As Filed Pursuant to Rule 424(b)(5)
Registration No. 333-230535
The information in this
preliminary prospectus supplement is not complete and may be changed. A registration statement relating to these securities has
been filed with the Securities and Exchange Commission and is effective. This preliminary prospectus supplement and the accompanying
prospectus are not an offer to sell these securities and they are not soliciting an offer to buy these securities in any jurisdiction
where the offer or sale is not permitted.
SUBJECT TO COMPLETION,
DATED JULY 27, 2020
PROSPECTUS SUPPLEMENT
(To Prospectus Dated April 8, 2019)

NEMAURA MEDICAL INC.
Shares
of Common Stock
Warrants to Purchase up to Shares
of Common Stock
We are offering shares
of our common stock, par value $0.001 per share, and warrants to purchase up to shares
of our common stock (each a “Warrant”). Each share of our common stock is being sold together with of
a Warrant, each whole Warrant to purchase one share of our common stock. The Warrants will have an exercise price of $ per share,
will be immediately exercisable and will expire on the five-year anniversary of the original issuance date. The shares of our
common stock and Warrants are immediately separable and will be issued separately, but will be purchased together in this offering.
The shares of our common stock issuable from time to time upon exercise of the Warrants are also being offered pursuant to this
prospectus supplement and the accompanying prospectus.
Our common stock is listed on the Nasdaq
Capital Market under the symbol "NMRD." On July 24, 2020, the last reported sale price of our common stock on the Nasdaq
Capital Market was $9.47 per share. There is no established public trading market for the Warrants, and we do not expect a market
to develop. We do not intend to apply for listing of the Warrants on any securities exchange or other nationally recognized trading
system. Without an active trading market, the liquidity of the Warrants will be limited.
Investing
in our securities involves risks. See "Risk Factors" beginning on page S-9 of this prospectus supplement and the
risk factors incorporated by reference into this prospectus supplement and the accompanying prospectus.
|
|
|
Per Share and
Warrant
|
|
Total
|
|
|
Public offering price
|
|
$
|
|
|
$
|
|
|
|
Underwriting discount (1)
|
|
$
|
|
|
$
|
|
|
|
Proceeds to us (before expenses)
|
|
$
|
|
|
$
|
|
|
|
|
(1)
|
See section entitled “Underwriting” beginning on page S-23 for additional information regarding the compensation payable to the underwriters.
|
Neither the Securities and Exchange
Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy
of this prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
The securities offered
hereby are expected to be ready for delivery on or about ,
2020 against payment in immediately available funds.
Sole Book-Running Manager
Kingswood Capital Markets
a division of Benchmark Investments, Inc.
The date
of this prospectus supplement is ,
2020
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
|
|
|
|
Page
|
|
|
ABOUT THIS PROSPECTUS SUPPLEMENT
|
|
|
S-1
|
|
|
PROSPECTUS SUPPLEMENT SUMMARY
|
|
|
S-2
|
|
|
THE OFFERING
|
|
|
S-8
|
|
|
RISK FACTORS
|
|
|
S-9
|
|
|
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
|
|
S-13
|
|
|
USE OF PROCEEDS
|
|
|
S-14
|
|
|
DILUTION
|
|
|
S-14
|
|
|
DESCRIPTION OF SECURITIES BEING OFFERED
|
|
|
S-15
|
|
|
CAPITALIZATION
|
|
|
S-17
|
|
|
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF OUR COMMON STOCK
|
|
|
S-18
|
|
|
UNDERWRITING
|
|
|
S-24
|
|
|
INCORPORATION BY REFERENCE OF CERTAIN DOCUMENTS
|
|
|
S-29
|
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
|
|
S-30
|
|
|
LEGAL MATTERS
|
|
|
S-30
|
|
|
EXPERTS
|
|
|
S-30
|
|
PROSPECTUS
|
ABOUT THIS PROSPECTUS
|
|
|
2
|
|
|
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
|
|
2
|
|
|
PROSPECTUS SUMMARY
|
|
|
3
|
|
|
THE OFFERING
|
|
|
3
|
|
|
OUR COMPANY
|
|
|
4
|
|
|
RISK FACTORS
|
|
|
6
|
|
|
USE OF PROCEEDS
|
|
|
6
|
|
|
RATIO OF EARNINGS TO FIXED CHARGES
|
|
|
7
|
|
|
DESCRIPTIONS OF THE SECURITIES WE MAY OFFER
|
|
|
7
|
|
|
PLAN OF DISTRIBUTION
|
|
|
19
|
|
|
LEGAL MATTERS
|
|
|
21
|
|
|
EXPERTS
|
|
|
21
|
|
|
LIMITATION ON LIABILITY AND DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
|
|
|
21
|
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
|
|
21
|
|
|
INCORPORATION BY REFERENCE OF CERTAIN DOCUMENTS
|
|
|
22
|
|
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement
and the accompanying base prospectus form part of a registration statement on Form S-3 that we filed with the Securities and Exchange
Commission, which we refer to as the SEC, using a "shelf" registration process. This document contains two parts. The
first part consists of this prospectus supplement, which provides you with specific information about this offering. The second
part, the accompanying base prospectus, provides more general information, some of which may not apply to this offering. Generally,
when we refer only to the "prospectus," we are referring to both parts combined. This prospectus supplement may add,
update or change information contained in the accompanying base prospectus. To the extent that any statement we make in this prospectus
supplement is inconsistent with statements made in the accompanying base prospectus or any documents incorporated by reference
herein or therein, the statements made in this prospectus supplement will be deemed to modify or supersede those made in the accompanying
base prospectus and such documents incorporated by reference herein and therein.
In this prospectus
supplement, "Nemaura," the "Company," "we," "us," "our" and similar terms refer
to Nemaura Medical Inc., a Nevada corporation and its consolidated subsidiaries. References to our "common stock" refer
to the common stock, par value $0.001 per share, of Nemaura Medical Inc.
All references in this
prospectus supplement to our consolidated financial statements include, unless the context indicates otherwise, the related notes.
The industry and market
data and other statistical information contained in the documents we incorporate by reference in the prospectus are based on management's
own estimates, independent publications, government publications, reports by market research firms or other published independent
sources, and, in each case, are believed by management to be reasonable estimates. Although we believe these sources are reliable,
we have not independently verified the information.
You should rely only
on the information contained in or incorporated by reference in this prospectus supplement, the accompanying base prospectus and
in any free writing prospectus that we have authorized for use in connection with this offering. We have not, and the underwriters
have not, authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information,
you should not rely on it. You should assume that the information in this prospectus supplement, the accompanying base prospectus,
the documents incorporated by reference in the accompanying base prospectus, and in any free writing prospectus that we have authorized
for use in connection with this offering, is accurate only as of the date of those respective documents. Our business, financial
condition, results of operations and prospects may have changed since those dates. You should read this prospectus supplement,
the accompanying base prospectus, the documents incorporated by reference in the accompanying base prospectus, and any free writing
prospectus that we have authorized for use in connection with this offering, in their entirety before making an investment decision.
You should also read and consider the information in the documents to which we have referred you in the sections of the accompanying
base prospectus entitled "Where You Can Find More Information" and "Incorporation by Reference of Certain Documents."
We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale
is not permitted.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights
selected information contained elsewhere in this prospectus supplement, the accompanying prospectus and in the documents we incorporate
by reference. This summary does not contain all of the information you should consider before investing in our common stock. You
should read this entire prospectus supplement and the accompanying prospectus carefully, especially the risks of investing in our
common stock discussed under “Risk Factors” beginning on page S-8 of this prospectus supplement, page 5 of the accompanying
prospectus and page __ of our Annual Report on Form 10-K for the year ended March 31, 2020, which is incorporated by reference
in this prospectus supplement, along with our consolidated financial statements and notes to those consolidated financial statements
and the other information incorporated by reference in this prospectus supplement and the accompanying prospectus, before making
an investment decision.
About Nemaura
Overview
We are a medical technology company
developing sugarBEAT®, a non-invasive, affordable and flexible continuous glucose monitoring system for adjunctive use by persons
with diabetes. SugarBEAT consists of a disposable adhesive skin-patch connected to a rechargeable wireless transmitter that displays
glucose readings at regular five minute intervals via a mobile app. SugarBEAT works by extracting glucose from the skin into a
chamber in the patch that is in direct contact with an electrode-based sensor. The transmitter sends the raw data to a mobile app
where it is processed by an algorithm and displayed as a glucose reading, with the ability to track and trend the data over days,
weeks and months. While sugarBEAT requires once per day calibration by the patient using a blood sample obtained by a
finger stick, we believe sugarBEAT will be adopted by non-insulin dependent persons with diabetes alongside insulin-injecting
persons with diabetes who all perform multiple daily finger sticks to manage their disease.
CE approval was granted by the European
Notified Body BSI in May 2019, allowing the product to be made available for commercial sale. The company commenced a phase 1 launch
whereby devices were made available to limited cohorts of users to gauge their feedback so that any fine-tuning could be completed
prior to a mass market launch. A mass-market launch is due to commence in the UK in the coming months via its UK licensee DB Ethitronix,
whereby the sugarBEAT will be available via a number of subscription models that are yet to be determined. In July 2020, Nemaura
filed a PMA application with the FDA to use sugarBEAT as an adjunct to finger prick testing for blood glucose trending. In addition
it has been pursuing the possibility of launching the sugarBEAT under the FDA Wellness guidance which would preclude it from an
FDA assessment and allow the product to be launched in the US for Wellness use, to provide prompts and educate users on factors
affecting their blood sugar profiles. Further to discussions with the FDA Nemaura established that sugarBEAT can be classified
under the Wellness guidance when it is used according to the FDA Wellness guidance notes. Nemaura is now expediting a commercial
launch in the US under the Wellness guidance. Nemaura is now expediting its planning of a commercial launch in the US.
We believe there are additional applications
for sugarBEAT and the underlying BEAT technology platform, which may include:
|
|
–
|
a web-server accessible by physicians and diabetes professionals to track the condition remotely, thereby reducing healthcare costs and managing the condition more effectively;
|
|
|
–
|
a complete virtual doctor that monitors a person's vital signs and transmits results via the web;
|
|
|
–
|
other patches using the BEAT technology platform to measure alternative analytes, including lactate, uric acid, lithium and drugs. This would be a step-change in the monitoring of conditions, particularly in the hospital setting. Lactate monitoring is currently used to determine the relative fitness of professional athletes and we completed preliminary studies demonstrating the application of the BEAT technology for continuous lactate monitoring;
|
|
|
–
|
a continuous temperature monitoring system which could have various applications, including use for individuals to monitor their temperature in connection with diagnosis and monitoring of symptoms of novel coronavirus (COVID-19); and
|
|
|
–
|
monitoring disease progression in COVID-19 patients using continuous lactate monitoring (CLM).
|
Our Business Strategy
We intend to lead in the discovery,
development and commercialization of innovative and targeted diagnostic medical devices that improve disease monitoring, management
and overall patient care. Specifically, we intend to focus on the monitoring of molecules that can be drawn out through the skin
non-invasively using our technology platform. In addition to glucose, such molecules may include lactic acid monitoring and the
monitoring of prescription drugs and blood biomarkers that may help in the diagnosis, prevention or management of diseases, such
as diabetes. We plan to take the following steps to implement our broad business strategy. Our key commercial strategies post-approval
will first be implemented in Europe and then in parts of the Middle East and Asia, and then the U.S., as follows:
|
|
–
|
Commercialize sugarBEAT in the United Kingdom and Republic of Ireland with Dallas Burston Pharma (Jersey) Limited, with whom we have an exclusive marketing rights agreement for these two countries.
|
We have also signed a full commercial
agreement with Dallas Burston Ethitronix (Europe) Limited in May 2018 for all other European territories as part of an equal joint
venture agreement. The joint venture intends to seek sub-license rights opportunities to one or more leading companies
in the diabetes monitoring space, to leverage their network, infrastructure and resources.
Dallas Burston (Jersey) Limited
was founded by Dr. Dallas Burston, MBBS, an entrepreneur who has founded and sold several companies specializing in marketing pharmaceuticals.
For example, in 1999, he sold 49% of Ashbourne Pharmaceuticals to HSBC Private Equity for £32 million and Bartholomew-Rhodes
to Galen Ltd. for £19.8 million. More recently, in 2015, he sold DB Ashbourne Limited, a provider of off-patent branded pharmaceuticals
for the UK market, to Ethypharm. At the time of the sale, DB Ashbourne Limited was estimated to have annual revenue of approximately
£90 million.
|
|
–
|
Establish licensing or joint venture agreements with other parties to market sugarBEAT in other geographies. We are in detailed discussions and negotiations with several other parties worldwide for licensing or joint venture agreements for the sale of the sugarBEAT device and have signed commercial agreements with TP MENA for the Gulf Cooperation Council, and Al-Danah Medical for Qatar.
|
|
|
–
|
Submit FDA application for approval of sugarBEAT. The application is currently in progress and expected to be submitted by June 30, 2020.
|
|
|
–
|
Expand the indications for which the sugarBEAT device may be used. We believe that the sugarBEAT device may offer significant benefits as compared to those found in the non-acute setting for the monitoring of other diseases. This includes monitoring of lactic acid for performance athletics, and the monitoring of drugs. We have completed initial proof of concept for lactate monitoring and now plan to explore the route to commercialization for well-being applications in athletic performance training, and plan to undertake further clinical programs to support clinical use of the device for lactate monitoring.
|
|
|
–
|
Expand our product pipeline through our proprietary platform technologies, acquisitions and strategic licensing arrangements. We intend to leverage our proprietary platform technologies to grow our portfolio of product candidates for the diagnosis of diabetes and other diseases. In addition, we intend to license our product and acquire products and technologies that are consistent with our research and development and business focus and strategies. This may include drug delivery products for the improved management of diabetes, for example improved insulin injector systems, and/or combination drug products for diabetes related drugs.
|
Product Development
Management has extensive experience
in regulatory and clinical development of diagnostic medical devices. We intend to take advantage of this experience in the field
of diagnostic medical devices in an attempt to increase the probability of product approval. The overall regulatory process
for diagnostic medical devices for diabetes is currently similar to those governing other diagnostic devices. The timelines are
shorter than, for example, when new drugs or completely invasive diagnostic devices are trialed in clinics. We have successfully
tested and evaluated the device for its clinical output, in this case the accuracy and safety with which it can trend blood glucose
levels, based on which CE approval was granted by the Notified Body BSI, and we are currently in the process of preparing a submission
to the U.S. FDA. As we continue to raise funds for marketing the device in some European Union territories, we also intend to seek
collaborations with future licensees and marketing partners to achieve our product development and meet our projected milestones.
The table below provides our current estimate of our timeline:
Product Development and Commercialization
Timelines
|
|
|
|
|
Milestone
|
Target Start Date
|
Target Completion Date
|
|
Completion of clinical studies in Type 1 and Type 2 diabetic subjects to define final device claims and for submission for CE Mark approval with final device claims.
|
July 2017
|
Completed
|
|
Scale up of commercial sensor/patch manufacturing
(Scale up means we have started looking at larger scales
- sufficient for product launch in the UK. It refers to the manufacturing process for sensors.)
|
January 2017
|
Ongoing
|
|
Scale up of device (transmitter) manufacturing
|
January 2017
|
Ongoing
|
|
CE Mark for body worn transmitter device
|
August 2018
|
Completed
|
|
Commercial launch in the UK, followed by major territories in Europe
|
July - September 2020
|
Staggered launch
|
|
U.S. FDA PMA Submission
|
June 2020
|
June 2020
|
|
Commercial launch of proBEATÔ in the U.S.
|
October - December 2020
|
October - December 2020
|
Intellectual Property
Our trade secrets, trademarks, and patents filed, granted
and pending are as follows
|
IP: Patent (Core Claim), Know-how,7 Trademark Pending
|
|
Expiration Date
|
|
Jurisdictions in which Granted/ Issued
|
|
Jurisdictions in which
|
|
Ongoing Royalty or Milestone Payments
|
|
|
|
|
|
|
|
|
|
|
|
Patent: Cumulative Measurement of an Analyte (1)
|
|
May 20, 2032
|
|
Australia, France, Germany, Italy, Poland, Spain, Netherlands, UK, Brazil, China, India, Japan, U.S.
|
|
Canada, Qatar, UAE
|
|
None. Internal development
|
|
Skin prep Patch (2)
|
|
December 2, 2038
|
|
PCT Filed
|
|
To be determined at national stages
|
|
None. Internal development
|
|
Membrane Anchor sensor (3)
|
|
December 9, 2038
|
|
PCT Filed
|
|
To be determined at national stages
|
|
None. Internal development
|
|
Sensor Calibration algorithm (4)
|
|
October 27, 2039
|
|
UK Application filed, PCT due Oct 2020
|
|
To be determined at national stages
|
|
None. Internal development
|
|
Sample extraction (5)
|
|
March 9, 2040
|
|
UK Application filed, PCT due March 2021
|
|
To be determined at national stages
|
|
None. Internal development
|
|
Know-how: Sensor Formulation
|
|
N/A
|
|
Trade Secret
|
|
N/A
|
|
None. Internal development
|
|
Trademark: BEAT
|
|
Renewal due in 2026
|
|
UK, China, EU, India, Japan
|
|
Canada
|
|
None. Internal development
|
|
Trademark: sugarBEAT
|
|
Renewal due in 2025
|
|
UK, Australia, Switzerland, China, Egypt, EU, Israel, India, Iran, Japan, North Korea, Morocco, Mexico, Norway, New Zealand, Russia, Singapore, Tunisia, Turkey, U.S.
|
|
Canada
|
|
None. Internal development
|
(1) This patent provides a formula
for calculating the amount of glucose extracted over a defined period of time by deducting the difference between two readings
to allow rapid sensing without needing to deplete the analyte being measured.
