UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (date of earliest event reported): February 9, 2021
Heat Biologics, Inc.
(Exact name of registrant as specified in charter)
Delaware
(State or other jurisdiction of incorporation)
| |
001-35994
| 26-2844103
|
(Commission File Number)
| (IRS Employer Identification No.)
|
627 Davis Drive, Suite 400
Morrisville, North Carolina 27560
(Address of principal executive offices and zip code)
(919) 240-7133
(Registrant’s telephone number including area code)
N/A
(Former Name and Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of registrant under any of the following provisions:
| | |
| ¨
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
| | |
| ¨
| Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12)
|
| | |
| ¨
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
| | |
| ¨
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of each class
| Trading Symbol(s)
| Name of each exchange on which registered
|
Common Stock, $0.0002 par value per share
| HTBX
| The Nasdaq Stock Market
(The Nasdaq Capital Market)
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
| |
Emerging growth company ¨
| |
If an emerging growth company, indicate by checkmark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01. Regulation FD Disclosure.
On February 9, 2021, Heat Biologics, Inc. (the “Company”) issued a press release announcing positive interim data from its ongoing fully enrolled Phase 2 trial of HS-110, in combination with Bristol-Myers Squibb’s (BMS) OPDIVO® (nivolumab) in advanced non-small cell lung cancer (NSCLC). A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
In addition, the Company will be making several presentations to investors over the next several weeks. In connection with the presentations, the Company intends to discuss the investor presentation, which is furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information in this Item 7.01, in the press release attached as Exhibit 99.1 and in the investor presentation attached as Exhibit 99.2 to this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01, in the press release attached as Exhibit 99.1 and in the investor presentation attached as Exhibit 99.2 to this Current Report on Form 8-K shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
The press release attached as Exhibit 99.1 and investor presentation attached as Exhibit 99.2 to this Current Report on Form 8-K include “safe harbor” language pursuant to the Private Securities Litigation Reform Act of 1995, as amended, indicating that certain statements contained therein are “forward-looking” rather than historical.
The Company undertakes no duty or obligation to update or revise the information contained in this Current Report on Form 8-K, although it may do so from time to time if its management believes it is appropriate. Any such updating may be made through the filing of other reports or documents with the Securities and Exchange Commission, through press releases or through other public disclosures.
Item 8.01. Other Events.
On February 9, 2021, the Company issued a press release announcing positive interim data from its ongoing fully enrolled Phase 2 trial of HS-110 in combination with Bristol-Myers Squibb’s (BMS) OPDIVO® (nivolumab) in advanced non-small cell lung cancer (NSCLC).
Substantial survival benefit was observed in a cohort of previously treated, checkpoint inhibitor naïve patients with advanced NSCLC (Cohort A, N = 47). A median progression free survival (PFS) of 1.8 months and a median overall survival (OS) of 24.6 months was observed with a median follow-up time of 19.4 months. The one-year survival rate of Cohort A is 61.7%. The median OS data was 12.2 months and the 1-year survival rate was 50.7% in previously treated, advanced NSCLC patients who received nivolumab as a single agent, according to published data of the BMS CheckMate 057 study.
For NSCLC patients who had previously been treated with a checkpoint inhibitor and whose disease had subsequently progressed (Cohort B, N = 68), a median PFS of 2.8 months and median OS of 11.9 months was observed with a median follow-up time of 11.9 months. Published data from other studies reported median OS of 6.8 to 9.0 months for NSCLC patients treated with chemotherapies after PD-(L)1 progression.
As of this data cut, 30% of the patients in Cohort A and 26% of the patients in Cohort B are still alive. HS-110 has a favorable safety profile and has been administered in approximately 200 patients to date. As of this data cut, there have been no treatment-related serious adverse reactions. A review of immune-related adverse events reported in the study raised no safety concerns. The data to date demonstrate that combination of HS-110 and nivolumab is well-tolerated.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
The following exhibits are furnished with this Current Report on Form 8-K.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | |
Dated: February 9, 2021
| HEAT BIOLOGICS, INC.
|
| |
|
|
|
| By:
| /s/ Jeffrey Wolf
|
| Name:
| Jeffrey Wolf
|
| Title:
| Chairman, President and
Chief Executive Officer
|
EXHIBIT 99.1
![[htbx_ex99z1001.jpg]](https://www.sec.gov/Archives/edgar/data/1476963/000155335021000101/htbx_ex99z1001.jpg)
Heat Biologics Announces Positive Interim Survival Data
from Ongoing HS-110 Phase 2 Non-Small Cell Lung Cancer Trial
· Median overall survival of 24.6 months in previously treated checkpoint inhibitor naïve non-small cell lung cancer patients who received HS-110 in combination with nivolumab
Durham, NC – February 9, 2021 – Heat Biologics, Inc. (“Heat”) (NASDAQ: HTBX), a clinical-stage biopharmaceutical company focused on developing first-in-class therapies to modulate the immune system, including multiple oncology product candidates and a novel COVID-19 vaccine, today reported positive interim data of the Company's fully-enrolled Phase 2 trial of HS-110, in combination with Bristol-Myers Squibb’s (BMS) OPDIVO® (nivolumab) in advanced non-small cell lung cancer (NSCLC). HS-110 is an “off-the-shelf” allogeneic cell-based therapy designed to activate patients’ immune system against multiple cancer testis antigens to elicit a diverse and robust immune response against tumor cells.
Substantial survival benefit was observed in a cohort of previously treated, checkpoint inhibitor naïve patients with advanced NSCLC (Cohort A, N = 47). A median progression free survival (PFS) of 1.8 months and a median overall survival (OS) of 24.6 months was observed with a median follow-up time of 19.4 months. The one-year survival rate of Cohort A is 61.7%. The median OS data was 12.2 months and the 1-year survival rate was 50.7% in previously treated, advanced NSCLC patients who received nivolumab as a single agent, according to published data of the BMS CheckMate 057 study1. Our data suggests that addition of HS-110 to a checkpoint inhibitor has the potential to improve survival benefit for checkpoint inhibitor naïve NSCLC patients.
