FDA Issues Warning Letters to Two Breast-Implant Manufacturers
March 19 2019 - 5:13PM
Dow Jones News
By Maria Armental
U.S. health regulators have issued warning letters to two
manufacturers of silicone gel-filled breast implants, citing
failures to assess long-term safety and risks.
The Food and Drug Administration said Johnson and Johnson's
(JNJ) Mentor Worldwide failed to address regulators' concerns about
patient enrollment and data inconsistencies and that Sientra Inc.
(SIEN) failed to address agency concerns about study progress.
Company representatives didn't immediately comment Tuesday
afternoon.
"Post-approval requirements are critical to ensuring the safety
and effectiveness of the medical products we regulate," FDA
Commissioner Scott Gottlieb said in a statement.
Write to Maria Armental at maria.armental@wsj.com
(END) Dow Jones Newswires
March 19, 2019 16:58 ET (20:58 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
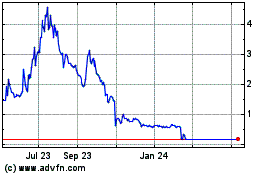
Sientra (NASDAQ:SIEN)
Historical Stock Chart
From Mar 2024 to Apr 2024
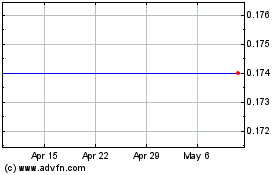
Sientra (NASDAQ:SIEN)
Historical Stock Chart
From Apr 2023 to Apr 2024