. The following description does not purport to be complete and is qualified in its entirety by reference to the
Merger Agreement. You should refer to the full text of the Merger Agreement for details of the merger and the terms and conditions of the Merger Agreement.
The Merger Agreement contains representations and warranties that Celladon and Merger Sub, on the one hand, and Eiger, on the other hand, have made to one
another as of specific dates. These representations and warranties have been made for the benefit of the other parties to the Merger Agreement and may be intended not as statements of fact but rather as a way of allocating the risk to one of the
parties if those statements prove to be incorrect. In addition, the assertions embodied in the representations and warranties are qualified by information in confidential disclosure schedules exchanged by the parties in connection with signing the
Merger Agreement. While Celladon and Eiger do not believe that these disclosure schedules contain information required to be publicly disclosed under the applicable securities laws, other than information that has already been so disclosed, the
disclosure schedules do contain information that modifies, qualifies and creates exceptions to the representations and warranties set forth in the attached Merger Agreement. Accordingly, you should not rely on the representations and warranties as
current characterizations of factual information about Celladon or Eiger, because they were made as of specific dates, may be intended merely as a risk allocation mechanism between Celladon and Merger Sub, and Eiger and are modified by the
disclosure schedules.
General
Under the Merger Agreement, Celladon Merger Sub, Inc., or Merger Sub, a wholly owned subsidiary of Celladon formed by Celladon in connection with the merger,
will merge with and into Eiger, with Eiger surviving as a wholly owned subsidiary of Celladon.
Merger Consideration
Immediately prior to the closing of the financing contemplated by the Subscription Agreement, each share of Eiger preferred stock outstanding at such time will
be converted into shares of Eiger common stock at a ratio determined in accordance with the Eiger certificate of incorporation then in effect, and each outstanding Eiger warrant to purchase equity securities will be automatically exercised
immediately prior to the closing of the merger. At the effective time of the merger, all outstanding shares of Eiger common stock, and all outstanding options and warrants to purchase Eiger common stock, respectively, will convert into the right to
receive Celladon common stock as follows:
| |
• |
|
each share of Eiger common stock outstanding immediately prior to the effective time of the merger (excluding shares of Eiger common stock held as treasury stock or held by Eiger, Merger Sub or any subsidiary of Eiger)
will automatically be converted into the right to receive a number of shares of Celladon common stock equal to the exchange ratio, described below, subject to adjustment to account for the proposed 1-for-15 reverse stock split; |
| |
• |
|
each option to purchase shares of Eiger common stock outstanding and unexercised immediately prior to the effective time of the merger will be assumed by Celladon and will become an option, subject to vesting, to
purchase that number of shares of the common stock of Celladon multiplied by the exchange ratio (and rounding the resulting number down to the nearest whole number), at an exercise price equal to the per share exercise price of such Eiger option
divided by the exchange ratio subject to adjustment to account for the proposed 1-for-15 reverse stock split; and |
119
| |
• |
|
each warrant to purchase shares of Eiger preferred stock or Eiger common stock not terminated or exercised at or prior to the effective time of the merger, or automatically exercised immediately prior to the
consummation of the merger, will be assumed by Celladon and will become a warrant to purchase that number of shares of the common stock of Celladon multiplied by the exchange ratio, at an exercise price equal to the per share exercise price of such
Eiger warrant multiplied by the exchange ratio, subject to adjustment to account for the proposed 1-for-15 reverse stock split. All outstanding Eiger warrants are anticipated to be automatically exercised immediately prior to consummation of the
merger. |
Exchange Ratio
The exchange ratio is calculated using a formula intended to allocate to the existing Eiger securityholders (on a fully diluted basis and assuming a cashless
exercise of options and warrants, referred to as Eiger fully-diluted outstanding shares) a percentage of the combined company based on the relative valuations of:
| |
• |
|
$55 million plus the proceeds obtained by Eiger in the pre-closing financing for Eiger; and |
| |
• |
|
$26.75 million for Celladon on a fully diluted basis, disregarding underwater options and assuming a cashless exercise of all other options and warrants, referred to as Celladon fully-diluted outstanding shares.
|
The exchange ratio formula is the quotient obtained by dividing the Eiger merger shares (as defined below) by the Eiger fully-diluted
outstanding shares, where:
| |
• |
|
Eiger merger shares is the product determined by multiplying the post-closing Celladon shares (as defined below) by the Eiger allocation percentage (as defined below). |
| |
• |
|
Post-closing Celladon shares is the quotient determined by dividing the Celladon fully-diluted outstanding shares by the Celladon allocation percentage (as defined below). |
| |
• |
|
Eiger allocation percentage is the quotient determined by dividing (i) the sum of the aggregate value (as defined below) minus $26.75 million by (ii) the aggregate value. |
| |
• |
|
Aggregate value is the sum of (i) $81.75 million plus (ii) the aggregate gross cash proceeds received by Eiger from the pre-closing financing (including a conversion of promissory notes of up to $6.0 million
in principal amount issued in connection therewith). |
| |
• |
|
Celladon allocation percentage is the quotient determined by dividing $26.75 million by the aggregate value. |
The Merger Agreement does not include a price-based termination right, so there will be no adjustment to the total number of shares of Celladon common stock
that Eiger stockholders, optionholders and warrantholders will be entitled to receive for changes in the market price of Celladon common stock. Accordingly, the market value of the shares of Celladon common stock issued pursuant to the merger will
depend on the market value of the shares of Celladon common stock at the time the merger closes, and could vary significantly from the market value on the date of this proxy statement/prospectus/information statement.
No fractional shares of Celladon common stock will be issuable pursuant to the merger to Eiger stockholders. Instead, each Eiger stockholder who would
otherwise be entitled to receive a fraction of a share of Celladon common stock, after aggregating all fractional shares of Celladon common stock issuable to such stockholder, will be entitled to receive in cash the dollar amount, rounded to the
nearest whole cent, without interest, determined by multiplying such fraction by the closing price of a share of Celladon common stock as quoted on The NASDAQ Global Market, on the date the merger becomes effective.
The Merger Agreement provides that, at the effective time of the merger, Celladon will deposit with an exchange agent acceptable to Celladon and Eiger stock
certificates representing the shares of Celladon common stock issuable to the Eiger stockholders and a sufficient amount of cash to make payments in lieu of fractional shares.
120
The Merger Agreement provides that, promptly after the effective time of the merger, the exchange agent will mail
to each record holder of Eiger capital stock immediately prior to the effective time of the merger a letter of transmittal and instructions for surrendering and exchanging the record holder’s Eiger stock certificates for shares of Celladon
common stock. Upon surrender of an Eiger stock certificate for exchange to the exchange agent, together with a duly signed letter of transmittal and such other documents as the exchange agent or Celladon may reasonably require, the Eiger stock
certificate surrendered will be cancelled and the holder of the Eiger stock certificate will be entitled to receive the following:
| |
• |
|
a certificate representing the number of whole shares of Celladon common stock that such holder has the right to receive pursuant to the provisions of the Merger Agreement; and |
| |
• |
|
cash in lieu of any fractional share of Celladon common stock. |
At the effective time of the merger, all
holders of certificates representing shares of Eiger common stock or Eiger preferred stock that were outstanding immediately prior to the effective time of the merger will cease to have any rights as stockholders of Eiger. In addition, no transfer
of Eiger common stock or Eiger preferred stock after the effective time of the merger will be registered on the stock transfer books of Eiger.
If any
Eiger stock certificate has been lost, stolen or destroyed, Celladon may, in its discretion, and as a condition to the delivery of any shares of Celladon common stock, require the owner of such lost, stolen or destroyed certificate to deliver an
affidavit claiming such certificate has been lost, stolen or destroyed and post a bond indemnifying Celladon against any claim suffered by Celladon related to the lost, stolen or destroyed certificate or any Celladon common stock issued in exchange
for such certificate as Celladon may reasonably request.
From and after the effective time of the merger, until it is surrendered, each certificate that
previously evidenced Eiger common stock or Eiger preferred stock will be deemed to represent only the right to receive shares of Celladon common stock and cash in lieu of any fractional share of Celladon common stock. Celladon will not pay dividends
or other distributions on any shares of Celladon common stock to be issued in exchange for any unsurrendered Eiger stock certificate until the Eiger stock certificate is surrendered as provided in the Merger Agreement.
Treatment of Celladon Stock Options and Warrants
As of the effective time of the reverse stock split, Celladon will adjust and proportionately decrease the number of shares of Celladon’s common stock
reserved for issuance upon exercise of, and adjust and proportionately increase the exercise price of, all options and warrants to acquire Celladon’s common stock at a fifteen (15) to one (1) ratio. All stock options and warrants to
acquire shares of Celladon’s common stock that are outstanding immediately prior to the effective time of the merger will remain outstanding following the effective time of the merger. In addition, as of the effective time of the reverse stock
split, Celladon will adjust and proportionately decrease the total number of shares of Celladon’s common stock that may be the subject of future grants under Celladon’s stock option plans at a fifteen (15) to one (1) ratio.
Treatment of Eiger Stock Options and Warrants
At the effective time of the merger, each option to purchase Eiger common stock that is outstanding and unexercised immediately prior to the effective time of
the merger under the Eiger 2009 Equity Incentive Plan, whether or not vested, will be converted into an option to purchase Celladon common stock. Celladon will assume the Eiger 2009 Equity Incentive Plan. All other options to purchase Eiger common
stock will be cancelled immediately prior to the effective time of the merger. All rights with respect to Eiger common stock under Eiger options assumed by Celladon will be converted into rights with respect to Celladon common stock. Accordingly,
from and after the effective time of the merger, each Eiger stock option assumed by Celladon may be exercised for such number of shares of Celladon common stock as is determined by multiplying the number of shares of Eiger common stock subject to
the option by the exchange ratio and rounding that result down to the
121
nearest whole number of shares of Celladon common stock. The per share exercise price of the converted option will be determined by dividing the existing exercise price of the option by the
exchange ratio and rounding that result up to the nearest whole cent. Any restrictions on the exercise of any Eiger option assumed by Celladon will continue following the conversion and the term, exercisability, vesting schedules and other
provisions of assumed Eiger options will generally remain unchanged; provided, that any Eiger options assumed by Celladon may be subject to adjustment to reflect changes in Celladon capitalization after the effective time of the merger and that the
Celladon board of directors will succeed to the authority of the Eiger board of directors with respect to each assumed Eiger option.
Eiger has issued
warrants to purchase shares of its capital stock. Each outstanding warrant to purchase shares of Eiger equity securities not terminated or exercised at or prior to the effective time of the merger, or automatically exercised immediately prior to the
consummation of the merger, will be assumed by Celladon at the effective time of the merger in accordance with its terms and will become a warrant to purchase shares of Celladon common stock. The number of shares of Celladon common stock subject to
each assumed warrant will be determined by multiplying the number of shares of Eiger common stock issuable upon conversion of the shares of Eiger preferred stock issuable upon exercise of such warrant that were subject to such warrant prior to the
effective time of the merger by the exchange ratio and rounding that result down to the nearest whole number of shares of Celladon common stock. The per share exercise price for the Celladon common stock issuable upon exercise of each of the assumed
warrants will be determined by dividing the per share exercise price of the Eiger share subject to each warrant as in effect exercised immediately prior to the effective time of the merger by the exchange ratio and rounding that result up to the
nearest whole cent. All outstanding Eiger warrants are anticipated to be automatically exercised immediately prior to consummation of the merger.
Directors and Executive Officers of Celladon Following the Merger
Pursuant to the Merger Agreement, all of the directors of Celladon will resign
at or prior to the effective time of the merger. Prior to the effective time of the merger, the board of directors of Celladon will elect seven designees selected by Eiger to serve as members of the board of directors of Celladon effective upon
closing of the merger in staggered classes to be designated by Eiger prior to the closing. The Eiger designees in the aggregate are expected to satisfy the requisite independence requirements for the board of directors of Celladon, as well as the
sophistication and independence requirements for the required committees pursuant to NASDAQ listing requirements. It is anticipated that the Eiger designees will include David A. Cory, Thomas J. Dietz, Edgar G. Engleman, Jeffrey S. Glenn and Nina
Kjellson, plus two additional nominees. Effective as of the effective time of the merger, the Celladon board of directors will appoint each of the following as officers of Celladon:
|
|
|
| Name |
|
Title |
| David A. Cory, RPH, MBA |
|
President and Chief Executive Officer |
| James H. Welch |
|
Chief Financial Officer |
| Joanne Quan, MD |
|
Chief Medical Officer |
| Eduardo Martins, MD, DPhil |
|
Senior Vice President, Liver and Infectious Diseases |
| James P. Shaffer, MBA |
|
Chief Business Officer |
Amendments to the Amended and Restated Certificate of Incorporation of Celladon
Stockholders of record of Celladon common stock on the record date for the Celladon special meeting will also be asked to approve the amendment to the amended
and restated certificate of incorporation of Celladon to effect the proposed 1-for-15 reverse stock split and the amendment to the amended and restated certificate of incorporation of Celladon to change the name of the corporation from
“Celladon Corporation” to “Eiger BioPharmaceuticals, Inc.” upon consummation of the merger, each of which requires the affirmative vote of holders of a majority of the outstanding common stock on the record date for the Celladon
special meeting.
122
Conditions to the Completion of the Merger
Each party’s obligation to complete the merger is subject to the satisfaction or waiver by each of the parties, at or prior to the merger, of various
conditions, which include the following:
| |
• |
|
the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, must have been declared effective by the SEC in accordance with the Securities Act and must not be
subject to any stop order or proceeding, or any proceeding threatened by the SEC, seeking a stop order; |
| |
• |
|
there must not have been issued any temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the merger by any court of competent jurisdiction or other governmental
entity of competent jurisdiction, and no law, statute, rule, regulation, ruling or decree shall be in effect which has the effect of making the consummation of the merger illegal; |
| |
• |
|
the holders of a majority of the shares of outstanding Eiger common stock and preferred stock, voting together as one class, and holders of 60% of the shares of Eiger preferred stock must have adopted and approved the
Merger Agreement and the merger, and the holders of a majority of the outstanding shares of Celladon common stock must have approved the merger, the issuance of Celladon common stock in the merger and the reverse stock split; |
| |
• |
|
the existing shares of Celladon common stock must have been continually listed on The NASDAQ Global Market through the closing of the merger, and the shares of Celladon common stock to be issued in the merger must be
approved for listing on The NASDAQ Global Market (subject to official notice of issuance) as of the effective time of the merger; |
| |
• |
|
any waiting period applicable to the consummation of the merger under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, or HSR Act, must have expired or been terminated, and there must not be in
effect any voluntary agreement by any party to the Merger Agreement and the U.S. Federal Trade Commission, the U.S. Department of Justice or any foreign governmental body, pursuant to which such party has agreed not to consummate the merger for any
period of time; |
| |
• |
|
there must not be any legal proceeding pending, or overtly threatened in writing by an official of any governmental body in which such governmental body indicates that it intends to conduct any legal proceeding or take
any action: |
| |
• |
|
challenging or seeking to restrain or prohibit the consummation of the merger; |
| |
• |
|
relating to the merger and seeking to obtain from Celladon, Merger Sub, or Eiger any damages or other relief that may be material to Celladon or Eiger; |
| |
• |
|
seeking to prohibit or limit in any material and adverse respect a party to the Merger Agreement’s ability to vote, transfer, receive dividends with respect to or otherwise exercise ownership rights with respect to
the stock of Celladon; |
| |
• |
|
that would materially and adversely affect the right or ability of Celladon or Eiger to own the assets or operate the business of Celladon or Eiger; or |
| |
• |
|
seeking to compel Eiger, Celladon, or any subsidiary of Celladon to dispose of or hold separate any material assets as a result of the merger. |
In addition, each party’s obligation to complete the merger is further subject to the satisfaction or waiver by that party of the following additional
conditions:
| |
• |
|
all representations and warranties of the other party in the Merger Agreement must be true and correct on the date of the Merger Agreement and on the
closing date of the merger with the same force and effect as if made on the date on which the merger is to be completed or, if such representations and warranties address matters as of a particular date, then as of that particular date, except where
the |
123
| |
failure of these representations and warranties to be true and correct, individually or in the aggregate, would not reasonably be expected to have a material adverse effect on the other party;
|
| |
• |
|
the other party to the Merger Agreement must have performed or complied with in all material respects all covenants and obligations in the Merger Agreement required to be performed or complied with by it on or before
the closing of the merger; and |
| |
• |
|
the other party must have delivered certain certificates and other documents required under the Merger Agreement for the closing of the merger. |
In addition, the obligation of Celladon and Merger Sub to complete the merger is further subject to the satisfaction or waiver of the following conditions:
| |
• |
|
Eiger must have delivered to Celladon written resignations of the officers and directors of Eiger that are not continuing as officers and directors of Eiger following the merger; |
| |
• |
|
the financing contemplated by the Subscription Agreement must have been consummated and Eiger must have received the proceeds from such financing pursuant to the terms and conditions set forth in the Subscription
Agreement; |
| |
• |
|
there shall have been no effect, change, event, circumstance, or development that is or could reasonably be expected to be materially adverse to, or has or could reasonably be expected to have or result in a material
adverse effect on the business, condition (financial or otherwise), capitalization, assets, operations or financial performance of Eiger and its subsidiaries, taken as a whole, or the ability of Eiger to complete the merger or any of the other
transactions contemplated by the Merger Agreement or to perform any of its covenants or obligations under the Merger Agreement in all material respects, each referred to as a material adverse effect as it relates to Eiger. The Merger Agreement
provides that certain events shall not be considered a material adverse change to Eiger, including without limitation: |
| |
• |
|
any rejection by a governmental body of a registration or filing by Eiger relating to intellectual property owned, licensed or controlled by Eiger; |
| |
• |
|
any change in the cash position of Eiger which results from operations in the ordinary course of business; |
| |
• |
|
any effect, change event, circumstance, or development resulting from the announcement or pendency of the merger; |
| |
• |
|
conditions generally affecting the industries in which Eiger participates or the U.S. or global economy or capital markets as a whole, to the extent that such conditions do not have a disproportionate impact on Eiger;
|
| |
• |
|
any failure by Eiger to meet internal projections or forecasts or third party revenue or earnings predictions for any period ending on or after the date of this Agreement (but not the effect causing or contributing to
any such failure); |
| |
• |
|
The announcement or performance of the obligations of Eiger pursuant to the Merger Agreement or the announcement or anticipated consummation of the merger or the financing contemplated by the Subscription Agreement;
|
| |
• |
|
any change in accounting requirements or principles or any change in applicable laws; or |
| |
• |
|
any natural disaster or any acts of terrorism or war; |
| |
• |
|
certain agreements between Eiger and certain stockholders must have been terminated; and |
| |
• |
|
Eiger must have effected a conversion of its preferred stock into common stock and conversion of all of its outstanding convertible debt, immediately prior to the consummation of the transactions contemplated under the
Merger Agreement. |
124
In addition, the obligation of Eiger to complete the merger is further subject to the satisfaction or waiver of
the following conditions:
| |
• |
|
Celladon must have delivered to Eiger written resignations of the officers and directors of Celladon and caused the new board members of Celladon, specified in the Merger Agreement, to be elected; |
| |
• |
|
either the principal executive officer or the principal financial officer of Celladon must have provided, with respect to any document filed with the SEC on or after November 18, 2015, any necessary certification
required under Rule 13a-14 under the Exchange Act, as amended; |
| |
• |
|
the Celladon Net Cash shall be greater than or equal to $24 million. Net Cash means (a) the sum of Celladon’s cash and cash equivalents, marketable securities, accounts, interest and other receivables (to the
extent determined to be collectible), and deposits (to the extent refundable to Celladon), in each case as of the close of business on the last business day prior to the date of determination, determined in a manner consistent with the manner in
which such items were historically determined and in accordance with Celladon’s audited financial statements and unaudited interim balance sheet, minus (b) the sum of Celladon’s accounts payable and accrued expenses (other than
accrued expenses listed below), in each case as of such date and determined in a manner consistent with the manner in which such items were historically determined and in accordance with Celladon’s audited financial statements and unaudited
interim balance sheet, minus (c) the cash cost of any unpaid change of control payments or severance payments that are or become due to any current or former employee of Celladon, minus (d) the cash cost of any accrued and unpaid retention
payments due to any employee of Celladon as of the closing date, minus (e) any remaining unpaid fees and expenses as of such date for which Celladon is liable incurred by Celladon in connection with the Merger Agreement and the merger or
otherwise, minus (f) any bona fide current liabilities payable in cash, in each case to the extent not cancelled at or prior to the determination date, minus (g) any unpaid amounts with respect to the directors and officers insurance
policy to be purchased by Celladon, minus (h) any fees and expenses payable by Celladon to the auditors in connection with the determination of Net Cash, minus (i) the cash cost of any unpaid retention payment amounts due under any
insurance policy with respect to any litigation against Celladon, minus (j) the net cash obligation of Celladon (i.e., remaining contractual payments owed less contractual receipts expected) with respect to certain leased premises plus
(k) the amount of any reimbursement owed to Celladon in connection with the merger; |
| |
• |
|
there shall have been no effect, change, event, circumstance, or development that is or could reasonably be expected to be materially adverse to, or has or could reasonably be expected to have or result in a material
adverse effect on the business, condition (financial or otherwise), capitalization, assets, operations or financial performance of Celladon and its subsidiaries, taken as a whole, or the ability of Celladon to complete the merger or any of the other
transactions contemplated by the Merger Agreement or to perform any of its covenants or obligations under the Merger Agreement in all material respects, each referred to as a material adverse effect as it relates to Celladon. The Merger Agreement
provides that certain events shall not be considered a material adverse change to Celladon, including without limitation: |
| |
• |
|
conditions generally affecting the industries in which Celladon participates or the U.S. or global economy or capital market as a whole; |
| |
• |
|
any effect, change, event, circumstance, or development resulting from the announcement or pendency of the merger; |
| |
• |
|
any failure by Celladon to meet internal projections or forecasts or third parties revenues predictions for any period ending on or after November 18, 2015 or any change in the stock price or trading volume of
Celladon independent of any other event that would be deemed to have a material adverse change to Celladon; |
| |
• |
|
the execution, announcement or performance of the obligations by Celladon under the Merger Agreement or the announcement or performance of the merger by Celladon; |
125
| |
• |
|
the resignation or termination of any officer or director of Celladon; or |
| |
• |
|
any natural disaster or any act of terrorism, sabotage, military action or war or escalation or worsening of any of the foregoing. |
Representations and Warranties
The Merger Agreement contains customary representations and warranties of Celladon and Eiger for a transaction of this type relating to, among other things:
| |
• |
|
corporate organization and power, and similar corporate matters; |
| |
• |
|
financial statements and with respect to Celladon, documents filed with the SEC and the accuracy of information contained in those documents; |
| |
• |
|
material changes or events; |
| |
• |
|
real property and leaseholds; |
| |
• |
|
the validity of material contracts to which the parties or their subsidiaries are a party and any violation, default or breach to such contracts; |
| |
• |
|
regulatory compliance, permits and restrictions; |
| |
• |
|
employee and labor matters and benefit plans; |
| |
• |
|
legal proceedings and orders; |
| |
• |
|
authority to enter into the Merger Agreement and the related agreements; |
| |
• |
|
transactions with affiliates; |
| |
• |
|
votes required for completion of the merger and approval of the proposals that will come before the Celladon special meeting and that will be the subject of the Eiger stockholder written consent; |
| |
• |
|
except as otherwise specifically identified in the Merger Agreement, the fact that the consummation of the merger would not contravene or require the consent of any third party; |
| |
• |
|
bank accounts, receivables and deposits; |
| |
• |
|
any brokerage or finder’s fee or other fee or commission in connection with the merger; |
| |
• |
|
with respect to Eiger, disclosures related to the Subscription Agreement; |
| |
• |
|
with respect to Celladon, the valid issuance in the merger of the Celladon common stock; and |
| |
• |
|
the inapplicability of Section 203 of the DGCL. |
The representations and warranties are, in many
respects, qualified by materiality and knowledge, and will not survive the merger, but their accuracy forms the basis of one of the conditions to the obligations of Celladon and Eiger to complete the merger.
126
No Solicitation
Each of Celladon and Eiger agreed that, except as described below, Celladon and Eiger and any of their respective subsidiaries will not, nor will either party
or any of its subsidiaries authorize or permit any of the officers, directors, employees, investment bankers, attorneys, accountants, representatives, consultants or other agents retained by it or any of its subsidiaries to, directly or indirectly:
| |
• |
|
solicit, initiate, encourage, induce or knowingly facilitate the communication, making, submission or announcement of, any “acquisition proposal,” as defined below, or inquiry, indication of interest or
request for information that could reasonably be expected to lead to an acquisition proposal, or take any action that could reasonably be expected to lead to an acquisition proposal or an inquiry, indication of interest or request for information
that could reasonably be expected to lead to an acquisition proposal; |
| |
• |
|
furnish any information with respect to it to any person in connection with or in response to an acquisition proposal or inquiry, indication of interest or request for information that could reasonably be expected to
lead to an acquisition proposal; |
| |
• |
|
engage in discussions or negotiations with any person with respect to any acquisition proposal or inquiry, indication of interest or request for information that could reasonably be expected to lead to an acquisition
proposal; |
| |
• |
|
approve, endorse or recommend an acquisition proposal; |
| |
• |
|
execute or enter into any letter of intent or similar document or any contract contemplating or otherwise relating to an acquisition transaction; or |
| |
• |
|
grant any waiver or release under any confidentiality, standstill or similar agreement, other than to either Celladon or Eiger. |
An “acquisition proposal” means any offer or proposal, whether written or oral contemplating or otherwise relating to any “acquisition
transaction,” as defined below.
An “acquisition transaction” means the following:
| |
• |
|
any merger, consolidation, amalgamation, share exchange, business combination, issuance or acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or similar transaction: in which
Celladon, Eiger or Merger Sub is a constituent corporation, in which any individual, entity, governmental entity, or “group,” as defined under applicable securities laws, directly or indirectly acquires beneficial or record ownership of
securities representing more than 15% of the outstanding securities of any class of voting securities of Celladon, Eiger or Merger Sub or any of their subsidiaries or in which Celladon, Eiger or Merger Sub or any of their subsidiaries issues
securities representing more than 15% of the outstanding voting securities of any class of voting securities of such party or any of its subsidiaries; |
| |
• |
|
any sale, lease, exchange, transfer, license, acquisition or disposition of any business or assets that constitute 15% or more of the consolidated book value or the fair market value of the assets of Celladon, Eiger or
Merger Sub and their subsidiaries, taken as a whole; and |
| |
• |
|
any liquidation or dissolution of Celladon, Eiger or Merger Sub. |
127
However, before obtaining the applicable Celladon or Eiger stockholder approvals required to consummate the
merger, each party may furnish nonpublic information regarding such party to, and may enter into discussions or negotiations with, any third party in response to a bona fide written acquisition proposal made or received after the date of the Merger
Agreement, which such party’s board of directors determines in good faith, after consultation with a nationally recognized independent financial advisor and its outside legal counsel, constitutes or is reasonably likely to result in a
“superior offer,” as defined below, if:
| |
• |
|
neither such party nor any representative of such party has breached the no solicitation provisions of the Merger Agreement described above; |
| |
• |
|
such party’s board of directors concludes in good faith, based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to result in a breach of the fiduciary duties of such
board of directors under applicable legal requirements; |
| |
• |
|
such party gives the other party at least five business days’ prior notice of the identity of the third party and of that party’s intention to furnish information to, or enter into discussions or negotiations
with, such third party before furnishing any information or entering into discussions or negotiations with such third party; |
| |
• |
|
such party receives from the third party an executed confidentiality agreement containing provisions at least as favorable to such party as those contained in the confidentiality agreement between Celladon and Eiger;
and |
| |
• |
|
at least five business days’ prior to the furnishing of any information to a third party, such party furnishes the same information to the other party to the extent not previously furnished. |
A “superior offer” means an unsolicited, bona fide written offer by a third party to enter into a merger, consolidation, amalgamation, share
exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction as a result of which either Celladon’s or Eiger’s stockholders
prior to such transaction in the aggregate cease to own at least 50% of the voting securities of the entity surviving or resulting from such transaction, or the ultimate parent entity thereof, or in which a person or “group,” as defined
under applicable securities laws, directly or indirectly acquires beneficial or record ownership of securities representing 50% or more of the party’s capital stock or a sale, lease, exchange transfer, license, acquisition or disposition of any
business or other disposition of at least 50% of the assets of the party or its subsidiaries, taken as a whole, in a single transaction or a series of related transactions that was not obtained or made as a direct or indirect result of a breach, or
violation, of the Merger Agreement, and is on terms and conditions that the board of directors of the party receiving the offer determines in its reasonable good faith judgment, after obtaining and taking into account such matters as the board of
directors deems relevant following consultation with its outside legal counsel and financial advisor:
| |
• |
|
is reasonably likely to be more favorable, from a financial point of view, to that party’s stockholders than the terms of the merger; and |
| |
• |
|
is reasonably capable of being consummated. |
An offer will not be a superior offer if any financing required
to consummate the transaction contemplated by such offer is not committed and is not reasonably capable of being obtained by such third party or if the consummation of such transaction is contingent on any such financing being obtained.
The Merger Agreement also provides that each party will promptly advise the other of the status and terms of, and keep the other party fully informed with
respect to, any acquisition proposal or any inquiry, indication of interest or request for information that could reasonably be expected to lead to an acquisition proposal or any change or proposed change to that acquisition proposal or inquiry,
indication of interest or request for information that could reasonably be expected to lead to an acquisition proposal.
128
Meetings of Stockholders
Celladon is obligated under the Merger Agreement to call, give notice of and hold a special meeting of its stockholders for the purposes of considering the
issuance of shares of Celladon common stock and the merger.
Eiger is obligated under the Merger Agreement to obtain written consents of its stockholders
sufficient to adopt the Merger Agreement thereby approving the merger and related transactions within two business days of the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, being
declared effective by the SEC.
Covenants; Conduct of Business Pending the Merger
Eiger agreed that it will conduct its business in the ordinary course in accordance with past practices and in compliance with all applicable laws,
regulations, and certain contracts, and to take other agreed-upon actions. Eiger also agreed that, subject to certain limited exceptions, without the consent of Celladon, it will not, during the period prior to closing of the merger:
| |
• |
|
declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock; or repurchase, redeem or otherwise reacquire any shares of capital stock or other securities
(except for shares of common stock from terminated employees); |
| |
• |
|
amend the certificate of incorporation, bylaws or other charter or organizational documents of Eiger, or effect or been a party to any merger, consolidation, share exchange, business combination, recapitalization,
reclassification of shares, stock split, reverse stock split or similar transaction; |
| |
• |
|
sell, issue or grant, or authorize the issuance of, or make any commitments to do any of the foregoing, other than as contemplated by the Merger Agreement: any capital stock or other security (except for options or
common stock issued to Eiger employees or consultants or shares of Eiger common stock issued upon the valid exercise of options); any option, warrant or right to acquire any capital stock or any other security; or any instrument convertible into or
exchangeable for any capital stock or other security; |
| |
• |
|
form any subsidiary or acquire any equity interest or other interest in any other entity; |
| |
• |
|
lend money to any person; other than in the ordinary course of business, incur or guarantee any indebtedness for borrowed money; issue or sell any debt securities or options, warrants, calls or other rights to acquire
any debt securities; guarantee any debt securities of others; or make any capital expenditure or commitment in excess of $5,000; |
| |
• |
|
adopt, establish or enter into any employee plan; cause or permit any employee plan to be amended other than as required by law; or pay any bonus or make any profit-sharing or similar payment to, or increase the amount
of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors, officers or employees, subject to certain exceptions; |
| |
• |
|
enter into any material transaction outside the ordinary course of business; |
| |
• |
|
acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its assets or properties, or grant any encumbrance with respect to such assets or properties, except in the ordinary course of
business consistent with past practices; |
| |
• |
|
make, change or revoke any material tax election; file any material amendment to any tax return; adopt or change any accounting method in respect of
taxes; change any annual tax accounting period; enter into any tax allocation agreement, tax sharing agreement or tax indemnity agreement, other than commercial contracts entered into in the ordinary course of business with vendors, customers or
landlords; enter into any closing agreement with respect to any tax; settle or compromise any claim, notice, audit report or assessment in respect of material taxes; apply for or enter into any ruling from any tax authority with respect to taxes;
surrender any right to claim a material tax refund; or consent to |
129
| |
any extension or waiver of the statute of limitations period applicable to any material tax claim or assessment; |
| |
• |
|
enter into, amend or terminate any material contract, subject to certain exceptions; or |
| |
• |
|
make any material change to, or agree to change, the pricing or royalties or other payments set or charged by Eiger or any of its subsidiaries to its customers or licensees or agree to materially increase pricing or
royalties or other payments to an existing licensor of intellectual property for no additional consideration to Eiger. |
Celladon agreed that
it will conduct its business in the ordinary course consistent with past practices and in compliance with all applicable laws, regulations and certain contracts, and to take other agreed-upon actions. Celladon also agreed that, subject to certain
limited exceptions, without the consent of Eiger, it will not, during the period prior to the closing of the merger:
| |
• |
|
declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock; or repurchase, redeem or otherwise reacquire any shares of capital stock or other securities
(except for shares of common stock from terminated employees of Celladon); |
| |
• |
|
except for contractual commitments in place at the time of the Merger Agreement, sell, issue or grant, or authorize the issuance of: any capital stock or other security (except for Celladon common stock issued upon the
valid exercise of outstanding Celladon options); any option, warrant or right to acquire any capital stock or any other security; or any instrument convertible into or exchangeable for any capital stock or other security; |
| |
• |
|
amend the certificate of incorporation, bylaws or other charter or organizational documents of Celladon, or effect or become a party to any merger, consolidation, share exchange, business combination, recapitalization,
reclassification of shares, stock split, reverse stock split or similar transaction except as related to the proposed transactions under the Merger Agreement; |
| |
• |
|
form any subsidiary other than Merger Sub or acquire any equity interest or other interest in any other entity; |
| |
• |
|
lend money to any person; other than in the ordinary course of business, incur or guarantee any indebtedness for borrowed money; issue or sell any debt securities or options, warrants, calls or other rights to acquire
any debt securities; guarantee any debt securities of others; or make any capital expenditure or commitment; |
| |
• |
|
adopt, establish or enter into any Celladon employee plan; cause or permit any Celladon employee plan to be amended other than as required by law or in order to make amendments for the purposes of Section 409A of
the Code, subject to prior review and approval (with such approval not to be unreasonably withheld) by Eiger; other than in the ordinary course of business, pay any bonus or make any profit-sharing or similar payment to, or increase the amount of
the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors or employees or increase the severance or change of control benefits offered to any current or new service providers; provided,
that, Celladon may pay full yearly bonuses and other severance and retention payments to its employees in connection with their termination of employment; |
| |
• |
|
enter into any material transaction outside the ordinary course of business, subject to certain exceptions; |
| |
• |
|
acquire any material asset, except in the ordinary course of business consistent with past practices; |
| |
• |
|
make, change or revoke any material tax election; file any material amendment to any tax return; adopt or change any accounting method in respect of
taxes; change any annual tax accounting period; enter into any tax allocation agreement, tax sharing agreement or tax indemnity agreement, other than commercial contracts entered into in the ordinary course of business with vendors, customers or
|
130
| |
landlords; enter into any closing agreement with respect to any tax; settle or compromise any claim, notice, audit report or assessment in respect of material taxes; apply for or enter into any
ruling from any tax authority with respect to taxes; surrender any right to claim a material tax refund; or consent to any extension or waiver of the statute of limitations period applicable to any material tax claim or assessment;
|
| |
• |
|
enter into any material contract; or |
| |
• |
|
make any material change to, or agree to change, the pricing or royalties or other payments set or charged by Celladon or any of its subsidiaries to its customers or licensees or agree to change pricing or royalties or
other payments set or charged by persons who have licensed intellectual property to Celladon or any of its subsidiaries. |
Other Agreements
Each of Celladon and Eiger has agreed to use its commercially reasonable efforts to:
| |
• |
|
file or otherwise submit all applications, notices, reports and other documents reasonably required to be filed with a governmental entity with respect to the merger; |
| |
• |
|
take all actions necessary to complete the merger; |
| |
• |
|
coordinate with the other in preparing and exchanging information and promptly provide the other with copies of all filings or submissions made in connection with the merger; |
| |
• |
|
obtain all consents, approvals or waivers reasonably required in connection with the transactions contemplated by the Merger Agreement; |
| |
• |
|
lift any injunction prohibiting, or any other legal bar to, the merger or other transactions contemplated by the Merger Agreement; and |
| |
• |
|
consult and agree with each other about any public statement either will make concerning the merger, subject to certain exceptions. |
Celladon and Eiger agreed that:
| |
• |
|
Celladon will use commercially reasonable efforts (i) to maintain the listing of its common stock on The NASDAQ Global Market, (ii) prepare and submit notification form with respect to and obtain approval for
listing on The NASDAQ Global Market of Celladon the shares of its common stock to be issued in the merger; |
| |
• |
|
for a period of six years after the closing of the merger, Celladon will indemnify each of the directors and officers of Celladon and Eiger to the fullest extent permitted under the DGCL and will maintain
directors’ and officers’ liability insurance for the directors and officers of Celladon and Eiger; and |
| |
• |
|
Celladon will purchase an insurance policy for six years effective as of the closing, which maintains the directors’ and officers’ liability insurance policies maintained by Celladon. |
Termination of the Merger Agreement
The Merger Agreement may be terminated at any time before the completion of the merger, whether before or after the required stockholder approvals to complete
the merger have been obtained, as set forth below:
| |
• |
|
by mutual written consent duly authorized by the board of directors of each of Celladon and Eiger; |
| |
• |
|
by either Celladon or Eiger if the merger has not been consummated by March 31, 2016; provided, however, that this right to terminate the Merger
Agreement will not be available to any party whose action or failure to act has been a principal cause of the failure of the merger to occur on or before such date and such action or failure to act constitutes a breach of the Merger Agreement, and
this right to terminate will not be available for an additional 60 days upon request of either party if the waiting |
131
| |
period under HSR Act has not expired, if applicable, or a request for additional information has been made by any government authority, or in the event that the SEC has not declared effective the
registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, by such date; |
| |
• |
|
by Celladon or Eiger if a court of competent jurisdiction or governmental entity has issued a final and nonappealable order, decree or ruling or taken any other action that permanently restrains, enjoins or otherwise
prohibits the merger; |
| |
• |
|
by Celladon if Eiger did not obtain the written consent of a requisite number of its stockholders necessary to adopt the Merger Agreement and approve the merger and related matters within two business days of the
registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, becoming effective, but this right to terminate the Merger Agreement will not be available to Celladon once Eiger obtains such approval;
|
| |
• |
|
by Celladon or Eiger if the stockholders of Celladon do not approve the merger or the issuance of Celladon common stock in the merger at the Celladon special meeting (including any adjournments and postponements
thereof), but Celladon may not terminate the Merger Agreement pursuant to this provision if the failure to obtain the approval of Celladon stockholders was caused by the action or failure to act of Celladon and such action or failure to act
constitutes a material breach by Celladon of the Merger Agreement; |
| |
• |
|
by Eiger, at any time prior to the approval by Celladon’s stockholders of the merger and the issuance of the shares of Celladon common stock pursuant to the merger, if: |
| |
• |
|
the Celladon board of directors fails to recommend that the stockholders of Celladon vote to approve the merger and the issuance of Celladon common stock or withdraws or modifies its recommendation in a manner adverse
to Eiger; |
| |
• |
|
Celladon fails to include in this proxy statement/prospectus/information statement such recommendation; |
| |
• |
|
Celladon fails to hold the Celladon special meeting within 60 days after the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, is declared effective under the
Securities Act, other than to the extent that such registration statement is subject to a stop order or proceeding, or threatened proceeding by the SEC, seeking a stop order with respect to such registration statement, in which case such 60-day
period will be tolled for so long as such stop order remains in effect or proceeding or threatened proceeding remains pending; |
| |
• |
|
the Celladon board of directors approves, endorses or recommends any acquisition proposal, as defined in the section entitled “The Merger Agreement—No Solicitation” in this proxy
statement/prospectus/information statement; |
| |
• |
|
Celladon enters into any letter of intent or similar document or any contract relating to any acquisition proposal, other than a confidentiality agreement permitted pursuant to the Merger Agreement; or
|
| |
• |
|
Celladon or any director, officer or agent of Celladon willfully and intentionally breaches the no solicitation provisions set forth in the Merger Agreement (each of the above clauses is referred to as a Celladon
triggering event); |
| |
• |
|
by Celladon, at any time prior to the adoption of the Merger Agreement by the stockholders of Eiger, if: |
| |
• |
|
the Eiger board of directors fails to recommend that the Eiger stockholders vote to adopt the Merger Agreement thereby approving the merger or withdraws or modifies its recommendation in a manner adverse to Celladon;
|
132
| |
• |
|
Eiger fails to include in this proxy statement/prospectus/information statement such recommendation; |
| |
• |
|
the Eiger board of directors approves, endorses or recommends any acquisition proposal, as defined in the section entitled “The Merger Agreement—No Solicitation” in this proxy
statement/prospectus/information statement; |
| |
• |
|
Eiger enters into any letter of intent or similar document or any contract relating to any acquisition proposal, other than a confidentiality agreement permitted pursuant to the Merger Agreement; or |
| |
• |
|
Eiger or any director, officer or agent of Eiger willfully and intentionally breaches the no solicitation provisions set forth in the Merger Agreement (each of the above clauses is referred to as an Eiger triggering
event); or |
| |
• |
|
by Celladon or Eiger if the other party has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of the other party has become
inaccurate, in either case such that the conditions to the closing of the merger would not be satisfied as of time of such breach or inaccuracy, but if such breach or inaccuracy is curable, then the Merger Agreement will not terminate pursuant to
this provision as a result of a particular breach or inaccuracy until the earlier of the expiration of a 30-day period after delivery of written notice of such breach or inaccuracy and the breaching party ceasing to exercise commercially reasonable
efforts to cure such breach, if such breach has not been cured; |
| |
• |
|
by Celladon, at any time, if all other closing conditions under the Merger Agreement have been satisfied and/or waived by Celladon, except for the financing contemplated by the Subscription Agreement, and Celladon has
provided written notice to Eiger that it is prepared to consummate the closing of the merger upon the consummation of the financing and Eiger has not obtained the financing contemplated by the Subscription Agreement within five days of receipt of
such notice; or |
| |
• |
|
by Celladon, at any time prior to the approval of the issuance of the shares of Celladon common stock pursuant to the Merger Agreement upon entering into a definitive agreement if all the following conditions are met:
|
| |
• |
|
such agreement provides for the consummation of a transaction that is on terms and conditions that the Celladon board of directors determines, in its reasonable good faith judgment, after obtaining and taking into
account such matters that the Celladon board of directors deems relevant following consultation with its outside legal counsel and financial advisor, if any, is more reasonably likely to be more favorable, from a financial point of view, to
Celladon’s stockholders, than the terms of the merger and is reasonably capable of being consummated (and if any financing is required, only if the financing is committed and is reasonably capable of being obtained), with any such transaction
referred to herein as a permitted alternative transaction; |
| |
• |
|
Celladon complies with its obligations under the Merger Agreement, including its obligations with respect to the no-solicitation covenants; |
| |
• |
|
Celladon provided Eiger a written notice of its intention to enter into a permitted alternative transaction at least five business days in advance with certain details included in such notice; |
| |
• |
|
the Celladon board of directors determines in good faith, after consultation with its outside legal counsel that the permitted alternative transaction satisfies the criteria described above as permitted alternative
transaction and that the failure to enter into such transaction would result in a breach of its fiduciary duties under applicable legal requirements; |
| |
• |
|
Celladon pays Eiger the termination fee of $3.0 million; and |
| |
• |
|
a copy of the agreement contemplating such permitted alternative transaction is delivered to Eiger. |
133
Termination Fee
Fee payable by Celladon
Celladon must pay Eiger a
termination fee of $3.0 million if:
| |
• |
|
the Merger Agreement is terminated by either Celladon or Eiger because the stockholders of Celladon do not approve the merger or the issuance of Celladon common stock in the merger at the Celladon special meeting
(including any adjournments and postponements thereof) and an acquisition proposal, as defined above in the section entitled “The Merger Agreement—No Solicitation,” with respect to Celladon was publicly announced, disclosed or
otherwise communicated to the board of directors of Celladon prior to the Celladon special meeting and Celladon enters into a definitive agreement for, or consummates, an acquisition transaction, as defined above in the section entitled “The
Merger Agreement—No Solicitation,” that results or would result in any third party beneficially owning securities of Celladon representing more than 50% of the voting power of the outstanding securities of Celladon or owning or exclusively
licensing tangible or intangible assets representing more than 50% of the fair market value of the income-generating assets of Celladon and its subsidiaries, taken as a whole, within 12 months of the termination; |
| |
• |
|
the Merger Agreement is terminated by Eiger at any time prior to the approval of the merger and the issuance of Celladon common stock in the merger by the stockholders of Celladon because of a Celladon triggering event,
as defined above in the section entitled “The Merger Agreement—Termination,” and an acquisition proposal, as defined above in the section entitled “The Merger Agreement—No Solicitation,” with respect to Celladon was
publicly announced, disclosed or otherwise communicated to the board of directors of Celladon prior to the Celladon special meeting; or |
| |
• |
|
the Merger Agreement is terminated by Celladon in connection with entering into a permitted alternative transaction (pursuant to the terms described above in the section entitled “The Merger
Agreement—Termination”) |
Celladon must reimburse Eiger for expenses incurred by Eiger in connection with the Merger Agreement and
the transactions contemplated thereby, up to a maximum of $1.0 million, if:
| |
• |
|
the Merger Agreement is terminated by either Celladon or Eiger because the stockholders of Celladon do not approve the merger or the issuance of Celladon common stock in the merger at the Celladon special meeting
(including any adjournments and postponements thereof); |
| |
• |
|
the Merger Agreement is terminated by Eiger because of a Celladon triggering event, as defined above in the section entitled “The Merger Agreement—Termination”; |
| |
• |
|
the Merger Agreement is terminated by Eiger because Celladon or Merger Sub has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or
warranty of Celladon or Merger Sub has become inaccurate, in either case such that the conditions to the closing of the merger would not be satisfied as of time of such breach or inaccuracy, but if such breach or inaccuracy is curable, then the
Merger Agreement will not terminate pursuant to this provision as a result of a particular breach or inaccuracy until the earlier of the expiration of a 30-day period after delivery of written notice of such breach or inaccuracy and Celladon or
Merger Sub ceasing to exercise commercially reasonable efforts to cure such breach, if such breach has not been cured; |
| |
• |
|
the Merger Agreement is terminated by Celladon in connection with entering into a permitted alternative transaction, as defined above in the section entitled “The Merger Agreement – Termination”; or
|
| |
• |
|
in the event of a failure by Eiger to consummate the transactions described in the Merger Agreement solely because there is a Celladon material adverse effect, as defined above in the section entitled “The Merger
Agreement—Termination.” |
134
If Eiger is entitled to reimbursement for expenses and the $3.0 million termination fee, Celladon’s
liability is capped at $3.0 million and in no event will Celladon be required to pay Eiger any amount in excess of $3.0 million in the event of termination of the Merger Agreement.
Fee payable by Eiger
Eiger must pay Celladon a
termination fee of $3.0 million if:
| |
• |
|
the Merger Agreement is terminated by Celladon because Eiger did not obtain the written consent of the requisite number of its stockholders necessary to adopt the Merger Agreement thereby approving the merger and
related matters within two business days of the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, being declared effective by the U.S. Securities and Exchange Commission and an acquisition
proposal, as defined above in the section entitled “The Merger Agreement—No Solicitation,” with respect to Eiger was publicly announced, disclosed or otherwise communicated to the board of directors of Eiger and Eiger enters into a
definitive agreement for, or consummates, an acquisition transaction, as defined above in the section entitled “The Merger Agreement—No Solicitation,” that results or would result in any third party beneficially owning securities of
Eiger representing more than 50% of the voting power of the outstanding securities of Eiger or owning or exclusively licensing tangible or intangible assets representing more than 50% of the fair market value of the income-generating assets of Eiger
and its subsidiaries, taken as a whole, within 12 months of the termination; |
| |
• |
|
the Merger Agreement is terminated by Celladon at any time prior to the adoption of the Merger Agreement, and the approval of the merger and related matters by the stockholders of Eiger because of an Eiger triggering
event, as defined above in the section entitled “The Merger Agreement—Termination”; or |
| |
• |
|
the Merger Agreement is terminated by Celladon because Eiger has not obtained the financing contemplated by the Subscription Agreement and all other conditions are satisfied or waived and Celladon is prepared to
consummate the closing of the merger. |
Eiger must reimburse Celladon for expenses incurred by Celladon in connection with the Merger
Agreement and the transactions contemplated thereby, up to a maximum of $1.0 million, if:
| |
• |
|
the Merger Agreement is terminated by Celladon because Eiger did not obtain the written consent of the requisite number of its stockholders necessary to adopt the Merger Agreement thereby approving the merger and
related matters within two business days of the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, being declared effective by the U.S. Securities and Exchange Commission; |
| |
• |
|
the Merger Agreement is terminated by Celladon at any time prior to the adoption of the Merger Agreement, and approval of the merger and related matters by the stockholders of Eiger because of an Eiger triggering event,
as defined above in the section entitled “The Merger Agreement—Termination”; |
| |
• |
|
the Merger Agreement is terminated by Celladon because Eiger has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of Eiger
has become inaccurate, in either case such that the conditions to the closing of the merger would not be satisfied as of time of such breach or inaccuracy, but if such breach or inaccuracy is curable, then the Merger Agreement will not terminate
pursuant to this provision as a result of a particular breach or inaccuracy until the earlier of the expiration of a 30-day period after delivery of written notice of such breach or inaccuracy and the breaching party ceasing to exercise commercially
reasonable efforts to cure such breach, if such breach has not been cured; |
| |
• |
|
the Merger Agreement is terminated by Celladon because all other closing conditions under the Merger Agreement have been satisfied and/or waived by
Celladon, except for the financing contemplated by |
135
| |
the Subscription Agreement, and Celladon has provided written notice to Eiger that it is prepared to consummate the closing of the merger upon the consummation of such financing and Eiger has not
obtained such financing within five days of receipt of such notice; or |
| |
• |
|
in the event of a failure by Celladon to consummate the transactions described in the Merger Agreement solely because there is an Eiger material adverse effect, as defined above in the section entitled “The Merger
Agreement—Termination.” |
If Celladon is entitled to reimbursement for expenses and the $3.0 million termination fee, Eiger’s
liability is capped at $3.0 million and in no event will Eiger be required to pay Celladon any amount in excess of $3.0 million in the event of termination of the Merger Agreement.
Amendment
The Merger Agreement
may be amended by the parties at any time, except that after the Merger Agreement has been adopted and approved by the stockholders of Celladon or Eiger, no amendment which by law requires further approval by the stockholders of Celladon or Eiger,
as the case may be, shall be made without such further approval.
136
AGREEMENTS RELATED TO THE MERGER
Subscription Agreement
On
November 18, 2015, prior to the execution of the Merger Agreement, Eiger entered into the Subscription Agreement with certain current stockholders of Eiger and certain new investors in Eiger pursuant to which Eiger agreed to sell, and the
purchasers listed therein agreed to purchase in the financing, an aggregate of 26,329,818 shares of Eiger common stock at a purchase price of $1.5002 per share prior to the closing of the merger for an aggregate purchase price of $39.5 million,
including the conversion of $6.0 million in outstanding convertible debt and warrants pursuant to the bridge loan discussed below. The merger is conditioned upon the closing of the financing contemplated by the Subscription Agreement.
The Subscription Agreement contains representations and warranties of Eiger comparable to the representations and warranties of Eiger in the Merger Agreement.
The Subscription Agreement also contains customary representations and warranties of the purchasers.
Each purchaser’s obligation to purchase shares
of Eiger common stock from Eiger pursuant to the Subscription Agreement is subject to the satisfaction or waiver of certain conditions, including:
| |
• |
|
Eiger’s representations and warranties in the Subscription Agreement being true and correct in all respects as of November 18, 2015, and as of the closing date for the financing, subject to certain exceptions;
|
| |
• |
|
Eiger having performed, satisfied and complied in all material respects with all covenants, agreements and conditions required to be performed, satisfied or complied with by it; |
| |
• |
|
the absence of any statute, rule, regulation, executive order, decree, ruling or injunction that prohibits the consummation of the sale of the shares; |
| |
• |
|
as notified by Eiger, each of the conditions to the consummation of the merger set forth in the Merger Agreement having been satisfied or waived and the parties to the Merger Agreement being ready, willing and able to
consummate the merger immediately after the closing of the financing on the terms and conditions set forth therein (provided that no condition may be waived by Eiger without the prior written consent of the purchasers holding or having the right to
acquire 75% of the shares to be purchased at the closing unless such waiver of condition, in the reasonable and good faith determination of such purchasers would not reasonably be expected to be adverse to the interests of any purchaser or that
would not reasonably be expected to have a materially adverse effect on the value of such purchaser’s investment in the shares, in which event, such prior written consent of such purchasers will not be required for such waiver of condition);
and |
| |
• |
|
the SEC having declared effective the registration statement of which this proxy statement/prospectus/information statement is a part and no stop order suspending the effectiveness of the registration statement of which
this proxy statement/prospectus/information statement is a part having been issued and remain pending. |
Eiger’s obligation to sell
shares of Eiger common stock to each purchaser pursuant to the Subscription Agreement is subject to the satisfaction or waiver of certain conditions, including:
| |
• |
|
the representations and warranties made by such purchaser being true and correct in all material respects as of the date when made or specified, and as of the closing date, subject to certain exceptions;
|
| |
• |
|
such purchaser having performed, satisfied and complied in all material respects with all covenants, agreements and conditions required to be performed, satisfied or complied with by such purchaser; |
| |
• |
|
the absence of any statute, rule, regulation, executive order, decree, ruling or injunction that prohibits the consummation of the sale of the shares; |
137
| |
• |
|
such purchaser’s delivery to Eiger of certain items at or prior to the closing; |
| |
• |
|
as notified by Eiger, each of the conditions to the consummation of the merger set forth in the Merger Agreement having been satisfied or waived and the parties to the Merger Agreement being ready, willing and able to
consummate the merger immediately after the closing of the financing on the terms and conditions set forth therein; and |
| |
• |
|
the actual subscription amount for each investor under the Subscription Agreement, either in cash or conversion of outstanding Bridge Notes, as applicable, has been released to Eiger in accordance with the Subscription
Agreement. |
The representations and warranties contained in the Subscription Agreement will terminate at the closing of the financing and
only the agreements and covenants that by their terms survive the closing of the financing will survive.
The Subscription Agreement may be amended and
its provisions waived by Eiger, the purchasers holding or having the right to acquire 75% of the shares to be purchased at the closing, or then outstanding, and Celladon, provided that in no event will Celladon unreasonably withhold, condition or
delay its consent to any such waiver or amendment.
At any time prior to the closing of the financing, the Subscription Agreement may be terminated by any
purchaser (with respect to itself only) by the mutual written consent of Eiger and the purchaser, provided however, that prior to the termination of the Merger Agreement, Eiger and any purchaser will not terminate such purchaser’s obligation
under the Subscription Agreement without the prior consent of Celladon which consent will not be unreasonably withheld, conditioned or delayed by Celladon. The Subscription Agreement may also be terminated by any purchaser (with respect to itself
only) if the closing of the financing has not been consummated on or prior to 5:00 p.m., New York City time, on March 31, 2016 (or, in certain instances, the date 60 days thereafter). In addition, Eiger or any purchaser (with respect to itself
only) may terminate the Subscription Agreement if the purchase and sale of the shares pursuant to the Subscription Agreement would violate any nonappealable order, degree or judgment of any governmental authority having competent jurisdiction.
Celladon is an express third-party beneficiary of the amendment and termination provisions of the Subscription Agreement and is entitled to specifically
enforce such provisions. In addition, upon the satisfaction or waiver of the closing conditions, Celladon will be an express third-party beneficiary of the Subscription Agreement and will be entitled to specifically enforce its terms, including the
obligations of the parties to sell and purchase the shares of Eiger common stock.
Bridge Loan
On November 12, 2015, certain of the purchasers under the Subscription Agreement agreed to loan Eiger $6.0 million in a bridge loan in order to provide
the financing required by Eiger to complete the merger. In connection with the bridge loan, Eiger issued unsecured convertible promissory notes and warrants exercisable for shares of Eiger’s equity securities, referred to here as the Bridge
Notes and Bridge Warrants.
The Bridge Notes accrue interest at a rate of 6.0% per year and have a maturity date of March 31, 2016. In the event
Eiger consummates the sale of its equity securities, either in the form of preferred stock or common stock, for aggregate proceeds of at least $25.0 million (excluding the conversion of the Bridge Notes) which is referred to as a Qualified
Financing, the outstanding principal and all accrued interest under the Bridge Notes will automatically convert into the class of equity securities issued in such qualified financing at the price per share of such equity securities sold in such
qualified financing. In the event Eiger consummates a change of control (as defined in the Bridge Notes) while the Bridge Notes are outstanding, each holder of a Bridge Note will be repaid an amount equal to one hundred and twenty percent
(120.0%) of the outstanding principal and accrued interest
138
under the Bridge Notes concurrent with the closing of such change of control. Eiger may not prepay the Bridge Notes and no term of the Bridge Notes may be amended or waived without the consent of
Eiger and the holders of 66.67% of the outstanding principal amount of the Bridge Notes.
In connection with the bridge loan, Eiger also issued Bridge
Warrants exercisable for shares of the equity securities issued by Eiger in any qualified financing as described in the Bridge Notes, or if no qualified financing is consummated, then into shares of Eiger’s common stock. The exercise price of
the equity securities issuable under the Bridge Warrants is $0.01 per share. The number of shares of equity securities exercisable pursuant to the Bridge Warrants equals fifteen percent (15.0%) of the principal amount of such holder’s
Bridge Note divided by (a) the price per share of the equity securities sold by Eiger in any qualified financing (as defined in the Bridge Notes) or, (b) if no qualified financing is consummated, the price per shares of equity securities
sold by Eiger in its next bona fide equity financing with aggregate proceeds to Eiger of at least $1.0 million, provided, however, if no qualified financing, or next financing has occurred by January 1, 2017, then the per share price Eiger last
sold shares of its Series A-1 Preferred Stock. The Bridge Warrants have a term of five (5) years from the date of issuance and terminate unless otherwise exercised immediately prior to the consummation of any change of control (as defined in
the Bridge Notes), provided, however, that in the event Eiger consummates any a merger or share exchange with a third party (referred to as a public merger party) then currently subject to the reporting requirements under the Exchange Act, where
shares of the outstanding stock of Eiger is exchanged for common stock of the public merger party, (a “public merger”), then the Bridge Warrants will be deemed to be automatically exercised immediately prior to the consummation of such
public merger and the aggregate exercise price under such Bridge Warrant will be paid, first by cancellation of interest under the Bridge Notes and, to the extent not fully exercised, any remainder will be exercised pursuant to the Bridge Warrants
net exercise provision. Any term of the Bridge Warrants may be amended or waived by the agreement of Eiger and the holders of 66.67% of the principal amount subscribed for under the Bridge Notes.
In the event Eiger consummates the transactions contemplated by the Subscription Agreement in connection with the closing of the merger, the outstanding
principal amount and all accrued interest (other than the portion of the interest used to automatically exercise the Bridge Warrants) under the Bridge Notes will convert into shares of Eiger’s common stock at a price of $1.5002 per share and
the Bridge Warrants will be automatically exercised for shares of Eiger’s common stock in exchange for cancellation of interest under the Bridge Notes equal to the aggregate exercise price for the share of common stock issuable under the Bridge
Warrants. To the extent any holder of a Bridge Note is also a party to the Subscription Agreement, the total subscription amount for such purchasers will be reduced by the aggregate amount of such purchaser’s outstanding principal and accrued
interest (to the extent such interest is not used for the automatic exercise of the Bridge Warrants) under such purchaser’s Bridge Note at the time of the closing of the financing contemplated by the Subscription Agreement.
Support Agreements and Written Consent
In order to induce Celladon to enter into the Merger Agreement, the Eiger directors, officers and certain affiliated stockholders are parties to support
agreements with Celladon pursuant to which, among other things, each of these stockholders agreed, solely in its capacity as a stockholder, to vote all of its shares of Eiger capital stock in favor of the adoption of the Merger Agreement, the
approval of any proposal to adjourn or postpone the meeting to a later date, if there are not sufficient votes for the adoption of the Merger Agreement on the date on which such meeting is held, and any other matter necessary to consummate the
transactions contemplated by the Merger Agreement that are considered and voted upon by Eiger’s stockholders, and against any “Acquisition Proposal,” as defined in the Merger Agreement. These Eiger stockholders also granted Celladon
an irrevocable proxy to their respective Eiger capital stock in accordance with the support agreements. These Eiger stockholders may vote their shares of Eiger capital stock on all other matters not referred to in such proxy.
The parties to the support agreements with Celladon are: Eiger Group International, Inc.; InterWest Partners X L.P.; Vivo Ventures Fund VI, L.P.; Vivo
Ventures VI Affiliates Fund, L.P.; and the officers and directors of Eiger.
139
Without giving effect to the shares of common stock issuable under the Subscription Agreement or upon the
conversion and exercise of the Bridge Notes and Bridge Warrants, the stockholders of Eiger that are parties to support agreements with Celladon owned an aggregate of 2,461,505 shares of Eiger common stock and 26,754,308 shares of Eiger preferred
stock, representing approximately 88.7% of the outstanding shares of Eiger capital stock on an as converted to common stock basis, and 89.8% of the outstanding shares of Eiger’s preferred stock, in each case as of December 31, 2015. Following
the effectiveness of the registration statement of which this proxy statement/prospectus/information statement is a part and pursuant to the Merger Agreement, stockholders of Eiger holding a sufficient number of shares to adopt the Merger Agreement
and approve the merger and related transaction will execute written consents providing for such adoption and approval. Therefore, holders of the number of shares of Eiger stock required to adopt the Merger Agreement and approve the merger and
related transactions are contractually obligated to adopt the Merger Agreement and are expected to adopt the Merger Agreement via written consent.
Under these support agreements, subject to certain exceptions, such stockholders also have agreed not to sell or transfer Eiger capital stock and securities
held by them, or any voting rights with respect thereto, until the earlier of the termination of the Merger Agreement or the completion of the merger. To the extent that any such sale or transfer is permitted pursuant to the exceptions included in
the support agreement, each person to whom any shares of Eiger capital stock or securities are so sold or transferred must agree in writing to be bound by the terms and provisions of the support agreement.
In addition, in order to induce Eiger to enter into the Merger Agreement, the Celladon executive officers and directors are parties to support agreements with
Eiger pursuant to which, among other things, each of these persons agreed, solely in his or her capacity as a stockholder, to vote all of his or her shares of Celladon common stock, if any, in favor of the merger and the issuance of Celladon common
stock in the merger pursuant to the Merger Agreement, the adoption of the Merger Agreement if submitted for adoption, the approval of any proposal to adjourn or postpone the meeting to a later date, if there are not sufficient votes for the merger
and the issuance of Celladon common stock in the merger pursuant to the Merger Agreement on the date on which such meeting is held, and any other matter necessary to consummate the transactions contemplated by the Merger Agreement that are
considered and voted upon by Celladon’s stockholders and against any “Acquisition Proposal,” as defined in the Merger Agreement. These Celladon stockholders also granted Eiger an irrevocable proxy to their respective shares in
accordance with these supporting agreements. These Celladon stockholders may vote their shares of Celladon common stock on all other matters not referred to in such proxy.
As of December 31, 2015, the directors and executive officers of Celladon that are parties to these support agreements owned an aggregate of 82,500 shares of
Celladon common stock representing 0.3% of the outstanding Celladon common stock.
Under these support agreements, subject to certain exceptions,
such stockholders also have agreed not to sell or transfer Celladon common stock and securities held by them until the earlier of the termination of the Merger Agreement or the completion of the merger. To the extent that any such sale or transfer
is permitted pursuant to the exceptions included in the support agreement, each person to whom any shares of Celladon common stock or securities are so sold or transferred must agree in writing to be bound by the terms and provisions of the support
agreement.
140
MATTERS BEING SUBMITTED TO A VOTE OF CELLADON STOCKHOLDERS
Celladon Proposal No. 1: Approval of the Merger and the Issuance of Common Stock in the Merger
At the Celladon special meeting, Celladon stockholders will be asked to approve the merger and the issuance of Celladon common stock pursuant to the Merger
Agreement. Immediately following the merger, it is expected that Eiger stockholders, warrantholders and optionholders will own approximately 78% of the fully-diluted common stock of Celladon, with existing Celladon stockholders and optionholders
holding approximately 22% of the fully-diluted common stock of Celladon.
The terms of, reasons for and other aspects of the Merger Agreement, the merger
and the issuance of Celladon common stock pursuant to the Merger Agreement are described in detail in the other sections in this proxy statement/prospectus/information statement.
Required Vote; Recommendation of Board of Directors
Presuming a quorum is present, the affirmative vote of the holders of a majority of the shares of Celladon common stock having voting power present in person
or represented by proxy at the Celladon special meeting is required for approval of Celladon Proposal No. 1. Each of Proposal Nos. 1, 2 and 3 are conditioned upon each other. Therefore, the merger cannot be consummated without the approval
of Proposal Nos. 1, 2 and 3.
THE CELLADON BOARD OF DIRECTORS RECOMMENDS THAT THE CELLADON STOCKHOLDERS VOTE “FOR” CELLADON PROPOSAL NO.
1 TO APPROVE THE MERGER AND THE ISSUANCE OF CELLADON COMMON STOCK PURSUANT TO THE MERGER AGREEMENT.
141
Celladon Proposal No. 2: Approval of the Amendment to the Amended and Restated
Certificate of Incorporation of Celladon Effecting the 1-for-15 Reverse Stock Split
General
At the Celladon special meeting, Celladon stockholders will be asked to approve the amendment to the amended and restated certificate of incorporation of
Celladon effecting a reverse stock split of the issued shares of Celladon common stock, at a ratio of 1-for-15. Upon the effectiveness of the amendment to the amended and restated certificate of incorporation of Celladon effecting the reverse stock
split, or the split effective time, the issued shares of Celladon common stock immediately prior to the split effective time will be reclassified into a smaller number of shares such that a Celladon stockholder will own one new share of Celladon
common stock for each fifteen shares of issued common stock held by that stockholder immediately prior to the split effective time.
If Celladon Proposal
No. 2 is approved, the reverse stock split would become effective in connection with the closing of the merger.
The Celladon board of directors may
determine to effect the reverse stock split, if it is approved by the stockholders, even if the other proposals to be acted upon at the meeting are not approved, including the merger and the issuance of shares of Celladon common stock pursuant to
the Merger Agreement.
The form of the amendment to the amended and restated certificate of incorporation of Celladon to effect the reverse stock split,
as more fully described below, will effect the reverse stock split but will not change the number of authorized shares of common stock or preferred stock, or the par value of Celladon common stock or preferred stock.
Purpose
The Celladon board of directors approved
the proposal approving the amendment to the amended and restated certificate of incorporation of Celladon effecting the reverse stock split for the following reasons:
| |
• |
|
the board of directors believes effecting the reverse stock split may be an effective means of maintaining the listing of the combined company’s post-merger common stock and avoiding a delisting of Celladon common
stock from The NASDAQ Global Market in the future; and |
| |
• |
|
the board of directors believes a higher stock price may help generate investor interest in Celladon and help Celladon attract and retain employees. |
If the reverse stock split successfully increases the per share price of Celladon common stock, the Celladon board of directors believes this increase may
increase trading volume in Celladon common stock and facilitate future financings by Celladon.
NASDAQ Requirements for Listing on The NASDAQ Global
Market
Celladon common stock is listed on The NASDAQ Global Market under the symbol “CLDN.” Celladon intends to file an initial listing
application under the reverse merger rules with NASDAQ to seek listing on The NASDAQ Global Market upon the closing of the merger.
According to NASDAQ
rules, an issuer must, in a case such as this, apply for initial inclusion following a transaction whereby the issuer combines with a non-NASDAQ entity, resulting in a change of control of the issuer and potentially allowing the non-NASDAQ entity to
obtain a NASDAQ listing. Accordingly, the listing standards of The NASDAQ Global Market will require Celladon to have, among other things, a $4.00 per share minimum bid price upon the closing of the merger. Therefore, the reverse stock split may be
necessary in order to consummate the merger.
142
One of the effects of the reverse stock split will be to effectively increase the proportion of authorized shares
which are unissued relative to those which are issued. This could result in Celladon’s management being able to issue more shares without further stockholder approval. For example, before the reverse stock split, Celladon’s authorized but
unissued shares immediately prior to the closing of the merger would be approximately 176.1 million compared to shares issued of approximately 23.9 million. If Celladon effects the reverse stock split using a 1-for-15 ratio, its authorized but
unissued shares immediately prior to the closing of the merger would be approximately 198.4 million compared to shares issued of approximately 1.6 million. The reverse stock split will not affect the number of authorized shares of Celladon
common stock and preferred stock which will continue to be authorized pursuant to the certificate of incorporation of Celladon, thus the reverse stock split will have the effect of increasing the number of authorized but unissued shares of
Celladon’s common stock. There are no shares of Celladon preferred stock currently outstanding. Celladon currently has no plans, commitments, arrangements, understandings or agreements to issue shares, other than in connection with the merger,
and to satisfy obligations under the Celladon warrants and employee stock options from time to time as these warrants and options are exercised. The additional authorized shares of common stock will provide the combined company with the flexibility
to consider and respond to future business opportunities and needs as they arise, including but not limited to, equity offerings; financings; potential strategic transactions, including mergers, acquisitions and business combinations; stock
dividends; stock splits; grants under equity compensation plans; and other general corporate transactions.
Potential Increased Investor Interest
On , 2016, Celladon
common stock closed at $ per share. An investment in Celladon common stock may not appeal to brokerage firms that are reluctant to recommend lower priced securities to their clients. Investors may also
be dissuaded from purchasing lower priced stocks because the brokerage commissions, as a percentage of the total transaction, tend to be higher for such stocks. Moreover, the analysts at many brokerage firms do not monitor the trading activity or
otherwise provide coverage of lower priced stocks. Also, the Celladon board of directors believes that most investment funds are reluctant to invest in lower priced stocks.
There are risks associated with the reverse stock split, including that the reverse stock split may not result in an increase in the per share price of
Celladon common stock.
Celladon cannot predict whether the reverse stock split will increase the market price for Celladon common stock. The history of
similar stock split combinations for companies in like circumstances is varied. There is no assurance that:
| |
• |
|
the market price per share of Celladon common stock after the reverse stock split will rise in proportion to the reduction in the number of shares of Celladon common stock outstanding before the reverse stock split;
|
| |
• |
|
the reverse stock split will result in a per share price that will attract brokers and investors who do not trade in lower priced stocks; |
| |
• |
|
the reverse stock split will result in a per share price that will increase the ability of Celladon to attract and retain employees; or |
| |
• |
|
the market price per share will either exceed or remain in excess of the $1.00 minimum bid price as required by NASDAQ Stock Market LLC for continued listing, or that Celladon will otherwise meet the requirements of
NASDAQ Stock Market LLC for inclusion for trading on The NASDAQ Global Market, including the $4.00 minimum bid price upon the closing of the merger. |
The market price of Celladon common stock will also be based on performance of Celladon and other factors, some of which are unrelated to the number of shares
outstanding. If the reverse stock split is effected and the market price of Celladon common stock declines, the percentage decline as an absolute number and as a
143
percentage of the overall market capitalization of Celladon may be greater than would occur in the absence of a reverse stock split. Furthermore, the liquidity of Celladon common stock could be
adversely affected by the reduced number of shares that would be outstanding after the reverse stock split.
Principal Effects of the Reverse Stock
Split
The amendment to the amended and restated certificate of incorporation of Celladon effecting the reverse stock split is set forth in
Annex D to this proxy statement/prospectus/information statement.
The reverse stock split will be effected simultaneously for all outstanding
shares of Celladon common stock. The reverse stock split will affect all of the Celladon stockholders uniformly and will not affect any stockholder’s percentage ownership interests in Celladon, except to the extent that the reverse stock split
results in any of the Celladon stockholders owning a fractional share. Common stock issued pursuant to the reverse stock split will remain fully paid and nonassessable. The reverse split does not affect the total proportionate ownership of Celladon
following the merger. The reverse stock split will not affect Celladon continuing to be subject to the periodic reporting requirements of the Exchange Act.
Procedure for Effecting Reverse Stock Split and Exchange of Stock Certificates
If the Celladon stockholders approve the amendment to the amended and restated certificate of incorporation of Celladon effecting the reverse stock split, and
if the Celladon board of directors still believes that a reverse stock split is in the best interests of Celladon and its stockholders, Celladon will file the amendment to the amended and restated certificate of incorporation with the Secretary of
State of the State of Delaware at such time as the Celladon board of directors has determined to be the appropriate split effective time. The Celladon board of directors may delay effecting the reverse stock split without resoliciting stockholder
approval. Beginning at the split effective time, each certificate representing pre-split shares will be deemed for all corporate purposes to evidence ownership of post-split shares.
As soon as practicable after the split effective time, stockholders will be notified that the reverse stock split and/or corporate name change have been
effected. Celladon expects that the Celladon transfer agent will act as exchange agent for purposes of implementing the exchange of stock certificates. Holders of pre-split shares will be asked to surrender to the exchange agent certificates
representing pre-split shares in exchange for certificates representing post-split shares in accordance with the procedures to be set forth in a letter of transmittal to be sent by Celladon. In the event that Celladon Proposal No. 3 is approved
by Celladon, the certificates reflecting the post-split shares will also reflect the change of the Celladon corporate name to “Eiger BioPharmaceuticals, Inc.” No new certificates will be issued to a stockholder until such stockholder has
surrendered such stockholder’s outstanding certificate(s) together with the properly completed and executed letter of transmittal to the exchange agent. Any pre-split shares submitted for transfer, whether pursuant to a sale or other
disposition, or otherwise, will automatically be exchanged for post-split shares. Stockholders should not destroy any stock certificate(s) and should not submit any certificate(s) unless and until requested to do so.
Fractional Shares
No fractional shares will be
issued in connection with the reverse stock split. Stockholders of record who otherwise would be entitled to receive fractional shares because they hold a number of pre-split shares not evenly divisible by the number of pre-split shares for which
each post-split share is to be reclassified, will be entitled, upon surrender to the exchange agent of certificates representing such shares, to a cash payment in lieu thereof at a price equal to the fraction to which the stockholder would otherwise
be entitled multiplied by the closing price of the common stock on The NASDAQ Global Market on the first trading day immediately following the split effective time. The ownership of a fractional interest will not give the holder thereof any voting,
dividend, or other rights except to receive payment therefor as described herein.
144
By approving the amendment to the amended and restated certificate of incorporation of Celladon effecting the
reverse stock split, stockholders will be approving the combination of fifteen shares of Celladon common stock into one share of Celladon common stock.
Stockholders should be aware that, under the escheat laws of the various jurisdictions where stockholders reside, where Celladon is domiciled, and where the
funds will be deposited, sums due for fractional interests that are not timely claimed after the effective date of the split may be required to be paid to the designated agent for each such jurisdiction, unless correspondence has been received by
Celladon or the exchange agent concerning ownership of such funds within the time permitted in such jurisdiction. Thereafter, stockholders otherwise entitled to receive such funds will have to seek to obtain them directly from the state to which
they were paid.
Potential Anti-Takeover Effect
Although the increased proportion of unissued authorized shares to issued shares could, under certain circumstances, have an anti-takeover effect, for example,
by permitting issuances that would dilute the stock ownership of a person seeking to effect a change in the composition of the Celladon board of directors or contemplating a tender offer or other transaction for the combination of Celladon with
another company, the reverse stock split proposal is not being proposed in response to any effort of which Celladon is aware to accumulate shares of Celladon common stock or obtain control of Celladon, other than in connection with the merger, nor
is it part of a plan by management to recommend a series of similar amendments to the Celladon board of directors and stockholders. Other than the proposals being submitted to the Celladon stockholders for their consideration at the Celladon special
meeting, the Celladon board of directors does not currently contemplate recommending the adoption of any other actions that could be construed to affect the ability of third parties to take over or change control of Celladon. For more information,
please see the section entitled “Risk Factors—Risks Related to Ownership of Celladon’s Common Stock,” and “Description of Celladon Capital Stock—Anti-Takeover Effects of Provisions of Celladon Charter Documents and
Delaware Law.”
Material U.S. Federal Income Tax Consequences of the Reverse Stock Split
The following is a discussion of the material U.S. federal income tax consequences of the reverse stock split to holders of Celladon common stock, but does not
purport to be a complete analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local, or foreign tax laws are not discussed. This discussion is based on the
Code, U.S. Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the IRS in effect as of the date of the merger. These authorities may change or be subject to differing
interpretations. Any such change may be applied retroactively in a manner that could adversely affect a holder of Celladon common stock.
This discussion
is limited to holders who hold their Celladon common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax
consequences relevant to the particular circumstances of a Celladon common stockholder, including the impact of the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to holders of Celladon
common stock that are subject to particular rules, including, without limitation:
| |
• |
|
persons subject to the alternative minimum tax; |
| |
• |
|
persons whose functional currency is not the U.S. dollar; |
| |
• |
|
persons holding Celladon common stock as part of a hedge, straddle, or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| |
• |
|
persons who are not U.S. Holders; |
| |
• |
|
banks, insurance companies, and other financial institutions; |
145
| |
• |
|
real estate investment trusts or regulated investment companies; |
| |
• |
|
brokers, dealers, or traders in securities; |
| |
• |
|
partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| |
• |
|
tax-exempt organizations or governmental organizations; |
| |
• |
|
persons deemed to sell Celladon common stock under the constructive sale provisions of the Code; |
| |
• |
|
persons who hold or receive Celladon common stock pursuant to the exercise of any employee stock options or otherwise as compensation; and |
| |
• |
|
tax-qualified retirement plans. |
This discussion is limited to holders of Celladon common stock that are U.S.
Holders. For purposes of this discussion, a “U.S. Holder” is a beneficial owner of Celladon common stock that, for U.S. federal income tax purposes, is or is treated as:
| |
• |
|
an individual who is a citizen or resident of the United States; |
| |
• |
|
a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| |
• |
|
an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| |
• |
|
a trust if either a court within the United States is able to exercise primary supervision over the administration of such trust and one or more United States persons (within the meaning of Section 7701(a)(30) of
the Code) have the authority to control all substantial decisions of such trust, or the trust has a valid election in effect under applicable Treasury Regulations to be treated as a United States person for U.S. federal income tax purposes.
|
If an entity treated as a partnership for U.S. federal income tax purposes holds Celladon common stock, the tax treatment of a partner in
the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding Celladon common stock and the partners in such partnerships should
consult their tax advisors regarding the U.S. federal income tax consequences to them.
In addition, the following discussion does not address the tax
consequences of the reverse stock split under state, local and foreign tax laws. Furthermore, the following discussion does not address any tax consequences of transactions effectuated before, after or at the same time as the reverse stock split,
whether or not they are in connection with the reverse stock split.
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE
U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE REVERSE STOCK SPLIT ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR
UNDER ANY APPLICABLE INCOME TAX TREATY.
Tax Consequences of the Reverse Stock Split
The reverse stock split should constitute a “recapitalization” for U.S. federal income tax purposes. As a result, a U.S. Holder of Celladon common
stock generally should not recognize gain or loss upon the reverse stock split, except with respect to cash received in lieu of a fractional share of Celladon common stock, as discussed below. A U.S. Holder’s aggregate tax basis in the shares
of Celladon common stock received pursuant to the reverse stock split should equal the aggregate tax basis of the shares of the Celladon common stock surrendered
146
(excluding any portion of such basis that is allocated to any fractional share of Celladon common stock), and such U.S. Holder’s holding period in the shares of Celladon common stock
received should include the holding period in the shares of Celladon common stock surrendered. Treasury Regulations provide detailed rules for allocating the tax basis and holding period of the shares of Celladon common stock surrendered to the
shares of Celladon common stock received in a recapitalization pursuant to the reverse stock split. U.S. Holders of shares of Celladon common stock acquired on different dates and at different prices should consult their tax advisors regarding the
allocation of the tax basis and holding period of such shares.
Cash in Lieu of Fractional Shares
A U.S. Holder of Celladon common stock that receives cash in lieu of a fractional share of Celladon common stock pursuant to the reverse stock split should
recognize capital gain or loss in an amount equal to the difference between the amount of cash received and the U.S. Holder’s tax basis in the shares of Celladon common stock surrendered that is allocated to such fractional share of Celladon
common stock. Such capital gain or loss should be long-term capital gain or loss if the U.S. Holder’s holding period for Celladon common stock surrendered exceeded one year at the effective time of the reverse stock split.
Information Reporting and Backup Withholding
A U.S.
Holder of Celladon common stock may be subject to information reporting and backup withholding on cash paid in lieu of fractional shares in connection with the reverse stock split. A U.S. Holder of Celladon common stock will be subject to backup
withholding if such holder is not otherwise exempt and such holder does not provide its taxpayer identification number in the manner required or otherwise fails to comply with applicable backup withholding tax rules.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be refunded or allowed as a credit against a U.S.
Holder of Celladon common stock’s federal income tax liability, if any, provided the required information is timely furnished to the IRS. U.S. Holders of Celladon common stock should consult their tax advisors regarding their qualification for
an exemption from backup withholding and the procedures for obtaining such an exemption.
Required Vote; Recommendation of Board of Directors
The affirmative vote of holders of a majority of the shares of Celladon common stock having voting power outstanding on the record date for the
Celladon special meeting is required to approve the amendment to the amended and restated certificate of incorporation of Celladon effecting a 1-for-15 reverse stock split of Celladon common stock. Each of Proposal Nos. 1, 2 and 3 are conditioned
upon each other. Therefore, the merger cannot be consummated without the approval of Proposal Nos. 1, 2 and 3.
THE CELLADON BOARD OF DIRECTORS
RECOMMENDS THAT CELLADON STOCKHOLDERS VOTE “FOR” CELLADON PROPOSAL NO. 2 TO APPROVE THE AMENDMENT TO THE AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF CELLADON EFFECTING THE 1-FOR-15 REVERSE STOCK SPLIT.
147
Celladon Proposal No. 3: Approval of Name Change
At the Celladon special meeting, holders of Celladon stock will be asked to approve the amendment to the amended and restated certificate of incorporation of
Celladon to change the name of the corporation from “Celladon Corporation” to “Eiger BioPharmaceuticals, Inc.” by filing the amendment to the amended and restated certificate of incorporation at the effective time of the merger.
The primary reason for the corporate name change is that management believes this will allow for brand recognition of Eiger product candidates and product candidate pipeline following the consummation of the merger. Celladon management believes that
the current name will no longer accurately reflect the business of Celladon and the mission of Celladon subsequent to the consummation of the merger.
Required Vote; Recommendation of Board of Directors
The affirmative vote of holders of a majority of the shares of Celladon common stock having voting power outstanding on the record date for the Celladon
special meeting is required to approve the amendment to the amended and restated certificate of incorporation to change the name “Celladon Corporation” to “Eiger BioPharmaceuticals, Inc.” Each of Proposal Nos. 1, 2 and 3 are
conditioned upon each other. Therefore, the merger cannot be consummated without the approval of Proposal Nos. 1, 2 and 3.
THE CELLADON BOARD OF
DIRECTORS RECOMMENDS THAT CELLADON STOCKHOLDERS VOTE “FOR” CELLADON PROPOSAL NO. 3 TO APPROVE THE NAME CHANGE.
148
Celladon Proposal No. 4: Approval of Possible Adjournment of the Celladon
Special Meeting
If Celladon fails to receive a sufficient number of votes to approve Celladon Proposal Nos. 1, 2, and 3, Celladon may propose to
adjourn the Celladon special meeting, for a period of not more than 30 days, for the purpose of soliciting additional proxies to approve Celladon Proposal Nos. 1, 2 and 3. Celladon currently does not intend to propose adjournment at the Celladon
special meeting if there are sufficient votes to approve Celladon Proposal Nos. 1, 2, and 3.
Required Vote; Recommendation of Board of Directors
The affirmative vote of the holders of a majority of the shares of Celladon common stock having voting power present in person or represented
by proxy at the Celladon special meeting is required to approve the adjournment, if necessary, of the Celladon special meeting for the purpose of soliciting additional proxies to approve Celladon Proposal Nos. 1, 2, and 3. Each of Proposal Nos.
1, 2 and 3 are conditioned upon each other. Therefore, the merger cannot be consummated without the approval of Proposal Nos. 1, 2 and 3.
THE
CELLADON BOARD OF DIRECTORS RECOMMENDS THAT THE CELLADON STOCKHOLDERS VOTE “FOR” CELLADON PROPOSAL NO. 4 TO ADJOURN THE SPECIAL MEETING, IF NECESSARY, TO SOLICIT ADDITIONAL PROXIES IF THERE ARE NOT SUFFICIENT VOTES IN FAVOR OF CELLADON
PROPOSAL NOS. 1, 2 AND 3.
149
CELLADON BUSINESS
Overview
Celladon is a biotechnology company that has
been focused on the development of cardiovascular gene therapy. Celladon’s lead product candidate, MYDICAR® (AAV1/SERCA2a) has been under investigation as a treatment for patients with
heart failure with reduced ejection fraction, or HFrEF, also referred to as systolic heart failure. Historically, Celladon has devoted substantially all of its research, development and clinical efforts and financial resources toward the development
of MYDICAR.
Celladon evaluated MYDICAR in a 250-patient randomized, double-blind, placebo-controlled multinational Phase 2b trial in patients with HFrEF
referred to as the CUPID 2 trial. Celladon un-blinded the results from the active observation period in April 2015 and announced that the CUPID 2 trial did not meet its primary and secondary endpoints. In light of the negative CUPID 2 results,
Celladon’s board of directors suspended further research and development activities for MYDICAR and Celladon’s pre-clinical programs and implemented cost-cutting measures including several reductions in workforce and the termination of
certain contracts related to MYDICAR and other programs. Celladon’s continuing development activities are currently limited to the oversight of the long-term follow up period in the CUPID 2 trial, which is expected to continue through February
2016.
Celladon’s suspended research and development activities are as follows:
| |
• |
|
MYDICAR-HFrEF—the majority of Celladon’s research and development resources were focused on Celladon’s CUPID 2 trial, commercialization and manufacturing preparations, clinical trials and
other work needed to submit MYDICAR for regulatory approval in the United States and Europe. |
| |
• |
|
Stem Cell Factor Program—Celladon’s research and development activities for this indication related primarily to the preclinical testing of the membrane-bound form of the Stem Cell Factor gene, or mSCF,
in myocardial infarction porcine models. |
| |
• |
|
Small Molecule Program—Celladon’s research and development expenses for its small molecule program related primarily to identification and pre-clinical testing of small molecule SERCA2 enzyme
modulators. |
Also as a consequence of the negative results from the CUPID 2 trial, Celladon’s board of directors began evaluating its
strategic opportunities to maximize stockholder value, including the possibility of seeking a merger, a sale of the company or all or some of its assets and/or distributing some or all of Celladon’s remaining cash through either a dividend or a
liquidation of Celladon.
In May 2015, Celladon engaged Wedbush, as its exclusive financial advisor in connection with a potential merger, reorganization
or other business combination transaction or potential alternatives thereto, including a liquidation and dissolution of Celladon. Wedbush was selected by Celladon due to its substantial experience with the healthcare industry and transactions
similar to this transaction. Working with Wedbush and Celladon’s legal advisors, Celladon conducted a process of identifying and evaluating potential strategic combinations, or other transactions, with biotechnology companies. On
November 18, 2015, Celladon, Merger Sub and Eiger entered into a Merger Agreement, pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and
into Eiger, with Eiger becoming a wholly-owned subsidiary of Celladon and the surviving corporation of the merger. If the merger is completed, the business of Celladon will become the business of Eiger as described in this proxy
statement/prospectus/information statement under the caption “Eiger Business.”
If the merger is not completed, Celladon will reconsider its
strategic alternatives and could pursue one of the following courses of action, which Celladon currently believes to be the most likely alternatives if the merger with Eiger is not completed:
| |
• |
|
Pursue another strategic transaction. Celladon may resume its process of evaluating a potential merger, reorganization or other business combination transaction. |
150
| |
• |
|
Dissolve and liquidate its assets. If Celladon does not believe it can find a suitable alternate merger partner, Celladon may dissolve and liquidate its assets. Celladon would be required to pay all of its
debts and contractual obligations, and to set aside certain reserves for potential future claims, and there can be no assurances as to the amount or timing of available cash remaining to distribute to stockholders after paying the Celladon
obligations and setting aside funds for reserves. |
Celladon cannot predict whether or to what extent it might resume drug development
activities, or what its future cash needs would be for any such activities.
Manufacturing
Following the negative results from the CUPID 2 trial, Celladon terminated its commercial manufacturing agreements and currently does not have any
manufacturing capabilities.
Competition
The
biotechnology and pharmaceutical industries in which Celladon operates are subject to rapid change and are characterized by intense competition to develop new technologies and proprietary products. As a biotechnology company, Celladon faces
potential competition from many different sources, including larger and better-funded pharmaceutical companies. Celladon’s potential competitors also include academic institutions, government agencies and research institutions.
Intellectual Property
Historically, Celladon has strived
to protect and enhance the proprietary technologies that it believes are important to Celladon’s business, and seek to obtain and maintain patents for any patentable aspects of Celladon’s products or product candidates, their methods of
use and any other inventions that are important to the development of Celladon’s business.
Celladon is the owner or licensee of a portfolio of
patents and patent applications and possesses know-how and trade secrets which protect various aspects of Celladon’s historical business. The patent families comprising Celladon’s patent portfolio are primarily focused on MYDICAR for the
treatment of heart failure and are generally directed to certain genes, AAV vectors and methods of delivering such AAV vectors to cells, methods of delivery to myocardial cells and processes to manufacture Celladon’s product candidates.
Following the negative CUPID 2 results Celladon has discontinued the prosecution of certain patents and patent applications in Celladon’s patent portfolio.
Trademarks
Celladon has registered the trademark
“MYDICAR” in the United States for use in connection with a biological product, namely, a gene transfer product composed of a recombinant AAV vector for medical use.
License Agreements
Sublicense Agreement and
Amended and Restated License Agreement with AmpliPhi
Sublicense Agreement
In June 2012, Celladon entered into a sublicense agreement, or the AmpliPhi Sublicense, with AmpliPhi Biosciences Corporation, or AmpliPhi, pursuant to which
AmpliPhi sublicensed to Celladon certain rights under a separate agreement, the UPenn Agreement, which AmpliPhi entered into in 2009 with the Trustees of the University of Pennsylvania, or UPenn. Under the terms of the agreement, Celladon obtained
an exclusive, worldwide sublicense from AmpliPhi under certain UPenn patents related to AAV1 vectors for the development, manufacture, use and sale of companion diagnostics to MYDICAR. Celladon has the right to grant sublicenses to
151
its affiliates and third-party collaborators under the agreement solely for research, development or other non-commercial purposes, or as reasonably necessary, to its manufacturers or
distributors, provided that Celladon remains primarily liable and such downstream sublicenses are consistent with the terms of Celladon’s agreement with AmpliPhi and prohibit further sublicensing. In addition, Celladon is required to use
commercially reasonable efforts to meet certain developmental, regulatory and commercial milestones with respect to companion diagnostics under the agreement. Following the decision to not pursue additional previously planned development activities
with MYDICAR and its companion diagnostic, Celladon may not currently be in compliance with these milestone requirements and may in the future choose to terminate the agreement. While Celladon has sole control over the development and
commercialization of companion diagnostics under the agreement, AmpliPhi has the first right to prosecute and maintain the licensed patents, subject to Celladon’s right to consult with AmpliPhi with regard to such prosecution and maintenance
upon Celladon’s reasonable request.
In consideration for the sublicense granted to Celladon under the agreement, Celladon paid to AmpliPhi a
sublicense initiation fee of $310,000, and Celladon is obligated to pay to AmpliPhi an annual sublicense maintenance fee of $310,000. Celladon is also required to pay to AmpliPhi a low single-digit percentage royalty based on net sales of any
companion diagnostic covered by a licensed patent sold by Celladon, Celladon’s affiliates or its sublicensees. Celladon’s royalty obligations continue on a companion diagnostic-by-companion diagnostic and country-by-country basis until the
expiration of the last-to-expire valid claim in a licensed patent covering the applicable companion diagnostic in such country. Finally, Celladon is obligated to pay to AmpliPhi all royalty and milestone payments that become due and payable by
AmpliPhi to UPenn under the UPenn Agreement as a result of Celladon’s exercise of the sublicense granted under Celladon’s agreement with AmpliPhi, including a low single-digit tiered percentage royalty on net sales of any companion
diagnostic sold by Celladon, Celladon’s affiliates or its sublicensees, which royalty is separate from and in addition to the royalty payable to AmpliPhi described above, and up to an aggregate of $850,000 in potential milestone payments per
product covered by the licensed patents.
Celladon may unilaterally terminate the agreement upon 30 days’ written notice to AmpliPhi. Absent early
termination, the agreement will automatically terminate upon the expiration of the last-to-expire licensed patent, which is expected to occur in 2019.
Amended and Restated License Agreement
Celladon entered
into an amended and restated license agreement with AmpliPhi concurrently with the AmpliPhi Sublicense that both amended the terms of the license agreement which Celladon entered into with AmpliPhi in 2009 and terminated Celladon’s
manufacturing agreement with AmpliPhi which Celladon entered into in 2009. Under the agreement, Celladon obtained an exclusive, worldwide license under certain patents and know-how related to AmpliPhi’s AAV vector and manufacturing technology
for the development, manufacture, use and sale of MYDICAR. Celladon has the right to grant sublicenses to Celladon’s affiliates and third-party collaborators under the agreement for research, development or other non-commercial purposes, or as
reasonably necessary, to Celladon’s manufacturers or distributors, provided that Celladon remains primarily liable and such sublicenses comply with the terms of Celladon’s agreement with AmpliPhi and prohibit further sublicensing. In
addition, Celladon has agreed to use commercially reasonable efforts to meet certain diligence milestones with respect to the development and commercialization of at least one product covered by the UPenn patent rights licensed to AmpliPhi by UPenn
under the UPenn Agreement. Following the decision to not pursue additional previously planned development activities with MYDICAR and its companion diagnostic, Celladon may not currently be in compliance with these milestone requirements. While
Celladon has sole control over development and commercialization of products covered by the licensed patents, AmpliPhi has the first right to prosecute and maintain the licensed patents, subject to Celladon’s right to consult with AmpliPhi with
regard to such prosecution and maintenance upon Celladon’s reasonable request.
During the term of the agreement, Celladon is obligated to pay to
AmpliPhi all royalty and milestone payments that become due and payable by AmpliPhi to UPenn under the UPenn Agreement as a result of Celladon’s exercise of the sublicense granted under its agreement with AmpliPhi. This includes a low
single-digit tiered
152
percentage royalty on net sales of MYDICAR and any other product covered by the licensed patents sold by Celladon, Celladon’s affiliates or its sublicensees, and up to $850,000 in milestone
payments upon the achievement of certain developmental and regulatory milestones related to MYDICAR and any other product covered by the licensed patents.
The agreement does not provide either party with termination rights and does not have a provision for expiration or automatic termination.
License Agreement with AdVec
In February 2009,
Celladon entered into a license agreement with AdVec, Inc., or AdVec, under which Celladon obtained a non-exclusive, worldwide license to use and acquire from AdVec’s distributor certain human embryo kidney cells transformed by Adenovirus 5
DNA, or 293 Cells, and certain AdVec know-how related to 293 Cells for use in testing of MYDICAR for lot release. In consideration for the rights granted to Celladon under the agreement, Celladon is obligated to pay to AdVec an annual license
maintenance fee of $5,000.
Either party may terminate the agreement upon written notice of the other party’s insolvency or bankruptcy or upon the
other party’s breach of the agreement if such breach remains uncured after 60 days of receipt of written notice of such breach. Absent early termination, the agreement will remain in effect until the tenth anniversary of the effective date.
Thereafter, the agreement will automatically renew for successive five-year terms unless either party notifies the other party in writing at least 90 days prior to the end of any such five-year term of its election not to renew the agreement.
Non-Exclusive License Agreement with AskBio
In
January 2008, Celladon entered into a non-exclusive license agreement with AskBio, LLC, or AskBio, a wholly owned subsidiary of Asklepios Biopharmaceutical Inc., under which Celladon obtained a non-exclusive, worldwide license under certain patents
related to recombinant AAV vectors to develop, manufacture, use and sell MYDICAR. Celladon has the right to grant sublicenses to third parties under the agreement provided that such sublicenses are entered into pursuant to a written sublicense
agreement containing terms consistent with Celladon’s agreement with AskBio.
In consideration for the rights granted to Celladon under the
agreement, Celladon paid to AskBio license fee payments of $150,000 in the aggregate. In addition, Celladon is obligated to pay to AskBio an annual maintenance fee of $100,000. Upon commercialization of any product utilizing the licensed patents,
Celladon will also be required to pay to AskBio a low single-digit percentage royalty on net sales of such products, including MYDICAR. Celladon’s royalty obligations continue on a product-by-product and country-by-country basis until the
expiration of the last-to-expire valid claim in a licensed patent covering the applicable product in such country, which is expected to be in 2021. Celladon is also obligated to reimburse AskBio for up to an aggregate of $355,000 per licensed
product upon the achievement of certain clinical, regulatory and sales milestones that may become due and payable by AskBio under a separate agreement between AskBio and the University of North Carolina at Chapel Hill from 2003.
Celladon may unilaterally terminate the agreement upon 180 days’ written notice to AskBio. Either party may terminate the agreement for the other
party’s material breach of the agreement if such breach is not cured after 30 days of receiving written notice of such breach. Absent early termination, the agreement will continue in effect until the expiration of Celladon’s royalty
payment obligations under the agreement.
Exclusive Patent License with Enterprise Management Partners
On July 18, 2014, Celladon and Enterprise Management Partners, LLC, or Enterprise, entered into an Assignment and License Agreement, pursuant to which
Enterprise granted to Celladon an exclusive, worldwide license and the assignment of patents held by Enterprise relating to certain gene therapy applications of mSCF for the treatment of cardiac ischemia. Celladon has the right to grant sublicenses
to third parties under the agreement.
153
In consideration for the rights granted to Celladon under the agreement, Celladon paid an upfront fee to
Enterprise of $160,000. Celladon is also obligated to pay to Enterprise a milestone payment in the amount of $1,000,000 upon the grant to Celladon, or an affiliate or sublicensee of Celladon’s, of the first regulatory approval in the United
States of a product that is covered by the licensed patents. In addition, Celladon is required to pay to Enterprise a 2% royalty on net sales of products sold by Celladon or by Celladon’s affiliates or sublicensees that are covered by the
licensed patents. Celladon’s royalty obligations continue on a product-by-product and country-by-country basis until the expiration of the last-to-expire valid claim in the licensed patents covering a licensed product in such country. Celladon
may unilaterally terminate the agreement upon written notice to Enterprise. Enterprise may terminate the agreement in the event of Celladon’s material breach of the agreement if such breach remains uncured for 90 days following receipt of
written notice of such breach. Absent early termination, the agreement will automatically terminate upon the expiration of the last-to-expire of the licensed patents containing a valid claim.
Government Regulation
Biological products, including
gene therapy products, are subject to regulation under the Federal Food, Drug, and Cosmetic Act, or FD&C Act, and the Public Health Service Act, or PHS Act, and other federal, state, local and foreign statutes and regulations. Both the FD&C
Act and the PHS Act and their corresponding regulations govern, among other things, the testing, manufacturing, safety, purity, potency, efficacy, labeling, packaging, storage, record keeping, distribution, reporting, advertising and other
promotional practices involving biological products. FDA approval must be obtained before clinical testing of a biological product begins, and each clinical trial protocol for a gene therapy product is reviewed by the FDA and, in some instances, the
U.S. National Institutes of Health, or NIH, through its Recombinant DNA Advisory Committee, or RAC. FDA approval also must be obtained before marketing of biological products. The process of obtaining regulatory approvals and the subsequent
compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. To date, the FDA has never approved a gene therapy product for commercial sale. Within the
FDA, the Center for Biologics Evaluation and Research, or CBER, regulates gene therapy products, CDRH regulates companion diagnostics, and the Office of Combination Products, or OCP, issues classification and jurisdiction assignments for medical
products. Specifically, OCP determines how combination products, such as biologic/medical device combination products, will be regulated and which FDA Center or Lead Center (e.g., CBER or CDRH) will regulate the product.
CBER works closely with the NIH and its RAC, which makes recommendations to the NIH on gene therapy issues and engages in a public discussion of scientific,
safety, ethical and societal issues related to proposed and ongoing gene therapy protocols. The FDA and the NIH have published guidance documents with respect to the development and submission of gene therapy protocols. The FDA also has published
guidance documents related to, among other things, gene therapy products in general, their preclinical assessment, observing subjects involved in gene therapy studies for delayed adverse events, potency testing and chemistry, manufacturing and
control information in gene therapy INDs.
Ethical, social and legal concerns about gene therapy, genetic testing and genetic research could result in
additional regulations. Federal and state agencies, congressional committees and foreign governments have expressed interest in further regulating biotechnology. New government requirements may be established that could delay or prevent regulatory
approval of product candidates under development. It is impossible to predict whether legislative changes will be enacted, regulations, policies or guidance changed, or interpretations by agencies or courts changed, or what the impact of such
changes, if any, may be.
Government Regulation Outside of the United States
In addition to regulations in the United States, biotechnology companies are subject to a variety of regulations in other jurisdictions governing, among other
things, clinical trials and any commercial sales and distribution of products. Because biologically sourced raw materials are subject to unique contamination risks, their use may be restricted in some countries.
154
The requisite approvals from regulatory authorities in foreign countries must be obtained prior to the
commencement of clinical trials or marketing of a product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the Investigational New Drug
Application prior to the commencement of human clinical trials. In the European Union, for example, a Clinical Trial Application, or CTA, must be submitted to each country’s national health authority and an independent ethics committee, much
like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all
cases, the clinical trials are conducted in accordance with good clinical practice, or GCP, and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of an investigational biological product under European Union regulatory systems, a company must submit a marketing
authorization application. For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary
from country to country. In all cases, again, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
Research and Development Expenses
Celladon’s
research and development expenses were $22.7 million, $16.9 million and $13.3 million for the years ended December 31, 2014, 2013 and 2012, and $21.8 million and $15.5 million for the nine months ended September 30, 2015 and 2014,
respectively.
Employees
As of December 31,
2015, Celladon had three full-time employees, consisting of executive staff in support of the proposed merger. None of Celladon’s employees are covered by collective bargaining agreements and Celladon considers relations with its employees to
be good.
Properties
Celladon’s
corporate headquarters are currently located at 12707 High Bluff Drive, Suite 200, San Diego, California 92130 in an executive office facility where Celladon leases approximately 605 square feet of office space. The lease for this office space
expires in February 2016 and is extendable on a month-by-month basis.
Legal Proceedings
In July 2015, following Celladon’s announcements of the negative CUPID 2 data and the suspension of further research and development activities and the
subsequent declines of the price of Celladon’s common stock, three putative securities class action complaints (captioned Fialkov v. Celladon Corporation, Case No. 15-cv-1458-AJB-DHB, Lorusso v. Celladon Corporation, Case
No. 15-cv-1501-L-JLB and Jacobs v. Celladon Corporation, Case No. 15-cv-1529-AJB-MDD ) were filed in the U.S. District Court for the Southern District of California against Celladon and certain of Celladon’s current and former
officers. The complaints generally allege that the defendants violated Sections 10(b) and 20(a) of the Exchange Act by making materially false and misleading statements regarding the clinical trial program for MYDICAR, thereby artificially inflating
the price of Celladon’s common stock. The complaints seek unspecified monetary damages and other relief, including attorneys’ fees. On September 1, 2015, six stockholders (or groups of stockholders) filed motions to consolidate the
three putative securities class actions and to appoint lead plaintiffs (the “Motions to Consolidate”). A hearing
155
on the Motions to Consolidate was held on December 3, 2015. On December 9, 2015, the Court consolidated the three putative securities class actions and appointed a lead plaintiff to
represent the putative class. Celladon expects the lead plaintiff to file a consolidated complaint. It is possible that additional suits will be filed, or allegations made by stockholders, with respect to these same or other matters and also naming
Celladon and/or Celladon’s officers and directors as defendants. Celladon believes that it has meritorious defenses and intends to defend these lawsuits vigorously. Due to the early stage of these proceedings, Celladon is not able to predict or
reasonably estimate the ultimate outcome or possible losses relating to these claims.
156
EIGER BUSINESS
Overview
Eiger is a clinical stage biopharmaceutical
company focused on bringing to market novel products for the treatment of orphan diseases. Since its founding in 2008, Eiger has worked with investigators at Stanford University and has evaluated a number of potential development candidates from
pharmaceutical companies to comprise a pipeline of novel product candidates. Eiger’s resulting pipeline includes three Phase 2 candidates addressing four distinct orphan diseases. The programs have several aspects in common: the disease targets
represent conditions of high medical need which are inadequately treated by current standard of care; the therapeutic approaches are supported by an understanding of disease biology and mechanism as elucidated by Eiger’s academic research
relationships; prior clinical experience with the product candidates guides an understanding of safety; and the development paths leverage the experience and capabilities of Eiger’s experienced, commercially focused management team. The
pipeline includes Sarasar® (lonafarnib) for hepatitis delta virus, or HDV, exendin (9-39) for severe hypoglycemia and Bestatin™ (ubenimex) for pulmonary arterial hypertension, or
PAH, and lymphedema. Eiger plans to deliver Phase 2 data on all four programs over the course of the next one to three years beginning in 2016.
Eiger’s current project timelines, planned development and regulatory pathways are illustrated below. As discussed above, prior clinical experience by
Eiger’s licensors with the product candidates has supported and guided Eiger’s understanding of safety in advancing these products in its clinical development programs. Specifically, Eiger in-licensed lonafarnib from Merck Sharp &
Dohme Corp, or Merck, in 2010, and licensed ubenimex from Nippon Kayaku Co., Ltd., or Nippon Kayaku, in 2015. Eiger has relied upon Merck and Nippon Kayaku’s prior Phase 1 clinical data, manufacturing and experience with these two molecules to
file the Eiger INDs and proceed directly into Phase 2 clinical trials following authorization by the FDA.
Pipeline
Timeline
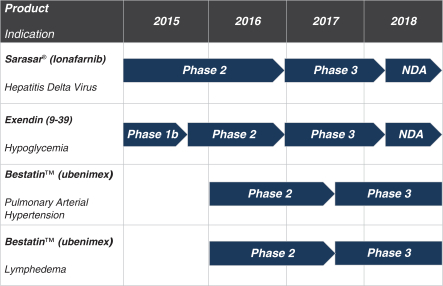
Note: All dates represent Eiger’s current expectations. Actual timing may vary.
Eiger’s product candidate pipeline includes four Phase 2 programs:
| |
• |
|
Lonafarnib is an orally bioavailable, small molecule in Phase 2 clinical trials for HDV infection and is Eiger’s most advanced program. HDV is
the most severe form of viral hepatitis for which there is |
157
| |
currently no cure and no approved therapy. Chronic HDV infection can lead to a rapid progression to liver cirrhosis, a greater likelihood of developing liver cancer, and has the highest fatality
rate of all the hepatitis infections. |
Eiger in-licensed lonafarnib from Merck in 2010. Lonafarnib blocks the
production of HDV virus particles by inhibiting a key step, called prenylation, in the virus life cycle. To date, over 50 HDV infected patients have been dosed with lonafarnib across international Phase 2 clinical trials. Lonafarnib has demonstrated
dose-related activity in reducing HDV viral load both as a monotherapy and in combination with other agents. Lonafarnib boosted with ritonavir has demonstrated a reduction in HDV viral loads by two logs and three logs at four weeks and eight weeks,
respectively. Lonafarnib boosted with ritonavir and combined with pegylated interferon alpha, or PEG-IFN-alpha, has demonstrated a reduction in HDV viral loads by 99.9%, or up to three logs, in four weeks. Multiple Phase 2 studies of lonafarnib are
ongoing with endpoints of clearance of HDV virus and sustained virologic response, or SVR. The most common adverse events experienced with lonafarnib to date are gastrointestinal-related and include nausea, vomiting, and diarrhea.
Lonafarnib has been granted orphan drug designation by the U.S. Food and Drug Administration, or the FDA, and European Medicines Agency, or
EMA. The U.S. Orphan Drug Act, or ODA, provides for granting special status to a drug or biological product to treat a rare disease or condition. Orphan drug designation qualifies the sponsor of the drug for various development incentives. Orphan
drug designation also provides for a period of market exclusivity or protection against generic entry. The potential market for HDV therapies in the United States and Western Europe is growing due to increased migration from regions where the
disease is endemic, primarily from Eastern Europe, the Middle East, and Asia.
| |
• |
|
Exendin (9-39) is the second most advanced product candidate in Eiger’s pipeline, and Eiger is developing this candidate as a treatment for hyperinsulinemic hypoglycemia associated with bariatric surgery.
Hyperinsulinemic hypoglycemia associated with bariatric surgery is a debilitating and potentially life-threatening condition for which there is currently no approved therapy. This disorder leads to frequent symptomatic hypoglycemia, where blood
sugar levels are below 50 mg/dL, and results in glucose concentrations low enough to cause seizures, altered mental status, loss of consciousness, and even death. Gastric bypass procedures are widely performed and are increasing for medically
complicated obesity, including obesity due to Type 2 diabetes. |
To date, research at Stanford University has generated
results demonstrating clinical proof of concept in 18 patients suffering from gastric bypass surgery-induced hypoglycemia indicating that exendin (9-39) can potentially prevent post-prandial hypoglycemia in affected patients. Exendin (9-39) is a
glucagon-like peptide-1, or GLP-1, receptor antagonist and has the potential to compete with endogenous GLP-1 and prevent excess insulin release. These data were generated using both intravenous and subcutaneous, or SQ, formulation delivery
developed by Stanford University. Pharmacokinetics from this Phase 2 SQ study indicate that the SQ formulation could enable once or twice a day pre-prandial dosing. Eiger is developing its own SQ formulation and plans to initiate a Phase 2
dose-ranging trial in affected patients with its exendin (9-39) SQ formulation in the second quarter of 2016. Eiger intends to seek orphan drug designation for exendin (9-39).
| |
• |
|
Eiger’s third product candidate is ubenimex for PAH. PAH a life-threatening disease characterized by increased pulmonary vascular resistance, heart failure and premature death. |
Ubenimex is a well-characterized, oral, small-molecule inhibitor of leukotriene A4
hydrolase, or LTA4H, the enzyme responsible for converting the inflammatory mediator leukotriene A4, or LTA4, to leukotriene B4, or LTB4. Results of a preclinical study published in Science
Translational Medicine (Tian, W. et al. “Blocking Macrophage Leukotriene B4 Prevents Endothelial Injury and Reverses Pulmonary Hypertension,” Sci Transl Med, 2013; 5:1) by Stanford University researchers have demonstrated that both LTB4 and LTA4H are elevated in animal models of PAH and human PAH disease. In that study, elevated LTB4 caused inflammation resulting in arteriole occlusion and
158
hypertension in animal models of PAH. Targeted pharmacologic inhibition of LTB4, including ubenimex, reversed PAH disease in treated rat
animal models; obstructed arterioles opened, cardiac function improved, and the animals survived. Based on the findings in these models that pathological inflammation may be important in the etiology of PAH, Eiger believes that ubenimex is an
attractive candidate for clinical development. In December 2015, ubenimex was granted orphan drug designation by the FDA for the treatment of PAH. Eiger intends to begin enrollment in a Phase 2 clinical trial of ubenimex in patients with PAH in the
first quarter of 2016.
Ubenimex was licensed from Nippon Kayaku, and Eiger has exclusive rights in the United States, Europe and
certain other countries to develop ubenimex for PAH as well as other inflammatory diseases involving LTB4. Ubenimex has been marketed in Japan and other countries outside of Eiger’s
licensed territory by Nippon Kayaku for over 25 years for a different indication.
| |
• |
|
Eiger’s fourth program involves clinical development of ubenimex in lymphedema, which is a state of vascular functional insufficiency in which decreased clearance of interstitial fluid through the lymphatic
vasculature leads to edema formation and to progressive, debilitating architectural alterations in skin and supporting tissues. There is no approved pharmacologic therapy. The current standard of therapy involves compression garments.
|
Researchers at Stanford University have demonstrated for the first time that LTB4 is elevated in both animal models of lymphedema as well as human lymphedema and that elevated LTB4 is associated with tissue inflammation
and impaired lymphatic function. In that research, applying inhibitors of LTB4 promoted physiologic lymphatic repair and reversed lymphedema in treated animals. Eiger is seeking orphan drug
designation for ubenimex in lymphedema. Eiger intends to begin enrollment in a Phase 2 clinical trial of ubenimex in patients with lymphedema in the first half of 2016.
Eiger believes that its approach to clinical development enables achievement of early clinical signals in its Phase 2 programs and potentially reduces
clinical risks and costs inherent in the drug discovery and development process. Eiger has a highly experienced management team whose members have, in the course of their prior employment, participated in the bringing of more than 20 product
candidates through regulatory approval and into commercialization. Eiger plans to leverage its management team’s breadth and depth of experience in clinical and regulatory drug development as well as market development and commercialization to
identify potentially promising product candidates to address unmet medical needs.
Eiger’s current product candidate pipeline has been created by
in-licensing from pharmaceutical companies as well as Stanford University. With its focus on orphan diseases, Eiger’s strategy is to acquire and retain some or all commercialization rights to its products in significant territories to diversify
risk, identify a rapid regulatory pathway to approval and minimize the development investment in order to maximize long-term value for its stockholders. Over time, depending upon the data and potential market opportunity, Eiger expects to establish
a commercial organization, which Eiger believes can be targeted and cost effective for selected, promising orphan disease designated programs. Eiger plans to balance these interests with opportunities to enhance stockholder value through
partnerships and other strategic relationships.
An orphan drug designation in the United States refers to a designation by the U.S. Food and Drug
Administration, or FDA, granting seven years of marketing and treatment exclusivity to a product to treat a rare disease or condition (a disease affecting fewer than 200,000 people) that is clinically superior or otherwise makes a major contribution
to patient care in a specified indication or rare disease or condition. In the European Union, the European Medicines Agency, or EMA, grants orphan drug designation with ten years of market exclusivity for diseases that are life-threatening or
chronically debilitating and have a prevalence in the European Union of not more than 5 in 10,000, or it is otherwise unlikely that marketing would generate returns sufficient to justify the investment. Other countries have similar opportunities for
exclusivity with respect to products addressing unmet medical needs.
159
Business Model and Management Team
Eiger plans to continue evaluating in-licensing opportunities in order to enhance its pipeline and leverage its business development, clinical development,
regulatory and commercial expertise. Eiger believes its management team has the capability and experience to continue to execute this model. Eiger’s management team has worked in other private and public biotechnology companies such as
Prestwick Pharmaceuticals, New River Pharmaceuticals, Clinical Data, CoTherix and InterMune, each of which was acquired by a larger pharmaceutical industry company. Eiger’s management also has previous work experience, in some cases working
together, at pharmaceutical companies, including The Upjohn Company, Glaxo, Glaxo Wellcome, Glaxo Smith Kline, Arena Pharmaceuticals, Alza (Johnson and Johnson), Halozyme, Clinical Data Inc., New River Pharmaceuticals, Genentech and Gilead Sciences.
Eiger’s Strategy
Eiger’s mission is to
identify, develop, and, directly or through collaborations, bring to market novel products that receive orphan drug designation for the treatment of rare diseases or conditions. Eiger currently has a diverse portfolio of well-characterized product
candidates with the potential to address diseases for which the unmet medical need is high, the biology for treatment is believed to be understood, and for which an effective therapy is not available. Eiger’s goal is to be a leader in the
development and commercialization of novel therapeutics for serious unmet medical needs in orphan diseases. Eiger’s focus to achieve this goal will be to utilize its experience and capabilities to:
| |
• |
|
Advance its existing product candidates through late-stage clinical trials, generating meaningful clinical results; |
| |
• |
|
Work with U.S. and international regulatory authorities for expeditious, efficient development pathways toward registration; |
| |
• |
|
Prepare for commercialization of each program; |
| |
• |
|
Use Eiger’s industry relationships and experience to source, evaluate and in-license well-characterized product candidates to continue pipeline development; and |
| |
• |
|
Identify potential commercial or distribution partners for Eiger’s products in relevant territories. |
Eiger’s Product Candidates
Lonafarnib in HDV
Lonafarnib, brand named Sarasar®, is a small molecule that Eiger in-licensed from
Merck in 2010 that Eiger is advancing for the treatment of HDV infection. Lonafarnib is a well-characterized, orally active inhibitor of farnesyl transferase, an enzyme involved in modification of proteins through a process called prenylation. HDV
uses this prenylation process inside host liver cells to complete a key step in its life cycle. Lonafarnib inhibits the prenylation step of HDV replication inside liver cells and blocks the virus life cycle at the stage of assembly. Since
prenylation is carried out by a host enzyme, there is a higher barrier to develop viral resistance mutations to lonafarnib therapy. Eiger has generated clinical results in over 50 patients in Phase 2 trials, across international study sites,
demonstrating rapid decreases in HDV viral loads and no resistance. Eiger is actively recruiting patients in additional Phase 2 trials of longer duration using lonafarnib in combination with other antiviral therapies including ritonavir and
PEG-IFN-alpha, with a goal of addressing HDV.
Hepatitis Delta Overview
About Hepatitis Delta
Hepatitis delta infection is caused
by HDV a small circular ribonucleic acid, or RNA, virus that expresses only one protein, the hepatitis delta antigen, or HDAg. There are two forms of HDAg, small and large. Together, these two forms of HDAg and the single-stranded RNA genome are
surrounded by a lipid envelope, which is
160
embedded with Hepatitis B Virus, or HBV, derived surface antigen, or HBsAg, proteins. HDV does not encode its own envelope proteins and must acquire them from HBV during the final steps of
replication. Hence natural HDV infections always occur in the presence of a co-existing HBV infection. HBsAg is the only element of HBV relied upon by HDV. HDV replication can occur independently of HBV replication.
Hepatitis delta is the most severe form of viral hepatitis. Hepatitis delta can be acquired either by co-infection (a simultaneous co-infection with HDV and
HBV) or by super-infection (infection of someone already harboring a chronic HBV infection). Both co-infection and superinfection with HDV result in more severe complications compared to infection with HBV alone. These complications include a
greater likelihood of experiencing liver failure in acute infections and a rapid progression to liver cirrhosis, with an increased chance of developing liver cancer in chronic infections. HDV has the highest fatality rate of all the hepatitis
infections at up to 20%. Although HDV/HBV simultaneous co-infection in adults usually resolves completely, in some cases it can become fulminant, or rapidly severe, hepatitis. In the case of super-infections, the predominant form of HDV, HDV
super-infection leads to a more severe form of disease than chronic HBV mono-infection. In a study published in 1987 in the Journal of Infectious Diseases (Fattovich, G. et al. “Influence of Hepatitis delta Virus Infection on Progression to
Cirrhosis in Chronic Hepatitis Type B,” J Infect Dis, 1987; 155:931), histological liver deterioration was observed in 77% of HBV patients co-infected with HDV over a 15-year follow-up period, versus 30% of patients infected with HBV alone
(p<0.01). In a 2013 study of chronic HBV patients published in the Journal of Gastroenterology and Hepatology (Gish, R. et al. “Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern
California,” J Gastroenterol Hepatol, 2013; 28(9):1521), cirrhosis was present in 73% of HBV patients co-infected with HDV, compared to only 22% of those infected with HBV alone. Patients co-infected with HDV are more than twice-as-likely to
develop liver-related complications, cirrhosis, or require liver transplants than matched patients infected with HBV alone.
HDV is generally spread
through exchange of body fluids either sexually or through contact with infected blood. Globally, it is estimated that between 4.3% and 5.7% of the 250 million worldwide chronic HBV population, or 15 to 20 million people, are infected with
HDV. The prevalence of HDV in patients infected with chronic HBV is even higher in certain regions, including certain parts of Mongolia, China, Russia, Central Asia, Pakistan, Turkey, Africa, and South America, with an HDV prevalence as high as 60%
being reported in HBV-infected patients in Mongolia and Pakistan. The prevalence of HDV has recently begun to increase in Western Europe and the United States due to migration from countries with high infection rates.
The Role of HDV Screening in Identifying Patients Who May Benefit From Lonafarnib
There are diagnostic tests in use today in clinical laboratories to detect anti-HDV antibodies in serum. These tests are currently able to detect acute HDV
infections after four weeks, but they are poor tests for active HDV infections. These infections are best detected by reverse transcriptase-polymerase chain reaction, or RT-PCR, assays for genomic RNA. These assays yield a quantitative assessment of
the number of viral particles or viral load in serum. A commercial assay for quantitative HDV RNA is currently available in Europe. A commercial assay for quantitative HDV RNA is not yet available in the United States.
Eiger has developed an HDV RNA quantitative RNA assay that has been calibrated using the World Health Organization HDV standard provided by the Paul Erhlich
Institute in Germany. Eiger has used this assay to quantitate HDV RNA in its Phase 2 trials. Eiger is facilitating transfer of its HDV RNA assay into commercial laboratories. By increasing the number of assays performed, Eiger believes it can
increase the number of patients who can be identified and who will potentially benefit from an HDV therapy such as lonafarnib.
In Eiger’s initial
discussions with payers, these payers have indicated that they would be willing to reimburse healthcare providers for HDV assays that are carried out sequentially following a positive HBsAg positive test for HBV. Eiger is in the process of
transferring its assay to U.S. commercial labs and anticipates this assay being available on testing menus by the first half of 2016. If, due to Eiger’s efforts and growing awareness of HDV as a health issue for HBV patients, screening for HDV
becomes more widespread, this will have the effect of increasing the pool of patients who would be eligible for, and might benefit from, lonafarnib.
161
Current Therapy for HDV
Currently, there is no FDA approved therapy for hepatitis delta infection. The American Association for the Study of Liver Diseases, or the AASLD, guidelines
suggest treatment of chronic hepatitis delta infections with IFN-alpha, but this therapeutic regime usually requires injections of IFN-alpha over a prolonged period. In clinical trials of IFN-alpha or PEG-IFN-alpha, between 25% and 33% of HDV
infected patients were able to clear their infections after a minimum of 48 weeks of therapy, with some requiring two years of therapy. However, long-term therapy with IFN-alpha is known to be associated with numerous adverse events and tolerability
is a significant problem for some of these patients. HBV nucleoside analogs that inhibit HBV genome replication are ineffective against HDV since they are ineffective in suppressing the expression HBsAg. Other antiviral therapies have been tested,
but none have been shown to be effective against HDV infection.
HDV Replication and Prenylation
After HDV enters a target cell hepatocyte, the genome is translocated to the nucleus where genome replication occurs and the two forms of HDAg small delta
antigen, or SHDAg, and large delta antigen, or LHDAg, are produced. The newly formed HDV genome and the small and large delta antigen must acquire a lipid envelope from HBV to complete the assembly process. An important interaction between HDV and
HBV proteins has been shown to depend on the presence of the last four amino acids of the large delta antigen, comprising a CXXX box motif, where C represents cysteine and X denotes any other amino acid. This amino acid sequence is required for
LHDAg to be prenylated by a host enzyme which covalently attaches a 15-carbon prenyl lipid (farnesyl-moiety) to the cysteine of the CXXX box. Prenylation of the large delta antigen renders it more lipophilic, promotes its association with HBsAg and
is essential for initiating the HDV particle formation process. Eiger’s approach involves targeting a host process called prenylation, or protein farnesylation, that has been shown to be essential for the last steps in HDV replication, the
assembly and release of new virus progeny.
In the 1980’s farnesyltransferase inhibitors were developed by multiple pharmaceutical companies for
oncology indications. Addition of a farnesyl or prenyl lipid group to the Ras protein, or Ras, a well-known and important regulator of cellular proliferation, allows for membrane association. Once membrane bound, Ras may then be activated. The
importance of activated Ras in tumor development was demonstrated by sequence analyses of tumors from patients where up to 30% have mutations involved Ras. Several prenylation inhibitors were developed in oncology and taken into the clinic and in
some cases through late-stage clinical development. However these programs did not lead to approvals, due to a lack of compelling efficacy. The class-related, dose-limiting toxicity has been gastrointestinal side effects including nausea, vomiting,
and diarrhea.
Published studies conducted by Stanford University researchers demonstrated that farnesyltransferase inhibitors can block HDV viral
production both in cellular experiments and in HDV transgenic mice. Targeting prenylation or farnesyl transferase, a host target, significantly reduces the likelihood of HDV developing resistance to escape effects of antiviral therapy. Viruses
mutate quickly and there is a higher rate of mutations in viral replication compared to mammalian cell division. However, no matter how much HDV may mutate, these changes do not alter the host process of prenylation which HDV requires to complete
packaging. Thus, targeting a host prenylation process provides what Eiger believes to be a higher barrier to resistance. Identification of clinic-ready farnesylation inhibitors has allowed Eiger to test this concept in humans.
Eiger’s Solution: Lonafarnib for HDV
Lonafarnib
is a well-characterized, orally active inhibitor of farnesyl transferase. Lonafarnib inhibits the prenylation step of HDV replication inside liver cells and blocks the ability of the virus to multiply. Since prenylation is a host process, not under
control of HDV, and lonafarnib inhibits prenylation, Eiger believes that there is also a potentially higher barrier to resistance with lonafarnib therapy. Lonafarnib has been granted orphan drug designation in Europe and the United States, and
lonafarnib in combination with ritonavir has been granted Fast Track designation from FDA for the treatment of chronic HDV infections. Eiger is currently conducting three Phase 2 clinical trials: LOWR
162
HDV—2 (Ankara, Turkey), LOWR HDV—3 (NIH) and LOWR HDV—4 (Hannover, Germany). Eiger has filed and received approval for a U.S. IND and is conducting LOWR HDV—4 under this IND.
