SK Bioscience and GlaxoSmithKline's Covid-19 Vaccine Meets Co-Primary Objectives
April 29 2022 - 4:23AM
Dow Jones News
By Cecilia Butini
GlaxoSmithKline PLC said Friday that a Covid-19 vaccine
developed with South Korea-based SK Bioscience Co. has met its
co-primary objectives in a Phase 3 study, demonstrating superior
antibody levels compared with AstraZeneca PLC's shot, which was
chosen as a control vaccine.
The British pharmaceutical major said that the vaccine, which is
called SKYCovione and uses Glaxo's adjuvant technology, showed a
clinically acceptable safety profile, with most postvaccine adverse
reactions being mild or moderate.
SK Bioscience will apply for emergency-use listing with the
World Health Organization and authorization at individual
regulatory agencies around the world. The company had already
signed an advance purchase agreement with South Korea for a total
of 10 million doses, Glaxo said.
Write to Cecilia Butini at cecilia.butini@wsj.com
(END) Dow Jones Newswires
April 29, 2022 04:08 ET (08:08 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
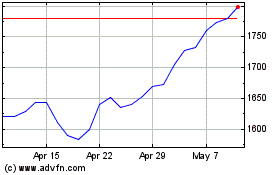
Gsk (LSE:GSK)
Historical Stock Chart
From Mar 2024 to Apr 2024
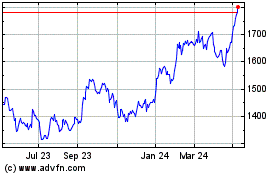
Gsk (LSE:GSK)
Historical Stock Chart
From Apr 2023 to Apr 2024