Filed Pursuant to Rule 253(g)(1)
Commission File No. 024-11819
OFFERING CIRCULAR
Bioadaptives, Inc.
200,000,000 Shares of Common Stock
By this Offering Circular, Bioadaptives, Inc., a Delaware corporation, is offering for sale a maximum of 200,000,000 shares of its common stock (the “Offered Shares”), at a fixed price of $0.005 per share, pursuant to Tier 2 of Regulation A of the United States Securities and Exchange Commission (the “SEC”). A minimum purchase of $10,000 of the Offered Shares is required in this offering; any additional purchase must be in an amount of at least $1,000. This offering is being conducted on a best-efforts basis, which means that there is no minimum number of Offered Shares that must be sold by us for this offering to close; thus, we may receive no or minimal proceeds from this offering. All proceeds from this offering will become immediately available to us and may be used as they are accepted. Purchasers of the Offered Shares will not be entitled to a refund and could lose their entire investments. Please see the “Risk Factors” section, beginning on page 4, for a discussion of the risks associated with a purchase of the Offered Shares.
This offering commenced on May 16, 2022; this offering will terminate at the earliest of (a) the date on which the maximum offering has been sold, (b) the date which is one year from this offering being qualified by the SEC or (c) the date on which this offering is earlier terminated by us, in our sole discretion. (See “Plan of Distribution”).
| Title of Securities Offered | | Number of Shares | | | Price to Public | | | Commissions (1) | | Proceeds to Company (2) | |
| Common Stock | | | 200,000,000 | | | $ | 0.005 | | | $ -0- | | $ | 1,000,000 | |
| | (1) | We may offer the Offered Shares through registered broker-dealers and we may pay finders. However, information as to any such broker-dealer or finder shall be disclosed in an amendment to this Offering Circular. |
| | (2) | Does not account for the payment of expenses of this offering estimated at $20,000. See “Plan of Distribution.” |
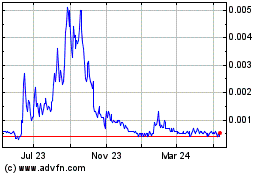
Our common stock is quoted in the over-the-counter under the symbol “BDPT” in the OTC Pink marketplace of OTC Link. On May 13, 2022, the closing price of our common stock was $0.005755 per share.
Investing in the Offered Shares is speculative and involves substantial risks. You should purchase such securities only if you can afford a complete loss of your investment. See Risk Factors, beginning on page 4, for a discussion of certain risks that you should consider before purchasing any of the Offered Shares.
THE SEC DOES NOT PASS UPON THE MERITS OF, OR GIVE ITS APPROVAL TO, ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE SEC. HOWEVER, THE SEC HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
The use of projections or forecasts in this offering is prohibited. No person is permitted to make any oral or written predictions about the benefits you will receive from an investment in Offered Shares.
No sale may be made to you in this offering, if you do not satisfy the investor suitability standards described in this Offering Circular under “Plan of Distribution—State Law Exemption and Offerings to ‘Qualified Purchasers’” (page 14). Before making any representation that you satisfy the established investor suitability standards, we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
This Offering Circular follows the disclosure format of Form S-1, pursuant to the General Instructions of Part II(a)(1)(ii) of Form 1-A.
The date of this Offering Circular is May 16, 2022.
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this Offering Circular includes some statements that are not historical and that are considered forward-looking statements. Such forward-looking statements include, but are not limited to, statements regarding our development plans for our business; our strategies and business outlook; anticipated development of our company; and various other matters (including contingent liabilities and obligations and changes in accounting policies, standards and interpretations). These forward-looking statements express our expectations, hopes, beliefs and intentions regarding the future. In addition, without limiting the foregoing, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words anticipates, believes, continue, could, estimates, expects, intends, may, might, plans, possible, potential, predicts, projects, seeks, should, will, would and similar expressions and variations, or comparable terminology, or the negatives of any of the foregoing, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this Offering Circular are based on current expectations and beliefs concerning future developments that are difficult to predict. We cannot guarantee future performance, or that future developments affecting our company will be as currently anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements.
All forward-looking statements attributable to us are expressly qualified in their entirety by these risks and uncertainties. These risks and uncertainties, along with others, are also described below in the Risk Factors section. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. You should not place undue reliance on any forward-looking statements and should not make an investment decision based solely on these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
OFFERING CIRCULAR SUMMARY
The following summary highlights material information contained in this Offering Circular. This summary does not contain all of the information you should consider before purchasing our common stock. Before making an investment decision, you should read this Offering Circular carefully, including the Risk Factors section and the consolidated financial statements and the notes thereto. Unless otherwise indicated, the terms “the Company,” “Bioadaptives,” “we”, “us” and “our” refer and relate to Bioadaptives, Inc., a Delaware corporation, including its subsidiary corporation, MORO2, Inc., a Nevada corporation.
Our Company
Our company was incorporated under the laws of the State of Delaware on April 19, 2013, as APEX 8 Inc. Our corporate name changed to Bioadaptives, Inc. on September 25, 2013.
We are a research and development company. Our current focus is on products that improve health and wellness. These products include dietary supplements, specialty food and beverage items, and proprietary methods of optimizing the bioelectromagnetic availability of foods and beverages. Our base of intellectual property and products include solutions in the form of devices and nutraceuticals that are designed to aid in cognition, focus, fatigue reduction, weight control, improved overall emotional and physical wellness, healing, and anti-aging. The original products were designed for and tested on humans and we have now found that many of those original products, when optimized for the species, work as well or better for pets such as horses, dogs and cats.
Offering Summary
| Securities Offered | | The Offered Shares, 200,000,000 shares of common stock, are being offered by our company. |
| Offering Price Per Share | | $0.005 per Offered Share. |
| Shares Outstanding Before This Offering | | 67,367,483 shares of common stock issued and outstanding as of the date of this Offering Circular. |
| Shares Outstanding After This Offering | | 267.367.483 shares of common stock issued and outstanding, assuming a maximum offering hereunder. |
| Minimum Number of Shares to Be Sold in This Offering | | None |
| Investor Suitability Standards | | The Offered Shares are being offered and sold only to “qualified purchasers” (as defined in Regulation A under the Securities Act). “Qualified purchasers” include: (a) “accredited investors” under Rule 501(a) of Regulation D and (b) all other investors so long as their investment in the Offered Shares does not represent more than 10% of the greater of their annual income or net worth (for natural persons), or 10% of the greater of annual revenue or net assets at fiscal year-end (for non-natural persons). |
| Market for our Common Stock | | Our common stock is quoted in the over-the-counter market under the symbol “BDPT” in the OTC Pink marketplace of OTC Link. |
| Termination of this Offering | | This offering will terminate at the earliest of (a) the date on which the maximum offering has been sold, (b) the date which is one year from this offering being qualified by the SEC and (c) the date on which this offering is earlier terminated by us, in our sole discretion. (See “Plan of Distribution”). |
| Use of Proceeds | | We will apply the proceeds of this offering for marketing and advertising, inventory, testing and studies, professional and regulatory fees, research and development and working capital. (See “Use of Proceeds”). |
| Risk Factors | | An investment in the Offered Shares involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investments. You should carefully consider the information included in the Risk Factors section of this Offering Circular, as well as the other information contained in this Offering Circular, prior to making an investment decision regarding the Offered Shares. |
| Corporate Information | | Our principal executive offices are located at 2620 Regatta Drive, Suite 102, Las Vegas, Nevada 89128; our telephone number is (702) 659-8829; our corporate website is located at www.bioadaptives.com. No information found on our company’s website is part of this Offering Circular. |
Continuing Reporting Requirements Under Regulation A
We are required to file periodic and other reports with the SEC, pursuant to the requirements of Section 13(a) of the Securities Exchange Act of 1934. Our continuing reporting obligations under Regulation A are deemed to be satisfied, as long as we comply with our Section 13(a) reporting requirements. As a Tier 2 issuer under Regulation A, we will be required to file with the SEC a Form 1-Z (Exit Report Under Regulation A) upon the termination of this offering.
RISK FACTORS
An investment in the Offered Shares involves substantial risks. You should carefully consider the following risk factors, in addition to the other information contained in this Offering Circular before purchasing any of the Offered Shares. The occurrence of any of the following risks might cause you to lose a significant part of your investment. The risks and uncertainties discussed below are not the only ones we face, but do represent those risks and uncertainties that we believe are most significant to our business, operating results, prospects and financial condition. The risk factors discussed below cover not only our current products, product candidates and relationships, but also the risks we expect to encounter when and if we add new product candidates and approved products to our proprietary portfolio, which new products, if added, we expect to be a various stages of pre-clinical and perhaps clinical development.
Risks Associated with the COVID-19 Pandemic
It is possible that the Coronavirus (“COVID-19”) pandemic could cause long-lasting stock market volatility and weakness, as well as long-lasting recessionary effects on the United States and/or global economies. Should the negative economic impact caused by the COVID-19 pandemic result in continuing long-term economic weakness in the United States and/or globally, our ability to expand our business would be severely negatively impacted. It is possible that our company would not be able to sustain during any such long-term economic weakness.
Risks Related to Our Company
There is a substantial doubt about our ability to continue as a going concern. The report of our independent auditors that accompanies our consolidated financial statements includes an explanatory paragraph indicating there is a substantial doubt about our ability to continue as a going concern, citing our need for additional capital for the future planned expansion of our activities and to service our ordinary course activities (which may include servicing of indebtedness). The inclusion of a going concern explanatory paragraph in the report of our independent auditors will make it more difficult for us to secure additional financing or enter into strategic relationships on terms acceptable to us, if at all, and likely will materially and adversely affect the terms of any financing that we might obtain. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty.
We have incurred significant losses in prior periods, and losses in the future could cause the quoted price of our common stock to decline or have a material adverse effect on our financial condition, our ability to pay our debts as they become due, and on our cash flows. To date, we have generated a minimal level of revenues from our operations, and we have incurred significant losses in prior periods. For the years ended December 31, 2021 and 2020, we incurred a net loss of $1,049,948 and $847,244, respectively, and, as of such dates, we had an accumulated deficit of $6,656,109 and $5,606,161, respectively.
Additionally, we had net cash used in operating activities of $158,651 and $106,889 for the years ended December 31, 2021 and 2020, respectively. At December 31, 2021, we had a working capital deficit of $1,152,748 and a shareholders’ deficit of $1,093,039. At December 31, 2020, we had a working capital deficit of $1,378,785 and a shareholders’ deficit of $1,378,785. Any losses in the future could cause the quoted price of our common stock to decline or have a material adverse effect on our financial condition, our ability to pay our debts as they become due, and on our cash flows.
At December 31, 2021, and as of the date of this Offering Circular, we do not have sufficient cash resources or current assets to pay our obligations. This circumstance represents a significant risk to our business and shareholders and results in: (a) making it more difficult for us to satisfy our obligations; (2) impeding us from obtaining additional financing in the future for working capital, capital expenditures and general corporate purposes; and (3) making us more vulnerable to a downturn in our business and limits our flexibility to plan for, or react to, changes in our business.
Our cash expenses are large relative to our cash resources and cash flow. At December 31, 2021, we had $82,936 of cash resources and continue to have limited cash resources available to us. Consequently, we have been required either to sell new shares of our common stock or convertible promissory notes to raise the cash necessary to pay ongoing expenses and to make new investments, which actions could lead to continuing dilution in the interest of our existing shareholders.
We will require additional capital to fund our operations and if we do not obtain additional capital, we may be required to substantially limit our operations. Our business does not presently generate the cash needed to finance our current and anticipated operations and we need to obtain additional financing, including from this offering, to finance our operations, until such time that we are able to conduct profitable revenue-generating activities.
Our quarter-to-quarter performance may vary substantially, and this variance, as well as general market conditions, may cause our stock price to fluctuate greatly and even potentially expose us to litigation. We have been unable to generate significant revenues under our current business plan and we cannot accurately estimate future revenue and operating expenses based on historical performance. Our quarterly operating results may vary significantly based on many factors, including:
| | - | Fluctuating demand for our current and future products; |
| | - | Announcements or implementation by our competitors of new products; |
| | - | Amount and timing of our costs related to our marketing efforts or other initiatives; |
| | - | Timing and amounts relating to the expansion of our operations; |
| | - | Our ability to enter into, renegotiate or renew key agreements; |
| | - | Timing and amounts relating to the expansion of our operations; or |
| | - | Economic conditions specific to our industry, as well as general economic conditions. |
Our current and future expense estimates are based, in large part, on estimates of future revenue, which is difficult to predict. We expect to make significant operating and capital expenditures in connection with the development of our plan of business. We may be unable to, or may elect not to, adjust spending quickly enough to offset any unexpected revenue shortfall. If our increased expenses were not accompanied by increased revenue in the same quarter, our quarterly operating results would be harmed.
There are risks and uncertainties encountered by under-capitalized companies. As an under-capitalized company, we are unable to offer assurance that we will be able to overcome our lack of capital, among other challenges.
We may never earn a profit in future financial periods. Because we lack a successful operating history, we are unable to offer assurance that we will ever earn a profit in future financial periods.
If we are unable to manage future expansion effectively, our business may be adversely impacted. In the future, we may experience rapid growth in our operations, which could place a significant strain on our company’s infrastructure, in general, and our internal controls and other managerial, operating and financial resources, in particular. If we are unable to manage future expansion effectively, our business would be harmed. There is, of course, no assurance that we will enjoy rapid development in our business.
We currently depend on the efforts of our Chief Executive Officer; the loss of this executive officer could disrupt our operations and adversely affect the further development of our business. Our success in establishing implementing our business strategies will depend, primarily, on the continued service of our Chief Executive Officer, Edward E. Jacobs, Jr., MD. The loss of service of Dr. Jacobs, for any reason, could seriously impair our ability to execute our business plan, which could have a materially adverse effect on our business and future results of operations. We have entered into an employment agreement with Dr. Jacobs. (See “Executive Compensation —Employment Agreements”. We have not purchased any key-man life insurance.
If we are unable to recruit and retain key personnel, our business may be harmed. If we are unable to attract and retain key personnel, our business may be harmed. Our failure to enable the effective transfer of knowledge and facilitate smooth transitions with regard to our key employees could adversely affect our long-term strategic planning and execution.
Our business strategies are not based on independent market studies. We have not commissioned any independent market studies with respect to the industries in which we compete or intend to compete. Rather, our plans for implementing our business and achieving profitability are based on the experience, judgment and assumptions of our management. If these assumptions prove to be incorrect, we may not be successful in expanding our business.
Our Board of Directors may change our policies without shareholder approval. Our policies, including any policies with respect to investments, leverage, financing, growth, debt and capitalization, will be determined by our Board of Directors or officers to whom our Board of Directors delegates such authority. Our Board of Directors will also establish the amount of any dividends or other distributions that we may pay to our shareholders. Our Board of Directors or officers to which such decisions are delegated will have the ability to amend or revise these and our other policies at any time without shareholder vote. Accordingly, our shareholders will not be entitled to approve changes in our policies, which policy changes may have a material adverse effect on our financial condition and results of operations.
We are subject to the risks frequently experienced by smaller reporting companies. The likelihood of our success must be considered in light of the risks frequently encountered by smaller reporting companies. These risks include our potential inability to:
| | - | Establish product sales, marketing capabilities and establish and maintain markets for our current and future products; |
| | - | Identify, attract, retain, and motivate qualified personnel; |
| | - | Attract sufficient capital resources to develop our business. |
Our limited operating history makes it difficult for you to evaluate our prospects and future performance. Our business operations have only a limited history upon which an evaluation of our prospects and future performance can be made. Our company’s operations are subject to all business risks associated with development stage enterprises. The likelihood of our company’s success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with the establishment and expansion of a business, operation in a competitive industry and the continued development of advertising and promotions strategies. We believe it is likely that we will continue to sustain losses throughout the next twelve months. We cannot assure you that we will ever operate profitably.
Competition from large, well-financed competitors may hinder development of our existing markets, as well as prevent us from expanding our markets. Each of the industries in which we compete, the nutritional and dietary supplement industries, both for humans and animals, the medical device industry and the beverage industry, are highly competitive, rapidly evolving and subject to constant change. The number of competitors in each of these industries is continually expanding. Substantially all of our potential competitors can be expected to possess:
| | - | greater financial, technical, personnel, promotional and marketing resources; |
| | - | longer operating histories; |
| | - | greater name recognition; and |
| | - | larger consumer bases. |
We face competition in the health food channel from a limited number of large nationally known companies, multi-level marketers, private label brands and many smaller distributors of dietary and nutrition supplements and in the mass-market distribution channel and others. Mass-market chains represent substantial sources of income for our competitors and the mass-market merchants often support their own labels at the expense of other brands. As such, the growth of our brands within food, drug, and general mass-market merchants is highly competitive and uncertain. If we cannot compete effectively, we may not be profitable.
We believe that existing industry competitors are likely to continue to expand their product offerings. Moreover, because there are few, if any, substantial barriers to entry, we expect that new competitors are likely to enter both markets. We cannot be certain that we will be able to compete successfully in this extremely competitive market.
Our business could be affected by changes in governmental regulation. Federal, state and local laws and regulations governing food products and nutritional supplements are broad in scope and are subject to evolving interpretations, which could require us to incur substantial costs associated with compliance. In addition, violations of these laws, actual or alleged, could disrupt the Company’s planned business and adversely affect our financial condition and results of operations. In addition, it is possible that additional or revised Federal, state and local laws and regulations may be enacted in the future governing the mining industry. There can be no assurance that the Company will be able to comply with any such laws and regulations and its failure to do so could significantly harm our business, financial condition and results of operations.
Risks Related to a Purchase of the Offered Shares
We may seek capital that may result in shareholder dilution or that may have rights senior to those of our common stock, including the Offered Shares. From time to time, we may seek to obtain additional capital, either through equity, equity-linked or debt securities. The decision to obtain additional capital will depend on, among other factors, our business plans, operating performance and condition of the capital markets. If we raise additional funds through the issuance of equity, equity-linked or debt securities, those securities may have rights, preferences or privileges senior to the rights of our common stock, which could negatively affect the market price of our common stock or cause our shareholders to experience dilution. (See “Dilution—Investment Dilution”).