For NSCLC patients who had previously been treated with a checkpoint inhibitor and whose disease had subsequently progressed (Cohort B, N = 68), a median PFS of 2.8 months and median OS of 11.9 months was observed with a median follow-up time of 11.9 months. NSCLC patients whose disease progresses following checkpoint inhibitor therapy have limited treatment options2. Published data from other studies reported median OS of 6.8 to 9.0 months for NSCLC patients treated with chemotherapies after PD-(L)1 progression3,4. Our data of HS-110 in combination with nivolumab in Cohort B suggests potential treatment benefit for NSCLC patients whose disease progressed following treatment with PD-(L)1 therapy.
As of this data cut, 30% of the patients in Cohort A and 26% of the patients in Cohort B are still alive. HS-110 has a favorable safety profile and has been administered in approximately 200 patients to date. As of this data cut, there have been no treatment-related serious adverse reactions. A review of immune-related adverse events reported in the study raised no safety concerns. The data to date demonstrate that combination of HS-110 and nivolumab is well-tolerated.
“We are thrilled to report this latest positive survival data from our Phase 2 trial of HS-110, in combination with Bristol-Myers Squibb’s OPDIVO® (nivolumab) in advanced non-small cell lung cancer demonstrating HS-110’s broad potential for providing multiple treatment options to NSCLC patients,” stated Jeff Wolf, Chief Executive Officer of Heat Biologics. “HS-110 is the lead candidate in our portfolio of therapeutic products and vaccines utilizing Heat’s gp96 technology platform and showcases the broad utility of this platform for NSCLC and potentially other types of cancer. We are currently evaluating possible Phase 3 registration pathways for HS-110 in combination with a checkpoint inhibitor and intend to review these plans with the FDA as well as potential partners.”
About Heat Biologics, Inc.
Heat Biologics is a biopharmaceutical company focused on developing first-in-class therapies to modulate the immune system. The company's gp96 platform is designed to activate immune responses against cancer or infectious diseases. The Company has multiple product candidates in development leveraging the gp96 platform, including HS-110, which has completed enrollment in its Phase 2 trial, and a COVID-19 vaccine program in preclinical development. In addition, Heat is also developing a pipeline of proprietary immunomodulatory antibodies and cell-based therapies, including PTX-35 and HS-130 in Phase 1 clinical trials.
For more information, please visit: www.heatbio.com, and also follow us on Twitter.
Forward Looking Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 on our current expectations and projections about future events. In some cases, forward-looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes," "estimates," and similar expressions. These statements are based upon current beliefs, expectation, and assumptions and include statements such as the potential of the addition of HS-110 to a checkpoint inhibitor to improve survival benefit for checkpoint inhibitor naïve NSCLC patients, the potential treatment benefit of HS-110 in combination with nivolumab for NSCLC patients whose disease progressed following treatment with PD-(L)1 therapy, HS-110’s broad potential for providing multiple treatment options to NSCLC patients, possible Phase 3 registration pathways of HS-110 in combination with a checkpoint inhibitor and intended discussion of these plans with the FDA as well as potential partners and the broad utility of this platform for NSCLC and potentially other types of cancer. These statements are subject to a number of risks and uncertainties, many of which are difficult to predict, including the ability of HS-110 when added to a checkpoint inhibitor to improve survival benefit for checkpoint inhibitor naïve NSCLC patients, the ability of HS-110 in combination with nivolumab to have a potential treatment benefit for NSCLC patients whose disease progressed following treatment with PD-(L)1 therapy, the ability of HS-110’s to provide multiple treatment options to NSCLC patients, the ability of Heat to successfully design a registrational pathway for HS-110, the ability of Heat’s platform to have utility for NSCLC and potentially other types of cancer, Heat's vaccine platform to provide protection against COVID-19, the ability of Heat's therapies to perform as designed,
to demonstrate safety and efficacy, as well as results that are consistent with prior results, the ability to enroll patients and complete the clinical trials on time and achieve desired results and benefits, especially in light of COVID-19, Heat's ability to obtain regulatory approvals for commercialization of product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to Heat's ability to promote or commercialize its product candidates for specific indications, acceptance of its product candidates in the marketplace and the successful development, marketing or sale of products, Heat's ability to maintain its license agreements, the continued maintenance and growth of its patent estate, its ability to establish and maintain collaborations, its ability to obtain or maintain the capital or grants necessary to fund its research and development activities, its ability to continue to maintain its listing on the Nasdaq Capital Market and its ability to retain its key scientists or management personnel, and the other factors described in Heat's most recent annual report on Form 10-K filed with the SEC, and other subsequent filings with the SEC. The information in this release is provided only as of the date of this release, and Heat undertakes no obligation to update any forward-looking statements contained in this release based on new information, future events, or otherwise, except as required by law.
Reference
1 Borghaei et al. Five-Year Outcomes from the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol. 2021 Jan 15.
2 NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer Version 2.2021-Dec 15, 2020.
3 Costantini et al. Efficacy of next treatment received after nivolumab progression in patients with advanced nonsmall cell lung cancer. ERJ Open Res. 2018 Apr 20;4(2):00120-2017.
4 Schvartsman et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017 Oct;112:90-95.
Media and Investor Relations Contact
David Waldman
+1 919 289 4017
investorrelations@heatbio.com
EXHIBIT 99.2

