 Ibelieveinmusic
4 months ago
Ibelieveinmusic
4 months ago
ASCEND Cardiovascular to Market and Distribute Ventripoint's AI-powered Heart-Scanning Technology to North American Hospitals and Clinics
V.VPT | 1 day ago
(TheNewswire)
Ventripoint Diagnostics Ltd.
Highlights of agreement
ASCEND’s IT software is used by 50,000 hospitals, clinics, and caregivers.
Initial two-year term for North American markets.
Ventripoint will be integrated into ASCEND’s IT for cardiovascular care.
Toronto, Ontario – TheNewswire - December 12, 2023 - Ventripoint Diagnostics Ltd. ("Ventripoint" or the "Company"), (TSXV:VPT)(OTC:VPTDF), now bringing its AI-powered heart-scanning technology to the world’s hospitals, is pleased to announce it has entered into a global distribution and marketing agreement with ASCEND Cardiovascular (“ASCEND”).
ASCEND Cardiovascular is a leader in offering cutting-edge diagnostic, and imaging technologies and workflows to improve cardiac health care. ASCEND is trusted by AGFA Healthcare, Sectra, Oracle/ Cerner, and other major players in the healthcare system. Its technology stack has been integrated into the workflows of 1,000+ top-ranked healthcare facilities and 600+ health systems, representing a user base of 50,000 who serve millions of heart patients.
ASCEND will market and distribute Ventripoint’s AI-powered heart-scanning technology, which turns ultrasound images of the heart into MRI-quality heart images. Using AI, and the images from existing ultrasound systems of any brand, Ventripoint creates these MRI-quality heart images at a fraction of the cost and time needed for more traditional heart MRIs. “After many years of careful research and development, and rigorous in-hospital testing, Ventripoint’s heart-imaging technology with ASCEND’s proven support is ready for the world’s hospitals and heart patients,” said Dr. Alvira Macanovic, President & CEO of Ventripoint. “With this ASCEND partnership I am encouraged we will win the confidence of hospitals and cardiologists across North America and beyond.”
“We are looking forward to the partnership between Ventripoint Diagnostics and ASCEND Cardiovascular. Our aim is that by working together, we can ultimately provide reliable and effective tools for clinicians on a global scale,” stated Jeffrey Soble, MD, ASCEND’s President and CEO.
The global distribution agreement has an initial two-year term, with ASCEND as the official reseller. ASCEND will market and sell the Ventripoint VMS+ system as part of its inclusive solution, as well as integration with ASCEND’s cardiovascular IT platform.
Ventripoint will be providing support and training to ASCEND on the use and capabilities of the VMS+ during the term of the agreement. The collaboration between Ventripoint and ASCEND will reduce time to market and overall effort to provide new software solutions to the market.
“Combining these two products creates an end-to-end solution rivaling the best technology offered on the market, said Dr. Macanovic. “Our partnership includes joint research and development initiatives to optimize current technologies and exploratory projects to identify new treatment possibilities, reduce waiting times, and ensure a cost-effective addition to the heart healthcare system.”
Ventripoint, now commercializing its technology, has won accolades from leading hospitals, and the British Heart Foundation and has drawn international media attention. As the relationship between Ventripoint and ASCEND Cardiovascular continues to evolve, both companies remain committed to driving innovation and excellence in cardiovascular health technology.
About Ventripoint Diagnostics Ltd.
Ventripoint has become an industry leader in the application of AI (Artificial Intelligence) to echocardiography. Ventripoint's VMS products are powered by its proprietary KBR technology, which is the result of a decade of development and provides accurate volumetric cardiac measurements equivalent to MRI. This affordable, gold-standard alternative allows cardiologists greater confidence in the management of their patients. Providing better care to patients serves as a springboard and basic standard for all of Ventripoint's products that guide our future developments. In addition, VMS+ is versatile and can be used with all ultrasound systems from any vendor supported by regulatory market approvals in the U.S., Europe, and Canada. Learn more: www.ventripoint.com.