We have approximately $462,000 in currently convertible debt instruments, the existence and/or conversion of which could cause a reduction in the market price for our common stock. As of the date of this Offering Circular, we have approximately $462,000 in currently convertible debt instruments, the conversion terms of which require share issuances at below-market prices. All such shares constitute an overhang on the market for our common stock and, if and when issued, will be issued without transfer restrictions, pursuant to certain exemptions from registration, and could reduce prevailing market prices for our common stock. Also, in the future, we may also issue securities in connection with our obtaining needed capital or an acquisition transaction. The amount of shares of our common stock issued in connection with any such transaction could constitute a material portion of our then-outstanding shares of common stock.
Our failure to reserve sufficient shares of common stock could be considered an event of default. We have existing convertible promissory notes with a covenant to reserve sufficient shares of common stock with our transfer agent for the potential conversion of these securities. As of the date of this Offering Circular, the calculated shares issuable under the assumed conversion of the promissory notes is greater than the amount of shares that we have reserved with respect to such convertible promissory notes. As a result, the holders of such convertible promissory notes could declare an event of default and the principal and accrued interest would become immediately due and payable. Additionally, the holders of such convertible promissory notes have additional remedies, including penalties against our company.
Shares eligible for future sale may adversely affect the market. From time to time, certain of our shareholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act, subject to certain limitations. In general, a non-affiliate stockholder who has satisfied a six-month holding period may, under certain circumstances, sell its shares, without limitation. Any substantial sale of our common stock pursuant to Rule 144, pursuant to any resale prospectus or pursuant to this Offering Circular may have a material adverse effect on the market price of our common stock.
We do not intend to pay dividends on our common stock. We intend to retain earnings, if any, to provide funds for the implementation of our business strategy. We do not intend to declare or pay any dividends in the foreseeable future. Therefore, there can be no assurance that holders of our common stock will receive cash, stock or other dividends on their shares of our common stock, until we have funds which our Board of Directors determines can be allocated to dividends.
Our common stock has been, and may in the future be, a “Penny Stock” and subject to specific rules governing its sale to investors. The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to our Common Stock, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a person’s account for transactions in penny stocks; and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person; and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination; and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors sell shares of our common stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
There is limited trading activity in our common stock and there is no assurance that an active market will develop in the future. Although our common stock is currently quoted on the OTC Pink marketplace of OTC Link (an interdealer electronic quotation system operated by OTC Markets Group, Inc.) under the symbol “BDPT”, trading of our common stock may be extremely sporadic. For example, several days may pass before any shares may be traded. As a result, an investor may find it difficult to dispose of, or to obtain accurate quotations of the price of our common stock. There can be no assurance that a more active market for our common stock will develop, or if one should develop, there is no assurance that it will be sustained. This severely limits the liquidity of our common stock, and would likely have a material adverse effect on the market price of our common stock and on our ability to raise additional capital.
The market for our common stock may be volatile; you could lose all or part of your investment in the Offered Shares. The market price of our common stock may fluctuate substantially and will depend on a number of factors many of which are beyond our control and may not be related to our operating performance. These fluctuations could cause you to lose all or part of your investment in the Offered Shares, since you might be unable to sell your Offered Shares at or above the price you pay for the Offered Shares. Factors that could cause fluctuations in the market price of our common stock include, but are not limited to, the following:
| | - | price and volume fluctuations in the overall stock market from time to time; |
| | - | volatility in the market prices and trading volumes of sports-related stocks; |
| | - | changes in operating performance and stock market valuations of other sports-related companies generally, or those in our industry, in particular; |
| | - | sales of shares of our common stock by us or our shareholders; |
| | - | failure of securities analysts to maintain coverage of us, changes in financial estimates by securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors; |
| | - | the financial projections we may provide to the public, any changes in those projections or our failure to meet those projections; |
| | - | announcements by us or our competitors of new products or services; |
| | - | the public’s reaction to our press releases, other public announcements and filings with the SEC; |
| | - | rumors and market speculation involving us or other companies in our industry; |
| | - | actual or anticipated changes in our operating results or fluctuations in our operating results; |
| | - | actual or anticipated developments in our business, our competitors’ businesses or the competitive landscape generally; |
| | - | litigation involving us and/or our industry, or investigations by regulators into our operations or those of our competitors; |
| | - | developments or disputes concerning our intellectual property or other proprietary rights; |
| | - | announced or completed acquisitions of businesses or technologies by us or our competitors; |
| | - | new laws or regulations or new interpretations of existing laws or regulations applicable to our business; |
| | - | changes in accounting standards, policies, guidelines, interpretations or principles; |
| | - | any significant change in our management; and |
| | - | general economic conditions and slow or negative growth of our markets. |
In addition, in the past, following periods of volatility in the overall market and the market price of a particular company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
Compliance with the reporting requirements of federal securities laws can be expensive. We are a public reporting company in the United States, and accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, and the compliance obligations of the Sarbanes-Oxley Act. The costs of preparing and filing annual and quarterly reports and other required information with the SEC, furnishing audited reports to shareholders and preparing any registration statements from time to time, if any, are substantial.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for us to retain or attract qualified officers and directors, which could adversely affect the management of our business and our ability to obtain or retain listing of our common stock. We may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. While certain board and committee requirements may not apply to us as an OTC listed company, we intend to explore voluntarily complying with some of these requirements. We may have difficulty attracting and retaining directors with the requisite qualifications. If we are unable to attract and retain qualified officers and directors, the management of our business and our ability to obtain or retain listing of our shares of common stock on any stock exchange (assuming we elect to seek and are successful in obtaining such listing) could be adversely affected.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or detect fraud, and, consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our common stock. We must maintain effective internal controls to provide reliable financial reports and detect fraud. We have been assessing our internal controls to identify areas that need improvement. We are in the process of implementing changes to internal controls, but have not yet completed implementing these changes. Failure to implement these changes to our internal controls or any others that it identifies as necessary to maintain an effective system of internal controls could harm our operating results and cause investors to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price of our stock.
A material weakness in internal controls may remain undetected for a longer period, because of our exemption from the auditor attestation requirements under Section 404(b) of Sarbanes-Oxley. Our Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Our management’s report was not subject to attestation by our registered public accounting firm, pursuant to rules of the SEC that permit us to provide only management’s attestation with respect thereto. As a result, any material weakness in our internal controls may remain undetected for a longer period.
Our Certificate of Incorporation allows for our board to create new series of preferred stock without further approval by our shareholders, which could adversely affect the rights of the holders of our common stock, including purchasers of the Offered Shares. Our board of directors has the authority to issue shares of our preferred stock, with such relative rights and preferences as the board of directors may determine, without further shareholder approval. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation and the right to receive dividend payments before dividends are distributed to the holders of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing shareholders.
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law (DGCL), which may, unless certain criteria are met, prohibit large shareholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
You will suffer dilution in the net tangible book value of the Offered Shares you purchase in this offering. If you acquire any Offered Shares, you will suffer immediate dilution, due to the lower book value per share of our common stock compared to the purchase price of the Offered Shares in this offering. (See “Dilution”).
Future issuances of debt securities and equity securities could negatively affect the market price of shares of our common stock and, in the case of equity securities, may be dilutive to existing shareholders. In the future, we may issue debt or equity securities or incur other financial obligations, including stock dividends. Upon liquidation, it is possible that holders of our debt securities and other loans and preferred stock would receive a distribution of our available assets before common shareholders. We are not required to offer any such additional debt or equity securities to existing shareholders on a preemptive basis. Therefore, additional common stock issuances, directly or through convertible or exchangeable securities, warrants or options, would dilute the holdings of our existing common shareholders and such issuances, or the perception of such issuances, could reduce the market price of shares of our common stock.
As an issuer of penny stock, the protection provided by the federal securities laws relating to forward-looking statements does not apply to us. Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection, in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
DILUTION
Dilution in net tangible book value per share to purchasers of our common stock in this offering represents the difference between the amount per share paid by purchasers of the Offered Shares in this offering and the pro forma as adjusted net tangible book value per share immediately after completion of this offering. In this offering, dilution is attributable primarily to our relatively low net tangible book value per share.
If you purchase Offered Shares in this offering, your investment will be diluted to the extent of the difference between your purchase price per Offered Share and the net pro forma as adjusted tangible book value per share of our common stock after this offering. Our net tangible book value as of December 31, 2021, was $(1,093,039) or $(0.02) per share.
| Assuming the Sale of 100% of the Offered Shares | | | |
| Offering price per share | | $ | 0.005 | |
| Net tangible book value per share as of December 31, 2021 | | $ | (0.020 | ) |
| Increase in net tangible book value per share after giving effect to this offering | | $ | 0.020 | |
| Pro forma net tangible book value per share as of December 31, 2021 | | $ | (0.000 | ) |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | | $ | 0.005 | |
| | | | | |
| Assuming the Sale of 75% of the Offered Shares | | | | |
| Offering price per share | | $ | 0.005 | |
| Net tangible book value per share as of December 31, 2021 | | $ | (0.02 | ) |
| Increase in net tangible book value per share after giving effect to this offering | | $ | 0.020 | |
| Pro forma net tangible book value per share as of December 31, 2021 | | $ | (0.000 | ) |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | | $ | 0.005 | |
| | | | | |
| Assuming the Sale of 50% of the Offered Shares | | | | |
| Offering price per share | | $ | 0.005 | |
| Net tangible book value per share as of December 31, 2021 | | $ | (0.02 | ) |
| Increase in net tangible book value per share after giving effect to this offering | | $ | 0.020 | |
| Pro forma net tangible book value per share as of December 31, 2021 | | $ | (0.000 | ) |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | | $ | 0.005 | |
| | | | | |
| Assuming the Sale of 25% of the Offered Shares | | | | |
| Offering price per share | | $ | 0.005 | |
| Net tangible book value per share as of December 31, 2021 | | $ | (0.02 | ) |
| Increase in net tangible book value per share after giving effect to this offering | | $ | 0.010 | |
| Pro forma net tangible book value per share as of December 31, 2021 | | $ | (0.010 | ) |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | | $ | 0.015 | |
USE OF PROCEEDS
The table below sets forth the estimated proceeds we would derive from this offering, assuming the sale of 25%, 50%, 75% and 100% of the Offered Shares and assuming the payment of no sales commissions or finder’s fees. There is, of course, no guaranty that we will be successful in selling any of the Offered Shares in this offering.
| | | Assumed Percentage of Offered Shares Sold in This Offering | |
| | | | 25% | | | | 50% | | | | 75% | | | | 100% | |
| Offered Shares sold | | | 50,000,000 | | | | 100,000,000 | | | | 150,000,000 | | | | 200,000,000 | |
| Gross proceeds | | $ | 250,000 | | | $ | 500,000 | | | $ | 750,000 | | | $ | 1,000,000 | |
| Offering expenses | | | 20,000 | | | | 20,000 | | | | 20,000 | | | | 20,000 | |
| Net proceeds | | $ | 230,000 | | | $ | 480,000 | | | $ | 730,000 | | | $ | 980,000 | |
The table below sets forth the manner in which we intend to apply the net proceeds derived by us in this offering, assuming the sale of 25%, 50%, 75% and 100% of the Offered Shares. All amounts set forth below are estimates.
Use of Proceeds for Assumed Percentage of Offered Shares Sold in This Offering
| | | | 25% | | | | 50% | | | | 75% | | | | 100% | |
| | | | | | | | | | | | | | | | | |
| Marketing and Advertising | | $ | 80,500 | | | $ | 168,000 | | | $ | 255,500 | | | $ | 343,000 | |
| Inventory | | | 46,000 | | | | 96,000 | | | | 146,000 | | | | 196,000 | |
| Testing and Studies | | | 23,000 | | | | 48,000 | | | | 73,000 | | | | 98,000 | |
| Professional and Regulatory Fees | | | 23,000 | | | | 48,000 | | | | 73,000 | | | | 98,000 | |
| Research and Development | | | 23,000 | | | | 48,000 | | | | 73,000 | | | | 98,000 | |
| Working Capital | | | 34,500 | | | | 72,000 | | | | 109,500 | | | | 147,000 | |
| TOTAL | | $ | 230,000 | | | | 480,000 | | | $ | 730,000 | | | $ | 980,000 | |
We reserve the right to change the foregoing use of proceeds, should our management believe it to be in the best interest of our company. The allocations of the proceeds of this offering presented above constitute the current estimates of our management and are based on our current plans, assumptions made with respect to the industries in which we currently or, in the future, expect to operate, general economic conditions and our future revenue and expenditure estimates.
Investors are cautioned that expenditures may vary substantially from the estimates presented above. Investors must rely on the judgment of our management, who will have broad discretion regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will depend upon numerous factors, including market conditions, cash generated by our operations (if any), business developments and the rate of our growth. We may find it necessary or advisable to use portions of the proceeds of this offering for other purposes.
In the event we do not obtain the entire offering amount hereunder, we may attempt to obtain additional funds through private offerings of our securities or by borrowing funds. Currently, we do not have any committed sources of financing.
PLAN OF DISTRIBUTION
In General
Our company is offering a maximum of 200,000,000 Offered Shares on a best-efforts basis, at a fixed price of $0.005 per Offered Share; any funds derived from this offering will be immediately available to us for our use. There will be no refunds. This offering will terminate at the earliest of (a) the date on which the maximum offering has been sold, (b) the date which is one year from this offering being qualified by the SEC or (c) the date on which this offering is earlier terminated by us, in our sole discretion.
There is no minimum number of Offered Shares that we are required to sell in this offering. All funds derived by us from this offering will be immediately available for use by us, in accordance with the uses set forth in the Use of Proceeds section of this Offering Circular. No funds will be placed in an escrow account during the offering period and no funds will be returned, once an investor’s subscription agreement has been accepted by us.
We intend to sell the Offered Shares in this offering through the efforts of our Chief Executive Officer, Edward E. Jacobs, Jr., MD. Dr. Jacobs will not receive any compensation for offering or selling the Offered Shares. We believe that Dr. Jacobs is exempt from registration as a broker-dealer under the provisions of Rule 3a4-1 promulgated under the Securities Exchange Act of 1934 (the Exchange Act). In particular, Dr. Jacobs:
| | - | is not subject to a statutory disqualification, as that term is defined in Section 3(a)(39) of the Securities Act; and |
| | - | is not to be compensated in connection with his participation by the payment of commissions or other remuneration based either directly or indirectly on transactions in securities; and |
| | - | is not an associated person of a broker or dealer; and |
| | - | meets the conditions of the following: |
| | - | primarily performs, and will perform at the end of this offering, substantial duties for us or on our behalf otherwise than in connection with transactions in securities; and |
| | - | was not a broker or dealer, or an associated person of a broker or dealer, within the preceding 12 months; and |
| | - | did not participate in selling an offering of securities for any issuer more than once every 12 months other than in reliance on paragraphs (a)(4)(i) or (iii) of Rule 3a4-1 under the Exchange Act. |
As of the date of this Offering Circular, we have not entered into any agreements with selling agents for the sale of the Offered Shares. However, we reserve the right to engage FINRA-member broker-dealers. In the event we engage FINRA-member broker-dealers, we expect to pay sales commissions of up to 8.0% of the gross offering proceeds from their sales of the Offered Shares. In connection with our appointment of a selling broker-dealer, we intend to enter into a standard selling agent agreement with the broker-dealer pursuant to which the broker-dealer would act as our non-exclusive sales agent in consideration of our payment of commissions of up to 8.0% on the sale of Offered Shares effected by the broker-dealer.
Procedures for Subscribing
If you are interested in subscribing for Offered Shares in this offering, please submit a request for information by e-mail to Dr. Jacobs, at: drted@bioadaptives.com; all relevant information will be delivered to you by return e-mail. Thereafter, should you decide to subscribe for Offered Shares, you are required to follow the procedures described in the subscription agreement included in the delivered information, which are:
| | - | Electronically execute and deliver to us a subscription agreement; and |
| | - | Deliver funds directly by check or by wire or electronic funds transfer via ACH to our specified bank account. |
Right to Reject Subscriptions
After we receive your complete, executed subscription agreement and the funds required under the subscription agreement have been transferred to us, we have the right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies from rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions
Conditioned upon our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the Offered Shares subscribed. Once you submit the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All accepted subscription agreements are irrevocable.
This Offering Circular will be furnished to prospective investors upon their request via electronic PDF format and will be available for viewing and download 24 hours per day, 7 days per week on our company’s page on the SEC’s website: www.sec.gov.
An investor will become a shareholder of our company and the Offered Shares will be issued, as of the date of settlement. Settlement will not occur until an investor's funds have cleared and we accept the investor as a shareholder.
By executing the subscription agreement and paying the total purchase price for the Offered Shares subscribed, each investor agrees to accept the terms of the subscription agreement and attests that the investor meets certain minimum financial standards. (See “State Qualification and Investor Suitability Standards” below).
An approved trustee must process and forward to us subscriptions made through IRAs, Keogh plans and 401(k) plans. In the case of investments through IRAs, Keogh plans and 401(k) plans, we will send the confirmation and notice of our acceptance to the trustee.
Minimum Purchase Requirements
You must initially purchase at least $10,000 of the Offered Shares in this offering. If you have satisfied the minimum purchase requirement, any additional purchase must be in an amount of at least $1,000.