Heat Biologics NASDAQ: HTBX CORPORATE PRESENTATION FEBRUARY 2021
1

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward - looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended . In some cases, these forward - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward - looking statements contain these words . They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, autoimmune diseases and infectious diseases , our planned discovery and development of a COVID - 19 vaccine, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to complete clinical trials and make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us . By their nature, forward - looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated . Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward - looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31 , 2019 , our quarterly reports on Form 10 - Q for the subsequent quarters and our other subsequent filings with the Securities and Exchange Commission (collectively, our “SEC Filings”) . In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this presentation, they may not be predictive of results or developments in future periods . Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law . 2

Snapshot of Heat Biologics (Nasdaq: HTBX) • US - based biopharmaceutical company developing potential first - in - class immunotherapy products • HS - 110, an “off - the - shelf” cell - based immunotherapy product that has the potential to improve PD - (L)1 therapy - Ongoing Phase 2 program demonstrates positive survival data in PD - (L)1 naïve and PD - (L)1 progressor patients • HS - 130 is the first allogeneic, off - the - shelf, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance activation of dormant immune signals - Phase 1 in solid tumors currently enrolling • COVID - 19 vaccine program aims to engineer multiple viral protein regions into our gp96 platform - Target to generate long - term innate and adaptive immune responses; currently in preclinical development • PTX - 35 for T - cell activation and co - stimulation - Phase 1 trial in solid tumors currently enrolling - Preclinical synergy with anti - PD - (L)1 when combined with antigen - driven immunotherapies • Experienced management team with proven track record advancing oncology drugs to the market 3