(2) This patent describes a device
and method for preparing the skin for extraction of glucose.
(3) This patent provides a device
and method for interfacing a solid substrate to the sensor electrode to reduce background noise signals.
(4) This patent describes an algorithm
for calibrating the raw data to provide glucose readings.
(5) This patent describes a device
and method for extracting glucose from the skin.
RECENT DEVELOPMENT
Continuous glucose monitoring
(CGM) device
In February 2020,
Nemaura announced that following discussions with the FDA, Nemaura had established that it may sell its CGM product with a digital
service offering in the U.S. without FDA approval as a non-medical wellbeing application. Nemaura further announced that it intends
to commence sales of this product under the brand proBEAT in the U.S. in Q4 2020. The product offering will enable users to wear
the CGM device from which data will be sent to Nemaura’s servers in the cloud, from where the big data will be processed
to provide users with educational material and insights into factors that can affect their sugar levels and tips for healthy lifestyle
and diet, with a view to helping pre-diabetics and diabetics alike live healthier lives.
Note Purchase
Note Purchase Agreement
On April 15, 2020, the Company entered
into a note purchase agreement (the “Note Purchase Agreement”) by and among the Company, DDL, TCL and Chicago Venture
Partners, L.P. (the “Investor”).
Pursuant to the terms of the Note
Purchase Agreement, the Company agreed to issue and sell to the Investor and the Investor agreed to purchase from the Company a
secured promissory note (the “Secured Note”) in the original principal amount of $6,015,000. In consideration thereof,
on April 15, 2020 (the closing date), (i) the Investor (a) paid $1,000,000 in cash, (b) issued to the Company (1) Investor Note
#1 in the principal amount of $2,000,000 (“Investor Note #1”), and (2) Investor Note #2 in the principal amount of
$2,000,000 (“Investor Note #2” and together with Investor Note #1, the “Investor Notes”), and (ii) the
Company delivered the Secured Note on behalf of the Company, to the Investor, against delivery of the Purchase Price. For these
purposes, the “Purchase Price” means the Investor’s initial cash purchase price, together with the sum of the
initial principal amounts of the Investor Notes.
The Secured Note is secured by the
Collateral (as hereinafter defined). The Secured Note carries an original issue discount (“OID”) of $1,000,000. In
addition, the Company agreed to pay $15,000 to the Investor to cover the Investor’s legal fees, accounting costs, due diligence,
monitoring and other transaction costs incurred in connection with the purchase and sale of the Secured Note (the “Transaction
Expense Amount”), all of which amount is included in the initial principal balance of the Secured Note. The Purchase Price
for the Secured Note is $5,000,000, computed as follows: $6,015,000 original principal balance, less the OID, less the Transaction
Expense Amount.
The borrowing period is 24 months
and the Company shall pay the outstanding balance and all fees on maturity. A monitoring fee equal to 0.833% of the outstanding
balance will automatically be added to the outstanding balance on the first day of each month. The debt less the discount will
be accreted over the term of the Note using the effective interest method.
Security Agreement
On April 15, 2020, the Company entered
into the Security Agreement by the Company, DDL and TCL, in favor of the Investor (the “Security Agreement”). Pursuant
to the terms of the Security Agreement, the Company entered into the Security Agreement and granted the Investor a first-priority
security interest in all rights, title, interest, claims and demands of the Company in and to all of the Company’s patents
and all other proprietary rights, and all rights corresponding to the Company’s patents throughout the world, now owned and
existing, and all replacements, proceeds, products and accessions thereof.
ATM Offering
On October 19, 2018, the Company
entered into an Equity Distribution Agreement (the “Agreement”) with Maxim Group LLC as sales agent (“Maxim”),
pursuant to which the Company may offer and sell, from time to time, through Maxim up to $20,000,000 in shares of its common stock,
par value $0.001 per share.
On March 4, 2020, the Company and
Maxim entered into an amendment (the “Amendment”) to the Agreement, pursuant to which the parties agreed, that notwithstanding
anything in the Agreement to the contrary, the Agreement will remain in full force and effect without a specific time-period term,
provided that either the Company or Maxim may terminate the Agreement upon ten (10) days’ prior written notice to the other
party. No other changes to the Agreement were made by the Amendment.
Subsequent to March 31, 2020, and
through July 24, 2020, the Company raised gross proceeds of $4,174,090 for the issuance of 401,230 shares of common stock through
Maxim.
Exercise of Warrants
Subsequent to March 31, 2020 and
through July 24, 2020, the Company raised gross proceeds of $394,503 from the exercise of warrants and the issuance of 37,933 shares
at $10.40 per share.
Impact of COVID-19
Since
the outbreak of COVID-19 in March 2020 resulting in national social distancing and people working from home, COVID-19 resulted
in delays in our product development, manufacturing and commercialization of our innovative and targeted diagnostic medical
devices that improve disease monitoring, management and overall patient care.
As
a medical device manufacturer, public health crises, such as the widespread outbreaks of infectious diseases like the COVID-19
pandemic, may negatively impact our operations. Health concerns and significant changes in political or economic conditions caused
by such outbreaks can cause significant reductions in demand for routine diagnostic testing and medical device procedures or increased
difficulty in serving customers, disrupt manufacturing and supply chains, and negatively affect our operations as well as the operations
of our suppliers, distributors and other third-party partners. Furthermore, such widespread outbreaks may impact the broader economies
of affected countries, including negatively impacting economic growth, the proper functioning of financial and capital markets,
foreign currency exchange rates, and interest rates. Due to uncertainties regarding the duration and impact of the current COVID-19
pandemic, we are unable to predict the extent to which the COVID-19 pandemic may have a material effect on our business, financial
condition or results of operations.
Notwithstanding,
the Company does not anticipate that COVID-19 will have any long-term detrimental effect on the Company’s success. While
key suppliers have not been accessible throughout the whole period of the outbreak, we have been able to be flexible in our priorities
and respond favorably to the challenges faced during the outbreak. We
have also seen a surge in the uptake of technologies for remote and patient self- monitoring, which therefore potentially enhances
the prospects for the likes of the Company and its CGM product and planned digital healthcare offering.
Employee equity compensation plan
The Company adopted the Nemaura Medical
Inc., Omnibus Incentive Plan (the “Plan”) effective May 15, 2020. The Plan authorized 1,000,000 shares of common stock
for issuance under the Plan for future grants.
Submission of PMA Application
for sugarBeat®
In July 2020, the Company submitted
its PMA application for sugarBEAT® to the United States Food and Drug Administration (FDA).
Recent announcements
of two potential applications of the BEAT technology platform in the management of COVID-19.
Continuous
Temperature Monitoring
Several diseases
including COVID-19 are characterized by fever (an increase in body temperature) meaning that temperature monitoring is a vital
tool in detection/ diagnosis and consequently in preventing the spread of such diseases. Measuring body temperature can also be
used to track the course of a disease so as to allow doctors to analyse the effectiveness of treatments and thus proactively adapt
to improve outcomes. Monitoring body temperature can also assist in other areas not related to diseases such as helping a woman
track her ovulation cycle and hence can be a powerful ally in helping couples understand the fertility cycle and giving them the
best chance of conception.
In order to monitor
temperature at the standard that is fit for clinical use requires electronic components and circuitry that are of a very high quality,
precision, and accuracy. The Company’s platform has all of these features by default given it measures electrical currents
from a sensor that are as small as a billionth of an ampere. The Company is adapting the device for continuous temperature monitoring
for all of the above applications. It anticipates a product launch in early to mid-2021 (in Europe initially) pending regulatory
approvals.
Continuous
Lactate Monitoring
Increased lactate
levels have been consistently associated with morbidity and mortality in a wide range of disease states for many years. The Company
recently issued a presentation summarizing several independently published articles indicating that there is an increase in lactic
acid levels in patients with COVID-19, (https://nemauramedical.com/wp-content/uploads/2020/07/BEAT-CLM-in-Covid19-July-2020-1.pdf).
The measurement of blood lactic acid levels therefore serves to potentially provide a means of tracking disease progression, including
progression and severity of COVID-19 infections. The Company’s existing CE mark approved device sugarBEAT for glucose monitoring
has already been demonstrated as being suited for lactic acid monitoring, within changes only to the sensor chemistry and the algorithm
and the information depicted on a smart device app. The Company is working towards regulatory approval of this device in 2021 for
continuous lactic acid monitoring in critical care setting including the monitoring of disease progression in COVID-19.
License from Healthimation
As indicated above
under “-Continuous glucose monitoring (CGM) device”, the Company intends to be launching by the end of 2020 a subscription
based digital wellness program incorporating its non-invasive continuous glucose monitor in the USA under the brand proBEATTM.
The Company has recently been in
discussions with and entered into a letter of intent to acquire key assets from Healthimation Inc., which possesses technology
developed at the Joslin Institute, which is affiliated to Harvard University. The key assets include and relate to a behavioural
change program that has been clinically validated over a 12 year period to demonstrate that it can lead to clinically significant
outcomes in diabetic patients through a series of programs intended to help with diet and weight loss. The key assets also include
several on-going contracts of Healthimation Inc. with third party licensees. The Company believes the acquisition of the key assets
of Healthimation Inc. to be a valuable addition to its existing assets, and will allow the Company to provide a compelling digital
subscription offering, which the Company anticipates will also lead to reimbursement from employers as well as healthcare insurers.
The acquisition is intended to be completed by the end of August 2020, and proBEATTM launched in the USA by the end of 2020.
CORPORATE INFORMATION
Our principal executive offices are
located at 57 West 57th Street New York, NY 10019. Our website is located at www.nemauramedical.com and our telephone
number is + 1 646-416-8000. Information found on, or accessible through, our website is not a part of, and is not incorporated
into, this prospectus supplement, and you should not consider it part of the prospectus supplement.
THE OFFERING
|
|
|
|
Common Stock offered by us
|
shares of our common stock.
|
|
|
|
|
Total common stock outstanding after the offering (1)
|
shares of common stock.
|
|
|
|
|
Warrants offered by us
|
Warrants to purchase up to shares
of common stock. Each share of our common stock is being sold together with
of a Warrant, each whole Warrant to purchase one share of our common stock. Each of the Warrants will have an exercise price of
$ per share, will be immediately exercisable and will expire on the fifth anniversary of the original issue date.
This prospectus supplement also related to
the offering of the shares of common stock issuable upon exercise of the Warrants. The exercise price of the Warrants and the number
of shares into which the Warrants may be exercised are subject to adjustment in certain circumstances. See “Description of
Securities Being Offered” on page S-15.
|
|
|
|
|
Use of proceeds
|
We intend to use the net proceeds from this offering for general corporate purposes, which include, but are not limited to, the commercial launch of a subscription based service for the US under the Wellness category, Lactate monitor development for launch, glucose monitoring product launch in Europe and the development of a second generation of sugarBeat. See "Use of Proceeds" on page S-14.
|
|
|
|
|
Risk factors
|
An investment in our shares of common stock is highly speculative and involves a number of risks. You should carefully consider the information contained in the "Risk Factors" section beginning on page S-9 of this prospectus supplement, and elsewhere in this prospectus supplement and the base prospectus, and the information we incorporate by reference, before making your investment decision.
|
|
|
|
|
Nasdaq Capital Market symbol
|
Our common stock is listed on the Nasdaq Capital Market under the symbol "NMRD." There is no established public trading market for the Warrants, and we do not expect a market to develop. We do not intend to apply for listing of the Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Warrants will be limited.
|
__________________________________
|
(1)
|
The number of shares of common stock
to be outstanding after this offering is based on 21,299,711 shares of common stock outstanding on July 24, 2020. The
number of shares of common stock excludes:
|
|
|
●
|
|
147,637 shares of common stock issuable upon the exercise of warrants outstanding with a weighted average exercise price of $10.40 per share;
|
|
|
●
|
|
240,000 units, each consisting of one share of common stock, par value $0.001 per share, together with one warrant to purchase one share of common stock at an exercise price equal to $10.40, per share, in a public offering;
|
|
|
●
|
|
9,710 shares of common stock issuable upon the exercise of options outstanding with a weighted average exercise price of $13.00 per share; and
|
|
|
●
|
|
______ shares of common stock issuable upon the exercise of the Warrants offered hereby.
|
RISK FACTORS
An investment
in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully
the risks described below and discussed under the sections captioned "Risk Factors" contained in our Annual Report on
Form 10-K for the year ended March 31, 2020, which are incorporated by reference into this prospectus supplement and the accompanying
base prospectus in their entirety, together with other information in this prospectus supplement, the accompanying base prospectus,
the information and documents incorporated by reference herein and therein, and in any free writing prospectus that we have authorized
for use in connection with this offering. If any of these risks actually occurs, our business, financial condition, results of
operations or cash flow could be seriously harmed. This could cause the trading price of our common stock to decline, resulting
in a loss of all or part of your investment.
Risks Related to our Business
Our business, financial condition and
results of operations may be materially adversely affected by global health epidemics, including the recent COVID-19 outbreak.
Outbreaks of epidemic,
pandemic, or contagious diseases such as COVID-19, could have an adverse effect on our business, financial condition, and results
of operations. The spread of COVID-19 from China to other countries has resulted in the World Health Organization declaring the
outbreak of COVID-19 as a global pandemic. Any resulting financial impact cannot be reasonably estimated at this time. The extent
to which the COVID-19 impacts our results will depend on future developments, which are highly uncertain and cannot be predicted,
including new information which may emerge concerning the severity of the coronavirus and the actions taken globally to contain
the coronavirus or treat its impact, among others. Existing insurance coverage may not provide protection for all costs that may
arise from all such possible events. We are still assessing our business operations and the impact COVID-19 may have on our results
and financial condition, but there can be no assurance that this analysis will enable us to avoid part or all of any impact from
the spread of COVID-19 or its consequences, including downturns in business sentiment generally or in our sector in particular.
The
impact of COVID-19 is not expected to have any long-term detrimental effect on the Company’s success. While key suppliers
have not been accessible throughout the whole period of the outbreak, we have been able to be flexible in our priorities and respond
favorably to the challenges faced during the outbreak. We
have also seen a surge in the uptake of technologies for remote and patient self- monitoring, which therefore potentially enhances
the prospects for the likes of Nemaura Medical and its CGM product and planned digital healthcare offering.
There can be no
assurances that we will be able to successfully launch our continuous temperature monitoring system to assist with diagnosis and
monitor disease progression in COVID-19 patients using continuous lactate monitoring (CLM).
Although
we believe we will be able to do so, there can be no assurances that we will be able to launch our continuous temperature monitoring
system to assist with diagnosis or monitor disease progression in COVID-19 patients using continuous lactate monitoring (CLM).
If we are unable to successfully launch our continuous temperature monitoring system to assist with diagnosis or monitor disease
progression in COVID-19 patients, we will not be able to move forward our planned short term business transition of launching our
continuous temperature monitoring system to assist with diagnosis and using our applications for monitoring disease progression
in COVID-19 patients using CLM. Additionally, time spent attempting to launch our continuous temperature monitoring system to assist
with diagnosis and monitor disease progression in COVID-19 would affect our ability to perform testing and upgrades for our other
products and could have an adverse impact on business operations.
Even if the Company
is able to successfully launch our continuous temperature monitoring system to assist with diagnosis or monitor disease progression
in COVID-19 patients using continuous lactate monitoring (CLM), there can be no assurances that we will generate revenue.
The
Company has not had any material conversations or entered into any agreements with a third party regarding launching our continuous
temperature monitoring system to assist with diagnosis or the performance of COVID-19 monitoring and there is no guarantee that
we will ever enter into any such agreements. As a result, despite our potential ability to launch our continuous temperature monitoring
system to assist with diagnosis or monitor disease progression in COVID-19, there can be no assurances that we will be able to
commercialize such ability to generate any revenue.
The public may
not ultimately adopt our continuous temperature monitoring system to assist with the diagnosis and monitoring of symptoms of COVID-19
or adopt our continuous lactate monitoring system (CLM) to monitor disease progression in COVID -19 patients.
There
are currently no vaccines or drugs approved to treat or prevent COVID-19. Although there are investigational COVID-19 vaccines
and treatments under development, these investigational products are in the early stages of product development and have not yet
been fully tested for safety or effectiveness.
The
U.S. Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) issued warning letters to a number of companies
for selling fraudulent COVID-19 products. These products are unapproved drugs that pose significant risks to patient health and
violate federal law. The FDA and FTC are taking this action as part of their response in protecting consumers during the global
COVID-19 outbreak from companies preying on such consumers by promoting products with fraudulent prevention and treatment claims.
The FDA is particularly concerned that products that claim to cure, treat or prevent serious diseases like COVID-19 may cause consumers
to delay or stop appropriate medical treatment, leading to serious and life-threatening harm.
In light of potential
distrust of the public and regulatory enforcement agencies regarding the effectiveness of products that claim to cure, treat or
prevent COVID-19, the public may not ultimately adopt our continuous temperature monitoring system to assist with the diagnosis
and monitoring of symptoms of COVID-19 or adopt our continuous lactate monitoring system (CLM) to monitor disease progression in
COVID -19 patients, which could significantly impact our business and prospects.
Risks Related to this Offering
Our management will have broad
discretion over the use of the net proceeds from this offering, you may not agree with how we use the proceeds and the proceeds
may not be invested successfully.
Our management will
have broad discretion over the use of proceeds from this offering, and we could spend the proceeds from this offering in ways with
which you may not agree or that do not yield a favorable return. We intend to use the net proceeds from this offering for general
corporate purposes, which include, but are not limited to, the commercial launch of a subscription based service for the US under
the Wellness category, Lactate monitor development for launch, glucose monitoring product launch in Europe and the development
of a second generation of sugarBeat. As of the date of this prospectus supplement, we cannot specify with certainty all of the
particular uses of the proceeds from this offering. Accordingly, our management will have broad discretion as to the use of the
net proceeds from this offering and could use them for purposes other than those contemplated at the time of commencement of this
offering. Accordingly, you will be relying on the judgment of our management with regard to the use of these net proceeds, and
you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately.