Lonafarnib has never been approved or commercialized for any indication.
Fast Track designation from FDA is a process to facilitate the development and
expedite the review of drugs to treat serious conditions and fill an unmet medical need. A drug that receives Fast Track designation may be eligible to receive more frequent meetings with FDA to discuss the drug’s development, more frequent
written communication from FDA about the design of proposed clinical trials and biomarkers, eligibility for Accelerated Approval and Priority Review, if relevant criteria are met, and eligibility to have Biologics License Applications, or BLAs, and
New Drug Applications, or NDAs, reviewed on a rolling basis.
Lonafarnib Clinical Data
Eiger in-licensed lonafarnib from Merck in 2010, and has relied upon Merck’s prior Phase 1 clinical experience with lonafarnib to understand safety. In
addition, Eiger conducted a Phase 1 study with goals including: evaluating the effects of multiple-dosing of an antacid (proton pump inhibitor or H2-receptor antagonist) on the systemic absorption of a single dose of lonafarnib, and evaluating the
effects of multiple-dosing of lonafarnib on the inhibition potential of the cytochrome P450 enzyme, CYP2C19. The Phase 1 study results demonstrated a weak effect on systemic absorption of lonafarnib following administration of an antacid, which
Eiger believes reduces the risk for the use of an antacid by patients treated with lonafarnib to manage possible dyspepsia during treatment. These Phase 1 study results also demonstrated a weak effect of lonafarnib on the systemic absorption of a
sensitive CYP2C19 substrate, which Eiger further believes reduces the potential need for the use of concomitant medications that are metabolized by CYP2C19 by patients treated with lonafarnib.
Merck conducted four Phase 1 studies in 85 healthy volunteers to study food effect (study P00042), absorption, metabolism and excretion (study P00260),
ketoconazole drug interaction (study P00393), and the effect of age and gender (study P02673).
In study P00042, administration of lonafarnib with food
decreased the rate and extent of lonafarnib absorption in healthy subjects when administered as a single 100 mg dose of lonafarnib. The relative oral bioavailability of lonafarnib for subjects that ate prior to receiving lonafarnib compared to those
that fasted prior to receiving lonarfib was 48% based on measuring the maximum serum concentration, or Cmax, and 77% based measuring the concentration of the lonafarnib in the blood plasma over
time, or AUC. However, administration of lonafarnib with food did not have a significant effect on lonafarnib bioavailability in subjects following multiple-dose administration. In addition, inter-subject variability was lower (~16%) following
multiple-dose administration with food. Given the apparent lower incidence of gastrointestinal side effects and the lower inter-subject variability, results from P00042 study support dosing lonafarnib with food.
Study P00260 was an absorption, metabolism and excretion study conducted in healthy volunteers following single-dose administration of lonafarnib.
Drug-derived radioactivity was primarily excreted via the feces. Mean cumulative excretion of radioactivity was 61% in feces and less than 1% in urine up to 24 hours post-dose. Metabolite profiles in plasma, urine, and feces showed that lonafarnib
was metabolized extensively. The common metabolic pathways included oxidation, dehydrogenation, and combinations of these two processes. The results of in vivo metabolic profiling and in vitro metabolism studies indicate that no
human-specific lonafarnib metabolites are formed.
Study P00393 was a two-way crossover study that was conducted in 16 healthy volunteers exploring
the interaction of lonafarning with ketoconazole, an anti-fungal medication. Co-administration of single-dose lonafarnib (50 mg) and multiple doses of ketoconazole (200 mg BID for 5 days) resulted in
an approximately five-fold increase in lonafarnib exposures, and an increase in the mean elimination half-life from 2.68 hours to 3.99 hours. Administration of lonafarnib with ketoconazole was also associated with lower inter-subject variability
than when lonafarnib was administered with a placebo.
163
Study P02673 was a single-center, single-dose study conducted in 48 healthy volunteers (24 males and
24 females). Twenty-four of the subjects were between the ages of 18 and 45 and the other 24 subjects were 65 years old or older. Each subject received a single 100 mg dose of lonafarnib in the morning after a standardized meal. PK data
suggested that lonafarnib exposures were higher in female subjects (44% higher) as compared to male subjects. Additionally, lonafarnib exposures in subjects 65 years old or older were approximately 59% higher as compared to the younger healthy
subject population. Young male subjects had the lowest lonafarnib systemic exposures; AUC values in male subjects between the ages of 18 and 45 were approximately 50% lower than female and elderly subjects.
To date, lonafarnib has been tested in three Phase 2 trials in over 50 HDV infected patients.
NIH Clinical Proof-of-Concept Phase 2a Study
The
National Institutes of Health, or the NIH, conducted a 14 patient, double blind, placebo-controlled, proof of concept study, which was the first ever to evaluate lonafarnib in patients infected with HDV. Patients either received lonafarnib 100 mg
(group 1) or lonafarnib 200 mg (group 2) twice daily, or BID, for 28 days with 6 months’ follow-up. Both groups enrolled six treatment participants and two placebo participants. The two placebo patients from group 1 later received open-label
lonafarnib as group 2 participants. Doses of 100 mg and 200 mg of lonafarnib administered BID demonstrated a dose dependent decrease in viral loads of 0.73 and 1.54 log decline, respectively, in 28 days. The results were published in The Lancet
Infectious Diseases Journal in 2015.
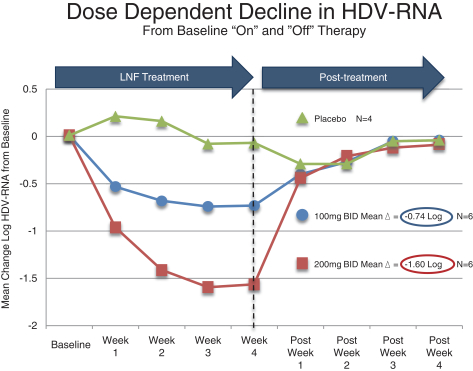
As shown in the table above, statistically significant decreases in HDV RNA viral load were demonstrated by both the 100 mg
of lonafarnib BID (p<0.04) and 200 mg of lonafarnib BID (p<0.0001) active groups versus the placebo. A statistically significant correlation between increasing lonafarnib serum levels and decreasing HDV RNA viral loads was also demonstrated.
The 100 mg twice daily dose was well-tolerated while GI intolerability such as nausea and diarrhea was experienced in the 200 mg twice daily dose. No resistant variants were identified from population-based sequencing of HDV infected patients after
28 days of treatment with lonafarnib.
164
A p-value is a statistical measure of the probability that the difference in two values could have occurred by
chance. The smaller the p-value, the greater the statistical significance and confidence in the result. Typically, results are considered statistically significant if they have a p-value less than 0.05, meaning that there is less than a one-in-20
likelihood that the observed results occurred by chance. The FDA requires that sponsors demonstrate the effectiveness and safety of their product candidates through the conduct of adequate and well-controlled studies in order to obtain marketing
approval. Typically, the FDA requires a p-value of less than 0.05 to establish the statistical significance of a clinical trial, although there are no laws or regulations requiring that clinical data be statistically significant, or that require a
specific p-value, in order for the FDA to grant approval.
LOWR HDV—1 (LOnafarnib With and without
Ritonavir) Phase 2 Study
The LOWR HDV—1 trial studied lonafarnib in 15 subjects who were enrolled into one of five groups
comprised of three patients in each group, three groups receiving different doses lonafarnib monotherapy, lonafarnib in combination with ritonavir, and a group receiving lonafarnib in connection with PEG-IFN-alpha.
In lonafarnib monotherapy treatment groups, increasing the dosage of lonafarnib from 100 mg three times a day to 200 mg twice a day to 300 mg twice a day led
to greater reductions in viral loads. Viral loads were reduced, after 28 days, from a 1.2 log decline in the patients dosed with 100 mg three times a day to 1.6 log decline in patients dosed with 200 mg twice a day to a 2.0 log decline in the
patients dosed with 300 mg of lonafarnib twice a day. However, doses greater than 100 mg BID led to increasing gastrointestinal, or GI, intolerability and were not considered to be ideal for longer term dosing.
In the lonafarnib-ritonavir combination arm of LOWR HDV—1, 100 mg of lonafarnib BID was combined with 100 mg of ritonavir once daily. Ritonavir is a
pharmacokinetic, or PK, enhancer known to inhibit the metabolism of lonafarnib, allowing lower doses of lonafarnib to be administered, while resulting in higher systemic concentrations of lonafarnib, and presumably higher liver concentrations of
lonafarnib.
The addition of 100 mg of ritonavir once daily to 100 mg lonafarnib BID led to a four- to five-fold increase in the serum concentration of
lonafarnib in treated patients compared to lonafarnib alone. This dose combination led to a greater reduction in viral load, compared to monotherapy treatment with 100 mg lonafarnib BID, with a mean decrease of 2.4 logs and 3.2 logs after 28 days
and 56 days, respectively. Importantly, when therapy was discontinued the viral loads rebounded, which Eiger believes indicates that lonafarnib treatment was eliciting an antiviral effect.
165
The addition of 180 mcg of PEG-IFN-alpha once weekly to 100 mg lonafarnib BID was also more active in
reducing HDV RNA versus either agent alone. This dose combination led to a greater reduction in viral load, compared to monotherapy treatment with 100 mg lonafarnib BID, with a mean decrease of 1.8 logs and 3.0 logs after 28 days and 56 days,
respectively. Importantly, when therapy was discontinued the viral loads rebounded. The mean change in HDV RNA for the patients receiving eight weeks of treatment of 100 mg lonafarnib BID in combination with ritonavir and 100 mg lonafarnib BID in
combination with PEG-IFN-alpha is shown below. LOWR HDV—1 was a dose-finding study conducted on a total of 15 patients, with three patients in each treatment arm. The study did not include a placebo arm and, as such, statistical significance
could not be determined.
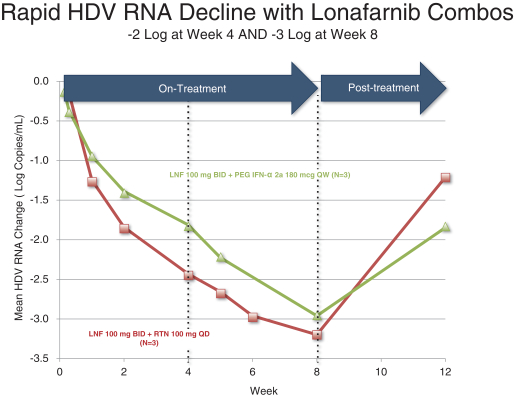
166
Liver enzymes are often elevated during infections with viral hepatitis, a sign of damage being done to liver
cells. In both lonafarnib combination cohorts, all HDV patients enrolled had elevated alanine aminotransferase, or ALT, liver enzymes prior to receiving any treatment. By the end of eight weeks of combination therapy with lonafarnib and ritonavir or
lonafarnib and PEG-IFN-alpha, all patients’ ALT liver enzymes normalized or trended toward normal while on therapy. The figures below show the ALT levels, measured in units per liter, or U/L, for each of the patients receiving lonafarnib in
combination with ritonavir and lonafarnib in combination with PEG-IFN-alpha.


167
The results of LOWR HDV—1 study generated the most encouraging results to date in reducing HDV RNA in
patients infected with hepatitis delta. In the three patients receiving lonafarnib in combination with ritonavir and the three patients receiving lonafarnib in combination with PEG-IFN-alpha, Eiger observed decreases in HDV RNA viral load of
approximately 3.2 logs and 3.0 logs after eight weeks of treatment, respectively. For comparison, and as shown in the figure below, published data from the HIDIT-2 trial of PEG-IFN-alpha in 91 HDV infected patients demonstrated a mean decline in HDV
RNA of approximately 1.6 logs and 2.7 logs after 8 weeks and 48 weeks, respectively. The HIDIT-2 (Hep-Net International Delta Hepatitis International Trial-II) was a multicenter randomized trial studying effects of PEG-IFN plus tenofovir in chronic
HDV patients, and is the largest clinical study to date in HDV. The HIDIT-2 trial was conducted on 91 patients, whereas the LOWR HDV—1 study was conducted on an aggregate of 15 patients, with three patients per treatment arm. If the LOWR
HDV—1 trial were conducted on a larger group of patients, the mean HDV RNA decline may differ from the 3.2 log and 3.0 log declines after eight weeks of treatment observed in the three patient arms receiving lonafarnib combination treatment in
the LOWR HDV—1 trial. However, based on clinical results to date, Eiger expects all patients who are treated with lonafarnib to show a viral load response.

LOWR HDV—2 (LOnafarnib With Ritonavir) Phase 2 Study
LOWR HDV—2 is an ongoing “dose finding” trial to explore lonafarnib doses in combination with ritonavir and/or PEG-IFN-alpha. The goal
of LOWR HDV—2 is to identify the lowest tolerable and effective dose of lonafarnib for longer duration therapy. Eiger believes that dosing durations of at least 24 weeks may be necessary to clear HDV RNA and to achieve SVR for HDV infection.
Twenty-seven subjects were enrolled into one of nine groups of different lonafarnib with ritonavir and/or PEG-IFN combinations for 12 to 24 weeks (n=3
per group) as follows: Group 1: LNF 100 mg bid and RTN 50 mg bid; Group 2: LNF 100 mg bid and RTN 100 mg qd; Group 3: LNF 150 mg qd and RTN 100 mg qd; Group 4: LNF 100 mg qd and RTN 100 mg qd; Group 5: LNF 75 mg bid and RTN 100 mg bid; Group 6: LNF
50 mg bid and RTN 100 mg bid; Group 7: LNF 50 mg bid and RTN 100 mg bid and PEG-IFN 180 mcg qw; Group 8: LNF 25 mg bid and RTN 100 mg bid; and Group 9: LNF 25 mg bid, RTN 100 mg bid and PEG-IFN 180 mcg qw. A low dose of lonafarnib 50 mg BID in
combination with ritonavir 100 mg BID has proven to be tolerable and allowed
168
for the addition of PEG-IFN-alpha. This triple dose combination has achieved the most robust mean HDV RNA viral load declines of greater than 3 logs after 28 days. Tolerability with this regimen
was reported by investigators and patients to be acceptable, enabling more patients to remain on therapy for periods sufficient to possibly clear HDV RNA. Eiger plans to carry out dosing durations of up to 6 months.
Eiger does not believe it has identified the lowest effective dose of lonafarnib. Dosing cohorts are ongoing in LOWR HDV—2 with doses of lonafarnib 25 mg
BID in combination with ritonavir 100 mg BID with/and without PEG-IFN-alpha. Tolerability with these regimens has also been reported by the investigator and the patients to be acceptable, enabling more patients to remain on therapy for periods
sufficient to possibly clear HDV RNA. Eiger plans to carry out dosing for a longer duration.
LOWR HDV—2 is an open label study and results will be
presented over time as the study completes in the second quarter of 2016.
LOWR HDV—3 Phase 2 Trial
LOWR HDV—3 is an ongoing “duration” study to explore lonafarnib doses in combination with ritonavir for six months. Eiger’s Phase 2
placebo-controlled trial will test once daily doses of lonafarnib of 50 mg, 75 mg and 100 mg in combination with ritonavir 100 mg once a day for a duration of six months in 21 HDV patients, with seven HDV patients in each group. The goals of LOWR
HDV—3 include dose ranging and longer duration therapy to determine reduction in HDV RNA, including the potential for clearance of HDV RNA.
Eiger completed enrollment of 21 patients at the NIH for LOWR HDV—3, and plans to follow patients for an additional six months post-treatment to assess
long-term suppression of HDV viral loads. Initial data is expected in late 2016.
LOWR HDV—4 Phase 2 Trial
LOWR HDV—4 is a “dose titration” study to explore escalating lonafarnib dosed in combination with ritonavir for 6 months. The study is a Phase 2
open label trial in which patients will be given a starting dose of lonafarnib 50 mg BID in combination with ritonavir 100 mg BID. Patients demonstrating good tolerability will be allowed to titrate up to lonafarnib 75 mg BID at the
investigator’s discretion. The fifteen HDV patients will be dosed for a duration of six months, followed by an additional six month post-treatment evaluation to assess long-term suppression of HDV viral loads. The goals of LOWR HDV—4
include the exploration of upward dose titration and longer duration therapy to determine reduction in HDV RNA in this time period, including the potential for clearance of HDV RNA. As noted above, Eiger believes that dosing durations of at least 24
weeks may be necessary to clear HDV RNA and to achieve SVR for HDV infection. Eiger began enrolling patients at the Hannover Medical Center in the fourth quarter of 2015 and results are planned to be presented over the course of the study in 2016.
Potential for Registration in HDV for Lonafarnib
Eiger’s goal in developing lonafarnib is to reduce viral load in such a manner as to achieve clearance of the virus to SVR, the point where, upon
withdrawal of the therapy, the infection does not return. Evidence that academic investigators have gathered suggests that combinations of lonafarnib with other antiviral agents may hold promise for longer duration treatment and sustained, long-term
reduction of viral load.
Even if patients do not achieve SVR, Eiger believes that treatment with lonafarnib in combination with other antiviral agents
may contribute to long-term benefit for patients, which may represent an alternative path to regulatory approval. In a study published in Plos One in 2014 (Romeo, R. et al. “High Serum Levels of HDV RNA Are Predictors of Cirrhosis and Liver
Cancer in Patients with Chronic Hepatitis Delta,” Plos One, 2014; 9:1), high serum levels of HDV were found to be a predictor of cirrhosis and liver cancer development. In a study
169
published in Gastroenterology in 2004 (Farci, P. et al. “Long-Term Benefit of Interferon Therapy of Chronic Hepatitis D: Regression of Advanced Hepatic Fibrosis,” Gastroenterol, 2004;
126:1740), researchers demonstrated that lower frequencies of clinical events, leading to improvements in overall liver health and reductions in the rates of developing hepatic complications, could be achieved in HDV infected patients who were
treated with PEG-IFN-alpha and who experienced as little as 2 log declines in viral load. A 2014 Hepatology study by Heidrich suggests that transient suppression of HDV replication improves the clinical long-term outcome, as not a single patient in
their study with a posttreatment week 24 HDV RNA response experienced a clinical event. Eiger believes that these studies suggest that eradication of HDV RNA may not be necessary to achieve a substantial clinical benefit and improve long-term
outcomes. Lower doses of lonafarnib, alone and in combination with other antiviral agents, have demonstrated tolerability for longer duration and possibly chronic dosing.
Exendin (9-39) for Hyperinsulinemic Hypoglycemia Following Bariatric Surgery
Exendin (9-39) is the second most advanced product candidate in Eiger’s pipeline. Exendin (9-39) is a glucagon-like peptide-1, or GLP-1, receptor
antagonist. GLP-1 is a gastrointestinal hormone released after meals that binds to GLP-1 receptors, releasing insulin and lowering blood glucose levels. Exendin (9-39) blocks GLP-1 from binding to the GLP-1 receptor, preventing the steep fall in
glucose levels. Eiger is developing exendin (9-39) as a treatment for hyperinsulinemic hypoglycemia, which is low levels of glucose in the bloodstream caused by excess levels of insulin in the blood, associated with bariatric surgery, including
gastric bypass surgery. This form of hypoglycemia is a debilitating and potentially life-threatening condition. Gastric bypass procedures are widely performed and are increasing in frequency for medically complicated obesity, including obesity due
to Type 2 diabetes. There is no approved therapy for gastric bypass induced hypoglycemia and the unmet medical need is high.
Stanford University
researchers have demonstrated clinical proof of concept in 18 patients suffering from gastric bypass surgery induced hypoglycemia that exendin (9-39) can prevent an exaggerated fall in blood sugar following a meal, or post-prandial hypoglycemia, in
affected patients. Data has been generated using both intravenous delivery and a novel SQ formulation delivery. Pharmacokinetics indicate that the SQ formulation could enable once or twice a day pre-prandial dosing. Eiger plans to initiate a
Phase 2 dose-ranging trial in affected patients with Eiger’s exendin (9-39) SQ formulation in the second quarter of 2016.
Hyperinsulinemic Hypoglycemia Disease Overview
As the
use of bariatric surgical procedures has increased worldwide, a new post-surgical complication, hyperinsulinemic hypoglycemia, has been increasingly diagnosed and reported in the procedures that involve reducing the size of the stomach with a
vertical sleeve gastrectomy or by resecting and re-routing the small intestine to a small stomach pouch (Roux-en-Y). This disorder leads to frequent symptomatic hypoglycemia, often resulting in glucose concentrations low enough to cause seizures,
altered mental status, loss of consciousness, cognitive dysfunction, disability and death. Quality of life can be severely diminished, and many patients cannot care for themselves or others, work, drive, or be left alone. There is no approved
treatment for this condition. Severe cases have historically been surgically managed with near-total to total pancreatectomy, which results in insulin dependent diabetes and is associated with up to a 2-9% surgical mortality risk.
Research suggests that elevated GLP-1 may play an important role in hyperinsulinemic hypoglycemia in post-bariatric surgery patients. Surgically-altered
nutrient transit, such as bariatric surgery, causes enhanced secretion of GLP-1 leading to elevated insulin secretion. This effect may play a primary role in the early resolution of Type 2 diabetes after surgery. A number of synthetic agonist
analogs of GLP-1, or agonists, have been approved for the treatment of Type 2 diabetes including Byetta™ (exenatide), Victoza™ (liraglutide), and Trulicity™ (dulaglutide). These drugs, all agonists, bind to the GLP-1 receptor and
stimulate release of insulin. In patients with hyperinsulinemic hypoglycemia, an excess secretion of GLP-1 results in excess insulin release and leads to severe debilitating hypoglycemia. GLP-1 receptor antagonists have the potential to compete with
endogenous GLP-1 and prevent excess insulin release.
170
Approximately 150,000 to 200,000 bariatric surgical procedures are performed each year in the United States, and
another 125,000 are performed each year in Europe.
Eiger’s Solution: Exendin (9-39)
Exendin (9-39) is a well-characterized, competitive antagonist of GLP-1 at its receptor. Exendin (9-39) is a 31 amino acid fragment of exenatide, a
commercially available GLP-1 agonist, brand named Byetta™ and used in the treatment of type 2 diabetes. Exendin (9-39) blocks the GLP-1 receptor and leads to reduced levels of insulin secreted by the pancreas. While exenatide has been approved
for the treatment of type 2 diabetes, exendin (9-39), as a new molecular entity, has never been approved or commercialized for any indication.
Clinical Data to Date
Stanford University
researchers have demonstrated in two placebo-controlled clinical trials with exendin (9-39) that pharmacologic blockade of the GLP-1 receptor prevents hypoglycemia in affected patients, and Eiger believes that it may represent the first targeted
medical treatment for patients with post-bariatric hypoglycemia. In these two single-dose studies, exendin (9-39) was tolerated with no observed side-effects. These Phase 1b studies were conducted under an investigator-initiated IND for the study of
exendin (9-39) for bariatric surgery-induced hyperinsulinemic hypoglycemia at Stanford University.
The first clinical proof of concept study, a Phase 1b
clinical trial, was a double-blinded crossover study of eight patients with hyperinsulinemic hypoglycemia conducted at Stanford. Four patients were dosed with exendin (9-39) IV infusion and another four patients received a placebo infusion following
an oral glucose tolerance test, or OGTT. The trial assessed patient blood glucose levels and the rate of glucose rise and fall. Hypoglycemia was defined as glucose levels falling below 50 mg/dL. Physicians who treat hyperinsulinemic hypoglycemia
recognize that the steeper the glucose rise and fall, the worsening of all symptoms including cognitive function and dumping syndrome. After dosing was completed, patients were sent home for a 1-7 day washout period and then crossed over to either
placebo or exendin (9-39) until a total of 8 patients were treated with exendin (9-39).
171
Each of the patients treated with exendin (9-39) mirrored glucose levels of healthy patients, with none of
their glucose levels falling below 50 mg/dL. In contrast, every patient who received the placebo had a steep glucose fall, became hypoglycemic with glucose levels falling below 50 mg/dL, and had to be rescued with IV dextrose. The chart below shows
the mean glucose levels of eight patients in the above trial. Patients receiving exendin (9-39) and the placebo infusion completed severity-grade questionnaires every 30 minutes during the 180 minute OGTT
period. The severity-grade questionnaires showed that, on average, severe hyperinsulinemic hypoglycemic patients who received an IV infusion of exendin (9-39) experienced fewer and less severe hypoglycemic symptoms compared to the group receiving
the placebo infusion (p<0.001). While symptoms reported by subjects during the glucose rise (from T=0 to peak glucose) were unchanged by exendin (9-39) infusion, symptoms reported during the glucose fall period (from peak to nadir glucose) were
reduced (p<0.001).
Exendin (9-39) IV Infusion Study Results
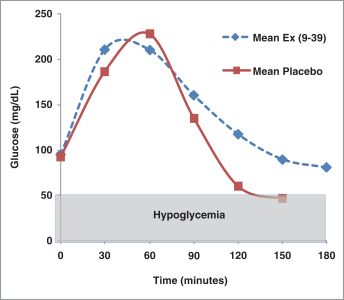
The second clinical proof of concept study, a Phase 2b clinical trial, was a single ascending dose, or SAD, study using
an SQ formulation in eight patients with hyperinsulinemic hypoglycemia. Patients were dosed with a novel immediate release SQ formulation of exendin (9-39) prior to an OGTT. The study assessed patient blood glucose levels as well as the rate of
glucose rise and fall across all doses. Eiger expects the results of this study will be published in the second quarter of 2016.
Eiger intends to
begin a Phase 2 clinical dose-ranging trial in the second half of 2016 using repeat dosing over multiple days with the immediate release SQ injection of exendin (9-39).
172
Ubenimex for Pulmonary Arterial Hypertension
Ubenimex is a well-characterized, oral, small-molecule inhibitor of leukotriene A4 hydrolase, or LTA4H, the enzyme responsible for converting leukotriene A4, or LTA4, to leukotriene B4, or LTB4. LTB4 is a naturally occurring molecule involved in inflammation. Ubenimex
has been marketed in Japan by Nippon Kayaku for over 25 years as an adjunct to chemotherapy agents to extend survival and to maintain remission after treatment for acute non-lymphocytic leukemia in adults.
Results of a preclinical study published in Science Translational Medicine (Tian, W. et al. “Blocking Macrophage Leukotriene B4 Prevents Endothelial
Injury and Reverses Pulmonary Hypertension,” Sci Transl Med, 2013; 5:1) by Stanford University researchers demonstrated that both LTB4 and LTA4H are elevated in animal models of PAH and human PAH disease. Macrophages, a type of white blood cell that ingests foreign materials, accumulate around small arterioles of the lungs and synthesize
excess LTB4. This causes programmed cell death, or apoptosis, of cells that line the interior surface of the pulmonary artery, pulmonary artery endothelial cells. Additionally, this causes
proliferation and an increase in volume, or hypertrophy, of pulmonary arterial smooth muscle cells. Elevated LTB4 causes inflammation resulting in blockage of the arteries, or arteriole
occlusion, and hypertension in animal models of PAH. Targeted pharmacologic inhibition of LTB4, including ubenimex, reversed PAH disease in all treated animals; obstructed arterioles opened,
cardiac function improved, and the animals survived. Eiger therefore believes that ubenimex is a potential therapeutic candidate for treatment of PAH where pathological inflammation is believed to be important in the etiology of the disease.
All currently approved agents for PAH were originally developed as vasodilators, drugs that dilate blood vessels. Inflammation is now recognized as a
primary component of PAH disease, which can lead to obstructed arterioles, vasoconstriction, and worsening cardiac function. Work published by Stanford researchers in Science Translational Medicine (Tian, W. et al. “Blocking Macrophage
Leukotriene B4 Prevents Endothelial Injury and Reverses Pulmonary Hypertension,” Sci Transl Med, 2013; 5:1) discusses a potentially novel therapeutic approach to PAH that may address the inflammatory component of PAH with the potential for
disease modification. Eiger plans to conduct a clinical trial to explore if blocking the effects of LTB4 may be a useful new treatment for PAH. Eiger has filed and received approval for a U.S.
IND and plans to begin enrolling a Phase 2 clinical trial in early 2016.
Pulmonary Arterial Hypertension Disease Overview
PAH is a type of high blood pressure that affects the arteries in the lungs and the right side of the heart. PAH begins when tiny arteries in the lungs, called
pulmonary arterioles, become narrowed, blocked or destroyed. This makes it harder for blood to flow through the lungs, and raises pressure within the arteries in the lungs. As the pressure builds, the heart’s lower right chamber, or right
ventricle, must work harder to pump blood through the lungs, causing the heart muscle to weaken and eventually fail. PAH is a progressive, life-threatening illness that meets criteria for orphan drug designation in the United States, European Union,
and Japan.
Current treatments for PAH
Initial treatments developed and used for PAH focus on reduction of hypertension with agents such as diuretics, calcium channel blockers, increasing cardiac
output with agents such as digoxin, and various anticoagulation therapies. These therapies are all generic agents. A number of therapies specifically approved for PAH, such as prostacyclin agonists, phosphodiesterase 5, or PDE5, inhibitors,
guanylate cyclase stimulators, and endothelin receptor antagonists, target mechanisms that induce vasodilation. These therapies together represent approximately a $4 billion annual market in the United States and Europe. Prostanoids such as
epoprostenol, treprostinil and iloprost are stable versions of vasodilators that are naturally produced by the body and help compensate for low levels of prostacyclin production in some patients. PDE5 inhibitors such as sildenafil and tadalafil also
work as vasodilators by increased signaling through the nitric oxide pathway. Other stimulators of this pathway include guanylate cyclase stimulators such as riociguat. Endothelin is a natural vasoconstrictor
173
which binds to the endothelin receptors to elicit vasoconstriction. Antagonists of the endothelin receptor such as ambrisentan, bosentan, and macitentan have been approved for the treatment of
PAH. Despite their premium pricing, these drugs are all considered to be palliative and not disease-modifying. Specifically, these drugs do not address the underlying causes of the disease, especially in PAH patients with connective tissue diseases,
or CTD, or PAH patients with inflammation, highlighting the need for novel therapeutic approaches. An estimated 30,000 PAH patients and 15,000 PAH patients receive pharmacologic therapy in the United States and Europe, respectively.
Preclinical LTB4 Data in PAH
In animal models of PAH, LTB4 was significantly elevated in both broncho-alveolar lavage fluid and in
serum, suggesting that LTB4 may play a key role in development of the pathology associated with PAH.
174
Significantly elevated levels of LTB4 were also observed
in serum from PAH patients, with the highest levels in patients with systemic sclerosis-related PAH, or SSc-PAH, identifying a link between the pathology of the animal model of PAH disease and human PAH disease.
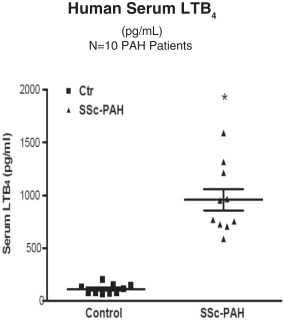
In animal models of PAH, right ventricular systolic pressure is greatly elevated and there is hypertrophy of the right
ventricle 21 days after induction of the disease. Treatment with ubenimex for 14 days reversed these effects, normalizing ventricular pressure and right ventricular size.
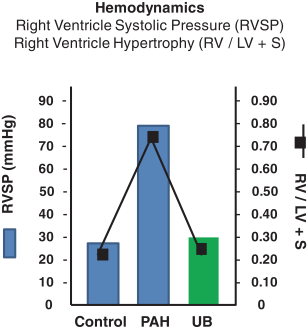
175
The activity of ubenimex in this PAH animal model is more pronounced when overall survival is examined. All of
the animals tested in this model that received ubenimex survived until at least day 35, whereas none of the untreated animals survived.
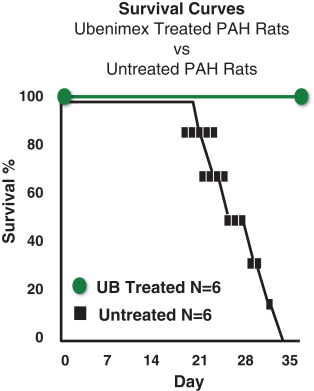
Improvements in pressures and survival with ubenimex were seen in three distinct animal models of PAH disease: SU5416
induced-PAH in athymic rats, SU5416-induced PAH in hypoxia, and monocrotaline-induced PAH. Activity of ubenimex in treated animals correlated with LTB4 levels in the model.
Eiger’s Planned Solution: Ubenimex for PAH
Ubenimex is a well-characterized, oral, small-molecule, dual-inhibitor of aminopeptidase and LTA4H, the
enzyme responsible for catalyzing the committed step in the formation of the proinflammatory mediator LTB4. Ubenimex is approved in Japan as an adjunct to chemotherapy agents to extend survival
and to maintain remission after treatment for acute non-lymphocytic leukemia in adults. Ubenimex has been used for over 25 years in Japan and remains commercially available through Nippon Kayaku. In December 2015, the FDA granted orphan drug
designation to ubenimex for the treatment of PAH. Ubenimex is not approved for any indication in the United States or Europe.
Clinical Plan
Eiger in-licensed ubenimex from Nippon Kayaku in 2015 and has relied on Nippon Kayaku’s prior Phase 1 clinical data and experience with ubenimex to
understand safety. Nippon Kayaku conducted four Phase 1 studies in healthy subjects and cancer patients to study metabolite determination, metabolism and excretion, drug absorption, and a pharmacokinetic study in lymphoma patients.
In the metabolite determination study, ubenimex was rapidly absorbed following oral administration of single doses ranging from 10 mg to 200 mg,
reaching a maximum serum level between 30 minutes and three hours after
176
dosing. Mean peak concentrations after 30 mg, 100 mg and 200 mg were 2.9 µg/ml at one hour, 2.5 µg/ml at three hours, and 7.4 µg/ml at two hours,
respectively.
In the metabolism and excretion study, 84% to 94% of the administered doses of ubenimex was recovered in urine within 24 hours of
dosing.
In the absorption study, prolonged administration of ubenimex to cancer patients showed rapid absorption of the drug and maximum peak levels
which ranged from 30 minutes to three hours in most patients. In a small study of eight patients receiving 30 mg of ubenimex daily, delayed a-phase decrease, an initial phase of rapid
decrease of concentration of the drug in the plasma, was observed in patients with renal cancer compared to patients with bladder cancer, suggesting that clearance of ubenimex may be slower in patients with impaired renal function. The
pharmacokinetics did not appear to change over time with repeated administration of ubenimex.
In a Phase 1b study performed in bone marrow transplant
lymphoma patients in the United States, PK evaluation was performed in groups of ten patients receiving 10 mg of ubenimex QD, 30 mg of ubenimex QD, 30 mg of ubenimex three times a day, or TID, or 60 mg of ubenimex TID, in each
case for up to 60 days. The mean AUC and Cmax increased with increasing doses of 10 mg, 30 mg, 90 mg or 180 mg ubenimex daily. At all doses, no accumulation was
apparent over the six days.
Eiger completed a pre-IND meeting at FDA where Eiger discussed both Phase 2 and Phase 3 clinical development plans for
ubenimex in patients with PAH. Eiger subsequently filed an IND with FDA which was approved to proceed. Eiger’s planned Phase 2 trial for ubenimex in PAH will be called LIBERTY (A Randomized, Double-BLInd, Placebo-Controlled Study of uBenimex in
PatiEnts with PulmonaRy ArTerial HYpertension) and is planned to enroll a total of approximately 45 patients with PAH in multiple centers. The trial will assess activity of ubenimex combined with standard of care treatment for PAH versus placebo
combined with standard of care treatment for PAH. The primary endpoint will be a measure of change in pulmonary vascular resistance, or PVR, with secondary endpoints based on hemodynamic changes and exercise tolerance tests, including a six minute
walk. Based on the proposed mechanism of action of ubenimex as a potential anti-proliferative, anti-inflammatory and disease modifying agent, dosing in the LIBERTY trial will be six months, which Eiger believes will be sufficient time to demonstrate
activity. Eiger plans to initiate enrollment in LIBERTY beginning in the first quarter of 2016.
Ubenimex for Lymphedema
A study conducted at Stanford University demonstrated that LTB4 is elevated in both animal models of
lymphedema and human lymphedema. Elevated LTB4 is associated with tissue inflammation and impaired lymphatic function. Targeted pharmacologic inhibition of LTB4 promotes physiologic lymphatic repair and reverses lymphedema disease in treated animals.
Researchers at Stanford demonstrated a novel function of LTB4 in the pathogenesis of lymphedema
suggesting that blocking the effects of LTB4 may be a promising and potentially safe new therapeutic strategy for this disease. Eiger intends to conduct a clinical study to explore if blocking
the effects of LTB4 may be useful as a new treatment for lymphedema.
Lymphedema Disease
Overview
Lymphedema is the build-up of fluid in soft body tissues when the lymph system has been damaged or blocked. It is characterized by
swelling due to abnormal transport of lymphatic fluid and thickening or hardening of the skin in affected areas. As fluid builds up, swelling occurs, usually in an arm or a leg, but can also affect other parts of the body. Lymphedema often causes
long-term physical, psychological and social problems for patients and significantly impacts quality of life. There are currently no approved pharmacological treatments for lymphedema and the unmet medical need is high.
177
Lymphedema can be either primary, meaning it is congenital or occurs on its own, or secondary, meaning it is
caused by another disease or condition. Primary lymphedema is caused by the absence of certain lymph vessels at birth or by abnormalities in the lymphatic vessels. It can be divided into three forms, depending on age of onset. The prevalence of
primary lymphedema is less than 200,000 in the United States and less than 5 in 10,000 in the European Union, and expected to be eligible for orphan drug designation by regulatory authorities. Secondary Lymphedema usually develops as a result of a
blockage or interruption that alters the flow of lymph through the lymphatic system and can develop from an infection, malignancy, surgery, scar tissue formation, trauma, radiation, or other cancer treatment.
Primary lymphedema and secondary lymphedema can both be debilitating disorders with negative impact on quality of life and a large unmet medical need exists
for an effective therapy. There is no approved pharmacologic treatment for Lymphedema. Available treatments include compression garments, massage and exercise. Several agents such as coumarin have been tested in investigator-initiated clinical
trials but have shown no clinical efficacy.
Preclinical LTB4 Data in Lymphedema
An animal model of lymphedema was used to mimic the physiological changes seen in lymphedema patients. In this model, acquired lymphedema was
surgically induced in the tails of mice through the ablation of lymphatic trunks. As the tail volume increases, there is an accumulation of fibroblasts, fat cells and skin cells in the tail, and poor clearance of immune cells from the tail. As
lymphedema is established in this model, the levels of LTB4 in serum rise significantly. For surgical controls (sham animals), skin incision alone was performed without lymphatic cautery.
Normal controls did not go under any surgical manipulation. When serum from human lymphedema patients was examined, the LTB4 levels were also significantly (p<0.0001) elevated compared to
normal controls (control n=17, lymphedema patients n=8).

178
In animal models, ubenimex significantly reduced tail volume (p<0.0001, sham n=13, saline n=14, ubenimex
n=14). Sham surgery (placebo surgery) is a faked surgical intervention that omits the step thought to be therapeutically necessary. In clinical trials of surgical interventions, sham surgery is an important scientific control. This is because it
isolates the specific effects of the treatment as opposed to the incidental effects caused by anesthesia, the incisional trauma, pre- and postoperative care, and the patient’s perception of having had a regular operation. Thus, sham surgery
serves an analogous purpose to placebo drugs, neutralizing biases such as the placebo effect.
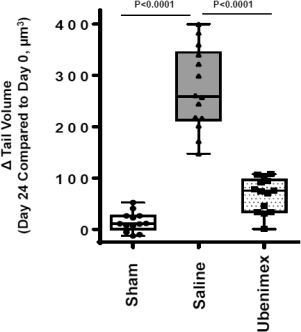
Ubenimex reversed lymphedema-induced tissue remodeling in animal models. Thickness of both the epidermis and dermis were
reduced.
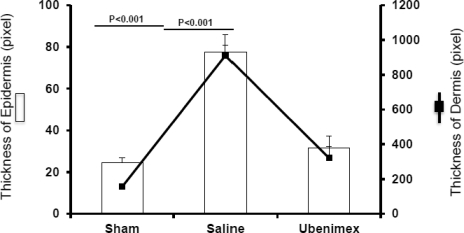
179
Eiger’s Planned Solution: Ubenimex for Lymphedema
Clinical Plan
Eiger in-licensed ubenimex from
Nippon Kayaku in 2015 and has relied on Nippon Kayaku’s prior Phase 1 clinical data and experience with ubenimex to understand safety. Eiger filed an additional IND for ubenimex in lymphedema with FDA in December 2015, which was approved to
proceed. Eiger’s Phase 2 clinical proof of concept trial for ubenimex in lymphedema will be called ULTRA (Ubenimex Lymphedema Trial to Restore Activity). The trial is expected to enroll approximately 40
patients at Stanford with a goal to assess activity of ubenimex versus placebo. The primary endpoint is planned to be a measure of change in skin fold thickness from baseline. Secondary endpoints are expected to include change in limb volume from
baseline and patient reported outcomes, including quality of life. Based on the proposed mechanism of action of ubenimex, as a potential anti-proliferative and a potential disease modifying agent, dosing in the planned trial is expected to be six
months, which Eiger believes represents sufficient time to demonstrate activity. Subject to FDA feedback, Eiger plans to initiate enrollment in in its Phase 2 clinical proof of concept trial in early 2016.
Manufacturing
Eiger currently contracts with third
parties for the manufacturing of all of its product candidates for preclinical and clinical studies and intends to do so in the future. Eiger does not own or operate manufacturing facilities for the production of clinical trial quantities of its
product candidates and has no plans to build its own clinical or commercial scale manufacturing capabilities. Eiger believes that the use of contracted manufacturing organization, or CMOs, eliminates the need for Eiger to directly invest in
manufacturing facilities and equipment and additional staff. Although Eiger relies on contract manufacturers, Eiger’s personnel and consultants have extensive manufacturing experience overseeing its CMOs.
To date, Eiger’s third-party manufacturers have met the manufacturing requirements for the product candidates. Eiger expects third-party manufacturers to
be capable of providing sufficient quantities of its product candidates to meet anticipated full scale commercial demands but has not assessed these capabilities beyond the supply of clinical material. Eiger plans to identify commercial contract
manufacturers as it moves its product candidates to Phase 3 clinical trials. Eiger believes there are alternate sources of manufacturing that could be identified and enabled to satisfy its clinical and commercial requirements, however Eiger cannot
be certain that identifying and establishing alternative relationships with such sources can be successful, cost effective, or completed on a timely basis without significant delay in the development or commercialization of its product candidates.
Lonafarnib
The drug product for lonafarnib
Phase 2 clinical studies for the treatment of HDV was manufactured by Merck. Merck is currently in the process of transferring 50 kilograms, or kg, of drug substance and the manufacturing technology for lonafarnib drug substance and drug product to
Eiger. Eiger believes that the 50 kg of drug substance are sufficient to provide drug product for Phase 3 pivotal studies. Eiger is in the process of selecting CMOs to manufacturer lonafarnib drug substance and drug product for future clinical
studies and commercial supply.
Ubenimex
Nippon Kayaku manufactures the drug substance and drug product for ubenimex Phase 2 clinical studies for the treatment of PAH and lymphedema. Upon completion
of Phase 2 clinical studies in PAH and lymphedema, Nippon Kayaku will transfer the manufacturing technology to Eiger and its CMOs, who will supply Phase 3 clinical trial materials and, if successful, commercial materials.
Exendin 9-39
The drug product for exendin (9-39)
for the treatment of hyperinsulinemic hypoglycemia for Phase 2 clinical studies are manufactured by a third party CMO.
180
Intellectual Property
Eiger strives to protect those proprietary technologies it believes are important to its business. Eiger seeks and maintains, where available, patent
protection for its product candidates including: composition of matter, method(s) of use, and process patents covering manufacture and/or formulation. Eiger has also licensed patents and patent applications that cover certain of its product
candidates and/or their manufacture, use, or formulation. Eiger also relies on regulatory exclusivity, including orphan drug designation and New Chemical Entity, or NCE, exclusivities, as well as trade secrets and carefully monitors its proprietary
information to protect all aspects of its business.
Eiger plans to continue to expand its intellectual property portfolio by filing patent applications
on new dosage forms, methods of treatment, and compositions of matter for its product candidates. Eiger files and prosecutes patent applications in the United States and Europe, and when appropriate, additional countries, including Japan, Korea and
China.
Eiger’s success will depend significantly upon its ability to: (i) obtain and maintain patents and other exclusivity protections for
commercially important technology, inventions and know-how related to its business; (ii) prosecute its patent applications to issue as patents and defend and enforce its patents; (iii) maintain its licenses to use intellectual property
owned by others; (iv) preserve the confidentiality of its trade secrets, and (v) operate without infringing the valid and enforceable patents and other proprietary rights of others. In addition to maintaining its existing proprietary
assets, Eiger seeks to strengthen its proprietary positions when economically reasonable to do so. Eiger’s ability to augment its proprietary position relies on its: (i) know-how; (ii) ability to access technological innovations, and
(iii) ability to in-license technology when appropriate.
The patent positions of pharmaceutical/biotechnology companies like Eiger are generally
uncertain and involve complex legal, scientific, and factual issues. In addition, the scope claimed in a patent application can be significantly reduced before any patent issues. After issuance of a patent application, if the issued patent is
challenged, then the courts can redefine the scope of the patent, including by invalidating it or rendering it unenforceable in its entirety. Consequently, Eiger does not know with certainty whether patents will issue in each country where it or its
licensor’s file patent applications, or if those patent applications, if ever issued, will issue with claims that cover Eiger’s product candidates, or, even if they do whether the patent or its relevant claims will remain enforceable upon
challenge. Accordingly, Eiger cannot predict with certainty whether the patent applications it is currently pursuing will issue as patents in a particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary
protection from potential competitors to make any of Eiger’s products commercially successful. Any of Eiger’s patents, including already issued in-licensed patents or any patents that may issue to Eiger or its licensors in the future could
potentially be challenged, narrowed, circumvented, or invalidated by third parties.
Because newly filed patent applications in the United States Patent
and Trademark Office, or the USPTO, and certain other patent offices are maintained in secrecy for a minimum of 18 months, and because publications of discoveries in the scientific or patent literature often lag far behind the actual discoveries
themselves, Eiger cannot be certain of the priority of its inventions covered by pending patent applications. Moreover, Eiger may have to participate in interference proceedings declared by the USPTO to determine priority of invention, although
patent applications filed after 2013 are given priority based on first to file in the U.S. The date of an invention is typically not publicly disclosed. Also, while Eiger is not currently participating in any interferences or post-grant challenge
proceedings, such as patent oppositions and patent litigation, that seek to invalidate the patentability of patents before or after they issue, respectively, Eiger may have to participate in such proceedings in the future. Such proceedings could
result in substantial cost, even if the eventual outcome is favorable to Eiger.
The term of individual patents depends upon the legal term of the patents
in the countries where they are issued. In most countries, the standard patent term for inventions relating to human drugs and their formulation and use is 20 years from the date of filing the first non-provisional patent or international
application under the Patent Cooperation Treaty of 1970, or the PCT.
181
Patent Protection of Eiger’s Product Candidates
Eiger’s product candidates and/or their uses in one or more indications of interest to Eiger are covered by in-licensed patents and patent applications
and by Eiger’s own patent applications.
Lonafarnib. Eiger has in-licensed from Merck a portfolio of patents that will
expire before the anticipated launch date of the lonafarnib product candidate. Eiger has a PCT application that claims the use of lonafarnib in combination with ritonavir and/or optionally other drugs for the treatment of HDV infection. Eiger
anticipates that this PCT will mature into patent applications in at the United States, the European Patent Office, or the EPO, and Japan. Any patents that issue from this PCT will expire in 2035, but a patent term extension (as described below) of
up to 5 years is available in the United States, and Eiger expects lonafarnib to be eligible for this additional protection. In addition, Eiger expects lonafarnib to be eligible for NCE and lonafarnib has been granted orphan drug designation by the
FDA and the EMA in this indication, which respectively provide five, seven and ten years of regulatory exclusivity. The PCT is an international patent law treaty that provides a unified procedure for filing patent applications to protect inventions
in each of its contracting states. Thus, a single PCT application can be converted into a patent application in any of the more than 145 PCT contracting states, and is considered a simple, cost-effective means for seeking patent protection in
numerous regions or countries. This nationalization (converting into an application in any of the contracting states) typically occurs 18 months after the PCT application filing date. An applicant must undertake prosecution within the allotted time
in each of the contracting states or on a regional basis if it determines to undertake patent issuance in protection in such country or territory. Eiger has not yet determined the countries in which it will pursue potential patent protection, but
even if Eiger determines to make such filings its efforts may not result in the issuance of one or more patents as a result of such filing. Even if no patents issue from this PCT, Eiger has two U.S. provisional applications, both of which were filed
in April 2015, relating to combination dose forms of lonafarnib and ritonavir.
Exendin (9-39). Eiger has in-licensed from
Stanford two U.S. provisional applications that claim the use of exendin (9-39) and other agents in the treatment of hyperinsulinemic hypoglycemia, including in post-bariatric surgery hypoglycemia. Eiger anticipates filing a PCT application in 2016
claiming priority to these two provisional applications. Any patents that issue from this PCT will expire in 2036, but patent term extension of up to 5 years is available in the United States, and Eiger expects exendin (9-39) to be eligible for this
additional protection. In addition, Eiger expects exendin (9-39) to be eligible for NCE and orphan drug designation exclusivity, which respectively provide five and seven years of regulatory exclusivity in the United States.
Ubenimex.
PAH. Eiger has in-licensed from Stanford an allowed U.S. patent application and a pending EPO application that claim the use of ubenimex
and other agents in the treatment of PAH; a continuation application of the allowed U.S. application (which Eiger expects to issue in 2016) will also be filed this year. An allowed U.S. application is a patent application which has been given a
Notice of Allowance from the Patent Office, indicating that it will issue as a patent upon payment of fees and satisfaction of other formal requirements. Eiger has also in-licensed from Nippon Kayaku the exclusive right outside Asia to access its
regulatory dossier for ubenimex, which Eiger believes to be a significant competitive advantage. The patent that issues from the allowed U.S. application (and from any patent that issues in the EPO or from any U.S. continuation) will expire in 2033,
but may be extended as described below.
Lymphedema. Eiger has in-licensed from Stanford a pending provisional U.S. application
that claims the use of ubenimex in the treatment of lymphedema. Eiger has also in-licensed from Nippon Kayaku the exclusive right outside Asia to access its regulatory dossier for ubenimex. Eiger anticipates filing a PCT application in 2016 claiming
priority to this and any other provisional applications Eiger may file in the interim. Any patents that issue from this PCT will expire in 2036, but may be extended as described below.
Regulatory Exclusivity and Patent Term Extension. If ubenimex is approved in any indication, it would be entitled to NCE exclusivity,
which would provide for five years of regulatory exclusivity for the treatment of
182
such indication. In addition, the FDA has granted orphan drug designation to ubenimex for the treatment of PAH and Eiger is seeking orphan drug designation for ubenimex for the treatment of
lymphedema. Orphan drug designation provides, or in the case of ubenimex for the treatment of lymphedema, if obtained, would provide seven years of regulatory exclusivity for each indication. However, patent term extension, as described below, will
potentially be available only for the first of the two indications, PAH and lymphedema, to be approved.
Patent Term
In the United States, the patent term for an FDA-approved drug may be eligible for a patent term extension, or a PTE. The Hatch-Waxman Act of 1984 permits
restoration of a portion of the patent term of a U.S. patent as compensation for the patent term lost during product development and the FDA regulatory review process if approval of the application for the product is the first permitted commercial
marketing of a drug or biological product containing the active ingredient. The length of the PTE is based on the length of time it takes for the drug to complete the pre-market regulatory approval requirements. A credit of 50% of the time spent in
development is credited up to a maximum five year extension and the PTE cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent per approved drug may be extended and a patent can
only be extended once; thus, even if a single patent is applicable to multiple products, it can only be extended based on one product. Similar provisions may be available in Europe and certain other foreign jurisdictions to extend the term of a
patent that covers an approved drug. In Europe, through the European Medicines Agency, there is a period of ten years of regulatory data exclusivity from the time of approval if the centralized procedure is used, however under the centralized
procedure this term would run concurrently with period of exclusivity provided by the patent. When possible, depending upon the length of clinical trials and other factors involved in the filing of NDAs for its products, Eiger expects to apply for
PTEs for patents covering its product candidates and their methods of use both in the United States and any foreign jurisdiction where available. There is no guarantee, however, that the applicable authorities will agree to grant extensions, and if
granted, what the length of those extensions will be.
Other Proprietary Rights and Processes
Eiger also relies on trade secret protection for some of its confidential and proprietary information. Although Eiger takes steps to protect its proprietary
information and trade secrets, including through contractual means with its employees and consultants, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to Eiger’s
trade secrets and disclose its technology. If these events happen, Eiger may not be able to meaningfully protect its trade secrets. It is Eiger’s policy to require its employees, consultants, outside scientific collaborators, sponsored
researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with Eiger. These agreements provide that all confidential information concerning Eiger’s business, scientific,
development or financial affairs that are either developed or made known to the individual during the course of the individual’s relationship with Eiger are to be kept confidential and not disclosed to third parties except in specific
circumstances. Eiger’s agreements with employees also provide that all inventions conceived by the employee in the course of employment with Eiger or based on the employee’s use of Eiger’s confidential information are Eiger’s
exclusive property or that Eiger has an exclusive royalty free license to use such technology.
Competition
The biopharmaceutical industry is highly competitive. Eiger faces competition from major pharmaceutical companies, specialty pharmaceutical companies and
biotechnology companies worldwide. Given the significant unmet medical need for novel therapies to treat chronic hepatitis delta infection, post-bariatric surgery-induced hyperinsulinemic hypoglycemia, PAH and lymphedema , these conditions are where
various treatments from many companies are used and where many public and private universities and research organizations are actively engaged in the discovery, research and development of product candidates. As a result, there are and will likely
continue to be extensive resources invested in the discovery and development of new products to treat these unmet medical needs. Eiger anticipates facing intense and increasing competition as new products enter the market and advanced technologies
become available.
183
In addition, there are numerous multinational pharmaceutical companies and large biotechnology companies
currently marketing or pursuing the development of products or product candidates targeting the same indications as Eiger’s product candidates. Many of Eiger’s competitors, either alone or with strategic partners, have or will have
substantially greater financial, technical and human resources than Eiger. Accordingly, Eiger’s competitors may be more successful than Eiger in developing or marketing products and technologies that are more effective, safer or less costly.
Additionally, Eiger’s competitors may obtain regulatory approval for their products more rapidly and may achieve more widespread market acceptance. Accelerated mergers and acquisitions activity in the biotechnology and pharmaceutical industries
may result in even more resources being concentrated among a smaller number of Eiger’s competitors. These companies also compete with Eiger in recruiting and retaining qualified scientific and management personnel, establishing clinical study
sites and patient registration for clinical studies and acquiring technologies complementary to, or necessary for, Eiger’s programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through
collaborative arrangements with large and established companies.
Eiger’s potential competitors and the related stage of development of their product
candidates in target indications is as follows:
| |
• |
|
Hepatitis delta virus: Replicor, Inc. (Phase 2), Hepatera Ltd (Phase 2) and Alnylam Pharmaceutical, Inc. (preclinical), Arbutus Biopharma (preclinical), Arrowhead Research Corporation (preclinical); |
| |
• |
|
Hyperinsulinemic hypoglycemia : Xoma Corporation (Phase 1); |
| |
• |
|
Pulmonary arterial hypertension: Gilead Sciences, Inc. (Phase 2), Reata Pharmaceuticals, Inc. (Phase 2), AADi LLC (Phase 1) and United Therapeutics Corporation (Phase 1), Arena Pharmaceuticals (Phase 2); and
|
| |
• |
|
Lymphedema: Novartis (Phase 2). |
There are other therapies that are used or may be used for Eiger’s
targeted indications, however Eiger does not believe that these therapies are potentially curative for Eiger’s targeted indications. For example, there are a number of therapies used for symptomatic relief of PAH such as calcium channel
blockers and diuretics as well as vasodilators. Other products in clinical development or marketed for other indications may be used in competition with Eiger’s product candidates if Eiger is able to identify potential market opportunities of
interest. For example, HDV has not been generally identified as a target for development compared to hepatitis B or hepatitis C, and products on the market or in development for those indications may potentially be tested in HDV as the understanding
of the potential medical need for therapies in this indication become more widely understood.
Eiger believes that the key competitive factors that will
affect the development and commercial success of its product candidates are efficacy, safety and tolerability profile, convenience in dosing, product labeling, cost-effectiveness, price, the level of generic competition and the availability of
reimbursement from the government and other third-parties. Eiger’s commercial opportunity could be reduced or eliminated for any of its products if its competitors have products that are approved earlier than Eiger’s product candidates or
are superior compared to Eiger’s product candidates or if Eiger’s product candidates do not result in an improvement in condition compared to those other products.
License and Asset Purchase Agreements
License
Agreement with Merck
In September 2010, Eiger entered a License Agreement with Merck Corporation (acquired by Merck & Co., Inc.), or
Merck, dated September 3, 2010, or the Merck License Agreement.
Under the Merck License Agreement, Merck granted Eiger an exclusive, worldwide
license to develop, manufacture, and sell products containing the compounds Sarasar™ (lonafarnib) for the treatment of all human viruses except certain specified viruses such as hepatitis B and hepatitis C alone.
184
Eiger is responsible for the manufacture, development, and commercialization of the product at its cost and
expense, and is obligated to use commercially reasonable efforts to develop and commercialize the product in the licensed field; provided however, that Merck agreed to provide sufficient product, free of charge, for Eiger to complete the proof of
concept trial.
Under the Merck License Agreement, Eiger provided Merck a $500,000 equity interest in EB Pharma, LLC, its wholly-owned subsidiary, which
has an obligation to make development milestone payments in aggregate of up to $27.0 million, and pay a tiered royalty, ranging from mid single to low double digits, based on increasing tiered levels of aggregate annual net sales of all licensed
products.
The Merck License will continue for so long as Eiger owes royalty payments to Merck under the agreement. Each party has the right to terminate
the Merck License Agreement for the other party’s uncured material breach or bankruptcy. Merck also has the right to terminate the agreement if Eiger discontinues development and commercialization of lonafarnib for a specified period of time.
In addition, Eiger has the right to terminate the agreement, with notice, for any reason.
Asset Purchase Agreement with Eiger Group International,
Inc.
In December 2010, Eiger entered into an Asset Purchase Agreement with Eiger Group International, Inc., or EGI, dated December 8, 2010,
or the EGI APA. Dr. Jeffrey Glenn is the sole owner of EGI.
Under the EGI APA, Eiger purchased all intellectual property rights regarding the use of
farnesyl transferase inhibitors as anti-viral agents and methods to treat viral infection with those inhibitors. Eiger also purchased all intellectual property rights regarding the use of inhibitors of prenylation, prenyl cysteine methyltransferase,
and a specified protease as anti-viral agents and methods to treat viral infection with those inhibitors. Eiger is obligated to use commercially reasonable efforts to develop and commercialize the licensed products in major markets.
Under the EGI APA, Eiger paid EGI an upfront payment of $350,000. Additionally, Eiger is obligated to pay EGI a low single-digit royalty based on aggregate
annual net sales of products developed using the intellectual property. Eiger’s obligation to pay EGI royalties on a product ends when that product is no longer sold in a country.
The term of the EGI APA extends until expiration of all payment obligations, and Eiger may terminate the agreement upon notice to EGI. EGI may terminate the
EGI APA if Eiger fails to use commercially reasonable efforts to develop and commercialize licensed products. In addition, each party may terminate the EGI APA for the other party’s uncured material breach or bankruptcy. In the event of any
termination, other than termination by Eiger for EGI’s breach, Eiger will assign the purchased assets back to EGI.
License Agreement with
Janssen Pharmaceutica NV
In December 2014, Eiger, through its wholly-owned subsidiary EB Pharma, LLC, or EBP, entered a License Agreement, or the
Janssen License Agreement, with Janssen Pharmaceutica NV, or Janssen, dated December 19, 2014.
Under the Janssen License Agreement, Janssen granted
Eiger an exclusive, worldwide, license to develop, manufacture, and sell products containing the compound tipifarnib for all therapeutic and diagnostic uses in humans, including any such uses for human virology diseases, but excluding oncology
diseases.
Eiger is responsible for the development of at least one product in a major market country and for commercialization of products in all
countries where necessary authorization is obtained, both at Eiger’s cost and expense. Eiger may manufacture, develop, and commercialize the products itself or it may grant one or more sublicenses for such purposes. However, for a period of
time following completion of the proof of concept trial, Janssen has a first right of negotiation for an exclusive license back from Eiger to develop and commercialize tipifarnib in any country in the world.
185
Under the Janssen License Agreement, Eiger is obligated to make development milestone payments in aggregate of up
to $38.0 million, sales milestone payments in aggregate of up to $65.8 million, and pay a tiered royalty, ranging from the mid single to low double digits, based on aggregate annual net sales of all licensed products. If Eiger grants a sublicense,
Eiger is obligated to pay Janssen a portion of the sublicensing income received.
The Janssen License Agreement will continue for so long as Eiger owes
royalty payments to Janssen under the agreement or for so long as there is a valid patent claim under the agreement, whichever is longer. Both parties have the right to terminate the agreement for the other party’s uncured material breach of
the agreement or for the other party’s bankruptcy. Janssen also has the right to terminate the agreement if Eiger fails to meet certain specified diligence obligations. In addition, Eiger has the right to terminate the agreement without cause
at any time.
License Agreement with Nippon Kayaku Co., Ltd.
In May 2015, Eiccose, LLC, or Eiccose, and Nippon Kayaku Co., Ltd, or NK, entered into a License Agreement, or NK License, dated May 1, 2015 pursuant to
which NK granted Eiccose an exclusive license to develop, manufacture, and sell ubenimex outside certain identified Asia countries, including Japan, for the treatment of PAH and other inflammatory disease involving leukotriene B4. Eiccose assigned
the NK License to Eiger as part of the Eiccose asset purchase described below.
Under the NK License, Eiger is responsible for the development and
commercialization of ubenimex in its territory at its cost and expense. Eiger will purchase ubenimex for development and commercial use from NK at agreed transfer prices under a separate supply agreement, but Eiger has the option to manufacture and
supply the product for Phase 3 studies and/or commercial use. If Eiger exercises the manufacturing option, NK will transfer the manufacture of the product to Eiger or Eiger’s contract manufacturer, at Eiger’s cost and expense, and Eiger
will pay NK a running, mid, single-digit royalty on the net sales of ubenimex sold in Eiger’s territory or, if the parties agree, a lump-sum payment, for the use of NK’s manufacturing know-how.
Under the NK License, Eiger also granted back to NK an exclusive license to develop, manufacture, and sell ubenimex for the treatment of PAH and other
inflammatory disease involving leukotriene B4 in the Asia countries comprising the NK territory. NK is responsible for the development and commercialization of ubenimex in the licensed indications in its territory at its own cost and expense. NK
will pay Eiger a running, mid, single-digit royalty on net sales of ubenimex in the specified indications in NK’s territory.
The NK License
Agreement will continue for so long as the parties and their sublicensees continue to develop and commercialize ubenimex for the treatment of PAH and other inflammatory disease involving leukotriene B4. Both parties have the right to terminate the
agreement for the other party’s uncured material breach, and NK also has the right to terminate the agreement if Eiger fails to meet certain specified diligence obligations. In addition, the parties may terminate the agreement if further
development of the product is commercially, financially, or otherwise not advisable.
Asset Purchase Agreement with Tracey McLaughlin and Colleen
Craig
In September 2015, Eiger entered into an Asset Purchase Agreement with two individuals, Drs. Tracey McLaughlin and Colleen Craig, or the
Sellers, dated September 25, 2015, or the Exendin APA.
Under the Exendin APA, Eiger purchased all intellectual property rights from the Sellers,
including an assignment of a license agreement with Stanford which covered exclusive rights with respect to the compound exendin. Under the assigned Stanford exclusive license agreement, Eiger is obligated to pay Stanford a low, single-digit royalty
on net sales.
Under the Exendin APA, Eiger is obligated to pay to each of the Sellers milestone payments in aggregate up to $1.0 million and a low,
single-digit royalty based on aggregate annual net sales of all products developed based on exendin. Eiger also agreed to retain each of the Sellers pursuant to a consulting agreement with a term of one year, subject to annual renewal.
186
Asset Purchase Agreement with Eiccose, LLC
In October 2015, Eiger entered into an Asset Purchase Agreement, or the Eiccose APA, with Eiccose. David Cory, the President, Chief Executive Officer and a
director of Eiger, is a managing member and significant equity interest holder of Eiccose.
Under the Eiccose APA, Eiger purchased all intellectual
property rights with respect to ubenimex from Eiccose. Specifically, under the Eiccose APA, Eiccose assigned to Eiger the exclusive license agreement regarding ubenimex between Eiccose and Nippon Kayaku Co., Ltd., or the NK License. Eiger also
purchased intellectual property rights related to Stanford Docket S11-438—Pulmonary Arterial Hypertension and Stanford Docket S14-323—Lymphedema, and Eiccose assigned to Eiger the exclusive license agreements between Eiccose and the Board
of Trustees of the Leland Stanford Junior University regarding these two Stanford Dockets, or the Stanford Licenses.
Under the Eiccose APA, Eiger also
paid to Eiccose a total of $119,673, representing reimbursement of certain specified expenses, including payments and accrued amounts owed under the Stanford Licenses for previously incurred patent expenses and costs related to the negotiation and
assignment of the Stanford Licenses. Under the terms of the Eiccose APA, at the closing of the next round of financing pursuant to which Eiger sells shares of its preferred stock (or if there is no preferred stock, then common stock) resulting in
gross proceeds to Eiger of at least $25.0 million, Eiger committed to issue to Eiccose the number of fully vested shares of Eiger’s common stock equal to 1.75% of the total number of Eiger’s outstanding capital stock following the first
closing of such financing, which is expected to be issued upon the closing of the merger. Under the terms of the Eiccose APA, Eiger is further required to pay Eiccose milestone payments totaling up to $10.0 million after achievement of specified
milestones. Eiger is also required to pay Eiccose royalties at a rate in the low single digits based on the net sales of the first pharmaceutical product sold to an independent third party that contains or uses ubenimex.
Exclusive Agreement with the Board of Trustees of the Leland Stanford Junior University—Lymphedema
In October 2015, as part of the assets Eiger purchased from Eiccose, Eiger acquired and was assigned an Exclusive Agreement, or the Stanford Lymphedema
Agreement, between Eiccose and the Board of Trustees of the Leland Stanford Junior University, or Stanford, dated October 27, 2015.
Under the
Stanford Lymphedema Agreement, Stanford granted Eiger an exclusive, worldwide license under specified patent rights related to the treatment of lymphedema, to manufacture, use, and sell products covered by the licensed patents for all uses.
Eiger is responsible for the development and commercialization of any products under the license at its cost and expense, and is obligated to use commercially
reasonable efforts to achieve certain specified milestones. In consideration of the license, Eiger paid to Stanford a low, single-digit equity interest and is obligated to make milestone payments in aggregate of up to $1.5 million as well as a low,
single-digit royalty on net sales of any products.
Stanford may terminate the agreement for Eiger’s uncured material breach or bankruptcy. Stanford
also has the right to terminate the agreement if Eiger fails to develop and commercialize products in accordance with certain specified diligence obligations. Eiger has the right to terminate the agreement without cause at any time.
Exclusive Agreement with the Board of Trustees of the Leland Stanford Junior University—PAH
In October 2015, as part of the assets Eiger purchased from Eiccose, Eiger also acquired an Exclusive Agreement between Eiccose and Stanford, dated May 1,
2015, or the Stanford PAH Agreement.
187
Under the Stanford PAH Agreement, Stanford granted Eiger an exclusive, worldwide license under specified patent
rights related to the treatment of PAH and improved right ventricle function, to manufacture, use, and sell products covered by the licensed patents for all uses. Stanford and other non-profit research institutions retain the right to practice under
the licensed patents for any non-profit purpose.
Eiger is responsible for the development and commercialization of the products at its cost and expense,
and is obligated to use commercially reasonable efforts to achieve certain specified milestones. Eiger may satisfy these requirements itself, through its affiliates, or through granting one or more sublicenses. Eiger is obligated to give Stanford a
low, single-digit equity interest, make milestone payments in aggregate of up to $1.5 million, and pay a low, single-digit royalty on net sales of the products. If Eiger grants a sublicense, it will pay Stanford a portion of the sublicensing income
received.
Stanford may terminate the agreement for Eiger’s uncured material breach or bankruptcy. Stanford also has the right to terminate the
agreement if Eiger fails to develop and commercialize products in accordance with certain specified diligence obligations. Eiger has the right to terminate the agreement, with notice, for any reason.
Government Regulations and Product Approvals
Government
authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, packaging, storage, recordkeeping, labeling, advertising,
promotion, distribution, marketing, import and export of pharmaceutical products such as those Eiger is developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with
applicable statutes and regulations, require the expenditure of substantial time and financial resources.
FDA Approval Process
All of Eiger’s current product candidates are subject to regulation in the United States by the FDA under the Federal Food, Drug, and Cosmetic Act, or FDC
Act, and its implementing regulations. The FDA subjects drugs to extensive pre and post market regulation. Failure to comply with the FDC Act and other federal and state statutes and regulations may subject a company to a variety of administrative
or judicial sanctions, such as FDA refusal to approve pending NDAs, withdrawal of approvals, clinical holds, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil
penalties or criminal penalties.
FDA approval is required before any new unapproved drug or dosage form, including a new use of a previously approved
drug, can be marketed in the United States. The process required by the FDA before a new drug may be marketed in the United States is long, expensive, and inherently uncertain. Drug development in the United States typically involves completion of
preclinical laboratory and animal tests, submission to the FDA of an Investigational New Drug application, or IND, which must become effective before clinical testing may commence, approval by an independent institutional review board, or IRB, at
each clinical site before each trial may be initiated, performance of adequate and well controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought, submission to the FDA of
an NDA, satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced, and FDA review and approval of the NDA. Developing the data to satisfy FDA pre-market approval requirements typically
takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product, disease or indication.
Preclinical tests include laboratory evaluation of the product’s chemistry, formulation, and toxicity, as well as animal studies to characterize and
assess the potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practice, or GLP, regulations. These preclinical results are submitted to
the FDA as part of an IND along with other
188
information, including information about the product’s chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long term preclinical studies including reproductive
toxicity and carcinogenicity may be initiated or continue after the IND is submitted.
An IND must become effective before United States clinical trials
may begin. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the IND automatically
becomes effective and the clinical trial proposed in the IND may begin. If the FDA does raise any concerns or questions and places the clinical trial on a clinical hold, the IND sponsor and the FDA must resolve any outstanding concerns before the
clinical trial can begin. As a result, a submission of an IND may not result in FDA authorization to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product
development.
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of a qualified
investigator. Clinical trials must be conducted: (i) in compliance with federal regulations, including good clinical practice, or GCP, requirements for conducting, monitoring, recording and reporting the results of clinical trials, in order to
ensure that the data and results are scientifically credible and accurate and that the trial subjects are adequately informed of the potential risks of participating in clinical trials; and (ii) with protocols that detail, among other things,
the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of
the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that
the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be
submitted to and approved by an IRB at each study site before the study commences at that site and the IRB must monitor the clinical trial until it is completed. An IRB may also require the clinical trial at that site to be halted, either
temporarily or permanently, for failure to comply with the IRB’s requirements or if the drug candidate has been associated with unexpected serious harm to patients, or the IRB may impose other conditions. The study sponsor or the FDA may also
suspend or discontinue a clinical trial at any time on various grounds, including a determination that the subjects are being exposed to an unacceptable health risk.
Clinical trials to support an NDA for marketing approval are typically conducted in three sequential phases, although there is leeway to overlap or combine
these phases.
| |
• |
|
Phase 1. The drug candidate is initially introduced into healthy human subjects or patients with the target disease or condition, and is tested to assess safety, dosage tolerance,
pharmacokinetics and pharmacological activity, and, when possible, to ascertain evidence of efficacy. The drug candidate may also be tested in patients with severe or life-threatening diseases to gain an early indication of its effectiveness.
|
| |
• |
|
Phase 2. The trials are conducted using a limited patient population for the purposes of preliminarily determining the effectiveness of the drug in that particular indication, ascertaining
dosage tolerance, discerning the optimal dosage, and identifying possible adverse effects and safety risks. |
| |
• |
|
Phase 3. If a compound demonstrates evidence of efficacy and has an acceptable safety profile in the Phase 2 clinical trials, then Phase 3 clinical trials are undertaken to obtain additional
information from an expanded and diverse patient population, at multiple, geographically dispersed clinical trial sites, in randomized controlled studies often with a double-blind design to maximize the reproducibility of the study
results. Typically, a minimum of two positive Phase 3 clinical trials are submitted to support the product’s marketing application. These Phase 3 clinical trials are intended to provide sufficient data demonstrating evidence of the efficacy and
safety of the drug such that the FDA can evaluate the overall benefit-risk of the drug and provide adequate information for the labeling and package insert for the drug. Trials conducted outside of the United States under similar, GCP-compliant
conditions in accordance with local applicable laws may also be acceptable to FDA in support of product approval. |
189
Sponsors of clinical trials for investigational drugs must publicly disclose certain clinical trial information,
including detailed trial design. These requirements are subject to specific timelines and apply to most Phase 3 clinical trials of FDA-regulated products.
In some cases, FDA may condition approval of an NDA for a product candidate on the sponsor’s agreement to conduct additional clinical trials after NDA
approval. In other cases, a sponsor may voluntarily conduct additional clinical trials post approval to gain more information about the drug. Such post approval trials are typically referred to as Phase 4 clinical trials.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events
occur. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a
trial may move forward at designated check points based on access to certain data from the study. Phase 1, Phase 2, Phase 3 and Phase 4 clinical trials may not be completed successfully within any specified period, or at all.
Concurrent with clinical trials, companies usually finalize a process for manufacturing the drug in commercial quantities in accordance with current good
manufacturing practice, or cGMP, requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and, among other things, the manufacturer must develop methods for testing the identity,
strength, quality and purity of the final drug product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over
its shelf life.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA requesting approval to market the drug for
one or more specified indications. FDA review and approval of the NDA is required before marketing of the product may begin in the United States. The NDA must include the results of all preclinical, clinical, and other testing, including negative or
ambiguous results as well as positive findings, together with other detailed information including compilation of data relating to the product’s pharmacology, chemistry, manufacture, and controls. The NDA must also contain extensive
manufacturing information. The FDA reviews an NDA to determine, among other things, whether a drug is safe and effective for its intended use. The cost of preparing and submitting an NDA is substantial. Under federal law, the submission of most NDAs
is subject to both a substantial application user fee and annual product and establishment user fees. The sum of these fees may total several million dollars and they are typically increased annually.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold
determination that the application is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional
information. Once the submission is accepted for filing, the FDA begins an in-depth review.
Under the Prescription Drug User Fee Act, or PDUFA,
guidelines that are currently in effect, the FDA has agreed to certain performance goals in the review of NDAs. Standard NDAs are generally reviewed within ten months of filing, or twelve months from submission. Although FDA often meets its user fee
performance goals, the FDA can extend these timelines if necessary, and FDA review may not occur on a timely basis. The FDA usually refers applications for novel drugs, or drugs that present difficult questions of safety or efficacy, to an advisory
committee—a panel of independent experts, typically including clinicians and other scientific experts—for review, evaluation, and a recommendation as to whether the application should be approved and under what conditions. The FDA is not
bound by the recommendation of the advisory committee, but it generally follows its recommendations. Before approving an NDA, the FDA will typically inspect one, or more, clinical sites to assure compliance with GCP. Additionally, the FDA will
inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve an application unless it verifies that compliance with cGMP requirements is satisfactory and that the manufacturing processes and facilities are
adequate to assure consistent
190
production of the product within required specifications. The FDA will not approve a drug unless the application contains data showing substantial evidence that it is safe and effective in the
indication studied.
After the FDA evaluates the NDA and conducts its inspections, it issues either an approval letter or a complete response letter. A
complete response letter generally outlines the deficiencies contained in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application, including potentially significant, expensive
and time-consuming requirements related to clinical trials, nonclinical studies or manufacturing. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials are
not always conclusive, and the FDA may interpret data differently than Eiger does. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the application, the FDA will typically issue an approval
letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of additional information requested. FDA approval is never guaranteed. The FDA may refuse to approve an NDA if applicable regulatory criteria
are not satisfied.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. The
approval for a drug may be significantly more limited than requested in the application, including limitations on the specific diseases and dosages or the indications for use, which could restrict the commercial value of the product. The FDA may
also require that certain contraindications, warnings, or precautions be included in the product’s package insert, or labeling.
In addition, as a
condition of approval, the FDA may require a risk evaluation and mitigation strategy, or REMS, to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guidelines, communication plans for healthcare
professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing-including dispensing only under certain circumstances, special monitoring, and the
use of patient registries. The requirement for a REMS or use of a companion diagnostic with a drug can materially affect the potential market and profitability of the drug. Moreover, product approval may require, as a condition of approval,
substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. The FDA may also condition approval on, among other things, changes to proposed labeling or development of adequate controls and specifications.
Once granted, product approvals may be withdrawn if compliance with regulatory standards are not maintained or problems are identified following initial
marketing. In addition, after approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
Orphan Drugs
Under the Orphan Drug Act, the FDA
may grant an orphan drug designation to products intended to treat a rare disease or condition—generally one that affects fewer than 200,000 individuals in the United States. Orphan drug designation must be requested before submitting the NDA.
After the FDA grants orphan drug designation, the FDA publicly discloses the drug’s identity and its intended orphan use. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval
process. The first NCE to be approved to treat a disease with FDA’s orphan drug designation is entitled to a seven-year period of marketing exclusivity in the United States for that product, for that indication. During the seven-year
exclusivity period, the FDA may not approve any other applications to market the same drug for the same orphan indication, regardless of patent status, except in limited circumstances, such as a showing of clinical superiority to the product with
orphan exclusivity or if the FDA finds that the holder of the orphan exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or
191
condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different chemical/biological entity for the same disease or condition. An orphan
drug designation also does not preclude the same drug from being developed for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research expenses and a waiver of the NDA application
user fee.
Advertising and Promotion
Drugs
manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing post-approval regulatory requirements. For instance, the FDA closely regulates the post-approval marketing, labeling, advertising and promotion of drugs,
including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the Internet. Failure to comply with these requirements can
result in adverse publicity as well as significant penalties, including the issuance of warning letters directing a company to correct any deviations from the FDA’s standards. The FDA may also impose a requirement that future advertising and
promotional materials be pre-cleared by the FDA, and the company may face federal and/or state civil and criminal investigations and prosecutions.
Drugs
may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing
processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application,
and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs. Obtaining new indication is an important part of managing the life cycle of the drug.
Adverse Event Reporting and cGMP Compliance
Recordkeeping, adverse event reporting and the submission of periodic reports are required following the FDA’s approval of an NDA. The FDA also may
require post-marketing testing or Phase 4 clinical trials, REMS, or surveillance to monitor the effects of an approved drug. In addition, the FDA may place conditions on an approval that could restrict the distribution or use of the product.
Furthermore, manufacture, packaging, labeling, storage and distribution procedures must continue to conform to cGMPs after approval. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and
certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies to assess compliance with ongoing regulatory requirements, including cGMPs. Changes to the manufacturing process are strictly regulated
and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP requirements and impose reporting and documentation requirements upon the sponsor and any
third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality control to maintain compliance with cGMPs. Failure to comply with the
statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil penalties. Eiger cannot be certain
that it or its present or future third-party manufacturers or suppliers will be able to comply with the cGMP regulations and other ongoing FDA regulatory requirements. If Eiger or its present or future third-party manufacturers or suppliers are not
able to comply with these requirements, the FDA may halt Eiger’s clinical trials, require Eiger to recall a drug from distribution or withdraw approval of the NDA for that drug. Regulatory authorities may also withdraw product approvals,
request product recalls, or impose marketing restrictions through labeling changes or product removals upon discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with
manufacturing processes.
192
Companion Diagnostics
In vitro diagnostics, or IVDs, are a type of medical device that are intended to detect diseases, conditions, infections, biomarkers, or the presence of
specific genetic alleles. If the safe and effective use of a drug requires an IVD, the FDA generally will demand clearance or approval of the companion diagnostic at the same time they approve the therapeutic. The FDA has required in vitro
companion diagnostics to obtain premarket clearance or approval simultaneously with drug approval if the diagnostic is intended to identify those patients most likely to respond to drug treatment. Accordingly, a required companion diagnostic has
the potential to delay approval of the drug.
In the United States, the FDC Act and its implementing regulations, and other federal and state statutes and
regulations govern, among other things, the design and development, preclinical and clinical testing, premarket clearance or approval, registration and listing, manufacturing, labeling, storage, advertising and promotion, sales and distribution,
export and import, and post-market surveillance of medical devices, including IVDs. Unless an exemption applies, diagnostic tests require marketing clearance or approval from the FDA prior to commercial distribution. The two primary types of FDA
marketing authorization applicable to a medical device are premarket notification, also called 510(k) clearance, and approval of a premarket approval application, or PMA. The FDA classifies all medical devices into one of three classes. Devices
deemed to pose lower risk are categorized as either Class I or II, which requires the manufacturer to submit to the FDA a 510(k) pre-market notification requesting clearance of the device for commercial distribution in the United States, unless an
exemption applies. Devices deemed by the FDA to pose the greatest risk, such as life sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously 510(k)-cleared device are categorized as Class
III, requiring a PMA.
To obtain 510(k) clearance for a medical device, a pre-market notification must be submitted to the FDA demonstrating that the
proposed device is substantially equivalent to a previously 510(k)-cleared device or a device that was in commercial distribution before May 28, 1976 for which FDA has not yet called for the submission of a PMA, or the device must be one that
has been reclassified from Class III to either Class II or I. The 510(k) clearance process usually takes from three to twelve months from the date the application is submitted and filed with the FDA, but may take significantly longer and clearance
is never assured. Although many 510(k) pre-market notifications are cleared without clinical data, in some cases, the FDA requires significant clinical data to support substantial equivalence. In reviewing a pre-market notification, the FDA may
request additional information, including clinical data, which may significantly prolong the review process. After a device receives 510(k) clearance, any subsequent modification of the device that could significantly affect its safety or
effectiveness, or that would constitute a major change in its intended use, will require a new 510(k) clearance or could require pre-market approval. The FDA requires each manufacturer to make this determination initially, but the FDA may review any
such decision and may disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA may require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance
or a PMA is obtained.
PMAs must be supported by valid scientific evidence, which typically requires extensive data, including technical, preclinical,
clinical and manufacturing data, to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device. For diagnostic tests, a PMA typically includes data regarding analytical and clinical validation studies. As part of its
review of the PMA, the FDA will conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the Quality System Regulation, or QSR, which requires manufacturers to follow design, testing, control,
documentation and other quality assurance procedures. FDA review of an initial PMA is required by statute to take between six to ten months, although the process typically takes longer, and may require several years to complete. If the FDA
evaluations of both the PMA and the manufacturing facilities are favorable, the FDA will either issue an approval letter or an approvable letter, which usually contains a number of conditions that must be met in order to secure the final approval of
the PMA. If the FDA’s evaluation of the PMA or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. A not approvable letter will outline the deficiencies in the application
193
and, where practical, will identify what is necessary to make the PMA approvable. The FDA may also determine that additional clinical trials are necessary, in which case the PMA approval may be
delayed for several months or years while the trials are conducted and then the data submitted in an amendment to the PMA. Once granted, PMA approval may be withdrawn by the FDA if compliance with post approval requirements, conditions of approval
or other regulatory standards is not maintained or problems are identified following initial marketing.
Other Healthcare Laws and Compliance
Requirements
In the United States, Eiger’s activities are potentially subject to regulation by federal, state, and local authorities in
addition to the FDA. These other agencies include, without limitation, the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice and individual U.S. Attorney
offices within the Department of Justice, as well as state and local governments. Such agencies enforce a variety of laws, including without limitation, anti-kickback and false claims laws, data privacy and security laws, and physician payment
transparency laws.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any
remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any good, facility, item
or service reimbursable, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value. The Anti-Kickback Statute has been interpreted
to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common
activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not
qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality
of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement
involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated.
Additionally, the
intent standard under the Anti-Kickback Statute was amended by the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or the ACA, to a stricter standard such that a person or
entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, ACA codified case law that a claim including items or services resulting from a violation of the
federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil False Claims
Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to or approval by the federal government or knowingly making, using or causing to be made or used
a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. Several pharmaceutical and other healthcare
companies have been prosecuted under these laws for, among other things, allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for
causing false claims to be submitted because of the companies’ marketing of products for unapproved, and thus non-reimbursable, uses. In addition, the civil monetary penalties statute imposes penalties against any person or entity that, among
other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
194
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created new federal criminal
statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material
fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the Anti-Kickback Statute, ACA broadened the reach of certain criminal healthcare
fraud statutes created under HIPAA by amending the intent requirement such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Also, many states have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items
and services reimbursed under Medicaid and other state programs.
Eiger may be subject to data privacy and security regulation by both the federal
government and the states in which Eiger conducts its business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, including the final Omnibus Rule
published on January 25, 2013, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s security standards directly
applicable to business associates, defined as service providers of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also
created four new tiers of civil monetary penalties and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated
with pursuing federal civil actions. In addition, many state laws govern the privacy and security of health information in certain circumstances, many of which differ from HIPAA and each other in significant ways and may not have the same effect,
thus complicating compliance efforts.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians
and other healthcare providers. ACA imposed, among other things, new annual reporting requirements for covered manufacturers for certain payments and “transfers of value” provided to physicians and teaching hospitals, as well as ownership
and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interests may result in
civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for “knowing failures.” Covered manufacturers were required to begin collecting data on August 1, 2013 and submit
reports on aggregate payment data to the government for the first reporting period (August 1, 2013—December 31, 2013) by March 31, 2014, and were required to report detailed payment data for the first reporting period and submit legal
attestation to the completeness and accuracy of such data by June 30, 2014. Thereafter, covered manufacturers must submit reports by the 90th day of each subsequent calendar year. In addition, certain states require implementation of commercial
compliance programs and compliance with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, impose restrictions on marketing practices, and/or tracking and
reporting of gifts, compensation and other remuneration or items of value provided to physicians and other healthcare professionals and entities.
If
Eiger’s operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to it, Eiger may be subject to penalties, including potentially significant criminal, civil and/or administrative
penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of
its operations, any of which could adversely affect Eiger’s ability to operate its business and its results of operations.
195
International Regulation
In addition to regulations in the United States, a variety of foreign regulations govern clinical trials, commercial sales, and distribution of drugs. Whether
or not Eiger obtains FDA approval for a drug, Eiger or its collaborators must obtain approval of the drug by the comparable regulatory authorities of foreign countries before commencing clinical trials or marketing of the drug in those countries.
The approval process varies from country to country and the time to approve may be longer or shorter than that required for FDA approval. Further, to the extent that any of Eiger’s products are sold in a foreign country, Eiger may be subject to
additional foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws and implementation of corporate compliance programs and reporting of payments
or transfers of value to healthcare professionals.
Pharmaceutical Coverage, Pricing and Reimbursement
In the United States and other countries, patients who are prescribed treatments for their conditions and providers performing the prescribed services
generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use Eiger’s products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost
of its products. Sales of any products for which Eiger receives regulatory approval for commercial sale will therefore depend in part on the availability of reimbursement from third-party payors, including government health administrative
authorities, managed care providers, private health insurers, and other organizations.
The process for determining whether a third-party payor will
provide coverage for a drug product typically is separate from the process for setting the price of a drug product or for establishing the reimbursement rate that the payor will pay for the drug product once coverage is approved. Third-party payors
may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all of the FDA-approved drugs for a particular indication. A decision by a third-party payor not to cover Eiger’s product
candidates could reduce physician utilization of Eiger’s products once approved and have a material adverse effect on Eiger’s sales, results of operations and financial condition. Moreover, a third-party payor’s decision to provide
coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable Eiger to maintain price levels sufficient to realize an appropriate return on
Eiger’s investment in product development. Additionally, coverage and reimbursement for drug products can differ significantly from payor to payor. One third-party payor’s decision to cover a particular medical product or service does not
ensure that other payors will also provide coverage for the medical product or service, or will provide coverage at an adequate reimbursement rate. As a result, the coverage determination process will require Eiger to provide scientific and clinical
support for the use of its products to each payor separately and will be a time-consuming process.
The containment of healthcare costs has become a
priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. Third-party payors are increasingly challenging the prices charged for medical products and services, examining the medical necessity and
reviewing the cost-effectiveness of drug products and medical services, in addition to questioning safety and efficacy. If these third-party payors do not consider Eiger’s products to be cost-effective compared to other available therapies,
they may not cover Eiger’s products after FDA approval or, if they do, the level of payment may not be sufficient to allow Eiger to sell its products at a profit.
In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls,
restrictions on coverage and reimbursement and requirements for substitution of generic products. By way of example, in the United States, ACA contains provisions that may reduce the profitability of drug products. The ACA, among other things,
increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate Program to utilization of prescriptions for individuals enrolled in Medicaid managed care plans, imposed
196
mandatory discounts for certain Medicare Part D beneficiaries and subjected manufacturers to new annual fees based on pharmaceutical companies’ share of sales to federal healthcare programs.
Eiger expects that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in
reduced demand for Eiger’s products once approved or additional pricing pressures.
Other legislative changes have been proposed and adopted since
ACA was enacted. On August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The
Joint Select Committee did not recommend and Congress did not enact legislation to reduce the deficit by at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs.
This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and will remain in effect through 2024 unless additional Congressional action is taken. On January 2, 2013,
President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers.
Eiger expects that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal
and state governments will pay for healthcare products and services, which could result in reduced demand for Eiger’s products once approved or additional pricing pressures.
Employees
As of December 31, 2015, Eiger had a
total of ten full-time employees in the United States, five of whom were primarily engaged in research and development activities and five of whom were engaged in general management and administration. Five of Eiger’s employees have either an
M.D. or a Ph.D. None of Eiger’s employees are represented by a labor union or subject to a collective bargaining agreement. Eiger has never experienced any work stoppage and considers its relations with its employees to be good.
Facilities
As of December 31, 2015, Eiger
conducted all of its operations, other than its outsourced operations, at its 1,570 square foot leased office space located at 350 Cambridge Avenue, Suite 350, Palo Alto, CA 94306. The term of the lease is for 36 months and expires on March 31,
2018. In January 2016, Eiger entered into a sublease agreement to sublease an additional 4,029 square feet of office space located in Palo Alto, California. The term of the sublease commences when the premises are delivered to Eiger and expires on
March 30, 2017.
Legal Proceedings
Eiger is not
currently a party to any material legal proceedings.
197
CELLADON MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of financial condition and results of operations should be read together with the section entitled “Selected
Historical and Unaudited Pro Forma Condensed Combined Financial Information and Data—Selected Historical Financial Consolidated Data of Celladon” in this proxy statement/prospectus/information statement and the consolidated financial
statements of Celladon and accompanying notes appearing elsewhere in this proxy statement/prospectus/information statement. This discussion of the Celladon financial condition and results of operations contains certain statements that are not
strictly historical and are “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995 and involve a high degree of risk and uncertainty. Actual results may differ materially from those
projected in the forward-looking statements due to other risks and uncertainties that exist in the Celladon operations, development efforts and business environment, including those set forth in the section entitled “Risk Factors—Risks
Related to Celladon” in this proxy statement/prospectus/information statement, the other risks and uncertainties described in the section entitled “Risk Factors” in this proxy statement/prospectus/information statement and the other
risks and uncertainties described elsewhere in this proxy statement/prospectus/information statement. All forward-looking statements included in this proxy statement/prospectus/information statement are based on information available to Celladon as
of the date hereof, and Celladon assumes no obligation to update any such forward-looking statement.
Overview
Celladon is a biotechnology company that has been focused on the development of cardiovascular gene therapy. Following the negative results from the Phase 2b
clinical trial of its lead product candidate, MYDICAR (AAV1/SERCA2a), during the second quarter of 2015, Celladon initiated a process of evaluating its strategic opportunities to maximize shareholder value, including the possibility of a merger or
sale of the company, and suspended further research and development activities. On November 18, 2015, Celladon entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Eiger BioPharmaceuticals,
Inc., or Eiger, pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, a wholly owned subsidiary of Celladon will merge with and into Eiger, with Eiger becoming a
wholly-owned subsidiary of Celladon and the surviving corporation of the merger. Celladon’s ability to close the proposed merger with Eiger entails numerous significant risks and uncertainties, including the risks and uncertainties set forth in
the section entitled “Risk Factors.”
Celladon is currently in the long-term follow-up stage of a 250-patient randomized, double-blind,
placebo-controlled multinational Phase 2b trial that was designed to evaluate MYDICAR in patients with heart failure for reduced ejection fraction, or HFrEF (also referred to as systolic heart failure). This Phase 2b trial is referred to as the
CUPID 2 trial. CUPID 2 evaluated a single, one-time, intracoronary infusion of the cardiovascular gene therapy agent MYDICAR versus placebo, in each case added to a maximal, optimized heart failure drug and device regimen. Celladon completed
enrollment of CUPID 2 in February 2014 and un-blinded the results from the active observation period in late April 2015.
On April 26, 2015, Celladon
announced that the CUPID 2 trial did not meet its primary and secondary endpoints and failed to show any treatment effect. Following Celladon’s un-blinding of the CUPID 2 data, Celladon implemented cost-cutting measures, including a reduction
in workforce and termination of certain contracts related to MYDICAR, and subsequently decided to not pursue additional previously planned development activities.
During the second quarter of 2015, Celladon’s board of directors approved, in two phases, an aggregate reduction of approximately 70% of its peak
workforce of 34 employees as of April 30, 2015 in order to reduce operating expenses and conserve cash resources. The majority of employees included in this workforce reduction were separated during the second quarter of 2015, with the
remainder separated during the third quarter of 2015.
198
Celladon also committed to retention payments payable to certain key employees if such employees remain employed by Celladon until December 31, 2015 or are terminated by Celladon without
cause prior to such date.
On June 23, 2015, Celladon’s board of directors approved the voluntary prepayment of the outstanding amounts due
under its Loan and Security Agreement with Hercules Technology III, L.P. and Hercules Technology Growth Capital, Inc. (as agent and as a lender, and together with Hercules Technology III, L.P., the “Lenders”) dated July 31, 2014 (the
“Loan Agreement”), with such prepayment to be effected on August 3, 2015 (the “Prepayment Date”). On the Prepayment Date, Celladon paid the Lenders: (i) the $10.0 million outstanding principal balance, (ii) $0.1
million in accrued and unpaid interest, and (iii) an end of term charge of $1.8 million for a total payment of $11.8 million.
On September 10,
2015, Celladon entered into a Sublease Termination and Settlement Agreement (the “Termination Agreement”) with Brandes Investment Partners, L.P., or Brandes, providing for the termination of that certain Sublease Agreement by and between
Celladon and Brandes dated May 28, 2014 (the “Lease”). Pursuant to the Termination Agreement, the parties agreed to terminate the Lease, effective October 31, 2015, which the parties subsequently extended to November 13,
2015 (the “Effective Date”). Celladon agreed to pay an early termination fee of $950,000 to Brandes in consideration of Brandes’ entry into the Termination Agreement and release of Celladon from any further base rent or other payment
obligations that would otherwise arise pursuant to the Lease after the Effective Date. At this time, Celladon’s current development activities are limited to the oversight of the long-term follow up period in the CUPID 2 trial, which is
expected to continue through February 2016.
Historically, Celladon has devoted substantially all of its resources to research and development efforts
relating to its product candidates, including conducting clinical trials and developing manufacturing capabilities, in-licensing related intellectual property, providing general and administrative support for these operations and protecting its
intellectual property. Celladon does not have any products approved for sale and have not generated any revenue from product sales or other sources. From Celladon’s inception through September 30, 2015, it has funded its operations
primarily through the sales of equity and debt securities totaling approximately $208.3 million.
Celladon has incurred net losses in each year since its
inception. As of September 30, 2015, Celladon had an accumulated deficit of approximately $186.1 million. Substantially all of its net losses, including those incurred during the periods presented in this report, have resulted from costs
incurred in connection with its research and development programs and from general and administrative costs associated with its operations.
Celladon
cannot predict whether and to what extent it will resume drug development activities, and what its future cash needs would be for any such activities.
If
the proposed merger with Eiger is not completed and Celladon is unable to seek an appropriate alternate use for its remaining assets, the board of directors may decide to pursue a dissolution and liquidation of the company. In such an event, the
amount of cash available for distribution to Celladon stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and contingent liabilities.
Celladon’s future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
| |
• |
|
the timing and completion of the proposed merger with Eiger; |
| |
• |
|
the extent to which it elects to pursue drug development activities in the future; and |
| |
• |
|
the costs associated with litigation, including the costs incurred in defending against claims made in the three putative class action complaints filed in July 2015 following Celladon’s announcements regarding the
negative CUPID 2 data and suspension of further research and development activities and the subsequent decline of the price of its common stock. |
199
Financial Operations Overview
Research and Development Expenses
Prior to the
suspension of further research and clinical development activities, Celladon devoted substantially all of its resources to research and development efforts relating to its product candidates, including conducting clinical trials, developing
manufacturing capabilities, in-licensing related intellectual property, providing general and administrative support for these operations and protecting its intellectual property. Celladon recognizes research and development expenses as they are
incurred. Celladon’s research and development expenses have consisted primarily of:
| |
• |
|
salaries and related overhead expenses, which include stock-based compensation and benefits for personnel in research and development functions; |
| |
• |
|
fees paid to contract manufacturers for commercial scale-up activities; |
| |
• |
|
fees paid to consultants and contract research organizations, or CROs, including in connection with preclinical studies and clinical trials and other related clinical trial fees, such as for investigator grants, patient
screening, laboratory work, clinical trial material management and statistical compilation and analysis; |
| |
• |
|
costs related to acquiring and manufacturing clinical trial materials, including continued testing such as process validation and stability of drug product; |
| |
• |
|
costs related to compliance with regulatory requirements; and |
| |
• |
|
payments related to licensed products and technologies. |
From Celladon’s inception through
September 30, 2015, it has incurred approximately $136.5 million in research and development expenses, of which it estimates $129.8 million relates to its development of MYDICAR. Celladon’s direct research and development expenses have
consisted principally of external costs, such as fees paid to investigators, consultants, central laboratories and CROs, in connection with clinical trials, developing manufacturing capabilities and costs related to acquiring and manufacturing
clinical trial materials. Prior to Celladon’s reductions in force including all its research and development staff, Celladon typically used its employee and infrastructure resources across multiple research and development programs. Celladon
expects its research and development expenses to decrease compared to prior periods through the completion of the CUPID 2 trial in the first quarter of 2016 due to a reduction in workforce, the suspension of further research and development
activities and reduced facility space and rent.
MYDICAR-HFrEF
Prior to the suspension of further research and clinical development activities, the majority of Celladon’s research and development resources were
focused on the CUPID 2 trial, commercialization and manufacturing preparations, clinical trials and other work needed to submit MYDICAR for regulatory approval in the United States and Europe.
MYDICAR-PAH
Prior to the suspension of further research
and clinical development activities, Celladon’s research and development expenses for MYDICAR for PAH related primarily to the preclinical testing in porcine models of PAH.
Stem Cell Factor Program
Prior to the suspension of
further research and clinical development activities, Celladon’s research and development expenses for its stem cell factor program related primarily to the preclinical testing of the membrane-bound form of the Stem Cell Factor gene, or mSCF,
in myocardial infarction porcine models.
200
Small Molecule Program
Prior to the suspension of further research and clinical development activities, Celladon’s research and development expenses for the small molecule
program related primarily to identification and pre-clinical testing of small molecule SERCA2 enzyme modulators.
General and Administrative
Expenses
General and administrative expenses have consisted primarily of salaries and related costs for employees in executive, finance, legal and
administration, corporate development and administrative support functions, including stock-based compensation expenses and benefits. Other significant general and administrative expenses have included accounting and legal services, expenses
associated with obtaining and maintaining patents, the cost of various consultants, occupancy costs and information systems costs. Celladon expects its general and administrative expenses to decrease compared to prior periods for the foreseeable
future due to a reduction in workforce, suspended activities related to pre-commercial planning and reduced facility space and rent.
Restructuring
Charges
In light of the CUPID 2 results and following analysis of the CUPID 2 data, Celladon implemented two reductions in workforce starting in
the second quarter of 2015 to reduce operating expenses and conserve cash resources while it evaluated its strategic alternatives. Celladon has also committed to retention payments payable to certain key employees if such employees remain with the
company until December 31, 2015 or are terminated by Celladon without cause prior to such date. The restructuring charges consisting of severance and retention commitments are expected to be fully settled in 2015. Also included in restructuring
charges were asset impairments related to certain equipment used in the MYDICAR manufacturing process and early termination fees incurred upon the termination of certain facility subleases. Celladon may incur additional charges in the future for
additional restructuring activities.
Other Income (Expense)
Other income consists primarily of interest income earned on Celladon’s cash, cash equivalents and investments. Celladon expects its interest income to
decrease as it reduces its investment balance to fund current operations. Other expense consists primarily of the accretion of debt discount and interest charges on prior debt agreements and the change in the fair value of outstanding warrant
liability prior to its reclassification to stockholders’ equity in February 2014 in connection with the closing of Celladon’s initial public offering. In August 2015, Celladon prepaid the outstanding amounts due under its Loan and Security
Agreement and recorded the debt discount balance as interest expense in its financial statements.
Critical Accounting Policies and Estimates
Celladon’s management’s discussion and analysis of its financial condition and results of operations is based on its consolidated financial
statements, which it has prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of Celladon’s consolidated financial statements requires it to make estimates and assumptions that
affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of its consolidated financial statements, as well as the reported expenses during the reported periods. Celladon evaluates
these estimates and judgments on an ongoing basis. Celladon bases its estimates on historical experience and on various other factors that it believes are reasonable under the circumstances, the results of which form the basis for making judgments
about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
201
Clinical Trial Accruals
As part of the process of preparing Celladon’s consolidated financial statements, Celladon is required to estimate its expenses resulting from its
obligations under contracts with vendors and consultants and clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations which vary from contract to contract and may
result in payment flows that do not match the periods over which materials or services are provided to Celladon under such contracts. Celladon’s clinical trial accrual is dependent upon the timely and accurate reporting of CROs and other
third-party vendors.
Celladon’s objective is to reflect the appropriate clinical trial expenses in its consolidated financial statements by matching
those expenses with the period in which services and efforts are expended. Celladon accounts for these expenses according to the progress of the trial as measured by patient progression and the timing of various aspects of the trial. Celladon
determines accrual estimates through discussion with applicable personnel and outside service providers as to the progress or state of completion of clinical trials, or the services completed. During the course of a clinical trial, Celladon adjusts
the rate of clinical trial expense recognition if actual results differ from the estimates. Celladon makes estimates of its accrued expenses as of each balance sheet date in its financial statements based on facts and circumstances known at that
time. Although Celladon does not expect that its estimates will be materially different from amounts actually incurred, Celladon’s understanding of status and timing of services performed relative to the actual status and timing of services
performed may vary and may result in its reporting amounts that are too high or too low for any particular period. Through September 30, 2015, there had been no material adjustments to prior period estimates of accrued expenses for clinical
trials. However, due to the nature of estimates, Celladon cannot assure you that it will not make changes to its estimates in the future as it becomes aware of additional information about the status or conduct of its clinical trials.
Stock-Based Compensation
Stock-based compensation
expense represents the grant date fair value of employee equity grants recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis, net of estimated forfeitures. For awards with performance-based
milestones, the expense is recorded over the remaining service period after the point when the achievement of the milestone is probable or the performance condition has been achieved.
Celladon accounts for awards granted to non-employees using the fair-value approach. These awards are subject to periodic revaluation over their vesting
terms.
Celladon estimates the fair value of stock options granted to employees and non-employees using the Black-Scholes option pricing model, which
requires the input of highly subjective assumptions, including (a) the risk-free interest rate, (b) the expected volatility of Celladon’s stock, (c) the expected term of the award and (d) the expected dividend yield. Until
Celladon’s recently completed initial public offering, there was no public market for the trading of its common stock. Due to this fact and a lack of company specific historical and implied volatility data, Celladon has based its estimate of
expected volatility on the historical volatility of a group of similar companies that are publicly traded. For these analyses, Celladon has selected companies with comparable characteristics to it, including enterprise value, risk profiles, position
within the industry and with historical share price information sufficient to meet the expected life of the stock-based awards. Celladon computes the historical volatility data using the daily closing prices for the selected companies’ shares
during the equivalent period of the calculated expected term of the stock-based awards. Celladon will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes
available. Celladon has estimated the expected life of its employee stock options using the “simplified” method, whereby, the expected life equals the average of the vesting term and the original contractual term of the option. The
risk-free interest rate is based on U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award being valued.
202
Results of Operations
Comparison of the Three Months Ended September 30, 2015 and 2014
The following table summarizes results of operations for the three months ended September 30, 2015 and 2014 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months
Ended September 30, |
|
|
Increase /
(Decrease) |
|
| |
|
2015 |
|
|
2014 |
|
|
| Research and development |
|
$ |
738 |
|
|
$ |
5,316 |
|
|
$ |
(4,578 |
) |
| General and administrative |
|
|
2,533 |
|
|
|
2,815 |
|
|
|
(282 |
) |
| Restructuring charges |
|
|
1,781 |
|
|
|
— |
|
|
|
1,781 |
|
| Total other (expense) income |
|
|
(1,344 |
) |
|
|
(227 |
) |
|
|
(1,117 |
) |
Research and Development Expenses
Research and development expenses were $0.7 million and $5.3 million for the three months ended September 30, 2015 and 2014, respectively. The decrease of
approximately $4.6 million was primarily due to a decrease of $1.2 million in expenses incurred during the third quarter of 2015 associated with drug substance manufacturing scale-up as a result of suspension of MYDICAR manufacturing activities,
$1.1 million in stock based compensation and $0.8 million in compensation costs due to a reduction in workforce including all the research and development staff, $0.9 million in clinical and preclinical costs, and $0.6 million in consulting and
various other expenses. Celladon anticipates a further reduction in its research and development expenses for the foreseeable future due to the suspension of further development of MYDICAR and other pre-clinical programs and the reduction in
workforce as it evaluates its strategic alternatives.
General and Administrative Expenses
General and administrative expenses were $2.5 million and $2.8 million for the three months ended September 30, 2015 and 2014, respectively. The decrease
of approximately $0.3 million was due to a decrease of $0.3 million in compensation costs due to a reduction in workforce as compared to the same period in 2014, $0.2 million in pre-commercial planning costs due to the suspension of pre-commercial
planning activities, $0.2 million in patent related costs, and $0.4 million in rent, recruiting and other administrative expenses offset by an increase of $0.3 million in consulting costs and $0.2 million in legal and various other public company
costs due to additional service fees incurred as a result of strategic planning activities, $0.2 million in depreciation expense as a result of a change in the estimated useful life of certain assets, and $0.1 million in stock based compensation.
Celladon expects its general and administrative expenses to decrease in the foreseeable future due to a reduction in workforce and the suspended activities related to pre-commercial planning efforts.
Restructuring Charges
Restructuring charges were $1.8
million and zero for the three months ended September 30, 2015 and 2014, respectively. In light of the CUPID 2 results and following analysis of the CUPID 2 data in the second quarter of 2015, the board of directors, in two phases prior to
September 30, 2015, approved an approximately 70% aggregate reduction of Celladon’s peak workforce of 34 employees to reduce operating expenses and conserve cash resources. Celladon has also committed to retention payments payable to
certain key employees if such employees remain with Celladon until December 31, 2015 or are terminated by Celladon without cause prior to such date. Celladon suspended further research or development of its MYDICAR (AAV1/SERCA2a) program and
its pre-clinical programs. The charges incurred during the three months ended September 30, 2015, included $1.1 million in facility lease termination costs, $0.6 million related to retention payment costs and $0.1 million related to employee
severance costs. In connection with the restructuring activities executed to date, Celladon anticipates recording $0.3 million in charges related to retention payments in the fourth quarter of 2015. Celladon
203
has committed to approximately $3.8 million in aggregate charges related to employee severance and related costs which are expected to be settled in 2015. Celladon estimates that employee actions
implemented to date will result in gross quarterly savings of approximately $1.5 million, which will be realized within research and development and within general and administrative. Celladon began to realize these savings in the second quarter of
2015 and expects to fully realize these savings by the end of 2015. Celladon may incur additional charges in the future in connection with future restructuring activities.
Other (Expense) Income
Other expense was $1.3 million
and $0.2 million for the three months ended September 30, 2015 and 2014, respectively. Other expense for the three months ended September 30, 2015 consisted primarily of $1.3 million of expense related to the accelerated accretion of debt
discount and interest charges on the term loan that was prepaid in full in August 2015. Other expense for the three months ended September 30, 2014 consisted primarily of $0.3 million of expense related to the accretion of debt discount and
interest charges on the prior term loan offset by $44,000 in interest and other income.
Comparison of the Nine Months Ended September 30, 2015
and 2014
The following table summarizes results of operations for the nine months ended September 30, 2015 and 2014 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
|
Increase /
(Decrease) |
|
| |
|
2015 |
|
|
2014 |
|
|
| Research and development |
|
$ |
21,757 |
|
|
$ |
15,515 |
|
|
$ |
6,242 |
|
| General and administrative |
|
|
10,805 |
|
|
|
6,545 |
|
|
|
4,260 |
|
| Restructuring charges |
|
|
4,862 |
|
|
|
— |
|
|
|
4,862 |
|
| Total other (expense) income |
|
|
(2,243 |
) |
|
|
(452 |
) |
|
|
(1,791 |
) |
Research and Development Expenses
Research and development expenses were $21.8 million and $15.5 million for the nine months ended September 30, 2015 and 2014, respectively. The increase
of approximately $6.2 million was primarily due to an increase of $7.0 million in expenses incurred during the first nine months of 2015 associated with drug substance manufacturing scale-up prior the negative results of the CUPID 2 trial, $0.9
million in preclinical costs and $0.1 million in rent expense, offset by a decrease of $1.4 million in clinical expenses due to completion of CUPID 2 clinical trial during the first quarter of 2015, a decrease of $0.2 million in stock-based
compensation and $0.2 million in cash compensation costs due to reduction in force including all the research and development staff compared to the same period in 2014. Celladon anticipates a further reduction in its research and development
expenses for the foreseeable future due to the suspension of development of MYDICAR and other pre-clinical programs and the reduction in workforce.
General and Administrative Expenses
General and
administrative expenses were $10.8 million and $6.5 million for the nine months ended September 30, 2015 and 2014, respectively. The increase of approximately $4.3 million was primarily due an increase of $1.3 million in stock-based
compensation due to additional grants, $1.2 million in pre-commercial planning efforts, $1.0 million in compensation costs related to both an increase in staff through May 2015 compared to the same period in the prior year and separation charges
associated with the departure of Celladon’s chief executive officer, $1.0 million in consulting and public company related costs, and $0.2 million in depreciation expense offset by a decrease of $0.4 million in recruiting, investor relations
and various other administrative costs. Celladon expects its general and administrative expenses to decrease in the foreseeable future due to a reduction in workforce and the suspended activities related to pre-commercial planning efforts.
204
Restructuring Charges
Restructuring charges were $4.9 million and zero for the nine months ended September 30, 2015 and 2014, respectively. In light of the CUPID 2 results and
following analysis of the CUPID 2 data in the second quarter of 2015, Celladon’s board of directors, in two phases prior to September 30, 2015, approved an approximately 70% aggregate reduction of its peak workforce of 34 employees to
reduce operating expenses and conserve cash resources. Celladon has also committed to retention payments payable to certain key employees if such employees remain with Celladon until December 31, 2015 or are terminated by Celladon without cause
prior to such date. Celladon suspended further research or development of the MYDICAR (AAV1/SERCA2a) program and the pre-clinical programs. The charges incurred during the nine months ended September 30, 2015, included $2.4 million related to
employee severance costs, which impacted 25 employees, $1.1 million related to retention payment charges, $1.1 million in facility lease termination costs and $0.2 million in asset impairment charges. In connection with the restructuring activities
executed to date, Celladon anticipates recording $0.3 million in charges related to retention payments in the fourth quarter of 2015. Celladon has committed to approximately $3.8 million in aggregate charges related to employee severance and related
costs which are expected to be settled in 2015. Celladon estimates that employee actions implemented to date will result in gross quarterly savings of approximately $1.5 million, which will be realized within research and development and within
general and administrative. Celladon began to realize these savings in the second quarter of 2015 and expect to fully realize these savings by the end of 2015. Celladon may incur additional charges in the future in connection with future
restructuring activities.
Other Expense
Other
expense was $2.2 million and $0.5 million for the nine months ended September 30, 2015 and 2014, respectively. Other expense for the nine months ended September 30, 2015 consisted primarily of $2.3 million of expense related to the
accelerated accretion of debt discount and interest charges on Celladon’s term loan which was prepaid in full in August 2015 offset by $0.1 million in interest income. Other expense for the nine months ended September 30, 2014 consisted
primarily of $0.3 million of expense related to the accretion of debt discount and interest charges on Celladon’s prior term loan and $0.2 million related to an increase in the fair value of the warrant liability prior to conversion to equity
upon the closing of Celladon’s initial public offering.
Comparison of the Years Ended December 31, 2014 and 2013
The following table summarizes results of operations for the years ended December 31, 2014 and 2013 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years Ended
December 31, |
|
|
Increase /
(Decrease) |
|
| |
|
2014 |
|
|
2013 |
|
|
| Research and development |
|
$ |
22,676 |
|
|
$ |
16,927 |
|
|
$ |
5,749 |
|
| General and administrative |
|
|
10,342 |
|
|
|
3,037 |
|
|
|
7,305 |
|
| Total other income (expense) |
|
|
(835 |
) |
|
|
(127 |
) |
|
|
(708 |
) |
Research and Development Expenses
Research and development expenses were $22.7 million and $16.9 million for the years ended December 31, 2014 and 2013, respectively. The increase of
approximately $5.7 million was due primarily to an increase of $5.7 million in expenses during 2014 associated with the drug substance manufacturing scale-up, $1.7 million in personnel costs related to an increase in headcount, $0.6 million in
non-clinical studies related to MYDICAR, $0.4 million in stock-based compensation, $0.3 million in consulting, regulatory and other costs offset by a decrease of $3.0 million in clinical costs due to the completion of enrollment in the CUPID2 trial
in the first quarter of 2014. Celladon expects that its overall research and development expenses will increase in 2015 as it initiates additional clinical trials and continue manufacturing scale-up activities.
205
General and Administrative Expenses
General and administrative expenses were $10.3 million and $3.0 million for the years ended December 31, 2014 and 2013, respectively. The increase of
approximately $7.3 million was due primarily to an increase of $2.5 million in compensation expense related to an increase in headcount, $2.1 million in costs associated with operating as a publicly traded company, including investor relations,
legal, audit, insurance, taxes and director fees, $1.5 million in stock-based compensation, $0.7 million in marketing costs and $0.5 million in patent, office and other costs. Celladon expects that its general and administrative expenses will
increase as it continues to operate as a public company, including costs to comply with corporate governance and internal controls, and as it adds personnel to support product commercialization efforts.
Other Expense
Other expense was $0.8 million and $0.1
million for the years ended December 31, 2014 and 2013, respectively. The other expense for the year ended December 31, 2014 consisted primarily of $0.7 million of expense related to the accretion of debt discount and interest charges on
Celladon’s term loan, $0.2 million increase in fair value of the warrant liability prior to reclassification to equity upon Celladon’s initial public offering and $29,000 foreign currency exchange loss offset by $0.1 million in interest
income on Celladon’s investments. The other expense for the year ended December 31, 2013 consisted primarily of $0.2 million of other expense related to an increase in the fair value of the outstanding warrant liability and $45,000 of
interest expense related to the amortization of debt discount on the outstanding convertible debt, offset by $0.1 million of interest income on its investments and $25,000 foreign currency exchange gain.
Comparison of the Years Ended December 31, 2013 and 2012
The following table summarizes results of operations for the years ended December 31, 2013 and 2012 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years Ended
December 31, |
|
|
Increase /
(Decrease) |
|
| |
|
2013 |
|
|
2012 |
|
|
| Research and development |
|
$ |
16,927 |
|
|
$ |
13,314 |
|
|
$ |
3,613 |
|
| General and administrative |
|
|
3,037 |
|
|
|
2,631 |
|
|
|
406 |
|
| Total other income (expense) |
|
|
(127 |
) |
|
|
74 |
|
|
|
(201 |
) |
Research and Development Expenses
Research and development expenses were $16.9 million and $13.3 million for the years ended December 31, 2013 and 2012, respectively. The increase of
approximately $3.6 million was due primarily to an increase of $4.5 million in expenses during 2013 associated with the increase in enrollment of patients in the CUPID 2 clinical trial, $0.8 million associated with the transfer of Celladon’s
manufacturing process to Lonza and $1.5 million in compensation related to an increase in headcount and stock-based compensation, offset by a charge of $3.2 million which occurred during the year ended December 31, 2012 related to the purchase
of intangible assets from AmpliPhi Biosciences Corporation, or AmpliPhi, relating to the development of MYDICAR.
General and Administrative Expenses
General and administrative expenses were $3.0 million and $2.6 million for the years ended December 31, 2013 and 2012, respectively. The increase
of approximately $0.4 million was due primarily to an increase in compensation expense related to an increase in headcount and professional fees associated with transitioning Celladon into a public company, offset by a reduction in outside legal
services due to the completion of the establishment of Celladon’s former European subsidiary, Celladon Europe, and legal costs associated with licensing activities in 2012.
206
Other Income (Expense)
Other income (expense) was $(0.1) million and $74,000 for the years ended December 31, 2013 and 2012, respectively. The other expense for the year ended
December 31, 2013 consisted primarily of $0.2 million of other expense related to an increase in the fair value of Celladon’s outstanding warrant liability and $45,000 of interest expense related to the amortization of debt discount on
Celladon’s outstanding convertible debt, offset by $0.1 million of interest income on Celladon’s investments and $25,000 foreign currency exchange gain. The other income for the year ended December 31, 2012 consisted primarily of
interest income on Celladon’s investments offset by interest expense on outstanding convertible debt.
Liquidity and Capital Resources
Celladon has incurred net losses each year since inception and as of September 30, 2015, Celladon had an accumulated deficit of approximately $186.1
million. Celladon anticipates that it will continue to incur net losses for the foreseeable future. Celladon expects that its research and development and general and administrative expenses will decrease for the foreseeable future due to a
reduction in workforce, suspended activities related to pre-commercial planning and the suspension of further development of MYDICAR and other pre-clinical programs. Should Celladon choose to continue drug development, Celladon expects that it may
need additional capital to fund its operations, which it may obtain through one or more public or private equity offerings, debt financings, government or other third-party funding, strategic alliances and licensing or collaboration arrangements. On
August 3, 2015, Celladon prepaid the outstanding amounts due under its loan facility with Hercules, including the $10.0 million principal borrowed in 2014. Upon the prepayment in August 2015, Celladon’s obligations, covenants, debts and
liabilities under the loan facility were satisfied in full and Hercules’ commitments to extend further credit to Celladon were terminated.
Since
Celladon’s inception through September 30, 2015, it has funded its operations primarily through the sale of its equity and debt securities. As of September 30, 2015, Celladon had cash and cash equivalents of approximately $37.1
million. Cash in excess of immediate requirements is invested in accordance with Celladon’s investment policy, primarily with a view to liquidity and capital preservation.
The following table summarizes cash flows for the periods indicated (in thousands):
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
| Net cash provided by (used in): |
|
|
|
|
|
|
|
|
| Operating activities |
|
$ |
(36,733 |
) |
|
$ |
(19,374 |
) |
| Investing activities |
|
|
70,369 |
|
|
|
(71,010 |
) |
| Financing activities |
|
|
(10,979 |
) |
|
|
96,848 |
|
|
|
|
|
|
|
|
|
|
| Net increase in cash and cash equivalents |
|
$ |
22,657 |
|
|
$ |
6,464 |
|
|
|
|
|
|
|
|
|
|
Operating activities
Net
cash used in operating activities of $36.7 million during the nine months ended September 30, 2015 was primarily a result of the net loss of $39.7 million. The primary difference between the net loss and the cash used in operating activities
was $3.2 million of stock-based compensation, $1.8 million of non-cash interest related to the accretion of debt discount on the prior term loan with Hercules, $0.4 million of depreciation expense, $0.2 million of asset impairment charges, $0.2
million amortization of premiums paid on investment securities, $0.1 million of deferred rent charges, and $2.7 million of changes in Celladon’s operating assets and liabilities.
Net cash used in operating activities of $19.4 million during the nine months ended September 30, 2014, was primarily a result of the net loss of $22.5
million. The primary difference between the net loss and the cash used
207
in operating activities was $2.2 million of stock-based compensation, $0.2 million related to the change in fair value of the warrant liability, $0.2 million of non-cash interest related to the
accretion of debt discount on Celladon’s prior debt arrangements, $0.2 million amortization of premiums paid on investment securities, $0.2 million of changes in Celladon’s operating assets and liabilities and $0.1 million of depreciation
expense. In connection with Celladon’s initial public offering which was completed in February 2014, the warrant liability was reclassified to additional paid-in capital, the outstanding principal and accrued interest on Celladon’s
convertible debt was converted into shares of Celladon’s common stock and the unamortized debt discount related to the convertible debt was charged to expense.
Investing Activities
Net cash provided by investing
activities of $70.4 million during the nine months ended September 30, 2015 was primarily a result of $70.3 million in the net maturities of investments used to fund Celladon’s operating activities and $0.3 million in proceeds from the
sale of property and equipment. Net cash used by investing activities of $71.0 million during the nine months ended September 30, 2014 was primarily a result of purchases of investment securities of $88.7 million offset by $18.2 million net
maturities of investments. In 2015 and 2014, amounts of $0.2 million and $0.5 million, respectively, were also used to purchase property and equipment.
Financing Activities
Net cash used in financing
activities of $11.0 million during the nine months ended September 30, 2015 consisted of $11.8 million prepayment in full of the prior term loan and related costs offset by $0.8 million in proceeds received upon the exercise of employee and
consultant stock options. Net cash provided by financing activities of $96.8 million during the nine months ended September 30, 2014 consisted primarily of $94.3 million in proceeds received and $7.4 million in costs paid in connection with
Celladon’s public offerings, $9.7 million in net borrowings under its prior term loan, $0.1 million in proceeds upon the exercise of warrants in exchange for common stock and $0.1 million in proceeds from the sale of shares under
Celladon’s employee stock purchase plan.
Future Funding Requirements
To date, Celladon has not generated any revenue from product sales. Celladon does not know when, or if, it will generate any revenue from product sales.
Celladon expects that its expenses will decrease for the foreseeable future due to a reduction in workforce, suspended activities related to pre-commercial planning and the suspension of certain research and development activities. Celladon
anticipates that it would need additional capital to fund research and development operations, if resumed in the future.
Based upon Celladon’s
current operating plan, it believe that its existing cash and cash equivalents will enable it to fund its operations for at least the next 12 months. Celladon is currently evaluating various strategic alternatives for the use of its existing
resources. Celladon has based its planning estimates on assumptions that may prove to be wrong, and it may use its available capital resources sooner than it currently expects.
Celladon’s future capital requirements, both near and long-term, will depend on many factors, including, but not limited to:
| |
• |
|
the timing and completion of the proposed merger with Eiger; |
| |
• |
|
the extent to which it elects to pursue drug development activities in the future; and |
| |
• |
|
the costs associated with litigation, including the costs incurred in defending against claims made in the three putative class action complaints filed in July 2015 following Celladon’s announcements regarding the
negative CUPID 2 data and suspension of further research and development activities and the subsequent decline of the price of Celladon’s common stock. |
208
Depending upon Celladon’s future business prospects and developments, it expects to finance its operating
activities through existing cash and cash equivalents, public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing
arrangements or a combination of these approaches. To the extent that Celladon raises additional capital through the sale of equity or convertible debt securities, the ownership interests of the common stockholders will be diluted, and the terms of
these securities may include liquidation or other preferences that adversely affect the rights of the common stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting Celladon’s ability to
take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If Celladon raises additional funds through government or other third-party funding, marketing and distribution arrangements or other
collaborations, or strategic alliances or licensing arrangements with third parties, Celladon may have to relinquish valuable rights to its technologies, future revenue streams, research programs or product candidates or to grant licenses on terms
that may not be favorable to Celladon.
Contractual Obligations and Commitments
The following table summarizes Celladon’s contractual obligations at September 30, 2015 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Payments due by period |
|
| |
|
Total |
|
|
Less
than
1 year |
|
|
1 – 3
Years |
|
|
3 – 5
Years |
|
|
More
than
5 years |
|
| Operating lease obligations |
|
$ |
214 |
|
|
$ |
106 |
|
|
$ |
108 |
|
|
$ |
— |
|
|
$ |
— |
|
During 2015 Celladon prepaid its term loan in full and terminated several facility lease contracts. At September 30,
2015, operating lease obligations consisted of future rent payments under two San Diego facility lease contracts.
Off-Balance Sheet Arrangements
During the periods presented Celladon did not have, nor does it currently have, any off-balance sheet arrangements as defined under the rules of the
SEC.
209
QUANTITATIVE AND QUALITATIVE DISCLOSURES
ABOUT CELLADON MARKET RISK
As of
September 30, 2015, Celladon had market risk exposure related to its cash and cash equivalents. Historically, Celladon has invested its excess cash in highly liquid short-term investments such as money market funds. Changes in interest rates
affect the investment income Celladon earns on its investments and therefore impacts its cash flows and results of operations.
Celladon does not believe
that its cash and cash equivalents have significant risk of default or illiquidity. While Celladon believes its cash and cash equivalents do not contain excessive risk, it cannot provide absolute assurance that in the future its investments will not
be subject to adverse changes in market value. In addition, Celladon maintains significant amounts of cash and cash equivalents at one or more financial institutions that are in excess of federally insured limits.