About ASCEND Cardiovascular
ASCEND Cardiovascular is a leader in innovating cardiovascular solutions that empower the provider community to improve cardiovascular care. Designed with openness in mind, our solutions integrate with EHRs, medical devices, and other systems to deliver seamless workflows that span procedure types and modalities. A complete cardiovascular solution, ASCEND provides structured reporting, image visualization, collaboration, and analytics that improve efficiency, outcomes, and ROI. With decades of experience and a practicing cardiologist at our helm, the ASCEND team brings unparalleled “know how” in cardiology workflow, collaboration, and IT offering limitless opportunities to improve clinical, operational, and quality performance. Learn more: www.ascendcv.com.
For further information, please contact:
Jonathan Robinson CFA
JRobinson@oakhillfinancial.ca
(416) 669-1001
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this news release.
 imjake
4 years ago
imjake
4 years ago
More News with attachments probably on the website: https://www.ventripoint.com
Dear Friend;
It has been a long time coming but we are back! or almost there as you will read in the attached news release. Here is some background:
1. The Company asked to have its stock halted December 12, 2019 - we were NOT suspended by the Exchange.
2. We received approval to stay halted until we completed the $1,220,000 private placement, which we did last Friday. No one expected it to take so long but COVID-19 disrupted everyone’s plans.
3. The Company actually benefited overall from the pandemic, which you will read about, but briefly we had more access to cardiologist and received a lot of government support (like many other companies). This allowed us to make major changes to almost every aspect of the VMS+3.0 to lower the barrier to adoption, reduce manufacturing costs, accelerate analysis and rewrite the entire source code. The key initiatives attachment is a long read (5 pages), but worth the time.
4. The major advantage of all this work is we no longer have to go to a hospital to install the machine, calibrate the probes and train the staff. It is all done remotely now. You will read about all the advantages this approach has created for us and our customers.
5. The hospitals, while focused on COVID, did continue in their processes to acquire a VMS+3.0 and so we have 11 major hospitals ready to acquire a system as soon as they re-open their echo labs. This is starting to happen as hospitals have to start dealing with regular cardiac patients.
6. We now know COVID wrecks havoc with the heart, especially the right side, and so cardiologists are very interested in our capabilities as they fear the heart problems induced by COVID could go on for decades.
7. Lots more news to come and lots more information on what we have been doing and why it is important on our revamped website and in the addendum..
8. We are in the final stages of the TSXV Exchange review to allow us to resume trading and so I expect that will happen very soon.
I am very thankful for all the people who encouraged me to keep going. It has not been a lot of fun, but we are in a very good place right now - a way ahead of where we were before we restructured. Our team has done remarkable things in the last 9 months and the responses we are seeing from cardiologists are reflecting that we have finally gotten through all the barriers to adoption with a device that is user-friendly and fits perfectly into the workflow of an echocardiography lab. I look forward to telling you all about the wonderful effects doctors with the VMS+3.0 are having on their patients, as well as how well the company is progressing to build value for its shareholders.
As usual, I thank you for your support and interest and if you have any questions or comments, email, text, call.
Regards,
Dr. George Adams ICD.D
Executive-Chairman
Ventripoint Diagnostics Ltd.
 imjake
4 years ago
imjake
4 years ago
Here is the email I received just now:
Attached is the news release for today documenting we have closed the second tranche of $495,000 of the private placement to complete the round (which we announced many months ago) with total gross proceeds of $1,220,000.
Here is some background:
1. The Company halted trading and ceased operations in December 2019 to restructure with a new Board and the return of Dr. Adams as acting CEO.
2. The Company closed a first tranche of the $1,220,000 financing February 9 ,2020 with proceeds of $725,000 and resumed operations with all key employees returning to work.
3. In March 2020, COVID-19 pandemic reached Canada and Company ceased manufacturing and fulfillment of orders to hospitals as hospitals were focused on COVID-19 operations.
4. The Company has been focused on eliminating barriers to adoption and enabling remote installation and training.
5. As of Sept 11, 2020 the Company has closed its financing with the remaining $495,000 of proceeds and now has the funds to fulfill its existing and forthcoming purchase orders (more about this in next NR).
