J&J Says Covid-19 Vaccine Batch Didn't Meet Quality Standards
March 31 2021 - 7:26PM
Dow Jones News
By Peter Loftus and Thomas M. Burton
Johnson & Johnson said one batch of its new Covid-19 vaccine
didn't meet quality standards at a contract manufacturer, and the
doses weren't distributed.
J&J said Wednesday the batch never advanced to the filling
and finish stages of its manufacturing process, and that the
quality lapse wouldn't affect its ability to supply the U.S. with
100 million doses by the end of May.
J&J didn't disclose the nature of the quality lapse or how
many doses were affected, but said it arose from quality checks
during the start-up phase of manufacturing. The company said it
shared information about the issue with the U.S. Food and Drug
Administration.
The FDA is investigating, according to a person familiar with
the matter.
The New York Times reported Wednesday that the J&J doses
were ruined due to an accidental mix-up of ingredients at Emergent
BioSolutions Inc., the contract manufacturer working with J&J.
The Times reported that about 15 million doses were ruined.
Emergent declined to comment.
Write to Peter Loftus at peter.loftus@wsj.com and Thomas M.
Burton at tom.burton@wsj.com
(END) Dow Jones Newswires
March 31, 2021 19:11 ET (23:11 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
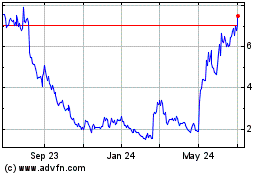
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Mar 2024 to Apr 2024
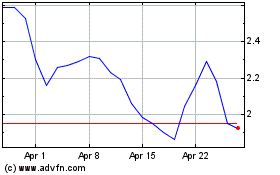
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Apr 2023 to Apr 2024