Product Pipeline 4 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 + CTAs (Cell Therapy) NSCLC HS - 130 OX40L (Cell Therapy) Solid Tumors COVID - 19 Vaccine gp96 + Viral Antigens (Cell Therapy) COVID - 19 PTX - 35 TNFRSF25 (mAb) Solid Tumors

Product Pipeline 5 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 + CTAs (Cell Therapy) NSCLC HS - 130 OX40L (Cell Therapy) Solid Tumors COVID - 19 Vaccine gp96 + Viral Antigens (Cell Therapy) COVID - 19 PTX - 35 TNFRSF25 (mAb) Solid Tumors

HS - 110 Overview • HS - 110 is a Phase 2 cell - based immunotherapy administered in combination with PD - (L)1 therapy to improve clinical outcomes for NSCLC patients - Allogeneic cells with engineered gp96 to present multiple cancer testis antigens - Selectively activate CD8+ “killer” T cells - gp96 can up - regulate T - cell co - stimulation and maturation of antigen presenting cells (APCs) • PD - (L)1 is approved for multiple cancers and combination approaches may enhance survival benefits • Combination of HS - 110 and PD - (L)1 therapy may benefit patients in multiple treatment settings 6 References: Strbo et al 2013. Immunologic research; Strbo et al 2013. Journal of immunology; Yifei Wang et al. 2018. J Immunol; Heat Biologics Internal data; ‡ Shukuya & Carbone 2016. Journal of Thoracic Oncology, Vol.11 No.7: 976 - 988

H eat Biologics’ gp96 Platform Activating the Immune System • Function of heat shock protein gp96: - Potent mucosal adaptive memory inducer - Chaperones antigens (pathogens or tumor) to the immune system - Activates B cell response and drives antigen - specific CD4+ and CD8+ T cel l activation • platform • Leverages gp96’s role as a natural molecular warning system • Engineered to secrete viral antigens bound to gp96 • Off - the - shelf allogeneic cell vaccine • Feasible for large scale manufacturing • Amenable to stockpiling • Broad applications in infectious diseases and cancer • Lead product in Phase 2 trial for NSCLC 7

H eat Biologics’ gp96 Platform Activating the Immune System • Function of heat shock protein gp96: - Potent mucosal adaptive memory inducer - Chaperones antigens (pathogens or tumor) to the immune system - Activates B cell response and drives antigen - specific CD4+ and CD8+ T cel l activation • Key features of Heat’s gp96 platform - Leverages gp96’s role as a natural molecular warning system - Engineered to secrete antigens bound to gp96 - Off - the - shelf allogeneic cell vaccine - Feasible for large scale manufacturing - Amenable to stockpiling - Broad applications in infectious diseases and cancer • Lead product in Phase 2 trial for NSCLC 8

Synergy of HS - 110 with PD - 1 Inhibitor B16F10 Syngeneic Mouse Melanoma Model • B16F10 mouse model is a very aggressive tumor model and is resistant to anti - PD1 treatment • S ynergistic anti - tumor - growth activity of mouse HS - 110 with anti - PD1 was demonstrated as compared to either agent individually • Anti - PD1 or HS - 110 as a single agent did not significantly inhibit tumor growth 9 1 3 5 7 9 11 13 15 17 19 0 100 200 300 Days from Tumor Innoculation T u m o r S i z e ( m m 2 ) Vehicle (PBS) anti-PD1 mHS-110 + anti-PD1 mHS-110 Treatment Initiation

HS - 110 Trial Schema Cohorts A and B Patients receive weekly HS - 110 ( 1 x 10 7 cells) intradermally for 18 weeks via 5 simultaneous injections of 0 . 1 ml each, and biweekly nivolumab 240 mg IV until disease progression or unacceptable toxicity . 10

