Payment of Filing Fee (Check the appropriate box):
| | | | | | | | |
ý | No fee required. |
o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies: |
| (2) | Aggregate number of securities to which transaction applies: |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0‑11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| (4) | Proposed maximum aggregate value of transaction: |
| (5) | Total fee paid: |
o | Fee paid previously with preliminary materials. |
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount Previously Paid: |
| | |
| (2) | Form, Schedule or Registration Statement No.: |
| | |
| (3) | Filing Party: |
| | |
| (4) | Date Filed:
|
On May 10, 2022, Biohaven Pharmaceutical Holding Company Ltd. (the “Company”) made the following social media posts on LinkedIn, Twitter and Facebook.
Disclosure Notice
This release contains forward-looking information about Pfizer’s proposed acquisition of the Company, the Company’s related spin-off of its development stage pipeline compounds, the Company’s commercial and pipeline portfolio, including rimegepant and zavegepant, expected best-in-class and growth potential, and Pfizer’s Internal Medicine portfolio and growth potential, including their potential benefits, that involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Risks and uncertainties include, among other things, risks related to the satisfaction or waiver of the conditions to closing the proposed acquisition (including the failure to obtain necessary regulatory approvals and failure to obtain the requisite vote by the Company’s shareholders) in the anticipated timeframe or at all, including the possibility that the proposed acquisition does not close; the possibility that competing offers may be made; risks related to the ability to realize the anticipated benefits of the proposed acquisition, including the possibility that the expected benefits from the acquisition will not be realized or will not be realized within the expected time period; the risk that the businesses will not be integrated successfully; disruption from the transaction making it more difficult to maintain business and operational relationships; risks related to diverting management’s attention from the Company’s ongoing business operation; negative effects of this announcement or the consummation of the proposed acquisition on the market price of Pfizer’s common stock, the Company’s common shares and/or their respective operating results; significant transaction costs; unknown liabilities; the risk of litigation and/or regulatory actions related to the proposed acquisition, spin-off or the Company’s business; risks and costs related to the implementation of the separation of Biohaven Research Ltd., including timing anticipated to complete the separation and any changes to the configuration of the businesses included in the separation if implemented; the risk that the integration of the Company and Pfizer will be more difficult, time consuming or costly than expected; other business effects and uncertainties, including the effects of industry, market, business, economic, political or regulatory conditions; future exchange and interest rates; changes in tax and other laws, regulations, rates and policies; future business combinations or disposals; the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as the possibility of unfavorable new clinical data and further analyses of existing clinical data; risks associated with interim data; the risk that clinical trial data are subject to differing interpretations and assessments by regulatory authorities; whether regulatory authorities will be satisfied with the design of and results from the clinical studies; whether and when drug applications may be filed in particular jurisdictions for rimegepant or zavegepant or any other investigational products; whether and when any such applications may be approved by regulatory authorities, which will depend on myriad factors, including making a determination as to whether the product's benefits outweigh its known risks and determination of the product's efficacy and, if approved, whether rimegepant, zavegepant or any such other products will be commercially successful; decisions by regulatory authorities impacting labeling, manufacturing processes, safety and/or other matters that could affect the availability or commercial potential of rimegepant, zavegepant or any such other products; uncertainties regarding the impact of COVID-19; and competitive developments.
You should carefully consider the foregoing factors and the other risks and uncertainties that affect the businesses of Pfizer and the Company described in the “Risk Factors” and “Forward-Looking Information and Factors That May Affect Future Results” sections of their respective Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and other documents filed by either of them from time to time with the U.S. Securities and Exchange Commission (the “SEC”), all of which are available at www.sec.gov. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and Pfizer and the Company assume no obligation to, and do not intend to, update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise, unless required by law. Neither Pfizer nor the Company gives any assurance that it will achieve its expectations.
Additional Information and Where to Find It
In connection with the proposed transaction, the Company will be filing documents with the SEC, including preliminary and definitive proxy statements relating to the proposed transaction. The definitive proxy statement will be mailed to the Company’s shareholders in connection with the proposed transaction. This communication is not a substitute for the proxy statement or any other document that may be filed by the Company with the SEC. BEFORE MAKING ANY VOTING DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PRELIMINARY AND DEFINITIVE PROXY STATEMENTS AND ANY OTHER DOCUMENTS TO BE FILED WITH THE SEC IN CONNECTION WITH THE PROPOSED TRANSACTION OR INCORPORATED BY REFERENCE IN THE PROXY STATEMENT WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Any vote in respect of resolutions to be proposed at the Company’s shareholder meeting to approve the proposed transaction or other responses in relation to the proposed transaction should be made only on the basis of the information contained in the Company’s proxy statement. Investors and security holders may obtain free copies of these documents (when they are available) and other related documents filed with the SEC at the SEC’s web site at www.sec.gov or on the Company’s website at https://www.biohavenpharma.com/investors.
No Offer or Solicitation
This communication is for information purposes only and is not intended to and does not constitute, or form part of, an offer, invitation or the solicitation of an offer or invitation to purchase, otherwise acquire, subscribe for, sell or otherwise dispose of any securities, or the solicitation of any vote or approval in any jurisdiction, pursuant to the proposed transaction or otherwise, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law.
Participants in the Solicitation
The Company and certain of its directors, executive officers and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the Company’s directors and executive officers is set forth in its proxy statement for its 2022 annual meeting of shareholders, which was filed with the SEC on March 11, 2022. Other information regarding participants in the proxy solicitations in connection with the proposed transaction, and a description of any interests that they have in the proposed transaction, by security holdings or otherwise, will be included in the proxy statement described above. These documents are available free of charge at the SEC’s web site at www.sec.gov and by going to the Company’s website at https://www.biohavenpharma.com/investors.
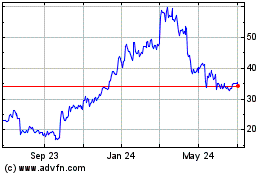
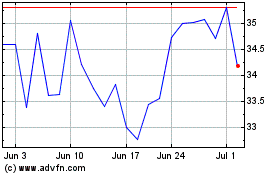
Biohaven (NYSE:BHVN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Biohaven (NYSE:BHVN)
Historical Stock Chart
From Apr 2023 to Apr 2024