6. The Company is in a “Resumption Review” with the TSXV Exchange and expects to be trading in the next few days and will provide a comprehensive corporate update prior to the resumption of trading.
As usual, if you have questions call me.
Regards,
Dr. George Adams ICD.D
Executive-Chairman
Ventripoint Diagnostics Ltd.
 imjake
4 years ago
imjake
4 years ago
Doesn't look good for the company. Here is their last press release: Toronto, Ontario, December 19, 2019 – Ventripoint Diagnostics Ltd. (“Ventripoint” or the “Company”, TSXV:VPT, OTCQB:VPTDF) wishes to clarify the news release of December 17, 2019.
The Company is not in receivership and it has not made an assignment under the Bankruptcy and Insolvency Act. The Company is organizing a private placement and expects to resume normal operations in the new year."
That, of course, was 8 months ago so they may be in bankruptcy by now. The 12/17/19 release describes their funding plan which must not have happened. Getting involved with China is BAD news especially when you have no money to defend your property. I will try to contact Adams and see if there is anything salvageable with this stock.
 teq0904
5 years ago
teq0904
5 years ago
Dear Friend;
As I wrote yesterday the FDA clearance (their word for approval) could come any day and it came late yesterday. Here is the news release and some background:
1. The US market is one of the largest single markets for medical devices in the world. We estimate that it would take 16,000 VMS+3.0 to deal with all the patients who get echocardiograms per year. This number is growing at 7% per year (1,000 system per year more each year) with an expectation of 25M people over 65 years by 2030.
2. We are now approved to market the VMS+3.0 so we can contact several major centres who have indicated they wanted to know as soon as the VMS+3.0 was clearance for clinical use.
3. The label remains the same that the VMS+3.0 can be used on "any patient where the results are warrants or desired”, which means all the attending doctor only needs is to desire the analysis be done to help with the diagnosis or monitoring.
4. I remind you the VMS+3.0 is a portable device which can be added to any existing 2D ultrasound machine (GE, Phillips, Siemens, Toshiba, Samsung, Mindray…) and be operational in a few minutes. It now allows for patient movement during the exam and has a continuous user guide for the exam as well as the analysis. These features have made it very user friendly.
5. In addition, it is inexpensive to manufacture, which will allow us to offer it as “razor-razor blade” or per use sale to accelerate adoption.
6. FDA clearance helps us worldwide as everyone knows how difficult it is to get FDA approval.
7. This is a major milestone for the company, which has been a long time coming, but has finally arrived. The company is undervalued at $6M market cap. If we were a pharmaceutical company with a FDA-approved orphan drug for a $1B market we would be trading at 10-20 times this market cap. The news today should tell people we are a company with a large market opportunity.
 teq0904
5 years ago
teq0904
5 years ago
Update on HC approval. Mazankowski Hospital study due any day now, and we know that it is going to be positive since they presented at the VPT booth on the weekend.
Attached is a news release announcing the Health Canada approval for the next-generation VMS+3.0. Here is some background:
1. We started to develop the VMS+3.0 18 months ago as soon as the VMS+ was approved based upon the upgrades doctors, sonographers and patients requested.
2. The first step was to invent a completely new tracking system, which we have now filed a patent on and allows for patient movement during the scanning and collection of 2D images. Even though the exam is only 2-3 minuets, patients still found it difficult to remain motionless. Sonographers were also challenged in getting some of the views without repositioning the patient. This is a major upgrade to the usability the system.
3. We also included a step-by-step guide into the exam procedure to allow sonographers to be sure they acquired all the necessary views. This guide also has the recommended location for the anatomical landmarks for the analysis of the views and so again the doctor knows exactly what is required in each view.
4. As we reported earlier, the human factors study showed 100% of clinicians were able to be quickly trained and remembered how to use this new guided process. This will greatly improve the consistency of the results, which up until now were so variable that doctors do not even look back at the previous echocardiogram. They have told us many times, it would be great to be able to compare exams over time.
5. From business perspective, the VMS+3.0 is much cheaper and easier to manufacture. As announced we will be assembling it in house and therefore have cancelled the contract manufacturers for the VMS+.
