FDA Finds Poor Conditions at Contractor's Plant for Making J&J's Covid-19 Vaccine
April 21 2021 - 10:29AM
Dow Jones News
By Peter Loftus
A contract manufacturing plant that was helping make Johnson
& Johnson's Covid-19 vaccine didn't keep clean, sanitary
conditions and failed to take proper steps to prevent
contamination, federal health regulators said Wednesday.
The U.S. Food and Drug Administration, which inspected the plant
owned by contract manufacturer Emergent BioSolutions Inc., said it
completed the inspection on Tuesday and would work with Emergent to
address the findings from the inspection.
Production of J&J's vaccine at the Emergent plant has been
paused, and material already manufactured will undergo additional
testing before it is released for potential distribution, the FDA
said.
"We will not allow the release of any product until we feel
confident that it meets our expectations for quality," said FDA
Acting Commissioner Janet Woodcock and Peter Marks, who heads the
FDA department overseeing vaccines.
The Emergent plant recently had a contamination-related mishap
that ruined a batch of the main ingredient for J&J's Covid-19
vaccine. No doses were distributed from the failed batch, and the
FDA hasn't authorized doses using material from the Emergent plant
to be distributed.
The mishap led to J&J taking over responsibility for
producing its vaccine at the Emergent plant.
(END) Dow Jones Newswires
April 21, 2021 10:14 ET (14:14 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
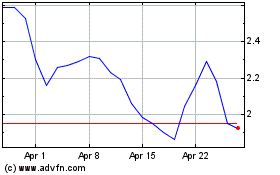
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Mar 2024 to Apr 2024
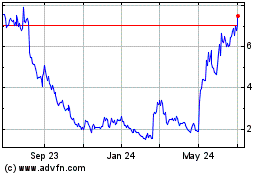
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Apr 2023 to Apr 2024