It is possible that, pending their use, we may invest the net proceeds in a way that does not yield a favorable, or any, return
for our company.
If you purchase shares of common
stock in this offering, you will suffer immediate and substantial dilution in the book value of your investment.
The price per share
of our common stock in this offering may exceed the net tangible book value per share of our common stock outstanding prior to
this offering. Therefore, if you purchase shares of our common stock in this offering, you may pay a price per share that substantially
exceeds our net tangible book value per share after this offering. See "Dilution" for a more detailed discussion of the
dilution you may incur in connection with this offering.
There may be future sales or other
dilution of our equity, which may adversely affect the market price of our common stock.
We are not
generally restricted from issuing additional common stock, or any securities that are convertible into or exchangeable for,
or that represent the right to receive, common stock. The issuance of any additional common stock or preferred stock or
securities convertible into, exchangeable for, or that represent the right to receive, common stock, or the exercise of such
securities, could be substantially dilutive to holders of our common stock. After this offering, we will
have shares
of common stock outstanding based on 21,299,711 shares of common stock outstanding on July 24, 2020. The market price of our
common stock could decline as a result of this offering, sales of our common stock made after this offering or the perception
that such sales could occur. Because our decision to issue securities in any future offering will depend on market conditions
and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of future offerings. Thus,
our stockholders bear the risk of future offerings reducing the market price of our common stock and diluting their
shareholdings in us.
A large number of shares may be
sold in the market following this offering, which may depress the market price of our common stock.
Sales of a substantial
number of shares of our common stock in the public market following this offering could cause the market price of our common stock
to decline. If there are more shares of common stock offered for sale than buyers are willing to purchase, then the market price
of our common stock may decline to a market price at which buyers are willing to purchase the offered shares of common stock and
sellers remain willing to sell the shares. All of the shares sold in this offering will be freely tradable without restriction
or further registration under the Securities Act.
Because we do not intend to declare
cash dividends on our shares of common stock in the foreseeable future, stockholders must rely on appreciation of the value of
our common stock for any return on their investment.
We have never declared
or paid cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation
and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. In addition,
the terms of any future debt agreements may preclude us from paying dividends. As a result, we expect that only appreciation of
the price of our common stock, if any, will provide a return to investors in this offering for the foreseeable future.
Our common stock may be delisted
from The Nasdaq Capital Market if we cannot maintain compliance with Nasdaq’s continued listing requirements.
Our common stock
is listed on the Nasdaq Capital Market. There are a number of continued listing requirements that we must satisfy in order
to maintain our listing on the Nasdaq Capital Market.
On July 15, 2019, we received
a deficiency letter from the Listing Qualifications Department of The Nasdaq Stock Market stating that, for the preceding 30 consecutive
business days, the closing bid price for our common stock was below the minimum $1.00 per share requirement (“Minimum Bid
Price Rule”) for continued inclusion on The Nasdaq Capital Market pursuant to Nasdaq Listing Rule 5550(a)(2). In accordance
with Nasdaq Rule 5810(c)(3)(A), we were provided a period of 180 calendar days, or until January 11, 2020, to regain compliance
with the Minimum Bid Price Rule. On November 27, 2019, our Board of Directors approved (i) a reverse stock split of the issued
and outstanding shares of our common stock, at a ratio of 1-for-10 shares (“Reverse Stock Split), and (ii) a decrease of
our authorized number of shares of common stock on the same basis from 420,000,000 shares of common stock to 42,000,000 shares
of common stock (“Decrease in Authorized Common Stock”). On November 27, 2019, we filed a Certificate of Change with
the Secretary of State of Nevada to effect the Reverse Stock Split and Decrease in Authorized Common Stock. The Reverse Stock Split
became effective on the Nasdaq Capital Market at the open of market on December 5, 2019. On December 19, 2019, we received a letter
from the Nasdaq Stock Market stating that because our common stock maintained a closing bid price at or above $1.00 per share
for a minimum of ten (10) consecutive business days, we regained compliance with the Minimum Bid Price Rule for continued listing
on the Nasdaq Capital Market, as set forth in Nasdaq Listing Rule 5550(a)(2), and that the matter is now closed.
On April 2, 2020, the Company
received a written notice (the “Notice”) from staff of Listing Qualifications of The Nasdaq Stock Market LLC (“Nasdaq”)
indicating that the Company has not complied with the requirements of Nasdaq Listing Rule 5620(a) of Nasdaq’s listing rules
due to its failure to hold an annual meeting of stockholders within 12 months of the end of the Company’s fiscal year ended
December 31, 2018. The Company had 45 calendar days to submit a plan to regain compliance and, if Nasdaq accepted the plan, Nasdaq
could grant an exception of up to 180 calendar days from the fiscal year end, or until September 28, 2020, to regain compliance.
As discussed with Nasdaq, the Company filed and mailed its proxy materials relating to its annual meeting on April 10, 2020 and
held such annual meeting on May 15, 2020. As a result, the Company regained compliance with Nasdaq Listing Rule 5620(a), and that
matter is now closed.
We cannot assure you our
securities will meet the continued listing requirements to be listed on Nasdaq in the future. If Nasdaq delists our common stock
from trading on its exchange, we could face significant material adverse consequences including:
|
|
·
|
a limited availability of market quotations for our securities;
|
|
|
·
|
a determination that our common stock is a “penny stock” which will require brokers trading in our common stock to adhere to more stringent rules and possibly resulting in a reduced level of trading activity in the secondary trading market for our common stock;
|
|
|
·
|
a limited amount of news and analyst coverage for our company; and
|
|
|
·
|
a decreased ability to issue additional securities or obtain additional financing in the future.
|
If we fail to maintain
compliance with all applicable continued listing requirements for the Nasdaq Capital Market and Nasdaq determines to delist our
common stock, the delisting could adversely affect the market liquidity of our common stock, our ability to obtain financing to
repay debt and fund our operations.
If our common stock is delisted from
the Nasdaq and the price of our common stock declines below $5.00 per share, our common stock would come within the definition
of “penny stock”.
Transactions in securities
that are traded in the United States that are not traded on Nasdaq or on other securities exchange by companies, with net tangible
assets of $5,000,000 or less and a market price per share of less than $5.00, may be subject to the “penny stock”
rules. The market price of our common stock is currently more than $5.00 per share. If our common stock is delisted from the Nasdaq
and the price of our common stock declines below $5.00 per share and our net tangible assets remain $5,000,000 or less, our common
stock would come within the definition of “penny stock”.
Under these penny stock rules, broker-dealers
that recommend such securities to persons other than institutional accredited investors:
|
|
·
|
must make a special written suitability determination for the purchaser;
|
|
|
·
|
receive the purchaser’s written agreement to a transaction prior to sale;
|
|
|
·
|
provide the purchaser with risk disclosure documents which identify risks associated with investing in “penny stocks” and which describe the market for these “penny stocks” as well as a purchaser’s legal remedies; and
|
|
|
·
|
obtain a signed and dated acknowledgment from the purchaser demonstrating that the purchaser has actually received the required risk disclosure document before a transaction in a “penny stock” can be completed.
|
As a result of these requirements,
if our common stock is at such time subject to the “penny stock” rules, broker-dealers may find it difficult to effectuate
customer transactions and trading activity in these shares in the United States may be significantly limited. Accordingly, the
market price of the shares may be depressed, and investors may find it more difficult to sell the shares.
Our common stock may be affected
by limited trading volume and may fluctuate significantly.
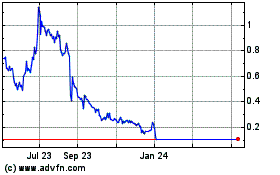
Our common stock
is traded on the Nasdaq Capital Market. Although an active trading market has developed for our common stock, there can be no assurance
that an active trading market for our common stock will be sustained. Failure to maintain an active trading market for our common
stock may adversely affect our shareholders’ ability to sell our common stock in short time periods, or at all. Our common
stock has experienced, and may experience in the future, significant price and volume fluctuations, which could adversely affect
the market price of our common stock.
There is no public market for the warrants.
There is no established
public trading market for the warrants being offered in this offering and we do not expect a market to develop. In addition, we
do not intend to apply for listing of the warrants on any securities exchange or automated quotation system. Without an active
market, investors in this offering may be unable to readily sell the warrants.
The warrants may be dilutive to
holders of our common stock.
The ownership interest
of the existing holders of our common stock will be diluted to the extent the warrants offered in this offering or other outstanding
warrants are exercised. Prior to this offering, 147,637 shares of common stock are issuable upon the exercise of warrants outstanding
with a weighted average exercise price of $10.40 (aggregate amount of $1,535,425). The shares of our common stock underlying our
outstanding warrants represented approximately 0.69% of our common stock outstanding as of July 24, 2020.
Holders of warrants purchased in
this offering will have no rights as common stockholders until such holders exercise their warrants and acquire our common stock.
Until holders of
warrants acquire shares of our common stock upon exercise of the warrants, such holders will have no rights with respect to the
shares of our common stock underlying such warrants. Upon exercise of the warrants, the holders thereof will be entitled to exercise
the rights of common stockholders only as to matters for which the record date occurs after the exercise date.
SPECIAL NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus supplement and our SEC
filings that are incorporated by reference into this prospectus supplement contain or incorporate by reference forward-looking
statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities
Exchange Act of 1934, as amended (the "Exchange Act"). All statements, other than statements of historical fact, included
or incorporated by reference in this prospectus supplement regarding our development of our strategy, future operations, future
financial position, projected costs, prospects, plans and objectives of management are forward-looking statements. Forward-looking
statements may include, but are not limited to, statements about:
|
|
•
|
|
any statements of the plans, strategies and objectives of management for future operations;
|
|
|
•
|
|
any statements concerning proposed new products, services or developments;
|
|
|
•
|
|
any statements regarding future economic conditions or performance;
|
|
|
•
|
|
our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others;
|
|
|
•
|
|
our estimates regarding the sufficiency of our cash resources and our need for additional funding;
|
|
|
•
|
|
our intended use of the net proceeds from the offerings of shares of common stock under this prospectus supplement;
|
|
|
•
|
|
any statement that our business, financial condition and results of operations may be materially adversely affected by global health epidemics, including the recent COVID-19; and
|
|
|
•
|
|
any statement regarding the effectiveness of our continuous temperature monitoring system to assist with the diagnosis and monitoring of symptoms of COVID-19 or the effectiveness of our continuous lactate monitoring system (CLM) to monitor disease progression in COVID -19 patients.
|
The words "believe,"
"anticipate," "design," "estimate," "plan," "predict," "seek," "expect,"
"intend," "may," "could," "should," "potential," "likely," "projects,"
"continue," "will," and "would" and similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain these identifying words. Forward-looking statements reflect our
current views with respect to future events, are based on assumptions and are subject to risks and uncertainties. We cannot guarantee
that we actually will achieve the plans, intentions or expectations expressed in our forward-looking statements and you should
not place undue reliance on these statements. There are a number of important factors that could cause our actual results to differ
materially from those indicated or implied by forward-looking statements. These important factors include those discussed under
the heading "Risk Factors" contained or incorporated in this prospectus supplement and the accompanying prospectus and
any free writing prospectus we may authorize for use in connection with a specific offering. These factors and the other cautionary
statements made in this prospectus supplement and the accompanying prospectus should be read as being applicable to all related
forward-looking statements whenever they appear in this prospectus supplement and the accompanying prospectus. Except as required
by law, we do not assume any obligation to update any forward-looking statement. We disclaim any intention or obligation to update
or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
USE OF PROCEEDS
We estimate that the net
proceeds we will receive from this offering will be approximately $ million (or approximately $ million if
the underwriter exercises its option to purchase additional shares in full), in each case, after deducting the estimated underwriting
discount and estimated offering expenses payable by us.
We intend to use
the net proceeds from this offering for general corporate purposes, which include, but are not limited to, the commercial launch
of a subscription based service for the US under the Wellness category, Lactate monitor development for launch, glucose monitoring
product launch in Europe and the development of a second generation of sugarBeat.
We have not determined
the amount of net proceeds to be used specifically for such purposes and, as a result, management will retain broad discretion
over the allocation of net proceeds. The occurrence of unforeseen events or changed business conditions could result in the application
of the net proceeds from this offering in a manner other than as described in this prospectus supplement. Pending the use of any
net proceeds, we expect to invest the net proceeds in interest-bearing, marketable securities.
DILUTION
If you invest in
our securities in this offering, your interest in our common stock will be diluted immediately to the extent of the difference
between the combined public offering price per share and related Warrant and the as adjusted net tangible book value per share
of our common stock immediately after this offering.
The net tangible
book value of our common stock as of March 31, 2020, was approximately $(2,264,796) or approximately (0.11) per share. Net tangible
book value per share represents the amount of our total tangible assets, excluding goodwill and intangible assets, less total liabilities,
divided by the total number of shares of our common stock outstanding. Dilution per share to new investors represents the difference
between the combined offering price per share and related warrant paid by purchasers in this offering and the net tangible book
value per share of our common stock immediately following the completion of this offering.
After giving effect
to the sale of shares of
our common stock and Warrants to purchase shares
of common stock in this offering at the combined public offering price of $ per
share and related Warrant, assuming no exercise of the Warrants offered hereby, no value is attributed to such Warrants and such
Warrants are classified as and accounted for as equity, and after deducting the underwriting discount and estimated offering
expenses, our as-adjusted net tangible book value as of March 31, 2020 would have been approximately $ or
approximately $ per share. This represents an immediate
increase in net tangible book value of approximately $ per
share to our existing stockholders and an immediate dilution in as-adjusted net tangible book value of approximately $ per
share new investors participating in this offering, as illustrated by the following table:
|
|
|
|
|
|
|
|
|
Public offering price per share and related Warrant
|
|
|
|
|
$
|
|
|
|
Net tangible book
value per share as of March 31, 2020
|
|
$
|
(0.11)
|
|
|
|
|
|
|
Increase in net tangible book value per share attributable to this offering
|
|
|
|
|
|
$
|
|
|
|
As-adjusted net tangible book value per share as of March 31, 2020, after giving effect to this offering
|
|
|
|
|
|
$
|
|
|
|
Dilution per share to new investors participating in this offering
|
|
|
|
|
|
$
|
|
|
The table above is
based on 20,850,848 shares of common stock outstanding as of March 31, 2020, and excludes, as of March 31, 2020, the following:
|
|
●
|
|
147,637 shares of common stock issuable upon the exercise of warrants outstanding with a weighted average exercise price of $10.40 per share;
|
|
|
●
|
|
240,000 units, each consisting of one share of common stock, par value $0.001 per share, together with one warrant to purchase one share of common stock at an exercise price equal to $10.40, per share, in a public offering;
|
|
|
●
|
|
9,710 shares of common stock issuable upon the exercise of options outstanding with a weighted average exercise price of $13.00 per share; and
|
|
|
●
|
|
______ shares of common stock issuable upon the exercise of the Warrants offered hereby.
|
To the extent that
after March 31, 2020, any outstanding warrants were or are exercised, or we otherwise issued or issue additional shares of common
stock in the future at prices per share below the price per share for any shares sold in this offering, there will be further dilution
to new investors.
DESCRIPTION OF SECURITIES BEING OFFERED
Common Stock
The material terms and
provisions of our common stock are described under the caption “Description of Securities We May Offer” starting on
page 9 of the accompanying prospectus.
Warrants
The following summary of
certain terms and provisions of the Warrants is not complete and is subject to, and qualified in its entirety by, the provisions
of the Warrants. Prospective investors should carefully review the terms and provisions of the form of Warrant for a complete description
of the terms and conditions of the Warrants.
Duration and Exercise
Price
Each Warrant will have
an initial exercise price of $ per share. The Warrants are exercisable
immediately upon issuance and will expire on the fifth anniversary of the date they first become exercisable. The exercise price
and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends,
stock splits, reorganizations or similar events affecting our common stock and the exercise price. The Warrants will be issued
separately from the common stock included in this offering. of a Warrant to purchase one share of our common stock will be sold
with each share of common stock purchased in this offering.
Cashless Exercise
If, at the time a holder
exercises its Warrants, a registration statement registering the issuance of the shares of common stock underlying the Warrants
under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment
otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder will be permitted
to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula
set forth in the Warrants.
Exercisability
The Warrants will be exercisable,
at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in
full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed
above). A holder (together with its affiliates) may not exercise any portion of a Warrant to the extent that the holder would own
more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days' prior notice from
the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder's Warrants up
to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage
ownership is determined in accordance with the terms of the Warrants. Purchasers of Warrants in this offering may also elect prior
to the issuance of Warrants to have the initial exercise limitation set at 9.99% of our outstanding common stock.
Fractional Shares
No fractional shares of
common stock will be issued upon the exercise of the Warrants. Rather, the number of shares of common stock to be issued will be
rounded to the nearest whole number.
Transferability
Subject to applicable laws,
the warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing
There is no established
public trading market for the Warrants being issued in this offering and we do not intend to list the Warrants on any securities
exchange or nationally recognized trading system. The common stock issuable upon exercise of the Warrants is currently listed on
the Nasdaq Capital Market.
Right as a Stockholder
Except as otherwise provided
in the Warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the Warrants do not
have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Warrants.