6. More importantly, the VMS+3.0 is much smaller and so is portable. Our sale people can take it with them on all sales calls, set it up in minutes and allow potential customers to use it at their sites immediately. We will be doing these in Canada starting next week now that we have approval.
7. We also have hospitals and cardiac clinics in Canada waiting to submit the paperwork to acquire the VMS+3.0 and we have already notified them we can now supply the VMS+3.0 immediately. We will be announcing these installations as they happen.
8. Overall, the VMS+3.0 is smaller, easier to use, allows patient movement and promotes consistency. This is the tool everyone has been asking for and we look forward to demonstrating it to cardiac centres in Canada.
 teq0904
5 years ago
teq0904
5 years ago
Dear Friend;
Attached is a news release, which announces our successful Human Factors Study with operators to verify the user-friendly and easy-of-use of the VMS+3.0. Here is some background:
1. Recently, we announced the filing with Health Canada, Europe and US-FDA for approves to market the VMS+3.0. All agencies have accepted our submissions as complete and they are under substantive technical review at this time.
2. As a new part of all medical device submissions is a “Human Factors Study” to verify that the software can be easily operated and understood by users. We conducted a study with clinicians wherein we trained them on the VMS+3.0 and then sent them away. A few days later they returned and were tested to see if they could operate the VMS+3.0 without assistance. We were overjoyed to have 100% success. Everyone easily was able to operate the system.
3. This study proves the the system is easily learned and not easily forgotten. The study participants even commented on how intuitive the system was and how it was obvious to them even in the training how to use the VMS+3.0.
4. The “ease-of-use” part is one feature of the upgraded system along with the guided procedure and no more need to keep the patient motionless. These are the features users of the VMS+2.0 had requested.
5. From a business perspective, the VMS+3.0 is much cheaper and easier to manufacture. As a result, we have established manufacturing for the VMS+3.0 in our headquarters. The lower mill cost will have a significant impact on our margins. The device is much smaller and is now portable. This makes it possible for our sales people to take it with them and do an on-site evaluation. These on-site evaluations are also what potential customers have been asking for. They want to be able to scan a patient and reconstruct a heart before committing to initiate the process to purchase the VMS+3.0
6. We are looking forward to exhibiting at the American Society of Echocardiography next week in Portland, Oregon. This is the largest conference of the year which is dedicated solely to cardiac ultrasound. The Mazankowski Heart Institute has a presentation on the VMS+ at the conference and will be reporting on their study of the reduction or elimination of contrast media in technically-challenging patients, which have “unreadable” conventional 2D echocardiograms. We believe the VMS+ can “read” the “unreadable” studies and there will be no need for contrast media. We await the report of their independent clinical assessment.
 teq0904
5 years ago
teq0904
5 years ago
Today's email Attached is a News Release announcing we have filed in Canada, Europe and USA for approval of the VMS+3.0. Here is some background:
1. These applications document the VMS+3.0, with the new tracking system and updated software, is equivalent to the VMS+. We are not asking for a change in label so from a regulatory standpoint these are relatively minor changes. From a user standpoint the changes, which allow for patient movement, easier placement of the VMS+3.0 within the echo suite and guided exams and analysis, are major advancements.
2. The last time we submitted to these agencies for approval for the VMS+, we received Heath Canada approval in a week, CE mark in 2 weeks and FDA in 6 weeks. That application was a change in labelling (from RV-only to whole heart analysis and some hardware changes). While it is not always possible to predict the behaviour of these agencies, we are hopeful a similar timeline will be achieved.
3. We have also completed a human factors study wherein sonographers were trained on the VMS+3.0 for 3 hours and then tested the next day to to see if they could operate the system correctly. All 13 passed with 100% and commented how intuitively obvious it was to operate the VMS+3.0. More on this in a later news release.
4. Our sales people have been chomping at the bit to get out and sell the VMS+3.0 and have lined up a number of demonstrations, as the new system is portable and can be set up in a few minutes. On-site evaluations are now possible anywhere in the world. We have major sites waiting for the VMS+3.0 to be approved so they can immediately evaluate it and conduct clinical studies to show its value in patient care and cost reductions.
