Biofrontera: FDA Approves BF-RhodoLED XL Lamp; ADRs Up
October 22 2021 - 10:56AM
Dow Jones News
By Michael Dabaie
Biofrontera AG said the U.S. Food and Drug Administration
approved the BF-RhodoLED XL red-light source for photodynamic
therapy, sending American depositary receipts of the German company
higher.
FDA granted the approval as a combination approval with the
company's prescription drug Ameluz. The new, larger BF-RhodoLED XL
was approved in combination with Ameluz for the treatment of mild
and moderate actinic keratoses--rough scaly patches--on the face
and scalp. The new PDT lamp enables the illumination of larger
areas, enabling the simultaneous treatment of several actinic
keratoses distant from each other.
ADRs were up 9%, to $5.99, in morning trading.
Ahead of the approval by the FDA, the new lamp was protected by
several patent applications.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
October 22, 2021 10:41 ET (14:41 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
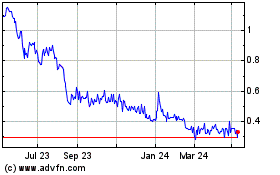
Biofrontera (TG:B8F)
Historical Stock Chart
From Mar 2024 to Apr 2024
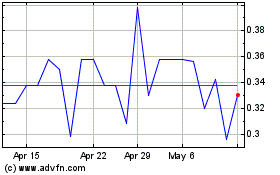
Biofrontera (TG:B8F)
Historical Stock Chart
From Apr 2023 to Apr 2024