Fundamental Transaction
If
a fundamental transaction occurs, then the successor entity will succeed to, and be substituted for us, and may exercise every
right and power that we may exercise and will assume all of our obligations under the Warrants with the same effect as if such
successor entity had been named in the Warrant itself. If holders of our common stock are given a choice as to the securities,
cash or property to be received in a fundamental transaction, then the holder shall be given the same choice as to the consideration
it receives upon any exercise of the Warrant following such fundamental transaction. In addition, in certain circumstances, upon
a fundamental transaction, the holder will have the right to require us to repurchase its Warrant at its fair value using the Black
Scholes option pricing formula; provided, however, that, if the fundamental transaction is not within our control, including not
approved by our board of directors, then the holder shall only be entitled to receive the same type or form of consideration (and
in the same proportion), at the Black Scholes value of the unexercised portion of the Warrant, that is being offered and paid to
the holders of our common stock in connection with the fundamental transaction.
Rights
as a Stockholder
Except
as otherwise provided in the Warrants or by virtue of such holder’s ownership of our common stock, the holder of a Warrant
does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises
the warrant.
Amendment
and Waiver
The
Warrants may be modified or amended or the provisions thereof waived with the written consent of our company and the holders of
at least a majority of the common stock issuable upon the exercise of the then-outstanding Warrants (determined without giving
effect to the exercise limitation provisions of the Warrants); provided such modification, amendment or waiver applies to all of
the then-outstanding warrants.
CAPITALIZATION
The following
table sets forth our audited consolidated capitalization as of March 31, 2020 and our capitalization as of March 31, 2020 on an
as-adjusted basis, based on a public offering price of $ per share of common stock
and accompanying Warrant (excluding any proceeds that may be received, and shares of common stock that may be issued, upon exercise
of the Warrants). You should read the following table in conjunction with “Use of Proceeds” in this prospectus supplement
and our consolidated financial statements and the notes thereto incorporated by reference in this prospectus supplement and the
accompanying prospectus.
|
|
|
As of March 31, 2020
|
|
|
|
|
Actual
|
|
|
As Adjusted (1)
|
|
|
|
|
(audited)
|
|
|
|
|
|
Cash
|
|
$
|
106,107
|
|
|
$
|
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Series A Convertible preferred stock, $0.001 par value, 200,000 shares authorized and 0 shares issued and outstanding at March 31, 2020 on an actual basis and on an as adjusted basis, respectively.
|
|
|
-
|
|
|
|
-
|
|
|
Common stock, $0.001 par value, 42,000,000 shares authorized and 20,850,848 shares and _______ shares issued and outstanding at March 31, 2020 on an actual basis and on an as adjusted basis, respectively.
|
|
|
20,851
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
16,589,272
|
|
|
|
|
|
|
Accumulated deficit
|
|
|
(17,586,075
|
)
|
|
|
|
|
|
Accumulated other comprehensive loss
|
|
|
(336,992
|
)
|
|
|
|
|
|
Total stockholders’ equity (deficit)
|
|
|
(1,312,994
|
)
|
|
|
|
|
|
Total capitalization
|
|
$
|
1,220,023
|
|
|
$
|
|
|
|
(1)
|
The number of shares of common stock to be outstanding after the offering is based on 20,850,848, which is the number of shares outstanding on March 31, 2020, assumes no exercise by the underwriters of their option to purchase up to an additional shares of common stock and Warrants to purchase shares of common stock to cover over-allotments, if any, and excludes:
|
|
|
●
|
|
147,637 shares of common stock issuable upon the exercise of Warrants outstanding with a weighted average exercise price of $10.40 per share;
|
|
|
●
|
|
240,000 units, each consisting of one share of common stock, par value $0.001 per share, together with one Warrant to purchase one share of common stock at an exercise price equal to $10.40, per share, in a public offering;
|
|
|
●
|
|
9,710 shares of common stock issuable upon the exercise of options outstanding with a weighted average exercise price of $13.00 per share; and
|
|
|
●
|
|
______ shares of common stock issuable upon the exercise of the Warrants offered hereby.
|
|
|
|
|
|
|
MATERIAL U.S. FEDERAL
TAX CONSIDERATIONS FOR HOLDERS OF OUR COMMON STOCK AND WARRANTS
The following discussion
describes the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our common stock and
Warrants acquired in this offering. This discussion is based on the current provisions of the Internal Revenue Code of 1986, as
amended, referred to as the Code, existing and proposed U.S. Treasury regulations promulgated thereunder, and administrative rulings
and court decisions in effect as of the date hereof, all of which are subject to change at any time, possibly with retroactive
effect. No ruling has been or will be sought from the Internal Revenue Service, or IRS, with respect to the matters discussed below,
and there can be no assurance the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership
or disposition of our common stock, or Warrants, or that any such contrary position would not be sustained by a court.
We assume in this discussion
that the shares of our common stock, or Warrants will be held as capital assets (generally, property held for investment). This
discussion does not address all aspects of U.S. federal income taxes, does not discuss the potential application of the Medicare
contribution tax, the alternative minimum tax and does not deal with state or local taxes, U.S. federal gift and estate tax laws,
except as specifically provided below with respect to non-U.S. holders, or any non-U.S. tax consequences that may be relevant to
holders in light of their particular circumstances. This discussion also does not address the special tax rules applicable to particular
holders, such as:
|
|
•
|
financial institutions;
|
|
|
•
|
brokers or dealers in securities;
|
|
|
•
|
tax-exempt organizations;
|
|
|
•
|
regulated investment companies;
|
|
|
•
|
owners that hold our common stock or Warrants as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment;
|
|
|
•
|
controlled foreign corporations, passive foreign investment companies, or corporations that accumulate earnings to avoid U.S. federal income tax; and
|
|
|
•
|
certain U.S. expatriates.
|
In addition, this discussion
does not address the tax treatment of partnerships or other pass-through entities or persons who hold our common stock or Warrants
through partnerships or other entities which are pass-through entities for U.S. federal income tax purposes. A partner in a partnership
or other pass-through entity that will hold our common stock or Warrants should consult his, her or its own tax advisor regarding
the tax consequences of the ownership and disposition of our common stock or Warrants through a partnership or other pass-through
entity, as applicable.
This discussion of
U.S. federal income tax considerations is for general information purposes only and is not tax advice. Prospective investors should
consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring,
holding and disposing of our common stock and Warrants.
For the purposes of this
discussion, a "U.S. Holder" means a beneficial owner of our common stock or Warrants that is for U.S. federal income
tax purposes (a) an individual citizen or resident of the United States, (b) a corporation (or other entity taxable as
a corporation for U.S. federal income tax purposes), created or organized in or under the laws of the United States, any state
thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless
of its source, or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and
one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) have the authority to control all substantial
decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a
U.S. person. A "Non-U.S. Holder" is, for U.S. federal income tax purposes, a beneficial owner of common stock or Warrants
that is not a U.S. Holder or a partnership for U.S. federal income tax purposes.
Tax Cuts and
Jobs Act
Under tax legislation
signed into law in December 2017 commonly known as the Tax Cuts and Jobs Act of 2017, U.S. Holders that use an accrual method of
accounting for tax purposes and have certain financial statements generally will be required to include certain amounts in income
no later than the time such amounts are taken into account as revenue in such financial statements. The application of this rule
thus may require the accrual of income earlier than would be the case under the general tax rules described below, although the
precise application of this rule is unclear at this time. This rule is effective for taxable years beginning after December 31,
2017. U.S. Holders that use an accrual method of accounting should consult with their tax advisors regarding the potential applicability
of this legislation to their particular situation.
Allocation
of Purchase Price of Common Unit
For U.S. federal income
tax purposes, each Common Unit will be treated as an "investment unit" consisting of one share of common stock and a
warrant to acquire one share of our common stock. The purchase price for each investment unit will be allocated between these two
components in proportion to their relative fair market values at the time the unit is purchased by the holder. This allocation
of the purchase price for each unit will establish the holder's initial tax basis for U.S. federal income tax purposes in the share
of common stock and the Warrant included in each unit. The separation of the share of common stock and the Warrant included in
each unit should not be a taxable event for U.S. federal income tax purposes. Each holder should consult his, her or its own tax
advisor regarding the allocation of the purchase price for a unit.
Tax Considerations
Applicable to U.S. Holders
Exercise
and Expiration of Warrants
In general, a U.S. Holder
will not recognize gain or loss for U.S. federal income tax purposes upon exercise of a Warrant. The U.S. Holder will take a tax
basis in the shares acquired on the exercise of a Warrant equal to the exercise price of the Warrant, increased by the U.S. Holder's
adjusted tax basis in the Warrant exercised (as determined pursuant to the rules discussed above). The U.S. Holder's holding period
in the shares of our common stock acquired on exercise of the Warrant will begin on the date of exercise of the Warrant, and will
not include any period for which the U.S. Holder held the Warrant.
In certain limited circumstances,
a U.S. Holder may be permitted to undertake a cashless exercise of Warrants into our common stock. The U.S. federal income tax
treatment of a cashless exercise of Warrants into our common stock is unclear, and the tax consequences of a cashless exercise
could differ from the consequences upon the exercise of a Warrant described in the preceding paragraph. U.S. Holders should consult
their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of Warrants.
The lapse or expiration
of a Warrant will be treated as if the U.S. Holder sold or exchanged the Warrant and recognized a capital loss equal to the U.S.
Holder's tax basis in the Warrant. The deductibility of capital losses is subject to limitations.
Certain
Adjustments to and Distributions on Warrants
Under Section 305
of the Code, an adjustment to the number of shares of common stock issued on the exercise of the Warrants, or an adjustment to
the exercise price of the Warrants, may be treated as a constructive distribution to a U.S. Holder of the Warrants if, and to the
extent that, such adjustment has the effect of increasing such U.S. Holder's proportionate interest in our "earnings and profits"
or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution
of cash or other property to our shareholders). An adjustment made pursuant to a bona fide reasonable adjustment formula that has
the effect of preventing dilution should generally not be considered to result in a constructive distribution. Any such constructive
distribution would be taxable whether or not there is an actual distribution of cash or other property to the holders of Warrants.
In certain circumstances, if we were to make a distribution in cash or other property with respect to our common stock after the
issuance of the Warrants, then we may make a corresponding distribution to a Warrant holder. The taxation of a distribution received
with respect to a Warrant is unclear. It is possible such a distribution would be treated as a distribution (or constructive distribution),
although other treatments are possible. For more information regarding the tax considerations related to distributions, see the
discussion below regarding "Distributions". U.S. Holders should consult their tax advisors regarding the proper treatment
of any adjustments to the Warrants and any distributions with respect to the Warrants.
Distributions
As discussed above, we
currently anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do
not intend to pay cash dividends in respect of our common stock in the foreseeable future. In the event that we do make distributions
on our common stock to a U.S. Holder, those distributions generally will constitute dividends for U.S. tax purposes to the extent
paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Distributions
in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces,
but not below zero, a U.S. Holder's adjusted tax basis in our common stock. Any remaining excess will be treated as gain realized
on the sale or exchange of our common stock as described below under the section titled " – Disposition of Our
Common Stock or Warrants."
Disposition
of Our Common Stock or Warrants
Upon a sale or other
taxable disposition of our common stock or Warrants, a U.S. Holder generally will recognize capital gain or loss in an amount equal
to the difference between the amount realized and the U.S. Holder's adjusted tax basis in the common stock or Warrants. Capital
gain or loss will constitute long-term capital gain or loss if the U.S. Holder's holding period for the common stock or Warrants
exceeds one year. The deductibility of capital losses is subject to certain limitations. U.S. Holders who recognize losses with
respect to a disposition of our common stock or Warrants should consult their own tax advisors regarding the tax treatment of such
losses.
Information
Reporting and Backup Reporting
Information reporting
requirements generally will apply to payments of dividends (including constructive dividends) on the common stock and Warrants
and to the proceeds of a sale or other disposition of common stock and Warrants paid by us to a U.S. Holder unless such U.S. Holder
is an exempt recipient, such as a corporation. Backup withholding will apply to those payments if the U.S. Holder fails to provide
the holder's taxpayer identification number, or certification of exempt status, or if the holder otherwise fails to comply with
applicable requirements to establish an exemption.
Backup withholding is
not an additional tax. Rather, any amounts withheld under the backup withholding rules will be allowed as a refund or a credit
against the U.S. Holder's U.S. federal income tax liability provided the required information is timely furnished to the IRS. U.S.
Holders should consult their own tax advisors regarding their qualification for exemption from information reporting and backup
withholding and the procedure for obtaining such exemption.
Tax
Considerations Applicable To Non-U.S. Holders
Exercise
and Expiration of Warrants
In general, a Non-U.S.
Holder will not recognize gain or loss for U.S. federal income tax purposes upon the exercise of Warrants into shares of common
stock. The U.S. federal income tax treatment of a cashless exercise of Warrants into our common stock is unclear. A Non-U.S. Holder
should consult his, her, or its own tax advisor regarding the U.S. federal income tax consequences of a cashless exercise of Warrants.
The expiration
of a Warrant will be treated as if the Non-U.S. Holder sold or exchanged the Warrant and recognized a capital loss equal to the
Non-U.S. Holder's tax basis in the Warrant. However, a Non-U.S. Holder will not be able to utilize a loss recognized upon expiration
of a Warrant against the Non-U.S. Holder's U.S. federal income tax liability unless the loss is effectively connected with the
Non-U.S. Holder's conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable
to a permanent establishment or fixed base in the United States) or is treated as a U.S.-source loss and the Non-U.S. Holder is
present 183 days or more in the taxable year of disposition and certain other conditions are met.
Certain
Adjustments to and Distributions on Warrants
As described under " –
U.S. Holders – Certain Adjustments to Warrants", an adjustment to the Warrants could result in a constructive distribution
to a Non-U.S. Holder, which would be treated as described under "Distributions" below, and the tax treatment of distributions
on the warrants is unclear. Any resulting withholding tax attributable to deemed dividends would be collected from other amounts
payable or distributable to the Non-U.S. Holder. Non-U.S. Holders should consult their tax advisors regarding the proper treatment
of any adjustments to and distributions on the Warrants.
Distributions
As discussed above, we
currently anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do
not intend to pay cash dividends in respect of our common stock in the foreseeable future. In the event that we do make distributions
on our common stock to a Non-U.S. Holder, those distributions generally will constitute dividends for U.S. federal income tax purposes
as described in " – U.S. Holders – Distributions".
Any distribution (including
constructive distributions) on our common stock that is treated as a dividend paid to a Non-U.S. Holder that is not effectively
connected with the holder's conduct of a trade or business in the United States will generally be subject to withholding tax at
a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and the Non-U.S.
Holder's country of residence. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required
to provide the applicable withholding agent with a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate
form, certifying the Non-U.S. Holder's entitlement to benefits under that treaty. Such form must be provided prior to the payment
of dividends and must be updated periodically. If a Non-U.S. Holder holds stock through a financial institution or other agent
acting on the holder's behalf, the holder will be required to provide appropriate documentation to such agent. The holder's agent
may then be required to provide certification to the applicable withholding agent, either directly or through other intermediaries.
If you are eligible for a reduced rate of U.S. withholding tax under an income tax treaty, you should consult with your own tax
advisor to determine if you are able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate
claim for a refund with the IRS.
We generally are not
required to withhold tax on dividends paid (or constructive dividends deemed paid) to a Non-U.S. Holder that are effectively connected
with the holder's conduct of a trade or business within the United States (and, if required by an applicable income tax treaty,
are attributable to a permanent establishment or fixed base that the holder maintains in the United States) if a properly executed
IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial
institution or other agent, to the applicable withholding agent). In general, such effectively connected dividends will be subject
to U.S. federal income tax on a net income basis at the regular graduated rates applicable to U.S. persons. A corporate Non-U.S.
Holder receiving effectively connected dividends may also be subject to an additional "branch profits tax," which is
imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the
corporate Non-U.S. Holder's effectively connected earnings and profits, subject to certain adjustments.
See also the sections
below titled " – Backup Withholding and Information Reporting" and " – Foreign Accounts"
for additional withholding rules that may apply to dividends paid to certain foreign financial institutions or non-financial foreign
entities.
Disposition of Our
Common Stock or Warrants
Subject to the discussions
below under the sections titled " – Backup Withholding and Information Reporting" and " –
Foreign Accounts," a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax with respect
to gain realized on a sale or other disposition of our common stock or Warrants unless:
|
|
•
|
the gain is effectively connected with the Non-U.S. Holder's conduct of a trade or business in the United States, and if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States; in these cases, the Non-U.S. Holder will be taxed on a net income basis at the regular graduated rates and in the manner applicable to U.S. persons, and if the Non-U.S. Holder is a corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply;
|
|
|
•
|
the Non-U.S. Holder is a nonresident alien present in the United States for 183 days or more in the taxable year of the disposition and certain other requirements are met, in which case the Non-U.S. Holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence) on the net gain derived from the disposition, which may be offset by certain U.S.-source capital losses of the Non-U.S. Holder, if any; or
|
|
|
•
|
our common stock constitutes a U.S. real property interest because we are, or have been at any time during the five-year period preceding such disposition (or the Non-U.S. Holder's holding period of the common stock or Warrants, if shorter), a "U.S. real property holding corporation," unless our common stock is regularly traded on an established securities market and the Non-U.S. Holder held no more than 5% of our outstanding common stock, directly or indirectly, during the shorter of the five-year period ending on the date of the disposition or the period that the Non-U.S. Holder held our common stock. Special rules may apply to the determination of the 5% threshold in the case of a holder of a Warrant. Non-U.S. Holders are urged to consult their own tax advisors regarding the effect of holding our Warrants on the calculation of such 5% threshold. Generally, a corporation is a "U.S. real property holding corporation" if the fair market value of its "U.S. real property interests" (as defined in the Code and applicable regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we believe that we are not currently, and we do not anticipate becoming, a "U.S. real property holding corporation" for U.S. federal income tax purposes. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. Non-U.S. Holders are urged to consult their own tax advisors regarding the U.S. federal income tax considerations that could result if we are, or become, a "U.S. real property holding corporation".
|
See the sections titled
" – Backup Withholding and Information Reporting" and " – Foreign Accounts" for additional
information regarding withholding rules that may apply to proceeds of a disposition of our common stock or Warrants paid to foreign
financial institutions or non-financial foreign entities.