State Law Exemption and Offerings to “Qualified Purchasers”
The Offered Shares are being offered and sold only to “qualified purchasers” (as defined in Regulation A under the Securities Act). As a Tier 2 offering pursuant to Regulation A under the Securities Act, this offering will be exempt from state “Blue Sky” law review, subject to certain state filing requirements and anti-fraud provisions, to the extent that the Offered Shares offered hereby are offered and sold only to “qualified purchasers”. “Qualified purchasers” include: (a) “accredited investors” under Rule 501(a) of Regulation D and (b) all other investors, so long as their investment in Offered Shares does not represent more than 10% of the greater of their annual income or net worth (for natural persons), or 10% of the greater of annual revenue or net assets at fiscal year-end (for non-natural persons). Accordingly, we reserve the right to reject any investor’s subscription in whole or in part for any reason, including if we determine, in our sole and absolute discretion, that such investor is not a “qualified purchaser” for purposes of Regulation A. We intend to offer and sell the Offered Shares to qualified purchasers in every state of the United States.
Issuance of Offered Shares
Upon settlement, that is, at such time as an investor’s funds have cleared and we have accepted an investor’s subscription agreement, we will either issue such investor’s purchased Offered Shares in book-entry form or issue a certificate or certificates representing such investor’s purchased Offered Shares.
Transferability of the Offered Shares
The Offered Shares will be generally freely transferable, subject to any restrictions imposed by applicable securities laws or regulations.
Advertising, Sales and Other Promotional Materials
In addition to this Offering Circular, subject to limitations imposed by applicable securities laws, we expect to use additional advertising, sales and other promotional materials in connection with this offering. These materials may include information relating to this offering, articles and publications concerning industries relevant to our business operations or public advertisements and audio-visual materials, in each case only as authorized by us. In addition, the sales material may contain certain quotes from various publications without obtaining the consent of the author or the publication for use of the quoted material in the sales material. Although these materials will not contain information in conflict with the information provided by this Offering Circular and will be prepared with a view to presenting a balanced discussion of risk and reward with respect to the Offered Shares, these materials will not give a complete understanding of our company, this offering or the Offered Shares and are not to be considered part of this Offering Circular. This offering is made only by means of this Offering Circular and prospective investors must read and rely on the information provided in this Offering Circular in connection with their decision to invest in the Offered Shares.
DESCRIPTION OF SECURITIES
General
Our authorized capital stock consists of 750,000,000 shares of common stock, $.0001 par value per share, and 10,000,000 shares of preferred stock, $.0001 par value per share, of which 4,000,0000 shares have been designated “Series A Convertible Preferred Stock” and 6,000,000 shares of which have been designated “Series B Convertible Preferred Stock.”
As of the date of this Offering Circular, there were 67,367,483 shares of our common stock issued and outstanding held by 13,629 holders of record; 1,600,000 shares of our Series A Convertible Preferred Stock issued and outstanding held by three (3) holders of record; no shares of our Series B Convertible Preferred Stock issued and outstanding; and 146,079,867 shares of our common stock reserved for the issuance upon the conversion of outstanding convertible instruments.
Common Stock
The holders of our common stock are entitled to one vote per share on all matters submitted to a vote of the shareholders, including the election of directors. Generally, all matters to be voted on by shareholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of our common stock that are present in person or represented by proxy. Except as otherwise provided by law, amendments to our Certificate of Incorporation generally must be approved by a majority of the votes entitled to be cast by all outstanding shares of our common stock. Our Certificate of Incorporation does not provide for cumulative voting in the election of directors. Holders of our common stock will be entitled to such cash dividends as may be declared from time to time by the Board from funds available. Holders of our common stock have no preemptive rights to purchase shares of our common stock. The issued and outstanding shares of our common stock are not subject to any redemption provisions and are not convertible into any other shares of our capital stock. Upon our liquidation, dissolution or winding up, the holders of our common stock will be entitled to receive pro rata all assets available for distribution to such holders.
Series A Convertible Preferred Stock
The following description of the preferences, rights and limitations associated with our Series A Convertible Preferred Stock (the “Preferred Stock”) is qualified in its entity by the Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock (the “Series A Designation”) that is included as Exhibit 2.4 in the Offering Statement of which this Offering Circular forms a part. Capitalized terms in this section have the same meanings ascribed to them in the Series A Designation.
Purchase or Exchange; Segregation of Preferred Stock and Acquired Assets. The Preferred Stock may be used by the Corporation for the purpose of acquiring tangible and intangible assets from any qualified person (an “Acquired Asset”). The Preferred Stock may also be exchanged with creditors, with each share issued to them for payment of or in exchange for partial or full and complete release of the Corporation’s recorded, acknowledged debt. Such acquisitions and exchanges shall be subject to the requirement that at least 85% of the Preferred Stock shares shall be issued in exchange for the acquisition of assets and no more than 15% shall be exchanged for release of debt. The Preferred Stock shall not be offered or sold for consideration other than an Acquired Asset or recorded, acknowledged debt.
To the extent practicable, the Corporation will establish procedures to segregate each Acquired Asset by a separate Accounting Unit. Nothing in this Certificate of Designation shall prevent the Corporation from commonly managing the Acquired Assets and pooling or combining such for management purposes or for the subsequent sale, lease or license thereof, provided that the Initial Holder’s rights to redeem an Acquired Asset, as set out herein, and the Corporation’ rights to make payment or exchange some other asset or right in exchange for surrender of such right, shall be fixed by this Certificate of Designation.
Purchase of an Acquired Asset by use of Preferred Shares or other consideration (or a combination thereof) and the number of Preferred Shares to be offered therefore shall be subject to the discretion and reasonable judgment of the Board.
Voting Rights. The Preferred Stock shall have voting rights only as follows: (a) Upon conversion into Common Stock, as Common Stock; (b) in the event the Initial Holder sells, assigns or otherwise surrenders ownership of the Preferred Shares to any person not under the control of the Initial Holder, the voting privileges attaching to these Preferred Shares shall be one (1) vote per share; (c) prior to an event described in (a) or (b), in the event the Corporation seeks to sell, lease, mortgage or license an Acquired Asset, the Initial Holder shall have the right to one-hundred (100) votes for each share of Preferred Stock and, if the Acquired Asset is sold, then such voting rights until the Preferred Stock is sold or converted; (d) prior to an event described in (a) or (b), in the event the Corporation requires shareholder approval for (1) a sale, lease, exchange or mortgage of all or substantially all of its assets, or (2) an issuance of shares that would result in a Change of Control Transaction, the Initial Holder shall have the right to one-hundred (100) votes for each share of Preferred Stock.
Dividend Rights. The Company anticipates it will negotiate a royalty payment to the seller that will be paid from revenues generated from sales of products (or product lines) purchased with the Preferred Stock. As a result, the Preferred Stock shall have no dividend rights prior to conversion.
Liquidation. Upon any liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary (a "Liquidation"), the Preferred Stock will be converted into common stock and the Holders shall not be entitled to receive a liquidation preference.
Conversion. Holders of Preferred Stock shall have the following rights with respect to the conversion of the Preferred Stock into shares of Common Stock: each and every share of Preferred Stock may, at the option of the Holder, be converted into five (5) fully paid and non-assessable shares of Common Stock, upon notice to the Corporation. No notice shall be made (or made effective) until six (6) months after the issuance of the Preferred Stock to the Holder except in the event of a Liquidation, whereupon the conversion will occur immediately.
Restrictions and Limitation. In addition to any other rights provided by law, so long as any of the Preferred Stock remains issued and outstanding, the Corporation shall not, without first obtaining the affirmative vote or written consent of the Holder(s): (a) alter, modify, amend or repeal (whether by merger or otherwise) any of the terms of this Certificate of Designation in any way; (b) authorize or issue shares of stock of any class or any bonds, debentures, notes or other obligations convertible into or exchangeable for, or having option rights to purchase, any shares of stock of the Corporation having any preference or priority as to dividends, assets, or other rights; (c) alter or change the preferences, rights, privileges or power of, or the restrictions provided for the benefit of any Preferred Stock to include any preference or priority as to dividends payable from the Series A Royalty Pool, assets, or other right superior to or on a parity with any such preference or priority of the Preferred Stock; (d) re-classify any class or series of any Capital Stock hereafter created junior to the Preferred Stock into shares having any preference or priority as to dividends payable from the Series A Royalty Pool, assets or other right superior to or on a parity with any such preference or priority of the Preferred Stock.
Voting Rights as a Class; Shareholder Consent; Limitations. The voting rights of the Preferred Stock shall be exercised by the class collectively. If there is more than one Holder of the Preferred Stock, then each of them shall have a single vote per share with respect to any matter requiring consent and a majority of votes shall determine whether the Preferred Stock, as a class, will provide any required consent at the request of the directors, such that a shareholder meeting will not be required to effect corporate action. Holders of the Preferred Stock will not be permitted to offer shareholder proposals or resolutions by proxy or otherwise but will be permitted, at their sole option, to appoint a single (1) director to serve the Corporation. Voting rights otherwise granted in the Series A Designation shall be exercised by the Holder individually.
Series B Convertible Preferred Stock
The following description of the preferences, rights and limitations associated with our Series B Convertible Preferred Stock (the “Preferred Stock”) is qualified in its entity by the Certificate of Designation of Preferences, Rights and Limitations of Series B Convertible Preferred Stock (the “Series B Designation”) that is included as Exhibit 2.7 in the Offering Statement of which this Offering Circular forms a part. Capitalized terms in this section have the same meanings ascribed to them in the Series B Designation.
Purchase or Exchange; Segregation of Preferred Stock and Acquired Assets. The Preferred Stock may be used by the Corporation for the purpose of acquiring tangible and intangible assets from any qualified person (an "Acquired Asset"). The Preferred Stock shall not be offered or sold for consideration other than an Acquired Asset. To the extent practicable, the Corporation will establish procedures to segregate each Acquired Asset by a separate Accounting Unit. Nothing in this Certificate of Designation shall prevent the Corporation from commonly managing the Acquired Assets and pooling or combining such for management purposes or for the subsequent sale, lease or license thereof, provided that the Initial Holder's rights to redeem an Acquired Asset, as set out herein, and the Corporation's rights to make payment or exchange some other asset or right in exchange for surrender of such right, shall be fixed by this Certificate of Designation. Purchase of an Acquired Asset by use of Preferred Shares or other consideration (or a combination thereof) and the number of Preferred Shares to be offered therefore shall be subject to the discretion and reasonable judgment of the Board.
Voting Rights. The Series B Convertible Preferred Stock shall have voting rights in or at any meeting of the Shareholders called for a vote, or in any circumstance where consent of the Shareholders is called for. The Holder shall have the right to a number of votes equal to one hundred (100) times the total number of shares of Series B Convertible Preferred Stock Common Stock held at the time of such meeting or solicitation of consent. The right to such number of votes shall not be limited by the total number of common shares issued, outstanding or authorized.
Dividend Rights. The Preferred Stock shall have no dividend rights prior to conversion.
Liquidation. Upon any liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary (a "Liquidation"), the Preferred Stock will be converted in accordance with the ratios set out in Section 6 and the Holders shall not be entitled to receive a liquidation preference.
Conversion. Holders of Preferred Stock shall have the following rights with respect to the conversion of the Preferred Stock into shares of Common Stock: each and every share of Preferred Stock may, at the option of the Holder, be converted into ten (10) fully paid and non-assessable shares of Common Stock, upon notice to the Corporation. No notice shall be made (or made effective) until six (6) months after the issuance of the Preferred Stock to the Holder except in the event of a Liquidation, whereupon the conversion will occur immediately.
Voting Rights as a Class; Shareholder Consent; Limitations. The voting rights of the Preferred Stock shall be exercised by the class collectively. If there is more than one Holder of the Preferred Stock, then each of them shall have a single vote per share with respect to any matter requiring consent and a majority of votes shall determine whether the Preferred Stock, as a class, will provide any required consent at the request of the directors, such that a shareholder meeting will not be required to effect corporate action. Holders of the Preferred Stock will not be permitted to offer shareholder proposals or resolutions by proxy or otherwise but will be permitted, at their sole option, to appoint a single (1) director to serve the Corporation. Voting rights otherwise granted in the Series B Designation shall be exercised by the Holder individually.
Convertible Promissory Notes
As of December 31, 2021, we had outstanding convertible promissory notes in an aggregate principal amount of $416,450. The table below sets forth summary information with respect to such convertible promissory notes.
| Description of Terms | | Principal Balance at 12/31/21 | | | Principal Balance at 12/31/20 | |
| Originated in 2018 During the year ended December 31, 2018, the Company issued a total principal amount of $426,000 in convertible notes for cash proceeds of $426,000. The terms of these convertible notes are summarized, as follows: Term, two years; Annual interest rate, 12%; Convertible at the option of the holders at any time; Conversion prices are based on 50% discount to market value for the common stock based on a 4-week weekly average of the closing price. These notes are in default. | | $ | 311,000 | | | $ | 311,000 | |
| Originated in 2019 During the year ended December 31, 2019, the Company issued a total principal amount of $73,500 in convertible notes for cash proceeds of $67,000. The terms of these convertible notes are summarized, as follows: Term, one year; Annual interest rate, 10%; Convertible at 180 days from issuance; Conversion prices are based on a 42% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. | | | --- | | | | 13,000 | |
| Originated in 2020 During the year ended December 31, 2020, the Company issued a total principal amount of $86,000 in convertible notes for cash proceeds of $80,000. The terms of these convertible notes are summarized, as follows: Term, one year; Annual interest rate, 10%; Convertible at 180 days from issuance; Conversion prices are 39% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. | | | --- | | | | 86,000 | |
| Originated in 2021 During the year ended December 31, 2021, the Company issued a total principal amount of $272,500 in convertible note for cash proceeds of $255,000. The terms of these convertible notes are summarized, as follows: Term, one year; Annual interest rate, 10%; Convertible at 180 days from issuance; Conversion prices are based on 39% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. | | | 105,450 | | | | --- | |
| Total Convertible Notes | | $ | 416,450 | | | $ | 410,000 | |
Dividend Policy
We have never declared or paid any dividends on our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. As a result, we do not anticipate paying any cash dividends in the foreseeable future.
Shareholder Meetings
Our bylaws provide that special meetings of shareholders may be called only by our Board of Directors, the chairman of the board or our president, or as otherwise provided under Delaware law.
Transfer Agent
We have retained the services of Madison Stock Transfer Inc., 2500 Coney Island Avenue, Sub Level, Brooklyn, New York 11223, as the transfer agent for our common stock.
BUSINESS
History
Our company was incorporated under the laws of the State of Delaware on April 19, 2013, as APEX 8 Inc. Our corporate name changed to Bioadaptives, Inc. on September 25, 2013.
We are a research and development company. Our current focus is on products that improve health and wellness. These products include dietary supplements, specialty food items, and proprietary methods of optimizing the bioelectromagnetic availability of foods and beverages. Our base of intellectual property and products include solutions in the form of devices and nutraceuticals that are designed to aid in cognition, focus, fatigue reduction, weight control, improved overall emotional and physical wellness, healing, and anti-aging. The original products were designed for and tested on humans and we have now found that many of those original products, when optimized for the species, work as well or better for pets such as horses, dogs, and cats.
Our principal executive offices are located at 2620 Regatta Drive, Suite 102, Las Vegas, Nevada 89128; our telephone number is (702) 659-8829; our corporate website is located at www.bioadaptives.com. No information found on our company’s website is part of this Offering Circular.
Change in Control
On October 2, 2017, the Board of Directors appointed Kim Southworth and Dr. Edward E. Jacobs, Jr., MD as directors and Barry K. Epling resigned as Chairman. FHI donated 9,628.568 shares of the Company’s common stock to the Breath of Life Foundation, a Section 501(c)(3) non-profit organization. At that time, these shares constituted 51.84% of the Company’s issued and outstanding shares and, as part of the grant, Breath of Life granted the Company’s Board of Directors an irrevocable proxy to vote them. With these actions, Barry K. Epling terminated his relationship with the Company as an owner (direct or indirect), officer or director.
Core Business
BioAdaptives’ core business is to investigate, market and distribute natural plant- and algal-based products that improve health and wellness for humans and animals, with an emphasis on pain relief and anti-aging properties. In connection with this work in nutraceuticals, the Company has also entered into a marketing agreement for an FDA-cleared Class II medical device, the Lung Flute™, and is exploring agreements with another medical device manufacturer; the owners of intellectual property relating to medical devices and processes; and marketing companies associated with these manufacturers and owners.
The Company’s current products include dietary supplements using natural ingredients and proprietary methods of optimizing the availability of nutrients in foods and beverages. The human products are designed to promote lung health, boost immune defense, aid memory, cognition, and focus, assist in sleep and fatigue reduction, provide pain relief and healing, and improve overall emotional and physical wellness. The science behind our human products has also been found to be effective for performance enhancement and pain relief for horses and dogs as well as providing improvements in appearance.
Our current product line for humans includes PrimiCell®, PluriPain®, PrimiLungs™, PrimiLive® and PrimiSleep™. We also market the Lung Flute™ (redesignated as the Lung Cleanser™) and PrimiLungs™ product in our Lung Armor™ packaging, emphasizing the anti-viral properties of the nutraceutical and general respiratory health benefits from use of the device. PrimiLive® is a nootropic formulation that enhances mental clarity and endurance; PrimiSleep™ is a natural soporific that aids relaxation and sleep quality. We acquired the licenses for these products during 2021, and we have begun a marketing campaign for them as well.
Our animal products include Canine Regen® and Equine Regen® for dogs and horses, along with versions of the base formulations, including an Equine All-in-One® formulation evolved from Equine Regen® to trainers, horse owners and boarding stables. Anecdotal and testimonial reports express the opinion that the equine products provide significant relief from lung bleeding during extreme exercise, improved coat and mane appearance, as well as more healthy hoof growth.
While we continue to investigate and acquire nutraceutical products for humans and animals, all of our current activities are reliant on marketing and distributing products developed and owned by others. We do not own the formulations for our key products and manufacture and market them under an agreement with the developer that requires payment of a royalty and license agreements.