5. With the MAJOR conference of the year the American Society of Echocardiography (ASE) coming up on 3 weeks, this is excellent timing to launch the VMS+3.0, which we demonstrated the prototype at Euroecho and a few regional conferences events already. This will be its first exhibition at an international event specific to ultrasound analysis of the heart.
6. At the ASE, the Edmonton Heart Centre will be presenting the results of their study of the ability of the VMS+ to analyze “unreadable” echo exams of the left ventricle (LV) and avoid the need for injection of contrast media to obtain a result. This will spotlight the VMS+ and its cost reduction potential at the conference. More on this in a later news release.
 teq0904
5 years ago
teq0904
5 years ago
Dear Friend; Attached is a news release. Here is some background:
1. The VMS+ with analysis of all 4 chambers of the heart using 2D or 3D ultrasound was approved a year ago by the US-FDA. Ongoing consultation with the cardiology community identified ways to improve the VMS+ to make it more user-friendly as well as to reduce the cost of production. The result was the development of the VMS+3.0.
2. A key novel component was the creation and patenting of a tracking system based on modern microcircuits and advanced positioning software. This new tracking system was also established to allow patient movement during the exam, which was a major source of error and the most common complaint from sonographers, who usually move the patient to get the best window for scanning. Since the Company now manufactures the new tracking system, the cost is a fraction of the off-the-shelf system we purchased from an outside vendor and used in all previous VMS versions.
3. The Company has completed a usability study with 13 cardiac sonographers and they unanimously found the VMS+3.0 intuitively easy to learn and use. More on this in a future news release.
4. The next step is to complete the submissions to the US-FDA, Health Canada and Europe. These are minor submissions as we are not asking for a change in the label, simply updating them on the change in technology and sending them the data to show equivalency.
5. We anticipate the approvals will take a few weeks and so the sales team are gearing up to close some sales based on the delivery of the VMS+3.0. This is good timing as the MAJOR conference of the year is the Annual Congress of the American Society of Echocardiography and this year it is in Portland, June 21-25. While it is unlikely we would have US-FDA clearance for the ASE, we will likely have Canada and Europe and the congress is attended by cardiologists from all over the world.
6. The new tracking system will make it much easier to integrate the VMS+3.0 into an existing 2D ultrasound device and we are assessing various devices to see which device and company would be the best partner. Again more on this later.
I know it has been a long haul, but making cardiac diagnosis and treatment better for all and especially for children is worth it. I believe the new VMS+3.0 is the clinical tool cardiologists have been waiting for.

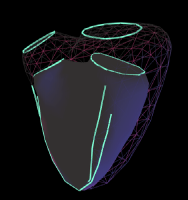
 Ibelieveinmusic
4 months ago
Ibelieveinmusic
4 months ago
 supervalue
2 years ago
supervalue
2 years ago
 supervalue
2 years ago
supervalue
2 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 supervalue
3 years ago
supervalue
3 years ago
 imjake
3 years ago
imjake
3 years ago
 imjake
4 years ago
imjake
4 years ago
 imjake
4 years ago
imjake
4 years ago
 banderas76
4 years ago
banderas76
4 years ago
 imjake
4 years ago
imjake
4 years ago
 banderas76
4 years ago
banderas76
4 years ago
 imjake
4 years ago
imjake
4 years ago
 banderas76
4 years ago
banderas76
4 years ago
 imjake
4 years ago
imjake
4 years ago
 banderas76
4 years ago
banderas76
4 years ago
 imjake
4 years ago
imjake
4 years ago
 imjake
4 years ago
imjake
4 years ago
 teq0904
4 years ago
teq0904
4 years ago
 teq0904
4 years ago
teq0904
4 years ago
 teq0904
4 years ago
teq0904
4 years ago
 teq0904
4 years ago
teq0904
4 years ago
 UCParadise
4 years ago
UCParadise
4 years ago
 teq0904
4 years ago
teq0904
4 years ago
 xwing229
4 years ago
xwing229
4 years ago
 teq0904
4 years ago
teq0904
4 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
 teq0904
5 years ago
teq0904
5 years ago