As of September 30, 2015, all of Celladon’s short-term investments had matured and it did not have any investments, nor did it have any outstanding
indebtedness for amounts borrowed. Accordingly, a 10% change in interest rates from the interest rates on September 30, 2015 would not have had a material effect on Celladon’s financial condition.
Celladon has ongoing clinical trial agreements denominated in euros. Celladon does not participate in any foreign currency hedging activities and it does not
have any other derivative financial instruments. Celladon did not recognize any significant exchange rate losses during the nine month period ended September 30, 2015. A 10% change in the euro-to-dollar exchange rate on September 30, 2015
would not have had a material effect on Celladon’s results of operations or financial condition.
Inflation generally affects Celladon by increasing
its cost of labor and clinical trial costs. Celladon does not believe that inflation has had a material effect on its results of operations during the periods presented.
210
EIGER MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of Eiger’s financial condition and results of operations together with the section entitled
“Selected Historical and Unaudited Pro Forma Condensed Combined Financial Data—Selected Historical Financial Data of Eiger” and Eiger’s consolidated financial statements and related notes included elsewhere in this proxy
statement/prospectus/information statement. This discussion and other parts of this proxy statement/prospectus/information statement contain forward-looking statements that involve risks and uncertainties, such as its plans, objectives,
expectations, intentions and beliefs. Eiger’s actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those
identified below and those discussed in the section entitled “Risk Factors” included elsewhere in this proxy statement/prospectus/information statement.
Overview
Eiger is a clinical stage biopharmaceutical
company focused on bringing to market novel product candidates for the treatment of orphan diseases. Since its founding in 2008, Eiger has worked with investigators at Stanford University and has evaluated a number of potential development
candidates from pharmaceutical companies to comprise a pipeline of novel product candidates. Eiger’s resulting pipeline includes three Phase 2 candidates addressing four distinct orphan diseases. The programs have several aspects in common: the
disease targets represent conditions of high medical need which are inadequately treated by current standard of care; the therapeutic approaches are supported by an understanding of disease biology and mechanism as elucidated by Eiger’s
academic research relationships; prior clinical experience with the product candidates guides an understanding of safety; and the development paths leverage the experience and capabilities of Eiger’s experienced, commercially focused management
team. The pipeline includes Sarasar® (lonafarnib) for HDV, exendin (9-39) for severe hypoglycemia and Bestatin™ (ubenimex) for PAH and
lymphedema. Eiger plans to deliver Phase 2 data on all four programs over the course of the next one to three years beginning in 2016.
Eiger has no
products approved for commercial sale and has not generated any revenue from product sales. From inception to September 30, 2015, Eiger has raised net cash proceeds of approximately $22.6 million, primarily from private placements of
convertible preferred stock and bridge financings.
Eiger has never been profitable and has incurred operating losses in each year since inception.
Eiger’s net losses were $1.5 million, $1.0 million and $6.3 million for the years ended December 31, 2014 and 2013 and for the nine months ended September 30, 2015, respectively. As of September 30, 2015, Eiger had an accumulated
deficit of $22.2 million. Substantially all of its operating losses resulted from expenses incurred in connection with its research and development programs and from general and administrative costs associated with its operations.
Eiger expects to incur significant expenses and increasing operating losses for at least the next several years as it initiates and continues the clinical
development of, and seeks regulatory approval for, its product candidates and adds personnel necessary to operate as a public company with an advanced clinical candidate pipeline of products. In addition, operating as a publicly traded company would
involve the hiring of additional financial and other personnel, upgrading its financial information systems and incurring costs associated with operating as a public company. Eiger expects that its operating losses will fluctuate significantly from
quarter to quarter and year to year due to timing of clinical development programs and efforts to achieve regulatory approval.
As of September 30,
2015, Eiger had cash of $2.1 million. In November 2015, Eiger received $6.0 million in financing under bridge promissory notes which is due and payable on March 31, 2016. Eiger’s current capital resources are not sufficient to fund its
planned operations for a 12 month period without the merger and financing contemplated by the Merger Agreement being completed, and therefore, raise substantial doubt about its ability
211
to continue as a going concern. Eiger will continue to require substantial additional capital to continue its clinical development and potential commercialization activities. Accordingly, Eiger
will need to raise substantial additional capital to continue to fund its operations. The amount and timing of its future funding requirements will depend on many factors, including the pace and results of its clinical development efforts. Failure
to raise capital as and when needed, on favorable terms or at all, would have a negative impact on its financial condition and its ability to develop its product candidates.
Recent Events
On November 18, 2015,
Eiger entered into an Agreement and Plan of Merger and Reorganization, or the Merger Agreement, with Celladon Corporation, or Celladon, pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth in the
Merger Agreement, that a wholly owned subsidiary of Celladon will merge with and into Eiger, with Eiger becoming a wholly-owned subsidiary of Celladon and the surviving corporation of the merger. At the closing of the merger, each outstanding share
of Eiger’s common stock will be converted into the right to receive approximately 1.32 pre-split shares of common stock of Celladon (or 0.09 shares post-split), as well as the payment of cash in lieu of fractional shares. Immediately following
the effective time of the Merger, Celladon equity holders are expected to own approximately 22% of the outstanding capital stock of the combined company on a fully diluted basis after giving effect to its pre-Merger financing activities referred to
below, with Eiger’s preexisting equity holders expected to own approximately 45% and the participants in the pre-Merger financing expected to receive approximately 33% for their investment.
Prior to Eiger’s entry into the Merger Agreement, certain third parties, including Eiger’s existing stockholders entered into agreements with Eiger
pursuant to which such parties have agreed, subject to the terms and conditions of such agreements, to purchase, prior to consummation of the merger, shares of its capital stock and conversion of its note payable referred to below into such shares
upon the merger for an aggregate purchase price of approximately $33.5 million. The consummation of the transactions contemplated by such agreements is conditioned upon the satisfaction or waiver of the conditions set forth in the Merger Agreement.
In November 2015, Eiger entered into a note and warrant purchase agreement with three investors, including two existing holders of its convertible
preferred stock and one of the purchasers in the financing contemplated prior to the consummation of the merger, which includes the issuance of notes payable in the aggregate principal amount of $6.0 million and the issuance of warrants to purchase
Eiger equity securities. The notes bear interest of 6.0% per annum. The warrants entitle each investor to purchase equity securities for a number of shares equal 15.0% of the principal borrowed from such investor, or 17.5% of the principal
borrowed from such investor in the event that Eiger does not consummate a reverse merger with a third party then currently reporting under the Securities Act of 1933 and Exchange Act of 1934 by February 28, 2016, divided by the per share price
of the equity securities sold in Eiger’s next equity financing that results in total proceeds to Eiger of not less than $25.0 million, with an exercise price of $0.01 for each share purchased under the warrants. The warrants are exercisable for
the type of equity securities issued by Eiger in a qualified financing as described in the notes, or if no qualified financing is consummated, then into shares of Eiger’s common stock. All principal and interest is due March 31, 2016.
However, prior to March 31, 2016, the outstanding balance on the note plus unpaid accrued interest is automatically converted into common stock or preferred stock sold in Eiger’s next equity financing that results in total proceeds to
Eiger of not less than $25.0 million, which would be satisfied by the financing contemplated prior to the consummation of the merger. If Eiger does not consummate a reverse merger or next equity financing that results in total proceeds of at least
$25.0 million, then the per share price to determine the number of shares under the warrant is the per share price from Eiger’s last financing under which it sold shares of its Series A-1 Preferred Stock. In addition, in the event that 50% of
the voting power of its stockholders is transferred in a transaction or series of transactions prior to March 31, 2016 (other than the merger), Eiger will repay the investors 120% of the outstanding principal plus unpaid accrued interest upon
such event.
212
Financial Operations Overview
Research and Development Expenses
Research and
development expenses represent costs incurred to conduct research and development, such as the development of Eiger’s product candidates. Eiger recognizes all research and development costs as they are incurred. Research and development
expenses consist primarily of the following:
| |
• |
|
expenses incurred under agreements with consultants and clinical trial sites that conduct research and development activities on its behalf; |
| |
• |
|
laboratory and vendor expenses related to the execution of clinical trials; |
| |
• |
|
contract manufacturing expenses, primarily for the production of clinical supplies; and |
| |
• |
|
internal costs that are associated with activities performed by Eiger’s research and development organization and generally benefit multiple programs. These costs are not separately allocated by product candidate.
Unallocated internal research and development costs consist primarily of: |
| |
• |
|
personnel costs, which include salaries, benefits and stock-based compensation expense; |
| |
• |
|
allocated facilities and other expenses, which include expenses for rent and maintenance of facilities and depreciation expense; and |
| |
• |
|
regulatory expenses and technology license fees related to development activities. |
The largest component of
Eiger’s operating expenses has historically been the investment in research and development activities. However, Eiger does not allocate internal research and development costs, such as salaries, benefits, stock-based compensation expense and
indirect costs to product candidates on a program-specific basis. The following table shows Eiger’s research and development expenses for the years ended December 31, 2014 and 2013 and for the nine months ended September 30, 2015 and
2014:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
|
|
Nine Months Ended
September 30, |
|
| |
|
2014 |
|
|
2013 |
|
|
2015 |
|
|
2014 |
|
| |
|
(in thousands) |
|
| Product candidates: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lonafarnib |
|
$ |
515 |
|
|
$ |
241 |
|
|
$ |
3,509 |
|
|
$ |
267 |
|
| Ubenimex |
|
|
— |
|
|
|
— |
|
|
|
330 |
|
|
|
— |
|
| Other clinical programs and research related costs |
|
|
19 |
|
|
|
103 |
|
|
|
— |
|
|
|
19 |
|
| Internal research and development costs |
|
|
110 |
|
|
|
82 |
|
|
|
654 |
|
|
|
34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total research and development expenses |
|
$ |
644 |
|
|
$ |
426 |
|
|
$ |
4,493 |
|
|
$ |
320 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eiger expects research and development expenses will increase in the future as Eiger advances its product candidates into and
through clinical trials and pursues regulatory approvals, which will require a significant investment in regulatory support and contract manufacturing and inventory build-up related costs. In addition, Eiger continues to evaluate opportunities to
acquire or in-license other product candidates and technologies, which may result in higher research and development expenses due to license fee and/or milestone payments.
The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. Eiger may never succeed in timely developing
and achieving regulatory approval for its product candidates. The probability of success of Eiger’s product candidates may be affected by numerous factors, including clinical data, competition, intellectual property rights, manufacturing
capability and commercial viability. As a result, Eiger is unable to determine the duration and completion costs of Eiger’s development projects or when and to what extent Eiger will generate revenue from the commercialization and sale of any
of its product candidates.
213
General and Administrative Expenses
General and administrative expenses consist of personnel costs, allocated expenses and expenses for outside professional services, including legal, audit and
accounting services. Personnel costs consist of salaries, benefits and stock-based compensation. Allocated expenses consist of facilities and other expenses, which include direct and allocated expenses for rent and maintenance of facilities,
depreciation expense and other supplies. Eiger expects to incur additional expenses as a result of becoming a public company following completion of the merger, including expenses related to compliance with the rules and regulations of the SEC and
NASDAQ, additional insurance, investor relations and other administrative expenses and professional services.
Critical Accounting Policies and
Estimates
Eiger’s management’s discussion and analysis of financial condition and results of operations is based on its consolidated
financial statements, which have been prepared in accordance with GAAP. The preparation of these financial statements requires Eiger to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing
basis, Eiger evaluates these estimates and judgments. Eiger bases its estimates on historical experience and on various assumptions that Eiger believes to be reasonable under the circumstances. These estimates and assumptions form the basis for
making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially from these estimates. Eiger believes that the accounting
policies discussed below are critical to understanding its historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
Accrued Research and Development Expenses
Eiger
records accrued expenses for estimated costs of its research and development activities conducted by external service providers, which include the conduct of clinical and contract formulation and manufacturing activities. Eiger records the estimated
costs of development activities based upon the estimated amount of services provided but not yet invoiced, and includes these costs in accrued liabilities in the consolidated balance sheet and within development expense in the consolidated statement
of operations and comprehensive loss. These costs are a significant component of Eiger’s research and development expenses. Eiger records accrued expenses for these costs based on the estimated amount of work completed and in accordance with
agreements established with these external service providers.
Eiger estimates the amount of work completed through discussions with internal personnel
and external service providers as to the progress or stage of completion of the services and the agreed-upon fee to be paid for such services. Eiger makes significant judgments and estimates in determining the accrued balance in each reporting
period. As actual costs become known, Eiger adjusts their accrued estimates.
Stock-based Compensation
Eiger recognizes stock-based awards to employees and directors, including stock options, based on the fair value on the grant date using the Black-Scholes
option pricing model. The related stock-based compensation is recognized as expense on a straight line-basis over the employee’s or director’s requisite service period (generally the vesting period). Noncash stock compensation expense is
based on awards ultimately expected to vest and is reduced by an estimate for future forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates.
Eiger accounts for stock-based compensation arrangements with non-employees using a fair value approach. The fair value of options granted to non-employees is
measured using the Black-Scholes option pricing model reflecting similar assumptions for employees except that the expected term is based on the options’ remaining contractual term instead of the simplified method in each of the reported
periods. The compensation costs of these arrangements are subject to remeasurement over the vesting terms as earned.
214
In determining the fair value of the stock-based awards, Eiger uses the Black-Scholes option-pricing model and
assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
Fair Value of
Common Stock. The fair value of the shares of common stock underlying stock options has historically been determined by Eiger’s board of directors. In order to determine the fair value of the common stock at the time of grant of the option,
the board of directors considered, among other things, valuations performed by an independent third-party. Because there has been no public market for its common stock, the board of directors exercised reasonable judgment and considered a number of
objective and subjective factors to determine the best estimate of the fair value of Eiger’s common stock, including important developments in its operations, sales of convertible preferred stock, actual operating results and financial
performance, the conditions in the life sciences industry and the economy in general, the stock price performance and volatility of comparable public companies, and the lack of liquidity of its common stock, among other factors.
Expected Term. Eiger’s expected term represents the period that their stock-based awards are expected to be outstanding and is
determined using the simplified method (based on the mid-point between the vesting date and the end of the contractual term) for employee options and the contractual term for non-employee options.
Expected Volatility. Since Eiger is privately held and does not have any trading history for its common stock, the expected volatility
was estimated based on the average volatility for comparable publicly traded biotechnology companies over a period equal to the expected term of the stock option grants. The comparable companies were chosen based on their similar size, or stage in
the life cycle.
Risk-Free Interest Rate. The risk-free interest rate is based on the U.S. Treasury zero coupon issues in effect at
the time of grant for periods corresponding with the expected term of option.
Expected Dividend. Eiger has never paid dividends on
its common stock and has no plans to pay dividends on its common stock. Therefore, Eiger used an expected dividend yield of zero.
For the years ended
December 31, 2014 and 2013, stock-based compensation expense was $27,000 and $61,000, respectively. For the nine months ended September 30, 2015 and 2014, stock-based compensation expense was $73,000 and $5,000, respectively. As of
September 30, 2015, Eiger had $2.1 million of total unrecognized stock-based compensation costs, net of estimated forfeitures, which it expects to recognize over a weighted-average period of 3.85 years.
Results of Operations
Comparison of the nine
months ended September 30, 2015 and 2014
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
|
Dollar
Change |
|
| |
|
2015 |
|
|
2014 |
|
|
| |
|
(in thousands) |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
4,493 |
|
|
$ |
320 |
|
|
$ |
4,173 |
|
| General and administrative |
|
|
1,768 |
|
|
|
403 |
|
|
|
1,365 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
6,261 |
|
|
|
723 |
|
|
|
5,538 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(6,261 |
) |
|
|
(723 |
) |
|
|
(5,538 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(6,261 |
) |
|
$ |
(723 |
) |
|
$ |
(5,538 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
215
Research and development
Research and development expenses increased by $4.2 million, or 1,304%, to $4.5 million for the nine months ended September 30, 2015 from $0.3 million for
the nine months ended September 30, 2014. The increase was due to a $1.0 million milestone payment in May 2015 related to the Merck license agreement related to the achievement of a development milestone related to clinical trials, $0.5 million
related to a drug interaction study, $0.4 million related to purchase of drug product for clinical trials, $0.2 million related to the issuance of common stock in connection with the exendin asset purchase agreement, an increase of $0.9 million
in costs to third party consultants and regulatory expenses related to clinical trials for lonafarnib, an increase of $0.6 million in other research and development activities and an increase of $0.6 million in personnel-related costs due to
additional headcount in support of Eiger’s research and development activities.
General and administrative
General and administrative expenses increased by $1.4 million, or 339%, to $1.8 million for the nine months ended September 30, 2015 from $0.4 million for
the nine months ended September 30, 2014. The increase was due to an increase of $0.8 million in legal and accounting services in connection with various business development activities, including a potential acquisition and patent related
matters, $0.5 million in personnel-related costs due to increase in headcount, and $0.1 million in facility costs due to the new office facility leased in March 2015.
Comparison of the years ended December 31, 2014 and 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Year Ended
December 31, |
|
|
Dollar
Change |
|
| |
|
2014 |
|
|
2013 |
|
|
| |
|
(in thousands) |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
644 |
|
|
$ |
426 |
|
|
$ |
218 |
|
| General and administrative |
|
|
872 |
|
|
|
526 |
|
|
|
346 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
1,516 |
|
|
|
952 |
|
|
|
564 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(1,516 |
) |
|
|
(952 |
) |
|
|
(564 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(1,516 |
) |
|
$ |
(952 |
) |
|
$ |
(564 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
Research and development expenses increased by $0.2 million, or 51%, to $0.6 million for the year ended December 31, 2014 from $0.4 million for the year
ended December 31, 2013. The increase was due to an increase of $0.2 million in connection with the lonafarnib Phase 2 clinical trials as enrollment increased in 2014 as did the related activities.
General and administrative
General and administrative
expenses increased by $0.3 million or 66%, to $0.9 million for the year ended December 31, 2014 from $0.5 million for the year ended December 31, 2013. The increase was primarily due to an increase of $0.3 million in legal and professional
service costs related to patent and regulatory matters.
216
Liquidity and Capital Resources
Sources of Liquidity
Since inception through
September 30, 2015, Eiger’s operations have been financed primarily by net cash proceeds of $22.6 million from the sale of its convertible preferred stock. As of September 30, 2015, Eiger had $2.1 million in cash and an accumulated
deficit of $22.2 million. Eiger expects that its research and development and general and administrative expenses will increase, and, as a result, Eiger anticipates that it will continue to incur increasing losses in the foreseeable future.
Therefore, Eiger will need to raise additional capital to fund its operations, which may be through the issuance of additional equity, including in connection with the contemplated merger, and potentially through borrowings.
Note and Warrant Purchase Agreement
In November
2015, Eiger entered into a note and warrant purchase agreement with three investors, including two holders of the its convertible preferred stock, which includes the issuance of notes payable in the aggregate principal amount of $6.0 million and the
issuance of warrants to purchase Eiger equity securities. The notes bear interest of 6.0% per annum. The warrants entitle each investor to purchase equity securities for a number of shares equal 15% of the principal borrowed from such investor,
or 17.5% of the principal borrowed from such investor in the event that Eiger does not consummate a reverse merger with a third party then currently reporting under the Securities Act of 1933 and Exchange Act of 1934 by February 28, 2016,
divided by the per share price of equity securities sold in Eiger’s next equity financing that results in total proceeds to Eiger of not less than $25.0 million with an exercise price of $0.01 for each share of common stock purchased under the
warrants. The warrants are exerciseable for the type of equity securities issued by Eiger in a qualified financing as described in the notes, or if no qualified financing is consummated then into shares of Eiger’s common stock. All principal
and interest is due March 31, 2016. However, prior to March 31, 2016, the outstanding balance on the note plus unpaid accrued interest is automatically converted into common stock or preferred stock sold in their next equity financing that
results in total proceeds to Eiger of not less than $25.0 million, which would be satisfied by the financing contemplated prior to the consummation of the merger. If Eiger does not consummate a reverse merger or next equity financing that results in
total proceeds of at least $25.0 million as discussed immediately above, then the per share price to determine the number of shares under the warrant is the per share price from Eiger’s last financing under which it sold shares of its Series
A-1 Preferred Stock. In addition, in the event that 50% of the voting power of its stockholders is transferred in a transaction or series of transactions prior to March 31, 2016 (other than the merger), Eiger will repay the investors 120% of
the outstanding principal plus unpaid accrued interest upon such event.
Cash Flows
The following table summarizes Eiger’s cash flows for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Year Ended
December 31, |
|
|
Nine Months Ended
September 30, |
|
| |
|
2014 |
|
|
2013 |
|
|
2015 |
|
|
2014 |
|
| |
|
(in thousands) |
|
| Cash used in operating activities |
|
$ |
(1,280 |
) |
|
$ |
(856 |
) |
|
$ |
(5,815 |
) |
|
$ |
(631 |
) |
| Cash used in investing activities |
|
|
(2 |
) |
|
|
(2 |
) |
|
|
(27 |
) |
|
|
(1 |
) |
| Cash provided by financing activities |
|
|
1,915 |
|
|
|
750 |
|
|
|
7,201 |
|
|
|
1,590 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net increase/(decrease) in cash |
|
$ |
633 |
|
|
$ |
(108 |
) |
|
$ |
1,359 |
|
|
$ |
958 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities
Cash used in operating activities for the nine months ended September 30, 2015 was $5.8 million, consisting of a net loss of $6.3 million, which was
offset by noncash charges of $0.2 million for issuance of common stock
217
related to an asset purchase agreement, $0.1 million for stock-based compensation expense and net changes in operating assets and liabilities of $0.2 million. The change in Eiger’s net
operating assets and liabilities was primarily due to an increase in cash used for prepaid expenses and other current assets of $0.3 million related to sponsorship of a conference and prepayments related to clinical trials and studies in Q3 2015.
This increase was offset by increases of $0.4 million and $0.1 million in accounts payable and accrued liabilities, respectively, due to increase in research and development activities.
Cash used in operating activities for the nine months ended September 30, 2014 was $0.6 million, consisting of a net loss of $0.7 million, which was
offset by net changes in operating assets and liabilities of $0.1 million. The change in Eiger’s net operating assets and liabilities was due primarily to an increase $0.1 million in accrued liabilities related to the increase in research and
development activities.
Cash used in operating activities for the year ended December 31, 2014 was $1.3 million, consisting of a net loss of $1.5
million, which was offset by net changes in operating assets and liabilities of $0.2 million. The change in Eiger’s net operating assets and liabilities was due primarily to an increase of $0.2 million in accrued liabilities due to an increase
in research and development activities.
Cash used in operating activities for the year ended December 31, 2013 was $0.9 million, consisting of a net
loss of $1.0 million, which was offset by noncash charges of $0.1 million for stock-based compensation expense.
Cash flows from investing
activities
Cash used in investing activities for all periods presented was related to purchases of property and equipment, primarily related to
office and computer equipment.
Cash flows from financing activities
Cash provided by financing activities for all periods presented was related to proceeds from the issuance of convertible preferred stock, net of issuance
costs.
Future Funding Requirements
Eiger has
not generated any revenue from product sales. Eiger does not know when, or if, it will generate any revenue from product sales. Eiger does not expect to generate any revenue from product sales unless and until it obtains regulatory approval for and
commercializes any of its other product candidates. At the same time, Eiger expects its expenses to increase in connection with its ongoing development and manufacturing activities, particularly as Eiger continues the research, development,
manufacture and clinical trials of, and seeks regulatory approval for, its product candidates. Immediately prior to the closing of the merger, Eiger expects to receive proceeds of $33.5 million from the financing contemplated to close
contemporaneously with the Merger Agreement. Upon the closing of the merger, Eiger expects to incur additional costs associated with operating as a public company. In addition, subject to obtaining regulatory approval of any of its product
candidates, Eiger anticipates that it will need substantial additional funding in connection with its continuing operations.
As of September 30,
2015, Eiger had cash of $2.1 million. In November 2015, Eiger issued $6.0 million in aggregate principal amount of notes payable to certain of its existing stockholders and entered into the Merger Agreement with Celladon. Eiger’s present
capital resources are not sufficient to fund its planned operations for a 12-month period without the merger and financing contemplated by the Merger Agreement being completed, and therefore, raises substantial doubt about Eiger’s ability to
continue as a going concern.
Until Eiger can generate a sufficient amount of product revenue to finance its cash requirements, it expects to finance its
future cash needs primarily through the issuance of additional equity, including in connection with the contemplated merger, and potentially through borrowings, and strategic alliances with partner companies. To the
218
extent that Eiger raises additional capital through the issuance of additional equity or convertible debt securities, the ownership interest of Eiger’s stockholders will be diluted, and the
terms of these securities may include liquidation or other preferences that adversely affect the rights of existing stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting Eiger’s
ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If Eiger raises additional funds through marketing and distribution arrangements or other collaborations, strategic alliances or
licensing arrangements with third parties, Eiger may have to relinquish valuable rights to its technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to Eiger. If Eiger is
unable to raise additional funds through equity or debt financings when needed, Eiger may be required to delay, limit, reduce or terminate its product development or commercialization efforts or grant rights to develop and market product candidates
to third parties that Eiger would otherwise prefer to develop and market itself.
Contractual Obligations and Other Commitments
Eiger does not have fixed contractual obligations as of December 31, 2014.
Leases
In March 2015, Eiger entered into a
non-cancellable facility lease agreement for an office facility in Palo Alto, California. The lease commenced on April 1, 2015 and expires 36 months after the commencement date. The lease has a two year renewal option prior to expiration and
includes rent escalation clauses through the lease term. Eiger provided a security deposit of $21,000 as collateral for the lease. As of September 30, 2015, the remaining minimum lease payments was $281,000.
License Agreements
Under various license
agreements, Eiger will be required to make milestone payments and pay royalties and other amounts to third parties.
Merck License Agreement
In September 2010, Eiger entered into an exclusive license agreement with Merck which provides Eiger with the exclusive right to develop and commercialize
lonafarnib in human antiviral indications other than against certain identified viruses. As of September 30, 2015, Eiger was obligated to pay Merck up to an aggregate of $26.0 million in development milestones and will be required to pay tiered
royalties based on aggregate annual net sales of all licensed products from ranging from mid-single to low double-digit royalties on net sales.
Janssen License Agreement
In December 2014, Eiger,
through its wholly-owned subsidiary, EB Pharma LLC entered into a license agreement with Janssen Pharmaceutica NV, or Janssen, with respect to the exclusive right to develop and commercialize tipifarnib. In connection with this license agreement,
Eiger is obligated to make development milestone payments in aggregate of up to $38.0 million, sales milestone payments in aggregate up to $65.8 million, and will be required to pay tiered royalties based on aggregate annual net sales of all
licensed products from ranging mid-single to low double-digit royalties on net sales.
Asset Purchase Agreements
EGI License Agreement
In December 2010, Eiger entered
into an asset purchase agreement with Eiger Group International, Inc., or EGI, to purchase the intellectual property related to the use of farnesyl transferase inhibitors as anti-viral agents and
219
methods to treat viral infections with those inhibitors and inhibitors of prenylation, prenyl cysteine methyltranferase and a protease that removes the XXX tripeptide from the CXXX polypeptide
following prenylation. Eiger will pay a low single digit royalty on aggregate annual net sales if Eiger has not recouped the development costs of any drug product that incorporates clemizole. Once the costs have been recouped, Eiger will pay a low
single digit royalty of future aggregate annual net sales if there is no generic competition for the product and a low single digit royalty of future aggregate annual net sales if there is generic competition for the product. Within the first ten
years after commercialization, Eiger may make a one-time payment of $500,000 each for the three types of product related to such intellectual property that would reduce the payment term for the three products to the tenth anniversary of the first
commercial sale. The obligation to pay royalties expires on a country-by-country and product-by-product basis on the later of either when the product is no longer sold in any country or the earliest of the tenth anniversary of the first commercial
sale of the product.
Exendin Asset Purchase Agreement
In September 2015, Eiger entered into an asset purchase agreement with two individuals, Drs. Tracey McLaughlin and Coleen Craig, or the Sellers, to purchase
the intellectual property rights and an assignment of an exclusive license agreement from the Board of Trustees of the Leland Stanford Junior University, or Stanford, related to the compound exendin. In relation to this asset purchase agreement,
Eiger is obligated to pay to each of the two individuals milestone payments in aggregate up to $1.0 million and low single digit royalties based on aggregate annual net sales of all products developed based upon exendin. Under the related Stanford
University exclusive license agreement assigned as part of the asset purchase, Eiger is additionally obligated to pay Stanford low single digit royalties on net sales.
Eiccose Asset Purchase Agreement
In October 2015, Eiger
entered into an asset purchase agreement with Eiccose, LLC, or Eiccose, which is owned in significant part by Eiger’s chief executive officer, to purchase the intellectual property related to the treatment of PAH treatment of lymphedema and
products containing ubenimex for the treatment of PAH. Eiger is obligated to pay to Eiccose an aggregate of $10.0 million in connection with future sales of commercial sale of the product and low single digit royalties on aggregate annual net sales
following the first commercial sale of any product.
In addition, as a result of this agreement, Eiger assumed the license agreements Eiccose had
previously entered into. These include two license agreements with Stanford for the treatment of PAH and lymphedema, respectively, and a license agreement with Nippon Kayaku Co., Ltd. In connection with the each license agreement with Stanford,
Eiger is obligated to make milestone payments in aggregate of $500,000 for each contract, increasing annual license maintenance fees ranging from $10,000 to $75,000 over the term of each license agreement and royalty payments in low single digits on
annual net sales after the first commercial sale of a product under each license. Additionally, as part of the agreement, Nippon is obligated to make a payment for royalties in the low single digits of sales to Eiger.
Contract Manufacturing Arrangement
In September
2015, Eiger began using a contract manufacturing organization for the production of its Phase 2 clinical trial materials and issued a non-cancelable purchase order to the contract manufacturer for approximately $1.8 million. Eiger has paid $0.6
million of this commitment in November 2015 with the remaining balance to be paid 30 days after the delivery of the material, which is scheduled for January 2016.
Other Contracts
Eiger enters into contracts in
the normal course of business with various third parties for preclinical research studies, clinical trials, testing and other services. These contracts generally provide for termination upon notice, and therefore Eiger believes that its
non-cancelable obligations under these agreements are not material.
220
Off-Balance Sheet Arrangements
Eiger has not entered into any off-balance sheet arrangements and do not have any holdings in variable interest entities.
Recent Accounting Pronouncements
In June 2014, the
Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest
Entities Guidance in Topic 810, Consolidation. ASU 2014-10 simplifies the accounting guidance by removing all incremental financial reporting requirements for development stage entities. The amendments related to the elimination of the
inception-to-date information and other disclosure requirement of Topic 915 should be applied retrospectively, and are effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. Eiger early adopted
this guidance and accordingly, there is no inception to date information presented in its consolidated financial statements.
In August 2014, the FASB
issued ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. ASU 2014-15 requires management to evaluate relevant conditions, events and certain management plans that are known or reasonably knowable
that when, considered in the aggregate, raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued, for both annual and interim periods. ASU 2014-15
also requires certain disclosures around management’s plans and evaluation, as well as the plans, if any, that are intended to mitigate those conditions or events that will alleviate the substantial doubt. ASU 2014-15 is effective for fiscal
years ending after December 15, 2016. Eiger does not anticipate the adoption of ASU 2014-15 to have a material impact on its consolidated financial statements and related disclosures.
Internal Control over Financial Reporting
During the
audit of Eiger’s consolidated financial statements for the years ended December 31, 2014 and 2013, a material weakness was identified in Eiger’s internal control over financial reporting. Under standards established by the Public
Company Accounting Oversight Board, a material weakness is a deficiency or combination of deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of Eiger’s annual or
interim financial statements will not be prevented or detected and corrected on a timely basis. The material weakness that was identified related to a lack of sufficient accounting resources and personnel that limits Eiger’s ability to
adequately segregate duties, establish defined accounting policies and procedures and perform timely reviews of account reconciliations.
221
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT EIGER MARKET RISK
As of September 30, 2015, Eiger had cash of $2.1 million, which consisted of bank deposits. Such interest-earning instruments carry a degree of
interest rate risk; however, historical fluctuations of interest income have not been significant. Eiger has not been exposed nor does it anticipate being exposed to material risks due to changes in interest rates. A hypothetical 10% change in
interest rates during any of the periods presented would not have had a material impact on Eiger’s consolidated financial statements.
Eiger has an
ongoing agreement for the manufacturing of one of its product candidates denominated in yen. Eiger does not participate in any foreign currency hedging activities and it does not have any other derivative financial instruments. Eiger did not
recognize any significant exchange rate losses during the nine month period ended September 30, 2015. A 10% change in the yen-to-dollar exchange rate on September 30, 2015 would not have had a material effect on Eiger’s results of
operations or financial condition.
222
MANAGEMENT FOLLOWING THE MERGER
Executive Officers and Directors
Resignation of Current Executive Officers of Celladon
Pursuant to the Merger Agreement, all of the current executive officers of Celladon will resign immediately prior to the completion of the merger and the Eiger
executive officers will remain in their current rolls post-merger.
Executive Officers and Directors of the Combined Company Following the Merger
The Celladon board of directors is currently composed of four directors. Pursuant to the Merger Agreement, all of the directors of Celladon will
resign at or prior to the effective time of the merger. As of the effective time of the merger, the board of directors will initially consist of the five directors currently serving on the Eiger board of directors. Additionally, prior to the
effective time of the merger, but to be effective as of the effective time of the merger, the Celladon board of directors will appoint two additional designees selected by Eiger (with such designees expected to satisfy the requisite independence
requirements, as well as the sophistication and independence requirements for the required committees, pursuant to NASDAQ’s listing standards). All of the directors will serve in staggered classes to be designated by Eiger prior to closing.
Following the merger, the management team of Celladon is expected to be composed of the management team of Eiger. The following table lists the
names, ages as of January 1, 2016 and positions of the individuals who are expected to serve as executive officers and directors of Celladon upon completion of the merger:
|
|
|
|
|
|
|
| Name |
|
Age |
|
|
Position(s) |
| Executive Officers |
|
|
|
|
|
|
| David Cory, R.Ph. |
|
|
52 |
|
|
President, Chief Executive Officer and Director |
| James Welch |
|
|
58 |
|
|
Chief Financial Officer |
| Joanne Quan, M.D. |
|
|
52 |
|
|
Chief Medical Officer |
| James Shaffer |
|
|
49 |
|
|
Chief Business Officer |
| Eduardo Martins, M.D., D.Phil |
|
|
53 |
|
|
Senior Vice President, Liver and Infectious Disease |
| Non-Employee Directors |
|
|
|
|
|
|
| Edgar Engleman, M.D. |
|
|
69 |
|
|
Director |
| Nina Kjellson |
|
|
41 |
|
|
Director |
| Jeffrey Glenn, M.D., Ph.D. |
|
|
53 |
|
|
Director |
| Thomas Dietz, Ph.D. |
|
|
52 |
|
|
Director |
Executive Officers
David Cory, R.Ph. David Cory has been the President and Chief Executive Officer of Eiger since 2009. Prior to working at Eiger, Mr. Cory was
Chief Executive Officer of DiObex from 2007 to 2008 and President and Chief Operating Officer at Prestwick Pharmaceuticals from 2004 to 2006. Mr. Cory was Co-Founder and Acting Chief Commercial Officer at CoTherix in 2003 and Senior Vice
President of Sales and Marketing at InterMune from 2000 to 2003. Previously, Mr. Cory held positions of increasing responsibility in Commercial Operations at Glaxo, Glaxo Wellcome, and Glaxo Smith Kline. Mr. Cory earned a B.S. in Pharmacy
from the University of Cincinnati, College of Pharmacy and an M.B.A. from the University of Maryland University College.
Eiger believes
Mr. Cory’s qualifications to sit on the board of directors include his extensive management experience in the biopharmaceutical industry.
James Welch. James Welch has been the Chief Financial Officer of Eiger since August 2015. Mr. Welch has over 20 years of experience as Chief
Financial Officer at both public and private companies including at Virobay Inc. from 2014 to 2015, at AcelRx Pharmaceuticals, Inc. from 2010 to 2014, at Cerimon Pharmaceuticals, Inc.
223
from 2006 to 2010, at Rigel Pharmaceuticals, Inc. from 1999 to 2006, and at Biocircuits Corporation from 1992 to 1998. Mr. Welch graduated from Whitworth College with a B.A. in
Business Administration and from Washington State University with an M.B.A. in Finance.
Joanne Quan, M.D. Joanne Quan has
been the Chief Medical Officer of Eiger since April 2015. Prior to joining Eiger, Dr. Quan was Vice President, New Product Clinical Development at InterMune from 2014 to 2015. Previously, she was Vice President, Clinical Development at Arena
Pharmaceuticals from 2012 to 2014. Prior to this, Dr. Quan held scientific, clinical and regulatory positions of increasing responsibility at BioMarin Pharmaceuticals from 2011 to 2012, Bayhill Therapeutics from 2008 to 2011, at Alza
Corporation (Johnson and Johnson) from 2005 to 2008, at Genentech from 2000 to 2005, and at PathoGenesis Corporation from 1996 to 2000. Dr. Quan received a B.A. in Molecular Biology at the University of California, Berkeley and an M.D. at
Stanford University School of Medicine. She completed a residency in Internal Medicine at Massachusetts General Hospital and a fellowship in Pulmonary and Critical Care Medicine at the University of Washington, Seattle.
James Shaffer. James Shaffer has been the Chief Business Officer of Eiger since September 2015, having previously served as a consultant to Eiger from
August 2014 through September 2015. Prior to his time at Eiger, Mr. Shaffer was Vice President and Chief Commercial Officer at Halozyme Therapeutics from 2011 to 2014 and Executive Vice President and Chief Commercial Officer at Clinical Data
Inc. from 2007 to 2011. Prior to those positions, Mr. Shaffer held a series of different sale and product related positions of increasing responsibility at multiple pharmaceutical companies. Mr. Shaffer earned a B.S. in Agriculture
Economics and a M.B.A. from the Ohio State University.
Eduardo Martins, M.D., D.Phil. Eduardo Martins has been the Senior Vice President, Liver
and Infectious Diseases of Eiger since November 2015. Priror to this position, Dr. Martins was the Senior Director, Medical Affairs for Hepatitis at Gilead Sciences from December 2010 to October 2015, Senior International Medical Leader for
Pegasys (pegylated interferon alfa-2a) at Genentech, a member of the Roche Group, from August 2009 to December 2010, and head of the Office of International Development at Genentech from December 2008 to August 2009. Prior to joining Genentech,
Dr. Martins worked in positions of increasing responsibility at Dynavax Technologies from March 2006 to December 2008, at InterMune from February 2005 to February 2006, at SciClone Pharmaceuticals from July 1999 to January 2005, primarily
focused on vaccines and therapeutics for viral hepatitis. Dr. Martins received an M.D. from the Medical School, Federal University of Rio De Janeiro, Brazil and a Ph.D. in Immunology of Liver Diseases from the University of Oxford (D.Phil.),
United Kingdom. He completed specialty training in Gastroenterology and Hepatology at John Radcliffe Hospital, Oxford, United Kingdom.
Non-Employee
Directors
Edgar Engleman, M.D. Edgar Engleman has been a member of Eiger’s board of directors since his appointment in 2008.
Dr. Engleman is a founding member and Managing Partner of Vivo Capital, LLC, founded in 1996. Dr. Engleman has been a Professor of Pathology and Medicine at Stanford University School of Medicine since 1978, where he oversees the Stanford
Blood Center and his own immunology research group. Dr. Engleman currently serves on the boards of several private biotechnology companies and two public companies, Capnia and RegenX. He has co-founded a number of biopharmaceutical companies
including Cetus Immune in 1980, Genelabs in 1983, Dendreon in 1992 and Medeor in 2013. He received his B.A. from Harvard University and his M.D. from Columbia University School of Medicine. He also completed residency training in internal medicine
at the University of California, San Francisco, and training in immunology, rheumatology and transfusion medicine at Stanford University School of Medicine.
Eiger believes Dr. Engleman’s qualifications to sit on the board of directors include his medical and research backgrounds and extensive experience
in the biopharmaceutical industry.
224
Nina Kjellson. Nina Kjellson has been a member of Eiger’s board of directors since her appointment in
2008. Ms. Kjellson is currently a general partner at Canaan Partners, a venture capital firm. Prior to joining Canaan Partners, Ms. Kjellson held various roles at InterWest Partners, a venture capital firm, from 2002 to September 2015,
most recently as a general partner. Prior to joining InterWest, Ms. Kjellson was an investment manager at Bay City Capital, a life sciences merchant bank from 1999 to 2000, and a research associate at Oracle Partners, a healthcare-focused hedge
fund. From 1997 to 1999, Ms. Kjellson conducted health policy and survey research with the Kaiser Family Foundation. Ms. Kjellson currently also serves on the board of directors of Lycera Corp., Ocera Therapeutics Inc., and Welltok, Inc.
Ms. Kjellson received a B.S. in human biology from Stanford University.
Eiger believes Ms. Kjellson’s qualifications to sit on the board
of directors include her extensive experience in venture capital and biopharmaceutical industries.
Jeffrey Glenn, M.D., Ph.D. Jeffrey Glenn has
been a member of Eiger’s board of directors since his appointment in 2008. Dr. Glenn has been an Associate Professor of Medicine, Division of Gastroenterology & Hepatology, and Microbiology & Immunology at Stanford
University School of Medicine since 2000, and the Director of the Center for Hepatitis and Liver Tissue Engineering since 2006. Dr. Glenn is also the scientific founder of Eiger. Dr. Glenn earned an A.B. in biochemistry and French
civilization form University of California, Berkeley and both a M.D. and Ph.D. in biochemistry from University of California, San Francisco. He also completed an internal medicine residency and a gastroenterology fellowship at Stanford University
Medical Center.
Eiger believes Dr. Glenn’s qualifications to sit on the board of directors include his medical and research backgrounds.
Thomas Dietz, Ph.D. Thomas Dietz has been a member of Celladon’s board of directors since his appointment in October 2015. Dr. Dietz has
served as Chairman and CEO of Waypoint Holdings, LLC, a financial services firm, since December 2010. Dr. Dietz was previously co-CEO and then CEO and a director of Pacific Growth Equities, LLC, an investment bank and institutional brokerage
firm, from 2004 to January 2009, when the firm was acquired by Wedbush Securities, a financial services firm. Dr. Dietz subsequently served as head of the investment banking division at Wedbush until November 2010. Dr. Dietz joined Pacific
Growth in 1993 and served in various roles, including senior roles in equities research and investment banking, prior to taking the CEO role there. Previously, Dr. Dietz was a member of the research faculty in the Department of Medicine,
University of California, San Francisco and the VA Medical Center. Dr. Dietz holds a Ph.D. in molecular biology and biochemistry from Washington University in St. Louis.
Eiger believes Dr. Dietz’s qualifications to sit on the board of directors include his medical and research backgrounds and extensive finance and
executive experience in the financial services industry.
Composition of the Board of Directors
The board of directors of Celladon currently consists of four directors divided into three staggered classes, each class serving three-year terms. The
staggered structure of the board of directors will remain in place for the combined company board of directors following completion of the merger. At the most recent annual meeting of stockholders of Celladon held in 2015, Class II directors were
elected. As a result, the term of the Class II directors of the combined company will expire upon the election and qualification of successor directors at the annual meeting of stockholders in 2018, with the terms of the Class III directors and
Class I directors expiring upon the election and qualification of successor directors at the annual meetings of stockholders to be held in 2016 and 2017, respectively.
The director classes for Celladon are currently as follows:
| |
• |
|
Class I directors: Peter Honig, M.D., M.P.H.; |
225
| |
• |
|
Class II directors: Michael Narachi; and |
| |
• |
|
Class III directors: Gregg Alton and Graham Cooper. |
Pursuant to the Merger Agreement, all of the directors of
Celladon will resign at or prior to the effective time of the merger. As of the effective time of the merger, the board of directors will consist of seven directors, five of whom are currently serving on the Eiger board of directors. Additionally,
prior to the effective time of the merger, but to be effective as of the effective time of the merger, the Celladon board of directors will appoint two additional designees selected by Eiger (with such designees expected to satisfy the requisite
independence requirements, as well as the sophistication and independence requirements for the required committees, pursuant to NASDAQ’s listing standards). All of the directors will serve in staggered classes to be designated by Eiger prior to
closing.
There are no family relationships among any of the current Celladon directors and executive officers, and there are no family relationships
among any of the proposed post-merger company directors and executive officers.
The division of the board of directors into three classes with staggered
three-year terms may delay or prevent a change of management or a change of control of Celladon, or, following the completion of the merger, the combined company.
Director Independence
The Celladon board of
directors has determined that each of its current directors is independent as defined under The NASDAQ Stock Market listing standards. The Celladon board of directors has also determined that each current member of the Nominating and Corporate
Governance Committee is independent as defined under The NASDAQ Stock Market listing standards, and that each current member of the Audit Committee and Compensation Committee is independent as defined under The NASDAQ Stock Market listing standards
and applicable SEC rules. In making this determination, Celladon’s board of directors found that none of these directors had a material or other disqualifying relationship with Celladon.
Based upon information requested from and provided by each proposed director concerning his or her background, employment and affiliations, including family
relationships, other than David Cory by virtue of his position as Chief Executive Officer of Eiger, the Eiger board of directors has determined that each of the Eiger director designees anticipated to serve on the board of directors of the combined
company as of the effective time of the merger is independent as defined under The NASDAQ Stock Market listing standards. Eiger anticipates that the directors who will be appointed to the Compensation Committee and the Nominating and Governance
Committee will satisfy the independence standards for such committees established by the SEC and The NASDAQ Stock Market listing standards, as applicable. With respect to the Audit Committee, Eiger anticipates that the directors who will be
appointed will satisfy the independence standards for such committee established by Rule 10A-3 under the Exchange Act, the SEC and The NASDAQ Stock Market listing standards, as applicable. In making such determination, the relationships that each
such director has with Celladon or Eiger and all other facts and circumstances deemed relevant in determining their independence have been and will be considered.
Committees of the Board of Directors
The Celladon board of directors currently has, and after completion of the merger the combined organization will continue to have, an Audit Committee, a
Compensation Committee and a Nominating and Corporate Governance Committee.
Audit Committee
The Audit Committee of the board of directors was established by Celladon’s board of directors in accordance with Section 3(a)(58)(A) of the Exchange
Act to oversee Celladon’s corporate accounting and financial reporting
226
processes and audits of its financial statements. For this purpose, the Audit Committee performs several functions, including, among other things:
| |
• |
|
evaluating the performance, independence and qualifications of Celladon’s independent auditors and determining whether to retain its existing independent auditors or engage new independent auditors;
|
| |
• |
|
reviewing and approving the engagement of Celladon’s independent auditors to perform audit services and any permissible non-audit services; |
| |
• |
|
monitoring the rotation of partners of Celladon’s independent auditors on its engagement team as required by law; |
| |
• |
|
prior to engagement of any independent auditor, and at least annually thereafter, reviewing relationships that may reasonably be thought to bear on their independence, and assessing and otherwise taking the appropriate
action to oversee the independence of Celladon’s independent auditor; |
| |
• |
|
reviewing Celladon’s annual and quarterly financial statements and reports, including the disclosures contained under the caption “Celladon Management’s Discussion and Analysis of Financial Condition and
Results of Operations,” and discussing the statements and reports with its independent auditors and management; |
| |
• |
|
reviewing with Celladon’s independent auditors and management significant issues that arise regarding accounting principles and financial statement presentation and matters concerning the scope, adequacy and
effectiveness of Celladon’s financial controls; |
| |
• |
|
reviewing with management and Celladon’s auditors any earnings announcements and other public announcements regarding material developments; |
| |
• |
|
establishing procedures for the receipt, retention and treatment of complaints received by Celladon regarding financial controls, accounting or auditing matters and other matters; |
| |
• |
|
preparing the report that the SEC requires in Celladon’s annual proxy statement; |
| |
• |
|
reviewing and providing oversight of any related-person transactions in accordance with Celladon’s related-person transaction policy and reviewing and monitoring compliance with legal and regulatory
responsibilities, including Celladon’s code of business conduct and ethics; |
| |
• |
|
reviewing Celladon’s major financial risk exposures, including the guidelines and policies to govern the process by which risk assessment and risk management is implemented; |
| |
• |
|
reviewing on a periodic basis Celladon’s investment policy; and |
| |
• |
|
reviewing and evaluating on an annual basis the performance of the Audit Committee, including compliance of the Audit Committee with its charter. |
The Audit Committee of the combined organization is expected to retain these duties and responsibilities following completion of the merger.
Celladon’s management has the primary responsibility for its consolidated financial statements and the reporting process including its system of internal
accounting and financial controls.
Celladon’s Audit Committee currently consists of Mr. Cooper, who serves as its chairman, Dr. Honig and
Mr. Narachi. The Celladon board of directors reviews the NASDAQ listing standards definition of independence for Audit Committee members on an annual basis and has determined that all current members of Celladon’s Audit Committee are
independent (as independence is currently defined in Rule 5605(c)(2)(A) of the NASDAQ listing standards and Rule 10A-3 of the Exchange Act).
The Celladon
board of directors has also determined that Mr. Cooper qualifies as an “audit committee financial expert,” as defined in applicable SEC rules. The Celladon board of directors made a qualitative assessment of
227
Mr. Cooper’s level of knowledge and experience based on a number of factors, including his formal education and experience in financial roles.
Eiger believes that, after the completion of the merger, the composition of the Audit Committee will meet the requirements for independence under, and the
Compensation Committee will comply with, any applicable requirements of the rules and regulations of The NASDAQ Stock Market LLC and the SEC.
Compensation Committee
The Compensation Committee
of the board of directors acts on behalf of the board to review, adopt or recommend for adoption, and oversee Celladon’s compensation strategy, policies, plans and programs. For this purpose, the Compensation Committee performs several
functions, including, among other things:
| |
• |
|
reviewing, modifying and approving (or if it deems appropriate, making recommendations to the full board of directors regarding) Celladon’s overall compensation strategy and policies; |
| |
• |
|
reviewing, modifying and approving (or if it deems appropriate, making recommendations to the full board of directors regarding) the compensation and other terms of employment of Celladon’s executive officers;
|
| |
• |
|
reviewing and making recommendations to the full board of directors regarding performance goals and objectives relevant to the compensation of Celladon’s executive officers and assessing their performance against
these goals and objectives; |
| |
• |
|
reviewing and approving (or if it deems it appropriate, making recommendations to the full board of directors regarding) the equity incentive plans, compensation plans and similar programs advisable for Celladon, as
well as modifying, amending or terminating existing plans and programs; |
| |
• |
|
evaluating risks associated with Celladon’s compensation policies and practices and assessing whether risks arising from Celladon’s compensation policies and practices for its employees are reasonably likely
to have a material adverse effect on Celladon; |
| |
• |
|
reviewing and making recommendations to the full board of directors regarding the type and amount of compensation to be paid or awarded to Celladon’s non-employee board members; |
| |
• |
|
establishing policies with respect to votes by Celladon’s stockholders to approve executive compensation to the extent required by Section 14A of the Exchange Act and, if applicable, determining
Celladon’s recommendations regarding the frequency of advisory votes on executive compensation; |
| |
• |
|
reviewing and assessing the independence of compensation consultants, legal counsel and other advisors as required by Section 10C of the Exchange Act; |
| |
• |
|
administering Celladon’s equity incentive plans; |
| |
• |
|
establishing policies with respect to equity compensation arrangements; |
| |
• |
|
reviewing the competitiveness of Celladon’s executive compensation programs and evaluating the effectiveness of Celladon’s compensation policy and strategy in achieving expected benefits to Celladon;
|
| |
• |
|
reviewing, modifying and approving (or if it deems appropriate, making recommendations to the full board of directors regarding) the terms of any employment agreements, severance arrangements, change of control
protections and any other compensatory arrangements for Celladon’s executive officers; |
| |
• |
|
reviewing the adequacy of its charter on a periodic basis; |
| |
• |
|
reviewing with management and approving Celladon’s disclosures under the caption “Compensation Discussion and Analysis” in Celladon’s periodic reports or proxy statements to be filed with the SEC, to
the extent such caption is included in any such report or proxy statement; |
228
| |
• |
|
preparing the report that the SEC requires in Celladon’s annual proxy statement; and |
| |
• |
|
reviewing and assessing on an annual basis the performance of the Compensation Committee. |
The Compensation
Committee of the combined organization is expected to retain these duties and responsibilities following completion of the merger.
Celladon’s
Compensation Committee currently consists of Mr. Narachi, who serves as its chairman, and Mr. Alton. Both members of the Compensation Committee are independent as independence is currently defined in Rule 5605(d)(2)(A) of the NASDAQ
listing standards and Rule 10C-1 of the Exchange Act.
Eiger believes that, after the completion of the merger, the composition of the Compensation
Committee will meet the requirements for independence under, and the Compensation Committee will comply with, any applicable requirements of the rules and regulations of The NASDAQ Stock Market LLC and the SEC.
Nominating and Corporate Governance Committee
The
Nominating and Corporate Governance Committee of the board of directors is responsible for identifying, reviewing and evaluating candidates to serve as directors of Celladon (consistent with criteria approved by the Board), reviewing and evaluating
incumbent directors, selecting or recommending to the Board for selection candidates for election to the board of directors, making recommendations to the Board regarding the membership of the committees of the Board, assessing the performance of
the Board, and developing a set of corporate governance principles for Celladon. The responsibilities of the Nominating and Corporate Governance Committee relating to the nomination of directors include, among other things, the following:
| |
• |
|
considering and approving all nominees for membership on Celladon’s board of directors, including the slate of nominees to be proposed by Celladon’s board of directors to Celladon’s stockholders for
election at an annual meeting of stockholders and any nominees to be elected or appointed by the board to fill interim director vacancies; |
| |
• |
|
evaluating all proposed director nominees; and |
| |
• |
|
assessing incumbent directors before recommending re-nomination. |
The Nominating and Corporate Governance
Committee believes that candidates for director should have certain minimum qualifications, including the ability to read and understand basic financial statements and having the highest personal integrity and ethics. The Nominating and Corporate
Governance Committee also intends to consider such factors as possessing relevant expertise upon which to be able to offer advice and guidance to management, having sufficient time to devote to the affairs of Celladon, demonstrated excellence in his
or her field, having the ability to exercise sound business judgment and having the commitment to rigorously represent the long-term interests of Celladon’s stockholders. However, the Nominating and Corporate Governance Committee retains the
right to modify these qualifications from time to time. Candidates for director nominees are reviewed in the context of the current composition of the board, the operating requirements of Celladon and the long-term interests of stockholders. In
conducting this assessment, the Nominating and Corporate Governance Committee typically considers diversity, age, skills and such other factors as it deems appropriate, given the current needs of the board and Celladon, to maintain a balance of
knowledge, experience and capability. In the case of incumbent directors whose terms of office are set to expire, the Nominating and Corporate Governance Committee reviews these directors’ overall service to Celladon during their terms,
including the number of meetings attended, level of participation, quality of performance and any other relationships and transactions that might impair the directors’ independence. In the case of new director candidates, the Nominating and
Corporate Governance Committee also determines whether the nominee is independent for NASDAQ purposes, which determination is based upon applicable NASDAQ listing standards, applicable SEC rules and regulations and the advice of counsel, if
necessary. The Nominating and Corporate Governance Committee then uses its network of contacts to compile a list of potential candidates, but may also engage, if it deems appropriate, a professional search firm. The Nominating and Corporate
Governance
229
Committee conducts any appropriate and necessary inquiries into the backgrounds and qualifications of possible candidates after considering the function and needs of the board. The Nominating and
Corporate Governance Committee meets to discuss and consider the candidates’ qualifications and then selects a nominee by majority vote which is typically recommended to the full board.
The Nominating and Corporate Governance Committee will consider director candidates recommended by stockholders. The Nominating and Corporate Governance
Committee does not intend to alter the manner in which it evaluates candidates, including the minimum criteria set forth above, based on whether or not the candidate was recommended by a stockholder. Stockholders who wish to recommend individuals
for consideration by the Nominating and Corporate Governance Committee to become nominees for election to the board may do so by delivering a written recommendation to the Nominating and Corporate Governance Committee at the following address: c/o
Celladon Corporation, 12707 High Bluff Drive, Suite 200, San Diego, California 92130, Attn: Secretary, no later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the
preceding year’s annual meeting. Submissions must include the name and address of Celladon stockholder on whose behalf the submission is made; the number of Company shares that are owned beneficially by such stockholder as of the date of the
submission; the full name of the proposed candidate; a description of the proposed candidate’s business experience for at least the previous five years; complete biographical information for the proposed candidate; and a description of the
proposed candidate’s qualifications as a director. Any such submission must be accompanied by the written consent of the proposed nominee to be named as a nominee and to serve as a director if elected.
The responsibilities of the Nominating and Corporate Governance Committee relating to corporate governance include, among other things, the following:
| |
• |
|
consideration and assessing the independence of members of the board of directors; |
| |
• |
|
developing, as appropriate, and recommending to the board the governance principles applicable to Celladon; |
| |
• |
|
overseeing the evaluation of the board; |
| |
• |
|
recommending director nominees for each committee of the board; |
| |
• |
|
considering questions of possible conflicts of interest as such questions arise; and |
| |
• |
|
making recommendations to the board regarding committee organization, membership, function and effectiveness. |
The Nominating and Corporate Governance Committee of the combined organization is expected to retain these duties and responsibilities following completion of
the merger.
The Nominating and Corporate Governance Committee currently consists of Mr. Alton, who serves as its chairman, Mr. Cooper and
Dr. Honig. All members of the Nominating and Corporate Governance Committee are independent (as independence is currently defined in Rule 5605(a)(2) of the NASDAQ listing standards).
Eiger believes that, after the completion of the merger, the composition of the Nominating and Corporate Governance Committee will meet the requirements for
independence under, and the Compensation Committee will comply with, any applicable requirements of the rules and regulations of The NASDAQ Stock Market LLC and the SEC.
The board of directors of Celladon may from time to time establish other committees.
230
2015 Eiger Director Compensation
The table below shows all compensation earned by or paid to Eiger’s non-employee directors during the year ended December 31, 2015.
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
|
Fees Earned or
Paid in Cash |
|
|
Option
Awards |
|
|
Total |
|
| Ed Engleman |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
| Nina Kjellson |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
| Thomas J. Dietz, Ph.D. |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
| Jeffrey S. Glenn, M.D., Ph.D |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
Upon completion of the merger, the Eiger board of directors intends to establish a compensation program for non-employee
directors.
Compensation Committee Interlocks and Insider Participation
Composition of the Compensation Committee for the combined company has not yet been determined. Following completion of the merger, each member designated by
Eiger and appointed to the Compensation Committee is expected to be an “outside” director as that term is defined in Section 162(m) of the Internal Revenue Code, a “non-employee” director within the meaning of Rule 16b-3 of
the rules promulgated under the Exchange Act and independent within the meaning of the independent director guidelines of The NASDAQ Stock Market. None of the proposed combined company’s executive officers serve as a member of the board of
directors or compensation committee of any entity that has one or more executive officers who is proposed to serve on the combined company’s board of directors or Compensation Committee following the merger.
Executive Compensation
This section discusses the material components of the executive compensation program offered to Eiger’s named executive officers identified below.
As noted above, Celladon is an “emerging growth company” within the meaning of the JOBS Act, and has elected to comply with the reduced
compensation disclosure requirements available to emerging growth companies under the JOBS Act.
2015 Summary Compensation Table
The following table provides information regarding Eiger’s named executive officers during the fiscal year ended December 31, 2014 and 2015. For the
management of the combined company after the closing of the merger, see “Management Following the Merger—Executive Officers and Directors—Executive Officers and Directors of the Combined Company Following the Merger.” These
individuals are referred to elsewhere in this proxy statement/prospectus/information statement as the “named executive officers” of Eiger.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name and Principal Position |
|
Year |
|
|
Salary |
|
|
Bonus |
|
|
Option
Awards(1) |
|
|
All Other
Compensation |
|
|
Total |
|
| David A. Cory |
|
|
2015 |
|
|
$ |
354,375 |
|
|
|
— |
|
|
|
$931,057 |
|
|
|
$78,000(2) |
|
|
$ |
1,363,432 |
|
| President and Chief Executive Officer |
|
|
2014 |
|
|
$ |
192,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
192,000 |
|
| James Shaffer(3) |
|
|
2015 |
|
|
$ |
100,000 |
|
|
|
— |
|
|
$ |
328,609 |
|
|
$ |
200,000 |
(4) |
|
$ |
628,609 |
|
| Chief Business Officer |
|
|
2014 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
87,500 |
(4) |
|
$ |
87,500 |
|
| Joanne Quan(5) |
|
|
2015 |
|
|
$ |
239,829 |
|
|
|
— |
|
|
$ |
328,609 |
|
|
|
— |
|
|
$ |
568,438 |
|
| Chief Medical Officer |
|
|
2014 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
231
| (1) |
The amounts in the “Option Awards” column reflect the aggregate grant date fair value of stock options granted during the calendar year computed in accordance with the provisions of Accounting Standards
Codification (ASC) 718, Compensation—Stock Compensation. The assumptions that Eiger used to calculate these amounts are discussed in the notes to the unaudited interim condensed consolidated financial statements of Eiger included
elsewhere in this proxy statement/prospectus/information statement. These amounts do not reflect the actual economic value that will be realized by the named executive officer upon the vesting of the stock options, the exercise of the stock options,
or the sale of the common stock underlying such stock options. |
| (2) |
Represents an amount otherwise owed by Mr. Cory to Eiger in respect of certain company awards that was waived by the board of directors of Eiger in recognition of Mr. Cory’s services in 2015.
|
| (3) |
Mr. Shaffer became an employee of Eiger in September 2015. From August 2014 through September 2015, Mr. Shaffer served as a consultant to Eiger. |
| (4) |
Represents consulting fees earned by Mr. Shaffer in respect of strategic and business consulting services provided by Mr. Shaffer from August 2014 through September 2015, prior to Mr. Shaffer becoming an
employee of Eiger. The amount reflected for Mr. Shaffer for 2014 includes $75,000 earned in 2014, which was paid in 2015. |
| (5) |
Ms. Quan became an employee of Eiger in April 2015. |
Narrative Disclosure to Summary Compensation
Table
The primary element of compensation for Eiger’s named executive officers are base salary and long-term, equity-based compensation
awards. The named executive officers also participate in employee benefit plans and programs that Eiger offers to its other full-time employees on the same basis.
Base Salary
The base salary payable to Eiger’s
named executive officers is intended to provide a fixed component of compensation that reflects the executive’s skill set, experience, role and responsibilities. During the fiscal year ended December 31, 2014, Mr. Cory worked part
time for Eiger and received a salary of $192,000.
Health, Welfare and Additional Benefits
Eiger’s named executive officer is eligible to participate in Eiger’s employee benefit plans and programs, including medical and dental benefits,
flexible spending accounts, long-term care benefits, and short- and long-term disability and life insurance, to the same extent as its other full-time employees, subject to the terms and eligibility requirements of those plans.
Although Eiger does not have a formal policy with respect to the grant of equity incentive awards to its executive officers or any formal equity ownership
guidelines applicable to them, Eiger believes that equity grants provide its executives with a strong link to Eiger’s long-term performance, create an ownership culture and help to align the interests of Eiger’s executives and its
stockholders. In addition, Eiger believes that equity grants with a time-based vesting feature promote executive retention because this feature incentivizes executive officers to remain in Eiger’s employment during the vesting period. In 2015,
Eiger granted Mr. Cory an option to purchase 849,000 shares of Eiger common stock, Mr. Shaffer an option to purchase 300,000 shares of Eiger common stock and Ms. Quan an option to purchase 300,000 shares of its common stock.
232
2015 Outstanding Equity Awards at Year-End
The following table presents the outstanding equity awards held by Eiger’s named executive officers as of December 31, 2015.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
|
Vesting
Commencement
Date |
|
|
Number of
securities
underlying
unexercised
options
exercisable |
|
|
Number of
securities
underlying
unexercised
options
unexercisable |
|
|
Option
Exercise
price |
|
|
Option
Expiration
date |
|
| David A. Cory |
|
|
09/24/2013 |
|
|
|
649,999 |
|
|
|
0 |
|
|
$ |
0.12 |
|
|
|
09/24/2023 |
|
|
|
|
09/24/2013 |
(1) |
|
|
112,500 |
|
|
|
87,500 |
|
|
$ |
0.12 |
|
|
|
09/24/2023 |
|
|
|
|
09/22/2015 |
(2) |
|
|
0 |
|
|
|
849,000 |
|
|
$ |
0.18 |
|
|
|
09/22/2025 |
|
| James Shaffer |
|
|
09/01/2015 |
(2) |
|
|
0 |
|
|
|
300,000 |
|
|
$ |
0.18 |
|
|
|
09/22/2025 |
|
| Joanne Quan |
|
|
04/14/2015 |
(2) |
|
|
0 |
|
|
|
300,000 |
|
|
$ |
0.18 |
|
|
|
09/22/2025 |
|
| (1) |
The option vests as to 1/48 of the shares in monthly installments measured from September 24, 2013. |
| (2) |
Twenty-five percent of the shares subject to the option vest on the first anniversary of the vesting |
commencement date, and the remainder vests in 36 equal monthly installments thereafter.
Employment and Severance Agreements
Eiger entered
into an employment agreement with Mr. Cory in December 2008. The agreement is for an unspecified term and entitles Mr. Cory to an initial annual base salary of $320,000. Mr. Cory’s current annual base salary is $320,000. The
agreement also provides that he will be eligible to receive a bonus of up to 30% of base salary based upon his performance and the attainment of company objectives. Pursuant to the terms of the agreement, Mr. Cory is subject to certain
confidentiality obligations and is obligated to sign and comply with an agreement relating to proprietary information and inventions. Further provisions of the agreement are discussed below in the section entitled, “Potential Payments Upon
Termination of Employment or Change in Control.”
Eiger entered into an offer letter agreement with Mr. Shaffer in September 2015. The agreement
is for an
unspecified term and entitles Mr. Shaffer to an initial annual base salary of $300,000. The agreement also provides that Mr. Shaffer will be
eligible to receive a bonus of up to 35% of base salary based upon his performance and the attainment of company objectives. Pursuant to the terms of the agreement, Mr. Shaffer is subject to certain confidentiality obligations and is obligated to
sign and comply with an agreement relating to proprietary information and inventions. Further provisions of the agreement are discussed below in the section entitled, “Potential Payments Upon Termination of Employment or Change in
Control.”
Eiger entered into an offer letter agreement with Ms. Quan in April 2015. The agreement is for an
unspecified term and entitles Ms. Quan to an initial annual base salary of $335,000. The agreement also provides that Ms. Quan will be eligible to receive a
bonus of up to 30% of base salary based upon her performance and the attainment of company objectives. Pursuant to the terms of the agreement, Ms. Quan is subject to certain confidentiality obligations and is obligated to sign and comply with an
agreement relating to proprietary information and inventions. Further provisions of the agreement are discussed below in the section entitled, “Potential Payments Upon Termination of Employment or Change in Control.”
Potential Payments Upon Termination of Employment or Change in Control
Pursuant to the terms of his employment agreement, upon termination of his employment without cause or upon a change in control of Eiger that requires a move
of the company over 60 miles or results in a substantial reduction in his responsibilities or compensation, Mr. Cory receives 12 months of base salary and COBRA coverage, as well as accelerated vesting of his equity awards.
Pursuant to the terms of his offer letter agreement, upon a change in control of Eiger that requires a move of the company over 50 miles or results in a
substantial reduction in his responsibilities or compensation, Mr. Shaffer receives 12 months of base salary, six months of COBRA coverage and the unvested portion of his equity awards
233
will immediately vest in full. After the first six months of Mr. Shaffer’s employment by Eiger, upon termination of his employment without cause, Mr. Shaffer receives six months of base
salary and COBRA coverage, and 50% of the unvested portion of his equity awards will become vested.
Pursuant to the terms of her offer letter agreement,
upon a change in control of Eiger that requires a move of the company over 50 miles or results in a substantial reduction in her responsibilities or compensation, Ms. Quan receives 12 months of base salary, six months of COBRA coverage and the
unvested portion of her equity awards will immediately vest in full. After the first year of Ms. Quan’s employment by Eiger, upon termination of her employment without cause, Ms. Quan receives six months of base salary and COBRA coverage, and
50% of the unvested portion of her equity awards will become vested.
Employment Benefits Plan
Eiger’s 2009 Equity Incentive Plan
The Eiger
BioPharmaceuticals, Inc. 2009 Equity Incentive Plan, or the 2009 plan, was adopted by Eiger’s board of directors in May 2009, approved by Eiger’s stockholders in July 2009, and amended and restated in April 2011 and September 2015. The
2009 plan provides for the grant of incentive stock options, nonstatutory stock options, stock appreciation rights, awards of restricted stock and restricted stock units. Eiger’s employees, directors and consultants are eligible to
receive awards under the 2009 plan; however, incentive stock options may only be granted to Eiger’s employees. A maximum of 3,867,792 shares of Eiger’s common stock are authorized for issuance under the 2009 plan.
The terms of each award granted under the 2009 plan are set forth in the applicable award agreement.
Pursuant to the terms of the 2009 plan, Eiger’s board of directors (or a committee delegated by Eiger’s board of directors) administers the plan
and, subject to any limitations set forth in the plan and has the ability to:
| |
• |
|
determine the persons to be granted awards; |
| |
• |
|
determine the type, terms and number of awards to be granted; |
| |
• |
|
determine the fair market value applicable to an award; |
| |
• |
|
construe and interpret the 2009 plan and awards granted thereunder, and settles related controversies; |
| |
• |
|
determine whether an award’s exercisability and/or vesting will be accelerated; |
| |
• |
|
delegate authority to an officer to make grants of a designated number of awards to individuals other than the officer, provided that the board will determine fair market value with respect to the award;
|
| |
• |
|
suspend or terminate the plan and amend the plan and awards granted thereunder, with certain exceptions; |
| |
• |
|
with the consent of option holders, reduce the exercise price of options, exchange options for other awards or take other actions that are treated as a repricing under generally accepted accounting principles; and
|
| |
• |
|
generally exercise the powers deemed necessary or expedient to promote the best interests of the company that are not in conflict with the provisions of the 2009 plan and awards granted under the 2009 plan.
|
In the event of a change in Eiger’s common stock without the receipt of consideration by Eiger (through merger, consolidation,
reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or other transaction not involving
the receipt of consideration by Eiger), the Eiger’s board of directors will proportionately and appropriately adjust the class and maximum number of securities subject to the 2009 plan and any share limits in the 2009 plan, and the class and
number of securities and price per share of stock subject to outstanding awards.
234
In the event of certain specified significant corporate transactions, the plan administrator has the discretion
to take any of the following actions with respect to stock awards:
| |
• |
|
arrange for the assumption, continuation or substitution of a stock award by a surviving or acquiring entity or parent company; |
| |
• |
|
accelerate the vesting of the stock award and provide for its termination prior to the effective time of the corporate transaction; and |
| |
• |
|
make a payment equal to the excess of (1) the value of the property the participant would have received upon exercise of the stock award over (2) the exercise price or strike price otherwise payable in
connection with the stock award. |
Eiger’s plan administrator is not obligated to treat all stock awards, even those that are of the
same type, in the same manner.
Celladon Equity Benefit Plans
Celladon 2013 Equity Incentive Plan
Celladon’s board
of directors adopted the Celladon Corporation 2013 Equity Incentive Plan (the “2013 Plan”) in September 2013 and Celladon’s stockholders approved the 2013 Plan in October 2013, which became effective on January 29, 2014, the date
of the final prospectus for Celladon’s initial public offering. In October 2013, Celladon’s board of directors approved an amendment to the 2013 Plan, which Celladon’s stockholders approved in November 2013. In January 2014,
Celladon’s board of directors again approved an amendment to the 2013 Plan, which Celladon’s stockholders approved in January 2014.
No person
may be granted stock awards covering more than 3,000,000 shares of Celladon’s common stock under the 2013 Plan during any calendar year pursuant to stock options, stock appreciation rights and other stock awards whose value is determined by
reference to an increase over an exercise or strike price of at least 100% of the fair market value on the date the stock award is granted. Additionally, no person may be granted in a calendar year a performance stock award covering more than
3,000,000 shares of Celladon’s common stock or a performance cash award having a maximum value in excess of $3,000,000. Such limitations are designed to help assure that any deductions to which Celladon would otherwise be entitled with respect
to such awards will not be subject to the $1,000,000 limitation on the income tax deductibility of compensation paid to any covered executive officer imposed by Section 162(m) of the Code.
Celladon’s board of directors, or a duly authorized committee thereof, has the authority to administer the 2013 Plan. Celladon’s board of directors
may also delegate to one or more of its officers the authority to (1) designate employees (other than other officers) to be recipients of certain stock awards, and (2) determine the number of shares of common stock to be subject to such
stock awards. Subject to the terms of the 2013 Plan, Celladon’s board of directors or the authorized committee, referred to herein as the plan administrator, determines recipients, dates of grant, the numbers and types of stock awards to be
granted and the terms and conditions of the stock awards, including the period of their exercisability and vesting schedule applicable to a stock award. Subject to the limitations set forth below, the plan administrator also determines the exercise
price, strike price or purchase price of awards granted and the types of consideration to be paid for the award.
The plan administrator has the authority
to modify outstanding awards under the 2013 Plan. Subject to the terms of the 2013 Plan, the plan administrator has the authority to reduce the exercise, purchase or strike price of any outstanding stock award, cancel any outstanding stock award in
exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
The plan administrator determines the term of stock options granted under the 2013 Plan, up to a maximum of ten years. Unless the terms of an
optionholder’s stock option agreement provides otherwise, if an optionholder’s service relationship with Celladon, or any of its affiliates, ceases for any reason other than disability, death or
235
cause, the optionholder may generally exercise any vested options for a period of three months following the cessation of service. The option term may be extended in the event that exercise of
the option following such a termination of service is prohibited by applicable securities laws or Celladon’s insider trading policy. If an optionholder’s service relationship with Celladon or any of its affiliates ceases due to disability
or death, or an optionholder dies within a certain period following cessation of service, the optionholder or a beneficiary may generally exercise any vested options for a period of 12 months in the event of disability and 18 months in the event of
death. In the event of a termination for cause, options generally terminate immediately upon the termination of the individual for cause. In no event may an option be exercised beyond the expiration of its term.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option is determined by the plan administrator and may include
(1) cash, check, bank draft or money order, (2) a broker-assisted cashless exercise, (3) the tender of shares of Celladon’s common stock previously owned by the optionholder, (4) a net exercise of the option if it is a
non-qualified stock option, or NSO, and (5) other legal consideration approved by the plan administrator.
Unless the plan administrator provides
otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to a domestic relations order. An optionholder may designate a beneficiary, however, who may exercise the option following the
optionholder’s death.
The aggregate fair market value, determined at the time of grant, of Celladon’s common stock with respect to Incentive
Stock Options, or ISOs, that are exercisable for the first time by an optionholder during any calendar year under all of Celladon’s stock plans may not exceed $100,000. Options or portions thereof that exceed such limit are generally treated as
NSOs. No ISO may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of Celladon’s total combined voting power or that of any of Celladon’s affiliates unless (1) the option
exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and (2) the term of the ISO does not exceed five years from the date of grant.
The 2013 Plan permits the grant of performance-based stock and cash awards that may qualify as performance-based compensation that is not subject to the
$1,000,000 limitation on the income tax deductibility of compensation paid to a covered executive officer imposed by Section 162(m) of the Code. To help assure that the compensation attributable to performance-based awards will so qualify,
Celladon’s compensation committee can structure such awards so that stock or cash will be issued or paid pursuant to such award only after the achievement of certain pre-established performance goals during a designated performance period.
The plan administrator may grant other awards based in whole or in part by reference to Celladon’s common stock. The plan administrator will set the
number of shares under the stock award and all other terms and conditions of such awards.
In the event that there is a specified type of change in
Celladon’s capital structure, such as a stock split or recapitalization, appropriate adjustments will be made to (1) the class and maximum number of shares reserved for issuance under the 2013 Plan, (2) the class and maximum number of
shares by which the share reserve may increase automatically each year, (3) the class and maximum number of shares that may be issued upon the exercise of ISOs, (4) the class and maximum number of shares subject to stock awards that can be
granted in a calendar year (as established under the 2013 Plan pursuant to Section 162(m) of the Code) and (5) the class and number of shares and exercise price, strike price, or purchase price, if applicable, of all outstanding stock
awards.
In the event of certain specified significant corporate transactions, the plan administrator has the discretion to take any of the following
actions with respect to stock awards:
| |
• |
|
arrange for the assumption, continuation or substitution of a stock award by a surviving or acquiring entity or parent company; |
| |
• |
|
arrange for the assignment of any reacquisition or repurchase rights held by Celladon to the surviving or acquiring entity or parent company; |
236
| |
• |
|
accelerate the vesting of the stock award and provide for its termination prior to the effective time of the corporate transaction; |
| |
• |
|
arrange for the lapse of any reacquisition or repurchase right held by Celladon; |
| |
• |
|
cancel or arrange for the cancellation of the stock award in exchange for such cash consideration, if any, as Celladon’s board of directors may deem appropriate; or |
| |
• |
|
make a payment equal to the excess of (1) the value of the property the participant would have received upon exercise of the stock award over (2) the exercise price otherwise payable in connection with the
stock award. |
Celladon’s plan administrator is not obligated to treat all stock awards, even those that are of the same type, in the
same manner.
Under the 2013 Plan, a corporate transaction is generally the consummation of (1) a sale or other disposition of all or substantially
all of Celladon’s consolidated assets, (2) a sale or other disposition of at least 90% of Celladon’s outstanding securities, (3) a merger, consolidation or similar transaction following which Celladon is not the surviving
corporation, or (4) a merger, consolidation or similar transaction following which Celladon is the surviving corporation but the shares of Celladon’s common stock outstanding immediately prior to such transaction are converted or exchanged
into other property by virtue of the transaction.
The plan administrator may provide, in an individual award agreement or in any other written agreement
between a participant and Celladon that the stock award will be subject to additional acceleration of vesting and exercisability in the event of a change of control. For example, certain of Celladon’s employees may receive an award agreement
that provides for vesting acceleration upon the individual’s termination without cause or resignation for good reason (including a material reduction in the individual’s base salary, duties, responsibilities or authority, or a material
relocation of the individual’s principal place of employment with Celladon) in connection with a change of control. Under the 2013 Plan, a change of control is generally (1) the acquisition by a person or entity of more than 50% of
Celladon’s combined voting power other than by merger, consolidation or similar transaction; (2) a consummated merger, consolidation or similar transaction immediately after which Celladon’s stockholders cease to own more than 50% of
the combined voting power of the surviving entity; or (3) a consummated sale, lease or exclusive license or other disposition of all or substantially of Celladon’s consolidated assets.
Celladon 2012 Equity Incentive Plan
Celladon’s
board of directors and stockholders approved the Celladon Corporation 2012 Equity Incentive Plan (the “2012 Plan”) which became effective in January 2012, and was further amended by Celladon’s board of directors and stockholders in
October 2013. As of December 31, 2015, there were outstanding stock awards under the 2012 Plan covering a total of 130,660 shares of Celladon’s common stock.
No additional awards will be granted under the 2012 Plan, and all awards granted under the 2012 Plan that are repurchased, forfeited, expire or are cancelled
will become available for grant under the 2013 Plan in accordance with its terms.
The plan administrator has the authority to modify outstanding awards
under the 2012 Plan. Subject to the terms of the 2012 Plan, the plan administrator has the authority to reduce the exercise, purchase or strike price of any outstanding stock award, cancel any outstanding stock award in exchange for new stock
awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Unless the plan administrator provides otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to
a domestic relations order. An optionholder may designate a beneficiary, however, who may exercise the option following the optionholder’s death.
In
the event that there is a specified type of change in Celladon capital structure, such as a stock split or recapitalization, appropriate adjustments will be made to (a) the class and maximum number of shares reserved
237
for issuance under the 2012 Plan, (b) the class and maximum number of shares that may be issued upon the exercise of ISOs and (c) the class and number of shares and exercise price,
strike price, or purchase price, if applicable, of all outstanding stock awards.
Unless otherwise provided in a stock award agreement or other written
agreement between Celladon and a participant, in the event of certain specified significant corporate transactions, the plan administrator has the discretion to take any of the following actions with respect to stock awards:
| |
• |
|
arrange for the assumption, continuation or substitution of a stock award by a surviving or acquiring entity or parent company; |
| |
• |
|
arrange for the assignment of any reacquisition or repurchase rights held by Celladon to the surviving or acquiring entity or parent company; |
| |
• |
|
accelerate the vesting of the stock award and provide for its termination prior to the effective time of the corporate transaction; |
| |
• |
|
arrange for the lapse of any reacquisition or repurchase right held by Celladon; |
| |
• |
|
cancel or arrange for the cancellation of the stock award in exchange for such cash consideration, if any, as Celladon’s board of directors may deem appropriate; or |
| |
• |
|
make a payment equal to the excess of (a) the value of the property the participant would have received upon exercise of the stock award over (b) the exercise price otherwise payable in connection with the
stock award. |
Celladon’s plan administrator is not obligated to treat all stock awards, even those that are of the same type, in the
same manner.
Under the 2012 Plan, a corporate transaction is generally defined as the consummation of (1) a sale or other disposition of all or
substantially all of Celladon’s consolidated assets, (2) a sale or other disposition of at least 90% of Celladon’s outstanding securities, (3) a merger, consolidation or similar transaction following which Celladon is not the
surviving corporation, or (4) a merger, consolidation or similar transaction following which Celladon is the surviving corporation but the shares of its common stock outstanding immediately prior to such transaction are converted or exchanged
into other property by virtue of the transaction.
Celladon 2001 Stock Option Plan
Celladon’s board of directors and stockholders approved the Celladon Corporation 2001 Stock Option Plan (the “2001 Plan”) which became effective
in December 2001 and was subsequently amended most recently in March 2008. The 2001 Plan terminated and no further awards were granted under the 2001 Plan upon the effective date of the 2012 Plan. As of December 31, 2015, there were outstanding
stock awards under the 2001 Plan covering a total of 1,441 shares of Celladon’s common stock.
In the event that there is a specified type of
change in Celladon’s capital structure, such as a stock split or recapitalization, appropriate adjustments will be made to (1) the class and maximum number of shares reserved for issuance under the 2001 Plan, (2) the class and maximum
number of shares that may be issued upon the exercise of ISOs, and (3) the class and number of shares and exercise price, strike price, or purchase price, if applicable, of all outstanding stock options.
In the event of certain change of control events, outstanding stock options may be assumed or substituted for substantially equivalent stock options by the
surviving or acquiring corporation. If any surviving or acquiring corporation fails to assume or substitute such stock options, all outstanding stock options will terminate effective as of the date of the change of control. However, not all options
will automatically terminate if Celladon’s board of directors otherwise provides for such options in the event of a change of control triggered by the direct or indirect sale or exchange by Celladon’s stockholders of more than 50% of its
voting stock, where Celladon is the surviving or continuing corporation and immediately after such sale or exchange less than 50% of the total combined voting power of Celladon’s voting stock is held by another corporation or corporations that
are
238
members of an affiliated group. In addition, the plan administrator may provide for special vesting acceleration in an individual award agreement or in any other written agreement between a
participant and Celladon.
Under the 2001 Plan, a change of control is generally defined as an ownership change event where Celladon’s stockholders
immediately before such event do not retain immediately thereafter, direct or indirect beneficial ownership of more than 50% of the total combined voting power of outstanding voting securities of Celladon or the corporation or other entity to which
Celladon’s assets were transferred, as applicable, in substantially the same proportions as their ownership of shares of Celladon’s voting stock immediately before such event. An ownership change event is generally defined as (1) the
sale or exchange by Celladon’s stockholders of more than 50% of Celladon’s voting stock, (2) a merger or consolation in which Celladon is a party, (3) the sale, exchange or transfer or all or substantially all of Celladon’s
assets or (4) Celladon’s liquidation or dissolution.
Celladon 2013 Employee Stock Purchase Plan
Celladon’s board of directors adopted the 2013 Employee Stock Purchase Plan (the “Celladon ESPP”) in September 2013 and Celladon’s
stockholders approved the Celladon ESPP in October 2013. The Celladon ESPP became effective on January 29, 2014, the date of Celladon’s final prospectus for its initial public offering. The purpose of the ESPP is to retain the services of
new employees and secure the services of new and existing employees while providing incentives for such individuals to exert maximum efforts toward Celladon’s success and that of its affiliates.
Celladon’s board of directors has delegated its authority to administer the Celladon ESPP to Celladon’s compensation committee. The Celladon ESPP is
implemented through a series of offerings of purchase rights to eligible employees. Under the Celladon ESPP, Celladon may specify offerings with durations of not more than 27 months, and may specify shorter purchase periods within each offering.
Each offering has one or more purchase dates on which shares of Celladon’s common stock will be purchased for employees participating in the offering. An offering may be terminated under certain circumstances.
Generally, all regular employees, including executive officers, employed by Celladon or by any of its designated affiliates, may participate in the Celladon
ESPP and may contribute, normally through payroll deductions, up to 15% of their earnings for the purchase of Celladon’s common stock under the Celladon ESPP. Unless otherwise determined by Celladon’s board of directors, common stock will
be purchased for accounts of employees participating in the Celladon ESPP at a price per share equal to the lower of (1) 85% of the fair market value of a share of Celladon’s common stock on the first date of an offering or (2) 85% of
the fair market value of a share of Celladon’s common stock on the date of purchase.
In the event that there occurs a change in Celladon’s
capital structure through such actions as a stock split, merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, liquidating dividend,
combination of shares, exchange of shares, change in corporate structure or similar transaction, Celladon’s board of directors will make appropriate adjustments to (1) the number of shares reserved under the Celladon ESPP, (2) the
maximum number of shares by which the share reserve may increase automatically each year and (3) the number of shares and purchase price of all outstanding purchase rights.
In the event of certain significant corporate transactions, including the consummation of: (1) a sale of all Celladon’s assets, (2) the sale or
disposition of 90% of Celladon’s outstanding securities, (3) a merger or consolidation where Celladon does not survive the transaction and (4) a merger or consolidation where Celladon survives the transaction but the shares of
Celladon’s common stock outstanding immediately prior to such transaction are converted or exchanged into other property by virtue of the transaction, any then-outstanding rights to purchase Celladon’s stock under the Celladon ESPP may be
assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such purchase rights, then the
participants’ accumulated payroll contributions will be used to purchase shares of Celladon’s common stock within ten business days prior to such corporate transaction, and such purchase rights will terminate immediately.
239
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Described below are any transactions occurring since January 1, 2012 and any currently proposed transactions to which Eiger was a party and in which
| |
• |
|
The amounts involved exceeded or will exceed $120,000; and |
| |
• |
|
A director, executive officer, holder of more than 5% of the outstanding capital stock of Eiger, or any member of such person’s immediate family had or will have a direct or indirect material interest.
|
Bridge Financing
In November 2015,
Eiger entered into a convertible note and warrant purchase agreement, or the Eiger Bridge Financing, in which Eiger issued (i) convertible promissory notes, or the Eiger Bridge Notes, for an aggregate principal amount of $6.0 million and
(ii) warrants exercisable for shares of Eiger’s equity securities at a purchase price of $0.01 per share. The Eiger Bridge Notes accrue interest at a rate of 6% per year and have a maturity date of March 31, 2016. For a
discussion of the terms of the warrants and the number of shares of Eiger capital stock issuable upon the exercise of such warrants, see “Agreements Related to the Merger—Bridge Loan.”
The following table summarizes the participation in the Eiger Bridge Financing by holders of more than 5% of Eiger’s capital stock and their affiliates.
|
|
|
|
|
| Name |
|
Aggregate Loan
Amount |
|
| InterWest Partners X, L.P(1) |
|
$ |
2,000,000 |
|
| Entities affiliated with Vivo Ventures Fund VI, L.P.(2) |
|
$ |
2,000,000 |
|
| (1) |
Nina Kjellson is a member of Eiger’s board of directors who has been designated by InterWest Partners X, L.P. |
| (2) |
Includes Vivo Ventures Fund VI, L.P. and Vivo Ventures VI Affiliates Fund L.P. Edgar Engleman is a member of Eiger’s board of directors who has been designated by Vivo Ventures Fund VI, L.P. |
Series A-1 Preferred Stock Financing
In April 2011,
Eiger entered into a Series A-1 preferred stock purchase agreement, pursuant to which Eiger issued and sold, in a series of closings, an aggregate of 24,935,950 shares of Eiger’s Series A-1 Preferred Stock at a price of $0.58 per share.
The following table summarizes the participation in the Series A-1 convertible preferred stock financing by holders of more than 5% of Eiger’s capital
stock and their affiliates.
|
|
|
|
|
|
|
|
|
| Name |
|
Shares of Series A-1
Preferred |
|
|
Aggregate
Purchase Price Paid |
|
| InterWest Partners X, L.P.(1) |
|
|
11,189,654 |
|
|
$ |
6,489,999.32 |
|
| Entities Affiliated with Vivo Ventures(2) |
|
|
11,189,654 |
|
|
$ |
6,489,999.32 |
|
| The Board of Trustees of the Leland Stanford Junior University |
|
|
2,543,595 |
|
|
$ |
1,475,285.10 |
|
| (1) |
Nina Kjellson is a member of Eiger’s board of directors who has been designated by InterWest Partners X, L.P. |
| (2) |
Consists of (a) 11,108,277 shares purchased by Vivo Ventures Fund VI, L.P. and (b) 81,377 shares purchased by Vivo Ventures VI Affiliates Fund, L.P. Edgar Engleman is a member of Eiger’s board of
directors who has been designated by Vivo Ventures Fund VI, L.P. |
240
Subscription Agreement
Certain holders of more than 5% of Eiger’s capital stock, are parties to the Subscription Agreement and have agreed to purchase shares of Eiger common
stock for an aggregate purchase price in excess of $120,000. For more information regarding the Subscription Agreement, please see the section entitled “Agreements Related to the Merger—Subscription Agreement” in this proxy
statement/prospectus/information statement. The table below sets forth the number of shares of Eiger common stock they have agreed to purchase and the aggregate purchase price for such shares.
|
|
|
|
|
|
|
|
|
| Name of Purchaser |
|
Aggregate
Purchase
Price |
|
|
Number of
Shares |
|
| InterWest Partners X, L.P.(1) |
|
$ |
7,000,000 |
|
|
|
4,666,044 |
|
| Entities Affiliated with Vivo Ventures Fund VI, L.P.(2) |
|
$ |
7,000,000 |
|
|
|
4,666,044 |
|
| (1) |
Nina Kjellson is a member of Eiger’s board of directors who has been designated by InterWest Partners X, L.P. |
| (2) |
Consists of (a) 4,632,111 shares to be purchased by Vivo Ventures Fund VI, L.P. and (b) 33,933 shares to be purchased by Vivo Ventures VI Affiliates Fund, L.P. Edgar Engleman is a member of Eiger’s board of
directors who has been designated by Vivo Ventures Fund VI, L.P. |
Eiccose Asset Purchase Agreement
In October 2015, Eiger entered into an Asset Purchase Agreement with Eiccose. David Cory, the President, Chief Executive Officer and a director of Eiger, is a
managing member and significant equity interest holder of Eiccose. For information regarding the Asset Purchase Agreement with Eiccose, please see the section entitled “Eiger Business—License and Asset Purchase
Agreements—Asset Purchase Agreement with Eiccose, LLC.”
Director and Executive Officer Compensation
For information regarding the compensation of Eiger’s directors and executive officers, please see the section entitled “Management Following the
Merger—Director Compensation” in this proxy statement/prospectus/information statement.
Policy for Approval of Related Person Transactions
While Eiger does not have a formal written policy or procedure for the review, approval or ratification of related party transactions, Eiger’s
board of directors reviews and considers the interests of its directors, executive officers and principal stockholders in its review and consideration of transactions and forms committees of non-interested directors when it determines that the
formation of such committees is appropriate under the circumstances.
241
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
The following unaudited pro forma condensed combined financial statements give effect to the merger between Celladon and Eiger and were prepared in accordance
with generally accepted accounting principles in the United States, or GAAP. For accounting purposes, Eiger is considered to be acquiring Celladon in the merger. Eiger was determined to be the accounting acquirer based upon the terms of the merger
and other factors including: (i) Eiger security holders will own approximately 78% of the combined company immediately following the closing of the merger, (ii) Eiger directors will hold all board seats in the combined company, and
(iii) Eiger management will hold all key positions in the management of the combined company. The transaction will be accounted for under the acquisition method of accounting under GAAP. Under the acquisition method of accounting for the
purpose of these unaudited pro forma condensed combined financial statements, management of Celladon and Eiger have determined a preliminary estimated purchase price, calculated as described in Note 2 to these unaudited pro forma condensed combined
financial statements. The net tangible and intangible assets acquired and liabilities assumed in connection with the transaction are recorded at their estimated acquisition date fair values. A final determination of these estimated fair values will
be based on the actual net tangible and intangible assets of Celladon that exist as of the date of completion of the transaction.
The unaudited pro forma
condensed combined balance sheet as of September 30, 2015 assumes that the merger took place on September 30, 2015 and combines the historical balance sheets of Celladon and Eiger as of September 30, 2015. The unaudited pro forma
condensed combined statement of operations for the nine months ended September 30, 2015 assumes that the merger took place as of January 1, 2015, and combines the historical results of Celladon and Eiger for the nine months ended
September 30, 2015. The unaudited pro forma condensed combined statement of operations for the year ended December 31, 2014 assumes that the merger took place as of January 1, 2014, and combines the historical results of Celladon and
Eiger for the year ended December 31, 2014. The historical financial statements of Celladon and Eiger, which are provided elsewhere in this proxy statement/prospectus/information statement, have been adjusted to give pro forma effect to events
that are (i) directly attributable to the merger, (ii) factually supportable, and (iii) with respect to the statements of operations, expected to have a continuing impact on the combined results.
The unaudited pro forma condensed combined financial statements are based on the assumptions and adjustments that are described in the accompanying notes. The
unaudited pro forma condensed combined financial statements and pro forma adjustments have been prepared based on preliminary estimates of fair value of assets acquired and liabilities assumed. Differences between these preliminary estimates and the
final acquisition accounting will occur and these differences could have a material impact on the accompanying unaudited pro forma condensed combined financial statements and the combined company’s future results of operations and financial
position. The actual amounts recorded as of the completion of the merger may differ materially from the information presented in these unaudited pro forma combined financial statements as a result of the amount, if any, of capital raised by Eiger
between entering the Merger Agreement and closing of the merger; the amount of cash used by Celladon’s operations between the signing of the Merger Agreement and the closing of the merger; the timing of closing of the merger; and other changes
in the Celladon assets and liabilities that occur prior to the completion of the merger.
The unaudited pro forma condensed combined financial statements
do not give effect to the potential impact of current financial conditions, regulatory matters, operating efficiencies or other savings or expenses that may be associated with the acquisition. The unaudited pro forma condensed combined financial
statements have been prepared for illustrative purposes only and are not necessarily indicative of the financial position or results of operations in future periods or the results that actually would have been realized had Celladon and Eiger been a
combined company during the specified period. The unaudited pro forma condensed combined financial statements, including the notes thereto, should be read in conjunction with the Celladon and Eiger historical audited financial statements for the
year ended December 31, 2014 and the unaudited condensed financial statements for the nine months ended September 30, 2015 included elsewhere in this proxy statement/prospectus/information statement.
242
Unaudited Pro Forma Condensed Combined Balance Sheet
September 30, 2015
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Celladon |
|
|
Eiger |
|
|
Pro Forma Merger
Adjustments |
|
|
|
|
Pro Forma
Combined |
|
| Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
37,092 |
|
|
$ |
2,136 |
|
|
$ |
39,500 |
|
|
D |
|
$ |
78,728 |
|
| Prepaid expenses and other current assets |
|
|
1,001 |
|
|
|
333 |
|
|
|
— |
|
|
|
|
|
1,334 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
38,093 |
|
|
|
2,469 |
|
|
|
39,500 |
|
|
|
|
|
80,062 |
|
| Property and equipment, net |
|
|
133 |
|
|
|
26 |
|
|
|
— |
|
|
|
|
|
159 |
|
| Other assets |
|
|
10 |
|
|
|
21 |
|
|
|
— |
|
|
|
|
|
31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
38,236 |
|
|
$ |
2,516 |
|
|
$ |
39,500 |
|
|
|
|
$ |
80,252 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Liabilities and stockholders’ equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued expenses |
|
$ |
1,188 |
|
|
$ |
758 |
|
|
$ |
390 |
|
|
H |
|
$ |
5,036 |
|
|
|
|
|
|
|
|
|
|
|
|
2,700 |
|
|
I |
|
|
|
|
| Accrued restructuring charges |
|
|
609 |
|
|
|
— |
|
|
|
2,887 |
|
|
G |
|
|
3,496 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
1,797 |
|
|
|
758 |
|
|
|
5,977 |
|
|
|
|
|
8,532 |
|
| Other noncurrent liabilities |
|
|
29 |
|
|
|
2 |
|
|
|
— |
|
|
|
|
|
31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
1,826 |
|
|
|
760 |
|
|
|
5,977 |
|
|
|
|
|
8,563 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible preferred stock |
|
|
|
|
|
|
22,567 |
|
|
|
(22,567 |
) |
|
C |
|
|
— |
|
| Common stock |
|
|
24 |
|
|
|
— |
|
|
|
4 |
|
|
B |
|
|
104 |
|
|
|
|
|
|
|
|
|
|
|
|
39 |
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
35 |
|
|
D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
F |
|
|
|
|
| Additional paid-in capital |
|
|
222,492 |
|
|
|
1,399 |
|
|
|
(186,106 |
) |
|
A |
|
|
101,102 |
|
|
|
|
|
|
|
|
|
|
|
|
(4 |
) |
|
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22,528 |
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
39,465 |
|
|
D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,329 |
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1 |
) |
|
F |
|
|
|
|
| Accumulated deficit |
|
|
(186,106 |
) |
|
|
(22,210 |
) |
|
|
186,106 |
|
|
A |
|
|
(29,517 |
) |
|
|
|
|
|
|
|
|
|
|
|
(1,330 |
) |
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,887 |
) |
|
G |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(390 |
) |
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,700 |
) |
|
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total stockholders’ equity |
|
|
36,410 |
|
|
|
1,756 |
|
|
|
33,523 |
|
|
|
|
|
71,689 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total liabilities and stockholders’ equity |
|
$ |
38,236 |
|
|
$ |
2,516 |
|
|
$ |
39,500 |
|
|
|
|
$ |
80,252 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited pro forma condensed combined financial statements.
243
Unaudited Pro Forma Condensed Combined Statement of Operations
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For Nine Months Ended September 30, 2015 |
|
| |
|
Celladon |
|
|
Eiger |
|
|
Pro Forma Merger
Adjustment |
|
|
|
|
Pro Forma
Combined |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
21,757 |
|
|
$ |
4,493 |
|
|
$ |
— |
|
|
|
|
$ |
26,250 |
|
| General and administrative |
|
|
10,805 |
|
|
|
1,768 |
|
|
|
(602 |
) |
|
J |
|
|
11,971 |
|
| Restructuring charges |
|
|
4,862 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
4,862 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
37,424 |
|
|
|
6,261 |
|
|
|
(602 |
) |
|
|
|
|
43,083 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(37,424 |
) |
|
|
(6,261 |
) |
|
|
602 |
|
|
|
|
|
(43,083 |
) |
| Interest income |
|
|
60 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
60 |
|
| Interest expense |
|
|
(2,302 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
(2,302 |
) |
| Other expense |
|
|
(1 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
(1 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(39,667 |
) |
|
$ |
(6,261 |
) |
|
$ |
602 |
|
|
|
|
$ |
(45,326 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic and diluted net loss per share |
|
$ |
(1.66 |
) |
|
$ |
(2.82 |
) |
|
|
|
|
|
|
|
$ |
(0.70 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average common share outstanding—basic and diluted |
|
|
23,830,668 |
|
|
|
2,222,561 |
|
|
|
38,625,173 |
|
|
B, F |
|
|
64,678,402 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited pro forma condensed combined financial statements.
Unaudited Pro Forma Condensed Combined Statement of Operations
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For Year Ended December 31, 2014 |
|
| |
|
Celladon |
|
|
Eiger |
|
|
Pro Forma Merger
Adjustment |
|
|
|
|
Pro Forma
Combined |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
22,676 |
|
|
$ |
644 |
|
|
$ |
— |
|
|
|
|
$ |
23,320 |
|
| General and administrative |
|
|
10,342 |
|
|
|
872 |
|
|
|
— |
|
|
|
|
|
11,214 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
33,018 |
|
|
|
1,516 |
|
|
|
— |
|
|
|
|
|
34,534 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(33,018 |
) |
|
|
(1,516 |
) |
|
|
— |
|
|
|
|
|
(34,534 |
) |
| Interest income |
|
|
118 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
118 |
|
| Interest expense |
|
|
(741 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
(741 |
) |
| Other income expense |
|
|
(29 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
(29 |
) |
| Change in fair value of warrant liability |
|
|
(183 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
(183 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(33,853 |
) |
|
$ |
(1,516 |
) |
|
$ |
— |
|
|
|
|
$ |
(35,369 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic and diluted net loss per share |
|
$ |
(1.82 |
) |
|
$ |
(0.68 |
) |
|
|
|
|
|
|
|
$ |
(0.84 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average common share outstanding—basic and diluted |
|
|
18,603,605 |
|
|
|
2,214,958 |
|
|
|
21,447,245 |
|
|
B, F |
|
|
42,265,808 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited pro forma condensed combined financial statements.
244
NOTES TO THE UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION
1. Description of Transaction and Basis of Presentation
Description of Transaction
On
November 18, 2015, Eiger entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with Celladon, pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth
in the Merger Agreement, that a wholly owned subsidiary of Celladon will merge with and into Eiger, with Eiger becoming a wholly-owned subsidiary of Celladon and the surviving corporation following the completion of the merger. At the closing of the
merger, each outstanding share of Eiger’s common stock will be converted into the right to receive approximately 1.32 pre-split shares of common stock of Celladon (or 0.09 shares post-split), as well as the payment of cash in lieu of
fractional shares. Immediately following the effective time of the merger, Celladon equity holders are expected to own approximately 22% of the outstanding capital stock of the combined company on a fully diluted basis after giving effect to its
pre-merger financing activities referred to below, with Eiger security holders owning approximately 78% of the combined company immediately following the closing of the merger. Prior to Eiger’s entry into the Merger Agreement, certain third
parties, including Eiger’s existing stockholders entered into agreements with Eiger pursuant to which such parties have agreed, subject to the terms and conditions of such agreements, to purchase prior to consummation of the Merger shares of
its capital stock and conversion of its note payable into such shares upon the Merger for an aggregate purchase price of approximately $39.5 million, which includes the $6.0 million in convertible promissory notes issued in November 2015. The
consummation of the transactions contemplated by such agreements is conditioned upon the satisfaction or waiver of the conditions set forth in the Merger Agreement.
Basis of Presentation
The unaudited pro forma
condensed combined financial statements were prepared in accordance with the regulations of the Securities and Exchange Commission (SEC). The unaudited pro forma condensed combined balance sheet as of September 30, 2015 is presented as if the
merger had been completed on September 30, 2015. The unaudited pro forma condensed combined statement of operations for the nine months ended September 30, 2015 combines the unaudited historical statements of operations of Celladon and
Eiger for their respective nine month period ended September 30, 2015, and gives pro forma effect to the merger as if it had been completed on January 1, 2015. The unaudited pro forma condensed combined statement of operations for the year
ended December 31, 2014 combines the audited historical statements of operations of Celladon and Eiger for their respective year ended December 31, 2014, and gives pro forma effect to the merger as if it had been completed on
January 1, 2014. Based on the terms of the merger, Eiger is deemed to be the acquiring company for accounting purposes and the transaction will be accounted for under the acquisition method of accounting in accordance with accounting principles
generally accepted in the United States, or GAAP. Accordingly, assets and liabilities of Eiger will be recorded as of the merger closing date at their respective carrying value and assets and liabilities of Celladon will be recorded as of the merger
closing date at their fair value. Under the acquisition method of accounting for the purpose of these unaudited pro forma financial statements, management of Eiger and Celladon have determined a preliminary estimated purchase price, calculated as
described in Note 2 to these unaudited pro forma condensed combined financial statements. The net tangible assets acquired and liabilities assumed in connection with the transaction are at their estimated acquisition date fair values. A final
determination of these estimated fair values will be based on the actual net tangible assets of Celladon that exist as of the date of completion of the transaction.
To the extent there are significant changes to the Company’s business following completion of the merger, the assumptions and estimates set forth in the
unaudited pro forma condensed combined financial statements could change significantly. Accordingly, the pro forma purchase price adjustments are subject to further adjustments as additional information becomes available and as additional analyses
are conducted following the completion of the merger. There can be no assurances that these additional analyses will not result in material changes to the estimates of fair value.
245
2. Preliminary Purchase Price
The preliminary estimated purchase price of the merger, and the estimated fair value of the net assets acquired, is $36.4 million, using Celladon’s share
price for its common stock of $1.52 as of the close of business on December 3, 2015 and its 23,916,021 shares of common stock outstanding as of September 30, 2015.
Management of Eiger has preliminarily concluded that the proposed merger is a business combination and will apply the acquisition method of accounting. Under
the acquisition method of accounting, the total purchase price is allocated to the acquired tangible and intangible assets and assumed liabilities of Celladon based on their estimated fair values as of the proposed merger closing date. The excess of
the purchase price over the fair value of assets acquired and liabilities assumed, if any, is allocated to goodwill.
A preliminary allocation of the
total preliminary estimated purchase price, as shown above, to the acquired assets and assumed liabilities of Celladon based on the estimated fair values as of September 30, 2015 is as follows (in thousands):
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
37,092 |
|
| Prepaid and other current assets |
|
|
1,001 |
|
| Property and equipment, net |
|
|
133 |
|
| Other assets |
|
|
10 |
|
| Current liabilities |
|
|
(1,797 |
) |
| Non-current liabilities |
|
|
(29 |
) |
|
|
|
|
|
| Net acquired tangible assets |
|
|
36,410 |
|
|
|
|
|
|
| Estimated total purchase price allocation |
|
$ |
36,410 |
|
|
|
|
|
|
The allocation of the estimated purchase price is preliminary because the proposed merger has not yet been completed. The
purchase price allocation will remain preliminary until Eiger management determines the fair values of assets acquired and liabilities assumed. The final determination of the purchase price allocation is anticipated to be completed as soon as
practicable after completion of the merger and will be based on the fair values of the assets acquired and liabilities assumed as of the merger closing date. The final amounts allocated to assets acquired and liabilities assumed could differ
significantly from the amounts presented in the unaudited pro forma condensed combined financial statements.
3. Pro Forma Adjustments
Pro forma adjustments are necessary to reflect the acquisition consideration exchanged and to adjust amounts related to the tangible assets and liabilities of
Celladon to reflect the preliminary estimate of their fair values, and to reflect the impact on the statements of operations of the Merger as if the companies had been combined during the periods presented therein. The pro forma adjustments included
in the unaudited pro forma condensed combined financial statements are as follows:
| |
A. |
To reflect the elimination of Celladon’s historical stockholders’ equity balances, including accumulated deficit, and to reflect the estimated fair value of Celladon’s net assets at the close of the
merger referred to in Note 2 above. |
| |
B. |
To reflect the reclassification Eiger’s par value of common stock and additional paid-in capital in connection with the exchange of Eiger’s common stock for Celladon’s common stock. |
| |
C. |
To reflect the conversion of Eiger’s convertible preferred stock to Celladon common stock. |
| |
D. |
To reflect the $33.5 million capital raise by Eiger and the conversion of $6.0 million in aggregate principal of Eiger’s convertible notes into equity, both to be completed prior to the closing of the merger.
|
246
| |
E. |
To record the issuance of additional shares of Eiger common stock prior to the closing of the merger related to certain top off rights related to the asset purchase agreement with Eiccose. |
| |
F. |
To reflect the automatic exercise of warrants completed before closing the merger. |
| |
G. |
To record $2.9 million of severance liabilities in relation to November 2015 workforce reduction of Celladon as of September 30, 2015. |
| |
H. |
To record $0.4 million of cash bonus payable to three executives of Celladon related to the Merger for the assumed milestones achievement that are related to the Merger, such as filing of Form S-4, as of
September 30, 2015. |
| |
I. |
To record $2.7 million of estimated transaction costs that were not incurred as of September 30, 2015. |
| |
J. |
To reflect the elimination of transaction related costs to date. |
247
DESCRIPTION OF CELLADON CAPITAL STOCK
Celladon’s amended and restated certificate of incorporation authorizes Celladon to issue up to 200,000,000 shares of common stock, $0.001 par value, and
10,000,000 shares of preferred stock, $0.001 par value.
As of December 31, 2015, there were outstanding:
| |
• |
|
23,954,375 shares of common stock; |
| |
• |
|
zero shares of preferred stock; |
| |
• |
|
options exercisable for up to 1,097,960 shares of common stock; and |
| |
• |
|
warrants exercisable for up to 152,735 shares of common stock. |
The following description of Celladon’s
capital stock is not complete and is subject to and qualified in its entirety by Celladon’s amended and restated certificate of incorporation and amended and restated bylaws, each filed as an exhibit to Celladon’s Current Report on Form
8-K filed with the SEC on February 10, 2014, and by the relevant provisions of the Delaware General Corporation Law.
Common Stock
Voting
Celladon’s common stock is entitled to
one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors, and does not have cumulative voting rights. Accordingly, the holders of a majority of the shares of Celladon’s
common stock entitled to vote in any election of directors can elect all of the directors standing for election.
Dividends
Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of common stock are entitled to receive dividends, if any,
as may be declared from time to time by Celladon’s board of directors out of legally available funds. Celladon has never paid cash dividends and has no present intention to pay cash dividends.
Liquidation
In the event of Celladon’s
liquidation, dissolution or winding up, holders of Celladon’s common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of Celladon’s debts and other
liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock.
Rights
and Preferences
Holders of Celladon’s common stock have no preemptive, conversion or subscription rights, and there are no redemption or
sinking fund provisions applicable to Celladon’s common stock. The rights, preferences and privileges of the holders of Celladon’s common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any
series of Celladon’s preferred stock that Celladon may designate and issue in the future.
Fully Paid and Nonassessable
All of Celladon’s outstanding shares of common stock are fully paid and nonassessable.
248
Preferred Stock
Celladon’s board of directors has the authority, without further action by the stockholders, to issue up to 10,000,000 shares of preferred stock in one or
more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions
thereon and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Celladon’s board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power
or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring
or preventing a change in Celladon’s control that may otherwise benefit holders of Celladon’s common stock and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock. As of
December 31, 2015, there were no shares of preferred stock outstanding and Celladon has no current plans to issue any shares of preferred stock.
Stock Options
As of December 31, 2015, there were
1,097,960 shares of common stock issuable upon the exercise of outstanding stock options, at a weighted-average exercise price of $14.06 per share.
Warrants
As of December 31, 2015, there were 152,735
shares of common stock issuable upon the exercise of outstanding warrants at an exercise price of $5.61 per share.
These warrants provide for
cashless exercise at the option of the holder, and also contain provisions for the adjustment of the number of shares issuable upon the exercise of the warrant in the event of stock splits, recapitalizations, reclassifications and consolidations.
These warrants will expire in October 2018.
Registration Rights
Certain holders of Celladon’s common stock, or their transferees, are entitled to the registration rights described below with respect to registration of
the resale of such shares under the Securities Act pursuant to an amended and restated investors’ rights agreement by and among Celladon and certain of its stockholders. The amended and restated investors’ rights agreement will be
terminated upon the consummation of the merger, and the demand, piggyback and Form S-3 registration rights discussed below will terminate at that time.
Demand Registration Rights
Upon the written
request from the holders of 25% of the registrable securities (excluding registrable securities derived from Celladon’s former Junior preferred stock) then outstanding that Celladon file a registration statement under the Securities Act with an
anticipated aggregate price to the public of at least $5.0 million, Celladon will be obligated to notify all holders of registrable securities of such request and to use Celladon’s reasonable best efforts to register the sale of all registrable
securities that holders may request to be registered. Celladon is not required to effect more than two registration statements which are declared or ordered effective, subject to certain exceptions. Celladon may postpone the filing of a registration
statement for up to 90 days once in any 12-month period if in the good faith judgment of Celladon’s board of directors such registration would be detrimental to Celladon.
249
Form S-3 Registration Rights
If Celladon is eligible to file a registration statement on Form S-3, holders of registrable securities have the right to demand that Celladon file a
registration statement on Form S-3 so long as the aggregate amount of securities to be sold under the registration statement on Form S-3 is at least $3.0 million, subject to specified exceptions, conditions and limitations.
“Piggyback” Registration Rights
If
Celladon registers any securities for public sale, holders of registration rights will have the right to include their shares in the registration statement. The underwriters of any underwritten offering will have the right to limit the number of
shares having registration rights to be included in the registration statement, but not below 33% of the total number of shares included in the registration statement, except this offering in which the holders have waived any and all rights to have
their shares included.
Expenses of Registration
Generally, Celladon is required to bear all registration and selling expenses incurred in connection with the demand, piggyback and Form S-3 registrations
described above, other than underwriting discounts and commissions.
Expiration of Registration Rights
As noted above, the demand, piggyback and Form S-3 registration rights discussed above will terminate upon the consummation of the merger. If the merger is not
consummated, the demand, piggyback and Form S-3 registration rights discussed above will terminate seven years following the closing of Celladon’s initial public offering or, (i) as to a given holder of registrable securities, at such
earlier time as the holder’s registrable securities, taken together with any registrable securities held by such holder’s affiliates, constitute less than 1% of Celladon’s outstanding common stock and such holder is able to sell of
such holder’s registrable securities in a single 90-day period under Rule 144 of the Securities Act, or (ii) as to any securities otherwise registrable pursuant to the exercise of the foregoing registration rights, at such time as the
securities are sold (a) to the public, either through a registration statement or under Rule 144 of the Securities Act, or (b) in a private transaction in which the registration rights are not also transferred.
Anti-Takeover Effects of Provisions of Celladon Charter Documents and Delaware Law
Delaware Anti-Takeover Law
Celladon is subject to
Section 203 of the Delaware General Corporation Law, or Section 203. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a
period of three years after the date of the transaction in which the person became an interested stockholder, unless:
| |
• |
|
prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
|
| |
• |
|
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding upon consummation of the transaction, excluding for purposes of determining the number of shares outstanding
(1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be
tendered in a tender or exchange offer; or |
250
| |
• |
|
on or subsequent to the consummation of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative
vote of at least 66 2⁄3% of the outstanding voting
stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| |
• |
|
any merger or consolidation involving the corporation and the interested stockholder; |
| |
• |
|
any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| |
• |
|
subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested
stockholder; |
| |
• |
|
subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; and |
| |
• |
|
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the
corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Amended and Restated Certificate of
Incorporation and Amended and Restated Bylaws
Provisions of Celladon’s amended and restated certificate of incorporation and amended and
restated bylaws may delay or discourage transactions involving an actual or potential change in Celladon’s control or change in Celladon’s management, including transactions in which stockholders might otherwise receive a premium for their
shares or transactions that Celladon’s stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of Celladon’s common stock. Among other things, Celladon’s amended
and restated certificate of incorporation and amended and restated bylaws:
| |
• |
|
permit Celladon’s board of directors to issue up to 10,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other
change in Celladon’s control); |
| |
• |
|
provide that the authorized number of directors may be changed only by resolution adopted by a majority of the board of directors; |
| |
• |
|
provide that the board of directors or any individual director may only be removed with cause and the affirmative vote of the holders of at least 66 2⁄3% of the voting power of all of Celladon’s then outstanding common stock; |
| |
• |
|
provide that all vacancies, including newly created directorships, may, except as otherwise required by law or subject to the rights of holders of preferred stock as designated from time to time, be filled by the
affirmative vote of a majority of directors then in office, even if less than a quorum; |
| |
• |
|
divide Celladon’s board of directors into three classes; |
| |
• |
|
require that any action to be taken by Celladon’s stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
| |
• |
|
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner
and also specify requirements as to the form and content of a stockholder’s notice; |
251
| |
• |
|
do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election,
if they should so choose); |
| |
• |
|
provide that special meetings of Celladon’s stockholders may be called only by the chairman of the board, Celladon’s Chief Executive Officer or by the board of directors pursuant to a resolution adopted by a
majority of the total number of authorized directors (whether or not there exists any vacancies); and |
| |
• |
|
provide that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (1) any derivative action or proceeding brought on Celladon’s behalf, (2) any action asserting a claim
of breach of a fiduciary duty owed by any of Celladon’s directors or officers to Celladon or its stockholders, (3) any action asserting a claim against Celladon arising pursuant to any provision of the Delaware General Corporation Law or
Celladon’s certificate of incorporation or bylaws or (4) any action asserting a claim against Celladon governed by the internal affairs doctrine. |
The amendment of any of these provisions, with the exception of the ability of Celladon’s board of directors to issue shares of preferred stock and
designate any rights, preferences and privileges thereto, would require the affirmative vote of the holders of at least 662/3% of the voting
power of all of Celladon’s then outstanding common stock.
NASDAQ Global Market Listing
Celladon’s common stock is currently listed on The NASDAQ Global Market under the symbol “CLDN.”
Transfer Agent and Registrar
The transfer agent and
registrar for Celladon’s common stock is American Stock Transfer & Trust Company, LLC. The transfer agent and registrar’s address is 6201 15th Avenue, Brooklyn, New York 11219.
252
COMPARISON OF RIGHTS OF HOLDERS OF CELLADON STOCK AND EIGER STOCK
Both Celladon and Eiger are incorporated under the laws of the State of Delaware and, accordingly, the rights of the stockholders of each are
currently, and will continue to be, governed by the DGCL. If the merger is completed, Eiger stockholders will become stockholders of Celladon, and their rights will be governed by the DGCL, the bylaws of Celladon and, assuming Celladon Proposal Nos.
2 and 3are approved by the Celladon stockholders at the Celladon special meeting, and the amendments to the amended and restated certificate of incorporation of Celladon attached to this proxy statement/prospectus/information statement as Annex
D and Annex E, respectively.
The table below summarizes the material differences between the current rights of Eiger stockholders under the
Eiger amended and restated certificate of incorporation and bylaws and the rights of Celladon stockholders, post-merger, under the Celladon amended and restated certificate of incorporation and bylaws, each as amended, as applicable, and as in
effect immediately following the merger.
While Celladon and Eiger believe that the summary tables cover the material differences between the rights of
their respective stockholders prior to the merger and the rights of Celladon stockholders following the merger, these summary tables may not contain all of the information that is important to you. These summaries are not intended to be a complete
discussion of the respective rights of Celladon and Eiger stockholders and are qualified in their entirety by reference to the DGCL and the various documents of Celladon and Eiger that are referred to in the summaries. You should carefully read this
entire proxy statement/prospectus/information statement and the other documents referred to in this proxy statement/prospectus/information statement for a more complete understanding of the differences between being a stockholder of Celladon or
Eiger before the merger and being a stockholder of Celladon after the merger. Celladon has filed copies of its current amended and restated certificate of incorporation and bylaws with the SEC and will send copies of the documents referred to in
this proxy statement/prospectus/information statement to you upon your request. Eiger will also send copies of its documents referred to in this proxy statement/prospectus/information statement to you upon your request. See the section entitled
“Where You Can Find More Information” in this proxy statement/prospectus/information statement.
Current Eiger
Rights Versus Celladon Rights Post-Merger
|
|
|
|
|
| Provision |
|
Eiger (Pre-Merger) |
|
Celladon (Post-Merger) |
| Elections;Voting; Procedural Matters |
|
|
|
| Authorized Capital Stock |
|
The amended and restated certificate of incorporation of Eiger authorizes the issuance of up to 68,000,000 of common stock, $0.0001 par value per share, and 30,787,500 shares of preferred stock, $0.0001 par value per share, of which
5,187,500 shall be designated “Series A Preferred Stock,” and 25,600,000 shall be designated “Series A-1 Preferred Stock.” |
|
The amended and restated certificate of incorporation of Celladon authorizes the issuance of up to 210,000,000 shares, of which 200,000,000 shares are common stock, each having a par value of $0.001, and 10,000,000 shares are
preferred stock, each having a par value of $0.001. |
|
|
|
| Number of Directors |
|
The amended and restated certificate of incorporation and Voting Agreement of Eiger sets the number of directors at five. |
|
The amended and restated certificate of incorporation and bylaws of Celladon currently provide that the number of directors that shall constitute the whole board of directors shall be fixed exclusively by one
or |
253
|
|
|
|
|
| Provision |
|
Eiger (Pre-Merger) |
|
Celladon (Post-Merger) |
|
|
|
|
more resolutions adopted from time to time by a majority of the board of directors. |
|
|
|
| Stockholder Nominations and Proposals |
|
The amended and restated certificate of incorporation and bylaws of Eiger do not provide for procedures with respect to stockholder proposals or director nominations. |
|
The bylaws of Celladon provide that in order for a stockholder to make a director nomination or propose business at an annual meeting of stockholders, the stockholder must give timely written notice to the Celladon secretary, which
must be received not more than 120 calendar days before and not less than 90 calendar days before the one year anniversary of the date of the previous year’s annual meeting (with certain adjustments if no annual meeting was held the previous
year or the date of the annual meeting is changed by more than 30 days from the first anniversary of the preceding year’s annual meeting). |
|
|
|
| Classified Board of Directors |
|
The amended and restated certificate of incorporation of Eiger does not provide for the division of the board of directors into staggered classes. |
|
The amended and restated certificate of incorporation of Celladon provides that the directors comprising the board of directors shall be divided into three staggered classes, with each class serving three-year terms. |
|
|
|
| Removal of Directors |
|
The bylaws of Eiger provide that directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except that the directors elected by the holders of a
particular class or series of stock may be removed without cause only by vote of the holders of a majority of the outstanding shares of such class or series. |
|
Under the amended and restated certificate of incorporation of Celladon, a director may be removed at any time with cause by the affirmative vote of the holders of 66 2⁄3% of the voting power of all then-outstanding shares of capital stock entitled to vote at an election of directors. |
|
|
|
| Special Meeting of the Stockholders |
|
The bylaws of Eiger provide that special meetings of stockholders may be called at any time by the board of directors or the president. |
|
The amended and restated certificate of incorporation and bylaws of Celladon provide that a special meeting of the stockholders may be called by the chairman of the board of directors, the chief executive officer or president, by
the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors. |
254
|
|
|
|
|
| Provision |
|
Eiger (Pre-Merger) |
|
Celladon (Post-Merger) |
| Cumulative Voting |
|
The Eiger amended and restated certificate of incorporation and bylaws do not allow cumulative voting rights in the election of its directors. |
|
The Celladon amended and restated certificate of incorporation and bylaws do not have a provision granting cumulative voting rights in the election of its directors. |
|
|
|
| Vacancies |
|
The amended and restated certificate of incorporation and bylaws of Eiger provide that any vacancy on the board of directors may be filled by vote of a majority of the directors then in office, although less than a quorum, or by a
sole remaining director. The amended and restated certificate of incorporation further provides that where a vacancy occurs among the directors elected by the holders of a class or series of stock, the holders of shares of such class or series may
override the board of directors’ action to fill such vacancy by voting for their own designee to fill such vacancy at a meeting or by written consent. |
|
The amended and restated certificate of incorporation and bylaws of Celladon provide that any vacancy or newly created directorships on the board of directors will be filled only by the affirmative vote of a majority of the
directors in office, even though less than a quorum of the board of directors and not by the stockholders. |
|
|
|
| Voting Stock |
|
Under the amended and restated certificate of incorporation of Eiger, the holders of common stock are entitled to one vote for each share of stock held by them and holders of preferred stock are entitled to one vote for each share
of common stock into which such share of preferred stock is convertible; provided that holders of preferred stock, voting as a separate class, are entitled to elect two directors, and holders of common stock, voting as a separate class, are entitled
to elect two director. |
|
Under the Celladon bylaws, the holders of voting stock are entitled to vote on each matter properly submitted to the stockholders at a meeting of the stockholders and shall be entitled to cast one vote in person or by proxy for each
share of voting stock held by them respectively as of the record date fixed by the secretary at least 10 days before the meeting of the Stockholders. |
|
|
|
| Voting Agreement |
|
The Amended and Restated Voting Agreement entered into as of April 15, 2011 between Eiger and certain of its stockholders, or the Voting Agreement, provides for the election of two director nominees by holders of Eiger’s
preferred stock; two director nominees by the holders of Eiger’s common stock, and one director who shall be acceptable to the holders of a majority of the share of Eiger’s common stock and the holders of 60% of the shares of Eiger’s
preferred stock then outstanding. |
|
Celladon does not have a voting agreement or similar agreement with any of its stockholders in place. |
255
|
|
|
|
|
| Provision |
|
Eiger (Pre-Merger) |
|
Celladon (Post-Merger) |
| Drag Along |
|
Under the Voting Agreement, if the board of directors and the holders of at least 60% of the then outstanding Preferred Stock of Eiger approve a change in control transaction, each stockholder party to the Voting Agreement is
required to vote in favor of such transaction or sell their shares, as applicable. |
|
Celladon does not have drag along terms in place. |
|
|
|
| Stockholder Action by Written Consent |
|
The bylaws of Eiger provide that any action required or permitted to be taken at any annual or special meeting of stockholders may be taken without a meeting, without prior notice and without a vote, if a consent in writing, setting
forth the action so taken, is signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote on such action were
present and voted. |
|
The amended and restated certificate of incorporation and bylaws of Celladon specify that no action shall be taken by the stockholders except at an annual or special meeting of the stockholders and that no action shall be taken by
the stockholders by written consent. |
|
|
|
| Notice of Stockholder Meeting |
|
The bylaws of Eiger provide that notices of all meetings shall state the place, if any, date and time of the meeting and the means of remote communications, if any, by which stockholders and proxyholders may be deemed to be present
in person and vote at such meeting. The notice of a special meeting shall state, in addition, the purpose or purposes for which the meeting is called. The bylaws of Eiger provide that notice of each meeting of stockholders shall be given not less
than 10 nor more than 60 days before the date of the meeting to each stockholder entitled to vote at such meeting. |
|
Under the bylaws of Celladon, written notice of each stockholder meeting must specify the place, if any, date and hour of the meeting, the means of remote communication, if any, by which stockholders and proxy holders may be deemed
to be present in person and vote at such meeting, and, in the case of a special meeting, the purposes for which the meeting is called. Notice shall be given not less than 10 nor more than 60 calendar days before the date of the meeting to each
stockholder entitled to vote at such meeting. |
|
|
|
| Conversion Rights and Protective Provisions |
|
The amended and restated certificate of incorporation of Eiger provides that each holder of shares of Series A Preferred Stock shall have the right to convert such shares into shares of common stock at any time in accordance with
the amended and restated certificate of incorporation. In addition, upon the closing of the sale |
|
The amended and restated certificate of incorporation of Celladon does not provide that holders of Celladon stock shall have preemptive, conversion or other protective
rights. |
256
|
|
|
|
|
| Provision |
|
Eiger (Pre-Merger) |
|
Celladon (Post-Merger) |
|
|
of shares of common stock in a firm-commitment underwritten public offering resulting in at least $30 million of proceeds or the date and time, or occurrence of an event, specified by the holders of 60% of the outstanding shares of
Preferred Stock, all outstanding shares shall be converted into shares of common stock. Eiger may not amend the amended and restated certificate of incorporation in a manner that materially alters or changes the rights, preferences or privileges of
the Preferred Stock so as to affect them adversely in a manner different than other classes without the written consent or affirmative vote of 60% of the outstanding shares of Preferred Stock. |
|
|
|
|
|
| Right of First Refusal |
|
The bylaws of Eiger provide that stockholders wishing to transfer any shares of stock shall first provide Eiger with the right to purchase such shares. |
|
Celladon does not have a right of first refusal in place. |
|
| Indemnification of Officers and Directors and Advancement of Expenses; Limitation on Personal Liability |
|
|
|
| Indemnification |
|
The amended and restated certificate of incorporation of Eiger provides that Eiger shall indemnify its directors and officers to the fullest extent permitted by applicable law. |
|
The amended and restated certificate of incorporation and bylaws of Celladon provide that Celladon shall indemnify its directors and officers to the fullest extent permitted by the DGCL or any other applicable law. Under its bylaws,
Celladon will not be required to indemnify any director or officer in connection with any proceeding initiated by such person unless the proceeding was authorized by the Celladon board of directors, expressly required by law, or is provided for by
the corporation. Under the bylaws of Celladon, such rights shall not be exclusive of any other rights acquired by directors and officers, including by agreement. |
|
|
|
| Advancement of Expenses |
|
The bylaws of Eiger provides that Eiger shall pay the expenses incurred by a director or officer in defending any proceeding in advance of its final disposition, provided, that, to the |
|
The bylaws of Celladon provide that Celladon will advance expenses to any director or officer prior to the final disposition of the proceeding, provided, however, that
such |
257
|
|
|
|
|
| Provision |
|
Eiger (Pre-Merger) |
|
Celladon (Post-Merger) |
|
|
extent required by law, such payment of expenses in advance of the final disposition of the proceeding shall be made only upon receipt of an undertaking by the director or officer to repay all amounts advanced if it should be
ultimately determined that such director or officer is not entitled to be indemnified |
|
advancements shall be made only upon receipt of an undertaking by such director or officer to repay all amounts advanced if it should be ultimately determined that such director or officer is not entitled to indemnification under
the bylaws of Celladon or otherwise. |
|
|
|
| Dividends |
|
|
|
|
|
|
|
| Declaration and Payment of Dividends |
|
The amended and restated certificate of incorporation of Eiger provides that holders of Series A Preferred Stock shall be entitled, if, when and as declared by the board of directors, to dividends of $0.1280 per share per annum and
Series A-1 Preferred Stock shall be entitled to dividends of $0.0464 per share per annum. |
|
The bylaws of Celladon provide that, subject to any restrictions contained in the DGCL or the amended and restated certificate of incorporation of Celladon, the board of directors may declare and pay dividends upon the shares of
capital stock. Dividends may be paid in cash, in property, or in shares of capital stock. The board of directors may set aside out of any funds of the corporation available for dividends reserves for any proper purposes, including equalizing
dividends, repairing or maintaining corporate property and meeting contingencies, and may abolish any such reserve. |
|
| Amendments to Certificate of Incorporation or Bylaws |
|
|
|
| General Provisions |
|
The amended and restated certificate of incorporation of Eiger may not be amended in a manner that materially alters or changes the rights, preferences or privileges of the Preferred Stock so as to affect them adversely in a manner
different than other classes without the written consent or affirmative vote of 60% of the outstanding shares of Preferred Stock. The bylaws of Eiger may be amended by the board of directors. |
|
The amended and restated certificate of incorporation of Celladon may be amended in any manner otherwise permitted by law, with the exception that under the amended and restated certificate of incorporation of Celladon, Article V
(relating to the composition of and vacancies on the board of directors, election and removal of directors, alterations and amendments to bylaws, actions by written consent and stockholder meetings), Article VI (relating to indemnification of
directors and officers), and Article VII (relating to amendment of certain provisions of the certificate of incorporation) require the affirmative vote of the holders of 66 2⁄3% of the voting power of the |
258
|
|
|
|
|
| Provision |
|
Eiger (Pre-Merger) |
|
Celladon (Post-Merger) |
|
|
|
|
outstanding shares of voting stock, voting together as a single class. The amended and restated certificate of incorporation and bylaws of Celladon provide that the board of directors is expressly authorized to make, alter or repeal
the bylaws; provided, however, that the bylaws may be rescinded, altered, amended or repealed in any respect by the affirmative vote of the holders of at least
66 2⁄3% of the voting power of the outstanding shares of voting stock. |
259
PRINCIPAL STOCKHOLDERS OF CELLADON
Except where specifically noted, the following information and all other information contained in this proxy statement/prospectus/information statement do
not give effect to the proposed 1-for-15 reverse stock split described in Celladon Proposal No. 2.