Federal
Estate Tax
Common stock owned or
treated as owned by an individual who is not a citizen or resident of the United States (as specially defined for U.S. federal
estate tax purposes) at the time of death will be included in the individual's gross estate for U.S. federal estate tax purposes
and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty provides otherwise.
The foregoing may also apply to Warrants. A Non-U.S. Holder should consult his, her, or its own tax advisor regarding the U.S.
federal estate tax consequences of the ownership or disposition of shares of our common stock and Warrants.
Backup
Withholding and Information Reporting
We must report annually
to the IRS and to each Non-U.S. Holder the gross amount of the distributions (including constructive distributions) on our common
stock or Warrants paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. Holders may have
to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in
order to avoid backup withholding at the applicable rate, currently 24%, with respect to dividends (or constructive dividends)
on our common stock or Warrants. Generally, a holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN
(or other applicable Form W-8) or otherwise meets documentary evidence requirements for establishing that it is a Non-U.S.
Holder, or otherwise establishes an exemption. Dividends paid to Non-U.S. Holders subject to withholding of U.S. federal income
tax, as described above under the heading "Dividends," will generally be exempt from U.S. backup withholding.
Information reporting
and backup withholding generally will apply to the proceeds of a disposition of our common stock or Warrants by a Non-U.S. Holder
effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a Non-U.S. Holder
and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding
will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States
through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office
of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected
through a U.S. office of a broker. Non-U.S. Holders should consult their own tax advisors regarding the application of the information
reporting and backup withholding rules to them.
Copies of information
returns may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or is incorporated under
the provisions of a specific treaty or agreement.
Backup withholding is
not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded
or credited against the Non-U.S. Holder's U.S. federal income tax liability, if any, provided that an appropriate claim is timely
filed with the IRS.
Foreign
Accounts
The Foreign Account Tax
Compliance Act, or FATCA, generally imposes a 30% withholding tax on dividends (including constructive dividends) on, and gross
proceeds from the sale or other disposition of, our common stock and Warrants if paid to a non-U.S. entity unless (i) if the
non-U.S. entity is a "foreign financial institution," the non-U.S. entity undertakes certain due diligence, reporting,
withholding, and certification obligations, (ii) if the non-U.S. entity is not a "foreign financial institution,"
the non-U.S. entity identifies certain of its U.S. investors, if any, or (iii) the non-U.S. entity is otherwise exempt under
FATCA.
Withholding under FATCA
generally applies to payments of dividends (including constructive dividends) on our common stock and Warrants. An intergovernmental
agreement between the United States and an applicable foreign country may modify the requirements described in this section. Under
certain circumstances, a holder may be eligible for refunds or credits of the tax. Holders should consult their own tax advisors
regarding the possible implications of FATCA on their investment in our common stock or Warrants.
The preceding discussion
of material U.S. federal tax considerations is for information only. It is not tax advice. Prospective investors should consult
their own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding
and disposing of our common stock or Warrants, including the consequences of any proposed changes in applicable laws.
UNDERWRITING
We are offering the shares
of common stock and Warrants described in this prospectus supplement and the accompanying prospectus through the underwriter listed
below. Kingswood Capital Markets, a division of Benchmark Investments, Inc., is acting as the sole book-running manager of this
offering. The underwriter named below has agreed to buy, subject to the terms of the underwriting agreement, the number of securities
listed opposite its name below. The underwriter is committed to purchase and pay for all of the securities if any are purchased,
other than those securities covered by the over-allotment option described below.
|
Underwriters
|
|
Number of
Shares of
Common Stock and accompanying Warrants
|
|
|
|
Kingswood Capital Markets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
The underwriter has advised
us that it proposes to offer the shares of common stock and Warrants to the public at a combined offering price of $_____. The
underwriter proposes to offer the shares of common stock and Warrants to certain dealers at the same price less a concession of
not more than $_____. After the offering, these figures may be changed by the underwriter.
The common stock and
Warrants sold in this offering are expected to be ready for delivery on or about July __, 2020, against payment in immediately
available funds. The underwriter may reject all or part of any order.
We have granted to the
underwriter an option to purchase up to an additional _____ shares of common stock and/or Warrants from us at the same price to
the public, and with the same underwriting discount, as set forth in the table below. The underwriter may exercise this option
any time during the 45-day period after the date of this prospectus supplement, but only to cover over-allotments, if any. To the
extent the underwriter exercises the option, the underwriter will become obligated, subject to certain conditions, to purchase
the shares of common stock and/or Warrants for which it exercises the option.
The table below summarizes
the underwriting discounts that we will pay to the underwriter. These amounts are shown assuming both no exercise and full exercise
of the over-allotment option. In addition to the underwriting discount, we have agreed to pay up to $40,000 of the fees and expenses
of the underwriter, which may include the fees and expenses of counsel to the underwriter. The fees and expenses of the underwriter
that we have agreed to reimburse are not included in the underwriting discounts set forth in the table below. The underwriting
discount and reimbursable expenses the underwriter will receive were determined through arms’ length negotiations between
us and the underwriter.
|
|
|
|
Per
Share and
Accompanying
Warrant
|
|
|
|
Total with no Over-Allotment
|
|
|
Total with Over-Allotment
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
|
|
Underwriting discount
|
|
$
|
|
|
|
$
|
|
|
|
|
|
Proceeds, before expenses, to us
|
|
$
|
|
|
|
$
|
|
|
|
|
We estimate that
the total expenses of this offering, excluding underwriting discounts, will be $_____. This includes $____ of the fees and expenses
of the underwriter. These expenses are payable by us.
We also have agreed to indemnify the
underwriter against certain liabilities, including civil liabilities under the Securities Act of 1933, as amended, or to contribute
to payments that the underwriter may be required to make in respect of those liabilities.
No Sales of Similar
Securities
We and each of our directors and officers
have agreed not to offer, sell, agree to sell, directly or indirectly, or otherwise dispose of any shares of common stock or any
securities convertible into or exchangeable for shares of common stock without the prior written consent of the underwriter for
a period of 90 days after the date of this prospectus supplement. These lock-up agreements provide limited exceptions and their
restrictions may be waived at any time by the underwriter.
Right of First Refusal
If this offering is consummated, subject
to a minimum of $15,000,000 in gross proceeds being raised, we have agreed, subject to certain conditions, limitations and exceptions,
to provide Kingswood Capital Markets, a division of Benchmark Investments, Inc. with a right of first refusal to act as the sole
investment banker, sole book-runner, and/or sole placement agent, at its sole discretion, for each and every future public and
private equity and debt offering, including all equity linked financings during the period of 12 months after the date the Offering
is completed.
Price Stabilization,
Short Positions and Penalty Bids
To facilitate this offering,
the underwriter may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock during and
after the offering. Specifically, the underwriter may over-allot or otherwise create a short position in our common stock for its
own account by selling more shares of common stock than we have sold to the underwriter. The underwriter may close out any short
position by either exercising its option to purchase additional shares or purchasing shares in the open market.
In addition, the underwriter
may stabilize or maintain the price of our common stock by bidding for or purchasing shares in the open market and may impose penalty
bids. If penalty bids are imposed, selling concessions allowed to broker-dealers participating in this offering are reclaimed if
shares previously distributed in this offering are repurchased, whether in connection with stabilization transactions or otherwise.
The effect of these transactions may be to stabilize or maintain the market price of our common stock at a level above that which
might otherwise prevail in the open market. The imposition of a penalty bid may also affect the price of our common stock to the
extent that it discourages resales of our common stock. The magnitude or effect of any stabilization or other transactions is uncertain.
These transactions may be effected on the Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
In connection with this
offering, the underwriter and selling group members may also engage in passive market making transactions in our common stock on
the Nasdaq Capital Market. Passive market making consists of displaying bids on the Nasdaq Capital Market limited by the prices
of independent market makers and effecting purchases limited by those prices in response to order flow. Rule 103 of Regulation
M promulgated by the Securities and Exchange Commission limits the amount of net purchases that each passive market maker may make
and the displayed size of each bid. Passive market making may stabilize the market price of our common stock at a level above that
which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriter make
any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have
on the price of our common stock. In addition, neither we nor the underwriter make any representation that the underwriter will
engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Electronic Delivery of Prospectus
In connection with this
offering, the underwriter or certain of the securities dealers may distribute prospectuses by electronic means, such as e-mail.
In addition, the underwriter may facilitate Internet distribution for this offering to certain of its Internet subscription customers.
The underwriter may allocate a limited number of securities for sale to its online brokerage customers. An electronic prospectus
is available on the Internet websites maintained by any such underwriter. Other than the prospectus in electronic format, the information
on the websites of the underwriter is not part of this prospectus supplement or the accompanying prospectus.
Listing
Our common stock is listed
on the Nasdaq Capital Market under the symbol “NMRD.”
Transfer Agent and
Registrar
The transfer agent and registrar for
our common stock is Nevada Agency and Transfer Company.
Notice to Prospective Investors in
the European Economic Area
In relation to each member
state of the European Economic Area that has implemented the Prospectus Directive (each, a relevant member state), with effect
from and including the date on which the Prospectus Directive is implemented in that relevant member state (the relevant implementation
date), an offer of our securities described in this prospectus may not be made to the public in that relevant member state other
than:
|
|
•
|
to any legal entity which is a qualified investor as defined in the Prospectus Directive;
|
|
|
•
|
to fewer than 100 or, if the relevant member state has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the relevant Dealer or Dealers nominated by us for any such offer; or
|
|
|
•
|
in any other circumstances falling within Article 3(2) of the Prospectus Directive,
|
provided that no such
offer of shares of our securities shall require us or the underwriter to publish a prospectus pursuant to Article 3 of the
Prospectus Directive.
For purposes of this
provision, the expression an "offer of securities to the public" in any relevant member state means the communication
in any form and by any means of sufficient information on the terms of the offer and our securities to be offered so as to enable
an investor to decide to purchase or subscribe for any securities, as the expression may be varied in that member state by any
measure implementing the Prospectus Directive in that member state, the expression "Prospectus Directive" means Directive
2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the relevant member
state) and includes any relevant implementing measure in the relevant member state, and the expression "2010 PD Amending Directive"
means Directive 2010/73/EU.
We and the underwriter
have not authorized and do not authorize the making of any offer of our securities through any financial intermediary on our or
their behalf, other than offers made by the underwriter with a view to the final placement of our securities as contemplated in
this prospectus. Accordingly, no purchaser of our securities, other than the underwriter, is authorized to make any further offer
of our securities on behalf of us or the underwriter.
Notice
to Prospective Investors in the United Kingdom
This prospectus is only
being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of
Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5)
of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, or the Order, or (ii) high net worth entities,
and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (each
such person being referred to as a relevant person).
This prospectus and its
contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients
to any other persons in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely
on this document or any of its contents.
Resale
Restrictions
The offer and sale of
the securities in Canada is being made on a private placement basis only and is exempt from the requirement to prepare and file a
prospectus under applicable Canadian securities laws. Any resale of Securities acquired by a Canadian investor in this offering
must be made in accordance with applicable Canadian securities laws, which may vary depending on the relevant jurisdiction, and
which may require resales to be made in accordance with Canadian prospectus requirements, a statutory exemption from the prospectus
requirements, in a transaction exempt from the prospectus requirements or otherwise under a discretionary exemption from the prospectus
requirements granted by the applicable local Canadian securities regulatory authority. These resale restrictions may under certain
circumstances apply to resales of the Securities outside of Canada.
Representations
of Purchasers
Each Canadian investor
who purchases the securities will be deemed to have represented to the issuer and to each dealer from whom a purchase confirmation
is received, as applicable, that the investor (i) is purchasing as principal, or is deemed to be purchasing as principal in
accordance with applicable Canadian securities laws, for investment only and not with a view to resale or redistribution; (ii) is
an "accredited investor" as such term is defined in section 1.1 of National Instrument 45-106 Prospectus Exemptions
("NI 45-106") or, in Ontario, as such term is defined in section 73.3(1) of the Securities Act (Ontario); and
(iii) is a "permitted client" as such term is defined in section 1.1 of National Instrument 31-103 Registration
Requirements, Exemptions and Ongoing Registrant Obligations.
Taxation
and Eligibility for Investment
Any discussion of taxation
and related matters contained in this document does not purport to be a comprehensive description of all of the tax considerations
that may be relevant to a Canadian investor when deciding to purchase the securities and, in particular, does not address any Canadian
tax considerations. No representation or warranty is hereby made as to the tax consequences to a resident, or deemed resident,
of Canada of an investment in the securities or with respect to the eligibility of the securities for investment by such investor
under relevant Canadian federal and provincial legislation and regulations.
Rights
of Action for Damages or Rescission
Securities legislation
in certain of the Canadian jurisdictions provides certain purchasers of securities pursuant to an offering memorandum, including
where the distribution involves an "eligible foreign security" as such term is defined in Ontario Securities Commission
Rule 45-501 Ontario Prospectus and Registration Exemptions and in Multilateral Instrument 45-107 Listing Representation
and Statutory Rights of Action Disclosure Exemptions, as applicable, with a remedy for damages or rescission, or both, in addition
to any other rights they may have at law, where the offering memorandum, or other offering document that constitutes an offering
memorandum, and any amendment thereto, contains a "misrepresentation" as defined under applicable Canadian securities
laws. These remedies, or notice with respect to these remedies, must be exercised or delivered, as the case may be, by the purchaser
within the time limits prescribed under, and are subject to limitations and defences under, applicable Canadian securities legislation.
In addition, these remedies are in addition to and without derogation from any other right or remedy available at law to the investor.
Language of Documents
Upon receipt of this
document, each Canadian investor hereby confirms that it has expressly requested that all documents evidencing or relating in any
way to the sale of the Securities described herein (including for greater certainty any purchase confirmation or any notice) be
drawn up in the English language only. Par la réception de ce document, chaque investisseur canadien confirme par les
présentes qu'il a expressément exigé que tous les documents faisant foi ou se rapportant de quelque manière
que ce soit à la vente des valeurs mobilières décrites aux présentes (incluant, pour plus de certitude,
toute confirmation d'achat ou tout avis) soient rédigés en anglais seulement.
INCORPORATION BY REFERENCE OF CERTAIN DOCUMENTS
The SEC allows us to "incorporate
by reference" in this prospectus supplement and the accompanying base prospectus certain information we file with the SEC,
which means that we may disclose important information in this prospectus supplement and the accompanying base prospectus by referring
you to the document that contains the information. The information incorporated by reference is considered to be an integral part
of this prospectus supplement and the accompanying base prospectus, and information that we file later with the SEC will automatically
update and supersede this information. We incorporate by reference the documents listed below and any future filings made with
the SEC under Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, until the termination
of the offering:
|
|
·
|
our Current Reports on Form 8-K filed with the SEC on April
8, 2020, April
21, 2020, May
15, 2020, June
30, 2020, July 7, 2020, July 21, 2020 and July 27, 2020.
|
|
|
·
|
the description of our common stock contained in our Form 8-A12B filed with the SEC on January 19, 2018, including any amendment or report filed for the purpose of updating that description; and
|
|
|
·
|
all documents filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act on or after the date of this prospectus and before we stop offering the securities covered by this prospectus and any accompanying prospectus supplement.
|
Notwithstanding the foregoing,
information and documents that we elect to furnish, but not file, or have furnished, but not filed, with the SEC in accordance
with SEC rules and regulations is not incorporated into this prospectus supplement and the accompanying base prospectus and does
not constitute a part hereof.
Upon written or oral request,
at no cost we will provide to each person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or
all of the information that has been incorporated by reference in the prospectus but not delivered with the prospectus. Inquiries
should be directed to:
57 West 57th Street
Manhattan, New York 10019
Attention: Chief Financial Officer
(646) 416-8000
In addition, you may access
these filings on our website at www.nemauramedical.com.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the information
reporting requirements of the Securities Exchange Act of 1934, as amended. Accordingly, we file annual, quarterly and current reports,
proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC's
web site at www.sec.gov. You may also read and copy any document we file at the SEC's public reference rooms located at 100 F Street,
N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms and their
copy charges. Also, using our website, www.nemauramedical.com, you can access electronic copies of documents we file with the SEC,
including our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K, and any amendments
to those reports, free of charge. Information on our website is not incorporated by reference in this prospectus supplement or
the accompanying base prospectus.
LEGAL MATTERS
The validity of the shares
of common stock offered under this prospectus supplement and the accompanying base prospectus will be passed upon for us by Anthony
L.G., PLLC, West Palm Beach, Florida. The underwriters are being represented by Ellenoff Grossman & Schole LLP, New York,
New York.
EXPERTS
The consolidated financial
statements for the years ended March 31, 2020 and 2019 have been incorporated in this prospectus supplement by reference to the
Annual Report on Form 10-K for the year ended March 31, 2020, have been so incorporated in reliance on the report of Mayer Hoffman
McCann P.C., an independent registered public accounting firm, given on the authority of said firm as experts in accounting and
auditing.
PROSPECTUS

$250,000,000
Common Stock
Preferred Stock
Warrants
Debt Securities
Units
__________________
We may offer and sell, from time to time in
one or more offerings the following securities:
|
·
|
shares of common stock, par value $0.001 per share;
|
|
·
|
shares of preferred stock, par value $0.001 per share;
|
|
·
|
warrants to purchase shares of our common stock, preferred stock and/or debt securities;
|
|
·
|
debt securities consisting of senior notes, subordinated notes or debentures;
|
|
·
|
units consisting of a combination of the foregoing securities; or
|
|
·
|
any combination of these securities.
|
We may offer and sell up to $250,000,000 in
the aggregate of the securities identified above from time to time in one or more offerings. This prospectus provides a general
description of the securities that we may offer. However, this prospectus may not be used to offer or sell our securities unless
accompanied by a prospectus supplement relating to the offered securities. Each time that we offer securities under this prospectus,
we will provide the specific terms of the securities offered, including the public offering price, in a related prospectus supplement.
