Axcella Announces Upcoming Later-Breaker Presentation at The Digital International Liver Congress 2020
August 20 2020 - 7:30AM
Business Wire
Axcella (Nasdaq: AXLA), a
clinical-stage biotechnology company focused on leveraging
endogenous metabolic modulators (EMMs) to pioneer a new approach
for treating complex diseases and improving health, today announced
an upcoming late-breaker poster presentation at The Digital
International Liver Congress™ 2020 (EASL), which is being held
virtually from August 27 to 29. This presentation will discuss key
data from AXA1125-003, a placebo-controlled, randomized, multi-arm
clinical study assessing the impact of AXA1125 and AXA1957 on
safety, tolerability and effects on structures and functions of the
liver in 102 adult subjects with non-alcoholic fatty liver disease
(NAFLD).
Details of the presentation are as follows:
Title: Multifactorial Effects of AXA1125 and AXA1957
Observed on Markers of Metabolism, Inflammation and Fibrosis: A
16-Week Randomized Placebo-Controlled Study in Subjects With
Nonalcoholic Fatty Liver Disease (NAFLD) With and Without Type 2
Diabetes (T2D)
Abstract Number: 4470
Presenter: Stephen A. Harrison, M.D., Medical Director of
Pinnacle Clinical Research in San Antonio, TX, visiting professor
of Hepatology at the University of Oxford, UK
Session: Late-Breaker Poster
Date/Time: August 28, 9:30 a.m. to 7:30 p.m. CEST
About Endogenous Metabolic Modulators (EMMs)
EMMs are a broad family of molecules, including amino acids,
that regulate human metabolism. Axcella is developing a range of
novel product candidates that are comprised of multiple EMMs
engineered in distinct combinations and ratios to simultaneously
impact multiple metabolic pathways to modify the root causes of
various complex diseases and improve health.
About Axcella’s Clinical Studies
Each of the company’s clinical studies to date are or have been
conducted as non-investigational new drug application (IND)
clinical studies under U.S. Food and Drug Administration
regulations and guidance supporting research with food. These
studies evaluate product candidates for safety, tolerability and
effects on the normal structures and functions in humans, including
in individuals with disease. They are not designed or intended to
evaluate a product candidate’s ability to diagnose, cure, mitigate,
treat or prevent a disease. If Axcella decides to further develop a
product candidate as a potential therapeutic, as is the case with
AXA1665 and AXA1125, any subsequent clinical studies will be
conducted under an IND.
Internet Posting of Information
Axcella uses its website, www.axcellahealth.com, as a means of
disclosing material nonpublic information and for complying with
its disclosure obligations under Regulation FD. Such disclosures
will be included on the company’s website in the “Investors and
News” section. Accordingly, investors should monitor such portions
of the company’s website, in addition to following its press
releases, SEC filings and public conference calls and webcasts.
About Axcella
Axcella is a clinical-stage biotechnology company focused on
leveraging endogenous metabolic modulators (EMMs) to pioneer a new
approach for treating complex diseases and improving health. The
company’s product candidates are comprised of EMMs and their
derivatives that are engineered in distinct combinations and ratios
to simultaneously impact multiple biological pathways. Axcella’s
pipeline includes lead therapeutic candidates for non-alcoholic
steatohepatitis (NASH) and the reduction in risk of overt hepatic
encephalopathy (OHE) recurrence. Additional muscle- and
blood-related programs are in earlier-stage development. For more
information, please visit www.axcellahealth.com.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20200820005003/en/
Company/Investor Contact Jason Fredette jfredette@axcellahealth.com 857.320.2236
Media Contact Azeem Zeekrya HDMZ azeem.zeekrya@hdmz.com 312-506-5244
Axcella Health (NASDAQ:AXLA)
Historical Stock Chart
From Mar 2024 to Apr 2024
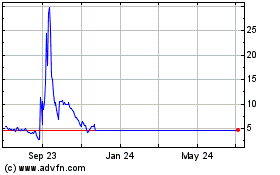
Axcella Health (NASDAQ:AXLA)
Historical Stock Chart
From Apr 2023 to Apr 2024