The following table sets forth certain
information with respect to the beneficial ownership of Celladon common stock as of December 31, 2015 (except where otherwise indicated) for:
| |
• |
|
each person, or group of affiliated persons, who are known by Celladon to beneficially own more than 5% of the outstanding shares of Celladon common stock; |
| |
• |
|
each of the Celladon directors as of December 31, 2015; |
| |
• |
|
each of the Celladon named executive officers, as identified in Celladon’s definitive proxy statement filed with the SEC on April 30, 2015; and |
| |
• |
|
all of the current directors and executive officers of Celladon as a group. |
The number of shares
beneficially owned by each entity, person, director or executive officer is determined under the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership
includes any shares as to which the individual has the sole or shared voting power or investment power and also any shares that the individual has the right to acquire within 60 days of December 31, 2015, through the exercise of any stock option or
other right. Unless otherwise indicated, each person has sole investment and voting power, or shares such powers with his or her spouse, with respect to the shares set forth in the following table.
260
The percentage of ownership is based on 23,954,375 shares of common stock outstanding on December 31, 2015,
adjusted as required by the rules promulgated by the SEC to determine beneficial ownership. Celladon does not know of any arrangements, including any pledge by any person of securities of Celladon, the operation of which may at a subsequent date
result in a change of control of Celladon. Unless otherwise noted, the address of each director and current and former executive officer of Celladon is c/o Celladon Corporation, 12707 High Bluff Drive, Suite 200, San Diego, California 92130.
|
|
|
|
|
|
|
|
|
| |
|
Beneficial Ownership |
|
| Beneficial Owner |
|
Number of Shares |
|
|
Percent of Total |
|
| Greater than 5% stockholders |
|
|
|
|
|
|
|
|
| Entities Affiliated with EcoR1 Capital, LLC(1)
409 Illinois Street San
Francisco, CA 94158 |
|
|
2,843,170 |
|
|
|
11.9 |
% |
| Pfizer Inc.(2)
c/o Pfizer Venture Investments
235 E. 42nd Street New
York, NY 10017 |
|
|
1,828,495 |
|
|
|
7.6 |
% |
| Lundbeckfond Invest A/S(3)
Vestagervej 17 DK-2900
Hellerup Denmark |
|
|
1,774,349 |
|
|
|
7.4 |
% |
| Entities Affiliated with Boxer Capital, LLC(4)
440 Stevens Ave., Suite 100
Solana Beach, CA 92075 |
|
|
1,399,991 |
|
|
|
5.8 |
% |
| GBS Bioventures IV(5)
Level 5, 71 Collins Street
Melbourne, Vic 3000
Australia |
|
|
1,348,798 |
|
|
|
5.6 |
% |
|
|
|
| Directors and Named Executive Officers |
|
|
|
|
|
|
|
|
| Krisztina M. Zsebo, Ph.D.(6) |
|
|
659 |
|
|
|
* |
|
| Elizabeth E. Reed(7) |
|
|
48,002 |
|
|
|
* |
|
| Paul B. Cleveland(8) |
|
|
— |
|
|
|
* |
|
| Gregg Alton(9) |
|
|
34,705 |
|
|
|
* |
|
| Graham Cooper(10) |
|
|
34,705 |
|
|
|
* |
|
| Michael Narachi(11) |
|
|
106,341 |
|
|
|
* |
|
| Peter Honig, M.D.(12) |
|
|
15,854 |
|
|
|
* |
|
| All current executive officers and directors as a group (7 persons)(13) |
|
|
397,431 |
|
|
|
1.6 |
% |
| (1) |
This information is based on the Schedule 13G filed with the SEC on June 30, 2015 jointly by EcoR1 Capital, LLC, or EcoR1, Oleg Nodelman and EcoR1 Capital Fund Qualified, L.P., or Qualified Fund. EcoR1 is the
general partner and investment adviser of investment funds, including Qualified Fund. Mr. Nodelman is the control person of EcoR1. EcoR1 and Mr. Nodelman have shared voting and investment power over 2,843,170 shares, and Qualified Fund has
shared voting and investment power over 1,905,208 shares. |
| (2) |
This information is based on the Schedule 13G filed with the SEC on February 14, 2014 by Pfizer Inc. Includes 42,659 shares of common stock issuable upon the exercise of warrants, all of which are exercisable.
|
| (3) |
This information is based on the Schedule 13G filed with the SEC on February 23, 2015. Includes 28,666 shares issuable upon the exercise of warrants, all of which are exercisable. |
| (4) |
This information is based on the Schedule 13G filed with the SEC on December 24, 2015 jointly by Boxer Capital, LLC (“Boxer Capital”), Boxer
Asset Management Inc. (“Boxer Management”) and Joe Lewis. Boxer Management is the managing member and majority owner of Boxer Capital. Joe Lewis is the sole |
261
| |
indirect owner and controls Boxer Management. Boxer Capital, Boxer Management and Joe Lewis share voting and investment power over all 1,399,991 shares. |
| (5) |
This information is based on the Schedule 13G filed with the SEC on April 20, 2015 by GBS BioVentures IV. Includes 53,807 shares of common stock issuable upon the exercise of warrants, all of which are exercisable.
|
| (6) |
Includes 659 shares that Dr. Zsebo has the right to acquire from Celladon within 60 days of December 31, 2015 pursuant to the exercise of stock options. Dr. Zsebo’s employment terminated as of
May 29, 2015. |
| (7) |
Includes 47,833 shares that Ms. Reed has the right to acquire from Celladon within 60 days of December 31, 2015 pursuant to the exercise of stock options. |
| (8) |
Mr. Cleveland’s employment terminated as of November 19, 2015. |
| (9) |
Consists of 34,705 shares that Mr. Alton has the right to acquire from Celladon within 60 days of December 31, 2015 pursuant to the exercise of stock options. |
| (10) |
Consists of 34,705 shares that Mr. Cooper has the right to acquire from Celladon within 60 days of December 31, 2015 pursuant to the exercise of stock options. |
| (11) |
Includes 43,841 shares that Mr. Narachi has the right to acquire from Celladon within 60 days of December 31, 2015 pursuant to the exercise of stock options. |
| (12) |
Consists of 15,854 shares that Dr. Honig has the right to acquire from Celladon within 60 days of December 31, 2015 pursuant to the exercise of stock options. |
| (13) |
Includes only current directors and executive officers serving in such capacity on the date of the table. Consists of the shares described in footnotes (7) and (9) through (12) and (i) 24,712 shares
of common stock and (ii) 133,112 shares issuable upon the exercise of stock options within 60 days of December 31, 2015 held by executive officers who are not listed in the table above. |
262
PRINCIPAL STOCKHOLDERS OF EIGER
The following table and the related notes present information on the beneficial ownership of shares of Eiger’s capital stock as of December 31, 2015 by:
| |
• |
|
each director of Eiger; |
| |
• |
|
each executive officer of Eiger; |
| |
• |
|
all of Eiger’s current directors and executive officers as a group; and |
| |
• |
|
each stockholder known by Eiger to beneficially own more than five percent of its common stock on an as converted basis. |
The number of shares owned, total shares beneficially owned and the percentage of common stock beneficially owned below assumes, in each case, the conversion
of all 29,810,950 shares of Eiger Series A Preferred Stock and Series A-1 Preferred Stock into common stock as of December 31, 2015 and a total of 32,941,615 shares of Eiger common stock outstanding as of December 31, 2015.
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of
common stock that may be acquired by an individual or group within 60 days of December 31, 2015, pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual
or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table.
Except
as indicated in footnotes to this table, Eiger believes that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided
to Eiger by such stockholders. Unless otherwise indicated, the address for each stockholder listed is: c/o Eiger Biopharmaceuticals, Inc., 350 Cambridge Avenue, Suite 350, Palo Alto, CA 94306.
|
|
|
|
|
|
|
|
|
| |
|
Beneficial Ownership |
|
| Beneficial Owner |
|
Number of Shares |
|
|
Percent of Total |
|
| Greater than 5% stockholders |
|
|
|
|
|
|
|
|
| InterWest Partners X, L.P.(1)
2710 Sand Hill Road, Second Floor
Menlo Park, CA 94025 |
|
|
13,377,154 |
|
|
|
40.6 |
% |
| Entities affiliated with Vivo Ventures Fund VI, L.P.(2)
575 High Street, Suite 201
Palo Alto, CA 94301 |
|
|
13,377,154 |
|
|
|
40.6 |
% |
| Entities Affiliated with the Board of Trustees of the Leland Stanford Junior University (PVF)(3)
Direct Investments
Stanford Management Company
635 Knight Way Stanford,
CA 94305-7297 |
|
|
3,108,820 |
|
|
|
9.4 |
% |
| Eiger Group International, Inc.(4)
2061 Webster Street Palo
Alto, CA 94301 |
|
|
1,726,024 |
|
|
|
5.2 |
% |
263
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Number of Shares |
|
|
Options and
Warrants
Exercisable
Within 60
Days |
|
|
Approximate
Percent Owned |
|
| Directors and Executive Officers |
|
|
|
|
|
|
|
|
| David A. Cory |
|
|
649,999 |
|
|
|
120,833 |
|
|
|
2.3 |
% |
| James Welch |
|
|
— |
|
|
|
— |
|
|
|
* |
|
| Joanne Quan |
|
|
— |
|
|
|
— |
|
|
|
* |
|
| James Shaffer |
|
|
— |
|
|
|
— |
|
|
|
* |
|
| Eduardo Martins |
|
|
— |
|
|
|
— |
|
|
|
* |
|
| Ed Engleman(2) |
|
|
13,377,154 |
|
|
|
— |
|
|
|
40.6 |
% |
| Nina Kjellson(1) |
|
|
13,377,154 |
|
|
|
— |
|
|
|
40.6 |
% |
| Jeffrey Glenn(4) |
|
|
1,741,506 |
|
|
|
— |
|
|
|
5.2 |
% |
| Thomas Dietz |
|
|
|
|
|
|
|
|
|
|
|
|
| All current executive officers and directors as a group (9 persons) |
|
|
29,145,813 |
|
|
|
120,833 |
|
|
|
88.5 |
% |
| * |
Represents beneficial ownership of less than 1% of the shares of Common Stock. |
| (1) |
Represents 13,377,154 shares of common stock issuable upon conversion of preferred stock held by InterWest Partners X, LP. InterWest Management Partners X, LLC has sole voting and investment control over the shares
owned by InterWest X, LP. The Managing Directors and Venture Members of InterWest Management Partners X, LLC have shared voting and investment control over the shares owned by InterWest Partners X, LP. The managing directors of InterWest Management
Partners X, LLC are Bruce A. Cleveland, Philip T. Gianos, W. Stephen Holmes, Gilbert H. Kilman, Arnold L. Oronsky and Douglas A. Pepper and its venture members are Keval Desai and Khaled A. Nasr. Each of the foregoing individuals disclaims
beneficial ownership of the shares owned by InterWest Partners X, LP, except to the extent of their pro rata partnership interest therein. Nina Kjellson is a member of InterWest Management Partners X, LLC. Nina Kjellson disclaims beneficial
ownership of the shares owned by InterWest Partners X, LP, except to the extent of her pro rata partnership interest therein. |
| (2) |
Vivo Ventures Fund VI, L.P. directly holds 13,279,868 shares of common stock issuable upon conversion of preferred stock. Vivo Ventures VI Affiliates Fund, L.P. directly holds 97,286 shares of common stock issuable upon
conversion of preferred stock. Vivo Ventures VI, LLC, the sole general partner of both Vivo Ventures Fund VI, L.P. and Vivo Ventures VI Affiliates Fund, L.P., has shared voting power and shared investment power over such securities, may be deemed to
beneficially own such shares, and disclaims beneficial ownership of the shares except to the extent of its pecuniary interests therein. Dr. Engleman, one of Eiger’s board members, is a managing partner at Vivo Ventures VI, LLC, the general
partner of both Vivo Ventures Fund VI, L.P. and Vivo Ventures VI Affiliates Fund, L.P., Dr. Englemanhas shared voting or investment power over the shares held by Vivo Ventures Fund VI, L.P. and Vivo Ventures VI Affiliates Fund, L.P. and
disclaims beneficial ownership of these shares except to the extent of any pecuniary interest therein. |
| (3) |
Consists of 127,725 shares held directly by The Board of Trustees of the Leland Stanford Junior University (OTL) and 2,981,095 shares held directly by The Board of Trustees of the Leland Stanford Junior University
(PVF). The Board of Trustees of the Leland Stanford Junior University has voting and dispositive power with respect to these shares. |
| (4) |
Includes 1,726,024 shares held by Eiger Group International. Jeffrey Glenn is the Chief Executive Officer of Eiger Group International, Inc. Dr. Glenn has sole power to vote and sole power to dispose of shares
directly owned by Eiger Group International, Inc. |
264
LEGAL MATTERS
Pillsbury Winthrop Shaw Pittman LLP will pass upon the validity of the Celladon common stock offered by this proxy statement/prospectus/information statement.
The material U.S. federal income tax consequences of the merger will be passed upon for Celladon by Pillsbury Winthrop Shaw Pittman LLP and for Eiger by Cooley LLP.
EXPERTS
The
consolidated financial statements of Celladon at December 31, 2014 and 2013, and for each of the three years in the period ended December 31, 2014, included in the Proxy Statement of Celladon Corporation, which is referred to and made a
part of this Prospectus and Registration Statement, have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report appearing elsewhere herein, and are included in reliance upon such
report given on the authority of such firm as experts in accounting and auditing.
The consolidated financial statements of Eiger as of December 31,
2014 and 2013, and for each of the years in the two-year period ended December 31, 2014, have been included herein in reliance upon the report of KPMG LLP, independent registered public accounting firm, appearing elsewhere herein, and upon the
authority of said firm as experts in accounting and auditing.
The audit report covering the December 31, 2014 consolidated financial statements of
Eiger contains an explanatory paragraph that states that Eiger’s recurring losses from operations and accumulated deficit raise substantial doubt about the entity’s ability to continue as a going concern. The consolidated financial
statements do not include any adjustments that might result from the outcome of that uncertainty.
WHERE YOU
CAN FIND MORE INFORMATION
Celladon files annual, quarterly and special reports, proxy statements and other information are with the SEC. You may read
and copy any reports, statements or other information that Celladon files at the SEC public reference room in at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms.
Celladon SEC filings are also available to the public from commercial document retrieval services and on the website maintained by the SEC at http://www.sec.gov. Reports, proxy statements and other information concerning Celladon also may be
inspected at the offices of the National Association of Securities Dealers, Inc., Listing Section, 1735 K Street, Washington, D.C. 20006.
As of the date
of this proxy statement/prospectus/information statement, Celladon has filed a registration statement on Form S-4 to register with the SEC the Celladon common stock that Celladon will issue to Eiger stockholders in the merger. This proxy
statement/prospectus/information statement is a part of that registration statement and constitutes a prospectus of Celladon, as well as a proxy statement of Celladon for its special meeting and an information statement for the purpose of Eiger for
its written consent.
Celladon has supplied all information contained in this proxy statement/prospectus/information statement relating to Celladon and
Eiger has supplied all information contained in this proxy statement/prospectus/information statement relating to Eiger.
If you would like to request
documents from Celladon or Eiger, please send a request in writing or by telephone to either Celladon or Eiger at the following addresses:
|
|
|
| Celladon Corporation
12707 High Bluff Drive, Suite 200
San Diego, CA 92130 Telephone:
(858) 350-4355 Attn: Investor Relations |
|
Eiger Biopharmaceuticals, Inc.
350 Cambridge Avenue, Suite 350
Palo Alto, CA 94306 Telephone:
(650) 272-6138 Attn: David Cory |
265
If you are a Celladon stockholder and would like additional copies, without charge, of this proxy
statement/prospectus/information statement or if you have questions about the merger, including the procedures for voting your shares, you should contact Celladon’s proxy solicitor:
ADVANTAGE PROXY
(877)
870-8565 (toll free)
(206) 870-8565 (collect)
TRADEMARK NOTICE
Celladon Corporation is an unregistered trademark of Celladon in the United States and other jurisdictions. MYDICAR is a registered trademark of Celladon in
the United States. Eiger Biopharmaceuticals, Inc. is a registered and unregistered trademark of Eiger in the United States and other jurisdictions. Other third-party logos and product/trade names are registered trademarks or trade names of their
respective companies.
OTHER MATTERS
Stockholder Proposals
Celladon stockholders are entitled to present proposals for action at a forthcoming meeting if they comply with the requirements of Celladon bylaws and the
rules established by the SEC under the Exchange Act. Under these requirements, to be considered for inclusion in Celladon’s proxy materials for Celladon’s 2016 annual meeting, stockholder proposals must be submitted in writing by
January 7, 2016, to the attention of the Secretary of Celladon Corporation, 12707 High Bluff Drive, Suite 200, San Diego, California 92130. For stockholder proposals (including director nominations) at Celladon’s 2016 annual meeting that
are not to be included in Celladon’s proxy materials for the 2016 annual meeting, such proposals must be submitted between February 5, 2016 and March 6, 2016, unless the 2016 annual meeting of stockholders is initially scheduled to be
held more than 30 days before or after June 4, 2016, in which case the notice must be received no later than the 10th day following the day on which public announcement of the annual meeting date is first made. Stockholders are also advised to
review Celladon’s bylaws, which contain additional requirements relating to advance notice of stockholder proposals and director nominations.
266
Celladon Corporation
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2014 and 2013 and Nine Months Ended September 30, 2015 and 2014
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Celladon Corporation
We have audited the accompanying consolidated balance sheets of Celladon Corporation, as of December 31, 2014 and 2013, and the related consolidated
statements of operations and comprehensive income (loss), stockholders’ (deficit) equity, and cash flows for the years ended December 31, 2014, 2013 and 2012. These financial statements are the responsibility of the Company’s
management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the
standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were
not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the
circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis,
evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our
audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material
respects, the consolidated financial position of Celladon Corporation at December 31, 2014 and 2013, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in
conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
San Diego, California
March 31, 2015
F-2
Celladon Corporation
Consolidated Balance Sheets
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
| |
|
December 31 |
|
| |
|
2014 |
|
|
2013 |
|
| Assets |
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
14,435 |
|
|
$ |
7,903 |
|
| Short-term investments |
|
|
70,513 |
|
|
|
10,467 |
|
| Prepaid expenses and other assets |
|
|
3,135 |
|
|
|
180 |
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
88,083 |
|
|
|
18,550 |
|
| Property and equipment, net |
|
|
763 |
|
|
|
308 |
|
| Other assets |
|
|
264 |
|
|
|
2,296 |
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
89,110 |
|
|
$ |
21,154 |
|
|
|
|
|
|
|
|
|
|
| Liabilities, preferred stock and stockholders’ equity (deficit) |
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable and accrued expenses |
|
$ |
5,803 |
|
|
$ |
2,908 |
|
| Accrued clinical expenses |
|
|
731 |
|
|
|
1,478 |
|
| Accrued interest |
|
|
71 |
|
|
|
14 |
|
| Current portion of long-term obligations |
|
|
1 |
|
|
|
— |
|
| Convertible notes, net of discount |
|
|
— |
|
|
|
1,044 |
|
| Warrant liability |
|
|
— |
|
|
|
1,116 |
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
6,606 |
|
|
|
6,560 |
|
| Term loan, net of discount |
|
|
10,102 |
|
|
|
— |
|
| Non-current liabilities |
|
|
298 |
|
|
|
37 |
|
| Commitments and contingencies (Note 5) |
|
|
|
|
|
|
|
|
| Series A-1 redeemable convertible preferred stock, $0.0001 par value: |
|
|
|
|
|
|
|
|
| Authorized shares—none and 135,826,497 at December 31, 2014 and 2013, respectively; issued and outstanding shares—none
and 127,140,530 at December 31, 2014 and 2013, respectively; liquidation preference—none and $114,172 at December 31, 2014 and 2013, respectively |
|
|
— |
|
|
|
60,098 |
|
| Convertible preferred stock, $0.0001 par value: |
|
|
|
|
|
|
|
|
| Authorized shares—none and 12,138,080 at December 31, 2014 and 2013, respectively; issued and outstanding shares—none
and 12,138,080 at December 31, 2014 and 2013, respectively; liquidation preference—none and $5,450 at December 31, 2014 and 2013, respectively |
|
|
— |
|
|
|
5,450 |
|
| Stockholders’ equity (deficit): |
|
|
|
|
|
|
|
|
| Preferred stock, $0.001 par value; authorized shares—10,000,000 and none at December 31, 2014 and 2013, respectively; no
shares issued and outstanding |
|
|
— |
|
|
|
— |
|
| Common stock, $0.001 par value; authorized shares—200,000,000 and 180,000,000 at December 31, 2014 and 2013, respectively;
issued and outstanding—23,490,737 and 884,179 at December 31, 2014 and 2013, respectively |
|
|
23 |
|
|
|
— |
|
| Additional paid-in capital |
|
|
218,528 |
|
|
|
61,593 |
|
| Accumulated other comprehensive (loss) income |
|
|
(8 |
) |
|
|
2 |
|
| Accumulated deficit |
|
|
(146,439 |
) |
|
|
(112,586 |
) |
|
|
|
|
|
|
|
|
|
| Total stockholders’ equity (deficit) |
|
|
72,104 |
|
|
|
(50,991 |
) |
|
|
|
|
|
|
|
|
|
| Total liabilities, preferred stock and stockholders’ equity (deficit) |
|
$ |
89,110 |
|
|
$ |
21,154 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-3
Celladon Corporation
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years Ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
|
2012 |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
22,676 |
|
|
$ |
16,927 |
|
|
$ |
13,314 |
|
| General and administrative |
|
|
10,342 |
|
|
|
3,037 |
|
|
|
2,631 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
33,018 |
|
|
|
19,964 |
|
|
|
15,945 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(33,018 |
) |
|
|
(19,964 |
) |
|
|
(15,945 |
) |
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
| Interest income |
|
|
118 |
|
|
|
69 |
|
|
|
35 |
|
| Interest expense |
|
|
(741 |
) |
|
|
(59 |
) |
|
|
(108 |
) |
| Other (expense) income |
|
|
(29 |
) |
|
|
25 |
|
|
|
147 |
|
| Change in fair value of warrant liability |
|
|
(183 |
) |
|
|
(162 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Consolidated net loss |
|
|
(33,853 |
) |
|
|
(20,091 |
) |
|
|
(15,871 |
) |
| Net loss attributable to non-controlling interest |
|
|
— |
|
|
|
96 |
|
|
|
154 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss attributable to Celladon Corporation |
|
|
(33,853 |
) |
|
|
(19,995 |
) |
|
|
(15,717 |
) |
| Accretion to redemption value of redeemable convertible preferred stock |
|
|
— |
|
|
|
— |
|
|
|
(343 |
) |
| Change in fair value of non-controlling interest |
|
|
— |
|
|
|
(3,105 |
) |
|
|
(154 |
) |
| Deemed dividend |
|
|
— |
|
|
|
(856 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss attributable to common stockholders |
|
$ |
(33,853 |
) |
|
$ |
(23,956 |
) |
|
$ |
(16,214 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
| Unrealized (loss) gain on investments |
|
|
(10 |
) |
|
|
(7 |
) |
|
|
9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Comprehensive loss |
|
$ |
(33,863 |
) |
|
$ |
(20,098 |
) |
|
$ |
(15,862 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(1.82 |
) |
|
$ |
(27.09 |
) |
|
$ |
(19.74 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted-average shares outstanding, basic and diluted |
|
|
18,603,605 |
|
|
|
884,179 |
|
|
|
821,568 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-4
Celladon Corporation
Consolidated Statements of Preferred Stock and Stockholders’ Deficit
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Series A-1
Redeemable
Convertible Preferred
Stock |
|
|
Convertible
Preferred Stock |
|
|
Special
Voting Stock |
|
|
Common Stock |
|
|
Additional
Paid-in
Capital |
|
|
Accumulated
Other
Comprehensive
Income (Loss) |
|
|
Accumulated
Deficit |
|
|
Total
Stockholders’
Equity
(Deficit) |
|
| |
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2011 |
|
|
— |
|
|
$ |
— |
|
|
|
39,186,807 |
|
|
$ |
56,282 |
|
|
|
— |
|
|
$ |
— |
|
|
|
2,798 |
|
|
$ |
5,895 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
(76,874 |
) |
|
$ |
(70,979 |
) |
| Issuance of common stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
55 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Conversion of Series A, B, B-1 and C preferred stock to common stock |
|
|
— |
|
|
|
— |
|
|
|
(39,186,807 |
) |
|
|
(56,282 |
) |
|
|
— |
|
|
|
— |
|
|
|
31,374 |
|
|
|
56,282 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
56,282 |
|
| Issuance of preferred stock and common stock in connection with conversion of debt and accrued interest |
|
|
15,160,301 |
|
|
|
6,807 |
|
|
|
12,138,080 |
|
|
|
5,450 |
|
|
|
— |
|
|
|
— |
|
|
|
849,952 |
|
|
|
2,188 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,188 |
|
| Delaware reincorporation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(64,168 |
) |
|
|
64,168 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Issuance of special voting stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Issuance of Series A-1 preferred stock, net of $343 of offering costs |
|
|
101,263,824 |
|
|
|
45,124 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Accretion to redemption value of redeemable convertible preferred stock |
|
|
— |
|
|
|
343 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(252 |
) |
|
|
(91 |
) |
|
|
— |
|
|
|
— |
|
|
|
(343 |
) |
| Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
55 |
|
|
|
243 |
|
|
|
— |
|
|
|
— |
|
|
|
298 |
|
| Change in fair value of redeemable non-controlling interest |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(154 |
) |
|
|
— |
|
|
|
— |
|
|
|
(154 |
) |
| Consolidated net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(15,871 |
) |
|
|
(15,871 |
) |
| Net loss attributable to redeemable non-controlling interest |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
154 |
|
|
|
154 |
|
| Unrealized gain on investment securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
9 |
|
|
|
— |
|
|
|
9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2012 |
|
|
116,424,125 |
|
|
|
52,274 |
|
|
|
12,138,080 |
|
|
|
5,450 |
|
|
|
1 |
|
|
|
1 |
|
|
|
884,179 |
|
|
|
— |
|
|
|
64,166 |
|
|
|
9 |
|
|
|
(92,591 |
) |
|
|
(28,416 |
) |
| Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,388 |
|
|
|
— |
|
|
|
— |
|
|
|
1,388 |
|
| Change in fair value of redeemable non-controlling interest |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,105 |
) |
|
|
— |
|
|
|
— |
|
|
|
(3,105 |
) |
| Share exchange related to non-controlling interest and special voting stock |
|
|
10,716,405 |
|
|
|
7,824 |
|
|
|
— |
|
|
|
— |
|
|
|
(1 |
) |
|
|
(1 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Deemed dividend |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(856 |
) |
|
|
— |
|
|
|
— |
|
|
|
(856 |
) |
| Consolidated net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(20,091 |
) |
|
|
(20,091 |
) |
| Net loss attributable to redeemable non-controlling interest |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
96 |
|
|
|
96 |
|
| Unrealized loss on investment securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(7 |
) |
|
|
— |
|
|
|
(7 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2013 |
|
|
127,140,530 |
|
|
$ |
60,098 |
|
|
|
12,138,080 |
|
|
$ |
5,450 |
|
|
|
— |
|
|
$ |
— |
|
|
|
884,179 |
|
|
$ |
— |
|
|
$ |
61,593 |
|
|
$ |
2 |
|
|
$ |
(112,586 |
) |
|
$ |
(50,991 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-5
Celladon Corporation
Consolidated Statements of Preferred Stock and Stockholders’ Deficit
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Series A-1
Redeemable
Convertible
Preferred
Stock |
|
|
Convertible
Preferred Stock |
|
|
Special
Voting Stock |
|
|
Common Stock |
|
|
Additional
Paid-in
Capital |
|
|
Accumulated
Other
Comprehensive
Income (Loss) |
|
|
Accumulated
Deficit |
|
|
Total
Stockholders’
Equity
(Deficit) |
|
| |
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2013 |
|
|
127,140,530 |
|
|
|
60,098 |
|
|
|
12,138,080 |
|
|
|
5,450 |
|
|
|
— |
|
|
|
— |
|
|
|
884,179 |
|
|
|
— |
|
|
|
61,593 |
|
|
|
2 |
|
|
|
(112,586 |
) |
|
|
(50,991 |
) |
| Impact of initial public offering |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Initial public offering of common stock, net of $6,342 in offering costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,325,000 |
|
|
|
6 |
|
|
|
44,252 |
|
|
|
— |
|
|
|
— |
|
|
|
44,258 |
|
| Conversion of convertible notes into common stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
139,644 |
|
|
|
— |
|
|
|
1,117 |
|
|
|
— |
|
|
|
— |
|
|
|
1,117 |
|
| Conversion of convertible preferred stock into common stock |
|
|
(127,140,530 |
) |
|
|
(60,098 |
) |
|
|
(12,138,080 |
) |
|
|
(5,450 |
) |
|
|
— |
|
|
|
— |
|
|
|
11,151,192 |
|
|
|
11 |
|
|
|
65,537 |
|
|
|
— |
|
|
|
— |
|
|
|
65,548 |
|
| Warrant liability reclassification |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,299 |
|
|
|
— |
|
|
|
— |
|
|
|
1,299 |
|
| Common stock issuance upon exercise of warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
25,481 |
|
|
|
— |
|
|
|
143 |
|
|
|
— |
|
|
|
— |
|
|
|
143 |
|
| Public offering of common stock, net of $3,011 of offering costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,600,000 |
|
|
|
5 |
|
|
|
40,684 |
|
|
|
— |
|
|
|
— |
|
|
|
40,689 |
|
| Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,319 |
|
|
|
— |
|
|
|
— |
|
|
|
3,319 |
|
| Exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
340,220 |
|
|
|
1 |
|
|
|
406 |
|
|
|
— |
|
|
|
— |
|
|
|
407 |
|
| Issuance of common stock under employee stock purchase plan |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
25,021 |
|
|
|
— |
|
|
|
178 |
|
|
|
— |
|
|
|
— |
|
|
|
178 |
|
| Consolidated net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(33,853 |
) |
|
|
(33,853 |
) |
| Unrealized loss on investment securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(10 |
) |
|
|
— |
|
|
|
(10 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2014 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
23,490,737 |
|
|
$ |
23 |
|
|
$ |
218,528 |
|
|
$ |
(8 |
) |
|
$ |
(146,439 |
) |
|
$ |
72,104 |
|
See accompanying notes.
F-6
Celladon Corporation
Consolidated Statements of Cash Flows
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years Ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
|
2012 |
|
| Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Consolidated net loss |
|
$ |
(33,853 |
) |
|
$ |
(20,091 |
) |
|
$ |
(15,871 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Depreciation |
|
|
153 |
|
|
|
67 |
|
|
|
64 |
|
| Stock-based compensation |
|
|
3,319 |
|
|
|
1,388 |
|
|
|
298 |
|
| Noncash interest expense |
|
|
388 |
|
|
|
59 |
|
|
|
108 |
|
| Amortization of investment premium |
|
|
393 |
|
|
|
255 |
|
|
|
124 |
|
| Change in fair value of warrant liability |
|
|
183 |
|
|
|
162 |
|
|
|
— |
|
| Loss on disposal of property and equipment |
|
|
1 |
|
|
|
— |
|
|
|
— |
|
| Deferred rent |
|
|
74 |
|
|
|
17 |
|
|
|
28 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Prepaid expenses and other assets |
|
|
(2,860 |
) |
|
|
104 |
|
|
|
(266 |
) |
| Accounts payable and accrued expenses |
|
|
2,935 |
|
|
|
1,843 |
|
|
|
878 |
|
| Other liabilities |
|
|
8 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash used in operating activities |
|
|
(29,259 |
) |
|
|
(16,196 |
) |
|
|
(14,637 |
) |
| Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Purchases of investment securities |
|
|
(90,659 |
) |
|
|
(17,860 |
) |
|
|
(26,751 |
) |
| Proceeds from maturities of investment securities |
|
|
30,210 |
|
|
|
28,801 |
|
|
|
4,966 |
|
| Purchases of property and equipment |
|
|
(739 |
) |
|
|
(87 |
) |
|
|
(48 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash provided by (used in) investing activities |
|
|
(61,188 |
) |
|
|
10,854 |
|
|
|
(21,833 |
) |
| Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance of common stock |
|
|
95,028 |
|
|
|
— |
|
|
|
— |
|
| Proceeds from issuance of preferred stock, net |
|
|
— |
|
|
|
— |
|
|
|
45,140 |
|
| Proceeds from issuance of exchangeable shares |
|
|
— |
|
|
|
— |
|
|
|
4,814 |
|
| Proceeds from issuance of convertible debt |
|
|
— |
|
|
|
1,097 |
|
|
|
— |
|
| Repayment of convertible debt |
|
|
— |
|
|
|
— |
|
|
|
(111 |
) |
| Costs paid in connection with common stock offerings |
|
|
(7,661 |
) |
|
|
(1,693 |
) |
|
|
— |
|
| Proceeds from borrowing under term loan |
|
|
10,000 |
|
|
|
— |
|
|
|
— |
|
| Costs paid in connection with term loan |
|
|
(387 |
) |
|
|
— |
|
|
|
— |
|
| Other |
|
|
(1 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash provided by (used in) financing activities |
|
|
96,979 |
|
|
|
(596 |
) |
|
|
49,843 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net increase (decrease) in cash and cash equivalents |
|
|
6,532 |
|
|
|
(5,938 |
) |
|
|
13,373 |
|
| Cash and cash equivalents, beginning of period |
|
|
7,903 |
|
|
|
13,841 |
|
|
|
468 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents, end of period |
|
$ |
14,435 |
|
|
$ |
7,903 |
|
|
$ |
13,841 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Supplemental disclosure of cash flow information |
|
|
|
|
|
|
|
|
|
|
|
|
| Interest paid |
|
$ |
282 |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Supplemental schedule of noncash investing and financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion of convertible debt and accrued interest for Series A-1 and Junior preferred and common stock |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
14,430 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Share exchange related to non-controlling interest and special voting stock |
|
$ |
— |
|
|
$ |
7,824 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Deemed dividend |
|
$ |
— |
|
|
$ |
856 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued purchases of property and equipment |
|
$ |
23 |
|
|
$ |
166 |
|
|
$ |
— |
|
| Conversion of convertible preferred stock into common stock |
|
$ |
65,548 |
|
|
$ |
— |
|
|
$ |
— |
|
| Conversion of convertible notes into common stock |
|
$ |
1,117 |
|
|
$ |
— |
|
|
$ |
— |
|
| Warrant liability reclassification to equity |
|
$ |
1,299 |
|
|
$ |
— |
|
|
$ |
— |
|
| Capital expenditures funded by capital lease borrowings |
|
$ |
12 |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-7
Celladon Corporation
Notes to Consolidated Financial Statements
1. Organization and Summary of Significant Accounting Policies
Organization
Celladon Corporation (Celladon or the
Company) was incorporated in California on December 21, 2000 (inception) and reincorporated in Delaware in April 2012. The Company is a clinical-stage biotechnology with industry-leading expertise in the development of cardiovascular gene
therapy. The Company applies its leadership position in the field of gene therapy and calcium dysregulation to develop novel therapies for diseases with tremendous unmet medical needs and characterized by an underlying SERCA enzyme deficiency.
As of December 31, 2014, the Company has devoted substantially all of its efforts to product development, raising capital and building infrastructure and
has not generated revenues from its planned principal operations.
Use of Estimates
The Company’s consolidated financial statements are prepared in accordance with U.S. generally accepted accounting principles (GAAP). The preparation of
the Company’s consolidated financial statements requires it to make estimates and assumptions that impact the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in the Company’s
consolidated financial statements and accompanying notes. The most significant estimates in the Company’s consolidated financial statements relate to the fair value of equity awards and clinical trial expense accruals. Although these estimates
are based on the Company’s knowledge of current events and actions it may undertake in the future, actual results may ultimately materially differ from these estimates
Public Offerings
In February 2014, the Company
completed its initial public offering of 6,325,000 shares of common stock at an offering price of $8.00 per share, which included the exercise by the underwriters of their option to purchase 825,000 additional shares of common stock. The Company
received net proceeds of $44.3 million after deducting underwriting discounts and commission and offering expenses payable by the Company, including $1.7 million in offering costs paid by the Company prior to December 31, 2013. In connection
with the initial public offering, all outstanding shares of convertible preferred stock were converted into shares of common stock, the outstanding principal and accrued interest on the Company’s outstanding convertible notes were converted
into shares of common stock and the unamortized debt discount related to the convertible notes was charged to expense, warrants to purchase shares of Series A-1 preferred stock were converted into warrants to purchase common stock, the warrant
liability was reclassified to additional paid-in capital, and the Company’s certificate of incorporation was amended and restated to authorize 200,000,000 shares of common stock and 10,000,000 shares of undesignated preferred stock. See Note 7
for additional information.
In August 2014, the Company completed an underwritten public offering of 4,600,000 shares of common stock at an offering
price of $9.50 per share, which included the exercise by the underwriters of their option to purchase 600,000 additional shares of common stock. The Company received net proceeds of $40.7 million after deducting underwriting discounts and commission
and offering expenses payable by the Company. See Note 7 for additional information.
Principles of Consolidation
On April 27, 2012, Celladon formed a subsidiary, Celladon Europe B.V. (Celladon Europe), a Dutch limited liability company, for the purpose of managing
the new capital investment made by Cooperatief LSP IV U.A.
F-8
(LSP) related to Celladon’s Series A-1 preferred stock (see Note 2). From its inception to June 6, 2013 the subsidiary was 90% owned by Celladon and from June 6, 2013 to
December 29, 2014 the subsidiary was wholly owned by Celladon. Celladon Europe was dissolved on December 30, 2014. The financial statements of Celladon Europe are consolidated with those of the Company. All intercompany transactions and
balances were eliminated in consolidation. The U.S. dollar was the functional currency of Celladon Europe. The Company remeasured Celladon Europe’s assets and liabilities related to monetary assets and liabilities to the U.S. dollar and
recorded the net gains or losses resulting from remeasurement in other income (expense) in the consolidated statements of operations and comprehensive loss. During all periods presented, the Company did not record any material gains or losses from
remeasurement.
Segment Reporting
Operating
segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance.
The Company views its operations and manages its business in one operating segment.
Cash and Cash Equivalents
Cash and cash equivalents consists primarily of readily available checking, money market accounts and money market funds. The Company considers all highly
liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents.
Investment Securities
Investment securities primarily consist of investment grade corporate debt securities. The Company classifies all investment securities as
available-for-sale. Investments with maturity dates greater than 12 months from the end of each reporting period are classified as long-term. Investment securities are carried at fair value, with the unrealized gains and losses reported as a
component of other comprehensive income (loss) in stockholders’ equity (deficit) until realized. Realized gains and losses from the sale of investment securities, if any, are determined on a specific identification basis. A decline in the
market value of any investment security below cost that is determined to be other than temporary will result in an impairment charge to earnings and a new cost basis for the security is established. No such impairment charges were recorded for any
period presented. As of December 31, 2014 and December 31, 2013, none of the investment securities have been in an unrealized loss position for more than 12 months. Premiums and discounts are amortized or accreted over the life of the
related security as an adjustment to yield using the straight-line method and are included in interest income. Interest income is recognized when earned.
The following table sets forth the composition of the Company’s investment securities (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As of December 31, 2014 |
|
Maturity in Years |
|
|
Amortized
Cost |
|
|
Unrealized |
|
|
Fair
Value |
|
| |
|
|
Gains |
|
|
Losses |
|
|
| Corporate debt securities |
|
|
Less than 1 year |
|
|
$ |
70,521 |
|
|
$ |
— |
|
|
$ |
(8 |
) |
|
$ |
70,513 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As of December 31, 2013 |
|
Maturity in Years |
|
|
Amortized
Cost |
|
|
Unrealized |
|
|
Fair
Value |
|
| |
|
|
Gains |
|
|
Losses |
|
|
| Corporate debt securities |
|
|
Less than 1 year |
|
|
$ |
10,465 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
10,467 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-9
Concentration of Credit Risk
Financial instruments, which potentially subject the Company to significant concentration of credit risk, consist primarily of cash, cash equivalents and
investment securities. The Company has established guidelines regarding diversification of investments and their maturities, which are designed to maintain principal and maximize liquidity. The Company maintains deposits in federally insured
financial institutions in excess of federally insured limits. The Company has not experienced any losses in such accounts and management believes that the Company is not exposed to significant credit risk due to the financial position of the
depository institutions in which those deposits are held.
Property and Equipment
Property and equipment are recorded at cost and depreciated using the straight-line method over the estimated useful lives of the assets (generally three to
five years) and generally consist of furniture and fixtures, computers, and office equipment. Repairs and maintenance costs are charged to expense as incurred.
Impairment of Long-Lived Assets
Long-lived assets
consist primarily of property and equipment. An impairment loss is recorded if and when events and circumstances indicate that assets might be impaired and the undiscounted cash flows estimated to be generated by those assets are less than the
carrying amount of those assets. While the Company’s current and historical operating losses and negative cash flows are indicators of impairment, management believes that future cash flows to be received support the carrying value of its
long-lived assets and, accordingly, has not recognized any impairment losses since inception.
Clinical Trial Accruals
As part of the process of preparing its financial statements, the Company is required to estimate its expenses resulting from its obligations under contracts
with vendors and consultants and clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations which vary from contract to contract and may result in payment flows that do
not match the periods over which materials or services are provided to the Company under such contracts. The Company’s objective is to reflect the appropriate trial expenses in its financial statements by matching those expenses with the period
in which services and efforts are expended. The Company accounts for these expenses according to the progress of the trial as measured by patient progression and the timing of various aspects of the trial. The Company determines accrual estimates
through financial models taking into account discussion with applicable personnel and outside service providers as to the progress or state of consummation of trials, or the services completed. During the course of a clinical trial, the Company
adjusts its rate of clinical expense recognition if actual results differ from its estimates. The Company makes estimates of its accrued expenses as of each balance sheet date in its financial statements based on the facts and circumstances known at
that time. Although the Company does not expect its estimates to be materially different from amounts actually incurred, its understanding of the status and timing of services performed relative to the actual status and timing of services performed
may vary and may result in the Company reporting amounts that are too high or too low for any particular period. Through December 31, 2014, there have been no material adjustments to the Company’s prior period estimates of accrued expenses
for clinical trials. The Company’s clinical trial accrual is dependent upon the timely and accurate reporting of contract research organizations and other third-party vendors.
Preferred Stock Warrant Liability
The Company had
issued freestanding warrants to purchase shares of its convertible preferred stock. The fair value of these warrants is classified as a current liability at December 31, 2013 in the accompanying consolidated balance sheets since the underlying
redeemable convertible preferred stock is classified as temporary equity at
F-10
December 31, 2013 in the accompanying consolidated balance sheets instead of in stockholders’ equity (deficit) in accordance with authoritative guidance for the classification and
measurement of redeemable securities. The warrants were recorded at fair value using the Black-Scholes option pricing model with any changes in fair value being recognized as a component of other income (expense) in the accompanying consolidated
statements of operations and comprehensive loss. Upon completion of the Company’s initial public offering in February 2014, the warrants no longer required liability accounting and the then fair value of the warrant liability was reclassified
into equity
Deferred Rent
Deferred rent
consists of the difference between cash payments and the recognition of rent expense on a straight-line basis for the facility the Company occupies. The Company’s lease for its facility provides for fixed increases in minimum annual rental
payments. The total amount of rental payments due over the lease term is being charged to rent expense ratably over the life of the lease.
Preferred Stock
The Company classifies preferred
stock that is redeemable or subject to liquidation outside of the Company’s control outside of permanent equity. For preferred stock that is contractually redeemable outside of the Company’s control, the carrying value is increased to its
redemption value by accretion in the period of issuance. In the absence of retained earnings, these accretion charges are recorded against additional paid-in capital.
Research and Development Costs
Research and
development expenses consist primarily of salaries and related overhead expenses; fees paid to consultants and contract research organizations; costs related to acquiring and manufacturing clinical trial materials; costs related to compliance with
regulatory requirements; and maintenance and license payments related to licensed product candidates and technologies. All research and development costs are expensed as incurred.
Patent Costs
Costs related to filing and pursuing
patent applications are recorded as general and administrative expense and expensed as incurred since recoverability of such expenditures is uncertain.
Stock-Based Compensation
Stock-based compensation
expense represents the cost of the grant date fair value of employee stock option grants and stock purchases under the Employee Stock Purchase Plan (ESPP) recognized over the requisite service period of the awards (usually the vesting period) on a
straight-line basis, net of estimated forfeitures. The Company estimates the fair value of the awards using the Black-Scholes option pricing model.
The
Company accounts for stock options granted to non-employees using the fair value approach. These option grants are subject to periodic revaluation over their vesting terms.
Income Taxes
The Company accounts for income
taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax
assets and liabilities are determined on the basis of the differences between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect
F-11
for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the
enactment date.
The Company recognizes net deferred tax assets to the extent that management believes these assets are more likely than not to be
realized. In making such a determination, management considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results
of recent operations. If management determines that the Company would be able to realize its deferred tax assets in the future in excess of their net recorded amount, management would make an adjustment to the deferred tax asset valuation allowance,
which would reduce the provision for income taxes.
The Company records uncertain tax positions on the basis of a two-step process whereby
(1) management determines whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition
threshold, management recognizes the largest amount of tax benefit that is more than 50% likely to be realized upon ultimate settlement with the related tax authority. The Company recognizes interest and penalties related to unrecognized tax
benefits within income tax expense. Any accrued interest and penalties are included within the related tax liability.
Comprehensive Loss
Comprehensive loss is defined as a change in equity during a period from transactions and other events and circumstances from non-owner sources.
The Company’s only component of other comprehensive loss is unrealized gains (losses) on investment securities. Comprehensive loss has been reflected in the consolidated statements of operations and comprehensive loss and as a separate
component of the statements of stockholders’ deficit for all periods presented.
Net Loss Per Share Attributable to Common Stockholders
Basic net loss per share attributable to common stockholders is calculated by dividing the net loss attributable to common stockholders by the
weighted-average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share attributable to common stockholders is computed by dividing the net loss attributable to common
stockholders by the weighted-average number of common share equivalents outstanding for the period determined using the treasury-stock method. Dilutive common stock equivalents are comprised of convertible preferred stock and rights to acquire
convertible preferred stock (non-controlling interest), warrants for the purchase of common stock and options outstanding under the Company’s stock option plans. For all periods presented, there is no difference in the number of shares used to
calculate basic and diluted shares outstanding due to the Company’s net loss position.
Potentially dilutive securities not included in the
calculation of diluted net loss per share attributable to common stockholders because to do so would be anti-dilutive are as follows (in common stock equivalent shares):
|
|
|
|
|
|
|
|
|
| |
|
Years Ended December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Redeemable convertible preferred stock |
|
|
— |
|
|
|
10,179,372 |
|
| Convertible preferred stock |
|
|
— |
|
|
|
971,820 |
|
| Warrants for convertible preferred stock |
|
|
— |
|
|
|
231,821 |
|
| Warrants for common stock |
|
|
206,340 |
|
|
|
702 |
|
| Common stock options |
|
|
2,408,634 |
|
|
|
1,543,667 |
|
|
|
|
|
|
|
|
|
|
|
|
|
2,614,974 |
|
|
|
12,927,382 |
|
|
|
|
|
|
|
|
|
|
F-12
Recent Accounting Pronouncements
In July 2013, the FASB issued Accounting Standards Update (ASU) No. 2013-11, Presentation of an Unrecognized Tax Benefit When a Net Operating Loss
Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists. ASU 2013-11 provides explicit guidance on the financial statement presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a
tax credit carryforward exists. The guidance is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013, with an option for early adoption. On January 1, 2014, the Company adopted
this standard, which had no impact on its financial position or results of operations.
In June 2014, the FASB issued Accounting Standards Update (ASU)
No. 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation. ASU 2014-10 a) eliminates the
requirement for development stage entities to present inception-to-date information in the statements of income, cash flows and shareholder equity, b) amends Topic 275 to clarify that the risk and uncertainty disclosure requirements apply to
entities that have not commenced principal operations, c) eliminates the exception related to the sufficiency of equity at risk for development stage entities from the guidance on variable interest entities in paragraph 810-10-15-16 to increase
consistency in application of consolidated guidance across all entities and d) removes the definition of development stage entities from the Master Glossary of the Accounting Standards Codification. The amendments in this Update are to be
applied retrospectively except for the clarification to Topic 275, which shall be applied prospectively. The guidance is effective for fiscal years, and interim periods within those years, beginning after December 15, 2014, with an option for
early adoption. The Company adopted this guidance prior to filing this Annual Report on Form 10-K for the year ended December 31, 2014. The adoption of ASU 2014-10 impacted disclosure only and did not have any impact on the Company’s
financial position or results of operations.
In June 2014, the FASB issued ASU No. 2014-12, Accounting for Share-Based Payments When the Terms of
an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period, which clarifies that entities should treat performance targets that can be met after the requisite service period of a share-based payment award as
performance conditions that affect vesting. This standard is effective for annual reporting periods ending after December 15, 2015 and interim periods within those annual periods. Early adoption is permitted. The adoption of this guidance is
expected to have no impact on the Company’s financial position or results of operations.
In August 2014, the FASB issued ASU No. 2014-15,
Presentation of Financial Statements Going Concern, which requires management to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosure in certain circumstances. This standard is effective
for annual reporting periods ending after December 15, 2016 and interim periods thereafter. Early adoption is permitted. The adoption of this guidance is expected to have no impact on the Company’s financial position or results of
operations.
In November 2014, the FASB issued ASU No. 2014-16, Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in
the Form of a Share is More Akin to Debt or Equity, which requires the use of the whole instrument approach in determining the nature of a host contract in a hybrid instrument. This standard is effective for annual reporting periods ending after
December 15, 2015 and interim periods within those annual periods. Early adoption is permitted. The adoption of this guidance is expected to have no impact on the Company’s financial position or results of operations.
2. Celladon Europe B.V.
In April 2012 and June 2012, LSP
invested an aggregate of $4.8 million in Celladon Europe. In exchange for the investment, the Company issued LSP one share of Special Preferred Voting stock and Celladon Europe issued LSP 1,999 non-voting B shares. The 1,999 B shares were
exchangeable into 10,716,405 shares of the Company’s
F-13
Series A-1 preferred stock at the option of LSP. The Company determined that the investment held by LSP in Celladon Europe should be classified as a redeemable non-controlling interest, as the
shares of Celladon Europe were not in-substance common stock. In-substance common stock is an investment in an entity that has risk and reward characteristics that are substantially similar to that entity’s common stock. Due to the liability
characteristics associated with the shares of Celladon Europe held by LSP, the Company concluded that the investor’s shares were not substantially similar to common stock. The liability characteristics included the investor’s put rights,
which provided the investor with the ability to exchange its shares in Celladon Europe for Series A-1 preferred stock of the Company.
The redeemable
non-controlling interest was initially valued using the fair value of the Series A-1 preferred stock. At each reporting period, the Company adjusted the carrying value of the redeemable non-controlling interest by the net loss attributable to the
redeemable non-controlling interest. Any difference between the fair value and the adjusted carrying value of the redeemable non-controlling interest was recorded as an adjustment to additional paid-in capital and presented as a component of net
loss attributable to common stockholders in the accompanying consolidated statements of operations and comprehensive loss.
From April 2012 through
June 6, 2013, LSP owned approximately 10% of Celladon Europe.
On June 6, 2013, LSP delivered a notice to exchange its 1,999 B shares of
Celladon Europe for 10,716,405 shares of the Company’s Series A-1 preferred stock. Concurrently, the one share of outstanding Special Preferred Voting stock was cancelled. As of June 6, 2013, the redeemable non-controlling interest was
adjusted to fair value and reclassified to Series A-1 preferred stock on the accompanying consolidated balance sheet.
During the years ended
December 31, 2013 and 2012, the Company adjusted the loss attributable to common stockholders as a result of increases in the fair value of the redeemable non-controlling interest of approximately $3.1 million and $0.2 million, respectively.
The increases in fair value increased the loss attributable to common stockholders.
In May 2014, the Company completed the transfer of the open clinical
site contracts from Celladon Europe to Celladon and on December 30, 2014, Celladon Europe was dissolved. Upon dissolution, final administrative fees were settled and cash of approximately $0.1 million was transferred from Celladon Europe to
Celladon.
3. Balance Sheet Details
Property and
equipment consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
| |
|
As of December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Office furniture and other equipment |
|
$ |
881 |
|
|
$ |
555 |
|
| Leasehold improvements |
|
|
246 |
|
|
|
— |
|
| Accumulated depreciation |
|
|
(364 |
) |
|
|
(247 |
) |
|
|
|
|
|
|
|
|
|
|
|
$ |
763 |
|
|
$ |
308 |
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
| |
|
As of December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Accounts payable |
|
$ |
3,293 |
|
|
$ |
1,397 |
|
| Accrued compensation |
|
|
1,909 |
|
|
|
664 |
|
| Accrued other |
|
|
596 |
|
|
|
839 |
|
| Current portion of deferred rent |
|
|
5 |
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
|
|
$ |
5,803 |
|
|
$ |
2,908 |
|
|
|
|
|
|
|
|
|
|
F-14
4. Fair Value Measurements
The Company’s financial instruments primarily consist of cash and cash equivalents, investment securities, accounts payable and accrued liabilities. The
carrying value of these financial instruments generally approximates fair value due to their short-term nature. Investment securities, warrant liabilities and redeemable non-controlling interest are recorded at fair value. Based on the borrowing
rates currently available to the Company for loans with similar terms, which is considered a Level 2 input, the Company believes that the fair value of its convertible debt approximates its carrying value.
The accounting guidance defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and
liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction
between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the
accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1: Observable inputs such as quoted prices in active markets;
Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and
Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions
As of December 31, 2014 and 2013, cash and cash equivalents consist primarily of bank deposits with third-party financial institutions and highly liquid
money market securities with original maturities at date of purchase of 90 days or less and are stated at cost which approximate fair value and are classified within the Level 1 designation discussed above. Marketable securities are recorded at fair
value, defined as the exit price in the principal market in which the Company would transact, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants.
Level 2 securities are valued using quoted market prices for similar instruments, non-binding market prices that are corroborated by observable market data, or discounted cash flow techniques and include the Company’s investments in corporate
debt securities and commercial paper. Financial assets and liabilities that are measured or disclosed at fair value on a recurring basis, and are classified within the Level 3 designation, include the warrant liability and redeemable non-controlling
interest. None of the Company’s non-financial assets and liabilities are recorded at fair value on a non-recurring basis. No transfers between levels have occurred during the periods presented.
Cash equivalents measured at fair value as of December 31, 2014 and 2013, are all classified within Level 1. Below is a summary of assets and liabilities
measured at fair value (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Fair Value Measurements at
Reporting Date Using |
|
| |
|
As of
December 31,
2014 |
|
|
Quoted Prices
in Active
Markets for
Identical
Assets
(Level 1) |
|
|
Significant
Other
Observable
Inputs
(Level 2) |
|
|
Significant
Unobservable
Inputs
(Level 3) |
|
| Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Corporate debt securities |
|
$ |
70,513 |
|
|
$ |
— |
|
|
$ |
70,513 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-15
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Fair Value Measurements at
Reporting Date Using |
|
| |
|
As of
December 31,
2013 |
|
|
Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) |
|
|
Significant
Other
Observable
Inputs
(Level 2) |
|
|
Significant
Unobservable
Inputs
(Level 3) |
|
| Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Corporate debt securities |
|
$ |
10,467 |
|
|
$ |
— |
|
|
$ |
10,467 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes |
|
$ |
1,044 |
|
|
|
— |
|
|
|
— |
|
|
$ |
1,044 |
|
| Warrant liability |
|
|
1,116 |
|
|
|
— |
|
|
|
— |
|
|
|
1,116 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
2,160 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
2,160 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company determined the fair value of the convertible notes utilizing an estimated cost of debt for comparable venture
backed and mezzanine financings.
The fair value per share of the Company’s underlying Series A-1 preferred stock was used to determine the fair
value of the redeemable non-controlling interest and the warrant liability. As of February 4, 2014, December 31, 2013, October 15, 2013 (issuance date of Series A-1 warrants), June 6, 2013 (exchange date of exchangeable
shares) and December 31, 2012, the fair value of the Series A-1 preferred stock was $0.64, $0.64, $0.91, $0.73 and $0.449, respectively. The fair value of the Series A-1 preferred stock was determined using either an option pricing model, a
hybrid option pricing and probability weighted expected return model or, in the case of the February 4, 2014 and December 31, 2013 values, derived from the Company’s IPO price. The key inputs into the models included the probability
and timing of expected liquidity event dates, discount rates and the selection of appropriate market comparable transactions and multiples to apply to the Company’s various historical and forecasted operational metrics.
In addition to the fair value of the underlying Series A-1 preferred stock, the following assumptions were used in the Black-Scholes option pricing model to
determine the fair value of the preferred stock warrant liability:
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
October 15,
2013 |
|
|
December 31,
2013 |
|
|
February 4,
2014 |
|
| Risk-free interest rate |
|
|
1.37 |
% |
|
|
1.58 |
% |
|
|
1.58 |
% |
| Expected volatility |
|
|
79 |
% |
|
|
82 |
% |
|
|
82 |
% |
| Expected term (in years) |
|
|
5.0 |
|
|
|
4.8 |
|
|
|
4.7 |
|
| Expected dividend yield |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
0.0 |
% |
F-16
The following table provides a reconciliation of all liabilities measured at fair value using Level 3 significant
unobservable inputs (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Redeemable
Non-Controlling
Interest |
|
|
Convertible
Notes |
|
|
Warrant
Liability |
|
| Balance at December 31, 2011 |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
| Issuance of shares of redeemable non-controlling interest |
|
|
4,814 |
|
|
|
— |
|
|
|
— |
|
| Net loss attributable to redeemable non-controlling interest |
|
|
(154 |
) |
|
|
— |
|
|
|
— |
|
| Change in fair value |
|
|
154 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2012 |
|
|
4,814 |
|
|
|
— |
|
|
|
— |
|
| Issuance of warrants in connection with note and warrant purchase agreement |
|
|
— |
|
|
|
— |
|
|
|
954 |
|
| Issuance of debt |
|
|
— |
|
|
|
999 |
|
|
|
— |
|
| Net loss attributable to redeemable non-controlling interest |
|
|
(96 |
) |
|
|
— |
|
|
|
— |
|
| Changes in fair value |
|
|
3,105 |
|
|
|
45 |
|
|
|
162 |
|
| Exchange of redeemable non-controlling interest for Series A-1 preferred stock |
|
|
(7,823 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2013 |
|
|
— |
|
|
|
1,044 |
|
|
|
1,116 |
|
| Changes in fair value |
|
|
— |
|
|
|
53 |
|
|
|
183 |
|
| Reclassification to equity upon initial public offering |
|
|
— |
|
|
|
— |
|
|
|
(1,299 |
) |
| Conversion to common stock upon initial public offering |
|
|
— |
|
|
|
(1,097 |
) |
|
|
— |
|
| Balance at December 31, 2014 |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. Commitments and Contingencies
Note and Warrant Purchase Agreement
In October
2013, the Company entered into a note and warrant purchase agreement with certain existing investors for the sale of up to an aggregate of $1,097,017 of convertible promissory notes (the 2013 Notes) and warrants exercisable to purchase shares of
Series A-1 Preferred Stock (the 2013 Warrants).
The terms of the 2013 Notes provided for their automatic conversion (including accrued interest) upon a
qualified initial public offering or private placement of equity securities into common stock or other equity securities issued in such private placement at a conversion price per share equal to the initial public offering price or per share
purchase price to investors in the private placement. The conversion of the 2013 Notes in the event of a qualified initial public offering or private placement of equity was deemed to be the predominant settlement mechanism. As this predominant
settlement mechanism provided for the settlement of a fixed monetary amount in a variable number of equity instruments, the Company concluded that it was appropriate to recognize the 2013 Notes at fair value. The Company valued the 2013 Notes
utilizing an estimated cost of debt for comparable venture backed and mezzanine financings. The initial fair value of the 2013 Notes was approximately $1.0 million. Upon completion of the Company’s initial public offering in February 2014, the
2013 Notes plus approximately $20,000 of accrued interest automatically converted into 139,644 shares of common stock.
The 2013 Warrants were initially
accounted for as liabilities with subsequent changes in fair value recognized within the consolidated statement of operations. The Company determined that the initial fair value of the 2013 Warrants was $1.0 million. The fair value of the 2013
Warrants was derived from the probability weighted expected return model the Company used to value its common stock. Upon completion of the Company’s initial public offering in February 2014, the warrants no longer require liability accounting
and the then fair value of the warrant liability was reclassified into equity. The warrants also became exercisable for an aggregate of 231,821 shares of common stock at an exercise price of $5.61 per share.
F-17
The initial recognition of the 2013 Notes and 2013 Warrants at fair value resulted in a deemed dividend in the
amount of $0.9 million that was accounted for as additional net loss attributable to common stockholders.
Sublicense Agreement and Amended and
Restated License Agreement with AmpliPhi
Sublicense Agreement
In June 2012, the Company entered into a sublicense agreement (the AmpliPhi Sublicense) with AmpliPhi Biosciences Corporation (AmpliPhi), pursuant to which
AmpliPhi sublicensed to the Company certain rights under a separate agreement which AmpliPhi entered into in 2009 with the Trustees of University of Pennsylvania (UPenn). Under the terms of the AmpliPhi Sublicense, the Company obtained an exclusive,
worldwide sublicense from AmpliPhi under certain UPenn patents related to AAV1 vectors for the development, manufacture, use and sale of companion diagnostics to MYDICAR. In addition, the Company is required to use commercially reasonable efforts to
meet certain developmental, regulatory and commercial milestones with respect to companion diagnostics under the agreement. The Company is currently in compliance with these milestone requirements. In consideration for the sublicense granted to the
Company under the agreement, the Company paid to AmpliPhi a sublicense initiation fee of $310,000, and the Company is obligated to pay to AmpliPhi an annual sublicense maintenance fee of $310,000. The Company is also required to pay to AmpliPhi a
low single-digit percentage royalty based on net sales of any companion diagnostic covered by a licensed patent sold by the Company, its affiliates or its sublicensees. The Company’s royalty obligations continue on a companion
diagnostic-by-companion diagnostic and country-by-country basis until the expiration of the last-to-expire valid claim in a licensed patent covering the applicable companion diagnostic in such country. Finally, the Company is obligated to pay to
AmpliPhi all royalty and milestone payments that become due and payable by AmpliPhi to UPenn under AmpliPhi’s agreement with UPenn as a result of the Company’s exercise of the sublicense granted under the Company’s agreement with
AmpliPhi, including a low single-digit tiered percentage royalty on net sales of any companion diagnostic sold by the Company, its affiliates or its sublicensees, which royalty is separate from and in addition to the royalty payable to AmpliPhi
described above, and up to an aggregate of $850,000 in potential milestone payments per product covered by the licensed patents.
The Company may
unilaterally terminate the agreement upon 30 days’ written notice to AmpliPhi. Absent early termination, the agreement will automatically terminate upon the expiration of the last-to-expire licensed patent, which is expected to be in 2019.
The Company has recorded research and development expense related to sublicense fees under the agreement of $0.3 million, $0.3 million and $0.3 million,
respectively, for the years ended December 31, 2014, 2013 and 2012. Through December 31, 2014, no milestone obligations were incurred under the agreement.
Amended and Restated License Agreement
The Company
entered into an amended and restated license agreement with AmpliPhi concurrently with the AmpliPhi Sublicense that both amended the terms of the license agreement which the Company entered into with AmpliPhi in 2009 and terminated its manufacturing
agreement with AmpliPhi which the Company entered into in 2009. Under the agreement, the Company obtained an exclusive, worldwide license under certain patents and know-how related to AmpliPhi’s AAV vector and manufacturing technology for the
development, manufacture, use and sale of MYDICAR. In addition, the Company has agreed to use commercially reasonable efforts to meet certain diligence milestones with respect to the development and commercialization of at least one product covered
by the UPenn patent rights licensed to AmpliPhi by UPenn under the Company’s agreement with UPenn.
The Company is currently in compliance with these
milestone requirements. During the term of the agreement, the Company is not obligated to make annual license or maintenance payments, but is obligated to pay to AmpliPhi all royalty and milestone payments that become due and payable by AmpliPhi to
UPenn under AmpliPhi’s agreement with UPenn as a result of the Company’s exercise of the sublicense granted under the
F-18
Company’s agreement with AmpliPhi. This includes a low single-digit tiered percentage royalty on net sales of MYDICAR and any other product covered by the licensed patents sold by the
Company, its affiliates or its sublicensees, and up to $850,000 in milestone payments upon the achievement of certain developmental and regulatory milestones related to MYDICAR and any other product covered by the licensed patents. Through
December 31, 2014, no milestone obligations were incurred under the agreement. The agreement does not provide either party with termination rights and does not have a provision for expiration or automatic termination. In addition, the Company
paid $3.2 million in exchange for certain intangible assets associated with the license agreement that the Company acquired from AmpliPhi in June 2012, which were expensed as in-process research and development during the year ended
December 31, 2012.
Exclusive Patent License with the Regents of the University of Minnesota
In May 2009, the Company entered into an exclusive patent license agreement with the Regents of the University of Minnesota (UMinn) under which it obtained an
exclusive license to UMinn’s joint ownership interest in a patent application related to screening technology for isolation of small molecule modulators of SERCA enzymes. The agreement does not encompass a manufacturing agreement.
The Company has agreed to meet certain performance milestones under the agreement, the deadline for which may be extended at the Company’s request
provided that the Company has used commercially reasonable efforts to achieve such milestones by the applicable deadlines. The Company is currently in compliance with these milestone requirements. The Company has the first right to prosecute and
maintain the applicable patent family.
The Company made an upfront payment to UMinn of $120,000. In addition, the Company is obligated to pay to UMinn an
annual license fee of $120,000. The annual license fee will increase to $325,000 if the Company (1) undergoes a change of control, (2) assigns the agreement, any of its rights or obligations under the agreement or as joint ownership
interest in the licensed technology, (3) receives a certain amount in license and sublicense revenues under the agreement, (4) files an investigational new drug application, or IND, new drug application, biologic license application or
orphan drug application (or a foreign equivalent of any such application) for a product covered by the licensed technology, or (5) enters into any agreement with a third party to market or use the licensed technology, subject to certain
exceptions.
The Company may unilaterally terminate the agreement upon 90 days’ written notice to UMinn. UMinn may terminate the agreement upon 10
days’ written notice to the Company upon the Company’s insolvency or for its breach of the agreement if such breach remains uncured for 90 days after the Company receives notice of such breach, or 30 days in the case of a non-payment
breach. Absent early termination, the agreement will automatically terminate upon the expiration of all active claims in any licensed patent or patent application, which is expected to occur no earlier than January 2030.
The Company has recorded research and development expense related to license and annual maintenance fees under the agreement of $0.1 million, $0.1 million,
and $0.1 million, respectively, for the years ended December 31, 2014, 2013 and 2012. Through December 31, 2014, no milestone obligations were incurred under the agreement.
Material Transfer and Exclusivity Agreement
In
February 2014, the Company and Les Laboratoires Servier (Servier) entered into a material transfer and exclusivity agreement, pursuant to which the Company agreed to transfer to Servier samples of certain proprietary compounds from the
Company’s small molecule SERCA2b modulator program and granted to Servier a non-exclusive, non-sublicensable, royalty-free license to conduct certain studies of the samples for the purpose of evaluating Servier’s interest in negotiating a
potential license and research collaboration agreement with the Company relating to small molecule SERCA2b modulators (Compounds), for the treatment of type 2 diabetes and other metabolic diseases.
F-19
Although the evaluation period under this Agreement has expired, the Company is in the process of completing
certain pre-clinical studies of these compounds in coordination with Servier and Servier is continuing to evaluate its potential interest in this program.
Under the terms of the agreement, the Company also granted to Servier the exclusive right to negotiate for an exclusive, royalty-bearing license to develop
and commercialize Compounds, and products containing Compounds, in the field of type 2 diabetes and other metabolic diseases, or the field, solely outside of the United States and its territories and possessions on the terms and conditions set forth
in the agreement and other commercially reasonable terms to be negotiated in good faith by the parties and set forth in a definitive license and research collaboration agreement.
License Agreement with Enterprise
On
July 18, 2014, the Company and Enterprise Partners Management, LLC (Enterprise), an affiliate of Enterprise Partners Venture Capital, entered into an Assignment and License Agreement (the Enterprise License Agreement), pursuant to which
Enterprise granted to the Company an exclusive, worldwide license and the assignment of patents held by Enterprise relating to certain gene therapy applications of the membrane-bound form of the Stem Cell Factor gene (mSCF) for treatment of cardiac
ischemia. The Company has the right to grant sublicenses to third parties under the Enterprise License Agreement. Entities affiliated with Enterprise beneficially owned more than 10% of Celladon’s stock as of the date the Enterprise License
Agreement was executed.
In consideration for the rights granted to the Company under the Enterprise License Agreement, the Company paid an upfront fee to
Enterprise of $160,000. The Company is also obligated to pay to Enterprise a milestone payment in the amount of $1,000,000 upon the grant to the Company, a Company affiliate or a Company sublicensee of the first regulatory approval in the United
States of a product that is covered by the licensed patents. In addition, the Company is required to pay to Enterprise a 2% royalty on net sales of products sold by the Company, Company affiliates and Company sublicensees that are covered by the
licensed patents. The Company’s royalty obligations continue on a product-by-product and country-by-country basis until the expiration of the last-to-expire valid claim in the licensed patents covering a licensed product in such country.
The Company may unilaterally terminate the Enterprise License Agreement upon written notice to Enterprise. Enterprise may terminate the agreement in the event
of the Company’s material breach of the Enterprise License Agreement if such breach remains uncured for 90 days following receipt of written notice of such breach. Absent early termination, the Enterprise License Agreement will automatically
terminate upon the expiration of the last-to-expire of the licensed patents containing a valid claim.
Other License Agreements
The Company has entered into various license agreements pursuant to which the Company acquired certain intellectual property. Pursuant to each agreement the
Company paid a license fee and reimbursed historical patent costs. Additionally, under each agreement, the Company may be required to pay annual maintenance fees, royalties, milestone payments and sublicensing fees. Each of the license agreements is
generally cancelable by the Company, given appropriate prior written notice. Minimum annual payments to maintain these cancelable licenses total an aggregate of approximately $0.2 million and potential future milestone payments total an aggregate of
approximately $3.3 million. The Company has recorded research and development expense related to license and annual maintenance fees under the agreements of $0.2 million for each of the years ended December 31, 2014, 2013 and 2012.
Through December 31, 2014, the Company has recorded research and development expense of $0.1 million related to milestone obligations incurred under the
agreements.
F-20
Leases
The Company leases office space in San Diego, California under long-term operating leases that expire in October 2017 and September 2021. On July 1, 2014,
the Company relocated its San Diego office to another location in San Diego and is subleasing the prior space. The Company also has short-term leases for satellite office space in Seattle, Washington and housing accommodation in San Diego that
expire in 2015. Rent expense was $0.3 million, $0.1 million and $0.1 million for the years ended December 31, 2014, 2013 and 2012, respectively. In March 2015, the Company entered into a short-term lease for approximately 7,000 additional
square feet of office space in Seattle, Washington that expires in June 2016.
The future minimum annual rental commitments under the lease obligations
and sublease rental receipts at December 31, 2014 are as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Lease
Obligations |
|
|
Sublease
Rental
Receipts |
|
|
Total |
|
| Year ending December 31: |
|
|
|
|
|
|
|
|
|
|
|
|
| 2015 |
|
$ |
466 |
|
|
$ |
(75 |
) |
|
$ |
391 |
|
| 2016 |
|
|
487 |
|
|
|
(77 |
) |
|
|
410 |
|
| 2017 |
|
|
485 |
|
|
|
(67 |
) |
|
|
418 |
|
| 2018 |
|
|
414 |
|
|
|
— |
|
|
|
414 |
|
| 2019 |
|
|
426 |
|
|
|
— |
|
|
|
426 |
|
| Thereafter |
|
|
776 |
|
|
|
— |
|
|
|
776 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
$ |
3,054 |
|
|
$ |
(219 |
) |
|
$ |
2,835 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6. Long-Term Obligations
Hercules Loan Agreement
On July 31, 2014, the
Company entered into a Loan and Security Agreement (the Loan Agreement) with Hercules Technology III, L.P. and Hercules Technology Growth Capital, Inc. (as agent and as a lender, and together with Hercules Technology III, L.P., the Lenders) under
which the Company may borrow up to $25.0 million in two tranches (the Loan).
The Company borrowed the first tranche of $10.0 million on August 1,
2014 and paid a facility charge to the Lenders of $150,000 in addition to $37,500 previously paid to the Lenders as a commitment fee. The Company plans to use the proceeds of the Loan to provide additional funding for the development of MYDICAR, for
other development programs in its pipeline and for general corporate purposes. The second tranche of up to $15.0 million can be drawn through June 30, 2015 (amended from May 31, 2015), but only if the Company has provided the Lenders with
notice that data from the Company’s Phase 2b clinical trial for MYDICAR supports the continued development of MYDICAR for its Breakthrough Therapy designation to either a Phase 3 clinical trial or for registration for approval, as reasonably
determined by the Company’s senior management and board of directors (the Milestone). Upon funding of the second tranche of the Loan, the Company will be required to pay a facility charge to the Lenders of $100,000.
The interest rate for each tranche will be calculated at a rate equal to the greater of either (i) 8.25% plus the prime rate as reported from time to
time in The Wall Street Journal minus 5.25%, and (ii) 8.25%. Payments under the Loan Agreement are interest only until August 1, 2015 (which will be extended until February 1, 2016 if the Company achieves the Milestone on or before
June 30, 2015) (the Amortization Date) followed by equal monthly payments of principal and interest through the scheduled maturity date on February 1, 2018 (the Loan Maturity Date). In addition, a final payment equal to $1,750,000 will be
due at such time as the Loan is prepaid or becomes due and payable as specified in the Loan Agreement. The Company’s obligations under the Loan Agreement are secured by a security interest in substantially all of its assets, excluding its
intellectual property but including the proceeds from the sale, licensing or disposition of its intellectual property. The Company’s intellectual property is also subject to customary negative covenants.
F-21
If the Company prepays the loan prior to maturity, it will pay the Lenders a prepayment charge, based on a
percentage of the then outstanding principal balance, equal to 1.5% if the prepayment occurs prior to the Amortization Date.
Subject to certain
conditions and limitations set forth in the Loan Agreement, including ownership limitations of the Lenders, the Company has the right to convert up to $3.0 million of scheduled principal installments of the Loan into shares of the Company’s
common stock, provided such shares must be freely tradable. The number of shares of common stock that would be issued upon conversion would be equal to the number determined by dividing (x) the principal amount to be paid in shares of common
stock by (y) $16.33.
The Loan Agreement includes customary representations, warranties and covenants (affirmative and negative) of the Company,
including restrictive covenants that limit the Company’s ability to: incur additional indebtedness; encumber the collateral securing the Loan; acquire, own or make investments; repurchase or redeem stock or other equity securities; declare or
pay any cash dividend or make a cash distribution on any class of stock or other equity interest; transfer a material portion of the Company’s assets; acquire other businesses; and merge or consolidate with or into any other business
organization. The Loan Agreement does not however include any financial maintenance covenants. The Loan Agreement also includes standard events of default, including payment defaults, breaches of covenants following any applicable cure period, a
material impairment in the perfection or priority of the Lenders’ security interest or in the value of the collateral, and events relating to bankruptcy or insolvency. Upon the occurrence of an event of default, a default interest rate of an
additional 5% may be applied to the outstanding Loan, and the Lenders may declare all outstanding obligations immediately due and payable and take such other actions as are set forth in the Loan Agreement.
Capital Lease
In 2014 the Company entered into a
capital lease arrangement for office equipment in the Company’s San Diego, California office.
Contractual Payments and Carrying-Value
Reconciliation
As of December 31, 2014, future contractual principal and final fee payments on the Company’s debt and capital lease
obligations are as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| Year ending December 31: |
|
Total |
|
|
Term
Loan |
|
|
Capital
Lease |
|
| 2015 |
|
$ |
1 |
|
|
$ |
— |
|
|
$ |
1 |
|
| 2016 |
|
|
3,432 |
|
|
|
3,430 |
|
|
|
2 |
|
| 2017 |
|
|
4,052 |
|
|
|
4,049 |
|
|
|
3 |
|
| 2018 |
|
|
4,275 |
|
|
|
4,271 |
|
|
|
4 |
|
| 2019 |
|
|
2 |
|
|
|
— |
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
$ |
11,762 |
|
|
$ |
11,750 |
|
|
$ |
12 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table provides a reconciliation of the Company’s future contractual principal and final fee payments on its
debt and capital lease obligations to the reported carrying value as of December 31, 2014 (in thousands):
|
|
|
|
|
| Total loan debt and capital lease obligations |
|
$ |
11,762 |
|
| Less: Debt discount |
|
|
(1,659 |
) |
|
|
|
|
|
| Total carrying value: |
|
|
10,103 |
|
|
|
|
|
|
| Less: Carrying value of current portion of long-term obligations |
|
|
(1 |
) |
|
|
|
|
|
| Carrying value of long-term obligations, less current portion |
|
|
10,102 |
|
|
|
|
|
|
F-22
Interest expense for the years ended December 31, 2014, 2013 and 2012 was $0.7 million, $0.1 million and
$0.1 million, respectively. Interest expense in 2014 related mainly to the Hercules Loan Agreement and interest expense in prior years related mainly to convertible debt outstanding in those periods.
7. Preferred Stock and Stockholders’ Equity (Deficit)
Preferred Stock
In addition to its redeemable
convertible preferred stock, the Company’s convertible preferred stock has been classified as temporary equity at December 31, 2013 on the accompanying consolidated balance sheets instead of in stockholders’ deficit in accordance with
authoritative guidance for the classification and measurement of potentially redeemable securities whose redemption is based upon certain change in control events that are outside of the control of the Company, including liquidation, sale or
transfer of control of the Company. On February 4, 2014, in connection with the Company’s initial public offering, all outstanding shares of convertible preferred stock were converted into 11,151,192 shares of the Company’s common
stock.
The authorized, issued and outstanding shares of preferred stock by series are as follows (in thousands, except share amounts):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Shares
Authorized |
|
|
Shares
Outstanding |
|
|
Liquidation
Preference |
|
|
Redemption
Amount |
|
| As of December 31, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock |
|
|
10,000,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As of December 31, 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Redeemable convertible preferred stock: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Series A-1 |
|
|
135,826,497 |
|
|
|
127,140,530 |
|
|
$ |
114,172 |
|
|
$ |
57,086 |
|
| Convertible preferred stock: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Junior preferred stock |
|
|
12,138,080 |
|
|
|
12,138,080 |
|
|
|
5,450 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
147,964,577 |
|
|
|
139,278,610 |
|
|
$ |
119,622 |
|
|
$ |
57,086 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock and Common Stock Warrants
In February 2014, the Company completed its initial public offering in which it sold 6,325,000 shares of common stock at a public offering price of $8.00 per
share.
The proceeds received and costs incurred in connection with the Company’s initial public offering, shown in the period received or paid, were
as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Year ended December 31, |
|
| |
|
Total |
|
|
2014 |
|
|
2013 |
|
| Gross proceeds (including over-allotment) |
|
$ |
50,600 |
|
|
$ |
50,600 |
|
|
$ |
— |
|
| Underwriting discounts and commissions |
|
|
(3,542 |
) |
|
|
(3,542 |
) |
|
|
— |
|
| Offering costs |
|
|
(2,800 |
) |
|
|
(1,107 |
) |
|
|
(1,693 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net proceeds |
|
$ |
44,258 |
|
|
$ |
45,951 |
|
|
$ |
(1,693 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition, each of the following occurred on February 4, 2014 in connection with the Company’s initial public
offering:
| |
• |
|
Series A-1 redeemable convertible preferred stock outstanding (127,140,530 shares) and Junior preferred convertible stock outstanding (12,138,080 shares) were converted into 10,179,372 and 971,820 shares of the
Company’s common stock, respectively; |
F-23
| |
• |
|
the outstanding principal balance of $1,097,017 and accrued interest of $20,000 on convertible promissory notes converted into 139,644 shares of the Company’s common stock; and |
| |
• |
|
warrants to purchase 2,895,570 shares of Series A-1 preferred stock were converted into warrants to purchase 231,821 shares of the Company’s common stock and the warrant liability was reclassified to additional
paid-in capital. |
In August 2014, the Company completed an underwritten public offering in which it sold 4,600,000 shares of common stock at
a public offering price of $9.50 per share. The proceeds received and costs incurred in connection with this offering, shown in the period received or paid, were as follows (in thousands):
|
|
|
|
|
| |
|
2014 |
|
| Gross proceeds (including option to purchase additional shares) |
|
$ |
43,700 |
|
| Underwriting discounts and commissions |
|
|
(2,622 |
) |
| Offering costs |
|
|
(389 |
) |
|
|
|
|
|
| Net proceeds |
|
$ |
40,689 |
|
|
|
|
|
|
The following table summarizes the fully exercisable warrants outstanding for the purchase of common stock as of
December 31, 2014 and 2013:
|
|
|
|
|
|
|
|
|
|
|
|
|
| December 31, |
|
|
Exercise
Price |
|
|
Expiration
Date |
| 2014 |
|
|
2013 |
|
|
|
| |
— |
|
|
|
80 |
|
|
$ |
224.82 |
|
|
January 2015 |
| |
— |
|
|
|
622 |
|
|
$ |
12.49 |
|
|
October 2016 |
| |
206,340 |
|
|
|
— |
|
|
$ |
5.61 |
|
|
October 2018 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
206,340 |
|
|
|
702 |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Stock Options
Options granted under the Company’s equity incentive plans generally expire no more than 10 years from the date of grant and generally vest and become
exercisable over a period not to exceed four years, as determined by the Company’s board of directors. Recipients of stock options are eligible to purchase shares of the Company’s common stock at an exercise price equal to no less than the
estimated fair market value of such stock on the date of grant.
Prior Plans
In December 2001, the Company adopted its 2001 Stock Option Plan (the 2001 Plan) and in January 2012 adopted its 2012 Equity Incentive Plan (the 2012 Plan, and
together with the 2001 Plan, the Prior Plans). The Prior Plans have terminated and no further shares may be granted under the Prior Plans.
2013
Equity Incentive Plan
In October 2013, the Company’s stockholders approved the 2013 Equity Incentive Plan, as amended (2013 Plan), which
became effective in February 2014. Under the 2013 Plan, the Company may grant stock options, stock appreciation rights, restricted stock, restricted stock units, performance-based stock awards and other awards to individuals who are then employees,
officers, non-employee directors or consultants of the Company and its affiliates. Additionally, the 2013 Plan provides for the grant of performance cash awards. Initially, the aggregate number of shares of common stock that may be issued pursuant
to stock awards under the 2013 Plan is the sum of (1) 1,473,738 shares, plus (2) the number of shares (not to exceed 1,569,905 shares) (i) the 26,294 shares reserved for issuance under the 2012 Plan at the time the 2013 Plan became
effective, and (ii) any shares subject
F-24
to outstanding stock options or other stock awards that were granted under the 2012 Plan or 2001 Plan that are forfeited, terminate, expire or are otherwise not issued. Additionally, the number
of shares of common stock reserved for issuance under the 2013 Plan will automatically increase on January 1 of each year, beginning on January 1, 2015 and continuing through and including January 1, 2023, by 5% of the total number of
shares of the Company’s capital stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares determined by the Company’s board of directors.
A summary of the Company’s stock option activity under the Prior Plans and 2013 Plan is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Options |
|
|
Weighted-
Average
Exercise
Price Per
Share |
|
|
Weighted-
Average
Remaining
Contractual
Term |
|
|
Aggregate
Intrinsic
Value
(in 000’s) |
|
| Outstanding at December 31, 2013 |
|
|
1,543,667 |
|
|
$ |
3.19 |
|
|
|
8.66 |
|
|
$ |
9,128 |
|
| Granted |
|
|
1,240,673 |
|
|
|
10.61 |
|
|
|
|
|
|
|
|
|
| Exercised |
|
|
(340,220 |
) |
|
|
1.20 |
|
|
|
|
|
|
|
|
|
| Canceled |
|
|
(35,486 |
) |
|
|
10.93 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding at December 31, 2014 |
|
|
2,408,634 |
|
|
$ |
7.18 |
|
|
|
8.53 |
|
|
$ |
31,031 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Options exercisable at December 31, 2014 |
|
|
1,263,695 |
|
|
$ |
4.02 |
|
|
|
7.78 |
|
|
$ |
20,877 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Options exercisable, vested and expected to vest at December 31, 2014 |
|
|
2,408,634 |
|
|
$ |
7.18 |
|
|
|
8.53 |
|
|
$ |
31,031 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The weighted-average grant date fair value of employee options granted during the years ended December 31, 2014, 2013 and
2012 was $7.36, $7.27 and $0.75 per share, respectively. The aggregate intrinsic value of options exercised during the year ended December 31, 2014 was approximately $3.9 million. There were no options exercised in the years prior to the
Company’s initial public offering in 2014.
2013 Employee Stock Purchase Plan
In October 2013, the Company’s stockholders approved the 2013 Equity Stock Purchase Plan (ESPP) which became effective in February 2014. Initially, the
ESPP authorizes the issuance of 165,732 shares of common stock pursuant to purchase rights granted to the Company’s employees or to employees of any of the Company’s designated affiliates. The number of shares of common stock reserved for
issuance will automatically increase on January 1 of each calendar year, from January 1, 2015 through January 1, 2023 by the least of (1) 1% of the total number of shares of the Company’s common stock outstanding on
December 31 of the preceding calendar year, (2) 384,307 shares, or (3) a number determined by the Company’s board of directors that is less than (1) and (2). During the year ended December 31, 2014, the Company recorded
stock-based compensation expense of approximately $0.1 million related to the ESPP.
Stock-Based Compensation Expense
The weighted-average assumptions used in the Black-Scholes option pricing model to determine the fair value of the employee stock option grants were as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of December 31, |
|
| |
|
2014 |
|
|
2013 |
|
|
2012 |
|
| Risk-free interest rate |
|
|
1.90 |
% |
|
|
1.62 |
% |
|
|
2.29 |
% |
| Expected volatility |
|
|
80 |
% |
|
|
79 |
% |
|
|
84 |
% |
| Expected term (in years) |
|
|
6.0 |
|
|
|
5.6 |
|
|
|
5.9 |
|
| Expected dividend yield |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
0.0 |
% |
F-25
Risk-free interest rate
The Company bases the risk-free interest rate assumption on observed interest rates appropriate for the expected term of the stock option grants.
Expected volatility
The expected volatility assumption
is based on volatilities of a peer group of similar companies whose share prices are publicly available. The peer group was developed based on companies in the biotechnology industry.
Expected term
The expected term represents the period of
time that options are expected to be outstanding. Because the Company does not have historical exercise behavior, it determines the expected life assumption using the simplified method, which is an average of the contractual term of the option and
its vesting period.
Expected dividend yield
The
Company bases the expected dividend yield assumption on the fact that it has never paid cash dividends and has no present intention to pay cash dividends.
The allocation of stock-based compensation for all equity awards is as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
As of December 31, |
|
| |
|
2014 |
|
|
2013 |
|
|
2012 |
|
| Research and development |
|
$ |
1,712 |
|
|
$ |
1,264 |
|
|
$ |
222 |
|
| General and administrative |
|
|
1,607 |
|
|
|
124 |
|
|
|
76 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
3,319 |
|
|
$ |
1,388 |
|
|
$ |
298 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2014 the unrecognized compensation cost related to outstanding employee options was $8.9 million and
is expected to be recognized as expense over approximately 3.0 years.
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance as of December 31, 2014 and 2013 is as follows:
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Granted and outstanding under the Plans |
|
|
2,408,634 |
|
|
|
1,543,667 |
|
| Available for grant under the 2012 Plan |
|
|
294,845 |
|
|
|
26,294 |
|
| Available for issuance under Employee Stock Purchase Plan |
|
|
140,711 |
|
|
|
— |
|
| Common stock warrants issued and outstanding |
|
|
206,340 |
|
|
|
702 |
|
| Convertible preferred stock warrants issued and outstanding |
|
|
— |
|
|
|
231,821 |
|
| Convertible preferred stock |
|
|
— |
|
|
|
11,151,192 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3,050,530 |
|
|
|
12,953,676 |
|
|
|
|
|
|
|
|
|
|
F-26
8. Income Taxes
The following is a reconciliation of the expected statutory federal income tax provision to the actual income tax provision (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
|
2012 |
|
| Tax computed at federal statutory rate |
|
$ |
(11,510 |
) |
|
$ |
(6,831 |
) |
|
$ |
(5,396 |
) |
| State income tax, net of federal benefit |
|
|
(1,517 |
) |
|
|
(987 |
) |
|
|
(907 |
) |
| Non-deductible interest |
|
|
20 |
|
|
|
20 |
|
|
|
36 |
|
| Other permanent items |
|
|
1,676 |
|
|
|
756 |
|
|
|
214 |
|
| Research credits |
|
|
(557 |
) |
|
|
(728 |
) |
|
|
(93 |
) |
| Remove (restore) DTA for NOL and Credits—IRC 382 |
|
|
— |
|
|
|
(12,666 |
) |
|
|
2,135 |
|
| State Taxes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Uncertain tax position |
|
|
(1,125 |
) |
|
|
859 |
|
|
|
— |
|
| Valuation allowance |
|
|
13,013 |
|
|
|
19,577 |
|
|
|
4,011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Provision (benefit) for income taxes |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The components of the Company’s deferred tax assets are summarized as follows (in thousands):
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Deferred tax assets: |
|
|
|
|
|
|
|
|
| Net operating loss carryforwards |
|
$ |
31,484 |
|
|
$ |
19,288 |
|
| Research credits |
|
|
1,649 |
|
|
|
1,092 |
|
| Capitalized R&D |
|
|
5,410 |
|
|
|
6,391 |
|
| Other |
|
|
2,049 |
|
|
|
803 |
|
|
|
|
|
|
|
|
|
|
| Deferred tax assets |
|
|
40,592 |
|
|
|
27,574 |
|
| Valuation allowance |
|
|
(40,592 |
) |
|
|
(27,574 |
) |
|
|
|
|
|
|
|
|
|
| Net deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
The Company has established a valuation allowance for all deferred tax assets (DTA) including those for new operating loss and
tax credit carryforwards. A valuation allowance of approximately $40.6 million of which approximately $13.0 million relates to 2014, has been recognized to offset the deferred tax assets, as realization of such assets is uncertain.
At December 31, 2014, the Company had federal and California net operating loss (NOL) carryforwards of approximately $82.2 million and $77.0 million,
respectively. The federal NOL carryforwards will begin to expire in 2027 unless previously utilized, and the state NOL carryforwards have already begun to expire, and will continue to do so, unless utilized. At December 31, 2014, the Company
had federal and state research tax credits each of $1.3 million. The federal research tax credits begin to expire in 2032 unless previously utilized. The California research credit will carry forward indefinitely until utilized.
Utilization of the NOL and research tax credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that have
occurred or that could occur in the future, as required by Section 382 of the Code, as well as similar state and foreign provisions. These ownership changes may limit the amount of NOL and research tax credit carryforwards that can be utilized
annually to offset future taxable income and tax, respectively. In general, an “ownership change” as defined by Section 382 of the Code results from a transaction or series of transactions over a three-year period resulting in an
ownership change of more than 50 percentage points of the outstanding stock of a company by certain stockholders or public groups.
F-27
The Company completed a study to assess whether an ownership change, as defined by Section 382 of the Code,
had occurred from the Company’s formation through December 31, 2014. Based upon this study, the Company determined that several ownership changes had occurred. Accordingly, the Company has reduced its deferred tax assets related to the
federal and state NOL carryforwards and the federal research tax credit carryforwards that are anticipated to expire unused as a result of these ownership changes. These tax attributes have been excluded from the deferred tax assets with a
corresponding reduction in the valuation allowance with no net effect on income tax expense or the effective tax rate. Future ownership changes may further limit the Company’s ability to utilize its remaining tax attributes.
The Company adopted the provisions of Financial Accounting Standards Board (FASB) ASC 740-10 Income Taxes, relating to accounting for uncertain tax
positions on July 1, 2009.
The following table summarized the activity related to the Company’s unrecognized tax benefits (in thousands):
|
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Balance beginning of the year |
|
$ |
1,709 |
|
|
$ |
— |
|
| Increase related to prior year tax positions |
|
|
(1,361 |
) |
|
|
681 |
|
| Increase related to current year tax positions |
|
|
314 |
|
|
|
1,028 |
|
|
|
|
|
|
|
|
|
|
| Balance at end of year |
|
$ |
662 |
|
|
$ |
1,709 |
|
|
|
|
|
|
|
|
|
|
There were no unrecognized tax benefits prior to 2013. Approximately $0.7 million of the unrecognized tax benefits would
reduce the Company’s annual effective tax rate, if recognized, subject to the valuation allowance. It is not anticipated that there will be significant change in the unrecognized tax benefits over the next 12 months.
Due to the net operating loss carryforwards, the U.S. federal and state returns are open to examination by the Internal Revenue Service and significant state
and foreign jurisdictions for all years beginning with the inception of the Company. The Company’s policy is to recognize interest expense and penalties related to income tax matters as a component of income tax expense. There was no interest
and penalties associated with uncertain tax positions as of December 31, 2014.
9. Employee Benefits
All employees of the Company are eligible to participate in the 401(k) Plan. The 401(k) matching contributions, if any, are determined by the Company at its
sole discretion. During the years ended December 31, 2014, 2013 and 2012, the Company made matching contributions totaling $0.3 million, $0.1 million, $0.1 million, respectively.
10. Subsequent Events
Novasep Agreement
On March 20, 2015, the Company entered into a Development, Manufacturing and Supply Agreement (the “Manufacturing Agreement”) with
Novasep, Inc. (“Novasep”) which superseded the Letter Agreement dated December 19, 2014 by and between the Company and Novasep. Under the terms of the Manufacturing Agreement, the parties agreed to continue the work initiated under
the Letter Agreement, including the work necessary to prepare for the potential manufacture of MYDICAR drug substance (AAV1/SERCA2a) at the facilities of Novasep’s affiliate Henogen in Europe (the “Novasep Facility”). Pursuant to the
Manufacturing Agreement (and as previously agreed in the Letter Agreement), in exchange for payments from the Company to Novasep totaling up to €4,750,000, Novasep agreed to (i) conduct the engineering design work for facility
modifications that would be necessary for the manufacture of MYDICAR drug substance, (ii) undertake initial process and analytical transfer and initial scale-up work in support of such potential future commercial
F-28
manufacturing of MYDICAR drug substance, and (iii) allocate the resources and capacity necessary for the foregoing activities. The parties have also agreed to proceed with the additional
process transfer, engineering/construction, scale-up and development activities necessary for future production of MYDICAR drug substance in accordance with current Good Manufacturing Practices (“GMP”), and agreed to terms of a commercial
supply arrangement with a term through at least December 31, 2018, with extension options through 2020 in favor of the Company. The Company has the right to terminate the Manufacturing Agreement, exercisable for a specified period of time
following the un-blinding of the data from the Company’s Phase 2b clinical trial of MYDICAR (CUPID 2), if the Company concludes in good faith that the CUPID 2 data is such that the Company does not require production of MYDICAR drug substance
at the Novasep Facility. The Company expects to un-blind the data from the CUPID 2 trial in late April 2015.
Unless the Company exercises the post CUPID
2 data termination right described above, the Company will be obligated to (i) fund Novasep’s modifications to the Novasep Facility through time- and event-triggered milestone payments, (ii) make additional payments for the
development services to be performed by Novasep, and (iii) commit to purchase a specified number of batches of MYDICAR drug substance (or make minimum payments with respect to any such batches that are not purchased) through 2018 (if the
Company elects that the Novasep Facility be operated as a multi-product facility) or through 2019 (if the Company elects to have the Novasep Facility dedicated to MYDICAR drug substance production during the term of the Manufacturing Agreement).
In addition to the above-described post CUPID 2 data termination right, the Company has the right to terminate the Manufacturing Agreement (i) at
will on or before March 31, 2016, (ii) following the shut-down or non-production of the Novasep Facility for a specified period of time, or (iii) upon Novasep’s debarment. Additionally, each party may terminate the Manufacturing
Agreement upon uncured material breach thereof by the other party, upon the other party’s insolvency or bankruptcy, or in the event of a continuing force majeure preventing performance. Upon any termination of the Manufacturing Agreement by
Celladon following the expiration of the post CUPID 2 data termination right either for convenience or for any reason other than material breach of the Manufacturing Agreement, shut-down or non-production of the Novasep Facility for a period
extending longer than six months, or Novasep’s insolvency, the Company is obligated to pay previously-unreimbursed amounts incurred by Novasep and specified termination fees as set forth in the Manufacturing Agreement.
11. Selected Quarterly Financial Data (Unaudited)
The
following financial information reflects all normal recurring adjustments, which are, in the opinion of management, necessary for a fair statement of the results of the interim periods. Summarized quarterly data for fiscal 2014 and 2013 are as
follows (in thousands, except per share data):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
First
Quarter |
|
|
Second
Quarter |
|
|
Third
Quarter |
|
|
Fourth
Quarter |
|
| 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
$ |
6,924 |
|
|
$ |
7,005 |
|
|
$ |
8,131 |
|
|
$ |
10,958 |
|
| Consolidated Net loss |
|
|
(7,162 |
) |
|
|
(6,992 |
) |
|
|
(8,358 |
) |
|
|
(11,341 |
) |
| Basic and diluted net loss per share |
|
$ |
(0.60 |
) |
|
$ |
(0.38 |
) |
|
$ |
(0.40 |
) |
|
$ |
(0.49 |
) |
| 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
$ |
3,472 |
|
|
$ |
4,992 |
|
|
$ |
5,523 |
|
|
$ |
5,977 |
|
| Consolidated Net loss |
|
|
(3,530 |
) |
|
|
(4,929 |
) |
|
|
(5,453 |
) |
|
|
(6,179 |
) |
| Basic and diluted net loss per share |
|
$ |
(3.99 |
) |
|
$ |
(8.98 |
) |
|
$ |
(6.17 |
) |
|
$ |
(7.96 |
) |
F-29
Celladon Corporation
Consolidated Balance Sheets
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
| |
|
September 30,
2015 |
|
|
December 31,
2014 |
|
| |
|
(unaudited) |
|
|
|
|
| Assets |
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
37,092 |
|
|
$ |
14,435 |
|
| Short-term investments |
|
|
— |
|
|
|
70,513 |
|
| Prepaid expenses and other assets |
|
|
1,001 |
|
|
|
3,135 |
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
38,093 |
|
|
|
88,083 |
|
| Property and equipment, net |
|
|
133 |
|
|
|
763 |
|
| Other assets |
|
|
10 |
|
|
|
264 |
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
38,236 |
|
|
$ |
89,110 |
|
|
|
|
|
|
|
|
|
|
| Liabilities and stockholders’ equity |
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable and accrued expenses |
|
$ |
1,025 |
|
|
$ |
5,803 |
|
| Accrued restructuring charges |
|
|
609 |
|
|
|
— |
|
| Accrued clinical expenses |
|
|
163 |
|
|
|
731 |
|
| Accrued interest |
|
|
— |
|
|
|
71 |
|
| Current portion of long-term obligations |
|
|
— |
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
1,797 |
|
|
|
6,606 |
|
| Long-term obligations, net of discount |
|
|
— |
|
|
|
10,102 |
|
| Non-current liabilities |
|
|
29 |
|
|
|
298 |
|
| Commitments and contingencies (Note 5) |
|
|
|
|
|
|
|
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
|
| Preferred stock, $0.001 par value; authorized shares—10,000,000 at September 30, 2015 and December 31, 2014,
respectively; no shares issued and outstanding |
|
|
— |
|
|
|
— |
|
| Common stock, $0.001 par value; authorized shares—200,000,000 at September 30, 2015 and December 31, 2014, respectively;
issued and outstanding—23,916,021 and 23,490,737 at September 30, 2015 and December 31, 2014, respectively |
|
|
24 |
|
|
|
23 |
|
| Additional paid-in capital |
|
|
222,492 |
|
|
|
218,528 |
|
| Accumulated other comprehensive loss |
|
|
— |
|
|
|
(8 |
) |
| Accumulated deficit |
|
|
(186,106 |
) |
|
|
(146,439 |
) |
|
|
|
|
|
|
|
|
|
| Total stockholders’ equity |
|
|
36,410 |
|
|
|
72,104 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities and stockholders’ equity |
|
$ |
38,236 |
|
|
$ |
89,110 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-30
Celladon Corporation
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended
September 30, |
|
|
Nine Months Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
|
2015 |
|
|
2014 |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
738 |
|
|
$ |
5,316 |
|
|
$ |
21,757 |
|
|
$ |
15,515 |
|
| General and administrative |
|
|
2,533 |
|
|
|
2,815 |
|
|
|
10,805 |
|
|
|
6,545 |
|
| Restructuring charges |
|
|
1,781 |
|
|
|
— |
|
|
|
4,862 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
5,052 |
|
|
|
8,131 |
|
|
|
37,424 |
|
|
|
22,060 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(5,052 |
) |
|
|
(8,131 |
) |
|
|
(37,424 |
) |
|
|
(22,060 |
) |
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest income |
|
|
6 |
|
|
|
36 |
|
|
|
60 |
|
|
|
65 |
|
| Interest expense |
|
|
(1,347 |
) |
|
|
(271 |
) |
|
|
(2,302 |
) |
|
|
(330 |
) |
| Other income (expense) |
|
|
(3 |
) |
|
|
8 |
|
|
|
(1 |
) |
|
|
(4 |
) |
| Change in fair value of warrant liability |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(183 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Consolidated net loss |
|
$ |
(6,396 |
) |
|
$ |
(8,358 |
) |
|
$ |
(39,667 |
) |
|
$ |
(22,512 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Unrealized gain on investments |
|
|
— |
|
|
|
(26 |
) |
|
|
8 |
|
|
|
(10 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Comprehensive loss |
|
$ |
(6,396 |
) |
|
$ |
(8,384 |
) |
|
$ |
(39,659 |
) |
|
$ |
(22,522 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(0.27 |
) |
|
$ |
(0.40 |
) |
|
$ |
(1.66 |
) |
|
$ |
(1.32 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted-average shares outstanding, basic and diluted |
|
|
23,915,361 |
|
|
|
20,752,895 |
|
|
|
23,830,668 |
|
|
|
16,999,766 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-31
Celladon Corporation
Consolidated Statements of Cash Flows
(in thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
| Cash flows from operating activities |
|
|
|
|
|
|
|
|
| Consolidated net loss |
|
$ |
(39,667 |
) |
|
$ |
(22,512 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities |
|
|
|
|
|
|
|
|
| Depreciation |
|
|
371 |
|
|
|
110 |
|
| Asset impairments |
|
|
192 |
|
|
|
— |
|
| Stock-based compensation |
|
|
3,183 |
|
|
|
2,178 |
|
| Noncash interest expense |
|
|
1,807 |
|
|
|
190 |
|
| Amortization of investment premium |
|
|
194 |
|
|
|
163 |
|
| Change in fair value of warrant liability |
|
|
— |
|
|
|
183 |
|
| Loss on disposal of property and equipment |
|
|
2 |
|
|
|
1 |
|
| Deferred rent |
|
|
(117 |
) |
|
|
84 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Prepaid expenses and other assets |
|
|
2,239 |
|
|
|
(689 |
) |
| Accounts payable and accrued expenses |
|
|
(4,937 |
) |
|
|
910 |
|
| Other liabilities |
|
|
— |
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
| Net cash used in operating activities |
|
|
(36,733 |
) |
|
|
(19,374 |
) |
| Cash flows from investing activities |
|
|
|
|
|
|
|
|
| Purchases of investment securities |
|
|
— |
|
|
|
(88,661 |
) |
| Proceeds from maturities of investment securities |
|
|
70,327 |
|
|
|
18,200 |
|
| Purchases of property and equipment |
|
|
(235 |
) |
|
|
(549 |
) |
| Proceeds from sale of property and equipment |
|
|
277 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Net cash provided by (used in) investing activities |
|
|
70,369 |
|
|
|
(71,010 |
) |
| Cash flows from financing activities |
|
|
|
|
|
|
|
|
| Proceeds from issuance of common stock |
|
|
783 |
|
|
|
94,578 |
|
| Costs paid in connection with common stock offering |
|
|
— |
|
|
|
(7,396 |
) |
| Proceeds from borrowing under term loan |
|
|
— |
|
|
|
10,000 |
|
| Repayment of term loan |
|
|
(10,000 |
) |
|
|
— |
|
| Costs paid in connection with term loan |
|
|
(1,750 |
) |
|
|
(334 |
) |
| Other |
|
|
(12 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Net cash provided by (used in) financing activities |
|
|
(10,979 |
) |
|
|
96,848 |
|
|
|
|
|
|
|
|
|
|
| Net increase in cash and cash equivalents |
|
|
22,657 |
|
|
|
6,464 |
|
| Cash and cash equivalents, beginning of period |
|
|
14,435 |
|
|
|
7,903 |
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents, end of period |
|
$ |
37,092 |
|
|
$ |
14,367 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-32
Celladon Corporation
Notes to Consolidated Financial Statements
(Unaudited)
1. Basis of Presentation,
Organization and Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited consolidated financial statements of Celladon Corporation (Celladon or the Company) should be read in conjunction with the audited
financial statements and notes thereto as of and for the year ended December 31, 2014 included in the Company’s Annual Report on Form 10-K (Annual Report) filed with the Securities and Exchange Commission (SEC). The accompanying financial
statements have been prepared in accordance with U.S. generally accepted accounting principles (GAAP) for interim financial information and in accordance with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, since they
are interim statements, the accompanying financial statements do not include all of the information and notes required by GAAP for complete financial statements. In the opinion of management, the accompanying financial statements reflect all
adjustments (consisting of normal recurring adjustments) that are necessary for a fair statement of the financial position, results of operations and cash flows for the interim periods presented. Interim results are not necessarily indicative of
results for a full year. The preparation of the Company’s consolidated financial statements requires it to make estimates and assumptions that impact the reported amounts of assets, liabilities and expenses and the disclosure of contingent
assets and liabilities in the Company’s consolidated financial statements and accompanying notes. The most significant estimates in the Company’s consolidated financial statements relate to the fair value of equity awards and clinical
trial expense accruals. Although these estimates are based on the Company’s knowledge of current events and actions it may undertake in the future, actual results may ultimately materially differ from these estimates and assumptions.
Organization
Celladon was incorporated in
California on December 21, 2000 (inception) and reincorporated in Delaware in April 2012. The Company is a biotechnology company that has been focused on the development of cardiovascular gene therapy. As a consequence of the negative results
from the Phase 2b clinical trial of its lead product candidate, MYDICAR (AAV1/SERCA2a), the Company is evaluating its strategic opportunities to maximize shareholder value, including the possibility of seeking a merger, sale of the Company or all or
some of its assets, and/or a liquidation. At this time, the Company’s current development activities are limited to the oversight of the long-term follow up period in the CUPID 2 trial, which is expected to continue through February 2016.
As of September 30, 2015, the Company has devoted substantially all of its efforts to product development, raising capital and building infrastructure
and has not generated revenues from product sales or other sources.
Principles of Consolidation
The financial statements of the Company’s former subsidiary Celladon Europe B.V. (Celladon Europe) were consolidated with those of the Company through
Celladon Europe’s dissolution on December 30, 2014. All intercompany transactions and balances were eliminated in consolidation.
Investment Securities
Investment securities
primarily consist of investment grade corporate debt securities. The Company classifies all investment securities as available-for-sale. Investments with maturity dates greater than 12 months from the end of each reporting period are classified as
long-term. Investment securities are carried at fair value, with the unrealized gains and losses reported as a component of other comprehensive income (loss) in stockholders’
F-33
equity (deficit) until realized. Realized gains and losses from the sale of investment securities, if any, are determined on a specific identification basis. A decline in the market value of any
investment security below cost that is determined to be other than temporary will result in an impairment charge to earnings and a new cost basis for the security is established. No such impairment charges were recorded for any period presented. As
of December 31, 2014, none of the investment securities had been in an unrealized loss position for more than 12 months. Premiums and discounts are amortized or accreted over the life of the related security as an adjustment to yield using the
straight-line method and are included in interest income. Interest income is recognized when earned. During the third quarter of 2015 the Company’s investment securities matured and the Company held the proceeds as cash equivalents. As of
September 30, 2015, the Company had no investment securities.
The following table sets forth the composition of the Company’s investment
securities (in thousands) as of December 31, 2014:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Unrealized |
|
|
|
|
| As of December 31, 2014 |
|
Maturity in Years |
|
|
Amortized
Cost |
|
|
Gains |
|
|
Losses |
|
|
Fair
Value |
|
| Corporate debt securities |
|
|
Less than 1 year |
|
|
$ |
70,521 |
|
|
$ |
— |
|
|
$ |
(8 |
) |
|
$ |
70,513 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss Per Share Attributable to Common Stockholders
Basic and diluted net loss per common share is calculated by dividing net loss applicable to common stockholders by the weighted-average number of common
shares outstanding during the period, without consideration for common stock equivalents. Potentially dilutive shares, which include convertible preferred stock and rights to acquire convertible preferred stock (non-controlling interest), warrants
for the purchase of common stock and options outstanding under the Company’s equity incentive plans, are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is
dilutive.
Potentially dilutive securities not included in the calculation of diluted net loss per share attributable to common stockholders because to do
so would be anti-dilutive are as follows (in common stock equivalent shares):
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
| Warrants for common stock |
|
|
152,735 |
|
|
|
206,340 |
|
| Common stock options and restricted stock units |
|
|
1,527,098 |
|
|
|
2,527,067 |
|
|
|
|
|
|
|
|
|
|
|
|
|
1,679,833 |
|
|
|
2,733,407 |
|
|
|
|
|
|
|
|
|
|
Recent Accounting Pronouncements
In April 2015, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) No. 2015-03,
Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs. The amendments in this ASU require that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a
direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The amendments in this ASU are effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods
within those fiscal years. Early adoption of the amendments is permitted. The new guidance shall be applied on a retrospective basis, wherein the balance sheet of each individual period presented should be adjusted to reflect the period-specific
effects of applying the new guidance. The Company is currently evaluating the impact of the adoption of this standard on its consolidated financial statements.
F-34
In August 2014, the FASB issued ASU 2014-15, which defined management’s responsibility to evaluate whether
there is substantial doubt about an entity’s ability to continue as a going concern and to provide related disclosure. ASU 2014-15 defined the term substantial doubt and requires an assessment for a period of one year after the date of the
issuance of the financial statements. It requires certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans and requires an express statement and other disclosures when substantial doubt is not
alleviated. The guidance becomes effective for reporting periods beginning after December 15, 2016, with early adoption permitted. The Company does not believe that the adoption of this guidance will have a material impact on its consolidated
financial statements.
2. Balance Sheet Details
Prepaid expenses and other assets consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
| |
|
September 30,
2015 |
|
|
December 31,
2014 |
|
| Prepaid clinical expenses(1) |
|
$ |
388 |
|
|
$ |
— |
|
| Prepaid other expenses |
|
|
546 |
|
|
|
756 |
|
| Commercial manufacturing costs(2) |
|
|
— |
|
|
|
1,751 |
|
| Other receivables |
|
|
67 |
|
|
|
628 |
|
|
|
|
|
|
|
|
|
|
|
|
$ |
1,001 |
|
|
$ |
3,135 |
|
|
|
|
|
|
|
|
|
|
| (1) |
During the second half of 2015, following the reduction in workforce initiatives (see Note 7), the Company prepaid certain contract research organizations, or CROs, to manage most aspects of the ongoing CUPID2 trial,
including the future payments to clinical sites for patient costs. Additionally, the Company prepaid $0.4 million to CROs in October 2015. |
| (2) |
The commercial manufacturing costs consisted mainly of design and engineering services for commercial drug manufacturing capabilities. The Company determined that it was probable that it would not complete the
commercial manufacturing project in light of the CUPID 2 clinical data announced in April 2015 (see Note 7). The Company therefore recorded the costs accumulated as of December 31, 2014 and activity in 2015 as a period expense in the
consolidated financial statements in the nine month period ended September 30, 2015. |
Property and equipment consist of the following
(in thousands):
|
|
|
|
|
|
|
|
|
| |
|
September 30,
2015 |
|
|
December 31,
2014 |
|
| Office furniture and other equipment(1) |
|
$ |
174 |
|
|
$ |
881 |
|
| Leasehold improvements(2) |
|
|
246 |
|
|
|
246 |
|
| Accumulated depreciation(1)(2) |
|
|
(287 |
) |
|
|
(364 |
) |
|
|
|
|
|
|
|
|
|
|
|
$ |
133 |
|
|
$ |
763 |
|
|
|
|
|
|
|
|
|
|
| (1) |
Following the CUPID 2 trial results and the decision to not pursue additional previously planned development activities with MYDICAR, the Company sold certain MYDICAR manufacturing assets in the third quarter of 2015.
The assets had a historical cost of $0.8 million and accumulated depreciation of $0.3 million. The Company had recognized an impairment charge of $0.2 million in the second quarter of 2015 related to the assets which had reduced the net asset value
to $0.3 million, the amount of cash proceeds received (see Note 7). |
| (2) |
In September 2015, in light of the scale-down of certain operations, the Company terminated a sublease agreement effective November 13, 2015, as
amended, in order to reduce the Company’s office space and corresponding rent obligations. The sublease was originally scheduled to expire in September 2021. The Company revised the estimated depreciable life of the leasehold improvements
associated with the facility |
F-35
| |
to end on November 13, 2015. The change in estimated life resulted in an acceleration of depreciation expense in the amount of $0.1 million in the third quarter of 2015. The net book value
of the leasehold improvements was $0.1 million at September 30, 2015. |
Accounts payable and accrued expenses consist of the following
(in thousands):
|
|
|
|
|
|
|
|
|
| |
|
September 30,
2015 |
|
|
December 31,
2014 |
|
| Accounts payable |
|
$ |
228 |
|
|
$ |
3,293 |
|
| Accrued compensation |
|
|
93 |
|
|
|
1,909 |
|
| Accrued other |
|
|
547 |
|
|
|
596 |
|
| Current portion of deferred rent |
|
|
157 |
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
$ |
1,025 |
|
|
$ |
5,803 |
|
|
|
|
|
|
|
|
|
|
3. Fair Value Measurements
The Company’s financial instruments primarily consist of cash and cash equivalents, accounts payable and accrued liabilities and historically have
included investment securities. The carrying value of these financial instruments generally approximates fair value due to their short-term nature. Investment securities are recorded at fair value.
The accounting guidance defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and
liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction
between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the
accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1: Observable inputs such as quoted prices in active markets;
Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and
Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions
As of September 30, 2015 and December 31, 2014, cash and cash equivalents consist primarily of bank deposits with third-party financial institutions
and highly liquid money market securities with original maturities at date of purchase of 90 days or less and are stated at cost which approximate fair value and are classified within the Level 1 designation discussed above. Marketable securities
are recorded at fair value, defined as the exit price in the principal market in which the Company would transact, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between
market participants. Level 2 securities are valued using quoted market prices for similar instruments, non-binding market prices that are corroborated by observable market data, or discounted cash flow techniques and include the Company’s
investments in corporate debt securities and commercial paper. Financial liabilities that were measured or disclosed at fair value on a recurring basis, and were classified within the Level 3 designation, included the warrant liability and
convertible notes prior to their conversion to equity upon the Company’s initial public offering in February 2014. None of the Company’s non-financial assets and liabilities are recorded at fair value on a non-recurring basis. No transfers
between levels have occurred during the periods presented.
F-36
Cash equivalents measured at fair value as of September 30, 2015 and December 31, 2014 are all
classified within Level 1. As of September 30, 2015, the Company had no investment securities. Below is a summary of other assets and liabilities measured at fair value (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Fair Value Measurements at
Reporting Date Using |
|
| |
|
As of
December 31,
2014 |
|
|
Quoted
Prices
in Active
Markets
for
Identical
Assets
(Level 1) |
|
|
Significant
Other
Observable
Inputs
(Level 2) |
|
|
Significant
Unobservable
Inputs
(Level 3) |
|
| Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Corporate debt securities |
|
$ |
70,513 |
|
|
$ |
— |
|
|
$ |
70,513 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. Commitments and Contingencies
Sublicense Agreement and Amended and Restated License Agreement with AmpliPhi
Sublicense Agreement
In June 2012, the Company entered
into a sublicense agreement (the AmpliPhi Sublicense) with AmpliPhi Biosciences Corporation (AmpliPhi), pursuant to which AmpliPhi sublicensed to the Company certain rights under a separate agreement which AmpliPhi entered into in 2009 with the
Trustees of University of Pennsylvania (UPenn). Under the terms of the AmpliPhi Sublicense, the Company obtained an exclusive, worldwide sublicense from AmpliPhi under certain UPenn patents related to AAV1 vectors for the development, manufacture,
use and sale of companion diagnostics to MYDICAR. In addition, the Company is required to use commercially reasonable efforts to meet certain developmental, regulatory and commercial milestones with respect to companion diagnostics under the
agreement. Following the decision to not pursue additional previously planned development activities with MYDICAR and its companion diagnostic, the Company may not currently be in compliance with these milestone requirements and may in the future
choose to terminate the agreement. In consideration for the sublicense granted to the Company under the agreement, the Company paid to AmpliPhi a sublicense initiation fee of $310,000, and the Company is obligated to pay to AmpliPhi an annual
sublicense maintenance fee of $310,000. The Company is also required to pay to AmpliPhi a low single-digit percentage royalty based on net sales of any companion diagnostic covered by a licensed patent sold by the Company, its affiliates or its
sublicensees. The Company’s royalty obligations continue on a companion diagnostic-by-companion diagnostic and country-by-country basis until the expiration of the last-to-expire valid claim in a licensed patent covering the applicable
companion diagnostic in such country. Finally, the Company is obligated to pay to AmpliPhi all royalty and milestone payments that become due and payable by AmpliPhi to UPenn under AmpliPhi’s agreement with UPenn as a result of the
Company’s exercise of the sublicense granted under the Company’s agreement with AmpliPhi, including a low single-digit tiered percentage royalty on net sales of any companion diagnostic sold by the Company, its affiliates or its
sublicensees, which royalty is separate from and in addition to the royalty payable to AmpliPhi described above, and up to an aggregate of $850,000 in potential milestone payments per product covered by the licensed patents.
The Company may unilaterally terminate the agreement upon 30 days’ written notice to AmpliPhi. Absent early termination, the agreement will automatically
terminate upon the expiration of the last-to-expire licensed patent, which is expected to be in 2019.
The Company recorded $0.3 million in research and
development expense in each of the nine month periods ended September 30, 2015 and September 30, 2014. Through September 30, 2015, no milestone obligations were incurred under the agreement.
F-37
Amended and Restated License Agreement
The Company entered into an amended and restated license agreement with AmpliPhi concurrently with the AmpliPhi Sublicense that both amended the terms of the
license agreement which the Company entered into with AmpliPhi in 2009 and terminated its manufacturing agreement with AmpliPhi which the Company entered into in 2009. Under the agreement, the Company obtained an exclusive, worldwide license under
certain patents and know-how related to AmpliPhi’s AAV vector and manufacturing technology for the development, manufacture, use and sale of MYDICAR. In addition, the Company has agreed to use commercially reasonable efforts to meet certain
diligence milestones with respect to the development and commercialization of at least one product covered by the UPenn patent rights licensed to AmpliPhi by UPenn under the Company’s agreement with UPenn. Following the decision to not pursue
additional previously planned development activities with MYDICAR and its companion diagnostic, the Company may not currently be in compliance with these milestone requirements.
During the term of the agreement, the Company is not obligated to make annual license or maintenance payments, but is obligated to pay to AmpliPhi all royalty
and milestone payments that become due and payable by AmpliPhi to UPenn under AmpliPhi’s agreement with UPenn as a result of the Company’s exercise of the sublicense granted under the Company’s agreement with AmpliPhi. This includes a
low single-digit tiered percentage royalty on net sales of MYDICAR and any other product covered by the licensed patents sold by the Company, its affiliates or its sublicensees, and up to $850,000 in milestone payments upon the achievement of
certain developmental and regulatory milestones related to MYDICAR and any other product covered by the licensed patents. Through September 30, 2015, no milestone obligations were incurred under the agreement. The agreement does not provide
either party with termination rights and does not have a provision for expiration or automatic termination.