Such prospectus supplement may add to, update or change information contained in this prospectus. To the extent there is a conflict
between the information contained in this prospectus, on the one hand, and the information contained in any prospectus supplement,
on the other hand, you should rely on the information in the prospectus supplement. You should read this prospectus and any applicable
prospectus supplement together with additional information described under the headings “Where You Can Find More Information”
and “Information Incorporated By Reference” before making your investment decision.
These securities may be sold directly by us,
through dealers or agents designated from time to time, to or through underwriters or through a combination of these methods. See
“Plan of Distribution” in this prospectus for additional information on methods of sale. We may also describe the plan
of distribution for any particular offering of our securities in a prospectus supplement. If any agents, underwriters or dealers
are involved in the sale of any securities in respect of which this prospectus is being delivered, we will disclose their names
and the nature of our arrangements with them in that prospectus supplement. The net proceeds we expect to receive from any such
sale will also be included in the prospectus supplement.
Our common stock is traded on the NASDAQ Capital
Market under the ticker symbol “NMRD.” The closing price of our common stock on March 26, 2019 was $1.05 per share.
The aggregate market value of our outstanding
common equity held by non-affiliates, or public float, was $77,306,177, based on 207,648,416 shares of common stock outstanding
as of March 26, 2019, of which 66,130,177 shares were held by non-affiliates, and a per share price of $1.169 based on the closing
sale price of our common stock on February 26, 2019 (within 60 days prior to the date of filing). Therefore, as of March 26, 2019,
the aggregate market value of our common equity held by non-affiliates was more than $75,000,000, as calculated in accordance with
General Instruction I.B.1 of Form S-3.
An investment in our securities involves
a high degree of risk. See the sections entitled “Risk Factors” included in our most recent Annual Report on Form 10-K
and in any subsequent Quarterly Report on Form 10-Q, which are incorporated by reference into this prospectus, as well as in any
prospectus supplement related to a specific offering we make pursuant to this prospectus. You should carefully read this entire
prospectus together with any related prospectus supplement and the information incorporated by reference into both before you make
your investment decision.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
This prospectus may not be used to sell securities
unless accompanied by a prospectus supplement.
The date of this prospectus is April 8, 2019.
TABLE OF CONTENTS
|
|
PAGE
|
|
|
ABOUT THIS PROSPECTUS
|
2
|
|
|
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
2
|
|
|
PROSPECTUS SUMMARY
|
3
|
|
|
THE OFFERING
|
3
|
|
|
OUR COMPANY
|
3
|
|
|
RISK FACTORS
|
5
|
|
|
USE OF PROCEEDS
|
5
|
|
|
RATIO OF EARNINGS TO FIXED CHARGES
|
6
|
|
|
DESCRIPTIONS OF THE SECURITIES WE MAY OFFER
|
6
|
|
|
PLAN OF DISTRIBUTION
|
18
|
|
|
LEGAL MATTERS
|
20
|
|
|
EXPERTS
|
20
|
|
|
LIMITATION ON LIABILITY AND DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
|
20
|
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
20
|
|
|
INFORMATION INCORPORATED BY REFERENCE
|
21
|
|
ABOUT THIS PROSPECTUS
This prospectus is part of a registration
statement that we filed with the Securities and Exchange Commission (the “SEC”) using a “shelf” registration
process. Under this shelf registration process, we may offer from time to time securities having a maximum aggregate offering price
of $250,000,000. Each time we offer securities, we will prepare and file with the SEC a prospectus supplement that describes the
specific amounts, prices and terms of the securities we offer. The prospectus supplement also may add, update or change information
contained in this prospectus or the documents incorporated herein by reference. You should read carefully both this prospectus
and any prospectus supplement together with additional information described below under “Where You Can Find More Information”
and “Information Incorporated By Reference.”
This prospectus does not contain all
the information provided in the registration statement we filed with the SEC. For further information about us or our securities
offered hereby, you should refer to that registration statement, which you can obtain from the SEC or directly from us as described
below under “Where You Can Find More Information.”
You should rely only on the information
contained or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized any other person
to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely
on it. This prospectus is not an offer to sell securities, and it is not soliciting an offer to buy securities, in any jurisdiction
where the offer or sale is not permitted. You should assume that the information appearing in this prospectus or any prospectus
supplement, as well as information we have previously filed with the SEC and incorporated by reference, is accurate as of the date
of those documents only. Our business, financial condition, results of operations and prospects may have changed since those dates.
We may sell securities through underwriters
or dealers, through agents, directly to purchasers or through any combination of these methods. We and our agents reserve the sole
right to accept or reject in whole or in part any proposed purchase of securities. The prospectus supplement, which we will prepare
and file with the SEC each time we offer securities, will set forth the names of any underwriters, agents or others involved in
the sale of securities, and any applicable fee, commission or discount arrangements with them. See “Plan of Distribution.”
In this prospectus, unless otherwise indicated, “our company,” “we,” “us” or “our”
refer to Nemaura Medical Inc., a Nevada corporation, and its consolidated subsidiaries.
SPECIAL NOTE REGARDING FORWARD-LOOKING
STATEMENTS
Statements in this prospectus and in
the documents incorporated by reference in this prospectus contain forward-looking statements within the meaning of Section 27A
of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended,
or the Exchange Act. Any statements contained herein, other than statements of historical fact, including statements regarding
the progress and timing of our product development programs; our future opportunities; our business strategy, future operations,
anticipated financial position, future revenues and projected costs; our management’s prospects, plans and objectives; and
any other statements about our management’s future expectations, beliefs, goals, plans or prospects constitute forward-looking
statements. Examples of such statements are those that include words such as “may,” “assume(s),”
“forecast(s),” “position(s),” “predict(s),” “strategy,” “will,” “expect(s),”
“estimate(s),” “anticipate(s),” “believe(s),” “project(s),” “intend(s),”
“plan(s),” “budget(s),” “potential,” “continue” and variations thereof. However,
the words cited as examples in the preceding sentence are not intended to be exhaustive and any statements contained in this prospectus
regarding matters that are not historical facts may also constitute forward-looking statements.
Because these statements implicate risks
and uncertainties, as well as certain assumptions, actual results may differ materially from those expressed or implied by such
forward-looking statements. Factors that could cause actual results to differ materially include, but are not limited to, those
risks identified under “Risk Factors” in our most recent annual report on Form 10-K and our quarterly reports on Form
10-Q and from time to time in our other filings with the SEC. The information in this prospectus or any prospectus supplement speaks
only as of the date of that document and the information incorporated herein by reference speaks only as of the date of the document
incorporated by reference. Except as required by law, we undertake no obligation to update any forward-looking statement, whether
as a result of new information, future events or otherwise. Forward-looking statements include our plans and objectives for future
operations, including plans and objectives relating to our products and our future economic performance. Assumptions relating to
the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions as well
as future business decisions, including any acquisitions, mergers, dispositions, joint ventures, investments and any other business
development transactions we may enter into in the future. The amounts of time and money required to successfully complete development
and commercialization of our technologies as well as any evolution of or shift in our business plans, or to execute any future
strategic options are difficult or impossible to predict accurately and may involve factors that are beyond our control. Although
we believe that the assumptions underlying the forward-looking statements contained herein are reasonable, any of those assumptions
could prove inaccurate and, therefore, we cannot assure you that the results contemplated in any of the forward-looking statements
contained herein will be realized.
Based on the significant uncertainties
inherent in the forward-looking statements described herein, the inclusion of any such statement should not be regarded as a representation
by us or any other person that our objectives or plans will be achieved. Accordingly, you should not place undue reliance on these
forward-looking statements.
PROSPECTUS SUMMARY
This prospectus summary highlights
certain information about our company and other information contained elsewhere in this prospectus or in documents incorporated
by reference. This summary does not contain all of the information that you should consider before making an investment decision.
You should carefully read the entire prospectus, any prospectus supplement, including the section entitled “Risk Factors”
and the documents incorporated by reference into this prospectus, before making an investment decision.
THE OFFERING
This prospectus is part of a registration
statement that we filed with the SEC utilizing a shelf registration process. Under this shelf registration process, we may sell
any combination of:
|
·
|
debt securities, in one or more series;
|
|
·
|
warrants to purchase any of the securities listed above; and/or
|
|
·
|
units consisting of one or more of the foregoing,
|
in one or more offerings up to a total
dollar amount of $250,00,000. This prospectus provides you with a general description of the securities we may offer. Each time
we sell securities, we will provide a prospectus supplement that will contain specific information about the terms of that specific
offering and include a discussion of any risk factors or other special considerations that apply to those securities. The prospectus
supplement may also add, update or change information contained in this prospectus. You should read both this prospectus and any
prospectus supplement together with the additional information described under the heading “Where You Can Find More Information.”
OUR COMPANY
Product Development
We are a medical technology company engaged
in developing our proprietary product candidate, sugarBEAT®, as a non-invasive, affordable and flexible glucose trending device
for use by persons with diabetes and pre-diabetics. The sugarBEAT® device consists of a daily disposable adhesive skin-patch
connected to a rechargeable transmitter, with an app displaying glucose readings at five minute intervals for periods up to 24
hours. In addition, sugarBEAT® may be used by insulin using persons with diabetes as an adjunctive glucose monitoring device
when calibrated by a finger stick reading. The sugarBEAT® device works by extracting glucose from the skin into a chamber in
the patch that is in direct contact with an electrode based sensor. The transmitter sends the raw data to a mobile app where it
is processed by a proprietary algorithm.
In December 2017, we completed a European
clinical trial programme for sugarBEAT® where we evaluated 525 patient days across 75 Type 1 and Type 2 diabetic patients.
Based on the results of that study, we have applied for a priority CE Mark review and are currently awaiting CE approval to allow
sugarBEAT® to be sold in the European Union as a medical device. CE approval is disclosed by the use of the CE Mark, a manufacturers'
declaration that the product meets the requirements of the applicable European laws.
We have also completed human factors
studies in the United States to support a Food and Drug Administration (the “FDA”) submission, for approval to market
sugarBEAT® in the United States, and are currently in the process of finalizing the application for the FDA submission.
We previously developed a wristwatch-based
version of sugarBEAT® for which we obtained CE approval in February 2016. However, since then we have further developed sugarBEAT®,
improving the sensor technology and miniaturizing the electronics and incorporating Bluetooth connectivity. We believe there
are additional applications for the sugarBEAT® device and the underlying BEAT technology platform, which may include:
|
·
|
web-server accessible by physicians and diabetes professionals to track the condition remotely, thereby reducing healthcare costs and managing the condition more effectively;
|
|
·
|
other patches using the BEAT technology platform to measure alternative analytes, including lactate, uric acid, lithium and drugs. This would be a step-change in the monitoring of conditions, particularly in the hospital setting. Lactate monitoring is also used to determine the relative fitness of professional athletes.
|
The manufacture and sale of CE certified
medical devices are controlled and governed by guidelines stipulated in the International Organisation for Standardisation (ISO),
more specifically ISO13485; sugarBEAT will be manufactured and marketed according to ISO13485 quality standards.
Our management has extensive experience
in regulatory and clinical development of diagnostic medical devices. We intend to take advantage of this experience in the field
of diagnostic medical devices in an attempt to increase the probability of product approval. The overall regulatory process for
diagnostic medical devices for diabetes is currently similar to those governing other diagnostic devices. The device has successfully
completed clinical programs demonstrating the safety and efficacy of the system, and data has been published on the company website.
As we continue to raise funds for marketing the device on CE approval, we are also actively engaged in discussions with multiple
parties for commercialization of the product in the United States and other territories around the world, subject to regulatory
approvals in each respective territory.
Our Business Strategy
We intend to lead in the discovery, development
and commercialization of innovative and targeted diagnostic medical devices that improve disease monitoring, management and overall
patient care. Specifically, we intend to focus on the monitoring of molecules that can be drawn out through the skin non-invasively
using our technology platform. In addition to glucose, such molecules may include lactic acid monitoring and the monitoring of
prescription drugs and blood biomarkers that may help in the diagnosis, prevention or management of diseases such as diabetes.
We plan to take the following steps to implement our broad business strategy. Our key commercial strategies post-approval will
first be implemented in Europe and then in parts of the Middle East and Asia, and then the U.S., as follows:
|
·
|
Commercialize sugarBEAT® in the United Kingdom and Republic of Ireland with Dallas Burston Pharma (Jersey) Limited, with whom we have an exclusive marketing rights agreement for these two countries. We have also signed a full commercial agreement with Dallas Burston Ethitronix (Europe) Limited in May 2018 for all other European territories as part of an equal joint venture agreement. The joint venture intends to seek sub-license rights opportunities to one or more leading companies in the diabetes monitoring space, to leverage their network, infrastructure and resources. Dallas Burston (Jersey) Limited was founded by Dr. Dallas Burston, MBBS, an entrepreneur who has founded and sold several companies specializing in marketing pharmaceuticals. For example, in 1999, he sold 49% of Ashbourne Pharmaceuticals to HSBC Private Equity for £32 million and Bartholomew-Rhodes to Galen Ltd. for £19.8 million. More recently, in 2015, he sold DB Ashbourne Limited, a provider of off-patent branded pharmaceuticals for the UK market, to Ethypharm. At the time of the sale, DB Ashbourne Limited was estimated to have revenue of approximately £90 million;
|
|
·
|
Establish licensing or joint venture agreements with other parties to market sugarBEAT® in other geographies. We are in detailed discussions and negotiations with several other parties worldwide for licensing or joint venture agreements for the sale of the sugarBEAT® device and have signed commercial agreements for product distribution in the GCC region with TPMENA and for Qatar with Al-Danah Medical.
|
|
·
|
Compile data for U.S. FDA submission. The clinical program and Human factors usability studies to support the application have been completed, and the application is in the process of being compiled.
|
|
·
|
Expand the indications for which the sugarBEAT® device may be used. We believe that the sugarBEAT® device may offer significant benefits as compared to those found in the non-acute setting for the monitoring of other diseases. This includes monitoring of lactic acid for performance athletics, and the monitoring of drugs. We intend to complete initial proof of concept in laboratory settings followed by a clinical program for such applications; and
|
|
·
|
Expand our product pipeline through our proprietary platform technologies, acquisitions and strategic licensing arrangements. We intend to leverage our proprietary platform technologies to grow our portfolio of product candidates for the diagnosis of diabetes and other diseases. In addition, we intend to license our product and acquire products and technologies that are consistent with our research and development and business focus and strategies. This may include drug delivery products for the improved management of diabetes, for example improved insulin injector systems, and/or combination drug products for diabetes related drugs.
|
Corporate Information
We are a holding company that was incorporated
under the laws of the State of Nevada in December 2013. We currently own one hundred percent (100%) of Region Green Limited, a
British Virgin Islands corporation. Region Green Limited currently owns one hundred percent (100%) of the stock in Dermal Diagnostic
(Holdings) Limited, an England and Wales corporation. Dermal Diagnostics (Holdings) Limited currently owns one hundred percent
(100%) of the stock in Dermal Diagnostics Limited, an England and Wales corporation, and one hundred percent (100%) of the stock
in Trial Clinic Limited, an England and Wales corporation.
Our principal executive offices are located
at The Advanced Technology Centre, Oakwood Drive, Loughborough, Leicestershire, LE113QF, UK. Our website is located at www.nemauramedical.com
and our telephone number is + 44 1509 222912. Information found on, or accessible through, our website is not a part of, and is
not incorporated into, this prospectus, and you should not consider it part of the prospectus or part of any prospectus supplement.
RISK FACTORS
Our business is influenced by many factors
that are difficult to predict and that involve uncertainties that may materially affect operating results, cash flows, and financial
condition. Before making an investment decision, you should carefully consider these risks, including those set forth in the “Risk
Factors” section of our most recent Annual Report on Form 10-K filed with the SEC, as revised or supplemented by our Quarterly
Reports on Form 10-Q filed with the SEC since the filing of our most recent Annual Report on Form 10-K, all of which are incorporated
by reference into this prospectus. You should also carefully consider any other information we include or incorporate by reference
in this prospectus or include in any applicable prospectus supplement. Each of the risks described in these sections and documents
could materially and adversely affect our business, financial condition, results of operations and prospects, and could result
in a partial or complete loss of your investment.
USE OF PROCEEDS
Except as otherwise stated in the applicable
prospectus supplement, we intend to use the net proceeds from the sale of the securities covered by this prospectus for general
corporate purposes, which may include, but are not limited to, working capital, capital expenditures and research and development
expenditures, product launch, product inventory, establishment of sales and marketing teams, and potential new manufacture facilities.
The precise amount, use and timing of the application of such proceeds will depend upon our funding requirements and the availability
and cost of other capital. Additional information on the use of net proceeds from an offering of securities covered by this prospectus
may be set forth in the prospectus supplement relating to the specific offering.
RATIO OF EARNINGS TO FIXED CHARGES
Any time debt securities are offered
pursuant to this prospectus, we will provide a table setting forth our ratio of earnings to fixed charges on a historical basis
in the applicable prospectus supplement, if required.
DESCRIPTIONS OF THE SECURITIES WE MAY
OFFER
The descriptions of the securities contained
in this prospectus, together with any applicable prospectus supplement, summarize all the material terms and provisions of the
various types of securities that we may offer. We will describe in the applicable prospectus supplement relating to a particular
offering the specific terms of the securities offered by that prospectus supplement. We will indicate in the applicable prospectus
supplement if the terms of the securities differ from the terms we have summarized below. We will also include in the prospectus
supplement information, where applicable, material United States federal income tax considerations relating to the securities.
