KalVista Pharma Hits Targeted Number of Patients in Sebetralstat Trial
November 13 2023 - 7:13AM
Dow Jones News
By Chris Wack
KalVista Pharmaceuticals said Monday it has hit a milestone in
its clinical trial for its lead program sebetralstat, in
development as a potential oral on-demand therapy for hereditary
angioedema.
The clinical-stage pharmaceutical company has achieved the
targeted number of on-treatment attacks required to complete the
phase 3 trial.
The trial is a cross-over study in which patients are intended
to treat a total of three attacks: one each with 300 mg
sebetralstat, 600 mg sebetralstat and placebo, given in a
randomized sequence.
KalVista expects topline data in early 2024, remaining on track
for a New Drug Application submission to the U.S. Food and Drug
Administration in the first half of 2024. The company also expects
to file for approval in the European Union and Japan later in
2024.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
November 13, 2023 06:58 ET (11:58 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
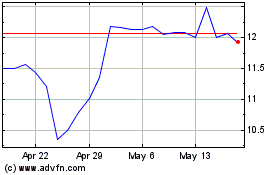
KalVista Pharmaceuticals (NASDAQ:KALV)
Historical Stock Chart
From Apr 2024 to May 2024
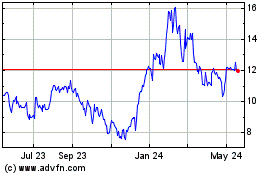
KalVista Pharmaceuticals (NASDAQ:KALV)
Historical Stock Chart
From May 2023 to May 2024