Roche's At-Home Covid-19 Test Gets Emergency-Use Authorization in US
December 27 2021 - 1:57AM
Dow Jones News
By Cristina Roca
Roche Holding AG said late Friday that its at-home Covid-19 test
has been granted emergency-use authorization by the U.S. Food and
Drug Administration and will be available across the country from
January.
The Swiss pharmaceutical holding said the kit produces accurate
results in as little as 20 minutes for all known variants of
concern of the virus, including Omicron.
The test was prioritized by the FDA based on the ability of
Roche and SD Biosensor Inc.--with which Roche has a global
distribution agreement--to delivery large quantities and increase
manufacturing to meet future demand. Roche has the capacity to
produce tens of millions of tests a month, it said.
Write to Cristina Roca at cristina.roca@wsj.com
(END) Dow Jones Newswires
December 27, 2021 01:42 ET (06:42 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
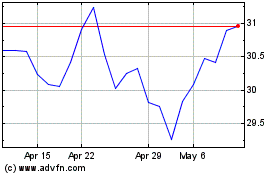
Roche (QX) (USOTC:RHHBY)
Historical Stock Chart
From Mar 2024 to Apr 2024
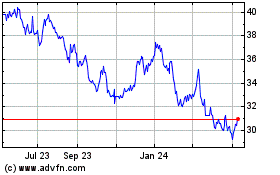
Roche (QX) (USOTC:RHHBY)
Historical Stock Chart
From Apr 2023 to Apr 2024