We have entered into an exclusive licensing right for a patented oxygenated water that can be utilized for multiple purposes. These include functional beverages to promote healthy activity and enhance sports performance, as a component in topical nutraceuticals and cosmetics, and for a variety of medical conditions that may be improved with more efficient oxygenation. The Company envisions utilizing this oxygenated water in combination with other natural ingredients to form a new class of nutraceuticals with advanced beneficial effects.
We are reliant on direct and indirect sales of the Primi and Pluri lines for humans and the Regen and All-in-One animal products for revenues, neither of which have yet to produce any significant revenue. We have very limited experience in marketing and have yet to develop reliable sales expectations and forecasting.
Market and Marketing
We market our science-based, quality nutraceuticals to a broad base of the population in the U.S., and are exploring marketing prospects in Asia, Australasia, the Middle East, and Europe. The Company’s current target markets also include equine and canine companion animals and equine competitors in the U.S., Australasia, and the Middle East.
During June 2021, we commenced use of social media professionals and existing connections to create awareness about our human products’ benefits. The initial results were not satisfactory and a recast, animal product-specific program that followed later in 2021, similarly did not achieve the results wanted. We are developing affiliate marketing opportunities and recognized that a broader campaign using traditional advertising, advertorials, along with social media, and more contemporary marketing tools is necessary. One of the chief reasons for this offering is to generate sales through improved marketing.
Our basic products have not changed, except for refinements and improvements, and we will continue to emphasize the unique qualities, use and function of our nutraceuticals. We intend to create market share in our target demographic by (i) emphasizing the benefits of our proprietary algal-based, all-natural, stimulant-free, non-GMO ingredients that combine with proven Traditional Chinese Medicine and Ayurvedic botanicals into science-based formulations, (ii) investigating additional products in response to market demand and testing, and (iii) utilizing our marketing operation to act as its sales and distribution arm to seek additional channels for sales coverage.
As noted above, we entered into licensing agreements for the PrimiLive® and Primisleep™ products during 2021. While these products are all-natural botanical formulations, as are our other nutraceutical products, they represent a departure from our existing product lines because they are targeted to specific markets: PrimiLive® is a nootropic, intended to improve concentration and mental acuity; our initial marketing efforts are oriented toward e-gamers. PrimiSleep™ is intended for use as an aid to relaxation, with an emphasis on improving the quality of sleep. We believe these products can be used in a complementary manner, to maintain on-task endurance and then to unwind and recover from such activities.
The Lung Flute™, (redesignated the Lung Cleanser ™) especially when used with PrimiLungs™, shows great promise. The device is an FDA-cleared Class II medical device that employs user-generated low frequency acoustic waves to loosen lung secretions for expulsion. In short, the device helps users clear their lungs. In conjunction with the anti-viral function of PrimiLungs™, the Lung Armor™ package presents great opportunities in view of current fears of a spreading pandemic.
In addition, we are investigating the use of an advanced formulation of PluriPain® targeted toward the symptoms of pre-menstrual syndrome and menstrual pain. Anecdotal and testimonial reports have long noted that users obtain relief from these symptoms with use of the PluriPain® product, and we have made adjustments and additions to the formulation to address these symptoms. Early reports regarding the use of Pluripain® for these symptoms are promising, and we expect marketing efforts to emphasize the benefit of this new product.
With regard to animal products, in 2020, the Company formed the Livestock Impact Division. President of the Division is Bruce Colclasure, a National Cutting Horse Association champion who owns and operates the Flying C Bar Ranch and is the breeder and trainer of over 80 NCHA champion cutting horses. Mr. Colclasure uses and endorses our Equine All-in-One®, Equine Regen® and Equine Regen® Plus products and provides valuable feedback and testimonials regarding its function.
In light of the short fall of our social media campaigns, in 2021, we commenced a marketing affiliate outreach program, directly contacting the principals of horse clubs and associations, offering discounts, samples and other inducements, seeking to develop “product champion” and “maven” relationships. We have contacted principals in organizations with thousands of members and many more thousands of horses. We have distributed samples and marketing materials to approximately forty principals and influencers in regional and breed-specific equine associations and anticipate useful feedback from these marketing initiatives in 1Q and 2Q 2022. Our expectation is that use of the samples by these principals will demonstrate improvements in appearance and performance and that they will, in turn, recommend the product to their members. We have delayed contracting for print and on-line ads with certain of these organizations, as well as traditional magazine and other print placements, pending the results of our sample program, which we expect to learn in 1Q and 2Q 2022.
The Company believes that the population growth in the seasoned and geriatric demographic cohort presents a unique opportunity. The World Health Organization has stated that the 60 years and over population segment will more than double from 11% to over 22% between 2000 and 2050, with the absolute number of people aged sixty and over expected to increase to 2 billion within the same period. The Company also recognizes the rising buying power and interests of the Millennials in wellness products and their choice of communication medium being social media and internet. It intends to establish a major focus to capture the anticipated growth in this sector. We believe that our social media promotion program will eventually gain traction and generate revenues, and are actively seeking a workable, efficient formula to grow consumer engagement and product sales.
The Company believes that international sales represent a significant future growth opportunity as aging population growth outside North America exceeds 1 billion people. The Company plans to aggressively pursue international sales by adding salespeople to its marketing effort, including use of social media influencers, developing a network in high-growth APAC regions, and continuing its efforts to register products and trademarks in attractive foreign markets.
Manufacturing
All of the Company’s nutraceutical products are considered dietary supplements or natural foods, and we carefully avoid making health, drug or disease cure claims that could trigger regulatory compliance issues and affect our ability to market BioAdaptives products. Our active ingredients are all plant- or algal-based and sourced worldwide from reputable suppliers who employ stringent compliance and sustainable agriculture practices or operate gmp facilities.
We contract with manufacturers that utilize pharmaceutical grade facilities to assemble and package our products, all of which is subject to our inspection and approval. Fulfillment of retail internet and direct-to-reseller orders are conducted from our warehouse facilities. BioAdaptives actively investigates new products, techniques and novel applications of existing products or technology in our research. The Company’s research work has centered on investigations of all-natural supplement formulations that activate primitive cells, including stem cells and their derivatives, and natural ingredients that encourage stem cell proliferation.
With regard to medical devices, we purchase the Lung Flute™ from a company affiliated with the patent holder. We do not expect to develop any direct capability to manufacture medical devices for numerous reasons, including a lack of capital and the fact that amortized cost of such facilities, if we were to construct or acquire them, is generally far higher compared to the cost of purchasing a finished product.
With the new agreement to pursue the Oxygenated water technology, the Company has now also extended into the beverage arena. We anticipate intend to be marketing this, and other infused beverages, with the oxygenated technology to the hospitality industry and also via retail stores and our own direct internet sales.
Competition
Each of the industries in which we compete, the nutritional and dietary supplement industries, both for humans and animals, the medical device industry and the beverage industry, are highly competitive, rapidly evolving and subject to constant change. The number of competitors in each of these industries is continually expanding. Substantially all of our potential competitors can be expected to possess:
| | - | greater financial, technical, personnel, promotional and marketing resources; |
| | - | longer operating histories; |
| | - | greater name recognition; and |
| | - | larger consumer bases. |
We face competition in the health food channel from a limited number of large nationally known companies, multi-level marketers, private label brands and many smaller distributors of dietary and nutrition supplements and in the mass-market distribution channel and others. Mass-market chains represent substantial sources of income for our competitors and the mass-market merchants often support their own labels at the expense of other brands. As such, the growth of our brands within food, drug, and general mass-market merchants is highly competitive and uncertain. If we cannot compete effectively, we may not be profitable.
We believe that existing industry competitors are likely to continue to expand their product offerings. Moreover, because there are few, if any, substantial barriers to entry, we expect that new competitors are likely to enter both markets. We cannot be certain that we will be able to compete successfully in this extremely competitive market.
Governmental Regulation
Federal, state and local laws and regulations governing food products and nutritional supplements are broad in scope and are subject to evolving interpretations, which could require us to incur substantial costs associated with compliance. In addition, violations of these laws, actual or alleged, could disrupt the Company’s planned business and adversely affect our financial condition and results of operations. In addition, it is possible that additional or revised Federal, state and local laws and regulations may be enacted in the future governing the mining industry. There can be no assurance that the Company will be able to comply with any such laws and regulations and its failure to do so could significantly harm our business, financial condition and results of operations.
Properties
The Company currently maintains a physical address at 2620 Regatta Dr, Suite 102, Las Vegas, Nevada 89128, and mailing address at its contracted warehouse/fulfillment facility at 4385 Cameron Street, Suite B, Las Vegas, Nevada 89103. The Company does not currently maintain any other office facilities and does not anticipate the need for maintaining office facilities at any time in the foreseeable future.
Employees
Currently, we have seven employees, including our executive officers, of which three are full-time employees, and four of which are part-time employees. Our employees are not represented by any labor union or any collective bargaining arrangement with respect to their employment with us. We have never experienced any work stoppages or strikes as a result of labor disputes. We believe that our employee relations are good.
MANAGEMENTS DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Cautionary Statement
The following discussion and analysis should be read in conjunction with our financial statements and related notes, beginning on page F-1 of this Offering Circular.
Our actual results may differ materially from those anticipated in the following discussion, as a result of a variety of risks and uncertainties, including those described under Cautionary Statement Regarding Forward-Looking Statements and Risk Factors. We assume no obligation to update any of the forward-looking statements included herein.
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the JOBS Act). As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| | · | Only two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly reduced Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure. |
| | · | Reduced disclosure about our executive compensation arrangements. |
| | · | Not having to obtain non-binding advisory votes on executive compensation or golden parachute arrangements. |
| | · | Exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.07 billion in annual revenue, we have more than $700 million in market value of our stock held by non-affiliates, or we issue more than $1 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced burdens. We have taken advantage of these reduced reporting burdens herein, and the information that we provide may be different than what you might get from other public companies in which you hold stock.
Results of Operations
Year Ended December 31, 2021, compared to the Year Ended December 31, 2020. We had a net loss of $(1,049,948) for the year ended December 31, 2021, which was $202,704 more than the net loss of $(847,244) for the year ended December 31, 2020. The change in our results over the two periods is primarily due to an increase in operating and other expenses and a positive adjustment to the value of our derivative liabilities.
In conjunction with our company’s business plan, we have expended considerable effort and financial resources to the implementation of our business plan. During the year ended December 31, 2021, we used cash in our operations of $158,651 compared to the year ended December 31, 2020, when we used cash in our operations of $106,889. During both years, the use of cash in our operations was principally funded through convertible debt as disclosed in the accompanying financial statement footnotes and advances from shareholders.
The following table summarizes key items of comparison and their related increase (decrease) for the year ended December 31, 2021 and 2020:
| | | 2021 | | | 2020 | | | Changes | |
| Revenue | | $ | 19,776 | | | $ | 16,327 | | | $ | 3,449 | |
| Cost of Revenue | | | 9,671 | | | | 10,232 | | | | (561 | ) |
| Operating Expenses | | | 646,186 | | | | 260,421 | | | | 385,765 | |
| Other income (expenses) | | | (413,867 | ) | | | (592,918 | ) | | | (179,051 | ) |
| Net Income (loss) | | | (1,049,948 | ) | | | (847,244 | ) | | | 202,704 | |
Revenue. Our revenues have been derived entirely from product sales.
Cost of Sales. Our cost of sales is primarily derived from contract manufacturing expenses and shipping and handling expenses related to customer fulfillment. We also expense small amounts for marketing expenses, which includes the cost of samples or products provided for promotional purposes and website content development.
Operating Expenses. Our general, administrative and professional fees are largely attributable to office, rent, advertising, consultants and transfer agent, legal, accounting and audit fees related to our reporting requirements as a public company as well as stock-based compensation for officers, directors and consultants.
Other Income (Expense). We reported an unrealized loss on marketable securities of $(254) and $(328) for the years ended December 31, 2021 and 2020, respectively. We reported interest expense of $(272,394) and $(230,328) for the years ended December 31, 2021, and 2020, respectively. We reported change in fair value of derivative liabilities of $(90,219) and $(362,262) for the years ended December 31, 2021, and 2020, respectively. We reported loss on settlement of $(51,000) and $-0- for the years ended December 31, 2021, and 2020, respectively.
Net Loss. As a result of our operating expenses, we reported a net loss of ($1,049,948) and ($847,244) for the years ended December 31, September 2021 and 2020, respectively.
Earnings (Loss) Per Share. Earnings (Loss) per share for the years ended December 31, 2021 and 2020, were $(0.03) and $(0.04), respectively, based on the weighted-average shares issued and outstanding at the end of each respective period.
We anticipate that future expenditure levels will remain relatively consistent until such time that we fully implement our current business plan, at which time our expenses and working capital requirements may increase significantly. We do not expect to generate any meaningful revenue or incur operating expenses for purposes other than fulfilling the obligations of a reporting company under the Exchange Act, unless and until such time that our company begins meaningful operations.
Plan of Operation
In General. We believe that the proceeds of this offering will satisfy our cash requirements for at least the next twelve months.
Subject to financing and various regulatory approvals, we intends to (1) market our existing products as described below, (2) continue research and investigation activities to identify new products or markets and improve our existing products and marketing strategies; and (3) seek strategic or complimentary acquisitions in our current market space or, if indicated, others.
Core Business. BioAdaptives’ core business is to investigate, market and distribute natural plant- and algal-based products that improve health and wellness for humans and animals, with an emphasis on pain relief and anti-aging properties. In connection with this work in nutraceuticals, the Company has also entered into a marketing agreement for an FDA-cleared Class II medical device, the Lung Flute™, and is exploring agreements with another medical device manufacturer; the owners of intellectual property relating to medical devices and processes; and marketing companies associated with these manufacturers and owners.
The Company’s current products include dietary supplements using natural ingredients and proprietary methods of optimizing the availability of nutrients in foods and beverages. The human products are designed to promote lung health, boost immune defense, aid memory, cognition, and focus, assist in sleep and fatigue reduction, provide pain relief and healing, and improve overall emotional and physical wellness. The science behind our human products has also been found to be effective for performance enhancement and pain relief for horses and dogs as well as providing improvements in appearance.
Our current product line for humans includes PrimiCell®, PluriPain®, PrimiLungs™, PrimiLive® and PrimiSleep™. We also market the Lung Flute™ (redesignated as the Lung Cleanser™) and PrimiLungs™ product in our Lung Armor™ packaging, emphasizing the anti-viral properties of the nutraceutical and general respiratory health benefits from use of the device. PrimiLive® is a nootropic formulation that enhances mental clarity and endurance; PrimiSleep™ is a natural soporific that aids relaxation and sleep quality. We acquired the licenses for these products during 2021, and we have begun a marketing campaign for them as well.
Our animal products include Canine Regen® and Equine Regen® for dogs and horses, along with versions of the base formulations, including an Equine All-in-One® formulation evolved from Equine Regen® to trainers, horse owners and boarding stables. Anecdotal and testimonial reports express the opinion that the equine products provide significant relief from lung bleeding during extreme exercise, improved coat and mane appearance, as well as more healthy hoof growth.
While we continue to investigate and acquire nutraceutical products for humans and animals, all of our current activities are reliant on marketing and distributing products developed and owned by others. We do not own the formulations for our key products and manufacture and market them under an agreement with the developer that requires payment of a royalty and license agreements.
We have entered into an exclusive licensing right for a patented oxygenated water that can be utilized for multiple purposes. These include functional beverages to promote healthy activity and enhance sports performance, as a component in topical nutraceuticals and cosmetics, and for a variety of medical conditions that may be improved with more efficient oxygenation. The Company envisions utilizing this oxygenated water in combination with other natural ingredients to form a new class of nutraceuticals with advanced beneficial effects.
We are reliant on direct and indirect sales of the Primi and Pluri lines for humans and the Regen and All-in-One animal products for revenues, neither of which have yet to produce any significant revenue. We have very limited experience in marketing and have yet to develop reliable sales expectations and forecasting.
Market and Marketing. We market our science-based, quality nutraceuticals to a broad base of the population in the U.S., and are exploring marketing prospects in Asia, Australasia, the Middle East, and Europe. The Company’s current target markets also include equine and canine companion animals and equine competitors in the U.S., Australasia, and the Middle East.
During June 2021, we commenced use of social media professionals and existing connections to create awareness about our human products’ benefits. The initial results were not satisfactory and a recast, animal product-specific program that followed later in 2021, similarly did not achieve the results wanted. We are developing affiliate marketing opportunities and recognized that a broader campaign using traditional advertising, advertorials, along with social media, and more contemporary marketing tools is necessary. One of the chief reasons for this offering is to generate sales through improved marketing.
Our basic products have not changed, except for refinements and improvements, and we will continue to emphasize the unique qualities, use and function of our nutraceuticals. We intend to create market share in our target demographic by (i) emphasizing the benefits of our proprietary algal-based, all-natural, stimulant-free, non-GMO ingredients that combine with proven Traditional Chinese Medicine and Ayurvedic botanicals into science-based formulations, (ii) investigating additional products in response to market demand and testing, and (iii) utilizing our marketing operation to act as its sales and distribution arm to seek additional channels for sales coverage.
As noted above, we entered into licensing agreements for the PrimiLive® and PrimiSleep™ products during 2021. While these products are all-natural botanical formulations, as are our other nutraceutical products, they represent a departure from our existing product lines because they are targeted to specific markets: PrimiLive® is a nootropic, intended to improve concentration and mental acuity; our initial marketing efforts are oriented toward e-gamers. PrimiSleep™ is intended for use as an aid to relaxation, with an emphasis on improving the quality of sleep. We believe these products can be used in a complementary manner, to maintain on-task endurance and then to unwind and recover from such activities.
The Lung Flute™, (redesignated the Lung Cleanser ™) especially when used with PrimiLungs™, shows great promise. The device is an FDA-cleared Class II medical device that employs user-generated low frequency acoustic waves to loosen lung secretions for expulsion. In short, the device helps users clear their lungs. In conjunction with the anti-viral function of PrimiLungs™, the Lung Armor™ package presents great opportunities in view of current fears of a spreading pandemic.
