UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. )
Filed by the Registrant ☒
Filed by a Party other than the Registrant ☐
Check the appropriate box:
|
☐
|
Preliminary Proxy Statement
|
|
☐
|
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
|
|
☐
|
Definitive Proxy Statement
|
|
☒
|
Definitive Additional Materials
|
|
☐
|
Soliciting Material under §240.14a-12
|
ACORDA THERAPEUTICS, INC.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
|
☐
|
Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11.
|
|
|
(1)
|
Title of each class of securities to which transaction applies:
|
|
|
(2)
|
Aggregate number of securities to which transaction applies:
|
|
|
(3)
|
Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
|
|
|
(4)
|
Proposed maximum aggregate value of transaction:
|
|
☐
|
Fee paid previously with preliminary materials.
|
|
☐
|
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing.
|
|
|
(1)
|
Amount Previously Paid:
|
|
|
(2)
|
Form, Schedule or Registration Statement No.:
|

CONTACT:
Tierney Saccavino
(917) 783-0251
tsaccavino@acorda.com
FOR IMMEDIATE RELEASE
Acorda Therapeutics Announces Adjournment of Special Meeting of Stockholders
Scheduled to Reconvene August 28, 2020 at 9:00 a.m. Eastern Time at Company Headquarters to Vote on Proposal One
ARDSLEY, NY – July 31, 2020 – Acorda Therapeutics, Inc. (Nasdaq: ACOR) convened its Special Meeting of Stockholders on July 31, 2020, and stockholders approved proposals to authorize the Company’s Board of Directors to implement a reverse stock split, and to adjourn the meeting to provide stockholders with additional time to vote on Proposal One to approve an increase to the number of authorized shares of common stock. The Special Meeting will be reconvened on Friday, August 28, 2020 at 9:00 a.m. Eastern Time at the Company’s principal executive office located at 420 Saw Mill River Road, Ardsley, NY 10502. The sole matter of business before the reconvened Special Meeting will be Proposal One.
Stockholders have thus far strongly supported Proposal One. At the time the meeting was convened on July 31, 2020, approximately 80% of the shares that had been voted on Proposal One had been voted in its favor. However, the favorable votes were less than the absolute majority of all outstanding shares on the record date needed for approval. Proposals Two and Three were approved at the Special Meeting. Each of these proposals are described in the Company’s definitive proxy statement for the Special Meeting, which was filed with the Securities and Exchange Commission on July 6, 2020.
The record date for determining stockholders eligible to vote on Proposal One at the Special Meeting remains June 29, 2020.
Acorda strongly encourages any eligible stockholder that has not yet voted their shares, or provided voting instructions to their broker or other record holder, to do so promptly. No action is required by any stockholder who has previously delivered a proxy and who does not wish to revoke or change that proxy.
Shares may be voted via the Internet or by telephone. Questions should be addressed to the Company’s proxy solicitor: Innisfree M&A Incorporated TOLL-FREE, at 1-877-717-3929 from 10:00 a.m. – 6:00 p.m. Eastern Time on Monday – Friday, and from 10:00 a.m. – 2:00 p.m. Eastern Time on Saturdays.
Additional Information
This communication may be deemed to be solicitation material in respect of Proposal One. On July 6, 2020, Acorda Therapeutics, Inc. filed a definitive proxy statement with the Securities and Exchange Commission (“Commission”) in connection with the Special Meeting. STOCKHOLDERS ARE URGED TO READ CAREFULLY AND IN THEIR ENTIRETY THE PROXY STATEMENT AND ANY OTHER SOLICITING MATERIALS THAT ARE FILED WITH THE COMMISSION WHEN THEY BECOME AVAILABLE BECAUSE THESE DOCUMENTS CONTAIN IMPORTANT INFORMATION ABOUT THE COMPANY AND THE PROPOSAL TO BE VOTED UPON. The Company’s proxy statement and any other solicitation materials filed by the Company with the Commission can be obtained free of charge at the Commission’s website at www.sec.gov and at the investor relations section of the Company’s website at www.acorda.com. Stockholders may also write to the Company at the following address to request copies of these materials: Acorda Therapeutics, Inc., 420 Saw Mill River Road, Ardsley, NY 10502 Attention: Communications Department. The Company, its directors and certain of its officers and employees will be participants in the solicitation of proxies from stockholders in respect of the Special Meeting. The Company has also engaged Innisfree M&A Incorporated to aid in the solicitation of proxies. Detailed information regarding the identity of participants, and their respective interests in the Company by security holdings or otherwise, are set forth in the definitive proxy statement for the Special Meeting. The contents of the websites referenced above are not deemed to be incorporated by reference into the proxy statement.