Exclusive Patent License with the
Regents of the University of Minnesota
In May 2009, the Company entered into an exclusive patent license agreement with the Regents of the
University of Minnesota (UMinn) under which it obtained an exclusive license to UMinn’s joint ownership interest in a patent application related to screening technology for isolation of small molecule modulators of SERCA enzymes. The agreement
did not encompass a manufacturing agreement. The Company suspended further development of the small molecule program in the first half of 2015 and, after review of its strategic alternatives, cancelled the patent license agreement in September 2015.
The Company recorded $0.1 million in research and development expense in each of the nine month periods ended September 30, 2015 and September 30, 2014. No milestone obligations were incurred under the agreement.
Material Transfer and Exclusivity Agreement
In
February 2014, the Company and Les Laboratoires Servier (Servier) entered into a material transfer and exclusivity agreement, pursuant to which the Company agreed to transfer to Servier samples of certain proprietary compounds from the
Company’s small molecule SERCA2b modulator program and granted to Servier a non-exclusive, non-sublicensable, royalty-free license to conduct certain studies of the samples for the purpose of evaluating Servier’s interest in negotiating a
potential license and research collaboration agreement with the Company relating to small molecule SERCA2b modulators (Compounds), for the treatment of type 2 diabetes and other metabolic diseases. In 2015 the Company concluded certain pre-clinical
studies in coordination with Servier and the evaluation period has expired.
License Agreement with Enterprise
On July 18, 2014, the Company and Enterprise Partners Management, LLC (Enterprise), an affiliate of Enterprise Partners Venture Capital, entered into an
Assignment and License Agreement (the Enterprise License Agreement), pursuant to which Enterprise granted to the Company an exclusive, worldwide license and the assignment of patents held by Enterprise relating to certain gene therapy applications
of the membrane-bound
F-38
form of the Stem Cell Factor gene (mSCF) for treatment of cardiac ischemia. The Company has the right to grant sublicenses to third parties under the Enterprise License Agreement. Entities
affiliated with Enterprise beneficially owned more than 10% of the Company’s stock as of the date the Enterprise License Agreement was executed.
In
consideration for the rights granted to the Company under the Enterprise License Agreement, the Company paid an upfront fee to Enterprise of $160,000. The Company is also obligated to pay to Enterprise a milestone payment in the amount of $1,000,000
upon the grant to the Company, a Company affiliate or a Company sublicensee of the first regulatory approval in the United States of a product that is covered by the licensed patents. In addition, the Company is required to pay to Enterprise a 2%
royalty on net sales of products sold by the Company, Company affiliates and Company sublicensees that are covered by the licensed patents. The Company’s royalty obligations continue on a product-by-product and country-by-country basis until
the expiration of the last-to-expire valid claim in the licensed patents covering a licensed product in such country.
The Company may unilaterally
terminate the Enterprise License Agreement upon written notice to Enterprise. Enterprise may terminate the agreement in the event of the Company’s material breach of the Enterprise License Agreement if such breach remains uncured for 90 days
following receipt of written notice of such breach. Absent early termination, the Enterprise License Agreement will automatically terminate upon the expiration of the last-to-expire of the licensed patents containing a valid claim.
Other License Agreements
The Company has entered
into various license agreements pursuant to which the Company acquired certain intellectual property. Pursuant to each agreement the Company paid a license fee and reimbursed historical patent costs. Additionally, under each agreement, the Company
may be required to pay annual maintenance fees, royalties, milestone payments and sublicensing fees. Each of the license agreements is generally cancelable by the Company, given appropriate prior written notice. The Company cancelled certain license
agreements following the CUPID 2 clinical trial results in April 2015 (see Note 7). Minimum annual payments to maintain these cancelable licenses total an aggregate of approximately $0.1 million and potential future milestone payments total an
aggregate of approximately $0.9 million. The Company has recorded research and development expense related to license and annual maintenance fees under the agreements of $0.2 million and $0.2 million for the nine month periods ended
September 30, 2015 and September 30, 2014, respectively.
Through September 30, 2015, the Company has recorded research and development
expense of $0.1 million related to milestone obligations incurred under the agreements.
Leases
The Company leases office space in San Diego, California under a long-term operating lease that expires in October 2017 and a short-term operating lease that
expires in January 2016. In September 2015, in light of the scale-down of certain operations, the Company terminated a sublease agreement for office space effective November 13, 2015, as amended, and paid an early termination fee of
approximately $1.0 million. The subleased office space was in San Diego, California and the sublease was originally scheduled to expire in September 2021. In the third quarter of 2015 the Company also terminated its Seattle, Washington leases and
paid an early termination fee of $0.2 million. Rent expense for the three months and nine months ended September 30, 2015 was $31,000 and $0.3 million, respectively, which expense included $0.1 million for the acceleration of deferred rent due
to the early termination of the sublease agreements. Rent expense for the three months and nine months ended September 30, 2014 was $94,000 and $136,000, respectively. Future minimum payments under the long-term operating leases net of
contractual sublease payments total $51,000.
F-39
5. Stockholders’ Equity
Common Stock Warrants
The following table
summarizes the fully exercisable warrants outstanding for the purchase of common stock as of September 30, 2015 and December 31, 2014:
|
|
|
|
|
|
|
September 30,
2015 |
|
December 31,
2014 |
|
Exercise
Price |
|
Expiration
Date |
| 152,735 |
|
206,340 |
|
$5.61 |
|
October 2018 |
| |
|
|
|
|
|
|
Stock Options
Options granted under the Company’s equity incentive plans generally expire no more than ten years from the date of grant and generally vest and become
exercisable over a period not to exceed four years, as determined by the Company’s board of directors. Recipients of stock options are eligible to purchase shares of the Company’s common stock at an exercise price equal to no less than the
estimated fair market value of such stock on the date of grant. The Company has also granted inducement stock options outside an equity incentive plan that are subject to the terms and conditions of the Company’s 2013 Equity Incentive Plan.
Prior Plans
In December 2001, the Company
adopted its 2001 Stock Option Plan (the 2001 Plan) and in January 2012 adopted its 2012 Equity Incentive Plan (the 2012 Plan, and together with the 2001 Plan, the Prior Plans). The Prior Plans have terminated and no further shares may be granted
under the Prior Plans.
2013 Equity Incentive Plan
The 2013 Equity Incentive Plan became effective in February 2014. Under the 2013 Equity Incentive Plan, the Company may grant stock options, stock appreciation
rights, restricted stock, restricted stock units (RSUs), performance-based stock awards and other awards to individuals who are then employees, officers, non-employee directors or consultants of the Company and its affiliates. Additionally, the 2013
Equity Incentive Plan provides for the grant of performance cash awards. The number of shares of common stock reserved for issuance under the 2013 Equity Incentive Plan will automatically increase on January 1 of each year continuing through
and including January 1, 2023 by 5% of the total number of shares of the Company’s capital stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares determined by the Company’s board of
directors.
A summary of the Company’s stock option and RSU activity is as follows:
|
|
|
|
|
| |
|
Options and
RSUs |
|
| Outstanding at December 31, 2014 |
|
|
2,408,634 |
|
| Granted |
|
|
1,342,750 |
|
| Exercised |
|
|
(383,011 |
) |
| Cancelled |
|
|
(1,841,275 |
) |
|
|
|
|
|
| Outstanding at September 30, 2015 |
|
|
1,527,098 |
|
|
|
|
|
|
2013 Employee Stock Purchase Plan
The 2013 Equity Stock Purchase Plan (ESPP) became effective in January 2014. The number of shares of common stock reserved for issuance will automatically
increase on January 1 of each calendar year through
F-40
January 1, 2023 by the least of (1) 1% of the total number of shares of the Company’s common stock outstanding on December 31 of the preceding calendar year, (2) 384,307
shares, or (3) a number determined by the Company’s board of directors that is less than (1) and (2).
Stock-Based Compensation
Expense
The allocation of stock-based compensation for all equity awards is as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months
Ended
September 30, |
|
|
Nine Months
Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
|
2015 |
|
|
2014 |
|
| Research and development(1) |
|
$ |
(663 |
) |
|
$ |
391 |
|
|
$ |
929 |
|
|
$ |
1,175 |
|
| General and administrative |
|
|
645 |
|
|
|
520 |
|
|
|
2,254 |
|
|
|
1,003 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
(18 |
) |
|
$ |
911 |
|
|
$ |
3,183 |
|
|
$ |
2,178 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1) |
During the three months ended September 30, 2015, following the reduction in workforce initiatives (see Note 7), the Company reversed the previously recorded expense for unvested options of terminated employees
aggregating $0.8 million. |
As of September 30, 2015 the unrecognized compensation cost related to outstanding employee options was $7.3
million and is expected to be recognized as expense over approximately 2.9 years.
6. Long-Term Obligations
Hercules Loan Agreement
On July 31, 2014, the
Company entered into a Loan and Security Agreement (the Loan Agreement) with Hercules Technology III, L.P. and Hercules Technology Growth Capital, Inc. (as agent and as a lender, and together with Hercules Technology III, L.P., the Lenders) under
which up to $25.0 million was available for the Company to borrow in two tranches (the Loan).
The Company borrowed the first tranche of $10.0 million on
August 1, 2014. The Loan accrued interest at a rate equal to the greater of either (i) 8.25% plus the prime rate as reported from time to time in The Wall Street Journal minus 5.25%, and (ii) 8.25%. Contractual payments under the Loan
Agreement were interest only until August 1, 2015 followed by equal monthly payments of principal and interest, through the scheduled maturity date on February 1, 2018. In addition, a final payment equal to $1,750,000 was due at such time
as the Loan was prepaid or became due and payable in full as specified in the Loan Agreement.
The second tranche of up to $15.0 million was available to
be drawn through June 30, 2015, but only if the Company provided the Lenders with notice that data from the Company’s Phase 2b clinical trial for MYDICAR supported the continued development of MYDICAR for its Breakthrough Therapy
designation to either a Phase 3 clinical trial or for registration for approval, as reasonably determined by the Company’s senior management and board of directors (the Milestone). In April 2015, the Company’s senior management and board
of directors determined that the Company did not achieve the Milestone (see Note 7). Accordingly, the Company could not draw down the second tranche of $15.0 million.
In June 2015 the Company announced it would prepay the outstanding amounts due under the Loan Agreement and on August 3, 2015, the Company paid the
Lenders (i) the $10,000,000 outstanding principal balance, (ii) $75,625 in accrued and unpaid interest, and (iii) an end of term charge of $1,750,000, for a total payment of $11,825,625. Upon the prepayment on August 3, 2015, the
Company’s obligations, covenants, debts and liabilities under the Loan Agreement were satisfied in full and the Lender’s commitments to extend further credit to the Company were terminated.
F-41
Capital Lease
In 2014 the Company entered into a capital lease arrangement for office equipment in the Company’s San Diego, California office. The Company was obligated
to make 60 payments of approximately $600. In September 2015 the Company purchased and obtained title to the office equipment. At September 30, 2015, the Company had no capital lease obligations.
7. Restructuring charges
On April 26, 2015, the
Company announced that its Phase 2b CUPID 2 trial did not meet its primary and secondary endpoints. No safety issues were noted. In light of the CUPID 2 results and following analysis of the CUPID 2 data, the Company’s board of directors, in
two phases prior to September 30, 2015, approved an approximately 70% aggregate reduction of the Company’s peak workforce of 34 employees to reduce operating expenses and conserve cash resources. The Company has also committed to retention
payments payable to certain key employees if such employees remain with the Company until December 31, 2015 or are terminated by the Company without cause prior to such date.
Restructuring charges for each period were as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months
Ended
September 30, |
|
|
Nine Months
Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
|
2015 |
|
|
2014 |
|
| Employee severance and related costs |
|
$ |
675 |
|
|
$ |
— |
|
|
$ |
3,543 |
|
|
$ |
— |
|
| Facility lease termination costs |
|
|
1,127 |
|
|
|
— |
|
|
|
1,127 |
|
|
|
— |
|
| Asset impairments |
|
|
(21 |
) |
|
|
— |
|
|
|
192 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total restructuring and asset impairment charges |
|
$ |
1,781 |
|
|
$ |
— |
|
|
$ |
4,862 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accrued restructuring activity during the nine months ended September 30, 2015 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Employee
Severance
and
Related Costs |
|
|
Facility
Lease
Termination
Costs |
|
|
Total |
|
| Accrued restructuring balance as of December 31, 2014 |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
| Additional accruals |
|
|
3,543 |
|
|
|
1,127 |
|
|
|
4,670 |
|
| Cash payments |
|
|
(2,934 |
) |
|
|
(1,127 |
) |
|
|
(4,061 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued restructuring balance as of September 30, 2015 |
|
$ |
609 |
|
|
$ |
— |
|
|
$ |
609 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company recorded the additional accruals as restructuring charges in the consolidated statements of operations. The
accrued restructuring balance as of September 30, 2015, is presented as a current liability in the consolidated balance sheets and is expected to be paid within the fourth quarter of 2015. The charges incurred during the nine months ended
September 30, 2015, included $2.4 million related to employee severance costs, which impacted 25 employees who were notified of their termination prior to September 30, 2015, $1.1 million related to retention payment accruals, and $1.1
million related to facility lease termination costs. The Company expects to record an additional $0.3 million related to retention payments in the fourth quarter of 2015.
Following the announcement on June 26, 2015, that the Company had suspended further research and development of its MYDICAR programs, the Company
determined that certain equipment used in the MYDICAR manufacturing process was impaired and an asset impairment charge of $0.2 million was recorded to restructuring charges in the consolidated statements of operations for the nine months ended
September 30, 2015. The equipment was subsequently sold in September 2015.
F-42
The Company may incur additional charges in connection with future restructuring activities.
8. Litigation
In July 2015, following the Company’s
announcements of the negative CUPID 2 data and the suspension of further research and development activities and the subsequent declines of the price of the Company’s common stock, three putative securities class action complaints (captioned
Fialkov v. Celladon Corporation, Case No. 15-cv-1458-AJB-DHB, Lorusso v. Celladon Corporation, Case No. 15-cv-1501-L-JLB and Jacobs v. Celladon Corporation, Case No. 15-cv-1529-AJB-MDD) were
filed in the U.S. District Court for the Southern District of California against the Company and certain of the Company’s current and former officers. The complaints generally allege that the defendants violated Sections 10(b) and 20(a) of the
Securities Exchange Act of 1934 by making materially false and misleading statements regarding the clinical trial program for MYDICAR, thereby artificially inflating the price of the Company’s common stock. The complaints seek unspecified
monetary damages and other relief, including attorneys’ fees. The Company expects the court to consolidate the three putative securities class actions and to appoint a lead plaintiff to represent the putative class. The Company then expects the
lead plaintiff to file a consolidated complaint. It is possible that additional suits will be filed, or allegations made by stockholders, with respect to these same or other matters and also naming the Company and/or the Company’s officers and
directors as defendants. The Company believes that it has meritorious defenses and intends to defend these lawsuits vigorously. Due to the early stage of these proceedings, the Company is not able to predict or reasonably estimate the ultimate
outcome or possible losses relating to these claims.
F-43
Eiger BioPharmaceuticals, Inc.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2014 and 2013 and Nine Months Ended September 30, 2015 and 2014
F-44
Independent Auditors’ Report
The Board of Directors
Eiger BioPharmaceuticals, Inc.:
Report on the Financial Statements
We have audited the
accompanying consolidated financial statements of Eiger BioPharmaceuticals, Inc. and its subsidiaries, which comprise the consolidated balance sheets as of December 31, 2014 and 2013, and the related consolidated statements of operations and
comprehensive loss, stockholders’ equity, and cash flows for the years then ended, and the related notes to the consolidated financial statements.
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with U.S. generally accepted
accounting principles; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of consolidated financial statements that are free from material misstatement, whether due to
fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with auditing
standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures
selected depend on the auditors’ judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal
control relevant to the entity’s preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the
effectiveness of the entity’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by
management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained
is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Eiger
BioPharmaceuticals, Inc. and its subsidiaries as of December 31, 2014 and 2013, and the results of their operations and their cash flows for the years then ended in accordance with U.S. generally accepted accounting principles.
Emphasis of Matter
The accompanying consolidated
financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has an accumulated
deficit that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that
might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
/s/ KPMG
LLP
San Francisco, California
December 11, 2015
F-45
Eiger BioPharmaceuticals, Inc.
Consolidated Balance Sheets
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Assets |
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
| Cash |
|
$ |
776,797 |
|
|
$ |
144,766 |
|
| Prepaid expenses and other current assets |
|
|
31,999 |
|
|
|
18,170 |
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
808,796 |
|
|
|
162,936 |
|
| Property and equipment, net |
|
|
7,678 |
|
|
|
13,077 |
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
816,474 |
|
|
$ |
176,013 |
|
|
|
|
|
|
|
|
|
|
| Liabilities and Stockholders’ Equity |
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
59,267 |
|
|
$ |
19,266 |
|
| Accrued liabilities |
|
|
226,262 |
|
|
|
51,279 |
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
285,529 |
|
|
|
70,545 |
|
|
|
|
|
|
|
|
|
|
| Commitments and contingencies |
|
|
|
|
|
|
|
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
|
| Convertible preferred stock, $0.0001 par value: 17,787,500 and 14,687,500 shares authorized as of December 31, 2014 and 2013,
respectively; 17,358,845 and 14,035,555 shares issued and outstanding as of December 31, 2014 and 2013, respectively; liquidation preference of $15,040,630 and $13,613,122 as of December 31, 2014 and 2013, respectively |
|
|
15,366,286 |
|
|
|
13,451,674 |
|
| Common stock, $0.0001 par value, 24,400,000 and 20,500,000 shares authorized as of December 31, 2014 and 2013, respectively;
2,214,958 shares issued and outstanding as of December 31, 2014 and 2013 respectively |
|
|
221 |
|
|
|
221 |
|
| Additional paid-in capital |
|
|
1,113,483 |
|
|
|
1,086,724 |
|
| Accumulated deficit |
|
|
(15,949,045 |
) |
|
|
(14,433,151 |
) |
|
|
|
|
|
|
|
|
|
| Total stockholders’ equity |
|
|
530,945 |
|
|
|
105,468 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities and stockholders’ equity |
|
$ |
816,474 |
|
|
$ |
176,013 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the consolidated financial statements.
F-46
Eiger BioPharmaceuticals, Inc.
Consolidated Statements of Operations and Comprehensive Loss
|
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
643,552 |
|
|
$ |
426,366 |
|
| General and administrative |
|
|
872,342 |
|
|
|
526,497 |
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
1,515,894 |
|
|
|
952,863 |
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(1,515,894 |
) |
|
|
(952,863 |
) |
|
|
|
|
|
|
|
|
|
| Net loss and comprehensive loss |
|
$ |
(1,515,894 |
) |
|
$ |
(952,863 |
) |
|
|
|
|
|
|
|
|
|
| Net loss per share, basic and diluted |
|
$ |
(0.68 |
) |
|
$ |
(0.43 |
) |
|
|
|
|
|
|
|
|
|
| Shares used in computing net loss per share, basic and diluted |
|
|
2,214,958 |
|
|
|
2,214,958 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the consolidated financial statements.
F-47
Eiger BioPharmaceuticals, Inc.
Consolidated Statements of Stockholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Convertible
Preferred Stock |
|
|
Common Stock |
|
|
Additional
Paid-In
Capital |
|
|
Accumulated
Deficit |
|
|
Total
Stockholders’
Equity |
|
| |
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
|
| Balance at December 31, 2012 |
|
|
12,732,875 |
|
|
$ |
12,701,575 |
|
|
|
2,214,958 |
|
|
$ |
221 |
|
|
$ |
1,026,039 |
|
|
$ |
(13,480,288 |
) |
|
$ |
247,547 |
|
| Issuance of Series A-1 convertible preferred stock, net of $5,455 in issuance costs |
|
|
1,302,680 |
|
|
|
750,099 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
750,099 |
|
| Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
60,685 |
|
|
|
— |
|
|
|
60,685 |
|
| Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(952,863 |
) |
|
|
(952,863 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2013 |
|
|
14,035,555 |
|
|
|
13,451,674 |
|
|
|
2,214,958 |
|
|
|
221 |
|
|
|
1,086,724 |
|
|
|
(14,433,151 |
) |
|
|
105,468 |
|
| Issuance of Series A-1 convertible preferred stock, net of $12,897 in issuance costs |
|
|
3,323,290 |
|
|
|
1,914,612 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,914,612 |
|
| Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
26,759 |
|
|
|
— |
|
|
|
26,759 |
|
| Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,515,894 |
) |
|
|
(1,515,894 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2014 |
|
|
17,358,845 |
|
|
$ |
15,366,286 |
|
|
|
2,214,958 |
|
|
$ |
221 |
|
|
$ |
1,113,483 |
|
|
$ |
(15,949,045 |
) |
|
$ |
530,945 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the consolidated financial statements.
F-48
Eiger BioPharmaceuticals, Inc.
Consolidated Statements of Cash Flows
|
|
|
|
|
|
|
|
|
| |
|
Year Ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Cash Flows From Operating Activities |
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(1,515,894 |
) |
|
$ |
(952,863 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Depreciation |
|
|
7,680 |
|
|
|
7,234 |
|
| Stock-based compensation |
|
|
26,759 |
|
|
|
60,685 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Prepaid expenses and other current assets |
|
|
(13,829 |
) |
|
|
(5,205 |
) |
| Accounts payable |
|
|
40,001 |
|
|
|
16,410 |
|
| Accrued liabilities |
|
|
174,983 |
|
|
|
16,868 |
|
|
|
|
|
|
|
|
|
|
| Net cash used in operating activities |
|
|
(1,280,300 |
) |
|
|
(856,871 |
) |
|
|
|
|
|
|
|
|
|
| Cash Flows From Investing Activities |
|
|
|
|
|
|
|
|
| Purchase of property and equipment |
|
|
(2,281 |
) |
|
|
(1,742 |
) |
|
|
|
|
|
|
|
|
|
| Net cash used in investing activities |
|
|
(2,281 |
) |
|
|
(1,742 |
) |
|
|
|
|
|
|
|
|
|
| Cash Flows From Financing Activities |
|
|
|
|
|
|
|
|
| Proceeds from issuance of convertible preferred stock, net of issuance costs |
|
|
1,914,612 |
|
|
|
750,099 |
|
|
|
|
|
|
|
|
|
|
| Net cash provided by financing activities |
|
|
1,914,612 |
|
|
|
750,099 |
|
|
|
|
|
|
|
|
|
|
| Net increase (decrease) in cash |
|
|
632,031 |
|
|
|
(108,514 |
) |
| Cash, beginning of year |
|
|
144,766 |
|
|
|
253,280 |
|
|
|
|
|
|
|
|
|
|
| Cash, end of year |
|
$ |
776,797 |
|
|
$ |
144,766 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the consolidated financial statements.
F-49
Eiger Biopharmaceuticals, Inc.
Notes to Consolidated Financial Statements
1. Organization and Basis of Presentation
Eiger
BioPharmaceuticals, Inc. (the “Company”) was incorporated in the State of Delaware on November 6, 2008. The Company is a clinical-stage biopharmaceutical company committed to bringing to market novel products for the treatment of
orphan diseases. The Company has built a diverse portfolio of well-characterized product candidates with the potential to address diseases for which the unmet medical need is high, the biology for treatment is clear, and for which an effective
therapy is urgently needed. The Company’s principal operations are based in Palo Alto, California and it operates in one segment.
Need for
Additional Liquidity
The consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of
assets and satisfaction of liabilities in the normal course of business. In the course of its development activities, the Company has sustained operating losses and expects such losses to continue over the next several years. The Company’s
ultimate success depends on the outcome of its research and development activities. The Company has incurred net losses from operations since inception and has an accumulated deficit of $15.9 million as of December 31, 2014. While the Company
has obtained $6.0 million in cash proceeds from a bridge financing completed in November 2015 (See Note 10), the Company expects to incur additional losses in the future to conduct product research and development and recognizes the need raise
additional capital to fully implement its business plan. The Company intends to raise additional capital through the issuance of additional equity, including in connection with the reverse merger discussed in Note 10, and potentially through
borrowings, and strategic alliances with partner companies. However, if such financing are not available timely and at adequate levels, the Company will need to reevaluate its operating plans. The failure to obtain sufficient funds on acceptable
terms when needed could have a material adverse effect on the Company’s business, results of operations, future cash flows and financial conditions. These factors raise substantial doubt about the Company’s ability to continue as a going
concern. Management is currently pursuing financing alternatives. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis of Presentation and Consolidation
The
consolidated financial statements include the accounts of Eiger BioPharmaceuticals, Inc. and its wholly owned subsidiaries, EB Pharma LLC and Eiger BioPharmaceuticals Europe Limited, and have been prepared in conformity with accounting principles
generally accepted in the United States of America, or U.S. GAAP. All intercompany balances and transactions have been eliminated in consolidation.
2.
Summary of Significant Accounting Policies
Use of Estimates
The accompanying financial statements have been prepared in accordance with U.S. GAAP. The preparation of financial statements in conformity with U.S. GAAP
requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during
the reporting period. On an ongoing basis, the Company evaluates its estimates, including those related to clinical trial accrued liabilities, income taxes, and stock-based compensation. The Company bases its estimates on historical experience and
on various other market-specific and relevant assumptions that the Company believes to be reasonable under the circumstances. Actual results could differ from those estimates.
F-50
Concentrations of Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consists of cash. The Company’s cash is held by a financial
institution in the United States. Amounts on deposit may at times exceed federally insured limits. Management believes that the financial institution is financially sound, and accordingly, minimal credit risk exists with respect to the financial
institution.
The Company relies on one major contract manufacturer organization to develop and commercialize pharmaceutical products for the treatment of
pulmonary arterial hypertension, or PAH. If the single source supplier fails to satisfy the Company’s requirements on a timely basis, it could suffer delays in its clinical development programs and activities, which could adversely affects its
operating results.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation expense is computed using the straight-line method over the estimated
useful lives of the assets. Depreciation begins at the time the asset is placed into service. Maintenance and repairs are charged to operations as incurred.
The useful lives of the property and equipment are as follows:
|
|
|
|
|
| Research and development equipment |
|
|
5 years |
|
| Computer equipment |
|
|
3 years |
|
Impairment of Long-Lived Assets
The Company evaluates its long-lived assets, including property and equipment, for impairment whenever events or changes in circumstances indicate that the
carrying value of these assets may not be recoverable. The Company assesses the recoverability of long-lived assets by determining whether or not the carrying value of such assets will be recovered through undiscounted expected future cash flows. If
the asset is considered to be impaired, the amount of any impairment is measured as the difference between the carrying value and the fair value of the impaired asset. Through December 31, 2014, the Company has not written down any long-lived
assets as a result of impairment.
Accrued Research and Development Costs
The Company accrues for estimated costs of research and development activities conducted by third-party service providers, which include the conduct of
preclinical and clinical studies, and contract manufacturing activities. The Company records the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced, and includes these
costs in accrued liabilities in the balance sheets and within research and development expense in the statements of operations and comprehensive loss. These costs are a significant component of the Company’s research and development expenses.
The Company accrues for these costs based on factors such as estimates of the work completed and in accordance with agreements established with its third-party service providers. The Company makes significant judgments and estimates in determining
the accrued liabilities balance in each reporting period. As actual costs become known, the Company adjusts its accrued liabilities.
Research and
Development Costs
Research and development costs are expensed as incurred and consist of stock-based compensation expense, lab supplies and
allocated facility costs, as well as fees paid to third parties that conduct certain research and development activities on the Company’s behalf. Amounts incurred in connection with license agreements are also included in research and
development expense.
F-51
Fair Value Measurements
Fair value accounting is applied for all financial assets and liabilities that are recognized or disclosed at fair value in the consolidated financial
statements on a recurring basis (at least annually). Financial instruments include cash, accounts payable and accrued liabilities that approximate fair value due to their relatively short maturities.
Assets and liabilities recorded at fair value on a recurring basis in the balance sheets are categorized based upon the level of judgment associated with the
inputs used to measure their fair values. Fair value is defined as the exchange price that would be received for an asset or an exit price that would be paid to transfer a liability in the principal or most advantageous market for the asset or
liability in an orderly transaction between market participants on the measurement date. The authoritative guidance on fair value measurements establishes a three-tier fair value hierarchy for disclosure of fair value measurements as follows:
Level 1: Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date;
Level 2: Inputs are observable, unadjusted quoted prices in active markets for similar assets or liabilities, unadjusted quoted prices
for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the related assets or liabilities; and
Level 3: Unobservable inputs that are significant to the measurement of the fair value of the assets or liabilities that are supported
by little or no market data.
As of December 31, 2014 and 2013, the Company does not have any assets or liabilities recorded at fair value on a
recurring basis.
Stock-Based Compensation
Stock-based awards to employees and directors, including stock options, are recorded at fair value as of the grant date using the Black-Scholes option pricing
model and recognized as expense on a straight line-basis over the employee’s or director’s requisite service period (generally the vesting period). Noncash stock compensation expense is based on awards ultimately expected to vest and is
reduced by an estimate for future forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates.
Stock-based awards and stock options issued to nonemployee consultants are recorded at fair value and remeasured at the end of each period as they vest using
the Black-Scholes option pricing model. Expense is recognized over the vesting period which is generally the same as the service period.
Income
Taxes
The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are
determined based on the differences between the financial reporting and the tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company
must then assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. Due to the
Company’s lack of earnings history, the net deferred tax assets have been fully offset by a valuation allowance.
The Company recognizes benefits of
uncertain tax positions if it is more likely than not that such positions will be sustained upon examination based solely on their technical merits, as the largest amount of benefit that is more likely than not to be realized upon the ultimate
settlement. The Company’s policy is to recognize interest
F-52
and penalties related to the underpayment of income taxes as a component of income tax expense or benefit. To date, there have been no interest or penalties charged in relation to unrecognized
tax benefits.
Net Loss per Share
Basic net
loss per share is calculated by dividing the net loss by the weighted average number of shares of common stock outstanding during the period without consideration of common stock equivalents. Since the Company was in a loss position for all periods
presented, diluted net loss per share is the same as basic net loss per share for all periods as the inclusion of all potential common shares outstanding would have been anti-dilutive.
Recent Accounting Pronouncements
In June 2014,
the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest
Entities Guidance in Topic 810, Consolidation. ASU 2014-10 simplifies the accounting guidance by removing all incremental financial reporting requirements for development stage entities. The amendments related to the elimination of the
inception-to-date information and other disclosure requirement of Topic 915 should be applied retrospectively, and are effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. The Company early
adopted this guidance and accordingly, there is no inception to date information presented in these consolidated financial statements.
In August 2014,
the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. ASU 2014-15 requires management to evaluate relevant conditions, events and certain management plans that are known or
reasonably knowable that when, considered in the aggregate, raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued, for both annual and interim
periods. ASU 2014-15 also requires certain disclosures around management’s plans and evaluation, as well as the plans, if any, that are intended to mitigate those conditions or events that will alleviate the substantial doubt. ASU 2014-15 is
effective for fiscal years ending after December 15, 2016. The Company does not anticipate the adoption of ASU 2014-15 to have a material impact on its consolidated financial statements and related disclosures.
3. Balance Sheet Components
Property and
Equipment, Net
Property and equipment, net consist of the following:
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Lab equipment |
|
$ |
35,651 |
|
|
$ |
35,651 |
|
| Office equipment |
|
|
4,023 |
|
|
|
1,742 |
|
|
|
|
|
|
|
|
|
|
| Total property and equipment |
|
|
39,674 |
|
|
|
37,393 |
|
| Less: accumulated depreciation |
|
|
(31,996 |
) |
|
|
(24,316 |
) |
|
|
|
|
|
|
|
|
|
| Property and equipment, net |
|
$ |
7,678 |
|
|
$ |
13,077 |
|
|
|
|
|
|
|
|
|
|
Depreciation expense for the years ended December 31, 2014 and 2013 was $7,680 and $7,234, respectively.
F-53
Accrued Liabilities
Accrued liabilities consist of the following:
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Accrued consulting costs |
|
$ |
100,000 |
|
|
$ |
— |
|
| Accrued contract research costs |
|
|
80,515 |
|
|
|
5,840 |
|
| Accrued legal fees |
|
|
18,324 |
|
|
|
28,324 |
|
| Accrued vacation |
|
|
25,673 |
|
|
|
17,115 |
|
| Accrued other |
|
|
1,750 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Total accrued liabilities |
|
$ |
226,262 |
|
|
$ |
51,279 |
|
|
|
|
|
|
|
|
|
|
4. License Agreements
Merck License Agreement
In September 2010, the
Company entered into an exclusive license agreement with Schering Corporation, subsequently acquired by Merck & Co., Inc., or Merck, which provides the Company with the exclusive right to develop and commercialize Sarasar/Lonafarnib. As
consideration for such exclusive right, the Company issued 312,500 shares of Series A convertible preferred stock with a fair value of $500,000 when the agreement was executed in September 2010. In addition, the Company is obligated to pay Merck up
to an aggregate of $27.0 million in development milestones and will be required to pay tiered royalties based on aggregate annual net sales of all licensed products ranging from mid-single to low double-digit royalties on net sales. The
Company’s obligation to pay royalties to Merck expires on a country-by-country and product-by-product basis on the later of either the expiration of the last to expire patent assigned to the Company under the agreement, which is estimated to be
in December 2016 or the earliest of the tenth anniversary of the first commercial sale of the product. Through the year ended December 31, 2014, the Company had not reached any of the milestone events. In May 2015, the first regulatory
milestone was achieved and the Company paid the related milestone payment of $1.0 million to Merck.
EGI License Agreement
In December 2010, the Company entered into an asset purchase agreement with Eiger Group International, Inc., or EGI, which is owned by the Company’s
founder who is a stockholder of the Company, whereby the Company purchased all of the assets related to the use of farnesyl transferase inhibitors as anti-viral agents and methods to treat viral infections with those inhibitors and inhibitors of
prenylation, prenyl cysteine methyltranferase and a protease that removes the XXX tripeptide from the CXXX polypeptide following prenylation including any related intellectual property to EGI. The Company paid EGI an upfront payment of $350,000 when
the agreement was executed in December 2010. Additionally, the Company will pay a low single digit royalty of future aggregate annual net sales if the Company has not recouped the development costs of any drug product that incorporates clemizole.
Once the costs have been recouped, the Company will pay a low single digit royalty of future aggregate annual net sales if there is no generic competition for the product and a low single digit royalty of future aggregate annual net sales if there
is generic competition for the product. Within the first ten years after commercialization, the Company may make a one-time payment of $500,000 for each contract for the three types of product related to such intellectual property that would reduce
the payment term for the three products to the tenth anniversary of the first commercial sale. The obligation to pay royalties expires on a country-by-country and product-by-product basis on the later of either when the product is no longer sold in
any country or the earliest of the tenth anniversary of the first commercial sale of the product. As of December 31, 2014, the product has not achieved regulatory approval.
In November 2012, the Company entered into an agreement with EGI whereby the Company sold all of the assets related to the compound clemizole, including any
related intellectual property. EGI will pay to the
F-54
Company a high single digit royalty on future aggregate annual net sales, subject to certain reductions and exceptions. EGI’s obligation to pay royalties expires on a country-by-country and
product-by-product basis on the later of either expiration of the last to expire patent sold to EGI under the agreement or the earliest of the tenth anniversary of the first commercial sale of the product. As of December 31, 2014, the product
has not achieved regulatory approval.
Janssen License Agreement
The Company entered into a license agreement with Janssen Pharmaceutica NV, or Janssen, in December 2014. In connection with this license agreement, the
Company is obligated to make development milestone payments in aggregate of up to $38.0 million, sales milestone in aggregate up to $65.8 million and will be required to pay tiered royalties based on aggregate annual net sales of all licensed
products ranging from mid-single to low double-digit royalties of net sales. As of December 31, 2014, the product has not reached any development milestones nor achieved regulatory approval.
5. Stockholders’ Equity
Convertible Preferred
Stock
The Company has the following series of convertible preferred stock outstanding as of December 31, 2014 and 2013:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| December 31, 2014 |
|
Shares
Authorized |
|
|
Shares
Issued and
Outstanding |
|
|
Issuance
Price and
Conversion
Price
(Per Share) |
|
|
Net Carrying
Value |
|
|
Liquidation
Preference |
|
| Series A |
|
|
5,187,500 |
|
|
|
4,875,000 |
|
|
$ |
1.60 |
|
|
$ |
7,668,451 |
|
|
$ |
7,800,000 |
|
| Series A-1 |
|
|
12,600,000 |
|
|
|
12,483,845 |
|
|
$ |
0.58 |
|
|
|
7,697,835 |
|
|
|
7,240,630 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17,787,500 |
|
|
|
17,358,845 |
|
|
|
|
|
|
$ |
15,366,286 |
|
|
$ |
15,040,630 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| December 31, 2013 |
|
Shares
Authorized |
|
|
Shares
Issued and
Outstanding |
|
|
Issuance
Price and
Conversion
Price
(Per Share) |
|
|
Net Carrying
Value |
|
|
Liquidation
Preference |
|
| Series A |
|
|
5,187,500 |
|
|
|
4,875,000 |
|
|
$ |
1.60 |
|
|
$ |
7,668,451 |
|
|
$ |
7,800,000 |
|
| Series A-1 |
|
|
9,500,000 |
|
|
|
9,160,555 |
|
|
$ |
0.58 |
|
|
|
5,783,223 |
|
|
|
5,313,122 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14,687,500 |
|
|
|
14,035,555 |
|
|
|
|
|
|
$ |
13,451,674 |
|
|
$ |
13,113,122 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Significant provisions of the preferred stock are as follows:
Voting Rights: The holders of outstanding convertible preferred and common stock vote together as a single class, except with respect to
certain matters that require a separate class vote, and are entitled to the number of votes equal to the number of shares of common stock into which such shares of the applicable preferred stock could then be converted.
Dividends: The Series A preferred stock and Series A-1 preferred stock shall be entitled to receive dividends (on a pari passu basis) at
a rate per annum of $0.128 per share and $0.0464 per share, respectively, subject to adjustment for stock splits, combinations, reorganizations and the like, when and if declared by the Board of Directors. After payment of the dividends on the
Preferred Stock, any additional declared dividends shall be distributed between the holders of preferred stock and common stock on a pro-rata as-converted basis. No rights shall accrue to the holders of the convertible preferred stock if dividends
are not declared and any dividends declared are noncumulative. No dividends have been declared or paid through December 31, 2014.
F-55
Liquidation: In the event of (1) a liquidation, dissolution or winding up of the
Company, (2) the acquisition of the Company by means of any transaction or series of related transactions (including, without limitation, any reorganization, merger or consolidation) provided that the applicable transaction shall not be deemed
a liquidation unless the Company’s stockholders constituted immediately prior to such transaction hold less than 50% of the voting power of the surviving or acquiring entity, (3) the closing of the transfer (whether by merger,
consolidation, or otherwise) of 50% or more of the outstanding common stock of the Company on one transaction or series of related transactions, or (4) the sale of substantially all of the assets of the Company, the holders of preferred stock
shall be the first to be paid out of the assets available for distribution, an amount per share equal to the issue price of the applicable preferred stock, subject to adjustment for stock splits, combinations, reorganizations and the like, plus any
dividends declared but unpaid. The holders of 60% of the outstanding convertible preferred stock, voting together as a single class on an as converted basis, may elect to waive the treatment of any of the occurrences of a liquidation.
Conversion: Each share of preferred stock is convertible into a number of shares of common stock equal to its issuance price divided by
its conversion price at any time, automatically in the event of an initial public offering with minimum proceeds of $30.0 million and a price per share of $4.80, subject to adjustment for stock splits, combinations, reorganizations and the like, or
upon the election of the holders of at least 60% of the outstanding preferred stock.
The conversion price is subject to adjustment in the
event of a stock split, stock dividend, corporate reorganization and the like. In addition, in the event that common stock is issued for an amount less than the conversion price of the Series A and Series A-1 preferred stock in effect immediately
prior to such issuance, the conversion price of the Series A and A-1 preferred stock will be reduced to the issuance price of such common stock. As of December 31, 2014, the conversion ratio was 1-for-1.
Redemption: Convertible preferred stock is not redeemable.
Common Stock
The holders of the Company’s
common stock have one vote for each share of common stock. Common stockholders are entitled to dividends when, as, and if declared by the Board of Directors, subject to the prior rights of the preferred stockholders. As of December 31, 2014, no
dividends had been declared by the Board of Directors.
The Company had reserved shares of common stock for issuance as follows:
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Convertible preferred stock, on as-converted basis |
|
|
17,358,845 |
|
|
|
14,035,555 |
|
| Options issued and outstanding |
|
|
1,209,999 |
|
|
|
929,999 |
|
| Options available for future grants |
|
|
337,793 |
|
|
|
617,793 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
18,906,637 |
|
|
|
15,583,347 |
|
|
|
|
|
|
|
|
|
|
F-56
6. Stock Option Plan
In 2009, the Company adopted the 2009 Equity Incentive Plan (the “Plan”). Under the Plan, shares of the Company’s common stock have been
reserved for the issuance of stock options to employees, directors, and consultants under terms and provisions established by the Board of Directors. A total of 1,547,792 shares were reserved for issuance under the Plan at December 31, 2014.
Under the terms of the Plan, options may be granted at an exercise price not less than fair market value. For employees holding more than 10% of the voting rights of all classes of stock, the exercise prices for incentive and nonstatutory stock
options may not be less than 110% of fair market value, as determined by the Board of Directors. The terms of options granted under the Plan may not exceed ten years. The vesting schedule of newly issued option grants is typically four years. The
following summarizes option activity under the Plan:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Shares
Available
for Grant |
|
|
Number
of Options |
|
|
Weighted-
Average
Exercise
Price
Per Option |
|
|
Aggregate
Intrinsic
Value |
|
| Balance, December 31, 2012 |
|
|
1,547,792 |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
|
| Options granted |
|
|
(929,999 |
) |
|
|
929,999 |
|
|
$ |
0.12 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2013 |
|
|
617,793 |
|
|
|
929,999 |
|
|
$ |
0.12 |
|
|
|
|
|
| Options granted |
|
|
(280,000 |
) |
|
|
280,000 |
|
|
$ |
0.12 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance Outstanding, December 31, 2014 |
|
|
337,793 |
|
|
|
1,209,999 |
|
|
$ |
0.12 |
|
|
$ |
60,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercisable, December 31, 2014 |
|
|
|
|
|
|
900,624 |
|
|
$ |
0.12 |
|
|
$ |
45,031 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vested and expected to vest, December 31, 2014 |
|
|
|
|
|
|
1,209,999 |
|
|
$ |
0.12 |
|
|
$ |
60,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The aggregate intrinsic values of options outstanding, exercisable, vested and expected to vest were calculated as the
difference between the exercise price of the options and the estimated fair value of the Company’s common stock, as determined by the Board of Directors, as of December 31, 2014.
The total grant date fair value of employee options that vested during the years ended December 31, 2014 and 2013 was $70,404 and $60,573, respectively.
The weighted-average grant date fair value of employee options granted during the year ended December 31, 2013 was $0.09 per option. There were no
employee options granted during the year ended December 31, 2014.
As of December 31, 2014, the weighted-average remaining contractual life was
8.92 years and 8.99 years for exercisable options and vested and expected to vest options, respectively.
Stock Options Granted to Employees
There were no stock options granted to employees during the year ended December 31, 2014. During the year ended December 31, 2013, the
Company granted employees stock options for 929,999 shares.
The fair value of stock option awards to employees was estimated at the date of grant using a
Black-Scholes option-pricing model with the following assumptions:
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
| |
|
2014 |
|
|
2013 |
| Expected term (in years) |
|
|
— |
|
|
5.00 – 6.02 |
| Volatility |
|
|
— |
|
|
95.47% – 97.62% |
| Risk-free interest rate |
|
|
— |
|
|
1.44% – 1.75% |
| Dividend yield |
|
|
— |
|
|
— |
F-57
In determining the fair value of the stock-based awards, the Company uses the Black-Scholes option-pricing model
and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
Fair
Value of Common Stock: The fair value of the shares of common stock underlying stock options has historically been determined by the Company’s board of directors. In order to determine the fair value of the common stock at the time of grant
of the option, the board of directors considered, among other things, valuations performed by an independent third-party. Because there has been no public market for the Company’s common stock, the board of directors exercised reasonable
judgment and considered a number of objective and subjective factors to determine the best estimate of the fair value of the Company’s common stock, including important developments in the Company’s operations, sales of convertible
preferred stock, actual operating results and financial performance, the conditions in the life sciences industry and the economy in general, the stock price performance and volatility of comparable public companies, and the lack of liquidity of the
Company’s common stock, among other factors.
Expected Term: The Company’s expected term represents the period that the
Company’s stock-based awards are expected to be outstanding and is determined using the simplified method (based on the mid-point between the vesting date and the end of the contractual term for employee options).
Expected Volatility: Since the Company is privately held and does not have any trading history for its common stock, the expected
volatility was estimated based on the average volatility for comparable publicly traded biotechnology companies over a period equal to the expected term of the stock option grants. The comparable companies were chosen based on their similar size, or
stage in the life cycle.
Risk-Free Interest Rate: The risk-free interest rate is based on the U.S. Treasury zero coupon issues in
effect at the time of grant for periods corresponding with the expected term of option.
Expected Dividend: The Company has never
paid dividends on its common stock and has no plans to pay dividends on its common stock. Therefore, the Company used an expected dividend yield of zero.
Stock Options Granted to Non-Employees
The
Company grants stock options to certain consultants in exchange for services rendered. During the year ended December 31, 2014, the Company granted consultants stock options for 280,000 shares. There were no stock options granted to
non-employees during the year ended December 31, 2013. Stock-based compensation expense related to stock options granted to non-employees is recognized as the stock options are earned and will fluctuate as the estimated fair value of the common
stock fluctuates until the awards vest. The Company believes that the estimated fair value of the stock options is more readily measurable than the fair value of the services rendered.
The fair value of stock option awards to non-employees was estimated at the date of grant using a Black-Scholes option-pricing model using similar assumptions
for employees except that the expected term is based on the options’ remaining contractual term instead of the simplified method. The following assumptions were used to fair value the stock options awards for the year ended December 31,
2014 for non-employees:
|
|
|
| Remaining contractual term (in years) |
|
8.75 – 9.67 |
| Volatility |
|
87.53% – 94.17% |
| Risk-free interest rate |
|
2.17% – 2.69% |
| Dividend yield |
|
— |
F-58
Stock-Based Compensation Expense
Total stock-based compensation recognized for options granted to employees and non-employees was as follows:
|
|
|
|
|
|
|
|
|
| |
|
Year Ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Research and development |
|
$ |
12,528 |
|
|
$ |
— |
|
| General and administrative |
|
|
14,231 |
|
|
|
60,685 |
|
|
|
|
|
|
|
|
|
|
| Total stock-based compensation expense |
|
$ |
26,759 |
|
|
$ |
60,685 |
|
|
|
|
|
|
|
|
|
|
As of December 31, 2014, the total unrecognized compensation expense related to unvested employee options, net of
estimated forfeitures, was $16,427, which the Company expects to recognize over an estimated weighted average period of 2.73 years.
7. Income Taxes
No provision for income taxes was recorded for the years ended December 31, 2014 and 2013. The Company has incurred net operating losses for all
the periods presented. The Company has not reflected any benefit of such net operating loss carryforwards in the accompanying consolidated financial statements. The Company has established a full valuation allowance against its deferred tax assets
due to the uncertainty surrounding the realization of such assets.
The effective tax rate of the provision for income taxes differs from the federal
statutory rate as follows:
|
|
|
|
|
|
|
|
|
| |
|
Year Ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Federal statutory income tax rate |
|
|
34.00 |
% |
|
|
34.00 |
% |
| State income taxes, net of federal benefit |
|
|
6.14 |
|
|
|
6.24 |
|
| Federal and state tax credits |
|
|
4.30 |
|
|
|
5.83 |
|
| Change in valuation allowance |
|
|
(44.19 |
) |
|
|
(43.48 |
) |
| Stock-based compensation |
|
|
(0.23 |
) |
|
|
(2.54 |
) |
| Other, net |
|
|
(0.02 |
) |
|
|
(0.05 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
— |
% |
|
|
— |
% |
|
|
|
|
|
|
|
|
|
The components of the deferred tax assets and liabilities are as follows:
|
|
|
|
|
|
|
|
|
| |
|
As of December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Deferred tax assets: |
|
|
|
|
|
|
|
|
| Net operating loss carryforwards |
|
$ |
4,871,000 |
|
|
$ |
4,149,000 |
|
| Tax credits |
|
|
426,000 |
|
|
|
323,000 |
|
| Depreciation and amortization |
|
|
990,000 |
|
|
|
1,156,000 |
|
| Accruals and reserves |
|
|
17,000 |
|
|
|
7,000 |
|
|
|
|
|
|
|
|
|
|
| Gross deferred tax assets |
|
|
6,304,000 |
|
|
|
5,635,000 |
|
| Valuation allowance |
|
|
(6,304,000 |
) |
|
|
(5,635,000 |
) |
|
|
|
|
|
|
|
|
|
| Net deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Due to the Company’s lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance as
of December 31, 2014 and 2013. The valuation allowance increased by $669,000 and $415,000 during the years ended December 31, 2014 and 2013, respectively.
F-59
As of December 31, 2014, the Company had approximately $12.2 million and $12.4 million, respectively, of
federal and state operating loss carryforwards available to reduce future taxable income that will begin to expire in 2030 for federal and state tax purposes.
As of December 31, 2014, the Company also had research and development tax credit carryforwards of approximately $184,522 and $212,238, respectively, for
federal and state purposes available to offset future taxable income tax. If not utilized, the federal carryforwards will expire in various amounts beginning in 2028, and the state credits can be carried forward indefinitely.
Utilization of the net operating loss carryforwards may be subject to a substantial annual limitation due to the ownership change limitations provided by the
Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization. An analysis to determine the limitation of the net operating loss
carryforwards has not been performed.
Uncertain Tax Positions
A reconciliation of the Company’s unrecognized tax benefits for the years ended December 31, 2014 and 2013 is as follows:
|
|
|
|
|
|
|
|
|
| |
|
Year Ended
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Balance at beginning of year |
|
$ |
97,000 |
|
|
$ |
95,000 |
|
| Additions based on tax positions related to current year |
|
|
2,000 |
|
|
|
2,000 |
|
|
|
|
|
|
|
|
|
|
| Balance at end of year |
|
$ |
99,000 |
|
|
$ |
97,000 |
|
|
|
|
|
|
|
|
|
|
The Company does not expect the unrecognized tax benefits to change significantly over the next 12 months.
Interest and penalties are zero, and the Company’s policy is to account for interest and penalties in tax expense on the statement of operations and
comprehensive loss. The Company files income tax returns in the U.S. federal and California tax jurisdictions. All periods since inception are subject to examination by U.S. federal and California tax jurisdictions.
8. Net Loss per Share
Basic net loss per share is
computed by dividing the net loss by the weighted-average number of common shares outstanding. Diluted net loss per share is computed similarly to basic net loss per share except that the denominator is increased to include the number of additional
common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Diluted net loss share is the same as basic net loss per common share, since the effects of potentially
dilutive securities are antidilutive.
As of December 31, 2014 and 2013, potentially dilutive securities include:
|
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
| |
|
2014 |
|
|
2013 |
|
| Convertible preferred stock |
|
|
17,358,845 |
|
|
|
14,035,555 |
|
| Options to purchase common stock |
|
|
1,209,999 |
|
|
|
929,999 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
18,568,844 |
|
|
|
14,965,554 |
|
|
|
|
|
|
|
|
|
|
F-60
9. Related Party Transactions
For the year ended December 31, 2013, the Company reimbursed its Chief Executive Officer $60,000 for the use of his private residence that was being used
as the Company’s primary office. There were no such reimbursements for the year ended December 31, 2014.
For the years ended December 31,
2014 and 2013, the Company paid consulting expenses to the Company’s founder, who is also a stockholder of Company, of approximately $44,550 and $30,740, respectively, which is included in research and development expenses.
As disclosed in Note 4, the Company entered into license agreements with EGI, which is owned by the founder of the Company.
10. Subsequent Events
The Company has evaluated, for
potential recognition and disclosure, events that occurred from the balance sheet date through December 11, 2015, the date the financial statements were available to be issued.
Lease Agreement
In March 2015, the Company
entered into a non-cancelable facility lease agreement for an office facility in Palo Alto, California. The lease commenced on April 1, 2015 and expires 36 months after the commencement date. The lease has one two year renewal option prior to
expiration and includes rent escalation clauses through the lease term.
Future aggregate minimum lease payments under the non-cancelable operating lease
is as follows:
|
|
|
|
|
| Year ending December 31, |
|
Amounts |
|
| 2015 |
|
$ |
81,248 |
|
| 2016 |
|
|
110,767 |
|
| 2017 |
|
|
114,090 |
|
| 2018 |
|
|
28,732 |
|
|
|
|
|
|
| Total |
|
$ |
334,837 |
|
|
|
|
|
|
Purchase of Intellectual Property
In September 2015, the Company entered into an asset purchase agreement with two individuals, Drs. Tracey McLaughlin and Colleen Craig, or the Sellers, whereby
the Company purchased all of the assets related to the compound exendin including any related intellectual property from the Sellers and also entered into a consulting agreement with the Sellers as part of the agreement. The Company issued 175,708
shares of common stock that were valued at $212,607 and 527,124 options to purchase common stock with an exercise price of $0.18 per share when the agreement was executed in September 2015. Of the 527,124 options to purchase shares of common stock,
175,708 shares vest monthly over four years as services are provided by the Sellers and 351,416 vest upon the earlier of the first commercial sale of the product or the approval of new drug application by the U.S. Food and Drug Administration.
Additionally, at the next equity financing round, including a reverse merger, each Seller will receive top-up options so that the Seller’s total options represent 1% of the total number of the Company’s issued and outstanding shares of
capital stock. The top-up options consist of both time-vested and milestone-vested options. The fair value of the time-vested options will be recognized as non-employee share-based compensation expense as the awards vest over time, with the unvested
portion revalued each period. The fair value of the milestone-vested options will be recognized as research and development expense when the earliest milestone is achieved.
F-61
The Company is also obligated to pay milestone payments to each Seller in aggregate up to $1.0 million.
Additionally, the Company is obligated to pay each of the Sellers royalties of low single digits based on aggregate annual net sales, subject to certain reductions and exceptions. The Company’s obligation to pay royalties expires on the
expiration of the last to expire patent assigned to the Company under the agreement. Additionally, the Company has assumed the license agreement the Sellers had previously entered into with the Board of Trustees of the Stanford. The Company is
obligated to pay a royalty to Stanford in the low single digits on annual net sales after the first commercial sale.
In October 2015, the Company entered
into an asset purchase agreement with Eiccose, LLC, or Eiccose, which is owned by the Company’s chief executive officer, whereby Eiccose sold all of the assets related to the treatment of pulmonary arterial hypertension, or PAH, treatment of
lymphedema and products containing ubenimex for the treatment of PAH including any related intellectual property from Eiccose. The Company made a payment to Eiccose of $119,673 representing reimbursement of certain previously incurred expenses,
including payments and accrued amounts owed to The Leland Stanford University in connection with the Lymphedema License Agreement and the PAH License Agreement. At the closing of the next round of financing pursuant to which the Company sells shares
of its preferred stock (or if there is no preferred stock, then common stock) resulting in gross proceeds to the Company of at least $25,000,000, the Company will issue to Eiccose that number of fully vested shares of the Company’s common stock
equal to 1.75% of the total number of the Company’s outstanding capital stock. The Company is obligated to pay to Eiccose an aggregate of $10.0 million in connection with future sales of commercial sale of the product and royalties in the low
single digits based on aggregate annual net sales following the first commercial sale of any product.
In addition, as a result of this agreement, the
Company has assumed the license agreements Eiccose had previously entered into. These include the license agreement with the Board of Trustees of the Leland Stanford Junior University, or Standford, for the treatment of PAH, the license agreement
with Stanford for the treatment of lymphedema and the license agreement with Nippon Kayaku Co., Ltd, or Nippon. Stanford is a holder of both Series A and Series A-1 preferred shares of the Company and is considered to be a related party. In
connection with the each license agreement with Stanford, the Company is obligated to make milestone payments in aggregate of $500,000 for each contract, increasing annual license maintenance fees ranging from $10,000 to $75,000 over the term of
each license agreement and royalty payments in low single digits on annual net sales after the first commercial sale. Additionally, as part of the agreement, Nippon is obligated to make a payment for royalties in the low single digits of sales to
the Company.
Equity Transactions
In February
2015, the Company’s Board of Directors amended the Company’s certificate of incorporation to increase the number of shares of common stock and preferred stock that the Company is authorized to issue by 3,000,000 shares and 3,000,000
shares, respectively, to a total of up to 27,400,000 shares of common stock and 20,787,500 shares of preferred stock, respectively.
In February 2015, the
Company issued 2,586,206 shares of Series A-1 preferred stock for proceeds of $1,499,999, net of issuance costs of $8,256.
In March 2015, the
Company’s Board of Directors amended the Company’s certificate of incorporation to increase the number of shares of common stock and preferred stock that the Company is authorized to issue by 10,000,000 shares and 10,000,000 shares,
respectively, to a total of up to 37,400,000 shares of common stock and 30,787,500 shares of preferred stock, respectively.
In March 2015, the Company
issued 9,865,899 shares of Series A-1 preferred stock for proceeds of $5,722,221, net of issuance costs of $13,310.
In May 2015, the Company granted
720,000 employee options with an exercise price of $0.12, vesting period of four years.
F-62
In September 2015, the Company’s Board of Directors increased the number of shares of common stock reserved
for issuance under the Plan by 2,320,000 shares to an aggregate of 3,867,792 shares.
In September 2015, the Company granted 1,185,726 employee and
527,124 non-employees options with an exercise price of $0.18 per share with a vesting period of four years.
In November 2015, the Company’s Board
of Directors amended the Company’s certificate of incorporation to increase the number of shares of common stock that the Company is authorized to issue by 30,600,000 shares, to a total of up to 68,000,000 shares of common stock.
Contract Manufacturing Arrangement
In September
2015, the Company began using a contract manufacturing organization for the production of its clinical trial materials and issued a non-cancelable purchase order to the contract manufacturer for $1.8 million. The Company has paid $0.6 million of
this commitment in November 2015 with the remaining balance to be paid upon delivery of the material, which is scheduled for January 2016.
Note and
Warrant Purchase Agreement
In November 2015, the Company entered into a note and warrant purchase agreement with three investors, including two
holders of the Company’s convertible preferred stock, which includes the issuance of notes payable in the aggregate principal amount of $6.0 million and the issuance of warrants to purchase equity securities. The notes bear interest of
6.0% per annum. The warrants to entitle each investor to purchase equity securities for a number of shares equal 15% of the principal borrowed from such investor, or 17.5% of the principal borrowed from such investor in the event that the
Company does not consummate a reverse merger with a third party then currently reporting under the Securities Act of 1933 and Exchange Act of 1934 by February 28, 2016, divided by the per share price of the equity securities sold in the
Company’s next equity financing that results in total proceeds to the Company of not less than $25.0 million, with an exercise price of $0.01 for each share purchased under the warrants. The warrants are exercisable for the type of equity
securities issued by the Company in a qualified financing as described in the notes, or if no qualified financing is consummated, then into shares of the Company’s common stock. All principal and interest is due March 31, 2016. However,
prior to March 31, 2016, the outstanding balance on the note plus unpaid accrued interest is automatically converted into common stock or preferred stock sold in the Company’s next equity financing that results in total proceeds to the
Company of not less than $25.0 million. In addition, in the event that 50% of the voting power of the Company’s stockholders is transferred in a transaction or series of transactions prior to March 31, 2016, the Company will repay the
investors 120% of the outstanding principal plus unpaid accrued interest upon such event.
Option Modification
In May 2015, the Company modified 759,999 fully vested employee and non-employee stock options, whereby the Company forgave the exercise price of $0.12 per
share.
Merger Agreement
The Company entered
into a definitive Merger Agreement with Celladon Corporation, or Celladon, on November 18, 2015, in which the stockholders of the Company would become the majority owners of Celladon and the operations of the two parties would be combined. The
proposed merger remains subject to certain conditions, including the approval of the Celladon stockholders. If approved, upon closing of the transaction, Celladon will be renamed Eiger BioPharmaceuticals, Inc.
F-63
Eiger BioPharmaceuticals, Inc.
Condensed Consolidated Balance Sheets
|
|
|
|
|
|
|
|
|
| |
|
September 30,
2015 |
|
|
December 31,
2014 |
|
| |
|
(Unaudited) |
|
|
|
|
| Assets |
|
| Current assets: |
|
|
|
|
|
|
|
|
| Cash |
|
$ |
2,136,130 |
|
|
$ |
776,797 |
|
| Prepaid expenses and other current assets |
|
|
332,907 |
|
|
|
31,999 |
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
2,469,037 |
|
|
|
808,796 |
|
| Property and equipment, net |
|
|
26,176 |
|
|
|
7,678 |
|
| Other assets |
|
|
20,724 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
2,515,937 |
|
|
$ |
816,474 |
|
|
|
|
|
|
|
|
|
|
| Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
433,904 |
|
|
$ |
59,267 |
|
| Accrued liabilities |
|
|
324,467 |
|
|
|
226,262 |
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
758,371 |
|
|
|
285,529 |
|
| Other liabilities |
|
|
1,641 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
760,012 |
|
|
|
285,529 |
|
|
|
|
|
|
|
|
|
|
| Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
| Stockholders’ equity |
|
|
|
|
|
|
|
|
| Convertible preferred stock, $0.0001 par value: 30,787,500 and 17,787,500 shares authorized as of September 30, 2015 (unaudited)
and December 31, 2014, respectively; 29,810,950 and 17,358,845 shares issued and outstanding as of September 30, 2015 (unaudited) and December 31, 2014, respectively; liquidation preference of $22,262,851 and $15,040,630 as of
September 30, 2015 and December 31, 2014, respectively |
|
|
22,566,940 |
|
|
|
15,366,286 |
|
| Common stock, $0.0001 par value, 37,400,000 and 24,400,000 shares authorized as of September 30, 2015 (unaudited) and
December 31, 2014, respectively; 3,060,665 and 2,214,958 shares issued and outstanding as of September 30, 2015 (unaudited) and December 31, 2014, respectively |
|
|
306 |
|
|
|
221 |
|
| Additional paid-in capital |
|
|
1,398,503 |
|
|
|
1,113,483 |
|
| Accumulated deficit |
|
|
(22,209,824 |
) |
|
|
(15,949,045 |
) |
|
|
|
|
|
|
|
|
|
| Total stockholders’ equity |
|
|
1,755,925 |
|
|
|
530,945 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities and stockholders’ equity |
|
$ |
2,515,937 |
|
|
$ |
816,474 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited interim condensed consolidated financial statements.
F-64
Eiger BioPharmaceuticals, Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
| Research and development |
|
$ |
4,492,792 |
|
|
$ |
319,974 |
|
| General and administrative |
|
|
1,767,987 |
|
|
|
402,637 |
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
6,260,779 |
|
|
|
722,611 |
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(6,260,779 |
) |
|
|
(722,611 |
) |
|
|
|
|
|
|
|
|
|
| Net loss and comprehensive loss |
|
$ |
(6,260,779 |
) |
|
$ |
(722,611 |
) |
|
|
|
|
|
|
|
|
|
| Net loss per share, basic and diluted |
|
$ |
(2.82 |
) |
|
$ |
(0.33 |
) |
|
|
|
|
|
|
|
|
|
| Shares used in computing net loss per share, basic and diluted |
|
|
2,222,561 |
|
|
|
2,214,958 |
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited interim condensed consolidated financial statements.
F-65
Eiger BioPharmaceuticals, Inc.
Condensed Consolidated Statements of Cash Flows
(Unaudited)
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
| Cash Flows From Operating Activities |
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(6,260,779 |
) |
|
$ |
(722,611 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Depreciation |
|
|
8,184 |
|
|
|
5,656 |
|
| Stock-based compensation |
|
|
72,498 |
|
|
|
4,498 |
|
| Issuance of common stock for a license and asset agreement |
|
|
212,607 |
|
|
|
— |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Prepaid expenses and other current assets |
|
|
(300,908 |
) |
|
|
4,388 |
|
| Other assets |
|
|
(20,724 |
) |
|
|
— |
|
| Accounts payable |
|
|
374,637 |
|
|
|
21,299 |
|
| Accrued liabilities |
|
|
98,205 |
|
|
|
56,785 |
|
| Other liabilities |
|
|
1,641 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Net cash used in operating activities |
|
|
(5,814,639 |
) |
|
|
(629,985 |
) |
|
|
|
|
|
|
|
|
|
| Cash Flows From Investing Activities |
|
|
|
|
|
|
|
|
| Purchase of property and equipment |
|
|
(26,682 |
) |
|
|
(1,349 |
) |
|
|
|
|
|
|
|
|
|
| Net cash used in investing activities |
|
|
(26,682 |
) |
|
|
(1,349 |
) |
|
|
|
|
|
|
|
|
|
| Cash Flows From Financing Activities |
|
|
|
|
|
|
|
|
| Proceeds from issuance of convertible preferred stock, net of issuance costs |
|
|
7,200,654 |
|
|
|
1,589,657 |
|
|
|
|
|
|
|
|
|
|
| Net cash provided by financing activities |
|
|
7,200,654 |
|
|
|
1,589,657 |
|
|
|
|
|
|
|
|
|
|
| Net increase in cash |
|
|
1,359,333 |
|
|
|
958,323 |
|
| Cash, beginning of period |
|
|
776,797 |
|
|
|
144,766 |
|
|
|
|
|
|
|
|
|
|
| Cash, end of period |
|
$ |
2,136,130 |
|
|
$ |
1,103,089 |
|
|
|
|
|
|
|
|
|
|
| Supplemental Disclosure of Non-Cash Financing Activities: |
|
|
|
|
|
|
|
|
| Issuance of common stock in connection with a license and asset agreement |
|
$ |
212,607 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited interim condensed consolidated financial statements.
F-66
Eiger Biopharmaceuticals, Inc.
Notes to Unaudited Interim Condensed Consolidated Financial Statements
| 1. |
Organization and Basis of Presentation |
Eiger BioPharmaceuticals, Inc. (the “Company”) was
incorporated in the State of Delaware on November 6, 2008. The Company is a clinical-stage biopharmaceutical company committed to bringing to market novel products for the treatment of orphan diseases. The Company has built a diverse portfolio
of well-characterized product candidates with the potential to address diseases for which the unmet medical need is high, the biology for treatment is clear, and for which an effective therapy is urgently needed. The Company’s principal
operations are based in Palo Alto, California and it operates in one segment.
Need for Additional Liquidity
The unaudited interim condensed consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and
satisfaction of liabilities in the normal course of business. In the course of its development activities, the Company has sustained operating losses and expects such losses to continue over the next several years. The Company’s ultimate
success depends on the outcome of its research and development activities. The Company has incurred net losses from operations since inception and has an accumulated deficit of $22.2 million as of September 30, 2015. The Company received $6.0
million in cash proceeds from a bridge financing completed in November 2015 (See Note 11). Management expects to incur additional losses in the future to conduct product research and development and recognizes the need to raise additional capital to
fully implement its business plan. The Company intends to raise additional capital through the issuance of additional equity, including in connection with the reverse merger discussed in Note 11, and potentially through borrowings, and strategic
alliances with partner companies. However, if such financing is not available timely and at adequate levels, the Company will need to reevaluate its operating plans. The failure to obtain sufficient funds on acceptable terms when needed could have a
material adverse effect on the Company’s business, results of operations, future cash flows and financial condition. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management is currently
pursuing financing alternatives. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis of Presentation and Consolidation
The
unaudited interim condensed consolidated financial statements include the accounts of Eiger BioPharmaceuticals, Inc. and its wholly owned subsidiaries, EB Pharma LLC and Eiger BioPharmaceuticals Europe Limited, and have been prepared in conformity
with accounting principles generally accepted in the United States, or U.S. GAAP. All intercompany balances and transactions have been eliminated in consolidation.
Unaudited Interim Consolidated Financial Statements
The interim condensed consolidated balance sheet as of September 30, 2015, and the condensed consolidated statements of operations and comprehensive loss
and cash flows for the nine months ended September 30, 2015 and 2014 are unaudited. The unaudited interim consolidated financial statements have been prepared on the same basis as the annual consolidated financial statements and reflect, in the
opinion of management, all adjustments of a normal and recurring nature that are necessary for the fair presentation of the Company’s financial position as of September 30, 2015 and its results of operations and cash flows for the nine
months ended September 30, 2015 and 2014. The results of operations for the nine months ended September 30, 2015 are not necessarily indicative of the results to be expected for the year ending December 31, 2015 or for any other
future annual or interim period. The condensed consolidated balance sheet as of December 31, 2014 included herein was derived from the audited consolidated financial statements as of that date. These condensed consolidated financial statements
should be read in conjunction with the Company’s audited consolidated financial statements included elsewhere in this prospectus.
F-67
| 2. |
Summary of Significant Accounting Policies |
Use of Estimates
The accompanying unaudited interim condensed consolidated financial statements have been prepared in accordance with U.S. GAAP. The preparation of consolidated
financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated
financial statements and the reported amounts of expenses during the reporting period. On an ongoing basis, the Company evaluates its estimates, including those related to clinical trial accrued liabilities, fair value of assets and liabilities and
common stock, income taxes, and stock-based compensation. The Company bases its estimates on historical experience and on various other market-specific and relevant assumptions that the Company believes to be reasonable under the circumstances.
Actual results could differ from those estimates.
Concentrations of Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consists of cash. The Company’s cash is held by a financial
institution in the United States. Amounts on deposit may at times exceed federally insured limits. Management believes that the financial institution is financially sound, and accordingly, minimal credit risk exists with respect to the financial
institution.
The Company relies on one major contract manufacturer organization to develop and commercialize pharmaceutical products for the treatment of
PAH. If the single source supplier fails to satisfy the Company’s requirements on a timely basis, it could suffer delays in its clinical development program, which could adversely affects its operating results.
Accrued Research and Development Costs
The
Company accrues for estimated costs of research and development activities conducted by third-party service providers, which include the conduct of preclinical and clinical studies, and contract manufacturing activities. The Company records the
estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced, and include these costs in accrued liabilities in the balance sheets and within research and development expense in the
statements of operations and comprehensive loss. These costs are a significant component of the Company’s research and development expenses. The Company accrues for these costs based on factors such as estimates of the work completed and in
accordance with agreements established with its third-party service providers. The Company makes significant judgments and estimates in determining the accrued liabilities balance in each reporting period. As actual costs become known, the Company
adjusts its accrued liabilities.
Fair Value Measurements
Fair value accounting is applied for all financial assets and liabilities that are recognized or disclosed at fair value in the consolidated financial
statements on a recurring basis (at least annually). Financial instruments include cash, accounts payable and accrued liabilities that approximate fair value due to their relatively short maturities.
Assets and liabilities recorded at fair value on a recurring basis in the balance sheets are categorized based upon the level of judgment associated with the
inputs used to measure their fair values. Fair value is defined as the exchange price that would be received for an asset or an exit price that would be paid to transfer a liability in the principal or most advantageous market for the asset or
liability in an orderly transaction between market participants on the measurement date. The authoritative guidance on fair value measurements establishes a three-tier fair value hierarchy for disclosure of fair value measurements as follows:
Level 1: Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date;
F-68
Level 2: Inputs are observable, unadjusted quoted prices in active markets for similar
assets or liabilities, unadjusted quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of
the related assets or liabilities; and
Level 3: Unobservable inputs that are significant to the measurement of the fair value of
the assets or liabilities that are supported by little or no market data.
As of September 30, 2015 and December 31, 2014, the Company does not
have any assets or liabilities recorded at fair value on a recurring basis.
Net Loss per Share
Basic net loss per share is calculated by dividing the net loss by the weighted average number of shares of common stock outstanding during the period without
consideration of common stock equivalents. Since the Company was in a loss position for all periods presented, diluted net loss per share is the same as basic net loss per share for all periods as the inclusion of all potential common shares
outstanding would have been anti-dilutive.
| 3. |
Balance Sheet Components |
Property and Equipment, Net
Property and equipment, net consist of the following:
|
|
|
|
|
|
|
|
|
| |
|
September 30, |
|
|
December 31, |
|
| |
|
2015 |
|
|
2014 |
|
| |
|
(Unaudited) |
|
|
|
|
| Lab equipment |
|
$ |
35,651 |
|
|
$ |
35,651 |
|
| Office equipment |
|
|
30,705 |
|
|
|
4,023 |
|
|
|
|
|
|
|
|
|
|
| Total property and equipment |
|
|
66,356 |
|
|
|
39,674 |
|
| Less: accumulated depreciation |
|
|
(40,180 |
) |
|
|
(31,996 |
) |
|
|
|
|
|
|
|
|
|
| Property and equipment, net |
|
$ |
26,176 |
|
|
$ |
7,678 |
|
|
|
|
|
|
|
|
|
|
Depreciation expense for the nine months ended September 30, 2015 and 2014 was $8,184 and $5,656, respectively.
Accrued Liabilities
Accrued liabilities consist
of the following:
|
|
|
|
|
|
|
|
|
| |
|
September 30, |
|
|
December 31, |
|
| |
|
2015 |
|
|
2014 |
|
| |
|
(Unaudited) |
|
|
|
|
| Accrued consulting costs |
|
$ |
4,141 |
|
|
$ |
100,000 |
|
| Accrued contract research costs |
|
|
203,286 |
|
|
|
80,515 |
|
| Accrued vacation |
|
|
62,327 |
|
|
|
25,673 |
|
| Accrued legal fees |
|
|
39,944 |
|
|
|
18,324 |
|
| Accrued other |
|
|
14,769 |
|
|
|
1,750 |
|
|
|
|
|
|
|
|
|
|
| Total accrued liabilities |
|
$ |
324,467 |
|
|
$ |
226,262 |
|
|
|
|
|
|
|
|
|
|
F-69
Merck License Agreement
In September 2010, the Company entered into an exclusive license agreement with Schering Corporation, subsequently acquired by Merck, which provides the
Company with the exclusive right to develop and commercialize Sarasar/Lonafarnib. As consideration for such exclusive right, the Company issued 312,500 shares of Series A convertible preferred stock with a fair value of $500,000 when the agreement
was executed in September 2010.
During the nine months ended September 30, 2015, the first regulatory milestone under the arrangement was achieved
and the Company paid the related milestone payment of $1.0 million to Merck. As of September 30, 2015, the Company is obligated to pay Merck up to an aggregate of $26.0 million in development milestones and will be required to pay tiered
royalties based on aggregate annual net sales of all licensed products ranging from mid-single to low double-digit royalties on net sales.
| 5. |
Asset Purchase Agreement |
In September 2015, the Company entered into an asset purchase agreement with
two individuals, Drs. Tracey McLaughlin and Colleen Craig, or Sellers, whereby the Company purchased all of the assets related to the compound exendin including any related intellectual property from the Sellers and also entered into a consulting
agreement with the Sellers as part of the agreement. The Company issued 175,708 shares of common stock that were valued at $212,607 and 527,124 options to purchase common stock with an exercise price of $0.18 per share when the agreement was
executed in September 2015. Of the 527,124 options to purchase common stock, 175,708 shares vest monthly over four years as services are provided by the Sellers and 351,416 vest upon the earlier of the first commercial sale of the product or the
approval of new drug application by the U.S. Food and Drug Administration. Additionally, at the next equity financing round, including a reverse merger, each Seller will receive top-up options so that the Seller’s total options represent 1% of
the total number of the Company’s issued and outstanding shares of capital stock. The top-up options consist of both time-vested and milestone-vested options. The fair value of the time-vested options will be recognized as non-employee
share-based compensation expense as the awards vest over time, with the unvested portion revalued each period. The fair value of the milestone-vested options will be recognized as research and development expense when the earliest milestone is
achieved. The Company is also obligated to pay milestone payments in aggregate up to $1.0 million to each Seller. Additionally, the Company is obligated to pay each Seller royalties of low single digits based on aggregate annual net sales, subject
to certain reductions and exceptions. The Company’s obligation to pay royalties expires on the expiration of the last to expire patent assigned to the Company under the agreement. Additionally, the Company has assumed the license agreement the
Sellers had previously entered into with the Board of Trustees of the Leland Stanford Junior University, Stanford. The Company is obligated to pay a royalty to Stanford in the low single digits on annual net sales after the first commercial sale.
Convertible Preferred Stock
In February and March 2015, the Company’s Board of Directors amended the Company’s certificate of incorporation to increase the number of shares of
common stock and preferred stock that the Company is authorized to issue by a total of 13,000,000 shares and 13,000,000 shares, respectively, to a total of up to 37,400,000 shares of common stock and 30,787,500 shares of preferred stock.
As of September 30, 2015 (unaudited), convertible preferred stock consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| September 30, 2015 |
|
Shares
Authorized |
|
|
Shares
Outstanding |
|
|
Issuance Price
per Share |
|
|
Carrying
Values |
|
|
Liquidation
Preference |
|
| Series A |
|
|
5,187,500 |
|
|
|
4,875,000 |
|
|
$ |
1.60 |
|
|
$ |
7,668,451 |
|
|
$ |
7,800,000 |
|
| Series A-1 |
|
|
25,600,000 |
|
|
|
24,935,950 |
|
|
$ |
0.58 |
|
|
|
14,898,489 |
|
|
|
14,462,851 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
30,787,500 |
|
|
|
29,810,950 |
|
|
|
|
|
|
$ |
22,566,940 |
|
|
$ |
22,262,851 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-70
As of December 31, 2014, convertible preferred stock consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| December 31, 2014 |
|
Shares
Authorized |
|
|
Shares
Outstanding |
|
|
Issuance Price
per Share |
|
|
Carrying
Values |
|
|
Liquidation
Preference |
|
| Series A |
|
|
5,187,500 |
|
|
|
4,875,000 |
|
|
$ |
1.60 |
|
|
$ |
7,668,451 |
|
|
$ |
7,800,000 |
|
| Series A-1 |
|
|
12,600,000 |
|
|
|
12,483,845 |
|
|
$ |
0.58 |
|
|
|
7,697,835 |
|
|
|
7,240,630 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17,787,500 |
|
|
|
17,358,845 |
|
|
|
|
|
|
$ |
15,366,286 |
|
|
$ |
15,040,630 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There were no changes to the significant provisions of the Series A Preferred Stock and Series A-1 Preferred Stock since
December 31, 2014.
Common Stock
Common
stockholders are entitled to dividends when, as and if declared by the Board of Directors, subject to the prior rights of the preferred stockholders. As of September 30, 2015, no dividends had been declared by the Board of Directors.
As of September 30, 2015, the Company had reserved shares of common stock for issuance as follows (unaudited):
|
|
|
|
|
| Convertible preferred stock, on as-converted basis |
|
|
29,810,950 |
|
| Options issued and outstanding |
|
|
2,972,860 |
|
| Options available for future grants |
|
|
224,933 |
|
|
|
|
|
|
| Total |
|
|
33,008,743 |
|
|
|
|
|
|
In September 2015, the Company’s Board of Directors increased the number of
shares of common stock reserved for issuance under the 2009 Equity Incentive Plan (the “Plan”) by 2,320,000 shares to an aggregate of 3,867,792 shares.
The following summarizes option activity under the Plan:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Shares
Available
for Grant |
|
|
Number
of Options |
|
|
Weighted-
Average
Exercise
Price
Per
Option |
|
|
Aggregate
Intrinsic
Value |
|
| Balance Outstanding, December 31, 2014 |
|
|
337,793 |
|
|
|
1,209,999 |
|
|
$ |
0.12 |
|
|
|
|
|
| Additional shares authorized (unaudited) |
|
|
2,320,000 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
| Options granted (unaudited) |
|
|
(2,432,860 |
) |
|
|
2,432,860 |
|
|
$ |
0.18 |
|
|
|
|
|
| Options exercised (unaudited) |
|
|
— |
|
|
|
(669,999 |
) |
|
$ |
0.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance Outstanding, September 30, 2015 (unaudited) |
|
|
224,933 |
|
|
|
2,972,860 |
|
|
$ |
0.17 |
|
|
$ |
3,094,446 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercisable, September 30, 2015 (unaudited) |
|
|
|
|
|
|
315,000 |
|
|
$ |
0.12 |
|
|
$ |
343,350 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vested and expected to vest, September 30, 2015 (unaudited) |
|
|
|
|
|
|
2,972,860 |
|
|
$ |
0.17 |
|
|
$ |
3,094,446 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-71
The aggregate intrinsic values of options outstanding, exercisable, vested and expected to vest were calculated
as the difference between the exercise price of the options and the estimated fair value of the Company’s common stock, as determined by the Board of Directors, as of September 30, 2015.
The total grant date fair value of employee options that vested during the nine months ended September 30, 2015 and 2014 was $18,876 and $65,084,
respectively.
The weighted-average grant date fair value of employee options granted during the nine months ended September 30, 2015 was $1.10 per
option. There were no employee option grants during the nine months ended September 30, 2014.
As of September 30, 2015, the weighted-average
remaining contractual life was 8.65 years and 9.72 years for exercisable options and vested and expected to vest options, respectively.
Option
Modification
In May 2015, the Company modified 759,999 fully vested employee and non-employee stock options, whereby the Company forgave the
exercise price of $0.12 per share. As a result of this modification, the Company recognized incremental expense related to stock-based compensation of $43,983 during the nine months ended September 30, 2015.
Stock Options Granted to Employees
The fair value
of stock option awards to employees was estimated at the date of grant using a Black-Scholes option-pricing model with the following assumptions:
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
|
| |
|
2015 |
|
2014
|
|
| |
|
(Unaudited) |
|
| Expected term (in years) |
|
5.00 – 6.08 |
|
|
— |
|
| Volatility |
|
77.58% – 97.62% |
|
|
— |
|
| Risk-free interest rate |
|
1.44% – 1.75% |
|
|
— |
|
| Dividend yield |
|
— |
|
|
— |
|
Stock Options Granted to Non-Employees
The Company grants stock options to certain consultants in exchange for services rendered. During the nine months ended September 30, 2015, the Company
granted consultants stock options for 527,124 shares. There were no stock options granted to non-employees during the nine months ended September 30, 2014. Stock-based compensation expense related to stock options granted to non-employees is
recognized as the stock options are earned and will fluctuate as the estimated fair value of the common stock fluctuates until the awards vest. The Company believes that the estimated fair value of the stock options is more readily measurable than
the fair value of the services rendered.
The fair value of stock option awards to non-employees was estimated at the date of grant using a Black-Scholes
option-pricing model with the following assumptions:
|
|
|
|
|
|
|
| |
|
Nine Months Ended September 30, |
|
| |
|
2015 |
|
2014
|
|
| |
|
(Unaudited) |
|
| Expected term (in years) |
|
8.00 – 10.00 |
|
|
— |
|
| Volatility |
|
86.16% – 89.32% |
|
|
— |
|
| Risk-free interest rate |
|
1.73% – 2.23% |
|
|
— |
|
| Dividend yield |
|
— |
|
|
— |
|
F-72
Stock-Based Compensation Expense
Total stock-based compensation recognized for options granted to employees and non-employees was as follows:
|
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
| |
|
(Unaudited) |
|
| Research and development |
|
$ |
11,854 |
|
|
$ |
— |
|
| General and administrative |
|
|
60,644 |
|
|
|
4,498 |
|
|
|
|
|
|
|
|
|
|
| Total stock-based compensation expense |
|
$ |
72,498 |
|
|
$ |
4,498 |
|
|
|
|
|
|
|
|
|
|
As of September 30, 2015, the total unrecognized compensation expense related to unvested employee options, net of
estimated forfeitures, was $2.1 million, which the Company expects to recognize over an estimated weighted average period of 3.85 years.
Basic net loss per share is computed by dividing the net loss by the
weighted-average number of common shares outstanding. Diluted net loss per share is computed similarly to basic net loss per share except that the denominator is increased to include the number of additional common shares that would have been
outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Diluted net loss share is the same as basic net loss per common share, since the effects of potentially dilutive securities are
antidilutive.
As of September 30, 2015 and 2014, potentially dilutive securities include:
|
|
|
|
|
|
|
|
|
| |
|
September 30, |
|
| |
|
2015 |
|
|
2014 |
|
| |
|
(Unaudited) |
|
| Convertible preferred stock |
|
|
29,810,950 |
|
|
|
16,794,177 |
|
| Options to purchase common stock |
|
|
2,972,860 |
|
|
|
929,999 |
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
32,783,810 |
|
|
|
17,724,176 |
|
|
|
|
|
|
|
|
|
|
| 9. |
Related Party Transactions |
In connection with the license agreement the Company holds with Stanford,
Stanford owns Series A and Series A-1 preferred shares of the Company. As of September 30, 2015, the Company owed $14,149 to Stanford, which is recorded in accounts payable.
For the nine months ended September 30, 2015 and 2014, the Company paid consulting expenses to the Company’s founder, who is also a stockholder of
Company, of $1,159 and $1,339, respectively, which is included in research and development expenses.
| 10. |
Commitments and Contingencies |
Lease Agreement
In March 2015, the Company entered into a non-cancelable facility lease agreement for an office facility in Palo Alto, California. The lease commenced on
April 1, 2015 and expires 36 months after the commencement date. The lease has one two year renewal option prior to expiration and includes rent escalation clauses through the lease term. Rent increases are recognized as deferred rent and are
amortized on a straight-line basis over the term
F-73
of the lease. The Company has provided a security deposit of $20,724 as collateral for the lease, which is included in other assets in the Company’s consolidated balance sheet as of
September 30, 2015.
Future aggregate minimum lease payments under the non-cancelable operating leases are as follows:
|
|
|
|
|
| Year ending December 31, |
|
Amounts |
|
| 2015 (remaining three months) |
|
$ |
27,083 |
|
| 2016 |
|
|
110,767 |
|
| 2017 |
|
|
114,090 |
|
| 2018 |
|
|
28,732 |
|
|
|
|
|
|
| Total |
|
$ |
280,672 |
|
|
|
|
|
|
Rent expense was $97,432 and $31,500 for the nine months ended September 30, 2015 and 2014, respectively.
Contract Manufacturing Arrangement
In September
2015, the Company began using a contract manufacturing organization for the production of its clinical trial materials and issued a non-cancelable purchase order to the contract manufacturer for $1.8 million. The Company has paid $0.6 million of
this commitment in November 2015 with the remaining balance to be paid upon delivery of the material, which is scheduled for January 2016.
The Company has evaluated, for potential recognition and disclosure, events that
occurred from the balance sheet date through December 11, 2015, the date the financial statements were available to be issued.
Asset Purchase
Agreement
In October 2015, the Company entered into an asset purchase agreement with Eiccose, LLC., or Eiccose, which is owned by the
Company’s chief executive officer, whereby Eiccose sold all of the assets related to the treatment of pulmonary arterial hypertension, or PAH, treatment of lymphedema and products containing ubenimex for the treatment of PAH including any
related intellectual property from Eiccose. The Company made a payment to Eiccose of $119,673 representing reimbursement of certain previously incurred expenses, including payments and accrued amounts owed to the Board of Trustees of the Stanford in
connection with the Lymphedema License Agreement and the PAH License Agreement. At the closing of the next round of financing pursuant to which the Company sells shares of its preferred stock (or if there is no preferred stock, then common stock)
resulting in gross proceeds to the Company of at least $25,000,000, the Company will issue to Eiccose that number of fully vested shares of the Company’s common stock equal to 1.75% of the total number of the Company’s outstanding capital
stock. The Company is obligated to pay to Eiccose an aggregate of $10.0 million in connection with future sales of commercial sale of the product and royalties in the low single digits based on aggregate annual net sales following the first
commercial sale of any product.
In addition, as a result of this agreement, the Company has assumed the license agreements Eiccose had previously entered
into. These include the license agreement with Stanford for the treatment of PAH, the license agreement with Stanford for the treatment of lymphedema and the license agreement with Nippon Kayaku Co., Ltd, or Nippon. Stanford is a holder of both
Series A and Series A-1 preferred shares of the Company and is considered to be a related party. In connection with the each license agreement with Stanford, the Company is obligated to make milestone payments in aggregate of $500,000 for each
contract, increasing annual license maintenance fees ranging from $10,000 to $75,000 over the term of each license agreement and royalty payments in low single digits on annual net sales after the first commercial sale of a product under each
license.
F-74
Additionally, as part of the agreement, Nippon is obligated to make a payment for royalties in the low single digits of sales to the Company.
Note and Warrant Purchase Agreement
In November 2015,
the Company entered into a note and warrant purchase agreement with three investors, including two holders of the Company’s convertible preferred stock, which includes the issuance of notes payable in the aggregate principal amount of $6.0
million and the issuance of warrants to purchase equity securities. The warrants entitle each investor to purchase equity securities for a number of shares equal 15% of the principal borrowed from such investor, or 17.5% of the principal borrowed
from such investor in the event that the Company does not consummate a reverse merger with a third party then currently reporting under the Securities Act of 1933 and Exchange Act of 1934 by February 28, 2016, divided by the per share price of
the equity securities sold in the Company’s next equity financing that results in total proceeds to the Company of not less than $25.0 million, with an exercise price of $0.01 for each share of equity securities purchased under the warrants.
The warrants are exercisable for the type of equity securities issued by the Company in a qualified financing as described in the notes, or if no qualified financing is consummated, then into shares of the Company’s common stock. All principal
and simple interest of 6.0% per annum is due March 31, 2016. However, prior to March 31, 2016, the outstanding balance on the note plus unpaid accrued interest is automatically converted into common stock or preferred stock sold in
the Company’s next equity financing that results in total proceeds to the Company of not less than $25.0 million. In addition, in the event that 50% of the voting power of the Company’s stockholders is transferred in a transaction or
series of transactions prior to March 31, 2016, the Company will repay the investors 120% of the outstanding principal plus unpaid accrued interest upon such event.
Merger Agreement
The Company entered into a
definitive Merger Agreement with Celladon Corporation, or Celladon, on November 18, 2015, in which the stockholders of the Company would become the majority owners of Celladon and the operations of the two parties would be combined. The
proposed merger remains subject to certain conditions, including the approval of the Celladon stockholders. If approved, upon closing of the transaction, Celladon will be renamed Eiger BioPharmaceuticals, Inc.
Equity Transactions
In November 2015, the
Company’s Board of Directors amended the Company’s certificate of incorporation to increase the number of shares of common stock that the Company is authorized to be issued by 30,600,000 shares, to a total of up to 68,000,000 shares of
common stock.
F-75
Annex A
EXECUTION VERSION
AGREEMENT
AND PLAN OF MERGER AND REORGANIZATION
THIS AGREEMENT AND PLAN OF MERGER AND REORGANIZATION (this
“Agreement”) is made and entered into as of November 18, 2015, by and among CELLADON CORPORATION, a Delaware corporation (“Celladon”), CELLADON MERGER SUB,
INC., a Delaware corporation (“Merger Sub”), and EIGER BIOPHARMACEUTICALS, INC., a Delaware corporation (“Eiger”). Certain capitalized terms used in this
Agreement are defined in Exhibit A.
RECITALS
A. Celladon and Eiger intend to effect a merger of Merger Sub into Eiger (the “Merger”) in accordance
with this Agreement and the DGCL. Upon consummation of the Merger, Merger Sub will cease to exist, and Eiger will become a wholly-owned subsidiary of Celladon.
B. The Parties intend, by approving resolutions authorizing this Agreement, to adopt this Agreement as a plan of reorganization within the
meaning of Section 368(a) of the Code, and to cause the Merger to qualify as a reorganization under the provisions of Section 368(a) of the Code and the Treasury Regulations promulgated thereunder.
C. The Board of Directors of Celladon (i) has determined that the Merger is fair to, and in the best interests of, Celladon and its
stockholders, (ii) has deemed advisable and approved this Agreement, the Merger, the issuance of shares of Celladon Common Stock to the stockholders of Eiger pursuant to the terms of this Agreement, the change of control of Celladon, and the
other actions contemplated by this Agreement, (iii) has approved the Reverse Split; and (iv) has determined to recommend that the stockholders of Celladon vote to approve the issuance of shares of Celladon Common Stock to the stockholders
of Eiger pursuant to the terms of this Agreement, the change of control of Celladon, the Reverse Split and such other actions as contemplated by this Agreement.
D. The Board of Directors of Merger Sub (i) has determined that the Merger is fair to, and in the best interests of, Merger Sub and its
sole stockholder, (ii) has deemed advisable and approved this Agreement, the Merger, and the other actions contemplated by this Agreement, and (iii) has determined to recommend that the stockholder of Merger Sub vote to adopt this
Agreement and thereby approve the Merger and such other actions as contemplated by this Agreement.
E. The Board of Directors of Eiger
(i) has determined that the Merger is advisable and fair to, and in the best interests of, Eiger and its stockholders, (ii) has deemed advisable and approved this Agreement, the Merger and the other transactions contemplated by this
Agreement, and (iii) has determined to recommend that the stockholders of Eiger vote to adopt this Agreement and thereby approve the Merger and such other actions as contemplated by this Agreement.
F. In order to induce Celladon to enter into this Agreement and to cause the Merger to be consummated, the officers, directors
and stockholders of Eiger listed on Schedule A hereto are executing concurrently with the execution and delivery of this Agreement support agreements in favor of Celladon in the form substantially attached hereto as Exhibit B (the
“Eiger Stockholder Support Agreements”).
G. In order to induce Eiger to enter into
this Agreement and to cause the Merger to be consummated, the officers and directors of Celladon listed on Schedule B hereto are executing support agreements in favor of Eiger
A-1
concurrently with the execution and delivery of this Agreement in the form substantially attached hereto as Exhibit C (the “Celladon Stockholder Support
Agreements”).
H. It is expected that within two (2) Business Days after the Form S-4
Registration Statement is declared effective under the Securities Act, the holders of shares of Eiger Capital Stock sufficient to adopt and approve this Agreement and the Merger as required under the DGCL and Eiger’s Certificate of
Incorporation and Bylaws will execute and deliver an action by written consent adopting this Agreement, in a form reasonably acceptable to Celladon, in order to obtain the Required Eiger Stockholder Vote (each, an “Eiger Stockholder
Written Consent” and collectively, the “Eiger Stockholder Written Consents”).
I.
Immediately prior to the execution and delivery of this Agreement, and as a condition of the willingness of Celladon to enter into this Agreement, certain investors have executed the Subscription Agreement with Eiger pursuant to which such investors
have agreed to purchase certain shares of Eiger Common Stock prior to the Closing in connection with the Eiger Pre-Closing Financing.
AGREEMENT
The parties to
this Agreement, intending to be legally bound, agree as follows:
| |
Section 1. |
DESCRIPTION OF TRANSACTION |
1.1 Structure of the Merger. Upon the terms and
subject to the conditions set forth in this Agreement, at the Effective Time, Merger Sub shall be merged with and into Eiger, and the separate existence of Merger Sub shall cease. Eiger will continue as the surviving corporation in the Merger (the
“Surviving Corporation”).
1.2 Effects of the Merger. The Merger shall have the effects set forth in
this Agreement and in the applicable provisions of the DGCL. As a result of the Merger, Eiger will become a wholly-owned subsidiary of Celladon.
1.3 Closing; Effective Time. Unless this Agreement is earlier terminated pursuant to the provisions of Section 9.1, and
subject to the satisfaction or waiver of the conditions set forth in Sections 6, 7 and 8, the consummation of the Merger (the “Closing”) shall take place at the offices of Pillsbury Winthrop Shaw Pittman
LLP, 12255 El Camino Real, Suite 300, San Diego, California, as promptly as practicable (but in no event later than the second Business Day following the satisfaction or waiver of the last to be satisfied or waived of the conditions set forth in
Sections 6, 7 and 8, other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of each of such conditions), or at such other time, date and place as Celladon
and Eiger may mutually agree in writing. The date on which the Closing actually takes place is referred to as the “Closing Date.” At the Closing, the Parties hereto shall cause the Merger to be consummated by executing and
filing with the Secretary of State of the State of Delaware a Certificate of Merger with respect to the Merger, satisfying the applicable requirements of the DGCL and in a form reasonably acceptable to Celladon and Eiger (the “Certificate
of Merger”). The Merger shall become effective at the time of the filing of such Certificate of Merger with the Secretary of State of the State of Delaware or at such later time as may be specified in such Certificate of Merger with the
consent of Celladon and Eiger (the time as of which the Merger becomes effective being referred to as the “Effective Time”).
1.4 Certificate of Incorporation and Bylaws; Directors and Officers. At the Effective Time:
(a) the Certificate of Incorporation of the Surviving Corporation shall be amended and restated in its entirety to read identically to
the Certificate of Incorporation of Merger Sub as in effect immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such Certificate of Incorporation;
A-2
(b) the Certificate of Incorporation of Celladon shall be the Certificate of Incorporation
of Celladon immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such Certificate of Incorporation; provided, however, that at the Effective Time, Celladon shall file one or more amendments to
its Certificate of Incorporation to (i) change the name of Celladon to “Eiger Biopharmaceuticals, Inc.,” (ii) effect the Reverse Split to the extent applicable, and (iii) make such other changes as are mutually agreeable to
Celladon and Eiger;
(c) the Bylaws of the Surviving Corporation shall be amended and restated in their entirety to read
identically to the Bylaws of Merger Sub as in effect immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such Bylaws;
(d) the directors and officers of Celladon, each to hold office in accordance with the Certificate of Incorporation and Bylaws of
Celladon, shall be as set forth in Section 5.14; and
(e) the directors and officers of the Surviving Corporation, each
to hold office in accordance with the Certificate of Incorporation and Bylaws of the Surviving Corporation, shall be the directors and officers of Celladon as set forth in Section 5.14, after giving effect to the provisions of
Section 5.14.
1.5 Conversion of Shares and Issuance of Warrants.
(a) At the Effective Time, by virtue of the Merger and without any further action on the part of Celladon, Merger Sub, Eiger or any
stockholder of Eiger:
(i) any shares of Eiger Common Stock or Eiger Preferred Stock held as treasury stock or held or owned by
Eiger, Merger Sub or any Subsidiary of Eiger immediately prior to the Effective Time shall be canceled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor; and
(ii) subject to Section 1.5(c), each share of Eiger Common Stock outstanding immediately prior to the Effective Time
(excluding shares to be canceled pursuant to Section 1.5(a)(i) and excluding Dissenting Shares) shall be converted solely into the right to receive a number of shares of Celladon Common Stock equal to the Exchange Ratio.
(b) If any shares of Eiger Common Stock outstanding immediately prior to the Effective Time are unvested or are subject to a repurchase
option or the risk of forfeiture under any applicable restricted stock purchase agreement or other agreement with Eiger, then the shares of Celladon Common Stock issued in exchange for such shares of Eiger Common Stock will to the same extent be
unvested and subject to the same repurchase option or risk of forfeiture, and the certificates representing such shares of Celladon Common Stock shall accordingly be marked with appropriate legends. Eiger shall take all actions that may be necessary
to ensure that, from and after the Effective Time, Celladon is entitled to exercise any such repurchase option or other right set forth in any such restricted stock purchase agreement or other agreement.
(c) No fractional shares of Celladon Common Stock shall be issued in connection with the Merger, and no certificates or scrip for any
such fractional shares shall be issued. Any holder of Eiger Common Stock who would otherwise be entitled to receive a fraction of a share of Celladon Common Stock (after aggregating all fractional shares of Celladon Common Stock issuable to such
holder) shall, in lieu of such fraction of a share and upon surrender by such holder of a letter of transmittal in accordance with Section 1.8 and accompanying documents as required therein, be paid in cash the dollar amount (rounded to
the nearest whole cent), without interest, determined by multiplying such fraction by the closing price of a share of Celladon Common Stock on The NASDAQ Global Market (or such other NASDAQ market on which the Celladon Common Stock then trades) on
the date the Merger becomes effective.
(d) All Eiger Options outstanding immediately prior to the Effective Time under the 2009
Plan and all Eiger Warrants outstanding immediately prior to the Effective Time shall be exchanged for options to purchase Celladon Common Stock or warrants to purchase Celladon Common Stock, as applicable, in accordance with
Section 5.5.
A-3
(e) Each share of Common Stock, $0.001 par value per share, of Merger Sub issued and
outstanding immediately prior to the Effective Time shall be converted into and exchanged for one validly issued, fully paid and nonassessable share of Common Stock, $0.001 par value per share, of the Surviving Corporation. Each stock certificate of
Merger Sub evidencing ownership of any such shares shall, as of the Effective Time, evidence ownership of such shares of Common Stock of the Surviving Corporation.
(f) If, between the date of this Agreement and the Effective Time, the outstanding shares of Eiger Capital Stock or Celladon Common
Stock shall have been changed into, or exchanged for, a different number of shares or a different class, by reason of any stock dividend, subdivision, reclassification, recapitalization, split (including the Reverse Split), combination or exchange
of shares, the Exchange Ratio shall be correspondingly adjusted to provide the holders of Eiger Common Stock, Eiger Preferred Stock, Eiger Options and Eiger Warrants the same economic effect as contemplated by this Agreement prior to such event.
1.6 Calculation of Net Cash
(a) For the purposes of this Agreement, the “Determination Date” shall be the date that is ten
(10) calendar days prior to the anticipated date for Closing, as agreed upon by Celladon and Eiger at least ten (10) calendar days prior to the Celladon Stockholders’ Meeting (the “Anticipated Closing Date”).
Within five (5) calendar days following the Determination Date, Celladon shall deliver to Eiger a schedule (the “Net Cash Schedule”) setting forth, in reasonable detail, Celladon’s good faith, estimated calculation
of Net Cash (using an estimate of Celladon’s accounts payable and accrued expenses, in each case as of the Anticipated Closing Date and determined in a manner substantially consistent with the manner in which such items were determined for
Celladon’s most recent SEC filings) (the “Net Cash Calculation”) as of the Anticipated Closing Date prepared and certified by Celladon’s Chief Financial Officer (or if there is no Chief Financial Officer, the
principal accounting officer for Celladon). Celladon shall make the work papers and back-up materials used or useful in preparing the Net Cash Schedule, as reasonably requested by Eiger, available to Eiger and, if requested by Eiger, its accountants
and counsel at reasonable times and upon reasonable notice.
(b) Within three (3) calendar days after Celladon delivers the Net Cash
Schedule (the “Response Date”), Eiger shall have the right to dispute any part of such Net Cash Schedule by delivering a written notice to that effect to Celladon (a “Dispute Notice”). Any Dispute
Notice shall identify in reasonable detail the nature of any proposed revisions to the Net Cash Calculation.
(c) If on or prior to the
Response Date, (i) Eiger notifies Celladon in writing that it has no objections to the Net Cash Calculation or (ii) Eiger fails to deliver a Dispute Notice as provided in Section 1.6(b), then the Net Cash Calculation as set
forth in the Net Cash Schedule shall be deemed to have been finally determined for purposes of this Agreement and to represent the Net Cash at the Determination Date for purposes of this Agreement.
(d) If Eiger delivers a Dispute Notice on or prior to the Response Date, then Representatives of Celladon and Eiger shall promptly meet and
attempt in good faith to resolve the disputed item(s) and negotiate an agreed-upon determination of Net Cash, which agreed upon Net Cash amount shall be deemed to have been finally determined for purposes of this Agreement and to represent the Net
Cash at the Determination Date for purposes of this Agreement.
(e) If Representatives of Celladon and Eiger are unable to negotiate an
agreed-upon determination of Net Cash at the Determination Date pursuant to Section 1.6(d) within three (3) calendar days after delivery of the Dispute Notice (or such other period as Celladon and Eiger may mutually agree upon),
then Celladon and Eiger shall jointly select an independent auditor of recognized national standing (the “Accounting Firm”) to resolve any remaining disagreements as to the Net Cash Calculation. Celladon shall promptly
deliver to the Accounting Firm the work papers and back-up materials used in preparing the Net Cash Schedule, and Celladon and Eiger shall use commercially reasonable efforts to cause the Accounting Firm to make its determination within ten
A-4
(10) calendar days of accepting its selection. Eiger and Celladon shall be afforded the opportunity to present to the Accounting Firm any material related to the unresolved disputes and to
discuss the issues with the Accounting Firm; provided, however, that no such presentation or discussion shall occur without the presence of a Representative of each of Eiger and Celladon. The determination of the Accounting Firm shall be
limited to the disagreements submitted to the Accounting Firm. The determination of the amount of Net Cash made by the Accounting Firm shall be deemed to have been finally determined for purposes of this Agreement and to represent the Net Cash at
the Determination Date for purposes of this Agreement, and the Parties shall delay the Closing until the resolution of the matters described in this Section 1.6(e). The fees and expenses of the Accounting Firm shall be allocated between
Celladon and Eiger in the same proportion that the disputed amount of the Net Cash that was unsuccessfully disputed by such Party (as finally determined by the Accounting Firm) bears to the total disputed amount of the Net Cash amount (and for the
avoidance of doubt the fees and expenses to be paid by Celladon shall reduce the Net Cash). If this Section 1.6(e) applies as to the determination of the Net Cash at the Determination Date described in Section 1.6(a), upon
resolution of the matter in accordance with this Section 1.6(e), the Parties shall not be required to determine Net Cash again even though the Closing Date may occur later than the Anticipated Closing Date, except that either Party may
request a redetermination of Net Cash if the Closing Date is more than ten (10) Business Days after the Anticipated Closing Date.
1.7 Closing of Eiger’s Transfer Books. At the Effective Time: (a) all shares of Eiger Common Stock outstanding immediately
prior to the Effective Time shall be treated in accordance with Section 1.5(a), and all holders of certificates representing shares of Eiger Common Stock and Eiger Preferred Stock that were outstanding immediately prior to the Effective
Time shall cease to have any rights as stockholders of Eiger; and (b) the stock transfer books of Eiger shall be closed with respect to all shares of Eiger Common Stock and Eiger Preferred Stock outstanding immediately prior to the Effective
Time. No further transfer of any such shares of Eiger Common Stock shall be made on such stock transfer books after the Effective Time. If, after the Effective Time, a valid certificate previously representing any shares of Eiger Common Stock,
including any valid certificate representing any shares of Eiger Preferred Stock previously converted into shares of Eiger Common Stock in connection with the Preferred Stock Conversion, outstanding immediately prior to the Effective Time (an
“Eiger Stock Certificate”) is presented to the Exchange Agent or to the Surviving Corporation, such Eiger Stock Certificate shall be canceled and shall be exchanged as provided in Sections 1.5 and 1.8.
1.8 Surrender of Certificates.
(a) On or prior to the Closing Date, Celladon and Eiger shall agree upon and select a reputable bank, transfer agent or trust company
to act as exchange agent in the Merger (the “Exchange Agent”). At the Effective Time, Celladon shall deposit with the Exchange Agent: (i) certificates representing the shares of Celladon Common Stock issuable pursuant to
Section 1.5(a) and (ii) cash sufficient to make payments in lieu of fractional shares in accordance with Section 1.5(c). The shares of Celladon Common Stock and cash amounts so deposited with the Exchange Agent, together
with any dividends or distributions received by the Exchange Agent with respect to such shares, are referred to collectively as the “Exchange Fund.”
(b) At or before the Effective Time, Eiger will deliver to Celladon a true, complete and accurate listing of all record holders of
Eiger Stock Certificates at the Effective Time, including the number and class of shares of Eiger Capital Stock held by such record holder, and the number of shares of Celladon Common Stock such holder is entitled to receive pursuant to
Section 1.5. Promptly after the Effective Time, the Parties shall cause the Exchange Agent to mail to the Persons who were record holders of Eiger Stock Certificates immediately prior to the Effective Time: (i) a letter of
transmittal in customary form and containing such provisions as Celladon may reasonably specify (including a provision confirming that delivery of Eiger Stock Certificates shall be effected, and risk of loss and title to Eiger Stock Certificates
shall pass, only upon delivery of such Eiger Stock Certificates to the Exchange Agent); and (ii) instructions for effecting the surrender of Eiger Stock Certificates in exchange for certificates representing Celladon Common Stock. Upon
surrender of an Eiger Stock Certificate to the Exchange Agent for exchange, together with a duly executed letter of transmittal and such other documents as may be reasonably required by the Exchange Agent or Celladon: (A) the holder of such
Eiger Stock Certificate
A-5
shall be entitled to receive in exchange therefor a certificate representing the number of whole shares of Celladon Common Stock that such holder has the right to receive pursuant to the
provisions of Section 1.5(a) (and cash in lieu of any fractional share of Celladon Common Stock pursuant to the provisions of Section 1.5(c)); and (B) the Eiger Stock Certificate so surrendered shall be canceled. Until
surrendered as contemplated by this Section 1.8(b), each Eiger Stock Certificate shall be deemed, from and after the Effective Time, to represent only the right to receive shares of Celladon Common Stock (and cash in lieu of any
fractional share of Celladon Common Stock). If any Eiger Stock Certificate shall have been lost, stolen or destroyed, Celladon may, in its discretion and as a condition precedent to the delivery of any shares of Celladon Common Stock, require the
owner of such lost, stolen or destroyed Eiger Stock Certificate to provide an applicable affidavit with respect to such Eiger Stock Certificate and post a bond indemnifying Celladon against any claim suffered by Celladon related to the lost, stolen
or destroyed Eiger Stock Certificate or any Celladon Common Stock issued in exchange therefor as Celladon may reasonably request.
(c)
No dividends or other distributions declared or made with respect to Celladon Common Stock with a record date after the Effective Time shall be paid to the holder of any unsurrendered Eiger Stock Certificate with respect to the shares of
Celladon Common Stock that such holder has the right to receive in the Merger until such holder surrenders such Eiger Stock Certificate or an affidavit of loss or destruction in lieu thereof in accordance with this Section 1.8 (at which
time such holder shall be entitled, subject to the effect of applicable abandoned property, escheat or similar laws, to receive all such dividends and distributions, without interest).
(d) Any portion of the Exchange Fund that remains undistributed to holders of Eiger Stock Certificates as of the date 180 days after
the Closing Date shall be delivered to Celladon upon demand, and any holders of Eiger Stock Certificates who have not theretofore surrendered their Eiger Stock Certificates in accordance with this Section 1.8 shall thereafter look only
to Celladon for satisfaction of their claims for Celladon Common Stock, cash in lieu of fractional shares of Celladon Common Stock and any dividends or distributions with respect to shares of Celladon Common Stock.
(e) Each of the Exchange Agent, Celladon and the Surviving Corporation shall be entitled to deduct and withhold from any consideration
deliverable pursuant to this Agreement to any holder of any Eiger Stock Certificate such amounts as are required to be deducted or withheld from such consideration under the Code or under any other applicable Legal Requirement and shall be entitled
to request any reasonably appropriate Tax forms, including Form W-9 (or the appropriate Form W-8, as applicable), from any recipient of payments hereunder. To the extent such amounts are so deducted or withheld, and remitted to the appropriate
taxing authority, such amounts shall be treated for all purposes under this Agreement as having been paid to the Person to whom such amounts would otherwise have been paid.
(f) No party to this Agreement shall be liable to any holder of any Eiger Stock Certificate or to any other Person with respect to any
shares of Celladon Common Stock (or dividends or distributions with respect thereto) or for any cash amounts delivered to any public official pursuant to any applicable abandoned property law, escheat law or similar Legal Requirement.
1.9 Appraisal Rights.
(a) Notwithstanding any provision of this Agreement to the contrary, shares of Eiger Capital Stock that are outstanding immediately
prior to the Effective Time and which are held by stockholders who have exercised and perfected appraisal rights for such shares of Eiger Capital Stock in accordance with the DGCL (collectively, the “Dissenting Shares”) shall
not be converted into or represent the right to receive the per share amount of the merger consideration described in Section 1.5 attributable to such Dissenting Shares. Such stockholders shall be entitled to receive payment of the
appraised value of such shares of Eiger Capital Stock held by them in accordance with the DGCL, unless and until such stockholders fail to perfect or effectively withdraw or otherwise lose their appraisal rights under the DGCL. All Dissenting Shares
held by stockholders who shall have failed to perfect or who effectively shall have withdrawn or lost their right to appraisal of such shares of Eiger
A-6
Capital Stock under the DGCL shall thereupon be deemed to be converted into and to have become exchangeable for, as of the Effective Time, the right to receive the per share amount of the merger
consideration attributable to such Dissenting Shares upon their surrender in the manner provided in Section 1.5.
(b)
Eiger shall give Celladon prompt written notice of any demands by dissenting stockholders received by Eiger, withdrawals of such demands and any other instruments served on Eiger and any material correspondence received by Eiger in connection
with such demands.
1.10 Further Action. If, at any time after the Effective Time, any further action is determined by the
Surviving Corporation to be necessary or desirable to carry out the purposes of this Agreement or to vest the Surviving Corporation with full right, title and possession of and to all rights and property of Eiger, then the officers and directors of
the Surviving Corporation shall be fully authorized, and shall use their commercially reasonable efforts (in the name of Eiger, in the name of Merger Sub and otherwise) to take such action.
1.11 Tax Consequences. For federal income tax purposes, the Merger is intended to constitute a reorganization within the meaning of
Section 368(a) of the Code and the Treasury Regulations promulgated thereunder. The parties to this Agreement adopt this Agreement as a “plan of reorganization” within the meaning of Section 1.368-2(g) of the Treasury
Regulations.
| |
Section 2. |
REPRESENTATIONS AND WARRANTIES OF EIGER |
Eiger represents and warrants to
Celladon and Merger Sub as follows, except as set forth in the written disclosure schedule delivered by Eiger to Celladon (the “Eiger Disclosure Schedule”). The Eiger Disclosure Schedule shall be arranged in sections and
subsections corresponding to the numbered and lettered sections and subsections contained in this Section 2. The disclosures in any section or subsection of the Eiger Disclosure Schedule shall qualify other sections and subsections in
this Section 2 to the extent it is reasonably clear from a reading of the disclosure that such disclosure is applicable to such other sections and subsections. The inclusion of any information in the Eiger Disclosure Schedule (or any
update thereto) shall not be deemed to be an admission or acknowledgment, in and of itself, that such information is required by the terms hereof to be disclosed, is material, has resulted in or would result in an Eiger Material Adverse Effect, or
is outside the Ordinary Course of Business.
2.1 Subsidiaries; Due Organization; Etc.
(a) Eiger has no Subsidiaries, except for the Entities identified in Part 2.1(a) of the Eiger Disclosure Schedule; and neither Eiger
nor any of the other Entities identified in Part 2.1(a) of the Eiger Disclosure Schedule owns any capital stock of, or any equity interest of any nature in, any other Entity, other than the Entities identified in Part 2.1(a) of the Eiger Disclosure
Schedule. Eiger has not agreed nor is obligated to make, nor is bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Eiger has not, at any time, been a general
partner of, or has otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
(b) Each of Eiger and the Eiger Subsidiaries is a corporation duly organized, validly existing and in good standing under the laws of
the jurisdiction of its incorporation and has all necessary power and authority: (i) to conduct its business in the manner in which its business is currently being conducted; (ii) to own and use its assets in the manner in which its assets
are currently owned and used; and (iii) to perform its obligations under all Contracts by which it is bound.
(c) Each of
Eiger and the Eiger Subsidiaries is qualified to do business as a foreign corporation, and is in good standing, under the laws of all jurisdictions where the nature of its business requires such qualification other than in jurisdictions where the
failure to be so qualified individually or in the aggregate would not be reasonably expected to have an Eiger Material Adverse Effect.
A-7
2.2 Certificate of Incorporation; Bylaws; Charters and Codes of Conduct. Eiger has
delivered to Celladon accurate and complete copies of the certificate of incorporation, bylaws and other charter and organizational documents, including all currently effective amendments thereto, for Eiger and each Eiger Subsidiary. Part 2.2 of the
Eiger Disclosure Schedule lists, and Eiger has delivered to Celladon, accurate and complete copies of: (a) the charters of all committees of Eiger’s board of directors; and (b) any code of conduct or similar policy adopted by Eiger or
by the board of directors, or any committee of the board of directors, of Eiger. Neither Eiger nor any Eiger Subsidiary has taken any action in breach or violation in any material respect of any of the material provisions of its certificate of
incorporation, bylaws and other charter and organizational documents nor is in breach or violation in any material respect of any of the material provisions of its certificate of incorporation, bylaws and other charter and organizational documents.
2.3 Capitalization, Etc.
(a) The authorized capital stock of Eiger as of the date of this Agreement consists of (i) 37,400,000 shares of Eiger Common
Stock, par value $0.0001 per share, of which 3,130,665shares have been issued and are outstanding as of the date of this Agreement, and (ii) 30,787,500 shares of preferred stock, par value $0.0001 per share (the “Eiger Preferred
Stock”), of which (A) 5,187,500 shares have been designated Series A Preferred Stock, 4,875,000 of which shares of Series A Preferred Stock are outstanding as of the date of this Agreement and (B) 25,600,000 shares have been
designated Series A-1 Preferred Stock (the “Series A-1 Preferred Stock”), 24,935,950 shares of which are issued and outstanding. Except as set forth in Part 2.3(a) of the Eiger Disclosure Schedule, the authorized capital
stock of Eiger as of immediately prior to the Closing shall consist of (i) 68,000,000 shares of Eiger Common Stock, 59,271,433 shares of which will be issued and outstanding, (ii) warrants to purchase 590,241 shares of Eiger Common Stock
and (iii) 30,787,500 shares of Eiger Preferred Stock, of which 5,187,500 shares will have been designated Series A Preferred Stock and 25,600,000 shares will have been designated Series A-1 Preferred Stock, none of which shares of Eiger
Preferred Stock will be issued and outstanding. Eiger does not hold any shares of its capital stock in its treasury. All of the outstanding shares of Eiger Common Stock and Eiger Preferred Stock have been duly authorized and validly issued, and are
fully paid and nonassessable. Except as set forth in Part 2.3(a) of the Eiger Disclosure Schedule, none of the outstanding shares of Eiger Common Stock or Eiger Preferred Stock is entitled or subject to any preemptive right, right of participation,
right of maintenance or any similar right and none of the outstanding shares of Eiger Common Stock or Eiger Preferred Stock is subject to any right of first refusal in favor of Eiger. Except as contemplated herein or as set forth in Part 2.3(a) of
the Eiger Disclosure Schedule, there is no Eiger Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to),
any shares of Eiger Common Stock or Eiger Preferred Stock. Eiger is not under any obligation, nor is it bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Eiger
Common Stock or other securities. Part 2.3(a) of the Eiger Disclosure Schedule accurately and completely lists all repurchase rights held by Eiger with respect to shares of Eiger Common Stock (including shares issued pursuant to the exercise of
stock options) and Eiger Preferred Stock, and specifies each holder of Eiger Common Stock or Eiger Preferred Stock, the date of purchase of such Eiger Common Stock or Eiger Preferred Stock, the number of shares of Eiger Common Stock or Eiger
Preferred Stock subject to such repurchase rights, the purchase price paid by such holder, the vesting schedule under which such repurchase rights lapse, and whether the holder of such Eiger Common Stock or Eiger Preferred Stock filed an election
under Section 83(b) of the Code with respect to such Eiger Common Stock or Eiger Preferred Stock within thirty (30) days of purchase. Each share of Eiger Preferred Stock is convertible into one share of Eiger Common Stock.
(b) Except for the Eiger 2009 Equity Incentive Plan (the “2009 Plan”), and except as set forth in Part 2.3(b)
of the Eiger Disclosure Schedule, Eiger does not have any stock option plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person. Eiger has reserved 3,867,792 shares of Eiger Common Stock
for issuance under the 2009 Plan. Of such reserved shares of Eiger Common Stock, 739,999 shares have been issued pursuant to the exercise of outstanding options, options to purchase 2,902,860 shares have been granted and are currently outstanding,
and 224,933 shares of Eiger
A-8
Common Stock remain available for future issuance pursuant to the 2009 Plan. Part 2.3(b) of the Eiger Disclosure Schedule sets forth the following information with respect to each Eiger Option
outstanding as of the date of this Agreement: (A) the name of the optionee; (B) the number of shares of Eiger Common Stock subject to such Eiger Option at the time of grant; (C) the number of shares of Eiger Common Stock subject to
such Eiger Option as of the date of this Agreement; (D) the exercise price of such Eiger Option; (E) the date on which such Eiger Option was granted; (F) the applicable vesting schedule, including the number of vested and unvested
shares; (G) the date on which such Eiger Option expires; and (H) whether such Eiger Option is an “incentive stock option” (as defined in the Code) or a non-qualified stock option. Eiger has made available to Celladon an accurate
and complete copy of the 2009 Plan and forms of all stock option agreements approved for use thereunder. No vesting of Eiger Options will accelerate in connection with the closing of the Contemplated Transactions.
(c) Except for the outstanding Eiger Options as set forth in Section 2.3(b), for the warrants identified on Part 2.3(c) of
the Eiger Disclosure Schedule (the “Eiger Warrants”) or as set forth on Part 2.3(c) of the Eiger Disclosure Schedule, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently
exercisable) to acquire any shares of the capital stock or other securities of Eiger or any of its Subsidiaries; (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the
capital stock or other securities of Eiger or any of its Subsidiaries; (iii) stockholder rights plan (or similar plan commonly referred to as a “poison pill”) or Contract under which Eiger or any of its Subsidiaries is or may become
obligated to sell or otherwise issue any shares of its capital stock or any other securities; or (iv) condition or circumstance that may give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person
is entitled to acquire or receive any shares of capital stock or other securities of Eiger or any of its Subsidiaries. There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with
respect to Eiger or any of its Subsidiaries.
(d) All outstanding shares of Eiger Common Stock and Eiger Preferred Stock, as well
as all options, warrants and other securities of Eiger, have been issued and granted in material compliance with (i) all applicable securities laws and other applicable Legal Requirements and (ii) all requirements set forth in applicable
Contracts. Eiger has delivered to Celladon accurate and complete copies of all Eiger Warrants.
2.4 Financial Statements.
(a) Part 2.4(a) of the Eiger Disclosure Schedule includes true and complete copies of (i) Eiger’s audited consolidated
balance sheets at December 31, 2013 and December 31, 2014, (ii) the Eiger Unaudited Interim Balance Sheet, (iii) Eiger’s audited consolidated statements of income, cash flow and stockholders’ equity for the years ended
December 31, 2013 and December 31, 2014, and (iv) Eiger’s unaudited statements of income, cash flow and shareholders’ equity for the nine months ended September 30, 2015 (collectively, the “Eiger
Financials”). The Eiger Financials (i) were prepared in accordance with United States generally accepted accounting principles (“GAAP”) (except as may be indicated in the footnotes to such Eiger Financials
and that unaudited financial statements may not have notes thereto and other presentation items that may be required by GAAP and are subject to normal and recurring year-end adjustments that are not reasonably expected to be material in amount)
applied on a consistent basis unless otherwise noted therein throughout the periods indicated and (ii) fairly present the financial condition and operating results of Eiger and its consolidated Subsidiaries as of the dates and for the periods
indicated therein.
(b) Each of Eiger and its Subsidiaries maintains a system of internal accounting controls designed to provide
reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with
GAAP and to maintain asset accountability; (iii) access to assets is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability for assets is compared with the existing
assets at reasonable intervals and appropriate action is taken with respect to any differences.
A-9
Eiger and each of its Subsidiaries maintains internal control over financial reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with GAAP.
(c) Part 2.4(c) of the Eiger Disclosure Schedule lists, and
Eiger has delivered to Celladon accurate and complete copies of the documentation creating or governing, all securitization transactions and “off-balance sheet arrangements” (as defined in Item 303(c) of Regulation S-K under the
Exchange Act) effected by Eiger or any of its Subsidiaries since January 1, 2010.
(d) Since January 1, 2010, there have
been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer or general counsel of Eiger,
Eiger’s Board of Directors or any committee thereof. Since January 1, 2010, neither Eiger nor its independent auditors have identified (i) any significant deficiency or material weakness in the system of internal accounting controls
utilized by Eiger and the Eiger Subsidiaries, (ii) any fraud, whether or not material, that involves Eiger’s management or other employees who have a role in the preparation of financial statements or the internal accounting controls
utilized by Eiger and the Eiger Subsidiaries or (iii) any claim or allegation regarding any of the foregoing.