In addition, we are investigating the use of an advanced formulation of PluriPain® targeted toward the symptoms of pre-menstrual syndrome and menstrual pain. Anecdotal and testimonial reports have long noted that users obtain relief from these symptoms with use of the PluriPain® product, and we have made adjustments and additions to the formulation to address these symptoms. Early reports regarding the use of Pluripain® for these symptoms are promising, and we expect marketing efforts to emphasize the benefit of this new product.
With regard to animal products, in 2020, the Company formed the Livestock Impact Division. President of the Division is Bruce Colclasure, a National Cutting Horse Association champion who owns and operates the Flying C Bar Ranch and is the breeder and trainer of over 80 NCHA champion cutting horses. Mr. Colclasure uses and endorses our Equine All-in-One®, Equine Regen® and Equine Regen® Plus products and provides valuable feedback and testimonials regarding its function.
In light of the short fall of our social media campaigns, in 2021, we commenced a marketing affiliate outreach program, directly contacting the principals of horse clubs and associations, offering discounts, samples and other inducements, seeking to develop “product champion” and “maven” relationships. We have contacted principals in organizations with thousands of members and many more thousands of horses. We have distributed samples and marketing materials to approximately forty principals and influencers in regional and breed-specific equine associations and anticipate useful feedback from these marketing initiatives in 1Q and 2Q 2022. Our expectation is that use of the samples by these principals will demonstrate improvements in appearance and performance and that they will, in turn, recommend the product to their members. We have delayed contracting for print and on-line ads with certain of these organizations, as well as traditional magazine and other print placements, pending the results of our sample program, which we expect to learn in 1Q and 2Q 2022.
The Company believes that the population growth in the seasoned and geriatric demographic cohort presents a unique opportunity. The World Health Organization has stated that the 60 years and over population segment will more than double from 11% to over 22% between 2000 and 2050, with the absolute number of people aged sixty and over expected to increase to 2 billion within the same period. The Company also recognizes the rising buying power and interests of the Millennials in wellness products and their choice of communication medium being social media and internet. It intends to establish a major focus to capture the anticipated growth in this sector. We believe that our social media promotion program will eventually gain traction and generate revenues, and are actively seeking a workable, efficient formula to grow consumer engagement and product sales.
The Company believes that international sales represent a significant future growth opportunity as aging population growth outside North America exceeds 1 billion people. The Company plans to aggressively pursue international sales by adding salespeople to its marketing effort, including use of social media influencers, developing a network in high-growth APAC regions, and continuing its efforts to register products and trademarks in attractive foreign markets.
Manufacturing. All of the Company’s nutraceutical products are considered dietary supplements or natural foods, and we carefully avoid making health, drug or disease cure claims that could trigger regulatory compliance issues and affect our ability to market BioAdaptives products. Our active ingredients are all plant- or algal-based and sourced worldwide from reputable suppliers who employ stringent compliance and sustainable agriculture practices or operate gmp facilities.
We contract with manufacturers that utilize pharmaceutical grade facilities to assemble and package our products, all of which is subject to our inspection and approval. Fulfillment of retail internet and direct-to-reseller orders are conducted from our warehouse facilities. BioAdaptives actively investigates new products, techniques and novel applications of existing products or technology in our research. The Company’s research work has centered on investigations of all-natural supplement formulations that activate primitive cells, including stem cells and their derivatives, and natural ingredients that encourage stem cell proliferation.
With regard to medical devices, we purchase the Lung Flute™ from a company affiliated with the patent holder. We do not expect to develop any direct capability to manufacture medical devices for numerous reasons, including a lack of capital and the fact that amortized cost of such facilities, if we were to construct or acquire them, is generally far higher compared to the cost of purchasing a finished product.
With the new agreement to pursue the Oxygenated water technology, the Company has now also extended into the beverage arena. We anticipate intend to be marketing this, and other infused beverages, with the oxygenated technology to the hospitality industry and also via retail stores and our own direct internet sales.
Liquidity and Capital Resources
At December 31, 2021, we had $82,936 in cash and a working capital deficit of $1,152,748 compared to $4,587 in cash and a working capital deficit of $1,378,785 at December 31, 2020.
We must obtain additional capital, including in this offering, in order to implement our full business plan. There is no assurance that we will ever possess sufficient capital with which to implement our full business plan. (See “Risk Factors—Risks Related to Our Company”).
While it is the belief of our management that our current finance partners and shareholders will be able provide sufficient working capital necessary to support and preserve the integrity of the corporate entity, there is no assurance that such will be the case. In addition, our company is subject to future economic trends and the growth of our business operations, if any, for our majority stockholder to have the resources available to support our company. Should such financing not be obtained, we have no other ready source of capital. Consequently, there is substantial doubt about our ability to continue as a going concern.
Regardless of whether our company’s cash assets prove to be adequate our operational needs, we might seek to compensate service providers through issuances of our common stock in lieu of cash payments.
Convertible Promissory Notes
As of December 31, 2021, we had outstanding convertible promissory notes in an aggregate principal amount of $416.450. The table below sets forth summary information with respect to such convertible promissory notes.
| Description of Terms | | Principal Balance at 9/30/21 | | | Principal Balance at 12/31/20 | |
| Originated in 2018 During the year ended December 31, 2018, the Company issued a total principal amount of $426,000 in convertible notes for cash proceeds of $426,000. The terms of these convertible notes are summarized, as follows: Term, two years; Annual interest rate, 12%; Convertible at the option of the holders at any time; Conversion prices are based on 50% discount to market value for the common stock based on a 4-week weekly average of the closing price. These notes are in default. | | $ | 311,000 | | | $ | 311,000 | |
| Originated in 2019 During the year ended December 31, 2019, the Company issued a total principal amount of $73,500 in convertible notes for cash proceeds of $67,000. The terms of these convertible notes are summarized, as follows: Term, one year; Annual interest rate, 10%; Convertible at 180 days from issuance; Conversion prices are based on a 42% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. | | | --- | | | | 13,000 | |
| Originated in 2020 During the year ended December 31, 2020, the Company issued a total principal amount of $86,000 in convertible note for cash proceeds of $80,000. The terms of these convertible notes are summarized, as follows: Term, one year; Annual interest rate, 10%; Convertible at 180 days from issuance; Conversion prices are 39% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. | | | --- | | | | 86,000 | |
| Originated in 2021 During the nine months ended December 31, 2021, the Company issued a total principal amount of $222,500 in convertible note for cash proceeds of $205,000. The terms of these convertible notes are summarized, as follows: Term, one year; Annual interest rate, 10%; Convertible at 180 days from issuance; Conversion prices are based on 39% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. | | | 105,450 | | | | --- | |
| Total Convertible Notes | | $ | 416,450 | | | $ | 410,000 | |
Critical Accounting Policies
Our financial statements and related public financial information are based on the application of accounting principles generally accepted in the United States (“GAAP”). GAAP requires the use of estimates; assumptions, judgments and subjective interpretations of accounting principles that have an impact on the assets, liabilities, revenue and expense amounts reported. These estimates can also affect supplemental information contained in our external disclosures including information regarding contingencies, risk and financial condition. We believe our use of estimates and underlying accounting assumptions adhere to GAAP and are consistently and conservatively applied. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ materially from these estimates under different assumptions or conditions. We continue to monitor significant estimates made during the preparation of our financial statements.
Our significant accounting policies are summarized in Note 2 of our financial statements, which begin on page F-1 of this Offering Circular. While all these significant accounting policies impact our financial condition and results of operations, we view certain of these policies as critical. Policies determined to be critical are those policies that have the most significant impact on our financial statements and require management to use a greater degree of judgment and estimates. Actual results may differ from those estimates. Our management believes that given current facts and circumstances, it is unlikely that applying any other reasonable judgments or estimate methodologies would cause effect on our results of operations, financial position or liquidity for the periods presented in this report.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
The following table sets forth the names and ages of our company’s current directors and executive officers.
| Name | | Age | | Position(s) |
| Edward E. Jacobs, Jr., MD | | 80 | | Director and Chief Executive Officer |
| Robert W. Ellis | | 79 | | Director and President |
| Charles Townsend | | 75 | | Director, Chief Operating Officer and Chief Financial Officer |
Our Directors serve until the earlier occurrence of the election of his successor at the next meeting of shareholders, death, resignation or removal by the Board of Directors. Officers serve at the discretion of our Board of Directors. There are no family relationships between or among our directors and executive officers.
Certain information regarding the backgrounds of each of our officers and directors is set forth below.
Edward E. Jacobs, Jr., Director and Chief Executive Officer. Dr. Edward E. Jacobs, Jr., a graduate of Princeton University and Harvard Medical school, is a biotechnology consultant with over 25 years’ experience in biopharmaceutical and medical device development, as well as, 35 years of teaching and direct patient care. Dr. Jacobs has participated in drug development process from discovery through animal and human studies, including regulatory support for FDA and international regulatory affairs, strategic planning, and investor relations.
Dr. Jacobs has extensive clinical operations experience, having conducted, as chief clinical investigator, more than 15 human trials in the US, Europe, Eastern Europe, and the Republic of South Africa. He has also served as a medical monitor and liaison for clinical investigators involved with international trials with responsibility for regulatory compliance.
After completing surgical training at Harvard and research positions at the National Heart, Lung and Blood Institute, Bethesda, Maryland, and at Saint Thomas’ Hospital Medical School, London, Dr. Jacobs was a member of the Harvard Medical School staff, combining teaching with clinical practice and research. He has also served on the Scientific Advisory Board of the Armenise-Harvard Foundation and the Publications Committee for the New England Journal of Medicine
Scientifically, Dr. Jacobs has made original observations in the field of tissue oxygenation therapy and water modification. His current focus is on natural products for animal and human use, anti-ageing strategy, and primitive cell biology.
Dr. Jacobs is an author of more than 40 scientific publications and is the holder of four patents.
Robert W. Ellis, Director and President. Robert Ellis brings more than forty years of business management experience to the Company. He has worked extensively in senior positions across a variety of industry disciplines, including aerospace, electronics, communications, and international marketing. Mr. Ellis has been an executive officer in private and public companies, both major entities, start-ups, and emerging entities. He has an Accounting Degree from the University of Illinois and is a CPA.
Charles Townsend, Director, Chief Operating Officer and Chief Financial Officer. Mr. Townsend brings over 30 years of experience in operations and enterprise marketing to the BioAdaptives team. Mr. Townsend has a Bachelor of Arts degree in accounting from the University of Texas and an MBA from the University of North Texas. He served as an officer in the U.S. Navy Submarine Force prior to private business.
Mr. Townsend has been cost accounting manager, comptroller, and Chief Financial Officer for several private and public companies, including NYSE and OTC companies. Mr. Townsend has been personally responsible for growing and servicing corporate and individual customer bases for software companies in the telecommunications, home health care and security industries, as well as developing investment banking relationships with institutional finance partners for purposes of acquisitions, mergers and corporate funding requirements. Most recently, Mr. Townsend served as a Census Field Manager for the US Department of Commerce 2020 national census.
Advisory Board
The Company uses an Advisory Board to assist the officers and directors with regard to specific scientific and administrative matters. These advisors did not meet during the period ended December 31, 2020, and we did not call on them due to a lack of need.
The following table sets forth the names and ages of the current members of our Advisory Board.
| Name | | Age | | Position(s) |
| Dr. Jun Gu, M.D., Ph.D. | | 56 | | Medical Advisor |
| Dr. Antonina Nabokova, M.D. | | 58 | | Medical Advisor |
Dr. Jun Gu has extensive experience in laboratory toxicology work and is frequently called upon as an expert in toxicology and pharmacology. He received his medical degree (M.D.) in 1986 from the Second Military Medicine University in Shanghai, China, and a Ph.D. in pharmacology from Shanghai Medical College at Fudan University in Shanghai, China in 1993.
Dr. Antonina Nabokova M.D has over 11 years’ experience in clinical trials and managing development of clinical operations in areas such as psychiatry, orphan indication, and urology. Dr. Nabokova currently maintains an office as the head of Representative office of SynteractHCR Deutschland GmbH, in Moscow, Russia. She holds an M.D. from Leningrad Medical University and also obtained a certificate in cardiology from Leningrad Medical University.
Conflicts of Interest
Certain of our officers and directors are engaged in other businesses, either individually or through partnerships and corporations in which they have an interest, hold an office or serve on a board of directors. As a result, certain conflicts of interest may arise between our company and these officers and directors. We will attempt to resolve such conflicts of interest in favor of our company. Our officers and directors are accountable to us and our shareholders as fiduciaries, which requires that these officers and directors exercise good faith and integrity in handling our company’s affairs. A shareholder may be able to institute legal action on behalf of our company or on behalf of itself and other similarly situated shareholders to recover damages or for other relief in cases of the resolution of conflicts is in any manner prejudicial to us.
Corporate Governance
We do not have a separate Compensation Committee, Audit Committee or Nominating Committee. These functions are conducted by our Board of Directors. During the year ended December 31, 2021, our Board of Directors held no meetings and took action by written consent in lieu of a meeting on three occasions.
Independence of Board of Directors
None of our directors is independent, within the meaning of definitions established by the SEC or any self-regulatory organization. We are not currently subject to any law, rule or regulation requiring that all or any portion of our Board of Directors include independent directors.
Shareholder Communications with Our Board of Directors
Our company welcomes comments and questions from our shareholders. Shareholders should direct all communications to our Chief Executive Officer, Edward E. Jacobs, Jr,, MD, at our executive offices. However, while we appreciate all comments from shareholders, we may not be able to respond individually to all communications. We will attempt to address shareholder questions and concerns in our press releases and documents filed with the SEC, so that all shareholders have access to information about us at the same time. Dr. Jacobs collects and evaluates all shareholder communications. All communications addressed to our directors and executive officers will be reviewed by those parties, unless the communication is clearly frivolous.
Code of Business Conduct
Our Board of Directors has not adopted a Code of Business Conduct for our company.
EXECUTIVE COMPENSATION
In General
Currently, our management is unable to estimate accurately when, if ever, our company will possess sufficient capital, whether derived from sales revenues, this offering or otherwise, for the payment of salaries to our management.
As of the date of this Offering Circular, there are no annuity, pension or retirement benefits proposed to be paid to officers, directors or employees of our company, pursuant to any presently existing plan provided by, or contributed to, our company.
Compensation Summary
We have employment agreements with our Chief Executive Officer, President and Chief Financial Officer, each of whom was to be compensated by stock grants accrued monthly based on current share prices or block grants during the period ended December 31, 2020. In consideration of the Company’s financial performance and lack of liquidity during this period, each of our officers waived compensation and we adjusted compensation accordingly. Also by agreement, we canceled shares previously issued to the Chief Executive Officer.
The following table summarizes information concerning the compensation awarded, paid to or earned by, our executive officers.
| Name and Principal Position | | Fiscal Year Ended 12/31 | | Salary ($) | | Bonus ($) | | Stock Awards ($) | | | Option Awards ($) | | Non-Equity Incentive Plan Compensation ($) | | Non-qualified Deferred Compensation Earnings ($) | | All Other Compen- sation ($) | | Total ($) | |
| Edward E. Jacobs, Jr, MD Chief Executive Officer | | 2021 2020 | | --- --- | | --- --- | | | 0(3,409,091 0(2,438,525 | ) ) | | --- --- | | --- --- | | --- --- | | --- --- | | --- --- | |
| Robert W. Ellis President | | 2021 2020 | | --- --- | | --- --- | | | 0(3,641,217 0(332,788 | ) ) | | --- --- | | --- --- | | --- --- | | --- --- | | --- --- | |
| Charles Townsend Chief Operating Officer and Chief Financial Officer | | 2021 2020 | | --- --- | | --- --- | | | 0(3,694,479) --- | | | --- --- | | --- --- | | --- --- | | --- --- | | --- --- | |
| Ronald Lambrecht Former Chief Financial Officer | | 2021 2020 | | 1,000 --- | | --- --- | | --- 0(250,000) | | | --- --- | | --- --- | | --- --- | | --- --- | | 1,000 --- | |
Outstanding Option Awards
The following table provides certain information regarding unexercised options to purchase common stock, stock options that have not vested and equity-incentive plan awards outstanding as of the date of this Offering Circular, for each named executive officer.
| | Option Awards | | | Stock Awards | |
| Name | | Number of Securities Underlying Unexercised Options (#) Exercisable | | | Number of Securities Underlying Unexercised Options (#) Unexercisable | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | | | Option Exercise Price ($) | | | Option Expiration Date | | | Number of Shares or Units of Stock That Have Not Vested (#) | | | Market Value of Shares or Units of Stock That Have Not Vested ($) | | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | |
| Edward E. Jacobs, Jr., MD | | | --- | | | | --- | | | | --- | | | | --- | | | | n/a | | | | --- | | | | n/a | | | | --- | | | | --- | |
| Robert W. Willis | | | --- | | | | --- | | | | --- | | | | --- | | | | n/a | | | | --- | | | | n/a | | | | --- | | | | --- | |
| Charles Townsend | | | --- | | | | --- | | | | --- | | | | --- | | | | n/a | | | | --- | | | | n/a | | | | --- | | | | --- | |
Employment Agreements
We have entered into employment agreements with our current executive officers.
Edward E. Jacobs, Jr., MD. In June 2018, the Company entered into an employment agreement with Dr. Edward E. Jacobs, Jr., our CEO, for a base compensation of $10,000 monthly and 100,000 shares of common stock, then valued at $27,500. In October 2018, the agreement was amended to a base compensation is $7,000 in cash or equivalent in common stock. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. During years ended December 31, 2020 and 2019, the Company recorded Stock based compensation of $42,000 and $84,000, respectively.
Robert W. Ellis. In August 2019, the Company entered into an employment agreement with Robert W. Ellis, our president, for a base compensation of $5,000 in cash monthly and 250,000 shares to be issued on August 31, 2020, then valued at $26,375. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. During the years ended December 31, 2020 and 2019, the Company recorded Stock based compensation of $13,188 and $8,792 and accrued liabilities of $10,000 and $5,000, respectively.