Δ Heat Biologics Cohort A interim results as of November 2020 data cut. Median follow - up time = 19.45 months. * Borghaei et al 2021. J Clin Oncol Α Please note Heat Biologics’ trial did not have a comparative nivolumab only arm. Published data in green is historical data and not HS - 110 data. Δ Heat Biologics Cohort B interim results as of November 2020 data cut . Median follow - up time = 11.94 months. † C onstatini et al 2018 ERJ Open Research ‡ Schvartsman et al 2017 Lung Cancer. Α Please note Heat Biologics’ trial did not have a chemotherapy only arm. Published data in green is historical data and not HS - 110 data. Cohort A: 2+ line Checkpoint Inhibitor (CPI) naïve patients Cohort B: 2+ line patients that progressed after CPI Clinical Proof - of - Concept Achieved HS - 110 in Combination with Nivolumab • HS - 110 in combination with nivolumab compares favorably with published data Α • Two 2+ line NSCLC settings are under evaluation: - 2+ line Checkpoint Inhibitor (CPI) naïve patients - 2+ line patients that progressed after CPI • Potential registration strategies in combination with a PD - (L)1 - Frontline treatment for NSCLC patients - NSCLC patients who progressed after prior PD - (L)1 treatment 11

Overall Survival (%) Months Cohort A: CPI naïve pts treated by HS - 110 + Nivolumab at > 2L 12 Δ Heat Biologics Cohort A interim results as of November 2020 data cut. Median follow - up time = 19.45 months. * Borghaei et al 2021. J Clin Oncol Α Please note Heat Biologics’ trial did not have a comparative nivolumab only arm. Published data in green is historical data and not HS - 110 data. Overall Survival (OS)
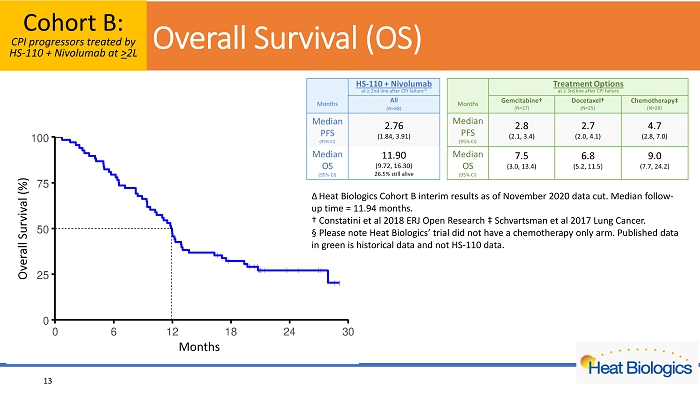
Overall Survival (OS) Cohort B: CPI progressors treated by HS - 110 + Nivolumab at > 2L 13 Overall Survival (%) Months Δ Heat Biologics Cohort B interim results as of November 2020 data cut . Median follow - up time = 11.94 months. † C onstatini et al 2018 ERJ Open Research ‡ Schvartsman et al 2017 Lung Cancer. Α Please note Heat Biologics’ trial did not have a chemotherapy only arm. Published data in green is historical data and not HS - 110 data.

Product Pipeline 14 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 + CTAs (Cell Therapy) NSCLC HS - 130 OX40L (Cell Therapy) Solid Tumors COVID - 19 Vaccine gp96 + Viral Antigens (Cell Therapy) COVID - 19 PTX - 35 TNFRSF25 (mAb) Solid Tumors

HS - 130 Overview • HS - 130 is the first allogeneic, off - the - shelf, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance activation of dormant immune signal - Leverage HS - 110 clinical experience and manufacturing know - how - Addition of OX40L fusion protein to extend and expand T cell memory • Mechanism of action offers broad market potential • Phase 1 in solid tumors currently enrolling • Heat Biologics has worldwide rights 15