We may sell from time to time, in one or more offerings:
|
·
|
shares of our common stock;
|
|
·
|
shares of our preferred stock;
|
|
·
|
warrants to purchase shares of our common stock, shares of our preferred stock and/or debt securities;
|
|
·
|
debt securities consisting of senior notes, subordinated notes or debentures; or
|
|
·
|
units consisting of a combination of the foregoing securities.
|
Capital Stock
General
The following descriptions of common
and preferred stock, together with the additional information we include in any applicable prospectus supplement, summarizes the
material terms and provisions of the common stock that we may offer under this prospectus but is not intended to be complete. For
the full terms of our common and preferred stock, please refer to our articles of incorporation, as amended from time to time,
and our bylaws, as amended from time to time. The Nevada Revised Statutes may also affect the terms of these securities. While
the terms we have summarized below will apply generally to any future common or preferred stock that we may offer, we will describe
the specific terms of any series of these securities in more detail in the applicable prospectus supplement. If we so indicate
in a prospectus supplement, the terms of any common or preferred stock we offer under that prospectus supplement may differ from
the terms of our outstanding capital stock that we describe below.
As of March 21, 2019, our authorized
capital stock consists of 420,000,000 shares of common stock, par value $0.001 per share, of which 207,648,416 shares were issued
and outstanding as of March 21, 2019, and 200,000 shares of preferred stock, par value $0.01, of which no shares were issued and
outstanding as of March 21, 2019. The authorized and unissued shares of both common and preferred stock are available for issuance
without further action by our stockholders, unless such action is required by applicable law, the NASDAQ Capital Market, or the
rules of any other stock exchange on which our securities may be listed. Unless approval of our stockholders is so required, our
board of directors will not seek stockholder approval for the issuance and sale of either our common stock or preferred stock.
Common Stock
The holders of our common stock are entitled
to one vote per share. Any action required to be taken by the holders of our common stock at a meeting may, without prior notice,
by taken by written consent in lieu of a meeting if the consent has been signed by the minimum number of holders of common stock
required to approve such action.
In addition, the holders of our common
stock will be entitled to receive ratably such dividends, if any, as may be declared by our board of directors out of legally available
funds; however, the current policy of our board of directors is to retain earnings, if any, for operations and growth. Upon liquidation,
dissolution or winding-up, the holders of our common stock will be entitled to share ratably in all assets that are legally available
for distribution. The holders of our common stock will have no preemptive, subscription, redemption or conversion rights. The holders
of our common stock do not have cumulative rights in the election of directors. The rights, preferences and privileges of holders
of our common stock are subject to, and may be adversely affected by, the rights of the holders of our preferred stock.
Our common stock is listed on the NASDAQ
Capital Market under the symbol “NMRD.” The transfer agent and registrar for our common stock is Nevada Agency and
Transfer Company. Its address is 50 West Liberty Street, Suite 880, Reno NV 89501, and its telephone number is (775) 322-0626.
Preferred Stock
Our board of directors may determine, in
its sole discretion, the powers, designations, preferences, and relative participation, optional or other rights, if any, and the
qualifications, limitations or restrictions thereof, including dividend rights, conversion rights, voting rights, redemption rights,
liquidation preference, sinking fund terms and the number of shares. The rights, preferences, privileges and restrictions of the
preferred stock of each series will be fixed by the certificate of designation relating to that series.
In October 2017, we filed with the Nevada
Secretary of State a Certificate of Designation for up to 200,000 shares of Series A convertible preferred stock. The holders of
the Series A preferred stock have rights superior to the holders of our common stock as to the distributions of assets upon our
liquidation, dissolution or winding up, whether voluntary or involuntary. The Series A convertible preferred stock shall automatically
convert to shares of common stock at a ratio of 1000-for-1, i.e. each share of Series A preferred stock shall convert into 1000
shares of common stock, when the following conditions are met: (a) the sugarBEAT® device has received CE regulatory approval;
(b) retail sales of sugarBEAT® have commenced and (c) such retail sales have exceeded $5 million. Holders of Series A preferred
stock may voluntarily convert their shares after February 7, 2018 at the conversion ratio then in effect, subject to adjustment
for any stock splits, combinations, dividends, distributions, or mergers and acquisitions.
The holders of the Series A convertible
preferred stock are entitled to vote, as a class, on matters on all matters voted on by the holders of our common stock. Each share
of Series A convertible preferred stock is entitled to that number of votes equal to the number of shares of common stock the Series
A preferred stock is convertible into at the time the vote is taken. The holders of the Series A convertible preferred stock
shall also vote, as a class, on all matters that may adversely impact their rights and preferences. The Series A convertible preferred
stock is not eligible for dividend payments and we have no right to redeem these preferred shares. Holders of the Series A convertible
preferred stock may transfer their shares without our consent.
As of March 21, 2019, there were no shares
of Series A convertible preferred stock issued and outstanding.
With respect to any future issuances of
preferred stock made pursuant to this prospectus and an applicable prospectus supplement, the prospectus supplement will specify
the following: the maximum number of shares; the designation of the shares; the annual dividend rate, if any, and whether the dividend
is fixed or variable; the price and terms and conditions for redemption, if any; the liquidation preference, if any; any sinking
fund or similar provision; the terms and conditions, if any, for conversion and exchange of the preferred stock into any other
class or classes of our capital stock or any other of our securities or assets; and voting rights.
The future issuance of shares of preferred
stock will affect, perhaps adversely, the rights of holders of our common stock. While we cannot state the actual effects of such
issuance until our board of directors determines the specific rights attached to the preferred stock to be issued, these effects
could include: restricting dividends on the common stock; diluting the voting power of the common stock; impairing the liquidation
rights of our common stock; and delaying or preventing changes in our control or management.
Description of Certain Provisions of Nevada Law
As a Nevada corporation, we are subject
to the provisions of the Nevada Revised Statutes, some of which have an anti-takeover effect.
For example, Sections 78.378 to 78.3793
of the Nevada Revised Statutes, which are referred to as the Control Share Statute, restrict the ability of individuals and groups
from acquiring one-fifth or more of the voting shares of a Nevada corporation that has 200 or more stockholders of record, at least
100 of whom have addresses in Nevada, from exercising the voting rights of the acquired shares, absent required stockholder approval
of the share acquisition transaction or an opt out election by the corporation. The prohibition on the voting of the acquired shares
is limited to three years after acquisition. To avoid the voting restriction, the acquisition of a controlling interest must be
approved by both (a) the holders of a majority of the voting power of the corporation, and (b) if the acquisition would adversely
alter or change any preference or any relative or other right given to any other class or series of outstanding shares, the holders
of the majority of each class or series affected, excluding those shares as to which any interested stockholder exercises voting
rights, and the approval must specifically include the conferral of such voting rights. Although we have not opted out of this
statute, a corporation alternatively may expressly elect not to be governed by the provisions in either its articles of incorporation
or its bylaws. Additionally, in the face of potential control share transaction, a corporation, if it has not opted out of the
statutory provisions, may opt out of the control share statute by amending its articles of incorporation or its bylaws prior to
the 10th day following the acquisition of a controlling interest by an acquiring person.
We are also subject to Sections 78.411
to 78.444 of the Nevada Revised Statutes, which are referred to as the Business Combination Statute. This statute is designed to
limit acquirers of voting stock of a corporation from effecting a business combination without the consent of the stockholders
or board of directors. The statute provides that specified persons who, together with their affiliates and associates, own, or
within two years did own, 10% or more of the outstanding voting stock of a Nevada corporation with at least 200 stockholders of
record cannot engage in specified business combinations with a Nevada corporation for a period of two years after the date on which
the person became an interested stockholder, unless (a) the business combination or the transaction by which the person first became
an interested stockholder was approved by the Nevada corporation’s board of directors before the person first became an interested
stockholder, or (b) the combination is approved by the board and, at or after that time, the combination is approved at an annual
or special meeting of the stockholders by the affirmative vote of 60% or more of the voting power of the disinterested stockholders.
The foregoing is a summary of certain provisions
of Nevada law and does not purport to be complete and is qualified in its entirety by reference to the Nevada Revised Statutes.
Warrants
The following description, together with
the additional information we may include in any applicable prospectus supplement, summarizes the material terms and provisions
of the warrants that we may offer under this prospectus and any related warrant agreement and warrant certificate. While the terms
summarized below will apply generally to any warrants that we may offer, we will describe the specific terms of any series of warrants
in more detail in the applicable prospectus supplement. If we indicate in the prospectus supplement, the terms of any warrants
offered under that prospectus supplement may differ from the terms described below. Specific warrant agreements will contain additional
important terms and provisions
As of March 21, 2019, we had warrants
outstanding to purchase 10,000,000 and 1,880,704 shares of our common stock at exercise prices of $0.50 per share and $1.04
per share, respectively. These warrants have been incorporated by reference as Exhibits 4.2 and 4.7, respectively, to the registration
statement that includes this prospectus.
General
We may issue warrants for the purchase
of common stock, preferred stock and/or debt securities in one or more series. We may issue warrants independently or together
with common stock and/or debt securities, and the warrants may be attached to or separate from these securities.
We will evidence each series of warrants
by warrant certificates that we may issue under a separate agreement. We may enter into a warrant agreement with a warrant agent.
Each warrant agent may be a bank that we select which has its principal office in the United States. We may also choose to act
as our own warrant agent. We will indicate the name and address of any such warrant agent in the applicable prospectus supplement
relating to a particular series of warrants.
We will describe in the applicable prospectus supplement the
terms of the series of warrants, including:
|
·
|
the offering price and aggregate number of warrants offered;
|
|
·
|
if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security;
|
|
·
|
if applicable, the date on and after which the warrants and the related securities will be separately transferable;
|
|
·
|
in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise;
|
|
·
|
in the case of warrants to purchase common stock, the number or amount of shares of common stock, purchasable upon the exercise of one warrant and the price at which and currency in which these shares may be purchased upon such exercise;
|
|
·
|
the manner of exercise of the warrants, including any cashless exercise rights;
|
|
·
|
the warrant agreement under which the warrants will be issued;
|
|
·
|
the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants; anti-dilution provisions of the warrants, if any;
|
|
·
|
the terms of any rights to redeem or call the warrants;
|
|
·
|
any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants;
|
|
·
|
the dates on which the right to exercise the warrants will commence and expire or, if the warrants are not continuously exercisable during that period, the specific date or dates on which the warrants will be exercisable;
|
|
·
|
the manner in which the warrant agreement and warrants may be modified;
|
|
·
|
the identities of the warrant agent and any calculation or other agent for the warrants;
|
|
·
|
federal income tax consequences of holding or exercising the warrants;
|
|
·
|
the terms of the securities issuable upon exercise of the warrants;
|
|
·
|
any securities exchange or quotation system on which the warrants or any securities deliverable upon exercise of the warrants may be listed or quoted; and
|
|
·
|
any other specific terms, preferences, rights or limitations of or restrictions on the warrants.
|
Before exercising their warrants, holders
of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including:
|
·
|
in the case of warrants to purchase debt securities, the right to receive payments of principal of, or premium, if any, or interest on, the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture; or
|
|
·
|
in the case of warrants to purchase common stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any.
|
Exercise of Warrants
Each warrant will entitle the holder
to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the
applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants
may exercise the warrants at any time up to 5:00 P.M. Eastern Time on the expiration date that we set forth in the applicable prospectus
supplement. After the close of business on the expiration date, unexercised warrants will become void.
Holders of the warrants may exercise
the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information,
and paying the required exercise price by the methods provided in the applicable prospectus supplement. We will set forth on the
reverse side of the warrant certificate, and in the applicable prospectus supplement, the information that the holder of the warrant
will be required to deliver to the warrant agent.
Upon receipt of the required payment
and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other
office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise.
If fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate
for the remaining amount of warrants.
Enforceability of Rights by Holders of Warrants
Any warrant agent will act solely as
our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any
holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent
will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any
duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant
may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action the
holder’s right to exercise, and receive the securities purchasable upon exercise of, its warrants in accordance with their
terms.
Warrant Agreement Will Not Be Qualified Under Trust Indenture
Act
No warrant agreement will be qualified
as an indenture, and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders
of warrants issued under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
Governing Law
Each warrant agreement and any warrants
issued under the warrant agreements will be governed by New York law.
Calculation Agent
Any calculations relating to warrants
may be made by a calculation agent, an institution that we appoint as our agent for this purpose. The prospectus supplement for
a particular warrant will name the institution that we have appointed to act as the calculation agent for that warrant as of the
original issue date for that warrant, if any. We may appoint a different institution to serve as calculation agent from time to
time after the original issue date without the consent or notification of the holders. The calculation agent’s determination
of any amount of money payable or securities deliverable with respect to a warrant will be final and binding in the absence of
manifest error.
Debt Securities
The following description, together with
the additional information we include in any applicable prospectus supplement, summarizes the material terms and provisions of
the debt securities that we may offer under this prospectus. While the terms we have summarized below will generally apply to any
future debt securities we may offer under this prospectus, we will describe the particular terms of any debt securities that we
may offer in more detail in the applicable prospectus supplement. The terms of any debt securities we offer under a prospectus
supplement may differ from the terms we describe below. As of the date of this prospectus, we have no outstanding registered debt
securities.
We will issue senior notes under a senior
indenture, which we will enter into with the trustee to be named in the senior indenture. We will issue subordinated notes under
a subordinated indenture, which we will enter into with the trustee to be named in the subordinated indenture. We have filed forms
of these documents as exhibits to the registration statement of which this prospectus is a part. We use the term “indentures”
to refer to both the senior indenture and the subordinated indenture.
The indentures will be qualified under
the Trust Indenture Act of 1939. References to the Trust Indenture Act of 1939 include all amendments thereto. We use the term
“debenture trustee” to refer to either the senior trustee or the subordinated trustee, as applicable.
The following summaries of material provisions
of the senior notes, the subordinated notes and the indentures are subject to, and qualified in their entirety by reference to,
all the provisions of the indenture applicable to a particular series of debt securities, and all supplements thereto. We urge
you to read the applicable prospectus supplement(s) related to the debt securities that we sell under this prospectus, as well
as the complete indentures that contain the terms of the debt securities. Except as we may otherwise indicate, the terms of the
senior and the subordinated indentures are identical.
General
The terms of each series of debt securities
will be established by or pursuant to a resolution of our board of directors and set forth or determined in the manner provided
in an officers’ certificate or by a supplemental indenture. Debt securities may be issued in separate series without limitation
as to aggregate principal amount. We may specify a maximum aggregate principal amount for the debt securities of any series.
In addition, the particular terms of
each series of debt securities will be described in a prospectus supplement relating to such series, including any pricing supplement.
The prospectus supplement will set forth, among other things:
|
·
|
the principal amount being offered, and, if a series, the total amount authorized and the total amount outstanding;
|
|
·
|
any limit on the amount that may be issued;
|
|
·
|
whether or not we will issue the series of debt securities in global form and, if so, the terms and who the depositary will be;
|
|
·
|
whether and under what circumstances, if any, we will pay additional amounts on any debt securities held by a person who is not a U.S. person for tax purposes, and whether we can redeem the debt securities if we have to pay such additional amounts;
|
|
·
|
the annual interest rate, which may be fixed or variable, or the method for determining the rate, the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates;
|
|
·
|
the terms of the subordination of any series of subordinated debt, if applicable;
|
|
·
|
the place where payments will be payable;
|
|
·
|
restrictions on transfer, sale or other assignment, if any;
|
|
·
|
our right, if any, to defer payment of interest and the maximum length of any such deferral period;
|
|
·
|
the date, if any, after which, the conditions upon which, and the price at which we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions, and any other applicable terms of those redemption provisions;
|
|
·
|
the date, if any, on which, and the price at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable;
|
|
·
|
whether the indenture will restrict our ability and/or the ability of our subsidiaries to, among other things:
|
|
·
|
incur additional indebtedness;
|
|
·
|
issue additional securities;
|
|
·
|
pay dividends and make distributions in respect of our capital stock and the capital stock of our subsidiaries;
|
|
·
|
place restrictions on our subsidiaries’ ability to pay dividends, make distributions or transfer assets;
|
|
·
|
make investments or other restricted payments, sell or otherwise dispose of assets;
|
|
·
|
enter into sale-leaseback transactions;
|
|
·
|
engage in transactions with stockholders and affiliates, issue or sell stock of our subsidiaries; or
|
|
·
|
effect a consolidation or merger;
|
|
·
|
whether the indenture will require us to maintain any interest coverage, fixed charge, cash flow-based, asset-based or other financial ratios;
|
|
·
|
information describing any book-entry features;
|
|
·
|
provisions for a sinking fund purchase or other analogous fund, if any;
|
|
·
|
whether the debt securities are to be offered at a price such that they will be deemed to be offered at an “original issue discount” as defined in paragraph (a) of Section 1273 of the Internal Revenue Code;
|
|
·
|
the procedures for any auction and remarketing, if any; the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple thereof; if other than dollars, the currency in which the series of debt securities will be denominated;
|
|
·
|
and any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, including any events of default that are in addition to those described in this prospectus or any covenants provided with respect to the debt securities that are in addition to those described above, and any terms that may be required by us or advisable under applicable laws or regulations or advisable in connection with the marketing of the debt securities.
|
Conversion or Exchange Rights
We will set forth in the prospectus supplement
the terms on which a series of debt securities may be convertible into or exchangeable for common stock or other securities of
ours or a third party, including the conversion or exchange rate, as applicable, or how it will be calculated, and the applicable
conversion or exchange period. We will include provisions as to whether conversion or exchange is mandatory, at the option of the
holder or at our option. We may include provisions pursuant to which the number of our securities or the securities of a third
party that the holders of the series of debt securities receive upon conversion or exchange would, under the circumstances described
in those provisions, be subject to adjustment, or pursuant to which those holders would, under those circumstances, receive other
property upon conversion or exchange, for example in the event of our merger or consolidation with another entity.