Charles Townsend. In June 2021, the Company entered into an employment agreement with Charles Townsend, our chief operating officer and chief financial officer, for a base monthly compensation of $8,333 payable in shares of our common stock (unless we achieve sufficient cash flow) and 100,000 shares issued as a signing bonus.
Outstanding Equity Awards
Our Board of Directors has made no equity awards and no such award is pending.
Long-Term Incentive Plans
We currently have no employee incentive plans.
Director Compensation
None of our directors receives compensation for his serving as a director.
INSERT E
| | | Share Ownership Before This Offering | | | Share Ownership After This Offering | | | Effective Voting Power After This Offering | |
| Name of Shareholder | | Number of Shares Beneficially Owned | | | % Beneficially Owned(1) | | | Number of Shares Beneficially Owned | | | % Beneficially Owned(2) | | | Number of Shares Entitled to Vote | | | % of Total Shares Voting (3) | |
| Common Stock | | | | | | | | | | | | | | | | | | |
| Executive Officers and Directors | | | | | | | | | | | | | | | | | | |
| Dr. Edward E. Jacobs, Jr. | | | 10,239,5 | (4) | | 13.01 | % | | | 10,239,5 | (4) | | 3.55 | % | | | 171,868,0 | (5) | | 40.22 | % |
| Robert W. Ellis | | | 0 | | | | 0 | % | | | 0 | | | | 0 | % | | | 0 | | | | 0 | % |
| Charles Townsend | | | 100,000 | | | | * | | | | 100,000 | | | | * | | | | 100,000 | | | | * | |
| Officers and directors, as a group (3 persons) | | | 10,339,531 | (4) | | | 13.13 | % | | | 10,239,531 | (4) | | | 3.58 | % | | | 171,968,099 | (5) | | | 40.24 | % |
| 5% Owners | | | | | | | | | | | | | | | | | | | | | | | | |
| Breath of Life Foundation(6) | | | 9,628,568 | | | | 12.23 | % | | | 9,628,568 | | | | 3.34 | % | | | 0 | (5) | | | 0 | % |
| Series A Preferred Stock(7) | | | | | | | | | | | | | | | | | | | | | | | | |
| Dr. Edward E. Jacobs, Jr. | | | 600,000 | | | | 37.50 | % | | | 600,000 | | | | 37.50 | % | | | 160,000,000 | | | | 38.73 | % |
| Essence Now Ltd.(8) | | | 500,000 | | | | 31.25 | % | | | 500,000 | | | | 31.25 | % | | | 0 | (9) | | | 0 | % |
| Essence Worldwide Ltd.(8) | | | 500,000 | | | | 31.25 | % | | | 500,000 | | | | 31.25 | % | | | 0 | (9) | | | 0 | % |
| * | Less than 1%. |
| (1) | Based on 78,729,120 shares outstanding, which includes (a) 67,367,483 issued shares, (b) 8,000,000 unissued shares that underlie shares of Series A Convertible Preferred Stock convertible within 60 days of the date of this Offering Circular and (c) 3,361,637 shares that underlie convertible promissory notes convertible within 60 days of the date of this Offering Circular, before this offering. |
| (2) | Based on 288,709,120 shares outstanding, which includes (a) 267,367,483 issued shares, assuming the sale of all of the Offered Shares, (b) 8,000,000 unissued shares that underlie shares of Series A Convertible Preferred Stock convertible within 60 days of the date of this Offering Circular and (c) 13,341,637 shares that underlie convertible promissory notes convertible within 60 days of the date of this Offering Circular, after this offering. |
| (3) | Based on 427,367,483 shares voting, which includes (a) 267,367,483 issued shares, assuming the sale of all of the Offered Shares, and (b) 160,000,000 shares associated with the Series A Convertible Preferred Stock that are assumed to be issued for purposes of voting on corporate matters. |
| (4) | 8,000,000 of these shares are unissued, but underlie shares of Series A Convertible Preferred Stock convertible within 60 days of the date of this Offering Circular. |
| (5) | Includes (a) 9,628,568 shares owned beneficially of record by Breath of Life Foundation, which has irrevocably assigned the voting rights to all of such shares to our Board of Directors (Dr. Jacobs, as Chairman of the Board, control the vote of such shares), and (b) 160,000,000 shares associated with the Series A Convertible Preferred Stock that are assumed to be issued for purposes of voting on corporate matters and which are owned by Dr. Jacobs. |
| (6) | The Address of this Entity is 5124 Creasy Drive, Joelton, Tennessee 37080. Joel S. Mills, Esq. is the Administrator and act as sole officer and director of this entity. |
| (7) | Each shares of Series A Convertible Preferred Stock has 100 votes. Dr. Jacobs will, therefore, be able to control the management and affairs of our company, as well as matters requiring the approval by our shareholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets, and any other significant corporate transaction. In addition, the outstanding shares of Series A Convertible Preferred Stock are convertible, at any time, into 8,000,000 shares of our common stock. |
| (8) | The address of this entity is: 4385 Cameron Street, Las Vegas, Nevada 89103. Michele Sheriff is the manager of this entity. |
| (9) | The voting rights associated with all of these shares have been irrevocably assigned to our Board of Directors (Dr. Jacobs, as Chairman of the Board, control the vote of such shares). |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
License Agreements
On October 21, 2013, our company entered into a license agreement (“LA #1”) with Ferris Holding, Inc. (“FHI”), a company owned by our former control person, Barry K. Epling, whereby we obtained a license to certain technologies owned by FHI (the “Subject Technologies”). On the same date, our company entered into a second license agreement (“LA #1) with FHI, which related to certain proprietary products of owned by FHI (the “Subject Products”). By their respective terms, LA#1 and LA #2 were to have expired after six months, unless renewed by mutual agreement.
Since our company’s current management assumed control in October 2017, no transactions have occurred pursuant to LA#1 and LA#2, and, since their assuming control, our current management considered LA#1 and LA#2 to have been terminated by our prior management. However, to establish clearly such circumstance, our management issued a letter on April 26, 2022, to FHI confirming that, prior to the October 2, 2017, change-in-control transaction (see “Changes in Control – Irrevocable Voting Proxy”), LA#1 and LA#2 had been terminated. FHI has not indicated to the contrary.
Changes in Control - Irrevocable Voting Proxy
June 2013 Change in Control. On June 21, 2013, the Company’s sole officer, director and shareholder, Richard Chiang, sold 10,000,000 shares of the Company’s common stock, constituting 100% of its issued and outstanding shares, to Ferris Holding Inc., a Nevada corporation (“FHI”) for a purchase price of $40,000. Effective the same date, Mr. Chiang or the Company appointed Barry K. Epling, who was the sole shareholder of FHI, as Chairman of the Board of Directors, and Gerald A. Epling as its President, Chief Executive Officer, Secretary, Chief Financial Officer and a director; Mr. Chiang then resigned. On September 25, 2013, the Company changed its name to BioAdaptives, Inc.
October 2017 Change in Control. On October 2, 2017, there occurred a change in control of our company. This change-in-control transaction was initiated by our former control person, Barry K. Epling, in accordance with a “penny stock bar” imposed against him and was consummated with the intention that Mr. Epling would no longer be involved in our company On October 2, 2017, the Board of Directors appointed Kim Southworth and Dr. Edward E Jacobs, Jr., MD as directors and Mr. Barry Epling resigned as Chairman. FHI donated 9,628.568 shares of the Company’s common stock to the Breath of Life Foundation, a Section 501(c)(3) non-profit organization. At that time, these shares constituted 51.84% (and now 14.29%) of the Company’s issued and outstanding shares and, as part of the grant, Breath of Life granted the Company’s Board of Directors an irrevocable proxy to vote them. With these actions, Mr.Barry Epling effectively terminated his relationship with the Company as an owner (direct or indirect), officer or director.
Until April 26, 2022, however, Mr. Barry Epling remained as President and a Director of Breath of Life Foundation, which continues to own beneficially 9,628,568 shares of our common stock. Effective April 26, 2022, the then-current officers and directors of Breath of Life Foundation, including Mr. Barry Epling, either resigned or were removed, and control of Breath of Life Foundation was transferred to Joel S. Mills, Esq., a Nashville, Tennessee-based attorney who now serves as the sole officer and director of Breath of Life Foundation. This action served to sever any relationship, direct or indirect, between Breath of Life Foundation and Mr. Barry Epling and, thus, between our company and Mr. Barry Epling, it having been our company’s course of conduct that Mr. Barry Epling had no actual involvement in our company, following the October 2017 change-in-control transaction
Issuances of Series A Convertible Preferred Stock
BioScience Associates, a firm founded and managed by Dr. Edward E. Jacobs, Jr., our Chief Executive Officer, loaned the company $74,715.25. $24,000 of this debt was converted to 600,000 shares of Series A Preferred Stock, in November 2020. Dr. Jacobs now owns these shares.
During 2021, we entered into three license and royalty agreements for human and animal nutraceutical products, which we currently market. These agreements are for a period of one year and we issued a total of 1,000,000 shares of Series A Convertible Preferred Stock valued at $193,500 in consideration of these licenses. 500,000 of these shares of Series A Convertible Preferred Stock were issued to Essence Now, Ltd.; the voting rights associated with these shares have been assigned to Dr. Jacobs. 500,000 of these shares of Series A Convertible Preferred Stock were issued to Essence Worldwide, Ltd.; the voting rights associated with these shares have been assigned to Dr. Jacobs.
Employment Agreements
We have entered into employment agreements with our current executive officers.
Edward E. Jacobs, Jr., MD. In June 2018, the Company entered into an employment agreement with Dr. Edward E. Jacobs, Jr., our CEO, for a base compensation of $10,000 monthly and 100,000 shares of common stock, then valued at $27,500. In October 2018, the agreement was amended to a base compensation is $7,000 in cash or equivalent in common stock. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. During years ended December 31, 2020 and 2019, the Company recorded Stock based compensation of $42,000 and $84,000, respectively.
Robert W. Ellis. In August 2019, the Company entered into an employment agreement with Robert W. Ellis, our president, for a base compensation of $5,000 in cash per monthly and 250,000 shares to be issued on August 31, 2020, then valued at $26,375. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. During the years ended December 31, 2020 and 2019, the Company recorded Stock based compensation of $13,188 and $8,792 and accrued liabilities of $10,000 and $5,000, respectively.
Charles Townsend. In June 2021, the Company entered into an employment agreement with Charles Townsend, our chief operating officer and chief financial officer, for a base monthly compensation of $8,333 payable in shares of our common stock (unless we achieve sufficient cash flow) and 100,000 shares issued as a signing bonus.
Ronald Lambrecht. In September 2019, the Company entered into an employment agreement with Ronald Lambrecht, our former Chief Financial Officer, for a base compensation of $5,000, or equivalent in common stock, monthly and 100,000 shares to be issued on September 30, 2020, then valued at $11,010. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. We extended the period of the original agreement and the services to be provided will commence on January 2, 2020. During the year ended December 31, 2020, the Company recorded Stock based compensation of $37,340. Mr. Lambrecht retired from our company in December 2021.
Stock Cancellation
In December 2021, Ronald Lambrecht, our former Chief Financial Officer, redeemed, in connection with his retirement, all 232,788 shares of our common stock owned by him, in exchange for a cash payment of $1,000.
LEGAL MATTERS
Certain legal matters with respect to the Offered Shares offered by this Offering Circular will be passed upon by Newlan Law Firm, PLLC, Flower Mound, Texas.
WHERE YOU CAN FIND MORE INFORMATION
We have filed an offering statement on Form 1-A with the SEC under the Securities Act with respect to the common stock offered by this Offering Circular. This Offering Circular, which constitutes a part of the offering statement, does not contain all of the information set forth in the offering statement or the exhibits and schedules filed therewith. For further information with respect to us and our common stock, please see the offering statement and the exhibits and schedules filed with the offering statement. Statements contained in this Offering Circular regarding the contents of any contract or any other document that is filed as an exhibit to the offering statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the offering statement. The offering statement, including its exhibits and schedules, may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549, and copies of all or any part of the offering statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains an Internet website that contains all information regarding companies that file electronically with the SEC. The address of the website is www.sec.gov.
INDEX TO FINANCIAL STATEMENTS
Bioadaptives, Inc.
Consolidated Financial Statements
Years Ended December 31, 2021 and 2020
BIOADAPTIVES, INC
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021, AND 2020
| TABLE OF CONTENT | | PAGE | |
| | | | |
| Report of Independent Registered Public Accounting Firm (PCAOB ID: 5041) | | | | |
| Consolidated Balance Sheet | | | | |
| Consolidated Statements of Operations | | | | |
| Consolidated Statements of Stockholders’ Deficit | | | | |
| Consolidated Statements of Cash Flows | | | | |
| Notes to Consolidated Financial Statements | | | | |
Report of Independent Registered Public Accounting Firm
To the shareholders and the board of directors of BioAdaptives, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BioAdaptives, Inc. as of December 31, 2021 and 2020, the related statements of operations, stockholders' equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has suffered recurring losses from operations and has a significant accumulated deficit. In addition, the Company continues to experience negative cash flows from operations. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/S/ BF Borgers CPA PC
BF Borgers CPA PC
We have served as the Company's auditor since 2018
Lakewood, CO
March 30, 2022
BIOADAPTIVES, INC.
CONSOLIDATED BALANCE SHEETS
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| | | | | | | |
| ASSETS | | | | | | |
| Current Assets: | | | | | | |
| Cash | | $ | 82,936 | | | $ | 4,587 | |
| Prepaid expense | | | 1,000 | | | | - | |
| Marketable securities | | | 190 | | | | 444 | |
| Inventory | | | 4,750 | | | | 13,815 | |
| Total Current Assets | | | 88,876 | | | | 18,846 | |
| | | | | | | | | |
| License, net | | | 59,709 | | | | - | |
| TOTAL ASSETS | | $ | 148,585 | | | $ | 18,846 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' DEFICIT | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities | | | 247,750 | | | | 87,561 | |
| Derivative liabilities | | | 557,042 | | | | 827,119 | |
| Current portion of convertible notes - net of discount of $13,333 and $4,764 | | | 403,117 | | | | 405,236 | |
| Note payable - related party | | | 33,715 | | | | 77,715 | |
| Total Current Liabilities | | | 1,241,624 | | | | 1,397,631 | |
| | | | | | | | | |
| Total Liabilities | | | 1,241,624 | | | | 1,397,631 | |
| | | | | | | | | |
| Stockholders' Deficit: | | | | | | | | |
| Preferred stock, ($.0001 par value, 10,000,000 shares authorized: | | | | | | | | |
| Series A Preferred Stock 4,000,000 shares designated: 1,600,000 and 0 issued and outstanding, respectively | | | 160 | | | | - | |
| Series B Preferred Stock 6,000,000 shares designated; no share issued and outstanding | | | - | | | | - | |
| Common stock ($.0001 par value, 750,000,000 shares authorized; 50,819,780 and 21,591,942 shares issued and outstanding, and 10,000 and 762,390 issuable, respectively) | | | 5,082 | | | | 2,159 | |
| Additional paid-in capital | | | 5,557,828 | | | | 4,225,217 | |
| Accumulated deficit | | | (6,656,109 | ) | | | (5,606,161 | ) |
| Total Stockholders' Deficit | | | (1,093,039 | ) | | | (1,378,785 | ) |
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | | $ | 148,585 | | | $ | 18,846 | |
The accompanying notes are an integral part of these consolidated financial statements.
BIOADAPTIVES, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| | | Years ended December 31, | |
| | | 2021 | | | 2020 | |
| | | | | | | |
| Revenues | | $ | 19,776 | | | $ | 16,327 | |
| Cost of revenue | | | 9,671 | | | | 10,232 | |
| Gross Profit | | | 10,105 | | | | 6,095 | |
| | | | | | | | | |
| Operating Expenses | | | | | | | | |
| General and administrative | | | 268,268 | | | | 72,409 | |
| Professional fees | | | 80,227 | | | | 48,369 | |
| Stock based compensation | | | 163,900 | | | | 139,643 | |
| Amortization of license | | | 133,791 | | | | - | |
| Total Operating Expenses | | | 646,186 | | | | 260,421 | |
| | | | | | | | | |
| Other Income (Expense) | | | | | | | | |
| Unrealized loss on marketable securities | | | (254 | ) | | | (328 | ) |
| Interest expense | | | (272,394 | ) | | | (230,328 | ) |
| Change in fair value of derivative liabilities | | | (90,219 | ) | | | (362,262 | ) |
| Loss on settlement of debt | | | (51,000 | ) | | | - | |
| Total Other Expense | | | (413,867 | ) | | | (592,918 | ) |
| | | | | | | | | |
| Loss before income taxes | | | (1,049,948 | ) | | | (847,244 | ) |
| | | | | | | | | |
| Net Loss | | $ | (1,049,948 | ) | | $ | (847,244 | ) |
| | | | | | | | | |
| Net Loss Per Common Share: | | | | | | | | |
| Basic and Diluted | | $ | (0.03 | ) | | $ | (0.04 | ) |
| | | | | | | | | |
| Weighted Average Number of Common Shares Outstanding: | | | | | | | | |
| Basic and Diluted | | | 33,742,920 | | | | 20,387,798 | |
The accompanying notes are an integral part of these consolidated financial statements.
