Gilead Gets FDA Approval for Remdesivir for Some Non-Hospitalized Patients
January 21 2022 - 7:16PM
Dow Jones News
By Josh Beckerman
Gilead Sciences Inc.'s remdesivir, commonly used to treat
hospitalized Covid-19 patients, has received Food and Drug
Administration approval for some non-hospitalized patients.
Gilead said the expanded indication allows the drug to be
administered in qualified outpatient settings that can provide
daily intravenous infusions over three consecutive days.
The FDA also expanded the pediatric emergency use authorization
to include non-hospitalized pediatric patients younger than 12 who
are at high risk of disease progression.
Gilead said "while we continue to advance remdesivir to benefit
more patients in multiple settings, we are also advancing our
investigational oral compounds. These are based on the same
antiviral mechanism of action as remdesivir and a Phase 1 trial for
our oral COVID-19 antiviral, GS-5245 is now under way."
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
January 21, 2022 19:01 ET (00:01 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
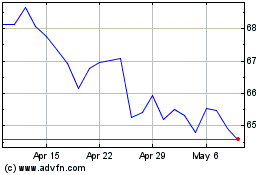
Gilead Sciences (NASDAQ:GILD)
Historical Stock Chart
From Mar 2024 to Apr 2024
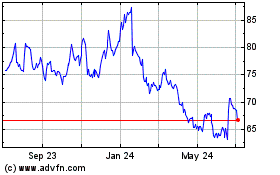
Gilead Sciences (NASDAQ:GILD)
Historical Stock Chart
From Apr 2023 to Apr 2024