Addex Therapeutics, Janssen Gets FDA Approval to Begin Proof of Concept Study
January 21 2021 - 6:43AM
Dow Jones News
By Chris Wack
Addex Therapeutics Ltd. said its partner, Janssen
Pharmaceuticals Inc., has received the U.S. Food and Drug
Administration's Investigational New Drug approval to begin a Phase
2a proof of concept study for a treatment for patients with
epilepsy.
The clinical-stage pharmaceutical company said the first patient
is expected to be treated during the second quarter of 2021.
Addex said the multi-center study will assess the efficacy,
safety, tolerability and pharmacokinetics of adjunctive
JNJ-40411813 administration in patients with focal onset seizures
with suboptimal response to levetiracetam.
The primary objective of the study is to evaluate the efficacy
of JNJ-40411813 in combination with levetiracetam using a
time-to-event endpoint.
Addex Therapeutics shares were up 65% to $17.79 in premarket
trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
January 21, 2021 06:28 ET (11:28 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
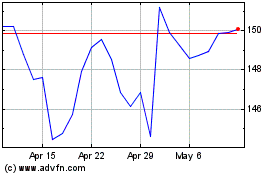
Johnson and Johnson (NYSE:JNJ)
Historical Stock Chart
From Mar 2024 to Apr 2024
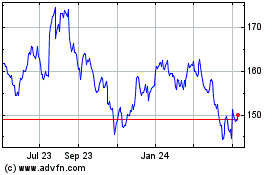
Johnson and Johnson (NYSE:JNJ)
Historical Stock Chart
From Apr 2023 to Apr 2024