BIOADAPTIVES, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ DEFICIT
| | | | | | | | | Additional | | | | | | | |
| | | Series A Preferred stock | | | Common stock | | | paid-in | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | capital | | | Deficit | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2019 | | | - | | | | - | | | | 18,938,769 | | | | 1,894 | | | | 3,917,147 | | | | (4,758,917 | ) | | | (839,876 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common stock issued for conversion of debt | | | - | | | | - | | | | 2,253,173 | | | | 225 | | | | 168,467 | | | | - | | | | 168,692 | |
| Common stock issued for service | | | - | | | | - | | | | 400,000 | | | | 40 | | | | 47,075 | | | | - | | | | 47,115 | |
| Common stock issued for service - related party | | | - | | | | - | | | | 558,693 | | | | 56 | | | | 71,944 | | | | - | | | | 72,000 | |
| Stock based compensation | | | - | | | | - | | | | - | | | | - | | | | 20,528 | | | | - | | | | 20,528 | |
| Cancellation of common stock - officers | | | | | | | | | | | (558,693 | ) | | | (56 | ) | | | 56 | | | | - | | | | - | |
| Net loss for the period | | | - | | | | - | | | | - | | | | - | | | | - | | | | (847,244 | ) | | | (847,244 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2020 | | | - | | | $ | - | | | | 21,591,942 | | | $ | 2,159 | | | $ | 4,225,217 | | | $ | (5,606,161 | ) | | $ | (1,378,785 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Series A preferred stock issued for settlement of debt - related party | | | 600,000 | | | | 60 | | | | - | | | | - | | | | 74,940 | | | | - | | | | 75,000 | |
| Series A preferred stock issued for license fee | | | 1,000,000 | | | | 100 | | | | - | | | | - | | | | 193,400 | | | | - | | | | 193,500 | |
| Common stock issued for conversion of debt | | | - | | | | - | | | | 28,580,228 | | | | 2,858 | | | | 853,769 | | | | - | | | | 856,627 | |
| Common stock issued for service | | | - | | | | - | | | | 1,000,000 | | | | 100 | | | | 163,800 | | | | - | | | | 163,900 | |
| Cancellation of common stock - officers | | | | | | | | | | | (352,390 | ) | | | (35 | ) | | | 35 | | | | - | | | | - | |
| Debts forgiveness - related party | | | - | | | | - | | | | - | | | | - | | | | 46,667 | | | | - | | | | 46,667 | |
| Net loss for the period | | | - | | | | - | | | | - | | | | - | | | | - | | | | (1,049,948 | ) | | | (1,049,948 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 1,600,000 | | | $ | 160 | | | | 50,819,780 | | | $ | 5,082 | | | $ | 5,557,828 | | | $ | (6,656,109 | ) | | $ | (1,093,039 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
BIOADAPTIVES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOW
| | | Years ended December 31, | |
| | | 2021 | | | 2020 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | |
| Net loss | | $ | (1,049,948 | ) | | $ | (847,244 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Stock-based compensation | | | 163,900 | | | | 47,115 | |
| Stock-based compensation - related party | | | - | | | | 92,528 | |
| Change in fair value of derivative liabilities | | | 90,219 | | | | 362,262 | |
| Amortization of license | | | 133,791 | | | | - | |
| Amortization of debt discount | | | 216,931 | | | | 179,843 | |
| Loss on settlement of debt | | | 51,000 | | | | - | |
| Unrealized gain on investments in marketable securities | | | 254 | | | | 328 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Inventory | | | 9,065 | | | | (2,687 | ) |
| Prepaid expense and other current assets | | | (1,000 | ) | | | - | |
| Accounts payable and accrued liabilities | | | 227,137 | | | | 60,966 | |
| Net Cash Used in Operating Activities | | | (158,651 | ) | | | (106,889 | ) |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | |
| Proceeds from Notes payable | | | - | | | | 9,800 | |
| Repayments of notes payable | | | - | | | | (18,352 | ) |
| Proceed from related party | | | 1,623 | | | | - | |
| Repayment to related party | | | (1,623 | ) | | | - | |
| Proceeds from notes payable - related party | | | - | | | | 27,715 | |
| Repayment of notes payable - related party | | | (20,000 | ) | | | - | |
| Proceeds from convertible notes | | | 257,000 | | | | 80,000 | |
| Net Cash Provided by Financing Activities | | | 237,000 | | | | 99,163 | |
| | | | | | | | | |
| Net change in cash | | | 78,349 | | | | (7,726 | ) |
| Cash at beginning of period | | | 4,587 | | | | 12,313 | |
| Cash at end of period | | $ | 82,936 | | | $ | 4,587 | |
| | | | | | | | | |
| SUPPLEMENTAL CASH FLOW INFORMATION: | | | | | | | | |
| Cash paid for income taxes | | $ | - | | | $ | - | |
| Cash paid for interest | | $ | 16,424 | | | $ | 1,079 | |
| | | | | | | | | |
| NON-CASH INVESTING AND FINANCING ACTIVITIES | | | | | | | | |
| Derivative liability recognized as debt discount | | $ | 205,000 | | | $ | - | |
| Issuance of common stock for conversion of debt | | $ | 856,627 | | | $ | 168,692 | |
| Issuance Series A preferred stock for license fee | | $ | 193,500 | | | $ | - | |
| Issuance Series A preferred stock for settlement of debt - related party | | $ | 60 | | | $ | - | |
| Debts forgiveness - related party | | $ | 46,667 | | | $ | - | |
| Cancellation of common stock | | $ | 35 | | | $ | 56 | |
The accompanying notes are an integral part of these consolidated financial statements.
BIOADAPTIVES, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS AND HISTORY
Description of business
BioAdaptives, Inc. (“BioAdaptives” or the ”Company”) was incorporated in Delaware on April 19, 2013, under the name Apex 8, Inc. Shortly afterwards, the Company’s control person sold his interest; new owners appointed management and changed its name to BioAdaptives, Inc. The Company acquired assets relating to the investigation, development and marketing of nutraceutical products; equipment designed to improve the bioavailability of nutrients in humans and animals; and licenses for specific products. It commenced investigation of the role of various botanicals in primitive cell development and proliferation, including certain algae along with herbs used in Traditional Chinese Medicine and Ayurvedic Practice. In the course of this investigation, BioAdaptives identified several potential human and animal products. The Company terminated further work on the equipment and products licensed in its early stages to concentrate on these products, for both human and animals.
We currently market and distribute natural plant- and algal-based products that improve health and wellness for humans and animals, with an emphasis on pain relief and anti-aging properties. The Company’s current products include dietary supplements for humans developed with our knowledge of natural foods. These products are designed to aid memory, cognition and focus; assist in sleep and fatigue reduction; provide pain relief and healing; and improve overall emotional and physical wellness. The science behind our products has proven to be effective for performance enhancement and pain relief for horses and dogs as well as providing improvements in appearance and we have developed products to utilize these advances.
Our current product line includes PrimiCell®, PluriPain® and PrimiLungs™ for humans, and Canine Regen® and Equine Regen® for dogs and horses, along with Equine All-in-One® and a related Booster. The All-in-One products combine minerals, vitamins, amino acids and other botanicals along with the Regen® compounds and have demonstrated improved performance and appearance in competition and show animals. All of these products are sold under licensing and manufacturing agreements with third parties. While we continue to investigate our own nutraceutical products for humans and animals, most of our current activities are reliant on marketing and distributing products developed and owned by others.
On May 22, 2019, the Company moved its corporate office to 2620 Regatta Drive, Suite 102, Las Vegas, NV 89128, but maintained fulfillment facilities at 4385 Cameron Street, Suite B, Las Vegas, NV 89103.
COVID-19
A novel strain of coronavirus (COVID-19) was first identified in December 2019, and subsequently declared a global pandemic by the World Health Organization on March 11, 2020. As a result of the outbreak, many companies have experienced disruptions in their operations and in markets served. The Company has instituted some and may take additional temporary precautionary measures intended to help ensure the well-being of its employees and minimize business disruption. The Company considered the impact of COVID-19 on the assumptions and estimates used and determined that there were no material adverse impacts on the Company’s results of operations and financial position on December 31, 2021. The full extent of the future impacts of COVID-19 on the Company’s operations is uncertain. A prolonged outbreak could have a material adverse impact on financial results and business operations of the Company, including the timing and ability of the Company to collect accounts receivable and the ability of the Company to continue to provide high quality services to its clients. The Company is not aware of any specific event or circumstance that would require an update to its estimates or judgments or a revision of the carrying value of its assets or liabilities as of the date of issuance of this Annual Report on Form 10-K. These estimates may change, as new events occur, and additional information is obtained.
2. SUMMARY OF SIGNIFICANT POLICIES
Basis of Presentation
The Company represents its consolidated financial statements were prepared in accordance with US GAAP and the rules of the Securities and Exchange Commission and that, in the opinion of management, all adjustments, consisting of normal recurring adjustments, necessary for a fair presentation of financial position and the results of operations for the interim period presented have been reflected herein. The results of operations are historical and not necessarily indicative of the results to be expected for any future period.
Use of estimates
The preparation of consolidated financial statements in conformity with US GAAP requires the Company to make estimates and judgments that affect the reported amounts of assets and liabilities, revenues and expenses, and related disclosures of contingent assets and liabilities. These estimates and judgments are based on historical information, information that is currently available to the Company, and on various other assumptions that the Company believes to be reasonable under the circumstances. Actual results could differ from those estimates.
Cash and cash equivalents
Cash and cash equivalents consist of cash and short-term investments with original maturities of less than 90 days. Cash equivalents are placed with high credit quality financial institutions and are primarily in money market funds. The carrying value of those investments approximates fair value. As of December 31, 2021, and 2020, the Company had $82,936 and $4,587 cash equivalents, respectively.
Investment Securities
Equity securities are classified as available for sale. All available for sale securities are classified as current assets as they are available to support the Company’s current operating needs in the next 12 months.
In accordance with Accounting Standards Codification (“ASC”) 320-10, “Investments-Debt and Equity Securities,” the Company evaluates its securities portfolio for other-than-temporary impairment (“OTTI”) throughout the year. Each investment that has a fair value less than the book value is reviewed on a quarterly basis by management. Management considers at a minimum the following factors that, both individually or combination, could indicate that the decline is other-than-temporary: (a) the Company has the intent to sell the security; (b) it is more likely than not that it will be required to sell the security before recovery; and (c) the Company does not expect to recover the entire amortized cost basis of the security. Among the factors that are considered in determining intent is a review of capital adequacy, interest rate risk profile and liquidity at the Company. An impairment charge is recorded against individual securities if the review described above concludes that the decline in value is other-than-temporary.
Earnings (loss) per share
Basic earnings (loss) per common share is computed by dividing net income (loss) available to common shareholders by the weighted-average number of shares of common stock outstanding during the period. Diluted earnings per common share is computed by dividing income available to common shareholders by the weighted-average number of shares of common stock outstanding during the period increased to include the number of additional shares of common stock that would have been outstanding if potentially dilutive securities had been issued.
For the years ended December 31, 2021 and 2020, the following common stock equivalents were excluded from the computation of diluted net loss per share as the result of the computation was anti-dilutive.
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| | | (Shares) | | | (Shares) | |
| Series A Preferred Stock | | | 8,000,000 | | | | - | |
| Convertible notes | | | 57,513,358 | | | | 36,247,341 | |
| Total | | | 65,513,358 | | | | 36,247,341 | |
Revenue recognition
Revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements:
| | • | identify the contract with a customer; |
| | • | identify the performance obligations in the contract; |
| | • | determine the transaction price; |
| | • | allocate the transaction price to performance obligations in the contract; and |
| | • | recognize revenue as the performance obligation is satisfied. |
Cost of revenue
Cost of revenue includes the inventory purchased from a related party.
Inventory
Inventories, consisting of products available for sale, are primarily accounted for using the first-in-first-out (“FIFO”) method and are valued at the lower of cost or market value. Inventories on hand are evaluated on an on-going basis to determine if any items are obsolete or in excess of future market needs. Items determined to be obsolete are reserved for. As of December 31, 2021, and 2020, the Company determined that no reserve was required.
Stock-based compensation
The Company accounts for stock-based compensation arrangements with employees, nonemployee directors and consultants using a fair value method, which requires the recognition of compensation expense for costs related to all stock-based payments, including stock options, on a straight-line basis over the requisite service period in the Company’s consolidated statements of operations. The fair value method requires the Company to estimate the fair value of stock-based payment awards on the date of grant.
Financial Instruments and Fair Value Measurements
As defined in ASC 820” Fair Value Measurements,” fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (exit price). The Company utilizes market data or assumptions that market participants would use in pricing the asset or liability, including assumptions about risk and the risks inherent in the inputs to the valuation technique. These inputs can be readily observable, market corroborated, or generally unobservable. The Company classifies fair value balances based on the observability of those inputs. ASC 820 establishes a fair value hierarchy that prioritizes the inputs used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurement) and the lowest priority to unobservable inputs (level 3 measurement).
The following table summarizes fair value measurements by level as of December 31, 2021, and 2020, measured at fair value on a recurring basis:
Fair Value Measurements as of December 31, 2021, Using:
| | | Total Carrying Value | | | Quoted Market Prices in Active | | | Significant Other | | | Significant Unobservable | |
| | | as of | | | Markets | | | Observable Inputs | | | Inputs | |
| | | December 31, 2021 | | | (Level 1) | | | (Level 2) | | | (Level 3) | |
| Assets: | | | | | | | | | | | | |
| Equity Securities | | $ | 190 | | | $ | 190 | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| Liabilities | | | | | | | | | | | | | | | | |
| Derivative liabilities | | $ | 557,042 | | | $ | - | | | $ | - | | | $ | 557,042 | |
Fair Value Measurements as of December 31, 2020, Using:
| | | Total Carrying Value as of December 31, | | | Quoted Market Prices in Active Markets | | | Significant Other Observable Inputs | | | Significant Unobservable Inputs | |
| | | 2020 | | | (Level 1) | | | (Level 2) | | | (Level 3) | |
| Assets: | | | | | | | | | | | | |
| Equity Securities | | $ | 444 | | | $ | 444 | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| Liabilities | | | | | | | | | | | | | | | | |
| Derivative liabilities | | $ | 827,119 | | | $ | - | | | $ | - | | | $ | 827,119 | |
Derivative Financial Instruments
The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. We evaluate all of our financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. For stock-based derivative financial instruments, the Company used a Black Scholes valuation model to value the derivative instruments at inception and on subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the balance sheet date.
Concentration of credit risk
Financial instruments that potentially expose the Company to significant concentrations of credit risk consist principally of cash. The Company places its cash with financial institutions with high credit ratings.
Income taxes
The Company records income taxes under the asset and liability method, whereby deferred tax assets and liabilities are recognized based on the future tax consequences attributable to temporary differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and attributable to operating loss and tax credit carryforwards. Accounting standards regarding income taxes requires a reduction of the carrying amounts of deferred tax assets by a valuation allowance, if based on the available evidence, it is more likely than not that such assets will not be realized. Accordingly, the need to establish valuation allowances for deferred tax assets is assessed at each reporting period based on a more- likely-than-not realization threshold. This assessment considers, among other matters, the nature, frequency and severity of current and cumulative losses, forecasts of future profitability, the duration of statutory carryforward periods, the Company’s experience with operating loss and tax credit carryforwards not expiring unused, and tax planning alternatives.
The Company recorded valuation allowances on the net deferred tax assets. Management will reassess the realization of deferred tax assets based on the accounting standards for income taxes each reporting period. To the extent that the financial results of operations improve, and it becomes more likely than not that the deferred tax assets are realizable, the Company will be able to reduce the valuation allowance.
Significant judgment is required in evaluating the Company’s tax positions and determining its provision for income taxes. During the ordinary course of business, there are many transactions and calculations for which the ultimate tax determination is uncertain. Accounting standards regarding uncertainty in income taxes provides a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount which is more than 50% likely, based solely on the technical merits, of being sustained on examinations. The Company considers many factors when evaluating and estimating its tax positions and tax benefits, which may require periodic adjustments, and which may not accurately anticipate actual outcomes.
3. GOING CONCERN
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred losses since inception and had an accumulated deficit of $6,656,109 as of December 31, 2021. The Company requires capital for its contemplated operational and marketing activities. The Company’s ability to raise additional capital through the future issuances of common stock is unknown. Obtaining additional financing, successful development of the Company’s contemplated plan of operations, and the transition, ultimately, to profitable operations are necessary for the Company to continue operations. The ability to successfully resolve these factors raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements of the Company do not include any adjustments that may result from the outcome of these aforementioned uncertainties.
In order to mitigate the risk related with this uncertainty, the Company plans to issue additional shares of common stock for cash and services during the next 12 months.
4. MARKETABLE SECURITIES
Equity securities as of December 31, 2021, and 2020, were comprised of 105,736 shares of common stock of Hemp, Inc. (HEMP.PK) recorded at fair value of $190 and $444, respectively.
5. ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
Accounts payable and accrued liabilities aa of December 31, 2021 and 2020 consists of the following:
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| Accounts payable | | $ | 1,750 | | | $ | 5,374 | |
| Credit card | | | - | | | | 7,759 | |
| Accrued salary | | | 166,666 | | | | - | |
| Accrued interest | | | 68,341 | | | | 49,583 | |
| Accrued liabilities | | | 10,993 | | | | 24,845 | |
| | | $ | 247,750 | | | $ | 87,561 | |
6. CONVERTIBLE NOTES
Convertible notes as of December 31, 2021 and 2020 consist of the following:
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| Convertible Notes - originated in April 2018 | | $ | 95,000 | | | $ | 95,000 | |
| Convertible Notes - originated in June 2018 | | | 166,000 | | | | 166,000 | |
| Convertible Notes - originated in October 2018 | | | 50,000 | | | | 50,000 | |
| Convertible Notes - issued fiscal year 2019 | | | - | | | | 13,000 | |
| Convertible Notes - issued fiscal year 2020 | | | - | | | | 86,000 | |
| Convertible Notes - issued fiscal year 2021 | | | 105,450 | | | | - | |
| Total convertible notes payable | | | 416,450 | | | | 410,000 | |
| | | | | | | | | |
| Less: Unamortized debt discount | | | (13,333 | ) | | | (4,764 | ) |
| Total convertible notes | | | 403,117 | | | | 405,236 | |
| | | | | | | | | |
| Less: current portion of convertible notes | | | 403,117 | | | | 405,236 | |
| Long-term convertible notes | | $ | - | | | $ | - | |
For the years ended December 31, 2021 and 2020, the interest expense on convertible notes was $53,660 and $46,779, respectively. As of December 31, 2021, and 2020, the accrued interest was $63,100 and $46,145, respectively.