2.5 Absence of
Changes. Except as set forth on Part 2.5 of the Eiger Disclosure Schedule, between December 31, 2014 and the date of this Agreement and except as otherwise expressly contemplated by this Agreement:
(a) there has not been any Eiger Material Adverse Effect or an event or development that would, individually or in the aggregate,
reasonably be expected to have an Eiger Material Adverse Effect;
(b) there has not been any material loss, damage or destruction
to, or any material interruption in the use of, any of the assets or business of Eiger or any Eiger Subsidiary (whether or not covered by insurance);
(c) Eiger has not: (i) declared, accrued, set aside or paid any dividend or made any other distribution in respect of any shares
of capital stock; or (ii) repurchased, redeemed or otherwise reacquired any shares of capital stock or other securities except for the repurchase or reacquisition of shares pursuant to Eiger rights arising upon an individual’s termination
as an employee, director or consultant;
(d) Eiger has not sold, issued or granted, or authorized the issuance of: (i) any
capital stock or other security (except for Eiger Common Stock issued upon the valid exercise of outstanding Eiger Options); (ii) any option, warrant or right to acquire any capital stock or any other security (except for the Eiger Options
identified in Part 2.3(b) of the Eiger Disclosure Schedule); or (iii) any instrument convertible into or exchangeable for any capital stock or other security (except for the Eiger Options identified in Part 2.3(b) of the Eiger Disclosure
Schedule);
(e) there has been no amendment to the certificate of incorporation, bylaws or other charter or organizational
documents of Eiger or any Eiger Subsidiary, and neither Eiger nor any Eiger Subsidiary has effected or been a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split,
reverse stock split or similar transaction;
(f) Eiger has not amended or waived any of its rights under, or exercised its
discretion to permit the acceleration of vesting under any provision of: (i) the 2009 Plan; (ii) any Eiger Option or any Contract evidencing or relating to any Eiger Option; (iii) any restricted stock purchase agreement; or
(iv) any other Contract evidencing or relating to any equity award (whether payable in cash or stock);
(g) Neither Eiger nor
any Eiger Subsidiary has formed any Subsidiary or acquired any equity interest or other interest in any other Entity;
A-10
(h) Neither Eiger nor any Eiger Subsidiary has: (i) lent money to any Person;
(ii) incurred or guaranteed any indebtedness; (iii) issued or sold any debt securities or options, warrants, calls or other rights to acquire any debt securities; (iv) guaranteed any debt securities of others; or (v) made any
capital expenditure or commitment in excess of $100,000;
(i) Neither Eiger nor any Eiger Subsidiary has changed any of its
accounting methods, principles or practices;
(j) Neither Eiger nor any Eiger Subsidiary has made, changed or revoked any material
Tax election, filed any material amendment to any Tax Return, adopted or changed any accounting method in respect of Taxes, changed any annual Tax accounting period, entered into any Tax allocation agreement, Tax sharing agreement or Tax indemnity
agreement, other than commercial contracts entered into in the Ordinary Course of Business with vendors, customers or landlords, entered into any closing agreement with respect to any Tax, settled or compromised any claim, notice, audit report or
assessment in respect of material Taxes, applied for or entered into any ruling from any Tax authority with respect to Taxes, surrendered any right to claim a material Tax refund, or consented to any extension or waiver of the statute of limitations
period applicable to any material Tax claim or assessment;
(k) Neither Eiger nor any Eiger Subsidiary has commenced or settled any
Legal Proceeding;
(l) Neither Eiger nor any Eiger Subsidiary has entered into any material transaction outside the Ordinary Course
of Business;
(m) Neither Eiger nor any Eiger Subsidiary has acquired any material assets nor sold, leased or otherwise irrevocably
disposed of any of its material assets or properties, nor has any Encumbrance been granted with respect to such assets or properties, except for Encumbrances of immaterial assets in the Ordinary Course of Business consistent with past practices;
(n) there has been no entry into, amendment or termination of any Eiger Material Contract;
(o) there has been no (i) material change in pricing or royalties or other payments set or charged by Eiger or any Eiger
Subsidiary to its customers or licensees, (ii) agreement by Eiger or any Eiger Subsidiary to change pricing or royalties or other payments set or charged by persons who have licensed Intellectual Property to Eiger or any Eiger Subsidiary, or
(iii) material change in pricing or royalties or other payments set or charged by persons who have licensed Intellectual Property to Eiger or any Eiger Subsidiary; and
(p) Neither Eiger nor any Eiger Subsidiary has negotiated, agreed or committed to take any of the actions referred to in clauses
“(c)” through “(o)” above (other than negotiations between the Parties to enter into this Agreement).
2.6 Title to
Assets. Each of Eiger and the Eiger Subsidiaries owns, and has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests in, all tangible properties or assets and equipment used or held for use in its
business or operations or purported to be owned by it, including: (a) all assets reflected on the Eiger Unaudited Interim Balance Sheet; and (b) all other assets reflected in the books and records of Eiger or any Eiger Subsidiary as being
owned by Eiger or such Eiger Subsidiary. All of said assets are owned by Eiger or an Eiger Subsidiary free and clear of any Encumbrances, except for: (i) any lien for current Taxes not yet due and payable or for Taxes that are being contested
in good faith and for which adequate reserves have been made on the Eiger Unaudited Interim Balance Sheet; (ii) minor liens that have arisen in the Ordinary Course of Business and that do not (in any case or in the aggregate) materially detract
from the value of the assets subject thereto or materially impair the operations of Eiger or any Eiger Subsidiary; and (iii) liens listed in Part 2.6 of the Eiger Disclosure Schedule.
A-11
2.7 Real Property; Leasehold. Neither Eiger nor any Eiger Subsidiary owns any real
property or any interest in real property, except for the leaseholds created under the real property leases identified in Part 2.7 of the Eiger Disclosure Schedule which are in full force and effect and with no existing default thereunder.
2.8 Intellectual Property.
(a) Eiger, directly or through an Eiger Subsidiary, owns, or has the right to use, and has the right to bring actions for the
infringement of, all Eiger IP Rights, except for any failure to own or have the right to use, or have the right to bring actions that would not reasonably be expected to have an Eiger Material Adverse Effect.
(b) Part 2.8(b) of the Eiger Disclosure Schedule is an accurate, true and complete listing of all Eiger Registered IP.
(c) Part 2.8(c) of the Eiger Disclosure Schedule accurately identifies (i) all Eiger IP Rights licensed to Eiger or any Eiger
Subsidiary (other than (I) any non-customized software that (A) is so licensed solely in executable or object code form pursuant to a non-exclusive, internal use software license and other Intellectual Property associated with such
software and (B) is not incorporated into, or material to the development, manufacturing, or distribution of, any of Eiger’s or any Eiger Subsidiary’s products or services and (II) any Intellectual Property licensed ancillary to the
purchase or use of equipment, reagents or other materials); (ii) the corresponding Eiger Contracts pursuant to which such Eiger IP Rights are licensed to Eiger or any Eiger Subsidiary; and (iii) whether the license or licenses granted to
Eiger or any Eiger Subsidiary are exclusive or non-exclusive.
(d) Part 2.8(d)(i) of the Eiger Disclosure Schedule accurately
identifies each Eiger Contract pursuant to which any Person has been granted any license under, or otherwise has received or acquired any right (whether or not currently exercisable) or interest in, any Eiger IP Rights. Except as identified in Part
2.8(d)(ii) of the Eiger Disclosure Schedule, Eiger is not bound by, and no Eiger IP Rights are subject to, any Contract containing any covenant or other provision that in any way limits or restricts the ability of Eiger or any Eiger Subsidiary to
use, exploit, assert or enforce any Eiger IP Rights anywhere in the world, in each case as would materially limit the business of Eiger.
(e) Except as identified in Part 2.8(e) of the Eiger Disclosure Schedule, to the Knowledge of Eiger, Eiger or one of its Subsidiaries
exclusively owns all right, title, and interest to and in Eiger IP Rights (other than Eiger IP Rights (i) exclusively and non-exclusively licensed to Eiger or one of its Subsidiaries, as identified in Part 2.8(c) of the Eiger Disclosure
Schedule, (ii) any non-customized software that (A) is so licensed solely in executable or object code form pursuant to a non-exclusive, internal use software license and other Intellectual Property associated with such software and
(B) is not incorporated into, or material to the development, manufacturing, or distribution of, any of Eiger’s or any Eiger Subsidiary’s products or services, and (iii) any Intellectual Property licensed ancillary to the
purchase or use of equipment, reagents or other materials) free and clear of any Encumbrances (other than those Encumbrances granted pursuant to the Eiger Contracts listed in Part 2.8(d) of the Eiger Disclosure Schedule). Without limiting the
generality of the foregoing:
(i) All documents and instruments necessary to register or apply for or renew registration of all
Eiger Registered IP have been validly executed, delivered and filed in a timely manner with the appropriate Governmental Body except for any such failure, individually or collectively, that would not reasonably be expected to have an Eiger Material
Adverse Effect.
(ii) Each Person who is or was an employee or contractor of Eiger or any Eiger Subsidiary and who is or was
involved in the creation or development of any Eiger IP Rights has signed a valid, enforceable agreement containing an assignment of such Intellectual Property to Eiger or such Subsidiary and confidentiality provisions protecting trade secrets and
confidential information of Eiger and its Subsidiaries. To the Knowledge of Eiger and its Subsidiaries, no current or former stockholder, officer, director, or employee of Eiger or any of
A-12
its Subsidiaries has any claim, right (whether or not currently exercisable), or interest to or in any Eiger IP Rights. To the Knowledge of Eiger and its Subsidiaries, no employee of Eiger or any
or any Eiger Subsidiary is (a) bound by or otherwise subject to any Contract restricting him or her from performing his or her duties for Eiger or such Subsidiary or (b) in breach of any Contract with any former employer or other Person
concerning Eiger IP Rights or confidentiality provisions protecting trade secrets and confidential information comprising Eiger IP Rights.
(iii) No funding, facilities or personnel of any Governmental Body were used, directly or indirectly, to develop or create, in whole
or in part, any Eiger IP Rights in which Eiger or any of its Subsidiaries has an ownership interest.
(iv) Eiger and each of its
Subsidiaries has taken reasonable steps to maintain the confidentiality of and otherwise protect and enforce its rights in all proprietary information that Eiger or such Subsidiary holds, or purports to hold, as a trade secret.
(v) Neither Eiger nor any of its Subsidiaries has assigned or otherwise transferred ownership of, or agreed to assign or otherwise
transfer ownership of, any Eiger IP Rights to any other Person.
(vi) To the Knowledge of Eiger and its Subsidiaries, the Eiger IP
Rights constitute all Intellectual Property necessary for Eiger and its Subsidiaries to conduct its business as currently conducted and planned to be conducted.
(f) Eiger has delivered, or made available to Celladon, a complete and accurate copy of all Eiger IP Rights Agreements. Neither Eiger
nor any Eiger Subsidiary is a party to any Contract (A) pursuant to which the execution, delivery and performance of this Agreement and the consummation of the Contemplated Transactions will constitute a breach, or (B) as a result of such
execution, delivery and performance of this Agreement and the consummation of the Contemplated Transactions will cause the forfeiture or termination of or Encumbrance upon, or the grant of any license or other right to, or give rise to a right of
forfeiture or termination of or Encumbrance upon, any Eiger IP Rights or impair the right of Eiger or the Surviving Corporation and its Subsidiaries to use, sell or license any Eiger IP Rights or portion thereof, except for the occurrence of any
such breach, forfeiture, termination, Encumbrance, grant or impairment that would not individually or in the aggregate, reasonably be expected to result in an Eiger Material Adverse Effect. With respect to each of the Eiger IP Rights Agreements:
(i) each such agreement is valid and binding on Eiger or its Subsidiaries, as applicable, and in full force and effect; (ii) Eiger has not received any written notice of termination or cancellation under such agreement, or received any
written notice of breach or default under such agreement, which breach has not been cured or waived; and (iii) neither Eiger nor its Subsidiaries, and to the Knowledge of Eiger, no other party to any such agreement, is in breach or default
thereof in any material respect.
(g) The manufacture, marketing, license, sale or intended use of any product or technology
currently licensed or sold or under development by Eiger or any of its Subsidiaries (i) does not violate any license or agreement between Eiger or its Subsidiaries and any third party, and, to the Knowledge of Eiger and its Subsidiaries,
(ii) does not infringe or misappropriate any Intellectual Property right of any other party, which infringement or misappropriation would reasonably be expected to have an Eiger Material Adverse Effect. Eiger has disclosed in correspondence to
Celladon the third-party patents and patent applications found during all freedom to operate searches that were conducted by Eiger or its Subsidiaries related to any product or technology currently licensed or sold or under development by Eiger or
its Subsidiaries. To the Knowledge of Eiger and its Subsidiaries, no third party is infringing upon, or violating any license or agreement with Eiger or its Subsidiaries relating to, any Eiger IP Rights. There is no current or pending challenge,
claim or Legal Proceeding (including opposition, interference or other proceeding in any patent or other government office) contesting the validity, ownership or right to use, sell, license or dispose of any Eiger IP Rights, nor has Eiger or any of
its Subsidiaries received any written notice asserting that any Eiger IP Rights or the proposed use, sale, license or disposition thereof conflicts with or infringes or misappropriates or will conflict with or infringe or misappropriate the rights
of any other Person.
A-13
(h) Each item of Eiger IP Rights that is Eiger Registered IP is and at all times has been
filed and maintained in compliance with all applicable Legal Requirements and all filings, payments and other actions required to be made or taken to maintain such item of Eiger Registered IP in full force and effect have been made by the applicable
deadline, except for any failure to perform any of the foregoing, individually or collectively, that would not reasonably be expected to have an Eiger Material Adverse Effect.
(i) No trademark (whether registered or unregistered) or trade name owned, used, or applied for by Eiger or any of its Subsidiaries
conflicts or interferes with any trademark (whether registered or unregistered) or trade name owned, used, or applied for by any other Person. None of the goodwill associated with or inherent in any trademark (whether registered or unregistered) in
which Eiger or any of its Subsidiaries has or purports to have an ownership interest has been impaired as determined by Eiger or any of its Subsidiaries in accordance with GAAP.
(j) Except as set forth in the Contracts listed on Parts 2.8(c) or 2.8(d) of the Eiger Disclosure Schedule, (i) neither Eiger nor
any of its Subsidiaries is bound by any Contract to indemnify, defend, hold harmless, or reimburse any other Person with respect to any Intellectual Property infringement, misappropriation, or similar claim, and (ii) neither Eiger nor any of
its Subsidiaries has ever assumed, or agreed to discharge or otherwise take responsibility for, any existing or potential liability of another Person for infringement, misappropriation, or violation of any Intellectual Property right, which
assumption, agreement or responsibility remains in force as of the date of this Agreement.
2.9 Agreements, Contracts and
Commitments. Part 2.9 of the Eiger Disclosure Schedule identifies, except for Eiger Contracts set forth in Part 2.13 of the Eiger Disclosure Schedule:
(a) each Eiger Contract relating to any bonus, deferred compensation, severance, incentive compensation, pension, profit-sharing or
retirement plans, or any other employee benefit plans or arrangements;
(b) each Eiger Contract relating to the employment of, or
the performance of employment-related services by, any Person, including any employee, consultant or independent contractor, not terminable by Eiger or its Subsidiaries on ninety (90) days’ notice without liability, except to the extent
general principles of wrongful termination law may limit Eiger’s, Eiger’s Subsidiaries’ or such successor’s ability to terminate employees at will;
(c) each Eiger Contract relating to any agreement or plan, including any stock option plan, stock appreciation right plan or stock
purchase plan, any of the benefits of which will be increased, or the vesting of benefits of which will be accelerated, by the occurrence of any of the Contemplated Transactions (either alone or in conjunction with any other event, such as
termination of employment), or the value of any of the benefits of which will be calculated on the basis of any of the Contemplated Transactions;
(d) each Eiger Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business
other than indemnification agreements between Eiger and any of its respective officers or directors;
(e) each Eiger Contract
relating to any agreement, contract or commitment containing any covenant limiting the freedom of Eiger, its Subsidiaries or the Surviving Corporation to engage in any line of business or compete with any Person;
(f) each Eiger Contract relating to any agreement, contract or commitment relating to capital expenditures and involving obligations
after the date of this Agreement in excess of $100,000 and not cancelable without penalty;
A-14
(g) each Eiger Contract relating to any agreement, contract or commitment currently in
force relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(h) each Eiger
Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or instruments relating to the borrowing of money or extension of credit in excess of $100,000 or creating any material
Encumbrances with respect to any assets of Eiger or any Eiger Subsidiary or any loans or debt obligations with officers or directors of Eiger;
(i) each Eiger Contract relating to (i) any distribution agreement (identifying any that contain exclusivity provisions);
(ii) any agreement involving provision of services or products with respect to any pre-clinical or clinical development activities of Eiger (iii) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, development
or other agreement currently in force under which Eiger or its Subsidiaries has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which Eiger or its Subsidiaries has continuing obligations
to develop any Intellectual Property that will not be owned, in whole or in part, by Eiger or such Eiger Subsidiary; or (iv) any Contract currently in force to license any third party to manufacture or produce any Eiger product, service or
technology or any Contract currently in force to sell, distribute or commercialize any Eiger products or service except agreements with distributors or sales representatives in the Ordinary Course of Business;
(j) each Eiger Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing
advisory services to Eiger in connection with the Contemplated Transactions; or
(k) any other agreement, contract or commitment
(i) which involves payment or receipt by Eiger or its Subsidiaries under any such agreement, contract or commitment of $100,000 or more in the aggregate or obligations after the date of this Agreement in excess of $100,000 in the aggregate, or
(ii) that is material to the business or operations of Eiger and its Subsidiaries.
Eiger has delivered to Celladon accurate and complete (except for
applicable redactions thereto) copies of all Eiger Material Contracts, including all amendments thereto. There are no Eiger Material Contracts that are not in written form. Except as set forth on Part 2.9 of the Eiger Disclosure Schedule, neither
Eiger nor any of its Subsidiaries has, nor to Eiger’s Knowledge, as of the date of this Agreement has any other party to an Eiger Material Contract, breached, violated or defaulted under, or received notice that it has breached, violated or
defaulted under, any of the terms or conditions of any of the agreements, contracts or commitments to which Eiger or its Subsidiaries is a party or by which it is bound of the type described in clauses (a) through (k) above (any such
agreement, contract or commitment, an “Eiger Material Contract”) in such manner as would permit any other party to cancel or terminate any such Eiger Material Contract, or would permit any other party to seek damages which
would reasonably be expected to have an Eiger Material Adverse Effect. As to Eiger and its Subsidiaries, as of the date of this Agreement, each Eiger Material Contract is valid, binding, enforceable and in full force and effect, subject to:
(i) laws of general application relating to bankruptcy, insolvency and the relief of debtors; and (ii) rules of law governing specific performance, injunctive relief and other equitable remedies. The consummation of the Contemplated
Transactions shall not result in any material payment or payments becoming due from Eiger, any Eiger Subsidiary, the Surviving Corporation or Celladon to any Person under any Eiger Contract or give any Person the right to terminate or alter the
provisions of any Eiger Contract. No Person is renegotiating, or has a right pursuant to the terms of any Eiger Material Contract to change, any material amount paid or payable to Eiger under any Eiger Material Contract or any other material term or
provision of any Eiger Material Contract.
2.10 Liabilities. As of the date hereof, neither Eiger nor any Eiger Subsidiary has any
liability, indebtedness, obligation, expense, claim, deficiency, guaranty or endorsement of any kind, whether accrued, absolute, contingent, matured, unmatured or other (whether or not required to be reflected in the financial statements in
accordance with GAAP) (each a “Liability”), except for: (a) Liabilities identified as such in the
A-15
“liabilities” column of the Eiger Unaudited Interim Balance Sheet; (b) normal and recurring current Liabilities that have been incurred by Eiger or its Subsidiaries since the date
of the Eiger Unaudited Interim Balance Sheet in the Ordinary Course of Business and which are not in excess of $100,000 in the aggregate; (c) Liabilities for performance in the Ordinary Course of Business of obligations of Eiger or any Eiger
Subsidiary under Eiger Contracts, including the reasonably expected performance of such Eiger Contracts in accordance with their terms (which would not include, for example, any instances of breach or indemnification); (d) Liabilities incurred
in connection with this Agreement and the Subscription Agreement; and (e) Liabilities listed in Part 2.10 of the Eiger Disclosure Schedule.
2.11 Compliance; Permits; Restrictions.
(a) Eiger and each Eiger Subsidiary are, and since January 1, 2010 have been, in compliance in all material respects with all
applicable Legal Requirements. No investigation, claim, suit, proceeding, audit or other action by any Governmental Body or authority is pending or, to the Knowledge of Eiger, threatened against Eiger or any Eiger Subsidiary, nor has any
Governmental Body or authority indicated to Eiger an intention to conduct the same. There is no agreement, judgment, injunction, order or decree binding upon Eiger or any Eiger Subsidiary which (i) has or would reasonably be expected to have
the effect of prohibiting or materially impairing any business practice of Eiger or any Eiger Subsidiary, any acquisition of material property by Eiger or any Eiger Subsidiary or the conduct of business by Eiger or any Eiger Subsidiary as currently
conducted, (ii) may have an adverse effect on Eiger’s ability to comply with or perform any covenant or obligation under this Agreement, or (iii) may have the effect of preventing, delaying, making illegal or otherwise interfering
with the Merger or any of the Contemplated Transactions.
(b) Eiger and the Eiger Subsidiaries hold all required Governmental
Authorizations which are material to the operation of the business of Eiger (the “Eiger Permits”) as currently conducted. Part 2.11(b) of the Eiger Disclosure Schedule identifies each Eiger Permit. Each of Eiger and each
Eiger Subsidiary is in material compliance with the terms of the Eiger Permits. No action, proceeding, revocation proceeding, amendment procedure, writ, injunction or claim is pending or, to the Knowledge of Eiger, threatened, which seeks to revoke,
limit, suspend, or materially modify any Eiger Permit. The rights and benefits of each material Eiger Permit will be available to the Surviving Corporation immediately after the Effective Time on terms substantially identical to those enjoyed by
Eiger and its Subsidiaries as of the date of this Agreement and immediately prior to the Effective Time.
(c) There are no
proceedings pending or threatened with respect to an alleged violation by Eiger or any of its Subsidiaries of the Federal Food, Drug, and Cosmetic Act (“FDCA”), Food and Drug Administration (“FDA”)
regulations adopted thereunder, the Controlled Substance Act or any other similar Legal Requirements promulgated by the FDA or other comparable Governmental Body responsible for regulation of the development, clinical testing, manufacturing, sale,
marketing, distribution and importation or exportation of drug products (“Drug Regulatory Agency”).
(d)
Eiger and each of its Subsidiaries holds all required Governmental Authorizations issuable by any Drug Regulatory Agency necessary for the conduct of the business of Eiger or such Subsidiary as currently conducted, and development, clinical
testing, manufacturing, marketing, distribution and importation or exportation, as currently conducted, of any of its products or product candidates (the “Eiger Product Candidates”) (collectively, the “Eiger
Regulatory Permits”), and no such Eiger Regulatory Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner, other than immaterial adverse modifications. Eiger and each
Eiger Subsidiary is in compliance in all material respects with the Eiger Regulatory Permits and has not received any written notice or other written communication from any Drug Regulatory Agency regarding (A) any material violation of or
failure to comply materially with any term or requirement of any Eiger Regulatory Permit or (B) any revocation, withdrawal, suspension, cancellation, termination or material modification of any Eiger Regulatory Permit. Except for the
information and files identified in Part 2.11(d) of the Eiger Disclosure Schedule, Eiger has made available to Celladon all information
A-16
requested by Celladon in Eiger’s or its Subsidiaries’ possession or control relating to the Eiger Product Candidates and the development, clinical testing, manufacturing, importation
and exportation of the Eiger Product Candidates, including complete copies of the following (to the extent there are any): (x) adverse event reports; clinical study reports and material study data; inspection reports, notices of adverse
findings, warning letters, filings and letters and other written correspondence to and from any Drug Regulatory Agency; and meeting minutes with any Drug Regulatory Agency; and (y) similar reports, material study data, notices, letters,
filings, correspondence and meeting minutes with any other Governmental Authority.
(e) All clinical, pre-clinical and other
studies and tests conducted by or on behalf of, or sponsored by, Eiger or its Subsidiaries or in which Eiger or its Subsidiaries or their respective current products or product candidates, including the Eiger Product Candidates, have participated
were, and if still pending are being, conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance with the applicable regulations of the Drug Regulatory Agencies and other applicable
Legal Requirements, including 21 C.F.R. Parts 50, 54, 56, 58 and 312. Since January 1, 2010, neither Eiger nor any of its Subsidiaries has received any notices, correspondence or other communications from any Drug Regulatory Agency requiring,
or to the Knowledge of Eiger threatening to initiate, the termination or suspension of any clinical studies conducted by or on behalf of, or sponsored by, Eiger or any of its Subsidiaries or in which Eiger or any of its Subsidiaries or their
respective current products or product candidates, including the Eiger Product Candidates, have participated.
(f) Neither Eiger
nor any of the Eiger Subsidiaries is the subject of any pending, or to the Knowledge of Eiger or the Eiger Subsidiaries, threatened investigation in respect of its business or products by the FDA pursuant to its “Fraud, Untrue Statements of
Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Knowledge of Eiger or any of the Eiger Subsidiaries, neither Eiger nor any of the Eiger
Subsidiaries has committed any acts, made any statement, or failed to make any statement, in each case in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal
Gratuities” Final Policy, and any amendments thereto. None of Eiger, any of its Subsidiaries or any of their respective officers, employees or agents has been convicted of any crime or engaged in any conduct that could result in a debarment or
exclusion (i) under 21 U.S.C. Section 335a or (ii) any similar applicable Legal Requirement. To the Knowledge of Eiger, no debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or
products are pending or threatened against Eiger, any Eiger Subsidiary or any of their respective officers, employees or agents.
2.12
Tax Matters.
(a) Eiger and each Eiger Subsidiary have timely filed all federal income Tax Returns and other material Tax
Returns that they were required to file under applicable Legal Requirements. All such Tax Returns were correct and complete in all material respects and have been prepared in material compliance with all applicable Legal Requirements. Neither Eiger
nor any Eiger Subsidiary is currently the beneficiary of any extension of time within which to file any Tax Return. No claim has ever been made by an authority in a jurisdiction where Eiger or any Eiger Subsidiary does not file Tax Returns that it
is subject to taxation by that jurisdiction.
(b) All material Taxes due and owing by Eiger or any Eiger Subsidiary on or before
the date hereof (whether or not shown on any Tax Return) have been paid. The unpaid Taxes of Eiger and any Eiger Subsidiary have been reserved for on the Eiger Unaudited Interim Balance Sheet in accordance with GAAP. Since the date of the Eiger
Unaudited Interim Balance Sheet, neither Eiger nor any Eiger Subsidiary has incurred any Liability for Taxes outside the Ordinary Course of Business or otherwise inconsistent with past custom and practice.
(c) Eiger and each Eiger Subsidiary have withheld and paid all Taxes required to have been withheld and paid in connection with any
amounts paid or owing to any employee, independent contractor, creditor, stockholder or other third party.
A-17
(d) There are no Encumbrances for Taxes (other than Taxes not yet due and payable or Taxes
that are being contested in good faith and for which adequate reserves have been made on Eiger’s Unaudited Interim Balance Sheet) upon any of the assets of Eiger or any Eiger Subsidiary.
(e) No material deficiencies for Taxes with respect to Eiger or any Eiger Subsidiary have been claimed, proposed or assessed by any
Governmental Body in writing. There are no pending (or, based on written notice, threatened) audits, assessments or other actions for or relating to any liability in respect of Taxes of Eiger or any Eiger Subsidiary. No issues relating to Taxes of
Eiger or any Eiger Subsidiary were raised by the relevant Tax authority in any completed audit or examination that would reasonably be expected to result in a material amount of Taxes in a later taxable period. Eiger has delivered or made available
to Celladon complete and accurate copies of all federal income Tax and all other material Tax Returns of Eiger and each Eiger Subsidiary (and predecessors of each) for all taxable years remaining open under the applicable statute of limitations, and
complete and accurate copies of all examination reports and statements of deficiencies assessed against or agreed to by Eiger and each Eiger Subsidiary (and predecessors of each), with respect to federal income Tax and all other material Taxes.
Neither Eiger nor any Eiger Subsidiary (or any of their predecessors) has waived any statute of limitations in respect of Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency, nor has any request been made in
writing for any such extension or waiver.
(f) All material elections with respect to Taxes affecting Eiger or any Eiger Subsidiary
as of the date hereof are set forth on Schedule 2.12(f). Neither Eiger nor any Eiger Subsidiary (i) has consented at any time under former Section 341(f)(1) of the Code to have the provisions of former Section 341(f)(2) of the
Code apply to any disposition of the assets of Eiger or any Eiger Subsidiary; (ii) has agreed, or is required, to make any adjustment under Section 481(a) of the Code by reason of a change in accounting method or otherwise;
(iii) has made an election, or is required, to treat any of its assets as owned by another Person for Tax purposes or as a tax-exempt bond financed property or tax-exempt use property within the meaning of Section 168 of the Code;
(iv) has acquired or owns any assets that directly or indirectly secure any debt the interest on which is tax exempt under Section 103(a) of the Code; (v) has made or will make a consent dividend election under Section 565
of the Code; (vi) has elected at any time to be treated as an S corporation within the meaning of Sections 1361 or 1362 of the Code; or (vii) has made any of the foregoing elections or is required to apply any of the foregoing rules under
any comparable provision of state, local or foreign law.
(g) Neither Eiger nor any Eiger Subsidiary has been a United States real
property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(h) Neither Eiger nor any Eiger Subsidiary is a party to any Tax allocation, Tax sharing or similar agreement (including indemnity
arrangements), other than commercial contracts entered into in the Ordinary Course of Business with vendors, customers and landlords.
(i) Neither Eiger nor any Eiger Subsidiary has ever been a member of an affiliated group filing a consolidated, combined or unitary Tax
Return (other than a group the common parent of which is Eiger) for federal, state, local or foreign Tax purposes. Neither Eiger nor any Eiger Subsidiary has any Liability for the Taxes of any Person (other than Eiger and any Eiger Subsidiary) under
Treasury Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign law), as a transferee or successor, by Contract, or otherwise.
(j) Neither Eiger nor any Eiger Subsidiary has distributed stock of another Person, or has had its stock distributed by another Person,
in a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code.
(k) Neither Eiger nor any Eiger Subsidiary will be required to include any item of income in, or exclude any item of deduction from,
taxable income for any period (or any portion thereof) ending after the Closing Date as a result of any (i) installment sale or other open transaction disposition made on or prior to the
A-18
Closing Date, or (ii) agreement with any Tax authority (including any closing agreement described in Section 7121 of the Code or any similar provision of state, local or foreign law)
made or entered into on or prior to the Closing Date.
(l) Neither Eiger nor any Eiger Subsidiary is a partner for Tax purposes
with respect to any joint venture, partnership, or, to the Knowledge of Eiger, other arrangement or contract which is treated as a partnership for Tax purposes.
(m) Neither Eiger nor any Eiger Subsidiary has entered into any transaction identified as a “listed transaction” for purposes
of Treasury Regulations Sections 1.6011-4(b)(2) or 301.6111-2(b)(2).
(n) Neither Eiger nor any Eiger Subsidiary has taken any
action, or has any knowledge of any fact or circumstance, that could reasonably be expected to prevent the transactions contemplated hereby, including the Merger, from qualifying as a reorganization within the meaning of Section 368(a) of the
Code.
2.13 Employee and Labor Matters; Benefit Plans.
(a) The employment of each of the Eiger and Eiger Subsidiary employees is terminable by Eiger or the applicable Eiger Subsidiary at
will (or otherwise in accordance with general principles of wrongful termination law). Eiger has made available to Celladon accurate and complete copies of all employee manuals and handbooks, disclosure materials, policy statements and other
materials relating to the employment of Eiger Associates to the extent currently effective and material.
(b) To the Knowledge of
Eiger, no officer or Key Employee of Eiger or any Eiger Subsidiary intends to terminate his or her employment with Eiger or the applicable Eiger Subsidiary, nor has any such officer or Key Employee threatened or expressed in writing any intention to
do so.
(c) Neither Eiger nor any Eiger Subsidiary is a party to or bound by, nor has a duty to bargain under, any collective
bargaining agreement or other Contract with a labor organization representing any of its employees, and there are no labor organizations representing, purporting to represent or, to the Knowledge of Eiger, seeking to represent any employees of Eiger
or any Eiger Subsidiary.
(d) There has never been, nor has there been any threat of, any strike, slowdown, work stoppage, lockout,
job action, union organizing activity, question concerning representation or any similar activity or dispute, affecting Eiger or any Eiger Subsidiary.
(e) Neither Eiger nor any Eiger Subsidiary is or has been engaged in any unfair labor practice within the meaning of the National Labor
Relations Act. There is no Legal Proceeding, claim, labor dispute or grievance pending or, to the Knowledge of Eiger, threatened or reasonably anticipated relating to any employment contract, privacy right, labor dispute, wages and hours, leave of
absence, plant closing notification, workers’ compensation policy, long-term disability policy, harassment, retaliation, immigration, employment statute or regulation, safety or discrimination matter involving any Eiger Associate, including
charges of unfair labor practices or discrimination complaints. Part 2.13(e) of the Eiger Disclosure Schedule lists all written and all non-written employee benefit plans (as defined in Section 3(3) of ERISA) and all bonus, equity-based,
incentive, deferred compensation, retirement or supplemental retirement, profit sharing, severance, golden parachute, vacation, cafeteria, dependent care, medical care, employee assistance program, education or tuition assistance programs and other
similar fringe or employee benefit plans, programs or arrangements, including any employment or executive compensation or severance agreements, written or otherwise, which are currently in effect relating to any present or former employee or
director of Eiger or any Eiger Subsidiary (or any trade or business (whether or not incorporated) which is an Eiger Affiliate) or which is maintained by, administered or contributed to by, or required to be contributed to by, Eiger, any Eiger
Subsidiary or any Eiger Affiliate, or under which Eiger or any Eiger Subsidiary or any Eiger Affiliate has any current or may incur liability after the date hereof (each, an “Eiger Employee Plan”).
A-19
(f) With respect to Eiger Options granted pursuant to the 2009 Plan, (i) each Eiger
Option intended to qualify as an “incentive stock option” under Section 422 of the Code so qualifies, (ii) each grant of an Eiger Option was duly authorized no later than the date on which the grant of such Eiger Option was by
its terms to be effective (the “Grant Date”) by all necessary corporate action, including, as applicable, approval by the board of directors of Eiger (or a duly constituted and authorized committee thereof) and any required
stockholder approval by the necessary number of votes or written consents, and the award agreement governing such grant (if any) was duly executed and delivered by each party thereto, (iii) each Eiger Option grant was made in accordance with
the terms of the 2014 Plan and all other applicable laws and regulatory rules or requirements and (iv) the per share exercise price of each Eiger Option was equal to the fair market value of a share of Eiger Common Stock on the applicable Grant
Date.
(g) Each Eiger Employee Plan that is intended to be qualified under Section 401(a) of the Code has received a favorable
determination with respect to such qualified status from the Internal Revenue Service. To the Knowledge of Eiger, nothing has occurred that would reasonably be expected to adversely affect the qualified status of any such Eiger Employee Plan or the
exempt status of any related trust.
(h) Each Eiger Employee Plan has been maintained in compliance, in all material respects, with
its terms and, both as to form and operation, with all applicable Legal Requirements, including the Code and ERISA.
(i) Neither
Eiger nor any Eiger Subsidiary has engaged in any transaction in violation of Sections 404 or 406 of ERISA or any “prohibited transaction,” as defined in Section 4975(c)(1) of the Code, for which no exemption exists under
Section 408 of ERISA or Section 4975(c)(2) or (d) of the Code, or has otherwise violated the provisions of Part 4 of Title I, Subtitle B of ERISA. Neither Eiger nor any Eiger Subsidiary has knowingly participated in a violation of
Part 4 of Title I, Subtitle B of ERISA by any plan fiduciary of any Eiger Employee Plan subject to ERISA and neither Eiger nor any Eiger Subsidiary has been assessed any civil penalty under Section 502(l) of ERISA.
(j) No Eiger Employee Plan is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, and neither Eiger nor
any Eiger Subsidiary or Eiger Affiliate has ever maintained, contributed to or partially or completely withdrawn from, or incurred any obligation or liability with respect to, any such plan. No Eiger Employee Plan is a Multiemployer Plan, and
neither Eiger nor any Eiger Subsidiary or Eiger Affiliate has ever contributed to or had an obligation to contribute, or incurred any liability in respect of a contribution, to any Multiemployer Plan.
(k) No Eiger Employee Plan provides for medical or death benefits beyond termination of service or retirement, other than
(i) pursuant to COBRA or an analogous state law requirement or (ii) death or retirement benefits under an Eiger Employee Plan qualified under Section 401(a) of the Code.
(l) Neither Eiger nor any Eiger Subsidiary is a party to any Contract that has resulted or would reasonably be expected to result,
separately or in the aggregate, in the payment of (i) any “excess parachute payment” within the meaning of section 280G of the Code and (ii) any amount the deduction for which would be disallowed under Section 162(m) of the
Code.
(m) To the Knowledge of Eiger, no payment pursuant to any Eiger Employee Plan or other arrangement to any “service
provider” (as such term is defined in Section 409A of the Code and the United States Treasury Regulations and IRS guidance thereunder) to Eiger or any Eiger Subsidiary, including the grant, vesting or exercise of any stock option, would
subject any Person to tax pursuant to Section 409A(1) of the Code, whether pursuant to the transactions contemplated by this Agreement or otherwise.
(n) Eiger and each of its Subsidiaries has complied with all state and federal laws applicable to employees, including COBRA, FMLA,
CFRA, HIPAA, the Women’s Health and Cancer Rights Act of 1998, the
A-20
Newborn’s and Mothers’ Health Protection Act of 1996, and any similar provisions of state law applicable to its employees. To the extent required under HIPAA and the regulations issued
thereunder, Eiger and each of its Subsidiaries has, prior to the Closing Date, performed all obligations under the medical privacy rules of HIPAA (45 C.F.R. Parts 160 and 164), the electronic data interchange requirements of HIPAA (45 C.F.R. Parts
160 and 162), and the security requirements of HIPAA (45 C.F.R. Part 142). Neither Eiger nor any of its Subsidiaries has any unsatisfied obligations to any employees or qualified beneficiaries pursuant to COBRA, HIPAA or any state law governing
health care coverage or extension.
(o) Eiger and each of its Subsidiaries is in material compliance with all applicable foreign,
federal, state and local laws, rules and regulations respecting employment, employment practices, terms and conditions of employment, worker classification, tax withholding, prohibited discrimination, equal employment, fair employment practices,
meal and rest periods, immigration status, employee safety and health, wages (including overtime wages), compensation and hours of work, and in each case, with respect to employees: (i) has withheld and reported all amounts required by law or
by agreement to be withheld and reported with respect to wages, salaries and other payments to employees, (ii) is not liable for any arrears of wages, severance pay or any Taxes or any penalty of any material amount for failure to comply with
any of the foregoing, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any governmental authority, with respect to unemployment compensation benefits, social security or other
benefits or obligations for employees (other than routine payments to be made in the normal course of business and consistent with past practice). There are no actions, suits, claims or administrative matters pending, threatened or reasonably
anticipated against Eiger or any of its Subsidiaries relating to any employee, employment agreement or Eiger Employee Plan. There are no pending or threatened or reasonably anticipated claims or actions against Eiger, any of its Subsidiaries, any
Eiger trustee or any trustee of any Subsidiary under any worker’s compensation policy or long-term disability policy. Neither Eiger nor any Subsidiary thereof is party to a conciliation agreement, consent decree or other agreement or order with
any federal, state or local agency or governmental authority with respect to employment practices.
(p) Part 2.13(p) of the
Disclosure Schedule lists all liabilities of Eiger or any of its Subsidiaries to any employee that result from the termination by Eiger or any of its Subsidiaries of such employee’s employment or provision of services, a change of control of
Eiger, or a combination thereof. Neither Eiger nor any of its Subsidiaries has any material liability with respect to any misclassification of: (a) any Person as an independent contractor rather than as an employee, (b) any employee leased
from another employer or (c) any employee currently or formerly classified as exempt from overtime wages. Neither Eiger nor any Subsidiary has taken any action which would constitute a “plant closing” or “mass layoff” within
the meaning of the WARN Act or similar state or local law, issued any notification of a plant closing or mass layoff required by the WARN Act or similar state or local law, or incurred any liability or obligation under WARN or any similar state or
local law that remains unsatisfied. No terminations of employees of Eiger or any of its Subsidiaries prior to the Closing would trigger any notice or other obligations under the WARN Act or similar state or local law.
(q) With respect to each Eiger Employee Plan, Eiger has made available to Celladon a true and complete copy of, to the extent
applicable, (i) such Eiger Employee Plan, (ii) the three (3) most recent annual reports (Form 5500) as filed with the Internal Revenue Service, (iii) each currently effective trust agreement related to such Eiger Employee Plan,
(iv) the most recent summary plan description for each Eiger Employee Plan for which such description is required, along with all summaries of material modifications, amendments, resolutions and all other material plan documentation related
thereto in the possession of Eiger, and (v) the most recent Internal Revenue Service determination or opinion letter or analogous ruling under foreign law issued with respect to any Eiger Employee Plan.
2.14 Environmental Matters. Eiger and each Eiger Subsidiary is in compliance with all applicable Environmental Laws, which compliance
includes the possession by Eiger of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof. Neither Eiger nor any Eiger Subsidiary has received since
January 1, 2010 any written notice or other
A-21
communication (in writing or otherwise), whether from a Governmental Body, citizens group, employee or otherwise, that alleges that Eiger or any Eiger Subsidiary is not in compliance with any
Environmental Law, and, to the Knowledge of Eiger, there are no circumstances that may prevent or interfere with Eiger’s or any of its Subsidiaries’ compliance with any Environmental Law in the future. To the Knowledge of Eiger:
(i) no current or prior owner of any property leased or controlled by Eiger or any of its Subsidiaries has received since January 1, 2010 any written notice or other communication relating to property owned or leased at any time by Eiger
or any of its Subsidiaries, whether from a Governmental Body, citizens group, employee or otherwise, that alleges that such current or prior owner or Eiger or any of its Subsidiaries is not in compliance with or has violated any Environmental Law
relating to such property and (ii) neither it nor any of its Subsidiaries has any material liability under any Environmental Law.
2.15 Insurance.
(a)
Eiger has delivered to Celladon accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of Eiger and each Eiger
Subsidiary. Each of such insurance policies is in full force and effect and Eiger and each Eiger Subsidiary are in compliance with the terms thereof. Other than customary end of policy notifications from insurance carriers, since January 1,
2010, neither Eiger nor any Eiger Subsidiary has received any notice or other communication regarding any actual or possible: (i) cancellation or invalidation of any insurance policy; (ii) refusal or denial of any coverage, reservation of
rights or rejection of any material claim under any insurance policy; or (iii) material adjustment in the amount of the premiums payable with respect to any insurance policy. There is no pending workers’ compensation or other claim under
or based upon any insurance policy of Eiger or any Eiger Subsidiary. All information provided to insurance carriers (in applications and otherwise) on behalf of Eiger and each Eiger Subsidiary is accurate and complete. Eiger and each Eiger
Subsidiary have provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding pending or threatened against Eiger or any Eiger Subsidiary, and no such carrier has issued a denial of coverage or a reservation of
rights with respect to any such Legal Proceeding, or informed Eiger or any Eiger Subsidiary of its intent to do so.
(b) Eiger has
delivered to Celladon accurate and complete copies of the existing policies (primary and excess) of directors’ and officers’ liability insurance maintained by Eiger and each Eiger Subsidiary as of the date of this Agreement (the
“Existing Eiger D&O Policies”). Part 2.15(b) of the Eiger Disclosure Schedule accurately sets forth the most recent annual premiums paid by Eiger and each Eiger Subsidiary with respect to the Existing Eiger D&O
Policies.
2.16 Legal Proceedings; Orders.
(a) There is no pending Legal Proceeding, and, to the Knowledge of Eiger, no Person has threatened in writing to commence any Legal
Proceeding: (i) that involves Eiger or any of its Subsidiaries, any Eiger Associate (in his or her capacity as such) or any of the material assets owned or used by Eiger or its Subsidiaries; or (ii) that challenges, or that may have the
effect of preventing, delaying, making illegal or otherwise interfering with, the Merger or any of the other Contemplated Transactions. To the Knowledge of Eiger, no event has occurred, and no claim, dispute or other condition or circumstance
exists, that will, or that would reasonably be expected to, give rise to or serve as a basis for the commencement of any such Legal Proceeding.
(b) There is no order, writ, injunction, judgment or decree to which Eiger or any Eiger Subsidiary, or any of the material assets owned
or used by Eiger or any Eiger Subsidiary, is subject. To the Knowledge of Eiger, no officer or other Key Employee of Eiger or any Eiger Subsidiary is subject to any order, writ, injunction, judgment or decree that prohibits such officer or other
employee from engaging in or continuing any conduct, activity or practice relating to the business of Eiger or any Eiger Subsidiary or to any material assets owned or used by Eiger or any Eiger Subsidiary.
2.17 Authority; Binding Nature of Agreement. Eiger and each Eiger Subsidiary has all necessary corporate power and authority to enter
into and to perform its obligations under this Agreement. The Board of
A-22
Directors of Eiger (at one or more meetings duly called and held) has: (a) determined that the Merger is advisable and fair to and in the best interests of Eiger and its stockholders;
(b) duly authorized and approved by all necessary corporate action, the execution, delivery and performance of this Agreement and the transactions contemplated hereby, including the Merger; and (c) recommended the adoption and
approval of this Agreement by the holders of Eiger Common Stock and Eiger Preferred Stock and directed that this Agreement and the Merger be submitted for consideration by Eiger’s stockholders in connection with the solicitation of the Required
Eiger Stockholder Vote. This Agreement has been duly executed and delivered by Eiger and, assuming the due authorization, execution and delivery by Celladon, constitutes the legal, valid and binding obligation of Eiger, enforceable against Eiger in
accordance with its terms, subject to: (i) laws of general application relating to bankruptcy, insolvency and the relief of debtors; and (ii) rules of law governing specific performance, injunctive relief and other equitable remedies.
Prior to the execution of the Eiger Stockholder Support Agreements, the Board of Directors of Eiger approved the Eiger Stockholder Support Agreements and the transactions contemplated thereby.
2.18 Inapplicability of Anti-takeover Statutes. The Board of Directors of Eiger has taken and will take all actions necessary to ensure
that the restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the execution, delivery and performance of this Agreement and the Eiger Stockholder Support Agreements and to the
consummation of the Merger and the other Contemplated Transactions. No other state takeover statute or similar Legal Requirement applies or purports to apply to the Merger, this Agreement, the Eiger Stockholder Support Agreements or any of the other
Contemplated Transactions.
2.19 Vote Required. The affirmative vote of (i) a majority of the shares of Eiger Preferred
Stock and Common Stock, voting together as a single class; (ii) at least 60% of the shares of Eiger Preferred Stock, and (iii) a majority of the shares of Eiger Common Stock, voting together as a single class, in each case, as outstanding
on the record date for the Eiger Stockholder Written Consent and entitled to vote thereon (the “Required Eiger Stockholder Vote”), is the only vote of the holders of any class or series of Eiger Capital Stock necessary to
adopt or approve this Agreement and approve the Merger and the matters set forth in Section 5.2(a).
2.20 Non-Contravention;
Consents. Subject to compliance with the HSR Act and any foreign antitrust Legal Requirement, obtaining the Required Eiger Stockholder Vote for the applicable Contemplated Transactions and the filing of the Certificate of Merger required by the
DGCL, neither (x) the execution, delivery or performance of this Agreement by Eiger, nor (y) the consummation of the Merger or any of the other Contemplated Transactions, will directly or indirectly (with or without notice or lapse of
time):
(a) contravene, conflict with or result in a violation of (i) any of the provisions of the certificate of
incorporation, bylaws or other charter or organizational documents of Eiger, or (ii) any resolution adopted by the stockholders, the board of directors or any committee of the board of directors of Eiger;
(b) contravene, conflict with or result in a material violation of, or give any Governmental Body or other Person the right to
challenge the Merger or any of the other Contemplated Transactions or to exercise any remedy or obtain any relief under, any Legal Requirement or any order, writ, injunction, judgment or decree to which Eiger or its Subsidiaries, or any of the
assets owned or used by Eiger or its Subsidiaries, is subject;
(c) contravene, conflict with or result in a material violation of
any of the terms or requirements of, or give any Governmental Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by Eiger or its Subsidiaries or that otherwise relates to the
business of Eiger or its Subsidiaries or to any of the assets owned or used by Eiger or its Subsidiaries;
(d) contravene, conflict
with or result in a violation or breach of, or result in a default under, any provision of any Eiger Contract, or give any Person the right to: (i) declare a default or exercise any remedy under any Eiger Contract; (ii) a rebate,
chargeback, penalty or change in delivery schedule under any such Eiger
A-23
Contract; (iii) accelerate the maturity or performance of any Eiger Contract; or (iv) cancel, terminate or modify any term of any Eiger Contract, except, in the case of any Eiger
Material Contract, any non-material breach, default, penalty or modification and, in the case of all other Eiger Contracts, any breach, default, penalty or modification that would not result in an Eiger Material Adverse Effect or default;
(e) result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by Eiger or its
Subsidiaries (except for minor liens that will not, in any case or in the aggregate, materially detract from the value of the assets subject thereto or materially impair the operations of Eiger); or
(f) result in, or increase the likelihood of, the transfer of any material asset of Eiger or its Subsidiaries to any Person.
Except (i) for any Consent set forth on Part 2.20 of the Eiger Disclosure Schedule under any Eiger Contract, (ii) the approval of this Agreement and
the Contemplated Transactions by Eiger’s stockholders, (iii) the filing of the Certificate of Merger with the Secretary of State of the State of Delaware pursuant to the DGCL, (iv) any required filings under the HSR Act and any
foreign antitrust Legal Requirement and (v) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable federal and state securities laws, neither Eiger nor any of its
Subsidiaries was, is or will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (x) the execution, delivery or performance of this Agreement or (y) the consummation of
the Merger or any of the other Contemplated Transactions.
2.21 Bank Accounts; Receivables.
(a) Part 2.21(a) of the Eiger Disclosure Schedule provides accurate information with respect to each account maintained by or for the
benefit of Eiger or any Eiger Subsidiary at any bank or other financial institution, including the name of the bank or financial institution, the account number, the balance as of November 3, 2015 and the names of all individuals authorized to
draw on or make withdrawals from such accounts.
(b) All existing accounts receivable of Eiger or any Eiger Subsidiary (including
those accounts receivable reflected on the Eiger Unaudited Interim Balance Sheet that have not yet been collected and those accounts receivable that have arisen since the date of the Eiger Unaudited Interim Balance Sheet and have not yet been
collected) (i) represent valid obligations of customers of Eiger or any Eiger Subsidiary arising from bona fide transactions entered into in the Ordinary Course of Business, and (ii) are current and are expected to be collected in full
when due, without any counterclaim or set off, net of applicable reserves for bad debts on the Eiger Unaudited Interim Balance Sheet.
2.22 No Financial Advisor. Except as set forth on Part 2.22 of the Eiger Disclosure Schedule, no broker, finder or investment banker is
entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Merger or any of the other Contemplated Transactions based upon arrangements made by or on behalf of Eiger
or any of its Subsidiaries.
2.23 Subscription Agreement. The Subscription Agreement has not been amended or modified in any manner
prior to the date of this Agreement. Neither Eiger nor, to the Knowledge of Eiger, any of its Affiliates has entered into any agreement, side letter or other arrangement relating to the Eiger Pre-Closing Financing, or the transactions contemplated
by the Subscription Agreement, other than as set forth in the Subscription Agreement. The respective obligations and agreements contained in the Subscription Agreement have not been withdrawn or rescinded in any respect. The Subscription Agreement
is in full force and effect and represents a valid, binding and enforceable obligation of Eiger and, to the Knowledge of Eiger, of each other party thereto, subject to the qualification that such enforceability may be limited by bankruptcy,
insolvency, reorganization or other laws of general application relating to or affecting rights of creditors. No event has occurred which, with or without
A-24
notice, lapse of time or both, would constitute a breach or default on the part of Eiger or, to the Knowledge of Eiger, any other party thereto, under the Subscription Agreement. To the Knowledge
of Eiger as of the date hereof, no party thereto will be unable to satisfy on a timely basis any term of the Subscription Agreement. There are no conditions precedent related to the consummation of the Eiger Pre-Closing Financing contemplated by the
Subscription Agreement, other than the satisfaction or waiver of the conditions expressly set forth in Article 5 of the Subscription Agreement. To the Knowledge of Eiger as of the date hereof, the Eiger Pre-Closing Financing will be made available
to Eiger prior to the consummation of the Merger.
2.24 Disclosure. The information supplied by Eiger and each Eiger Subsidiary for
inclusion in the Proxy Statement/Prospectus/Information Statement (including any Eiger Financials) will not, as of the date of the Proxy Statement/Prospectus/Information Statement or as of the date such information is prepared or presented,
(i) contain any statement that is inaccurate or misleading with respect to any material facts or (ii) omit to state any material fact necessary in order to make such information, in the light of the circumstances under which such
information is provided, not false or misleading.
| |
Section 3. |
REPRESENTATIONS AND WARRANTIES OF CELLADON AND MERGER SUB |
Celladon and
Merger Sub represent and warrant to Eiger as follows, except as set forth in the written disclosure schedule delivered by Celladon to Eiger (the “Celladon Disclosure Schedule”). The
Celladon Disclosure Schedule shall be arranged in sections and subsections corresponding to the numbered and lettered sections and subsections contained in this Section 3. The disclosures in any section or subsection of the Celladon
Disclosure Schedule shall qualify other sections and subsections in this Section 3 to the extent it is reasonably clear from a reading of the disclosure that such disclosure is applicable to such other sections and subsections. The
inclusion of any information in the Celladon Disclosure Schedule (or any update thereto) shall not be deemed to be an admission or acknowledgment, in and of itself, that such information is required by the terms hereof to be disclosed, is material,
has resulted in or would result in a Celladon Material Adverse Effect, or is outside the Ordinary Course of Business.
3.1 Subsidiaries; Due Organization; Etc.
(a) Other than Merger Sub, Celladon has no Subsidiaries and does not own any capital stock of, or any equity interest of any nature in,
any other Entity. Celladon has not agreed, nor is obligated to make nor bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Celladon has not been, at any time, a
general partner of, or otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
(b) Celladon is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its
incorporation and has all necessary power and authority: (i) to conduct its business in the manner in which its business is currently being conducted; (ii) to own and use its assets in the manner in which its assets are currently owned and
used; and (iii) to perform its obligations under all Contracts by which it is bound.
(c) Celladon is qualified to do business
as a foreign corporation, and is in good standing, under the laws of all jurisdictions where the nature of its business requires such qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate
would not be reasonably expected to have a Celladon Material Adverse Effect.
3.2 Certificate of Incorporation; Bylaws; Charters and
Codes of Conduct. Celladon has delivered to Eiger accurate and complete copies of the certificate of incorporation, bylaws and other charter and organizational documents, including all amendments thereto, for Celladon. Part 3.2 of the Celladon
Disclosure Schedule lists, and Celladon has delivered to Eiger, accurate and complete copies of: (a) the charters of all committees of Celladon’s board of directors; and (b) any code of conduct or similar policy adopted by Celladon
A-25
or by the board of directors, or any committee of the board of directors, of Celladon. Celladon has not taken any action in breach or violation of any of the provisions of its certificate of
incorporation, bylaws and other charter and organizational documents nor is in breach or violation of any of the material provisions of its certificate of incorporation, bylaws and other charter and organizational documents.
3.3 Capitalization, Etc.
(a) The authorized capital stock of Celladon consists of (i) 200,000,000 shares of Celladon Common Stock, par value $0.001 per
share, of which 23,916,021 shares were issued and outstanding as of October 31, 2015 (the “Capitalization Date”), and (ii) 10,000,000 shares of preferred stock, par value $0.001 per share, of which no shares are
issued and outstanding as of the Capitalization Date. Celladon does not hold any shares of its capital stock in its treasury. All of the outstanding shares of Celladon Common Stock have been duly authorized and validly issued, and are fully paid and
nonassessable. None of the outstanding shares of Celladon Common Stock is entitled or subject to any preemptive right, right of participation, right of maintenance or any similar right. None of the outstanding shares of Celladon Common Stock is
subject to any right of first refusal in favor of Celladon, other than early exercise rights and rights of repurchases in favor of Celladon with respect to such early exercise rights. Except as contemplated herein and except as identified on Part
3.3(a)(i) of the Celladon Disclosure Schedule, there is no Celladon Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right
with respect to), any shares of Celladon Common Stock. Celladon is not under any obligation, nor is bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Celladon Common
Stock or other securities. Part 3.3(a)(ii) of the Celladon Disclosure Schedule accurately and completely describes all repurchase rights held by Celladon with respect to shares of Celladon Common Stock (including shares issued pursuant to the
exercise of stock options) and specifies which of those repurchase rights are currently exercisable.
(b) Except for the Celladon
2013 Employee Stock Purchase Plan, the Celladon 2013 Equity Incentive Plan, the Celladon 2012 Equity Incentive Plan and the Celladon 2001 Stock Option Plan (collectively, the “Celladon Stock Plans”), or except
as set forth on Part 3.3(b) of the Celladon Disclosure Schedule, Celladon does not have any stock option plan or any other plan, program, agreement or arrangement providing for any equity or equity-based compensation for any Person. Part 3.3(b) of
the Celladon Disclosure Schedule sets forth the aggregate number of Celladon Options outstanding and a weighted average exercise price of such options. Celladon has made available to Eiger accurate and complete copies of all stock option plans
pursuant to which Celladon has ever granted stock options, the forms of all stock option agreements evidencing such options and evidence of board and stockholder approval of the Celladon Stock Plans and any amendments thereto.
(c) Except for the outstanding Celladon Options as set forth in Section 3.3(b), for the warrants identified in
Celladon’s most recent Quarterly Report on Form 10-Q filed with the SEC as of the date hereof (the “Celladon Warrants”) or as set forth on Part 3.3(c) of the Celladon Disclosure Schedule, there is no:
(i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of the capital stock or other securities of Celladon or any of its Subsidiaries; (ii) outstanding security,
instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock or other securities of Celladon or any of its Subsidiaries; (iii) stockholder rights plan (or similar plan commonly referred to
as a “poison pill”) or Contract under which Celladon or any of its Subsidiaries is or may become obligated to sell or otherwise issue any shares of its capital stock or any other securities; or (iv) condition or circumstance that may
give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or other securities of Celladon or any of its Subsidiaries. There are no
outstanding or authorized stock appreciation, phantom stock, profit participating or other similar rights with respect to Celladon.
(d) All outstanding shares of Celladon Common Stock and options, warrants and other securities of Celladon have been issued and granted
in compliance with (i) all applicable securities laws and other applicable
A-26
Legal Requirements, and (ii) all requirements set forth in applicable Contracts. Except as identified on Part 3.3(c) of the Celladon Disclosure Schedule, there are no Warrants to purchase
capital stock of Celladon outstanding on the date of this Agreement.
3.4 SEC Filings; Financial Statements.
(a) Celladon has made available to Eiger accurate and complete copies of all registration statements, proxy statements, Certifications
(as defined below) and other statements, reports, schedules, forms and other documents filed by Celladon with the SEC since January 1, 2014 (the “Celladon SEC Documents”), other than such documents that can
be obtained on the SEC’s website at www.sec.gov. Except as set forth on Part 3.4(a) of the Celladon Disclosure Schedule, all material statements, reports, schedules, forms and other documents required to have been filed by Celladon or
its officers with the SEC have been so filed on a timely basis. As of the time it was filed with the SEC (or, if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing), each of the Celladon SEC
Documents complied in all material respects with the applicable requirements of the Securities Act or the Exchange Act (as the case may be) and, to Celladon’s Knowledge, as of the time they were filed, none of the Celladon SEC Documents
contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading.
The certifications and statements required by (A) Rule 13a-14 under the Exchange Act and (B) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act) relating to the Celladon SEC Documents (collectively, the
“Certifications”) are accurate and complete and comply as to form and content with all applicable Legal Requirements. As used in this Section 3, the term “file” and variations thereof shall
be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC.
(b) The financial statements (including any related notes) contained or incorporated by reference in the Celladon SEC Documents:
(i) complied as to form in all material respects with the published rules and regulations of the SEC applicable thereto; (ii) were prepared in accordance with GAAP (except as may be indicated in the notes to such financial statements or,
in the case of unaudited financial statements, as permitted by Form 10-Q of the SEC, and except that the unaudited financial statements may not contain footnotes and are subject to normal and recurring year-end adjustments that are not reasonably
expected to be material in amount) applied on a consistent basis unless otherwise noted therein throughout the periods indicated; and (iii) fairly present the consolidated financial position of Celladon as of the respective dates thereof and
the results of operations and cash flows of Celladon for the periods covered thereby. Other than as expressly disclosed in the Celladon SEC Documents filed prior to the date hereof, there has been no material change in Celladon’s accounting
methods or principles that would be required to be disclosed in Celladon’s financial statements in accordance with GAAP. The books of account and other financial records of Celladon and each of its Subsidiaries are true and complete in all
material respects.
(c) Celladon’s auditor has at all times since the date of enactment of the Sarbanes-Oxley Act been:
(i) a registered public accounting firm (as defined in Section 2(a)(12) of the Sarbanes-Oxley Act); (ii) to the knowledge of Celladon, “independent” with respect to Celladon within the meaning of Regulation S-X under the
Exchange Act; and (iii) to the knowledge of Celladon, in compliance with subsections (g) through (l) of Section 10A of the Exchange Act and the rules and regulations promulgated by the SEC and the Public Company Accounting
Oversight Board thereunder.
(d) From January 1, 2010, through the date hereof, Celladon has not received any comment letter
from the SEC or the staff thereof or any correspondence from NASDAQ or the staff thereof relating to the delisting or maintenance of listing of the Celladon Common Stock on the NASDAQ Global Market. Celladon has not disclosed any unresolved comments
in its SEC Documents.
(e) Since January 1, 2010, there have been no formal internal investigations regarding financial
reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief
A-27
executive officer or chief financial officer of Celladon, Celladon’s Board of Directors or any committee thereof, other than ordinary course audits or reviews of accounting policies and
practices or internal controls required by the Sarbanes-Oxley Act.
(f) Celladon is in compliance in all material respects with the
applicable provisions of the Sarbanes-Oxley Act and the applicable listing and governance rules and regulations of the NASDAQ Global Market.
(g) Celladon maintains a system of internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the
Exchange Act) that is sufficient to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, including policies and procedures sufficient
to provide reasonable assurance (i) that Celladon maintains records that in reasonable detail accurately and fairly reflect Celladon’s transactions and dispositions of assets, (ii) that transactions are recorded as necessary to permit
preparation of financial statements in accordance with GAAP, (iii) that receipts and expenditures are made only in accordance with authorizations of management and Celladon’s Board of Directors, and (iv) regarding prevention or timely
detection of the unauthorized acquisition, use or disposition of Celladon’s assets that could have a material effect on Celladon’s financial statements. Celladon has evaluated the effectiveness of Celladon’s internal control over
financial reporting and, to the extent required by applicable law, presented in any applicable Celladon SEC Document that is a report on Form 10-K or Form 10-Q (or any
amendment thereto) its conclusions about the effectiveness of the internal control over financial reporting as of the end of the period covered by such report or amendment based on such evaluation. Celladon has disclosed to Celladon’s auditors
and the Audit Committee of Celladon’s Board of Directors (and made available to Eiger a summary of the significant aspects of such disclosure) (A) all significant deficiencies and material weaknesses in the design or operation of internal
control over financial reporting that are reasonably likely to adversely affect Celladon’s ability to record, process, summarize and report financial information and (B) any fraud, whether or not material, that involves management or other
employees who have a significant role in Celladon’s internal control over financial reporting. Except as disclosed in the Celladon SEC Documents filed prior to the date hereof, Celladon has not identified any material weaknesses in the design
or operation of Celladon’s internal control over financial reporting. Since December 31, 2014, there have been no material changes in Celladon’s internal control over financial reporting.
(h) Celladon’s “disclosure controls and procedures” (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act)
are reasonably designed to ensure that all information (both financial and non-financial) required to be disclosed by Celladon in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the
time periods specified in the rules and forms of the SEC, and that all such information is accumulated and communicated to Celladon’s management as appropriate to allow timely decisions regarding required disclosure and to make the
Certifications.
3.5 Absence of Changes. Except as set forth on Part 3.5 of the Celladon Disclosure Schedule, between
September 30, 2015 and the date of this Agreement and except as otherwise expressly contemplated by this Agreement:
(a) there
has not been any Celladon Material Adverse Effect or an event or development that would, individually or in the aggregate, reasonably be expected to have a Celladon Material Adverse Effect;
(b) there has not been any material loss, damage or destruction to, or any material interruption in the use of, any of the material
assets or business of Celladon (whether or not covered by insurance);
(c) Celladon has not: (i) declared, accrued, set aside
or paid any dividend or made any other distribution in respect of any shares of capital stock; or (ii) repurchased, redeemed or otherwise reacquired any shares of capital stock or other securities;
(d) Celladon has not sold, issued or granted, or authorized the issuance of: (i) any capital stock or other security (except for
Celladon Common Stock issued upon the valid exercise of outstanding Celladon Options);
A-28
(ii) any option, warrant or right to acquire any capital stock or any other security (except for Celladon Options identified in Part 3.3(b) of the Celladon Disclosure Schedule); or
(iii) any instrument convertible into or exchangeable for any capital stock or other security;
(e) Celladon has not amended
or waived any of its rights under, or exercised its discretion to permit the acceleration of vesting under any provision of: (i) any of the Celladon Stock Plans; (ii) any Celladon Option or any Contract evidencing or relating to any
Celladon Option; (iii) any restricted stock purchase agreement; or (iv) any other Contract evidencing or relating to any equity award (whether payable in cash or stock);
(f) there has been no amendment to the certificate of incorporation, bylaws or other charter or organizational documents of Celladon,
and Celladon has not effected or been a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction;
(g) Celladon has not formed any Subsidiary (other than Merger Sub) or acquired any equity interest or other interest in any other
Entity;
(h) Celladon has not: (i) lent money to any Person; (ii) incurred or guaranteed any indebtedness for borrowed
money; (iii) issued or sold any debt securities or options, warrants, calls or other rights to acquire any debt securities; (iv) guaranteed any debt securities of others; or (v) made any capital expenditure or commitment in excess of
$50,000 individually or $100,000 in the aggregate;
(i) Celladon has not, other than in the Ordinary Course of Business:
(i) adopted, established or entered into any Celladon Employee Plan; (ii) caused or permitted any Celladon Employee Plan to be amended other than as required by law; or (iii) paid any bonus or made any profit-sharing or similar
payment to, or increased the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors or employees;
(j) Celladon has not made, changed or revoked any material Tax election, filed any material amendment to any Tax Return, adopted or
changed any accounting method in respect of Taxes, changed any annual Tax accounting period, entered into any Tax allocation agreement, Tax sharing agreement or Tax indemnity agreement, other than commercial contracts entered into in the Ordinary
Course of Business with vendors, customers or landlords, entered into any closing agreement with respect to any Tax, settled or compromised any claim, notice, audit report or assessment in respect of material Taxes, applied for or entered into any
ruling from any Tax authority with respect to Taxes, surrendered any right to claim a material Tax refund, or consented to any extension or waiver of the statute of limitations period applicable to any material Tax claim or assessment;
(k) Celladon has not commenced or settled any Legal Proceeding;
(l) Celladon has not entered into any material transaction outside the Ordinary Course of Business;
(m) Celladon has not acquired any material asset nor sold, leased or otherwise irrevocably disposed of any of its material assets or
properties, nor has any Encumbrance been granted with respect to such assets or properties, except in the Ordinary Course of Business consistent with past practices;
(n) there has been no entry into, amendment or termination of any Celladon Material Contract;
(o) there has been no (i) material change in pricing or royalties or other payments set or charged by Celladon to its customers or
licensees, (ii) agreement by Celladon to change pricing or royalties or other payments set or charged by persons who have licensed Intellectual Property to any of Celladon, or (iii) as of the
A-29
date of this Agreement, material change in pricing or royalties or other payments set or charged by persons who have licensed Intellectual Property to Celladon; and
(p) Celladon has not negotiated, agreed or committed to take any of the actions referred to in clauses”(c)” through
“(o)” above (other than negotiations between the Parties to enter into this Agreement).
3.6 Intellectual Property.
(a) Celladon owns, or has the right to use, all Intellectual Property listed on Part 3.6(a) of the Celladon Disclosure Schedule
(“Celladon IP Rights”), except for any failure to own or have the right to use, or have the right to bring actions that would not reasonably be expected to have a Celladon Material Adverse Effect.
(b) Part 3.6(b) of the Celladon Disclosure Schedule is an accurate, true and complete listing of all Celladon Registered IP.
(c) Celladon has delivered, or made available to Eiger, a complete and accurate copy of all Celladon IP Rights Agreements. Celladon is
not a party to any Contract (A) pursuant to which the execution, delivery and performance of this Agreement and the consummation of the Contemplated Transactions will constitute a breach, or (B) as a result of such execution, delivery and
performance of this Agreement and the consummation of the Contemplated Transactions will cause the forfeiture or termination of or Encumbrance upon, or the grant of any license or other right to, or give rise to a right of forfeiture or termination
of or Encumbrance upon, any Celladon IP Rights or impair the right of Celladon or the Surviving Corporation to use, sell or license any Celladon IP Rights or portion thereof, except for the occurrence of any such breach, forfeiture, termination,
Encumbrance, grant or impairment that would not individually or in the aggregate, reasonably be expected to result in a Celladon Material Adverse Effect. With respect to each of the Celladon IP Rights Agreements: (i) each such agreement is
valid and binding on Celladon and in full force and effect; (ii) Celladon has not received any notice of termination or cancellation under such agreement, or received any notice of breach or default under such agreement, which breach has not
been cured or waived; and (iii) neither Celladon nor, to the Knowledge of Celladon, any other party to such agreement is in breach or default thereof in any material respect.
(d) Except as set forth on Part 3.6(d) of the Celladon Disclosure Schedule, neither the manufacture, marketing, license, sale or
intended use of any product or technology currently licensed or sold or under development by Celladon violates any license or agreement between Celladon and any third party or to the Knowledge of Celladon, infringes or misappropriates any valid
Intellectual Property right of any other party, which violation, infringement or misappropriation would reasonably be expected to have a Celladon Material Adverse Effect. Celladon has disclosed in correspondence to Eiger the third-party patents and
patent applications found during all freedom to operate searches that were conducted by Celladon related to any product or technology currently under development by Celladon. To the Knowledge of Celladon, no third party is infringing upon, or
violating any license or agreement with Celladon or relating to, any Celladon IP Rights.
(e) There is no current or pending
challenge, claim or Legal Proceeding (including any opposition, interference or other proceeding in any patent or other government office) contesting the validity, ownership or right to use, sell, license or dispose of any Celladon IP Rights, nor
has Celladon received any written notice asserting that any Celladon IP Rights or the proposed use, sale, license or disposition thereof conflicts with or infringes or misappropriates or will conflict with or infringe or misappropriate the rights of
any other party.
(f) To the Knowledge of Celladon, (i) no trademark (whether registered or unregistered) or trade name owned,
used, or applied for by Celladon conflicts or interferes with any trademark (whether registered or unregistered) or trade name owned, used, or applied for by any other Person and (ii) none of the goodwill associated with or inherent in any
trademark (whether registered or unregistered) in which Celladon has or purports to have an ownership interest has been impaired.
A-30
(g) Except as may be set forth in the Contracts listed on Part 3.6(g) of the Celladon
Disclosure Schedule (i) Celladon is not bound by any Contract to indemnify, defend, hold harmless, or reimburse any other Person with respect to any Intellectual Property infringement, misappropriation, or similar claim, and (ii) Celladon
has never assumed, or agreed to discharge or otherwise take responsibility for, any existing or potential liability of another Person for infringement, misappropriation, or violation of any Intellectual Property right.
3.7 Agreements, Contracts and Commitments. Part 3.7 of the Celladon Disclosure Schedule identifies:
(a) each Celladon Contract relating to any bonus, deferred compensation, severance, incentive compensation, pension, profit-sharing or
retirement plans, or any other employee benefit plans or arrangements;
(b) each Celladon Contract relating to the employment of,
or the performance of employment-related services by, any Person, including any employee, consultant or independent contractor, or entity providing employment related, consulting or independent contractor services, not terminable by Celladon on
ninety (90) days’ notice without liability, except to the extent general principles of wrongful termination law may limit Celladon’s ability to terminate employees at will;
(c) each Celladon Contract relating to any agreement or plan, including any stock option plan, stock appreciation right plan or stock
purchase plan, any of the benefits of which will be increased, or the vesting of benefits of which will be accelerated, by the occurrence of any of the Contemplated Transactions (either alone or in conjunction with any other event, such as
termination of employment) or the value of any of the benefits of which will be calculated on the basis of any of the Contemplated Transactions;
(d) each Celladon Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business
other than indemnification agreements between Celladon and any of its officers or directors;
(e) each Celladon Contract relating
to any agreement, contract or commitment containing any covenant limiting the freedom of Celladon or the Surviving Corporation to engage in any line of business or compete with any Person;
(f) each Celladon Contract relating to any agreement, contract or commitment relating to capital expenditures and involving obligations
after the date of this Agreement in excess of $100,000 and not cancelable without penalty;
(g) each Celladon Contract relating to
any agreement, contract or commitment currently in force relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(h) each Celladon Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other
agreements or instruments relating to the borrowing of money or extension of credit in excess of $100,000 or creating any Encumbrances with respect to any assets of Celladon or any loans or debt obligations with officers or directors of Celladon;
(i) each Celladon Contract relating to the following if currently in force and if the obligations of Celladon under such Celladon
Contract after the date of this Agreement are in excess of $50,000 in the aggregate (except that no dollar threshold shall apply to any Celladon Contract that, to the Knowledge of Celladon, includes most favored pricing arrangements, exclusivity
provisions, non-competition, non-solicitation or other similar limitations which could adversely affect or apply to the conduct of current clinical development programs or other operations of Eiger following the Closing (except the business of
Celladon as conducted prior to the date hereof)): (i) any distribution agreement (identifying any that contain exclusivity provisions); (ii) any agreement involving provision of services or products with respect to any pre-clinical or
clinical development activities of Celladon; (iii) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, development or other
A-31
agreement currently in force under which Celladon has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which Celladon has continuing
obligations to develop any Intellectual Property that will not be owned, in whole or in part, by Celladon; or (iv) any Contract to license any third party to manufacture or produce any product, service or technology of Celladon or any Contract
to sell, distribute or commercialize any products or service of Celladon, except agreements in the Ordinary Course of Business;
(j)
each Celladon Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing advisory services to Celladon in connection with the Contemplated Transactions; or
(k) any other agreement, contract or commitment which is not terminable at will (with no penalty or payment) by Celladon and
(i) which involves payment or receipt by Celladon after the date of this Agreement under any such agreement, contract or commitment of more than $50,000 in the aggregate, or obligations after the date of this Agreement in excess of $50,000 in
the aggregate, or (ii) that is material to the business or operations of Celladon.
Celladon has delivered or made available to Eiger
accurate and complete (except for applicable redactions thereto) copies of all Celladon Material Contracts, including all amendments thereto. There are no Celladon Material Contracts that are not in written form. Except as set forth on Part 3.7 of
the Celladon Disclosure Schedule, neither Celladon nor, to the Knowledge of Celladon, as of the date of this Agreement, any other party to a Celladon Material Contract (as defined below) has breached, violated or defaulted under, or received notice
that it has breached, violated or defaulted under, any of the terms or conditions of any of the agreements, contracts or commitments to which Celladon is a party or by which it is bound of the type described in clauses (a) through
(k) above (any such agreement, contract or commitment, a “Celladon Material Contract”) in such manner as would permit any other party to seek damages which would reasonably be
expected to have a Celladon Material Adverse Effect. The consummation of the Contemplated Transactions shall not (either alone or upon the occurrence of additional acts or events) result in any material payment or payments becoming due from Celladon
or the Surviving Corporation to any Person under any Celladon Contract.
3.8 Liabilities. As of the date hereof,
Celladon has no Liability except for: (i) Liabilities identified as such in the Celladon Unaudited Interim Balance Sheet; (ii) normal and recurring current Liabilities that have been incurred by Celladon since the date of the Celladon
Unaudited Interim Balance Sheet in the Ordinary Course of Business; (iii) Liabilities for performance in the Ordinary Course of Business of obligations of Celladon under Celladon Contracts, including the reasonably expected performance of such
Celladon Contracts in accordance with their terms (which would not include, for example, any instances of breach or indemnification); (iv) Liabilities described in Part 3.8 of the Celladon Disclosure Schedule; and (v) Liabilities incurred
in connection with the Contemplated Transactions.
3.9 Compliance; Permits; Restrictions.
(a) Celladon since January 1, 2010 has complied in all material respects with, is not in material violation of, and has not
received any written notices of alleged or actual material violation with respect to, any foreign, federal, state or local statute, law or regulation, including all applicable Legal Requirements. No investigation, claim, suit, proceeding, audit or
other action by any Governmental Body or authority is pending or, to the Knowledge of Celladon, threatened against Celladon, nor has any Governmental Body or authority indicated to Celladon an intention to conduct the same. There is no agreement,
judgment, injunction, order or decree binding upon Celladon which (i) has or could reasonably be expected to have the effect of prohibiting or materially impairing any business practice of Celladon, any acquisition of material property by
Celladon or the conduct of business by Celladon as currently conducted and planned to be conducted, (ii) may have an adverse effect on Celladon’s ability to comply with or perform any covenant or obligation under this Agreement or
(iii) may have the effect of preventing, delaying, making illegal or otherwise interfering with the Merger or any of the Contemplated Transactions.
A-32
(b) Celladon holds all Governmental Authorizations which are material to the operation of
its business (collectively, the “Celladon Permits”) as currently conducted and planned to be conducted. Part 3.9(b) of the Celladon Disclosure Schedule identifies each Celladon Permit. Celladon is in material compliance with
the terms of the Celladon Permits. No action, proceeding, revocation proceeding, amendment procedure, writ, injunction or claim is pending or, to the Knowledge of Celladon, threatened, which seeks to revoke, limit, suspend, or materially modify any
Celladon Permit. The rights and benefits of each material Celladon Permit will be available to the Surviving Corporation immediately after the Effective Time on terms substantially identical to those enjoyed by Celladon as of the date of this
Agreement and immediately prior to the Effective Time.
(c) There are no proceedings pending or, to the Knowledge of Celladon,
threatened with respect to an alleged material violation by Celladon of the FDCA, FDA regulations adopted thereunder, or any other similar Legal Requirements promulgated by a Drug Regulatory Agency.
(d) Celladon holds all required Governmental Authorizations issuable by any Drug Regulatory Agency necessary for the conduct of its
business as currently conducted, and, as applicable, development, clinical testing and manufacturing as currently conducted of any of its product candidates (the “Celladon Product Candidates”) (the “Celladon
Regulatory Permits”) and no such Celladon Regulatory Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any materially adverse manner. Celladon has not received any written notice or
other written communication from any Drug Regulatory Agency regarding any revocation, withdrawal, suspension, cancellation, termination or material modification of any Celladon Regulatory Permit. Except for the information and files identified in
Part 3.9(d) of the Celladon Disclosure Schedule, Celladon has made available to Eiger all information in its possession or control relating to the Celladon Product Candidates and the development, clinical testing, manufacturing, importation and
exportation of the Celladon Product Candidates, including complete copies of the following (to the extent there are any): (x) adverse event reports; clinical study reports and material study data; and inspection reports, notices of adverse
findings, warning letters, filings and letters and other written correspondence to and from any Drug Regulatory Agency; and meeting minutes with any Drug Regulatory Agency; and (y) similar reports, material study data, notices, letters,
filings, correspondence and meeting minutes with any other Governmental Authority.
(e) All clinical, pre-clinical and other
studies and tests conducted by or on behalf of, or sponsored by, Celladon or in which Celladon or its products or product candidates have participated were conducted in all material respects in accordance with standard medical and scientific
research procedures and in compliance with the applicable regulations of the Drug Regulatory Agencies and other applicable Legal Requirements, including 21 C.F.R. Parts 50, 54, 56, 58 and 312.
(f) Celladon is not the subject of any pending, or to the Knowledge of Celladon, threatened investigation in respect of its business or
products by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Knowledge of Celladon,
Celladon has not committed any acts, made any statement, or failed to make any statement, in each case in respect of its business or products that would violate FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal
Gratuities” Final Policy, and any amendments thereto. None of Celladon or any of its officers, employees or agents has been convicted of any crime or engaged in any conduct that could result in a material debarment or exclusion (i) under
21 U.S.C. Section 335a or (ii) any similar applicable Legal Requirement. To the Knowledge of Celladon, no material debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are
pending or threatened against Celladon or any of its officers, employees or agents.
3.10 Tax Matters.
(a) Celladon has timely filed all federal income Tax Returns and other material Tax Returns that it was required to file under
applicable Legal Requirements. All such Tax Returns were correct and complete in all
A-33
material respects and have been prepared in material compliance with all applicable Legal Requirements. No claim has ever been made by an authority in a jurisdiction where Celladon does not file
Tax Returns that it is subject to taxation by that jurisdiction.
(b) All material Taxes due and owing by Celladon on or before the
date hereof (whether or not shown on any Tax Return) have been paid. The unpaid Taxes of Celladon have been reserved for on the Celladon Unaudited Interim Balance Sheet in accordance with GAAP. Since the date of the Celladon Unaudited Interim
Balance Sheet, Celladon has not incurred any Liability for Taxes outside the Ordinary Course of Business or otherwise inconsistent with past custom and practice.
(c) Celladon has withheld and paid all Taxes required to have been withheld and paid in connection with any amounts paid or owing to
any employee, independent contractor, creditor, stockholder or other third party.
(d) There are no Encumbrances for Taxes (other
than Taxes not yet due and payable or Taxes that are being contested in good faith and for which adequate reserves have been made on Celladon’s Unaudited Interim Balance Sheet) upon any of the assets of Celladon.
(e) No material deficiencies for Taxes with respect to Celladon have been claimed, proposed or assessed by any Governmental Body in
writing. There are no pending (or, based on written notice, threatened) audits, assessments or other actions for or relating to any liability in respect of Taxes of Celladon. No issues relating to Taxes of Celladon were raised by the relevant Tax
authority in any completed audit or examination that would reasonably be expected to result in a material amount of Taxes in a later taxable period. Celladon has delivered or made available to Eiger complete and accurate copies of all federal income
Tax and all other material Tax Returns of Celladon for all taxable years remaining open under the applicable statute of limitations, and complete and accurate copies of all examination reports and statements of deficiencies assessed against or
agreed to by Celladon with respect to federal income Tax and all other material Taxes. Celladon has not waived any statute of limitations in respect of Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency, nor has
any request been made in writing for any such extension or waiver.
(f) All material elections with respect to Taxes affecting
Celladon as of the date hereof are set forth on Schedule 3.10(f). Celladon (i) has not consented at any time under former Section 341(f)(1) of the Code to have the provisions of former Section 341(f)(2) of the Code apply to any
disposition of the assets of Celladon; (ii) has not agreed, or is not required, to make any adjustment under Section 481(a) of the Code by reason of a change in accounting method or otherwise; (iii) has not made an election, or is not
required, to treat any of its assets as owned by another Person for Tax purposes or as a tax-exempt bond financed property or tax-exempt use property within the meaning of Section 168 of the Code; (iv) has not acquired or doesn’t own
any assets that directly or indirectly secure any debt the interest on which is tax exempt under Section 103(a) of the Code; (v) has not made or will not make a consent dividend election under Section 565 of the Code; (vi) has
not elected at any time to be treated as an S corporation within the meaning of Sections 1361 or 1362 of the Code; or (vii) has not made any of the foregoing elections or is not required to apply any of the foregoing rules under any comparable
provision of state, local or foreign law.
(g) Celladon has not been a United States real property holding corporation within the
meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(h)
Celladon is not a party to any Tax allocation, Tax sharing or similar agreement (including indemnity arrangements), other than commercial contracts entered into in the Ordinary Course of Business with vendors, customers and landlords.
(i) Celladon has never been a member of an affiliated group filing a consolidated, combined or unitary Tax Return (other than a group
the common parent of which is Celladon) for federal, state, local or foreign Tax
A-34
purposes. Celladon has no Liability for the Taxes of any Person (other than Celladon) under Treasury Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign law),
as a transferee or successor, by Contract or otherwise.
(j) Celladon has not distributed stock of another Person, nor had its
stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code.
(k) Celladon is not a partner for Tax purposes with respect to any joint venture, partnership, or, to the Knowledge of Celladon, other
arrangement or contract which is treated as a partnership for Tax purposes.
(l) Celladon will not be required to include any item
of income in, or exclude any item of deduction from, taxable income for any period (or any portion thereof) ending after the Closing Date as a result of any (i) installment sale or other open transaction disposition made on or prior to the
Closing Date, or (ii) agreement with any Tax authority (including any closing agreement described in Section 7121 of the Code or any similar provision of state, local or foreign law) made or entered into on or prior to the Closing Date.
(m) Celladon has not entered into any transaction identified as a “listed transaction” for purposes of Treasury
Regulations Sections 1.6011-4(b)(2) or 301.6111-2(b)(2).
(n) Celladon has not taken any action, nor has any knowledge of any fact
or circumstance, that could reasonably be expected to prevent the transactions contemplated hereby, including the Merger, from qualifying as a reorganization within the meaning of Section 368(a) of the Code.
3.11 Employee and Labor Matters; Benefit Plans.
(a) The employment of Celladon’s employees is terminable by Celladon at will (or otherwise in accordance with general principles
of wrongful termination law). Celladon has made available to Eiger accurate and complete copies of all employee manuals and handbooks, disclosure materials, policy statements and other materials relating to the employment of Celladon Associates to
the extent currently effective and material.
(b) Celladon is not a party to, bound by, or has a duty to bargain under, any
collective bargaining agreement or other Contract with a labor organization representing any of its employees, and there are no labor organizations representing, purporting to represent or, to the Knowledge of Celladon, seeking to represent any
employees of Celladon.
(c) Part 3.11(c) of the Celladon Disclosure Schedule lists all written and describes all non-written
employee benefit plans (as defined in Section 3(3) of ERISA) and all bonus, equity-based, incentive, deferred compensation, retirement or supplemental retirement, profit sharing, severance, golden parachute, vacation, cafeteria, dependent care,
medical care, employee assistance program, education or tuition assistance programs and other similar fringe or employee benefit plans, programs or arrangements, including any employment or executive compensation or severance agreements, written or
otherwise, which are currently in effect relating to any present or former employee or director of Celladon (or any trade or business (whether or not incorporated) which is a Celladon Affiliate) or which is maintained by, administered or contributed
to by, or required to be contributed to by, Celladon, or any Celladon Affiliate, or under which Celladon or any Celladon Affiliate has incurred or may incur any liability (each, an “Celladon Employee Plan”). Part 3.11(c) of
the Celladon Disclosure Schedule sets forth all amounts owed to any employee or consultant of Celladon, under any severance arrangement with Celladon, as a result of the consummation of the Merger or the Contemplated Transactions.
(d) With respect to each Celladon Employee Plan, Celladon has made available to Eiger a true and complete copy of, to the extent
applicable, (i) such Celladon Employee Plan, (ii) the three (3) most recent annual reports (Form 5500) as filed with the Internal Revenue Service, (iii) each currently effective trust agreement
A-35
related to such Celladon Employee Plan, (iv) the most recent summary plan description for each Celladon Employee Plan for which such description is required, along with all summaries of
material modifications, amendments, resolutions and all other material plan documentation related thereto in the possession of Celladon, and (v) the most recent Internal Revenue Service determination or opinion letter or analogous ruling under
foreign law issued with respect to any Celladon Employee Plan.
(e) Each Celladon Employee Plan that is intended to be qualified
under Section 401(a) of the Code has received a favorable determination with respect to such qualified status from the Internal Revenue Service. To the Knowledge of Celladon, nothing has occurred that would reasonably be expected to adversely
affect the qualified status of any such Celladon Employee Plan or the exempt status of any related trust.
(f) Each Celladon
Employee Plan has been maintained in compliance, in all material respects, with its terms and, both as to form and operation, with all applicable Legal Requirements, including the Code and ERISA.
(g) No Celladon Employee Plan is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, and neither Celladon
nor any Celladon Affiliate has ever maintained, contributed to or partially or completely withdrawn from, or incurred any obligation or liability with respect to, any such plan. No Celladon Employee Plan is a Multiemployer Plan, and neither Celladon
nor any Celladon Affiliate has ever contributed to or had an obligation to contribute, or incurred any liability in respect of a contribution, to any Multiemployer Plan. No Celladon Employee Plan is a Multiple Employer Plan.
(h) No Celladon Employee Plan (other than to the extent set forth in an employment, retention, change in control, deferred compensation
or severance agreement or arrangement between Celladon and any present or former employee or director) provides for medical or death benefits beyond termination of service or retirement, other than (i) pursuant to COBRA or an analogous state
law requirement or (ii) death or retirement benefits under an Celladon Employee Plan qualified under Section 401(a) of the Code.
(i) With respect to Celladon Options granted pursuant to the Celladon Stock Plans, (i) each Celladon Option intended to qualify as
an “incentive stock option” under Section 422 of the Code so qualifies, (ii) each grant of a Celladon Option was duly authorized no later than the date on which the Grant Date by all necessary corporate action, including, as
applicable, approval by the board of directors of Celladon (or a duly constituted and authorized committee thereof or, as applicable, an officer of Celladon duly authorized and designated under the applicable Celladon Stock Plan and in accordance
with applicable laws) and any required stockholder approval by the necessary number of votes or written consents, and the award agreement governing such grant (if any) was duly executed and delivered by each party thereto, (iii) each Celladon
Option grant was made in accordance with the terms of the Celladon Stock Plans, the Exchange Act and all other applicable laws and regulatory rules or requirements, including the rules of the Nasdaq Global Market and any other exchange on which
Celladon securities are traded, (iv) the per share exercise price of each Celladon Option was equal to the fair market value of a share of Celladon Common Stock on the applicable Grant Date and (v) each such Celladon Option grant was
properly accounted for in accordance with GAAP in the financial statements (including the related notes) of Celladon and disclosed in Celladon filings with the Securities and Exchange Commission in accordance with the Exchange Act and all other
applicable laws. Celladon has not knowingly granted, and there is no and has been no policy or practice of Celladon of granting, Celladon Options prior to, or otherwise coordinate the grant of Celladon Options with, the release or other public
announcement of material information regarding Celladon or its results of operations or prospects.
(j) To the Knowledge of
Celladon, no Celladon Options, stock appreciation rights or other equity-based awards issued or granted by Celladon are subject to the requirements of Code Section 409A. To the Knowledge of Celladon, each “nonqualified deferred
compensation plan” (as such term is defined under Section 409A(d)(1) of the Code and the guidance thereunder) under which Celladon makes, is obligated to make or promises to make, payments (each, a “409A Plan”)
complies in all material respects, in both form and operation, with the
A-36
requirements of Code Section 409A and the guidance thereunder. No payment to be made under any 409A Plan is, or to the Knowledge of Celladon will be, subject to the penalties of Code
Section 409A(a)(1).
(k) Celladon is in compliance with all of its bonus, commission and other compensation plans and has paid
any and all amounts required to be paid under such plans, including any and all bonuses and commissions (or pro rata portion thereof) that may have accrued or been earned through the calendar quarter preceding the Effective Time, and is not liable
for any payments, taxes or penalties for failure to comply with any of the terms or conditions of such plans or the laws governing such plans.
(l) Celladon has complied with all state and federal laws applicable to employees, including but not limited to COBRA, FMLA, CFRA,
HIPAA, the Women’s Health and Cancer Rights Act of 1998, the Newborn’s and Mothers’ Health Protection Act of 1996, and any similar provisions of state law applicable to its employees. Celladon has no unsatisfied obligations to any of
its employees or qualified beneficiaries pursuant to COBRA, HIPAA or any state law governing health care coverage or extension.
(m)
Celladon is in material compliance with all applicable foreign, federal, state and local laws, rules and regulations respecting employment, employment practices, terms and conditions of employment, worker classification, tax withholding,
prohibited discrimination, equal employment, fair employment practices, meal and rest periods, immigration status, employee safety and health, wages (including overtime wages), compensation, and hours of work, and in each case, with respect to
employees: (i) has withheld and reported all amounts required by law or by agreement to be withheld and reported with respect to wages, salaries and other payments to employees, (ii) is not liable for any arrears of wages, severance pay or
any Taxes or any penalty of any material amount for failure to comply with any of the foregoing, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any governmental authority, with
respect to unemployment compensation benefits, social security or other benefits or obligations for employees (other than routine payments to be made in the normal course of business and consistent with past practice). There are no actions, suits,
claims or administrative matters pending, threatened or reasonably anticipated against Celladon relating to any employee, employment agreement or Celladon Employee Plan. There are no pending or threatened or reasonably anticipated claims or actions
against Celladon or any Celladon trustee under any worker’s compensation policy or long-term disability policy. Celladon is not party to a conciliation agreement, consent decree or other agreement or order with any federal, state, or local
agency or governmental authority with respect to employment practices. Part 3.11(m) of the Disclosure Schedule lists all liabilities of Celladon to any of its employees that result from the termination by Celladon of such employee’s employment
or provision of services, a change of control of Celladon, or a combination thereof. Celladon has no material liability with respect to any misclassification of: (a) any Person as an independent contractor rather than as an employee,
(b) any employee leased from another employer, or (c) any employee currently or formerly classified as exempt from overtime wages. Celladon has not taken any action which would constitute a “plant closing” or “mass
layoff” within the meaning of the WARN Act or similar state or local law, issued any notification of a plant closing or mass layoff required by the WARN Act or similar state or local law, or incurred any liability or obligation under WARN or
any similar state or local law that remains unsatisfied. No terminations of employees of Celladon prior to the Closing would trigger any notice or other obligations under the WARN Act or similar state or local law.
(n) There has never been, nor has there been any threat of, any strike, slowdown, work stoppage, lockout, job action, union organizing
activity, question concerning representation or any similar activity or dispute, affecting Celladon. No event has occurred, and no condition or circumstance exists, that might directly or indirectly be likely to give rise to or provide a basis for
the commencement of any such strike, slowdown, work stoppage, lockout, job action, union organizing activity, question concerning representation or any similar activity or dispute.
(o) Celladon is not, nor has been, engaged in any unfair labor practice within the meaning of the National Labor Relations Act. There
is no Legal Proceeding, claim, labor dispute or grievance pending or, to the
A-37
Knowledge of Celladon, threatened or reasonably anticipated relating to any employment contract, privacy right, labor dispute, wages and hours, leave of absence, plant closing notification,
workers’ compensation policy, long-term disability policy, harassment, retaliation, immigration, employment statute or regulation, safety or discrimination matter involving any Celladon Associate, including charges of unfair labor practices or
discrimination complaints.
(p) There is no contract, agreement, plan or arrangement to which Celladon or any Celladon Affiliate is
a party or by which it is bound to compensate any of its employees for excise taxes paid pursuant to Section 4999 of the Code.
(q) Celladon is not a party to any Contract that has resulted or would reasonably be expected to result, separately or in the
aggregate, in the payment of (i) any “excess parachute payment” within the meaning of section 280G of the Code and (ii) any amount the deduction for which would be disallowed under Section 162(m) of the Code.
3.12 Environmental Matters. Celladon is in compliance with all applicable Environmental Laws, which compliance includes the possession
by Celladon of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof. Celladon has not received since January 1, 2010 any written notice or other
communication (in writing or otherwise), whether from a Governmental Body, citizens group, employee or otherwise, that alleges that Celladon is not in compliance with any Environmental Law, and, to the Knowledge of Celladon, there are no
circumstances that may prevent or interfere with Celladon’s compliance with any Environmental Law in the future. To the Knowledge of Celladon: (i) no current or prior owner of any property leased or controlled by Celladon has received
since January 1, 2010 any written notice or other communication relating to property owned or leased at any time by Celladon, whether from a Governmental Body, citizens group, employee or otherwise, that alleges that such current or prior owner
or Celladon is not in compliance with or violated any Environmental Law relating to such property and (ii) Celladon has no material liability under any Environmental Law.
3.13 Insurance.
(a)
Celladon made available to Eiger accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of Celladon. Each of such
insurance policies is in full force and effect and Celladon is in compliance with the terms thereof. Other than customary end of policy notifications from insurance carriers, since January 1, 2010, Celladon has not received any notice or other
communication regarding any actual or possible: (i) cancellation or invalidation of any insurance policy; (ii) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy; or
(iii) material adjustment in the amount of the premiums payable with respect to any insurance policy. There is no pending workers’ compensation or other claim under or based upon any insurance policy of Celladon. All information provided
to insurance carriers (in applications and otherwise) on behalf of Celladon is accurate and complete. Celladon has provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding pending or threatened in writing
against Celladon, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed Celladon of its intent to do so.
(b) Celladon has made available to Eiger accurate and complete copies of the existing policies (primary and excess) of directors’
and officers’ liability insurance maintained by Celladon as of the date of this Agreement (the “Existing Celladon D&O Policies”). Part 3.13(b) of the Celladon Disclosure Schedule accurately sets forth the most recent
annual premiums paid by Celladon with respect to the Existing Celladon D&O Policies.
3.14 Transactions with Affiliates. Except
as set forth in the Celladon SEC Documents filed prior to the date of this Agreement, since the date of Celladon’s last proxy statement filed in 2015 with the SEC, no event has occurred that would be required to be reported by Celladon pursuant
to Item 404 of Regulation S-K promulgated by the SEC. Part 3.14 of the Celladon Disclosure Schedule identifies each Person who is (or who may be deemed to be) an Affiliate of Celladon as of the date of this Agreement.
A-38
3.15 Legal Proceedings; Orders.
(a) Except as set forth in Part 3.15 of the Celladon Disclosure Schedule, there is no pending Legal Proceeding, and, to the Knowledge
of Celladon, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves Celladon or any Celladon Associate (in his or her capacity as such) or any of the material assets owned or used by Celladon; or (ii) that
challenges, or that may have the effect of preventing, delaying, making illegal or otherwise interfering with, the Merger or any of the other Contemplated Transactions. To the Knowledge of Celladon, no event has occurred, and no claim, dispute or
other condition or circumstance exists, that will, or that would reasonably be expected to, give rise to or serve as a basis for the commencement of any such Legal Proceeding. With regard to any Legal Proceeding set forth on Part 3.15 of the
Celladon Disclosure Schedule, Celladon has provided Eiger or its counsel all pleadings and material written correspondence related to such Legal Proceeding, all insurance policies and material written correspondence with brokers and insurers related
to such Legal Proceedings and other information material to an assessment of such Legal Proceeding. Celladon has an insurance policy or policies that is expected to cover such Legal Proceeding and has complied with the requirements of such insurance
policy or policies to obtain coverage with respect to such Legal Proceeding under such insurance policy or policies.
(b) There is
no order, writ, injunction, judgment or decree to which Celladon or any of the assets owned or used by Celladon is subject. To the Knowledge of Celladon, no officer or other Key Employee of Celladon is subject to any order, writ, injunction,
judgment or decree that prohibits such officer or other employee from engaging in or continuing any conduct, activity or practice relating to the business of Celladon or to any material assets owned or used by Celladon.
3.16 Authority; Binding Nature of Agreement. Each of Celladon and Merger Sub has all necessary corporate power and authority to enter
into and to perform its obligations under this Agreement. Each of the Boards of Directors of Celladon and Merger Sub (at meetings duly called and held) has: (a) determined that the Merger is advisable and fair to and in the best interests of
such Party and its stockholders; (b) duly authorized and approved by all necessary corporate action, the execution, delivery and performance of this Agreement and the transactions contemplated hereby, including the Merger; and
(c) recommended the adoption and approval of this Agreement by the holders of Celladon Common Stock and directed that this Agreement and the issuance of shares of Celladon Common Stock in the Merger be submitted for consideration by
Celladon’s stockholders at the Celladon Stockholders’ Meeting. This Agreement has been duly executed and delivered by Celladon and Merger Sub and, assuming the due authorization, execution and delivery by Eiger, constitutes the legal,
valid and binding obligation of Celladon or Merger Sub (as applicable), enforceable against each of Celladon and Merger Sub in accordance with its terms, subject to: (i) laws of general application relating to bankruptcy, insolvency and the
relief of debtors; and (ii) rules of law governing specific performance, injunctive relief and other equitable remedies. Prior to the execution of the Celladon Stockholder Support Agreements, the Board of Directors of Celladon approved the
Celladon Stockholder Support Agreements and the transactions contemplated thereby.
3.17 Inapplicability of Anti-takeover Statutes.
The Boards of Directors of Celladon and Merger Sub have taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the
execution, delivery and performance of this Agreement and the Celladon Stockholder Support Agreements and to the consummation of the Merger and the other Contemplated Transactions. No other state takeover statute or similar Legal Requirement applies
or purports to apply to the Merger, this Agreement, the Celladon Stockholder Support Agreements or any of the other Contemplated Transactions.
3.18 Vote Required. The affirmative vote of the holders of a majority of the outstanding shares of Celladon Common Stock is the only
vote of the holders of any class or series of Celladon’s capital stock necessary to approve the Merger, the issuance of Celladon Common Stock in the Merger and the Reverse Split (the “Required Celladon Stockholder
Vote”).
A-39
3.19 Non-Contravention; Consents. Subject to compliance with the HSR Act and any foreign
antitrust Legal Requirement, obtaining the Required Celladon Stockholder Vote for the applicable Contemplated Transactions and the filing of the Certificate of Merger required by the DGCL, neither (x) the execution, delivery or performance of
this Agreement by Celladon or Merger Sub, nor (y) the consummation of the Merger or any of the other Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(a) contravene, conflict with or result in a violation of (i) any of the provisions of the certificate of incorporation, bylaws or
other charter or organizational documents of Celladon or Merger Sub, or (ii) any resolution adopted by the stockholders, the board of directors or any committee of the board of directors of Celladon or Merger Sub;
(b) contravene, conflict with or result in a violation of, or give any Governmental Body or other Person the right to challenge the
Merger or any of the other Contemplated Transactions or to exercise any remedy or obtain any relief under, any Legal Requirement or any order, writ, injunction, judgment or decree to which Celladon or any of the assets owned or used by Celladon is
subject;
(c) contravene, conflict with or result in a violation of any of the terms or requirements of, or give any Governmental
Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by Celladon or that otherwise relates to the business of Celladon or to any of the assets owned or used by Celladon;
(d) contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Celladon
Contract, or give any Person the right to: (i) declare a default or exercise any remedy under any Celladon Contract; (ii) a rebate, chargeback, penalty or change in delivery schedule under any such Celladon Contract; (iii) accelerate
the maturity or performance of any Celladon Contract; or (iv) cancel, terminate or modify any term of any Celladon Contract; except, in the case of any Celladon Material Contract, any non-material breach, default, penalty or modification and in
the case of all other Celladon Contracts, any breach, default, penalty or modification that would not result in a Celladon Material Adverse Effect;
(e) result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by Celladon (except for
minor liens that will not, in any case or in the aggregate, materially detract from the value of the assets subject thereto or materially impair the operations of Celladon); or
(f) result in, or increase the likelihood of, the transfer of any material asset of Celladon to any Person.
Except (i) for any Consent set forth on Part 3.19 of the Celladon Disclosure Schedule under any Celladon Contract, (ii) the approval of the Merger
and the issuance of shares of Celladon Common Stock in the Merger, (iii) the filing of the Certificate of Merger with the Secretary of State of the State of Delaware pursuant to the DGCL, (iv) the filing of an amendment to Celladon’s
certificate of incorporation to effect the Reverse Split, (v) any required filings under the HSR Act, any foreign antitrust Legal Requirement and (vi) such consents, waivers, approvals, orders, authorizations, registrations, declarations
and filings as may be required under applicable federal and state securities laws, Celladon was not, is not, nor will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with
(x) the execution, delivery or performance of this Agreement, or (y) the consummation of the Merger or any of the other Contemplated Transactions.
3.20 Bank Accounts; Deposits.
(a) Part 3.20(a) of the Celladon Disclosure Schedule provides accurate information with respect to each account maintained by or for
the benefit of Celladon at any bank or other financial institution, including the name of the bank or financial institution, the account number, the balance as of October 31, 2015 and the names of all individuals authorized to draw on or make
withdrawals from such accounts.
A-40
(b) All existing accounts receivable of Celladon (including those accounts receivable
reflected on the Celladon Unaudited Interim Balance Sheet that have not yet been collected and those accounts receivable that have arisen since the date of the Celladon Unaudited Interim Balance Sheet and have not yet been collected)
(i) represent valid obligations of customers of Celladon arising from bona fide transactions entered into in the Ordinary Course of Business, and (ii) are current and collectible in full when due, without any counterclaim or set off, net
of applicable reserves for bad debts on the Celladon Unaudited Interim Balance Sheet. All deposits of Celladon (including those set forth on the Celladon Unaudited Interim Balance Sheet) which are individually more than $25,000 or more than $50,000
in the aggregate are fully refundable to Celladon.
3.21 No Financial Advisor. Except as set forth on Part 3.21 of the Celladon
Disclosure Schedule, no broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Merger or any of the other Contemplated
Transactions based upon arrangements made by or on behalf of Celladon.
3.22 Valid Issuance. The Celladon Common Stock to be issued
in the Merger will, when issued in accordance with the provisions of this Agreement be validly issued, fully paid and nonassessable.
3.23 Code of Ethics. Celladon has adopted a code of ethics, as defined by Item 406(b) of Regulation S-K of the SEC, for senior
financial officers, applicable to its principal executive officer, principal financial officer, controller or principal accounting officer, or persons performing similar functions. Celladon has promptly disclosed any change in or waiver of
Celladon’s code of ethics with respect to any such persons, as required by Section 406(b) of the Sarbanes-Oxley Act. To the Knowledge of Celladon, there have been no violations of provisions of Celladon’s code of ethics by any such
persons.
3.24 Title to Assets. Celladon owns, and has good and valid title to, or, in the case of leased properties and assets,
valid leasehold interests in, all tangible properties or assets and equipment used or held for use in its business or operations or purported to be owned by it.
3.25 Real Property; Leasehold. Celladon does not own any real property or any interest in real property, except for the leaseholds
created under the real property leases identified in Part 3.25 of the Celladon Disclosure Schedule, which are in full force and effect and with no existing default thereunder.
| |
Section 4. |
CERTAIN COVENANTS OF THE PARTIES |
4.1 Access and Investigation. Subject to
the terms of the Confidentiality Agreement which the Parties agree will continue in full force following the date of this Agreement, during the period commencing on the date of this Agreement and ending at the Effective Time (the
“Pre-Closing Period”), upon reasonable notice each Party shall, and shall use commercially reasonable efforts to cause such Party’s Representatives to: (a) provide the other Party and such other Party’s
Representatives with reasonable access during normal business hours to such Party’s Representatives, personnel and assets and to all existing books, records, Tax Returns, work papers and other documents and information relating to such Party
and its Subsidiaries; (b) provide the other Party and such other Party’s Representatives with such copies of the existing books, records, Tax Returns, work papers, product data, and other documents and information relating to such Party
and its Subsidiaries, and with such additional financial, operating and other data and information regarding such Party and its Subsidiaries as the other Party may reasonably request; and (c) permit the other Party’s officers and other
employees to meet, upon reasonable notice and during normal business hours, with the chief financial officer and other officers and managers of such Party responsible for such Party’s financial statements and the internal controls of such Party
to discuss such matters as the other Party may deem necessary or appropriate in order to enable the other Party to satisfy its obligations under the Sarbanes-Oxley Act and the rules and regulations relating thereto. Without limiting the generality
of any of the foregoing, during the Pre-Closing Period, each Party shall promptly make available to the other Party copies of:
(i)
the unaudited monthly consolidated balance sheets of such Party as of the end of each calendar month and the related unaudited monthly consolidated statements of operations, statements of stockholders’
A-41
equity and statements of cash flows for such calendar month, which shall be delivered within twenty days after the end of such calendar month, or such longer periods as the Parties may agree to
in writing;
(ii) all material operating and financial reports prepared by such Party for its senior management, including sales
forecasts, marketing plans, development plans, discount reports, write-off reports, hiring reports and capital expenditure reports prepared for its management;
(iii) any written materials or communications sent by or on behalf of a Party to its stockholders;
(iv) any material notice, document or other communication sent by or on behalf of a Party to any party to any Celladon Material
Contract or Eiger Material Contract, as applicable, or sent to a Party by any party to any Celladon Material Contract or Eiger Material Contract, as applicable (other than any communication that relates solely to routine commercial transactions
between such Party and the other party to any such Celladon Material Contract or Eiger Material Contract, as applicable, and that is of the type sent in the Ordinary Course of Business and consistent with past practices);
(v) any notice, report or other document filed with or otherwise furnished, submitted or sent to any Governmental Body on behalf of a
Party in connection with the Merger or any of the Contemplated Transactions;
(vi) any non-privileged notice, document or other
communication sent by or on behalf of, or sent to, a Party relating to any pending or threatened Legal Proceeding involving or affecting such Party; and
(vii) any material notice, report or other document received by a Party from any Governmental Body.
Notwithstanding the foregoing, any Party may restrict the foregoing access to the extent that any Legal Requirement applicable to such Party requires such
Party to restrict or prohibit access to any of such Party’s properties or information.
4.2 Operation of Celladon’s
Business.
(a) Except as set forth on Part 4.2(a) of the Celladon Disclosure Schedule, during the Pre-Closing Period:
(i) Celladon shall conduct its business and operations: (A) in the Ordinary Course of Business but, as reasonably deemed appropriate and with the prior written consent of Eiger (which shall not be unreasonably withheld, conditioned or
delayed), with a view towards potentially winding down its historical clinical development programs and related operations; and (B) in compliance with all applicable Legal Requirements and the requirements of all Contracts that constitute
Celladon Material Contracts; (ii) Celladon shall continue to pay outstanding accounts payable and other current Liabilities (including payroll) when due and payable; and (iii) Celladon shall promptly notify Eiger of: (A) any notice or
other communication from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (B) any Legal Proceeding against, relating to, involving or otherwise affecting Celladon
that is commenced, or, to the Knowledge of Celladon, threatened against, Celladon after the date of the Merger Agreement; and (C) any notice or other communication from any Person alleging that any payment or other obligation is or will be owed
to such Person at any time before or after the date of this Agreement, except for invoices or other communications related to agreements or dealings in the Ordinary Course of Business or payments or obligations identified in this Agreement,
including the Celladon Disclosure Schedule.
(b) During the Pre-Closing Period, Celladon shall promptly notify Eiger in writing, by
delivering an updated Celladon Disclosure Schedule, of: (i) the discovery by Celladon of any event, condition, fact or circumstance that occurred or existed on or prior to the date of this Agreement and that caused or constitutes a material
inaccuracy in any representation or warranty made by Celladon in this Agreement in a manner that causes the conditions set forth in Section 8.1 not to be satisfied; (ii) any event, condition, fact or circumstance
A-42
that occurs, arises or exists after the date of this Agreement and that would cause or constitute a material inaccuracy in any representation or warranty made by Celladon in this Agreement in a
manner that causes the conditions set forth in Section 8.1 not to be satisfied if: (A) such representation or warranty had been made as of the time of the occurrence, existence or discovery of such event, condition, fact or
circumstance; or (B) such event, condition, fact or circumstance had occurred, arisen or existed on or prior to the date of this Agreement; (iii) any material breach of any covenant or obligation of Celladon; and (iv) any event,
condition, fact or circumstance that would reasonably be expected to make the timely satisfaction of any of the conditions set forth in Section 6, 7 or 8 impossible or materially less likely. Without limiting the generality
of the foregoing, Celladon shall promptly advise Eiger in writing of any Legal Proceeding or material, written claim threatened, commenced or asserted against or with respect to, or otherwise affecting, Celladon or, to the Knowledge of Celladon, any
director, officer or Key Employee of Celladon. No notification given to Eiger pursuant to this Section 4.2(b) shall change, limit or otherwise affect any of the representations, warranties, covenants or obligations of Celladon or any of
its Subsidiaries contained in this Agreement or the Celladon Disclosure Schedule for purposes of Section 8.1.
4.3
Operation of Eiger’s Business.
(a) Except as set forth on Part 4.3(a) of the Eiger Disclosure Schedule, during the
Pre-Closing Period: (i) each of Eiger and its Subsidiaries shall conduct its business and operations: (A) in the Ordinary Course of Business and in accordance with past practices and with respect to recently acquired assets currently part
of the business of Eiger, consistent with customary practice for the manufacture and development of such assets; and (B) in compliance with all applicable Legal Requirements and the requirements of all Contracts that constitute Eiger Material
Contracts; and (ii) each of Eiger and its Subsidiaries shall use reasonable efforts to keep available the services of its current Key Employees, officers and other employees and maintain its relations and goodwill with all suppliers, customers,
landlords, creditors, licensors, licensees, employees and other Persons having material business relationships with Eiger or its Subsidiaries; (iii) continue to make regularly scheduled payments on its existing debt when due and payable (and
not make any prepayments), if any; (iv) continue to pay outstanding accounts payable and other current Liabilities (including payroll) when due and payable; and (v) promptly notify Celladon of: (A) any notice or other communication
from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (B) any Legal Proceeding against, relating to, involving or otherwise affecting Eiger or any of its
Subsidiaries that is commenced, or, to the Knowledge of Eiger, threatened against, Eiger or any of its Subsidiaries; and (C) any notice or other communication from any Person alleging that any payment or other obligation is or will be owed to
such Person at any time before or after the date of this Agreement, except for invoices or other communications related to agreements or dealings in the Ordinary Course of Business or payments or obligations identified in this Agreement, including
the Eiger Disclosure Schedule.
(b) During the Pre-Closing Period, Eiger shall promptly notify Celladon in writing, by delivery of
an updated Eiger Disclosure Schedule, of: (i) the discovery by Eiger of any event, condition, fact or circumstance that occurred or existed on or prior to the date of this Agreement and that caused or constitutes a material inaccuracy in any
representation or warranty made by Eiger in this Agreement in a manner that causes the conditions set forth in Section 7.1 not to be satisfied; (ii) any event, condition, fact or circumstance that occurs, arises or exists after the
date of this Agreement and that would cause or constitute a material inaccuracy in any representation or warranty made by Eiger in this Agreement in a manner that causes the conditions set forth in Section 7.1 not to be satisfied if:
(A) such representation or warranty had been made as of the time of the occurrence, existence or discovery of such event, condition, fact or circumstance; or (B) such event, condition, fact or circumstance had occurred, arisen or existed
on or prior to the date of this Agreement; (iii) any material breach of any covenant or obligation of Eiger; and (iv) any event, condition, fact or circumstance that would reasonably be expected to make the timely satisfaction of any of
the conditions set forth in Section 6, 7 or 8 impossible or materially less likely. Without limiting the generality of the foregoing, Eiger shall promptly advise Celladon in writing of any Legal Proceeding or material,
written claim threatened in writing, commenced or asserted against or with respect to, or otherwise affecting, Eiger or any of its Subsidiaries or, to the Knowledge of
A-43
Eiger, any director, officer or Key Employee of Eiger or any of its Subsidiaries. No notification given to Celladon pursuant to this Section 4.3(b) shall change, limit or otherwise
affect any of the representations, warranties, covenants or obligations of Eiger contained in this Agreement or the Eiger Disclosure Schedule for purposes of Section 7.1.
4.4 Negative Obligations.
(a) Except (i) as expressly contemplated or permitted by this Agreement, (ii) as set forth in Part 4.4(a) of the Celladon
Disclosure Schedule, or (iii) with the prior written consent of Eiger, at all times during the period commencing with the execution and delivery of this Agreement and continuing until the earlier to occur of the termination of this Agreement
pursuant to Section 9 and the Effective Time, Celladon shall not, nor shall it cause or permit any of its Subsidiaries to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or made any other distribution in respect of any shares of its capital stock; or
repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except for shares of Celladon Common Stock from terminated employees of Celladon);
(ii) except for contractual commitments in place at the time of this Agreement as listed in Part 4.4(a)(ii) of the Celladon Disclosure
Schedule, sell, issue or grant, or authorize the issuance of: (i) any capital stock or other security (except for shares of Celladon Common Stock issued upon the valid exercise of Celladon Options or Celladon Warrants outstanding as of the date
of this Agreement); (ii) any option, warrant or right to acquire any capital stock or any other security; or (iii) any instrument convertible into or exchangeable for any capital stock or other security;
(iii) amend the certificate of incorporation, bylaws or other charter or organizational documents of Celladon, or effect or be a party
to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except as related to the Contemplated Transactions;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity;
(v) lend money to any Person; other than in the Ordinary Course of Business, incur or guarantee any indebtedness for borrowed money;
issue or sell any debt securities or options, warrants, calls or other rights to acquire any debt securities; guarantee any debt securities of others; or make any capital expenditure or commitment;
(vi) adopt, establish or enter into any Celladon Employee Plan; cause or permit any Celladon Employee Plan to be amended other than as
required by law or in order to make amendments for the purposes of Section 409A of the Code, subject to prior review and approval (with such approval not to be unreasonably withheld, conditioned or delayed) by Eiger; other than in the Ordinary
Course of Business, pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors or employees; or
increase the severance or change of control benefits offered to any current or new service providers, provided, that Celladon may pay those severance and retention payments owed under existing Celladon Employee Plans scheduled on Part 3.11(m)
of the Celladon Disclosure Schedule to its current or former employees in connection with their termination of employment;
(vii)
enter into any material transaction outside the Ordinary Course of Business, except any transactions with a view towards potentially winding down Celladon’s historical clinical development programs and related operations as reasonably
deemed appropriate by Celladon and with the prior written consent of Eiger (which shall not be unreasonably withheld, conditioned or delayed);
(viii) acquire any material asset, except in the Ordinary Course of Business consistent with past practices;
A-44
(ix) make, change or revoke any material Tax election; file any material amendment to any
Tax Return; adopt or change any accounting method in respect of Taxes; change any annual Tax accounting period; enter into any Tax allocation agreement, Tax sharing agreement or Tax indemnity agreement, other than commercial contracts entered into
in the Ordinary Course of Business with vendors, customers or landlords; enter into any closing agreement with respect to any Tax; settle or compromise any claim, notice, audit report or assessment in respect of material Taxes; apply for or enter
into any ruling from any Tax authority with respect to Taxes; surrender any right to claim a material Tax refund; or consent to any extension or waiver of the statute of limitations period applicable to any material Tax claim or assessment;
(x) enter into any Celladon Material Contract;
(xi) materially change pricing or royalties or other payments set or charged by Celladon to its customers or licensees; agree to
materially increase pricing or royalties or other payments set or charged by persons who have licensed Intellectual Property to Celladon; or as of the date of this Agreement, materially increase pricing or royalties or other payments set or charged
by persons who have licensed Intellectual Property to Celladon; or
(xii) agree, resolve or commit to do any of the foregoing.
(b) Except (i) as expressly contemplated or permitted by this Agreement, (ii) as set forth on Part 4.4(b) of the Eiger
Disclosure Schedule, or (iii) with the prior written consent of Celladon, at all times during the period commencing with the execution and delivery of this Agreement and continuing until the earlier to occur of the termination of this Agreement
pursuant to Section 9 and the Effective Time, Eiger shall not, nor shall it cause or permit any of its Subsidiaries to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock; or
repurchase, redeem or otherwise reacquire any shares of capital stock or other securities (except for shares of Eiger Common Stock from terminated employees of Eiger);
(ii) amend the certificate of incorporation, bylaws or other charter or organizational documents of Eiger, or effect or be a party to
any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction;
(iii) sell, issue or grant, or authorize the issuance of, or make any commitments to do any of the foregoing, other than as
contemplated by the Contemplated Transactions: (i) any capital stock or other security (except for shares of Eiger Common Stock issued upon the valid exercise of Eiger Options or Eiger Warrants outstanding as of the date of this Agreement);
(ii) any option, warrant or right to acquire any capital stock or any other security; or (iii) any instrument convertible into or exchangeable for any capital stock or other security;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity;
(v) lend money to any Person; other than in the Ordinary Course of Business, incur or guarantee any indebtedness for borrowed money;
issue or sell any debt securities or options, warrants, calls or other rights to acquire any debt securities; guarantee any debt securities of others; or make any capital expenditure or commitment in excess of $5,000;
(vi) other than in the Ordinary Course of Business, and in observance of common practice for a similarly situated company:
(A) adopt, establish or enter into any Eiger Employee Plan; (B) cause or permit any Eiger Employee Plan to be amended other than as required by law; or (C) pay any bonus or made any profit-sharing or similar payment to, or increase
the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors, officers or employees;
(vii) enter into any material transaction outside the Ordinary Course of Business;
A-45
(viii) acquire any material asset nor sell, lease other otherwise irrevocably dispose of
any of its assets or properties, or grant any Encumbrance with respect to such assets or properties, except in the Ordinary Course of Business;
(ix) make, change or revoke any material Tax election; file any material amendment to any Tax Return; adopt or change any accounting
method in respect of Taxes; change any annual Tax accounting period; enter into any Tax allocation agreement, Tax sharing agreement or Tax indemnity agreement, other than commercial contracts entered into in the Ordinary Course of Business with
vendors, customers or landlords; enter into any closing agreement with respect to any Tax; settle or compromise any claim, notice, audit report or assessment in respect of material Taxes; apply for or enter into any ruling from any Tax authority
with respect to Taxes; surrender any right to claim a material Tax refund; or consent to any extension or waiver of the statute of limitations period applicable to any material Tax claim or assessment;
(x) enter into, amend or terminate any Eiger Material Contract other than in the Ordinary Course of Business with respect to the
business as currently being conducted; provided such amendment or termination shall not include winding down any of Eiger’s current clinical development programs and related operations;
(xi) materially change pricing or royalties or other payments set or charged by Eiger or any Eiger Subsidiary to its customers or
licensees; or agree to materially increase pricing or royalties or other payments to an existing licensor of Intellectual Property for no additional consideration to Eiger; or
(xii) agree, resolve or commit to do any of the foregoing.
4.5 No Solicitation.
(a) Each Party agrees that neither it nor any of its Subsidiaries shall, nor shall it nor any of its Subsidiaries authorize or permit
any of the officers, directors, employees, investment bankers, attorneys, accountants, Representatives, consultants or other agents retained by it or any of its Subsidiaries to directly or indirectly: (i) solicit, initiate, encourage, induce or
knowingly facilitate the communication, making, submission or announcement of any Acquisition Proposal or Acquisition Inquiry or take any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry;
(ii) furnish any information regarding such Party to any Person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry; (iii) engage in discussions or negotiations with any Person with respect to any
Acquisition Proposal or Acquisition Inquiry; (iv) approve, endorse or recommend any Acquisition Proposal (subject to Sections 5.2 and 5.3); (v) execute or enter into any letter of intent or similar document or any Contract
contemplating or otherwise relating to any Acquisition Transaction; or (vi) grant any waiver or release under any confidentiality, standstill or similar agreement (other than to the other Party); provided, however, that,
notwithstanding anything contained in this Section 4.5(a), prior to the adoption and approval of this Agreement by a Party’s stockholders (i.e., the Required Eiger Stockholder Vote, in the instance of Eiger, or the Required
Celladon Stockholder Vote, in the instance of Celladon), such Party may furnish nonpublic information regarding such Party to, and enter into discussions or negotiations with, any Person in response to a bona fide written Acquisition Proposal, which
such Party’s Board of Directors determines in good faith, after consultation with a nationally recognized independent financial advisor, if any, and its outside legal counsel, constitutes, or is reasonably likely to result in, a Superior Offer
(and is not withdrawn) if: (A) neither such Party nor any Representative of such Party shall have breached this Section 4.5; (B) the Board of Directors of such Party concludes in good faith based on the advice of outside legal
counsel, that the failure to take such action is reasonably likely to result in a breach of the fiduciary duties of the Board of Directors of such Party under applicable Legal Requirements; (C) at least five (5) Business Days prior to
furnishing any such nonpublic information to, or entering into discussions with, such Person, such Party gives the other Party written notice of the identity of such Person and of such Party’s intention to furnish nonpublic information to, or
enter into discussions with, such Person; (D) such Party receives from such Person an executed confidentiality agreement containing provisions (including nondisclosure provisions, use restrictions, non-solicitation provisions, no hire
provisions and “standstill” provisions) at least as favorable to such Party as
A-46
those contained in the Confidentiality Agreement; and (E) at least five (5) Business Days prior to furnishing any such nonpublic information to such Person, such Party furnishes such
nonpublic information to the other Party (to the extent such nonpublic information has not been previously furnished by such Party to the other Party). Without limiting the generality of the foregoing, each Party acknowledges and agrees that, in the
event any Representative of such Party (whether or not such Representative is purporting to act on behalf of such Party) takes any action that, if taken by such Party, would constitute a breach of this Section 4.5 by such Party, the
taking of such action by such Representative shall be deemed to constitute a breach of this Section 4.5 by such Party for purposes of this Agreement.
(b) If any Party or any Representative of such Party receives an Acquisition Proposal or Acquisition Inquiry at any time during the
Pre-Closing Period, then such Party shall promptly (and in no event later than 24 hours after such Party becomes aware of such Acquisition Proposal or Acquisition Inquiry) advise the other Party orally and in writing of such Acquisition Proposal or
Acquisition Inquiry (including the identity of the Person making or submitting such Acquisition Proposal or Acquisition Inquiry, and the terms thereof). Such Party shall keep the other Party informed in all material respects with respect to the
status and terms of any such Acquisition Proposal or Acquisition Inquiry and any modification or proposed modification thereto. In addition to the foregoing, each Party shall provide the other Party with at least five (5) Business Days’
written notice of a meeting of its board of directors (or any committee thereof) at which its board of directors (or any committee thereof) is reasonably expected to consider an Acquisition Proposal or Acquisition Inquiry it has received.
(c) Each Party shall immediately cease and cause to be terminated any existing discussions, negotiations and communications with any
Person that relate to any Acquisition Proposal or Acquisition Inquiry as of the date of this Agreement and cause the destruction or return of any nonpublic information provided to such Person.
| |
Section 5. |
ADDITIONAL AGREEMENTS OF THE PARTIES |
5.1 Registration Statement; Proxy
Statement/Prospectus/Information Statement.
(a) As promptly as practicable after the date of this Agreement, the Parties shall
prepare and cause to be filed with the SEC the Proxy Statement/Prospectus/Information Statement and Celladon shall prepare and cause to be filed with the SEC the Form S-4 Registration Statement, in which the Proxy Statement/Prospectus/Information
Statement will be included as a prospectus. Celladon covenants and agrees that the Proxy Statement/Prospectus/Information Statement, including any pro forma financial statements included therein (and the letter to stockholders, notice of meeting and
form of proxy included therewith), will not, at the time that the Proxy Statement/Prospectus/Information Statement or any amendment or supplement thereto is filed with the SEC or is first mailed to the stockholders of Celladon, at the time of the
Celladon Stockholders’ Meeting and at the Effective Time, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of
the circumstances under which they were made, not misleading. Notwithstanding the foregoing, Celladon makes no covenant, representation or warranty with respect to statements made in the Proxy Statement/Prospectus/Information Statement (and the
letter to stockholders, notice of meeting and form of proxy included therewith), if any, based on information furnished in writing by Eiger specifically for inclusion therein. Each of the Parties shall use commercially reasonable efforts to cause
the Form S-4 Registration Statement and the Proxy Statement/Prospectus/Information Statement to comply with the applicable rules and regulations promulgated by the SEC, to respond promptly to any comments of the SEC or its staff and to have the Form
S-4 Registration Statement declared effective under the Securities Act as promptly as practicable after it is filed with the SEC. Each of the Parties shall use commercially reasonable efforts to cause the Proxy Statement/Prospectus/Information
Statement to be mailed to Celladon’s stockholders as promptly as practicable after the Form S-4 Registration Statement is declared effective under the Securities Act. Each Party shall promptly furnish to the other Party all information
concerning such Party and such Party’s subsidiaries and such Party’s stockholders that may be required or reasonably requested in connection with any action contemplated by this Section 5.1. If any event relating to
A-47
Eiger occurs, or if Eiger becomes aware of any information, that should be disclosed in an amendment or supplement to the Form S-4 Registration Statement or the Proxy
Statement/Prospectus/Information Statement, then Eiger shall promptly inform Celladon thereof and shall cooperate fully with Celladon in filing such amendment or supplement with the SEC and, if appropriate, in mailing such amendment or supplement to
Celladon’s stockholders.