Consolidation, Merger or Sale
The indentures in the forms filed as
exhibits to the registration statement of which this prospectus is a part do not contain any covenant that restricts our ability
to merge or consolidate, or sell, convey, transfer or otherwise dispose of all or substantially all of our assets. However, any
successor of ours or the acquirer of such assets must assume all of our obligations under the indentures and the debt securities.
If the debt securities are convertible
for our other securities, the person with whom we consolidate or merge or to whom we sell all of our property must make provisions
for the conversion of the debt securities into securities that the holders of the debt securities would have received if they had
converted the debt securities before the consolidation, merger or sale.
Events of Default under the Indenture
The following are events of default under
the indentures in the forms initially filed as exhibits to the registration statement with respect to any series of debt securities
that we may issue:
|
·
|
if we fail to pay interest when due and payable and our failure continues for 90 days and the time for payment has not been extended or deferred;
|
|
·
|
if we fail to pay the principal, sinking fund payment or premium, if any, when due and payable and the time for payment has not been extended or delayed;
|
|
·
|
if we fail to observe or perform any other covenant contained in the debt securities or the indentures, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive notice from the debenture trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and
|
|
·
|
if specified events of bankruptcy, insolvency or reorganization occur.
|
If an event of default with respect to
debt securities of any series occurs and is continuing, other than an event of default specified in the last bullet point above,
the debenture trustee or the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series,
by notice to us in writing, and to the debenture trustee if notice is given by such holders, may declare the unpaid principal of,
premium, if any, and accrued interest, if any, due and payable immediately. If an event of default specified in the last bullet
point above occurs with respect to us, the principal amount of and accrued interest, if any, of each issue of debt securities then
outstanding shall be due and payable without any notice or other action on the part of the debenture trustee or any holder.
The holders of a majority in principal
amount of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series
and its consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless
we have cured the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the indentures,
if an event of default under an indenture shall occur and be continuing, the debenture trustee will be under no obligation to exercise
any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of
debt securities, unless such holders have offered the debenture trustee reasonable indemnity. The holders of a majority in principal
amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting
any proceeding for any remedy available to the debenture trustee, or exercising any trust or power conferred on the debenture trustee,
with respect to the debt securities of that series, provided that:
|
·
|
the direction so given by the holder is not in conflict with any law or the applicable indenture; and
|
|
·
|
subject to its duties under the Trust Indenture Act of 1939, the debenture trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding.
|
A holder of the debt securities of any
series will only have the right to institute a proceeding under the indentures or to appoint a receiver or trustee, or to seek
other remedies if:
|
·
|
the holder has given written notice to the debenture trustee of a continuing event of default with respect to that series;
|
|
·
|
the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, and such holders have offered reasonable indemnity, to the debenture trustee to institute the proceeding as trustee; and
|
|
·
|
the debenture trustee does not institute the proceeding and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer.
|
|
|
|
These limitations do not apply to a suit
instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt
securities.
We will periodically file statements
with the debenture trustee regarding our compliance with specified covenants in the indentures.
Modification of Indenture; Waiver
We and the debenture trustee may change
an indenture without the consent of any holders with respect to specific matters, including:
|
·
|
to fix any ambiguity, defect or inconsistency in the indenture;
|
|
·
|
to comply with the provisions described above under “-Consolidation, Merger or Sale”;
|
|
·
|
to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act of 1939;
|
|
·
|
to evidence and provide for the acceptance of appointment by a successor trustee;
|
|
·
|
to provide for uncertificated debt securities and to make all appropriate changes for such purpose;
|
|
·
|
to add to, delete from, or revise the conditions, limitations and restrictions on the authorized amount, terms or purposes of issuance, authorization and delivery of debt securities or any series, as set forth in the indenture;
|
|
·
|
to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided under “-General” to establish the form of any certifications required to be furnished pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities;
|
|
·
|
to add to our covenants such new covenants, restrictions, conditions or provisions for the protection of the holders, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default, or to surrender any of our rights or powers under the indenture; or
|
|
·
|
to change anything that does not materially adversely affect the interests of any holder of debt securities of any series.
|
In addition, under the indentures, the
rights of holders of a series of debt securities may be changed by us and the debenture trustee with the written consent of the
holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected.
However, we and the debenture trustee may only make the following changes with the consent of each holder of any outstanding debt
securities affected:
|
·
|
extending the fixed maturity of the series of debt securities;
|
|
·
|
reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable upon the redemption of any debt securities; or
|
|
·
|
reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification or waiver.
|
Discharge
Each indenture provides that we can elect
to be discharged from our obligations with respect to one or more series of debt securities, except that the following obligations,
among others, survive until the maturity date or the redemption date:
|
·
|
register the transfer or exchange of debt securities of the series;
|
|
·
|
replace stolen, lost or mutilated debt securities of the series;
|
|
·
|
maintain paying agencies;
|
|
·
|
hold monies for payment in trust; and
|
|
·
|
appoint any successor trustee;
|
and the following obligations survive
the maturity date or the redemption date:
|
·
|
recover excess money held by the debenture trustee; and
|
|
·
|
compensate and indemnify the debenture trustee.
|
As more fully set forth in the indentures,
in order to exercise our rights to be discharged, we must either deliver for cancellation all securities of a series to the debenture
trustee or must deposit with the debenture trustee money or government obligations sufficient to pay all the principal of, any
premium, if any, and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of
each series only in fully registered form without coupons and, unless we otherwise specify in the applicable prospectus supplement,
in denominations of $1,000 and any integral multiple thereof. The indentures provide that we may issue debt securities of a series
in temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository
Trust Company, New York, New York, known as DTC, or another depositary named by us and identified in a prospectus supplement with
respect to that series. See “Legal Ownership of Securities” for a further description of the terms relating to any
book-entry securities.
At the option of the holder, subject
to the terms of the indentures and the limitations applicable to global securities described in the applicable prospectus supplement,
the holder of the debt securities of any series can exchange the debt securities for other debt securities of the same series,
in any authorized denomination and of like tenor and aggregate principal amount.
Subject to the terms of the indentures
and the limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities
may present the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed
thereon duly executed if so required by us or the security registrar, at the office of the security registrar or at the office
of any transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder presents
for transfer or exchange, we will make no service charge for any registration of transfer or exchange, but we may require payment
of any taxes or other governmental charges.
We will name in a board resolution the
security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities.
We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in
the office through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of
payment for the debt securities of each series.
If we elect to redeem the debt securities
of any series, we will not be required to:
|
·
|
issue, register the transfer of, or exchange any debt securities of any series being redeemed in part during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and ending at the close of business on the day of the mailing; or
|
|
·
|
register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part.
|
Information Concerning the Debenture Trustee
The debenture trustee, other than during
the occurrence and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically
set forth in the applicable indenture. Upon an event of default under an indenture, the debenture trustee must use the same degree
of care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the debenture
trustee is under no obligation to exercise any of the powers given it by the indentures at the request of any holder of debt securities
unless it is offered reasonable security and indemnity against the costs, expenses and liabilities that it might incur.
Payment and Paying Agents
Unless we otherwise indicate in the applicable
prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in
whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record
date for the interest.
We will name in the applicable board
resolution any other paying agents that we initially designate for the debt securities of a particular series. We will maintain
a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or
the debenture trustee for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed
at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder
of the debt security thereafter may look only to us for payment thereof.
Governing Law
The indentures and the debt securities
will be governed by and construed in accordance with the laws of the State of New York, except to the extent that the Trust Indenture
Act of 1939 is applicable.
Subordination of Subordinated Debt
Securities
The subordinated debt securities will
be subordinate and junior in priority of payment to certain of our other indebtedness to the extent described in a prospectus supplement.
The indentures in the forms initially filed as exhibits to the Registration Statement of which this prospectus is a part do not
limit the amount of indebtedness that we may incur, including senior indebtedness or subordinated indebtedness, and do not limit
us from issuing any other debt, including secured debt or unsecured debt.
Units
We may issue units comprised of one or
more of the other securities described in this prospectus or in any prospectus supplement in any combination. Each unit will be
issued so that the holder of the unit is also the holder, with the rights and obligations of a holder, of each security included
in the unit. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held
or transferred separately, at any time or at any time before a specified date or upon the occurrence of a specified event or occurrence.
The applicable prospectus supplement
will describe:
|
·
|
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
·
|
any unit agreement under which the units will be issued;
|
|
·
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and
|
|
·
|
whether the units will be issued in fully registered or global form.
|
PLAN OF DISTRIBUTION
We may sell the securities being offered
pursuant to this prospectus to or through underwriters, through dealers, through agents, or directly to one or more purchasers
or through a combination of these methods. The applicable prospectus supplement will describe the terms of the offering of the
securities, including:
|
·
|
the name or names of any underwriters, if, and if required, any dealers or agents;
|
|
·
|
the purchase price of the securities and the proceeds we will receive from the sale;
|
|
·
|
any underwriting discounts and other items constituting underwriters’ compensation;
|
|
·
|
any discounts or concessions allowed or re-allowed or paid to dealers; and
|
|
·
|
any securities exchange or market on which the securities may be listed or traded.
|
We may distribute the securities from
time to time in one or more transactions at:
|
·
|
a fixed price or prices, which may be changed;
|
|
·
|
market prices prevailing at the time of sale;
|
|
·
|
prices related to such prevailing market prices; or
|
Only underwriters named in the prospectus
supplement are underwriters of the securities offered by the prospectus supplement.
If underwriters are used in an offering,
we will execute an underwriting agreement with such underwriters and will specify the name of each underwriter and the terms of
the transaction (including any underwriting discounts and other terms constituting compensation of the underwriters and any dealers)
in a prospectus supplement. The securities may be offered to the public either through underwriting syndicates represented by managing
underwriters or directly by one or more investment banking firms or others, as designated. If an underwriting syndicate is used,
the managing underwriter(s) will be specified on the cover of the prospectus supplement. If underwriters are used in the sale,
the offered securities will be acquired by the underwriters for their own accounts and may be resold from time to time in one or
more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time
of sale. Any public offering price and any discounts or concessions allowed or re-allowed or paid to dealers may be changed from
time to time. Unless otherwise set forth in the prospectus supplement, the obligations of the underwriters to purchase the offered
securities will be subject to conditions precedent, and the underwriters will be obligated to purchase all of the offered securities,
if any are purchased.
We may grant to the underwriters options
to purchase additional securities to cover over-allotments, if any, at the public offering price, with additional underwriting
commissions or discounts, as may be set forth in a related prospectus supplement. The terms of any over- allotment option will
be set forth in the prospectus supplement for those securities.
If we use a dealer in the sale of the
securities being offered pursuant to this prospectus or any prospectus supplement, we will sell the securities to the dealer, as
principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time
of resale. The names of the dealers and the terms of the transaction will be specified in a prospectus supplement.
We may sell the securities directly or
through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will
describe any commissions we will pay the agent in the prospectus supplement.
We may authorize agents or underwriters
to solicit offers by institutional investors to purchase securities from us at the public offering price set forth in the prospectus
supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will
describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus
supplement.
In connection with the sale of the securities,
underwriters, dealers or agents may receive compensation from us or from purchasers of the securities for whom they act as agents,
in the form of discounts, concessions or commissions. Underwriters may sell the securities to or through dealers, and those dealers
may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from the purchasers
for whom they may act as agents. Underwriters, dealers and agents that participate in the distribution of the securities, and any
institutional investors or others that purchase securities directly for the purpose of resale or distribution, may be deemed to
be underwriters, and any discounts or commissions received by them from us and any profit on the resale of the common stock by
them may be deemed to be underwriting discounts and commissions under the Securities Act. No FINRA member firm may receive compensation
in excess of that allowable under FINRA rules, including Rule 5110, in connection with the offering of the securities.
We may provide agents, underwriters and
other purchasers with indemnification against particular civil liabilities, including liabilities under the Securities Act, or
contribution with respect to payments that the agents, underwriters or other purchasers may make with respect to such liabilities.
Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business.
To facilitate the public offering of
a series of securities, persons participating in the offering may engage in transactions that stabilize, maintain, or otherwise
affect the market price of the securities. This may include over-allotments or short sales of the securities, which involves the
sale by persons participating in the offering of more securities than have been sold to them by us. In addition, those persons
may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing
penalty bids, whereby selling concessions allowed to underwriters or dealers participating in any such offering may be reclaimed
if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may
be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open
market. Such transactions, if commenced, may be discontinued at any time. We make no representation or prediction as to the direction
or magnitude of any effect that the transactions described above, if implemented, may have on the price of our securities.
Unless otherwise specified in the applicable
prospectus supplement, any common stock sold pursuant to a prospectus supplement will be eligible for trading on the NASDAQ Capital
Market. Any underwriters to whom securities are sold by us for public offering and sale may make a market in the securities, but
such underwriters will not be obligated to do so and may discontinue any market making at any time without notice.
In order to comply with the securities
laws of some states, if applicable, the securities offered pursuant to this prospectus will be sold in those states only through
registered or licensed brokers or dealers. In addition, in some states securities may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and
complied with.
LEGAL MATTERS
The validity of the issuance of the securities
offered hereby will be passed upon for us by Anthony L.G., PLLC, West Palm Beach, Florida. As appropriate, legal counsel representing
the underwriters, dealers or agents will be names in the accompanying prospectus supplement and may opine to certain legal matters.
EXPERTS
The
consolidated financial statements incorporated in this prospectus by reference to the Annual Report on Form 10-K for the year ended
March 31, 2018, have been so incorporated in reliance on the report of Crowe LLP, an independent registered public accounting firm,
and have been given on the authority of such firm as experts in accounting and auditing.
LIMITATION ON LIABILITY AND DISCLOSURE
OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our directors and officers are indemnified
by our bylaws against amounts actually and necessarily incurred by them in connection with the defense of any action, suit or proceeding
in which they are a party by reason of being or having been directors or officers of the company. Our amended articles of incorporation
provide that none of our directors or officers shall be personally liable for damages for breach of any fiduciary duty as a director
or officer involving any act or omission of any such director or officer. Insofar as indemnification for liabilities arising under
the Securities Act may be permitted to such directors, officers and controlling persons pursuant to the foregoing provisions, or
otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the
Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other
than the payment by us of expenses incurred or paid by such director, officer or controlling person in the successful defense of
any action, lawsuit or proceeding, is asserted by such director, officer or controlling person in connection with the securities
being registered, we will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court
of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities
Act and will be governed by the final adjudication of such issue.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus and any subsequent prospectus
supplements do not contain all of the information in the registration statement. We have omitted from this prospectus some parts
of the registration statement as permitted by the rules and regulations of the SEC. Statements in this prospectus concerning any
document we have filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to
be comprehensive and are qualified in their entirety by reference to these filings. In addition, we file annual, quarterly and
current reports, proxy statements and other information with the SEC. The SEC also maintains a website that contains reports, proxy
and information statements and other information that we file electronically with the SEC, including us. The SEC’s website
can be found at http://www.sec.gov. In addition, we make available on or through our website copies of these reports as soon as
reasonably practicable after we electronically file or furnished them to the SEC. Our website can be found at http://www.nemauramedical.com.
The content contained in, or that can be accessed through, our website is not a part of this prospectus.
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to "incorporate
by reference" in this prospectus certain information we have filed and will file with the SEC, which means that we may disclose
important information in this prospectus by referring you to the document that contains the information. The information incorporated
by reference is considered to be an integral part of this prospectus, and information that we file later with the SEC will automatically
update and supersede this information. We incorporate by reference the documents listed below:
|
·
|
our Annual Report on Form 10-K for the fiscal year ended March 31, 2018, filed with the SEC on June 12, 2018;
|
|
·
|
our Quarterly Reports on Form 10-Q for the fiscal quarters ended June 30, 2018, September 30, 2018 and December 31, 2018, filed with the SEC on August 9, 2018, November 6, 2018 and February 11, 2019, respectively;
|
|
·
|
our Current Reports on Form 8-K filed with the SEC on June 27, 2018, August 6, 2018, October 19, 2018, December 26, 2018 and February 8, 2019, respectively, and our Current Report on Form 8-K/A filed with the SEC on August 9, 2018;
|
|
·
|
the description of our common stock contained in our Form 8-A12B filed with the SEC on January 19, 2018, including any amendment or report filed for the purpose of updating that description; and
|
|
·
|
all documents filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act on or after the date of this prospectus and before we stop offering the securities covered by this prospectus and any accompanying prospectus supplement.
|
Notwithstanding the foregoing, information
and documents that we elect to furnish, but not file, or have furnished, but not filed, with the SEC in accordance with SEC rules
and regulations is not incorporated into this prospectus and does not constitute a part hereof.
You may access these filings on our website
at www.nemauramedical.com. The information on our website is not incorporated by reference and is not considered part of this prospectus.
Also, upon written or oral request, at no cost we will provide to each person, including any beneficial owner, to whom a
prospectus is delivered, a copy of any or all of the information that has been incorporated by reference in the prospectus but
not delivered with the prospectus. Inquiries should be directed to:
Advanced Technology Innovation Centre
Loughborough University Science and Enterprise
Parks
5 Oakwood Drive
Loughborough, Leicestershire LE11 3QF United
Kingdom
Attn: Chief Financial Officer
+ 44 1509 222912
Shares of Common Stock
Warrants to Purchase up to
Shares of Common Stock

NEMAURA MEDICAL INC.
PROSPECTUS SUPPLEMENT
Sole Book-Running Manager
Kingswood Capital Markets
a division of Benchmark Investments, Inc.
,
2020
Nemaura Medical (NASDAQ:NMRD)
Historical Stock Chart
From Mar 2024 to Apr 2024
Nemaura Medical (NASDAQ:NMRD)
Historical Stock Chart
From Apr 2023 to Apr 2024