Emergent BioSolutions Announces Publication of CHIKV VLP Phase 2 Safety and Immunogenicity Data in The Lancet Infectious Diseases
June 21 2022 - 6:30AM

Emergent BioSolutions Inc. (NYSE:EBS) announced today that two-year
persistence data from its phase 2 clinical study evaluating the
safety and immunogenicity of the company’s investigational
chikungunya virus virus-like particle (CHIKV VLP) vaccine candidate
were published in The Lancet Infectious Diseases. The article,
Safety and immunogenicity of PXVX0317, an aluminum
hydroxide-adjuvanted chikungunya virus-like particle vaccine: a
randomised, double-blind, parallel-group, phase 2 trial, is
available online.
“Emergent is pleased that results from our CHIKV VLP phase 2
study have been published and shared with the scientific community
in The Lancet Infectious Diseases, a highly esteemed peer-reviewed
journal,” said Chris Cabell, M.D., chief medical officer and SVP
clinical development at Emergent BioSolutions. “Publication of this
body of work highlights the importance of developing ways to
address Chikungunya disease, a public health threat for which no
vaccine or treatment exists.”
Summary of published resultsThis randomized,
double-blind, parallel-group, phase 2 study was conducted at three
U.S. sites with 415 participants. Eligible participants were
healthy CHIKV-naïve adults aged 18–45 years. The primary endpoint
was the geometric mean titer of anti-CHIKV neutralizing antibody on
day 57 (28 days after the last vaccination). Safety was also
assessed.
Emergent’s CHIKV VLP vaccine candidate was well tolerated and
induced a robust and durable serum neutralizing antibody immune
response against the Chikungunya virus up to two years. A single
dose, 40 μg injection of adjuvanted CHIKV VLP is being further
investigated in phase 3 clinical trials (clinicaltrials.gov
identifiers NCT05072080 and NCT05349617).
A Comment, which provides expert opinion on a published article,
was subsequently published by the journal, and noted that the
“adjuvanted CHIKV VLP vaccine candidate is a welcome step forward.”
i
About the Chikungunya virusChikungunya virus is
spread to people by infected mosquitoes. Symptoms include fever,
joint pain, headache, muscle pain, joint swelling or rash. The
geographic distribution of CHIKV has expanded to more than 100
countries and territories worldwide.
About Emergent BioSolutionsAt Emergent, our
mission is to protect and enhance life. For over 20 years, we’ve
been at work defending people from things we hope will never
happen—so we are prepared, just in case they ever do. We provide
solutions for complex and urgent public health threats through a
portfolio of vaccines and therapeutics that we develop and
manufacture for governments and consumers. We also offer a range of
integrated contract development and manufacturing services for
pharmaceutical and biotechnology customers. To learn more about how
we plan to protect or enhance 1 billion lives by 2030, visit
our website and follow us on LinkedIn, Twitter,
and Instagram.
Safe Harbor StatementThis press release
includes forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Any statements,
other than statements of historical fact, including statements
regarding our ability to fill the need for an approved vaccine to
prevent chikungunya disease, the effectiveness of the product
candidate, and executing on our development program, are
forward-looking statements. These forward-looking statements are
based on our current intentions, beliefs and expectations regarding
future events. We cannot guarantee that any forward-looking
statement will be accurate. Investors should realize that if
underlying assumptions prove inaccurate or unknown risks or
uncertainties materialize, actual results could differ materially
from our expectations. Investors are, therefore, cautioned not to
place undue reliance on any forward-looking statement. Any
forward-looking statement speaks only as of the date of this press
release, and, except as required by law, we do not undertake to
update any forward-looking statement to reflect new information,
events or circumstances.
There are a number of important factors that could cause the
company's actual results to differ materially from those indicated
by such forward-looking statements, including the success of the
planned development program; the timing of and ability to obtain
and maintain regulatory approvals for the product candidate; and
our commercialization, marketing and manufacturing capabilities.
The foregoing sets forth many, but not all, of the factors that
could cause actual results to differ from our expectations in any
forward-looking statement. Investors should consider this
cautionary statement, as well as the risk factors identified in our
periodic reports filed with the SEC, when evaluating our
forward-looking statements.
Investor Contact:Robert G.
BurrowsVice President, Investor
Relations240-631-3280BurrowsR@ebsi.com
Media Contact:Matt HartwigSenior
Director, Media Relationsmediarelations@ebsi.com
____________________________
i Stephenson K, Novel chikungunya vaccine shows
promise for durable protection, Lancet Infect
Dis. 2022; (published online June 13)
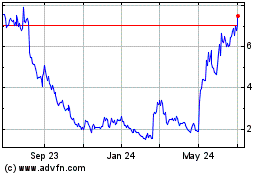
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Mar 2024 to Apr 2024
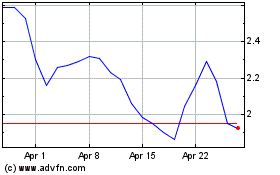
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Apr 2023 to Apr 2024