The Company recognized amortization expense related to the debt discount of $216,931 and $179,843 for the years ended December 31, 2021 and 2020, respectively, which is included in interest expense in the statements of operation.
Conversion
During the year ended December 31, 2021, the Company converted notes with principal amounts of $271,050 and accrued interest of $20,281 into 28,580,228 shares of common stock. The corresponding derivative liability at the date of conversion of $565,296 was credited to additional paid in capital.
Convertible Notes – Issued during the year ended December 31, 2018
During the year ended December 31, 2018, the Company issued a total principal amount of $426,000 in convertible notes for cash proceeds of $426,000. The convertible notes were also provided with a total of 107,000 common shares valued at $22,210. The terms of these convertible notes are summarized as follows:
| | • | Term two years; |
| | | |
| | • | Annual interest rates 12%; |
| | | |
| | • | Convertible at the option of the holders at any time |
| | | |
| | • | Conversion prices are based on 50% discount to market value for the common stock based on a 4-week weekly average of the closing price. |
Convertible Notes – Issued during the year ended December 31, 2019
During the year ended December 31, 2019, the Company issued a total principal amount of $73,500 in convertible notes for cash proceeds of $67,000. The terms of convertible notes are summarized as follows:
| | • | Term one years; |
| | | |
| | • | Annual interest rates 10%; |
| | | |
| | • | Convertible at 180 days from issuance |
| | | |
| | • | Conversion prices are based on a 42% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. |
During the year ended December 31, 2021, the unpaid principal of $13,000 and accrued interest of $9,781converted into 2,476,196 shares of common stock.
Convertible Notes – Issued during the year ended December 31, 2020
During the year ended December 31, 2020, the Company issued a total principal amount of $86,000 in convertible note for cash proceeds of $80,000. The terms of convertible note are summarized as follows:
| | · | Term one year; |
| | | |
| | · | Annual interest rates 10%; |
| | | |
| | · | Convertible at 180 days from issuance |
| | | |
| | · | Conversion prices are 39% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. |
During the year ended December 31, 2021, the unpaid principal of $86,000 and accrued interest of $4,300 converted into 8,316,677 shares of common stock.
Convertible Notes - Issued during the year ended December 31, 2021
During the year ended December 31, 2021, the Company issued a total principal amount of $222,500 in convertible note for cash proceeds of $205,000. The terms of convertible note are summarized as follows:
| | • | Term one year; |
| | | |
| | • | Annual interest rates 10%; |
| | | |
| | • | Convertible at 180 days from issuance |
| | | |
| | • | Conversion prices are based on 39% discount to the lowest trading price during the 20-trading day period ending on the latest complete training day prior to the conversion date. |
During the year ended December 31, 2021, the Company converted principal of $172,050 and accrued interest of $6,200 into 17,787,355 shares of common stock.
The Company valued the conversion feature using the Black-Scholes pricing model. The fair value of the derivative liability for all the notes that became convertible, including the notes issued in prior years, during the year ended December 31, 2021 amounted to $358,697, and $205,000 of the value assigned to the derivative liability was recognized as a debt discount to the notes while the balance of $153,697 was recognized as a “day 1” derivative loss.
The Company valued the conversion feature using the Binomial pricing model. The fair value of the derivative liability for all the notes that became convertible, including the notes issued in prior years, during the year ended December 31, 2020 amounted to $239,593, and $107,000 of the value assigned to the derivative liability was recognized as a debt discount to the notes while the balance of $132,593 was recognized as a “day 1” derivative loss.
7. DERIVATIVE LIABILITIES
The Company analyzed the conversion option for derivative accounting consideration under ASC 815, Derivatives and Hedging, and hedging, and determined that the instrument should be classified as a liability since the conversion option becomes effective at issuance resulting in there being no explicit limit to the number of shares to be delivered upon settlement of the above conversion options. The Company accounts for warrants as a derivative liability due to there being no explicit limit to the number of shares to be delivered upon settlement of all conversion options.
Fair Value Assumptions Used in Accounting for Derivative Liabilities.
ASC 815 requires we assess the fair market value of derivative liability at the end of each reporting period and recognize any change in the fair market value as other income or expense item.
The Company determined our derivative liabilities to be a Level 3 fair value measurement and used the Black-Scholes pricing model to calculate the fair value as of December 31, 2021. The Black-Scholes model requires six basic data inputs: the exercise or strike price, time to expiration, the risk-free interest rate, the current stock price, the estimated volatility of the stock price in the future, and the dividend rate. Changes to these inputs could produce a significantly higher or lower fair value measurement. The fair value of each convertible note is estimated using the Black-Scholes valuation model.
For the years ended December 31, 2021 and 2020, the estimated fair values of the liabilities measured on a recurring basis are as follows:
| | | Year Ended | | | Year ended | |
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| Expected term | | 0.02- 0.51 years | | | 0.01- 0.51 years | |
| Expected average volatility | | 128% - 315% | | | 117% - 317% | |
| Expected dividend yield | | | - | | | | - | |
| Risk-free interest rate | | 0.03% - 0.07% | | | 0.05% - 0.19% | |
The following table summarizes the changes in the derivative liabilities during the years ended December 31, 2021 and 2020
| Balance - December 31, 2019 | | $ | 464,024 | |
| | | | | |
| Addition of new derivatives recognized as debt discounts | | | 107,000 | |
| Addition of new derivatives recognized as options compensation | | | - | |
| Addition of new derivatives recognized as loss on derivatives | | | 132,593 | |
| Settled on issuance of common stock | | | (106,167 | ) |
| Reclassification from APIC to derivative due to tainted instruments | | | - | |
| Loss on change in fair value of the derivative | | | 229,669 | |
| Balance - December 31, 2020 | | $ | 827,119 | |
| | | | | |
| Addition of new derivatives recognized as debt discounts | | | 205,000 | |
| Addition of new derivatives recognized as loss on derivatives | | | 153,697 | |
| Settled on issuance of common stock | | | (565,296 | ) |
| (Gain) on change in fair value of the derivative | | | (63,478 | ) |
| Balance - December 31, 2021 | | $ | 557,042 | |
The aggregate (gain) loss on derivatives during the year ended December 31, 2021 and 2020 was as follows.
| | | Years ended | |
| | | December 31, | |
| | | 2021 | | | 2020 | |
| Day one loss due to derivative liabilities on convertible notes | | $ | 153,697 | | | $ | 132,593 | |
| (Gain) loss on change in fair value of the derivative liabilities | | | (63,478 | ) | | | 229,669 | |
| | | $ | 90,219 | | | $ | 362,262 | |
8. NOTES PAYABLE
During the year ended December 31, 2020, the Company issued notes payable of $9,800 to a third party. The term is 6 months. During the year ended December 31, 2020, the Company recognized interest expense of $1,079 and fully repaid $14,840.
9. STOCKHOLDERS’ EQUITY
Preferred Stock
On January 24, 2022, the Board of Directors of the Company’s, approved for an increase in the number of authorized shares of the Company’s preferred stock from 5,000,000 shares to 10,000,000 shares.
The Company is authorized to issue 10,000,000 shares of $.0001 par value preferred stock, of which 4,000,000 have been designated as Series A Preferred Stock and 6,000,000 have been designated as Series B Preferred Stock.
Series A Preferred Stock
On February 6, 2020, the Company established its Series A Preferred Stock, par value $.0001, by filing a Certificate of Designation with the Delaware Secretary of State. The Company’s board exercised “blank check” authority to establish classes of preferred stock without approval by shareholders under provision of its original Articles of Incorporation and has designated 4,000,000 shares of Series A Preferred Stock.
The Company may use the Series A Preferred Stock for purpose of asset acquisition or in satisfaction of recognized debt; they are not otherwise available for sale. The Series A Preferred Stock have enhanced voting privileges under certain circumstances; the collective right to appoint elect one director, at the Holders’ option; and conversion-to-common rights at a 5:1 ratio.
During the year ended December 31, 2021, the Company issued 1,600,000 shares of series A preferred Stock as follows;
| | • | 600,000 shares of Series A Preferred Stock valued at $75,000 for settlement of related party debt |
| | • | 1,000,000 shares of Series A Preferred Stock valued at $193,500 for consideration of licenses. |
Series B Preferred Stock
On January 24, 2022, the Company established its Series B Preferred Stock, par value $.0001, by filing a Certificate of Designation with the Delaware Secretary of State. The Company’s board exercised “blank check” authority to establish classes of preferred stock without approval by shareholders under provision of its original Articles of Incorporation and has designated 6,000,000 shares of Series B Preferred Stock.
The Company may use the Series B Preferred Stock for purpose of asset acquisition or in satisfaction of recognized debt; they are not otherwise available for sale. The Series B Preferred Stock have enhanced voting privileges (100:1); the collective right to appoint elect one director, at the Holders’ option; and conversion-to-common rights at a 10:1 ratio.
As of the date of this filing, no shares of Series B Preferred Stock are issued and outstanding.
Common Stock
On January 24, 2022, the holder of a majority of the Company’s outstanding voting stock, approved for an increase in the number of authorized shares of the Company’s common stock from 200,000,000 shares to 750,000,000 shares.
As of December 31, 2021, and 2020, there were 50,809,780 and 20,829,552 shares of the Company’s common stock issued and outstanding, respectively. In addition, as of December 31, 2021 and 2020, there were 10,000 shares and 762,390 shares of the Company’s common stock issuable, respectively.
Fiscal year 2021
During the year ended December 31, 2021, the Company issued 28,580,228 shares of common stock for conversion of debt of $856,627.
During the year ended December 31, 2021, the Company issued 1,000,000 shares of common stock valued at $163,900 for service.
During the year ended December 31, 2021, the Company cancelled 352,390 shares of common stock related to our officer’s compensation.
Fiscal year 2020
On February 6, 2020, the Company’s board and a majority of its shareholders approved an amendment to the Company’s Articles of Incorporation to increase the authorized number of shares of its common stock, par value .0001, from 100,000,000 shares to 200,000,000 shares.
During the year ended December 31, 2020, the Company issued 2,253,173 shares of common stock for conversion of debt of $62,525.
During the year ended December 31, 2020, the Company recorded 558,693 common stock issuable valued at $72,000 based on an employment agreement – related party transaction. During the year ended December 31, 2020, all our management have waived their compensation due to COVID-19 related slow-downs and other factors and none of these shares were issued.
During the year ended December 31, 2020, the Company recorded 400,000 common stock issuable valued at $47,115 for services.
Warrant
During the year ended December 31, 2018, the Company entered into an agreement with consultant to provide the Company with consulting services in exchange for 2-year warrant to purchase 200,000 shares of common stock with an exercise price of $0.1 per share. The Company recognized a warrant expense of $52,365, as stock-based compensation and additional paid-in capital. The Company determined that the warrants qualify for derivative accounting as a result of the related issuance of the convertible note on in April 2018, which had no express limit on the number of shares to be delivered upon future settlement of the conversion options. This warrant expired unexercised during the period ended December 31, 2020.
A summary of activity during the year ended December 31,2020 follows:
| | | Warrants Outstanding | |
| | | | | | Weighted Average | |
| | | Shares | | | Exercise Price | |
| | | | | | | |
| Outstanding, December 31, 2019 | | | 200,000 | | | $ | 0.10 | |
| Granted | | | - | | | | - | |
| Exercised | | | - | | | | - | |
| Expired | | | (200,000 | ) | | | 0.10 | |
| Outstanding, December 31, 2020 | | | - | | | $ | - | |
10. RELATED PARTY TRANSACTIONS
Notes payable – related party
During the year ended December 31, 2021 and 2020, the Company issued notes for a total principal amount of $0 and $27,715 to a company owned by our CEO, respectively. The notes bear 4% interest and have terms of 3 and 6 months.
During the year ended December 31, 2021, the Company issued 600,000 shares of Series A Preferred Stock valued at $75,000 for settlement of $24,000 notes. As a result, the Company recorded a loss on settlement of debt of $51,000.
During the year ended December 31, 2021 and 2020, the Company repaid notes payable to a related party of $20,000 and $0 and recognized interest of $1,803 and $2,628, respectively
As of December 31, 2021, and 2020, the Company recorded notes payable - related party of $33,715 and $77,715 and accrued interest of $5,241 and $3,438, respectively. The note is a 4% interest bearing promissory note that the term is 1 year.
Employee agreements
Year ended December 2021
Effective May 31, 2021, the Company entered into an Employment Contract with Charles Townsend to serve as its Chief Operating Officer. The Contract provides for a 12-month term and for payment of an annual salary of $100,000, payable in Restricted Stock Units calculated based on the closing market price of the Company’s shares as of the effective date. Mr. Townsend was also appointed as a director.
Effective May 31, 2021, the Company entered into an Employment Contract with Robert Ellis, to continue his service as the Company’s President. The Contract provides for a 12-month term and for payment of an annual salary of $100,000, payable in Restricted Stock Units calculated based on the closing market price of the Company’s shares each quarter. Mr. Ellis was also appointed as a director.
Effective May 31, 2021, the Company entered into an Employment Contract with Ronald Lambrecht, to continue his service as its Chief Financial Officer. The Contract provides for a 12-month term and for payment of an annual salary of $80,000, payable in Restricted Stock Units calculated based on the closing market price of the Company’s shares each quarter. On December 31, 2021, the Company signed a separation agreement and general release and waiver with Ronald Lambrecht which both parties agreed to terminate the Employment Agreement effective on December 31, 2021. Ronald Lambrecht confirmed by receiving $1,000 at the time, he shall be entitled to no further compensation from the Company and also Ronald Lambrecht agreed to surrender all of his Restricted Stock Units under the Company’s Incentive Plan. The Company recognized accrued salary payable of $46,667 as additional paid -in- capital.
During the year ended December 31, 2021, the Company accrued salary of $213,333 and was forgiven $46,667. As of December 31, 2021, the Company owes salary of $166,667.
Year ended December 31, 2020
In June 2018, the Company entered into an employment agreement with Dr. Edwards E. Jacobs, Jr., our CEO, for a base compensation of $10,000 monthly and 100,000 shares of common stock, then valued at $27,500. In October 2018, the agreement was amended to a base compensation is $7,000 in cash or equivalent in common stock. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. During year ended December 31, 2021, the Company recorded Stock based compensation of $42,000.
In August 2019, the Company entered into an employment agreement with Robert W. Ellis, our president, for a base compensation of $5,000 in cash per monthly and 250,000 shares to be issued on August 31, 2020, then valued at $26,375. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. During the year ended December 31, 2021, the Company recorded Stock based compensation of $13,188 and $8,792 and accrued liabilities of $10,000.
In September 2019, the Company entered into an employment agreement with Ronald Lambrecht, our Chief Financial Officer, for a base compensation of $5,000, or equivalent in common stock, monthly and 100,000 shares to be issued on September 30, 2020, then valued at $11,010. All shares to be issued during fiscal year 2020 were waived due to COVID-19 related slow-downs and other factors. We extended the period of the original agreement and the services to be provided will commence on January 2, 2020. During the year ended December 31, 2020, the Company recorded Stock based compensation of $37,340.
11. PROVISION FOR INCOME TAXES
The Company provides for income taxes under ASC 740,” Income Taxes.” Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recorded based on the differences between the financial statement and tax basis of assets and liabilities and the tax rates in effect when these differences are expected to reverse. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
The provision for income taxes differs from the amounts which would be provided by applying the statutory federal income tax rate of 21% to the net loss before provision for income taxes for the following reasons:
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| Net operating loss | | $ | (155,988 | ) | | $ | (64,079 | ) |
| Valuation allowance | | | 155,988 | | | | 64,079 | |
| Income tax expense per books | | $ | - | | | $ | - | |
The cumulative tax effect at the expected rate of 21% of significant items comprising our net deferred tax amount is as follows:
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| NOL Carryover | | $ | 426,096 | | | $ | 270,108 | |
| Valuation allowance | | | (426,096 | ) | | | (270,108 | ) |
| Net deferred tax asset | | $ | - | | | $ | - | |
Due to the change in ownership provisions of the Income Tax laws of United States of America, net operating loss carry forwards of approximately $1,286,000 for federal income tax reporting purposes are subject to annual limitations. When a change in ownership occurs, net operating loss carry forwards may be limited as to use in future years.
12. SUBSEQUENT EVENTS
On January 26, 2022, the Company amended its articles of incorporation to increase its authorized common shares from 200,000,000 to 750,000,000; this increase will be effective March 19, 2022. Also on January 26, 2022, the Company’s directors authorized the establishment of its Series B Preferred Stock. The Series B can be used to purchase intellectual property and other assets and has enhanced voting and conversion privileges. There are no shares of Series B Preferred Stock outstanding as of the date of this filing.
On February 2, 2022, the Company entered into a Patent Purchase Agreement and Consulting Agreement with Thomas J. Mohr. Under the Agreement, the Company has the exclusive option to purchase Mohr’s U.S. Patent No. 9,783,432B (the “Patent”), which covers technology used in enhancing the capability of water to hold significantly larger amounts of oxygen, for a two-year period. The Agreement furthermore allows the Company a two-year license to use the technology covered by the Patent, including for further development of oxygenated water products for consumers. In exchange for the license, the Company will pay Mohr a royalty, scaled to total product sales.
Bioadaptives (PK) (USOTC:BDPT)
Historical Stock Chart
From Apr 2024 to May 2024
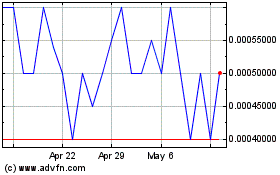
Bioadaptives (PK) (USOTC:BDPT)
Historical Stock Chart
From May 2023 to May 2024