(b) Prior to the Effective Time, Celladon shall use commercially reasonable efforts to obtain all
regulatory approvals needed to ensure that the Celladon Common Stock to be issued in the Merger (to the extent required) shall be registered or qualified or exempt from registration or qualification under the securities law of every jurisdiction of
the United States in which any registered holder of Eiger Capital Stock has an address of record on the record date for determining the stockholders entitled to notice of and to vote pursuant to the Eiger Stockholder Written Consent;
provided, however, that Celladon shall not be required: (i) to qualify to do business as a foreign corporation in any jurisdiction in which it is not now qualified; or (ii) to file a general consent to service of process in
any jurisdiction.
(c) Eiger shall reasonably cooperate with Celladon and provide, and require its Representatives, advisors,
accountants and attorneys to provide, Celladon and its Representatives, advisors, accountants and attorneys, with all true, correct and complete information regarding Eiger that is required by law to be included in the Form S-4 Registration
Statement or reasonably requested from Eiger to be included in the Form S-4 Registration Statement. Without limiting the foregoing, Eiger will use commercially reasonable efforts to cause to be delivered to Celladon a letter of Eiger’s
independent accounting firm, dated no more than two (2) Business Days before the date on which the Form S-4 Registration Statement becomes effective (and reasonably satisfactory in form and substance to Celladon), that is customary in scope and
substance for letters delivered by independent public accountants in connection with registration statements similar to the S-4 Registration Statement.
5.2 Eiger Stockholder Written Consent.
(a) Promptly after the S-4 Registration Statement shall have been declared effective under the Securities Act, and in any event no
later than two (2) Business Days thereafter, Eiger shall obtain the approval by written consent from certain of those Eiger stockholders sufficient for the Required Eiger Stockholder Vote in lieu of a meeting pursuant to Section 228 of the
DGCL for purposes of (i) adopting this Agreement and approving the Merger, and all other transactions contemplated hereby, including the Preferred Stock Conversion, (ii) acknowledging that the approval given thereby is irrevocable and that
such stockholder is aware of its rights to demand appraisal for its shares pursuant to Section 262 of the DGCL, a copy of which was attached thereto, and that such stockholder has received and read a copy of Section 262 of the DGCL, and
(iii) acknowledging that by its approval of the Merger it is not entitled to appraisal rights with respect to its shares in connection with the Merger and thereby waives any rights to receive payment of the fair value of its capital stock under
the DGCL. Under no circumstances shall Eiger assert that any other approval or consent is necessary by its stockholders to approve the Merger or this Agreement.
(b) Eiger agrees that, subject to Section 5.2(c): (i) Eiger’s Board of Directors shall recommend that
Eiger’s stockholders vote to adopt and approve this Agreement and the Merger and shall use commercially reasonable efforts to solicit such approval within the time set forth in Section 5.2(a) (the recommendation of Eiger’s
Board of Directors that Eiger’s stockholders vote to adopt and approve this Agreement being referred to as the “Eiger Board Recommendation”); and (ii) the Eiger Board Recommendation shall not be withdrawn or
modified in a manner adverse to Celladon, and no resolution by the Board of Directors of Eiger or any committee thereof to withdraw or modify the Eiger Board Recommendation in a manner adverse to Celladon shall be adopted or proposed.
(c) Notwithstanding anything to the contrary contained in Section 5.2(b), at any time prior to the approval of this
Agreement by the Required Eiger Stockholder Vote, Eiger’s Board of Directors may withhold,
A-48
amend, withdraw or modify the Eiger Board Recommendation in a manner adverse to Celladon if, but only if, Eiger’s Board of Directors determined in good faith, based on such matters as it
deems relevant following consultation with its outside legal counsel, that the failure to withdraw, withhold, amend, or modify such recommendation would result in a breach of its fiduciary duties under applicable Legal Requirements; provided,
that Celladon receives written notice from Eiger confirming that Eiger’s Board of Directors has determined to change its recommendation at least five (5) Business Days in advance of the Eiger Board Recommendation being so withdrawn,
withheld, amended or modified in a manner adverse to Celladon.
(d) Eiger’s obligation to solicit the consent of its
stockholders to sign the Eiger Stockholder Written Consent in accordance with Section 5.2(a) shall not be limited or otherwise affected by the commencement, disclosure, announcement or submission of any Superior Offer or other
Acquisition Proposal, or by any withdrawal or modification of the Eiger Board Recommendation.
5.3 Celladon Stockholders’
Meeting.
(a) Celladon shall take all action necessary under applicable Legal Requirements to call, give notice of and hold a
meeting of the holders of Celladon Common Stock to vote on the Merger, the issuance of Celladon Common Stock in the Merger and the Reverse Split (such meeting, the “Celladon Stockholders’ Meeting”). The Celladon
Stockholders’ Meeting shall be held as promptly as practicable after the Form S-4 Registration Statement is declared effective under the Securities Act. Celladon shall take reasonable measures to ensure that all proxies solicited in connection
with the Celladon Stockholders’ Meeting are solicited in compliance with all applicable Legal Requirements.
(b) Celladon
agrees that, subject to Section 5.3(c): (i) Celladon’s Board of Directors shall recommend that the holders of Celladon Common Stock vote to approve the Merger, the issuance of Celladon Common Stock in the Merger and the Reverse
Split and shall use commercially reasonable efforts to solicit such approval within the timeframe set forth in Section 5.3(a) above, (ii) the Proxy Statement/Prospectus/Information Statement shall include a statement to the effect
that the Board of Directors of Celladon recommends that Celladon’s stockholders vote to approve the Merger, the issuance of Celladon Common Stock in the Merger and the Reverse Split (the recommendation of Celladon’s Board of Directors that
Celladon’s stockholders vote to approve the Merger, the issuance of Celladon Common Stock in the Merger and the Reverse Split being referred to as the “Celladon Board Recommendation”); and (iii) the Celladon Board
Recommendation shall not be withdrawn or modified in a manner adverse to Eiger, and no resolution by the Board of Directors of Celladon or any committee thereof to withdraw or modify the Celladon Board Recommendation in a manner adverse to Eiger
shall be adopted or proposed.
(c) Notwithstanding anything to the contrary contained in Section 5.3(b), at any time
prior to the approval of the issuance of Celladon Common Stock in the Merger by the stockholders of Celladon by the Required Celladon Stockholder Vote, Celladon’s Board of Directors may withhold, amend, withdraw or modify the Celladon Board
Recommendation in a manner adverse to Eiger or recommend any Acquisition Transaction (collectively a “Celladon Board Adverse Recommendation Change”) if, but only if, Celladon’s Board of Directors determines in good
faith, based on such matters as it deems relevant following consultation with its outside legal counsel, that the failure to withhold, amend, withdraw or modify such recommendation would result in a breach of its fiduciary duties under applicable
Legal Requirements; provided, that Eiger receives written notice from Celladon confirming that Celladon’s Board of Directors intends to change its recommendation at least five (5) Business Days in advance of the Celladon Board
Recommendation being withdrawn, withheld, amended or modified in a manner adverse to Eiger. Such notice shall describe in reasonable details the reasons for such intention and if such reasons are related to a Superior Offer, then also specifying the
material terms and conditions of such Superior Offer, including the identity of the Person making such offer (and attaching the most current and complete version of any written agreement or other document relating thereto).
(d) Celladon’s obligation to call, give notice of and hold the Celladon Stockholders’ Meeting in accordance with
Section 5.3(a) shall not be limited or otherwise affected by the commencement, disclosure,
A-49
announcement or submission of any Superior Offer or Acquisition Proposal, or by any withdrawal or modification of the Celladon Board Recommendation.
(e) Nothing contained in this Agreement shall prohibit Celladon or its Board of Directors from (i) taking and disclosing to the
stockholders of Celladon a position as contemplated by Rule 14e-2(a) under the Exchange Act or complying with the provisions of Rule 14d-9 under the Exchange Act (other than Rule 14d-9(f) under the Exchange Act), (ii) making any disclosure to
the stockholders of Celladon if the Celladon Board of Directors determines in good faith, after consultation with its outside legal counsel, that the failure to make such disclosure would be inconsistent with its fiduciary duties to the stockholders
of Celladon under applicable Legal Requirements, and (iii) making a “stop, look and listen” communication to the stockholders of Celladon pursuant to Rule 14d-9(f) under the Exchange Act, provided, however, that (A) in the
case of each of the foregoing clauses “(i)” and “(ii),” any such disclosure or public statement shall be deemed to be a Celladon Board Adverse Recommendation Change subject to the terms and conditions of this Agreement unless
Celladon’s Board of Directors reaffirms the Celladon Board Recommendation in such disclosure or public statement or within seven (7) Business Days of such disclosure or public statement; (B) in the case of clause “(iii),”
any such disclosure or public statement shall be deemed to be a Celladon Board Adverse Recommendation Change subject to the terms and conditions of this Agreement unless Celladon’s Board of Directors reaffirms the Celladon Board Recommendation
in such disclosure or public statement or within seventeen (17) Business Days of such disclosure or public statement; and (C) Celladon shall not affect a Celladon Board Adverse Recommendation Change unless specifically permitted pursuant
to the terms of Section 5.3(c).
5.4 Regulatory Approvals. Each Party shall use commercially reasonable efforts to file or
otherwise submit, as soon as practicable after the date of this Agreement, all applications, notices, reports and other documents reasonably required to be filed by such Party with or otherwise submitted by such Party to any Governmental Body with
respect to the Merger and the other Contemplated Transactions, and to submit promptly any additional information requested by any such Governmental Body. Without limiting the generality of the foregoing, the Parties shall, promptly after the date of
this Agreement, prepare and file, if any, (a) the notification and report forms required to be filed under the HSR Act and (b) any notification or other document required to be filed in connection with the Merger under any applicable
foreign Legal Requirement relating to antitrust or competition matters. Eiger and Celladon shall respond as promptly as is practicable to respond in compliance with: (i) any inquiries or requests received from the Federal Trade Commission or
the Department of Justice for additional information or documentation; and (ii) any inquiries or requests received from any state attorney general, foreign antitrust or competition authority or other Governmental Body in connection with
antitrust or competition matters.
5.5 Eiger Options and Warrants.
(a) Subject to Section 5.5(c), at the Effective Time, each Eiger Option that is outstanding and unexercised immediately
prior to the Effective Time under the 2009 Plan, whether or not vested, shall be converted into and become an option to purchase Celladon Common Stock, and Celladon shall assume the 2009 Plan and each such Eiger Option in accordance with the terms
(as in effect as of the date of this Agreement) of the 2009 Plan and the terms of the stock option agreement by which such Eiger Option is evidenced. All rights with respect to Eiger Common Stock under Eiger Options assumed by Celladon shall
thereupon be converted into rights with respect to Celladon Common Stock. Accordingly, from and after the Effective Time: (i) each Eiger Option assumed by Celladon may be exercised solely for shares of Celladon Common Stock; (ii) the
number of shares of Celladon Common Stock subject to each Eiger Option assumed by Celladon shall be determined by multiplying (A) the number of shares of Eiger Common Stock that were subject to such Eiger Option, as in effect immediately prior
to the Effective Time, by (B) the Exchange Ratio and rounding the resulting number down to the nearest whole number of shares of Celladon Common Stock; (iii) the per share exercise price for the Celladon Common Stock issuable upon exercise
of each Eiger Option assumed by Celladon shall be determined by dividing (A) the per share exercise price of Eiger Common Stock subject to such Eiger Option, as in effect immediately prior to the Effective Time, by (B) the Exchange Ratio
and rounding the
A-50
resulting exercise price up to the nearest whole cent; and (iv) any restriction on the exercise of any Eiger Option assumed by Celladon shall continue in full force and effect and the term,
exercisability, vesting schedule and other provisions of such Eiger Option shall otherwise remain unchanged; provided, however, that: (A) to the extent provided under the terms of an Eiger Option, such Eiger Option assumed by Celladon in
accordance with this Section 5.5(a) shall, in accordance with its terms, be subject to further adjustment as appropriate to reflect any stock split, division or subdivision of shares, stock dividend, reverse stock split, consolidation of
shares, reclassification, recapitalization or other similar transaction with respect to Celladon Common Stock subsequent to the Effective Time; and (B) Celladon’s Board of Directors or a committee thereof shall succeed to the authority and
responsibility of Eiger’s Board of Directors or any committee thereof with respect to each Eiger Option assumed by Celladon. Notwithstanding anything to the contrary in this Section 5.5(a), the conversion of each Eiger Option
(regardless of whether such option qualifies as an “incentive stock option” within the meaning of Section 422 of the Code) into an option to purchase shares of Celladon Common Stock shall be made in a manner consistent with Treasury
Regulation Section 1.424-1, such that the conversion of an Eiger Option shall not constitute a “modification” of such Eiger Option for purposes of Section 409A or Section 424 of the Code.
(b) Celladon shall file with the SEC, no later than 60 days after the Effective Time, a registration statement on Form S-8, if
available for use by Celladon, relating to the shares of Celladon Common Stock issuable with respect to Eiger Options assumed by Celladon in accordance with Section 5.5(a).
(c) Subject to Section 5.5(d), at the Effective Time, each Eiger Warrant that is outstanding and unexercised immediately
prior to the Effective Time (for the avoidance of doubt, excluding Eiger Warrants that are deemed to have been automatically exercised pursuant to their terms as a result of the consummation of the Merger), if any, shall be converted into and become
a warrant to purchase Celladon Common Stock and Celladon shall assume each such Eiger Warrant in accordance with its terms. All rights with respect to Eiger Common Stock or Eiger Preferred Stock under Eiger Warrants assumed by Celladon shall
thereupon be converted into rights with respect to Celladon Common Stock. Accordingly, from and after the Effective Time: (i) each Eiger Warrant assumed by Celladon may be exercised solely for shares of Celladon Common Stock; (ii) the
number of shares of Celladon Common Stock subject to each Eiger Warrant assumed by Celladon shall be determined by multiplying (A) the number of shares of Eiger Common Stock, or the number of shares of Eiger Common Stock issuable upon
conversion of the shares of Eiger Preferred Stock issuable upon exercise of the Eiger Warrant, as applicable, that were subject to such Eiger Warrant immediately prior to the Effective Time by (B) the Exchange Ratio and rounding the resulting
number down to the nearest whole number of shares of Celladon Common Stock; (iii) the per share exercise price for the Celladon Common Stock issuable upon exercise of each Eiger Warrant assumed by Celladon shall be determined by dividing the
per share exercise price of Eiger Common Stock or Eiger Preferred Stock subject to such Eiger Warrant, as in effect immediately prior to the Effective Time, by the Exchange Ratio and rounding the resulting exercise price up to the nearest whole
cent; and (iv) any restriction on any Eiger Warrant assumed by Celladon shall continue in full force and effect and the term and other provisions of such Eiger Warrant shall otherwise remain unchanged.
(d) Prior to the Effective Time, Eiger shall take all actions that may be necessary (under the Eiger Stock Option Plans, the Eiger
Warrants and otherwise) to effectuate the provisions of this Section 5.5 and to ensure that, from and after the Effective Time, holders of Eiger Options and Eiger Warrants have no rights with respect thereto other than those specifically
provided in this Section 5.5.
5.6 Employee Benefits. Celladon and Eiger shall cause Celladon to comply with terms of
any employment, severance, retention, change of control, or similar agreement specified on Part 3.11(c) of the Celladon Disclosure Schedule as being applicable to this Section 5.6, subject to the provisions of such agreements.
5.7 Indemnification of Officers and Directors.
(a) From the Effective Time through the sixth anniversary of the date on which the Effective Time occurs, each of Celladon and the
Surviving Corporation shall, jointly and severally, indemnify and hold harmless
A-51
each person who is now, or has been at any time prior to the date hereof, or who becomes prior to the Effective Time, a director or officer of Celladon or Eiger (the “D&O
Indemnified Parties”), against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees and disbursements (collectively, “Costs”),
incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that the D&O Indemnified Party is or was a director or officer
of Celladon or Eiger, whether asserted or claimed prior to, at or after the Effective Time, to the fullest extent permitted under the DGCL for directors or officers of Delaware corporations. Each D&O Indemnified Party will be entitled to
advancement of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation from each of Celladon and the Surviving Corporation, jointly and severally, upon receipt by Celladon or the Surviving Corporation from the
D&O Indemnified Party of a request therefor; provided, that any person to whom expenses are advanced provides an undertaking, to the extent then required by the DGCL, as applicable, to repay such advances if it is ultimately determined
that such person is not entitled to indemnification.
(b) The Certificate of Incorporation and Bylaws of each of Celladon and the
Surviving Corporation shall contain, and Celladon shall cause the Certificate of Incorporation and Bylaws of the Surviving Corporation to so contain, provisions no less favorable with respect to indemnification, advancement of expenses and
exculpation of present and former directors and officers of each of Celladon and Eiger than are presently set forth in the Certificate of Incorporation and Bylaws of Celladon and Eiger, as applicable, which provisions shall not be amended, modified
or repealed for a period of six years’ time from the Effective Time in a manner that would adversely affect the rights thereunder of individuals who, at or prior to the Effective Time, were officers or directors of Celladon or Eiger.
(c) Celladon shall purchase an insurance policy with an effective date as of the Closing which maintains in effect for six years from
the Closing the current directors’ and officers’ liability insurance policies maintained by Celladon (provided that Celladon may substitute therefor policies of at least the same coverage containing terms and conditions that are not
materially less favorable).
(d) Celladon shall pay all expenses, including reasonable attorneys’ fees, that may be incurred
by the persons referred to in this Section 5.7 in connection with their enforcement of their rights provided in this Section 5.7.
(e) The provisions of this Section 5.7 are intended to be in addition to the rights otherwise available to the current and
former officers and directors of Celladon and Eiger by law, charter, statute, bylaw or agreement, and shall operate for the benefit of, and shall be enforceable by, each of the D&O Indemnified Parties, their heirs and their representatives.
(f) In the event Celladon or the Surviving Corporation or any of their respective successors or assigns (i) consolidates with or
merges into any other person and shall not be the continuing or surviving corporation or entity of such consolidation or merger, or (ii) transfers all or substantially all of its properties and assets to any person, then, and in each such case,
proper provision shall be made so that the successors and assigns of Celladon or the Surviving Corporation, as the case may be, shall succeed to the obligations set forth in this Section 5.7. Celladon shall cause the Surviving
Corporation to perform all of the obligations of the Surviving Corporation under this Section 5.7.
5.8 Additional
Agreements. The Parties shall use commercially reasonable efforts to cause to be taken all actions necessary to consummate the Merger and make effective the other Contemplated Transactions. Without limiting the generality of the foregoing, each
Party to this Agreement: (i) shall make all filings and other submissions (if any) and give all notices (if any) required to be made and given by such Party in connection with the Merger and the other Contemplated Transactions; (ii) shall
use commercially reasonable efforts to obtain each Consent (if any) reasonably required to be obtained (pursuant to any applicable Legal Requirement or Contract, or otherwise) by such Party in connection with the Merger or any of the other
Contemplated
A-52
Transactions or for such Contract to remain in full force and effect; (iii) shall use commercially reasonable efforts to lift any injunction prohibiting, or any other legal bar to, the
Merger or any of the other Contemplated Transactions; and (iv) shall use commercially reasonable efforts to satisfy the conditions precedent to the consummation of this Agreement.
5.9 Disclosure. Without limiting any of either Party’s obligations under the Confidentiality Agreement, each Party shall not, and
shall not permit any of its Subsidiaries or any Representative of such Party to, issue any press release or make any disclosure (to any customers or employees of such Party, to the public or otherwise) regarding the Merger or any of the other
Contemplated Transactions unless: (a) the other Party shall have approved such press release or disclosure in writing; or (b) such Party shall have determined in good faith, upon the advice of outside legal counsel, that such disclosure is
required by applicable Legal Requirements and, to the extent practicable, before such press release or disclosure is issued or made, such Party advises the other Party of, and consults with the other Party regarding, the text of such press release
or disclosure; provided, however, that each of Eiger and Celladon may make any public statement in response to specific questions by the press, analysts, investors or those attending industry conferences or financial analyst conference calls,
so long as any such statements are consistent with previous press releases, public disclosures or public statements made by Eiger or Celladon in compliance with this Section 5.9.
5.10 Listing. Celladon shall use its commercially reasonable efforts: (i) maintain its existing listing on the NASDAQ
Global Market and to obtain approval of the listing of the combined company on the NASDAQ Global Market; (ii) without derogating from the generality of the requirements of clause “(i)” and to the extent required by the rules and
regulations of NASDAQ, to (x) prepare and submit to NASDAQ a notification form for the listing of the shares of Celladon Common Stock to be issued in the Merger and (y) to cause such shares to be approved for listing (subject to notice of
issuance); and (iii) to the extent required by Nasdaq Marketplace Rule 5110, to file an initial listing for the Celladon Common Stock on NASDAQ (the “Nasdaq Listing Application”) and to cause such Nasdaq Listing
Application to be conditionally approved prior to the Effective Time. Eiger will cooperate with Celladon as reasonably requested by Celladon with respect to the Nasdaq Listing Application and promptly furnish to Celladon all information concerning
Eiger and its stockholders that may be required or reasonably requested in connection with any action contemplated by this Section 5.10.
5.11 Tax Matters.
(a) Celladon, Merger Sub and Eiger shall use their respective commercially reasonable efforts to cause the Merger to qualify, and agree
not to, and not to permit or cause any affiliate or any Subsidiary to, take any actions or cause any action to be taken which would reasonably be expected to prevent the Merger from qualifying, as a “reorganization” under
Section 368(a) of the Code.
(b) This Agreement is intended to constitute, and the parties hereto hereby adopt this Agreement
as, a “plan of reorganization” within the meaning of Section 1.368-2(g) of the Treasury Regulations. The Parties shall treat and shall not take any tax reporting position inconsistent with the treatment of the Merger as a
reorganization within the meaning of Section 368(a) of the Code for U.S. federal, state and other relevant Tax purposes, unless otherwise required pursuant to a “determination” within the meaning of Section 1313(a) of the Code.
5.12 Legends. Celladon shall be entitled to place appropriate legends on the certificates evidencing any shares of Celladon Common
Stock to be received in the Merger by equityholders of Eiger who may be considered “affiliates” of Celladon for purposes of Rules 144 and 145 under the Securities Act reflecting the restrictions set forth in Rules 144 and 145 and to issue
appropriate stop transfer instructions to the transfer agent for Celladon Common Stock.
5.13 Cooperation. Each Party shall
cooperate reasonably with the other Party and shall provide the other Party with such assistance as may be reasonably requested for the purpose of facilitating the performance by each Party of its respective obligations under this Agreement and to
enable the combined entity to continue to meet its obligations following the Closing.
A-53
5.14 Directors and Officers. Celladon and Eiger shall obtain and deliver to the other
Party at or prior to the Effective Time the resignation of each officer and director of Celladon or Eiger who is not continuing as an officer or director of Celladon or the Surviving Corporation, as applicable, following the Effective Time. All of
the directors of Celladon shall resign at or prior to and effective as of the Effective Time. Prior to the Effective Time, but to be effective at the Effective Time, the Board of Directors of Celladon shall (i) elect seven (7) designees
selected by Eiger (with such designees, in the aggregate, expected to satisfy the requisite independence requirements for the Board of Directors of Celladon, as well as the sophistication and independence requirements for the required committees of
the Board of Directors of Celladon, pursuant to NASDAQ’s listing standards), each to serve as a member of the Board of Directors of Celladon in staggered classes to be agreed upon by the Parties prior to the Closing Date, (ii) appoint each
of the individuals set forth on Part 5.14(a) of the Eiger Disclosure Schedule as officers of Celladon and (iii) appoint each of the directors set forth on Part 5.14(b) of the Eiger Disclosure Schedule to the committees of the Board of Directors
of Celladon set forth opposite his or her name (with such director, in the aggregate, expected to satisfy the sophistication and independence requirements for the required committees of the Board of Directors of Celladon pursuant to NASDAQ’s
listing standards).
5.15 Section 16 Matters. Prior to the Effective Time, Celladon shall take all such steps as may be
required to cause any acquisitions of Celladon Common Stock and any options to purchase Celladon Common Stock resulting from the Merger and the other transactions contemplated by this Agreement, by each individual who is reasonably expected to
become subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Celladon, to be exempt under Rule 16b-3 promulgated under the Exchange Act.
5.16 Reverse Split. Celladon shall submit to the holders of Celladon Common Stock at the Celladon Stockholders’ Meeting a proposal
to approve and adopt an amendment to the Celladon Certificate of Incorporation to authorize the Board of Directors of Celladon to effect a reverse stock split of all outstanding shares of Celladon Common Stock at a reverse stock split ratio in the
range mutually agreed to by Celladon and Eiger (the “Reverse Split”).
5.17 Preferred
Stock. Eiger shall take all action required to effect the conversion of Eiger Preferred Stock into Eiger Common Stock prior to the Closing Date.
5.18 Termination of Certain Agreements and Rights. Eiger shall use its commercially reasonable efforts to terminate at or prior
to the Effective Time, those agreements set forth on Schedule C (collectively, the “Investor Agreements”).
5.19 Allocation Certificate. Eiger will prepare and deliver to Parent at least two (2) Business Days prior to the Closing
Date a certificate signed by the Chief Financial Officer and Secretary of Eiger in a form reasonably acceptable to Celladon which sets forth a true and complete list of the holders of shares of Eiger Common Stock, Eiger Options and Eiger Warrants as
of immediately prior to the Effective Time (including with an assumed consummation of the Eiger Pre-Closing Financing) and the number of shares of Eiger Common Stock owned and/or underlying the Eiger Options or Eiger Warrants held by such holders
(the “Allocation Certificate”).
5.20 Disclosure of Liabilities. For purposes of the computation of Net
Cash pursuant to Section 1.6, on or prior to the Determination Date, Celladon shall provide Eiger with a list of all Liabilities of Celladon as of the Determination Date which are individually in excess of $25,000 or in excess of
$100,000 in the aggregate, that had not previously been disclosed to Eiger in the Celladon Disclosure Schedules.
A-54
| |
Section 6. |
CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY |
The obligations of each Party to
effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or, to the extent permitted by applicable law, the written waiver by each of the Parties, at or prior to the Closing, of
each of the following conditions:
6.1 Effectiveness of Registration Statement. The Form S-4 Registration Statement shall have
become effective in accordance with the provisions of the Securities Act, and shall not be subject to any stop order or proceeding (or threatened proceeding by the SEC) seeking a stop order with respect to the Form S-4 Registration Statement.
6.2 No Restraints. No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of
the Merger shall have been issued by any court of competent jurisdiction or other Governmental Body of competent jurisdiction and remain in effect, and there shall not be any Legal Requirement which has the effect of making the consummation of the
Merger illegal.
6.3 Stockholder Approval. This Agreement, the Merger and the other transactions contemplated by this Agreement
shall have been duly adopted and approved by the Required Eiger Stockholder Vote, and the Merger, the issuance of the Celladon Common Stock in the Merger and the Reverse Split shall have been duly approved by the Required Celladon Stockholder Vote.
6.4 Regulatory Matters. Any waiting period applicable to the consummation of the Merger under the HSR Act shall have
expired or been terminated, and there shall not be in effect any voluntary agreement between Celladon, Merger Sub and/or Eiger, on the one hand, and the Federal Trade Commission, the Department of Justice or any foreign Governmental Body, on the
other hand, pursuant to which such Party has agreed not to consummate the Merger for any period of time; provided, that neither Eiger, on the one hand, nor Celladon or Merger Sub, on the other hand, shall enter into any such voluntary
agreement without the written consent of all Parties.
6.5 No Governmental Proceedings Relating to Contemplated Transactions or Right
to Operate Business. There shall not be any Legal Proceeding pending, or overtly threatened in writing by an official of a Governmental Body in which such Governmental Body indicates that it intends to conduct any Legal Proceeding or take any
other action: (a) challenging or seeking to restrain or prohibit the consummation of the Merger or any of the other Contemplated Transactions; (b) relating to the Merger or any of the other Contemplated Transactions and seeking to obtain
from Celladon, Merger Sub or Eiger any damages or other relief that may be material to Celladon or Eiger; (c) seeking to prohibit or limit in any material and adverse respect a Party’s ability to vote, transfer, receive dividends with
respect to or otherwise exercise ownership rights with respect to the Stock of Celladon; (d) that would materially and adversely affect the right or ability of Celladon or Eiger to own the assets or operate the business of Celladon or Eiger; or
(e) seeking to compel Eiger or Celladon (or any of their respective Subsidiaries) to dispose of or hold separate any material assets as a result of the Merger or any of the other Contemplated Transactions.
6.6 Listing. The existing shares of Celladon Common Stock shall have been continually listed on the NASDAQ Global Market as of and from
the date of this Agreement through the Closing Date, and the shares of Celladon Common Stock to be issued in the Merger shall be approved for listing (subject to official notice of issuance) on the NASDAQ Global Market as of the Effective Time.
A-55
| |
Section 7. |
ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF CELLADON AND MERGER SUB |
The
obligations of Celladon and Merger Sub to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by Celladon, at or prior to the Closing, of each of the
following conditions:
7.1 Accuracy of Representations. The representations and warranties of Eiger contained in this Agreement
shall have been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on the Closing Date except (A) in each case, or in the aggregate, where the
failure to be true and correct would not reasonably be expected to have an Eiger Material Adverse Effect, or (B) for those representations and warranties which address matters only as of a particular date (which representations shall have been
true and correct, subject to the qualifications as set forth in the preceding clause (A), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or
modification to the Eiger Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded).
7.2 Performance of Covenants. Each of the covenants and obligations in this Agreement that Eiger is required to comply with or to
perform at or prior to the Closing shall have been complied with and performed by Eiger in all material respects.
7.3 Agreements and
Other Documents. Celladon shall have received the following agreements and other documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer and Chief Financial Officer of Eiger confirming that the conditions set forth
in Sections 7.1, 7.2, 7.4, 7.6, 7.7, 7.8 and 7.9 have been duly satisfied;
(b)
certificates of good standing (or equivalent documentation) of Eiger in its jurisdiction of organization and the various foreign jurisdictions in which it is qualified, certified charter documents, a certificate as to the incumbency of officers
and the adoption of resolutions of the board of directors of Eiger authorizing the execution of this Agreement and the consummation of the Contemplated Transactions to be performed by Eiger hereunder;
(c) written resignations in forms satisfactory to Celladon, dated as of the Closing Date and effective as of the Closing, executed by
the officers and directors of Eiger who will not be officers or directors of the Surviving Corporation pursuant to Section 5.14 hereof; and
(d) the Allocation Certificate.
7.4 Eiger Pre-Closing Financing. The Eiger Pre-Closing Financing shall have been consummated and Eiger shall have received the proceeds
of the Eiger Pre-Closing Financing on the terms and conditions set forth in the Subscription Agreement.
7.5 FIRPTA Certificate.
Celladon shall have received from Eiger a form of notice to the Internal Revenue Service in accordance with the requirements of Treasury Regulation Section 1.897-2(h) and in form and substance reasonably acceptable to Celladon along with
written authorization for Celladon to deliver such notice form to the Internal Revenue Service on behalf of Eiger upon the Closing.
7.6 No Eiger Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Eiger Material Adverse Effect
that is continuing.
7.7 Termination of Investor Agreements. The Investor Agreements shall have been terminated.
A-56
7.8 Debt Conversion. Eiger shall have effected a conversion of all of its outstanding
convertible indebtedness.
7.9 Preferred Stock Conversion. Eiger shall have effected a conversion of all shares of Eiger
Preferred Stock into shares of Eiger Common Stock immediately prior to the Effective Time (the “Preferred Stock Conversion”).
| |
Section 8. |
ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF EIGER |
The obligations of Eiger to
effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written wavier by Eiger, at or prior to the Closing, of each of the following conditions:
8.1 Accuracy of Representations. The representations and warranties of Celladon and Merger Sub contained in this Agreement shall have
been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on the Closing Date except (A) in each case, or in the aggregate, where the failure to
be true and correct would not reasonably be expected to have a Celladon Material Adverse Effect, or (B) for those representations and warranties which address matters only as of a particular date (which representations shall have been true and
correct, subject to the qualifications as set forth in the preceding clause (A), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to
the Celladon Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded).
8.2
Performance of Covenants. All of the covenants and obligations in this Agreement that either Celladon or Merger Sub is required to comply with or to perform at or prior to the Closing shall have been complied with and performed in all material
respects.
8.3 Documents. Eiger shall have received the following documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer and Chief Financial Officer of Celladon confirming that the conditions set
forth in Sections 8.1, 8.2, 8.4, 8.5, 8.6 and 8.7 have been duly satisfied;
(b)
certificates of good standing of each of Celladon and Merger Sub in its jurisdiction of organization and the various foreign jurisdictions in which it is qualified, certified charter documents, certificates as to the incumbency of officers and
the adoption of resolutions of its board of directors authorizing the execution of this Agreement and the consummation of the Contemplated Transactions to be performed by Celladon and Merger Sub hereunder;
(c) written resignations in forms satisfactory to Eiger, dated as of the Closing Date and effective as of the Closing executed by the
officers and directors of Celladon who are not to continue as officers or directors of Celladon pursuant to Section 5.14 hereof; and
(d) a certificate duly executed by the Chief Executive Officer and Chief Financial Officer of Celladon setting forth the number of
Celladon Outstanding Shares and each component thereof (broken down by outstanding shares, options and other securities).
8.4 Board of
Directors. Celladon shall have caused the Board of Directors of Celladon to be constituted as set forth in Section 5.14 of this Agreement effective as of the Effective Time.
8.5 No Celladon Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Celladon Material Adverse
Effect that is continuing.
A-57
8.6 Sarbanes-Oxley Certifications. Neither the principal executive officer nor the
principal financial officer of Celladon shall have failed to provide, with respect to any Celladon SEC Document filed (or required to be filed) with the SEC on or after the date of this Agreement, any necessary certification in the form required
under Rule 13a-14 under the Exchange Act and 18 U.S.C. §1350.
8.7 Net Cash Threshold. The Celladon Net Cash shall be greater
than or equal to $24,000,000.
9.1 Termination. This Agreement may be terminated prior to the
Effective Time (whether before or after adoption of this Agreement by Eiger’s stockholders and whether before or after approval of the Merger and issuance of Celladon Common Stock in the Merger by Celladon’s stockholders, unless otherwise
specified below):
(a) by mutual written consent duly authorized by the Boards of Directors of Celladon and Eiger;
(b) by either Celladon or Eiger if the Merger shall not have been consummated by March 31, 2016 (subject to possible extension as
provided in this Section 9.1(b), the “End Date”); provided, however, that the right to terminate this Agreement under this Section 9.1(b) shall not be available to Eiger, on the one hand, or
to Celladon and Merger Sub, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure of the Merger to occur on or before the End Date and such action or failure to act constitutes a breach of this
Agreement; and provided, further, that, in the event the waiting period under the HSR Act has not expired or a request for additional information has been made by any Governmental Authority, or in the event that the SEC has not
declared effective under the Securities Act the Form S-4 Registration Statement by the date which is sixty (60) days prior to the End Date, then either Eiger or Celladon shall be entitled to extend the End Date for an additional sixty
(60) days;
(c) by either Celladon or Eiger if a court of competent jurisdiction or other Governmental Body shall have issued
a final and nonappealable order, decree or ruling, or shall have taken any other action, having the effect of permanently restraining, enjoining or otherwise prohibiting the Merger;
(d) by Celladon if the Required Eiger Stockholder Vote shall not have been obtained within two (2) Business Days of the Form S-4
Registration Statement becoming effective in accordance with the provisions of the Securities Act; provided, however, that once the Required Eiger Stockholder Vote has been obtained, Celladon may not terminate this Agreement pursuant
to this Section 9.1(d);
(e) by either Celladon or Eiger if (i) the Celladon Stockholders’ Meeting (including
any adjournments and postponements thereof) shall have been held and completed and Celladon’s stockholders shall have taken a final vote on the Merger and the issuance of shares of Celladon Common Stock in the Merger and (ii) the Merger
and the issuance of Celladon Common Stock in the Merger shall not have been approved at the Celladon Stockholders’ Meeting (or any adjournment or postponement thereof) by the Required Celladon Stockholder Vote; provided, however,
that the right to terminate this Agreement under this Section 9.1(e) shall not be available to Celladon where the failure to obtain the Required Celladon Stockholder Vote shall have been caused by the action or failure to act of Celladon
and such action or failure to act constitutes a material breach by Celladon of this Agreement;
(f) by Eiger (at any time prior to
the approval of the Merger and the issuance of Celladon Common Stock in the Merger by the Required Celladon Stockholder Vote) if a Celladon Triggering Event shall have occurred;
(g) by Celladon (at any time prior to the approval of the Merger by the Required Eiger Stockholder Vote) if an Eiger Triggering Event
shall have occurred;
A-58
(h) by Eiger, upon a breach of any representation, warranty, covenant or agreement on the
part of Celladon or Merger Sub set forth in this Agreement, or if any representation or warranty of Celladon or Merger Sub shall have become inaccurate, in either case such that the conditions set forth in Section 8.1 or
Section 8.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate, provided, that if such inaccuracy in Celladon’s or Merger Sub’s
representations and warranties or breach by Celladon or Merger Sub is curable by Celladon or Merger Sub, then this Agreement shall not terminate pursuant to this Section 9.1(h) as a result of such particular breach or inaccuracy until
the earlier of (i) the expiration of a thirty (30) day period commencing upon delivery of written notice from Celladon or Merger Sub to Eiger of such breach or inaccuracy and (ii) Celladon or Merger Sub (as applicable) ceasing to
exercise commercially reasonable efforts to cure such breach (it being understood that this Agreement shall not terminate pursuant to this Section 9.1(h) as a result of such particular breach or inaccuracy if such breach by Celladon or
Merger Sub is cured prior to such termination becoming effective);
(i) by Celladon, upon a breach of any representation, warranty,
covenant or agreement on the part of Eiger set forth in this Agreement, or if any representation or warranty of Eiger shall have become inaccurate, in either case such that the conditions set forth in Section 7.1 or
Section 7.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate, provided, that if such inaccuracy in Eiger’s representations and warranties or
breach by Eiger is curable by Eiger then this Agreement shall not terminate pursuant to this Section 9.1(i) as a result of such particular breach or inaccuracy until the earlier of (i) the expiration of a thirty (30) day period
commencing upon delivery of written notice from Eiger to Celladon of such breach or inaccuracy and (ii) Eiger ceasing to exercise commercially reasonable efforts to cure such breach (it being understood that this Agreement shall not terminate
pursuant to this Section 9.1(i) as a result of such particular breach or inaccuracy if such breach by Eiger is cured prior to such termination becoming effective);
(j) by Celladon, at any time, if (i) all conditions in Sections 6 and 8 have been satisfied (other than those
conditions that by their nature are to be satisfied by actions taken at the Closing), and remain so satisfied and (ii) Celladon irrevocably confirms by written notice to Eiger that (A) each of the conditions in Section 7, other
than the condition set forth in Section 7.4, has been satisfied or that Celladon is willing to waive any such conditions that have not been satisfied (other than those conditions that by their nature are to be satisfied by actions taken
at the Closing) and (B) it is prepared to consummate the Closing upon satisfaction of the condition set forth in Section 7.4 (i.e., consummation of the Eiger Pre-Closing Financing); provided, that this Agreement shall
not terminate pursuant to this Section 9.1(j) unless the condition set forth in Section 7.4 has not been satisfied within five (5) calendar days after delivery of the written notice from Celladon to Eiger pursuant to
clause (ii) of this Section 9.1(j); or
(k) by Celladon, at any time prior to the approval of the issuance of
Celladon Common Stock in the Merger by the stockholders of Celladon by the Required Celladon Stockholder Vote and following compliance with all of the requirements set forth in the proviso to this Section 9.1(k), upon Celladon entering
into a definitive agreement that provides for the consummation of a transaction that satisfies the requirements of clause (b) of the definition of a Superior Offer (a “Permitted Alternative Agreement”); provided,
however, that Celladon shall not enter into any Permitted Alternative Agreement unless: (i) Eiger shall have received written notice from Celladon of Celladon’s intention to enter into such Permitted Alternative Agreement at least
five (5) Business Days in advance, with such notice describing in reasonable detail the reasons for such intention as well as the material terms and conditions of such Permitted Alternative Agreement, including the identity of the counterparty
together with copies of the then current draft of such definitive agreement and all related agreements, (ii) Celladon shall have complied with its obligations under Section 4.5, (iii) the Celladon Board of Directors shall have
determined in good faith, after consultation with its outside legal counsel, that (1) the subject transaction of such Permitted Alternative Agreement satisfies the requirements of clause (b) of the definition of a Superior Offer and
(2) the failure to enter into such Permitted Alternative Agreement would result in a breach of its fiduciary duties under applicable Legal Requirements, (iv) Celladon shall concurrently pay to Eiger the Eiger
A-59
Termination Fee in accordance with Section 9.3(b)(i), and (v) a copy of such Permitted Alternative Agreement and all related agreements, exhibits, schedules and other documents
shall have been delivered to Eiger.
The Party desiring to terminate this Agreement pursuant to this Section 9.1 (other than pursuant to
Section 9.1(a)) shall give a notice of such termination to the other Party specifying the provisions hereof pursuant to which such termination is made and the basis therefor described in reasonable detail.
9.2 Effect of Termination. In the event of the termination of this Agreement as provided in Section 9.1, this Agreement
shall be of no further force or effect; provided, however, that (i) this Section 9.2, Section 9.3, and Section 10 shall survive the termination of this Agreement and shall remain in full force
and effect, and (ii) the termination of this Agreement shall not relieve any Party for its fraud or from any liability for any willful and material breach of any representation, warranty, covenant, obligation or other provision contained in
this Agreement.
9.3 Expenses; Termination Fees.
(a) Except as set forth in this Section 9.3, all fees and expenses incurred in connection with this Agreement and the
Contemplated Transactions shall be paid by the Party incurring such expenses, whether or not the Merger is consummated; provided, however, that Celladon and Eiger shall share equally all fees and expenses, other than attorneys’
and accountants’ fees and expenses, incurred in relation to the filings by the Parties under any filing requirement under the HSR Act and any foreign antitrust Legal Requirement applicable to this Agreement and the transactions contemplated
hereby; and provided, further, that Celladon and Eiger shall also share equally all fees and expenses incurred in relation to the printing (e.g., paid to a financial printer) and filing with the SEC of the Form S-4 Registration
Statement (including any financial statements and exhibits) and any amendments or supplements thereto.
(b) (i) If (x) this
Agreement is terminated by Celladon or Eiger pursuant to Section 9.1(e) or (f), (y) at any time before the Celladon Stockholders’ Meeting an Acquisition Proposal with respect to Celladon shall have been publicly
announced, disclosed or otherwise communicated to Celladon’s Board of Directors and (z) in the event this Agreement is terminated pursuant Section 9.1(e), within twelve (12) months after the date of such termination,
Celladon enters into a definitive agreement with respect to a Subsequent Transaction or consummates a Subsequent Transaction, then Celladon shall pay to Eiger, within ten (10) Business Days after termination (or, if applicable, upon such entry
into a definitive agreement and/or consummation of a Subsequent Transaction), a nonrefundable fee in an amount equal to $3,000,000 (the “Eiger Termination Fee”), in addition to any amount payable to Eiger pursuant to
Sections 9.3(c) or 9.3(e);
(ii) If (x) this Agreement is terminated by Celladon pursuant to
Section 9.1(d) or (g), (y) at any time before obtaining the Required Eiger Stockholder Vote an Acquisition Proposal with respect to Eiger shall have been publicly announced, disclosed or otherwise communicated to Eiger’s
Board of Directors, and (z) in the event this Agreement is terminated pursuant Section 9.1(d), within 12 months after the date of such termination, Eiger enters into a definitive agreement with respect to a Subsequent Transaction or
consummates a Subsequent Transaction, then Eiger shall pay to Celladon, within ten (10) Business Days after termination (or, if applicable, upon such entry into a definitive agreement and/or consummation of a Subsequent Transaction), a
nonrefundable fee in an amount equal to $3,000,000 (the “Celladon Termination Fee”), in addition to any amount payable to Celladon pursuant to Sections 9.3(d) or 9.3(e);
(iii) If this Agreement is terminated by Celladon pursuant to Section 9.1(j), then Eiger shall pay to Celladon, within ten
(10) Business Days after termination, the Celladon Termination Fee, in addition to any amount payable to Celladon pursuant to Sections 9.3(d) or 9.3(e); or
(iv) If this Agreement is terminated by Celladon pursuant to Section 9.1(k), then Celladon shall pay to Eiger, concurrent
with such termination, the Eiger Termination Fee, in addition to any amount payable to Eiger pursuant to Sections 9.3(d) or 9.3(e).
A-60
(c) (i) If this Agreement is terminated by Eiger pursuant to Sections 9.1(e),
(f) or (h), or (ii) if this Agreement is terminated by Celladon pursuant to Section 9.1(e) or (k), or (iii) in the event of a failure of Eiger to consummate the transactions to be consummated at the
Closing solely as a result of a Celladon Material Adverse Effect as set forth in Section 8.5 (provided, that at such time all of the other conditions precedent to Celladon’s obligation to close set forth in Sections 6
and 7 of this Agreement have been satisfied by Eiger, are capable of being satisfied by Eiger or have been waived by Celladon), then Celladon shall reimburse Eiger for all reasonable fees and expenses incurred by Eiger in connection with this
Agreement and the transactions contemplated hereby, including (x) all fees and expenses incurred in connection with the preparation, printing and filing, as applicable, of the Form S-4 Registration Statement (including any preliminary materials
related thereto and all amendments and supplements thereto, as well as any financial statements and schedules thereto) and (y) all fees and expenses incurred in connection with the preparation and filing under any filing requirement of any
Governmental Authority applicable to this Agreement and the transactions contemplated hereby (such expenses, including (x) and (y) above, collectively, the “Third Party Expenses”), up to a maximum of
$1,000,000, by wire transfer of same-day funds within ten (10) Business Days following the date on which Eiger submits to Celladon true and correct copies of reasonable documentation supporting such Third Party Expenses; provided,
however, that such Third Party Expenses shall not include any amounts for a financial advisor to Eiger except for reasonably documented out-of-pocket expenses otherwise reimbursable by Eiger to such financial advisor pursuant to the terms of
Eiger’s engagement letter or similar arrangement with financial advisor. Notwithstanding the foregoing, if Eiger is entitled to reimbursement for Third Party Expenses and the Eiger Termination Fee, Celladon’s liability shall be capped at
an amount equal to the Eiger Termination Fee and in no event shall Celladon be required to pay Eiger any amount in excess of the Eiger Termination Fee in the event of termination of this Agreement.
(d) (i) If this Agreement is terminated by Celladon pursuant to Sections 9.1(d), (g), (i) or
(j) or (ii) in the event of a failure of Celladon to consummate the transactions to be consummated at the Closing solely as a result of an Eiger Material Adverse Effect as set forth in Section 7.6 (provided, that
at such time all of the other conditions precedent to Eiger’s obligation to close set forth in Sections 6 and 8 of this Agreement have been satisfied by Celladon, are capable of being satisfied by Celladon or have been waived by
Eiger), then Eiger shall reimburse Celladon for all Third Party Expenses incurred by Celladon up to a maximum of $1,000,000 (the “Celladon Expense Reimbursement”), by wire transfer of same-day funds within ten
(10) Business Days following the date on which Celladon submits to Eiger true and correct copies of reasonable documentation supporting such Third Party Expenses; provided, however, that such Third Party Expenses shall not include
any amounts for a financial advisor to Celladon except for reasonably documented out-of-pocket expenses otherwise reimbursable by Celladon to such financial advisor pursuant to the terms of Celladon’s engagement letter or similar arrangement
with financial advisor. Notwithstanding the foregoing, if Celladon is entitled to the Celladon Expense Reimbursement and the Celladon Termination Fee, Eiger’s liability shall be capped at an amount equal to the Celladon Termination Fee and in
no event shall Eiger be required to pay Celladon any amount in excess of the Celladon Termination Fee in the event of termination of this Agreement.
(e) If either Party fails to pay when due any amount payable by such Party under Section 9.3(b), (c) or
(d), then (i) such Party shall reimburse the other Party for reasonable costs and expenses (including reasonable fees and disbursements of counsel) incurred in connection with the collection of such overdue amount and the enforcement by
the other Party of its rights under this Section 9.3, and (ii) such Party shall pay to the other Party interest on such overdue amount (for the period commencing as of the date such overdue amount was originally required to be paid
and ending on the date such overdue amount is actually paid to the other Party in full) at a rate per annum equal to the “prime rate” (as announced by Bank of America or any successor thereto) in effect on the date such overdue amount was
originally required to be paid.
(f) The Parties agree that the payment of the fees and expenses set forth in this
Section 9.3, subject to Section 9.2, shall be the sole and exclusive remedy of each Party following a termination of this Agreement under the circumstances described in this Section 9.3, it being understood that
in no event shall either Celladon or Eiger be required to pay fees or damages payable pursuant to this Section 9.3 on more than one occasion. Subject
A-61
to Section 9.2, the payment of the fees and expenses set forth in this Section 9.3, and the provisions of Section 10.11, each of the Parties and their
respective Affiliates shall have no liability, shall not be entitled to bring or maintain any other claim, action or proceeding against the other, shall be precluded from any other remedy against the other, at law or in equity or otherwise, and
shall not seek to obtain any recovery, judgment or damages of any kind against the other (or any partner, member, stockholder, director, officer, employee, Subsidiary, affiliate, agent or other representative of such Party) in connection with or
arising out of the termination of this Agreement, any breach by any Party giving rise to such termination or the failure of the Merger and the other Contemplated Transactions to be consummated. Each of the Parties acknowledges that (i) the
agreements contained in this Section 9.3 are an integral part of the Contemplated Transactions, (ii) without these agreements, the Parties would not enter into this Agreement and (iii) any amount payable pursuant to this
Section 9.3 is not a penalty, but rather is liquidated damages in a reasonable amount that will compensate the Parties in the circumstances in which such amount is payable.
| |
Section 10. |
MISCELLANEOUS PROVISIONS |
10.1 Non-Survival of Representations and Warranties.
The representations and warranties of Eiger, Merger Sub and Celladon contained in this Agreement or any certificate or instrument delivered pursuant to this Agreement shall terminate at the Effective Time, and only the covenants that by their terms
survive the Effective Time and this Section 10 shall survive the Effective Time.
10.2 Amendment. This Agreement
may be amended with the approval of the respective Boards of Directors of Eiger, Merger Sub and Celladon at any time (whether before or after the adoption and approval of this Agreement by Eiger’s stockholders or before or after the approval of
the Merger or issuance of shares of Celladon Common Stock in the Merger); provided, however, that after any such adoption and approval of this Agreement by a Party’s stockholders, no amendment shall be made which by law requires
further approval of the stockholders of such Party without the further approval of such stockholders. This Agreement may not be amended except by an instrument in writing signed on behalf of each of Eiger, Merger Sub and Celladon.
10.3 Waiver.
(a)
No failure on the part of any Party to exercise any power, right, privilege or remedy under this Agreement, and no delay on the part of any Party in exercising any power, right, privilege or remedy under this Agreement, shall operate as a waiver
of such power, right, privilege or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise thereof or of any other power, right, privilege or remedy.
(b) No Party shall be deemed to have waived any claim arising out of this Agreement, or any power, right, privilege or remedy under
this Agreement, unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered on behalf of such Party; and any such waiver shall not be applicable or have any effect
except in the specific instance in which it is given.
10.4 Entire Agreement; Counterparts; Exchanges by Facsimile. This
Agreement and the other agreements referred to in this Agreement constitute the entire agreement and supersede all prior agreements and understandings, both written and oral, among or between any of the Parties with respect to the subject matter
hereof and thereof; provided, however, that the Confidentiality Agreement shall not be superseded and shall remain in full force and effect in accordance with its terms. This Agreement may be executed in several counterparts, each of
which shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Agreement (in counterparts or otherwise) by all Parties by facsimile or electronic transmission in .PDF format shall be
sufficient to bind the Parties to the terms and conditions of this Agreement.
10.5 Applicable Law; Jurisdiction. This Agreement
shall be governed by, and construed in accordance with, the laws of the State of Delaware, regardless of the laws that might otherwise govern under applicable
A-62
principles of conflicts of laws. In any action or suit between any of the Parties arising out of or relating to this Agreement or any of the Contemplated Transactions: (a) each of the
Parties irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the state and federal courts located in the State of Delaware; (b) if any such action or suit is commenced in a state court, then, subject
to applicable Legal Requirements, no Party shall object to the removal of such action or suit to any federal court located in the District of Delaware; and (c) each of the Parties irrevocably waives the right to trial by jury.
10.6 Attorneys’ Fees. In any action at law or suit in equity to enforce this Agreement or the rights of any of the parties under
this Agreement, the prevailing Party in such action or suit shall be entitled to receive a reasonable sum for its attorneys’ fees and all other reasonable costs and expenses incurred in such action or suit.
10.7 Assignability; No Third Party Beneficiaries. This Agreement shall be binding upon, and shall be enforceable by and inure
solely to the benefit of, the parties hereto and their respective successors and assigns; provided, however, that neither this Agreement nor any of a Party’s rights or obligations hereunder may be assigned or delegated by such Party
without the prior written consent of the other Party, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s prior written consent shall be void and of no
effect. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person (other than the parties hereto and the D&O Indemnified Parties to the extent of their respective rights pursuant to Section 5.7)
any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
10.8 Notices. Any notice or other
communication required or permitted to be delivered to any Party under this Agreement shall be in writing and shall be deemed properly delivered, given and received when delivered by hand, by registered mail, by courier or express delivery service
or by facsimile to the address or facsimile telephone number set forth beneath the name of such Party below (or to such other address or facsimile telephone number as such Party shall have specified in a written notice given to the other parties
hereto):
if to Celladon or Merger Sub:
Celladon Corporation
12707 High
Bluff Drive, Suite 200
San Diego, CA 92130
Telephone No.: (858) 350-4355
Attention: Chief Executive Officer
with a copy to:
Pillsbury
Winthrop Shaw Pittman LLP
12255 El Camino Real, Suite 300
San Diego, California 92130
Telephone: (858) 509-4000
Fax: (858) 509-4010
Attention: Mike Hird
if to
Eiger:
Eiger Pharmaceuticals, Inc.
PO Box 430
San Carlos, CA 94070
Telephone No.: (415) 203-0934
Facsimile No.: (650) 849-7400
Attention: Chief Executive Officer
A-63
with a copy to:
Cooley LLP
3175 Hanover Street
Palo Alto, California 94304-1130
Telephone No.: (650) 843-5000
Facsimile No.: (650) 849-7400
E-Mail: gsato@cooley.com
Attention: Glen Sato
10.9
Cooperation. Each Party agrees to cooperate fully with the other Party and to execute and deliver such further documents, certificates, agreements and instruments and to take such other actions as may be reasonably requested by the other Party
to evidence or reflect the Contemplated Transactions and to carry out the intent and purposes of this Agreement.
10.10
Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions of this Agreement or the validity
or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or provision of this Agreement is invalid or unenforceable, the
Parties hereto agree that the court making such determination shall have the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision that is valid and enforceable and
that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the prior
sentence, the Parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or
unenforceable term or provision.
10.11 Other Remedies; Specific Performance. Except as otherwise provided herein, any and all
remedies herein expressly conferred upon a Party will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise
of any other remedy. The Parties hereto agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly
agreed that the Parties shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof in any court of the United States or any state having jurisdiction, this
being in addition to any other remedy to which they are entitled at law or in equity, and each of the Parties hereto waives any bond, surety or other security that might be required of any other Party with respect thereto.
10.12 Construction.
(a) For purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the
masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include masculine and feminine genders.
(b) The Parties hereto agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting Party
shall not be applied in the construction or interpretation of this Agreement.
(c) As used in this Agreement, the words
“include” and “including,” and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
A-64
(d) Except as otherwise indicated, all references in this Agreement to
“Sections,” “Exhibits” and “Schedules” are intended to refer to Sections of this Agreement and Exhibits and Schedules to this Agreement, respectively.
(e) The bold-faced headings contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this
Agreement and shall not be referred to in connection with the construction or interpretation of this Agreement.
[Remainder of
page intentionally left blank]
A-65
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed as of the date
first above written.
|
|
|
| CELLADON CORPORATION |
|
|
|
| By: |
|
/s/ Paul Cleveland |
| Name: |
|
Paul Cleveland |
| Title: |
|
President and Chief Executive Officer |
|
|
|
| CELLADON MERGER SUB, INC. |
|
|
|
| By: |
|
/s/ Fredrik Wiklund |
| Name: |
|
Fredrik Wiklund |
| Title: |
|
President and Chief Executive Officer |
|
|
|
| EIGER BIOPHARMACEUTICALS, INC. |
|
|
|
| By: |
|
/s/ David A. Cory |
| Name: |
|
David A. Cory |
| Title: |
|
President and Chief Executive Officer |
|
|
|
| Schedules: |
|
|
|
|
| Schedule A |
|
Persons Executing Eiger Stockholder Support Agreements |
| Schedule B |
|
Persons Executing Celladon Stockholder Support Agreements |
| Schedule C |
|
Investor Agreements |
|
|
| Exhibits: |
|
|
|
|
| Exhibit A |
|
Definitions |
| Exhibit B |
|
Form of Eiger Stockholder Support Agreement |
| Exhibit C |
|
Form of Celladon Stockholder Support Agreement |
| Exhibit D |
|
Subscription Agreement |
A-66
EXHIBIT A
CERTAIN DEFINITIONS
For
purposes of the Agreement (including this Exhibit A):
“2009 Plan” shall have
the meaning set forth in Section 2.3(b).
“409A Plan” shall have the meaning set forth in Section
3.11(j).
“Acquisition Inquiry” shall mean, with respect to a Party, an inquiry,
indication of interest or request for information (other than an inquiry, indication of interest or request for information made or submitted by Eiger, on the one hand, or Celladon, on the other hand, to the other Party) that could reasonably be
expected to lead to an Acquisition Proposal with such Party.
“Acquisition
Proposal” shall mean, with respect to a Party, any offer or proposal, whether written or oral (other than an offer or proposal made or submitted by or on behalf of Eiger or any of its Affiliates, on the one hand, or by or
on behalf of Celladon or any of its Affiliates, on the other hand, to the other Party) contemplating or otherwise relating to any Acquisition Transaction with such Party.
“Acquisition Transaction” shall mean any transaction or series of
transactions involving:
| |
• |
|
any merger, consolidation, amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar
transaction: (i) in which a Party is a constituent corporation; (ii) in which a Person or “group” (as defined in the Exchange Act and the rules promulgated thereunder) of Persons directly or indirectly acquires beneficial or
record ownership of securities representing more than 15% of the outstanding securities of any class of voting securities of a Party or any of its Subsidiaries; or (iii) in which a Party or any of its Subsidiaries issues securities representing
more than 15% of the outstanding securities of any class of voting securities of such Party or any of its Subsidiaries; provided, however, in the case of Eiger, the Eiger Pre-Closing Financing shall not be an “Acquisition
Transaction;” |
| |
• |
|
any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for 15% or more of the consolidated book value or the fair market value of the
assets of a Party and its Subsidiaries, taken as a whole; or |
| |
• |
|
any liquidation or dissolution of a Party. |
“Affiliates” shall have the meaning for such term as used in Rule 145 under
the Securities Act.
“Agreement” shall mean the Agreement and Plan of Merger and Reorganization to
which this Exhibit A is attached, as it may be amended from time to time.
“Business Day” shall mean any
day other than a day on which banks in the State of New York are authorized or obligated to be closed.
“Capitalization
Date” shall have the meaning set forth in Section 3.3(a).
“Celladon” shall have the meaning
set forth in the Preamble.
“Celladon Affiliate” shall mean any Person that is (or at any relevant time was) under
common control with Celladon within the meaning of Sections 414(b), (c), (m) and (o) of the Code, and the regulations issued thereunder.
“Celladon Associate” shall mean any current or former employee, independent contractor, officer or director of
Celladon or any Celladon Affiliate.
AA-1
“Celladon Board of Directors” shall mean the board of directors of
Celladon.
“Celladon Board Recommendation” shall have the meaning set forth in Section 5.3(b).
“Celladon Board Adverse Recommendation Change” shall have the meaning set forth in Section 5.3(c).
“Celladon Common Stock” shall mean the Common Stock, $0.001 par value per share, of Celladon.
“Celladon Contract” shall mean any Contract: (a) to which Celladon or any of its Subsidiaries is a party;
(b) by which Celladon or any of its Subsidiaries or any Celladon IP Rights or any other asset of Celladon or any of its Subsidiaries is or may become bound or under which Celladon has, or may become subject to, any obligation; or (c) under
which Celladon or any of its Subsidiaries has or may acquire any right or interest.
“Celladon Disclosure
Schedule” shall have the meaning set forth in Section 3.
“Celladon Employee Plan” shall
have the meaning set forth in Section 3.11(f).
“Celladon IP Rights” shall have the meaning set forth in
Section 3.6(b).
“Celladon IP Rights Agreement” shall mean any instrument or agreement governing, related
or pertaining to any Celladon IP Rights.
“Celladon Material Adverse Effect” shall mean any Effect that,
considered together with all other Effects that have occurred prior to the date of determination of the occurrence of the Celladon Material Adverse Effect, is or could reasonably be expected to be or to become materially adverse to, or has or could
reasonably be expected to have or result in a material adverse effect on: (a) the business, condition (financial or otherwise), capitalization, assets, operations or financial performance of Celladon; or (b) the ability of Celladon to
consummate the Merger or any of the other Contemplated Transactions or to perform any of its covenants or obligations under the Agreement in all material respects; provided, however, that Effects from the following shall not be deemed
to constitute (nor shall Effects from any of the following be taken into account in determining whether there has occurred) a Celladon Material Adverse Effect: (i) conditions generally affecting the industries in which Celladon participates or
the United States or global economy or capital markets as a whole, to the extent that such conditions do not have a disproportionate impact on Celladon; (ii) any failure by Celladon to meet internal projections or forecasts or third party
revenue or earnings predictions for any period ending (or for which revenues or earnings are released) on or after the date of this Agreement or any change in the price or trading volume of Celladon Common Stock (it being understood, however, that
any Effect causing or contributing to any such failure to meet projections or predictions or any change in stock price or trading volume may constitute a Celladon Material Adverse Effect and may be taken into account in determining whether a
Celladon Material Adverse Effect has occurred); (iii) the execution, delivery, announcement or performance of the obligations under this Agreement or the announcement, pendency or anticipated consummation of the Merger or the Eiger Pre-Closing
Financing; (iv) the resignation or termination of any officer or director; (v) any natural disaster or any acts of terrorism, sabotage, military action or war or any escalation or worsening thereof; or (vi) any changes (after the date
of this Agreement) in GAAP or applicable Legal Requirements.
“Celladon Material Contract” shall have the meaning
set forth in Section 3.7.
“Celladon Options” shall mean options or other rights to purchase shares of
Celladon Common Stock issued or granted by Celladon.
“Celladon Permits” shall have the meaning set forth in
Section 3.9(b).
AA-2
“Celladon Product Candidates” shall have the meaning set forth in
Section 3.9(d).
“Celladon Registered IP” shall mean all Celladon IP Rights that are registered, filed or
issued under the authority of, with or by any Governmental Body, including all patents, registered copyrights and registered trademarks and all applications for any of the foregoing.
“Celladon Regulatory Permits” shall have the meaning set forth in Section 3.9(d).
“Celladon SEC Documents” shall have the meaning set forth in Section 3.4(a).
“Celladon Stock Plans” shall have the meaning set forth in Section 3.3(b).
“Celladon Stockholder Support Agreements” shall have the meaning set forth in the recitals.
“Celladon Stockholders’ Meeting” shall have the meaning set forth in Section 5.3(a).
“Celladon Termination Fee” shall have the meaning set forth in Section 9.3(b)(ii).
A “Celladon Triggering Event” shall be deemed to have occurred if:
(i) the Board of Directors of Celladon shall have failed to recommend that Celladon’s stockholders vote to approve the Merger, the issuance of Celladon Common Stock in the Merger and the Reverse Split or shall for any reason have withdrawn
or shall have modified in a manner adverse to Eiger the Celladon Board Recommendation; (ii) Celladon shall have failed to include in the Proxy Statement/Prospectus/Information Statement the Celladon Board Recommendation; (iii) Celladon
shall have failed to hold the Celladon Stockholders’ Meeting within sixty (60) days after the Form S-4 Registration Statement is declared effective under the Securities Act (other than to the extent that the Form S-4 Registration Statement
is subject to any stop order or proceeding (or threatened proceeding by the SEC) seeking a stop order with respect to the Form S-4 Registration Statement, in which case such sixty (60) day period shall be tolled for the earlier of sixty
(60) days or so long as such stop order remains in effect or proceeding or threatened proceeding remains pending); (iv) the Board of Directors of Celladon shall have approved, endorsed or recommended any Acquisition Proposal;
(v) Celladon shall have entered into any letter of intent or similar document or any Contract relating to any Acquisition Proposal (other than a confidentiality agreement permitted pursuant to Section 4.5); or (vi) Celladon or
any director, officer or agent of Celladon shall have willfully and intentionally breached the provisions set forth in Section 4.5.
“Celladon Unaudited Interim Balance Sheet” shall mean the unaudited consolidated balance sheet of Celladon included in
Celladon’s Report on Form 10-Q filed with the SEC for the period ended September 30, 2015.
“Celladon
Warrants” shall have the meaning set forth in Section 3.3(c).
“Certificate of Merger” shall
have the meaning set forth in Section 1.3.
“Certifications” shall have the meaning set forth in Section
3.4(a).
“Closing” shall have the meaning set forth in Section 1.3.
“Closing Date” shall have the meaning set forth in Section 1.3.
“COBRA” means the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended, as set forth in
Section 4980B of the Code and Part 6 of Title I of ERISA.
“Code” shall mean the Internal Revenue Code of
1986, as amended.
AA-3
“Confidentiality Agreement” shall mean the Confidentiality Agreement
dated June 16, 2015, between Eiger and Celladon.
“Consent” shall mean any approval, consent, ratification,
permission, waiver or authorization (including any Governmental Authorization).
“Contemplated Transactions” shall
mean the Merger and the other transactions and actions contemplated by the Agreement.
“Contract” shall, with
respect to any Person, mean any written agreement, contract, subcontract, lease (whether real or personal property), mortgage, understanding, arrangement, instrument, note, option, warranty, purchase order, license, sublicense, insurance policy,
benefit plan or legally binding commitment or undertaking of any nature to which such Person is a party or by which such Person or any of its assets are bound or affected under applicable law.
“Costs” shall have the meaning set forth in Section 5.7(a).
“D&O Indemnified Parties” shall have the meaning set forth in Section 5.7(a).
“DGCL” shall mean the General Corporation Law of the State of Delaware.
“Dissenting Shares” shall have the meaning set forth in Section 1.9(a).
“Drug Regulatory Agency” shall have the meaning set forth in Section 2.11(c).
“Effect” shall mean any effect, change, event, circumstance, or development.
“Effective Time” shall have the meaning set forth in Section 1.3.
“Eiger” shall have the meaning set forth in the Preamble.
“Eiger Affiliate” shall mean any Person that is (or at any relevant time was) under common control with Eiger within
the meaning of Sections 414(b), (c), (m) and (o) of the Code, and the regulations issued thereunder.
“Eiger
Associate” shall mean any current or former employee, independent contractor, officer or director of Eiger or any Eiger Affiliate.
“Eiger Board of Directors” shall mean the board of directors of Eiger.
“Eiger Board Recommendation” shall have the meaning set forth in Section 5.2(b).
“Eiger Capital Stock” shall mean the Eiger Common Stock and the Eiger Preferred Stock.
“Eiger Common Stock” shall mean the Common Stock, $0.0001 par value per share, of Eiger.
“Eiger Contract” shall mean any Contract: (a) to which Eiger or any of its Subsidiaries is a Party; (b) by
which Eiger or any Eiger Subsidiary or any Eiger IP Rights or any other asset of Eiger or its Subsidiaries is or may become bound or under which Eiger or any Eiger Subsidiary has, or may become subject to, any obligation; or (c) under which
Eiger or Eiger Subsidiary has or may acquire any right or interest.
AA-4
“Eiger Disclosure Schedule” shall have the meaning set forth in
Section 2.
“Eiger Employee Plan” shall have the meaning set forth in Section 2.13(e).
“Eiger Financials” shall have the meaning set forth in Section 2.4(a).
“Eiger IP Rights” shall mean all Intellectual Property owned, licensed or controlled by Eiger or any of its
Subsidiaries that is necessary or used in the business of Eiger and its Subsidiaries as presently conducted or as presently proposed to be conducted.
“Eiger IP Rights Agreement” shall mean any instrument or agreement governing, related or pertaining to any Eiger IP
Rights.
“Eiger Material Adverse Effect” shall mean any Effect that, considered together with all other Effects
that have occurred prior to the date of determination of the occurrence of the Eiger Material Adverse Effect, is or could reasonably be expected to be materially adverse to, or has or could reasonably be expected to have or result in a material
adverse effect on: (a) the business, condition (financial or otherwise), capitalization, assets (including Intellectual Property), operations or financial performance of Eiger and its Subsidiaries taken as a whole; or (b) the ability of
Eiger to consummate the Merger or any of the other Contemplated Transactions or to perform any of its covenants or obligations under the Agreement in all material respects; provided, however, that Effects from the following shall not
be deemed to constitute (nor shall Effects from any of the following be taken into account in determining whether there has occurred) an Eiger Material Adverse Effect: (i) any rejection by a Governmental Body of a registration or filing by
Eiger relating to the Eiger IP Rights; (ii) any change in the cash position of Eiger which results from operations in the Ordinary Course of Business; (iii) conditions generally affecting the industries in which Eiger and its Subsidiaries
participate or the United States or global economy or capital markets as a whole, to the extent that such conditions do not have a disproportionate impact on Eiger and its Subsidiaries taken as a whole; (iv) any failure by Eiger or any of its
Subsidiaries to meet internal projections or forecasts or third party revenue or earnings predictions for any period ending (or for which revenues or earnings are released) on or after the date of this Agreement (it being understood, however, that
any Effect causing or contributing to any such failure to meet projections or predictions may constitute an Eiger Material Adverse Effect and may be taken into account in determining whether an Eiger Material Adverse Effect has occurred);
(v) the execution, delivery, announcement or performance of the obligations under this Agreement or the announcement, pendency or anticipated consummation of the Merger or the Eiger Pre-Closing Financing; (vi) any natural disaster or any
acts of terrorism, sabotage, military action or war or any escalation or worsening thereof; or (vi) any changes (after the date of this Agreement) in GAAP or applicable Legal Requirements.
“Eiger Material Contract” shall have the meaning set forth in Section 2.9.
“Eiger Options” shall mean options or other rights to purchase shares of Eiger Common Stock issued or granted by
Eiger.
“Eiger Permits” shall have the meaning set forth in Section 2.11(b).
“Eiger Pre-Closing Financing” means an acquisition of Eiger Common Stock to be consummated prior to the Closing
pursuant to the Subscription Agreement with aggregate gross cash proceeds to Eiger of at least $30,000,000 (not including any conversion of promissory notes in connection therewith).
“Eiger Preferred Stock” shall have the meaning set forth in Section 2.3(a).
“Eiger Product Candidates” shall have the meaning set forth in Section 2.11(d).
AA-5
“Eiger Registered IP” shall mean all Eiger IP Rights that are registered,
filed or issued under the authority of, with or by any Governmental Body, including all patents, registered copyrights and registered trademarks and all applications for any of the foregoing.
“Eiger Regulatory Permits” shall have the meaning set forth in Section 2.11(d).
“Eiger Stock Certificate” shall have the meaning set forth in Section 1.7.
“Eiger Stockholder Support Agreements” shall have the meaning set forth in the recitals.
“Eiger Stockholder Written Consent” shall have the meaning set forth in the recitals.
“Eiger Termination Fee” shall have the meaning set forth in Section 9.3(b)(i).
An “Eiger Triggering Event” shall be deemed to have occurred if: (i) the Board of Directors of Eiger shall have
failed to recommend that Eiger’s stockholders vote to approve the Merger or shall for any reason have withdrawn or shall have modified in a manner adverse to Celladon the Eiger Board Recommendation; (ii) Eiger shall have failed to include
in the Proxy Statement/Prospectus/Information Statement the Eiger Board Recommendation; (iii) the Board of Directors of Eiger shall have approved, endorsed or recommended any Acquisition Proposal; (iv) Eiger shall have entered into any
letter of intent or similar document or any Contract relating to any Acquisition Proposal (other than a confidentiality agreement permitted pursuant to Section 4.5); or (v) Eiger or any director, officer or agent of Eiger shall have
willfully and intentionally breached the provisions set forth in Section 4.5 of the Agreement.
“Eiger Unaudited
Interim Balance Sheet” shall mean the unaudited consolidated balance sheet of Eiger and its consolidated Subsidiaries as of September 30, 2015, provided to Celladon prior to the date of this Agreement.
“Eiger Warrants” shall have the meaning set forth in Section 2.3(c).
“Encumbrance” shall mean any lien, pledge, hypothecation, charge, mortgage, security interest, encumbrance, claim,
infringement, interference, option, right of first refusal, preemptive right, community property interest or restriction of any nature (including any restriction on the voting of any security, any restriction on the transfer of any security or other
asset, any restriction on the receipt of any income derived from any asset, any restriction on the use of any asset and any restriction on the possession, exercise or transfer of any other attribute of ownership of any asset).
“Entity” shall mean any corporation (including any non-profit corporation), partnership (including any general
partnership, limited partnership or limited liability partnership), joint venture, estate, trust, company (including any company limited by shares, limited liability company or joint stock company), firm, society or other enterprise, association,
organization or entity, and each of its successors.
“Environmental Law” means any federal, state, local or
foreign Legal Requirement relating to pollution or protection of human health or the environment (including ambient air, surface water, ground water, land surface or subsurface strata), including any law or regulation relating to emissions,
discharges, releases or threatened releases of Hazardous Materials, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials.
“ERISA” shall mean the Employee Retirement Income Security Act of 1974, as amended.
“Exchange Act” shall mean the Securities Exchange Act of 1934, as amended.
“Exchange Agent” shall have the meaning set forth in Section 1.8(a).
AA-6
“Exchange Fund” shall have the meaning set forth in Section
1.8(a).
“Exchange Ratio” shall mean, subject to Section 1.5(f), the quotient obtained by dividing
the Eiger Merger Shares by the Eiger Outstanding Shares, where:
| |
• |
|
“Aggregate Value” means the sum of (i) $81,750,000 plus (ii) the aggregate gross cash proceeds received by Eiger from the Eiger Pre-Closing Financing (including a
conversion of promissory notes of up to $6,000,000 in principal amount issued in connection therewith). |
| |
• |
|
“Celladon Allocation Percentage” means the quotient determined by dividing: $26,750,000 by the Aggregate Value. |
| |
• |
|
“Celladon Outstanding Shares” means, subject to Section 1.5(f), the total number of shares of Celladon Common Stock outstanding immediately prior to the Effective Time expressed on a
fully diluted and as-converted to Celladon Common Stock basis, but assuming, without limitation, (i) with respect to Celladon Options, the cashless exercise solely of those Celladon Options outstanding as
of immediately prior to the Effective Time that are in-the-money (and otherwise disregarding the Celladon Options), (ii) the cashless exercise of all Celladon Warrants outstanding as of immediately prior to the Effective Time; and
(iii) the issuance of shares of Celladon Capital Stock in respect of all other options, warrants or rights to receive such shares (assuming cashless exercise in the case of options, warrants and other similar rights), whether conditional or
unconditional and including any options, warrants or rights triggered by or associated with the consummation of the Merger. |
| |
• |
|
“Eiger Allocation Percentage” means the quotient determined by dividing (i) the sum of the Aggregate Value minus $26,750,000 by (ii) the Aggregate Value.
|
| |
• |
|
“Eiger Merger Shares” means the product determined by multiplying the Post-Closing Celladon Shares by the Eiger Allocation Percentage. |
| |
• |
|
“Eiger Outstanding Shares” means the total number of shares of Eiger Capital Stock outstanding immediately prior to the Effective Time expressed on a fully diluted and as-converted to Eiger Common Stock basis and assuming, without limitation, (i) the exercise of all Eiger Options and Eiger Warrants outstanding as of immediately prior to the Effective Time, (ii) the
consummation of the Eiger Pre-Closing Financing, (iii) the conversion of all of Eiger’s outstanding convertible indebtedness (including a conversion of promissory notes of up to $6,000,000 in principal amount issued in connection with the
Eiger Pre-Closing Financing) and (iv) the issuance of shares of Eiger Capital Stock in respect of all other options, warrants or rights to receive such shares, whether conditional or unconditional and including any options, warrants or rights
triggered by or associated with the consummation of the Eiger Pre-Closing Financing or the Merger. |
| |
• |
|
“Post-Closing Celladon Shares” mean the quotient determined by dividing the Celladon Outstanding Shares by the Celladon Allocation Percentage. |
“Existing Eiger D&O Policies” shall have the meaning set forth in Section 2.15(b).
“Existing Celladon D&O Policies” shall have the meaning set forth in Section 3.13(b).
“FDA” shall have the meaning set forth in Section 2.11(c).
“FDCA” shall have the meaning set forth in Section 2.11(c).
“Form S-4 Registration Statement” shall mean the registration statement on Form S-4 to be filed with the SEC by
Celladon registering the public offering and sale of Celladon Common Stock to some or all holders of Eiger Common Stock in the Merger, including all shares of Celladon Common Stock to be issued in exchange for all other shares of Eiger Common Stock
in the Merger, as said registration statement may be amended prior to the time it is declared effective by the SEC.
AA-7
“GAAP” shall have the meaning set forth in Section 2.4(a).
“Governmental Authority” means any court or tribunal, governmental, quasi-governmental or regulatory body,
administrative agency or bureau, commission or authority or other body exercising similar powers or authority.
“Governmental
Authorization” shall mean any: (a) permit, license, certificate, franchise, permission, variance, exceptions, orders, clearance, registration, qualification or authorization issued, granted, given or otherwise made available by or
under the authority of any Governmental Body or pursuant to any Legal Requirement; or (b) right under any Contract with any Governmental Body.
“Governmental Body” shall mean any: (a) nation, state, commonwealth, province, territory, county, municipality,
district or other jurisdiction of any nature; (b) federal, state, local, municipal, foreign or other government; (c) governmental or quasi-Governmental Authority of any nature (including any governmental division, department, agency,
commission, instrumentality, official, ministry, fund, foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any Taxing authority); or (d) self-regulatory organization (including
the NASDAQ Stock Market).
“Grant Date” shall have the meaning set forth in Section 2.13(f).
“Hazardous Materials” shall mean any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive,
corrosive, ignitable or flammable chemical, or chemical compound, or hazardous substance, material or waste, whether solid, liquid or gas, that is subject to regulation, control or remediation under any Environmental Law, including crude oil or any
fraction thereof, and petroleum products or by-products.
“HSR Act” shall mean the Hart-Scott-Rodino Antitrust
Improvements Act of 1976, as amended.
“Intellectual Property” shall mean (a) United States, foreign and
international patents, patent applications, including provisional applications, statutory invention registrations, invention disclosures and inventions, (b) trademarks, service marks, trade names, domain names, URLs, trade dress, logos and
other source identifiers, including registrations and applications for registration thereof, (c) copyrights, including registrations and applications for registration thereof, and (d) software, formulae, customer lists, trade secrets,
know-how, confidential information and other proprietary rights and intellectual property, whether patentable or not.
“Investor Agreements” shall have the meaning set forth in Section 5.18.
“IRS” shall mean the United States Internal Revenue Service.
“Key Employee” shall mean, with respect to the Eiger or Celladon, an executive officer or any employee that reports
directly to the Board of Directors or Chief Executive Officer or Chief Operating Officer.
“Knowledge” means, with
respect to an individual, that such individual is actually aware of the relevant fact or such individual would reasonably be expected to know such fact in the ordinary course of the performance of the individual’s employee or professional
responsibility. Any Person that is an Entity shall have Knowledge if any officer or director of such Person as of the date such knowledge is imputed has Knowledge of such fact or other matter.
“Legal Proceeding” shall mean any action, suit, litigation, arbitration, proceeding (including any civil, criminal,
administrative, investigative or appellate proceeding), hearing, inquiry, audit, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Body or any arbitrator or
arbitration panel.
AA-8
“Legal Requirement” shall mean any federal, state, foreign, material
local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by
or under the authority of any Governmental Body (or under the authority of the NASDAQ Stock Market or the Financial Industry Regulatory Authority).
“Liability” shall have the meaning set forth in Section 2.10.
“Merger” shall have the meaning set forth in the recitals.
“Merger Sub” shall have the meaning set forth in the Preamble.
“Multiemployer Plan” shall mean (A) a “multiemployer plan,” as defined in Section 3(37) or
4001(a)(3) of ERISA, or (B) a plan which if maintained or administered in or otherwise subject to the laws of the United States would be described in paragraph (A).
“Multiple Employer Plan” shall mean (A) a “multiple employer plan” within the meaning of
Section 413(c) of the Code or Section 3(40) of ERISA, or (B) a plan which if maintained or administered in or otherwise subject to the laws of the United States would be described in paragraph (A).
“Nasdaq Listing Application” shall have the meaning set forth in Section 5.10.
“Net Cash” shall mean (a) the sum of Celladon’s cash and cash equivalents,
marketable securities, accounts, interest and other receivables (to the extent determined to be collectible), and deposits (to the extent refundable to Celladon), in each case as of the close of business on the last Business Day prior to the date of
determination, determined in a manner consistent with the manner in which such items were historically determined and in accordance with Celladon’s Audited Financial Statements and Unaudited Interim Balance Sheet, minus
(b) the sum of Celladon’s accounts payable and accrued expenses (other than accrued expenses listed below), in each case as of such date and determined in a manner consistent with the manner in which such items were historically determined
and in accordance with Celladon’s Audited Financial Statements and Unaudited Interim Balance Sheet, minus (c) the cash cost of any unpaid change of control payments or severance payments that are or become due to any current
or former employee of Celladon, minus (d) the cash cost of any accrued and unpaid retention payments due to any employee of Celladon as of the Closing Date, minus (e) any remaining unpaid fees and expenses
(including any attorney’s, accountant’s, financial advisor’s or finder’s fees) as of such date for which Celladon or any of its Subsidiaries is liable incurred by Celladon or any of its Subsidiaries in connection with this
Agreement and the Contemplated Transactions or otherwise, minus (f) any bona fide current liabilities payable in cash, in each case to the extent not cancelled at or prior to the Determination Date, minus
(g) any unpaid amounts payable by Celladon in satisfaction of its obligations under Section 5.7(c) for the period after the Closing, minus (h) any fees and expenses payable by Celladon pursuant to
Section 1.6(e), minus (i) the cash cost of any unpaid retention payment amounts due under any insurance policy with respect to any Legal Proceeding against Celladon or Merger Sub (including the Legal Proceedings set
forth on Part 3.15 of the Celladon Disclosure Schedule), minus (j) the net cash obligation of Celladon (i.e., remaining contractual payments owed less contractual receipts expected) with respect to its leased premises at
12760 High Bluff Drive in San Diego plus (k) the amount of any reimbursement owed to Celladon pursuant to Section 9.3(a).
“Net Cash Calculation” shall have the meaning set forth in Section 1.6(a).
“Net Cash Schedule” shall have the meaning set forth in Section 1.6(a).
“Ordinary Course of Business” shall mean, in the case of each of Eiger and Celladon and for all periods, such actions
taken in the ordinary course of its normal operations and consistent with its past practices, and for periods following the date of this Agreement consistent with its operating plans delivered to the other Party; provided, however,
that the Ordinary Course of Business for Celladon shall also include activities in connection with potentially winding down of all its historical clinical development programs and related operations.
AA-9
“Party” or “Parties” shall mean Eiger, Merger Sub
and Celladon.
“Person” shall mean any individual, Entity or Governmental Body.
“Pre-Closing Period” shall have the meaning set forth in Section 4.1.
“Preferred Stock Conversion” shall have the meaning set forth in Section 7.9.
“Proxy Statement/Prospectus/ Information Statement” shall mean the proxy statement/prospectus/information statement to
be sent to Eiger’s stockholders in connection with the approval of this Agreement and the Merger (by signing the Eiger Stockholder Written Consent) and to Celladon’s stockholders in connection with the Celladon Stockholders’ Meeting.
“Representatives” shall mean directors, officers, other employees, agents, attorneys, accountants, advisors and
representatives.
“Required Eiger Stockholder Vote” shall have the meaning set forth in Section 2.19.
“Required Celladon Stockholder Vote” shall have the meaning set forth in Section 3.18.
“Reverse Split” shall have the meaning set forth in Section 5.16.
“Sarbanes-Oxley Act” shall mean the Sarbanes-Oxley Act of 2002, as it may be amended from time to time.
“SEC” shall mean the United States Securities and Exchange Commission.
“Securities Act” shall mean the Securities Act of 1933, as amended.
“Shareholder” shall mean each stockholder of Eiger, and “Shareholders” shall mean all
stockholders of Eiger, in each case as determined immediately prior to the Effective Time.
“Subscription
Agreement” means the Subscription Agreement attached hereto as Exhibit D, among Eiger and the Persons named therein, pursuant to which such Persons have agreed to purchase the number of shares of Eiger Common Stock set forth
therein in connection with the Eiger Pre-Closing Financing.
“Subsequent Transaction” shall mean any Acquisition
Transaction that results or would result in any third party beneficially owning securities of a Party representing more than fifty percent (50%) of the voting power of the outstanding securities of a Party or owning or exclusively licensing
tangible or intangible assets representing more than fifty percent (50%) of the fair market value of the income-generating assets of a Party and its Subsidiaries, taken as a whole.
An entity shall be deemed to be a “Subsidiary” of another Person if such Person directly or indirectly owns or
purports to own, beneficially or of record, (a) an amount of voting securities of other interests in such entity that is sufficient to enable such Person to elect at least a majority of the members of such entity’s board of directors or
other governing body, or (b) at least 50% of the outstanding equity, voting, beneficial or financial interests in such Entity.
“Superior Offer” shall mean a bona fide written offer by a third party to enter into (i) a merger, consolidation,
amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction as a result of which either (A) the Party’s
stockholders prior to such transaction in the aggregate cease to own at least 50% of
AA-10
the voting securities of the entity surviving or resulting from such transaction (or the ultimate parent entity thereof) or (B) in which a Person or “group” (as defined in the
Exchange Act and the rules promulgated thereunder) directly or indirectly acquires beneficial or record ownership of securities representing 50% or more of the Party’s capital stock or (ii) a sale, lease, exchange transfer, license,
acquisition or disposition of any business or other disposition of at least 50% of the assets of the Party or its Subsidiaries, taken as a whole, in a single transaction or a series of related transactions that (in each case of the foregoing clauses
“(i)” and “(ii)”): (a) was not obtained or made as a direct or indirect result of a breach of (or in violation of) this Agreement (including Section 4.5); and (b) is on terms and conditions that the Board of
Directors of Celladon or Eiger, as applicable, determines, in its reasonable, good faith judgment, after obtaining and taking into account such matters that its Board of Directors deems relevant following consultation with its outside legal counsel
and financial advisor, if any: (x) is reasonably likely to be more favorable, from a financial point of view, to Celladon’s stockholders or Eiger’s stockholders, as applicable, than the terms of the Merger; and (y) is reasonably
capable of being consummated; provided, however, that any such offer shall not be deemed to be a “Superior Offer” if any financing required to consummate the transaction contemplated by such offer is not committed and is not
reasonably capable of being obtained by such third party, or if the consummation of such transaction is contingent on any such financing being obtained.
“Surviving Corporation” shall have the meaning set forth in Section 1.1.
“Tax” shall mean any federal, state, local, foreign or other tax, including any income tax, franchise tax, capital
gains tax, gross receipts tax, value-added tax, surtax, estimated tax, unemployment tax, national health insurance tax, excise tax, ad valorem tax, transfer tax, stamp tax, sales tax, use tax, property tax, business tax, withholding tax, payroll
tax, customs duty, alternative or add-on minimum or other tax of any kind whatsoever, and including any fine, penalty, addition to tax or interest, whether disputed or not.
“Tax Return” shall mean any return (including any information return), report, statement, declaration, estimate,
schedule, notice, notification, form, election, certificate or other document or information, and any amendment or supplement to any of the foregoing, filed with or submitted to, or required to be filed with or submitted to, any Governmental Body in
connection with the determination, assessment, collection or payment of any Tax or in connection with the administration, implementation or enforcement of or compliance with any Legal Requirement relating to any Tax.
“Third Party Expenses” shall have the meaning set forth in Section 9.3(c).
“Treasury Regulations” shall mean the United States Treasury regulations promulgated under the Code.
AA-11
Annex B

Wedbush Securities Inc.
Two Embarcadero Center
Suite 600
San Francisco, CA 94111
November 16, 2015
Board of Directors
Celladon Corporation
11988 El Camino Real, Suite 650
Suite 650
San Diego, CA 92130
Members of the Board:
We understand that Celladon Corporation
(“Celladon”) proposes to enter into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) by and among Celladon, Curie Merger Sub Inc. (“Merger Sub”), and Eiger BioPharmaceuticals, Inc.
(“Eiger”), pursuant to which, among other things, Merger Sub will be merged with and into Eiger with Eiger continuing as the surviving corporation and becoming a wholly-owned subsidiary of Celladon (the “Merger”).
Pursuant to the Merger Agreement, and as more fully set forth in the Merger Agreement, each share of common stock, par value $0.0001 per share (“Eiger
Common Stock”), outstanding immediately prior to the effective time of the Merger, excluding shares to be canceled pursuant to Section 1.5(a)(i) of the Merger Agreement and Dissenting Shares (as defined in the Merger Agreement), shall be
converted into the right to receive a number of shares of common stock, par value $0.001 per share (“Celladon Common Stock”), of Celladon equal to the Exchange Ratio (as defined in the Merger Agreement). The terms and conditions of the
Merger are set forth in more detail in the Merger Agreement.
You have asked us whether, in our opinion as investment bankers as of the date hereof, the
Exchange Ratio in connection with the Merger, as provided in the Merger Agreement, is fair to Celladon from a financial point of view.
Wedbush Securities
Inc. (“Wedbush”) is an investment banking firm and member of The New York Stock Exchange and other principal stock exchanges in the United States, and is regularly engaged as part of its business in the valuation of businesses and their
securities in connection with mergers and acquisitions, negotiated underwritings, private placements, secondary distributions of listed and unlisted securities, and valuations for corporate, estate and other purposes.
For purposes of this opinion and in connection with our review, we have, among other things: (1) reviewed a draft of the Merger Agreement dated
November 13, 2015; (2) reviewed certain publicly available business and financial information relating to Celladon and Eiger, respectively; (3) reviewed certain internal information, primarily financial in nature, furnished to us by
the managements of Celladon and Eiger, respectively, and approved for our use by Celladon; (4) reviewed certain publicly available information with respect to other companies in the biopharmaceutical industry that we believe to be comparable in
certain respects to Eiger; and (5) considered the financial terms, to the extent publicly available, of selected recent business combinations and initial public offerings involving companies in the biopharmaceutical industry that we believe to
be comparable in certain respects to Eiger, in whole or in part, and to the Merger. In addition, we have held discussions with the
B-1
management of Celladon and Eiger concerning their views as to the financial and other information described above. In addition to the foregoing, we have conducted such other analyses and
examinations and considered such other financial, economic and market criteria as we deem appropriate to arrive at our opinion.
In rendering this
opinion, we have relied upon and assumed, without independent verification, the accuracy and completeness of all information that was publicly available or was furnished to or discussed with us by Celladon, Eiger or any other party to the Merger
Agreement or otherwise reviewed by us. With respect to information provided to or reviewed by us, we have been advised by the management of Celladon and Eiger that such information was reasonably prepared on bases reflecting the best currently
available estimates and judgments of the management of Celladon or Eiger, as applicable. We express no view as to the reasonableness of such financial information or the assumptions on which it was based.
We have further relied on the assurances of management of Celladon that they are unaware of any facts that would make the information provided to us
incomplete or misleading. Except for certain estimates of liabilities expected to be incurred by Celladon in connection with a potential liquidation of Celladon prepared by management of Celladon, we have not made or been provided with any
independent evaluations or appraisals of any of the assets, properties, liabilities (including any contingent, derivative or off-balance-sheet assets or liabilities) or securities, nor have we made any physical inspection of the properties or
assets, of Celladon or Eiger or any of their respective subsidiaries. Further, as you are aware, Eiger’s management did not provide us with, and we did not otherwise have access to, financial forecasts regarding Eiger’s business, other
than certain expense forecasts for the three years ended December 31, 2018, and, accordingly we did not perform either a discounted cash flow analysis or any multiples-based analyses with respect to Eiger. We did not evaluate the solvency or
fair value of Celldon, Eiger or any of their respective subsidiaries (or the impact of the transaction thereon) under any law relating to bankruptcy, insolvency or similar matters.
Our opinion is based on economic, market and other conditions as in effect on, and the information made available to us as of, the date hereof. We have also
relied on the accuracy and completeness of Celladon’s and Eiger’s representations and warranties in the Merger Agreement, without regard to any qualifications that may be set forth in disclosure schedules or any other such qualifications.
In addition, we have assumed that the Merger will be consummated in accordance with the terms set forth in the Merger Agreement without any waiver, amendment or delay of any terms or conditions that would be material to our analysis. Representatives
of Celladon have advised us, and we have further assumed that the final terms of the Merger Agreement will not differ from the terms set forth in the draft we have reviewed in any respect material to our analysis. In addition, we have assumed that
Eiger’s contemplated issuance of at least $30,000,000 of Eiger Common Stock will be consummated prior to the Merger in accordance with the terms of the Merger Agreement. Events occurring after the date hereof could materially affect the
assumptions used in preparing this opinion. We have not undertaken any obligation to reaffirm or revise this opinion or otherwise comment upon any events occurring after the date hereof.
We are not legal, tax or regulatory advisors and do not express any opinion as to any tax or other consequences that may arise from the Transactions, nor does
our opinion address any legal, regulatory or accounting matters, as to which we understand that Celladon has obtained such advice as it deemed necessary from qualified professionals. We are financial advisors only and have relied upon, without
independent verification, the assessment of Celladon and Eiger and their legal, tax or regulatory advisors with respect to legal, tax or regulatory matters. We have assumed that the Merger will have the tax effects contemplated by the Merger
Agreement.
In rendering this opinion, we express no opinion as to the amount or nature of any compensation to any officers, directors, or employees of
Celladon, or any class of such persons, whether relative to the Exchange Ratio or otherwise, or with respect to the fairness of any such compensation. We are not opining as to the merits of the Merger as compared with any alternative transactions or
strategies that may be available to Celladon. At your direction, we have not been asked to, nor do we, offer any opinion as to the terms, other than Exchange Ratio to the extent expressly specified herein, of the Merger Agreement or the form of the
Merger. Nor do we express any
B-2
opinion with respect to the terms of any other agreement entered into or to be entered into in connection with the Merger. We express no opinion as to the price at which shares of Celladon Common
Stock may trade at any time subsequent to the announcement or consummation of the Merger. We have also assumed that all governmental, regulatory or other consents and approvals necessary for the consummation of the Merger will be obtained without
imposition of any terms or conditions that would be material to our analysis.
Celladon has agreed to pay Wedbush fees for its services as financial
advisor in connection with the Merger. A portion of such fees becomes payable upon delivery of this opinion and the substantial portion of such fees will become payable upon consummation of the Merger. In addition, Celladon has agreed to reimburse
us for all reasonable out-of-pocket expenses incurred by us and to indemnify us for certain liabilities arising out of our engagement. We have in the past received compensation from Celladon relating to investment banking services in connection with
Celladon’s initial public offering in January 2014 and a follow-on equity offering in August 2014. We may also provide investment banking and financial advisory services to Celladon, Eiger and their respective affiliates in the future for which
we would expect to receive compensation.
In the ordinary course of our business, we and our affiliates may actively trade the Celladon Common Stock or
other instruments or obligations of Celladon for our own account and for the accounts of our customers and, accordingly, we may at any time hold a long or short position in the Celladon Common Stock or such instruments or obligations of Celladon.
This opinion is for the benefit and use of the board of directors of Celladon (in its capacity as such) in connection with its evaluation of the Merger
and does not constitute a recommendation to the board of directors of Celladon as to how to act or to any holder of Celladon Common Stock or any other person as to how such holder or other person should vote with respect to the Merger or any other
matter. This opinion may not be used for any other purpose without our prior written consent in each instance, except as expressly provided for in the engagement letter dated as of May 28, 2015 between Celladon and Wedbush.
This opinion was approved by a fairness committee at Wedbush in accordance with the requirements of FINRA Rule 5150.
Based upon and subject to the foregoing, it is our opinion that, as of the date hereof, the Exchange Ratio in connection with the Merger, as provided in the
Merger Agreement, is fair to Celladon from a financial point of view.
Very truly yours,
Wedbush Securities Inc.
B-3
Annex C
SECTION 262 OF THE DELAWARE GENERAL CORPORATION LAW
§262 Appraisal rights.
(a) Any stockholder
of a corporation of this State who holds shares of stock on the date of the making of a demand pursuant to subsection (d) of this section with respect to such shares, who continuously holds such shares through the effective date of the merger
or consolidation, who has otherwise complied with subsection (d) of this section and who has neither voted in favor of the merger or consolidation nor consented thereto in writing pursuant to § 228 of this title shall be entitled to an
appraisal by the Court of Chancery of the fair value of the stockholder’s shares of stock under the circumstances described in subsections (b) and (c) of this section. As used in this section, the word “stockholder” means a
holder of record of stock in a corporation; the words “stock” and “share” mean and include what is ordinarily meant by those words; and the words “depository receipt” mean a receipt or other instrument issued by a
depository representing an interest in 1 or more shares, or fractions thereof, solely of stock of a corporation, which stock is deposited with the depository.
(b) Appraisal rights shall be available for the shares of any class or series of stock of a constituent corporation in a merger or consolidation to be
effected pursuant to § 251 (other than a merger effected pursuant to § 251(g) of this title and, subject to paragraph (b)(3) of this section, § 251(h) of this title), § 252, § 254, § 255, § 256, § 257, §
258, § 263 or § 264 of this title:
(1) Provided, however, that, except as expressly provided in § 363(b) of this title, no
appraisal rights under this section shall be available for the shares of any class or series of stock, which stock, or depository receipts in respect thereof, at the record date fixed to determine the stockholders entitled to receive notice of the
meeting of stockholders to act upon the agreement of merger or consolidation, were either: (i) listed on a national securities exchange or (ii) held of record by more than 2,000 holders; and further provided that no appraisal rights shall
be available for any shares of stock of the constituent corporation surviving a merger if the merger did not require for its approval the vote of the stockholders of the surviving corporation as provided in § 251(f) of this title.
(2) Notwithstanding paragraph (b)(1) of this section, appraisal rights under this section shall be available for the shares of any class or
series of stock of a constituent corporation if the holders thereof are required by the terms of an agreement of merger or consolidation pursuant to §§ 251, 252, 254, 255, 256, 257, 258, 263 and 264 of this title to accept for such stock
anything except:
a. Shares of stock of the corporation surviving or resulting from such merger or consolidation, or depository receipts in
respect thereof;
b. Shares of stock of any other corporation, or depository receipts in respect thereof, which shares of stock (or
depository receipts in respect thereof) or depository receipts at the effective date of the merger or consolidation will be either listed on a national securities exchange or held of record by more than 2,000 holders;
c. Cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a. and b. of this section;
or
d. Any combination of the shares of stock, depository receipts and cash in lieu of fractional shares or fractional depository receipts
described in the foregoing paragraphs (b)(2)a., b. and c. of this section.
(3) In the event all of the stock of a subsidiary Delaware
corporation party to a merger effected under § 251(h), § 253 or § 267 of this title is not owned by the parent immediately prior to the merger, appraisal rights shall be available for the shares of the subsidiary Delaware
corporation.
(4) In the event of an amendment to a corporation’s certificate of incorporation contemplated by § 363(a) of
this title, appraisal rights shall be available as contemplated by § 363(b) of this title, and the procedures of this section, including those set forth in subsections (d) and (e) of this section, shall apply as nearly as
C-1
practicable, with the word “amendment” substituted for the words “merger or consolidation,” and the word “corporation” substituted for the words “constituent
corporation” and/or “surviving or resulting corporation.”
(c) Any corporation may provide in its certificate of incorporation that
appraisal rights under this section shall be available for the shares of any class or series of its stock as a result of an amendment to its certificate of incorporation, any merger or consolidation in which the corporation is a constituent
corporation or the sale of all or substantially all of the assets of the corporation. If the certificate of incorporation contains such a provision, the procedures of this section, including those set forth in subsections (d) and (e) of
this section, shall apply as nearly as is practicable.
(d) Appraisal rights shall be perfected as follows:
(1) If a proposed merger or consolidation for which appraisal rights are provided under this section is to be submitted for approval at a
meeting of stockholders, the corporation, not less than 20 days prior to the meeting, shall notify each of its stockholders who was such on the record date for notice of such meeting (or such members who received notice in accordance with §
255(c) of this title) with respect to shares for which appraisal rights are available pursuant to subsection (b) or (c) of this section that appraisal rights are available for any or all of the shares of the constituent corporations, and
shall include in such notice a copy of this section and, if 1 of the constituent corporations is a nonstock corporation, a copy of § 114 of this title. Each stockholder electing to demand the appraisal of such stockholder’s shares shall
deliver to the corporation, before the taking of the vote on the merger or consolidation, a written demand for appraisal of such stockholder’s shares. Such demand will be sufficient if it reasonably informs the corporation of the identity of
the stockholder and that the stockholder intends thereby to demand the appraisal of such stockholder’s shares. A proxy or vote against the merger or consolidation shall not constitute such a demand. A stockholder electing to take such action
must do so by a separate written demand as herein provided. Within 10 days after the effective date of such merger or consolidation, the surviving or resulting corporation shall notify each stockholder of each constituent corporation who has
complied with this subsection and has not voted in favor of or consented to the merger or consolidation of the date that the merger or consolidation has become effective; or
(2) If the merger or consolidation was approved pursuant to § 228, § 251(h), § 253, or § 267 of this title, then either a
constituent corporation before the effective date of the merger or consolidation or the surviving or resulting corporation within 10 days thereafter shall notify each of the holders of any class or series of stock of such constituent corporation who
are entitled to appraisal rights of the approval of the merger or consolidation and that appraisal rights are available for any or all shares of such class or series of stock of such constituent corporation, and shall include in such notice a copy
of this section and, if 1 of the constituent corporations is a nonstock corporation, a copy of § 114 of this title. Such notice may, and, if given on or after the effective date of the merger or consolidation, shall, also notify such
stockholders of the effective date of the merger or consolidation. Any stockholder entitled to appraisal rights may, within 20 days after the date of mailing of such notice or, in the case of a merger approved pursuant to § 251(h) of this
title, within the later of the consummation of the tender or exchange offer contemplated by § 251(h) of this title and 20 days after the date of mailing of such notice, demand in writing from the surviving or resulting corporation the appraisal
of such holder’s shares. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares. If such notice
did not notify stockholders of the effective date of the merger or consolidation, either (i) each such constituent corporation shall send a second notice before the effective date of the merger or consolidation notifying each of the holders of
any class or series of stock of such constituent corporation that are entitled to appraisal rights of the effective date of the merger or consolidation or (ii) the surviving or resulting corporation shall send such a second notice to all such
holders on or within 10 days after such effective date; provided, however, that if such second notice is sent more than 20 days following the sending of the first notice or, in the case of a merger approved pursuant to § 251(h) of this
title, later than the later of the consummation of the tender or exchange offer contemplated
C-2
by § 251(h) of this title and 20 days following the sending of the first notice, such second notice need only be sent to each stockholder who is entitled to appraisal rights and who has
demanded appraisal of such holder’s shares in accordance with this subsection. An affidavit of the secretary or assistant secretary or of the transfer agent of the corporation that is required to give either notice that such notice has been
given shall, in the absence of fraud, be prima facie evidence of the facts stated therein. For purposes of determining the stockholders entitled to receive either notice, each constituent corporation may fix, in advance, a record date that shall be
not more than 10 days prior to the date the notice is given, provided, that if the notice is given on or after the effective date of the merger or consolidation, the record date shall be such effective date. If no record date is fixed and the notice
is given prior to the effective date, the record date shall be the close of business on the day next preceding the day on which the notice is given.
(e)
Within 120 days after the effective date of the merger or consolidation, the surviving or resulting corporation or any stockholder who has complied with subsections (a) and (d) of this section hereof and who is otherwise entitled to
appraisal rights, may commence an appraisal proceeding by filing a petition in the Court of Chancery demanding a determination of the value of the stock of all such stockholders. Notwithstanding the foregoing, at any time within 60 days after the
effective date of the merger or consolidation, any stockholder who has not commenced an appraisal proceeding or joined that proceeding as a named party shall have the right to withdraw such stockholder’s demand for appraisal and to accept the
terms offered upon the merger or consolidation. Within 120 days after the effective date of the merger or consolidation, any stockholder who has complied with the requirements of subsections (a) and (d) of this section hereof, upon written
request, shall be entitled to receive from the corporation surviving the merger or resulting from the consolidation a statement setting forth the aggregate number of shares not voted in favor of the merger or consolidation and with respect to which
demands for appraisal have been received and the aggregate number of holders of such shares. Such written statement shall be mailed to the stockholder within 10 days after such stockholder’s written request for such a statement is received by
the surviving or resulting corporation or within 10 days after expiration of the period for delivery of demands for appraisal under subsection (d) of this section hereof, whichever is later. Notwithstanding subsection (a) of this section,
a person who is the beneficial owner of shares of such stock held either in a voting trust or by a nominee on behalf of such person may, in such person’s own name, file a petition or request from the corporation the statement described in this
subsection.
(f) Upon the filing of any such petition by a stockholder, service of a copy thereof shall be made upon the surviving or resulting
corporation, which shall within 20 days after such service file in the office of the Register in Chancery in which the petition was filed a duly verified list containing the names and addresses of all stockholders who have demanded payment for their
shares and with whom agreements as to the value of their shares have not been reached by the surviving or resulting corporation. If the petition shall be filed by the surviving or resulting corporation, the petition shall be accompanied by such a
duly verified list. The Register in Chancery, if so ordered by the Court, shall give notice of the time and place fixed for the hearing of such petition by registered or certified mail to the surviving or resulting corporation and to the
stockholders shown on the list at the addresses therein stated. Such notice shall also be given by 1 or more publications at least 1 week before the day of the hearing, in a newspaper of general circulation published in the City of Wilmington,
Delaware or such publication as the Court deems advisable. The forms of the notices by mail and by publication shall be approved by the Court, and the costs thereof shall be borne by the surviving or resulting corporation.
(g) At the hearing on such petition, the Court shall determine the stockholders who have complied with this section and who have become entitled to appraisal
rights. The Court may require the stockholders who have demanded an appraisal for their shares and who hold stock represented by certificates to submit their certificates of stock to the Register in Chancery for notation thereon of the pendency of
the appraisal proceedings; and if any stockholder fails to comply with such direction, the Court may dismiss the proceedings as to such stockholder.
(h)
After the Court determines the stockholders entitled to an appraisal, the appraisal proceeding shall be conducted in accordance with the rules of the Court of Chancery, including any rules specifically governing appraisal proceedings. Through such
proceeding the Court shall determine the fair value of the shares exclusive
C-3
of any element of value arising from the accomplishment or expectation of the merger or consolidation, together with interest, if any, to be paid upon the amount determined to be the fair value.
In determining such fair value, the Court shall take into account all relevant factors. Unless the Court in its discretion determines otherwise for good cause shown, interest from the effective date of the merger through the date of payment of the
judgment shall be compounded quarterly and shall accrue at 5% over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the effective date of the merger and the date of payment of the
judgment. Upon application by the surviving or resulting corporation or by any stockholder entitled to participate in the appraisal proceeding, the Court may, in its discretion, proceed to trial upon the appraisal prior to the final determination of
the stockholders entitled to an appraisal. Any stockholder whose name appears on the list filed by the surviving or resulting corporation pursuant to subsection (f) of this section and who has submitted such stockholder’s certificates of
stock to the Register in Chancery, if such is required, may participate fully in all proceedings until it is finally determined that such stockholder is not entitled to appraisal rights under this section.
(i) The Court shall direct the payment of the fair value of the shares, together with interest, if any, by the surviving or resulting corporation to the
stockholders entitled thereto. Payment shall be so made to each such stockholder, in the case of holders of uncertificated stock forthwith, and the case of holders of shares represented by certificates upon the surrender to the corporation of the
certificates representing such stock. The Court’s decree may be enforced as other decrees in the Court of Chancery may be enforced, whether such surviving or resulting corporation be a corporation of this State or of any state.
(j) The costs of the proceeding may be determined by the Court and taxed upon the parties as the Court deems equitable in the circumstances. Upon application
of a stockholder, the Court may order all or a portion of the expenses incurred by any stockholder in connection with the appraisal proceeding, including, without limitation, reasonable attorney’s fees and the fees and expenses of experts, to
be charged pro rata against the value of all the shares entitled to an appraisal.
(k) From and after the effective date of the merger or consolidation,
no stockholder who has demanded appraisal rights as provided in subsection (d) of this section shall be entitled to vote such stock for any purpose or to receive payment of dividends or other distributions on the stock (except dividends or
other distributions payable to stockholders of record at a date which is prior to the effective date of the merger or consolidation); provided, however, that if no petition for an appraisal shall be filed within the time provided in subsection
(e) of this section, or if such stockholder shall deliver to the surviving or resulting corporation a written withdrawal of such stockholder’s demand for an appraisal and an acceptance of the merger or consolidation, either within 60 days
after the effective date of the merger or consolidation as provided in subsection (e) of this section or thereafter with the written approval of the corporation, then the right of such stockholder to an appraisal shall cease. Notwithstanding
the foregoing, no appraisal proceeding in the Court of Chancery shall be dismissed as to any stockholder without the approval of the Court, and such approval may be conditioned upon such terms as the Court deems just; provided, however that this
provision shall not affect the right of any stockholder who has not commenced an appraisal proceeding or joined that proceeding as a named party to withdraw such stockholder’s demand for appraisal and to accept the terms offered upon the merger
or consolidation within 60 days after the effective date of the merger or consolidation, as set forth in subsection (e) of this section.
(l) The
shares of the surviving or resulting corporation to which the shares of such objecting stockholders would have been converted had they assented to the merger or consolidation shall have the status of authorized and unissued shares of the surviving
or resulting corporation.
C-4
Annex D
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF
CELLADON CORPORATION
Celladon Corporation, a corporation organized and existing under the General Corporation Law of the State of Delaware (the
“Corporation”), DOES HEREBY CERTIFY:
FIRST: The original Certificate of Incorporation of the Corporation was filed with
the Secretary of State of the State of Delaware on February 24, 2012, under the name Celladon Corporation.
SECOND: The Amendment of
the Amended and Restated Certificate of Incorporation of the Corporation in the form set forth in the following resolution has been duly adopted in accordance with the provisions of Sections 228 and 242 of the General Corporation Law of the State of
Delaware by the directors and stockholders of the Corporation:
RESOLVED, that Article IV of the
Amended and Restated Certificate of Incorporation as presently in effect be, and the same hereby is, amended to add the following Section D:
D. Immediately upon the filing of this Certificate of Amendment of Amended and Restated Certificate of Incorporation with the Secretary
of State of the State of Delaware each one (1) share of the Corporation’s Common Stock outstanding immediately prior to such filing shall be automatically reclassified into one-fifteenth (1/15) of one share of the Corporation’s
Common Stock. The aforementioned reclassification shall be referred to collectively as the “Reverse Split.”
The Reverse Split shall occur without any further action on the part of the Corporation or stockholders of the Corporation and whether or not
certificates representing such stockholders’ shares prior to the Reverse Split are surrendered for cancellation. No fractional interest in a share of Common Stock shall be deliverable upon the Reverse Split. All shares of Common Stock
(including fractions thereof) issuable upon the Reverse Split held by a holder prior to the Reverse Split shall be aggregated for purposes of determining whether the Reverse Split would result in the issuance of any fractional share. Any fractional
share resulting from such aggregation upon the Reverse Split shall be rounded down to the nearest whole number. Each holder who would otherwise be entitled to a fraction of a share of Common Stock upon the Reverse Split (after aggregating all
fractions of a share to which such stockholder would otherwise be entitled) shall, in lieu thereof, be entitled to receive a cash payment in an amount equal to the fraction to which the stockholder would otherwise be entitled multiplied by the
closing price of the Corporation’s Common Stock as reported on The NASDAQ Global Market on the first trading day immediately following the filing of this Certificate of Amendment of Amended and Restated Certificate of Incorporation with the
Secretary of State of the State of Delaware. The Corporation shall not be obliged to issue certificates evidencing the shares of Common Stock outstanding as a result of the Reverse Split unless and until the certificates evidencing the shares held
by a holder prior to the Reverse Split are either delivered to the Corporation or its transfer agent, or the holder notifies the Corporation or its transfer agent that such certificates have been lost, stolen or destroyed and executes an agreement
satisfactory to the Corporation to indemnify the Corporation from any loss incurred by it in connection with such certificates.
THIRD:
The Certificate of Amendment of the Amended and Restated Certificate of Incorporation so adopted reads in full as set forth above and is hereby incorporated herein by this reference. All other provisions of the Amended and Restated Certificate of
Incorporation remain in full force and effect.
D-1
IN WITNESS WHEREOF, the Corporation has caused this Certificate to be signed by its Chief
Executive Officer this day of , 2016.
|
|
|
| CELLADON CORPORATION |
|
|
| By: |
|
|
|
|
Fredrik Wiklund, Chief Executive Officer |
D-2
Annex E
CERTIFICATE OF AMENDMENT OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF
CELLADON CORPORATION
Celladon Corporation, a corporation organized and existing under the General Corporation Law of the State of Delaware (the
“Corporation”), DOES HEREBY CERTIFY:
FIRST: The original Certificate of Incorporation of the Corporation was filed with
the Secretary of State of the State of Delaware on February 24, 2012, under the name Celladon Corporation.
SECOND: The Amendment of
the Amended and Restated Certificate of Incorporation of the Corporation in the form set forth in the following resolution has been duly adopted in accordance with the provisions of Sections 228 and 242 of the General Corporation Law of the State of
Delaware by the directors and stockholders of the Corporation:
RESOLVED, that Article I of the Amended and Restated
Certificate of Incorporation as presently in effect be, and the same hereby is, amended and restated to read in its entirety as follows:
The name of this corporation is Eiger BioPharmaceuticals, Inc. (the “Company”).
THIRD: The Certificate of Amendment of the Amended and Restated Certificate of Incorporation so adopted reads in full as set forth above and
is hereby incorporated herein by this reference. All other provisions of the Amended and Restated Certificate of Incorporation remain in full force and effect.
IN WITNESS WHEREOF, the Corporation has caused this Certificate to be signed by its Chief Executive Officer this day
of , 2016.
|
|
|
| CELLADON CORPORATION |
|
|
| By: |
|
|
|
|
Fredrik Wiklund, Chief Executive Officer |
E-1
PART II
INFORMATION NOT REQUIRED IN PROXY STATEMENT/PROSPECTUS/INFORMATION STATEMENT
| Item 20. |
Indemnification of Directors and Officers |
Subsection (a) of Section 145
of the General Corporation Law of the State of Delaware, or the DGCL, empowers a corporation to indemnify any person who was or is a party or who is threatened to be made a party to any threatened, pending or completed action, suit or proceeding,
whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at
the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in
settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the
corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the person’s conduct was unlawful.
Subsection (b) of Section 145 empowers a corporation to indemnify any person who was or is a party or is threatened to be made a party to any
threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person acted in any of the capacities set forth above, against expenses (including
attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the
best interests of the corporation, except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of
Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity
for such expenses which the Court of Chancery or such other court shall deem proper.
Section 145 further provides that to the extent a director or
officer of a corporation has been successful on the merits or otherwise in the defense of any action, suit or proceeding referred to in subsections (a) and (b) of Section 145, or in defense of any claim, issue or matter therein, such
person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith; that indemnification provided for by Section 145 shall not be deemed exclusive of any other
rights to which the indemnified party may be entitled; and the indemnification provided for by Section 145 shall, unless otherwise provided when authorized or ratified, continue as to a person who has ceased to be a director, officer, employee
or agent and shall inure to the benefit of such person’s heirs, executors and administrators. Section 145 also empowers the corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee
or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such
person and incurred by such person in any such capacity, or arising out of his status as such, whether or not the corporation would have the power to indemnify such person against such liabilities under Section 145.
Section 102(b)(7) of the DGCL provides that a corporation’s certificate of incorporation may contain a provision eliminating or limiting the
personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, provided that such provision shall not eliminate or limit the liability of a director (i) for any breach of
the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the DGCL or
(iv) for any transaction from which the director derived an improper personal benefit.
II-1
The Celladon amended and restated certificate of incorporation contains provisions that eliminate, to the maximum
extent permitted by the DGCL, the personal liability of directors and executive officers for monetary damages for breach of their fiduciary duties as a director or officer. The Celladon amended and restated certificate of incorporation and bylaws
provide that Celladon shall indemnify its directors and executive officers and may indemnify its employees and other agents to the fullest extent permitted by the DGCL.
Celladon entered into indemnification agreements with its directors and executive officers, in addition to the indemnification provided for in its amended and
restated certificate of incorporation and bylaws, and intends to enter into indemnification agreements with any new directors and executive officers in the future.
Celladon has purchased and intends to maintain insurance on behalf of any person who is or was a director or officer of Celladon against any loss arising from
any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusion.
Pursuant to the terms of the Merger
Agreement, for six years from the effective time of the merger, Celladon must indemnify each individual who is at the effective date of the merger a director or officer of Celladon against claims, costs and damages incurred as a result of such
director or officer serving as a director or officer of Celladon, to the fullest extent permitted under the DGCL. Each such person will also be entitled to advancement of expenses incurred in the defense of such claims, provided that such person
provides an undertaking required by applicable law to repay such advancement if it is ultimately determined that such person is not entitled to indemnification. Celladon must also purchase an insurance policy, effective as of the closing of the
merger, which maintains in effect for six years following the closing of the merger the current director’s and officer’s liability insurance policies maintained by Celladon.
| Item 21. |
Exhibits and Financial Statement Schedules |
(a) Exhibit Index
A list of exhibits filed with this registration statement on Form S-4 is set forth on the Exhibit Index and is incorporated herein by reference.
(b) Financial Statements
The financial statements filed with
this registration statement on Form S-4 are set forth on the Financial Statement Index and is incorporated herein by reference.
(a) The undersigned registrant hereby undertakes as follows:
(1) That prior to any public reoffering of the securities registered hereunder through use of a proxy statement/prospectus/information
statement which is a part of this registration statement, by any person or party who is deemed to be an underwriter within the meaning of Rule 145(c), the issuer undertakes that such reoffering proxy statement/prospectus/information statement will
contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other items of the applicable form.
(2) That every proxy statement/prospectus/information statement (i) that is filed pursuant to paragraph (a)(1) immediately preceding, or
(ii) that purports to meet the requirements of Section 10(a)(3) of the Securities Act and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the registration statement and
will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities
offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-2
(3) To respond to requests for information that is incorporated by reference into this proxy
statement/prospectus/information statement pursuant to Item 4 10(b), 11, or 13 of this Form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This
includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request.
(4) To supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved
therein, that was not the subject of and included in the registration statement when it became effective.
(b) Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange
Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of
expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being
registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as
expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by
the undersigned, thereunto duly authorized in the city of San Diego, State of California, on the 22nd day of January, 2016.
|
|
|
| Celladon Corporation |
|
|
| By: |
|
/s/ Fredrik Wiklund |
|
|
Fredrik Wiklund
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Act, this report has been signed by the
following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
|
|
|
|
| Signature |
|
Title |
|
Date |
|
|
|
| /s/ Fredrik Wiklund
Fredrik Wiklund |
|
President and Chief Executive Officer
(Principal Executive Officer) |
|
January 22, 2016 |
|
|
|
| /s/ Andrew Jackson
Andrew Jackson |
|
Chief Financial Officer (Principal Financial
and Accounting Officer) |
|
January 22, 2016 |
|
|
|
| *
Michael Narachi |
|
Chairman of the Board of Directors |
|
January 22, 2016 |
|
|
|
| *
Gregg Alton |
|
Director |
|
January 22, 2016 |
|
|
|
| *
Graham Cooper |
|
Director |
|
January 22, 2016 |
|
|
|
| *
Peter Honig M.D., M.P.H. |
|
Director |
|
January 22, 2016 |
|
|
|
|
|
| * By: |
|
/s/ Fredrik Wiklund
|
|
|
Fredrik Wiklund
Attorney-in-fact |
II-4
EXHIBIT INDEX
|
|
|
| Exhibit
Number |
|
Description of Document
|
|
|
| 2.1 |
|
Agreement and Plan of Merger and Reorganization, dated as of November 18, 2015, by and among Celladon Corporation, Celladon Merger Sub, Inc., and Eiger BioPharmaceuticals, Inc. (incorporated by reference to Exhibit 2.1 to the
Current Report on Form 8-K filed with the SEC on November 19, 2015). |
|
|
| 2.2 |
|
Form of Support Agreement, by and between Celladon Corporation and certain directors, officers and affiliated stockholders of Eiger BioPharmaceuticals, Inc. (incorporated by reference to Exhibit 2.2 to the Current Report on Form 8-K
of Celladon Corporation filed with the SEC on November 19, 2015). |
|
|
| 2.3 |
|
Form of Support Agreement, by and between Eiger BioPharmaceuticals, Inc. and directors and executive officers of Celladon Corporation (incorporated by reference to Exhibit 2.3 to the Current Report on Form 8-K filed with the SEC on
November 19, 2015). |
|
|
| 2.4* |
|
Subscription Agreement, dated as of November 18, 2015, by and between Eiger BioPharmaceuticals, Inc. and each purchaser listed on Annex A thereto. |
|
|
| 3.1 |
|
Amended and Restated Certificate of Incorporation of Celladon Corporation (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K of Celladon Corporation, filed with the SEC on February 10, 2014). |
|
|
| 3.2 |
|
Amended and Restated Bylaws of Celladon Corporation (incorporated by reference to Exhibit 3.2 to the Current Report on Form 8-K, filed with the SEC on February 10, 2014). |
|
|
| 4.1 |
|
Form of Common Stock Certificate of Celladon Corporation (incorporated by reference to Exhibit 4.1 to the Registration Statement on Form S-1, as amended (File No. 333-191688), originally filed with the SEC on October 11,
2013). |
|
|
| 4.2 |
|
Amended and Restated Investor Rights Agreement by and among Celladon Corporation and certain of its stockholders, dated February 4, 2014 (incorporated by reference to Exhibit 4.2 to the Registration Statement on Form S-1, as
amended (File No. 333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 4.3 |
|
Form of Warrant to Purchase Common Stock issued to participants in Celladon Corporation’s Convertible Debt and Warrant financing, dated October 15, 2013 (incorporated by reference to Exhibit 4.3 to the Registration
Statement on Form S-1, as amended (File No. 333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 5.1* |
|
Opinion of Pillsbury Winthrop Shaw Pittman LLP regarding the validity of the securities. |
|
|
| 8.1 |
|
Opinion of Pillsbury Winthrop Shaw Pittman LLP regarding tax matters. |
|
|
| 8.2 |
|
Opinion of Cooley LLP regarding tax matters. |
|
|
| 10.1+ |
|
Form of Indemnity Agreement by and between Celladon Corporation and its directors and officers (incorporated by reference to Exhibit 10.1 to the Registration Statement on Form S-1, as amended (File No. 333-191688), originally filed
with the SEC on October 11, 2013). |
|
|
| 10.2+ |
|
Celladon Corporation 2001 Stock Option Plan and Form of Notice of Grant of Stock Option, Stock Option Agreement and Stock Option Exercise Notice thereunder (incorporated by reference to Exhibit 10.2 to the Registration Statement on
Form S-1, as amended (File No. 333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 10.3+ |
|
Celladon Corporation 2012 Equity Incentive Plan and Form of Stock Option Grant Notice, Option Agreement and Notice of Exercise thereunder (incorporated by reference to Exhibit 10.3 to the Registration Statement on Form S-1, as
amended (File No. 333-191688), originally filed with the SEC on October 11, 2013). |
|
|
|
| Exhibit
Number |
|
Description of Document
|
|
|
| 10.4+ |
|
Celladon Corporation 2013 Equity Incentive Plan and Form of Stock Option Grant Notice, Option Agreement and Notice of Exercise thereunder (incorporated by reference to Exhibit 10.4 to the Registration Statement on Form S-1, as
amended (File No. 333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 10.5+ |
|
Celladon Corporation 2013 Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.5 to the Registration Statement on Form S-1, as amended (File No. 333-191688), originally filed with the SEC on October 11,
2013). |
|
|
| 10.6+ |
|
Celladon Corporation Non-Employee Director Compensation Policy (incorporated by reference to Exhibit 10.6 to the Registration Statement on Form S-1, as amended (File No. 333-191688), originally filed with the SEC on October 11,
2013). |
|
|
| 10.7+ |
|
Employment Agreement by and between Celladon Corporation and Fredrik Wiklund, dated September 3, 2013, as amended (incorporated by reference to Exhibit 10.10 to the Registration Statement on Form S-1, as amended (File No.