Product Pipeline 16 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 + CTAs (Cell Therapy) NSCLC HS - 130 OX40L (Cell Therapy) Solid Tumors COVID - 19 Vaccine gp96 + Viral Antigens (Cell Therapy) COVID - 19 PTX - 35 TNFRSF25 (mAb) Solid Tumors

gp96 Platform for Infectious Disease • gp96 platform demonstrated activity in animal models in multiple infectious diseases - Significant mucosal protection against simian immunodeficiency virus (SIV) in non - human primates - Induction of Zika - specific CD8+ T cells in mouse - No pathological changes in placenta or fetus - Elevation of malaria - specific CD8+ T cells in mouse • Multiple grants received to utilize gp96 platform for various infectious diseases - National Institute of Health (NIH) - Department of Defense (DoD) - Florida Department of Health • Heat Biologics leverages the body of work done to date to develop our COVID - 19 vaccine program 17 Reference: Strbo et al 2013 J Immunol. 2013 March 15; 190(6): 2495 – 2499 Strbo et al 2016 J Immunol May 1, 2016, 196 (1 Supplement) 146.10 Strbo et al 2018 J Immunol May 1, 2018, 200 (1 Supplement) 180.19 Env - spec IgG & IgA Antigen Secreting Cells (per 10 6 PBMCs) Induction of Humoral Immune Response by gp96 SIV Ig Vaccines

Key Differentiation of gp96 Platform • Heat’s gp96 platform - based products evaluated in 250+ patients to date - HS - 110 (Phase 2) demonstrated favorable safety profile and clinical activity in combination with PD - 1 inhibitors for treatment of NSCLC • Potential first - in - class for infectious disease - Based on human cells engine ered to secrete gp96 - bound antigens - Platform designed to be antigen - specific and pathogen - specific - Aim to activate both B and T cell responses at the point of pathogen entry - Preclinical work using gp96 platform includes SIV/ HIV, malaria and zika • Heat’s COVID - 19 vaccine program utilizes the gp96 platform - Leverages natural immune process to induce long - lasting memory responses 18 gp 96 P LATFORM * N O A NTI - V ECTOR I MMUNITY x N O V IRAL A CTIVATION x N O I NTEGRATION OF F OREIGN DNA INTO H OST G ENOME x A CTIVATION O F T C ELLS x A CTIVATION OF B C ELLS x H IGH I MMUNOGENICITY x I NDUCTION OF M UCOSAL I MMUNITY x L ONG - T ERM M EMORY R ESPONSE x *Target product profile for infectious disease

Heat Biologics’ COVID - 19 Vaccine Program • Leverages our proprietary gp96 platform to activate the immune system • Designed to elicit long - lasting immune response against SARS - CoV - 2 virus • We plan to collaborate with companies, researchers, government agencies and funding organizations to accelerate our COVID - 19 vaccine program 19

Heat Biologics COVID - 19 Vaccine Program Summary of Preclinical Data To Date • Heat Biologics’ COVID - 19 vaccine utilizes gp96 technology and incorporate s full length Spike protein • Preclinical data demonstrated polyfunctional, polyepitope Spike protein - specific T cell responses as well as memory responses • IND - enabling activities in progress 20 - C DNA - gp96 - Ig KDEL CH2,CH3 - g 1 TM TM N - - C N - DNA - CoV - 2 - S AD - 100 C ell Line S1 subunit S2 subunit SARS - C oV - 2 Spike protein Endogenous gp96 Secretory form gp96 - Ig - C N - Transfect Transfect H eat Biologic COVID - 19 Vaccine Design Fisher et al 2021. Front. Immunol. https://doi.org/10.3389/fimmu.2020.602254

Product Pipeline 21 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 + CTAs (Cell Therapy) NSCLC HS - 130 OX40L (Cell Therapy) Solid Tumors COVID - 19 Vaccine gp96 + Viral Antigens (Cell Therapy) COVID - 19 PTX - 35 TNFRSF25 (mAb) Solid Tumors

PTX - 35 Overview • Potential first - in - class T cell co - stimulator targeting TNFRSF25, with preferential specificity to expand antigen - specific “memory” CD8+ T cells - Phase 1 trial in solid tumors currently enrolling • Broad market potential - Activity demonstrated in multiple preclinical in vivo colon, lung and breast cancer models • Synergistic combination with immunotherapies including HS - 110 and checkpoint inhibitors • Awarded a $15.2M grant to fund Phase 1 clinical development • Worldwide rights licensed by Heat Biologics 22

