By Jared S. Hopkins
Researchers have begun giving healthy volunteers in the U.S. an
experimental coronavirus vaccine developed by Pfizer Inc. and
partner BioNTech SE, the latest study exploring a potential defense
against the respiratory disease.
Researchers at the New York University Grossman School of
Medicine in Manhattan and the University of Maryland School of
Medicine in Baltimore said Tuesday they began injecting people with
the first of four vaccine candidates from Pfizer and Germany's
BioNTech.
The clinical trial will help the researchers evaluate whether
the candidates are safe, which produces the strongest immune
response that could fend off the coronavirus and what the dose
should be. Pfizer plans to advance the candidate that proves most
promising. Testing of the vaccine candidates in Germany began last
month.
Results from the 360-person study in the U.S. could come as
early as next month, but the vaccine will still need to undergo
additional testing in more patients, said Kathrin Jansen, Pfizer's
head of vaccine research and development.
Pfizer will track the progress of the study to pick the most
promising vaccine candidate, Dr. Jansen said.
The plan is to "weed out, weed out, weed out, focus on what's
good and move on," Dr. Jansen said. "It's a quick elimination."
A vaccine could be ready for emergency use as early as the fall
if testing indicates it works safely, Pfizer Chief Executive Albert
Bourla told The Wall Street Journal last week, though the company
would keep studying it in clinical trials.
The U.S. government has the authority to grant limited use of a
vaccine or drug during a health emergency, before testing is
complete.
Several potential coronavirus vaccines have entered human
testing, including candidates from Moderna Inc. and Oxford
University.
The vaccine efforts have advanced quickly compared with typical
vaccine R&D timelines, though some experts have expressed
concern over whether testing will be rigorous enough to adequately
assess if the shots can safely protect people from contracting the
coronavirus.
"The question is, what steps are you skipping?" said Paul Offit,
director of the Vaccine Education Center at Children's Hospital of
Philadelphia, who developed a vaccine for a common disease in young
children called rotavirus that causes diarrhea and vomiting.
Adding to the challenges of deciphering whether a coronavirus
vaccine works, researchers say, is uncertainty about how immunity
develops against the virus.
Without that knowledge, researchers are relying on their
understanding of previous coronaviruses and antibodies that
neutralize viruses, said Kirsten Lyke, a professor of medicine at
the University of Maryland School of Medicine, who is helping lead
the Pfizer trial.
The Pfizer and BioNTech vaccine will initially be studied in
adults 18 to 55 years old, and eventually will enroll older
volunteers, Pfizer said.
After receiving the first dose, patients will receive a second
dose three weeks later. Pfizer will test other doses later on,
including a single shot, Dr. Jansen said.
Four out of every five study subjects will get the vaccine,
while the remaining subjects will take a placebo.
The vaccine uses a gene-based technology known as messenger RNA.
Messenger RNA, or mRNA, carries instructions from DNA to the body's
cells to make certain proteins. An mRNA vaccine has never been
approved to prevent any infectious disease.
Mark Mulligan, director of the Vaccine Center at NYU Langone
Health, said he doesn't expect challenges enrolling patients.
"People, frankly, are sick of this virus and they want to do
whatever they can to fight back," he said. "The public recognizes
that in order to start returning to normalcy, we've got to get
people protected, and vaccines hold the greatest hope for
that."
Melissa Honkanen, 42 years old, of New York City, enrolled in
the study after hearing about it from her husband, a pediatrician
at NYU Langone, because she wanted to be helpful.
"It's really hard to see all of this happening," said Ms.
Honkanen, a yoga instructor. "We're in a world where we can't hug
each other or be social and active."
Aside from working on a vaccine, Pfizer is also trying to
develop an antiviral treatment that could begin testing this
summer, and it is testing whether rheumatoid-arthritis drug Xeljanz
helps fight Covid-19, the disease caused by the coronavirus.
Pfizer plans to soon manufacture the vaccines at its facilities
in Massachusetts, Michigan and Missouri, as well as Puurs, Belgium,
with additional locations to come.
Write to Jared S. Hopkins at jared.hopkins@wsj.com
(END) Dow Jones Newswires
May 05, 2020 06:59 ET (10:59 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
Pfizer (NYSE:PFE)
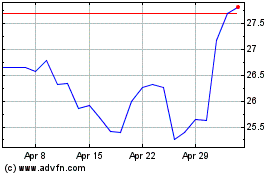
Historical Stock Chart
From Mar 2024 to Apr 2024
Pfizer (NYSE:PFE)
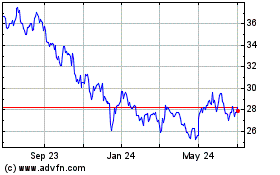
Historical Stock Chart
From Apr 2023 to Apr 2024