Advaxis to Host Business Update Conference Call on January 15, 2019
January 08 2019 - 8:00AM
Business Wire
Advaxis, Inc. (NASDAQ: ADXS), a late-stage
biotechnology company focused on the discovery, development and
commercialization of immunotherapy products, announces that the
Company will host a business update call on Tuesday, January 15,
2019.
Advaxis’ senior management will host a conference call to
provide a business update and to discuss fiscal year 2018 financial
results, which will be released on January 10, 2019. The conference
call and live audio webcast will begin at 11:00 a.m. Eastern time
on Tuesday, January 15, 2019.
Conference Call & Webcast
InformationWHEN: Tuesday, January 15, 2019 at 11:00 a.m.
Eastern timeDOMESTIC DIAL-IN: (844) 348-6133INTERNATIONAL DIAL-IN:
(631) 485-4564CONFERENCE ID: 4862946WEBCAST:
ir.advaxis.com/events-presentations
For those unable to participate in the live conference call or
webcast, a digital recording will be available beginning January
15, 2019 two hours after the close of the conference call. To
access the recording, please dial (855) 859-2056 for domestic
callers or (404) 537-3406 for international callers and provide the
operator with the conference ID: 4862946. In addition, an audio
webcast will be archived on the Company’s website for a period of
time at www.advaxis.com.
About Advaxis, Inc.
Advaxis, Inc. is a late-stage biotechnology company focused on
the discovery, development and commercialization of
proprietary Lm-based antigen delivery products. These
immunotherapies are based on a platform technology that utilizes
live attenuated Listeria monocytogenes (Lm) bioengineered to
secrete antigen/adjuvant fusion proteins. These Lm-based
strains are believed to be a significant advancement in
immunotherapy as they integrate multiple functions into a single
immunotherapy and are designed to access and direct antigen
presenting cells to stimulate anti-tumor T cell immunity, activate
the immune system with the equivalent of multiple adjuvants, and
simultaneously reduce tumor protection in the tumor
microenvironment to enable the T cells to eliminate tumors. Advaxis
has four franchises in various stages of clinical and preclinical
development: HPV-associated cancers, neoantigen therapy,
hotspot/cancer antigens and prostate cancer.
To learn more about Advaxis, visit www.advaxis.com and connect
on Twitter, LinkedIn, Facebook and YouTube.
Advaxis Forward-Looking Statement
Some of the statements included in this press release may be
forward-looking statements that involve a number of risks and
uncertainties. For those statements, we claim the protection of the
safe harbor for forward-looking statements contained in the Private
Securities Litigation Reform Act of 1995. The factors that could
cause our actual results to differ materially include: the success
and timing of our clinical trials, including subject accrual; our
ability to avoid any clinical holds; our ability to obtain and
maintain regulatory approval and/or reimbursement of our product
candidates for marketing; our ability to obtain the appropriate
labeling of our products under any regulatory approval; our plans
to develop and commercialize our products; the successful
development and implementation of our sales and marketing
campaigns; the size and growth of the potential markets for our
product candidates and our ability to serve those markets; our
ability to successfully compete in the potential markets for our
product candidates, if commercialized; regulatory developments in
the United States and other countries; the rate and degree of
market acceptance of any of our product candidates; new products,
product candidates or new uses for existing products or
technologies introduced or announced by our competitors and the
timing of these introductions or announcements; market conditions
in the pharmaceutical and biotechnology sectors; our available
cash; the accuracy of our estimates regarding expenses, future
revenues, capital requirements and needs for additional financing;
our ability to obtain additional funding; our ability to obtain and
maintain intellectual property protection for our product
candidates; the success and timing of our preclinical studies
including IND-enabling studies; the timing of our IND submissions,
the ability to get FDA approval for study amendments, the timing of
data read-outs, the ability of our product candidates to
successfully perform in clinical trials; our ability to initiate,
enroll, and execute pilots and clinical trials; our ability to
maintain collaborations; our ability to manufacture and the
performance of third-party manufacturers; the performance of our
clinical research organizations, clinical trial sponsors and
clinical trial investigators; our ability to successfully implement
our strategy; and other risk factors identified from time to time
in our reports filed with the SEC. Any forward-looking
statements set forth in this press release speak only as of the
date of this press release. We do not intend to update any of these
forward-looking statements to reflect events or circumstances that
occur after the date hereof.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20190108005532/en/
Investors:LHA Investor RelationsMiriam Weber Miller,
(212) 838-3777mmiller@lhai.com
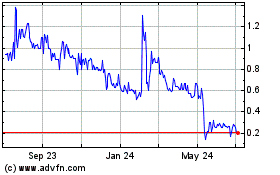
Ayala Pharmaceuticals (QX) (USOTC:ADXS)
Historical Stock Chart
From Mar 2024 to Apr 2024
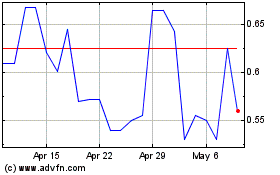
Ayala Pharmaceuticals (QX) (USOTC:ADXS)
Historical Stock Chart
From Apr 2023 to Apr 2024