Synergy between anti - PD - (L)1 and TNFRSF25 Agonism In Combination with Antigen - driven Immunotherapy TNFRSF25 agonism by PTX - 35 may provide additional help to expand antigen specific CD8+ T - cells Checkpoint blockade reverses immune suppression but sometimes this is not enough Antigen - driven tumor cell killing augmented by PTX - 35 - costimulation Figure adapted from Upal Basu Roy webinar, Lungevity blog, 2019 23

PTX - 35 Demonstrated Anti - Tumor Activities Synergy with Checkpoint Inhibition and Antigen - driven Immunotherapies 24 • Tumor growth inhibition (therapeutic setting) - Antigen is required for synergy between PTX - 35 and anti - PD - 1 inhibitor 0 5 10 15 20 0 50 100 150 200 250 300 350 Days post tumor inoculation Treatment started on Day 4 post tumor inoculation D i a m e t e r ( m m 2 ) Tumor Antigens + mPTX-35 Vehicle (PBS) mPTX-35 anti-PD-1 Tumor Antigens + anti-PD-1 mPTX-35 + anti-PD-1 Tumor Antigens Tumor Antigens + mPTX-35 + anti-PD-1 mPTX-35 refers to the mouse surrogate IgG1-PTX-35 mPTX-35: 1mg/kg, bi-weekly anti-PD-1: 200μg/mouse, every 3 days
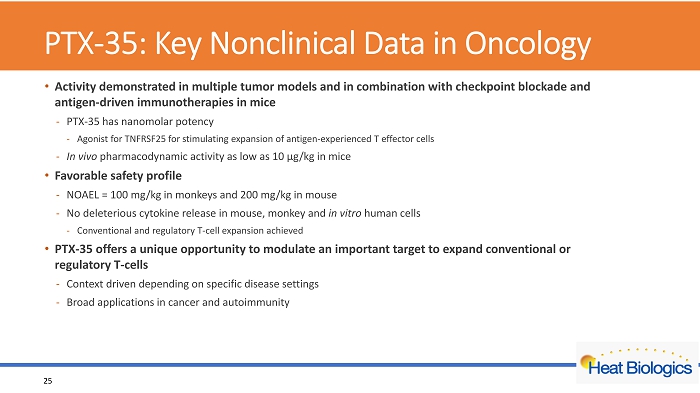
PTX - 35: Key Nonclinical Data in Oncology 25 • Activity demonstrated in multiple tumor models and in combination with checkpoint blockade and antigen - driven immunotherapies in mice - PTX - 35 has nanomolar potency - Agonist for TNFRSF25 for stimulating expansion of antigen - experienced T effector cells - In vivo pharmacodynamic activity as low as 10 µg/kg in mice • Favorable safety profile - NOAEL = 100 mg/kg in monkeys and 200 mg/kg in mouse - No deleterious cytokine release in mouse, monkey and in vitro human cells - Conventional and regulatory T - cell expansion achieved • PTX - 35 offers a unique opportunity to modulate an important target to expand conventional or regulatory T - cells - Context driven depending on specific disease settings - Broad applications in cancer and autoimmunity

Snapshot of Heat Biologics (Nasdaq: HTBX) • US - based biopharmaceutical company developing potential first - in - class immunotherapy products • HS - 110, an “off - the - shelf” cell - based immunotherapy product that has the potential to improve PD - (L)1 therapy - Ongoing Phase 2 program demonstrates positive survival data in PD - (L)1 naïve and PD - (L)1 progressor patients • HS - 130 is the first allogeneic, off - the - shelf, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance activation of dormant immune signals - Phase 1 in solid tumors currently enrolling • COVID - 19 vaccine program aims to engineer multiple viral protein regions into our gp96 platform - Target to generate long - term innate and adaptive immune responses; currently in preclinical development • PTX - 35 for T - cell activation and co - stimulation - Phase 1 trial in solid tumors currently enrolling - Preclinical synergy with anti - PD - (L)1 when combined with antigen - driven immunotherapies • Experienced management team with proven track record advancing oncology drugs to the market 26
This regulatory filing also includes additional resources:
htbx_ex99z2.pdf
Heat Biologics (NASDAQ:HTBX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Heat Biologics (NASDAQ:HTBX)
Historical Stock Chart
From Apr 2023 to Apr 2024