About Acorda Therapeutics
Acorda Therapeutics develops therapies to restore function and improve the lives of people with neurological disorders. INBRIJA™ (levodopa inhalation powder) is approved for intermittent treatment of OFF episodes in adults with Parkinson’s disease treated with carbidopa/levodopa. INBRIJA is not to be used by patients who take or have taken a nonselective monoamine oxidase inhibitor such as phenelzine or tranylcypromine within the last two weeks. INBRIJA utilizes Acorda’s innovative ARCUS® pulmonary delivery system, a technology platform designed to deliver medication through inhalation. Acorda also markets the branded AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg.
Forward-Looking Statements
This press release includes forward-looking statements. All statements, other than statements of historical facts, regarding management's expectations, beliefs, goals, plans or prospects should be considered forward-looking. These statements are subject to risks and uncertainties that could cause actual results to differ materially, including: we may not be able to successfully market INBRIJA or any other products under development; the COVID-19 pandemic, including related quarantines and travel restrictions, and the potential for the illness to affect our employees or consultants or those that work for other companies we rely upon, could have a material adverse effect on our business operations or product sales; we may need to raise additional funds to finance our operations, repay outstanding indebtedness or satisfy other obligations, and we may not be able to do so on acceptable terms or at all; risks associated with complex, regulated manufacturing processes for pharmaceuticals, which could affect whether we have sufficient commercial supply of INBRIJA to meet market demand; third party payers (including governmental agencies) may not reimburse for the use of INBRIJA or our other products at acceptable rates or at all and may impose restrictive prior authorization requirements that limit or block prescriptions; competition for INBRIJA, AMPYRA and other products we may develop and market in the future, including increasing competition and accompanying loss of revenues in the U.S. from generic versions of AMPYRA (dalfampridine) following our loss of patent exclusivity; the ability to
realize the benefits anticipated from acquisitions, among other reasons because acquired development programs are generally subject to all the risks inherent in the drug development process and our knowledge of the risks specifically relevant to acquired programs generally improves over time; the risk of unfavorable results from future studies of INBRIJA (levodopa inhalation powder) or from our other research and development programs, or any other acquired or in-licensed programs ; the occurrence of adverse safety events with our products; the outcome (by judgment or settlement) and costs of legal, administrative or regulatory proceedings, investigations or inspections, including, without limitation, collective, representative or class action litigation; failure to protect our intellectual property, to defend against the intellectual property claims of others or to obtain third party intellectual property licenses needed for the commercialization of our products; and failure to comply with regulatory requirements could result in adverse action by regulatory agencies.
These and other risks are described in greater detail in our filings with the Securities and Exchange Commission. We may not actually achieve the goals or plans described in our forward-looking statements, and investors should not place undue reliance on these statements. Forward-looking statements made in this press release are made only as of the date hereof, and we disclaim any intent or obligation to update any forward-looking statements as a result of developments occurring after the date of this press release.
###
Acorda Therapeutics (NASDAQ:ACOR)
Historical Stock Chart
From Mar 2024 to Apr 2024
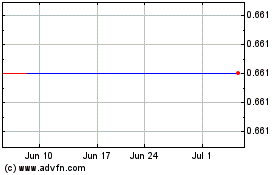
Acorda Therapeutics (NASDAQ:ACOR)
Historical Stock Chart
From Apr 2023 to Apr 2024