333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 10.8+ |
|
Employment Agreement by and between Celladon Corporation and Krisztina M. Zsebo, Ph.D., dated August 30, 2013, as amended (incorporated by reference to Exhibit 10.11 to the Registration Statement on Form S-1, as amended (File
No. 333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 10.9+ |
|
Letter Agreement by and between Celladon Corporation and Gregg Huber Alton, dated August 30, 2013 (incorporated by reference to Exhibit 10.12 to the Registration Statement on Form S-1, as amended (File No. 333-191688),
originally filed with the SEC on October 11, 2013). |
|
|
| 10.10+ |
|
Letter Agreement by and between Celladon Corporation and Graham Cooper, dated September 2, 2013 (incorporated by reference to Exhibit 10.13 to the Registration Statement on Form S-1, as amended (File No. 333-191688), originally
filed with the SEC on October 11, 2013). |
|
|
| 10.11 |
|
Office Lease by and between Celladon Corporation and Arden Realty, Inc., dated March 6, 2012, as amended (incorporated by reference to Exhibit 10.14 to the Registration Statement on Form S-1, as amended (File No. 333-191688),
originally filed with the SEC on October 11, 2013). |
|
|
| 10.12† |
|
Non-Exclusive License Agreement by and between Celladon Corporation and AskBio, LLC, dated January 15, 2008 (incorporated by reference to Exhibit 10.17 to the Registration Statement on Form S-1, as amended (File No.
333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 10.13 |
|
License Agreement by and between Celladon Corporation and AdVec Inc., dated February 24, 2009 (incorporated by reference to Exhibit 10.18 to the Registration Statement on Form S-1, as amended (File No. 333-191688), originally
filed with the SEC on October 11, 2013). |
|
|
| 10.14† |
|
Amended and Restated License Agreement by and between Celladon Corporation and AmpliPhi Biosciences Corporation, dated June 27, 2012 (incorporated by reference to Exhibit 10.21 to the Registration Statement on Form S-1, as
amended (File No. 333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 10.15† |
|
Sublicense Agreement by and between Celladon Corporation and AmpliPhi Biosciences Corporation, dated June 27, 2012 (incorporated by reference to Exhibit 10.22 to the Registration Statement on Form S-1, as amended (File No.
333-191688), originally filed with the SEC on October 11, 2013). |
|
|
| 10.16+ |
|
Letter Agreement by and between Celladon Corporation and Michael Narachi, dated October 16, 2013 (incorporated by reference to Exhibit 10.24 to the Registration Statement on Form S-1, as amended (File No. 333-191688),
originally filed with the SEC on October 11, 2013). |
|
|
|
| Exhibit
Number |
|
Description of Document
|
|
|
| 10.17 |
|
Assignment and License Agreement by and between Celladon Corporation and Enterprise Management Partners, LLC dated July 18, 2014 (incorporated by reference to Exhibit 99.1 to the Current Report on Form 8-K filed with the SEC on
July 21, 2014). |
|
|
| 10.18+ |
|
Employment Agreement by and between Celladon Corporation and Paul Cleveland, dated May 28, 2014 (incorporated by reference to Exhibit 10.28 to the Registration Statement on Form S-1 (File No. 333-197720), originally filed
with the SEC on July 30, 2014). |
|
|
| 10.19+ |
|
Employment Agreement by and between Celladon Corporation and Elizabeth Reed, dated May 30, 2014 (incorporated by reference to Exhibit 10.29 to the Registration Statement on Form S-1 (File No. 333-197720), originally filed
with the SEC on July 30, 2014). |
|
|
| 10.20+ |
|
Summary of Retention Program of Celladon Corporation (incorporated by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q, filed with the SEC on August 11, 2015). |
|
|
| 10.21+ |
|
Amendment to Employment Agreement, dated May 27, 2015, by and between Celladon Corporation and Andrew Jackson (incorporated by reference to Exhibit 10.2 to the Quarterly Report on
Form 10-Q, filed with the SEC on August 11, 2015). |
|
|
| 10.22+ |
|
Amendment to Employment Agreement, dated May 29, 2015, by and between Celladon Corporation and Andrew Jackson (incorporated by reference to Exhibit 10.3 to the Quarterly Report on
Form 10-Q, filed with the SEC on August 11, 2015). |
|
|
| 10.23+ |
|
Amendment to Employment Agreement, dated May 29, 2015, by and between Celladon Corporation and Paul Cleveland (incorporated by reference to Exhibit 10.4 to the Quarterly Report on Form 10-Q, filed with the SEC on
August 11, 2015). |
|
|
| 10.24+ |
|
Amendment to Employment Agreement, dated May 27, 2015, by and between Celladon Corporation and Elizabeth Reed (incorporated by reference to Exhibit 10.5 to the Quarterly Report on Form 10-Q, filed with the SEC on
August 11, 2015). |
|
|
| 10.25+ |
|
Amendment to Employment Agreement, dated May 27, 2015, by and between Celladon Corporation and Fredrik Wiklund (incorporated by reference to Exhibit 10.6 to the Quarterly Report on
Form 10-Q, filed with the SEC on August 11, 2015). |
|
|
| 10.26+ |
|
Amendment to Employment Agreement, dated May 27, 2015, by and between Celladon Corporation and Krisztina M. Zsebo (incorporated by reference to Exhibit 10.10 to the Quarterly Report on Form 10-Q, filed with the SEC on
August 11, 2015). |
|
|
| 10.27+ |
|
Release Agreement, dated May 29, 2015, by and between Celladon Corporation and Krisztina M. Zsebo (incorporated by reference to Exhibit 10.11 to the Quarterly Report on Form 10-Q, filed with the SEC on August 11,
2015). |
|
|
| 10.28+ |
|
Amendment to Employment Agreement, dated October 20, 2015, by and between Celladon Corporation and Paul Cleveland (incorporated by reference to Exhibit 10.2 to the Quarterly Report on Form 10-Q, filed with the SEC on
November 9, 2015). |
|
|
| 10.29+ |
|
Amendment to Employment Agreement, dated October 20, 2015, by and between Celladon Corporation and Andrew Jackson (incorporated by reference to Exhibit 10.3 to the Quarterly Report on Form 10-Q, filed with the SEC on
November 9, 2015). |
|
|
| 10.30+ |
|
Amendment to Employment Agreement, dated October 20, 2015, by and between Celladon Corporation and Elizabeth Reed (incorporated by reference to Exhibit 10.5 to the Quarterly Report on Form 10-Q, filed with the SEC on
November 9, 2015). |
|
|
| 10.31+ |
|
Amendment to Employment Agreement, dated October 20, 2015, by and between Celladon Corporation and Fredrik Wiklund (incorporated by reference to Exhibit 10.6 to the Quarterly Report on Form 10-Q, filed with the SEC on
November 9, 2015). |
|
|
|
| Exhibit
Number |
|
Description of Document
|
|
|
| 10.32+ |
|
Bonus Agreement, dated as of November 18, 2015, by and between Celladon Corporation and Fredrik Wiklund (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, filed with the SEC on November 19,
2015). |
|
|
| 10.33+ |
|
Bonus Agreement, dated as of November 18, 2015, by and between Celladon Corporation and Andrew Jackson (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, filed with the SEC on November 19,
2015). |
|
|
| 10.34+ |
|
Bonus Agreement, dated as of November 18, 2015, by and between Celladon Corporation and Elizabeth Reed (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K, filed with the SEC on November 19,
2015). |
|
|
| 10.35*+ |
|
Merger Incentive Bonus Agreement, effective as of November 18, 2015, by and between Celladon Corporation and Fredrik Wiklund. |
|
|
| 10.36*+ |
|
Merger Incentive Bonus Agreement, effective as of November 18, 2015, by and between Celladon Corporation and Andrew Jackson. |
|
|
| 10.37*+ |
|
Merger Incentive Bonus Agreement, effective as of November 18, 2015, by and between Celladon Corporation and Elizabeth Reed. |
|
|
| 10.38* |
|
Lease, dated as of March 19, 2015 by and between JTC, a California general partnership and Eiger BioPharmaceuticals, Inc. |
|
|
| 10.39*+ |
|
Offer Letter, dated as of December 5, 2008, by and between Eiger BioPharmaceuticals, Inc. and David Cory. |
|
|
| 10.40*+ |
|
Offer Letter, dated as of August 10, 2015, by and between Eiger BioPharmaceuticals, Inc. and James Welch. |
|
|
| 10.41*+ |
|
Offer Letter, dated as of July 31, 2015, by and between Eiger BioPharmaceuticals, Inc. and James Shaffer. |
|
|
| 10.42*+ |
|
Offer Letter, dated as of April 3, 2015, by and between Eiger BioPharmaceuticals, Inc. and Joanne Quan. |
|
|
| 10.43*+ |
|
Offer Letter, dated as of October 1, 2015, by and between Eiger BioPharmaceuticals, Inc. and Eduardo Martins. |
|
|
| 10.44*+ |
|
Eiger BioPharmaceuticals, Inc. 2009 Equity Incentive Plan and Form of Notice of Grant of Stock Option, Stock Option Agreement and Stock Option Exercise Notice thereunder. |
|
|
| 10.45*† |
|
Asset Purchase Agreement, effective as of December 8, 2010, by and between Eiger BioPharmaceuticals, Inc. and Eiger Group International, Inc. |
|
|
| 10.46*† |
|
Asset Purchase Agreement, dated September 25, 2015, by and between Eiger BioPharmaceuticals, Inc. and Tracey McLaughlin and Colleen Craig. |
|
|
| 10.47*† |
|
Asset Purchase Agreement, dated October 29, 2015, by and between Eiccose, LLC and Eiger BioPharmaceuticals, Inc. |
|
|
| 10.48*† |
|
Exclusive Agreement, dated May 1, 2015, by and between Eiccose, LLC and the Board of Trustees of the Leland Stanford Junior University. |
|
|
| 10.49*† |
|
Exclusive Agreement, dated October 27, 2015, by and between Eiccose, LLC and the Board of Trustees of the Leland Stanford Junior University. |
|
|
| 10.50*† |
|
License Agreement, dated September 3, 2010, by and between Eiger BioPharmaceuticals, Inc. and Merck Corporation. |
|
|
|
| Exhibit
Number |
|
Description of Document
|
|
|
| 10.51*† |
|
License Agreement, effective as of December 19, 2014, by and between EB Pharma, LLC and Janssen Parmaceutica NV. |
|
|
| 10.52*† |
|
License Agreement, dated as of May 1, 2015, by and between Eiccose, LLC and Nippon Kayaku Co., Ltd. |
|
|
| 10.53 |
|
Sublease Agreement, dated as of January 8, 2016, by and between Baker Hughes Oilfield Operations, Inc. and Eiger BioPharmaceuticals, Inc. |
|
|
| 21.1* |
|
List of subsidiaries. |
|
|
| 23.1 |
|
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm to Celladon Corporation. |
|
|
| 23.2 |
|
Consent of KPMG LLP, Independent Registered Public Accounting Firm to Eiger BioPharmaceuticals, Inc. |
|
|
| 23.3* |
|
Consent of Pillsbury Winthrop Shaw Pittman LLP (included in Exhibit 5.1 hereto). |
|
|
| 23.4 |
|
Consent of Pillsbury Winthrop Shaw Pittman LLP (included in Exhibit 8.1 hereto). |
|
|
| 23.5 |
|
Consent of Cooley LLP (included in Exhibit 8.2 hereto). |
|
|
| 24.1* |
|
Power of attorney (included on the signature page to this Registration Statement). |
|
|
| 99.1* |
|
Form of Proxy Card for the Celladon Corporation Special Meeting of Stockholders. |
|
|
| 99.2* |
|
Consent of Wedbush Securities Inc. |
|
|
| 99.3(a) |
|
Proposed Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Celladon Corporation (included as Annex D to the proxy statement/prospectus/information statement forming a part of this Registration
Statement). |
|
|
| 99.3(b) |
|
Proposed Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Celladon Corporation (included as Annex E to the proxy statement/prospectus/information statement forming a part of this Registration
Statement). |
|
|
| 99.4* |
|
Consent of David A. Cory to be named as director. |
|
|
| 99.5* |
|
Consent of Thomas J. Dietz to be named as director. |
|
|
| 99.6* |
|
Consent of Edgar G. Engleman to be named as director. |
|
|
| 99.7* |
|
Consent of Jeffrey S. Glenn to be named as director. |
|
|
| 99.8* |
|
Consent of Nina Kjellson to be named as director. |
|
|
| 101* |
|
The following materials from the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and the Registrant’s Quarterly Report on Form 10-Q for the quarter ending September 30, 2015,
formatted in Extensible Business Reporting Language (XBRL) includes: (i) Condensed Consolidated Balance Sheets at September 30, 2015 and December 31, 2014, (ii) Condensed Consolidated Statements of Operations and Comprehensive Loss for the
Three and Nine Months Ended September 30, 2015 and 2014, (iii) Condensed Consolidated Statements of Cash Flows for the Nine Months Ended September 30, 2015 and 2014 and (iv) Notes to Condensed Consolidated Financial Statements. |
| + |
Indicates management contract or compensatory plan. |
| † |
Confidential treatment has been requested or granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
Exhibit 8.1

January 22, 2016
Celladon Corporation
12707 High Bluff Drive, Suite 200
San Diego, CA 92130
Ladies and Gentlemen:
We have acted as counsel to Celladon Corporation, a Delaware corporation (the “Company”), in connection with the proposed merger of
Celladon Merger Sub, Inc., a wholly owned subsidiary of the Company and a Delaware corporation (“Merger Sub”), with and into Eiger BioPharmaceuticals, Inc., a Delaware corporation (“Eiger”), with Eiger continuing as the surviving
corporation and as a wholly owned subsidiary of Celladon, pursuant to the Agreement and Plan of Merger and Reorganization, dated as of November 18, 2015 (the “Agreement”), by and among the Company, Merger Sub and Eiger. In connection
therewith, we have assisted in the preparation and filing by the Company with the Securities and Exchange Commission of the Registration Statement on Form S-4 (the “Registration Statement”) under the Securities Act of 1933, as amended (the
“Securities Act”), with respect to the registration of 5.7 million shares of the Company’s common stock.
We hereby
confirm to you that the discussion set forth in the Registration Statement under the caption “The Merger—Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger,” insofar as it relates to U.S. federal income
tax law and legal conclusions with respect thereto, is our opinion, subject to the qualifications and limitations set forth therein and herein.
In rendering this opinion, we expressly assume that the transaction will be consummated in accordance with the provisions of the Agreement and
as described in the Registration Statement, and no transaction or condition described therein and affecting this opinion will be waived by any party.
The foregoing opinion is limited to the U.S. federal income tax matters addressed herein, and no other opinions are rendered with respect to
other U.S. federal tax matters or to any issues arising under the tax laws of any other country, or any state or locality. This opinion is expressed as of the date hereof, and we disclaim any undertaking to advise you of subsequent changes relating
to matters considered herein or of any subsequent changes in applicable law.

CELLADON CORPORATION
JANUARY
26, 2016
PAGE
2
We hereby consent to the filing of this opinion as an exhibit to the Registration Statement
and to the use of our name therein. In giving such consent, we do not thereby admit that we are within the category of persons whose consent is required under Section 7 of the Securities Act.
Very truly yours,
/s/ Pillsbury Winthrop Shaw Pittman LLP
Exhibit 8.2

January 22, 2016
Eiger
BioPharmaceuticals, Inc.
P.O. Box 430
San Carlos, CA 94070
Ladies and Gentlemen:
We have acted as
counsel to Eiger BioPharmaceuticals, Inc., a Delaware corporation (the “Company”), in connection with the transactions described in the Registration Statement on Form S-4 originally filed with the Securities and Exchange Commission on
December 14, 2015, as amended through the date hereof (the “Registration Statement”) of which this exhibit is a part. All section references, unless otherwise indicated, are to the United States Internal Revenue Code of 1986, as
amended (the “Code”). Capitalized terms not defined herein have the meanings set forth in the Registration Statement.
In
preparing this opinion, we have examined and relied upon the Registration Statement, including the Prospectus included therein, and such other documents as we have deemed necessary or appropriate in order to enable us to render this opinion. In our
examination of documents, we have assumed the authenticity of original documents, the accuracy of copies, the genuineness of signatures, and the legal capacity of signatories. We have also assumed that the transactions described in the Registration
Statement will be consummated in accordance with the description in the Registration Statement.
In rendering this opinion, we have
assumed without investigation or verification that the facts set forth in the Registration Statement are true, correct and complete in all material respects; that any representation in any of the documents referred to herein that is made “to
the best of the knowledge and belief” (or similar qualification) of any person or party are true, correct and complete without such qualification; and that, as to all matters for which a person or entity has represented that such person or
entity is not a party to, does not have, or is not aware of, any plan, intention, understanding or agreement, there is no such plan, intention, understanding or agreement. Any inaccuracy in, or breach of, any of the aforementioned statements,
representations or assumptions could adversely affect our opinion.
Our opinion is based on existing provisions of the Code, Treasury
Regulations, judicial decisions, and rulings and other pronouncements of the Internal Revenue Service as in effect on the date of this opinion, all of which are subject to change (possibly with retroactive effect) or reinterpretation. No assurances
can be given that a change in the law on which our opinion is based or the interpretation thereof will not occur or that such change will not affect the opinion expressed herein. We undertake no responsibility to advise of any such developments in
the law.
3175 HANOVER STREET, PALO ALTO, CA 94304-1130 T: (650) 843-5000 F: (650)
849-7400 WWW.COOLEY.COM

Eiger BioPharmaceuticals, Inc.
January 22, 2016
Page Two
Based on our examination of the foregoing items and subject to the limitations, qualifications, assumptions and caveats set forth herein, we
confirm that the statements in the Registration Statement under the heading “The Merger—Considerations with Respect to U.S. Federal Income Tax Consequences of the Merger,” subject to the limitations and qualifications described
therein, insofar as they relate to matters of United States federal income tax law, constitute our opinion of the material United States federal income tax consequences of the Merger.
No opinion is expressed as to any matter not discussed herein.
We hereby consent to the use of our name under the heading “The Merger—Considerations with Respect to U.S. Federal Income Tax
Consequences of the Merger,” and “Legal Matters” in the Registration Statement and to the filing of this opinion as an exhibit to the Registration Statement.
|
|
|
|
|
Sincerely, |
|
|
|
|
COOLEY LLP |
|
|
| By: |
|
/s/ Mark Windfeld-Hansen |
|
|
Mark Windfeld-Hansen |
3175 HANOVER STREET, PALO ALTO, CA 94304-1130 T: (650) 843-5000 F:
(650) 849-7400 WWW.COOLEY.COM
Exhibit 10.53
SUBLEASE AGREEMENT
THIS SUBLEASE AGREEMENT (this “Sublease”) is made and entered into effective as of the 8th day of January,
2016, by and between BAKER HUGHES OILFIELD OPERATIONS, INC., a California corporation (“Sublessor”), and EIGER BIOPHARMACEUTICALS, INC., a Delaware corporation (“Sublessee”). Capitalized
terms used herein and not otherwise defined shall have the meanings ascribed to such terms in the Lease (hereinafter defined).
WITNESSETH:
WHEREAS,
Leland Z. Wiesner and Maria C. Horton, as Landlord, and Sublessor, as Tenant, entered into that certain Lease dated as of April 1, 2012 (the “Lease”), covering and describing that certain parcel of real property located
at 366 Cambridge Avenue, Palo Alto, Santa Clara County, California, which land is described as Santa Clara County Assessor’s Parcel Number 124-32-003, together with all improvements thereon, including, without limitation, the approximately
4,029 square foot building located thereon (collectively, the “Leased Premises”), as more particularly described in the Lease, a true and correct copy of which, including all exhibits thereto, is attached hereto as Exhibit A
and made a part hereof for all purposes;
WHEREAS, Sublessor desires to sublease to Sublessee and Sublessee desires to Sublease from
Sublessor the Leased Premises, together with all improvements thereto installed or constructed by or on behalf of Sublessor (hereinafter the “Subleased Premises”);
NOW, THEREFORE, in consideration of the mutual covenants contained herein and other good and valuable consideration the receipt and
sufficiency of which are acknowledged and confessed by all parties, Sublessor and Sublessee hereby agree as follows:
1. Subleased
Premises. Sublessor hereby subleases to Sublessee, and Sublessee hereby subleases from Sublessor, the Subleased Premises, for the term and rental hereinafter specified. No parking spaces are included with the Premises.
2. Term. Subject to and on the terms and conditions hereinafter set forth, the term of this Sublease (the “Sublease
Term”) shall commence on the date (“Commencement Date”) which is the later of (i) the date a fully executed copy of this Sublease and the Landlord’s consent set forth below are delivered to Sublessee
(ii) the date that the Subleased Premises are delivered by Sublessor to Sublessee in broom clean condition (the “Effective Date”), and shall expire on March 30, 2017.
3. Sublessee’s Rights and Obligations.
(a) General Rights and Obligations. Except for the “Lease Summary” (other than Section IV., “Operating Expenses”
paragraph, which is included) and Lease Sections 1.1, 1.2(a), 1.5, 2, 2.1, 2.2, 3.1, 3.3, 3.5, 6.3(e), 11.3, 11.4, 22, 26, and 27, and Exhibits B, C, D, E and F, and to the extent not otherwise inconsistent with the agreements and understandings
expressed in this Sublease or applicable only to the original parties to the Lease, the terms, provisions, covenants and conditions of the Lease are hereby incorporated herein by reference on the following understandings:
1
(i) The term “Landlord” as used in the incorporated provisions of the Lease
shall refer to Sublessor hereunder (unless the context otherwise requires), its successors and assigns, and the term “Tenant” as used therein shall refer to Sublessee hereunder, its successor and assigns. The term “Lease” as used
therein shall refer to this Sublease. The term “Premises” as used therein shall refer to the Subleased Premises. The term ‘Term” as used therein shall refer to the Sublease Term.
(ii) In any case where the Landlord reserves in the Lease the right to enter the Premises, said right shall inure to the benefit of
both Landlord and Sublessor.
(iii) Sublessee agrees to perform and comply with the terms, provisions, covenants and conditions of
the Lease applicable during the Sublease Term and not to do or suffer or permit anything to be done which would result in a default under or cause the Lease to be terminated or forfeited; provided that nothing in this section shall be construed to
obligate Sublessee to perform any obligation which Sublessor failed to perform prior to the Commencement Date, nor shall Sublessee have any obligation to remove any Tenant Alterations or any Changes which were made by Sublessor prior to the
Commencement Date of this Sublease.
(iv) Sublessee shall have no right to exercise any options or rights of first refusal of any
kind available to Sublessor under the Lease or to exercise any rights of control or termination under the Lease, as all of the same are retained by the Sublessor.
(v) With respect to work, services repairs, repainting, restoration or the performance of other obligations required of Landlord under
the Lease, if any, Sublessor’s sole obligation, with respect thereto, shall be to request the same, on request in writing by Sublessee, and to assist Sublessee in obtaining the same from Landlord. If Landlord so permits, Sublessee shall have
the right to request the same directly from Landlord and to take all such action as Sublessee deems appropriate to obtain such services from Landlord. Sublessor agrees to cooperate with Sublessee in any such proceedings, but at Sublessee’s sole
cost and expense.
(vi) Notwithstanding anything to the contrary herein contained, Sublessee shall, in no event, be deemed to have
any rights hereunder In excess of those rights granted to the Sublessor, as Tenant, under the Lease.
(b) Rent.
Sublessee covenants and agrees to pay to Sublessor during the Sublease Term, without any right of offset or counterclaim, fixed triple net base rent in the amount of One Hundred Ninety-three Thousand Three Hundred Ninety-two and
No1100 Dollars ($193,392.00) per annum, payable in equal monthly installments of Sixteen Thousand One Hundred Sixteen and No/100 Dollars ($16,116.00) each for the Subleased Premises; provided, however, that no rent shall be due for the first thirty
(30) days of the Sublease Term (the “Free Rent Period”). The day after the end of the Free Rent Period is referred to herein as the “Rent Commencement Date”, The first installment of rent for the
period following the Rent Commencement Date, in the amount of $16,116.00, shall be delivered to Sublessor upon Sublessee’s execution of this Sublease, with subsequent
2
payments of rent being due and payable without notice or demand on or before the first day of each calendar month throughout the Sublease Term. Rent for the first month of the Sublease Term
following the end of the Free Rent Period shall be prorated to the extent that the Sublease Term does not begin on the first day of a calendar month. For example, upon execution of this Sublease, Sublessee shall pay first installment of rent for the
period following the Rent Commencement Date, in the amount of $16,116.00. If the Commencement Date were to be March 15, 2016, then the Rent Commencement Date would be April 14, 2016, A portion of the initial rent payment of $16,116.00
would be applied to the period from April 14 through April 30, the balance would be credited to the rent due in May, and Sublessee would pay on May 1, 2015, the balance due for the month of May. All such payments shall be paid to
Sublessor in lawful money of the United States of America at the address of Sublessor shown herein or to such other party or at such other place as Sublessor may designate from time to time in a written notice to Sublessee. In addition to the base
rent provided for above, Sublessee agrees to pay to Sublessor additional rental in an amount equal to all additional rental, payable by Sublessor under the Lease for the Leased Premises.
(c) Utilities. Utilities shall be provided as set forth in the Lease. Unless caused by Sublessor, no interruption of utility service
shall be construed as either a constructive or an actual eviction of Sublessee, nor work an abatement of the rent due under this Sublease, nor relieve Sublessee from fulfilling any covenant or condition of this Sublease.
(d) Other Financial Obligations. Sublessee further agrees to and shall pay to Sublessor on or before the date the same are due under
the Lease, without any right of offset or counterclaim, any and all other sums that become due from or are required to be paid by Sublessor, as Tenant with respect to the Subleased Premises, under the Lease for periods after the Effective Date
including, without limitation, sums due pursuant to or arising out of indemnity obligations, additional rent, holdover rent, repair, insurance, maintenance, restoration or replacement, but excluding any charges caused by any breach of the Lease
caused by Sublessor and not caused or contributed to by Sublessee, and excluding any charges relating to the removal of Tenant Improvements or Changes made by Sublessor.
(e) Security Deposit. Sublessee shall pay to Sublessor upon execution of this Sublease, a security deposit (the
“Security Deposit”) of $16,116.00. Upon the occurrence of any default which is not cured within the applicable cure period under this Sublease, Sublessor may, from time to time, without prejudice to any other
remedy, use the Security Deposit to the extent necessary to pay or satisfy any damage, injury, expense or liability caused to Sublessor by any default hereunder, however, the Security Deposit shall not be considered a measure of Sublessor’s
damages for any default by Sublessee. No interest shall be paid on the Security Deposit and Landlord shall be entitled to commingle the Security Deposit with its general funds. Provided no default then exists hereunder (or following the cure by
Sublessee of any default), the amount of the Security Deposit then held by Sublessor shall be returned to Sublessee within thirty (30) calendar days following the last day of the Sublease Term. Tenant hereby waives the provisions of California
Civil Code Section 1950.7(c).
(f) Insurance. Sublessee shall, at Sublessee’s sole cost and expense, obtain and keep in
force during the Term of this Sublease all insurance required to be
3
maintained by the “Tenant” under Section 11 of the Lease. All policies carried by Sublessee shall name Sublessor and Landlord as additional Insureds and shall
be primary over any insurance that may be carried by Sublessor and Landlord.
(g) Use; Keys. Sublessee may use the
Premises only for general office and other directly related legal uses of a technology company and no other purpose. Sublessor shall provide keys to the Subleased Premises, and Sublessee shall have the
right to re-key the Subleased Premises, at Sublessee’s sole cost and expense; provided, however, that the same shall be considered an Alteration under the Lease.
4. Sublessors Obligations. Sublessor agrees to and shall fully and timely perform all of its duties and obligations under the Lease not
assigned to Sublessee, including, without limitation, the payment of all rent and other sums due under the Lease. Sublessor agrees to and shall refrain from entering into any amendment to or modification of the Lease that would materially conflict
with the rights granted to Sublessee under this Sublease without the prior written consent of Sublessee, which consent shall not be unreasonably withheld, delayed or conditioned. Sublessor hereby covenants not to exercise any options or rights of
first refusal of any kind available to Sublessor under the Lease Sublessee agrees not to do or suffer or permit anything to be done which would result In a default under or cause the Lease to be terminated or forfeited.
5. Condition and Surrender of Subleased Premises.
(a) Condition of Subleased Premises; Signage Removal. Sublessor shall remove its signage on the front doors of the building, at
Sublessor’s sole cost and expense, within five (5) days of the Commencement Date of this Sublease. Sublessee acknowledges, by its execution of this Sublease, that it has inspected the Subleased Premises and has satisfied itself as to the
condition of same and that it accepts the Subleased Premises “AS IS” and “WHERE IS” and with all faults, and, except as expressly set forth in this Sublease, without representation or warranty of any kind, expressed, implied,
statutory or otherwise, including specifically, without limitation, any warranty as to habitability, suitability, merchantability, condition or fitness for a particular purpose. Without limiting the foregoing, Sublessee shall be responsible for any
cost and expense of any alterations, improvements and changes necessary to ensure the compliance of the Subleased Premises with the Americans with Disabilities Act and any similar state or local laws (collectively, the
“Acts”), but only to the extent such obligations arise during the Sublease Term, and only to the extent that such obligations are assigned to the Tenant under the Lease.
(b) Surrender of Subleased Premises. Sublessee shall assume sole and full responsibility for compliance with all terms and provisions
of the Lease relating to the use, occupancy, repair, maintenance and condition of the Subleased Premises during the Sublease Term, including without limitation, those concerning the condition of the Subleased Premises at the expiration of the Lease,
except to the extent of any Tenant Improvements or Changes installed by Sublessor,
6. Alterations and Additions. Sublessee
shall not install any trade fixtures in, make any alterations in or make any additions to the Subleased Premises without (i) the prior written consent of Sublessor, which consent may be withheld in Sublessor’s sole
4
discretion and (ii) fully complying with all relevant terms of the Lease. Any such work by Sublessee shall be at its sole cost and expense and shall be performed promptly and diligently
prosecuted to completion in a good and workmanlike manner in accordance with all applicable laws, permits, the Lease and this Sublease, and without the creation of any liens on the Subleased Premises.
7. Assignment or Sublease. Sublessee shall not have the right or power to assign this Sublease or to sublet all or any portion of the
Subleased Premises without first obtaining the written consent of Sublessor, which consent may be withheld in Sublessor’s sole discretion, and otherwise complying with the provisions of the Lease applicable to assignment or sublease. Any
attempted assignment or sublease by Sublessee in violation of this Sublease or the Lease shall be null and void and shall, at the election of Sublessor, constitute a default by Sublessee under this Sublease. In the event that Sublessor consents to
an assignment or sublease of this Sublease, Sublessor shall have the right to require such successor to Sublessee to pay to Sublessor all Additional Rent under the Lease.
8. Hazardous Materials.
(a) Sublessee shall not create, collect, store, treat, dispose of or cause to be released or otherwise discharged any Hazardous
Materials (hereinafter defined) on the Subleased Premises except in such minute quantities as are found in everyday cleaning supplies, office supplies and equipment and household pesticides, in all cases in compliance with all applicable federal,
state or local laws dealing with health, safety and/or the environment (“Environmental Laws”) and shall notify Sublessor within twenty-four (24) hours after discovering or being informed of the presence of any Hazardous
Materials on the Premises either in violation of Environmental Laws or in greater than minute quantities. Sublessor represents and warrants that it has not received any notice of any violations of Environmental Laws with respect to the Subleased
Premises or the presence of Hazardous Materials in the Subleased Premises
(b) Sublessee agrees to and does hereby
defend, indemnify and hold harmless Sublessor, Its directors, officers, shareholders, employees, representatives, agents, successors and assigns from and against any and all loss, cost, expense, fines, damages, penalties, personal injuries, death or
other liability (including strict liability), which may now or in the future (whether during or after the term of this Sublease) be paid, Incurred or suffered by or asserted against Sublessor by any person or entity or governmental agency for, with
respect to, or as a direct or indirect result of, (a) acts or omissions of Sublessee, its agents, employees, directors, officers, shareholders, contractors, representatives, or invitees, on or in connection with the Subleased Premises and
(b) any and all breaches of the covenants, representations and warranties set forth in this Section. The covenants and indemnifications contained in this Section shall survive the expiration or other termination of this Sublease.
(c) “Hazardous Materials” shall mean all asbestos, polychlorinated biphenyls,
petroleum products, radioactive substances, and other substances the use, disposal, ownership, sale or manufacture of which is prohibited or otherwise regulated by Environmental Laws.
5
9. Indemnification.
(a) It is specifically agreed that Sublessee shall not be responsible for the discharge and performance of the duties and obligations
required to be performed and/or discharged In connection with the Lease prior to the effective date hereof. In such regard, Sublessor agrees to indemnify, defend and hold Sublessee harmless from and against any and all loss, cost, expense or
liability (including, without limitation, attorneys’ fees, accountants’ fees, court costs and interest) resulting from any claims or causes of action existing in favor of or asserted by any party arising out of or relating to
Sublessor’s failure to perform any duties or obligations under the Lease prior to the effective date hereof, or caused by Sublessor’s breach of this Agreement.
(b) Sublessee agrees to indemnify, defend and hold harmless Sublessor from and against any and all loss, cost, liability, expense and
damages, Including, without limitation, attorneys’ fees and court costs, incurred by Sublessor in connection with the Subleased Premises during the Sublease Term, including, without limitation, any and all liability under any indemnity of
Landlord contained in the Lease, except as expressly limited by the terms of this Agreement, including, without limitation, Sections 5(b) and 9(a).
10. Default. If Sublessee fails to pay when due any rent or other sum payable under this Sublease or If Sublessee fails to perform or
observe any other covenant, term, provision or condition of this Sublease, and such failure continues for five (5) business days after written notice from Sublessor to Sublessee describing such failure, Sublessor shall be entitled to all the
rights and remedies available to Landlord under the Lease following the occurrence of an Event of Default by Tenant thereunder, as well as any other remedies available under California law. If Sublessee fails to make any payment or cure or commence
to cure any default (other than a failure to pay money) under this Sublease within five (5) business days after written notice from Sublessor to Sublessee specifying such failure or default in detail, Sublessor, without being under any
obligation to do so and without thereby waiving such default, may make such payment and/or remedy such other default for the account of Sublessee, Sublessor being hereby granted the unconditional right and license to enter the Subleased Premises for
such purpose, and thereupon Sublessee agrees to and shall pay to Sublessor, immediately upon demand, all reasonable costs, expenses and disbursements incurred by Sublessor in taking such remedial action. Additionally, Sublessor shall have all rights
and remedies available to it under applicable law for a breach of this Sublease.
11. Quiet Enjoyment. Sublessee shall peacefully
have, hold and enjoy the Subleased Premises, subject to the terms and conditions of this Sublease, provided that Sublessee timely and fully performs all of its covenants, duties and obligations under this Sublease and the Lease.
12. Waiver of Subrogation Rights. Anything contained in this Sublease or the Lease to the contrary notwithstanding, Sublessor and
Sublessee each waive any and all rights of recovery, claim, action or cause of action against the other, its agents, officers, or employees, for any loss or damage that may occur to the Subleased Premises or the improvements of which the Subleased
Premises are a part, or any personal property of such party therein, by reason of fire, the elements, or any other cause which could be insured against under the terms of any policy of insurance required under the Lease or this
6
Sublease, regardless of cause or origin, including negligence of the other, its agents, officers or employees, and covenant that no insurer shall hold any right of subrogation against the others.
To the extent that any part of this Section 12 would void or otherwise invalidate any policy of insurance of any party hereto, such portions of this Section 12 shall be of no effect. SUBLESSOR AND SUBLESSEE EACH RECOGNIZE AND
AGREE THAT THIS SECTION 12 WAIVES AND RELEASES THE OTHER PARTY FOR LIABILITY RELATING TO SUCH PARTY’S OWN NEGLIGENCE.
13.
Governing Law. This Sublease shall be governed by and construed in accordance with the internal laws of the State of California, without regard to the conflicts of laws principles thereof.
14. Notices. Any notice or other communication to any party required or permitted to be given under this Sublease must be in writing
and shall be effectively given if hand delivered or sent by a nationally recognized overnight courier, or sent by United States mail, postage prepaid, certified or registered, return receipt requested, to the following addresses:
|
|
|
| If to Sublessee: |
|
Eiger Bio Pharmaceuticals, Inc. |
|
|
at the Subleased Premises
Attn: James Welch |
|
|
| If to Sublessor: |
|
Baker Hughes Incorporated 2929 Allen
Parkway, Suite 2100 Houston, Texas 77019-2118 Attention: Vice
President - Real Estate & Facilities |
|
|
| If to Landlord: |
|
Leland Wiesner & Maria Horton c/a
Premier Properties 172 University Avenue Palo Alto,
California |
Any notice mailed shall be deemed to have been given on the second business day following the date of deposit of such
item in a depository of the United States Postal Service. Notice effected by hand delivery or courier shall be deemed to have been given at the time of actual delivery. Any party shall have the right to change its address to which notices shall
thereafter be sent by giving the other parties notice thereof.
15. Furniture. Sublessor hereby subleases to Sublessee all
furniture and furnishings contained in the Subleased Premises as of the Commencement Date and listed on Exhibit B (the “FF&E”) for the Sublease Term in consideration of the rent already reserved in
Section 3(b). Sublessor shall surrender the FF&E to Sublessor at the end of the Sublease Term in as good a condition as when received, reasonable wear and tear and damage due to casualty excepted. Sublessee understands that the reception
area furnishings, including without limitation, the desk, couches and table, are owned by Sublessor and will be removed prior to the Commencement Date.
[Remainder of Page Intentionally Left Blank]
7
16. Successors and Assigns. This Sublease shall be binding upon and shall inure to the
benefit of Sublessor, Sublessee and Landlord and their respective successors and permitted assigns, subject to the limitations set forth herein.
EXECUTED as of the date first set forth above.
|
|
|
| SUBLESSOR: |
| BAKER HUGHES OILFIELD OPERATIONS, INC. |
|
|
|
|
|
| By |
|
/s/ Lee Whitley |
| Name: |
|
Lee Whitley |
|
|
Vice President |
|
|
|
| SUBLESSEE: |
| EIGER BIOPHARMACEUTICALS, INC. |
|
|
|
|
|
| By |
|
/s/ Jim Welch |
| Name: |
|
Jim Welch |
| Title: |
|
CFO |
8
LANDLORD’S CONSENT TO SUBLEASE
The undersigned (collectively “Landlord”) hereby consents to this Sublease as required by the Lease, and represents that
(i) the undersigned are the owners of the real property of which the Subleased Premises are a part, and (ii) that they have the full right and authority to enter into this Consent, (iii) that no third party consents to the Sublease
are required and (iv) that, as of the date hereof, Tenant has not made any Tenant Improvements or Changes required to be removed by Tenant at the end of the Lease term. Landlord further agrees to and shall provide written notice to Sublessee of
any default by Sublessor under the Lease and allow Sublessee an additional period of five (5) days after the later to occur of (a) the date Sublessee is so notified by Landlord of a default by Sublessor, or (b) the date any applicable
grace period contained in the Lease expires, to cure such default. Landlord acknowledges that, to the best of Landlord’s knowledge, no default presently exists under the Lease of obligations to be performed by Sublessor and the Lease is in full
force and effect.
|
|
|
|
|
LANDLORD: |
|
|
| Dated: 12/16/15 |
|
/s/ Leland Z. Weisner |
|
|
Leland Z. Wiesner |
|
|
| Dated: 12/16/15 |
|
/s/ Maria C. Horton |
|
|
Maria C. Horton |
9
EXHIBIT A
Lease
10
366 CAMBRIDGE AVENUE, PALO ALTO, CA
LEASE SUMMARY
The
following information is incorporated into the terms of the attached Lease.
| I. |
LANDLORD: Leland Z. Wiesner and Maria C. Horton |
TENANT: Baker Hughes
Oilfield Operations, Inc., a California corporation.
| II. |
PREMISES: In accordance with Paragraph 1.1, the “Premises” consists of an approximate 4,029 square foot commercial office building located at 366 Cambridge Ave., Palo Alto, California.
|
LEASE TERM: In accordance with Paragraph 2.1, the Term of the Lease shall
commence on the “Commencement Date” determined under Paragraph 2.1 and shall continue for sixty (60) months from the Rent Commencement Date.
OPTION TO EXTEND: In accordance with Paragraph 2.2 and Exhibit C, Tenant shall have one (1) option to extend the Term of the
Lease for a period of sixty (60) months (the “Extended Term”). The Base Rent during the Extended Term shall be as set forth in Exhibit D.
BASE RENT: Base Rent shall be due in accordance with Paragraph
3.1 and the schedule attached as Exhibit B.
SECURITY DEPOSIT: In accordance with Paragraph 3.3, Tenant shall provide a total
deposit of Eighteen-thousand One Hundred Thirty and No/100 Dollars (518,130.00) less a credit of Five-thousand Six Hundred Eighty No/100 Dollars ($5,680.00) from an existing lease deposit. Tenant shall pay to Landlord the remaining security
deposit or Twelve-thousand Four Hundred Fifty No/100 Dollars ($12,450.00) contemporaneously with the first payment of the Base Rent. OPERATING EXPENSES: In accordance with Paragraph 32, Tenant shall reimburse its “Allocable Share” of
Taxes, Insurance, and maintenance and operation of the Premises, the Building, and the Property.
RENT COMMENCEMENT DATE: In
accordance with Paragraph 3.5, Base Rent and Additional Rent shall commence to be payable upon the “Rent Commencement Date”.
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
V. EXECUTION: The undersigned Landlord and Tenant agree to the provisions of this Lease,
including the attached Exhibits A—F (which are incorporated herein by this reference).
|
|
|
| “Tenant” |
|
“Landlord” |
|
|
| BAKER HUGHES OILFIELD |
|
|
| OPERATIONS, INC., a California
corporation |
|
|
|
|
|
|
|
|
|
|
|
| By: |
|
/s/ David E. Emerson |
|
|
|
/s/ Leland Z. Wiesner |
| Name: |
|
David E. Emerson |
|
|
|
Leland Wiesner |
| Title: |
|
Vice President |
|
|
|
|
|
|
|
|
|
| Date: |
|
May 4, 2012 |
|
|
|
Date: |
|
5/12/2012 |
|
|
|
|
|
|
|
|
|
|
/s/ Maria C. Horton |
|
|
|
|
|
|
Maria C. Horton |
|
|
|
|
|
|
|
|
|
|
|
Date: |
|
May 8, 2012 |
|
|
|
|
|
| Tenant’s Initials |
|
2 |
|
Landlord’s Initials LW |
Table of Contents
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Page |
|
| 1. |
|
PREMISES |
|
|
1 |
|
|
|
1.1 |
|
Description of Premises |
|
|
1 |
|
|
|
1.2 |
|
Condition of Premises |
|
|
1 |
|
|
|
1.3 |
|
Surrender of Premises |
|
|
2 |
|
|
|
1.4 |
|
No Parking |
|
|
2 |
|
|
|
1.5 |
|
Furniture, Fixtures, and Equipment |
|
|
2 |
|
| 2. |
|
TERM |
|
|
2 |
|
|
|
2.1 |
|
Commencement Date |
|
|
2 |
|
|
|
2.2 |
|
Option to Extend |
|
|
2 |
|
| 3. |
|
RENT |
|
|
2 |
|
|
|
3.1 |
|
Payment of Rent |
|
|
2 |
|
|
|
3.2 |
|
Additional Rent |
|
|
3 |
|
|
|
3.3 |
|
Security Deposit |
|
|
4 |
|
|
|
3.4 |
|
Late Payment: Interest |
|
|
4 |
|
|
|
3.5 |
|
Rent Commencement Date |
|
|
4 |
|
| 4. |
|
USES |
|
|
4 |
|
|
|
4.1 |
|
Premises |
|
|
4 |
|
|
|
4.2 |
|
Exterior |
|
|
4 |
|
|
|
4.3 |
|
Hazardous Materials |
|
|
4 |
|
| 5. |
|
ALTERATIONS AND ADDITIONS |
|
|
5 |
|
|
|
5.1 |
|
Alterations and Additions |
|
|
5 |
|
| 6. |
|
MAINTENANCE AND REPAIR |
|
|
6 |
|
|
|
6.1 |
|
Tenant’s Obligations |
|
|
6 |
|
|
|
6.2 |
|
Landlord’s Obligations |
|
|
6 |
|
|
|
6.3 |
|
Tenant’s Obligation to Reimburse |
|
|
6 |
|
| 7. |
|
TAXES |
|
|
8 |
|
|
|
7.1 |
|
Tenant’s Personal Property Taxes |
|
|
8 |
|
|
|
7.2 |
|
Real Property Taxes |
|
|
8 |
|
|
|
7.3 |
|
Definition |
|
|
8 |
|
|
|
7.4 |
|
Supplemental Assessments |
|
|
8 |
|
| 8. |
|
UTILITIES AND SERVICES |
|
|
8 |
|
1
Table of Contents
(continued)
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
Page |
|
|
|
|
|
8.1 |
|
Utilities and Services |
|
|
8 |
|
|
|
|
|
8.2 |
|
No Liability Due to interruption |
|
|
9 |
|
| |
9. |
|
|
INDEMNITY BY TENANT |
|
|
9 |
|
| |
10. |
|
|
TENANT’S WAIVER OF CLAIMS |
|
|
9 |
|
| |
11. |
|
|
INSURANCE |
|
|
9 |
|
|
|
|
|
11.1 |
|
Tenant’s Liability Insurance |
|
|
9 |
|
|
|
|
|
11.2 |
|
Tenant’s Property Insurance |
|
|
9 |
|
|
|
|
|
11.3 |
|
Landlord’s Liability insurance |
|
|
9 |
|
|
|
|
|
11.4 |
|
Landlord’s Property Insurance |
|
|
10 |
|
|
|
|
|
11.5 |
|
Mutual Waiver of Subrogation |
|
|
10 |
|
|
|
|
|
11.6 |
|
Insurance Policies |
|
|
10 |
|
|
|
|
|
11.7 |
|
Insurance Premiums and Deductibles are “Operating Expenses” |
|
|
10 |
|
| |
12. |
|
|
DAMAGE OR DESTRUCTION |
|
|
10 |
|
|
|
|
|
12.1 |
|
The Building |
|
|
10 |
|
|
|
|
|
12.2 |
|
Tenant’s Property |
|
|
11 |
|
| |
13. |
|
|
CONDEMNATION |
|
|
11 |
|
| |
14. |
|
|
ADVERTISEMENTS AND SIGNS |
|
|
12 |
|
| |
15. |
|
|
ENTRY BY LANDLORD |
|
|
12 |
|
| |
16. |
|
|
ASSIGNMENT AND SUBLETTING |
|
|
12 |
|
|
|
|
|
16.1 |
|
Landlord’s Consent Required |
|
|
12 |
|
|
|
|
|
16.2 |
|
Documentation |
|
|
12 |
|
|
|
|
|
16.3 |
|
Terms and Conditions |
|
|
12 |
|
|
|
|
|
16.4 |
|
Landlord’s Remedies |
|
|
13 |
|
|
|
|
|
16.5 |
|
Bonus Rent |
|
|
13 |
|
| |
17. |
|
|
DEFAULT |
|
|
13 |
|
|
|
|
|
17.1 |
|
Event of Default |
|
|
13 |
|
|
|
|
|
17.2 |
|
Remedies |
|
|
13 |
|
|
|
|
|
17.3 |
|
No Relief From Forfeiture After Default |
|
|
14 |
|
|
|
|
|
17.4 |
|
Landlord’s Right to Perform Tenant’s Obligations |
|
|
14 |
|
|
|
|
|
17.5 |
|
Remedies Not Exclusive |
|
|
14 |
|
|
|
|
|
17.6 |
|
Termination and Surrender |
|
|
14 |
|
|
|
|
|
17.7 |
|
Landlord’s Default |
|
|
15 |
|
|
|
|
|
17.8 |
|
Landlord Liability |
|
|
15 |
|
2
Table of Contents
(continued)
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
Page |
|
|
|
|
|
17.9 |
|
Tenant’s Bankruptcy |
|
|
15 |
|
| |
18. |
|
|
EFFECT OF CONVEYANCE |
|
|
17 |
|
| |
19. |
|
|
INSTRUMENTS REQUIRED BY LENDER |
|
|
17 |
|
| |
20. |
|
|
TENANT’S ESTOPPEL CERTIFICATE |
|
|
17 |
|
| |
21. |
|
|
SUBORDINATION AND ATTORNMENT |
|
|
17 |
|
| |
22. |
|
|
NOTICES |
|
|
18 |
|
| |
23. |
|
|
NO ACCORD AND SATISFACTION / NO WAIVER |
|
|
18 |
|
|
|
|
|
23.1 |
|
No Accord and Satisfaction |
|
|
18 |
|
|
|
|
|
23.2 |
|
No Waiver |
|
|
18 |
|
| |
24. |
|
|
HOLDING OVER |
|
|
18 |
|
| |
25. |
|
|
GENERAL PROVISIONS |
|
|
18 |
|
|
|
|
|
25.1 |
|
Entire Agreement and Written Modification |
|
|
18 |
|
|
|
|
|
25.2 |
|
Timeliness |
|
|
19 |
|
|
|
|
|
25.3 |
|
Captions |
|
|
19 |
|
|
|
|
|
25.4 |
|
California Law |
|
|
19 |
|
|
|
|
|
25.5 |
|
Partial Invalidity |
|
|
19 |
|
|
|
|
|
25.6 |
|
Joint and Several Liability |
|
|
19 |
|
|
|
|
|
25.7 |
|
Binding on Successors |
|
|
19 |
|
|
|
|
|
25.8 |
|
Authority to Act |
|
|
19 |
|
|
|
|
|
25.9 |
|
No Light, Air or View Easement |
|
|
19 |
|
|
|
|
|
25.10 |
|
No Brokers |
|
|
19 |
|
|
|
|
|
25.11 |
|
Force Majeure |
|
|
19 |
|
|
|
|
|
25.12 |
|
Attorneys’ Fees |
|
|
20 |
|
| |
26. |
|
|
NO RELIANCE ON LELAND WIESNER AS LEGAL COUNSEL |
|
|
20 |
|
| |
27. |
|
|
TERMINATION OF EXISTING LEASE |
|
|
20 |
|
|
|
|
|
SIGNATURES |
|
|
30 |
|
|
|
|
|
|
|
|
EXHIBITS |
|
|
|
|
|
|
|
|
|
|
|
|
A |
|
PREMISES |
|
|
|
|
|
|
|
|
A-1 |
|
LEGAL DESCRIPTION OF PROPERTY |
|
|
|
|
|
|
|
|
B |
|
BASE RENT ADJUSTMENT SCHEDULE |
|
|
|
|
|
|
|
|
C |
|
OPTION TO EXTEND TERM |
|
|
|
|
|
|
|
|
D |
|
BASE RENT DURING THE EXTENDED TERM |
|
|
|
|
|
|
|
|
E |
|
PREMISES FFE |
|
|
|
|
|
|
|
|
F |
|
REMODEL PROJECT |
|
|
|
|
3
366 CAMBRIDGE AVENUE, PALO ALTO, CA
LEASE
This Lease is
dated, for reference purposes only, as of April 1, 2012 and is made and entered into by and between “Landlord” and “Tenant” as these terms are defined in the “Lease Summary”, which Lease Summary constitutes and Is
numbered as pages 1 and 2 of this Lease.
1. Premises.
1.1 Description of Premises. Landlord leases the “Premises” (as described herein and in the Lease Summary) to Tenant
for Tenant’s exclusive use, and Tenant leases the Premises from Landlord for the Term, at the rental, and upon all of the conditions set forth in the Lease including the Commercial Lease Summary. The Premises consist of a) the entire building
(the “Building”) located at 366 Cambridge Ave., Palo Alto, California (as shown on the attached Exhibit A) and b) the entire property on which the Building is located which is Santa Clara County Assessor’s Parcel #124-32.003 (the
Property”). The legal description of the Property is attached as Exhibit A-1. The Building shall be deemed to consist of four-thousand twenty-nine (4,029) square feet (the “Rentable Square Footage”) and no subsequent
re-measurement of the Building shall change the Base Rent payable hereunder or Tenant’s Allocable Share. The term “Tenant’s Allocable Share,” as used herein, shall mean the rentable square footage of the Premises divided by the
Rentable Square Footage. Tenant’s Allocable Share is equal to one hundred percent (100%) at the commencement of this Lease and so long as Tenant leases the entire Property will remain one hundred percent (100%).
1.2 Condition of Premises.
a. Landlord shall deliver the Premises (including, without limitation, roof, HVAC, electrical, plumbing, and lighting) to Tenant in
good working condition (excluding existing furniture, cabinets, phone equipment, network equipment, access control equipment, data and telecommunications wiring which shall be delivered in “as-is” condition without any warranty). In the
event Tenant provides Landlord with written notice of any non-compliance with this Paragraph 1.2.a. within sixty (60) days after the Commencement Date, then Landlord, at its cost, shall complete such required repair as soon as practical
following receipt of such notice.
b. Except for those warranties and Landlord obligations specifically set forth in this
Lease and any latent defects, Tenant shall accept the Premises In “as-is” condition.
c. Tenant represents that it
has completed all necessary due diligence to determine that the Premises are suitable for its use and that the condition of the Premises is acceptable.
d. Any Tenant Improvements constructed by Tenant shall be in accordance with requirements of the Americans with Disabilities Act, any
legislation adopted by the State of California relating In any way to access to non-residential properties and applicable building codes, including the life-safety provisions thereof, and any other similar state or local laws (the “Acts”);
provided, however, that such obligation shall not require Tenant to upgrade other areas of the Premises including, but not limited to, upgrading restrooms, installing elevators and installing ramps or other path of travel improvements not part of
Tenant’s planned construction, but required as a result of any triggered retrofitting of the Premises. Landlord shall be responsible for any such requirements and shall indemnify, defend and hold Tenant harmless from and against any such costs
and expenses as well as for fines, penalties and other damages (including, without limitation, attorneys’ fees, accountants’ fees, court costs and interest) in connection therewith.
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
1.3 Surrender of Premises. Upon termination or expiration of the Lease, Tenant
shall surrender the Premises to Landlord in the same condition as existed on the Commencement Date as improved with the Tenant Improvements and/or any Changes as to which Landlord has not reserved the right to require removal pursuant to
Section 5, subject to reasonable wear and tear, loss by fire or other casualty, and loss by condemnation, except that all articles of personal property and all business and trade fixtures owned or installed by Tenant, at its expense, at the
Premises shall remain the property of Tenant, and may be removed by Tenant at any time during the Term of this Lease. If Tenant falls to remove all of its personal property or business or trade fixtures from the Premises at the expiration of the
Lease, Landlord may, without liability to Tenant, store or dispose of Tenant’s personal property or business or trade fixtures in accordance with the provisions of the California Civil Code.
1.4 No Parking. There is no parking located on the Property and therefore no parking is included under the Lease.
1.5 Furniture, Fixtures, and Equipment. During the Term of the Lease, at no additional charge, Tenant shall be entitled to use
all of the furniture, fixtures, and equipment which belong to Landlord and are located within the Premises (the “Premises FFE”). Tenant shall be required to maintain the Premises FFE in accordance with Paragraph 6.1.
2. Term. The Term of the Lease shall commence upon the “Commencement Date” (as defined in Paragraph 2.1 below) and continue for sixty
(60) months from the Rent Commencement Date. If the Rent Commencement Date is other than the first day of a calendar month, then the Lease Term shall continue for sixty (60) months from the first day of the first full calendar month
following the Rent Commencement Date.
2.1 Commencement Date. The Commencement Date shall be deemed April 1, 2012 so
long as Landlord provides Tenant access and exclusive use of the Premises within Forty-eight (45) hours of Tenant’s request for exclusive use and access to the Premises after the execution of the Lease. If Landlord does not provide Tenant
access and exclusive use as provided herein, the Commencement Date shall be the date when Landlord provides exclusive use and access of the Premises to Tenant.
2.2 Option to Extend. In accordance with Exhibit C, Tenant shall have one (1) option to extend the Term of the Lease for a
period of sixty (60) months (the “Extended Term”). The Base Rent during the Extended Term shall be as set forth in Exhibit D.
3.
Rent.
3.1 Payment of Rent. As of the Rent Commencement Date, Tenant shall pay to Landlord the Base Rent,
as stated in the Lease Summary, without deduction, offset, prior notice or demand, In advance on the first day of each calendar month of the Term of this Lease. Base Rent shall be payable in lawful money of the United States to Landlord at such
place as Landlord may designate. Tenant’s obligation to pay Base Rent for any partial month shall be prorated on the basis of a thirty (30) day month. Base Rent shall be due In accordance with the schedule attached as Exhibit B.
Notwithstanding the foregoing, within ten (10) business days of the execution of this Lease, Tenant shall pay eighteen thousand one hundred thirty dollars ($18,130.00), less any partial payment of rent already made, to Landlord as the Base Rent
for the thirty (30) day period commencing with the Rent Commencement Date.
|
|
|
|
|
| Tenant’s Initials |
|
2 |
|
Landlord’s Initials LW |
3.2 Additional Rent.
(a) As of the Rent Commencement Date, Tenant shall pay to Landlord during the term hereof, In addition to the Base Rent, as additional
rent (the “Additional Rent”);
(i) Tenant’s “Allocable Share” of “Operating Expenses” (as set
forth in Paragraph 6.3(b) herein);
(ii) Tenant’s Allocable Share of all Real Property Taxes relating to the Property, as set
forth In Paragraph 7.2 herein;
(iii) Tenant’s Allocable Share of Insurance premiums as set forth In Paragraph 11.7; and
(iv) All charges, costs and expenses which Tenant is required to pay hereunder, together with all late charges, Interest, costs and
expenses including attorneys’ fees, that may accrue in the event of Tenant’s failure to pay any amount, and all damages, reasonable costs and expenses which Landlord may Incur by reason of Tenant’s default or breach of this Lease.
(b) In the event of nonpayment by Tenant of Additional Rent, Landlord shall have all the rights and remedies with respect thereto
as Landlord has for nonpayment of Base Rent.
(c) From and after the Rent Commencement Date, Tenant shall pay to Landlord on the
first day of each calendar month of the Lease Term an amount reasonably estimated by Landlord to be the monthly amounts attributable to clauses (I), (II), and (iii) of Paragraph 3.2(a) (collectively, “Estimated Expense
Reimbursements”). The Estimated Expense Reimbursements may be adjusted by Landlord’s providing thirty (30) days written notice to Tenant of the changed Estimated Expense Reimbursements. Within ninety (90) days following the end
of each calendar year Landlord shall furnish Tenant a statement of the actual expenses incurred by Landlord in the calendar year and the payments made by Tenant with respect to such period (such statement of actual expense reimbursements is referred
to herein as the “Landlord’s Statement”). If Tenant’s payments are less than the amount of the actual expenses properly allocable to Tenant, Tenant shall pay Landlord the deficiency within thirty (30) days after delivery of
such statement. If Tenant’s payments exceed the actual expenses properly allocable to Tenant, Landlord shall offset the excess against the Base Rent and Additional Rent next thereafter to become due to Landlord; provided that If the Lease Term
shall have expired, Landlord shall refund the excess to Tenant within thirty (30) days. Tenant shall have the right to audit financial records of Landlord with respect to expense reimbursements for the Premises as set forth in Paragraph 3.2(a)
of the Lease to verify expense reimbursements for a period of up to six (6) months following receipt of Landlord’s Statement. Tenant shall not have the right to Inspect or audit any other financial records of Landlord. Such audit shall be
conducted at Landlord’s offices by Tenant or a certified public accountant on Tenant’s behalf (so long as such auditor is not compensated based on the value of any claims or savings resulting from such audit), during normal business hours,
and on no less than ten (10) days prior written notice. Tenant shall make any claim to Landlord for an adjustment to the Landlord’s Statement within thirty (30) days of any audit of Landlord’s Statement. Further, Tenant must
initiate any legal action with respect to expense reimbursements within twelve (12) months of receipt of the Landlord’s Statement or Tenant shall have waived any right to adjustment or reduction of expense reimbursements paid by Tenant to
Landlord for any such period covered by the Landlord’s Statement.
|
|
|
|
|
| Tenant’s Initials |
|
3 |
|
Landlord’s Initials LW |
(d) Tenant’s Allocable Share shall not be modified as the result of any modification
in any calculations or change in useable, rentable or gross square footage of the Building.
(e) Base Rent and Additional Rent
shall be referred lo collectively as “Rent”.
3.3 Security Deposit. Upon execution of the Lease, Tenant shall
deposit the Security Deposit with Landlord, in the amount set forth in the Lease Summary, as security for the full and faithful performance by Tenant of every term and covenant of this Lease. In the event of an “Event of Default” by Tenant
(as defined in Section 17.1), Landlord may use or apply any portion of the Security Deposit to cure the default or to compensate Landlord for its damages from the default, in which event Tenant shall deposit with Landlord the sum necessary to
restore the Security Deposit to Its original amount within ten (10) days following Landlord’s written demand. Tenant shall not be entitled to any interest on the Security Deposit, and Landlord shall be entitled to commingle the Security
Deposit with its general funds. Tenant hereby waives the provisions of California Civil Code Section 1950.7(c).
3.4 Late
Payment: Interest. If any installment of Rent, Base Rent, Additional Rent, or any other amount due from Tenant is not received by Landlord thin ten business (10) days after receipt of written notice the due date, Tenant shall pay to
Landlord for each late payment, as liquidated damages, an amount equal to six percent (6%) of the overdue amount to compensate Landlord for reasonably foreseeable processing and accounting charges, and any charges that may be incurred by
Landlord with regard to any financing related to the Premises, and Landlord and Tenant agree that the foregoing is a reasonable amount to compensate Landlord for costs incurred as a result of any late payment by Tenant. Acceptance by Landlord of any
liquidated damage payment or interest related to Tenant’s late payment charge shall not constitute a waiver by Landlord of Tenant’s default with respect to any delinquent payment by Tenant.
3.5 Rent Commencement Date. Base Rent and Additional Rent shall commence to be payable upon the “Rent Commencement
Date” which shall be the Commencement Date.
4. Uses.
4.1 Premises. Tenant may only use the Premises for general office use, and related incidental uses (Including research and
development) and for no other use without Landlord’s consent. Tenant will engage in no activity on the Premises that would, in the reasonable judgment of any insurer of the Premises, increase the premium for Landlord’s insurance or cause
insurance for the Premises to be canceled. Tenant will comply with all applicable laws and governmental regulations pertaining to its use of the Premises. Tenant will not cause any excessive loads to be placed upon the floor slabs or the walls of
the Premises by the placement of its furnishings or equipment or otherwise. Tenant will commit no nuisance or waste on the Premises and will not cause any unreasonable odors, noise, smoke, vibration, electronic emissions, or any other item to
emanate from the Premises so as to damage the Property or the property of any person.
4.2 Exterior. Trash and recycling
containers may be stored outside of the Building only in areas specifically identified by Landlord. No other materials may be stored outside of the Building by Tenant or Landlord.
4.3 Hazardous Materials.
|
|
|
|
|
| Tenant’s Initials |
|
4 |
|
Landlord’s Initials LW |
(a) Tenant shall not cause or permit to be discharged by any of its employees, agents or
representatives under Its direct control from or about the Premises or the Building any materials identified by any federal, state, or local governmental body or agency as hazardous materials (collectively, “Hazardous Materials”). Tenant
shall at its sole expense comply with all applicable governmental rules, regulations, codes, ordinances, statutes and other requirements respecting Hazardous Materials in connection with Tenant’s activities on or about the Premises. Tenant
shall at its sole cost perform all clean-up and remedial actions that: (i) are required of Tenant by any governmental authority; and (ii) pertain to any discharge of such materials by Tenant.
(b) Tenant shall indemnify and hold Landlord harmless from all costs, claims, judgments, losses, demands, causes of action, proceedings
or hearings, Including Landlord’s attorneys’ fees and court costs, relating to the storage, placement or use of Hazardous Materials by Tenant on or about the Premises. Tenant shall reimburse Landlord for (i) losses in or reductions to
rental income resulting from Tenant’s use, storage, or disposal of Hazardous Materials; (ii) all costs of clean-up or other alterations to the Premises necessitated by Tenant’s use, storage, or disposal of Hazardous Materials; and
(iii) any diminution in the fair market value of the Property caused by Tenant’s use, storage, or disposal of Hazardous Materials. The obligations of Tenant under this Paragraph 4.3 shall survive the expiration of the Lease term.
(c) Landlord shall be responsible for all investigation, remediation, monitoring, and any costs associated with any Hazardous Materials
released, discharged, used, stored or present at the Premises because of any action or failure to act by: (I) Landlord, or its agents, employees, representatives, or contractors; (ii) any other tenants in the property of which the Premises
are a part; or (iii) any other individuals or entities. The provisions of this Paragraph 4.3(c) shall apply regardless of the timing of the release, discharge or discovery of Hazardous Materials.
(d) Landlord shall indemnify, defend and hold Tenant harmless from all costs, claims, fines, judgments, losses, demands, causes of
action, proceedings or hearings, including Tenant’s attorneys’ fees and court costs, arising in connection with any remediation required by governmental authority in connection with any Hazardous Materials present on the Premises at the
Commencement Date, or released, discharged, used or stored by Landlord, its agents, employees or contractors on, before or after the Commencement Date, and Landlord’s failure to comply with Its responsibilities and obligations under this
Paragraph 4.3. The obligations of Landlord under this Paragraph 4.3 shall survive the expiration of the Lease term.
5. Alterations and
Additions.
5.1 Alterations and Additions. Tenant shall not make any alteration, addition or utility
installation (collectively “Changes” or “Tenant improvements”) to the Premises without Landlord’s prior written consent which consent shall not be unreasonably withheld. Notwithstanding the immediately preceding sentence,
Tenant shall have the right to make interior, non-structural alterations within the Premises without Landlord’s approval, provided that the Changes do not exceed Five-Thousand Dollars ($5,000) in cost per project. If Tenant chooses to perform a
Change without Landlord’s consent, Landlord shall have the right to require Tenant at Tenant’s sole expense to remove such Change (and perform such remedial repairs which may be required as a result of such removal) upon the expiration or
earlier termination of the Lease. Prior to commencing to perform work for any Changes, Tenant shall provide Landlord not less than ten (10) days prior written notice specifying the Changes to be performed and the area of the Premises affected
by the Changes so that Landlord may post on the Premises appropriate notices of non-responsibility on the Building and Premises. Tenant shall obtain all necessary governmental permits and approvals prior to commencing work for any Changes. Tenant
shall comply with all building codes and other
|
|
|
|
|
| Tenant’s Initials |
|
5 |
|
Landlord’s Initials LW |
governmental requirements. All Changes and Tenant Improvements, including, but not limited to, wall and floor coverings and cabinetry, but excluding trade fixtures and furniture, shall become
part of the Premises and shall belong to Landlord and, upon expiration of the Lease, shall be surrendered by Tenant to Landlord. Notwithstanding the foregoing, Landlord shall have the right, at the time it provides approval for any proposed Tenant
Improvements and/or any proposed Change, to require that Tenant shall remove the same from the Premises, at Tenant’s expense, upon the expiration of the Lease and repair all damage occasioned by such removal. Tenant shall hold Landlord harmless
and shall indemnify Landlord from mechanics’ liens and any other liability related to any Changes performed by Tenant.
6. Maintenance and
Repair.
6.1 Tenant’s Obligations.
(a) Except for any elements of the Premises which Landlord Is obligated to maintain and repair pursuant to Paragraph 6.2 below, Tenant,
at its sole cost and at all times during the Term of the Lease or any extension thereof, shall clean, maintain and repair the interior non-structural portions of the Premises and every part thereof, including, without limitation the interior
plumbing and electrical systems (including light bulb and ballast repair and replacement) and the Premises FFE; and
(b) If Tenant
refuses or neglects to make repairs and/or maintain the Premises or any part thereof, or any other area required to be maintained by Tenant, in a manner and within a time period reasonably satisfactory to Landlord, Landlord shall have the right,
upon giving Tenant reasonable written notice of its election to do so, to make such repairs or perform such maintenance on behalf of and for the account of Tenant. In such event the cost of such work shall be paid by Tenant promptly upon
Landlord’s presentation of reasonable evidence of the costs actually incurred.
(c) If Tenant requests that Landlord perform
repairs to the Premises that are not required to be performed by Landlord in accordance with the Lease, Landlord, at its sole discretion, may choose to perform such requested repair and Tenant shall reimburse the actual cost of such repair as an
Operating Expense within thirty (30) calendar days of receiving a detailed invoice of the repairs made.
6.2 Landlord’s
Obligations. Subject to Tenant’s reimbursement obligations pursuant to Paragraph 6.3, and the provisions of this Lease dealing with damage or destruction and condemnation, Landlord shall repair and maintain in good working order the
following elements of the Building and Property:
(a) the roof, roof membrane, and all structural portions of the Premises and the
Building;
(b) the Building’s main water, sewer, and electrical service (including exterior utility lines and conduits but
excluding interior plumbing and electrical systems and equipment within the Premises which shall be maintained by Tenant in accordance with Paragraph 6.1 above);
(c) the HVAC systems serving the Premises; and
(d) the exterior surfaces (including exterior windows), sidewalks and landscaping of the Building.
|
|
|
|
|
| Tenant’s Initials |
|
6 |
|
Landlord’s Initials LW |
Tenant hereby waives any benefits of any applicable existing or future Law, including the provisions of
California Civil Code Sections 1932(1), 1941 and 1942, that allows a tenant to make repairs at its landlord’s expense.
6.3
Tenant’s Obligation to Reimburse.
(a) Tenant shall pay Tenant’s Allocable Share of all “Operating
Expenses” (as defined below) as may be paid or Incurred by Landlord during the Term of this Lease following the Rent Commencement Date. All Operating Expenses shall be prorated as of the Rent Commencement Date and Expiration Date to reflect any
portion of a calendar year occurring within the Lease Term.
(b) The term “Operating Expenses” shall mean all costs and
disbursements which Landlord shall pay or become obligated to pay in connection with the Taxes described in Paragraph 7.2, the Insurance described in Paragraphs 11.3 and 11.4 below, and the maintenance, repair and operation of the Property,
including, but not limited to all labor, materials, supplies and services, including the cost of all maintenance contracts, used or consumed In performing Landlord’s maintenance obligations hereunder provided such costs are Incurred for the
purpose of maintaining the Building. Operating Expenses shall also Include wages and salaries of all employees, accounting personnel, and consultants engaged in the operation, maintenance and security of the Building, Including taxes, insurance and
benefits relating thereto, prorated to the extent attributable to their involvement in the operation, maintenance and security of the Building (as compared to other projects in which they are involved). Operating Expenses shall also include all
costs and disbursements which Landlord shall pay or become obligated to pay in connection with the maintenance, repair and operation of the outside area of the Building, including landscaping costs. Operating Expenses shall also include a fee for
general and administrative expenses (the “Property Management Fee”) equal to three percent (3%) of the Gross Rent payable hereunder shall be included as an Operating Expense.
(c) In addition to the foregoing, Tenant shall reimburse Landlord in full for (I) any and all maintenance and repair expenses
incurred by Landlord and provided exclusively for Tenant and/or the Premises, and/or (ii) any damages to the Premises or the Building which are caused by Tenant, Its agents, employees or contractors but not repaired by Tenant or covered by
Insurance carried or required to be carried by Landlord pursuant to Paragraph 11.2.
(d) Notwithstanding anything to the contrary
in the Lease, if any of Tenant’s maintenance obligations under Paragraph 6.1 or Tenant’s reimbursement obligations under this Paragraph 6.3 would require Tenant to pay all or any portion of any charge which could be treated as a capital
improvement under generally accepted accounting principles, then the cost of such improvements shall be amortized over the useful life of the improvement (as reasonably determined by Landlord in accordance with generally accepted accounting
principles) and Landlord shall Inform Tenant of the monthly amortization payment required to so amortize such costs, and shall also provide Tenant with the information upon which such determination was made.
(e) Landlord shall complete to Tenants’ reasonable satisfaction the repairs and improvements described on Exhibit F “Remodel
Project” attached hereto and made a part hereof for all purposes. Landlord shall work in good faith to complete the project in a timely manner; however, in the event such repairs and improvements are not completed by July 1, 2012, Tenant
shall have the right to take over the Remodel Project and complete using their own contractors and resources. In the event Tenant takes over the Remodel Project, Tenant shall only pay to Landlord the reasonable cost of the work completed to
Tenant’s reasonable satisfaction by Landlord’s
|
|
|
|
|
| Tenant’s Initials |
|
7 |
|
Landlord’s Initials LW |
contractors, not exceeding the Project Budget as defined below. In any event, both the Landlord and Tenant agree that the Remodel Project is mentioned within this lease merely for convenience and
is not a material aspect of the lease and consequently under no circumstances shall serve as a basis for terminating the lease or changing any aspects of the lease by either the Tenant or Landlord whether the Tenant or Landlord partially completes
or fully completes the Remodel Project to the complete or partial satisfaction of either the Tenant or Landlord. Should the Tenant decide not to take over the Remodel Project and instead allow the Landlord to complete the project, the Landlord shall
pay all labor, materials, and costs associated with the repairs and improvements. Tenant agrees to reimburse Landlord the actual cost of such repairs and improvements, not to exceed $20,000.00 (the “Project Budget”). Reimbursement by
Tenant shall occur fifteen business days after Tenant (I) receives detailed line itemed invoices for the repairs and improvements that were paid by the Landlord and (ii) the repairs and improvements were completed to Tenant’s
reasonable satisfaction. Landlord shall bear any amount for the repairs and improvements that exceeds the Project Budget. Landlord shall be responsible to engage and supervise all contractors. The contractors completing the repairs and Improvements
shall take reasonable precautions to not interfere with Tenant’s business operations at the Premises. In the event the contractors are interfering with Tenant’s business operations, the contractors shall work during Tenant’s
non-business office hours to complete the repairs and improvements.
(f) Notwithstanding anything to the contrary in the Lease, in
no event shall Tenant have any obligation to perform or to pay directly, or to reimburse Landlord for all, or any portion, of the following repairs, maintenance, improvements, replacements, premiums, claims, losses, fees, charges, costs and expenses
(collectively, “Costs”): (I) excepting insurance deductibles, costs occasioned by fire, acts of God, or other casualties, or by the exercise of the power of eminent domain; (ii) costs for which Landlord has a right of
reimbursement from others; (iii) costs to comply with any underwriter’s requirement or Law applicable to the Premises on the Commencement Date; (iv) depreciation, amortization or other expense reserves; (v) interest, charges and
fees incurred on debt payments, on mortgages and rent underground leases; and (vi) costs incurred to investigate the presence of any Hazardous Material, costs to respond to any claim of Hazardous Material contamination or damage, costs to
remove any Hazardous Material from the Premises, and any judgments or other costs incurred In connection with any Hazardous Material exposure or releases, except to the extent caused by the storage, use or disposal of the Hazardous Material In
question by Tenant.
7. Taxes.
7.1 Tenant’s Personal Property Taxes. Tenant shall pay prior to delinquency all taxes, license fees, and public charges
assessed or levied against Tenant, Tenant’s estate in this Lease or Tenant’s leasehold improvements, trade fixtures, furnishings, equipment and other personal property.
7.2 Real Property Taxes. Tenant shall pay Tenant’s Allocable Share of “Real Property Taxes” (as defined in
Paragraph 7.3 below) during the Lease Term. Tenant’s liability to pay Real Property Taxes shall be prorated on the basis of a 365-day year to account for any fractional portion of a tax year included in the lease term at the commencement or
expiration hereof.
7.3 Definition. The term “Real Property Taxes” shall mean all taxes, general and special
assessments, and other charges imposed by any taxing authority and collection of rental income therefrom (excepting only estate taxes, inheritance taxes, and includes all entities having taxing or assessment authority by law or by virtue of any
recorded instrument binding on the owner of the Property.
|
|
|
|
|
| Tenant’s Initials |
|
8 |
|
Landlord’s Initials LW |
7.4 Supplemental Assessments. Tenant shall be liable for Tenant’s Allocable
Share of any supplemental assessments levied against the Property which are applicable to any portion of the Term of the Lease, applying the maximum permitted allocation period. Tenant’s liability for supplemental assessments which are
applicable to the Term of the Lease shall survive the expiration or earlier termination of the Term of the Lease for a period of one (1) year. Tenant shall pay Landlord such amounts within thirty (30) days of Tenant’s receipt of
Landlord’s invoice for supplemental assessments.
8. Utilities and Services.
8.1 Utilities and Services. All utilities or services for the Premises (including, without limitation charges for gas,
electricity, cable, telephone, Internet, security system monitoring, and any other utilities or services metered or charged to the Premises) shall be registered in the name of Tenant and Tenant shall pay directly for the costs of all such utilities
or services. Landlord shall not be responsible for providing any security protection for all or any portion of the Property and Tenant shall at its own expense provide or obtain any security services that it desires.
8.2 No Liability Due to interruption. No failure or Interruption of any utilities or service shall entitle Tenant to terminate
this Lease or to withhold rent or other sums payable by Tenant. Landlord shall not be liable to Tenant for any failure or interruption of any utilities or services unless caused by the gross negligence or willful misconduct of Landlord. Tenant
hereby waives any benefits of any applicable existing or future Law, including, but not limited to the provisions of California Civil Code Section 1932(1), permitting the termination of this Lease due to such interruption, failure or inability
to provide services and/or utilities.
9. Indemnity by Tenant. Tenant shall indemnify, defend and hold Landlord harmless from and against
any and all claims, demands, liabilities, and expenses, including attorneys’ fees, arising from: (I) Tenant’s use of the Premises, the Building and the Property; (Ii) any action permitted, or any omission to act, in or about the
Premises and Property (Including areas exterior to the Building such as the sidewalks and alleys) by Tenant or its agents, employees, contractors or invitees; (iii) for any costs required to be paid by Tenant under Section 17 of the Lease
or (iv) from any injury to person or property arising out of any act or omission of Tenant or its agents, employees, contractors or invitees. Notwithstanding the terms of the immediately preceding sentence, Tenant’s indemnity shall not be
applicable to the extent any such claims, demands, liabilities and expenses arise out of the gross negligence or willful misconduct of Landlord or any agent, employee or representative of Landlord. In the event any action or proceeding shall be
brought against Landlord by reason of any claim encompassed by the provisions of this Paragraph 9, Tenant shall defend the claim at Tenant’s expense by counsel reasonably satisfactory to Landlord or any agent, employee or representative of
Landlord.
10. Tenant’s Waiver of Claims. Tenant hereby waives any claims against Landlord far injury to Tenant’s business or any
loss of income therefrom, for damage to Tenant’s property, or for injury or death of any other person in or about the Premises or Property from any cause whatsoever, except to the extent caused by gross negligence or willful misconduct on the
part of Landlord or any agent, employee or representative of Landlord.
11. Insurance.
11.1 Tenant’s Liability Insurance. Tenant shall, at its expense, obtain and keep in force during the term of this Lease a
policy of commercial public liability insurance insuring Landlord and Tenant against any liability arising out of the operation of Tenant’s business and the use, occupancy
|
|
|
|
|
| Tenant’s Initials |
|
9 |
|
Landlord’s Initials LW |
or maintenance of the Premises. Such insurance policy shall have a combined single limit for both bodily injury and property damage in an amount not less than Five Million Dollars ($5,000,000).
The policy shall contain cross liability endorsements (to the extent available on commercially reasonable terms) protecting Landlord and shall insure performance by Tenant of the indemnity provisions of Paragraph 9 above. The limits of said
Insurance shall not limit the liability of Tenant hereunder. Tenant may satisfy the requirement herein by insuring under its global blanket liability policy subject to Tenant’s standard deductible under such policy.
11.2 Tenant’s Property Insurance. Intentionally deleted.
11.3 Landlord’s Liability insurance. Subject to Tenant’s obligation to reimburse in accordance with Paragraph
3.2(a)(iii), Landlord shall maintain a policy of commercial general liability insurance in an amount of not less than Five Million Dollars ($5,000,000) insuring Landlord (and such other entities as designated by Landlord) against liability for
personal injury, bodily Injury or death and damage to property occurring or resulting from an occurrence in, on, or about the Premises with such coverage as Landlord may from time to time deem advisable.
11.4 Landlord’s Property Insurance. Subject to Tenant’s obligation to reimburse in accordance with Paragraph
3.2(a)(iii), Landlord shall maintain a policy or policies of insurance covering loss or damage to the Premises, Including pro action from rental loss and coverage for operating expenses resulting from loss or damage to the Building, and such other
hazards (including earthquake insurance if Landlord, at its sole discretion, decides to carry such coverage) as are normally insured in the industry in such amounts and with such coverage as Landlord deems advisable, but in no event for less than
the full replacement cost of the Premises (except for earthquake coverage for which no minimum coverage is required). Landlord may insure the tenant improvements in the Premises, without such insurance requiring Landlord to restore the tenant
improvements in accordance with Paragraph 12 of this Lease. All proceeds under such policies shall be payable exclusively to Landlord. Landlord’s policy shall separately waive subrogation in accordance with Paragraph 11.5.
11.5 Mutual Waiver of Subrogation. Each of Tenant and Landlord waive, and shall use their best efforts to cause their respective
insurers to similarly waive, any and all rights of recovery against the other, or against the officers, employees, partners, agents and representatives of the other, for loss of or damage to the property of the waiving party or the property of
others under its control, to the extent such losses are covered by policies required to be carried under this Lease.
11.6 Insurance
Policies. All of Tenant’s Insurance shall be primary insurance written in a form reasonably satisfactory to Landlord by companies reasonably acceptable to Landlord and shall specifically provide by endorsements reasonably acceptable to
Landlord that such policies shall: (I) not be subject to cancellation or other change except after at least thirty (30) days’ prior written notice to Landlord; (ii) be primary insurance; (iii) specifically waive subrogation
in accordance with Paragraph 11.5 of this Lease. All liability policies maintained by Tenant hereunder shall name Landlord and Landlord’s property management company as additional insured parties. Copies of the policies or certificates
evidencing the policies, together with satisfactory evidence of payment of premiums shall be deposited with Landlord on or prior to the Commencement Dale, and upon each renewal of such policies, which shall be effected not less than thirty
(30) days prior to the expiration date of the term of such coverage.
11.7 Insurance Premiums and Deductibles are
“Operating Expenses”. Premiums and deductibles (related to casualty or damage that occurred during the Term of the Lease) for any insurance (except for the portion of any insurance deductibles in excess of ten thousand dollars
|
|
|
|
|
| Tenant’s Initials |
|
10 |
|
Landlord’s Initials LW |
($10,000) per occurrence) obtained by Landlord pursuant to Paragraphs 11.3 and 11.4 payable in connection with such Insurance shall be Operating Expenses. Landlord may obtain such insurance for
the Premises separately, or together with other buildings and improvements which Landlord elects to insure together under blanket policies of insurance. In such case, Tenant shall be liable only for such portion of the premiums of such blanket
policies as are allocable to the Premises as reasonably determined by the insurer of Landlord.
12. Damage or Destruction.
12.1 The Building. Except as otherwise provided in this Paragraph 12, if any portion of the Building that Landlord is obligated
to maintain is damaged or destroyed by any cause, if such damage is required to be insured against pursuant to Paragraph 11 above, and if Insurance proceeds are sufficient to pay for the full replacement of the Premises, then this Lease will not
terminate and Landlord will cause such damage to be repaired with reasonable diligence, subject to delays in the obtaining and disbursement of insurance proceeds and delays in repair caused by inclement weather, governmental action or inaction, and
shortage of materials or services. From the date of the casualty until the restoration is completed, Tenant’s obligation to pay Rent shall be abated to the extent the damage and repair period interfere with Tenant’s use of the Premises. if
the damage is not required to be insured against, or if the damage occurs within the last nine (9) months of the Lease Term, either party may, at its option exercised by written notice to the other within thirty (30) days of the date of
the damage, elect to terminate this Lease as of the date of damage, and if neither party so elects, Landlord shall complete the repair at its expense. Within thirty days following the date on which Landlord learns of any damage to the Premises,
Landlord shall notify Tenant in writing as to the estimated time for repairing the damage. If Landlord reasonably estimates that the time required for repair of the Premises will exceed six (6) months, then either Landlord or Tenant shall be
entitled to terminate this Lease as of the date of the damage by delivering written notice of termination to the other party within ten (10) days of Landlord’s determination of the time required for repair. If Landlord elects or is
required to repair the Premises in accordance with the terms of this Lease, Rent will abate in the manner described above, and, other than the obligation to repair stated above, and Landlord shall have no liability to Tenant related to the damage to
the Premises. If the estimated time for completion of repairs exceeds six (6) months, or if the repairs are not completed within six (6) months (regardless of the time estimated for completion of the repairs), Tenant shall have the right to
terminate this Lease by delivering written notice thereof to Landlord within thirty (30) days after receipt of the estimate, or the expiration of the six (6) month period, as the case may be, with any such termination effective as of the
date of the damage. In the event Landlord elects to terminate this Lease pursuant to this Paragraph 12, Tenant shall have the right, within ten (10) days after receipt of such notice, to agree in writing on a basis satisfactory to Landlord to
pay for the entire cost of repairing such damage less only the amount of Insurance proceeds, if any received by Landlord, in which event the notice of termination shall be ineffective and this Lease shall continue in full force and effect, and
Landlord shall proceed to make such repairs as soon as reasonably possible. If Tenant does not give such notice within ten (10) days, the Lease shall be terminated pursuant to such notice of termination by Landlord. Landlord and Tenant each
hereby waive the provisions of California Civil Code Sections 1932(2), 1933(4) and any other applicable existing or future Law permitting the termination of a lease agreement in the event of damage or destruction under any circumstances other than
as provided in this Article 12.
12.2 Tenant’s Property. Landlord’s obligation to rebuild or restore shall not
include Tenant’s personal property, trade fixtures, equipment, merchandise, leasehold improvements paid for by Tenant, Tenants trade fixtures, equipment and other personal property or Changes made by Tenant to the Premises. Landlord may insure
tenant improvements at the Premises without this obligating Landlord to restore the tenant improvement portion of the Premises for the benefit of Tenant provided, however, that any insurance proceeds actually received for damage to any Tenant
Improvements, shall be used for their replacement.
|
|
|
|
|
| Tenant’s Initials |
|
11 |
|
Landlord’s Initials LW |
13. Condemnation. if any part of the Premises shall be taken for any public, or quasi-public use,
under any statute or by right of eminent domain or purchase in lieu thereof, and a part thereof remains which is suitable for the operation of Tenant’s business, this Lease shall, as to the part so taken, terminate as of the date Tenant can no
longer occupy the portion of the Premises taken, and the rent payable hereunder shall be adjusted so that Tenant shall be required to pay for the remainder of the Lease term only such portion of such rent as the value of the part remaining after
such taking bears to the value of the entire Premises prior to such taking. If all of the Premises, or such part thereof shall be taken so that there does not remain a portion suitable for the operation of Tenant’s business, this Lease shall
terminate as of the date Tenant can no longer occupy the portion of the Premises taken. Notwithstanding the foregoing, if any taking materially lessens the ability of Tenant to conduct its business at the Premises, Tenant may terminate this Lease.
All compensation awarded upon any taking hereunder shall belong exclusively to the Landlord. Notwithstanding any provision to the contrary contained herein, Tenant shall have the right to make a separate claim against the appropriate governmental
authority for condemnation proceeds allocable to the unamortized costs of the leasehold improvements made at the cost of Tenant, the removal of Tenant’s trade fixtures or removable personal property, and relocation expenses if and only to the
extent that such separate claim does not diminish Landlord’s condemnation award.
14. Advertisements and Signs. Tenant shall not place
or maintain any sign, advertisement, notice or other marking whether temporary or permanent about the Premises or on the Building without the approval of the City of Palo Alto and the prior written consent of Landlord. The Landlord’s consent
shall not be unreasonably withheld or delayed. Any sign or advertisement placed by Tenant with Landlord’s approval shall be at Tenant’s sole expense.
15. Entry by Landlord. On not less than twenty-four (24) hours prior written notice, Landlord and its agents shall have the right to enter
and Inspect the Premises, to show the Premises to prospective tenants or purchasers or others, to post notices of non-responsibility or other legal notices, to make repairs, alterations or additions to any portion of the Building, provided such
repairs are performed in a manner that will cause the least disturbance to Tenant and the Premises. Landlord retains the right to post one or more “For Lease” signs on an exterior wall of the Premises during the last six months of the
initial lease term or any extended term unless Tenant has exercised its right to extend the term of the Lease. Tenant shall have the right to also place signs on the Premises regarding the disposition of Tenant’s business, such as *…
moving to new location”, subject to reasonable approval by Landlord.
16. Assignment and Subletting.
16.1 Landlord’s Consent Required. Tenant shall not voluntarily or by operation of law assign, transfer, mortgage, sublet,
or otherwise transfer or encumber all or any part of Tenant’s interest in the Lease or in the Premises, without Landlord’s prior written consent, which shall not be unreasonably withheld or delayed. It shall be reasonable for Landlord to
deny consent if, for example and without limiting Landlord’s right to make other reasonable objections, (I) the use to be made of the Premises by the proposed assignee or sublessee would be prohibited by any other term of this Lease; or
(ii) the character, reputation and financial condition of the proposed assignee or sublessee are not reasonably satisfactory to Landlord. Notwithstanding anything to the contrary in this Lease, and without any effect upon Section 16.5
hereof, Tenant may, without Landlord’s prior written consent and without constituting an assignment under this Section 16.1 or sublease hereunder, sublet the Premises or assign this Lease to (a) an entity controlling, controlled by or
under common
|
|
|
|
|
| Tenant’s Initials |
|
12 |
|
Landlord’s Initials LW |
control with Tenant, (b) an entity related to Tenant by merger, consolidation, nonbankruptcy reorganization, or government action, or (c) a purchaser of a substantial portion of
Tenant’s assets. A sale or transfer of Tenant’s capital stock shall not be deemed an assignment, subletting or any other transfer of this Lease or the Premises. Permitted Transfers shall be limited to transferees with financial capacity no
less than that of Tenant at the time of the proposed transfer.
16.2 Documentation. Prior to any assignment or sublease,
Tenant shall (a) provide to Landlord the proposed assignee’s or sublessee’s name, address, financial statements for the previous thirty-six (36) months, (If available) and copies of all documents relating to Tenant’s
proposed assignment or sublease, and (b) shall specify all monies and other consideration to be received by Tenant for such assignment or sublease. Within fifteen (15) days after the receipt of such documentation, Landlord shall either
(a) consent in writing to the proposed assignment or sublease subject to the terms and conditions hereinafter set forth, or (b) notify Tenant in writing that Landlord refuses such consent and specifically identifies its reasons for denial.
In the event Landlord fails to deliver such written notice within the required period, consent to the proposed sublease or assignment shall be deemed approved.
16.3 Terms and Conditions. In connection with any proposed assignment or sublease, Tenant shall reimburse up to One Thousand
Dollars (61,000) to Landlord for costs actually incurred by Landlord for analysis and documentation related to any proposed assignment or sublease regardless of whether Landlord consents to such assignment or sublease. Each assignment or
sublease shall be in form satisfactory to Landlord and shall be subject and subordinate to the provisions of this Lease. Once approved by Landlord, such assignment or sublease shall not be modified without Landlord’s prior written consent. Each
assignee or sublessee shall agree to perform all of the obligations of Tenant hereunder and shall acknowledge that the termination of this Lease shall, at Landlord’s sole election, constitute a termination of every such assignment or sublease.
Notwithstanding any assignment or sublease, Tenant shall remain primarily liable for all obligations and liabilities of Tenant under this Lease. Landlord may accept Rent from a proposed assignee or sublessee without waiving its right to withhold
consent to a proposed assignment or subletting.
16.4 Landlord’s Remedies. Any assignment or sublease without
Landlord’s prior written consent where such consent is required shall at Landlord’s election be void, and shall constitute a default under this Lease. The consent by Landlord to any assignment or sublease shall not constitute a waiver of
the provisions of this Paragraph 16 with respect to any subsequent assignment or sublease.
16.5 Bonus Rent. If Tenant
subleases or assigns the Lease and collects payment for such sublease or assignment in an amount exceeds the total Tenant Is required to pay to Landlord (“Tenant’s Obligation”) for the period of the sublease or assignment, Tenant
shall pay to Landlord an amount equal to fifty percent (50%) of the “Bonus Rent” which shall be the amount by which the payment by the subtenant or assignee exceeds the sum of the following: Tenant’s Obligation plus brokerage
commissions actually paid by Tenant for such sublease or assignment .
17. Default.
17.1 Event of Default. The occurrence of any of the following events (an “Event of Default”) shall constitute a
default and breach of this Lease by Tenant:
(a) The failure by Tenant to make any payment of Rent or any other required payment, as
and when due, and removal of any mechanics liens, if such failure shall not have been cured within ten (10) business days after written notice from Landlord;
|
|
|
|
|
| Tenant’s Initials |
|
13 |
|
Landlord’s Initials LW |
(b) The failure by Tenant to provide an Estoppel Certificate or any other instrument
required to be delivered by Tenant if such failure shall not have been cured within ten (10) business days after written notice from Landlord;
(c) The failure by Tenant to perform any other term, covenant or condition contained in this Lease if such failure shall have continued
for thirty (30) days after written notice from Landlord; provided that where any such failure cannot reasonably be cured within a thirty (30) day period, Tenant shall not be in default if Tenant commences to cure the failure within the
thirty (30) day period and thereafter diligently pursues all reasonable efforts to complete the work necessary to cure the failure.
(d) Tenant’s assignment of its assets for the benefit of its creditors; the filing of a petition by or against Tenant, where such
action is not dismissed within sixty (60) days, seeking adjudication or reorganization under the Bankruptcy Code; the appointment of a receiver to take possession of, or a levy by way of attachment or execution upon, substantially all of
Tenant’s assets at the Premises.
17.2 Remedies. Upon any Event of Default, Landlord shall have the following remedies,
in addition to all other remedies now or hereafter provided by law or equity, subject to Landlord’s obligation to mitigate damages:
(a) Landlord shall be entitled to keep this Lease In full force and effect and Landlord may enforce all of its rights and remedies under
this Lease, including the right to recover rent and other sums as they become due, plus interest at the highest rate then allowed by law, from the due date of each Installment of rent or other sum until paid; or
(b) Landlord may terminate Tenant’s right to possession by giving Tenant written notice of termination, whereupon this Lease and
all of Tenant’s rights in the Premises shall terminate. Any termination under this paragraph shall not release Tenant from the payment of any sum then due Landlord or from any claim for damages or rent accrued.
In the event this Lease Is terminated pursuant to this Paragraph 17.2(b), Landlord may recover from Tenant all damages incurred by Landlord by
reason of Tenant’s default, including but not limited to: (i) The cost of recovering possession of the Premises; (i1) Expenses of relettIng, including necessary renovation and alteration of the Premises; (Ili) Reasonable attorneys’
fees, any real estate commissions actually paid and that portion of any leasing commission paid by Landlord applicable to the unexpired term of this Lease; (iv) The worth at the time of award of the unpaid rent which had been earned at the time
of termination; (v) The worth at the time of award of the amount by which the unpaid rent which would have been earned after termination until the time of award exceeds the amount of such rental loss for the same period that Tenant proves could
have been reasonably avoided; (vi) The worth at the time of award of the amount by which the unpaid rent for the balance of the term after the time of award exceeds the amount of such rental loss for the same period that Tenant proves could be
reasonably avoided; and (vii) Any other amount necessary to compensate Landlord for all the detriment proximately caused by Tenant’s failure to perform Tenant’s obligations under this Lease, or which in the ordinary course of things
would be likely to result therefrom.
The “worth at the time of award” of the amounts referred to in subparagraphs (iv) and
(v) of this Paragraph 17.2(b) shall be computed by allowing interest at the Interest Rate. The “worth at the time of award” of the amount referred to in subparagraph (vi) of this Paragraph 17.2(b) shall be computed by discounting
such amount at the discount rale of the Federal Reserve Bank of San Francisco at the time of award plus one percent (1%). The term “rent” as used in this paragraph shall include alI sums required to be paid by Tenant to Landlord pursuant
to the terms of this Lease.
|
|
|
|
|
| Tenant’s Initials |
|
14 |
|
Landlord’s Initials LW |
17.3 No Relief From Forfeiture After Default. Tenant waives all rights of
redemption or relief from forfeiture under California Code of Civil Procedure Sections 1174 and 1179, and under any other present or future law, in the event Tenant is evicted or Landlord otherwise lawfully takes possession of the Premises by reason
of any Event of Default.
17.4 Landlord’s Right to Perform Tenant’s Obligations. If Tenant shall at any time fail
to perform any obligation required of Tenant hereunder, and provided Tenant has been provided a thirty (30) day written notice from Landlord concerning such obligation, then Landlord may, at Its option, perform such obligation to the extent
Landlord deems desirable, and may pay any and all expenses incidental thereto and employ counsel. No such action by Landlord shall be deemed a waiver by Landlord of any of Landlord’s rights or remedies, or a release of Tenant from performance
of such obligation. All sums so paid by Landlord shall be due and payable by Tenant to Landlord on the day immediately following Landlord’s payment thereof. Landlord shall have the same rights and remedies for the nonpayment of any such sums as
for default by Tenant In the payment of rent.
17.5 Remedies Not Exclusive. No remedy or election hereunder shall be deemed
exclusive but shall, wherever possible, be cumulative with all other remedies available.
17.6 Termination and Surrender. No
act or conduct of Landlord, including, without limitation, efforts to relet the Premises, an action in unlawful detalner or service of notice upon Tenant or surrender of possession by Tenant pursuant to such notice or action, shall extinguish the
liability of Tenant to pay rent or other sums due hereunder or terminate this Lease, unless Landlord notifies Tenant in writing of Landlord’s election to terminate this Lease. No act or conduct of Landlord, Including the acceptance of the keys
to the Premises, other than a written acknowledgment of acceptance of surrender signed by Landlord, shall be deemed to be or constitute an acceptance of the surrender of the Premises by Tenant prior to the expiration of the Lease term. The surrender
of this Lease by Tenant, voluntarily or otherwise, shall, at Landlord’s option, operate as an assignment to Landlord of any and all existing assignments and subleases, or Landlord may elect to terminate any or all of such assignments and
subleases by notifying the assignees and sublessees of its election within fifteen (15) days after such surrender.
17.7
Landlord’s Default. In the event of any failure by Landlord to perform any of Landlord’s obligations under this Lease, Tenant will give Landlord written notice specifying such default with particularity, and Landlord shall
thereupon have thirty (30)-days in which to cure any such default. Unless and until Landlord fails to so cure any default after such notice, Tenant shall not have any remedy or cause of action by reason thereof, If a default by Landlord remains
uncured after the expiration of the thirty (30) day period (except for obligations of Landlord which reasonably require greater than thirty (30) days to fulfill, and provided Landlord has Initialed performance of any such obligation within
such thirty (30) day period end has thereafter diligently acted to fulfill any such obligation), then Tenant shall have the right, as Tenant’s sole and exclusive remedies, to either (i) bring an action for damages, or (ii) cure
such default and all reasonable sums expended by Tenant in effecting such cure, together with interest thereon from the date of such expenditure at the Interest Rate, shall be due and payable by Landlord to Tenant within thirty (30) days of
Tenant’s written demand accompanied by reasonable documentation substantiating such sums. Notwithstanding anything to the contrary contained herein, If Landlord shall be obligated to perform any repair or maintenance which is of an emergency
nature, then Landlord shall commence all such repair and maintenance work as soon as reasonably possible upon its receipt of written or oral notice or upon its actual knowledge.
|
|
|
|
|
| Tenant’s Initials |
|
15 |
|
Landlord’s Initials LW |
17.8 Landlord Liability. If Landlord shall fail to perform any obligation under
this Lease, and as a consequence thereof, or if on any other claim by Tenant concerning the Premises or this Lease, Tenant shall recover a money Judgment against Landlord, then such Judgment shall be satisfied only out of Landlord’s estate in
the Premises, including sales proceeds, insurance proceeds, and rent, and Landlord shall have no personal or further liability whatsoever with respect to any such default or Judgment.
17.9 Tenant’s Bankruptcy. In the event of Tenant’s bankruptcy which is not dismissed within sixty (60) days of
filing, Landlord at its option may, in addition to all other rights and remedies provided In the Lease at law or in equity, terminate this Lease by giving written notice lo Tenant. If termination of this Lease shall be stayed by order of any court
having jurisdiction over any bankruptcy or insolvency proceeding or by federal or state statute, then, following the expiration of any such stay, or if Tenant or Tenant as debtor-in-possession or the trustee appointed in any such proceeding (being
collectively referred to as “Tenant” only for the purposes of this Paragraph 17.9) shall fail to assume Tenant’s obligations under this Lease within the period prescribed therefor by law or within fifteen (15) days after entry of
the order for relief or as may be allowed by the court, or if Tenant shall fail to provide adequate protection of Landlord’s right, title and Interest in and to the Premises or adequate assurance of the complete and continuous future
performance of Tenant’s obligation under this Lease, Landlord, to the extent permitted by law or by leave of the court having Jurisdiction over such proceeding, shall have the right, at its election, to terminate this Lease on fifteen
(15) days’ notice to Tenant and upon the expiration of said fifteen (15) day period this Lease shall cease and expire as aforesaid and Tenant shall Immediately quit and surrender the Premises as aforesaid. Upon the termination of this
Lease as provided above, Landlord, without notice, may re-enter and repossess the Premises using such force for that purpose as may be necessary without being liable to indictment, prosecution or damages therefor and may dispossess Tenant by summary
proceedings or otherwise. For the purposes of this Lease, adequate protection of Landlord’s right, title and interest in and to the Premises, and adequate assurance of the complete and continuous future performance of Tenant’s obligations
under this Lease, shall include, without limitation, the following requirements:
Tenant shall comply with all of its obligations under
this Lease, including, without limitation that: (i) Tenant pay to Landlord, on the first day of each month occurring subsequent to the entry of such order, or the effective date of such stay, a sum equal to the amount by which the Premises
diminished in value during the immediately preceding monthly period, but, in no event, shall such amount be less than the aggregate Base Annual Rent, Common Area Expenses and Additional Charges payable for such monthly period; (ii) Tenant
continue to use the Premises in the manner originally required by the Lease; (iii) Landlord be permitted to supervise the performance of Tenant’s obligations under this Lease; (iv) Tenant pay to Landlord within fifteen (15) days
after entry of such order or the effective date of such stay, as partial adequate protection against future diminution in value of the Premises and adequate assurance of the complete and continuous future performance of Tenant’s obligations
under this Lease, an additional security deposit in an amount acceptable to Landlord; (v) Tenant has and will continue to have unencumbered assets after the payment of all secured obligations and administrative expenses to assure Landlord that
sufficient funds will be available to fulfill the obligations of Tenant under this Lease; (vi) if Tenant assumes this Lease and proposes to assign the same (pursuant to Title 11 U.S.C. 365, or as the same may be amended) to any person who shall
have made a bona fide offer to accept an assignment of this Lease on terms acceptable to such court having competent Jurisdiction over Tenant’s estate, then notice of such proposed assignment, setting forth (1) the name and address of such
person, (2) all of the terms and conditions of such offer, and (3) the adequate assurance to be provided Landlord to assure such person’s future performance under this Lease, including, without limitation, the assurances referred to
in Title 11 U.S.C. 365(b)(3), as it may be amended, shall be given to Landlord by Tenant no later
|
|
|
|
|
| Tenant’s Initials |
|
16 |
|
Landlord’s Initials LW |
than fifteen (15) days after receipt by Tenant of such offer, but in any event no later than thirty (30) days prior to the dale that Tenant shall make application to such court for
authority and approval to enter into such assignment and assumption, and Landlord shall thereupon have the prior right and option, to be exercised by notice to Tenant given at any time prior to the effective date of such proposed assignment, to
accept, or to cause Landlord’s designee to accept, an assignment of this Lease upon the same terms and conditions and for the same consideration, If any as the bona fide offer made by such person less any brokerage commissions which may be
payable out of the consideration to be paid by such person for the assignment of this Lease; and (vii) if Tenant assumes this Lease and proposes to assign the same, and Landlord does not exercise its option as provided In subparagraph
(f) hereinabove, Tenant hereby agrees that: (a) such assignee shall have a net worth not less that the net worth of Tenant as of the Commencement Date, or such Tenant’s obligations under this Lease shall be unconditionally guaranteed
by a person having a net worth equal to Tenant’s net worth as of the Commencement Dale; (b)such assignee shall not use the Premises other than as permitted under this Lease; (c) such assignee shall assume in writing all of the terms,
covenants and conditions of this Lease; and (d) if such assignee makes any payment to Tenant, or for Tenant’s account, for the right to assume this Lease (including, without limitation, any lump sum payment, installment payment or payment
in the nature of rent over and above the Base Annual Rent, Common Area Expenses and Additional Charges payable under this Lease), Tenant shall pay over to Landlord one-half of any such payment.
Tenant hereby agrees that (a) all obligations that accrue under this Lease (including, but not limited to Tenant’s obligation to pay
rent), from and after the date that a petition is filed or other action is commenced under the Bankruptcy Code or the Insolvency Laws (an “Action-) shall be timely performed exactly as provided in this Lease and any failure to so perform shall
be harmful and prejudicial to Landlord; (b) any and all rents that accrue from and after the date that an Action is commenced and that are not paid as required by this Lease shall, in the amount of such rents, constitute administrative expense
claims allowable under the Bankruptcy Code with priority of payment at least equal to that of any other actual and necessary expenses incurred after the commencement of the Action; (c) any extension of the time period within which Tenant may
assume or reject the Lease without an obligation to cause all obligations under the Lease to be performed as and when required under the Lease, and/or any extension of the time within which Tenant must cure all defaults and compensate Landlord for
all pecuniary losses that extends beyond the date of assumption of the Lease shall be harmful and prejudicial to Landlord; (d) any proposed assignment of the Lease to an assignee: (I) that will not use the Premises specifically in
accordance with the Lease, or (ii) that does not possess financial condition, operating performance and experience characteristics equal to or better than the financial condition, operating performance and experience of Tenant as of the date of
this Lease, shall be harmful and prejudicial to Landlord; and (e) the rejection (or deemed rejection) of the Lease for any reason whatsoever shall constitute cause for immediate relief from the automatic stay provisions of the Bankruptcy Code,
and Tenant stipulates that such automatic stay shall be lifted immediately and possession of the Premises will be delivered to Landlord immediately without the necessity of any further action by Landlord. No provision of this Lease shall be deemed a
waiver of Landlord’s rights or remedies under the Bankruptcy Code, the Insolvency Laws, or applicable law to oppose any assumption and/or assignment of this Lease, to require timely performance of Tenant’s obligations under this Lease, or
to regain possession of the Premises as a result of the failure of Tenant to comply with the terms and conditions of this Lease or the Bankruptcy Code.
18. Effect of Conveyance. The term “Landlord” as used in this Lease, means only the current owner(s) of the Building. In the event of
any sale or other transfer of the Building, the transferor shall be deemed to be relieved of all obligations of the Landlord under the terms of this Lease from and after the date of such sale or other transfer, and the transferee shall be deemed to
have assumed and agreed to perform all obligations of Landlord under the Lease arising from and after the date of sale or other transfer.
|
|
|
|
|
| Tenant’s Initials |
|
17 |
|
Landlord’s Initials LW |
19. Instruments Required by Lender. Within ten (10) business days written request from
Landlord, Tenant shall execute and deliver to Landlord, such instruments, including a current statement of Tenant’s financial condition, as may be reasonably required by any mortgagee or holder of a deed of trust or other encumbrance related to
the Premises. Landlord shall keep and protect all such Information as confidential except as necessary to obtain debt or equity financing.
20.
Tenant’s Estoppel Certificate. Within ten (10) business days after written request of Landlord, Tenant shall, from time to time, deliver a duly executed and factually accurate estoppel certificate to Landlord in a form
reasonably satisfactory to Landlord and Tenant.
21. Subordination and Attornment. Tenant agrees that this Lease may, at the option of
Landlord, be subject and subordinate to any mortgage, deed of trust, any other Instrument of security, or ground lease which is now or hereafter placed on the Premises, provided that, with respect to any such instrument over which this Lease would
otherwise have priority, such subordination is conditioned upon the mortgagee, beneficiary, other secured party or ground lessor executing and delivering a Non-Disturbance Agreement In a form reasonably required by such mortgagee, beneficiary or
other party recognizing all rights of Tenant hereunder. Subject to Landlord’s providing an executed Non-Disturbance Agreement, in reasonably acceptable form, the foregoing subordination described herein is hereby made effective without any
further act of Tenant. In addition, following the mutual execution and delivery of this Lease Landlord shall make commercially reasonable efforts to obtain a Non-Disturbance Agreement for the benefit of Tenant from the beneficiary under any
mortgage, deed of trust, any other instrument of security, or ground lease encumbering the Building as of the date of this Lease. Tenant shall, at any time hereafter, on demand, execute any instruments, releases, or other documents that may be
required by any mortgagee, mortgagor, or trustor or beneficiary under any security instrument for the purpose of subjecting and subordinating this Lease to the lien of such instrument and Tenant shall agree to reasonable modifications to the form of
Non-Disturbance Agreement so long as such modifications do not diminish Tenant’s rights under this Lease. Tenant shall attorn to any third party purchasing or otherwise acquiring the Premises at any sale or other proceeding or pursuant to the
exercise of any rights, powers or remedies under any instruments of security or ground leases now or hereafter encumbering all or any part of the Premises, as if such third party had been named as Landlord under this Lease. Tenant may encumber or
finance its moveable fixtures and equipment installed on the Premises and its inventory, and no such encumbrance or financing shall be deemed an assignment by Tenant hereunder. In connection with any such encumbrance or financing, Landlord agrees to
execute and deliver an owner’s waiver and consent thereto In a form reasonably acceptable to Landlord and Tenant’s tender, provided Tenant is not In default of its obligations hereunder.
22. Notices. All notices, demands or requests to be given to Tenant or Landlord shall be in writing, delivered personally or by commercial
courier or by United States mail, postage prepaid, certified return receipt requested and addressed (a) to Tenant at Baker Hughes Oilfield Operations, Inc. c/a Vice President of Real Estate and Facilities, 2929 Allen Parkway, Suite 2100,
Houston, TX 77019 or (b) to Landlord at c/o Premier Properties, 172 University Ave„ Palo Alto, CA. Any notice shall be deemed to have been received on the date of actual delivery of such notice. Either party may change the address for
notice under the Lease on ten (10) days prior written notice to the other party.
23. No Accord and Satisfaction / No Waiver.
|
|
|
|
|
| Tenant’s Initials |
|
18 |
|
Landlord’s Initials LW |
23.1 No Accord and Satisfaction. No payment by Tenant or receipt by Landlord of an
amount that is less than the full amount of Rent or any other amount payable by Tenant then due to Landlord shall be deemed to be other than a partial payment applicable to the earliest amount payable to Landlord. No endorsement or statement on any
check or any letter accompanying any payment of Rent or any other amount payable to Landlord shall be deemed an accord and satisfaction of any Tenant obligation to Landlord, and Landlord may accept any check or payment without prejudice to
Landlord’s right to receive full payment of any Rent or other amounts payable to Landlord, including reservation of all of Landlord’s rights with respect to Rent or any other payment by Tenant.
23.2 No Waiver. Any Inaction by Landlord or Tenant with respect to any Event of Default or any other breach of the Lease shall
not be deemed to be a waiver of such Event of Default or breach of the Lease, regardless of Landlord’s or Tenant’s knowledge of such Event of Default or breach of the Lease. Landlord or Tenant may, at any time, pursue any and all remedies
provided for in the Lease, and otherwise as permitted by law.
24. Holding Over. This Lease shall terminate without further notice at the
expiration of the lease term. If the Tenant remains in possession after the expiration of the lease term with the express written consent of Landlord, such occupancy shall be construed to be a tenancy from month to month, at a monthly rental of
equal to the last applicable Rent, and shall otherwise be on the terms and conditions herein specified. Should Tenant remain in possession of the Premises without the Landlord’s consent, such possession shall be construed as a tenancy at
sufferance art a rental rate equal to 125% of the last applicable Rent.
25. General Provisions.
25.1 Entire Agreement and Written Modification. This Lease, together with its exhibits constitutes the entire agreement between
the parties. Except as may be specifically set forth in writing in this Lease, neither Landlord nor Tenant has relied on any representation or warranty by either Landlord or Tenant, or any agent of Landlord or Tenant, with respect to the Lease or
the Building. The Lease may not be modified orally or in any manner other than by an agreement in writing signed by all of the parties hereto or their respective successors in interest.
25.2 Timeliness. Time is of the essence with respect to the performance of each provision of this Lease In which time of
performance is a factor. Whenever the provisions of this Lease provide that the consent of the party must be obtained, except as otherwise specifically provided, such party agrees to act reasonably and in a timely manner in determining whether to
grant or withhold its consent.
25.3 Captions. The captions of the numbered paragraphs of this Lease are inserted solely for
convenience and shall have no effect upon the construction or interpretation of the Lease.
25.4 California Law. This Lease
shall be construed and interpreted in accordance with the laws of the State of California with Santa Clara County as venue for any Action related to the Lease.
25.5 Partial Invalidity. If any provision of this Lease is held by a court of competent jurisdiction to be invalid, void, or
unenforceable, the remainder of the provisions of this Lease shall, nonetheless, continue in full force and effect.
|
|
|
|
|
| Tenant’s Initials |
|
19 |
|
Landlord’s Initials LW |
25.6 Joint and Several Liability. If either Landlord or Tenant is more than one
person or entity, each such person or entity shall be jointly and severally liable for all applicable obligations of either Landlord or Tenant. Tenant and Landlord each warrant and represent that the party signing this Lease on behalf of each has
authority to enter into this Lease and to bind Tenant and Landlord, respectively, to the terms, covenants and conditions contained herein. Each party shall deliver to the other, upon request, all documents reasonably requested by the other
evidencing such authority, Including a copy of all corporate resolutions, consents or minutes reflecting the authority of persons or parties to enter into agreements on behalf of such party.
25.7 Binding on Successors. Subject to the provisions of Section 16 and Section 18, the covenants and conditions of
this Lease shall apply to and be binding upon the parties hereto and their respective successors in interest.
25.8 Authority to
Act. Each of the individuals executing the Lease hereby represent and warrant that he or she has all necessary power and authority to execute and deliver this Lease on behalf of the entity for which he or she is signing.
25.9 No Light, Air or View Easement. Any diminution or shutting off of light, air or view by any structure which may be erected
on lands adjacent to or in the vicinity of the Building shall in no way affect this Lease, entitle Tenant to any reduction of Rent or impose any liability upon Landlord
25.10 No Brokers. Neither Landlord nor Tenant is represented by a broker and no commissions shall be payable to any broker in
accordance with this transaction. Each party hereby agrees to indemnify and hold the other harmless from any claims arising from its dealings with any broker or agent.
25.11 Force Majeure. If either party shall be delayed or prevented from the performance of any act required under the Lease by
reason of acts of God, strikes, inability to procure materials, restrictive governmental laws or regulations, delay by the other party, or other cause without fault and beyond the control of the party obligated to perform (financial inability
excepted), then upon notice to the other party, the performance of such act shall be excused for the period of the delay, and the period for the performance of such act shall be extended for a period equal to the period of such delay; provided,
however, the party so delayed or prevented from performing shall exercise good faith efforts to remedy any such cause of delay or cause preventing performance. Notwithstanding the foregoing, this Section shall not cause any delay In the Rent
Commencement Date or allow Tenant to delay making any payment hereunder when due.
25.12 Attorneys’ Fees. If any action
or proceeding at law or in equity, or an arbitration proceeding (collectively, an “Action”), shall be brought to recover any rent under this Lease, or for or on account of any breach of or to enforce or interpret any of the terms of this
Lease, or for the recovery of possession of the Premises, the prevailing party shall be entitled to recover from the other party as a part of the Action, its reasonable attorneys’ fees and costs and expenses incurred related to the Action.
“Prevailing Party” shall mean, without limitation, the party who brings an Action against the other part, If such Action is dismissed upon payment by a party of the sums allegedly due or upon the performance of the covenants allegedly
breached, or if the party Initiating an Action obtains substantially the relief sought by it In such Action, whether or not such Action proceeds to a final judgment or determination.
26. No Reliance on Leland Wiesner as Legal Counsel. Each of the parties executing this Lease acknowledges that Leland Wiesner, the managing
member of Esquire Advisors has advised
|
|
|
|
|
| Tenant’s Initials |
|
20 |
|
Landlord’s Initials LW |
each party that he is an active, licensed member of the Bar of the State of California. None of the parties have sought from or retied upon Leland Wiesner for legal advice, counsel or opinions,
or for tax advice or counsel. Both Landlord and Tenant each further represent and warrant for the benefit of Leland Wiesner as a third-party beneficiary, that they have been advised to retain independent legal counsel with respect to this Lease and
the transactions contemplated in this Lease.
27. Termination of Existing Lease. Upon the Commencement Date of the Lease, Geomechanics
International, Inc. and Tenant’s existing lease with Landlord dated September 25, 2011 for the Premises at 366 Cambridge Ave., Suites 210, 209, 208, 207 Palo Alto, CA. shall be terminated and of no force and effect.
IN WITNESS WHEREOF, Landlord and Tenant have executed this Lease on the dates specified below immediately adjacent to their respective
signatures. 6livery of this Lease to Landlord, duly executed by Tenant, constitutes an offer by Tenant to lease the Premises as herein set forth, and under no circumstances shall such delivery be deemed to create an option or reservation to tease
the Premises for the benefit of Tenant. This Lease shall only become effective and binding upon execution of this Lease by Landlord and delivery of a signed copy to Tenant.
|
|
|
| “Tenant” |
|
“Landlord” |
|
|
| BAKER HUGHES OILFIELD |
|
|
| OPERATIONS, INC., a California
corporation |
|
|
|
|
|
|
|
|
|
|
|
| By: |
|
/s/ David E. Emerson |
|
|
|
/s/ Leland Z. Wiesner |
| Name: |
|
David E. Emerson |
|
|
|
Leland Wiesner |
| Title: |
|
Vice President |
|
|
|
|
|
|
|
|
|
| Date: |
|
May 4, 2012 |
|
|
|
Date: |
|
5/8/2012 |
|
|
|
|
|
|
|
|
|
|
/s/ Maria C. Horton |
|
|
|
|
|
|
Maria C. Horton |
|
|
|
|
|
|
|
|
|
|
|
Date: |
|
May 8, 2012 |
|
|
|
|
|
| Tenant’s Initials |
|
21 |
|
Landlord’s Initials LW |
EXHIBIT A
PREMISES

|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
EXHIBIT A-1
Legal Description of the Property
All
that certain Real Property in the City of Palo Alto, County of Santa Clara, State of California, described as follows:
All of Lot 30, in block 15, as
shown upon chat certain Map entitled, “Map or Evergreen Park”, which Map was filed for record in the Office of the Recorder of the County of Santa Clara. State of California, on August 12. 1904 in Book P-3 of Maps, at Page 92.
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
EXHIBIT B
Base Rent Schedule
| 1. |
Base Rent Schedule: During the Initial Term of the Lease, Base Rent shall be due in accordance with the following schedule: |
|
|
|
|
|
| Period |
|
Base Rent / Month |
|
| Rent Commencement Date—Month 12 |
|
$ |
18,131 |
|
| Month 13—Month 24 |
|
$ |
18,674 |
|
| Month 26—Month 36 |
|
$ |
19,235 |
|
| Month 37—Month 48 |
|
$ |
19,812 |
|
| Month 49—Month 60 |
|
$ |
20,406 |
|
For ease of reference, the successive months of the Lease Term are referred to in the above schedule by a
number, with Month 1 meaning the first full calendar month of the Term following the Rent Commencement Date, Month 12 being the twelfth full calendar month of the Term following the Rent Commencement Date, and so on.
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
EXHIBIT C
OPTION TO EXTEND TERM
| 1. |
Options. At the expiration of the initial term hereof (the “Expiration Date”), Tenant may extend the Term of this Lease for one (1) additional period for a term of sixty (60) months
commencing immediately following the Expiration Date (the “Extended Term”). Tenant shall be required to provide Landlord with six (6) months advance written notice of its intention to exercise its option to extend and failure by
Tenant to provide written notice of exercising of any option when written notice is due shall constitute a waiver of Tenant’s right to exercise such option. Landlord shall not be required to provide Tenant with notice of the expiration of
Tenant’s exercise of option period. In no event shall any purported exercise of any option by Tenant be effective if Tenant is in default of any material monetary term, covenant, agreement or obligation on its part under this Lease during the
period from the date Tenant exercised its option hereunder up to and including the commencement of the Extended Term. The Extended Term shall be upon all of the terms and conditions hereof, except that the Base Rent shall be as set forth in Exhibit
D. |
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
EXHIBIT D
Base Rent during the Extended Term
| 1. |
Initial Base Rent During the Extended Term. Upon Tenant’s exercising of its Option to Extend the Term of this Lease, the monthly Base Rent for the Extended Term shall initially be the fair market rent
for the Premises (the “FMR”) but in no event fess than the Base Rent during the final month of the immediately preceding term. When Landlord and Tenant are called upon to determine the FMR, Landlord and Tenant shall make best business
efforts to agree upon the FMR. In the event the parties fail to agree upon the FMR on or before one hundred fifty (150) days prior to the date upon which the Base Rent was scheduled to adjust to the FMR, the FMR shall be determined by appraisal
in the manner hereinafter set forth. |
In the event it becomes necessary under this paragraph to determine the FMR by
appraisal, one hundred twenty (120) days prior to the date upon which the Base Rent was scheduled to adjust to the FMR, Landlord and Tenant each shall appoint a real estate appraiser who shall a) be a member of the Northern California Chapter
of the Appraisal Institute, b) hold an MAI designation and c) be experienced in the appraisal of rental value for commercial properties In the Palo Alto area, and such appraisers shall each determine the FMR for the Premises taking into account the
use of the Premises, the Rentable Square Footage, the value of the Premises Including any “Tenant Improvements” and “Changes” (as they are herein defined) and the amenities provided by the Building, the annual adjustments of Base
Rent provided for hereinabove, and prevailing comparable rentals; provided, however, that in their determination of the FMR shall not take into account Improvements to the Premises provided or installed by Tenant which Tenant would be entitled to
remove upon expiration of the term pursuant to the terms of this Lease. Such appraisers shall, within forty-five (45) business days after their appointment, complete their appraisals and submit their appraisal reports to Landlord and Tenant. If
the FMR of the Premises established in the two (2) appraisals varies by five percent (5%) or less of the higher rental, the average of two shall be controlling. If said FMR varies by more than five percent (5%) of the higher rental,
said appraisers, within ten (10) days after submission of the last appraisal, shall appoint a third appraiser who shall be a member of the Northern California Chapter of the Appraisal Institute and who shall be similarly qualified and
experienced. Such third appraiser shall, within forty-five (45) business days after his appointment, determine by appraisal the FMR of the Premises, taking into account the same factors referred to above, and submit his appraisal report to
Landlord and Tenant. The FMR determined by the third appraiser for the Premises shall be averaged with whichever of the other two appraised values is closest to that determined by the third appraiser, and said average shall be the FMR used to
determine Base Rent pursuant to the preceding paragraph. If either Landlord or Tenant fails to appoint an appraiser, the appraisal submitted by the appraiser properly appointed and timely submitting his appraisal shall be controlling. If the two
appraisers appointed by Landlord and Tenant are unable to agree upon a third appraiser within the required period in accordance with the foregoing, application shall be made within twenty (20) days thereafter by either Landlord or Tenant to the
Northern California Chapter of the Appraisal Institute, which shall appoint an MAI member of said institute willing to serve as appraiser. The cost of all appraisals under this paragraph shall be borne equally by Landlord and Tenant.
| 2. |
Base Rent Adjustments during the Extended Term. The Base Rent during the Extended Term shall be increased by three percent (3%) on each anniversary of the date of commencement of the Extended Term.
|
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
Exhibit E
Premises FFE
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
Exhibit F
Remodel Project
| (1) |
Landlord agrees to remove the wall between the wailing area and office #3 (the “Combined Room”); |
| (2) |
Replace the flooring in the Combined Room with file that is approved in writing by Tenant; |
| (3) |
Repair any sheetrock damage and electrical outlets/lines In the Combined Room; |
| (4) |
Ensure that there are sufficient electrical outlets for Tenant’s use within the Combined Room. |
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
EXHIBIT B
Furniture, Fixtures & Equipment
|
|
|
|
|
| Tenant’s Initials |
|
1 |
|
Landlord’s Initials LW |
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the reference to our firm under the caption “Experts” and to the use of our report dated March 31, 2015, included in the Proxy
Statement of Celladon Corporation that is made part of Amendment No. 1 to the Registration Statement (Form S-4 No. 333-208521) and Prospectus of Celladon Corporation for the registration of 5,700,000 shares of its common stock.
/s/ Ernst & Young LLP
San Diego, California
January 21, 2016
Exhibit 23.2
Consent of Independent Registered Public Accounting Firm
The Board of Directors
Eiger BioPharmaceuticals, Inc.:
We consent to the use of our report dated December 11, 2015, with respect to the consolidated balance sheets of Eiger BioPharmaceuticals, Inc. as of
December 31, 2014 and 2013, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2014, included herein and
to the reference to our firm under the heading “Experts” in the proxy statement/prospectus/information statement.
Our report dated December 11,
2015 contains an explanatory paragraph that states that the Company has suffered recurring losses from operations and has an accumulated deficit, which raise substantial doubt about its ability to continue as a going concern. The consolidated
financial statements do not include any adjustments that might result from the outcome of that uncertainty.
/s/ KPMG
LLP
San Francisco, CA
January 21, 2016
CELLADON CORP (NASDAQ:CLDN)
Historical Stock Chart
From Mar 2024 to Apr 2024
CELLADON CORP (NASDAQ:CLDN)
Historical Stock Chart
From Apr 2023 to Apr 2024